Abstract
Background/Aims:
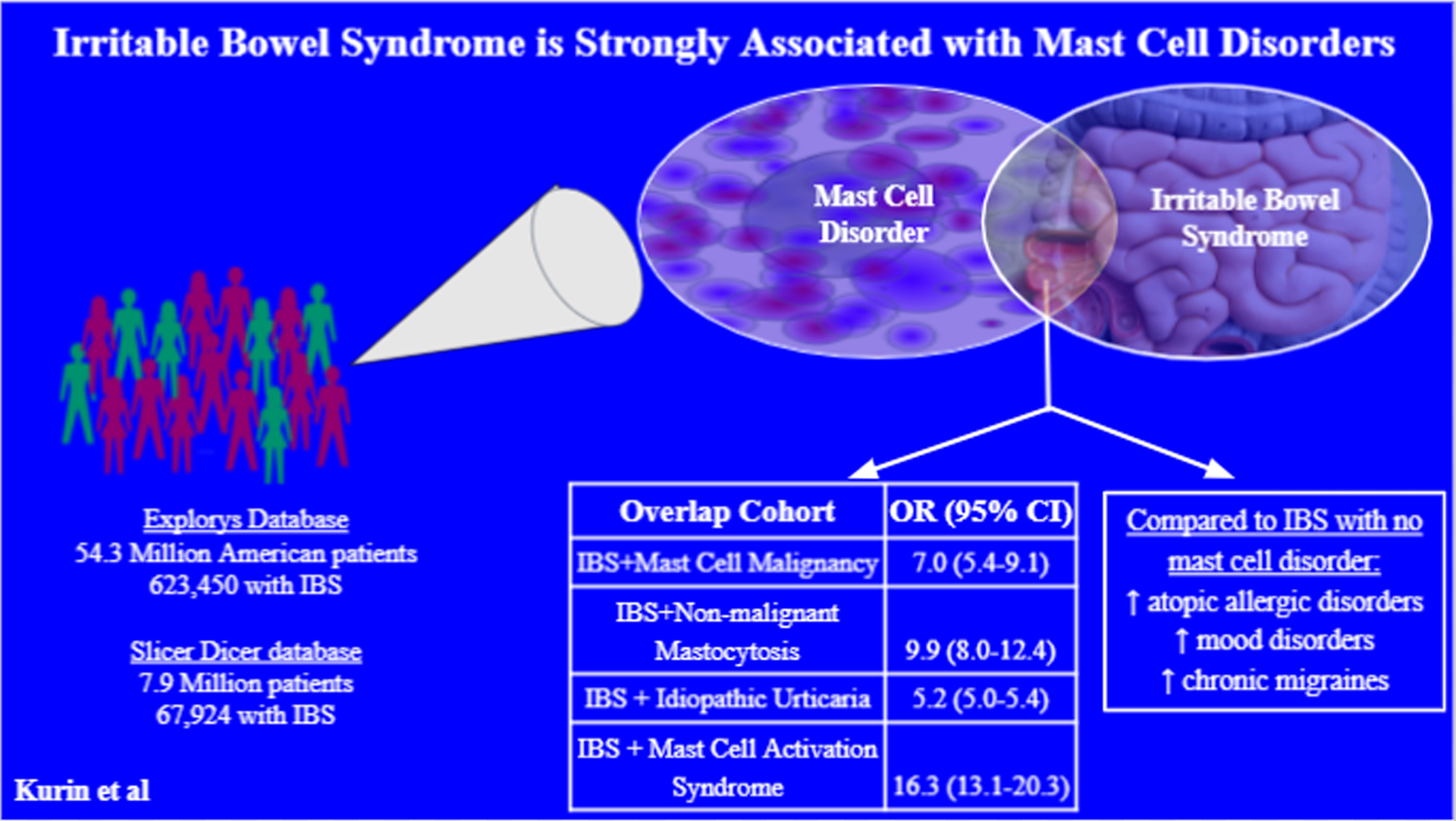
Mounting evidence supports a mechanistic association between irritable bowel syndrome (IBS) symptoms and mast cell hyperactivity. Yet, association between IBS and mast cell disorders (MCDs) has not been studied. We examined this association using two large databases and verified with manual chart review.
Methods:
The IBM Watson Health Explorys database (Somers, NY), an aggregate of electronic health record (EHR) data from over two dozen US healthcare systems, and Epic’s SlicerDicer tool, a self-service tool containing de-identified data from the Epic EHR, were used to identify patients with IBS and MCDs. Patients with organic gastrointestinal disease or diseases associated with secondary mast cell hyperproliferation were excluded. Results were verified with manual chart review from two academic centers.
Key Results:
Up to 4% of IBS patients had a comorbid MCD. IBS was strongly associated with all MCDs. The strongest association was between IBS and mast cell activation syndrome (OR 16.3; 95% CI 13.1–20.3). Odds ratios for IBS+urticaria, IBS+idiopathic urticaria, IBS+non-malignant mastocytosis and IBS+mast cell malignancy ranged from 4.5 to 9.9. Patients from each of these overlap cohorts were predominantly female, and the overlap occurred with all IBS subtypes. Thorough endoscopic evaluation and comorbid mood disorders and migraines are more common in the overlap cohorts than in IBS alone
Conclusions/Inferences:
In a large US database encompassing >53 million patients over >20 years, patients with IBS are at least 4 times more likely to have a MCD than the general population. Further study of mast cell involvement in the pathogenesis of IBS is warranted.
Keywords: irritable bowel syndrome, mast cells, mastocytosis, chronic urticaria, urticaria
Introduction:
Irritable bowel syndrome (IBS) is a heterogenous disorder of brain-gut interaction characterized by abdominal pain and altered bowel habits.1 IBS remains very common, though with the more stringent Rome IV criteria2 its prevalence is estimated at 4–5% of the population.3,4 Due to frequent office and hospital visits, diagnostic procedures and medication consumption, IBS is thought to cost the US healthcare system more than $20 billion annually.5
The pathophysiology of IBS remains incompletely understood. In part, IBS symptoms occur due to visceral hypersensitivity.6 The mechanism underlying visceral hypersensitivity and symptom production in IBS is multifactorial and likely varies between patients.7 There is growing evidence that one important mechanism of visceral hypersensitivity in IBS is the release of mast cell (MC) mediators in close proximity to the afferent nerve endings of the intestinal wall.8,9 This process may be augmented by food antigens, potentially explaining post-prandial symptoms.10 Besides the contribution to visceral hypersensitivity, MCs may also cause IBS symptoms via alterations in motility such as increased segmental bowel contractions due to depolarization of afferent myenteric neurons leading to contraction of longitudinal muscles11. The latter may lead to either constipation or diarrhea. Lastly, MCs may also contribute to increased intestinal permeability due to their effects on epithelial tight junctions12.
