Abstract
Purinergic transmitters such as adenosine, ADP, ATP, UTP, and UDP-glucose play important roles in a wide range of physiological processes, including the sleep-wake cycle, learning and memory, cardiovascular function, and the immune response. Moreover, impaired purinergic signaling has been implicated in various pathological conditions such as pain, migraine, epilepsy, and drug addiction. Examining the function of purinergic transmission in both health and disease requires direct, sensitive, non-invasive tools for monitoring structurally similar purinergic transmitters; ideally, these tools should have high spatial and temporal resolution in in vivo applications. Here, we review the recent progress with respect to the development and application of new methods for detecting purinergic transmitters, focusing on optical tools; in addition, we provide discussion regarding future perspectives.
Keywords: Purinergic transmitters, ATP, Adenosine, Sensors, Imaging
1. Introduction
Purines and pyrimidines are essential components in living cells, where they mediate energy conversion and form the basis for synthesizing nucleic acids. In addition to these intracellular functions, some purines and pyrimidines such as ATP, adenosine, UTP, and UDP-glucose—known collectively as purinergic transmitters—also play a key role in extracellular signaling via purinergic receptors (Ralevic and Burnstock, 1998). Among these transmitters, adenosine (Ado) and ATP are arguably two of the most thoroughly studied.
As early as 1929, Alan Drury and Albert Szent-Györgyi found that an intravenous injection of Ado induced sleep in animals (Drury and Szent-Györgyi, 1929), providing the first physiological description for this nucleotide. Subsequently, a role for ATP in chemical transmission was identified in sensory nerve endings (Holton and Holton, 1954; Holton, 1959), later giving rise to the concept of purinergic transmission after ATP was identified as the transmitter involved in non-adrenergic, non-cholinergic inhibitory nerves in guinea pig intestinal smooth muscle (Burnstock, 1972).
After decades of research, it is now widely accepted that ATP can function as a neurotransmitter (Pankratov et al., 2006), gliotransmitter (Maienschein et al., 1999; Zhang et al., 2003), or co-transmitter (Burnstock, 2007; Jo and Schlichter, 1999) upon release from secretory vesicles such as synaptic vesicles, glial vesicles, and lysosomes (Zhang et al., 2007) via exocytosis (MacDonald et al., 2006). In addition, ATP can be released from cells via connexin/pannexin hemichannels (Cotrina et al., 1998), anion channels (Sabirov and Okada, 2005) and voltage-gated ion channels (Ma et al., 2018; Taruno et al., 2013). Extracellular Ado is believed to be generated by the breakdown of extracellular nucleotides, particularly ATP and ADP (Chen et al., 2013), via ecto-nucleotidases (Zimmermann et al., 2012), or by the direct efflux of Ado through the so-called equilibrative nucleoside transporters (ENTs) (Lovatt et al., 2012); whether Ado functions as a neurotransmitter released via the exocytosis of synaptic vesicle is currently under debate (Corti et al., 2013; Dale and Frenguelli, 2009). In the extracellular space, Ado and ATP perform distinct functions by activating P1 receptors and P2 receptors, respectively. The P1 receptor family consists of the A1, A2A, A2B, and A3 G protein‒coupled receptors (GPCRs), whereas P2 receptors include the ionotropic P2X receptor (Khakh and North, 2006) and the G protein‒ coupled metabotropic P2Y receptor. Both P1 and P2 receptors are widely expressed and have been implicated in a variety of physiological processes (Burnstock, 2008; Chen et al., 2013), including controlling the sleep-wake cycle (Huang et al., 2005; Porkka-Heiskanen et al., 1997), learning and memory (Dias et al., 2013), cardiovascular function (Shryock and Belardinelli, 1997), and the immune response (Eltzschig et al., 2012), as well as pathological processes, including pain (Hasko et al., 2008), epilepsy (Boison, 2012; During and Spencer, 1992; Engel et al., 2016), ischemia–reperfusion (Eltzschig and Eckle, 2011), drug addiction (Dunwiddie, 1999), and neurodegenerative disorders (Schwarzschild et al., 2006).
In addition to the broad effects that Ado and ATP have on various systems, functions of other purinergic transmitters are also identified. For example, ADP plays an important role in platelet aggregation by acting at P2Y1 receptor (Jin et al., 1998); UTP has been found to play a role in nociception in the central (Li et al., 2014; Ren et al., 2016) and enteric (Hockley et al., 2016) nervous systems and is an agonist of P2Y receptors; UDP-glucose may induce inflammatory effects by acting at the P2Y14 receptor (Lazarowski and Harden, 2015).
Despite the significant contributions to our understanding of purinergic signaling by many researchers over the past several decades, many key questions remain, including which purinergic transmitters are released—and where—under physiological and pathological conditions, how purinergic transmitters are converted from one to another, and details regarding their spatial and temporal release patterns. Answering these questions requires a detection method that provides high spatial and temporal resolution, as well as high molecular precision in order to monitor the dynamics of structurally similar purinergic transmitters, ideally in living animals. Here, we review the technologies that are currently available for detecting purinergic transmitters, including classic non-optical methods and recently developed optical methods. The molecular, pharmacological, and functional profiles of purinergic signaling have been reviewed by other groups (Burnstock, 2008; Chen et al., 2013) and are not discussed here in detail.
2. Non-optical methods for probing purinergic transmitters
2.1. Electrophysiology
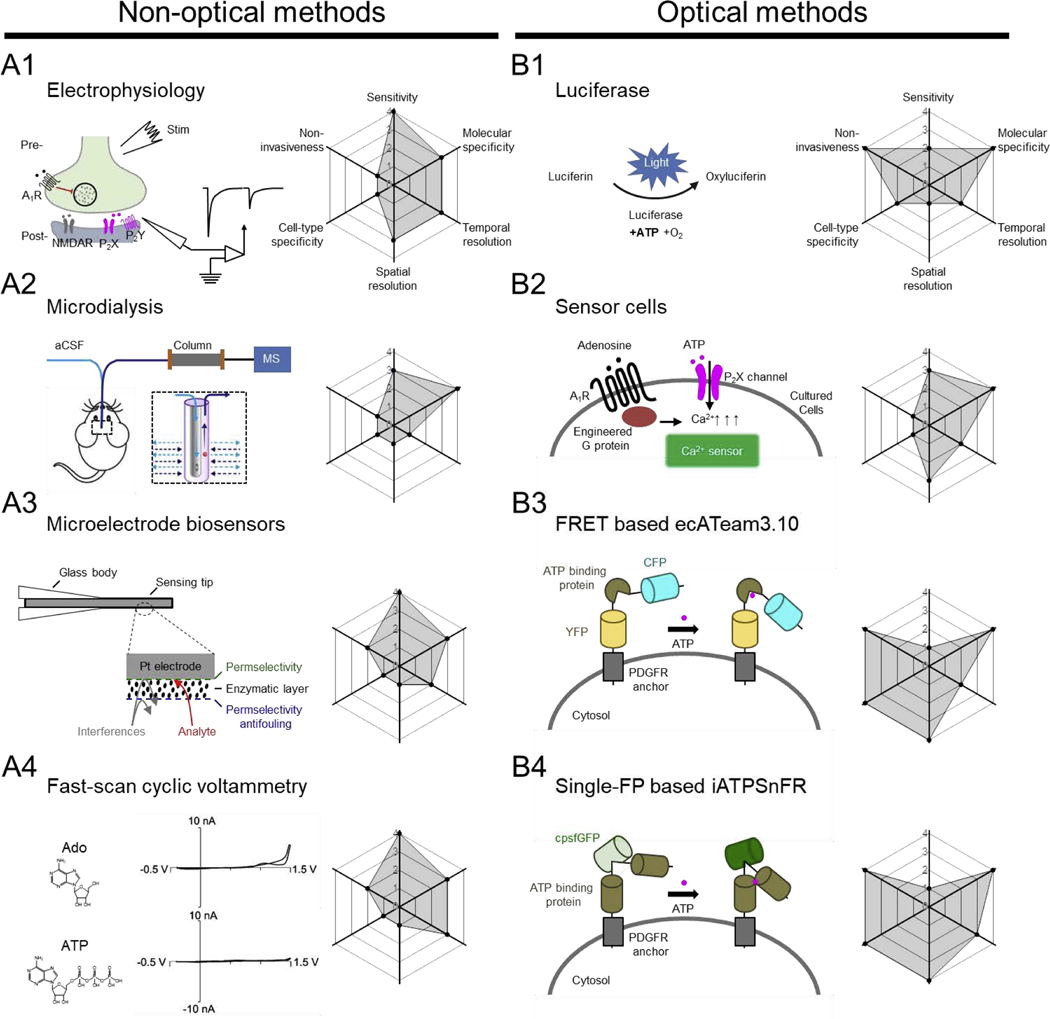
