Abstract
Simple Summary
Angiogenesis, the formation of new blood vessels from preexisting ones, is a complex and demanding biological process that plays an important role in physiological, as well as pathological conditions, including cancer. During tumor growth, the induction of angiogenesis allows tumor cells to grow, invade, and metastasize. Recent evidence supports endothelial cell metabolism as a critical regulator of angiogenesis. However, whether and how tumor endothelial cells rewire their metabolism in cancer remains elusive. In this review, we discussed the metabolic signatures of tumor endothelial cells and their symbiotic, competitive, and mechanical metabolic interactions with tumor cells. We also discussed the recent works that may provide a rationale for attractive metabolic targets and strategies for developing specific therapies against tumor angiogenesis.
Abstract
Cancer is a leading cause of death worldwide. If left untreated, tumors tend to grow and spread uncontrolled until the patient dies. To support this growth, cancer cells need large amounts of nutrients and growth factors that are supplied and distributed to the tumor tissue by the vascular system. The aberrant tumor vasculature shows deep morphological, molecular, and metabolic differences compared to the blood vessels belonging to the non-malignant tissues (also referred as normal). A better understanding of the metabolic mechanisms driving the differences between normal and tumor vasculature will allow the designing of new drugs with a higher specificity of action and fewer side effects to target tumors and improve a patient’s life expectancy. In this review, we aim to summarize the main features of tumor endothelial cells (TECs) and shed light on the critical metabolic pathways that characterize these cells. A better understanding of such mechanisms will help to design innovative therapeutic strategies in healthy and diseased angiogenesis.
Keywords: tumor vasculature, tumor endothelial cells, endothelial metabolism in cancer
1. Introduction
1.1. Blood Vessel Formation
The vascular tree is a dense network of closed blood vessels that branch out along the entire body. This vascular network carries oxygen and nutrients to the tissues and drains waste products [1]. Although blood vessels in our body are very different in shape and size, all share the same structure, such as a monolayer of endothelial cells (ECs) that delimits their walls. Under physiological conditions, an adult vasculature is composed of quiescent ECs that, under specific circumstances, can reactivate cell proliferation and migration, becoming activated proliferating ECs. This reactivation and the formation of new blood vessels from pre-existing vessels, is a process known as angiogenesis [2]. This process occurs in physiological as well as pathological conditions [3]. In addition to embryonic development, angiogenesis takes place in adult tissues such as during wound repair and the female reproductive cycle. Angiogenesis can also occur during tumor growth, and the induction of angiogenesis within solid tumors is a cancer hallmark [4]. The discovery of the vascular endothelial growth factors (VEGFs) [5], and therefore the existence of surface receptors capable of binding such ligands, which, due to their surface localization, are easily druggable, has led to the development of biological anti-angiogenic drugs capable of targeting these receptors [6]. Nevertheless, the addition of these drugs to chemotherapy has shown modest effects on patient survival: tumors often escape treatments and regain angiogenic capacity or gain access to the bloodstream by co-opting non-malignant vessels within themselves [7], resulting in a worse prognosis. This led to a paradigm shift in anti-angiogenic therapeutic strategies, from starving the tumor by destroying tumor blood vessels to “normalize” the tumor vasculature in order to better deliver anti-tumor drugs [8]. Another piece of the puzzle came from the discovery that tumor ECs are highly dependent on metabolic processes, such as glycolysis [9]. This observation opened the innovative hypothesis of being able to target the ECs’ metabolism to normalize the vasculature of tumors and thus prevent their growth [10,11]. Understanding tumor EC metabolism is mandatory to better design therapeutic strategies.
1.2. Tumor Vasculature
Tumor cells shape the tumor microenvironment (TME) [12]. Rapidly growing solid tumors (e.g., carcinomas) incorporate various cell types such as fibroblasts and immune cells into their parenchyma to secrete factors that confer survival benefits [13]. As the volume of the tumor grows, the diffusion of oxygen (O2) decreases making the center of mass hypoxic and necrotic. Low oxygen levels activate the hypoxia inducible factor (HIF), which in turn leads to the expression of growth factors such as VEGF that trigger angiogenesis [14]. This implicates that tumor mass volume is critical for the angiogenic switch [15,16]. The uncontrolled hyper-release of pro-angiogenic factors causes abnormal vessel growth. Unlike the normal vasculature, which has a high hierarchical organization in vascular beds (veins, arteries, and capillaries), the tumor vasculature appears as a disorganized tangle of vessels [17] often with a blind end. Tumor endothelial cells (TECs), unlike normal endothelial cells (NECs), can grow in multilayers along the vessel wall [18]. Moreover, the reduction of cellular junctions [19], the decrease in mural cells (pericytes and smooth muscle cells) [20], and a strong compromise of the basement membrane determine vessel leakage. This generates an increase in the interstitial fluids pressure that in turn causes the collapse of the less resistant vessels [21], while the blood flow within the tumor vessels becomes chaotic [22]. The microhemorrhages of these vessels allow the deposition of fibrin, which acts as an anchor for the tumor cells [23]. In addition, the increase in stiffness of the parenchyma, in part, due to vessel leakage, makes the tumor poorly perfused and resistant to chemotherapy treatments.
The vascular supply required by cancer cells to support their growth can be provided by the hijacking of normal vessels from surrounding tissues, a process known as co-option [7], or by the formation of new vessels within the tumor. The classic sprouting angiogenesis model foresees that in response to pro-angiogenic cues, such as VEGF, FGF, ANG-2, and several other chemokines, the basement membrane is locally degraded by metalloproteases (MMPs) and the surrounding pericytes detach from the vessel wall. This allows the ECs to resume proliferative activity. An EC engages an invasive phenotype, extending filopodia and lamellipodia towards the chemokine gradient, and driving the sprouting of the extending blood vessel [24].
1.3. Molecular Aspects of Tumor Endothelial Cells (TECs)
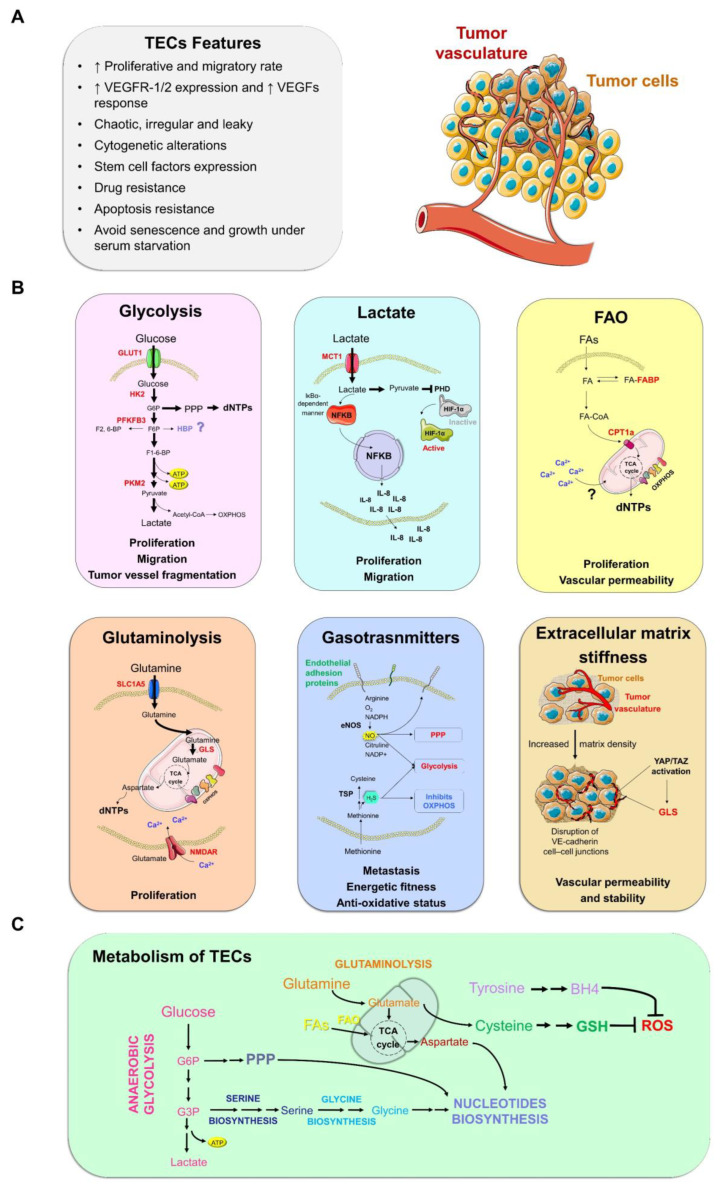
The structural differences of the tumor vessels are reflected both at the cellular and molecular level on the morphological and physiological alterations of the ECs (Figure 1A).
Figure 1.
Overview of tumor endothelial cell (TEC) metabolism. (A) Phenotypically, TECs show a high replication rate coupled to invasiveness, over-expression of receptors, and pro-angiogenic growth factors such as the vascular endothelial growth factor/vascular endothelial growth factor receptor 2 (VEGF/VEGFR2) axis, cytogenetic alterations, stemness and chemotherapy resistance, the ability to grow in serum-free media, and reduced senescence and apoptosis. (B) The main metabolic pathways reported so far in the remodeling of endothelial cell metabolism in cancer context. Proliferating TECs strongly depend on glycolysis and glucose uptake to produce adenosine triphosphate (ATP). Glycolysis provides the energy necessary to guide TEC proliferation and migration; lactate present in tumor microenvironment is imported into TECs through monocarboxylate transporter 1 (MCT-1) and can fuel vessel sprouting by two ways. By activating the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) pathway that up-regulates interleukin 8 (IL-8) and leads to the IL-8 self-stimulation, or by converting into pyruvate that inhibits prolyl hydroxylase (PHD2) activating hypoxia-inducible factor 1 alpha (HIF1α); Fatty acids oxidation (FAO) provides intermediate metabolites and biomass to support sprouting vessels. The EC-specific deletion of both carnitine palmitoyltransferase 1a and 2 (CPT1a and CPT2, respectively), genes that regulate the carnitine shuttle, cause vessel leakage; gasotransmitters such as hydrogen sulfide (H2S) and nitric oxide (NO) play a pleiotropic role by altering the proteome of cells and their pro-angiogenic action affects metabolism; extracellular matrix stiffness promotes tumor vessels formation by yes-associated protein (YAP)/transcriptional coactivator with PDZ-binding motif (TAZ) activation and leads to angiogenesis. The enzymes/transporters directly involved in tumor angiogenesis are highlighted in red. (C) Metabolic interaction within individual pathways in TECs. The Figure was partly generated using Servier Medical Art.Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/, accessed on 30 March 2022). ↑: High. GLUT1: glucose transporter 1; HK2: hexokinase 2; PPP: pentose phosphate pathway; HBP: hexosamine biosynthetic pathway; G6P: glucose-6-phosphate; PFKFB3: 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3; F2,6-BP: Fructose 2,6-bisphosphate; F6P: fructose 6-phosphate; F1,6-BP: fructose 1,6-bisphosphate; OXPHOS: oxidative phosphorylation; FAs: fatty acids; FABP: fatty acid binding protein; TCA cycle: tricaboxylic cycle; dNTPs: deoxyribonucleoside triphosphates; SLC1A5: solute carrier family 1 member 5; GLS: glutaminase; NMDAR: N-methyl-D-aspartate receptor; eNOS: endothelial nitric oxide synthase; ROS: reactive oxygen species; BH4: tetrahydrobiopterin; GSH: glutathione.
