ABSTRACT
T cell engaging therapies, like CAR-T cells and T cell engagers, redirect T cells toward tumor cells, facilitating the formation of a cytotoxic synapse and resulting in subsequent tumor cell killing. T cell receptor or CAR-T downstream signaling triggers a release of pro-inflammatory cytokines, which can induce a Cytokine Release Syndrome (CRS). The incidence of CRS is still hardly predictable among individuals and remains one of the major dose-limiting safety liabilities associated with on-target activity of T cell engaging therapies. This emphasizes the need to elaborate mitigation strategies, which reduce cytokine release while retaining efficacy. Here, we review pre-clinical and clinical approaches applied for the management of CRS symptoms in the context of T cell engaging therapies, highlighting the use of tyrosine kinase inhibitors as an emerging mitigation strategy. In particular, we focus on the effects of Bruton’s tyrosine kinase (BTK), Src family including Lck, mammalian target of rapamycin (mTOR) and Janus tyrosine kinase (JAK) inhibitors on T cell functionality and cytokine release, to provide a rationale for their use as mitigation strategies against CRS in the context of T cell engaging therapies.
KEYWORDS: Chimeric antigen receptor-(CAR-) T cells, T cell engagers, CD3 bispecific antibody, cytokine release syndrome (CRS), cytokines, tyrosine kinase inhibitors
1. Introduction
In the field of cancer immunotherapy, redirecting T cell cytotoxicity toward tumor cells is a promising approach for the treatment of various types of cancer. For this purpose, two main strategies are currently developed; one involving T cell genetic modification with chimeric antigen receptors (CAR) and the other one using T cell engaging bispecific antibodies linking the CD3ε chain of the T cell receptor (TCR) to the targeted tumor antigen.1−6 The CD19 bispecific T cell engager (BiTE) blinatumomab and the TCR-based gp100-peptide MHC specific T cell engaging ImmTAC tebentafusp are approved for the treatment of acute lymphocytic leukemia (ALL) and metastatic uveal melanoma, respectively.7,8 We have previously described several T cell bispecific antibodies (TCBs) including CEA-TCB (cibisatamab) directed against CEA positive solid tumors, CD20-TCB (glofitamab) indicated for B cell malignancies, WT1-TCB, a TCR-like TCB that recognizes a WT1 derived peptide presented by HLA-A02 on AML cells, and BCMA-TCB for the treatment of multiple myeloma.1–3,9–12 In the field of CAR-T cells several CD19-targeted CAR-T cell products Kymriah (tisagenlecleucel); Yescarta, (axicabtagene ciloleucel); Tecartus (brexucabtagene autoleucel), Breyanzi (lisocabtagene maraleucel) are approved for ALL and/or B cell malignancies.13,14 The BCMA-targeted CAR-T cell products Abecma (idecabtagene vicleucel) and Carvykti (ciltacabtagene autoleucel) are also approved for the treatment of multiple myeloma.15,16 Both T cell engagers and CAR-T cells are showing remarkable clinical efficacy, particularly to cure hematological tumors, opening an avenue for approval of other T cell engaging therapies.17,18 Consequently, various CAR-T cell products and T cell engagers against a wide spectrum of solid tumors and hematological tumors are under development and hold a great promise.19,20
As part of their natural mechanism of action, on-target activity of T cell engaging therapies can lead to strong release of pro-inflammatory cytokines that can potentially induce a Cytokine Release Syndrome (CRS).21–24 CRS may also occur after viral infection including COVID-19 or after treatment with other classes of immunotherapies including the super agonist anti-CD28 antibody TGN1412.25,26 The hallmark of CRS is a cytokine storm associated with an over-activation of the immune system which can cause symptoms including fever, hypotension and respiratory deficiency and, in the worst case, multi-organ failure.25,27,28 In the context of T cell engaging therapies, the cytokine release cascade is initiated by T cell activation and then amplified by myeloid and T cell-derived cytokines.28–33 This may further mediate endothelial cell activation and vascular leakage in various tissues and organs, manifesting symptoms including hypotension and hypoxia.28,34,35
The ASTCT classifies CRS into different grades based on clinical symptoms (fever, hypotension, and hypoxia). The management of grade 2 and higher grade CRS requires patient hospitalization and the use of vasopressors, oxygen flow, high-dose glucocorticoids, and/or IL-6 R blockade to alleviate symptoms.27 To improve patient well-being, and reduce the cost associated to hospitalization, there is a need to anticipate the occurrence of grade 2 or higher grade CRS by implementing early interventions.36 In the specific case of T cell engagers, step-up or fractionated dosing schedules are used in the clinic to lower the risk of first-infusion cytokine storm that may be observed after flat-dose administration.37 Nevertheless, CRS still remains a frequent dose-limiting safety liability associated with on-target activity of T cell engagers.
The severity and onset of CRS are likely to vary across the types and formats of the T cell engaging therapies (e.g. CAR-T cell vs. T cell engagers, costimulatory domain, binder affinity), and to differ between hematological and solid tumor indications.3,4,5,24,32,38 Whereas CRS incidence was lower for first-generation CAR-T cells, CRS is more commonly reported with second-generation CAR-T cells, which include a co-stimulatory domain.39 Even though T cell activation is the common trigger of cytokine release, the onset of CRS comes later for CAR-T cells than for T cell engagers as a consequence of the slower kinetics of cytokine release.35 For CAR-T cells, the peak of cytokine release typically occurs a few days after infusion and is associated with CAR-T cell proliferation and activation.24,38 For T cell engagers, the peak of cytokine release can occur within hours after the first infusion depending on the affinity of the CD3 and tumor antigen binder, and is reduced after repeated treatments.40,41 For CAR-T cells, CRS management may be more challenging than for T cell engagers, as their activity cannot be stopped by simple dose-interruption. Regarding the variability between cancer indications, circulating cytokines may possibly be found at high levels during the treatment of hematological tumors with T cell engaging therapies, due to unrestricted on-target activity on peripheral B cells and in lymphoid organs. In solid tumor indications, cytokine release is rather expected to occur locally in the tumor microenvironment, unless the targeted tumor antigen is also expressed in healthy tissues. Additionally, the tumor load may also contribute the severity of CRS.42 Altogether, this supports the need to establish CRS mitigation strategies specific to each different class of T cell engaging therapies and indications.
In this review, we will first discuss the current management of CRS with glucocorticoids and/or tocilizumab and the development of novel targeted approaches toward specific cytokine pathways. We will then dive into the promising use of tyrosine kinase inhibitors targeting Src family, BTK, JAK, and mTOR kinases downstream of TCR activation to prevent T-cell derived cytokine release while retaining T cell engaging therapy efficacy.
2. Glucocorticoids
Glucocorticoids like methylprednisolone or dexamethasone are the most commonly used agents for the mitigation of CRS induced by T cell engaging therapies (Figure 1). They suppress inflammatory reactions and are effective in reducing CRS symptoms.4,27,28,43–45 In preclinical models, they were shown to retain the efficacy of a GPC3 TRAB T cell engager.4 In the clinic, the transient use of high-dose glucocorticoids for the management or prophylaxis of CRS was reported not to interfere with complete response rate after treatment with BiTE antibodies 46 or CAR-T cells.47 In addition to preventing CRS, glucocorticoids can also cross the blood–brain barrier (BBB) and could mitigate neurotoxicity risks associated with T cell engaging therapies against hematological targets.48 However, it is still controversial whether longer term exposure to glucocorticoids might have an inhibitory effect on treatment efficacy. Indeed, they are known to suppress T cell function and T cell infiltration in solid tumors, especially when used at high dose for the mitigation of CRS.49,50 Besides, some patients are refractory to glucocorticoids, emphasizing the need to explore alternative approaches.4
Figure 1.
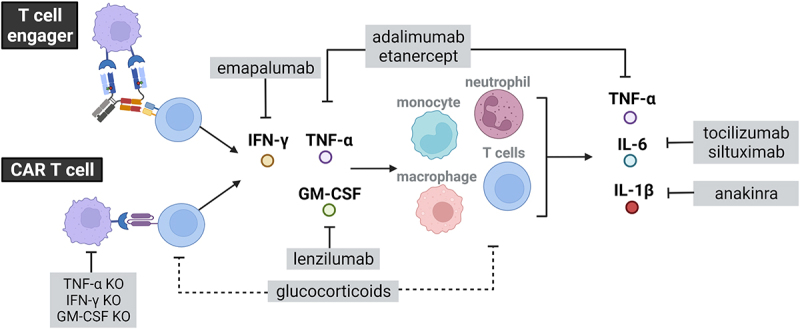
Schematic representation of glucocorticoids and cytokine targeted approaches used for the mitigation of cytokine release induced by T cell engaging therapies.
Glucocorticoids and tocilizumab are the most commonly used interventions for CRS. Unlike tocilizumab, which blocks IL-6 R, siltuximab blocks IL-6 and may prevent neurotoxicity in addition to CRS by stopping IL-6 from crossing the blood–brain barrier. Besides, In vitro and in vivo studies have suggested novel cytokine targeted approaches for the mitigation of CRS. Those are directed against pro-inflammatory cytokines including IL-1β (anakinra), TNF-α (Adalimumab/etanercept), IFN-γ (emapulamab), or GM-CSF (lenzimumab). Along those lines, TNF-α, IFN-γ and GM-CSF knock-out can be engineered in CAR-T cells to prevent CRS. Pre-treatment with targeted antibodies depleting and/or masking the tumor. Created with BioRender.com
3. Cytokine targeted approaches
3.1. Blockade of myeloid-derived cytokines – IL-6, IL-6 R, IL-1 R blockade
After treatment with T cell engagers, IL-6 and IL-1β are released by myeloid cells, downstream of T cell derived cytokines and are described as key cytokines involved in the pathophysiology of CRS30,32,33,51 (Figure 1). IL-6 levels were reported to correlate with the severity of CRS, highlighting the role of IL-6 in mediating CRS symptoms.24 Tocilizumab is a monoclonal antibody that competitively prevents the binding of IL-6 to its receptor IL-6 R. It was first approved for the treatment of rheumatoid arthritis before being successfully used in the clinic to mitigate CRS symptoms induced by CAR-T cells (BLA 125276/S-114).27 Based on these data, tocilizumab has been approved for the treatment of CAR T cell-induced cytokine release syndrome. Norelli et al. and Giavridis et al. developed mouse models mimicking CAR-T cell-induced CRS and showed that IL-1 R and IL-6 R blockade prevented CD19 CAR-T cells induced-CRS but that only IL-1 R blockade protected the mice from neurological adverse events.29,31 The IL-1 blocking fusion protein anakinra was shown to be an efficient drug for the mitigation of both CRS and neurotoxicity.50,52 Siltuximab is a monoclonal antibody that binds IL-6 with a higher affinity than tocilizumab for IL-6 R. One advantage of siltuximab over tocilizumab may be that it prevents IL-6 to cross the BBB, and therefore could mitigate CAR-T cell-induced neurotoxicity. Nevertheless, this hypothesis remains to be proven.48
3.2. Blockade of T- cell derived cytokines – TNF-α, GM-CSF, and IFN-γ blockade
As the cascade of cytokines mediated by T cell engaging therapies is initiated by on-target T cell activation and cytokine release, there is a rationale to block upstream T cell-derived cytokines for the mitigation of CRS (Figure 1).
