Abstract
Adding chondroitin sulfate (CS) to collagen scaffolds has been shown to improve outcomes for articular cartilage tissue engineering. Instead of physical entrapment or chemical crosslinking of CS within a scaffold, this study investigated the use of CS with attached collagen-binding peptides (termed CS-SILY). This method better recapitulates aspects of native cartilage while retaining CS within a collagen type I and II blend (Col I/II) hydrogel. CS retention, average fibril diameter, and mechanical properties were altered by varying the number of SILY peptides attached to the CS backbone. When mesenchymal stromal cells (MSCs) were encapsulated within the scaffolds, the addition of CS-SILY molecules resulted in higher sulfated glycosaminoglycan production, and these results suggest that CS-SILY promotes MSC differentiation into chondrocytes. Taken together, our study shows the promise of adding a CS-SILY molecule to a Col I/II hydrogel with encapsulated MSCs to promote cartilage repair.
Keywords: extracellular matrix, fibrillogenesis, osteoarthritis, glycosaminoglycan, chondrogenic differentiation, biomimetic, mesenchymal stem cells
Graphical Abstract
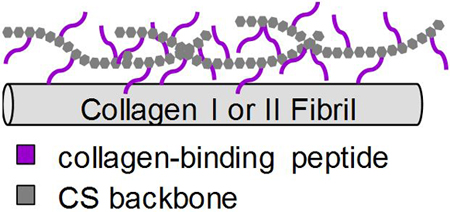
INTRODUCTION
The loss of articular cartilage incurred as osteoarthritis progresses is a burden both physically and financially. Each year, over one million Americans undergo total joint replacement,1, 2 which is the medically indicated treatment for osteoarthritis of the knee or hip in patients for whom pain is no longer treatable using drugs and viscosupplements. Because articular cartilage is avascular and contains a low concentration of cells, it cannot repair itself.3 Current surgical options for focal cartilage defects are invasive and include osteochondral grafts, autologous chondrocyte implantation, and marrow stimulation.4, 5 However, these treatments incur long rehabilitation times and often result in fibrocartilage tissue, which has mechanical properties inferior to native cartilage.6 In order to better mimic native tissue, we take a tissue engineering approach, which combines cells, a scaffold, and bioactive factors for implantation into a cartilage defect.7 The choice of scaffold and addition of extracellular matrix molecules are two ways to enhance the chondrogenesis of mesenchymal stromal cells (MSCs), a promising cell source that differentiates into chondrocytes under certain environmental conditions.7, 8
Articular cartilage is predominantly composed of collagen type II fibrils, which comprise the 3D architecture of the tissue, and proteoglycans such as aggrecan. Aggrecan contains a large number of sulfated glycosaminoglycans (GAGs), such as chondroitin sulfate (CS), and attaches, along with link protein, to a central filament of hyaluronic acid (HA) to form supramolecular complexes.9 These supramolecular complexes, which are trapped within the cartilage matrix, are negatively-charged molecules, are present in high concentration in cartilage, and result in water influx and retention within cartilage.9, 10 The retention of water gives cartilage a high compressive strength and aids in joint lubrication.
For tissue engineered scaffolds, collagen is an attractive option due to its biocompatibility and ubiquity in tissues.11 Collagen type I, which is widely available, is the collagen type most often used for cartilage engineering even though collagen type II is the predominant collagen type in native cartilage. Early studies saw promising repair when MSCs were differentiated into chondrocytes in collagen type I hydrogels.12, 13 However, these studies demonstrated that clinical limitations of collagen I hydrogels include poor integration with surrounding tissue and surface splitting, fibrillation, and thinning.12 Furthermore, collagen type II hydrogels are superior in promoting the differentiation of encapsulated MSCs to chondrocytes compared to collagen type I hydrogels.14, 15
A scaffold with a collagen type I to collagen type II ratio of 3:1 (this formulation is hereafter referred to as Col I/II gels) was developed and characterized previously by our laboratory to harness the biological activity of collagen type II and the superior gelation of collagen type I.16 Although the blends were able to retain CS and HA post-polymerization, less than 45% of the original CS and 25% of the original HA added was incorporated in the Col I/II hydrogels. The Col I/II hydrogels were evaluated for chondrogenic differentiation of MSCs in vitro and cartilage repair potential in vivo.17 The Col I/II hydrogel showed potential for regeneration since it promoted integration with surrounding tissue and provided favorable conditions for cartilage repair, but there was room for improvement. The current study augments the cartilage-promoting abilities of these hydrogels by investigating strategies that recapitulate aspects of native cartilage and result in better retention of CS.
There are many examples in which the addition of CS improved scaffolds for articular cartilage tissue engineering using either encapsulated chondrocytes or MSCs. CS stimulated the production of proteoglycans by chondrocytes when added to the medium.18 Adding CS to chitosan resulted in scaffolds that retained the chondrocyte phenotype and promoted chondrocyte deposition of GAG and collagen type II.19 Photocrosslinking CS with polyvinyl alcohol formed a hydrogel that also maintained the rounded shape of encapsulated chondrocytes.20 When CS was crosslinked within poly(ethylene glycol) (PEG) hydrogels, embedded chondrocytes produced higher levels of GAG and collagen accumulation,21 upregulated gene expression of aggrecan and link protein,22 and deposited more collagen type II in vivo22 compared to chondrocytes in control PEG gels. Similar effects were seen with MSCs. Copolymerizing CS within PEG hydrogels upregulated cartilage-specific genes and downregulated hypertrophic genes, such as Col X, of encapsulated MSCs.23, 24 Furthermore, combining dynamic compression with CS in these PEG-based gels strongly supported MSC differentiation into chondrocytes and inhibited hypertrophy.25 Of particular interest, crosslinked chondroitin sulfate and collagen type II hydrogels with seeded MSCs resulted in better repair of in vivo cartilage defects after one month compared to collagen type II hydrogels alone.26
To incorporate CS within our Col I/II hydrogels, we utilized technology previously developed by our laboratory. Previous work covalently attached matrix-binding peptides to glycosaminoglycan backbones to mimic aggrecan,27–30 lubricin,31, 32 and decorin.33–37 In particular, a collagen-binding peptide (RRANAALKAGELYKSILYGSG), which was named SILY, attached to a dermatan sulfate (DS) backbone, was designed to mimic decorin, a small proteoglycan that is associated with collagen fibrils.33–35, 37 The decorin mimic has been shown to bind to collagen and inhibit collagen degradation mediated by MMP-1 and MMP-13.33, 37 Importantly, decorin has been shown to play an integral role in cartilage matrix organization and mechanics.38 To facilitate incorporation of CS in our collagen type I/II blend hydrogels, this study uses a similar strategy by conjugating different amounts of the SILY peptide to functional groups on CS to create a collagen-binding glycan (named CS-SILY).
Overall, this study investigates how the addition of SILY peptides to a CS backbone enhances the retention of CS in a Col I/II blend hydrogel over time and alters network structure and mechanical properties. SILY-hydrazide peptides were attached to a CS backbone through coupling to -COOH using 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC), and the number of peptides was varied to create 3 different molecules: CS-10SILY, CS-15SILY, and CS-20SILY with 10, 15, and 20 denoting the number of SILY peptides attached to CS. The in vitro chondrogenic differentiation of bone marrow-derived MSCs embedded within Col I/II gels and supplemented with either CS or CS-SILY molecules was also examined.
MATERIALS AND METHODS
Reagents
Unless otherwise specified, all materials were purchased from Sigma-Aldrich (St. Louis, MO).
Molecule Preparation
The collagen-binding proteoglycan mimetic was synthesized using EDC chemistry to conjugate the SILY peptide-hydrazide (China Peptide Company) to a chondroitin-6-sulfate (CS) (40 kDa, Seikagaku, Tokyo, Japan) backbone creating an amide bond. Using an 8 M urea solution and 0.05 mM EDC, the carboxyl groups on the CS backbone were activated for 20 minutes at room temperature and pH 4.5. The CS backbone was then functionalized by reacting 10% molar excess of the desired molar ratio of SILY peptide-hydrazide (10:1, 15:1, or 20:1 ratio of peptide/CS) overnight with shaking. The pH was then altered to 8 to stop the reaction. The molecules were purified using size exclusion chromatography on an AKTA fast protein liquid chromatography (FPLC) unit (GE Healthcare, Piscataway, NJ) with Bio-Scale Mini Bio-Gel columns packed with polyacrylamide beads (Bio-Rad Laboratories, Hercules, CA) and then freeze dried. A Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA) was used to confirm the final concentration of peptide attachment to the CS backbone using a standard curve based on peptide absorbance at 280 nm. Three different molecules (CS-10SILY, CS-15SILY, and CS-20SILY) were created. All of these molecules had measured molar ratios of SILY peptide to CS within ±10% of the desired molar ratio (Table 1).
Table 1.
Expected and Actual Molar Ratio of SILY Peptides Conjugated to Chondroitin Sulfate
| Name | Expected Molar Ratio of SILY to CS | Actual Molar Ratio of SILY to CS |
|---|---|---|
| CS-10SILY | 10:1 | 10.08:1 |
| CS-15SILY | 15:1 | 14.14:1 |
| CS-20SILY | 20:1 | 18.40:1 |
Hydrogel Preparation
The collagen type I and II blend hydrogels were prepared using a protocol described in detail in Kilmer et al.17 A stock solution of collagen type II from lyophilized chicken sternum (Sigma-Aldrich, Saint Louis, MO) was prepared at a concentration of 11 mg/mL in 20 mM acetic acid. The concentration of the collagen type II stock solution was measured after sterile filtration using a bicinchoninic acid (BCA) assay (Pierce, Rockford, IL). Using the post-sterilization concentration, the collagen type II was then diluted to a stock concentration of 8 mg/mL in 20 mM acetic acid prior to use. A 3:1 collagen type I to collagen type II (Col I/II) was created by combining acid-solubilized collagen type I from rat tail (Corning, Corning, NY) with the stock solution of collagen type II. A neutralization solution was prepared to raise the pH of the solutions to 7.4 with the addition of 10x phosphate-buffered saline (PBS), 1 M NaOH, and 1x PBS and diluted to a final concentration of 4 mg/mL total collagen. To the base hydrogel with no added CS (No Trt), 10 μM of CS, CS-10SILY, CS-15SILY, or CS-20SILY was added in neutralization solution with 1x PBS.
