Abstract
Drug resistance is still an obstacle in cancer therapy, leading to the failure of tumor treatment. The emergence of tumor drug resistance has always been a main concern of oncologists. Therefore, overcoming tumor drug resistance and looking for new strategies for tumor treatment is a major focus in the field of tumor research. Natural products serve as effective substances against drug resistance because of their diverse chemical structures and pharmacological effects. We reviewed the signaling pathways involved in the development of tumor drug resistance, including Epidermal growth factor receptor (EGFR), Renin-angiotensin system (Ras), Phosphatidylinositol-3-kinase/protein kinase B (PI3K/Akt), Wnt, Notch, Transforming growth factor-beta (TGF-β), and their specific signaling pathway inhibitors derived from natural products. This can provide new ideas for the prevention of drug resistance in cancer therapy.
Keywords: tumor, drug resistance, signaling pathway, mechanism
1. Introduction
Tumors continue to be a threat to human health, despite the many therapies available to treat malignant tumors. At present, the treatment of tumors includes chemotherapy, radiotherapy, surgical treatment, and immunotherapy [1]. Although tumor treatments are constantly improved, chemotherapy remains the most common method of tumor treatment [2]. However, in the process of using combination or single targeted chemotherapies, there are many drug-related side effects [3]. As treatment continues, tumors often become resistant to chemotherapeutic compounds. Currently, tumor-targeted inhibitors and cytotoxic drugs use one or several specific biomolecules to activate corresponding signaling pathways and exert antitumor effects. Thus, tumor resistance generation is associated with the reactivation or inhibition of these signaling pathways. The mechanisms of tumor resistance are complex [4]. Resistance is primarily manifested in the following ways [5]. (1) Tumor cells can modify tumor suppressor targets or their downstream signaling pathway proteins through the endocrine pathway to generate tumor drug resistance [6]. (2) Tumor cells may have enhanced DNA repair capacity, conferring resistance to platinum compounds or poly (ADP-ribose) polymerase (PARP) inhibitors [7]. (3) Loss of tumor-specific antigens can lead to resistance to immunotherapy [8]. (4) ATP-binding box transporter family members, ATP-binding cassette superfamily B member 1 (ABCB1), ATP-binding cassette subfamily C member 1 (ABCC1), and ATP-binding cassette transporter of subfamily G members (ABCG2) promote drug efflux, leading to tumor drug resistance [9]. (5) Cancer stem cells (CSCs) have a low division rate, which can upregulate the expression of ATP binding box transporter family members, improve DNA repairability, and induce tumor drug resistance [10]. (6) When cell polarity and cell contact are lost in epithelial cells, specific epithelial markers (such as E-cadherin and cytokeratin) are reduced, and mesenchymal characteristics (such as increased cell motility, vimentin, N-cadherin, fibronectin, and matrix metalloproteinase) increase, activating ABC transporters and regulating anti-apoptotic pathways, thus participating in tumor resistance to cytotoxic drugs, tyrosine kinase inhibitors, and endocrine therapy [11].
Certain signaling pathways play important roles in the development of drug resistance. For example, the epidermal growth factor receptor (EGFR) signaling pathway can activate the Ras/Raf/mitogen-activated protein kinase (MEK)/extracellular signal-regulated kinase (ERK) or phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) cascades to regulate cell growth, differentiation, adhesion, and migration [11,12]. Abnormal regulation of EGFR signaling is closely related to drug resistance in cancer [13]. Studies have reported that mutant EGFR can activate the mitogen-activated protein kinase (MAPK) signaling pathway and intersect with the Akt pathway to induce non-small cell lung cancer (NSCLC) resistance to AZD929. When MAPK signaling is activated, lung cancer patients with fibroblast growth factor receptor (FGFR)1 mutations can become resistant to FGFR inhibitors [14]. MEK is a component of the Ras signaling pathway, and when MEK is activated, the EGFR-Ras-Raf signaling pathway is activated, which can eventually lead to tumor resistance to MEK inhibitors [15]. MEK activates the ErbB3 signaling pathway, leading to gefitinib resistance in lung cancer [16]. In addition to EGFR activation of Ras signaling, EGFR activation of PI3K signaling also plays an important role in inducing drug resistance in tumors [17]. PI3K signaling was found to be activated in acute leukemia cells resistant to chemotherapeutic drugs. When PI3K and MAPK pathways are jointly activated, melanoma can induce the resistance of Raf mutations to MAPK inhibitors [18]. Other signaling pathways, such as the Wnt signaling pathway, can also induce the generation of tumor drug resistance [19]. It was found that abnormal Wnt signaling can upregulate ABCB1 and ABCG2, inducing NSCLC resistance to cisplatin [20]. Furthermore, notch signaling, by upregulating ABCB1, can induce doxorubicin resistance in prostate cancer cells [21]. The inhibition of Notch-1 signaling restores the sensitivity of breast cancer cells and prostate cancer cells to doxorubicin and docetaxel [22].
Chemotherapy is a conventional treatment for cancer. However, cancer cells are resistant to almost all types of chemotherapeutic and targeted drugs, and approximately 80–90% of deaths in cancer patients are directly or indirectly attributed to resistance, making it a considerable challenge in cancer treatment [23]. Fortunately, natural products with different chemical structures and pharmacological effects are effective substances against drug resistance [24]. Natural products reverse drug resistance by regulating proteins involved in resistance, targeting non-apoptotic cell death, and inducing other types of non-apoptotic cell death [25]. Many natural products have very strong drug resistance reversal properties, among which are alkaloids, flavonoids, phenylpropanoids, and terpenoids (Table 1). For example, matrine can improve the sensitivity of resistant cancer cells to chemotherapeutic drugs by reactivating apoptosis and inhibiting drug efflux [26]; Tetrandrine can fight the multiple-drug resistance (MDR) of cancer cells by downregulating the expression of ABCB1 transporters [27]; quercetin can reverse the resistance of paclitaxel-resistant prostate cancer cells in vitro by reversing activation of the androgen receptor and PI3K/Akt signaling [28]. Osthole reverses the chemotherapeutic resistance of cisplatin-resistant cervical cancer cells by inactivating the PI3K/AKT pathway [29]; and ginsenoside Rg3 can inhibit the mTOR signaling axis of SOX2, PI3K/Akt, and miR-429 to reduce the resistance to cisplatin [30].
Table 1.
Natural products in preventing tumor drug resistance.
| Natural Products | Molecular Structure | Cancer | Signaling Pathway |
|---|---|---|---|
| 1. Alkaloids | |||
| Matrine |
|
Lung cancer | PI3K/AKT/mTOR |
| Breast cancer | Wnt/β-catenin | ||
| Hepatocellular carcinoma | PI3K/AKT/mTOR, AKT/GSK3/β-catenin | ||
| Ovarian cancer | MAPK/ERK PI3K/AKT/mTOR |
||
| Urothelial bladder carcinoma | VEGF/PI3K/AKT | ||
| Tetrandrine |
|
Ovarian cancer | β-catenin/c-myc/cyclin D1 |
| Pancreatic cancer | PI3K/AKT/mTOR | ||
| Gastric cancer | AKT/mTOR | ||
| Bladder cancer | AMPK/mTOR | ||
| Neferine |
|
Ovarian cancer | mTOR/p70S6K, MAPK/JNK |
| Lung cancer | PI3K/AKT/mTOR | ||
| Dauricine |
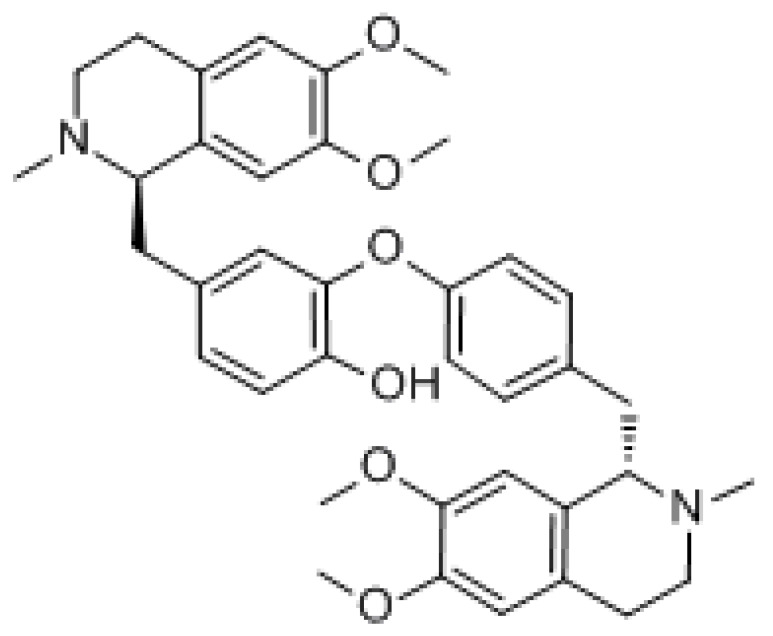
|
Renal cell carcinoma | Cell cycle |
| Melanoma | Src/STAT3 | ||
| Hepatic cell carcinoma | miR-199aHK2/PKM2 | ||
| Cepharanthine |
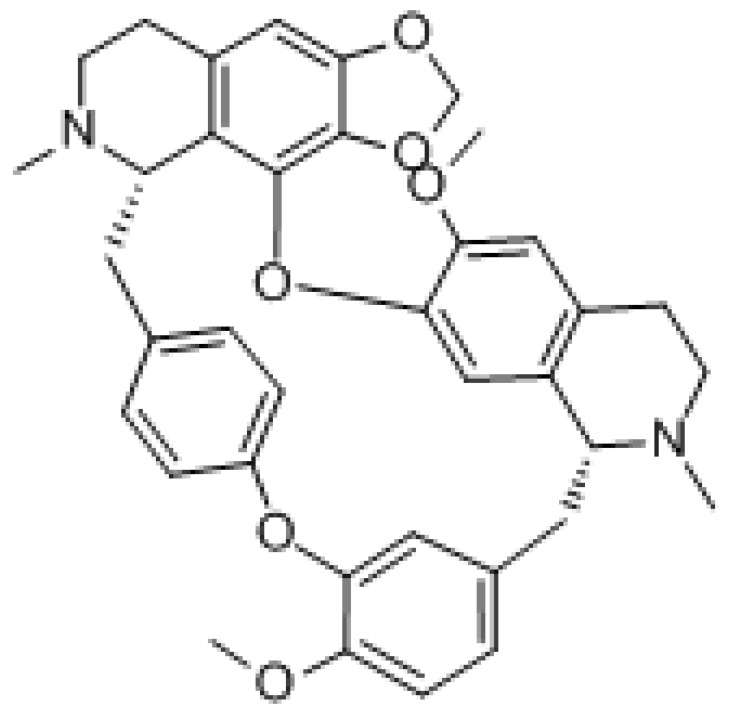
|
Breast cancer | Akt/mTOR |
| Ovarian cancer | PI3K/AKT | ||
| Solanine |
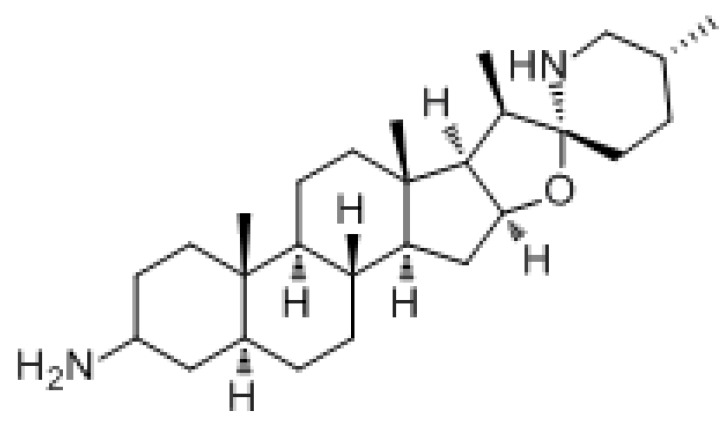
|
Endometrial cancer | PI3K/AKT |
| 2. Flavonoid | |||
| Meletin |
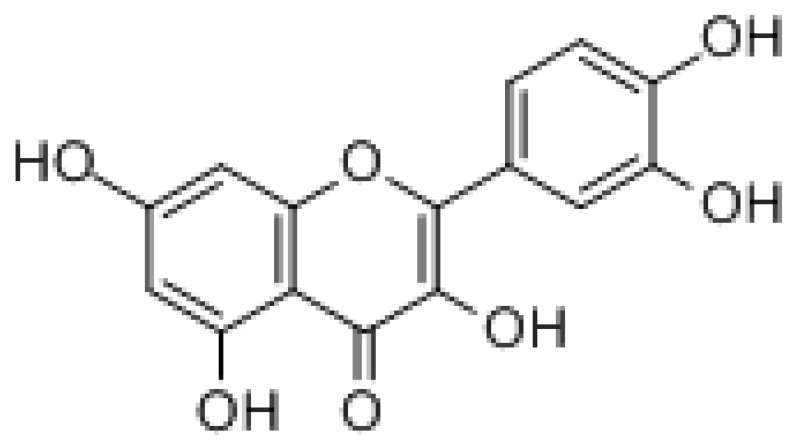
|
Prostate cancer | PI3K/AKT |
| Colon cancer | Notch-1 | ||
| Curcumin |
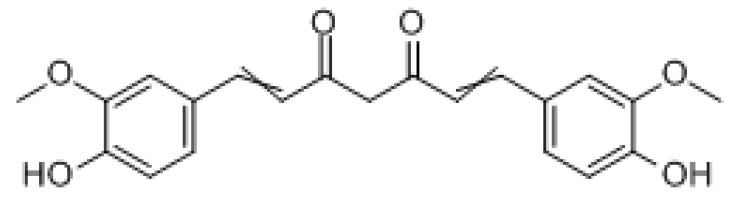
|
Hepatocellular carcinoma | PI3K/AKT/mTOR |
| Lung cancer | PI3K/AKT | ||
| Colorectal cancer | TGF-β/Smad2/3 | ||
| 3.Terpene | |||
| Ginsenoside |
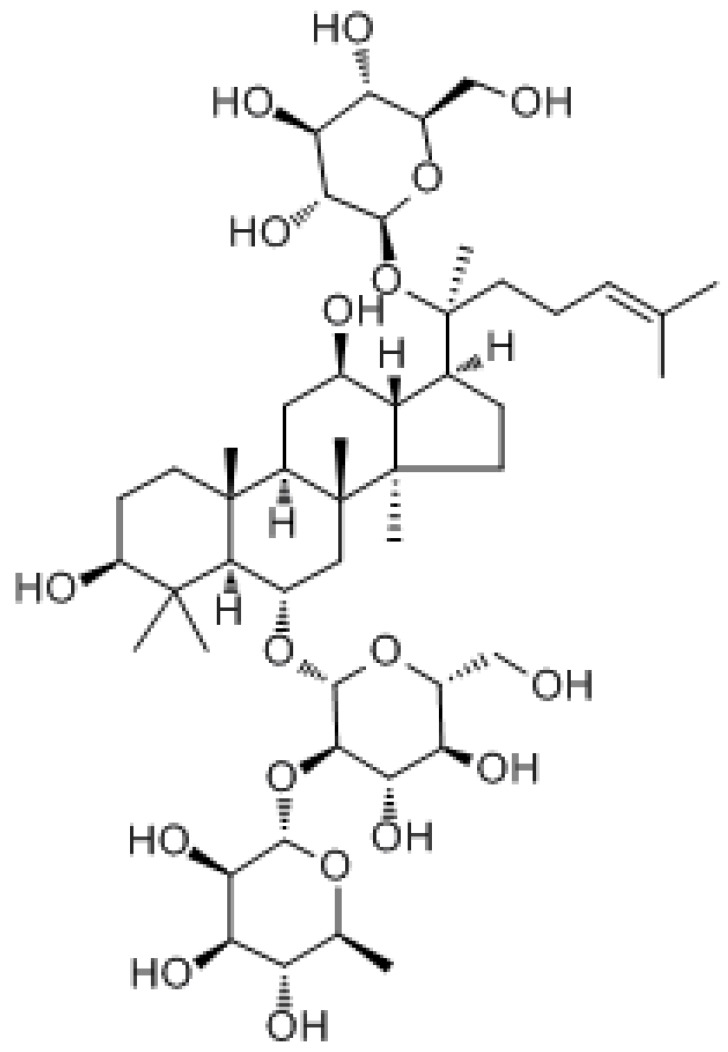
|
Gastric cancer | PI3K/AKT/mTOR |
| Glioblastoma | Wnt/β-catenin | ||
| Soil bastard saponin |
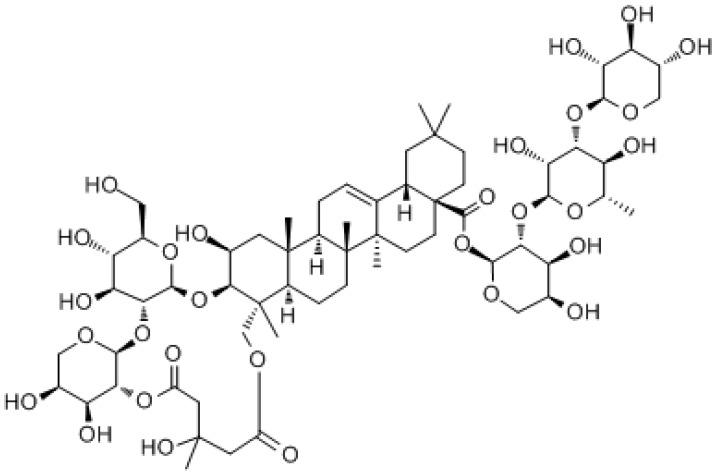
|
Colorectal cancer | Wnt/β-catenin signaling pathway |
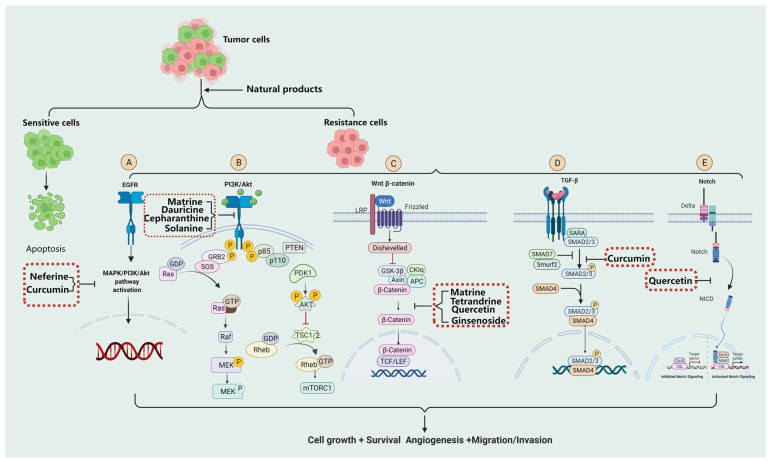
Here, we reviewed the molecular mechanisms of tumor drug resistance induced by EGFR, PI3K/Akt, Ras/MAPK, Wnt/β-catenin, Notch, Transforming growth factor-beta (TGF-β), and combinations of these signaling pathway inhibitors derivatives from natural products to overcome tumor resistance, provide new ideas and strategies for the rational clinical selection of antitumor drugs, and therefore improve patient quality of life and survival.
