Abstract
Objective:
Traumatic brain injury (TBI) is associated with elevated rates of cardiovascular disease (CVD), and both CVD and TBI are risk factors for dementia. We investigated whether CVD and its risk factors underlie the association between TBI and dementia.
Materials and Methods:
Cox proportional hazards models among 195,416 Veterans Health Administration patients age 55+ with TBI and a no-TBI, age/sex/race-matched comparison sample (97,708 per group).
Results:
Veterans +TBI were more likely to have any CVD diagnosis (24% vs 36% p=<0.001) or risk factor (83 vs. 90% p<0.001) compared to -TBI. During follow-up (mean ~7 years), 12.0% of Veterans with TBI only (HR: 2.17 95% CI 2.09–2.25), and 10.3% with CVD only developed dementia (HR 1.21 95% CI 1.15–1.28), compared to 6.5% with neither. There was an additive association between TBI and CVD on dementia risk (HR 2.51, 95% CI 2.41–2.61). Among those +TBI (+/−CVD), risk was minimally attenuated by adjustment for CVD/CVD risk factors (unadjusted HR: 2.38, 95% CI: 2.31–2.45; adjusted HR: 2.17, 95% CI 2.10–2.23).
Conclusions:
Older veterans with TBI have increased prevalence of CVD/CVD risk factors. TBI and CVD had an additive statistical association, with dementia risk increased by ~2.5-fold. However, CVD accounted for little of the association between TBI and dementia. More research is needed to understand mechanisms of TBI-dementia and inform clinical guidelines post-TBI.
Keywords: traumatic brain injury, dementia, cardiovascular disease, veterans
INTRODUCTION
Traumatic brain injury (TBI) is common and debilitating [1, 2], and is associated with several adverse outcomes, particularly among older adults [3, 4]. Of particular public health significance, TBI, including mild TBI [5], is a risk factor for dementia [6–12]. However, the etiology and mechanisms underlying the relationship between TBI and dementia risk are largely unknown. One possible link between TBI and increased risk for dementia is cardiovascular disease (CVD) as individuals with history of TBI have a higher burden of CVD [13–15], and CVD is a well-documented risk factor for dementia [16–18].
However, the relationship between TBI and CVD is not yet well-understood. Older adults who experience a TBI have high rates of preexisting CVD and CVD risk factors [15], which may increase TBI risk through vulnerability to falls [19]. TBI may also increase risk for, or even cause, CVD: TBI exposure has been shown to increases risk for subsequent CVD compared to individuals without TBI [14], and vascular damage is a commonly-reported outcome of TBI due to molecular changes causing chronic inflammation and damage to the blood brain barrier [13, 20].
Although CVD is an established risk factor for cognitive decline and dementia [16–18], it is unknown how CVD and TBI together may contribute to risk of dementia. CVD may explain the association between TBI and dementia; exacerbate the effects of TBI, including dementia risk; or could have an effect on dementia risk independent from TBI (i.e., additive effect). In addition to addressing possible mechanisms linking TBI to dementia, understanding how TBI and CVD together increase risk for dementia has important implications for the clinical management of patients with TBI.
Veterans are a group at high risk for TBI and may be particularly vulnerable to developing dementia [21]. Therefore, our objective was to study a large, diverse, nationally representative cohort of older veterans to investigate whether CVD explains the association between TBI and dementia or whether they have additive or synergistic effects.
METHODS
Standard Protocol Approvals
All study procedures were approved by institutional review boards at the University of California, San Francisco, San Francisco Veterans Affairs Medical Center, and US Army Medical Research and Materiel Command Human Research Protection Office. Informed consent was waived because of the use of deidentified archival data.
Study Population
We sourced data from two nationwide Veterans Health Administration (VHA) system databases: the inpatient and outpatient visits database (National Patient Care Database [NPCD]) and the Vital Status File. Using these databases, we identified all VHA patients 55 years of age or older who received a TBI diagnosis between October 1, 2002 and September 30, 2019. TBI was defined using the Defense and Veterans Brain Injury Center list of International Classification of Disease, Ninth and Tenth Revisions (ICD-9 and 10) Codes for TBI surveillance coded in inpatient or outpatient visits.
