Abstract
Background:
Depressive symptoms in patients with cancer are associated with poor quality of life and decreased survival. While inflammation is reliably associated with depression in otherwise healthy individuals, the association in patients with cancer remains unclear. Given the high prevalence of cancer-related inflammation, we aimed to establish the relationship between inflammation and depression in cancer patients based on extant literature.
Methods:
A systematic review and meta-analysis was performed using PRISMA 2020 guidelines and registered under Prospero ID CRD42021226743. Three databases were searched including PubMed, the Cochrane Library, and PsycINFO using the following criteria for inclusion: 1) measurement of a peripheral inflammatory marker; 2) use of a validated tool/scale to measure depression; and 3) a cancer diagnosis. Risk of publication bias was assessed by Funnel plot and Egger test.
Results:
Seventy-three studies were included in the systematic review and 54 studies (n= 5,017) were included in meta-analyses. Associations with depressive symptoms were significant for peripheral blood IL-6 (SMD 0.59; 95% CI, 0.35-0.82), I2=57.9%; TNF (SMD 0.73; 95% CI, 0.35-1.11), I2=74.1%; and C-reactive protein (CRP) (SMD 0.57; 95% CI, 0.27-0.87), I2=0%. IL-5, IL-13, albumin, and neutrophil-to-lymphocyte ratio were associated with depressive symptoms but based on fewer studies. Most cancer settings were represented; number of studies per inflammatory marker varied from one to 52.
Conclusions:
Although peripheral inflammatory markers were unevenly studied, the most studied markers (IL-6, TNF, CRP) were associated with depressive symptoms in cancer patients and may be useful for management of depressive symptoms in the cancer setting.
Keywords: inflammation, Depressive symptoms, C-Reactive Protein, Tumor Necrosis Factor, Interleukin-6, Depression, Cancer, meta-analysis
Precis:
Peripheral blood inflammatory markers (IL-6, TNF, CRP) were associated with depressive symptoms in various cancer settings. Although further studies are warranted, these findings may help identify and manage depressive symptoms in patients with cancer.
Lay Summary:
Peripheral blood inflammatory markers (IL-6, TNF, CRP) were associated with depressive symptoms in various cancer settings. Although further studies are warranted, these findings may help identify and manage depressive symptoms in patients with cancer.
Introduction:
Increasing data suggest that inflammation may play a role in depression.1 Medically healthy depressed patients reliably exhibit increases in inflammatory markers in the peripheral blood and cerebrospinal fluid.2,3 In addition, treatment of patients with cancer or infectious diseases with inflammatory cytokines as well as administration of other inflammatory stimuli including endotoxin and typhoid vaccination to healthy volunteers induces depressive symptoms.4 Moreover, inhibition of inflammation by anti-cytokine and other anti-inflammatory agents has been shown to reduce depressive symptoms in several patient populations.5,6
Patients with cancer experience numerous neuropsychiatric symptoms including depression, which is associated with poor quality of life and decreased survival.7,8 Cancer-related depression occurs in approximately 25% of patients, and 6-13% of cancer patients meet diagnostic criteria for major depression cross-sectionally.9,10 High rates of depression in patients with cancer contrasts strikingly with a 10% lifetime prevalence of depression in community non-cancer settings.11 Of note, depression is often under-diagnosed and inadequately treated despite its ubiquitous presence across cancer subtypes and the cancer trajectory.12 This shortcoming is even more pronounced in the context of health inequities due to socioeconomic and racial factors.13
One pathway to depression in cancer patients may be inflammation. Cancer and its treatment are often associated with increased circulating levels of biomarkers of inflammation.14 To date, several variables limit a cohesive understanding of inflammation and depression in the cancer context. Studies have generally relied on disparate markers of inflammation, unclear measurement of depression, and various settings in which the association was evaluated (e.g., during or after cancer treatments), making it challenging to assess the extent to which depression may be associated with inflammation in cancer patients.
Accordingly, the goals of this systematic review and meta-analysis were to 1) identify peripheral inflammatory biomarkers that are associated with depressive symptoms and which markers warrant further exploration; 2) assess which cancer types (e.g., breast, lung, prostate cancers), stage (e.g., localized versus metastatic cancer) and settings (e.g., receiving radiation or systemic therapy, before and after surgery) have been most thoroughly studied; 3) evaluate the consistency of depressive symptom measurement: and 4) assess the association between inflammation and depression based on the primary study outcome (i.e., depression versus non-depression primary outcome). We hypothesized that multiple measures of inflammation would be associated with depressive symptoms in patients with both localized and metastatic cancers, regardless of primary study outcome and that the relationship would be quantifiable.
Materials and Methods
This study was comprised of a systemic review and between-group meta-analyses of studies that evaluated the association between inflammation and depression in patients with cancer. We complied with the Preferred Reported Items for Systematic Reviews and Meta-Analysis (PRISMA) 2020 and registered under Prospero ID CRD42021226743.15 The literature search, title/abstract review, and data extraction were independently performed by two authors (DM, MD).
Search Methods
This research was conducted following the PRISMA 2020 guidelines.15 We developed our search strategy with an experienced medical sciences librarian (RO). On November 23rd, 2020, three databases were searched: PubMed (See Supplement 1), the Cochrane Library (Wiley), and PsycINFO (Ovid) to identify potentially relevant studies. The search had three main categories, combined using the AND operator: (1) depression (2) inflammation and (3) cancer. In PubMed, we used the Cochrane Handbook filter for excluding animal-only studies.16 We searched for articles in all available languages and with no date or publication type restrictions. We saved all references to the citation management software EndNote (Clarivate Analytics), removed duplicates following the Bramer Method,17 and screened all references using the systematic review management software Covidence.18 An updated search using the same strategy on PubMed was performed on January 15th, 2022.
Eligibility criteria
We included descriptive or observational studies (i.e., non-interventional studies) where measures of depression and inflammation were valid, and a statistical inference between these variables was made. Observational studies were defined as using data from an existing database, a cross-sectional study, a case series, a case-control design, a design with historical controls or a cohort design. Observational designs may lack the experimental element of a random allocation to an intervention and rely on studies of association between changes or differences in one characteristic (e.g., an exposure or intervention) and changes or differences in an outcome of interest. The studies had to include patients who were diagnosed with cancer (e.g., localized, metastatic, or after completion of cancer therapies). The primary outcome did not have to be depression, but depressive symptoms had to be measured by a validated depression scale. Observational studies that allowed for ongoing anticancer treatments were allowed. However, cancer treatments that directly targeted the immune system or inflammation were excluded (e.g., interferon and IL-2 studies) as were symptom intervention studies that could directly influence either inflammation or depression (e.g., exercise, antidepressant, psychotherapy studies).
Measures
The primary measure was depression or depressive symptoms evaluated with inflammation. Only validated measures of depressive symptoms were included. Measures validated for the identification of depressive symptoms have been developed to be administered in various populations of patients to identify depressive symptoms. Comparators included inflammatory markers as defined by inclusion criteria. Also, statistical inference had to be presented in the article. Articles that measured depression and inflammation without inferential statistical analyses were excluded.
Data extraction
The initial literature search was done by one author (DM). Two authors (DM/MD) then retrieved and independently screened full-text articles. Conflicts over inclusion were resolved through discussion. Data were extracted by two authors (DM/MD) and checked by other authors. Data extraction included a quality assessment in addition to bibliographical data, description of participants, description of any intervention and control group, psychometric data collected and outcomes.
Quality Assessment
A modified Downs and Black Scale was adapted for observational studies and carried out by two authors (DM/MD). Intervention questions were excluded leaving 16 of 27 total questions.19 This modified version of 17 points maintained the same original scale sections: study reporting; internal validity/bias and confounding; and external validity. Each paper was assigned a quality grade of “excellent” (15-17), “good” (12-14), “fair” (9-11), or “poor” (<9).
Risk of Publication Bias Assessment
Studies that were included in the meta-analysis were evaluated for risk of publication bias using funnel plot analyses and Egger tests. Funnel plot asymmetry was tested with a rank correlation test to ascertain publication bias.
Data Qualitative and Quantitative Syntheses and Analyses
Study characteristics were analyzed to assess differences in number and types of inflammatory markers, depression measures, cancer settings, demographics, and study conclusions. Separate meta-analyses were performed for individual inflammatory biomarkers if data were available from two or more studies. To explore potential sources of heterogeneity, inflammatory markers that were evaluated by 10 or more studies were assessed for sub-group differences based on the following: 1) localized versus metastatic cancer and 2) a primary endpoint of depression versus a primary endpoint of a variable other than depression (but with a validated depression scale as a secondary endpoint). Due to different measurement methods and anticipated heterogeneity, effects sizes were converted and reported as Cohen’s d and then calculated by an estimated standardized mean difference (SMD) for each inflammatory marker using random effects modeling conducted by STATA 16.20 Heterogeneity or inconsistency was evaluated across studies using the I2 statistic, with a value of up to 25% as low, up to 50% as medium, and 75% and greater as high heterogeneity. In addition, the Cochran’s Q statistic was calculated for significant heterogeneity across studies. If studies were longitudinal, only baseline data were used to avoid skewed meta-analysis from inclusion of more than one effect size from the same study per inflammatory marker. Of note, each meta-analysis only counted studies one time.
Forest plots were generated for each model to visualize the relative contribution of each study to the SMD. Funnel plots with trim and fill explored potential publication biases. Asymmetry was assessed visually, as Egger’s test of asymmetry could be inaccurate with analyses of less than 10 studies.
