Abstract
Long-lived proteins (LLPs) are susceptible to the accumulation of both enzymatic and spontaneous post-translational modifications (PTMs). A prominent PTM observed in LLPs is covalent protein-protein crosslinking. In this study we examined aged human lenses and found several proteins to be crosslinked at Glu and Gln residues. This new covalent bond involves the amino group of Lys or an α-amino group. A number of these crosslinks were found in intermediate filament proteins. Such crosslinks could be reproduced experimentally by incubation of Glu- or Gln-containing peptides and their formation were consistent with an amino group attacking a glutarimide intermediate. These findings show that both Gln and Glu residues can act as sites for spontaneous covalent cross-linking in LLPs and they provide a mechanistic explanation for an otherwise puzzling observation, that a major fraction of Aβ in the human brain is crosslinked via Glu 22 and the N-terminal amino group.
Keywords: Protein crosslinks, Human lens, Alzheimer’s disease, Abeta, Long-lived proteins, Age
Introduction
Through recent advances in the analysis of nuclear bomb test-derived 14C, pulse-chase experiments and high-resolution mass spectrometry, it has become apparent that many cells within the human body are long-lived [1–3]. Some cells, such as lens fibre cells exist for a lifetime [4], as do most neurons [3, 5]. Cells in other human organs can also be very long-lived. For example, human cardiomyocyte turnover is <1% per year in adults [6] and intestinal cells are, on average, 15.9 years old [3]. The pancreas has been described as a “mosaic” in that the organ is composed of different types of cells, with some such as beta cells being newly synthesised, whereas others such as alpha, delta and stellate cells appear to be as old as neurons [1]. Within these long-lived cells, a number of proteins show little turnover with some such as nucleoporins [7] and lens proteins existing for a lifetime [4, 8].
Due to their extensive dwell time in the body, long-lived proteins (LLPs) accumulate many post-translational modifications (PTMs). Some modifications are generated enzymatically, for example, phosphorylation and citrullination. Reactive metabolites, such as sugars can covalently modify LLPs without the need for enzymes [9]. Generally less studied has been the spontaneous breakdown of amino acids. These processes can generate reactive intermediates, and multiple products, some of which ultimately form covalent protein-protein crosslinks [10, 11].
While processes such as those leading to advanced glycation end-products (AGE) cross-linked proteins have been well studied [9], other mechanisms of crosslinking are currently poorly characterised. As part of an ongoing collaborative study to identify novel sites of protein-protein crosslinks in the human lens, we have elucidated several novel mechanisms by which amino acid residues in LLPs can breakdown leading to protein-protein crosslinking [10, 12, 13]. Recently, Asn/Asp residues in LLPs from the human lens were found to initiate protein-protein crosslinking via a succinimide (Asu) intermediate that is also involved in deamidation, isomerisation and racemisation of Asn and Asp residues [14].
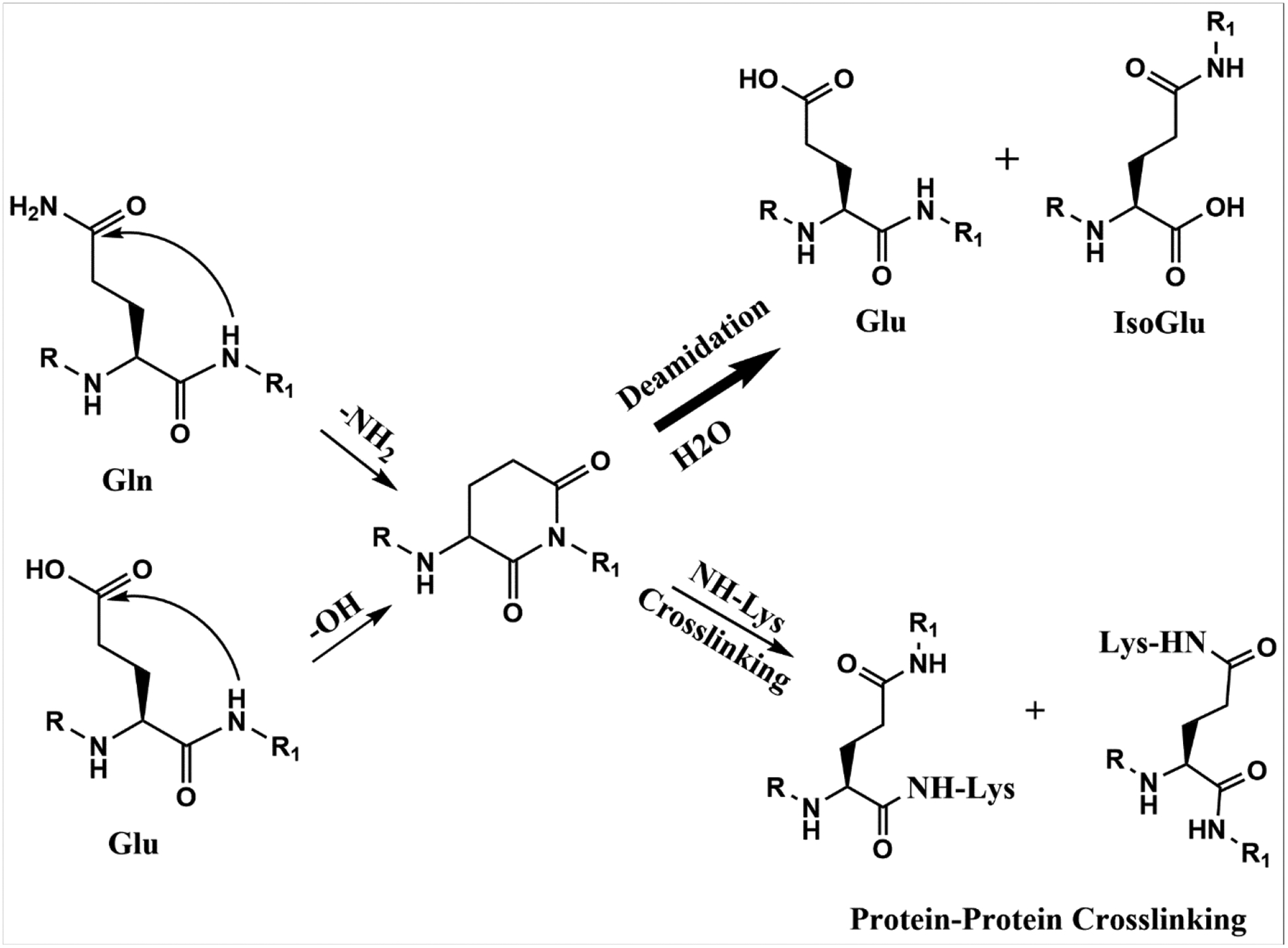
Gln residues are thought to undergo deamidation analogously through a six-membered glutarimide (Gsu) intermediate [15, 16] (See figure 1). While data supporting this mechanism are incomplete, recent evidence of isoGlu in proteins from the human brain and lens suggests Gln deamidation can occur via this process [16, 17]. Utilizing proteomic analysis we examined human lenses and found evidence of protein crosslinking at several Gln and Glu sites. By use of model peptides, the process of spontaneous Gln/Glu crosslink formation was investigated.
Figure 1. Proposed mechanisms of Gln deamidation and Glu breakdown in polypeptides.