To identify a comparison sample of veterans without TBI, we first selected all veterans aged 55 years and older evaluated at VHA facilities during the study period. We excluded veterans with prevalent dementia during the 2-year baseline period (defined as 2 years prior to TBI diagnosis or a randomly selected date within the study period for Veterans without TBI) and those with less than one year of follow-up. We then performed 1:1 matching based on age, sex, and race (white vs. non-white) resulting in 97,708 veterans with TBI and 97,708 veterans without TBI. Dementia was defined using the VA Dementia Steering Committee’s recommended list of ICD-9 and 10 codes (2016 version)[22] or a prescription for dementia medication (donepezil, memantine, rivastigmine, galantamine).
Demographic information (age, sex, race/ethnicity) was collected from VHA inpatient or outpatient files. Zip codes and 2012 US Census data were used to categorize veterans’ residences into educational and income categories (for education, 25% or less of the adult population has a bachelor’s degree or higher vs. more than 25%; income was categorized into median income tertiles). Post-traumatic stress disorder (PTSD), depression, and cardiovascular risk factors (diabetes mellitus, obesity/overweight, current tobacco use, hypertension, and hypercholesteremia) were defined using ICD-9 and 10 codes assessed during the 2-year period prior to the TBI diagnosis or random selection date. Cardiovascular disease (CVD) was defined as having an ICD 9 or 10 code for any of the following: heart failure, atrial fibrillation, stroke/transient ischemic attack or coronary artery disease (using the VA Informatics and Computing Infrastructure [VINCI] phenotype library definition of myocardial infarction, cardiac arrest, coronary arteriosclerosis, or coronary artery bypass grafting procedure codes).
Baseline characteristics of the age, sex, and race matched veterans with and without TBI were compared using t tests for continuous variables and chi square tests for categorical variables. We used Cox proportional hazard regression models to determine whether TBI was associated with greater risk of dementia with censoring at the date of the last medical encounter and age as the timescale. Models were unadjusted and then adjusted for confounding factors selected a priori in steps for 1) education, depression and PTSD; and 2) education, depression, PTSD, any cardiovascular risk factor, and any CVD diagnosis. We also completed a sensitivity analysis additionally adjusting for incident CVD risk factors and diagnoses occurring during follow-up. We also repeated our analyses using Fine-Grey proportional hazards models to account for the competing risk of death. Results are reported as hazard ratios (HR) with 95% confidence intervals (CI). We separately tested for the presence of an interaction between TBI and any CVD on risk of dementia. Additionally, we examined whether TBI and any CVD only, or TBI and any CVD in combination, were associated with greater risk of dementia. Cumulative incidence of dementia as a function of TBI and CVD diagnoses was examined graphically.
Standard statistical and graphical techniques were used to assess proportional hazards assumptions for all final models. Statistical significance was set at p<.05 (two-sided). SAS version 9.4 and STATA/MP version 16.1 were used for all analyses. The data are derived from VHA electronic health records and contain protected health information; therefore, the data cannot be placed in a public repository. Please contact the authors for additional details regarding the process of accessing these data.
RESULTS
Veterans had a mean age of 67 years (SD 9.31) at baseline; 6% were female and 80% were white. Although we included veterans with TBIs across the severity spectrum, approximately 80% of participants had injuries categorized as mild. Baseline characteristics of veterans with and without TBI are shown in Table 1. Veterans with TBI were much more likely than those without TBI to have any CVD diagnosis (36% vs 24%, p<0.001) or any cardiovascular risk factors (90% vs 83%). Thirty-two percent of veterans with TBI had a diagnosis of diabetes mellitus compared to 28% without TBI, and 78% with TBI had a diagnosis of hypertension compared to 70% without TBI (p<0.001 for both). Additionally, 22% of veterans with TBI compared to 15% of veterans without TBI currently used tobacco at baseline, and 21% of veterans with TBI vs. 17% of those without were categorized as overweight or obese (p<0.001 for both). Veterans with TBI were also almost twice as likely than those without TBI to have depression (29% vs. 15%%, p<0.001) and PTSD (19% vs. 10%). Moreover, education and income differed between veterans with and without TBI, such that those with TBI were slightly better educated and more likely to live in less wealthy ZIP codes compared to those without TBI.
