Here, Eisemann and Wechsler-Reya review recent preclinical and clinical studies that have identified mechanisms of immune evasion in medulloblastoma, and highlight possible therapeutic interventions that may give new hope to medulloblastoma patients and their families.
Keywords: CAR T cell, antigen presentation, immune checkpoint, immunotherapy, medulloblastoma, myeloid cell, oncolytic virus, pediatric brain tumor, vaccination
Abstract
Medulloblastoma is an aggressive brain tumor that occurs predominantly in children. Despite intensive therapy, many patients die of the disease, and novel therapies are desperately needed. Although immunotherapy has shown promise in many cancers, the low mutational burden, limited infiltration of immune effector cells, and immune-suppressive microenvironment of medulloblastoma have led to the assumption that it is unlikely to respond to immunotherapy. However, emerging evidence is challenging this view. Here we review recent preclinical and clinical studies that have identified mechanisms of immune evasion in medulloblastoma, and highlight possible therapeutic interventions that may give new hope to medulloblastoma patients and their families.
Medulloblastoma is among the most common malignant brain tumors in children, with an estimated 450 new cases per year in the United States (Ostrom et al. 2018). Progress in diagnosis and treatment has led to an increased 10-yr survival rate of 65% in 2015 (Ostrom et al. 2018) compared with 44% in 1984 (Hughes et al. 1988). Molecular profiling of medulloblastoma has identified four major subgroups of the disease: the Wingless (WNT) and Sonic hedgehog (SHH) subgroups, which are driven by deregulation of the eponymous signaling pathways; group 3, characterized by overexpression of MYC; and group 4, whose genetic signature is more heterogeneous, without a common genetic driver (Taylor et al. 2012; Juraschka and Taylor 2019). The stratification of medulloblastoma into subclasses is of great clinical relevance, as the identification of oncogenic drivers may enable the use of subgroup-specific targeted therapies (Robinson et al. 2012) and de-escalation strategies for treatment of tumors with a better prognosis (NCT01878617, NCT02724579, and EORTC-1634-BTG). Despite this progress, up to 40% of patients remain affected with an incurable recurrent and metastatic form of this disease, which is the primary cause of death. Moreover, patients who do survive suffer from severe long-term side effects, including cerebrovascular disease; visual, auditory, and other neurosensory impairments; neurocognitive deficits; and increased incidence of secondary malignancies (for review, see Chevignard et al. 2017). Thus, the development of more effective therapies with less toxicity is essential. Here we review what is known about interactions between medulloblastoma and the immune system, as well as efforts to develop immunotherapies against this disease.
The immune system provides a potent defense against pathogens and can also identify and eradicate malignant cells. This concept was first postulated by the Nobel prize-winning scientist Paul Ehrlich in the early 20th century (Ehrlich 1909; for review, see Galon and Bruni 2020), 50 yr after Rudolf Virchow had discovered the first immune infiltrates in cancer specimens (Virchow 1863; Galon and Bruni 2020). At about the same time, without understanding the underlying mechanisms, the surgeon William Coley successfully treated cancer patients with injections of heat-inactivated bacteria, laying the foundation for modern immunotherapy (Coley 1893; Galon and Bruni 2020). However, it was several decades before experimental evidence supported the hypothesis of immune surveillance (Burnet 1957). One key observation was that inbred mice could be immunized against syngeneic transplants of tumors induced by chemical carcinogens or viruses, establishing the existence of tumor antigens that mark tumor cells for immune-mediated elimination (Old and Boyse 1966). This was further corroborated by observations of immune cell infiltrates correlating with improved prognosis of cancer patients (Dunn et al. 2004). Since then, numerous studies have identified immune cells that are involved in antitumor immunity. Accumulating evidence suggests that the adaptive and innate immune systems cooperate to recognize and kill tumor cells. However, in medulloblastoma and other brain tumors, a variety of factors impede the antitumor activity of these cells and thwart efforts to use the immune system as a mode of therapy. These factors are discussed below.
Barriers to effective immunotherapy of brain tumors
Currently, the FDA has approved at least 55 viral and adoptive cell therapies, immune modulators, vaccines, and targeted antibody-based immunotherapies for >20 cancer types (The Cancer Research Institute, https://www.cancerresearch.org/en-us/immunotherapy/treatment-types). Unfortunately, the development of immunotherapies for brain tumor patients has remained challenging, and their clinical implementation is clearly lagging behind other cancer types. For pediatric patients suffering from brain tumors, there is no FDA-approved immunotherapeutic option yet. Tumor immunology research has identified various barriers to an effective immune response that remain challenges for the development of immunotherapies for medulloblastoma.
Lack of infiltrating immune cells
Slight variations in the composition of immune infiltrates between the different subgroups of medulloblastoma have been reported (Bockmayr et al. 2018; Grabovska et al. 2020; Riemondy et al. 2022). However, all medulloblastoma tumors are characterized by low numbers of infiltrating immune cells. Some studies have indicated that medulloblastomas harbor even fewer immune cells than glioblastoma (Griesinger et al. 2013; Bockmayr et al. 2018), which has been described as a hostile “immunologic desert with rare to nonexistent infiltrating immune effector cells” (Reardon et al. 2017). The number of T cells present in medulloblastoma tumors was found to be not statistically higher than in pediatric control tissue (Griesinger et al. 2013). In particular, the low percentage of effector cells such as granzyme B-expressing CD8+ T cells and natural killer (NK) cells suggests a very low level of active antitumor immune responses in medulloblastoma (Bockmayr et al. 2018; Vermeulen et al. 2018).
The blood–brain barrier tightly controls immune cell infiltration
The limited immune infiltration in medulloblastoma might have several causes. The blood–brain barrier (BBB), which is made up of endothelial cells, pericytes, and astrocytic endfeet, serves to protect the brain from pathogens, toxins, and other harmful factors in the blood. It also represents a physical barrier for immune cells. The classical concept of the brain as an immune-privileged organ that is protected from immune surveillance has been called into question over the past decade (Carson et al. 2006). Increasing evidence has supported the existence of CNS immune surveillance; however, it remains a highly controlled process granting only limited access to immune cells (for review, see Carson et al. 2006; Engelhardt et al. 2017).
Despite restricted immune cell trafficking across the BBB under physiological conditions, there may be an increase in trafficking under pathological conditions due to induced leakiness or disruption of the barrier (Weiss et al. 2009). However, with the exception of WNT tumors, most medulloblastomas show large regions of intact BBB (Phoenix et al. 2016), suggesting sustained limited immune cell infiltration. Moreover, reactive astrocytes have been reported to form perivascular scar-like barriers that serve to restrict the infiltration of leukocytes in an experimental autoimmune encephalomyelitis model (Voskuhl et al. 2009). It remains to be determined whether reactive astrocytes can also function as a physical barrier to immune cell infiltration in medulloblastoma.
Therapy-induced immunosuppression
Chemotherapy and radiotherapy are part of the standard of care for medulloblastoma and are intended to target rapidly dividing cancer cells. However, this form of treatment does not exclusively kill cancer cells, but can also cause collateral damage to immune cells. A recent study investigated how radiotherapy and chemotherapy affect circulating T cells in medulloblastoma patients and found that treated patients exhibited CD4+ T-cell lymphopenia (Gururangan et al. 2017). Moreover, medulloblastoma treatment commonly includes application of corticosteroids to relieve symptoms such as headache and nausea caused by edema, hydrocephalus, increased intracranial pressure, and compression of the fourth ventricle (for review, see De Braganca and Packer 2013). However, a common side effect of steroids is immunosuppression. As a result, steroid-treated patients with melanoma or nonsmall cell lung cancer brain metastasis have been shown to respond less efficiently to immunotherapy (Margolin 2012; Kotecha et al. 2019). Thus, it can be presumed that in addition to radiotherapy and chemotherapy, corticosteroid treatment induces systemic immunosuppression and reduced antitumor immunity in medulloblastoma patients.
Low mutational load
A high mutational load correlates with higher numbers of neoantigens that can elicit tumor-directed T-cell responses (Rizvi et al. 2015) and, accordingly, tumors with a high mutation burden have been found to respond more efficiently to immune checkpoint blockade compared with tumors with a low or intermediate number of mutations (Rizvi et al. 2015; Goodman et al. 2017). Pediatric tumors, and in particular brain tumors like medulloblastoma, have a relatively low mutation rate (Parsons et al. 2011; Chalmers et al. 2017). Thus, it has been concluded that medulloblastoma is minimally immunogenic and unlikely to respond to therapeutic approaches that promote the endogenous T-cell immune response, such as immune checkpoint inhibition (Landi et al. 2018).
Dysfunctional antigen presentation
Beyond the expression of immunogenic antigens, a key determinant of tumor immunogenicity is presentation of these antigens by professional antigen-presenting cells (APCs) and tumor cells.
APCs, such as dendritic cells, macrophages, microglia, and B cells, have been reported to infiltrate medulloblastoma tumors. However, medulloblastoma and atypical teratoid/rhabdoid tumors (ATRT) showed the lowest infiltration of APCs among all brain tumors tested (Bockmayr et al. 2018). It has not been explored whether medulloblastoma-associated APCs are functional and which APC type is the predominant one in inducing antimedulloblastoma T-cell responses. Microglia, as brain-resident macrophages, may represent the most abundant APCs in brain tumors. There is little evidence that microglia migrate out of the CNS to present antigens to naïve T cells in lymph nodes, suggesting that T cells are initially primed by a different type of APC. Once activated T cells enter the brain, microglia can act as local APCs to modulate and enhance T-cell activity. There are no data available on whether microglia can promote antimedulloblastoma immune responses; however, studies on other neuropathologies, such as experimental rodent autoimmune encephalomyelitis, have reported inefficient antigen presentation (Ford et al. 1996; Mundt et al. 2019) and showed that microglia are dispensable for T-cell entry into the brain and for local reactivation of T cells (Mundt et al. 2019). In mouse models of glioma, microglia exhibit a reduced capacity to process or present antigens, up-regulate MHC class II, and stimulate T cells (Badie et al. 2002; Schartner et al. 2005; Qian et al. 2018). Interestingly, single-cell sequencing of myeloid cells isolated from a glioblastoma model revealed that the extent of MHC class II up-regulation in microglia is sex-dependent (Ochocka et al. 2021).
In summary, little is known about the function of microglia as APCs in medulloblastoma, but it appears that efficient antigen presentation and T-cell activation in the brain tumor setting may rely on other APCs. Of note, these APCs can be suppressed in their maturation and antigen presentation capacity as well (Herber et al. 2010; Cubillos-Ruiz et al. 2015).
In addition to the presentation of tumor antigens by APCs to activate T cells, antigens must be presented by MHC class I on tumor cells for these cells to be recognized and eliminated by CD8+ T cells. In a process called immunoediting (Dunn et al. 2004), CD8+ T cells may progressively clear immunogenic MHC class I-presenting tumor cells, resulting in the selection of tumor cells that have a deficiency in the antigen presentation machinery. The loss of MHC class I presentation on the tumor cell surface is a common mechanism of immune escape and has been reported for medulloblastoma (Raffaghello et al. 2007; Vermeulen et al. 2018).
Tumor-induced immunosuppression
Medulloblastoma cells can escape NK cell attack
Although MHC class I is necessary to activate CD8+ T cells, it acts as a negative regulator of natural killer cells. Thus, tumors lacking MHC class I could show increased susceptibility to NK cell-mediated elimination. However, recent studies of medulloblastoma have demonstrated minimal infiltration of NK cells (Haberthur et al. 2016; Vermeulen et al. 2018). Moreover, NK cell activation depends on positive signals from target cells, and tumor cells often down-regulate these signals to evade NK cell attack. For example, low expression of NKG2D ligands on tumor cells, as well as low expression of the cognate receptor NKG2D on NK cells, hinders NK cell activation in medulloblastoma (Haberthur et al. 2016). Mechanistically, medulloblastomas may inhibit NKG2D expression and NK cell cytotoxicity by release of the immunosuppressive cytokine transforming growth factor β (TGFβ) (Gate et al. 2014; Bockmayr et al. 2018; Powell et al. 2019), as shown for other cancer entities including glioma (Friese et al. 2004; Crane et al. 2010). In a PDX model of glioblastoma, TGFβ release by glioblastoma stem cells was triggered by cell–cell interactions with adoptively transferred human NK cells, resulting in NK cell dysfunction and enhanced tumor growth (Shaim et al. 2021). TGFβ also suppresses T-cell proliferation, T-cell cytotoxicity, and antigen presentation and recruits regulatory T cells (Tregs) (for review, see Flavell et al. 2010), creating a hostile, immunosuppressive microenvironment.