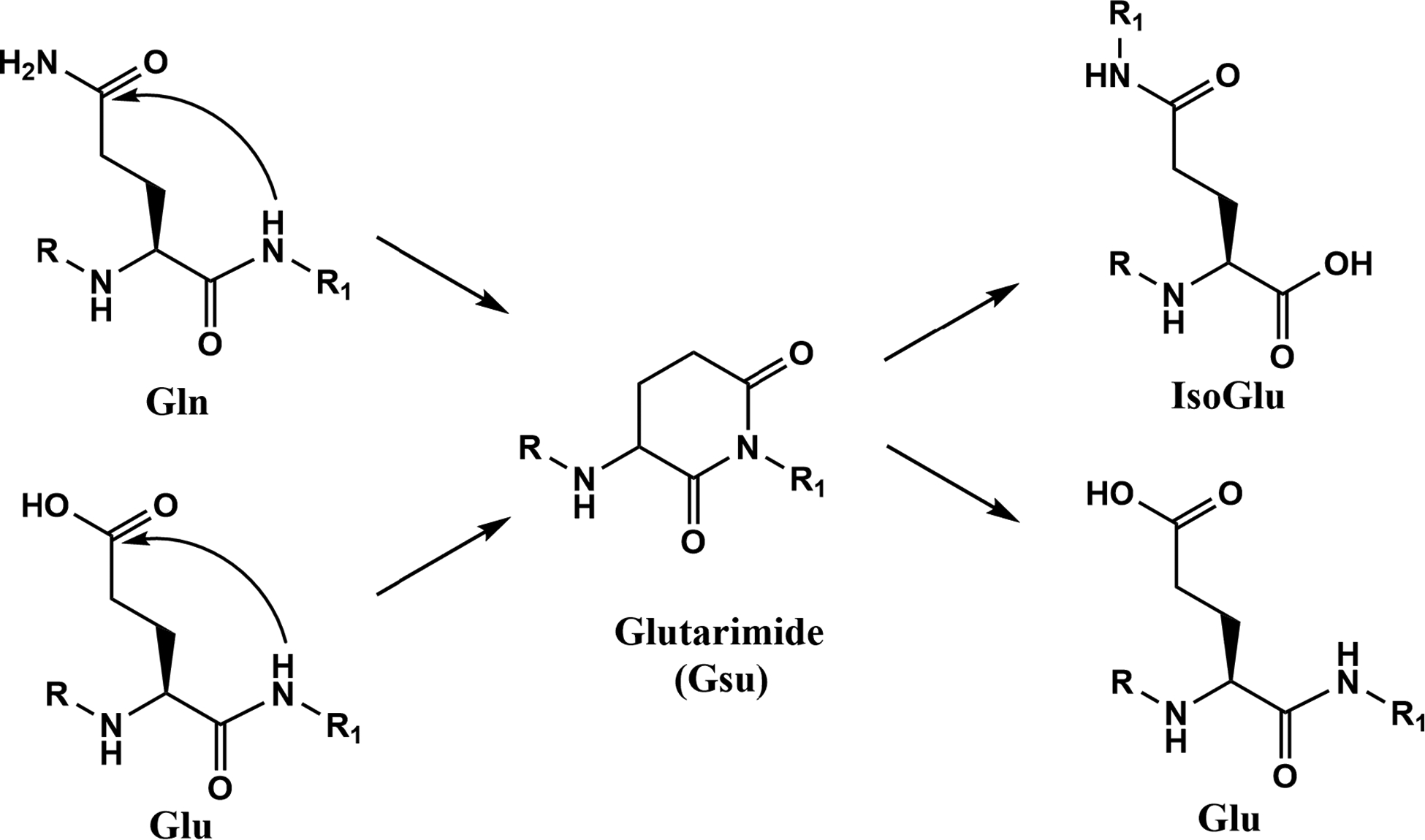
Gln deamidation is thought to proceed via a Gsu intermediate as is the isomerisation of Glu. Depending on the site of Gsu hydrolysis, either Glu or isoGlu can be formed. Direct hydrolysis of the side chain Gln amide may also occur but this route of deamidation is observed more frequently at low pH [16].
Methods
Lens protein extraction
Frozen human lenses were obtained from NDRI (Philadelphia, PA). All lenses were isolated from the donor no later than 8 hours post mortem and shipped on dry ice. Human lens work was conducted in compliance with the Declaration of Helsinki. The lenses were classified as normal or cataract lenses by the eye bank. All lenses received were stored at −80°C until use.
To identify crosslinked peptides, the urea-insoluble fraction from the nucleus region of a cataract lens aged 71y were prepared and digested by trypsin (Thermo Fisher Scientific, Rockford, IL) as described previously [18]. Tryptic peptides were fractionated by offline SCX as described previously [9] and peptides were step-eluted sequentially from SCX resins with 40%, 60% and 100% buffer B (5 mM potassium phosphate buffer containing 30% ACN, 350 mM KCl, pH 2.5) balanced with buffer A (5 mM potassium phosphate buffer containing 30% ACN, pH 2.5). Peptides from 10 μg of total protein were fractioned using 1 mg of SCX resins (Luna SCX, 5 μm, 100 Å media, Phenomenex, Torrance, CA). 40%−100% buffer B eluates were directly analyzed by one-dimensional LC-MS/MS. The amount of sample loaded on the column corresponded to 1 μg of the original digest for 40% buffer B eluate and 2–3 μg original digest for 60–100% buffer B eluates. In addition, urea-soluble fractions (USF) from a 19 y and 48 y normal lens and a 57 y cataract lens were also prepared as described previously [19].
LC-MS/MS
For LC-MS/MS analysis, tryptic peptides were separated on a one-dimensional fused silica capillary column (250 mm × 100 μm) packed with Phenomenex Jupiter resin (3 μm mean particle size, 300 Å pore size). An 82-minute gradient was performed, consisting of the following: 0–77 min, 2–45% ACN (0.1% formic acid); 77–82 min, 45–98% ACN (0.1% formic acid) balanced with 0.1% formic acid. The eluate was directly infused into a Q Exactive Plus instrument (Thermo Scientific, San Jose, CA) with a nanoelectrospray ionization source. A data-dependent acquisition method consisted of MS1 acquisition (R=70,000), using an MS AGC target value of 3e6, followed by up to 20 MS/MS scans (R=17,500) of the most abundant ions detected in the preceding MS scan. The MS2 AGC target value was set to 1e5, with a maximum ion time of 100 ms, and an intensity threshold of 4e4. HCD collision energy was set to 28, dynamic exclusion was set to 15 s.
Data Analysis
For crosslinked peptide identification, the raw data were searched using pLink 2.0 [20] to generate candidate crosslinked peptides. The crosslinker setting was set to search for crosslinking of Lys and Gln residues with ammonia loss and Lys and Glu residues with water loss. Other parameters included trypsin-specific cleavage with a static modification of carbamidomethylation of cysteine, variable modification of oxidation of methionine, acetylation of protein N-terminus and deamidation of Asn, precursor mass deviation of <5 ppm, and fragment ions mass deviation<10 ppm. Manual analysis was then performed to identify crosslinked peptides from the list of candidate peptides generated from pLink. During manual verification, the presence of a series of b- or y-ions from both peptides were required to be considered as a correct identification.
Incubation of Glu and Gln peptides
Ac-YAQTT and Ac-YAETT (1 mg/mL) were incubated at 60 °C in 50mM MOPS buffer at pH 6.7, 7.4 and 8.0. Ac-FAEAA and Ac-FAEPA were incubated at 60 °C in 50mM MOPS buffer at pH 6.7. Aliquots were injected onto an Agilent 1100 HPLC using an Aeris RP-HPLC column (2.6 μ, XB-C18, 100 × 2.1 mm, Phenomenex). The elution gradient was as follows: 2% ACN, 0.1% TFA 5 min, 20% ACN, 0.1% TFA 15 mins, 40% ACN, 0.1%TFA 20 min, 25 min 80%ACN, 0.1% TFA, 35 min 80% ACN, 0.1% TFA, 30.1 min 2% ACN, 0.1%TFA, 40 min 2% ACN, 0.1%TFA.
Breakdown of Ac-YAQTT, Ac-YAETT, Ac-FAEAA and Ac-FAEPA was monitored at 216nm and 280nm. Peaks were collected and identified by tandem mass spectrometry on an LTQ Fusion orbitrap (Thermo Scientific, San Jose, CA). The above experiments were repeated with peptides incubated in the presence of a 5-molar excess of either phenylethylamine (PE) or the dipeptide GlyAla in 50mM MOPS 6.7 at 60 °C. All experiments were performed in triplicate.
Methylation of Glu
Attempts to promote the formation of a Gsu intermediate were undertaken using a Glu containing peptide Ac-YAETT. The peptide (2 mg) was initially dissolved in 50uL of 100% MeOH and acidified with a drop of concentrated HCl and incubated at RT for 30 min. Samples were dried down under vacuum and reconstituted in 0.1% TFA. Peptide containing Ac-YA(Glu-OCH3)TT were isolated by HPLC using an Aeris peptide RP-HPLC column (2.6 μ, XB-C18, 100 × 2.1 mm, Phenomenex) using the same gradient as shown above. All peptides containing methylated Glu residues were confirmed by tandem mass spectrometry and dried down before further experiments.
