Abstract
Treatments that elevate NAD+ levels have been found to improve oocyte quality in mice, cattle, and pigs, suggesting that NAD+ is vital during oocyte maturation. This study aimed to examine the influence of different NAD+ biosynthetic pathways on oocyte quality by inhibiting key enzymes. Porcine oocytes from small antral follicles were matured for 44 h in a defined maturation system supplemented with 2-hydroxynicotinic acid [2-HNA, nicotinic acid phosphoribosyltransferase (NAPRT) inhibitor], FK866 [nicotinamide phosphoribosyltransferase (NAMPT) inhibitor], or gallotannin [nicotinamide mononucleotide adenylyltransferase (NMNAT) inhibitor] and their respective NAD+ pathway modulators (nicotinic acid, nicotinamide, and nicotinamide mononucleotide, respectively). Cumulus expansion was assessed after 22 h of maturation. At 44 h, maturation rates were determined and mature oocytes were fixed and stained to assess spindle formation. Each enzyme inhibitor reduced oocyte maturation rate and adversely affected spindle formation, indicating that NAD+ is required for meiotic spindle assembly. Furthermore, NAMPT and NMNAT inhibition reduced cumulus expansion, whereas NAPRT inhibition affected chromosomal segregation. Treating oocytes with gallotannin and nicotinamide mononucleotide together showed improvements in spindle width, while treating oocytes with 2-HNA and nicotinic acid combined showed an improvement in both spindle length and width. These results indicate that the salvage pathway plays a vital role in promoting oocyte meiotic progression, while the Preiss-Handler pathway is essential for spindle assembly.
Keywords: In vitro maturation, Meiotic spindle, NAD+ precursors, Oocyte, Pig
The acquisition of developmental competence in mammalian oocytes is driven by nuclear and cytoplasmic remodelling during oocyte maturation [1, 2]. Poor oocyte maturation rates and limited oocyte developmental potential hinder the efficiency of embryo production programs owing to the associated high embryo mortality rates [3, 4]. Nuclear maturation is characterized by condensation of chromatin and alignment of chromosomes along the metaphase plate, together with polymerization of tubulin into microtubules that form the meiotic spindle [5]. Cytoplasmic maturation refers to the redistribution of cortical granules and the rearrangement of mitochondria and other organelles throughout the oocyte cytoplasm [6, 7]. Both stages can occur independently in mice [8, 9], whereas cytoplasmic maturation ceases upon completion of nuclear maturation in other species, such as horses and pigs. Therefore, the oocyte must remain in meiotic arrest until cytoplasmic maturation is almost complete to adequately support fertilization and embryo development [9,10,11]. Abnormalities in oocytes that appear to have successfully reached metaphase II (MII) may include chromosomal aberrations caused by improper spindle function and assembly [12, 13] as well as incorrect or incomplete redistribution of mitochondria and cortical granules [14,15,16].
Meiotic spindle assembly is an important aspect of oocyte maturation, and hence, the quality of oocytes. Assembly of the spindle apparatus is vital for proper chromosome segregation during meiotic divisions, and hence for genomic integrity [17]. Aberrant spindles and misaligned chromosomes in oocytes may result in aneuploidy [18, 19], the leading cause of spontaneous abortion and pregnancy loss in women [20]. Porcine oocytes matured in vitro display significantly shorter spindles compared to their in vivo counterparts [21], whereas 50% of equine oocytes matured in vitro display spindle anomalies, including monopolar and multipolar spindles, and an increase in spindle length and width, relative to in vivo-sourced oocytes [22]. In addition, aged oocytes often display a greater incidence of spindle anomalies, which have been ameliorated by nicotinamide adenine dinucleotide (NAD+) precursor treatments [12, 23,24,25]. Sirtuins are NAD+-dependent deacetylases implicated in histone and chromatin modifications [26, 27], mitotic progression [28], microtubule dynamics [29], oxidative stress and mitochondrial function [30,31,32]. Inhibition of Sirtuins in oocytes has previously resulted in a block in meiotic progression, multipolar and malformed spindles, and displacement of chromosomes along the M-plate [13, 33,34,35,36].
Pregnancy loss and higher rates of congenital abnormalities in mice have been attributed to NAD+ deficiency [37]. Recent studies focusing on ameliorating the detrimental effects of aging on oocyte quality have revealed that NAD+ biosynthesis can be enhanced by nicotinamide mononucleotide (NMN) and nicotinic acid (NA) supplementation [12, 23,24,25]. The addition of NMN to drinking water of aged mice [12, 18] and in vitro supplementation of aged mouse oocytes with NMN [24] resulted in the reversion of meiotic spindle defects. The synthesis of NAD+ can occur via three separate pathways that feed in on each other: the Preiss-Handler pathway, which starts with the absorption of dietary NA; the salvage pathway, which salvages nicotinamide (NAM) that is produced as a result of NAD+ consumption; and de novo synthesis from the essential amino acid tryptophan (Fig. 1). Inhibition of nicotinamide phosphoribosyltransferase (NAMPT) in the salvage pathway by treatment with FK866 slowed meiotic progression and reduced the incidence of blastocyst formation in mice, whereas inhibiting nicotinic acid phosphoribosyl transferase (NAPRT) with 2-hydroxynicotinic acid (2-HNA) had little effect on mouse embryo development in vitro [12]. Gallotannin treatment has previously been shown to inhibit all three isoforms of nicotinamide mononucleotide adenylyltransferase (NMNAT) in cultured human cells [38] and exerts dose-dependent effects on porcine oocyte maturation possibly due to of its antioxidant properties [39, 40].
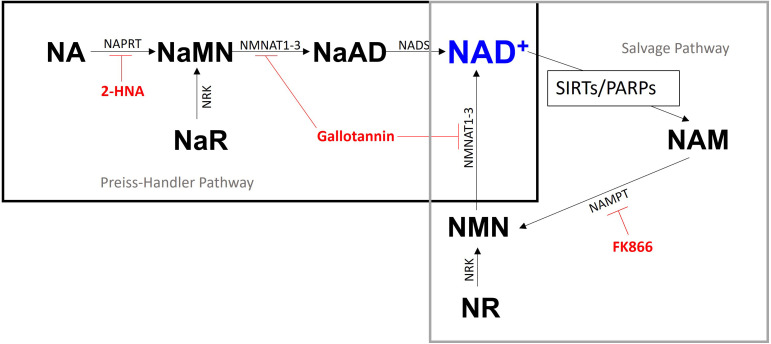
Fig. 1.
