Abstract
Background and study aims Quality measures were established to develop standards to help assess quality of care, yet variation in endoscopy exists. We performed a systematic review to assess the overall quality of evidence cited in formulating quality measures in endoscopy.
Methods A systematic search was performed on multiple databases from inception until November 15, 2020, to examine the quality measures proposed by all major societies. Quality measures were assessed for their level of quality evidence and categorized as category A (guideline-based), category B (observational studies) or category C (expert opinion). They were also examined for the type of measure (process, structure, outcome), the quality, measurability, review, existing conflicts of interest (COI), and patient participation of the quality measure.
Results An aggregate total of 214 quality measures from nine societies (15 manuscripts) were included and analyzed. Of quality measures in endoscopy, 71.5 %, 23.8 %, and 4.7 % were based on low, moderate, and high quality of evidence, respectively. The proportion of high-quality evidence across societies was significantly different ( P = 0.028). Of quality measures, 76 % were quantifiable, 18 % contained patient-centric outcomes, and 7 % reported outcome measures. None of the organizations reported on patient involvement or external review, six disclosed existing COI, and 40 % were published more than 5 years ago.
Conclusions Quality measures are important to standardize clinical practice. Because over 70 % of quality measures in endoscopy are based on low-quality evidence, further studies are needed to improve the overall quality to effectively set a standard, reduce variation, and improve care in endoscopic practice.
Introduction
Quality measures are used to provide a standardized metric by which the overall quality of healthcare being offered to patients can be assessed 1 . In general, they can be used to assess characteristics of care (structural), the delivery of care (process), or the results of care (outcomes). In particular, these measures can be developed in relation to diagnostics, management, patient prevention, or administration function 2 . While quality measures can be used as a means to identify those providing high-quality care and thus provide a mechanism to reward those for this practice, they can also be used as a means to penalize those who fail to meet the expected standards 3 .
Owing to the variation in colorectal cancer screening recommendations, lower gastrointestinal endoscopy was one of the first areas of endoscopy to directly address quality 2 3 4 5 6 . As such, numerous potential measures of quality in lower endoscopy have been identified, and as a consequence, many professional societies have published recommendations on performance measures 2 3 4 . The united aim was to propose quality and safety procedures and indicators to facilitate quality improvement in digestive endoscopy units 2 3 4 5 . However, these recommendations are country-specific and not always evidence-based, which has limited their wider adoption 2 4 7 . Hence, while the goal of guidelines and position statements are to reduce variation in practice and standards between individual endoscopists and centers, data assessing the quality of evidence supporting quality measures are lacking 1 8 9 10 .
Despite attempts at using quality measures to standardize healthcare, significant variation in clinical practice remains 11 . When evaluating the reasons for non-adherence to guideline recommendations, some report a lack of confidence in guidelines due to the lack of high-quality evidence supporting many of the recommendations 11 . Similarly, the ability for quality metrics to effectuate change in clinical practice, standardize care, and improve the quality of care when they are based on lower-quality evidence is not proven to provide improved patient outcomes in long-term longitudinal studies 1 8 9 10 11 12 13 .
We, therefore, conducted a systematic review of the quality measures in endoscopy proposed by international medicine, oncology, surgical, gastrointestinal, and endoscopy societies to assess the overall quality of evidence and COI cited in formulating these quality measures.
Methods
Search strategy and data
A systematic literature search was performed on the PubMed/MEDLINE, EMBASE, and Web of Science databases using Mesh terms; “endoscopy,” “digestive endoscopy,” “gastrointestinal endoscopy,” “quality standards,” “quality measures,” “quality indicators,” and “quality metrics” in different combinations to generate a comprehensive and up-to-date list of articles on November 15, 2020. In addition, major international medicine, gastrointestinal, and endoscopy society websites were also examined for the presence of endoscopy-specific quality measures. Moreover, in all manuscripts identified, citations were examined for relevant papers. This identified a total of 407 manuscripts. After screening for relevance and excluding studies that: 1) reviewed quality measures; 2) only discussed adherence to quality measures; and/or 3) did not discuss the presence of endoscopy-specific quality measures, 15 manuscripts, totaling nine task forces/groups of societies remained and were included in the final analysis. Manuscripts were not limited by age, date, or language written. All studies were screened by two authors (SW and MB) and any disagreement was resolved by mutual discussion and by consulting a third author (JDF) via a modified Delphi system 14 . The methodological protocol herein was established a priori as we followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) to conduct our systematic review ( Fig. 1 ) 15 16 .
Fig. 1.
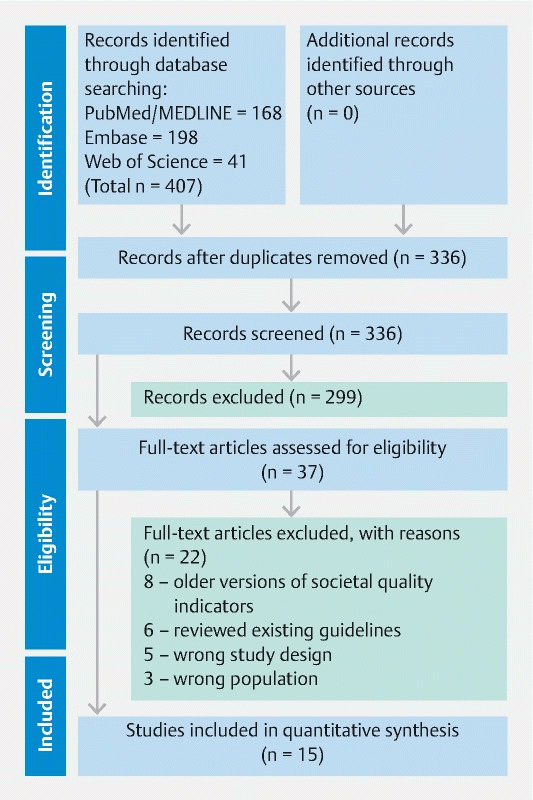
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement diagram delineating the process by which manuscripts were screened and ultimately included.
Quality measures
All quality measures were examined for: 1) the type of measure—structural, process, or outcome related; 2) the grading methods used; 3) the supporting quality of evidence behind the inclusion of the quality measure; 4) whether the quality measure can be numerically measured; 5) if the measure was externally reviewed; 6) if there was inclusion of patients in the development of the measure; 7) if the measure reported the presence of any conflicts of interest (COI); 8) if the measure could impact patient outcomes; and 9) its age from publication.
Levels of evidence
Given the diversity of grading systems internationally, we formulated levels of evidence based on the GRADE and ABC(D) models of level of evidence used in the development of clinical practice guidelines and prior studies assessing guideline quality 1 8 9 10 12 13 .