Ac-YA(Glu-OCH3)TT was incubated in 50mM MOPS 6.7 for 72 hrs at 60 °C. Formation of Gsu was monitored by HPLC using the method highlighted above. As the Gsu was unstable and could be only isolated in tiny quantities, the interaction of Ac-YA(Glu- OCH3)TT with free amino groups was examined. Ac-YA(Glu-OCH3)TT was incubated with a five molar excess of PE in 50 mM MOPS pH 6.7 at 60 °C. Aliquots were removed at selected times and analysed by RP-HPLC. Peaks corresponding to the peptides cross-linked with PE were identified by mass spectrometry.
Results
Identification of protein-protein crosslinking in human lenses
Previously we have reported sites of protein-protein crosslinking at Asn and Asp residues in human lenses [21]. These irreversible protein-protein crosslinks were found to be mediated by a five-membered succinimide intermediate that is also intimately involved in the processes of deamidation, isomerisation and racemisation. Since Gln deamidation has been implied to occur via an analogous mechanism [16] (see figure 1), we investigated tryptic peptides of human lens proteins for evidence of Gln or Glu crosslinking. Software for crosslinked peptide identification provided candidate crosslinked peptides. Together with manual verification and De Novo sequencing, we identified 8 crosslinked peptides through Gln residues and two crosslinked peptides through Glu residues. These peptides can be found in Table 1. The crosslinked peptides identified in human lenses indicated that Gln can crosslink with ε-amino group of lysine or α-amino group of a protein N-terminus. One crosslink was formed between Q329 and the N-terminus of C65 of phakinin. The tandem mass spectrum for this crosslinked peptide can be found in Figure 1. Phakinin 65–72 (CIGGLGAR) is a non-tryptic peptide and C65 is a new N-terminus formed after protein truncation. Literature precedents have shown the peptide bond on the N-terminal side of cysteine can undergo spontaneous scission [22, 23]. To confirm the presence of this new N-terminus, a signal corresponding to the linear phakinin 65–72 was searched for and detected. The tandem mass spectrum for this linear peptide can be found in the supplemental. The involvement of an α-amino group suggested a nonenzymatic mechanism of crosslinking.
Table 1.
Sites of Gln/Glu crosslinks identified in the human lens
| Site of Gln Crosslink | Site of Lys or N-terminal | [MH]+Exp | [MH]+Cal | Error (ppm) |
|---|---|---|---|---|
| Filensin: VELQAQ*TTTLEQAIK | Phakinin: ALK*R | 2142.2129 | 2142.2151 | 1.02 |
| Phakinin: HWHDMELQ*NLGAVVGR | Filensin: K*EQYEHADEASR | 3306.5345 | 3306.5406 | 1.84 |
| αB: KQ*VSGPER | αA: VQDDFVEIHGK*HNER | 2705.339 | 2705.3413 | 0.85 |
| βB1: Q*WHLEGSFPVLATEPPK | Filensin: EK*VR | 2448.300812 | 2448.3034 | 1.06 |
| βB2: DMQWHQR | βB2: TDSLSSLRPIKVDSQEHK | 3022.4835 | 3022.4799 | 1.85 |
| Phakinin: VELHNTSCQVQ*SLQAETESLR | Phakinin: *CIGGLGAR | 3214.5579 | 3214.5578 | 0.04 |
| αB: Q#VSGPER | αB: LEK#DR | 1414.7259 | 1414.7285 | 1.83 |
| αA: VQ#DDFVEIHGK | αB: EEK#PAVTAAPK | 2409.2251 | 2409.2296 | 1.85 |
| Site of Glu Crosslink | Site of Lys | |||
| αB: VLGDVIE*VHGK | αB: K*YR | 1612.9141 | 1612.917 | 1.82 |
| γD: HYE*CSSDHPNLQPYLS | γD: G(carbamyl)K*ITLYEDR | 3221.5063 | 3221.5069 | 1.34 |
Asterisks indicate the residues that are involved in crosslinking.
Breakdown of Gln
In the past, protein-protein crosslinking between Gln and Lys residues has been attributed to the enzymatic action of transglutaminase [24]. However transglutaminase has been reported to be localized to the epithelial layer of the human lens, [25] which is consistent with the lack of other enzyme activities in the lens nucleus due to thermal denaturation over our lifetime [26]. To understand the reason for the formation of Gln/Lys crosslinks in the absence of transglutaminase and also linkage via α-amino groups, model studies were undertaken using synthetic peptides.
The synthetic peptide sequence AQTT contains the Gln crosslinking site observed in filensin (Table 1) and this peptide was synthesised with the addition of a Tyr residue on the N-terminus to aid in HPLC detection and the α-amino group was blocked by acetylation. Ac-YAQTT was incubated in 50 mM MOPS at pH 6.7 and the breakdown of Ac-YAQTT monitored by HPLC (See figure 3). After two weeks of incubation, several breakdown products were detected and these were identified by mass spectrometry. Two peaks eluting at 17.2 min and 17.4 min were found to correspond to deamidation of Ac-YAQTT. By comparison with standards corresponding to Ac-YAETT and Ac-YA(isoGlu)TT, the earlier eluting peak was identified as Ac-YA(isoGlu)TT. The MS/MS of synthetic Ac-YA(isoGlu)TT also showed some diagnostic ions that allowed confirmation of its identity (data not shown). A low abundance peak was observed eluting close to Ac-YAETT at 17.8 mins. Upon isolation and mass spectral analysis it was revealed to have a mass of 608.25 Da that corresponded to the loss of 17 Da from the predicted parent peptide mass. Tandem mass spectrometry analysis showed it to be consistent with a cyclic Gsu peptide (Figure 4). Concurrently with deamidation of Ac-YAQTT, a broad peak eluting at 14.5min was detected (Figure 3). Analysis by mass spectrometry confirmed this peak to be Ac-YAQ (data not shown), indicating that cleavage on the C-terminal side of the Gln residue had also occurred.
Figure 3. HPLC profiles of Ac-YAQTT and methylated Ac-YAETT incubated in the presence of PE. Methylation increases glutarimide formation.
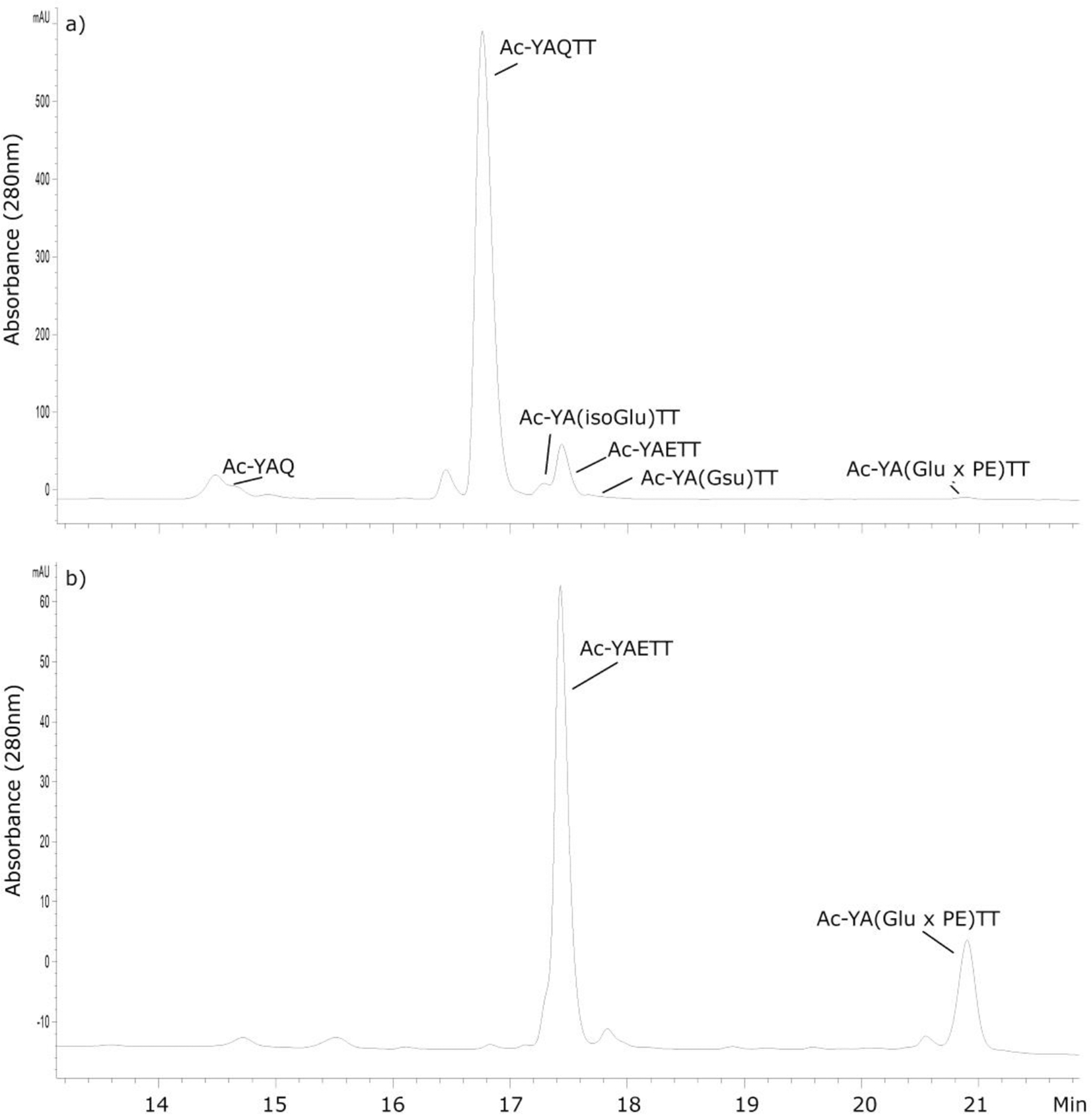
a) HPLC profile of Ac-YAQTT after 100 days of incubation with a 5-molar excess of PE at 60 °C, pH 6.7. b) HPLC profile of Ac-YA(E-OCH3)TT incubated for 7 days at 60°C, pH 6.7. HPLC peaks were identified by tandem mass spectrometry together with the elution time of synthetic standards. HPLC detection was at 280nm. Glutarimide (Gsu); Glu crosslinked with PE; (Glu × PE).