Results:
Systematic Review Results
We identified 4,275 papers after duplicates were removed. The screening process excluded 3,795 leaving 480 studies evaluated by full text assessment (Figure 1). The most common reasons for exclusion were evidence of intervention interference with inflammation (n=164), no validated depression outcome (n=80), or study design (e.g., non-human study) (n=107).
Figure 1:
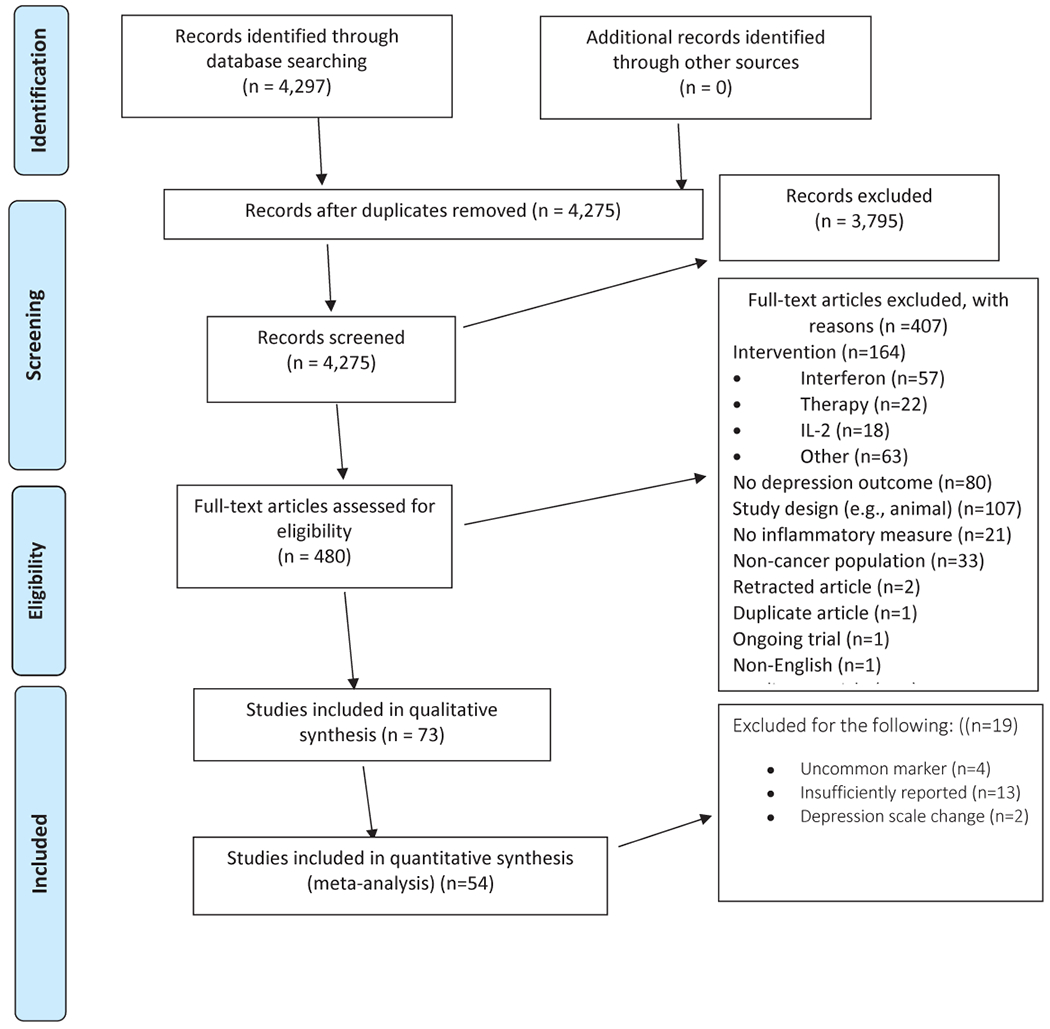
PRISMA Flow Diagram
Seventy-three studies were included in the qualitative synthesis and 54 in the quantitative synthesis (Figure 1). The included studies comprised 6,864 research participants. Nineteen studies were not included in the meta-analyses because the inflammatory biomarker was only reported by one study (n=4), the reported values were insufficiently described (n=13) or the study used altered or non-standardized depression scales (n=2). Overall, 5,017 research participants contributed to the quantitative synthesis (73.1%).
Studies were gathered from 1998 to 2022 with 57 studies (77%) published after 2010. Most commonly, studies originated from the United States (n=32), China (n=10), Brazil (n=4), U.K. (n=4), Germany (n=3), Canada (n=2), Japan (n=2), Taiwan (n=2), and the remaining 12 studies originated from individual countries. The most common tumor types were breast cancer (n=27), mixed solid tumors (n=15), lung cancer (n=10), colorectal carcer (n=5), ovarian cancer (n=4), acute myelogenous leukemia (n=2), gastric cancer (n=2), and testicular cancer (n=2). Several cancer types were represented by only one study and included the following cancers: astrocytoma, hepatobiliary, hepatocellular carcinoma, melanoma, pancreatic, prostate, nasopharyngeal, and renal cell carcinoma. Over half of the studies only included patients with localized or treated cancers (n=39) and the other half included patients with metastatic cancers (n=34). Of the localized cancers, 12 studies analyzed patients in after completion of cancer therapeis (at least one year from last treatment) (30%), eight studies in the pre/post-operative setting (20%), and six studies of patients undergoing radiation therapy (15%).
Study designs were most commonly cross-sectional (n=37), prospective cohort (n=15), case-control (n=13), and longitudinal with repeated measures (n=8). Depression was the primary outcome or endpoint in 43 studies (58%). The most frequently used depression measures were the Hospital Anxiety Depression Scale-Depression (HADS-D) (n=17), Center for Epidemiological Studies Depression (CES-D) (n=18), and the Hamilton Depression Rating Scale (HAM-D) (n=13) (Table 1). Depression caseness was evaluated in 12 studies (18%) while 46 studies evaluated depressive symptoms as a continuous dependent variable (62%) and 15 evaluated both (20%). Seven studies (9%) considered survival outcomes, and four studies included an analysis of depression and survival.21–24
Table 1:
Measures of depression and inflammation.
| Measures of Depression | Cytokine Inflammatory Markers | Non-cytokine Inflammatory markers |
|---|---|---|
| 1. Center for Epidemiological Studies Depression (CES-D), n=18 2. Hospital Anxiety Depression Scale-Depression (HADS-D), n=17 3. Hamilton Depression Rating Scale (HAM-D), n=13 4. Beck Depression Inventory (BDI), n=8 5. Structural Clinical Interview for DSM (Diagnostic & Statistical Manual for Mental Disorders) (SCID), n=6 6. Profile of Mood States (POMS), n=2 7. Hospital Anxiety Depression Scale (HADS), n=3 (full scale) 8. Patient Health Questionnaire-9 (PHQ-9), n=4 9. Depression Self-Rating Scale (DSD), n=2 10. Inventory of Depressive Symptomatology Self Report (IDS-SR), n=2 11. Patient-Reported Outcomes Measurement Information Systems (PROMIS™) Depression, n=1 |
1. IL-6, n=52 2. TNF, n=34 3. IL-1b, n=17 4. IL-10, n=14 5. IFN-gamma, n=12 6. IL-2, n=9 7. IL-5, n=8 8. IL-8, n=8 9. IL-4, n=7 10. IL-17, n=4 11. IL-1a, n=3 12. IL-12, n=3 13. IL-12p70, n=3 14. IL-1ra, n=3 15. IL-13, n=3 16. TGF-β, n=3 17. sTNFr, n=3 18. TGF-α, n=2 19. sIL-2, n=2 20. s-IL-6, n=2 |
1. C-reactive protein, n=18 2. Albumin, n=3 3. NLR, n=2 4. GM-CSF, n=1 5. ESR, n=1 6. Fibrinogen, n=1 7. D-dimer, n=1 8. Monocyte, n=1 chemoattractant protein 1 9. Fractaline, n=1 |
Abbreviations: ESR, erythrocyte stimulating factor; GM-CSF, granulocyte macrophage colony stimulating factor; IFN-gamma, interferon gamma; NLR, neutrophil to lymphocyte ratio; IL, interleukin; sIL-2, soluble interleukin-2; sTNFr, soluble tumor necrosis factor receptor; TGF, tumor growth factor; TNF, tumor necrosis factor
The most frequent measures of inflammation were IL-6 (n=52), TNF (n=34), CRP (n=18), IL-1beta (n=17), IL-10 (n=14), and IFN-gamma (n=12) (Table 1). On average, 4.1 (SD 3.2) inflammatory markers were evaluated per study (median =3). Seventeen studies evaluated only one inflammatory marker (23%), and 22 studies evaluated five or more inflammatory markers (30%).
Quality Assessment:
The average score was 12.5 (SD 3.1) belonging to the ‘good’ category with no quality differences among studies included in the quantitative analysis.
Notable Cancer Settings and Endpoints:
Surgical Settings:
Pre-operatively, five out of six studies (83%) found an association between depression and inflammatory markers before curative-intent surgery.25–28 Post-operatively, three of four studies (75%) revealed an association between depressive symptoms and inflammation.29–32
End of life:
Four studies evaluated patients with advanced cancer who were receiving palliative care without cancer directed treatment.33–36 Two of the four studies (50%) found a positive association between depressive symptoms and inflammation.34,36
During cancer treatments:
Five of six studies (83%) found an association between depression and inflammation while patients were receiving chemotherapy.37–42 All four studies of patients receiving radiation therapy revealed an association between depressive symptoms and inflammation.43–46
Survivorship:
Twelvet studies were in the survivorship setting with nine studies involving patients with breast cancer,47–50 46,51–54 in addition to other studies of testicular, hepatobiliary, and non-small cell lung cancers.23,55,56 Seven of the 12 studies (58%) found an association between inflammation and depressive symptoms.
