Abstract
The prognosis of patients with severe cases of COVID-19 is poor; thus, biomarkers for earlier prediction of COVID-19 progression are vital. We measured levels of five lung injury-related biomarkers, SP-D, KL-6, presepsin, kallistatin and stratifin, in serum samples collected serially during hospitalization from 31 patients with mild/moderate or severe/critical COVID-19 pneumonia, and their predictive performances were compared. Like the previously reported presepsin, a new biomarker candidate, stratifin, was significantly elevated with the onset of severe or critical symptoms in COVID-19 patients and decreased with symptom improvement. Notably, changes in stratifin and presepsin levels were distinctly earlier than those in SP-D, KL-6 and even SpO2/FiO2 values. Furthermore, serum levels of these biomarkers were significantly higher at the pre-severe stage (before the start of oxygen support) of patients who eventually advanced to severe/critical stages than in the patients who remained at the mild/moderate stage. These results were confirmed in an independent cohort, including 71 mild/moderate and 14 severe/critical patients, for whom the performance of stratifin and presepsin in discriminating between mild/moderate and pre-severe conditions of COVID-19 patients was superior to that of the SpO2/FiO2 ratio. Therefore, we concluded that stratifin and presepsin could be used as prognostic biomarkers for severe COVID-19 progression.
Keywords: COVID-19, Predictive biomarker, Stratifin, Presepsin
1. Introduction
Coronavirus disease-2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus (SARS-CoV-2), has dominated health concerns worldwide since early 2020. As of Jan. 12, 2022, more than 315 million cases including over 5.5 million deaths had been reported (https://coronavirus.jhu.edu/). COVID-19 shows a wide range of clinical manifestations, including fever (83–99%), cough (59–82%), fatigue (44–70%), anorexia (40–84%), shortness of breath (31–40%) and myalgias (11–35%). Other symptoms are headache, diarrhea, nausea and vomiting as well as loss of smell (anosmia) and taste (ageusia).1
The World Health Organization (WHO) has classified the patients who test positive for SARS-CoV-2 into 4 grades depending on the severity of their symptoms: mild, moderate, severe and critical. “Mild” includes symptomatic patients without evidence of viral pneumonia or hypoxia. “Moderate” covers cases with clinical signs of pneumonia (fever, cough, dyspnea, fast breathing) but no signs of severe pneumonia such as SpO2 < 90% on room air. “Severe” describes patients with severe pneumonia plus at least one of the followings: respiratory rate >30 breaths/min, severe respiratory distress, or SpO2 < 90% on room air. “Critical” is defined as cases with acute respiratory distress syndrome (ARDS) bearing PaO2/FiO2 ≤ 300 mmHg. About 40%, 40%, 15% and 5% of COVID-19 patients develop mild, moderate, severe and critical disease, respectively. The Japanese case definition criteria differ slightly from those of the WHO.2 The mild category is the same, but moderate is divided into two sub-grades, I and II. Moderate I is defined as patients with respiratory failure and signs of pneumonia with SpO2 of 93%–96% at atmosphere while Moderate II describes cases with respiratory failure for whom SpO2 is below 93% and oxygen therapy is necessary. Severe patients need treatment by mechanical ventilation or extracorporeal membrane oxygenation (ECMO) and/or admission into an intensive care unit. Early diagnosis is crucial for proper treatment of COVID-19 with agents such as sotrovimab or molnupiravir to avoid aggravation.
Pneumonia is diagnosed by X-ray radiation or more properly a computerized tomography (CT) scan. However, use of the same equipment for patients with COVID-19 and those with other diseases increases the risk of infection spread. Instead, if novel blood biomarker were found that could reflect lung conditions or even predict the development of severe pneumonia, rapid diagnosis at bedside might greatly improve treatment decision making. Thus, potential biomarkers have been explored and reported using blood samples from COVID-19 patients. A meta-analysis identified lymphopenia, thrombocytopenia, and elevation of any of the following biomarkers as significantly predictive of outcomes in COVID-19 hospitalized patients: CRP, procalcitonin, creatine kinase, aspartate aminotransferase, alanine aminotransferase, creatinine, D-dimer and lactate dehydrogenase.3 However, most of these candidate biomarkers are non-specific to lung disease; they may reflect damage to other organs or diseases. Biomarkers that reflect lung pathophysiology would be more useful in diagnosing the severity of COVID-19.
There are 2 biomarkers used in the diagnosis of lung diseases. Krebs von den Lungen 6 (KL-6) is a mucin-like, high-molecular-weight sialoglycoprotein expressed in alveolar and is bronchiolar epithelial cells, and is elevated in blood during interstitial lung disease (ILD).4 KL-6 levels were higher in severe and critical COVID-19 patients than in mild cases, and were directly associated with the extent of pulmonary lesions on CT scan.5 KL-6 levels were correlated with PaO2/FiO2 ratio, and higher in unfavorable outcome (subsequently died) patients than favorable outcome (survived) patients, in the blood from the COVID-19 patients collected at hospital admission.6 In addition, patients with fibrotic lung alterations with COVID-19 had high serum KL-6 concentrations than those with non-fibrotic ones.7
Surfactant protein D (SP-D) is a large hydrophilic glycoprotein classified as a collectin, mainly produced by alveolar type 2 cells and used as a biomarker for ILD including idiopathic pulmonary fibrosis.8 Blood SP-D levels were higher in COVID-19 patients with acute respiratory distress syndrome (ARDS) than in patients without ARDS, and were significantly correlated with the PaO2/FiO2 ratio.9 , 10 In addition, serum SP-D levels were significantly higher in severe COVID-19 patients than in mild cases, and they decreased with recovery from the acute phase.11 SP-D levels were also correlated with the CT image score (calculated by formula from the presence of ground glass opacities, patchy shadows, pleural effusions and other findings).
