Abstract
Unprecedented nanoemulsion formulations (NE) of Jasminum humile and Jasminum grandiflorum essential oils (EO) were prepared, and examined for their cytotoxic and antiviral activities. NE characterization and stability examination tests were performed to ensure formula stability. The antiviral activity was determined against hepatitis A (HAV) and herpes simplex type-1 (HSV-1) viruses using MTT assay, while the cytotoxic potential was determined against liver (HepG-2), breast (MCF-7), leukemia (THP-1) cancer cell lines and normal Vero cells. Statistical significance was determined in comparison with doxorubicin as cytotoxic and acyclovir as antiviral standard drugs. GC-MS analysis indicated twenty four compounds in the EO of J. humile and seventeen compounds in the EO of J. grandiflorum. Biological investigations of pure EOs revealed weak cytotoxic and antiviral effects. Nevertheless, their NE formulations exhibited high biological value as cytotoxic and antiviral agents. NE formulations also showed feasible selectivity index for the viral-infected and cancer cells (especially HepG-2) than normal Vero cells. Both nanoemulsions showed lower IC50 than standard doxorubicin against HepG-2 (26.65 and 22.58 vs. 33.96 μg/mL) and MCF-7 (36.09 and 36.19 vs. 52.73 μg/mL), respectively. The study results showed the dramatic effect of nanoemulsion preparation on the biological activity of EOs and other liposoluble phytopharmaceuticals.
Keywords: Jasminum, humile, grandiflorum, nanoemulsion, HAV, HSV-1, HepG-2, MCF-7, THP-1
1. Introduction
Jasminum is a genus of flowering plants (approximately 600 species), belonging to family Oleaceae, usually used in perfume industries and ornamental purposes due to their bright colored flowers and characteristic fragrances. Traditionally, the essential oils (EOs) of different Jasminum species were used in aromatherapy for the treatment of diarrhea, fever, abdominal spasms, conjunctivitis, skin inflammations, bronchial asthma and uterine hemorrhage [1]. Nowadays, several reports investigated the biological activities of the EOs of several Jasminum species. Jaminum sambac EO was reported to exhibit antibacterial activity and to suppress puerperal lactation [2] in addition to its antidepressant and mood uplifting properties [3]. Jasminum officinale EO was reported to have antiviral activity against hepatitis B virus [4]. Only few studies investigated the biological activities of Jasminum grandiflorum EO, much less Jasminum humile EO. The EO of Jasminum grandiflorum was examined for its antimicrobial activity against Gram-positive and Gram-negative bacteria as well as candidiasis [5]. Jasminum grandiflorum was reported to have cytotoxic activity against brain cancer cell line [6].
In recent times, there has been more interest in the nanoscience and nanotechnologies applications. Nanotechnology modifies and develops the properties of drugs by converting them into their nanoparticles. It has numerous applications including drug delivery and disease diagnostics [7]. The small molecule-based nanotechnology may reduce the side effects, enhance the potency, and deliver drugs in a well-targeted manner through increasing their permeability and retention effects [8]. As droplets decrease in size, reaching nano-diameter; the biological activity of lipophilic constituents prepared as nanoemulsions increases. This is due to the higher surface area obtained, and the easier transport of the effective drugs through bio-membranes [9]. Although several previous studies were reported about the biological activities of the EOs of different Jasminum species, there are no reports, as far as we know, investigating the potency of their nanoemulsion preparations on the biological effects.
In 2020, breast cancer was found to be first in line among cancer diagnosed cases. Liver cancer was one of the top three, in the list of cancer-related deaths [10]. Both cancer types are pervasive in Egypt, ranking as first and second leading causes of cancer cases [11], while leukemia was responsible for 35.6% of diagnosed cancer cases among children [12]. In this study, chemical profiling was performed for the EOs of two oleaceous plants; Jasminum humile L. and Jasminum grandiflorum L. using GC-MS analysis technique. Moreover, nanoemulsion formulations were prepared for both EOs (JhEO and JgEO), then the pure EOs and NE formula were examined biologically for their cytotoxic and antiviral effects to investigate the possible activity enhancement by nanoemulstion preparation. The cytotoxic effects were examined against HepG-2, MCF-7 and THP-1 human cancer cell lines in comparison with doxorubicin as a standard. Statistical significance was determined and normal Vero cells were used to evaluate their selectivity towards cancer cells. The antiviral activities were also investigated using MTT assay against hepatitis A virus (HAV) and herpes simplex virus type 1 (HSV-1) and results were compared to the standard drug acyclovir.
2. Materials and Methods
2.1. Plant Materials
The fresh flowers of Jasminum humile L. and Jasminum grandiflorum L. (250 gm, each) were collected in the early morning during May 2021 from the botanical garden of Kafr EL-Sheikh university, Egypt and were attested by Dr. Ibrahim Mashaly, Professor of Ecology, Faculty of Science, Mansoura University, Egypt. The essential oils (EOs) were extracted by hydro-distillation for almost 8 h; according to the European pharmacopoeia (European Pharmacopoeia, 1975) using Clevenger’s cohobation apparatus to produce 2 mL and 2.5 mL, respectively of yellow colored EOs. The collected oils were dehydrated over anhydrous sodium sulfate and then they were stored in sealed vials at low temperature for further (GC/MS) analysis and biological investigations.
2.2. Capillary Gas Chromatography-Mass Spectrometry (GC/MS) Analysis
The GC/MS analysis was carried out at the faculty of postgraduate studies for advanced sciences (the central laboratory), Beni Suef. Egypt. Identification of the components was confirmed by comparing their mass spectral fragmentation patterns and retention indices with the previously reported literature [13,14,15,16,17] as well as the mass spectral NIST/ChemStation database.
2.3. Preparation of Nanoemulsion Formulations
JhEO-NE and JgEO-NE formula were prepared by mixing 1% w/w of each EO with 8% w/w glycerol monoacetate (GMA). Tween 80 (surfactant) was mixed with labrosol (co-surfactant) in an equal ratio, and the mixture (30% w/w) was added to EO-GMA. Finally water (61% w/w) was dropped in order to obtain an apparent and clear NE [18]. The prepared JhEO-NE and JgEO-NE formula were subjected to thermodynamic stability studies and self-nanoemulsification efficiency tests according to the International Conference for Harmonization (ICH) guidelines. Freshly prepared formula were packaged in glass bottles and subjected to different storage conditions of refrigeration at (5 ± 3 °C) and different ambient conditions over a period of 3 months. Physical assessment of the samples was achieved by visual inspection of phase separation, color and/or odor change and pH measurements at zero time (freshly prepared formula), as well as after storage periods of 1, 2 and 3 months. The particle size, polydispersity index (PDI) and zeta potential (ZP) were also measured (using Malvern Zetasizer Nanoseries, Malvern Instruments Limited, UK).
2.4. Determination of Sample Cytotoxicity on Cancer Cells
Cytotoxic potential was measured against normal Vero cells (ATCC; CCL81-22), as well as HepG-2 (ATCC; HB-8065), MCF-7 (ATCC; HTB-22), and THP-1 (ATCC; TIB-202) human cancer cell lines, obtained from American Type Culture Collection (Rockville, MD, USA), using a microplate 3-(4,5-dimethythiazole-2yl)-2,5-diphenyl-tetrazolium bromide (MTT) method. Experiments were repeated for three times with doxorubicin (Sigma, USA) as the positive control and 0.1% DMSO media as the negative control. Cells were grown in DMEM supplemented with 10% FBS, 100 µg/mL of streptomycin, 100 units/mL of penicillin, 0.07% NaHCO3 and 2 mM L-glutamine and maintained at 37 °C in humidified 5% CO2 atmosphere. The cultures were maintained at 37 °C in a humidified 5% CO2 atmosphere.
