Abstract
In 1995, journalist Gary Taubes published an article in Science titled “Epidemiology faces its limits,” which questioned the utility of nonrandomized epidemiologic research and has since been cited more than 1000 times. He highlighted numerous examples of research topics he viewed as having questionable merit. Studies have since accumulated for these associations. We systematically evaluated current evidence of 53 example associations discussed in the article. Approximately one-quarter of those presented as doubtful are now widely viewed as causal based on current evaluations of the public health consensus. They include associations between alcohol consumption and breast cancer, residential radon exposure and lung cancer, and the use of tanning devices and melanoma. This history should inform current debates about the reproducibility of epidemiologic research results.
Many examples used in Taubes’ influential 1995 article to denigrate epidemiology now have important public health implications.
INTRODUCTION
In July of 1995, journalist Taubes (1) published an influential news article in Science titled, “Epidemiology faces its limits.” The main emphasis was that epidemiology was “straying beyond the limits of the possible no matter how carefully the studies are done” because nonrandomized studies are so “plagued with biases, uncertainties, and methodological weaknesses that they may be inherently incapable of accurately discerning… weak associations.” The science of epidemiology, Taubes argued, studied a “mind-numbing array of potential disease-causing agents,” yielding an onslaught of “constitutionally contradictory” results.
The article prompted an immediate defense by the epidemiologic community (2–7), but together, these defenses have been cited eightfold less often than the original Taubes paper. Therefore, despite these contemporary objections, the Taubes article has exerted a sustained influence on epidemiologists’ self-impression and the impression of epidemiology in the wider scientific community. It has been cited more than 1000 times (fig. S1), with most citations relying on the article to cast doubt on the value of epidemiologic research (8). Examples of papers published in 2019–2021 (of 59 citations during this time period) cite the 1995 Taubes article to support the following contentions:
1) “Identifying major risk factors for disease can lead to misleading results when studying large numbers of variables interacting at multiple social, spatial, and temporal scales” (9)
2) “In [evidence-based medicine], observational studies are taken to suffer from different biases and confounding” (10)
3) “[epidemiology is among the fields] where serious concerns and debates about low reproducibility have been raised” (11)
4) “[…] the vast majority of epidemiological literature has important limitations, which are magnified when assessing low-dose effects” (12)
In the article by Taubes, he highlighted a substantial number of associations between exposures and outcomes as having questionable merit. In his introduction to a table listing many of these example associations, Taubes wrote, “Are these dangers real? As the saying goes, you be the judge.” To our knowledge, no one has taken up the challenge. Twenty-five years after the publication of this influential article, we systematically evaluated the epidemiologic evidence surrounding each of Taubes’ example associations. For each, we searched for a recent and representative meta-analysis of the posited association and whether there have been public health recommendations by credible authorities made to address it.
RESULTS
Updated causal evaluations
We identified 70 example associations in the Taubes (1995) paper. We excluded 14 duplicate associations and 3 associations that were too vaguely worded for causal evaluation (e.g., “a few drugs and cancer”). Thus, 53 associations remained for assessment of causality (table S1). We obtained a recent and representative meta-analysis for 49 of these associations. We did not identify meta-analyses related to the use of phenoxy herbicides on lawns and malignant lymphoma in dogs, having shorter or longer than average menstrual cycles and breast cancer, or the use of the antihypertension medication reserpine and breast cancer. We obtained a credible consensus statement for evaluating causality for 51 associations. No consensus statements from authoritative public health organizations were found for having shorter or longer than average menstrual cycles and breast cancer or the use of the antihypertension medication reserpine and breast cancer. These two associations were automatically deemed indeterminate.
Table 1 presents comparisons of the assumed causal evaluation by Taubes (1995) and our group (2021). Full results with identified meta-analyses and consensus statements by association are included in the Supplementary Materials (table S2, A to WW). Among these, 11 example associations were provided by Taubes as evidence of causal associations, and our group concurs. They include the association between smoking and lung cancer, human papillomavirus and cancer, and ionizing radiation and cancer. One association (saccharine and bladder cancer) was presented as an example of a noncausal association, and our group concurs. The remaining 41 associations were suggested by Taubes as likely false-positive associations. Of these, our group considered 11 (27%) as causal and 5 (12%) as noncausal based on public health statements by authoritative bodies. For example, the associations between alcohol consumption and breast cancer, residential radon exposure and lung cancer, and use of tanning devices and melanoma were suggested by Taubes as false positives and are now considered causal. On the basis of our updated assessment, examples of noncausal associations include those between coffee and pancreatic cancer and induced abortion and breast cancer. The remaining 25 (61%) associations were deemed indeterminate by our group. These included the association between hair dyes and several hematologic malignancies, eating red meat and breast cancer, and drinking chlorinated tap water and bladder cancer.
Table 1. Updated causal evaluations (2021) for abstracted associations discussed in Taubes paper (1995).
Full results with identified meta-analyses and consensus statements are included in the Supplementary Materials. ACOG, American College of Obstetricians and Gynecologists; AHA, American Heart Association; APHA, American Public Health Association; AUA, American Urological Association; FDA, U.S. Food and Drug Administration; IARC, International Agency for Research on Cancer; NCI, U.S. National Cancer Institute; WCRF/AICR, World Cancer Research Fund/American Institute for Cancer Research.
| Exposure | Outcome |
Taubes causal evaluation
(1995) |
Updated causal
evaluation (2021) |
Evaluation by authoritative
bodies |
| Human papillomavirus | Cancer | Causal | Causal | IARC: group 1 carcinogen |
| Ionizing radiation | Cancer | Causal | Causal | IARC: group 1 carcinogen |
| Hepatitis virus | Cancer | Causal | Causal | IARC: group 1 carcinogen |
| Smoking | Lung cancer | Causal | Causal | IARC: group 1 carcinogen |
| Cigarette smoke | Cancer | Causal | Causal | IARC: group 1 carcinogen |
| Sunlight | Skin cancer | Causal | Causal | IARC: group 1 carcinogen |
| Alcohol | Cancer | Causal | Causal | IARC: group 1 carcinogen |
| Asbestos | Cancer | Causal | Causal | IARC: group 1 carcinogen |
| Occupational steel (coke-oven) exposure | Lung cancer | Causal | Causal | IARC: group 1 carcinogen |
| Early childbirth (maternal age) | Breast cancer | Causal | Causal | WCRF/AICR: established cause of breast cancer |
| Human T cell leukemia virus | Cancer | Causal | Causal | IARC: group 1 carcinogen |
| Obesity | Esophageal cancer | Indeterminate | Causal | IARC: group 1 carcinogen |
| Cigarette smoke | Pancreatic cancer | Indeterminate | Causal | IARC: group 1 carcinogen |
| Lengthy occupational exposure to dioxin (2,3,7,8-tetrachlorodibenzodioxin) |
All cancers | Indeterminate | Causal | IARC: group 1 carcinogen |
| Alcohol | Breast cancer | Indeterminate | Causal | IARC: group 1 carcinogen |
| Residential radon | Lung cancer | Indeterminate | Causal | IARC: group 1 carcinogen |
| Eating red meat | Colon cancer | Indeterminate | Causal | IARC: group 2A probably carcinogenic to humans |
| High birthweight | Breast cancer | Indeterminate | Causal | WCRF/AICR: “There is strong evidence that factors that lead to a greater birthweight, or its consequences, increase the risk of premenopausal breast cancer” |
| Oral contraceptive use | Breast cancer | Indeterminate | Causal | IARC: group 1 carcinogen for estrogen-progestogen oral contraceptives (combined) and breast cancer |
| Sun lamp use | Melanoma | Indeterminate | Causal | IARC: group 1 carcinogen |
| Eating processed meats | Colon cancer | Indeterminate | Causal | IARC: group 1 carcinogen |
| Breastfeeding | Childhood leukemia/brain cancer |
Indeterminate | Causal | NCI: “Being breastfed and having been exposed to routine childhood infections are both associated with a lowered risk of developing childhood leukemia” |
| High-alcohol mouthwash | Mouth cancer | Indeterminate | Indeterminate | FDA: “The available data do not support a causal relationship between the use of alcohol-containing mouthrinses and oral cancer” |
| Electromagnetic fields | Childhood leukemia/brain cancer |
Indeterminate | Indeterminate | IARC: group 2B possibly carcinogenic to humans for limited evidence in relation to childhood leukemia. Inadequate evidence for all other cancers. |
| Traffic density | Childhood leukemia/brain cancer |
Indeterminate | Indeterminate | IARC: “[...] consistent association between exposure to benzene and AML for children, and coherence with findings for adult AML and benzene exposure, but could not rule out chance, bias, and confounding as alternative explanations” |
| High-cholesterol diet | Rectal cancer in men | Indeterminate | Indeterminate | WCRF/AICR 2018 Colorectal Cancer Report: limited to no conclusion for cholesterol |
| Douching | Cervical cancer | Indeterminate | Indeterminate | APHA: linked with cervical cancer but difficult to determine causality |
| Occupational stress | Colorectal cancer | Indeterminate | Indeterminate | NCI: “Although stress can cause a number of physical health problems, the evidence that it can cause cancer is weak” |
| Smoking | Fatal breast cancer | Indeterminate | Indeterminate | Komen: “Growing evidence suggests smoking lowers the chances of survival for women with breast cancer” |
| Hair dyes | Myeloma | Indeterminate | Indeterminate | IARC: group 3 not classifiable |
| Chlorinated tap water | Bladder cancer | Indeterminate | Indeterminate | IARC: group 3 not classifiable as to its carcinogenicity to humans; there was inadequate evidence of carcinogenicity in both humans and animals for chlorinated drinking water |
| Eating yogurt | Ovarian cancer | Indeterminate | Indeterminate | WCRF/AICR 2014 Ovarian Cancer Report: limited to no conclusion for milk and dairy products |
| Hair dyes | Lymphoma | Indeterminate | Indeterminate | IARC: group 3 not classifiable |
| Electromagnetic fields | Brain cancer | Indeterminate | Indeterminate | IARC: group 2B possibly carcinogenic to humans for limited evidence in relation to childhood leukemia. Inadequate evidence for all other cancers. |
| Hair dyes | Leukemia | Indeterminate | Indeterminate | IARC: group 3 not classifiable |
| Smoking | Breast cancer | Indeterminate | Indeterminate | IARC evaluation noted a positive association but did not state that tobacco smoking was a cause of breast cancer. |
| Diet high in saturated fat | Lung cancer | Indeterminate | Indeterminate | WCRF/AICR 2017 Lung Cancer Report: limited to no conclusion for total fat and animal fat |
| Electromagnetic fields | Leukemia | Indeterminate | Indeterminate | IARC: group 2B possibly carcinogenic to humans for limited evidence in relation to childhood leukemia. Inadequate evidence for all other cancers. |
| Fat intake | Breast cancer | Indeterminate | Indeterminate | WCRF/AICR 2017 Breast Cancer Report: limited to no conclusion for fats and oils, vegetable fat, fatty acid composition, trans fatty acids, cholesterol |
| Maternal smoking | Childhood leukemia/brain cancer |
Indeterminate | Indeterminate | IARC: Limited evidence in humans for tobacco smoking and childhood leukemia (in smokers’ children); “[...] a fairly consistent association of paternal tobacco smoking with childhood cancers is beginning to emerge, which is stronger in studies with more specific exposure assessments” |
| Eating red meat | Breast cancer | Indeterminate | Indeterminate | WCRF/AICR 2017 Breast Cancer Report: limited to no conclusion for red and processed meat |
| Electromagnetic fields | Breast cancer | Indeterminate | Indeterminate | IARC: group 2B possibly carcinogenic to humans for limited evidence in relation to childhood leukemia. Inadequate evidence for all other cancers. |
| Coffee | Heart disease | Indeterminate | Indeterminate | AHA: “Moderate coffee drinking (1–2 cups/day) does not seem to be harmful” |
| Consuming olive oil | Breast cancer | Indeterminate | Indeterminate | WCRF/AICR 2017 Breast Cancer Report: limited to no conclusion for fats and oils |
| Use of phenoxy herbicides on lawns | Malignant lymphoma in dogs |
Indeterminate | Indeterminate | IARC: There is limited evidence in experimental animals for the carcinogenicity of 2,4-dichlorophenoxyacetic acid |
| Having shorter or longer than average menstrual cycles |
Breast cancer | Indeterminate | Indeterminate | None identified |
| Antihypertensive medication reserpine | Breast cancer | Indeterminate | Indeterminate | None identified |
| Coffee | Pancreatic cancer | Indeterminate | Not causal | IARC: There is evidence suggesting lack of carcinogenicity |
| Vasectomy | Prostate cancer | Indeterminate | Not causal | AUA: “Clinicians do not need to routinely discuss prostate cancer […] in prevasectomy counseling of patients because vasectomy is not a risk factor for these conditions. Standard (evidence strength: grade B)” |
| Breast self-examination | Breast cancer mortality | Indeterminate | Not causal | ACOG: “Breast self- examination is not recommended in average- risk women because there is a risk of harm from false-positive test results and a lack of evidence of benefit.” |
| Abortion | Breast cancer | Indeterminate | Not causal | ACOG: no causal relationship |
| Dichlorodiphenyltrichloroethane | Breast cancer | Indeterminate | Not causal | IARC: no association overall; however, the potential influence of age at exposure to dichlorodiphenyltrichloroethane in relation to risk of breast cancer remains of interest |
| Saccharine | Bladder cancer | Not causal | Not causal | IARC: group 3 not classifiable |
Summary-relative risks by causal evaluation status
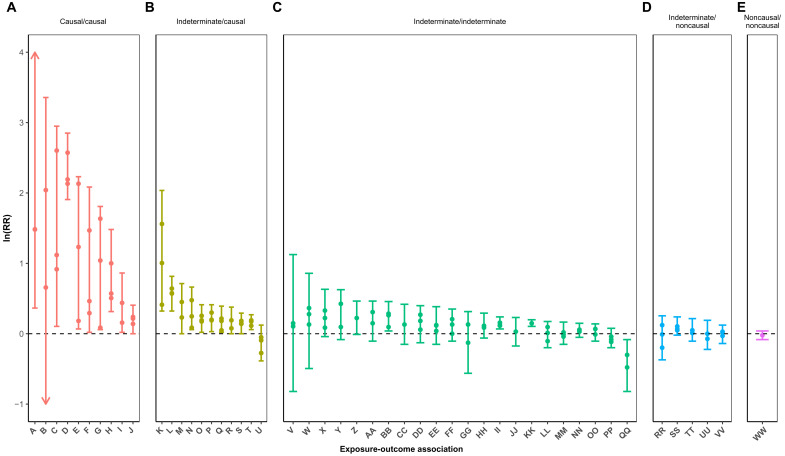
Figure 1 examines the selected meta-analytic estimates by causal evaluation status. Meta-analytic estimates for the association between human T cell leukemia virus and cancer were not included in the figure as the estimates were not summary-relative risks. Meta-analyses for ionizing radiation and cancer were included, but estimates are summary excess-relative risks. Meta-analytic estimates were most robust for associations deemed causal by both Taubes (1995) and our group (2021). Meta-analytic estimates were more modest and closer to the null for associations Taubes (1995) deemed indeterminate and for those that our group (2021) determined as causal, with the exception of the association between obesity and esophageal cancer.
