Abstract
Skeletal muscle atrophy is a hallmark of severe spinal cord injury (SCI) that is precipitated by the neural insult and paralysis. Additionally, other factors may influence muscle loss, including systemic inflammation, low testosterone, low insulin-like growth factor (IGF)-1, and high-dose glucocorticoid treatment. The signaling cascades that drive SCI-induced muscle loss are common among most forms of disuse atrophy and include ubiquitin-proteasome signaling and others. However, differing magnitudes and patterns of atrophic signals exist after SCI versus other disuse conditions and are accompanied by endogenous inhibition of IGF-1/PI3K/Akt signaling, which combine to produce exceedingly rapid atrophy. Several well-established anabolic agents, including androgens and myostatin inhibitors, display diminished ability to prevent SCI-induced atrophy, while ursolic acid and β2-agonists more effectively attenuate muscle loss. Strategies combining physical rehabilitation regimens to reload the paralyzed limbs with drugs targeting the underlying molecular pathways hold the greatest potential to improve muscle recovery after severe SCI.
Keywords: MAFbx, Atrogin-1, MuRF1, FOXO, Ubiquitin, mTOR, Hypertrophy, Atrophy, Anabolic, Spinal cord injury, Disuse, Unloading, Denervation, Paralysis, Paralyzed, Muscle, Musculoskeletal, Testosterone, Testosterone replacement therapy, Androgen, Insulin-like growth factor 1, Igf-1, Myostatin, Activin IIb receptor, Transforming growth factor beta, TGF beta, Beta 2 agonist, Clenbuterol, Formoterol, Ursolic acid, SS-31, Elamipretide, Antioxidant, Epicatechin, Acteoside, Verbascoside, Activity-based physical therapy, Functional electrical stimulation, Neuromuscular electrical stimulation, Bodyweight-supported treadmill training
Introduction
In the United States, ~80,000—120,000 individuals are living with a severe motor-complete spinal cord injury (SCI) [1], which induces immediate and permanent paralysis in muscles innervated below the spinal lesion. Rapid skeletal muscle atrophy is a hallmark of severe SCI that is precipitated by the neural insult and the resulting neuromuscular impairment, with 25—60% lower muscle cross-sectional area (CSA) and muscle fiber (f)CSA in paralyzed muscles 3—6 months post-injury [2]. Changes in the molecular signaling cascades that regulate muscle size are distinct after severe SCI, with muscle loss being more rapid than in other disuse conditions, such as hindlimb immobilization [3] or sciatic transection [4]. Therefore, pharmacologic strategies intending to limit SCI-induced muscle loss must target the initiating atrophy pathways in the paralyzed limbs, while also addressing systemic physiologic consequences of SCI that have the potential to exacerbate muscle loss and/or inhibit muscle recovery. This mini-review provides overviews of the SCI muscle phenotype, the molecular signaling pathways, and secondary factors that influence muscle atrophy, and recent pharmacologic approaches to lessen muscle loss in the paralyzed limbs after severe SCI.
2. Pathophysiology of skeletal muscle loss following spinal cord injury
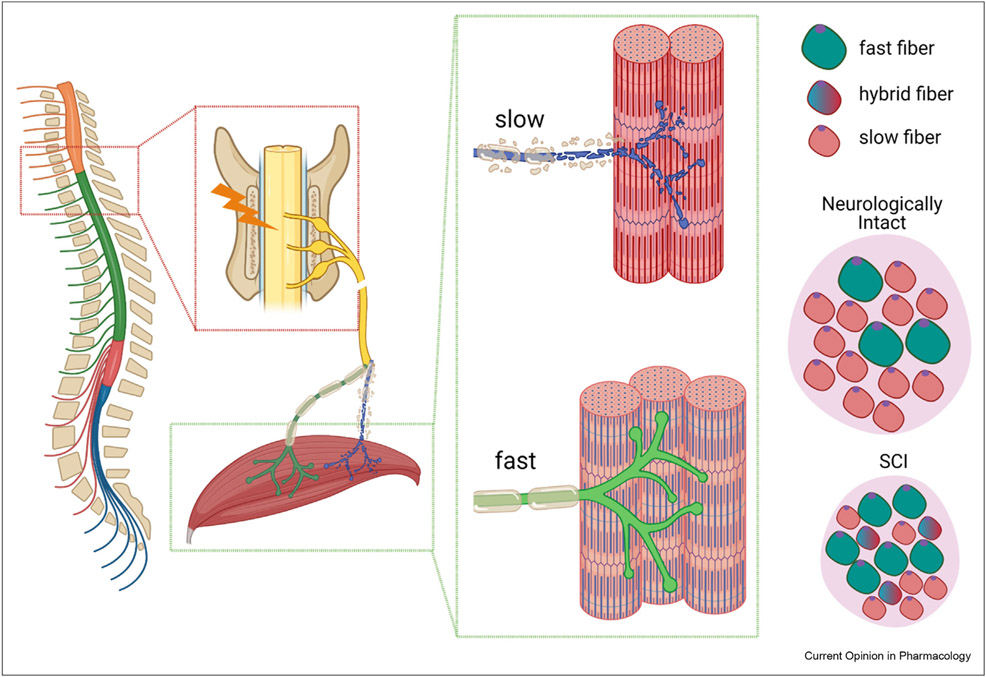
Severe SCI results in impaired neural drive, motoneuron atrophy, and pathologic changes to the neuromuscular junction that combine to produce low muscle force generating capacity and/or paralysis [2]. Collectively, these deficits impact the rapid muscle atrophy and the development of the SCI muscle phenotype, which is characterized by mitochondrial dysfunction, a slow-oxidative to fast-glycolytic muscle fiber-type transition, and the development of muscle fibrosis (Figure 1) [2,5]. The atrophic signals that initiate muscle loss after SCI are thought to be common among most forms of disuse atrophy and include the ubiquitin-proteasome and transforming growth factor (TGF) β/Smad-3 signaling pathways, among others [6]. However, after severe SCI the rate of muscle loss is more rapid than in other