Abstract
Multiple sclerosis (MS) is a progressive neuromuscular disorder characterized by demyelination of neurons of the central nervous system (CNS). The pathogenesis of the disorder is described as an autoimmune attack targeting the myelin sheath of nerve cell axons in the CNS. Available treatments only reduce the risk of relapse, prolonging the remissions of neurological symptoms and halt the progression of the disorder. Among the new ways of targeting neurological disorders, including MS, there is modulation of gut microbiota since the link between gut microbiota has been rethought within the term gut-brain axis. Gut microbiota is known to help the body with essential functions such as vitamin production and positive regulation of immune, inflammatory, and metabolic pathways. High consumption of saturated fatty acids, gluten, salt, alcohol, artificial sweeteners, or antibiotics is the responsible factor for causing gut dysbiosis. The latter can lead to dysregulation of immune and inflammatory pathways, which eventually results in leaky gut syndrome, systemic inflammation, autoimmune reactions, and increased susceptibility to infections. In modern medicine, scientists have mostly focused on the modulation of gut microbiota in the development of novel and effective therapeutic strategies for numerous disorders, with probiotics and prebiotics being the most widely studied in this regard. Several pieces of evidence from preclinical and clinical studies have supported the positive impact of probiotic and/or prebiotic intake on gut microbiota and MS. This review aims to link gut dysbiosis with the development/progression of MS, and the potential of modulation of gut microbiota in the therapeutics of the disease.
Keywords: Gut microbiota, gut dysbiosis, multiple sclerosis, probiotics, prebiotics, synbiotics
1. INTRODUCTION
Multiple sclerosis (MS) is a neuromuscular disorder characterized by demyelination of neurons of the central nervous system (CNS), mostly affecting the adult population (20 to 40 years of age). MS is one of the most common causes of progressive physical disability in several countries. It is estimated that about 2.5 million people have been affected by MS around the globe. Furthermore, women are more prone to the occurrence and progression of the disease, with a female to male ratio of 2:1 [1, 2]. The various symptoms associated with MS, such as walking impairment, motor abnormalities, spasticity, fatigue, pain, and cognitive impairment, result in a significant decrease in the patients’ overall quality of life [3-5]. Most patients (85-90%) have a relapsing course from the onset that is characterized by relapses and remissions of neurological symptoms associated with areas of CNS inflammation, still in more than 50% of the untreated patients, gradual worsening independent of acute attacks is seen after two decades [6, 7].
The pathogenesis of MS is best described as an autoimmune attack targeting the myelin sheath in CNS, which leads to demyelination of nerve cell axons. The inflammatory cells involved in MS include T lymphocytes, microglia, and macrophages. While the pathogenic causes of MS are still unknown, the development of the disease has been documented as progressing due to the overlapping role of genetic, environmental, and infectious factors [8-10]. Significant progress in the treatment of MS was achieved in 1993, when interferon first became available. Current therapeutic options have been expanded to 11 disease-modifying therapies approved by the European Medicine Agency (EMA) in the form of both oral and injectable formulations, with a primary focus on immunomodulatory agents. On the other hand, available therapies have only aimed at reducing the risk of relapse and halting the progression of disease, and none of them can cure MS [6].
For the mentioned reasons, the new therapeutic options for MS treatment are of great interest. The necessary modulation of immune and inflammatory pathways can be realized through the influence on gut microbiota. Gut microbiota is an assortment of microbes inhabiting the length and width of the gastrointestinal (GI) tract, and their complex interaction with the immune system is required to maintain gut homeostasis. Gut dysbiosis provides a road map for the development of both intestinal and extra-intestinal disorders, including autoimmune disorders, and thus supplementation with probiotics and prebiotics can protect against a number of disorders [11, 12]. Among the nutraceuticals, the demand for which has interestingly been increasing over the past few years, there are those modulating gut microbiota, namely dietary fibers, polyunsaturated fatty acids (PUFAs), vitamins, polyphenols and spices, and especially probiotics and prebiotics [13]. In this review, we have focused on the role of gut dysbiosis in the cause and progression of MS and the potential of probiotics and prebiotics used in the treatment of the disorder.
2. GUT MICROBIOTA
The human body is colonized by a vast number of micro-organisms, including bacteria, viruses, fungi, archaea and protozoans, living in coexistence with their host and termed as normal flora or microbiota [14]. The concept of the human microbiota was first introduced to the scientific community by Joshua Lederberg, who defined it as “the ecological community of symbiotic and pathogenic microorganisms that literally share our body space and have been all but ignored as determinants of health and disease” [15]. He gave a detailed explanation of the concept in 2000 [16], while the history of the term appearance as well as the distinction between the terms “microbiome” and “microbiota” was further discussed with references to the other scientists’ role [17]. Bacteria are most widely studied for their beneficial role in the human microbiota while viruses, fungi, archaea, and protozoans are not particularly known for any advantageous effects to their host. The microbiota colonizes every part of the human body that is exposed to the external environment, including the skin, genitourinary, respiratory and GI tracts, with the human body having been estimated to normally contain about 1014 bacterial cells, while the number of human cells present in the body is ranging between 1012 to 1014 cells [18, 19], for instance, the revised estimation by Sender et al. 2016 [20] reported that the number of bacterial cells present in the human body is 3.8 × 1013 in comparison to human cells (3.0 × 1013). Of the human organs, the GI tract is the most heavily colonized, with the colon alone containing about 70% of all microbes present in the body [14, 21, 22].
The most common examples of gut microbiota are Bacteroidetes and Firmicutes phyla. Other phyla that exist in the gut microbiota include Actinobacteria, Proteobacteria, Fusobacteria and Verrucomicrobia [23]. Gut microbiota promote GI tract morphogenesis (including villus architecture, crypt depth, stem cell proliferation, blood vessel density, mucus layer properties and maturation of mucosa-associated lymphoid tissues, and the insufficient development of these structures was seen in germ-free animals), help their host in regulating digestion and increased energy production, mainly through the availability of additional calories from otherwise indigestible oligosaccharides or promotion of nutrient uptake by modulation of absorptive capacity of the intestinal epithelium and ultimate nutrient metabolism (the role of microbiota in obesity development in humans remains controversial). The metabolism of dietary fiber to short-chain fatty acids (SCFAs) is of great importance as well as modulation of the uptake and deposition of dietary lipids through the support of lipoprotein lipase activity, upregulation of the expression of colipase (a cofactor of the pancreatic lipase). Besides, upregulation of a Na+/glucose cotransporter is possible within the intestinal epithelium. Moreover, the microbiota-dependent regulation of host energy balance is supposed through GPR41, a G protein-coupled receptor (GPCR) that binds SCFAs, and peptide YY, an enteroendocrine hormone [14, 21, 22]. Gut microbiota provide the breakdown of various polyphenols such as hydroxycinnamic acids and flavonoids, with final active products being available for the host systemic circulation and realization of metabolic, antimicrobial, antioxidative effects in the different host tissues [16]. Microbial cells produce vitamin B12 essential for cell metabolism and vitamin K essential for blood clotting. The restriction of harmful pathogens growth through competitive exclusion, consumption of nutrient sources, and production of antimicrobial substances as well as through modulating the body’s immune system, is also an important gut microbiota function [13, 24]. There are several factors, such as a high-fat diet (HFD) that can cause imbalances in the composition of the gut microbiota as well as in the integrity of the intestinal epithelial layer [25]. Numerous strategies have been proposed to regulate imbalances in the composition of gut microbiota, including the use of probiotics and prebiotics [26].
In 2001, the Food and Agricultural Organization of the United Nations (FAO) and the World Health Organization (WHO) defined probiotics as “living micro-organisms, [which] when administered in adequate amounts can modulate gut microbiota and confer beneficial effects to the host” [27]. Probiotics have been shown to enhance intestinal epithelial integrity, protect against gut barrier disruption, regulate the immune system in GI mucosa and inhibit pathogenic bacterial growth [28, 29]. Lactobacillus, Bifidobacterium and Saccharomyces species are common examples of probiotics [30]. Among nutritional components, dietary fibres are the most important factors for the growth and/or activity of the gut microbiome, as they may act as a direct substrate for the microbiota or support them via by-products of digestion such as SCFAs. The beneficial role of dietary fibres is mostly attributed to their viscosity, solubility and fermentability [31].
3. PREBIOTICS
Prebiotics are a subclass of dietary fibres and are defined as supplements or foods that selectively enhance the favorable indigenous probiotic bacteria in term of growth and/or activity [32]. They are basically resistant to the action of gastric acid and digestive enzymes [33]. A broad list of prebiotics exists, with various origins and chemical properties, but the most common are fructans (fructo-oligosaccharides (FOS) and inulin) and galacto-oligosaccharides (GOS) [34]. Dietary sources rich in prebiotics include soybeans, raw oats, unrefined wheat and barley, yacon, non-digestible carbohydrates, and inulin type fructans from traditional Chinese medicines (TCM) [35].
The chemical structure of fructans consists of a linear chain of fructose with β (2 →1) linkage. The degree of polymerization (DP) of inulin is about 60, while the DP of FOS is less than 10. As previously reported, fructans can selectively stimulate lactic acid bacteria, however recent studies suggest that the chain length of fructans is an important determinant to select bacteria for their fermentation, and thus can also directly or indirectly stimulate other bacterial species [36, 37]. GOS is the product of lactose extension and are categorized in two sub-groups; one group having excess galactose at C3, C4 or C6 and the second group being manufactured by enzymatic trans-glycosylation from lactose, which is also known as trans-galacto-oligosaccharides (TOS). GOS can enhance the growth and/or activity of Lactobacilli and Bifidobacteria to a great extent. To lesser degree, they can also stimulate some other species of phyla Bacteroidetes and Firmicutes [36, 38, 39].
Other examples of prebiotics include resistant starch and non-carbohydrate entities (flavonoids). Resistant starch confers health benefits by producing high levels of butyrate, and studies have supported its high selectivity for Firmicutes [40-42]. Evidence from in vitro and in vivo studies has supported the stimulation of lactic acid bacteria with flavanols [43]. In short, prebiotics modulate the composition and function of gut microbiota, and support the survival and growth of probiotic bacteria during the passage through the upper GIT in an adequate number, with little or no stimulation of other microorganisms [44]. The combination of probiotics and prebiotics that act synergistically to promote healthy GI bacteria is known as synbiotics, a concept introduced by Gibson and Roberfroid [45]. Furthermore, in the development of synbiotics one must consider that probiotics must exert greater effect in the presence of prebiotics, compared to probiotics used alone [46].
4. GUT MICROBIOTA MODULATION: HOPES AND THREATS
The importance of the intestines in human health was first recognized centuries ago, as postulated by Hippocrates in 400 B.C. “death sits in the bowels” and “bad digestion is the root of all evil” [47]. Several factors such as antibiotic intake, physical and psychological stresses, diet components, altered GIT peristalsis and radiation can induce dysbiosis in the gut microbiome, which may lead to impaired functioning of gut microbiota in maintaining host wellness and eventually result in a diverse range of local or systemic disorders [48]. In the last 15 years, the impact of normal flora present in the GI tract has been considered as a potential preventive and therapeutic strategy for a number of diseases ranging from intestinal disorders to metabolic and nervous system disorders [49].
The intestinal immune system is constantly exposed to foreign antigens which are tolerated for the most part, however if the body fails to tolerate them, these may precipitate the development of immune related disorders [50]. Gut microbiota play an important role in shaping intestinal immune responses. Selected strains of beneficial microbes exert potent effects on the immune system and keep the immunity of the body in balance. Probiotics regulate pro- and anti-inflammatory responses and modify dendritic cell phenotypes and secreted cytokines. They can also suppress T helper 2 (Th2) cells, T helper 17 (Th17) cells and related cytokines such as IL-17 [51-53]. Of particular interest, bacterial metabolites such as SCFAs have been reported to regulate immune cells, namely, induce neutrophils migration to inflammatory sites and enhance their ability of phagocytosis, regulate the T cell function through two mechanisms. The first mechanism is realized through the GPCR associated pathways, for instance, GPR43can be efficiently activated by acetate, propionate, butyrate and other SCFAs. Within the colonic T cells, this activation induces differentiation and enhances the FOXP3 expression. The second mechanism presupposes inhibition of histone deacetylases (HDAC) activity with the subsequent facilitation of the conversion of naïve T cells into Th1 or Th17 depending on cytokine milieu. HDAC suppression is also involved in the inhibition of proinflammatory cytokines production by intestinal macrophages. Besides, SCFAs were also thought to induce intestinal IgA production of B cells. All these effects provide the modulation of intestinal mucosal immunity, together with SCFAs’ role in intestinal barrier integrity maintenance through upregulating the expression of the tight junction as well as inducing epithelial cell secretion of IL-18, antimicrobial peptides, and mucin [54]. The positive role of beneficial microbes has been reported in multiple GIT disorders. They can be useful in constipation by increasing gut motility through direct stimulation of intestinal smooth muscle cells [55]. They can also eradicate H. pylori and thus can protect against H. pylori infections and related complications [56].