Chondroitin Sulfate Diffusion from Hydrogel
A dimethyl methylene blue (DMMB) assay was used to measure the amount of CS, in the form of CS or a CS-SILY molecule, retained in a 50 µL hydrogel over time. Hydrogels (n = 4), which were allowed to polymerize in a 96 well plate for either 3 or 12 hours at 37°C, were created with 10 µM of either CS, CS-10SILY, CS-15SILY, or CS-20SILY. After polymerization, all hydrogels were washed with 1x PBS for 5 minutes, and hydrogels were freeze-dried each day from day 0 to 7. As previously described, hydrogels were digested at 60°C for 24 hours with 125 μg/mL of activated papain solution in a papain digestion buffer (5 mM ethylenediaminetetraacetic acid (EDTA), 5 mM L-cysteine (Alfa Aesar, Ward Hill, MA), and 100 mM NaH2PO4).39 The digested product was then freeze-dried and resuspended in autoclaved water. A 20 µL aliquot of digested hydrogel construct was combined with 30 µL of water and 250 µL DMMB dye solution. The absorbance of the solution was then measured at 525 nm. A standard curve was created using either CS, CS-10SILY, CS-15SILY, or CS-20SILY depending on the molecule that was added prior to polymerization. The percentage of encapsulated CS was calculated by comparing the amount of CS retained in the gel over time with the amount of CS added to the hydrogel.
Rheology
Frequency sweeps were performed on an ARG2 rheometer (TA instruments, New Castle, DE) using a 20 mm cone geometry. Hydrogels (n = 3) were polymerized on a Teflon coated microscope slide (Tekdon, Myakka City, FL) using a volume of 150 uL. The frequency was varied from 0.01 to 10 Hz with a controlled stress of 0.5 Pa.
Cryoscanning Electron Microscopy (Cryo-SEM)
The same hydrogel treatments used in the diffusion experiments were analyzed using Cryo-SEM (Nova NanoSEM, FEI, Hillsboro, OR), and 75 uL of each hydrogel (n = 3) was polymerized at 37°C on a machined stage. The stages with polymerized hydrogels were moved into a stage holder and then flash frozen in liquid nitrogen slush. The samples were fractured in a Gatan Alto 2500 prechamber (Gatan Inc., Pleasanton, CA). Samples were sublimated for between 10 and 15 minutes at −90°C and sputter-coated with platinum for 120 seconds. The samples were then imaged on the microscope cryostage at −140oC. Fibrils from three images per sample were measured with the FIJI image processing package (National Institutes of Health, Bethesda, MD) using a previously described protocol.16 Fibril diameter measurements (n ≥ 270) were analyzed by blinded observers who measured the fibril diameter of 10 fibers in 9 images of each treatment. The percent porosity and number of pores was calculated using the Diameter J plugin on FIJI to segment the image and calculate the void in 9 images per treatment.
Stem Cell Encapsulation
Mesenchymal stromal cells were isolated from the bone marrow of New Zealand White rabbits following a previously described protocol.17 Briefly, bone marrow was collected from both femurs and humeri of skeletally mature New Zealand White rabbits (Covance, Princeton, NJ) following a protocol that was approved by the Purdue Animal Care and Use Committee (PACUC). Bone marrow was aspirated using an 18-gauge needle that was percutaneously inserted into the intertrochanteric fossa of the femur and the greater tubercle of the humerus. The marrow from each rabbit was pooled, centrifuged, and resuspended in maintenance medium (low-glucose Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (Lonza, Walkersville, MD) and 1% penicillin-streptomycin). Autoclaved water was added to lyse the red blood cells. Upon centrifugation, the cells were plated and incubated at 37°C with 5% CO2. The first medium change was performed after four days of culture following a 1x PBS wash step, and subsequent medium changes were every three days. The cells were subcultured after 2.5 weeks upon reaching 70% - 80% confluency.
MSCs were resuspended in collagen pre-polymerization solutions at a cell density of 5 × 106 cells/mL. The pre-polymerization solution contained either the neutralization solution discussed in the hydrogel preparation section or the addition of CS in the form of 10 µM CS, CS-10SILY, CS-15SILY, or CS-20SILY in neutralization solution. The hydrogels, which were made of 50 μL of solution, were polymerized in 96 well plates at 37°C for 3 hours before the addition of chondrogenic medium. Defined chondrogenic medium (CM) was formulated with high-glucose DMEM supplemented with 1% ITS+Premix (BD Biosciences, San Jose, CA), 1% penicillin-streptomycin, 1 mM sodium pyruvate, 50 µM proline, 4 mM L-glutamine, 50 µg/mL ascorbic acid, 100 nM dexamethasone, and 10 ng/mL transforming growth factor-β3 (TGF-β3) (Peprotech, Rocky Hill, NJ). For GAG, DNA, and collagen analysis, cell-hydrogel constructs were cultured for up to 4 weeks with 3 medium changes each week. For collagen analysis of the medium, medium aliquots from each medium change were pooled together from each week. Hydrogels were maintained in free-floating conditions. Cell-hydrogel constructs were digested by papain before DNA and GAG quantification.
Sulfated GAG Production and DNA Analysis
Using a Hoechst dye, DNA was measured as previously described,40 and a standard curve of calf thymus DNA was created. The cell-hydrogel construct (n = 4) was added to a Hoechst dye solution, and the fluorescence was read at an excitation wavelength of 340 nm and an emission wavelength of 465 nm. Sulfated GAG content (n = 4) was measured using a DMMB assay where 20 µL of the papain-digested hydrogel constructs were diluted with 30 µL of water and 250 µL DMMB dye solution. The absorbance of the solution was read at 525 nm. A standard curve was created using chondroitin sulfate from shark cartilage (Seikagaku, Tokyo, Japan).
Collagen Analysis
Using a Biocolor Soluble Collagen Assay kit (Carrickfergus, Northern Ireland), collagen from cell-hydrogel constructs and media aliquots was measured using a Sircol dye reagent and following the manufacturer’s instructions. The cell-hydrogel constructs (n =3) were freeze dried and resuspended in a 0.1 mg/mL solution of pepsin in 0.5 mM acetic acid. The scaffold samples were incubated overnight at 4°C in the acid-pepsin extraction solution. The acid-pepsin extraction step was not required for media aliquots. Once the collagen-dye complex precipitated out from unbound dye, the pellet was resuspended. The absorbance was measured at a wavelength of 555 nm. A standard curve was created using collagen type I/II in either 20 mM acetic acid or chondrogenic medium depending on whether cell-hydrogel or medium samples, respectively, were analyzed.
Gene Expression
The 50 µL hydrogels (n = 6) were washed with PBS then homogenized in lysis buffer and β-mercaptoethanol using a syringe needle. The NucleoSpin RNA kit from Macherey-Nagel (Bethlehem, PA) was used to isolate RNA. A High-Capacity cDNA Reverse Transcription kit from Applied Biosystems (Foster City, CA) was used to synthesize complementary DNA from the isolated RNA. Relative expression levels were measured using qRT-PCR with the primer sequences (Table S1) for collagen type I, II, and X, aggrecan, SOX9, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Integrated DNA Technologies, Skokie, IL). The samples were heated for 10 minutes at 95°C followed by 40 cycles for 15 seconds at 95°C, 15 seconds at 55 or 60°C, and 40 seconds at 68°C. All values were normalized to GAPDH levels. The differences in gene expression were calculated relative to negative controls using the ΔΔCt method.41
Statistics
All data is shown as a mean with error bars showing one standard deviation with statistical analysis evaluated in GraphPad Prism (GraphPad Software Inc.). Single factor analysis of variance (ANOVA) and Tukey post hoc tests were performed for the CS encapsulation, rheology experiments, GAG production, collagen content in cell culture media, and collagen content in cell-hydrogel constructs. To compare GAG and collagen content in hydrogel constructs between days 21 and 28, an unpaired t-test with two tails was used except for GAG content for hydrogels with CS-15SILY, which did not pass normality and used the Mann-Whitney test. A general linear model with nested factors and Tukey post hoc tests were performed to analyze the fibril diameter, percent porosity, and pore number data. Single factor analysis of variance (ANOVA) and Tukey’s post hoc tests were performed for gene expression analyses of collagen type II at 1 week, SOX9 at 1 week, aggrecan at 2 weeks, and SOX9 at 2 weeks. Due to unequal variances, ANOVA and Games Howell post hoc tests were performed for gene expression analyses of collagen type I and collagen type X at both time points. A Box-Cox transformation was used to analyze data for gene expression analyses of aggrecan at 1 week and collagen type II at 2 weeks. An α level of 0.05 was selected for statistical significance in all statistical tests.
RESULTS
Addition of CS-SILY molecules enhanced CS retention.
Col I/II hydrogels were polymerized for 3 hours with either 10 µM of CS, CS-10SILY, CS-15SILY, or CS-20SILY. A DMMB assay was used to determine the amount of CS encapsulated and retained in the hydrogel from 0 to 7 days. Immediately after a 3-hour polymerization period and a PBS wash, there was a statistically higher amount of CS encapsulated in the CS-20SILY hydrogels (73.7 ± 6.6%) compared to the CS-10SILY (55.6 ± 9.0%) and CS (55.5 ± 6.0%) hydrogels (Figure 1). However, there was no statistical difference between the CS-20SILY and CS-15SILY (67.6 ± 4.9%) treatments. The CS and CS-10SILY hydrogels took 2 and 4 days, respectively, to reach <20% of the original amount of CS encapsulated. The CS-15SILY and CS-20SILY hydrogels did not reach <20% of the original amount of CS encapsulated in the 7-day period analyzed. After 7 days, there was no statistical difference between the amounts of CS encapsulated in the CS-15SILY (20.8 ± 4.8%) and the CS-20SILY (21.8 ± 3.9%) hydrogels, and these values were statistically higher than the CS-10SILY (10.0 ± 1.4%) or CS (0.8 ± 0.9%) hydrogels. Similar results were obtained for col I/II gels polymerized for 12 hours (Figure S1).
Figure 1.