Murine TECs isolated from different human tumor xenografts showed a high proliferative and migratory rate, a greater response to VEGFs, and a higher expression of VEGF receptors-1/2 compared to NECs [25]. Furthermore, these cells show cytogenetic alterations [26] and overexpress stem cell factors such as Sca-1 (stem cell antigen-1) [25] and MDR1 (multidrug resistance-1) [27], which increase their resistance to several types of chemotherapeutic agents such as paclitaxel and 5-fluorouracil [27,28]. As cancer cells, the human TECs from patients are resistant to apoptosis, do not undergo senescence, and can grow under serum starvation [28].
At the molecular level, from a single-cell RNA sequencing (scRNA-seq) analysis conducted on TECs isolated from lung cancer patients, it emerged that, compared to NECs, TECs show a strong enriched signature in some signaling pathways such as the MYC pathway and the PI3K/Akt/mTOR pathway [29]. The pivotal role of MYC during both blood vessel formation in embryo development (vasculogenesis) and the angiogenic switch is supported by in vivo evidence [30]. It has also been proven that the PI3K/Akt/mTOR pathway plays a key role in tumor angiogenesis [31]. Surprisingly, Lambrechts and colleagues found a strong down-regulation of the inflammatory response in TECs [29].
A broader transcriptomic analysis, conducted on ECs from human (hTEC), murine (mTEC), and human cell culture (hcTEC) lung cancers, improved the molecular characterization of cellular phenotypes and identified new TEC markers in active sprouting [32]. It also emerged that the genes displaying higher expression in sprouting TECs are those encoding collagens (e.g., COL4A1, COL4A2, and COL18A1) and collagen-modifying enzymes (e.g., PXDN and PLOD1) [32]. This suggests that the extracellular matrix synthesis pathways, and in particular collagen synthesis, may represent a new target for striking growing tumor vessels.
2. Endothelial Metabolism during Angiogenesis
Adult vasculature is composed of quiescent ECs, also called phalanx cells. The quiescence state can be locally lost in favor of a proliferative state under pro-angiogenic stimuli. The current model of sprouting angiogenesis predicts that proliferating ECs sprout from a pre-existing vessel to anastomose with another newly formed sprouting vessel. Each sprout is guided by a tip cell, which is an activated EC cell, followed by so-called stalk cells [33]. This functional subdivision of ECs, based on the role played in angiogenesis, shows molecular and metabolic signatures [34].
Metabolism plays a crucial role in maintaining the quiescent state [35]. In particular, the forkhead box O (FOXO1) transcription factor has been reported as the gatekeeper of endothelial quiescence. FOXO1 suppresses the MYC pathway and down-regulates both glycolysis and oxidative phosphorylation (OXPHOS) [35]. Additionally, FOXO1 enhances branched-chain amino acid catabolites, shutting down ECs’ proliferation and angiogenesis in an MYC-independent manner [35]. Moreover, quiescent EC fatty acid consumption through β-oxidation is threefold greater than in proliferating ECs [36]. This is not related to the ATP production, which in any case remains mainly dependent on glycolysis, but serves as an anaplerotic reaction to fuel the NADPH production [36]. Understanding the molecular–metabolic mechanisms that trigger and maintain Ecs’ quiescence could provide a new toolbox for anti-angiogenic therapies. From this perspective, metabolically-induced quiescence could be used to impair tumor growth.
Following an imbalance between pro- and anti-angiogenic factors, phalanx cells can reactivate the cell cycle and become proliferating. Specialization in tip and stalk cells is a dynamic state that is dependent on receptors expressed on the EC surface [37]. The proliferating EC that most activates VEGF-VEGFR1/2 signaling is selected as the tip and, through overexpression in the NOTCH ligand DLL4, leads to the lateral inhibition of the other ECs, which then acquire the stalk phenotype [38]. Tip cells are highly dependent on glycolysis to produce the energy needed to support sprouting [9]. Despite the strong dependence on glycolytic flux, proliferating ECs cannot abolish OXPHOS. Respiratory chain complex III disruptions inhibits angiogenesis in vivo [39]. However, mitochondrial respiration reduces EC proliferation, but not migratory activity, which is the phenotypic hallmark of tip cells, suggesting a non-primary role of OXPHOS in tip–stalk differentiation. On the other hand, the endothelium-specific knockout of glutamine synthetase (GLUL) impairs angiogenesis by inhibiting migration [40]. Thus, the amino acid (AA) metabolism could be the main metabolic actor in promoting the tip identity.
3. Rewiring of Endothelial Metabolism in Cancer
3.1. Glycolysis
ECs utilize anaerobic glycolytic metabolism to produce most of the ATP rather than OXPHOS, regardless of living in an oxygen-rich environment [9]. Indeed, ECs produce around 80% of ATP via anaerobic glycolysis entering into the TCA cycle only 1% of glucose-derived pyruvate [41], lactate being the metabolic end-product of glycolysis in ECs. Under pro-angiogenic stimuli in a tumor context, the endothelial glycolytic rate increases and different glycolytic enzymes (e.g., hexokinase 2 (HK2) and pyruvate kinase (PK)) relocate into migratory cell structures, such as lamellipodia and filopodia, favoring cell migration [9]. Consistently, the endothelium-specific deletion of the SLC2A1 gene, encoding glucose transporter type 1 (GLUT1), the main transporter of glucose uptake, reduces the formation of new blood vessels [42].
Analyses of scRNA-seq have shown that glycolysis-related genes are overexpressed, compared to NECs, in the transcriptomic signature of TECs (Table 1) [29,43]. Therefore, ECs within tumors appear to be more dependent on glycolytic flux (Figure 1B,C). Both genetic ablation and the pharmacological inhibition [44] of 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase-3 (PFKFB3), a key enzyme in glycolysis regulation, inhibit blood vessel formation in tumor xenografts (Table 1). In addition, the PFKFB3 haplodeficiency or blockade induces tumor vessel normalization by tightening the EC barrier and promoting pericyte quiescence (Table 1) [11]. Moreover, FGF2, another proangiogenic factor, has been shown to increase glycolytic flux and regulate the glycolytic enzyme HK2 [45] via the MYC pathway [29]. It is interesting to note that OXPHOS factors are still expressed at high levels in TECs (Table 1) [29] and the inhibition of mitochondrial respiration prevents tumor angiogenesis [46]. These data reinforce the knowledge that OXPHOS serves to feed the growth of the tumor vasculature by anaplerosis more than producing energy.
Table 1.
Direct in vivo evidence of TEC metabolic reprogramming that impairs tumor vasculature and consequently cancer growth.
| Metabolic Pathway | Molecular Mechanism | Tumor Type | Reference |
|---|---|---|---|
| Glycolysis | PFKFB3 inhibition or haploinsufficiency normalize tumor vessel reducing glycolysis. | Melanoma, pancreatic tumor. | [11,44] |
| Glycolytic genes over-expression. | NSCLC, Melanoma. |
[29,43] | |
| Lactate | VEGF-A induces carbonic anhydrase 2 (CAII), which reduces lactate acidosis in the tumor environment and enhances TEC survival. | Melanoma. | [49] |
| Cancer-produced lactate enter ECs by monocarboxylate transporter (MCT-1) and fuels angiogenesis through NF-κB pathway, leading to the autocrine stimulation of IL-8. | Colorectal, breast cancer. | [50] | |
| Within TEC, lactate can be converted into pyruvate, which exerts a negative feedback on PHD2 triggering angiogenesis by HIF1α activation. | Pulmonary Lewis lung carcinoma. | [51] | |
| OXPHOS | Mitochondrial respiration is necessary to sustain tumor angiogenesis. | Colorectal cancer, melanoma. | [46] |
| OXPHOS genes over-expression. | NSCLC. | [29] | |
| Hypoxia | EC-specific HIF1α deletion prevents autocrine signaling of VEGF, leading to reduction of vasculature. | Pulmonary Lewis lung carcinoma. | [52] |
| PHD2 haploinsufficiency in ECs reduces glycolysis normalizing tumor vasculature. | Melanoma. | [53] | |
| H2S | CBS silencing and pharmacological inhibition of CTH in cancer cells, the major H2S producers, reduce tumor angiogenesis. | Breast cancer. | [54] |
| CTH knockdown reduces lymphangiogenesis. | Prostate cancer. | [55] | |
| CBS silencing reduces vasculature. | Colon cancer. | [55] | |
| ECM stiffness | Pharmacological inhibition of the stiffness of the ECM reduces tumor angiogenesis. | Breast cancer. | [56] |
| EC-specific knockout of YAP/TAZ results in impaired tumor and tumor vessel growth. | Melanoma. | [57] |
While the effect of different glycolytic enzymes (e.g., PFKFB3, HK2, and PKM2) has been addressed in tumor angiogenesis, the role of glycolytic-branching pathways still remains largely unexplored, including hexosamine biosynthesis (HBP) and the pentose phosphate pathway (PPP) in an angiogenic cancer context. Although, it has been reported that there is an increased level of the PPP metabolite, sedoheptulose-7-phosphate (S7P) (Figure 1C), in TECs compared with NECs, but whether and how PPP is regulated during tumor angiogenesis is still unclear [11]. Moreover, recently a novel role of PPP in smooth muscle cell recruitment by regulating elastin production in zebrafish and mouse models in developmental angiogenesis has been published [47], but the potential role of this PPP controlling vessel normalization is yet to be elucidated. On the other hand, it has been described that the inhibition of HBP using azaserine increases flux throughout the TCA cycle and PPP, showing an interlink between these pathways in ECs [48], but the role of HBP in tumor angiogenesis remains unexplored.
3.2. Lactate
The high consumption of glucose followed by an incomplete oxidation within the tumor, known as anaerobic glycolysis, leads to the production of lactate. The release of lactate by tumor cells into the extracellular environment causes the acidification of TME and, thus, hypoxia and acidification always go together in tumors. The decrease in pH due to the accumulation of lactic acid, a toxic condition for NECs, stimulates TEC proliferation [49]. The expression of monocarboxylate transporter 1 (MCT1) in TECs allows to uptake lactate from the TME. Lactate flow in TECs activates the NF-kB pathway (Figure 1B), leading to the release of IL-8 that stimulates angiogenesis [50]. The pharmacological inhibition of MCT1 has been shown to reduce angiogenesis both in vitro and in vivo through the suppression of HIF-1α levels [51] (Figure 1B,C). This finding indicates that lactate is one of the driving forces of metabolic remodeling and tumor angiogenesis (Table 1).
3.3. Fatty Acid Oxidation
Lipids come from the diet or can be produced endogenously by the cells. In the endothelium, the blockade of both the lipogenesis [52] and lipolysis [53] cause angiogenesis defects. Specifically, ECs uptake fatty acids (FAs) by CD36 [54] and fatty acid transport proteins (FATPs) [55]. Pro-angiogenic signals, such as VEGF-B, increase the FAs flow by inducing the expression of FATPs [55], while the lymphatic endothelial cells (LEC)-specific deletion of CD36 alters the VE-cadherin junction and the integrity of the lymphatic vessels [56]. The accumulation of free FAs within the ECs triggers ROS generation compromising the integrity of the endothelium and triggering an inflammatory process [57]. Therefore, FAs should be continuously stored in membranes, degraded to produce energy and biomass, or esterified to proteins. Inside the cell, fatty acid-binding proteins (FABPs) bind and sort the FAs to the right compartment [58]. In ECs, VEGF signaling induces FABP4 expression [59], leading to vessel sprouting.