Fig. 1. Selected meta-analytic estimates were strongest for associations deemed causal by both Taubes (1995) and our group (2021) and were more modest for associations deemed indeterminate by Taubes (1995) and causal by our group (2021).
Meta-analytic estimates by causal evaluation (Taubes’ 1995 Assessment/Updated 2021 Assessment) for (A) causal/causal, (B) indeterminate/causal, (C) indeterminate/indeterminate, (D) indeterminate/noncausal, and (E) noncausal/noncausal. Arrows indicate that estimate is beyond the y-axis range. Points indicate meta-analytic estimates, and segment end caps indicate minimum and maximum values of the 95% confidence intervals (CIs). Key: A, human papillomavirus and cancer; B, ionizing radiation and cancer; C, hepatitis and cancer; D, smoking and lung cancer; E, cigarette smoke and cancer; F, sunlight and skin cancer; G, alcohol and cancer; H, asbestos and cancer; I, occupational steel (coke-oven) exposure and lung cancer; J, early childbirth (maternal age) and breast cancer; K, obesity and esophageal cancer; L, cigarette smoking and pancreatic cancer; M, lengthy occupational dioxin (2,3,7,8-tetrachlorodibenzodioxin) and cancer; N, alcohol and breast cancer; O, residential radon and lung cancer; P, eating red meat and colon cancer; Q, birthweight and breast cancer; R, oral contraceptive use and breast cancer; S, sun lamp use and melanoma; T, eating processed meat and colon cancer; U, breastfeeding and brain cancer/leukemia in children; V, high-alcohol mouthwash and mouth cancer; W, electromagnetic fields and brain cancer/leukemia in children; X, traffic density and brain cancer/leukemia in children; Y, high-cholesterol diet and rectal cancer; Z, douching and cervical cancer; AA, occupational stress and colorectal cancer; BB, smoking and fatal breast cancer; CC, hair dyes and myeloma; DD, drinking chlorinated tap water and bladder cancer; EE, eating yogurt and ovarian cancer; FF, hair dyes and lymphoma; GG, electromagnetic fields and brain cancer; HH, hair dyes and leukemia; II, smoking and breast cancer; JJ, diet high in saturated fat and lung cancer (among nonsmokers); KK, electromagnetic fields and leukemia; LL, fat intake and breast cancer; MM, maternal smoking and brain cancer/leukemia in children; NN, eating red meat and breast cancer; OO, electromagnetic fields and breast cancer; PP, coffee and heart disease; QQ, olive oil and breast cancer; RR, coffee and pancreatic cancer; SS, vasectomy and prostate cancer; TT, breast self-examination and breast cancer mortality; UU, abortion and breast cancer; VV, dichlorodiphenyltrichloroethane and breast cancer; WW, saccharine and bladder cancer.
DISCUSSION
Twenty-five years ago, Gary Taubes’ widely cited report in Science questioned the utility of epidemiologic research. To bolster his case, Taubes selected numerous examples of research topics that he viewed as unlikely to have public health implications, building an argument for the “inherent incapability” of epidemiologic studies to draw causal inferences for weak associations [risk ratios (RRs) < 3]. Research has since accumulated about these example topics. We evaluated the current evidence regarding Taubes’ selected examples, finding that 11 of the 41 associations (~27%) suggested in his initial paper to be false positives are now widely viewed as causal based on current evaluations of the public health consensus.
With the exception of the association between obesity and esophageal cancer, none of the associations deemed indeterminate in 1995 and subsequently classified as causal by our assessment reached Taubes’ cited threshold (RR ≥ 3) for “serious consideration.” However, each association had sufficient scientific data to support one or more consensus statements from reputable national and international agencies, including the U.S. National Cancer Institute (NCI), U.S. Centers for Disease Control and Prevention (CDC), International Agency for Research on Cancer (IARC), and World Health Organization (WHO)—supporting the value of epidemiologic research in identifying even modest associations.
The example of the association between alcohol consumption and breast cancer risk is illustrative. Taubes (1) quotes an epidemiologist as saying “nobody is convinced of the breast cancer–alcohol connection ‘except Walt Willett.’” The U.S. NCI and the IARC both now describe alcohol drinking as a known cause of breast cancer. Taubes (1) also casts doubt on the association between exposure to radon and lung cancer risk. Homebuyers across the United States routinely have homes tested for radon, and high levels abated because the association is considered causal. We wonder whether those who continue to cite Taubes (1995) as a critique of the science of epidemiology are aware that one-quarter of the associations selected by Taubes to erroneously illustrate the limits reached by epidemiology now have sufficient causal evidence and corresponding public health policies to reduce their risks.
Our group deemed 25 of the original examples indeterminate, reflecting a lack of public health consensus regarding causality (causal or noncausal) for these associations and consistent with Taubes’ previous assessment. Many studies of the proposed associations were too specific for judgment, limiting consensus. For example, IARC concluded that extremely low-frequency magnetic fields are possibly carcinogenic to humans due to evidence of an increased risk of childhood leukemia at high levels of magnetic fields, but associations between electromagnetic fields and other cancers are unclear (13). Other associations have sufficient emerging evidence (e.g., automotive benzene, a byproduct of traffic-related air pollution, and childhood cancer) that authoritative bodies have already made recommendations to limit exposure (14). Causal associations for many examples are limited by important confounding factors, which may be challenging to disentangle. For instance, the association between high-cholesterol diet and rectal cancer risk may be confounded by certain foods or food groups. Similarly, current smoking and drinking may confound the association between mouthwash and oral cancer (15). Inconsistencies observed for douching and cervical cancer may arise because individuals with certain characteristics (i.e., lower education, multiple sexual partners, and poverty) are also at a greater risk of sexually transmitted infections and bacterial vaginosis; they may douche secondary to infection-related symptoms rather than as a part of their normal hygienic practice (16). Additional data on these topics would be prudent to further explore causality.
Our efforts to update the example associations cited by Taubes were limited by two factors. In many instances, the 1995 examples were vague: Did Taubes mean “fatal breast” cancer as de novo metastatic disease or breast cancer–specific mortality? Still, others were too specific: drinking >3.3 liters of fluid (particularly chlorinated tap water) per day and bladder cancer. Thus, the 53 identified associations and causal evaluations are based on our own interpretations of Taubes’ 1995 report. For some associations, there was wide variation in meta-analytic estimates, limiting comparability. This was, in part, due to different meta-analyses using different exposure definitions, cut points, or outcomes. Our analysis of the meta-analytic estimates was not intended to be exhaustive. We selected up to three meta-analyses per association to examine patterns in the strength of associations by causal evaluation—not to represent all meta-analyses for these associations or estimate causal effects. Consensus statements by authoritative groups were based on the state of the science and viewpoints of the experts at the time these exposures were reviewed. Thus, they could change as new data become available. Nonetheless, we took a systematic approach to identifying and summarizing the example associations put forth by Taubes, considering both meta-analyses and authoritative consensus statements.
Inherent in the title and a common theme throughout Taubes’ 1995 article is that epidemiology is “stretched to its limits or beyond” and “at the edge of what can be done” (1). However, epidemiology has experienced tremendous innovation over the past few decades. The proliferation of large prospective cohorts and cohort consortia has allowed for better study of rare exposures and outcomes with adequate power rather than relying on retrospective case-control studies, which were the target of much of Taubes’ criticisms. These cohorts and consortia also allow for replication of studies across independent populations and in different contexts, which can help inform the impact of confounding (for example) on associations (confounding by health care access would presumably be less concerning in countries with universal health care).
Advances in technology have resulted in an explosion of data and the ability to readily link data across sources such as electronic health records, biobanks, vital records, disease surveillance registries, claims and administrative data, geospatial data, and mobile data. These can be used on their own for conducting research (e.g., studies using nationwide health registries or administrative databases) or linked to enrich cohort or case-control study data.
Technological advances such as wearable devices for health and environmental monitoring and omics data have and will continue to improve exposure assessment—which Taubes (1) describes as “the most pernicious” bias that plagues epidemiologic study of risk factors. For example, ongoing studies are examining the use of distinct somatic mutational signatures to identify past exposures (17). One of the aims of the Sherlock-Lung study is to examine mutational signatures of over 2000 lung cancers in never smokers with the goal of linking them to known risk factors such as radon, secondhand tobacco smoke, and air pollution (18). These technologically advanced exposure assessment tools raise new challenges and are often prohibitive for large-scale epidemiologic studies. However, if proven useful, then methods already exist to integrate these exposure assessment tools by conducting case-control studies nested within cohorts, validation studies, and/or quantitative bias analyses (19). With regard to outcome assessment, molecular and omics data have greatly accelerated our understanding of the heterogeneity of diseases. For example, molecular classification and gene expression–based assays are already part of routine clinical care for breast cancer (20) and are increasingly incorporated in studies of risk factors to elucidate differential risks by subtype (21).