2. The Signaling Pathways in Tumor Drug Resistance
It has been well established that signaling pathways interact to form complex regulatory networks. Signaling pathways are a key factor in maintaining the homeostasis of the intracellular environment [31]. Many signaling pathways play important roles in tumor transformation, metastasis, inhibition of apoptosis, and tumor stem cells [32,33]. Abnormal activation or inhibition of one or more signaling pathways can lead to tumorigenesis or induce drug resistance [34]. At present, chemotherapy remains the main treatment for tumors [35]. Mutations of the targets of chemotherapeutic drugs, or modifications of the signaling pathway components involved, can reduce drug efficacy so that the tumor develops resistance to the chemotherapeutic drugs [36]. The mechanisms of tumor resistance to chemotherapeutic drugs are still very complex [37]. Several researchers report that EGFR, Ras/MAPK, PI3K/Akt, Notch, Wnt/β-catenin, TGF-β, and Notch pathways all play important roles in the process of tumor drug resistance [38,39].
2.1. EGFR
EGFR is a tyrosine kinase encoded by the EGFR gene, which is widely expressed in normal tissues [40]. In recent years, epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) have shifted from empirical cytotoxic chemotherapy to molecular-targeted therapy [41]. At present, three generations of EGFR-TKIs (gefitinib and erlotinib, dacomitinib and afatinib, and osimertinib) have been used in clinical practice and achieved positive results [42]. However, most patients develop drug resistance after 6–13 months of treatment with EGFR-TKIs [43]. It has been shown that molecular-targeted drugs such as EGFR-TKIs are in two different states: “on-target” and “off-target,” and the molecular mechanisms of tumor resistance to these states are different [44]. When the drug is in the “on-target” state, an EGFR mutation is the main cause of tumor resistance to the drug [45]. There are four members of the human EGFR receptor (HER) receptor tyrosine kinase family, EGFR (HER1 or ErbB1), HER2 (neu/ErbB2), HER3 (ErbB3), and HER4 (ErbB4) [46]. The EGFR mutations occur only on four exons, exons 18–21, with deletion, insertion, and missense point mutations. When exon 19 is deleted (Del19) or leucine (L) at position 858 of exon 21 is mutated to arginine (R) (L858R), impaired endocytosis of EGFR and increased tyrosine phosphorylation result [47]. The mutation of threonine (T) at position 790 in exon 20 to methionine (M) (T790M) is another important mutation commonly seen in clinical resistance to TKIs. Studies have shown that the T790M mutation causes acquired resistance to first- and second-generation TKIs in approximately 50–60% of patients [48]. Threonine 790 in exon 20 is the “gatekeeper” of the kinase structure, and when it is replaced by methionine, the EGFR kinase region undergoes conformational changes, rendering the TKI drug unable to access the active center of the tyrosine kinase and weakening reversible binding to the TKI drugs, which induces tumor resistance [49]. With advances in sequencing technology, scientists have also identified new mutation sites, such as L747S, D761Y, and T854A, which also reduce the binding interactions between the drug and EGFR-TKI to induce tumor resistance [50]. However, the mechanisms underlying drug resistance due to mutations remain unclear [51].
When the drug is in the “off-target” state, tumor resistance to the drug is primarily associated with signaling pathway interactions [52], such as between hepatocyte growth factor (HGF)/c-MET, vascular endothelial growth factor (VEGF), insulin-like growth factor 1 (IGF1), signal transducers and activators of transcription (STAT)3, and paracrine pathways [53]. c-MET is a receptor tyrosine kinase for a hepatocyte growth factor (HGF). It was found that 5–10% of lung cancer patients develop resistance to TKIs due to c-MET gene amplification [54]. This amplification, leading to ErbB3-dependent PI3K activation, contributes to tumor resistance to gefitinib [55]. HGF can induce resistance to gefitinib in lung adenocarcinoma patients with EGFR mutations by phosphorylating c-MET and activating the PI3K/Akt signaling pathway through an ErbB3-independent pathway [56]. HGF/c-MET also interacts with the Src/STAT3/FAK/ERK signaling pathway to induce resistance to platinum-based drugs in tumor cells [57]. Cancer-associated fibroblast-derived HGF can activate c-Met/PI3K/Akt and glucose-regulated protein 78 (GRP78) signaling pathways to promote tumor cell proliferation and induce drug resistance in tumor cells [58]. VEGF is an indispensable cytokine in angiogenesis, and VEGF signaling is mainly mediated by VEGF receptor 2 (VEGFR2). Studies have shown that in the microenvironment of interleukin (IL)-23-mediated tumor inflammation, the STAT3/VEGF pathway activates to induce resistance to doxorubicin in small-cell lung cancer (SCLC) cells [59]. IGF1 can promote tumor growth. The biological activity of IGF1 is primarily mediated by the IGF1 receptor (IGF1R). The IGF1R is a heterotetramer composed of tyrosine kinases located on the cell surface. The key factor in resistance to afatinib in NSCLC patients carrying the T790M mutation is the activation of the IGF1R signaling pathway [60]. Osimertinib-treated lung cancer patients were found to have increased IGF2 expression, and IGF2 autocrine-mediated IGF1R pathway activation is one of the causes of osimertinib resistance in lung cancer patients [61]. The STAT family is closely related to drug resistance production in NSCLC. The STAT3/zinc finger E-box-binding homeobox 1 (ZEB1) signaling axis was found to be a key factor in reversing resistance to gefitinib in NSCLC [62]. Long non-coding RNA MALAT1 positively regulates STAT3 and fructosyltransferase 4 (FUT4) activity and induces paclitaxel resistance in A549 lung cancer cells [63].
2.2. Ras/MAPK
Ras is a member of the small GTPase family and is the switch controlling GDP and GTP conversion. Functional mutations in Ras are one of the main causes of tumorigenesis [64]. The human Ras family includes four homologous Ras proteins: H-Ras, N-Ras, and two K-Ras splice variants (K-Ras4A and K-Ras4B), and are encoded by three RAS genes, HRAS, NRAS, and KRAS. Across all tumors caused by Ras mutations, K-Ras, N-Ras, and H-Ras mutations account for 85%, 12%, and 3%, respectively [64]. Studies have shown that oncogenic K-Ras mutations are closely associated with tumor resistance to platinum drugs [65]. In NSCLC, K-Ras mutations can induce nuclear factor-erythroid factor 2-related factor 2 (NRF2) gene transcription through TPE response elements, upregulating the NRF2 pathway, leading to excessive activation of an antioxidative stress pathway, reducing cisplatin-induced reactive oxygen species (ROS) generation in tumor cells, reducing the mortality of tumor cells, and therefore causing tumor cells to become resistant to cisplatin [66]. K-Ras mutations can also cause hyperactivation of the PI3K-AKT pathway, inhibit apoptosis, and increase cell proliferation [67].
Ras mutations are not only a cause of tumor resistance to platinum drugs, but also a common cause of tumor resistance to TKIs [68]. TKIs are widely used in the treatment of patients with clinical tumors, including renal cell cancer (RCC), colorectal cancer, acute myeloid leukemia (AML), and NSCLC [69]. The main mechanism of action of TKIs is to inhibit the phosphorylation sites within the protein, thus preventing them from exerting their kinase activity against downstream effectors [70]. Tumor resistance to TKIs occurs mainly by mutations in tyrosine kinases (RTKs) themselves or by mutations in downstream pathway proteins [71].
Tyrosine kinase 3 (FLT3) is very commonly mutated in AML, with FLT3 mutations present in approximately 40% of cytogenetically normal AML patients. Between 20 and 30% of patients with AML have FLT3-internal tandem duplication (ITD) mutations, and such patients have a high tumor recurrence rate and poor prognosis. Tumor cells can promote cell proliferation, inhibit apoptosis, and induce resistance to TKIs through the MAPK, STAT5, and PI3K pathways [72]. Therefore, scientists have developed many FLT3 inhibitors, such as gefitinib, clolani, and middolin, based on the active conformation of FLT3 [73,74]. Gefitinib and middoorin received FDA approval while Kolani is in a phase II trial [75,76]. Ras mutations can be detected in 30% of patients with resistance to gefitinib. Since Ras mutations can activate the MAPK and PI3K-AKT pathways, some tumor patients are also resistant to these novel FLT3 inhibitors [71].
2.3. PI3K/Akt
The PI3K/Akt/mTOR pathway is an important regulatory pathway for physiological activities such as cell proliferation, cell cycle, and apoptosis, and the hyperactivation of this signaling pathway plays an important role in cancer progression and cancer drug resistance [77]. Abnormal activation of the PI3K/Akt/mTOR pathway has been found to be one of the most important reasons for the development of tumor resistance to chemotherapy [78]. When Akt, the core protein of the PI3K/Akt/mTOR pathway, is activated by phosphorylation, activated Akt stimulates the serine/threonine-protein kinase PAK1, and PAK1 then upregulates the expression of the anti-apoptotic protein Bcl-2. PAK1 also promotes phosphorylation of Bad for release from the mitochondrial membrane to the cytoplasm and inhibits Bax transfer from the cytoplasm to mitochondria, attenuating the proapoptotic function of Bax and Bad, thus inhibiting tumor cell apoptosis and promoting resistance to chemotherapeutic drugs [79]. Tropicrine inhibited phosphorylation of Akt and resensitized multidrug-resistant breast cancer cells to chemotherapeutic drugs [80]. The activated PI3K/Akt pathway can then activate the NF-kB pathway, which results in increased intracellular protein synthesis, increased energy reserves, and rapid cell growth, favoring the formation of multidrug resistance in tumor cells [81]. Simultaneous activation of the PI3K/Akt/mTOR pathway and the MAPK signaling pathway can induce paclitaxel resistance in gastric cancer [82]. The mTOR mutation E2419K also contributes to tumor resistance to EGFR-TKI [83]. Studies have shown that the production of multidrug resistance in breast cancer cells is also closely related to Akt overexpression and phosphatase and tensin homolog (PTEN)/EMT/Akt activation [84,85]. Moreover, microRNA also modulates the PTEN/PI3K/Akt pathway to induce resistance of tumor cells to chemotherapeutic drugs [86]. For example, miR-132 and miR-212 regulate PTEN, leading to resistance to doxorubicin in breast cancer cells [87].
2.4. Wnt/β-Catenin
The Wnt signaling pathway is a very conserved pathway that is essential for cell fate and embryonic development in multicellular organisms. The Wnt genes are involved in cell proliferation, migration, apoptosis, and differentiation [88]. There are three main Wnt pathways: The β-catenin-dependent classical pathway, the planar cell polarity non-classical pathway, and the Wnt-Ca2+ non-classical pathway [89]. In the classical pathway, various factors leading to the massive accumulation of β-catenin in the cytoplasm and entry into the nucleus are key to activating Wnt/β-catenin signaling. Β-catenin promotes transcription of downstream target genes of the Wnt pathway [90]. The Wnt/β-catenin signaling pathway (classical Wnt pathway) is abnormally activated in tumor tissues and cells. The increased β-catenin activity was found to induce carboplatin resistance in A2780 cells [91]. However, the downregulation of β-catenin expression prevented β-catenin from entering the nucleus, which effectively increased the sensitivity of ovarian cancer cells to chemotherapeutic drugs and reversed resistance to platinum chemotherapeutic drugs [92]. Activation of the PI3K/Akt signaling pathway and an increase in nuclear β-catenin upregulated hypoxia-inducible factor 1 (HIF-1) expression and induced glucose metabolic reprogramming, conferring resistance to fluorouracil (5-FU) in colorectal cancer [93]. In colorectal cancer tissues, carnitine palmitoyltransferase 2 (CPT2) expression is downregulated, activating the ROS/Wnt/β-catenin pathway and inducing oxaliplatin resistance in colorectal cancer [94]. Xenograft tumor model studies from carboplatin-resistant breast cancer patients found that canonical Wnt/β-catenin signaling activation upregulated the expression of tumor stem cell markers and promoted tumor resistance to carboplatin. Inhibition of the Wnt/β-catenin signaling pathway restored the sensitivity of tumor tissue to carboplatin [95] and reversed the resistance to enzalutamide in prostate cancer [96]. Studies have shown that frameshift mutations in TCF7, a positive transcriptional regulator in the WNT/β-catenin signaling pathway, can induce abnormal WNT/β-catenin signaling, activate glycogen synthase kinase-3 (GSK3), and induce resistance to gedatolisib in colon cancer cells [97]. Moreover, small RNA can also regulate the Wnt/β-catenin signaling pathway to promote or inhibit the production of tumor resistance to chemotherapeutic drugs. For example, MiR-624-5p upregulates nod-like receptor family pyrin domain-containing protein 3 (NLRP3) expression through the EMT/IL-1/Wnt/β-catenin signaling pathway and promotes gemcitabine resistance in ovarian cancer [98]. MiR-331-3p activates Wnt/β-catenin through the suppressor of tumorigenicity 7 protein-like (ST7L) and promotes resistance to chemotherapeutic drugs in prostate cancer [99]. The targeting of miR-199b-3p to cysteine-rich transmembrane BMP regulator 1 (CRIM1) inhibits Wnt/β-catenin signaling and reverses resistance to cetuximab in colorectal cancer [100]. Both the ubiquitin-conjugating enzyme 2 proteins UBE2S and UBE2M can activate the Wnt/β-catenin signaling pathway, promoting resistance to olaparib in ovarian cancer and 5-FU resistance in colorectal cancer, respectively [101,102].
2.5. Notch
Notch signaling is a conserved ligand-receptor signaling pathway that plays a critical role in cell proliferation, survival, apoptosis, and differentiation, affecting the development and function of many organs [103]. Among the numerous targets of Notch are proteins that play a key role in tumor development and progression, such as the hairy and enhance of split (HES) family, hairy/enhancer of spit related with YRPW motif (HEY), nuclear factor-kappa B (NF-κB), VEGF, mTOR, cyclin D1, c-myc, p21, p27, and Akt [103].
Studies have shown that the Notch pathway regulates tumor stem cell formation, promotes the epithelial–mesenchymal transition (EMT), and participates in the formation of tumor resistance to chemotherapeutic drugs [104]. EMT plays an important role in the paclitaxel resistance process in cervical cancer. Notch1 expression was elevated in paclitaxel-resistant cervical cancer cells compared to non-resistant cells, and silencing the NOTCH1 gene reversed EMT, indicating that Notch1 is involved in the paclitaxel resistance process in cervical cancer [105]. Notch signaling is activated and is a key factor in developing resistance to endocrine therapy in estrogen receptor (ER)-positive (ER+) breast cancer. Endocrine therapy is the primary treatment modality in patients with ER+ breast cancer, and the main drugs are tamoxifen, aromatase inhibitors, and fulvestrant [106]. Tamoxifen and fulvestrant, competitive inhibitors of 17-estradiol, can directly bind to the ligand-binding domain of estradiol and inhibit 17-estradiol-mediated ER signaling in cancer cells. Aromatase inhibitors can reduce the aromatase-mediated local androgen synthesis of 17-estradiol, indirectly targeting the ER signaling pathway [107]. It was found that 17-estradiol-mediated estrogen synthesis maintains Notch activity at low levels, while antagonistic estrogen activates Notch signaling. Mutations in the ER hormone-binding domain, such as Y537S, can cause resistance to endocrine therapy in ER+ breast cancer [107]. Knockdown of Notch1 decreased cell proliferation and increased the sensitivity of prostate cells to benzalotamide, indicating that Notch1 signaling plays an important role in the production of enzalutamide resistance in prostate cancer, and inhibition of Notch1 signaling restored the sensitivity of resistant cells to enzalutamide [108].