Table 1.
Baseline characteristics according to traumatic brain injury (TBI) status among older veterans
| No TBI (n=97,708) |
Any TBI (n=97,708) |
P | |
|---|---|---|---|
|
| |||
| Demographic | |||
| Age, y, mean (SD) | 66.91 (9.3) | 66.91 (9.3) | -- |
| Female, n (%) | 5,690 (5.8) | 5,690 (5.8) | -- |
| White | 77,372 (79.2) | 77,372 (79.2) | -- |
| >25% college-educated zip code | 46,034 (47.1) | 46,953 (48.1) | <.001 |
| Low median income tertile | 32,349 (33.1) | 33,107 (33.9) | <.001 |
| Any CVD Risk Factor | 81,553 (83.5) | 87,921 (90.0) | <.001 |
| Current tobacco use | 14,334 (14.7) | 21,320 (21.8) | <.001 |
| Diabetes mellitus | 26,993 (27.6) | 30,744 (31.5) | <.001 |
| Obesity/overweight | 16,087 (16.5) | 20,012 (20.5) | <.001 |
| Hypertension | 68,772 (70.4) | 76,528 (78.3) | <.001 |
| Hypercholesterolemia | 59,744 (61.2) | 64,044 (65.6) | <.001 |
| Any CVD Diagnosis | 23,184 (23.7) | 34,794 (35.6) | <.001 |
| Coronary artery disease (CAD) | 15,598 (16.0) | 19,275 (19.7) | <.001 |
| Heart Failure | 4,252 (4.4) | 8,049 (8.2) | <.001 |
| Atrial Fibrillation | 4,821 (4.9) | 9,135 (9.4) | <.001 |
| Stroke/transient ischemic attack | 5,634 (5.8) | 15,816 (16.2) | <.001 |
| Psychiatric | |||
| Depression | 14,311 (14.7) | 28,273 (28.9) | <.001 |
| Post-traumatic stress disorder | 9,232 (9.5) | 18,756 (19.2) | <.001 |
SD, standard deviation, CVD, cardiovascular disease
Overall, 10.8% of veterans developed a dementia diagnosis over follow-up (mean 6.6 years, range 1–18 years) with veterans with TBI developing dementia at a higher rate (14.3%) compared to those without TBI (7.4%). The unadjusted risk of dementia was almost two and a half times as high for veterans with TBI compared to those without TBI (HR: 2.38, 95% CI: 2.31–2.45). After adjustment for education, depression and PTSD, the HR was slightly attenuated to 2.21 (95% CI 2.15–2.28). After further adjustment for any CVD diagnoses and any CVD risk factor, the adjusted hazard for dementia was 2.17 (95%, CI 2.10–2.23). Results were similar using Fine-Grey proportional hazards models accounting for the competing risk of death (unadjusted, HR: 2.29, 95% CI 2.23–2.36; fully adjusted HR: 2.08, 95% CI 2.02–2.14). About 33% of veterans who did not have CVD risk factors or a CVD diagnosis at baseline developed incident CVD or risk factors during follow-up; 35% of those with TBI and 31% of those with no TBI. Our sensitivity analysis adjusting for incident CVD risk factors and diagnoses led to similar results (fully adjusted HR: 2.18, 95% CI 2.12–2.25).