Regulatory T cells suppress antitumor immune responses
Tregs control the activity of effector immune cells by secreting anti-inflammatory cytokines such as TGFβ and interleukin-10 (IL-10), removing costimulatory signals from APCs by CTLA4-mediated trogocytosis (Gu et al. 2012) or trans-endocytosis (processes in which plasma membrane fragments are transferred between cells) (Qureshi et al. 2011), or consumption of interleukin-2, limiting the availability of this stimulatory cytokine for effector T cells (Pandiyan et al. 2007). Tumors may benefit from Treg-mediated immunosuppression, as in some cancer entities Tregs have been correlated with tumor grade (El Andaloussi and Lesniak 2007), decreased survival (Curiel et al. 2004), and resistance to immune checkpoint inhibition (Amoozgar et al. 2021). Treg infiltration has been described for medulloblastoma (Gate et al. 2014; Bockmayr et al. 2018; Vermeulen et al. 2018; Grabovska et al. 2020) and can be facilitated by tumor-derived TGFβ (Gate et al. 2014). TGFβ drives the conversion of CD4+ T cells to Tregs, which in turn secrete high levels of TGFβ, generating a feed-forward loop that may ensure the continued presence of immunosuppressive Tregs in the tumor. Elevated Treg infiltration in medulloblastoma tumors may also be therapy-induced, as Treg expansion has been observed in the blood of treated medulloblastoma patients (Gururangan et al. 2017) as well as in an experimental in vitro setting using mTOR inhibitor-treated medulloblastoma cell lines (Folgiero et al. 2016).
Reactive astrocytes and their tumor-promoting potential
A recent study of melanoma brain metastasis found that STAT3-positive reactive astrocytes can act as a physical barrier, shielding the lesion from lymphocytes and repressing the activity of CD8+ T cells, presumably by expression of PD-L1 and VEGF-A, which drive T-cell exhaustion (Priego et al. 2018). In medulloblastoma, there is substantial cross-talk between astrocytes and microglia and other myeloid cells. A recent study has elegantly shown that SHH medulloblastoma cells can transdifferentiate into interleukin-4-secreting astrocytes, which stimulate microglia to release insulin-like growth factor 1 (IGF1) and IL-10 (Yao et al. 2020). The study focused on IGF1, demonstrating an essential role for this growth factor in tumor development and progression. The effect of IL-10 in medulloblastoma is yet to be determined, but it is commonly reported to function as an immunosuppressive cytokine that substantially represses antitumor immunity by inhibiting APCs (for review, see Widodo et al. 2021). Furthermore, medulloblastoma-associated astrocytes, especially in group 3 medulloblastoma, have recently been shown to produce high levels of the tumor-promoting chemokine CCL2 (Liu et al. 2020). CCL2 signaling in medulloblastoma cells has been shown to promote leptomeningeal metastasis (Garzia et al. 2018) and to maintain stem-like properties and proliferation of disseminated tumor cells (Liu et al. 2020). CCL2 is also a very potent chemoattractant for numerous immune cells, including myeloid cells (for review, see Gschwandtner et al. 2019), and has been shown to facilitate infiltration of bone marrow-derived macrophages into SHH medulloblastoma lesions (Maximov et al. 2019).
Medulloblastoma-associated myeloid cells can adopt an immunosuppressive phenotype
The role of myeloid cells in medulloblastoma is controversial, with some studies identifying an antitumoral function and others reporting a tumor-promoting function for these cells. The tumoricidal function of medulloblastoma-associated macrophages was postulated based on the observation that their depletion in a model of SHH medulloblastoma (the NeuroD2-SmoA1 mouse) resulted in enhanced tumor growth (Maximov et al. 2019). Moreover, blockade of the surface molecule CD47 on tumor cells led to macrophage-mediated phagocytosis in a patient-derived xenograft (PDX) model (Gholamin et al. 2017). These studies highlight the capacity of tumor-associated myeloid cells to impair the growth and survival of medulloblastoma cells.
However, there is also strong evidence for a tumor-supportive function of medulloblastoma-associated myeloid cells. Gene expression profiling of human medulloblastoma samples showed that SHH tumors, which are characterized by high myeloid infiltration, are enriched for an M2-like gene expression profile, which is associated with immunosuppressive functions of myeloid cells (Margol et al. 2015). Consistently, Margol et al. (2015) suggested an inverse correlation of CD163 expression (an M2-like marker) and survival of SHH medulloblastoma patients, but highlighted the need for validation with a larger sample size. In group 4 medulloblastoma patients, a high infiltration of monocytes has also been associated with poor prognosis (Grabovska et al. 2020). This notion of a tumor-promoting macrophage function was further affirmed by two recent studies. In a mouse model of SHH medulloblastoma (Atoh1-SmoM2), Tan et al. (2021) showed delayed tumor growth and prolonged mouse survival in response to pharmacological inhibition of colony-stimulating factor 1 receptor (CSF1R or CD115), which depletes microglia and macrophages. Likewise, Dang et al. (2021) reported increased infiltration of immunosuppressive monocyte-derived macrophage cells in response to radiotherapy in another mouse model of SHH medulloblastoma (Ptch1+/−; Tp53−/−). Coculture of these tumor-infiltrating monocyte-derived Ly6Chi/Ccr2hi myeloid cells with ex vivo stimulated T cells resulted in significantly decreased T-cell proliferation. In vivo, knockout of Ccr2 (which encodes the receptor for CCL2) decreased monocyte-derived macrophage infiltration in irradiated tumors and resulted in increased CD8+ T-cell levels. Despite increased intratumoral CD8+ T-cell numbers, no survival benefit was reported, most likely due to drastically increased numbers of tumor-associated neutrophils. These neutrophils are characterized by elevated IL-10 signaling and resemble granulocytic myeloid-derived suppressor cells (MDSCs), indicating an immunoregulating phenotype that could compensate for the loss of immunosuppressive monocyte-derived macrophages in this model (Dang et al. 2021). The presence of MDSCs has also been reported for another SHH-associated medulloblastoma model, where the investigators show that MDSCs increase Treg infiltration and reduce numbers of effector T cells in the tumor (Abad et al. 2014).
In line with the putative immunosuppressive phenotype of medulloblastoma-associated macrophages, infiltrating myeloid cells have been identified as the predominant source of PD-L1 expression in mouse models of SHH and group 3 medulloblastoma (Pham et al. 2016). Binding of PD-L1 to PD-1 on effector T cells promotes T-cell exhaustion and leads to immune escape of tumor cells (Freeman et al. 2000; Dong et al. 2002).
In summary, medulloblastoma tumors are sparsely infiltrated by immune cells and seem to have a broad repertoire of mechanisms for evading the few immune cells that do find their way into the tumor. However, the field of medulloblastoma immunology is relatively unexplored, and emerging evidence indicates that medulloblastoma cells can be susceptible to immune-mediated attack. Below, we discuss the approaches that have been used to enhance the antimedulloblastoma immune response and present findings from other cancer types that might be relevant for the future development of immunotherapies for medulloblastoma.
Approaches to overcoming immune response barriers
Corticosteroid substitution and metronomic chemotherapy can minimize therapy-induced immunosuppression
In medulloblastoma patients, corticosteroids are administered in order to relieve edema-related symptoms. Steroid treatment is often accompanied by immunosuppression, which could contribute to the lack of therapy response and relapse. However, multiple studies have demonstrated that corticosteroids can be replaced with bevacizumab, an anti-VEGF monoclonal antibody that seems highly effective in the control of peritumoral edema in brain tumor patients (Liu et al. 2009; Nagpal et al. 2011; Banks et al. 2019). Combination therapy using bevacizumab and anti-CTLA-4 in metastatic melanoma was associated with enhanced effector immune cell infiltration, increased numbers of circulating memory T cells, and a more favorable outcome compared with anti-CTLA-4 treatment alone (Hodi et al. 2014). The high cost of anti-VEGF therapy represents a financial burden for the health care system, limiting its clinical use. However, combining costly immunotherapies with corticosteroids that likely reduce the efficiency of these very same therapies may be equally challenging from an ethical and financial standpoint. At least in clinical studies where the efficacy of immunotherapies is evaluated, the use of immunosuppressive steroids should be avoided.
Conventional chemotherapy and radiotherapy have been associated with systemic immunosuppression as well. Metronomic treatment, which involves the application of chemotherapeutic drugs in lower but more frequent doses, has been proposed to have milder side effects and, moreover, to enhance antitumor immune responses (Wu and Waxman 2018). On the one hand, this might be due to the lower overall toxicity that also spares effector immune cells; on the other hand, metronomic therapy has also been shown to reduce the numbers of immunosuppressive immune cells such as Tregs and MDSCs in glioma (Banissi et al. 2009; Peereboom et al. 2019). Moreover, the metronomic administration of certain chemotherapeutic drugs induces an immunogenic tumor cell death, which is characterized by the release of inflammatory factors such as HMGB1 and ATP and exposure of phagocytosis-stimulating calreticulin, greatly increasing the efficacy of antitumor responses (Wang et al. 2018). A recent report describing a metronomic antiangiogenic combination treatment of patients with recurrent medulloblastoma has shown promising preliminary results (Slavc et al. 2018). The combination of metronomic therapy with immune checkpoint blockade has not been tested yet in medulloblastoma, but it is possible that these approaches may have synergistic effects.
Enhancing trafficking of immune cells into tumors
Although the BBB represents a physical barrier to immune cells, the CNS is constantly surveilled by immune cells. T cells can patrol perivascular and leptomeningeal compartments until local recognition of their specific antigen initiates their infiltration into the brain parenchyma, which is normally sealed off by the glia limitans (for review, see Engelhardt and Ransohoff 2012). In addition, peripherally activated T cells have been shown to traverse the BBB. Peripheral presentation of brain tumor antigens and T-cell activation has been reported for brain-draining lymph nodes (Calzascia et al. 2005). Despite the lack of conventional lymphatic vessels in the brain parenchyma, evidence has emerged that the glymphatic system, a draining system that clears metabolic by-products from the brain, as well as recently discovered meningeal lymphatic vessels, allows drainage of soluble factors and immune cells from the brain to peripheral lymph nodes (Louveau et al. 2015; Hu et al. 2020).
Increased leukocyte trafficking into the brain has been reported in certain pathological conditions. Activated T cells have been shown to alter the BBB characteristics, allowing for recruitment and entry of immune cells across the glia limitans into the brain parenchyma (for review, see Engelhardt et al. 2017). Disruption of the BBB can also be caused by radiotherapy, factors secreted by tumor cells (Phoenix et al. 2016), or, as observed in glioblastoma, mechanical dislocation of astrocytic endfeet from the vasculature by tumor cells (Watkins et al. 2014). A recent study investigated the BBB phenotype in medulloblastoma subgroups and demonstrated that WNT-driven tumors have aberrant fenestrated vasculature, which facilitates the influx of chemotherapeutics and immune cells (Phoenix et al. 2016). Paradoxically, WNT tumors secrete large amounts of Wnt inhibitors, including Wnt inhibitory factor 1 (WIF1) and Dickkopf 1 (DKK1), which act in a paracrine fashion on adjacent endothelial cells to impair BBB integrity (Phoenix et al. 2016). In contrast, SHH tumors do not secrete Wnt inhibitors and overall show a more intact BBB (Phoenix et al. 2016). Although it might be tempting to induce BBB permeability by administering Wnt inhibitors, this therapeutic strategy requires careful investigation, as recent studies have shown that Wnt signaling is beneficial in non-WNT medulloblastoma tumors. For example, activation of Wnt signaling in SHH, group 3, and group 4 tumors decreased proliferation and prolonged survival in medulloblastoma mouse models (Pöschl et al. 2014; Manoranjan et al. 2020). Thus, administration of Wnt inhibitors could exacerbate tumor growth.
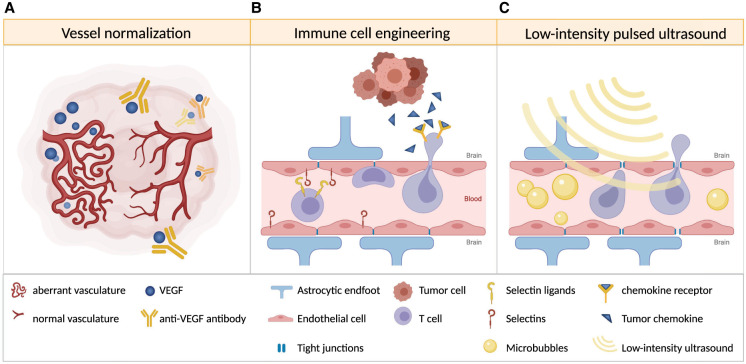
Another approach to therapeutically disrupting the BBB is the application of low-intensity pulsed ultrasound (LIPU). The ultrasound-induced oscillation of intravenously administered microbubbles results in a temporary opening of endothelial tight junctions and increased transcytosis (Sabbagh et al. 2021). Whereas LIPU alone showed no significant effect in a glioblastoma mouse model, the combination of LIPU with anti-PD-1 immune checkpoint blockade or chimeric antigen receptor T (CAR-T) cell therapy led to enhanced antibody penetration into the brain, a significantly higher CAR-T cell infiltration, and prolonged survival of treated mice (Sabbagh et al. 2021). Thus, combining this technique with other immunotherapies could significantly enhance treatment efficacy (see Fig. 1).
Figure 1.
Strategies to enhance immune cell trafficking into brain tumors. (A) Vessel normalization induced by anti-VEGF antibody therapy can improve immune cell infiltration. (B) Immune cells engineered to express selectin ligands and chemokine receptors that match tumor-derived chemokines may show increased homing to and extravasation at the tumor site. (C) Low-intensity pulsed ultrasound temporarily opens the blood–brain barrier and may allow increased immune cell infiltration.