2.6. TGF-β
Dysregulation of the TGF-β-signaling pathway leads to many diseases, including cancer. Although the activation of TGF-β signaling in healthy non-cancer and early cancer cells induced effective cell cycle arrest, elevated TGF-β expression and TGF-β receptor activation of intracellular signaling are observed in many cancers [109]. Cohort studies of tumor patients suggest that patients whose TGF-β pathway is activated in vivo have poor outcomes [110]. Activation of this pathway in tumor cells induces EMT [111]. In addition to its importance in migration, EMT has a role in resistance to chemotherapy [112,113]. TGF-β is mainly produced by tumor cells and stromal cells where it promotes tumor angiogenesis and therefore tumorigenesis. This suggests that TGF-β signaling changes during tumor progression from tumor suppression to tumor promotion. TGF-β has been found to induce tumor resistance to chemotherapy through multiple pathways [114]. For example, the miRNA-mediated activation of the TGF-β signaling pathway can increase DNA repair capacity, leading to tumor drug resistance [115]. Tumor cells undergoing autophagy upregulate E3 ubiquitin ligase and promote the degradation of SMAD7, thus activating TGFβ/SMAD signaling, inducing EMT, and promoting tumor cell proliferation and migration, which produces resistance to chemotherapeutics [116,117]. TGF-β/SMAD signaling activation directly induces G1 cell cycle arrest in tumor cells, leading to tumor proliferating cancer cells (TPCs) entering a quiescent state, protecting cancer cells from cellular DNA damage caused by 5-FU, and therefore inducing drug resistance [118]. In docetaxel-resistant and paclitaxel-resistant triple-negative breast cancer cells, aurora kinase A (AURKA) is highly expressed in the cells, where it mediates TGF-β-induced EMT and produces resistance to these drugs [119].
The immune system can accurately identify and remove malignant tumor cells. However, tumor cells alter or reduce the expression of tumor-specific antigens, upregulate immune checkpoint proteins, and alter the expression of cytokines to promote immune evasion [120]. TGF-β can inhibit the cytotoxicity and activation of immune cells, including macrophages, neutrophils, bone marrow-derived suppressor cells (MDSC), natural killer (NK) cells, and dendritic cells (DCs) and T cells, and inhibit immune cell function [121]. Tumor cells can secrete TGF-β, which directly induces the transformation of NK cells into innate lymphoid cell type 1 (ILC1) cells lacking cytotoxic function, inhibits the cytotoxicity mediated by the NK cell receptor NKG2D, promotes immune escape of tumor cells, and induces immune tolerance of tumor cells [121]. In the immunosuppressed microenvironment, glycoprotein A repetitions predominant (GARP) is highly expressed, which can induce the binding of dormant TGF-β to integrin αvβ8 on the cell membrane; the activated TGF-β is released, causing the immune escape of tumor cells [122]. Specific inhibition of TGF-β1 in Treg cells highly expressing GARP overcame drug resistance to programmed cell death protein 1 (PD1)/programmed cell death ligand 1 (PD-L1) blockers in tumor patients [123]. TGF-β also inhibits Th2 cells, which mediate tumor immunity. By blocking TGF-β signaling in CD4+ T cells, Th2 cells secrete IL-4, which reshapes the tumor immune microenvironment and suppresses tumor growth [124,125]. TGF-β1 can induce high expression of PD1 and PD-L1 in T cells and tumor cells, respectively, thus impairing the antitumor activity of T cells and promoting tumor immune evasion [126]. Although the major mechanisms of resistance to cancer immunotherapy have not been fully established, the inhibition of TGF-β signaling and modifying the tumor microenvironment may overcome tumor resistance to PD1\PD-L1 blockers [127,128].
3. Natural Products in Preventing Tumor Drug Resistance
3.1. Alkaloids
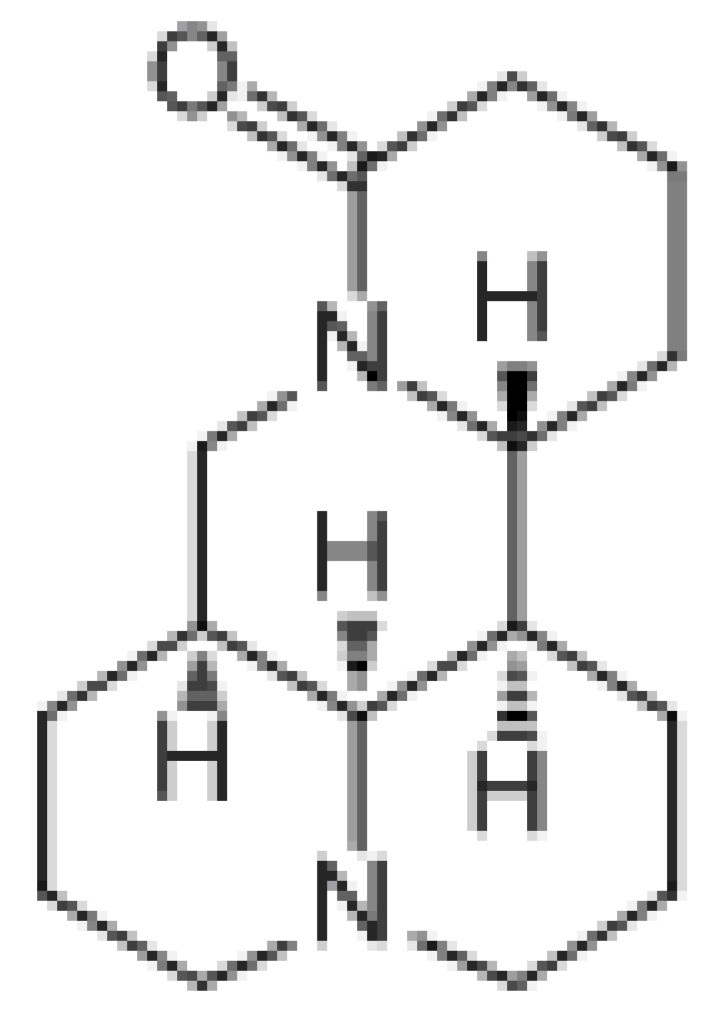
3.1.1. Matrine
Matrine is a class of tetracyclic quinoline alkaloids extracted from plants in the genus Sophora with the formula C15H24N2O, and a molecular weight of 248.36 g/mol. The antitumor activity of matrine is mainly manifested by inhibiting cancer cell proliferation, blocking the cell cycle, inducing apoptosis, and inhibiting cancer cell metastasis. At the same time, matrine can reverse the resistance and reduce the toxicity of anticancer drugs [129]. Matrine and its compounds were reported to inhibit the growth and proliferation of various cancer cells through the induction of apoptosis and cell cycle arrest [130,131]. It can also inhibit tumor cell migration, invasion, and adhesion by downregulating the expression of certain active oncogenes [132,133]. Furthermore, it was found that the resistance reversal action of low doses of matrine can be improved by structural modification, such as the introduction of a thiophene structure in matrine [134]. Studies have found that matrine can reverse lung cancer [135], mammary cancer [80], and bladder cancer [136] resistance. Matrine can induce apoptosis in lung cancer cells by regulating the PI3K/AKT/mTOR signaling pathway, and simultaneously downregulating the expression of apoptosis inhibitory protein [135]. Matrine can also inhibit the expression of VEGF and the proliferation of breast cancer cells by regulating the Wnt/β-catenin signaling pathway [137]. Some matrine derivatives can also inhibit the proliferation of hepatocellular carcinoma (HCC) cells through the PI3K/AKT/mTOR and AKT/GSK3/β-catenin signaling pathway [138].
It was found that matrine exerts antitumor effects in drug-resistant ovarian cancer cells in vitro and in vivo by downregulating MAPK/ERK, PI3K/Akt, and Akt/mTOR signaling [139]. It shows a dose- and time-dependent growth inhibition by inducing increased G0/G1 and decreased S and G2/M phases in A2780 and SKOV3 cells, with a molecular mechanism associated with the upregulation of p21 and downregulation of cyclin D1 and CDK4 [140]. When studying the therapeutic effect of matrine on colorectal cancer (CRC), it was found that matrine could upregulate p21, p27, and pGSK-3 in a dose-dependent manner and downregulate CDK6, cyclinD1, and cyclin E, also in a dose-dependent manner [141]. Moreover, it has been found that nontoxic concentrations of matrine sensitized multidrug-resistant K562 cells in two ways, namely, the reactivation of apoptosis and inhibition of drug efflux [142]. Matrine can downregulate the phosphorylation of NF-κB, restore pro-apoptotic factors, and inhibit anti-apoptotic factors, thus promoting endogenous apoptosis [143]. Matrine can also induce mitochondrial damage by promoting the proapoptotic genes Bax, Bid, Bad, and Bim, and downregulating the apoptosis-inhibiting genes Bcl-2, Mcl-1, and Bcl-XL to promote endogenous Apoptosis. Additionally, matrine can downregulate ABCB1 expression, resulting in reduced drug efflux, which may also be related to the inhibition of NF-κB and facilitate the intrinsic apoptotic pathway by downregulating a downstream antiapoptotic factor Bcl-2 [80,142]. Finally, matrine significantly reversed the resistance of oxaliplatin-resistant HT-29/OXA cells with a dose-dependent increase in the sensitivity of HT-29/OXA cells to oxaliplatin [144]. Furthermore, it was shown that matrine combined with cisplatin inhibited urothelial bladder carcinoma (UBC) cells through the downregulation of the VEGF/PI3K/Akt signaling pathway. In the combination treatment, matrine improved the sensitivity of UBC cells to cisplatin and reduced the dose of cisplatin, thus potentially attenuating its side effects, suggesting that matrine may be a new option for UBC combination therapy [136].
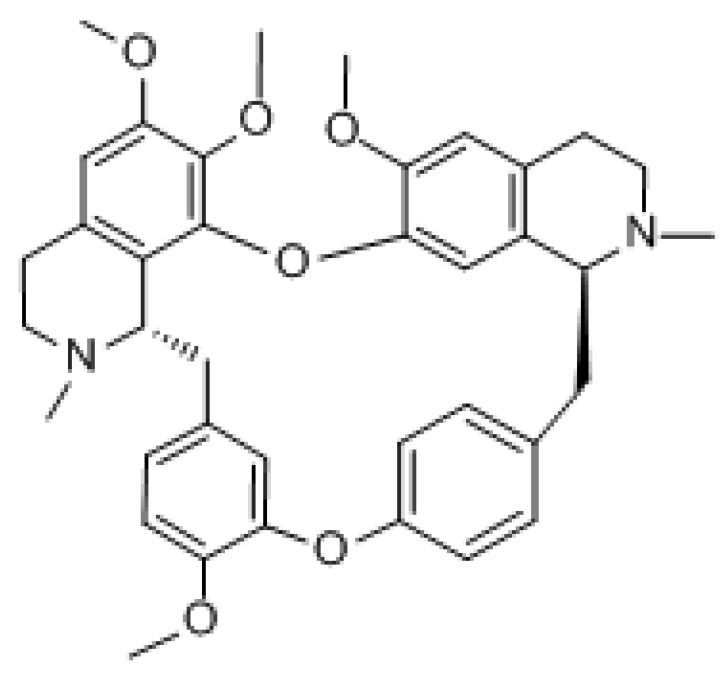
3.1.2. Tetrandrine
Tetrandrine (TET) is a class of dibenzyl isoquinoline alkaloids extracted from Stephania tetrandra with the molecular formula C38H42N2O6 and molecular weight of 622.76 g/mol. It mainly acts by regulating molecular signaling pathways, inducing apoptosis in cancer cells, promoting cell cycle arrest, and increasing cell autophagy. It has been reported that TET induced apoptosis at high concentrations and autophagy at low concentrations [145]. Studies have shown that it contributes to many types of cancer treatment, such as lung, breast, colon, and cervical cancer [146,147,148,149]. Although it exhibits mild side effects such as diarrhea, myelosuppression, and mucositis, it has been shown to be nontoxic to normal cells under in vivo experimental conditions. Otherwise, it did not change the pharmacokinetic parameters in in vivo assays, an advantage of its use over other compounds [145]. It was found that TET may block gefitinib-induced autophagic flow by inhibiting lysosomes and therefore enhance the sensitivity of PC14 cells to gefitinib. Because autophagy provides a large source of energy and material for cancer cell growth, the inhibition of autophagy may lead to cancer cell growth arrest [150]. Furthermore, TET can counter drug resistance and multidrug resistance in MDR1 gene-transfected cancer cells by downregulating ABCB1 transporter expression and consequently increasing the intracellular concentration of chemotherapeutic drugs [27].
TET was found to enhance SKOV3/paclitaxel cell sensitivity to paclitaxel by inducing apoptosis and cell cycle arrest in ovarian cancer therapy. Furthermore, TET could enhance the antitumor effects of paclitaxel in SKOV3/paclitaxel cells by inhibiting the β-catenin/c-myc/cyclin D1 signaling pathway [151]. In the treatment of pancreatic cancer, the powder promoted apoptosis and autophagy, thus restoring the chemosensitivity of gemcitabine-resistant human pancreatic cancer cells to gemcitabine. The proapoptotic effects of TET are associated with the reduction of survivin expression and downregulation of PI3K/Akt/mTOR signaling pathway activity [152]. In the treatment of gastric cancer, the alkali upregulated the caspase cascade proteins (cleaving PARP, caspase-3, and caspase-9) and inhibited the phosphorylation of Akt/mTOR, resulting in significant apoptosis of human gastric cancer cells [153]. In leukemia treatment, PPA induced apoptosis through caspase cascade regulation, cell cycle arrest, MAPK activation, and PI3K/Akt/mTOR signaling modification, showing cytotoxic effects on glucocorticoid-resistant human leukemia Jurkat T cells [154]. In bladder cancer treatment, TET induced autophagy in human bladder cancer cells by regulating AMPK/mTOR signaling, which favors apoptosis induction, suggesting that it may be a potential anticancer candidate for the treatment of bladder cancer [155].
3.1.3. Ligustrazine
Ligustrazine (TMP) is an alkaloid monomer isolated from Ligusticum xiong. The formula is C8H12N2, with a molecular weight of 136.19 g/mol. However, ligustrazine is chemically unstable with a half-life of approximately 1.5 h, which partly limits its potential as a cancer therapeutic agent and, therefore, drug delivery systems compatible with it are being developed [156]. Previous studies have found that ligustrazine reduced the risk of multidrug resistance in chemotherapy and inhibited the proliferation and metastasis of various types of cancer cells, such as ovarian and liver cancer [157]. Studies have shown that ligustrazine can promote the antitumor effects of paclitaxel. Ligustrazine combined with paclitaxel inhibited angiogenesis by inhibiting the ERK1/2 and Akt pathways and promoted tumor cell apoptosis. Ligustrazine can decrease MRP1, GST, and BCL-2 to reverse multidrug resistance in human bladder cancer [158].
Furthermore, ligustrazine enhanced the antitumor effects of paclitaxel in vivo and somewhat attenuated the toxicity of paclitaxel [159]. It was found that K562 sensitivity and subsequent cytotoxicity of A02 cells (doxorubicin-resistant) in the presence of ligustrazine derivatives were enhanced due to doxorubicin accumulation in cells [160]. In addition, after ligustrazine and Danshensu (DSS) combine to form the conjugate DT-010, the complex reversed doxorubicin multidrug resistance in MCF-7/doxorubicin-resistant human breast cancer cells at non-cytotoxic concentrations. This effect may be related to P-gp inhibition [161]. Finally, it has been shown that ligustrazine suppresses retinoblastoma growth by regulating CXCR4 expression, and it has been established that this effect is related to cell density. Therefore, ligustrazine is as a potential drug candidate for the treatment of retinoblastoma [162].
