Abstract
Objective:
To determine the effectiveness of physiotherapy interventions for postprostatectomy erectile dysfunction and climacturia.
Data sources:
Multiple databases were searched from database inception to February 2019.
Review methods:
Randomized controlled trials comparing physiotherapy interventions to control were included.
Results:
The search yielded 127 potentially relevant articles; seven met the inclusion criteria and were included in the review. Meta-analysis of two studies revealed a statistically significant effect of pelvic floor muscle training (PFMT) plus biofeedback compared to the no treatment control group for erectile function at the12-month follow-up period (risk ratio (RR) = 3.65, 95% confidence interval (CI) = 1.02–13.05; P = 0.05). Data from one small study (n = 31) identified a greater number of men reporting improved climacturia in the PFMT plus electrical stimulation group compared to the no treatment control group, and the overall effect was significant (RR = 15.60, 95% CI = 0.95–254.91; P = 0.05). Meta-analyses of two studies found no statistically significant differences between groups receiving PFMT and no treatment control for erectile function or climacturia at long-term follow-up.
Conclusions:
PFMT augmented with biofeedback improves erectile function after prostatectomy. Data from a single study found PFMT combined with electrical stimulation to be beneficial for postprostatectomy climacturia. However, electrical stimulation is recommended for terminally ill people only. The effect of PFMT alone on postprostatectomy erectile dysfunction and climacturia remains inconclusive. However, this is likely to be affected by the participant adherence and physiotherapy supervision. High-quality trials providing intensive supervision and due consideration of adherence factors are recommended.
Keywords: Climacturia, erectile dysfunction, prostatectomy, sexual dysfunction
Introduction
The second most common cancer among men is prostate cancer. 1 Incidence of prostate cancer increases rapidly after the age of 50 years. 2 Radical prostatectomy is a surgical procedure to remove the prostate gland and the surrounding tissues. Radical prostatectomy is associated with erectile dysfunction (inability to obtain and maintain adequate erection for sexual intercourse) and climacturia (urine leakage during ejaculation). 3 Recovery of erectile function following prostatectomy ranges from 12 to 24 months.4,5 Lack of active intervention in the recovery period results in flaccidity, and prolonged flaccid state is reported to cause irreversible damage to the cavernous tissue. 5 Postprostatectomy urinary incontinence has been associated with development of climacturia,3,6–8 and urinary incontinence following prostatectomy has been identified as a potential predictor of climacturia in several studies.3,6,8
In men, the pelvic floor muscles that are active during sexual intercourse for penile erection and ejaculation are the ischiocavernosus and the bulbospongiosus;9,10 atrophy of ischiocavernosus muscle partly contributes to erectile dysfunction. 10 Conservative therapies that have been proposed for penile rehabilitation include pelvic floor muscle training, electrical stimulation, and biofeedback. However, the efficacy of these conservative therapies for sexual dysfunction following prostatectomy is not known.
To the best of our knowledge, there are no meta-analyses on the efficacy of physiotherapy interventions for erectile function and climacturia following prostatectomy. The efficacy of physiotherapy interventions for improving erectile dysfunction and climacturia is therefore not known. The objective of this review was to determine the effectiveness of physiotherapy interventions in comparison to controls for improving erectile function and climacturia after radical prostatectomy or transurethral resection of tumor.
Methods
This systematic review was developed and is reported in accordance with the Preferred Reporting Items for Systematic Review and Meta-analyses guidelines. 11 An extensive Ovid Medline, EMBASE, Web of Science, EBSCO, PubMed, PEDro, and Scopus search was performed from database inception to February 2019, using the following search terms: prostatectomy, sexual dysfunction, physiotherapy intervention, and randomized controlled trials. Reference lists of relevant studies were hand searched for any other potentially relevant articles. No limits were placed on language or publication year. A detailed description of the search is provided in Supplemental Appendix 1. Study screening and selection were performed independently by two review authors. Conflicts were resolved by discussion between the review authors until consensus was reached. A third reviewer (PK) was consulted for unresolved conflicts.
Studies were eligible for inclusion if they (1) were randomized controlled trials, pilot randomized controlled trials, randomized cross-over (if data available prior to cross-over), cluster trials, or unpublished work; (2) compared physiotherapy interventions consisting of exercise and electrotherapy modalities such as electrical stimulation (a technique used to elicit a muscle contraction using electrical impulses) and biofeedback (instrument that allows detection of electrical signals from muscles and provides feedback reinforcing information via auditory or visual signals) 12 with either no treatment, sham, placebo, usual care, or active control; and (3) used self-reported recovery of climacturia or at least one of the following outcomes for erectile function: the International Index of Erectile Function, Self-reported Erectile Function, the Sexual Health Inventory in Men, or the Quality of Erection Questionnaire. In urology, physiotherapy interventions including exercise (pelvic floor muscle training), biofeedback, and electrical stimulation are provided by physiotherapists and other professionals such as physicians and nurses. Therefore, studies were not excluded on the basis of who delivered the intervention. Studies of quasi-experimental design were excluded. Studies comparing active interventions (electrical stimulation/biofeedback to sham electrical stimulation/biofeedback, and pelvic floor muscle training to electrical stimulation/biofeedback) were also excluded. For this review, we considered men who received only verbal/written instructions or lifestyle advice but no formal pelvic floor muscle training as no treatment controls.
