Abstract
Objective
The dysregulation of stress-related networks due to chronic symptoms such as severe worry and/or rumination is one of the putative pathways linking anxiety in late-life with cognitive decline and increased cardiovascular burden. Symptoms such as severe worry or rumination respond poorly to standard treatment and drive the morbidity associated with anxiety in older adults. We assessed if any of the neural networks anchored in the stressrelated regions of interest (ROIs) are associated with distinct anxiety phenotypes (worry, rumination and global anxiety).
Methods
We recruited older participants (over 50 years of age) with varying levels of worry (N=91) to undergo resting state fMRI. We computed seed-based connectivity for each ROI: the bed nucleus of the stria terminalis (BNST), the paraventricular nucleus of the hypothalamus (PVN), habenula, and amygdala. We limited our connectivity analyses to extracted regions for each seeded ROI-based network based on their canonical networks in 1000 participants (Neurosynth). Using connectivity and clinical factors, we fit cross-validated elastic net models to predict scores on Penn State Worry Questionnaire, Rumination Subscale Questionnaire, Hamilton Anxiety Rating Scale, and Perceived Stress Scale.
Results
We identified several distinct connectivity patterns that predict anxiety phenotypes’ severity. Greater worry was associated with greater PVN-subgenual anterior cingulate cortex, parahippocampal (PHC), and olfactory and amygdala-PHC connectivity. Greater global anxiety was associated with lower amygdala-superior temporal gyrus connectivity. Greater perceived stress was associated with lower amygdala-inferior temporal gyrus and amygdala-fusiform gyrus connectivity.
Conclusions
Our study suggests that various late-life anxiety phenotypes (worry, global anxiety, rumination) may be associated with varying functional connectivity related to stress and emotion regulation. This may aid in the development of future targeted interventions.
Keywords: Late Life, Anxiety, Worry, Stress, Connectivity, Resting State
Introduction
Anxiety disorders are the most common psychiatric disorders in older adults, with up to 15% prevalence in community samples and up to 28% prevalence in clinical samples (1, 2). Anxiety is a broad concept covering several distinct phenotypes such as global anxiety, worry, and - partially - rumination. Global anxiety includes both psychological anxiety (mental agitation and distress) and somatic anxiety (cardiovascular, respiratory and gastrointestinal symptoms) (3). Unlike global anxiety, worry tends to be specifically associated with apprehension about future events (4). In contrast, rumination is related to apprehension over past events (4). While worry and rumination remain distinct constructs, they both stem from persistent repetitive thoughts (5).
Anxiety disorders often have profound behavioral and mood effects, impacting overall functioning and carrying a significant personal and public health burden, both in terms of years-lived-with disability and costs associated with the illness (6). Particularly, late-life anxiety disorders are related to increased morbidity and mortality through higher cardiovascular disease burden and considerable cognitive decline (7, 8). Over the last decade, multiple studies have reported an independent effect of various anxiety phenotypes (e.g., worry, rumination, global anxiety) on aging. More specifically, the independent effect of anxiety symptoms on increasing the risk of cognitive decline has been documented in several recent studies, including longitudinal studies indicating an increased association between amyloid burden and anxiety symptoms in cognitively normal older adults (9), and an association between anxiety symptoms and greater decline in executive function (10).
One of the putative pathways through which anxiety may contribute to aging involves the effect of dysregulated neural networks in inducing chronic, exaggerated stress responses. This poorly calibrated stress response is long-lasting and has chronic effects reverberating through multiple regulatory systems including the HPA axis, autonomic nervous system, and immune system (11–13). The highly vigilant state induced by chronic anxiety phenotypes produces chronic level of activation for cardiovascular, HPA and immune systems, which becomes consequential for brain aging (14) through heightened cerebrovascular burden, as well as greater tau and amyloid burden (15–18).
However, the unique neural signature of each anxiety phenotype is unclear. The distinctive neural signatures of different anxiety phenotypes have rarely been explored and are not well understood due, in part, to the heterogeneity in measures of anxiety, which add considerable variability in imaging analyses. There seems to be a considerable correlation but also distinct phenomenological features among the anxiety phenotypes. Treatment response also differs; for example, global anxiety responds better to regular pharmacological and psychotherapeutic approaches, while severe worry is more difficult to treat (7). Given the world’s aging population and poorer response of phenotypes like severe worry to standard pharmaco- and psychotherapy in late life (19), a better understanding of the neurobiological correlates of late-life anxiety phenotypes would allow for the development of mechanistically informed interventions such as targeted brain stimulation techniques (e.g., transcranial magnetic stimulation).
Our previous work on emotion dysregulation in late-life anxiety (20), combined with recent animal and human studies (21, 22), identified a specific set of regions of interest (ROI) associated both with anxiety as well as stress regulation in the elderly. Worry and rumination, in particular, have been shown to lead to sustained emotional distress and prolonged physiological arousal in advance of (worry) and following (rumination) stressors (23).
The association of anxiety phenotypes with stress-related networks is not well understood. In this work, we evaluated if resting state functional connectivity could serve as a marker of vulnerability for anxiety and its phenotypes, including global anxiety, worry, and rumination, as well as stress. We explored the interface between networks anchored in ROIs involved in anxiety and stress responses in late life. We have chosen four of the most salient ROIs involved in anxiety and stress responses - the amygdala, the bed nucleus of stria terminalis (BNST), the paraventricular nucleus (PVN) and the habenula (24). The amygdala, a central limbic structure with an essential role in information integration from cortical and thalamic sensory inputs (25), is considered a key structure in both stress responses and anxiety circuitry (26). The BNST acts as a point of convergence between the limbic structures and PVN, receiving and coordinating upstream influences and modulating HPA output in response to stressors (27). The PVN is located at the nexus of multiple systems sensitive to external threat and internal physiological state (28). The PVN interprets stress-activated signaling and acts as a primary driver of the HPA axis response. We included the habenula, an epithalamic nuclear complex implicated in emotion, reward modulation, and flexible decision making, due to its extensive connections with the forebrain and the midbrain, and the more recent data pointing toward its involvement in anxiety disorders (29).
In the current study, we used an elastic net regression to predict if any of the networks anchored in the stress-related ROIs are associated with distinct anxiety phenotypes, namely worry, rumination, and global anxiety. Due to the focus on the stress-anxiety relationships, we also included measures of chronic perceived stress. We hypothesized that worry and rumination, but not global anxiety, would be associated with greater functional connectivity in PVN- and amygdala-based networks. We also hypothesized that these associations would be distinctive from the effect of perceived stress on the stress-related neural networks.