MCs are understood to be the primary component of the low-grade inflammatory infiltrate that has been noted in patients with IBS11. The trigger for the predominance of MCs in the gastrointestinal (GI) tract of patients with IBS requires further elucidation, but food antigens13, GI infections14 and stress-induced release of corticotropin releasing factor15 may contribute. Several studies have shown that some patients with IBS, as much as >75% of patients,9 have an increase in intestinal MCs. Increased numbers of MCs have been found throughout the bowel, including the jejunum,16 terminal ileum,17,18 colon,9,19,20 and rectum.17 While some studies have not replicated this finding,21,22 a recent meta-analysis demonstrated an increase in MC counts in the left colon of patients with IBS compared to controls.23 Further, whether or not they demonstrated an increase in MC count among IBS patients, some studies also found increased MC degranulation, including higher levels of MC mediators such as histamine and tryptase, among patients with IBS.9,22,24,25 MC stabilizers and inhibitors of MC mediators continue to be studied as therapy for IBS with mixed results.21,24,26 Common MC mediators and symptom production that has been associated with them are listed in Table 1.
Table 1.
Common Mast Cell Mediators and their Associated Symptoms.
| Mast Cell Mediator | Associated Symptoms |
|---|---|
| Tryptase | Easy bruising and bleeding, fatigue, myalgias, vertigo, flushing, diarrhea, edema |
| Histamine | urticaria, pruritis, anaphylaxis, diarrhea, angioedema, headache, hypotension |
| Proteoglycans | Bleeding |
| Prostaglandin D2 | headache, brain fog, abdominal pain, nausea, bronchoconstriction |
| Platelet activation factor | cardiac arrhythmia, bronchoconstriction, urticaria, abdominal pain |
| Interleukins | inflammation |
| Tumor necrosis factor alpha | fatigue |
| Leukotrienes | bronchoconstriction, mucous production |
Despite this growing recognition of the role of MCs in the pathogenesis of IBS symptoms, the association of IBS with disorders of MC proliferation or activity has not been studied. Mast cell disorders (MCDs) are a heterogenous group of conditions that share a similar clinical presentation of symptoms of MC activation such as urticaria, flushing, angioedema, shortness of breath, tachycardia, vomiting, and diarrhea.27 They are subdivided according to the presence or absence of a clonal population of MCs and based on the presence or absence of a trigger to MC activation. Therefore, although we use the general term MCDs to describe these disorders, they are of variable pathogenesis. In this manuscript, the term MCD is meant to encompass disorders that involve a clonal population of MCs, such as mastocytosis (MA), as well as those that involve inappropriately increased MC activation without presence of a clonal population, such as chronic spontaneous urticaria (CSU) and idiopathic MC activation syndrome (MCAS).
MCDs are traditionally subdivided into primary, idiopathic, and secondary subtypes28. Primary MCDs are disorders of increased MC activity due to a clonal population of MCs29. The most common primary MCD is MA, with an estimated prevalence of 0.01%.27 In a rare variant of MA, the clonal population of MCs can be malignant, and present as a sarcoma with infiltrative and metastatic potential,30 or a leukemia.31 The most common GI symptoms of mastocytosis are abdominal pain, bloating, nausea and diarrhea32.
Idiopathic MCDs have increased MC activity without a clonal population or increased number of MCs, and without an identifiable trigger for the MC activity. Some authors have recently suggested that the term “Non-clonal MC mediator disorders” may more effectively characterize this group of disorders28 The most common idiopathic MCDs are CSU and MCAS, though not all experts agree that CSU should fall under this category. The prevalence of CSU is approximately 0.5–1% of the population33. GI symptoms are similar to MA, but gastroesophageal reflux disease (GERD) symptoms have been demonstrated to be especially common in this population34. While MCAS is thought to be a common and underdiagnosed disorder35, its exact prevalence remains unknown36. In CSU, the cutaneous symptom of urticaria predominates the clinical presentation, whereas in MCAS, the symptoms are typically systemic and include flushing, hypotension, and GI discomfort.37
Using a large database, verified by manual chart review, we determined the association between IBS and the primary and idiopathic MCDs. We also characterized the demographics, comorbidities, and pharmacologic and procedural exposures of the patients with both disorders.
Materials and Methods:
Database
We retrospectively analyzed a large commercial database (IBM Watson Health Explorys Inc, Somers, NY). The Explorys database is an aggregate of de-identified electronic health record (EHR) data from dozens of US healthcare systems. The database collects several categories of data including past medical history, vital signs, findings, procedures, encounter diagnoses and medications prescribed. It is programmed to map encounter diagnosis, typically entered in the underlying EHR as international classification of disease (ICD) codes to the Systematized Nomenclature of Medicine – Clinical Terms (SNOMED–CT) codes. Explorys data is automatically updated from the underlaying EHRs frequently, typically once per day.38 Explorys is a Health Insurance Portability and Accountability Act (HIPAA) and Health Information Technology for Economic and Clinical Health (HITECH) compliant platform.39 To protect patient identities,, Explorys rounds all counts to the nearest 10, and treats all cell counts <10 as equivalent to zero.