Another determinant of immune infiltration is recruitment or homing of immune cells to the tumor site. Generally, activated lymphocytes migrate from the lymph nodes along a gradient of chemoattractants to their target site, and this requires expression of the appropriate chemokine receptors on lymphocytes. The subsequent extravasation of these cells into tumor tissue is facilitated by expression of selectins and integrins by immune and endothelial cells, which facilitate tethering, rolling, arrest, and diapedesis. Thus, mismatching of chemokine receptors expressed by lymphocytes and chemokines released by the tumor, down-regulation of adhesion molecules, and aberrant vasculature may all contribute to poor homing of effector immune cells (for review, see Slaney et al. 2014). With this in mind, efforts are being made to engineer immune cells to overcome these challenges and to adoptively transfer them back into cancer patients. For example, cell surface glycoengineering is being used to enforce expression and modification of E-selectin ligands in T cells to enhance their extravasation at the target site (see Fig. 1; Mondal et al. 2019). Several studies have shown that ectopic expression of chemokine receptors matching tumor-derived chemokines enhances tumor infiltration of CAR-T cells (Di Stasi et al. 2009; Craddock et al. 2010; Moon et al. 2011). Medulloblastoma cells as well as medulloblastoma-associated reactive astrocytes secrete the chemoattractant CCL2 (Garzia et al. 2018; Maximov et al. 2019; Liu et al. 2020), but its corresponding receptor CCR2 is poorly expressed on activated T cells; thus, ectopic CCR2 expression in adoptively transferred CAR-T cells may substantially improve their homing to medulloblastoma tumors (see Fig. 1), as has been reported for neuroblastoma (Craddock et al. 2010) and mesothelioma (Moon et al. 2011) models.
In addition to the aforementioned ability of bevacizumab to relieve severe edema in brain tumor patients, anti-VEGF therapy may also enhance immune cell infiltration by promoting tumor vessel normalization (see Fig. 1). The administration of low, vascular-normalizing doses of bevacizumab has been shown to result in restoration of functional tumor vessels and increased infiltration of T cells in a breast cancer model (Huang et al. 2012). In a mouse model of glioblastoma, vessel normalization has been shown to prolong survival in combination with immune checkpoint inhibition, which has limited therapeutic efficacy as a monotherapy in this disease (Di Tacchio et al. 2019). Importantly, in addition to blood vessel normalization, lymphangiogenesis has also been shown to positively impact immune infiltration. Two recent studies demonstrated that ectopic VEGF-C expression by glioblastoma cells (or other cells in the brain and meninges) resulted in increased lymphangiogenesis in the meninges and enabled enhanced T-cell infiltration in mouse models of glioblastoma. This approach, combined with immune checkpoint blockade, led to tumor regression and significantly prolonged survival of the mice (Hu et al. 2020; Song et al. 2020).
Medulloblastomas still express neoantigens despite their low mutational load
A high tumor mutation burden has been suggested to contribute to immunogenicity and to predict response to immunotherapy. Concordantly, a recent study has found a sustained response to anti-PD-1 therapy in pediatric cancer patients with an exceptionally high load of mutations and microsatellite insertion–deletion events due to germline DNA mismatch repair deficiency or polymerase proofreading deficiency (Das et al. 2022). However, this correlation found in ultrahypermutated tumors does not necessarily extrapolate to other cancers, as recently shown by a large-scale study by Touat et al. (2020). The investigators showed that chemotherapy-induced hypermutation in glioblastomas does not promote an immune response following anti-PD-1 immunotherapy (Touat et al. 2020). Another recent study identified low mutational burden as a common feature of immunotherapy-responsive recurrent glioblastoma tumors (Gromeier et al. 2021). Although the investigators note that this unexpected correlation may not be causal, it still calls into question the assumption that a high mutational burden is required for an efficient antitumor immune response. Rather, it supports the idea that it is not the quantity but the quality of neoantigens and the ability to present them that determine the antitumor immune response.
Medulloblastomas are rarely ultrahypermutated and are usually characterized by a rather low mutation burden (Parsons et al. 2011). Nevertheless, neoantigen expression has been identified in medulloblastoma tumors. These neoantigens were able to elicit a T-cell response in vitro (Blaeschke et al. 2019) and a humoral response in patients (Behrends et al. 2003). Neoantigens can be derived from errors at the genetic level, such as point or frameshift mutations, deletions, and fusions, and at the transcriptional or post-transcriptional level, such as frameshifts generated by errors in transcription of microsatellites and missplicing of exons (Shen et al. 2019). The latter study by Shen et al. (2019) has intriguingly demonstrated a high load of RNA-based neoantigens in glioblastoma, a low mutation rate cancer, which elicited overall immune responses similar to neoantigens from other high mutation cancers.
Recent studies have revealed aberrations in the splicing and translational machinery of medulloblastomas: SHH subgroup-specific mutations in U1 spliceosomal small nuclear RNA have been associated with enhanced alternative splicing, which not only activates oncogenes and inactivates tumor suppressor genes like PTCH1 but has also been suggested to result in generation of neoepitopes (Suzuki et al. 2019). SHH tumors have also been shown to be enriched for mutations in the elongator complex protein 1, which results in loss of elongator-dependent U34 tRNA modifications (Waszak et al. 2020). The absence of these modifications results in translational inefficiency of AA-ending codons and protein misfolding and aggregation. Consequently, the translation of proteins rich in AA-ending codons is down-regulated, whereas proteins that encode the same amino acids by AG-ending codons are translationally up-regulated. In addition to aberrantly translated proteins, phosphorylated proteins can serve as potent tumor-associated antigens. Leukemia cells show increased MHC class I presentation of phospho-peptides derived from signaling molecules with well-established roles as oncogenic drivers such as MAP kinases, Myc, and Gfi1. Many of these phospho-peptides have been shown to induce a T-cell response (Cobbold et al. 2013). A recent study described a profound increase in global tyrosine phosphorylation in group 4 medulloblastomas (Forget et al. 2018). Thus, similar to leukemia, phospho-peptides could function as tumor antigens in medulloblastoma.
The identification of medulloblastoma neoantigens could enable the promotion of tumor targeting T-cell responses, either by vaccination with tumor-specific epitopes or by engineering CAR-T cells expressing tumor antigen-specific TCRs.
Therapeutic approaches to increase antigen presentation
It has been suggested that there is minimal infiltration of APCs in medulloblastoma. Nonetheless, a positive correlation of dendritic cell expression markers and survival of group 3 and 4 patients suggests a beneficial role of this cell type in medulloblastoma (Bockmayr et al. 2018). Successful approaches to increasing the number of APCs in medulloblastoma have been reported. In mouse models of SHH medulloblastoma and glioblastoma, Flores et al. (2018) have demonstrated that adoptively transferred CCR2+ hematopoietic stem cells (HSCs) migrate to the tumor site and differentiate into dendritic cells. These dendritic cells then home to draining lymph nodes where they prime T cells by presentation of tumor antigens. The transfer of CCR2+ HSCs together with anti-PD-1 treatment or adoptive T-cell transfer resulted in significantly prolonged survival of the animals (Flores et al. 2018).
Adoptive transfer or vaccination with dendritic cells that were loaded ex vivo with tumor RNA or peptides has shown promising effects in some cancer types (Lesterhuis et al. 2006; Kantoff et al. 2010; Mitchell et al. 2015). One study investigated the effect of tumor lysate-loaded dendritic cells in 45 children with recurrent malignant brain tumors. Patients with high-grade glioma and atypical teratoid–rhabdoid tumors responded favorably to the vaccine treatment, whereas no clear benefit was achieved for the five medulloblastoma patients who were included in the study (Ardon et al. 2010). In contrast, preclinical studies in group 3 and SHH medulloblastoma models have indicated that dendritic cell vaccination is beneficial when combined with the autologous transfer of ex vivo propagated T cells (Flores et al. 2018; Flores et al. 2019). This approach is currently being evaluated in a clinical trial for patients with recurrent medulloblastoma (NCT01326104, Re-MATCH) (see Table 1 for an overview of clinical trials of medulloblastoma).
Table 1.
Ongoing and recently completed medulloblastoma immunotherapy trialsa
In addition to APC infiltration, antigen uptake, processing, and presentation are essential for a potent antitumor response. However, tumor antigen presentation might be inefficient and fail to elicit antitumor T-cell responses. One immunotherapeutic approach whose success presumably relies on the release of large quantities of tumor antigens is oncolytic virus therapy. Oncolytic viruses can selectively replicate in tumor cells until they burst and release more infectious particles, which spread within the tumor. While the virus-mediated lysis of tumor cells was initially considered the primary mode of action, stimulation of the host antitumor immune response is now considered an essential aspect of oncolytic virus therapy (for review, see Davola and Mossman 2019). Virus-induced lysis of tumor cells releases cytosolic damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs), endogenous danger signals that attract and activate the innate immune system. Importantly, cell lysis also releases large quantities of tumor and viral antigens that are phagocytosed and presented by recruited APCs, eliciting an adaptive antitumor immune response (see Fig. 2). New generations of oncolytic viruses are equipped with genes that encode immune checkpoint inhibitors such as soluble PD-1-IgG Fc (Wang et al. 2020) or immune cell-recruiting or -stimulating factors such as IL-12, GM-CSF, OX40L, or Flt3L (Liu et al. 2003; Barnard et al. 2012; Jiang et al. 2017; Saha et al. 2017). In medulloblastoma, several preclinical studies have been performed analyzing the efficacy of oncolytic viruses (Lal et al. 2018; Thompson et al. 2018). A recent study of G207, a genetically engineered herpes simplex virus (HSV-1), demonstrated efficient elimination of murine group 3 medulloblastoma tumors without induction of systemic toxicity in an immunocompetent, HSV-1-sensitive mouse strain (Bernstock et al. 2020). G207 (NCT03911388) and other oncolytic viruses such as modified measles virus (NCT02962167), poliovirus (NCT03043391), and wild-type reovirus (NCT02444546) are currently being investigated in clinical trials for medulloblastoma (see Table 1).
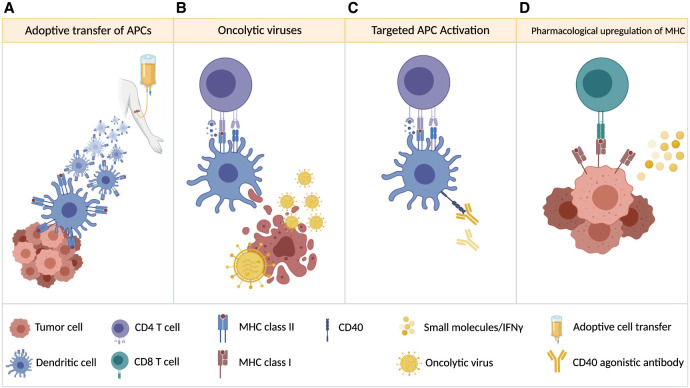
Figure 2.
Strategies to increase antigen presentation. (A) Autologous dendritic cells, pulsed with total tumor RNA or lysate, are infused into the patient. (B) Oncolytic viruses replicate in tumor cells, which ultimately leads to tumor cell lysis and release of tumor-associated antigens, damage-associated molecular patterns (DAMPs), and pathogen-associated molecular patterns (PAMPs) that can enhance antigen presentation. (C) Targeted activation of APCs (for example, by agonistic anti-CD40 antibody treatment) enhances APC activity and antigen presentation. (D) Antigen presentation by tumor cells may be enhanced by treating with molecules (e.g., IFNγ) that up-regulate MHC class I expression and presentation.
Another approach to increase the efficacy of antigen presentation is targeted stimulation of CD40 using agonistic monoclonal antibodies (see Fig. 2). Activation of CD40 on APCs induces up-regulation of costimulatory molecules, production of cytokines, increased expression of MHC, and prosurvival genes (Suek et al. 2019). Although direct cytotoxic effects on tumor cells have also been reported, experiments in immunodeficient and immunocompetent mice suggest that the prolonged survival of anti-CD40-treated glioblastoma-bearing mice is due to its immune stimulatory effect (Shoji et al. 2016). Currently, an agonistic CD40 targeting antibody is being evaluated in a clinical trial in patients with medulloblastoma and other pediatric brain tumors (NCT03389802).
APCs are crucial for the initiation of T-cell-mediated tumor rejection, yet the final cytotoxic T-cell-induced tumor cell killing relies on MHC presentation by tumor cells. The genetic loss or transcriptional, translational, or post-translational suppression of MHC class I in tumor cells (for review, see Dhatchinamoorthy et al. 2021) is a frequent event in immune escape and has been reported for medulloblastoma (Raffaghello et al. 2007). Studies in cancer cell lines have shown that the suppression of MHC class I expression is often reversible and can be restored by histone deacetylase inhibitors (Khan et al. 2008), DNA methyltransferase inhibitors (Serrano et al. 2001), γ interferon (IFNγ) (Zhang et al. 2019), or SMAC mimetics (see Fig. 2; Gu et al. 2021). More preclinical studies will be required to determine whether these measures can also sensitize MHC class I-low or -negative brain tumor cells to CD8+ T-cell-mediated tumor cell rejection.