Methods
Participants and Study Design
We recruited 110 older participants (>50 years) as a part of an ongoing study (Functional Neuroanatomy Correlates of Worry in Older Adults - FINA). As described by Karim et al. (8), participants were recruited from the community of the greater Pittsburgh area through in person recommendations, online/television/radio advertisements, and flyers. The study was approved by the University of Pittsburgh Institutional Review Board and all participants provided written informed consent prior to participating.
In accordance with the NIMH RDoC (Research Domain Criteria) framework, we recruited participants with varying levels of worry ranging from low to severe. Categorical diagnoses were assessed using the structured clinical interview for DSM-V (SCID) administered by trained interviewers and confirmed by a psychiatrist. Notably, since we consider worry and other anxiety phenotypes to be a spectrum which can appear across multiple different diagnoses, our analyses did not include splitting participants into groups based on categorical diagnoses. However, the categorical diagnoses of the sample included in secondary analyses are reported under Statistical Analysis. The use of antidepressants was allowed if prescribed primarily for sleep or non-psychiatric indications (e.g., peripheral neuropathy) at low doses: amitriptyline (50mg/day), doxepin (50mg/day), trazodone (100mg/day), imipramine (50mg/day). All other antidepressants were tapered off and, if necessary, an adequate washout (e.g., six weeks for fluoxetine) was supervised by a psychiatrist.
Exclusion criteria for all participants were: diagnosis of autism spectrum disorder, intellectual development disorder, major neurocognitive disorder, psychosis, bipolar disorder, cerebrovascular accident, multiple sclerosis, vasculitis, or significant head trauma, presence of Axis II disorders, increased suicide risk, drug/alcohol abuse within the last six months, use of antidepressants within the last 5–14 days, use of high doses of benzodiazepines (defined as greater than equivalent of 2mg of lorazepam), uncorrected vision problems, below 6th grade level of reading, and/or any MRI contraindications.
Assessments
We collected standard demographic information including age, sex, race, and education, as well as illness severity [cumulative illness rating scale for geriatrics (CIRSG)] (30), and cognitive function [modified-mini mental state exam (3MSE)]) (31).
Major neurocognitive disorders were diagnosed using a comprehensive battery including the Repeatable Battery for the Assessment of Neuropsychological Status (R-BANS) (32), the sorting and verbal fluency tests of the Delis-Kaplan Executive Function System (D-KEFS) (33), Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) (34), and the Performance Assessment of Self-Care Skills (PASS) (35). Additionally, we used a 3MSE cutoff of 86 to determine gross cognitive impairment.
We collected the following clinical assessments: worry [Penn State Worry Questionnaire (PSWQ)] (36), overall anxiety [Hamilton Anxiety Rating Scale (HARS)]) (37), depression [Montgomery-Asberg Depression Rating Scale(MADRS)] (38), rumination [Rumination Subscale from Response Styles Questionnaire (RSQ)] (39), neuroticism [Neuroticism subscale from the Five Factor Inventory (FFI)] (40), perceived stress [Cohen’s Perceived Stress Scale (PSS)] (41), and the habitual use of cognitive reappraisal and suppression subscale [Emotion Regulation Questionnaire (ERQ)] (42).
MRI Data Acquisition
All participants underwent a 1-hour MRI using a 3T Siemens MAGNETOM Prisma scanner with a 32-channel head coil at the Magnetic Resonance Research Center (MRRC) at the University of Pittsburgh. T1-weighted magnetization prepared rapid gradient echo (MPRAGE) images were collected with GeneRalized Autocalibrating Partial Parallel Acquisition (GRAPPA) with acceleration factor R=2 using the following specifications: repetition time (TR)/echo time (TE): 2400/2.22ms, flip angle 4°, 0.8 mm3 voxel size with 0.4mm slice gap, and a 320×300 field of view with 208 slices, for a total time of 6.63 minutes. T2-weighted Sampling Perfection with Application optimized Contrasts using different flip angle Evolution (SPACE) were collected with GRAPPA (R=2) with the following specifications: TR/TE: 3200/5.63ms, flip angle 120°, 0.8 mm3 voxel size without a slice gap, and a 320×300 field of view with 208 slices, for a total time of 5.95 minutes.
Resting state blood-oxygen-level-dependent (BOLD) images were collected using echo-planar imaging with multiband acceleration factor of 5 and with the following specifications: TR/TE: 1000/30 ms, 2.3 mm3 isotropic voxel size with a 2.3 mm slice gap, and a 96×96 field of view with 60 slices for a total of 8 minutes. Participants were instructed to fixate on a white crosshair on a black background and stay awake.
Structural Processing
Structural data were processed using a statistical parametric mapping toolbox (SPM 12) in MatLab 2018b (MathWorks, Natick, MA). All interpolation was done with a 4th degree B-spline and the similarity metric used for co-registration between different image types was normalized mutual information. Processing included the following procedures: (a) co-registration of the T2-SPACE image to the MPRAGE using affine registration; (b) unified segmentation was used for bias correction, image normalization, and tissue classification. This process generates a deformation field that can be used to normalize images to MNI space. To counter the effect of white matter hyperintensities and improve gray matter classification, the number of Gaussians used to classify white matter was adjusted to two. We computed an intracranial volume mask by thresholding gray matter, white matter, and cerebrospinal fluid (CSF) probability maps by 0.1 then using image filling (imfill) and image close (imclose) in MatLab to generate a final cleaned up mask. This was used to skull-strip the MPRAGE image in native space.
Resting State Preprocessing
Functional data were preprocessed using a combination of SPM12 and in-house scripts in MatLab 2018b. Preprocessing included the following procedures: (a) slice time correction using the temporally middle slice as the reference; (b) motion correction via rigid body co-registration to the mean; (c) co-registration to the skull stripped MPRAGE, where the mean functional image was used to calculate the affine transformation; (d) normalization to MNI space using the structural deformation from structural processing with 2 mm isotropic resolution; and (e) smoothing with an 8mm FWHM Gaussian kernel. We then conducted wavelet despiking to remove spike artifacts and filtering by regressing out six motion parameters, and five principal components of white matter and cerebrospinal fluid, a series of sinusoids corresponding to unwanted frequencies outside of the band-pass in resting state (i.e., a band-pass filter 0.008–0.15 Hz). By doing this in a single regression in one step, we do not reintroduce artifact/noise into our signal.