Patient selection
We used the search tool on the Explorys database to identify an aggregated cohort of adult patients, ≥ 18 years, diagnosed with IBS and one of the MCDs from 1999–2020. In order to include all available MCDs in the Explorys database, the browse diagnosis feature in the Explore module of Explorys was used with the SNOMED-CT term “MCD”. The “Browse more specific term” feature identified three more specific SNOMED-CT terms encompassed within the term “MCD”: “Mast cell malignancy (MCM)”, “Non malignant MC disease”, and “Systemic MC disease”. Using the “Browse more specific” feature to further search the SNOMED-CT hierarchy on each of these terms individually, we found “Mast cell malignancy” encompassed the specific disorders “Malignant MC tumor”, and “MC leukemia” as even more specific SNOMED-CT terms. As both of these are primary MCDs with a clonal population of MCs, we chose to keep these grouped together in one cohort by using the more general SNOMED-CT search term of “MCM”.
Next, the SNOMED-CT term “Non malignant MC disease” encompassed the more specific SNOMED-CT terms “Cutaneous MA”, and “Indolent systemic MA”. Lastly, the SNOMED-CT term “Systemic MC disease” encompassed the more specific SNOMED-CT terms “Systemic MA with associated clonal hematological non-MC lineage disease”, and “Aggressive lymphadenopathic MA with eosinophilia”. We excluded the former as it related to a non-MC lineage disease. We decided to create one search combining the remaining three specific SNOMED-CT terms (“Cutaneous MA”, “Indolent systemic MA”, and “Aggressive lymphadenopathic MA with eosinophilia”), as these are all forms of non-malignant mastocytosis. We labeled this group “Non-malignant MA” (NMM). Thus, the MCM and NMM groups encompass all primary MCDs available in the Explorys database.
After deciding on these SNOMED-CT search terms for the primary MCDs, we determined appropriate SNOMED-CT search terms for the most common idiopathic MCD, CSU. Since there is no SNOMED-CT diagnosis codes for “CSU” or “chronic idiopathic urticaria (IU),” we created two separate searches for comparison, using the more sensitive SNOMED-CT diagnosis “urticaria”, and the more specific diagnosis “IU” while excluding all patients with SNOMED-CT diagnosis codes “acute urticaria”, “urticaria pigmentosa”, “urticaria medicamentosa”, and “papular dermatitis of pregnancy” from both searches. In order to prevent any overlap of patients between cohorts, when performing each search we excluded all patients from the other cohorts. For example, when performing the search for MCM, all patients who met inclusion criteria for NMM or “urticaria” were excluded.
For all searches, we used the exclusion attributes feature of Explorys to exclude patients with any disorder known to cause secondary MCD that had a SNOMED-CT diagnosis, including “inflammatory bowel disease”, “celiac disease”, “hyperthyroidism”, “hypothyroidism”, “systemic lupus erythematosus”, “Sjogren’s syndrome”, “rheumatoid arthritis”, “cryoglobulinemia”, “cryopyrin associated periodic syndrome”, “autoimmune vasculitis,” “autoimmune skin disease,” and “autoimmune connective tissue disorder”.
As there is neither a SNOMED-CT diagnosis code for MCAS nor a way to approximate it using other SNOMED-CT diagnosis codes, we identified patients with IBS and MCAS codes as encounter diagnoses by searching SlicerDicer, a self-service tool for obtaining de-identified customized EHR data in the Epic EHR, from two local academic centers during the same time period. We applied the same exclusion criteria as in the Explorys searches.
Using the above searches, we used the population attributes feature of Explorys to create five distinct aggregated cohorts (Figure 1):
Figure 1.
Search methodology to arrive at final overlap cohorts.
patients with IBS and MCM (IBS+MCM)
patients with IBS and NMM (IBS+NMM)
patients with IBS and idiopathic urticaria (IBS+IU)
patients with IBS and urticaria (IBS+U)
patients with IBS and MCAS (IBS+MCAS)
A list of SNOMED-CT codes and corresponding ICD-9 and ICD-10 codes used in our study is available in Table 2.
Table 2:
SNOMED-CT and corresponding ICD-9 and ICD-10 Codes.
| Disease | SNOMED-CT code | ICD-9 code | ICD-10 code |
|---|---|---|---|
| Irritable Bowel Syndrome | 10743008 | 564.1 | K58 |
| Urticaria | 126485001 | 708.9 | L50.0-L50.9 |
| Idiopathic Urticaria | 42265009 | 708.1 | L50.1 |
| Mast Cell Malignancy | 397009000 | 202.6 | C96.2 |
| Non-malignant Mastocytosis | 397008008, 70910003, 397012002 | N/A | D47.01, D47.02 |
| Mast Cell Activation Syndrome | N/A | N/A | D89.42 |
SNOMED-CT Systematized nomenclature of medicine - Clinical terms. ICD International classification of disease.
Data collection
The descriptive data of each cohort except IBS+MCAS was collected using the Explore browse cohort feature of Explorys. The descriptive data of the IBS+MCAS cohort was obtained from Epic’s SlicerDicer tool. We collected demographic information such as gender, race, type of health insurance, and obesity status. The latter was defined by body mass index (BMI) 30–34.99 documented as a vital sign. The full range of BMI categories was not collected as some patients may be documented in multiple BMI categories as their BMI changes over time, which would make the results difficult to interpret. We attempted to determine the prevalence of each IBS subtype among the cohorts by browsing each cohort for prevalence of symptoms diarrhea and constipation. We also collected procedural history - such as history of endoscopic procedures, allergy testing, and skin biopsies - all procedures pertinent to IBS and MCDs. Additionally, we collected medication exposures, with a focus on medications typically used to manage GI symptoms and symptoms of MC activation. Finally, we determined the prevalence in each cohort of comorbidities known to be associated with IBS such as mood disorders, migraine headaches, interstitial cystitis and fibromyalgia,40
We also obtained data for the IBS+MCAS cohort using more detailed, patient-identifiable data from one of our institutions. With institutional review board (IRB) approval, we obtained a list of all patients whose provider entered the ICD-10 code for MCAS (D89.42) as a diagnostic code for a clinical encounter at one of our institutions from January 2017 to December 2019. We manually reviewed the charts of these patients to search for comorbid IBS. We performed further chart review of those with IBS to characterize their clinical features such as their IBS subtype, their most prominent symptoms of IBS and of their MCD, allergies, comorbid conditions (including psychiatric illness, atopic and allergic disorders, autoimmune diseases, pulmonary disease, and other GI diseases).
