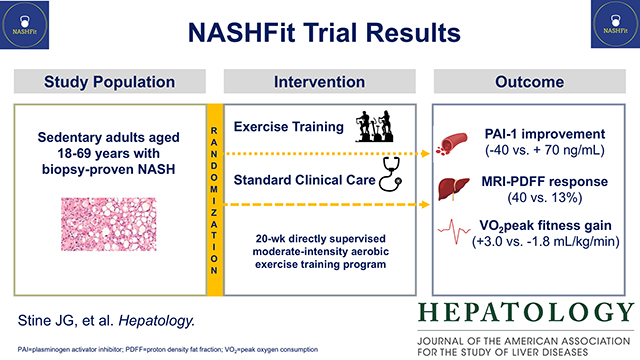
Abstract
Background & Aims:
Nonalcoholic steatohepatitis (NASH) is a common disease that is associated with increased rates of thromboembolism. While exercise training can lessen thrombotic risk in patients with vascular disease, it is unknown whether similar findings are observed in patients with NASH.
Approach & Results:
We conducted a 20-week, randomized controlled clinical trial involving patients with biopsy-confirmed NASH. Patients were randomly assigned (2:1 ratio) to receive either an exercise training program or standard clinical care. The primary end point was change in plasminogen activator inhibitor one (PAI-1) level, an established thrombotic biomarker. Twenty-eight patients were randomly assigned (18 exercise training and 10 standard clinical care). PAI-1 level was significantly decreased by exercise training when compared to standard clinical care (−40+/− 100 vs. +70 +/− 63 ng/mL, p=0.02). Exercise training decreased magnetic resonance imaging proton density fat fraction (MRI-PDFF) (−4.7 +/− 5.6 vs. 1.2 +/− 2.8% absolute liver fat, p=0.01); 40% of exercise subjects had ≥30% relative reduction in MRI-PDFF (histologic response threshold) compared to 13% for standard of care (p<0.01). Exercise training improved fitness (VO2peak) (+3.0 +/− 5.6 vs. −1.8 +/− 5.1 mL/kg/min, p=0.05) in comparison to standard clinical care.
Conclusions:
This clinical trial showed that independent of weight loss or dietary change, exercise training resulted in a significantly greater decrease in thrombotic risk than standard clinical care in patients with NASH, in parallel with MRI-PDFF reduction and improvement in fitness. Future studies are required to determine if exercise training can directly impact patient outcomes and lower rates of thromboembolism.
Keywords: nonalcoholic fatty liver disease, fatty liver, physical activity, fitness, thrombosis
Graphical Abstract
Nonalcoholic fatty liver disease (NAFLD) is a leading cause of chronic liver disease worldwide that is independently associated with inferior survival.(1, 2) In the setting of adiposity and insulin resistance, NAFLD develops through a multi-hit pathogenesis leading to fat accumulation in the liver. NAFLD is an umbrella term covering a spectrum of histologic features including NASH, the progressive form.(3) If untreated, NASH can lead to advanced liver disease including the possibility of fibrosis, cirrhosis, hepatocellular carcinoma and/or the need for liver transplantation.(4, 5)
Because currently available pharmacologic therapies have limited efficacy and a strong side effect profile, they are not regulatory-agency approved.(6) This places increased importance on using lifestyle modification with dietary change and increased physical activity to treat NAFLD and NASH.(7) Physical activity, including exercise training, has many favorable effects on the liver including a reduction in liver fat and when coupled with modest weight loss, offers the possibility to halt or reverse liver fibrosis.(7, 8)
However, the effect of NASH or the benefit of physical activity is not solely limited to the liver. Patients with NASH have multiple extrahepatic manifestations including abnormalities in the hemostatic system, which lead to increased rates of arterial and venous thromboembolism.(9–12) Primary, secondary and tertiary hemostasis are all abnormal in NASH.(9) Plasminogen activator inhibitor (PAI)-1 is the best described prohemostatic factor in NASH and is an established biomarker of clotting risk.(9, 13, 14) PAI-1 plays a crucial role in fibrinolysis or tertiary hemostasis;(15) abnormally high levels prevent clot breakdown. A small decrease in PAI-1 can improve fibrinolysis and lessen the risk of venous thromboembolism by up to 60%.(16, 17) Importantly, exercise training can greatly reduce PAI-1 and activate fibrinolysis in healthy persons and in those with vascular disease.(18–23) Whether aerobic exercise training improves fibrinolysis by lowering PAI-1 in patients with NASH remains unknown. We conducted a randomized controlled clinical trial to investigate the effect of exercise training on clotting risk in sedentary patients with biopsy-confirmed NASH.
Patients and Methods
Patients and Study Design
The NASH Fitness Intervention in Thrombosis (NASHFit) Trial was a single center, randomized controlled clinical trial (NCT03518294) designed to enroll 42 patients aged 18–69 years with NASH conducted at Penn State Milton S. Hershey Medical Center and the Penn State College of Medicine. The study design has been reported previously.(24) The study included sedentary (individuals performing ≥90 min of physical activity each week were excluded) adult men and women with biopsy-confirmed NASH according to the NASH Clinical Research Network (CRN) histological scoring system.(25) Key exclusion criteria included a hemoglobin A1c (HbA1c) >9% at screening, causes of chronic liver disease other than NASH, excessive alcohol consumption and inability to complete regular exercise training. Full eligibility criteria were previously published.(24) Patients were randomized 2:1 to intervention with exercise training or a standard of care control using a list generated by computer randomization (REDCap, Vanderbilt University) and in blocks of six.(26) Both study groups received standard dietary counseling. Subjects in the exercise training group completed five moderate-intensity (heart rate corresponding to 45–55% VO2peak) aerobic exercise sessions per week, each lasting 30 minutes. All sessions were directly supervised by a study Exercise Physiologist. Exercise intensity was monitored real-time using a wearable fitness activity tracker (FitBit Charge HR2, FitBit, Inc, San Francisco, CA) with heart rate monitor to ensure compliance with the intended exercise prescription. Exercise training was performed over 20 weeks. Standard of care control subjects were instructed to continue their current clinical care at the direction of their treating clinicians.
All patients provided written informed consent and the study protocol was approved by the local institutional review board. The study was conducted in compliance with the Declaration of Helsinki, Good Clinical Practice guidelines and local regulatory requirements. The study was designed and conducted by the principal investigator in collaboration with study sub-investigators. The principal investigator collected the data and monitored study conduct. All authors had access to the data, participated in the data interpretation and vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol. All authors reviewed and approved the final manuscript.
Study Assessments
Markers of Hemostasis
All three phases of hemostasis were assessed at baseline and again at week 20 with the following tests: ADAMTS-13, Antithrombin, D-dimer, Factor VII, Factor VIII, Fibrinogen, International Normalized Ratio (INR), PAI-1, platelet count, Protein S, Protein C, and von Willebrand Factor. Using fresh whole blood, a dynamic assessment of the entire coagulation cascade was performed by thromboelastography (TEG).
Liver Histology
Liver biopsies were obtained within six months prior to trial enrollment. A single blinded liver pathologist performed standard histological assessment which included confirmation of NASH diagnosis, liver fibrosis stage and NAFLD activity score (NAS) (including individual components of steatosis, inflammation and hepatocyte ballooning) according to NASH CRN criteria.(25)
Liver steatosis and volume
Liver steatosis was quantified by magnetic resonance imaging-proton density fat fraction (MRI-PDFF) at baseline and week 20 (Siemens 3T PrismaFit system).
Serum Markers
Fasting blood samples were collected at screening and again at week 20 for clinical laboratory values which included adiponectin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), cytokeratin (CK)-18, ferritin, glucose, hemoglobin A1c, high-density lipoprotein (HDL), insulin level, low-density lipoprotein (LDL), white blood cell count.
Fitness and Body Composition Testing
Symptom-limited graded cardiopulmonary fitness testing was performed on a treadmill at baseline and at week 20 to determine maximal oxygen uptake (VO2peak) using the Bruce protocol.(27, 28) Body composition was assessed by dual-energy X-ray absorptiometry (General Electric Healthcare Lunar iDXA® DXA scanner) and skin-fold measurement from seven standard sites (Harpenden Skinfold Caliper, Baty International) at baseline and at week 20.
Dietary composition
At baseline and again at the end of the intervention period, dietary composition was measured for all subjects using a 24-hour dietary recall form and food frequency questionnaire. Mediterranean-diet informed counseling reinforcing consumption of foods low in saturated fat, high in fiber and low in sodium and avoidance or processed foods and fructose containing beverages was also provided by a study Registered Dietitian at both time points. Additionally, at the baseline visit, subjects in each group were given individual caloric goals determined by the Mifflin St. Jeor equation. Subjects in the intervention group were instructed to self-report their daily dietary intake via the secure FitBit application. Total energy, macronutrient (fat, protein and carbohydrate), fiber and sodium intake were calculated by the Fitbit application. Following review of this information, weekly individualized education and counseling were provided by a study Registered Dietitian using the same framework.
Health-related quality of life (HRQOL)
Subjects completed the Patient-Reported Outcomes Measurement Information System (PROMIS) Computerized Adaptive Testing instrument via tablet computer (iPad) at enrollment, monthly and again at week 20 to assess changes in nine standard domains of health.