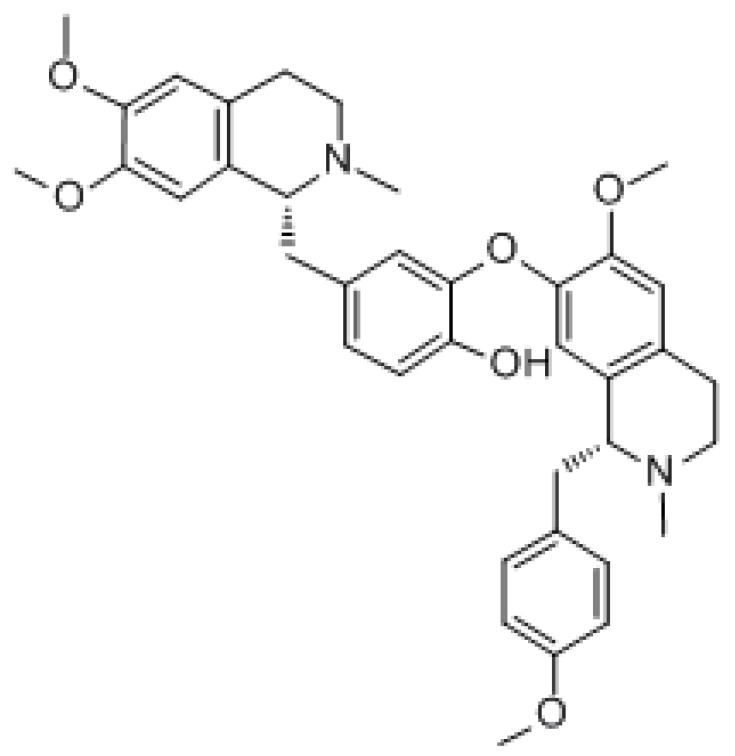
3.1.4. Neferine
Lotus heart base is a dibenzylisoquinoline alkaloid isolated from the green seed embryo of the lotus flower. The formula is C38H44N2O6, and the molecular weight is 624.778 g/mol. A very important feature of neferine activity is that it is cytotoxic to hepatoma cells but does not damage normal human hepatocytes. Neferine can effectively inhibit the proliferation of multidrug-resistant cancer cells and induce autophagy [163]. Previous studies have shown that the lotus heart base has certain efficacy in many types of cancer, such as lung cancer [164], neuroblastoma [165], and ovarian cancer [166]. Pretreatment with neferine can activate the MAPK/mTOR pathway to combat cisplatin-induced apoptosis and regulate autophagy [167]. Some studies have reported that neferine activates the Ryanodine receptor through the AMPK/mTOR-dependent pathway, thus inducing autophagy and releasing calcium ions [168]. It has been demonstrated that neferine can inhibit angiogenesis in ovarian cancer by inhibiting the mTOR/p70S6K signaling pathway while inducing autophagy and inhibiting M2 macrophage polarization [166].
In addition, it was found that the natural alkaloid neferine can promote apoptosis and induce the cell cycle by inducing JNK and p38 MAPK phosphorylation and downregulating PI3K and NF-κB signaling, enabling CD44+, and therefore inhibiting the proliferation and migration of CSCs. Neferine treatment not only inhibited CD44+ CSC activity, but it also inhibited the survival of androgen-insensitive PC3 and androgen-sensitive LNCaP cells. This suggests that neferine may be eradicating prostate cancer (PCa) cells and CD44+ CSC and may inhibit metabolism. By acting on CSC, it can effectively combat the development of drug resistance and disease recurrence [169]. Mitomycin C is a known antitumor drug that, upon binding to neferine, continuously activates the p38 MAPK pathway in a ROS-dependent manner, thereby inducing apoptosis in cervical cancer cells [170]. It also induced G1 phase growth arrest in osteosarcoma cells, Hep3B cells, and adenocarcinoma cells, and induced autophagy in PC-12 cells, human ovarian cancer cells, and Hep3B cells. The mechanisms inducing growth arrest are increased stability of p21 and phosphorylation at Ser130, whereas autophagy induction involves the inhibition of PI3K/Akt/mTOR signaling in A549 cells, and the activation of p38 MAPK/JNK in human ovarian cancer cells. Studies have shown that neferine cooperates with anticancer compounds (such as doxorubicin, paclitaxel, cisplatin, and vincristine) in many cancer cells, thus improving efficacy and combating chemotherapy resistance. Neferine combined with isoliensinine could downregulate the cell survival protein expression (PI3K/pAkt/mTOR) and activate mitochondria-mediated apoptosis by upregulating Bax, cytochrome c, caspase-3, and PARP cleavage expression while downregulating BCl-2 expression in cisplatin-resistant colon stem cells (CSCs) [171]. In addition, neferine can enhance the sensitivity of some types of cancer cells to chemotherapeutic drugs and reduce epithelial–mesenchymal transitions (EMT) due to chemoresistance against liver cancer cells. This fully illustrates the resistance reversal activity of neferine [172].
3.1.5. Dauricine
Dauricine is a dibenzylisoquinoline alkaloid found in pueraria with the formula C38H44N2O6 and a molecular weight of 624.77 g/mol. It has potent antitumor activity, including the induction of apoptosis and overcoming drug resistance of tumor cells. Dauricine was found to reduce the survival of renal cell carcinoma (RCC) cells and induced cell cycle arrest in the G0/G1 phase and induced apoptosis through the intrinsic pathway [173]. Among these mechanisms, the former was associated with the downregulation of cyclin D1, CDK2, and CDK4 and the upregulation of p21. The latter was associated with the activation of caspase-9, caspase-3, and the downregulation of anti-apoptotic Bcl-2 protein expression. Moreover, dauricine also inhibited PI3K/Akt signaling activation and exerted antitumor effects [174]. In addition, it inhibited melanoma cell proliferation and strongly induced melanoma cell death by inhibiting the phosphorylation and activation of the Src protein and the activation of downstream signals (e.g., STAT3 protein) [175]. Dauricine downregulated the expression of hexokinase 2 (HK2) and pyruvate kinase M2 (PKM2). HK2 and PKM2 can be directly targeted by miR-199a. Dauricine dose-dependently increased miR-199a expression in HCC cells. This means dauricine suppressed glycolysis through miR-199aHK2/PKM2, increasing the sensitivity of hepatoma cells to chemotherapeutic drugs [176].
3.1.6. Cepharanthine
Cepharanthine is an alkaloid extracted from Stephania with the formula C37H38N5O6, and a molecular weight of 606.71 g/mol. It exhibits multiple antitumor pharmacological effects, such as the induction of apoptosis, inhibition of angiogenesis, metastasis, and reversal of multidrug resistance [177,178,179]. Cepharanthine can induce autophagy by inhibiting Akt/mTOR signaling in human breast cancer MCF-7 and MDA-MB-231 cells, and stimulating AMPK-mTOR-dependent autophagy to induce apoptosis-tolerant cell death [180]. Furthermore, cepharanthine can induce apoptosis not only by upregulating the expression of initiator caspases such as caspase-8 and 9, but also by increasing the expression of effector caspases such as caspase-3 and 6 through caspase cascade regulation. Cepharanthine also activates MAPK and modifies PI3K/Akt/mTOR signaling pathway to show cytotoxic effects on glucocorticoid-resistant leukemic Jurkat T cells by increasing the phosphorylation of JNK and p38 [154]. It has been shown that cepharanthine hydrochloride can reverse P-gp-mediated multidrug resistance in A2780/paclitaxel cells. Furthermore, the mechanism by which galentin hydrochloride induced the reversal of multidrug resistance in human ovarian cancer may be related to the inhibition of the PI3K/Akt signaling pathway [181]. Mutant p53 is a major reason for the ineffectiveness of many anticancer drugs. It has been shown that cepharanthine alone in vitro and in vivo or in combination with 5-FU effectively controlled the growth of HT-29 human colorectal cancer cells carrying mutant p53. Cepharanthine with 5-FU also induced apoptosis and significantly upregulated BAK and cleaved PARP expression in tumor tissues. At the same time, cepharanthine prevented 5-FU-induced breast cancer drug-resistant protein and multidrug resistance-associated protein 1 (MRP1) expression [182].
3.1.7. Solanine
Solanine is a type of toxic steroidal glycoside alkaloid. It is a toxic substance produced by potatoes after germination, and when they become green or ulcerated. It can exert anticancer effects by inhibiting cell proliferation, inducing apoptosis, blocking the cell cycle, inducing autophagy, enhancing chemoradiation, inhibiting epithelial–mesenchymal transformation, inhibiting tumor metastasis, and inhibiting angiogenesis, such as in liver cancer, breast cancer, liver cancer, pancreatic cancer, and colorectal cancer [183]. Studies have shown that serine inhibits the growth of transplanted hepatocellular carcinoma (HCC) in mice and reduces the CD4+CD25+Foxp3+ proportion of Treg and the expression levels of Foxp3 and TGFβ mRNA, and enhanced the body’s antitumor immune response by inhibiting the TGF/SMAD signaling pathway [184]. Furthermore, it reduced the expression and activity of Akt and ER, and this inhibitory effect may contribute to inactivated PI3K/Akt and ER signaling pathways in human endometrial cancer cells [185]. In addition, solanine enhanced the sensitivity of esophageal cancer cells to chemotherapy through the miR-138/survivin pathway [186].
3.2. Flavonoid
3.2.1. Quercetin
Quercetin is a flavonoid, widely found in the flowers, leaves, and fruits of many plants, such as onions, apples, and hawthorn. The formula is C15H10O7 with a molecular weight of 302.24 g/mol. Quercetin can exert anticancer effects in various ways, such as through its antioxidant, antiproliferative, cell cycle arrest, apoptosis induction, and inhibition of migration effects [187]. It was found that quercetin was able to reverse drug resistance in paclitaxel-resistant prostate cancer cells in vitro by reversing the activation of the androgen receptor and PI3K/Akt signaling pathway in prostate cancer treatment [28]. Quercetin could significantly increase PI3K/AKT/mTOR axis and endurance gemcitabine-induced cytotoxicity in MIA Paca-2 and MIA Paca-2GEMR cells [188]. In the treatment of colon cancer, when quercetin was combined with ionizing radiation (IR) therapy, colon cancer stem cells as target cells can have antitumor effects by inhibiting Notch-1 signaling [189]. Alternating consumption of quercetin and beta-glucan has been reported to reduce mortality in a colon cancer mouse model [190]. In addition, the combination of quercetin and heparin-bound cytokine (MK) can significantly promote apoptosis by downregulating the expression of PI3K/PTEN, MAPK, and NF-B signaling, leading to G1 cell cycle arrest and the inhibition of PC3 and CD44+/CD133+ cell migration, thus effectively eliminating cancer and tumor CSCs. Furthermore, the downregulation of MK expression with quercetin binding limits CD44+/CD133+ migration and the development progression of tumor cells as well as PCa cells [191]. It has been shown that quercetin can enhance the efficacy of the chemotherapeutic agents BEL/5-FU with ABCB1, ABCC1, and ABCC2 overexpression by blocking FZD7/β-catenin signaling. Thus, quercetin can act as an MDR reversal agent mediated by ABCB1 or ABCC1/2 [192].
3.2.2. Curcumin
Curcumin is an Indian dietary polyphenol derived from turmeric roots, which includes three major bioactive components, namely curcumin, demethoxy curcumin (DMC), and didemethoxycurcumin at a ratio of 77:17:3. The molecular formula is C21H20O6 with a molecular weight of 368.39 g/mol. Curcumin is a highly potential alternative therapy for lung cancer with fewer side effects. It can exert its anticancer effects in lung cancer by modulating various molecular targets, signaling pathways, epigenetic alterations, and microRNA expression. However, the bioavailability of curcumin is relatively low, and its clinical application is limited to some extent [193]. In HCC treatment, curcumin enhanced the chemosensitivity of HCC cells to 5-FU in vitro, increased the apoptosis rate, arrested the cell cycle in the G2/M phase, and blocked PI3k/AKT/mTOR signaling by inhibiting the phosphorylation of PI3k and its downstream protein kinase. Curcumin also significantly sensitized H22 cells to 5-FU, enabling it to inhibit tumor growth in vivo [194]. In lung cancer treatment, when the PI3K-Akt pathway is inactivated, curcumin (DMC) enhanced the sensitivity of drug-resistant lung cancer cells to cisplatin by downregulating the expression of thymidine phosphorylase and RCC1 [195]. In addition, Didemethoxylated curcumin may increase the sensitivity of cisplatin-resistant lung cancer cells to chemotherapy by inhibiting CA916798 (an over-expressed MDR protein) and PI3K/AKT/mTOR signaling, thus exerting antitumor activity and reversing MDR [196]. Studies have shown that curcumin can induce apoptosis by reducing Akt and mTOR phosphorylation, thereby inhibiting the PI3K/Akt/mTOR pathway in A549 and H1299 NSCLC cells [197,198,199]. In colorectal cancer treatment, curcumin can reverse oxaliplatin resistance by inhibiting TGF-β/Smad2/3 signaling in vitro and in vivo [200]. Furthermore, HER2 is a transmembrane receptor with tyrosine kinase activity associated with cell proliferation, survival, metastasis, and drug resistance, and its overexpression is frequently seen in various types of human cancers, such as bladder cancer. DMC can induce apoptosis in bladder cancer cells with HER2 overexpression, via the degradation of HER2 and inhibition of the PI3K/Akt pathway [201]. In glioma therapy, the miR-145/SOX2-Wnt/β-catenin axis plays a key role in DMC-mediated glioma stem cell (GSC) inhibition, and upregulation of miR-145 can effectively enhance the effect of DMC against GSC; therefore, this may be a new therapeutic target for GSC resistance [202].
3.3. Terpene
3.3.1. Ginsenoside
Ginsenoside is a triterpenoid isolated from ginseng that can exert antitumor effects through various mechanisms, including the induction of apoptosis, inhibition of proliferation, metastasis, angiogenesis, and activation of immunity [203]. The use of ginsenoside Rg3 may contribute to reducing toxicity and improving chemosensitivity in cancer combination therapy [204]. It was found that ginsenoside Rg3 reduced cisplatin resistance by upregulating miR-429 to inhibit the SOX2 and PI3K/Akt/mTOR signaling axes in gastric cancer treatment [30]. In pancreatic cancer treatment, ginsenoside Rg3 regulated the survival of pancreatic cancer cells via the PI3K/Akt/mTOR pathway [205]. In osteosarcoma treatment, ginsenoside Rg3 inhibited the proliferation and migration of osteosarcoma cells and induced apoptosis by reducing the protein expression of Bcl 2 and the PI3K/AKT pathway [206]. In glioblastoma treatment, ginsenoside Rg3 inhibited O6-methylguanine-DNA-methyltransferase (MGMT) by regulating the Wnt/β-catenin pathway and significantly enhanced the sensitivity of glioma to TMZ chemotherapy. Additionally, we found that 20(S)-ginsenoside-Rg3 significantly inhibited the epithelial–stromal transition process in glioma cells [207]. It was found that in taxol-resistant human nasopharyngeal carcinoma cells, ginsenosides Rg1 exerted antitumor activity by activating autophagic cell death, apoptosis, endogenous ROS production, S phase cell cycle arrest, and inhibition of the m-TOR/PI3K/AKT signaling pathway [208]. In addition, ginsenoside Rk1 reduced cell viability and colony formation, and triggered lactate dehydrogenase (LDH) leakage, G0/G1 phase arrest, and apoptosis. Furthermore, the ROS/PI3K/Akt signaling pathway was involved in Rk1-induced MDA-MB-231 cell death. These results suggest that ginsenoside Rk1 may be a potential antitumor agent for triple-negative breast cancer [209].
3.3.2. Soil Bastard Saponin
Soil bastard saponin (TBM) is a terpenoid isolated from Porcini, whose main pharmacological active components are triterpenoid saponins, including TBM-I, II, and III. TBMs can inhibit cell growth and proliferation through various signaling pathways, such as ROS/cytochrome C/caspase-3, ROS/MAPK, MAPK-JNK, MAPK/p38, PI3K/Akt/mTOR, p53/MDM2, NF-κB, VEGF-A/VEGFR-2/ERK, MEK/ERK, Wnt/β-catenin, CXCL12-CXCR4, and Akt-mTOR-eEF-2K to induce cell differentiation, apoptosis, and autophagy, and inhibit inflammation, angiogenesis, invasion, and metastasis. Combination treatment with earth shellfish mother saponin has shown curative effects for drug-resistant colorectal cancer cells (CRC) [210,211].
4. Summary
Most of the research on cancer drug-resistance mechanisms is displayed in experimental animals. However, drug resistance at the cell level has not been confirmed in the clinic. Tumor multi-drug resistance pathogenesis is complex, so the actual clinical significance of many mechanisms is unclear. The best means to study tumor drug resistance is to obtain human tumor tissue for research. In recent years, high-throughput technology in China has made great progress in the field of biomedicine, using high-precision technology, combined with modern pharmacology, pharmacodynamics, molecular biology, and other new technology, from the single-cell level of tumor molecular mechanisms.
However, natural products show some problems, such as poor solubility, poor permeability, low bioavailability, instability in biological milieu, and extensive first-pass metabolism in drug delivery systems. Some delivery strategies, exosomes or nanotechnology, have attempted to overcome these limitations, rediscovering new benefits associated with these natural products. Exosomes or nanotechnology can certainly enhance the pharmacokinetics and therapeutic index of natural active natural products and improve their performance in therapy.
Chinese medicine application has a long history in China, with rich resources and pharmacological effects. We reviewed the signaling pathways involved in the development of tumor drug resistance, and their specific signaling pathway inhibitors derived from natural products (Figure 1). This provides a theoretical basis for drug resistance and expanding the range of candidate compounds to all natural products, including traditional Chinese medicine (TCM) extracts, TCM compound preparations, and derivatives, in order to find new and efficient tumor multidrug resistance treatments using a new direction, bringing a new dawn.
Figure 1.
Molecular mechanisms of tumor drug resistance induced by EGFR, PI3K/Akt, Wnt/β-catenin, TGF-β, and combinations of these signaling pathway inhibitors’ derivatives from natural products to overcome tumor resistance. (A) EGFR signaling pathway and its inhibitors’ derivatives from natural products. (B) PI3K/Akt signaling pathway and its inhibitors derivatives from natural products. (C) Wnt/β-catenin signaling pathway and its inhibitors’ derivatives from natural products. (D)TGF-β signaling pathway and its inhibitors’ derivatives from natural products. (E) Notch signaling pathway and its inhibitors’ derivatives from natural products.