Figure 4. MS/MS spectrum of Ac-YA(Gsu)TT.
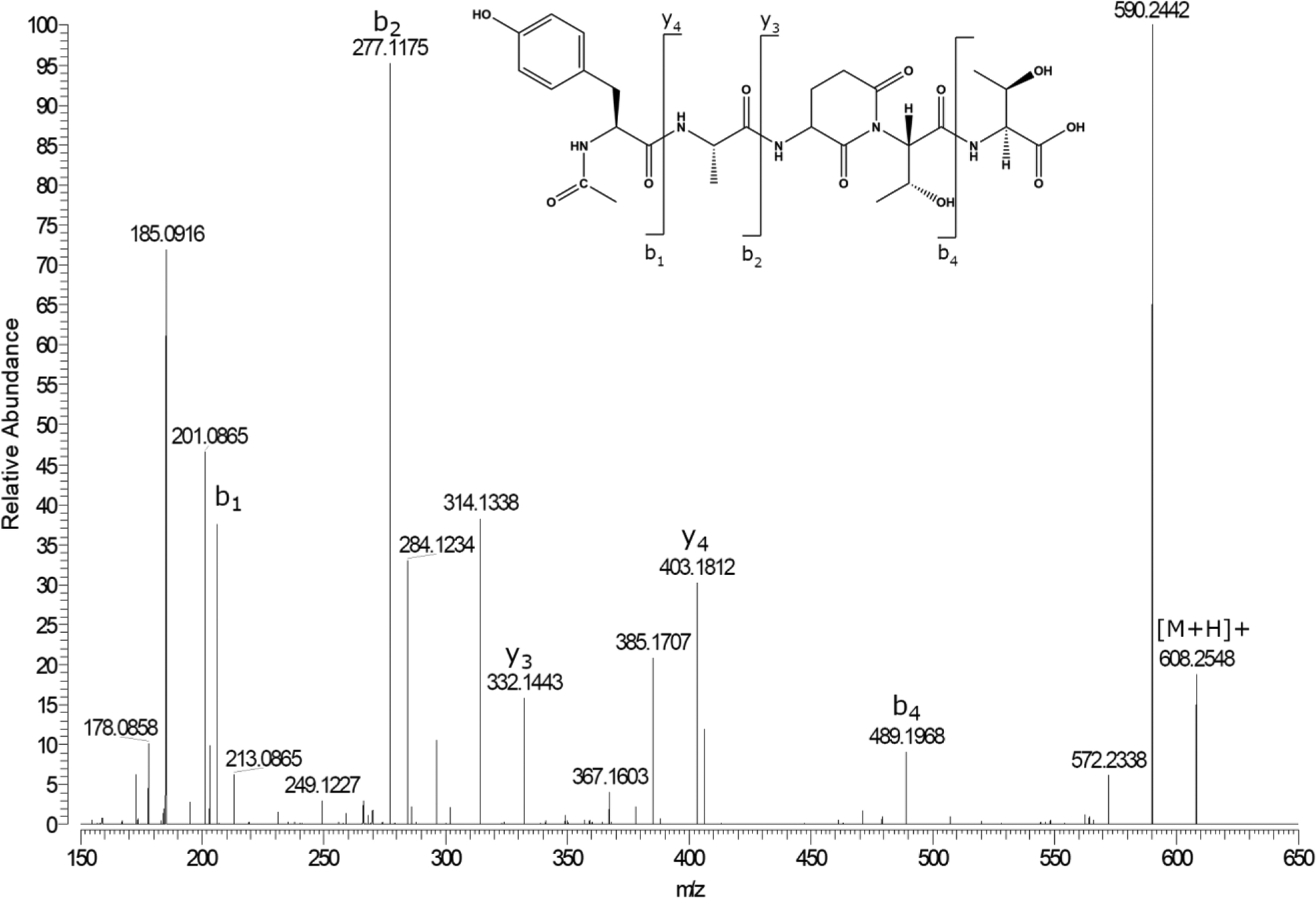
Ac-YA(Gsu)TT was isolated by HPLC at a retention time of 17.8 minutes(Fig 3).
Deamidation of Gln
As alluded to previously, spontaneous crosslinking involving an Asn residue occurs via the same succinimide intermediate that is involved in Asn deamidation and this process is pH-dependent [19]. To determine if Gln residues may also deamidate in a similar pH-dependent manner, deamidation was examined as a function of pH. Three pHs were employed to monitor deamidation of Gln; pH 6.7 corresponding to the pH of the lens nucleus, pH 7.4 the pH of blood and extracellular fluid and an elevated pH of 8.0 to accelerate deamidation of Gln. Deamidation occurred more readily at pH 8.0, being ~1.5 faster than at pH 7.4 and ~2.5 fold faster than pH 6.7 (Figure 5). These results suggest that, as is the case with Asn, deamidation of Gln residues may occur via a similar cyclic intermediate, in this case a Gsu. Rates were comparable to what has been reported previously for deamidation of Gln residues [16], but were considerably slower than those observed for Asn deamidation [21].
Figure 5. Deamination of Ac-YAQTT is dependent on pH.