Endpoints
The primary study endpoint was change in serum PAI-1 level (Invitrogen/Thermo Fisher Scientific, Waltham, MA) after 20 weeks. Supportive secondary endpoints included other markers of primary, secondary and tertiary hemostasis as well as dynamic coagulation system assessment with TEG. Other secondary endpoints included changes from baseline to week 20 in liver steatosis measured by MRI-PDFF, liver volume, liver enzyme levels, inflammatory markers, exploratory biomarker levels (including adiponectin and CK-18), glucose metabolism, lipid levels, body composition, cardiopulmonary fitness, dietary composition (macronutrients, sodium content, fiber), HRQOL, blood pressure, heart rate, hip and waist circumference, cardiovascular risk, physical activity level and sleep quality.
Safety endpoints included adverse events after the start of the exercise training intervention and clinical assessments. Selected events were adjudicated by the study Principal Investigator and reviewed by an independent medical monitor. A complete list of the trial endpoints was previously published.(24) Modifications to the original study protocol are also included in the Supplementary Appendix, including the provision to complete exercise sessions using telehealth during the COVID-19 pandemic based on local research restrictions.
Statistical Analysis
The study sample size was based on the assumption that at least a 23% reduction in PAI-1 (25ng/mL) would be observed following exercise training(19, 22, 29, 30) and that 0% reduction would be observed in the standard of care control. After accounting for 20% drop-out,(31) we determined that enrollment of 42 patients would be needed to evaluate the primary endpoint with 80% statistical power. This is well beyond the sample size reported by previous studies investigating our secondary endpoints of interest.(32–36)
During research restrictions imposed during the coronavirus disease 2019 (COVID-19) pandemic, the study Data Safety and Monitoring Board (DSMB) recommended an interim analysis be performed after 2/3 study enrollment. Conditional power based on the accrued sample size was 0.96, providing justification for not taking the trial to completion.(37) Consequently, the DSMB unanimously recommended early termination of the trial given completion of the primary outcome.
The efficacy analyses included all patients who were randomized, whereas the safety analysis included all standard of care control patients and only those exercise training patients who completed at least one exercise session.
The main analyses of the primary endpoint were performed with the use of paired t-tests. Both between-group and within-group comparisons were performed. Two-sided p-values of <0.05 (equivalent to a one-sided level of 0.025) indicated statistical significance. 95% confidence intervals for odds ratios were calculated, but without adjustments for multiple comparisons. SAS (Cary, NC) Version 9.4 was used for all statistical analysis.
The secondary endpoints are presented as means with standard deviations. Both between-group and within-group comparisons were performed. Continuous endpoints were analyzed with the use of paired t-tests and categorical endpoints by Chi-squared and Fisher’s exact test where appropriate.
Using similar statistical methods, clinically important subgroup analyses were performed to compare MRI-PDFF responders (≥30% relative reduction in MRI-PDFF) to non-responders(38) and exercise responders (≥10% gain in VO2peak) to non-responders.(39)
Liver fibrosis stage and NAS were analyzed with the use of an analysis of covariance (ANCOVA) model.
Results
Patient Characteristics
From May 2018 through February 2021, 31 patients were screened for this study. Three were excluded after screening (two declined and one was excluded because they were actively smoking) (Figure 1). Twenty-eight patients were randomly assigned to exercise training (18 patients) or standard of care control (10 patients). In total, 24 patients (86%) completed the trial (e.g., seen for their final scheduled visit) with two patients in each group not completing the trial (each subject expressed a personal choice to discontinue study participation); less subject drop-out was encountered than projected by the original sample size estimate. Information for the primary and secondary outcomes was available for all 24 patients who completed the trial in order for intention to treat analysis to be performed. Additionally, exercise adherence was excellent. Overall exercise session completion rate was 82%. Moreover, 89% of exercise subjects met the a priori definition of exercise adherence (completion of ≥80% of exercise sessions).
Figure 1.
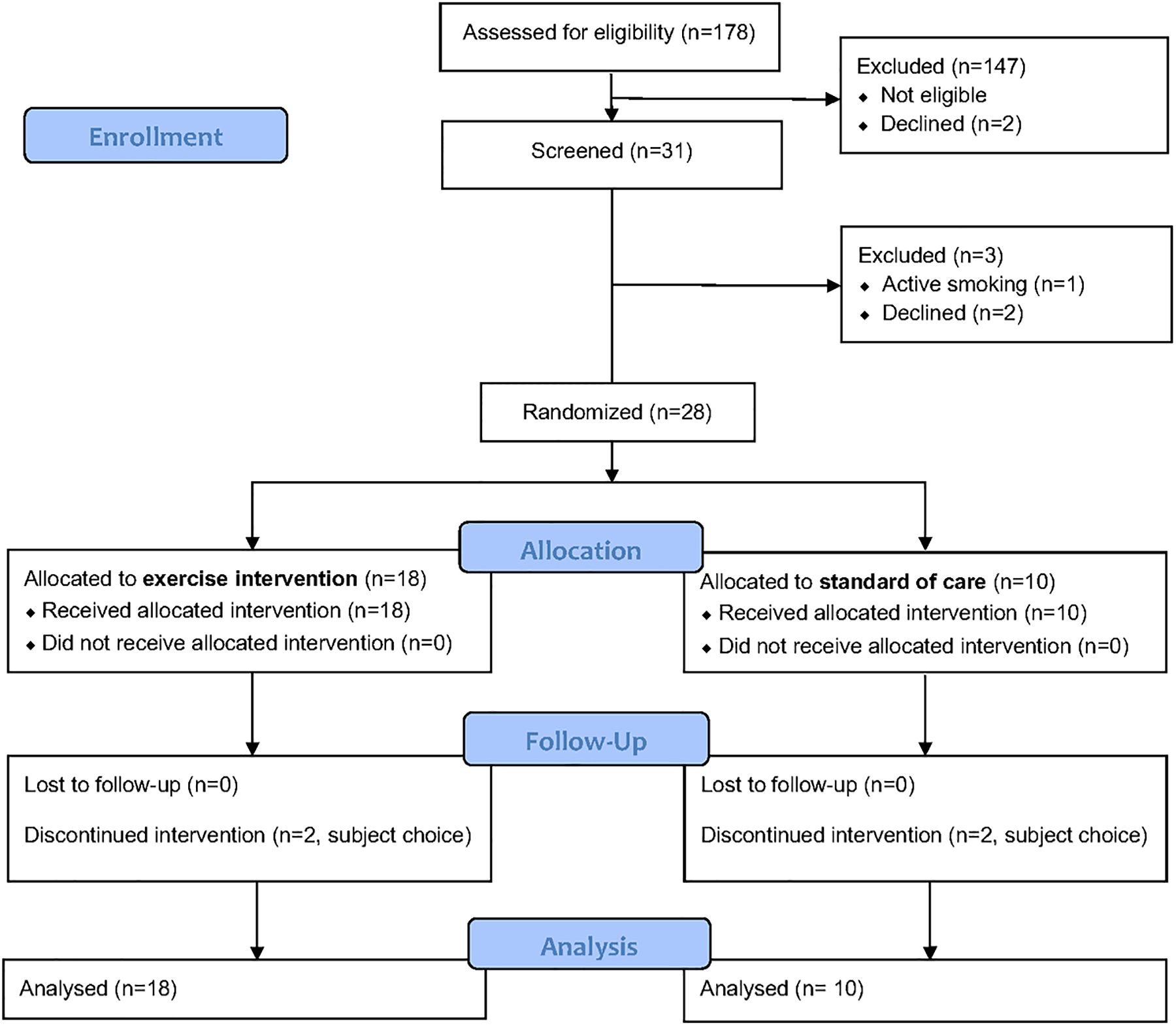
NASHFit Trial CONSORT Diagram
The majority of the patients were White (92%), were women (61%) and were hypertensive (68%). Diabetes was less common (39%). The mean age was 50 years (range 25 to 69 years), the mean body weight was 102.1 kg and the mean body mass index (BMI) was 34.6 kg/m2. Seventy-nine percent of patients were obese. A total of 17 patients (61%) had stage F0/F1 fibrosis, six (21%) had stage F2, four (14%) had stage F3 and one (4%) had stage F4. Mean NAS was 5.2.
Demographic and baseline clinical characteristics were similar between the two trial groups (Table 1). Specifically, the groups were well matched for weight, BMI, waist/hip circumference and histologic NASH activity, including NAS and liver fibrosis stage.
Table 1.