The Preiss-Handler and salvage pathways for NAD+ biosynthesis. Nicotinic acid (NA) is the initial metabolite in the Preiss-Handler pathway and is converted to NAD+ through a series of enzymatic reactions. The NAD+ synthesised is consumed by Sirtuins and poly-ADP-ribose polymerases, releasing nicotinamide (NAM) which can then be recycled back into NAD+ via the salvage pathway. Inhibitors for the two pathways utilised in this study are represented in red and the metabolites upstream of these inhibitors are utilised as NAD+ pathway modulators with the exception of nicotinic acid adenine dinucleotide (NaAD). NA, nicotinic acid; NaMN, nicotinic acid mononucleotide; NaAD, nicotinic acid adenine dinucleotide; NAM, nicotinamide; NMN, nicotinamide mononucleotide; NAD, nicotinamide adenine dinucleotide; NR, nicotinamide riboside; NaR, nicotinic acid riboside. NaPRT, nicotinic acid phosphoribosyltransferase; NMNAT, nicotinamide mononucleotide adenylyltransferase; NADS, nicotinamide nicotinamide phosphoribosyltransferase; SIRTs, Sirtuins; PARPs, poly ADP-ribose polymerases; NRK, nicotinamide riboside kinase
The aim of this study was to determine the role of NAD+ during porcine oocyte maturation by inhibiting the key enzymes of the Preiss-Handler and salvage pathways. We hypothesized that the inhibition of NAD+ biosynthesis via treatment with NAPRT, NAMPT, and NMNAT inhibitors would increase the incidence of spindle defects, and that the addition of NAD+ intermediates during the inhibitory treatments would restore, at least partially, spindle formation and chromosome alignment.
Materials and Methods
Chemicals and media preparation
All chemicals and reagents were purchased from Sigma-Aldrich (Australia) unless otherwise specified. The medium used for washing the cumulus oocyte complexes (COCs) was HEPES-buffered 199 (H199) medium (Gibco, Grand Island, NY, USA) supplemented with polyvinyl alcohol (PVA; 1 mg/ml), glutamax (1 mM; Gibco), penicillin G (75 µg/ml; P-3032), and streptomycin sulfate (50 µg/ml; S-9137). The in vitro maturation (IVM) media used was porcine oocyte medium (POM) [41], which consisted of sodium chloride (NaCl; 108 mM; S-5886), potassium chloride (KCl; 10 mM; P-5405), sodium bicarbonate (NaHCO3; 25 mM; S-5761), penicillin G (75 µg/ml), streptomycin sulfate (50 µg/ml), magnesium sulfate (MgSO4; 0.4 mM; M-1880), potassium phosphate (K2PO4; 0.35 mM; P-5655), glucose (4 mM; G-7021), sodium pyruvate (0.2 mM; P-5280), calcium lactate (2 mM; C-8356), glutamax (1 mM), hypotaurine (5 mM; H-1384) minimum essential medium (MEM) amino acids (20 µl/ml; 50x; Gibco; 11130-051), MEM non-essential amino acids (10 µl/ml; 100x; Gibco; 11140-060), L-cysteine (0.6 mM; C-7352) and PVA (3 mg/ml), and was adjusted to a pH of 7.5–7.55 and an osmolarity of 286 mOsm. The POM used for the first 22 h of IVM also contained 1 mM dibutyryl cyclic adenosine monophosphate (db-cAMP; D-0627). The NAD+ metabolites nicotinic acid (N4126), nicotinamide (72340), and nicotinamide mononucleotide and the inhibitors 2-hydroxynicotinic acid (2-HNA; 251054), FK866 (F8557), and gallotannin (403040) were utilized as NAD+ biosynthetic pathway modulators in this study. Stock solutions of NMN (10 mM), NA (20 mM), NAM (50 mM), FK866 (50 mM), and gallotannin (10 mM) were prepared by dissolving in Milli-Q water. A stock solution of 2-HNA (100 mM) was prepared by dissolving in formic acid. Small aliquots (20 µl) of metabolites and inhibitors were stored at –20°C until use.
Ovary collection and in-vitro maturation
Ovaries were collected from prepubertal gilts at a local abattoir and transported to the laboratory in saline solution supplemented with antibiotic-antimycotic (3 ml; 100 × solution; Gibco; 15240-062) that was maintained at 38°C. Ovaries were placed in fresh saline solution (EBOS; BHAHF7123) supplemented with antibiotic-antimycotic and kept at 38°C until aspiration (within 1 h of returning from the abattoir). Small antral follicles (1–3 mm in diameter) were aspirated into warm vacutainer tubes via a 21 G needle using a vacuum pump at a flow rate of 1 l/min. COCs were recovered from the aspirated material using a stereomicroscope, and oocytes with evenly distributed cytoplasm and a minimum of three layers of cumulus cells were selected for IVM. The COCs were washed twice in H199 medium, then once in POM supplemented with db-cAMP, and incubated in a four-well NUNC dish (Nunc A/S, Roskilde, Denmark) with POM media containing db-cAMP and supplemented with the respective treatments for 22 h at 38.5°C in a humidified atmosphere of 6% CO2 in air. After 22 h of IVM, the oocytes were transferred to a separate four-well NUNC dish with POM medium (without db-cAMP) supplemented with the respective treatments and incubated for an additional 22 h. Following maturation, oocytes were denuded of cumulus cells by the addition of hyaluronidase (1 mg/ml) followed by gentle aspiration using a narrow bore pipette.
Assessment of cumulus expansion
At 22 h of IVM, the degree of cumulus expansion for each COC was assessed according to a subjective scoring system on a scale of 0–4 as described previously [42]. The average score (0.0–4.0) for each group in each replicate was then calculated to obtain a value referred to as the “cumulus expansion index” (CEI) [43].
Immunofluorescent meiotic spindle staining
This procedure was performed at room temperature. Denuded metaphase II (MII) oocytes were fixed in 4% paraformaldehyde in phosphate buffered saline (PBS) for 30 min, permeabilized in 1% Triton X-100 in PBS for 20 min, and blocked using 1% bovine serum albumin (BSA; MP Biomedicals, Auckland, New Zealand) in PBS for 1 h. Then, the oocytes were washed three times in wash buffer (0.1% Tween-20 and 0.01% Triton-X100 in PBS) and incubated with FITC-conjugated mouse monoclonal anti-α-tubulin antibody (1:200 in wash buffer; F2168) in the dark for 1 h, followed by three 10 min washes in wash buffer. Oocytes were then incubated in PBS supplemented with 10 µg/ml Hoechst33342 and 1% BSA for 10 min in the dark, they were then washed three times with 1% BSA in PBS, and mounted onto glass slides in 5 µl of ProLong Diamond Antifade (P36961; Life Technologies Corporation, Eugene, OR, USA). A Nikon A1R microscope (Nikon Corporation; Tokyo, Japan) was used to visualize the meiotic spindle of each oocyte using a Z-stack at 100x magnification and Huygens software (version 20.10; Scientific Volume Imaging B.V. Hilversum, The Netherlands) was used to measure spindle length, spindle width and metaphase plate (M-plate) width. Spindle length was defined as the distance between spindle poles and spindle width was defined as the width of the spindle at the plane of the metaphase plate (M-plate). M-plate width was defined as the distance between the outermost edges of the DNA perpendicular to the plane of the M-plate (Fig. 2). Spindles were classified as normal or abnormal based on data obtained from the image. Typical barrel-shaped bipolar spindles with well-organized microtubules and condensed chromosomes that were tightly aligned along the M-plate were considered normal. Spindles with disordered microtubule fibers, multipolar spindles, and spindles with lagging or disorganized chromosomes were considered abnormal (representative images of normal and aberrant spindles are shown in Fig. 3).