The results of the meta-analysis of randomized controlled trials support the use of probiotics in GIT inflammatory conditions, namely in patients with ulcerative colitis; they provide additional benefit in inducing remission and can exert the similar effect as 5-aminosalicylic acid on maintaining the remission, without any additional adverse events [57]. It has been well established that gut microbiota modulates energy homeostasis of the host (the microbiota-dependent influence on GPR41 and peptide YY dependent processes was mentioned above, as well as significant alteration of glucose and lipid metabolism). Among numerous results concerning the role of microbiota in obesity and diabetes, it is worthwhile to mention that the role of the Western diet in promoting an obesogenic gut microbiota is supported by the data from in vivo studies and is under further research in clinics. The causal role of the composition of gut microbiota in the progression of insulin resistance to type 2 diabetes is supposed. Moreover, in several short-term randomized controlled trials, the beneficial effect of prebiotics and probiotics was proven by the criteria of insulin sensitivity, inflammatory markers, postprandial incretins, and glucose tolerance [58-60]. The role of the inflammatory cascade in diabetes and associated
complications has been shown in some studies [61, 62]. While investigating the effects of probiotics on inflammatory markers in an animal model of diabetes, it was noted that Lactobacillus casei consumption results in decreasing IL-6 plasma level, which was significantly elevated in the untreated diabetic group [63]. It was supposed that, since IL-6 is a major cytokine of the acute phase response (and its elevation was also seen in patients with diabetes), its modulation can have a role in the prevention of the inflammatory complications of diabetes, namely the inflammatory response of the endothelial vasculature. IL-6-mediated progression of insulin resistance is also principally possible (still not supported by the results of the study cited, which has not shown any positive dynamics in glycemia). IL-6 is involved in regulating the hepatic synthesis of C-reactive protein (CRP) as a sensitive biomarker of inflammatory processes activation, and this biomarker was also downregulated by Lactobacillus casei consumption. The prebiotic also prevented infiltration of neutrophils in the portal tract [63]. The potential role of gut microbes in psychiatric and neurological disorders has also been supported by various studies, including in depression, anxiety, autism spectrum disorder, autoimmune encephalomyelitis, and neurodegenerative disorders [64-66]. Studies have shown improvements in motor dysfunction from neurological disorders due to the consumption of probiotics [67].
5. GUT DYSBIOSIS IN MS
The reassessment of the significance of gut microbiota enables the identification of new ways of counteracting neurological disorders. The link between the gut microbiome and the CNS, a concept first described at the beginning of modern physiology, has recently obtained fruitful developments. Expressive headlines such as “A healthy gut and a healthy brain” [68] or “Mind-altering with the gut” [69] are no longer astonishing. The term microbiota-gut-brain axis was proposed to describe these relationships [70, 71], and is under intensive research now in numerous fields, including that of neurodegenerative disorders. Together with the well-known links between microbiota and brain through the vagus nerve and enteric nervous system, as well as through immune system-mediated mechanisms, microbial metabolites such as SCFAs, branched-chain amino acids, and peptidoglycans are of great importance, together with tryptophan metabolism [72]. The pathways connecting the brain and gut are elucidated in a comprehensive review [73], and are illustrated in Fig. 1.
Fig. (1).
Gut-brain axis: pathways connecting the brain and gut. The gut microbiota may affect CNS via immunoregulation pathway, endocrine regulation pathway and neuronal regulation pathway. Short-chain fatty acids (SCFAs); antigen-presenting cell (APC); hypothalamic-pituitary-adrenal axis (HPA axis). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
The neural regulation pathway includes the secretion and regulation by gut microbiota of neurotransmitters of the CNS and peripheral nervous system, the release of cytokines from stimulated intestinal lymphocytes with influence on the endocrine or paracrine systems and consequently on the CNS, the secretion of many neuroactive and immunotropic substances by gut microbiota, namely γ-aminobutyric acid, histamine, serotonin, dopamine, and others [73]. The ability of bacteria to produce and/or consume a wide range of mammalian neurotransmitters is summarized in the review [74] and it can be noted that different species of Bifidobacterium and Lactobacterium can produce γ-aminobutyric acid [74] while the improvement of metabolic processes and normalization depressive-like behavior were seen in a mouse model of metabolic syndrome after γ-aminobutyric acid-producing strains [75] were added to drinking water. Dopamine is synthetized by Bacillus subtilis, Escherichia coli (K-12), Hafnia alvei, Morganella morganii, Proteus vulgaris etc., serotonin - by Escherichia coli (K-12), Hafnia alvei (NCIMB, 11999), Lactobacillus plantarum (FI8595), Lactococcus lactis subsp. cremoris (MG 1363), Morganella morganii (NCIMB, 10466), Streptococcus thermophilus etc., histamine-by different species of Lactobacillus and Lactococcus, Citrobacter freundii, Hafnia alvei (NCIMB, 11999), Morganella morganii (NCIMB, 10466), Streptococcus thermophiles (NCFB2392) etc. [74].
The endocrine regulation pathway is described as the result of glucocorticoids, mineralocorticoids, or catecholamines (released after the stress-induced reaction of the hypothalamic-pituitary-adrenal axis) influencing the composition of the gut microbiota, with possible disturbances of barrier functions and immune responses. Dysbiosis under the influence of stress was confirmed in vivo, and autoimmune diseases, including MS are generally associated with shifts in the function of the hypothalamic-pituitary-adrenal (HPA) axis. The immunoregulation pathway presupposes the role of gut microbiota in antigen presenting in regulating the production of cytokines and the function of lymphocytes. The production of SCFAs such as acetate (produced by gut anaerobes in humans), propionate (produced by Bacteroidetes), and butyrate (produced by Firmicutes), can modify the immune response and participate in the development of inflammatory processes in the CNS. The metabolic system regulation pathway is connected with the release of immune antigens (peptidoglycan, lipopolysaccharide) from the gut microbiota as well as polysaccharide A. The synthesis of cross-reactive antibodies (mainly IgA) may also be stimulated by gut bacteria [73].
As illustrated in Fig. 2, several factors contribute to disorders in the integrity of tight junctions and the increase in permeability of the gut barrier, such as western diets, saturated fatty acids, salt, gluten, alcohol, food additives (especially emulsifiers and artificial sweeteners) and antibiotics. Nonsteroidal anti-inflammatory drugs (NSAIDs) are also known to contribute to this disturbance, as well as stress-induced reactions with the activation of the corticotropin-releasing factor - mast cell axis. The protective effect on the gut barrier is inherent in calorie restriction or fasting, SCFAs, vitamins D and A, polyphenols, omega-3 polyunsaturated fatty acids, zinc, mucoprotectors and certainly in prebiotics and probiotics [76]. The influence of the aforementioned substances disturbing the gut barrier may be realized either directly or by modulation of the composition of gut microbiota. This situation is associated with a decrease in overall microbial diversity and an increment in the Firmicutes/Bacteroidetes ratio. The consequence of dysbiosis is an increase in lipopolysaccharide levels and in the Th17/Treg ratio, which triggers intestinal inflammation and contributes to a disorder of the integrity of the tight junctions. The latter leads to the inflammatory basis for neurodegenerative disorders [76, 77].
Fig. (2).
Factors responsible for gut dysbiosis. The imbalance between several factors may lead to gut dysbiosis, which eventually results in leaky gut syndrome, systemic inflammation, immune dysregulation, and increased susceptibility to certain infections. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
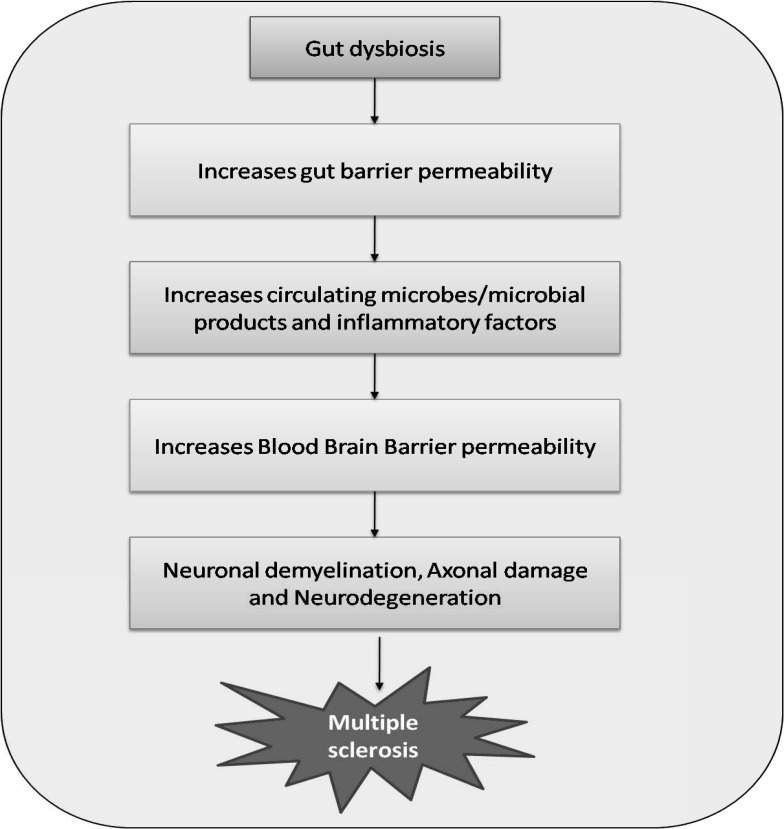
The significance of increased gut permeability and alterations in gut microbiota is emphasized in the main pathophysiological mechanisms of MS (Fig. 3), such as increases in oxidative and nitrosative stress, pro-inflammatory immune responses (especially Th17 cell proliferation and activation), alteration in tryptophan metabolism and depressed mental states [78]. There are data directly confirming the development of leaky gut syndrome in patients with MS. Increased intestinal permeability was registered in these patients [79], and highly significant increases in IgA and IgG antibodies were found against gliadin and gluten [80]. It has recently been suggested that alterations in the regulation of local melatonergic pathways represent an important link between CNS disorders in MS and gut microbiota, as well as gut permeability. Decreasing serotonin availability as a precursor for melatoninergic pathways is considered to lead to the increased frequency of depression at the different stages of MS (and the changes in the gut are evident in the early stages of MS, including in pediatric patients) [78].
Fig. (3).
Gut dysbiosis, a potential contributor to the pathogenesis of Multiple sclerosis. Imbalance in gut microbiota may increase gut barrier permeability, circulating microbes/microbial products and inflammatory factors, which eventually results in increased blood-brain barrier permeability, neuronal demyelination, axonal damage, and neurodegeneration. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Evidence also supports the breakdown of the blood-brain barrier (BBB) linked to the presence of gut-derived molecules and cells in the bloodstream. The lipopolysaccharides from gram-negative bacteria and lipoteichoic acid from gram-positive bacteria can bind to the Toll-like receptors (TLR2 and TLR4) expressed by the brain endothelial cells. Activation of these receptors is a well-known response to antigens with the initiation of pro-inflammatory transcription factor NF-κB and the MAP kinase pathway. Antibodies and pro-inflammatory cytokines may also have a role in the breakdown of the BBB, as well as T cells which can cross the BBB after activation, which may happen with the participation of gut-associated lymphoid tissues. Activated Th17 and IL-17A can disrupt the BBB [76, 81, 82]. In this context, it is important to mention that the recent evidence supports the role of the dysregulation of the BBB and transendothelial migration of activated leukocytes among the earliest cerebrovascular abnormalities in MS [83].