Baseline comparisons between Control and Exercise participants
| Control (n=10) | Exercise (n=18) | p-value | |
|---|---|---|---|
| Demographics | |||
| Age, yrs | 45.0 (10.2) | 52.9 (11.5) | 0.082 |
| Female sex, n (%) | 5 (50.0) | 12 (66.7) | 0.444 |
| BMI, kg/m2 | 35.1 (4.9) | 34.3 (4.9) | 0.659 |
| Alcohol consumption (g/day) | 2.1 (4.6) | 0.7 (1.3) | 0.362 |
| Metabolic risk | |||
| Comorbidities, n (%) | |||
| Diabetes | 5 (50.0) | 6 (33.3) | 0.444 |
| Hyperlipidemia | 7 (70.0) | 11 (61.1) | 0.703 |
| Hypertension | 5 (50.0) | 14 (77.8) | 0.210 |
| Medication use, n (%) | |||
| Cholesterol lowering (all) | 4 (40.0) | 8 (44.4) | 0.999 |
| Statin | 4 (40.0) | 6 (33.3) | 0.999 |
| Fibrate | 0 (0.0 0 | 1 (5.6) | 0.999 |
| Antihyperglycemic (all) | 5 (50.0) | 7 (38.9) | 0.698 |
| Metformin | 3 (30.0) | 5 (27.8) | 0.999 |
| Sulfonylurea | 1 (10.0) | 1 (5.6) | 0.999 |
| GLP-1 | 0 (0.0) | 1 (5.6) | 0.999 |
| SGLT2 | 1 (10.0) | 0 (0.0) | 0.357 |
| Insulin | 1 (10.0) | 2 (11.1) | 0.999 |
| Antihypertensive (all) | 5 (50.0) | 14 (77.8) | 0.210 |
| Diuretic | 0 (0.0) | 5 (27.8) | 0.128 |
| ACE/ARB | 4 (40.0) | 4 (22.2) | 0.400 |
| BB | 2 (20.0) | 3 (16.7) | 0.999 |
| CCB | 0 (0.0) | 2 (11.1) | 0.524 |
| Aspirin 81mg/d | 0 (0.0) | 2 (11.1) | 0.524 |
| Hemoglobin A1c, % | 6.3 (1.2) | 6.3 (1.2) | 0.961 |
| HOMA-IR | 9.5 (13.8) | 13.8 (11.3) | 0.365 |
| VO2peak, mL/kg/min | 23.9 (5.4) | 20.3 (5.1) | 0.096 |
| NASH phenotyping | |||
| Vitamin E, n (%) | 2 (20.0) | 3 (16.7) | 0.999 |
| Liver fat (MRI-PDFF), % | 22.5 (10.5) | 20.4 (7.7) | 0.553 |
| NAS | 5.2 (0.6) | 5.2 (1.0) | 0.928 |
| Steatosis | 2.5 (1.0) | 3.0 (2.0) | 0.396 |
| Lobular inflammation | 1.0 (1.0) | 1.5 (2.0) | 0.341 |
| Hepatocyte ballooning | 1.0 (2.0) | 1. 0 (1.0) | 0.327 |
| Fibrosis stage, n (%) | 0.125 | ||
| 0 | 1 (10.00%) | 2 (11.11%) | |
| 1 | 4 (40.00%) | 10 (55.56%) | |
| 2 | 4 (40.00%) | 2 (11.11%) | |
| 3 | 0 (0%) | 4 (22.22%) | |
| 4 | 1 (10.00%) | 0 (0%) | |
ACE=angiotensin converting enzyme; ARB=angiotensin receptor blocker; BB=beta blocker; BMI=body mass index; CCB=calcium channel blocker; GLP=glucagon like peptide; HOMA-IR=homeostatic model assessment for insulin resistance; MRI=magnetic resonance imaging; NAFLD=nonalcoholic fatty liver disease; NAS=NAFLD Activity Score; PDFF=proton density fat fraction; SGLT=sodium-glucose transport protein; VO2=oxygen consumption
Randomization was upheld as there were no significant differences between the exercise intervention group and the standard of care control
Continuous variables reported as mean +/− SD,
No subjects were taking pioglitazone or obeticholic acid
Efficacy
Exercise Training vs. Standard Clinical Care
PAI-1 level was significantly decreased for the exercise training group when compared to the standard of care control group (−40+/− 100 vs. +70 +/− 63 ng/mL, p=0.02) (Figure 2). Other markers of hemostasis as well as global coagulation testing did not show any significant between group differences (Table S1).
Figure 2. Change in PAI-1 comparing exercise training to standard clinical care.
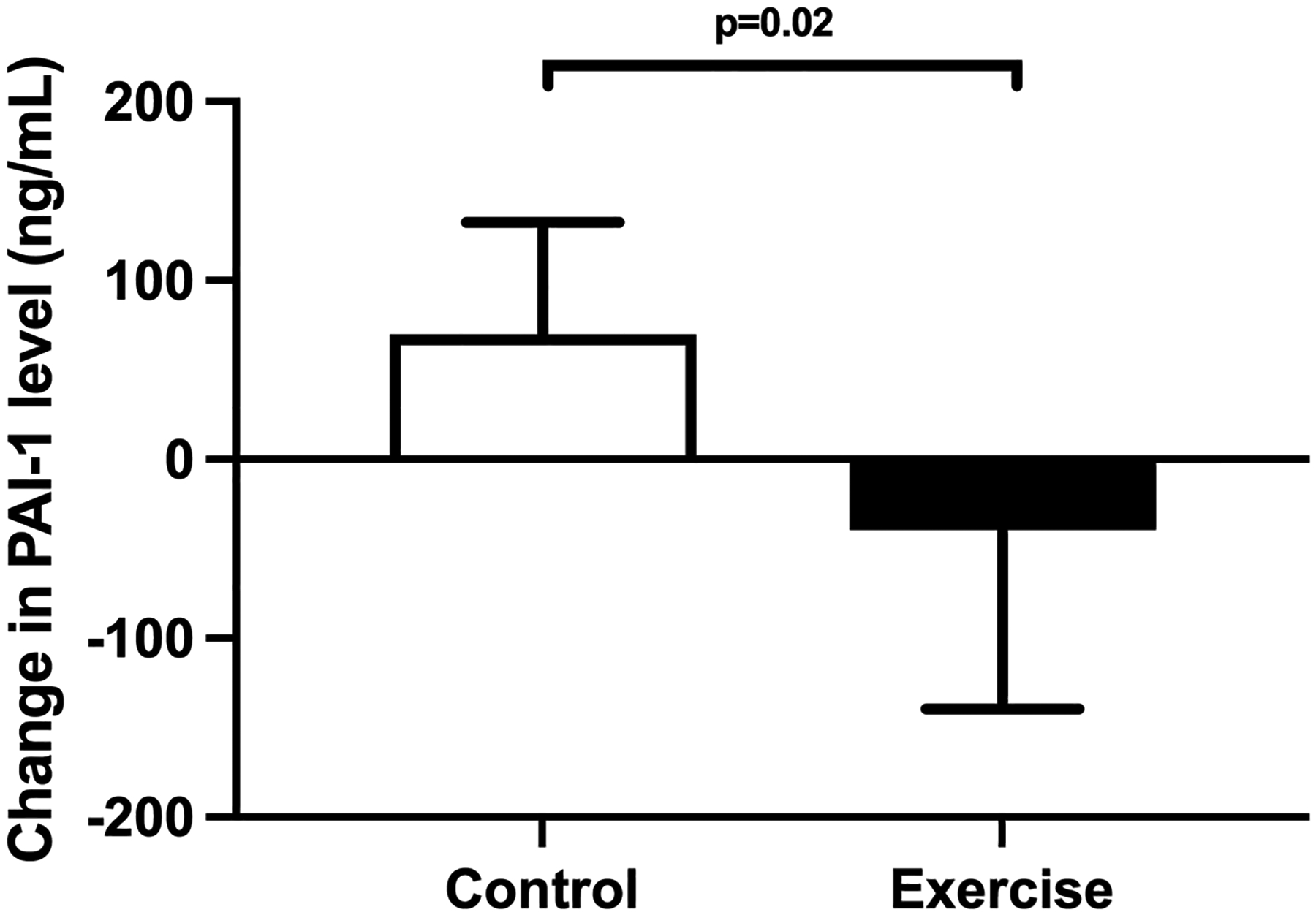
PAI-1 level was significantly decreased for the exercise training group when compared to the standard of care control group (−40+/− 100 vs. +70 +/− 63 ng/mL, p=0.02)
Exercise training decreased liver fat as measured by MRI-PDFF (−4.7 +/− 5.6 vs. 1.2 +/− 2.8% absolute liver fat, p=0.01) (Figure 3). Forty percent of exercise subjects had ≥30% relative reduction in MRI-PDFF, the threshold for histologic response,(38) compared to 13% of control subjects. Changes were also observed for exploratory biomarker CK-18 when comparing exercise intervention to standard of care control patients (−62 +/− 45 vs. +71 +/− 143, p=0.07), however this was not statistically significant between groups (Table 2).
Figure 3. Change in MRI-PDFF measured liver fat comparing exercise training to standard clinical care.
