Abstract
The purpose of this study was to verify the influence of physical activity level on the length of hospital stay in older men recovered from COVID-19. In total, 126 older men diagnosed with COVID-19 were admitted to the hospital between September and December 2020. Among them, 70 survived, of which 39 older men were included in the study. Within 30 days after discharge, patients answered the International Physical Activity Questionnaire to measure their physical activity level through phone contact, with questions corresponding to the week before symptom onset. Clinical and laboratorial data from admission, days between onset of symptoms and admission, length of stay, computed tomography abnormalities, and the need for the intensive care unit were collected. The groups (active × sedentary) were compared using the Student t test or Mann–Whitney test for quantitative data and chi-square test was used for categorical data. There is no difference between the groups in characteristics of admission (p > 0.05), except by potassium level. Active older men had a shorter length of stay (6.50 ± 3.46 vs 11.48 ± 7.63 days; p = 0.03), disease duration (15.71 ± 4.84 vs 21.09 ± 7.69 days; p = 0.02), and lower frequency of lung damage when compared to their sedentary counterparts. In conclusion, being physically active prior to infection can attenuate length of hospital stay in older men with COVID-19.
Keywords: Hospitalization, Exercise, Aging, Coronavirus, Questionnaire
Introduction
Novel Coronavirus (SARS-CoV-2), which causes the systemic disease COVID-19, has become a major public health problem around the world, since the outbreak in Wuhan, China, in December 2019. In March 2022, almost two years later, approximately 6 million people died from this disease around the world. Concomitantly, several studies were developed to understand the disease, risk factors, and prognostic indices [1, 2].
In general, COVID-19 is an acute curable disease with a mortality rate of about 2%. The risk of mortality increases for adults over 60 years and people diagnosed with chronic diseases [1]. When severe, COVID-19 causes massive alveolar damage and progressive respiratory failure, which can lead to acute respiratory distress syndrome (ARDS), very similar to another coronavirus pneumonia, such as acute respiratory syndrome [3].
People with severe symptoms of the disease may present functional declines at the cardiorespiratory level, requiring hospitalization, in a clinical bed or intensive care unit (ICU) for days, weeks, or months, as well as requiring the use of invasive mechanical ventilation (to reestablish respiratory function), especially in the presence of ARDS [1].
Several measures to protect and contain the number of cases have been imposed, mainly social distancing [4]. However, social distancing can induce a relevant physical activity level decline, which increases sedentary behavior and the resulting negative health consequences [5–7]. In addition, before the COVID-19 outbreak and social distancing measures, there was already a high prevalence of sedentary behavior in Brazil and in the world [8].
Previous studies have shown that physically active people have a lower risk of cardiorespiratory disease [9] and low-grade inflammation [10]. Furthermore, physically active people have a lower incidence and days of duration of viral infections [11]. Regular exercise induces antiatherogenic adaptations, increases insulin sensitivity, improves cardiac autonomic activation, and improves immune function. Some of these benefits are provided through the release of muscle-derived myokines during exercise, although all mechanisms are not completely understood [11–13].
Some conditions are related to a greater risk of developing critical disease, mostly older age, obesity, and/or previous comorbidities [14, 15], which could determine a greater length of stay in hospital. Similarly, laboratory examinations and clinical features on admission are related to severe cases [16, 17]. Furthermore, some studies that have investigated the relationship of physical activity and COVID-19 outcomes, and they showed greater risk of hospitalization and/or severe cases in sedentary people when compared to physically active [18–20], but among hospitalized patients, the benefits were not observed [21]. Nevertheless, an association between physical activity level and COVID-19 outcomes could be showed in stratify group, especially in individuals with a higher risk, such as elderly men.
However, to our knowledge, there are no data on whether a higher physical activity level in older men who were infected with COVID-19 and needed hospital admission is related to faster recovery compared to sedentary older men. Therefore, the purpose of this study was to verify the influence of physical activity level and length of stay in older men recovered from COVID-19.
Materials and methods
Subjects
This is a descriptive, cross-sectional and quantitative study. Older men diagnosed with COVID-19 who were admitted to clinical or intensive care units at the University Hospital of the State University of Ponta Grossa (HU-UEPG) between September and December 2020 participated in the study. Therefore, 126 men over 60 years were admitted to HU-UEPG after being diagnosed with COVID-19 by quantitative PCR viral test (qPCR) or blood test (serology) and remained hospitalized for at least one day. The exclusion criteria were: (1) patients who died, (2) with cognitive impairment or diseases, (3) with memory disturbances, and (4) with absence of phone contact after six unsuccessful calls within 30 days.