As the essential feature of the BBB, microglia regulate the pro-inflammatory activity of astrocytes by the release of VEGF-B (pro-inflammatory factor) and TGF-α (alleviating inflammatory responses in this case), with activated astrocytes having a destructive effect on neurons. In this context, the influence of the gut microbiota is possible through the restoration of VEGF-B / TGF-α balance, and it has been shown in the generally used experimental autoimmune encephalomyelitis model of MS in mice that the metabolites of dietary tryptophan produced by microbiota are able to exert such an effect. The result is the limitation of pro-inflammatory programs in microglia and further suppression of the activation of detrimental astrocytes, with the involvement of the aryl hydrocarbon receptor being of great importance. Besides, SCFAs may also modulate microglia and astrocyte activity [76, 84-86]. Thus, butyrate was found to ameliorate demyelination in an in vivo model and exert a direct protective influence on oligodendrocytes in vivo [75]. Sodium butyrate, being an HDAC inhibitor, is widely investigated on brain injury models and the study [87] defines restoration of the blood-brain barrier as the main mechanism of its action with the upregulation of tight junction-associated proteins expression. The recent review [88] emphasizes the possibilities of SCFAs crossing the BBB considering the abundant expression of H+-coupled monocarboxylate transporter in endothelial cells. The brain uptake of SCFAs, including butyrate, after parenteral administration in rats, was demonstrated long ago [89], but even more significant data are those indicating the higher concentration of SCFAs including butyrate in human brain tissue compared to that in plasma [90].
As summarized in review [73], a decrease in butyrate (as Faecalibacterium metabolite) and lipid 654 (Bacteroides metabolite) are important links in MS development, while an increment is observed in the content of proinflammatory cytokines in serum, CD4+CD25+ regulatory T cells (targeting the brain and spinal cord), and toxins B and D (C. perfringens) [70]. All these changes present promising targets for pharmacological intervention, including the use of probiotics and prebiotics.
Furthermore, the role of the endogenous cannabinoid system in the integration of gut microbiota and neuroinflammation (including that in MS) has been addressed recently [91]. This system, which contributes to the maintenance of homeostasis, and related bioactive lipids are involved in metabolic, GI and neuroimmune regulatory mechanisms at multiple central and peripheral levels. In particular, a structural convergence was suggested between the endocannabinoids and microbiota-encoded metabolites, such as active, long-chain N-acyl amide able to interact with GPCR in a similar way as endocannabinoids do. Activation of these receptors by microbial N-acyl amides in humans is mostly localized in the immune cells within the gut, thus supporting the possibility of neuroinflammatory processes modulation within the gut-brain axis. The experimental data supporting this concept were obtained in a model of MS by Mestre et al. 2018 [91], while there is a large body of data showing a reduced severity of MS in experimental models after the activation of the endogenous cannabinoid system. The role of the endogenous cannabinoid system in the control of gut barrier function as well as BBB permeability is also being discussed [91]. Besides, an influence on the Toll-like receptors and suppression of neuroinflammation has also been reported for docosanoic acid, some probiotics, and prebiotics [68, 75].
Within the gut-brain axis concept, the contribution of the GI peptides is also of high significance. Peptidergic growth factors as cytoprotectors with multi-directed action are supposed to beneficially affect CNS-disorders [92]. Thus, a stable gastric pentadecapeptide BPC 157 has demonstrated high efficacy in the cuprizone-induced MS model, consistently decreasing nerve damage in all damaged areas (and especially in those areas that otherwise were most affected), counteracted cerebellar ataxia and impaired forelimb function [93].
Together with the studies elucidating the different mechanisms of function and modulation of the microbiota-gut-brain axis, there are studies of particular interest addressing the relevance of changes in the gut microbiota for the progression of MS, through regulation of the adaptive autoimmune responses [94, 95]. These studies have shown that transplantation of fecal content from patients with MS to germ-free mice leads to the worsening of the course of experimental autoimmune encephalomyelitis. Akkermansia muciniphila and Acinetobacter calcoaceticus (the increase in these species was significantly associated with the course of MS) induced proinflammatory reactions, while Parabacteroides distasonis (reduced in patients with MS) stimulated IL-10+FoxP3+ Tregs. These results are also supported by in vitro findings in human peripheral blood mononuclear cells and clearly determine microbiota as a target for MS treatment [84, 94, 95].
Fecal microbial transplantation is considered to be a promising intervention to ameliorate gut dysbiosis in MS patients (nevertheless, additional data in the form of clinical research with special attention to treatment design are needed) [96]. Up till now, it has been shown (in a limited quantity of patients) that this intervention can lead to the amelioration of neurological symptoms and improvement in quality of life [70, 97].
The decrease in anti-inflammatory pathways associated with changes in certain bacterial species is also considered to be significant [70, 98, 99]. Thus, reduced proportions of Faecalibacterium, Eubacterium rectale, Corynebacterium, and Fusobacteria were seen in MS patients compared to healthy controls [70, 100]. The reduced quantity of Faecalibacterium leads to a reduced level of butyrate production, which is the prerequisite for a decrease in Treg cells, APCs, and the release of pro-inflammatory cytokines [70, 98]. Lowered levels of Bacteroides stercoris and Bacteroides coprocola were registered in MS patients; moreover, a negative correlation between the number of Prevotella copri and the risk of MS development was established [101]. The shifts in the microbiota-gut-brain axis in MS are also supported by clinical findings. Clostridium perfringens toxins B and D, which are not detectable in healthy volunteers, are supposed to contribute to proinflammatory changes in the retina by affecting barrier veins or by binding to vascular system receptors [70, 102]. The ability of these toxins to induce a lack of movement coordination or blurred vision has been confirmed [98]. The significant decrease in serum concentration of Bacteroidetes species’ metabolite lipid 654 is even suggested to be a MS biomarker [70, 103]. The evidence regarding gut microbiota disturbances in MS compared with healthy controls is summarized in Table 1.
Table 1.
Gut microbiota disturbances (families, genera, and species) in multiple sclerosis compared with healthy controls.
| Increased | References | Decreased | References |
|---|---|---|---|
| Escherichia | [70] | Faecalibacterium | [70, 100, 101, 104] |
| Shigella | [70] | Eubacterium (E. rectale) | [70, 101, 104] |
| Clostridium | [70, 100, 101, 104, 105] | Corynebacterium | [70] |
| Firmicutes | [70] |
Fusobacteria (genus Fusobacterium or Leptotrichia) |
[70, 106] |
| Blautia | [104, 107] | Bacteroides | [70, 104, 107] |
| Dorea | [104, 107] | Parabacteroides | [104, 107] |
| Streptococcus | [101, 104] | Prevotella | [101, 104, 107, 108] |
| Ruminococcus | [100, 104] | Butyricimonas | [104, 108] |
| Acinetobacter | [94, 104] | Lachnospiraceae | [104, 106] |
| Bifidobacterium | [101, 104, 106] | Ruminococcaceae | [104, 106] |
| Eggerthella | [101, 104] | Lactobacillus | [104, 107] |
| Pseudomonas | [104, 107] | Coprobacillus | [104, 107] |
| Mycoplana | [104, 107] | Erysipelotrichaceae | [104, 107] |
| Haemophilus | [104, 107] | Veillonellaceae | [104, 107] |
| Bilophila | [104, 106] | Collinsella | [104, 107, 108] |
| Sutterella | [101, 104] | Adlercreutzia | [104, 107] |
| Akkermansia | [94, 95, 104, 108] | Slackia | [104, 108] |
6. SYNBIOTICS AND MS
The data supported above substantiate the expediency of probiotic and prebiotic use in MS. The direct evaluation of such interventions was intensively studied experimentally. Great progress has been made in the development of experimental approaches to study neurodegeneration [109]. Animal models allow the linking of microbiome shifts and microglia-associated changes to the development of neurogenesis and myelination disorders [72]. For this reason, the attention of most researchers is focused on research at the level of the whole organism, and relatively few works elucidate certain mechanisms of MS counteraction in vitro studies. Thus, it has been shown that Streptococcus thermophilus favorably modulates cytokine secretion against MS peptide in mice (mouse spleen cells immunized with agonist MBP83-99 peptide). A significant increment in the expression of anti-inflammatory IL-4, IL-5, IL-10 together with the reduced secretion of pro-inflammatory IL-1β and IFN-γ were registered [110].
6.1. Experimental Studies of Synbiotics on In vivo Models of MS
The data concerning microflora modulation on in vivo models of MS are summarized in Table 2. Most of these data were obtained on a model of experimental autoimmune encephalomyelitis (EAE). Despite certain limitations (such as variability of disease susceptibility, severity, and course depending on the species and strain choice, and, if rodents are used, the differences from MS course in clinics are the questionable involvement of CD8+ cells, insufficient axonal loss, strain dependence of white matter or grey matter demyelination severity), this model is considered to be adequate in its representation of principal links of MS pathogenesis and has been widely used in studying inflammation, CNS penetration, demyelination, axonopathy, and neuron loss mediated by immune cells [111].
Table 2.
