Abstract
Recombinant strains of Pseudomonas putida KT2440 carrying genetic expression cassettes with xylene oxygenase- and styrene monooxygenase-encoding genes on their chromosomes could be induced in shaking-flask experiments to specific activities that rivaled those of multicopy-plasmid-based Escherichia coli recombinants. Such strains maintained the introduced styrene oxidation activity in continuous two-liquid-phase cultures for at least 100 generations, although at a lower level than in the shaking-flask experiments. The data suggest that placement of target genes on the chromosome might be a suitable route for the construction of segregationally stable and highly active whole-cell biocatalysts.
Biotransformations provide access to asymmetric oxidations which are difficult to achieve by purely chemical methods (1, 35). The required reduced cofactors can best be generated in whole-cell biocatalysts, and we have described Escherichia coli recombinants that synthesize Pseudomonas monooxygenases for the production of (S)-styrene oxide, a potentially important chiral building block in organic synthesis, from styrene in two-liquid-phase fed-batch processes (23, 26, 41). The corresponding genes were expressed via pBR322-derived multicopy plasmid vectors based on the alk regulatory system of Pseudomonas oleovorans (23, 26), where the positive regulator protein AlkS activates transcription from the cognate promoter alkBp (Fig. 1A) (39). A continuous production process would eliminate periods of low productivity and prevent the drop in the number of viable cells (and consequently a drop in volumetric productivities) when a two-liquid-phase culture enters stationary phase (9, 10). Continuous cultures frequently suffer from limited genetic stability of highly active biocatalysts (10, 11, 13, 19, 43). Plasmid-located recombinant genes in growing cells can be subject to structural or segregational instability. A number of possible solutions for segregational instability have been proposed (2, 12, 17, 21, 27). One way to eliminate the possibility of segregational instability completely might be to place the recombinant genes on the chromosome of a suitable host strain, for example via mini-Tn5 transposons (16). As the transposase is lost during transposition with this system, the recombinant gene remains stably integrated, which has made this system a very attractive model system for engineering microorganisms for environmental, medical, and metabolic engineering applications (4, 8, 20, 28, 30, 36–38). Furthermore, tools to efficiently remove (antibiotic) selection markers (18), thereby facilitating commercial utilization of the resulting strain and biomass disposal, are available. Since such recombinants carry only one to a few gene copies (14), it remains to be shown whether such strains—lacking the opportunity to capitalize on gene dosage effects—produce sufficient activities for practical application as biocatalysts in the production of fine chemicals. In this report, we investigated whether placing genetic cassettes that contain the elements of the alk regulatory system together with monooxygenase genes onto the chromosome of E. coli JM101 or P. putida KT2440 led to stable whole-cell biocatalysts with high specific activities.
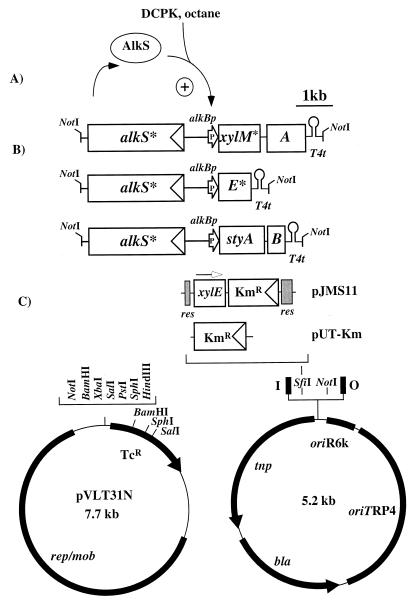
FIG. 1.