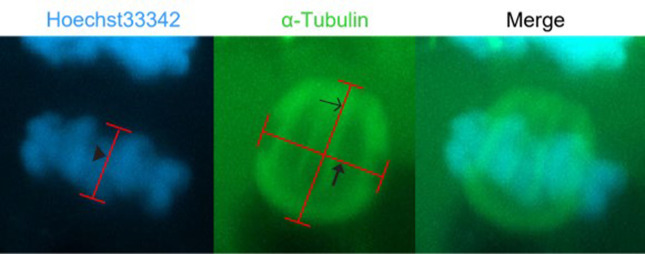
Fig. 2.
Representative images of spindle and metaphase plate measurements. Spindle length was calculated by the distance between spindle poles, the spindle width was calculated by the width of the microtubules across the metaphase plate, and metaphase plate width was calculated by the distance between the edges of the DNA along the spindle length axis. The α-tubulin is stained green with a FITC-conjugated antibody and the DNA is stained blue with Hoechst33342. Arrow head indicates M-Plate width, open arrow indicates spindle length and closed arrow indicates spindle width.
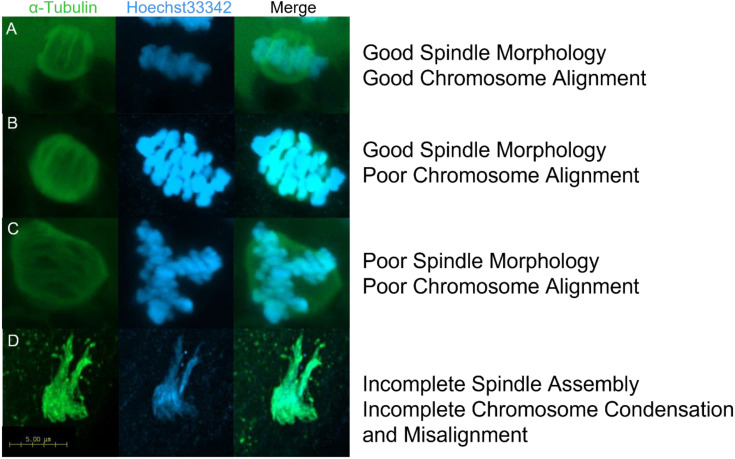
Fig. 3.
Representative images of spindle assembly and chromosome alignment in MII oocytes. A) oocytes displaying barrel-shaped spindles with well organised microtubule fibres and highly condensed DNA tightly aligned along the metaphase plate (normal spindles), B) barrel-shaped spindles with well organised microtubule fibres with highly condensed DNA loosely aligned along the metaphase plate (aberrant spindles), C) Disorganised spindles with well-defined microtubule fibres and chromosomes not aligned along the metaphase plate (aberrant spindles), D) incomplete spindle assembly with no discernible microtubule structure and uncondensed DNA with no discernible structure (aberrant spindles). The α-tubulin is stained green with a FITC-conjugated antibody and the DNA is stained blue with Hoechst33342.
Experimental design
All treatments were performed over the entire duration of IVM, and each experiment was replicated at least thrice. In all experiments, cumulus expansion was assessed at 22 h of IVM. After 44 h of IVM, mature oocytes were fixed and stained to assess spindle assembly and chromosome alignment.
Experiment 1: Effect of inhibiting NA utilization on oocyte maturation
To determine the optimum dose of 2-HNA, COCs were randomly allocated to groups and matured in media containing 0, 0.01, 0.1 or 1.0 mM 2-HNA (approximately 30 COCs per group in each replicate). Based on these results, 1.0 mM 2-HNA was used in subsequent experiments. The results of a previous study showed that 200 µM NA was optimal for oocyte maturation [44]. To determine the effect of inhibiting NA utilization by the Preiss-Handler pathway, COCs were randomly allocated to groups and matured in media containing no supplement (control), 200 µM NA, 1.0 mM 2-HNA or NA, and 2-HNA combined (approximately 30 COCs per group in each replicate). An equal volume of the inhibitor vehicle (formic acid) was added to the control and NA-treated groups.
Experiment 2: Effect of inhibiting NAM utilization on oocyte maturation
To determine the optimum dose of FK866, COCs were randomly allocated to groups and matured in media containing 0, 1, 10, or 100 nM FK866 (approximately 30 COCs per group in each replicate). Based on these results, 10 nM FK866 was used in the subsequent experiments. The results of a previous study showed that 5 µM NAM was optimal for oocyte maturation [44]. To determine the effect of inhibiting NAM utilization by the salvage pathway, COCs were randomly allocated to groups and matured in media containing no supplement (control), 5 µM NAM, 10 nM FK866, or NAM and FK866 combined (approximately 30 COCs per group in each replicate).
Experiment 3: Effect of inhibiting NMN utilization on oocyte maturation
To determine the optimum dose of gallotannin, COCs were randomly allocated to groups and matured in media containing 0, 0.55, 5.5, or 55 µM gallotannin (approximately 30 COCs per group in each replicate). Based on these results, 55 µM gallotannin was used in subsequent experiments. The results of a previous study showed that 100 µM NMN was optimal for oocyte maturation [12]. To determine the effect of inhibiting NMN utilization by the salvage pathway, COCs were randomly allocated to groups and matured in media containing no supplement (control), 100 µM NMN, 55 µM gallotannin or NMN, and gallotannin combined (approximately 30 COCs per group in each replicate).
Statistical analyses
R (version 3.6.1; 2019; Vienna, Austria) was used to assess differences in oocyte maturation rates following treatment. The proportional data were subjected to a logit transformation using the car package (version 3.0-3) before being subjected to a residual maximum likelihood regression using the lmerTest package (version 3.1-0), with the treatment group as a factor and replicate as a random effect. The cumulus expansion data were subjected to a Kruskal-Wallis rank sum test using the stats package (version 3.5.3), followed by a post-hoc Dunn test using the Bonferroni method from the dunn.test package (version 1.3.5) to determine differences between treatment groups. Spindle length, spindle width, M-plate diameter, M-plate width, and spindle classification data were also subjected to residual maximum likelihood regression with the treatment group as a factor and replicate as a random effect. When a p-value ≤ 0.05 was obtained, the data was further subjected to a post hoc Tukey’s test using the emmeans package (version 1.3.5.1) in R to identify significant differences between treatment groups.
