Abstract
Background:
Hypertensive disorders of pregnancy (HDP), including gestational hypertension and preeclampsia, are associated with an increased risk of CVD.
Objectives:
To evaluate associations between HDP and long-term CVD and identify the proportion of the association mediated by established CVD risk factors.
Methods:
Parous participants without CVD in the Nurses’ Health Study II (n=60,379) were followed for incident CVD from first birth through 2017. Cox proportional hazards models estimated hazard ratios (HR) and 95% confidence intervals (CI) for the relationship between HDP and CVD, adjusting for potential confounders, including pre-pregnancy body mass index, smoking, and parental history of CVD. To evaluate the proportion of the association jointly accounted for by chronic hypertension, hypercholesterolemia, type 2 diabetes, and changes in BMI, we used the difference method.
Results:
Women with HDP in first pregnancy had a 63% higher rate of CVD (CI:1.37–1.94) compared to women with normotensive pregnancies. This association was mediated by established CVD risk factors (proportion mediated=64%). The increased rate of CVD was higher for preeclampsia (HR=1.72, CI:1.42–2.10) than gestational hypertension (HR=1.41, CI:1.03–1.93). Established CVD risk factors accounted for 57% of the increased rate of CVD for preeclampsia but 84% for gestational hypertension (both p<0.0001).
Conclusions:
Established CVD risk factors arising after pregnancy explained most (84%) of the increased risk of CVD conferred by gestational hypertension and 57% of the risk among women with preeclampsia. Screening for chronic hypertension, hypercholesterolemia, type 2 diabetes, and overweight/obesity after pregnancy may be especially helpful in CVD prevention among women with a history of HDP.
Keywords: Preeclampsia, Pregnancy, Cardiovascular Disease, Cardiovascular Disease Risk Factors
Condensed Abstract:
In this prospective cohort study of 60,379 women followed for a median of 34 years after first birth, hypertensive disorders of pregnancy (HDP), including gestational hypertension and preeclampsia, were associated with cardiovascular disease (CVD) events. This relationship persisted with adjustment for shared risk factors, including pre-pregnancy body mass index, smoking, and family history, and was largely accounted for by the subsequent development of established CVD risk factors. Screening for chronic hypertension, hypercholesterolemia, type 2 diabetes, and overweight/obesity after pregnancy may be especially helpful in CVD prevention among women with a history of HDP.
Short tweet (paper summary):
Established risk factors (chronic HTN, elev chol, T2DM, BMI) accounted for the majority of the 63% higher rate of CVD after a hypertensive pregnancy.
INTRODUCTION
New-onset hypertensive disorders of pregnancy (HDP; gestational hypertension and preeclampsia) occur in approximately 15% of parous women and are consistently associated with a two-fold increased risk of cardiovascular disease (CVD) and premature CVD-related mortality, compared to women with a history of normotensive pregnancy (1,2). However, few studies of incident CVD after hypertensive pregnancies have adjusted for shared pre-pregnancy risk factors, such as body mass index (BMI) (3–5), or had a mean/median follow-up of more than 30 years (4,6–9).
Women with a history of HDP have elevated risks of chronic hypertension, hypercholesterolemia, and type 2 diabetes mellitus (T2DM), and the American Heart Association (AHA) and American College of Cardiology endorse preeclampsia as a risk-enhancing factor for hypercholesterolemia (10,11). However, the extent to which the relationship between HDP and CVD events is mediated by these established CVD risk factors remains less clear. Previous studies have examined the role of individual mediators but no study has examined the joint contribution of chronic hypertension, hypercholesterolemia, T2DM, and BMI in mediating the relationship between HDP and CVD (5,12,13). The AHA has recognized gestational hypertension and preeclampsia as risk factors for CVD and encouraged clinicians to evaluate cardiovascular risk by screening women for these adverse pregnancy outcomes since 2011 (14). Longitudinal investigation of CVD incidence after a hypertensive pregnancy that examines the role of intermediate CVD risk factors is essential to inform screening practices and clinical recommendations for women with a history of HDP.
With up to over 50 years of follow-up after first birth and longitudinal collection of health-related behaviors, reproductive history, and incident CVD risk factors and events, we examined the association between HDP and CVD, controlling for pre-pregnancy confounders, and examined mediation by subsequent CVD risk factors—chronic hypertension, hypercholesterolemia, T2DM, and changes in BMI—in the Nurses’ Health Study II (NHSII).
METHODS
Cohort Description
The NHSII is an ongoing cohort of 116,429 female U.S. registered nurses aged 25–42 at enrollment in 1989. Participants are followed prospectively through biennial questionnaires, which ascertain information on health-related behaviors, medication use, and incident disease. The average active follow-up rate for each questionnaire is over 90%. This study protocol was approved by the Institutional Review Boards of Brigham and Women’s Hospital and the Harvard T.H. Chan School of Public Health (protocol number: 1999P003389).
Hypertensive Disorders of Pregnancy
The 2009 biennial questionnaire captured complete pregnancy history, including adverse pregnancy outcomes and gestation length. Participants self-reported HDP as “pregnancy-related high blood pressure” (gestational hypertension) or “pre-eclampsia/toxemia.” Since this questionnaire did not ascertain chronic hypertension during pregnancy, the analysis focused on new-onset hypertension in pregnancy (gestational hypertension and preeclampsia). A validation study among 462 NHSII participants who self-reported preeclampsia on biennial questionnaires from 1991 to 2001 demonstrated that 89% had medical record evidence of preeclampsia (10). The primary analysis focused on HDP in first pregnancy (normotension [ref], gestational hypertension, preeclampsia) since HDP predominantly occurs during first pregnancies (15) and to avoid potential bias induced by selective fertility (wherein the decision to pursue subsequent pregnancies is dependent on previous pregnancy outcomes) (16). A secondary analysis examined HDP exposure across all lifetime pregnancies, including ever and recurrent HDP (see Supplemental Text for details).
Cardiovascular Disease Events
Participants reported history of physician-diagnosed “myocardial infarction (MI) or angina” or “stroke (cerebrovascular accident) or transient ischemic attack” on the 1989 baseline questionnaire. Subsequent biennial questionnaires captured incident CVD events. Participants or next of kin approved medical record access for validation of incident events. MI events were confirmed using the World Health Organization criteria of symptoms plus either diagnostic electrocardiographic results or elevated cardiac-specific enzymes (17,18). Fatal coronary artery disease (CAD) events were confirmed using hospital records, autopsy, or death certificates among individuals with evidence of prior CAD. Stroke was confirmed using National Survey of Stroke criteria, requiring neurological deficit with rapid or sudden onset that persisted for more than 24 hours or until death (19). Strokes discovered through radiologic imaging alone and cerebrovascular pathology resulting from infection, trauma, or malignancy (“silent” strokes) were not included. CVD events, including CAD (MI or fatal CAD) and stroke, that satisfied these criteria upon medical record review were considered definite. CVD events for which medical records could not be obtained or for which permission was not granted but were confirmed by the participant or a relative were considered probable. Definite and probable CVD cases comprised the outcome of interest.
Covariates
In 1989, participants reported race/ethnicity, current height and weight, weight at age 18, physical activity, parental history of MI before age 60, medical history (including chronic hypertension and diabetes not during pregnancy), and history of smoking, alcohol consumption, and oral contraceptive use. Biennial questionnaires after 1989 updated health-related behaviors and additionally ascertained diet, an expanded personal medical history (including hypercholesterolemia), and the nurse’s parents’ education level, history of stroke before age 60, and ages at and causes of death.
BMI (kg/m2) was calculated from height and weight at age 18 and at each biennial questionnaire. For ages at which weight was not reported, BMI was derived based on reported weights and incorporating somatograms at ages 20, 30, and 40 (10). Diet was summarized in quintiles using the 2010 Alternative Healthy Eating Index dietary quality score, derived from food frequency questionnaires (20). Self-reported weight at age 18, current height, diet, and physical activity have been shown to be reliable in previous validation studies (see Supplemental Methods). Pre-pregnancy information was drawn from the biennial questionnaire immediately before the first pregnancy. Since most first pregnancies (83%) occurred before NHSII enrollment, information about health-related behavior in high school and within varying age ranges from 13 through 42 years reported at baseline was used to assign pre-pregnancy values for women with first births before 1989.
Hypercholesterolemia was defined as self-report of hypercholesterolemia or cholesterol-lowering medication use (collected since 1999). Incident diabetes was confirmed through a supplemental questionnaire, which collected information on symptoms, diagnostic tests, and treatment. Diabetes cases were classified into categories established by the National Diabetes Data Group and American Diabetes Association, as described elsewhere (21–23). Participants reported the year of diagnosis for incident physician-diagnosed conditions within three categories. For chronic hypertension and hypercholesterolemia, the midpoint of each date range was used as the year of diagnosis. For T2DM, year of diagnosis came from the supplemental questionnaire. Medical record validation has previously demonstrated the accuracy of nurse participants’ self-report of chronic hypertension (sensitivity: 94%), hypercholesterolemia (confirmation: 86%), and T2DM (confirmation: ≥98%) (24–26).