2.5. Determination of Antiviral Activity
Hepatitis A and Herpes simplex-1 viruses were provided by Dr. Mohammed Ali, Virology Lab., Faculty of Medicine, Al-Azhar University, Egypt. Cells were grown in DMEM supplemented with 10% FBS, 100 µg/mL of streptomycin, 100 units/mL of penicillin, 0.07% NaHCO3 and 2 mM L-glutamine and maintained at 37 °C in humidified 5% CO2 atmosphere. The antiviral assay was performed using a microplate 3-(4,5-dimethythiazole-2yl)-2,5-diphenyl-tetrazolium bromide (MTT) method [19,20]. Experiments were repeated for three times with acyclovir (Sigma-Aldrich, St. Louis, MO, USA) as the positive control and 0.1% DMSO media as the negative control.
2.6. MTT Protocol
A 96 well tissue culture plate was inoculated with 1 × 105 cells/ml (100 µL/well) and incubated at 37 °C for 24 h to develop a complete monolayer sheet. Growth medium was decanted from 96 well micro titer plates after confluent sheet of cells were formed, cell monolayer was washed twice with wash media. Two-fold dilutions of tested samples were made in RPMI medium with 2% serum (maintenance medium). 0.1 mL of each dilution was tested in different wells leaving 3 wells as control, receiving only maintenance medium. The plate was incubated at 37 °C and examined. Cells were checked for any physical signs of toxicity e.g., partial or complete loss of the monolayer, rounding, shrinkage, or cell granulation. The cytotoxic potential was investigated utilizing doses of (1000, 500, 250, 125, 62.5, 31.25, and 15.62 µg/mL), while the antiviral activity was assessed by applying (31.25, 15.62, and 7.81 µg/mL) of each sample.
MTT solution was prepared (5 mg/mL in PBS) (Bio Basic Inc.; Markham, Ontario, Canada) and 20 µL MTT solution were added to each well. A shaking table was used (150 rpm for 5 min) to thoroughly mix the MTT into the media and incubated (37 °C, 5% CO2) for 1–5 h to allow the MTT to be metabolized. Media was dumped off and the plate was dried on paper towels—if necessary—to remove residues. Formazan (MTT metabolic product) was resuspended in 200 µL DMSO, then placed on a shaking table −150 rpm for 5 min—to thoroughly mix the formazan into the solvent. Optical density was measured at 560 nm (and subtract background at 620 nm), which is directly correlated with cell quantity.
2.7. Calculation of the Selectivity Index (SI)
Selectivity indices (SI) were obtained after dividing CC50 (the half maximal inhibitory concentration of normal Vero cells) by the specific IC50 of cancer cells and viral infected cells. Selectivity indices are used to evaluate the cytotoxic potential and antiviral activity relative to the normal cells toxicity; where high (SI) indicates high potency and low cellular toxicity [21,22,23].
2.8. Statistical Analysis
All statistical analyses were performed using GraphPad Prism version 9.2.0 to calculate the half-maximal inhibitory concentration (IC50) and the half-maximal cytotoxic concentration (CC50) where the level of significance was set at (p > 0.05). Quantitative data were expressed as mean ± standard deviation (SD). GraphPad Prism version 9.2.0 was also used to create multiple bar charts of the antiviral activity and cell viability.
3. Results and Discussion
3.1. GC-MS Analysis of the Essential Oil
Freshly collected flowers of J. grandiflorum yielded 0.8% v/w of a clear, faint yellow, lighter than water, essential oil. The oil components together with the percentage and retention indices are shown in (Table 1). Seventeen compounds (97.7%) were identified in the oil. Esters and sesquiterpenes, are the main constituents, accounting for 49.4% and 25.7%, respectively. Esters are represented by benzyl acetate (32.4%), benzyl benzoate (7.4%), methyl anthranilate (2.5%), benzyl salicylate (2.5%), (3Z)-hexenyl benzoate (1.1%), (Z)-methyl jasmonate (1.8%) and (Z)-methyl epijasmonate (1.6%) as the main constituents, while sesquiterpenes are represented by (Z)-nerolidol (11.9%), (E, E)-α-farnesene (7.606%) and (Z)-caryophyllene (6.2%) as the main constituents.
Table 1.
GC-MS profiling of the essential oils obtained from freshly collected flowers of Jasminum humile L. and Jasminum grandiflorum L.
| Tentative Identification | R.R.I. calc. a | R.R.I. lit. b | Content % | Base Peak | M. Wt. | M. Formula | ||
|---|---|---|---|---|---|---|---|---|
| J. humile | J. grandiflorum | |||||||
| 1 | Linalool | 1095 | 1096 | 17.2 | 3.6 | 71.1 | 154.0 | C10H18O |
| 2 | Benzyl acetate | 1161 | 1162 | - | 32.4 | 108.0 | 150.0 | C9H10O2 |
| 3 | Carvone | 1240 | 1243 | 2.2 | - | 82.0 | 150.0 | C10H14O |
| 4 | Methyl anthranilate | 1338 | 1337 | 2.1 | 2.5 | 119.1 | 151.1 | C8H9NO2 |
| 5 | (Z)-jasmone | 1390 | 1392 | 6.6 | 8.5 | 79.1 | 164.0 | C11H16O |
| 6 | (Z)-caryophyllene | 1407 | 1408 | 5.6 | 6.2 | 41.1 | 204.0 | C15H24 |
| 7 | (E, E)-α-farnesene | 1506 | 1505 | 6.9 | 7.6 | 41.1 | 204.0 | C15H24 |
| 8 | (Z)-nerolidol | 1532 | 1532 | 5.0 | 11.9 | 69.1 | 222.0 | C15H26O |
| 9 | (3Z)-hexenyl benzoate | 1567 | 1566 | 7.4 | 1.1 | 105.1 | 204.0 | C13H16O2 |
| 10 | n-hexadecane | 1601 | 1600 | - | 1.3 | 57.1 | 226.0 | C16H34 |
| 11 | epi-α-cadinol | 1639 | 1640 | 3.0 | - | 161.1 | 222.0 | C15H26O |
| 12 | epi-α-muurolol | 1642 | 1642 | 2.9 | - | 43.1 | 222.0 | C15H26O |
| 13 | (Z)-methyl jasmonate | 1648 | 1649 | - | 1.8 | 83.1 | 224.0 | C13H20O3 |
| 14 | (Z)-methyl epijasmonate | 1679 | 1679 | - | 1.6 | 83.1 | 224.0 | C13H20O3 |
| 15 | (2E, 6Z)-farnesol | 1714 | 1715 | 2.5 | - | 69.1 | 222.0 | C15H26O |
| 16 | Benzyl benzoate | 1759 | 1759 | 6.9 | 7.4 | 105.1 | 212.0 | C14H12O2 |
| 17 | (2E, 6E)-farnesyl acetate | 1845 | 1846 | 2.0 | - | 69.1 | 264.0 | C17H28O2 |
| 18 | Benzyl salicylate | 1864 | 1865 | 2.6 | 2.5 | 91.1 | 228.0 | C14H12O3 |
| 19 | n-nonadecane | 1899 | 1900 | - | 1.2 | 57.1 | 268.0 | C19H40 |
| 20 | Phytol | 1941 | 1943 | - | 3.5 | 71.1 | 296.0 | C20H40O |
| 21 | Isophytol | 1947 | 1947 | 2.4 | - | 71.1 | 296.0 | C20H40O |
| 22 | Geranyl benzoate | 1957 | 1959 | 1.7 | - | 105.1 | 258.1 | C17H22O2 |
| 23 | Hexadecanoic acid | 1960 | 1960 | 3.4 | 1.2 | 41.1 | 256.0 | C16H32O2 |
| 24 | Methyl linoleate | 2084 | 2085 | 3.1 | - | 67.1 | 294.1 | C19H34O2 |
| 25 | n-heneicosane | 2099 | 2100 | - | 3.4 | 57.1 | 296.0 | C21H44 |
| 26 | Oleic acid | 2141 | 2142 | 1.6 | - | 41.1 | 282.1 | C18H34O2 |
| 27 | Phytol acetate | 2218 | 2218 | 1.5 | - | 43.1 | 338.1 | C22H42O2 |
| 28 | Tricosane | 2300 | 2300 | 2.8 | - | 57.1 | 324.0 | C23H48 |
| 29 | Tetracosane | 2400 | 2400 | 2.2 | - | 57.1 | 338.1 | C24H50 |
| 30 | Hexacosane | 2600 | 2600 | 4.6 | - | 57.1 | 366.1 | C26H54 |
| 31 | Nonacosane | 2900 | 2900 | 1.8 | - | 57.1 | 408.0 | C29H60 |
a: Relative retention indices obtained experimentally in this study. b: Relative retention indices reported in previous literature [13].