Inhibition of tumor-induced immunosuppression
Immune checkpoint blockade
Immune checkpoint proteins, including the intensively studied surface proteins PD-1 and CTLA-4, decrease the proliferation and activity of T cells by inducing cell cycle arrest and down-regulation of T-cell receptor signaling, by promoting death of activated T cells, and by suppressing prosurvival signals and cytokine secretion (Krummel and Allison 1996; Freeman et al. 2000; Dong et al. 2002). Their blockade has emerged as a promising immunotherapy in a variety of cancer types (see Fig. 3). Several approaches have been taken to achieve immune checkpoint inhibition in brain tumors. Dorand et al. (2016) have shown that Cdk5 deletion in mouse SHH medulloblastoma tumors abrogates the IFNγ-induced up-regulation of the PD-1 ligand PD-L1 in tumor cells, and this results in CD4+ T-cell-mediated tumor rejection, suggesting Cdk5 as a therapeutic target. Although medulloblastomas are generally characterized by the presence of very few PD-L1-positive tumor cells, a preclinical study has demonstrated a modest response of murine group 3 medulloblastoma tumors to anti-PD-1 monoclonal antibody treatment (Pham et al. 2016). Similarly, nonsmall cell lung cancer patients scored as tumor PD-L1-null have been found to respond to anti-PD-L1 therapy (Bocanegra et al. 2019). The study has identified circulating immune cells, predominantly of myeloid origin, that express PD-L1 and whose number significantly correlates with clinical responses to immune checkpoint inhibition (Bocanegra et al. 2019). Finally, two recent studies used various combinations of PD-L1 knockout and wild-type cancer cells and mouse recipients to demonstrate that Cd274 (which encodes PD-L1) expression by host APCs, and not by tumor cells, determines the response to anti-PD-L1 and anti-PD-1 therapy (Lin et al. 2018; Tang et al. 2018).
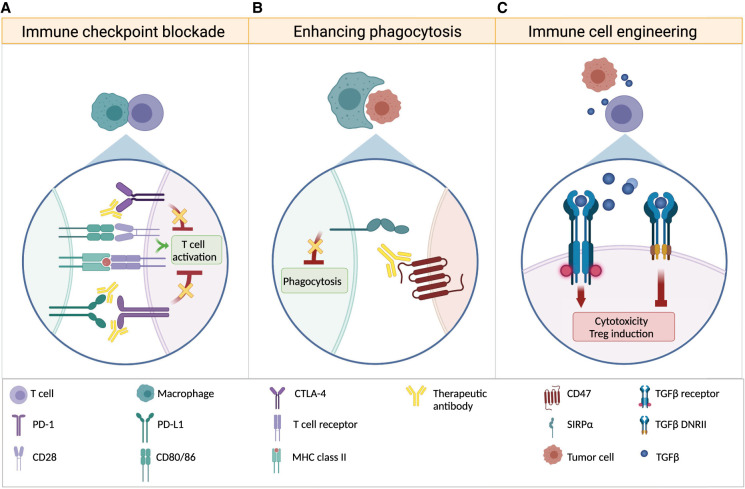
Figure 3.
Strategies to overcome immune suppression. (A) Monoclonal antibodies block immune checkpoint proteins that serve as intrinsic negative regulators of immune responses, allowing for continued immune cell activity. Antibodies directed against CTLA-4, PD-1, or its ligand PD-L1 inhibit the suppression of activated T cells. (B) CD47 is expressed on tumor cells and, upon binding to SIRPα on macrophages, prevents phagocytosis. Anti-CD47 antibodies block this interaction, allowing tumor cell phagocytosis by macrophages. (C) Genetic engineering can render immune cells resistant to tumor cell-induced suppression. Expression of a dominant-negative TGFβ receptor on immune cells prevents TGFβ-induced cytotoxicity as well as differentiation of Tregs and sequesters soluble TGFβ, preventing immunosuppressive TGFβ signaling in endogenous immune cells.
It is important to note that PD-1 activation can also be initiated by PD-L2. However, its expression in medulloblastoma has not been examined, with the exception of a study that found low PD-L2 expression in two medulloblastoma cell lines (Martin et al. 2018). This underscores the complexity of immune checkpoints and suggests that PD-L1 expression in tumor specimens cannot necessarily predict responses to anti-PD-1 treatment.
Comparatively little is known about CTLA-4 expression on medulloblastoma-infiltrating T cells. Bockmayr et al. (2018) have described the microenvironment of human group 3 and group 4 tumors as having an increased immunosuppressive gene signature characterized by PD-L1 and CTLA-4 expression. No CTLA-4 expression was detected on infiltrating CD3+ T cells in mouse models of SHH and group 3 medulloblastoma (Pham et al. 2016). Accordingly, anti-CTLA-4 monoclonal antibody treatment showed no effect on survival (Pham et al. 2016).
Recent studies have identified several other inhibitory checkpoint molecules, including LAG3, TIM-3, B7-H3, TIGIT, and VISTA, most of which are being investigated as therapeutic targets (Kelly et al. 2020). Likewise, the activation of T-cell stimulatory molecules like ICOS, GITR, and OX40 have been suggested to enhance antitumor responses. However, preclinical and clinical data on these second-generation immune checkpoint inhibitors or activators in medulloblastoma tumors are lacking. A detailed review of immune checkpoint modulation in brain tumors is provided in Kelly et al. (2020).
Despite limited knowledge about immune checkpoint expression and function in medulloblastoma, several clinical trials have been initiated to investigate the safety and efficacy of anti-PD-1, PD-L1, and CTLA-4 monoclonal antibody therapy in medulloblastoma patients (NCT03173950, NCT02359565, and NCT03130959) (see Table 1).
Overcoming the immunosuppressive milieu
Given that tumor cells generate an immune-hostile environment by releasing immunosuppressive molecules, one therapeutic approach is to equip immune cells with resistance to the anti-inflammatory milieu before their adoptive transfer into the patient. Along these lines, first attempts have been made to render NK and T cells insensitive to immune-suppressive TGFβ secreted by medulloblastoma cells. The exogenous expression of a dominant-negative TGFβ receptor type II (TGFβRII DNR) in NK cells restored their cytotoxic activity in in vitro coculture experiments with TGFβ-secreting medulloblastoma cells (Powell et al. 2019). As the engineered NK cells sequestered TGFβ, the investigators suggested that the use of TGFβRII DNR-expressing NK cells might not only have therapeutic benefits due to restoration of tumor-directed cytotoxicity but could also help reverting the immunosuppressive environment in medulloblastomas, improving the efficacy of other immune cells.
Abrogation of TGFβ signaling can also promote antitumor activity of other immune cell types, particularly T cells (for review, see Thomas and Massagué 2005). A mouse model of medulloblastoma that expresses the TGFβRII DNR under the control of a CD4 promoter has shown enhanced antitumor immune responses and a clear survival benefit compared with TGFβ wild-type receptor littermates (Gate et al. 2014). TGFβ signaling blockade in T cells not only reduced the number of medulloblastoma-infiltrating Tregs but also promoted CD8+ T-cell differentiation and formation of T-cell memory (Gate et al. 2014).
Thus, engineering immune cells for TGFβ signaling resistance and their subsequent adoptive transfer may be one option to enhance antitumor responses (see Fig. 3). Alternatively, small molecule inhibitors or neutralizing antibodies specific for TGFβ could be applied to abrogate tumor-induced immunosuppression. The safety and efficacy of TGFβ inhibitors as well as of TGFβ-resistant CAR-T cells are currently being investigated in clinical trials for other solid cancers (for review, see Patel et al. 2019; Huang et al. 2021), including studies that combine TGFβ inhibition with immune checkpoint blockade (NCT02423343 and NCT02734160). Furthermore, as TGFβ signaling is a known driver of SHH and group 3 medulloblastoma progression and metastasis (Aref et al. 2013; Ferrucci et al. 2018; Morabito et al. 2019; Sarić et al. 2020), its therapeutic targeting might have additional benefits in inhibiting tumor growth.
Other ongoing clinical trials that target the immune-suppressive medulloblastoma milieu include assessment of indoximod, an indoleamine 2,3-dioxygenase (IDO) inhibitor (NCT02502708), and a humanized monoclonal antibody against placental growth factor (PlGF; NCT02748135). IDO1 inhibition results in reduced catalytic depletion of tryptophan, an amino acid that is critical for T-cell activity and survival. In glioblastoma mouse models, IDO1 inhibition has been shown to restore antitumor immune responses in a multimodal treatment approach with immune checkpoint blockade (Wainwright et al. 2014) or checkpoint blockade and radiotherapy (Ladomersky et al. 2018). PlGF is a member of the VEGF family and may be a good therapeutic target, as it has been reported to suppress the immune system by inhibition of dendritic cell maturation (Lin et al. 2007) and, moreover, to promote medulloblastoma growth and metastasis (Snuderl et al. 2013).
The extent to which macrophages are suitable as therapeutic targets is controversial. As discussed above, several studies have indicated an immunosuppressive function of medulloblastoma-associated myeloid cells, which makes them an attractive target in cancer therapy. However, there is also increasing evidence indicating no or even adverse effects of myeloid cell depletion from medulloblastoma tumors. The pharmacological CSF1R inhibition in a syngeneic mouse model of SHH medulloblastoma (Ptch1+/−; Trp53−/−) and in a group 3 PDX model (grown in immune-compromised mice) did reduce the number of tumor-associated macrophages; however, no effect on survival or tumor progression was observed (Crotty et al. 2021). Interestingly, as opposed to the above-mentioned studies, the CSF1R-inhibited SHH mouse model showed reduced numbers of T-cell infiltrates. Moreover, other studies have shown that macrophages and other myeloid cells can have a beneficial effect, and their depletion may even enhance tumor growth. Gholamin et al. (2017) described a high phagocytic potential of medulloblastoma-associated macrophages that was unleashed by blocking the surface molecule CD47 on tumor cells (see Fig. 3), and this resulted in elimination of primary and metastatic tumor cells in a medulloblastoma PDX model. Consistent with this, another study has demonstrated a beneficial effect of medulloblastoma-associated macrophages, as their depletion from SHH (NeuroD2-SmoA1) tumors by Ccr2 knockout, CSF1R inhibition, or clodrosome administration promoted tumor progression (Maximov et al. 2019). More studies are needed to investigate the conditions that determine tumor-promoting or tumor-opposing functions of medulloblastoma-associated macrophages. Until then, re-education of medulloblastoma-associated myeloid cells to become tumoricidal, rather than nonspecific depletion of these cells, may be a more effective therapeutic strategy.
Re-education, reprogramming, or repolarization of tumor-associated myeloid cells involves loss of tumor-promoting and immunosuppressive signatures and acquisition of proinflammatory signatures. In a mouse model of proneural glioblastoma, CSF1R inhibition was reported to reprogram tumor-associated macrophages from M2-like tumor-promoting to more tumor phagocytic cells, resulting in significant inhibition of tumor growth and prolonged survival (Pyonteck et al. 2013). However, clinical trials in glioblastoma patients have not confirmed the beneficial effects of CSF1R inhibition observed in preclinical mouse models (ClinicalTrials.gov identifier NCT01790503, https://clinicaltrials.gov/ct2/show/results/NCT01790503; Butowski et al. 2016). Another promising strategy is the inhibition of the γ isoform of phosphoinositide-3-kinase (PI3Kγ), which is predominantly expressed by myeloid cells. Pharmacological inhibition of PI3Kγ in mouse models of glioblastoma changed the cytokine profile of tumor-associated myeloid cells, which sensitized tumors to antiangiogenic (Rivera et al. 2015) or temozolomide (Li et al. 2021) therapy. In particular, Li et al. (2021) found that glioblastoma-associated microglia induced MYC expression in tumor cells by secretion of IL-11, which was abrogated by inhibition of PI3Kγ. The effect of PI3Kγ inhibition in medulloblastoma, and in particular in MYC-driven medulloblastoma, remains to be determined.
CAR-T cells have gained interest as an alternative to pharmacological intervention in the reprogramming of the tumor microenvironment. A recent study used CAR-T cells for the selective targeting of folate receptor β (FRβ)-positive, tumor-promoting myeloid cells in mouse models of ovarian cancer, colon cancer, and melanoma. Specific depletion of the FRβ+ macrophage subpopulation led to increased numbers of classical/inflammatory monocytes and endogenous CD8+ T cells that displayed an activated phenotype, resulting in delayed tumor growth and prolonged survival (Rodriguez-Garcia et al. 2021). More studies will be required to identify markers of tumor-promoting myeloid populations in medulloblastoma in order to apply the CAR-T cell technology for the reprogramming of the medulloblastoma microenvironment.