Results
Experiment 1: Inhibiting NA utilization impairs spindle formation and chromosome segragation
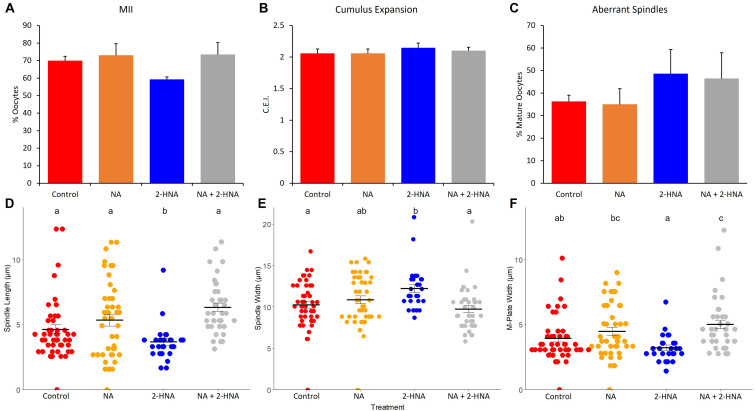
The addition of 0.1 mM 2-HNA reduced the proportion of oocytes that reached the MII stage compared to the control and 0.01 mM treatment groups (52.77 ± 0.02 vs. 66.77 ± 0.02 and 66.21 ± 0.05%, respectively; P < 0.05), but was not different from that of the 1.0 mM treatment group (64.89 ± 0.03%; P > 0.05). There was no difference in the cumulus expansion index between any treatment group (range 1.97 ± 0.07 to 2.16 ± 0.07 C.E.I.; P > 0.05). There was also no effect of NA and 2-HNA, alone or in combination, on the percentage of oocytes that attained MII (P > 0.05; Fig. 4A) or on the cumulus expansion index between the treatments (P > 0.05; Fig. 4B).
Fig. 4.
The effects of NA (200 µM) and 2HNA (1.0 mM) alone and in combination on A) oocyte maturation, B) cumulus expansion, C) the proportion of oocytes with aberrant spindles, D) spindle length, E) spindle width, and F) M-plate width. Treatments labelled with different letters indicate statistical differences.
The spindle assessment results of Experiment 1 are shown in Fig. 4. Oocytes in the 2-HNA (n = 26) treatment group had shorter spindles than those in the control (n = 47), NA (n = 45), and NA+2-HNA (n = 39) treatment groups (Fig. 4D; P < 0.05). The spindles of 2-HNA treated oocytes were also wider than those of the control and NA+2-HNA treated oocytes (Fig. 4E; P < 0.05), and the spindles of the NA-treated oocytes were of intermediate width. Additionally, chromosomes were more tightly aligned along the M-plate in the 2-HNA treatment group than in the NA and NA+2-HNA treatment groups (P < 0.05), but were not different from the control (Fig. 4F; P > 0.05). There were no differences in the proportion of aberrant spindles between any of the treatment groups (Fig. 4C; P > 0.05).
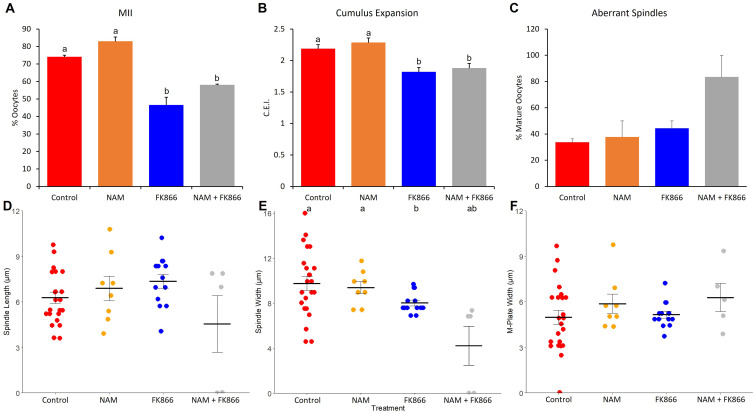
Experiment 2: Inhibiting NAM utilization inhibits oocyte maturation and impairs cumulus expansion and spindle formation
The addition of 100 nM FK866 to the maturation media reduced the proportion of oocytes that reached the MII stage compared to the control and 1 nM treatment groups (31.11 ± 9.49 vs. 67.62 ± 0.48 and 73.31 ± 5.35%, respectively; P < 0.01), but was not different from that in the 10 nM treatment group (44.17 ± 9.61%; P > 0.05). The addition of FK866 at all concentrations inhibited cumulus expansion compared with the control (0.90 ± 0.31 to 0.97 ± 0.21 vs. 1.43 ± 0.79 C.E.I.; P < 0.001). The proportion of oocytes that reached the MII stage was greater in the control and the NAM groups than in the FK866 and NAM+FK866 groups (P < 0.001 and 0.01, respectively; Fig. 5A). Additionally, the cumulus expansion in the FK866 and NAM+FK866 treatment groups was less than that in the control and the NAM treatment groups (P < 0.05; Fig. 5B).
Fig. 5.
The effects of NAM (5 µM) and FK866 (100 nM) alone and in combination on A) oocyte maturation, B) cumulus expansion, C) the proportion of oocytes with aberrant spindles, D) spindle length, E) spindle width, and F) M-plate width. Treatments labelled with different letters indicate statistical differences.
The spindle assessment results of Experiment 2 are shown in Fig. 5. Oocytes treated with FK866 (n = 13) had narrower spindles than those of the control (n = 23) and NAM (n = 8) groups (P < 0.05), but they did not differ from those of the NAM+FK866 (n = 5) group (Fig. 5E; P > 0.05). There was no difference in spindle length (Fig. 5D; P > 0.05) or M-plate width (Fig. 5F; P > 0.05) between the treatment groups. Furthermore, while there were no significant differences in the proportion of aberrant spindles between any of the treatment groups, oocytes in the NAM+FK866 treatment group tended to have a higher proportion of aberrant spindles compared to all other treatments (Fig. 5C; P < 0.1).