Efficacy of microflora modulation in in vivo models of multiple sclerosis
| Species and Strain of Laboratory Animals | Model | Intervention | Main Findings | Refs. | |||
|---|---|---|---|---|---|---|---|
| Lactobacilli | |||||||
| C57BL/6 mice | EAE (immunization with a synthetic peptide from myelin oligodendrocyte glycoprotein in CFA, administration of pertussis toxin) |
Lactobacillus paracasei and L. plantarum in a therapeutic regimen or before and after immunization |
Delayed progression and reversal of the clinical and histological signs of the disease. The mechanism is associated with attenuation of pro-inflammatory Th1 and Th17 cytokines followed by IL-10 induction in MLNs, spleen, and blood. Monotherapy with the strains used in the study appeared to be inefficient. |
[70, 115, 116] | |||
| C57BL/6 Mice |
EAE (MOG35-55)1 |
Lactobacilli (three strains, L. paracasei DSM 13434, L. plantarum DSM 15312 and DSM 15313) before and after induction of the model |
Reduction of clinical and histological signs of the disease, that correlated with attenuation of pro-inflammatory Th1 and Th17 cytokines followed by IL-10 induction in MLNs, spleen, and blood. Monotherapy with the strains used in the study appeared to be inefficient. |
[73, 116] | |||
| SJL/J mice | EAE (PLP139-151)2 | Lactobacillus helveticus SBT2171 intraperitoneally before and after induction of the model | A significant decrease in the incidence and clinical signs, reduction in the quantity of Th17 cells in the spinal cord associated with downregulation of IL-6 production and the subsequent Th17 differentiation and spinal cord infiltration. | [117] | |||
| Lewis rat | Immunization with MBP72-85 in Difco’s incomplete adjuvant |
Lactobacillus casei 393, or commercially available products containing Lactobacillus casei DN 114-001 or Lactobacillus rhamnosus or Lactobacillus casei given orally seven times during a 44-day experiment |
In all cases, the total disease burden was reduced (27—63%), maximal disease score and time of onset (but not the duration of disease) were decreased. Most notably, in the other experiments on SJL/J mice, no efficacy was seen in any of the lactobacilli preparations. |
[73, 118] | |||
| SJL/J and C57BL/6 Mice |
EAE (PLP139-151) 2 in SJL/J mice and EAE (MOG35-55) 1 in C57BL/6 mice |
Lactobacillus casei strain Shirota before and after induction of the model | Absence of exacerbation of neurological symptoms, tendency towards improvement of neurological symptoms in the SJL/J mouse. Enhanced production of IL-10 and an increase in the percentage of CD4(+) CD25(+) T regulatory cells. Transient upregulation of IL-17 production by antigen-stimulated lymphocytes, confirming that IL-17 responses at peripheral sites may not always result in a worsening of autoimmune diseases. |
[114] | |||
| C57BL/6 mice | EAE (MOG35-55) 1 |
Lactobacillus reuteri DSM 17938 before and after immunization | Reduction of TH1/TH17 cells and their associated cytokines IFN-γ/IL-17, restoration of the diversity of gut microbiota. | [119] | |||
| C57BL/6 mice | Cuprizone-induced model | Lactobacillus casei strain T2 (IBRC-M10783), oral administration of 1 × 109 CFU/ml probiotic for 4 weeks before or after induction of the model, the combined treatment with vitamin D (20 IU/day) after induction of the model was also evaluated. | Improvement of the motor behaviors in the Y-maze test (all regimens of treatment), decrease in IL-17 serum level (especially after the combined treatment), reversal of cuprizone-induced IDO gene expression and miR-155 expression, reversal of the decrease in miR-25 expression. It is supposed that the probiotic it seems that can shift responses from Th17 to Tregs and play a role in the recovery of demyelination processes. |
[120] | |||
| Lewis rats | Immunization with guinea pig myelin basic protein with CFA | Lactobacillus casei Shirota started at the age of 2 weeks, induction of the model at the age of 7 weeks | Increased the duration of clinical symptoms, adjuvation is supposed for both Th1 and Th2 responses. |
[73, 121] | |||
| Species and Strain of Laboratory Animals | Model | Intervention | Main Findings | Refs. | |||
| Lactococcus | |||||||
| C57BL/6 mice | EAE (MOG35-55) 1 |
Recombinant Lactococcus lactis strain that produces and releases LPS-free Hsp65 orally in a prophylactic regimen | Decrease in inflammatory cell infiltration and injury signs in the spinal cord associated with reduced IL-17 and increased IL-10 production in mesenteric lymph nodes and spleen. The significant increase in the number of natural and inducible CD4+Foxp3+ regulatory T (Treg) cells and CD4+LAP+ Tregs expressing the membrane-bound TGF-β - in spleen, inguinal and mesenteric lymph nodes, and spinal cord. |
[73, 122] | |||
| Bifidobacteria | |||||||
| Lewis rats (LEW/HanHsD) | Immunization with guinea pig myelin basic protein (MBP) with CFA | Bifidobacterium animalis started at the age of 2 weeks, induction of the model at the age of 7 weeks | Significant reduction in the duration of clinical symptoms, normalization of body weight dynamics. | [73, 121] | |||
| Bacteroides fragilis | |||||||
| SJL/J mice | EAE (PLP139-151) 2 |
Bacteroides fragilis producing a bacterial capsular polysaccharide Ag (decolonization after the destruction of gut bacteria with a combination of antibiotics) | Resistance to the development of the disease with protection against CNS demyelination. Activated rates of conversion of CD4+ T cells into IL-10- producing Foxp3+ Treg cells, thus shifting the balance towards the anti-inflammatory cytokines. |
[73, 123] | |||
| C57BL/6 mice | EAE (MOG35-55) 1 |
Zwitterionic capsular polysaccharide A of Bacteroides fragilis before and after induction of the model | Delayed onset and progression of the disease, enhanced levels of anti-inflammatory cytokines. The mechanism of action is associated with the expansion of CD4 T cells. |
[73, 124, 125] | |||
| Pediococcus acidilactici | |||||||
| C57BL/6 mice | EAE (MOG35-55) 1 |
Pediococcus acidilactici R037 (heat-killed) from 14 days before immunization | Decreased severity of the disease, inhibition of the antigen-specific production of inflammatory cytokines associated with primary induction of Foxp3(-) IL10-producing T regulatory type 1 (Tr1) cells in mesenteric lymph nodes. | [73, 126] | |||
| Escherichia coli strain Nissle | |||||||
| C57/BL6J mice | EAE (MOG35-55) 1 | Escherichia coli strain Nissle 1917 | Reduction in the severity of the disease, suppression of secretion of inflammatory cytokines and an activated production of the anti-inflammatory cytokine IL-10 by autoreactive CD4 T cells, both in peripheral lymph nodes and CNS. Modulation of activation and/or differentiation of T cells, thus influencing their migration from the periphery to the CNS is supposed. | [127] | |||
| SJL or C57BL / 6 mice | EAE (MOG35-55) 1 or EAE (PLP139-151) 2 |
Zwitterionic capsular polysaccharide A of Bacteroides fragilis in prophylactic or therapeutic regimen | Reduction in the severity of the disease, the increase in CD103 expressing dendritic cells that accumulated in the cervical lymph nodes. The mechanism is attributed to the enhancement in the conversion of CD4+ T cells into IL-10- producing Foxp3+ Treg cells. |
[73, 128] | |||
| Prevotella histicola | |||||||
| HLA-DR3.DQ8 double-transgenic mice lacking MHC class II genes |
Immunization with PLP91-110 | Prevotella histicola starting 7 days after immunization, 7 doses on alternate days | Decrease in pro-inflammatory Th1 and Th17 cells and increase in the frequencies of CD4+FoxP3+ regulatory T cells, tolerogenic dendritic cells, and suppressive macrophages substantiating the favorable modulation of systemic immune responses. | [73, 129] | |||
| Species and Strain of Laboratory Animals | Model | Intervention | Main Findings | Refs. | |||
| Combined probiotics | |||||||
| C57BL/6 mice | EAE (MOG35-55) 1 |
IRT5 containing Lactobacillus casei, Lactobacillus acidophilus, Lactobacillus reuteri, Bifidobacterium bifidum, and Streptococcus thermophilus in a prophylactic regimen | Delayed onset and less severe course of the disease, inhibition of the pro-inflammatory Th1/Th17 polarization, while inducing IL10+ producing or/and Foxp3+ regulatory T cells, both in the peripheral immune system and at the site of inflammation. |
[70, 115, 130] | |||
| C57BL/6 mice | EAE (MOG35-55) 1 |
Lactobacillus plantarum A7, Bifidobacterium animalis PTCC 1631or a mixture of both strains orally daily for 22 days beginning simultaneously with induction of the disease | Delay in the time of disease onset, decrease in mononuclear infiltration into the CNS, enhancement of the population of CD4+CD25+Foxp3+-expressing T-cells in the lymph nodes and the spleen. These effects were more significant when the combination of both strains was used. The inhibition of the disease associated cytokines and increment in anti-inflammatory cytokines, favoring Th2 and Treg differentiation via up-regulation of Foxp3 and GATA3 in the brain and spleen, inhibition of the differentiation of Th1 and Th17 cells. |
[131] | |||
| Lewis rats | Immunization with guinea pig MBP or other antigens | Lactobacillus casei strain Shirota and Bifidobacterium breve strain Yakult before and after induction of the model in different regimens | Absence of autoimmune disease exacerbation after the use of both strains, in one of the regimens - tendency to suppression of neurological symptoms. |
[114] | |||
| Additional data obtained using vaccination approaches | |||||||
| SJL mice | EAE (PLP139-151) 2 |
Vaccination with Salmonella expressing E. coli colonization factor antigen I fimbriae, with further transfer of isolated CD25+ and CD25−CD4+ T cells to naive mice |
Reduced development and progression of the disease, suppression of the development and expansion of Th1 and Th17 cells, normalization of cytokine balance. It was ascertained that CFA/I fimbriae offer an alternative strategy to adapt Treg cells and modulate the expansion of myelin specific Treg cells to suppress autoimmune disease. |
[73, 132, 133] | |||
| SJL/J mice | EAE (PLP139-151) 2 |
Vaccination with Salmonella-CFA/I vaccine expressing functional CFA/I fimbriae from Escherichia coli in a therapeutic regimen. | Reduction in disease severity and duration, enhanced production of TGF-β, (not IL-10), the optimal induction of the protective T(reg) cells in conjunction with immune deviation by Th2 cells. | [73, 134] | |||
1EAE (MOG35-55) - experimental autoimmune encephalomyelitis induced by immunization with MOG35-55 peptide in CFA and administration of Pertussis toxin. 2EAE (PLP139-151) - experimental autoimmune encephalomyelitis induced by immunization with PLP139-151 peptide in CFA and administration of Pertussis toxin.
The Cuprizone-induced model is characterized by oligodendrocyte dysfunction and is a straightforward model used to investigate brain-intrinsic inflammatory responses, together with demyelination/remyelination processes. In regard to the latter, it is even described as an ideal model [111], taking into account its simplicity, high reliability and reproducibility. Still, the variable degree of demyelination depending on the strain, age, and sex of the laboratory animals is reported, together with lack of immune system involvement (seen in the other toxin-induced demyelination models), including the absence of CD8+ cells and B cells involvement, as well as lack of inflammatory lesions within the brain. Great prospects are supposed for the models using cuprizone in combination with actively induced EAE, resulting in inflammatory forebrain lesions [111]. Unfortunately, the experimental data on probiotics efficacy evaluation on the cuprizone-induced model are scarce. The study [112] is of great interest in this context, demonstrating that inflammatory responses during remyelination depend upon the microbiota, whereas the regenerative responses of oligodendrocyte progenitor cells are largely independent of it. The study cited above has demonstrated the prospects of GI peptides use in MS just on cuprizone-induced model [93].
Proceeding with the aim of this review, the evidence regarding antibiotic treatments on the mentioned models is not summarized here. It is stressed that the effects of probiotics on the mice with experimental autoimmune encephalomyelitis are strain-dependent, either stimulating or suppressing clinical symptoms [73, 113]. In some cases, neutrality was revealed, and the absence of exacerbation was emphasized [114]. Indeed, the multifactorial pathways associated with the microbiota-gut-brain axis (described above) presuppose the influence of the individual strain as well as the timing of such intervention on the further development of the disease.
6.2. Clinical Studies of Synbiotics Efficacy in MS
The aspects described above also substantiate a need for the clinical evaluation of such treatments. The data from clinical research which addressed the evaluation of microflora modulation in patients with MS are summarized in
Table 3. Thus, the existing evidence supports the great significance of the microbiome as a potential target for pharmacological intervention, as well as high prospects for microbiome modulation and correction in neurodegenerative diseases including MS. Fascinating results are expected from this field in the future.
Table 3.
Efficacy of microflora modulation by combined probiotics in multiple sclerosis - results of the clinical studies.