Engineering CAR-T cells to overcome limitations of endogenous T cells
Endogenous T cells engineered to express chimeric antigen receptors against common tumor antigens represent an important pillar of immunotherapy. A major advantage of the chimeric antigen receptor is that it does not require tumor antigen presentation by MHC class I and interaction with costimulatory ligands, but instead can be designed against any surface protein expressed by tumor cells. In medulloblastoma, several promising CAR-T cell targets have been identified. Preclinical studies have shown high efficacy of HER2-directed (Ahmed et al. 2007; Nellan et al. 2018; Donovan et al. 2020), EPHA2-directed (Donovan et al. 2020), and B7-H3-directed (Majzner et al. 2019) CAR-T cells. To overcome CAR-T cell resistance by intratumoral antigen heterogeneity, trivalent CAR-T cells have been developed that are able to target tumor cells that express any one of three target genes. Although multivalent HER-2/IL13Rα2/EPHA2 CAR-T have proved more efficacious than their monovalent CAR-T cell counterpart in glioblastoma xenografts (Bielamowicz et al. 2018), this superiority has not been shown in PDX models of group 3 medulloblastoma (Donovan et al. 2020).
Additional modifications such as overexpression of surface proteins and immune stimulatory cytokines that improve T-cell homing, expansion, and persistence or equipping cells with resistance against tumor-induced immunosuppression may augment antitumor responses. As encouraging as engineered immune cells such as CAR-T cells may seem, their safety must be carefully evaluated. Cell engineering may harbor unknown risks; for example, expression of the TGFβRII DNR in T cells has been shown to cause lymphoproliferative disorders in mice (Lucas et al. 2000; Ishigame et al. 2013). Identified risks can be overcome by further modifications, such as an inducible TGFβRII DNR expression regulated by TCR activation, coupling TGF-β resistance to tumor responsiveness (Hartley and Abken 2019).
Currently, multiple clinical trials are dedicated to assessing the safety and efficacy of CAR-T cells directed against HER2 (NCT03500991), the tumor-specific variant EGFR806 (NCT03638167), and B7-H3 (NCT04185038) in medulloblastoma and other pediatric brain tumors.
Future perspectives
Multiple studies have shown that medulloblastoma tumors largely lack effector immune cells. Like many other brain tumors, medulloblastoma might be protected from immune infiltration by the fact that the immune surveillance of the central nervous system is tightly controlled by the BBB and is restricted to specific subsets of immune cells. Although our knowledge of the BBB and its common disruption by tumor growth has remained very limited, immune cell infiltration seems to be continuously impaired in the tumor setting. The limited numbers of tumor-infiltrating immune cells will eventually encounter a hostile environment that by various means either suppresses, kills, or polarizes them toward a tumor-promoting phenotype. Although these facts suggest that medulloblastoma may be refractory to endogenous antitumor immune responses, there are intriguing data indicating great potential of intervening strategies. Slight but effective changes in chemotherapy and radiotherapy regimens and use of anti-VEGF instead of corticosteroids have been shown to spare immune cells, enabling them to participate in antitumor immune responses. Engineering of immune cells, particularly T cells, may enable their enhanced and persistent tumor infiltration by overexpression of tumor-matching chemokine receptors or cell adhesion molecules. Several strategies are being established to increase tumor antigen release (for example, by oncolytic viruses) and enhance antigen presentation by APCs and tumor cells themselves. Furthermore, the immunosuppressive environment created by the tumor has been shown to be reversible; for example, by targeting anti-inflammatory molecules such as TGFβ or by immune checkpoint inhibition. Moreover, T-cell engineering enables the equipment of T cells with resistance against tumor-initiated immunosuppression and with an MHC class I-independent T-cell receptor.
Despite great successes of individual preclinical studies, clinical trials of various brain and other solid tumors exhibiting immunotherapy resistance have highlighted the need for more detailed mechanistic studies on interactions between immune cells and tumor cells in order to develop novel therapies and to predict and prevent immunotherapy resistance. One critical approach to bypassing resistance may be combining several of the above-described strategies to initiate, enhance, and maintain antitumor responses. With more preclinical and clinical studies analyzing potent and safe combinations of immunotherapeutic agents, immunotherapy might offer a durable cure for medulloblastoma patients.
Acknowledgments
We thank members of the Wechsler-Reya laboratory, and especially Theophilos Tzaridis, for the helpful discussions and feedback on this review. We also gratefully acknowledge support from National Institutes of Health grants P30-CA030199, U01-CA253547, R01-CA159859, R01-NS096368, and R35-NS122339; the Remission Alliance against Brain Tumors; Padres Pedal the Cause; the V Foundation for Cancer Research; Alex's Lemonade Stand Foundation; William's Superhero Fund; and the McDowell Charity Trust. Figures were created with BioRender.com.
Footnotes
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.349538.122.
Freely available online through the Genes & Development Open Access option.
Competing interest statement
The authors declare no competing interests.
References
- Abad C, Nobuta H, Li J, Kasai A, Yong WH, Waschek JA. 2014. Targeted STAT3 disruption in myeloid cells alters immunosuppressor cell abundance in a murine model of spontaneous medulloblastoma. J Leukoc Biol 95: 357–367. 10.1189/jlb.1012531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed N, Ratnayake M, Savoldo B, Perlaky L, Dotti G, Wels WS, Bhattacharjee MB, Gilbertson RJ, Shine HD, Weiss HL, et al. 2007. Regression of experimental medulloblastoma following transfer of HER2-specific T cells. Cancer Res 67: 5957–5964. 10.1158/0008-5472.CAN-06-4309 [DOI] [PubMed] [Google Scholar]
- Amoozgar Z, Kloepper J, Ren J, Tay RE, Kazer SW, Kiner E, Krishnan S, Posada JM, Ghosh M, Mamessier E, et al. 2021. Targeting Treg cells with GITR activation alleviates resistance to immunotherapy in murine glioblastomas. Nat Commun 12: 2582. 10.1038/s41467-021-22885-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardon H, De Vleeschouwer S, Van Calenbergh F, Claes L, Kramm CM, Rutkowski S, Wolff JE, Van Gool SW. 2010. Adjuvant dendritic cell-based tumour vaccination for children with malignant brain tumours. Pediatr Blood Cancer 54: 519–525. 10.1002/pbc.22319 [DOI] [PubMed] [Google Scholar]
- Aref D, Moffatt CJ, Agnihotri S, Ramaswamy V, Dubuc AM, Northcott PA, Taylor MD, Perry A, Olson JM, Eberhart CG, et al. 2013. Canonical TGF-β pathway activity is a predictor of SHH-driven medulloblastoma survival and delineates putative precursors in cerebellar development. Brain Pathol 23: 178–191. 10.1111/j.1750-3639.2012.00631.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badie B, Bartley B, Schartner J. 2002. Differential expression of MHC class II and B7 costimulatory molecules by microglia in rodent gliomas. J Neuroimmunol 133: 39–45. 10.1016/S0165-5728(02)00350-8 [DOI] [PubMed] [Google Scholar]
- Banissi C, Ghiringhelli F, Chen L, Carpentier AF. 2009. Treg depletion with a low-dose metronomic temozolomide regimen in a rat glioma model. Cancer Immunol Immunother 58: 1627–1634. 10.1007/s00262-009-0671-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks PD, Lasocki A, Lau PKH, Sandhu S, McArthur G, Shackleton M. 2019. Bevacizumab as a steroid-sparing agent during immunotherapy for melanoma brain metastases: a case series. Health Sci Rep 2: e115. 10.1002/hsr2.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard Z, Wakimoto H, Zaupa C, Patel AP, Klehm J, Martuza RL, Rabkin SD, Curry WT Jr. 2012. Expression of FMS-like tyrosine kinase 3 ligand by oncolytic herpes simplex virus type I prolongs survival in mice bearing established syngeneic intracranial malignant glioma. Neurosurgery 71: 741–748; discussion 748. 10.1227/NEU.0b013e318260fd73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrends U, Schneider I, Rössler S, Frauenknecht H, Golbeck A, Lechner B, Eigenstetter G, Zobywalski C, Müller-Weihrich S, Graubner U, et al. 2003. Novel tumor antigens identified by autologous antibody screening of childhood medulloblastoma cDNA libraries. Int J Cancer 106: 244–251. 10.1002/ijc.11208 [DOI] [PubMed] [Google Scholar]
- Bernstock JD, Vicario N, Li R, Nan L, Totsch SK, Schlappi C, Gessler F, Han X, Parenti R, Beierle EA, et al. 2020. Safety and efficacy of oncolytic HSV-1 G207 inoculated into the cerebellum of mice. Cancer Gene Ther 27: 246–255. 10.1038/s41417-019-0091-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielamowicz K, Fousek K, Byrd TT, Samaha H, Mukherjee M, Aware N, Wu MF, Orange JS, Sumazin P, Man TK, et al. 2018. Trivalent CAR T cells overcome interpatient antigenic variability in glioblastoma. Neuro Oncol 20: 506–518. 10.1093/neuonc/nox182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaeschke F, Paul MC, Schuhmann MU, Rabsteyn A, Schroeder C, Casadei N, Matthes J, Mohr C, Lotfi R, Wagner B, et al. 2019. Low mutational load in pediatric medulloblastoma still translates into neoantigens as targets for specific T-cell immunotherapy. Cytotherapy 21: 973–986. 10.1016/j.jcyt.2019.06.009 [DOI] [PubMed] [Google Scholar]
- Bocanegra A, Fernandez-Hinojal G, Zuazo-Ibarra M, Arasanz H, Garcia-Granda MJ, Hernandez C, Ibañez M, Hernandez-Marin B, Martinez-Aguillo M, Lecumberri MJ, et al. 2019. PD-L1 expression in systemic immune cell populations as a potential predictive biomarker of responses to PD-L1/PD-1 blockade therapy in lung cancer. Int J Mol Sci 20: 1631. 10.3390/ijms20071631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockmayr M, Mohme M, Klauschen F, Winkler B, Budczies J, Rutkowski S, Schüller U. 2018. Subgroup-specific immune and stromal microenvironment in medulloblastoma. Oncoimmunology 7: e1462430. 10.1080/2162402X.2018.1462430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnet M. 1957. Cancer; a biological approach. I. The processes of control. Br Med J 1: 779–786. 10.1136/bmj.1.5022.779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butowski N, Colman H, De Groot JF, Omuro AM, Nayak L, Wen PY, Cloughesy TF, Marimuthu A, Haidar S, Perry A, et al. 2016. Orally administered colony stimulating factor 1 receptor inhibitor PLX3397 in recurrent glioblastoma: an ivy foundation early phase clinical trials consortium phase II study. Neuro Oncol 18: 557–564. 10.1093/neuonc/nov245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzascia T, Masson F, Di Berardino-Besson W, Contassot E, Wilmotte R, Aurrand-Lions M, Rüegg C, Dietrich PY, Walker PR. 2005. Homing phenotypes of tumor-specific CD8 T cells are predetermined at the tumor site by crosspresenting APCs. Immunity 22: 175–184. 10.1016/j.immuni.2004.12.008 [DOI] [PubMed] [Google Scholar]
- Carson MJ, Doose JM, Melchior B, Schmid CD, Ploix CC. 2006. CNS immune privilege: hiding in plain sight. Immunol Rev 213: 48–65. 10.1111/j.1600-065X.2006.00441.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, Schrock A, Campbell B, Shlien A, Chmielecki J, et al. 2017. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med 9: 34. 10.1186/s13073-017-0424-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevignard M, Câmara-Costa H, Doz F, Dellatolas G. 2017. Core deficits and quality of survival after childhood medulloblastoma: a review. Neurooncol Pract 4: 82–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbold M, De La Peña H, Norris A, Polefrone JM, Qian J, English AM, Cummings KL, Penny S, Turner JE, Cottine J, et al. 2013. MHC class I-associated phosphopeptides are the targets of memory-like immunity in leukemia. Sci Transl Med 5: 203ra125. 10.1126/scitranslmed.3006061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coley WB. 1893. The treatment of malignant tumors by repeated inoculations of erysipelas: with a report of ten original cases. Am J Med Sci 105: 487–510. 10.1097/00000441-189305000-00001 [DOI] [PubMed] [Google Scholar]