The prophylactic blockade of TNF-α resulted in a significant decrease in IL-6 and IL-1β release after treatment with HER2-T cell dependent bispecific antibody (TDB), both in in vitro co-culture of PBMCs and HER2-expressing cells and in immunocompetent MMTV-HER2 transgenic mice.32 These findings were confirmed in vitro for other T cell bispecific antibodies (Tyrp1-TCB, CEA-TCB, and FolR-TCB).33 The prophylactic blockade of TNF-α appears as promising strategy to control the activation of myeloid cells and the release of IL-6 and IL-1β.30,32,33 Etanercept was used to mitigate CRS in a patient treated with BCMA CAR-T cells who had elevated serum TNF-α levels.53 However, TNF-α is also known to induce upregulation of ICAM and VCAM, favoring T cell infiltration in the tumors and its blockade may interfere with anti-tumor efficacy of T cell engaging therapies targeted against solid tumors.3
Similarly, the blockade of CAR-T cell-derived GM-SCF prevented cytokine release in co-culture of CD19 CAR-T cells with tumor cells. In vivo, GM-CSF CRISPR/Cas9-knockout in CD19 CAR-T cells or the combination of CD19 CAR-T cells with anti-GM-CSF antibody (lenzilumab) prevented cytokine release and neurotoxicity in mice engrafted with acute lymphoblastic leukemia (ALL) patient-derived xenograft, while retaining CAR-T cell efficacy.54,55
The blockade of IFN-γ with emapalumab or the IFN-γ knock-out in CAR-T cells prevented the activation of pro-inflammatory macrophages together with the associated cytokine release.51,56,57 While these studies did not show a negative impact on the efficacy of IFN-γ KO CAR-T cells, other studies showed that IFN-γ blockade decreased the accumulation of CD8+ T cells within tumors after treatment with an anti-HER2xCD3 TDB. In particular, the blockade of IFN-γ prevented the release of essential chemokines involved in T cell recruitment. Therefore, IFN-γ blockade may ultimately negatively affect anti-tumor efficacy when combined with T cell engaging therapies, especially when targeted against solid tumors where T cell infiltration plays a major role in response.58
On-target activity of T-cell engaging therapies results in T cell activation and cytokine release, which initiate the cytokine storm. Therefore, a strategy to reduce strong release of pro-inflammatory cytokines on first infusion consists in covering the targeted antigen with a masking antibody (Figure 2). The pre-treatment with anti-CAIX monoclonal antibody competed with CAIX CAR-T cells, reducing on-target CAR-T cell activity and cytokine release.59 Another example is the pre-treatment with obinutzumab (Gazyva) to avoid CRS occurrence in patients with hematological malignancies treated with the CD20xCD3 TCB glofitamab. By de-bulking peripheral B cells and competing with glofitamab for CD20 binding, the pre-treatment with obinutuzumab decreases on-target cytokine release while retaining a profound anti-tumor efficacy.3,11,37,60 Pre-treatment with obinutuzumab or other anti-CD20 antibodies may also reduce CRS induced by CD19-targeted CAR-T cells or CD3 bispecific antibodies. However, it was not yet evaluated more broadly, for example, in patients with large peripheral tumor load like in chronic lymphocytic leukemia (CLL).
Figure 2.
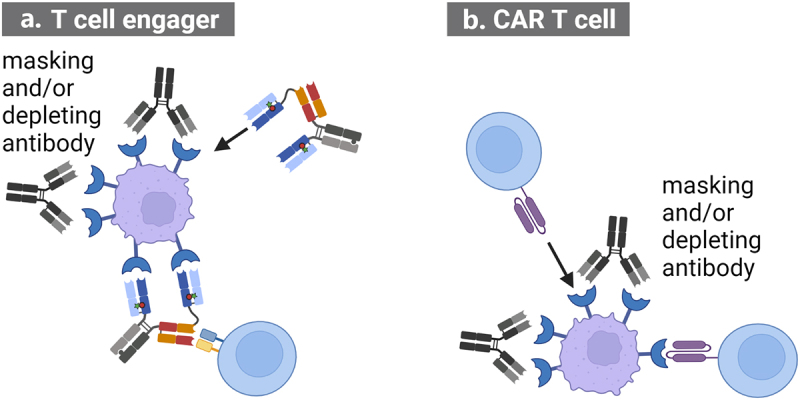
Pre-treatment with antibodies depleting tumor cells and competing for target binding with A. T cell engager or B. CAR T cells can be used to mitigate CRS.
When used prior to treatment with T cell engaging therapies, these masking and/or depleting antibodies reduce on-target T cell activation resulting in a lower release of cytokines on first infusion. Created with BioRender.com
4. Engineering of T cell engager format
The therapeutic index of T cell engagers can also be improved by engineering lower affinity CD3 binders that trigger less cytokine release while retaining efficient in vivo anti-tumor efficacy.61–64 In vitro, lower CD3 binder affinity is associated with reduced tumor cell killing and reduced cytokine release.61,62 In vivo, a low-affinity CD3 binder retains efficacy while reducing cytokine release and ensuring optimal bio-distribution and accumulation in the tumor.65 Additionally, Dang et al. described that a lower affinity CD3 binder does not trigger activation and proliferation of immunosuppressive Tregs, preventing their infiltration in tumor tissues.40 Altogether, CD3 engagement on TCR appears more sensitive to trigger signaling pathways involved in T cell cytotoxicity than cytokine release. Consequently, fine-tuning of CD3 binder affinity is an approach to lower CRS occurrence while retaining T cell-mediated cytotoxicity. In this context, new formats of TCBs are currently being engineered and evaluated in an attempt to increase their tolerability by avoiding off-tumor activity. One approach relies on protease-activated TCBs where masking of the anti-CD3 Fab fragment with an anti-idiotypic mask was proven to enhance selectivity and safety of TCBs, as the mask needs to be cleaved by tumor-specific proteases to activate the TCB.66 Another approach relies on pH-dependent TCBs engineered with the conditionally active biologic (CAB) technology, where the CD3 binder is only active under acidic intra-tumoral pH and remains inactive in healthy tissues.67,68
5. Modular CAR-T cells and safety switches
For CAR-T cell therapies, CRS and safety management is more challenging than for T cell engagers, as their activity cannot be stopped by simple dose-interruption. The use of modular CAR-T cells and their respective adaptor molecules may allow to control CAR-T cell activity by dose titration of the CAR adaptor molecule. Depending on the exposure of the CAR adaptor, dose interruption of the molecule may not result in a prompt switch-off of CAR-T cell activity.69 To achieve a faster switch-off, counter-CAR adaptors can be applied to neutralize the adaptor molecule. Further safety switches have been developed to stop the activity and proliferation of CAR-T cells in rare cases of severe toxicity. One example is the development of STOP-CAR-T cells where the administration of a small-molecule drug can inactivate their functions.70 Along those lines, Zheng et al. reviewed the use of small molecule-based safety switches that aim to provide pharmacological control over CAR-T cell activity.71 Jan et al. engineered lenalidomide OFF switch degradable CAR-T cells and lenalidomide ON switch split CAR-T cells. Those chemical genetic switches in CAR-T cells rapidly and reversibly control CAR T cell activity and degradation to mitigate toxicities associated with CAR-T cell treatment. Recently, SNIP CAR-T cells were engineered with a protease-based platform to control CAR-T cell activity with an FDA approved small molecule, allowing to switch-off CAR activity by dose interruption of the drug.72 This novel design also circumvents on-target off-tumor activity and rapid T cell exhaustion, which may be observed with classical CAR-T cells.
By interacting with downstream signaling pathways of CAR activation, kinase inhibitors may represent attractive approaches to switch-off CAR-T cell activity.
6. The use of kinase inhibitors for CRS mitigation
Recent screening of kinase inhibitors identified compounds able to enhance or suppress functionality of CAR-T cells or T cells following stimulation with CD3 bispecific antibodies.73–76 Here, we will attempt to decipher the effects of the main FDA-approved tyrosine kinase inhibitors including Bruton’s tyrosine kinase (BTK), BCR-Abl, mammalian target of rapamycin (mTOR) and JAK/STAT inhibitors, on T cell functionality and cytokine release, to provide a rationale for their use as mitigation strategy against CRS (Figure 3). The clinical interventions using tyrosine kinase inhibitors for the mitigation of CRS in the context of CAR-T cell or CD3 bispecific antibody therapies are summarized in Table 1.
Figure 3.