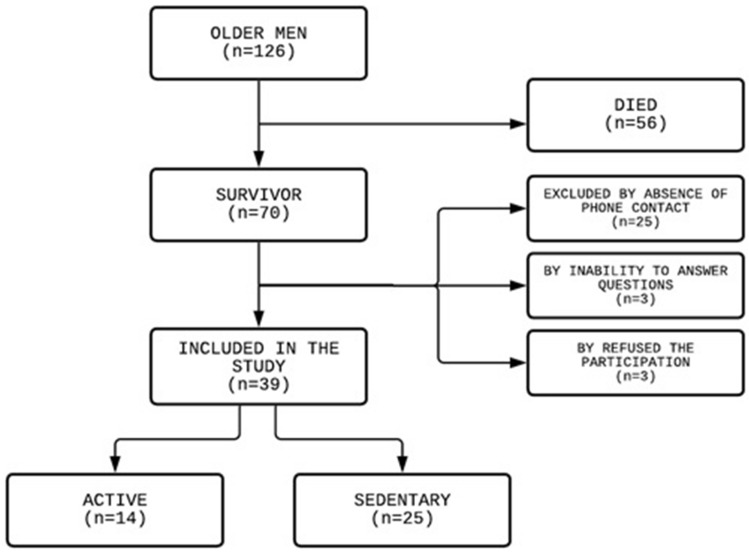
Among the 126 older men admitted, 87 were excluded (56 died, 25 due to absence of phone contact, 3 due to inability to answer questions, and 3 refused to participate) (Fig. 1). At the end, 39 older men were included in the study.
Fig. 1.
Flowchart of study design
The procedures were approved by Human Research Ethics Committee of State University of Ponta Grossa (protocol: 40051120.8.0000.0105) and all participants were informed about the aim and study protocols and signed an informed consent form.
Study design
Within 30 days after discharge, the patients answered a physical activity level questionnaire through phone contact. During the phone contact, the patients were asked to answer International Physical Activity Questionnaire (IPAQ) questions corresponding to the week before their COVID-19 symptoms began. Furthermore, clinical data and laboratorial results from hospital admission, days between onset of symptoms and admission, length of stay, computed tomography (CT) abnormalities, and the necessity for an ICU stay were collected.
IPAQ
The instrument used to assess the practice of physical activity before the hospitalization period was the IPAQ Long-Portuguese version, validated for Brazilians [22]. However, only Sections 2 (transport) and 4 (leisure) were used, to avoid overestimating physical activity level [23]. Following the script previously established and adapted for phone contact, all patients answered questions corresponding to the week before their COVID-19 symptoms began, to collect data from a regular week. Subsequently patients were classified and separated into two groups: active (≥ 150 min.week−1 total activity or ≥ 600 MET min.week−1) and sedentary (those who did not meet the criteria to be considered active) [24].
Admission data
Hospital admission data were collected from electronic medical records and divided into two categories: clinical characteristics and laboratorial results. Data collected on clinical characteristics were: age, weight, height, heart rate, respiratory rate, body temperature, blood pressure, oxygen therapy, and comorbidities which was considered previous diagnosed chronic disease. Laboratorial exams were: complete hemogram, blood gas analysis, C-reactive protein, d-dimer, creatinine, urea, potassium, and sodium.
Outcomes
The primary outcomes of the study were length of stay in hospital and total duration of disease. Additionally, the period from the onset of symptoms until admission was recorded. The secondary outcomes were need for the intensive care unit, medications used, and CT abnormalities. CT findings were considered: peripheral, bilateral ground glass opacity with or without consolidation or visible intralobular lines; multifocal ground glass opacity of rounded morphology with or without consolidation or visible intralobular lines; reverse halo or other findings of organizing pneumonia [25]. The exams were evaluated by a radiologist and were considered mild CT-abnormalities: < 25%, moderate CT-abnormalities: 25–50%, and accentuated CT-abnormalities: > 50%.
Statistical analysis
Normal distribution was verified with the Shapiro–Wilk test. Quantitative variables are represented by mean and standard deviation. The groups (active × sedentary) were compared using the Student t test for normally distributed data and Mann–Whitney test for non-normally distributed data. Categorical variables are represented by absolute and relative frequency. To compare groups, the chi-square test was used and the Fisher exact test when necessary. The data analysis was performed using SPSS v.25 software and significance level was set at p < 0.05.
Results
Clinical characteristics of the patients, comorbidities, oxygen therapy on admission to the hospital, and laboratorial exams are presented in Table 1. There were no differences between the groups in these data (p > 0.05), except for potassium level, which was higher in the active group (4.50 ± 0.20 mEq/L vs 4.30 ± 0.5 mEq/L; p = 0.04).
Table 1.