Exclusions
Analyses were restricted to women who completed the 2009 biennial questionnaire, which permitted dating of first birth and assignment of HDP exposure (n=76,840). After applying additional exclusion criteria (Figure 1), 60,379 parous women free of CVD before first pregnancy were retained in the primary analysis. For the mediation analysis, we further restricted to 57,974 women at risk for the potential mediators of interest. The analysis of lifetime HDP started follow-up at age 40 (by which point 95% of women had experienced their final pregnancy) and was restricted to 57,137 parous women with their final pregnancy before age 40 and who remained free of CVD at age 40.
Figure 1: Flow diagram for the primary, mediation, and secondary analyses.
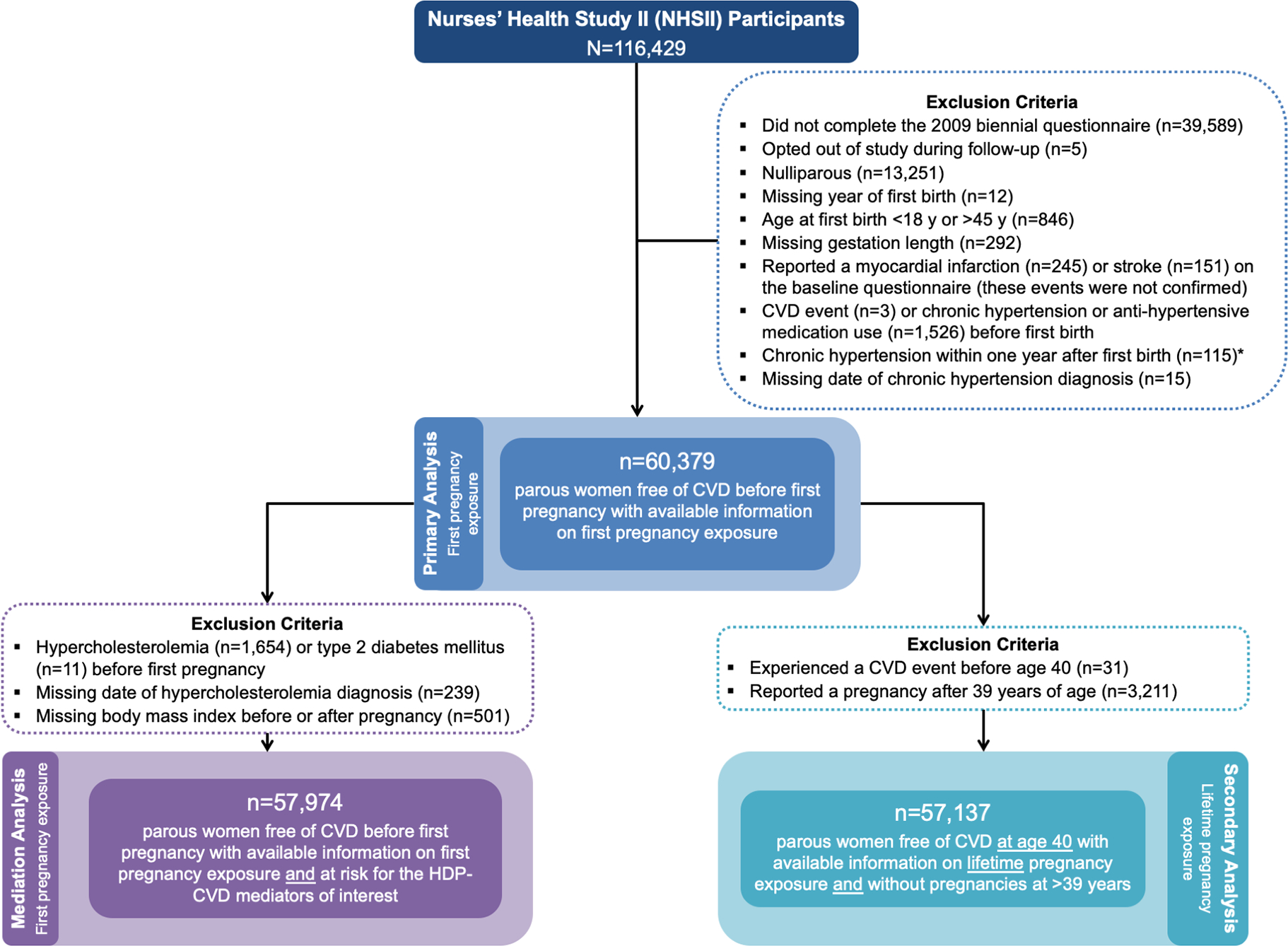
* Women reporting chronic hypertension within one year after first birth (n=115) were excluded to prevent misclassification of exposure (undetected chronic hypertension before pregnancy may be incorrectly captured as incident chronic hypertension directly following pregnancy) and since the exact date of diagnosis was unknown, which prevented definitive determination as to whether the chronic hypertension arose before, during, or after pregnancy.
Statistical Analysis
Characteristics of the analytic population at first pregnancy and at 1989 NHSII enrollment were standardized to the age distribution of the population and summarized by HDP status in first pregnancy (Table 1). Cox proportional hazards models estimated hazard ratios (HR) and 95% confidence intervals (CI) for the association between HDP and CVD. Women contributed person-time to the analysis from first birth until confirmed CVD, death, last returned questionnaire, or 2017 (Figure 2).
Table 1.
Age-standardized characteristics of Nurses’ Health Study II participants by hypertensive status at first pregnancy
| Hypertensive Disorder in First Pregnancy Status | |||
|---|---|---|---|
| Normotensive Pregnancy n=54,756 (90.7%) | Gestational Hypertension n=1,789 (3.0%) | Preeclampsia n=3,834 (6.4%) | |
|
| |||
| Age at first birth, years, mean (SD)* | 27.0 (4.6) | 28.2 (4.9) | 27.0 (4.8) |
| Age at NHSII enrollment (1989), years, mean (SD)* | 35.1 (4.7) | 34.3 (4.7) | 34.5 (4.6) |
| Nulliparous at NHSII enrollment | 16 | 18 | 16 |
| Race/ethnicity | |||
| White | 94 | 95 | 94 |
| Black | 1 | 1 | 1 |
| Hispanic/Latina | 2 | 1 | 2 |
| Asian | 1 | 1 | 1 |
| Other/Multi-racial | 2 | 2 | 2 |
| Participant mother’s years of education >12 years | 32 | 32 | 32 |
| Participant father’s years of education >12 years | 38 | 35 | 38 |
| Strenuous physical activity, at ages 18–22 years | |||
| Never | 28 | 29 | 27 |
| 10–12 months/year | 11 | 11 | 11 |
| Pre-pregnancy body mass index, kg/m2, mean (SD) | 21.8 (3.5) | 23.3 (4.4) | 22.9 (4.2) |
| Pre-pregnancy body mass index ≥30 kg/m2 | 2 | 7 | 6 |
| Pre-pregnancy type 2 diabetes mellitus† | <1 | 0 | 0 |
| Pre-pregnancy hypercholesterolemia | 3 | 3 | 4 |
| Parental history of MI/fatal CAD or stroke before age 60 | 20 | 24 | 24 |
| Alternative Healthy Eating Index (AHEI) score‡ | |||
| Lowest quintile (unhealthy) | 20 | 23 | 22 |
| Highest quintile (healthy) | 19 | 20 | 18 |
| Pre-pregnancy smoking status | |||
| Never | 68 | 70 | 68 |
| Past | 10 | 9 | 10 |
| Current | 22 | 21 | 22 |
| Pre-pregnancy alcohol intake | |||
| None | 26 | 27 | 28 |
| ≤1 drink/week | 37 | 36 | 36 |
| 2–6 drinks/week | 29 | 29 | 28 |
| ≥1 drink/day | 8 | 8 | 8 |
| Pre-pregnancy oral contraceptive use | |||
| Never | 22 | 21 | 19 |
| <2 years | 25 | 26 | 27 |
| 2–<4 years | 22 | 21 | 22 |
| ≥4 years | 31 | 32 | 32 |
| Preterm delivery (<37 weeks) in first birth | 8 | 8 | 16 |
| Final parity§ | |||
| 1 birth | 16 | 21 | 22 |
| 2 births | 49 | 48 | 49 |
| 3 births | 26 | 23 | 23 |
| ≥4 births | 9 | 8 | 7 |
Percentages unless otherwise noted. Values are means (SD) or percentages and are standardized to the age distribution of the study population. Values of polytomous variables may not sum to 100% due to rounding.