Fresh flowers of J. humile yielded 1.0% v/w of a clear, faint yellow, lighter than water, essential oil. The oil components together with the percentage and retention indices are shown in (Table 1). Twenty four compounds (98.0%) were identified in the oil. Esters, monoterpenes and sesquiterpenes are the main constituents, accounting for 27.4%, 26.0% and 26.0%, respectively. Esters are represented by (3Z)-hexenyl benzoate (7.429%), benzyl benzoate (6.9%), methyl linoleate (3.1%), benzyl salicylate (2.6%), methyl anthranilate (2.1%), (2E, 6E)-farnesyl acetate (2.0%), geranyl benzoate (1.7%) and phytol acetate (1.5%) as the main constituents. Monoterpenes are represented by linalool (17.2%), (Z)-jasmone (6.6%) and carvone (2.2%) as the main constituents. Sesquiterpenes are represented by (E, E)-α-farnesene (6.9%), (Z)-caryophyllene (5.6%), (Z)-nerolidol (5.0%), epi-α-cadinol (3.0%), epi-α-muurolol (2.9%) and (2E, 6Z)-farnesol (2.5%) as the main constituents.
3.2. Characterization of Nanoemuslion Formulations (Physical Characterization)
JhEO-NE and JgEO-NE formula passed thermodynamic stability as well as self-nanoemulsification efficiency tests. There was no observed phase separation or physical changes, in color, odor, or pH, over the storage period of 3 months at refrigeration conditions (5 ± 3 °C). They had negative ZP charges of (−18.63 and −229.67), nanometric sizes of (8.03 ± 0.45 and 12.09 ± 0.81 nm) and low PDI values of (0.30 ± 0.04 and 0.27 ± 0.03), respectively.
3.3. Cytotoxic Concentrations of Tested Samples on Normal Cells
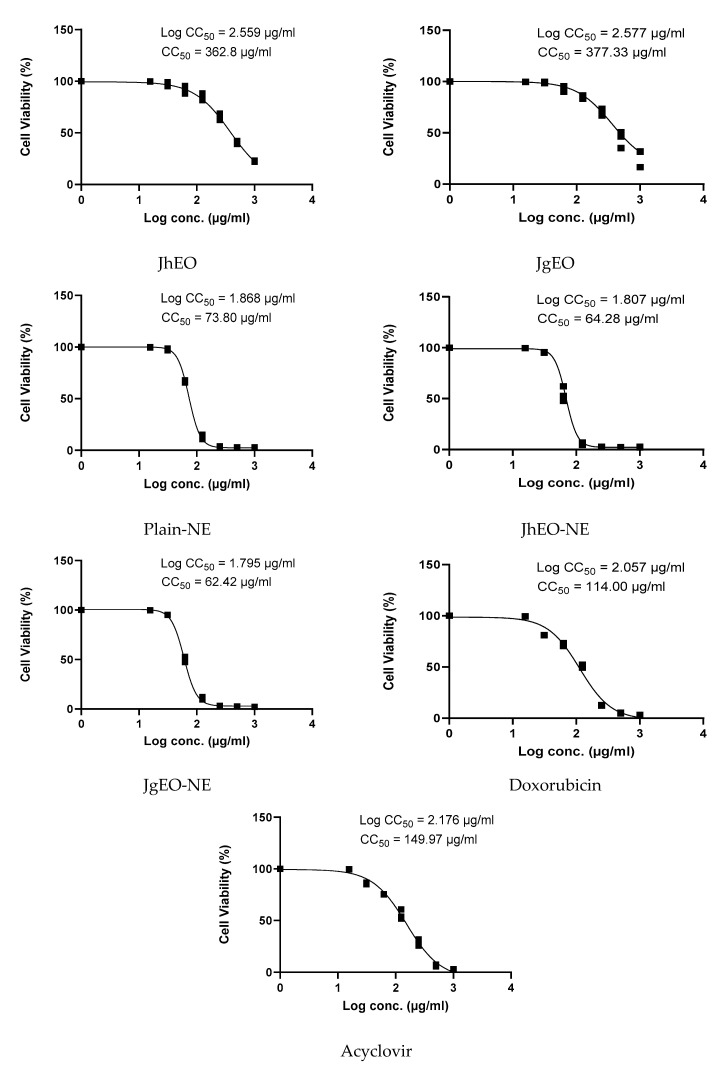
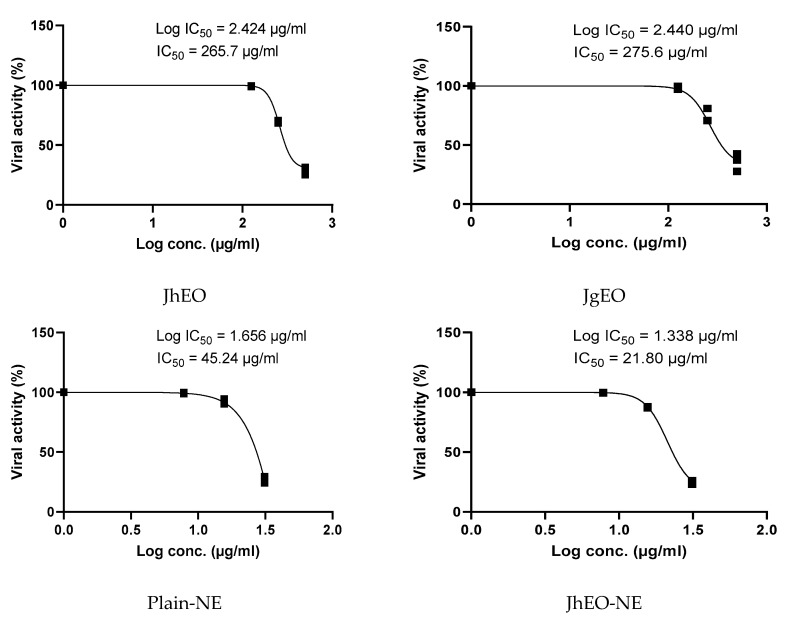
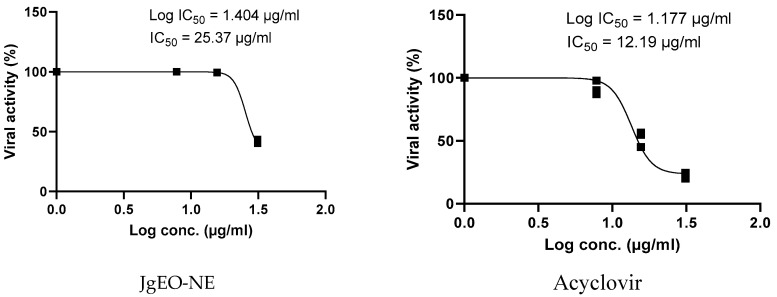
The half maximal cytotoxic concentration (CC50) for the essential oils (EOs) and the nanoemuslion formulatios of Jasminum grandiflorum L. (JgEO-NE) and Jasminum humile L. (JhEO-NE) was assessed on VERO cells using MTT assay (Table 2 and Table 3). Dose-response curves are shown in (Figure 1). Both EOs (JgEO and JhEO) showed CC50 values of 362.83 and 377.33 μg/mL, respectively. Plain-NE showed CC50 of 73.80 μg/mL. Nanoemulsion formula showed lower cytotoxicity where JhEO-NE showed CC50 of 64.28 μg/mL, while JgEO-NE showed CC50 of 62.42 μg/mL.