In recent years, causal inference methods have come to the forefront of epidemiology (22) with increasing use of causal diagrams [e.g., directed acyclic graphs (23)] and study designs [e.g., negative control (24–27) and Mendelian randomization (MR) studies (28, 29), difference-in-difference methods (30–32), and regression discontinuity designs (33, 34)] to rule out noncausal associations. For example, MR studies, which use genetic variants as instrumental variables, have provided additional insight into the role of alcohol consumption and the risk of cancer with compelling evidence for head/neck and esophageal cancer (35–37), although evidence from three recent MR studies for breast cancer is less clear (38–40). MR studies attempt to address issues of confounding and reverse causation but require certain assumptions be met (as do all statistical and epidemiologic methods), can require large sample sizes for genetic variant(s) that explain only a small proportion of variation in an exposure, and may be subject to other biases (41, 42). Thus, casual evaluation of risk factors, especially for those where randomized controlled trials are infeasible or unethical, will continue to require synthesizing evidence across various sources [see textbox 3 from Krieger and Davey Smith (22) for triangulating evidence from eight different epidemiologic study designs for the example of smoking and low birthweight]. This triangulation of evidence is necessary to overcome the shortcomings of all study types from mechanistic studies and cohort studies to randomized controlled trials and meta-analyses.
Many of the associations selected by Taubes as examples to denigrate epidemiologic research have proven to have important public health implications—as evidenced by policy recommendations from reputable national and international agencies to reduce risks arising from the associations. The utility of epidemiologic research in this regard is all the more impressive when one remembers that the associations were selected because Taubes thought they would prove to be false positives. Twenty-five years later, epidemiology has reached beyond its limits. This history should inform current debates about the rigor and reproducibility of epidemiologic research results.
MATERIALS AND METHODS
The project was led by two doctorally trained epidemiologists with faculty appointments (T.L.L. and L.E.M.), with most of the work completed by a subset of doctoral students in the epidemiology department at Emory University’s Rollins School of Public Health. This team drafted a protocol, reviewed, revised, and registered it with the Center for Open Science (https://osf.io/4sfrb).
Protocol for identification of meta-analyses and consensus statements by authoritative bodies
Briefly, the Taubes paper was split into pages, and each page was independently evaluated by two reviewers who identified and abstracted each example epidemiologic association mentioned on the page. The work of the two reviewers was evaluated by a third independent reviewer, and any discrepancies were resolved by consensus among the three.
Each association was then assigned, at random, to a pair of reviewers to search for recent meta-analyses and a consensus statement that address the association. Three reviewers were assigned for each association. Two reviewers independently identified relevant search terms and combinations of search terms from National Library of Medicine Medical Subject Headings (NLM MeSH) for use in identifying meta-analyses and consensus statements. A third reviewer submitted terms and noted discrepancies. All three reviewers worked to achieve agreement on final search strings.
Meta-analyses
Each reviewer was assigned to search a single database: PubMed [including term “meta-analysis” (publication type)], Cochrane, PROSPERO (meta-analysis filter), or Embase (review filter). Relevant titles and abstracts on the associations of interest—published after 31 July 1995—were entered into Mendeley reference management software (43). We included one meta-analysis published before 31 July 1995 for the association between saccharine and bladder cancer because there were no later meta-analyses. Full text review was completed by two independent reviewers, and a third reviewer noted discrepancies. All reviewers worked to achieve consensus on the inclusion of up to three meta-analyses per association—prioritizing the degree the meta-analysis addressed the association of interest, the quality of the meta-analysis, and year of publication, in that order. Because many of the example associations in the paper by Taubes (1) were not specific in defining the exposure or outcome, we also prioritized meta-analyses that were aligned with the public health consensus statements. For instance, for associations where the outcome was described by Taubes (1) as cancer, we prioritized meta-analyses where the outcome was a cancer site that has been assessed by an expert working group for its relation with an exposure—such as cervical cancer for the association between human papillomavirus and cancer. For selected studies, each reviewer documented the following information: association of interest, first author’s last name and publication year, title of article, quote with study objective (generally abstracted from the body of paper), study designs included, number of studies included, final conclusion, pooled measure of association(s) and confidence interval(s) (CI), and any relevant additional information.
To visualize the strength of associations by causal evaluation, we plotted the log of the summary estimate and the minimum and maximum values of the corresponding 95% CIs from up to three meta-analyses for each exposure-outcome association. In several meta-analyses, separate summary estimates for different exposure measurements, outcomes, and subgroups were provided. We selected one summary estimate (and corresponding 95% CI) per meta-analysis that we deemed representative of the exposure-outcome association of interest. If the exposure-outcome association described by Taubes (1) was nonspecific (e.g., alcohol and cancer), then we aimed to select meta-analytic estimates used by authoritative bodies for issuing scientific evaluations/policy recommendations—or were in accordance with them.
Consensus statements
Each reviewer was tasked to identify and list disease-specific public health governmental and nongovernmental organizations (national or international) listed within the first 10 pages of the Google search results. In addition, the following general public health governmental and nongovernmental organizations were searched if they were not included in the first 10 pages of Google search results: IARC, U.S. CDC, WHO, American Public Health Association (APHA), U.S. Environmental Protection Agency, U.S. Agency for Toxic Substances and Disease Registry, U.S. Preventive Services Task Force, and the U.S. National Academy of Medicine. For each identified organization, it was determined whether the organization had a webpage dedicated to scientific evaluations, policy statements, guidelines, or recommendations. If confirmed, then that page was searched for statements relevant to the association of interest. If no such page could be located, then the outcome of interest was searched to identify specific evaluations, policy statements, guidelines, or recommendations. At least two independent reviewers compiled statements—published after 31 July 1995—that addressed the associations of interest and cited evidence from meta-analyses or individual studies. A third independent reviewer noted discrepancies, and all reviewers worked to achieve consensus. For each source, reviewers documented the following information: association of interest, organization and link to statement, title of the statement, author (if applicable), year of publication, and final evaluation or recommendation(s)—citing quotations from the statement.
Protocol for causal determination
The abstracted information was reviewed by two new randomly assigned evaluators to determine whether the association was viewed as causal. Independently, reviewers classified each association as causal, noncausal, or indeterminate based on the criteria below. A third reviewer identified discrepancies, which were resolved by consensus among all reviewers.
Consensus statements were primarily used for determining causality. We prioritized consensus statements that were based on comprehensive reviews conducted by a group of expert scientists for establishing causality (e.g., IARC monographs). Associations where the meta-analysis was not accompanied by a consensus statement, or accompanied by a vague consensus statement, were assessed for causality based on the following guidelines:
1) Consistency of results (number of replicated studies across multiple populations)
2) Dose response (e.g., examine associations in quartiles of exposure)
3) Demonstration of results in a randomized trial
4) Biological plausibility (basic science supportive of causal mechanism)
5) Temporal sequence (association measured using cohort or other study design in which exposure was measured before outcome)
Associations without any published meta-analyses or consensus statements were classified as indeterminate. Final determination of whether the association should be considered causal, noncausal, or indeterminate was made taking all guidelines into account.
Acknowledgments
Funding: This work was supported in part by the U.S. National Library of Medicine grant R01LM013049 (to T.L.L.), NCI grant F31CA239566 (to L.J.C.), National Centre for Advancing Translational Sciences grant TL1TR002540 (to L.J.C.).
Author contributions: Conceptualization: L.E.M., A.B.A., J.M.B., D.B., C.A.B., L.J.C., A.A.F., D.C.G., M.B.H., E.W.H., S.H., A.M.H., J.A.K., M.A.K., K.L., V.C.L., A.A.M., K.J.M., K.N.N., Z.S.Q., K.R.-D., S.S., M.P.S., I.Y.S., J.P.S., K.K.S., M.E.V.D., and T.L.L. Methodology: L.E.M., A.B.A., J.M.B., D.B., C.A.B., L.J.C., A.A.F., D.C.G., M.B.H., E.W.H., S.H., A.M.H., J.A.K., M.A.K., K.L., V.C.L., A.A.M., K.J.M., K.N.N., Z.S.Q., K.R.-D., S.S., M.P.S., I.Y.S., J.P.S., K.K.S., M.E.V.D., and T.L.L. Investigation: L.E.M., M.L.M., A.B.A., J.M.B., D.B., J.B., C.M.B., C.A.B., L.J.C., A.A.F., D.C.G., M.B.H., E.W.H., S.H., K.R.V.H., A.M.H., J.A.K., M.A.K., K.L., V.C.L., A.A.M., L.M.M., K.J.M., K.N.N., Z.S.Q., K.R.-D., S.S., M.P.S., I.Y.S., J.P.S., K.K.S., M.V.-L., M.E.V.D., K.J.V., and T.L.L. Visualization: M.L.M. Funding acquisition: T.L.L. Project administration: T.L.L. and L.E.M. Supervision: T.L.L. and L.E.M. Writing (original draft): L.E.M., T.L.L., and M.L.M. Writing (review and editing): L.E.M., M.L.M., A.B.A., J.M.B., D.B., J.B., C.M.B., C.A.B., L.J.C., A.A.F., D.C.G., M.B.H., E.W.H., S.H., K.R.V.H., A.M.H., J.A.K., M.A.K., K.L., V.C.L., A.A.M., L.M.M., K.J.M., K.N.N., Z.S.Q., K.R.-D., S.S., M.P.S., I.Y.S., J.P.S., K.K.S., M.V.-L., M.E.V.D., K.J.V., and T.L.L.
Competing interests: T.L.L. is a member of the Methods Advisory Council for Amgen, for which he receives consulting fees and travel support. C.A.B. is an employee of and holds stock with Amgen Inc. (1 Amgen Center Dr., Thousand Oaks, CA 91320). S.S. is an employee of ViiV Healthcare; contribution to this study was completed before joining ViiV Healthcare. All other authors declare that they have no competing interests.
Data and materials availability: All data and code used in the analysis and needed to evaluate the conclusions in the paper are available on GitHub (https://github.com/mmaliniak/Taubes_project) and Zenodo (10.5281/zenodo.6338809).
Supplementary Materials
This PDF file includes:
Supplementary Abbreviations
Fig. S1
Tables S1 and S2A–WW
References
REFERENCES AND NOTES
- 1.Taubes G., Epidemiology faces its limits. Science 269, 164–169 (1995). [DOI] [PubMed] [Google Scholar]
- 2.Willett W., Greenland S., MacMahon B., Trichopoulos D., Rothman K., Thomas D., Thun M., Weiss N., The discipline of epidemiology. Science 269, 1325–1326 (1995). [DOI] [PubMed] [Google Scholar]
- 3.Trichopoulos D., The discipline of epidemiology. Science 269, 1326 (1995). [DOI] [PubMed] [Google Scholar]
- 4.Trichopoulos D., The future of epidemiology. BMJ 313, 436–437 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saah A. J., The discipline of epidemiology. Science 269, 1327 (1995). [DOI] [PubMed] [Google Scholar]
- 6.Smith A. H., Depicting epidemiology. Science 270, 1743–1744 (1995). [PubMed] [Google Scholar]
- 7.Wynder E. L., Invited commentary: Response to science article, “Epidemiology faces its limits”. Am. J. Epidemiol. 143, 747–748 (1996). [DOI] [PubMed] [Google Scholar]
- 8.A. W. Harzing, “Publish or perish” (2016); https://harzing.com/resources/publish-or-perish.
- 9.Hubbard A., Trostle J., Cangemi I., Eisenberg J. N. S., Countering the curse of dimensionality: Exploring data-generating mechanisms through participant observation and mechanistic modeling. Epidemiology 30, 609–614 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jukola S., On the evidentiary standards for nutrition advice. Stud. Hist. Philos. Biol. Biomed. Sci. 73, 1–9 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Hardwicke T. E., Ioannidis J. P. A., Petitions in scientific argumentation: Dissecting the request to retire statistical significance. Eur. J. Clin. Invest. 49, e13162 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Calabrese E. J., LNT and cancer risk assessment: Its flawed foundations part 1: Radiation and leukemia: Where LNT began. Environ. Res. 197, 111025 (2021). [DOI] [PubMed] [Google Scholar]
- 13.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, Non-Ionizing Radiation, Part 1: Static and Extremely Low-Frequency (ELF) Electric and Magnetic Fields (International Agency for Research on Cancer, 2002), vol. 80. [PMC free article] [PubMed] [Google Scholar]
- 14.“Cancer prevention during early life” (CDC, 2021); www.cdc.gov/cancer/dcpc/prevention/childhood.htm.