There was no evidence of an interaction between TBI and any CVD diagnosis on dementia risk (p for interaction, 0.12). Table 2 shows the unadjusted and adjusted associations between TBI only and dementia, any CVD diagnosis only and dementia, or TBI in addition to any CVD diagnosis with dementia. Compared with Veterans with neither exposure (6.5%), veterans with TBI only, CVD only, or both TBI and CVD had higher rates of incident dementia during follow-up (with incident dementia rates ranging from 12.0% for TBI only to 18.4% for both diagnoses). TBI and CVD were both associated with increased risk of dementia in a model adjusted for education, depression, PTSD and cardiovascular risk factors (TBI only HR: 2.17, 95% CI 2.09–2.25; CVD only HR: 1.21, 95% CI 1.15–1.28). Veterans with TBI plus any CVD diagnosis had the highest risk of dementia (HR: 2.51, 95% CI 2.41–2.61), suggesting the presence of an independent, additive statistical association. Results of sensitivity analyses adjusting for both baseline and incident CVD and CVD risk factors were very similar: TBI only HR 2.23, 95% CI 2.12–2.35; CVD only HR 1.08, 95% CI 1.02–1.13; TBI plus CVD HR 2.33, 95% CI 2.22–2.43). The cumulative incidence of dementia diagnosis among veterans with TBI, any CVD diagnosis, or both is shown in Figure 1.
Table 2.
The association between TBI and CVD and risk of dementia from Cox proportional hazards models
| No. (%) |
HR (95% CI) |
|||
|---|---|---|---|---|
| Dementia | Unadjusted | Adjusted |
||
| Model 1 | Model 2 | |||
| Neither | 4,864 (6.5) | ref | ref | ref |
| TBI only | 7,555 (12.0) | 2.35 (2.27–2.44) | 2.19 (2.11–2.27) | 2.17 (2.09–2.25) |
| CVD only | 2,378 (10.3) | 1.26 (1.20–1.32) | 1.23 (1.17–1.29) | 1.21 (1.15–1.28) |
| TBI and CVD | 6,398 (18.4) | 2.83 (2.72–2.94) | 2.59 (2.49–2.69) | 2.51 (2.41–2.61) |
TBI, traumatic brain injury, CVD, cardiovascular disease, HR, hazard ratio, CI, confidence interval, PTSD, post-traumatic stress disorder. Unadjusted and adjusted analyses included Veterans with TBI matched 1:1 on age, race and sex. Model 1 adjusted for education, PTSD and depression. Model 2 adjusted for education, PTSD, depression and CVD risk factors (diabetes mellitus, obesity/ overweight, current tobacco use, hypertension, and hypercholesterolemia).
Figure 1.
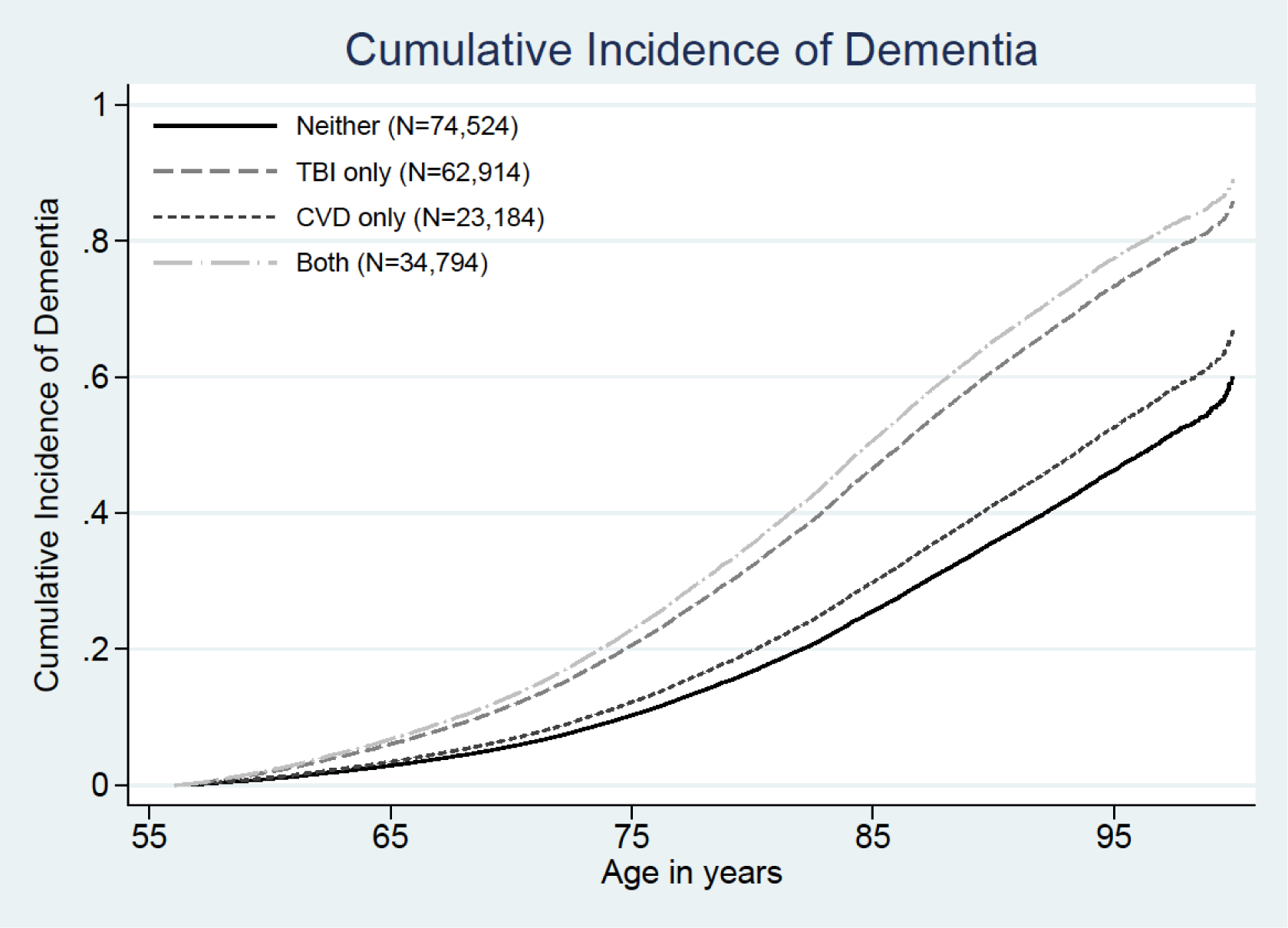