Author Contributions
C.X. conceived and designed the manuscript. C.X. and C.Y. were involved in writing and revision of the manuscript. Z.M., C.L., S.Y. and Y.C. participated in drawing the pictures and review the manuscript. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds curcumin are available from the authors.
Funding Statement
This work was supported by grants from the Science and Technology Bureau of Foshan (No. FS0AA-KJ218-1301-0008 and no. FS0AA-KJ819-4901-0082).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Deng J., Zhou M., Liao T., Kuang W., Xia H., Yin Z., Tan Q., Li Y., Song S., Zhou E., et al. Targeting Cancer Cell Ferroptosis to Reverse Immune Checkpoint Inhibitor Therapy Resistance. Front. Cell Dev. Biol. 2022;10:818453. doi: 10.3389/fcell.2022.818453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dasari S., Njiki S., Mbemi A., Yedjou C.G., Tchounwou P.B. Pharmacological Effects of Cisplatin Combination with Natural Products in Cancer Chemotherapy. Int. J. Mol. Sci. 2022;23:1532. doi: 10.3390/ijms23031532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang W., Wang Y., Xu S., Qiao H., Cheng H., Wang L., Liu S., Tian Q., Wang R., Wang H., et al. Design, synthesis, and tumor drug resistance reversal activity of novel hederagenin derivatives modified by nitrogen-containing heterocycles. Eur. J. Med. Chem. 2022;232:114207. doi: 10.1016/j.ejmech.2022.114207. [DOI] [PubMed] [Google Scholar]
- 4.Wang X., Zhou Y., Ding K. Roles of exosomes in cancer chemotherapy resistance, progression, metastasis and immunity, and their clinical applications (Review) Int. J. Oncol. 2021;59:1–18. doi: 10.3892/ijo.2021.5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y., Yu C., Deng W. Roles and mechanisms of adipokines in drug resistance of tumor cells. Eur. J. Pharmacol. 2021;899:174019. doi: 10.1016/j.ejphar.2021.174019. [DOI] [PubMed] [Google Scholar]
- 6.Morales M., de la Fuente M., Martín-Folgar R. BPA and its analogues (BPS and BPF) modify the expression of genes involved in the endocrine pathway and apoptosis and a multi drug resistance gene of the aquatic midge Chironomus riparius (Diptera) Environ. Pollut. 2020;265:114806. doi: 10.1016/j.envpol.2020.114806. [DOI] [PubMed] [Google Scholar]
- 7.van Waardenburg R., Yang E.S. Targeting DNA repair pathways to overcome cancer drug resistance. Cancer Drug Resist. 2021;4:837–841. doi: 10.20517/cdr.2021.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jabbarzadeh Kaboli P., Salimian F., Aghapour S., Xiang S., Zhao Q., Li M., Wu X., Du F., Zhao Y., Shen J., et al. Akt-targeted therapy as a promising strategy to overcome drug resistance in breast cancer—A comprehensive review from chemotherapy to immunotherapy. Pharmacol. Res. 2020;156:104806. doi: 10.1016/j.phrs.2020.104806. [DOI] [PubMed] [Google Scholar]
- 9.Gomes B.C., Honrado M., Armada A., Viveiros M., Rueff J., Rodrigues A.S. ABC Efflux Transporters and the Circuitry of miRNAs: Kinetics of Expression in Cancer Drug Resistance. Int. J. Mol. Sci. 2020;21:2985. doi: 10.3390/ijms21082985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y., Wang Z., Ajani J.A., Song S. Drug resistance and Cancer stem cells. Cell Commun. Signal. 2021;19:19. doi: 10.1186/s12964-020-00627-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan G., Liu Y., Shang L., Zhou F., Yang S. EMT-associated microRNAs and their roles in cancer stemness and drug resistance. Cancer Commun. 2021;41:199–217. doi: 10.1002/cac2.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cascone T., Morelli M.P., Ciardiello F. Small molecule epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors in non-small cell lung cancer. Ann. Oncol. 2006;17((Suppl. 2)):ii46–ii48. doi: 10.1093/annonc/mdj921. [DOI] [PubMed] [Google Scholar]
- 13.Wang K., Xu K., Leng X., Han Y., Fang Q. miRNA-9 Inhibits Proliferation and Migration of Lung Squamous Cell Carcinoma Cells by Regulating NRSF/EGFR. Technol. Cancer Res. Treat. 2020;19:1533033820945807. doi: 10.1177/1533033820945807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mizuuchi H., Suda K., Murakami I., Sakai K., Sato K., Kobayashi Y., Shimoji M., Chiba M., Sesumi Y., Tomizawa K., et al. Oncogene swap as a novel mechanism of acquired resistance to epidermal growth factor receptor-tyrosine kinase inhibitor in lung cancer. Cancer Sci. 2016;107:461–468. doi: 10.1111/cas.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pietrobono S., Gagliardi S., Stecca B. Non-canonical Hedgehog Signaling Pathway in Cancer: Activation of GLI Transcription Factors Beyond Smoothened. Front. Genet. 2019;10:556. doi: 10.3389/fgene.2019.00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.An S.M., Lei H.M., Ding X.P., Sun F., Zhang C., Tang Y.B., Chen H.Z., Shen Y., Zhu L. Interleukin-6 identified as an important factor in hypoxia- and aldehyde dehydrogenase-based gefitinib adaptive resistance in non-small cell lung cancer cells. Oncol. Lett. 2017;14:3445–3454. doi: 10.3892/ol.2017.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jing C., Jin Y.H., You Z., Qiong Q., Jun Z. Prognostic value of amphiregulin and epiregulin mRNA expression in metastatic colorectal cancer patients. Oncotarget. 2016;7:55890–55899. doi: 10.18632/oncotarget.10151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eroglu B., Pang J., Jin X., Xi C., Moskophidis D., Mivechi N.F. HSF1-Mediated Control of Cellular Energy Metabolism and mTORC1 Activation Drive Acute T-Cell Lymphoblastic Leukemia Progression. Mol. Cancer Res. 2020;18:463–476. doi: 10.1158/1541-7786.MCR-19-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Izumi D., Toden S., Ureta E., Ishimoto T., Baba H., Goel A. TIAM1 promotes chemoresistance and tumor invasiveness in colorectal cancer. Cell Death Dis. 2019;10:267. doi: 10.1038/s41419-019-1493-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rapp J., Jaromi L., Kvell K., Miskei G., Pongracz J.E. WNT signaling—lung cancer is no exception. Respir. Res. 2017;18:167. doi: 10.1186/s12931-017-0650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sosa Iglesias V., Giuranno L., Dubois L.J., Theys J., Vooijs M. Drug Resistance in Non-Small Cell Lung Cancer: A Potential for NOTCH Targeting. Front. Oncol. 2018;8:267. doi: 10.3389/fonc.2018.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y.W., Zhang W., Ma R. Bioinformatic identification of chemoresistance-associated microRNAs in breast cancer based on microarray data. Oncol. Rep. 2018;39:1003–1010. doi: 10.3892/or.2018.6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan R., Hou Y., Sun W., Yu J., Liu X., Niu Y., Lu J.J., Chen X. Natural products to prevent drug resistance in cancer chemotherapy: A review. Ann. N. Y. Acad. Sci. 2017;1401:19–27. doi: 10.1111/nyas.13387. [DOI] [PubMed] [Google Scholar]
- 24.Wu L.M., Liao X.Z., Zhang Y., He Z.R., Nie S.Q., Ke B., Shi L., Zhao J.F., Chen W.H. Parthenolide Augments the Chemosensitivity of Non-small-Cell Lung Cancer to Cisplatin via the PI3K/AKT Signaling Pathway. Front. Cell Dev. Biol. 2020;8:610097. doi: 10.3389/fcell.2020.610097. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Talib W.H., Alsayed A.R., Barakat M., Abu-Taha M.I., Mahmod A.I. Targeting Drug Chemo-Resistance in Cancer Using Natural Products. Biomedicines. 2021;9:1353. doi: 10.3390/biomedicines9101353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao B., Li B., Liu Q., Gao F., Zhang Z., Bai H., Wang Y. Effects of matrine on the proliferation and apoptosis of vincristine-resistant retinoblastoma cells. Exp. Ther. Med. 2020;20:2838–2844. doi: 10.3892/etm.2020.8992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liao D., Zhang W., Gupta P., Lei Z.N., Wang J.Q., Cai C.Y., Vera A.A., Zhang L., Chen Z.S., Yang D.H. Tetrandrine Interaction with ABCB1 Reverses Multidrug Resistance in Cancer Cells Through Competition with Anti-Cancer Drugs Followed by Downregulation of ABCB1 Expression. Molecules. 2019;24:4383. doi: 10.3390/molecules24234383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu X., Yang F., Chen D., Zhao Q., Chen D., Ping H., Xing N. Quercetin reverses docetaxel resistance in prostate cancer via androgen receptor and PI3K/Akt signaling pathways. Int. J. Biol. Sci. 2020;16:1121–1134. doi: 10.7150/ijbs.41686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su J., Zhang F., Li X., Liu Z. Osthole promotes the suppressive effects of cisplatin on NRF2 expression to prevent drug-resistant cervical cancer progression. Biochem. Biophys. Res. Commun. 2019;514:510–517. doi: 10.1016/j.bbrc.2019.04.021. [DOI] [PubMed] [Google Scholar]
- 30.Wang X., He R., Geng L., Yuan J., Fan H. Ginsenoside Rg3 Alleviates Cisplatin Resistance of Gastric Cancer Cells Through Inhibiting SOX2 and the PI3K/Akt/mTOR Signaling Axis by Up-Regulating miR-429. Front. Genet. 2022;13:823182. doi: 10.3389/fgene.2022.823182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dou Y., Jiang X., Xie H., He J., Xiao S. The Jun N-terminal kinases signaling pathway plays a “seesaw” role in ovarian carcinoma: A molecular aspect. J. Ovarian Res. 2019;12:99. doi: 10.1186/s13048-019-0573-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao L., Ouyang Y., Li R., Zhang X., Gao X., Lin S., Wang X. Icaritin Inhibits Migration and Invasion of Human Ovarian Cancer Cells via the Akt/mTOR Signaling Pathway. Front. Oncol. 2022;12:843489. doi: 10.3389/fonc.2022.843489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carcereny E., Morán T., Capdevila L., Cros S., Vilà L., de Los Llanos Gil M., Remón J., Rosell R. The epidermal growth factor receptor (EGRF) in lung cancer. Transl. Respir. Med. 2015;3:1. doi: 10.1186/s40247-015-0013-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu X., Shen X., Qu J., Straubinger R.M., Jusko W.J. Proteomic Analysis of Combined Gemcitabine and Birinapant in Pancreatic Cancer Cells. Front. Pharm. 2018;9:84. doi: 10.3389/fphar.2018.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang L., Sui M., Wang X. miR-338-3p suppresses the malignancy of T-cell lymphoblastic lymphoma by downregulating HOXA. Mol. Med. Rep. 2019;20:2127–2134. doi: 10.3892/mmr.2019.10451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maleki Dana P., Sadoughi F., Asemi Z., Yousefi B. The role of polyphenols in overcoming cancer drug resistance: A comprehensive review. Cell. Mol. Biol. Lett. 2022;27:1. doi: 10.1186/s11658-021-00301-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zaki M., Hairat S., Aazam E.S. Scope of organometallic compounds based on transition metal-arene systems as anticancer agents: Starting from the classical paradigm to targeting multiple strategies. RSC Adv. 2019;9:3239–3278. doi: 10.1039/C8RA07926A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmad R., Dhawan P., Singh A.B. Cancer Stem Cell and Gastrointestinal Cancer: Current Status, Targeted Therapy and Future Implications. Biochem. Pharmacol. Open Access. 2016;5:202. doi: 10.4172/2167-0501.1000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Podar K., Tai Y.T., Hideshima T., Vallet S., Richardson P.G., Anderson K.C. Emerging therapies for multiple myeloma. Expert Opin. Emerg. Drugs. 2009;14:99–127. doi: 10.1517/14728210802676278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Passaro A., Jänne P.A., Mok T., Peters S. Overcoming therapy resistance in EGFR-mutant lung cancer. Nat. Cancer. 2021;2:377–391. doi: 10.1038/s43018-021-00195-8. [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto Y., Kodama K., Maniwa T., Takeda M. Surgical resection of advanced non-small cell lung cancer after a response to EGFR-TKI: Presentation of two cases and a literature review. J. Cardiothorac. Surg. 2017;12:98. doi: 10.1186/s13019-017-0668-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ikeuchi H., Hirose T., Ikegami M., Takamochi K., Suzuki K., Mano H., Kohsaka S. Preclinical assessment of combination therapy of EGFR tyrosine kinase inhibitors in a highly heterogeneous tumor model. Oncogene. 2022;41:2470–2479. doi: 10.1038/s41388-022-02263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao Y., Wang H., He C. Drug resistance of targeted therapy for advanced non-small cell lung cancer harbored EGFR mutation: From mechanism analysis to clinical strategy. J. Cancer Res. Clin. Oncol. 2021;147:3653–3664. doi: 10.1007/s00432-021-03828-8. [DOI] [PubMed] [Google Scholar]
- 44.Iommelli F., De Rosa V., Terlizzi C., Fonti R., Camerlingo R., Stoppelli M.P., Stewart C.A., Byers L.A., Piwnica-Worms D., Del Vecchio S. A Reversible Shift of Driver Dependence from EGFR to Notch1 in Non-Small Cell Lung Cancer as a Cause of Resistance to Tyrosine Kinase Inhibitors. Cancers. 2021;13:2022. doi: 10.3390/cancers13092022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee T.F., Tseng Y.C., Chang W.C., Chen Y.C., Kao Y.R., Chou T.Y., Ho C.C., Wu C.W. YAP1 is essential for tumor growth and is a potential therapeutic target for EGFR-dependent lung adenocarcinomas. Oncotarget. 2017;8:89539–89551. doi: 10.18632/oncotarget.19647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harvey R.D., Adams V.R., Beardslee T., Medina P. Afatinib for the treatment of EGFR mutation-positive NSCLC: A review of clinical findings. J. Oncol. Pharm. Pract. 2020;26:1461–1474. doi: 10.1177/1078155220931926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen Y.F., Yuan A., Cho K.H., Lu Y.C., Kuo M.Y., Chen J.H., Chang Y.C. Functional evaluation of therapeutic response of HCC827 lung cancer to bevacizumab and erlotinib targeted therapy using dynamic contrast-enhanced and diffusion-weighted MRI. PLoS ONE. 2017;12:e0187824. doi: 10.1371/journal.pone.0187824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang J., Sun N., Lee Y.T., Ni Y., Koochekpour R., Zhu Y., Tseng H.R., Wang S., Jiang L., Zhu H. A circulating tumor cell-based digital assay for the detection of EGFR T790M mutation in advanced non-small cell lung cancer. J. Mater. Chem. B. 2020;8:5636–5644. doi: 10.1039/D0TB00589D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahmadzada T., Kao S., Reid G., Boyer M., Mahar A., Cooper W.A. An Update on Predictive Biomarkers for Treatment Selection in Non-Small Cell Lung Cancer. J. Clin. Med. 2018;7:153. doi: 10.3390/jcm7060153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hondelink L.M., Jebbink M., von der Thüsen J.H., Cohen D., Dubbink H.J., Paats M.S., Dingemans A.C., de Langen A.J., Boelens M.C., Smit E.F., et al. Real-World Approach for Molecular Analysis of Acquired EGFR Tyrosine Kinase Inhibitor Resistance Mechanisms in NSCLC. JTO Clin. Res. Rep. 2021;2:100252. doi: 10.1016/j.jtocrr.2021.100252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keretsu S., Ghosh S., Cho S.J. Molecular Modeling Study of c-KIT/PDGFRα Dual Inhibitors for the Treatment of Gastrointestinal Stromal Tumors. Int. J. Mol. Sci. 2020;21:8232. doi: 10.3390/ijms21218232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang H.D., Jiang L.H., Zhong S.L., Li J., Sun D.W., Hou J.C., Wang D.D., Zhou S.Y., Tang J.H. The role of long non-coding RNAs in drug resistance of cancer. Clin. Genet. 2021;99:84–92. doi: 10.1111/cge.13800. [DOI] [PubMed] [Google Scholar]