Statistical Analysis:
We calculated period prevalence by dividing the aggregate number in the cohort by the total number of patients in the database. The odds ratio (OR) and 95% confidence interval (CI) were calculated using the MedCalc statistical software41 for a case-control design.
We performed bivariate analysis to compare the demographics, procedure history, medication exposures and comorbid conditions of each of the five cohorts to the remainder of the total IBS population, using the Pearson Chi-square test. Statistical significance was assumed for two-tailed alpha of p<0.05.
Verification of Data:
We used manual chart review when possible to verify the database data. For the four cohorts identified on Explorys (IBS+MCM, IBS+NMM, IBS+IU, IBS+U), we used the identical search criteria to identify the aggregated cohorts limited to only patients from each of our institutions. With IRB approval, we used Explorys to identify the patients at our own institutions meeting our search criteria. We then performed manual chart review on these patients to confirm the diagnoses of IBS and MCD, characterized patients by the same clinical features outlined above for the manually reviewed patients with IBS and MCAS. Patients were considered confirmed to have IBS if the primary care provider (PCP) or gastroenterologist listed this encounter diagnosis in the assessment and plan section of an EHR note and provided any management plan. An encounter diagnosis of MCD was confirmed the same way, only the documentation must have been documented by a PCP, dermatologist, allergist/immunologist, or rheumatologist.
IRB approval was obtained individually from each institution for both the database and manual chart review components of the study.
Results
There were over 58.6 million adult patients in the Explorys database during the study period. After incorporating our exclusion criteria, there were 54.3 million eligible patients. Of these, 623,450 (1.1%) had IBS, and 488,390 (0.9%) had a MCD (urticaria/MA/MCM), the overwhelming majority of whom had urticaria (N= 486,720; 0.9%). 0.04% (N=23,590) of all patients had both IBS and at least one MCD (urticaria/MA/MCM), meaning that nearly 4% of IBS patients had an overlapping MCD. When urticaria was replaced with IU (IU/MA/MCM) 39,900 (0.07%) patients had a MCD, and 2,320 (0.004%) had both IBS and a MCD. Per this search, 0.4% of the IBS population have an overlapping MCD (IU/MA/MCM).
Of the approximately 8 million adults in SlicerDicer, 67,924 (0.8%) had IBS. Of the IBS population, 92 (0.1%) patients had overlapping MCAS.
We created four overlap cohorts from the Explorys data: IBS+U (N=23,440), IBS+IU (N=2170), IBS+MA (N=90), IBS+MCM (N=60). We created the fifth overlap cohort, IBS+MCAS (N=92) from SlicerDicer. There was a strong association between IBS and all 5 MCDs (Table 3). The strongest association was between IBS and MCAS (OR 16.3; 95% CI 13.1–20.3). The weakest association, between IBS and urticaria (OR 4.5; 95% CI 4.3–4.5), still had an odds ratio (OR)>4.0. The ORs for IBS+IU, IBS+MA and IBS+MCM ranged from 5.2 to 9.9.
Table 3.
Association of MCDs with IBS.
| MCD | Population of MCD with IBS N(%) | Total IBS without MCD N(%) | Population of MCD Without IBS N(%) | Total population without IBS or MCD N(%) | OR (95% CI) |
|---|---|---|---|---|---|
| Urticaria | 23,440 (4.8) | 600,010 (96.2) | 463,280 (95.2) | 53,187,850 (98.0) | 4.5 (4.3–4.5) |
| MCM | 60 (7.5) | 623,390 (100.0) | 740 (92.5) | 53,654,150 (98.8) | 7.0 (5.4–9.1) |
| NMM | 90 (10.3) | 623,360 (100.0) | 780 (89.7) | 53,654,110 (98.8) | 9.9 (8.0–12.4) |
| IU | 2,170 (5.7) | 621,280 (99.7) | 36,060 (94.3) | 53,618,770 (98.7) | 5.2 (5.0–5.4) |
| MCAS | 92 (12.3) | 67,832 (99.9) | 655 (87.7) | 7,861,523 (99.1) | 16.3 (13.1–20.3) |
MCD - mast cell disorder; IBS - irritable bowel syndrome; MCM - mast cell malignancy; NMM - Non-malignant mastocytosis; IU - idiopathic urticaria; MCAS - mast cell activation syndrome
Diagnostic codes for any IBS subtype were infrequently found on Explorys and thus the true frequency of the IBS subtypes in each of our cohorts is unknown. However, with the exception of the IBS+MCAS cohort, patients in most of the overlapping cohorts were more likely to have diarrhea than constipation (Table 4). Besides the IBS+MCM cohort, most of the overlap cohorts had a strong female predominance, most carried private insurance, and many were obese (Table 5).
Table 4.
Prevalence of Diarrhea and Constipation in the overlap cohorts.
| IBS-related symptom | IBS+ Urticaria (N=23,440); N (%) | IBS+MCM (N=60); N (%) | IBS+NMM (N=90); N (%) | IBS+IU (N=2170); N(%) | IBS+MCAS (N=90); N(%) |
|---|---|---|---|---|---|
| Diarrhea | 11,540 (49) | 30 (50) | 40 (44) | 1,050 (48) | 20 (22) |
| Constipation | 8,090 (35) | 20 (33) | 30 (33) | 700 (32) | 26 (29) |
MCD mast cell disorder; IBS irritable bowel syndrome; MCM mast cell malignancy; NMM Non-malignant mastocytosis; IU idiopathic urticaria; MCAS mast cell activation syndrome
Table 5.
