Abstract
Tumor hypoxia renders treatments ineffective that are directly (e.g., radiotherapy and photodynamic therapy) or indirectly (e.g., chemotherapy) dependent on tumor oxygenation. This study introduces a ruthenium compound as a light-responsive anticancer agent that is water soluble, has minimal dark cytotoxicity, is active at concentrations as low as 170 pM in ~18.5% O2 normoxia and near 10 nM in 1% O2 hypoxia, and exhibits phototherapeutic indices as large as >500,000 in normoxia and >5,800 in 1% O2 hypoxia using broadband visible and monochromatic blue light treatments. These are the largest values reported to date for any compound class. We highlight the response in four different cell lines to improve rigor and reproducibility in the identification of promising clinical candidates.
Tumor hypoxia presents a major barrier in cancer therapy,1 being highly correlated with poor outcomes in patients. Hypoxia enhances chemoresistance of cancer cells through a variety of mechanisms. First, hypoxia can negatively impact both the delivery and uptake of drugs. Second, some chemotherapeutics actually require oxygen to generate the reactive oxygen species (ROS) that ultimately lead to cytotoxicity. Third, many cancer drugs are preferentially active against highly proliferating cells, and the nutrient deprivation caused by hypoxia reduces cellular proliferation and thus the efficacy of such agents. Finally, hypoxia can induce cellular adaptations at the transcriptional level that further compromise the efficacy of chemotherapy by promoting cell survival and upregulating certain resistance pathways.
In a similar vein, radiotherapy and other ROS-dependent treatment modalities such as photodynamic therapy (PDT) can be rendered ineffective by hypoxia. PDT combines a photosensitizer, light, and oxygen to destroy tumors and tumor vasculature. Its inherent selectivity for cancer tissues (through spatiotemporal delivery of light) and ability to induce an antitumor immune response2,3 make PDT an attractive adjuvant or alternative therapy for patients that have exhausted other options. Given that some of the most aggressive and drug-resistant cancers are characterized by hypoxia and that PDT requires oxygen, there is motivation to develop light-triggered compounds that exploit oxygen-independent mechanisms for their cytotoxic effects against cancer cells.4 As a result, the concept of photochemotherapy (PCT) has emerged as an alternative to PDT.
The idea behind PCT is that molecules can be designed to undergo stoichiometric photoreactions to generate reactive intermediates and/or products that should ultimately result in cell death in the absence of oxygen.5–8 The pioneering concept preceded any standardized methods for evaluating photocytotoxic effects in hypoxia. Developing such methods has been challenging, especially with regard to maintaining hypoxia during the irradiation step and also due to the absence of compounds that are substantially hypoxia-active. However, progress in this area is materializing9–18 as the potential of PCT and the possibility that PCT agents could overcome the issues of hypoxia begin to be realized.
The systems proposed for PCT usually contain a metal ion and are often based on Ru(II) coordinated to at least one strain-inducing ligand. The resulting complexes are distorted and readily undergo photoinduced ligand loss from triplet metal-centered (3MC) excited states. The assumption is that the ligand-deficient Ru(II) center and/or the liberated ligand may be cytotoxic. The idea that solvated Ru(II) centers could potentiate cell death through DNA interactions in a manner analogous to the mechanism of action of cisplatin was first suggested for distorted bis-heteroleptic dimmine systems.5–8 Photosubstitution as a means of effecting cytotoxicity has also been extended to Ru(II) tris-heteroleptics containing a labile monodentate ligand.19–21 However, these seminal studies did not evaluate photocytotoxicity in hypoxia for the reasons outlined.
Inspired by the idea of dual-action PCT-PDT agents,22,23 we (in collaboration with the Glazer Group) designed several families of Ru(II) bis-heteroleptic complexes that combined two strain-inducing ligands (6,6′-dimethyl-2,2′-bipyridine, dmb) with a third imidazo[4,5-f][1,10]phenanthroline (IP) ligand tethered to an aromatic or polyaromatic R groups.9,10,24 We reasoned that an appropriate R group would facilitate the formation of singlet oxygen (1O2) in the presence of oxygen, and the increased steric bulk at the Ru(II) center afforded by two strain-inducing ligands (rather than just one) would lead to even lower-lying 3MC states and thus favor photoinduced ligand loss (in the absence of oxygen). Although we did achieve some of the largest phototherapeutic indices (PIs) under hypoxia with visible light, these were still less than 20 and certainly nowhere near what would be needed to generate clinical interest in these PCT agents.9,10
Herein, we explore two novel Ru(II) complexes (1 and 2) of the type rac-[Ru(LL)2(IP-4T)](Cl)2, where LL=6,6′-dmb or 2,9-dimethyl-1,10-phenanthroline (2,9-dmp) and IP-4T is IP appended with 2,2′:5′,2″:5″,2‴-quaterthiophene (Chart 1). We investigate whether the two compounds can generate 1O2 and also undergo photoinduced ligand loss under cell-free conditions. We also determine whether they have the capacity to elicit potent photocytotoxic effects in both normoxia and importantly in hypoxia against a number of unrelated cancer cell lines. This preliminary study also probes whether the identities of the strain-inducing ligands have a role to play in determining the overall photocytotoxicity. Surprisingly, we find that photoinduced ligand loss likely does not contribute to the photocytotoxicity exerted by these compounds.
Chart 1.