Function of the alk regulatory system and structure of the genetic elements used in this study. (A) The regulator protein AlkS is activated by interaction with DCPK or n-octane and initiates transcription from alkBp. (B) NotI fragments of plasmids pSPZ2MA, pSPZ2AB, and pSPZ2E for the synthesis of xylene oxygenase, styrene monooxygenase, and catechol-2,3-dioxygenase, respectively. The drawing is to scale. Asterisks indicate that the wild-type gene has been engineered to eliminate internal NdeI sites. Arrows indicate heterologous promoters, and triangles within boxes indicate homologous promoters. T4t, phage T4 transcriptional terminator. (C) Plasmids that received the NotI cassettes shown in panel B. The drawing in the upper part is to the same scale as in panel B. The open arrow indicates the direction of transcription of the promoterless xylE gene. The two cassettes shown are inserted in the SfiI site of the basic vector and give rise to the two mini-transposon delivery plasmids pJMS11 and pUT-Km. The grey boxes represent the two res sequences that are the substrate of the RP4 resolvase. The mini-transposon is defined by the I and O ends (black boxes).
Analysis of E. coli mini-Tn5 mutants.
The 6.4-kb NotI fragment of pSPZ2MA containing alkS* alkBp-xylM*A (the asterisks indicate genes where internal NdeI sites have been removed) (Fig. 1B) (26) was transferred to the mini-Tn5 delivery plasmid pJMS11 (Fig. 1C) (24), and the resulting mini-transposon was delivered to E. coli JM101 cells (31) by a triparental mating with E. coli HB101(RK600) as the helper as described previously (18). We could readily isolate strains that were resistant to kanamycin but sensitive to ampicillin, indicating true transposition events (7). The transconjugants were grown in 100 ml of M9* mineral medium supplemented with 100 μl of US* trace element solution, 100 μl of a 1% (wt/vol) thiamine hydrochloride solution, and 0.5% (wt/vol) glucose as the carbon source as described previously (26). The cultures were induced at an optical density at 450 nm of around 0.4 by the addition of 0.05% (vol/vol) dicyclopropylketone (DCPK; Aldrich, Buchs, Switzerland) and at regular intervals subjected to a whole-cell styrene oxide formation assay as described previously (25). However, we failed to detect any styrene oxide formation activity with these strains in our assay (detection limit, 0.1 U · g of cells [dry weight]−1, 1 U being defined as the enzymatic activity that forms 1 μmol of styrene oxide in 1 min. Previous attempts to produce xylene oxygenase in E. coli JM101 from the pBR322-derived expression plasmid pSPZ3 had resulted in maximum specific activities of 91 U · g of cells (dry weight)−1 (26), suggesting that the >900-fold-reduced level of specific activity in the present experiment might be—at least in part—a function of the vastly lower gene dosage in the E. coli transconjugant.
Efficient alk-based expression of oxygenases in E. coli depends on copy number.
To investigate the reduced level of activity, we constructed a 5.1-kb genetic cassette that was identical to the one described above but that carried xylE* instead of xylM*A (Fig. 1B), so that transconjugant strains would produce catechol-2,3-dioxygenase, which is easy to assay in vitro even in small amounts (22). To obtain an xylE gene devoid of internal NdeI sites but with an NdeI site on the start codon which was compatible with sites in the alk-based expression plasmids (26), we performed a first PCR with one primer that annealed at the 5′ end of the gene and introduced the NdeI site on the start codon together with a new BamHI site further upstream (5′ CATGAGGATCCAAGAGGTGACCATATGAACAAAGGTG 3′, where the BamHI site is underlined, the NdeI site is in italics, and the xylE start codon is in boldface type) and with a second primer that primed inside xylE and thereby silently mutated the internal NdeI site (5′ GGCACAGCCATACGCCATCAGATC 3′, where the mutagenic nucleotide is underlined). The resulting 300-bp fragment served as the first primer of a second PCR, which