FAs are burned into the mitochondria through the fatty acid β-oxidation (FAO) pathway to generate energy and biomass. The rate-limiting step of this catabolic pathway is the import of FAs by carnitine palmitoyltransferase 1a (CPT1a) (Figure 1B,C). The endothelium-specific deletion of CPT1a reduces ECs proliferation [55]. Through the conditional knockout of endothelial carnitine palmitoyltransferase II (CPT2), an enzyme that works in synergy with CPT1a to regulate the carnitine shuttle, it has been shown that FAO is an endothelial-to-mesenchymal-transition (EndoMT) regulator [60]. The EndoMT of ECs promotes tumor aggressiveness by facilitating dissemination and rendering the vasculature insensitive to anti-VEGFR treatments [61]. Thus EC-specific up-regulation of the FAO pathway should antagonize EndoMT, ameliorating the survival rate. Thus, the up-regulation of the FAO pathway specifically in the ECs should antagonize EndoMT, ameliorating the survival rate.
3.4. Non-Essential Amino Acids
In recent years it has been become increasingly evident that amino acid (AA) metabolism also plays a pivotal role in regulating angiogenesis [62]. AAs had traditionally been classified as nutritionally essential (EAA, indispensable) or non-essential (NEAA, dispensable) depending on whether it is de novo synthesized or not. In this section we will discuss the new findings regarding tumor vasculature formation and NEAAs metabolism.
3.4.1. Glutamine–Glutamate
Glutamine is the most abundant NEAA (~700 µM) in blood plasma being an indisputable source of nitrogen and carbon to support biosynthesis, energy, and anti-oxidant defense for the cellular homeostasis [63]. Despite that glutamine is a NEAA, ECs are not able to survive in absence of glutamine [64]. Indeed, ECs display a glutaminase (GLS) activity about 20-fold higher than lymphocytes, which have a high potential for rapid cell division [65]. Different works have described the crucial role of GLS in physiological angiogenesis showing that glutaminolysis fuels proliferation more than migration in ECs [64,66]. GLS blockade causes a drop of tricarboxilic acid (TCA) cycle intermediates and a subsequent decrease in macromolecular biosynthesis that leads to proliferation arrest [64,67].
Endothelial glutamine metabolism reprogramming has been related to different tumor extrinsic factors. Kaposi’s Sarcoma-associated Herpesvirus (KSHV), an oncogenic virus and the etiologic agent of Kaposi’s Sarcoma, induces glutaminolysis in ECs. Specifically, KSHV activates the MYC/MondoA-network to up-regulate the glutamine transporter, SLC1A5, leading to an increased glutamine up-take [68] (Figure 1B). Moreover, advanced tumors produce excessive amounts of TGFβ1, a cytokine associated with specific aspects of tumor progression including epithelial–mesenchymal transition (EMT), tissue invasion, and metastasis [69], which is able to induce GLS expression and promote endothelial cell sprouting by Raf/MEK/ERK activation [70]. Furthermore, it has been recently described that glutaminolysis drives tumor angiogenesis by controlling endothelial growth factor receptor 2 (VEGFR2) and fibroblast growth factor receptor 1(FGFR1) translation via mTORC1 activation [71] (Figure 1C).
Compared with glutamine, the concentration of glutamate in human plasma is lower (around 50 μM). Together with glycine and serine, glutamate is able to activate N-methyl-D-aspartate receptors (NMDAR) after binding the GRIN2D subunit, facilitating the cellular calcium influx [72] (Figure 1B). Interestingly, it has been shown that targeting GRIN2D in colorectal cancer by a vaccination approach leads to an inhibition of tumor growth and vascularization [73]. In addition, it has been shown that ECs utilize NMDAR1 for vasculature formation in gliomas, indicating a novel biological aspect of glutamate signaling in tumor angiogenesis [74]. Moreover, glutamate can be converted into glutamine by the glutamine synthetase enzyme (GLUL). ECs show negligible GLUL activity [68]. Eelen et al. have shown that the genetic deletion of Glul in ECs impairs vessel sprouting during vascular development, while minimally affecting healthy quiescent ECs. Mechanistically, GLUL knockdown reduces membrane localization and activation of the GTPase RHOJ, thereby inducing actin stress fibers and impeding endothelial cell motility [40]. Remaining unexplored is the role of GLUL in tumor angiogenesis.
3.4.2. Aspartate–Asparagine
Aspartate participates in many reactions, including nucleotide and protein synthesis [75]. Due to its low concentration in blood (0–15 μM) [76], aspartate synthesis is crucial for cell survival. Aspartate biosynthesis is driven largely by glucose- or glutamine-dependent refilling of the TCA cycle to replenish mitochondrial oxaloacetate (OAA), which is subsequently converted to aspartate through the activity of mitochondrial glutamic-oxaloacetic transaminase 2 (GOT2). In humans, GOTs exist as two distinct isoenzymes: cytoplasmic GOT1 and mitochondrial GOT2. Both enzymes catalyze the same reaction albeit with different kinetics, share a sequence homology of ~45%, and are thought to have evolved from a common ancestral gene [76]. It is known that GOTs have multiple metabolic functions, including maintenance of the nicotinamide adenine dinucleotide/reduced nicotinamide adenine dinucleotide (NAD+/NADH) ratio in cells, α-keto acids production, gluconeogenesis, hydrogen sulfide (H2S) production via the CAT/3-mercaptopyruvate sulfotransferase pathway, and aspartate synthesis [77].
Even though the role of GOTs in physiological and pathological angiogenesis has been never investigated, several pieces of evidence suggest a crucial role of these enzymes in tumor angiogenesis. First, it has been shown that human recombinant VEGF-A induces the up-regulation of GOT2 in a study of chicken microarray analyses [78]. Second, an increased GOT2 level was addressed in angiogenic compared to non-angiogenic brain tumors in human glioblastoma xenograft studies [79]. Third, the endothelial-specific genetic ablation of mitochondrial complex III impairs tumor angiogenesis associated with a significant decrease in aspartate levels [39]. Fourth, it has been shown that short- and long-term hypoxia decreases aspartate availability in microvascular ECs [80]. Finally, the deletion of GOT1 in TECs blocks vessel formation in plug angiogenesis assays [71].
Asparagine, a proteogenic AA, is present in circulation (~100 μM) [81], and it can be synthesized by asparagine synthetase (ASNS), which converts aspartate and glutamine to asparagine and glutamate in an ATP-dependent reaction [82]. The enzyme is ubiquitous in mammals, but basal expression is relatively low in tissues other than the exocrine pancreas [83]. Human ASNS activity is highly regulated in response to cell stress, primarily by increased transcription [83]. ASNS expression and asparagine metabolism have received considerable attention in transformed cells, beginning with the observation that childhood acute lymphoblastic leukemia is susceptible to treatment by the infusion of bacterial asparaginase (ASNase) [84]. The role of asparagine metabolism has not been as extensively investigated in tumor angiogenesis. However, it has been shown that asparagine is crucial in glutamine-deprived ECs to reactivate mTOR signaling and protein biosynthesis [67].
3.4.3. Serine
Serine is implicated in numerous metabolic processes such as antioxidant defense, one-carbon metabolism, and de novo nucleotide synthesis [85] (Figure 1C). Indeed, many cancers show an up-regulation of the biosynthetic enzymes involved in serine synthesis [86]. In fact, it has been reported that phosphoglycerate dehydrogenase (PHGDH) expression accelerates tumor growth in mouse models of melanoma and breast cancer; however, whether this acquired fitness advantage may induce the angiogenic process by secreting serine, remains obscure. Ten years ago, Maralani and colleagues showed that a serine pre-treatment (0.1–3.2 mM) protects ECs from hydrogen peroxide-mediated cell cytotoxicity and leads to significant induction of NRF2 activity, HO-1 expression, and NOx production [87]. Accordingly, it was reported that mouse neonates with EC-specific PHGDH deficiency suffer lethal vascular defects, due to reduced EC proliferation and survival. This phenotype is associated with insufficient heme production and consequently with elevated ROS levels due to cellular serine depletion [88]. In addition, recently it has been reported that dimethyl fumarate, a drug with known anti-angiogenic properties, inhibits the serine synthesis pathway by blocking PHGDH activity [89]. Despite serine having an active role in angiogenesis, its function in tumor angiogenesis is still poorly understood.
3.4.4. Glycine
Glycine is involved in both angiogenesis and anti-angiogenesis. During angiogenic development, low concentrations of glycine promotes intersegmental vessel formation (ISVs), whereas high concentrations reduce angiogenesis in zebrafish embryos [90].
In a tumor context, glycine has been reported to suppress tumor angiogenesis. In fact, it was reported that glycine (100 μM) inhibited angiogenesis by more than 50% in a chorioallantoic membrane (CAM) assay. Dietary glycine supplementation reduces blood vessels in a fibrin Z-chamber assay and tumor angiogenesis in a tumor Z-chamber (fibrin with R3230 mammary adenocarcinoma cells) through the reduction of iNOS expression [91]. Moreover, tumor growth and vessel density were decreased in rats fed with 5% glycine compared without it in a WAG-Rij/CC-531 rat model of metastatic colorectal cancer [92]. On the other hand, glycine promotes angiogenesis in other pathological contexts. For example, in a hindlimb ischemia model, glycine treatment significantly enhanced neovascularization promoting the recovery of vascular flow via the GlyT1-glycine-mTOR-VDAC1 axis pathway [93]. Furthermore, glycine prevents the apoptosis of rat sinusoidal endothelial cells caused by Blc-2 decrease following VEGF deprivation [94]. The seemingly inconsistent results of these studies on glycine may be related to differences in the species, tissues, and cells used, or may be attributed to different approaches (e.g., culture conditions, doses and time of stimulation, animal models, and disease models). However, we cannot discard glycine as a novel target for angiogenic and anti-angiogenic therapy.
3.4.5. Cysteine
Cysteine is a nutritional semi-essential AA that is present mainly in the form of cystine (Cys–Cys dimmer) in the extracellular space [95,96]. The maintenance of relatively low concentrations of cysteine in the body is critical because cysteine is toxic at high levels. Yet, the level of cysteine must be high enough to allow its use for other purposes, such as the formation of glutathione (GSH) and the synthesis of proteins (Figure 1C) [97]. In culture, cysteine seems to be indispensable for EC growth and survival [98]. The uptake of cystine in ECs is Na+-independent and inhibited competitively by glutamate being mediated by system xCT [99]. Recently, it has been revealed that xCT and its substrate glutamate specifically operate on ECs and promote neoangiogenesis [74]. Thus, targeting xCT expression and glutamate secretion in gliomas provides a novel therapeutic roadmap for normalizing tumor angiogenesis [74]. In addition, EC treatment with erastin, an inhibitor of xCT, at a non-lethal level promotes EC proliferation, migration, and vessel-like structures formation, concomitantly with a reduction of GSH and an increase in ROS. The dual therapy using propranolol, which reverts the erastin-dependent activation of ECs, and chrysin to induce cytotoxic to cancer cells, has been proposed [100].
On the other hand, the heterodimeric transporter LAT1/4F2hc mediates the transport of essential (histidine, isoleucine, methionine, tryptophan, phenylalanine, leucine, cysteine, and tyrosine) and non-essential (glutamine) AAs across the cell membrane, the affinity of EAAs for LAT1 being higher compared NEAAs [101]. In human pancreatic ductal adenocarcinoma xenograft mouse models, a significant increase in LAT1 expression in TECs compared with NECs has been reported [102]. Molecularly, LAT1 is crucial to support angiogenesis-mediated amino acid transport, which is indispensable for the VEGF-A-dependent activation of mTORC1.
Cysteine seems to be an indispensable AA for ECs survival, reflecting a high requirement for antioxidants to protect ECs from oxidative stress [98]. In addition, it has been described that in tumor cells, cysteine activates mTORC1 through the GCN2/ATF4/SESN2 axis, inducing cell growth [103]. The replenishment of cellular GSH with thiol-amino acids counteracts the growth-inhibitory effect of TGF-β1 in ECs through a currently undefined mechanism [104]. These findings support the crucial role of cysteine in both the survival and growth of ECs.