| Study Sample | Probiotics | Main Findings | Refs. | ||
|---|---|---|---|---|---|
| n=9 in the treatment group, patients with relapsing-remitting MS, n=13 in the healthy control group (pilot study) The dynamics of the studied parameters was determined in each group (prior to, at discontinuation of therapy, and 3 months thereafter) |
Probiotic mixture VSL3 containing 3×1011/g of viable lyophilized bacteria including four strains of Lactobacillus (Lactobacillus paracasei DSM 24734, Lactobacillus plantarum DSM 24730, Lactobacillus acidophilus DSM 24735, and Lactobacillus delbrueckii subspecies bulgaricus DSM 24734), three strains of Bifidobacterium (Bifidobacterium longum DSM 24736, Bifidobacterium infantis DSM 24737, and Bifidobacterium breve DSM 24732), and one strain of Streptococcus (Streptococcus thermophilus DSM 24731). | Increased abundance of many species predominated by Lactobacillus, Streptococcus, and Bifidobacterium was seen in both healthy controls and MS patients. In controls, the significant increase in the relative abundance of these strains resulted in the reduced evenness (as the measure of representation of all species) due to the possible competitive interaction. At the immune level, the probiotic effect was predominantly seen on monocytes and dendritic cells. The decreased frequency of intermediate monocytes (CD14highCD16low) was seen in MS subjects (9.07% vs. 7.58%, p = 0.039), while decreased mean fluorescence intensity of costimulatory marker CD80 on classical monocytes was registered in controls (88 vs. 80.6, p=0.048). These changes were considered by the authors as an anti-inflammatory peripheral innate immune response, as well as the decreased mean fluorescence intensity of human leukocyte antigen-antigen D related (HLA-DR) on myeloid derived dendritic cells (CD45+LIN− CD11c+) following administration of the probiotic (1890 vs. 1510, p = 0.016) in MS patients. These immunomodulatory effects did not persist after discontinuation of the probiotic. |
[135] | ||
| Study Sample | Probiotics | Main Findings | Refs. | ||
| n=30 per group, patients with relapsing-remitting MS (receiving interferon beta-1α therapy), comparison of the results of probiotic treatment with the results in placebo group |
Probiotic containing Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum and Lactobacillus fermentum (each 2×109 CFU/g) daily for 12 weeks |
The improvement in expanded disability status scale dynamics was registered after the probiotic use compared with placebo (-0.3 ± 0.6 vs.+0.1 ± 0.3, p = 0.001), as well as inflammatory factors such as high-sensitivity CRP serum level (-1.3 ± 3.5 vs. +0.4 ± 1.4 mg/mL, p = 0.01). These parameters were considered as the primary outcomes. The positive changes in the secondary outcomes were also seen, including mental health parameters - Beck Depression Inventory (-5.6 ± 4.9 vs. -1.1 ± 3.4, p < 0.001), general health questionnaire (9.1 ± 6.2 vs. 2.6 ± 6.4, p < 0.001) and depression anxiety and stress scale (16.5 ± 12.9 vs. 6.2 ± 11.0, p = 0.001). The additional benefits were the improvements of the metabolic profile, among which the serum insulin and the calculated values of assessment-estimated insulin resistance, beta cell function was the most favorably changed (in all cases p≤0.001). |
[136] | ||
| n=24 per group, patients with relapsing-remitting MS (receiving interferon beta-1α therapy), comparison of the results of probiotic treatment with the results in placebo group |
Probiotic containing Bifidobacterium infantis, Bifidobacterium lactis, Lactobacillus reuteri, Lactobacillus casei, Lactobacillus plantarum and Lactobacillus fermentum (each 2 × 109 CFU/d) for 16 weeks |
The positive effect was reached after the probiotic use in the primary outcomes, namely the expanded disability status scale dynamics (-0.52 ± 0.04 vs. +0.17 ± 0.07, p < 0.001), as well as the reduction in IL-6 (-0.2 ± 0.1 vs. 0.07 ± 0.08, pg/mL; P = 0.01) and a significant increase in IL-10 (+0.46 ± 0.16 vs. -0.3 ± 0.22, pg/mL; p< 0.001) plasma levels. These shifts were considered as balancing the inflammatory and anti-inflammatory responses. The other biomarker of inflammatory processes activation - high-sensitivity CRP level in serum - was also decreased (p = 0.03). Among the secondary outcomes pronounced decrease was observed in the plasma concentration of MDA (-0.31 ± 0.75 vs. +0.15 ± 0.79, µmol/L; p < 0.001) as well as 8-hydroxy-2′-deoxyguanosine (-6.72 ± 2.03 vs. +3.15 ± 1.57, ng/mL; P < 0.001) in the probiotic compared to placebo group. These oxidative stressors are expected to be significant for the symptoms reduction in MS patients. The mental health parameters (as the secondary outcomes) were also improved by the probiotic, namely Beck Depression Inventory (-5.08 ± 0.71 vs. -2.62 ± 0.78, p = 0.026), general health questionnaire-28 (-6.7 ± 1.17 vs.-3.04 ± 1.13, p = 0.03) and depression, anxiety, and stress scale (-12.5 ± 1.81 vs. -3.33 ± 2.26, P = 0.003). |
[137] | ||
| n=20 per group, patients with relapsing-remitting MS, comparison of the results of probiotic treatment with the results in placebo group |
Probiotic containing Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum, and Lactobacillus fermentum (each 2 × 109 CFU /g) |
Compared with placebo, probiotic supplementation downregulated gene expression of the pro-inflammatory cytokines IL-8 (p < 0.001) and TNF-α mRNA (p < 0.001) in peripheral blood mononuclear cells of patients with MS. | [138] | ||
CONCLUSION
MS is a progressive neuromuscular disorder characterized by the loss of the myelin sheath of neurons. Because of its complicated etiologies, effective therapeutic strategies for MS have been of great interest for decades. The concept of gut microbial modulation is being actively explored, and several pieces of evidence exist that support the link between alterations in gut microbial composition with certain pathologies. The relationship between gut microbiota composition and neurodegenerative disorders, including MS has become a novel and promising field of research that could provide insights into the understanding of pathological mechanisms of the disease and could offer effective therapeutic alternatives. Numerous options are known to modulate gut microbiota for correction of pathogenic dysbiosis, but the intake of probiotics is the most widely studied and one of the most effective ways. Consumption of probiotics alone or in combination with prebiotics (synbiotics) can improve gut dysbiosis and thus provide a positive impact on the leaky gut syndrome, systemic inflammation, immune regulation, and infection susceptibility, which may result in the improvement of MS pathogenesis as evidenced by several in vivo studies and clinical trials. However, clinical trials on larger scales are suggested to explore the efficacy and safety profile of different species of probiotics in human populations as well as to establish certain schemes of probiotics treatment as part of regular or alternative treatment of MS or its prophylaxis.
ACKNOWLEDGEMENTS
We thank Dr. Owen Perring for English language editing.
LIST OF ABBREVIATIONS
- APC
Antigen Presenting Cell
- BBB
Blood brain barrier
- CNS
Central nervous system
- CRP
C-reactive protein
- DP
Degree of polymerization
- EMA
European Medicine Agency
- FAO
Food and Agricultural Organization of the United Nations
- FOS
Fructo-oligosaccharides
- GIT
Gastro-intestinal tract
- GOS
Galacto- oligosaccharides
- GPCR
G protein-coupled receptor
- HDAC
histone deacetylases
- HFD
High fat diet
- HPA axis
Hypothalamic-pituitary-adrenal axis
- IFN-γ
Interferon gamma
- IgAs
Immunoglobulins A
- MAP kinase
Mitogen activated protein kinase
- MS
Multiple sclerosis
- NF-κB
Nuclear factor kappa light chain enhancer of activated B cells
- NSAIDs
Nonsteroidal anti-inflammatory drugs
- PPAR-γ
Peroxisome proliferator activated receptor gamma
- PUFAs
Polyunsaturated fatty acids
- SCFAs
Short chain fatty acids
- TCM
Traditional Chinese medicines
- TGF-α
Transforming growth factor alpha
- TLR
Toll-like receptors
- TOS
Trans-galacto- oligosaccharides
- Treg
Regulatory T cells
- VEGF-B
Vascular endothelial growth factor B
- WHO
World Health Organization
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Hughes A.J., Dunn K.M., Chaffee T. Sleep disturbance and cognitive dysfunction in multiple sclerosis: a systematic review. Curr. Neurol. Neurosci. Rep. 2018;18(1):2. doi: 10.1007/s11910-018-0809-7. [DOI] [PubMed] [Google Scholar]
- 2.Tardieu M., Banwell B., Wolinsky J.S., Pohl D., Krupp L.B. Consensus definitions for pediatric MS and other demyelinating disorders in childhood. Neurology. 2016;87(9) Suppl. 2:S8–S11. doi: 10.1212/WNL.0000000000002877. [DOI] [PubMed] [Google Scholar]
- 3.Calandri E., Graziano F., Borghi M., Bonino S. Depression, positive and negative affect, optimism and health-related quality of life in recently diagnosed multiple sclerosis patients: The role of identity, sense of coherence, and self-efficacy. J Happiness Stud. 2018;19(1):277–295. doi: 10.1007/s10902-016-9818-x. [DOI] [Google Scholar]
- 4.Yazgan Y.Z., Tarakci E., Tarakci D., Ozdincler A.R., Kurtuncu M. Comparison of the effects of two different exergaming systems on balance, functionality, fatigue, and quality of life in people with multiple sclerosis: A randomized controlled trial. Mult. Scler. Relat. Disord. 2019;39:101902. doi: 10.1016/j.msard.2019.101902. [DOI] [PubMed] [Google Scholar]
- 5.Khan H., Sureda A., Belwal T., Çetinkaya S., Süntar İ., Tejada S., Devkota H.P., Ullah H., Aschner M. Polyphenols in the treatment of autoimmune diseases. Autoimmun. Rev. 2019;18(7):647–657. doi: 10.1016/j.autrev.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montalban X., Gold R., Thompson A.J., Otero-Romero S., Amato M.P., Chandraratna D., Clanet M., Comi G., Derfuss T., Fazekas F., Hartung H.P., Havrdova E., Hemmer B., Kappos L., Liblau R., Lubetzki C., Marcus E., Miller D.H., Olsson T., Pilling S., Selmaj K., Siva A., Sorensen P.S., Sormani M.P., Thalheim C., Wiendl H., Zipp F. ECTRIMS/EAN Guideline on the pharmacological treatment of people with multiple sclerosis. Mult. Scler. 2018;24(2):96–120. doi: 10.1177/1352458517751049. [DOI] [PubMed] [Google Scholar]
- 7.Confavreux C., Vukusic S. Natural history of multiple sclerosis: a unifying concept. Brain. 2006;129(Pt 3):606–616. doi: 10.1093/brain/awl007. [DOI] [PubMed] [Google Scholar]
- 8.Musella A., Fresegna D., Rizzo F.R., Gentile A., De Vito F., Caioli S., Guadalupi L., Bruno A., Dolcetti E., Buttari F., Bullitta S., Vanni V., Centonze D., Mandolesi G. ‘Prototypical’ proinflammatory cytokine (IL-1) in multiple sclerosis: role in pathogenesis and therapeutic targeting. Expert Opin. Ther. Targets. 2020;24(1):37–46. doi: 10.1080/14728222.2020.1709823. [DOI] [PubMed] [Google Scholar]
- 9.Meira M., Sievers C., Hoffmann F., Bodmer H., Derfuss T., Kuhle J., Haghikia A., Kappos L., Lindberg R.L. PARP-1 deregulation in multiple sclerosis. Mult. Scler. J. Exp. Transl. Clin. 2019;5(4):2055217319894604. doi: 10.1177/2055217319894604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azimi G., Ranjbaran F., Arsang-Jang S., Ghafouri-Fard S., Mazdeh M., Sayad A., Taheri M. Upregulation of VEGF-A and correlation between VEGF-A and FLT-1 expressions in Iranian multiple sclerosis patients. Neurol. Sci. 2020;41(6):1459–1465. doi: 10.1007/s10072-019-04234-2. [DOI] [PubMed] [Google Scholar]
- 11.Carding S., Verbeke K., Vipond D.T., Corfe B.M., Owen L.J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 2015;26(1):26191. doi: 10.3402/mehd.v26.26191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Oliveira G.L.V., Leite A.Z., Higuchi B.S., Gonzaga M.I., Mariano V.S. Intestinal dysbiosis and probiotic applications in autoimmune diseases. Immunology. 2017;152(1):1–12. doi: 10.1111/imm.12765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghani U., Bukhari S., Ullah S., Rafeeq H., Saeed M., Amjad A., Hussain M., Akmal A., Zahra F., Qasim F. A review on nutraceuticals as a therapeutic agents. Int. J. Biosci. 2019;15(5):326–340. [Google Scholar]
- 14.Sekirov I., Russell S.L., Antunes L.C.M., Finlay B.B. Gut microbiota in health and disease. Physiol. Rev. 2010;90(3):859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 15.Lederberg J., McCray A.T. Ome Sweet Omics--A genealogical treasury of words. Scientist. 2001;15(7):8. [Google Scholar]
- 16.Lederberg, J. Infectious history. Science. 2000;288(5464):287–293. doi: 10.1126/science.288.5464.287. [DOI] [PubMed] [Google Scholar]
- 17.Eisen J. What does the term microbiome mean? 2015.