Although KL-6 and SP-D could be promising biomarkers in the diagnosis of COVID-19 severity, more characterization is necessary, especially for their potential use as predictive markers for future disease development. It is noteworthy that serum SP-D levels were elevated in the patients with community-acquired bacterial pneumonia at the similar levels as with COVID-1912 or even higher in the pandemic influenza patients than in COVID-19 patients.13 Thus, novel diagnostic, predictive and prognostic biomarkers reflecting lung injury states should be explored for COVID-19 patients. We previously reported that serum presepsin (P-SEP), a soluble CD14 subtype and a novel biomarker in sepsis, was elevated earlier than KL-6 in 3 COVID-19 patients who progressed from moderate to severe status.13 In addition, increased blood P-SEP has been suggested to be associated with COVID-19 severity or ARDS.14 , 15
In this study, we additionally focused on stratifin (SFN), the sigma type of the 14-3-3 protein family which has shown enhanced expression in some lung cancer types,16 and kallistatin (KAL), a tissue kallikrein-binding protein and a serine proteinase inhibitor that has been related to inflammation and fibrosis.17 We recently found that these proteins are highly variable in the blood of patients at the onset of drug-induced interstitial lung disease with the diffuse alveolar damage (DAD) pattern, a major pathological patten of ARDS (Research Square, preprint, https://doi.org/10.21203/rs.3.rs-690487/v1). Therefore, we hypothesized that SFN and KAL levels may also change in the blood of patients with COVID-19.
In the present study, we assessed SP-D, KL-6, P-SEP, SFN and KAL as predictive biomarkers for COVID-19 aggravation and its severity using patients testing positive for SARS-CoV-2.
2. Materials and methods
2.1. Ethics and human subjects
All work performed in this study was approved by the ethics committees of National Institute of Health Sciences, Nagoya City University East Medical Center, Saitama Medical University Hospital, and Self-Defense Forces Central Hospital and written informed consents were obtained from patients. Patients testing positive for SARS-CoV-2 RNA or antigen using officially approved in vitro diagnostics were enrolled at Nagoya City University East Medical Center from May 2020 to May 2021.
In this study, the COVID-19 severity was classified into four stages, “mild”, “moderate”, “severe”, and “critical”, by the respiratory specialists with reference to the Guidelines of the Diagnosis and Treatment of Novel Coronavirus issued by Ministry of Health, Labour and Welfare, Japan as follows: mild, lack of respiratory symptoms, no findings of pneumonia and oxygen saturation levels (SpO2) ≥ 96%; moderate, findings of low grade pneumonia and 93% < SpO2 < 96% (corresponding to moderate I in the Japanese guideline); severe, requiring oxygen administration (defined as administration ≥2 days in this study) and SpO2 ≤ 93% (mostly corresponding to moderate II in the Japanese guideline); and critical, requiring invasive ventilation and/or admission to the Intensive Care Unit (ICU) (corresponding to severe in the Japanese guideline).2 If there was a difference in severity between SpO2 value and clinical status, the clinical status was considered more important for classification. Further inclusion criteria were as follows: age >18 years, within 2 weeks from symptom onset (except for asymptomatic patients).
In the early phase of this study, as cohort-1, 31 patients with COVID-19 pneumonia were enrolled from whom a total 206 serially collected samples were analyzed to compare biomarker performances of SFN, KAL, P-SEP, SP-D and KL-6. In cohort-2, 344 samples from 85 additional enrolled patients were collected and used for validation of the new biomarker candidate SFN as well as P-SEP.
2.2. Biomarker assay
SFN was measured using an in-house ELISA, using two commercially available anti-SFN mouse monoclonal antibodies, the primary capture antibody (clone CS112-2A8; Merck, Kenilworth, NJ, USA) and the secondary detection antibody (clone 3c3; Sigma–Aldrich, St. Louis, MO, USA). An E. coli recombinant human SFN (NKMAX, Seongnam, Korea) was used as a standard. Primary antibody-absorbed Nunc MaxiSorp 96-well microtiter plates (Thermo Fischer Scientific, Waltham, MA, USA) were blocked with the SuperBlock reagent (Thermo Fischer Scientific). Fifty μL of samples diluted with General Serum Diluent (Immunochemistry Technologies, Bloomington, MN, USA) and 50 μL of the assay buffer (SuperBlock reagent containing 2 M KCL and 50 μg/mL of the HBR-1 heterophilic antibody blocking reagent [Scantibodies Laboratory, Santee, CA, USA]) were mixed in the plates and incubated for 2 h. Following three washings, the SuperBlock reagent containing biotin-labeled secondary detection antibody was added and plates were incubated for 1 h. After washing, the plates were reacted with 10% SuperBlock/PBS solution containing Streptavidin-Poly HRP40 (Stereospecific Detection Technologies, Baesweiler, Germany). SFN was then quantitatively measured using the QuantaRed Enhanced Chemifluorescent HRP Substrate (Thermo Fischer Scientific) by the Nivo microplate reader (PerkinElmer, Germany). Serum KAL was measured using DuoSet ELISA (R&D Systems), according to the manufacturer's instructions. The research ELISA reagents for SFN and KAL were confirmed to meet the criteria of the Japanese Guidelines on Bioanalytical Method Validation (Ligand Binding Assay) in Pharmaceutical Development (https://www.pmda.go.jp/files/000206208.pdf).
SP-D was measured by an in vitro diagnostic SP-D kit, YAMASA EIA II ELISA plate (Yamasa, Chiba, Japan) with the Nivo microplate reader. Serum KL-6 levels were measured by a Nanopia KL-6 sassay (Sekisui Medical, Tokyo, Japan) with an autoanalyzer (Hitachi LABOSPECT 008, Tokyo, Japan), and serum P-SEP levels were measured by a chemiluminescence enzyme immunoassay (CLEIA) presepsin kit (LSI Medience, Tokyo, Japan) with the routine analyzer LABOSPECT008 (Hitachi High-Technologies, Tokyo, Japan).
2.3. Statistical analysis
In group comparisons of patient demographics, the Kruskal–Wallis test was used for continuous variables and the chi-square test or Fisher's Exact test was used for categorical variables, as appropriate. When a significant difference was found by the analysis of variance, Dunn's multiple comparison test was applied. Differences between two groups of biomarkers were calculated by the Mann–Whitney U test. The diagnostic performance of each candidate was evaluated based on the AUC values of ROC curves. Spearman's nonparametric analysis was used to evaluate the correlation between variables. All data were analyzed using the following software: GraphPad PRISM (version 8.4.3; GraphPad Software, San Diego, CA, USA) and Microsoft Excel.