3.4.6. Tyrosine
Mammals synthesize tyrosine from the EAA phenylalanine in a reaction catalyzed by phenylalanine hydroxylase. This enzyme hydroxylates phenylalanine to tyrosine and is the rate-limiting step in phenylalanine catabolism. Phenylketonuria (PKU) is an autosomal-recessive inborn error of phenylalanine (Phe) catabolism, caused by the deficiency of phenylalanine hydroxylase [105]. It has been reported that patients with PKU show endothelial dysfunction [105] and increased aortic stiffness compared to healthy controls [106], changes that could be associated with a secondary deficit in tetrahydrobiopterin (BH4) (Figure 1C) [107]. Consistently, the oral supplementation with Phe is able to enhance endogenous BH4 biosynthesis through the GCH1–GFRP protein complex, elevates nitrite levels, reduces vascular ROS levels, and improves endothelium-dependent vascular relaxation in a spontaneously hypertensive rat model [108]. Furthermore, it has been demonstrated that BH4 synthesis via either the pterin salvage or the de novo pathway, promotes endothelial cell proliferation, migration, and tubule formation in cultures and induces angiogenesis in tumor xenografts [108].
3.5. Metabolic Interactions between Tumor Tissue and Endothelial Cells
Tumors exhibit an altered metabolism compared to non-transformed tissues [109]. The discovery and characterization of tumor reprogrammed metabolisms may provide opportunities to predict endothelial behavior and prevent tumor angiogenesis by targeting tumor–endothelial metabolic interactions.
Symbiotic and competitive metabolic interactions between tumor cells and microenvironmental cells have been reported in various cancers. For example, pancreatic stellate cells (PSCs) utilize SLC1A4 to exchange and maintain extracellular alanine levels. Moreover, pancreatic ductal adenocarcinoma (PDAC) cells up-regulate SLC38A2 and increase their alanine demand. PDACs lacking SLC38A2 fail to concentrate intracellular alanine and undergo a profound metabolic crisis resulting in a tumor growth impairment [110]. In addition, metabolites profiling of melanoma interstitial fluids reveals uridine diphosphate as a potent immune modulator capable of limiting tumor growth by increasing CD4+CD25+FoxP3− cells [111]. Lastly, targeting GLUL in cancer-associated fibroblasts induces tumor regression in an orthotopic ovarian cancer model [112]. However, it remains unanswered whether it could be possible target stromal metabolic pathways to find therapeutic opportunities to block tumor angiogenesis.
Recently, the impact of breast cancer subtypes on LEC metabolism and lymphangiogenesis was studied. Specifically, LECs co-cultured with breast cancer cell lines increased glycolysis. Moreover, lactate levels were significantly increased, which correlated to the up-regulation of lactate metabolism enzymes and transporters [113].
Emerging data have identified cooperative and competitive relationships between cancer cells and TECs. Indeed, it has been shown that blocking the heme exporter Feline Leukemia Virus subgroup C Receptor 1a (FLVCR1a) in TECs causes ketone bodies (KBs) accumulation. TEC-derived KBs can be secreted producing a metabolic rewiring in the cancer cells [114]. In addition, it has been reported that tumor-associated macrophages (TAMs) can up-regulate the expression of REDD1, a negative regulator of mTOR, under hypoxic conditions. REDD1-deficient TAMs are highly glycolytic cells that out-compete ECs for glucose usage, which thwarts vascular hyperactivation and promotes the formation of quiescent vascular junctions. Tuning down glycolysis in REDD1 knockout TAMs re-establishes abnormal angiogenesis and metastases [115].
3.6. Gasotransmitters: Signals in the Air
Not all biologically active molecules have a solid nature. In fact, there are volatile molecules capable of arousing functional responses in cells and tissues. These molecules, known as gasotransmitters, can transduce signals and therefore influence cell activities [116]. An increasing body of evidence is starting to shed light on the roles played by gasotransmitters, for example in the tumor context [117]. It is interesting to note that all known gasotransmitters are in some way linked to the physiology of the vascular endothelium. This is not surprising since the vascular system has evolved to facilitate gas exchange between tissues, but this makes ECs an excellent model for investigating and dissecting the gasotransmitter pathways. Here, we will briefly discuss the role of major gasotransmitters on endothelial cell metabolism, a new emerging, and still largely unexplored field.
Nitric oxide (NO) is a gas produced by enzymes called nitric oxide synthases (NOSs), which convert arginine into citrulline in a reaction involving oxygen and various coenzymes such as NADPH and BH4. The NOS family contains neuronal NOS (nNOS/NOS1), inducible NOS (iNOS/NOS2), and endothelial NOS (eNOS/NOS3). In ECs, both eNOS and iNOS are under the control of VEGF [118]. eNOS full knockout mice show a strong impairment in vascular development [119]. Gene therapy aimed at overexpressing eNOS in an ischemic hindlimb rat model improves the perfusion of the tissue [120]. NO produced by NOS acts in an autocrine and paracrine manner on the cells of the vascular system leading to the direct activation of some signaling pathways [121] or through post-translational modifications such as S-nitrosylation [122]. In ECs, the S-nitrosylation reduces the reserve respiratory capacity, while stimulating glycolysis [123] (Figure 1B). The supplementation of ECs with NO-donors leads to an increase in glucose uptake coupled to GLUT1 up-regulation, and enhances HK activity [124]. This indicates that S-nitrosylation, NO-induced, acts not only as an actor of metabolic reprogramming, but also as a possible driver of glycolytic metabolism during angiogenesis. However, it has recently been found that eNOS inhibits the glycolytic enzyme PKM2 through S-nitrosylation, which leads to an increase in PPP activity to generate reducing equivalents. By this mechanism, NO protects the vasculature from oxidative stress and exerts anti-inflammatory effects [125].
Another gasotrasmitter is the hydrogen sulphyde (H2S). H2S is an endogenous product of sulfur AA metabolism (Figure 1B). It is released during the transsulfuration pathway (TSP), the metabolic pathway that leads to the de novo synthesis of cysteine from the methionine by cystathionine β-synthase (CBS) and cystathionine γ-lyase (CTH) [126]. Cancer cells express high levels of CBS and CTH and their genetic or pharmacological inhibition leads to a reduction in tumor vasculature [125,126,127], suggesting that the TSP metabolic reprogramming may be a promising therapeutic strategy for solid tumors (Table 1). Finally, in ECs, H2S enhances glucose uptake and glycolytic ATP production, and at the same time inhibits the complex IV (Figure 1B), leading to the reduction of mitochondrial OXPHOS [128]. Taken together, these data indicate TSP as a powerful driver of angiogenesis and a putative target for anti-angiogenic therapy.
Not only can the presence of a gas have biological effects on metabolism, but also its shortage. The best known and studied case is the lack of oxygen (O2), or hypoxia. All multi-cellular living organisms consume O2 to support their own energy request. However, when in each tissue, O2 level-decreased cells must adapt their metabolic needs to new conditions. At the molecular level, the cell senses and transduces the signal through the hypoxia-inducible factor pathway [129]. HIF-1α deletion in ECs impairs vessel growth within cancers [129], highlighting the essential role of hypoxia in tumor angiogenesis. Surprisingly, PHD2 haploinsufficiency in ECs bears tumor vascular normalization, resulting in a better oxygenation and metastases reduction in a tumor xenograft model [130]. The transcriptomic analysis of hypoxic ECs has shown the importance of HIF-1α in up-regulating the glycolytic factors and down-regulating the mitochondrial metabolism [131]. In addition, glycolysis-associated genes such as SLC2A3, PFKFB3, and HK2 belong to the early hypoxia-responsive genes [78,131].
3.7. Extracellular Matrix Stiffness and Angiogenesis
The niche in which tumors develop and grow often becomes a fibrotic and extremely rigid microenvironment, referred as desmoplastic stroma. The stiffness is associated with the cancer aggressiveness [132]. This stiffness enhancement is due to the progressive remodeling of the extracellular matrix (ECM) induced by the tumor [132,133]. In addition, to being embedded in a rigid desmoplastic stroma, highly aggressive tumors are usually highly vascularized [3]. Consistent with this, it has been reported that the ECM stiffening is able to trigger tumor angiogenesis (Figure 1B) [134]. The mechanical forces acting inside a tumor play a central role in the formation of new vessels and can act in different ways. For example, it has been reported that mechanical tension is able to induce the activation of the VEGFR2 [135]. While the pressure induced by the stiffening of the matrix causes the destruction of the cell–cell endothelial junctions [134], leading to the release and activation of YAP/TAZ that regulates both physiological and tumor angiogenesis [136] (Table 1). These factors are downstream targets of the VEGF pathway and are necessary to induce the migratory phenotype in sprouting ECs [137,138]. Furthermore, a proteomic analysis based on quantitative mass spectrometry showed how ECs cultured into different matrix stiffnesses promote metastasis through a CCN1-dependent mechanism [139]. CCN1 signaling promotes the adhesion between endothelial and tumor cells by inducing the expression of N-cadherin [139], thus facilitating the dissemination of cancer cells. It is interesting to note how during embryonic development the activation of YAP regulates GLUT1/2 expression, increasing the consumption of glucose and nucleotides synthesis [140]. While in breast cancer cells, matrix stiffness causes a metabolic shift from a predominantly glycolytic to OXPHOS and FAO metabolism [141]. On the other hand, in pulmonary arterial ECs and smooth muscle cells, an increase in glycolysis coupled by an increase in glutaminolysis to fuel proliferation was observed [142]. This strongly suggests that matrix stiffening can influence the metabolic reprogramming of ECs. Despite both the role of stiffness and metabolism in promoting angiogenesis being widely documented, there is still a lack of knowledge that connects these two fundamental aspects in the tumor context.
Another mechanical stress to which TECs are subjected is an irregular and turbulent flow that reduces tumor perfusion, increasing its malignancy. In general, the laminar flow into the adult vasculature helps to keep ECs quiescent by activating the cell cycle checkpoints through the induction of p53 and p21 [143]. A disturbed flow activates the mechano-transducers YAP/TAZ, promoting their dephosphorylation and translocation into the nucleus, where they propel proliferation and an inflammatory phenotype [144]. A low fluid shear stress promotes the adhesion and extravasation of cancer cells to the vascular endothelium [145]. Instead, a physiological-like flow activates the pro-quiescence factor KLF2, which represses PFKFB3 gene expression and inhibits glycolytic metabolism. However, it has been reported that an increased laminar flow in circulating cancer cells enhances the transcription factor ATOH8, which, in turn, induces the expression of the glycolytic enzyme HK2 [146]. This confers a proliferative advantage on metastatic cells. Laminar flow appears to play a dual role in tumor progression, therefore further investigations are needed to better dissect the mechanisms induced on the metabolism of endothelial and tumor cells.
4. Conclusions and Perspectives
Altogether these findings indicate that researchers are moving away from the only VEGF-oriented model of angiogenesis, and start to realize that endothelial metabolism cooperates with canonical signaling mechanisms to drive vascular morphogenesis and differentiation. The next challenge will be to reveal the functional crosstalk among all these conditions in normal and pathological conditions. This aspect is intriguing considering that most metabolic pathways regulate the classical angiogenic signaling pathways such as VEGF, FGF, or NOTCH signaling. Novel technologies, such as metabolic sensors (e.g., in vivo metabolite tracers) and single cell technology coupled to system biology are needed to spatially and temporally decode these mechanisms and comprehend how angiogenic signal networks are regulated in diseased angiogenic conditions. We strongly believe that all these aspects will lead to important and unforeseen advances in the tumor angiogenic field in the coming years.
