Abstract
This brief commentary aims to provide an overview of the available and relatively new precision management of reward deficiencies manifested as substance and behavioral disorders. Current and future advances, concepts, and the substantial evidential basis of this potential therapeutic and prophylactic treatment modality are presented. Precision Behavioral Management (PBM), conceptualized initially as Precision Addiction Management (PAM), certainly deserves consideration as an important modality for the treatment of impaired cognitive control in reward processing as manifested in people with neurobiologically expressed Reward Deficiency Syndrome (RDS).
Keywords: dopamine, hypodopaminergia, Genetic Addiction Risk Severity (GARS) test, pro-dopamine regulation (KB220), Restoregen ®
1. Precision Behavioral Management
The Precision Behavioral Management (PBM) platform is related to addiction medicine, with the first USA and foreign patents related to the accurate Genetic Addiction Risk Severity (GARS®) test. Blum and Noble (JAMA, 1990) [1] found the first confirmed association of the DRD2 gene A1 allele with severe alcoholism and other Reward Deficiency Syndrome (RDS) behaviors, and Blum et al. have developed the GARS test and the pro- dopamine regulator, a nutraceutical neuronutrient (Research ID Code KB220) [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102]. The basis of these addiction treatment interventions is research that identified a neurotransmitter network function within the Mesolimbic and Pre-Frontal Cortex (PFC) brain regions that regulates the final reward and motivational pathway of “wanting,” causing neuronal dopamine release (see Figure 1 and Figure 2) [103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126].
Figure 1.
Precision Behavioral Management (PBM) platform. Reprinted/adapted with permission from Ref. [127]. Gold et al. copyright 2021.
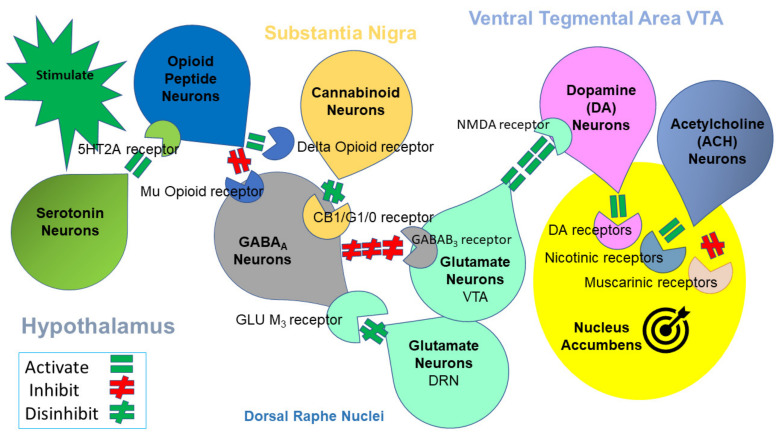
Figure 2.
Mesolimbic Brain Reward Cascade [128]. This cartoon illustrates the interaction of the known major neurotransmitter pathways involved in the Brain Reward Cascade (BRC). In the hypothalamus, environmental stimulation results in the release of serotonin, which in turn, via, for example, 5HT-2a receptors, activates (green equal sign) the subsequent release of opioid peptides from opioid peptide neurons. Then, Substantia Nigra, the opioid peptides move to possibly two different opioid receptors with different effects. One inhibits (red hash sign) through the mu-opioid receptor (possibly via enkephalin) to GABAA neurons. Another stimulates (green equal sign) cannabinoid neurons (the Anandamide and 2-archydonoglcerol, for example) through beta-endorphin-linked delta receptors, which inhibit GABAA neurons. In addition, when activated, cannabinoids, primarily 2-archydonoglerol, can indirectly disinhibit (green hash sign) GABAA neurons through the activation of G1/0 coupled to CB1 receptors. In the Dorsal Raphe Nuclei (DRN), glutamate neurons can then indirectly disinhibit GABAA neurons in the Substantia Nigra through activation of GLU M3 receptors (green hash sign). GABAA neurons, when disinhibited, will, in turn, powerfully (red hash signs) inhibit VTA glutaminergic drive via GABAB 3 receptors. At the Nucleus, Accumbens ACH neurons may stimulate both muscarinic (red hash) and nicotinic (green hash). Finally, glutamate neurons in the VTA will project to dopamine neurons through NMDA receptors (green equal sign) to preferentially release dopamine at the Nucleus Accumbens (NAc), shown as a bullseye, indicating a euphoria, or “wanting” response. The result is dopamine release; low release is (endorphin deficiency), and unhappiness is felt. General (healthy) happiness depends on the dopamine homeostatic tonic set point (with permission) [22]. Notably, various hypotheses have explained the findings that led to the modern known correlates of neurotransmitter interactions within this brain reward circuitry.
2. The Brain Reward Cascade
The cascading interaction of neurotransmitters and second messengers results in the correct release of dopamine within the NAc and across many brain regions. These regions are involved in motivation, cognition (memory), pleasure, stress reduction, drug reinstatement, decision making, recall, wellbeing, and cravings. The result is to provide homo sapiens with a usual happiness setpoint (i.e., dopamine homeostasis) [126], reflected in resting-state functional connectivity (rsFC) in neuroimaging studies [127].
In the neurophysiologic reward system, repeated frequent acute dopamine stimulation becomes chronic stimulation and leads to a dysfunctional hypodopaminergic state, rendering the reward system less responsive to natural reinforcers, a symptom of RDS [126,128]. The stimulation can be from euphorigenic substances, non-substances like gambling, or severe stressors like pain and anxiety. Chronic stimulation causes dopamine depletion (Hypodopaminergia). Reward deficiency results from depleted or hereditary hypodopaminergia, potentially reflected in a host of personality traits and mental and medical disorders that have been associated in genetic studies with dopamine depleting alleles. These symptoms and disorders create the diagnostic criteria for RDS and include, but are not limited to, novelty seeking, schizophrenia, obesity, chronic pain, post-traumatic stress disorder (PTSD), major depression, and attention deficit hyperactivity disorder (see Table 1) [129].
Table 1.
Reward Deficiency Syndrome criteria.
| Set 1. Criteria DSM5 Disorders | |
|---|---|
| A Present or Past Diagnosis or History of These Behavioral Disorders | |
| Substance Use Process Disorders | Disorders: Alcohol Use Disorder, Opioid Use Disorder, Cannabis Use Disorder, Sedative, Hypnotic, Anxiolytic Use Disorder, Cocaine Use Disorder, Amphetamine Use Disorder, Hallucinogen Use Disorder, Nicotine Use Disorder, Inhalant Use Disorder, Other, Unknown Substance Use Disorder Specifiers: Mild, Moderate, Severe, Early Remission (6–12 months), Sustained Remission (12 + months), in a Controlled Environment, on Maintenance Therapy |
| Process Disorders | Gambling, Sex, Other Specified Process Disorders |
| Depressive (and related) Disorders | Major Depression, Dysthymia, Disruptive Mood Dysregulation, SUD/Medication/Medical Condition Inducted Depressive Disorder, Disruptive Premenstrual Dysphoric Disorder |
| Anxiety Disorders | Generalized Anxiety Disorder, Social Anxiety, Panic Attack Disorder, Separation Anxiety, Selective Mutism, Specific Phobia, SUD/Medication/Medical Condition Inducted Anxiety |
| Trauma and Stress Disorders | Reactive Attachment, Disinhibited Social Engagement, Post-Traumatic Stress Disorder (PTSD), Acute Stress Disorders |
| Disruptive, Impulse Control, and Conduct Disorders | Oppositional Defiant Disorder, Intermittent Explosive Disorder, Conduct Disorder, Pyromania, Kleptomania |
| Personality Disorders | General Personality Disorder, Paranoid Personality Disorder, Schizoid/Schizotypal Personality Disorder, Anti-Social Personality Disorder, Borderline Personality Disorder, Histrionic Personality Disorder, Narcissistic, Personality Disorder, Avoidant Personality Disorder, Dependent Personality Disorder |
| Obsessive Compulsive Disorders and Related Disorders | Trichotillomania, Excoriation Disorder, SUD/Medical/Medication Inducted OCD Disorder, other Medical Condition, Induced Personality Disorder |
| Schizophrenic Disorders | Schizophrenia, Schizoaffective Disorder, Schizophreniform Disorder, Delusional disorder, Brief Psychotic Disorder, MH/Medical Catalonia, SUD/Medication/Medical Condition Inducted Psychotic Disorder |
| Dissociative Disorders | Dissociative Identity Disorder, Dissociative Amnesia, Depersonalization/Derealization Disorder |
| Other Not Otherwise Specified (NOS) Disorders | Gender Dysphoric Disorder Paraphilic Disorders |
| Spectrum Disorders | Attention Deficient Disorder, Attention Deficient/Hyperactivity Disorder, Tourette’s Syndrome, Autism |
| Set 2. Criteria | |
| Reported history of these symptoms: | |
| Novelty seeking | The y trait is associated with exploratory activity in response to novel stimulation, impulsive decision making, extravagance in approach to reward cues, quick loss of temper, and avoidance of frustration. |
| Impulsivity | The impulsivity construct includes at least two independent components: first, acting without an appropriate amount of deliberation, which may or may not be functional; and second, choosing short-term gains over long-term ones. |
| Difficulty feeling reward (Anhedonia) |
Either a reduced ability to experience pleasure or a diminished interest in engaging in pleasurable activities. |
| Motivational Anhedonia | A decrease in motivation to participate in pleasurable activities. |
| Rumination, Obsessive, and Intrusive Negative Thoughts | Possible causes and consequences, as opposed to its solutions. |
As alluded to above, reward deficiencies may also occur in the absence of dopaminergic stimulation by exogenous factors due to specific polymorphic alleles that alter the function of genes in the reward cascade. One example is the A1 allele of the D2 dopamine receptor gene that causes a reduced number of dopamine receptors in the mesolimbic NAc. An essential feature of RDS is the lack of integration between cognition, perception, and emotions occurring due to (1) substantial dopaminergic surges in reward, motivation, and learning centers leading to neuroplasticity in the striato-thalamic-frontal cortical loop, with ensuing top-down dissociation from the subcortical activity; (2) hypo-functionality of the excitatory glutamatergic afferents from the amygdala–hippocampus complex failing to produce bottom-up restraint of the striato-thalamic-frontal cortical loop [130,131,132,133,134].