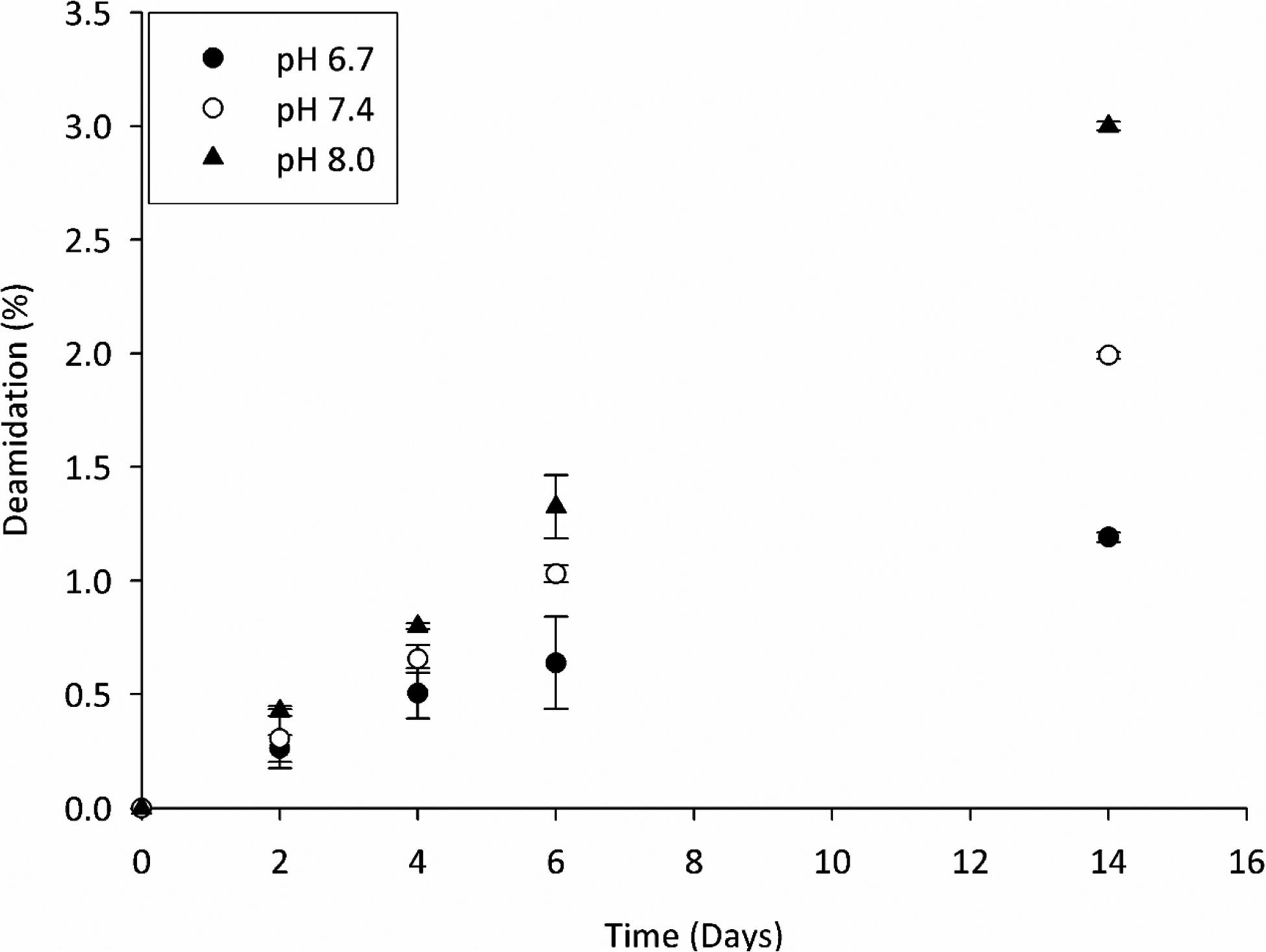
Deamidation at pH 6.7, pH 7.4 and pH 8.0 was determined by the combined HPLC peak areas of Ac-YAETT and Ac-YA(isoGlu)TT at 280nm. All experiments were in triplicate and time points are averages +/− SD. Samples were incubated at 60°C.
Breakdown of Glu residues
The stability of Ac-YAETT was investigated under the same conditions as used for Ac-YAQTT. Similar to Ac-YAQTT, breakdown of Ac-YAETT was slow when incubated at 60°C, in pH 6.7 buffer. Ac-YAETT displayed several breakdown products in low amounts after two weeks. These products corresponded to Ac-YA(isoGlu)TT and Ac-YAE. Formation of a peak consistent with Ac-YA(Gsu)TT was only detected by HPLC after 100 days incubation (Supplemental figure 3).
Spontaneous formation of Gln/Glu crosslinks
Both Ac-YAQTT and Ac-YAETT were incubated at 60 °C with a five-molar excess of a Lys mimic, phenylethylamine (PE) at pH 6.7. Formation of the Ac-YA(E × PE)TT was slow with a peak not initially visible by HPLC, although it was detected by mass spectrometry after 20 days of incubation. After 100 days of incubation, peaks corresponding to both Ac-YAQTT and Ac-YAETT crosslinked with PE were visible by HPLC and their identities were confirmed by mass spectrometry (Figures 3b & 6). The yield of the PE crosslink from both Ac-YAQTT and Ac-YAETT was estimated to be ~ 0.3% of the original peptide based on peak area after 100 days.
Figure 6. MS/MS spectrum of Ac-YA(Glu X PE)TT.
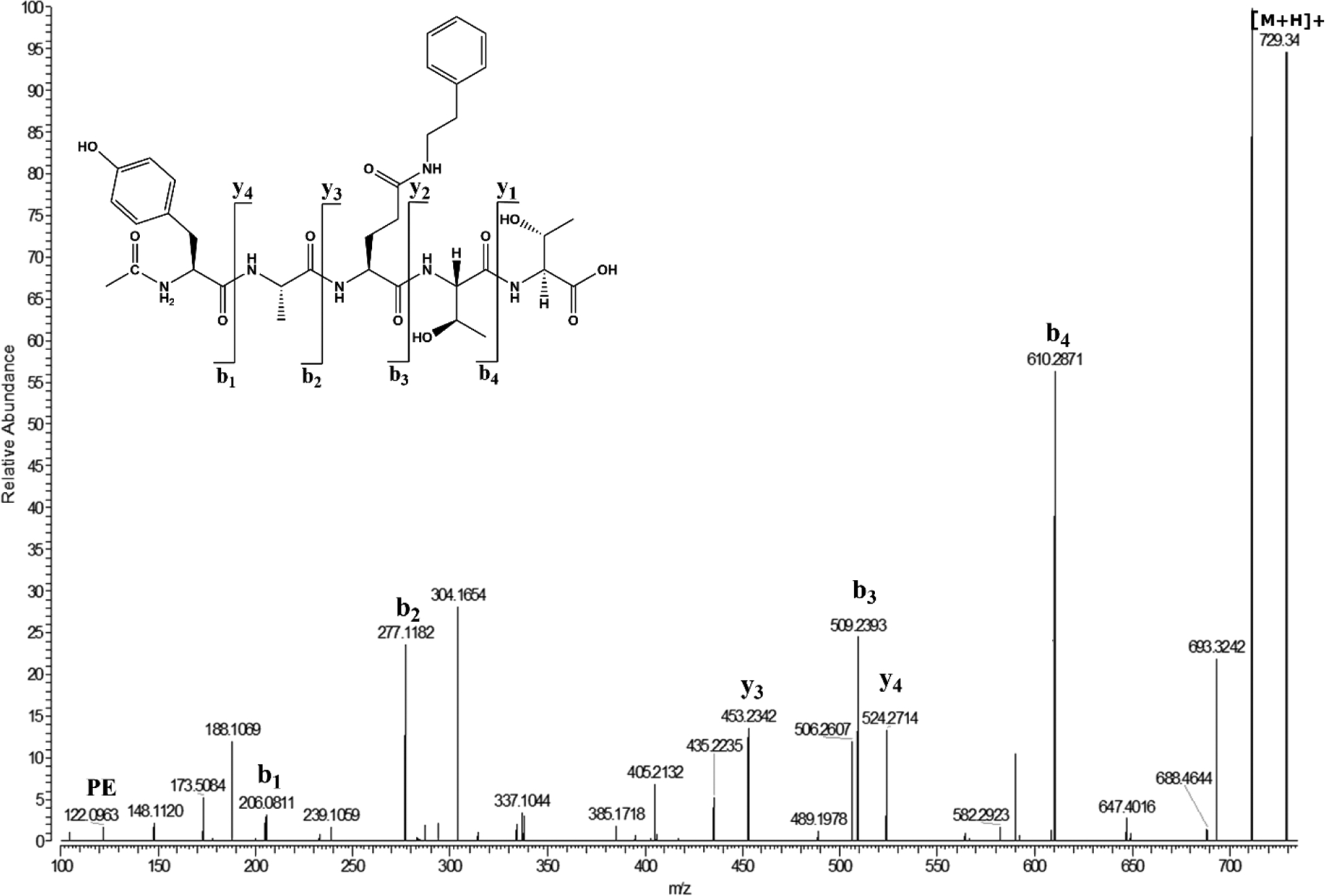
Ac-YAQTT was incubated with a five-molar excess of PE in 50mM MOPS buffer pH 6.7 at 60°C for 100 days and Ac-YA(Glu X PE)TT was isolated by HPLC. The same crosslinked compound was obtained by incubation of Ac-YAETT in the presence of PE, based on retention time and MS/MS spectra.