The Physiotherapy Evidence Database (PEDro) scale 13 and the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) tool 14 were used to rate the methodological quality and the quality of evidence, respectively. Two reviewers performed the methodological quality assessment and compared their results with the quality scores reported on the PEDro website (http://search.pedro.org.au/search). Discrepancies between reviewer scores and scores reported on PEDro were resolved by discussion with the third reviewer (PK). Studies scoring ⩾6 were considered high quality and studies scoring ⩽5 were considered low quality. 15
The quality of evidence (GRADE) was evaluated using GRADEpro software (version 3.6.1) (http://tech.cochrane.org/revman/other-resources/gradepro/download). The quality of evidence was categorized as either “high,” “moderate,” “low,” or “very low.” 16 The overall quality for an outcome measure was based on the lowest quality for the outcome. 17 Studies were rated across outcome measures for risk of bias (such as lack of concealment of allocation, lost to follow-up > 15%), 18 indirectness (use of surrogate outcome measures), 19 imprecision (minimum or no overlap of confidence interval (CI) across studies), 20 inconsistency (evidence of clinical or statistical heterogeneity (I > 50%)), 21 and publication bias (industry sponsored). 22 Given the nature of the intervention, studies were not downgraded for lack of participant blinding; however, studies were downgraded by one level for lack of either therapist or assessor blinding and by two levels for lack of therapist and assessor blinding.
Two independent reviewers extracted the following data from each included study: first author’s name and year of publication, study design, participant age (mean age and standard deviation or median and range), sample size per group, intervention and control, and results (n for dichotomous variable or mean and standard deviation data for continuous variable).
The meta-analysis was performed using RevMan 5.3 software. Separate meta-analyses were conducted for erectile function and climacturia. Studies reporting continuous data (mean and standard deviation) were pooled separately from studies reporting dichotomous (numbers and percentages) data. Studies comparing similar interventions (pelvic floor muscle training alone; pelvic floor muscle training alone plus electrical stimulation or biofeedback) and assessment time-points (immediately after the intervention (usually three months) and final follow-up (usually 12–15 months) were grouped together to obtain the pooled estimate of between-group differences. Treatment effect size and 95% CI were estimated for continuous data, whereas the risk ratio (RR) and 95% CI were calculated for dichotomous data. Statistical heterogeneity was determined using the chi-square test. Weighted mean differences (WMD) were calculated to obtain the pooled estimate utilizing a fixed effects model for low heterogeneity (I2 < 50%) or random effects model for high heterogeneity (I2 > 50%). 23 Statistical significance was established as P ⩽ 0.05.
Results
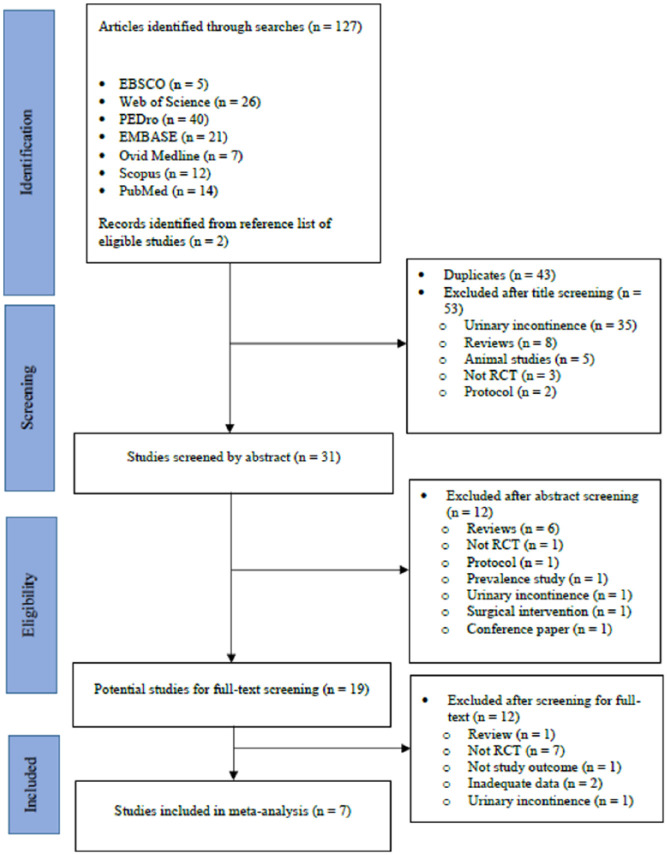
The search yielded 127 potentially relevant articles. Of these, seven met the inclusion criteria and were included in the review (excluded studies and reasons for exclusion are summarized in Supplemental Appendix 2). The review process and the reasons for exclusion at each stage are summarized in Figure 1. Study characteristics are summarized in Table 1. Seven included studies provided data for 1622 participants aged 47–90 years. Of the seven included studies, only three24–26 made participants visualize the movement of their penis and testicles upon contraction of the pelvic floor muscles.