3. Results
3.1. Patients
The samples analyzed in this study were collected from May 2020 to May 2021. Most of the samples were collected during the first to third waves of infection spread, when infection with the original strain of SARS-CoV-2 increased in Japan, and the early phase of the fourth wave, when the alpha variant virus increased.
For cohort-1, from a total of 31 patients with COVID-19, 225 serum samples were serially collected during hospitalization. The COVID-19 severities were classified at each sample collection point, and patients were categorized into mild/moderate (MM) and severe/critical (SC) groups based on their worst symptoms throughout the disease course (Table 1 ). Among 19 patients of the SC group, 12 had severe symptoms at the worst stage while 7 patients reached critical-stage disease. On the day of admission, 14 patients of the SC group were in the moderate stage and progressed to the severe (“moderate-to-severe”) or critical stage (“moderate-to-critical”), while 5 patients were already in the severe stage on admission. Twelve patients with mild or moderate symptoms that did not progress were classified into the MM group. No significant differences were found in sex, age, or duration from onset of symptoms to admission between the MM group and the SC group or among the MM group, and severe and critical subsets of the SC group (Table 1).
Table 1.
Characteristics of COVID-19 patients in this study.
| Mild/Moderate (MM) group | Severe/Critical (SC) group | p valuea | p valueb | |||
|---|---|---|---|---|---|---|
| Cohort_1 | Symptoms at worst stage [n] | Mild/Moderate [12] | Severe [12] | Critical [7] | – | – |
| Symptoms at start of hospital admission [n] | Mild [5], Moderate [7] | Moderate [9], Severe [3] | Moderate [5], Severe [2], Critical [0] | – | – | |
| Age [n]e | 50 (24–79) [12] | 57.5 (33–71) [12] | 55 (44–79) [7] | ns | ns | |
| Male/female | 7/5 | 9/3 | 6/1 | ns | ns | |
| Days between symptom onset and hospital admissione | 6 (1–11) [11] | 7 (2–11) [12] | 6 (4–9) [7] | ns | ns | |
| Samplesd | 32 | 78 | 96 | – | – | |
| Cohort_2 | Symptoms at worst stage [n] | Mild/Moderate [71] | Severe [10] | Critical [4] | – | – |
| Symptoms at start of hospital admission [n] | Mild [16], Moderate [55] | Moderate [4], Severe [6] | Moderate [2], Severe [2], Critical [0] | – | – | |
| Age [n]e | 49 (18–86) [71] | 52 (41–75) [10] | 76 (72–81) [4] | 0.011c | 0.021 | |
| Male/female | 42/29 | 9/1 | 3/1 | ns | ns | |
| Days between symptom onset and hospital admissione | 6 (0–11) [70] | 5 (3–12) [10] | 4 (2–10) [4] | ns | ns | |
| Samplesd | 219 | 58 | 67 | – | – | |
Differences in patient characteristics among the MM group, and severe and critical subsets of the SC group were tested using Kruskal–Wallis (age and days of symptoms before hospital admission) and chi-square (sex) tests.
Differences between the MM group and SC group were tested using Mann–Whitney U-test and Fisher's Exact (sex) tests.
p = 0.011, comparison of age between the MM group and the critical subset by Dunn's test.
Total number of samples taken at each symptom stages of the COVID-19 patients.
Data presented as median (range)
For cohort-2, which was entirely independent of cohort-1, a total of 85 patients were enrolled including 10 and 4 patients who progressed to severe and critical stages, respectively. In cohort-2, there was a significant difference (p = 0.011) in age distribution between the MM group and the critical subset, and indeed, it is generally well known that the risk of severe COVID19 is higher in older age. On the other hand, no significant differences were found in sex and durations from onset of symptoms to hospital admission between the MM and SC groups (Table 1).
3.2. Change of biomarker levels with COVID-19 progression
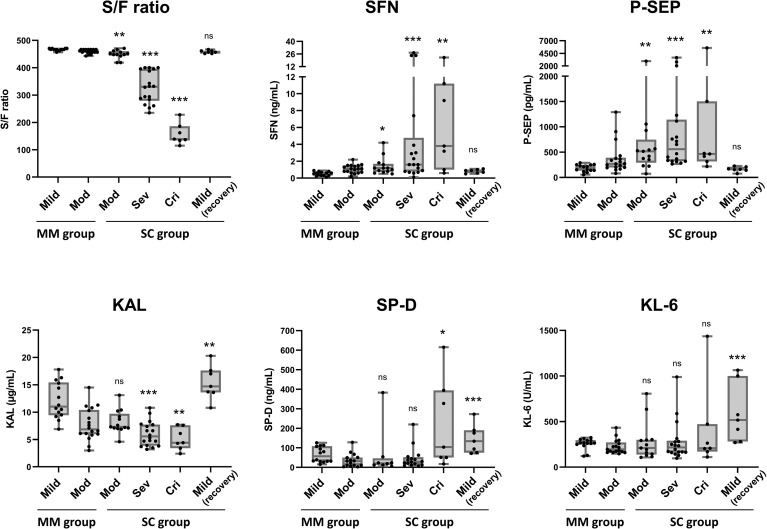
For cohort-1, we first measured the serum levels of SFN, KAL, P-SEP, KL-6 and SP-D. Fig. 1 shows the differences in distribution of biomarker levels by symptoms of the MM and SC groups. For the SC group, data from the first day of onset of moderate, severe or critical symptoms are shown because early diagnostic ability of the biomarkers is the main purpose of this study. The corresponding values are shown in Supplementary Table 1. The oxygenation capacity, or SpO2/FiO2 (S/F) ratio, one of the criteria for diagnosing the severity of lung impairment, showed a clear difference in the SC group. Of note, the serum SFN, as well as P-SEP, showed significantly higher levels on the first day of moderate-, severe- and critical-stage disease in the SC group (i.e., at least later advanced to severe or critical stage), and decreased dramatically in the recovery stage, when only mild symptom was present. KAL showed a significant decrease in the severe and critical stages, although no significant difference was found in the moderate stage of the SC group. KL-6 did not increase in the SC group, while SP-D did show increases in the critical stage but did not decrease in the condition of mild symptoms during the recovery phase.