Characteristics of participants classified as physically active and sedentary
| Clinical characteristics | Active (n = 14) | Sedentary (n = 25) | p |
|---|---|---|---|
| Age (y) | 67.36 ± 5.90 | 66.48 ± 5.33 | 0.52 |
| Weight (kg) | 82.14 ± 9.73 | 79.40 ± 12.30 | 0.48 |
| Height (m) | 1.69 ± 0.08 | 1.70 ± 0.07 | 0.69 |
| BMI (kg.m−2) | 28.71 ± 3.06 | 27.40 ± 4.05 | 0.16 |
| HR (bpm) | 75.86 ± 12.60 | 85.27 ± 14.24 | 0.05 |
| RR (bpm) | 19.85 ± 2.12 | 23.42 ± 7.70 | 0.27 |
| SBP (mmHg) | 132.00 ± 18.60 | 129.27 ± 23.18 | 0.47 |
| DBP (mmHg) | 81.00 ± 10.16 | 84.41 ± 12.69 | 0.51 |
| Body temperature (ºC) | 36.28 ± 0.53 | 36.44 ± 0.63 | 0.52 |
| Comorbidities | |||
| 0 (%) | 4 (28.57) | 11 (44.00) | 0.18 |
| 1–2 (%) | 10 (71.63) | 11 (44.00) | |
| 3+ (%) | 0 (0.00) | 3 (12.00) | |
| Most common comorbidities | |||
| Hypertension (%) | 7 (50.00) | 10 (40.00) | 0.55 |
| Diabetes (%) | 5 (37.70) | 6 (24.00) | 0.46 |
| Oxygen therapy admission | |||
| Room air (%) | 4 (28.57) | 6 (24.00) | 0.64 |
| Nasal cannula (%) | 5 (35.71) | 5 (20.00) | |
| Non-rebreather mask (%) | 4 (28.57) | 11 (44.00) | |
| Invasive ventilation (%) | 1 (7.14) | 3 (12.00) | |
| Laboratorial exams | |||
| SaO2 (%) | 93.54 ± 3.30 | 94.49 ± 4.15 | 0.09 |
| pH | 7.41 ± 0.05 | 7.43 ± 0.06 | 0.44 |
| pO2 (mmHg) | 69.55 ± 10.47 | 74.31 ± 17.56 | 0.44 |
| pCO2 (mmHg) | 34.09 ± 4.89 | 33.45 ± 4.35 | 0.63 |
| HCO3 (mEq/L) | 21.73 ± 2.79 | 22.02 ± 2.57 | 0.83 |
| C-reactive protein (mg/L) | 15.08 ± 11.18 | 15.49 ± 10.45 | 0.92 |
| d-dimer (ug/ml) | 2.18 ± 2.71 | 2.25 ± 2.80 | 0.68 |
| Creatinine (mg/dL) | 1.01 ± 0.29 | 1.32 ± 1.24 | 0.44 |
| Urea (mg/dL) | 57.36 ± 22.27 | 61.64 ± 42.81 | 0.78 |
| Potassium (mEq/L) | 4.50 ± 0.20 | 4.30 ± 0.56 | 0.04* |
| Sodium (mEq/L) | 135.93 ± 2.84 | 136.84 ± 3.94 | 0.45 |
| Hemoglobin (g/dL) | 13.89 ± 1.72 | 13.59 ± 2.08 | 0.65 |
| Leukocytes (1000/µl) | 8.15 ± 3.49 | 8.83 ± 3.34 | 0.63 |
| Platelets (1000/µl) | 249.85 ± 96.23 | 230.00 ± 68.13 | 0.29 |
Values in mean ± standard deviation or frequency
BMI body mass index, HR heart rate, RR respiratory rate, SBP systolic blood pressure, DBP diastolic blood pressure
*p < 0.05
Physical activity level of the groups is demonstrated in Table 2. As expected, the IPAQ (min.week−1) and MET (min.week−1) were significantly higher in the active group in comparison to the sedentary group (p < 0.001).
Table 2.
Physical activity level between the groups
| Physical activity level | Active (n = 14) | Sedentary (n = 25) | p |
|---|---|---|---|
| IPAQ (min.week−1) | 403.57 ± 521.36 | 27.80 ± 43.06 | < 0.001* |
| MET (min.week−1) | 1441.18 ± 1815.84 | 96.42 ± 148.53 | < 0.001* |
Values in mean ± standard deviation
*p < 0.05
The interval from symptom onset until admission to the hospital was equivalent between the groups (p = 0.72). The length of stay was lower in the active (6.50 ± 3.46 days) when compared to the sedentary group (11.48 ± 7.63 days; p = 0.03). Consequently, the total duration of disease was also lower in the active group (15.71 ± 4.84 days) vs. sedentary group (21.09 ± 7.69 days; p = 0.02) (Table 3).
Table 3.
Primary outcomes
| Outcomes | Active (n = 14) | Sedentary (n = 25) | p |
|---|---|---|---|
| Onset of symptom until admission (days) | 9.21 ± 3.53 | 9.61 ± 3.00 | 0.72 |
| Length of stay (days) | 6.50 ± 3.46 | 11.48 ± 7.63 | 0.03* |
| Disease duration (days) | 15.71 ± 4.84 | 21.09 ± 7.69 | 0.02* |
Values in mean ± standard deviation
*p < 0.05
In secondary outcomes, there were no differences between the groups in need for the intensive care unit and medications used (p > 0.05). The active group presented a lower frequency of moderate and severe lung abnormalities on the CT compared to the sedentary group (p = 0.01).