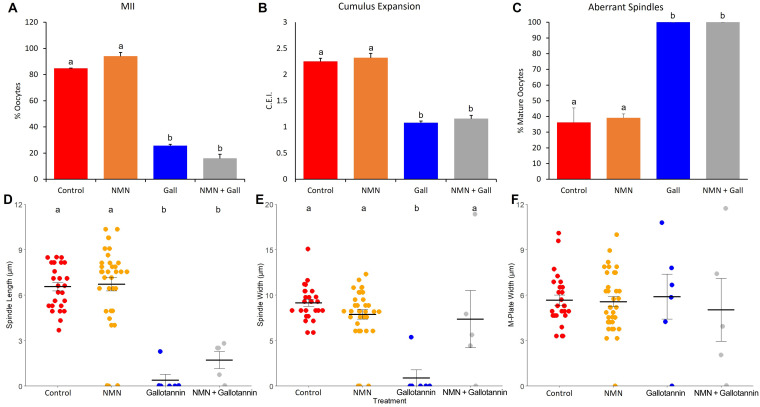
Experiment 3: Inhibiting NMN utilization inhibits oocyte maturation and impairs cumulus expansion and spindle formation
The addition of 55 µM gallotannin significantly reduced the proportion of oocytes that reached MII compared to all other treatment groups (22.36 ± 3.33 vs. 61.58 ± 8.89 to 81.54 ± 2.14%; P < 0.05). Oocytes from the 55 µM treatment group also exhibited reduced cumulus expansion compared with all other treatment groups (1.40 ± 0.07 vs. 1.84 ± 0.08 to 2.12 ± 0.09 C.E.I.; P < 0.0001). The addition of 55 µM gallotannin decreased the proportion of oocytes that reached the MII stage compared with the control and 100 µM NMN groups (P < 0.0001; Fig. 6A). The maturation rates of the gallotannin and NMN+gallotannin treatment groups did not differ significantly (P > 0.05). In addition, cumulus expansion in the gallotannin and NMN+gallotannin groups was less than that in the NMN and control groups (P < 0.0001; Fig. 6B).
Fig. 6.
The effects of NMN (100 µM) and gallotannin (55 µM) alone and in combination on A) oocyte maturation, B) cumulus expansion, C) the proportion of oocytes with aberrant spindles, D) spindle length, E) spindle width, and F) M-plate width. Treatments labelled with different letters indicate statistical differences.
The spindle assessment results of Experiment 3 are shown in Fig. 6. Oocytes of the gallotannin (n = 6) and NMN+gallotannin (n = 5) treatment groups had significantly shorter spindles than those of the control (n = 26) and NMN (n = 35) treatment groups (Fig. 6D; P < 0.001). The gallotannin-treated oocytes also had narrower spindles than those of the other treatment groups (Fig. 6E; P < 0.01). There were no differences in the M-plate width (Fig. 6F; P > 0.05) between any of the treatment groups. Furthermore, the proportion of oocytes with aberrant spindles was much greater in the gallotannin and NMN+gallotannin treatment groups than in the control and NMN treatment groups (Fig. 6C; P < 0.01).
Discussion
Using oocytes from small antral follicles as a model of poor oocyte quality, the results of this study show that NAD+ biosynthesis via the Preiss-Handler and salvage pathways is essential for oocyte maturation in pigs. We observed a reduction in oocyte maturation rates and abnormalities in chromosome segregation and spindle assembly when the Preiss-Handler and salvage pathways were inhibited by 2-HNA, FK866, and gallotannin, albeit to varying degrees. These findings add to the growing body of evidence indicating that NAD+ biosynthesis is crucial for oocyte maturation and spindle formation.
Inhibition of NAPRT in the Preiss-Handler pathway using 2-HNA had varying effects on the proportion of oocytes completing meiosis, but did not affect cumulus expansion, indicating that 2-HNA is a low-potency inhibitor [45]. This suggests that 2-HNA acts solely on the oocyte without perturbing cumulus cell function. The assembly of spindles during meiosis is an energy-expensive process, and inhibiting NAPRT hampers replenishment of the intracellular NAD+ pool [46], effectively reducing the activity of ATP synthase and hindering ATP production [45]. As such, inhibiting the Preiss-Handler pathway results in reduced ATP production that is utilized in spindle assembly during meiosis, which can potentially result in lagging chromosomes during anaphase and an increased incidence of aneuploidy [47, 48], leading to compromised embryo development. This study showed that inhibiting the Preiss-Handler pathway with 2-HNA reduced spindle length and increased spindle width compared to all other treatment groups, suggesting that spindle assembly was adversely affected by inhibition of the Preiss-Handler pathway. Unexpectedly, 2-HNA treated oocytes exhibited more tightly aligned chromosomes than all other groups, as reflected by the reduction in M-plate width, which may be attributed to the shorter and wider spindles. The changes in spindle parameters in response to 2-HNA exposure did not alter the incidence of aberrant spindle formation because these spindles were still mostly regarded as having a normal morphology.
The observed rescue in spindle length and width with the inclusion of NA may be attributed to the protective effects of NA under stress conditions through an increase in intracellular NAD+ levels [49, 50]. Previously, supplementation during IVM with NA was found to improve the developmental competence of bovine [51] and porcine oocytes [44, 52]. Confirmation that NAPRT is expressed in mouse oocytes [24] provides further evidence that the Preiss-Handler pathway is important for NAD+ biosynthesis during oocyte maturation. The observed spindle assembly rescue may be due to the utilization of alternative precursors such as nicotinamide riboside (NR), NMN, and NAM for NAD+ production. Recently, Yang et al. found that supplementing the drinking water of aged mice with NR increased intra-oocyte levels of NAD+ and decreased spindle anomalies [53]. Therefore, inhibition of the Preiss-Handler pathway appears to be compensated by other NAD+ biosynthetic pathways.
Inhibition of NAMPT in the salvage pathway using FK866 treatment significantly reduced the proportion of oocytes that reached MII and inhibited cumulus expansion. Additionally, a subtle effect on spindle width with the FK866 treatment resulting in narrower spindles was observed. These results highlight the importance of the salvage pathway in processes within the oocyte, as well as cumulus cell function in pigs, which may reflect a breakdown in gap junctions between the cumulus cells and the oocyte, as observed in bovine oocytes [54], leading to a block in meiotic progression in porcine oocytes. Bertoldo et al. [12] showed that NAMPT inhibition using FK866 impaired murine oocyte maturation by slowing the rate of germinal vesicle breakdown and polar body extrusion and inhibited cumulus expansion. Supplementation of medium with FK866 also markedly reduced NAD+ and ATP levels in mouse oocytes, which correlated with significantly longer spindles [55].
In contrast to the Preiss-Handler pathway, inhibition of the salvage pathway adversely affects both cumulus cell function and meiotic progression. Surprisingly, these oocytes that were still able to complete meiosis had a limited impact on spindle morphology, suggesting that they had a sufficient pool of NAD+ to support this process. The addition of NAM at a concentration that has previously been shown to improve the developmental potential of porcine oocytes [44] did not diminish the effects of NAPRT inhibition. Supplementation during IVM with a high concentration of NAM (5 mM) has previously been shown to reduce the rate of meiotic maturation in mouse and pig oocytes [34, 56]. As FK866 prevents the conversion of NAM to NMN, it is possible that NAM accumulated to detrimental concentrations within the oocyte and cumulus cells and that the additional NAM (5 µM) exacerbated the negative effects of the salvage pathway inhibition.