disuse conditions [3,4], likely because the magnitude and pattern of atrophic signals differ in response to SCI. For example, in rodents, mRNA expression of the muscle-specific E3 ubiquitin ligases muscle atrophy F-box (MAFbx or atrogin-1) and muscle ring finger-1 (MuRF1) are twofold to threefold higher after spinal transection vs. sciatic nerve transection, resulting in a two-fold increase in the muscle atrophy rate over the initial 7-d post-injury [4]. Thereafter, atrophy signals revert to the levels of sham-operated controls and muscle atrophy slows. Similarly, in persons with chronic complete SCI, muscle expression of MAFbx and MuRF1 was equal to or less than that of similarly aged able-bodied persons [7] and nuclear localization of forkhead box O (FOXO)1 and FOXO3a (atrophic transcription factors) and MAFbx were lower [8], suggesting that proteasomal degradation is not central to the sustained atrophy after SCI.
Figure 1.
Pathophysiology of skeletal muscle loss after severe spinal cord injury (SCI). SCI results in impaired neural drive, motor neuron atrophy, and pathological changes to the neuromuscular junction that combine to produce low muscle force generating capacity and/or paralysis. Collectively, these deficits impact the rapid rate of muscle atrophy and the repeated denervation–reinnervation cycles that influence the slow-oxidative to fast-glycolytic muscle fiber-type transition in paralyzed muscles. Figure was generated in BioRender.
Although atrophy signaling initiates muscle loss after severe SCI, reduced anabolic signaling likely contributes to the sustained muscle deficits. Key proteins involved in anabolic signaling are those downstream of the insulin-like growth factor (IGF)-1/PI3K/Akt pathway [9] and of other anabolic stimuli, with the nexus of protein synthesis in all cells being the intracellular kinase mechanistic target of rapamycin (mTOR). Once phosphorylated (p), mTOR targets and phosphorylates downstream proteins involved in translation initiation and efficiency that increases protein synthesis and, if sustained, produces muscle growth [10]. However, total and (p)mTOR decline within the muscle in persons over the initial 3—12 months post-SCI [11], likely contributing to lower anabolic signaling that persists for decades in this population [8]. Similarly, (p)PI3K, (p)Akt, and (p)mTOR levels are ~50—75% lower in the soleus muscle of rodent SCI models versus controls within only a few days to weeks post-injury [12,13].
The reasons why differing atrophic and anabolic signaling patterns exist after SCI versus other disuse conditions are unknown, although it is likely that secondary factors that occur in response to SCI are involved. For example, it is possible that the systemic inflammation that develops in response to the direct SCI trauma (e.g., car crash or fall) or to the subsequent surgical interventions [14] exacerbates muscle atrophy. In this regard, high-dose methylprednisolone (systemic glucocorticoid) is routinely administered to persons with severe SCI over the acute—subacute recovery phase to suppress inflammatory processes that influence the secondary injury cascade within the spinal cord, despite the questionable safety and efficacy of this regimen [15]. However, preclinical research indicates that high-dose glucocorticoid treatment directly stimulates muscle atrophy by initiating atrophic pathways and/or suppressing anabolic signaling [16] and that methylprednisolone exacerbates the increase in FOXO1, MAFbx, MuRF1, and REDD1 (an inhibitor of mTOR signaling) expression in response to SCI, along with the subsequent muscle loss [17]. Several hormonal irregularities that are associated with muscle atrophy (e.g., low testosterone and low IGF-1) also occur secondary to SCI [2,18]. These are important factors to consider because circulating testosterone [19] and IGF-1 [20] have both been positively correlated with thigh muscle CSA in persons with chronic complete SCI.