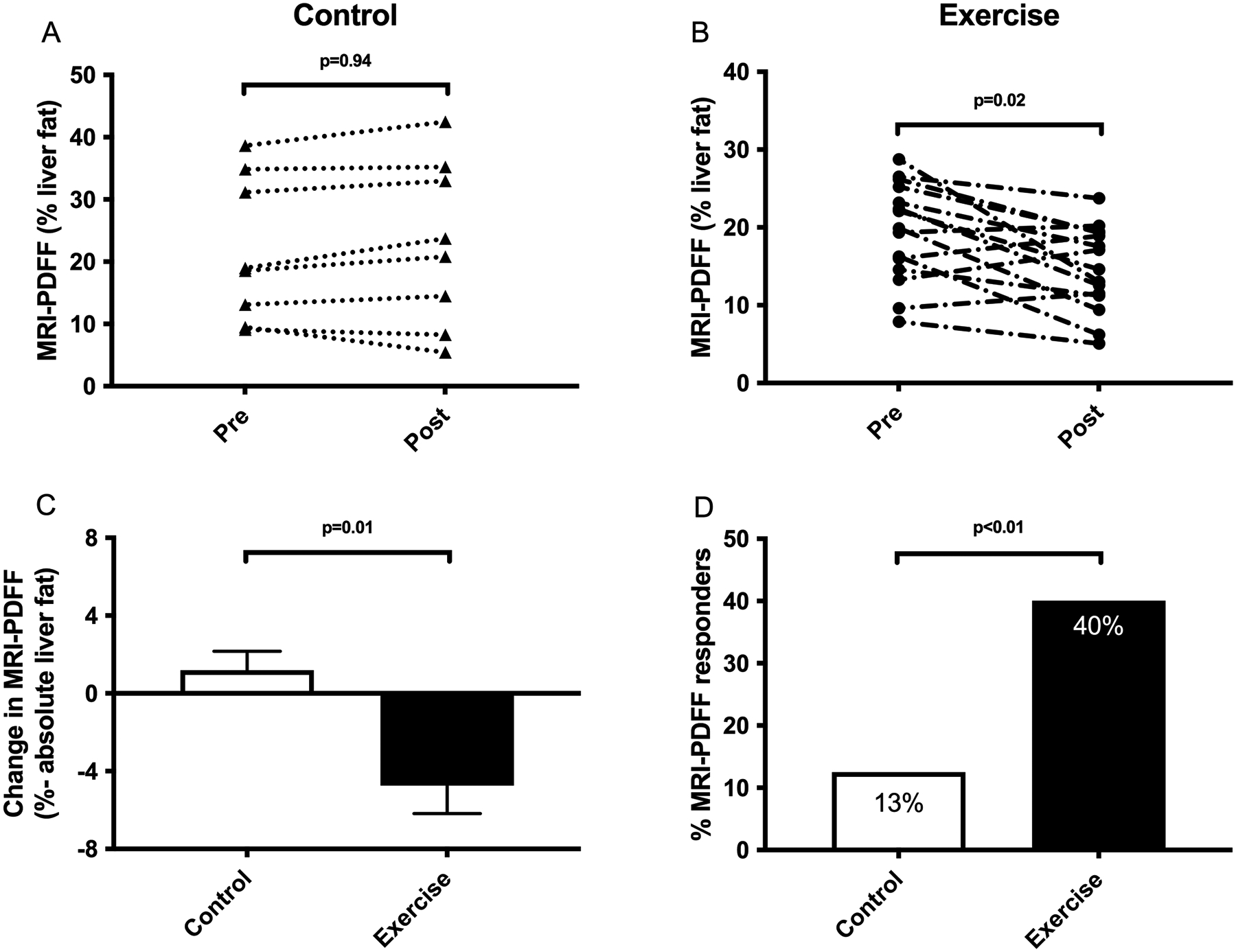
(A) No significant change was observed in MRI-PDFF in standard of care subjects. (B) MRI-PDFF significantly decreased following exercise training. (C) Exercise training decreased liver fat as measured by MRI-PDFF (−4.7 +/− 5.6 vs. 1.2 +/− 2.8% absolute liver fat, p=0.01). (D) 40% of exercise subjects had ≥30% relative reduction in MRI-PDFF, the threshold for histologic response, compared to 13% of standard of care subjects.
Table 2-.
Outcome measures: Non-invasive assessment of liver disease severity
| Control (n=10) | Exercise (n=18) | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | Post | Within group p-value | Baseline | Post | Within group p-value | Between group p-value |
|
| Clinical Decision Aids | |||||||
| NFS | −1.25 (1.80) | −1.46 (2.04) | 0.728 | −1.58 (0.93) | −1.52 (0.93) | 0.898 | 0.653 |
| FIB-4 | 1.67 (1.88) | 1.64 (2.26) | 0.696 | 1.24 (0.52) | 1.20 (0.52) | 0.120 | 0.861 |
| APRI index | 0.84 (0.75) | 0.72 (0.76) | 0.376 | 0.60 (0.27) | 0.46 (0.15) | 0.017 | 0.904 |
| AST/ALT | 0.84 (0.37) | 0.71 (0.32) | 0.641 | 0.74 (0.25) | 0.76 (0.22) | 0.915 | 0.779 |
| Serum Biomarkers | |||||||
| Adiponectin, ng/mL | 3,641.0 (2,004.2) | 3,482.3 (1,728.8) | 0.450 | 3,697.73 (3,727.0) | 1,065.1 (1,220.7) | 0.865 | 0.500 |
| CK18, IU/L | 125.6 (117.7) | 168.3 (197.6) | 0.279 | 425.3 (344.6) | 363.9 (334.2) | 0.012 | 0.074 |
| Imaging biomarkers | |||||||
| MRI-PDFF liver fat, % | 22.5 (13.3) | 23.8 (19.3) | 0.265 | 20.4 (7.7) | 14.7 (5.4) | 0.005 | 0.011 |
ALT=alanine aminotransferase; APRI=AST to platelet ratio; AST=aspartate aminotransferase; CK=cytokeratin; FIB-4=Fibrosis-4 index; MCID=minimal clinically important difference; MRI=magnetic resonance imaging; NAFLD=nonalcoholic fatty liver disease; NFS=NAFLD Fibrosis Score; PDFF=proton density fat fraction
MRI-PDFF was significantly improved by exercise training with an absolute reduction of −4.7% compared to an absolute gain of +1.2% for control subjects. Importantly, 40% of exercise subjects had ≥30% relative reduction in MRI-PDFF, the threshold for histologic response, compared to 13% of control subjects.
reported as mean +/− SD
Cardiorespiratory fitness improved more with exercise training as greater gain in VO2peak (+3.0 +/− 5.6 vs. −1.8 +/− 5.1 mL/kg/min, p=0.05) was observed in comparison to standard clinical care (Table 3). 44% of exercise subjects had ≥10% gain in VO2peak,(39) the threshold to improve overall mortality, compared to 0% of control subjects (Figure 4).
Table 3-.
Outcome measures: Anthropometry, Body composition and Cardiorespiratory fitness
| Control (n=10) | Exercise (n=18) | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | Post | Within group p-value | Baseline | Post | Within group p-value | Between group p-value | |
| Anthropometry | |||||||
| BMI, kg/m2 | 35.1 (4.9) | 35.0 (5.4) | 0.168 | 34.3 (4.9) | 32.8 (4.5) | 0.104 | 0.089 |
| Weight, kg | 108.6 (20.4) | 110.1 (23.4) | 0.896 | 96.8 (13.8) | 94.1 (12.3) | 0.546 | 0.007 |
| Weight loss goal, n (%) | |||||||
| >7–10% | 0 (0.0) | 1 (6.3) | 0.999 | ||||
| Waist circumference, in | 46.2 (4.8) | 46.3 (4.8) | 0.393 | 44.5 (4.0) | 42.8 (3.2) | 0.074 | 0.073 |
| Hip circumference, in | 45.8 (5.7) | 46.7 (6.3) | 0.001 | 45.7 (3.7) | 44.6 (4.4) | 0.163 | 0.005 |
| Waist to hip ratio | 1.01 (0.08) | 1.00 (0.08) | 0.247 | 0.97 (0.07) | 0.97 (0.07) | 0.824 | 0.385 |
| Skinfold thickness, mm | |||||||
| Abdominal | 24.2 (6.7) | 24.4 (5.7) | 0.622 | 20.4 (5.3) | 20.2 (4.8) | 0.406 | 0.940 |
| Triceps | 16.1 (7.9) | 16.2 (8.9) | 0.290 | 18.6 (6.6) | 19.0 (7.4) | 0.628 | 0.371 |
| Midaxillary | 20.9 (4.2) | 22.2 (6.2) | 0.477 | 29.4 (3.4) | 20.1 (4.0) | 0.892 | 0.546 |
| Body composition | |||||||
| VAT, lbs | 7.0 (2.4) | 7.2 (2.8) | 0.853 | 5.8 (1.9) | 5.2 (1.1) | 0.108 | 0.030 |
| SAT, lbs | 230.5 (42.3) | 233.7 (48.2) | 0.819 | 211.2 (28.5) | 200.5 (26.9) | 0.031 | 0.121 |
| VAT-SAT Ratio | 0.30 (0.05) | 0.30 (0.06) | 0.901 | 0.27 (0.07) | 0.20 (0.05) | 0.090 | 0.210 |
| Fat free (muscle) mass, lbs | 122.8 (35.6) | 127.6 (29.2) | 0.581 | 112.5 (24.9) | 111.7 (27.2) | 0.767 | 0.254 |
| Body fat, % | 41.9 (10.7) | 38.4(11.1) | 0.257 | 44.5 (7.4) | 42.3 (8.0) | 0.028 | 0.069 |
| Body fat, lbs | 98.6 (29.8) | 93.5 (35.2) | 0.961 | 94.6 (24.5) | 85.4 (22.0) | 0.021 | 0.025 |
| Android fat mass, % | 54.4 (7.5) | 51.3 (8.5) | 0.229 | 54.2 (6.0) | 51.0 (7.4) | 0.049 | 0.259 |
| Gynoid fat mass, % | 39.2 (11.0) | 25.6 (11.1) | 0.420 | 42.6 (8.6) | 40.6 (8.8) | 0.036 | 0.088 |
| Android to gynoid radio | 1.43 (0.21) | 1.48 (0.18) | 0.474 | 1.34 (0.24) | 1.29 (0.23) | 0.375 | 0.648 |
| Liver volume, cc | 2,584.0 (579.3) | 2,596.9 (569.9) | 0.395 | 2,304.3 (482.5) | 2,109.0 (411.4) | 0.010 | 0.021 |
| Cardiorespiratory fitness | |||||||
| Resting VO2, L/min | 3.5 (0.6) | 3.5 (0.6) | 0.441 | 3.7 (1.0) | 4.0 (1.1) | 0.463 | 0.812 |
| VO2peak, mL/kg/min | 23.9 (5.3) | 22.7 (3.2) | 0.347 | 20.3 (5.1) | 23.6 (6.3) | 0.051 | 0.054 |
| Resting HR, bpm | 72.9 (11.4) | 81.8 (12.1) | 0.308 | 78.1 (14.8) | 81.7 (13.6) | 0.13 | 0.198 |
BMI=body mass index; SAT=subcutaneous adipose tissue; VAT=visceral adipose tissue; VO2=oxygen uptake
Cardiorespiratory fitness improved with exercise training as greater gain in VO2peak +3.0 mL/kg/min vs. −1.8 mL/kg/min was observed in comparison to a −1.8 mL/kg/min reduction with standard clinical care. 44% of exercise subjects had ≥10% gain in VO2peak, compared to 0% of control subjects. This was seen in parallel with improvements in hip and waist circumference, total body fat, visceral adipose tissue and liver volume.