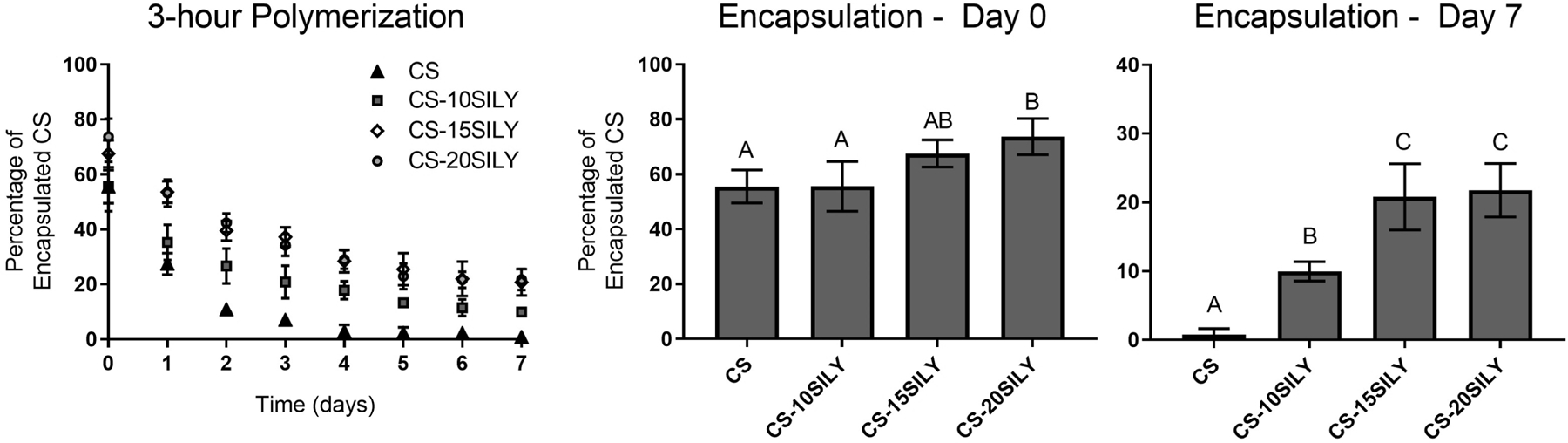
CS-SILY molecule increases retention in hydrogels compared to CS. Percentage of encapsulated CS retained in the hydrogel over time after a 3-hour polymerization. CS encapsulation after a wash (day 0) or 7-day period are shown. Single factor analysis of variance (ANOVA) and Tukey post hoc tests were performed (n = 4). Data that share the same letter do not have statistically significant differences (p > 0.05) whereas data that do not share the same letter have statistically significantly differences (p < 0.05). Data is represented as the mean ± the standard deviation.
Mechanical properties of the scaffold were altered with the addition of CS and CS-SILY molecules.
To better understand how the mechanical properties of a Col I/II hydrogel are altered with the addition of CS-SILY molecules, frequency sweeps were performed from 0.01 to 10 Hz (Figure S2). Hydrogels of only collagen (No Trt) were compared to hydrogels with added CS, CS-10SILY, CS-15SILY, or CS-20SILY. The frequency sweeps show that at 0.1 Hz, the No Trt, CS, CS-10SILY, CS-15SILY, and CS-20SILY hydrogels, respectively, had an average storage modulus (G’) of 144.8 ± 58.3, 280.6 ± 180.3, 201.1 ± 47.6, 493.9 ± 87.3, and 684.0 ± 63.2 Pa (Figure 2). At 1 Hz, the No Trt, CS, CS-10SILY, CS-15SILY, and CS-20SILY hydrogels, respectively, had an average G’ of 188.1 ± 73.0, 389.4 ± 262.0, 245.6 ± 74.0, 551.48 ± 132.3, and 763.5 ± 90.0 Pa (Figure 2). The G’ values at 0.01 and 10 Hz show similar trends (Figure S3). In all cases, the loss modulus trends followed the storage modulus trends (Figure 2). The No Trt, CS, and CS-10SILY hydrogels had statistically similar G’ values, and the CS-20SILY hydrogels had a statistically higher G’ value than those three groups but was statistically similar to CS-15SILY. Thus, a higher number of SILY peptides on the CS backbone increased the G’ value.
Figure 2.
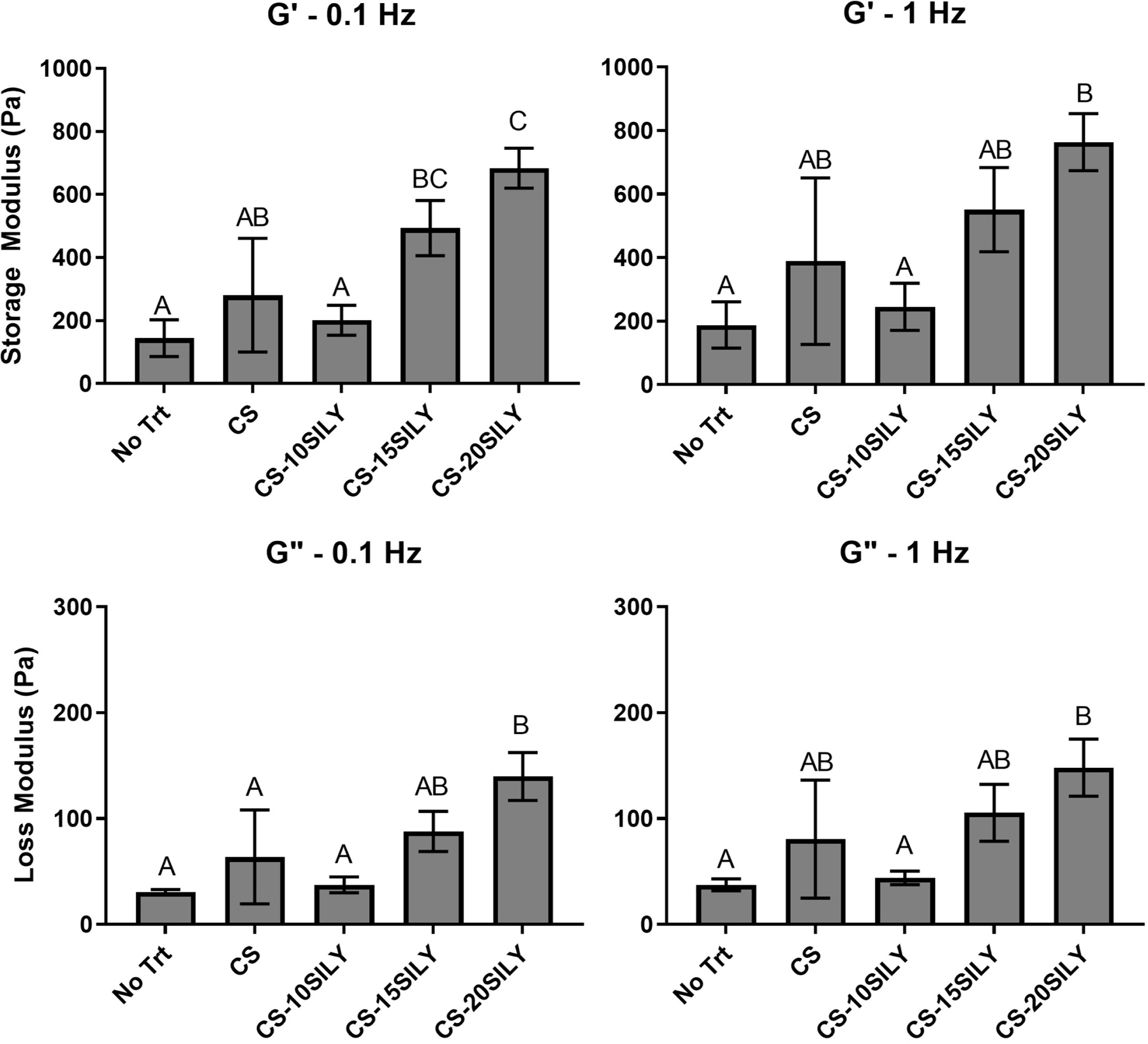
The storage modulus (G’) and loss modulus (G”) of hydrogels with the addition of CS or CS-SILY molecules. Frequency sweeps from 0.01 to 10 Hz were performed. Single factor analysis of variance (ANOVA) and Tukey post hoc tests were performed (n = 3) at 0.1 and 1 Hz. Data that share the same letter do not have statistically significant differences (p > 0.05) whereas data that do not share the same letter have statistically significantly differences (p < 0.05). Data is represented as the mean ± the standard deviation.
Addition of CS-SILY decreases the average fibril diameter.
Cryo-SEM was performed to investigate the network structure of hydrogels polymerized in the presence of CS or CS-SILY molecules, and a representative cryo-SEM image of each treatment is shown in Figure 3A. The distribution of collagen fibrils was analyzed, and the normalized frequency of fibril diameters is shown in Figure 3B. The No Trt and CS hydrogels had a wider distribution of fibril diameters of 50–300 nm and 25–300 nm, respectively (Figure 3B). In contrast, the fibril diameters in the hydrogels with CS-SILY molecules added had a fibril diameter distribution with less variation. The CS-10SILY, CS-15SILY, and CS-20SILY hydrogels had ranges of 75–250 nm, 25–150 nm, and 50–200 nm, respectively. Qualitatively, the fibrils look much thinner in the images where CS-SILY molecules are added (Figure 3A). ImageJ was used to calculate the average fibril diameter (Figure 3C). There were statistically smaller average fibril diameters for the CS-15SILY and CS-20SILY hydrogels compared to the collagen only (No Trt), CS, and CS-10SILY hydrogels. The average fibril diameters of the No Trt and CS hydrogels were statistically similar. The average fibril diameter of the CS-10SILY hydrogel was smaller than the No Trt and CS hydrogels but larger than the CS-15SILY and CS-20SILY hydrogels. There were no trends between the treatment groups when the percent porosity was analyzed using ImageJ (Figure 3D), and the addition of CS-SILY molecules increased the number of pores found in the hydrogels (Figure 3D).
Figure 3.