Table 2.
Cytotoxic activity of the nanoemulsion formulations of the essential oils obtained from J. humile (JhEO-NE) and J. grandiflorum (JgEO-NE) as well as the reference drug doxorubicin, against HepG2, MCF-7, and THP-1 cell lines, showing CC50, IC50, and SI.
| Sample | CC50 ± SD (µg/mL) |
Cytotoxic Activity | ||||||
|---|---|---|---|---|---|---|---|---|
| HepG-2 | MCF-7 | THP-1 | ||||||
| IC50 ± SD (µg/mL) | SI | IC50 ± SD (µg/mL) | SI | IC50 ± SD (µg/mL) | SI | |||
| Media | DMSO (-Ve Control) |
NT | NA | - | NA | - | NA | - |
| Pure EOs | J. humile | 362.83 ± 15.31 | 289.10 ± 5.61 | 1.26 | 304.13 ± 3.55 | 1.19 | 265.60 ± 10.85 | 1.37 |
| J. grandiflorum | 377.33 ± 10.82 | 324.90 ± 22.85 | 1.16 | 327.53 ± 6.06 | 1.15 | 286.37 ± 7.63 | 1.32 | |
| Nano-emulsion | J. humile | 64.28 ± 4.17 | 22.58 ± 0.86 | 2.85 | 36.19 ± 0.77 | 1.78 | 51.76 ± 6.68 | 1.24 |
| J. grandiflorum | 62.42 ± 2.07 | 26.65 ± 0.06 | 2.34 | 36.09 ± 1.44 | 1.73 | 54.41 ± 0.60 | 1.15 | |
| Standard | Doxorubicin | 114.00 ± 2.17 | 33.96 ± 2.03 | 3.36 | 52.73 ± 2.44 | 2.16 | 47.98 ± 2.35 | 2.38 |
NT: Nontoxic, NA: Not active.
Table 3.
Results of the antiviral assay of the nanoemulsion formulations of the essential oils obtained from Jasminum humile L. (JhEO-NE) and Jasminum grandiflorum L. (JgEO-NE) as well as the reference drug acyclovir showing CC50, MNTC, IC50, and selectivity indices (SI) against HAV and HSV-I viruses.
| Sample | CC50 ± SD (µg/mL) |
MNTC (µg/mL) |
HAV | HSV-1 | |||
|---|---|---|---|---|---|---|---|
| IC50 ± SD (µg/mL) |
SI | IC50 ± SD (µg/mL) |
SI | ||||
| Media | DMSO (-Ve Control) |
NT | NT | NA | - | NA | - |
| Pure EOs | J. humile | 362.83 ± 15.31 | 500 | 265.70 ± 4.62 | 1.36 | 275.33 ± 7.41 | 1.32 |
| J. grandiflorum | 377.33 ± 10.82 | 500 | 275.57 ± 7.51 | 1.37 | 299.63 ± 19.76 | 1.26 | |
| Nano-emulsion | J. humile | 64.28 ± 4.17 | 31.25 | 21.80 ± 1.17 | 2.94 | 18.49 ± 1.20 | 3.48 |
| J. grandiflorum | 62.42 ± 2.07 | 31.25 | 25.37 ± 1.26 | 2.45 | 31.34 ± 2.72 | 1.99 | |
| Standard | Acyclovir | 149.97 ± 5.46 | 31.25 | 15.04 ± 1.38 | 9.98 | 12.49 ± 1.98 | 12.00 |
NT: Nontoxic, NA: Not active. Bold value denotes the highest activity.
Figure 1.
CC50 of J. humile (JhEO) and J. grandiflorum (JgEO) pure essential oils, their nanoemulsion formulations (JhEO-NE) and (JgEO-NE), plain nanoemulsion formulation (Plain-NE) as well as the standard drugs doxorubicin and acyclovir against normal Vero cells.
3.4. Cytotoxic Activity of Tested Samples
The cytotoxic activity was evaluated for the two (EOs) as well as their nanoemulsion formulations against three cell lines; HepG-2, MCF-7 and THP-1. The Vero cell line is a non-tumorigenic cell line established from kidney cells of the African green monkey (Cercopitbecus aetbiops). It can be used for investigating cell growth, differentiation, and cytotoxicity against non-cancerous cells. This cell line is an excellent in vitro model for investigating cytotoxicity and carcinogenesis due to its well-defined growth pattern and behavior in culture [24,25,26]. Human hepatocellular carcinoma cells (HepG-2) are usually used as an in vitro model for assessing cytotoxicity against liver cancer cells. They preserve several genotypic and phenotypic features of hepatocytes. Their cytotoxicity assay is highly sensitive and specific [27]. MCF-7 is a classic in vitro model usually used for determining the cytotoxicity against breast cancer cells for having several mammary epithelium characteristics, e.g., estradiol processing, and presence of estrogen receptors (ER) in their cytoplasm [28]. THP-1 is a monocytic-derived cell line; commonly used to investigate immune system disorders, and cytotoxicity. Many reports investigated the cytotoxic potential of different drugs on THP-1 leukemia cells; as a model for acute myeloid leukemia [29,30,31,32,33,34,35].