Figure 1.
Flow diagram of searches and study selection.
Table 1.
Characteristics and PEDro methodological quality scores of included studies (n = 7).
| Study, PEDro quality, language of publication | Participants |
Intervention | Duration of intervention | EF, climacturia outcomes mean (SD)/number | |
|---|---|---|---|---|---|
| Study design, age a (years), surgery | Number (Exp/Con) | ||||
| Dorey et al.
24
PEDro: 6/10 English |
Randomized cross-over 59.5 (2.1) TURP |
25/25 | Exp: PFMT + BFB Con: No treatment for first three months. At three months, intervention similar to experimental group |
30-minute sessions once a week for five weeks. No information about who provided treatment |
EF (three months) Exp: 17.2 (9.7) Con: 8.4 (7.3) Score: not reported Climacturia NE |
| Fode et al.
27
PEDro: 5/10 English |
Parallel-group RCT Median (range): Exp: 62 (46–73) Con: 65 (49–76) RP |
30/38 | Exp: PFMT + PVS PVS: Amplitude: 2 mm; frequency: 100 Hz; Duration: 10 seconds of stimulation followed by a 10-second pause Con: One preoperative session of PFMT |
Daily stimulation for six weeks. No information about who provided treatment |
EF (three months) Exp: 5 Con: 4 EF (12 months) Exp: 16 Con: 12 Score ⩾ 18 Climacturia NE |
| Lin et al.
29
PEDro: 6/10 English |
Randomized cross-over 65.7 (6.12) RP |
35/27 | Exp: PFMT + BFB Con: No treatment for first three months. At three months, same treatment as for experimental group |
Two one-to-one sessions. No information about who provided treatment | EF (three months) Exp: 5.8 (2.3) Con: 5.0 (0.2) EF (12 months) Exp: 1 Con: 0 Score ⩾ 18 Climacturia NE |
| Glazener et al.
25
PEDro: 7/10 English |
Parallel-group RCT 62.4 (5.8) RP |
EF: 189/190 Climacturia: 135/139 |
Exp: PFMT alone Con: no treatment |
Four one-to-one sessions of PFMT by a physical therapist over a period of three months | EF (12 months) Exp: 84 Con: 85 Exp: 6.0 (3.3) Con: 6.5 (3.1) Score: NR. Study reports number of men not able to achieve any erection Climacturia (12 months) Exp: 109 Con: 109 |
| Glazener et al.
26
PEDro: 7/10 English |
Parallel-group RCT 68.2 (7.7) TURP |
EF: 177/178 Climaturia: 135/133 |
Exp: PFMT alone Con: No treatment |
Four one-to-one sessions of PFMT by a physical therapist over a period of three months | EF (12 months) Exp: 125 Con: 135 Exp: 4.2 (3.7) Con: 4.6 (3.9) Score: NR. Study reports number of men not able to achieve any erection Climacturia (12 months) Exp: 132 Con: 130 |
| Prota et al.
30
PEDro: 4/10 English |
Parallel-group RCT 62.4 (6.4) RP |
17/16 | Exp: PFMT + BFB Con: No treatment |
Once a week for three months by a physical therapist | EF six months Exp: 4 Con: 1 EF 12 months Exp: 8 Con: 2 Score ⩾ 20 Climacturia NE |
| Geraerts et al.
28
PEDro: 5/10 English |
Parallel-group RCT 61.1 (5.8) RP |
EF:14/16 Climacturia: 14/17 |
Exp: PFMT + ES ES: Frequency: 50 Hz; pulse duration: 600 µs Con: No treatment for first 15 months. At 15 months, similar treatment as for experimental group |
Once a week for six weeks followed by once every fortnight for another six weeks by therapist. | EF 15 months Exp: 4 Con: 5 Exp: 11.1 (8.8) Con: 9.3 (7.1) Score ⩾ 18 Climacturia (15 months) Exp: 6 Con: 0: |
BFB: biofeedback; Con: control group; EF: erectile function; ES: electrical stimulation; Exp: experimental group; NE: not evaluated; NR: not reported; PFMT: pelvic floor muscle training; PVS: penile vibratory stimulator; RP: radical prostatectomy; TURP: trans urethral resection of the prostate; RCT: randomized controlled trial.