Because the binding of ATP to P2X receptors induces an increase in cationic conductance, electrophysiological recording can be used to measure ATP by recording currents through P2X receptors (Fig. 1A1). Using the patch-clamp technique, ATP-induced P2X currents can be recorded in cells expressing endogenous P2X receptors or in “sniffer cells” that express P2X receptors (Hayashi et al., 2004; Zhang et al., 2019). In addition to ionotropic receptors, all purinergic transmitters identified to date also have one or more corresponding metabotropic G protein‒coupled receptors (GPCRs), which transduces receptor activation into downstream electrophysiological effects on target cells. For example, electrophysiological studies in guinea pig hippocampal slices revealed that NMDA-induced depression of evoked excitatory postsynaptic currents was abolished by the adenosine A1 receptor antagonist 8-cyclo-pentyltheophylline, suggesting that adenosine is released in an activity-dependent manner (Manzoni et al., 1994). Similarly, the binding of non-adenosine purinergic transmitters to P2Y receptors induces the G protein‒mediated opening or closing of downstream ion channels, giving rise to an electrophysiological change that can be recorded in target cells. Although electrophysiological methods are extremely sensitive, they typically have extremely low throughput. Moreover, because electrophysiological methods mainly detect the downstream effects of purinergic transmitters, pharmacology is often required in order to verify molecular specificity. Finally, although electrophysiology can achieve temporal resolution on the order of milliseconds, it does not provide a measure of the transmitter’s release kinetics. Thus, combining electrophysiology with direct detection methods would enable researchers to study both the release of purinergic transmitters and their downstream effects in target cells.
Fig. 1. Overview of select non-optical and optical methods for detecting purinergic transmitters.
In each panel, the principle (with example data, where indicated) behind for each method is shown at the left; the corresponding radar graph summarizing the method’s performance index (including sensitivity, molecular specificity, cell-type, non-invasiveness, spatial resolution, and temporal resolution) is shown at the right, with arbitrary units ranging from 0 to 4. Abbreviations: aCSF, artificial cerebrospinal fluid; Ado, adenosine; ATP, adenosine triphosphate; CFP, cyan fluorescent protein; FRET, fluorescence resonance energy transfer; IgK, light chain kappa; MS, mass spectrometry; NMDAR, N-methyl-D-aspartate receptor; PDGFR, platelet-derived growth factor receptor; YFP, yellow fluorescent protein. Schematic shown in (A3) adapted from (Dale and Frenguelli, 2012); data shown in (A4) adapted from (Swamy and Venton, 2007); schematic shown in (B3) adapted from (Conley et al., 2017); schematic shown in (B4) adapted from (Lobas et al., 2019).
2.2. Microdialysis
A widely used method for directly measuring purinergic transmitters is microdialysis (Fig. 1A2) (Bito et al., 1966; Porkka-Heiskanen et al., 1997), often in combination with high-performance liquid chromatography (HPLC) together with either ultraviolet (UV) absorption detector or mass spectrometry (MS). With this method, a microdialysis probe (~200 μm in diameter), containing physiological solution such as artificial cerebrospinal fluid (aCSF), is first implanted into the brain region(s) of interest (Porkka-Heiskanen et al., 2000), and extracellular small molecules such as purinergic transmitters are collected via passive diffusion through the semi-permeable membrane. The dialysates are then separated by HPLC and detected using MS or UV. Microdialysis has several clear advantages compared to other approaches. First, it can be used in living animals, including humans. Second, this approach has extremely high sensitivity for detecting purinergic transmitters (in the nanomolar range). Third, it can be used to detect multiple chemicals simultaneously in one sample. Finally, it can provide the absolute concentrations of multiple chemicals. On the other hand, microdialysis also has several drawbacks. First, the sampling rate is relatively slow, on the order of minutes; this low temporal resolution precludes the possibility of capturing the rapid dynamics of purinergic transmitters. In addition, purinergic transmitters can be metabolized during the lengthy sample preparation. A second disadvantage of microdialysis is that although the microdialysis probe can be quite small, it cannot provide subcellular spatial resolution or even cell-type specificity. Finally, implanting microdialysis probe(s) in the brain can cause tissue damage and/or neuroinflammation, thereby potentially altering the concentration of purinergic transmitters in the local microenvironment (Ohyama et al., 2019).
2.3. Amperometric biosensors
In order to detect purinergic transmitters in real time, researchers have developed a variety of electrochemistry-based methods using electrodes, including enzyme-based amperometric biosensors (Dale, 1998; Dale et al., 2000) and fast-scan cyclic voltammetry (FSCV) using carbon-fiber microelectrodes (Swamy and Venton, 2007). The basic principle of amperometric biosensors (Fig. 1A3) capitalizes on the metabolization of purinergic transmitters to create hydrogen peroxide (H2O2) via an enzymatic cascade; the resulting H2O2 can then be detected using an amperometer at the microelectrode’s surface (Dale and Frenguelli, 2012). For example, to detect ATP, the surface of the microelectrode is coated with the glycerol kinase and glycerol-3-phosphate oxidase (Llaudet et al., 2005). In the presence of ATP, glycerol is phosphorylated by glycerol kinase to produce glycerol-3-phosphate, which is then oxidized by glycerol-3-phosphate oxidase, generating H2O2. When glycerol is available at saturated levels, this biosensor provides a final readout that is proportional to the concentration of ATP. A similar strategy has been used to detect adenosine, in this case using three enzymes, adenosine deaminase, nucleotide phosphorylase, and xanthine oxidase (Dale and Frenguelli, 2012). During the enzymatic cascade, adenosine is first converted to inosine, then to hypoxanthine, and finally to xanthine, urate, and H2O2.