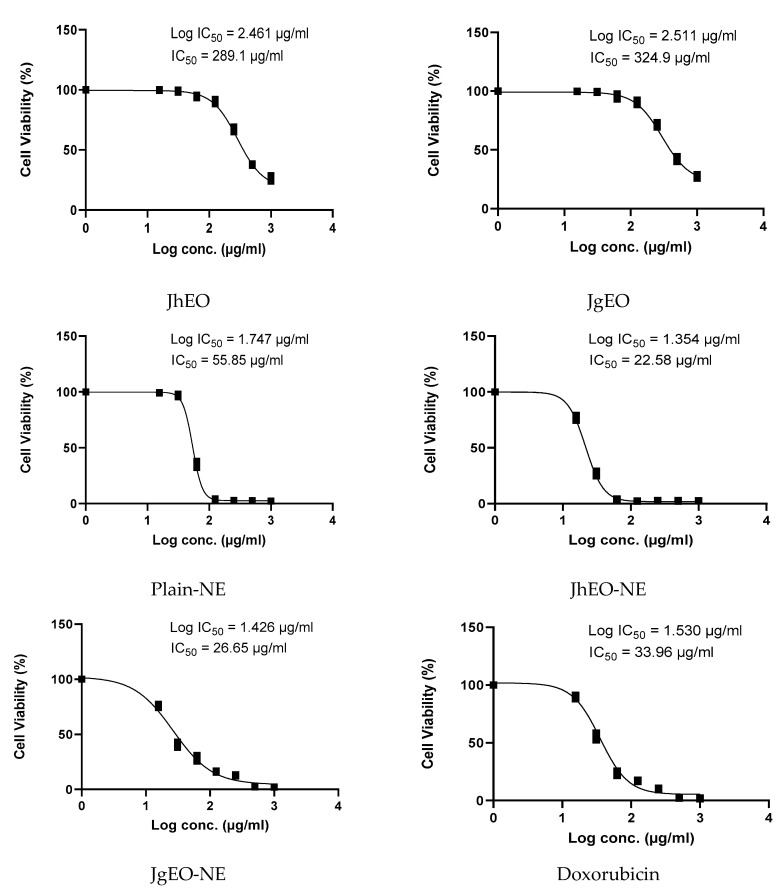
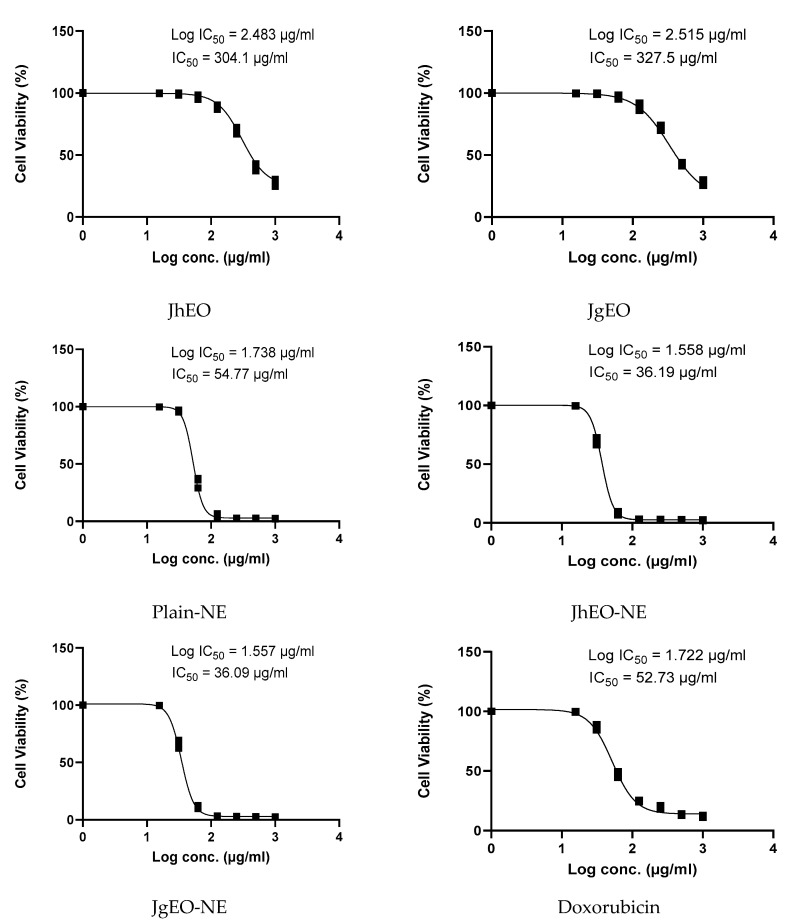
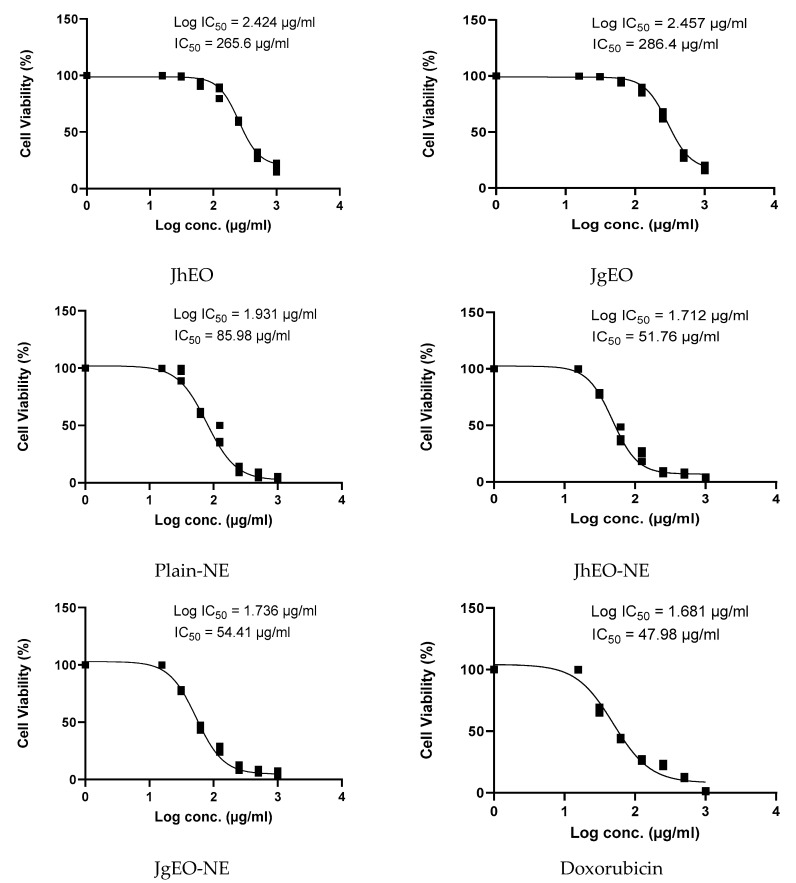
Dose-response curves are shown in (Figure 2, Figure 3 and Figure 4). Both (EOs) showed weak cytotoxic activity against the examined cell lines. JgEO showed IC50 value of 324.90 μg/mL against HepG-2, 327.53 μg/mL against MCF-7 and 286.37 μg/mL against THP-1 cells, while JhEO showed IC50 value of 289.10 μg/mL against HepG-2, 304.13 μg/mL against MCF-7 and 265.60 μg/mL against THP-1 cells. Yet, the prepared nanoemuslion formulations of the two (EOs) induced significant cytotoxic activity against all cell lines in a concentration-dependent manner (Figure S5–S7), and the results are depicted in (Table 2).
Figure 2.
IC50 of J. humile (JhEO) and J. grandiflorum (JgEO) pure essential oils, their nanoemulsion formulations (JhEO-NE) and (JgEO-NE), plain nanoemulsion formulation (Plain-NE) as well as standard doxorubicin against HepG-2 cell line.
Figure 3.
IC50 of J. humile (JhEO) and J. grandiflorum (JgEO) pure essential oils, their nanoemulsion formulations (JhEO-NE) and (JgEO-NE), plain nanoemulsion formulation (Plain-NE) as well as standard doxorubicin against MCF-7 cell line.
Figure 4.
IC50 of J. humile (JhEO) and J. grandiflorum (JgEO) pure essential oils, their nanoemulsion formulations (JhEO-NE) and (JgEO-NE), plain nanoemulsion formulation (Plain-NE) as well as standard doxorubicin against THP-1 cell line.
(JhEO-NE) and (JgEO-NE) significantly inhibited cell growth of the liver cancer cell line; HepG-2 at 250, 125, 62.5 and 31.25 μg/mL (compared to the reference drug doxorubicin) (Figure S3). Statistical significance was also observed for both nanoenulsion formulations against the breast cancer cell line; MCF-7 (Figure S4) at all the examined concentrations (1000, 500, 250, 125, 62.5 and 31.25 μg/mL). Only 3 concentrations (1000, 500 and 250 μg/mL) showed statistically significant inhibition of the cell viability of leukemia cell line; THP-1 (Figure S5).