Category A: High-quality of evidence: Based on clinical guidelines derived from randomized controlled trials
Category B: Moderate-quality of evidence: Based primarily on observational, population-based, or cross-sectional studies
Category C: Low-quality of evidence: Based primarily on expert opinion or small case-series with week evidence or high study heterogeneity.
When evidence was based on prior studies (moderate quality of evidence), these studies were analyzed for the methodology used. If the quality measure had no accompanying primary literature cited, it was subsequently placed in the category of low-quality evidence (i. e. expert opinion), as done in prior guideline quality studies 1 5 .
Review of the quality measures
All quality measures were reviewed by two authors (SW and MB) for determination of the type of measure, the supporting quality of evidence behind the measure, whether in fact it can be measured, if it was externally reviewed, if patients were included in its development, if it reported the presence of any COI, and if the measure could impact patient outcomes. COI that were determined to be relevant included being a part of an advisory board, speaker's bureau, and consulting or industry-sponsored continuing medical education activities (government and non-profit awards were not considered COI). If there was disagreement between the above authors with regards to data extraction, a third author (JDF) reviewed it using a modified Delphi system 14 .
Ethical considerations
Given the publicly available nature of these data, i. e. all recommendations were previously published and patients were not individually included, it is exempt from Institutional Review Board review. In addition, informed consent was not needed as these data were not obtained from study participants.
Data analysis
Quality measures were assessed for evidence quality and categorized as category A (guideline-based), category B (primarily observational/population-based studies), or category C (expert opinion). Statistical analysis was conducted in R using ANOVA, linear regression and chi-square or Kruskal-Wallis tests. A P = 0.05 was considered significant.
Results
Organizations involved and quality measures reported
The following nine (task forces/groups of) societies/organizations quality measures (comprising 15 manuscripts) were included in the final analysis: American Gastroenterological Association (AGA) 17 , American College of Gastroenterology and American Society of Gastrointestinal Endoscopy (ACG-ASGE) 18 19 20 21 22 23 , British Society of Gastroenterology and Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland (BSG-AUGIS) 24 , Canadian Association of Gastroenterology (CAG) 25 , European Society of Gastrointestinal Endoscopy (ESGE) 26 27 , Health Programme of the European Union (HPEU) 28 , National Colorectal Cancer Roundtable (NCCR) 29 , Sociedad Española de Patología Digestiva (SEPD) 30 , and the Spanish Society of Gastroenterology and Spanish Society of Gastrointestinal Endoscopy Working Group (SSG-SSGE) 31 .
A total of 183 distinct and an aggregate total of 214 quality measure recommendations were reviewed and included in this study from the 15 manuscripts as reported by the nine task forces/groups of societies/organizations: AGA reported 7, ACG-ASGE reported 36 quality measures, BSG-AUGIS reported 38, CAG reported 23, ESGE reported 44, HPEU reported 29, NCCR reported 4, SEPD reported 13, and SSG-SSGE reported 20 ( Table 1 ) 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 .
Table 1. Distinct quality measure recommendations listed by level of scientific evidence.
| High-quality evidence | Type | Patient-centric | Quantifiable |
| Exams should be performed only after adequate bowel preparation i. e. without any residual stool or liquid in the lumen that could mask any suspicious area | Process | Yes | Yes |
| Exams should be complete to the caecum and there should be slow, careful inspection of the colonic mucosa during withdrawal of the scope | Process | Yes | Yes |
| Where coeliac disease is suspected, a minimum of four biopsies should be taken, including representative specimens from the second part of the duodenum and at least one from the duodenal bulb | Process | No | Yes |
| Attention should be focused on preventing transmission of highly resistant organisms by duodenoscopes, in particular, on ensuring cleaning and HLD of the elevator mechanism and elevator wire channel | Process | No | No |
| General infection control principles should be complied with in the endoscopy unit | Structural | No | No |
| Use of standard precautions reduces the transmission of infection from patients to endoscopy personnel | Process | Yes | Yes |
| Adenoma detection rate | Outcome | Yes | Yes |
| Adenoma detection rate after positive FOBT | Outcome | No | Yes |
| Appropriate interval between colonoscopies | Process | Yes | Yes |
| Mean number of adenomas excised per colonoscopy procedure | Structural | No | Yes |
| Moderate-quality evidence | |||
| Endoscopy facilities should ensure that the services they provide are patient-centered | Structural | No | No |
| Patients can be divided into low, intermediate and high-risk groups with respect to their risk of developing advanced adenomas and cancer based on findings at baseline colonoscopy. The surveillance strategy can vary accordingly | Process | Yes | Yes |
| A readjustment of the strategy can be made based on findings at the first and subsequent surveillance examinations | Structural | Yes | No |
| Low risk. Patients with only one or two small (< 10 mm) adenomas are at low risk, and should be returned to the screening program | Process | No | No |
| Intermediate risk. Patients with three or four small adenomas or at least one adenoma of size ≥ 10 mm and < 20 mm are at intermediate risk | Process | Yes | Yes |
| Intermediate risk. Patients with three or four small adenomas or at least one adenoma of size ≥ 10 mm and < 20 mm are at intermediate risk and should be offered surveillance at 3-yearly intervals | Process | Yes | Yes |
| High risk. If either of the following is detected at any single examination (at baseline or follow-up): 5 or more adenomas, or an adenoma ≥ 20 mm, the patient is at high risk and an extra examination should be undertaken within 12 months, to check for missed synchronous lesions, before initiating 3-yearly surveillance. In the absence of evidence on the safety of stopping surveillance in the high-risk group, surveillance should continue | Process | Yes | Yes |
| High risk. If either of the following is detected at any single examination (at baseline or follow-up): 5 or more adenomas, or an adenoma ≥ 20 mm, after two consecutive normal exams, the interval can be extended to 5-yearly | Process | Yes | Yes |
| The risk stratification is based on accurate detection and complete removal of adenomas otherwise risk status will be underestimated | Process | No | No |
| Recommendations should not differ for patients with a family history who are found to have adenomas, unless it is suspected that they have one of the dominantly inherited conditions | Structural | No | No |
| New symptoms should be assessed on the basis that a recent clearance colonoscopy reduces the chance of advanced adenomas and cancers but does not eliminate the risk altogether | Process | No | No |
| By their nature locally removed pT1 cancers are high-risk lesions and therefore should undergo a surveillance strategy similar to the high-risk adenoma group | Structural | No | No |
| There is no evidence that patients in whom only small, distally located hyperplastic polyps are detected are at increased risk for colorectal cancer; therefore, they should be offered routine screening | Structural | No | No |
| A safety checklist should be completed before starting an esophagogastroduodenoscopy (EGD) | Process | No | Yes |
| Intravenous sedation and local anesthetic throat spray can be used in conjunction if required. Caution should be exercised in those at risk of aspiration | Process | No | No |
| Adequate mucosal visualization should be achieved by a combination of adequate air insufflation, aspiration and the use of mucosal cleansing techniques | Process | No | No |
| When no lesions are detected within a Barrett’s segment, biopsies should be taken in accordance with the Seattle protocol | Process | No | Yes |