3. Pharmacologic approaches to ameliorate muscle atrophy after SCI
No drugs are currently approved to counter muscle atrophy after severe SCI and no pharmacologic strategy has been definitively shown to attenuate muscle loss in the paralyzed limbs of persons with SCI; however, several promising preclinical strategies have been identified. The following sections discuss the most investigated anabolic agents in relation to SCI (i.e., androgens and β2-adrenergic agonists), along with several other promising pharmaceuticals. Because of the brevity of this mini-review, we focus on drugs that have been tested in persons with paralyzed limbs or in animal models with complete/severe SCI because muscle atrophy after incomplete SCI is less drastic, due to the presence of spared spinal tracts that permit voluntary musculoskeletal loading in the impaired limbs [2].
4. Androgens
Testosterone is the most abundant bioactive androgen within the circulation. Pharmacologic testosterone increases muscle mass in able-bodied hypogonadal men when administered in sufficient doses [21], either via direct androgen receptor engagement and/or indirectly via androgen-induced alterations in anabolic (e.g., IGF-1/PI3K/Akt) or catabolic signaling (e.g., myostatin/Smad-3) pathways [2]. In the 1950s, Cooper et al. [22] reported elevated urinary nitrogen excretion and a negative nitrogen balance that persisted for several months in persons with SCI and that high-dose testosterone (50—100 mg/day) normalized nitrogen balance by mitigating nitrogen excretion, suggesting that high-dose testosterone may limit muscle wasting after SCI. However, this possibility has yet to be verified, likely because high-dose testosterone produces several health risks, including prostate enlargement [23]. Alternatively, moderate-dose testosterone (5—10 mg/day for 12 months) was shown to increase whole body and lower extremity lean mass in a small cohort of hypogonadal men with chronic complete SCI [24], with improvements persisting for 6 months [25]. In contrast, low-dose testosterone (2—6 mg/day for 16 weeks) did not increase whole body lean mass, lower extremity muscle CSA [26], or muscle fCSA [27] in eugonadal and hypogonadal men with chronic complete SCI. These small trials did not observe prostate enlargement nor reported any serious adverse events. In comparison, some preclinical SCI studies reported that high-dose testosterone increased the mass of the prostate and of the levator ani-bulbocavernosus (LABC) muscle (involved in sexual function) and various hindlimb muscles [2], while others have reported that testosterone did not increase muscle mass or fCSA in the paralyzed hindlimbs [28-31]. The reasons for these inconsistencies are unknown, although androgen receptor expression is greater than threefold higher in the prostate and LABC (androgen-responsive tissues) versus soleus [30] and other non-androgen-responsive hindlimb muscles [2]. Regardless, testosterone has been shown to suppress muscle FOXO1, MAFbx, MuRF1, and REDD1 expression and lessen the excess atrophy associated with methylprednisolone treatment in a rodent spinal transection model [17]. Moreover, testosterone attenuated gastrocnemius muscle loss after spinal transection, when given in combination with nandrolone (non-5α-reducible androgen), with muscle preservation being associated with reduced ACVR2B (myostatin receptor) expression and reduced nuclear content of Smad2/3 (downstream effectors of myostatin signaling) [31]. In a rodent severe SCI model, high-dose testosterone with finasteride (US Food and Drug Administration (FDA)-approved 5α-reductase inhibitor) also lessened prostate enlargement versus testosterone alone and did not impede androgen-induced LABC growth [32], indicating that the 5α reduction of testosterone mediates prostate growth but not muscle growth. However, these preclinical findings remain to be verified in clinical trials.
5. β2-Adrenergic agonists
β2-agonists are traditionally used to treat bronchospasm resulting from asthma or chronic obstructive pulmonary disease (COPD) through smooth muscle relaxation and are categorized as short- or long-acting agonists, with treatment effects lasting 3—6 h or 12—24 h, respectively. Select β2-agonists also increase protein synthesis and suppress protein degradation in skeletal muscle by activating the PI3K/Akt/mTOR pathway and suppressing FOXO transcriptional activation of the ubiquitin-proteosome and autophagy—lysosome pathways [33]. In this regard, short-acting β2-agonist metaproterenol (80 mg/day for 4 weeks) improved muscle size and strength in a small cohort of men with muscle atrophy following SCI [34] and short-acting clenbuterol (2 μg/kg/day for 3 months) attenuated the reduction in type I and II fCSA by ~40% in persons with acute denervation due to traumatic cervical brachial plexus injury [35]. Additionally, in a mouse spinal transection model, short-acting clenbuterol (1 mg/kg/day) and high-dose testosterone produced additive improvement in hindlimb muscle fCSA when delivered for 1—8 weeks [36], although the signaling changes mediating this effect remain to be determined. Interestingly, in a mouse contusion SCI model, the long-acting μ2-agonist formoterol (0.3 mg/kg/day) did not prevent gastrocnemius muscle loss 3 days post-SCI, despite completely preventing myostatin mRNA induction and producing 100% higher muscle Igf1 expression, likely because formoterol did not prevent the rapid increase in MuRF1 protein nor the dramatic (p)Akt suppression after SCI [13]. In comparison, formoterol-treated mice displayed similar MuRF1 and (p)Akt protein levels to controls at 21 days and higher muscle mass versus untreated SCI mice [13]. It is important to note that considerable locomotor recovery occurred in formoterol-treated mice after SCI, which introduced hindlimb reloading. However, delaying formoterol treatment for 24-h post-SCI induced preservation of gastrocnemius mass in the absence of locomotor recovery [37]. Although promising, it remains unknown whether formoterol lessened fCSA atrophy in these studies. Regardless, the formoterol-induced locomotor improvements, muscle signaling changes, and muscle mass preservation appeared dependent on the β2-adrenergic receptor (ADRB2), as no neuromuscular improvements were observed in global Adbr2−/− knockout mice treated with formoterol after SCI [13]. The above-mentioned preclinical findings have not yet been verified in clinical trials, although formoterol is FDA approved to control COPD symptoms.
