Abstract
Aim
To examine the determinants and metabolic impact of the reduction in fasting and postload insulin levels after a low n‐6 to n‐3 polyunsaturated fatty acid (PUFA) ratio diet in obese youth.
Materials and Methods
Insulin secretion and clearance were assessed by measuring and modelling plasma insulin and C‐peptide in 17 obese youth who underwent a nine‐point, 180‐minute oral glucose tolerance test (OGTT) before and after a 12‐week, eucaloric low n‐6:n‐3 polyunsaturated fatty acid (PUFA) ratio diet. Hepatic fat content was assessed by repeated abdominal magnetic resonance imaging.
Results
Insulin clearance at fasting and during the OGTT was significantly increased after the diet, while body weight, glucose levels, absolute and glucose‐dependent insulin secretion, and model‐derived variables of β‐cell function were not affected. Dietary‐induced changes in insulin clearance positively correlated with changes in whole‐body insulin sensitivity and β‐cell glucose sensitivity, but not with changes in hepatic fat. Subjects with greater increases in insulin clearance showed a worse metabolic profile at enrolment, characterized by impaired insulin clearance, β‐cell glucose sensitivity, and glucose tolerance, and benefitted the most from the diet, achieving greater improvements in glucose‐stimulated hyperinsulinaemia, insulin resistance, and β‐cell function.
Conclusions
We showed that a 12‐week low n‐6:n‐3 PUFA ratio diet improves hyperinsulinaemia by increasing fasting and postload insulin clearance in obese youth, independently of weight loss, glucose concentrations, and insulin secretion.
Keywords: adolescents, β‐cell function, dietary lipids, fatty liver disease, glucose metabolism, insulin clearance, insulin metabolism, insulin resistance, insulin secretion, liver, obesity
1. INTRODUCTION
Hyperinsulinaemia, a result of reduced insulin clearance 1 , 2 , 3 and insulin hypersecretion, 1 , 4 is a key feature of insulin resistance that is affected by several factors such as hepatic response to insulin action, 5 , 6 , 7 , 8 dietary and circulating fat, 4 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 and ethnic background. 1 , 6 , 18 Reduced insulin clearance is associated with intrahepatic fat accumulation and hepatic insulin resistance in most ethnic groups 6 , 7 , 19 , 20 in a complex metabolic network that has been linked to β‐cell impairment and increased risk of prediabetes and type 2 diabetes. 2 , 21
A reduced dietary intake of omega‐3 (n‐3) polyunsaturated fatty acids (PUFA) with overconsumption of omega‐6 (n‐6) PUFA is associated with an adverse metabolic profile. 22 Recently, we have shown that a 12‐week normocaloric dietary intervention characterized by a low n‐6 to n‐3 PUFA ratio (4:1) reduced hepatic fat content by ~30% in obese youth with non‐alcoholic fatty liver disease (NAFLD), in the absence of body weight changes. 23 Remarkably, the diet also lowered plasma insulin levels at fasting and in response to an oral glucose tolerance test (OGTT) by ~25% and improved whole‐body insulin sensitivity by 34%, without affecting fasting or postload glucose concentrations. This suggests a potential role of the low n‐6:n‐3 PUFA ratio diet as a therapeutic strategy to reduce obesity‐associated hyperinsulinaemia by reducing hepatic fat.
Herein, we tested the hypothesis that a low n‐6:n‐3 PUFA ratio diet can modify the metabolic phenotype of obese youth with NAFLD by increasing insulin clearance, in the absence of significant changes in body weight and glucose tolerance. To achieve our aim, in a secondary analysis, we measured plasma C‐peptide levels during the frequently sampled OGTT and estimated variables of insulin secretion, β‐cell function, and insulin clearance by mathematical modelling of glucose, insulin, and C‐peptide data, in the participants who underwent the low n‐6:n‐3 PUFA ratio diet. 23
2. MATERIALS AND METHODS
2.1. Study design and participants
In the current study, newly generated data from our recent clinical trial 23 on the effects of a low n‐6:n‐3 PUFA ratio diet on intrahepatic fat content were modelled and analysed. A detailed description of the clinical trial design has been previously published. 23 Briefly, the trial was a single‐arm, unblinded, interventional longitudinal study in a multiethnic cohort of 17 obese youth with NAFLD recruited from the Yale Pediatric Obesity Clinic. The inclusion criteria for the study were body mass index equal or above the 95th percentile for age and sex and hepatic fat fraction (HFF%) measured by liver magnetic resonance imaging (MRI) of 5.5% or higher at the screening visit. Participants with diabetes, food allergy to fish, liver disease other than NAFLD, or who were taking medications affecting glucose, lipid, or hepatic metabolism, were excluded.
The study protocol was approved by the Human Investigations Committee of the Yale School of Medicine (NCT01556113). Participants provided assent and parents provided written informed consent to participate in the study before enrolment.
2.2. Study procedures
Participants underwent a 3‐hour OGTT at baseline and after 12 weeks of dietary intervention. Briefly, the diet consisted of a meal plan with low (4:1) n‐6 to n‐3 PUFA ratio, and 50%‐55% daily total calories from carbohydrate, 20% from protein, and 25%‐30% from fat, with saturated, monounsaturated, and polyunsaturated fats each comprising 8%‐10% of daily total caloric intake. To control for the confounding effect of weight loss, the meal plan was designed to be isocaloric to prestudy food intake. Further details on the dietary intervention have been previously reported. 23
2.3. Oral glucose tolerance test
Prior to the OGTT, all participants followed a weight‐maintenance diet consisting of at least 250 g of carbohydrates per day for 7 days. Participants were studied in the Yale Center for Clinical Investigation at 08:00 am after a 12‐hour overnight fast. Two baseline samples were obtained for measurements of fasting plasma glucose, insulin, and C‐peptide. Thereafter, flavoured glucose in a dose of 1.75 g per kilogram of body weight (up to a maximum of 75 g) was given orally, and blood samples were obtained every 10 minutes for the first 30 minutes, then every 30 minutes until 180 minutes for the measurement of plasma glucose, insulin, and C‐peptide. Glucose samples were processed at the bedside using a YSI2700‐STAT‐Analyzer (Yellow Springs Instruments, Yellow Springs, OH).