Age reported as mean and SD unless specified.
PEDro scores for included studies are reported in Table 1. The summary of findings generated by the GRADE profiler software is presented in Supplemental Table S1. Methodological (PEDro) quality of included studies was low to high with mean PEDro score of 5.7 out of 10. Based on the GRADE assessment, the quality of evidence for both the outcome measures ranged from “very low” to “moderate.”
The GRADE quality of evidence for the comparison, pelvic floor muscle training plus electrical stimulation versus no treatment, contributed by two studies was “very low.” However, the PEDro quality for these two studies was “low.” The discrepancy in the quality is due to one of the studies being downgraded for publication bias 27 in the GRADE system. Two25,26 studies that obtained a “high” quality PEDro rating were rated as “moderate” in the GRADE system. One study that obtained a “low” quality PEDro rating was rated as “very low” in the GRADE system. These discrepancies in quality rating are because studies were downgraded for additional criteria such as publication bias, inconsistency (methodological/clinical heterogeneity), and imprecision in the GRADE system but not in PEDro.
Pelvic floor muscle training plus electrical stimulation for erectile function and climacturia
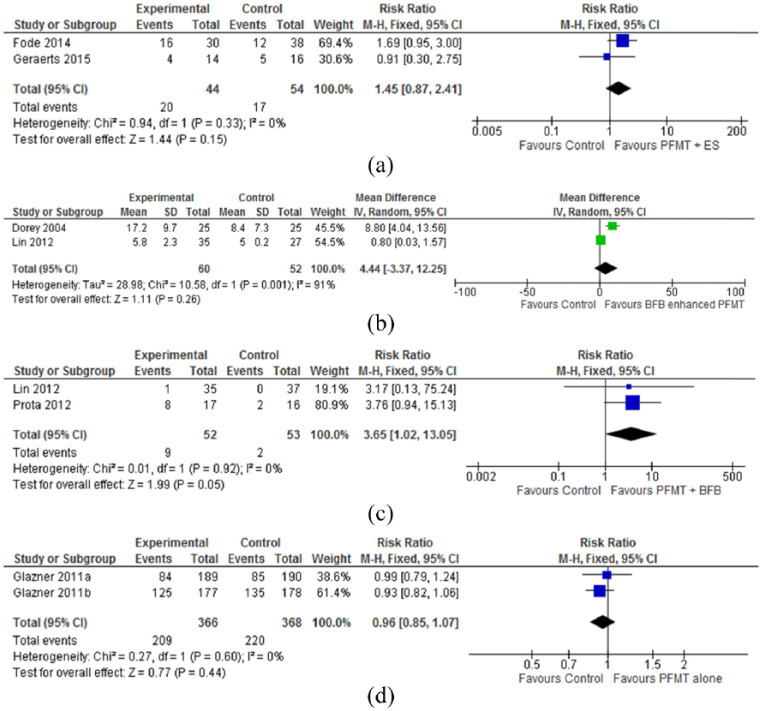
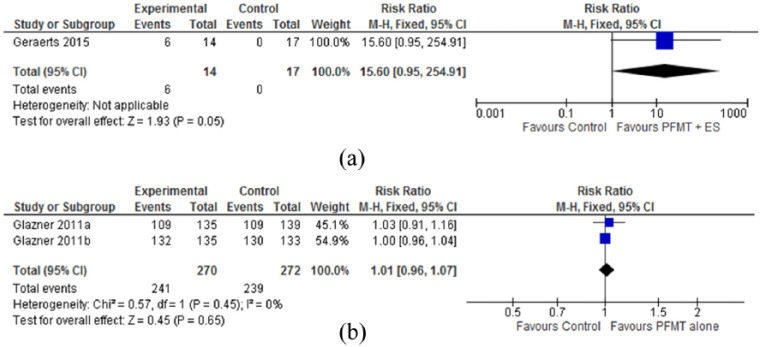
Meta-analyses of two27,28 methodologically low-quality, very low-grade studies with 98 participants found no statistically significant differences between groups receiving pelvic floor muscle training plus electrical stimulation and no treatment for erectile function (RR = 1.45, 95% CI = 0.87–2.41; P = 0.15) at the 12- to 15-month follow-up (Figure 2(a)). Data from one 28 small study (n = 31) of low-methodological and grade quality identified a greater number of men reporting improved climacturia in the pelvic floor muscle training plus electrical stimulation group compared to the no treatment control group (6/14 vs 0/16 in the control group), and the overall effect was significant (15.60, 95% CI = 0.95–254.91; P = 0.05; Figure 3(a)).
Figure 2.