Information for above characteristics was missing in n=6,934 for participant mother’s education, n=7,689 for participant father’s education, n=352 for pre-pregnancy smoking, n=351 for strenuous physical activity at 18–22 y, n=255 for pre-pregnancy alcohol intake, n=27,460 for AHEI diet score, and n=974 for pre-pregnancy oral contraceptive use
Value is not age adjusted
n=11 women (0.02%) developed type 2 diabetes mellitus prior to first pregnancy (all of whom had a normotensive first pregnancy)
Self-reported diet based on high school diet information (for women with first birth before 1991) or within 0–3 years before first birth if first birth was in 1991 or later
Final parity based on self-reported lifetime pregnancy history provided on the 2009 biennial questionnaire
Figure 2: Nurses’ Health Study II data collection timeline and analytic follow-up.
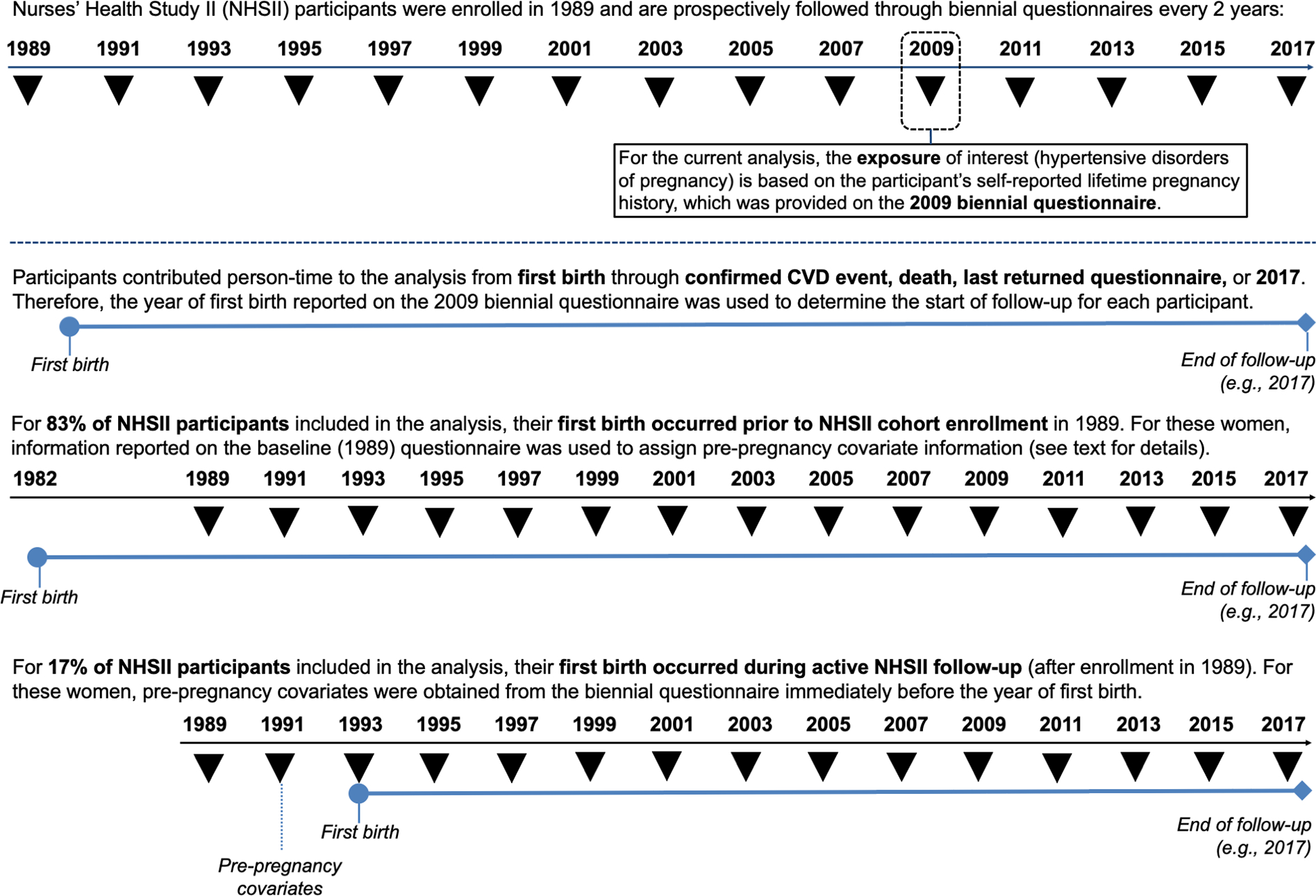
Ascertainment of exposure and assignment of pre-pregnancy covariate information for participants with their first birth before cohort enrollment and for those with their first birth during active follow-up.
We compared the distributions of age at and time to CVD development between HDP groups using log-rank tests. Multivariable models were adjusted for variables identified a priori as pre-pregnancy confounding variables: age at first birth, age at NHSII enrollment, race/ethnicity, parental education, strenuous physical activity at ages 18–22, parental history of CVD before age 60, and pre-pregnancy BMI, alcohol consumption, diet, smoking, oral contraceptive use, and hypercholesterolemia. (As only 11 women developed T2DM prior to first pregnancy, this was not included in multivariable adjustment.) Models for lifetime HDP additionally adjusted for final parity. Missing covariate data were addressed by missing indicators. To evaluate non-linear departures from proportional hazards, we used restricted cubic splines to conduct a non-parametric test of whether the HDP-CVD association was modified by time since first birth (27,28). As no non-linearity was revealed, we tested for linear departures through a likelihood ratio test, comparing nested models with and without multiplicative interaction terms between 1) gestational hypertension and time since first birth and 2) preeclampsia and time since first birth; no linear departures from proportional hazards were found (p=0.12). Multivariable-adjusted cumulative incidence curves for CVD were obtained at the mean and mode values of the continuous and categorical covariates, respectively, using the Breslow estimator.
To evaluate chronic hypertension, hypercholesterolemia, T2DM, and changes in BMI occurring after first pregnancy as potential mediators, we used the difference method, fitting models with and without these established CVD risk factors (29). Chronic hypertension, hypercholesterolemia, and T2DM were treated as time-dependent binary mediators and, once a woman developed a mediator, she was considered to have the mediator through end of follow-up. BMI was treated as a time-varying continuous mediator, updated over follow-up according to self-reported changes in weight. Mediation analysis requires the following assumptions: 1) no unmeasured exposure-outcome confounding, 2) no unmeasured mediator-outcome confounding, 3) no unmeasured exposure-mediator confounding, and 4) no mediator-outcome confounder affected by the exposure (30). To control for confounding of these relationships, we additionally adjusted for updated behaviors over follow-up in mediation models. We tested for the presence of interactions between each mediator and HDP using likelihood ratio tests of nested models with and without the interactions; no exposure-mediator interactions were found (all p-values>0.05). We used the SAS %mediate macro to calculate the proportion of the HDP-CVD jointly mediated by chronic hypertension, T2DM, hypercholesterolemia, and BMI (31,32). Several sensitivity analyses were conducted to examine the robustness of our findings (Supplemental Text). All analyses were conducted using SAS 9.4 (SAS Institute, Inc., Cary, NC).
RESULTS
Approximately 10% of women experienced HDP in their first pregnancy. First births occurred between 1964 and 2008 at an average age of 27.0 ± 4.7 years. Women with HDP in first pregnancy were similar to those with normotensive first pregnancies in demographics and health-related behaviors (Table 1). However, women with HDP were more than three times as likely to have a pre-pregnancy BMI ≥30 kg/m2 and were more likely to have a parent with a premature CVD event.
By the end of follow-up, when participants were a median age of 61 years (interquartile range [IQR]: 57, 64; range: 33–71) with a median follow-up of 34 years since first birth (IQR: 29, 40; range: 2–54), 1,074 (1.8%) women had experienced a first CVD event—560 CAD events (554 MIs and 6 fatal CAD) and 515 strokes (n=1 woman had both an MI and stroke). In fully adjusted models, women with HDP in first pregnancy had a 63% increased rate of CVD, compared to women with a normotensive first pregnancy (CI: 1.4–1.9; Table 2, Model 2). Adjustment for pre-pregnancy BMI, smoking, and parental history of CVD accounted for most of the modest attenuation between age, race/ethnicity, and parental education-adjusted and fully adjusted estimates. When we separately examined gestational hypertension and preeclampsia with CAD and stroke endpoints, there were significant associations between preeclampsia and CAD (HR=2.2, CI: 1.7, 2.8) and gestational hypertension and stroke (HR: 1.6, CI: 1.0–2.4) (Central Illustration). Further adjustment for updated smoking, diet, alcohol intake, physical activity, and oral contraceptive use after pregnancy, resulted in slight attenuation but did not alter conclusions (data not shown). Women with HDP in first pregnancy exhibited elevated rates of CVD, relative to women with normotensive first pregnancies, regardless of gestation length (Table 3).
Table 2.