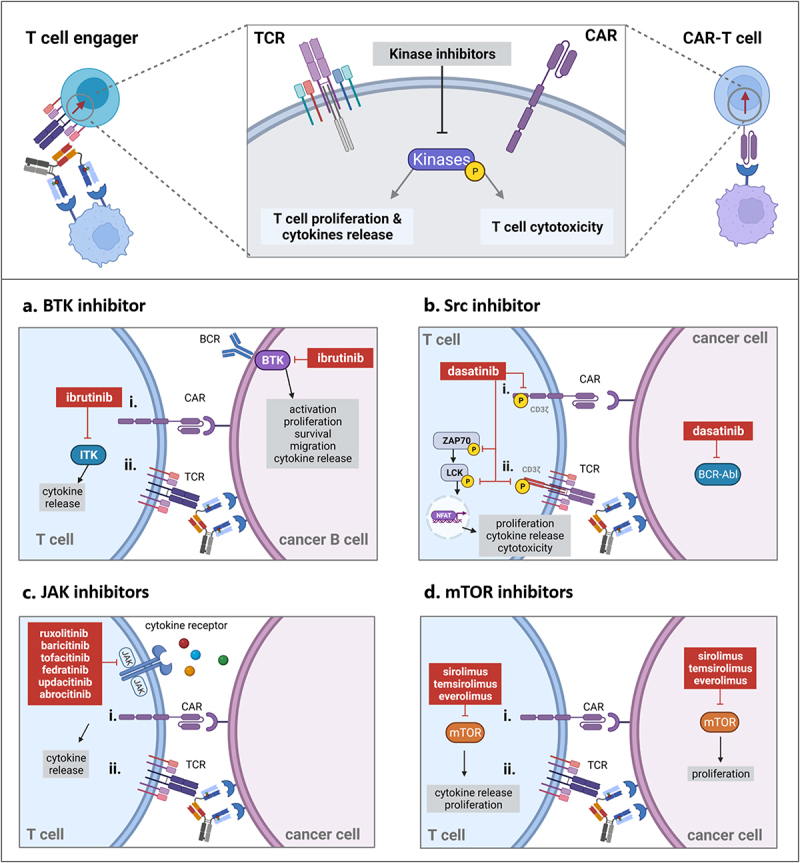
Kinase inhibitors target kinase-signaling pathways involved downstream of TCR or CAR activation and interfere with T cell proliferation, T cell cytokine release and/or T cell-mediated cytotoxicity. Panel A. The BTK inhibitor ibrutinib prevents phosphorylation of ITK kinases downstream of CAR (i) or TCR activation (ii) and of BTK kinases in tumor cells resulting in a reduction of cytokine release. The combination of ibrutinib with T cell engaging therapies directed against hematological tumors may increase treatment efficacy while preventing the risk of CRS. Panel B. Dasatinib blocks CD3ξ, ZAP70 and Lck kinases phosphorylation and NFAT-mediated gene transcription resulting in a reversible switch-off of T cell functionality for CAR-T cell (i) and T cell engagers (ii). BCR-Abl expressed in leukemia cells is a target of dasatinib. Dasatinib may be combined with T cell engaging therapies directed against acute lymphoblastic leukemia to decrease incidence of CRS. Panel C. JAK inhibitors prevent JAK phosphorylation downstream of various cytokine receptors and can reduce CAR T cell (i) or CD3 bispecific antibody-induced cytokine release (ii). They prevent cytokine release while retaining the efficacy of T cell engaging therapy. Panel D. mTOR inhibitors prevent mTOR signaling downstream of CAR (i) or TCR activation (ii) as well as mTOR signaling in tumor cells resulting in a reduction of cytokine release and cell proliferation. mTOR inhibitors retain cytotoxic properties of T cells and may be combined with T cell engaging therapies in indications where they exert direct anti-tumor efficacy. Created with BioRender.com
Table 1.
Summary of the clinical interventions with kinase inhibitors for the mitigation or prevention of CRS induced by T cell engaging therapies
| Study | Therapy | Disease | Results/comments |
|---|---|---|---|
| Gauthier et al., 2020 NCT01865617 |
CD19 CAR-T cells (CD28 and 4–1BB) + ibrutinib | CLL | Ibrutinib reduced CRS severity while retaining efficacy.
|
| Uy et al., 2019 NCT02152956 |
flotetuzumab + ruxolitinib | AML | Prophylaxis ruxolitinib modified the cytokine profile but did not resolve CRS symptoms induced by flotetuzumab.
|
| Wei et al., 2020 Chi CTR1900025419 |
CD22/CD19 CAR-T cells + ruxolitinib | Ph+ ALL | Ruxolitinib was used to treat glucocorticoid-refractory CRS. After dexamethasone treatment, ruxolitinib resolved CRS symptoms. This was associated with a reduction of cytokines, ferritin and CRP levels and no apparent effect on the CAR-T cell anti-leukemic activity. |
| Zi et al., 2021 NCT04303520 |
CD22/ CD19 CAR-T cells + ruxolitinib | Ph+ ALL | Ruxolitinib was used to treat glucocorticoid and tocilizumab-refractory CRS. After tocilizumab and methylprednisolone treatment, ruxolitinib resolved CRS symptoms. This was associated with a reduction of cytokines, ferritin and CRP levels and no apparent effect on the CAR-T cell anti-leukemic activity. |
| ChiCTR190002531 (patient 1) ISRCTN19144142 (Patient 2). |
CD7 CAR-T cells + ruxolitinib and etanercept | T ALL | Concomitant use of ruxolitinib and etanercept induced a change in cytokine profile but did not fully resolve CRS symptoms (administration of noradrenaline was sustained to mitigate CRS grade 3 in both patients) |
| Park et al., 2021 NCT04071366 |
CD19 CAR-T cells + itacitinib (JAK1 inhibitor) | r/r B cell malignancies | Ongoing study |
| Assi et al., 2017 77 | Ponatinib, bosutinib or dasatinib + blinatumomab | ALL | Two cases of grade 2 CRS (resolved with glucocorticoids and tocilizumab). One case was observed with dasatinib and the other with ponatinib. |
| King et al., 2019 78 | Ponatinib, imatinib, nilotinib or dasatinib + blinatumomab | ALL | Blinatumomab was used to eliminate remaining ALL cells in patients with MRD and spare toxicity associated to chemotherapy. CRS (grade 1–2) was observed in 3/11 patients |
| Foà et al., 2020 NCT0244768 |
Dasatinib + blinatumomab | Ph+ALL | Concomitant use of blinatumomab and dasatinib was safe and efficacious. |
6.1. BTK inhibitors
Ruella et al. showed that the combination of ibrutinib and CD19 CAR-T cells enhanced the killing of Mantle Cell Lymphoma (MCL) cells in vitro and led to profound and durable responses in NSG mice engrafted with MCL xenograft.79 In addition, ibrutinib co-treatment with CD19 CAR-T cells reduced cytokine release in the serum of these mice (IL-6, IFN-γ, TNF-α, IL-2, and GM-CSF).80 In vitro, escalating concentrations of ibrutinib (10 nmol/L, 100 nmol/L, and 1000 nmol/L) in the assay medium did not significantly reduce CD19-CAR T cell-derived cytokine release and functionality (grzB, Fas ligand, IFN-γ, perforin, and TRAIL). However, treatment of MCL cells with ibrutinib decreased cytokine levels in the cell culture supernatants, reflecting its cytotoxic activity toward cancer B cells.79
To investigate the mode of action of ibrutinib, Godwin et al. compared the effect of ibrutinib to the more specific BTK inhibitor acalabrutinib and to other IL-2-inducible kinase (ITK) and Src inhibitors using in vitro co-cultures of acute lymphoblastic leukemia (ALL) cell lines or acute myeloid leukemia (AML) cell lines with T cells in the presence of CD19/CD33xCD3 bispecific antibodies.81 Their results suggest that the mechanisms by which ibrutinib inhibits T cell cytotoxicity is unlikely to be mediated via BTK inhibition, but rather triggered by ITK inhibition downstream of TCR activation82 (Figure 3A).
At clinically relevant doses, the reduction in cytokine release observed with ibrutinib is probably mediated by a synergistic effect on tumor cell-derived cytokine release and on CD19 CAR-T cell-derived cytokine release. In addition, ibrutinib polarizes the different T-cell populations toward effector cells and reduces their exhaustion marker expression. This may enable a better functionality of CAR-T cells or T cells following stimulation with T cell engagers.83,84
In the clinic, Gauthier et al. reported that the combination of CD19 CAR-T cells with ibrutinib to treat CLL patients after ibrutinib failure was well tolerated and associated with lower CRS-associated cytokines in the serum and lower CRS severity.85 In terms of efficacy, the combination of CD19 CAR-T cells with ibrutinib lead to comparable response rates than achieved with CD19 CAR-T cell monotherapy.86 Additionally, ibrutinib reduced obinutuzumab-infusion-related reaction (IRR) in CLL patients, suggesting that it may reduce cytokine release associated with other classes of immunotherapies.87,88
6.2. BCR-Abl/Src family inhibitors
Weber et al. and Mestermann et al. described the use of the FDA-approved kinase inhibitor dasatinib as a rapid and reversible pharmacological ON/OFF switch for CAR-T cells.89,90 By inhibiting phosphorylation of CD3ξ, Lck and ZAP70 kinases downstream of the CAR construct, dasatinib can prevent CAR-T cell functionality and cytokine release (Figure 3B). The inhibitory effects of dasatinib being reversible, a temporary switch-off of activated CAR-T cells with dasatinib could prevent lethal CRS in a mouse model reproducing high-grade CRS, while maintaining long-term efficacy.89,90 Weber et al. also demonstrated that transient ON/OFF switches with dasatinib could prevent rapid CAR-T cell exhaustion and restore T cell functionality.91 Dasatinib was also shown to reversibly switch-off cytotoxicity and cytokine release from PBMCs that were pre-stimulated with HLA-A2 WT1-TCB or CEA-TCB.92 Furthermore, dasatinib stopped CD19-TCB-mediated B cell depletion and cytokine release in vivo in humanized NSG mice (Figure 3B).92,93 Based on these findings, the use of dasatinib was developed as a safety switch for HLA-A2 WT1-TCB, a TCR-like TCB, associated with the potential unpredictable recognition of WT1-similar peptides presented by MHC class-I on healthy cells. Another use of dasatinib could be to stop TCB activity in rare cases of life-threatening CRS where glucocorticoids may not be sufficient.92,93 Along those lines, the multi-pharmacologically targeted kinase inhibitor midostaurin was shown to switch-off T cell proliferation and cytokine release following stimulation with CD33-directed uniCAR and CD33 T cell engager at clinically relevant doses.94 The authors report that the inhibitory effects of midostaurin are likely driven through off-target inhibition of Lck and ZAP70 kinases, similarly to dasatinib.