Amperometric biosensors provide sufficient temporal resolution and nanomolar-range sensitivity for detecting ATP and/or adenosine dynamics in different brain regions, including the hippocampus (Wall and Dale, 2013) and cerebellum (Wall and Dale, 2007), and under a variety of physiological and pathological conditions (Dale and Frenguelli, 2009; Gourine et al., 2005). On the other hand, amperometric biosensors have relatively poor molecular specificity and can also detect intermediate products. For example, the adenosine amperometric biosensor is sensitive to both adenosine and its metabolite inosine. Therefore, a control experiment should be performed using a sensor that does not include the adenosine deaminase (Dale and Frenguelli, 2012). When measuring adenosine, the control sensor can be positioned close to the full biosensor in order to distinguish adenosine from similar compounds; the adenosine-specific signal can then be calculated by subtracting the control sensor’s signal from the full biosensor’s signal. Similarly, using an amperometric biosensor to measure ATP has this problem as well. In addition, as with other electrode-based sensors, placing the amperometric biosensors in the brain is invasive and can lead to tissue damage. Finally, amperometric biosensors usually have relatively limited spatial resolution and are unable to detect purinergic transmitter in specific cell types. In principle, if an enzymatic cascade catalyzes the bioconversion of purinergic transmitters other than adenosine or ATP, and if that cascade produces H2O2 at levels proportional to the original transmitter’s concentration, it should be possible to develop new amperometric biosensors that can detect a diverse number of purinergic transmitters; however, to the best of our knowledge, this method has only been used to detect adenosine and ATP.
2.4. Fast-scan cyclic voltammetry
In addition to amperometry, fast-scan cyclic voltammetry (FSCV) with carbon-fiber microelectrodes (Fig. 1A4) has also been used as an electrochemical method to directly monitor purinergic transmitters, including adenosine (Swamy and Venton, 2007). Traditionally, FSCV has been used to detect monoamine neuromodulators such as dopamine, norepinephrine, and serotonin (Bunin et al., 1998; Park et al., 2011), as monoamines can be readily oxidized, thereby giving rise to signature currents under a specific cyclic voltage scan range. Because adenosine is also an electroactive molecule that undergoes a series of two-electron oxidation steps, it can also be detected using FSCV (Nguyen and Venton, 2015). Compared to amperometric biosensors, FSCV electrodes have similar sensitivity (i.e., in the nanomolar range). Importantly, however, FSCV can achieve temporal resolution on the order of hundreds of milliseconds; thus, FSCV has been used to detect rapid activity-dependent adenosine release (Pajski and Venton, 2010), as well as spontaneous transient adenosine signals (Nguyen et al., 2014) in brain slices. With respect to specificity, adenosine metabolites such as inosine are electrochemically inactive to carbon-fiber electrodes; therefore, FSCV has higher specificity than amperometric biosensors (Nguyen and Venton, 2015). Moreover, although FSCV was successfully used for monitoring adenosine, it was still difficult to expand this method for measuring other purinergic transmitters. Finally, as with other electrode-based methods, FSCV lacks subcellular spatial resolution and cell-type specificity.
3. Optical methods
Compared to the conventional non-optical methods discussed above, optical imaging methods provide direct, sensitive, higher throughput, and long-term monitoring neurotransmitter dynamics in cultured cells, brain slices, and behaving animals in vivo. More importantly, the genetically-encoded optical sensor could be targeted to defined celltypes or subcellular compartments to achieve the cell-type specificity and/or subcellular spatial resolution. Compared with the classical electrophysiology recording, optical imaging methods are less invasive and therefore provide repeatable measurements of neurotransmitter dynamics from the same neuronal population. Therefore, optical methods are becoming increasingly popular. Several optical sensors have been developed for detecting both intracellular purine metabolites (Table 1) and extracellular purinergic transmitters (Table 2), and are discussed in further detail below.
Table 1.
Overview of optical sensors for detecting intracellular purine metabolites
| Sensor | Ligand | Binding protein / module | Optical reporter | ΔF/F0 or FRET/BRET ratio | Kd or EC50 | Kinetics | In vivo application | Ref. |
|---|---|---|---|---|---|---|---|---|
| Luciferase | ATP | No | Bioluminescence | N.D. | N.D. | N.D. | N.D. | (DeLuca and McElroy, 1974) |
| Syn-ATP | Presynaptic ATP | No | Bioluminescence | ~0.25 | 2.3 mM | N.D. | N.D. | (Rangaraju et al., 2014) |
| ParM-based sensor | ADP | Bacterial actin-like ParM | Chemical dyes | >3.5/~15 | 0.45/~30 μM | 0.65/9.5×104 M−1s−1 (τon),2.9 s−1 (τoff) | N.D. | (Kunzelmann and Webb, 2009) |
| FRET-Based chemosensor | Nucleoside polyphosphates | Binuclear zinc complex | Chemical dyes | ~33 | 0.1–1 μM | N.D. | N.D. | (Kurishita et al., 2010) |
| Perceval and PercevalHR | Cytosolic ATP/ADP ratio | Bacterial GlnKI | cpmVenus | Kr*: 0.5/3.5 | N.D. | N.D. | N.D. | (Berg et al., 2009; Tantama et al., 2013) |
| ATeam | Cytosolic ATP | Bacterial F0F1- ATP synthase | CFP/YFP | ~1.5 | 7.4 μM-3.3 mM | ~17μM/S (τon), 98ms (τoff) | N.D. | (Imamura et al., 2009) |
| ARSeNL | ATP | Bacterial F0F1- ATP synthase | mScarlet/NanoLuc | ~5 | ~1.1 mM | N.D. | Mice | (Min et al., 2019) |
| QUEEN | Cytosolic ATP | Bacterial F0F1- ATP synthase | cpEGFP | <4 (excitation ratio) | 7 μM | N.D. | N.D. | (Yaginuma et al., 2014) |
| iATPSnFR | ATP | Bacterial F0F1- ATP synthase | cpSFGFP | ~3.0 (?) | 138 μM, 350 μM | < 5s (τon), >1s (τoff) | N.D. | (Lobas et al., 2019) |
KR* represents a half-maximal signal change, which was used to quantify the ability of Perceval sensors to report ATP/ADP ratio. N.D., not determined.
Table 2.
Overview of optical sensors for detecting extracellular purinergic transmitters
| Sensor | Ligand | Binding protein / module | Optical reporter | ΔF/F0 or FRET/BRET ratio | Kd or EC50 | Kinetics | In vivo application | Ref. |
|---|---|---|---|---|---|---|---|---|
| pmeLUC | ATP | No | Bioluminescence | ~8 | > 10 μM | N.D. | Mice | (Morciano et al., 2017) |
| TANGO Assay | GPCR ligands | GPCRs | Reporter gene | >10 | ~ 1 nM | >8 h | N.D. | (Barnea et al., 2008) |
| Sniffer cells | Adenosine, ATP, or ADP | A1R, P2X, or P2Y | Ca2+ sensor | ~1.5 (?) for Ado | 8 0μM (Ado) | N.D. | N.D. | (Yamashiro et al., 2017) |
| ecATeam3.10 | ATP | Bacterial F0F1- ATP synthase | CFP/YFP | ~0.27 | ~11 mM | ~13–172 s (τon) | N.D. | (Conley et al., 2017) |
| A2AR-based FlAsH | Adenosine | A2AR | CFP/FlAsH | ~0.1 | Similar to WT A2AR (?) | 66–88 ms (τon) | N.D. | (Hoffmann et al., 2005) |
| P2X2-Cam | ATP | P2X2 | CFP/YFP | ~0.37 | ~14 μM | ~6 s (decay) | Zebrafish | (Richler et al., 2008) |
| iATPSnFR | ATP | Bacterial F0F1- ATP synthase | cpSFGFP | ~1.0 | 350 μM | <5 s (τon), >1 s (τoff) | N.D. | (Lobas et al., 2019) |
N.D., not determined.