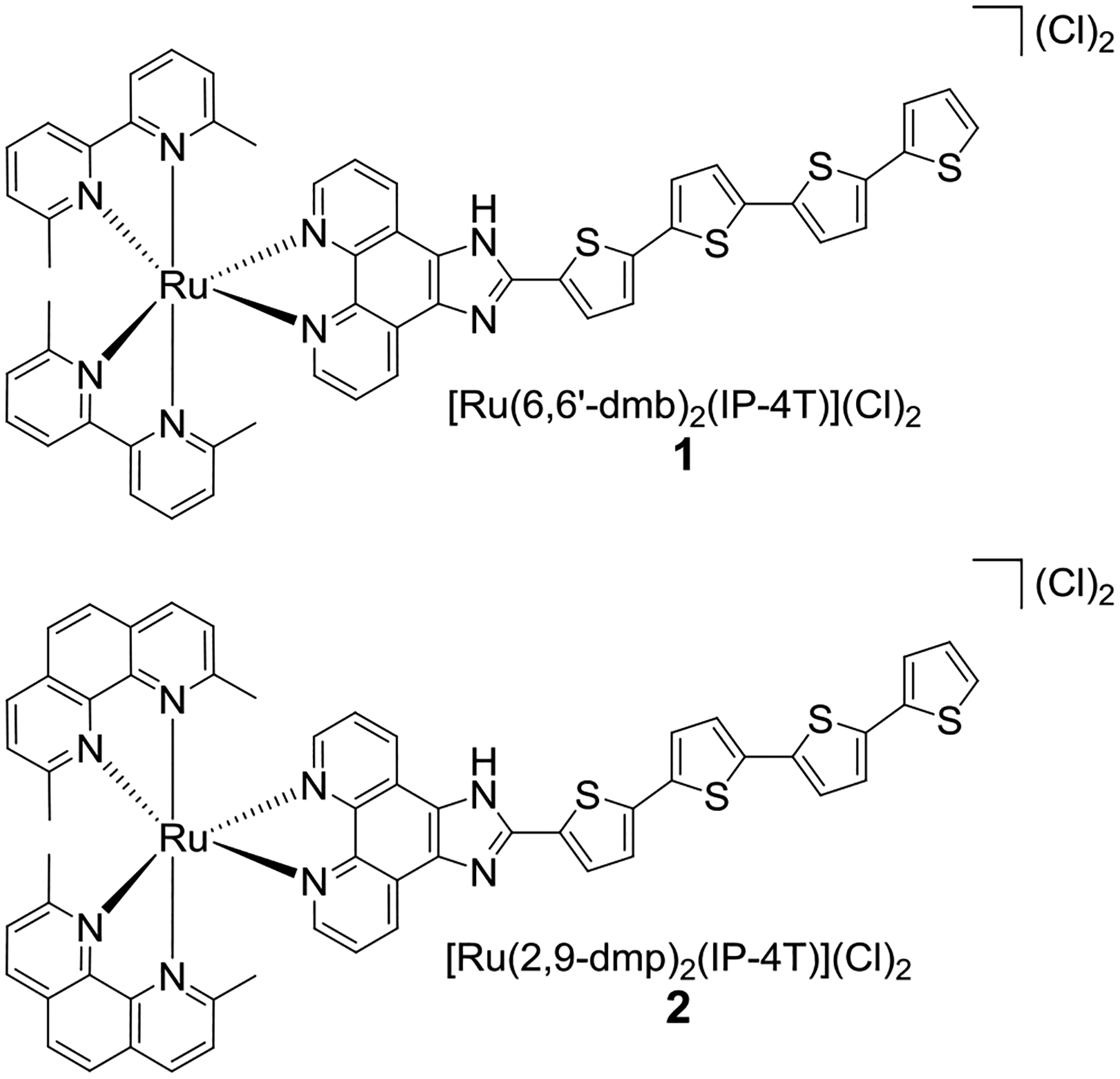
Molecular Structures of Complexes 1 and 2.
Complexes 1 and 2 were prepared under low-light conditions as racemic mixtures by reacting the precursor Ru(LL)2(Cl)2 (LL=6,6′-dmb or 2,9-dmp) with IP-4T in ethylene glycol with microwave irradiation at 180 °C. The compounds were isolated at their (PF6)− salts and purified with silica gel flash chromatography. They were then subjected to ion-exchange chromatography to form their corresponding Cl− salts, which underwent a final purification by size-exclusion chromatography. The details can be found in the Supporting Information (Figures S1–S11, Charts S1–S2).
The IP-4T ligand was incorporated for ROS generation owing to the relatively high 1O2 quantum yield of the 4T unit,25,26 while addition of the methyl groups to either bpy (2,2′-bipyridyl) or phen (1,10-phenanthroline) leads to complexes that undergo dissociation of this ligand upon irradiation with visible light.5,27 When combined around the Ru(II) center, IP-4T and the strain-inducing 6,6′-dmb or 2,9-dmp ligands do indeed partition their excited state reactivity between the two pathways.
When exposed to visible light, compound 1 has a 1O2 quantum yield of 43% while 2 exhibits an efficiency near 65%. Both compounds also undergo photoselective ligand loss of 6,6′-dmb or 2,9-dmp (Figure 1, S12–S19) regardless of whether oxygen is present. Their photosubstitution quantum yields are <1% (0.47% for 1 vs. 0.28% for 2). Despite low quantum efficiencies for this process, continued illumination leads to substantial photolysis through a process whereby 2 appears to have slower kinetics than 1. This photosubstitution profile is consistent with 2 having the larger 1O2 quantum yield.