In a lymphoma patient-derived xenograft model in huNSG mice, transient interventions with dasatinib on the first infusion with CD19-TCB strongly reduced cytokine release while minimally interfering with long-term anti-tumor efficacy.76 This supports the transient prophylactic use of dasatinib to prevent CRS after the first infusion with CD3 bispecific antibodies.
In the clinic, Foà et al. used dasatinib as induction therapy for 85 days in patients with acute lymphoblastic leukemia (ALL) followed by a consolidation therapy with concomitant treatment of blinatumomab and dasatinib.95,96 This approach was successful with an overall survival of 95% and a disease-free survival of 88% at a median follow-up of 18 months. Importantly, it was associated with few toxic effects. The combination of blinatumomab and dasatinib was associated with a safe and efficacious response. In the study described by Foà et al., the de-bulking of tumor cells using the BCR-Abl inhibitor dasatinib in the induction phase considerably reduced the tumor load. Therefore, the risk of CRS was reduced in the consolidation phase combining blinatumomab and dasatinib. Although the in vitro continuous exposure of dasatinib was shown to suppress blinatumomab-mediated T cell activity,97 it is likely that the in vivo PK/PD properties of dasatinib inducing rapid ON/OFF switches during the consolidation phase may explain the safety and efficacy profile of blinatumomab treatment. It can also be hypothesized that the transient ON/OFF switches with dasatinib may have even prevented rapid T cell exhaustion thereby prolonging T cell functionality, as recently described by Weber et al. in the field of CAR T cells.91 The trial reported by Foà et al. likely benefited from both the direct anti-tumor effect of dasatinib in the induction phase and its effect on cytokine release during the consolidation phase, making the treatment with blinatumomab well tolerated.
6.3. JAK inhibitors
Since many cytokines involved in cytokine release syndrome signal through the JAK/STAT pathways, the use of FDA-approved JAK inhibitors for the mitigation of CRS induced by T cell engaging therapies was investigated (Figure 3C).98–100
Kenderian et al. have shown that ruxolitinib may prevent CRS after CD123 CAR-T cell therapy using an acute myeloid leukemia xenograft mouse model. Mice treated with CD123 CAR-T cells in combination with ruxolitinib exhibited less severe weight loss and attenuated cytokine release. In this model, ruxolitinib retained treatment efficacy providing long-term survival.101 Additionally, the combination of ruxolitinib with CD19 CAR-T cells reduced in vitro proliferation and in vivo expansion while maintaining their therapeutic efficacy and decreasing cytokine release.102 Itacitinib, a JAK1 inhibitor, was also shown to prevent IL-6, IFN-γ, IL-2, and IL-8 release in co-culture of CD19+ lymphoma cells with CD19 CAR-T cells.103 Co-treatment with itacitinib did not impair CD19 CAR-T cell anti-tumor activity in immune-deficient NSG mice inoculated with CD19+ expressing Nalm6 lymphoma cells. Furthermore, itacitinib was shown to prevent release of IL-6 from macrophages after in vitro and in vivo LPS stimulation, showing that JAK1 inhibition directly affects myeloid-derived cytokine release.103
In the field of T cell engagers, the combination of FDA-approved JAK inhibitors (ruxolitinib, baricitinib, fedratinib, and tofacitinib) strongly reduced CEA-TCB and CD19-TCB-induced cytokine release while retaining in vitro activity at pharmacologically active doses.76 In vivo, ruxolitinib reduced CD19-TCB-mediated cytokine release in non-tumor bearing humanized NSG mice (huNSG).76 In a lymphoma patient-derived xenograft (PDX) model in huNSG mice, ruxolitinib prophylaxis minimally interfered with CD19-TCB anti-tumor efficacy.76 Altogether, these preclinical findings suggest that JAK inhibitors efficiently modulate the cytokine profile after T cell engaging therapies.
In the clinic, ruxolitinib was used for CRS management in a patient being refractory to glucocorticoids after treatment with CD22/CD19 CAR-T cell.104 Ruxolitinib was given after dexamethasone and reduced the body temperature, cytokine levels (IL-6, IL-8, IL-10, and TNF-α), ferritin and CRP levels while not influencing CAR-T cell anti-leukemic activity. In line with this, ruxolitinib combined to etanercept (anti-TNF-α) was used for the mitigation of grade 3 CRS developed in two patients after treatment with CD7 targeted universal CAR-T cells.105 Ruxolitinib reduced cytokine levels in the serum of the two patients. Nevertheless, noradrenaline treatment was continued, making it complicated to conclude on the effects of ruxolitinib in preventing CRS symptoms. In another study, Uy et al. reported that prophylactic treatment with ruxolitinib decreased cytokine secretion, but did not lead to discernable improvement in clinical severity of CRS in patients receiving flotetuzumab, emphasizing that the pan-JAK inhibitor ruxolitinib alone may not be sufficient to mitigate CRS.106 Itacitinib, a more selective JAK1 inhibitor, is currently being explored for the prevention of CD19 CAR-T cell induced CRS in patients with hematological malignancies.103,107 Additionally, the combination of ruxolitinib with other mitigating agents including tocilizumab or low-dose glucocorticoids might be beneficial to improve CRS symptoms management and remains to be explored pre-clinically.
6.4. mTOR inhibitors
A recent screening of 52 FDA-approved kinase inhibitors revealed that mTOR inhibitors reduced T cell proliferation and cytokine release following TCR activation via CD3 stimulation.76 In a co-culture of PBMCs and tumor cells, the FDA-approved mTOR inhibitors sirolimus, temsirolimus, and everolimus prevented T-cell mediated cytokine release while retaining tumor cell killing following treatment with CEA-TCB and CD19-TCB at pharmacologically active doses76 (Figure 3D). When compared side by side to JAK, Src inhibitors, and glucocorticoids, mTOR inhibitors appear to be the most potent kinase inhibitors, which strongly reduce in vitro cytokine release while preserving T cell killing.76 In vivo, sirolimus favorably prevented first infusion cytokine release and retained B cell depletion in non-tumor bearing huNSG treated with CD19-TCB.76 In a lymphoma patient-derived xenograft model in huNSG mice, transient treatment with sirolimus on first infusion with CD19-TCB retained long-term anti-tumor efficacy comparably to JAK, Src inhibitors and dexamethasone.76 Altogether, these data suggests that mTOR inhibitors may be the preferred candidates for prophylaxis of CRS. Since mTOR inhibitors are used as anti-tumor agents in various solid cancers, a combination with a solid-tumor targeted TCB may be of particular interest to prevent CRS while maintaining efficacy in such indications.108,109 Indeed, mTOR signaling is frequently dysregulated in various cancers, such as breast, prostate, lung, liver, and renal cell carcinomas. It was reported that the upregulation of mTOR signaling may promote growth factor receptor signaling, angiogenesis, glycolytic activity, lipid metabolism, cancer cell migration, and suppression of autophagy, resulting in tumor growth and progression.110,111 Esfahani et al. showed that sirolimus promoted allograft tolerance while retaining pembrolizumab-mediated anti-tumor activity in a melanoma patient undergoing kidney allograft rejection resulting from pembrolizumab treatment.112 This further supports targeting the mTOR pathway to mitigate inflammation-driven adverse events related to treatment with immunotherapies while retaining their efficacy.
7. Summary
There is a need of developing prophylactic mitigation strategies that would prevent the occurrence of grade 1 or higher grade CRS after treatment with T cell engaging therapies, thereby improving patient well-being and reducing treatment costs associated to hospitalization for symptoms management.
The idea of using targeted approaches using single anti-cytokine antibodies might be a way to retain other cytokines essential for anti-tumor efficacy of T cell engaging therapies, especially those targeting solid tumors. In such indications, various cytokines and chemokines are required for effective T cell infiltration. However, these targeted approaches may not be sufficient to prevent the rapid and massive cytokine storm caused by constructs engineered with high avidity binders toward the tumor-associated antigen and/or toward the CD3ε of the TCR.41 In such circumstances, the transient inhibition of the broad spectrum of cytokines and chemokines might more efficiently resolve CRS symptoms, as observed with glucocorticoids.
This sheds light on novel broader approaches, including the use of tyrosine kinase inhibitors, which interfere with signaling pathways downstream of TCR or CAR activation resulting in reduction of T cell-derived cytokine release.73,76,89,90,92 The BTK inhibitor ibrutinib is of particular interest for the mitigation of CRS associated with T cell engaging therapies against B cell malignancies like CLL or MCL, where BTK inhibition is pharmacologically active.86 When combined with CAR-T cells or T cell engagers, ibrutinib does not only prevent cytokine release but also exerts anti-tumor activity and related debulking effect, resulting in a safer and more efficacious treatment of CLL or MCL.79,81
The kinase inhibitor dasatinib was shown to suppress both cytokine release and T cell-mediated cytotoxicity following CAR or TCR activation.89,92 Therefore, it represents an attractive safety switch for the mitigation of severe CRS or other adverse events where glucocorticoids would not be sufficient to rapidly switch-off T cell functionality. In the clinical trial described by Foà et al., the BCR-Abl inhibitor dasatinib pre-depleted ALL tumor cells therefore reducing the risk of on-target CRS in the consolidation phase, where the combination of blinatumomab and dasatinib eliminated the residual tumor cells with few CRS events.95 During this step, dasatinib targeted Src and Lck kinases downstream of TCR activation by blinatumomab, preventing cytokine release and T cell-mediated cytotoxicity.97 Since the inhibitory properties of dasatinib are reversible, it most likely induced rapid ON/OFF switches that did not fully suppress blinatumomab activity and may have even hindered rapid T cell exhaustion.91
In contrast, the mTOR and JAK inhibitors were shown to suppress cytokine release while retaining T cell cytotoxicity after stimulation with CD3 bispecific antibodies.76 In the clinic, the JAK1/2 inhibitor ruxolitinib induced a change in cytokine profile, which was not sufficient to prevent CRS clinical signs, unless combined with glucocorticoids or other cytokine neutralizing antibodies.106 The side-by-side in vitro comparison of JAK and mTOR inhibitors reveals that the blockade of the mTOR pathway more broadly reduces cytokine release. Consequently, mTOR inhibitors represent attractive candidates for the mitigation of CRS. Nevertheless, this remains to be clinically tested.76
In the specific case of CD3 bispecific antibodies, these kinase inhibitors may be used as premedication on the first infusion for constructs at high risk for CRS. These compounds may not or only transiently interfere with treatment efficacy, benefiting from the combination of long PK/PD properties of large molecules with short PK/PD of small molecules. Another important feature of kinase inhibitors is their potential antitumor activity, which makes them ideal combination partners with T cell engaging therapies in the same cancer indications.