- 15.“Oral health care drug products for over-the-counter human use; antigingivitis/antiplaque drug products; establishment of a monograph” (Federal Register, 2003); www.federalregister.gov/documents/2003/05/29/03-12783/oral-health-care-drug-products-for-over-the-counter-human-use-antigingivitisantiplaque-drug-products.
- 16.Martino J. L., Vermund S. H., Vaginal douching: Evidence for risks or benefits to women’s health. Epidemiol. Rev. 24, 109–124 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alexandrov L. B., Kim J., Haradhvala N. J., Huang M. N., Tian Ng A. W., Wu Y., Boot A., Covington K. R., Gordenin D. A., Bergstrom E. N., Islam S. M. A., Lopez-Bigas N., Klimczak L. J., McPherson J. R., Morganella S., Sabarinathan R., Wheeler D. A., Mustonen V., Getz G., Rozen S. G., Stratton M. R.; PCAWG Consortium , The repertoire of mutational signatures in human cancer. Nature 578, 94–101 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landi M. T., Synnott N. C., Rosenbaum J., Zhang T., Zhu B., Shi J., Zhao W., Kebede M., Sang J., Choi J., Mendoza L., Pacheco M., Hicks B., Caporaso N. E., Abubakar M., Gordenin D. A., Wedge D. C., Alexandrov L. B., Rothman N., Lan Q., Garcia-Closas M., Chanock S. J., Tracing lung cancer risk factors through mutational signatures in never-smokers. Am. J. Epidemiol. 190, 962–976 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.T. L. Lash, M. P. Fox, A. K. Fink, Applying Quantitative Bias Analysis to Epidemiologic Data (Springer, 2009), vol. 533. [Google Scholar]
- 20.Litton J. K., Burstein H. J., Turner N. C., Molecular testing in breast cancer. Am. Soc. Clin. Oncol. Educ. Book 39, e1–e7 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Yang X. R., Chang-Claude J., Goode E. L., Couch F. J., Nevanlinna H., Milne R. L., Gaudet M., Schmidt M. K., Broeks A., Cox A., Fasching P. A., Hein R., Spurdle A. B., Blows F., Driver K., Flesch-Janys D., Heinz J., Sinn P., Vrieling A., Heikkinen T., Aittomäki K., Heikkilä P., Blomqvist C., Lissowska J., Peplonska B., Chanock S., Figueroa J., Brinton L., Hall P., Czene K., Humphreys K., Darabi H., Liu J., Van ‘t Veer L. J., van Leeuwen F. E., Andrulis I. L., Glendon G., Knight J. A., Mulligan A. M., O’Malley F. P., Weerasooriya N., John E. M., Beckmann M. W., Hartmann A., Weihbrecht S. B., Wachter D. L., Jud S. M., Loehberg C. R., Baglietto L., English D. R., Giles G. G., McLean C. A., Severi G., Lambrechts D., Vandorpe T., Weltens C., Paridaens R., Smeets A., Neven P., Wildiers H., Wang X., Olson J. E., Cafourek V., Fredericksen Z., Kosel M., Vachon C., Cramp H. E., Connley D., Cross S. S., Balasubramanian S. P., Reed M. W. R., Dörk T., Bremer M., Meyer A., Karstens J. H., Ay A., Park-Simon T.-W., Hillemanns P., Pérez J. I. A., Rodríguez P. M., Zamora P., Benítez J., Ko Y.-D., Fischer H.-P., Hamann U., Pesch B., Brüning T., Justenhoven C., Brauch H., Eccles D. M., Tapper W. J., Gerty S. M., Sawyer E. J., Tomlinson I. P., Jones A., Kerin M., Miller N., McInerney N., Anton-Culver H., Ziogas A., Shen C.-Y., Hsiung C.-N., Wu P.-E., Yang S.-L., Yu J.-C., Chen S.-T., Hsu G.-C., Haiman C. A., Henderson B. E., Le Marchand L., Kolonel L. N., Lindblom A., Margolin S., Jakubowska A., Lubiński J., Huzarski T., Byrski T., Górski B., Gronwald J., Hooning M. J., Hollestelle A., van den Ouweland A. M. W., Jager A., Kriege M., Tilanus-Linthorst M. M. A., Collée M., Wang-Gohrke S., Pylkäs K., Jukkola-Vuorinen A., Mononen K., Grip M., Hirvikoski P., Winqvist R., Mannermaa A., Kosma V.-M., Kauppinen J., Kataja V., Auvinen P., Soini Y., Sironen R., Bojesen S. E., Ørsted D. D., Kaur-Knudsen D., Flyger H., Nordestgaard B. G., Holland H., Chenevix-Trench G., Manoukian S., Barile M., Radice P., Hankinson S. E., Hunter D. J., Tamimi R., Sangrajrang S., Brennan P., McKay J., Odefrey F., Gaborieau V., Devilee P., Huijts P. E. A., Tollenaar R. A. E. M., Seynaeve C., Dite G. S., Apicella C., Hopper J. L., Hammet F., Tsimiklis H., Smith L. D., Southey M. C., Humphreys M. K., Easton D., Pharoah P., Sherman M. E., Garcia-Closas M., Associations of breast cancer risk factors with tumor subtypes: A pooled analysis from the breast cancer association consortium studies. J. Natl. Cancer Inst. 103, 250–263 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krieger N., Davey Smith G., The tale wagged by the DAG: Broadening the scope of causal inference and explanation for epidemiology. Int. J. Epidemiol. 45, 1787–1808 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Greenland S., Pearl J., Robins J. M., Causal diagrams for epidemiologic research. Epidemiology 10, 37–48 (1999). [PubMed] [Google Scholar]
- 24.Keyes K. M., Davey Smith G., Susser E., Commentary: Smoking in pregnancy and offspring health: Early insights into family-based and ‘negative control’ studies? Int. J. Epidemiol. 43, 1381–1388 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lipsitch M., Tchetgen Tchetgen E., Cohen T., Negative controls: A tool for detecting confounding and bias in observational studies. Epidemiology 21, 383–388 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith G. D., Negative control exposures in epidemiologic studies. Epidemiology 23, 351–352 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Flanders W. D., Klein M., Darrow L. A., Strickland M. J., Sarnat S. E., Sarnat J. A., Waller L. A., Winquist A., Tolbert P. E., A method for detection of residual confounding in time-series and other observational studies. Epidemiology 22, 59–67 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Didelez V., Sheehan N., Mendelian randomization as an instrumental variable approach to causal inference. Stat. Methods Med. Res. 16, 309–330 (2007). [DOI] [PubMed] [Google Scholar]
- 29.Smith G. D., Ebrahim S., Mendelian randomization: Prospects, potentials, and limitations. Int. J. Epidemiol. 33, 30–42 (2004). [DOI] [PubMed] [Google Scholar]
- 30.Meyer B. D., Natural and quasi-experiments in economics. J. Bus. Econ. Stat. 13, 151–161 (1995). [Google Scholar]
- 31.M. Lechner, “The estimation of causal effects by difference-in-difference methods,” University of St. Gallen Department of Economics working paper series 2010 (2010–28), Department of Economics, University of St. Gallen, October 2011; https://ideas.repec.org/p/usg/dp2010/2010-28.html.
- 32.Abadie A., Diamond A., Hainmueller J., Synthetic control methods for comparative case studies: Estimating the effect of California’s tobacco control program. J. Am. Stat. Assoc. 105, 493–505 (2010). [Google Scholar]
- 33.Oldenburg C. E., Moscoe E., Bärnighausen T., Regression discontinuity for causal effect estimation in epidemiology. Curr. Epidemiol. Rep. 3, 233–241 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.T. D. Cook, D. T. Campbell, W. Shadish, Experimental and Quasi-Experimental Designs for Generalized Causal Inference (Houghton Mifflin, 2002). [Google Scholar]
- 35.Pierce B. L., Kraft P., Zhang C., Mendelian randomization studies of cancer risk: A literature review. Curr. Epidemiol. Rep. 5, 184–196 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewis S. J., Smith G. D., Alcohol, ALDH2, and esophageal cancer: A meta-analysis which illustrates the potentials and limitations of a Mendelian randomization approach. Cancer Epidemiol. Biomarkers Prev. 14, 1967–1971 (2005). [DOI] [PubMed] [Google Scholar]
- 37.Boccia S., Hashibe M., Gallì P., De Feo E., Asakage T., Hashimoto T., Hiraki A., Katoh T., Nomura T., Yokoyama A., van Duijn C. M., Ricciardi G., Boffetta P., Aldehyde dehydrogenase 2 and head and neck cancer: A meta-analysis implementing a Mendelian randomization approach. Cancer Epidemiol. Biomarkers Prev. 18, 248–254 (2009). [DOI] [PubMed] [Google Scholar]
- 38.Ong J.-S., Derks E. M., Eriksson M., An J., Hwang L.-D., Easton D. F., Pharoah P. P., Berchuck A., Kelemen L. E., Matsuo K., Chenevix-Trench G., Hall P., Bojesen S. E., Webb P. M., MacGregor S., Evaluating the role of alcohol consumption in breast and ovarian cancer susceptibility using population-based cohort studies and two-sample Mendelian randomization analyses. Int. J. Cancer 148, 1338–1350 (2021). [DOI] [PubMed] [Google Scholar]
- 39.Zhu J., Jiang X., Niu Z., Alcohol consumption and risk of breast and ovarian cancer: A Mendelian randomization study. Cancer Genet. 245, 35–41 (2020). [DOI] [PubMed] [Google Scholar]
- 40.Larsson S. C., Carter P., Kar S., Vithayathil M., Mason A. M., Michaëlsson K., Burgess S., Smoking, alcohol consumption, and cancer: A Mendelian randomisation study in UK Biobank and international genetic consortia participants. PLOS Med. 17, e1003178 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swanson S. A., Tiemeier H., Ikram M. A., Hernán M. A., Nature as a trialist?: Deconstructing the analogy between Mendelian randomization and randomized trials. Epidemiology 28, 653–659 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glymour M. M., Tchetgen Tchetgen E. J., Robins J. M., Credible Mendelian randomization studies: Approaches for evaluating the instrumental variable assumptions. Am. J. Epidemiol. 175, 332–339 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gunn W., Mendeley: Enabling and understanding scientific collaboration. Inf. Serv. Use 34, 99–102 (2014). [Google Scholar]
- 44.Koshiol J., Lindsay L., Pimenta J. M., Poole C., Jenkins D., Smith J. S., Persistent human papillomavirus infection and cervical neoplasia: A systematic review and meta-analysis. Am. J. Epidemiol. 168, 123–137 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saulle R., Semyonov L., Mannocci A., Careri A., Saburri F., Ottolenghi L., Guerra F., La Torre G., Human papillomavirus and cancerous diseases of the head and neck: A systematic review and meta-analysis. Oral Dis. 21, 417–431 (2015). [DOI] [PubMed] [Google Scholar]
- 46.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, Human Papillomaviruses (International Agency for Research on Cancer, 2007), vol. 90. [Google Scholar]
- 47.“Cancers associated with human papillomavirus (HPV)” (CDC, 2021); www.cdc.gov/cancer/hpv/basic_info/cancers.htm.