Treatment effectiveness for erectile dysfunction: (a) PFMT plus ES for number of men reporting erectile function at 12–15 months follow-up; (b) PFMT plus BFB for erectile function at three months; (c) PFMT plus BFB versus no treatment control for number of men reporting erectile function at three months; and (d) PFMT versus no treatment for number of men reporting erectile function at 12 months follow-up.
BFB: biofeedback; ES: electrical stimulation; PFMT: pelvic floor muscle training.
Figure 3.
Treatment effectiveness for climacturia: (a) PFMT plus ES versus no treatment for number of men reporting improved climacturia at 15 months follow-up and (b) PFMT versus no treatment for number of men reporting improved climacturia at 12 months follow-up.
BFB: biofeedback; ES: electrical stimulation; PFMT: pelvic floor muscle training.
Pelvic floor muscle training plus biofeedback for erectile function
Meta-analyses of two24,29 methodologically high-quality, very low-grade studies (n = 122) found no significant differences between groups receiving pelvic floor muscle training plus biofeedback and no treatment for erectile function at three months post-intervention (4.44, 95% CI = 3.37–12.25; P = 0.26; Figure 2(b)). The pooled analysis of two studies,29,30 one of high methodological quality and the other of low-methodological quality, revealed a significant effect of pelvic floor muscle training plus biofeedback compared to the no treatment control for erectile function at the 12-month follow-up period (RR = 3.65, 95% CI = 1.02–13.05; P = 0.05; Figure 2(c)).
Pelvic floor muscle training alone for erectile function and climacturia
Meta-analyses of two 25 , 26 methodologically high-quality, moderate-grade studies with 734 participants found no statistically significant differences between groups receiving pelvic floor muscle training and no treatment control for erectile function (RR = 0.96, 95% CI = 0.85–1.07; P = 0.44; Figure 2(d)) or climacturia (RR = 1.01, 95% CI = 0.96–1.07; P = 0.65; Figure 3(b)) at the 12-month follow-up.
Discussion
The pooled analysis of high-quality, moderate-grade studies 25 , 26 revealed a non-significant effect for pelvic floor muscle training alone on erectile function and climacturia. However, when pelvic floor muscle training was augmented with biofeedback, a greater number of men reported improved erectile function in the intervention group compared to the control group at 12 months follow-up (17% vs 3% in the control group; Figure 2(c)). Nevertheless, the overall effect was of marginal significance; these results are supported by two studies29,30 of “high” quality and “very low” grade. Results at three months showed no significant effect of pelvic floor muscle training combined with biofeedback on erectile function. These findings indicate that men with postprostatectomy erectile dysfunction might benefit from long-term pelvic floor muscle training combined with biofeedback.
Less promising erectile function results supported by “low” methodological and “very low” grade evidence quality were obtained for pelvic floor muscle training supplemented with electrical stimulation compared to no treatment controls.27,28 Although a greater number of men reported improved climacturia in the pelvic floor muscle training plus electrical stimulation group compared with the no treatment control group 28 (42.8% vs 0% in the control group; Figure 3(b)), the overall effect was of marginal significance. Regardless, the safety of administrating electrical stimulation in the presence of cancer is still inconclusive. 31 Numerous studies have identified disseminated tumor cells in blood and bone marrow of men with prostate cancer;31–35 these cancer cells are reported to disseminate from the tumor early on.32,36 Although there is no empirical evidence for the spread of malignant cells by electrical stimulation, the current recommendation is to apply electrical stimulation to improve muscle mass and strength in terminally ill patients only. 31
Pelvic floor muscle training causes hypertrophy of pelvic floor muscles, increases muscle connective tissue strength, enhances awareness of muscles in the brain, and enables greater recruitment of active motor neurons. 37 However, success with pelvic floor muscle training is hampered by lack of adherence to training. Adherence to pelvic floor muscle training is influenced by patient- and therapy-related factors. 38 Patient-related factors to non-adherence include (1) low level of motivation, (2) perception of minimal benefit, and (3) forgetting to do exercises. 38 Therapy-related factors include (1) patient–therapist relationship (lack of connection and interaction with therapist) and (2) ineffective feedback of performance. 38 Of the seven included studies, only two27,29 tracked participant adherence with the exercises by making regular telephone calls to ensure they were performing exercises and to discuss barriers to performing exercises. Future trials evaluating effectiveness of pelvic floor muscle training are recommended to (1) make use of technology (Internet, mobile apps, etc.) and educational approaches to improve adherence to pelvic floor muscle training; 38 (2) use online electronic diaries instead of paper diaries (as patients who used electronic diaries are reported to be more compliant than those using paper diaries); 39 (3) make frequent telephone calls to remind patients to do the exercises; (4) arrange frequent visits with the intervention provider; and (5) make frequent assessments to inform participants the outcomes of exercises.