was performed together with a primer that annealed at the end of the xylE gene and introduced an EcoRI site (5′ AAAAAAGAATTCCCATCAGGTCAGCACGGTCATGAATCG 3′, where the EcoRI site is underlined and the xylE stop codon is in boldface type). The resulting 940-bp fragment was digested with BamHI and EcoRI and inserted into pSPZ1(+), reexcised as an NdeI/AscI fragment, and inserted into pSPZ2Not along the lines outlined earlier (26). In the resulting plasmid, pSPZ2E, the xylE* gene was available as a NotI-flanked alkS* alkBp-xylE* cassette analogous to the one carrying xylM*A (Fig. 1B). This cassette was transferred to pUT-Km (Fig. 1C) (6) for mini-transposon delivery and to pVLT31N (Fig. 1C) for expression from a multicopy number plasmid. Plasmid pVLT31N is a derivative of the broad-host-range vector pVLT31 (5), in which an additional NotI site was introduced by digesting pVLT31 with HpaI and SmaI and ligating the resulting fragment to an octameric DNA linker with the NotI recognition sequence. This process also led to the loss of the lacIq Ptac expression system present on pVLT31. E. coli JM101 transconjugants carrying the xylE* mini-transposon on the chromosome were readily isolated, grown in mineral medium with glucose as the carbon source as described above, and harvested 4 h after induction. All strains tested produced catechol-2,3-dioxygenase to levels measurable in cell extracts, up to a maximum of 0.9 U · mg of protein−1. E. coli JM101 transformants carrying the xylE* cassette in multicopy numbers on pVLT31N were grown in parallel in the presence of 12.5 μg of tetracycline · ml−1 and produced after induction of catechol-2,3-dioxygenase activity to 72 U · mg of protein−1 in cell extracts. This result suggested that in E. coli the specific activities of recombinant strains carrying our genetic cassettes were indeed a function of gene dosage, although the ratio of in vitro activities found for the catechol-2,3-dioxygenase was on the order of 80, which does not fully explain our inability to obtain E. coli transconjugants with xylene oxygenase activity. Interestingly, the ratio of activities is around four times the estimated ratio of gene copy numbers; RSF1010-based plasmids are usually present in a copy number of 20 (15), whereas mini-transposons are likely to insert only once into the chromosome (14).
Analysis of P. putida mini-Tn5 mutants.
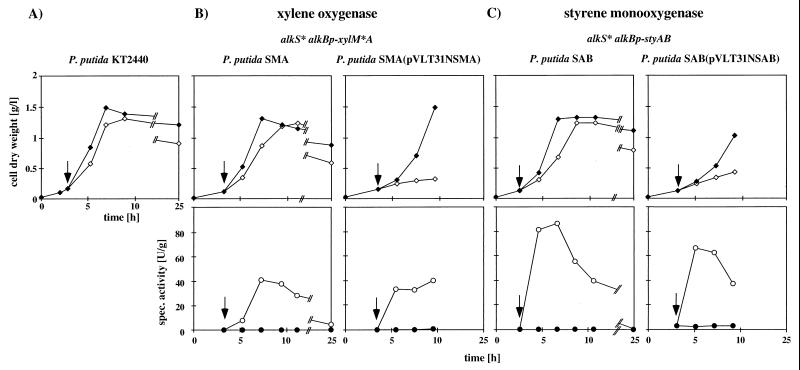
In wild-type P. oleovorans GPo1, the alk regulatory system is located on the low-copy-number catabolic OCT plasmid and membrane-located AlkB protein is still synthesized to 2% of total cell protein after induction (34). This result raised the possibility that in Pseudomonas strains alk-based monocopy constructs give significantly more active strains. We used the NotI cassettes of pSPZ2MA and pSPZ2AB (a 6.1-kb cassette carrying the genes of the styrene monooxygenase of Pseudomonas sp. strain VLB120 [Fig. 1B]) (23) on pJMS11 to construct P. putida KT2440 transconjugants. On pJMS11, the selection markers of the mini-transposons are flanked by res sites that are the substrate of the RP4 resolvase. After successful construction and analysis of the biocatalyst (see Fig. 3A), they can be easily excised by expressing the resolvase gene from a suicide plasmid (18). The transconjugants were used in shaking-flask experiments as described above but with 0.5% (wt/vol) citrate as the carbon source. P. putida SMA, a P. putida KT2440 derivative with alkS* alkBp-xylM*A in the chromosome, synthesized xylene oxygenase to levels of 41 U · g of cells (dry weight)−1 (Fig. 2B). P. putida SAB, with alkS* alkBp-styAB in the chromosome, synthesized styrene monooxygenase to specific activities of 86 U · g of cells (dry weight)−1 (Fig. 2C), which was even more than the 70 U · g of cells (dry weight)−1 found for E. coli JM101 recombinants expressing the styrene monooxygenase genes from the same cassette on the multicopy plasmid pSPZ10 (23). The same activities were also achieved with the strains P. putida SMAΔ and P. putida SABΔ, which