Demographic features of the overlap cohorts.
| Variable | IBS+ Urticaria (N=23440); N (%) | IBS+MCM (N=60); N (%) | IBS+NMM (N=90); N (%) | IBS+IU (N=2170); N(%) | IBS+MCAS (N=92); N (%) | Other IBS (N=599500); N (%) |
|---|---|---|---|---|---|---|
| Female | 19470 (83) | 40 (67) | 70 (78) | 1820 (84) | 78 (85) | 442770 (74) |
| Caucasian | 19080 (81) | 40 (67) | 80 (89) | 1740 (80) | 80 (87) | 488330 (81) |
| African American | 2440 (10) | 0 (0) | 0 (0) | 240 (11) | 4 (4) | 44190 (7) |
| Race unknown | 2940 (13) | 20 (33) | 10 (11) | 300 (14) | 0 | 71840 (12) |
| Medicare + Medicaid | 8040 (35) | 20 (33) | 20 (22) | 690 (32) | 25 (27) | 1848300 (30) |
| Private | 15380 (66) | 30 (50) | 60 (67) | 1520 (70) | n/a | 344270 (57) |
| Obese | 10130 (43) | 20 (33) | 30 (33) | 940 (43) | 60 (65) | 193180 (32) |
IBS irritable bowel syndrome; MCM mast cell malignancy; NMM Non-malignant mastocytosis; IU idiopathic urticaria; MCAS mast cell activation syndrome
Compared to the total population of IBS patients without MCDs (N=599,500), atopic and allergic diseases were more common in the overlap patients, as was antihistamine use (Table 6). Interestingly, comorbid mood disorders and history of migraines were more common in the overlap patients than the general IBS population (Table 6). Fibromyalgia was not well-documented on Explorys and due to extremely low reported prevalence could not be included in the analysis. Antidiarrheal use was low across all cohorts, while laxative use was high, especially among the overlap cohorts besides for IBS+MCAS (Table 6). Lastly the patients with IBS and MCD were more likely to have undergone endoscopic evaluation and have endoscopic biopsies taken compared to the remainder of the IBS population, though this was poorly reported for the IBS+MCAS cohort via SlicerDicer (Table 6).
Table 6.
Prevalence of clinical features of the overlap cohorts in aggregate, compared to the population of IBS with no overlapping MCD
| Variables | IBS+ Urticaria (N=23340); N (%) | IBS+MCM (N=60); N (%) | IBS+NMM (N=90); N (%) | IBS+IU (N=2170); N (%) | IBS+MCAS (N=92); N(%) | IBS without MCD (N=599,500); N (%) | |
|---|---|---|---|---|---|---|---|
| ComorbidCondition | Contact dermatitis | 9130 (39) (P<0.0001) * | 10 (17) | 20 (22) | 840 (39) * | 12 (13) | 84230 (14) |
| Allergic rhinitis | 11280 (48) * | 20 (33) * | 40 (44) * | 1210 (56) * | 36 (39) * | 149650 (25) | |
| Eczema | 9230 (39) * | 10 (17) * | 20 (22) | 870 (40) * | 12 (13) | 84890 (14) | |
| Allergic asthma (IgE mediated) | 1210 (5) * | <10 (<17) | <10 (<11) | 140 (6) | N/A | 11100 (2) | |
| Asthma | 7930 (34) * | 30 (50) * | 30 (33) * | 770 (35) * | 34 (37) * | 120440 (20) | |
| Food allergy | 3770 (16) * | 20 (33) * | 20 (22) * | 420 (19) * | 24 (26) * | 45230 (8) | |
| Drug allergy | 15560 (66) * | 50 (83) * | 60 (67) * | 1400 (65) * | 26 (28) * | 300600 (50) | |
| Angioedema | 1430 (6) * | <10 (<17) | 10 (11) | 310 (14) * | 7 (8) * | 4282 (<1) | |
| Major depressive disorder | 9010 (38) * | 20 (33) * | 30 (33) * | 850 (39) * | 35 (38) * | 159680 (27) | |
| Anxiety disorder | 12420 (53) * | 30 (50) * | 50 (56) * | 1150 (53) * | 58 (63) * | 219410 (37) | |
| Migraine | 6930 (30) * | 20 (33) * | 30 (33) * | 640 (29) * | 39 (42) * | 105560(18) | |
| Interstitial cystitis | 510 (2) * | 0 (0) | <10 (<11) | 50 (2) | N/A | 7280 (1) | |
| Procedure History | Delayed hypersensitivity skin test | 3160 (13) * | <10 (<17) | <10 (<11) | 370 (17) | N/A | 31530 (5) |
| Food IgE level | 540 (2) * | 0 (0) | 0 (0) | 90 (4) * | 0 (0) | 2070 (<1) | |
| Food IgG level | 110 (0) | <10 (<17) | 0 (0) | <10 (<1) | 0 (0) | 1210 (0) | |
| Colonoscopy | 11690 (50) * | 20 (33) | 30 (33) | 1110 (51) * | 5 (5) | 233060 (39) | |
| Upper endoscopy | 8120 (35) * | 30 (50) * | 30 (33) * | 790 (36) * | 0 (0) | 144510 (24) | |
| Biopsy of skin | 2630 (11) * | <10 (<17) | 10 (11) * | 320 (15) * | 0 (0) | 29510 (5) | |
| Endoscopic biopsy | 11250 (48) * | 30 (50) * | 30 (33) | 1100 (51) * | 0 (0) | 203950 (34) | |
| Medication Exposures | MC stabilizer | 1730 (7) * | 20 (33) * | 20 (22) * | 200 (9) * | 5 (5) | 16360 (3) |
| Antispasmodic | 13260 (57) * | 30 (50) | 40 (44) | 1200 (55) * | 0 (0) | 258910 (43) | |
| Antidiarrheal | 3140 (13) * | <10 (<17) | <10 (<11) | 270 (12) | 2 (2) * | 57980 (10) | |
| Prokinetic | 6170 (26) * | 20 (33) | 20 (22) | 530 (24) * | 2 (2) * | 98150 (16) | |
| Laxative | 13230 (56) * | 30 (50) * | 50 (56) * | 1180 (54) * | 16 (17) * | 258340 (43) | |
| Antiemetic | 15760 (67) * | 40 (67) | 50 (56) | 1390 (64) * | 17 (18) * | 310950 (52) | |
| Antihistamine | 20270 (86) * | 50 (83) * | 80 (89) * | 1920 (88) * | 20 (22) * | 383440 (64) | |
and bold font indicates P<0.05 for the difference between the overlap IBS+MCD cohort compared to IBS without MCD
MCD mast cell disorder; IBS irritable bowel syndrome; MCM mast cell malignancy; NMM Non-malignant mastocytosis; IU idiopathic urticaria; MCAS mast cell activation syndrome
Manual chart review
We identified 50 patients from one institution in the IBS+U cohort. Through manual chart review we were able to confirm 39 of the 50 had IBS based on our criteria, and 9 of the 50 were diagnosed with both IBS and an urticarial disorder. 3 of these 9 had forms of acute urticaria, while 6 had chronic urticaria. Of these 6 patients, 4 were female, 3 had IBS-D, 4 were diagnosed with IBS prior to developing urticaria, and 5 had comorbid mood disorders (Supplementary Table 1). Several patients had comorbid atopic diseases, migraines and GERD. Etiology of the chronic urticaria was idiopathic in 4 of the 6 patients, while one was cold-induced and one was thought to be autoimmune (Supplementary table 1). Nearly all of the remaining 41 patients who did not have an urticarial disorder had urticaria as a drug reaction.