Fig. 1.
Biomarker levels and COVID-19 severity. Data of cohort-1 are shown. The mild, moderate (Mod), severe (Sev) and critical (Cri) indicate stages of COVID-19 pneumonia symptom at sample taken. For the MM group, data of all samples taken during hospitalization are shown. For the SC group, data of samples taken on the first day of the indicated stages are shown. Mild (recovery) indicates a recovered stage within the post-severe stage in the SC group. The boxes indicate interquartile ranges (75% and 25%) and medians. Differences from the non-severe group by the Mann–Whitney U-test: ∗: p < 0.05, ∗∗: p < 0.01, ∗∗∗: p < 0.001.
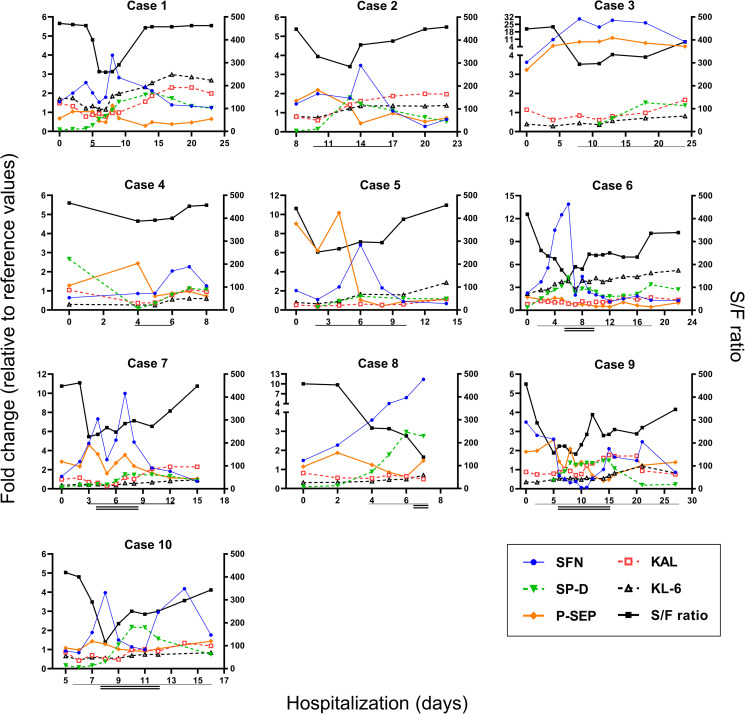
Fig. 2 shows the clinical course and fold changes relative to the reference values for these biomarkers in ten representative patients of the SC group (Cases 1–5, moderate-to-severe cases; Cases 6–10, moderate-to-critical cases). As the tentative reference values, the mean ± 2SD value for each biomarker level at the mild stage in the MM group was calculated (Supplementary Table 1). The calculated reference values, 323 pg/mL for P-SEP, 378 U/mL for KL-6 and 144 ng/mL for SP-D, were not far from those used in clinical practice: 300 pg/mL, 500 U/mL and 110 ng/mL, respectively.
Fig. 2.
Change in biomarker levels on consecutive days. Data of representative 10 patients who developed severe- and/or critical-stage disease from initial moderated status are shown (Cases 1–5: the moderate-to-severe cases, 6–10: the moderate-to-critical cases). The left Y axis shows the fold change value relative to the reference value of each biomarker (1.0 indicates the reference value). The reference values were determined as the upper (SFN, SP-D, KL-6. P-SEP) or lower (KAL) 2SD values from mean concentration for each biomarker at the mild stage in the MM group (ref. Supplementary Table 1). The right Y-axis shows the SpO2/FiO2 (S/F) ratio, while the X axis shows the number of days of hospitalization. Horizontal single and double lines indicate duration of severe stage (oxygen support) and critical stage (invasive ventilation), respectively.
In most cases, the serum levels of SFN, as well as P-SEP, were higher (>1 fold) than the reference values even in the early phase of hospitalization (Cases 1–3, 5, 6, 8 and 9, Fig. 2). SFN and P-SEP increased immediately with the severity progression of pneumonia symptoms and decreased along with improvement in symptoms (except for Case 10). Compared to SFN and P-SEP, the increase in SP-D tended to be delayed, and the changes in KAL and KL-6 were slight. SFN and P-SEP tended to increase earlier than the change in the S/F ratio (Cases 1, 3, 6, 7 and 8). These results suggest that continuous measurement of SFN may lead to early detection of COVID-19 progression, as we previously showed for P-SEP.13
3.3. Predictive performances for COVID-19 progression
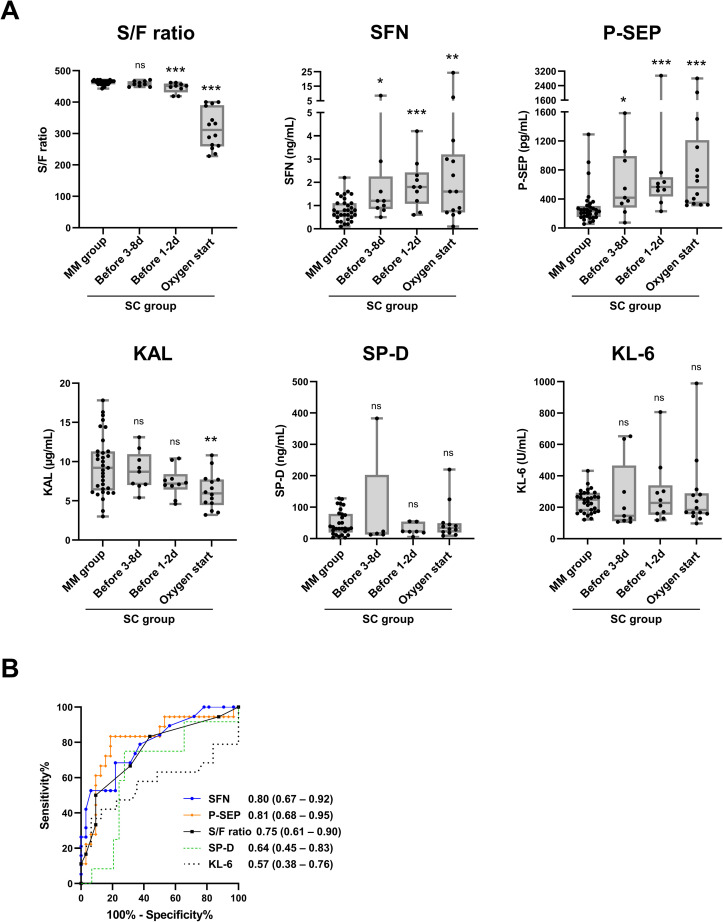
To verify the utility of each biomarker for predicting progression to later severe- or critical-stage COVID-19, we focused the moderate-to-severe and moderate-to-critical cases of the SC group, and statistically analyzed the biomarker levels before entering the severe (or critical) stage (termed “pre-severe stage”). As shown in Fig. 3 A, the levels of SFN and P-SEP in the pre-severe stage of the samples were significantly higher than those in the MM group, not just for 1–2 days but as early as 3 days or more before the start of oxygen support (first day of severe-stage disease). The S/F ratio showed a significant change 1–2 days before oxygen support, but not 3 days or more before. No significant changes in KAL, SP-D, or KL-6 values were observed in the pre-severe stage. A receiver operating characteristic analysis revealed that the area under curve (AUC) of SFN and P-SEP for predicting the severity of COVID-19 (all samples taken from the MM group during hospitalization vs. samples taken at the pre-severe stage in the SC group) were 0.80 and 0.81, respectively, which were higher than the AUC values of the S/F ratio (0.75), SP-D (0.64) and KL-6 (0.57). These data suggest that SFN and P-SEP would be useful prognostic biomarkers for later disease progression by discriminating between mild/moderate and pre-severe stages of COVID-19 pneumonia.