Survival:
Four studies found an association between depressive symptoms and decreased survival.22–24 Two of the four studies also evaluated the role of inflammation as a mediator of depressive symptoms and worsened survival in advanced cancer settings.22,24
Notable Inflammatory Markers included in only one study (excluded from meta-analysis):
Several unique inflammatory markers were reported in single studies Erythrocyte sedimentation rate (ESR),36 d-dimer,21 and fractaline57 showed a positive association with depression21,36 while fibrinogen was not associated with depressive symptoms.30
Meta-Analysis:
Meta-analyses were performed for individual inflammatory markers (Table 2). Descriptive data are provided for inflammatory markers represented by 10 or more studies (Table 3).
Table 2:
Results of individual meta-analyses of inflammatory markers and depressive symptoms in patients with cancer.
| Marker | # Studies (% of total) | N | Female | SMD (95% CI) | P | Q | (I2) |
|---|---|---|---|---|---|---|---|
| IL-6 | 40 (77%) | 3,349 | 2,313 (69%) | 0.59 (0.35-0.82) | <0.001 | 85.2* | 57.9% |
| TNF | 24(70%) | 2,294 | 1,591 (69%) | 0.73 (0.35-1.11) | <0.001 | 69.9* | 74.1%* |
| IL-1beta | 15 (88%) | 1,250 | 1072 (86%) | 0.44 (−0.05-0.92) | 0.08 | 33.1* | 61.3% |
| CRP | 13 (76%) | 1123 | 737 (66%) | 0.57 (0.27-0.87) | <0.001 | 4.7 | 0.0% |
| IL-10 | 11 (79%) | 961 | 565 (59%) | 0.09 (−0.41-0.23) | 0.67 | 7.56 | 1.32% |
| IFN-gamma | 9 (75%) | 921 | 463 (50%) | 0.11 (0.16-0.38) | 0.43 | 4.8 | 0.0% |
| IL-8 | 8(100%) | 637 | 317 (68%) | 0.11 (−0.001-0.22) | 0.05 | 6.6 | 0.0% |
| IL-4 | 6 (86%) | 345 | 242 (70%) | 0.03 (−0.37-0.44) | 0.93 | 1.4 | 0.0% |
| IL-2 | 6 (67%) | 601 | 304 (51%) | −0.28 (−1.01-0.46) | 0.46 | 16.2* | 68.2% |
| IL-5 | 5 (63%) | 316 | 242 (77%) | −0.69 (−1.14-−0.24) | <.001 | 1.3 | 0.0% |
| IL-12 | 4 (100%) | 260 | 219 (84%) | −0.01 (−0.35-0.33) | 0.96 | 4.3 | 37.4% |
| IL-17 | 4 (100%) | 448 | 419 (94%) | −0.04 (−0.35-0.44) | 0.83 | 1.97 | 0.0% |
| IL-1a | 3 (100%) | 604 | 77 (13%) | 0.38 (−.08-0.83) | 0.10 | 1.2 | 0.0% |
| TGF-β | 3 (100%) | 144 | 53 (37%) | 0.02 (−0.57-0.61) | 0.75 | 0.57 | 0.0% |
| IL-12p70 | 3 (100%) | 233 | 154 (66%) | 0.13 (−0.53-0.80) | 0.70 | 1.7 | 0.0% |
| Albumin | 3 (100%) | 267 | 95 (36%) | −0.67 (−1.12-−0.21) | <0.001 | 0.23 | 0.0% |
| IL-1ra | 3 (100%) | 273 | 202 (74%) | 0.71 (−0.39-1.82) | 0.21 | 1.7 | 0.0% |
| IL-13 | 3 (100%) | 231 | 202 (87%) | −0.56 (−1.04-−0.07) | 0.02 | 0.26 | 0.0% |
| NLR | 2 (100%) | 162 | 83 (51%) | 0.63 (0.15-1.11) | 0.01 | 0.62 | 0.0% |
| TGF-α | 2 (100%) | 156 | 78 (50%) | 0.35 (−0.82-1.52) | 0.56 | 0.01 | 0.0% |
| sIL-2 | 2 (100%) | 104 | 55 (53%) | 0.77 (−1.34-2.88) | 0.47 | 3.41 | 70.7% |
| sTNF-r | 2 (67%) | 131 | 121 (92%) | 0.13 (−1.83-2.10) | 0.90 | .01 | 0.0% |
| sIL-6 | 2 (100%) | 120 | 111 (93%) | 0.58 (−0.18-1.34) | 0.13 | .29 | 0.0% |
Abbreviations: IFN-gamma, interferon gamma; NLR, neutrophil to lymphocyte ratio; IL, interleukin; sIL-2, soluble interleukin-2; sTNFr, soluble tumor necrosis factor receptor; TGF, tumor growth factor; TNF, tumor necrosis factor
Table 3:
Characteristics of inflammatory biomarkers evaluated in more than 10 studies where sub-group analyses were included in the Meta-Analysis of inflammatory marker (IL-6, CRP, TNF-α, and IL-1β) and depression
| IL-6 | TNF | IL-1beta | CRP | |
|---|---|---|---|---|
| Number of studies | 40 | 24 | 15 | 13 |
| Number of participants | 3,349 | 2,294 | 1,250 | 1123 |
| Longitudinal Studies | n=9 (23%) | N=6 (25%) | N=1 (7%) | n=5 (38%) |
| Study Quality | 12.9 (SD 3.3) “good” | 12.1 (SD 3.0) “good” | 12.7 (SD 2.6) “good” | 13.5 (SD 3.1) “good” |
| # of markers tested per study | 4.3 (3.3) | 5.9 (SD 2.9) | 7.5 (SD 5.2) | 3.3 (SD 2.4) |
| Cancer Types (number of studies) |
Localized: Breast (12) CRC (1) HNSCC (1) prostate (1) ovarian (1) testicular (1) Lung (1) Metastatic: Breast (8) CRC (7) Esophagus (5) Gyn (3) HNSCC (1) Leukemia (2) Lung (10) Mixed (1) Ovarian (1) Pancreas (4) Prostate (1) Unknown (1) |
Localized: Breast (9) CRC (1) HNSCC (1) Ovarian (1) Prostate (1) Metastatic: CRC (1) GI (1) Gyn (1) Leukemia (2) Lung (3) Mixed (1) |
Localized: Breast (9) Lung (2) Metastatic Lung (2) pancreatic (1) GI (1) HCC (1) CRC (1) Mixed (1) |
Localized: Breast (4) CRC (1) HNSCC (1) Testicular (1) Lung (1) Metastatic: Lung (3) GI (1) Gyn (1) Leukemia (1) |
Abbreviations: CRC, colorectal cancer, CRP, C-reactive protein; GI, gastrointestinal cancer; Gyne, gynecological cancer; HCC, hepatocellular carcinoma; HNSCC, head and neck squamous cell carcinoma; IL-1beta, interleukin-1beta; IL-6, interleukin-6; TNF, tumor necrosis factor
IL-6:
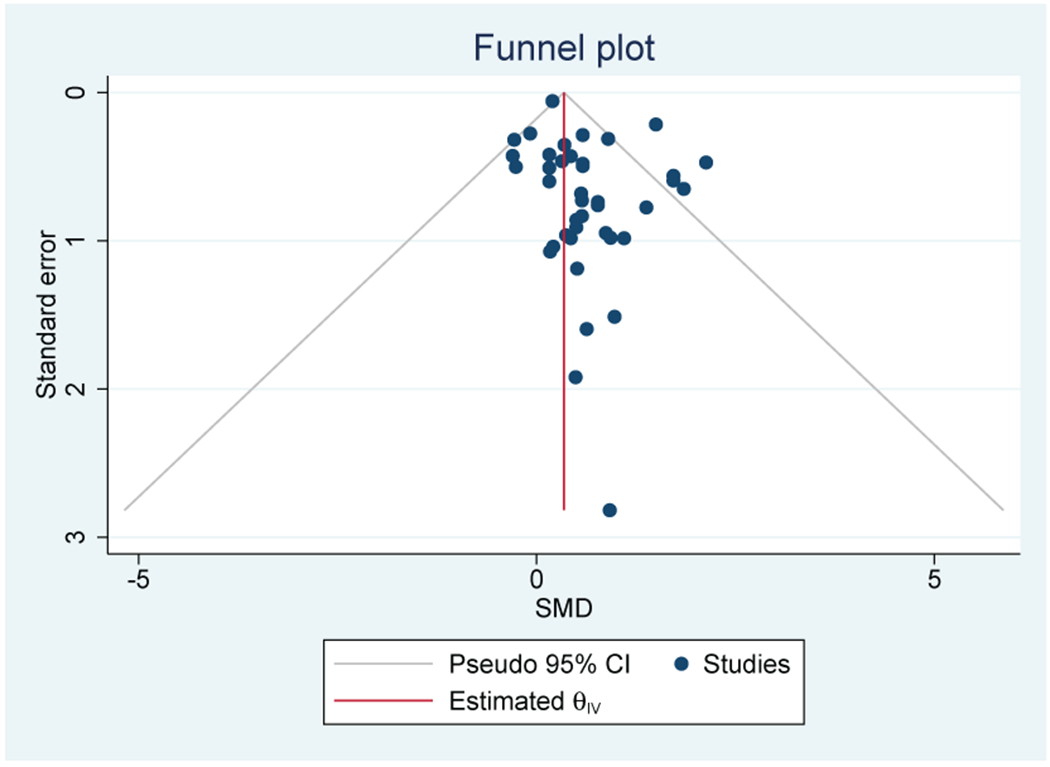
Forty out of 52 studies (76%) that evaluated IL-6 were included (n=3,349) (Table 2). Thirty-one out of 40 studies (78%) reported a positive association between IL-6 and depression confirmed by a medium to large effect size (SMD=0.59; 95% CI, 0.35-0.82) (Figure 2A). Heterogeneity was substantial (I2=57.9%). There was no evidence of publication bias observed on the Funnel Plot (Figure 2B) or Egger’s test. Breast (n=15), GI (n=6), mixed solid tumor (n=6), lung (n=6), and ovarian (n=5) were the most common cancer types.
