Abstract
Purpose of the review.
Clinical trials of adult cell therapy for chronic heart failure (HF) are often misrepresented in an unfairly negative light. Results are claimed to be “negative”, “incremental”, or “modest”. This common misconception is detrimental to medical progress and needs to be dispelled.
Recent findings.
Contrary to the false narrative of scientific and lay media, the outcome of recent trials of cell therapy for HF has been encouraging and even exciting. Specifically, with the exception of ALLSTAR, in the past 2 years several Phase II-III double-blind, randomized trials have yielded impressive results, demonstrating not just safety but also salubrious effects on cardiac function (MSC-HF) or clinical events (MSC-HF, CONCERT-HF, and DREAM-HF) for at least 1 year after a single administration of cells. Such outcomes were neither incremental nor minor, nor achievable with one dose of any other non-device therapy for HF.
Summary.
The oft-repeated assertion that cell therapy does not benefit patients with chronic HF is based on a misrepresentation of the literature and is contrary to the available scientific evidence. Although the mechanism of action of cell therapy is unclear, research on its use in HF should continue, as only rigorous, well-designed, Phase III trials can definitely confirm or refute its efficacy.
Keywords: Cell therapy, heart failure, stem cells, progenitor cells, cardiac repair
Introduction
The status of cell therapy for heart failure (HF) has been extensively reviewed [1]. The purpose of this article is to summarize the trials of cell therapy in heart failure (HF) that have been published (or presented) in the past 18 months.
Recent trials of cell therapy in HF
1. ALLSTAR.
ALLSTAR was a Phase II, randomized, double-blind, placebo controlled, multicenter trial of cardiosphere-derived cells (CDCs) in patients who had suffered a myocardial infarction (MI) in the previous 1–12 months and had LV dysfunction [2, 3]. A total of 142 patients were randomized 2:1 to intracoronary infusion of 25×106 allogeneic CDCs or placebo, at an average of 4.6 months after MI. The primary endpoint was the size of the LV scar, measured by MRI, at 12 months after treatment. However, the sponsor stopped the trial early, after an interim analysis of the data obtained at 6 months of follow-up revealed futility to meet the primary endpoint; thus, results at 12 months are not available. The results obtained at 6 months showed that there was no difference in scar size between the CDC-treated and the placebo groups. There was also no significant difference in LVEF, although there was a small decrease in LVEDV (−4.5 mL), LVESV (−4.8 mL), and plasma levels of NT-proBNP (−303 pg/mL) in the CDC-treated group vs. placebo. Thus, ALLSTAR failed to reproduce the results of CADUCEUS, an earlier Phase I, single-center, open-label, Phase I study of 31 patients [4].
Interpretation of ALLSTAR is problematic for many reasons. The primary endpoint was not assessed because the study was stopped before the follow-up was completed. The decision to report results at 6 months instead of 12 months was made post hoc. The study population was quite heterogeneous because it included patients with a recent MI (1–2 months) and with an old MI (> 6 months), and patients with both STEMI and NSTEMI. The average baseline LV EF was 40%, indicating that the degree of LV dysfunction was relatively minor. Important clinical data, including medications, presence of multi-vessel disease, comorbidities, diastolic dysfunction, and body mass index, were not collected consistently. There was no information on the type of MI in 58 of the 142 patients. Taken together, these issues make it difficult to interpret the results of ALLSTAR [2, 3] in a meaningful manner.
2. MSC-HF.
Some of the most encouraging results in the field of cell therapy for HF have come from MSC-HF, a Phase II, randomized, double-blind, placebo-controlled study of autologous bone marrow (BM)-MSCs in ischemic HF [5, 6]. The initial 6-month results were published a few years ago [6], and the final 4-year data in 2020 [5]. In this study, 60 patients with ischemic HF (NYHA class II-III; LV EF < 45% by MRI) were randomized 2:1 to transendocardial injection of autologous BM-MSCs or placebo and followed for 12 months; in addition, data on hospitalizations and survival were obtained at 4 years. The primary endpoint was the change in LVESV at 12 months. This endpoint was met: the trial showed a highly significant reduction in LVESV of 17 mL (P < 0.0002) at 12 months. In addition, MSC-treated patients had a highly significant improvement in LVEF (+ 6.2 units; P <0.0001), myocardial mass (+ 9.8 g; P = 0.09), and quality of life at 12 months (Figure 1). The data collected at 4 years of follow-up showed a significant reduction in the hospitalizations for angina in the MSC group, although there were no significant differences in overall hospitalization or survival [5].
Figure 1.