Crosslinking via Glutarimide.
The data obtained suggested that the crosslinking observed at Gln/Glu sites may be occurring via a Gsu intermediate. To test this, the Glu hydroxyl group from Ac-YAETT was methylated to render it a better leaving group and to encourage cyclisation. Purified Ac-YA(Glu-OCH3)TT was then incubated in 50mM MOPS pH 7.4 with aliquots taken over seven days. Analysis by HPLC revealed gradual hydrolysis of the methyl ester and the formation of Ac-YAETT which was confirmed by mass spectrometry. The observed low levels of Gsu formation, suggest that the Gsu intermediate may not be stable under these conditions and this factor limited further studies on the direct interaction of Gsu with an amine.
As shown in Figure 3b when Ac-YA(Glu-OCH3)TT was incubated for seven days in the presence of PE at pH 6.7 a peak was observed eluting at the same retention time as Ac-YA(E × PE)TT and its identification was confirmed by tandem mass spectrometry. By comparison after seven days of incubation under the same conditions using either Ac-YAQTT or Ac-YAETT no PE crosslink could be detected.
To further delineate the crosslinking mechanism, two peptides Ac-FAEAA and Ac-FAEPA were incubated in the presence of a dipeptide GlyAla. At neutral pH, α−amino groups will be better nucleophiles than the ε-amino group of Lys. With a C-terminal Pro in the sequence, the nitrogen atom of the peptide bond is unavailable for the formation of a Gsu (see Figure 1). After 20 days of incubation of Ac-FAEAA with GlyAla in 50mM MOPS pH 6.7, direct analysis of the incubation mixture by mass spectrometry showed Ac-FA(E × GA)AA had formed (Figure 7). By comparison, using Ac-FAEPA, there was no evidence of Ac-FA(E × GA)PA by either HPLC or direct analysis by mass spectrometry. Ac-FAEPA cleavage and formation of Ac-FAE were prominent however, consistent with spontaneous cleavage. Interestingly, a C-terminal crosslink of Ac-FAE to GlyAla was detected. (See supplemental figure 5) that is consistent with the formation of an unstable Glu anhydride. This process is presumably analogous to C-terminal crosslinking via Asp residues [13] and was not investigated further.
Figure 7. Incubation of Ac-FAEAA in the presence of GlyAla also leads to crosslink formation.
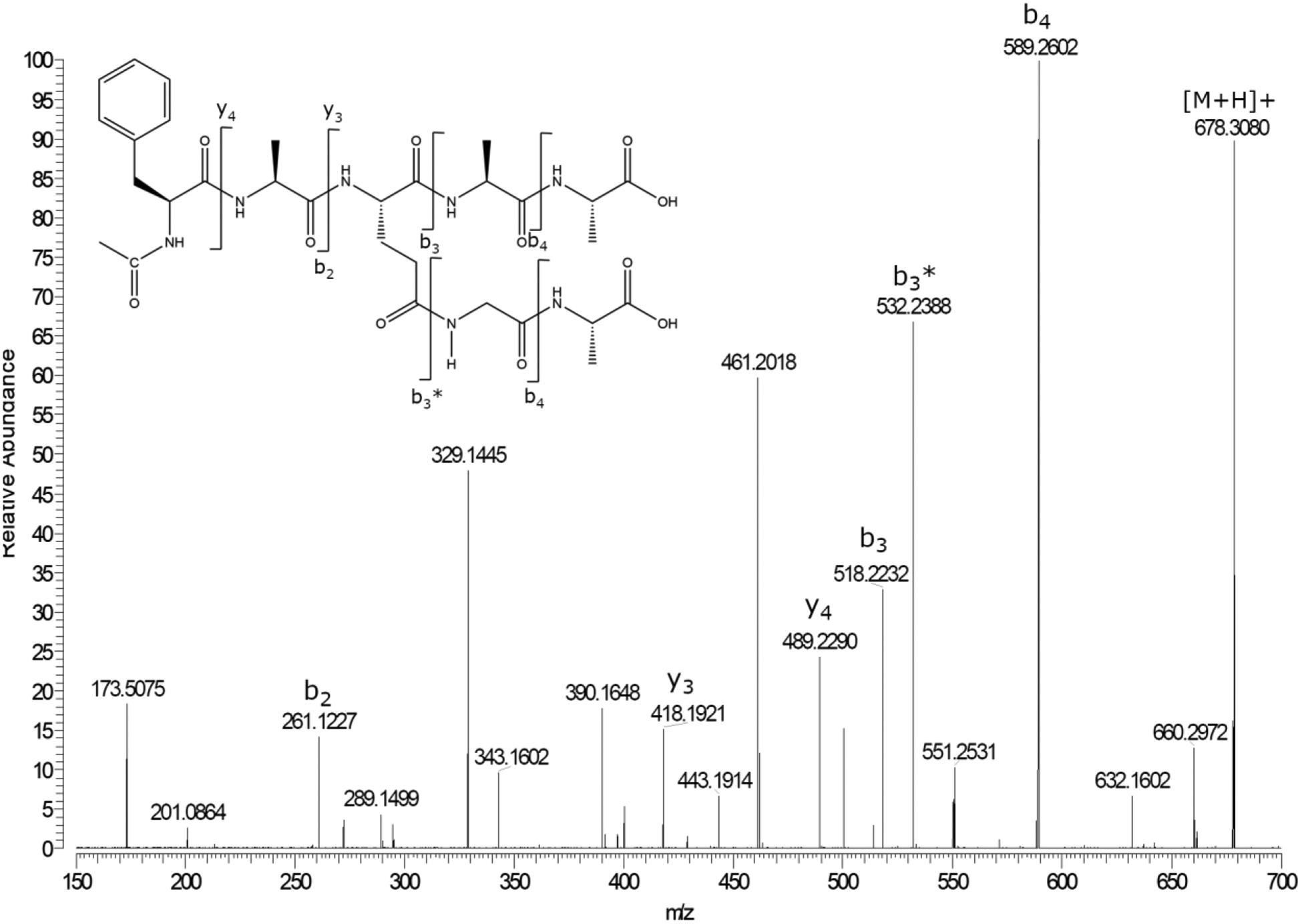
MS/MS spectrum of Ac-FA(Glu × GA)AA. Ac-FA(Glu X GA)AA formed after a 20 day incubation of Ac-FAEAA with 5-molar excess of GA, pH 6.7.
Discussion
This paper describes a spontaneous mechanism by which protein-protein crosslinking can occur at Gln and Glu residues. The mechanism appears to involve cyclisation of Gln and Glu residues forming a Gsu and this intermediate is formed when the peptide backbone amide nitrogen attacks the side chain carbonyl (See Figure 1). The resulting Gsu can then hydrolyse forming Glu and isoGlu, or it can react with free amines such as lysine or free α-amino groups forming an isopeptide bond between two proteins (Figure 8). Data from human lenses revealed several sites of crosslinking at Gln and Glu resides that were consistent with this mechanism (Table 1). These crosslinked sites were found primarily to involve long-lived cytosketelal proteins (filensin and phakinin) and lens crystallins. As expected, signals for these crosslinked peptides were normally low.
Figure 8. Proposed mechanism of spontaneous breakdown of glutamine and glutamic acid residues and crosslinking in LLPs.
Attack of the peptide bond nitrogen atom on the side chain carbonyl leads to the formation of a Gsu intermediate. In the case of Gln residues, this leads to deamidation. Hydrolysis of the Gsu will lead to formation of Glu and isoGlu. If a Lys amino or α-amino group are in proximity to the Gsu intermediate, an isopeptide crosslink can form.
Gln-Lys crosslinks in proteins have previously been attributed to the activity of transglutaminase [27]. The current study highlights an alternative mechanism by which they may form in long-lived proteins. Gln/Glu crosslinking observed in this study was detected primarily in the oldest region of the lens, the nucleus, where transglutaminase activity is absent [25]. Though we cannot completely rule out transglutaminase involvement, the fact that crosslinks were observed involving both an N-terminal amine, as well as Glu residues, and in model peptides that were incubated in the absence of enzymes, indicates that an alternative mechanism is leading to protein-protein crosslinking in the human lens.