| If squamous neoplasia is suspected, full assessment with enhanced imaging and/or Lugol’s chromo-endoscopy is required | Process | No | Yes |
| Biopsies from two different regions in the esophagus should be taken to rule out eosinophilic esophagitis in those presenting with dysphagia/food bolus obstruction, where an alternate cause is not found | Process | No | Yes |
| Where gastric or duodenal ulcers are identified, H. pylori should be tested and eradicated if positive | Process | No | Yes |
| The presence of gastric polyps should be recorded, with the number, size, location and morphology described, and representative biopsies taken | Process | No | Yes |
| In the event of reprocessing failure, the patient, the institution’s designated infection control personnel, local and/or state public health agencies, the FDA, the CDC, and the manufacturers of the involved equipment should be notified immediately | Structural | No | No |
| Frequency with which endoscopy is performed for an indication that is included in a published standard list of appropriate indications, and the indication is documented (priority indicator) | Process | No | Yes |
| Colonoscopy withdrawal time | Process | No | Yes |
| Cecal intubation rate | Process | No | Yes |
| Decontamination indicators | Structural | No | No |
| We recommend endoscopy services have policies and processes in place to assess the appropriateness of procedures against guidelines and take action when endoscopic procedures have been performed inappropriately | Structural | No | No |
| We recommend endoscopy services have procedures in place to assess the comfort of patients before, during, and after procedures | Structural | No | No |
| We recommend that endoscopy services provide an environment and have processes in place that ensure the privacy and dignity of patients is respected and maintained | Structural | No | No |
| Endoscopes should undergo HLD as recommended by governmental agencies and all pertinent professional organizations for the reprocessing of gastrointestinal endoscopes | Structural | No | Yes |
| The efficacy of manual cleaning and HLD is operator dependent, thus assignment of personnel responsible for endoscope reprocessing, extensive training of reprocessing personnel, process validation, and quality assurance is vital, and staff competency should be assessed at the very least on an annual basis | Structural | No | Yes |
| Low-quality evidence | |||
| For a patient to give a physician informed consent to perform an elective endoscopic procedure, the patient must be advised, in a timely fashion, of all relevant information about the procedure, its risks, benefits and alternatives, if any, and be given an opportunity to ask questions that the physician must answer | Process | No | No |
| Endoscopy facilities should meet or exceed defined operating standards, in all domains, consistent with accreditation under the appropriate national or regional standards | Structural | No | Yes |
| Endoscopic procedures are performed for an appropriate, clearly documented indication, consistent with current, evidence-based guidelines | Process | No | Yes |
| Endoscopy facilities should have the technical and personnel resources required by national and/or regional standards to complete all planned procedures safely and effectively | Structural | No | Yes |
| Endoscopy facilities should implement and monitor the effect of pre-procedure policies that ensure best practice | Structural | No | No |
| Endoscopy facilities should implement and monitor the effect of intraprocedural policies that ensure best practice | Structural | No | No |
| The endoscopy facility should implement and monitor the effects of policies for the discharge of patients that ensure best practice | Structural | Yes | Yes |
| Endoscopy facilities should ensure that there is a policy in place to notify patients of the need, and appropriate interval, for follow-up | Structural | Yes | No |
| All patients, on discharge, are given written information regarding the procedural findings, plans for treatment and follow-up, worrisome symptoms to watch for, and steps to be taken | Process | Yes | Yes |
| Endoscopy facilities should maintain a comprehensive quality improvement program incorporating formal, regular, scheduled review of performance reports | Structural | No | No |
| Endoscopy facilities should appoint a review committee to monitor and report back to management on adherence to and implementation of quality standards | Structural | No | No |
| Endoscopy facilities should systematically and regularly review current indicators of quality for all endoscopic procedures and implement appropriate responses | Structural | No | No |
| Endoscopy facilities should systematically and regularly review current indicators of safety for all endoscopic procedures and implement appropriate responses | Structural | No | No |
| Endoscopy facilities should provide high-quality education programs or opportunities for all staff | Structural | No | No |
| All endoscopy facility personnel in training should be supervised and their performance monitored regularly until they have achieved competency to perform specified routine and/or emergency procedures according to appropriate current standards | Process | No | Yes |
| All endoscopy facility personnel engaged, directly or indirectly, in endoscopy service delivery should be trained and certified as having competency to perform specified routine and/ or emergency procedures according to appropriate current standards | Structural | No | Yes |
| Endoscopists should regularly review their endoscopic practice and outcome data with the aim of continuous professional development | Structural | No | No |
| Endoscopists should be granted privileges to perform specified procedures based on a formal evaluation of their competence consistent with appropriate current standards | Process | No | No |
| Endoscopists’ privileges should be subject to formal, regular, scheduled review to ensure that renewal is based on documented competence to perform specified procedures consistent with appropriate current standards | Process | No | No |
| Endoscopic procedures should be reported in a standardized electronic format, including mandatory reporting fields, to provide full documentation of all necessary clinical and quality measures | Structural | No | No |
| Endoscopy facilities should implement policies to monitor and ensure the timeliness and completeness of procedure reporting | Structural | No | No |
| Endoscopy facilities should systematically and at least annually solicit patient feedback, report the results to the service and to the institution’s quality committee, and implement effective measures to address patients’ concerns | Structural | Yes | No |
| Intermediate risk. Patients with three or four small adenomas or at least one adenoma of size ≥ 10 mm and < 20 mm, after one negative exam, the interval for surveillance can be extended to 5 years. After two consecutive normal exams, the patient can return to routine screening | Process | Yes | Yes |
| High risk. If either of the following is detected at any single examination (at baseline or follow-up): 5 or more adenomas, or an adenoma ≥ 20 mm, in the absence of evidence on the safety of stopping surveillance in the high-risk group, surveillance should continue | Process | No | Yes |
| Patients with a failed colonoscopy should, if possible, undergo repeat colonoscopy or an alternative complete colonic examination, particularly if they are in the high-risk group | Process | Yes | No |
| The site of large sessile lesions removed piecemeal should be re-examined at 2 to 3 months. Small areas of residual tissue can then be treated endoscopically, with a further check for complete eradication within 3 months. India ink tattooing aids recognition of the site of excision at follow-up. If extensive residual lesion is seen, surgical resection must be considered, or alternatively, referral to a colonoscopist with special expertise in advanced endoscopic excision | Process | Yes | No |