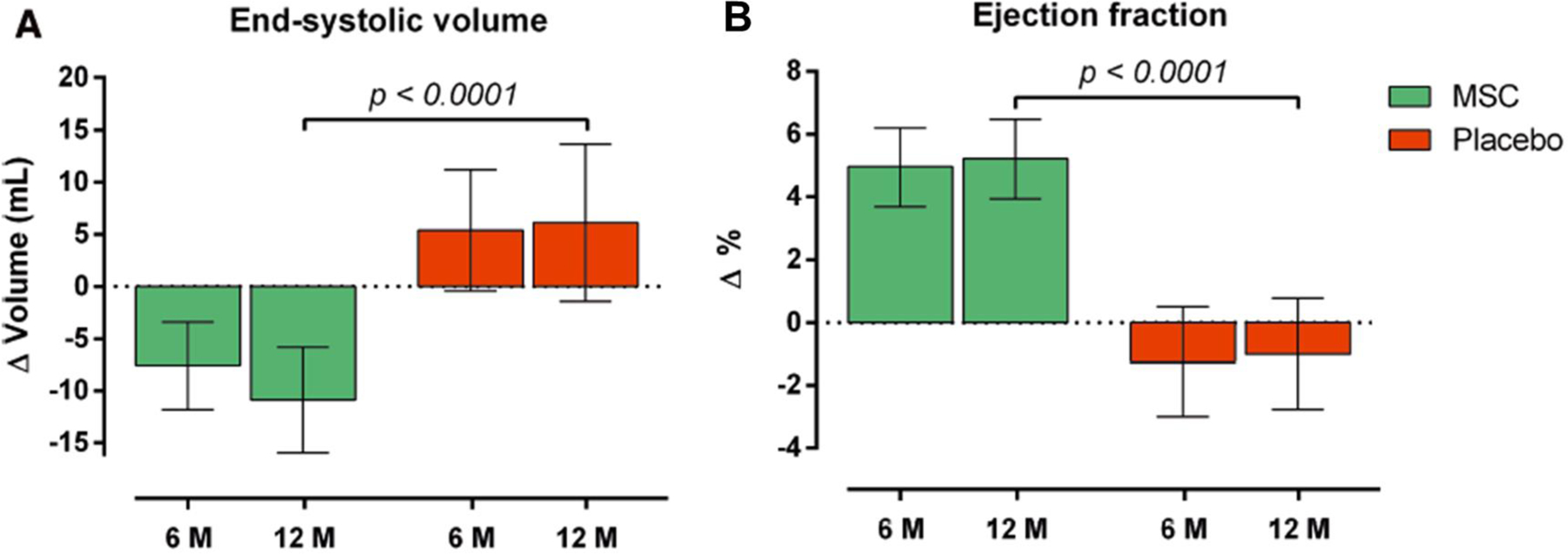
Effect of autologous BM-MSCs on LV end-systolic volume and ejection fraction in the MSC-HF trial. Reproduced with permission from [5].
Several features of MSC-HF should be underscored. First, this trial met its primary endpoint, thereby refuting misleading statements in the literature that no cell therapy study has met its primary endpoint in cardiac disease [7]. Secondly, the differences between MSC-treated and placebo patients were highly significant. Thirdly, the effects of a single injection of MSCs were long-lasting, being observed for at least 12 months and, in the case of hospitalization for angina, for at least 4 years after treatment. Finally, these impressive results were obtained despite the fact that this trial used autologous MSCs, which are now obsolete, since virtually all current trials of cell therapy use allogeneic cells [1]. Since allogeneic cell products are obtained from young, healthy donors, it seems very likely that they will be more effective than autologous products obtained from older patients with significant comorbidities - a concept supported by published clinical trials, such as POSEIDON [8] and POSEIDON-DCM [9]. If this is the case, the results of MSC-HF would be expected to be even more robust with the use of allogeneic MSCs.
Another interesting aspect of MSC-HF is that patients were given different amounts of MSCs, depending on the amount and quality of the BM that was harvested in each of them. This heterogeneity provided an opportunity to examine the dose-response relationship between cell number and outcome, which can rarely be done in clinical trials since the dose of cells is usually fixed. MSC-HF found that the greatest benefits (reduction in LVESV and LVEF) were observed in the upper tertile of cell doses (> 83×106 cells) and that the changes observed in this tertile were significantly greater than those observed in the lower tertile (< 43×106 cells). These data suggest a positive relationship between the number of MSCs injected and the clinical outcome; a conclusion that is also supported by the results of the TRIDENT study [10]. Based on the results of MSC-HF and TRIDENT, it appears that transendocardial doses of BM-MSCs should be in the 100–150×106 range.
3. CONCERT-HF.
CONCERT-HF was a Phase II, randomized, placebo-controlled, double blind, multicenter study of autologous BM-MSCs, CPCs, or both, in patients with ischemic HF [11, 12]. The study was conducted by the Cardiovascular Cell Therapy Research Network (CCTRN), a network that was sponsored by the NHLBI and that the NHLBI decided not to renew 2 years ago. CONCERT-HF was arguably the most rigorous clinical trial of cell therapy for HF conducted heretofore. The oversight of this study was extraordinarily rigorous, including a Protocol Review Committee, a Data Safety and Monitoring Board, a Steering Committee, a Protocol Development Committee, the local Institutional Review Boards, and the Food and Drug Administration. Developing the final protocol required several years of discussions and review of data and protocols by the various bodies.