Previously we reported a novel crosslink formed at internal Asn and Asp residues via an isopeptide bond with Lys residues [21]. It was determined that a succinimide intermediate that occurs spontaneously at both Asn and Asp residues could be attacked by the ε-amino group of Lys resulting in the formation of a covalent bond. We hypothesize that crosslinking at Gln/Glu residues occurs by an analogous mechanism. In this case, a 6-membered Gsu intermediate is attacked by the epsilon ε-amino group of Lys or free α-amino group, resulting in the formation of a protein-protein crosslink.
Although Glu/Gln crosslinking in model peptides was detected in low abundance and required extended incubations, human lens proteins have a lifetime for such processes to occur [4]. Methylation of the Glu hydroxyl group, employed to encourage the formation of Gsu intermediate (via addition of a better leaving group), resulted in a decreased time for crosslink formation in comparison to the non-methylated peptide (See figure 3). A Pro residue (which lacks an NH required for Gsu formation) on the C-terminal side of Glu eliminated the formation of the crosslink. These results are indicative that a Gsu is the likely intermediate involved in crosslink formation. It is feasible that cyclisation could also potentially occur via the peptide bond NH group on the N-terminal side of Gln/Glu residue attacking the Gln/Glu carbonyl group [28, 29]. The resultant 5-membered ring, in theory, could lead to crosslinking although no evidence was detected in conditions used in this study.
Unlike the case of Asp/Asn crosslinking, where it was possible to isolate the succinimide intermediates of peptides [21] in the current study, we were not able to isolate Gsu intermediates in quantities needed for additional studies. Presumably this reflects a much lower yield of the intermediate from Glu/Gln peptides, as well as increased lability of the Gsu. Despite this, there is significant evidence that a Gsu intermediate is involved in crosslinking.
This can be summarised as follows: The pH dependence of Gln deamidation (Figure 5) was similar to that of Asn [21] where the pathway is known to involve a cyclic intermediate [14]. Following extended incubation, both Glu and Gln peptides formed products whose MS/MS spectra were consistent with Gsu at the same site as the Glu and Gln residue. isoGlu peptides were formed from peptides Ac-YAQTT and Ac-YAETT (Figures 3 and 6) and these can only be formed via the cyclic intermediate. Furthermore, in the presence of PE (Figure 7) or dipeptides (Figure 8) crosslinks at these sites could be characterised and the structures of the crosslinked products were consistent with the attack of an amine upon a Gsu. All these observations are also in accord with existing data on Gln deamidation [16].
Both Gln and Asn residues in proteins are susceptible to deamidation, although Gln sites are generally more stable. This can be illustrated using the LLP γS-crystallin, that, over time in the human lens, accumulates many PTMs. When this crystallin was examined as a function of age, the extent of deamidation of Asn residues [30] was significantly greater than that of Gln residues [31]. This finding holds across the range of crystallins in the human lens where by age 60 on average, ~22% of Asn have been deamidated compared with ~ 6% Gln [32].
In agreement with these observations on LLPs, the results from peptide model studies showed deamidation of Gln to be a slow process (see figure 5). These findings imply that the formation of protein-protein crosslinks at Gln/Glu residues via a Gsu is likely to be minor in comparison to other spontaneous protein-protein crosslinking processes previously described [13, 19, 21].
Of particular interest was the finding that many of the Gln crosslinks involved intermediate filament proteins (Table 1). This is unlike the other spontaneous protein-protein crosslinks in the lens that we have elucidated previously [10, 13, 19, 21]. It may be that the structural alignments of side chains associated with individual polypeptide subunits in filaments may facilitate the crosslinks in this instance.
Spontaneous formation of such crosslinks may have importance outside the human lens. For example, covalently crosslinked dimers of Aβ account for a significant proportion of amyloid isolated from Alzheimer disease (AD)-affected brains [33, 34]. This crosslinked form is particularly neurotoxic [35]. Recently, in an impressive proteomic investigation, Brinkmalm et al [35] found that this crosslink involves Glu 22 covalently crosslinked to the N-terminal amino group of Asp 1 in Aβ. No mechanism for the formation of this novel crosslink was proposed in this paper, however, it is clear that such a crosslink can form between Glu and an N-terminal amine by spontaneous processes described in this paper (see figure 8). The proximity of Glu22 and Asp 1 in Aβ may explain why these crosslinks form [36].
In conclusion, this study has revealed a mechanism responsible for novel protein-protein crosslinking at Gln and Glu residues in long-lived proteins. Gln/Glu residues cyclize forming a six-membered cyclic intermediate, and nucleophilic attack by an ε-amine of Lys or an α-amino group upon this Gsu can result in the formation of an isopeptide bond. Since deamidation /isomerisation of Gln/Glu residues has been detected in other long-lived proteins such as those in the human brain [17] it raises the possibility that protein-protein crosslinking at Gln and Glu residues could take place in LLPs via these spontaneous mechanisms.
Supplementary Material
Figure 2.
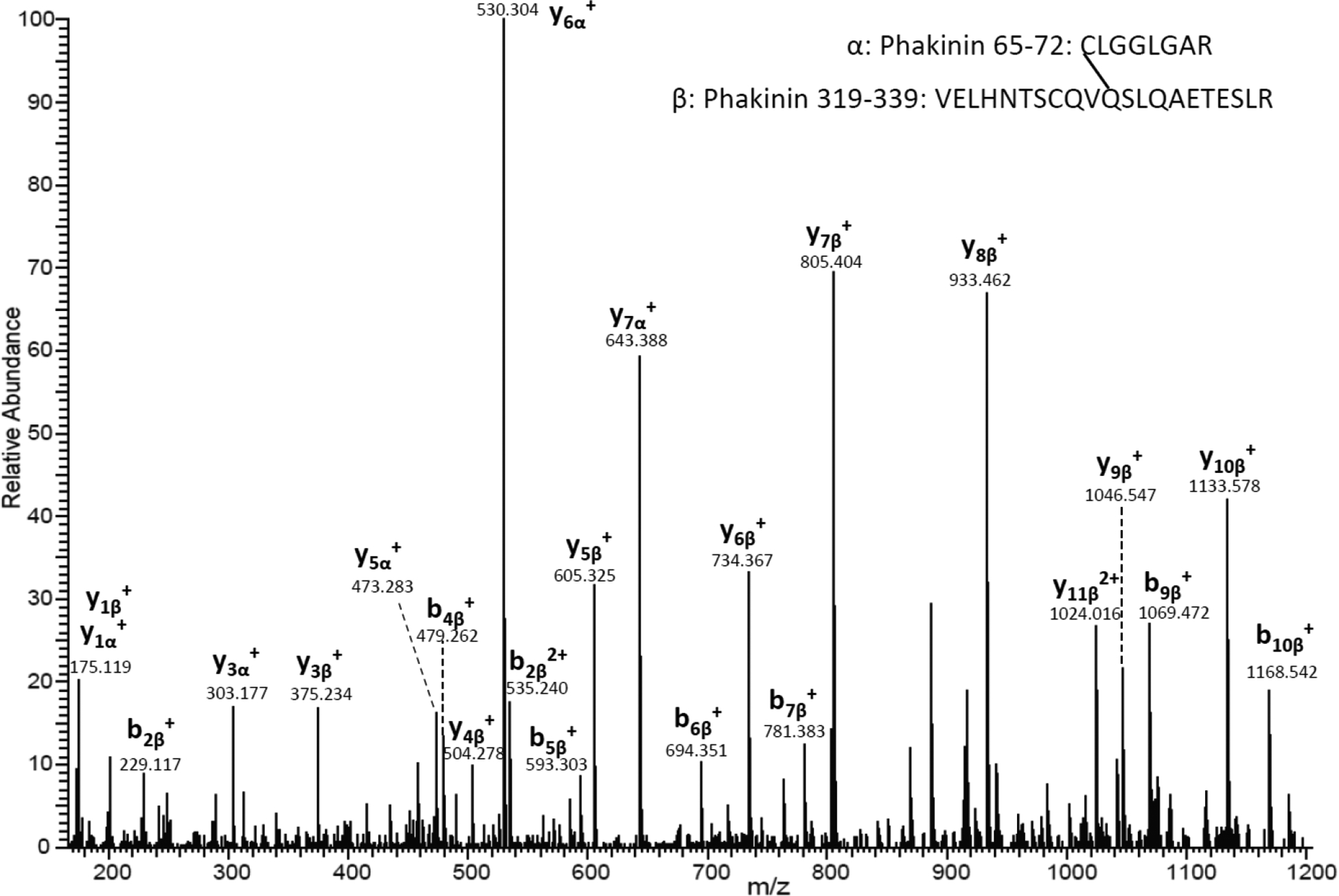
MS/MS spectrum of a crosslinked peptide detected in the tryptic digest of the lens USF. The tandem mass spectrum shows the crosslink between Gln 329 (VELHNTSCQVQ*SLQAETESLR) and α-amino of Cys65 (C*LGGLGAR) from Phakinin.