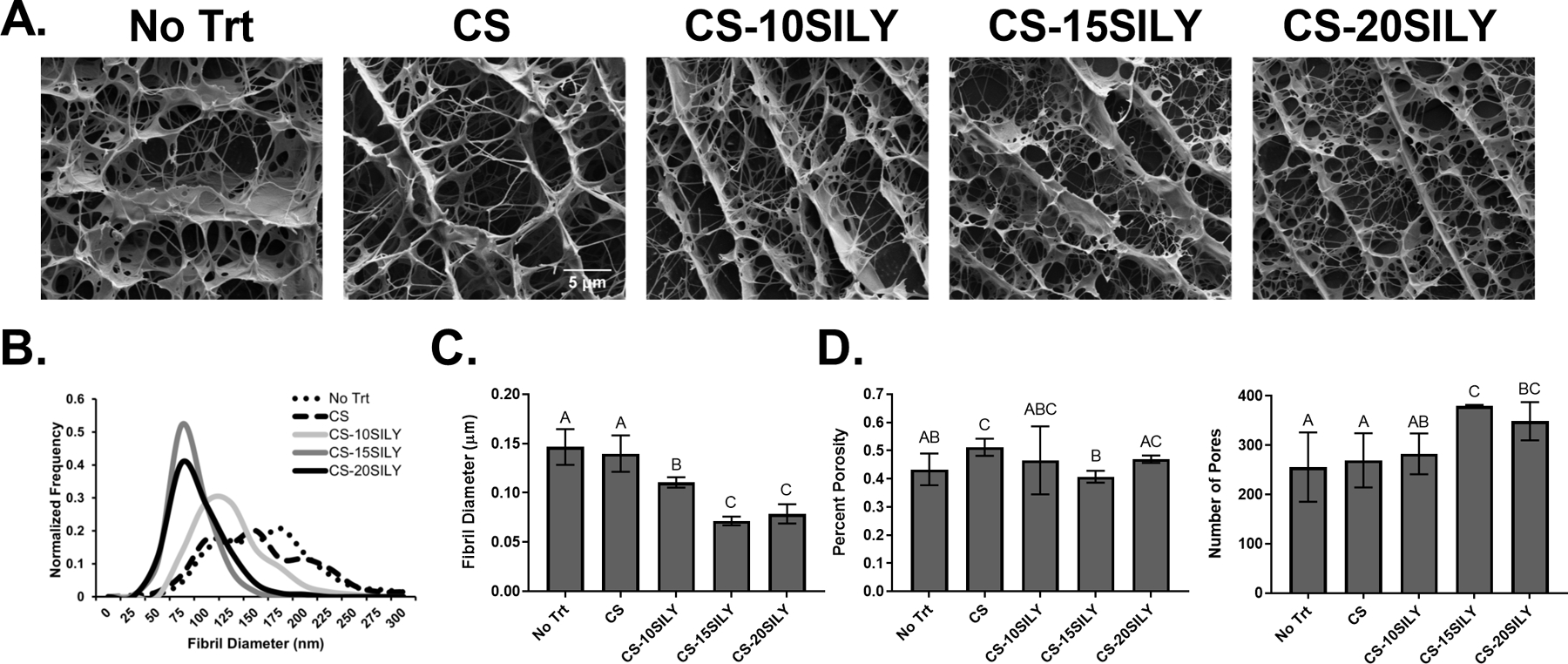
The effect of adding CS or CS-SILY molecules on collagen network structure. (A) Representative cyroSEM images for collagen hydrogels with no additional molecules (No Trt) or the addition of CS, CS-10SILY, CS-15SILY, or CS-20SILY. The scale bar represents 5 µm. (B) The fibril distributions are also represented as normalized frequency for each hydrogel formulation (n ≥ 270). (C) Average fibril diameter in hydrogels with added CS or CS-SILY. (D) The percent porosity (n = 9) and number of pores (n = 9) based on cryoSEM images. Data that share the same letter do not have statistically significant differences (p > 0.05) whereas data that do not share the same letter have statistically significantly differences (p < 0.05). Data is represented as the mean ± the standard deviation.
Addition of CS-SILY molecules increased sulfated GAG production and had no change on collagen content in scaffolds.
The sulfated GAG and DNA content in and dry weight of each scaffold, with or without added CS or CS-SILY, and encapsulated MSCs were measured after a 21- or 28-day culture period (Figures 4A,B and S4). At both 21 and 28 days, the overall GAG in scaffolds with any of the CS-SILY molecules was higher compared to scaffolds with no added CS (No Trt) or CS (Figure 4A,B). Furthermore, scaffolds with CS-SILY significantly increased the total amount of GAG between days 21 and 28 whereas no increase was seen in the control scaffolds. GAG production was also normalized to DNA content to understand the amount of GAG produced per cell (Figure S5A,B). Although all other treatments had a significant increase in GAG content compared to the scaffold with no added CS (No Trt), there were no statistical differences in the normalized GAG levels in scaffolds with added CS, CS-10SILY, CS-15SILY, or CS-20SILY when they were cultured for 21 days. After 28 days in culture, there was a statistically greater amount of normalized GAG in the scaffolds with any of the CS-SILY molecules added prior to fibrillogenesis compared to the scaffolds without CS (No Trt) or with added CS.
Figure 4.
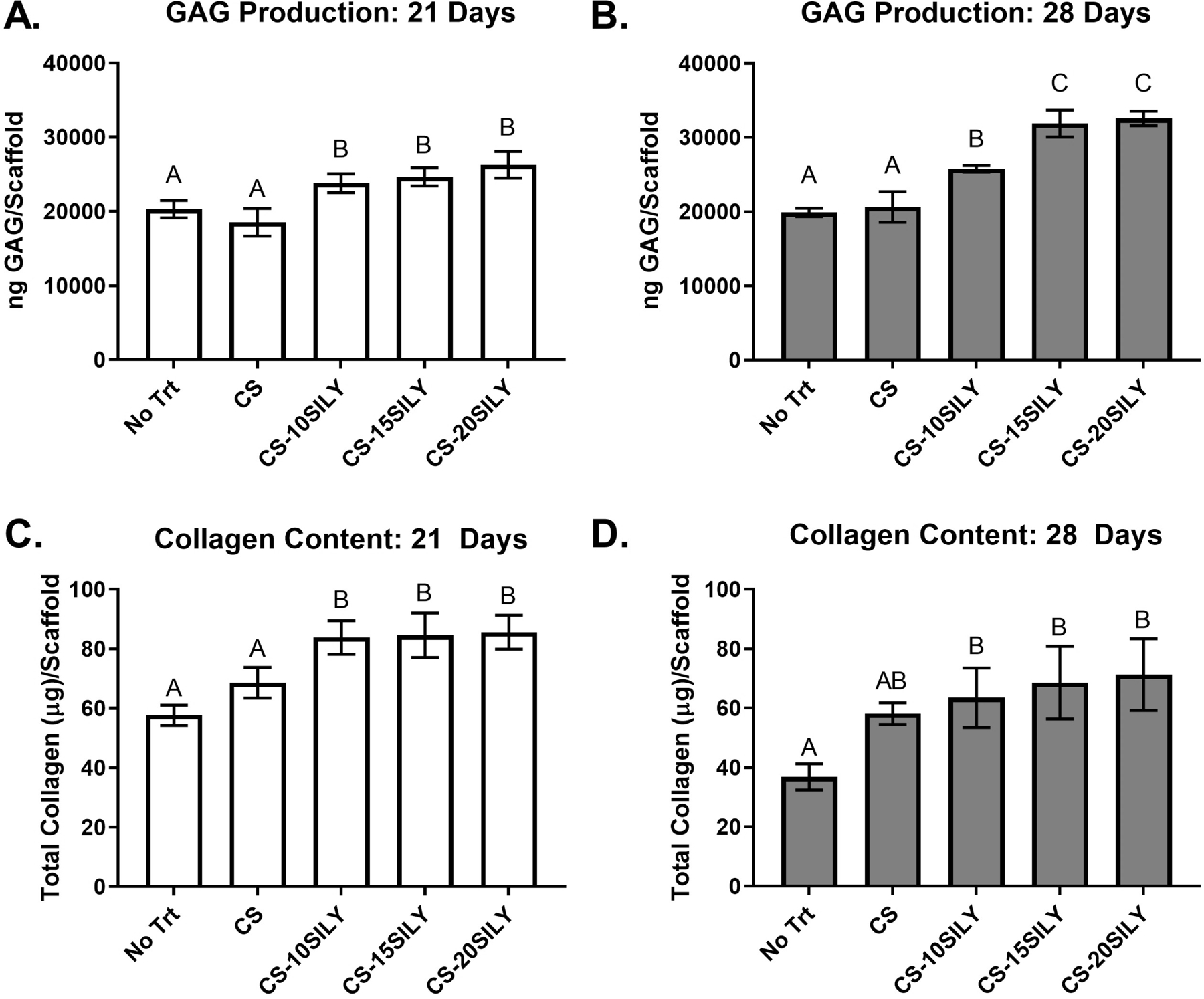
Overall sulfated GAG and collagen production per scaffold are upregulated in hydrogels with CS-SILY molecules. GAG production of the cell-hydrogel constructs (n = 4) with or with added CS or CS-SILY molecules after a (A) 21-day or (B) 28-day culture period. Total collagen of the cell-hydrogel constructs (n =3) with or with added CS or CS-SILY molecules after a (C) 21-day or (D) 28-day culture period. Values are expressed as mean ± standard deviation. ANOVA and Tukey’s post hoc tests were performed. Data that share the same letter do not have statistically significant differences (p > 0.05) whereas data that do not share the same letter have statistically significantly differences (p < 0.05).
The collagen content in each scaffold, with or without added CS or CS-SILY, and encapsulated MSCs was determined after a 21- or 28-day culture period (Figure 4C,D). After 21 days of culture, there was a statistically higher amount of collagen per scaffold when a CS-SILY molecule was added compared to when CS or no molecule (No Trt) was added (Figure 4C). After 28 days in culture, there was a statistically higher amount of collagen per scaffold when a CS-SILY molecule was added into the scaffold compared to the scaffolds that had no added CS (No Trt) (Figure 4D). However, there was no statistical difference between the scaffolds that had a CS-SILY molecule or CS added. The amount of collagen in each scaffold decreased between 21 and 28 days in hydrogels with no treatment, CS, or CS-10SILY, whereas there were no differences in hydrogels with CS-15SILY or CS-20SILY. There were no statistical differences in the total collagen content normalized to DNA in the hydrogels polymerized with or without CS or CS-SILY molecules (Figure S5C,D).
There was less collagen in cell culture media with CS-SILY molecules.