Hypertensive Disorders in First Pregnancy and Cardiovascular Disease
| Hypertensive Disorder in First Pregnancy Status | ||||
|---|---|---|---|---|
| Normotensive Pregnancy n=54,756 (90.7%) | Gestational Hypertension n=1,789 (3.0%) | Preeclampsia n=3,834 (6.4%) | Hypertensive Disorders of Pregnancy n=5,623 (9.3%) | |
|
|
||||
| CVD (CAD or Stroke) | ||||
| Cases/Person-Years | 920/1,885,474 | 41/57,900 | 113/128,840 | 154/186,740 |
| Excess cases per 100,000 person-years | --- | 22 | 39 | 34 |
| Median age at event (IQR), years* | 56 (50, 62) | 58 (52, 63) | 55 (47, 60) | 56 (48, 62) |
| Median time to event (IQR), years* | 35 (29, 40) | 32 (27, 37) | 34 (29, 39) | 33 (28, 38) |
| Model 1 | 1.00 (ref) | 1.55 (1.13, 2.12) | 1.87 (1.54, 2.28) | 1.78 (1.50, 2.11) |
| Model 2 | 1.00 (ref) | 1.41 (1.03, 1.93) | 1.72 (1.42, 2.10) | 1.63 (1.37, 1.94) |
| CAD | ||||
| Cases/Person-Years | 467/1,831,185 | 19/56,130 | 74/125,080 | 93/181,210 |
| Excess cases per 100,000 person-years | --- | 8 | 34 | 26 |
| Median age at event (IQR), years* | 56 (51, 61) | 61 (55, 65) | 55 (47, 61) | 57 (50, 62) |
| Median time to event (IQR), years* | 34 (28, 39) | 31 (26, 36) | 33 (28, 38) | 32 (27, 37) |
| Model 1 | 1.00 (ref) | 1.42 (0.90, 2.25) | 2.42 (1.90, 3.10) | 2.12 (1.70, 2.65) |
| Model 2 | 1.00 (ref) | 1.27 (0.80, 2.02) | 2.21 (1.73, 2.84) | 1.93 (1.54, 2.41) |
| Stroke | ||||
| Cases/Person-Years | 454/1,831,172 | 22/56,133 | 39/125,045 | 61/181,178 |
| Excess cases per 100,000 person-years | --- | 14 | 6 | 9 |
| Median age at event (IQR), years† | 57 (50, 62) | 56 (52, 62) | 54 (48, 60) | 55 (48, 61) |
| Median time to event (IQR), years† | 34 (28, 39) | 31 (26, 36) | 33 (28, 38) | 32 (27, 37) |
| Model 1 | 1.00 (ref) | 1.67 (1.09, 2.56) | 1.29 (0.93, 1.80) | 1.41 (1.08, 1.84) |
| Model 2 | 1.00 (ref) | 1.56 (1.01, 2.40) | 1.21 (0.87, 1.68) | 1.32 (1.00, 1.73)‡ |
CAD: coronary artery disease; CVD: cardiovascular disease; IQR: interquartile range. Values are HR and 95% CI unless otherwise indicated. Excess cases (rate differences) were calculated by subtracting the incidence (cases/person-years) in the unexposed from the incidence in the exposed.
Model 1 is adjusted for age at first birth (years), age at NHSII enrollment (years), race/ethnicity (Black, Hispanic/Latina, Asian, White [ref], other/multi-race), and parental education (<9, 9–11, 12, 13–15, ≥16 years [ref]).
Model 2 is additionally adjusted for physical activity at ages 18–22 (never, 1–3 [ref], 4–6, 7–9, 10–12 mo/yr), pre-pregnancy smoking (never [ref], past, current), pre-pregnancy BMI (<18.5, 18.5–24.9 [ref], 25–29.9, ≥30 kg/m2), pre-pregnancy alcohol consumption (none [ref], ≤1 drink/week, 2–6 drinks/week, ≥1 drink/day), pre-pregnancy Alternative Healthy Eating Index (AHEI) score (quintiles with the fifth quintile [ref] representing the healthiest diet category), pre-pregnancy oral contraceptive use (never [ref], <2, 2–<4, ≥4 years), pre-pregnancy hypercholesterolemia (no [ref], yes), and parental history of CAD and/or stroke before age 60 (CAD only for CAD model, stroke only for stroke model, CAD or stroke for CVD models; no [ref], yes)
P-value <0.0001 from a global test of the difference in the distribution of age at/time to CVD event between hypertensive disorder in first pregnancy exposure groups
P-value <0.05 from a global test of the difference in the distribution of age at/time to CVD event between hypertensive disorder in first pregnancy exposure groups
The hazard ratio for the association between stroke and hypertensive disorders of pregnancy was statistically significant (p=0.0467; CI: 1.004–1.726)
Central Illustration: Differential associations by hypertensive disorder of pregnancy and cardiovascular disease subtypes.
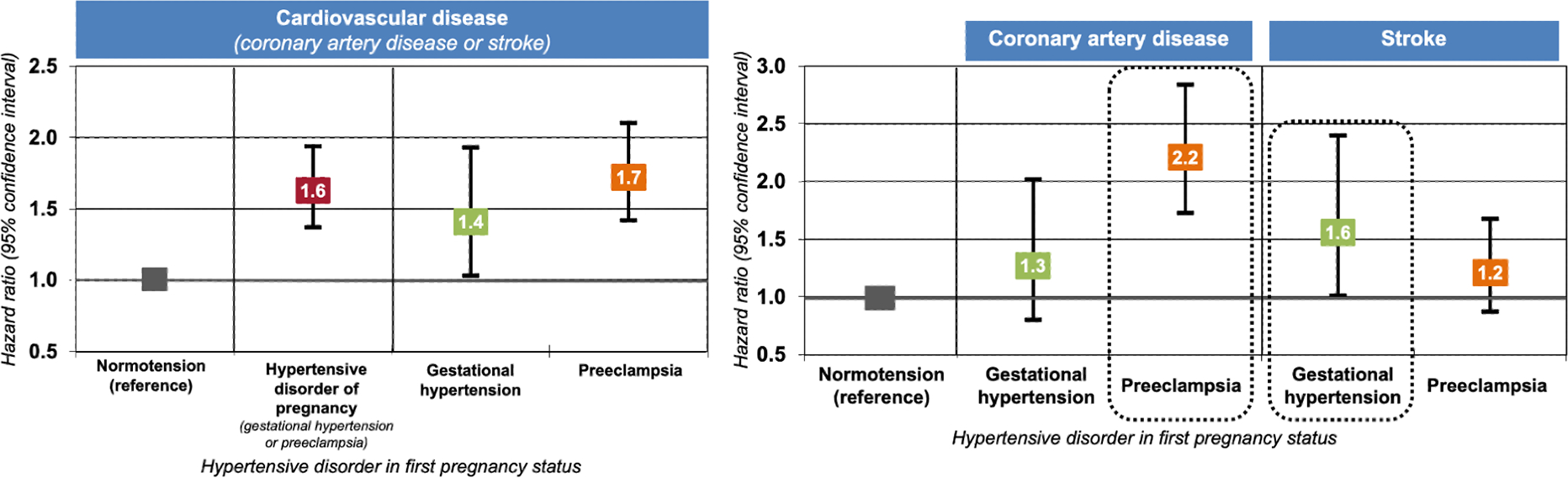
Hazard ratios and 95% confidence intervals (CI) were obtained from fully adjusted Cox proportional hazards models (Table 2, Model 2). The overall association between having a hypertensive disorder in first pregnancy and long-term maternal cardiovascular disease appeared to be driven by underlying associations between preeclampsia and coronary artery disease and between gestational hypertension and stroke.
Table 3.
Hypertensive Disorders in First Pregnancy and Cardiovascular Disease by Gestation Length
| Hypertensive Disorder in Pregnancy & Gestational Length at Delivery Status | |||||||
|---|---|---|---|---|---|---|---|
| Term (≥37 weeks) | Preterm (<37 weeks) | ||||||
|
|
|
||||||
| Normotensive Pregnancy 50,404 (83.5%) | Gestational Hypertension 1,643 (2.7%) | Preeclampsia 3,216 (5.3%) | HDP 4,859 (8.1%) | Gestational Hypertension 146 (0.2%) | Preeclampsia 618 (1.0%) | HDP 764 (1.3%) | |
|
|
|
||||||
| CVD (CAD or Stroke) | |||||||
| Cases/Person-years | 820/1,737,169 | 40/53,413 | 95/109,544 | 135/162,957 | 1/4,487 | 18/19,296 | 19/23,783 |
| Excess cases per 100,000 person-years | --- | 28 | 40 | 36 | *** | 46 | 33 |
| Model 1 | 1.00 (ref) | 1.69 (1.23, 2.33) | 1.89 (1.53, 2.34) | 1.83 (1.52, 2.19) | *** | 2.21 (1.39, 3.53) | 1.88 (1.19, 2.96) |
| Model 2 | 1.00 (ref) | 1.53 (1.11, 2.11) | 1.74 (1.40, 2.15) | 1.67 (1.39, 2.01) | *** | 2.04 (1.27, 3.25) | 1.74 (1.10, 2.75) |
CAD: coronary artery disease; CVD: cardiovascular disease; HDP: hypertensive disorders of pregnancy; PY: person-years. Hazard ratios and corresponding 95% confidence intervals are provided for Models 1 and 2. Excess cases (rate differences) were calculated by subtracting the incidence (cases/person-years) in the unexposed from the incidence in the exposed. Tests for effect modification by preterm delivery in the fully adjusted model (Model 2) through likelihood ratio tests, comparing models with and without multiplicative interaction terms between HDP and preterm delivery: p=0.31 (for the three-category HDP exposure) and p=0.87 (for the binary HDP exposure).