- 53.Dutta R., Mahato R.I. Recent advances in hepatocellular carcinoma therapy. Pharmacol. Ther. 2017;173:106–117. doi: 10.1016/j.pharmthera.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cabanos H.F., Hata A.N. Emerging Insights into Targeted Therapy-Tolerant Persister Cells in Cancer. Cancers. 2021;13:2666. doi: 10.3390/cancers13112666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mou L., Tian X., Zhou B., Zhan Y., Chen J., Lu Y., Deng J., Deng Y., Wu Z., Li Q., et al. Improving Outcomes of Tyrosine Kinase Inhibitors in Hepatocellular Carcinoma: New Data and Ongoing Trials. Front. Oncol. 2021;11:752725. doi: 10.3389/fonc.2021.752725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hardy-Werbin M., Del Rey-Vergara R., Galindo-Campos M.A., Moliner L., Arriola E. MET Inhibitors in Small Cell Lung Cancer: From the Bench to the Bedside. Cancers. 2019;11:1404. doi: 10.3390/cancers11101404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang W.C., Jang T.H., Tung S.L., Yen T.C., Chan S.H., Wang L.H. A novel miR-365-3p/EHF/keratin 16 axis promotes oral squamous cell carcinoma metastasis, cancer stemness and drug resistance via enhancing β5-integrin/c-met signaling pathway. J. Exp. Clin. Cancer Res. 2019;38:89. doi: 10.1186/s13046-019-1091-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Deying W., Feng G., Shumei L., Hui Z., Ming L., Hongqing W. CAF-derived HGF promotes cell proliferation and drug resistance by up-regulating the c-Met/PI3K/Akt and GRP78 signalling in ovarian cancer cells. Biosci. Rep. 2017;37:BSR20160470. doi: 10.1042/BSR20160470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hou C., Lu L., Liu Z., Lian Y., Xiao J. Resveratrol reduces drug resistance of SCLC cells by suppressing the inflammatory microenvironment and the STAT3/VEGF pathway. FEBS Open Bio. 2021;11:2256–2265. doi: 10.1002/2211-5463.13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee Y., Wang Y., James M., Jeong J.H., You M. Inhibition of IGF1R signaling abrogates resistance to afatinib (BIBW2992) in EGFR T790M mutant lung cancer cells. Mol. Carcinog. 2016;55:991–1001. doi: 10.1002/mc.22342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Manabe T., Yasuda H., Terai H., Kagiwada H., Hamamoto J., Ebisudani T., Kobayashi K., Masuzawa K., Ikemura S., Kawada I., et al. IGF2 Autocrine-Mediated IGF1R Activation Is a Clinically Relevant Mechanism of Osimertinib Resistance in Lung Cancer. Mol. Cancer Res. 2020;18:549–559. doi: 10.1158/1541-7786.MCR-19-0956. [DOI] [PubMed] [Google Scholar]
- 62.Liu Z., Ma L., Sun Y., Yu W., Wang X. Targeting STAT3 signaling overcomes gefitinib resistance in non-small cell lung cancer. Cell Death Dis. 2021;12:561. doi: 10.1038/s41419-021-03844-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ding Y., Zhen Z., Nisar M.A., Ali F., Din R.U., Khan M., Mughal T.A., Alam G., Liu L., Saleem M.Z. Sesquiterpene Lactones Attenuate Paclitaxel Resistance Via Inhibiting MALAT1/STAT3/ FUT4 Axis and P-Glycoprotein Transporters in Lung Cancer Cells. Front. Pharm. 2022;13:795613. doi: 10.3389/fphar.2022.795613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qiu Y., Wang Y., Chai Z., Ni D., Li X., Pu J., Chen J., Zhang J., Lu S., Lv C., et al. Targeting RAS phosphorylation in cancer therapy: Mechanisms and modulators. Acta Pharm. Sin. B. 2021;11:3433–3446. doi: 10.1016/j.apsb.2021.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alvisi M.F., Ganzinelli M., Linardou H., Caiola E., Lo Russo G., Cecere F.L., Bettini A.C., Psyrri A., Milella M., Rulli E., et al. Predicting the Role of DNA Polymerase β Alone or with KRAS Mutations in Advanced NSCLC Patients Receiving Platinum-Based Chemotherapy. J. Clin. Med. 2020;9:2438. doi: 10.3390/jcm9082438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shi Y., Fan S., Wu M., Zuo Z., Li X., Jiang L., Shen Q., Xu P., Zeng L., Zhou Y., et al. YTHDF1 links hypoxia adaptation and non-small cell lung cancer progression. Nat. Commun. 2019;10:4892. doi: 10.1038/s41467-019-12801-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li X., Fang X., Li S., Zhang W., Yang N., Cui Y., Huang H., Cai R., Lin X., Fu X., et al. A pharmacokinetic and safety study of a fixed oral dose of enzastaurin HCl in native Chinese patients with refractory solid tumors and lymphoma. Oncotarget. 2016;7:18585–18593. doi: 10.18632/oncotarget.7875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li X., Zhao Z., Yi S., Ma L., Li M., Liu M., Zhang Y., Liu G. Nuclear Klf4 accumulation is associated with cetuximab drug-resistance and predicts poor prognosis of nasopharyngeal carcinoma. J. Transl. Med. 2018;16:183. doi: 10.1186/s12967-018-1561-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Verougstraete N., Stove V., Verstraete A.G., Stove C.P. Therapeutic Drug Monitoring of Tyrosine Kinase Inhibitors Using Dried Blood Microsamples. Front. Oncol. 2022;12:821807. doi: 10.3389/fonc.2022.821807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yamaoka T., Kusumoto S., Ando K., Ohba M., Ohmori T. Receptor Tyrosine Kinase-Targeted Cancer Therapy. Int. J. Mol. Sci. 2018;19:3491. doi: 10.3390/ijms19113491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McMahon C.M., Ferng T., Canaani J., Wang E.S., Morrissette J., Eastburn D.J., Pellegrino M., Durruthy-Durruthy R., Watt C.D., Asthana S., et al. Clonal Selection with RAS Pathway Activation Mediates Secondary Clinical Resistance to Selective FLT3 Inhibition in Acute Myeloid Leukemia. Cancer Discov. 2019;9:1050–1063. doi: 10.1158/2159-8290.CD-18-1453. [DOI] [PubMed] [Google Scholar]
- 72.Moore A.R., Rosenberg S.C., McCormick F., Malek S. RAS-targeted therapies: Is the undruggable drugged. Nat. Rev. Drug Discov. 2020;19:533–552. doi: 10.1038/s41573-020-0068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aikawa T., Togashi N., Iwanaga K., Okada H., Nishiya Y., Inoue S., Levis M.J., Isoyama T. Quizartinib, a selective FLT3 inhibitor, maintains antileukemic activity in preclinical models of RAS-mediated midostaurin-resistant acute myeloid leukemia cells. Oncotarget. 2020;11:943–955. doi: 10.18632/oncotarget.27489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang H., Savage S., Schultz A.R., Bottomly D., White L., Segerdell E., Wilmot B., McWeeney S.K., Eide C.A., Nechiporuk T., et al. Clinical resistance to crenolanib in acute myeloid leukemia due to diverse molecular mechanisms. Nat. Commun. 2019;10:244. doi: 10.1038/s41467-018-08263-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Levis M. Midostaurin approved for FLT3-mutated AML. Blood. 2017;129:3403–3406. doi: 10.1182/blood-2017-05-782292. [DOI] [PubMed] [Google Scholar]
- 76.Galanis A., Ma H., Rajkhowa T., Ramachandran A., Small D., Cortes J., Levis M. Crenolanib is a potent inhibitor of FLT3 with activity against resistance-conferring point mutants. Blood. 2014;123:94–100. doi: 10.1182/blood-2013-10-529313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rascio F., Spadaccino F., Rocchetti M.T., Castellano G., Stallone G., Netti G.S., Ranieri E. The Pathogenic Role of PI3K/AKT Pathway in Cancer Onset and Drug Resistance: An Updated Review. Cancers. 2021;13:3949. doi: 10.3390/cancers13163949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li L., Zhang S., Xie D., Chen H., Zheng X., Pan D. Dual inhibitor of PI3K and mTOR (NVP-BEZ235) augments the efficacy of fluorouracil on gastric cancer chemotherapy. Oncol. Targets. 2018;11:6111–6118. doi: 10.2147/OTT.S172957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Adams J.M., Cory S. The BCL-2 arbiters of apoptosis and their growing role as cancer targets. Cell Death Differ. 2018;25:27–36. doi: 10.1038/cdd.2017.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou B.G., Wei C.S., Zhang S., Zhang Z., Gao H.M. Matrine reversed multidrug resistance of breast cancer MCF-7/ADR cells through PI3K/AKT signaling pathway. J. Cell. Biochem. 2018;119:3885–3891. doi: 10.1002/jcb.26502. [DOI] [PubMed] [Google Scholar]
- 81.Eberle J. Countering TRAIL Resistance in Melanoma. Cancers. 2019;11:656. doi: 10.3390/cancers11050656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen D., Lin X., Zhang C., Liu Z., Chen Z., Li Z., Wang J., Li B., Hu Y., Dong B., et al. Dual PI3K/mTOR inhibitor BEZ235 as a promising therapeutic strategy against paclitaxel-resistant gastric cancer via targeting PI3K/Akt/mTOR pathway. Cell Death Dis. 2018;9:123. doi: 10.1038/s41419-017-0132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yu H.A., Suzawa K., Jordan E., Zehir A., Ni A., Kim R., Kris M.G., Hellmann M.D., Li B.T., Somwar R., et al. Concurrent Alterations in EGFR-Mutant Lung Cancers Associated with Resistance to EGFR Kinase Inhibitors and Characterization of MTOR as a Mediator of Resistance. Clin. Cancer Res. 2018;24:3108–3118. doi: 10.1158/1078-0432.CCR-17-2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Knuefermann C., Lu Y., Liu B., Jin W., Liang K., Wu L., Schmidt M., Mills G.B., Mendelsohn J., Fan Z. HER2/PI-3K/Akt activation leads to a multidrug resistance in human breast adenocarcinoma cells. Oncogene. 2003;22:3205–3212. doi: 10.1038/sj.onc.1206394. [DOI] [PubMed] [Google Scholar]
- 85.Luo H., Cong S., Dong J., Jin L., Jiang D., Wang X., Chen Q., Li F. Paired-related homeobox 1 overexpression promotes multidrug resistance via PTEN/PI3K/AKT signaling in MCF-7 breast cancer cells. Mol. Med. Rep. 2020;22:3183–3190. doi: 10.3892/mmr.2020.11414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Si W., Shen J., Zheng H., Fan W. The role and mechanisms of action of microRNAs in cancer drug resistance. Clin. Epigenetics. 2019;11:25. doi: 10.1186/s13148-018-0587-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xie M., Fu Z., Cao J., Liu Y., Wu J., Li Q., Chen Y. MicroRNA-132 and microRNA-212 mediate doxorubicin resistance by down-regulating the PTEN-AKT/NF-κB signaling pathway in breast cancer. Biomed. Pharmacother. 2018;102:286–294. doi: 10.1016/j.biopha.2018.03.088. [DOI] [PubMed] [Google Scholar]
- 88.Zhou Y., Huang Y., Cao X., Xu J., Zhang L., Wang J., Huang L., Huang S., Yuan L., Jia W., et al. WNT2 Promotes Cervical Carcinoma Metastasis and Induction of Epithelial-Mesenchymal Transition. PLoS ONE. 2016;11:e0160414. doi: 10.1371/journal.pone.0160414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mo Y., Wang Y., Zhang L., Yang L., Zhou M., Li X., Li Y., Li G., Zeng Z., Xiong W., et al. The role of Wnt signaling pathway in tumor metabolic reprogramming. J. Cancer. 2019;10:3789–3797. doi: 10.7150/jca.31166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dhanasekaran R., Bandoh S., Roberts L.R. Molecular pathogenesis of hepatocellular carcinoma and impact of therapeutic advances. F1000 Res. 2016;5:879. doi: 10.12688/f1000research.6946.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nagaraj A.B., Joseph P., Kovalenko O., Singh S., Armstrong A., Redline R., Resnick K., Zanotti K., Waggoner S., DiFeo A. Critical role of Wnt/β-catenin signaling in driving epithelial ovarian cancer platinum resistance. Oncotarget. 2015;6:23720–23734. doi: 10.18632/oncotarget.4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Barghout S.H., Zepeda N., Xu Z., Steed H., Lee C.H., Fu Y. Elevated β-catenin activity contributes to carboplatin resistance in A2780cp ovarian cancer cells. Biochem. Biophys. Res. Commun. 2015;468:173–178. doi: 10.1016/j.bbrc.2015.10.138. [DOI] [PubMed] [Google Scholar]
- 93.Dong S., Liang S., Cheng Z., Zhang X., Luo L., Li L., Zhang W., Li S., Xu Q., Zhong M., et al. ROS/PI3K/Akt and Wnt/β-catenin signalings activate HIF-1α-induced metabolic reprogramming to impart 5-fluorouracil resistance in colorectal cancer. J. Exp. Clin. Cancer Res. 2022;41:15. doi: 10.1186/s13046-021-02229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li H., Chen J., Liu J., Lai Y., Huang S., Zheng L., Fan N. CPT2 downregulation triggers stemness and oxaliplatin resistance in colorectal cancer via activating the ROS/Wnt/β-catenin-induced glycolytic metabolism. Exp. Cell Res. 2021;409:112892. doi: 10.1016/j.yexcr.2021.112892. [DOI] [PubMed] [Google Scholar]
- 95.Abreu de Oliveira W.A., Moens S., El Laithy Y., van der Veer B.K., Athanasouli P., Cortesi E.E., Baietti M.F., Koh K.P., Ventura J.J., Amant F., et al. Wnt/β-Catenin Inhibition Disrupts Carboplatin Resistance in Isogenic Models of Triple-Negative Breast Cancer. Front. Oncol. 2021;11:705384. doi: 10.3389/fonc.2021.705384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang Z., Cheng L., Li J., Farah E., Atallah N.M., Pascuzzi P.E., Gupta S., Liu X. Inhibition of the Wnt/β-Catenin Pathway Overcomes Resistance to Enzalutamide in Castration-Resistant Prostate Cancer. Cancer Res. 2018;78:3147–3162. doi: 10.1158/0008-5472.CAN-17-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Park Y.L., Kim H.P., Cho Y.W., Min D.W., Cheon S.K., Lim Y.J., Song S.H., Kim S.J., Han S.W., Park K.J., et al. Activation of WNT/β-catenin signaling results in resistance to a dual PI3K/mTOR inhibitor in colorectal cancer cells harboring PIK3CA mutations. Int. J. Cancer. 2019;144:389–401. doi: 10.1002/ijc.31662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Alrashed M.M., Alharbi H., Alshehry A.S., Ahmad M., Aloahd M.S. MiR-624-5p enhances NLRP3 augmented gemcitabine resistance via EMT/IL-1β/Wnt/β-catenin signaling pathway in ovarian cancer. J. Reprod. Immunol. 2022;150:103488. doi: 10.1016/j.jri.2022.103488. [DOI] [PubMed] [Google Scholar]
- 99.Zhan T., Chen X., Tian X., Han Z., Liu M., Zou Y., Huang S., Chen A., Cheng X., Deng J., et al. MiR-331-3p Links to Drug Resistance of Pancreatic Cancer Cells by Activating WNT/β-Catenin Signal via ST7L. Technol. Cancer Res. Treat. 2020;19:1533033820945801. doi: 10.1177/1533033820945801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Han H., Li Y., Qin W., Wang L., Yin H., Su B., Yuan X. miR-199b-3p contributes to acquired resistance to cetuximab in colorectal cancer by targeting CRIM1 via Wnt/β-catenin signaling. Cancer Cell Int. 2022;22:42. doi: 10.1186/s12935-022-02460-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hu W., Li M., Chen Y., Gu X. UBE2S promotes the progression and Olaparib resistance of ovarian cancer through Wnt/β-catenin signaling pathway. J. Ovarian Res. 2021;14:121. doi: 10.1186/s13048-021-00877-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xu J., Lv G., Xu B., Jiang B. Overexpression of UBE2M through Wnt/β-Catenin signaling is associated with poor prognosis and chemotherapy resistance in colorectal cancer. Transl. Cancer Res. 2020;9:5614–5625. doi: 10.21037/tcr-20-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang Z., Li Y., Ahmad A., Azmi A.S., Banerjee S., Kong D., Sarkar F.H. Targeting Notch signaling pathway to overcome drug resistance for cancer therapy. Biochim. Biophys. Acta. 2010;1806:258–267. doi: 10.1016/j.bbcan.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Weber S., Koschade S.E., Hoffmann C.M., Dubash T.D., Giessler K.M., Dieter S.M., Herbst F., Glimm H., Ball C.R. The notch target gene HEYL modulates metastasis forming capacity of colorectal cancer patient-derived spheroid cells in vivo. BMC Cancer. 2019;19:1181. doi: 10.1186/s12885-019-6396-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sun T., Zhang D., Wang Z., Zhao B., Li Y., Sun X., Liu J., Wang X., Sheng J. Inhibition of the notch signaling pathway overcomes resistance of cervical cancer cells to paclitaxel through retardation of the epithelial-mesenchymal transition process. Environ. Toxicol. 2021;36:1758–1764. doi: 10.1002/tox.23296. [DOI] [PubMed] [Google Scholar]