Fig 2.
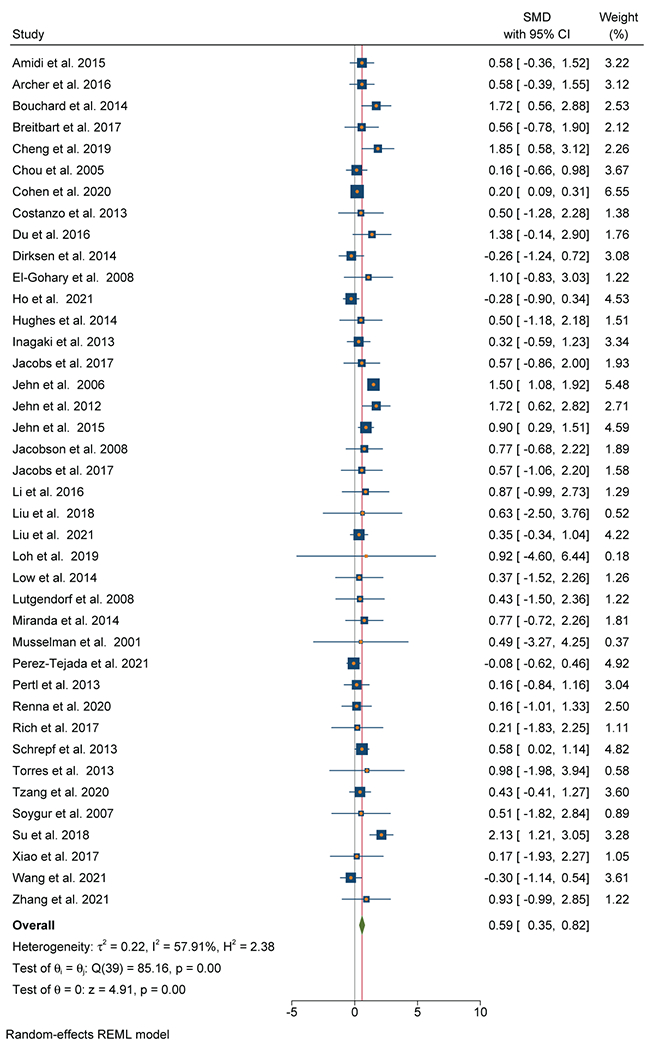
A: Forest Plot of IL-6 and depressive symptoms
B: Funnel Plot of studies of IL-6 and depressive symptoms in patients with cancer
Subgroup Analyses
Extent of disease (localized versus metastatic):
While both subgroups demonstrated a positive relationship between IL-6 and depression, effect sizes were significantly smaller for studies of localized cancer (n=19, SMD=0.38; 95% CI 0.09-0.68) and larger for studies that included metastatic cancer (n=21, SMD=0.90; 95% CI 0.58-1.22) (χ2=5.44, p=0.02).
Primary endpoint (depression versus other):
The difference between studies with depression primary endpoint (SMD=0.78; 95% CI 0.50-1.06) versus non-depression primary endpoint (SMD=0.18; 95% CI 0.08-0.29) was significant (χ2=15.24, p<.001).
TNF
Twenty-four out of 34 studies (71%) that evaluated TNF were included in the meta-analysis (n=1,576). An association between TNF and depressive symptoms was evident in 21 out of 24 studies (88%) and was confirmed by a large effect size (SMD=0.73; 95% CI, 0.35-1.11) (Figure 3A). There was substantial heterogeneity (I2=74.1%). There was no evidence of significant publication bias observed on the Funnel Plot (Figure 3B), which was verified by a non-significant Egger’s test. Breast (n=12), GI (n=4), and lung (n=4) were the most common cancer types.
Figure 3.
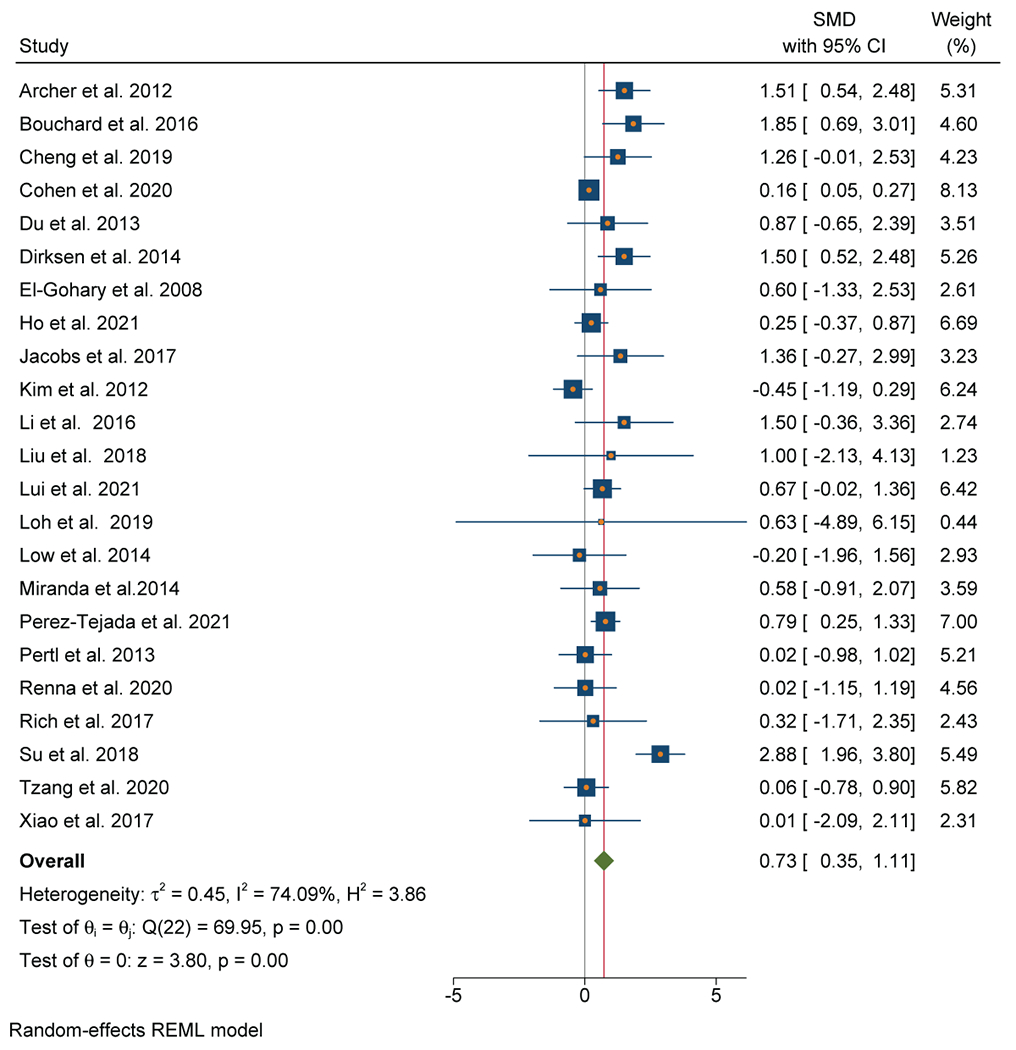
A: Forest plot of Tumor Necrosis Factor and depressive symptoms in patients with 3 cancer
B: Funnel Plot of Tumor Necrosis Factor and depressive symptoms in patients with 3 cancer
Subgroup Analyses
Extent of disease (localized versus metastatic):
No significant differences were observed between studies of localized cancer (n=13, SMD=0.71; 95% CI 0.19-1.22) and metastatic cancer (n=10, SMD=0.85; 95% CI 0.28-1.41) (χ2=0.13, p=0.71).
Primary endpoint (depression versus other):
No significant differences were observed between studies with depression as the primary endpoint (n=16, SMD=0.83; 95% CI 0.33-1.32) versus studies with non-depression primary endpoints (n=7, SMD=0.51; 95% CI 0.02-1.00) (χ2=0.79, p=0.38).