The above aberrations may be a target of neuromodulation with therapeutic interventions and prophylaxis of addictive and related disorders. PBM combines the GARS test that identifies RDS risk polymorphisms and is used to ascertain the neuropathways involved in the tested individual’s hypodopaminergic risk neurotransmission finite pathways. These pathways are used in an algorithm to select a neuronutrient formulation of KB220 for that individual to induce the desired dopamine homeostasis by balancing genetic and epigenetic (neuro-molecular) brain reward activity [134].
3. Genetic Addiction Risk Severity (GARS)
The GARS test uses saliva samples and polymerase chain reaction (PCR) to identify dysfunctional polymorphisms of reward genes [135]. Genes express proteins that determine neurotransmitter function [136]. People who have Single Nucleotide Polymorphisms (SNPs) of genes in their DNA that cause dysfunctional dopaminergic neurotransmission are at risk of RDS behaviors, including addictions. The development of the GARS test used the reward cascade of neurotransmission to identify eleven SNPs that cause hypodopaminergia from ten reward genes [137,138,139,140,141,142,143].
Hypo-dopaminergia refers to a reduced dopamine function in the brain reward circuitry. As stated in the paper and indicated by the interrelatedness of at least seven main neurotransmitter pathways involving synthesis, vesicular pre-neuronal storage, mitochondrial catabolism, synaptic catabolism, neuronal clearance via transporters, receptor affinity, and number, carrying any one of the eleven polymorphic alleles could explain low dopamine function (see Table 1). One example is the finding by Noble and Blum that a progressively reduced Bmax was found in subjects with A2/A2, A1/A2, and A1/A1 alleles of the DRD2 gene, with subjects with A2/A2 having the highest and subjects with A1/A1, the lowest mean values (see Table 2).
Table 2.
| Genetic Variant | Prime Function |
|---|---|
| G-Allele COMT | Carriers of this allele will have a high activity of synaptic dopamine (DA) reabsorption leading to a reduced interaction at DA receptors. |
| A-Allele of the DRD1 receptor gene | Carriers of this allele will have a reduced number of DRD1 receptors and lower DA function within the brain reward circuitry. The DRD1 receptor is involved in promoting normal DA function. |
| A1 variant of the DRD2 receptor gene | Carriers of this allele will have a reduced number of DRD2 receptors up to 40% and, as such, will have a lower DA function within the brain reward circuitry, especially at the Ventral Tegmental Area (VTA) Nucleus Accumbens. |
| C variant of the DRD3 | Carriers of this allele will have a reduced number of DRD3 receptors and have a lower DA function within the brain reward circuitry. Studies have found that this allele associates with risk for Alcohol, Cocaine, and Opioid Use Disorder as well as opioid dependence, especially in the African American population. |
| C variant of the DRD4 receptor gene | Carriers of this allele will have a reduced number of DRD4 receptors and have a lower DA function within the brain reward circuitry. The DRD4 gene is responsible for normal DA function within the mesolimbic reward cascade, and the C variant is highly associated with risk for ADHD and novelty seeking. |
| G-Allele of the OPRM1 receptor gene | Carriers of this allele will have a reduced number of Mu opioid receptors. Reduced Mu opioid receptors reduce GABA transmission at the Raphe Nuclei and Substania Nigra, leading to a reduced DA release at the VTA via altered inhibition of the normal Glutaminergic drive. |
| 9 R allele of the DAT1 gene | Carriers of this allele will have a high activity of synaptic dopamine (DA) reabsorption, leading to a reduced interaction at DA receptors. |
| S or LG allele of the 5-HTTLPR gene | Carriers of these alleles will have a high activity of synaptic serotonin reabsorption, leading to a reduced interaction at serotonin receptors. This paucity leads to a reduced serotonergic transmission at the hypothalamus in the mesolimbic system. The low serotonin activity results in a reduced interaction with the endogenous opioid peptides and, as such, a reduced inhibition at GABA sites. |
| 4 R variant of the MAOA gene | Carriers of this allele will have a high activity of mitochondrial catabolism of both serotonin and dopamine. The high activity will reduce the projection of these neurotransmitters to storage at the pre-neuron vesicles for further release when fired with an action potential. |
| 181 allele of the GABRB3 gene | Carriers of this allele will have an overexpressed GABRB3 that will lead to a higher GABA transmission at the VTA-Glutaminergic site, leading to hypodopaminergia. |
The GARS test examines the sum of many related polymorphisms instead of one gene alone to predict genetic risk for RDS behaviors. It is possible to find a significant association with the degree of risk for all addictive behaviors (RDS). In conjunction with the Institute of Behavioral Genetics at Colorado University, unpublished research compared the GARS test with the Addiction Severity Index (ASI) in 273 subjects from substance treatment centers to determine drug and alcohol risk severity. Alcohol risk severity p < 0.04 predicted with seven or more alleles, and drug risk severity p < 0.05 predicted with four or more alleles. Understanding the genetic antecedents to Alcohol Use Disorder (AUD) may help explain potential neuroplasticity in those dependent on alcohol or other substances of abuse [142].
4. Precision Behavioral Management (PBM) System
The PBM system uses the patented, commercially available GARS test with a nutraceutical Precision Neuronutrient-Research ID code: KB220 [143,144,145,146].
The GARS test identifies RDS risks and determines the neuropathways involved in hypodopaminergic risk to identify the neuronutrient formulation of KB220 via algorithm and create balanced genetic and epigenetic (neuro-molecular) brain function, the desired induction of dopamine homeostasis.
5. Cognitive Control of Reward Processing
This Special Issue is not only about risky behaviors (addiction) but also cognitive control of reward processing; here, we provide a few examples, emphasizing the role of dopamine in addiction and cognition. The neuronal release of dopamine in the limbic system relies on many neurotransmitters, peptides, and second messengers to impact the dopamine released at the NAc, which is critical to feeling good [127].
There are many examples of cognitive control of various aspects of drug and non-drug addictive behaviors. For example, the ability to resist the urge to eat is in part a function of the homeostatic functioning of neuronal circuits involved in top-down control to oppose the conditioned responses that predict reward from eating the food and the desire to eat the food. Moreover, imaging studies by Volkow’s group of this non-drug behavior have revealed that obese probands have impairments in dopaminergic pathways that regulate neuronal systems linked with reward sensitivity, conditioning, and control [147]. There is evidence that neuropeptides regulate energy balance (homeostatic processes) via the hypothalamus and subsequently modulate the activity of dopamine cells and their projections into the regions involved in the rewarding processes that modulate food intake.
Substance use disorders (SUDs), while effecting billions worldwide, are indeed preventable. Thousands of articles attest to the well-known effects of substance misuse that cause many physiological, molecular, and cellular changes in specific brain regions. Moreover, these neuroplastic changes have a role in seeking behaviors seen in substance and non-substance addictions [148,149].
Notably, many studies have focused on the dopamine neurons of the ventral tegmental area (VTA) and the regions where these neurons terminate: the striatum, the prefrontal cortex [148], and the amygdala. Specifically, decreases in dopamine receptors and transmission have been found in chronic users of psychostimulants [150], cannabis [151], opioids [152], alcohol [153], and nicotine [154].
Another example is the role of cognitive control and reward processing in Internet Gaming Disorder (IGD). There are indeed common neurochemical mechanisms that have been observed with both IGD and SUD [155]. Specifically, Functional Magnetic Resonance Imaging (fMRI) investigations of the resting state and measures of gray matter volume have shown that Internet game playing is associated with changes to brain regions responsible for attention and control, impulse control, motor function, emotional regulation, sensory-motor coordination [156], and stress processing [157]. Most interestingly, IGD was associated with reduced white matter density in brain regions involved in decision making, behavioral inhibition, and emotional regulation [158]. Playing videogames is also associated with changes in reward inhibitory mechanisms and loss of control [159]. In addition, Tain et al. [160] reported that D2 receptor activity is significantly associated with glucose metabolism in subjects with IGD, suggesting that D2/5-HT2A receptor-mediated dysregulation of the orbitofrontal cortex could underlie a mechanism for loss of control and compulsive behavior in IGD individuals.
There is also evidence from experiments that used lentivirus tools for over-expression or silencing of the dopamine transporter (DAT) gene in animals. Behavioral profiles that evaluated motivation and self-control compared to controls revealed significant differences. Specifically, DAT over-expressing rats showed increased impulsivity. The authors concluded that an attenuated dopaminergic tone following altered accumbal DAT function may subserve a sensation-seeker phenotype with vulnerability to lack of impulse control [161,162,163].
6. Summary
In summary [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,164,165,166]:
No matter what therapeutic strategy the clinical team chooses, a beneficial practice for treatment and recovery is genetic addiction risk testing and personalized induction of dopamine homeostasis based on genetic test results.
Indeed, the use of any treatment that reduces stress and enhances resting-state functional connectivity along the brain reward circuitry seems prudent.
From pre-authorization of very short-term use of opioids to reduce harm to cognitive behavioral therapy, trauma therapy, brain spotting, stress reduction, rsTMS to deep brain stimulation, and 12-stepping, the foundational induction, via epigenetics, of gentle up-regulation of dopaminergic function will be an evidenced basis for the induction of treatment (without complications or side effects) to keep people from any form of dopaminergic dysfunction.
Genetic addiction risk testing for patients attending a pain clinic provides information about the likelihood of a predisposition for Opioid Use Disorder (OUD) and enables the utilization of less addicting analgesia at the onset of treatment.