The additive association between traumatic brain injury (TBI) and cardiovascular disease (CVD) is illustrated by showing the cumulative incidence of dementia with age as the timescale for veterans with TBI only (dark grey), CVD only (dashed black), both TBI and CVD (light grey), or neither (solid black).
DISCUSSION
In this large, diverse, nationally representative cohort of older US veterans, we observed an increased prevalence of both CVD and cardiovascular risk factors among older veterans with TBI compared to those without TBI. Moreover, we found that TBI exposure was associated with more than a 2-fold increase in the risk for dementia, and CVD was also associated with increased dementia risk. However, the statistical association between TBI and dementia remained elevated after adjusting for CVD diagnoses and risk factors, suggesting that CVD and its risk factors do not account for much of the increased risk of dementia with TBI.
In spite of the documented connections between TBI and CVD and risk factors [13–15, 19], little prior research has explicitly addressed the impact of CVD on risk for dementia after TBI. Here, our results suggest a large increased risk of dementia after TBI [6–12] as well as a modest increase of dementia risk associated with CVD [16, 17]. However, we did not find that CVD or risk factors accounted for the association between TBI and risk for dementia, nor did we find an interaction between TBI and CVD on dementia risk. Instead, we observed an additive statistical association between TBI and CVD on risk of dementia. It is clear that more research is needed to understand mechanisms and causal pathways underlying the increased dementia diagnosis risk after TBI.
The reason for the elevated prevalence of CVD and CVD risk factors among those with TBI is unclear. Our results constitute a statistical association and do not establish a causal relationship between CVD and TBI. However, there are multiple pathways by which CVD and TBI appear be related. For example, TBI may trigger a complex molecular cascade that is not yet fully understood but that may lead to a number of central nervous system changes including arterial stiffness, chronic inflammation, and damage to the blood-brain barrier [23]. These neurovascular changes also increase risk for stroke [23]. TBI also increases risk of incident CVD, including coronary artery disease, arrythmias, heart failure, and stroke [14], perhaps by disturbing hemodynamics and interfering with coagulation pathways [24].