CONCERT-HF started with a pilot, open-label study that was mandated by the FDA to assess feasibility and safety [11, 12]. It included 18 patients who were randomized to either the combination of autologous MSCs and CPCs or standard of care, and were followed for 3 months. The main study consisted of 125 patients with ischemic HF enrolled at 7 CCTRN centers from November 2016 to October 2018. The average LV EF was 29%, the average scar size 19.4% of LV mass, and most patients (80%) were in NYHA class II, with 15% in class III. Patients were randomized 1:1:1:1 to the combination of MSCs and CPCs, MSCs alone, CPCs alone, or placebo (target doses, 150×106 MSCs and 5×106 CPCs). All patients underwent BM aspiration and right heart catheterization. In order to limit adverse events, endomyocardial biopsy was performed only in patients from whom CPCs were grown (MSCs + CPCs and CPCs alone groups); in the other 2 groups, a “sham biopsy” was performed to maintain patient blinding. All study products were injected transendocardially using the NOGA XP Mapping System. Efficacy endpoints included HF-related MACE (all-cause death, hospitalization for worsening HF, or HF exacerbation that did not require hospitalization); quality of life, assessed by the Minnesota Living With Heart Failure questionnaire (MLHFQ) score; LV function and structure, assessed by MRI; functional capacity, assessed as peak VO2 and 6-minute walking distance; and plasma levels of NT-proBNP.
Using an intention-to-treat analysis, the results showed a significant reduction in HF-MACE in patients receiving CPCs only or the combination of MSCs+CPCs, driven primarily by a reduction in hospitalization for worsening HF (Figure 2). Compared with placebo, there was a relative 77% reduction in HF-MACE in the CPCs alone group (P = 0.043) and a 68% reduction in the MSCs+CPCs group (P = 0.061). The results of the as-treated analysis were similar (Figure 3) (the as-treated analysis was conducted because in several patients the treatment administered was different from that of the group to which they were randomized). Interestingly, the as-treated analysis showed that the 15 untreated patients had a similar outcome to the placebo-treated patients, therefore corroborating the results of the study (Figure 3). Cox regression analysis showed a significant reduction in the incidence of HF-MACE in the CPC only group (hazard ratio vs. placebo, 0.20) and in the MSCs+CPCs group (hazard ratio, 0.26) (Figure 4). In addition, quality of life, as measured by the MLHFQ score, was significantly improved at 6 months and 12 months in the MSCs+CPCs group, and at 6 months in the MSCs alone group. There were no significant differences among groups in LV EF, LV volumes, scar size, peak VO2, 6-min walking distance, or NT-proBNP levels. Taken together, the results of CONCERT-HF suggest that in patients with chronic ischemic HF on maximal guideline-driven therapy, a single administration of autologous CPCs or MSCs has measurable beneficial effects over the ensuing 12 months, namely, a reduction in hospitalization for HF (with CPCs) and an improvement in quality of life (with MSCs). The best overall results were obtained by combining MSCs with CPCs: reduced HF-MACE and improved quality of life [11, 12].
Figure 2.
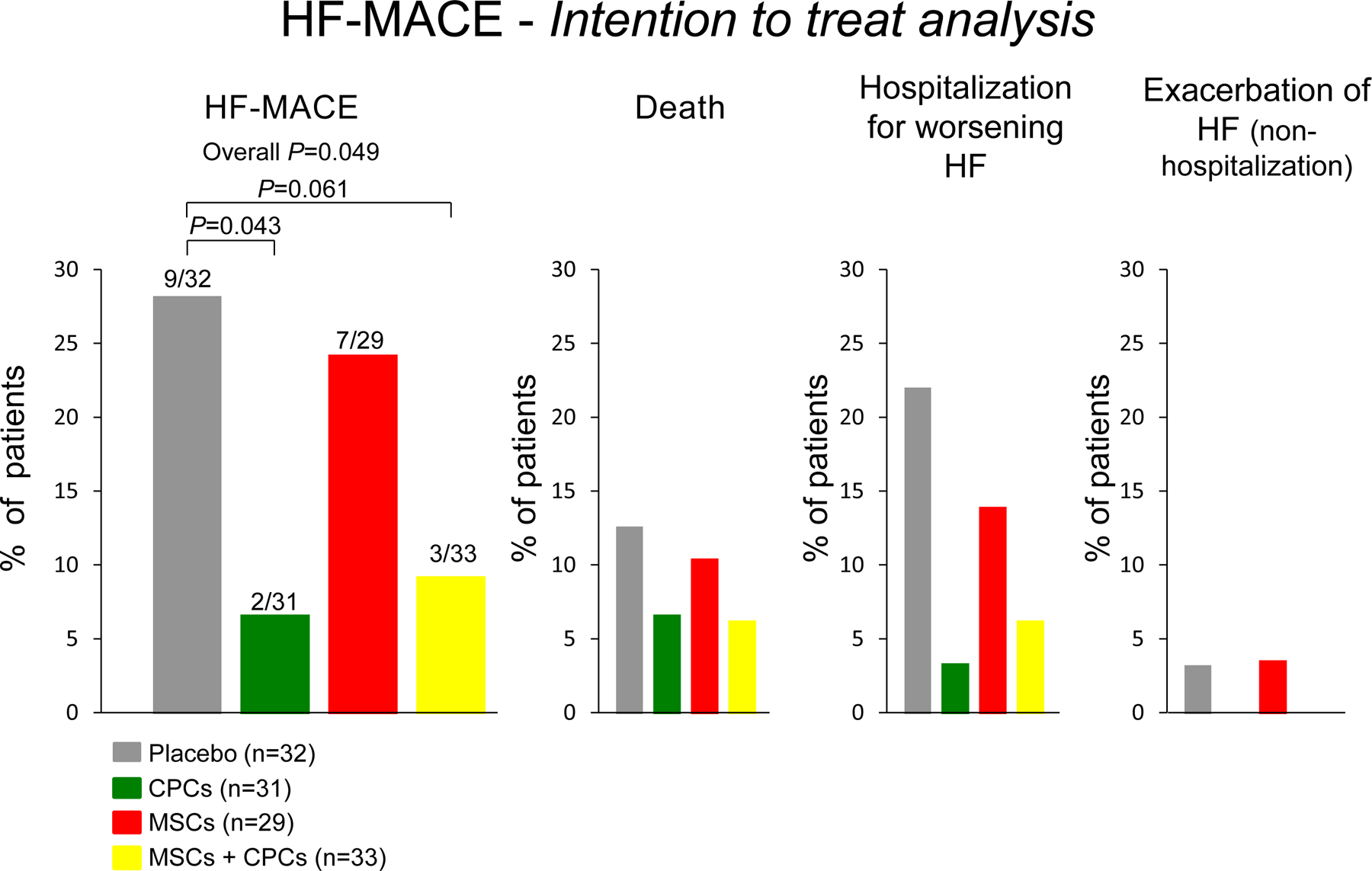
Effects of autologous c-kit-positive cardiac cells (CPCs), bone marrow mesenchymal stromal cells (MSCs), and their combination on HF-MACE in the CONCERT-HF trial: shown are results of intention-to-treat analysis. Reproduced with permission from [12].