differed from their parent strains only in that they had the transposon selection markers (the kanamycin resistance gene and the xylE gene) removed by expressing the RP4 resolvase gene from a suicide plasmid as described previously (reference 18 and data not shown). To investigate whether these numbers could be increased by increasing the copy number of the genetic cassettes, analogous to the situation in E. coli, we inserted the NotI cassettes with the genes for styrene monooxygenase and xylene oxygenase into pVLT31N. The resulting plasmids were conjugated into P. putida SMA and P. putida SAB with E. coli S17-1λpir as the host (33), after control experiments with the plasmids in unmodified P. putida KT2440 had shown that both plasmids were functional (data not shown). Generally, the maximum specific activities of the resulting strains were not higher (Fig. 2B and C). These results were in agreement with data reported by Yuste et al., who obtained similar results with an alkBp-lacZ fusion (42). Remarkably, although the specific activities did not change significantly upon provision of the cassettes in multiple copies, the growth behavior of the strains did: strains carrying the cassettes in multiple copies grew more slowly, while the strains carrying only the chromosomal copy showed that induction had only a little influence on growth (Fig. 2). Although this leaves unresolved the issue of the maximum achievable rate of styrene oxide formation in Pseudomonas and the nature of the bottleneck in the development of specific activity, the results indicate that by choosing a host different from E. coli, it is indeed possible with an alk-based regulatory system to construct a whole-cell biocatalyst that carries only one copy of the target genes and can still synthesize the encoded enzyme to a high specific activity. The results do not indicate whether the higher activities are due to an increased specific activities of the Pseudomonas-derived monooxygenases in a recombinant Pseudomonas host or due to an increased amount of formed protein, for example, because transcription from the alkBp promoter is more efficient in a P. putida than in an E. coli host.
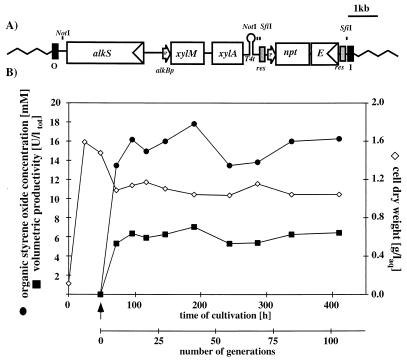
FIG. 3.
Continuous two-liquid-phase culture with P. putida SMA. (A) Genotype of P. putida SMA. The mini-transposon of pJMS-SMA (determined by the I and O ends [black boxes]) contained the alk expression cassette for the synthesis of xylene oxygenase, the selection marker npt for kanamycin resistance, and xylE for easy colorimetric selection. The selection marker is flanked by res sites (grey boxes), which are the substrate of the RP4 resolvase. Arrows indicate promoters (P). T4t, phage T4 transcriptional terminator. (B) Continuous culture (D = 0.2 h−1) of P. putida SMA on mineral medium with 0.5% (wt/vol) citrate as the carbon source. The arrow indicates the start of the organic feed of a mixture of long-chain alkanes containing 1% octane and 1% styrene, which accounted after equilibration of the system for 10% of total liquid volume. ltot, liter of total liquid volume; laq, liter of the aqueous phase.
FIG. 2.
Shaking-flask experiments with mineral medium and citrate as the carbon source with recombinant strains synthesizing xylene oxygenase or styrene monooxygenase. Upper graphs show growth behavior, and lower graphs show the development of specific activity after induction with 0.05% (vol/vol) DCPK. The point of induction is indicated by the arrow. (A) Influence of inducer on the growth of parent strain P. putida KT2440. No styrene oxidation activity was observed for this strain irrespective of the induction. (B) Expression of chromosomally located (left graphs) and chromosomally and plasmid located (right graphs) xylene oxygenase genes. The structure of the gene cassette is indicated on top. (C) Expression of chromosomally located (left graphs) and chromosomally and plasmid located (right graphs) styrene monooxygenase genes. Closed symbols, uninduced cultures; open symbols, induced cultures.