Given that the trends for collagen found in scaffolds differed depending on the value used to normalize the collagen, we sought to better understand collagen production and retention within the scaffolds. Thus, the mass of collagen in cell culture media, which was pooled together each week, was measured using a Sircol dye assay after a 7, 14, 21, or 28-day culture period (Figure 5). After 7 days in culture, there was statistically less collagen in the cell culture media removed from cell-hydrogel constructs with CS-10SILY or CS-15SILY compared to scaffolds with or without CS added. At all other time points, there were no statistical differences in the amount of collagen recovered from cell culture media in any of the treatments examined.
Figure 5.
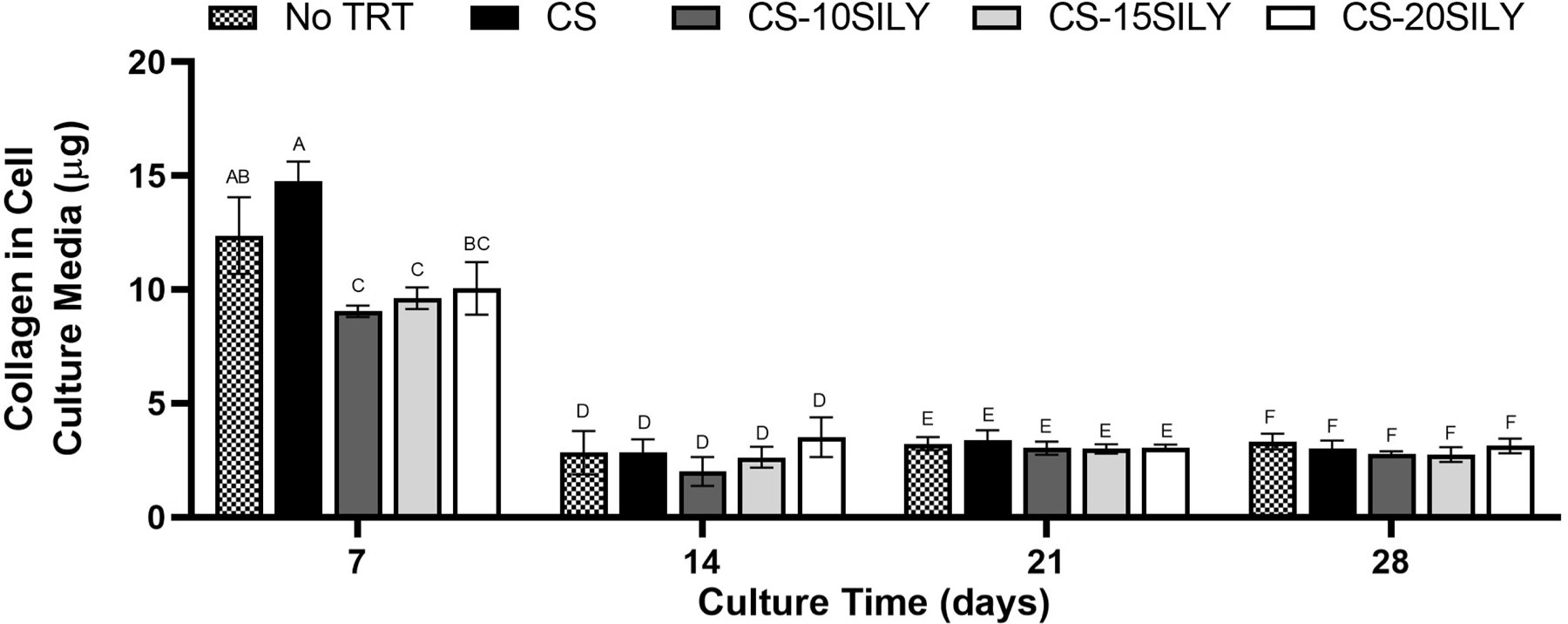
Less collagen was recovered in cell culture media in the scaffolds where a CS-SILY molecule was added. Collagen from media aliquots in which cell-hydrogel constructs, with or without added CS or CS-SILY molecules, were cultured. Medium aliquots were pooled together from each week and analyzed at 7, 14, 21, or 28 days. Collagen was measured using a Sircol dye assay kit. Values are expressed as mean ± standard deviation (n = 3). An ANOVA and Tukey’s post hoc tests were performed. Data that share the same letter do not have statistically significant differences (p > 0.05) whereas data that do not share the same letter have statistically significantly differences (p < 0.05).
CS-SILY upregulated chondrogenic genes.
Gene expression levels were measured at day 7 and 14 using qRT-PCR for MSCs cultured in Col I/II hydrogels with no additional treatment (No Trt) or CS, CS-10SILY, CS-15SILY, or CS-20SILY added (Figure 6). There was a slight upregulation in SOX9 gene expression with the addition of CS or CS-SILY molecules after 7 days in culture (Figure 6A). This increase was statistically significant for the scaffolds with CS-10SILY and CS compared to the scaffolds with no added treatment (No Trt). After 7 days in culture, there was also a statistical increase in aggrecan expression in samples with CS, CS-10SILY, or CS-15SILY compared to scaffolds with no added CS (No Trt) or CS-20SILY. There was no statistical difference between treatments in collagen type II or collagen type X expression at 7 days. There was statistically higher collagen I expression for the CS hydrogel compared to no CS (No Trt) or CS-20SILY hydrogel. There was a slight upregulation at 14 days of SOX9 expression in scaffolds with CS or CS-SILY. However, the only statistical difference was in scaffolds with CS and CS-15SILY compared to scaffolds with no added GAG (No Trt). Aggrecan gene expression was upregulated in the CS-10SILY hydrogels after 14 days compared to all other treatments. Scaffolds with added CS-20SILY had statistically higher collagen type II expression compared to scaffolds with CS-10SILY, CS-15SILY, or no added GAG treatment (No Trt). Finally, there were no statistical differences between treatments in collagen type I or collagen type X expression at 14 days.
Figure 6.
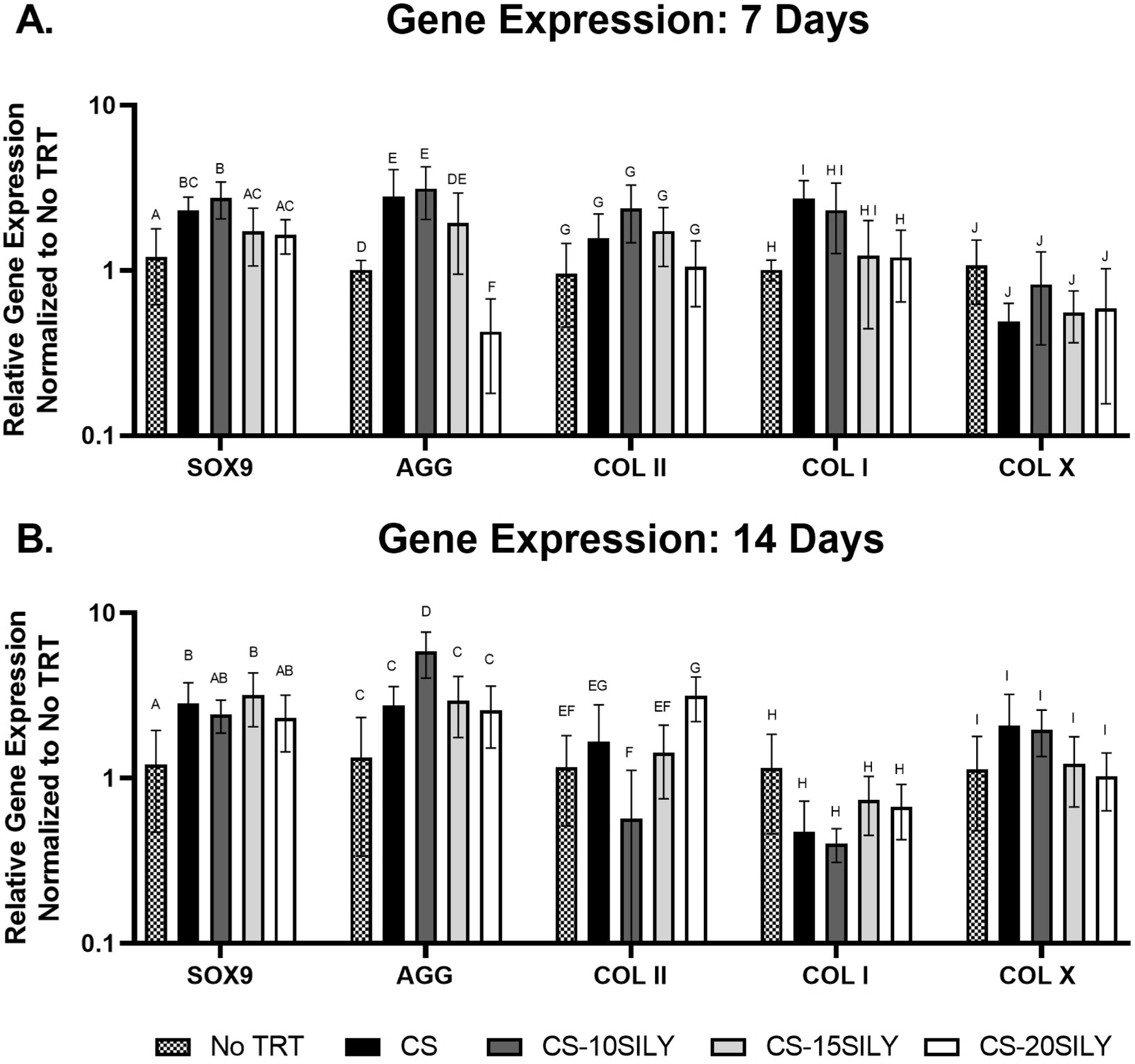
Relative gene expression of chondrogenic and collagen genes at days 7 and 14. ANOVA and Tukey’s post hoc tests were performed for gene expression analyses of collagen type II at 1 week, SOX9 at 1 week, aggrecan at 2 weeks, and SOX9 at 2 weeks. ANOVA and Games Howell post hoc tests were performed for gene expression analyses of collagen type I and collagen type X at both time points. A Box-Cox transformation was used to analyze data for gene expression analyses of aggrecan at 1 week and collagen type II at 2 weeks. Data that share the same letter do not have statistically significant differences (p > 0.05) whereas data that do not share the same letter have statistically significantly differences (p < 0.05).