Fig. 3.
Comparison of predictive performances for severe/critical COVID-19 patients. Data of cohort-1 are shown. A, S/F ratio, serum levels of SFN, P-SEP, KAL, SP-D, KL-6 in all samples from the MM group, and samples taken at the pre-severe stage (1–8 days before start of oxygen support) and the severe stage (start day of oxygen support) in the moderate-to-severe and moderate-to-critical cases of the SC group are shown. The boxes indicate interquartile ranges (75% and 25%) and medians. B, ROC curves in discrimination of the MM group samples and samples at the pre-severe stage (1–8 days before start of oxygen support) in the SC group. Numbers indicate the area under the curve (AUC) derived from ROC curves, and the 95% confidence intervals (95% CIs). Differences from the MM group by the Mann–Whitney U-test: ∗: p < 0.05, ∗∗: p < 0.01, ∗∗∗: p < 0.001.
3.4. Validation of SFN and P-SEP
To validate the prognostic performance of SFN and P-SEP, we analyzed samples of cohort-2 independent from cohort-1.
SFN levels in samples taken at the mild (n = 61) and moderate (n = 158) stages in the MM group of cohort-2 (mean ± SD, 0.6 ± 0.8 and 1.0 ± 1.7 ng/mL) were similar to those observed in cohort-1 (0.5 ± 0.2 and 1.1 ± 0.5 ng/mL), respectively (Supplementary Table 1). Also, serum P-SEP levels at the mild and moderate stage in the MM group of cohort-2 (mean ± SD, 283 ± 138 and 396 ± 322 ng/mL) were close to those observed in cohort-1 (183 ± 70 and 377 ± 308 ng/mL), respectively (Supplementary Table 1). On the first day of the moderate, severe and critical stages in the SC group, significant increases of serum SFN and P-SEP levels were observed with a decrease of the S/F ratio (Fig. 4 ), in line with the finding obtained from cohort-1 (Fig. 1A). Fig. 5 shows the prognostic performance of the S/F ratio, SFN and P-SEP for COVID-19 progression using cohort-2. The analyses highly reproduced the results obtained from cohort-1. Compared to the S/F ratio, serum SFN and P-SEP levels were more obviously changed at the pre-severe stage in the moderate-to-severe and moderate-to-critical cases (Fig. 5A). In ROC analysis, both SFN (AUC 0.84) and P-SEP (0.86) were superior to the S/F ratio (0.74) in discriminating samples taken at the pre-severe stage in the SC group from those taken from the MM group (Fig. 5B), in good agreement with the data from the cohort-1 data (Fig. 3B).
Fig. 4.
Validation of serum SFN and P-SEP levels using cohort-2. Serum S/F ratio, SFN levels and P-SEP levels for each symptom stage (mild, moderate (Mod), severe (Sev) and critical (Cri)) in the MM and SC groups. For the MM group, data of all samples taken during hospitalization are shown. For the SC group, data of samples taken at the first day of indicated stages are shown. The boxes indicate interquartile ranges (75% and 25%) and medians. Differences to the non-severe group by the Mann–Whitney U-test: ∗: p < 0.05, ∗∗: p < 0.01, ∗∗∗: p < 0.001.
Fig. 5.
Validation of prognostic performance for serum SFN and P-SEP using cohort-2. A, Serum S/F ratio, SFN levels and P-SEP levels in all samples from the MM group, and samples at the pre-severe stage (1–8 days before start of oxygen support) and severe stage (start day of oxygen support) in the moderate-to-severe and moderate-to-critical cases of the SC group. The boxes indicate interquartile ranges (75% and 25%) and medians. Differences from the MM group by the Mann–Whitney U-test: ∗: p < 0.05, ∗∗: p < 0.01, ∗∗∗: p < 0.001. B, ROC curves in discrimination of the MM group samples and samples at pre-severe stage in severe group.