Other than CVD, there are several additional proposed mechanistic pathways for the association between TBI and dementia. TBI appears to trigger neuropathological changes which may lead to dementia, through multiple pathways including the deposition of both tau and amyloid [25], and biomarkers of neuronal damage have been observed in the blood of TBI patients even many years after injury [26]. However, one study with brain autopsies has shown that individuals with TBI are at higher risk for Lewy body dementia and Parkinson’s disease neuropathology, not the neurofibrillary plaques and tangles that define Alzheimer disease [27]. Dementia diagnoses after TBI may also reflect cognitive impairment associated with the TBI-related structural damage rather than from the presence of a secondary neurodegenerative process [28]. This is supported by the accumulating evidence that greater severity of TBI is linked to higher risk of dementia [29]. Finally, repeated TBIs are associated with chronic traumatic encephalopathy (CTE), a condition characterized by a unique pattern of neuropathology detectable only by autopsy [30] and a loosely defined clinical syndrome that may include aggression, personality change, and cognitive impairment [31]. It is possible that CTE pathology alone or in combination with other aging processes could result in a clinical dementia presentation in some cases.
There are limitations to our study which impact the interpretation and generalizability of our results. While we carefully matched on the key variables of age, sex and race and adjusted for important confounders, all observational studies retain the risk of unmeasured confounding. We used ICD-9 and 10 codes as well as dementia medications in existing medical records for dementia diagnoses, which may result in less sensitive categorization of participants compared to studies in which participants are given a comprehensive dementia examination. Finally, results may not generalize to veterans who do not receive VA health care or to non-Veterans.
This is one of the first studies to examine the impact of CVD and CVD risk factors on risk for dementia diagnosis after TBI. Our primary finding was that, in a large, diverse, nationally representative sample of older veterans, TBI was associated with higher prevalence of CVD and risk factors. Yet, the statistical association between TBI and dementia diagnosis was not attenuated by adjustment for CVD risk factors or CVD, indicating that CVD does not seem to account for much of the risk for dementia after TBI. Our results also revealed an additive statistical association between TBI and CVD. Given the high prevalence of CVD in veterans with history of TBI, as well as their increased risk of dementia, these findings suggest that more research is needed to determine causal links among CVD, TBI, and dementia and to inform clinical guidelines for older Veterans post-TBI in order to optimize healthy cognitive aging.
Study Funding:
This work was supported by the Assistant Secretary of Defense for Health Affairs endorsed by the Department of Defense, through the Psychological Health/Traumatic Brain Injury Research Program Long-Term Impact of Military-Relevant Brain Injury Consortium (LIMBIC) Award/W81XWH-18-PH/TBIRP-LIMBIC under Awards No. W81XWH1920067 and W81XWH-13-2-0095, and by the U.S. Department of Veterans Affairs Awards No. I01 CX002097, I01 CX002096, I01 HX003155, I01 RX003444, I01 RX003443, I01 RX003442, I01 CX001135, I01 CX001246, I01 RX001774, I01 RX 001135, I01 RX 002076, I01 RX 001880, I01 RX 002172, I01 RX 002173, I01 RX 002171, I01 RX 002174, and I01 RX 002170. The U.S. Army Medical Research Acquisition Activity, 839 Chandler Street, Fort Detrick MD 21702-5014 is the awarding and administering acquisition office. Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the Department of Defense.
Dr. Yaffe was supported by NIA (K24 AG031155). Dr. Kornblith was supported by Department of Veterans Affairs Career Development Award (CDA-2) 1 IK2 RX003073-01A2.
Dr. Kornblith is a neuropsychologist and clinical researcher based at the San Francisco VA Medical Center. Her research, funded by VA Rehabilitation Research and Development, focuses on using technology to increase access to cognitive rehabilitation treatment for older Veterans with history of TBI. Other research and clinical interests include epidemiology of dementia, TBI and cognitive aging, and assessment and treatment of cognitive sequelae of TBI and acquired brain injury.
Dr. Bahorik is a research associate at University of California San Francisco, working on research projects at the San Francisco VA Medical Center and UCSF. Her research focuses on the cognitive and behavioral effects of substance abuse among older adults.
Ms. Li is a Sr. Statistician at Northern California Institute for Research and Education, working on research projects at the San Francisco VA Health Care System. She has significant experience in the management and analysis of large administrative datasets, in particular, VHA data.