| The decision to undertake each colonoscopic surveillance examination should depend not only on adenoma characteristics, but also on the patient's age and wishes, and the presence of significant comorbidity. The patient status should be established prior to attendance for each examination | Structural | No | No |
| The cut-off age for stopping surveillance is usually 75 years, but this should also depend upon patient wishes and comorbidity | Structural | No | No |
| Following cessation of surveillance, individuals should be returned to the population screening program | Structural | No | No |
| The potential benefit of supplementing colonoscopy exams with fecal occult blood testing is presumed to be too small to warrant double testing; therefore, it is recommended to stop fecal occult blood testing in individuals who are undergoing surveillance | Structural | No | No |
| For surveillance purposes, serrated adenomas (traditional serrated adenomas and mixed polyps with at least one adenomatous component) should be dealt with like any other adenoma; there are no data to suggest that different surveillance intervals are required | Structural | No | No |
| One or more large ( ≥ 10 mm) hyperplastic polyps or other non-neoplastic serrated lesions anywhere in the colon or multiple smaller lesions of these types in the proximal colon may confer an increased risk, but there are no data available to indicate appropriate surveillance intervals | Process | No | No |
| Every screening program should have a policy on surveillance. The policy may limit surveillance to the high-risk group if sufficient resources are not available to include people with lower risk | Structural | No | No |
| The responsibility of program management to assure the quality of screening services includes quality assurance of surveillance. For surveillance, the same principles, methods and standards of quality assurance apply that are elucidated elsewhere in the first edition of the European Guidelines | Structural | No | No |
| Adherence to the guidelines should be monitored | Structural | No | No |
| Surveillance histories should be documented and the results should be available for quality assurance | Structural | No | Yes |
| The occurrence of colorectal cancer in any individual in whom adenomas or pT1 cancers have been detected at a previous exam should be captured as an auditable outcome for any surveillance program | Structural | No | No |
| Patients should be assessed for fitness to undergo a diagnostic EGD | Process | No | Yes |
| Patients should receive appropriate information about the procedure before undergoing an EGD | Process | No | No |
| An appropriate time slot should be allocated dependent on procedure indications and patient characteristics | Structural | No | No |
| A checklist should be undertaken after completing an EGD, before the patient leaves the room | Process | No | Yes |
| Only an endoscopist with appropriate training and the relevant competencies should independently perform EGD | Structural | No | No |
| We suggest that endoscopists should aim to perform a minimum of 100 EGDs a year, to maintain a high-quality examination standard | Structural | No | Yes |
| UGI endoscopy should be performed with high-definition video endoscopy systems, with the ability to capture images and take biopsies | Structural | No | No |
| A complete EGD should assess all relevant anatomical landmarks and high-risk stations | Process | No | Yes |
| Photo documentation should be made of relevant anatomical landmarks and any detected lesions | Process | No | Yes |
| The quality of mucosal visualization should be reported | Process | No | Yes |
| It is suggested that the inspection time during a diagnostic EGD should be recorded for surveillance procedures, such as Barrett’s esophagus and gastric atrophy/intestinal metaplasia surveillance | Structural | No | Yes |
| Where a lesion is identified, this should be described using the Paris classification and targeted biopsies taken | Process | No | Yes |
| Endoscopy units should adhere to safe sedation practice | Structural | No | No |
| The length of a Barrett’s segment should be classified according to the Prague classification | Process | No | Yes |
| Where a lesion is identified within a Barrett’s segment, this should be described using the Paris classification and targeted biopsies taken | Process | No | Yes |
| Esophageal ulcers and esophagitis that is grade D or atypical in appearance, should be biopsied, with further evaluation in 6 weeks after PPI therapy | Process | Yes | Yes |
| The presence of an inlet patch should be photo-documented | Structural | No | Yes |
| The presence of a hiatus hernia should be documented and measured | Structural | No | Yes |
| Varices should be described according to a standardized classification | Process | No | Yes |
| Strictures should be biopsied to exclude malignancy before dilatation | Process | No | Yes |
| Gastric ulcers should be biopsied and re-evaluated after appropriate treatment, including H. pylori eradication where indicated, within 6–8 weeks | Process | No | Yes |
| Where there are endoscopic features of gastric atrophy or IM separate biopsies from the gastric antrum and body should be taken | Process | No | Yes |
| Where iron deficiency anemia is being investigated, separate biopsies from the gastric antrum and body should be taken, as well as duodenal specimens if coeliac serology is positive or has not been previously measured | Process | No | No |
| A malignant looking lesion should be described, photo-documented and a minimum of six biopsies taken | Process | Yes | Yes |
| After OGD readmission, mortality and complications should be audited | Structural | No | Yes |
| A report summarizing the endoscopy findings and recommendations should be produced and the key information provided to the patient before discharge | Process | No | Yes |
| A method for ensuring histological results are processed must be in place | Structural | No | No |
| Endoscopy units should audit rates of failing to diagnose cancer at endoscopy up to 3 years before an esophago-gastric cancer is diagnosed | Structural | Yes | Yes |
| Endoscopy units must have a qualified individual who directs their infection prevention plans | Structural | No | No |
| Frequency with which pre-procedure history and directed physical examination are performed and documented | Process | No | Yes |
| Frequency with which risk for adverse events is assessed and documented before sedation is started | Process | No | Yes |
| Frequency with which prophylactic antibiotics are administered only for selected settings in which they are indicated (priority indicator) | Process | No | Yes |
| Frequency with which a sedation plan is documented | Process | No | Yes |
| Frequency with which management of antithrombotic therapy is formulated and documented before the procedure (priority indicator) | Process | No | Yes |
| Frequency with which a team pause is conducted and documented | Process | No | Yes |
| Frequency with which photo documentation is performed | Process | No | Yes |
| Frequency with which patient monitoring among patients receiving sedation is performed and documented | Process | No | Yes |
| Frequency with which the doses and routes of administration of all medications used during the procedure are documented | Process | No | Yes |
| Frequency with which use of reversal agents is documented | Process | No | Yes |
| Frequency with which procedure interruption and premature termination because of oversedation or airway management issues is documented | Process | No | Yes |
| Frequency with which discharge from the endoscopy unit according to predetermined discharge criteria is documented | Process | No | Yes |
| Frequency with which patient instructions are provided | Process | No | Yes |
| Frequency with which the plan for pathology follow-up is specified and documented | Process | No | Yes |
| Frequency with which a complete procedure report is created | Process | No | Yes |
| Frequency with which immediate adverse events requiring interventions are documented | Process | No | Yes |
| Frequency with which immediate adverse events requiring interventions including hospitalization occur | Process | No | Yes |
| Frequency with which delayed adverse events leading to hospitalization or additional procedures or medical interventions occur within 14 days | Process | No | Yes |