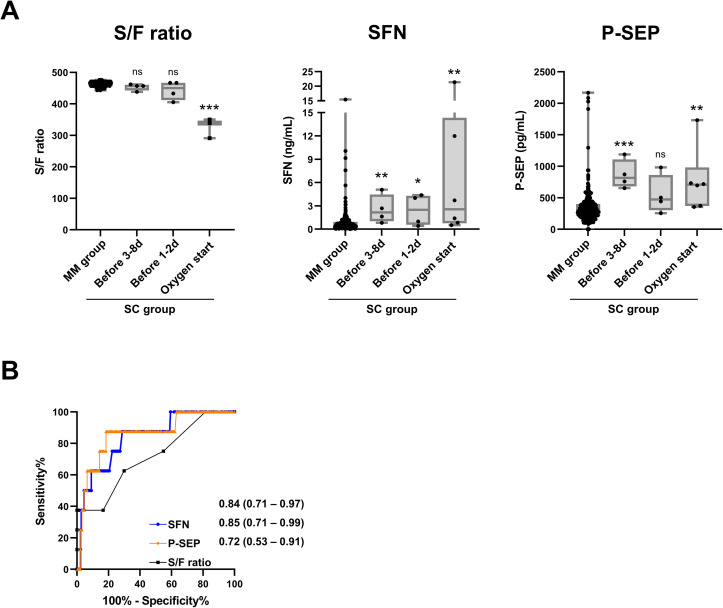
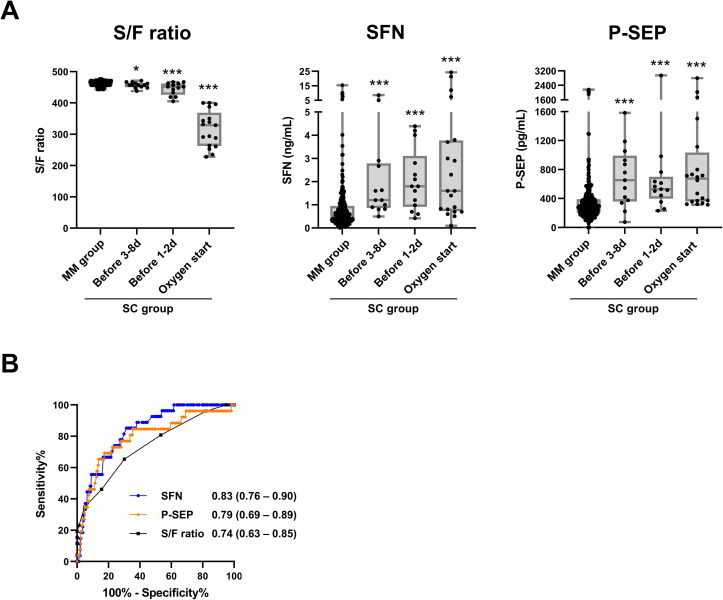
Finally, the prognostic performances of SFN and P-SEP were demonstrated using the combined cohort (with combined data from cohorts-1 and -2) (Fig. 6 ). Both serum SFN and P-SEP were obviously elevated at the pre-severe stage (Fig. 6A), and these protein levels showed a moderate correlation (Spearman's rank correlation coefficient [r s] = 0.506, p < 0.0001, Supplementary Fig. 1). The AUC values [95% CI] of these proteins in diagnosing the pre-severe stage were 0.83 [0.76–0.90] for SFN and 0.79 [0.69–0.89] for P-SEP, higher than that for the S/F ratio, 0.74 [0.63–0.85]. When the cutoff values of SFN and P-SEP for discriminating the pre-severe condition from the mild/moderate condition on COVID-19 patients were set at 0.81 ng/mL and 374 pg/mL, calculated from the result of Fig. 6B based on Youden's index, the diagnostic sensitivity and specificity were 81.5% and 70.1% for SFN, and 76.9% and 71.9% for P-SEP, respectively.
Fig. 6.
Combined analysis using cohort-1 and -2 on prognostic performance for serum SFN and P-SEP. A, Serum S/F ratio, SFN levels and P-SEP levels in all samples from the MM group, and samples at the pre-severe stage (1–8 days before start of oxygen support) and severe stage (start day of oxygen support) in the moderate-to-severe and moderate-to-critical cases of the SC group. The boxes indicate interquartile ranges (75% and 25%) and medians. Differences from the MM group by the Mann–Whitney U-test: ∗: p < 0.05, ∗∗: p < 0.01, ∗∗∗: p < 0.001. B, ROC curves in discrimination of the MM group samples and samples at pre-severe stage in severe group.
4. Discussion
COVID-19 is a respiratory disease with a wide range of manifestations, from asymptomatic to severe cases with ARDS.18 Although about 80% of patients with COVID-19 experience only mild or moderate symptoms and have a favorable prognosis, the remaining patients worsen to severe or critical stage, and their prognosis, largely driven by severe ARDS, is poor. Therefore, early detection of the severe COVID-19 cases is important.
In the present study, we analyzed five biomarkers for lung injury, SP-D, KL-6, P-SEP, KAL and SFN, all of which have been suggested to be related with ARDS or its typical histological pattern DAD, in serum samples collected serially from patients with COVID-19. Importantly, we found for the first time that serum SFN was significantly elevated in patients with severe COVID-19 compared to patients with mild or moderate symptoms. SFN, as well as P-SEP which has been suggested as a biomarker for severe COVID-19, increased immediately with the onset of moderate to severe symptoms in the SC group patients, and decreased along with improvement in symptoms. Most importantly in many patients with severe COVID-19, the change of SFN and P-SEP levels were observed early, before SP-D, KL-6 and even SpO2 changed. SFN and P-SEP showed good performance for discriminating between the pre-severe condition and mild/moderate condition in COVID-19 patients. Measurement of serum SFN and P-SEP in hospitalized COVID-19 patients may be useful for early prediction of prognosis for development of severe symptoms.
In addition, measurement of serum SFN and P-SEP may be useful in considering the timing of therapeutic drug administration. The main pathogenesis of COVID-19 consists of two distinct but overlapping pathologies: that of the SARS-CoV-2 virus and that of the host inflammation response.19 Antiviral or antibody drugs (e.g., molnupiravir and sotrovimab) have been suggested to be effective for patients in the early stages of disease onset, and anti-inflammatory drugs such as glucocorticoids have been suggested to be effective for patients who have progressed to the severe/critical stage.18 It has been pointed out that the beneficial effects of glucocorticoids in patients with severe COVID-19 patients are dependent on the selection of the right dose, at the right time, for the right patient.20 In fact, the RECOVERY clinical trial showed that administration of dexamethasone to patients before initiation of oxygen therapy did not improve outcomes.20 Our data showed that P-SEP and SFN could be biomarkers for detecting early lung injury in COVID-19 patients who are likely to proceed to the severe/critical stage. We suggest that patients with low SFN and P-SEP levels should only be followed up, while those who start to show elevations of these biomarkers in the mild/moderate stage should be treated with early antiviral drugs or antibody drugs optimal for the infected SARS-CoV-2 variant strains.