3.1. Optical sensors for detecting intracellular purine metabolites
Within the cytoplasm, purine metabolites—particularly ATP—serve as a universal and highly important source of cellular energy, and several optical methods have been developed in order to investigate how ATP affects cellular physiology. For example, firefly luciferase—an ATP-consuming enzyme that oxidizes the small-molecule substrate luciferin to produce a bioluminescent product—has been widely used to measure ATP both in vitro and in vivo. Importantly, luciferase can be expressed in specific target cells or even in subcellular organelles when fused with sequences that target the protein to specific structures such as the mitochondria or synaptic vesicles (Rangaraju et al., 2014); organelle-specific luciferase-based sensors have been discussed in detail elsewhere (Morciano et al., 2017). Unfortunately, luciferase can only be used to detect ATP, not other purine metabolites. Moreover, by detecting ATP, the enzymatic reaction itself consumes ATP, which inevitably leads to changes in cellular metabolism. Recently, Mathew Tantama and colleagues developed a ratiometric bioluminescence resonance energy transfer (BRET) sensor for detecting ATP; this system, called ARSeNL, uses the ATP-independent NanoLuc luciferase and the red fluorescent protein mScarlet as the donor and acceptor, respectively (Min et al., 2019). Using this sensor, they measured changes in energy metabolism both in vitro and in vivo. Because mammalian cells do not normally express bioluminescent proteins, luminescence-based methods usually have an extremely low background signal (Morciano et al., 2017); on the other hand, bioluminescence-based methods produce low numbers of photons and lack cellular-scale resolution, which can limit its general applicability.
Unlike bioluminescence-based methods, sensors based on fluorescent proteins can—at least in principle—provide higher spatial and temporal resolution with respect to imaging purinergic transmitters. A fluorescent sensor requires two components: i) a binding protein that specifically recognizes purinergic transmitters and generates a conformational change upon ligand binding, and ii) a fluorescent component that converts the conformational change into a fluorescent signal. To develop such a sensor, several proteins were covalently coupled to a fluorescent reporter dye that undergoes a change in fluorescence upon binding ATP or ADP, thereby providing a measure of ATP/ADP concentration (Kunzelmann and Webb, 2009, 2010; Vancraenenbroeck and Webb, 2015). Such chemical dye‒based methods provide an important tool for quantifying ATP with high sensitivity and high throughput; however, because it can be challenging to target these tools to specific cells, this method is generally limited.
To overcome this limitation, Gary Yellen and colleagues engineered Perceval—a fully genetically encoded fluorescent protein‒based sensor for imaging ATP—by inserting circularly permuted monomeric Venus (cpmVenus) into GlnK1, an ATP-binding bacterial protein that regulates ammonia transport (Berg et al., 2009). Like the native GlnK1 protein, Perceval measures the ATP/ADP ratio. However, because Perceval binding is saturated at a relatively low ATP/ADP ratio (< 5:1), its effectiveness is limited in mammalian cells. They therefore increased the sensor’s dynamic range so that it can still detect ATP/ADP ratios in healthy mammalian cells, which can reach 100:1. This optimized sensor, called PercevalHR (Tantama et al., 2013), produces a stronger signal, thus allowing researchers to measure the ATP/ADP ratio at the single-cell level; moreover, PercevalHR performs well using either one-photon or two-photon excitation. Unfortunately, however, both Perceval and PercevalHR are highly sensitive to pH; therefore, the fluorescence change that derives from the bona fide signal can be difficult to distinguish from an artifact. Moreover, the Perceval-based sensors can only be used to monitor cellular energy levels and would be difficult to use for directly quantifying specific purine metabolites.
To specifically visualize ATP levels within individual living cells, Hiroyuki Noji and colleagues generated a series of sensors based on fluorescence resonance energy transfer (FRET); these sensors, called ATeams, have specific apparent affinities ranging from 7.4 μM to 3.3 mM (Imamura et al., 2009). ATeams contain the epsilon subunit of bacterial F0F1-ATP synthase as the ATP-sensing protein “sandwiched” between cyan fluorescent protein (CFP) and yellow fluorescent protein (YFP). By targeting these sensors to specific subcellular compartments, Noji’s group successfully measured ATP levels within the mitochondrial matrix, cytoplasm, and nucleus in cultured HeLa cells. Unfortunately, because FRET efficiency is relatively low, the applications of ATeam are limited, particularly with in vivo preparations. In addition to their FRET-based ATeams, Hiroyuki Noji, Hiromi Imamura, and their colleagues developed an excitation ratio‒based sensor called QUEEN by inserting circularly permuted enhanced green fluorescent protein (cpEGFP) between two α-helices of the bacterial F0F1-ATP synthase epsilon subunit; they then used this sensor to measure ATP levels in E. coli cells (Yaginuma et al., 2014). Despite their advantages, both the FRET-based ATeam sensors and the excitation ratio‒based QUEEN sensors require customized equipment, have relatively slow imaging rates, and have a lower signal-to-noise ratio compared to single wavelength‒based sensors (Lobas et al., 2019).
Recently, Baljit Khakh, Loren Looger, and their colleagues developed a genetically encoded GFP-based single-wavelength ATP sensor called iATPSnFR by inserting circularly permutated super-folder GFP (cpSFGFP) in the epsilon subunit of the Bacillus PS3 F0F1-ATPase (Lobas et al., 2019). This newly developed sensor produces a bright, rapid, specific increase in fluorescence increase upon binding ATP (with peak ΔF/F0 values of ~3.9 using purified proteins); however, the low affinity of iATPSnFR for ATP (on the order of hundreds of micromolar) might not be sensitive enough for in vivo applications, particularly when measuring extracellular ATP.
3.2. Optical sensors for detecting extracellular purinergic transmitters
Several strategies have been used to develop optical sensors for detecting extracellular purinergic transmitters, including: i) targeting cytosolic sensors to the plasma membrane, and ii) using plasma membrane‒localized proteins to engineer new optical sensors. The resulting optical sensors can be based on either bioluminescence or fluorescence, and are discussed in detail below.
3.2.1. Bioluminescence-based sensors
As discussed above, luciferase-based bioluminescent sensors are widely used for measuring ATP (Fig. 1B1). To measure local ATP release from activated platelets, George Dubyak and colleagues attached a purified IgG binding domain‒tagged luciferase to the surface of cells expressing IgG (Beigi et al., 1999). Similarly, using luciferase either diluted in extracellular solution (Wang et al., 2000) or immobilized to beads (Zhang et al., 2008), Edward Yeung and colleagues successfully measured mechanical stimulation-evoked release of ATP from astrocytes in vitro. In addition, by fusing a leader sequence and glycosylphosphatidylinositol (GPI) lipid anchor derived from the folate receptor to luciferase, Francesco Di Virgilio, Paolo Pinton, and their colleagues designed a plasma membrane‒targeted luciferase called pmeLUC, which they then used to measure extracellular ATP dynamics both in cultured cells and in living animals injected with pmeLUC-expressing cells (Morciano et al., 2017). Again, although luciferase-based bioluminescent sensors have an extremely low background signal, their output yields relatively low numbers of photons, which reduces their feasibility for imaging purinergic transmitters at the cellular scale. Interestingly, the bioluminescent signal can be amplified by attaching the sensor to a downstream GPCR (Barnea et al., 2008); however, these so-called TANGO assays usually have poor temporal resolution (on the order of minutes to hours), making them less feasible for monitoring purinergic transmitters in real time.
3.2.2. Fluorescence-based sensors
Compared to bioluminescence-based sensors, fluorescence-based sensors have significantly higher brightness, which enables researchers to visualize purinergic transmitters with optical precision and/or millisecond temporal resolution. Therefore, fluorescence-based sensors are often used to analyze molecules and events that occur with rapid dynamics at a subcellular level using both in vitro and in vivo preparations. Here, we discuss the recent progress in fluorescence-based sensors for measuring purinergic transmitters, including cell-based sensors, FRET-based sensors, and single-wavelength sensors.