- 106.Felcher C.M., Bogni E.S., Kordon E.C. IL-6 Cytokine Family: A Putative Target for Breast Cancer Prevention and Treatment. Int. J. Mol. Sci. 2022;23:1809. doi: 10.3390/ijms23031809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.BeLow M., Osipo C. Notch Signaling in Breast Cancer: A Role in Drug Resistance. Cells. 2020;9:2204. doi: 10.3390/cells9102204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Farah E., Li C., Cheng L., Kong Y., Lanman N.A., Pascuzzi P., Lorenz G.R., Zhang Y., Ahmad N., Li L., et al. NOTCH signaling is activated in and contributes to resistance in enzalutamide-resistant prostate cancer cells. J. Biol. Chem. 2019;294:8543–8554. doi: 10.1074/jbc.RA118.006983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xue Y., Ning B., Liu H., Jia B. Construction of immune-related lncRNA signature to predict aggressiveness, immune landscape, and drug resistance of colon cancer. BMC Gastroenterol. 2022;22:127. doi: 10.1186/s12876-022-02200-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Guinney J., Dienstmann R., Wang X., de Reyniès A., Schlicker A., Soneson C., Marisa L., Roepman P., Nyamundanda G., Angelino P., et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015;21:1350–1356. doi: 10.1038/nm.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nieto M.A., Huang R.Y., Jackson R.A., Thiery J.P. EMT: 2016. Cell. 2016;166:21–45. doi: 10.1016/j.cell.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 112.Fischer K.R., Durrans A., Lee S., Sheng J., Li F., Wong S.T., Choi H., El Rayes T., Ryu S., Troeger J., et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature. 2015;527:472–476. doi: 10.1038/nature15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zheng X., Carstens J.L., Kim J., Scheible M., Kaye J., Sugimoto H., Wu C.C., LeBleu V.S., Kalluri R. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527:525–530. doi: 10.1038/nature16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang M., Zhang Y.Y., Chen Y., Wang J., Wang Q., Lu H. TGF-β Signaling and Resistance to Cancer Therapy. Front. Cell Dev. Biol. 2021;9:786728. doi: 10.3389/fcell.2021.786728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen L., Zhu Q., Lu L., Liu Y. MiR-132 inhibits migration and invasion and increases chemosensitivity of cisplatin-resistant oral squamous cell carcinoma cells via targeting TGF-β. Bioengineered. 2020;11:91–102. doi: 10.1080/21655979.2019.1710925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li H., Li J., Chen L., Qi S., Yu S., Weng Z., Hu Z., Zhou Q., Xin Z., Shi L., et al. HERC3-Mediated SMAD7 Ubiquitination Degradation Promotes Autophagy-Induced EMT and Chemoresistance in Glioblastoma. Clin. Cancer Res. 2019;25:3602–3616. doi: 10.1158/1078-0432.CCR-18-3791. [DOI] [PubMed] [Google Scholar]
- 117.Chen J., Ding Z.Y., Li S., Liu S., Xiao C., Li Z., Zhang B.X., Chen X.P., Yang X. Targeting transforming growth factor-β signaling for enhanced cancer chemotherapy. Theranostics. 2021;11:1345–1363. doi: 10.7150/thno.51383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brown J.A., Yonekubo Y., Hanson N., Sastre-Perona A., Basin A., Rytlewski J.A., Dolgalev I., Meehan S., Tsirigos A., Beronja S., et al. TGF-β-Induced Quiescence Mediates Chemoresistance of Tumor-Propagating Cells in Squamous Cell Carcinoma. Cell Stem. Cell. 2017;21:650–664.e8. doi: 10.1016/j.stem.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jalalirad M., Haddad T.C., Salisbury J.L., Radisky D., Zhang M., Schroeder M., Tuma A., Leof E., Carter J.M., Degnim A.C., et al. Aurora-A kinase oncogenic signaling mediates TGF-β-induced triple-negative breast cancer plasticity and chemoresistance. Oncogene. 2021;40:2509–2523. doi: 10.1038/s41388-021-01711-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kennedy L.B., Salama A. A review of cancer immunotherapy toxicity. CA Cancer J. Clin. 2020;70:86–104. doi: 10.3322/caac.21596. [DOI] [PubMed] [Google Scholar]
- 121.Batlle E., Massagué J. Transforming Growth Factor-β Signaling in Immunity and Cancer. Immunity. 2019;50:924–940. doi: 10.1016/j.immuni.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bouchard A., Collin B., Garrido C., Bellaye P.S., Kohli E. GARP: A Key Target to Evaluate Tumor Immunosuppressive Microenvironment. Biology. 2021;10:836. doi: 10.3390/biology10090836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.de Streel G., Bertrand C., Chalon N., Liénart S., Bricard O., Lecomte S., Devreux J., Gaignage M., De Boeck G., Mariën L., et al. Selective inhibition of TGF-β1 produced by GARP-expressing Tregs overcomes resistance to PD-1/PD-L1 blockade in cancer. Nat. Commun. 2020;11:4545. doi: 10.1038/s41467-020-17811-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li S., Liu M., Do M.H., Chou C., Stamatiades E.G., Nixon B.G., Shi W., Zhang X., Li P., Gao S., et al. Cancer immunotherapy via targeted TGF-β signalling blockade in TH cells. Nature. 2020;587:121–125. doi: 10.1038/s41586-020-2850-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu M., Kuo F., Capistrano K.J., Kang D., Nixon B.G., Shi W., Chou C., Do M.H., Stamatiades E.G., Gao S., et al. TGF-β suppresses type 2 immunity to cancer. Nature. 2020;587:115–120. doi: 10.1038/s41586-020-2836-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tang N., Cheng C., Zhang X., Qiao M., Li N., Mu W., Wei X.F., Han W., Wang H. TGF-β inhibition via CRISPR promotes the long-term efficacy of CAR T cells against solid tumors. JCI Insight. 2020;5:e133977. doi: 10.1172/jci.insight.133977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Martin C.J., Datta A., Littlefield C., Kalra A., Chapron C., Wawersik S., Dagbay K.B., Brueckner C.T., Nikiforov A., Danehy F.T., Jr., et al. Selective inhibition of TGFβ1 activation overcomes primary resistance to checkpoint blockade therapy by altering tumor immune landscape. Sci. Transl. Med. 2020;12:eaay8456. doi: 10.1126/scitranslmed.aay8456. [DOI] [PubMed] [Google Scholar]
- 128.Siewe N., Friedman A. TGF-β inhibition can overcome cancer primary resistance to PD-1 blockade: A mathematical model. PLoS ONE. 2021;16:e0252620. doi: 10.1371/journal.pone.0252620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang H., Chen L., Sun X., Yang Q., Wan L., Guo C. Matrine: A Promising Natural Product With Various Pharmacological Activities. Front. Pharm. 2020;11:588. doi: 10.3389/fphar.2020.00588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gu Y.Y., Chen M.H., May B.H., Liao X.Z., Liu J.H., Tao L.T., Man-Yuen Sze D., Zhang A.L., Mo S.L. Matrine induces apoptosis in multiple colorectal cancer cell lines in vitro and inhibits tumour growth with minimum side effects in vivo via Bcl-2 and caspase-3. Phytomedicine. 2018;51:214–225. doi: 10.1016/j.phymed.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 131.Rashid H.U., Xu Y., Muhammad Y., Wang L., Jiang J. Research advances on anticancer activities of matrine and its derivatives: An updated overview. Eur. J. Med. Chem. 2019;161:205–238. doi: 10.1016/j.ejmech.2018.10.037. [DOI] [PubMed] [Google Scholar]
- 132.Huang H., Wang Q., Du T., Lin C., Lai Y., Zhu D., Wu W., Ma X., Bai S., Li Z., et al. Matrine inhibits the progression of prostate cancer by promoting expression of GADD45B. Prostate. 2018;78:327–335. doi: 10.1002/pros.23469. [DOI] [PubMed] [Google Scholar]
- 133.Huang M., Xin W. Matrine inhibiting pancreatic cells epithelial-mesenchymal transition and invasion through ROS/NF-κB/MMPs pathway. Life Sci. 2018;192:55–61. doi: 10.1016/j.lfs.2017.11.024. [DOI] [PubMed] [Google Scholar]
- 134.Wei J., Liang Y., Wu L. Design, Synthesis, Molecular Docking, and Tumor Resistance Reversal Activity Evaluation of Matrine Derivative with Thiophene Structure. Molecules. 2021;26:417. doi: 10.3390/molecules26020417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xie W., Lu J., Lu Q., Wang X., Long H., Huang J., Guo Z. Matrine inhibits the proliferation and migration of lung cancer cells through regulation of the protein kinase B/glycogen synthase kinase-3β signaling pathways. Exp. Ther. Med. 2018;16:723–729. doi: 10.3892/etm.2018.6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Liao X.Z., Tao L.T., Liu J.H., Gu Y.Y., Xie J., Chen Y., Lin M.G., Liu T.L., Wang D.M., Guo H.Y., et al. Matrine combined with cisplatin synergistically inhibited urothelial bladder cancer cells via down-regulating VEGF/PI3K/Akt signaling pathway. Cancer Cell Int. 2017;17:124. doi: 10.1186/s12935-017-0495-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xiao X., Ao M., Xu F., Li X., Hu J., Wang Y., Li D., Zhu X., Xin C., Shi W. Effect of matrine against breast cancer by downregulating the vascular endothelial growth factor via the Wnt/β-catenin pathway. Oncol. Lett. 2018;15:1691–1697. doi: 10.3892/ol.2017.7519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liu Y., Qi Y., Bai Z.H., Ni C.X., Ren Q.H., Xu W.H., Xu J., Hu H.G., Qiu L., Li J.Z., et al. A novel matrine derivate inhibits differentiated human hepatoma cells and hepatic cancer stem-like cells by suppressing PI3K/AKT signaling pathways. Acta Pharmacol. Sin. 2017;38:120–132. doi: 10.1038/aps.2016.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang X., Hou G., Liu A., Xu H., Guan Y., Wu Y., Deng J., Cao X. Matrine inhibits the development and progression of ovarian cancer by repressing cancer associated phosphorylation signaling pathways. Cell Death Dis. 2019;10:770. doi: 10.1038/s41419-019-2013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang Z., Wang Y., Wang S., Meng X., Song F., Huo W., Zhang S., Chang J., Li J., Zheng B., et al. Coxsackievirus A6 Induces Cell Cycle Arrest in G0/G1 Phase for Viral Production. Front. Cell Infect. Microbiol. 2018;8:279. doi: 10.3389/fcimb.2018.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liu J., Guo Y., Cao J. Matrine triggers colon cancer cell apoptosis and G0/G1 cell cycle arrest via mediation of microRNA-22. Phytother. Res. 2020;34:1619–1628. doi: 10.1002/ptr.6626. [DOI] [PubMed] [Google Scholar]
- 142.Chen Z., Nishimura N., Okamoto T., Wada K., Naora K. Molecular Mechanism of Matrine from Sophora alopecuroides in the Reversing Effect of Multi-Anticancer Drug Resistance in K562/ADR Cells. Biomed Res. Int. 2019;2019:1269532. doi: 10.1155/2019/1269532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Scaria B., Sood S., Raad C., Khanafer J., Jayachandiran R., Pupulin A., Grewal S., Okoko M., Arora M., Miles L., et al. Natural Health Products (NHP’s) and Natural Compounds as Therapeutic Agents for the Treatment of Cancer; Mechanisms of Anti-Cancer Activity of Natural Compounds and Overall Trends. Int. J. Mol. Sci. 2020;21:8480. doi: 10.3390/ijms21228480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chen M.H., Gu Y.Y., Zhang A.L., Sze D.M., Mo S.L., May B.H. Biological effects and mechanisms of matrine and other constituents of Sophora flavescens in colorectal cancer. Pharmacol. Res. 2021;171:105778. doi: 10.1016/j.phrs.2021.105778. [DOI] [PubMed] [Google Scholar]
- 145.Bhagya B., Chandrasheka K.R. Tetrandrine and cancer—An overview on the molecular approach. Biomed. Pharmacother. Biomed. Pharmacother. 2018;97:624–632. doi: 10.1016/j.biopha.2017.10.116. [DOI] [PubMed] [Google Scholar]
- 146.Chen Z., Zhao L., Zhao F., Yang G., Wang J.J. Tetrandrine suppresses lung cancer growth and induces apoptosis, potentially via the VEGF/HIF-1α/ICAM-1 signaling pathway. Oncol. Lett. 2018;15:7433–7437. doi: 10.3892/ol.2018.8190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Guo Y., Pei X. Tetrandrine-Induced Autophagy in MDA-MB-231 Triple-Negative Breast Cancer Cell through the Inhibition of PI3K/AKT/mTOR Signaling. Evid. Based Complement. Altern. Med. 2019;2019:7517431. doi: 10.1155/2019/7517431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Juan T.K., Liu K.C., Kuo C.L., Yang M.D., Chu Y.L., Yang J.L., Wu P.P., Huang Y.P., Lai K.C., Chung J.G. Tetrandrine suppresses adhesion, migration and invasion of human colon cancer SW620 cells via inhibition of nuclear factor-κB, matrix metalloproteinase-2 and matrix metalloproteinase-9 signaling pathways. Oncol. Lett. 2018;15:7716–7724. doi: 10.3892/ol.2018.8286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang H., Xie B., Zhang Z., Sheng X., Zhang S. Tetrandrine suppresses cervical cancer growth by inducing apoptosis in vitro and in vivo. Drug Des. Dev. Ther. 2019;13:119–127. doi: 10.2147/DDDT.S187776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sato E., Ohta S., Kawakami K., Ikeda M., Takahashi T., Kobayashi S., Nomura M. Tetrandrine Increases the Sensitivity of Human Lung Adenocarcinoma PC14 Cells to Gefitinib by Lysosomal Inhibition. Anticancer Res. 2019;39:6585–6593. doi: 10.21873/anticanres.13874. [DOI] [PubMed] [Google Scholar]
- 151.Jiang L., Hou R. Tetrandrine Reverses Paclitaxel Resistance in Human Ovarian Cancer via Inducing Apoptosis, Cell Cycle Arrest Through β-Catenin Pathway. Oncol. Targets Ther. 2020;13:3631–3639. doi: 10.2147/OTT.S235533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Song J., Xu J., Guo J., Shang Y., Wang J., Wang T. The enhancement of Tetrandrine to gemcitabine-resistant PANC-1 cytochemical sensitivity involves the promotion of PI3K/Akt/mTOR-mediated apoptosis and AMPK-regulated autophagy. Acta Histochem. 2021;123:151769. doi: 10.1016/j.acthis.2021.151769. [DOI] [PubMed] [Google Scholar]
- 153.Bai X.Y., Liu Y.G., Song W., Li Y.Y., Hou D.S., Luo H.M., Liu P. Anticancer activity of tetrandrine by inducing pro-death apoptosis and autophagy in human gastric cancer cells. J. Pharm. Pharmacol. 2018;70:1048–1058. doi: 10.1111/jphp.12935. [DOI] [PubMed] [Google Scholar]
- 154.Xu W., Wang X., Tu Y., Masaki H., Tanaka S., Onda K., Sugiyama K., Yamada H., Hirano T. Tetrandrine and cepharanthine induce apoptosis through caspase cascade regulation, cell cycle arrest, MAPK activation and PI3K/Akt/mTOR signal modification in glucocorticoid resistant human leukemia Jurkat T cells. Chem. Biol. Interact. 2019;310:108726. doi: 10.1016/j.cbi.2019.108726. [DOI] [PubMed] [Google Scholar]
- 155.Kou B., Liu W., Xu X., Yang Y., Yi Q., Guo F., Li J., Zhou J., Kou Q. Autophagy induction enhances tetrandrine-induced apoptosis via the AMPK/mTOR pathway in human bladder cancer cells. Oncol. Rep. 2017;38:3137–3143. doi: 10.3892/or.2017.5988. [DOI] [PubMed] [Google Scholar]
- 156.Cheng L., Ma H., Shao M., Fan Q., Lv H., Peng J., Hao T., Li D., Zhao C., Zong X. Synthesis of folate-chitosan nanoparticles loaded with ligustrazine to target folate receptor positive cancer cells. Mol. Med. Rep. 2017;16:1101–1108. doi: 10.3892/mmr.2017.6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chen Z., Zhang C., Gao F., Fu Q., Fu C., He Y., Zhang J. A systematic review on the rhizome of Ligusticum chuanxiong Hort. (Chuanxiong) Food Chem. Toxicol. 2018;119:309–325. doi: 10.1016/j.fct.2018.02.050. [DOI] [PubMed] [Google Scholar]