6. Myostatin inhibitors
Myostatin (also known as growth and differentiation factor 8 (GDF-8)) is a member of the TGF-β superfamily and a muscle-derived negative regulator of muscle growth that acts via the activin IIB receptors [38]. Elevated myostatin gene expression has been observed in persons with chronic SCI [39] and in rodent SCI models several days post-injury [13]. Interestingly, in a rodent spinal transection model, administration of a soluble activin IIb receptor that inhibits myostatin (RAP-031, 10 mg/kg, 2×/week) increased whole body lean mass ~15% and increased mass of the fully loaded forelimb muscles ~20-40%, without attenuating muscle loss in the paralyzed hindlimbs [40]. Similarly, others have reported that pharmacologic myostatin inhibition did not attenuate muscle loss after sciatic nerve transection but prevented disuse (immobilization) muscle atrophy [41]. Collectively, these results suggest that intact innervation may be required for muscle growth in response to myostatin inhibition.
7. SS-31/Elamipretide
Mitochondrial reactive oxygen species (ROS) generation triggers muscle atrophy signaling in response to prolonged immobilization [42] and has been proposed a contributing factor to SCI-induced mitochondrial dysfunction and muscle atrophy [5]. SS-31, a mitochondrial-targeting tetrapeptide, prevents atrophy in response to immobilization and reduces markers of mitochondrial ROS and oxidative stress [42]. SS-31 has been shown to attenuate ROS levels, to reverse mitochondrial dysfunction, and to lessen lung edema and damage in a rodent model of SCI-induced lung injury [43]. However, SS-31 (5-mg/kg/day) did not lessen hindlimb muscle atrophy in mice after moderate contusion SCI [44]. This suggests that ROS generation may not contribute extensively to SCI-induced muscle atrophy and/or that locomotor recovery due to the moderate SCI may have confounded any positive benefits of SS-31.
8. Natural products
To identify novel small molecule muscle atrophy inhibitors Adams et al. developed an SCI-centric drug discovery strategy that (1) surveyed genome-wide mRNA expression patterns that were conserved across normal human and mouse muscle and that were altered in atrophic muscle collected after SCI or fasting and (2) searched for small molecules with established safety profiles that induced inverse mRNA patterns in human skeletal muscle cell lines [45]. This strategy identified ursolic acid (UA), a natural plant metabolite with previously unrecognized anabolic properties, which has since been shown to stimulate muscle growth in mice in an IGF-1-dependent manner and to lessen disuse atrophy, with effects dependent on repression of MAFbx and MuRF1 [46]. Interestingly, UA (200 mg/kg/day) lessened FOXO1 protein and MAFbx expression in the mouse soleus 1 week after moderate—severe SCI and prevented the SCI-induced suppression of (p)P13K, (p)Akt, (p)mTOR, and (p)70s6K for several weeks thereafter. This resulted in higher soleus masses in SCI+UA versus untreated SCI mice [12]. However, UA induced some locomotor recovery after SCI, which may have influenced these muscle responses. Although promising, it remains unknown whether UA preserved fCSA in this study or whether UA can improve muscle mass in the paralyzed limbs after severe SCI.
Epicatechin, a flavanol that is present in tea and other edible plants, has also been shown to improve muscle performance in several atrophy models [47]. Recently, epicatechin (1 mg/kg/day) was shown to lower ubiquitin and MuRF1 protein by 33—50% in mice within 7 days of spinal transection and to return ubiquitin, FOXO1, MAFbx, and MuRF1 to control levels within 30 days, which lessened muscle CSA and fCSA atrophy ~50% [48]. Similarly, acteoside (verbascoside), a phenylethanoid glycoside found in tea and other plants [49], was shown to stimulate skeletal muscle cell proliferation in culture by increasing secretion of pyruvate kinase isoform M2 [50]. In a mouse moderate—severe SCI, acteoside (0.1 mg, 3×/week) increased hindlimb muscle mass versus untreated SCI animals, when initiated 30 days post-SCI [50]. However, acteoside also improved hindlimb locomotor function, which likely influenced the observed findings. Although these natural compounds have shown promise in preclinical studies, their clinical efficacy remains to be established.