IL-1beta:
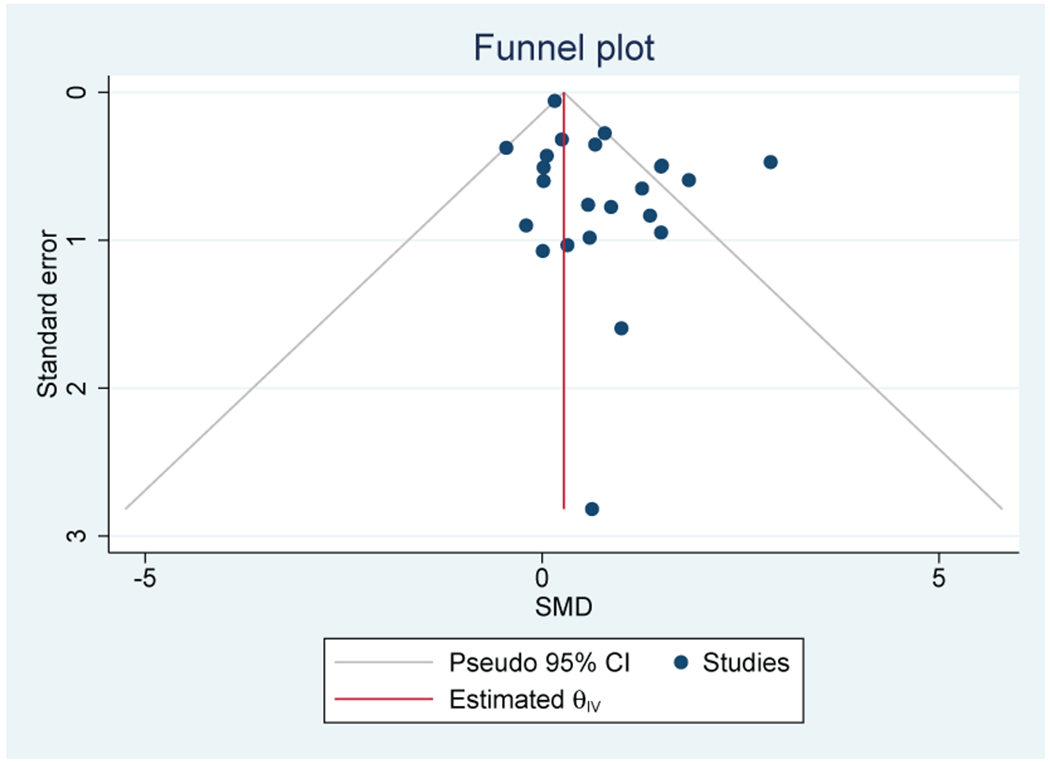
Fifteen out of 17 studies (88%) that evaluated IL-1beta were included in the meta-analysis (n=1,250). There was not a significant association between IL-1beta and depressive symptoms (SMD=0.44; 95% CI, −0.05-0.92) (Figure 4A). There was moderate heterogeneity (I2=61.3%). One outlier is seen on the Funnel plot, but bias was not confirmed by the Egger’s test (Figure 4B). Breast (n=9), GI (n=4), and lung (n=3) were the most common cancer types.
Figure 4.
A: Forest plot of Interleukin-1beta and depressive symptoms in patients with cancer
B: Funnel plot of Interleukin-1beta and depressive symptoms in patients with cancer
Subgroup Analyses
Extent of disease (localized versus metastatic):
No significant differences were observed between studies in the localized disease setting (n=9, SMD=0.40; 95% CI −0.32-1.12) and metastatic cancer (n=6, SMD=0.54; 95% CI −0.08 −1.15) (χ2 =0.39, p=0.82).
Primary endpoint (depression versus other):
No significant differences were observed between studies with depression as the primary endpoint (n=9, SMD=0.52; 95% CI −0.26-1.30) and non-depression endpoints (n=6, SMD=0.34; 95% CI −0.09-0.77) (χ2=0.17, p=0.68).
CRP
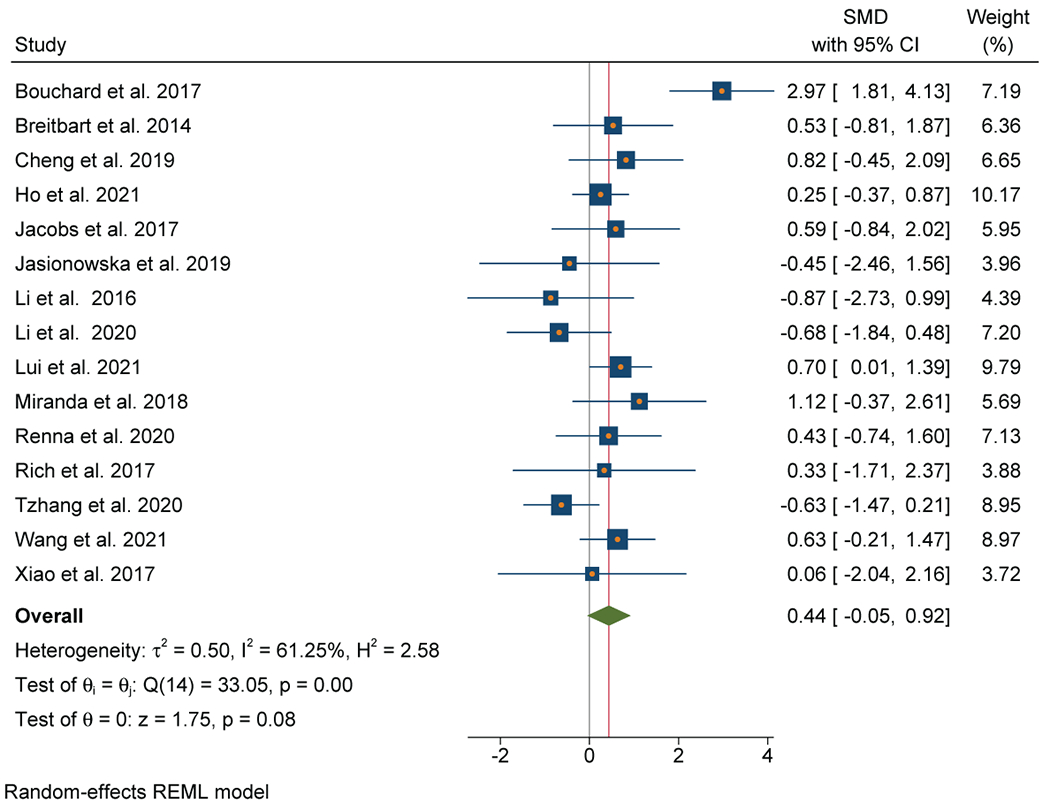
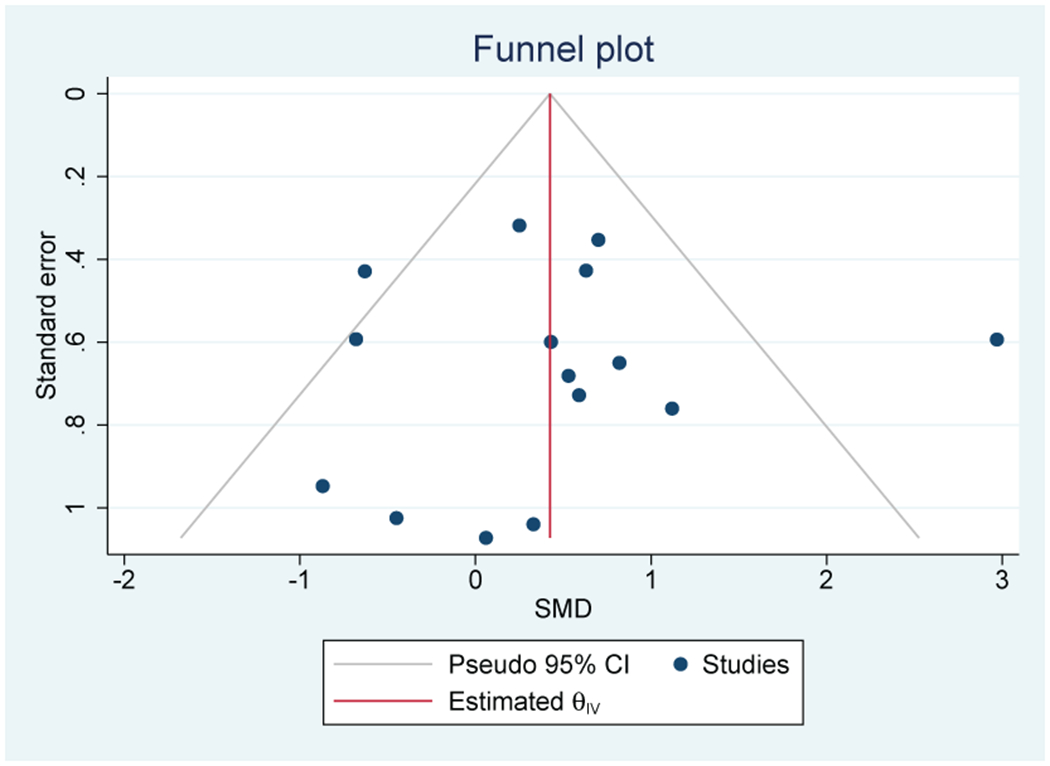
Thirteen out of 17 studies (76%) that evaluated CRP were included in the meta-analysis (n=1123). A positive association between inflammation and depressive symptoms was reported in 10 out of 13 individual studies (77%). Meta-analysis of these studies found a significant association between elevated CRP and depressive symptoms with a moderate effect size (SMD=0.57; 95% CI, 0.27-0.87) (Figure 5A). There was no evidence of heterogeneity (I2=0%). Publication bias was not observed on the Funnel Plot (Figure 5B) or by the Egger test. Lung (n=5), breast (n=4), and GI (n=2) cancers were the most common cancer types.
Figure 5.