Figure 1.
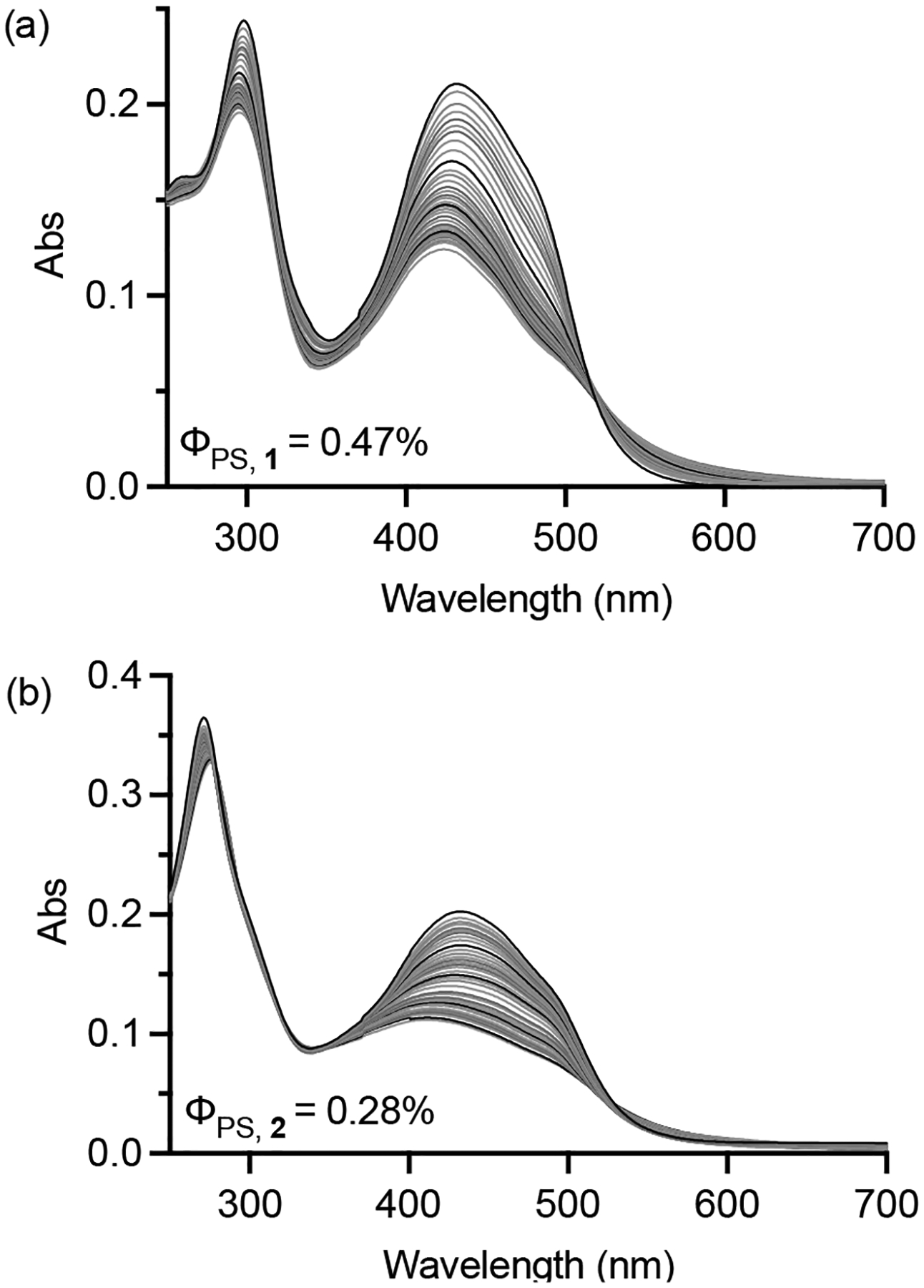
Photosubstitution of 1 and 2 (10 μM starting concentration) in water using 20 mW cm−2 broadband visible light. Indicated photosubstitution quantum yields (ΦPS) are differential, i.e., integrate spectral overlap between donor broadband light source and acceptor metal complex.
The proposed cell-free photophysical model of 1 and 2 involves excitation to singlet excited states that undergo rapid intersystem crossing to form their corresponding triplet excited states (Scheme 1). Some fraction of these triplet states dissipate their excess energy through photochemical substitution reactions or nonradiative decay involving the 3MC channel, while another sensitizes 1O2 from the lowest-lying 3IL/3ILCT states localized to the quaterthiophene. Since 1O2 production is catalytic, this pathway regenerates the ground state of 1 or 2, which can then be re-excited to start the process again. In principle, the solvated complexes can also absorb photons and participate in subsequent photochemical or photosensitization reactions.28 Of note, the 3ILCT state could also undergo electron transfer reactions that are typical of Type I photoprocesses or novel modes that are unique to oligothiophenes.29
Scheme 1.