Seed-Based Resting State Connectivity
We computed seed-based resting state connectivity maps for the BNST, PVN, habenula and left and right amygdala. The ROIs for the BNST, PVN and habenula were hand-drawn by an expert neuroanatomist (LB) in MNI space. The left and right amygdala ROIs were extracted using the Automated anatomical labeling atlas 3 (AAL3). Rather than investigating voxel-wise associations, we opted to identify canonical regions associated with each ROI. For each ROI, we found its center of mass and a coordinate in MNI space was extracted. We used center of mass coordinates to extract connectivity maps for each network based on their canonical networks in 1000 healthy participants as generated by Neurosynth. We downloaded and thresholded these maps (>0.1), separated regions using the AAL3, which resulted in a list of regions for each ROI. For each ROI, we extracted the average connectivity of each region that was identified via Neurosynth. Several temporal regions were excluded due to a poor signal to noise ratio (PVN – right temporal pole: middle temporal gyrus; PVN – left temporal lobe: superior temporal gyrus; left amygdala – right and left temporal pole: middle temporal gyrus; left amygdala – right and left middle temporal gyrus; right amygdala – right temporal pole: superior temporal gyrus; right amygdala – bilateral temporal pole: middle temporal gyrus). To identify highly collinear regions, we computed Pearson correlations between regions that were present on the left and right hemisphere. Specifically, for those regions that had a correlation of r > 0.7, we computed the average of the two regions and identified it instead as a bilateral region. On all remaining regions, we conducted a one-sample t-test and identified regions that had connectivity that was greater than zero after false-discovery rate (FDR) correction (p<0.05). For each ROI (BNST, PVN, habenula, and amygdala), this identified pairs of connectivity that were significantly correlated in a normative sample (Neurosynth.org) and in our sample.
Statistical Analysis
Prior to statistical analysis, there were 16 participants who were excluded from analysis: 9 participants had severe signal dropout via a manual quality check and 7 had preprocessing failures (normalization to MNI space failed). Additionally, 3 participants were excluded due to excessive motion, as defined by the art repair toolbox, https://github.com/Remi-Gau/art_repair, greater than 20% of the volumes. In addition, we computed 1.5x the interquartile range on our scales of interest (PSWQ, PSS, RSQ, and HARS). We identified the following number of participants as potential outliers on the scales of interest: 1 participant on PSS, 2 participants on RSQ, and 3 participants on HARS. However, given that these three measures did not have a normal distribution, we decided to include these people in the rest of the analysis, unless they were confirmed as outliers later in the analysis through using residuals from the elastic net models (see below). Thus, the analysis sample consisted of 91 participants. This cohort had 21 participants with current Generalized Anxiety Disorder (23%), 3 participants with current Post Traumatic Stress Disorder (3.3%), 7 participants with current Social Phobia (7.7%), 20 participants with any other current anxiety disorder (22%), and 5 participants with current Major Depressive Disorder (5.5%). Out of our final participant sample, 3 participants required tapering off antidepressants prior to study initiation (3.3%).
The n=91 participants had the following missing questionnaire data: education (four missing), CIRSG (seven missing), PSQW (one missing), HARS (two missing), MADRS (four missing), RSQ (eight missing), FFI-N (twelve missing), ERQ (nine missing) and PSS (nine missing). To handle missing data, we computed multiple imputation analysis using random forests with five imputations written in MICE package in R, with all variables imputed inside of their appropriate range (e.g., PSS ranges from 0–40).
We used the eNetXplorer package in R to build four cross-validated (5-fold cross-validation) elastic net models to predict participants’ scores on PSWQ, PSS, RSQ, and HARS. All the models had a combination of demographic and clinical variables, including age, sex, race, education, CIRSG, MADRS, ERQ suppression, ERQ reappraisal, FFI-N, and the other three questionnaires predicted in separate models (e.g., if the current model was predicting PSWQ, then RSQ, HARS, and PSS were included as independent variables). We computed 500 permutations for each model, and all the models used five-fold cross validation. For each model, we optimized over 50 values of lambda and alpha from 0–1 (10 values). In elastic net models, lambda controls the amount of regularization (where higher values indicate a greater penalty per additional non-zero independent variable) and alpha controls the mixing between ridge regression (alpha=0) and LASSO (alpha=1, least absolute shrinkage and selection operator). To maximize interpretability, we focused on choosing models closest to ridge regression that were significant.
Using the residuals from the eNetXplorer results, we confirmed suspected outliers on the scales of interest. In other words, the same participants who were outliers identified by looking at 1.5x the interquartile range ended up being outliers on the residuals as well. Thus, we removed the outliers, and reran the model with the following sample sizes: 91 participants for PSWQ, 90 for PSS, 89 for RSQ, and 88 for HARS. Thus, the following results are from the samples not including the identified outliers.
Results
Table 1 contains the demographic and clinical characterization of the participant sample. Our sample is consistent with the demographics of the greater Pittsburgh area. We found that multiple factors explained significant variance in PSWQ, PSS, RSQ and HARS (Table 2, Table 3, Figure 1).
Table 1.
Demographics of the Participant Sample.
| Variable name | Total sample (n=91) |
Number missing | Imputed mean | |
|---|---|---|---|---|
| Mean/N | SD/% | |||
| Age | 61.2 | 8.2 | 0 | |
| Sex, male | 37 | 40.7% | 0 | |
| Race | 0 | |||
| W | 80 | 87.9% | ||
| B | 10 | 11.0% | ||
| MR | 1 | 1.1% | ||
| Education | 15.8 | 2.5 | 4 | 15.8 |
| CIRSG | 4.1 | 3.6 | 7 | 4.1 |
| PSWQ | 49.6 | 14.5 | 1 | 49.8 |
| HARS | 7.9 | 6.9 | 2 | 8.0 |
| MADRS | 7.4 | 8.0 | 4 | 7.7 |
| RSQ | 38.0 | 13.0 | 8 | 37.6 |
| FFI-N | 20.1 | 9.9 | 12 | 20.4 |
| ERQ Reappraisal | 29.6 | 7.5 | 9 | 29.7 |
| ERQ Suppression | 14.0 | 5.3 | 9 | 14.0 |
| PSS | 15.4 | 8.1 | 9 | 15.4 |
Note. Race W: white, B: Black, MR: multiracial. CIRSG: Cumulative Illness Rating Scale for Geriatrics. PSWQ: Penn State Worry Questionnaire. HARS: Hamilton Anxiety Rating Scale. MADRS: Montgomery-Asberg Depression Rating Scale. RSQ: Rumination Subscale Questionnaire. FFI-N: Neuroticism subscale from the Five Factor Inventory. ERQ Reappraisal and Suppression: Reappraisal and Suppression subscales from the Emotion Regulation Questionnaire. PSS: Cohen’s Perceived Stress Scale.
Table 2.