Compared to standard doxorubicin, the nanoformulations (JhEO-NE) and (JgEO-NE) demonstrated lower IC50; i.e., higher cytotoxic activity against the two solid tumor cell lines; MCF-7 (showing 36.31 and 36.33 μg/mL, respectively) vs. (53.09 μg/mL for doxorubicin), and HepG-2 (showing 22.58 and 27.08 μg/mL, respectively) vs. (31.83 μg/mL for doxorubicin) (Figure S1). Statistical significance was determined for their IC50, indicating high statistical significance against MCF-7 along with HepG-2 cell lines. For leukemia (THP-1) cell line; (JhEO-NE) exhibited an IC50 value of (53.57 μg/mL), while (JgEO-NE) showed an IC50 value of (56.10 μg/mL) (Figure S1). Plain-NE exhibited an IC50 value of 55.85 μg/mL against HepG-2, 54.77 μg/mL against MCF-7 and 85.98 μg/mL against THP-1 cell lines. The cytotoxic potential of both EOs may be associated with (Z)-caryophyllene [36], oleic acid [37,38], and linalool; which has been previously reported to have cytotoxic activity against HepG-2 [39], MCF-7 [40], and THP-1 [31].
3.5. Antiviral Activity of Tested Samples
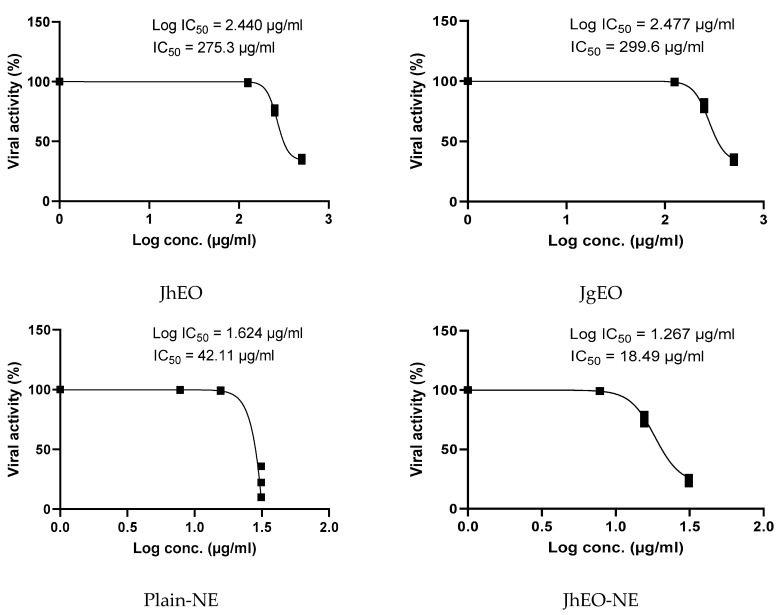
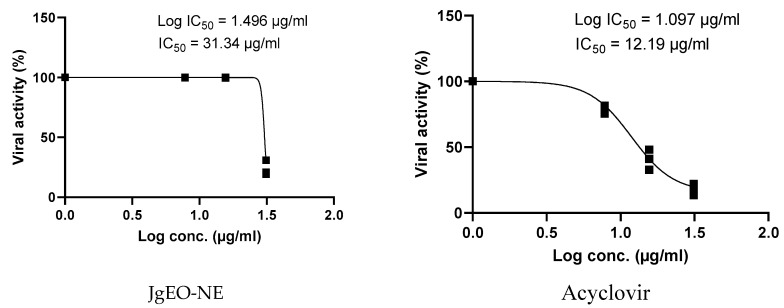
The antiviral activity was evaluated at the maximum non-toxic concentration (MNTC) against 100 tissue culture infectious dose TCID50/mL of HAV and HSV-1 viruses. Dose-response curves are shown in (Figure 5 and Figure 6). The examined (EOs) showed very weak antiviral activity against both viruses. JgEO revealed an IC50 value of 275.57 μg/mL against HAV and 299.63 μg/mL against HSV-1, while JhEO showed an IC50 value of 265.70 μg/mL against HAV and 275.33 μg/mL against HSV-1. However, the prepared nanoemuslion formulations of the two (EOs) induced significant antiviral activity against both viruses in a concentration-dependent manner (Figures S6 and S7), and the results are depicted in (Table 3). Statistical significance was determined for their antiviral activities in comparison with the standard drug acyclovir after using various concentrations (Figure S2).
Figure 5.
IC50 of J. humile (JhEO) and J. grandiflorum (JgEO) pure essential oils, their nanoemulsion formulations (JhEO-NE) and (JgEO-NE), plain nanoemulsion formulation (Plain-NE) as well as standard acyclovir against HAV virus.
Figure 6.
IC50 of J. humile (JhEO) and J. grandiflorum (JgEO) pure essential oils, their nanoemulsion formulations (JhEO-NE) and (JgEO-NE), plain nanoemulsion formulation (Plain-NE) as well as standard acyclovir against HSV-1 virus.
(JhEO-NE) demonstrated a lower IC50 i.e., a higher antiviral activity against both viruses. It exhibited an IC50 value of 21.80 μg/mL against HAV and 18.49 μg/mL against HSV-1, while (JgEO-NE) showed IC50 value of 25.37 μg/mL against HAV and 12.49 μg/mL against HSV-1. Plain-NE was also examined, showing IC50 values of 45.24 μg/mL against HAV and 42.11 μg/mL against HSV-1. Several constituents in the chemical composition of both EOs were reported to have antiviral activity, such as linalool [41], nerolidol [42], and straight chain hydrocarbon compounds (acyclic alkanes) e.g., tetracosane [43], and nonacosane [44].
4. Conclusions
GC-MS profiling showed the presence of twenty four compounds in the EO of J. humile and seventeen compounds in the EO of J. grandiflorum. A stable unprecedented nanoemulsion formulation was prepared for each EO. Biological investigation was performed for the pure EOs and NE formulations of both plants, examining their antiviral activity against HAV and HSV-1 viruses, as well as their cytotoxicity against HepG-2, MCF-7 and THP-1 cell lines.
As cytotoxic agents, both NE formula revealed much more activity and selectivity against solid tumor cell lines; HepG-2 and MCF-7 than the liquid tumor THP-1 cells. Their potency was higher and their selectivity index was comparative to that of the standard cytotoxic drug; doxorubicin. A Flow cytometric cell cycle analysis would be recommended; to measure the cells’ distribution in different stages of the cell cycle and evaluate cell proliferation in more detail. On the other hand; although their antiviral activity was enhanced (than the pure EOs), the standard antiviral drug -acyclovir- was more active than both nanoformulations against HAV and HSV-1.
Results showed clearly that NE formulations had much greater potency than pure EOs. Nanoemulsification highly increased the cytotoxic and antiviral activities of the pure EOs of the two plants which provides an excellent way to improve the biological activity of liposoluble natural agents. Moreover, NE formula could be a promising carrier for the drug administration of anticancer agents; which could enhance their efficacy, and reduce both dose and cost. These encouraging in-vitro results also recommend further in-vivo and clinical investigations of the phytopharmaceutical NE formulations as promising approach to enhance the essential oils cytotoxic and antiviral activities.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27113639/s1, Figure S1. Cytotoxic activity of various concentrations the nanoemulsion formulations of the essential oils obtained from Jasminum humile L. (JhEO-NE) and Jasminum grandiflorum L. (JgEO-NE) as well as standard doxorubicin against the leukemia cell line, HepG-2. Figure S2. Cytotoxic activity of various concentrations of the nanoemulsion formulations of the essential oils obtained from Jasminum humile L. (JhEO-NE) and Jasminum grandiflorum L. (JgEO-NE) as well as standard doxorubicin against the leukemia cell line, MCF-7. Figure S3. Cytotoxic activity of various concentrations of the nanoemulsion formulations of the essential oils obtained from Jasminum humile L. (JhEO-NE) and Jasminum grandiflorum L. (JgEO-NE) as well as standard doxorubicin against the leukemia cell line, THP-1. Figure S4. Antiviral activity of various concentrations of the nanoemulsion formulations of the essential oils obtained from Jasminum humile L. (JhEO-NE) and Jasminum grandiflorum L. (JgEO-NE) as well as standard doxorubicin against HAV virus. Figure S5. Antiviral activity of various concentrations of the nanoemulsion formulations of the essential oils obtained from Jasminum humile L. (JhEO-NE) and Jasminum grandiflorum L. (JgEO-NE) as well as standard doxorubicin against HSV-1 virus.