Supervised pelvic floor muscle training for at least three months has been shown to produce better outcomes than unsupervised training.37,40–42 However, only three of the included studies provided supervised training by a physiotherapist for three months.25,26,30 Future studies evaluating physiotherapy treatment effectiveness for the management of erectile dysfunction should provide adequate pelvic floor muscle training (for at least three months) by a trained physical therapist within the first few months postoperation.
The first step in pelvic floor muscle training is to identify and isolate the correct muscles; 37 contraction of the correct pelvic floor muscles leads to a scrotal lift and inward movement of the penis. 24 Visualization (with a mirror) is one way to ensure the correct muscles are contracting.24,43 However, four27–30 of the seven studies did not report having evaluated participants’ ability to contract pelvic floor muscles or instructing men to visualize the movement of the penis. Men with erectile dysfunction are required to time a voluntary contraction of the pelvic floor muscles during sexual activity to maintain penile hardness sufficient for vaginal penetration.25,44 Performing or timing a pelvic floor contraction during sexual activity is reported to increase the intracavernosal pressure to establish rigidity of the tumescent penis.25,44 However, only two studies 25 , 26 advised or taught men to perform voluntary contractions during sexual activity. Study protocols with inadequate supervision and advice could potentially lead to poor outcomes.
The comprehensive search strategy and use of psychometrically valid quality assessment tools are strengths of this review. Furthermore, language bias was eliminated by including studies published in all languages. The current systematic review has some limitations: (1) low quality and small sample size in included studies, (2) low number of studies included in the meta-analysis, and (3) some potentially relevant studies may have been missed either because of the search terms this review used or because they are indexed in databases not included in this review.
This systematic review found positive treatment effects for pelvic floor muscle training augmented with biofeedback for postprostatectomy erectile dysfunction. However, these results need to be considered with caution because meta-analysis was conducted using small number of studies (n = 2) of low to high methodological quality, very low-grade evidence. Data from one individual study found that pelvic floor muscle training augmented with electrical stimulation is beneficial for improving climacturia in men after prostatectomy. However, the safety of electrical stimulation for people with cancer remains uncertain. Phase 4 studies for identifying uncommon adverse effects are needed to test the safety of electrical stimulation in the presence of malignancy. The value of pelvic floor muscle training alone and in combination with therapies such as biofeedback and electrical stimulation for the management of erectile dysfunction and climacturia in men after prostatectomy remains uncertain. The evidence is limited; available evidence is of low quality. Therefore, rigorous, adequately powered, high-quality trials that comply with the Consolidated Standards of Reporting Trials (CONSORT) guidelines are required to produce a definitive answer. The effectiveness of pelvic floor muscle training and treatment success for improving erectile function in men cannot be investigated without due consideration of adherence factors. It is recommended that future studies evaluate strategies to increase adherence to a pelvic floor muscle training regimen. Future studies should include intensive supervision by a physiotherapist for at least three months and measures to evaluate the participants’ ability to contract their pelvic floor muscles prior to exercise prescription, and provide visual feedback for contraction instead of just verbal instructions.
Clinical messages.
Pelvic floor muscle training augmented with biofeedback improves erectile function in men after prostatectomy, but the evidence is limited.
Data from an individual study found pelvic floor muscle training combined with electrical stimulation to be beneficial for improving postprostatectomy climacturia.
However, electrical stimulation is only recommended for terminally ill patients.
Supplemental Material
Supplemental material, Supplemental_Material for Effectiveness of physiotherapy interventions for improving erectile function and climacturia in men after prostatectomy: a systematic review and meta-analysis of randomized controlled trials by Priya Kannan, Stanley J Winser, Lam Choi Ho, Leung C Hei, Lam C Kin, Garbien E Agnieszka and Leung HY Jeffrey in Clinical Rehabilitation
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Review registration: This systematic review is registered in the PROSPERO registry (CRD42017065255).
Supplemental material: Supplemental material for this article is available online.