Author Contributions
J.L., M.M.S. and R.E.O. wrote the manuscript; R.E.O. and M.M.S. drafted and revised the manuscript; J.L., prepared the table; and R.E.O. prepared the figure. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors have no conflict of interest to declare.
Funding Statement
The research of M.M.S. Lab was funded by European Research Council (ERC) Consolidator Grant-Rendox (ERC-CoG 647057) and AIRC (Associazione Italiana Ricerca sul Cancro) IG Grant 20119.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Potente M., Gerhardt H., Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 2.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 3.Lugano R., Ramachandran M., Dimberg A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2020;77:1745–1770. doi: 10.1007/s00018-019-03351-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Ferrara N., Chen H., Davis-Smyth T., Gerber H.P., Nguyen T.N., Peers D., Chisholm V., Hillan K.J., Schwall R.H. Vascular endothelial growth factor is essential for corpus luteum angiogenesis. Nat. Med. 1998;4:336–340. doi: 10.1038/nm0398-336. [DOI] [PubMed] [Google Scholar]
- 6.Hurwitz H., Fehrenbacher L., Novotny W., Cartwright T., Hainsworth J., Heim W., Berlin J., Baron A., Griffing S., Holmgren E., et al. Bevacizumab plus Irinotecan, Fluorouracil, and Leucovorin for Metastatic Colorectal Cancer. N. Engl. J. Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 7.Kuczynski E.A., Vermeulen P.B., Pezzella F., Kerbel R.S., Reynolds A.R. Vessel co-option in cancer. Nat. Rev. Clin. Oncol. 2019;16:469–493. doi: 10.1038/s41571-019-0181-9. [DOI] [PubMed] [Google Scholar]
- 8.Jain R.K. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 9.De Bock K., Georgiadou M., Schoors S., Kuchnio A., Wong B.W., Cantelmo A.R., Quaegebeur A., Ghesquière B., Cauwenberghs S., Eelen G., et al. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell. 2013;154:651–663. doi: 10.1016/j.cell.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 10.Schoors S., De Bock K., Cantelmo A.R., Georgiadou M., Ghesquière B., Cauwenberghs S., Kuchnio A., Wong B.W., Quaegebeur A., Goveia J., et al. Partial and transient reduction of glycolysis by PFKFB3 blockade reduces pathological angiogenesis. Cell Metab. 2014;19:37–48. doi: 10.1016/j.cmet.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Cantelmo A.R., Conradi L.-C., Brajic A., Goveia J., Kalucka J., Pircher A., Chaturvedi P., Hol J., Thienpont B., Teuwen L.-A., et al. Inhibition of the Glycolytic Activator PFKFB3 in Endothelium Induces Tumor Vessel Normalization, Impairs Metastasis, and Improves Chemotherapy. Cancer Cell. 2016;30:968–985. doi: 10.1016/j.ccell.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hinshaw D.C., Shevde L.A. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res. 2019;79:4557–4566. doi: 10.1158/0008-5472.CAN-18-3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sobierajska K., Ciszewski W.M., Sacewicz-Hofman I., Niewiarowska J. Endothelial Cells in the Tumor Microenvironment. In: Birbrair A., editor. Tumor Microenvironment: Non-Hematopoietic Cells. Springer International Publishing; Cham, Switzerland: 2020. pp. 71–86. [DOI] [PubMed] [Google Scholar]
- 14.Manalo D.J., Rowan A., Lavoie T., Natarajan L., Kelly B.D., Ye S.Q., Garcia J.G.N., Semenza G.L. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood. 2005;105:659–669. doi: 10.1182/blood-2004-07-2958. [DOI] [PubMed] [Google Scholar]
- 15.Folkman J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 16.Hanahan D., Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/S0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 17.Siemann D.W. The Unique Characteristics of Tumor Vasculature and Preclinical Evidence for its Selective Disruption by Tumor-Vascular Disrupting Agents. Cancer Treat. Rev. 2011;37:63–74. doi: 10.1016/j.ctrv.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aird W.C. Endothelial Cell Heterogeneity. Cold Spring Harb Perspect. Med. 2012;2:a006429. doi: 10.1101/cshperspect.a006429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hashizume H., Baluk P., Morikawa S., McLean J.W., Thurston G., Roberge S., Jain R.K., McDonald D.M. Openings between defective endothelial cells explain tumor vessel leakiness. Am. J. Pathol. 2000;156:1363–1380. doi: 10.1016/S0002-9440(10)65006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morikawa S., Baluk P., Kaidoh T., Haskell A., Jain R.K., McDonald D.M. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am. J. Pathol. 2002;160:985–1000. doi: 10.1016/S0002-9440(10)64920-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu M., Frieboes H.B., McDougall S.R., Chaplain M.A.J., Cristini V., Lowengrub J. The effect of interstitial pressure on tumor growth: Coupling with the blood and lymphatic vascular systems. J. Theor. Biol. 2013;320:131–151. doi: 10.1016/j.jtbi.2012.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baluk P., Hashizume H., McDonald D.M. Cellular abnormalities of blood vessels as targets in cancer. Curr. Opin. Genet. Dev. 2005;15:102–111. doi: 10.1016/j.gde.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Nagy J.A., Brown L.F., Senger D.R., Lanir N., Van de Water L., Dvorak A.M., Dvorak H.F. Pathogenesis of tumor stroma generation: A critical role for leaky blood vessels and fibrin deposition. Biochim. Biophys. Acta. 1989;948:305–326. doi: 10.1016/0304-419X(89)90004-8. [DOI] [PubMed] [Google Scholar]
- 24.Gerhardt H., Golding M., Fruttiger M., Ruhrberg C., Lundkvist A., Abramsson A., Jeltsch M., Mitchell C., Alitalo K., Shima D., et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsuda K., Ohga N., Hida Y., Muraki C., Tsuchiya K., Kurosu T., Akino T., Shih S.-C., Totsuka Y., Klagsbrun M., et al. Isolated tumor endothelial cells maintain specific character during long-term culture. Biochem. Biophys. Res. Commun. 2010;394:947–954. doi: 10.1016/j.bbrc.2010.03.089. [DOI] [PubMed] [Google Scholar]
- 26.Hida K., Hida Y., Amin D.N., Flint A.F., Panigrahy D., Morton C.C., Klagsbrun M. Tumor-associated endothelial cells with cytogenetic abnormalities. Cancer Res. 2004;64:8249–8255. doi: 10.1158/0008-5472.CAN-04-1567. [DOI] [PubMed] [Google Scholar]
- 27.Akiyama K., Ohga N., Hida Y., Kawamoto T., Sadamoto Y., Ishikawa S., Maishi N., Akino T., Kondoh M., Matsuda A., et al. Tumor endothelial cells acquire drug resistance by MDR1 up-regulation via VEGF signaling in tumor microenvironment. Am. J. Pathol. 2012;180:1283–1293. doi: 10.1016/j.ajpath.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 28.Xiong Y.-Q., Sun H.-C., Zhang W., Zhu X.-D., Zhuang P.-Y., Zhang J.-B., Wang L., Wu W.-Z., Qin L.-X., Tang Z.-Y. Human hepatocellular carcinoma tumor-derived endothelial cells manifest increased angiogenesis capability and drug resistance compared with normal endothelial cells. Clin. Cancer Res. 2009;15:4838–4846. doi: 10.1158/1078-0432.CCR-08-2780. [DOI] [PubMed] [Google Scholar]
- 29.Lambrechts D., Wauters E., Boeckx B., Aibar S., Nittner D., Burton O., Bassez A., Decaluwé H., Pircher A., Van den Eynde K., et al. Phenotype molding of stromal cells in the lung tumor microenvironment. Nat. Med. 2018;24:1277–1289. doi: 10.1038/s41591-018-0096-5. [DOI] [PubMed] [Google Scholar]
- 30.Baudino T.A., McKay C., Pendeville-Samain H., Nilsson J.A., Maclean K.H., White E.L., Davis A.C., Ihle J.N., Cleveland J.L. c-Myc is essential for vasculogenesis and angiogenesis during development and tumor progression. Genes Dev. 2002;16:2530–2543. doi: 10.1101/gad.1024602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamada K., Sasaki T., Koni P.A., Natsui M., Kishimoto H., Sasaki J., Yajima N., Horie Y., Hasegawa G., Naito M., et al. The PTEN/PI3K pathway governs normal vascular development and tumor angiogenesis. Genes Dev. 2005;19:2054–2065. doi: 10.1101/gad.1308805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goveia J., Rohlenova K., Taverna F., Treps L., Conradi L.-C., Pircher A., Geldhof V., de Rooij L.P.M.H., Kalucka J., Sokol L., et al. An Integrated Gene Expression Landscape Profiling Approach to Identify Lung Tumor Endothelial Cell Heterogeneity and Angiogenic Candidates. Cancer Cell. 2020;37:21–36.e13. doi: 10.1016/j.ccell.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 33.De Smet F., Segura I., De Bock K., Hohensinner P.J., Carmeliet P. Mechanisms of vessel branching: Filopodia on endothelial tip cells lead the way. Arterioscler. Thromb. Vasc. Biol. 2009;29:639–649. doi: 10.1161/ATVBAHA.109.185165. [DOI] [PubMed] [Google Scholar]
- 34.Kalucka J., de Rooij L.P.M.H., Goveia J., Rohlenova K., Dumas S.J., Meta E., Conchinha N.V., Taverna F., Teuwen L.-A., Veys K., et al. Single-Cell Transcriptome Atlas of Murine Endothelial Cells. Cell. 2020;180:764–779.e20. doi: 10.1016/j.cell.2020.01.015. [DOI] [PubMed] [Google Scholar]
- 35.Wilhelm K., Happel K., Eelen G., Schoors S., Oellerich M.F., Lim R., Zimmermann B., Aspalter I.M., Franco C.A., Boettger T., et al. FOXO1 couples metabolic activity and growth state in the vascular endothelium. Nature. 2016;529:216–220. doi: 10.1038/nature16498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalucka J., Bierhansl L., Conchinha N.V., Missiaen R., Elia I., Brüning U., Scheinok S., Treps L., Cantelmo A.R., Dubois C., et al. Quiescent Endothelial Cells Upregulate Fatty Acid β-Oxidation for Vasculoprotection via Redox Homeostasis. Cell Metab. 2018;28:881–894.e13. doi: 10.1016/j.cmet.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 37.Jakobsson L., Franco C.A., Bentley K., Collins R.T., Ponsioen B., Aspalter I.M., Rosewell I., Busse M., Thurston G., Medvinsky A., et al. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat. Cell Biol. 2010;12:943–953. doi: 10.1038/ncb2103. [DOI] [PubMed] [Google Scholar]
- 38.Moya I.M., Umans L., Maas E., Pereira P.N.G., Beets K., Francis A., Sents W., Robertson E.J., Mummery C.L., Huylebroeck D., et al. Stalk Cell Phenotype Depends on Integration of Notch and Smad1/5 Signaling Cascades. Dev. Cell. 2012;22:501–514. doi: 10.1016/j.devcel.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diebold L.P., Gil H.J., Gao P., Martinez C.A., Weinberg S.E., Chandel N.S. Mitochondrial complex III is necessary for endothelial cell proliferation during angiogenesis. Nat. Metab. 2019;1:158–171. doi: 10.1038/s42255-018-0011-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eelen G., Dubois C., Cantelmo A.R., Goveia J., Brüning U., DeRan M., Jarugumilli G., van Rijssel J., Saladino G., Comitani F., et al. Role of glutamine synthetase in angiogenesis beyond glutamine synthesis. Nature. 2018;561:63–69. doi: 10.1038/s41586-018-0466-7. [DOI] [PubMed] [Google Scholar]