Genetic testing should be the standard of care for all patients attending substance and non-substance (process addictions) dependency programs (i.e., inpatient, outpatient, and intensive outpatient programs).
Genetic addiction risk testing for early risk identification in children, especially if they have addiction issues in the family (for example, children of alcoholics), combined with precision pro-dopamine regulation prophylaxis, may attenuate or prevent addiction risk.
Coupling of KB220 precision variants with naltrexone to improve compliance [43].
Combat cannabis-induced anhedonia in adolescents and adults with RDS using “Precision Behavioral Management” (PBM) to provide precision KB220 formulations [144,146].
7. Conclusions
Billions worldwide are affected by SUDs and RDS, which are preventable with PBM, genetic testing for addiction risk, and pro-dopamine precision KB220 formulations selected to treat neurotransmitter deficits. The neurotransmitter deficits are caused by the tested person’s dysfunctional alleles, identified by their genetic test results. This required induction of “dopamine homeostasis” is recommended for frontline tertiary addiction treatment and relapse prevention.
While more in-depth and extensive double-blinded studies combining the GARS test with KB220 precision formulations are encouraged, we believe that this body of evidence presented to support PBM, an important therapeutic and prophylactic modality for the treatment of impaired cognitive control of reward processing as manifested in patients expressing RDS, deserves careful consideration.
Author Contributions
Conceptualization, K.B.; writing—original draft preparation, M.A.M. and K.B.; writing—review and editing, A.G., A.B., C.A.D., D.B., R.D.B., I.E., E.R.B., M.S.G. and K.B.; supervision, K.B.; project administration, A.G. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
K.B. is the inventor of GARS and KB220 variants and is credited with domestic and foreign issued and pending patents. K.B. has entered into an exclusive licensing agreement with Ivitalize, Inc. M.A.M. is a paid member of The Kenneth Blum Behavioral & Neurogenetic Institute. The other authors declare no conflict of interest.
Funding Statement
R.D.B. is the recipient of NIH R01NS073884, and K.B. and Marjorie Gondre-Lewis. (Howard University) are recipients of R41 MD012318/MD/NIMHD NIH HHS/United States.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Blum K., Noble E.P., Sheridan P.J., Montgomery A., Ritchie T., Jagadeeswaran P., Nogami H., Briggs A.H., Cohn J.B. Allelic association of human dopamine D2 receptor gene in alcoholism. JAMA. 1990;263:2055–2060. doi: 10.1001/jama.1990.03440150063027. [DOI] [PubMed] [Google Scholar]
- 2.Blum K., Wood R.C., Braverman E.R., Chen T.J., Sheridan P.J. The D2 dopamine receptor gene as a predictor of compulsive disease: Bayes’ theorem. Funct. Neurol. 1995;10:37–44. [PubMed] [Google Scholar]
- 3.Blum K., Lott L., Siwicki D., Fried L., Hauser M., Simpatico T., Baron D., Howeedy A., Badgaiyan R.D. Genetic addiction risk score (GARS™) as a predictor of substance use disorder: Identifying predisposition not diagnosis. Curr. Trends Med. Diagn. Methods. 2018;1 doi: 10.29011/CTMDM-101.100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blum K., Briggs A.H., Trachtenberg M.C., Delallo L., Wallace J.E. Enkephalinase inhibition: Regulation of ethanol intake in genetically predisposed mice. Alcohol. 1987;4:449–456. doi: 10.1016/0741-8329(87)90084-X. [DOI] [PubMed] [Google Scholar]
- 5.Chen A.L., Chen T.J., Waite R.L., Reinking J., Tung H.L., Rhoades P., Downs B.W., Braverman E., Braverman D., Kerner M., et al. Hypothesizing that brain reward circuitry genes are genetic antecedents of pain sensitivity and critical diagnostic and pharmacogenomic treatment targets for chronic pain conditions. Med. Hypotheses. 2009;72:14–22. doi: 10.1016/j.mehy.2008.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blum K., Bowirrat A., Baron D., Lott L., Ponce J.V., Brewer R., Siwicki D., Boyett B., Gondre-Lewis M.C., Smith D.E., et al. Biotechnical development of genetic addiction risk score (GARS) and selective evidence for inclusion of polymorphic allelic risk in substance use disorder (SUD) J. Syst. Integr. Neurosci. 2020;6:1–20. doi: 10.15761/JSIN.1000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blum K., Chen A.L.C., Thanos P.K., Febo M., Demetrovics Z., Dushaj K., Kovoor A., Baron D., Smith D.E., Roy A.K., III, et al. Genetic addiction risk score (GARS) ™, a predictor of vulnerability to opioid dependence. Front. Biosci. 2018;10:175–196. doi: 10.2741/e816. [DOI] [PubMed] [Google Scholar]
- 8.Blum K., Gondré-Lewis M.C., Modestino E.J., Lott L., Baron D., Siwicki D., McLaughlin T., Howeedy A., Krengel M.H., Oscar-Berman M., et al. Understanding the scientific basis of post-traumatic stress disorder (PTSD): Precision behavioral management overrides stigmatization. Mol. Neurobiol. 2019;56:7836–7850. doi: 10.1007/s12035-019-1600-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blum K., Siwicki D., Baron D., Modestino E.J., Badgaiyan R.D. The benefits of genetic addiction risk score (GARS™) and pro-dopamine regulation in combating suicide in the American Indian population. J. Syst. Integr. Neurosci. 2018;4 doi: 10.15761/JSIN.1000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blum K., Simpatico T., Badgaiyan R.D., Demetrovics Z., Fratantonio J., Agan G., Febo M., Gold M.S. Coupling neurogenetics (GARS™) and a nutrigenomic based dopaminergic agonist to treat reward deficiency syndrome (RDS): Targeting polymorphic reward genes for carbohydrate addiction algorithms. J. Reward Defic. Syndr. 2015;1:75–80. doi: 10.17756/jrds.2015-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fried L., Modestino E.J., Siwicki D., Lott L., Thanos P.K., Baron D., Badgaiyan R.D., Ponce J.V., Giordano J., Downs W.B., et al. Hypodopaminergia and “precision behavioral management” (PBM): It is a generational family affair. Curr. Pharm. Biotechnol. 2020;21:528–541. doi: 10.2174/1389201021666191210112108. [DOI] [PubMed] [Google Scholar]
- 12.Blum K., Gold M., Modestino E.J., Baron D., Boyett B., Siwicki D., Lott L., Podesta A., Roy A.K., Hauser M., et al. Would induction of dopamine homeostasis via coupling genetic addiction risk score (GARS®) and pro-dopamine regulation benefit benzodiazepine use disorder (BUD)? J. Syst. Integr. Neurosci. 2018;4 doi: 10.15761/JSIN.1000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blum K., Baron D., McLaughlin T., Gold M.S. Molecular neurological correlates of endorphinergic/dopaminergic mechanisms in reward circuitry linked to endorphinergic deficiency syndrome (EDS) J. Neurol. Sci. 2020;411:116733. doi: 10.1016/j.jns.2020.116733. [DOI] [PubMed] [Google Scholar]
- 14.Blum K., Baron D., Lott L., Ponce J.V., Siwicki D., Boyett B., Steinberg B., Modestino E.J., Fried L., Hauser M., et al. In search of reward deficiency syndrome (RDS)-free controls: The “holy grail” in genetic addiction risk testing. Curr. Psychopharmacol. 2020;9:7–21. doi: 10.2174/2211556008666191111103152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blum K., Madigan M.A., Fried L., Braverman E.R., Giordano J., Badgaiyan R.D. Coupling genetic addiction risk score (GARS) and pro dopamine regulation (KB220) to combat substance use disorder (SUD) Glob. J. Addict. Rehabil. Med. 2017;1:555556. doi: 10.19080/GJARM.2017.01.555556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blum K., Thanos P.K., Badgaiyan R.D., Febo M., Oscar-Berman M., Fratantonio J., Demotrovics Z., Gold M.S. Neurogenetics and gene therapy for reward deficiency syndrome: Are we going to the Promised Land? Expert Opin. Biol. Ther. 2015;15:973–985. doi: 10.1517/14712598.2015.1045871. [DOI] [PubMed] [Google Scholar]
- 17.Blum K., Chen T.J., Meshkin B., Waite R.L., Downs B.W., Blum S.H., Mengucci J.F., Arcuri V., Braverman E.R., Palomo T. Manipulation of catechol-O-methyl-transferase (COMT) activity to influence the attenuation of substance seeking behavior, a subtype of Reward Deficiency Syndrome (RDS), is dependent upon gene polymorphisms: A hypothesis. Med. Hypotheses. 2007;69:1054–1060. doi: 10.1016/j.mehy.2006.12.062. [DOI] [PubMed] [Google Scholar]
- 18.Blum K., Oscar-Berman M., Barh D., Giordano J., Gold M. Dopamine genetics and function in food and substance abuse. J. Genet. Syndr. Gene Ther. 2013;4:1000121. doi: 10.4172/2157-7412.1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blum K., Oscar-Berman M., Demetrovics Z., Barh D., Gold M.S. Genetic addiction risk score (GARS): Molecular neurogenetic evidence for predisposition to reward deficiency syndrome (RDS) Mol. Neurobiol. 2014;50:765–796. doi: 10.1007/s12035-014-8726-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blum K., Baron D., Hauser M., Henriksen S., Thanos P.K., Black C., Siwicki D., Modestino E.J., Downs B.W., Badgaiyan S., et al. Americas’ opioid/psychostimulant epidemic would benefit from general population early identification of genetic addiction risk especially in children of alcoholics (COAs) J. Syst. Integr. Neurosci. 2019;5:1–3. [PMC free article] [PubMed] [Google Scholar]