DISCUSSION
Due to the fact that CS is able to influence the fate of stem cells by acting as a biochemical cue and binding with growth factors,42 studies have attempted to retain CS in their tissue engineered cartilage constructs. This study investigates strategies to better retain, without the use of chemical crosslinking, matrix molecules such as CS to a scaffold material to better recapitulate aspects of native cartilage. To date, this study is the first to retain CS in a collagen-based scaffold by incorporating a CS molecule with attached collagen-binding peptides. The concentration of 10 μM of CS, which is equivalent to 0.4 mg/mL, was chosen to be within the range of CS concentrations found in the middle zone of human articular cartilage.43 The addition of the collagen-binding peptide to CS-15SILY or CS-20SILY allowed for >20% of the original amount of CS to be retained after 7 days whereas <1% of free CS remained in the same time period (Figures 1, S1). Similar results were seen for gels polymerized for 3 or 12 hours, and these findings support that, for cell encapsulation studies, a gelation time of 3 hours is sufficient before application of medium.
When GAGs such as HA or CS are added to collagen gels, the literature is inconsistent with regards to the resulting mechanical properties. One study found that the addition of 0.5 mg/mL HA or CS to collagen matrices at 5 mg/mL did not statistically change the compressive modulus.44 In contrast, a study of collagen type I hydrogels at 4 mg/mL observed a slight decrease in storage modulus when 0.2 mg/mL CS was added during polymerization.45 Another study using 1 mg/mL collagen hydrogels found that the G’ values initially increased with a maximum G’ value at 5 mg/mL CS and then decreased as CS concentrations increased to 20 mg/mL.46 In the current study, there was an increasing trend in the G’ value when the number of SILY peptides on the CS backbone was increased (Figures 2, S2, S3). We hypothesize that the increase in mechanical properties can be attributed to CS-SILY serving as a collagen crosslinker since each CS molecule can bind multiple collagen fibrils through the pendant SILY peptides. Thus, with a higher number of peptides grafted per CS molecule, the crosslink density can increase. When SILY peptides were grafted to dermatan sulfate (DS-SILY), a similar increase in G’ values was seen for collagen gels with DS-SILY, and a similar mechanism was proposed.33 It is important to note, however, that the stiffness of collagen hydrogels can also be modulated by various other fibril characteristics such as diameter, length, density, and orientation.47 Furthermore, it has been suggested that interactions between CS and collagen can directly alter the mechanics of individual collagen fibers through modulating the amount of collagen found in each fibril.46 Therefore, it is likely that crosslinking and gel structure play a role in the mechanical properties observed here.
Although the interactions of CS and proteins have been studied, there are discrepancies in the literature on how CS alters fibril size and fibrillogenesis rate.45, 48, 49 One study showed that the addition of CS to a collagen scaffold either increased or decreased fibril diameter depending on the buffer used.48 In contrast, Stuart and Panitch observed that adding CS to collagen I hydrogels shifted the fibril distribution away from large collagen fibrils.45 Douglas et al. also found that the addition of CS to either collagen type I or II caused fibrils to become thinner.49 They hypothesized that CS interactions with fibrils resulted in steric hindrance of fibril growth given that sugars can inhibit fibril formation by disrupting hydrogen-bonded water clusters that connect collagen helices.50 These last two studies of collagen fibril diameter are consistent with our results. The average fibril diameter was statistically smaller in the CS-15SILY and CS-20SILY gels, which both retained the highest amount of CS after one week, compared to the collagen only (No Trt), CS, and CS-10SILY hydrogels (Figure 3). A similar effect is seen in biology. Both decorin and biglycan, which are small leucine-rich proteoglycans (SLRPs), have been shown to control the degree of collagen incorporation into fibrils as well as collagen fibril diameter.51 The SLRPs compete with collagen monomers for binding to the growing fibrils and thus limit fibril size. Like the SLRPs, CS-SILY binds to collagen fibrils and is presumed to affect collagen fibril growth in a similar fashion.33 Thus, interactions of collagen with both CS and CS-SILY likely play a role in decreasing fibril diameter as the CS or CS-SILY concentration increases. If the amount of polymerized collagen and fibril length remain constant, a smaller fibril diameter would be expected to result in a smaller pore size as is qualitatively observed in the cryo-SEM pictures (Figure 3A). In addition to the role of increased crosslinking that likely occurs as the number of SILY peptides per CS increase, the smaller pore size could also contribute to the increased G’ values observed for CS-20SILY gels.
Other studies have reported that MSCs cultured in softer scaffolds were more likely to express cartilage-specific genes and produce higher levels of cartilage matrix molecules than MSCs cultured in stiffer scaffolds.52–57 Here, our results, which found that stiffer gels demonstrated increased chondrogenesis, are in conflict with these other studies; however, here, increased stiffness also correlated with increased CS content in the gel, which is known to support chondrogenesis.23–26, 58 In addition, the model of crosslinking differs from that of the other studies. Here, GAG crosslinking was achieved using peptides that bind transiently to collagen, whereas other published studies used covalent crosslinking to form the scaffolds. Cells will likely sense these mechanical environments differently leading to differing behavior. Regardless, in the gels studies here, increased CS content proved to be a stronger driving factor for chondrogenesis than decreased gel stiffness.
The DMMB assay measures the amount of sulfated GAGs, such as chondroitin sulfate, but does not detect unsulfated GAGs, such as hyaluronic acid. CS-SILY supported a greater amount of sulfated GAG per scaffold after 21 and 28 days of culture. Although we initially encapsulated 20 μg of CS, the CS-SILY molecules, which had the highest retention, had ≤45% (or ≤ 9 μg CS) retained after 7 days (Figure 1). At 21 and 28 days, all groups had ~20–30 μg of GAG per scaffold (Figure 4a,b). We can thus surmise that the encapsulated cells produced new GAGs during the culture period and that the CS-SILY molecules enhanced GAG production compared to CS or no treatment. Furthermore, the fact that only the scaffolds with CS-SILY had significant increases in GAG per scaffold between 21 and 28 days (Figure 4a,b) correlates with the significantly higher GAG production per cell for scaffolds with CS-SILY at 28 days (Figure S5b). These results suggest that CS, introduced to the Col I/II gels in the form of a CS-SILY molecule, is still bioactive when crosslinked through a collagen-binding peptide and that CS-SILY results in better bioactivity compared to CS because of its higher retention rates.
There is a decreased amount of collagen in control scaffolds compared to scaffolds with CS-SILY at 21 and 28 days (Figure 4c,d). These results correlated with the fact that, after 7 days of culture, more collagen is lost to the medium in the no CS (No Trt) and CS scaffolds compared to scaffolds with CS-SILY (Figure 5). We hypothesize that the increased collagen retention can be attributed to the additional collagen crosslinks that the CS-SILY molecules provide. A decrease in the mass of collagen in scaffolds with no CS (No Trt), CS, and CS-10SILY was seen from 21 to 28 days (Figure 4c,d), and these results correlate with the fact that collagen is continuously lost in the medium (Figure 5). Our previous study used similar collagen I/II gels (starting concentration of 4 mg/mL) with 5 μM CS and found that ~3 mg/mL of collagen was incorporated in the gel.16 Assuming that a similar amount of collagen is incorporated in this study, there is ~150 μg of collagen initially in the gels. Given that we detect ~40–80 μg of collagen in the scaffolds and see a continuous ~3–15 μg of collagen in the media, our results suggest that cells are remodeling and degrading collagen as they make more GAGs. Studies at longer time points may provide insight as to whether collagen accumulation in the scaffold will occur as cells continue to remodel the surrounding matrix.
Previous studies have added CS to collagen-based scaffolds to provide biochemical cues that promote cartilage matrix production.26, 58 In the current study, slight differences in the gene expression of cartilage specific genes were seen. Aggrecan gene expression was upregulated after 7 days in scaffolds with CS, CS-10SILY, or CS-15SILY compared to samples with no added GAG or CS-20SILY and in CS-10SILY hydrogels after 14 days compared to all other treatments. There was a statistical increase in expression of SOX9, which is considered the master transcription factor involved in chondrogenesis, after 7 days in the scaffolds with CS-10SILY and CS compared to scaffolds with no added GAG. These gene expression results are consistent with the idea that CS is still bioactive when conjugated to a collagen-binding peptide. Our study is consistent with one by van Susante et al. where collagen type I gels with CS resulted in more GAG production per scaffold at 14 days compared to collagen only gels, but no differences in proteoglycan gene expression by chondrocytes were seen after 14 days between the gel types.58 Chen et al. demonstrated that adding CS to collagen II hydrogels increased GAG and collagen II production by MSCs, upregulated chondrogenic gene expression, and enhanced in vivo cartilage repair.26 Differences in gene expression in response to CS may be a result of many factors such as cell source and type, collagen source and type, scaffold fabrication parameters, and method for crosslinking CS to the collagen scaffold. In fact, Chen and coworkers found that there was an optimal ratio of CS to collagen II and that increasing the ratio decreased GAG and collagen II production.26 Although the mechanism remains unclear, the addition of CS to PEG scaffolds has been shown to prevent or delay the further differentiation of MSCs to a hypertrophic phenotype, represented with the protein expression of collagen type X.24 However, if we assume gene and protein expression follow similar trends, our results differ since we saw no differences in collagen type X gene expression between our treatments investigated at either time point.
Overall, our results demonstrate that attaching SILY peptides to CS is an effective method for increasing retention in collagen hydrogels. The increased retention of CS-SILY supported enhanced chondrogenesis, increased collagen retention, and a higher production of GAGs, an important cartilage matrix component, within the scaffolds.
CONCLUSIONS
This study investigated the use of CS with attached collagen-binding peptides to retain, without the use of chemical crosslinking, matrix molecules and better recapitulate aspects of native cartilage. Since the CS retention, average fibril diameter, and mechanical properties are altered by the addition of different CS-SILY molecules, the properties of the desired Col I/II hydrogel can be tuned by adjusting the amount of SILY peptides attached to the CS backbone. Finally, the scaffolds that contained CS-10SILY, CS-15SILY, and CS-20SILY had higher sulfated glycosaminoglycan production, and this result suggests that the scaffolds containing a CS-SILY molecule supported better production of cartilage extracellular matrix. Taken together, these results suggest that the addition of a CS-SILY molecule to a collagen type I/II blend hydrogel with encapsulated MSCs has the potential to promote cartilage repair.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Purdue Davidson School of Chemical Engineering, William P. and Amanda C. Madar, and the National Institutes of Health [R01AR065398].