reported as mean +/− SD
Figure 4. Change in cardiorespiratory fitness comparing exercise training to standard clinical care.
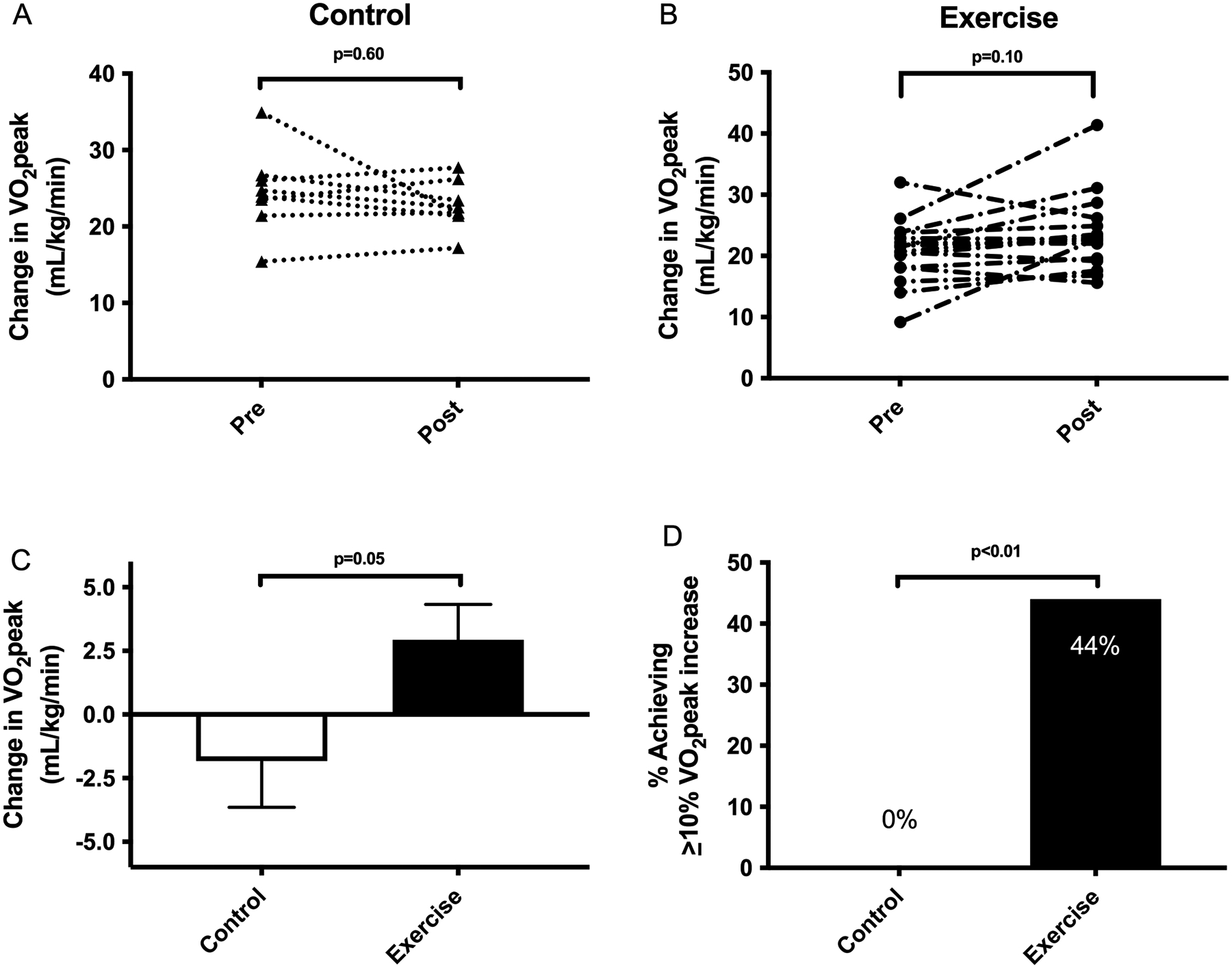
(A) No significant change was observed in VO2peak for standard of care subjects. (B) There was a trend towards significance in VO2peak improvement following exercise training. (C) Cardiorespiratory fitness improved more with exercise training as greater gain in VO2peak (+3.0 +/− 5.6 vs. −1.8 +/− 5.1 mL/kg/min, p=0.05) was observed in comparison to standard clinical care. (D) 44% of exercise subjects had ≥10% gain in VO2peak, the threshold to improve overall mortality, compared to 0% of standard clinical care subjects.
Modest weight loss was observed when comparing the exercise training group to the standard of care control group (−2.5 +/− 3.5 vs. +1.5 +/− 1.9 kg, p<0.01), however, no statistically significant change was observed in BMI. Only one exercise subject (6%) achieved the ≥7% threshold of weight loss to see histologic improvement vs. 0% in the standard of care control. No significant changes in dietary intake were observed (Table S2).
Hip circumference was reduced following exercise training (−0.7 +/− 1.8 vs. 1.1 +/− 0.6 inches, p<0.01) and a trend towards significance was observed in waist circumference change (−0.7 +/− 1.3 vs. +0.5 +/− 1.6, p=0.07) when compared to the standard of care control group (Fig S1). Body composition changes were observed for visceral adipose tissue (−0.6 +/− 0.8 vs. 0.0 +/− 0.4 pounds, p=0.03), and total body fat (−5.4 +/− 7.7 vs. +2.2 +/− 0.1 pounds, p=0.02) when comparing the two groups. Liver volume was reduced by exercise training (−7.0 +/− 9.2 vs. +2.7 +/− 6.5%, p=0.02) compared to the standard of care control.
Exercise training improved glucose metabolism (Fig S2) and lowered fasting glucose level (−14 +/− 32 vs. 20 +/− 43 mg/dL, p=0.04) and HbA1c (−0.4 +/− 0.6 vs. 0.4 +/−0.7%, p<0.01) compared to the control condition (Table 4).
Table 4-.