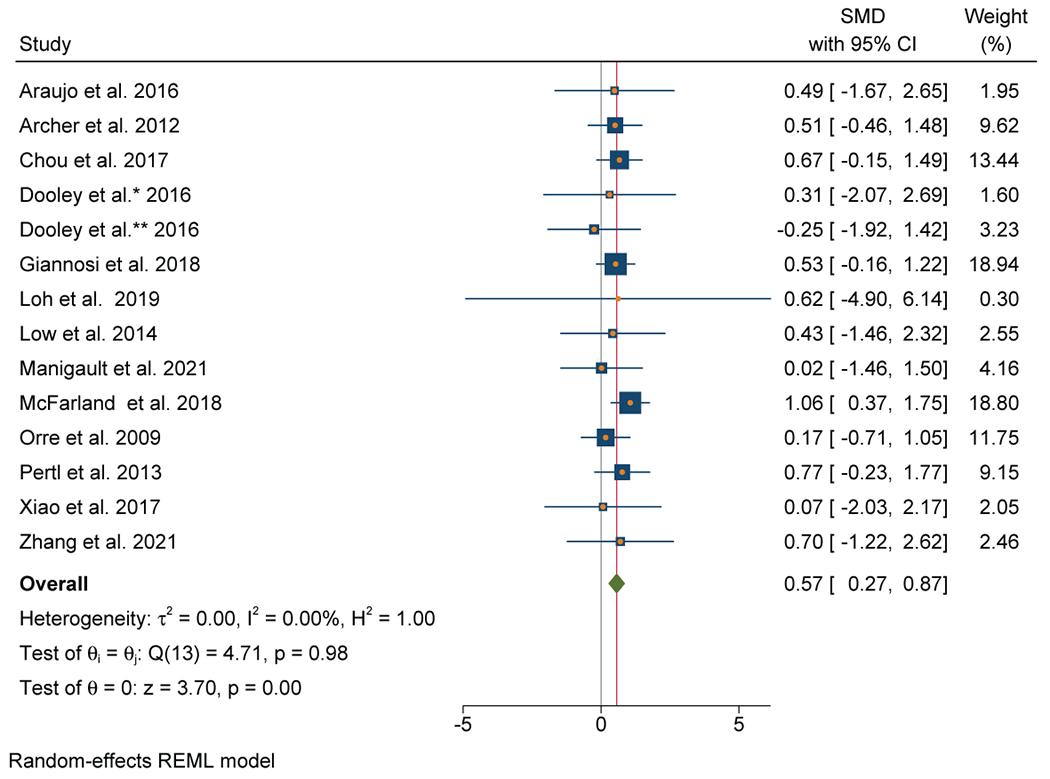
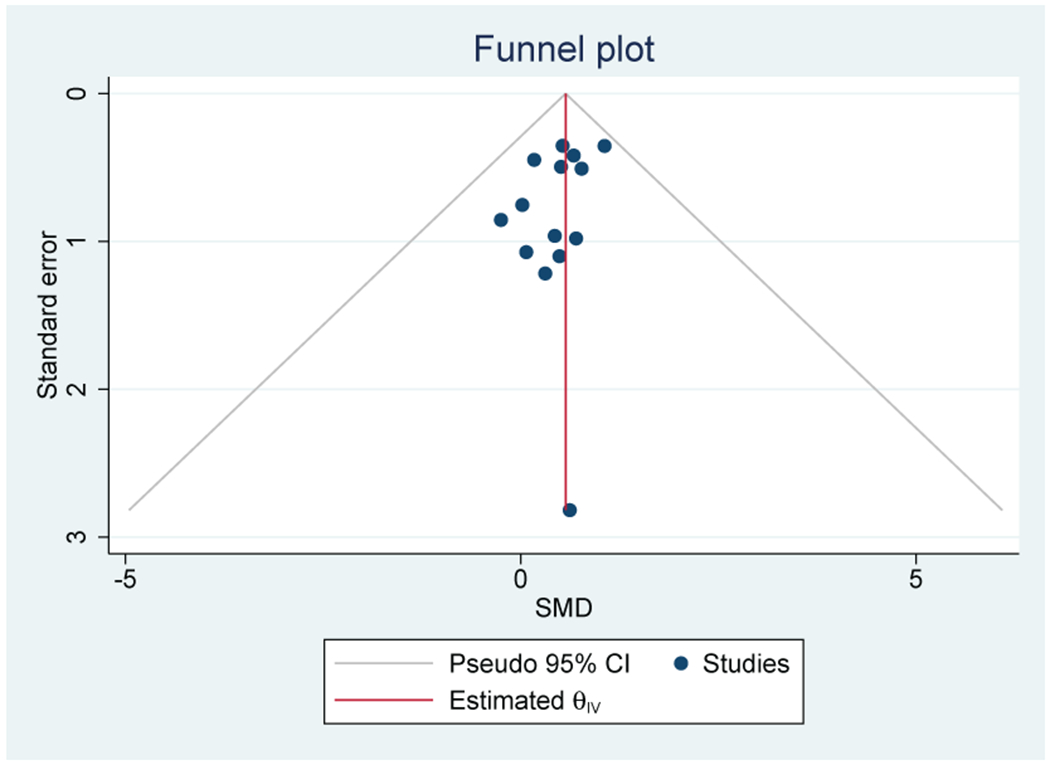
A: Forest plot of C-Reactive Protein and depressive symptoms in patients with cancer
B: Funnel plot of C - Reactive Protein and depressive symptoms in patients with cancer
Subgroup Analyses
Extent of disease (localized versus metastatic):
No significant differences were observed between studies limited to localized disease (n=8, SMD=0.36; 95% CI −0.08-0.80) versus studies including patients with metastatic disease (n=5, SMD=0.75; 95% CI 0.34-1.15) (χ2=1.56, p=0.21).
Primary endpoint (depression versus other):
No significant differences were observed based on primary endpoint (depression endpoint, n=7, SMD=0.68; 95% CI 0.34-1.02 versus non-depression endpoint, n=6, SMD=0.20; 95% CI −0.42-0.82) (χ2=1.74, p=0.19).
Inflammatory markers evaluated by less than ten studies:
The non-cytokine inflammatory markers neutrophil-to-lymphocyte ratio (NLR) and albumin were associated with depressive symptoms (SMD=0.63; 95% CI, 0.15-1.11, p=0.01; SMD=−0.67; 95% CI, −1.12-−0.21, p<.001, respectively) and demonstrated large effect sizes. NLR and albumin did not demonstrate significant heterogeneity. Cytokine markers IL-5 and IL-13 were associated with depressive symptoms (SMD=−0.69; 95% CI, −1.12—0.24, p<.001; SMD=−0.56; 95% CI, −1.04—0.07, p=.02, respectively) demonstrated by large effect sizes without significant heterogeneity. Cytokines IL-8 and IL-1 receptor antagonist (IL-1ra) demonstrated trends towards significance (Table 2). Several inflammatory markers demonstrated no association with depressive symptoms, but the number of meta-analyzed studies varied from two to nine.
Discussion
This is the first systematic review and meta-analysis of inflammation and depressive symptoms exclusively in patients with cancer. The peripheral blood inflammatory markers IL-6, TNF, and CRP were consistently associated with depressive symptoms as demonstrated by moderate to large effect sizes across multiple cancer populations and settings. Albeit in far fewer studies, large effect sizes with depressive symptoms were also demonstrated by IL-5, IL-13, NLR, and albumin. Study quality was consistently good across the primary meta-analyses of IL-6, TNF, IL-1beta and CRP. Taken together, these data provide robust evidence that inflammation is associated with depressive symptoms in patients with cancer and may play a role in understanding the unique biological association between cancer and depressive symptoms. At the same time, depression in patients with cancer may be contributing to inflammation in these settings.
In contrast to similar meta-analyses from studies on otherwise medically healthy depressed patients which have found significant but smaller effect sizes,58–61 the effect sizes of the current meta-analysis were moderate to large indicating that the relationship between inflammation and depression may be stronger and potentially more relevant for patients with cancer. Moreover, it should be noted that the studies included in this meta-analysis generally represented standard clinical settings and were not enriched for depressed patients as were previous meta-analyses in the psychiatric literature. Finally, while the overall heterogeneity of studies was moderate for IL-6 and TNF (and low for CRP) in our study, results from the literature in medically healthy depressed patients reveal reliably large heterogeneity in the relationship between the same inflammatory markers and depressive symptoms, justifying the use of random effects modeling and underlining the consistency of our findings with other work in this area.58,61 Differences in primary endpoint (depression as a primary versus secondary endpoint) and cancer setting (localized versus metastatic) did not explain heterogeneity.
Although the studies included in these analyses were descriptive in nature and cannot address cause and effect, increasing data have demonstrated a cause-and-effect relationship between peripheral inflammation and depression.1,59,60,62 Indeed, inflammatory markers predict the development of depressive symptoms in population-based studies,63,64 and administration of inflammatory stimuli induce depressive symptoms.1,4,65–68 Anti-inflammatory agents reduce depressive symptoms, especially in patients with autoimmune and inflammatory disorders.5,6 Mechanisms by which inflammation can affect neurotransmitter systems and neurocircuits in the brain have been determined in human and laboratory animal studies, and treatment targets involving inflammation itself and its downstream effects on the brain have been examined.1,69,70 Inflammation has been shown to alter monoamine metabolism and glutamate neurotransmission and thereby disrupt brain circuitry involved in motivation and motor activity as well as anxiety, arousal and alarm.1,3,71,72 Post hoc analyses of clinical trials using inflammatory markers to predict response suggest that drugs targeting serotonin (which are often used in the cancer setting) are less effective in depressed patients with increased inflammation (leading to high rates of treatment non-response to these conventional antidepressants), while the use of medications targeting dopamine may improve treatment response.73,74 In addition, motivational deficits as reflected by anhedonia (a core symptom of depression) appear to be uniquely responsive to anti-inflammatory treatments for depression.75–77 Finally, an increasing literature suggests that anti-inflammatory agents may have moderate efficacy in treating depressive symptoms.6,78 Thus, the association of inflammation with depression in cancer patients suggests that these patients may be less responsive to drugs that target serotonin, and those patients with symptoms of anhedonia (loss of interest or pleasure) may be more responsive to drugs that target inflammation and/or its downstream effects on dopamine.79 Furthermore, as indicated in the subgroup analyses, patients with metastatic disease may be especially at risk for inflammation-related depressive syndromes.