Figure 3.
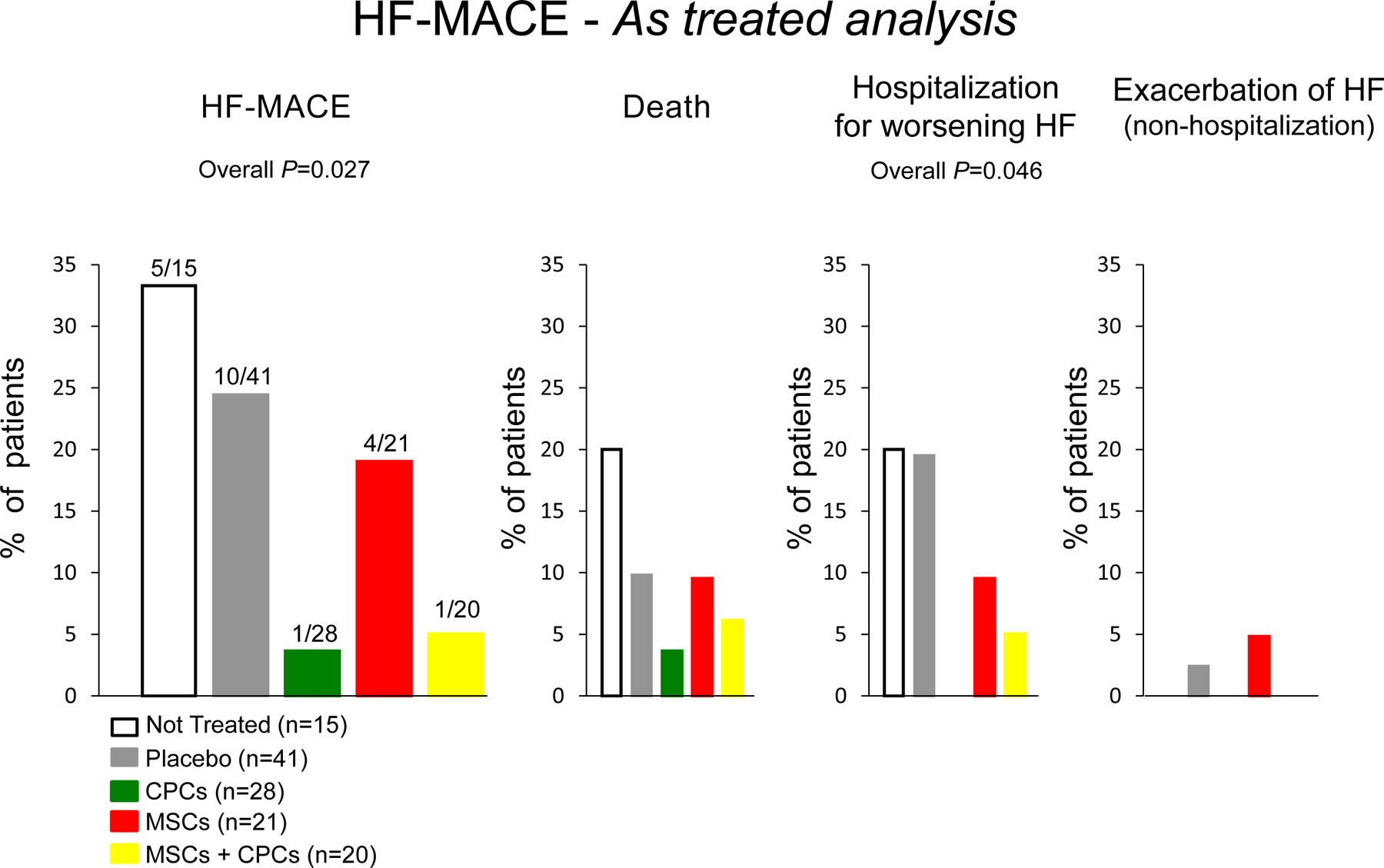
Effects of autologous c-kit-positive cardiac cells (CPCs), bone marrow mesenchymal stromal cells (MSCs), and their combination on HF-MACE in the CONCERT-HF trial: shown are results of as-treated analysis. Reproduced with permission from [12].