- 18.Goodsell D.S. The machinery of life. New York: Springer; 2009. [Google Scholar]
- 19.Alberts B., Johnson A., Lewis J., Raff M., Roberts K., Walter P. Molecular Biology of the Cell. 4th ed. New York: Garland Science; 2002. [Google Scholar]
- 20.Sender R., Fuchs S., Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016;14(8):e1002533. doi: 10.1371/journal.pbio.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sommer F., Bäckhed F. The gut microbiota--masters of host development and physiology. Nat. Rev. Microbiol. 2013;11(4):227–238. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- 22.Jandhyala S.M., Talukdar R., Subramanyam C., Vuyyuru H., Sasikala M., Nageshwar Reddy D. Role of the normal gut microbiota. World J. Gastroenterol. 2015;21(29):8787–8803. doi: 10.3748/wjg.v21.i29.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rinninella E., Raoul P., Cintoni M., Franceschi F., Miggiano G.A.D., Gasbarrini A., Mele M.C. What is the healthy gut microbiota composition? a changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019;7(1):14. doi: 10.3390/microorganisms7010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ho J.T., Chan G.C., Li J.C. Systemic effects of gut microbiota and its relationship with disease and modulation. BMC Immunol. 2015;16(1):21. doi: 10.1186/s12865-015-0083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cani P.D., Bibiloni R., Knauf C., Waget A., Neyrinck A.M., Delzenne N.M., Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 26.Bagarolli R.A., Tobar N., Oliveira A.G., Araújo T.G., Carvalho B.M., Rocha G.Z., Vecina J.F., Calisto K., Guadagnini D., Prada P.O., Santos A., Saad S.T.O., Saad M.J.A. Probiotics modulate gut microbiota and improve insulin sensitivity in DIO mice. J. Nutr. Biochem. 2017;50:16–25. doi: 10.1016/j.jnutbio.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 27.de Simone C. The unregulated probiotic market. Clin. Gastroenterol. Hepatol. 2019;17(5):809–817. doi: 10.1016/j.cgh.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 28.Bron P.A., Kleerebezem M., Brummer R-J., Cani P.D., Mercenier A., MacDonald T.T., Garcia-Ródenas C.L., Wells J.M. Can probiotics modulate human disease by impacting intestinal barrier function? Br. J. Nutr. 2017;117(1):93–107. doi: 10.1017/S0007114516004037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spinler J.K., Taweechotipatr M., Rognerud C.L., Ou C.N., Tumwasorn S., Versalovic J. Human-derived probiotic Lactobacillus reuteri demonstrate antimicrobial activities targeting diverse enteric bacterial pathogens. Anaerobe. 2008;14(3):166–171. doi: 10.1016/j.anaerobe.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gazerani P. Probiotics for Parkinson’s disease. Int. J. Mol. Sci. 2019;20(17):4121. doi: 10.3390/ijms20174121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Umu Ö.C.O., Rudi K., Diep D.B. Modulation of the gut microbiota by prebiotic fibres and bacteriocins. Microb. Ecol. Health Dis. 2017;28(1):1348886. doi: 10.1080/16512235.2017.1348886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng H.J., Guo J., Jia Q., Huang Y.S., Huang W.J., Zhang W., Zhang F., Liu W.J., Wang Y. The effect of probiotic and synbiotic supplementation on biomarkers of inflammation and oxidative stress in diabetic patients: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2019;142:303–313. doi: 10.1016/j.phrs.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 33.Patel S., Goyal A. 2012.
- 34.Roberfroid M., Gibson G.R., Hoyles L., McCartney A.L., Rastall R., Rowland I., Wolvers D., Watzl B., Szajewska H., Stahl B., Guarner F., Respondek F., Whelan K., Coxam V., Davicco M.J., Léotoing L., Wittrant Y., Delzenne N.M., Cani P.D., Neyrinck A.M., Meheust A. Prebiotic effects: metabolic and health benefits. Br. J. Nutr. 2010;104(S2) Suppl. 2:S1–S63. doi: 10.1017/S0007114510003363. [DOI] [PubMed] [Google Scholar]
- 35.Rovinaru C., Pasarin D. Application of microencapsulated synbiotics in fruit-based beverages. Probiotics Antimicrob. Proteins. 2020;12(2):764–773. doi: 10.1007/s12602-019-09579-w. [DOI] [PubMed] [Google Scholar]
- 36.Louis P., Flint H.J., Michel C. In: Microbiota of the human body. Schwiertz A., editor. Switzerland: Springer; 2016. How to manipulate the microbiota: prebiotics. pp. 119–142. [Google Scholar]
- 37.Scott K.P., Martin J.C., Duncan S.H., Flint H.J. Prebiotic stimulation of human colonic butyrate-producing bacteria and bifidobacteria, in vitro. FEMS Microbiol. Ecol. 2014;87(1):30–40. doi: 10.1111/1574-6941.12186. [DOI] [PubMed] [Google Scholar]
- 38.Gibson G.R., Scott K.P., Rastall R.A., Tuohy K.M., Hotchkiss A., Dubert-Ferrandon A., Gareau M., Murphy E.F., Saulnier D., Loh G. Dietary prebiotics: current status and new definition. Food Sci. Technol. Bull. Funct. Foods. 2010;7(1):1–19. doi: 10.1616/1476-2137.15880. [DOI] [Google Scholar]
- 39.Macfarlane G.T., Steed H., Macfarlane S. Bacterial metabolism and health-related effects of galacto-oligosaccharides and other prebiotics. J. Appl. Microbiol. 2008;104(2):305–344. doi: 10.1111/j.1365-2672.2007.03520.x. [DOI] [PubMed] [Google Scholar]
- 40.Davani-Davari D., Negahdaripour M., Karimzadeh I., Seifan M., Mohkam M., Masoumi S.J., Berenjian A., Ghasemi Y. Prebiotics: definition, types, sources, mechanisms, and clinical applications. Foods. 2019;8(3):92. doi: 10.3390/foods8030092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walker A.W., Ince J., Duncan S.H., Webster L.M., Holtrop G., Ze X., Brown D., Stares M.D., Scott P., Bergerat A., Louis P., McIntosh F., Johnstone A.M., Lobley G.E., Parkhill J., Flint H.J. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011;5(2):220–230. doi: 10.1038/ismej.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fuentes‐Zaragoza E., Sánchez‐Zapata E., Sendra E., Sayas E., Navarro C., Fernández‐López J., Pérez‐Alvarez J.A. Resistant starch as prebiotic: A review. Stärke. 2011;63(7):406–415. doi: 10.1002/star.201000099. [DOI] [Google Scholar]
- 43.Tzounis X., Rodriguez-Mateos A., Vulevic J., Gibson G.R., Kwik-Uribe C., Spencer J.P. Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study. Am. J. Clin. Nutr. 2011;93(1):62–72. doi: 10.3945/ajcn.110.000075. [DOI] [PubMed] [Google Scholar]
- 44.Markowiak P., Śliżewska K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients. 2017;9(9):1021. doi: 10.3390/nu9091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gibson G.R., Roberfroid M.B. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr. 1995;125(6):1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 46.Chapman C.M., Gibson G.R., Rowland I. Health benefits of probiotics: are mixtures more effective than single strains? Eur. J. Nutr. 2011;50(1):1–17. doi: 10.1007/s00394-010-0166-z. [DOI] [PubMed] [Google Scholar]
- 47.Kirchfeld F., Boyle W. Nature doctors: pioneers in naturopathic medicine. East Palestine, Ohio: Medicina Biologica; 1994. [Google Scholar]
- 48.Kho Z.Y., Lal S.K. The human gut microbiome–a potential controller of wellness and disease. Front. Microbiol. 2018;9:1835. doi: 10.3389/fmicb.2018.01835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cani P.D. Human gut microbiome: hopes, threats and promises. Gut. 2018;67(9):1716–1725. doi: 10.1136/gutjnl-2018-316723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rimoldi M., Chieppa M., Salucci V., Avogadri F., Sonzogni A., Sampietro G.M., Nespoli A., Viale G., Allavena P., Rescigno M. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat. Immunol. 2005;6(5):507–514. doi: 10.1038/ni1192. [DOI] [PubMed] [Google Scholar]
- 51.Round J.L., Mazmanian S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009;9(5):313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Foligne B., Zoumpopoulou G., Dewulf J., Ben Younes A., Chareyre F., Sirard J-C., Pot B., Grangette C. A key role of dendritic cells in probiotic functionality. PLoS One. 2007;2(3):e313. doi: 10.1371/journal.pone.0000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Owaga E., Hsieh R-H., Mugendi B., Masuku S., Shih C-K., Chang J-S. Th17 cells as potential probiotic therapeutic targets in inflammatory bowel diseases. Int. J. Mol. Sci. 2015;16(9):20841–20858. doi: 10.3390/ijms160920841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun M., Wu W., Liu Z., Cong Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J. Gastroenterol. 2017;52(1):1–8. doi: 10.1007/s00535-016-1242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Quigley E.M. 2007.
- 56.Oh B., Kim B.S., Kim J.W., Kim J.S., Koh S.J., Kim B.G., Lee K.L., Chun J. The effect of probiotics on gut microbiota during the Helicobacter pylori eradication: randomized controlled trial. Helicobacter. 2016;21(3):165–174. doi: 10.1111/hel.12270. [DOI] [PubMed] [Google Scholar]
- 57.Shen J., Zuo Z-X., Mao A-P. Effect of probiotics on inducing remission and maintaining therapy in ulcerative colitis, Crohn’s disease, and pouchitis: meta-analysis of randomized controlled trials. Inflamm. Bowel Dis. 2014;20(1):21–35. doi: 10.1097/01.MIB.0000437495.30052.be. [DOI] [PubMed] [Google Scholar]
- 58.Musso G., Gambino R., Cassader M. Obesity, diabetes, and gut microbiota: the hygiene hypothesis expanded? Diabetes Care. 2010;33(10):2277–2284. doi: 10.2337/dc10-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harsch I.A., Konturek P.C. The role of gut microbiota in obesity and type 2 and type 1 diabetes mellitus: new insights into “old” diseases. Med. Sci. (Basel) 2018;6(2):32. doi: 10.3390/medsci6020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim Y.A., Keogh J.B., Clifton P.M. Probiotics, prebiotics, synbiotics and insulin sensitivity. Nutr. Res. Rev. 2018;31(1):35–51. doi: 10.1017/S095442241700018X. [DOI] [PubMed] [Google Scholar]
- 61.Bertoni A.G., Burke G.L., Owusu J.A., Carnethon M.R., Vaidya D., Barr R.G., Jenny N.S., Ouyang P., Rotter J.I. Inflammation and the incidence of type 2 diabetes: the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care. 2010;33(4):804–810. doi: 10.2337/dc09-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goldberg R.B. Cytokine and cytokine-like inflammation markers, endothelial dysfunction, and imbalanced coagulation in development of diabetes and its complications. J. Clin. Endocrinol. Metab. 2009;94(9):3171–3182. doi: 10.1210/jc.2008-2534. [DOI] [PubMed] [Google Scholar]
- 63.Zarfeshani A., Khaza’ai H., Mohd Ali R., Hambali Z., Wahle K.W., Mutalib M.S. Effect of Lactobacillus casei on the production of pro-inflammatory markers in streptozotocin-induced diabetic rats. Probiotics Antimicrob. Proteins. 2011;3(3-4):168–174. doi: 10.1007/s12602-011-9080-9. [DOI] [PubMed] [Google Scholar]
- 64.Dinan T.G., Cryan J.F. Melancholic microbes: a link between gut microbiota and depression? Neurogastroenterol. Motil. 2013;25(9):713–719. doi: 10.1111/nmo.12198. [DOI] [PubMed] [Google Scholar]
- 65.Begum P.S., Madhavi G., Rajagopal S., Viswanath B., Razak M.A., Venkataratnamma V. Probiotics as functional foods: potential effects on human health and its impact on neurological diseases. Int. J. Nutr. Pharmacol. Neurol. Dis. 2017;7(2):23. doi: 10.4103/ijnpnd.ijnpnd_90_16. [DOI] [Google Scholar]