Author Contributions
Conceptualization, M.E.-N.; methodology, K.A.M.; software, K.A.M.; validation, A.E., M.E.-N., and M.-F.L.; data curation, A.E., M.E.-N., and M.-F.L.; writing-original draft preparation, K.A.M.; writing-review and editing, A.E., M.E.-N., and M.-F.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon request to the corresponding authors.
Conflicts of Interest
The authors declare that there is no conflict of interest.
Sample Availability
Samples are available upon request from the corresponding authors.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Neeraj Mourya N.M., Devendra Bhopte D.B., Rakesh Sagar R.S. A review on Jasminum sambac: A potential medicinal plant. Int. J. Indig. Herbs Drugs. 2017;2:13–16. [Google Scholar]
- 2.Kunhachan P., Banchonglikitkul C., Kajsongkram T., Khayungarnnawee A., Leelamanit W. Chemical composition, toxicity and vasodilatation effect of the flowers extract of Jasminum sambac (L.) Ait. “G. Duke of Tuscany”. Evid. Based Complement. Altern. Med. 2012;2012:1–7. doi: 10.1155/2012/471312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hongratanaworakit T. Stimulating effect of aromatherapy massage with jasmine oil. Nat. Prod. Commun. 2010;5:157–162. doi: 10.1177/1934578X1000500136. [DOI] [PubMed] [Google Scholar]
- 4.Zhao G., Yin Z., Dong J. Antiviral efficacy against hepatitis B virus replication of oleuropein isolated from Jasminum officinale L. var. grandiflorum. J. Ethnopharmacol. 2009;125:265–268. doi: 10.1016/j.jep.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 5.Jirovetz L., Buchbauer G., Schweiger T., Denkova Z., Slavchev A., Stoyanova A., Schmidt E., Geissler M. Chemical composition, olfactory evaluation and antimicrobial activities of Jasminum grandiflorum L. absolute from India. Nat. Prod. Commun. 2007;2:407–412. doi: 10.1177/1934578X0700200411. [DOI] [Google Scholar]
- 6.Manjunath C., Mahurkar N. In vitro cytotoxicity of cardamom oil, lemon oil, and jasmine oil on human skin, gastric, and brain cancer cell line. J. Cancer Res. Ther. 2021;17:62–68. doi: 10.4103/jcrt.JCRT_915_17. [DOI] [PubMed] [Google Scholar]
- 7.Kumar R., Nayak M., Sahoo G.C., Pandey K., Sarkar M.C., Ansari Y., Das V.N.R., Topno R.K., Madhukar M., Das P. Iron oxide nanoparticles based antiviral activity of H1N1 influenza A virus. J. Infect. Chemother. 2019;25:325–329. doi: 10.1016/j.jiac.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Z., Xiao Y., Xu L., Liu Y., Jiang G., Wang W., Li B., Zhu T., Tan Q., Tang L., et al. Glycyrrhizic acid nanoparticles as antiviral and anti-inflammatory agents for COVID-19 treatment. ACS Appl. Mater. Interfaces. 2021;13:20995–21006. doi: 10.1021/acsami.1c02755. [DOI] [PubMed] [Google Scholar]
- 9.Donsì F., Ferrari G. Essential oil nanoemulsions as antimicrobial agents in food. J. Biotechnol. 2016;233:106–120. doi: 10.1016/j.jbiotec.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization . Egypt-Global Cancer Observatory. World Health Organization; Geneva, Switzerland: 2020. [(accessed on 18 November 2021)]. Available online: https://gco.iarc.fr/today/data/factsheets/populations/818-egypt-fact-sheets.pdf. [Google Scholar]
- 12.Alburaiki T.A., Hany A.M., Riad K.F., Osman D.M. Association of parental, child, and environmental factors with the occurrence of childhood leukemia in Upper Egypt. J. High Inst. Pub. Health. 2021;51:10–18. doi: 10.21608/jhiph.2021.158653. [DOI] [Google Scholar]
- 13.Adams R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. 4th ed. Allured Publishing Corporation; Carol Stream, IL, USA: 2007. [Google Scholar]
- 14.Braun N.A., Kohlenberg B., Sim S., Meier M., Hammerschmidt F.-J. Jasminum flexile flower absolute from India—A detailed comparison with three other jasmine absolutes. Nat. Prod. Commun. 2009;4:1239–1250. doi: 10.1177/1934578X0900400917. [DOI] [PubMed] [Google Scholar]
- 15.Prakash O., Sahoo D., Rout P.K. Liquid CO2 extraction of Jasminum grandiflorum and comparison with conventional processes. Nat. Prod. Commun. 2012;7:89–92. doi: 10.1177/1934578X1200700131. [DOI] [PubMed] [Google Scholar]
- 16.Wei F.H., Chen F.L., Tan X.M. Gas chromatographic-mass spectrometric analysis of essential oil of Jasminum officinale L. var grandiflorum flower. Trop. J. Pharm. Res. 2015;14:149–152. doi: 10.4314/tjpr.v14i1.21. [DOI] [Google Scholar]
- 17.Zhou H.-C., Hou Z.-W., Wang D.-X., Ning J.-M., Wei S. Large scale preparation, stress analysis, and storage of headspace volatile condensates from Jasminum sambac flowers. Food Chem. 2019;286:170–178. doi: 10.1016/j.foodchem.2019.01.202. [DOI] [PubMed] [Google Scholar]
- 18.Aman R.M., Abu Hashim I.I., Meshali M.M. Novel clove essential oil nanoemulgel tailored by taguchi′s model and scaffold-based nanofibers: Phytopharmaceuticals with promising potential as cyclooxygenase-2 inhibitors in external inflammation. Int. J. Nanomed. 2020;15:2171–2195. doi: 10.2147/IJN.S246601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehrbod P., Safari H., Mollai Z., Fotouhi F., Mirfakhraei Y., Entezari H., Goodarzi S., Tofighi Z. Potential antiviral effects of some native Iranian medicinal plants extracts and fractions against influenza A virus. BMC Complement. Med. Ther. 2021;21:1–12. doi: 10.1186/s12906-021-03423-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang H.-X., Zeng M.-S., Ye Y., Liu J.-Y., Xu P.-P. Antiviral activity of puerarin as potent inhibitor of influenza virus neuraminidase. Phytother. Res. 2021;35:324–336. doi: 10.1002/ptr.6803. [DOI] [PubMed] [Google Scholar]
- 21.Oliveira P.F.D., Alves J.M., Damasceno J.L., Oliveira R.A.M., Dias H., Crotti A.E.M., Tavares D.C. Cytotoxicity screening of essential oils in cancer cell lines. Rev. Bras. Farmacogn. 2015;25:183–188. doi: 10.1016/j.bjp.2015.02.009. [DOI] [Google Scholar]
- 22.Tsemeugne J., Shinyuy L.M., Djeukoua S.K.D., Sopbue E.F., Ngemenya M.N. Evaluation of macrofilaricidal and microfilaricidal activities against Onchocerca ochengi and cytotoxicity of some synthesized azo compounds containing thiophene backbone. Parasitol. Res. 2021;120:2087–2094. doi: 10.1007/s00436-021-07162-3. [DOI] [PubMed] [Google Scholar]