Figure 4.
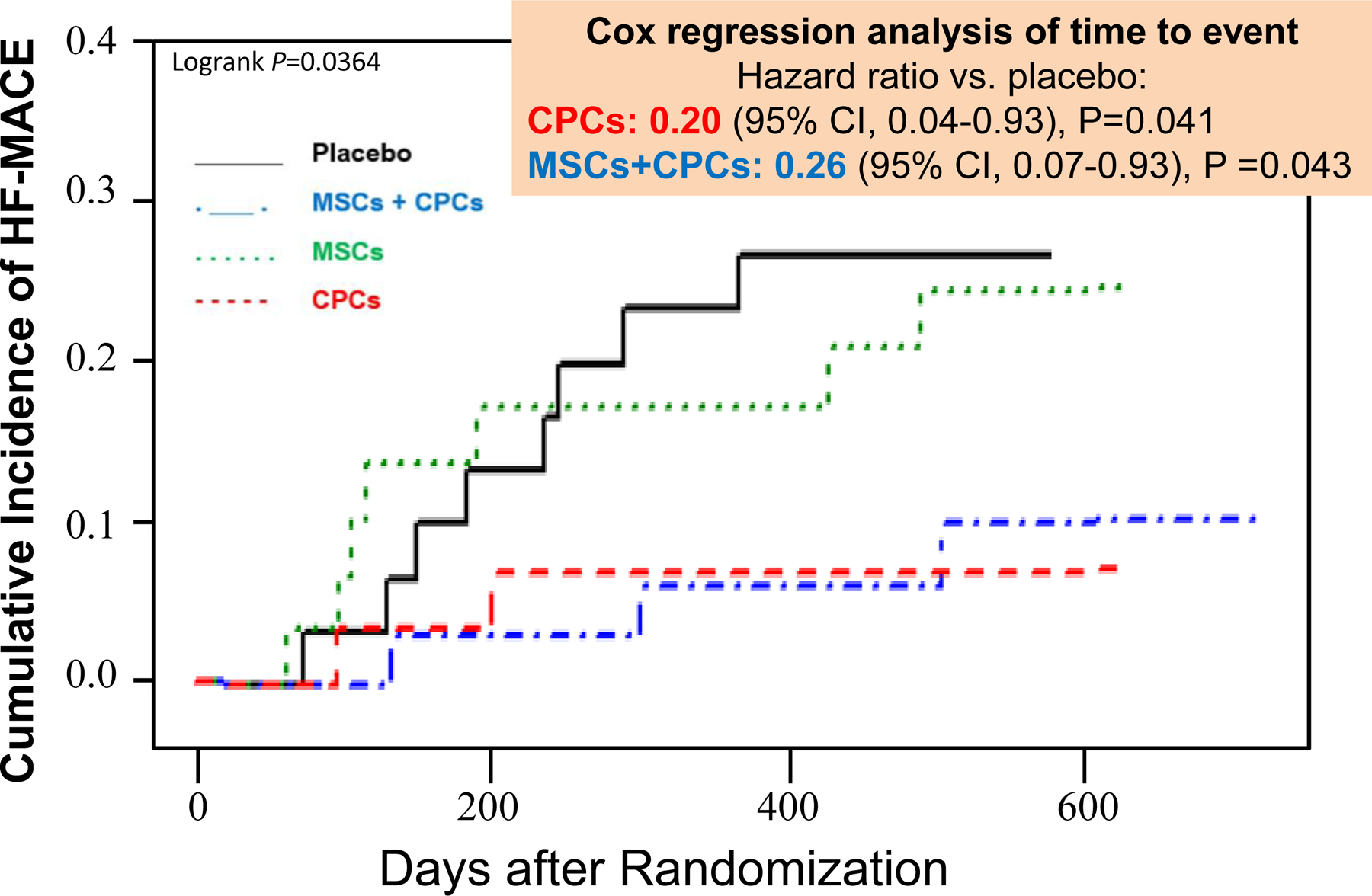
Effects of autologous c-kit-positive cardiac cells (CPCs), bone marrow mesenchymal stromal cells (MSCs), and their combination on cumulative incidence of HF-MACE in the CONCERT-HF trial: shown are results of intention-to-treat analysis. Reproduced with permission from [12].
CONCERT-HF was the first trial that used CPCs manufactured according to GMP standards.
Since neither CPCs nor MSCs improved LV function or reduced scar size, the mechanism of action of these cells remains unclear. However, improvement in clinical outcomes without an improvement in LV function or a reduction in scar size is consistent with other studies, such as TAC-HFT [13] and ixCELL-DCM [14] (Figure 5). It is possible that the beneficial effects of CPCs and MSCs may be related to their anti-inflammatory and immunomodulatory actions [15], consistent with the notion that chronic inflammation contributes to the progression of HF [16]. Other potential mechanisms include antifibrotic, proangiogenic, endothelial protective, or perhaps as-yet unknown actions of these cells [1, 15]. This is undoubtedly the most important question currently facing investigators in the field of cell therapy.
Figure 5.