2.4. Abdominal MRI
MRI studies were performed on a Siemens Sonata 1.5‐T system as previously described. 20 , 24 HFF% was measured using the two‐point Dixon as modified by Fishbein et al. 25
2.5. Biochemical analysis
For the purpose of this study, plasma C‐peptide levels were determined by ELISA using ALPCO‐Immunoassays (Salem, NH) with a 3.87% intra‐assay variability in frozen plasma samples. Plasma insulin was measured by a radioimmunoassay (Linco, St. Charles, MO) that has less than 1% cross‐reactivity with C‐peptide and proinsulin, as previously reported. 23
2.6. Measures of insulin sensitivity, secretion, and clearance
Whole‐body insulin sensitivity was assessed at fasting by the homeostasis model assessment (HOMA) index and during the OGTT by the oral glucose insulin sensitivity (OGIS) index 26 , 27 and the whole‐body insulin sensitivity index (WBISI). 28 Insulin secretion rate (ISR) was estimated by C‐peptide deconvolution. 29 Variables of β‐cell function were calculated by mathematical modelling of ISR and glucose concentrations during the OGTT, as previously reported. 30 , 31 , 32 The relationship between glucose and ISR is described as the sum of two components. The first component represents the dependence of ISR on absolute glucose concentration at any time point. The quasi‐linear dose–response function relating the two variables is described by a slope, named β‐cell glucose sensitivity, and by an intercept that can be described by the ISR at a fixed fasting plasma glucose of 5 mmol/L (ISR@5). The static dose–response function can be modulated over time by several factors (i.e. persistent hyperglycaemia, non‐glucose substrates, gastrointestinal hormones, and neurotransmitters), which are collectively modelled as a potentiation multiplying factor. The second insulin secretion component represents the dynamic dependence of ISR on the rate of change of glucose concentration and is named β‐cell rate sensitivity. Endogenous insulin clearance, which largely (~80%) reflects hepatic insulin clearance, was calculated at fasting as the ratio between fasting ISR and insulin concentration, and during the OGTT as the ratio between ISR and plasma insulin areas under the curve (AUC) (ISRAUC/insAUC). 6 A correction for the amount of released insulin that has not been cleared or has been cleared in excess during the OGTT was not applied for consistency with previous works, 6 and because of similar baseline and 3‐hour insulin concentrations (Figure 1A). The physiological percentage reduction of insulin clearance from fasting to fed conditions was calculated as the ratio between the estimated insulin clearance during the OGTT and at fasting (insulin clearanceOGTT/insulin clearancefasting).
FIGURE 1.
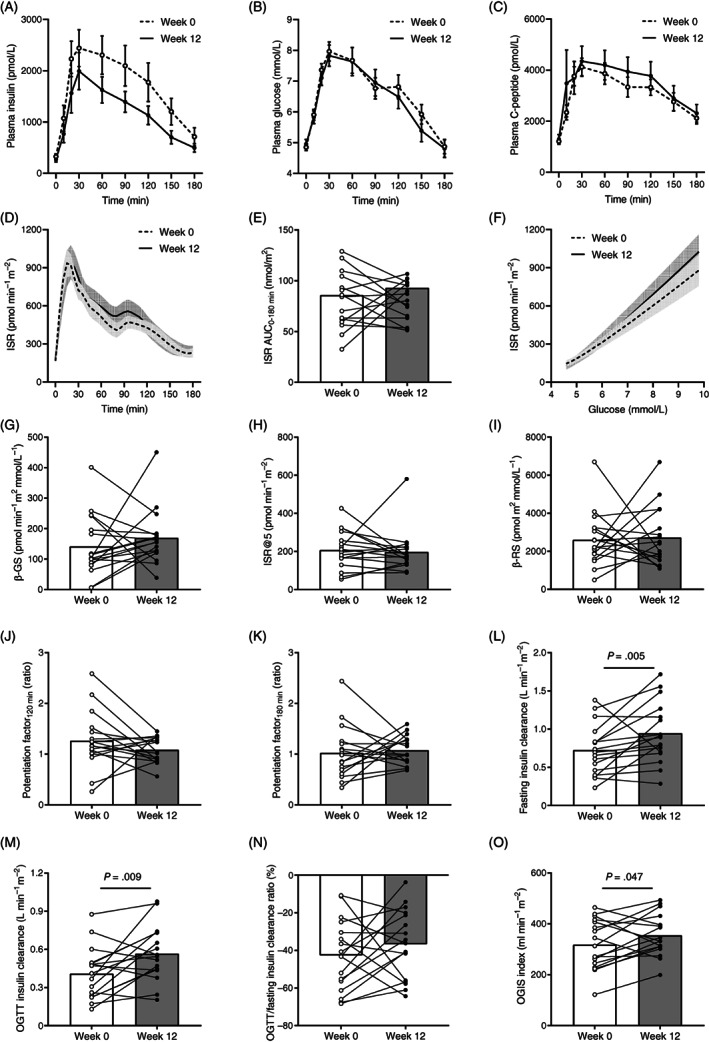
A, Plasma insulin, B, Glucose, and C, C‐peptide concentrations; D, Insulin secretion rate (ISR); E, Area under the 180‐minute curve (AUC0−180 min) of ISR; F, ISR relative to plasma glucose; G, β‐cell glucose sensitivity (β‐GS); H, ISR at a fixed fasting plasma glucose of 5 mmol/L (ISR@5); I, β‐cell rate sensitivity (β‐RS); potentiation factor ratio at J, 120 minutes, and K, 180 minutes; insulin clearance at L, Fasting, M, During the oral glucose tolerance test (OGTT), and N, Percentage reduction from fasting to OGTT; and O, Oral glucose insulin sensitivity (OGIS) index at baseline (week 0) and after the low n‐6:n‐3 polyunsaturated fatty acid ratio diet (week 12) in obese youth. Lines and columns indicate means. Error bars and shaded areas indicate SEM. Panels A and B are modified with permission from. 23 Group differences were tested using paired Wilcoxon signed‐rank test. P values less than .10 are reported
2.7. Statistical analysis
Categorical variables are reported as count (%). Continuous variables were tested for normality using the Shapiro–Wilk test and reported as mean ± SD if normally distributed or median [interquartile range] if not normally distributed, unless otherwise stated. Diet‐induced differences in insulin secretion, β‐cell function variables, and insulin clearance in all participants were analysed using paired Student's t tests or Wilcoxon signed‐rank tests as appropriate. A 25% difference in whole‐body insulin clearance was deemed clinically significant based on the following observations: (a) the Restoring Insulin Secretion (RISE) study reported a ~25% lower clearance in youths with prediabetes or recent‐onset type 2 diabetes, compared with the adults 33 ; and (b) our group observed that insulin clearance is ~20% lower in obese youth with impaired glucose tolerance (IGT) than obese youth with normal glucose tolerance (NGT) and preserved insulin sensitivity. 2 Thus we adopted a 25% difference in insulin clearance as a threshold to distinguish participants with marked or minor changes in insulin clearance induced by the diet (range +42% to +262% and −24% to +13%, respectively) (Figure S1). Group differences were analysed by two‐way ANOVA for repeated measures including group, time (0 week vs. 12 week), and group × time interaction as factors. Categorical variables were compared using the χ 2 test. Correlations of dietary‐induced changes in key metabolic variables were tested using Pearson's correlation. AUCs were calculated using the trapezoidal rule. Analyses were performed using JMP Pro 16.0 (SAS, Cary, NC) at a two‐sided α level of .05.