3.2.2.1. Cell-based sensors
Several cell-based sensors—also referred to as “sniffer” cells (Fig. 1B2)—have been developed for monitoring purinergic transmitters. With this method, a specific cell surface receptor that recognizes the purinergic transmitter of interest is expressed in the sniffer cells, which are then placed in close proximity to the target cells. For example, the Ca2+ dye Fluo-4-AM was loaded in the mouse glioma cell line GL261, which express high-sensitivity purinergic receptors, and these cells were then placed on the surface of a mouse brain slice and used to indirectly image ATP release; the Ca2+ signal measured in the sniffer cells was due specifically to the release of ATP, as it was blocked by the purinergic receptor blocker Reactive Blue-2 (Haas et al., 2006). A similar strategy was used to detect changes in extracellular adenosine by placing A1R-expressing and Fura-2 loaded HEK293T cells in the brain slice (Yamashiro et al., 2017). Although cell-based sensors can report specific purinergic transmitters with high sensitivity, they are not suitable for detecting the release of purinergic transmitters with high cell-type specificity or subcellular resolution. In addition, the sniffer cells would need to be implanted in specific tissues when used in living animals, making this approach potentially too invasive for use in in vivo applications.
3.2.2.2. FRET-based sensors
In principle, the above-mentioned limitations can be overcome using a genetically encoded probe, which allows researchers to monitor neuromodulator dynamics in specific cell types with subcellular spatial resolution. One such example is FRET-based sensors. For example, Martin Lohse and colleagues developed a CFP/FlAsH-based FRET sensor using the adenosine 2A receptor (A2AR) as the backbone (Hoffmann et al., 2005). This sensor, which has similar affinity for A2AR agonists compared to the wild-type A2AR, produces an ~10% change in the FRET ratio in response to a saturated concentration of adenosine. Similarly, Baljit Khakh and colleagues used the ATP receptor P2X2 as the detecting module in their FRET sensor P2X2-Cam for imaging ATP; specifically, they fused the P2X2 receptor with a YC3.1 tag, which contains CFP and YFP separated by the Ca2+ sensor Cameleon (Richler et al., 2008). The Cameleon domain undergoes a conformational change upon binding Ca2+, changing the relative positions of the CFP and YFP moieties, thereby causing an increase in the FRET ratio. The authors found that P2X2-Cam has an ~37% change in the FRET ratio in response to a saturated concentration of ATP, with an EC50 of approximately 13 μM. Unfortunately, because FRET-based sensors have a relatively low signal-to-noise ratio, they are less suitable for use in in vivo applications. Indeed, although P2X2-Cam was used in live zebrafish to measure the application of ATP-induced ratiometric changes, it has not been used to detect endogenous ATP release in vivo. In addition to using plasma membrane‒localized A2AR or P2X2 receptors to engineer optical sensors, cytosolic sensors could also be targeted to the plasma membrane for probing extracellular purinergic transmitters. For example, Mathew Tantama and colleagues re-engineered the FRETbased ATeam ATP sensor to be expressed on the cell surface, and the new ecAT3.10 sensor (Fig. 1B3) enabled probing ATP release in cultured cells but not in vivo (Conley et al., 2017).
3.2.2.3. Single-wavelength sensors
Compared to FRET-based sensors, single-wavelength sensors have a higher signal-to-noise ratio and are therefore more suitable for measuring endogenous neuromodulator dynamics in vivo. For example, in order to image extracellular ATP, the genetically encoded single-wavelength iATPSnFR (Fig. 1B4) was displayed at the extracellular side of the plasma membrane (Lobas et al., 2019). Despite their advantages, however, sensors based on bacterial binding proteins—including iATPSnFR—have relatively low ligand affinity, which limits their application, particularly with respect to in vivo preparations. Although the sensor’s affinity could theoretically be improved through molecular engineering, this can be highly time-consuming. Moreover, although this strategy has been used to develop ATP sensors, to the best of our knowledge it has not been used to develop sensors for other purinergic transmitters.
4. Future perspectives
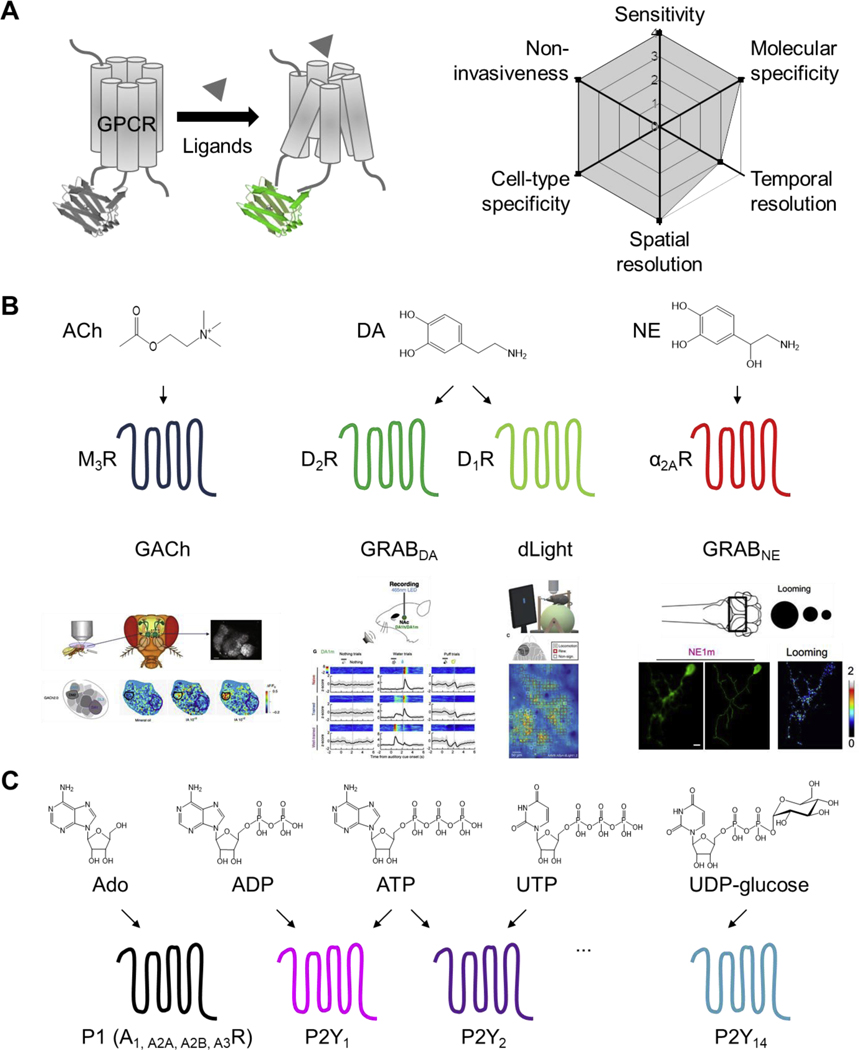
Despite significant advances over the past few decades, current methods for detecting purinergic transmitters are still limited in several respects, including poor spatial and/or temporal resolution, insufficient sensitivity, and/or low specificity; thus, a method that satisfies all of these criteria is needed. Yet the question remains: how to engineer a sensor that can be used to monitor structurally similar purinergic transmitters with high specificity and sensitivity? In fact, nature has already provided us with an important clue: in mammals, all purinergic transmitters identified to date bind at least one G protein‒coupled receptor. Thus, GPCRs provide an excellent scaffold for developing tools to detect purinergic transmitters. In this respect, a promising new strategy is to generate single-fluorophore GPCR Activation-Based (GRAB) sensors (Jing et al., 2019); indeed, this strategy has been used successfully to engineer sensors for detecting acetylcholine (Fig. 2A) (Jing et al., 2018), dopamine (Patriarchi et al., 2018; Sun et al., 2018), and norepinephrine (Feng et al., 2019). Importantly, these sensors have a wide dynamic range, rapid kinetics, single-cell resolution, and—most importantly—exquisite selectivity (Fig. 2B). In addition, most GRAB sensors have negligible coupling with their GPCR’s downstream signaling pathways, and have no detectable effects on cellular physiology (Wu et al., 2019). These sensors allow researchers to monitor neurotransmitter dynamics in vivo in a variety of organisms, including flies, fish, birds (Tanaka et al., 2018), and mice (Fig. 2C).