Dr. Peltz is a research associate at the Northern California Institute for Research and Education, working on research projects at the San Francisco VA Medical Center and UCSF. Her work focuses on clinical research and epidemiology of dementia, TBI, and modifiable risk factors.
Dr. Barnes is a Professor in the Departments of Psychiatry and Behavioral Sciences and Epidemiology & Biostatistics at UCSF and a Research Health Science Specialist at the San Francisco VA Medical Center. Her research focuses on identifying modifiable risk factors for cognitive impairment and dementia in older adults, developing dementia risk prediction models, and testing behavioral interventions to delay onset or slow progression of symptoms.
Dr. Yaffe is the Scola Endowed Chair and Vice Chair and Professor of Psychiatry, Neurology and Epidemiology at UCSF as well as the Chief of NeuroPsychiatry and Director of the Memory Evaluation Clinic at the San Francisco Veterans Affairs Medical Center. She is a leading expert in the epidemiology of dementia and cognitive aging. Dr. Yaffe’s work bridges neurology, psychiatry, and epidemiology with a primary focus on modifiable risk factors and dementia prevention.
Footnotes
Financial Disclosures:
Erica Kornblith: Reports no disclosures relevant to the manuscript.
Amber Bahorik: Reports no disclosures relevant to the manuscript.
Yixia Li: Reports no disclosures relevant to the manuscript.
Carrie B. Peltz: Reports no disclosures relevant to the manuscript.
Deborah E. Barnes: Reports no disclosures relevant to the manuscript.
Kristine Yaffe: Dr. Yaffe serves on Data Safety Monitoring Boards for Eli Lilly and several National Institute on Aging-sponsored studies, and serves on the board of directors for Alector, Inc.
Statistical Analysis conducted by Yixia Li, SFVA/NCIRE and Amber Bahorik, PhD, SFVA/NCIRE
References
- 1.Whiteneck GG, et al. , Prevalence of self-reported lifetime history of traumatic brain injury and associated disability: a statewide population-based survey. J head trauma rehabil, 2016. 31(1): p. E55–E62. [DOI] [PubMed] [Google Scholar]
- 2.Selassie AW, et al. , Incidence of long-term disability following traumatic brain injury hospitalization, United States, 2003. The Journal of head trauma rehabilitation, 2008. 23(2): p. 123–131. [DOI] [PubMed] [Google Scholar]
- 3.Thompson HJ, McCormick WC, and Kagan SH, Traumatic brain injury in older adults: epidemiology, outcomes, and future implications. Journal of the American Geriatrics Society, 2006. 54(10): p. 1590–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gardner RC, et al. , Geriatric traumatic brain injury: epidemiology, outcomes, knowledge gaps, and future directions. Journal of neurotrauma, 2018. 35(7): p. 889–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes DE, et al. , Association of mild traumatic brain injury with and without loss of consciousness with dementia in US military veterans. JAMA neurology, 2018. 75(9): p. 1055–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mortimer J, et al. , Head trauma as a risk factor for Alzheimer’s disease: a collaborative re-analysis of case-control studies. International journal of epidemiology, 1991. 20(Supplement_2): p. S28–S35. [DOI] [PubMed] [Google Scholar]
- 7.Plassman BL, et al. , Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology, 2000. 55(8): p. 1158–1166. [DOI] [PubMed] [Google Scholar]
- 8.Guo Z, et al. , Head injury and the risk of AD in the MIRAGE study. Neurology, 2000. 54(6): p. 1316–1323. [DOI] [PubMed] [Google Scholar]
- 9.Wang H-K, et al. , Population based study on patients with traumatic brain injury suggests increased risk of dementia. J Neurol Neurosurg Psychiatry, 2012. 83(11): p. 1080–1085. [DOI] [PubMed] [Google Scholar]
- 10.Gardner RC, et al. , Dementia risk after traumatic brain injury vs nonbrain trauma: the role of age and severity. JAMA neurology, 2014. 71(12): p. 1490–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee Y-K, et al. , Increased risk of dementia in patients with mild traumatic brain injury: a nationwide cohort study. PloS one, 2013. 8(5): p. e62422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nordström P, et al. , Traumatic brain injury and young onset dementia: a nationwide cohort study. Annals of neurology, 2014. 75(3): p. 374–381. [DOI] [PubMed] [Google Scholar]