- 21.Blum K., Downs B.W., Dushaj K., Li M., Braverman E.R., Fried L., Waite R., Demotrovics Z., Badgaiyan R.D. The benefits of customized DNA directed nutrition to balance the brain reward circuitry and reduce addictive behaviors. Precis. Med. 2016;1:18–33. [PMC free article] [PubMed] [Google Scholar]
- 22.Blum K., Modestino E.J., Neary J., Gondré-Lewis M.C., Siwicki D., Moran M., Hauser M., Braverman E.R., Baron D., Steinberg B., et al. Promoting precision addiction management (PAM) to combat the global opioid crisis. Biomed. J. Sci. Tech. Res. 2018;2:1–4. doi: 10.26717/BJSTR.2018.02.000738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen A.L., Blum K., Chen T.J., Giordano J., Downs B.W., Han D., Barh D., Braverman E.R. Correlation of the Taq1 dopamine D2 receptor gene and percent body fat in obese and screened control subjects: A preliminary report. Food Funct. 2012;3:40–48. doi: 10.1039/C1FO10089K. [DOI] [PubMed] [Google Scholar]
- 24.Blum K., Modestino E.J., Gondré-Lewis M.C., Neary J., Siwicki D., Hauser M., Barh D., Steinberg B., Badgaiyan R.D. Global opioid epidemic: Doomed to fail without genetically based precision addiction medicine (PAMTM): Lessons learned from America. Precis. Med. 2017;2:17–22. [PMC free article] [PubMed] [Google Scholar]
- 25.Thanos P.K., Hamilton J., O’Rourke J.R., Napoli A., Febo M., Volkow N.D., Blum K., Gold M. Dopamine D2 gene expression interacts with environmental enrichment to impact lifespan and behavior. Oncotarget. 2016;7:19111–19123. doi: 10.18632/oncotarget.8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blum K., Badgaiyan R.D., Agan G., Fratantonio J., Simpatico T., Febo M., Haberstick B.C., Smolen A., Gold M.S. Molecular genetic testing in reward deficiency syndrome (RDS): Facts and fiction. J. Reward Defic. Syndr. 2015;1:65–68. doi: 10.17756/jrds.2015-009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blum K., Chen A.L., Oscar-Berman M., Chen T.J., Lubar J., White N., Lubar J., Bowirrat A., Braverman E., Schoolfield J., et al. Generational association studies of dopaminergic genes in reward deficiency syndrome (RDS) subjects: Selecting appropriate phenotypes for reward dependence behaviors. Int. J. Environ. Res. Public Health. 2011;8:4425–4459. doi: 10.3390/ijerph8124425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blum K., Badgaiyan R.D., Dunston G.M., Baron D., Modestino E.J., McLaughlin T., Steinberg B., Gold M.S., Gondré-Lewis M.C. The DRD2 Taq1A A1 Allele may magnify the risk of Alzheimer’s in aging African-Americans. Mol. Neurobiol. 2018;55:5526–5536. doi: 10.1007/s12035-017-0758-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noble E.P., Blum K., Khalsa M.E., Ritchie T., Montgomery A., Wood R.C., Fitch R.J., Ozkaragoz T., Sheridan P.J., Anglin M.D., et al. Allelic association of the D2 dopamine receptor gene with cocaine dependence. Drug Alcohol Depend. 1993;33:271–285. doi: 10.1016/0376-8716(93)90113-5. [DOI] [PubMed] [Google Scholar]
- 30.Blum K., Febo M., Smith D.E., Roy AK 3rd Demetrovics Z., Cronjé F.J., Femino J., Agan G., Fratantonio J.L., Pandey S.C., Badgaiyan R.D., et al. Neurogenetic and epigenetic correlates of adolescent predisposition to and risk for addictive behaviors as a function of prefrontal cortex dysregulation. J. Child Adolesc. Psychopharmacol. 2015;25:286–292. doi: 10.1089/cap.2014.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen T.J., Blum K., Mathews D., Fisher L., Schnautz N., Braverman E.R., Schoolfield J., Downs B.W., Comings D.E. Are dopaminergic genes involved in a predisposition to pathological aggression? Hypothesizing the importance of “super normal controls” in psychiatricgenetic research of complex behavioral disorders. Med. Hypotheses. 2005;65:703–707. doi: 10.1016/j.mehy.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 32.Blum K., Noble E.P., Sheridan P.J., Montgomery A., Ritchie T., Ozkaragoz T., Fitch R.J., Wood R., Finley O., Sadlack F. Genetic predisposition in alcoholism: Association of the D2 dopamine receptor TaqI B1 RFLP with severe alcoholics. Alcohol. 1993;10:59–67. doi: 10.1016/0741-8329(93)90054-R. [DOI] [PubMed] [Google Scholar]
- 33.Moran M., Blum K., Ponce J.V., Lott L., Gondré-Lewis M.C., Badgaiyan S., Brewer R., Downs B.W., Fynman P., Weingarten A., et al. High genetic addiction risk score (GARS) in chronically prescribed severe chronic opioid probands attending multi-pain clinics: An open clinical pilot trial. Mol. Neurobiol. 2021;58:3335–3346. doi: 10.1007/s12035-021-02312-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bowirrat A., Chen T.J., Blum K., Madigan M., Bailey J.A., Chuan Chen A.L., Downs B.W., Braverman E.R., Radi S., Waite R.L., et al. Neuro-psychopharmacogenetics and neurological antecedents of posttraumatic stress disorder: Unlocking the mysteries of resilience and vulnerability. Curr. Neuropharmacol. 2010;8:335–358. doi: 10.2174/157015910793358123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blum K., Braverman E.R., Wood R.C., Gill J., Li C., Chen T.J., Taub M., Montgomery A.R., Sheridan P.J., Cull J.G. Increased prevalence of the Taq I A1 allele of the dopamine receptor gene (DRD2) in obesity with comorbid substance use disorder: A preliminary report. Pharmacogenetics. 1996;6:297–305. doi: 10.1097/00008571-199608000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Bowirrat A., Chen T.J., Oscar-Berman M., Madigan M., Chen A.L., Bailey J.A., Braverman E.R., Kerner M., Giordano J., Morse S., et al. Neuropsychopharmacology and neurogenetic aspects of executive functioning: Should reward gene polymorphisms constitute a diagnostic tool to identify individuals at risk for impaired judgment? Mol. Neurobiol. 2012;45:298–313. doi: 10.1007/s12035-012-8247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blum K., Gondré-Lewis M.C., Baron D., Thanos P.K., Braverman E.R., Neary J., Elman I., Badgaiyan R.D. Introducing precision addiction management of reward deficiency syndrome, the construct that underpins all addictive behaviors. Front. Psychiatry. 2018;9:548. doi: 10.3389/fpsyt.2018.00548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bisagno V., Cadet J.L. Expression of immediate early genes in brain reward circuitries: Differential regulation by psychostimulant and opioid drugs. Neurochem. Int. 2019;124:10–18. doi: 10.1016/j.neuint.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 39.Blum K., Oscar-Berman M., Dinubile N., Giordano J., Braverman E.R., Truesdell C.E., Barh D., Badgaiyan R. Coupling genetic addiction risk score (GARS) with electrotherapy: Fighting iatrogenic opioid dependence. J. Addict. Res. Ther. 2013;4:1000163. doi: 10.4172/2155-6105.1000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blum K., Oscar-Berman M., Blum S.H., Madigan M.A., Waite R.L., McLaughlin T., Barh D. Can genetic testing coupled with enhanced dopaminergic activation reduce recidivism rates in the workers compensation legacy cases? J. Alcohol Drug Depend. 2014;2:161. doi: 10.4172/2329-6488.1000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Downs B.W., Blum K., Baron D., Bowirrat A., Lott L., Brewer R., Boyett B., Siwicki D., Roy A.K., Podesta A., et al. Death by opioids: Are there non-addictive scientific solutions? J. Syst. Integr. Neurosci. 2019;5 doi: 10.15761/JSIN.1000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blum K., Febo M., Fried L., Baron D., Braverman E.R., Dushaj K., Li M., Demetrovics Z., Badgaiyan R.D. Pro-dopamine regulator—(KB220) to balance brain reward circuitry in reward deficiency syndrome (RDS) J. Reward Defic. Syndr. Addict. Sci. 2017;3:3–13. doi: 10.17756/jrdsas.2017-034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blum K., Modestino E.J., Badgaiyan R.D., Baron D., Thanos P.K., Elman I., Siwicki D., Febo M., Gold M.S. Analysis of evidence for the combination of pro-dopamine regulator (KB220PAM) and naltrexone to prevent opioid use disorder relapse. EC Psychol. Psychiatry. 2018;7:564–579. [PMC free article] [PubMed] [Google Scholar]