Acknowledgements:
The authors acknowledge the use of the UOW Mass Spectrometry User Resource and Research Facility (MSURRF), University of Wollongong and the Proteomics Core Facility of the Vanderbilt University Mass Spectrometry Research Center.
Funding sources and disclosure of conflicts of interest
Funding for this study was provided by National Institutes of Health by grants R01 EY024258 and P30 EY008126. The authors declare no conflict of interest
Data availability statement:
The mass spectrometry data generated during this study are openly available in the PRIDE database (Link will be added when available).
References
- 1.Arrojo EDR, et al. , Age Mosaicism across Multiple Scales in Adult Tissues. Cell Metab, 2019. 30(2): p. 343–351.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergmann O, et al. , The age of olfactory bulb neurons in humans. Neuron, 2012. 74(4): p. 634–9. [DOI] [PubMed] [Google Scholar]
- 3.Spalding KL, et al. , Retrospective birth dating of cells in humans. Cell, 2005. 122(1): p. 133–43. [DOI] [PubMed] [Google Scholar]
- 4.Lynnerup N, et al. , Radiocarbon Dating of the Human Eye Lens Crystallines Reveal Proteins without Carbon Turnover throughout Life. PLoS ONE, 2008. 3(1): p. e1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spalding KL, et al. , Dynamics of hippocampal neurogenesis in adult humans. Cell, 2013. 153(6): p. 1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergmann O, et al. , Dynamics of Cell Generation and Turnover in the Human Heart. Cell, 2015. 161(7): p. 1566–1575. [DOI] [PubMed] [Google Scholar]
- 7.Toyama B, et al. , Identification of Long-Lived Proteins Reveals Exceptional Stability of Essential Cellular Structures. Cell, 2013. 154(5): p. 971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fornasiero EF, et al. , Precisely measured protein lifetimes in the mouse brain reveal differences across tissues and subcellular fractions. Nature Communications, 2018. 9(1): p. 4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagaraj RH, et al. , High correlation between pentosidine protein crosslinks and pigmentation implicates ascorbate oxidation in human lens senescence and cataractogenesis. Proc Natl Acad Sci U S A, 1991. 88(22): p. 10257–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Z, et al. , Cleavage C-terminal to Asp leads to covalent crosslinking of long-lived human proteins. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics, 2019. 1867(9): p. 831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z, et al. , Human protein aging: modification and crosslinking through dehydroalanine and dehydrobutyrine intermediates. Aging Cell, 2014. 13(2): p. 226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z, et al. , Proteomics and phosphoproteomics analysis of human lens fiber cell membranes. Invest Ophthalmol Vis Sci, 2013. 54(2): p. 1135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedrich MG, et al. , Mechanism of protein cleavage at asparagine leading to protein–protein cross-links. Biochemical Journal, 2019. 476(24): p. 3817–3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geiger T and Clarke S, Deamidation, isomerization, and racemization at asparaginyl and aspartyl residues in peptides. Succinimide-linked reactions that contribute to protein degradation. J Biol Chem, 1987. 262(2): p. 785–94. [PubMed] [Google Scholar]
- 15.Capasso S, et al. , First evidence of spontaneous deamidation of glutamine residue via cyclic imide to α- and γ-glutamic residue under physiological conditions. Journal of the Chemical Society, Chemical Communications, 1991(23): p. 1667–1668. [Google Scholar]
- 16.Riggs DL, et al. , Analysis of Glutamine Deamidation: Products, Pathways, and Kinetics. Analytical Chemistry, 2019. 91: p. 13032–13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serra A, et al. , Characterization of Glutamine Deamidation by Long-Length Electrostatic Repulsion-Hydrophilic Interaction Chromatography-Tandem Mass Spectrometry (LERLIC-MS/MS) in Shotgun Proteomics. Anal Chem, 2016. 88(21): p. 10573–10582. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z, et al. , Cleavage C-terminal to Asp leads to covalent crosslinking of long-lived human proteins. Biochim Biophys Acta Proteins Proteom, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Z and Schey KL, Quantification of thioether-linked glutathione modifications in human lens proteins. Exp Eye Res, 2018. 175: p. 83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Z-L, et al. , A high-speed search engine pLink 2 with systematic evaluation for proteome-scale identification of cross-linked peptides. Nature Communications, 2019. 10(1): p. 3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedrich MG, et al. , Spontaneous cross-linking of proteins at aspartate and asparagine residues is mediated via a succinimide intermediate. Biochem J, 2018. 475(20): p. 3189–3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patchornik A and Sokolovsky M, Nonenzymatic Cleavages of Peptide Chains at the Cysteine and Serine Residues through their Conversion into Dehydroalanine. I. Hydrolytic and Oxidative Cleavage of Dehydroalanine Residues. Journal of the American Chemical Society, 1964. 86(6): p. 1206–1212. [Google Scholar]
- 23.Ebert C, Ebert G, and Rossmeissl G, On the specific cleavage of cysteine containing peptides and proteins. Adv Exp Med Biol, 1977. 86b: p. 205–11. [DOI] [PubMed] [Google Scholar]
- 24.Heck T, et al. , Enzyme-catalyzed protein crosslinking. Applied microbiology and biotechnology, 2013. 97(2): p. 461–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hidasi V, Adány R, and Muszbek L, Localization of transglutaminase in human lenses. Journal of Histochemistry & Cytochemistry, 1995. 43(11): p. 1173–1177. [DOI] [PubMed] [Google Scholar]
- 26.Zhu X, Korlimbinis A, and Truscott RJ, Age-dependent denaturation of enzymes in the human lens: a paradigm for organismic aging? Rejuvenation Res, 2010. 13(5): p. 553–60. [DOI] [PubMed] [Google Scholar]
- 27.Griffin M, Casadio R, and Bergamini CM, Transglutaminases: nature’s biological glues. The Biochemical journal, 2002. 368(Pt 2): p. 377–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kempkes LJM, et al. , Deamidation Reactions of Asparagine- and Glutamine-Containing Dipeptides Investigated by Ion Spectroscopy. Journal of The American Society for Mass Spectrometry, 2016. 27(11): p. 1855–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nalbone JM, et al. , Glutamic Acid Selective Chemical Cleavage of Peptide Bonds. Organic Letters, 2016. 18(5): p. 1186–1189. [DOI] [PubMed] [Google Scholar]
- 30.Hooi MYS, Raftery MJ, and Truscott RJW, Racemization of Two Proteins over Our Lifespan: Deamidation of Asparagine 76 in γS Crystallin Is Greater in Cataract than in Normal Lenses across the Age RangeRacemization and Cataract Formation. Investigative Ophthalmology & Visual Science, 2012. 53(7): p. 3554–3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hooi MY, Raftery MJ, and Truscott RJ, Age-dependent deamidation of glutamine residues in human gammaS crystallin: deamidation and unstructured regions. Protein Sci, 2012. 21(7): p. 1074–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hains PG and Truscott RJW, Age-Dependent Deamidation of Lifelong Proteins in the Human Lens. Investigative Ophthalmology & Visual Science, 2010. 51(6): p. 3107–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sergeant N, et al. , Truncated beta-amyloid peptide species in pre-clinical Alzheimer’s disease as new targets for the vaccination approach. J Neurochem, 2003. 85(6): p. 1581–91. [DOI] [PubMed] [Google Scholar]
- 34.Shankar GM, et al. , Amyloid-β protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nature Medicine, 2008. 14(8): p. 837–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brinkmalm G, et al. , Identification of neurotoxic cross-linked amyloid-β dimers in the Alzheimer’s brain. Brain, 2019. 142(5): p. 1441–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vivekanandan S, et al. , A partially folded structure of amyloid-beta(1–40) in an aqueous environment. Biochemical and Biophysical Research Communications, 2011. 411(2): p. 312–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The mass spectrometry data generated during this study are openly available in the PRIDE database (Link will be added when available).