| Frequency with which patient satisfaction data are obtained | Process | No | Yes |
| Frequency with which communication with referring providers is documented | Process | No | Yes |
| Endoscopy unit has a defined leadership structure | Structural | No | No |
| Process is in place to track each specific endoscope from storage, use, reprocessing, and back to storage | Structural | No | No |
| 1) Does the colonoscopy report include: a) depth of insertion, b) bowel preparation quality, c) patient tolerance of the procedure, d) description of polyps and whether they were removed or biopsied, e) pathology results for any biopsies, clear recommendations for Follow-up and/or surveillance (whether or not the exam was complete) | Process | Yes | Yes |
| Does the endoscopist have a high enough cecal intubation rate? | Process | No | Yes |
| Does the endoscopist have a high enough adenoma detection rate? | Process | Yes | Yes |
| Is the colonoscopy performed in a safe setting? | Structural | No | No |
| Endoscopist experience | Structural | No | No |
| Waiting time from positive FOBT to colonoscopy | Structural | No | Yes |
| Appropriate bowel cleansing | Process | Yes | Yes |
| Colon perforation rate | Outcome | Yes | Yes |
| Post-polypectomy bleeding rate | Outcome | Yes | Yes |
| Independent screening colonoscopy program | Structural | No | No |
| Record of complications | Structural | No | Yes |
| Endoscopic resection of pedunculated polyps and sessile/flat polyps | Outcome | Yes | Yes |
| Retrieval rate of resected polyps | Outcome | Yes | Yes |
| Colonoscopy complications due to lack of previous assessment | Outcome | No | Yes |
| Time slot allotted for colonoscopy | Structural | No | Yes |
| Polyp detection rate (PDR) | Outcome | Yes | Yes |
| Appropriate polypectomy technique | Process | No | No |
| Advanced imaging assessment | Process | No | No |
| Complication rate | Outcome | No | Yes |
| Patient experience | Outcome | Yes | Yes |
| Appropriate post-polypectomy surveillance recommendations | Process | Yes | Yes |
| We recommend endoscopy services be organized to acquire the necessary resources to deliver the service and to maximize utilization of these resources while maintaining high patient satisfaction, quality, and safety | Structural | No | No |
| We recommend that the endoscopy service carry out an assessment of the facilities and equipment required to deliver the service at least annually | Structural | No | No |
| We recommend that the endoscopy service has a planned program of inspection, calibration, and maintenance of its clinical equipment according to the manufacturers’ advice and relevant national regulations | Structural | No | Yes |
| We recommend that the endoscopy service has a plan to address shortfalls, plus replacement and purchase of facilities and equipment | Structural | No | No |
| We recommend that decontamination facilities, equipment, and processes meet national and/or European standards | Structural | No | No |
| We recommend key performance indicators are fed back to and discussed with endoscopists on a regular basis, and that corrective action for improvement, when indicated, with objectives are agreed with the individuals | Structural | No | No |
| We recommend that the endoscopy service ensures that, if corrective actions for improvement have been ineffective, new actions are agreed and implemented, and/or that the host organization quality and risk committee is informed of the continued underperformance | Structural | No | No |
| We recommend that it is made clear which diagnostic and therapeutic procedures endoscopists are competent in and allowed to perform in the service | Structural | No | No |
| We recommend endoscopy services identify potential risks to patients and staff and implement policies and procedures to mitigate them | Process | No | No |
| We recommend endoscopy services perform a root cause analysis of major events, such as missed cancers, unplanned admissions, and unexpected deaths following endoscopic procedures, and use the learning from the analysis to improve the service | Structural | No | No |
| We recommend endoscopy services have available, in written and electronic form, referral guidelines for all endoscopic procedures performed within the service that are based on regional and/or national guidelines | Structural | No | No |
| We recommend endoscopy services provide patients, prior to leaving the service, with the results of the procedure, the timing and mode of communication of pathology results, a plan of the next steps, and an explanation of what delayed complications can occur and what to do about them | Structural | No | No |
| We recommend information on comfort is reviewed and fed back to endoscopists and staff and, where appropriate, action is taken to improve patient comfort levels | Structural | No | No |
| We recommend that the endoscopy service undertakes regular reviews of staffing in relation to activity to identify gaps, and to improve the match between the skills of staff and the work undertaken | Process | No | No |
| We recommend that all new staff (including new endoscopists) undertake an induction and orientation program before working in the service | Process | No | No |
| We recommend the endoscopy service has methods in place to motivate staff to improve the service | Structural | No | No |
| We recommend there is a process for confidential reporting, with action being taken for abuse of endoscopy staff by patients or other staff, including endoscopists, in line with institutional policies | Process | No | No |
| We recommend the endoscopy service gathers patient feedback at least annually | Process | No | No |
| We recommend there is a process for reviewing patient complaints and suggestions | Process | No | No |
| We recommend that the service acts on both formal and informal feedback from patients to improve the service and to demonstrate it has addressed concerns when these are raised | Process | No | No |
| Complete colonoscopy | Process | Yes | Yes |
| Excision of all polyps smaller than 20 mm | Outcome | Yes | Yes |
| All polyps smaller than 20 mm excised in one fragment | Process | Yes | Yes |
| Biopsy sample collection in patients with chronic diarrhea | Process | No | Yes |
| Number and distribution of biopsies in patients with inflammatory bowel disease (IBD) | Process | No | Yes |
| Incidence of interval cancer after colonoscopy | Outcome | Yes | Yes |
| Barrett’s inspection time | Process | No | Yes |
| Neoplasia detection rate (NDR) – Barrett's | Process | No | Yes |
| Adequate number of biopsies | Process | No | Yes |
| Rates of H pylori testing and eradication | Process | No | Yes |
| Number of biopsies taken for suspected celiac disease | Process | No | Yes |
| Proximal and distal esophageal biopsies to evaluate for eosinophilic esophagitis | Process | No | Yes |
HLD, hypersensitivity lung disease; FOBT, fecal occult blood test; EGD, esophagogastroduodenoscopy; FDA, Food and Drug Administration; CDC, Centers for Disease Control and Prevention; UGI, upper gastrointestinal; PPI, proton pump inhibitor; PDR, poly detection rate; IBD, inflammatory bowel disease; NDR, neoplasia detection rate.
Types of measures
Fifty-three percent of quality measures reported process measures, 40 % reported structure measures, and 7 % reported outcome measures. The AGA only reported process-based measures. Sixty-nine percent of ACG-ASGE quality measures were process measures and 31 % were structure measures. Sixty-eight percent of BSG-AUGIS quality measures were process measures and 32 % were structure measures. 26 % of CAG quality measures were process measures and 74 % were structure measures. Forty-five percent of ESGE quality measures were process measures, 45 % were structure measures, and 10 % were outcome measures. 52 % of HPEU quality measures were process measures and 48 % were structure measures. Seventy-five percent of NCCR quality measures were process measures and 25 % were structure measures. Sixty-nine percent of SEPD quality measures were process measures, 8 % were structure measures, and 23 % were outcome measures. Fifteen percent of SSG-SSGE quality measures were process measures, 50 % were structure measures, and 35 % were outcome measures 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 .