In the most severe/critical cases, COVID-19 leads to ARDS.21 ARDS is characterized by acute and diffuse damage to the alveolar-capillary barrier. This condition is histologically known as DAD, which result in permanent damage to the alveolar epithelial cells and capillary endothelial cells.22 DAD progresses dynamically in a consistent and discrete manner: denudation and apoptosis of alveolar epithelia and hyaline membrane formation in the early exudative phase, fibroblast proliferation and type II pneumocyte hyperplasia in the mid proliferative (organizing) phase, and fibrosis and squamous cell metaplasia in the late fibrotic phase. They reflect the global mechanisms of wound repair, and are thought to be involved in cell cycle regulation and apoptosis.23 The dynamic change of phenotype and molecular mechanisms in DAD may be involved in the different timing of several proteins' expressions. Denudation and apoptosis of alveolar epithelia may be important early features of acute lung injury. The associations of DAD with activation of transcription factor p53, apoptosis and cell cycle arrest have been reported.24, 25, 26 SFN is also a direct transcriptional target of p53 and is induced in response to DNA damage, arresting progression from the G2/M phase of the cell cycle.27 We observed that SFN expression was increased in serum and tissues including alveolar type II epithelial cells from patients with drug-induced ILD or idiopathic interstitial pneumonias with DAD (Research Square, preprint, https://doi.org/10.21203/rs.3.rs-690487/v1). These findings lead us to speculate that SFN in severe COVID-19 patients may also be expressed in a p53-dependent manner and released from the lung tissue in early DAD, before serum SP-D or KL-6 are elevated.
The soluble CD14 protein subtype, P-SEP, is an established biomarker for sepsis, and is a candidate predictor for septic ARDS.28 In addition, patients with ARDS by severe community-acquired pneumonia had obviously higher plasma P-SEP levels than those without ARDS, and plasma P-SEP levels were significantly higher in non-survivors than in survivors at 28-day follow-up.29 Because P-SEP is produced from macrophages, and macrophages have been recognized as one of the main drivers in the physiopathology of DAD,30 this may be the origin of P-SEP release into blood of COVID-19 patients with severe lung symptoms. However, the exact mechanisms of increasing serum P-SEP levels by severe COVID-19 remain to be revealed.
In the SC group, serum SFN and P-SEP levels were continuously increased with the severity of pneumonia symptoms, but in some cases (Cases 6, 7, 9 and 10, Fig. 2), SFN and P-SEP levels were dramatically decreased after the first day (start of invasive ventilation) in the critical stage. It is unclear whether this decrease indicates a temporary improvement in symptoms or whether it is due to the invasive ventilator or drug administration. To clarify this point, further research is needed.
The major limitation of this study is the small size of both cohort-1 and cohort-2. Our data however was able to demonstrate that SFN and P-SEP has high potentials of being used as a predictive biomarker for severe COVID-19 progression, facilitating early proper treatment decisions to improve prognosis of patients by monitoring using this biomarker.
Relatively high values of both SFN and P-SEP were also observed in some mild/moderate patients (Fig. 6A). Of these patients, cancer and kidney dysfunction were found as background diseases. Several researches have described that P-SEP levels are increased in patients with chronic kidney disease (CKD)31, 32, 33; the correlation of CKD with SFN levels is less known. Given the small sample size of the present study, we could not make a linkage between these biomarker levels and patients' underlying diseases. Therefore, at this time, when predicting severe progression in COVID-19 patients using SFN or P-SEP, other clinical factors should be taken into account in these patients. However, if SFN or P-SEP can be used in the clinical setting to detect the early phase of severe COVID-19 with ARDS and those at risk of severe disease, it would represent an important step in decreasing mortality from severe COVID-19 and ARDS. Foreknowledge of the development of severe and critical symptoms could also help improve the management of COVID-19 hospital wards. Further validation studies are clearly necessary for validation of the analytical and clinical utility of SFN as well as P-SEP.
In conclusion, we explored prognostic biomarkers for early prediction of COVID-19 progression to severe or critical stages, and identified SFN and P-SEP as promising candidates.
Declaration of competing interest
The authors declare no conflicts of interest in this work.
Acknowledgments
Financial support was received in the form of grants from the Japan Agency for Medical Research and Development (nos. 20fk0108300 to NA, TM, CH, and YS).
Footnotes
Peer review under responsibility of Japanese Pharmacological Society.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jphs.2022.06.002.