Elastic Net Results for Four Anxiety Phenotypes: Worry (Measured by PSWQ), Stress (Measured by PSS), Rumination (Measured by RSQ), and Global Anxiety (Measured by HARS). Showing Significant Clinical and Demographic Measures.
| Feature | PSWQ |
PSS |
RSQ |
HARS |
|---|---|---|---|---|
| Mean β(SD) | Mean β(SD) | Mean β(SD) | Mean β(SD) | |
| MADRS | 0.70(0.004) **** | 0.39(0.002) **** | 0.47(0.004) *** | 0.38(0.001) **** |
| Neuroticism | 0.89(0.003) **** | 0.47(0.001) **** | 0.68(0.003) **** | 0.18(0.001) *** |
| PSS | 0.73(0.004) **** | 0.69(0.003) **** | 0.24(0.001) **** | |
| HARS | 0.69(0.006) **** | 0.39(0.003) **** | 0.45(0.004) *** | |
| RSQ | 0.75(0.004) **** | 0.44(0.002) **** | 0.17(0.001) ** | |
| PSWQ | 0.42(0.001) **** | 0.58(0.003) **** | 0.25(0.001) **** | |
| ERQ Suppression | 0.30(0.003) * | |||
| Age | −0.28(0.003) * |
Note. The results were obtained by building 4 separate elastic net models, one for each anxiety phenotype. We optimized over 50 values of lambda and 10 values of alpha over the range [0–1]. We ran 500 permutations using 5-fold cross validation for each model. P-values were used to assess statistical significance of mean non-zero feature coefficients and were obtained by running a model versus null comparison. Significance levels for p-values: 0.05 = *, 0.01 = **, 0.001 = ***, 0.0001 = ****. β values refer to mean of standardized regression coefficients over runs (n=500), weighed by non-zero frequency over folds (n=5). SD refers to standard deviation of feature coefficients over runs (n=500), weighted by non-zero frequency over folds (n=5). Abbreviations: PSWQ: Penn State Worry Questionnaire. PSS: Perceived Stress Scale. RSQ: Response Styles Questionnaire, HARS: Hamilton Anxiety Rating Scale. MADRS: MontgomeryAsberg Depression Rating Scale. Neuroticism: NEO Five Factor Inventory, Neuroticism Subscale. ERQ Suppression: Emotion Regulation Scale, Suppression Subscale.
Table 3.
Elastic Net Results for Three Anxiety Phenotypes: Worry (Measured by PSWQ), Stress (Measured by PSS), and Global Anxiety (Measured by HARS). Showing Significant Resting State Functional Connectivity Measures.
| Feature | PSWQ |
PSSQ |
HARSQ |
|---|---|---|---|
| Mean β(SD) | Mean β(SD) | Mean β(SD) | |
| PVN - Left Subgenual Anterior Cingulate Cortex | 0.33(0.004) * | ||
| PVN - Right Parahippocampal Gyrus | 0.37(0.004) * | ||
| PVN - Bilateral Olfactory Cortex | 0.32(0.004) * | ||
| Right Amygdala - Right Parahippocampal Gyrus | 0.32(0.004) * | ||
| Left Amygdala - Right Inferior Temporal Gyrus | −0.15(0.002) * | ||
| Left Amygdala - Left Inferior Temporal Gyrus | −0.17(0.002) * | ||
| Left Amygdala - Bilateral Fusiform Gyrus | −0.18(0.002) ** | ||
| Left Amygdala - Left Superior Temporal Gyrus | −0.11(0.001) * |
Note.
The results were obtained by building 4 separate elastic net models, one for each anxiety phenotype. Results for Rumination (Measured by RSQ) did not reach statistical significance. We optimized over 50 values of lambda and 10 values of alpha over the range [0–1]. We ran 500 permutations using 5-fold cross validation for each model. P-values were used to assess statistical significance of mean non-zero feature coefficients and were obtained by running a model versus null comparison. Significance levels for p-values: 0.05 = *, 0.01 = **, 0.001 = ***, 0.0001 = ****. β values refer to mean of standardized regression coefficients over runs (n=500), weighed by non-zero frequency over folds (n=5). SD refers to standard deviation of feature coefficients over runs (n=500), weighted by non-zero frequency over folds (n=5). Abbreviations: PSWQ: Penn State Worry Questionnaire. PSS: Perceived Stress Scale. RSQ: Response Styles Questionnaire, HARS: Hamilton Anxiety Rating Scale. PVN: Paraventricular Nucleus.
Figure 1. Elastic Net Results for Three Anxiety Phenotypes as Measured by PSWQ, PSS, and HARS.
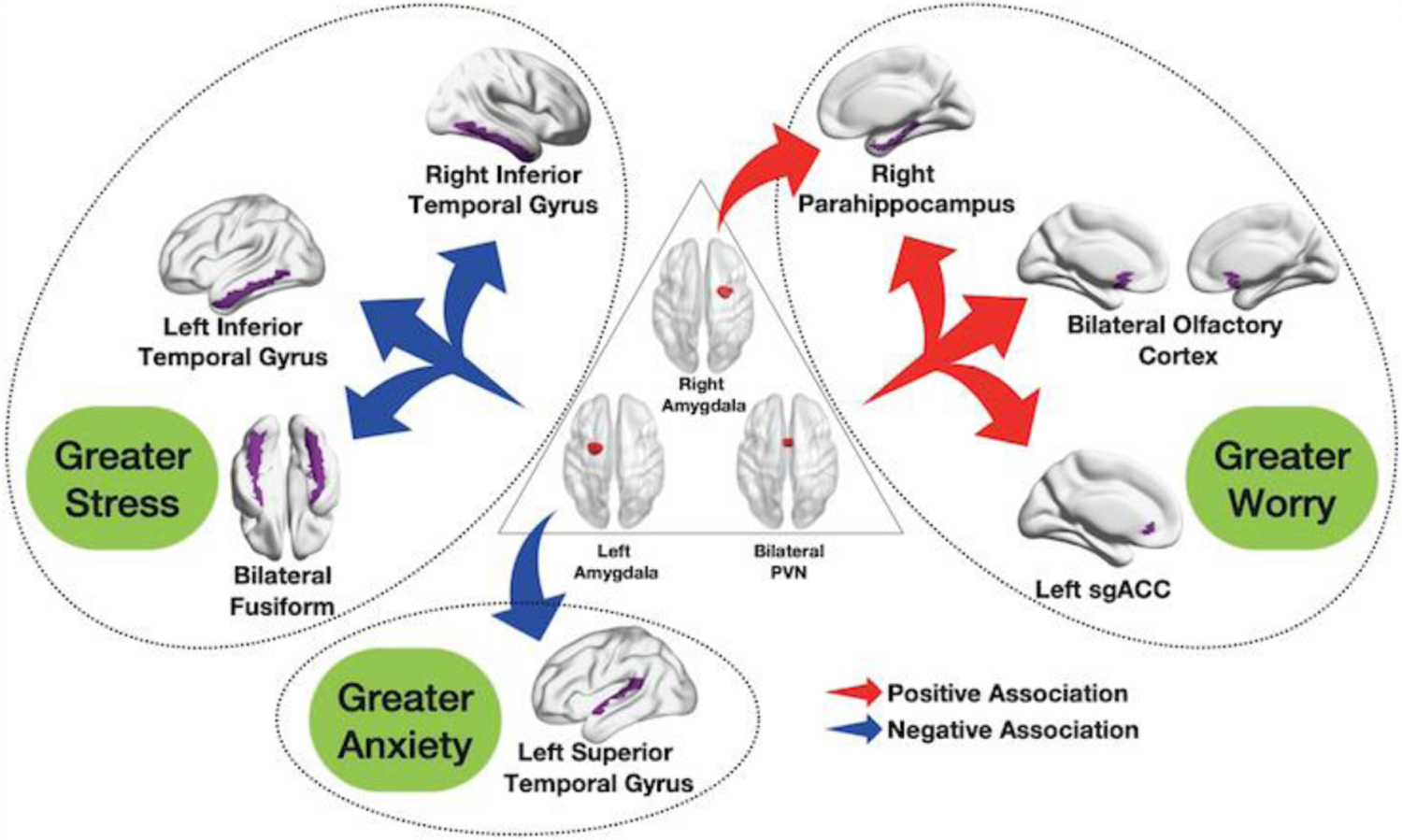
Increased functional connectivity between bilateral paraventricular nucleus (PVN) and the left subgenual anterior cingulate cortex, the bilateral olfactory cortex and the right parahippocampal gyrus, as well as increased connectivity between the right amygdala and the right parahippocampal gyrus were associated with higher worry as measured by Penn State Worry Questionnaire (PSWQ). Decreased functional connectivity between the left amygdala and the right and left inferior temporal gyrus as well as the bilateral fusiform gyrus was associated with higher perceived stress, as measured by Cohen’s Perceived Stress Scale (PSS). Decreased functional connectivity between the left amygdala and the left superior temporal gyrus was associated with increased global anxiety, as measured by Hamilton Anxiety Rating Scale (HARS).