Grading method used
Four organizations (BSG-AUGIS, CAG, ESGE, and SEPD) used the Grading of Recommendation Assessment Development and Evaluation (GRADE) system, one (SSG-SSGE) used the Center for Evidence Based Medicine (CEBM) from Oxford method, and four (AGA, ACG-ASGE, HPEU and NCCR) did not use a strict methodology or created their own methodology to formulate/provide evidence regarding their quality measures 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 .
Quality of levels of evidence
An aggregate total of 214 quality measure recommendations were analyzed for their quality of level of evidence. Of quality measures, 4.7 % (10) were category A, 23.8 % (51) were category B, and 71.5 % (153) were category C. Of these, there was disagreement among the two data extracting authors (SW and MB) regarding the level of evidence pertaining to 27 quality measures—of which 19 were resolved via mutual discussion, and the remaining eight by a third author (JDF). The breakdown by society on the quality of level of evidence is shown in Fig. 2 . The proportion of high-quality evidence across societies was significantly different ( P = 0.028) 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 .
Fig. 2.
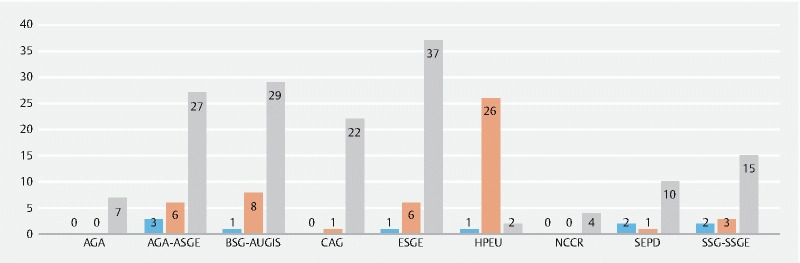
Clustered column chart depicting the graded evidence quality by society/organization. Blue represents high-quality evidence, orange represents moderate-quality evidence, and gray represents low-quality evidence.
Measurability
Fifty-seven percent of recommendations reported measurable/quantifiable outcomes and 43 % reported non-quantifiable outcomes. Ninety percent of category A quality measures recommendations were quantifiable, 75 % of category B quality measures were quantifiable, and 54 % of category C quality measures were quantifiable. Seventy-five percent of ASGE quality measures, 76 % of BSG-AUGIS, 30 % of CAG, 32 % of ESGE, 31 % of HPEU, 75 % of NCCR, 100 % of SEPD, and 65 % of SSG-SSGE quality measures were quantifiable, respectively 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 .
External review, patient participation, COI, and evidence cited
None of the nine organizations reported external review of their quality measures or included patients in the development of their quality measures. Six organizations (AGA, ACG-ASGE, CAG, ESGE, HPEU, and NCCR) reported the presence of a COI when it existed, whereas the remaining three did not. Five organizations (ACG-ASGE, ESGE, HPEU, SEPD and SSG-SSGE) cited evidence behind the grade assigned for their quality measures, whereas the remaining four did not 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 .
Effect on patient outcomes
Only 18 % of all quality measures were directed toward improving patient outcomes. None of AGA quality measures were directed toward improving patient outcomes. Three percent of ACG-ASGE, 8 % of BSG-AUGIS, 17 % of CAG, 11 % of ESGE, 38 % of HPEU, 50 % of NCCR, 46 % of SEPD, and 30 % of SSG-SSGE quality measures were patient outcome centric, respectively. Among process and outcome-based quality measures, 19 % and 78 % led to patient outcomes, respectively. Only 6 % of structure-based quality measures were directed toward patient outcomes 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 .
Age of publication
Forty percent of quality metrics were published more than 5 years ago (between 2010 and 2015), and 73 % were published more than 3 years ago (between 2010 and 2017). There were no significant associations between publication year and evidence quality ( P = 0.17). The distribution of evidence quality by publication year is represented in Fig. 3 .
Fig. 3.
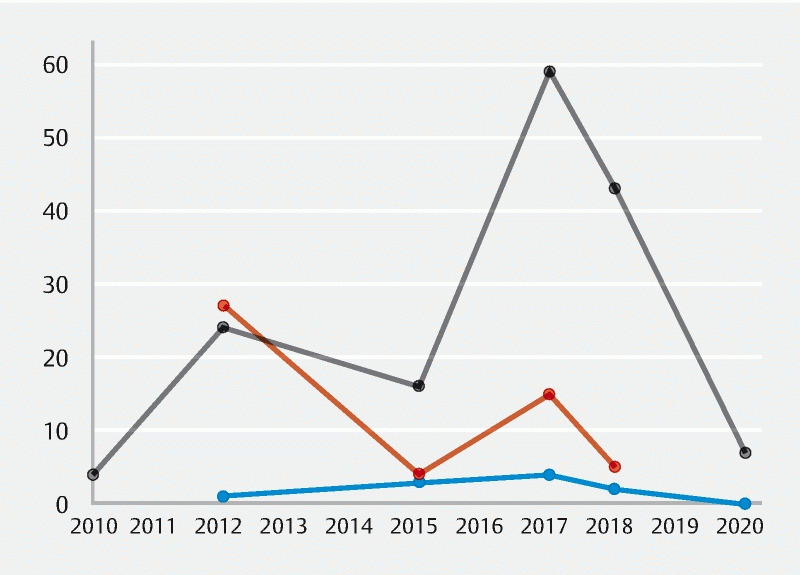
Scatter plot diagram depicting the graded evidence quality by the year the measure was published. Blue represents high-quality evidence, orange represents moderate-quality evidence, and gray represents low-quality evidence.
Discussion
Our study indicated that most (71.5 %) of the current quality measures in endoscopy are based on lower-quality levels of evidence. Additionally, only about half (57 %) of quality measure recommendations reported quantifiable outcomes, less than 10 % reported outcome measures, and less than 20 % of all quality measures were directed toward improving patient outcomes. Furthermore, many organizations did not include patients in the development of their quality measures, report on an external review of the guideline, include a strict grading system methodology, or report on the presence of existing COI. Finally, 40 % of quality metrics were published more than 5 years ago and 73 % more than 3 years ago.
With the publication of the Institution of Medicine (IOM) reports regarding quality of care in medicine, over the last decade, there has been a transformation toward a new found focus on the standardization of healthcare across different settings, including gastrointestinal endoscopy 32 33 . Significant efforts have been made by different national and international societies to regulate quality measures for endoscopy units and physicians performing endoscopy 2 3 4 5 6 7 . The notion behind these efforts is to provide practitioners with a standard (benchmark) to track and compare actual performance. Despite these efforts, there is a significant disparity noted between the actual recommendations and the evidence behind these judgments as evident from our systematic review. One of the biggest challenges encountered is the fact that more than 70 % of these metrics is centered around low-quality evidence.