We identified one patient from the same institution in the IBS+IU cohort. The other institution had 90 patients from the IBS+IU cohort. 20 of these were randomly selected for manual chart review, making a total of 21 IBS+IU patients manually reviewed (Supplementary Table 2). All 21 of them had true IBS and chronic IU. 8 of 21 had IBS-C, 7 had IBS-D and 6 had IBS-M. Many had multiple allergies and comorbid atopic disorders, while less had mood disorders, fibromyalgia and migraines.
The IBS+MA and IBS+MCM cohorts contained no patients from our institutions so manual chart review could not be performed.
Verification of data by manual chart review for the IBS+MCAS cohort from SlicerDicer was not completed due to a very low number of patients identified from our institution. Instead, we determined that 8 patients from one of our institutions were given the diagnostic code for MCAS over the study period. Manual chart review on these 8 patients revealed that 2 of them had comorbid IBS. One had IBS-D and one had IBS-C. Comorbid mood disorders, migraines and fibromyalgia were again identified, and drug and other allergies were prominent (Table 7).
Table 7.
Features of the IBS+MCAS patients identified via manual chart review.
| Pt number | Gender | IBS subtype | IBS symptoms | MCAS symptoms | Allergies | comorbidities | Order of presentation |
|---|---|---|---|---|---|---|---|
| 1 | female | IBS-D | abdominal pain, diarrhea | flushing | animal dander, dust, mold, pollen, corn, eggs, nut, soy. Mild- chamomile, dairy, gluten, amoxicillin, anefrin, bupivicaine, demerol, erythromycin, lidocaine, marcaine, meperidine, sulfa drugs | Sjogren’s syndrome, anemia, bronchitis | IBS first |
| 6 | female | IBS-C | hard stools, bleeding hemorrhoids | hives, angioedema, flushing | codeine, latex | anxiety, asthma, bipolar, fibromyalgia, GERD, migraine, Pott’s, Ehlers Danlos syndrome | IBS first |
IBS irritable bowel syndrome; IBS-D irritable bowel syndrome diarrhea; IBS-C irritable bowel syndrome constipation; MCAS mast cell activation syndrome; GERD gastroesophageal reflux disease
Discussion:
This is the first study to demonstrate a strong association between IBS and the primary and idiopathic MCDs. Primary MCDs occur due to a defect in the MC progenitor. This leads to uncontrolled production or activation of a clonal population of MCs that accumulates in the bone marrow and other tissues.27 When the accumulation is limited to the skin it is characterized as cutaneous, and when they accumulate in other tissues as well it is termed systemic. These are primarily diagnosed by histologic examination of the involved organ systems.42 On the other hand, idiopathic MCDs are defined by the presence of episodic symptoms of increased MC activation without a demonstrable clonal population of MCs or trigger of MC activation.
Secondary MCDs are defined by an increase in the production or activation of MCs that is triggered by a separate disease process.37 These are the most common forms of MCDs and can occur due to a variety of autoimmune, allergic, neoplastic, and environmental triggers.37 Due to confounding of the underlying etiologic disease, we excluded patients with these diagnoses from our study.
This newly discovered association between IBS and primary and idiopathic MCDs may support the growing body of evidence implicating MCs in the pathogenesis of IBS symptoms, particularly abdominal pain and visceral hypersensitivity. Our findings also suggest that the overlap with MCDs occurs with all subtypes of IBS, whether diarrhea or constipation predominant. This is an important finding since it has previously been demonstrated that MC numbers are increased in both IBS-D and IBS-C.23 The notion that MCs’ greatest impact on the pathophysiology of IBS is in the production of abdominal pain and visceral hypersensitivity without a prominent effect on gut motility8 is also consistent with our findings.
As expected, we found that patients with IBS and MCD were more likely to have other atopic diseases and were more likely to be prescribed antihistamines and MC stabilizers, compared to the remainder of the IBS population. More intriguing was the finding that comorbid mood disorders and migraines were more common in those with IBS and a MCD. This is not altogether surprising since MCs are present in large numbers in the brain and are implicated in psychiatric and neurologic disorders.43,44 In particular, MCDs have been strongly associated with autism spectrum disorder (ASD)28. MCDs may also present with neurologic symptoms that can mimic other neurologic or psychiatric disorders28. Further research is needed to better elucidate the interplay between MC activity in the brain and GI disorders.
Our study also showed that patients with IBS and MCDs were more likely to undergo endoscopic procedures and have endoscopic biopsies compared to the remainder of the IBS population. Part of this may be explained by the need for intestinal biopsies to diagnose or rule out intestinal involvement of the primary MCDs. When a MCD is suspected there is more likely to be an indication for endoscopic biopsy. However, this would not be relevant for the urticarial disorders, suggesting there may be another yet unknown explanation for the increased need for endoscopic biopsies in this cohort.
Ours is not the first study to investigate the association between IBS and urticaria. One Turkish study questioned a small number of patients with urticaria and healthy controls for symptoms of IBS based on Rome III criteria, and found that IBS was at least twice as common in patients with urticaria compared to controls.45 In our study the association was stronger, with IBS approximately 4–5 times as common in patients with urticaria. A larger scale study of over 11,000 patients that, like our study, was verified with manual chart review also found a positive association between IBS and urticaria.46
Atopic disease has also been associated with IBS. Several studies have shown a higher prevalence of IBS among patients with atopic dermatitis and asthma.47 48 49 A questionnaire study found the likelihood of IBS diagnosis ranged 2.5–4.0 times higher in patients with asthma, allergic eczema and allergic rhinitis.40 A recent meta-analysis of all studies investigating the association between IBS and asthma and found a positive association, though the OR was <2.0.50 The strength of association of IBS and these atopic disorders is closest to that of IBS and urticaria in our study, though our study shows an even stronger association. The association we found between IBS and the other primary and idiopathic MCDs is larger than anything that has been previously reported between IBS and urticarial or atopic disorders.