Outcome measures: Biochemistry, Lipids and Blood pressure
| Control (n=10) | Exercise (n=18) | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | Post | Within group p-value | Baseline | Post | Within group p-value | Between group p-value | |
| Biochemistry | |||||||
| Albumin, g/dL | 4.6 (0.3) | 4.6 (0.5) | 0.911 | 4.6 (0.3) | 4.7 (0.3) | 0.326 | 0.540 |
| Alkaline phosphatase, IU/L | 72.1 (37.3) | 86.3 (39.9) | 0.143 | 96.9 (46.5) | 89.9 (33.9) | 0.243 | 0.063 |
| ALT, IU/L | 54.7 (20.2) | 52.6 (15.7) | 0.311 | 61.5 (29.3) | 44.2 (16.1) | 0.002 | 0.251 |
| AST, IU/L | 44.4 (22.9) | 35.4 (13.9) | 0.272 | 42.9 (20.3) | 32.2 (10.6) | 0.008 | 0.671 |
| Ferritin, mcg/L | 264.7 (145.3) | 238.0 (156.1) | 0.617 | 128.4 (97.6) | 97.6 (77.2) | 0.002 | 0.724 |
| Glucose (fasting), mg/dL | 136.6 (53.5) | 155.8 (88.2) | 0.223 | 127.8 (34.5) | 116.9 (29.1) | 0.103 | 0.039 |
| Hemoglobin A1c, % | 6.3 (1.2) | 6.6 (1.7) | 0.109 | 6.3 (1.2) | 6.1 (0.8) | 0.021 | 0.006 |
| HOMA-IR | 9.5 (6.2) | 12.6 (14.9) | 0.511 | 13.8 (11.3) | 8.8 (6.5) | 0.142 | 0.160 |
| Insulin, IU/mL | 27.3 (14.5) | 31.1 (29.9) | 0.807 | 40.0 (27.3) | 32.0 (20.0) | 0.296 | 0.419 |
| WBC count, 109 | 6.3 (1.6) | 6.5 (1.6) | 0.518 | 7.1 (1.8) | 6.6 (1.7) | 0.310 | 0.247 |
| Lipids | |||||||
| Total cholesterol, mmol/L | 207.0 (33.8) | 184.0 (25.0) | 0.312 | 186.4 (46.4) | 187.0 (50.7) | 0.964 | 0.350 |
| LDL, mmol/L | 131.0 (36.6) | 109.2 (33.2) | 0.415 | 106.4 (39.2) | 108.6 (40.4) | 0.925 | 0.476 |
| HDL, mmol/L | 43.2 (10.5) | 29.6 (11.7) | 0.398 | 42.2 (8.2) | 44.0 (7.1) | 0.466 | 0.188 |
| Triglyceride, mmol/L | 242.8 (135.1) | 228.0 (108.2) | 0.988 | 188.8 (82.1) | 171.9 (76.0) | 0.995 | 0.987 |
| Cholesterol/HDL ratio | 4.8 (0.9) | 5.2 (2.1) | 0.530 | 4.5 (1.3) | 4.3 (1.1) | 0.381 | 0.931 |
| Blood pressure | |||||||
| SBP, mmHg | 133.4 (15.6) | 124.8 (6.8) | 0.017 | 127.3 (13.9) | 122.7 (12.9) | 0.206 | 0.274 |
| DBP, mmHg | 81.5 (9.6) | 82.4 (3.1) | 0.941 | 79.8 (9.6) | 77.6 (12.7) | 0.507 | 0.712 |
ALT=alanine aminotransferase; AST=aspartate aminotransferase, DBP=diastolic blood pressure; HDL= high density lipoprotein; HOMA-IR=homeostatic model assessment of insulin resistance; LDL=low density lipoprotein; SBP=systolic blood pressure; WBC=white blood cell
Glucose metabolism was favorably changed by exercise training with significant reductions in fasting glucose, hemoglobin A1c and a trend towards reduction in HOMA-IR.
reported as mean +/− SD
Health-related quality of life was improved by exercise training based on lessened pain interference and improved sleep quality (Table S3).
MRI-PDFF responders compared to non-responders
In general, MRI-PDFF responders (n=6) were similar to non-responders (n=9) in baseline characteristics (Table S4). MRI-PDFF responders were more likely to have a reduction in ALT (p<0.01), fasting glucose (p<0.01) and waist circumference (p=0.09) which were observed in parallel with gains in VO2peak (p=0.10).
Exercise responders compared to non-responders
Exercise responders (n=7) were similar to non-responders (n=9) in baseline characteristics with the exception of responders having less initial body fat (39.6 vs. 47.7%, p=0.03) but greater fasting glucose levels (150.7 vs. 113.9 mg/dL, p=0.03) (Table S5). Exercise responders had greater reduction in liver volume (p<0.01), MRI-PDFF (p=0.01), hemoglobin A1c (p=0.02), visceral adipose tissue (p=0.04) and BMI (p=0.09).
Histologic activity
No significant differences were observed when stratifying by liver fibrosis stage (Tables S6 and S7). Only patients with NAS 4 or 5 achieved exercise response with ≥10% improvement in VO2peak. No patients with NAS ≥6 achieved exercise response.
Safety
Nine adverse events were reported (seven not related or unlikely to be related to exercise intervention), only one of which was deemed a serious adverse event but was ultimately adjudicated to be unrelated to the trial as a standard of care control subject elected to have knee surgery to treat longstanding osteoarthritis. No adverse events occurred during VO2peak fitness testing.
Discussion
This is the first study to examine the effects of exercise training on clotting risk and its mediators in adults with NASH. Among patients with biopsy-proven NASH, 20-weeks of aerobic exercise training was superior to standard clinical care with respect to improving hemostasis because serum PAI-1 level was significantly decreased in response to exercise training. This was seen independent of significant weight loss, dietary change or baseline histologic activity and in parallel with improvement in MRI-PDFF, cardiorespiratory fitness, body composition, glucose metabolism, exploratory biomarkers and HRQOL. Importantly, exercise training led to greater rates of MRI-PDFF response at the threshold required for histologic response with 40% of exercise subjects achieving ≥30% relative reduction versus 13% of standard of care control subjects, similar to that reported by recent NASH drug trials that are advancing in trial phase towards hopeful future regulatory approval.(38, 40–42) Exercise training also achieved greater rates of VO2peak improvement at the threshold required to improve overall mortality, with 44% of exercise subjects improving VO2peak by ≥10% versus 0% of standard clinical care subjects. Taken together, these data provide further validation for prescribing exercise as medicine to treat all patients with NASH (with or without liver fibrosis), in order to improve liver-specific and extrahepatic outcomes.
Because there is no known cure or regulatory agency approved drug therapy for NASH, this illustrates the importance of using exercise as medicine. Current clinical guidelines from multiple leading academic societies(3, 43) focus broadly on lifestyle modification with dietary change and increased physical activity for all patients with NAFLD. However, it remains unclear as to how best prescribe exercise as a specific treatment. Currently, lifestyle modification is seen more as a vehicle to achieve the ≥7–10% weight-loss required for histologic improvement rather than a weight neutral intervention.
There is a growing body of evidence in support of weight-loss independent benefits of exercise training in patients with NAFLD and NASH, including a reduction in hepatic steatosis and liver inflammation, improvement in vascular endothelial function and increased cardiorespiratory fitness.(7, 44–46) With this trial, we found additional highly significant weight-loss independent benefits of exercise training, including improvements in hemostasis, favorable changes in body composition and improvement in glycemic control. Even more importantly, we found exercise training leads to both MRI-PDFF and fitness response at or above the thresholds which surrogate for histologic response and overall mortality respectively.
This is highly significant for three reasons. One, patients who progress in liver fibrosis stage, including those who develop cirrhosis with complications of end stage liver disease, develop increasing hemostatic abnormalities which ultimately lead to greater rates of arterial and venous thromboembolism.(9–12) Exercise training offers the promise to reverse and prevent clinically relevant clotting events, which would be expected to reduce morbidity and mortality.(47) Importantly, for each 1 ng/mL decrease in PAI-1, the risk of venous thromboembolism decreases by up to 60%.(16, 17) As a point of reference (albeit from a different population), an increase in PAI-1 to this level would exceed the accepted threshold for venous thromboembolism risk reduction expected for standard chemical prophylaxis in high-risk patients who are immobile. Moreover, for each 1 ng/mL decrease in PAI-1, the risk of future arterial thrombosis (e.g., myocardial infarction) decreases by 4%.(48) Two, it remains unknown if exercise training can improve liver fibrosis stage without significant weight loss. Given our findings that exercise training achieves MRI-PDFF relative reduction of at least 30% at much greater rates, these non-invasive imaging biomarker data routinely used in early-phase NASH drug trials suggest exercise training offers the potential to improve liver histology independent of weight loss and confirm a recent small pilot study by O’Gorman et al.(49) which was not limited solely to patients with NASH. Three, long-term patient outcomes in NASH clinical trials are lacking, as are short-term cardiovascular outcomes. To date, most NASH clinical trials, especially drug trials, focus exclusively on histologic improvement despite cardiovascular disease being the leading cause of death. Because cardiorespiratory fitness is strongly associated with cardiovascular disease and cardiovascular death, the findings that exercise training leads to considerable gains in cardiorespiratory fitness above the threshold to reduce overall and cardiovascular mortality, bears noting. If sustained, exercise training offers the promise to be as, if not more, effective at reducing both cardiovascular and overall mortality in patients with NAFLD and NASH than any pharmacologic treatment that may be developed in the future.
Previous clinical trials have provided conflicting evidence about how exercise training affects body composition, where reductions in visceral or subcutaneous adipose tissue were highly variable.(32, 34, 50) In this study, we demonstrate similar results to those published by Houghton et al.(32) who found visceral adipose tissue was significantly reduced after 12-weeks of exercise training. This is important because visceral adipose tissue contributes to liver disease progression by promoting the production and release of proinflammatory cytokines and adipokines which lead to hepatic de novo lipogenesis, inflammation and insulin resistance, all of which promote NAFLD development and disease progression to NASH.(51) Reduction in adipose tissue may also mediate PAI-reduction because PAI-1 is regularly synthesized in adipose tissue.(52)
Previous clinical trials in patients with NAFLD have also not demonstrated consistent improvement in glucose metabolism despite our longstanding understanding that physical activity and exercise training can improve glycemic control in patients with diabetes. In this study, we found significant improvement in fasting glucose, hemoglobin A1c and a trend towards improvement in insulin resistance following exercise training. This is important because better glycemic control would be expected to improve disease severity in NAFLD and NASH; a recent report from the NASH CRN(53) demonstrated that glycemic control predicts the severity of liver histology, including hepatocyte ballooning and liver fibrosis, providing further evidence that optimizing glycemic control offers promise to modify the risk of liver fibrosis progression in patients with NASH.