9. Future directions
Activity-based physical therapies (ABPTs) have been used to combat muscle atrophy and the deleterious muscle phenotype that develops following SCI [2]. For example, both bodyweight-supported treadmill training (BWSTT) and neuromuscular electrical stimulation (NMES) are known to increase muscle CSA in persons with chronic complete SCI and to facilitate a fast-glycolytic to slow-oxidative fiber-type conversion [2,5], although ABPT effectiveness wanes as injury severity increases and continual training is needed to maintain muscular gains. Given these limitations it seems relevant to assess pharmacologic adjuvants combined with established ABPTs. For example, in a rodent severe SCI model high-dose testosterone combined with quadrupedal (q)BWSTT (40 min/day, 5×/week) attenuated soleus fCSA atrophy, prevented the soleus slow-to-fast fiber-type transition, and maintained isolated muscle force production better than testosterone alone [28]. Similarly, in a rodent spinal transection model, a multimodal therapy involving high-dose testosterone with electrical stimulation (1.5 V, 40 Hz, 2 s:18 s on:off) suppressed MAFbx and MuRF1 expression better than testosterone alone and produced slightly better muscle recovery [29]. Moreover, in men with chronic complete SCI low-dose testosterone in combination with a 16-week NMES-based progressive resistance training protocol produced greater knee extensor CSA and fCSA than testosterone alone [26,27]. Collectively, these studies provide evidence that multimodal therapies combining ABPTs with pharmacologic adjuvants provide improved muscle recovery after severe SCI.
10. Conclusion
Numerous pharmacologic agents stimulate hypertrophy in fully innervated and loaded muscles. However, most anabolic agents display a diminished ability to lessen atrophy in the paralyzed limbs after severe SCI for yet to be identified reasons, although several possibilities exist. First, most anabolic drugs target specific signaling pathways but not the plethora of molecular changes in atrophic muscle after SCI, highlighting the need to elucidate the complexity of signaling pathways that drive SCI-induced muscle loss and to identify pharmaceuticals that target these pathways. Second, increased atrophy signaling coincides with reduced anabolic signaling after SCI, as detailed above, implying that effective drugs may need to suppress atrophy and simultaneously stimulate anabolic pathways, which has proven difficult in the absence of innervation and loading in the paralyzed limbs. Third, muscle atrophy occurs more rapidly after severe SCI than in other disuse conditions, suggesting that the ideal window to prevent muscle loss is limited. Given these possibilities, compounds that target the molecular signatures present in atrophic muscle after SCI appear to hold the greatest potential to lessen muscle loss and/or promote muscle recovery, especially when combined with established ABPTs that reload the paralyzed limbs.
Acknowledgements
This work was supported by the Department of Veterans Affairs Office of Research and Development, Rehabilitation Research and Development Service Merit Award #1I01RX002447-01 and PECASE #B9280-O to JEY and CDA-2 #IK2RX002781 to ZAG, and by resources provided by the North Florida/South Georgia Veterans Health System. The work reported herein does not represent the views of the US Department of Veterans Affairs or the US Government.
Footnotes
Conflict of interest statement
Nothing declared.
References
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.NSCISC. In National Spinal Cord Injury Statistical Center, facts and figures at a glance. Edited by Birmingham AL, University of Alabama at Birmingham; 2021. [Google Scholar]
- 2.Otzel DM, Lee J, Ye F, Borst SE, Yarrow JF: Activity-based physical rehabilitation with adjuvant testosterone to promote neuromuscular recovery after spinal cord injury. Int J Mol Sci 2018, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ye F, Baligand C, Keener JE, Vohra R, Lim W, Ruhella A, Bose P, Daniels M, Walter GA, Thompson F, et al. : Hindlimb muscle morphology and function in a new atrophy model combining spinal cord injury and cast immobilization. J Neurotrauma 2013, 30:227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeman RJ, Zhao J, Zhang Y, Zhao W, Wen X, Wu Y, Pan J, Bauman WA, Cardozo C: Differential skeletal muscle gene expression after upper or lower motor neuron transection. Pflügers Archiv 2009, 458:525–535. [DOI] [PubMed] [Google Scholar]