ORCID iDs: Priya Kannan  https://orcid.org/0000-0003-2583-9614
https://orcid.org/0000-0003-2583-9614
Stanley J Winser  https://orcid.org/0000-0002-8766-3688
https://orcid.org/0000-0002-8766-3688
References
- 1. Tal R, Alphs HH, Krebs P, et al. Erectile function recovery rate after radical prostatectomy: a meta-analysis. J Sex Med 2009; 6(9): 2538–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bashir MN. Epidemiology of prostate cancer. Asian Pac J Cancer Prev 2015; 16(13): 5137–5141. [DOI] [PubMed] [Google Scholar]
- 3. O’Neil BB, Presson A, Gannon J, et al. Climacturia after definitive treatment of prostate cancer. J Urol 2014; 191(1): 159–163. [DOI] [PubMed] [Google Scholar]
- 4. Bratu O, Oprea I, Marcu D, et al. Erectile dysfunction post-radical prostatectomy—a challenge for both patient and physician. J Med Life 2017; 10(1): 13–18. [PMC free article] [PubMed] [Google Scholar]
- 5. Dall’Era JE, Mills JN, Koul HK, et al. Penile rehabilitation after radical prostatectomy: important therapy or wishful thinking? Rev Urol 2006; 8(4): 209–215. [PMC free article] [PubMed] [Google Scholar]
- 6. Capogrosso P, Ventimiglia E, Cazzaniga W, et al. Orgasmic dysfunction after radical prostatectomy. World J Mens Health 2017; 35(1): 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nilsson AE, Carlsson S, Johansson E, et al. Orgasm-associated urinary incontinence and sexual life after radical prostatectomy. J Sex Med 2011; 8(9): 2632–2639. [DOI] [PubMed] [Google Scholar]
- 8. Frey A, Sonksen J, Jakobsen H, et al. Prevalence and predicting factors for commonly neglected sexual side effects to radical prostatectomies: results from a cross-sectional questionnaire-based study. J Sex Med 2014; 11(9): 2318–2326. [DOI] [PubMed] [Google Scholar]
- 9. Gratzke C, Angulo J, Chitaley K, et al. Anatomy, physiology, and pathophysiology of erectile dysfunction. J Sex Med 2010; 7(1 pt 2): 445–475. [DOI] [PubMed] [Google Scholar]
- 10. Lavoisier P, Roy P, Dantony E, et al. Pelvic-floor muscle rehabilitation in erectile dysfunction and premature ejaculation. Phys Ther 2014; 94(12): 1731–1743. [DOI] [PubMed] [Google Scholar]
- 11. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6(7): e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Watson T. Guidance for the clinical use of electrophysical agents. In: Watson T. (ed.) Electrotherapy: evidence-based practice. Amsterdam: Elsevier Health Sciences, 2008, pp.363–388. [Google Scholar]
- 13. Maher CG, Sherrington C, Herbert RD, et al. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther 2003; 83(8): 713–721. [PubMed] [Google Scholar]
- 14. Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction —GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011; 64(4): 383–394. [DOI] [PubMed] [Google Scholar]
- 15. Maher CG. A systematic review of workplace interventions to prevent low back pain. Aust J Physiother 2000; 46(4): 259–269. [DOI] [PubMed] [Google Scholar]
- 16. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336(7650): 924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ 2004; 328(7454): 1490–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guyatt GH, Oxman AD, Vist G, et al. GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias). J Clin Epidemiol 2011; 64(4): 407–415. [DOI] [PubMed] [Google Scholar]
- 19. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 8. Rating the quality of evidence—indirectness. J Clin Epidemiol 2011; 64(12): 1303–1310. [DOI] [PubMed] [Google Scholar]
- 20. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 6. Rating the quality of evidence—imprecision. J Clin Epidemiol 2011; 64(12): 1283–1293. [DOI] [PubMed] [Google Scholar]
- 21. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 7. Rating the quality of evidence—inconsistency. J Clin Epidemiol 2011; 64(12): 1294–1302. [DOI] [PubMed] [Google Scholar]
- 22. Guyatt GH, Oxman AD, Montori V, et al. GRADE guidelines: 5. Rating the quality of evidence—publication bias. J Clin Epidemiol 2011; 64(12): 1277–1282. [DOI] [PubMed] [Google Scholar]
- 23. Borenstein M, Hedges LV, Higgins JP, et al. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods 2010; 1(2): 97–111. [DOI] [PubMed] [Google Scholar]
- 24. Dorey G, Speakman M, Feneley R, et al. Randomised controlled trial of pelvic floor muscle exercises and manometric biofeedback for erectile dysfunction. Br J Gen Pract 2004; 54(508): 819–825. [PMC free article] [PubMed] [Google Scholar]
- 25. Glazener C, Boachie C, Buckley B, et al. Urinary incontinence in men after formal one-to-one pelvic-floor muscle training following radical prostatectomy or transurethral resection of the prostate (MAPS): two parallel randomised controlled trials. Lancet 2011. a; 378(9788): 328–337. [DOI] [PubMed] [Google Scholar]