3. RESULTS
To investigate the mechanisms of reduced insulinaemia after the low n‐6:n‐3 PUFA ratio diet (median insulin: −225 [955] pmol/L, P = .015; Figure 1A) in the absence of significant changes in glucose tolerance (mean glucose: −0.2 ± 0.2 mmol/L, P = .448; Figure 1B) and body weight (−0.5 ± 0.8 kg, P = .523), insulin kinetics was assessed by measuring and modelling plasma C‐peptide levels at enrolment (week 0) and after the 12‐week dietary intervention (week 12) (Figure 1C). Reduced plasma insulin levels during the OGTT were not associated with changes in absolute insulin secretion (Figure 1D,E), insulin secretion relative to plasma glucose (Figure 1F), and model‐derived variables of β‐cell function (Figure 1G‐K). Conversely, endogenous insulin clearance at fasting and during the OGTT was significantly increased at week 12 (Figure 1L,M), without changes in its physiological reduction from the fasting to the fed condition (Figure 1N). As compared with week 0, insulin sensitivity assessed by the OGIS index was increased by the diet at week 12 (Figure 1O).
In the whole cohort, changes in insulin sensitivity were associated with changes in insulin clearance (Figure 2A), whereas changes in HFF% were not associated with changes in insulin clearance (Figure 2B) or insulin sensitivity as measured with the OGIS index (Figure 2C). Furthermore, changes in insulin clearance correlated with changes in β‐cell glucose sensitivity (Figure 2D).
FIGURE 2.
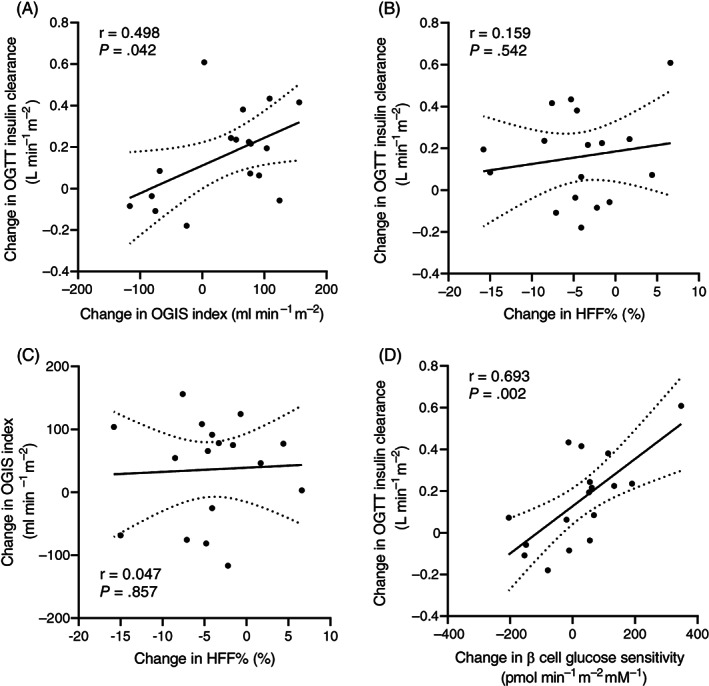
A, Correlation between changes in oral glucose insulin sensitivity (OGIS) index and changes in insulin clearance during the oral glucose tolerance test (OGTT); correlation between changes in hepatic fat fraction (HFF%), and B, Changes in insulin clearance during the OGTT, or C, Changes in OGIS index; and D, Correlation between changes in β‐cell glucose sensitivity and changes in insulin clearance during the OGTT. Changes were calculated as the difference between values measured after and before the 12‐week low n‐6:n‐3 polyunsaturated fatty acid ratio diet. Correlations were tested using Pearson's correlation. Areas between dotted lines indicate 95% confidence intervals of the best‐fit line
To identify predictors and determinants of dietary‐induced changes in insulin clearance, we analysed the clinical and metabolic characteristics of the 10 (59%) participants with a marked increase in postload insulin clearance and the seven (41%) participants with minor positive or negative changes (Tables 1 and 2). The two groups did not differ with respect to their baseline anthropometric characteristics (Table 1). However, adolescents with a marked increase in insulin clearance by week 12 had worse glucose tolerance, reduced β‐cell glucose sensitivity and insulin clearance, and numerically greater insulin resistance and glucose‐stimulated hyperinsulinaemia at enrolment (Figure 3), despite similar fasting glucose and insulin levels, compared with adolescents with minor changes in insulin clearance (Table 2).