Continuous two-liquid-phase cultivation with P. putida SMA.
To obtain a first indication of whether the potential for activity and stability could be realized with our transconjugants, we performed a continuous two-liquid-phase culture with P. putida SMA (Fig. 3B). We preferred to use this strain for the experiment at this stage over P. putida SMAΔ as it still allowed rapid identification of cells deriving from the inoculum due to the presence of the two selection markers. The details of the reactor system have been described previously (26). In short, a 3-liter stirred tank reactor with the temperature (30°C), pH (7.1, titration with 30% phosphoric acid and 4 M sodium hydroxide), and stirring speed (1,500 rounds per min) regulated and the airflow manually adjusted to 1 liter per min was placed on a balance (Bioengineering, Wald, Switzerland) which regulated an effluent pump that was activated above a predefined weight of the reactor. This pump then removed liquid from the reactor at around 10-fold the rate of the medium feed, leading to an oscillation in weight (and consequently of the dilution rate) of around 40 g (4%). A stationary-phase P. putida SMA preculture in 100 ml of M9* mineral medium supplemented with US* trace elements and 0.5% (wt/vol) citrate was pumped into the reactor, which contained 900 ml of M9* mineral medium with 2 ml of US* solution per liter and 0.5% citrate. The working volume was fixed to 1 liter, and mineral medium of identical composition was fed into the reactor at a dilution rate of 0.2 h−1. After 50 h, we started an organic feed that pumped an organic phase consisting of 1% (vol/vol of organic phase) n-octane (Acros, Geel, Belgium) as the inducer of the alk regulatory system and 1% (vol/vol of organic phase) styrene (99%; Fluka, Buchs, Switzerland) as the substrate for the xylene oxygenase dissolved in AL240 (Chemische Fabrik Schweizerhall, Schweizerhall, Switzerland) as the carrier solvent at a dilution rate of 0.02 h−1. AL240 is a mixture of iso-, cyclo-, and linear alkanes with a chain length of at least 13 carbon atoms and has no effect on bacterial growth (32). The aqueous feed was reduced to 0.18 h−1, and the weight limits for the effluent pump where adjusted accordingly. This led after equilibration to a volume portion of the organic phase of 10%. Analysis of the liquid phases in the reactor has been described previously (26). In the presence of the organic phase, the dry weight of cells stabilized at around 1.2 g per liter of aqueous phase, and styrene oxide accumulated in the organic phase to around 16 mM, which translated into a styrene oxide formation rate of 6 U per liter (liquid volume) or 5 U · g of cells (dry weight)−1 (Fig. 3). The activity was maintained until the end of the experiment 100 generations (350 h) after induction. However, this number was significantly smaller than the values obtained in the shaking-flask experiments or than the ca. 30 U · g of cells (dry weight)−1 that has been obtained with a pBR322-derived expression system in E. coli recombinants in two-liquid-phase fed-batch experiments (26). To investigate whether the smaller specific activities in the continuous culture were obtained with cells that had lost the ability to synthesize xylene monooxygenase to the high levels observed in the shaking-flask experiments, we removed aqueous phase from the reactor at three time points (50, 136, and 325 h after induction) and plated it on Luria-Bertani agar plates. Sets of five of the resulting colonies served as the starting cultures of new shaking-flask cultures in mineral medium with citrate as the carbon source as described above. In all three cases, the accumulated averaged specific activities 4 h after induction were within 10% of that of the original strain (results not shown), indicating that the relatively low activities were not due to genetic instability.