Discussion
The present study aimed to verify whether there is an influence of physical activity level on the length of stay in hospital due to COVID-19 in older men. The associations observed were that physically active older men spent less time in hospital, as well as which the disease duration was shorter when compared to their sedentary counterparts. Furthermore, physically active men demonstrated less pulmonary impairment on the CT.
To our knowledge, this is the first study to analyze the influence of physical activity level on the length of stay in older men recovered from COVID-19. This group was stratified due higher risk of death. It was observed that sedentary older men have a longer length of stay and total duration of disease than physically active. Woolcott et al. [26] also demonstrated that physically active older men tend to present a shorter length of stay in hospital when compared to sedentary older men, independent of previous chronic diseases. Langsetmo et al. [27] evaluated 1283 hospitalized older men and showed that a higher physical activity level was associated with a shorter total duration of acute/post-acute care stays. Furthermore, Grande et al. [28] observed in a meta-analysis that exercise reduced the severity of acute respiratory infection and number of symptom days, and Tavakol et al. [29] showed that physically active people have lower COVID-19 severity. These results indicate that an active lifestyle has beneficial effects in general diseases, respiratory infections, and now, in COVID-19. In a pandemic scenario, faster recovery is important to guarantee availability of clinical beds and reduce public health costs.
A shorter length of stay is related to faster clinical recovery [30] and a prolonged length of stay can be associated with functional, neuromuscular, and cardiorespiratory impairment [31, 32], due to the extended time (approximately 90–95%) sitting and on bed rest in hospital [33]. The present study observed that sedentary older men have prolonged clinical recovery. Previous studies evaluated the relationship between cardiorespiratory fitness and recovery, clinical complications, and mortality in older men undergoing major surgery. It was shown that cardiorespiratory fitness is a predictor of clinical complications, length of stay, and mortality [34–36]. Similarly, it was observed that exercise training before surgery reduced postoperative complications, length of stay, and hospital resource utilization [30, 37]. This evidence demonstrates that physically active people tend to have faster recovery after hospital admission. In the COVID-19 scenario, the present study corroborated these previous results and showed that physically active older men recovered more quickly.
No significant differences were found in most clinical characteristics of admission, comorbidities, oxygen therapy, and laboratorial exams, except for potassium level. Even with similar clinical characteristics at admission, the physically active group had a lower length of stay in hospital and total duration of disease. Immunological optimization provided by physical exercise seems to have been the factor that differentiated these individuals and promoted shorter hospital stays. Previous studies have observed that moderate exercise improves immune system function, such as lower levels of circulating inflammatory cytokines and increased neutrophil phagocytic activity, in both acute and chronic exercise [38–41]. In contrast, sedentary and fragile older people have a poor immunological response, reflecting in a higher risk of admission to the hospital and mortality [42–44]. Souza et al. [20] observed that physical activity levels are associated with a lower prevalence of COVID-19-related hospitalizations and reinforce protect role maintain active lifestyle. However, it is suggested that in patients who needed hospitalization could be more related to clinical characteristics, in which in our study it was not different, independent of physical activity level. Notwithstanding, to be physically active is auxiliary to avoid negative outcomes, length of stay and disease duration.
The influence of previous chronic diseases in harmful outcomes related to COVID-19 hospitalizations has been shown in other studies [1, 14]. In contrast, physically active people trend to have more stable chronic diseases, such as hypertension and diabetes [45–47], lowering the risks of hospitalization due to COVID-19 [19, 20]. The present study points to association between physical activity level and length of stay due to COVID-19 in older men matched by comorbidities. Therefore, it is plausible to hypothesize that to be physically active previous hospitalization would minimize the risks of problems related to uncontrolled chronic disease during hospitalization due to COVID-19 and to favor a better recovery, but this hypothesis still needs to be understood more (Table 4).
Table 4.
Secondary outcomes
| Outcomes | Active (n = 14) | Sedentary (n = 25) | p |
|---|---|---|---|
| Needed to ICU (%) | 1 (7.14) | 9 (36.00) | 0.06 |
| Dexamethasone (%) | 14 (100.00) | 25 (100.00) | 1 |
| Azithromycin (%) | 5 (35.71) | 6 (24.00) | 0.43 |
| CT-abnormalities# | |||
| Mild (%) | 7 (58.33) | 3 (12.00) | 0.01* |
| Moderate (%) | 2 (16.67) | 13 (52.00) | |
| Accentuated (%) | 3 (25.00) | 9 (36.00) | |
Values in absolute and relative frequency
ICU intensive care unit, CT computed tomography
*p < 0.05
#12 patients in active group were submitted to CT
Electrolyte disturbance is a multifactorial problem, and in a COVID-19 scenario can be related to severe cases of disease [48]. Serum potassium level can be influenced by a wide variety of factors, such as medicines (e.g., diuretics) and diet. Deficiency (< 3.5 mEq/L) or an excessive level (> 5.5 mEq/L) can cause adverse health consequences [49]. In the current study, it was observed that the potassium level was higher in active older men in comparison to sedentary older men, but values in both groups were within the normal range (4.5 ± 0.20 mEq/L vs 4.30 ± 0.56 mEq/L, respectively), which would not compromise the study results.