TABLE 1.
Clinical and metabolic characteristics of study participants stratified by dietary‐induced changes in postload insulin clearance at enrolment and after the 12‐week intervention
| Marked increase (n = 10) | Minor change (n = 7) | P | |||||
|---|---|---|---|---|---|---|---|
| Week 0 | Week 12 | Week 0 | Week 12 | Group | Time | Interaction | |
| Age (y) | 14.90 [6.60] | – | 12.40 [3.40] | – | Ns | – | – |
| Sex (n, %) | |||||||
| Boys | 4, 40.0% | – | 2, 28.6% | – | Ns | – | – |
| Girls | 6, 60.0% | 5, 71.4% | |||||
| Race or ethnicity (n, %) | |||||||
| Caucasian | 3, 30.0% | – | 4, 57.1% | – | Ns | – | – |
| African American | 2, 20.0% | 0, 0.0 % | |||||
| Hispanic | 5, 50.0% | 3, 42.9% | |||||
| Body weight (kg) | 88.4 ± 23.5 | 87.4 ± 24.1 | 94.5 ± 34.7 | 94.6 ± 34.4 | Ns | Ns | Ns |
| Body mass index (kg/m2) | 34.0 ± 7.3 | 33.4 ± 7.9 | 35.4 ± 8.5 | 35.2 ± 8.7 | Ns | Ns | Ns |
| Body composition | |||||||
| Fat mass (%) | 37.28 ± 14.67 | 41.26 ± 19.28 | 42.18 ± 17.97 | 39.10 ± 13.77 | Ns | Ns | Ns |
| Waist circumference (cm) | 113.2 ± 13.0 | 107.6 ± 17.2 | 113.4 ± 19.9 | 116.8 ± 16.0 | Ns | Ns | Ns |
| Waist‐to‐hip ratio (ratio) | 0.96 [0.07] | 0.98 [0.07] | 1.00 [0.12] | 1.00 [0.04] | Ns | Ns | Ns |
| HFF% (%) | 15.45 [19.10] | 10.85 [20.80] | 12.50 [5.90] | 8.00 [10.50] | Ns | .001 | Ns |
| VAT (cm2) | 83.9 ± 40.9 | 71.9 ± 37.3 | 79.2 ± 30.8 | 79.0 ± 33.2 | Ns | Ns | Ns |
| SAT (cm2) | 495.2 [303.2] | 496.1 [167.5] | 590.9 [435.6] | 669.1 [382.3] | Ns | Ns | Ns |
| Alanine aminotransferase (U/L) | 28 [27] | 13 [19] | 46 [43] | 22 [38] | Ns | .007 | .089 |
| Lipid profile | |||||||
| Total cholesterol (mg/dl) | 152.8 ± 17.6 | 143.9 ± 15.7 | 169.6 ± 29.8 | 153.4 ± 25.5 | Ns | .067 | Ns |
| HDL cholesterol (mg/dl) | 38.6 ± 4.7 | 38.8 ± 6.6 | 43.3 ± 10.7 | 39.1 ± 10.1 | Ns | .001 | .029 |
| LDL cholesterol (mg/dl) | 83.6 ± 10.3 | 80.8 ± 13.2 | 94.9 ± 26.0 | 86.4 ± 15.8 | Ns | Ns | Ns |
| Triglycerides (mg/dl) | 133 [133] | 100 [90] | 130 [208] | 111 [58] | Ns | Ns | Ns |
Note: Continuous variables are reported as median [interquartile range] or mean ± SD. Categorical variables are reported as count (%). Group differences were analysed by two‐way ANOVA for repeated measures including group, time, and group × time interaction as factors. P values less than .10 are reported.
Abbreviations: HDL, high‐density lipoprotein; HFF%, hepatic fat fraction; LDL, low‐density lipoprotein; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
TABLE 2.
Glucose tolerance and insulin metabolism at enrolment and after the 12‐week intervention in study participants stratified by dietary‐induced changes in postload insulin clearance
| Marked increase (n = 10) | Minor change (n = 7) | P | |||||
|---|---|---|---|---|---|---|---|
| Week 0 | Week 12 | Week 0 | Week 12 | Group | Time | Interaction | |
| Glucose control | |||||||
| Fasting glucose (mmol/L) | 4.82 [0.72] | 4.83 [0.34] | 4.86 [0.71] | 5.00 [0.44] | Ns | Ns | Ns |
| 2‐h glucose (mmol/L) | 6.76 [2.03] | 6.56 [1.19] | 6.16 [1.39] | 5.72 [0.89] | .021 | Ns | Ns |
| Mean glucose (mmol/L) | 7.20 [1.58] | 6.70 [0.90] | 6.33 [1.27] | 6.04 [1.58] | .034 | Ns | Ns |
| Fasting insulin (pmol/L) | 241 [106] | 169 [251] | 294 [192] | 249 [190] | Ns | Ns | Ns |
| Mean insulin (pmol/L) | 1518 [1003] | 999 [786] | 802 [703] | 934 [709] | .054 | Ns | .005 |
| β‐cell function | |||||||
| Fasting ISR (pmol m−2 min−1) | 183 [106] | 161 [110] | 179 [82] | 152 [99] | Ns | Ns | Ns |
| Total ISR (nmol/m2) | 75 [53] | 96 [39] | 91 [34] | 76 [22] | Ns | Ns | .092 |
| β‐GS (pmol min−1 m−2 mM−1) | 100 [39] | 134 [52] | 195 [143] | 169 [27] | .006 | Ns | .042 |
| ISR@5 (pmol min−1 m−2) | 189 [135] | 189 [84] | 239 [159] | 176 [59] | .086 | .002 | .005 |
| β‐RS (nmol m−2 mM−1) | 2005 [1102] | 1935 [1498] | 3052 [1617] | 2751 [2668] | Ns | Ns | Ns |
| Potentiation factor ratio | 1.18 ± 0.60 | 1.16 ± 0.24 | 0.81 ± 0.31 | 0.96 ± 0.31 | Ns | Ns | Ns |
| Insulin sensitivity | |||||||
| HOMA‐IR (unit) | 8.24 [7.01] | 6.13 [8.84] | 11.23 [7.39] | 8.91 [7.08] | Ns | Ns | Ns |
| OGIS (ml min−1 m−2) | 287 ± 93 | 364 ± 99 | 363 ± 92 | 342 ± 51 | .089 | Ns | .010 |
| WBISI (unit) | 1.03 [0.75] | 1.28 [1.23] | 1.44 [1.47] | 1.26 [2.16] | .087 | Ns | .048 |
| Insulin clearance | |||||||
| Fasting clearance (L min−1 m−2) | 0.65 ± 0.33 | 1.00 ± 0.47 | 0.84 ± 0.32 | 0.87 ± 0.26 | Ns | Ns | .034 |
| OGTT clearance (L min−1 m−2) | 0.30 ± 0.14 | 0.60 ± 0.21 | 0.56 ± 0.20 | 0.51 ± 0.23 | .002 | .046 | <.0001 |
Note: Data are reported as median [interquartile range] or mean ± SD. Group differences were analysed by two‐way ANOVA for repeated measures including group, time, and group × time interaction as factors. P values less than .10 are reported.