- 44.Blum K., Modestino E.J., Gondré-Lewis M., Downs B.W., Baron D., Steinberg B., Siwicki D., Giordano J., McLaughlin T., Neary J., et al. “Dopamine homeostasis” requires balanced polypharmacy: Issue with destructive, powerful dopamine agents to combat America’s drug epidemic. J. Syst. Integr. Neurosci. 2017;3 doi: 10.15761/JSIN.1000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blum K., Oscar-Berman M., Femino J., Waite R.L., Benya L., Giordano J., Borsten J., Downs W.B., Braverman E.R., Loehmann R., et al. Withdrawal from buprenorphine/naloxone and maintenance with a natural dopaminergic agonist: A cautionary note. J. Addict. Res. Ther. 2013;4 doi: 10.4172/2155-6105.1000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blum K., Febo M., Fried L., Li M., Dushaj K., Braverman E.R., McLaughlin T., Steinberg B., Badgaiyan R.D. Hypothesizing that neuropharmacological and neuroimaging studies of glutaminergic-dopaminergic optimization complex (KB220Z) are associated with “dopamine homeostasis” in reward deficiency syndrome (RDS) Subst. Use Misuse. 2017;52:535–547. doi: 10.1080/10826084.2016.1244551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blum K., Modestino E.J., Gondre-Lewis M., Chapman E.J., Neary J., Siwicki D., Baron D., Hauser M., Smith D.E., Roy A.K., et al. The Benefits of Genetic Addiction Risk Score (GARS™) Testing in Substance Use Disorder (SUD) Int. J. Genom. Data Min. 2018;2018:115. doi: 10.29011/2577-0616.000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Downs B.W., Blum K., Bagchi D., Kushner S., Bagchi M., Galvin J.M., Lewis M., Siwicki D., Brewer R., Boyett B., et al. Molecular neuro-biological and systemic health benefits of achieving dopamine homeostasis in the face of a catastrophic pandemic (COVID-19): A mechanistic exploration. J. Syst. Integr. Neurosci. 2020;7 doi: 10.15761/JSIN.1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schoenthaler S.J., Blum K., Fried L., Oscar-Berman M., Giordano J., Modestino E.J., Badgaiyan R. The effects of residential dual diagnosis treatment on alcohol abuse. J. Syst. Integr. Neurosci. 2017;3 doi: 10.15761/JSIN.1000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roy A.K., Bowirrat A., Smith D.E., Braverman E.R., Jalali R., Badgaiyan R.D., Baron D., Llanos-Gomez L., Barh D., Blum K. Neurobiology and Spirituality in Addiction Recovery. Acta Sci. Neurol. 2021;4:64–71. [PMC free article] [PubMed] [Google Scholar]
- 51.Blum K., Febo M., Badgaiyan R.D., Braverman E.R., Dushaj K., Li M., Demetrovics Z. Neuronutrient Amino-Acid Therapy Protects Against Reward Deficiency Syndrome: Dopaminergic Key to Homeostasis and Neuroplasticity. Curr. Pharm. Des. 2016;22:5837–5854. doi: 10.2174/1381612822666160719111346. [DOI] [PubMed] [Google Scholar]
- 52.Blum K., Noble E.P., Sheridan P.J., Finley O., Montgomery A., Ritchie T., Ozkaragoz T., Fitch R.J., Sadlack F., Sheffield D., et al. Association of the A1 allele of the D2 dopamine receptor gene with severe alcoholism. Alcohol. 1991;8:409–416. doi: 10.1016/0741-8329(91)90693-Q. [DOI] [PubMed] [Google Scholar]
- 53.Blum K., Febo M., Fahlke C., Archer T., Berggren U., Demetrovics Z., Dushaj K., Badgaiyan R.D. Hypothesizing Balancing Endorphinergic and Glutaminergic Systems to Treat and Prevent Relapse to Reward Deficiency Behaviors: Coupling D-Phenylalanine and N-Acetyl-L-Cysteine (NAC) as a Novel Therapeutic Modality. Clin. Med. Rev. Case Rep. 2015;2:76. doi: 10.23937/2378-3656/1410076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vitali M., Napolitano C., Berman M.O., Minuto S.F., Battagliese G., Attilia M.L., Braverman E.R., Romeo M., Blum K., Ceccanti M. Neurophysiological measures and alcohol use disorder (AUD): Hypothesizing links between clinical severity index and molecular neurobiological patterns. J. Addict. Res. Ther. 2016;5:182. doi: 10.4172/2155-6105.1000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blum K., Thanos P.K., Gold M.S. Dopamine and glucose, obesity, and reward deficiency syndrome. Front. Psychol. 2014;5:919. doi: 10.3389/fpsyg.2014.00919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duquette L.L., Mattiace F., Blum K., Waite R.L., Boland T., McLaughlin T., Dushaj K., Febo M., Badgaiyan R.D. Neurobiology of KB220Z-glutaminergic-dopaminergic optimization complex [GDOC] as a liquid nano: Clinical activation of brain in a highly functional clinician improving focus, motivation and overall sensory input following chronic intake. Clin. Med. Rev. Case Rep. 2016;3:104. doi: 10.23937/2378-3656/1410104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blum K., Oscar-Berman M., Giordano J., Downs B., Simpatico T., Han D., Femino J. Neurogenetic impairments of brain reward circuitry links to reward deficiency syndrome (RDS): Potential nutrigenomic induced dopaminergic activation. J. Genet. Syndr. Gene Ther. 2012;3:535–547. doi: 10.4172/2157-7412.1000e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blum K., Jacobs W., Modestino E.J., DiNubile N., Baron D., McLaughlin T., Siwicki D., Elman I., Moran M., Braverman E.R., et al. Insurance companies fighting the peer review empire without any validity: The case for addiction and pain modalities in the face of an American drug epidemic. SEJ Surg. Pain. 2018;1:1–11. [PMC free article] [PubMed] [Google Scholar]
- 59.McLaughlin T., Han D., Nicholson J., Steinberg B., Blum K., Febo M., Braverman E., Li M., Fried L., Badgaiyan R. Improvement of long-term memory access with a pro-dopamine regulator in an elderly male: Are we targeting dopamine tone? J. Syst. Integr. Neurosci. 2017;3 doi: 10.15761/JSIN.1000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blum K., Cadet J.L., Baron D., Badgaiyan R.D., Brewer R., Modestino E.J., Gold M.S. Putative COVID-19 induction of reward deficiency syndrome (RDS) and associated behavioral addictions with potential concomitant dopamine depletion: Is COVID-19 social distancing a double edged sword? Subst. Use Misuse. 2020;55:2438–2442. doi: 10.1080/10826084.2020.1817086. [DOI] [PubMed] [Google Scholar]
- 61.Archer T., Oscar-Berman M., Blum K., Gold M. Epigenetic Modulation of Mood Disorders. J. Genet. Syndr. Gene Ther. 2013;4:1000120. doi: 10.4172/2157-7412.1000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen T.J., Blum K., Waite R.L., Meshkin B., Schoolfield J., Downs B.W., Braverman E.E., Arcuri V., Varshavskiy M., Blum S.H., et al. Gene\Narcotic Attenuation Program attenuates substance use disorder, a clinical subtype of reward deficiency syndrome. Adv. Ther. 2007;24:402–414. doi: 10.1007/BF02849910. [DOI] [PubMed] [Google Scholar]
- 63.Blum K., Chen A.L., Giordano J., Borsten J., Chen T.J., Hauser M., Simpatico T., Femino J., Braverman E.R., Barh D. The addictive brain: All roads lead to dopamine. J. Psychoact. Drugs. 2012;44:134–143. doi: 10.1080/02791072.2012.685407. [DOI] [PubMed] [Google Scholar]
- 64.Blum K., Chen A.L., Chen T.J., Braverman E.R., Reinking J., Blum S.H., Cassel K., Downs B.W., Waite R.L., Williams L., et al. Activation instead of blocking mesolimbic dopaminergic reward circuitry is a preferred modality in the long term treatment of reward deficiency syndrome (RDS): A commentary. Theor. Biol. Med. Model. 2008;5:24. doi: 10.1186/1742-4682-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miller M., Chen A.L., Stokes S.D., Silverman S., Bowirrat A., Manka M., Manka D., Miller D.K., Perrine K., Chen T.J., et al. Early intervention of intravenous KB220IV—Neuroadaptagen amino-acid therapy (NAAT) improves behavioral outcomes in a residential addiction treatment program: A pilot study. J. Psychoact. Drugs. 2012;44:398–409. doi: 10.1080/02791072.2012.737727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blum K., Chen A.L., Chen T.J., Rhoades P., Prihoda T.J., Downs B.W., Waite R.L., Williams L., Braverman E.R., Braverman D., et al. LG839: Anti-obesity effects and polymorphic gene correlates of reward deficiency syndrome. Adv. Ther. 2008;25:894–913. doi: 10.1007/s12325-008-0093-z. [DOI] [PubMed] [Google Scholar]
- 67.Blum K., Chen T.J., Meshkin B., Downs B.W., Gordon C.A., Blum S., Mengucci J.F., Braverman E.R., Arcuri V., Varshavskiy M., et al. Reward deficiency syndrome in obesity: A preliminary cross-sectional trial with a Genotrim variant. Adv. Ther. 2006;23:1040–1051. doi: 10.1007/BF02850224. [DOI] [PubMed] [Google Scholar]
- 68.Blum K., Chen T.J., Meshkin B., Downs B.W., Gordon C.A., Blum S., Mangucci J.F., Braverman E.R., Arcuri V., Deutsch R., et al. Genotrim, a DNA-customized nutrigenomic product, targets genetic factors of obesity: Hypothesizing a dopamine-glucose correlation demonstrating reward deficiency syndrome (RDS) Med. Hypotheses. 2007;68:844–852. doi: 10.1016/j.mehy.2006.08.041. [DOI] [PubMed] [Google Scholar]
- 69.Downs B.W., Chen A.L., Chen T.J., Waite R.L., Braverman E.R., Kerner M., Braverman D., Rhoades P., Prihoda T.J., Palomo T., et al. Nutrigenomic targeting of carbohydrate craving behavior: Can we manage obesity and aberrant craving behaviors with neurochemical pathway manipulation by Immunological Compatible Substances (nutrients) using a Genetic Positioning System (GPS) Map? Med. Hypotheses. 2009;73:427–434. doi: 10.1016/j.mehy.2009.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen T.J., Blum K., Chen A.L., Bowirrat A., Downs W.B., Madigan M.A., Waite R.L., Bailey J.A., Kerner M., Yeldandi S., et al. Neurogenetics and clinical evidence for the putative activation of the brain reward circuitry by a neuroadaptagen: Proposing an addiction candidate gene panel map. J. Psychoact. Drugs. 2011;43:108–127. doi: 10.1080/02791072.2011.587393. [DOI] [PubMed] [Google Scholar]
- 71.Chen T.J., Blum K., Payte J.T., Schoolfield J., Hopper D., Stanford M., Braverman E.R. Narcotic antagonists in drug dependence: Pilot study showing enhancement of compliance with SYN-10, amino-acid precursors and enkephalinase inhibition therapy. Med. Hypotheses. 2004;63:538–548. doi: 10.1016/j.mehy.2004.02.051. [DOI] [PubMed] [Google Scholar]