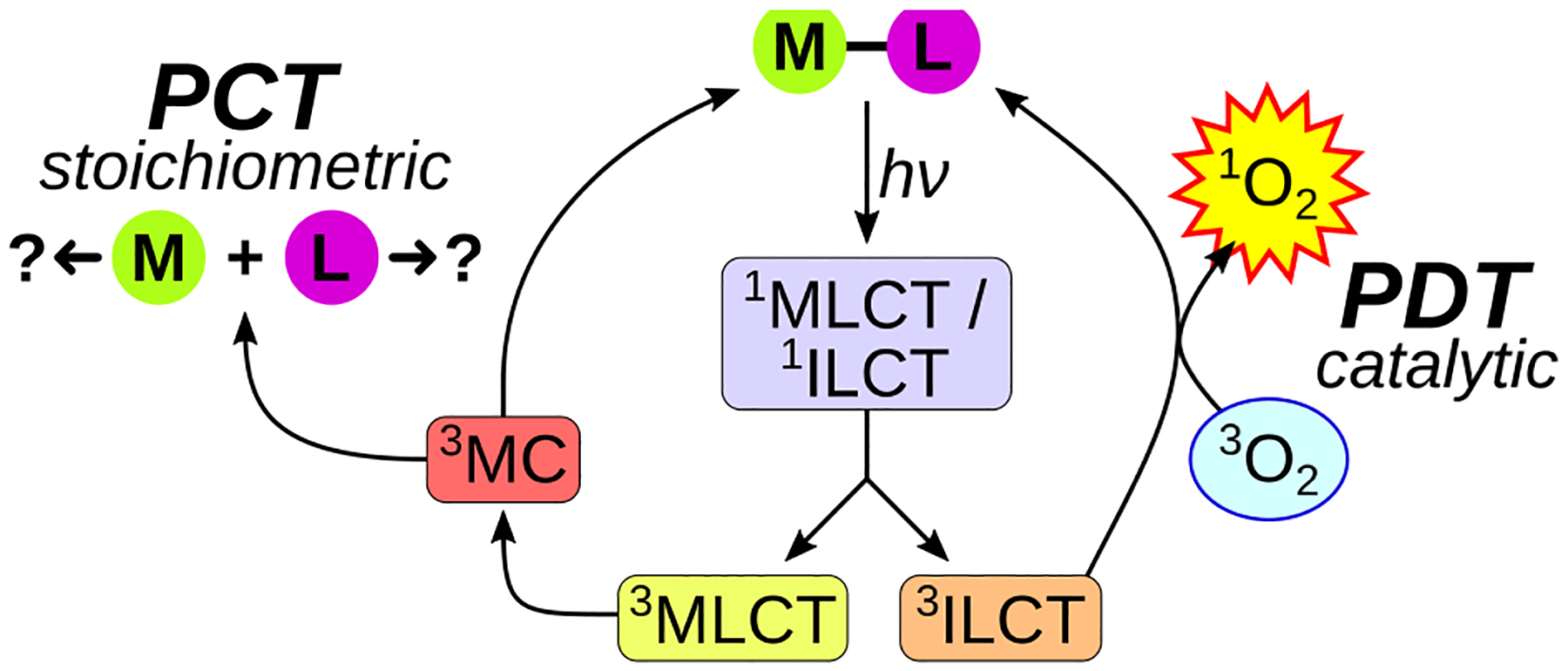
Competing excited state relaxation pathways.
Having shown that 1 and 2 can sensitize 1O2 and undergo photosubstitution, their (photo)cytotoxicity was examined in four different cell lines under both normoxia (18.5% O2) and hypoxia (1% O2). The detailed set-up for measuring dissolved O2 in the assays is illustrated in Figure S20. (Photo)cytotoxicity is reported as the effective concentration to reduce the cell viability by 50% (EC50) according to our established protocol,9–12 and the fold-enhancement in activity afforded by the light is reported as the PI. Various cell lines were tested to increase the rigor of the study: human melanoma (SKMEL28), highly pigmented mouse melanoma (B16F10), human lung (A549), and human breast (MCF7) cancer cells. The data is shown in Figure 2, S21, and S22 and tabulated in Tables S1 and S2. Light treatments were delivered at a fluence of 100 J cm−2 and irradiance of 18–24 mW cm−2 using single wavelength blue (453 nm), green (523 nm), or red (633 nm) LEDs with a bandwidth of approximately 19–43 nm or broadband visible (400–700 nm) LEDs. The spectral output of the light sources is shown in Figure S23, and their overlap with the absorption spectra of 1 and 2 is shown in Figure S24. The number of photons absorbed by the compounds based on this output is tabulated in Table S3.
Figure 2.