Abbreviations: β‐GS, β‐cell glucose sensitivity; β‐RS, β‐cell rate sensitivity; HOMA‐IR, homeostasis model assessment of insulin resistance; ISR, insulin secretion rate; ISR@5, ISR at a fixed fasting plasma glucose of 5 mmol/L; OGIS, oral glucose insulin sensitivity; OGTT, oral glucose tolerance test; WBISI, whole‐body insulin sensitivity index.
FIGURE 3.
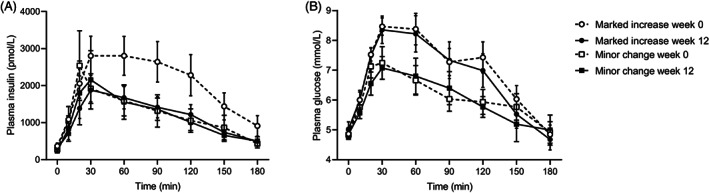
A, Plasma insulin, and B, Glucose concentrations in response to a 75‐g oral glucose tolerance test at baseline (week 0) and after the low n‐6:n‐3 polyunsaturated fatty acid ratio diet (week 12) in participants with marked increase (> 25% from baseline) or minor change (± 25%) in postload insulin clearance. Data are reported as mean ± SEM
The 12‐week diet induced similar reductions in HFF%, alanine aminotransferase, and fasting insulin secretion in the two groups (Table 1). Insulin sensitivity, β‐cell glucose sensitivity, and glucose‐stimulated hyperinsulinaemia improved after the diet only in the subjects with marked increases in insulin clearance (Table 2, Figure 3). Conversely, HDL cholesterol, ISR@5, and β‐cell glucose sensitivity declined in subjects with minor changes in insulin clearance (Tables 1 and 2).
4. DISCUSSION
Our findings show that a eucaloric dietary intervention high in n‐3 and low in n‐6 PUFA, compared with the ratio of a typical Western diet, can reduce hyperinsulinaemia by increasing fasting and postload insulin clearance in obese youth with NAFLD, in the absence of weight loss and change in model‐derived insulin secretory function. This effect could be related to dietary‐induced reductions in intrahepatic fat content and insulin resistance, as well as improvements in β‐cell function. To provide mechanistic insight and shed light on the heterogeneity across study participants, we stratified participants by percentage changes in insulin clearance induced by the diet. Those subjects with greater improvements (> 25%) in insulin clearance showed a worse metabolic profile at enrolment and benefitted more from the dietary intervention. In particular, improvements in β‐cell glucose sensitivity were observed in subjects with marked increases in insulin clearance and insulin sensitivity.
The reduction of postload insulin concentrations after the 12‐week intervention was not accompanied by a reduction of total insulin secretion, suggesting that the restored insulin clearance was mainly responsible for the reduced hyperinsulinaemia. This supports the hypothesis that insulin clearance plays a key role as a determinant of chronic hyperinsulinaemia in obese youth. In fact, insulin clearance is typically reduced in insulin‐resistant obese individuals, 34 , 35 even in the absence of dysglycaemia, with wide interindividual variability. The recent RISE study has shown that insulin clearance is almost 25% lower in youth with prediabetes or recent‐onset diabetes compared with the adults. 33 Given that hepatic insulin extraction, which accounts for the majority (~80%) of endogenous insulin clearance, showed a saturable process influenced by insulin delivery into the portal system, previous findings may be explained by the insulin hypersecretory state of the adolescents. 33 By contrast, the rise in insulin clearance after the low n‐6:n‐3 PUFA ratio diet was not dependent on lower insulin delivery rates, as insulin secretion tended to increase, rather than decrease, particularly in subjects with marked increases in insulin clearance (Table 2).
The beneficial effects of the low n‐6:n‐3 PUFA ratio diet on insulin clearance and sensitivity were more evident in adolescents at increased metabolic risk, characterized by impaired insulin clearance, β‐cell function, and glucose tolerance. Noteworthy, modifications in insulin clearance occurred in the absence of weight loss, but were related to changes in insulin sensitivity. This finding confirms the evidence that insulin extraction is more closely associated with insulin resistance than body weight per se 2 , 8 , 36 and proves the ability to achieve important metabolic benefits without the need for caloric restriction in this population at high risk for type 2 diabetes.