Several limitations in the available data are worthy of mention. Few studies in the current meta-analysis used standardized diagnostic criteria for depression or evaluated depression cases (e.g., SCID) while most studies measured depression symptom severity only. Self-report scales with continuous measures such as the HADS-D, CES-D, BDI, and HAM-D were most frequently employed. Scales commonly used in practice such as the PHQ-9 or the recommended PROMIS Depression scale were evaluated in only two studies. Although continuous scales instill confidence in the associations reported herein, the precision of the findings may be limited by the presence of multiple other neuropsychiatric symptoms resulting from inflammation, which are often included on depression scales but may not represent the diagnostic criteria for depression. The relationship between depressive symptoms and psychological distress, which is often measured in the oncology setting in place of depression, is not assessable. Moreover, wide variability in biomarker evaluation precludes any conclusions about biomarkers beyond IL-6, TNF, CRP, and IL-1beta, and there may be additional biomarkers of relevance that have yet to be adequately evaluated. Laboratory variability is assumed across studies as a possible source of error that could not be accounted for directly. While 27 unique inflammatory markers were evaluated in relation to depressive symptoms, only IL-6, TNF, IL-1beta and CRP were represented by more than eleven studies in meta-analyses. Of note, in subgroup analyses, no significant differences were found between studies on patients with localized versus metastatic disease or between studies where depression was the primary versus secondary endpoint. Nevertheless, these analyses should be interpreted with caution because of the limited number of studies included. In addition, we only considered baseline assessments in longitudinal studies (n=9). More longitudinal studies with repeated measures would provide important details on the stability of relationships between inflammatory markers and depression over time. 80 In terms of cancer types, gastrointestinal and prostate cancer were conspicuously missing; lung and breast cancers were more commonly represented along with rare cancer subtypes. Study settings were varied and provided multiple contexts in which the relationship appeared. Inflammation may mediate the relationship between depression and survival. Future studies should address this potential interaction.
In summary, the association between inflammation and depression in patients with cancer is robust based on the moderate to large effect sizes and high-quality studies that varied in cancer type and setting. Attention to cancer-related inflammation and depression may foster greater recognition of depression in cancer settings and enable cancer specific antidepressant treatments based on the increasing knowledgebase regarding the impact of inflammation on the brain. Finally, future studies employing longitudinal designs and varying cancer types and settings will inform a greater understanding of the relationship between inflammation and depression and promote further translational applications.
Supplementary Material
Funding:
P30 Cancer Center Grant: Craig Thompson Memorial Sloan Kettering
National Institutes of Health Loan Repayment Grant
Footnotes
Conflict of Interests: No conflict of interest reported by authors. We have reviewed and approved the manuscript as it is submitted and have no conflict of interest to declare.
References
- 1.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16(1):22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Felger JC, Haroon E, Patel TA, et al. What does plasma CRP tell us about peripheral and central inflammation in depression? Molecular psychiatry. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haroon E, Miller AH, Sanacora G. Inflammation, Glutamate, and Glia: A Trio of Trouble in Mood Disorders. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2017;42(1):193–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anisman H, Merali Z, Poulter MO, Hayley S. Cytokines as a precipitant of depressive illness: animal and human studies. Curr Pharm Des. 2005;11(8):963–972. [DOI] [PubMed] [Google Scholar]
- 5.Kappelmann N, Lewis G, Dantzer R, Jones PB, Khandaker GM. Antidepressant activity of anti-cytokine treatment: a systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Mol Psychiatry. 2018;23(2):335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kohler O, Benros ME, Nordentoft M, et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA psychiatry. 2014;71(12):1381–1391. [DOI] [PubMed] [Google Scholar]
- 7.Giese-Davis J, Collie K, Rancourt KM, Neri E, Kraemer HC, Spiegel D. Decrease in depression symptoms is associated with longer survival in patients with metastatic breast cancer: a secondary analysis. J Clin Oncol. 2011;29(4):413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26(6):971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krebber AM, Buffart LM, Kleijn G, et al. Prevalence of depression in cancer patients: a meta-analysis of diagnostic interviews and self-report instruments. Psychooncology. 2014;23(2):121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker J, Hansen CH, Martin P, et al. Prevalence, associations, and adequacy of treatment of major depression in patients with cancer: a cross-sectional analysis of routinely collected clinical data. The lancet Psychiatry. 2014;1(5):343–350. [DOI] [PubMed] [Google Scholar]
- 11.Lim GY, Tam WW, Lu Y, Ho CS, Zhang MW, Ho RC. Prevalence of Depression in the Community from 30 Countries between 1994 and 2014. Sci Rep. 2018;8(1):2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caruso R, Nanni MG, Riba M, et al. Depressive spectrum disorders in cancer: prevalence, risk factors and screening for depression: a critical review. Acta Oncol. 2017;56(2):146–155. [DOI] [PubMed] [Google Scholar]
- 13.Alcala HE. Differential mental health impact of cancer across racial/ethnic groups: findings from a population-based study in California. BMC Public Health. 2014;14:930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30(7):1073–1081. [DOI] [PubMed] [Google Scholar]
- 15.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.TC. C. Cochrane Handbook for Systematic Reviews of Interventions. 5.1 ed. London, UK: 2011. [Google Scholar]
- 17.Bramer WM, Giustini D, de Jonge GB, Holland L, Bekhuis T. De-duplication of database search results for systematic reviews in EndNote. J Med Libr Assoc. 2016;104(3):240–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kellermeyer L HB, Knight S. Covidence and Rayyan. Journal of the Medial Library Association 2018;106:580–583. [Google Scholar]
- 19.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stat Statistical Software Release 16 [computer program]. College Station, TX: StataCorp LLC; 2019. [Google Scholar]
- 21.Su JP, Liu HF, Zhang HL, He YJ, Nie Y. Effects of different degrees of depression on inflammatory response and immune function in patients with ovarian cancer. J Biol Regul Homeost Agents. 2018;32(5):1225–1230. [PubMed] [Google Scholar]
- 22.Cohen L, Cole SW, Sood AK, et al. Depressive symptoms and cortisol rhythmicity predict survival in patients with renal cell carcinoma: role of inflammatory signaling. PLoS One. 2012;7(8):e42324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steel JL, Geller DA, Gamblin TC, Olek MC, Carr BI. Depression, immunity, and survival in patients with hepatobiliary carcinoma. J Clin Oncol. 2007;25(17):2397–2405. [DOI] [PubMed] [Google Scholar]
- 24.McFarland DC, Saracino RM, Miller AH, Breitbart W, Rosenfeld B, Nelson C. Prognostic implications of depression and inflammation in patients with metastatic lung cancer. Future oncology (London, England). 2021;17(2):183–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu L, Pan Q, Lin R. Prevalence rate and influencing factors of preoperative anxiety and depression in gastric cancer patients in China: Preliminary study. J Int Med Res. 2016;44(2):377–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Low CA, Bovbjerg DH, Jenkins FJ, et al. Preoperative inflammatory biomarkers and neurovegetative symptoms in peritoneal carcinomatosis patients. Brain Behav Immun. 2014;42:65–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miranda DO, Anatriello E, Azevedo LR, et al. Elevated serum levels of proinflammatory cytokines potentially correlate with depression and anxiety in colorectal cancer patients in different stages of the antitumor therapy. Cytokine. 2018;104:72–77. [DOI] [PubMed] [Google Scholar]
- 28.Pertl MM, Hevey D, Boyle NT, et al. C-reactive protein predicts fatigue independently of depression in breast cancer patients prior to chemotherapy. Brain Behav Immun. 2013;34:108–119. [DOI] [PubMed] [Google Scholar]
- 29.Amidi A, Wu LM, Agerbaek M, et al. Cognitive impairment and potential biological and psychological correlates of neuropsychological performance in recently orchiectomized testicular cancer patients. Psychooncology. 2015;24(9):1174–1180. [DOI] [PubMed] [Google Scholar]
- 30.Araujo AS, Nogueira IC, Gomes Neto A, et al. The impact of lung cancer resection surgery on fibrinogen and C-reactive protein and their relationship with patients outcomes: A prospective follow up study. Cancer Biomark. 2016;16(1):47–53. [DOI] [PubMed] [Google Scholar]
- 31.Bouchard LC, Antoni MH, Blomberg BB, et al. Postsurgical Depressive Symptoms and Proinflammatory Cytokine Elevations in Women Undergoing Primary Treatment for Breast Cancer. Psychosom Med. 2016;78(1):26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fujita T, Nagayama A, Anazawa S. Circulating alpha-2-macroglobulin levels and depression scores in patients who underwent abdominal cancer surgery. J Surg Res. 2003;114(1):90–94. [DOI] [PubMed] [Google Scholar]
- 33.Inagaki M, Akechi T, Okuyama T, et al. Associations of interleukin-6 with vegetative but not affective depressive symptoms in terminally ill cancer patients. Support Care Cancer. 2013;21(8):2097–2106. [DOI] [PubMed] [Google Scholar]