Appendix ASupplementary data
The following is the Supplementary data to this article:
References
- 1.https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-2
- 2.https://www.mhlw.go.jp/content/000815065.pdf
- 3.Malik P., Patel U., Mehta D., et al. Biomarkers and outcomes of COVID-19 hospitalisations: systematic review and meta-analysis. BMJ Evid Based Med. 2021;26(3):107–108. doi: 10.1136/bmjebm-2020-111536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishikawa N., Hattori N., Yokoyama A., Kohno N. Utility of KL-6/MUC1 in the clinical management of interstitial lung diseases. Respir Investig. 2012;50(1):3–13. doi: 10.1016/j.resinv.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 5.d'Alessandro M., Cameli P., Refini R.M., et al. Serum KL-6 concentrations as a novel biomarker of severe COVID-19. J Med Virol. 2020;92(10):2216–2220. doi: 10.1002/jmv.26087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scotto R., Pinchera B., Perna F., et al. Serum KL-6 could represent a reliable indicator of unfavourable outcome in patients with COVID-19 pneumonia. Int J Environ Res Publ Health. 2021;18(4) doi: 10.3390/ijerph18042078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.d'Alessandro M., Bergantini L., Cameli P., et al. Serial KL-6 measurements in COVID-19 patients. Intern Emerg Med. 2021;16(6):1541–1545. doi: 10.1007/s11739-020-02614-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang K., Ju Q., Cao J., Tang W., Zhang J. Impact of serum SP-A and SP-D levels on comparison and prognosis of idiopathic pulmonary fibrosis: a systematic review and meta-analysis. Medicine (Baltim) 2017;96(23) doi: 10.1097/MD.0000000000007083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kerget B., Kerget F., Koçak A.O., et al. Are serum interleukin 6 and surfactant protein D levels associated with the clinical course of COVID-19? Lung. 2020;198(5):777–784. doi: 10.1007/s00408-020-00393-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tojo K., Yamamoto N., Mihara T., Abe M., Goto T. Distinct temporal characteristics of circulating alveolar epithelial and endothelial injury markers in ARDS with COVID-19. Crit Care. 2021;25(1):169. doi: 10.1186/s13054-021-03596-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tong M., Xiong Y., Zhu C., et al. Serum surfactant protein D in COVID-19 is elevated and correlated with disease severity. BMC Infect Dis. 2021;21(1):737. doi: 10.1186/s12879-021-06447-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alay H., Laloglu E. The role of angiopoietin-2 and surfactant protein-D levels in SARS-CoV-2-related lung injury: a prospective, observational, cohort study. J Med Virol. 2021;93(10):6008–6015. doi: 10.1002/jmv.27184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukada A., Kitagawa Y., Matsuoka M., et al. Presepsin as a predictive biomarker of severity in COVID-19: a case series. J Med Virol. 2021;93(1):99–101. doi: 10.1002/jmv.26164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kocyigit A., Sogut O., Durmus E., et al. Circulating furin, IL-6, and presepsin levels and disease severity in SARS-CoV-2-infected patients. Sci Prog. 2021;104(2_suppl) doi: 10.1177/00368504211026119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao J., Tan Y., Wang L., Shi Y. Discriminatory ability and prognostic evaluation of presepsin for sepsis-related acute respiratory distress syndrome. Sci Rep. 2020;10(1):9114. doi: 10.1038/s41598-020-66121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shiba-Ishii A. Significance of stratifin in early progression of lung adenocarcinoma and its potential therapeutic relevance. Pathol Int. 2021;71(10):655–665. doi: 10.1111/pin.13147. [DOI] [PubMed] [Google Scholar]
- 17.Chao J., Guo Y., Chao L. Protective role of endogenous kallistatin in vascular injury and senescence by inhibiting oxidative stress and inflammation. Oxid Med Cell Longev. 2018;2018 doi: 10.1155/2018/4138560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gandhi R.T., Lynch J.B., Del Rio C. Mild or moderate covid-19. N Engl J Med. 2020;383(18):1757–1766. doi: 10.1056/NEJMcp2009249. [DOI] [PubMed] [Google Scholar]
- 19.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39(5):405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horby P., Lim W.S., Emberson J.R., et al. Dexamethasone in hospitalized patients with covid-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polak S.B., Van Gool I.C., Cohen D., von der Thüsen J.H., van Paassen J. A systematic review of pathological findings in COVID-19: a pathophysiological timeline and possible mechanisms of disease progression. Mod Pathol. 2020;33(11):2128–2138. doi: 10.1038/s41379-020-0603-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Batah S.S., Fabro A.T. Pulmonary pathology of ARDS in COVID-19: a pathological review for clinicians. Respir Med. 2021;176 doi: 10.1016/j.rmed.2020.106239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin T.R., Nakamura M., Matute-Bello G. The role of apoptosis in acute lung injury. Crit Care Med. 2003;31(4 Suppl):S184–S188. doi: 10.1097/01.CCM.0000057841.33876.B1. [DOI] [PubMed] [Google Scholar]
- 24.Guinee D., Jr., Brambilla E., Fleming M., et al. The potential role of BAX and BCL-2 expression in diffuse alveolar damage. Am J Pathol. 1997;151(4):999–1007. [PMC free article] [PubMed] [Google Scholar]
- 25.Guinee D., Jr., Fleming M., Hayashi T., et al. Association of p53 and WAF1 expression with apoptosis in diffuse alveolar damage. Am J Pathol. 1996;149(2):531–538. [PMC free article] [PubMed] [Google Scholar]
- 26.Bardales R.H., Xie S.S., Schaefer R.F., Hsu S.M. Apoptosis is a major pathway responsible for the resolution of type II pneumocytes in acute lung injury. Am J Pathol. 1996;149(3):845–852. [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor W.R., Stark G.R. Regulation of the G2/M transition by p53. Oncogene. 2001;20(15):1803–1815. doi: 10.1038/sj.onc.1204252. [DOI] [PubMed] [Google Scholar]
- 28.Shimoyama Y., Umegaki O., Kadono N., Minami T. Presepsin values predict septic acute kidney injury, acute respiratory distress syndrome, disseminated intravascular coagulation, and shock. Shock. 2021;55(4):501–506. doi: 10.1097/SHK.0000000000001664. [DOI] [PubMed] [Google Scholar]
- 29.Liu B., Yin Q., Chen Y.X., Zhao Y.Z., Li C.S. Role of Presepsin (sCD14-ST) and the CURB65 scoring system in predicting severity and outcome of community-acquired pneumonia in an emergency department. Respir Med. 2014;108(8):1204–1213. doi: 10.1016/j.rmed.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Matthay M.A., Zemans R.L. The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol. 2011;6:147–163. doi: 10.1146/annurev-pathol-011110-130158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyoshi M., Inoue Y., Nishioka M., et al. Clinical evaluation of presepsin considering renal function. PLoS One. 2019;14(9) doi: 10.1371/journal.pone.0215791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagata T., Yasuda Y., Ando M., et al. Clinical impact of kidney function on presepsin levels. PLoS One. 2015;10(6) doi: 10.1371/journal.pone.0129159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobayashi S., Amano H., Terawaki H., Kawaguchi Y., Yokoo T. Prediction of presepsin concentrations through commensurate decline in kidney function in the elderly. Clin Chim Acta. 2020;500:1–9. doi: 10.1016/j.cca.2019.09.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.