- Craddock JA, Lu A, Bear A, Pule M, Brenner MK, Rooney CM, Foster AE. 2010. Enhanced tumor trafficking of GD2 chimeric antigen receptor T cells by expression of the chemokine receptor CCR2b. J Immunother 33: 780–788. 10.1097/CJI.0b013e3181ee6675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane CA, Han SJ, Barry JJ, Ahn BJ, Lanier LL, Parsa AT. 2010. TGF-β downregulates the activating receptor NKG2D on NK cells and CD8+ T cells in glioma patients. Neuro-Oncology 12: 7–13. 10.1093/neuonc/nop009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty EE, Smith SMC, Brasel K, Pakiam F, Girard EJ, Connor YD, Zindy F, Mhyre AJ, Roussel MF, Olson JM. 2021. Medulloblastoma recurrence and metastatic spread are independent of colony-stimulating factor 1 receptor signaling and macrophage survival. J Neurooncol 153: 225–237. 10.1007/s11060-021-03767-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubillos-Ruiz JR, Silberman PC, Rutkowski MR, Chopra S, Perales-Puchalt A, Song M, Zhang S, Bettigole SE, Gupta D, Holcomb K, et al. 2015. ER stress sensor XBP1 controls anti-tumor immunity by disrupting dendritic cell homeostasis. Cell 161: 1527–1538. 10.1016/j.cell.2015.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, et al. 2004. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 10: 942–949. 10.1038/nm1093 [DOI] [PubMed] [Google Scholar]
- Dang MT, Gonzalez MV, Gaonkar KS, Rathi KS, Young P, Arif S, Zhai L, Alam Z, Devalaraja S, To TKJ, et al. 2021. Macrophages in SHH subgroup medulloblastoma display dynamic heterogeneity that varies with treatment modality. Cell Rep 34: 108917. 10.1016/j.celrep.2021.108917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Sudhaman S, Morgenstern D, Coblentz A, Chung J, Stone SC, Alsafwani N, Liu ZA, Karsaneh OAA, Soleimani S, et al. 2022. Genomic predictors of response to PD-1 inhibition in children with germline DNA replication repair deficiency. Nat Med 28: 125–135. 10.1038/s41591-021-01581-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davola ME, Mossman KL. 2019. Oncolytic viruses: how ‘lytic’ must they be for therapeutic efficacy? Oncoimmunology 8: e1581528. 10.1080/2162402X.2019.1596006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Braganca KC, Packer RJ. 2013. Treatment options for medulloblastoma and CNS primitive neuroectodermal tumor (PNET). Curr Treat Options Neurol 15: 593–606. 10.1007/s11940-013-0255-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhatchinamoorthy K, Colbert JD, Rock KL. 2021. Cancer immune evasion through loss of MHC class I antigen presentation. Front Immunol 12: 636568. 10.3389/fimmu.2021.636568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Stasi A, De Angelis B, Rooney CM, Zhang L, Mahendravada A, Foster AE, Heslop HE, Brenner MK, Dotti G, Savoldo B. 2009. T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and antitumor activity in a Hodgkin tumor model. Blood 113: 6392–6402. 10.1182/blood-2009-03-209650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Tacchio M, Macas J, Weissenberger J, Sommer K, Bähr O, Steinbach JP, Senft C, Seifert V, Glas M, Herrlinger U, et al. 2019. Tumor vessel normalization, immunostimulatory reprogramming, and improved survival in glioblastoma with combined inhibition of PD-1, angiopoietin-2, and VEGF. Cancer Immunol Res 7: 1910–1927. 10.1158/2326-6066.CIR-18-0865 [DOI] [PubMed] [Google Scholar]
- Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al. 2002. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 8: 793–800. 10.1038/nm730 [DOI] [PubMed] [Google Scholar]
- Donovan LK, Delaidelli A, Joseph SK, Bielamowicz K, Fousek K, Holgado BL, Manno A, Srikanthan D, Gad AZ, Van Ommeren R, et al. 2020. Locoregional delivery of CAR T cells to the cerebrospinal fluid for treatment of metastatic medulloblastoma and ependymoma. Nat Med 26: 720–731. 10.1038/s41591-020-0827-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorand RD, Nthale J, Myers JT, Barkauskas DS, Avril S, Chirieleison SM, Pareek TK, Abbott DW, Stearns DS, Letterio JJ, et al. 2016. Cdk5 disruption attenuates tumor PD-L1 expression and promotes antitumor immunity. Science 353: 399–403. 10.1126/science.aae0477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn GP, Old LJ, Schreiber RD. 2004. The three Es of cancer immunoediting. Annu Rev Immunol 22: 329–360. 10.1146/annurev.immunol.22.012703.104803 [DOI] [PubMed] [Google Scholar]
- Ehrlich P. 1909. Ueber den jetzigen Stand der Karzinomforschung. Vortrag gehalten vor den Studenten der Amsterdamer Universitaet, Vereinigung fuer wissenschaftliche Arbeit 1 June 1908. Beitraege zur experimentellen Pathologie und Chemotherapie. Akademische Verlagsgesellschaft, Leipzig, Germany. [Google Scholar]
- El Andaloussi A, Lesniak MS. 2007. CD4+CD25+FoxP3+ T-cell infiltration and heme oxygenase-1 expression correlate with tumor grade in human gliomas. J Neurooncol 83: 145–152. 10.1007/s11060-006-9314-y [DOI] [PubMed] [Google Scholar]
- Engelhardt B, Ransohoff RM. 2012. Capture, crawl, cross: the T cell code to breach the blood–brain barriers. Trends Immunol 33: 579–589. 10.1016/j.it.2012.07.004 [DOI] [PubMed] [Google Scholar]
- Engelhardt B, Vajkoczy P, Weller RO. 2017. The movers and shapers in immune privilege of the CNS. Nat Immunol 18: 123–131. 10.1038/ni.3666 [DOI] [PubMed] [Google Scholar]
- Ferrucci V, de Antonellis P, Pennino FP, Asadzadeh F, Virgilio A, Montanaro D, Galeone A, Boffa I, Pisano I, Scognamiglio I, et al. 2018. Metastatic group 3 medulloblastoma is driven by PRUNE1 targeting NME1–TGF-β–OTX2–SNAIL via PTEN inhibition. Brain 141: 1300–1319. 10.1093/brain/awy039 [DOI] [PubMed] [Google Scholar]
- Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limón P. 2010. The polarization of immune cells in the tumour environment by TGFβ. Nat Rev Immunol 10: 554–567. 10.1038/nri2808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores CT, Wildes TJ, Drake JA, Moore GL, Dean BD, Abraham RS, Mitchell DA. 2018. Lin−CCR2+ hematopoietic stem and progenitor cells overcome resistance to PD-1 blockade. Nat Commun 9: 4313. 10.1038/s41467-018-06182-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores C, Wildes T, Dean BD, Moore G, Drake J, Abraham R, Gil J, Yegorov O, Yang C, Dean J, et al. 2019. Massive clonal expansion of medulloblastoma-specific T cells during adoptive cellular therapy. Sci Adv 5: eaav9879. 10.1126/sciadv.aav9879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folgiero V, Miele E, Carai A, Ferretti E, Alfano V, Po A, Bertaina V, Goffredo BM, Benedetti MC, Camassei FD, et al. 2016. IDO1 involvement in mTOR pathway: a molecular mechanism of resistance to mTOR targeting in medulloblastoma. Oncotarget 7: 52900–52911. 10.18632/oncotarget.9284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford AL, Foulcher E, Lemckert FA, Sedgwick JD. 1996. Microglia induce CD4 T lymphocyte final effector function and death. J Exp Med 184: 1737–1745. 10.1084/jem.184.5.1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forget A, Martignetti L, Puget S, Calzone L, Brabetz S, Picard D, Montagud A, Liva S, Sta A, Dingli F, et al. 2018. Aberrant ERBB4-SRC signaling as a hallmark of group 4 medulloblastoma revealed by integrative phosphoproteomic profiling. Cancer Cell 34: 379–395.e7. 10.1016/j.ccell.2018.08.002 [DOI] [PubMed] [Google Scholar]
- Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, et al. 2000. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 192: 1027–1034. 10.1084/jem.192.7.1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friese MA, Wischhusen J, Wick W, Weiler M, Eisele G, Steinle A, Weller M. 2004. RNA interference targeting transforming growth factor-β enhances NKG2D-mediated antiglioma immune response, inhibits glioma cell migration and invasiveness, and abrogates tumorigenicity in vivo. Cancer Res 64: 7596–7603. 10.1158/0008-5472.CAN-04-1627 [DOI] [PubMed] [Google Scholar]
- Galon J, Bruni D. 2020. Tumor immunology and tumor evolution: intertwined histories. Immunity 52: 55–81. 10.1016/j.immuni.2019.12.018 [DOI] [PubMed] [Google Scholar]
- Garzia L, Kijima N, Morrissy AS, De Antonellis P, Guerreiro-Stucklin A, Holgado BL, Wu X, Wang X, Parsons M, Zayne K, et al. 2018. A hematogenous route for medulloblastoma leptomeningeal metastases. Cell 172: 1050–1062.e14. 10.1016/j.cell.2018.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gate D, Danielpour M, Rodriguez J Jr, Kim G-B, Levy R, Bannykh S, Breunig JJ, Kaech SM, Flavell RA, Town T. 2014. T-cell TGF-β signaling abrogation restricts medulloblastoma progression. Proc Natl Acad Sci 111: E3458–E3466. 10.1073/pnas.1412489111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholamin S, Mitra SS, Feroze AH, Liu J, Kahn SA, Zhang M, Esparza R, Richard C, Ramaswamy V, Remke M, et al. 2017. Disrupting the CD47-SIRPα anti-phagocytic axis by a humanized anti-CD47 antibody is an efficacious treatment for malignant pediatric brain tumors. Sci Transl Med 9: eaaf2968. 10.1126/scitranslmed.aaf2968 [DOI] [PubMed] [Google Scholar]
- Goodman AM, Kato S, Bazhenova L, Patel SP, Frampton GM, Miller V, Stephens PJ, Daniels GA, Kurzrock R. 2017. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther 16: 2598–2608. 10.1158/1535-7163.MCT-17-0386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabovska Y, Mackay A, O'Hare P, Crosier S, Finetti M, Schwalbe EC, Pickles JC, Fairchild AR, Avery A, Cockle J, et al. 2020. Pediatric pan-central nervous system tumor analysis of immune-cell infiltration identifies correlates of antitumor immunity. Nat Commun 11: 4324. 10.1038/s41467-020-18070-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesinger AM, Birks DK, Donson AM, Amani V, Hoffman LM, Waziri A, Wang M, Handler MH, Foreman NK. 2013. Characterization of distinct immunophenotypes across pediatric brain tumor types. J Immunol 191: 4880–4888. 10.4049/jimmunol.1301966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromeier M, Brown MC, Zhang G, Lin X, Chen Y, Wei Z, Beaubier N, Yan H, He Y, Desjardins A, et al. 2021. Very low mutation burden is a feature of inflamed recurrent glioblastomas responsive to cancer immunotherapy. Nat Commun 12: 352. 10.1038/s41467-020-20469-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gschwandtner M, Derler R, Midwood KS. 2019. More than just attractive: how CCL2 influences myeloid cell behavior beyond chemotaxis. Front Immunol 10: 2759. 10.3389/fimmu.2019.02759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu P, Gao JF, D'Souza CA, Kowalczyk A, Chou KY, Zhang L. 2012. Trogocytosis of CD80 and CD86 by induced regulatory T cells. Cell Mol Immunol 9: 136–146. 10.1038/cmi.2011.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu SS, Zhang W, Wang X, Jiang P, Traugh N, Li Z, Meyer C, Stewig B, Xie Y, Bu X, et al. 2021. Therapeutically increasing MHC-I expression potentiates immune checkpoint blockade. Cancer Discov 11: 1524–1541. 10.1158/2159-8290.CD-20-0812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gururangan S, Reap E, Schmittling R, Kocak M, Reynolds R, Grant G, Onar-Thomas A, Baxter P, Pollack IF, Phillips P, et al. 2017. Regulatory T cell subsets in patients with medulloblastoma at diagnosis and during standard irradiation and chemotherapy (PBTC N-11). Cancer Immunol Immunother 66: 1589–1595. 10.1007/s00262-017-2051-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberthur K, Brennan K, Hoglund V, Balcaitis S, Chinn H, Davis A, Kreuser S, Winter C, Leary SES, Deutsch GH, et al. 2016. NKG2D ligand expression in pediatric brain tumors. Cancer Biol Ther 17: 1253–1265. 10.1080/15384047.2016.1250047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J, Abken H. 2019. Chimeric antigen receptors designed to overcome transforming growth factor-β-mediated repression in the adoptive T-cell therapy of solid tumors. Clin Transl Immunology 8: e1064. 10.1002/cti2.1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herber DL, Cao W, Nefedova Y, Novitskiy SV, Nagaraj S, Tyurin VA, Corzo A, Cho HI, Celis E, Lennox B, et al. 2010. Lipid accumulation and dendritic cell dysfunction in cancer. Nat Med 16: 880–886. 10.1038/nm.2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi FS, Lawrence D, Lezcano C, Wu X, Zhou J, Sasada T, Zeng W, Giobbie-Hurder A, Atkins MB, Ibrahim N, et al. 2014. Bevacizumab plus ipilimumab in patients with metastatic melanoma. Cancer Immunol Res 2: 632–642. 10.1158/2326-6066.CIR-14-0053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Deng Q, Ma L, Li Q, Chen Y, Liao Y, Zhou F, Zhang C, Shao L, Feng J, et al. 2020. Meningeal lymphatic vessels regulate brain tumor drainage and immunity. Cell Res 30: 229–243. 10.1038/s41422-020-0287-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Yuan J, Righi E, Kamoun WS, Ancukiewicz M, Nezivar J, Santosuosso M, Martin JD, Martin MR, Vianello F, et al. 2012. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci 109: 17561–17566. 10.1073/pnas.1215397109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C-Y, Chung C-L, Hu T-H, Chen J-J, Liu P-F, Chen C-L. 2021. Recent progress in TGF-β inhibitors for cancer therapy. Biomed Pharmacother 134: 111046. 10.1016/j.biopha.2020.111046 [DOI] [PubMed] [Google Scholar]