- 48.Ron E., Lubin J. H., Shore R. E., Mabuchi K., Modan B., Pottern L. M., Schneider A. B., Tucker M. A., Boice J. D. Jr., Thyroid cancer after exposure to external radiation: A pooled analysis of seven studies. Radiat. Res. 141, 259–277 (1995). [PubMed] [Google Scholar]
- 49.Cardis E., Vrijheid M., Blettner M., Gilbert E., Hakama M., Hill C., Howe G., Kaldor J., Muirhead C. R., Schubauer-Berigan M., Yoshimura T., Bermann F., Cowper G., Fix J., Hacker C., Heinmiller B., Marshall M., Thierry-Chef I., Utterback D., Ahn Y.-O., Amoros E., Ashmore P., Auvinen A., Bae J.-M., Bernar J., Biau A., Combalot E., Deboodt P., Sacristan A. D., Eklöf M., Engels H., Engholm G., Gulis G., Habib R. R., Holan K., Hyvonen H., Kerekes A., Kurtinaitis J., Malker H., Martuzzi M., Mastauskas A., Monnet A., Moser M., Pearce M. S., Richardson D. B., Rodriguez-Artalejo F., Rogel A., Tardy H., Telle-Lamberton M., Turai I., Usel M., Veress K., The 15-country collaborative study of cancer risk among radiation workers in the nuclear industry: Estimates of radiation-related cancer risks. Radiat. Res. 167, 396–416 (2007). [DOI] [PubMed] [Google Scholar]
- 50.Daniels R. D., Schubauer-Berigan M. K., A meta-analysis of leukaemia risk from protracted exposure to low-dose gamma radiation. Occup. Environ. Med. 68, 457–464 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, Ionizing Radiation, Part 1: X-and Gamma (γ)-Radiation, and Neutrons (International Agency for Research on Cancer, 2000), vol. 75. [Google Scholar]
- 52.Cho L. Y., Yang J. J., Ko K.-P., Park B., Shin A., Lim M. K., Oh J.-K., Park S., Kim Y. J., Shin H.-R., Yoo K.-Y., Park S. K., Coinfection of hepatitis B and C viruses and risk of hepatocellular carcinoma: Systematic review and meta-analysis. Int. J. Cancer 128, 176–184 (2011). [DOI] [PubMed] [Google Scholar]
- 53.Chen X., Wu F., Liu Y., Lou J., Zhu B., Zou L., Chen W., Gong J., Wang Y., Zhong R., The contribution of serum hepatitis B virus load in the carcinogenesis and prognosis of hepatocellular carcinoma: Evidence from two meta-analyses. Oncotarget 7, 49299–49309 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li M., Gan Y., Fan C., Yuan H., Zhang X., Shen Y., Wang Q., Meng Z., Xu D., Tu H., Hepatitis B virus and risk of non-Hodgkin lymphoma: An updated meta-analysis of 58 studies. J. Viral Hepat. 25, 894–903 (2018). [DOI] [PubMed] [Google Scholar]
- 55.Ryerson A. B., Eheman C. R., Altekruse S. F., Ward J. W., Jemal A., Sherman R. L., Henley S. J., Holtzman D., Lake A., Noone A.-M., Anderson R. N., Ma J., Ly K. N., Cronin K. A., Penberthy L., Kohler B. A., Annual Report to the Nation on the Status of Cancer, 1975-2012, featuring the increasing incidence of liver cancer. Cancer 122, 1312–1337 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, Biological Agents (International Agency for Research on Cancer, 2012), vol. 100B; www.ncbi.nlm.nih.gov/books/NBK304348/.
- 57.Gandini S., Botteri E., Iodice S., Boniol M., Lowenfels A. B., Maisonneuve P., Boyle P., Tobacco smoking and cancer: A meta-analysis. Int. J. Cancer 122, 155–164 (2008). [DOI] [PubMed] [Google Scholar]
- 58.Lee P. N., Forey B. A., Coombs K. J., Systematic review with meta-analysis of the epidemiological evidence in the 1900s relating smoking to lung cancer. BMC Cancer 12, 385 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ordóñez-Mena J. M., Schöttker B., Mons U., Jenab M., Freisling H., Bueno-de-Mesquita B., O’Doherty M. G., Scott A., Kee F., Stricker B. H., Hofman A., de Keyser C. E., Ruiter R., Söderberg S., Jousilahti P., Kuulasmaa K., Freedman N. D., Wilsgaard T., de Groot L. C., Kampman E., Håkansson N., Orsini N., Wolk A., Nilsson L. M., Tjønneland A., Pająk A., Malyutina S., Kubínová R., Tamosiunas A., Bobak M., Katsoulis M., Orfanos P., Boffetta P., Trichopoulou A., Brenner H.; Consortium on Health and Ageing: Network of Cohorts in Europe and the United States (CHANCES) , Quantification of the smoking-associated cancer risk with rate advancement periods: Meta-analysis of individual participant data from cohorts of the CHANCES consortium. BMC Med. 14, 62 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, Personal Habits and Indoor Combustions (International Agency for Research on Cancer, 2012), vol. 100E; www.ncbi.nlm.nih.gov/books/NBK304391/. [PMC free article] [PubMed]
- 61.Gandini S., Sera F., Cattaruzza M. S., Pasquini P., Picconi O., Boyle P., Melchi C. F., Meta-analysis of risk factors for cutaneous melanoma: II. Sun exposure. Eur. J. Cancer 41, 45–60 (2005). [DOI] [PubMed] [Google Scholar]
- 62.Dennis L. K., Vanbeek M. J., Beane Freeman L. E., Smith B. J., Dawson D. V., Coughlin J. A., Sunburns and risk of cutaneous melanoma: Does age matter? A comprehensive meta-analysis. Ann. Epidemiol. 18, 614–627 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Olsen C. M., Zens M. S., Green A. C., Stukel T. A., Holman C. D. J., Mack T., Elwood J. M., Holly E. A., Sacerdote C., Gallagher R., Swerdlow A. J., Armstrong B. K., Rosso S., Kirkpatrick C., Zanetti R., Bishop J. N., Bataille V., Chang Y.-M., Mackie R., Østerlind A., Berwick M., Karagas M. R., Whiteman D. C., Biologic markers of sun exposure and melanoma risk in women: Pooled case-control analysis. Int. J. Cancer 129, 713–723 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.“Sunlight” (National Cancer Institute, 2015); www.cancer.gov/about-cancer/causes-prevention/risk/sunlight.
- 65.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, Radiation (International Agency for Research on Cancer, 2012), vol. 100D; https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Radiation-2012.
- 66.Bagnardi V., Rota M., Botteri E., Tramacere I., Islami F., Fedirko V., Scotti L., Jenab M., Turati F., Pasquali E., Pelucchi C., Galeone C., Bellocco R., Negri E., Corrao G., Boffetta P., La Vecchia C., Alcohol consumption and site-specific cancer risk: A comprehensive dose-response meta-analysis. Br. J. Cancer 112, 580–593 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jayasekara H., MacInnis R. J., Room R., English D. R., Long-term alcohol consumption and breast, upper aero-digestive tract and colorectal cancer risk: A systematic review and meta-analysis. Alcohol Alcohol. 51, 315–330 (2016). [DOI] [PubMed] [Google Scholar]
- 68.Choi Y.-J., Myung S.-K., Lee J.-H., Light alcohol drinking and risk of cancer: A meta-analysis of cohort studies. Cancer Res. Treat. 50, 474–487 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.“Alcohol and cancer” (CDC, 2022); www.cdc.gov/cancer/alcohol/index.htm.
- 70.Camargo M. C., Stayner L. T., Straif K., Reina M., Al-Alem U., Demers P. A., Landrigan P. J., Occupational exposure to asbestos and ovarian cancer: A meta-analysis. Environ. Health Perspect. 119, 1211–1217 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lenters V., Vermeulen R., Dogger S., Stayner L., Portengen L., Burdorf A., Heederik D., A Meta-analysis of asbestos and lung cancer: Is better quality exposure assessment associated with steeper slopes of the exposure–response relationships? Environ. Health Perspect. 119, 1547–1555 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ngamwong Y., Tangamornsuksan W., Lohitnavy O., Chaiyakunapruk N., Scholfield C. N., Reisfeld B., Lohitnavy M., Additive synergism between asbestos and smoking in lung cancer risk: A systematic review and meta-analysis. PLOS ONE 10, e0135798 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, Arsenic, Metals, Fibres and Dusts (International Agency for Research on Cancer, 2012), vol. 100C; www.ncbi.nlm.nih.gov/books/NBK304375/. [PMC free article] [PubMed]
- 74.U.S. Environmental Protection Agency, Asbestos CASRN 1332-21-4 | DTXSID4023888; https://cfpub.epa.gov/ncea/iris2/chemicallanding.cfm?substance_nmbr=371.
- 75.Armstrong B., Hutchinson E., Unwin J., Fletcher T., Lung cancer risk after exposure to polycyclic aromatic hydrocarbons: A review and meta-analysis. Environ. Health Perspect. 112, 970–978 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Singh A., Kamal R., Ahamed I., Wagh M., Bihari V., Sathian B., Kesavachandran C. N., PAH exposure-associated lung cancer: An updated meta-analysis. Occup. Med. 68, 255–261 (2018). [DOI] [PubMed] [Google Scholar]
- 77.U.S. Environmental Protection Agency, Coke oven emissions CASRN NA | DTXSID7023984; https://cfpub.epa.gov/ncea/iris2/chemicalLanding.cfm?substance_nmbr=395.
- 78.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, Chemical Agents and Related Occupations (International Agency for Research on Cancer, 2012), vol. 100F; www.ncbi.nlm.nih.gov/books/NBK304416/. [PMC free article] [PubMed]
- 79.Ma H., Bernstein L., Pike M. C., Ursin G., Reproductive factors and breast cancer risk according to joint estrogen and progesterone receptor status: A meta-analysis of epidemiological studies. Breast Cancer Res. 8, R43 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reeves G. K., Pirie K., Green J., Bull D., Beral V., Reproductive factors and specific histological types of breast cancer: Prospective study and meta-analysis. Br. J. Cancer. 100, 538–544 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lambertini M., Santoro L., Del Mastro L., Nguyen B., Livraghi L., Ugolini D., Peccatori F. A., Azim H. A. Jr., Reproductive behaviors and risk of developing breast cancer according to tumor subtype: A systematic review and meta-analysis of epidemiological studies. Cancer Treat. Rev. 49, 65–76 (2016). [DOI] [PubMed] [Google Scholar]
- 82.“Reproductive history and cancer risk (National Cancer Institute, 2016); www.cancer.gov/about-cancer/causes-prevention/risk/hormones/reproductive-history-fact-sheet.
- 83.Diet, Nutrition, Physical Activity and Breast Cancer (World Cancer Research Fund/American Institute for Cancer Research, 2017), 124p. [Google Scholar]
- 84.Hoyo C., Cook M. B., Kamangar F., Freedman N. D., Whiteman D. C., Bernstein L., Brown L. M., Risch H. A., Ye W., Sharp L., Wu A. H., Ward M. H., Casson A. G., Murray L. J., Corley D. A., Nyrén O., Pandeya N., Vaughan T. L., Chow W.-H., Gammon M. D., Body mass index in relation to oesophageal and oesophagogastric junction adenocarcinomas: A pooled analysis from the International BEACON Consortium. Int. J. Epidemiol. 41, 1706–1718 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Turati F., Tramacere I., La Vecchia C., Negri E., A meta-analysis of body mass index and esophageal and gastric cardia adenocarcinoma. Ann. Oncol. 24, 609–617 (2013). [DOI] [PubMed] [Google Scholar]
- 86.Diet, Nutrition, Physical Activity and Oesophageal Cancer (World Cancer Research Fund/American Institute for Cancer Research, 2016), 62p. [Google Scholar]
- 87.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, Absence of Excess Body Fatness, vol. 16 of IARC Handbooks of Cancer Prevention (2018); https://publications.iarc.fr/Book-And-Report-Series/Iarc-Handbooks-Of-Cancer-Prevention/Absence-Of-Excess-Body-Fatness-2018.