- 72.Febo M., Blum K., Badgaiyan R.D., Baron D., Thanos P.K., Colon-Perez L.M., Demortrovics Z., Gold M.S. Dopamine homeostasis: Brain functional connectivity in reward deficiency syndrome. Front. Biosci. 2017;22:669–691. doi: 10.2741/4509. [DOI] [PubMed] [Google Scholar]
- 73.Blum K., Febo M., Badgaiyan R.D., Demetrovics Z., Simpatico T., Fahlke C., M O.-B., Li M., Dushaj K., Gold M.S. Common neurogenetic diagnosis and meso-limbic manipulation of hypodopaminergic function in reward deficiency syndrome (RDS): Changing the recovery landscape. Curr. Neuropharmacol. 2017;15:184–194. doi: 10.2174/1570159X13666160512150918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Blum K., Febo M., Thanos P.K., Baron D., Fratantonio J., Gold M. Clinically combating reward deficiency syndrome (RDS) with dopamine agonist therapy as a paradigm shift: Dopamine for dinner? Mol. Neurobiol. 2015;52:1862–1869. doi: 10.1007/s12035-015-9110-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Blum K., Febo M., McLaughlin T., Cronjé F.J., Han D., Gold S.M. Hatching the behavioral addiction egg: Reward deficiency solution system (RDSS)™ as a function of dopaminergic neurogenetics and brain functional connectivity linking all addictions under a common rubric. J. Behav. Addict. 2014;3:149–156. doi: 10.1556/JBA.3.2014.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Blum K., Thanos P.K., Oscar-Berman M., Febo M., Baron D., Badgaiyan R.D., Gardner E., Demetrovics Z., Fahlke C., Haberstick B.C., et al. Dopamine in the brain: Hypothesizing surfeit or deficit links to reward and addiction. J. Reward Defic. Syndr. 2015;1:95–104. doi: 10.17756/jrds.2015-016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Febo M., Blum K., Badgaiyan R.D., Perez P.D., Colon-Perez L.M., Thanos P.K., Ferris C.F., Kulkarni P., Giordano J., Baron D., et al. Enhanced functional connectivity and volume between cognitive and reward centers of naïve rodent brain produced by pro-dopaminergic agent KB220Z. PLoS ONE. 2017;12:e0174774. doi: 10.1371/journal.pone.0174774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Blum K., Febo M., Badgaiyan R.D. Fifty years in the development of a glutaminergic-dopaminergic optimization complex (KB220) to balance brain reward circuitry in reward deficiency syndrome: A pictorial. Austin Addict. Sci. 2016;1:1006. [PMC free article] [PubMed] [Google Scholar]
- 79.Blum K., Liu Y., Wang W., Wang Y., Zhang Y., Oscar-Berman M., Smolen A., Febo M., Han D., Simpatico T., et al. rsfMRI effects of KB220Z™ on neural pathways in reward circuitry of abstinent genotyped heroin addicts. Postgrad. Med. 2015;127:232–241. doi: 10.1080/00325481.2015.994879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cohen J.D., Blum K.I. Reward and decision. Neuron. 2002;36:193–198. doi: 10.1016/S0896-6273(02)00973-X. [DOI] [PubMed] [Google Scholar]
- 81.Blum K., Badgaiyan R.D., Braverman E.R., Dushaj K., Li M., Thanos P.K., Demetrovics Z., Febo M. Hypothesizing that, a pro-dopamine regulator (KB220Z) should optimize, but not hyper-activate the activity of trace amine-associated receptor 1 (TAAR-1) and induce anti-craving of psychostimulants in the long-term. J. Reward Defic. Syndr. Addict. Sci. 2016;2:14–21. doi: 10.17756/jrdsas.2016-023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Blum K., Whitney D., Fried L., Febo M., Waite R.L., Braverman E.R., Dushaj K., Li M., Giordano J., Demetrovics Z., et al. Hypothesizing that a pro-dopaminergic regulator (KB220z™ liquid variant) can induce “dopamine homeostasis” and provide adjunctive detoxification benefits in opiate/opioid dependence. Clin. Med. Rev. Case Rep. 2016;3:125. doi: 10.23937/2378-3656/1410125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Beitscher-Campbell H., Blum K., Febo M., Madigan M.A., Giordano J., Badgaiyan R.D., Braverman E.R., Dushaj K., Li M., Gold M.S. Pilot clinical observations between food and drug seeking derived from fifty cases attending an eating disorder clinic. J. Behav. Addict. 2016;5:533–541. doi: 10.1556/2006.5.2016.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McLaughlin T., Febo M., Badgaiyan R.D., Barh D., Dushaj K., Braverman E.R., Li M., Madigan M.A., Blum K. KB220Z™ a pro-dopamine regulator associated with the protracted, alleviation of terrifying lucid dreams. Can we infer neuroplasticity-induced changes in the reward circuit? J. Reward. Defic. Syndr. Addict. Sci. 2016;2:3–13. doi: 10.17756/jrdsas.2016-022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McLaughlin T., Blum K., Oscar-Berman M., Febo M., Demetrovics Z., Agan G., Fratantonio J., Gold M.S. Using the neuroadaptagen KB200z™ to ameliorate terrifying, lucid nightmares in RDS patients: The role of enhanced, brain-reward, functional connectivity and dopaminergic homeostasis. J. Reward Defic. Syndr. 2015;1:24–35. doi: 10.17756/jrds.2015-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McLaughlin T., Blum K., Oscar-Berman M., Febo M., Agan G., Fratantonio J.L., Simpatico T., Gold M.S. Putative dopamine agonist (KB220Z) attenuates lucid nightmares in PTSD patients: Role of enhanced brain reward functional connectivity and homeostasis redeeming joy. J. Behav. Addict. 2015;4:106–115. doi: 10.1556/2006.4.2015.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Steinberg B., Blum K., McLaughlin T., Lubar J., Febo M., Braverman E.R., Badgaiyan R.D. Low-resolution electromagnetic tomography (LORETA) of changed brain function provoked by pro-dopamine regulator (KB220z) in one adult ADHD case. Open J. Clin. Med. Case Rep. 2016;2:1121. [PMC free article] [PubMed] [Google Scholar]
- 88.McLaughlin T., Blum K., Steinberg B., Modestino E.J., Fried L., Baron D., Siwicki D., Braverman E.R., Badgaiyan R.D. Pro-dopamine regulator, KB220Z, attenuates hoarding and shopping behavior in a female, diagnosed with SUD and ADHD. J. Behav. Addict. 2018;7:192–203. doi: 10.1556/2006.6.2017.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Barh D., García-Solano M.E., Tiwari S., Bhattacharya A., Jain N., Torres-Moreno D., Ferri B., Silva A., Azevedo V., Ghosh P., et al. BARHL1 Is Downregulated in Alzheimer’s Disease and May Regulate Cognitive Functions through ESR1 and Multiple Pathways. Genes. 2017;8:245. doi: 10.3390/genes8100245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Blum K., Trachtenberg M.C., Elliott C.E., Dingler M.L., Sexton R.L., Samuels A.I., Cataldie L. Enkephalinase inhibition and precursor amino acid loading improves inpatient treatment of alcohol and polydrug abusers: Double-blind placebo-controlled study of the nutritional adjunct SAAVE. Alcohol. 1988;5:481–493. doi: 10.1016/0741-8329(88)90087-0. [DOI] [PubMed] [Google Scholar]
- 91.Blum K., McLaughlin T., Modestino E.J., Baron D., Bowirrat A., Brewer R., Steinberg B., Roy A.K., Febo M., Badgaiyan R.D., et al. Epigenetic Repair of Terrifying Lucid Dreams by Enhanced Brain Reward Functional Connectivity and Induction of Dopaminergic Homeostatic Signaling. Curr. Psychopharmacol. 2021;10 doi: 10.2174/2211556010666210215153513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brown R.J., Blum K., Trachtenberg M.C. Neurodynamics of relapse prevention: A neuronutrient approach to outpatient DUI offenders. J. Psychoact. Drugs. 1990;22:173–187. doi: 10.1080/02791072.1990.10472542. [DOI] [PubMed] [Google Scholar]
- 93.Blum K., Trachtenberg M.C., Ramsay J.C. Improvement of inpatient treatment of the alcoholic as a function of neurotransmitter restoration: A pilot study. Int. J. Addict. 1988;23:991–998. doi: 10.3109/10826088809058853. [DOI] [PubMed] [Google Scholar]
- 94.DeFrance J.F., Hymel C., Trachtenberg M.C., Ginsberg L.D., Schweitzer F.C., Estes S., Chen T.J., Braverman E.R., Cull J.G., Blum K. Enhancement of attention processing by Kantroll in healthy humans: A pilot study. Clin. Electroencephalogr. 1997;28:68–75. doi: 10.1177/155005949702800204. [DOI] [PubMed] [Google Scholar]
- 95.Blum K., Gold M.S. Neuro-chemical activation of brain reward meso-limbic circuitry is associated with relapse prevention and drug hunger: A hypothesis. Med. Hypotheses. 2011;76:576–584. doi: 10.1016/j.mehy.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 96.Blum K., Cadet J.L., Gold M.S. Psychostimulant use disorder emphasizing methamphetamine and the opioid-dopamine connection: Digging out of a hypodopaminergic ditch. J. Neurol. Sci. 2021;420:117252. doi: 10.1016/j.jns.2020.117252. [DOI] [PubMed] [Google Scholar]
- 97.Blum K., Futterman S., Wallace J.E., Schwertner H.A. Naloxone-induced inhibition of ethanol dependence in mice. Nature. 1977;265:49–51. doi: 10.1038/265049a0. [DOI] [PubMed] [Google Scholar]
- 98.Blum K., Braverman E.R., Holder J.M., Lubar J.F., Monastra V.J., Miller D., Lubar J.O., Chen T.J., Comings D.E. Reward deficiency syndrome: A biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. J. Psychoact. Drugs. 2000;32((Suppl. I–IV)):1–112. doi: 10.1080/02791072.2000.10736099. [DOI] [PubMed] [Google Scholar]