Fig. 2. A proposed toolbox for detecting purinergic transmitters.
(A) The principle behind our proposed GPCR Activation-Based (GRAB) sensors (left) for detecting transmitters, with the corresponding theoretical performance index (right). (B) A similar GRAB strategy was successfully used for in vivo monitoring of acetylcholine (ACh), dopamine (DA), and norepinephrine (NE) in behaving animals. (C) A proposed toolbox of GRAB sensors for monitoring various purinergic transmitters; the structure of each transmitter is shown at the top. Data shown in (B) adapted from (Jing et al., 2018), (Sun et al., 2018), (Patriarchi et al., 2018), and (Feng et al., 2019).
Given the wide variety of chemical-sensing GPCRs expressed in the nervous system, this strategy can likely be used to develop sensors for detecting a wide range of molecules, including purinergic transmitters (Fig. 2D). Moreover, by attaching fluorescent proteins with different excitation/emission profiles, multiple sensors with non-overlapping spectra can be developed and then used in combination to study the complex interplay between different purinergic transmitters. In this respect, the use of simultaneous multi-color imaging to measure the release of multiple purinergic transmitters in real time will provide important insights into how these purinergic transmitters function in the brain and other tissues in both health and disease.
Highlights.
Purinergic transmitters play critical roles in physiological and pathological processes.
Current methods for purinergic transmitter detection are thoroughly summarized.
New optical sensors are promising for monitoring purinergic transmitters in vivo.
Acknowledgments and declarations of interest
We thank the members of the Li lab for providing feedback on the manuscript. This work was supported by the Beijing Municipal Science & Technology Commission (Z181100001318002), the Beijing Brian Initiative of Beijing Municipal Science & Technology Commission (Z181100001518004), the Guangdong grant ‘Key technologies for treatment of brain disorders’ (grant 2018B030332001), the National Basic Research Program of China (973 Program; grant 2015CB856402), the General Program of National Natural Science Foundation of China (project 31671118 and project 31371442), the NIH BRAIN Initiative (NS103558), the Junior Thousand Talents Program of China, grants from the Peking-Tsinghua Center for Life Sciences and the State Key Laboratory of Membrane Biology at Peking University School of Life Sciences (to Y. L.). Z. W. is supported by the Boehringer Ingelheim-Peking University Postdoctoral Fellowship. Y. L. has filed patent applications, the value of which may be affected by this publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barnea G, Strapps W, Herrada G, Berman Y, Ong J, Kloss B, Axel R, Lee KJ, 2008. The genetic design of signaling cascades to record receptor activation. Proc Natl Acad Sci U S A 105, 64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beigi R, Kobatake E, Aizawa M, Dubyak GR, 1999. Detection of local ATP release from activated platelets using cell surface-attached firefly luciferase. American Journal of Physiology-Cell Physiology 276, C267–C278. [DOI] [PubMed] [Google Scholar]
- Berg J, Hung YP, Yellen G, 2009. A genetically encoded fluorescent reporter of ATP:ADP ratio. Nat Methods 6, 161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bito L, Davson H, Levin E, Murray M, Snider N, 1966. The concentrations of free amino acids and other electrolytes in cerebrospinal fluid, in vivo dialysate of brain, and blood plasma of the dog. J Neurochem 13, 1057–1067. [DOI] [PubMed] [Google Scholar]
- Boison D, 2012. Adenosine dysfunction in epilepsy. Glia 60, 1234–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunin MA, Prioleau C, Mailman RB, Wightman RM, 1998. Release and uptake rates of 5hydroxytryptamine in the dorsal raphe and substantia nigra reticulata of the rat brain. J Neurochem 70, 1077–1087. [DOI] [PubMed] [Google Scholar]
- Burnstock G, 1972. Purinergic nerves. Pharmacol Rev 24, 509–581. [PubMed] [Google Scholar]
- Burnstock G, 2007. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev 87, 659–797. [DOI] [PubMed] [Google Scholar]
- Burnstock G, 2008. Purinergic signalling and disorders of the central nervous system. Nat Rev Drug Discov 7, 575. [DOI] [PubMed] [Google Scholar]
- Chen J-F, Eltzschig HK, Fredholm BB, 2013. Adenosine receptors as drug targets—what are the challenges? Nat Rev Drug Discov 12, 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley JM, Radhakrishnan S, Valentino SA, Tantama M, 2017. Imaging extracellular ATP with a genetically-encoded, ratiometric fluorescent sensor. PLoS One 12, e0187481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti F, Cellai L, Melani A, Donati C, Bruni P, Pedata F, 2013. Adenosine is present in rat brain synaptic vesicles. Neuroreport 24, 982–987. [DOI] [PubMed] [Google Scholar]
- Cotrina ML, Lin JH, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, Naus CC, Nedergaard M, 1998. Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci U S A 95, 15735–15740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale N, 1998. Delayed production of adenosine underlies temporal modulation of swimming in frog embryo. J Physiol 511 ( Pt 1), 265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale N, Frenguelli BG, 2009. Release of adenosine and ATP during ischemia and epilepsy. Curr Neuropharmacol 7, 160–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale N, Frenguelli BG, 2012. Measurement of purine release with microelectrode biosensors. Purinergic Signal 8, 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale N, Pearson T, Frenguelli BG, 2000. Direct measurement of adenosine release during hypoxia in the CA1 region of the rat hippocampal slice. J Physiol 526 Pt 1, 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca M, McElroy WD, 1974. Kinetics of the firefly luciferase catalyzed reactions. Biochemistry 13, 921–925. [DOI] [PubMed] [Google Scholar]
- Dias RB, Rombo DM, Ribeiro JA, Henley JM, Sebastiao AM, 2013. Adenosine: setting the stage for plasticity. Trends Neurosci 36, 248–257. [DOI] [PubMed] [Google Scholar]
- Drury A, Szent-Györgyi A.v., 1929. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart 1. The Journal of physiology 68, 213–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie TV, 1999. Adenosine and Ethanol, The “Drunken” Synapse. Springer, pp. 119–133. [Google Scholar]
- During MJ, Spencer DD, 1992. Adenosine: a potential mediator of seizure arrest and postictal refractoriness. Ann Neurol 32, 618–624. [DOI] [PubMed] [Google Scholar]
- Eltzschig HK, Eckle T, 2011. Ischemia and reperfusion—from mechanism to translation. Nat Med 17, 1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig HK, Sitkovsky MV, Robson SC, 2012. Purinergic signaling during inflammation. N Engl J Med 367, 2322–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel T, Alves M, Sheedy C, Henshall DC, 2016. ATPergic signalling during seizures and epilepsy. Neuropharmacology 104, 140–153. [DOI] [PubMed] [Google Scholar]