- 41.Krützfeldt A., Spahr R., Mertens S., Siegmund B., Piper H.M. Metabolism of exogenous substrates by coronary endothelial cells in culture. J. Mol. Cell Cardiol. 1990;22:1393–1404. doi: 10.1016/0022-2828(90)90984-A. [DOI] [PubMed] [Google Scholar]
- 42.Veys K., Fan Z., Ghobrial M., Bouché A., García-Caballero M., Vriens K., Conchinha N.V., Seuwen A., Schlegel F., Gorski T., et al. Role of the GLUT1 Glucose Transporter in Postnatal CNS Angiogenesis and Blood-Brain Barrier Integrity. Circ. Res. 2020;127:466–482. doi: 10.1161/CIRCRESAHA.119.316463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rohlenova K., Goveia J., García-Caballero M., Subramanian A., Kalucka J., Treps L., Falkenberg K.D., de Rooij L.P.M.H., Zheng Y., Lin L., et al. Single-Cell RNA Sequencing Maps Endothelial Metabolic Plasticity in Pathological Angiogenesis. Cell Metab. 2020;31:862–877.e14. doi: 10.1016/j.cmet.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 44.Conradi L.-C., Brajic A., Cantelmo A.R., Bouché A., Kalucka J., Pircher A., Brüning U., Teuwen L.-A., Vinckier S., Ghesquière B., et al. Tumor vessel disintegration by maximum tolerable PFKFB3 blockade. Angiogenesis. 2017;20:599–613. doi: 10.1007/s10456-017-9573-6. [DOI] [PubMed] [Google Scholar]
- 45.Yu P., Wilhelm K., Dubrac A., Tung J.K., Alves T.C., Fang J.S., Xie Y., Zhu J., Chen Z., De Smet F., et al. FGF-dependent metabolic control of vascular development. Nature. 2017;545:224–228. doi: 10.1038/nature22322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coutelle O., Hornig-Do H.-T., Witt A., Andree M., Schiffmann L.M., Piekarek M., Brinkmann K., Seeger J.M., Liwschitz M., Miwa S., et al. Embelin inhibits endothelial mitochondrial respiration and impairs neoangiogenesis during tumor growth and wound healing. EMBO Mol. Med. 2014;6:624–639. doi: 10.1002/emmm.201303016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Facchinello N., Astone M., Audano M., Oberkersch R.E., Spizzotin M., Calura E., Marques M., Crisan M., Mitro N., Santoro M.M. Oxidative pentose phosphate pathway controls vascular mural cell coverage by regulating extracellular matrix composition. Nat. Metab. 2022;4:123–140. doi: 10.1038/s42255-021-00514-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moiz B., Garcia J., Basehore S., Sun A., Li A., Padmanabhan S., Albus K., Jang C., Sriram G., Clyne A.M. 13C Metabolic Flux Analysis Indicates Endothelial Cells Attenuate Metabolic Perturbations by Modulating TCA Activity. Metabolites. 2021;11:226. doi: 10.3390/metabo11040226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Annan D.A., Maishi N., Soga T., Dawood R., Li C., Kikuchi H., Hojo T., Morimoto M., Kitamura T., Alam M.T., et al. Carbonic anhydrase 2 (CAII) supports tumor blood endothelial cell survival under lactic acidosis in the tumor microenvironment. Cell Commun. Signal. 2019;17:169. doi: 10.1186/s12964-019-0478-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Végran F., Boidot R., Michiels C., Sonveaux P., Feron O. Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-κB/IL-8 pathway that drives tumor angiogenesis. Cancer Res. 2011;71:2550–2560. doi: 10.1158/0008-5472.CAN-10-2828. [DOI] [PubMed] [Google Scholar]
- 51.Sonveaux P., Copetti T., De Saedeleer C.J., Végran F., Verrax J., Kennedy K.M., Moon E.J., Dhup S., Danhier P., Frérart F., et al. Targeting the lactate transporter MCT1 in endothelial cells inhibits lactate-induced HIF-1 activation and tumor angiogenesis. PLoS ONE. 2012;7:e33418. doi: 10.1371/journal.pone.0033418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bruning U., Morales-Rodriguez F., Kalucka J., Goveia J., Taverna F., Queiroz K.C.S., Dubois C., Cantelmo A.R., Chen R., Loroch S., et al. Impairment of Angiogenesis by Fatty Acid Synthase Inhibition Involves mTOR Malonylation. Cell Metab. 2018;28:866–880.e15. doi: 10.1016/j.cmet.2018.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schoors S., Bruning U., Missiaen R., Queiroz K.C.S., Borgers G., Elia I., Zecchin A., Cantelmo A.R., Christen S., Goveia J., et al. Fatty acid carbon is essential for dNTP synthesis in endothelial cells. Nature. 2015;520:192–197. doi: 10.1038/nature14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Son N.-H., Basu D., Samovski D., Pietka T.A., Peche V.S., Willecke F., Fang X., Yu S.-Q., Scerbo D., Chang H.R., et al. Endothelial cell CD36 optimizes tissue fatty acid uptake. J. Clin. Investig. 2018;128:4329–4342. doi: 10.1172/JCI99315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hagberg C.E., Falkevall A., Wang X., Larsson E., Huusko J., Nilsson I., van Meeteren L.A., Samen E., Lu L., Vanwildemeersch M., et al. Vascular endothelial growth factor B controls endothelial fatty acid uptake. Nature. 2010;464:917–921. doi: 10.1038/nature08945. [DOI] [PubMed] [Google Scholar]
- 56.Cifarelli V., Appak-Baskoy S., Peche V.S., Kluzak A., Shew T., Narendran R., Pietka K.M., Cella M., Walls C.W., Czepielewski R., et al. Visceral obesity and insulin resistance associate with CD36 deletion in lymphatic endothelial cells. Nat. Commun. 2021;12:3350. doi: 10.1038/s41467-021-23808-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Inoguchi T., Li P., Umeda F., Yu H.Y., Kakimoto M., Imamura M., Aoki T., Etoh T., Hashimoto T., Naruse M., et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C—Dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49:1939–1945. doi: 10.2337/diabetes.49.11.1939. [DOI] [PubMed] [Google Scholar]
- 58.Smathers R.L., Petersen D.R. The human fatty acid-binding protein family: Evolutionary divergences and functions. Hum. Genom. 2011;5:170. doi: 10.1186/1479-7364-5-3-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Elmasri H., Karaaslan C., Teper Y., Ghelfi E., Weng M., Ince T.A., Kozakewich H., Bischoff J., Cataltepe S. Fatty acid binding protein 4 is a target of VEGF and a regulator of cell proliferation in endothelial cells. FASEB J. 2009;23:3865–3873. doi: 10.1096/fj.09-134882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xiong J., Kawagishi H., Yan Y., Liu J., Wells Q.S., Edmunds L.R., Fergusson M.M., Yu Z.-X., Rovira I.I., Brittain E.L., et al. A Metabolic Basis for Endothelial-to-Mesenchymal Transition. Mol. Cell. 2018;69:689–698.e7. doi: 10.1016/j.molcel.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Choi K.J., Nam J.-K., Kim J.-H., Choi S.-H., Lee Y.-J. Endothelial-to-mesenchymal transition in anticancer therapy and normal tissue damage. Exp. Mol. Med. 2020;52:781–792. doi: 10.1038/s12276-020-0439-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oberkersch R.E., Santoro M.M. Role of amino acid metabolism in angiogenesis. Vasc. Pharmacol. 2019;112:17–23. doi: 10.1016/j.vph.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 63.Zhang J., Pavlova N.N., Thompson C.B. Cancer cell metabolism: The essential role of the nonessential amino acid, glutamine. EMBO J. 2017;36:1302–1315. doi: 10.15252/embj.201696151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim B., Li J., Jang C., Arany Z. Glutamine fuels proliferation but not migration of endothelial cells. EMBO J. 2017;36:2321–2333. doi: 10.15252/embj.201796436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leighton B., Curi R., Hussein A., Newsholme E.A. Maximum activities of some key enzymes of glycolysis, glutaminolysis, Krebs cycle and fatty acid utilization in bovine pulmonary endothelial cells. FEBS Lett. 1987;225:93–96. doi: 10.1016/0014-5793(87)81137-7. [DOI] [PubMed] [Google Scholar]
- 66.Polet F., Feron O. Endothelial cell metabolism and tumour angiogenesis: Glucose and glutamine as essential fuels and lactate as the driving force. J. Intern. Med. 2013;273:156–165. doi: 10.1111/joim.12016. [DOI] [PubMed] [Google Scholar]
- 67.Huang H., Vandekeere S., Kalucka J., Bierhansl L., Zecchin A., Brüning U., Visnagri A., Yuldasheva N., Goveia J., Cruys B., et al. Role of glutamine and interlinked asparagine metabolism in vessel formation. EMBO J. 2017;36:2334–2352. doi: 10.15252/embj.201695518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sanchez E.L., Carroll P.A., Thalhofer A.B., Lagunoff M. Latent KSHV Infected Endothelial Cells Are Glutamine Addicted and Require Glutaminolysis for Survival. PLoS Pathog. 2015;11:e1005052. doi: 10.1371/journal.ppat.1005052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hargadon K.M. Dysregulation of TGFβ1 Activity in Cancer and Its Influence on the Quality of Anti-Tumor Immunity. J. Clin. Med. 2016;5:76. doi: 10.3390/jcm5090076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guo Y., Deng Y., Li X., Ning Y., Lin X., Guo S., Chen M., Han M. Glutaminolysis Was Induced by TGF-β1 through PP2Ac Regulated Raf-MEK-ERK Signaling in Endothelial Cells. PLoS ONE. 2016;11:e0162658. doi: 10.1371/journal.pone.0162658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oberkersch R.E., Pontarin G., Astone M., Spizzotin M., Arslanbaeva L., Tosi G., Panieri E., Ricciardi S., Allega M.F., Brossa A., et al. Aspartate metabolism in endothelial cells activates the mTORC1 pathway to initiate translation during angiogenesis. Dev. Cell. 2022;57:1241–1256.e8. doi: 10.1016/j.devcel.2022.04.018. [DOI] [PubMed] [Google Scholar]
- 72.Camp C.R., Yuan H. GRIN2D/GluN2D NMDA Receptor: Unique Features and Its Contribution to Pediatric Developmental and Epileptic Encephalopathy. Eur. Paediatr. Neurol. 2020;24:89–99. doi: 10.1016/j.ejpn.2019.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ferguson H.J.M., Wragg J.W., Ward S., Heath V.L., Ismail T., Bicknell R. Glutamate dependent NMDA receptor 2D is a novel angiogenic tumour endothelial marker in colorectal cancer. Oncotarget. 2016;7:20440–20454. doi: 10.18632/oncotarget.7812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen D., Fan Z., Rauh M., Buchfelder M., Eyupoglu I.Y., Savaskan N. ATF4 promotes angiogenesis and neuronal cell death and confers ferroptosis in a xCT-dependent manner. Oncogene. 2017;36:5593–5608. doi: 10.1038/onc.2017.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yoo H.C., Yu Y.C., Sung Y., Han J.M. Glutamine reliance in cell metabolism. Exp. Mol. Med. 2020;52:1496–1516. doi: 10.1038/s12276-020-00504-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Newgard C.B., An J., Bain J.R., Muehlbauer M.J., Stevens R.D., Lien L.F., Haqq A.M., Shah S.H., Arlotto M., Slentz C.A., et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ndrepepa G. Aspartate aminotransferase and cardiovascular disease—A narrative review. JLPM. 2020;6:1–17. doi: 10.21037/jlpm-20-93. [DOI] [Google Scholar]