- 99.Trachtenberg M.C., Blum K. Improvement of cocaine-induced neuromodulator deficits by the neuronutrient Tropamine. J. Psychoact. Drugs. 1988;20:315–331. doi: 10.1080/02791072.1988.10472501. [DOI] [PubMed] [Google Scholar]
- 100.Blum K., Allison D., Trachtenberg M.C., Williams R.W., Loeblich L.A. Reduction of both drug hunger and withdrawal against advice rate of cocaine abusers in a 30-day inpatient treatment program by the neuronutrient tropamine’. Curr. Ther. Res. 1988;43:1204–1214. [Google Scholar]
- 101.Blum K., Chen T.J.H., Downs B.W., Meshkin B., Blum S.H., Martinez Pons M., Mengucci J.F., Waite R.L., Arcuri V., Varshafski M., et al. Synaptamine (SG8839) an amino-acid enkephalinase inhibition nutraceutical improves recovery of alcoholics, a subtype of reward deficiency syndrome (RDS) Trends Appl. Sci. Res. 2007;2:132–138. [Google Scholar]
- 102.Mechelmans D.J., Strelchuk D., Doñamayor N., Banca P., Robbins T.W., Baek K., Voon V. Reward sensitivity and waiting impulsivity: Shift towards reward valuation away from action control. Int. J. Neuropsychopharmacol. 2017;20:971–978. doi: 10.1093/ijnp/pyx072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kótyuk E., Urbán R., Hende B., Richman M., Magi A., Király O., Barta C., Griffiths M.D., Potenza M.N., Badgaiyan R.D., et al. Development and validation of the Reward Deficiency Syndrome Questionnaire (RDSQ-29) J. Psychopharmacol. 2022;36:409–422. doi: 10.1177/02698811211069102. [DOI] [PubMed] [Google Scholar]
- 104.Mies G.W., Ma I., de Water E., Buitelaar J.K., Scheres A. Waiting and working for rewards: Attention-Deficit/Hyperactivity Disorder is associated with steeper delay discounting linked to amygdala activation, but not with steeper effort discounting. Cortex. 2018;106:164–173. doi: 10.1016/j.cortex.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 105.Jimura K., Chushak M.S., Braver T.S. Impulsivity and self-control during intertemporal decision making linked to the neural dynamics of reward value representation. J. Neurosci. 2013;33:344–357. doi: 10.1523/JNEUROSCI.0919-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Voon V., Irvine M.A., Derbyshire K., Worbe Y., Lange I., Abbott S., Morein-Zamir S., Dudley R., Caprioli D., Harrison N.A., et al. Measuring “waiting” impulsivity in substance Gold MSddictions and binge eating disorder in a novel analogue of rodent serial reaction time task. Biol. Psychiatry. 2014;75:148–155. doi: 10.1016/j.biopsych.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kazemi T., Huang S., Avci N.G., Waits C.M.K., Akay Y.M., Akay M. Investigating the influence of perinatal nicotine and alcohol exposure on the genetic profiles of dopaminergic neurons in the VTA using miRNA-mRNA analysis. Sci. Rep. 2020;10:15016. doi: 10.1038/s41598-020-71875-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Beran M.J., Evans T.A. Delay of gratification by chimpanzees (Pan troglodytes) in working and waiting situations. Behav. Processes. 2009;80:177–181. doi: 10.1016/j.beproc.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Barlow R.L., Gorges M., Wearn A., Niessen H.G., Kassubek J., Dalley J.W., Pekcec A. Ventral striatal D2/3 receptor availability is associated with impulsive choice behavior as well as limbic corticostriatal connectivity. Int. J. Neuropsychopharmacol. 2018;21:705–715. doi: 10.1093/ijnp/pyy030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schwing R., Weber S., Bugnyar T. Kea (Nestor notabilis) decide early when to wait in food exchange task. J. Comp. Psychol. 2017;131:269–276. doi: 10.1037/com0000086. [DOI] [PubMed] [Google Scholar]
- 111.Adriani W., Laviola G. Delay aversion but preference for large and rare rewards in two choice tasks: Implications for the measurement of self-control parameters. BMC Neurosci. 2006;7:52. doi: 10.1186/1471-2202-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Robinson E.S., Eagle D.M., Economidou D., Theobald D.E., Mar A.C., Murphy E.R., Robbins T.W., Dalley J.W. Behavioural characterisation of high impulsivity on the 5-choice serial reaction time task: Specific deficits in ‘waiting’ versus ‘stopping’. Behav. Brain Res. 2009;196:310–316. doi: 10.1016/j.bbr.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 113.Jacobsen T., Huss M., Fendrich M., Kruesi M.J., Ziegenhain U. Children’s ability to delay gratification: Longitudinal relations to mother-child attachment. J. Genet. Psychol. 1997;158:411–426. doi: 10.1080/00221329709596679. [DOI] [PubMed] [Google Scholar]
- 114.Berridge K.C. The debate over dopamine’s role in reward: The case for incentive salience. Psychopharmacology. 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- 115.Gardner E.L. Addiction and brain reward and antireward pathways. Adv. Psychosom. Med. 2011;30:22–60. doi: 10.1159/000324065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Koob G.F., Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat. Neurosci. 2005;8:1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- 117.Zhang Y., von Deneen K.M., Tian J., Gold M.S., Liu Y. Food addiction and neuroimaging. Curr. Pharm. Des. 2011;17:1149–1157. doi: 10.2174/138161211795656855. [DOI] [PubMed] [Google Scholar]
- 118.Berridge K.C. Wanting and liking: Observations from the neuroscience and psychology laboratory. Inquiry. 2009;52:378. doi: 10.1080/00201740903087359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tindell A.J., Smith K.S., Berridge K.C., Aldridge J.W. Dynamic computation of incentive salience: “Wanting” what was never liked. J. Neurosci. 2009;29:12220–12228. doi: 10.1523/JNEUROSCI.2499-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Peciña S., Cagniard B., Berridge K.C., Aldridge J.W., Zhuang X. Hyperdopaminergic mutant mice have higher “wanting” but not “liking” for sweet rewards. J. Neurosci. 2003;23:9395–9402. doi: 10.1523/JNEUROSCI.23-28-09395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sharot T., Shiner T., Brown A.C., Fan J., Dolan R.J. Dopamine enhances expectation of pleasure in humans. Curr. Biol. 2009;19:2077–2080. doi: 10.1016/j.cub.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kornetsky C. Brain-stimulation reward, morphine-induced oral stereotypy, and sensitization: Implications for abuse. Neurosci. Biobehav. Rev. 2004;27:777–786. doi: 10.1016/j.neubiorev.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 123.Dackis C.A., Gold M.S. New concepts in cocaine addiction: The dopamine depletion hypothesis. Neurosci. Biobehav. Rev. 1985;9:469–477. doi: 10.1016/0149-7634(85)90022-3. [DOI] [PubMed] [Google Scholar]
- 124.Di Chiara G., Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc. Natl. Acad. Sci. USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Elman I., Borsook D. Common brain mechanisms of chronic pain and addiction. Neuron. 2016;89:11–36. doi: 10.1016/j.neuron.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 126.Gold M.S., Baron D., Bowirrat A., Blum K. Neurological correlates of brain reward circuitry linked to opioid use disorder (OUD): Do homo sapiens acquire or have a reward deficiency syndrome? J. Neurol. Sci. 2020;418:117137. doi: 10.1016/j.jns.2020.117137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Elman I., Borsook D., Lukas S.E. Food intake and reward mechanisms in patients with schizophrenia: Implications for metabolic disturbances and treatment with second-generation antipsychotic agents. Neuropsychopharmacology. 2006;31:2091–2120. doi: 10.1038/sj.npp.1301051. [DOI] [PubMed] [Google Scholar]
- 128.Blum K., Sheridan P.J., Wood R.C., Braverman E.R., Chen T.J., Cull J.G., Comings D.E. The D2 dopamine receptor gene as a determinant of reward deficiency syndrome. J. R. Soc. Med. 1996;89:396–400. doi: 10.1177/014107689608900711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Elman I., Borsook D. Threat response system: Parallel brain processes in pain vis-à-vis fear and anxiety. Front. Psychiatry. 2018;9:29. doi: 10.3389/fpsyt.2018.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.LeDoux J.E., Pine D.S. Using neuroscience to help understand fear and anxiety: A two-system framework. Am. J. Psychiatry. 2016;173:1083–1093. doi: 10.1176/appi.ajp.2016.16030353. [DOI] [PubMed] [Google Scholar]
- 131.Murck H., Schubert M.I., Schmid D., Schüssler P., Steiger A., Auer D.P. The glutamatergic system and its relation to the clinical effect of therapeutic-sleep deprivation in depression - an MR spectroscopy study. J. Psychiatr. Res. 2009;43:175–180. doi: 10.1016/j.jpsychires.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 132.Salimpoor V.N., Benovoy M., Larcher K., Dagher A., Zatorre R.J. Anatomically distinct dopamine release during anticipation and experience of peak emotion to music. Nat. Neurosci. 2011;14:257–262. doi: 10.1038/nn.2726. [DOI] [PubMed] [Google Scholar]