Among the medications used in the patients, there were no differences between active and sedentary groups. All the patients used dexamethasone and there was no difference in azithromycin use. Dexamethasone is a corticosteroid used in a wide range of conditions due to its anti-inflammatory and immunosuppressant effects. In the COVID-19 scenario, this medicine is used in patients with inflammatory lung damage [50]. Azithromycin is an antibiotic used to treat chest infections, such as pneumonia. In the COVID-19 scenario, this medicine can be used in patients with bacterial infection associated with Sars-CoV-2 [51].
Previous studies have shown that physical inactivity is associated with a higher risk of hospitalization, severe COVID-19 outcomes, and death [19, 20, 29]. In the present study, there was no difference between the groups in the need for the ICU (p = 0.06). A plausible reason for the equality is that the men who died were excluded, since it would be impossible to collect their data. It is reasonable to speculate that the majority of people who died were sedentary, but the present study is unable to answer this affirmation. However, Sallis et al. [19] and Lee et al. [52], both with a larger database from US and South Korea, respectively, have demonstrated that physical activity was associated with a reduced risk for severe COVID-19 and death. Sallis et al. [19] observed inactive patients with COVID-19 had a great risk of death (OR 2.49, 95% CI 1.33–4.67) when compared with active patients. Similarly, Lee et al. [52] showed that physically active participants had a lower risk of COVID-19-related death (aRR 0.24; 95% CI 0.05–0.99) when compared to insufficiently active participants.
Sedentary older men have a higher frequency of moderate and severe abnormalities on the CT than active older men. Pulmonary damage is associated with COVID-19 severity. Both direct viral infection and inflammation due to cytokine storm can cause lung damage [53]. Some authors have demonstrated that physical exercise has an anti-inflammatory and immunomodulator effect [13], indicating a probable beneficial effect in infectious diseases. Higher physical activity level is associated with lower inflammatory markers, in turn, higher inflammatory markers level is associated with higher risk of cytokine storm,hence, it is probable that cytokine storm observed in COVID-19-severe patients would be attenuated in physically active individuals, and consequently in sedentary one, the lung damage would be more severe [54]. Thus, the present study suggests that physical activity level could be associated with severity of COVID-19, with a shorter length of stay in hospital due to lower lung damage. However, further studies are necessary to confirm this hypothesis.
Therefore, promoting actions that stimulate the practice of physical exercises under conditions of social distance in older men could help to reduce the negative consequences of the COVID-19 pandemic and in-hospital length of stay. Guidelines and strategies for practicing physical exercises at home have been widespread on social networks and have been the subject of study. The role of web-based physical activity interventions with online supervision is not a novelty, but it has gained a lot of notoriety during the pandemic [55]. Evidence shows that web-based physical interventions can help to modify behavior and adherence to physical exercises that are directly related to increased cardiorespiratory and neuromuscular capacity, such as walking, climbing stairs, and moderate- to high-intensity resistance exercises [56].
The present study has strengths and some limitations. These data were collected directly from the medical records, with the phone contact used only to identify physical activity level; however, this is an observational study, and the results should be interpreted with caution. Physical activity was not measured directly by a device (e.g., accelerometer); however, this is a retrospective measure, relating to the period before symptom onset. Moreover, the IPAQ has been widely used in studies and correlates with direct measures [57]. Besides that, in COVID-19 scenario, the non-standard use of IPAQ was performed in other studies [58, 20]. Furthermore, our sample is a stratified group and these results cannot be extrapolated for other populations (e.g., women and adult young men). Finally, there were samples losses because of the absence of phone contact, which limited inclusion of a larger sample.
Conclusion
In conclusion, being physically active prior to infection can be associated with lower length of stay in hospital and the resulting deleterious effects, and promote faster recovery in older men hospitalized with COVID-19; however, a cause–effect analysis is still necessary.
These findings are interesting in the pandemic scenario and reinforce the need to promote and evaluate physical activity, which can be used to screen and stratify risk populations. Furthermore, being physically active may be a tool to protect ourself and mitigate unfavorable outcomes after infection.
Acknowledgements
The authors are grateful to the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES) for the scholarship granted to the post-graduate student participating in the study.