Model 1 is adjusted for age at first birth (years), age at NHSII enrollment (years), race/ethnicity (Black, Hispanic/Latina, Asian, White [ref], other/multi-race), and parental education (<9, 9–11, 12, 13–15, ≥16 years [ref]).
Model 2 is additionally adjusted for physical activity at ages 18–22 (never, 1–3 [ref], 4–6, 7–9, 10–12 mo/yr), pre-pregnancy smoking (never [ref], past, current), pre-pregnancy BMI (<18.5, 18.5–24.9 [ref], 25–29.9, ≥30 kg/m2), pre-pregnancy alcohol consumption (none [ref], ≤1 drink/week, 2–6 drink/week, ≥1 drinks/day), pre-pregnancy Alternative Healthy Eating Index (AHEI) score (quintiles with the fifth quintile [ref] representing the healthiest diet category), pre-pregnancy oral contraceptive use (never [ref], <2, 2–<4, ≥4 years), pre-pregnancy hypercholesterolemia (no [ref], yes), and parental history of CAD and/or stroke before age 60 (CAD only for CAD model, stroke only for stroke model, CAD or stroke for CVD models; no [ref], yes).
These results are drawn from 6 different models—one model with hypertensive disorders in pregnancy and normotensive first pregnancies split out by term and preterm delivery (i.e. 4 exposure categories) and another model with preeclamptic, gestational hypertensive, and normotensive first pregnancies split out by term and preterm deliveries (i.e. 6 exposure categories); normotensive term served as the reference group. Results for women with normotensive preterm (n=4,352; 7.2%) are not shown above but were obtained within the same models: fully adjusted HR=1.41 (CI: 1.14–1.73).
Results not shown given only 1 CVD event among women with gestational hypertension and preterm delivery in first birth.
Women with HDP in first pregnancy also developed CVD slightly younger and sooner after their first birth than women with normotensive first pregnancies (Table 2). The increased rate of CVD among women with HDP in first pregnancy became statistically significant between 40–49 years of age, ranging between a 41–81% increased rate through age 69 (Supplemental Table 1).
Women with HDP exhibited a higher cumulative incidence of CVD that emerged approximately 10 years after first birth for women with preeclampsia and 30 years after first birth for women with gestational hypertension (Figure 3).
Figure 3: Multivariable-adjusted cumulative incidence of cardiovascular disease.
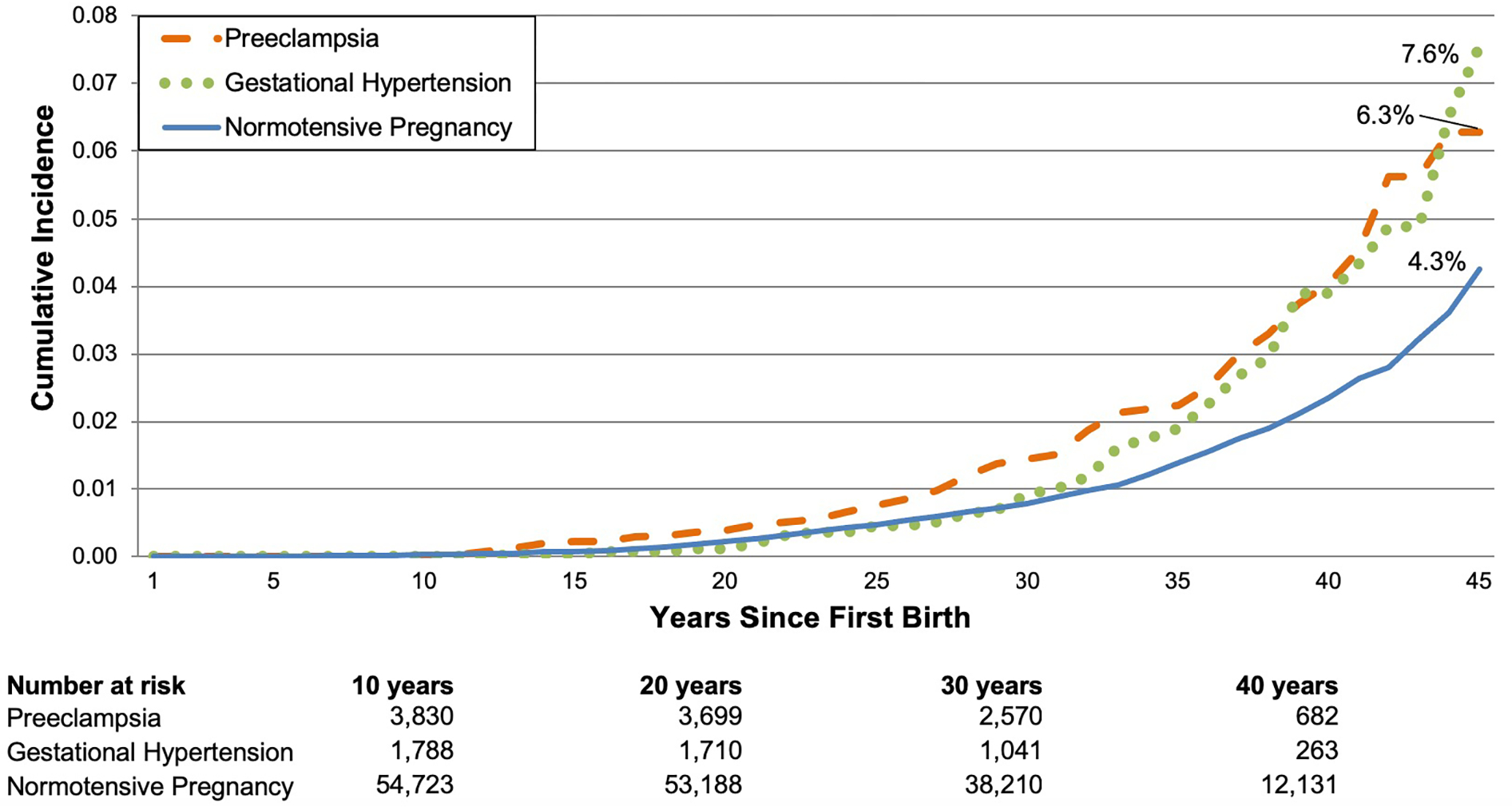
Curves were obtained at the mean and mode values of the following continuous and categorical covariates, respectively: age at first birth, age at NHSII enrollment, race/ethnicity, parental education, physical activity at ages 18–22, parental history of CVD <60, and pre-pregnancy smoking, body mass index, alcohol consumption, Alternative Healthy Eating Index score, oral contraceptive use, and hypercholesterolemia.
Twelve percent of women experienced at least one lifetime pregnancy characterized by HDP and 2.2% (n=1,265) experienced recurrent HDP (Table 4). Ever experiencing HDP was associated with a 63% higher rate of CVD (CI: 1.4–1.9) compared with women without HDP (Table 4, Model 3). Women with one pregnancy complicated by HDP had a 48% higher rate of CVD (CI: 1.2–1.8) while women with two or more HDP pregnancies had a 2.3-fold higher rate (CI: 1.7–3.1) compared to women with all normotensive pregnancies. Women with a history of one or more HDP pregnancies exhibited higher rates of CVD regardless of which pregnancies were complicated by HDP, although the highest relative risk was observed among women with recurrent HDP that impacted their first and then a second or later pregnancy (HR=2.5; CI: 1.8–3.3).
Table 4.