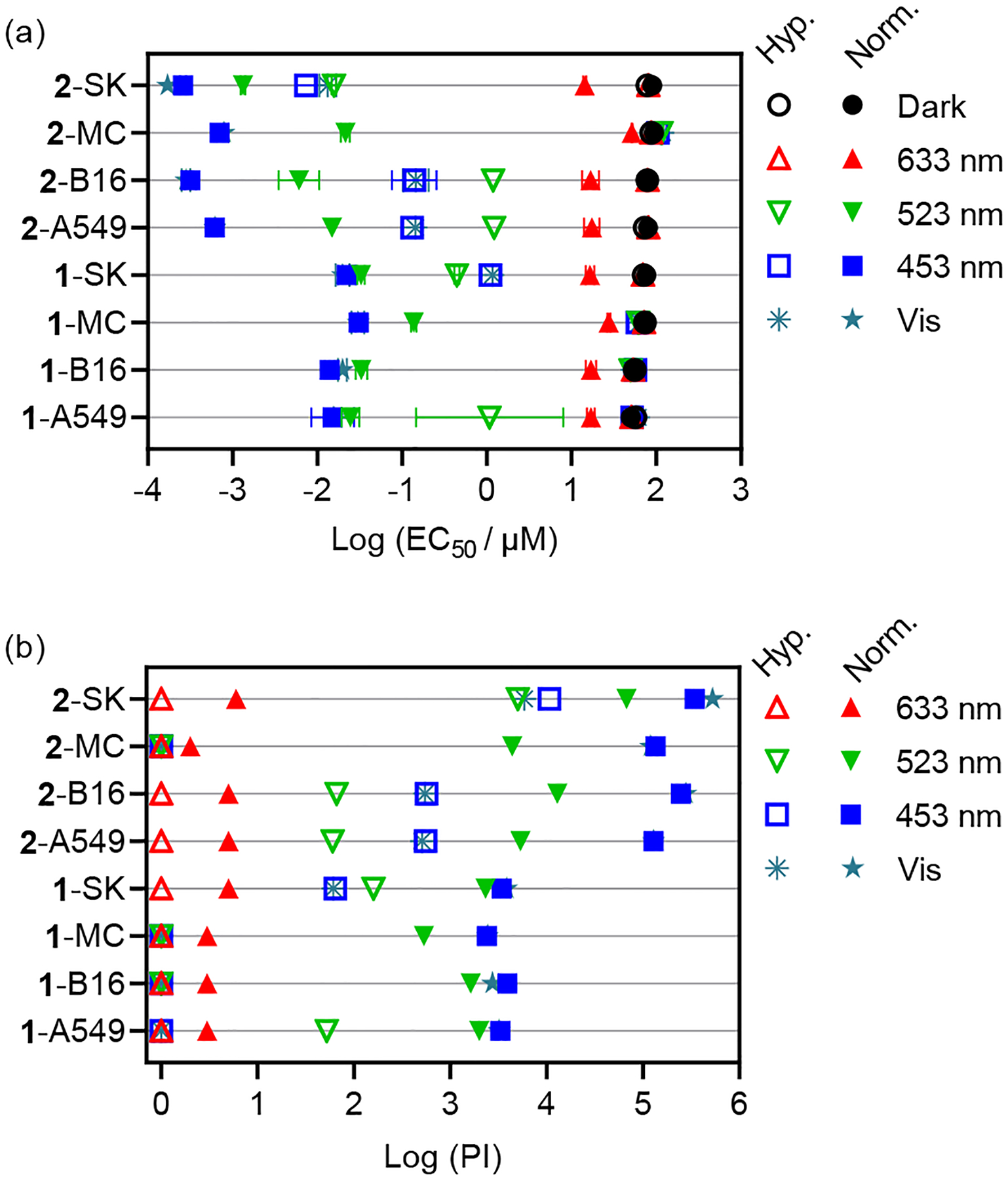
Summary of cytotoxicity (dark) and photocytotoxicity (100 J cm−2, ~20 mW cm−2) as (a) best-fit log (EC50±SEM) values and (b) phototherapeutic indices (PI; dark EC50/light EC50) for compounds 1 and 2. Cell lines are listed in order of A549, B16F10 (B16), MCF7 (MC), and SKMEL28 (SK). Unfilled symbols=1% O2; filled symbols=18.5–21% O2.
Compounds 1 and 2 are relatively nontoxic in the dark toward the selected cell lines, with EC50 values ≥50 μM regardless of cell line or oxygen tension. Compound 2 was less cytotoxic than 1 under all conditions, with EC50 values for 2 ranging from 78 to 94 μM in normoxia and 72 to 87 μM in hypoxia. The corresponding dark EC50 values for 1 were 50–76 μM in normoxia and 55–74 μM in hypoxia. A549 and B16F10 cell lines were slightly more sensitive to the compounds in the dark compared to SKMEL28 and MCF7 cells, but there was little difference in the two oxygen conditions for a given cell line and compound.
Both compounds are extremely photocytotoxic toward cancer cells in normoxia in the wavelength range where they absorb visible light, and are inactive with red light. Compound 2 yielded an EC50 value of 170 pM with visible light and a PI >105 (PI=527,000) with the SKMEL28 cell line. The EC50 values for 2 were subnanomolar (170–786 pM) with PIs of ~105 (119,500–527,000) for the cell lines that proved to be more resistant. Compound 1, although less active than 2, exhibited visible EC50 values ranging from about 15–30 nM in the four cell lines in normoxia, with PI values >103 (PI=2400–3800). This light-triggered cytotoxicity is unprecedented for PCT agents in normoxia tested under similar conditions.
Similar trends were obtained with blue light, and both compounds were still very photocytotoxic with green light at the activation wavelength currently being used for TLD1433 in the clinical trial.30,31 Compound 2 gave green EC50 values that were low nanomolar and PIs on the order of 103–104 and as high as 67,000. While the activity of 1 was attenuated, it was still in the nanomolar regime (EC50=30–140 nM; PI=534–2300) and more potent than many platforms.
Notably, compound 2 retained its photocytotoxicity toward three of the four cell lines in 1% hypoxia, with a visible EC50 value of 13 nM and PI of 5900 against SKMEL28 cells. In A549 and B16F10, these values were still in the nanomolar regime (EC50≈140 nM) and PIs in the 500–550 range. While these values were wavelength and cell-line dependent, 2 was equally effective with green light in the SKMEL28 cell line and still gave PIs ≥60 in A549 and B16F10. To our knowledge, 2 is the most potent hypoxia-active compound to date.