In addition to reducing cytokine release, these compounds may also induce epigenetic reprogramming of T cells, by interfering with signaling pathways downstream of TCR or CAR activation. One example is the use of dasatinib to prevent antigen-dependent T cell differentiation during the manufacturing of GRP78 CAR-T cells, by blocking CAR signaling post activation. As a result, GRP78 CAR-T cells effector functions were improved.113 Along those lines, the addition of PI3K inhibitor duvelisib during the manufacturing of CD19 CAR-T cells reprogrammed stem-cell like properties in the terminally differentiated T cells with exhausted phenotypes of CLL patients.114,115 An interesting aspect of kinase inhibitors to be further explored is their potential to reprogram T cells toward more functional phenotypes following CAR or TCR activation.
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Article highlights
The use of cytokine/cytokine receptor targeted antibodies for the mitigation of CRS
The use of target cell pre-depletion approaches and/or target cell masking antibodies
The engineering of CD3 binders of T cell engagers to reduce first infusion cytokine release
The emerging use of FDA-approved kinase inhibitors to prevent cytokine release following treatment with T cell engaging therapies
Disclosure statement
All the authors are employees of Roche. G. Leclercq, N. Steinhoff, H. Haegel, M. Bacac and C. Klein declare patents with Roche. H. Haegel, D. De Marco, M. Bacac and C, Klein declare ownership of Roche stock.
Data availability statement
Data are available on request.
References
- 1.Bacac M, Klein C, Umana P.. CEA TCB: a novel head-to-tail 2:1 T cell bispecific antibody for treatment of CEA-positive solid tumors. Oncoimmunol. 2016;5(8):e1203498. doi: 10.1080/2162402X.2016.1203498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bacac M, Fauti T, Sam J, Colombetti S, Weinzierl T, Ouaret D, Bodmer W, Lehmann S, Hofer T, Hosse RJ, et al. A novel carcinoembryonic antigen T-cell bispecific antibody (CEA TCB) for the treatment of solid tumors. Clin Cancer Res. 2016;22(13):3286–13. doi: 10.1158/1078-0432.CCR-15-1696. [DOI] [PubMed] [Google Scholar]
- 3.Bacac M, Colombetti S, Herter S, Sam J, Perro M, Chen S, Bianchi R, Richard M, Schoenle A, Nicolini V, et al. CD20-TCB with obinutuzumab pretreatment as next-generation treatment of hematologic malignancies. Clin Cancer Res. 2018;24(19):4785–4797. doi: 10.1158/1078-0432.CCR-18-0455. [DOI] [PubMed] [Google Scholar]
- 4.Ishiguro T, Sano Y, Komatsu SI, Kamata-Sakurai M, Kaneko A, Kinoshita Y, Shiraiwa H, Azuma Y, Tsunenari T, Kayukawa Y, et al. An anti-glypican 3/CD3 bispecific T cell-redirecting antibody for treatment of solid tumors. Sci Transl Med. 2017;9(410):eaal4291. doi: 10.1126/scitranslmed.aal4291. [DOI] [PubMed] [Google Scholar]
- 5.Depil S, Duchateau P, Grupp SA, Mufti G, Poirot L.. ‘Off-the-shelf’ allogeneic CAR T cells: development and challenges. Nat Rev Drug Discov. 2020;19(3):185–199. doi: 10.1038/s41573-019-0051-2. [DOI] [PubMed] [Google Scholar]
- 6.Ahmad A, Uddin S, Steinhoff M. CAR-T cell therapies: an overview of clinical studies supporting their approved use against acute lymphoblastic leukemia and large b-cell lymphomas. Int J Mol Sci. 2020;21(11):3906. doi: 10.3390/ijms21113906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goebeler M-E, Bargou R. Blinatumomab: a CD19/CD3 bispecific T cell engager (BiTE) with unique anti-tumor efficacy. Leukemia Lymphoma. 2016;57(5):1021–1032. doi: 10.3109/10428194.2016.1161185. [DOI] [PubMed] [Google Scholar]
- 8.Middleton MR, McAlpine C, Woodcock VK, Corrie P, Infante JR, Steven NM, Evans TRJ, Anthoney A, Shoushtari AN, Hamid O, et al. Tebentafusp, A TCR/anti-CD3 bispecific fusion protein targeting gp100, potently activated antitumor immune responses in patients with metastatic melanoma. Clin Cancer Res. 2020;26(22):5869–5878. doi: 10.1158/1078-0432.CCR-20-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klein C, Augsberger C, Xu W, Heitmüller C, Hanisch L, Sam J, Pulko V, Schönle A, Challier J, Carpy A, et al. Targeting Intracellular WT1 in AML utilizing a T cell bispecific antibody construct: augmenting efficacy through combination with lenalidomide. Blood. 2019;134(Supplement_1):4450. doi: 10.1182/blood-2019-130121. [DOI] [Google Scholar]
- 10.Augsberger C, Hänel G, Xu W, Pulko V, Hanisch LJ, Augustin A, Challier J, Hunt K, Vick B, Rovatti PE, et al. Targeting intracellular WT1 in AML with a novel RMF-peptide-MHC-specific T-cell bispecific antibody. Blood. 2021;138(25):2655–2669. doi: 10.1182/blood.2020010477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hutchings M, Morschhauser F, Iacoboni G, Carlo-Stella C, Offner FC, Sureda A, Salles G, Martínez-Lopez J, Crump M, Thomas DN, et al. Glofitamab, a novel, bivalent CD20-Targeting T-cell-engaging bispecific antibody, induces durable complete remissions in relapsed or refractory B-cell lymphoma: a phase I trial. J Clin Oncol offi Am J Clin Oncol. 2021;39(18):1959–1970. doi: 10.1200/JCO.20.03175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seckinger A, Delgado JA, Moser S, Moreno L, Neuber B, Grab A, Lipp S, Merino J, Prosper F, Emde M, et al. Target expression, generation, preclinical activity, and pharmacokinetics of the BCMA-T cell bispecific antibody EM801 for multiple myeloma treatment. Cancer Cell. 2017;31(3):396–410. doi: 10.1016/j.ccell.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Westin JR, Kersten MJ, Salles G, Abramson JS, Schuster SJ, Locke FL, Andreadis C. Efficacy and safety of CD19 -directed CAR-T cell therapies in patients with relapsed/refractory aggressive B-cell lymphomas: observations from the JULIET, ZUMA −1, and TRANSCEND trials. Am J Hematol. 2021;96(10):1295–1312. doi: 10.1002/ajh.26301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang M, Munoz J, Goy A, Locke FL, Jacobson CA, Hill BT, Timmerman JM, Holmes H, Jaglowski S, Flinn IW, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382(14):1331–1342. doi: 10.1056/NEJMoa1914347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teoh PJ, Chng WJ. CAR T-cell therapy in multiple myeloma: more room for improvement. Blood Cancer J. 2021;11(4):84. doi: 10.1038/s41408-021-00469-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berdeja JG, Madduri D, Usmani SZ, Jakubowiak A, Agha M, Cohen AD, Stewart AK, Hari P, Htut M, Lesokhin A, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet. 2021;398(10297):314–324. doi: 10.1016/S0140-6736(21)00933-8. [DOI] [PubMed] [Google Scholar]
- 17.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, Bader P, Verneris MR, Stefanski HE, Myers GD, et al. Tisagenlecleucel in children and young adults with b-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao J, Song Y, Liu D. Recent advances on blinatumomab for acute lymphoblastic leukemia. Exp Hematol Oncol. 2019;8(1):28. doi: 10.1186/s40164-019-0152-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goebeler M-E, Bargou RC. T cell-engaging therapies — Bites and beyond. Nat Rev Clin Oncol. 2020;17(7):418–434. doi: 10.1038/s41571-020-0347-5. [DOI] [PubMed] [Google Scholar]
- 20.Einsele H, Borghaei H, Orlowski RZ, Subklewe M, Roboz GJ, Zugmaier G, Kufer P, Iskander K, Kantarjian HM. The BiTE (Bispecific T-cell engager) platform: development and future potential of a targeted immuno-oncology therapy across tumor types. Cancer. 2020;126(14):3192–3201. doi: 10.1002/cncr.32909. [DOI] [PubMed] [Google Scholar]
- 21.Alexander Shimabukuro-Vornhagen PG, Subklewe M, Stemmler HJ, Schlößer HA, Schlaak M, Kochanek M, Böll B, von Bergwelt-Baildon MS. Cytokine release syndrome. J ImmunoTher Cancer. 2018 Jun 15;6(1):56. doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fitzgerald JC, Weiss SL, Maude SL, Barrett DM, Lacey SF, Melenhorst JJ, Shaw P, Berg RA, June CH, Porter DL, et al. Cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic leukemia. Crit Care Med. 2017;45(2):e124–e31. doi: 10.1097/CCM.0000000000002053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Obstfeld AE, Frey NV, Mansfield K, Lacey SF, June CH, Porter DL, Melenhorst JJ, Wasik MA. Cytokine release syndrome associated with chimeric-antigen receptor T-cell therapy: clinicopathological insights. Blood. 2017;130(23):2569–2572. doi: 10.1182/blood-2017-08-802413. [DOI] [PubMed] [Google Scholar]
- 24.Hay KA, Hanafi L-A, Li D, Gust J, Liles WC, Wurfel MM, López JA, Chen J, Chung D, Harju-Baker S, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood. 2017;130(21):2295–2306. doi: 10.1182/blood-2017-06-793141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karki R, Kanneganti T-D. The ‘cytokine storm’: molecular mechanisms and therapeutic prospects. Trends Immunol. 2021;42:681–705. doi: 10.1016/j.it.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.St Clair EW. The calm after the cytokine storm: lessons from the TGN1412 trial. J Clin Invest. 2008;118(4):1344–1347. doi: 10.1172/JCI35382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, Maus MV, Park JH, Mead E, Pavletic S, et al. ASTCT Consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625–638. doi: 10.1016/j.bbmt.2018.12.758. [DOI] [PubMed] [Google Scholar]