- 88.Lynch S. M., Vrieling A., Lubin J. H., Kraft P., Mendelsohn J. B., Hartge P., Canzian F., Steplowski E., Arslan A. A., Gross M., Helzlsouer K., Jacobs E. J., LaCroix A., Petersen G., Zheng W., Albanes D., Amundadottir L., Bingham S. A., Boffetta P., Boutron-Ruault M.-C., Chanock S. J., Clipp S., Hoover R. N., Jacobs K., Johnson K. C., Kooperberg C., Luo J., Messina C., Palli D., Patel A. V., Riboli E., Shu X.-O., Suarez L. R., Thomas G., Tjønneland A., Tobias G. S., Tong E., Trichopoulos D., Virtamo J., Ye W., Yu K., Zeleniuch-Jacquette A., Bueno-de-Mesquita H. B., Stolzenberg-Solomon R. Z., Cigarette smoking and pancreatic cancer: A pooled analysis from the pancreatic cancer cohort consortium. Am. J. Epidemiol. 170, 403–413 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.La Torre G., de Waure C., Specchia M. L., Nicolotti N., Capizzi S., Bilotta A., Clemente G., Ricciardi W., Does quality of observational studies affect the results of a meta-analysis?: The case of cigarette smoking and pancreatic cancer. Pancreas 38, 241–247 (2009). [DOI] [PubMed] [Google Scholar]
- 90.Zou L., Zhong R., Shen N., Chen W., Zhu B., Ke J., Lu X., Zhang T., Lou J., Wang Z., Liu L., Qi L., Miao X., Non-linear dose-response relationship between cigarette smoking and pancreatic cancer risk: Evidence from a meta-analysis of 42 observational studies. Eur. J. Cancer 50, 193–203 (2014). [DOI] [PubMed] [Google Scholar]
- 91.Leng L., Chen X., Li C.-P., Luo X.-Y., Tang N.-J., 2,3,7,8-Tetrachlorodibezo-p-dioxin exposure and prostate cancer: A meta-analysis of cohort studies. Public Health 128, 207–213 (2014). [DOI] [PubMed] [Google Scholar]
- 92.Xu J., Ye Y., Huang F., Chen H., Wu H., Huang J., Hu J., Xia D., Wu Y., Association between dioxin and cancer incidence and mortality: A meta-analysis. Sci. Rep. 6, 38012 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.“Lifestyle-related breast cancer risk factors” (American Cancer Society, 2021); www.cancer.org/cancer/breast-cancer/risk-and-prevention/lifestyle-related-breast-cancer-risk-factors.html.
- 94.Zhang Z.-L., Sun J., Dong J.-Y., Tian H.-L., Xue L., Qin L.-Q., Tong J., Residential radon and lung cancer risk: An updated meta- analysis of case-control studies. Asian Pac. J. Cancer Prev. 13, 2459–2465 (2012). [DOI] [PubMed] [Google Scholar]
- 95.Duan P., Quan C., Hu C., Zhang J., Xie F., Hu X., Yu Z., Gao B., Liu Z., Zheng H., Liu C., Wang C., Yu T., Qi S., Fu W., Kourouma A., Yang K., Nonlinear dose-response relationship between radon exposure and the risk of lung cancer: Evidence from a meta-analysis of published observational studies. Eur. J. Cancer Prev. 24, 267–277 (2015). [DOI] [PubMed] [Google Scholar]
- 96.Garzillo C., Pugliese M., Loffredo F., Quarto M., Indoor radon exposure and lung cancer risk: A meta-analysis of case-control studies. Transl. Cancer Res. 6, 1–10 (2017). [Google Scholar]
- 97.U.S. Environmental Protection Agency, “A citizen’s guide to Radon: The guide to protecting yourself and your family from Radon” (2012); www.epa.gov/sites/production/files/2016-02/documents/2012_a_citizens_guide_to_radon.pdf.
- 98.Norat T., Lukanova A., Ferrari P., Riboli E., Meat consumption and colorectal cancer risk: Dose-response meta-analysis of epidemiological studies. Int. J. Cancer 98, 241–256 (2002). [DOI] [PubMed] [Google Scholar]
- 99.Pham N. M., Mizoue T., Tanaka K., Tsuji I., Tamakoshi A., Matsuo K., Wakai K., Nagata C., Inoue M., Tsugane S., Sasazuki S.; Research Group for the Development and Evaluation of Cancer Prevention Strategies in Japan , Meat consumption and colorectal cancer risk: An evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn. J. Clin. Oncol. 44, 641–650 (2014). [DOI] [PubMed] [Google Scholar]
- 100.Vieira A. R., Abar L., Chan D. S. M., Vingeliene S., Polemiti E., Stevens C., Greenwood D., Norat T., Foods and beverages and colorectal cancer risk: A systematic review and meta-analysis of cohort studies, an update of the evidence of the WCRF-AICR Continuous Update Project. Ann. Oncol. 28, 1788–1802 (2017). [DOI] [PubMed] [Google Scholar]
- 101.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, Red Meat and Processed Meat (International Agency for Research on Cancer, 2018), vol. 114; www.ncbi.nlm.nih.gov/books/NBK507971/. [PubMed]
- 102.Diet, Nutrition, Physical Activity and Colorectal Cancer (Continuous Update Project Expert Report 2018, World Cancer Research Fund/American Institute for Cancer Research, 2018); www.wcrf.org/wp-content/uploads/2021/02/Colorectal-cancer-report.pdf.
- 103.Park S. K., Kang D., McGlynn K. A., Garcia-Closas M., Kim Y., Yoo K. Y., Brinton L. A., Intrauterine environments and breast cancer risk: Meta-analysis and systematic review. Breast Cancer Res. 10, R8 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xu X., Dailey A. B., Peoples-Sheps M., Talbott E. O., Li N., Roth J., Birth weight as a risk factor for breast cancer: A meta-analysis of 18 epidemiological studies. J. Womens Health 18, 1169–1178 (2009). [DOI] [PubMed] [Google Scholar]
- 105.“Birthweight and breast cancer risk” (Susan G Komen, 2021); www.komen.org/breast-cancer/facts-statistics/research-studies/topics/birthweight-and-breast-cancer-risk/.
- 106.Gierisch J. M., Coeytaux R. R., Urrutia R. P., Havrilesky L. J., Moorman P. G., Lowery W. J., Dinan M., McBroom A. J., Hasselblad V., Sanders G. D., Myers E. R., Oral contraceptive use and risk of breast, cervical, colorectal, and endometrial cancers: A systematic review. Cancer Epidemiol. Biomarkers Prev. 22, 1931–1943 (2013). [DOI] [PubMed] [Google Scholar]
- 107.Li L., Zhong Y., Zhang H., Yu H., Huang Y., Li Z., Chen G., Hua X., Association between oral contraceptive use as a risk factor and triple-negative breast cancer: A systematic review and meta-analysis. Mol. Clin. Oncol. 7, 76–80 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, Pharmaceuticals (International Agency for Research on Cancer, 2012), vol. 100A; www.ncbi.nlm.nih.gov/books/NBK304334/.
- 109.“Oral contraceptives (birth control pills) and cancer risk” (National Cancer Institute, 2018); www.cancer.gov/about-cancer/causes-prevention/risk/hormones/oral-contraceptives-fact-sheet.
- 110.International Agency for Research on Cancer Working Group on artificial ultraviolet (UV) light and skin cancer , The association of use of sunbeds with cutaneous malignant melanoma and other skin cancers: A systematic review. Int. J. Cancer 120, 1116–1122 (2007). [DOI] [PubMed] [Google Scholar]
- 111.Boniol M., Autier P., Boyle P., Gandini S., Cutaneous melanoma attributable to sunbed use: Systematic review and meta-analysis. BMJ 345, e4757 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Colantonio S., Bracken M. B., Beecker J., The association of indoor tanning and melanoma in adults: Systematic review and meta-analysis. J. Am. Acad. Dermatol. 70, 847–857.e1–18 (2014). [DOI] [PubMed] [Google Scholar]
- 113.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, Radiation (International Agency for Research on Cancer, 2012), vol. 100D; www.ncbi.nlm.nih.gov/books/NBK304366/.
- 114.Chan D. S. M., Lau R., Aune D., Vieira R., Greenwood D. C., Kampman E., Norat T., Red and processed meat and colorectal cancer incidence: Meta-analysis of prospective studies. PlOS ONE 6, e20456 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chiavarini M., Bertarelli G., Minelli L., Fabiani R., Dietary intake of meat cooking-related mutagens (HCAs) and risk of colorectal adenoma and cancer: A systematic review and meta-analysis. Nutrients 9, 514 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhao Z., Feng Q., Yin Z., Shuang J., Bai B., Yu P., Guo M., Zhao Q., Red and processed meat consumption and colorectal cancer risk: A systematic review and meta-analysis. Oncotarget 8, 83306–83314 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kwan M. L., Buffler P. A., Abrams B., Kiley V. A., Breastfeeding and the risk of childhood leukemia: A meta-analysis. Public Health Rep. 119, 521–535 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Martin R. M., Gunnell D., Owen C. G., Smith G. D., Breast-feeding and childhood cancer: A systematic review with metaanalysis. Int. J. Cancer 117, 1020–1031 (2005). [DOI] [PubMed] [Google Scholar]
- 119.Amitay E. L., Keinan-Boker L., Breastfeeding and childhood leukemia incidence: A meta-analysis and systematic review. JAMA Pediatr. 169, e151025 (2015). [DOI] [PubMed] [Google Scholar]
- 120.“Cancer in children and adolescents” (National Cancer Institute, 2021); www.cancer.gov/types/childhood-cancers/child-adolescent-cancers-fact-sheet.
- 121.U.S. Department of Health and Human Services, “Making the decision to breastfeed” (Office on Women’s Health, 2021); www.womenshealth.gov/breastfeeding/making-decision-breastfeed.
- 122.Gandini S., Negri E., Boffetta P., La Vecchia C., Boyle P., Mouthwash and oral cancer risk quantitative meta-analysis of epidemiologic studies. Ann. Agric. Environ. Med. 19, 173–180 (2012). [PubMed] [Google Scholar]
- 123.Boffetta P., Hayes R. B., Sartori S., Lee Y.-C. A., Muscat J., Olshan A., Winn D. M., Castellsagué X., Zhang Z.-F., Morgenstern H., Chen C., Schwartz S. M., Vaughan T. L., Wunsch-Filho V., Purdue M., Koifman S., Curado M. P., Vilensky M., Gillison M., Fernandez L., Menezes A., Daudt A. W., Schantz S., Yu G., D’Souza G., Haddad R. I., La Vecchia C., Hashibe M., Mouthwash use and cancer of the head and neck: A pooled analysis from the International Head and Neck Cancer Epidemiology Consortium. Eur. J. Cancer Prev. 25, 344–348 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.“Risk factors for oral cavity and oropharyngeal cancers” (American Cancer Society, 2021); www.cancer.org/cancer/oral-cavity-and-oropharyngeal-cancer/causes-risks-prevention/risk-factors.html.
- 125.Wartenberg D., Residential EMF exposure and childhood leukemia: Meta-analysis and population attributable risk. Bioelectromagnetics ( Suppl. 5), S86–S104 (2001). [DOI] [PubMed] [Google Scholar]
- 126.Kheifets L., Ahlbom A., Crespi C. M., Draper G., Hagihara J., Lowenthal R. M., Mezei G., Oksuzyan S., Schüz J., Swanson J., Tittarelli A., Vinceti M., Wunsch Filho V., Pooled analysis of recent studies on magnetic fields and childhood leukaemia. Br. J. Cancer 103, 1128–1135 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kheifets L., Ahlbom A., Crespi C. M., Feychting M., Johansen C., Monroe J., Murphy M. F. G., Oksuzyan S., Preston-Martin S., Roman E., Saito T., Savitz D., Schüz J., Simpson J., Swanson J., Tynes T., Verkasalo P., Mezei G., A pooled analysis of extremely low-frequency magnetic fields and childhood brain tumors. Am. J. Epidemiol. 172, 752–761 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Extremely Low Frequency Fields (Environmental Health Criteria Monograph 238, World Health Organization, 2007); www.who.int/publications-detail-redirect/9789241572385.
- 129.“Electromagnetic fields and cancer” (National Cancer Institute, 2019); www.cancer.gov/about-cancer/causes-prevention/risk/radiation/electromagnetic-fields-fact-sheet.