- 78.Exertier P., Javerzat S., Wang B., Franco M., Herbert J., Platonova N., Winandy M., Pujol N., Nivelles O., Ormenese S., et al. Impaired angiogenesis and tumor development by inhibition of the mitotic kinesin Eg5. Oncotarget. 2013;4:2302–2316. doi: 10.18632/oncotarget.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rajcevic U., Petersen K., Knol J.C., Loos M., Bougnaud S., Klychnikov O., Li K.W., Pham T.V., Wang J., Miletic H., et al. iTRAQ-based proteomics profiling reveals increased metabolic activity and cellular cross-talk in angiogenic compared with invasive glioblastoma phenotype. Mol. Cell. Proteom. 2009;8:2595–2612. doi: 10.1074/mcp.M900124-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cohen E.B., Geck R.C., Toker A. Metabolic pathway alterations in microvascular endothelial cells in response to hypoxia. PLoS ONE. 2020;15:e0232072. doi: 10.1371/journal.pone.0232072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pavlova N.N., Hui S., Ghergurovich J.M., Fan J., Intlekofer A.M., White R.M., Rabinowitz J.D., Thompson C.B., Zhang J. As Extracellular Glutamine Levels Decline, Asparagine Becomes an Essential Amino Acid. Cell Metab. 2018;27:428–438.e5. doi: 10.1016/j.cmet.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lomelino C.L., Andring J.T., McKenna R., Kilberg M.S. Asparagine synthetase: Function, structure, and role in disease. J. Biol. Chem. 2017;292:19952–19958. doi: 10.1074/jbc.R117.819060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Balasubramanian M.N., Butterworth E.A., Kilberg M.S. Asparagine synthetase: Regulation by cell stress and involvement in tumor biology. Am. J. Physiol. Endocrinol. Metab. 2013;304:E789–E799. doi: 10.1152/ajpendo.00015.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Egler R.A., Ahuja S.P., Matloub Y. L-asparaginase in the treatment of patients with acute lymphoblastic leukemia. J. Pharmacol. Pharmacother. 2016;7:62–71. doi: 10.4103/0976-500X.184769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang M., Vousden K.H. Serine and one-carbon metabolism in cancer. Nat. Rev. Cancer. 2016;16:650–662. doi: 10.1038/nrc.2016.81. [DOI] [PubMed] [Google Scholar]
- 86.Sullivan M.R., Mattaini K.R., Dennstedt E.A., Nguyen A.A., Sivanand S., Reilly M.F., Meeth K., Muir A., Darnell A.M., Bosenberg M.W., et al. Increased Serine Synthesis Provides an Advantage for Tumors Arising in Tissues Where Serine Levels Are Limiting. Cell Metab. 2019;29:1410–1421.e4. doi: 10.1016/j.cmet.2019.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maralani M.N., Movahedian A., Javanmard S.H. Antioxidant and cytoprotective effects of L-Serine on human endothelial cells. Res. Pharm. Sci. 2012;7:209–215. [PMC free article] [PubMed] [Google Scholar]
- 88.Vandekeere S., Dubois C., Kalucka J., Sullivan M.R., García-Caballero M., Goveia J., Chen R., Diehl F.F., Bar-Lev L., Souffreau J., et al. Serine Synthesis via PHGDH Is Essential for Heme Production in Endothelial Cells. Cell Metab. 2018;28:573–587.e13. doi: 10.1016/j.cmet.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 89.Ocaña M.C., Yang C., Bernal M., Martínez-Poveda B., Vu H.S., Cárdenas C., DeBerardinis R.J., Quesada A.R., Medina M.Á. The anti-angiogenic compound dimethyl fumarate inhibits the serine synthesis pathway and increases glycolysis in endothelial cells. bioRxiv. 2021 doi: 10.1101/2021.12.13.472337. [DOI] [Google Scholar]
- 90.Tsuji-Tamura K., Sato M., Fujita M., Tamura M. Glycine exerts dose-dependent biphasic effects on vascular development of zebrafish embryos. Biochem. Biophys. Res. Commun. 2020;527:539–544. doi: 10.1016/j.bbrc.2020.04.098. [DOI] [PubMed] [Google Scholar]
- 91.Amin K., Li J., Chao W.R., Dewhirst M.W., Haroon Z.A. Dietary glycine inhibits angiogenesis during wound healing and tumor growth. Cancer Biol. Ther. 2003;2:173–178. doi: 10.4161/cbt.2.2.280. [DOI] [PubMed] [Google Scholar]
- 92.Bruns H., Kazanavicius D., Schultze D., Saeedi M.A., Yamanaka K., Strupas K., Schemmer P. Glycine inhibits angiogenesis in colorectal cancer: Role of endothelial cells. Amino Acids. 2016;48:2549–2558. doi: 10.1007/s00726-016-2278-0. [DOI] [PubMed] [Google Scholar]
- 93.Guo D., Murdoch C.E., Xu H., Shi H., Duan D.D., Ahmed A., Gu Y. Vascular endothelial growth factor signaling requires glycine to promote angiogenesis. Sci. Rep. 2017;7:14749. doi: 10.1038/s41598-017-15246-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang Y., Ikejima K., Honda H., Kitamura T., Takei Y., Sato N. Glycine prevents apoptosis of rat sinusoidal endothelial cells caused by deprivation of vascular endothelial growth factor. Hepatology. 2000;32:542–546. doi: 10.1053/jhep.2000.16605. [DOI] [PubMed] [Google Scholar]
- 95.Yin J., Ren W., Yang G., Duan J., Huang X., Fang R., Li C., Li T., Yin Y., Hou Y., et al. L-Cysteine metabolism and its nutritional implications. Mol. Nutr. Food Res. 2016;60:134–146. doi: 10.1002/mnfr.201500031. [DOI] [PubMed] [Google Scholar]
- 96.Giuffrè A., Vicente J.B. Hydrogen Sulfide Biochemistry and Interplay with Other Gaseous Mediators in Mammalian Physiology. Oxid. Med. Cell. Longev. 2018;2018:6290931. doi: 10.1155/2018/6290931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stipanuk M.H., Londono M., Lee J.-I., Hu M., Yu A.F. Enzymes and metabolites of cysteine metabolism in nonhepatic tissues of rats show little response to changes in dietary protein or sulfur amino acid levels. J. Nutr. 2002;132:3369–3378. doi: 10.1093/jn/132.11.3369. [DOI] [PubMed] [Google Scholar]
- 98.Okumura N., Inoue R., Kakutani K., Nakahara M., Kinoshita S., Hamuro J., Koizumi N. Corneal Endothelial Cells Have an Absolute Requirement for Cysteine for Survival. Cornea. 2017;36:988–994. doi: 10.1097/ICO.0000000000001242. [DOI] [PubMed] [Google Scholar]
- 99.Miura K., Ishii T., Sugita Y., Bannai S. Cystine uptake and glutathione level in endothelial cells exposed to oxidative stress. Am. J. Physiol. 1992;262:C50–C58. doi: 10.1152/ajpcell.1992.262.1.C50. [DOI] [PubMed] [Google Scholar]
- 100.Lopes-Coelho F., Martins F., Hipólito A., Mendes C., Sequeira C.O., Pires R.F., Almeida A.M., Bonifácio V.D.B., Pereira S.A., Serpa J. The Activation of Endothelial Cells Relies on a Ferroptosis-Like Mechanism: Novel Perspectives in Management of Angiogenesis and Cancer Therapy. Front. Oncol. 2021;11:656229. doi: 10.3389/fonc.2021.656229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Puris E., Gynther M., Auriola S., Huttunen K.M. L-Type amino acid transporter 1 as a target for drug delivery. Pharm. Res. 2020;37:88. doi: 10.1007/s11095-020-02826-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Quan L., Ohgaki R., Hara S., Okuda S., Wei L., Okanishi H., Nagamori S., Endou H., Kanai Y. Amino acid transporter LAT1 in tumor-associated vascular endothelium promotes angiogenesis by regulating cell proliferation and VEGF-A-dependent mTORC1 activation. J. Exp. Clin. Cancer Res. 2020;39:266. doi: 10.1186/s13046-020-01762-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wu J., Yeung S.-C.J., Liu S., Qdaisat A., Jiang D., Liu W., Cheng Z., Liu W., Wang H., Li L., et al. Cyst(e)ine in nutrition formulation promotes colon cancer growth and chemoresistance by activating mTORC1 and scavenging ROS. Signal Transduct. Target. Ther. 2021;6:188. doi: 10.1038/s41392-021-00581-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Das S.K., White A.C., Fanburg B.L. Modulation of transforming growth factor-beta 1 antiproliferative effects on endothelial cells by cysteine, cystine, and N-acetylcysteine. J. Clin. Investig. 1992;90:1649–1656. doi: 10.1172/JCI116036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Azabdaftari A., van der Giet M., Schuchardt M., Hennermann J.B., Plöckinger U., Querfeld U. The cardiovascular phenotype of adult patients with phenylketonuria. Orphanet J. Rare Dis. 2019;14:213. doi: 10.1186/s13023-019-1188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hermida-Ameijeiras A., Crujeiras V., Roca I., Calvo C., Leis R., Couce M.-L. Arterial stiffness assessment in patients with phenylketonuria. Medicine. 2017;96:e9322. doi: 10.1097/MD.0000000000009322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sugiyama T., Levy B.D., Michel T. Tetrahydrobiopterin recycling, a key determinant of endothelial nitric-oxide synthase-dependent signaling pathways in cultured vascular endothelial cells. J. Biol. Chem. 2009;284:12691–12700. doi: 10.1074/jbc.M809295200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Heikal L., Starr A., Hussein D., Prieto-Lloret J., Aaronson P., Dailey L.A., Nandi M. l-Phenylalanine Restores Vascular Function in Spontaneously Hypertensive Rats Through Activation of the GCH1-GFRP Complex. JACC Basic Transl. Sci. 2018;3:366–377. doi: 10.1016/j.jacbts.2018.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lau A.N., Heiden M.G.V. Metabolism in the Tumor Microenvironment. Annu. Rev. Cancer Biol. 2020;4:26. doi: 10.1146/annurev-cancerbio-030419-033333. [DOI] [Google Scholar]