Declarations
Conflict of interest
The authors report no conflict of interest.
Ethical approval
This study was conducted in accordance with the recommendations from the Declaration of Helsinki.
Informed consent
All participants provided informed consent prior to their participation.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kannan S, Ali PSS, Sheeza A, et al. COVID-19 (novel coronavirus 2019)—recent trends. Eur Rev Med Pharmacol Sci. 2020;24:2006–2011. doi: 10.26355/eurrev_202002_20378. [DOI] [PubMed] [Google Scholar]
- 3.Campos NG, Costa RF. Pulmonary repercussions caused by the new Coronaviruses (COVID-19) and the use of invasive mechanical ventilation. J Health Biol Sci. 2020;8:1–3. doi: 10.12662/2317-3076jhbs.v8i1.3185.p1-3.2020. [DOI] [Google Scholar]
- 4.Chu DK, Akl EA, Duda S, et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395:1973–1987. doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costa CLA, Costa TM, Barbosa-Filho VC, et al. Influence of social distancing on the physical activity level during the COVID-19 pandemic. Rev Bras Ativ Fís Saúde. 2020;25:1–6. doi: 10.12820/rbafs.25e0123. [DOI] [Google Scholar]
- 6.Marques M, Gheller R, Henrique N, et al. Physical activity during the COVID-19 pandemic: a survey with adults in Northern Brazil. Rev Bras Ativ Fís Saúde. 2020;25:1–8. doi: 10.12820/rbafs.25e0151. [DOI] [Google Scholar]
- 7.Peçanha T, Goessler KF, Roschel H, et al. Social isolation during the COVID-19 pandemic can increase physical inactivity and the global burden of cardiovascular disease. Am J Physiol Heart Circ Physiol. 2020;318:1441–1446. doi: 10.1152/ajpheart.00268.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vigitel Brasil (2019) Vigilância dos fatores de risco e proteção para doenças crônicas por inquérito telefônico: estimativas sobre frequência e distribuição sociodemográfica de fatores de risco e proteção de doenças crônicas nas capitais dos 26 estados brasileiros e no Distrito Federal em 2019. Ministério da Saúde, Secretaria de Vigilância em Saúde (BR)
- 9.Thompson WT, Joy E, Jaworski CA, et al. Exercise is Medicine. Am J Lifestyle Med. 2020;14:511–523. doi: 10.1177/1559827620912192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki K. Chronic inflammation as an immunological abnormality and effectiveness of exercise. Biomolecules. 2019;223:1–11. doi: 10.3390/biom9060223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siu E, Campitelli MA, Kwong JC. Physical activity and Influenza-Coded outpatients visits, a population-based cohort study. PLoS ONE. 2012;7:1–7. doi: 10.1371/journal.pone.0039518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fiuza-Laces C, Santos-Lozano A, Joyner M, et al. Exercise benefits in cardiovascular disease: beyond attenuation of traditional risk factors. Nat Rev Cardiol. 2018;15:731–743. doi: 10.1038/s41569-018-0065-1. [DOI] [PubMed] [Google Scholar]
- 13.Pedersen BK, Saltin B. Exercise as medicine—evidence for prescribing exercise as therapy in 26 different chronic disease. Scand J Med Sci Sports. 2015;25:1–72. doi: 10.1111/sms.12581. [DOI] [PubMed] [Google Scholar]
- 14.Javanmardi F, Keshavarzi A, Akbari A, et al. Prevalence of underlying diseases in died cases of COVID-19: a systematic review and meta-analysis. PLoS ONE. 2020;15:1–13. doi: 10.1371/journal.pone.0241265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mesas AE, Redondo IC, Bueno CA, et al. Predictors of in-hospital COVID-19 mortality: a comprehensive systematic review and meta-analysis exploring differences by age, sex and health conditions. PLoS ONE. 2020;15:1–23. doi: 10.1371/journal.pone.0241742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng Z, Peng F, Xu B, et al. Risk factors of critical and mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020;81:17–25. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammer M, Kivimaki M, Gale CR, Batty G, D, Lifestyle risk factors, inflammatory mechanisms, and COVID-19 hospitalization: a community-based cohort study of 387,109 adults in UK. Brain, Behav Immun. 2020;87:184–187. doi: 10.1016/j.bbi.2020.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sallis R, Young DR, Tartof ST, et al. Physical inactivity is associated with a higher risk for severe COVID-19 outcomes: a study in 48440 adult patients. Br J Sports Med. 2021 doi: 10.1136/bjsports-2021-104080. [DOI] [PubMed] [Google Scholar]