Accumulation of intrahepatic fat has been associated with multiple metabolic abnormalities in large study cohorts, including reduced hepatic insulin clearance 6 , 19 , 37 , 38 , 39 , 40 and impaired insulin sensitivity. 6 , 40 , 41 , 42 However, we cannot definitively conclude that the reduction of intrahepatic fat content observed in this study was causally linked with the improvements in insulin clearance and insulin sensitivity as all the changes appeared to occur almost in parallel and given the lack of significant correlations between changes in liver fat content and either insulin clearance or insulin sensitivity. Plausible mechanisms linking these three metabolic alterations (i.e. liver steatosis, reduced insulin clearance, and insulin resistance) involve the carcino‐embryonic antigen‐related cell adhesion molecule 1 (CEACAM1), a transmembrane glycoprotein that promotes receptor‐mediated insulin uptake and degradation and downregulates fatty acid synthase (FAS) activity in the liver. 5 CEACAM1 expression in primary hepatocytes is inhibited by treatment with linoleic acid, the most abundant n‐6 PUFA. 43 Furthermore, removing n‐3 PUFA from the diet, but not n‐6 PUFA, induces hepatic insulin resistance and liver steatosis in mice by decreasing fatty acid oxidation and increasing de novo lipogenesis via a FAS‐mediated mechanism. 44 Thus it can be speculated that the low n‐6:n‐3 PUFA ratio diet may reduce liver steatosis and promote hepatic insulin sensitivity and clearance via a CEACAM1‐mediated mechanism; this hypothesis, however, will need further mechanistic studies to be explored.
Most previous studies support a positive effect of n‐3 PUFA on insulin secretion and β‐cell viability, 45 , 46 , 47 although contrasting evidence exists. 48 Herein, we describe a strong positive correlation between dietary‐induced changes in β‐cell glucose sensitivity and insulin clearance in obese youth. This result, consistent with some, 8 but not all, 4 , 49 previous findings in adults, supports a bidirectional relationship between insulin clearance and β‐cell function, so that reduced insulin clearance may represent an adaptive mechanism to mitigate the effects of β‐cell dysfunction, a key factor in diabetes progression, or primary changes in insulin clearance may be perceived by the β cells, which adjust their insulin secretory function accordingly.
The strengths of this study include the accurate estimation of insulin secretion and β‐cell function variables by C‐peptide modelling, the assessment of abdominal fat distribution using MRI, the longitudinal metabolic assessment using an eucaloric, child‐friendly nutritional intervention, and the young age of our patients, who are virtually free from major confounders that may affect glucose or insulin metabolism (e.g. alcohol consumption and tobacco smoking). Moreover, the low n‐6:n‐3 PUFA ratio diet provided us with a unique physiological model to examine in vivo the changes in metabolic phenotypes induced by modifications in plasma insulin levels and insulin clearance in the absence of changes in body weight, glucose tolerance, and insulin secretion.
Our study has also some limitations. The method used to evaluate insulin clearance provides an estimate of endogenous insulin clearance under less controlled but more physiological conditions compared with other methods using intravenous insulin and glucose infusions, and has been validated against model‐derived estimates of hepatic insulin clearance using hyperinsulinaemic‐euglycaemic clamp data in obese youths. 6 However, this method does not allow to dissect the peripheral from the hepatic components of insulin clearance. We also acknowledge that the derivation of insulin clearance, insulin sensitivity, and β‐cell function from the same OGTT data might not yield fully independent indices. Additionally, the use of OGTT‐derived insulin sensitivity indices does not allow the identification of peripheral versus hepatic insulin sensitivity contributions to the insulin‐resistant phenotype. The other limitations are the limited sample size and the short study duration, which cannot discern the transient effects of the dietary intervention from a persistent action on both fatty liver content and insulin kinetics, nor the longitudinal trajectory of insulin clearance, insulin sensitivity, and β‐cell function after the end of the intervention.
In conclusion, we showed that a 12‐week low n‐6:n‐3 PUFA ratio diet improves hyperinsulinaemia by increasing fasting and postload insulin clearance in insulin‐resistant obese youth, in the absence of significant changes in body weight, glucose tolerance, and average insulin secretion.
CONFLICT OF INTEREST
The authors have no conflicts of interest pertinent to this study.
AUTHOR CONTRIBUTIONS
DT: study design, data analysis, interpretation of results, and drafting of the manuscript. AG: data analysis, interpretation of results, and drafting of the manuscript. MAVN, SS, and ZL: enrolment of patients and data collection. BTG: data collection, data analysis, and manuscript editing. MS: diet design, nutritional assessment, and dietary instruction. AM and AEF: interpretation of results and manuscript editing. SC and NS: funding, study design, enrolment of patients, interpretation of results, manuscript drafting and editing, and study supervision.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14695.
Supporting information
Figure S1
ACKNOWLEDGEMENTS
The authors are grateful to the patients and their families as well as to the Yale Center for Genome Analysis, Yale Center for Clinical Investigation, Yale Core Lab, and Hospital Research Unit at the Yale New Haven Hospital. This study was funded by the National Institutes of Health (grants R01MD015974 and R01DK114504 to NS, grant R01DK111038 to SC) and the American Heart Association (grant 13SDG14640038 to NS). This work was also made possible by DK‐045735 to the Yale Diabetes Endocrinology Research Center and by Clinical and Translational Science Awards Grant UL1‐RR‐024139 from the National Center for Advancing Translational Sciences, a component of the NIH, and NIH Roadmap for Medical Research. The contents of this scientific contribution are solely the responsibility of the authors and do not necessarily represent the official view of the NIH. Open Access Funding provided by Universita degli Studi di Pisa within the CRUI‐CARE Agreement.
Tricò D, Galderisi A, Van Name MA, et al. A low n‐6 to n‐3 polyunsaturated fatty acid ratio diet improves hyperinsulinaemia by restoring insulin clearance in obese youth. Diabetes Obes Metab. 2022;24(7):1267‐1276. doi: 10.1111/dom.14695
Domenico Tricò and Alfonso Galderisi contributed equally to this work.
Funding information American Heart Association, Grant/Award Number: 13SDG14640038; National Institutes of Health, Grant/Award Numbers: DK‐045735, R01DK111038, R01DK114504, R01MD015974, UL1‐RR‐024139
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on reasonable request from the corresponding author.