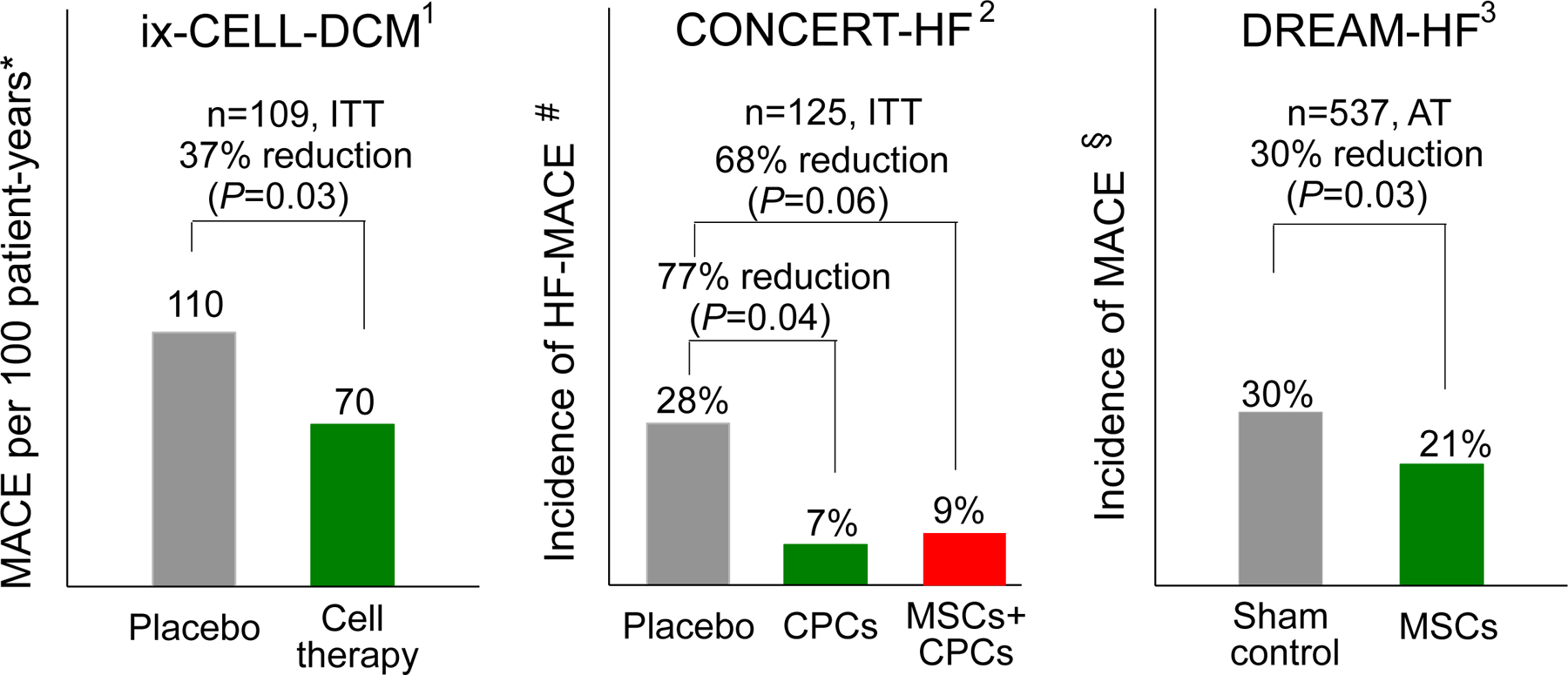
Randomized, double-blind, Phase II or III trials that have found improvement in a hard endpoint (reduction in MACE) in patients treated with cell therapy despite lack of improvement in LV function. Reproduced with permission from [1].
* MACE: all-cause death, cardiovascular hospitalization, or HF exacerbation
# HF-MACE: all-cause death, HF hospitalization, or HF exacerbation
§ MACE: cardiovascular death, MI, or stroke
ITT, intention-to-treat analysis; AT, as-treated analysis
1, ref. [14]
2, ref. [12]
3, ref. [17]
Despite the uncertainty regarding the mechanism of action, CONCERT-HF suggests that CPCs and MSCs have disease-modifying properties in ischemic HF. The results of CONCERT-HF are very encouraging and certainly warrant follow-up, larger Phase III trials to definitively examine the efficacy of MSCs and CPCs.
4. DREAM-HF.
The long-awaited results of DREAM-HF [17] have been recently presented in part [18], although at the time of this writing they have not been published. This is a very important study because it is the largest trial of cell therapy for HF to date. DREAM-HF was a randomized, double blind, sham-controlled, Phase III study that involved 59 centers in North America. The study population consisted of 537 patients with high-risk, chronic ischemic or non-ischemic HF (NYHA class II or III) on a stable, optimally tolerated guideline driven medical therapy for at least 1 month prior to randomization. The average LVEF was 28%. Patients were randomized to receive either transendocardial injection of 150 × 106 allogeneic BM-MSCs, which were immunoselected using anti-STRO-3 antibodies, or a sham catheterization procedure that did not include placebo. The duration of the follow-up varied because the study was event-driven, with a median of 30 months. The primary outcome was recurrent decompensated HF events.