- 66.Westfall S., Lomis N., Kahouli I., Dia S.Y., Singh S.P., Prakash S. Microbiome, probiotics and neurodegenerative diseases: deciphering the gut brain axis. Cell. Mol. Life Sci. 2017;74(20):3769–3787. doi: 10.1007/s00018-017-2550-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matsuzaki T., Saito M., Usuku K., Nose H., Izumo S., Arimura K., Osame M. A prospective uncontrolled trial of fermented milk drink containing viable Lactobacillus casei strain Shirota in the treatment of HTLV-1 associated myelopathy/tropical spastic paraparesis. J. Neurol. Sci. 2005;237(1-2):75–81. doi: 10.1016/j.jns.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 68.Bachner H.A. A healthy gut and a healthy brain: Implications for counseling and lifestyle. USA: American Counseling Association; 2015. pp. 1–11. [Google Scholar]
- 69.Kim N., Yun M., Oh Y.J., Choi H-J. Mind-altering with the gut: Modulation of the gut-brain axis with probiotics. J. Microbiol. 2018;56(3):172–182. doi: 10.1007/s12275-018-8032-4. [DOI] [PubMed] [Google Scholar]
- 70.Grochowska M., Laskus T., Radkowski M. Gut microbiota in neurological disorders. Arch. Immunol. Ther. Exp. (Warsz.) 2019;67(6):375–383. doi: 10.1007/s00005-019-00561-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Collins S.M., Surette M., Bercik P. The interplay between the intestinal microbiota and the brain. Nat. Rev. Microbiol. 2012;10(11):735–742. doi: 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- 72.Cryan J.F., O’Riordan K.J., Cowan C.S.M., Sandhu K.V., Bastiaanssen T.F.S., Boehme M., Codagnone M.G., Cussotto S., Fulling C., Golubeva A.V., Guzzetta K.E., Jaggar M., Long-Smith C.M., Lyte J.M., Martin J.A., Molinero-Perez A., Moloney G., Morelli E., Morillas E., O’Connor R., Cruz-Pereira J.S., Peterson V.L., Rea K., Ritz N.L., Sherwin E., Spichak S., Teichman E.M., van de Wouw M., Ventura-Silva A.P., Wallace-Fitzsimons S.E., Hyland N., Clarke G., Dinan T.G. The microbiota-gut-brain axis. Physiol. Rev. 2019;99(4):1877–2013. doi: 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- 73.Chu F., Shi M., Lang Y., Shen D., Jin T., Zhu J., Cui L. Gut microbiota in multiple sclerosis and experimental autoimmune encephalomyelitis: Current applications and future perspectives. Mediators Inflamm. 2018;2018(8168717):8168717. doi: 10.1155/2018/8168717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Strandwitz P. Neurotransmitter modulation by the gut microbiota. 2018. [DOI] [PMC free article] [PubMed]
- 75.Patterson E., Ryan P.M., Wiley N., Carafa I., Sherwin E., Moloney G., Franciosi E., Mandal R., Wishart D.S., Tuohy K., Ross R.P., Cryan J.F., Dinan T.G., Stanton C. Gamma-aminobutyric acid-producing lactobacilli positively affect metabolism and depressive-like behaviour in a mouse model of metabolic syndrome. Sci. Rep. 2019;9(1):16323. doi: 10.1038/s41598-019-51781-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Riccio P., Rossano R. Undigested food and gut microbiota may cooperate in the pathogenesis of neuroinflammatory diseases: A matter of barriers and a proposal on the origin of organ specificity. Nutrients. 2019;11(11):2714. doi: 10.3390/nu11112714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hills R.D., Jr, Pontefract B.A., Mishcon H.R., Black C.A., Sutton S.C., Theberge C.R. Gut microbiome: Profound implications for diet and disease. Nutrients. 2019;11(7):1613. doi: 10.3390/nu11071613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rodriguez M., Wootla B., Anderson G. Multiple sclerosis, gut microbiota and permeability: Role of tryptophan catabolites, depression and the driving down of local melatonin. Curr. Pharm. Des. 2016;22(40):6134–6141. doi: 10.2174/1381612822666160915160520. [DOI] [PubMed] [Google Scholar]
- 79.Yacyshyn B., Meddings J., Sadowski D., Bowen-Yacyshyn M.B. Multiple sclerosis patients have peripheral blood CD45RO+ B cells and increased intestinal permeability. Dig. Dis. Sci. 1996;41(12):2493–2498. doi: 10.1007/BF02100148. [DOI] [PubMed] [Google Scholar]
- 80.Reichelt K-L., Jensen D. IgA antibodies against gliadin and gluten in multiple sclerosis. Acta Neurol. Scand. 2004;110(4):239–241. doi: 10.1111/j.1600-0404.2004.00303.x. [DOI] [PubMed] [Google Scholar]
- 81.Wekerle H. Brain autoimmunity and intestinal microbiota: 100 trillion game changers. Trends Immunol. 2017;38(7):483–497. doi: 10.1016/j.it.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 82.Huppert J., Closhen D., Croxford A., White R., Kulig P., Pietrowski E., Bechmann I., Becher B., Luhmann H.J., Waisman A., Kuhlmann C.R.W. Cellular mechanisms of IL-17-induced blood-brain barrier disruption. FASEB J. 2010;24(4):1023–1034. doi: 10.1096/fj.09-141978. [DOI] [PubMed] [Google Scholar]
- 83.Ortiz G.G., Pacheco-Moisés F.P., Macías-Islas M.Á., Flores-Alvarado L.J., Mireles-Ramírez M.A., González-Renovato E.D., Hernández-Navarro V.E., Sánchez-López A.L., Alatorre-Jiménez M.A. Role of the blood-brain barrier in multiple sclerosis. Arch. Med. Res. 2014;45(8):687–697. doi: 10.1016/j.arcmed.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 84.Ochoa-Repáraz J., Kasper L.H. The microbiome and neurologic disease: Past and future of a 2-way interaction. Neurotherapeutics. 2018;15(1):1–4. doi: 10.1007/s13311-018-0604-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wekerle H. Brain inflammatory cascade controlled by gut-derived molecules. Nature. 2018;557(7707):642–643. doi: 10.1038/d41586-018-05113-0. [DOI] [PubMed] [Google Scholar]
- 86.Rothhammer V., Borucki D.M., Tjon E.C., Takenaka M.C., Chao C-C., Ardura-Fabregat A., de Lima K.A., Gutiérrez-Vázquez C., Hewson P., Staszewski O., Blain M., Healy L., Neziraj T., Borio M., Wheeler M., Dragin L.L., Laplaud D.A., Antel J., Alvarez J.I., Prinz M., Quintana F.J. Microglial control of astrocytes in response to microbial metabolites. Nature. 2018;557(7707):724–728. doi: 10.1038/s41586-018-0119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li H., Sun J., Wang F., Ding G., Chen W., Fang R., Yao Y., Pang M., Lu Z-Q., Liu J. Sodium butyrate exerts neuroprotective effects by restoring the blood-brain barrier in traumatic brain injury mice. Brain Res. 2016;1642:70–78. doi: 10.1016/j.brainres.2016.03.031. [DOI] [PubMed] [Google Scholar]
- 88.Silva Y.P., Bernardi A., Frozza R.L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front. Endocrinol. (Lausanne) 2020;11:25. doi: 10.3389/fendo.2020.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Oldendorf W.H. Carrier-mediated blood-brain barrier transport of short-chain monocarboxylic organic acids. Am. J. Physiol. 1973;224(6):1450–1453. doi: 10.1152/ajplegacy.1973.224.6.1450. [DOI] [PubMed] [Google Scholar]
- 90.Bachmann C., Colombo J-P., Berüter J. Short chain fatty acids in plasma and brain: quantitative determination by gas chromatography. Clin. Chim. Acta. 1979;92(2):153–159. doi: 10.1016/0009-8981(79)90109-8. [DOI] [PubMed] [Google Scholar]
- 91.Mestre L., Carrillo-Salinas F.J., Mecha M., Feliú A., Guaza C. Gut microbiota, cannabinoid system and neuroimmune interactions: New perspectives in multiple sclerosis. Biochem. Pharmacol. 2018;157:51–66. doi: 10.1016/j.bcp.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 92.Sikiric P., Seiwerth S., Rucman R., Kolenc D., Vuletic L.B., Drmic D., Grgic T., Strbe S., Zukanovic G., Crvenkovic D., Madzarac G., Rukavina I., Sucic M., Baric M., Starcevic N., Krstonijevic Z., Bencic M.L., Filipcic I., Rokotov D.S., Vlainic J. Brain-gut axis and pentadecapeptide BPC 157: theoretical and practical implications. Curr. Neuropharmacol. 2016;14(8):857–865. doi: 10.2174/1570159X13666160502153022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kliček R., Kolenc D., Šuran J., Drmić D., Brčić L., Aralica G., Sever M., Holjevac J., Radić B., Turudić T., Kokot A., Patrlj L., Rucman R., Seiwerth S., Sikiric P. Stable gastric pentadecapeptide BPC 157 heals cysteamine-colitis and colon-colon-anastomosis and counteracts cuprizone brain injuries and motor disability. J. Physiol. Pharmacol. 2013;64(5):597–612. [PubMed] [Google Scholar]
- 94.Cekanaviciute E., Yoo B.B., Runia T.F., Debelius J.W., Singh S., Nelson C.A., Kanner R., Bencosme Y., Lee Y.K., Hauser S.L., Crabtree-Hartman E., Sand I.K., Gacias M., Zhu Y., Casaccia P., Cree B.A.C., Knight R., Mazmanian S.K., Baranzini S.E. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc. Natl. Acad. Sci. USA. 2017;114(40):10713–10718. doi: 10.1073/pnas.1711235114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Berer K., Gerdes L.A., Cekanaviciute E., Jia X., Xiao L., Xia Z., Liu C., Klotz L., Stauffer U., Baranzini S.E., Kümpfel T., Hohlfeld R., Krishnamoorthy G., Wekerle H. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc. Natl. Acad. Sci. USA. 2017;114(40):10719–10724. doi: 10.1073/pnas.1711233114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wing A.C., Kremenchutzky M. Multiple sclerosis and faecal microbiome transplantation: are you going to eat that? Benef. Microbes. 2019;10(1):27–32. doi: 10.3920/BM2018.0029. [DOI] [PubMed] [Google Scholar]
- 97.Evrensel A., Ceylan M.E. Fecal microbiota transplantation and its usage in neuropsychiatric disorders. Clin. Psychopharmacol. Neurosci. 2016;14(3):231–237. doi: 10.9758/cpn.2016.14.3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Adamczyk-Sowa M., Medrek A., Madej P., Michlicka W., Dobrakowski P. Does the gut microbiota influence immunity and inflammation in multiple sclerosis pathophysiology? J. Immunol. Res. 2017;2017:7904821. doi: 10.1155/2017/7904821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Riccio P., Rossano R. Nutrition facts in multiple sclerosis. ASN Neuro. 2015;7(1):175909141456818. doi: 10.1177/1759091414568185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cantarel B.L., Waubant E., Chehoud C., Kuczynski J., DeSantis T.Z., Warrington J., Venkatesan A., Fraser C.M., Mowry E.M. Gut microbiota in multiple sclerosis: possible influence of immunomodulators. J. Investig. Med. 2015;63(5):729–734. doi: 10.1097/JIM.0000000000000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Miyake S., Kim S., Suda W., Oshima K., Nakamura M., Matsuoka T., Chihara N., Tomita A., Sato W., Kim S-W., Morita H., Hattori M., Yamamura T. Dysbiosis in the gut microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to clostridia XIVa and IV Clusters. PLoS One. 2015;10(9):e0137429. doi: 10.1371/journal.pone.0137429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Barnett M.H., Parratt J.D.E., Cho E-S., Prineas J.W. Immunoglobulins and complement in postmortem multiple sclerosis tissue. Ann. Neurol. 2009;65(1):32–46. doi: 10.1002/ana.21524. [DOI] [PubMed] [Google Scholar]