- 28.Shimabukuro-Vornhagen A, Gödel P, Subklewe M, Stemmler HJ, Schlößer HA, Schlaak M, Kochanek M, Böll B, von Bergwelt-Baildon MS. Cytokine release syndrome. J Immunother Cancer. 2018;6(1):56. doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norelli M, Camisa B, Barbiera G, Falcone L, Purevdorj A, Genua M, Sanvito F, Ponzoni M, Doglioni C, Cristofori P, et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med. 2018;24(6):739–748. doi: 10.1038/s41591-018-0036-4. [DOI] [PubMed] [Google Scholar]
- 30.Godbersen-Palmer C, Coupet TA, Grada Z, Zhang SC, Sentman CL. Toxicity induced by a bispecific T cell-redirecting protein is mediated by both T cells and myeloid cells in immunocompetent mice. J Immunol. 2020. Baltimore, Md: 1950;204(11):2973–2983. doi: 10.4049/jimmunol.1901401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giavridis T, van der Stegen SJC, Eyquem J, Hamieh M, Piersigilli A, Sadelain M. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med. 2018;24(6):731–738. doi: 10.1038/s41591-018-0041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J, Piskol R, Ybarra R, Chen YJ, Li J, Slaga D, Hristopoulos M, Clark R, Modrusan Z, Totpal K, et al. CD3 bispecific antibody-induced cytokine release is dispensable for cytotoxic T cell activity. Sci Transl Med. 2019;11(508). doi: 10.1126/scitranslmed.aax8861. [DOI] [PubMed] [Google Scholar]
- 33.Leclercq G, Servera LA, Danilin S, Challier J, Steinhoff N, Bossen C, Odermatt A, Nicolini V, Umaña P, Klein C, et al. Dissecting the mechanism of cytokine release induced by T-cell engagers highlights the contribution of neutrophils. OncoImmunol. 2022;11(1):2039432. doi: 10.1080/2162402X.2022.2039432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hong F, Shi M, Cao J, Wang Y, Gong Y, Gao H, Li Z, Zheng J, Zeng L, He A, et al. Predictive role of endothelial cell activation in cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic leukaemia. J Cell Mol Med. 2021;25(24):11063–11074. doi: 10.1111/jcmm.17029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morris EC, Neelapu SS, Giavridis T, Sadelain M. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat Rev Immunol. 2021;22(2):85–96. doi: 10.1038/s41577-021-00547-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rouce RH. The earlier the better: timely mitigation of CRS. Blood. 2019;134(24):2119–2120. doi: 10.1182/blood.2019003618. [DOI] [PubMed] [Google Scholar]
- 37.Carlo-Stella C, Khan C, Hutchings M, Offner FC, Morschhauser F, Bachy E, Crump M, Sureda A, Lacoboni G, Haioun C, et al. Glofitamab step-up dosing (SUD): updated efficacy data show high complete response rates in heavily pretreated relapsed/refractory (R/R) non-hodgkin lymphoma (NHL) patients (Pts). Cl Lymph Myelom Leuk. 2021;21:S394–S. doi: 10.1016/S2152-2650(21)01893-0. [DOI] [Google Scholar]
- 38.Subklewe M. BiTEs better than CAR T cells. Blood Adv. 2021;5(2):607–612. doi: 10.1182/bloodadvances.2020001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, Kamble RT, Bollard CM, Gee AP, Mei Z, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011;121(5):1822–1826. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dang K, Castello G, Clarke SC, Li Y, Balasubramani A, Boudreau A, Davison L, Harris KE, Pham D, Sankaran P, et al. Attenuating CD3 affinity in a PSMAxCD3 bispecific antibody enables killing of prostate tumor cells with reduced cytokine release. J ImmunoTher Cancer. 2021;9(6):e002488. doi: 10.1136/jitc-2021-002488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Staflin K, Zuch de Zafra CL, Schutt LK, Clark V, Zhong F, Hristopoulos M, Clark R, Li J, Mathieu M, Chen X, et al. Target arm affinities determine preclinical efficacy and safety of anti-HER2/CD3 bispecific antibody. JCI Insight. 2020;5(7). doi: 10.1172/jci.insight.133757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tedesco VE, Mohan C . Biomarkers for predicting cytokine release syndrome following CD19-targeted CAR T cell therapy. J Immunol. 2021; March 10:ji2001249. doi: 10.4049/jimmunol.2001249. [DOI] [PubMed] [Google Scholar]
- 43.Kamperschroer C, Shenton J, Lebrec H, Leighton JK, Moore PA, Thomas O. Summary of a workshop on preclinical and translational safety assessment of CD3 bispecifics. J Immunotoxicol. 2020;17:67–85 doi: 10.1080/1547691X.2020.1729902. [DOI] [PubMed] [Google Scholar]
- 44.Neelapu SS, Tummala S, Kebriaei P, Wierda W, Gutierrez C, Locke FL, Komanduri KV, Lin Y, Jain N, Daver N, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15(1):47–62. doi: 10.1038/nrclinonc.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khadka RH, Sakemura R, Kenderian SS, Johnson AJ. Management of cytokine release syndrome: an update on emerging antigen-specific T cell engaging immunotherapies. Immunother. 2019;11(10):851–857. doi: 10.2217/imt-2019-0074. [DOI] [PubMed] [Google Scholar]
- 46.Mori S, Nelson RJ, Patel RD, Ahmed WB. Low dose steroids can alleviate blinatumomab-associated toxicities without negatively impacting treatment efficacy. Blood. 2015;126(23):4875. doi: 10.1182/blood.V126.23.4875.4875. [DOI] [Google Scholar]
- 47.Liu S, Deng B, Yin Z, Pan J, Lin Y, Ling Z, Wu T, Chen D, Chang AH, Gao Z, et al. Corticosteroids do not influence the efficacy and kinetics of CAR-T cells for B-cell acute lymphoblastic leukemia. Blood Cancer J. 2020;10(2):15. doi: 10.1038/s41408-020-0280-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Riegler LL, Jones GP, Lee DW. Current approaches in the grading and management of cytokine release syndrome after chimeric antigen receptor T-cell therapy. Ther Clin Risk Manag. 2019;15:323–335. doi: 10.2147/TCRM.S150524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brandl C, Haas C, d’Argouges S, Fisch T, Kufer P, Brischwein K, Prang N, Bargou R, Suzich J, Baeuerle PA, et al. The effect of dexamethasone on polyclonal T cell activation and redirected target cell lysis as induced by a CD19/CD3-bispecific single-chain antibody construct. Cancer Immunol Immunother. 2007;56(10):1551–1563. doi: 10.1007/s00262-007-0298-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kauer J, Hörner S, Osburg L, Müller S, Märklin M, Heitmann JS, Zekri L, Rammensee H-G, Salih HR, Jung G, et al. Tocilizumab, but not dexamethasone, prevents CRS without affecting antitumor activity of bispecific antibodies. J ImmunoTher Cancer. 2020;8(1):e000621. doi: 10.1136/jitc-2020-000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hao Z, Li R, Meng L, Han Z, Hong Z. Macrophage, the potential key mediator in CAR-T related CRS. Exp Hematol Oncol. 2020;9(1):15. doi: 10.1186/s40164-020-00171-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Strati P, Ahmed S, Kebriaei P, Nastoupil LJ, Claussen CM, Watson G, Horowitz SB, Brown ART, Do B, Rodriguez MA, et al. Clinical efficacy of anakinra to mitigate CAR T-cell therapy–associated toxicity in large B-cell lymphoma. Blood Adv. 2020;4(13):3123–3127. doi: 10.1182/bloodadvances.2020002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang L, Wang S, Xu J, Zhang R, Zhu H, Wu Y, Zhu L, Li J, Chen L. Etanercept as a new therapeutic option for cytokine release syndrome following chimeric antigen receptor T cell therapy. Exp Hematol Oncol. 2021 Feb 19;10(1):16. doi: 10.1186/s40164-021-00209-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sterner RM, Sakemura R, Cox MJ, Yang N, Khadka RH, Forsman CL, Hansen MJ, Jin F, Ayasoufi K, Hefazi M, et al. GM-CSF inhibition reduces cytokine release syndrome and neuroinflammation but enhances CAR-T cell function in xenografts. Blood. 2019;133(7):697–709. doi: 10.1182/blood-2018-10-881722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sachdeva M, Duchateau P, Depil S, Poirot L, Valton J. Granulocyte-macrophage colony-stimulating factor inactivation in CAR T-cells prevents monocyte-dependent release of key cytokine release syndrome mediators. J Biol Chem. 2019;294(14):5430–5437. doi: 10.1074/jbc.AC119.007558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matthys P, Dillen C, Proost P, Heremans H, Van Damme J, Billiau A. Modification of the anti-CD3-induced cytokine release syndrome by anti-interferon-gamma or anti-interleukin-6 antibody treatment: protective effects and biphasic changes in blood cytokine levels. Eur J Immunol. 1993;23(9):2209–2216. doi: 10.1002/eji.1830230924. [DOI] [PubMed] [Google Scholar]
- 57.Bailey SR, Vatsa S, Larson RC, Bouffard AA, Scarfo I, Kann MC, Berger TR, Leick MB, Wehrli M, Schmidts A, et al. Blockade or deletion of IFNg reduces macrophage activation without compromising CAR-T function in hematologic malignancies. Blood Cancer Discovery. 2021;bloodcandisc.BCD-21-0181-E.2021. doi: 10.1158/2643-3230.BCD-21-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li J, Ybarra R, Mak J, Herault A, De Almeida P, Arrazate A, Ziai J, Totpal K, Junttila MR, Walsh KB, et al. IFNγ-induced chemokines are required for CXCR3-mediated T-cell recruitment and antitumor efficacy of anti-HER2/CD3 bispecific antibody. Clin Cancer Res. 2018;24(24):6447–6458. doi: 10.1158/1078-0432.CCR-18-1139. [DOI] [PubMed] [Google Scholar]