Overall, compound 1 was less active than compound 2 in both normoxia and in hypoxia and was completely inactive in hypoxia in three of the four cell lines. From this, it is clear that the identities of the strain-inducing coligands play a pivotal role in bioactivity although their liberated forms do not appear to be the source of cytotoxicity. Whether liberated ligands are responsible for the observed cytotoxicity in other systems has been a source of debate,32–34 and the reasons for their varied bioactivity is reviewed elsewhere.28 In our assay conditions, the free 6,6′-dmb and 2,9-dmp ligands were inactive in both normoxia and hypoxia (Figure S25, Table S4).
Compound 2 (despite extremely high potency under almost all conditions) was inactive in hypoxia in one of the four cell lines. This observation underscores the importance of testing the hypoxic response in multiple cell lines. In this limited study, we learned that SKMEL28 is the most sensitive cell line in hypoxia and MCF7 is the least. Therefore, an excellent test for the robustness of the response in 1% hypoxia is the MCF7 cell line, and a good test for the sheer magnitude of the response that is possible is SKMEL28 (at least for this compound class). We hesitate to speculate on the reason for the inactivity of 2 toward MCF7 cells in hypoxia without knowing the operative cellular mechanism(s) and whether it is distinct for this cell line. There could be a fundamental difference in cellular uptake/adhesion and/or localization, and this could in turn impact the operative mechanism(s). We do know that the lack of a hypoxic phototoxic response in MCF7 is reproducible and that the dark cytotoxicity is not affected. Expanded cell line studies with a larger number of hypoxia-active compounds are underway to gain a better understanding.
In summary, compound 2 demonstrates that the 2,9-dmp strain-inducing coligand is superior to 6,6′-dmb when combined with the IP-4T ligand and Ru(II). The relatively small difference in 1O2 quantum yields (43% for 1 vs. 65% for 2) cannot account for their large differences in potency in normoxia and the fact that 1 is almost inactive in hypoxia (except for in SKMEL28). Likewise, the cell-free photosubstitution quantum yields do not explain the extremely high potency of 2, given that the process is both less efficient and much slower for the 2,9-dmp ligand. From this we conclude that stoichiometric ligand dissociation does not contribute substantially to the observed photocytotoxic effects.
This new class of light-responsive, hypoxia-active agents incorporating the α-oligothienyl group may involve excited state pathways distinct from the 1O2 and photosubstitution pathways. The IP-4T ligand appears to be crucial for this inexplicable activity, but it is clear that the coligands attached to Ru(II) play a modulatory role. While Type I electron transfer pathways could be responsible and are not new concepts, the sheer magnitude of photocytotoxicity in both normoxia and hypoxia suggests reactivity that is unique to the longer oligothiophenes when they are incorporated into Ru(II) complexes with appropriate coligands. Strategic expansion of this family will use structure-activity relationships to gain mechanistic insight into these hypoxia-active agents, and detailed biological studies will aim to understand why certain cell lines are more sensitive than others to these compounds in hypoxia.
Supplementary Material
ACKNOWLEDGMENT
S.A.M. and C.G.C. thank the National Cancer Institute (NCI) of the National Institutes of Health (NIH) (award R01CA222227) for support. The content in this work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. S.A.M. and C.G.C. also thank the National Science Foundation (award 2102459) for partial support of this work. S.A.M. also thanks Dr. Daniel Todd as UNCG’s Triad Mass Spectrometry Facility manager and his assistants Jennifer Simpson and Diane Wallace. S.A.M. likewise thanks Dr. Franklin Moy (UNCG NMR facility manager) and Dr. Brian Edwards (UTA NMR facility manager) for experimental support and instrument maintenance.
Funding Sources
S.A.M. and C.G.C. thank the National Cancer Institute (NCI) of the National Institutes of Health (NIH) (award R01CA222227) for support. The content in this work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. S.A.M. and C.G.C. also thank the National Science Foundation (award 2102459) for partial support of this work.
Footnotes
The Supporting Information is available free of charge at http://pubs.acs.org: characterization data (1D and 2D NMR, HPLC, HRMS), photosubstitution methods and data, and (photo)biological data.
S.A.M. has a potential research conflict of interest due to a financial interest with Theralase Technologies, Inc. and PhotoDynamic, Inc. A management plan has been created to preserve objectivity in research in accordance with UTA policy.