- Hughes EN, Shillito J, Sallan SE, Loeffler JS, Cassady JR, Tarbell NJ. 1988. Medulloblastoma at the joint center for radiation therapy between 1968 and 1984. The influence of radiation dose on the patterns of failure and survival. Cancer 61: 1992–1998. [DOI] [PubMed] [Google Scholar]
- Ishigame H, Mosaheb MM, Sanjabi S, Flavell RA. 2013. Truncated form of TGF-βRII, but not its absence, induces memory CD8+ T cell expansion and lymphoproliferative disorder in mice. J Immunol 190: 6340–6350. 10.4049/jimmunol.1300397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Rivera-Molina Y, Gomez-Manzano C, Clise-Dwyer K, Bover L, Vence LM, Yuan Y, Lang FF, Toniatti C, Hossain MB, et al. 2017. Oncolytic adenovirus and tumor-targeting immune modulatory therapy improve autologous cancer vaccination. Cancer Res 77: 3894–3907. 10.1158/0008-5472.CAN-17-0468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juraschka K, Taylor MD. 2019. Medulloblastoma in the age of molecular subgroups: a review. J Neurosurg Pediatr 24: 353–363. 10.3171/2019.5.PEDS18381 [DOI] [PubMed] [Google Scholar]
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, et al. 2010. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 363: 411–422. 10.1056/NEJMoa1001294 [DOI] [PubMed] [Google Scholar]
- Kelly WJ, Giles AJ, Gilbert M. 2020. T lymphocyte-targeted immune checkpoint modulation in glioma. J Immunother Cancer 8: e000379. 10.1136/jitc-2019-000379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AN, Gregorie CJ, Tomasi TB. 2008. Histone deacetylase inhibitors induce TAP, LMP, Tapasin genes and MHC class I antigen presentation by melanoma cells. Cancer Immunol Immunother 57: 647–654. 10.1007/s00262-007-0402-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotecha R, Kim JM, Miller JA, Juloori A, Chao ST, Murphy ES, Peereboom DM, Mohammadi AM, Barnett GH, Vogelbaum MA, et al. 2019. The impact of sequencing PD-1/PD-L1 inhibitors and stereotactic radiosurgery for patients with brain metastasis. Neuro-Oncology 21: 1060–1068. 10.1093/neuonc/noz046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummel MF, Allison JP. 1996. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med 183: 2533–2540. 10.1084/jem.183.6.2533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladomersky E, Zhai L, Lenzen A, Lauing KL, Qian J, Scholtens DM, Gritsina G, Sun X, Liu Y, Yu F, et al. 2018. IDO1 inhibition synergizes with radiation and PD-1 blockade to durably increase survival against advanced glioblastoma. Clin Cancer Res 24: 2559–2573. 10.1158/1078-0432.CCR-17-3573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal S, Carrera D, Phillips JJ, Weiss WA, Raffel C. 2018. An oncolytic measles virus-sensitive group 3 medulloblastoma model in immune-competent mice. Neuro Oncol 20: 1606–1615. 10.1093/neuonc/noy089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi DB, Thompson EM, Ashley DM. 2018. Immunotherapy for pediatric brain tumors. Neuroimmunol Neuroinflamm 5: 29. 10.20517/2347-8659.2018.35 [DOI] [Google Scholar]
- Lesterhuis WJ, de Vries IJ, Schuurhuis DH, Boullart AC, Jacobs JF, de Boer AJ, Scharenborg NM, Brouwer HM, van de Rakt MW, Figdor CG, et al. 2006. Vaccination of colorectal cancer patients with CEA-loaded dendritic cells: antigen-specific T cell responses in DTH skin tests. Ann Oncol 17: 974–980. 10.1093/annonc/mdl072 [DOI] [PubMed] [Google Scholar]
- Li J, Kaneda MM, Ma J, Li M, Shepard RM, Patel K, Koga T, Sarver A, Furnari F, Xu B, et al. 2021. PI3Kγ inhibition suppresses microglia/TAM accumulation in glioblastoma microenvironment to promote exceptional temozolomide response. Proc Natl Acad Sci 118: e2009290118. 10.1073/pnas.2009290118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YL, Liang YC, Chiang BL. 2007. Placental growth factor down-regulates type 1 T helper immune response by modulating the function of dendritic cells. J Leukoc Biol 82: 1473–1480. 10.1189/jlb.0307164 [DOI] [PubMed] [Google Scholar]
- Lin H, Wei S, Hurt EM, Green MD, Zhao L, Vatan L, Szeliga W, Herbst R, Harms PW, Fecher LA, et al. 2018. Host expression of PD-L1 determines efficacy of PD-L1 pathway blockade-mediated tumor regression. J Clin Invest 128: 805–815. 10.1172/JCI96113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu BL, Robinson M, Han ZQ, Branston RH, English C, Reay P, McGrath Y, Thomas SK, Thornton M, Bullock P, et al. 2003. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther 10: 292–303. 10.1038/sj.gt.3301885 [DOI] [PubMed] [Google Scholar]
- Liu AK, Macy ME, Foreman NK. 2009. Bevacizumab as therapy for radiation necrosis in four children with pontine gliomas. Int J Radiat Oncol Biol Phys 75: 1148–1154. 10.1016/j.ijrobp.2008.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Sun Y, O'Brien JA, Franco-Barraza J, Qi X, Yuan H, Jin W, Zhang J, Gu C, Zhao Z, et al. 2020. Necroptotic astrocytes contribute to maintaining stemness of disseminated medulloblastoma through CCL2 secretion. Neuro Oncol 22: 625–638. 10.1093/neuonc/noz214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, et al. 2015. Structural and functional features of central nervous system lymphatic vessels. Nature 523: 337–341. 10.1038/nature14432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas PJ, Kim SJ, Melby SJ, Gress RE. 2000. Disruption of T cell homeostasis in mice expressing a T cell–specific dominant negative transforming growth factor β II receptor. J Exp Med 191: 1187–1196. 10.1084/jem.191.7.1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majzner RG, Theruvath JL, Nellan A, Heitzeneder S, Cui Y, Mount CW, Rietberg SP, Linde MH, Xu P, Rota C, et al. 2019. CAR T cells targeting B7-H3, a pan-cancer antigen, demonstrate potent preclinical activity against pediatric solid tumors and brain tumors. Clin Cancer Res 25: 2560–2574. 10.1158/1078-0432.CCR-18-0432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoranjan B, Venugopal C, Bakhshinyan D, Adile AA, Richards L, Kameda-Smith MM, Whitley O, Dvorkin-Gheva A, Subapanditha M, Savage N, et al. 2020. Wnt activation as a therapeutic strategy in medulloblastoma. Nat Commun 11: 4323. 10.1038/s41467-020-17953-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margol AS, Robison NJ, Gnanachandran J, Hung LT, Kennedy RJ, Vali M, Dhall G, Finlay JL, Erdreich-Epstein A, Krieger MD, et al. 2015. Tumor-associated macrophages in SHH subgroup of medulloblastomas. Clin Cancer Res 21: 1457–1465. 10.1158/1078-0432.CCR-14-1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolin K. 2012. Ipilimumab in a phase II trial of melanoma patients with brain metastases. Oncoimmunology 1: 1197–1199. 10.4161/onci.20687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AM, Nirschl CJ, Polanczyk MJ, Bell WR, Nirschl TR, Harris-Bookman S, Phallen J, Hicks J, Martinez D, Ogurtsova A, et al. 2018. PD-L1 expression in medulloblastoma: an evaluation by subgroup. Oncotarget 9: 19177–19191. 10.18632/oncotarget.24951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximov V, Chen Z, Wei Y, Robinson MH, Herting CJ, Shanmugam NS, Rudneva VA, Goldsmith KC, MacDonald TJ, Northcott PA, et al. 2019. Tumour-associated macrophages exhibit anti-tumoural properties in Sonic Hedgehog medulloblastoma. Nat Commun 10: 2410. 10.1038/s41467-019-10458-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DA, Batich KA, Gunn MD, Huang MN, Sanchez-Perez L, Nair SK, Congdon KL, Reap EA, Archer GE, Desjardins A, et al. 2015. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature 519: 366–369. 10.1038/nature14320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal N, Silva M, Castano AP, Maus MV, Sackstein R. 2019. Glycoengineering of chimeric antigen receptor (CAR) T-cells to enforce E-selectin binding. J Biol Chem 294: 18465–18474. 10.1074/jbc.RA119.011134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon EK, Carpenito C, Sun J, Wang LC, Kapoor V, Predina J, Powell DJ Jr, Riley JL, June CH, Albelda SM. 2011. Expression of a functional CCR2 receptor enhances tumor localization and tumor eradication by retargeted human T cells expressing a mesothelin-specific chimeric antibody receptor. Clin Cancer Res 17: 4719–4730. 10.1158/1078-0432.CCR-11-0351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morabito M, Larcher M, Cavalli FM, Foray C, Forget A, Mirabal-Ortega L, Andrianteranagna M, Druillennec S, Garancher A, Masliah-Planchon J, et al. 2019. An autocrine ActivinB mechanism drives TGFβ/activin signaling in group 3 medulloblastoma. EMBO Mol Med 11: e9830. 10.15252/emmm.201809830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundt S, Mrdjen D, Utz SG, Greter M, Schreiner B, Becher B. 2019. Conventional DCs sample and present myelin antigens in the healthy CNS and allow parenchymal T cell entry to initiate neuroinflammation. Sci Immunol 4: eaau8380. 10.1126/sciimmunol.aau8380 [DOI] [PubMed] [Google Scholar]
- Nagpal S, Harsh G, Recht L. 2011. Bevacizumab improves quality of life in patients with recurrent glioblastoma. Chemother Res Pract 2011: 602812. 10.1155/2011/602812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nellan A, Rota C, Majzner R, Lester-McCully CM, Griesinger AM, Mulcahy Levy JM, Foreman NK, Warren KE, Lee DW. 2018. Durable regression of medulloblastoma after regional and intravenous delivery of anti-HER2 chimeric antigen receptor T cells. J Immunother Cancer 6: 30. 10.1186/s40425-018-0340-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochocka N, Segit P, Walentynowicz KA, Wojnicki K, Cyranowski S, Swatler J, Mieczkowski J, Kaminska B. 2021. Single-cell RNA sequencing reveals functional heterogeneity of glioma-associated brain macrophages. Nat Commun 12: 1151. 10.1038/s41467-021-21407-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old LJ, Boyse EA. 1966. Specific antigens of tumors and leukemias of experimental animals. Med Clin North Am 50: 901–912. 10.1016/S0025-7125(16)33187-X [DOI] [PubMed] [Google Scholar]
- Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. 2018. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011–2015. Neuro Oncol 20: iv1–iv86. 10.1093/neuonc/noy131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. 2007. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol 8: 1353–1362. 10.1038/ni1536 [DOI] [PubMed] [Google Scholar]
- Parsons DW, Li M, Zhang X, Jones S, Leary RJ, Lin JC-H, Boca SM, Carter H, Samayoa J, Bettegowda C, et al. 2011. The genetic landscape of the childhood cancer medulloblastoma. Science 331: 435–439. 10.1126/science.1198056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Burga RA, Powell AB, Chorvinsky EA, Hoq N, McCormack SE, Van Pelt SN, Hanley PJ, Cruz CRY. 2019. Beyond CAR T cells: other cell-based immunotherapeutic strategies against cancer. Front Oncol 9: 196–196. 10.3389/fonc.2019.00196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peereboom DM, Alban TJ, Grabowski MM, Alvarado AG, Otvos B, Bayik D, Roversi G, McGraw M, Huang P, Mohammadi AM, et al. 2019. Metronomic capecitabine as an immune modulator in glioblastoma patients reduces myeloid-derived suppressor cells. JCI Insight 4: e130748. 10.1172/jci.insight.130748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham CD, Flores C, Yang C, Pinheiro EM, Yearley JH, Sayour EJ, Pei Y, Moore C, McLendon RE, Huang J, et al. 2016. Differential immune microenvironments and response to immune checkpoint blockade among molecular subtypes of murine medulloblastoma. Clin Cancer Res 22: 582–595. 10.1158/1078-0432.CCR-15-0713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phoenix TN, Patmore DM, Boop S, Boulos N, Jacus MO, Patel YT, Roussel MF, Finkelstein D, Goumnerova L, Perreault S, et al. 2016. Medulloblastoma genotype dictates blood brain barrier phenotype. Cancer Cell 29: 508–522. 10.1016/j.ccell.2016.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pöschl J, Bartels M, Ohli J, Bianchi E, Kuteykin-Teplyakov K, Grammel D, Ahlfeld J, Schüller U. 2014. Wnt/β-catenin signaling inhibits the Shh pathway and impairs tumor growth in Shh-dependent medulloblastoma. Acta Neuropathol 127: 605–607. 10.1007/s00401-014-1258-2 [DOI] [PubMed] [Google Scholar]