- 59.Lamers CH, Sleijfer S, van Steenbergen S, van Elzakker P, van Krimpen B, Groot C, Vulto A, den Bakker M, Oosterwijk E, Debets R, et al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol Ther J Am Soc Gene Ther. 2013;21(4):904–912. doi: 10.1038/mt.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morschhauser F, Carlo-Stella C, Offner F, Salles GA, Hutchings M, Iacoboni G, Fehler O, Hillebrand J, Schakaki M, Rojas J, et al. Dual CD20-targeted therapy with concurrent CD20-TCB and obinutuzumab shows highly promising clinical activity and manageable safety in relapsed or refractory B-cell non-hodgkin lymphoma: preliminary results from a phase Ib trial. Blood. 2019;134(2):134. doi: 10.1182/blood.2019000320. [DOI] [PubMed] [Google Scholar]
- 61.Haber L, Olson K, Kelly MP, Crawford A, DiLillo DJ, Tavaré R, Ullman E, Mao S, Canova L, Sineshchekova O, et al. Generation of T-cell-redirecting bispecific antibodies with differentiated profiles of cytokine release and biodistribution by CD3 affinity tuning. Sci Rep. 2021;11(1):14397. doi: 10.1038/s41598-021-93842-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang N, Patel H, Schneider IC, Kai X, Varshney AK, Zhou L. An optimal antitumor response by a novel CEA/CD3 bispecific antibody for colorectal cancers. Antibody Ther. 2021;4:90–100 doi: 10.1093/abt/tbab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trinklein ND, Pham D, Schellenberger U, Buelow B, Boudreau A, Choudhry P, Clarke SC, Dang K, Harris KE, Iyer S, et al. Efficient tumor killing and minimal cytokine release with novel T-cell agonist bispecific antibodies. mAbs. 2019;11(4):639–652. doi: 10.1080/19420862.2019.1574521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Poussin M, Sereno A, Wu X, Huang F, Manro J, Cao S, Carpenito C, Glasebrook A, Powell Jr DJ, Demarest SJ, et al. Dichotomous impact of affinity on the function of T cell engaging bispecific antibodies. J ImmunoTher Cancer. 2021;9(7):e002444. doi: 10.1136/jitc-2021-002444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mandikian D, Takahashi N, Lo AA, Li J, Eastham-Anderson J, Slaga D, Ho J, Hristopoulos M, Clark R, Totpal K, et al. Relative target affinities of T-cell-dependent bispecific antibodies determine biodistribution in a solid tumor mouse model. Mol Cancer Ther. 2018;17(4):776–785. doi: 10.1158/1535-7163.MCT-17-0657. Epub 2018 Jan 16. PMID: 29339550. [DOI] [PubMed] [Google Scholar]
- 66.Geiger M, Stubenrauch K-G, Sam J, Richter WF, Jordan G, Eckmann J, Hage C, Nicolini V, Freimoser-Grundschober A, Ritter M, et al. Protease-activation using anti-idiotypic masks enables tumor specificity of a folate receptor 1-T cell bispecific antibody. Nat Commun. 2020;11(1):3196. doi: 10.1038/s41467-020-16838-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang J, Xing C, Liu H, Cugnetti APG, Wheeler C, Lucas M, Frey G, Chang C, Boyle WJ, Short JM. Abstract 4560: conditionally active biologics (CAB): a novel class of molecules targeting solid tumors. Cancer Res. 2020;80(16 Supplement):4560. doi: 10.1158/1538-7445.AM2020-4560. [DOI] [Google Scholar]
- 68.Cugnetti APG, Liu H, Wang J, Xing C, Wheeler C, Lucas M, Chang C, Frey G, Boyle WJ, Short JM. Abstract 5698: novel conditionally active bispecific T cell engagers targeting solid tumors. Cancer Res. 15 August 2020;80(16 Supplement):5698. doi: 10.1158/1538-7445.AM2020-5698. [DOI] [Google Scholar]
- 69.Darowski D, Kobold S, Jost C, Klein C. Combining the best of two worlds: highly flexible chimeric antigen receptor adaptor molecules (CAR-adaptors) for the recruitment of chimeric antigen receptor T cells. mAbs. 2019;11(4):621–631. doi: 10.1080/19420862.2019.1596511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Giordano-Attianese G, Gainza P, Gray-Gaillard E, Cribioli E, Shui S, Kim S, Kwak M-J, Vollers S, Corria Osorio ADJ, Reichenbach P, et al. A computationally designed chimeric antigen receptor provides a small-molecule safety switch for T-cell therapy. Nat Biotechnol. 2020;38(4):426–432. doi: 10.1038/s41587-019-0403-9. [DOI] [PubMed] [Google Scholar]
- 71.Zheng Y, Nandakumar KS, Cheng K. Optimization of CAR-T cell-based therapies using small-molecule-based safety switches. J Med Chem. 2021;64(14):9577–9591. doi: 10.1021/acs.jmedchem.0c02054. [DOI] [PubMed] [Google Scholar]
- 72.Labanieh L, Majzner RG, Klysz D, Sotillo E, Fisher CJ, Vilches-Moure JG, Pacheco KZB, Malipatlolla M, Xu P, Hui JH, et al. Enhanced safety and efficacy of protease-regulated CAR-T cell receptors. Cell. 2022;185(10):1745–1763.e22. doi: 10.1016/j.cell.2022.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Amatya P, Cooper ML, Carter AJ, DiPersio JF. Identification of small molecule kinase inhibitors that potently and reversibly block chimeric antigen receptor T cell proliferation and cytotoxicity. Blood. 2019;134(Supplement_1):2068. doi: 10.1182/blood-2019-126165. [DOI] [Google Scholar]
- 74.Dufva O, Koski J, Maliniemi P, Ianevski A, Klievink J, Leitner J, Pölönen P, Hohtari H, Saeed K, Hannunen T, et al. Integrated drug profiling and CRISPR screening identify essential pathways for CAR T-cell cytotoxicity. Blood. 2020;135(9):597–609. doi: 10.1182/blood.2019002121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ramakrishna S, Davis KL. Supercharging your CAR. Blood. 2020;135:593–594. doi: 10.1182/blood.2019004469 [DOI] [PubMed] [Google Scholar]
- 76.Leclercq G, Haegel H, Toso A, Zimmermann T, Green L, Steinhoff N, Sam J, Pulko V, Schneider A, Giusti AM, et al. JAK and mTOR inhibitors prevent cytokine release while retaining T cell bispecific antibody in vivo efficacy. J ImmunoTher Cancer. 2022;10(1):e003766. doi: 10.1136/jitc-2021-003766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Assi R, Kantarjian H, Short NJ, Daver N, Takahashi K, Garcia-Manero G, DiNardo C, Burger J, Cortes J, Jain N, et al. Safety and efficacy of blinatumomab in combination with a tyrosine kinase inhibitor for the treatment of relapsed Philadelphia chromosome-positive leukemia. Clin Lymphoma Myeloma Leuk. 2017 Dec;17(12):897–901. doi: 10.1016/j.clml.2017.08.101. Epub 2017 Aug 18. PMID: 28927784. [DOI] [PubMed] [Google Scholar]
- 78.King AC, Pappacena JJ, Tallman MS, Park JH, Geyer MB. Blinatumomab administered concurrently with oral tyrosine kinase inhibitor therapy is a well-tolerated consolidation strategy and eradicates measurable residual disease in adults with Philadelphia chromosome positive acute lymphoblastic leukemia. Leuk Res. 2019;79:27–33. doi: 10.1016/j.leukres.2019.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ruella M, Kenderian SS, Shestova O, Fraietta JA, Qayyum S, Zhang Q, Maus MV, Liu X, Nunez-Cruz S, Klichinsky M, et al. The addition of the BTK inhibitor ibrutinib to anti-CD19 chimeric antigen receptor T cells (CART19) improves responses against mantle cell lymphoma. Clin Cancer Res. 2016;22(11):2684–2696. doi: 10.1158/1078-0432.CCR-15-1527. [DOI] [PubMed] [Google Scholar]
- 80.Ruella M, Kenderian SS, Shestova O, Klichinsky M, Melenhorst JJ, Wasik MA, Lacey SF, June CH, Gill S. Kinase inhibitor ibrutinib to prevent cytokine-release syndrome after anti-CD19 chimeric antigen receptor T cells for B-cell neoplasms. Leukemia. 2017;31(1):246–248. doi: 10.1038/leu.2016.262. [DOI] [PubMed] [Google Scholar]
- 81.Godwin CD, Bates OM, Garling EE, Beddoe ME, Laszlo GS, Walter RB. The Bruton’s tyrosine kinase inhibitor ibrutinib abrogates bispecific antibody-mediated T-cell cytotoxicity. Br J Haematol. 2020;189(1):e9–e13. doi: 10.1111/bjh.16406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dubovsky JA, Beckwith KA, Natarajan G, Woyach JA, Jaglowski S, Zhong Y, Hessler JD, Liu T-M, Chang BY, Larkin KM, et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood. 2013;122(15):2539–2549. doi: 10.1182/blood-2013-06-507947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mhibik M, Wiestner A, Sun C. Harnessing the effects of BTKi on T cells for effective immunotherapy against CLL. Int J Mol Sci. 2019;21(1):68. doi: 10.3390/ijms21010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Molina-Cerrillo J, Alonso-Gordoa T, Gajate P, Grande E. Bruton’s tyrosine kinase (BTK) as a promising target in solid tumors. Cancer Treat Rev. 2017;58:41–50. doi: 10.1016/j.ctrv.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 85.Gauthier J, Hirayama AV, Purushe J, Hay KA, Lymp J, Li DH, Yeung CCS, Sheih A, Pender BS, Hawkins RM, et al. Feasibility and efficacy of CD19-targeted CAR T cells with concurrent ibrutinib for CLL after ibrutinib failure. Blood. 2020;135(19):1650–1660. doi: 10.1182/blood.2019002936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kater AP, Melenhorst JJ. CAR-T and ibrutinib vs CLL: sequential or simultaneous?. Blood. 2020;135(19):1611–1612. doi: 10.1182/blood.2020005362. [DOI] [PubMed] [Google Scholar]