Greater worry, as measured by PSWQ, was associated with greater MADRS, FFI-N, PSS, HARS, and RSQ, as well as greater functional connectivity between the following regions: PVN - left subgenual anterior cingulate cortex, PVN - right parahippocampal gyrus, PVN - bilateral olfactory cortex, and right amygdala - right parahippocampal gyrus [out-of-bag correlation coefficient r(89)=0.55, permuted p-value < 0.0001, alpha=0, and lambda=98.1].
Greater stress, as measured by PSS, was associated with greater MADRS, FFI-N, HARS, RSQ, and PSWQ. It was also associated with lower connectivity in the following areas: left amygdala - right inferior temporal gyrus, left amygdala - left inferior temporal gyrus, and left amygdala - bilateral fusiform gyrus [out-of-bag correlation coefficient r(88)=0.64, permuted p-value < 0.0001, alpha=0, and lambda=52.6].
Greater rumination, as measured by RSQ, was associated with greater MADRS, FFI-N, PSWQ, HARS, ERQ Suppression and PSS. It was also associated with lower Age [out-of-bag correlation coefficient r(87)=0.50, permuted p-value < 0.0001, alpha=0, and lambda=70.6].
Greater global anxiety, as measured by HARS, was associated with greater MADRS, FFI-N, PSS, RSQ and PSWQ. It was also associated with lower left amygdala - left superior temporal gyrus connectivity [out-of-bag correlation coefficient r(86)=0.56, permuted p-value < 0.0001, alpha=0, and lambda=43.3].
Discussion
In the current study, we investigated if any of the networks anchored in the stress-related ROIs are associated with distinct anxiety phenotypes, namely worry, rumination, and global anxiety, as well as perceived stress. We found that all the anxiety phenotypes were associated with each other, as well as MADRS (depression severity) and FFI-N (Neuroticism). This was expected, as these scales capture many partially overlapping behavioral symptoms. In addition, higher rumination was predicted by higher ERQ Suppression scores, as well as lower age – a phenomenon that has been previously reported (43, 44).
Additionally, we identified several distinct connectivity patterns that are associated with anxiety phenotypes severity in older adults. Notably, greater PVN – left amygdala connectivity was associated with a higher worry severity in late life. Lower left amygdala connectivity was associated with both higher global anxiety and higher perceived stress. These results emphasize that different anxiety phenotypes have specific neural signatures.
Greater worry (as measured by PSWQ) was associated with higher PVN connectivity, linking one of the most pernicious anxiety phenotypes (severe worry) with functional networks involved in stress regulation. These results confirm our previous work on a different, smaller sample, pointing toward an association between PVN-amygdala connectivity and worry severity (20). Chronic, severe worry has been demonstrated to have a deleterious effect on both cardiovascular health and cognitive function. A ubiquitous syndrome hiding in the geriatric community (20% of older adults reports severe worry (45)), worry has been associated with diminished heart rate variability, higher risks of hypertension, coronary heart disease, stroke and heart failure (46, 47). In one of the few studies examining the effect of specific anxiety phenotypes on cognitive decline, Pietrzak et al. (48) reported that older adults with even mildly elevated worry symptoms performed worse on multiple cognitive measures and showed significant decline in memory at 2-year follow-up compared with low-worry older adults. Moreover, in our recent work, we have used a machine learning model to predict brain age, and we reported that worry and rumination were associated with accelerated aging, each point of the PSWQ adding 1.3 months to brain age (8). In addition, we found significant connectivity changes between the PVN and the bilateral olfactory cortex. Olfactory circuit connectivity changes have been previously reported in anxiety (49). Further, changes in olfaction are a reliable predictor of cognitive decline (50, 51) and they may be caused by or further exacerbated by severe worry.
Thus, our results point toward a possible mechanistic hypothesis for the effect of worry on aging. As a chronic phenomenon, worry converts the immediate psychological and physiological responses to daily stressors into prolonged physiological activation of the regulatory systems, leaving the individual in a prolonged state of “action preparation”. Worry has been shown to lead to sustained emotional distress and prolonged physiological arousal in advance of stressors (19). Gradually, this chronic activation becomes detrimental, accelerating the aging of both brain and body. The lack of significance for the rumination analysis may point toward shared mechanisms between worry and rumination, with worry having “an edge” over rumination as pointed out by our results.