REFERENCES
- (1).Hompland T; Fjeldbo CS; Lyng H Tumor Hypoxia as a Barrier in Cancer Therapy: Why Levels Matter. Cancers 2021, 13 (3), 499. 10.3390/cancers13030499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Falk-Mahapatra R; Gollnick SO Photodynamic Therapy and Immunity: An Update. Photochem. Photobiol 2020, 96, 550–559. 10.1111/php.13253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Alzeibak R; Mishchenko TA; Shilyagina NY; Balalaeva IV; Vedunova MV; Krysko DV Targeting Immunogenic Cancer Cell Death by Photodynamic Therapy: Past, Present and Future. J. Immunother. Cancer 2021, 9 (1), e001926. 10.1136/jitc-2020-001926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Bonnet S Why Develop Photoactivated Chemotherapy? Dalton Trans. 2018, 47 (31), 10330–10343. 10.1039/C8DT01585F. [DOI] [PubMed] [Google Scholar]
- (5).Howerton BS; Heidary DK; Glazer EC Strained Ruthenium Complexes Are Potent Light-Activated Anticancer Agents. J. Am. Chem. Soc 2012, 134 (20), 8324–8327. 10.1021/ja3009677. [DOI] [PubMed] [Google Scholar]
- (6).Wachter E; Heidary DK; Howerton BS; Parkin S; Glazer EC Light-Activated Ruthenium Complexes Photobind DNA and Are Cytotoxic in the Photodynamic Therapy Window. Chem. Commun 2012, 48 (77), 9649. 10.1039/c2cc33359g. [DOI] [PubMed] [Google Scholar]
- (7).Hidayatullah AN; Wachter E; Heidary DK; Parkin S; Glazer EC Photoactive Ru(II) Complexes With Dioxinophenanthroline Ligands Are Potent Cytotoxic Agents. Inorg. Chem 2014, 53 (19), 10030–10032. 10.1021/ic5017164. [DOI] [PubMed] [Google Scholar]
- (8).Wachter E; Glazer EC Mechanistic Study on the Photochemical “Light Switch” Behavior of [Ru(Bpy) 22+$. J. Phys. Chem. A 2014, 118 (45), 10474–10486. 10.1021/jp504249a. [DOI] [PubMed] [Google Scholar]
- (9).Roque J; Havrylyuk D; Barrett PC; Sainuddin T; McCain J; Colón K; Sparks WT; Bradner E; Monro S; Heidary D; Cameron CG; Glazer EC; McFarland SA Strained, Photoejecting Ru(II) Complexes That Are Cytotoxic Under Hypoxic Conditions. Photochem. Photobiol 2020, 96 (2), 327–339. 10.1111/php.13174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Cole HD; Roque JA; Lifshits LM; Hodges R; Barrett PC; Havrylyuk D; Heidary D; Ramasamy E; Cameron CG; Glazer EC; McFarland SA Fine-feature Modifications to Strained Ruthenium Complexes Radically Alter Their Hypoxic Anticancer Activity †. Photochem. Photobiol 2021, php.13395. 10.1111/php.13395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Roque III JA; Barrett PC; Cole HD; Lifshits LM; Shi G; Monro S; von Dohlen D; Kim S; Russo N; Deep G; Cameron CG; Alberto ME; McFarland SA Breaking the Barrier: An Osmium Photosensitizer with Unprecedented Hypoxic Phototoxicity for Real World Photodynamic Therapy. Chem. Sci 2020, 11, 9784–9806. 10.1039/D0SC03008B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Roque JA; Barrett PC; Cole HD; Lifshits LM; Bradner E; Shi G; von Dohlen D; Kim S; Russo N; Deep G; Cameron CG; Alberto ME; McFarland SA Os(II) Oligothienyl Complexes as a Hypoxia-Active Photosensitizer Class for Photodynamic Therapy. Inorg. Chem 2020, 59 (22), 16341–16360. 10.1021/acs.inorgchem.0c02137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Lameijer LN; Ernst D; Hopkins SL; Meijer MS; Askes SHC; Le Dévédec SE; Bonnet S A Red-Light-Activated Ruthenium-Caged NAMPT Inhibitor Remains Phototoxic in Hypoxic Cancer Cells. Angew. Chem. Int. Ed 2017, 56 (38), 11549–11553. 10.1002/anie.201703890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Lv Z; Wei H; Li Q; Su X; Liu S; Zhang KY; Lv W; Zhao Q; Li X; Huang W Achieving Efficient Photodynamic Therapy under Both Normoxia and Hypoxia Using Cyclometalated Ru(\textlessspan Style=“font-Variant:Small-Caps;”\textgreaterii\textless/Span\textgreater) Photosensitizer through Type I Photochemical Process. Chem. Sci 2018, 9 (2), 502–512. 10.1039/C7SC03765A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).van Rixel VHS; Ramu V; Auyeung AB; Beztsinna N; Leger DY; Lameijer LN; Hilt ST; Le Dévédec SE; Yildiz T; Betancourt T; Gildner MB; Hudnall TW; Sol V; Liagre B; Kornienko A; Bonnet S Photo-Uncaging of a Microtubule-Targeted Rigidin Analogue in Hypoxic Cancer Cells and in a Xenograft Mouse Model. J. Am. Chem. Soc 2019, 141 (46), 18444–18454. 10.1021/jacs.9b07225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Yu Q; Huang T; Liu C; Zhao M; Xie M; Li G; Liu S; Huang W; Zhao Q Oxygen Self-Sufficient NIR-Activatable Liposomes for Tumor Hypoxia Regulation and Photodynamic Therapy. Chem. Sci 2019, 10 (39), 9091–9098. 10.1039/C9SC03161H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Feng W; Gao C; Liu W; Ren H; Wang C; Ge K; Li S; Zhou G; Li H; Wang S; Jia G; Li Z; Zhang J A Novel Anticancer Theranostic Pro-Prodrug Based on Hypoxia and Photo Sequential Control. Chem Commun 2016, 52 (60), 9434–9437. 10.1039/C6CC02932A. [DOI] [PubMed] [Google Scholar]