- 130.Boothe V. L., Boehmer T. K., Wendel A. M., Yip F. Y., Residential traffic exposure and childhood leukemia: A systematic review and meta-analysis. Am. J. Prev. Med. 46, 413–422 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Carlos-Wallace F. M., Zhang L., Smith M. T., Rader G., Steinmaus C., Parental, in utero, and early-life exposure to benzene and the risk of childhood leukemia: A meta-analysis. Am. J. Epidemiol. 183, 1–14 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Filippini T., Hatch E. E., Rothman K. J., Heck J. E., Park A. S., Crippa A., Orsini N., Vinceti M., association between outdoor air pollution and childhood leukemia: A systematic review and dose-response meta-analysis. Environ. Health Perspect. 127, 46002 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, Benzene (International Agency for Research on Cancer, 2018), vol. 120; www.ncbi.nlm.nih.gov/books/NBK550157/.
- 134.Howe G. R., Aronson K. J., Benito E., Castelleto R., Cornée J., Duffy S., Gallagher R. P., Iscovich J. M., Deng-ao J., Kaaks R., Kune G. A., Kune S., Lee H. P., Lee M., Miller A. B., Peters R. K., Potter J. D., Riboli E., Slattery M. L., Trichopoulos D., Tuyns A., Tzonou A., Watson L. F., Whittemore A. S., Shu Z., The relationship between dietary fat intake and risk of colorectal cancer: Evidence from the combined analysis of 13 case-control studies. Cancer Causes Control 8, 215–228 (1997). [DOI] [PubMed] [Google Scholar]
- 135.Liu L., Zhuang W., Wang R.-Q., Mukherjee R., Xiao S.-M., Chen Z., Wu X.-T., Zhou Y., Zhang H.-Y., Is dietary fat associated with the risk of colorectal cancer? A meta-analysis of 13 prospective cohort studies. Eur. J. Nutr. 50, 173–184 (2011). [DOI] [PubMed] [Google Scholar]
- 136.“The cancer-cholesterol connection” (American Institute for Cancer Research, 2015); www.aicr.org/news/the-cancer-cholesterol-connection/.
- 137.Zhang J., Thomas A. G., Leybovich E., Vaginal douching and adverse health effects: A meta-analysis. Am. J. Public Health 87, 1207–1211 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.“Vaginal douching and adverse health outcomes” (American Public Health Association, 2007); www.apha.org/policies-and-advocacy/public-health-policy-statements/policy-database/2014/07/29/13/06/vaginal-douching-and-adverse-health-outcomes.
- 139.U.S. Department of Health and Human Services, “Douching” (Office on Women’s Health, 2021); www.womenshealth.gov/a-z-topics/douching#14.
- 140.Heikkilä K., Nyberg S. T., Theorell T., Fransson E. I., Alfredsson L., Bjorner J. B., Bonenfant S., Borritz M., Bouillon K., Burr H., Dragano N., Geuskens G. A., Goldberg M., Hamer M., Hooftman W. E., Houtman I. L., Joensuu M., Knutsson A., Koskenvuo M., Koskinen A., Kouvonen A., Madsen I. E. H., Hanson L. L. M., Marmot M. G., Nielsen M. L., Nordin M., Oksanen T., Pentti J., Salo P., Rugulies R., Steptoe A., Suominen S., Vahtera J., Virtanen M., Väänänen A., Westerholm P., Westerlund H., Zins M., Ferrie J. E., Singh-Manoux A., Batty G. D., Kivimäki M.; IPD-Work Consortium , Work stress and risk of cancer: Meta-analysis of 5700 incident cancer events in 116,000 European men and women. BMJ 346, f165 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yang T., Qiao Y., Xiang S., Li W., Gan Y., Chen Y., Work stress and the risk of cancer: A meta-analysis of observational studies. Int. J. Cancer 144, 2390–2400 (2019). [DOI] [PubMed] [Google Scholar]
- 142.“Psychological stress and cancer” (National Cancer Institute, 2012); www.cancer.gov/about-cancer/coping/feelings/stress-fact-sheet.
- 143.Bérubé S., Lemieux J., Moore L., Maunsell E., Brisson J., Smoking at time of diagnosis and breast cancer-specific survival: New findings and systematic review with meta-analysis. Breast Cancer Res. 16, R42 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang K., Li F., Zhang X., Li Z., Li H., Smoking increases risks of all-cause and breast cancer specific mortality in breast cancer individuals: A dose-response meta-analysis of prospective cohort studies involving 39725 breast cancer cases. Oncotarget 7, 83134–83147 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Duan W., Li S., Meng X., Sun Y., Jia C., Smoking and survival of breast cancer patients: A meta-analysis of cohort studies. Breast 33, 117–124 (2017). [DOI] [PubMed] [Google Scholar]
- 146.“Healthy lifestyle for breast cancer survivors” (Susan G Komen, 2021); www.komen.org/breast-cancer/survivorship/healthy-lifestyle/.
- 147.Takkouche B., Etminan M., Montes-Martínez A., Personal use of hair dyes and risk of cancer: A meta-analysis. JAMA 293, 2516–2525 (2005). [DOI] [PubMed] [Google Scholar]
- 148.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, Some Aromatic Amines, Organic Dyes, and Related Exposures (International Agency for Research on Cancer, 2010), vol. 99; www.ncbi.nlm.nih.gov/books/NBK385419/. [PMC free article] [PubMed]
- 149.“Hair dyes and cancer risk” (American Cancer Society, 2020); www.cancer.org/cancer/cancer-causes/hair-dyes.html.
- 150.Villanueva C. M., Fernández F., Malats N., Grimalt J. O., Kogevinas M., Meta-analysis of studies on individual consumption of chlorinated drinking water and bladder cancer. J. Epidemiol. Community Health 57, 166–173 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Costet N., Villanueva C. M., Jaakkola J. J. K., Kogevinas M., Cantor K. P., King W. D., Lynch C. F., Nieuwenhuijsen M. J., Cordier S., Water disinfection by-products and bladder cancer: Is there a European specificity? A pooled and meta-analysis of European case-control studies. Occup. Environ. Med. 68, 379–385 (2011). [DOI] [PubMed] [Google Scholar]
- 152.Bai Y., Yuan H., Li J., Tang Y., Pu C., Han P., Relationship between bladder cancer and total fluid intake: A meta-analysis of epidemiological evidence. World J. Surg. Oncol. 12, 223 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.U.S. Environmental Protection Agency, Chlorine CASRN 7782-50-5; https://cfpub.epa.gov/ncea/iris2/chemicalLanding.cfm?substance_nmbr=405.
- 154.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, Some Chemicals Present in Industrial and Consumer Products, Food and Drinking-Water (International Agency for Research on Cancer, 2013); www.ncbi.nlm.nih.gov/books/NBK373192/. [PMC free article] [PubMed]
- 155.Larsson S. C., Orsini N., Wolk A., Milk, milk products and lactose intake and ovarian cancer risk: A meta-analysis of epidemiological studies. Int. J. Cancer 118, 431–441 (2006). [DOI] [PubMed] [Google Scholar]
- 156.Genkinger J. M., Hunter D. J., Spiegelman D., Anderson K. E., Arslan A., Beeson W. L., Buring J. E., Fraser G. E., Freudenheim J. L., Goldbohm R. A., Hankinson S. E., Jacobs D. R., Koushik A., Lacey J. V., Larsson S. C., Leitzmann M., McCullough M. L., Miller A. B., Rodriguez C., Rohan T. E., Schouten L. J., Shore R., Smit E., Wolk A., Zhang S. M., Smith-Warner S. A., Dairy products and ovarian cancer: A pooled analysis of 12 cohort studies. Cancer Epidemiol. Biomark. Prev. 15, 364–372 (2006). [DOI] [PubMed] [Google Scholar]
- 157.Liu J., Tang W., Sang L., Dai X., Wei D., Luo Y., Zhang J., Milk, yogurt, and lactose intake and ovarian cancer risk: A meta-analysis. Nutr. Cancer 67, 68–72 (2015). [DOI] [PubMed] [Google Scholar]
- 158.Diet, Nutrition, Physical Activity and Ovarian Cancer (Continuous Update Project Expert Report 2018, World Cancer Research Fund/American Institute for Cancer Research); www.wcrf.org/wp-content/uploads/2021/02/ovarian-cancer-report.pdf.
- 159.Zhang Y., Sanjose S. D., Bracci P. M., Morton L. M., Wang R., Brennan P., Hartge P., Boffetta P., Becker N., Maynadie M., Foretova L., Cocco P., Staines A., Holford T., Holly E. A., Nieters A., Benavente Y., Bernstein L., Zahm S. H., Zheng T., Personal use of hair dye and the risk of certain subtypes of non-Hodgkin lymphoma. Am. J. Epidemiol. 167, 1321–1331 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Qin L., Deng H.-Y., Chen S.-J., Wei W., A meta-analysis on the relationship between hair dye and the incidence of non-Hodgkin’s lymphoma. Med. Princ. Pract. 28, 222–230 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.“Hair dyes and cancer risk” (National Cancer Institute, 2017); www.cancer.gov/about-cancer/causes-prevention/risk/myths/hair-dyes-fact-sheet.
- 162.Mezei G., Gadallah M., Kheifets L., Residential magnetic field exposure and childhood brain cancer: A meta-analysis. Epidemiology 19, 424–430 (2008). [DOI] [PubMed] [Google Scholar]
- 163.Kheifets L., Monroe J., Vergara X., Mezei G., Afifi A. A., Occupational electromagnetic fields and leukemia and brain cancer: An update to two meta-analyses. J. Occup. Environ. Med. 50, 677–688 (2008). [DOI] [PubMed] [Google Scholar]
- 164.Towle K. M., Grespin M. E., Monnot A. D., Personal use of hair dyes and risk of leukemia: A systematic literature review and meta-analysis. Cancer Med. 6, 2471–2486 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ambrosone C. B., Kropp S., Yang J., Yao S., Shields P. G., Chang-Claude J., Cigarette smoking, N-acetyltransferase 2 genotypes, and breast cancer risk: Pooled analysis and meta-analysis. Cancer Epidemiol. Biomarkers Prev. 17, 15–26 (2008). [DOI] [PubMed] [Google Scholar]
- 166.Gaudet M. M., Gapstur S. M., Sun J., Diver W. R., Hannan L. M., Thun M. J., Active smoking and breast cancer risk: Original cohort data and meta-analysis. J. Natl. Cancer Inst. 105, 515–525 (2013). [DOI] [PubMed] [Google Scholar]
- 167.Macacu A., Autier P., Boniol M., Boyle P., Active and passive smoking and risk of breast cancer: A meta-analysis. Breast Cancer Res. Treat. 154, 213–224 (2015). [DOI] [PubMed] [Google Scholar]
- 168.“Breast cancer: Potential risk factors” (American Cancer Society, 2021); www.cancer.org/cancer/breast-cancer/risk-and-prevention/factors-with-unclear-effects-on-breast-cancer-risk.html.
- 169.Yang J. J., Yu D., Takata Y., Smith-Warner S. A., Blot W., White E., Robien K., Park Y., Xiang Y.-B., Sinha R., Lazovich D., Stampfer M., Tumino R., Aune D., Overvad K., Liao L., Zhang X., Gao Y.-T., Johansson M., Willett W., Zheng W., Shu X.-O., Dietary fat intake and lung cancer risk: A pooled analysis. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 35, 3055–3064 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Diet, Nutrition, Physical Activity and Lung Cancer (Continuous Update Project Expert Report 2018, World Cancer Research Fund/American Institute for Cancer Research); www.wcrf.org/wp-content/uploads/2021/02/lung-cancer-report.pdf.