REFERENCES
- 1. Arslanian SA, Saad R, Lewy V, Danadian K, Janosky J. Hyperinsulinemia in african‐american children: decreased insulin clearance and increased insulin secretion and its relationship to insulin sensitivity. Diabetes. 2002;51(10):3014‐3019. [DOI] [PubMed] [Google Scholar]
- 2. Galderisi A, Polidori D, Weiss R, et al. Lower insulin clearance parallels a reduced insulin sensitivity in obese youths and is associated with a decline in beta‐cell function over time. Diabetes. 2019;68(11):2074‐2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Meistas MT, Margolis S, Kowarski AA. Hyperinsulinemia of obesity is due to decreased clearance of insulin. Am J Physiol. 1983;245(2):E155‐E159. [DOI] [PubMed] [Google Scholar]
- 4. Trico D, Natali A, Silva Arslanian AM, Ferrannini E. Identification, pathophysiology, and clinical implications of primary insulin hypersecretion in nondiabetic adults and adolescents. JCI Insight. 2018;3(24):e124912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Najjar Sonia M, Perdomo Germán. Hepatic insulin clearance: mechanism and physiology. Physiology. 2019;34(3):198‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Trico D, Galderisi A, Mari A, et al. Intrahepatic fat, irrespective of ethnicity, is associated with reduced endogenous insulin clearance and hepatic insulin resistance in obese youths: a cross‐sectional and longitudinal study from the Yale pediatric NAFLD cohort. Diabetes Obes Metab. 2020;22(9):1628‐1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Utzschneider KM, Kahn SE, Polidori DC. Hepatic insulin extraction in NAFLD is related to insulin resistance rather than liver fat content. J Clin Endocrinol Metab. 2019;104(5):1855‐1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bizzotto R, Tricò D, Natali A, et al. New insights on the interactions between insulin clearance and the Main glucose homeostasis mechanisms. Diabetes Care. 2021;44(9):2115‐2123. [DOI] [PubMed] [Google Scholar]
- 9. Wiesenthal SR, Sandhu H, McCall RH, et al. Free fatty acids impair hepatic insulin extraction in vivo. Diabetes. 1999;48(4):766‐774. [DOI] [PubMed] [Google Scholar]
- 10. Mittelman SD, van Citters GW, Kim SP, et al. Longitudinal compensation for fat‐induced insulin resistance includes reduced insulin clearance and enhanced beta‐cell response. Diabetes. 2000;49(12):2116‐2125. [DOI] [PubMed] [Google Scholar]
- 11. Dangardt F, Chen Y, Gronowitz E, Dahlgren J, Friberg P, Strandvik B. High physiological omega‐3 fatty acid supplementation affects muscle fatty acid composition and glucose and insulin homeostasis in obese adolescents. J Nutr Metab. 2012;2012:395757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lundsgaard AM, Sjøberg KA, Høeg LD, et al. Opposite regulation of insulin sensitivity by dietary lipid versus carbohydrate excess. Diabetes. 2017;66(10):2583‐2595. [DOI] [PubMed] [Google Scholar]
- 13. Suzuki R, Tamura Y, Takeno K, et al. Three days of a eucaloric, low‐carbohydrate/high‐fat diet increases insulin clearance in healthy non‐obese Japanese men. Sci Rep. 2019;9(1):3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Trico D, Trifirò S, Mengozzi A, et al. Reducing cholesterol and fat intake improves glucose tolerance by enhancing beta cell function in nondiabetic subjects. J Clin Endocrinol Metab. 2018;103(2):622‐631. [DOI] [PubMed] [Google Scholar]
- 15. Lundsgaard A‐M, Holm JB, Sjøberg KA, et al. Mechanisms preserving insulin action during high dietary fat intake. Cell Metab. 2019;29(1):50‐63. [DOI] [PubMed] [Google Scholar]
- 16. Tricò D, Moriconi D, Berta R, et al. Effects of low‐carbohydrate versus Mediterranean diets on weight loss, glucose metabolism, insulin kinetics and β‐cell function in morbidly obese individuals. Nutrients. 2021;13(4):1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nesti L, Mengozzi A, Trico D. Impact of nutrient type and sequence on glucose tolerance: physiological insights and therapeutic implications. Front Endocrinol. 2019;10:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bacha F, Gungor N, Lee S, Arslanian SA. Type 2 diabetes in youth: Are there racial differences in β‐cell responsiveness relative to insulin sensitivity? Pediatr Diabetes. 2012;13(3):259‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kotronen A, Juurinen L, Tiikkainen M, Vehkavaara S, Yki–Järvinen H. Increased liver fat, impaired insulin clearance, and hepatic and adipose tissue insulin resistance in type 2 diabetes. Gastroenterology. 2008;135(1):122‐130. [DOI] [PubMed] [Google Scholar]
- 20. Umano GR, Shabanova V, Pierpont B, et al. A low visceral fat proportion, independent of total body fat mass, protects obese adolescent girls against fatty liver and glucose dysregulation: a longitudinal study. Int J Obes. 2019;43(4):673‐682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gower BA, Granger WM, Franklin F, Shewchuk RM, Goran MI. Contribution of insulin secretion and clearance to glucose‐induced insulin concentration in African‐American and Caucasian children. J Clin Endocrinol Metab. 2002;87(5):2218‐2224. [DOI] [PubMed] [Google Scholar]
- 22. Trico D, Di Sessa A, Caprio S, et al. Oxidized derivatives of linoleic acid in pediatric metabolic syndrome: is their pathogenic role modulated by the genetic background and the gut microbiota? Antioxid Redox Signal. 2019;30(2):241‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Van Name MA, Savoye M, Chick JM, et al. A low ω‐6 to ω‐3 PUFA ratio (n‐6:n‐3 PUFA) diet to treat fatty liver disease in obese youth. J Nutr. 2020;150(9):2314‐2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leonetti S, Herzog RI, Caprio S, Santoro N, Tricò D. Glutamate‐serine‐glycine index: a novel potential biomarker in pediatric non‐alcoholic fatty liver disease. Children. 2020;7(12):270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fishbein MH, Gardner KG, Potter CJ, Schmalbrock P, Smith MA. Introduction of fast MR imaging in the assessment of hepatic steatosis. Magn Reson Imaging. 1997;15(3):287‐293. [DOI] [PubMed] [Google Scholar]