Low-quality evidence creates substantial variation in the actual delivery of healthcare. These measures are based either on expert opinion or small studies with considerable heterogeneity. Given there is less science supporting these measures or metrics, practitioners may opt to discard these recommendations and instead opt for personal judgment and anecdotal evidence to cater the need for their patients. While the existing studies that led to formulation of these low-quality evidence cannot be changed, there is certainly a need to undertake high-quality studies that will allow societies to strengthen these measures. In this vein, the authors understand that it is the role of a society to cover all aspects of a technique or procedure, even if some aspect has not been adequately evaluated with high-level of evidence. Thus, while we call for higher-quality studies, the authors congratulate the various international societies for their work, as it is indeed challenging to produce a quality measure when there is a low-level of evidence.
Another area that raises concern is the lack of strict grading methods when formulating these measures and guidelines. Four major societies did not employ a standard grading methodology. In addition, three societies did not report on COI. None of the societies included patients while formulating these recommendations. While not specifically developed for quality metrics, societies should still follow IOM standards similar to guideline development. The development process should be set a priori with a clear and transparent process that includes a standard methodology for grading evidence, reporting of all COI and how they will be handled, a process for external review of the manuscript, including a patient representative in the guideline panel, and report only quantifiable outcomes that are patient-centric 34 . The absence of current COI information among multiple guidelines is also notable. While the Institute of Medicine recommends that guideline panels should attempt to minimize COI, the disclosure of COI of panel members’ is crucial to mitigate any potential undue industry influence and improve transparency.
Ideally quality metrics can be used to implement benchmarks as quality measures to enhances the performance of endoscopy and specifically to improve patient outcomes. The successful implementation should result in improved efficiency, reliability, and cost-effectiveness in the endoscopy unit. Quality measures should be mandated in some form to standardize care delivered to patients. The National Quality Forum (NQF), a public-private organization created in 1999, in response to the Advisory Commission on Consumer Protection and Quality in the Healthcare Industry, advises Centers for Medicare & Medicaid Services on the selection of performance measures for federal health programs 35 . The agency maintains a database of quality measures and indicators for many procedures. Currently, no endoscopy related quality measures have been endorsed by the NQF, primarily due to the absence of high-quality evidence of improved outcomes. Measurement of some of the outcomes of endoscopy is inherently challenging for many reasons. Some of the outcomes may not become apparent for a long period of time (development of malignancy after adenoma detection) or may be dependent on patient characteristics (comorbidity, adherence and socioeconomic factors) and disease severity which may not be amenable to risk adjustment. In the absence of direct clinical outcomes, surrogate markers (e. g. adenoma detection rate, withdrawal time, cecal intubation rate, and surveillance intervals) have sometimes been utilized to reflect the quality of care as process-based or structural measures. But in the absence of high-quality evidence, the use of such surrogate measures remains subject to bias 36 .
The main limitation of the current systematic review is the significant variability in the reporting of these quality measures. Significant heterogeneity was observed in terms of both quality and quantity of metrics. Given the lack of uniformity in reporting outcomes i. e., some societies used standardized tools (such as GRADE and CEBM method), to attenuate this we provided a uniform perspective by using the pre-defined A, B, and C category system. Also, a few of the measures reported by societies were published 5 or more years ago. The authors acknowledge that age alone is not a fundamental limitation to guideline adherence so long as the evidence base is strong and the guideline panel has a method to provide up-to-date recommendations as new evidence emerges. Finally, guidelines and quality measures are also promoted by payors and regulatory groups, however, while these used to be publicly reported, there has been a shift toward societies increasingly recommending them and thus were not included to reflect clinician/provider-available data. Despite these, the major strengths of our systematic review were the inclusion of numerous data points, comparing nine distinct society/organizations, and encompassing an aggregate total of 214 quality measures. Outcome measures are the foundation of credible structural and process-based measures, and our study underscores the significance of outcomes-based research in quality measures in endoscopy.
In summary, majority of quality measures ( > 70 %) in endoscopy are based on low-quality evidence with significant heterogeneity observed in reporting from different societies/organizations. While there should be appreciation for the respective quality measures and as such we congratulate the numerous societies to make recommendations especially when data is scarce; our data calls for a need of high-quality studies examining patient-centered quality measures, the application of a standardized reporting method (such as GRADE), regular update of guidelines (based on newer evidence), as well as a strict adherence to protocol (COI disclosure, patient participation, etc.) for reporting quality measures in gastrointestinal endoscopy. We also suggest that societies focus primarily on the important metrics, namely—those that will be patient-centric and outcome driven—to best simplify the take-home measures that clinicians and endoscopy centers should strive to comply with.
Footnotes
Competing interests The authors declare that they have no conflict of interest.