- 110.Parker S.J., Amendola C.R., Hollinshead K.E.R., Yu Q., Yamamoto K., Encarnación-Rosado J., Rose R.E., LaRue M.M., Sohn A.S.W., Biancur D.E., et al. Selective Alanine Transporter Utilization Creates a Targetable Metabolic Niche in Pancreatic Cancer. Cancer Discov. 2020;10:1018–1037. doi: 10.1158/2159-8290.CD-19-0959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vecchio E., Caiazza C., Mimmi S., Avagliano A., Iaccino E., Brusco T., Nisticò N., Maisano D., Aloisio A., Quinto I., et al. Metabolites Profiling of Melanoma Interstitial Fluids Reveals Uridine Diphosphate as Potent Immune Modulator Capable of Limiting Tumor Growth. Front. Cell Dev. Biol. 2021;9:730726. doi: 10.3389/fcell.2021.730726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yang L., Achreja A., Yeung T.-L., Mangala L.S., Jiang D., Han C., Baddour J., Marini J.C., Ni J., Nakahara R., et al. Targeting Stromal Glutamine Synthetase in Tumors Disrupts Tumor Microenvironment-Regulated Cancer Cell Growth. Cell Metab. 2016;24:685–700. doi: 10.1016/j.cmet.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Acevedo-Acevedo S., Millar D.C., Simmons A.D., Favreau P., Cobra P.F., Skala M., Palecek S.P. Metabolomics revealed the influence of breast cancer on lymphatic endothelial cell metabolism, metabolic crosstalk, and lymphangiogenic signaling in co-culture. Sci. Rep. 2020;10:21244. doi: 10.1038/s41598-020-76394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Petrillo S., De Giorgio F., Kopecka J., Genova T., Fiorito V., Allocco A.L., Bertino F., Chiabrando D., Mussano F., Altruda F., et al. Endothelial Heme Dynamics Drive Cancer Cell Metabolism by Shaping the Tumor Microenvironment. Biomedicines. 2021;9:1557. doi: 10.3390/biomedicines9111557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wenes M., Shang M., Di Matteo M., Goveia J., Martín-Pérez R., Serneels J., Prenen H., Ghesquière B., Carmeliet P., Mazzone M. Macrophage Metabolism Controls Tumor Blood Vessel Morphogenesis and Metastasis. Cell Metab. 2016;24:701–715. doi: 10.1016/j.cmet.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 116.Szabo C. Gasotransmitters in cancer: From pathophysiology to experimental therapy. Nat. Rev. Drug Discov. 2016;15:185–203. doi: 10.1038/nrd.2015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kroll J., Waltenberger J. VEGF-A Induces Expression of eNOS and iNOS in Endothelial Cells via VEGF Receptor-2 (KDR) Biochem. Biophys. Res. Commun. 1998;252:743–746. doi: 10.1006/bbrc.1998.9719. [DOI] [PubMed] [Google Scholar]
- 118.Ha J.M., Jin S.Y., Lee H.S., Shin H.K., Lee D.H., Song S.H., Kim C.D., Bae S.S. Regulation of retinal angiogenesis by endothelial nitric oxide synthase signaling pathway. Korean J. Physiol. Pharmacol. 2016;20:533–538. doi: 10.4196/kjpp.2016.20.5.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Namba T., Koike H., Murakami K., Aoki M., Makino H., Hashiya N., Ogihara T., Kaneda Y., Kohno M., Morishita R. Angiogenesis Induced by Endothelial Nitric Oxide Synthase Gene Through Vascular Endothelial Growth Factor Expression in a Rat Hindlimb Ischemia Model. Circulation. 2003;108:2250–2257. doi: 10.1161/01.CIR.0000093190.53478.78. [DOI] [PubMed] [Google Scholar]
- 120.Arnold W.P., Mittal C.K., Katsuki S., Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3′:5′-cyclic monophosphate levels in various tissue preparations. Proc. Natl. Acad. Sci. USA. 1977;74:3203–3207. doi: 10.1073/pnas.74.8.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Thibeault S., Rautureau Y., Oubaha M., Faubert D., Wilkes B.C., Delisle C., Gratton J.-P. S-Nitrosylation of β-Catenin by eNOS-Derived NO Promotes VEGF-Induced Endothelial Cell Permeability. Mol. Cell. 2010;39:468–476. doi: 10.1016/j.molcel.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 122.Paik J.-Y., Lee K.-H., Ko B.-H., Choe Y.S., Choi Y., Kim B.-T. Nitric oxide stimulates 18F-FDG uptake in human endothelial cells through increased hexokinase activity and GLUT1 expression. J. Nucl. Med. 2005;46:365–370. [PubMed] [Google Scholar]
- 123.Siragusa M., Thöle J., Bibli S., Luck B., Loot A.E., de Silva K., Wittig I., Heidler J., Stingl H., Randriamboavonjy V., et al. Nitric oxide maintains endothelial redox homeostasis through PKM2 inhibition. EMBO J. 2019;38:e100938. doi: 10.15252/embj.2018100938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shibuya N., Mikami Y., Kimura Y., Nagahara N., Kimura H. Vascular Endothelium Expresses 3-Mercaptopyruvate Sulfurtransferase and Produces Hydrogen Sulfide. J. Biochem. 2009;146:623–626. doi: 10.1093/jb/mvp111. [DOI] [PubMed] [Google Scholar]
- 125.Erdélyi K., Ditrói T., Johansson H.J., Czikora Á., Balog N., Silwal-Pandit L., Ida T., Olasz J., Hajdú D., Mátrai Z., et al. Reprogrammed transsulfuration promotes basal-like breast tumor progression via realigning cellular cysteine persulfidation. Proc. Natl. Acad. Sci. USA. 2021;118:e2100050118. doi: 10.1073/pnas.2100050118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang Y.-H., Huang J.-T., Chen W.-L., Wang R.-H., Kao M.-C., Pan Y.-R., Chan S.-H., Tsai K.-W., Kung H.-J., Lin K.-T., et al. Dysregulation of cystathionine γ-lyase promotes prostate cancer progression and metastasis. EMBO Rep. 2019;20:e45986. doi: 10.15252/embr.201845986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Szabo C., Coletta C., Chao C., Módis K., Szczesny B., Papapetropoulos A., Hellmich M.R. Tumor-derived hydrogen sulfide, produced by cystathionine-β-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc. Natl. Acad. Sci. USA. 2013;110:12474–12479. doi: 10.1073/pnas.1306241110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Longchamp A., Mirabella T., Arduini A., MacArthur M.R., Das A., Treviño-Villarreal J.H., Hine C., Ben-Sahra I., Knudsen N.H., Brace L.E., et al. Amino Acid Restriction Triggers Angiogenesis via GCN2/ATF4 Regulation of VEGF and H2S Production. Cell. 2018;173:117–129.e14. doi: 10.1016/j.cell.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tang N., Wang L., Esko J., Giordano F.J., Huang Y., Gerber H.-P., Ferrara N., Johnson R.S. Loss of HIF-1α in endothelial cells disrupts a hypoxia-driven VEGF autocrine loop necessary for tumorigenesis. Cancer Cell. 2004;6:485–495. doi: 10.1016/j.ccr.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 130.Mazzone M., Dettori D., Leite de Oliveira R., Loges S., Schmidt T., Jonckx B., Tian Y.-M., Lanahan A.A., Pollard P., Ruiz de Almodovar C., et al. Heterozygous Deficiency of PHD2 Restores Tumor Oxygenation and Inhibits Metastasis via Endothelial Normalization. Cell. 2009;136:839–851. doi: 10.1016/j.cell.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Klomp J., Hyun J., Klomp J.E., Pajcini K., Rehman J., Malik A.B. Comprehensive transcriptomic profiling reveals SOX7 as an early regulator of angiogenesis in hypoxic human endothelial cells. J. Biol. Chem. 2020;295:4796–4808. doi: 10.1074/jbc.RA119.011822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Provenzano P.P., Inman D.R., Eliceiri K.W., Knittel J.G., Yan L., Rueden C.T., White J.G., Keely P.J. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008;6:11. doi: 10.1186/1741-7015-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Levental K.R., Yu H., Kass L., Lakins J.N., Egeblad M., Erler J.T., Fong S.F.T., Csiszar K., Giaccia A., Weninger W., et al. Matrix Crosslinking Forces Tumor Progression by Enhancing Integrin Signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bordeleau F., Mason B.N., Lollis E.M., Mazzola M., Zanotelli M.R., Somasegar S., Califano J.P., Montague C., LaValley D.J., Huynh J., et al. Matrix stiffening promotes a tumor vasculature phenotype. Proc. Natl. Acad. Sci. USA. 2017;114:492–497. doi: 10.1073/pnas.1613855114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liu J., Agarwal S. Mechanical Signals Activate Vascular Endothelial Growth Factor Receptor-2 To Upregulate Endothelial Cell Proliferation during Inflammation. J. Immunol. 2010;185:1215–1221. doi: 10.4049/jimmunol.0903660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shen Y., Wang X., Liu Y., Singhal M., Gürkaşlar C., Valls A.F., Lei Y., Hu W., Schermann G., Adler H., et al. STAT3-YAP/TAZ signaling in endothelial cells promotes tumor angiogenesis. Sci. Signal. 2021;14:eabj8393. doi: 10.1126/scisignal.abj8393. [DOI] [PubMed] [Google Scholar]
- 137.Wang X., Freire Valls A., Schermann G., Shen Y., Moya I.M., Castro L., Urban S., Solecki G.M., Winkler F., Riedemann L., et al. YAP/TAZ Orchestrate VEGF Signaling during Developmental Angiogenesis. Dev. Cell. 2017;42:462–478.e7. doi: 10.1016/j.devcel.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 138.Sakabe M., Fan J., Odaka Y., Liu N., Hassan A., Duan X., Stump P., Byerly L., Donaldson M., Hao J., et al. YAP/TAZ-CDC42 signaling regulates vascular tip cell migration. Proc. Natl. Acad. Sci. USA. 2017;114:10918–10923. doi: 10.1073/pnas.1704030114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Reid S.E., Kay E.J., Neilson L.J., Henze A.-T., Serneels J., McGhee E.J., Dhayade S., Nixon C., Mackey J.B.G., Santi A., et al. Tumor matrix stiffness promotes metastatic cancer cell interaction with the endothelium. EMBO J. 2017;36:2373–2389. doi: 10.15252/embj.201694912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cox A.G., Tsomides A., Yimlamai D., Hwang K.L., Miesfeld J., Galli G.G., Fowl B.H., Fort M., Ma K.Y., Sullivan M.R., et al. Yap regulates glucose utilization and sustains nucleotide synthesis to enable organ growth. EMBO J. 2018;37:e100294. doi: 10.15252/embj.2018100294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liu C., Li M., Dong Z.-X., Jiang D., Li X., Lin S., Chen D., Zou X., Zhang X.-D., Luker G.D. Heterogeneous microenvironmental stiffness regulates pro-metastatic functions of breast cancer cells. Acta Biomater. 2021;131:326–340. doi: 10.1016/j.actbio.2021.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bertero T., Oldham W.M., Cottrill K.A., Pisano S., Vanderpool R.R., Yu Q., Zhao J., Tai Y., Tang Y., Zhang Y.-Y., et al. Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. J. Clin. Investig. 2016;126:3313–3335. doi: 10.1172/JCI86387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lin K., Hsu P.-P., Chen B.P., Yuan S., Usami S., Shyy J.Y.-J., Li Y.-S., Chien S. Molecular mechanism of endothelial growth arrest by laminar shear stress. Proc. Natl. Acad. Sci. USA. 2000;97:9385–9389. doi: 10.1073/pnas.170282597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang K.-C., Yeh Y.-T., Nguyen P., Limqueco E., Lopez J., Thorossian S., Guan K.-L., Li Y.-S.J., Chien S. Flow-dependent YAP/TAZ activities regulate endothelial phenotypes and atherosclerosis. Proc. Natl. Acad. Sci. USA. 2016;113:11525–11530. doi: 10.1073/pnas.1613121113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Huang Q., Hu X., He W., Zhao Y., Hao S., Wu Q., Li S., Zhang S., Shi M. Fluid shear stress and tumor metastasis. Am. J. Cancer Res. 2018;8:763–777. [PMC free article] [PubMed] [Google Scholar]
- 146.Huang Q., Li S., Hu X., Sun M., Wu Q., Dai H., Tan Y., Sun F., Wang C., Rong X., et al. Shear stress activates ATOH8 via autocrine VEGF promoting glycolysis dependent-survival of colorectal cancer cells in the circulation. J. Exp. Clin. Cancer Res. 2020;39:25. doi: 10.1186/s13046-020-1533-0. [DOI] [PMC free article] [PubMed] [Google Scholar]