- 26. Mari A, Pacini G, Murphy E, Ludvik B, Nolan JJ. A model‐based method for assessing insulin sensitivity from the oral glucose tolerance test. Diabetes Care. 2001;24(3):539‐548. [DOI] [PubMed] [Google Scholar]
- 27. Mari A, Pacini G, Brazzale AR, Ahrén B. Comparative evaluation of simple insulin sensitivity methods based on the oral glucose tolerance test. Diabetologia. 2005;48(4):748‐751. [DOI] [PubMed] [Google Scholar]
- 28. Yeckel CW, Weiss R, Dziura J, et al. Validation of insulin sensitivity indices from oral glucose tolerance test parameters in obese children and adolescents. J Clin Endocrinol Metab. 2004;89(3):1096‐1101. [DOI] [PubMed] [Google Scholar]
- 29. Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C‐peptide levels. Comparison of individual and standard kinetic parameters for C‐peptide clearance. Diabetes. 1992;41(3):368‐377. [DOI] [PubMed] [Google Scholar]
- 30. Mari A, Ferrannini E. Beta‐cell function assessment from modelling of oral tests: an effective approach. Diabetes Obes Metab. 2008;10(Suppl 4):77‐87. [DOI] [PubMed] [Google Scholar]
- 31. Mengozzi A, Tricò D, Nesti L, et al. Disruption of fasting and post‐load glucose homeostasis are largely independent and sustained by distinct and early major beta‐cell function defects: a cross‐sectional and longitudinal analysis of the relationship between insulin sensitivity and cardiovascular risk (RISC) study cohort. Metabolism. 2020;105:154185. [DOI] [PubMed] [Google Scholar]
- 32. Galderisi A, Tricò D, Dalla Man C, et al. Metabolic and genetic determinants of glucose shape after Oral challenge in obese youths: a longitudinal study. J Clin Endocrinol Metab. 2020;105(2):534‐542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ehrmann DA, Temple KA, Rue A, et al. Metabolic contrasts between youth and adults with impaired glucose tolerance or recently diagnosed type 2 diabetes: I. observations using the hyperglycemic clamp. Diabetes Care. 2018;41(8):1696‐1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim Mee K, Reaven Gerald M, Kim Sun H. Dissecting the relationship between obesity and hyperinsulinemia: role of insulin secretion and insulin clearance. Obesity. 2017;25(2):378‐383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Valera Mora ME, Scarfone A, Calvani M, Greco AV, Mingrone G. Insulin clearance in obesity. J Am Coll Nutr. 2003;22(6):487‐493. [DOI] [PubMed] [Google Scholar]
- 36. Ramel A, Martinéz A, Kiely M, Morais G, Bandarra NM, Thorsdottir I. Beneficial effects of long‐chain n‐3 fatty acids included in an energy‐restricted diet on insulin resistance in overweight and obese European young adults. Diabetologia. 2008;51(7):1261‐1268. [DOI] [PubMed] [Google Scholar]
- 37. Matsubayashi Y, Yoshida A, Suganami H, et al. Role of fatty liver in the association between obesity and reduced hepatic insulin clearance. Diabetes Metab. 2018;44(2):135‐142. [DOI] [PubMed] [Google Scholar]
- 38. Bril F, Lomonaco R, Orsak B, et al. Relationship between disease severity, hyperinsulinemia, and impaired insulin clearance in patients with nonalcoholic steatohepatitis. Hepatology. 2014;59(6):2178‐2187. [DOI] [PubMed] [Google Scholar]
- 39. Kotronen A, Vehkavaara S, Seppälä‐Lindroos A, Bergholm R, Yki‐Järvinen H. Effect of liver fat on insulin clearance. Am J Physiol Endocrinol Metab. 2007;293(6):E1709‐E1715. [DOI] [PubMed] [Google Scholar]
- 40. Gastaldelli A, Cusi K, Pettiti M, et al. Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology. 2007;133(2):496‐506. [DOI] [PubMed] [Google Scholar]
- 41. Korenblat KM, Fabbrini E, Mohammed BS, Klein S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 2008;134(5):1369‐1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Seppala‐Lindroos A, Vehkavaara S, Häkkinen AM, et al. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab. 2002;87(7):3023‐3028. [DOI] [PubMed] [Google Scholar]
- 43. Ramakrishnan SK, Russo L, Ghanem SS, et al. Fenofibrate decreases insulin clearance and insulin secretion to maintain insulin sensitivity. J Biol Chem. 2016;291(46):23915‐23924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tomé D, Essaghir A, Demoulin JB, et al. Hepatic n‐3 polyunsaturated fatty acid depletion promotes steatosis and insulin resistance in mice: genomic analysis of cellular targets. PLoS ONE. 2011;6(8):e23365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Albert BB, Derraik JG, Brennan CM, et al. Higher omega‐3 index is associated with increased insulin sensitivity and more favourable metabolic profile in middle‐aged overweight men. Sci Rep. 2014;4:6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bi X, Li F, Liu S, et al. Omega‐3 polyunsaturated fatty acids ameliorate type 1 diabetes and autoimmunity. J Clin Invest. 2017;127(5):1757‐1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jacobo‐Cejudo MG, Valdés‐Ramos R, Guadarrama‐López AL, Pardo‐Morales RV, Martínez‐Carrillo BE, Harbige LS. Effect of n‐3 polyunsaturated fatty acid supplementation on metabolic and inflammatory biomarkers in type 2 diabetes mellitus patients. Nutrients. 2017;9(6):573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Holness MJ, Greenwood GK, Smith ND, Sugden MC. Diabetogenic impact of long‐chain ω‐3 fatty acids on pancreatic β‐cell function and the regulation of endogenous glucose production. Endocrinology. 2003;144(9):3958‐3968. [DOI] [PubMed] [Google Scholar]
- 49. Pivovarova O, Bernigau W, Bobbert T, et al. Hepatic insulin clearance is closely related to metabolic syndrome components. Diabetes Care. 2013;36(11):3779‐3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Data Availability Statement
The data that support the findings of this study are available on reasonable request from the corresponding author.