- Feng J, Zhang C, Lischinsky JE, Jing M, Zhou J, Wang H, Zhang Y, Dong A, Wu Z, Wu H, Chen W, Zhang P, Zou J, Hires SA, Zhu JJ, Cui G, Lin D, Du J, Li Y, 2019. A Genetically Encoded Fluorescent Sensor for Rapid and Specific In Vivo Detection of Norepinephrine. Neuron 102, 745–761 e748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Llaudet E, Dale N, Spyer KM, 2005. ATP is a mediator of chemosensory transduction in the central nervous system. Nature 436, 108. [DOI] [PubMed] [Google Scholar]
- Haas B, Schipke CG, Peters O, Sohl G, Willecke K, Kettenmann H, 2006. Activity-dependent ATP-waves in the mouse neocortex are independent from astrocytic calcium waves. Cereb Cortex 16, 237–246. [DOI] [PubMed] [Google Scholar]
- Hasko G, Linden J, Cronstein B, Pacher P, 2008. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov 7, 759–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S, Hazama A, Dutta AK, Sabirov RZ, Okada Y, 2004. Detecting ATP release by a biosensor method. Sci STKE 2004, pl14. [DOI] [PubMed] [Google Scholar]
- Hockley JR, Tranter MM, McGuire C, Boundouki G, Cibert-Goton V, Thaha MA, Blackshaw LA, Michael GJ, Baker MD, Knowles CH, 2016. P2Y receptors sensitize mouse and human colonic nociceptors. Journal of Neuroscience 36, 2364–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann C, Gaietta G, Bunemann M, Adams SR, Oberdorff-Maass S, Behr B, Vilardaga JP, Tsien RY, Ellisman MH, Lohse MJ, 2005. A FlAsH-based FRET approach to determine G protein-coupled receptor activation in living cells. Nat Methods 2, 171–176. [DOI] [PubMed] [Google Scholar]
- Holton FA, Holton P, 1954. The capillary dilator substances in dry powders of spinal roots; a possible role of adenosine triphosphate in chemical transmission from nerve endings. J Physiol 126, 124–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holton P, 1959. The liberation of adenosine triphosphate on antidromic stimulation of sensory nerves. J Physiol 145, 494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z-L, Qu W-M, Eguchi N, Chen J-F, Schwarzschild MA, Fredholm BB, Urade Y, Hayaishi O, 2005. Adenosine A 2A, but not A 1, receptors mediate the arousal effect of caffeine. Nat Neurosci 8, 858. [DOI] [PubMed] [Google Scholar]
- Imamura H, Nhat KP, Togawa H, Saito K, Iino R, Kato-Yamada Y, Nagai T, Noji H, 2009. Visualization of ATP levels inside single living cells with fluorescence resonance energy transferbased genetically encoded indicators. Proc Natl Acad Sci U S A 106, 15651–15656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Daniel JL, Kunapuli SP, 1998. Molecular basis for ADP-induced platelet activation II. The P2Y1 receptor mediates ADP-induced intracellular calcium mobilization and shape change in platelets. J Biol Chem 273, 2030–2034. [DOI] [PubMed] [Google Scholar]
- Jing M, Zhang P, Wang G, Feng J, Mesik L, Zeng J, Jiang H, Wang S, Looby JC, Guagliardo NA, Langma LW, Lu J, Zuo Y, Talmage DA, Role LW, Barrett PQ, Zhang LI, Luo M, Song Y, Zhu JJ, Li Y, 2018. A genetically encoded fluorescent acetylcholine indicator for in vitro and in vivo studies. Nat Biotechnol 36, 726–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing M, Zhang Y, Wang H, Li Y, 2019. G-protein-coupled receptor-based sensors for imaging neurochemicals with high sensitivity and specificity. J Neurochem 151, 279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo YH, Schlichter R, 1999. Synaptic corelease of ATP and GABA in cultured spinal neurons. Nat Neurosci 2, 241–245. [DOI] [PubMed] [Google Scholar]
- Khakh BS, North RA, 2006. P2X receptors as cell-surface ATP sensors in health and disease. Nature 442, 527. [DOI] [PubMed] [Google Scholar]
- Kunzelmann S, Webb MR, 2009. A biosensor for fluorescent determination of ADP with high time resolution. J Biol Chem 284, 33130–33138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzelmann S, Webb MR, 2010. A fluorescent, reagentless biosensor for ADP based on tetramethylrhodamine-labeled ParM. ACS Chem Biol 5, 415–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurishita Y, Kohira T, Ojida A, Hamachi I, 2010. Rational design of FRET-based ratiometric chemosensors for in vitro and in cell fluorescence analyses of nucleoside polyphosphates. J Am Chem Soc 132, 13290–13299. [DOI] [PubMed] [Google Scholar]
- Lazarowski ER, Harden TK, 2015. UDP-sugars as extracellular signaling molecules: cellular and physiologic consequences of P2Y14 receptor activation. Molecular pharmacology 88, 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lu Z. y., Yu L. h., Burnstock G, Deng X. m., Ma B, 2014. Inhibition of G protein-coupled P2Y 2 receptor induced analgesia in a rat model of trigeminal neuropathic pain. Molecular pain 10, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llaudet E, Hatz S, Droniou M, Dale N, 2005. Microelectrode biosensor for real-time measurement of ATP in biological tissue. Anal Chem 77, 3267–3273. [DOI] [PubMed] [Google Scholar]
- Lobas MA, Tao R, Nagai J, Kronschlager MT, Borden PM, Marvin JS, Looger LL, Khakh BS, 2019. A genetically encoded single-wavelength sensor for imaging cytosolic and cell surface ATP. Nat Commun 10, 711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovatt D, Xu Q, Liu W, Takano T, Smith NA, Schnermann J, Tieu K, Nedergaard M, 2012. Neuronal adenosine release, and not astrocytic ATP release, mediates feedback inhibition of excitatory activity. Proc Natl Acad Sci U S A 109, 6265–6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Taruno A, Ohmoto M, Jyotaki M, Lim JC, Miyazaki H, Niisato N, Marunaka Y, Lee RJ, Hoff H, 2018. CALHM3 is essential for rapid ion channel-mediated purinergic neurotransmission of GPCR-mediated tastes. Neuron 98, 547–561. e510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald PE, Braun M, Galvanovskis J, Rorsman P, 2006. Release of small transmitters through kiss-and-run fusion pores in rat pancreatic β cells. Cell Metabolism 4, 283–290. [DOI] [PubMed] [Google Scholar]
- Maienschein V, Marxen M, Volknandt W, Zimmermann H, 1999. A plethora of presynaptic proteins associated with ATP-storing organelles in cultured astrocytes. Glia 26, 233–244. [DOI] [PubMed] [Google Scholar]
- Manzoni OJ, Manabe T, Nicoll RA, 1994. Release of adenosine by activation of NMDA receptors in the hippocampus. Science 265, 2098–2101. [DOI] [PubMed] [Google Scholar]
- Min SH, French AR, Trull KJ, Tat K, Varney SA, Tantama M, 2019. Ratiometric BRET Measurements of ATP with a Genetically-Encoded Luminescent Sensor. Sensors (Basel) 19, 3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morciano G, Sarti AC, Marchi S, Missiroli S, Falzoni S, Raffaghello L, Pistoia V, Giorgi C, Di Virgilio F, Pinton P, 2017. Use of luciferase probes to measure ATP in living cells and animals. Nature protocols 12, 1542. [DOI] [PubMed] [Google Scholar]