Global anxiety (as measured by HARS) was predicted by lower connectivity between left amygdala and left superior temporal gyrus. These results may indicate a regulatory and inhibitory effect of the left superior temporal gyrus on the amygdala activity, supported by the consistent projections of the amygdala body to the superior temporal gyrus (52). Consequently, the lower amygdala-superior temporal connectivity may cause a release of amygdalar activity reflected in higher overall anxiety in older participants. The role of the superior temporal gyrus in several anxiety disorders has been described in pediatric (53), adult (54), and older adult (55) populations. Our results point to its specific role in the context of global anxiety - capturing most likely features related to social phobia (56) and post-traumatic stress (57) - thus further distinguishing global anxiety from severe worry.
We have included perceived stress in our analysis in order to explore if stress perception may explain the effect of anxiety phenotypes on stress-related networks. We found that the perception of stress is related to a relatively distinct neural pattern. Thus, greater perceived stress (as measured by PSS) was associated with lower connectivity between left amygdala and left and right inferior temporal gyrus, as well as left amygdala and bilateral fusiform gyrus. These results partially overlap with the global anxiety findings, and mirror previous reports in the literature, including altered amygdala-inferior temporal gyrus connectivity in social anxiety disorder (58), and altered amygdala-fusiform gyrus connectivity in post-traumatic stress disorder (59). As suggested by Liao and colleagues (58), changes in connectivity between the amygdala and the inferior temporal gyrus might form a neurobiological basis for attentional bias present in stress and some of the categorical anxiety disorders.
Understanding the neurobiological correlates of late-life anxiety phenotypes may lead to successful targeted interventions such as transcranial magnetic stimulation. Notably, a recent meta-analysis found that transcranial magnetic stimulation may be an effective treatment for anxiety and trauma-related disorders (60), both of which commonly encompass anxiety phenotypes described in this paper. Thus, targeted interventions that modulate specific networks, may help diminish the symptoms associated with specific anxiety phenotypes.
Our study has several limitations - as we examined an older population, our results may not be generalizable to younger cohorts; a larger sample may allow us to capture weaker albeit phenotype-specific associations. We conducted a cross-sectional study, thus we cannot make causal inferences based on reported associations. Conducting a longitudinal study in the future may help establish temporal sequence for observed findings. We fit elastic net models, which are optimized to include factors that are most predictive, and this may result in loss of generalizability. However, we conducted cross-validation and computed out-of-bag correlation coefficients to improve generalizability. Due to the overlap between emotion regulation and stress responsive neural circuits, we primarily focused on regions associated with stress, so other regions of the brain may correlate with anxiety phenotypes. The anxiety phenotypes have considerable overlap and are correlated (as confirmed in Table 2), which is why the elastic net regression was used to identify how connectivity explained unique variance in each anxiety phenotype. Finally, it is important to note that while the clinical scales used in this study are generally found to be reliable (61–63), and have well-reported internal consistency (64–66), they were designed to quantify behavioral symptoms. The correlation with neural results is only the first step toward the future development of scales optimized for the mapping of behavioral symptoms onto neuroanatomical circuits.
In conclusion, we presented new data describing distinct neurobiological correlates of late-life anxiety phenotypes and pointing toward a potential mechanistic pathway linking late-life worry with chronic, poorly regulated stress response. Our results suggest the need to aggressively treat severe worry throughout the lifespan and to develop mechanistically informed interventions to prevent the effect of severe worry on overall health.
What is the primary question addressed by this study?
In this work, we assessed if any of the neural networks anchored in the stress-related regions of interest are associated with distinct anxiety phenotypes (worry, rumination and global anxiety).
What is the main finding of this study?
We found distinct neurobiological correlates of late-life anxiety phenotypes which point toward a potential mechanistic pathway linking late-life worry with chronic, poorly regulated stress response.
What is the meaning of the finding?
Our results emphasize the importance of developing neurobiologically-grounded interventions to reduce the effects of late-life anxiety on overall health.
Acknowledgements:
The authors would like to acknowledge the staff of the Geriatric Psychiatry Neuroimaging Laboratory for their work and support.
Funding: This work was funded by NIMH R01MH108509, NIMH R01 MH 076079, NIMH R01 MH121619, NIA R01AG023651, R01GM113243, and NIA T32AG055381.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors report no conflicts of interest.
Previous Presentations Online Posters: Biological Psychiatry, May 2021; ADRD, June 2021
Authorship Contribution Statement: Antonija Kolobaric: Methodology, Data curation, Formal analysis, Writing - original draft, Writing - review & editing. Helmet T. Karim: Conceptualization, Methodology, Data curation, Funding acquisition, Writing - review & editing. Layla Banihashemi: Methodology, Writing - review & editing. Akiko Mizuno: Methodology, Writing - review & editing. Howard J. Aizenstein: Conceptualization, Methodology, Funding acquisition, Writing - review & editing. Carmen Andreescu: Conceptualization, Methodology, Data curation, Formal analysis, Funding acquisition, Writing - original draft, Writing - review & editing.