Inhibition of both the Preiss-Handler and salvage pathways using 55 µM gallotannin to inhibit NMNAT significantly reduced the proportion of oocytes that reached MII and inhibited cumulus cell expansion. Furthermore, spindles were significantly shorter and narrower, and all gallotannin-treated oocytes exhibited aberrant spindles. Yin et al. [40] also reported that at a high concentration (100 µg/mL), similar to that used here, exposure to gallotannin impeded porcine oocyte maturation and cumulus cell expansion. Remarkably, at a lower concentration (10 µg/mL), gallotannin treatment improved porcine oocyte maturation and cumulus cell expansion owing to the antioxidant properties of the compound [40]. As depleted NAD+ levels could not be replenished by another pathway, it is not surprising that spindle assembly was severely affected by gallotannin treatment. There was an apparent rescue in spindle width with the addition of NMN, although the spindles remained highly disorganized and individual fibers were not observed. Previously, supplementing the drinking water of aged mice with NMN restored spindle assembly, which was associated with increased levels of NAD+ in oocytes [12, 23], thus, providing further evidence of the importance of NAD+ biosynthesis via the salvage pathway during oocyte maturation. These results clearly indicate that replenishment of NAD+ throughout IVM via the Preiss-Handler and/or salvage pathways is essential for optimal meiotic maturation and cumulus cell function in porcine oocytes.
The results presented here can be attributed to a depletion of intra-oocyte NAD+ levels following inhibition of key enzymes in NAD+ biosynthetic pathways, resulting in a reduction in sirtuin activity. However, NAD+ levels and sirtuin expression within oocytes and cumulus cells during IVM were not investigated in this study. Additionally, this study only examined COCs from small antral follicles, which may respond differently to those from larger follicles due to a less advanced growth potential, especially where the follicle is on the path to atresia at the time of collection. Further studies are needed to fully elucidate the NAD+ precursor needs and optimal sirtuin activities of porcine oocytes and cumulus cells from follicles of different sizes. Assessing the development of embryos following in vitro fertilization of oocytes subjected to inhibition of each NAD+ biosynthetic pathway would also aid in determining the NAD+ requirements for optimal embryo development in pigs.
In conclusion, the present study describes the effects of inhibiting the Preiss-Handler and salvage pathways on oocyte maturation in pigs. These results suggest that the salvage pathway is more important for the production of NAD+ for meiotic progression and cumulus cell function. Inhibiting NAD+ biosynthesis via the Preiss-Handler pathway impaired spindle assembly and chromosome segregation, but had little effect on meiotic progression or cumulus expansion. In contrast, inhibition of NAD+ biosynthesis via the salvage pathway reduced cumulus expansion and meiotic progression. Inhibition of NAD+ biosynthesis via both pathways simultaneously blocked meiotic progression and severely affected cumulus expansion, spindle assembly, and chromosome segregation. Supplementing the maturation medium with NA rescued some aspects of spindle assembly, possibly by promoting NAD+ biosynthesis via an alternative compensatory pathway. It is evident that these pathways play vital roles in replenishing NAD+ levels in oocytes and follicular cells during oocyte maturation. Gaining a better understanding of the roles of these pathways during oocyte maturation will enable future improvements to maturation media, potentially increasing the pool of oocytes that can be used for in vitro embryo production programs in all species.
Conflict of interests
The authors declare no conflicts of interest.
Supplementary
Acknowledgments
The authors acknowledge Dr. Lindsay Wu and Dr. Michael Bertoldo for providing the NMN used in this study, and the technical and scientific assistance of Sydney Microscopy and Microanalysis, the University of Sydney node of Microscopy Australia. The authors would also thank the Australian Research Council for supplying funding for this research (LP160100824).
References
- 1.Albertini DF, Wickramasinghe D, Messinger S, Mattson BA, Plancha CE. Nuclear and cytoplasmic changes during oocyte maturation. In: Bavister BD (ed.), Preimplantation Embryo Development. Springer-Verlag, New York, 1993; 3–21. [Google Scholar]
- 2.Carneiro GF, Liu IKM, Hyde D, Anderson GB, Lorenzo PL, Ball BA. Quantification and distribution of equine oocyte cortical granules during meiotic maturation and after activation. Mol Reprod Dev 2002; 63: 451–458. [DOI] [PubMed] [Google Scholar]
- 3.Hinrichs K. The equine oocyte: factors affecting meiotic and developmental competence. Mol Reprod Dev 2010; 77: 651–661. [DOI] [PubMed] [Google Scholar]
- 4.Mohammadi-Sangcheshmeh A, Held E, Ghanem N, Rings F, Salilew-Wondim D, Tesfaye D, Sieme H, Schellander K, Hoelker M. G6PDH-activity in equine oocytes correlates with morphology, expression of candidate genes for viability, and preimplantative in vitro development. Theriogenology 2011; 76: 1215–1226. [DOI] [PubMed] [Google Scholar]
- 5.Soifer D. Factors regulating the presence of microtubules in cells. Ann N Y Acad Sci 1986; 466: 1–7. [DOI] [PubMed] [Google Scholar]
- 6.Grøndahl C, Hyttel P, Grøndahl ML, Eriksen T, Gotfredsen P, Greve T. Structural and endocrine aspects of equine oocyte maturation in vivo. Mol Reprod Dev 1995; 42: 94–105. [DOI] [PubMed] [Google Scholar]
- 7.Torner H, Alm H, Kanitz W, Goellnitz K, Becker F, Poehland R, Bruessow KP, Tuchscherer A. Effect of initial cumulus morphology on meiotic dynamic and status of mitochondria in horse oocytes during IVM. Reprod Domest Anim 2007; 42: 176–183. [DOI] [PubMed] [Google Scholar]
- 8.Eppig JJ. Coordination of nuclear and cytoplasmic oocyte maturation in eutherian mammals. Reprod Fertil Dev 1996; 8: 485–489. [DOI] [PubMed] [Google Scholar]