- 103.Farrokhi V., Nemati R., Nichols F.C., Yao X., Anstadt E., Fujiwara M., Grady J., Wakefield D., Castro W., Donaldson J., Clark R.B. Bacterial lipodipeptide, Lipid 654, is a microbiome-associated biomarker for multiple sclerosis. Clin. Transl. Immunology. 2013;2(11):e8. doi: 10.1038/cti.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Freedman S.N., Shahi S.K., Mangalam A.K. The “gut feeling”: Breaking down the role of gut microbiome in multiple sclerosis. Neurotherapeutics. 2018;15(1):109–125. doi: 10.1007/s13311-017-0588-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ventura R.E., Iizumi T., Battaglia T., Liu M., Perez-Perez G.I., Herbert J., Blaser M.J. Gut microbiome of treatment-naïve MS patients of different ethnicities early in disease course. Sci. Rep. 2019;9(1):16396. doi: 10.1038/s41598-019-52894-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tremlett H., Waubant E. The gut microbiota and pediatric multiple sclerosis: Recent findings. Neurotherapeutics. 2018;15(1):102–108. doi: 10.1007/s13311-017-0574-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen J., Chia N., Kalari K.R., Yao J.Z., Novotna M., Paz Soldan M.M., Luckey D.H., Marietta E.V., Jeraldo P.R., Chen X., Weinshenker B.G., Rodriguez M., Kantarci O.H., Nelson H., Murray J.A., Mangalam A.K. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci. Rep. 2016;6(1):28484. doi: 10.1038/srep28484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jangi S., Gandhi R., Cox L.M., Li N., von Glehn F., Yan R., Patel B., Mazzola M.A., Liu S., Glanz B.L., Cook S., Tankou S., Stuart F., Melo K., Nejad P., Smith K., Topçuolu B.D., Holden J., Kivisäkk P., Chitnis T., De Jager P.L., Quintana F.J., Gerber G.K., Bry L., Weiner H.L. Alterations of the human gut microbiome in multiple sclerosis. Nat. Commun. 2016;7(1):12015. doi: 10.1038/ncomms12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kipp M., van der Star B., Vogel D.Y.S., Puentes F., van der Valk P., Baker D., Amor S. Experimental in vivo and in vitro models of multiple sclerosis: EAE and beyond. Mult. Scler. Relat. Disord. 2012;1(1):15–28. doi: 10.1016/j.msard.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 110.Dargahi N., Matsoukas J., Apostolopoulos V. Streptococcus thermophilus ST285 alters pro-inflammatory to anti-inflammatory cytokine secretion against multiple sclerosis peptide in mice. Brain Sci. 2020;10(2):126. doi: 10.3390/brainsci10020126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bjelobaba I., Begovic-Kupresanin V., Pekovic S., Lavrnja I. Animal models of multiple sclerosis: Focus on experimental autoimmune encephalomyelitis. J. Neurosci. Res. 2018;96(6):1021–1042. doi: 10.1002/jnr.24224. [DOI] [PubMed] [Google Scholar]
- 112.McMurran C.E., Guzman de la Fuente A., Penalva R., Ben Menachem-Zidon O., Dombrowski Y., Falconer J., Gonzalez G.A., Zhao C., Krause F.N., Young A.M.H., Griffin J.L., Jones C.A., Hollins C., Heimesaat M.M., Fitzgerald D.C., Franklin R.J.M. The microbiota regulates murine inflammatory responses to toxin-induced CNS demyelination but has minimal impact on remyelination. Proc. Natl. Acad. Sci. USA. 2019;116(50):25311–25321. doi: 10.1073/pnas.1905787116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ezendam J., de Klerk A., Gremmer E.R., van Loveren H. Effects of Bifidobacterium animalis administered during lactation on allergic and autoimmune responses in rodents. Clin. Exp. Immunol. 2008;154(3):424–431. doi: 10.1111/j.1365-2249.2008.03788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kobayashi T., Kato I., Nanno M., Shida K., Shibuya K., Matsuoka Y., Onoue M. Oral administration of probiotic bacteria, Lactobacillus casei and Bifidobacterium breve, does not exacerbate neurological symptoms in experimental autoimmune encephalomyelitis. Immunopharmacol. Immunotoxicol. 2010;32(1):116–124. doi: 10.3109/08923970903200716. [DOI] [PubMed] [Google Scholar]
- 115.Forbes J.D., Van Domselaar G., Bernstein C.N. The gut microbiota in immune-mediated inflammatory diseases. Front. Microbiol. 2016;7:1081. doi: 10.3389/fmicb.2016.01081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lavasani S., Dzhambazov B., Nouri M., Fåk F., Buske S., Molin G., Thorlacius H., Alenfall J., Jeppsson B., Weström B. A novel probiotic mixture exerts a therapeutic effect on experimental autoimmune encephalomyelitis mediated by IL-10 producing regulatory T cells. PLoS One. 2010;5(2):e9009. doi: 10.1371/journal.pone.0009009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yamashita M., Ukibe K., Matsubara Y., Hosoya T., Sakai F., Kon S., Arima Y., Murakami M., Nakagawa H., Miyazaki T. Lactobacillus helveticus SBT2171 attenuates experimental autoimmune encephalomyelitis in mice. Front. Microbiol. 2018;8:2596. doi: 10.3389/fmicb.2017.02596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Borchers A.T., Selmi C., Meyers F.J., Keen C.L., Gershwin M.E. Probiotics and immunity. J. Gastroenterol. 2009;44(1):26–46. doi: 10.1007/s00535-008-2296-0. [DOI] [PubMed] [Google Scholar]
- 119.He B., Hoang T.K., Tian X., Taylor C.M., Blanchard E., Luo M., Bhattacharjee M.B., Freeborn J., Park S., Couturier J., Lindsey J.W., Tran D.Q., Rhoads J.M., Liu Y. Lactobacillus reuteri reduces the severity of experimental autoimmune encephalomyelitis in mice by modulating gut microbiota. Front. Immunol. 2019;10:385. doi: 10.3389/fimmu.2019.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Digehsara S.G., Esfandiari B., Karim E., Taheri S., Tajabadi-Ebrahimi M., Arasteh J. Effects of Lactobacillus casei Strain T2 (IBRC-M10783) on the modulation of Th17/Treg and evaluation of miR-155, miR-25, and IDO-1 expression in a cuprizone-induced C57BL/6 mouse model of demyelination. Inflammation. 2020;44(1):334–343. doi: 10.1007/s10753-020-01339-1. [DOI] [PubMed] [Google Scholar]
- 121.Ezendam J., van Loveren H. Lactobacillus casei Shirota administered during lactation increases the duration of autoimmunity in rats and enhances lung inflammation in mice. Br. J. Nutr. 2008;99(1):83–90. doi: 10.1017/S0007114507803412. [DOI] [PubMed] [Google Scholar]
- 122.Rezende R.M., Oliveira R.P., Medeiros S.R., Gomes-Santos A.C., Alves A.C., Loli F.G., Guimarães M.A.F., Amaral S.S., da Cunha A.P., Weiner H.L., Azevedo V., Miyoshi A., Faria A.M.C. Hsp65-producing Lactococcus lactis prevents experimental autoimmune encephalomyelitis in mice by inducing CD4+LAP+ regulatory T cells. J. Autoimmun. 2013;40:45–57. doi: 10.1016/j.jaut.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ochoa-Repáraz J., Kasper L.H. The influence of gut-derived CD39 regulatory T cells in CNS demyelinating disease. Transl. Res. 2017;179:126–138. doi: 10.1016/j.trsl.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang Y., Telesford K.M., Ochoa-Repáraz J., Haque-Begum S., Christy M., Kasper E.J., Wang L., Wu Y., Robson S.C., Kasper D.L., Kasper L.H. An intestinal commensal symbiosis factor controls neuroinflammation via TLR2-mediated CD39 signalling. Nat. Commun. 2014;5(1):4432. doi: 10.1038/ncomms5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang Y., Begum-Haque S., Telesford K.M., Ochoa-Repáraz J., Christy M., Kasper E.J., Kasper D.L., Robson S.C., Kasper L.H. A commensal bacterial product elicits and modulates migratory capacity of CD39(+) CD4 T regulatory subsets in the suppression of neuroinflammation. Gut Microbes. 2014;5(4):552–561. doi: 10.4161/gmic.29797. [DOI] [PubMed] [Google Scholar]
- 126.Takata K., Kinoshita M., Okuno T., Moriya M., Kohda T., Honorat J.A., Sugimoto T., Kumanogoh A., Kayama H., Takeda K., Sakoda S., Nakatsuji Y. The lactic acid bacterium Pediococcus acidilactici suppresses autoimmune encephalomyelitis by inducing IL-10-producing regulatory T cells. PLoS One. 2011;6(11):e27644. doi: 10.1371/journal.pone.0027644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Secher T., Kassem S., Benamar M., Bernard I., Boury M., Barreau F., Oswald E., Saoudi A. Oral administration of the probiotic strain Escherichia coli Nissle 1917 reduces susceptibility to neuroinflammation and repairs experimental autoimmune encephalomyelitis-induced intestinal barrier dysfunction. Front. Immunol. 2017;8:1096. doi: 10.3389/fimmu.2017.01096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ochoa-Repáraz J., Mielcarz D.W., Wang Y., Begum-Haque S., Dasgupta S., Kasper D.L., Kasper L.H. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. 2010;3(5):487–495. doi: 10.1038/mi.2010.29. [DOI] [PubMed] [Google Scholar]
- 129.Mangalam A., Shahi S.K., Luckey D., Karau M., Marietta E., Luo N., Choung R.S., Ju J., Sompallae R., Gibson-Corley K., Patel R., Rodriguez M., David C., Taneja V., Murray J. Human gut-derived commensal bacteria suppress CNS inflammatory and demyelinating disease. Cell Rep. 2017;20(6):1269–1277. doi: 10.1016/j.celrep.2017.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kwon H-K., Kim G-C., Kim Y., Hwang W., Jash A., Sahoo A., Kim J-E., Nam J.H. Im, S.H. Amelioration of experimental autoimmune encephalomyelitis by probiotic mixture is mediated by a shift in T helper cell immune response. Clin. Immunol. 2013;146(3):217–227. doi: 10.1016/j.clim.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 131.Salehipour Z., Haghmorad D., Sankian M., Rastin M., Nosratabadi R., Soltan Dallal M.M., Tabasi N., Khazaee M., Nasiraii L.R., Mahmoudi M. Bifidobacterium animalis in combination with human origin of Lactobacillus plantarum ameliorate neuroinflammation in experimental model of multiple sclerosis by altering CD4+ T cell subset balance. Biomed. Pharmacother. 2017;95:1535–1548. doi: 10.1016/j.biopha.2017.08.117. [DOI] [PubMed] [Google Scholar]
- 132.Jun S., Ochoa-Repáraz J., Zlotkowska D., Hoyt T., Pascual D.W. Bystander-mediated stimulation of proteolipid protein-specific regulatory T (Treg) cells confers protection against experimental autoimmune encephalomyelitis (EAE) via TGF-β. J. Neuroimmunol. 2012;245(1-2):39–47. doi: 10.1016/j.jneuroim.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jun S., Gilmore W., Callis G., Rynda A., Haddad A., Pascual D.W. A live diarrheal vaccine imprints a Th2 cell bias and acts as an anti-inflammatory vaccine. J. Immunol. 2005;175(10):6733–6740. doi: 10.4049/jimmunol.175.10.6733. [DOI] [PubMed] [Google Scholar]
- 134.Ochoa-Repáraz J., Riccardi C., Rynda A., Jun S., Callis G., Pascual D.W. Regulatory T cell vaccination without autoantigen protects against experimental autoimmune encephalomyelitis. J. Immunol. 2007;178(3):1791–1799. doi: 10.4049/jimmunol.178.3.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tankou S.K., Regev K., Healy B.C., Cox L.M., Tjon E., Kivisakk P., Vanande I.P., Cook S., Gandhi R., Glanz B., Stankiewicz J., Weiner H.L. Investigation of probiotics in multiple sclerosis. Mult. Scler. 2018;24(1):58–63. doi: 10.1177/1352458517737390. [DOI] [PubMed] [Google Scholar]
- 136.Kouchaki E., Tamtaji O.R., Salami M., Bahmani F., Daneshvar Kakhaki R., Akbari E., Tajabadi-Ebrahimi M., Jafari P., Asemi Z. Clinical and metabolic response to probiotic supplementation in patients with multiple sclerosis: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2017;36(5):1245–1249. doi: 10.1016/j.clnu.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 137.Salami M., Kouchaki E., Asemi Z., Tamtaji O.R. How probiotic bacteria influence the motor and mental behaviors as well as immunological and oxidative biomarkers in multiple sclerosis? A double blind clinical trial. J. Funct. Foods. 2019;52:8–13. doi: 10.1016/j.jff.2018.10.023. [DOI] [Google Scholar]
- 138.Tamtaji O.R., Kouchaki E., Salami M., Aghadavod E., Akbari E., Tajabadi-Ebrahimi M., Asemi Z. The effects of probiotic supplementation on gene expression related to inflammation, insulin, and lipids in patients with multiple sclerosis: A randomized, double-blind, placebo-controlled trial. J. Am. Coll. Nutr. 2017;36(8):660–665. doi: 10.1080/07315724.2017.1347074. [DOI] [PubMed] [Google Scholar]