- Nguyen MD, Lee ST, Ross AE, Ryals M, Choudhry VI, Venton BJ, 2014. Characterization of spontaneous, transient adenosine release in the caudate-putamen and prefrontal cortex. PLoS One 9, e87165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MD, Venton BJ, 2015. Fast-scan Cyclic Voltammetry for the Characterization of Rapid Adenosine Release. Comput Struct Biotechnol J 13, 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama K, Ikeshita Y, Fuchigami Y, Kawakami S, Nakashima MN, Nakashima M, 2019. Proteomic analysis and ATP assay reveal a positive effect of artificial cerebral spinal fluid perfusion following microdialysis sampling on repair of probe-induced brain damage. J Neurosci Methods 315, 1–5. [DOI] [PubMed] [Google Scholar]
- Pajski ML, Venton BJ, 2010. Adenosine release evoked by short electrical stimulations in striatal brain slices is primarily activity dependent. ACS Chem Neu 1, 775–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankratov Y, Lalo U, Verkhratsky A, North RA, 2006. Vesicular release of ATP at central synapses. Pflügers Archiv 452, 589–597. [DOI] [PubMed] [Google Scholar]
- Park J, Takmakov P, Wightman RM, 2011. In vivo comparison of norepinephrine and dopamine release in rat brain by simultaneous measurements with fast-scan cyclic voltammetry. J Neurochem 119, 932–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriarchi T, Cho JR, Merten K, Howe MW, Marley A, Xiong WH, Folk RW, Broussard GJ, Liang R, Jang MJ, Zhong H, Dombeck D, von Zastrow M, Nimmerjahn A, Gradinaru V, Williams JT, Tian L, 2018. Ultrafast neuronal imaging of dopamine dynamics with designed genetically encoded sensors. Science 360, eaat4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Strecker RE, McCarley RW, 2000. Brain site-specificity of extracellular adenosine concentration changes during sleep deprivation and spontaneous sleep: an in vivo microdialysis study. Neuroscience 99, 507–517. [DOI] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, McCarley RW, 1997. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science 276, 12651268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G, 1998. Receptors for purines and pyrimidines. Pharmacol Rev 50, 413–492. [PubMed] [Google Scholar]
- Rangaraju V, Calloway N, Ryan TA, 2014. Activity-driven local ATP synthesis is required for synaptic function. Cell 156, 825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren C, Gan X, Wu J, Qiu C-Y, Hu W-P, 2016. Enhancement of acid-sensing ion channel activity by metabotropic P2Y UTP receptors in primary sensory neurons. Purinergic Signal 12, 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richler E, Chaumont S, Shigetomi E, Sagasti A, Khakh BS, 2008. Tracking transmitter-gated P2X cation channel activation in vitro and in vivo. Nat Methods 5, 87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabirov RZ, Okada Y, 2005. ATP release via anion channels. Purinergic Signal 1, 311–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzschild MA, Agnati L, Fuxe K, Chen JF, Morelli M, 2006. Targeting adenosine A2A receptors in Parkinson’s disease. Trends Neurosci 29, 647–654. [DOI] [PubMed] [Google Scholar]
- Shryock JC, Belardinelli L, 1997. Adenosine and adenosine receptors in the cardiovascular system: biochemistry, physiology, and pharmacology. The American journal of cardiology 79, 2–10. [DOI] [PubMed] [Google Scholar]
- Sun F, Zeng J, Jing M, Zhou J, Feng J, Owen SF, Luo Y, Li F, Wang H, Yamaguchi T, Yong Z, Gao Y, Peng W, Wang L, Zhang S, Du J, Lin D, Xu M, Kreitzer AC, Cui G, Li Y, 2018. A Genetically Encoded Fluorescent Sensor Enables Rapid and Specific Detection of Dopamine in Flies, Fish, and Mice. Cell 174, 481–496 e419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swamy BK, Venton BJ, 2007. Subsecond detection of physiological adenosine concentrations using fast-scan cyclic voltammetry. Anal Chem 79, 744–750. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Sun F, Li Y, Mooney R, 2018. A mesocortical dopamine circuit enables the cultural transmission of vocal behaviour. Nature 563, 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantama M, Martinez-Francois JR, Mongeon R, Yellen G, 2013. Imaging energy status in live cells with a fluorescent biosensor of the intracellular ATP-to-ADP ratio. Nat Commun 4, 2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taruno A, Vingtdeux V, Ohmoto M, Ma Z, Dvoryanchikov G, Li A, Adrien L, Zhao H, Leung S, Abernethy M, 2013. CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature 495, 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vancraenenbroeck R, Webb MR, 2015. A Fluorescent, Reagentless Biosensor for ATP, Based on Malonyl-Coenzyme A Synthetase. ACS Chem Biol 10, 2650–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall MJ, Dale N, 2007. Auto-inhibition of rat parallel fibre-Purkinje cell synapses by activity-dependent adenosine release. J Physiol 581, 553–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall MJ, Dale N, 2013. Neuronal transporter and astrocytic ATP exocytosis underlie activity-dependent adenosine release in the hippocampus. J Physiol 591, 3853–3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Haydon PG, Yeung ES, 2000. Direct observation of calcium-independent intercellular ATP signaling in astrocytes. Anal Chem 72, 2001–2007. [DOI] [PubMed] [Google Scholar]
- Wu Z, Feng J, Jing M, Li Y, 2019. G protein-assisted optimization of GPCR-activation based (GRAB) sensors, Neural Imaging and Sensing 2019. 10865 vol. International Society for Optics and Photonics, p. 108650N. [Google Scholar]
- Yaginuma H, Kawai S, Tabata KV, Tomiyama K, Kakizuka A, Komatsuzaki T, Noji H, Imamura H, 2014. Diversity in ATP concentrations in a single bacterial cell population revealed by quantitative single-cell imaging. Sci Rep 4, 6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro K, Fujii Y, Maekawa S, Morita M, 2017. Multiple pathways for elevating extracellular adenosine in the rat hippocampal CA 1 region characterized by adenosine sensor cells. J Neurochem 140, 24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JM, Wang HK, Ye CQ, Ge W, Chen Y, Jiang ZL, Wu CP, Poo MM, Duan S, 2003. ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron 40, 971–982. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Liu B, Wu Q, Liu B, Li Y, Sun S, Wang Y, Wu X, Chai Z, Jiang X, Liu X, Hu M, Wang Y, Yang Y, Wang L, Kang X, Xiong Y, Zhou Y, Chen X, Zheng L, Zhang B, Wang C, Zhu F, Zhou Z, 2019. Differential Co-release of Two Neurotransmitters from a Vesicle Fusion Pore in Mammalian Adrenal Chromaffin Cells. Neuron 102, 173–183 e174. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Phillips GJ, Li Q, Yeung ES, 2008. Imaging localized astrocyte ATP release with firefly luciferase beads attached to the cell surface. Anal Chem 80, 9316–9325. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Chen G, Zhou W, Song A, Xu T, Luo Q, Wang W, Gu X. s., Duan S, 2007. Regulated ATP release from astrocytes through lysosome exocytosis. Nat Cell Bio 9, 945. [DOI] [PubMed] [Google Scholar]
- Zimmermann H, Zebisch M, Strater N, 2012. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal 8, 437–502. [DOI] [PMC free article] [PubMed] [Google Scholar]