- 87.Lujan JV, Lengerke-Diaz PA, Jacobs C, Moreno-Cortes EF, Ramirez-Segura CA, Choi MY, McCarthy C, Heinen A, Kipps TJ, Castro JE. Ibrutinib reduces obinutuzumab infusion-related reactions in patients with chronic lymphocytic leukemia and is associated with changes in plasma cytokine levels. Haematologica. 2020;105(1):e22–e5. doi: 10.3324/haematol.2018.212597. Epub 2019 May 2. PMID: 31048356; PMCID: PMC6939526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Greil R, Tedeschi A, Moreno C, Anz B, Larratt L, Simkovic M, Gill D, Gribben JG, Flinn IW, Wang Z, et al. Pretreatment with ibrutinib reduces cytokine secretion and limits the risk of obinutuzumab-induced infusion-related reactions in patients with CLL: analysis from the iLLUMINATE study. Ann Hematol. 2021;100(7):1733–1742. doi: 10.1007/s00277-021-04536-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mestermann K, Giavridis T, Weber J, Rydzek J, Frenz S, Nerreter T, Mades A, Sadelain M, Einsele H, Hudecek M, et al. The tyrosine kinase inhibitor dasatinib acts as a pharmacologic on/off switch for CAR T cells. Sci Transl Med. 2019;11(499):eaau5907. doi: 10.1126/scitranslmed.aau5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Weber EW, Lynn RC, Sotillo E, Lattin J, Xu P, Mackall CL. Pharmacologic control of CAR-T cell function using dasatinib. Blood Adv. 2019;3(5):711–717. doi: 10.1182/bloodadvances.2018028720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Weber EW, Parker KR, Sotillo E, Lynn RC, Anbunathan H, Lattin J, Good Z, Belk JA, Daniel B, Klysz D, et al. Transient rest restores functionality in exhausted CAR-T cells through epigenetic remodeling. Science (New York, NY). 2021;372(6537). doi: 10.1126/science.aba1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Leclercq G, Haegel H, Schneider A, Giusti AM, Marrer-Berger E, Boetsch C, Walz A-C, Pulko V, Sam J, Challier J, et al. Src/lck inhibitor dasatinib reversibly switches off cytokine release and T cell cytotoxicity following stimulation with T cell bispecific antibodies. J ImmunoTher Cancer. 2021;9(7):e002582. doi: 10.1136/jitc-2021-002582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Leclercq G, Haegel H, Schneider A, Berger EM, Walz A, Boetsch C, Pulko V, Ferlini C, Klein C. 653 Dasatinib as a rapid pharmacological ON/OFF switch for T cell bispecific antibody-induced T cell activation and cytokine release. J ImmunoTher Cancer. 2020;8(Suppl 3):A690–A. doi: 10.1136/jitc-2020-SITC2020.0653. [DOI] [Google Scholar]
- 94.Fasslrinner F, Arndt C, Koristka S, Feldmann A, Altmann H, von Bonin M, Schmitz M, Bornhäuser M, Bachmann M. Midostaurin abrogates CD 33-directed Uni CAR and CD 33- CD 3 bispecific antibody therapy in acute myeloid leukaemia. Br J Haematol. 2019;186(5):735–740. doi: 10.1111/bjh.15975. [DOI] [PubMed] [Google Scholar]
- 95.Foà R, Bassan R, Vitale A, Elia L, Piciocchi A, Puzzolo MC, Canichella M, Viero P, Ferrara F, Lunghi M, et al. Dasatinib-blinatumomab for Ph-positive acute lymphoblastic leukemia in adults. N Engl J Med. 2020;383(17):1613–1623. doi: 10.1056/NEJMoa2016272. [DOI] [PubMed] [Google Scholar]
- 96.Hoelzer D. Chemotherapy-free treatment — a new era in acute lymphoblastic leukemia?. N Engl J Med. 2020;383(17):1673–1674. doi: 10.1056/NEJMe2027937. [DOI] [PubMed] [Google Scholar]
- 97.Leonard JT, Kosaka Y, Malla P, LaTocha D, Lamble A, Hayes-Lattin B, Byrd K, Druker BJ, Tyner JW, Chang BH, et al. Concomitant use of a dual Src/ABL kinase inhibitor eliminates the in vitro efficacy of blinatumomab against Ph+ ALL. Blood. 2021;137(7):939–944. doi: 10.1182/blood.2020005655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Virtanen AT, Haikarainen T, Raivola J, Silvennoinen O. Selective JAKinibs: prospects in inflammatory and autoimmune diseases. BioDrugs. 2019;33(1):15–32. doi: 10.1007/s40259-019-00333-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huarte E, Peel MT, Verbist K, Fay BL, Bassett R, Albeituni S, Nichols KE, Smith PA. Ruxolitinib, a JAK1/2 inhibitor, ameliorates cytokine storm in experimental models of hyperinflammation syndrome. Front Pharmacol. 2021;12:650295. doi: 10.3389/fphar.2021.650295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hu X, li J, Fu M, Zhao X, Wang W. The JAK/STAT signaling pathway: from bench to clinic. Signal Transduction and Targeted Ther. 2021;6(1):402. doi: 10.1038/s41392-021-00791-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kenderian SS, Ruella M, Shestova O, Kim M, Klichinsky M, Chen F, Kengle N, Lacey S, Melenhorst J, June CH, et al. 2 - ruxolitinib prevents cytokine release syndrome after car T-cell therapy without impairing the anti-tumor effect in a xenograft model. Biol Blood Marrow Transplant. 2017;23(3, Supplement):S19–S20. doi: 10.1016/j.bbmt.2016.12.003. [DOI] [Google Scholar]
- 102.Xu N, Yang X-F, Xue S-L, Tan J-W, M-H L, Ye J, Lou X-Y, Yu Z, Kang L-Q, Yan Z-Q, et al. Ruxolitinib reduces severe CRS response by suspending CAR-T cell function instead of damaging CAR-T cells. Biochem Biophys Res Commun. 2022;595:54–61. doi: 10.1016/j.bbrc.2022.01.070. [DOI] [PubMed] [Google Scholar]
- 103.Huarte E, O’Connor RS, Peel MT, Nunez-Cruz S, Leferovich J, Juvekar A, Yang Y-O, Truong L, Huang T, Naim A, et al. Itacitinib (INCB039110), a JAK1 inhibitor, reduces cytokines associated with cytokine release syndrome induced by CAR T-cell therapy. Clin Cancer Res. 2020;26(23):6299–6309. doi: 10.1158/1078-0432.CCR-20-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wei S, Gu R, Xu Y, Liu X, Xing Y, Gong X, Zhou C, Liu B, Zhang G, Liu K, et al. Adjuvant ruxolitinib therapy relieves steroid-refractory cytokine-release syndrome without impairing chimeric antigen receptor-modified T-cell function. Immunother. 2020;12(14):1047–1052. doi: 10.2217/imt-2020-0116. [DOI] [PubMed] [Google Scholar]
- 105.Li S, Wang X, Yuan Z, Liu L, Luo L, Li Y, Wu K, Liu J, Yang C, Li Z, et al. Eradication of T-ALL cells by CD7 targeted universal CAR-T cells and initial test of ruxolitinib-based CRS management. Clin Cancer Res. 2021 Mar 1;.27(5):1242-1246. doi: 10.1158/1078-0432.CCR-20-1271. [DOI] [PubMed] [Google Scholar]
- 106.Uy GL, Rettig MP, Christ S, Aldoss I, Byrne MT, Erba HP, Arellano ML, Foster MC, Godwin JE, Ravandi F, et al. Prophylactic ruxolitinib for cytokine release syndrome (CRS) in relapse/refractory (R/R) AML patients treated with flotetuzumab. Blood. 2020;136(Supplement 1):19–21. doi: 10.1182/blood-2020-134612. [DOI] [Google Scholar]
- 107.Park JH, Frigault MJ, Maziarz RT, Naim A, Burke L, Tian C, Porter DL. Trial in progress: a phase 2, single-arm, open-label study of itacitinib (ITA) for the prevention of chimeric antigen receptor (CAR) T-cell–induced cytokine release syndrome (CRS). Biol Blood Marrow Transplant. 2020;26(3, Supplement):S269. doi: 10.1016/j.bbmt.2019.12.436. [DOI] [Google Scholar]
- 108.Xu T, Sun D, Chen Y, Ouyang L. Targeting mTOR for fighting diseases: a revisited review of mTOR inhibitors. Eur J Med Chem. 2020;199:112391. doi: 10.1016/j.ejmech.2020.112391. [DOI] [PubMed] [Google Scholar]
- 109.Thudium K, Gallo J, Bouillaud E, Sachs C, Eddy S, Cheung W. Bioavailability of everolimus administered as a single 5 mg tablet versus five 1 mg tablets: a randomized, open-label, two-way crossover study of healthy volunteers. Clin Pharmacol Adv Appl. 2015;7:11–17. doi: 10.2147/CPAA.S73472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hua H, Kong Q, Zhang H, Wang J, Luo T, Jiang Y. Targeting mTOR for cancer therapy. J Hematol Oncol. 2019;12(1):71. doi: 10.1186/s13045-019-0754-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xie J, Wang X, Proud CG. mTOR inhibitors in cancer therapy. F1000Research. 2016;5:2078. doi: 10.12688/f1000research.9207.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Esfahani K, Al-Aubodah TA, Thebault P, Lapointe R, Hudson M, Johnson NA, Baran D, Bhulaiga N, Takano T, Cailhier J-F, et al. Targeting the mTOR pathway uncouples the efficacy and toxicity of PD-1 blockade in renal transplantation. Nat Commun. 2019;10(1):4712. doi: 10.1038/s41467-019-12628-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hebbar N, Epperly R, Vaidya A, Thanekar U, Moore SE, Umeda M, Ma J, Patil SL, Langfitt D, Huang S, et al. CAR T cells redirected to cell surface GRP78 display robust anti-acute myeloid leukemia activity and do not target hematopoietic progenitor cells. Nat Commun. 2022;13(1):587. doi: 10.1038/s41467-022-28243-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Funk CR, Wang S, Chen KZ, Waller A, Sharma A, Edgar CL, Gupta VA, Chandrakasan S, Zoine JT, Fedanov A, et al. PI3Kδ/γ inhibition promotes human CART cell epigenetic and metabolic reprogramming to enhance antitumor cytotoxicity. Blood. 2022;139(4):523–537. doi: 10.1182/blood.2021011597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Peters FS, Kater AP. Increasing CART cell engine performance in CLL. Blood. 2022;139(4):473–474. doi: 10.1182/blood.2021013895. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on request.