- Powell AB, Yadavilli S, Saunders D, Van Pelt S, Chorvinsky E, Burga RA, Albihani S, Hanley PJ, Xu Z, Pei Y, et al. 2019. Medulloblastoma rendered susceptible to NK-cell attack by TGFβ neutralization. J Transl Med 17: 321. 10.1186/s12967-019-2055-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priego N, Zhu L, Monteiro C, Mulders M, Wasilewski D, Bindeman W, Doglio L, Martínez L, Martínez-Saez E, Ramón YCS, et al. 2018. STAT3 labels a subpopulation of reactive astrocytes required for brain metastasis. Nat Med 24: 1024–1035. 10.1038/s41591-018-0044-4 [DOI] [PubMed] [Google Scholar]
- Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, Olson OC, Quick ML, Huse JT, Teijeiro V, et al. 2013. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med 19: 1264–1272. 10.1038/nm.3337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian J, Luo F, Yang J, Liu J, Liu R, Wang L, Wang C, Deng Y, Lu Z, Wang Y, et al. 2018. TLR2 promotes glioma immune evasion by downregulating MHC class II molecules in microglia. Cancer Immunol Res 6: 1220–1233. [DOI] [PubMed] [Google Scholar]
- Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, Baker J, Jeffery LE, Kaur S, Briggs Z, et al. 2011. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science 332: 600–603. 10.1126/science.1202947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaghello L, Nozza P, Morandi F, Camoriano M, Wang X, Garrè ML, Cama A, Basso G, Ferrone S, Gambini C, et al. 2007. Expression and functional analysis of human leukocyte antigen class I antigen-processing machinery in medulloblastoma. Cancer Res 67: 5471–5478. 10.1158/0008-5472.CAN-06-4735 [DOI] [PubMed] [Google Scholar]
- Reardon DA, Wucherpfennig K, Chiocca EA. 2017. Immunotherapy for glioblastoma: on the sidelines or in the game? Discov Med 24: 201–208. [PubMed] [Google Scholar]
- Riemondy KA, Venkataraman S, Willard N, Nellan A, Sanford B, Griesinger AM, Amani V, Mitra S, Hankinson TC, Handler MH, et al. 2022. Neoplastic and immune single-cell transcriptomics define subgroup-specific intra-tumoral heterogeneity of childhood medulloblastoma. Neuro Oncol 24: 273–286. 10.1093/neuonc/noab135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera LB, Meyronet D, Hervieu V, Frederick MJ, Bergsland E, Bergers G. 2015. Intratumoral myeloid cells regulate responsiveness and resistance to antiangiogenic therapy. Cell Rep 11: 577–591. 10.1016/j.celrep.2015.03.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al. 2015. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348: 124–128. 10.1126/science.aaa1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson G, Parker M, Kranenburg TA, Lu C, Chen X, Ding L, Phoenix TN, Hedlund E, Wei L, Zhu X, et al. 2012. Novel mutations target distinct subgroups of medulloblastoma. Nature 488: 43–48. 10.1038/nature11213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Garcia A, Lynn RC, Poussin M, Eiva MA, Shaw LC, O'Connor RS, Minutolo NG, Casado-Medrano V, Lopez G, Matsuyama T, et al. 2021. CAR-T cell-mediated depletion of immunosuppressive tumor-associated macrophages promotes endogenous antitumor immunity and augments adoptive immunotherapy. Nat Commun 12: 877. 10.1038/s41467-021-20893-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbagh A, Beccaria K, Ling X, Marisetty A, Ott M, Caruso H, Barton E, Kong LY, Fang D, Latha K, et al. 2021. Opening of the blood–brain barrier using low-intensity pulsed ultrasound enhances responses to immunotherapy in preclinical glioma models. Clin Cancer Res 27: 4325–4337. 10.1158/1078-0432.CCR-20-3760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha D, Martuza RL, Rabkin SD. 2017. Macrophage polarization contributes to glioblastoma eradication by combination immunovirotherapy and immune checkpoint blockade. Cancer Cell 32: 253–267.e5. 10.1016/j.ccell.2017.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarić N, Selby M, Ramaswamy V, Kool M, Stockinger B, Hogstrand C, Williamson D, Marino S, Taylor MD, Clifford SC, et al. 2020. The AHR pathway represses TGFβ–SMAD3 signalling and has a potent tumour suppressive role in SHH medulloblastoma. Sci Rep 10: 148. 10.1038/s41598-019-56876-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schartner JM, Hagar AR, Van Handel M, Zhang L, Nadkarni N, Badie B. 2005. Impaired capacity for upregulation of MHC class II in tumor-associated microglia. Glia 51: 279–285. 10.1002/glia.20201 [DOI] [PubMed] [Google Scholar]
- Serrano A, Tanzarella S, Lionello I, Mendez R, Traversari C, Ruiz-Cabello F, Garrido F. 2001. Rexpression of HLA class I antigens and restoration of antigen-specific CTL response in melanoma cells following 5-aza-2′-deoxycytidine treatment. Int J Cancer 94: 243–251. 10.1002/ijc.1452 [DOI] [PubMed] [Google Scholar]
- Shaim H, Shanley M, Basar R, Daher M, Gumin J, Zamler DB, Uprety N, Wang F, Huang Y, Gabrusiewicz K, et al. 2021. Targeting the αv integrin/TGF-β axis improves natural killer cell function against glioblastoma stem cells. J Clin Invest 131: e142116. 10.1172/JCI142116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Zhang J, Lee H, Batista MT, Johnston SA. 2019. RNA transcription and splicing errors as a source of cancer frameshift neoantigens for vaccines. Sci Rep 9: 14184. 10.1038/s41598-019-50738-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji T, Saito R, Chonan M, Shibahara I, Sato A, Kanamori M, Sonoda Y, Kondo T, Ishii N, Tominaga T. 2016. Local convection-enhanced delivery of an anti-CD40 agonistic monoclonal antibody induces antitumor effects in mouse glioma models. Neuro-Oncology 18: 1120–1128. 10.1093/neuonc/now023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaney CY, Kershaw MH, Darcy PK. 2014. Trafficking of T cells into tumors. Cancer Res 74: 7168–7174. 10.1158/0008-5472.CAN-14-2458 [DOI] [PubMed] [Google Scholar]
- Slavc I, Peyrl A, Chocholous M, Reisinger D, Mayr L, Azizi A, Dieckmann K, Haberler C, Czech T. 2018. MBCL-27. Response of recurrent malignant childhood CNS tumors to a memmat based metronomic antiangiogenic combination therapy varies dependent on tumor type: experience in 71 patients. Neuro-Oncology 20: i122. 10.1093/neuonc/noy059.423 [DOI] [Google Scholar]
- Snuderl M, Batista A, Kirkpatrick ND, Ruiz de Almodovar C, Riedemann L, Walsh EC, Anolik R, Huang Y, Martin JD, Kamoun W, et al. 2013. Targeting placental growth factor/neuropilin 1 pathway inhibits growth and spread of medulloblastoma. Cell 152: 1065–1076. 10.1016/j.cell.2013.01.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song E, Mao T, Dong H, Boisserand LSB, Antila S, Bosenberg M, Alitalo K, Thomas JL, Iwasaki A. 2020. VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours. Nature 577: 689–694. 10.1038/s41586-019-1912-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suek N, Campesato LF, Merghoub T, Khalil DN. 2019. Targeted APC activation in cancer immunotherapy to enhance the abscopal effect. Front Immunol 10: 604. 10.3389/fimmu.2019.00604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Kumar SA, Shuai S, Diaz-Navarro A, Gutierrez-Fernandez A, De Antonellis P, Cavalli FMG, Juraschka K, Farooq H, Shibahara I, et al. 2019. Recurrent noncoding U1 snRNA mutations drive cryptic splicing in SHH medulloblastoma. Nature 574: 707–711. 10.1038/s41586-019-1650-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan IL, Arifa RDN, Rallapalli H, Kana V, Lao Z, Sanghrajka RM, Sumru Bayin N, Tanne A, Wojcinski A, Korshunov A, et al. 2021. CSF1R inhibition depletes tumor-associated macrophages and attenuates tumor progression in a mouse sonic Hedgehog-Medulloblastoma model. Oncogene 40: 396–407. 10.1038/s41388-020-01536-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Liang Y, Anders RA, Taube JM, Qiu X, Mulgaonkar A, Liu X, Harrington SM, Guo J, Xin Y, et al. 2018. PD-L1 on host cells is essential for PD-L1 blockade-mediated tumor regression. J Clin Invest 128: 580–588. 10.1172/JCI96061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MD, Northcott PA, Korshunov A, Remke M, Cho YJ, Clifford SC, Eberhart CG, Parsons DW, Rutkowski S, Gajjar A, et al. 2012. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol 123: 465–472. 10.1007/s00401-011-0922-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DA, Massagué J. 2005. TGF-β directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell 8: 369–380. 10.1016/j.ccr.2005.10.012 [DOI] [PubMed] [Google Scholar]
- Thompson EM, Brown M, Dobrikova E, Ramaswamy V, Taylor MD, McLendon R, Sanks J, Chandramohan V, Bigner D, Gromeier M. 2018. Poliovirus receptor (CD155) expression in pediatric brain tumors mediates oncolysis of medulloblastoma and pleomorphic xanthoastrocytoma. J Neuropathol Exp Neurol 77: 696–702. 10.1093/jnen/nly045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touat M, Li YY, Boynton AN, Spurr LF, Iorgulescu JB, Bohrson CL, Cortes-Ciriano I, Birzu C, Geduldig JE, Pelton K, et al. 2020. Mechanisms and therapeutic implications of hypermutation in gliomas. Nature 580: 517–523. 10.1038/s41586-020-2209-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen JF, Van Hecke W, Adriaansen EJM, Jansen MK, Bouma RG, Villacorta Hidalgo J, Fisch P, Broekhuizen R, Spliet WGM, Kool M, et al. 2018. Prognostic relevance of tumor-infiltrating lymphocytes and immune checkpoints in pediatric medulloblastoma. Oncoimmunology 7: e1398877. 10.1080/2162402X.2017.1398877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virchow R. 1863. Die krankhaften Geschwuelste. Hirschwald, Berlin, Germany. [Google Scholar]
- Voskuhl RR, Peterson RS, Song B, Ao Y, Morales LB, Tiwari-Woodruff S, Sofroniew MV. 2009. Reactive astrocytes form scar-like perivascular barriers to leukocytes during adaptive immune inflammation of the CNS. J Neurosci 29: 11511–11522. 10.1523/JNEUROSCI.1514-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright DA, Chang AL, Dey M, Balyasnikova IV, Kim CK, Tobias A, Cheng Y, Kim JW, Qiao J, Zhang L, et al. 2014. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PD-L1 in mice with brain tumors. Clin Cancer Res 20: 5290–5301. 10.1158/1078-0432.CCR-14-0514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y-J, Fletcher R, Yu J, Zhang L. 2018. Immunogenic effects of chemotherapy-induced tumor cell death. Genes Dis 5: 194–203. 10.1016/j.gendis.2018.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Kang X, Chen KS, Jehng T, Jones L, Chen J, Huang XF, Chen SY. 2020. An engineered oncolytic virus expressing PD-L1 inhibitors activates tumor neoantigen-specific T cell responses. Nat Commun 11: 1395. 10.1038/s41467-020-15229-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waszak SM, Robinson GW, Gudenas BL, Smith KS, Forget A, Kojic M, Garcia-Lopez J, Hadley J, Hamilton KV, Indersie E, et al. 2020. Germline elongator mutations in Sonic Hedgehog medulloblastoma. Nature 580: 396–401. 10.1038/s41586-020-2164-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins S, Robel S, Kimbrough IF, Robert SM, Ellis-Davies G, Sontheimer H. 2014. Disruption of astrocyte-vascular coupling and the blood–brain barrier by invading glioma cells. Nat Commun 5: 4196. 10.1038/ncomms5196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss N, Miller F, Cazaubon S, Couraud PO. 2009. The blood-brain barrier in brain homeostasis and neurological diseases. Biochim Biophys Acta 1788: 842–857. 10.1016/j.bbamem.2008.10.022 [DOI] [PubMed] [Google Scholar]
- Widodo SS, Dinevska M, Furst LM, Stylli SS, Mantamadiotis T. 2021. IL-10 in glioma. Br J Cancer 125: 1466–1476. 10.1038/s41416-021-01515-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Waxman DJ. 2018. Immunogenic chemotherapy: dose and schedule dependence and combination with immunotherapy. Cancer Lett 419: 210–221. 10.1016/j.canlet.2018.01.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M, Ventura PB, Jiang Y, Rodriguez FJ, Wang L, Perry JSA, Yang Y, Wahl K, Crittenden RB, Bennett ML, et al. 2020. Astrocytic trans-differentiation completes a multicellular paracrine feedback loop required for medulloblastoma tumor growth. Cell 180: 502–520.e19. 10.1016/j.cell.2019.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Kohli K, Black RG, Yao L, Spadinger SM, He Q, Pillarisetty VG, Cranmer LD, Van Tine BA, Yee C, et al. 2019. Systemic interferon-γ increases MHC class I expression and T-cell infiltration in cold tumors: results of a phase 0 clinical trial. Cancer Immunol Res 7: 1237–1243. 10.1158/2326-6066.CIR-18-0940 [DOI] [PMC free article] [PubMed] [Google Scholar]