Our study has multiple strengths inherent to the study design (e.g., direct supervision of all exercise sessions, control of additional exercise beyond that prescribed by the study protocol with remote monitoring using wearable fitness technology) and study population (e.g., limitation to solely biopsy-proven NASH). Possible limitations include the sample size (although this study is powered similar to previously published RCTs using an exercise intervention in patients with NAFLD), lack of allocation concealment, study population composed largely of early-stage NASH (61% were F0/F1) and non-Hispanic whites, lack of histologic endpoints (MRI-PDFF was used as a surrogate), lack of long-term clinical outcomes (cardiorespiratory fitness was used as a surrogate of mortality), inability to show global changes in hemostasis with TEG (likely due to the sample size, low-rates of baseline hyperfibrinolysis, measuring fibrinolysis at 30 minutes and not extending to a 60 minute window or even uncontrolled variation in pre-analytic variables) and a lack of statistically significant changes in exploratory serum biomarkers. Additionally, we do recognize that in the real-world setting, in-person training multiple days a week may not be feasible for every patient with NASH.
Conclusion
This clinical trial showed that independent of weight loss or dietary change, exercise training resulted in a significantly greater decrease in clotting risk than standard clinical care in patients with NASH. This was seen in parallel with MRI-PDFF reduction and improvement in cardiorespiratory fitness, both above the threshold required for histologic response and improvement in overall and cardiovascular mortality. Future studies are required to determine if exercise training can directly impact patient outcomes by lowering rates of thromboembolism, improving liver histology and/or lessening mortality.
Supplementary Material
Acknowledgments
No manuscript writing assistance was used in preparation of this work.
Grants and Financial Support:
This grant was funded in part by NIH grant L30 DK118601.
This project was also funded, in part, under a grant with the Pennsylvania Department of Health using Tobacco CURE Funds. The Department specifically disclaims responsibility for any analyses, interpretations or conclusion.
The study was supported by NIH/NCATS Grant UL1TR000127 and UL1TR002014.
Conflict of Interest:
Dr. Stine receives or has received research support from Grifols, Inc., Noom, Inc. and Novo Nordisk.
Dr. Loomba serves as a consultant or advisory board member for Arrowhead Pharmaceuticals, AstraZeneca, Bird Rock Bio, Boehringer Ingelheim, Bristol-Myer Squibb, Celgene, Cirius, CohBar, Conatus, Eli Lilly, Galmed, Gemphire, Gilead, Glympse bio, GNI, GRI Bio, Intercept, Ionis, Janssen Inc., Merck, Metacrine, Inc., NGM Biopharmaceuticals, Novartis, Novo Nordisk, Pfizer, Prometheus, Sanofi, Siemens, and Viking Therapeutics. In addition, his institution has received grant support from Allergan, Boehringer-Ingelheim, Bristol-Myers Squibb, Cirius, Eli Lilly and Company, Galectin Therapeutics, Galmed Pharmaceuticals, GE, Genfit, Gilead, Intercept, Grail, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, NuSirt, Pfizer, pH Pharma, Prometheus, and Siemens. He is also co-founder of Liponexus, Inc.
All other authors have no relevant conflicts of interest to report.
Abbreviations:
- ALT
alanine aminotransferase
- ANCOVA
analysis of covariance
- AST
aspartate aminotransferase
- BMI
body mass index
- CK
cytokeratin
- COVID19
coronavirus disease 2019
- CRN
clinical research network
- DSMB
data safety and monitoring board
- DXA
dual-energy x-ray absorptiometry
- FIT
fitness intervention in thrombosis
- HbA1c
hemoglobin A1c
- HDL
high-density lipoprotein
- HRQOL
health-related quality of life
- INR
international normalized ratio
- LDL
low-density lipoprotein
- MRI
magnetic resonance imaging
- NAFLD
nonalcoholic fatty liver disease
- NAS
NASH Activity Score
- NASH
nonalcoholic steatohepatitis
- PAI
plasminogen activator inhibitor
- PDFF
proton density fat fraction
- PROMIS
Patient-Reported Outcomes Measurement Information System
- TEG
thromboelastography
- VO2peak
maximal oxygen consumption
The NASHFit Study Team
Administrative Support: Cindy Strine, Rachel Wentzel
Biostatistical and Data Collection Support: Sara Marlin
Center for NMR Research: Chris Sica, Jeff Vesek
Co-investigators: Elaine Eyster, Lawrence Sinoway
Clinical Research Center: Kathy Bentz, Nancy Handley, Brenda Hershey Fell, Shirlynn Mottilla
Clinical Specimen Processing Core: Christine Christ, Susan George
Clinical Trials Office: Terry Novchich
Division of Gastroenterology and Hepatology: Megan Beyer, Kofi Clarke, Tiffany Myers, Martha Glading-Steinruck, Karen Krok, Thomas Ma, Thomas Riley III, Elizabeth Thompson, Heather Tressler
Institute for Personalized Medicine: James Broach, Trang Doan, Sue Patrick, Syndi Reed
Pathology Core Reference Laboratory: Chris Hamilton
Physical Medicine & Rehabilitation Research Laboratory: Kristin Slavoski
University Fitness Center: Deborah Tregea
Footnotes
Clinical trial registration number: NCT03518294
References
- 1.Younossi ZM. Non-alcoholic fatty liver disease - A global public health perspective. J Hepatol 2019;70:531–544. [DOI] [PubMed] [Google Scholar]
- 2.Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, Sebastiani G, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology 2017;65:1557–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, et al. The Diagnosis and Management of Nonalcoholic Fatty Liver Disease: Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology 2017. [DOI] [PubMed] [Google Scholar]
- 4.Loomba R, Lim JK, Patton H, El-Serag HB. AGA Clinical Practice Update on Screening and Surveillance for Hepatocellular Carcinoma in Patients with Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stine JG, Wentworth BJ, Zimmet A, Rinella ME, Loomba R, Caldwell SH, Argo CK. Systematic review with meta-analysis: risk of hepatocellular carcinoma in non-alcoholic steatohepatitis without cirrhosis compared to other liver diseases. Aliment Pharmacol Ther 2018;48:696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Majzoub AM, Nayfeh T, Barnard A, Munaganuru N, Dave S, Singh S, Murad MH, et al. Systematic review with network meta-analysis: comparative efficacy of pharmacologic therapies for fibrosis improvement and resolution of NASH. Aliment Pharmacol Ther 2021;54:880–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thorp A, Stine JG. Exercise as Medicine: The Impact of Exercise Training on Nonalcoholic Fatty Liver Disease. Current Hepatology Reports 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, Friedman SL, et al. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology 2015;149:367–378.e365; quiz e314–365. [DOI] [PubMed] [Google Scholar]
- 9.Spinosa M, Stine JG. Nonalcoholic Fatty Liver Disease-Evidence for a Thrombophilic State? Curr Pharm Des 2020;26:1036–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stine JG, Shah NL, Argo CK, Pelletier SJ, Caldwell SH, Northup PG. Increased risk of Portal Vein Thrombosis in Patients with Cirrhosis due to Non-Alcoholic Steatohepatitis (NASH). Liver Transpl 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stine JG, Argo CK, Pelletier SJ, Maluf DG, Caldwell SH, Northup PG. Advanced non-alcoholic steatohepatitis cirrhosis: A high-risk population for pre-liver transplant portal vein thrombosis. World J Hepatol 2017;9:139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stine JG, Niccum BA, Zimmet AN, Intagliata N, Caldwell SH, Argo CK, Northup PG. Increased risk of venous thromboembolism in hospitalized patients with cirrhosis due to non-alcoholic steatohepatitis. Clin Transl Gastroenterol 2018;9:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tjärnlund-Wolf A, Brogren H, Lo EH, Wang X. Plasminogen activator inhibitor-1 and thrombotic cerebrovascular diseases. Stroke 2012;43:2833–2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Targher G, Bertolini L, Rodella S, Lippi G, Franchini M, Zoppini G, Muggeo M, et al. NASH predicts plasma inflammatory biomarkers independently of visceral fat in men. Obesity (Silver Spring, Md.) 2008;16:1394–1399. [DOI] [PubMed] [Google Scholar]
- 15.Mertens I, Van Gaal LF. Obesity, haemostasis and the fibrinolytic system. Obes Rev 2002;3:85–101. [DOI] [PubMed] [Google Scholar]
- 16.Meltzer ME, Lisman T, de Groot PG, Meijers JC, le Cessie S, Doggen CJ, Rosendaal FR. Venous thrombosis risk associated with plasma hypofibrinolysis is explained by elevated plasma levels of TAFI and PAI-1. Blood 2010;116:113–121. [DOI] [PubMed] [Google Scholar]