- 9.Eppig JJ, Schultz RM, O’Brien M, Chesnel F. Relationship between the developmental programs controlling nuclear and cytoplasmic maturation of mouse oocytes. Dev Biol 1994; 164: 1–9. [DOI] [PubMed] [Google Scholar]
- 10.Gilchrist RB, Thompson JG. Oocyte maturation: emerging concepts and technologies to improve developmental potential in vitro. Theriogenology 2007; 67: 6–15. [DOI] [PubMed] [Google Scholar]
- 11.Mermillod P, Oussaid B, Cognié Y. Aspects of follicular and oocyte maturation that affect the developmental potential of embryos. J Reprod Fertil Suppl 1999; 54: 449–460. [PubMed] [Google Scholar]
- 12.Bertoldo MJ, Listijono DR, Ho WJ, Riepsamen AH, Goss DM, Richani D, Jin XL, Mahbub S, Campbell JM, Habibalahi A, Loh WN, Youngson NA, Maniam J, Wong ASA, Selesniemi K, Bustamante S, Li C, Zhao Y, Marinova MB, Kim LJ, Lau L, Wu RM, Mikolaizak AS, Araki T, Le Couteur DG, Turner N, Morris MJ, Walters KA, Goldys E, O’Neill C, Gilchrist RB, Sinclair DA, Homer HA, Wu LE. NAD+ repletion rescues female fertility during reproductive aging. Cell Reports 2020; 30: 1670–1681.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao Z, Zhang D, Tong X, Wang Y, Qi X, Ning W, Xu T, Gao D, Zhang L, Ma Y, Yu T, Zhang Y. Cumulus cell-derived and maternal SIRT6 differentially regulates porcine oocyte meiotic maturation. Theriogenology 2020; 142: 158–168. [DOI] [PubMed] [Google Scholar]
- 14.Eichenlaub-Ritter U, Winterscheidt U, Vogt E, Shen Y, Tinneberg HR, Sorensen R. 2-methoxyestradiol induces spindle aberrations, chromosome congression failure, and nondisjunction in mouse oocytes. Biol Reprod 2007; 76: 784–793. [DOI] [PubMed] [Google Scholar]
- 15.Delimitreva S, Zhivkova R, Isachenko E, Umland N, Nayudu PL. Meiotic abnormalities in in vitro-matured marmoset monkey (Callithrix jacchus) oocytes: development of a non-human primate model to investigate causal factors. Hum Reprod 2006; 21: 240–247. [DOI] [PubMed] [Google Scholar]
- 16.Goudet G, Bézard J, Duchamp G, Gérard N, Palmer E. Equine oocyte competence for nuclear and cytoplasmic in vitro maturation: effect of follicle size and hormonal environment. Biol Reprod 1997; 57: 232–245. [DOI] [PubMed] [Google Scholar]
- 17.Vogt E, Kirsch-Volders M, Parry J, Eichenlaub-Ritter U. Spindle formation, chromosome segregation and the spindle checkpoint in mammalian oocytes and susceptibility to meiotic error. Mutat Res 2008; 651: 14–29. [DOI] [PubMed] [Google Scholar]
- 18.Battaglia DE, Goodwin P, Klein NA, Soules MR. Influence of maternal age on meiotic spindle assembly in oocytes from naturally cycling women. Hum Reprod 1996; 11: 2217–2222. [DOI] [PubMed] [Google Scholar]
- 19.Schatten G, Simerly C, Schatten H. Microtubule configurations during fertilization, mitosis, and early development in the mouse and the requirement for egg microtubule-mediated motility during mammalian fertilization. Proc Natl Acad Sci USA 1985; 82: 4152–4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bond D, Chandley A. Aneuploidy. In: Oxford Monographs on Medical Genetics. Oxford University Press, Oxford, 1983; 198. [Google Scholar]
- 21.Ueno S, Kurome M, Ueda H, Tomii R, Hiruma K, Nagashima H. Effects of maturation conditions on spindle morphology in porcine MII oocytes. J Reprod Dev 2005; 51: 405–410. [DOI] [PubMed] [Google Scholar]
- 22.Franciosi F, Goudet G, Tessaro I, Papillier P, Dalbies-Tran R, Reigner F, Deleuze S, Douet C, Miclea I, Lodde V, Luciano AM. In vitro maturation affects chromosome segregation, spindle morphology and acetylation of lysine 16 on histone H4 in horse oocytes. Reprod Fertil Dev 2017; 29: 721–730. [DOI] [PubMed] [Google Scholar]
- 23.Miao Y, Cui Z, Gao Q, Rui R, Xiong B. Nicotinamide mononucleotide supplementation reverses the declining quality of maternally aged oocytes. Cell Reports 2020; 32: 107987. [DOI] [PubMed] [Google Scholar]
- 24.Wu X, Hu F, Zeng J, Han L, Qiu D, Wang H, Ge J, Ying X, Wang Q. NMNAT2-mediated NAD+ generation is essential for quality control of aged oocytes. Aging Cell 2019; 18: e12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang L, Hou X, Ma R, Moley K, Schedl T, Wang Q. Sirt2 functions in spindle organization and chromosome alignment in mouse oocyte meiosis. FASEB J 2014; 28: 1435–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma W, Zhang D, Hou Y, Li YH, Sun QY, Sun XF, Wang WH. Reduced expression of MAD2, BCL2, and MAP kinase activity in pig oocytes after in vitro aging are associated with defects in sister chromatid segregation during meiosis II and embryo fragmentation after activation. Biol Reprod 2005; 72: 373–383. [DOI] [PubMed] [Google Scholar]
- 27.Vaquero A, Scher M, Erdjument-Bromage H, Tempst P, Serrano L, Reinberg D. SIRT1 regulates the histone methyl-transferase SUV39H1 during heterochromatin formation. Nature 2007; 450: 440–444. [DOI] [PubMed] [Google Scholar]
- 28.Vaquero A, Scher MB, Lee DH, Sutton A, Cheng HL, Alt FW, Serrano L, Sternglanz R, Reinberg D. SirT2 is a histone deacetylase with preference for histone H4 Lys 16 during mitosis. Genes Dev 2006; 20: 1256–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dryden SC, Nahhas FA, Nowak JE, Goustin AS, Tainsky MA. Role for human SIRT2 NAD-dependent deacetylase activity in control of mitotic exit in the cell cycle. Mol Cell Biol 2003; 23: 3173–3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.North BJ, Marshall BL, Borra MT, Denu JM, Verdin E. The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell 2003; 11: 437–444. [DOI] [PubMed] [Google Scholar]
- 31.Kawamura Y, Uchijima Y, Horike N, Tonami K, Nishiyama K, Amano T, Asano T, Kurihara Y, Kurihara H. Sirt3 protects in vitro-fertilized mouse preimplantation embryos against oxidative stress-induced p53-mediated developmental arrest. J Clin Invest 2010; 120: 2817–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwak SS, Cheong SA, Yoon JD, Jeon Y, Hyun SH. Expression patterns of sirtuin genes in porcine preimplantation embryos and effects of sirtuin inhibitors on in vitro embryonic development after parthenogenetic activation and in vitro fertilization. Theriogenology 2012; 78: 1597–1610. [DOI] [PubMed] [Google Scholar]
- 33.Wang Q, Sun QY. Evaluation of oocyte quality: morphological, cellular and molecular predictors. Reprod Fertil Dev 2007; 19: 1–12. [DOI] [PubMed] [Google Scholar]