References
- 1.Bryant C, Jackson H, Ames D. The prevalence of anxiety in older adults: methodological issues and a review of the literature. J Affect Disord 2008;109(3):233–50. [DOI] [PubMed] [Google Scholar]
- 2.Skoog I. Psychiatric disorders in the elderly. Can J Psychiatry 2011;56(7):387–97. [DOI] [PubMed] [Google Scholar]
- 3.Andreescu C, Mennin D, Tudorascu D, Sheu LK, Walker S, Banihashemi L, et al. The many faces of anxiety-neurobiological correlates of anxiety phenotypes. Psychiatry Research: Neuroimaging 2015;234(1):96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong RY. Worry and rumination: differential associations with anxious and depressive symptoms and coping behavior. Behav Res Ther 2007;45(2):277–90. [DOI] [PubMed] [Google Scholar]
- 5.Segerstrom SC, Roach AR, Evans DR, Schipper LJ, Darville AK. The structure and health correlates of trait repetitive thought in older adults. Psychol Aging 2010;25(3):505–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porensky EK, Dew MA, Karp JF, Skidmore E, Rollman BL, Shear MK, et al. The burden of late-life generalized anxiety disorder: effects on disability, health-related quality of life, and healthcare utilization. Am J Geriatr Psychiatry 2009;17(6):473–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andreescu C, Lee S. Anxiety Disorders in the Elderly. Adv Exp Med Biol 2020;1191:561–76. [DOI] [PubMed] [Google Scholar]
- 8.Karim HT, Ly M, Yu G, Krafty R, Tudorascu DL, Aizenstein HJ, et al. Aging faster: worry and rumination in late life are associated with greater brain age. Neurobiol Aging 2021;101:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanseeuw BJ, Jonas V, Jackson J, Betensky RA, Rentz DM, Johnson KA, et al. Association of anxiety with subcortical amyloidosis in cognitively normal older adults. Mol Psychiatry 2020;25(10):2599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pietrzak RH, Scott JC, Neumeister A, Lim YY, Ames D, Ellis KA, et al. Anxiety symptoms, cerebral amyloid burden and memory decline in healthy older adults without dementia: 3-year prospective cohort study. Br J Psychiatry 2014;204:400–1. [DOI] [PubMed] [Google Scholar]
- 11.Llera SJ, Newman MG. Effects of worry on physiological and subjective reactivity to emotional stimuli in generalized anxiety disorder and nonanxious control participants. Emotion 2010;10(5):640. [DOI] [PubMed] [Google Scholar]
- 12.McEwen BS, Eiland L, Hunter RG, Miller MM. Stress and anxiety: structural plasticity and epigenetic regulation as a consequence of stress. Neuropharmacology 2012;62(1):3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stein DJ, Nesse RM. Threat detection, precautionary responses, and anxiety disorders. Neuroscience & Biobehavioral Reviews 2011;35(4):1075–9. [DOI] [PubMed] [Google Scholar]
- 14.Brosschot JF, Gerin W, Thayer JF. The perseverative cognition hypothesis: A review of worry, prolonged stress-related physiological activation, and health. Journal of psychosomatic research 2006;60(2):113–24. [DOI] [PubMed] [Google Scholar]
- 15.Lavretsky H, Siddarth P, Kepe V, Ercoli LM, Miller KJ, Burggren AC, et al. Depression and anxiety symptoms are associated with cerebral FDDNP-PET binding in middle-aged and older nondemented adults. The American Journal of Geriatric Psychiatry 2009;17(6):493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petkus AJ, Reynolds CA, Wetherell JL, Kremen WS, Pedersen NL, Gatz M. Anxiety is associated with increased risk of dementia in older Swedish twins. Alzheimer’s & Dementia 2016;12(4):399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pietrzak RH, Lim YY, Neumeister A, Ames D, Ellis KA, Harrington K, et al. Amyloid-β, anxiety, and cognitive decline in preclinical Alzheimer disease: a multicenter, prospective cohort study. JAMA psychiatry 2015;72(3):284–91. [DOI] [PubMed] [Google Scholar]
- 18.Pietrzak RH, Maruff P, Woodward M, Fredrickson J, Fredrickson A, Krystal JH, et al. “ Mild worry symptoms predict decline in learning and memory in healthy older adults: A 2-year prospective cohort study”: Erratum 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andreescu C, Varon D. New research on anxiety disorders in the elderly and an update on evidence-based treatments. Curr Psychiatry Rep 2015;17(7):53. [DOI] [PubMed] [Google Scholar]
- 20.Andreescu C, Sheu LK, Tudorascu D, Gross JJ, Walker S, Banihashemi L, et al. Emotion reactivity and regulation in late-life generalized anxiety disorder: functional connectivity at baseline and post-treatment. Am J Geriatr Psychiatry 2015;23(2):200–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacinto LR, Mata R, Novais A, Marques F, Sousa N. The habenula as a critical node in chronic stress-related anxiety. Exp Neurol 2017;289:46–54. [DOI] [PubMed] [Google Scholar]
- 22.Yoshino A, Okamoto Y, Sumiya Y, Okada G, Takamura M, Ichikawa N, et al. Importance of the Habenula for Avoidance Learning Including Contextual Cues in the Human Brain: A Preliminary fMRI Study. Front Hum Neurosci 2020;14:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brosschot JF, Gerin W, Thayer JF. The perseverative cognition hypothesis: a review of worry, prolonged stress-related physiological activation, and health. J Psychosom Res 2006;60(2):113–24. [DOI] [PubMed] [Google Scholar]
- 24.Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 2010;35(1):105–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Babaev O, Piletti Chatain C, Krueger-Burg D. Inhibition in the amygdala anxiety circuitry. Exp Mol Med 2018;50(4):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duval ER, Javanbakht A, Liberzon I. Neural circuits in anxiety and stress disorders: a focused review. Ther Clin Risk Manag 2015;11:115–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radley JJ, Johnson SB. Anteroventral bed nuclei of the stria terminalis neurocircuitry: Towards an integration of HPA axis modulation with coping behaviors - Curt Richter Award Paper 2017. Psychoneuroendocrinology 2018;89:239–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferguson AV, Latchford KJ, Samson WK. The paraventricular nucleus of the hypothalamus - a potential target for integrative treatment of autonomic dysfunction. Expert Opin Ther Targets 2008;12(6):717–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLaughlin I, Dani JA, De Biasi M. The medial habenula and interpeduncular nucleus circuitry is critical in addiction, anxiety, and mood regulation. J Neurochem 2017;142 Suppl 2:130–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salvi F, Miller MD, Grilli A, Giorgi R, Towers AL, Morichi V, et al. A manual of guidelines to score the modified cumulative illness rating scale and its validation in acute hospitalized elderly patients. J Am Geriatr Soc 2008;56(10):1926–31. [DOI] [PubMed] [Google Scholar]
- 31.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry 1987;48(8):314–8. [PubMed] [Google Scholar]
- 32.Randolph C. Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): Psychological Corporation; San Antonio, TX; 1998. [Google Scholar]