- 17.Tang J, Zhu W, Mei X, Zhang Z. Plasminogen activator inhibitor-1: a risk factor for deep vein thrombosis after total hip arthroplasty. J Orthop Surg Res 2018;13:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Womack CJ, Nagelkirk PR, Coughlin AM. Exercise-induced changes in coagulation and fibrinolysis in healthy populations and patients with cardiovascular disease. Sports Med 2003;33:795–807. [DOI] [PubMed] [Google Scholar]
- 19.Killewich LA, Macko RF, Montgomery PS, Wiley LA, Gardner AW. Exercise training enhances endogenous fibrinolysis in peripheral arterial disease. J Vasc Surg 2004;40:741–745. [DOI] [PubMed] [Google Scholar]
- 20.El-Sayed MS, El-Sayed Ali Z, Ahmadizad S. Exercise and training effects on blood haemostasis in health and disease: an update. Sports Med 2004;34:181–200. [DOI] [PubMed] [Google Scholar]
- 21.Francis RM, Romeyn CL, Coughlin AM, Nagelkirk PR, Womack CJ, Lemmer JT. Age and aerobic training status effects on plasma and skeletal muscle tPA and PAI-1. Eur J Appl Physiol 2014;114:1229–1238. [DOI] [PubMed] [Google Scholar]
- 22.el-Sayed MS. Effects of high and low intensity aerobic conditioning programs on blood fibrinolysis and lipid profile. Blood Coagul Fibrinolysis 1996;7:484–490. [DOI] [PubMed] [Google Scholar]
- 23.Sudi KM, Gallistl S, Trobinger M, Payerl D, Weinhandl G, Muntean W, Aigner R, et al. The influence of weight loss on fibrinolytic and metabolic parameters in obese children and adolescents. J Pediatr Endocrinol Metab 2001;14:85–94. [DOI] [PubMed] [Google Scholar]
- 24.Stine JG, Schreibman I, Navabi S, Kang M, Dahmus J, Soriano C, Rivas G, et al. Nonalcoholic steatohepatitis Fitness Intervention in Thrombosis (NASHFit): Study protocol for a randomized controlled trial of a supervised aerobic exercise program to reduce elevated clotting risk in patients with NASH. Contemp Clin Trials Commun 2020;18:100560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313–1321. [DOI] [PubMed] [Google Scholar]
- 26.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singhvi A, Sadowsky HS, Cohen A, Demzik A, VanWagner L, Rinella M, Levitsky J. Resting and Exercise Energy Metabolism After Liver Transplantation for Nonalcoholic Steatohepatitis. Transplant Direct 2017;3:e188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Austin P, Gerber L, Paik JM, Price JK, Escheik C, Younossi ZM. Aerobic capacity and exercise performance in nonalcoholic fatty liver disease. J Sports Med Phys Fitness 2019;59:1376–1388. [DOI] [PubMed] [Google Scholar]
- 29.Stratton JR, Chandler WL, Schwartz RS, Cerqueira MD, Levy WC, Kahn SE, Larson VG, et al. Effects of physical conditioning on fibrinolytic variables and fibrinogen in young and old healthy adults. Circulation 1991;83:1692–1697. [DOI] [PubMed] [Google Scholar]
- 30.de Geus EJ, Kluft C, de Bart AC, van Doornen LJ. Effects of exercise training on plasminogen activator inhibitor activity. Med Sci Sports Exerc 1992;24:1210–1219. [PubMed] [Google Scholar]
- 31.Steins Bisschop CN, Courneya KS, Velthuis MJ, Monninkhof EM, Jones LW, Friedenreich C, van der Wall E, et al. Control group design, contamination and drop-out in exercise oncology trials: a systematic review. PLoS One 2015;10:e0120996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Houghton D, Thoma C, Hallsworth K, Cassidy S, Hardy T, Burt AD, Tiniakos D, et al. Exercise Reduces Liver Lipids and Visceral Adiposity in Patients With Nonalcoholic Steatohepatitis in a Randomized Controlled Trial. Clin Gastroenterol Hepatol 2017;15:96–102.e103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hallsworth K, Thoma C, Hollingsworth KG, Cassidy S, Anstee QM, Day CP, Trenell MI. Modified high-intensity interval training reduces liver fat and improves cardiac function in non-alcoholic fatty liver disease: a randomized controlled trial. Clin Sci (Lond) 2015;129:1097–1105. [DOI] [PubMed] [Google Scholar]
- 34.Hallsworth K, Fattakhova G, Hollingsworth KG, Thoma C, Moore S, Taylor R, Day CP, et al. Resistance exercise reduces liver fat and its mediators in non-alcoholic fatty liver disease independent of weight loss. Gut 2011;60:1278–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shojaee-Moradie F, Cuthbertson DJ, Barrett M, Jackson NC, Herring R, Thomas EL, Bell J, et al. Exercise Training Reduces Liver Fat and Increases Rates of VLDL Clearance But Not VLDL Production in NAFLD. J Clin Endocrinol Metab 2016;101:4219–4228. [DOI] [PubMed] [Google Scholar]
- 36.Zelber-Sagi S, Buch A, Yeshua H, Vaisman N, Webb M, Harari G, Kis O, et al. Effect of resistance training on non-alcoholic fatty-liver disease a randomized-clinical trial. World J Gastroenterol 2014;20:4382–4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piantadosi S Clinical Trials: A Methodologic Perspective, Third Edition. Hoboken, NJ: John Wiley & Sons, Inc. 2017. [Google Scholar]
- 38.Stine JG, Munaganuru N, Barnard A, Wang JL, Kaulback K, Argo CK, Singh S, et al. Change in MRI-PDFF and Histologic Response in Patients with Nonalcoholic Steatohepatitis: A Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams PT. Physical fitness and activity as separate heart disease risk factors: a meta-analysis. Med Sci Sports Exerc 2001;33:754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loomba R, Neuschwander-Tetri BA, Sanyal A, Chalasani N, Diehl AM, Terrault N, Kowdley K, et al. Multicenter Validation of Association Between Decline in MRI-PDFF and Histologic Response in NASH. Hepatology 2020;72:1219–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel K, Harrison SA, Elkashab M, Trotter JF, Herring R, Rojter S, Kayali Z, et al. Cilofexor, a Nonsteroidal FXR Agonist, in Non-Cirrhotic Patients with Nonalcoholic Steatohepatitis: A Phase 2 Randomized Controlled Trial. Hepatology 2020;1. [DOI] [PubMed] [Google Scholar]
- 42.Harrison SA, Bashir MR, Guy CD, Zhou R, Moylan CA, Frias JP, Alkhouri N, et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. The Lancet 2019;394:2012–2024. [DOI] [PubMed] [Google Scholar]
- 43.EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016;64:1388–1402. [DOI] [PubMed] [Google Scholar]
- 44.Wang S-t, Zheng J, Peng H-w, Cai X-l, Pan X-t, Li H-q, Hong Q-z, et al. Physical activity intervention for non-diabetic patients with non-alcoholic fatty liver disease: a meta-analysis of randomized controlled trials. BMC Gastroenterology 2020;20:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sargeant JA, Gray LJ, Bodicoat DH, Willis SA, Stensel DJ, Nimmo MA, Aithal GP, et al. The effect of exercise training on intrahepatic triglyceride and hepatic insulin sensitivity: a systematic review and meta-analysis. Obes Rev 2018;19:1446–1459. [DOI] [PubMed] [Google Scholar]
- 46.Pugh CJ, Spring VS, Kemp GJ, Richardson P, Shojaee-Moradie F, Umpleby AM, Green DJ, et al. Exercise training reverses endothelial dysfunction in nonalcoholic fatty liver disease. Am J Physiol Heart Circ Physiol 2014;307:H1298–1306. [DOI] [PubMed] [Google Scholar]
- 47.Sogaard KK, Horvath-Puho E, Montomoli J, Vilstrup H, Sorensen HT. Cirrhosis is Associated with an Increased 30-Day Mortality After Venous Thromboembolism. Clin Transl Gastroenterol 2015;6:e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thögersen AM, Jansson JH, Boman K, Nilsson TK, Weinehall L, Huhtasaari F, Hallmans G. High plasminogen activator inhibitor and tissue plasminogen activator levels in plasma precede a first acute myocardial infarction in both men and women: evidence for the fibrinolytic system as an independent primary risk factor. Circulation 1998;98:2241–2247. [DOI] [PubMed] [Google Scholar]
- 49.O’Gorman P, Naimimohasses S, Monaghan A, Kennedy M, Melo AM, D NF, Doherty DG, et al. Improvement in histological endpoints of MAFLD following a 12-week aerobic exercise intervention. Aliment Pharmacol Ther 2020. [DOI] [PubMed] [Google Scholar]
- 50.Oh S, So R, Shida T, Matsuo T, Kim B, Akiyama K, Isobe T, et al. High-Intensity Aerobic Exercise Improves Both Hepatic Fat Content and Stiffness in Sedentary Obese Men with Nonalcoholic Fatty Liver Disease. Sci Rep 2017;7:43029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van der Poorten D, Milner KL, Hui J, Hodge A, Trenell MI, Kench JG, London R, et al. Visceral fat: a key mediator of steatohepatitis in metabolic liver disease. Hepatology 2008;48:449–457. [DOI] [PubMed] [Google Scholar]
- 52.Alessi MC, Peiretti F, Morange P, Henry M, Nalbone G, Juhan-Vague I. Production of plasminogen activator inhibitor 1 by human adipose tissue: possible link between visceral fat accumulation and vascular disease. Diabetes 1997;46:860–867. [DOI] [PubMed] [Google Scholar]
- 53.Alexopoulos AS, Crowley MJ, Wang Y, Moylan CA, Guy CD, Henao R, Piercy DL, et al. Glycemic Control Predicts Severity of Hepatocyte Ballooning and Hepatic Fibrosis in Nonalcoholic Fatty Liver Disease. Hepatology 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