- (18).Evans CL; Abu-Yousif AO; Park YJ; Klein OJ; Celli JP; Rizvi I; Zheng X; Hasan T Killing Hypoxic Cell Populations in a 3D Tumor Model with EtNBS-PDT. PLoS ONE 2011, 6 (8), e23434. 10.1371/journal.pone.0023434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Albani BA; Durr CB; Turro C Selective Photoinduced Ligand Exchange in a New Tris–Heteroleptic Ru(II) Complex. J. Phys. Chem. A 2013, 117 (50), 13885–13892. 10.1021/jp4085684. [DOI] [PubMed] [Google Scholar]
- (20).Knoll JD; Albani BA; Durr CB; Turro C Unusually Efficient Pyridine Photodissociation from Ru(II) Complexes with Sterically Bulky Bidentate Ancillary Ligands. J. Phys. Chem. A 2014, 118 (45), 10603–10610. 10.1021/jp5057732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Bahreman A; Cuello-Garibo J-A; Bonnet S Yellow-Light Sensitization of a Ligand Photosubstitution Reaction in a Ruthenium Polypyridyl Complex Covalently Bound to a Rhodamine Dye. Dalton Trans 2014, 43 (11), 4494–4505. 10.1039/C3DT52643G. [DOI] [PubMed] [Google Scholar]
- (22).Albani BA; Peña B; Leed NA; de Paula NABG; Pavani C; Baptista MS; Dunbar KR; Turro C Marked Improvement in Photoinduced Cell Death by a New Tris-Heteroleptic Complex with Dual Action: Singlet Oxygen Sensitization and Ligand Dissociation. J. Am. Chem. Soc 2014, 136 (49), 17095–17101. 10.1021/ja508272h. [DOI] [PubMed] [Google Scholar]
- (23).Toupin NP; Nadella S; Steinke SJ; Turro C; Kodanko JJ Dual-Action Ru(II) Complexes with Bulky π-Expansive Ligands: Phototoxicity without DNA Intercalation. Inorg. Chem 2020, 59 (6), 3919–3933. 10.1021/acs.inorgchem.9b03585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Sainuddin T; Pinto M; Yin H; Hetu M; Colpitts J; McFarland SA Strained Ruthenium Metal–Organic Dyads as Photocisplatin Agents with Dual Action. J. Inorg. Biochem 2016, 158, 45–54. 10.1016/j.jinorgbio.2016.01.009. [DOI] [PubMed] [Google Scholar]
- (25).Becker RS; Seixas de Melo J; Maçanita AL; Elisei F Comprehensive Evaluation of the Absorption, Photophysical, Energy Transfer, Structural, and Theoretical Properties of α-Oligothiophenes with One to Seven Rings. J Phys Chem 1996, 100, 18683–18695. 10.1021/jp960852e. [DOI] [Google Scholar]
- (26).de Melo JS; Silva LM; Arnaut LG; Becker RS Singlet and Triplet Energies of α-Oligothiophenes: A Spectroscopic, Theoretical, and Photoacoustic Study: Extrapolation to Polythiophene. J. Chem. Phys 1999, 111 (12), 5427–5433. 10.1063/1.479825. [DOI] [Google Scholar]
- (27).Havrylyuk D; Stevens K; Parkin S; Glazer EC Toward Optimal Ru(II) Photocages: Balancing Photochemistry, Stability, and Biocompatibility Through Fine Tuning of Steric, Electronic, and Physiochemical Features. Inorg. Chem 2020, 59 (2), 1006–1013. 10.1021/acs.inorgchem.9b02065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Hachey AC; Havrylyuk D; Glazer EC Biological Activities of Polypyridyl-Type Ligands: Implications for Bioinorganic Chemistry and Light-Activated Metal Complexes. Curr. Opin. Chem. Biol 2021, 61, 191–202. 10.1016/j.cbpa.2021.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Rajappa S; Gumaste VK Reactivity of Thiophenes, Oligothiophenes and Benzothiophenes. In Advances in Heterocyclic Chemistry; Elsevier, 2013; Vol. 108, pp 1–161. 10.1016/B978-0-12-404598-9.00001-8. [DOI] [Google Scholar]
- (30).Monro S; Colón KL; Yin H; Roque J; Konda P; Gujar S; Thummel RP; Lilge L; Cameron CG; McFarland SA Transition Metal Complexes and Photodynamic Therapy from a Tumor-Centered Approach: Challenges, Opportunities, and Highlights from the Development of TLD1433. Chem. Rev 2019, 119 (2), 797–828. 10.1021/acs.chemrev.8b00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).McFarland SA; Mandel A; Dumoulin-White R; Gasser G Metal-Based Photosensitizers for Photodynamic Therapy: The Future of Multimodal Oncology? Curr. Opin. Chem. Biol 2020, 56, 23–27. 10.1016/j.cbpa.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Azar DF; Audi H; Farhat S; El-Sibai M; Abi-Habib RJ; Khnayzer RS Phototoxicity of Strained Ru(II) Complexes: Is It the Metal Complex or the Dissociating Ligand? Dalton Trans. 2017, 46 (35), 11529–11532. 10.1039/C7DT02255G. [DOI] [PubMed] [Google Scholar]
- (33).Cuello-Garibo J-A; Meijer MS; Bonnet S To Cage or to Be Caged? The Cytotoxic Species in Ruthenium-Based Photoactivated Chemotherapy Is Not Always the Metal. Chem. Commun 2017, 53 (50), 6768–6771. 10.1039/C7CC03469E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Sun Y; Heidary DK; Zhang Z; Richards CI; Glazer EC Bacterial Cytological Profiling Reveals the Mechanism of Action of Anticancer Metal Complexes. Mol. Pharm 2018, 15 (8), 3404–3416. 10.1021/acs.molpharmaceut.8b00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