- 5.Gorgey AS, Witt O, O’Brien L, Cardozo C, Chen Q, Lesnefsky EJ, Graham ZA: Mitochondrial health and muscle plasticity after spinal cord injury. Eur J Appl Physiol 2019, 119:315–331. [DOI] [PubMed] [Google Scholar]
- 6.Drasites KP, Shams R, Zaman V, Matzelle D, D CS, D PG, C JS, Haque A, Banik NL: Pathophysiology, biomarkers, and therapeutic modalities associated with skeletal muscle loss following spinal cord injury. Brain Sci 2020:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yarar-Fisher C, Bickel CS, Kelly NA, Stec MJ, Windham ST, McLain AB, Oster RA, Bamman MM: Heightened TWEAK-NF-kappaB signaling and inflammation-associated fibrosis in paralyzed muscles of men with chronic spinal cord injury. Am J Physiol Endocrinol Metab 2016, 310:E754–E761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leger B, Senese R, Al-Khodairy AW, Deriaz O, Gobelet C, Giacobino JP, Russell AP: Atrogin-1, MuRF1, and FoXO, as well as phosphorylated GSK-3beta and 4E-BP1 are reduced in skeletal muscle of chronic spinal cord-injured patients. Muscle Nerve 2009, 40:69–78. [DOI] [PubMed] [Google Scholar]
- 9.Vassilakos G, Barton ER: Insulin-like growth factor I regulation and its actions in skeletal muscle. Comp Physiol 2018, 9:413–438. [DOI] [PubMed] [Google Scholar]
- 10.You JS, McNally RM, Jacobs BL, Privett RE, Gundermann DM, Lin KH, Steinert ND, Goodman CA, Hornberger TA: The role of raptor in the mechanical load-induced regulation of mTOR signaling, protein synthesis, and skeletal muscle hypertrophy. FASEB J 2019, 33:4021–4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lundell LS, Savikj M, Kostovski E, Iversen PO, Zierath JR, Krook A, Chibalin AV, Widegren U: Protein translation, proteolysis and autophagy in human skeletal muscle atrophy after spinal cord injury. Acta Physiol (Oxf) 2018, 223, e13051. [DOI] [PubMed] [Google Scholar]
- 12.Bigford GE, Darr AJ, Bracchi-Ricard VC, Gao H, Nash MS, Bethea JR: Effects of ursolic acid on sub-lesional muscle pathology in a contusion model of spinal cord injury. PloS One 2018, 13, e0203042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13..**. Scholpa NE, Simmons EC, Tilley DG, Schnellmann RG: beta2-adrenergic receptor-mediated mitochondrial biogenesis improves skeletal muscle recovery following spinal cord injury. Exp Neurol 2019, 322:113064. This study found that the long-acting β2-agonist formoterol attenuted hindlimb muscle loss and improved locomotor recovery in a mouse contusion SCI model and demonstrated that these effects were dependent upon the β2-adrendergic receptor (ADRB2).
- 14.Bank M, Stein A, Sison C, Glazer A, Jassal N, McCarthy D, Shatzer M, Hahn B, Chugh R, Davies P, et al. : Elevated circulating levels of the pro-inflammatory cytokine macrophage migration inhibitory factor in individuals with acute spinal cord injury. Arch Phys Med Rehabil 2015, 96:633–644. [DOI] [PubMed] [Google Scholar]
- 15.**. Liu Z, Yang Y, He L, Pang M, Luo C, Liu B, Rong L: High-dose methylprednisolone for acute traumatic spinal cord injury: a meta-analysis. Neurology 2019, 93:e841–e850. This meta-analysis reported that high-dose methylprednisolone was not associated with improved functional, improved sensory recovery, or lower in- hospital costs in persons with SCI but resulted in higher incidence of gastrointestinal hemorrhage and respiratory tract infections.
- 16.*. Fappi A, Neves JC, Sanches LN, Massaroto ESPV, Sikusawa GY, Brandao TPC, Chadi G, Zanoteli E: Skeletal muscle response to deflazacort, dexamethasone and methylprednisolone. Cells 2019, 8. This study demonstrates that various systemically administered glucocorticoids, including methylprednisolone, induce muscle loss by selectively activating several atrophic pathways and/or inhibiting anabolic signaling through the IGF-1 pathway.