- 20.Souza FR, Motta-Santos D, Soares DS, et al. Association of physical activity levels and the prevalence of COVID-19-associated hospitalization. J Sci Med Sport. 2021;24:913–918. doi: 10.1016/j.jsams.2021.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinto AJ, Goessler KF, Fernandes AL, et al. No independent associations between physical activity and clinical outcomes among hospitalized patients with moderate to severe COVID-19. J Sport Health Sci. 2021;10:690–696. doi: 10.1016/j.jshs.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsudo S, Araujo T, Matsudo V, et al. International Physical Activity Questionnaire (IPAQ): study of validity and reliability in Brazil. Rev Bras Ativ Fís Saúde. 2001;6:5–18. [Google Scholar]
- 23.Hallal PC, Gomez LF, Parra DC, et al. Lessons learned after 10 years of IPAQ use in Brazil and Colombia. J Phys Act Health. 2010;7:259–264. doi: 10.1123/jpah.7.s2.s259. [DOI] [PubMed] [Google Scholar]
- 24.Sjostrom M, Ainsworth B, Bauman A et al (2005) Guidelines for data processing and analysis of the International Physical Activity Questionnaire (IPAQ)—short and long forms
- 25.Simpson S, Kay FU, Abbara S, et al. Radiological society of North America expert consensus document on reporting chest CT findings related to COVID-19: endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA. Cardiothorac Imaging. 2020;2:1–10. doi: 10.1148/ryct.2020200152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woolcott JC, Ashe MC, Miller MC, et al. Does physical activity reduce seniors’ need for healthcare? A study of 24281 Canadians. Br J Sports Med. 2010;44:902–904. doi: 10.1136/bjsm.2008.057216. [DOI] [PubMed] [Google Scholar]
- 27.Langsetmo L, Bats AM, Cawthon PM, et al. The association between objectively measured physical activity and subsequent health care utilization in older men. J Gerontol A Biol Sci Med Sci. 2019;74:820–826. doi: 10.1093/gerona/glx191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grande AJ, Keogh J, Silva V, et al. Exercise versus no exercise for the occurrence, severity, and duration of acute respiratory infections (Review) Cochrane Database Syst Rev. 2020 doi: 10.1002/14651858.cd010596.pub.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tavakol Z, Ghannadi S, Tabesh MR, et al. Relationship between physical activity, healthy lifestyle and COVID-19 disease severity; a cross-sectional study. Z Gesundh Wiss. 2021 doi: 10.1007/s10389-020-01468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Assouline B, Cools E, Schorer R, et al. Preoperative exercise training to prevent postoperative pulmonary complications in adults undergoing major surgery: a systematic review and meta-analysis with trial sequential analysis. Ann Am Thorac Soc. 2020 doi: 10.1513/AnnalsATS.202002-183OC. [DOI] [PubMed] [Google Scholar]
- 31.Hoogerdujin JG, Grobbee DE, Schuurmans MJ. Prevention of functional decline in older hospitalized patients, nurses should play a key role in safe and adequate care. Int J Nurs Pract. 2014;20:106–113. doi: 10.1111/ijn.12134. [DOI] [PubMed] [Google Scholar]
- 32.Kortebein P, Symons TB, Ferrando A, et al. Functional impact of 10 days of bed rest in healthy older adults. J Gerontol A Biol Sci Med Sci. 2008;63:1076–1081. doi: 10.1093/gerona/63.10.1076. [DOI] [PubMed] [Google Scholar]
- 33.Brown CJ, Redden DT, Flood KL, et al. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57:1660–1665. doi: 10.1111/j.1532-5415.2009.02393.x. [DOI] [PubMed] [Google Scholar]
- 34.Snowden CP, Prentis J, Anderson HL, et al. Submaximal cardiopulmonary exercise testing predicts complications and hospital length of stay in patients undergoing major elective surgery. Ann Surg. 2010;251:531–535. doi: 10.1097/SLA.0b013e3181cf811d. [DOI] [PubMed] [Google Scholar]
- 35.Snowden CP, Prentis J, Jacques B, et al. Cardiorespiratory fitness predicts mortality and hospital length of stay after major elective surgery in older people. Ann Surg. 2013;257:999–1004. doi: 10.1097/SLA.0b013e31828dbac2. [DOI] [PubMed] [Google Scholar]
- 36.Tolchard S, Angell J, Pyke M, et al. Cardiopulmonary reserve as determined by cardiopulmonary exercise testing correlates with length of stay and predicts complications after radical cystectomy. BJU Int. 2015;115:554–561. doi: 10.1111/bju.12895. [DOI] [PubMed] [Google Scholar]
- 37.Garcia RS, Brage MIY, Moolhuyzen EG, et al. Functional and postoperative outcomes after preoperative exercise training in patients with lung cancer: a systematic review and meta-analysis. Interact Cardiovasc Thorac Surg. 2016;23:486–497. doi: 10.1093/icvts/ivw152. [DOI] [PubMed] [Google Scholar]