Ever and Recurrent Hypertensive Disorders of Pregnancy and Cardiovascular Disease
| Pregnancy History at Age 40 | N (%) | Cases/Person-years | Hazard ratio (95% CI) |
|||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||||
|
| ||||||
| Ever HDP* | 6,639 (11.6) | 180/137,586 | 1.78 (1.52, 2.10) | 1.63 (1.38, 1.92) | 1.63 (1.39, 1.92) | |
|
| ||||||
| Number of HDP pregnancies | ||||||
|
| ||||||
| 0 | 50,498 (88.4) | 824/1,084,133 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | |
| 1 | 5,374 (9.4) | 132/111,825 | 1.61 (1.34, 1.94) | 1.48 (1.23, 1.79) | 1.48 (1.23, 1.78) | |
| 2+ | 1,265 (2.2) | 48/25,761 | 2.53 (1.89, 3.39) | 2.26 (1.68, 3.04) | 2.28 (1.70, 3.07) | |
|
| ||||||
| First Pregnancy | Second or Later Pregnancies | |||||
|
| ||||||
| Normotensive | All Normotensive | 42,397 (74.2) | 670/906,026 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Normotensive | No further pregnancies | 8,101 (14.2) | 154/178,107 | 1.25 (1.04, 1.49) | 1.19 (0.99, 1.42) | 1.15 (0.96, 1.39) |
| Normotensive | Any HDP | 1,336 (2.3) | 38/28,147 | 1.80 (1.30, 2.50) | 1.65 (1.19, 2.29) | 1.67 (1.20, 2.32) |
| HDP | All Normotensive | 2,979 (5.2) | 63/61,140 | 1.46 (1.13, 1.89) | 1.36 (1.05, 1.77) | 1.36 (1.05, 1.76) |
| HDP | No further pregnancies | 1,178 (2.1) | 33/24,957 | 2.01 (1.41, 2.85) | 1.76 (1.23, 2.51) | 1.71 (1.20, 2.45) |
| HDP | Any HDP | 1,146 (2.0) | 46/23,342 | 2.78 (2.06, 3.74) | 2.45 (1.81, 3.32) | 2.45 (1.81, 3.31) |
CI: confidence interval; HDP: hypertensive disorder in pregnancy. This analysis of lifetime HDP started follow-up at age 40 and was restricted to 57,137 parous women, after excluding women who developed CVD before age 40 (n=31) and those with any pregnancies after age 39 (n=3,211). The three-category exposure variable for number of HDP pregnancies was derived from a sum of the HDP pregnancies across all lifetime pregnancies: 1) 0 HDP pregnancies, 2) 1 HDP pregnancy, 3) 2+ HDP pregnancies. The six-category exposure variable classified participants based on HDP status in first pregnancy and HDP status in second or later pregnancies: 1) Normotension in all pregnancies, 2) Normotension in first pregnancy, no additional pregnancies, 3) Normotension in first pregnancy, HDP (preeclampsia or gestational hypertension) in at least one later pregnancy, 4) HDP in first pregnancy, normotension in all subsequent pregnancies, 5) HDP in first pregnancy, no additional pregnancies, and 6) HDP in first pregnancy with recurrence in at least one later pregnancy. Women free from the endpoint at age 40 and with no births after age 39 contributed person-time to the models from age 40 on.
Model 1 is adjusted for age at first birth (years), age at NHSII enrollment (years), race/ethnicity (Black, Hispanic/Latina, Asian, White [ref], other/multi-race), and parental education (<9, 9–11, 12, 13–15, ≥16 years [ref]).
Model 2 is additionally adjusted for physical activity at ages 18–22 (never, 1–3 [ref], 4–6, 7–9, 10–12 mo/yr), pre-pregnancy smoking (never [ref], past, current), pre-pregnancy BMI (<18.5, 18.5–24.9 [ref], 25–29.9, ≥30 kg/m2), pre-pregnancy alcohol consumption (none [ref], ≤1 drink/week, 2–6 drinks/week, ≥1 drink/day), pre-pregnancy Alternative Healthy Eating Index (AHEI) score (quintiles with the fifth quintile [ref] representing the healthiest diet category), pre-pregnancy oral contraceptive use (never [ref], <2, 2–<4, ≥4 years), pre-pregnancy hypercholesterolemia (no [ref], yes), and parental history of CAD and/or stroke before age 60 (CAD only for CAD model, stroke only for stroke model, CAD or stroke for CVD models; no [ref], yes).
Model 3 is additionally adjusted for final parity (1–2 [ref], 3, 4+ births).
The reference group for the ever HDP exposure is no HDP (i.e., all normotensive pregnancies). Sample size, case count, and the hazard ratio and corresponding confidence interval for this group are equal to those shown under “Number of HDP Pregnancies” for 0 HDP pregnancies.
Comparing models for HDP and CVD with and without CVD risk factors developing after pregnancy, 63.8% (CI: 38.6–83.2; p<0.0001) of the association between HDP in first pregnancy and CVD was jointly mediated by the subsequent development of chronic hypertension, hypercholesterolemia, T2DM, and changes in BMI (Table 5). The proportion mediated (PM) by these factors was higher for gestational hypertension (PM=83.8%) than preeclampsia (PM=57.3%). All CVD risk factors contributed to mediation; however, chronic hypertension accounted for the largest individual proportion followed by changes in BMI, hypercholesterolemia, and T2DM. Chronic hypertension individually mediated 81% and 48% of the associations between gestational hypertension and preeclampsia with CVD, respectively. Among women with CVD events, 95% of those with gestational hypertension (n=39 of 41) and 89% of those with preeclampsia (n=101 of 113) developed chronic hypertension between first pregnancy and their CVD event.
Table 5.
Mediation of the HDP-CVD relationship by chronic hypertension, hypercholesterolemia, type 2 diabetes, and BMI changes
| Hypertensive Disorder in First Pregnancy Status | ||||
|---|---|---|---|---|
| Normotensive Pregnancy n=52,668 (90.9%) | Gestational Hypertension n=1,675 (2.9%) | Preeclampsia n=3,631 (6.3%) | Hypertensive Disorders of Pregnancy n=5,306 (9.2%) | |
|
|
||||
| Cases/Person-Years | 752/1,676,801 | 37/50,326 | 95/112,328 | 132/162,654 |
|
| ||||
| Without Mediators (Total Effect) | 1.00 (ref) | 1.52 (1.09, 2.12) | 1.73 (1.24, 2.42) | 1.67 (1.38, 2.01) |
| With Mediators (Direct Effect)* | 1.00 (ref) | 1.07 (0.77, 1.49) | 1.26 (0.90, 1.77) | 1.20 (1.00, 1.45) |
| Proportion mediated (95% CI) † | Ref | 83.8% (3.6, 99.9) | 57.3% (24.2, 84.9) | 63.8% (38.6, 83.2) |
| Individually mediated by: | ||||
| Chronic hypertension | Ref | 80.7% (6.1, 99.6) | 48.0% (21.9, 75.3) | 56.2% (35.2, 75.1) |
| Hypercholesterolemia | Ref | 19.2% (8.0, 39.2) | 15.3% (8.0, 27.4) | 16.2% (10.9, 23.4) |
| Type 2 diabetes mellitus | Ref | 13.1% (5.3, 29.0) | 9.1% (4.4, 18.0) | 10.1% (6.3, 15.7) |
| Changes in BMI | Ref | 30.4% (12.1, 58.1) | 23.0% (11.8, 40.2) | 24.8% (16.3, 35.8) |
BMI: body mass index; CVD: cardiovascular disease; HDP: hypertensive disorders of pregnancy. This mediation analysis was restricted to 57,974 parous women, after excluding women who had hypercholesterolemia (n=1,654) or type 2 diabetes mellitus (n=11) before first pregnancy, were missing date of hypercholesterolemia diagnosis (n=239), or missing body mass index (BMI; n=501). Hazard ratios and corresponding 95% confidence intervals are provided for the total and direct effects. Models are adjusted for age at first birth (years), age at NHSII enrollment (years), race/ethnicity (Black, Hispanic/Latina, Asian, White [ref], other/multi-race), parental education (<9, 9–11, 12, 13–15, ≥16 years [ref]), and the following pre-pregnancy variables updated over follow-up: physical activity (never, 1–3 (ref), 4–6, 7–9, 10–12 mo/yr), smoking (never (ref), past, current), BMI (<18.5, 18.5–24.9 (ref), 25–29.9, ≥30 kg/m2), alcohol consumption (none (ref), ≤1 drink/week, 2–6 drink/week, ≥1 drinks/day), Alternative Healthy Eating Index (AHEI) score (quintiles with the fifth quintile (ref) representing the healthiest diet category), oral contraceptive use (never (ref), <2, 2–<4, ≥4 years), hypercholesterolemia (no [ref], yes), and parental history of coronary artery disease (CAD) and/or stroke before age 60 (CAD only for CAD model, stroke only for stroke model, CAD or stroke for CVD models; no [ref], yes)
Direct effect obtained from a model including chronic hypertension, hypercholesterolemia, type 2 diabetes mellitus, and body mass index
Proportion of the association jointly mediated by the development of chronic hypertension, hypercholesterolemia, type 2 diabetes mellitus, and changes in body mass index after first pregnancy; p <0.0001 for all proportion mediated statistics. Additional inclusion of breastfeeding in these models did not increase the proportion mediated (data not shown).
Note: individual proportion mediated statistics may not sum to the joint proportion mediated statistic and may exceed 100% due to shared pathways, as an individual may develop multiple CVD risk factors between the HDP and CVD event and the presence of one risk factor may affect another.
Sensitivity Analyses
Sensitivity analyses to address the potential for outcome misclassification, immortal person-time bias, survival bias, and unmeasured confounding, and to examine an alternative method for handling missing data (multiple imputation by chained equations) did not materially alter results (Supplemental Text; Supplemental Tables 2 and 3).