One clear difference in the experimental protocol of shaking-flask experiments from that of continuous culture is the presence of a second phase and the mode of induction. While cells were induced by DCPK addition without an organic phase in shaking-flask experiments, induction in the continuous culture was achieved with octane dissolved in a carrier solvent of longer-chain alkanes. While 1% (vol/vol) octane was sufficient to induce fed-batch E. coli cultures efficiently (23, 26), this is not necessarily true for cultures with recombinants based on P. putida KT2440. It is also possible that the presence of the organic phase interfered with the accumulation of high specific activities. Although Pseudomonas strains in general are considered to be tolerant of organic solvents with a logP higher than 4 (29, 40), where P is the partition coefficient of the substance in a standard octanol-water system, and exceptional solvent resistances have been reported (3), the behavior of P. putida KT2440 in two-liquid-phase cultures has never been investigated in detail.
Taken together, the data presented here indicate that chromosomal integration of genes under a suitable regulatory system is a very useful route for constructing a whole-cell biocatalyst that is able to synthesize rather complex monooxygenases to high specific activities and that can maintain a constant activity for extended periods of cultivations in the presence of an organic phase. Further work will address the exploitation of the maximum specific activities of such recombinant strains in continuous cultivations.
REFERENCES
- 1.Besse P, Veschambre H. Chemical and biological synthesis of chiral epoxides. Tetrahedron. 1994;50:8885–8927. [Google Scholar]
- 2.Cheng C, Huang Y L, Yang S-T. A novel feeding strategy for enhanced plasmid stability and protein production in recombinant yeast fedbatch fermentation. Biotechnol Bioeng. 1997;56:23–31. doi: 10.1002/(SICI)1097-0290(19971005)56:1<23::AID-BIT3>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 3.de Bont J A M. Solvent-tolerant bacteria in biocatalysis. Trends Biotechnol. 1998;16:493–499. [Google Scholar]
- 4.de Lorenzo V. Designing microbial systems for gene expression in the field. Trends Biotechnol. 1994;12:365–371. doi: 10.1016/0167-7799(94)90037-X. [DOI] [PubMed] [Google Scholar]
- 5.de Lorenzo V, Eltis L, Kessler B, Timmis K N. Analysis of Pseudomonas gene products using lacIq/Ptrp-lac plasmids and transposons that confer conditional phenotypes. Gene. 1993;123:17–24. doi: 10.1016/0378-1119(93)90533-9. [DOI] [PubMed] [Google Scholar]
- 6.de Lorenzo V, Herrero M, Jakubzik U, Timmis K N. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J Bacteriol. 1990;172:6568–6572. doi: 10.1128/jb.172.11.6568-6572.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Lorenzo V, Timmis K N. Analysis and construction of stable phenotypes in Gram-negative bacteria with Tn5- and Tn10-derived minitransposons. Methods Enzymol. 1994;235:386–405. doi: 10.1016/0076-6879(94)35157-0. [DOI] [PubMed] [Google Scholar]
- 8.Erb R W, Eichner C A, Wagner-Döbler I, Timmis K N. Bioprotection of microbial communities from toxic phenol mixtures by a genetically designed pseudomonad. Nat Biotechnol. 1997;15:378–382. doi: 10.1038/nbt0497-378. [DOI] [PubMed] [Google Scholar]
- 9.Favre-Bulle O, Schouten T, Kingma J, Witholt B. Bioconversion of n-octane to octanoic acid by a recombinant Escherichia coli cultured in a two-liquid phase bioreactor. Bio/Technology. 1991;9:367–371. doi: 10.1038/nbt0491-367. [DOI] [PubMed] [Google Scholar]
- 10.Favre-Bulle O, Weenink E, Vos T, Preusting H, Witholt B. Continuous bioconversion of n-octane to octanoic acid by recombinant Escherichia coli (alk+) growing in a two-liquid-phase chemostat. Biotechnol Bioeng. 1993;41:263–272. doi: 10.1002/bit.260410213. [DOI] [PubMed] [Google Scholar]