- 133.Volkow N.D., Michaelides M., Baler R. The neuroscience of drug reward and addiction. Physiol. Rev. 2019;99:2115–2140. doi: 10.1152/physrev.00014.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Volkow N.D., Fowler J.S., Wolf A.P., Schlyer D., Shiue C.Y., Alpert R., Dewey S.L., Logan J., Bendriem B., Christman D., et al. Effects of chronic cocaine abuse on postsynaptic dopamine receptors. Am. J. Psychiatry. 1990;147:719–724. doi: 10.1176/ajp.147.6.719. [DOI] [PubMed] [Google Scholar]
- 135.Blum K., Raza A., Schultz T., Jalali R., Green R., Brewer R., Thanos P.K., McLaughlin T., Baron D., Bowirrat A., et al. Should we embrace the incorporation of genetically guided “dopamine homeostasis” in the treatment of reward deficiency syndrome (RSD) as a frontline therapeutic modality? Acta Sci. Neurol. 2021;4:17–24. [PMC free article] [PubMed] [Google Scholar]
- 136.Juknat A., Gao F., Coppola G., Vogel Z., Kozela E. miRNA expression profiles and molecular networks in resting and LPS-activated BV-2 microglia-effect of cannabinoids. PLoS ONE. 2019;14:e0212039. doi: 10.1371/journal.pone.0212039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Russo S.J., Nestler E.J. The brain reward circuitry in mood disorders. Nat. Rev. Neurosci. 2013;14:609–625. doi: 10.1038/nrn3381. Erratum in Nat. Rev. Neurosci. 2013, 14, 736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cooper S., Robison A.J., Mazei-Robison M.S. Reward circuitry in addiction. Neurotherapeutics. 2017;14:687–697. doi: 10.1007/s13311-017-0525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Walker D.M., Nestler E.J. Neuroepigenetics and addiction. Handb. Clin. Neurol. 2018;148:747–765. doi: 10.1016/B978-0-444-64076-5.00048-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Archer T., Oscar-Berman M., Blum K., Gold M. Neurogenetics and epigenetics in impulsive behaviour: Impact on reward circuitry. J. Genet. Syndr. Gene Ther. 2012;3:1000115. doi: 10.4172/2157-7412.1000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hansson A.C., Rimondini R., Neznanova O., Sommer W.H., Heilig M. Neuroplasticity in brain reward circuitry following a history of ethanol dependence. Eur. J. Neurosci. 2008;27:1912–1922. doi: 10.1111/j.1460-9568.2008.06159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Blum K., Bowirrat A., Lewis M.C., Simpatico T.A., Ceccanti M., Steinberg B., Modestino E.J., Thanos P.K., Baron D., McLaughlin T., et al. Exploration of epigenetic state hyperdopaminergia (Surfeit) and genetic trait hypodopaminergia (Deficit) during adolescent brain development. Curr. Psychopharmacol. 2021;10:181–196. doi: 10.2174/2211556010666210215155509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Blum K., Modestino E.J., Gondre-Lewis M.G., Baron D., Steinberg B., Thanos P.K., Downs W.B., Siwicki D., Lott L., Braverman E.R., et al. Pro-dopamine regulator (KB220) a fifty year sojourn to combat reward deficiency syndrome (RDS): Evidence based bibliography (annotated) [(accessed on 22 February 2022)];CPQ Neurol. Psychol. 2018 1 Available online: https://www.cientperiodique.com/journal/fulltext/CPQNP/1/2/13. [PMC free article] [PubMed] [Google Scholar]
- 144.Blum K., Oscar-Berman M., Braverman E.R., Febo M., Li M., Gold M.S. Enhancing brain pregnenolone may protect cannabis intoxication but should not be considered as an anti-addiction therapeutic: Hypothesizing dopaminergic blockade and promoting anti-reward. J. Reward Defic. Syndr. 2015;1:20–23. doi: 10.17756/jrds.2015-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gold M.S., Blum K., Febo M., Baron D., Modestino E.J., Elman I., Badgaiyan R.D. Molecular role of dopamine in anhedonia linked to reward deficiency syndrome (RDS) and anti- reward systems. Front. Biosci. 2018;10:309–325. doi: 10.2741/s518. [DOI] [PubMed] [Google Scholar]
- 146.Noble E.P., Blum K., Ritchie T., Montgomery A., Sheridan P.J. Allelic association of the D2 dopamine receptor gene with receptor-binding characteristics in alcoholism. Arch. Gen. Psychiatry. 1991;48:648–654. doi: 10.1001/archpsyc.1991.01810310066012. [DOI] [PubMed] [Google Scholar]
- 147.Volkow N.D., Wang G.J., Baler R.D. Reward, dopamine and the control of food intake: Implications for obesity. Trends Cogn. Sci. 2011;15:37–46. doi: 10.1016/j.tics.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tanabe J., Regner M., Sakai J., Martinez D., Gowin J. Neuroimaging reward, craving, learning, and cognitive control in substance use disorders: Review and implications for treatment. Br. J. Radiol. 2019;92:20180942. doi: 10.1259/bjr.20180942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Weele C.M.V., Siciliano C.A., Tye K.M. Dopamine tunes prefrontal outputs to orchestrate aversive processing. Brain Res. 2019;1713:16–31. doi: 10.1016/j.brainres.2018.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Richer K., Hamilton J., Delis F., Martin C., Fricke D., Yao R., Sajjad M., Blum K., Hadjiargyrou M., Komatsu D., et al. Chronic treatment and abstinence from methylphenidate exposure dose-dependently changes glucose metabolism in the rat brain. Brain Res. 2022;1780:147799. doi: 10.1016/j.brainres.2022.147799. [DOI] [PubMed] [Google Scholar]
- 151.Blum K., Kazmi S., Modestino E.J., Downs B.W., Bagchi D., Baron D., McLaughlin T., Green R., Jalali R., Thanos P.K., et al. A novel precision approach to overcome the “addiction pandemic” by incorporating genetic addiction risk severity (GARS) and dopamine homeostasis restoration. J. Pers. Med. 2021;11:212. doi: 10.3390/jpm11030212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Korb S., Götzendorfer S.J., Massaccesi C., Sezen P., Graf I., Willeit M., Eisenegger C., Silani G. Dopaminergic and opioidergic regulation during anticipation and consumption of social and nonsocial rewards. eLife. 2020;9:e55797. doi: 10.7554/eLife.55797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Di Chiara G. Alcohol and dopamine. Alcohol Health Res. World. 1997;21:108–114. [PMC free article] [PubMed] [Google Scholar]
- 154.Stefano G.B., Bianchi E., Guarna M., Fricchione G.L., Zhu W., Cadet P., Mantione K.J., Casares F.M., Kream R.M., Esch T. Nicotine, alcohol and cocaine coupling to reward processes via endogenous morphine signaling: The dopamine-morphine hypothesis. Med. Sci. Monit. 2007;13:RA91–RA102. [PubMed] [Google Scholar]
- 155.Weinstein A., Livny A., Weizman A. New developments in brain research of internet and gaming disorder. Neurosci. Biobehav. Rev. 2017;75:314–330. doi: 10.1016/j.neubiorev.2017.01.040. [DOI] [PubMed] [Google Scholar]
- 156.Green C.L., Nahhas R.W., Scoglio A.A., Elman I. Post-traumatic stress symptoms in pathological gambling: Potential evidence of anti-reward processes. J. Behav. Addict. 2017;6:98–101. doi: 10.1556/2006.6.2017.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Li Q., Wang Y., Yang Z., Dai W., Zheng Y., Sun Y., Liu X. Dysfunctional cognitive control and reward processing in adolescents with internet gaming disorder. Psychophysiology. 2020;57:e13469. doi: 10.1111/psyp.13469. [DOI] [PubMed] [Google Scholar]
- 158.Chamberlain S.R., Derbyshire K., Daws R.E., Odlaug B.L., Leppink E.W., Grant J.E. White matter tract integrity in treatment-resistant gambling disorder. Br. J. Psychiatry. 2016;208:579–584. doi: 10.1192/bjp.bp.115.165506. [DOI] [PubMed] [Google Scholar]
- 159.Wang L., Tian M., Zheng Y., Li Q., Liu X. Reduced loss aversion and inhibitory control in adolescents with internet gaming disorder. Psychol. Addict. Behav. 2020;34:484–496. doi: 10.1037/adb0000549. [DOI] [PubMed] [Google Scholar]
- 160.Tian M., Chen Q., Zhang Y., Du F., Hou H., Chao F., Zhang H. PET imaging reveals brain functional changes in internet gaming disorder. Eur. J. Nucl. Med. Mol. Imaging. 2014;41:1388–1397. doi: 10.1007/s00259-014-2708-8. [DOI] [PubMed] [Google Scholar]
- 161.Adriani W., Boyer F., Gioiosa L., Macrì S., Dreyer J.L., Laviola G. Increased impulsive behavior and risk proneness following lentivirus-mediated dopamine transporter over-expression in rats’ nucleus accumbens. Neuroscience. 2009;159:47–58. doi: 10.1016/j.neuroscience.2008.11.042. [DOI] [PubMed] [Google Scholar]
- 162.Zuckerman M., Kuhlman D.M. Personality and risk-taking: Common biosocial factors. J. Pers. 2000;68:999–1029. doi: 10.1111/1467-6494.00124. [DOI] [PubMed] [Google Scholar]
- 163.Badgaiyan R.D., Sinha S., Sajjad M., Wack D.S. Attenuated tonic and enhanced phasic release of dopamine in attention deficit hyperactivity disorder. PLoS ONE. 2015;10:e0137326. doi: 10.1371/journal.pone.0137326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.El Hayek S., Allouch F., Razafsha M., Talih F., Gold M.S., Wang K.K., Kobeissy F. Traumatic brain injury and methamphetamine: A double-hit neurological insult. J. Neurol. Sci. 2020;411:116711. doi: 10.1016/j.jns.2020.116711. [DOI] [PubMed] [Google Scholar]
- 165.Gold M.S. The role of alcohol, drugs, and deaths of despair in the U.S.’s falling life expectancy. MO Med. 2020;117:99–101. [PMC free article] [PubMed] [Google Scholar]
- 166.Oesterle T.S., Kolla B.P., Rummans T.A., Gold M.S. Medication-assisted therapies for opioid use disorders in patients with chronic pain. J. Neurol. Sci. 2020;411:116728. doi: 10.1016/j.jns.2020.116728. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.