- 17.Wu Y, Collier L, Pan J, Qin W, Bauman WA, Cardozo CP: Testosterone reduced methylprednisolone-induced muscle atrophy in spinal cord-injured rats. Spinal Cord 2012, 50:57–62. [DOI] [PubMed] [Google Scholar]
- 18.Yarrow JF, Cardozo CP: Effect of spinal cord injury and related conditions. In Zaidi M. Encyclopedia of bone biology, vol. 2. Oxford: Academic Press; 2020:429–448. [Google Scholar]
- 19.Abilmona SM, Sumrell RM, Gill RS, Adler RA, Gorgey AS: Serum testosterone levels may influence body composition and cardiometabolic health in men with spinal cord injury. Spinal Cord 2019, 57:229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorgey AS, Gater DR: Insulin growth factors may explain relationship between spasticity and skeletal muscle size in men with spinal cord injury. J Rehabil Res Dev 2012, 49:373–380. [DOI] [PubMed] [Google Scholar]
- 21.Skinner JW, Otzel DM, Bowser A, Nargi D, Agarwal S, Peterson MD, Zou B, Borst SE, Yarrow JF: Muscular responses to testosterone replacement vary by administration route: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle 2018, 9:465–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooper IS, Rynearson EH, Mac CC, Power MH: Testosterone propionate as a nitrogen-sparing agent after spinal cord injury. J Am Med Assoc 1951, 145:549–553. [DOI] [PubMed] [Google Scholar]
- 23.Jia H, Sullivan CT, McCoy SC, Yarrow JF, Morrow M, Borst SE: Review of health risks of low testosterone and testosterone administration. World J Clin Cases 2015, 3:338–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bauman WA, Cirnigliaro CM, La Fountaine MF, Jensen AM, Wecht JM, Kirshblum SC, Spungen AM: A small-scale clinical trial to determine the safety and efficacy of testosterone replacement therapy in hypogonadal men with spinal cord injury. Horm Metab Res 2011, 43:574–579. [DOI] [PubMed] [Google Scholar]
- 25.Bauman WA, La Fountaine MF, Cirnigliaro CM, Kirshblum SC, Spungen AM: Lean tissue mass and energy expenditure are retained in hypogonadal men with spinal cord injury after discontinuation of testosterone replacement therapy. J Spinal Cord Med 2015, 38:38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.*. Gorgey AS, Khalil RE, Gill R, Gater DR, Lavis TD, Cardozo CP, Adler RA: Low-dose testosterone and evoked resistance exercise after spinal cord injury on cardio-metabolic risk factors: an open-label randomized clinical trial. J Neurotrauma 2019, 36:2631–2645. This clinical trial demonstrated that low-dose testosterone (alone) did not alter total body lean mass or whole muscle CSA in persons with chronic complete SCI, while low-dose testosterone in combination with evoked neuromuscular electrical stimulation resistance training increased total body lean mass and muscle CSA.
- 27.**. Gorgey AS, Graham ZA, Chen Q, Rivers J, Adler RA, Lesnefsky EJ, Cardozo CP: Sixteen weeks of testosterone with or without evoked resistance training on protein expression, fiber hypertrophy and mitochondrial health after spinal cord injury. J Appl Physiol 2020, 128:1487–1496 (1985). This study demonstrated that low-dose testosterone (alone) did not alter muscle fCSA and muscle protein expression of GLUT4, total Akt or phosphorylated (p)Akt in persons with chronic complete SCI, while low-dose testosterone in combination with neuromuscular electrical stimulation resistance training increased muscle fCSA and expression of these proteins.
- 28.**. Yarrow JF, Kok HJ, Phillips EG, Conover CF, Lee J, Bassett TE, Buckley KH, Reynolds MC, Wnek RD, Otzel DM, et al. : Locomotor training with adjuvant testosterone preserves cancellous bone and promotes muscle plasticity in male rats after severe spinal cord injury. J Neurosci Res 2020, 98:843–868. This study demonstrated that high-dose testosterone prevented atrophy of the LABC muscle (involved in sexual function), constrained the slow-to-fast fiber-type transition, and preserved cancellous bone via antiresorptive actions in a rodent severe contusion SCI model, but did not influence soleus muscle fCSA or isolated muscle function. In comparison, high-dose testosterone combined with quadrupedal bodyweight-supported treadmill training increased LABC mass, attenuated soleus fCSA atrophy and muscle function deficits, and preserved cancellous bone volume by suppresing bone resorption and simultaneously stimulating bone formation.
- 29.Zhao W, Peng Y, Hu Y, Guo XE, Li J, Cao J, Pan J, Feng JQ, Cardozo C, Jarvis J, et al. : Electrical stimulation of hindlimb skeletal muscle has beneficial effects on sublesional bone in a rat model of spinal cord injury. Bone 2021, 144:115825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phillips EG, Beggs LA, Ye F, Conover CF, Beck DT, Otzel DM, Ghosh P, Bassit ACF, Borst SE, Yarrow JF: Effects of pharmacologic sclerostin inhibition or testosterone administration on soleus muscle atrophy in rodents after spinal cord injury. PloS One 2018, 13, e0194440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu Y, Zhao J, Zhao W, Pan J, Bauman WA, Cardozo CP: Nandrolone normalizes determinants of muscle mass and fiber type after spinal cord injury. J Neurotrauma 2012, 29:1663–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yarrow JF, Phillips EG, Conover CF, Bassett TE, Chen C, Teurlings T, Vasconez A, Alerte J, Prock H, Jiron JM, et al. : Testosterone plus finasteride prevents bone loss without prostate growth in a rodent spinal cord injury model. J Neurotrauma 2017, 34:2972–2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joassard OR, Durieux AC, Freyssenet DG: beta2-Adrenergic agonists and the treatment of skeletal muscle wasting disorders. Int J Biochem Cell Biol 2013, 45:2309–2321. [DOI] [PubMed] [Google Scholar]
- 34.Signorile JF, Banovac K, Gomez M, Flipse D, Caruso JF, Lowensteyn I: Increased muscle strength in paralyzed patients after spinal cord injury: effect of beta-2 adrenergic agonist. Arch Phys Med Rehabil 1995, 76:55–58. [DOI] [PubMed] [Google Scholar]
- 35.Jiang GL, Gu YD, Zhang LY, Shen LY, Yu C, Xu JG: Randomized, double-blind, and placebo-controlled trial of clenbuterol in denervated muscle atrophy. ISRN Pharm 2011, 2011:981254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ung RV, Rouleau P, Guertin PA: Effects of co-administration of clenbuterol and testosterone propionate on skeletal muscle in paraplegic mice. J Neurotrauma 2010, 27:1129–1142. [DOI] [PubMed] [Google Scholar]
- 37.*. Scholpa NE, Simmons EC, Crossman JD, Schnellmann RG: Time-to-treatment window and cross-sex potential of beta2-adrenergic receptor-induced mitochondrial biogenesis-mediated recovery after spinal cord injury. Toxicol Appl Pharmacol 2021, 411:115366. This study demonstrated that the long-acting β2-agonist formoterol improves locomotor function in a dose- and time-dependent manner in a mouse contusion SCI model and that the formoterol-induced attenuation of muscle loss does not require locomotor recovery.