- 34.Zhang L, Ma R, Hu J, Ding X, Xu Y. Sirtuin inhibition adversely affects porcine oocyte meiosis. PLoS One 2015; 10: e0132941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Emidio G, Falone S, Vitti M, D’Alessandro AM, Vento M, Di Pietro C, Amicarelli F, Tatone C. SIRT1 signalling protects mouse oocytes against oxidative stress and is deregulated during aging. Hum Reprod 2014; 29: 2006–2017. [DOI] [PubMed] [Google Scholar]
- 36.Han L, Ge J, Zhang L, Ma R, Hou X, Li B, Moley K, Wang Q. Sirt6 depletion causes spindle defects and chromosome misalignment during meiosis of mouse oocyte. Sci Rep 2015; 5: 15366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi H, Enriquez A, Rapadas M, Martin EMMA, Wang R, Moreau J, Lim CK, Szot JO, Ip E, Hughes JN, Sugimoto K, Humphreys DT, McInerney-Leo AM, Leo PJ, Maghzal GJ, Halliday J, Smith J, Colley A, Mark PR, Collins F, Sillence DO, Winlaw DS, Ho JWK, Guillemin GJ, Brown MA, Kikuchi K, Thomas PQ, Stocker R, Giannoulatou E, Chapman G, Duncan EL, Sparrow DB, Dunwoodie SL. NAD deficiency, congenital malformations, and niacin supplementation. N Engl J Med 2017; 377: 544–552. [DOI] [PubMed] [Google Scholar]
- 38.Berger F, Lau C, Dahlmann M, Ziegler M. Subcellular compartmentation and differential catalytic properties of the three human nicotinamide mononucleotide adenylyltransferase isoforms. J Biol Chem 2005; 280: 36334–36341. [DOI] [PubMed] [Google Scholar]
- 39.Lane RL, Whitaker BD. Melatonin and tannic acid supplementation in vitro improve fertilization and embryonic development in pigs. Anim Reprod 2018; 15: 118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yin Z, Sun JT, Cui HD, Jiang CQ, Zhang YT, Lee S, Liu ZH, Jin JX. Tannin supplementation improves oocyte cytoplasmic maturation and subsequent embryo development in pigs. Antioxidants 2021; 10: 1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoshioka K, Suzuki C, Tanaka A, Anas IMK, Iwamura S. Birth of piglets derived from porcine zygotes cultured in a chemically defined medium. Biol Reprod 2002; 66: 112–119. [DOI] [PubMed] [Google Scholar]
- 42.Vanderhyden BC, Caron PJ, Buccione R, Eppig JJ. Developmental pattern of the secretion of cumulus expansion-enabling factor by mouse oocytes and the role of oocytes in promoting granulosa cell differentiation. Dev Biol 1990; 140: 307–317. [DOI] [PubMed] [Google Scholar]
- 43.Grupen CG, Armstrong DT. Relationship between cumulus cell apoptosis, progesterone production and porcine oocyte developmental competence: temporal effects of follicular fluid during IVM. Reprod Fertil Dev 2010; 22: 1100–1109. [DOI] [PubMed] [Google Scholar]
- 44.Pollard CL, Gibb Z, Hawdon A, Swegen A, Grupen CG. Supplementing media with NAD+ precursors enhances the in vitro maturation of porcine oocytes. J Reprod Dev 2021; 67: 319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piacente F, Caffa I, Ravera S, Sociali G, Passalacqua M, Vellone VG, Becherini P, Reverberi D, Monacelli F, Ballestrero A, Odetti P, Cagnetta A, Cea M, Nahimana A, Duchosal M, Bruzzone S, Nencioni A. Nicotinic acid phosphoribosyltransferase regulates cancer cell metabolism, susceptibility to NAMPT inhibitors, and DNA repair. Cancer Res 2017; 77: 3857–3869. [DOI] [PubMed] [Google Scholar]
- 46.Zou Y, Wang A, Huang L, Zhu X, Hu Q, Zhang Y, Chen X, Li F, Wang Q, Wang H, Liu R, Zuo F, Li T, Yao J, Qian Y, Shi M, Yue X, Chen W, Zhang Z, Wang C, Zhou Y, Zhu L, Ju Z, Loscalzo J, Yang Y, Zhao Y. Illuminating NAD+ metabolism in live cells and in vivo using a genetically encoded fluorescent sensor. Dev Cell 2020; 53: 240–252.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Battaglia DE, Goodwin P, Klein NA, Soules MR. Influence of maternal age on meiotic spindle assembly in oocytes from naturally cycling women. Hum Reprod 1996; 11: 2217–2222. [DOI] [PubMed] [Google Scholar]
- 48.Schatten G, Simerly C, Schatten H. Microtubule configurations during fertilization, mitosis, and early development in the mouse and the requirement for egg microtubule-mediated motility during mammalian fertilization. Proc Natl Acad Sci USA 1985; 82: 4152–4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duarte-Pereira S, Pereira-Castro I, Silva SS, Correia MG, Neto C, da Costa LT, Amorim A, Silva RM. Extensive regulation of nicotinate phosphoribosyltransferase (NAPRT) expression in human tissues and tumors. Oncotarget 2016; 7: 1973–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galassi L, Di Stefano M, Brunetti L, Orsomando G, Amici A, Ruggieri S, Magni G. Characterization of human nicotinate phosphoribosyltransferase: Kinetic studies, structure prediction and functional analysis by site-directed mutagenesis. Biochimie 2012; 94: 300–309. [DOI] [PubMed] [Google Scholar]
- 51.Kafi M, Ashrafi M, Azari M, Jandarroodi B, Abouhamzeh B, Asl AR. Niacin improves maturation and cryo-tolerance of bovine in vitro matured oocytes: An experimental study. Int J Reprod Biomed 2019; 17: 621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Almubarak AM, Kim E, Yu IJ, Jeon Y. Supplementation with Niacin during in vitro maturation improves the quality of porcine embryos. Theriogenology 2021; 169: 36–46. . [DOI] [PubMed] [Google Scholar]
- 53.Yang Q, Cong L, Wang Y, Luo X, Li H, Wang H, Zhu J, Dai S, Jin H, Yao G, Shi S, Hsueh AJ, Sun Y. Increasing ovarian NAD+ levels improve mitochondrial functions and reverse ovarian aging. Free Radic Biol Med 2020; 156: 1–10. [DOI] [PubMed] [Google Scholar]
- 54.Xu D, He H, Liu D, Geng G, Li Q. A novel role of SIRT2 in regulating gap junction communications via connexin-43 in bovine cumulus-oocyte complexes. J Cell Physiol 2020; 235: 7332–7343. [DOI] [PubMed] [Google Scholar]
- 55.Wei Z, Greaney J, Loh WN, Homer HA. Nampt-mediated spindle sizing secures a post-anaphase increase in spindle speed required for extreme asymmetry. Nat Commun 2020; 11: 3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Riepsamen A, Wu L, Lau L, Listijono D, Ledger W, Sinclair DA, Homer H. Correction: nicotinamide impairs entry into and exit from meiosis I in mouse oocytes. PLoS One 2015; 10: e0130058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.