- 158.Wang S., Lei T., Zhang M. The Reversal Effect and Its Mechanisms of Tetramethylpyrazine on Multidrug Resistance in Human Bladder Cancer. PLoS ONE. 2016;11:e0157759. doi: 10.1371/journal.pone.0157759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zou L., Liu X., Li J., Li W., Zhang L., Li J., Zhang J. Tetramethylpyrazine Enhances the Antitumor Effect of Paclitaxel by Inhibiting Angiogenesis and Inducing Apoptosis. Front. Pharm. 2019;10:707. doi: 10.3389/fphar.2019.00707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zha G.F., Qin H.L., Youssif B., Amjad M.W., Raja M., Abdelazeem A.H., Bukhari S. Discovery of potential anticancer multi-targeted ligustrazine based cyclohexanone and oxime analogs overcoming the cancer multidrug resistance. Eur. J. Med. Chem. 2017;135:34–48. doi: 10.1016/j.ejmech.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 161.Zhou X., Wang A., Wang L., Yin J., Wang L., Di L., Hoi M.P., Shan L., Wu X., Wang Y. A Danshensu-Tetramethylpyrazine Conjugate DT-010 Overcomes Multidrug Resistance in Human Breast Cancer. Front. Pharm. 2019;10:722. doi: 10.3389/fphar.2019.00722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wu N., Xu L., Yang Y., Yu N., Zhang Z., Chen P., Zhang J., Tang M., Yuan M., Ge J., et al. Tetramethylpyrazine-mediated regulation of CXCR4 in retinoblastoma is sensitive to cell density. Mol. Med. Rep. 2017;15:2481–2488. doi: 10.3892/mmr.2017.6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kadioglu O., Law B., Mok S., Xu S.W., Efferth T., Wong V. Mode of Action Analyses of Neferine, a Bisbenzylisoquinoline Alkaloid of Lotus (Nelumbo nucifera) against Multidrug-Resistant Tumor Cells. Front. Pharm. 2017;8:238. doi: 10.3389/fphar.2017.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sivalingam K.S., Paramasivan P., Weng C.F., Viswanadha V.P. Neferine Potentiates the Antitumor Effect of Cisplatin in Human Lung Adenocarcinoma Cells Via a Mitochondria-Mediated Apoptosis Pathway. J. Cell. Biochem. 2017;118:2865–2876. doi: 10.1002/jcb.25937. [DOI] [PubMed] [Google Scholar]
- 165.Pham D.C., Chang Y.C., Lin S.R., Fuh Y.M., Tsai M.J., Weng C.F. FAK and S6K1 Inhibitor, Neferine, Dually Induces Autophagy and Apoptosis in Human Neuroblastoma Cells. Molecules. 2018;23:3110. doi: 10.3390/molecules23123110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhang Q., Li Y., Miao C., Wang Y., Xu Y., Dong R., Zhang Z., Griffin B.B., Yuan C., Yan S., et al. Anti-angiogenesis effect of Neferine via regulating autophagy and polarization of tumor-associated macrophages in high-grade serous ovarian carcinoma. Cancer Lett. 2018;432:144–155. doi: 10.1016/j.canlet.2018.05.049. [DOI] [PubMed] [Google Scholar]
- 167.Li H., Tang Y., Wen L., Kong X., Chen X., Liu P., Zhou Z., Chen W., Xiao C., Xiao P., et al. Neferine reduces cisplatin-induced nephrotoxicity by enhancing autophagy via the AMPK/mTOR signaling pathway. Biochem. Biophys. Res. Commun. 2017;484:694–701. doi: 10.1016/j.bbrc.2017.01.180. [DOI] [PubMed] [Google Scholar]
- 168.Law B., Michelangeli F., Qu Y.Q., Xu S.W., Han Y., Mok S., Dias I., Javed M.U., Chan W.K., Xue W.W., et al. Neferine induces autophagy-dependent cell death in apoptosis-resistant cancers via ryanodine receptor and Ca(2+)-dependent mechanism. Sci. Rep. 2019;9:20034. doi: 10.1038/s41598-019-56675-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Erdogan S., Turkekul K. Neferine inhibits proliferation and migration of human prostate cancer stem cells through p38 MAPK/JNK activation. J. Food Biochem. 2020;44:e13253. doi: 10.1111/jfbc.13253. [DOI] [PubMed] [Google Scholar]
- 170.Eid W., Abdel-Rehim W. Neferine Enhances the Antitumor Effect of Mitomycin-C in Hela Cells Through the Activation of p38-MAPK Pathway. J. Cell. Biochem. 2017;118:3472–3479. doi: 10.1002/jcb.26006. [DOI] [PubMed] [Google Scholar]
- 171.Manogaran P., Somasundaram B., Viswanadha V.P. Reversal of cisplatin resistance by neferine/isoliensinine and their combinatorial regimens with cisplatin-induced apoptosis in cisplatin-resistant colon cancer stem cells (CSCs) J. Biochem. Mol. Toxicol. 2022;36:e22967. doi: 10.1002/jbt.22967. [DOI] [PubMed] [Google Scholar]
- 172.Marthandam Asokan S., Mariappan R., Muthusamy S., Velmurugan B.K. Pharmacological benefits of neferine—A comprehensive review. Life Sci. 2018;199:60–70. doi: 10.1016/j.lfs.2018.02.032. [DOI] [PubMed] [Google Scholar]
- 173.Zhang Y.B., Fei H.X., Guo J., Zhang X.J., Wu S.L., Zhong L.L. Dauricine suppresses the growth of pancreatic cancer in vivo by modulating the Hedgehog signaling pathway. Oncol. Lett. 2019;18:4403–4414. doi: 10.3892/ol.2019.10790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhang S., Ren Y., Qiu J. Dauricine inhibits viability and induces cell cycle arrest and apoptosis via inhibiting the PI3K/Akt signaling pathway in renal cell carcinoma cells. Mol. Med. Rep. 2018;17:7403–7408. doi: 10.3892/mmr.2018.8732. [DOI] [PubMed] [Google Scholar]
- 175.Deng B., Jiang X.L., Tan Z.B., Cai M., Deng S.H., Ding W.J., Xu Y.C., Wu Y.T., Zhang S.W., Chen R.X., et al. Dauricine inhibits proliferation and promotes death of melanoma cells via inhibition of Src/STAT3 signaling. Phytother. Res. 2021;35:3836–3847. doi: 10.1002/ptr.7089. [DOI] [PubMed] [Google Scholar]
- 176.Li W., Qiu Y., Hao J., Zhao C., Deng X., Shu G. Dauricine upregulates the chemosensitivity of hepatocellular carcinoma cells: Role of repressing glycolysis via miR-199a:HK2/PKM2 modulation. Food Chem. Toxicol. 2018;121:156–165. doi: 10.1016/j.fct.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 177.Tang Z.H., Cao W.X., Guo X., Dai X.Y., Lu J.H., Chen X., Zhu H., Lu J.J. Identification of a novel autophagic inhibitor cepharanthine to enhance the anti-cancer property of dacomitinib in non-small cell lung cancer. Cancer Lett. 2018;412:1–9. doi: 10.1016/j.canlet.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 178.Rattanawong A., Payon V., Limpanasittikul W., Boonkrai C., Mutirangura A., Wonganan P. Cepharanthine exhibits a potent anticancer activity in p53-mutated colorectal cancer cells through upregulation of p21Waf1/Cip. Oncol. Rep. 2018;39:227–238. doi: 10.3892/or.2017.6084. [DOI] [PubMed] [Google Scholar]
- 179.Payon V., Kongsaden C., Ketchart W., Mutirangura A., Wonganan P. Mechanism of Cepharanthine Cytotoxicity in Human Ovarian Cancer Cells. Planta Med. 2019;85:41–47. doi: 10.1055/a-0706-7503. [DOI] [PubMed] [Google Scholar]
- 180.Gao S., Li X., Ding X., Qi W., Yang Q. Cepharanthine Induces Autophagy, Apoptosis and Cell Cycle Arrest in Breast Cancer Cells. Cell. Physiol. Biochem. 2017;41:1633–1648. doi: 10.1159/000471234. [DOI] [PubMed] [Google Scholar]
- 181.Huang C.Z., Wang Y.F., Zhang Y., Peng Y.M., Liu Y.X., Ma F., Jiang J.H., Wang Q.D. Cepharanthine hydrochloride reverses P-glycoprotein-mediated multidrug resistance in human ovarian carcinoma A2780/Taxol cells by inhibiting the PI3K/Akt signaling pathway. Oncol. Rep. 2017;38:2558–2564. doi: 10.3892/or.2017.5879. [DOI] [PubMed] [Google Scholar]
- 182.Unson S., Kongsaden C., Wonganan P. Cepharanthine combined with 5-fluorouracil inhibits the growth of p53-mutant human colorectal cancer cells. J. Asian Nat. Prod. Res. 2020;22:370–385. doi: 10.1080/10286020.2018.1564136. [DOI] [PubMed] [Google Scholar]
- 183.Yan X., Li M., Chen L., Peng X., Que Z.J., An H.M., Shen K.P., Hu B. α-Solanine inhibits growth and metastatic potential of human colorectal cancer cells. Oncol. Rep. 2020;43:1387–1396. doi: 10.3892/or.2020.7519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gao J., Ying Y., Wang J., Cui Y. Solanine Inhibits Immune Escape Mediated by Hepatoma Treg Cells via the TGFβ/Smad Signaling Pathway. Biomed Res. Int. 2020;2020:9749631. doi: 10.1155/2020/9749631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Karaboğa Arslan A.K., Yerer M.B. α-Chaconine and α-Solanine Inhibit RL95-2 Endometrium Cancer Cell Proliferation by Reducing Expression of Akt (Ser473) and ERα (Ser167) Nutrients. 2018;10:672. doi: 10.3390/nu10060672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wu J., Wang L., Du X., Sun Q., Wang Y., Li M., Zang W., Liu K., Zhao G. α-solanine enhances the chemosensitivity of esophageal cancer cells by inducing microRNA-138 expression. Oncol. Rep. 2018;39:1163–1172. doi: 10.3892/or.2018.6187. [DOI] [PubMed] [Google Scholar]
- 187.Busch C., Burkard M., Leischner C., Lauer U.M., Frank J., Venturelli S. Epigenetic activities of flavonoids in the prevention and treatment of cancer. Clin. Epigenetics. 2015;7:64. doi: 10.1186/s13148-015-0095-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lan C.Y., Chen S.Y., Kuo C.W., Lu C.C., Yen G.C. Quercetin facilitates cell death and chemosensitivity through RAGE/PI3K/AKT/mTOR axis in human pancreatic cancer cells. J. Food Drug Anal. 2019;27:887–896. doi: 10.1016/j.jfda.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Li Y., Wang Z., Jin J., Zhu S.X., He G.Q., Li S.H., Wang J., Cai Y. Quercetin pretreatment enhances the radiosensitivity of colon cancer cells by targeting Notch-1 pathway. Biochem. Biophys. Res. Commun. 2020;523:947–953. doi: 10.1016/j.bbrc.2020.01.048. [DOI] [PubMed] [Google Scholar]
- 190.Qi J., Yu J., Li Y., Luo J., Zhang C., Ou S., Zhang G., Yang X., Peng X. Alternating consumption of β-glucan and quercetin reduces mortality in mice with colorectal cancer. Food Sci. Nutr. 2019;7:3273–3285. doi: 10.1002/fsn3.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Erdogan S., Turkekul K., Dibirdik I., Doganlar O., Doganlar Z.B., Bilir A., Oktem G. Midkine downregulation increases the efficacy of quercetin on prostate cancer stem cell survival and migration through PI3K/AKT and MAPK/ERK pathway. Biomed. Pharmacother. 2018;107:793–805. doi: 10.1016/j.biopha.2018.08.061. [DOI] [PubMed] [Google Scholar]
- 192.Chen Z., Huang C., Ma T., Jiang L., Tang L., Shi T., Zhang S., Zhang L., Zhu P., Li J., et al. Reversal effect of quercetin on multidrug resistance via FZD7/β-catenin pathway in hepatocellular carcinoma cells. Phytomedicine. 2018;43:37–45. doi: 10.1016/j.phymed.2018.03.040. [DOI] [PubMed] [Google Scholar]
- 193.Wan Mohd Tajuddin W., Lajis N.H., Abas F., Othman I., Naidu R. Mechanistic Understanding of Curcumins Therapeutic Effects in Lung Cancer. Nutrients. 2019;11:2989. doi: 10.3390/nu11122989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Jin M., Kong L., Han Y., Zhang S. Gut microbiota enhances the chemosensitivity of hepatocellular carcinoma to 5-fluorouracil in vivo by increasing curcumin bioavailability. Phytother. Res. 2021;35:5823–5837. doi: 10.1002/ptr.7240. [DOI] [PubMed] [Google Scholar]
- 195.Lin C.Y., Hung C.C., Wang C., Lin H.Y., Huang S.H., Sheu M.J. Demethoxycurcumin sensitizes the response of non-small cell lung cancer to cisplatin through downregulation of TP and ERCC1-related pathways. Phytomedicine. 2019;53:28–36. doi: 10.1016/j.phymed.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 196.Wang H.J., Yang Z.X., Dai X.T., Chen Y.F., Yang H.P., Zhou X.D. Bisdemethoxycurcumin sensitizes cisplatin-resistant lung cancer cells to chemotherapy by inhibition of CA916798 and PI3K/AKT signaling. Apoptosis. 2017;22:1157–1168. doi: 10.1007/s10495-017-1395-x. [DOI] [PubMed] [Google Scholar]
- 197.Liu F., Gao S., Yang Y., Zhao X., Fan Y., Ma W., Yang D., Yang A., Yu Y. Antitumor activity of curcumin by modulation of apoptosis and autophagy in human lung cancer A549 cells through inhibiting PI3K/Akt/mTOR pathway. Oncol. Rep. 2018;39:1523–1531. doi: 10.3892/or.2018.6188. [DOI] [PubMed] [Google Scholar]
- 198.Wang A., Wang J., Zhang S., Zhang H., Xu Z., Li X. Curcumin inhibits the development of non-small cell lung cancer by inhibiting autophagy and apoptosis. Exp. Ther. Med. 2017;14:5075–5080. doi: 10.3892/etm.2017.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Liu F., Gao S., Yang Y., Zhao X., Fan Y., Ma W., Yang D., Yang A., Yu Y. Curcumin induced autophagy anticancer effects on human lung adenocarcinoma cell line A549. Oncol. Lett. 2017;14:2775–2782. doi: 10.3892/ol.2017.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Yin J., Wang L., Wang Y., Shen H., Wang X., Wu L. Curcumin reverses oxaliplatin resistance in human colorectal cancer via regulation of TGF-β/Smad2/3 signaling pathway. Oncol. Targets Ther. 2019;12:3893–3903. doi: 10.2147/OTT.S199601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kao C.C., Cheng Y.C., Yang M.H., Cha T.L., Sun G.H., Ho C.T., Lin Y.C., Wang H.K., Wu S.T., Way T.D. Demethoxycurcumin induces apoptosis in HER2 overexpressing bladder cancer cells through degradation of HER2 and inhibiting the PI3K/Akt pathway. Environ. Toxicol. 2021;36:2186–2195. doi: 10.1002/tox.23332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Qian C., Wang B., Zou Y., Zhang Y., Hu X., Sun W., Xiao H., Liu H., Shi L. MicroRNA 145 enhances chemosensitivity of glioblastoma stem cells to demethoxycurcumin. Cancer Manag. Res. 2019;11:6829–6840. doi: 10.2147/CMAR.S210076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sun M., Ye Y., Xiao L., Duan X., Zhang Y., Zhang H. Anticancer effects of ginsenoside Rg3 (Review) Int. J. Mol. Med. 2017;39:507–518. doi: 10.3892/ijmm.2017.2857. [DOI] [PubMed] [Google Scholar]
- 204.Nakhjavani M., Hardingham J.E., Palethorpe H.M., Tomita Y., Smith E., Price T.J., Townsend A.R. Ginsenoside Rg3: Potential Molecular Targets and Therapeutic Indication in Metastatic Breast Cancer. Medicines. 2019;6:17. doi: 10.3390/medicines6010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Jiang J., Yuan Z., Sun Y., Bu Y., Li W., Fei Z. Ginsenoside Rg3 enhances the anti-proliferative activity of erlotinib in pancreatic cancer cell lines by downregulation of EGFR/PI3K/Akt signaling pathway. Biomed. Pharmacother. 2017;96:619–625. doi: 10.1016/j.biopha.2017.10.043. [DOI] [PubMed] [Google Scholar]
- 206.Li Y., Lu J., Bai F., Xiao Y., Guo Y., Dong Z. Ginsenoside Rg3 Suppresses Proliferation and Induces Apoptosis in Human Osteosarcoma. Biomed Res. Int. 2018;2018:4306579. doi: 10.1155/2018/4306579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Chen Z., Wei X., Shen L., Zhu H., Zheng X. 20(S)-ginsenoside-Rg3 reverses temozolomide resistance and restrains epithelial-mesenchymal transition progression in glioblastoma. Cancer Sci. 2019;110:389–400. doi: 10.1111/cas.13881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Li W., Li G., She W., Hu X., Wu X. Targeted antitumor activity of Ginsenoside (Rg1) in paclitaxel-resistant human nasopharyngeal cancer cells are mediated through activation of autophagic cell death, cell apoptosis, endogenous ROS production, S phase cell cycle arrest and inhibition of m-TOR/PI3K/AKT signalling pathway. J. BUON. 2019;24:2056–2061. [PubMed] [Google Scholar]
- 209.Hong Y., Fan D. Ginsenoside Rk1 induces cell cycle arrest and apoptosis in MDA-MB-231 triple negative breast cancer cells. Toxicology. 2019;418:22–31. doi: 10.1016/j.tox.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 210.Islam M.S., Wang C., Zheng J., Paudyal N., Zhu Y., Sun H. The potential role of tubeimosides in cancer prevention and treatment. Eur. J. Med. Chem. 2019;162:109–121. doi: 10.1016/j.ejmech.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 211.Yan J., Dou X., Zhou J., Xiong Y., Mo L., Li L., Lei Y. Tubeimoside-I sensitizes colorectal cancer cells to chemotherapy by inducing ROS-mediated impaired autophagolysosomes accumulation. J. Exp. Clin. Cancer Res. 2019;38:353. doi: 10.1186/s13046-019-1355-0. [DOI] [PMC free article] [PubMed] [Google Scholar]