- 11.Friehs K, Reardon K F. Parameters influencing the productivity of recombinant E. coli cultivations. Adv Biochem Eng. 1993;48:53–77. doi: 10.1007/BFb0007196. [DOI] [PubMed] [Google Scholar]
- 12.Gerdes K. The parB (hok/sok) locus of plasmid R1: a general purpose plasmid stabilization system. Bio/Technology. 1988;6:1402–1405. [Google Scholar]
- 13.Glick B R, Whitney G K. Factors affecting the expression of foreign proteins in Escherichia coli. J Ind Microbiol. 1987;1:277–282. [Google Scholar]
- 14.Hansen L H, Sørensen S J, Jensen L B. Chromosomal insertion of the entire Escherichia coli lactose operon, into two strains of Pseudomonas, using a modified mini-Tn5 delivery system. Gene. 1997;186:167–173. doi: 10.1016/s0378-1119(96)00688-9. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto-Gotoh T, Franklin F C H, Nordheim A, Timmis K N. Specific-purpose plasmid cloning vectors. I. Low copy number, temperature sensitive, mobilization-defective pSC101-derived containment vectors. Gene. 1981;16:227–235. doi: 10.1016/0378-1119(81)90079-2. [DOI] [PubMed] [Google Scholar]
- 16.Herrero M, de Lorenzo V, Timmis K N. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J Bacteriol. 1990;172:6557–6567. doi: 10.1128/jb.172.11.6557-6567.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kidwell J, Kolibachuk D, Dennis D. High-level expression of lacZ under control of the tac or trp promoter using runaway replication vectors in Escherichia coli. Biotechnol Bioeng. 1996;50:108–114. doi: 10.1002/(SICI)1097-0290(19960405)50:1<108::AID-BIT12>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 18.Kristensen C S, Eberl L, Sánchez-Romero J M, Givskov M, Molin S, de Lorenzo V. Site-specific deletions of chromosomally located DNA segments with the multimer resolution system of broad-host-range plasmid RP4. J Bacteriol. 1995;177:52–58. doi: 10.1128/jb.177.1.52-58.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar P K R, Maschke H-E, Friehs K, Schügerl K. Strategies for improving plasmid stability in genetically modified bacteria in bioreactors. Trends Biotechnol. 1991;9:279–284. doi: 10.1016/0167-7799(91)90090-5. [DOI] [PubMed] [Google Scholar]
- 20.Munthali M T, Timmis K N, Díaz E. Restricting the dispersal of recombinant DNA: design of a contained biological catalyst. Bio/Technology. 1996;14:189–191. doi: 10.1038/nbt0296-189. [DOI] [PubMed] [Google Scholar]
- 21.Nakayama K, Kelly S M, Curtis R. Construction of an asd+ expression cloning vector: stable maintenance and high level expression of cloned genes in a Salmonella vaccine strain. Bio/Technology. 1988;6:693–697. [Google Scholar]
- 22.Nozaki M. Metapyrocatechase (Pseudomonas) Methods Enzymol. 1970;17:522–525. [Google Scholar]
- 23.Panke, S., M. G. Wubbolts, A. Schmid, and B. Witholt. Production of enantiopure styrene oxide by recombinant Escherichia coli synthesizing a two-component styrene monooxygenase. Submitted for publication. [DOI] [PubMed]
- 24.Panke S, Sánchez-Romero J M, de Lorenzo V. Engineering quasi-natural Pseudomonas putida strains for metabolism of toluene through an ortho-cleavage degradation pathway. Appl Environ Microbiol. 1998;64:748–751. doi: 10.1128/aem.64.2.748-751.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panke S, Witholt B, Schmid A, Wubbolts M G. Towards a biocatalyst for (S)-styrene oxide production: characterization of the styrene degradation pathway of Pseudomonas sp. strain VLB120. Appl Environ Microbiol. 1998;64:2032–2043. doi: 10.1128/aem.64.6.2032-2043.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Panke S, Witholt B, Wubbolts M G. An alkane-responsive expression system for the production of fine chemicals. Appl Environ Microbiol. 1999;65:2324–2332. doi: 10.1128/aem.65.6.2324-2332.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Porter R D, Black S, Pannuri S, Carlson A. Use of the Escherichia coli ssb gene to prevent bioreactor takeover by plasmidless cells. Bio/Technology. 1990;8:47–51. doi: 10.1038/nbt0190-47. [DOI] [PubMed] [Google Scholar]