- 26. Glazener C, Boachie C, Buckley B, Cochran C, Dorey G, Grant A, et al. Urinary incontinence in men after formal one-to-one pelvic-floor muscle training following radical prostatectomy or transurethral resection of the prostate (MAPS): two parallel randomised controlled trials. Lancet. 2011. b; 378(9788): 328–337. [DOI] [PubMed] [Google Scholar]
- 27. Fode M, Borre M, Ohl DA, et al. Penile vibratory stimulation in the recovery of urinary continence and erectile function after nerve-sparing radical prostatectomy: a randomized, controlled trial. BJU Int 2014; 114(1): 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Geraerts I, Van Poppel H, Devoogdt N, et al. Pelvic floor muscle training for erectile dysfunction and climacturia 1 year after nerve sparing radical prostatectomy: a randomized controlled trial. Int J Impot Res 2015; 28(1): 9–13. [DOI] [PubMed] [Google Scholar]
- 29. Lin Y-H, Yu T-J, Lin Wang HP, et al. Effects of early pelvic-floor muscle exercise for sexual dysfunction in radical prostatectomy recipients. Cancer Nurs 2012; 35(2): 106–114. [DOI] [PubMed] [Google Scholar]
- 30. Prota C, Gomes C, Ribeiro L, et al. Early postoperative pelvic-floor biofeedback improves erectile function in men undergoing radical prostatectomy: a prospective, randomized, controlled trial. Int J Impot Res 2012; 24(5): 174–178. [DOI] [PubMed] [Google Scholar]
- 31. Laakso E-L, Young C. Electrophysical agents (EPAs) for symptom control in cancer care–what is the evidence? Phys Ther Rev 2010; 15(4): 334–343. [Google Scholar]
- 32. Morgan TM, Lange PH, Porter MP, et al. Disseminated tumor cells in prostate cancer patients after radical prostatectomy and without evidence of disease predicts biochemical recurrence. Clin Cancer Res 2009; 15(2): 677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bianco FJ, Jr, Wood DP, Jr, Gomes de Oliveira J, et al. Proliferation of prostate cancer cells in the bone marrow predicts recurrence in patients with localized prostate cancer. Prostate 2001; 49(4): 235–242. [DOI] [PubMed] [Google Scholar]
- 34. Ellis WJ, Pfitzenmaier J, Colli J, et al. Detection and isolation of prostate cancer cells from peripheral blood and bone marrow. Urology 2003; 61(2): 277–281. [DOI] [PubMed] [Google Scholar]
- 35. Morgan TM, Lange PH, Vessella RL. Detection and characterization of circulating and disseminated prostate cancer cells. Front Biosci 2007; 12: 3000–3009. [DOI] [PubMed] [Google Scholar]
- 36. Klein CA, Blankenstein TJ, Schmidt-Kittler O, et al. Genetic heterogeneity of single disseminated tumour cells in minimal residual cancer. Lancet 2002; 360(9334): 683–689. [DOI] [PubMed] [Google Scholar]
- 37. Felicíssimo MF, Carneiro MM, Saleme CS, et al. Intensive supervised versus unsupervised pelvic floor muscle training for the treatment of stress urinary incontinence: a randomized comparative trial. Int Urogynecol J 2010; 21(7): 835–840. [DOI] [PubMed] [Google Scholar]
- 38. Frawley HC, McClurg D, Mahfooza A, et al. Health professionals’ and patients’ perspectives on pelvic floor muscle training adherence-2011 ICS State-of-the-Science Seminar research paper IV of IV. Neurourol Urodyn 2015; 34(7): 632–639. [DOI] [PubMed] [Google Scholar]
- 39. Marceau LD, Link C, Jamison RN, et al. Electronic diaries as a tool to improve pain management: is there any evidence? Malden, MA: Blackwell Publishing Inc, 2007. [DOI] [PubMed] [Google Scholar]
- 40. Zanetti MRD, Castro Rde A, Rotta AL, et al. Impact of supervised physiotherapeutic pelvic floor exercises for treating female stress urinary incontinence. Sao Paulo Med J 2007; 125(5): 265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Konstantinidou E, Apostolidis A, Kondelidis N, et al. Short-term efficacy of group pelvic floor training under intensive supervision versus unsupervised home training for female stress urinary incontinence: a randomized pilot study. Neurourol Urodyn 2007; 26(4): 486–491. [DOI] [PubMed] [Google Scholar]
- 42. Dumoulin C, Hay-Smith J. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database Syst Rev 2010; 1(1): CD005654. [DOI] [PubMed] [Google Scholar]
- 43. Dorey G, Glazener C, Buckley B, et al. Developing a pelvic floor muscle training regimen for use in a trial intervention. Physiotherapy 2009; 95(3): 199–209. [DOI] [PubMed] [Google Scholar]
- 44. Cohen D, Gonzalez J, Goldstein I. The role of pelvic floor muscles in male sexual dysfunction and pelvic pain. Sex Med Rev 2016; 4(1): 53–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_Material for Effectiveness of physiotherapy interventions for improving erectile function and climacturia in men after prostatectomy: a systematic review and meta-analysis of randomized controlled trials by Priya Kannan, Stanley J Winser, Lam Choi Ho, Leung C Hei, Lam C Kin, Garbien E Agnieszka and Leung HY Jeffrey in Clinical Rehabilitation