- 34.Jacobson CM, Rosenfeld B, Pessin H, Breitbart W. Depression and IL-6 blood plasma concentrations in advanced cancer patients. Psychosomatics. 2008;49(1):64–66. [DOI] [PubMed] [Google Scholar]
- 35.Kwekkeboom KL, Tostrud L, Costanzo E, et al. The Role of Inflammation in the Pain, Fatigue, and Sleep Disturbance Symptom Cluster in Advanced Cancer. J Pain Symptom Manage. 2018;55(5):1286–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodrigues AR, Trufelli DC, Fonseca F, de Paula LC, Giglio AD. Fatigue in Patients With Advanced Terminal Cancer Correlates With Inflammation, Poor Quality of Life and Sleep, and Anxiety/Depression. Am J Hosp Palliat Care. 2016;33(10):942–947. [DOI] [PubMed] [Google Scholar]
- 37.Burz C, Bojan A, Balacescu L, et al. Interleukin 8 as predictive factor for response to chemotherapy in colorectal cancer patients. Acta Clin Belg. 2021;76(2):113–118. [DOI] [PubMed] [Google Scholar]
- 38.Chou HL, Chao TY, Chen TC, et al. The Relationship Between Inflammatory Biomarkers and Symptom Distress in Lung Cancer Patients Undergoing Chemotherapy. Cancer Nurs. 2017;40(2):E1–E8. [DOI] [PubMed] [Google Scholar]
- 39.Giannousi Z, Gioulbasanis I, Pallis AG, et al. Nutritional status, acute phase response and depression in metastatic lung cancer patients: correlations and association prognosis. Support Care Cancer. 2012;20(8):1823–1829. [DOI] [PubMed] [Google Scholar]
- 40.Jehn CF, Flath B, Strux A, et al. Influence of age, performance status, cancer activity, and IL-6 on anxiety and depression in patients with metastatic breast cancer. Breast Cancer Res Treat. 2012;136(3):789–794. [DOI] [PubMed] [Google Scholar]
- 41.Jehn CF, Becker B, Flath B, et al. Neurocognitive function, brain-derived neurotrophic factor (BDNF) and IL-6 levels in cancer patients with depression. J Neuroimmunol. 2015;287:88–92. [DOI] [PubMed] [Google Scholar]
- 42.Jasionowska J, Talarowska M, Kalinka E, et al. Interleukin 1 level, cognitive performance, and severity of depressive symptoms in patients treated with systemic anticancer therapy: a prospective study. Croat Med J. 2019;60(2):166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Courtier N, Gambling T, Enright S, Barrett-Lee P, Abraham J, Mason MD. Psychological and immunological characteristics of fatigued women undergoing radiotherapy for early-stage breast cancer. Support Care Cancer. 2013;21(1):173–181. [DOI] [PubMed] [Google Scholar]
- 44.Dirksen SR, Kirschner KF, Belyea MJ. Association of symptoms and cytokines in prostate cancer patients receiving radiation treatment. Biol Res Nurs. 2014;16(3):250–257. [DOI] [PubMed] [Google Scholar]
- 45.Sephton SE, Dhabhar FS, Keuroghlian AS, et al. Depression, cortisol, and suppressed cell-mediated immunity in metastatic breast cancer. Brain Behav Immun. 2009;23(8):1148–1155. [DOI] [PubMed] [Google Scholar]
- 46.Torres MA, Pace TW, Liu T, et al. Predictors of depression in breast cancer patients treated with radiation: role of prior chemotherapy and nuclear factor kappa B. Cancer. 2013;119(11):1951–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bower JE, Ganz PA, Irwin MR, Kwan L, Breen EC, Cole SW. Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol. 2011;29(26):3517–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hughes S, Jaremka LM, Alfano CM, et al. Social support predicts inflammation, pain, and depressive symptoms: longitudinal relationships among breast cancer survivors. Psychoneuroendocrinology. 2014;42:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kesler S, Janelsins M, Koovakkattu D, et al. Reduced hippocampal volume and verbal memory performance associated with interleukin-6 and tumor necrosis factor-alpha levels in chemotherapy-treated breast cancer survivors. Brain Behav Immun. 2013;30 Suppl:S109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perez-Tejada J, Labaka A, Pascual-Sagastizabal E, Garmendia L, Iruretagoyena A, Arregi A. Predictors of psychological distress in breast cancer survivors: A biopsychosocial approach. Eur J Cancer Care (Engl). 2019;28(6):e13166. [DOI] [PubMed] [Google Scholar]
- 51.Xiao C, Miller AH, Felger J, Mister D, Liu T, Torres MA. Depressive symptoms and inflammation are independent risk factors of fatigue in breast cancer survivors. Psychol Med. 2017;47(10):1733–1743. [DOI] [PubMed] [Google Scholar]
- 52.Cohen M, Levkovich I, Katz R, Fried G, Pollack S. Low physical activity, fatigue and depression in breast cancer survivors: Moderation by levels of IL-6 and IL-8. Int J Psychophysiol. 2020;158:96–102. [DOI] [PubMed] [Google Scholar]
- 53.Perez-Tejada J, Aizpurua-Perez I, Labaka A, Vegas O, Ugartemendia G, Arregi A. Distress, proinflammatory cytokines and self-esteem as predictors of quality of life in breast cancer survivors. Physiol Behav. 2021;230:113297. [DOI] [PubMed] [Google Scholar]
- 54.Manigault AW, Ganz PA, Irwin MR, Cole SW, Kuhlman KR, Bower JE. Moderators of inflammation-related depression: a prospective study of breast cancer survivors. Transl Psychiatry. 2021;11(1):615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Orre IJ, Murison R, Dahl AA, Ueland T, Aukrust P, Fossa SD. Levels of circulating interleukin-1 receptor antagonist and C-reactive protein in long-term survivors of testicular cancer with chronic cancer-related fatigue. Brain Behav Immun. 2009;23(6):868–874. [DOI] [PubMed] [Google Scholar]
- 56.Liu M, Li Y, Liu X. Serum tumor necrosis factor-alpha, interleukin-1beta, interleukin-6, and interleukin-17 relate to anxiety and depression risks to some extent in non-small cell lung cancer survivor. Clin Respir J. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miranda DO, Anatriello E, Azevedo LR, et al. Fractalkine (C-X3-C motif chemokine ligand 1) as a potential biomarker for depression and anxiety in colorectal cancer patients. Biomed Rep. 2017;7(2):188–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71(2):171–186. [DOI] [PubMed] [Google Scholar]
- 59.Valkanova V, Ebmeier KP, Allan CL. CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. J Affect Disord. 2013;150(3):736–744. [DOI] [PubMed] [Google Scholar]
- 60.Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, Kivimaki M. Cumulative meta-analysis of interleukins 6 and 1beta, tumour necrosis factor alpha and C-reactive protein in patients with major depressive disorder. Brain Behav Immun. 2015;49:206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dowlati Y, Herrmann N, Swardfager W, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67(5):446–457. [DOI] [PubMed] [Google Scholar]
- 62.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature reviews Neuroscience. 2008;9(1):46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Osimo EF, Stochl J, Zammit S, Lewis G, Jones PB, Khandaker GM. Longitudinal population subgroups of CRP and risk of depression in the ALSPAC birth cohort. Compr Psychiatry. 2019;96:152143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mac Giollabhui N, Ng TH, Ellman LM, Alloy LB. The longitudinal associations of inflammatory biomarkers and depression revisited: systematic review, meta-analysis, and meta-regression. Molecular psychiatry. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Capuron L, Gumnick JF, Musselman DL, et al. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2002;26(5):643–652. [DOI] [PubMed] [Google Scholar]
- 66.Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol Psychiat. 2010;68(8):748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiat. 2009;66(5):407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21(2):153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Haroon E, Miller AH. Inflammation Effects on Brain Glutamate in Depression: Mechanistic Considerations and Treatment Implications. Curr Top Behav Neurosci. 2017;31:173–198. [DOI] [PubMed] [Google Scholar]
- 70.Miller AH, Haroon E, Felger JC. Therapeutic Implications of Brain-Immune Interactions: Treatment in Translation. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2017;42(1):334–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Felger JC, Li Z, Haroon E, et al. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Molecular psychiatry. 2016;21(10):1358–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miller AH, Haroon E, Raison CL, Felger JC. Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress Anxiety. 2013;30(4):297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jha MK, Minhajuddin A, Gadad BS, et al. Can C-reactive protein inform antidepressant medication selection in depressed outpatients? Findings from the CO-MED trial. Psychoneuroendocrinology. 2017;78:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Uher R, Tansey KE, Dew T, et al. An inflammatory biomarker as a differential predictor of outcome of depression treatment with escitalopram and nortriptyline. The American journal of psychiatry. 2014;171(12):1278–1286. [DOI] [PubMed] [Google Scholar]
- 75.Felger JC, Treadway MT. Inflammation Effects on Motivation and Motor Activity: Role of Dopamine. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2017;42(1):216–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee Y, Subramaniapillai M, Brietzke E, et al. Anti-cytokine agents for anhedonia: targeting inflammation and the immune system to treat dimensional disturbances in depression. Ther Adv Psychopharmacol. 2018;8(12):337–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Raison CL, Rutherford RE, Woolwine BJ, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70(1):31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Adzic M, Brkic Z, Mitic M, et al. Therapeutic Strategies for Treatment of Inflammation-related Depression. Curr Neuropharmacol. 2018;16(2):176–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Strawbridge R, Arnone D, Danese A, Papadopoulos A, Herane Vives A, Cleare AJ. Inflammation and clinical response to treatment in depression: A meta-analysis. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2015;25(10):1532–1543. [DOI] [PubMed] [Google Scholar]
- 80.Bravery B, Loughnan S, Murphy M. Depression treatment research in people with cancer does not reflect cancer prevalence: findings from a systematic review. Evid Based Ment Health. 2020;23(4):155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