- 38.Lee SJ: Targeting the myostatin signaling pathway to treat muscle loss and metabolic dysfunction. J Clin Invest 2021:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petrie MA, Suneja M, Faidley E, Shields RK: Low force contractions induce fatigue consistent with muscle mRNA expression in people with spinal cord injury. Phys Rep 2014, 2, e00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Graham ZA, Collier L, Peng Y, Saez JC, Bauman WA, Qin W, Cardozo CP: A soluble activin receptor IIB fails to prevent muscle atrophy in a mouse model of spinal cord injury. J Neurotrauma 2016, 33:1128–1135. [DOI] [PubMed] [Google Scholar]
- 41.MacDonald EM, Andres-Mateos E, Mejias R, Simmers JL, Mi R, Park JS, Ying S, Hoke A, Lee SJ, Cohn RD: Denervation atrophy is independent from Akt and mTOR activation and is not rescued by myostatin inhibition. Dis Model Mech 2014, 7:471–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hyatt H, Deminice R, Yoshihara T, Powers SK: Mitochondrial dysfunction induces muscle atrophy during prolonged inactivity: a review of the causes and effects. Arch Biochem Biophys 2019, 662:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu LL, Li MQ, He F, Zhou SB, Jiang W: Mitochondria targeted peptide attenuates mitochondrial dysfunction, controls inflammation and protects against spinal cord injury-induced lung injury. Cell Physiol Biochem 2017, 44:388–400. [DOI] [PubMed] [Google Scholar]
- 44.Graham ZA, DeBerry JJ, Cardozo CP, Bamman MM: A 50 kdyne contusion spinal cord injury with or without the drug SS-31 was not associated with major changes in muscle mass or gene expression 14 d after injury in young male mice. Phys Rep 2021, 9, e14751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kunkel SD, Suneja M, Ebert SM, Bongers KS, Fox DK, Malmberg SE, Alipour F, Shields RK, Adams CM: mRNA expression signatures of human skeletal muscle atrophy identify a natural compound that increases muscle mass. Cell Metabol 2011, 13:627–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adams CM, Ebert SM, Dyle MC: Use of mRNA expression signatures to discover small molecule inhibitors of skeletal muscle atrophy. Curr Opin Clin Nutr Metab Care 2015, 18:263–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li P, Liu A, Xiong W, Lin H, Xiao W, Huang J, Zhang S, Liu Z: Catechins enhance skeletal muscle performance. Crit Rev Food Sci Nutr 2020, 60:515–528. [DOI] [PubMed] [Google Scholar]
- 48.*. Gonzalez-Ruiz C, Cordero-Anguiano P, Morales-Guadarrama A, Mondragon-Lozano R, Sanchez-Torres S, Salgado-Ceballos H, Villarreal F, Meaney E, Ceballos G, Najera N: (−)-Epicatechin reduces muscle waste after complete spinal cord transection in a murine model: role of ubiquitin-proteasome system. Mol Biol Rep 2020, 47:8975–8985. This study demonstrated that the flavanol epicatechin lessened FOXO1a, MAFbx, and MuRF1 protein expression in muscle and attenuated muscle fCSA atrophy in a rodent spinal transection model.
- 49.Wu L, Georgiev MI, Cao H, Nahar L, El-Seedi HR, Sarker SD, Xiao J, Lu B: Therapeutic potential of phenylethanoid glycosides: a systematic review. Med Res Rev 2020, 40:2605–2649. [DOI] [PubMed] [Google Scholar]
- 50.Kodani A, Kikuchi T, Tohda C: Acteoside improves muscle atrophy and motor function by inducing new myokine secretion in chronic spinal cord injury. J Neurotrauma 2019, 36:1935–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]