- 28.Prieto M A, Kellerhals M B, Bozzato G B, Radnovic D, Witholt B, Kessler B. Engineering of stable recombinant bacteria for production of chiral medium-chain-length poly-3-hydroxyalkanoates. Appl Environ Microbiol. 1999;65:3265–3271. doi: 10.1128/aem.65.8.3265-3271.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rajagopal A N. Growth of gram-negative bacteria in the presence of organic solvents. Enzyme Microb Technol. 1996;19:606–613. [Google Scholar]
- 30.Ronchell M C, Ramos C, Jensen L B, Molin S, Ramos J L. Construction and behavior of biologically contained bacteria for environmental applications in bioremediation. Appl Environ Microbiol. 1995;61:2990–2994. doi: 10.1128/aem.61.8.2990-2994.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 32.Schmid A, Sonnleitner B, Witholt B. Medium chain length alkane solvent-cell transfer rates in two-liquid phase, Pseudomonas oleovorans cultures. Biotechnol Bioeng. 1998;60:10–23. doi: 10.1002/(sici)1097-0290(19981005)60:1<10::aid-bit2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 33.Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 34.Staijen I E, Hatzimanikatis V, Witholt B. The AlkB monooxygenase of Pseudomonas oleovorans: synthesis, stability and level in recombinant Escherichia coli and the native host. Eur J Biochem. 1997;244:462–470. doi: 10.1111/j.1432-1033.1997.00462.x. [DOI] [PubMed] [Google Scholar]
- 35.Stephenson G R. Asymmetric oxidation. In: Stephenson G R, editor. Advanced asymmetric synthesis. London, United Kingdom: Chapman & Hall; 1996. pp. 367–391. [Google Scholar]
- 36.Suarez A, Güttler A, Strätz M, Staendner L H, Timmis K N, Gúzman C A. Green fluorescent protein-based reporter systems for genetic analysis of bacteria including monocopy applications. Gene. 1997;196:69–74. doi: 10.1016/s0378-1119(97)00197-2. [DOI] [PubMed] [Google Scholar]
- 37.Suarez A, Staendner L H, Rohde M, Piatti G, Timmis K N, Guzman C A. Stable expression of pertussis toxin in Bordetella bronchiseptica under the control of a tightly regulated promoter. Appl Environ Microbiol. 1997;63:122–127. doi: 10.1128/aem.63.1.122-127.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tzachaschel B D, Gúzman C A, Timmis K N, de Lorenzo V. An Escherichia coli hemolysin transport-system based vector for the export of polypeptides: export of Shiga-like toxin IIeB subunit by Salmonella typhimurium aroA. Nat Biotechnol. 1996;14:765–769. doi: 10.1038/nbt0696-765. [DOI] [PubMed] [Google Scholar]
- 39.van Beilen J B, Wubbolts M G, Witholt B. Genetics of alkane oxidation by Pseudomonas oleovorans. Biodegradation. 1994;5:161–174. doi: 10.1007/BF00696457. [DOI] [PubMed] [Google Scholar]
- 40.Vermuë M, Sikkema J, Verheul A, Bakker R, Tramper J. Toxicity of homologous series of organic solvents for the Gram-positive bacteria Arthrobacter and Nocardia sp. and the gram-negative bacteria Acinetobacter and Pseudomonas sp. Enzyme Microb Technol. 1993;42:747–758. doi: 10.1002/bit.260420610. [DOI] [PubMed] [Google Scholar]
- 41.Wubbolts M G, Favre-Bulle O, Witholt B. Biosynthesis of synthons in two-liquid-phase media. Biotechnol Bioeng. 1996;52:301–308. doi: 10.1002/(SICI)1097-0290(19961020)52:2<301::AID-BIT10>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 42.Yuste L, Canosa I, Rojo F. Carbon-source-dependent expression of the PalkB promoter from the Pseudomonas oleovorans alkane degradation pathway. J Bacteriol. 1998;180:5218–5226. doi: 10.1128/jb.180.19.5218-5226.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zabriskie D W, Arcuri E J. Factors influencing productivity of fermentations employing recombinant microorganisms. Enzyme Microb Technol. 1986;8:706–717. [Google Scholar]