- 38.El-Kader SMA, Al-Shreef FM. Inflammatory cytokines and immune system modulation by aerobic versus resisted exercise training for elderly. Afr Health Sci. 2018;18:120–131. doi: 10.4314/ahs.v18i1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nieman DC, Wentz LM. The compelling link between physical activity and the body’s defense system. J Sport Health Sci. 2019;8:201–217. doi: 10.1016/j.jshs.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sellami M, Gasmi M, Denham J, et al. Effects of acute and chronic exercise on immunological parameters in the elderly aged: can physical activity counteract the effects of aging? Front Immunol. 2018;9:1–17. doi: 10.3389/fimmu.2018.02187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simpson RJ, Kunz H, Agha N, et al. Exercise and the regulation of immune functions. Prog Mol Biol Transl Sci. 2015;135:355–380. doi: 10.1016/bs.pmbts.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 42.Elovainio M, Hakulinen C, Pulkki-Raback L, et al. Contribuition of risk factors to excess mortality in isolated and lonely individuals: an analysis of data from the UK Biobank cohort study. Lance Public Health. 2017;6:260–266. doi: 10.1016/S2468-2667(17)30075-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferreira AO, Polonini HC, Dijkers ECF. Postulated adjuvant therapeutic strategies for COVID-19. J Pers Med. 2020;10:1–33. doi: 10.3390/jpm10030080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yao X, Hamilton RG, Wen N, et al. Frailty is associated with impairment of vaccine-induced antibody response and increase in post-vaccination influenza infection in community-dwelling older adults. Vaccine. 2011;23:2015–2021. doi: 10.1016/j.vaccine.2011.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pagan LU, Gomes MJ, Damatto RL, et al. Aerobic exercise during advance stage of uncontrolled arterial hypertension. Front Physiol. 2021;12:1–10. doi: 10.3389/fphys.2021.675778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paula TP, Viana LV, Neto ATZ, et al. Effects of the DASH diet and walking on blood pressure in patients with type 2 diabetes and uncontrolled hypertension: a randomized controlled trial. J Clin Hypertens. 2015;17:895–901. doi: 10.1111/jch.12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wake AD. Antidiabetic effects of physical activity: how it helps to control type 2 diabetes. Diabetes Metab Syndr Obes. 2020;13:2909–2923. doi: 10.2147/DMSO.S262289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lippi G, South AM, Henry BM. Electrolyte imbalances in patients with severe coronavirus disease 2019 (COVID-19) Ann Clin Biochem. 2020;57:262–265. doi: 10.1177/0004563220922255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Udensi UK, Tchounwou PB. Potassium homeostasis, oxidative stress, and human disease. Int J Clin Exp Physiol. 2017;43:111–122. doi: 10.4103/ijcep.ijcep_43_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Recovery Collaborative Group Dexamethasone in hospitalized patients with COVID-19—preliminary report. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Echeverría-Esnal D, Martin-Ontiyuelo C, Navarrete-Rouco ME, et al. Azithromycin in the treatment of COVID-19: a review. Expert Rev Anti Infect Ther. 2021;19:147–163. doi: 10.1080/14787210.2020.1813024. [DOI] [PubMed] [Google Scholar]
- 52.Lee SW, Lee J, Moon SY, et al. Physical activity and the risk of SARS-CoV-2 infection, severe COVID-19 illness and COVID-19 related mortality in South Korea: a nationwide cohort study. Br J Sports Med. 2021 doi: 10.1136/bjsports-2021-104203. [DOI] [PubMed] [Google Scholar]
- 53.Hu B, Huang S, Yin L. The cytokine storm and COVID-19. J Med Virol. 2021;93:250–256. doi: 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marino FE, Vargas NT, Skein M, et al. Metabolic and inflammatory health in SARS-CoV-2 and the potential role for habitual exercise in reducing disease severity. Inflamm Res. 2021 doi: 10.1007/s00011-021-01517-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matias TS, Dominski FH. The COVID-19 pandemic challenges physical activity with two emerging paradigms. Rev Bras Ativ Fís Saúde. 2020;25:1–6. doi: 10.12820/rbafs.25e0113. [DOI] [Google Scholar]
- 56.Jahangiry L, Farhangi MA, Shab-Bidar S, et al. Web-based physical activity interventions: a systematic review and meta-analysis of randomized controlled trials. Public Health. 2017;152:36–46. doi: 10.1016/j.puhe.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 57.Cleland C, Ferguson S, Ellis G, et al. Validity of the International Physical Activity Questionnaire (IPAQ) for assessing moderate-to-vigorous physical activity and sedentary behavior of older adults in the United Kingdom. BMC Med Res Methodol. 2018;18:1–12. doi: 10.1186/s12874-018-0642-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Castañeda-Babarro A, Arbillaga-Etxarri A, Gutiérrez-Santamaría B, et al. Physical activity change during COVID-19 confinement. Int J Environ Res Public Health. 2021;17:1–10. doi: 10.3390/ijerph17186878. [DOI] [PMC free article] [PubMed] [Google Scholar]