- 171.Turner L. B., A meta-analysis of fat intake, reproduction, and breast cancer risk: An evolutionary perspective. Am. J. Hum. Biol. 23, 601–608 (2011). [DOI] [PubMed] [Google Scholar]
- 172.Yang B., Ren X.-L., Fu Y.-Q., Gao J.-L., Li D., Ratio of n-3/n-6 PUFAs and risk of breast cancer: A meta-analysis of 274135 adult females from 11 independent prospective studies. BMC Cancer 14, 105 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Cao Y., Hou L., Wang W., Dietary total fat and fatty acids intake, serum fatty acids and risk of breast cancer: A meta-analysis of prospective cohort studies. Int. J. Cancer 138, 1894–1904 (2016). [DOI] [PubMed] [Google Scholar]
- 174.Zhou Y., Zhang S., Li Z., Zhu J., Bi Y., Bai Y., Wang H., Maternal benzene exposure during pregnancy and risk of childhood acute lymphoblastic leukemia: A meta-analysis of epidemiologic studies. PLOS ONE 9, e110466 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Huang Y., Huang J., Lan H., Zhao G., Huang C., A meta-analysis of parental smoking and the risk of childhood brain tumors. PLOS ONE 9, e102910 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Metayer C., Petridou E., Aranguré J. M. M., Roman E., Schüz J., Magnani C., Mora A. M., Mueller B. A., de Oliveira M. S. P., Dockerty J. D., McCauley K., Lightfoot T., Hatzipantelis E., Rudant J., Flores-Lujano J., Kaatsch P., Miligi L., Wesseling C., Doody D. R., Moschovi M.; MIGICCL Group, Orsi L., Mattioli S., Selvin S., Kang A. Y., Clavel J., Parental tobacco smoking and acute myeloid leukemia: The Childhood Leukemia International Consortium. Am. J. Epidemiol. 184, 261–273 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.IARC, “Agents classified by the IARC monographs, volumes 1–131” (IARC Monographs on the Identification of Carcinogenic Hazards to Humans, 2022); https://monographs.iarc.who.int/agents-classified-by-the-iarc/.
- 178.Wu J., Zeng R., Huang J., Li X., Zhang J., Ho J. C.-M., Zheng Y., Dietary protein sources and incidence of breast cancer: A dose-response meta-analysis of prospective studies. Nutrients 8, 730 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Anderson J. J., Darwis N. D. M., Mackay D. F., Celis-Morales C. A., Lyall D. M., Sattar N., Gill J. M. R., Pell J. P., Red and processed meat consumption and breast cancer: UK Biobank cohort study and meta-analysis. Eur. J. Cancer 90, 73–82 (2018). [DOI] [PubMed] [Google Scholar]
- 180.Farvid M. S., Stern M. C., Norat T., Sasazuki S., Vineis P., Weijenberg M. P., Wolk A., Wu K., Stewart B. W., Cho E., Consumption of red and processed meat and breast cancer incidence: A systematic review and meta-analysis of prospective studies. Int. J. Cancer 143, 2787–2799 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Chen C., Ma X., Zhong M., Yu Z., Extremely low-frequency electromagnetic fields exposure and female breast cancer risk: A meta-analysis based on 24,338 cases and 60,628 controls. Breast Cancer Res. Treat. 123, 569–576 (2010). [DOI] [PubMed] [Google Scholar]
- 182.Chen Q., Lang L., Wu W., Xu G., Zhang X., Li T., Huang H., A meta-analysis on the relationship between exposure to ELF-EMFs and the risk of female breast cancer. PLOS ONE 8, e69272 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zhang Y., Lai J., Ruan G., Chen C., Wang D. W., Meta-analysis of extremely low frequency electromagnetic fields and cancer risk: A pooled analysis of epidemiologic studies. Environ. Int. 88, 36–43 (2016). [DOI] [PubMed] [Google Scholar]
- 184.“Electromagnetic fields (EMF) and breast cancer risk” (Susan G Komen, 2021); www.komen.org/breast-cancer/facts-statistics/research-studies/topics/electromagnetic-fields-and-breast-cancer-risk/.
- 185.Ding M., Bhupathiraju S. N., Satija A., van Dam R. M., Hu F. B., Long-term coffee consumption and risk of cardiovascular disease: A systematic review and a dose-response meta-analysis of prospective cohort studies. Circulation 129, 643–659 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Larsson S. C., Drca N., Jensen-Urstad M., Wolk A., Coffee consumption is not associated with increased risk of atrial fibrillation: Results from two prospective cohorts and a meta-analysis. BMC Med. 13, 207 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Caldeira D., Martins C., Alves L. B., Pereira H., Ferreira J. J., Costa J., Caffeine does not increase the risk of atrial fibrillation: A systematic review and meta-analysis of observational studies. Heart 99, 1383–1389 (2013). [DOI] [PubMed] [Google Scholar]
- 188.“Caffeine and heart disease” (American Heart Association, 2014); www.heart.org/en/healthy-living/healthy-eating/eat-smart/nutrition-basics/caffeine-and-heart-disease.
- 189.Dietary Guidelines Advisory Committee, Scientific Report of the 2015 Dietary Guidelines Advisory Committee: Advisory Report to the Secretary of Health and Human Services and the Secretary of Agriculture (U.S. Department of Agriculture, Agricultural Research Service, 2015). [Google Scholar]
- 190.Pelucchi C., Bosetti C., Negri E., Lipworth L., La Vecchia C., Olive oil and cancer risk: An update of epidemiological findings through 2010. Curr. Pharm. Des. 17, 805–812 (2011). [DOI] [PubMed] [Google Scholar]
- 191.Xin Y., Li X.-Y., Sun S.-R., Wang L.-X., Huang T., Vegetable oil intake and breast cancer risk: A meta-analysis. Asian Pac. J. Cancer Prev. 16, 5125–5135 (2015). [DOI] [PubMed] [Google Scholar]
- 192.Yu X., Bao Z., Zou J., Dong J., Coffee consumption and risk of cancers: A meta-analysis of cohort studies. BMC Cancer 11, 96 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Turati F., Galeone C., Edefonti V., Ferraroni M., Lagiou P., La Vecchia C., Tavani A., A meta-analysis of coffee consumption and pancreatic cancer. Ann. Oncol. 23, 311–318 (2012). [DOI] [PubMed] [Google Scholar]
- 194.Nie K., Xing Z., Huang W., Wang W., Liu W., Coffee intake and risk of pancreatic cancer: An updated meta-analysis of prospective studies. Minerva Med. 107, 270–278 (2016). [PubMed] [Google Scholar]
- 195.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, Drinking Coffee, Mate, and Very Hot Beverages (International Agency for Research on Cancer, 2018), vol. 116; www.ncbi.nlm.nih.gov/books/NBK543953/. [PubMed]
- 196.Diet, Nutrition, Physical Activity and Pancreatic Cancer (Continuous Update Project Expert Report 2018, World Cancer Research Fund/American Institute for Cancer Research, 2018); www.wcrf.org/wp-content/uploads/2021/02/pancreatic-cancer-report.pdf.
- 197.Shang Y., Han G., Li J., Zhao J., Cui D., Liu C., Yi S., Vasectomy and prostate cancer risk: A meta-analysis of cohort studies. Sci. Rep. 5, 9920 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Bhindi B., Wallis C. J. D., Nayan M., Farrell A. M., Trost L. W., Hamilton R. J., Kulkarni G. S., Finelli A., Fleshner N. E., Boorjian S. A., Karnes R. J., The association between vasectomy and prostate cancer: A systematic review and meta-analysis. JAMA Intern. Med. 177, 1273–1286 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wu T., Duan X., Chen S., Hu C., Yu R., Cui S., Does vasectomy increase prostate cancer risk? An updated meta-analysis and systematic review of cohort studies. Int. J. Clin. Exp. Med. 11, 2978–2987 (2018). [Google Scholar]
- 200.Sharlip I. D., Belker A. M., Honig S., Labrecque M., Marmar J. L., Ross L. S., Sandlow J. I., Sokal D. C., Vasectomy: AUA guideline. J. Urol. 188, 2482–2491 (2012). [DOI] [PubMed] [Google Scholar]
- 201.“Prostate cancer risk factors” (American Cancer Society, 2020); www.cancer.org/cancer/prostate-cancer/causes-risks-prevention/risk-factors.html.
- 202.Hackshaw A. K., Paul E. A., Breast self-examination and death from breast cancer: A meta-analysis. Br. J. Cancer 88, 1047–1053 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kösters J. P., Gøtzsche P. C., Regular self-examination or clinical examination for early detection of breast cancer. Cochrane Database Syst. Rev. , CD003373 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.“Breast cancer risk assessment and screening in average-risk women” (Practice Bulletin 179, American College of Obstetricians and Gynecologists, 2017); www.acog.org/en/clinical/clinical-guidance/practice-bulletin/articles/2017/07/breast-cancer-risk-assessment-and-screening-in-average-risk-women.
- 205.“ACS breast cancer screening guidelines” (American Cancer Society, 2022); www.cancer.org/cancer/breast-cancer/screening-tests-and-early-detection/american-cancer-society-recommendations-for-the-early-detection-of-breast-cancer.html.
- 206.“Breast cancer screening (PDQ®)–Health professional version” (National Cancer Institute, 2022); www.cancer.gov/types/breast/hp/breast-screening-pdq.
- 207.Beral V., Bull D., Doll R., Peto R., Reeves G.; Collaborative Group on Hormonal Factors in Breast Cancer , Breast cancer and abortion: Collaborative reanalysis of data from 53 epidemiological studies, including 83?000 women with breast cancer from 16 countries. Lancet 363, 1007–1016 (2004). [DOI] [PubMed] [Google Scholar]
- 208.Huang Y., Zhang X., Li W., Song F., Dai H., Wang J., Gao Y., Liu X., Chen C., Yan Y., Wang Y., Chen K., A meta-analysis of the association between induced abortion and breast cancer risk among Chinese females. Cancer Causes Control 25, 227–236 (2014). [DOI] [PubMed] [Google Scholar]
- 209.Guo J., Huang Y., Yang L., Xie Z., Song S., Yin J., Kuang L., Qin W., Association between abortion and breast cancer: An updated systematic review and meta-analysis based on prospective studies. Cancer Causes Control 26, 811–819 (2015). [DOI] [PubMed] [Google Scholar]
- 210.“Abortion and breast cancer risk” (Susan G Komen, 2021), www.komen.org/breast-cancer/facts-statistics/research-studies/topics/abortion-and-breast-cancer-risk/.
- 211.“Induced abortion and breast cancer risk” (ACOG Committee Opinion 434, American College of Obstetricians and Gynecologists, 2009); www.acog.org/en/clinical/clinical-guidance/committee-opinion/articles/2009/06/induced-abortion-and-breast-cancer-risk.
- 212.López-Cervantes M., Torres-Sánchez L., Tobías A., López-Carrillo L., Dichlorodiphenyldichloroethane burden and breast cancer risk: A meta-analysis of the epidemiologic evidence. Environ. Health Perspect. 112, 207–214 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ingber S. Z., Buser M. C., Pohl H. R., Abadin H. G., Murray H. E., Scinicariello F., DDT/DDE and breast cancer: A meta-analysis. Regul. Toxicol. Pharmacol. 67, 421–433 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Park J.-H., Cha E. S., Ko Y., Hwang M.-S., Hong J.-H., Lee W. J., Exposure to dichlorodiphenyltrichloroethane and the risk of breast cancer: A systematic review and meta-analysis. Osong. Public Health Res. Perspect. 5, 77–84 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, DDT, Lindane, and 2,4-D (International Agency for Research on Cancer, 2018), vol. 113; www.ncbi.nlm.nih.gov/books/NBK507424/. [PubMed]
- 216.J. R. Roberts, J. R. Reigart, Recognition and Management of Pesticide Poisonings (U.S. Environmental Protection Agency, ed. 6, 2013); www.epa.gov/pesticide-worker-safety/recognition-and-management-pesticide-poisonings.
- 217.Elcock M., Morgan R. W., Update on artificial sweeteners and bladder cancer. Regul. Toxicol. Pharmacol. 17, 35–43 (1993). [DOI] [PubMed] [Google Scholar]
- 218.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, Some Chemicals that Cause Tumours of the Kidney or Urinary Bladder in Rodents and Some Other Substances (International Agency for Research on Cancer, 1999), vol. 73; www.ncbi.nlm.nih.gov/books/NBK402050/.
- 219.“Artificial sweeteners and cancer” (National Cancer Institute, 2005); www.cancer.gov/about-cancer/causes-prevention/risk/diet/artificial-sweeteners-fact-sheet).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Abbreviations
Fig. S1
Tables S1 and S2A–WW
References