There were no major adverse events except 1 incidence of LV perforation during LV mapping. Administration of MSCs did not reduce the primary endpoint. However, it did reduce pre-specified secondary endpoints, including a 65% reduction in non-fatal MI or non-fatal stroke across all patients (P = 0.001) and a 57% reduction in cardiac death in NYHA II patients (P = 0.044) but not in the total population or in NYHA III patients. MPC treatment resulted in a 33% reduction in the composite of cardiac death, non-fatal MI, or non-fatal stroke (P = 0.021), which appear to be entirely driven by patients with baseline elevated (≥ 2 mg/L) high-sensitivity C-reactive protein (hsCRP) (45% reduction, P = 0.012) [18].
The results of DREAM-HF are very encouraging. They show that a single administration of MSCs in patients with optimal guideline-driven therapy has beneficial effects on clinical outcome, including non-fatal MI, non-fatal stroke, and cardiac death after a median follow-up of 30 months. Although the mechanism is unclear, the MSCs used in this trial are known to have proangiogenic and anti-inflammatory properties [17]. It is possible that the beneficial effects observed in DREAM-HF reflect, at least in part, the ability of MSCs to reduce inflammation in large vessels throughout the body, thereby preventing MI and stroke, particularly since they were most apparent in patients with baseline inflammation (hsCRP ≥ 2 mg/L).
The results of DREAM-HF are important because, for the first time, a Phase III trial has demonstrated that cell therapy can beneficially affect hard clinical endpoints, such as cardiac death, MI, or stroke, in HF patients. It is noteworthy that these effects were observed after a single administration of cells and in patients who were on maximal guideline-directed medical therapy for HF. At present, DREAM-HF has not been published and some of the secondary endpoints, such as LV volumes and function, NT-proBNP, functional capacity, and quality of life have not been released. Despite these caveats, the results of DREAM-HF are provoking and warrant further investigation. Fortunately, the sponsor of DREAM-HF is planning a follow-up Phase III study targeted at patients with evidence of systemic inflammation (elevated hsCRP), in which the effect of MSCs on non-fatal MI, non-fatal stroke, and cardiac death will be the primary endpoint.
SENECA.
SENECA was the first study that used cell therapy in patients with HF caused by anthracycline-induced cardiomyopathy [19, 20]. This was a Phase I, randomized, placebo-controlled, double blind, multicenter study sponsored by the CCTRN. It randomized patients to allogeneic BM-MSCs or placebo, both of which were injected by the transendocardial route. The study met its primary endpoints of safety and feasibility. The trial was small (31 patients) and therefore not powered for efficacy. Nevertheless, there was a significant improvement in functional capacity (measured as 6-min walking distance) and quality of life (measured as MLHFQ score) in patients treated with MSCs [20]. Other endpoints, including LV function and volumes, scar size, NT-proBNP, were not significantly different between the 2 groups. The results of SENECA are consistent with those of CONCERT-HF [11, 12], ixCELL-DCM [14], and TAC-HFT [13], all of which suggest that cell therapy can produce clinical benefit without changes in LVEF. Overall, the results of SENECA are encouraging and warrant larger studies in patients with anthracycline-induced cardiomyopathy to assess the efficacy of cell therapy.
Conclusions
Contrary to the narratives that are frequently encountered in both scientific and lay publications, the results obtained heretofore in clinical trials of cell therapy in HF are encouraging and support continued investigation. Adult cell therapy is safe, and multiple randomized, placebo-controlled, double blind studies have indicated beneficial effects of a single dose of cells in HF. With the exception of devices, no other HF therapy has shown disease-modifying properties for 12 months after a single dose. It seems plausible that repeated doses would be even more effective. Thus, larger, rigorous trials are warranted. Given the epidemic proportions of HF and the limitations of current therapies, it would be unreasonable, and possibly unethical, to halt clinical investigation of cell therapy in this population.
Supplementary Material
Key Points.
Adult cell therapy is safe, and several randomized, double-blind, multicenter Phase II or III trials have demonstrated salubrious effects of cells for at least 1 year after a single administration in HF patients.
Although the mechanism of action is unclear, the beneficial effects of cell therapy in these trials were neither incremental nor minor.
Thus, research on the use of cell therapy in heart failure should continue, as only rigorous, well-designed, Phase III trials can definitely confirm or refute its efficacy.
Acknowledgements
This work was supported by National Institutes of Health grant P01 HL078825.
Footnotes
Conflicts of Interest
No conflicts
References
- 1. Bolli R, Solankhi M, Tang XL, Kahlon A. Cell Therapy in Patients with Heart Failure: A Comprehensive Review and Emerging Concepts. Cardiovasc Res. 2021. Apr 19;cvab135. doi: 10.1093/cvr/cvab135. * A comprehensive, detailed, and up-to-date review of all clinical trials of cell therapy in HF over the past 10 years.
- 2.Chakravarty T, Makkar RR, Ascheim DD, Traverse JH, Schatz R, DeMaria A, et al. ALLogeneic Heart STem Cells to Achieve Myocardial Regeneration (ALLSTAR) Trial: Rationale and Design. Cell Transplant. 2017;26(2):205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Makkar RR, Kereiakes DJ, Aguirre F, Kowalchuk G, Chakravarty T, Malliaras K, et al. Intracoronary ALLogeneic heart STem cells to Achieve myocardial Regeneration (ALLSTAR): a randomized, placebo-controlled, double-blinded trial. Eur Heart J. 2020;41(36):3451–8. [DOI] [PubMed] [Google Scholar]
- 4.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379(9819):895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mathiasen AB, Qayyum AA, Jørgensen E, Helqvist S, Kofoed KF, Haack-Sørensen M, et al. Bone marrow-derived mesenchymal stromal cell treatment in patients with ischaemic heart failure: final 4-year follow-up of the MSC-HF trial. Eur J Heart Fail. 2020;22(5):884–92. ** MSC-HF was a Phase II, randomized, double-blind, placebo-controlled study of autologous bone marrow-MSCs in ischemic HF. It showed a robust improvement in LV function and other clinical outcomes, with a follow-up of 4 years.