- 13.Hammond FM, et al. , Prevalence of medical and psychiatric comorbidities following traumatic brain injury. The Journal of Head Trauma Rehabilitation, 2019. 34(4): p. E1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nyam T-TE, et al. , Traumatic brain injury increases the risk of major adverse cardiovascular and cerebrovascular events: a 13-year, population-based study. World neurosurgery, 2019. 122: p. e740–e753. [DOI] [PubMed] [Google Scholar]
- 15.Kumar RG, et al. , Epidemiology of comorbid conditions among adults 50 years and older with traumatic brain injury. Journal of head trauma rehabilitation, 2018. 33(1): p. 15–24. [DOI] [PubMed] [Google Scholar]
- 16.Whitmer RA, et al. , Midlife cardiovascular risk factors and risk of dementia in late life. Neurology, 2005. 64(2): p. 277–281. [DOI] [PubMed] [Google Scholar]
- 17.Stampfer M, Cardiovascular disease and Alzheimer’s disease: common links. Journal of internal medicine, 2006. 260(3): p. 211–223. [DOI] [PubMed] [Google Scholar]
- 18.Eriksson UK, et al. , Non-Stroke Cardiovascular Disease and Risk of Alzheimer’s Disease and Dementia. Alzheimer disease associated disorders, 2010. 24(3): p. 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coronado VG, et al. , The CDC traumatic brain injury surveillance system: characteristics of persons aged 65 years and older hospitalized with a TBI. The Journal of head trauma rehabilitation, 2005. 20(3): p. 215–228. [DOI] [PubMed] [Google Scholar]
- 20.Caplan B, et al. , Epidemiology of comorbid conditions among adults 50 years and older with traumatic brain injury. Journal of head trauma rehabilitation, 2018. 33(1): p. 15–24. [DOI] [PubMed] [Google Scholar]
- 21.Barnes DE, et al. , Traumatic brain injury and risk of dementia in older veterans. Neurology, 2014. 83(4): p. 312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.US Department of Veterans Affiars (VA) Veterans Health Administration (VHA) Dementia Steering Committee (DSC). VHA dementia steering committee recommendations for dementia care in the VHA healthcare system 2016. 2016. [cited 2019 September 3]; Available from: https://www.va.gov/GERIATRICS/docs/VHA_DSC_RECOMMENDATIONS_SEPT_2016_9-12-16.pdf.
- 23.Salehi A, et al. , Response of the cerebral vasculature following traumatic brain injury. Journal of Cerebral Blood Flow, 2017. 37(7): p. 2320–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fletcher-Sandersjöö A, Maegele M, and Bellander B-M, Does complement-mediated hemostatic disturbance occur in traumatic brain injury? A literature review and observational study protocol. International journal of molecular sciences, 2020. 21(5): p. 1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramos-Cejudo J, et al. , Traumatic brain injury and Alzheimer’s disease: the cerebrovascular link. EBioMedicine, 2018. 28: p. 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peltz CB, et al. , Blood biomarkers of traumatic brain injury and cognitive impairment in older veterans. Neurology, 2020. 95(9): p. e1126–e1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crane PK, et al. , Association of traumatic brain injury with late-life neurodegenerative conditions and neuropathologic findings. JAMA neurology, 2016. 73(9): p. 1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peterson K, et al. , Evidence Brief: Traumatic Brain Injury and Dementia. 2019. [PubMed]
- 29.Barnes DE, et al. , Traumatic brain injury and risk of dementia in older veterans. JAMA Neurology, 2014. 83(4): p. 312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKee AC, et al. , The neuropathology of chronic traumatic encephalopathy. Brain pathology, 2015. 25(3): p. 350–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McAllister T and McCrea M, Long-term cognitive and neuropsychiatric consequences of repetitive concussion and head-impact exposure. Journal of athletic training, 2017. 52(3): p. 309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]