- 33.Delis DC, Kaplan E, Kramer JH. Delis-Kaplan executive function system 2001. [DOI] [PubMed] [Google Scholar]
- 34.Jorm A, Korten A. Assessment of cognitive decline in the elderly by informant interview. The British Journal of Psychiatry 1988;152(2):209–13. [DOI] [PubMed] [Google Scholar]
- 35.Holm MB, Rogers J, Hemphill-Pearson B. The performance assessment of self-care skills (PASS). Assessments in occupational therapy mental health 2008;2:101–10. [Google Scholar]
- 36.Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn State Worry Questionnaire. Behav Res Ther 1990;28(6):487–95. [DOI] [PubMed] [Google Scholar]
- 37.Hamilton M. The assessment of anxiety states by rating. British journal of medical psychology 1959;32(1):50–5. [DOI] [PubMed] [Google Scholar]
- 38.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979;134:382–9. [DOI] [PubMed] [Google Scholar]
- 39.Bagby RM, Rector NA, Bacchiochi JR, McBride C. The stability of the response styles questionnaire rumination scale in a sample of patients with major depression. Cognitive Therapy and Research 2004;28(4):527–38. [Google Scholar]
- 40.Costa PT, McCrae RR. Revised NEO personality inventory (NEO-PI-R) and Neo five-factor inventory (NEO-FFI): Psychological Assessment Resources; 1992. [Google Scholar]
- 41.Cohen S. Perceived stress in a probability sample of the United States 1988. [Google Scholar]
- 42.Gross JJ, John OP. Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. Journal of personality and social psychology 2003;85(2):348. [DOI] [PubMed] [Google Scholar]
- 43.Sütterlin S, Paap M, Babic S, Kübler A, Vögele C. Rumination and age: some things get better. Journal of aging research 2016;2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wenzlaff RM, Luxton DD. The role of thought suppression in depressive rumination. Cognitive Therapy and Research 2003;27(3):293–308. [Google Scholar]
- 45.Golden J, Conroy RM, Bruce I, Denihan A, Greene E, Kirby M, et al. The spectrum of worry in the community-dwelling elderly. Aging & mental health 2011;15(8):985–94. [DOI] [PubMed] [Google Scholar]
- 46.Emdin CA, Odutayo A, Wong CX, Tran J, Hsiao AJ, Hunn BH. Meta-Analysis of Anxiety as a Risk Factor for Cardiovascular Disease. Am J Cardiol 2016;118(4):511–9. [DOI] [PubMed] [Google Scholar]
- 47.Tully PJ, Cosh SM, Baumeister H. The anxious heart in whose mind? A systematic review and meta-regression of factors associated with anxiety disorder diagnosis, treatment and morbidity risk in coronary heart disease. J Psychosom Res 2014;77(6):439–48. [DOI] [PubMed] [Google Scholar]
- 48.Pietrzak RH, Maruff P, Woodward M, Fredrickson J, Fredrickson A, Krystal JH, et al. Mild worry symptoms predict decline in learning and memory in healthy older adults: a 2-year prospective cohort study. Am J Geriatr Psychiatry 2012;20(3):266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krusemark EA, Novak LR, Gitelman DR, Li W. When the sense of smell meets emotion: anxiety-state-dependent olfactory processing and neural circuitry adaptation. J Neurosci 2013;33(39):15324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sohrabi HR, Bates KA, Weinborn MG, Johnston AN, Bahramian A, Taddei K, et al. Olfactory discrimination predicts cognitive decline among community-dwelling older adults. Transl Psychiatry 2012;2:e118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Swan GE, Carmelli D. Impaired olfaction predicts cognitive decline in nondemented older adults. Neuroepidemiology 2002;21(2):58–67. [DOI] [PubMed] [Google Scholar]
- 52.Reichard RA, Subramanian S, Desta MT, Sura T, Becker ML, Ghobadi CW, et al. Abundant collateralization of temporal lobe projections to the accumbens, bed nucleus of stria terminalis, central amygdala and lateral septum. Brain Struct Funct 2017;222(4):1971–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Bellis MD, Keshavan MS, Shifflett H, Iyengar S, Dahl RE, Axelson DA, et al. Superior temporal gyrus volumes in pediatric generalized anxiety disorder. Biol Psychiatry 2002;51(7):553–62. [DOI] [PubMed] [Google Scholar]
- 54.Pico-Perez M, Radua J, Steward T, Menchon JM, Soriano-Mas C. Emotion regulation in mood and anxiety disorders: A meta-analysis of fMRI cognitive reappraisal studies. Prog Neuropsychopharmacol Biol Psychiatry 2017;79(Pt B):96–104. [DOI] [PubMed] [Google Scholar]
- 55.Karim HT, Tudorascu DL, Butters MA, Walker S, Aizenstein HJ, Andreescu C. In the grip of worry: cerebral blood flow changes during worry induction and reappraisal in late-life generalized anxiety disorder. Transl Psychiatry 2017;7(8):e1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bruhl AB, Delsignore A, Komossa K, Weidt S. Neuroimaging in social anxiety disorder-a meta-analytic review resulting in a new neurofunctional model. Neurosci Biobehav Rev 2014;47:260–80. [DOI] [PubMed] [Google Scholar]
- 57.Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci 2006;1071:67–79. [DOI] [PubMed] [Google Scholar]
- 58.Liao W, Qiu C, Gentili C, Walter M, Pan Z, Ding J, et al. Altered effective connectivity network of the amygdala in social anxiety disorder: a resting-state FMRI study. PLoS One 2010;5(12):e15238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sripada RK, King AP, Garfinkel SN, Wang X, Sripada CS, Welsh RC, et al. Altered restingstate amygdala functional connectivity in men with posttraumatic stress disorder. J Psychiatry Neurosci 2012;37(4):241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cirillo P, Gold AK, Nardi AE, Ornelas AC, Nierenberg AA, Camprodon J, et al. Transcranial magnetic stimulation in anxiety and trauma‐related disorders: A systematic review and meta-analysis. Brain and behavior 2019;9(6):e01284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beck JG, Stanley MA, Zebb BJ. Effectiveness of the Hamilton Anxiety Rating Scale with older generalized anxiety disorder patients. Journal of Clinical Geropsychology 1999;5(4):281–90. [Google Scholar]
- 62.Lee E-H. Review of the psychometric evidence of the perceived stress scale. Asian nursing research 2012;6(4):121–7. [DOI] [PubMed] [Google Scholar]
- 63.van Rijsoort S, Emmelkamp P, Vervaeke G. The Penn state worry questionnaire and the worry domains questionnaire: Structure, reliability and validity. Clinical Psychology & Psychotherapy: An International Journal of Theory & Practice 1999;6(4):297–307. [Google Scholar]
- 64.Beck JG, Stanley MA, Zebb BJ. Psychometric properties of the Penn State Worry Questionnaire in older adults. Journal of Clinical Geropsychology 1995;1(1):33–42. [Google Scholar]
- 65.Maier W, Buller R, Philipp M, Heuser I. The Hamilton Anxiety Scale: reliability, validity and sensitivity to change in anxiety and depressive disorders. Journal of affective disorders 1988;14(1):61–8. [DOI] [PubMed] [Google Scholar]
- 66.Nolen-Hoeksema S, Morrow J, Fredrickson BL. Response styles and the duration of episodes of depressed mood. Journal of abnormal psychology 1993;102(1):20. [DOI] [PubMed] [Google Scholar]