Footnotes
SUPPORTING INFORMATION
qRT-PCR primer sequences, CS retained in hydrogel after 12-hour polymerization, storage and loss moduli from a frequency sweep, storage moduli at selected frequencies, DNA mass and dry weight of hydrogels after 21 and 28 days of culture, normalized GAG and collagen amount after 21 and 28 days of culture
CONFLICTS OF INTEREST
A.P. is a co-inventor of the CS-SILY technology and is a founder of Symic Holdings, which has exclusive rights to the patent on this technology.
REFERENCES
- (1).Williams SN; Wolford ML; Bercovitz A Hospitalization for Total Knee Replacement among Inpatients Aged 45 and Over: United States, 2000–2010. NCHS Data Brief, No. 210 Hyattsville, MD: National Center for Health Statistics, 2015. [PubMed] [Google Scholar]
- (2).Wolford ML; Palso K; Bercovitz A Hospitalization for Total Hip Replacement among Inpatients Aged 45 and Over: United States, 2000–2010. NCHS Data Brief, No. 186 Hyattsville, MD: National Center for Health Statistics, 2015. [PubMed] [Google Scholar]
- (3).Huey DJ; Hu JC; Athanasiou KA Unlike Bone, Cartilage Regeneration Remains Elusive. Science 2012, 338 (6109), 917–921. DOI: 10.1126/science.1222454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).de l’Escalopier N; Anract P; Biau D Surgical Treatments for Osteoarthritis. Ann. Phys. Rehabil. Med 2016, 59 (3), 227–233. DOI: 10.1016/j.rehab.2016.04.003. [DOI] [PubMed] [Google Scholar]
- (5).Madry H; Grün UW; Knutsen G Cartilage Repair and Joint Preservation: Medical and Surgical Treatment Options. Dtsch. Arztebl. Int 2011, 108 (40), 669–677. DOI: 10.3238/arztebl.2011.0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Alford JW; Cole BJ Cartilage Restoration, Part 1: Basic Science, Historical Perspective, Patient Evaluation, and Treatment Options. Am. J. Sports Med 2005, 33 (2), 295–306. DOI: 10.1177/0363546504273510. [DOI] [PubMed] [Google Scholar]
- (7).Matsiko A; Levingstone TJ; O’Brien FJ Advanced Strategies for Articular Cartilage Defect Repair. Materials 2013, 6 (2), 637–668. DOI: 10.3390/ma6020637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Pittenger MF; Mackay AM; Beck SC; Jaiswal RK; Douglas R; Mosca JD; Moorman MA; Simonetti DW; Craig S; Marshak DR Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284 (5411), 143–147. DOI: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- (9).Hardingham TE; Fosang AJ Proteoglycans: Many Forms and Many Functions. FASEB J 1992, 6 (3), 861–870. DOI: 10.1096/fasebj.6.3.1740236. [DOI] [PubMed] [Google Scholar]
- (10).Chen S; Fu P; Wu H; Pei M Meniscus, Articular Cartilage and Nucleus Pulposus: A Comparative Review of Cartilage-Like Tissues in Anatomy, Development and Function. Cell Tissue Res 2017, 370 (1), 53–70. DOI: 10.1007/S00441-017-2613-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Spiller KL; Maher SA; Lowman AM Hydrogels for the Repair of Articular Cartilage Defects. Tissue Eng. Part B Rev 2011, 17 (4), 281–299. DOI: 10.1089/ten.teb.2011.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Wakitani S; Goto T; Pineda SJ; Young RG; Mansour JM; Caplan AI; Goldberg VM Mesenchymal Cell-Based Repair of Large, Full-Thickness Defects of Articular Cartilage. J. Bone Joint Surg 1994, 76 (4), 579–592. DOI: 10.2106/00004623-199404000-00013. [DOI] [PubMed] [Google Scholar]
- (13).Wakitani S; Imoto K; Yamamoto T; Saito M; Murata N; Yoneda M Human Autologous Culture Expanded Bone Marrow Mesenchymal Cell Transplantation for Repair of Cartilage Defects in Osteoarthritic Knees. Osteoarthritis Cartilage 2002, 10 (3), 199–206. DOI: 10.1053/joca.2001.0504. [DOI] [PubMed] [Google Scholar]
- (14).Bosnakovski D; Mizuno M; Kim G; Takagi S; Okumura M; Fujinaga T Chondrogenic Differentiation of Bovine Bone Marrow Mesenchymal Stem Cells (MSCs) in Different Hydrogels: Influence of Collagen Type II Extracellular Matrix on MSC Chondrogenesis. Biotechnol. Bioeng 2006, 93 (6), 1152–1163. DOI: 10.1002/bit.20828. [DOI] [PubMed] [Google Scholar]
- (15).Lu Z; Doulabi BZ; Huang C; Bank RA; Helder MN Collagen Type II Enhances Chondrogenesis in Adipose Tissue-Derived Stem Cells by Affecting Cell Shape. Tissue Eng. Part A 2010, 16 (1), 81–90. DOI: 10.1089/ten.tea.2009.0222. [DOI] [PubMed] [Google Scholar]
- (16).Vázquez-Portalatín N; Kilmer CE; Panitch A; Liu JC Characterization of Collagen Type I and II Blended Hydrogels for Articular Cartilage Tissue Engineering. Biomacromolecules 2016, 17 (10), 3145–3152. DOI: 10.1021/acs.biomac.6b00684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Kilmer CE; Battistoni CM; Cox A; Breur GJ; Panitch A; Liu JC Collagen Type I and II Blend Hydrogel with Autologous Mesenchymal Stem Cells as a Scaffold for Articular Cartilage Defect Repair. ACS Biomater. Sci. Eng 2020, 6 (6), 3464–3476. DOI: 10.1021/acsbiomaterials.9b01939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Huang D Effect of Extracellular Chondroitin Sulfate on Cultured Chondrocytes. J. Cell Biol 1974, 62 (3), 881–886. DOI: 10.1083/jcb.62.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Sechriest VF; Miao YJ; Niyibizi C; Westerhausen-Larson A; Matthew HW; Evans CH; Fu FH; Suh JK GAG-Augmented Polysaccharide Hydrogel: A Novel Biocompatible and Biodegradable Material to Support Chondrogenesis. J. Biomed. Mater. Res 2000, 49 (4), 534–541. DOI: . [DOI] [PubMed] [Google Scholar]
- (20).Bryant SJ; Davis-Arehart KA; Luo N; Shoemaker RK; Arthur JA; Anseth KS Synthesis and Characterization of Photopolymerized Multifunctional Hydrogels: Water-Soluble Poly(Vinyl Alcohol) and Chondroitin Sulfate Macromers for Chondrocyte Encapsulation. Macromolecules 2004, 37 (18), 6726–6733. DOI: 10.1021/ma0499324. [DOI] [Google Scholar]
- (21).Kim HD; Heo J; Hwang Y; Kwak S-Y; Park OK; Kim H; Varghese S; Hwang NS Extracellular-Matrix-Based and Arg-Gly-Asp-Modified Photopolymerizing Hydrogels for Cartilage Tissue Engineering. Tissue Eng. Part A 2015, 21 (3–4), 757–766. DOI: 10.1089/ten.tea.2014.0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Han M-E; Kim S-H; Kim HD; Yim H-G; Bencherif SA; Kim T-I; Hwang NS Extracellular Matrix-Based Cryogels for Cartilage Tissue Engineering. Int. J. Biol. Macromol 2016, 93B, 1410–1419. DOI: 10.1016/j.ijbiomac.2016.05.024. [DOI] [PubMed] [Google Scholar]
- (23).Steinmetz NJ; Bryant SJ Chondroitin Sulfate and Dynamic Loading Alter Chondrogenesis of Human MSCs in PEGHydrogels. Biotechnol. Bioeng 2012, 109 (10), 2671–2682. DOI: 10.1002/bit.24519. [DOI] [PubMed] [Google Scholar]
- (24).Varghese S; Hwang NS; Canver AC; Theprungsirikul P; Lin DW; Elisseeff J Chondroitin Sulfate Based Niches for Chondrogenic Differentiation of Mesenchymal Stem Cells. Matrix Biol 2008, 27 (1), 12–21. DOI: 10.1016/j.matbio.2007.07.002. [DOI] [PubMed] [Google Scholar]
- (25).Aisenbrey EA; Bryant SJ The Role of Chondroitin Sulfate in Regulating Hypertrophy During MSC Chondrogenesis in a Cartilage Mimetic Hydrogel under Dynamic Loading. Biomaterials 2019, 190–191, 51–62. DOI: 10.1016/j.biomaterials.2018.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Chen W-C; Wei Y-H; Chu I-M; Yao C-L Effect of Chondroitin Sulphate C on the In Vitro and In Vivo Chondrogenesis of Mesenchymal Stem Cells in Crosslinked Type II Collagen Scaffolds. J. Tissue Eng. Regen. Med 2013, 7 (8), 665–672. DOI: 10.1002/term.1463. [DOI] [PubMed] [Google Scholar]
- (27).Bernhard JC; Panitch A Synthesis and Characterization of an Aggrecan Mimic. Acta Biomater 2012, 8 (4), 1543–1550. DOI: 10.1016/j.actbio.2011.12.029. [DOI] [PubMed] [Google Scholar]
- (28).Sharma S; Lee A; Choi K; Kim K; Youn I; Trippel SB; Panitch A Biomimetic Aggrecan Reduces Cartilage Extracellular Matrix from Degradation and Lowers Catabolic Activity in Ex Vivo and In Vivo Models. Macromol. Biosci 2013, 13 (9), 1228–1237. DOI: 10.1002/mabi.201300112. [DOI] [PubMed] [Google Scholar]
- (29).Sharma S; Panitch A; Neu CP Incorporation of an Aggrecan Mimic Prevents Proteolytic Degradation of Anisotropic Cartilage Analogs. Acta Biomater 2013, 9 (1), 4618–4625. DOI: 10.1016/j.actbio.2012.08.041. [DOI] [PubMed] [Google Scholar]