- 6.Mathiasen AB, Qayyum AA, Jørgensen E, Helqvist S, Fischer-Nielsen A, Kofoed KF, et al. Bone marrow-derived mesenchymal stromal cell treatment in patients with severe ischaemic heart failure: a randomized placebo-controlled trial (MSC-HF trial). Eur Heart J. 2015;36(27):1744–53. [DOI] [PubMed] [Google Scholar]
- 7.A futile cycle in cell therapy. Nat Biotechnol. 2017;35(4):291. [DOI] [PubMed] [Google Scholar]
- 8.Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, et al. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. Jama. 2012;308(22):2369–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hare JM, DiFede DL, Rieger AC, Florea V, Landin AM, El-Khorazaty J, et al. Randomized Comparison of Allogeneic Versus Autologous Mesenchymal Stem Cells for Nonischemic Dilated Cardiomyopathy: POSEIDON-DCM Trial. J Am Coll Cardiol. 2017;69(5):526–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Florea V, Rieger AC, DiFede DL, El-Khorazaty J, Natsumeda M, Banerjee MN, et al. Dose Comparison Study of Allogeneic Mesenchymal Stem Cells in Patients With Ischemic Cardiomyopathy (The TRIDENT Study). Circ Res. 2017;121(11):1279–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolli R, Hare JM, March KL, Pepine CJ, Willerson JT, Perin EC, et al. Rationale and Design of the CONCERT-HF Trial (Combination of Mesenchymal and c-kit(+) Cardiac Stem Cells As Regenerative Therapy for Heart Failure). Circ Res. 2018;122(12):1703–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bolli R, Mitrani RD, Hare JM, Pepine CJ, Perin EC, Willerson JT, et al. A Phase II study of autologous mesenchymal stromal cells and c-kit positive cardiac cells, alone or in combination, in patients with ischaemic heart failure: the CCTRN CONCERT-HF trial. Eur J Heart Fail. 2021;23(4):661–74. ** CONCERT-HF was a Phase II, randomized, placebo-controlled, double blind, multicenter study of autologous c-kit-positive cardiac cells (CPCs), bone marrow mesenchymal stromal cells (BM-MSCs), or both, in patients with ischemic HF. This was the first trial that used CPCs manufactured according to GMP standards. It showed an impressive ~70% reduction in MACE (particularly rehospitalization for HF) after administration of CPCs with or without BM-MSCs.
- 13.Heldman AW, DiFede DL, Fishman JE, Zambrano JP, Trachtenberg BH, Karantalis V, et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. Jama. 2014;311(1):62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel AN, Henry TD, Quyyumi AA, Schaer GL, Anderson RD, Toma C, et al. Ixmyelocel-T for patients with ischaemic heart failure: a prospective randomised double-blind trial. Lancet. 2016;387(10036):2412–21. [DOI] [PubMed] [Google Scholar]
- 15.Wysoczynski M, Khan A, Bolli R. New Paradigms in Cell Therapy: Repeated Dosing, Intravenous Delivery, Immunomodulatory Actions, and New Cell Types. Circ Res. 2018;123(2):138–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy SP, Kakkar R, McCarthy CP, Januzzi JL Jr. Inflammation in Heart Failure: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75(11):1324–40. [DOI] [PubMed] [Google Scholar]
- 17.Borow KM, Yaroshinsky A, Greenberg B, Perin EC. Phase 3 DREAM-HF Trial of Mesenchymal Precursor Cells in Chronic Heart Failure. Circ Res. 2019;125(3):265–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kosyakovsky L Stem Cells Did Not Decrease Heart Failure Readmissions in DREAM-HF https://cardiologynownews.org/stem-cells-did-not-decrease-heart-failure-readmissions-in-dream-hf/. 2021:(November 15, 2021). ** Preliminary results of the long-awaited DREAM-HF trial, the largest trial of cell therapy for HF to date. DREAM-HF was a randomized, double blind, sham-controlled, Phase III study that involved 537 patients with chronic ischemic or non-ischemic HF. It showed a 33% reduction in the composite of cardiac death, non-fatal MI, or non-fatal stroke. DREAM-HF is important because, for the first time, a Phase III trial has demonstrated that cell therapy can beneficially affect hard clinical endpoints, such as cardiac death, MI, or stroke, in HF patients.
- 19.Bolli R, Hare JM, Henry TD, Lenneman CG, March KL, Miller K, et al. Rationale and Design of the SENECA (StEm cell iNjECtion in cAncer survivors) Trial. Am Heart J. 2018;201:54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bolli R, Perin EC, Willerson JT, Yang PC, Traverse JH, Henry TD, et al. Allogeneic Mesenchymal Cell Therapy in Anthracycline-Induced Cardiomyopathy Heart Failure Patients: The CCTRN SENECA Trial. J Am Coll Cardiol CardioOnc. 2020;2(4):581–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