DISCUSSION
Women with HDP in first pregnancy had a 63% higher rate of future CVD events compared to women with normotension, even after accounting for important shared risk factors, including pre-pregnancy BMI, smoking, and parental history of CVD. This elevated rate was largely explained by subsequent development of established CVD risk factors—chronic hypertension, hypercholesterolemia, T2DM, and changes in BMI—in the years after a hypertensive first pregnancy. The HDP-CVD relationship appeared to be driven by associations between preeclampsia and CAD and between gestational hypertension and stroke.
This study deepens our understanding of the relationship between HDP and long-term maternal CVD and highlights targets for potential risk reduction. Previous studies, largely unadjusted for pre-pregnancy cardiometabolic confounding factors, suggested an increased CVD risk of 1.7–2.7-fold in women with a history of HDP, depending on the specific exposure and outcome of interest, length of follow-up, and degree of adjustment (1). The longitudinal nature of the NHSII permitted thorough adjustment for pre-pregnancy risk factors, yet inclusion of pre-pregnancy demographic and behavioral variables only slightly attenuated hazard ratios for the relationship between HDP and CVD. We found that 64% of the increased CVD risk associated with HDP was explained by subsequent development of chronic hypertension, hypercholesterolemia, T2DM, and changes in BMI. The fact that chronic hypertension accounted for much of this association is consistent with previous mediation analyses (5,12,13). For example, an analysis among 220,024 women with a mean age of 57 years at baseline and followed for a median of 7 years in the UK Biobank found that chronic hypertension accounted for 64% of the increased risk of CAD among women with a history of HDP (13). The large degree of mediation observed in the HDP-CVD relationship may also partially explain why including HDP in an established CVD risk score does not appear to improve CVD prediction in women 40 years of age and older (33).
NHSII participants were followed for a median of 34 years after first birth and provided a complete reproductive history, which allowed examination of CVD risk associated with HDP exposure in any pregnancy. While history of HDP in any pregnancy increased a woman’s risk of CVD relative to women without HDP, recurrent HDP in two or more pregnancies was associated with a more than doubling of CVD risk—findings consistent with those from the Swedish Medical Birth Register (34).
Much of the previous literature has focused on preeclampsia or examined the hypertensive disorders jointly; with over 60,000 parous women, we were able to examine gestational hypertension and preeclampsia separately, yielding informative differential relationships. Women with a history of preeclampsia in first birth had an increased rate of CAD but not stroke whereas the opposite was true for women with a history of gestational hypertension, which conferred an increased rate of stroke but not CAD. This finding is consistent with a growing understanding that the HDP subtypes may represent different disease phenotypes rather than a spectrum of severity. The suggestion that gestational hypertension might be more strongly linked to stroke than preeclampsia in our data is consistent with findings from the Northern Finland Birth Cohort 1966 (4). Further, our mediation findings demonstrated that chronic hypertension accounted for a greater proportion of the association between gestational hypertension and CVD than that between preeclampsia and CVD, and we previously found women with gestational hypertension to have a higher risk of developing chronic hypertension than women with preeclampsia (10). Given these findings and the primacy of hypertension as a risk factor for stroke (35), it is not surprising to see the association between gestational hypertension and stroke in the current analysis.
While gestational hypertension appears to be a pure hypertensive phenotype, the pathophysiology underlying preeclampsia is more heterogeneous, stemming from abnormal placentation that results in endothelial dysfunction, systemic vascular impairment, vasoconstriction, and end-organ ischemia during pregnancy (36). In the years and decades following delivery, women with a history of preeclampsia exhibit vascular endothelial dysfunction, changes to cardiac structure and function, and increased premature vascular aging and subclinical atherosclerosis (36–41). Endothelial dysfunction may serve as a shared risk factor for both preeclampsia and CAD via inadequate vascularization of the uterus during pregnancy and of the myocardium later in life (40).
The primary study limitation is the potential for exposure misclassification, given nurse participants self-reported HDP. However, a NHSII validation study confirmed medical record evidence of preeclampsia for the majority of women who reported it. While HDP additionally include chronic hypertension and superimposed preeclampsia (42), this analysis focused on new-onset hypertension during pregnancy (gestational hypertension and preeclampsia), which is consistent with existing HDP-CVD literature (1). While we cannot rule out the potential for residual or unmeasured confounding (such as by social determinants of health), this study provides the most complete pre-pregnancy covariate adjustment currently available. Further, based on the calculated E-values, an unmeasured confounder would need to be associated with both HDP and CVD by a magnitude of 2.0–3.9-fold above and beyond the measured variables included in the model to explain away the observed associations. The only measured confounder within that range was pre-pregnancy obesity (HR=2.4, Model 2 for HDP-CVD), so it is unlikely that the observed associations were caused by an unmeasured confounder. Finally, NHSII participants are predominantly White nurses, and our findings should be generalized to other populations with some caution. In particular, non-Hispanic Black women have higher risks of HDP and CVD, relative to non-Hispanic White women, and rates of preeclampsia are increasing more rapidly among Black women than White women (43), yet it remains to be seen whether the magnitude of the HDP-CVD association differs among these and other women of color.
Women who develop HDP typically exhibit a subtle adverse cardiovascular risk profile before pregnancy, as demonstrated in this and previous studies (44). While this suggests the CVD risk trajectory precedes pregnancy, the experience of HDP itself may also induce vascular, endothelial, or organ damage that directly increases a woman’s risk of CVD (45,46). However, regardless of whether the HDP-CVD relationship is causal, HDP has the potential to be a powerful clinical risk marker. The “stress test” of pregnancy may help alert women and their providers to their underlying cardiovascular risk, creating an opportunity for primordial prevention of CVD risk factors (43,47,48). To leverage this window of opportunity, however, bridges need to be established between obstetric and primary care for risk communication, behavioral intervention, and follow-up; primary care providers and cardiologists should also be sure to obtain reproductive histories from their patients.
To our knowledge, this study presents the most complete control of pre-pregnancy confounding in the relationship between HDP and long-term CVD and is the first to estimate the proportion of this association jointly mediated by chronic hypertension, hypercholesterolemia, T2DM, and changes in BMI. Even after adjustment for pre-pregnancy confounders, HDP in first pregnancy remained associated with a 63% higher rate of CVD later in life. Over 80% of the increased risk of CVD among women with gestation hypertension was accounted for by the development of chronic hypertension after pregnancy. Although the majority of the preeclampsia-CVD association was jointly explained by established CVD risk factors, approximately 40% of the association remained unexplained; this suggests that preeclampsia may increase the risk of CVD through non-traditional and/or underrecognized risk factors. Our findings suggest that screening for and treatment of chronic hypertension, hypercholesterolemia, T2DM, and overweight/obesity following a pregnancy may delay, or even prevent, cardiovascular disease among women with a history of HDP.
Supplementary Material
Perspectives:
Competency in Medical Knowledge:
Hypertensive disorders of pregnancy, including gestational hypertension and pre-eclampsia, are associated with an increased risk of cardiovascular events that is incompletely explained by shared risk factors, including obesity, smoking, and family history.
Translational Outlook:
Research is needed to establish strategies to reduce long-term cardiovascular risk following hypertensive pregnancy.
Acknowledgements:
We thank the participants and staff of the Nurses’ Health Study II for their invaluable contributions. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding:
This research was funded by the following grants from the National Institutes of Health: U01 CA176726, R01 HL088521, R01 HL34594, and U01 HL145386. This work was supported by awards from the American Heart Association (12PRE9110014, 13GRNT17070022). JJS was supported by Training Grant T32HL098048 from the National Heart, Lung, and Blood Institute and by Training Grant T32HD060454 from the National Institute of Child Health and Human Development. LJT was supported by F31HL131222 from the National Heart, Lung, and Blood Institute under the Ruth L. Kirschstein National Research Service Award.