- (30).Sharma S; Vazquez-Portalatin N; Calve S; Panitch A Biomimetic Molecules Lower Catabolic Expression and Prevent Chondroitin Sulfate Degradation in an Osteoarthritic Ex Vivo Model. ACS Biomater. Sci. Eng 2016, 2 (2), 241–250. DOI: 10.1021/acsbiomaterials.5b00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Lawrence A; Xu X; Bible MD; Calve S; Neu CP; Panitch A Synthesis and Characterization of a Lubricin Mimic (mLub) to Reduce Friction and Adhesion on the Articular Cartilage Surface. Biomaterials 2015, 73, 42–50. DOI: 10.1016/j.biomaterials.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Twitchell C; Walimbe T; Liu JC; Panitch A Peptide-Modified Chondroitin Sulfate Reduces Coefficient of Friction at Articular Cartilage Surface. Curr. Res. Biotechnol 2020, 2, 16–21. DOI: 10.1016/j.crbiot.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Paderi JE; Panitch A Design of a Synthetic Collagen-Binding Peptidoglycan That Modulates Collagen Fibrillogenesis. Biomacromolecules 2008, 9 (9), 2562–2566. DOI: 10.1021/bm8006852. [DOI] [PubMed] [Google Scholar]
- (34).Paderi JE; Stuart K; Sturek M; Park K; Panitch A The Inhibition of Platelet Adhesion and Activation on Collagen During Balloon Angioplasty by Collagen-Binding Peptidoglycans. Biomaterials 2011, 32 (10), 2516–2523. DOI: 10.1016/j.biomaterials.2010.12.025. [DOI] [PubMed] [Google Scholar]
- (35).Scott RA; Paderi JE; Sturek M; Panitch A Decorin Mimic Inhibits Vascular Smooth Muscle Proliferation and Migration. PLoS One 2013, 8 (11), e82456. DOI: 10.1371/journal.pone.0082456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Scott RA; Panitch A Decorin Mimic Regulates Platelet-Derived Growth Factor and Interferon-g Stimulation of Vascular Smooth Muscle Cells. Biomacromolecules 2014, 15 (6), 2090–2103. DOI: 10.1021/bm500224f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Stuart K; Paderi J; Snyder PW; Freeman L; Panitch A Collagen-Binding Peptidoglycans Inhibit MMP Mediated Collagen Degradation and Reduce Dermal Scarring. PLoS One 2011, 6 (7), e22139. DOI: 10.1371/journal.pone.0022139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Han B; Li Q; Wang C; Patel P; Adams SM; Doyran B; Nia HT; Oftadeh R; Zhou S; Li CY; Liu XS; Lu XL; Enomoto-Iwamoto M; Qin L; Mauck RL; Iozzo RV; Birk DE; Han L Decorin Regulates the Aggrecan Network Integrity and Biomechanical Functions of Cartilage Extracellular Matrix. ACS Nano 2019, 13 (10), 11320–11333. DOI: 10.1021/acsnano.9B04477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Renner JN; Kim Y; Liu JC Bone Morphogenetic Protein-Derived Peptide Promotes Chondrogenic Differentiation of Human Mesenchymal Stem Cells. Tissue Eng. Part A 2012, 18 (23–24), 2581–2589. DOI: 10.1089/ten.tea.2011.0400. [DOI] [PubMed] [Google Scholar]
- (40).Kim YJ; Sah RL; Doong JY; Grodzinsky AJ Fluorometric Assay of DNA in Cartilage Explants Using Hoechst 33258. Anal. Biochem 1988, 174 (1), 168–176. DOI: 10.1016/0003-2697(88)90532-5. [DOI] [PubMed] [Google Scholar]
- (41).Bookout AL; Cummins CL; Mangelsdorf DJ; Pesola JM; Kramer MF High-Throughput Real-Time Quantitative Reverse Transcription PCR. In Current Protocols in Molecular Biology, John Wiley & Sons, New York, 2006; Vol. 73, pp 15.18.11–15.18.28. [DOI] [PubMed] [Google Scholar]
- (42).Nandini CD; Sugahara K Role of the Sulfation Pattern of Chondroitin Sulfate in Its Biological Activities and in the Binding of Growth Factors. Adv. Pharmacol 2006, 53, 253–279. DOI: 10.1016/S1054-3589(05)53012-6. [DOI] [PubMed] [Google Scholar]
- (43).Kuiper NJ; Sharma A A Detailed Quantitative Outcome Measure of Glycosaminoglycans in Human Articular Cartilage for Cell Therapy and Tissue Engineering Strategies. Osteoarthritis Cartilage 2015, 23 (12), 2233–2241. DOI: 10.1016/j.joca.2015.07.011. [DOI] [PubMed] [Google Scholar]
- (44).Matsiko A; Levingstone TJ; O’Brien FJ; Gleeson JP Addition of Hyaluronic Acid Improves Cellular Infiltration and Promotes Early-Stage Chondrogenesis in a Collagen-Based Scaffold for Cartilage Tissue Engineering. J. Mech. Behav. Biomed. Mater 2012, 11, 41–52. DOI: 10.1016/j.jmbbm.2011.11.012. [DOI] [PubMed] [Google Scholar]
- (45).Stuart K; Panitch A Influence of Chondroitin Sulfate on Collagen Gel Structure and Mechanical Properties at Physiologically Relevant Levels. Biopolymers 2008, 89 (10), 841–851. DOI: 10.1002/bip.21024. [DOI] [PubMed] [Google Scholar]
- (46).Yang Y.-l.; Sun C; Wilhelm ME; Fox LJ; Zhu J; Kaufman LJ Influence of Chondroitin Sulfate and Hyaluronic Acid on Structure, Mechanical Properties, and Glioma Invasion of Collagen I Gels. Biomaterials 2011, 32 (31), 7932–7940. DOI: 10.1016/j.biomaterials.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Xu Q; Torres JE; Hakim M; Babiak PM; Pal P; Battistoni CM; Nguyen M; Panitch A; Solorio L; Liu JC Collagen- and Hyaluronic Acid-Based Hydrogels and Their Biomedical Applications. Mater. Sci. Eng. R Rep 2021, 146, 100641. DOI: 10.1016/j.mser.2021.100641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Bierbaum S; Douglas T; Hanke T; Scharnweber D; Tippelt S; Monsees TK; Funk RHW; Worch H Collagenenous Matrix Coatings on Titatnium Implants Modified with Decorin and Chondroitin Sulfate: Characterization and Influence on Osteoblastic Cells. J. Biomed. Mater. Res. A 2006, 77A (3), 551–562. DOI: 10.1002/jbm.a.30572. [DOI] [PubMed] [Google Scholar]
- (49).Douglas T; Heinemann S; Mietrach C; Hempel U; Bierbaum S; Scharnweber D; Worch H Interactions of Collagen Types I and II with Chondroitin Sulfates A-C and Their Effect on Osteoblast Adhesion. Biomacromolecules 2007, 8 (4), 1085–1092. DOI: 10.1021/bm0609644. [DOI] [PubMed] [Google Scholar]
- (50).Kuznetsova N; Chi SL; Leikin S Sugars and Polyols Inhibit Fibrillogenesis of Type I Collagen by Disrupting Hydrogen-Bonded Water Bridges between the Helices. Biochemistry 1998, 37 (34), 11888–11895. DOI: 10.1021/bi980089+. [DOI] [PubMed] [Google Scholar]
- (51).Douglas T; Heinemann S; Bierbaum S; Scharnweber D; Worch H Fibrillogenesis of Collagen Types I, II, and III with Small Leucine-Rich Proteoglycans Decorin and Biglycan. Biomacromolecules 2006, 7 (8), 2388–2393. DOI: 10.1021/bm0603746. [DOI] [PubMed] [Google Scholar]
- (52).Bian L; Hou C; Tous E; Rai R; Mauck RL; Burdick JA The Influence of Hyaluronic Acid Hydrogel Crosslinking Density and Macromolecular Diffusivity on Human MSC Chondrogenesis and Hypertrophy. Biomaterials 2013, 34 (2), 413–421. DOI: 10.1016/j.biomaterials.2012.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Choi B; Kim S; Lin B; Wu BM; Lee M Cartilaginous Extracellular Matrix-Modified Chitosan Hydrogels for Cartilage Tissue Engineering. ACS Appl. Mater. Interfaces 2014, 6 (22), 20110–20121. DOI: 10.1021/am505723k. [DOI] [PubMed] [Google Scholar]
- (54).Irawan V; Sung T-C; Higuchi A; Ikoma T Collagen Scaffolds in Cartilage Tissue Engineering and Relevant Approaches for Future Development. Tissue Eng. Regen. Med 2018, 15 (6), 673–697. DOI: 10.1007/s13770-018-0135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Liu SQ; Tian Q; Hedrick JL; Po Hui JH; Ee PLR; Yang YY Biomimetic Hydrogels for Chondrogenic Differentiation of Human Mesenchymal Stem Cells to Neocartilage. Biomaterials 2010, 31 (28), 7298–7307. DOI: 10.1016/j.biomaterials.2010.06.001. [DOI] [PubMed] [Google Scholar]
- (56).Murphy CM; Matsiko A; Haugh MG; Gleeson JP; O’Brien FJ Mesenchymal Stem Cell Fate Is Regulated by the Composition and Mechanical Properties of Collagen-Glycosaminoglycan Scaffolds. J. Mech. Behav. Biomed. Mater 2012, 11, 53–62. DOI: 10.1016/j.jmbbm.2011.11.009. [DOI] [PubMed] [Google Scholar]
- (57).Toh WS; Lim TC; Kurisawa M; Spector M Modulation of Mesenchymal Stem Cell Chondrogenesis in a Tunable Hyaluronic Acid Hydrogel Microenvironment. Biomaterials 2012, 33 (15), 3835–3845. DOI: 10.1016/j.biomaterials.2012.01.065. [DOI] [PubMed] [Google Scholar]
- (58).van Susante JLC; Pieper J; Buma P; van Kuppevelt TH; van Beuningen H; van der Kraan PM; Veerkamp JH; van den Berg WB; Veth RPH Linkage of Chondroitin-Sulfate to Type I Collagen Scaffolds Stimulates the Bioactivity of Seeded Chondrocytes In Vitro. Biomaterials 2001, 22 (17), 2359–2369. DOI: 10.1016/s0142-9612(00)00423-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