None of these other studies investigated the association of IBS with primary MCDs or MCAS. Most of these prior studies were not of the US population, and most were small scale. The major strength of our study is the use of a large database involving hospital systems throughout the United States. Use of a large database is crucial in studying these associations involving several rare MCDs that would not have been possible to detect in sufficient numbers via manual chart review. Our study is also more robust than a typical database study due to our extensive use of exclusion criteria, our use of two different databases, our verification with manual chart review, and our development of a small IBS+MCAS cohort obtained completely through manual chart review from one of our institutions. The finding of a strong association between IBS and the primary and idiopathic MCDs was consistent across all of these methods of data collection.
One potential complicating factor is the fact that GI symptoms are a known part of the clinical presentation of MCDs. This raises the possibility that some of the overlap patients diagnosed with IBS may actually have had GI symptoms related to their MCD and were misdiagnosed with IBS. In this evolving field, further research is needed to clarify whether the MCD induces symptoms of IBS, whether they are two distinct disorders, or a combination of both. We attempted to resolve this issue during manual chart review by noting which disorder presented first, assuming that a diagnosis of IBS after a diagnosis of MCD had already been established would increase the likelihood that they are distinct disorders. We found that the majority of patients reviewed were diagnosed with IBS prior to MCD, leaving this question unresolved. We did also attempt to determine whether IBS symptoms responded to treatment of MCD but the overwhelming majority of patients were treated for IBS with a different provider than the one who treated the MCD, and almost none acknowledged any connection between the two disorders. Although this left us with insufficient data to answer this question, it highlights the need for further studies, such as ours, to not only answer these important questions, but to raise awareness of the association between these disorders.
We acknowledge that our study has limitations. Data from the Explorys database relies on encounter diagnostic coding from the encounter provider, rather than true diagnostic criteria, and is therefore susceptible to inaccuracy. Data obtained from SlicerDicer is at risk for similar inaccuracies. The prevalence of IBS, for example, across both of our databases was approximately 1%, substantially lower than its true prevalence. This is likely explained by the notion that only a portion of patients with IBS will have a medical encounter that results in the provider entering a diagnostic code of IBS for that encounter. The same would apply to MCDs and therefore we do not suspect that this limitation affected the veracity of our results. In fact, the mastocytosis cohort in this study made up a much smaller portion of the total population than the reported prevalence in the literature (0.0016% vs. 0.01%). Similarly, the prevalence of IU was 0.07% yet the reported prevalence of CSU is 0.5–1%. Further, we attempted to combat the possibility of inaccuracy by using robust exclusion criteria for our searches in order to eliminate patients with an organic GI disorder who were misdiagnosed with IBS, and patients with MC proliferation due to secondary causes. We also combatted this limitation by verifying data using manual chart review on patients who came from our institutions. Still, given the retrospective nature of the manual chart review component of the study, it is difficult to confirm these diagnoses with absolute certainty. This is especially true for the diagnosis of IBS given the evolving nature of diagnostic criteria for IBS over the two decades of our study period. There is also a possibility that a provider could enter a diagnostic code of IBS for a patient who does not meet diagnostic criteria for IBS, while this is less likely for the more specific and rare MCDs. This may be a confounding factor in our study as well.
The proportion of African American patients in the database and across all of our cohorts was lower than expected. It is possible this is due to disparities in access to healthcare, as patients must have had an encounter that resulted in a diagnostic code in a hospital system that contributes to the Explorys database to be included. Most hospitals that contribute are tertiary care centers.
Findings on manual chart review of the IBS+U cohort seemed disappointing. 11 of the 50 patients (22%) reviewed could not be confirmed to have IBS, meaning that the diagnosis of IBS was not specifically addressed by a gastroenterology or primary care provider in a note in the EHR. While this does not mean the diagnosis obtained from the Explorys database was incorrect, the documentation in our institution’s EHR was insufficient to confirm this diagnosis. This decreased the size of the cohort of patients reviewed manually, which is another limitation of the study.
Furthermore, when using “urticaria” as the search term (IBS+U), many patients captured did not have chronic idiopathic urticaria, but rather an urticarial drug reaction. This may suggest an increased risk of allergic drug reactions in patients with IBS, but did not allow for verification of our database results. When the search term “idiopathic urticaria” was used (IBS+IU), many fewer patients were captured, but all had IBS and chronic idiopathic urticaria. Therefore, while “urticaria” was sensitive but not specific enough, and “idiopathic urticaria” was specific but perhaps not sensitive enough, each had similar strong association with IBS. The combination of the two search terms does verify our findings for IBS and chronic idiopathic urticaria. Furthermore, since the database results are more likely to be accurate the more specific the diagnosis, verification by manual chart review is less crucial to confirm the accuracy of the strong association found between IBS+NMM and IBS+MCM. Therefore, that a lack of patients from our institutions prevented the use of manual chart review to verify the diagnoses of patients in these cohorts is not a major deficiency.
We also acknowledge that diagnostic criteria for MCDs, as well as their nomenclature and categorization are complex, have evolved over time, and may be described differently by different specialties or experts. While we do believe the MCDs included in this study covers the spectrum of MCDs, we recognize the possibility that there may be other MCDs not included in this study.
In conclusion, while the association of IBS to other atopic diseases has been previously demonstrated, we have shown a novel and very strong association between IBS and the primary and idiopathic MCDs, especially among Caucasian women. Further studies are needed to confirm that this association extends populations with a higher proportion of African Americans. This association lends credence to the role of MCs in the pathogenesis of IBS symptoms. We discovered that many providers are unaware of this association and IBS and MCDs are often treated independently without regard for possible overlapping symptomatology or response to treatment. Patients with IBS and suggestive symptoms should undergo investigation for a MCD, and further studies of MC inhibitors for the treatment of IBS are warranted. Moreover, special care should be taken to screen patients with both IBS and a MCD for comorbid mood disorders and migraines, as they are more prevalent in this cohort than the IBS population without a MCD.