Abbreviations:
- BMI
body mass index
- CAD
coronary artery disease
- CI
confidence interval
- CVD
cardiovascular disease
- HDP
hypertensive disorders of pregnancy
- HR
hazard ratio
- IQR
interquartile range
- MI
myocardial infarction
- NHSII
Nurses’ Health Study II
- T2DM
type 2 diabetes mellitus
Footnotes
Disclosures: There are no author relationships with industry or financial disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Grandi SM, Filion KB, Yoon S et al. Cardiovascular Disease-Related Morbidity and Mortality in Women With a History of Pregnancy Complications. Circulation 2019;139:1069–1079. [DOI] [PubMed] [Google Scholar]
- 2.Wang YX, Arvizu M, Rich-Edwards JW et al. Hypertensive Disorders of Pregnancy and Subsequent Risk of Premature Mortality. J Am Coll Cardiol 2021;77:1302–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cirillo PM, Cohn BA. Pregnancy complications and cardiovascular disease death: 50-year follow-up of the Child Health and Development Studies pregnancy cohort. Circulation 2015;132:1234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mannisto T, Mendola P, Vaarasmaki M et al. Elevated blood pressure in pregnancy and subsequent chronic disease risk. Circulation 2013;127:681–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leon LJ, McCarthy FP, Direk K et al. Preeclampsia and Cardiovascular Disease in a Large UK Pregnancy Cohort of Linked Electronic Health Records: A CALIBER Study. Circulation 2019;140:1050–1060. [DOI] [PubMed] [Google Scholar]
- 6.Mongraw-Chaffin ML, Cirillo PM, Cohn BA. Preeclampsia and cardiovascular disease death: prospective evidence from the child health and development studies cohort. Hypertension 2010;56:166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergman L, Nordlof-Callbo P, Wikstrom AK et al. Multi-Fetal Pregnancy, Preeclampsia, and Long-Term Cardiovascular Disease. Hypertension 2020;76:167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garovic VD, White WM, Vaughan L et al. Incidence and Long-Term Outcomes of Hypertensive Disorders of Pregnancy. J Am Coll Cardiol 2020;75:2323–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Havenon A, Delic A, Stulberg E et al. Association of Preeclampsia With Incident Stroke in Later Life Among Women in the Framingham Heart Study. JAMA Netw Open 2021;4:e215077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stuart JJ, Tanz LJ, Missmer SA et al. Hypertensive Disorders of Pregnancy and Maternal Cardiovascular Disease Risk Factor Development: An Observational Cohort Study. Ann Intern Med 2018;169:224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grundy SM, Stone NJ, Bailey AL et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018. [Google Scholar]
- 12.Haug EB, Horn J, Markovitz AR et al. Association of Conventional Cardiovascular Risk Factors With Cardiovascular Disease After Hypertensive Disorders of Pregnancy: Analysis of the Nord-Trondelag Health Study. JAMA Cardiol 2019;4:628–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Honigberg MC, Zekavat SM, Aragam K et al. Long-Term Cardiovascular Risk in Women With Hypertension During Pregnancy. J Am Coll Cardiol 2019;74:2743–2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mosca L, Benjamin EJ, Berra K et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women−-2011 update: a guideline from the american heart association. Circulation 2011;123:1243–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stuart JJ, Gray KJ, Rich-Edwards J, Roberts JM. Epidemiology of Hypertensive Disorders in Pregnancy. In: Taylor RN, Conrad KP, Davidge ST, Staff AC, Roberts JM, editors. Chesley’s Hypertensive Disorders in Pregnancy. 5th ed. San Diego: Elseview Science & Technology, 2021:27. [Google Scholar]
- 16.Wilcox AJ, Gladen BC. Spontaneous abortion: the role of heterogeneous risk and selective fertility. Early Human Development 1982;7:165–178. [DOI] [PubMed] [Google Scholar]
- 17.Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined--a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol 2000;36:959–69. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. Ischemic Heart Disease Registers: Report of the Fifth Working Group. Copenhagen: World Health Organization. 1971. [Google Scholar]
- 19.Walker AE, Robins M, Weinfeld FD. The National Survey of Stroke. Clinical findings. Stroke 1981;12:I13–44. [PubMed] [Google Scholar]
- 20.Chiuve SE, Fung TT, Rimm EB et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 2012;142:1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28:1039–57. [DOI] [PubMed] [Google Scholar]
- 22.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183–97. [DOI] [PubMed] [Google Scholar]
- 23.Rich-Edwards JW, Colditz GA, Stampfer MJ et al. Birthweight and the risk for type 2 diabetes mellitus in adult women. Ann Intern Med 1999;130:278–84. [DOI] [PubMed] [Google Scholar]
- 24.Forman JP, Curhan GC, Taylor EN. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension among young women. Hypertension 2008;52:828–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colditz GA, Martin P, Stampfer MJ et al. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol 1986;123:894–900. [DOI] [PubMed] [Google Scholar]
- 26.Manson JE, Colditz GA, Stampfer MJ et al. A prospective study of maturity-onset diabetes mellitus and risk of coronary heart disease and stroke in women. Arch Intern Med 1991;151:1141–7. [PubMed] [Google Scholar]
- 27.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med 1989;8:551–61. [DOI] [PubMed] [Google Scholar]
- 28.Govindarajulu US, Spiegelman D, Thurston SW, Ganguli B, Eisen EA. Comparing smoothing techniques in Cox models for exposure-response relationships. Stat Med 2007;26:3735–52. [DOI] [PubMed] [Google Scholar]
- 29.VanderWeele TJ. Causal mediation analysis with survival data. Epidemiology 2011;22:582–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.VanderWeele TJ. Explanation in Causal Inference: Methods for Mediation and Interaction. New York: Oxford University Press, 2015. [Google Scholar]
- 31.Hertzmark E PM, Spiegelman D. The SAS Mediate Macro. https://cdn1.sph.harvard.edu/wp-content/uploads/sites/271/2012/09/mediate_manual_2012_06_06.pdf, 2012.
- 32.Lin DY, Fleming TR, De Gruttola V. Estimating the proportion of treatment effect explained by a surrogate marker. Stat Med 1997;16:1515–27. [DOI] [PubMed] [Google Scholar]
- 33.Stuart JJ, Tanz LJ, Cook NR et al. Hypertensive Disorders of Pregnancy and 10-Year Cardiovascular Risk Prediction. J Am Coll Cardiol 2018;72:1252–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wikstrom AK, Haglund B, Olovsson M, Lindeberg SN. The risk of maternal ischaemic heart disease after gestational hypertensive disease. BJOG 2005;112:1486–91. [DOI] [PubMed] [Google Scholar]
- 35.Tsao CW, Aday AW, Almarzooq ZI et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation 2022;145:e153–e639. [DOI] [PubMed] [Google Scholar]
- 36.Ying W, Catov JM, Ouyang P. Hypertensive Disorders of Pregnancy and Future Maternal Cardiovascular Risk. J Am Heart Assoc 2018;7:e009382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zoet GA, Benschop L, Boersma E et al. Prevalence of Subclinical Coronary Artery Disease Assessed by Coronary Computed Tomography Angiography in 45- to 55-Year-Old Women With a History of Preeclampsia. Circulation 2018;137:877–879. [DOI] [PubMed] [Google Scholar]
- 38.Countouris ME, Villanueva FS, Berlacher KL, Cavalcante JL, Parks WT, Catov JM. Association of Hypertensive Disorders of Pregnancy With Left Ventricular Remodeling Later in Life. J Am Coll Cardiol 2021;77:1057–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ives CW, Sinkey R, Rajapreyar I, Tita ATN, Oparil S. Preeclampsia-Pathophysiology and Clinical Presentations: JACC State-of-the-Art Review. J Am Coll Cardiol 2020;76:1690–1702. [DOI] [PubMed] [Google Scholar]
- 40.Bokslag A, Franssen C, Alma LJ et al. Early-onset preeclampsia predisposes to preclinical diastolic left ventricular dysfunction in the fifth decade of life: An observational study. Plos One 2018;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.AbdelWahab MA, Farrag HM, Saied CE. 24-Hour blood pressure variability as a predictor of short-term echocardiographic changes in normotensive women with past history of preeclampsia/eclampsia. Pregnancy Hypertens 2018;13:72–78. [DOI] [PubMed] [Google Scholar]
- 42.ACOG Practice Bulletin. Clinical Management Guidelines for Obstetrician-Gynecologists. Gestational Hypertension and Preeclampsia. Obstet Gynecol 2019;133:e1–e25. [DOI] [PubMed] [Google Scholar]
- 43.Parikh NI, Gonzalez JM, Anderson CAM et al. Adverse Pregnancy Outcomes and Cardiovascular Disease Risk: Unique Opportunities for Cardiovascular Disease Prevention in Women: A Scientific Statement From the American Heart Association. Circulation 2021;143:e902–e916. [DOI] [PubMed] [Google Scholar]
- 44.Magnussen EB, Vatten LJ, Lund-Nilsen TI, Salvesen KA, Davey Smith G, Romundstad PR. Prepregnancy cardiovascular risk factors as predictors of pre-eclampsia: population based cohort study. BMJ 2007;335:978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harskamp RE, Zeeman GG. Preeclampsia: at risk for remote cardiovascular disease. Am J Med Sci 2007;334:291–5. [DOI] [PubMed] [Google Scholar]
- 46.Rodie VA, Freeman DJ, Sattar N, Greer IA. Pre-eclampsia and cardiovascular disease: metabolic syndrome of pregnancy? Atherosclerosis 2004;175:189–202. [DOI] [PubMed] [Google Scholar]
- 47.Rich-Edwards JW, McElrath TF, Karumanchi SA, Seely EW. Breathing life into the lifecourse approach: pregnancy history and cardiovascular disease in women. Hypertension 2010;56:331–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sattar N, Greer IA. Pregnancy complications and maternal cardiovascular risk: opportunities for intervention and screening? Bmj 2002;325:157–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