References
- 1.Weissman S, Goldowsky A, Mehta T I et al. Are quality metrics in inflammatory bowel disease rooted on substantial quality evidence? a systematic review. J Crohns Colitis. 2020;16:336–354. doi: 10.1093/ecco-jcc/jjaa123. [DOI] [PubMed] [Google Scholar]
- 2.Gurudu S R, Ramirez F C. Quality metrics in endoscopy. Gastroenterol Hepatol. 2013;9:228–233. [PMC free article] [PubMed] [Google Scholar]
- 3.Vadlamudi C, Brethauer S. Quality in endoscopy. Surg Clin North Am. 2020;100:1021–1047. doi: 10.1016/j.suc.2020.08.008. [DOI] [PubMed] [Google Scholar]
- 4.Minoli G, Meucci G, Prada A et al. Quality assurance and colonoscopy. Endoscopy. 1999;31:522–527. doi: 10.1055/s-1999-54. [DOI] [PubMed] [Google Scholar]
- 5.Ball J E, Osbourne J, Jowett S et al. Quality improvement programme to achieve acceptable colonoscopy completion rates: prospective before and after study. BMJ. 2004;329:665–667. doi: 10.1136/bmj.329.7467.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rex D K, Bond J H, Winawer S et al. Quality in the technical performance of colonoscopy and the continuous quality improvement process for colonoscopy: recommendations of the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2002;97:1296–1308. doi: 10.1111/j.1572-0241.2002.05812.x. [DOI] [PubMed] [Google Scholar]
- 7.Rex D K, Petrini J L, Baron T H et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2006;101:873–885. doi: 10.1111/j.1572-0241.2006.00673.x. [DOI] [PubMed] [Google Scholar]
- 8.Feuerstein J D, Akbari M, Gifford A E et al. Systematic review: the quality of the scientific evidence and conflicts of interest in international inflammatory bowel disease practice guidelines. Aliment Pharmacol Ther. 2013;37:937–946. doi: 10.1111/apt.12290. [DOI] [PubMed] [Google Scholar]
- 9.Sardar P, Giri J, Jaff M R et al. Strength of evidence underlying the american heart association/american college of cardiology guidelines on endovascular and surgical treatment of peripheral vascular disease. Circ Cardiovasc Interv. 2019;12:1–8. doi: 10.1161/CIRCINTERVENTIONS.118.007244. [DOI] [PubMed] [Google Scholar]
- 10.Duarte-Garcia A, Zamore R, Wong J B. The evidence basis for the American College of Rheumatology Practice Guidelines. JAMA Intern Med. 2018;178:146–148. doi: 10.1001/jamainternmed.2017.6680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wennberg J E. Unwarranted variations in healthcare delivery: implications for academic medical centres. BMJ. 2002;325:961–964. doi: 10.1136/bmj.325.7370.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vanclooster A, Cassiman D, Steenbergen W V et al. The quality of hereditary haemochromatosis guidelines : A comparative analysis. Clin Res Hepatol Gastroenterol. 2015;39:205–214. doi: 10.1016/j.clinre.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Brito J P, Domecq J P, Murad M H et al. The Endocrine Society Guidelines: when the confidence cart goes before the evidence horse. J Clin Endocrinol Metab. 2013;98:3246–3252. doi: 10.1210/jc.2013-1814. [DOI] [PubMed] [Google Scholar]
- 14.Eubank B H, Mohtadi N G, Lafave M R. Using the modified Delphi method to establish clinical consensus for the diagnosis and treatment of patients with rotator cuff pathology. BMC Med Res Methodol. 2016;16:56. doi: 10.1186/s12874-016-0165-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009 doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shea B J, Reeves B C, Wells G et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma P, Parasa S, Shaheen N. Developing quality metrics for upper endoscopy. gastroenterology. 2020;158:9–13. doi: 10.1053/j.gastro.2019.09.048. [DOI] [PubMed] [Google Scholar]
- 18.Rex D K, Schoenfeld P S, Cohen J et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2015;110:72–90. doi: 10.1038/ajg.2014.385. [DOI] [PubMed] [Google Scholar]
- 19.Park W G, Shaheen N J, Cohen J et al. Quality Indicators for EGD. Am J Gastroenterol. 2015;110:60–71. doi: 10.1038/ajg.2014.384. [DOI] [PubMed] [Google Scholar]
- 20.Rizk M K, Sawhney M S, Cohen J et al. Quality indicators common to all GI endoscopic procedures. Am J Gastroenterol. 2015;110:48–59. doi: 10.1038/ajg.2014.383. [DOI] [PubMed] [Google Scholar]
- 21.Calderwood A H, Day L W, Muthusamy V R et al. ASGE guideline for infection control during GI endoscopy. Gastrointest Endosc. 2018;87:1167–1179. doi: 10.1016/j.gie.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 22.Day L W, Cohen J, Greenwald D et al. Quality indicators for gastrointestinal endoscopy units. VideoGIE. 2017;26:119–140. doi: 10.1016/j.vgie.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park W G, Shaheen N J, Cohen J et al. Quality indicators for EGD. Gastrointest Endosc. 2015;81:17–30. doi: 10.1016/j.gie.2014.07.057. [DOI] [PubMed] [Google Scholar]
- 24.Beg S, Ragunath K, Wyman A et al. Quality standards in upper gastrointestinal endoscopy: a position statement of the British Society of Gastroenterology (BSG) and Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland (AUGIS) Gut. 2017;66:1886–1899. doi: 10.1136/gutjnl-2017-314109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Armstrong D, Barkun A, Bridges R et al. Canadian Association of Gastroenterology consensus guidelines on safety and quality indicators in endoscopy. Can J Gastroenterol. 2012;26:17–31. doi: 10.1155/2012/173739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaminski M F, Thomas-Gibson S, Bugajski M et al. Performance measures for lower gastrointestinal endoscopy: a European Society of Gastrointestinal Endoscopy (ESGE) Quality Improvement Initiative. Endoscopy. 2017;49:378–397. doi: 10.1055/s-0043-103411. [DOI] [PubMed] [Google Scholar]
- 27.Valori R, Cortas G, de Lange T et al. Performance measures for endoscopy services: a European Society of Gastrointestinal Endoscopy (ESGE) Quality Improvement Initiative. Endoscopy. 2018;50:1186–1204. doi: 10.1055/a-0755-7515. [DOI] [PubMed] [Google Scholar]
- 28.Atkin W S, Valori R, Kuipers E J et al. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First Edition-Colonoscopic surveillance following adenoma removal. Endoscopy. 2012;44:SE151–SE163. doi: 10.1055/s-0032-1309821. [DOI] [PubMed] [Google Scholar]
- 29.Fletcher R H, Nadel M R, Allen J I et al. The quality of colonoscopy services--responsibilities of referring clinicians: a consensus statement of the Quality Assurance Task Group, National Colorectal Cancer Roundtable. J Gen Intern Med. 2010;25:1230–1234. doi: 10.1007/s11606-010-1446-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grupo de Trabajo de “Indicadores de calidad en endoscopia” de la Sociedad Española de Patología Digestiva (SEPD) . Quality indicators in colonoscopy. The colonoscopy procedure. Rev Esp Enferm Dig. 2018;110:316–326. doi: 10.17235/reed.2018.5408/2017. [DOI] [PubMed] [Google Scholar]
- 31.Jover R, Herráiz M, Alarcón O et al. Clinical practice guidelines: quality of colonoscopy in colorectal cancer screening. Endoscopy. 2012;44:444–451. doi: 10.1055/s-0032-1306690. [DOI] [PubMed] [Google Scholar]
- 32.Chassin M R, Galvin R W. The urgent need to improve health care quality. Institute of Medicine National Roundtable on Health Care Quality. JAMA. 1998;280:1000–1005. doi: 10.1001/jama.280.11.1000. [DOI] [PubMed] [Google Scholar]
- 33.Institute of Medicine (US) Committee on Quality of Health Care in America . Washington (DC): National Academies Press (US); 2001. Crossing the Quality Chasm: A New Health System for the 21st Century. [PubMed] [Google Scholar]
- 34.Guyatt G H, Oxman A D, Vist G E et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lo B, Field M J, editors. Institute of Medicine (US) Committee on Conflict of Interest in Medical Research, Education, and Practice . Washington (DC): National Academies Press (US); 2009. Conflict of Interest in Medical Research, Education, and Practice. Conflicts of Interest and Development of Clinical Practice Guidelines. https://www.ncbi.nlm.nih.gov/books/NBK22928/ [PubMed]
- 36.Panzer R J, Gitomer R S, Greene W H et al. increasing demands for quality measurement. JAMA. 2013;310:1971–1980. doi: 10.1001/jama.2013.282047. [DOI] [PubMed] [Google Scholar]