- 23.Yousefbeyk F., Dabirian S., Ghanbarzadeh S., Eghbali Koohi D., Yazdizadeh P., Ghasemi S. Green synthesis of silver nanoparticles from Stachys byzantina K. Koch: Characterization, antioxidant, antibacterial, and cytotoxic activity. Part. Sci. Technol. 2021;40:219–232. doi: 10.1080/02726351.2021.1930302. [DOI] [Google Scholar]
- 24.Sun X.-Y., Gan Q.-Z., Ouyang J.-M. Size-dependent cellular uptake mechanism and cytotoxicity toward calcium oxalate on Vero cells. Sci. Rep. 2017;7:41949. doi: 10.1038/srep41949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonçalves E.M., Ventura C.Â., Yano T., Rodrigues Macedo M.L., Genari S.C. Morphological and growth alterations in Vero cells transformed by cisplatin. Cell Biol. Int. 2006;30:485–494. doi: 10.1016/j.cellbi.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Saurav K., Kannabiran K. Cytotoxicity and antioxidant activity of 5-(2,4-dimethylbenzyl)pyrrolidin-2-one extracted from marine Streptomyces VITSVK5 spp. Saudi J. Biol. Sci. 2012;19:81–86. doi: 10.1016/j.sjbs.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sahu S.C., Zheng J., Graham L., Chen L., Ihrie J., Yourick J.J., Sprando R.L. Comparative cytotoxicity of nanosilver in human liver HepG2 and colon Caco2 cells in culture. J. Appl. Toxicol. 2014;34:1155–1166. doi: 10.1002/jat.2994. [DOI] [PubMed] [Google Scholar]
- 28.Camarillo I.G., Xiao F., Madhivanan S., Salameh T., Nichols M., Reece L.M., Leary J.F., Otto K., Natarajan A., Ramesh A., et al. Electroporation-Based Therapies for Cancer: From Basics to Clinical Applications. 1st ed. Woodhead Publishing; Sawston, UK: 2014. Low and high voltage electrochemotherapy for breast cancer: An in vitro model study; pp. 55–102. [Google Scholar]
- 29.Liu J.-J., Zhang Y., Lin D.-J., Xiao R.-Z. Tanshinone IIA inhibits leukemia THP-1 cell growth by induction of apoptosis. Oncol. Rep. 2009;21:1075–1081. doi: 10.3892/or_00000326. [DOI] [PubMed] [Google Scholar]
- 30.Popovich D.G., Kitts D.D. Structure–function relationship exists for ginsenosides in reducing cell proliferation and inducing apoptosis in the human leukemia (THP-1) cell line. Arch. Biochem. Biophys. 2002;406:1–8. doi: 10.1016/S0003-9861(02)00398-3. [DOI] [PubMed] [Google Scholar]
- 31.Gu Y., Ting Z., Qiu X., Zhang X., Gan X., Fang Y., Xu X., Xu R. Linalool preferentially induces robust apoptosis of a variety of leukemia cells via upregulating p53 and cyclin-dependent kinase inhibitors. Toxicology. 2010;268:19–24. doi: 10.1016/j.tox.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 32.Kitts D.D., Popovich D.G., Hu C. Characterizing the mechanism for ginsenoside-induced cytotoxicity in cultured leukemia (THP-1) cells. Can. J. Physiol. Pharmacol. 2007;85:1173–1183. doi: 10.1139/Y07-099. [DOI] [PubMed] [Google Scholar]
- 33.Ayesh B.M., Abed A.A., Faris D.A. In vitro inhibition of human leukemia THP-1 cells by Origanum syriacum L. and Thymus vulgaris L. extracts. BMC Res Notes. 2014;7:1–6. doi: 10.1186/1756-0500-7-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawagoe H., Kawagoe R., Sano K. Targeted down-regulation of MLL-AF9 with antisense oligodeoxyribonucleotide reduces the expression of the HOXA7 and -A10 genes and induces apoptosis in a human leukemia cell line, THP-1. Leukemia. 2001;15:1743–1749. doi: 10.1038/sj.leu.2402262. [DOI] [PubMed] [Google Scholar]
- 35.Giri B., Gupta V.K., Yaffe B., Modi S., Roy P., Sethi V., Lavania S.P., Vickers S.M., Dudeja V., Banerjee S., et al. Pre-clinical evaluation of Minnelide as a therapy for acute myeloid leukemia. J. Transl. Med. 2019;17:1–9. doi: 10.1186/s12967-019-1901-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dahham S.S., Tabana Y.M., Iqbal M.A., Ahamed M.B.K., Ezzat M.O., Majid A.S.A., Majid A.M.S.A. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-caryophyllene from the essential oil of Aquilaria crassna. Molecules. 2015;20:11808–11829. doi: 10.3390/molecules200711808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DAILEY O.D., Wang X., Chen F., Huang G. Anticancer activity of branched-chain derivatives of oleic acid. Anticancer Res. 2011;31:3165–3169. [PubMed] [Google Scholar]
- 38.Ahmed S.A., Rahman A.A., Elsayed K.N.M., Abd El-Mageed H.R., Mohamed H.S., Ahmed S.A. Cytotoxic activity, molecular docking, pharmacokinetic properties and quantum mechanics calculations of the brown macroalga Cystoseira trinodis compounds. J. Biomol. Struct. Dyn. 2021;39:3855–3873. doi: 10.1080/07391102.2020.1774418. [DOI] [PubMed] [Google Scholar]
- 39.Usta J., Shatha S., Racha K., Yolla B., Omar R., Sawsan K., Karim E. Possible mediators underlying linalool effect on HepG-2 but not primary hepatocytes: Comparative study. Planta Med. 2011;77:1–11. doi: 10.1055/s-0031-1282415. [DOI] [Google Scholar]
- 40.Zhao Y., Meng X., Zeng Y., Wang C., Chen J., She Z. Linalool inhibits MCF-7 tumor growth in a xenograft model by apoptosis induction and immune modulation. Nat. Prod. Commun. 2021;16:1–9. doi: 10.1177/1934578X211015125. [DOI] [Google Scholar]
- 41.Chiang L.-C., Ng L.-T., Cheng P.-W., Chiang W., Lin C.-C. Antiviral activities of extracts and selected pure constituents of Ocimum basilicum. Clin. Exp. Pharmacol. Physiol. 2005;32:811–816. doi: 10.1111/j.1440-1681.2005.04270.x. [DOI] [PubMed] [Google Scholar]
- 42.Ryabchenko B., Tulupova E., Schmidt E., Wlcek K., Buchbauer G., Jirovetz L. Investigation of anticancer and antiviral properties of selected aroma samples. Nat. Prod. Commun. 2008;3:1085–1088. doi: 10.1177/1934578X0800300710. [DOI] [Google Scholar]
- 43.Chathuranga K., Weerawardhana A., Dodantenna N., Ranathunga L., Cho W.-K., Ma J.Y., Lee J.-S. Inhibitory effect of Sargassum fusiforme and its components on replication of respiratory syncytial virus in vitro and in vivo. Viruses. 2021;13:548. doi: 10.3390/v13040548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chidewe C., Castillo U.F., Sem D.S. Structural analysis and antimicrobial activity of chromatographically separated fractions of leaves of Sesamum angustifolium. J. Biol. Act. Prod. Nat. 2017;7:463–474. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon request to the corresponding authors.