Supplementary Material
Supplementary Table 1. Features of the IBS+U cohort by manual chart review.
Supplementary Table 2. Features of the IBS+IU patients identified via manual. chart review
Footnotes
Disclosures: None of the authors have any financial disclosures or conflicts of interest
Data Availability Statement:
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- 1.Lacy BE, Pimentel M, Brenner DM, et al. ACG clinical guideline: management of irritable bowel syndrome. Am J Gastroenterol. 2021;116(1):17–44. doi: 10.14309/ajg.0000000000001036 [DOI] [PubMed] [Google Scholar]
- 2.Mearin F, Lacy BE, Chang L, et al. Bowel Disorders. Gastroenterology. 2016. Feb 18;S0016–5085(16)00222–5. doi: 10.1053/j.gastro.2016.02.031 [DOI] [PubMed] [Google Scholar]
- 3.Palsson OS, Whitehead W, Törnblom H, Sperber AD, Simren M. Prevalence of rome IV functional bowel disorders among adults in the united states, canada, and the united kingdom. Gastroenterology. 2020;158(5):1262–1273.e3. doi: 10.1053/j.gastro.2019.12.021 [DOI] [PubMed] [Google Scholar]
- 4.Sperber AD, Bangdiwala SI, Drossman DA, et al. Worldwide prevalence and burden of functional gastrointestinal disorders, results of rome foundation global study. Gastroenterology. 2021;160(1):99–114.e3. doi: 10.1053/j.gastro.2020.04.014 [DOI] [PubMed] [Google Scholar]
- 5.American College of Gastroenterology Task Force on Irritable Bowel Syndrome, Brandt LJ, Chey WD, et al. An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol. 2009;104 Suppl 1:S1–35. doi: 10.1038/ajg.2008.122 [DOI] [PubMed] [Google Scholar]
- 6.Delvaux M Role of visceral sensitivity in the pathophysiology of irritable bowel syndrome. Gut. 2002;51 Suppl 1:i67–71. doi: 10.1136/gut.51.suppl_1.i67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allescher H-D, Storr M Is irritable bowel syndrome a rhinitis of the gut? Gastroenterology. 2011;140(7):2132–2136; discussion 2136. doi: 10.1053/j.gastro.2011.04.019 [DOI] [PubMed] [Google Scholar]
- 8.Wouters MM, Balemans D, Van Wanrooy S, et al. Histamine Receptor H1-Mediated Sensitization of TRPV1 Mediates Visceral Hypersensitivity and Symptoms in Patients With Irritable Bowel Syndrome. Gastroenterology. 2016;150(4):875–87.e9. doi: 10.1053/j.gastro.2015.12.034 [DOI] [PubMed] [Google Scholar]
- 9.Barbara G, Stanghellini V, De Giorgio R, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126(3):693–702. doi: 10.1053/j.gastro.2003.11.055 [DOI] [PubMed] [Google Scholar]
- 10.Aguilera-Lizarraga J, Florens MV, Viola MF, et al. Local immune response to food antigens drives meal-induced abdominal pain. Nature. 2021;590(7844):151–156. doi: 10.1038/s41586-020-03118-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wouters MM, Vicario M, Santos J. The role of mast cells in functional GI disorders. Gut. 2016;65(1):155–168. doi: 10.1136/gutjnl-2015-309151 [DOI] [PubMed] [Google Scholar]
- 12.Martínez C, Vicario M, Ramos L, et al. The jejunum of diarrhea-predominant irritable bowel syndrome shows molecular alterations in the tight junction signaling pathway that are associated with mucosal pathobiology and clinical manifestations. Am J Gastroenterol. 2012;107(5):736–746. doi: 10.1038/ajg.2011.472 [DOI] [PubMed] [Google Scholar]
- 13.Fritscher-Ravens A, Schuppan D, Ellrichmann M, et al. Confocal endomicroscopy shows food-associated changes in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology. 2014;147(5):1012–20.e4. doi: 10.1053/j.gastro.2014.07.046 [DOI] [PubMed] [Google Scholar]
- 14.Vicario M, González-Castro AM, Martínez C, et al. Increased humoral immunity in the jejunum of diarrhoea-predominant irritable bowel syndrome associated with clinical manifestations. Gut. 2015;64(9):1379–1388. doi: 10.1136/gutjnl-2013-306236 [DOI] [PubMed] [Google Scholar]
- 15.Alonso C, Guilarte M, Vicario M, et al. Acute experimental stress evokes a differential gender-determined increase in human intestinal macromolecular permeability. Neurogastroenterol Motil. 2012;24(8):740–746, e348. doi: 10.1111/j.1365-2982.2012.01928.x [DOI] [PubMed] [Google Scholar]
- 16.Guilarte M, Santos J, de Torres I, et al. Diarrhoea-predominant IBS patients show mast cell activation and hyperplasia in the jejunum. Gut. 2007;56(2):203–209. doi: 10.1136/gut.2006.100594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park JH, Rhee P-L, Kim HS, et al. Mucosal mast cell counts correlate with visceral hypersensitivity in patients with diarrhea predominant irritable bowel syndrome. J Gastroenterol Hepatol. 2006;21(1 Pt 1):71–78. doi: 10.1111/j.1440-1746.2005.04143.x [DOI] [PubMed] [Google Scholar]
- 18.Wang LH, Fang XC, Pan GZ. Bacillary dysentery as a causative factor of irritable bowel syndrome and its pathogenesis. Gut. 2004;53(8):1096–1101. doi: 10.1136/gut.2003.021154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Sullivan M, Clayton N, Breslin NP, et al. Increased mast cells in the irritable bowel syndrome. Neurogastroenterol Motil. 2000;12(5):449–457. doi: 10.1046/j.1365-2982.2000.00221.x [DOI] [PubMed] [Google Scholar]
- 20.Piche T, Saint-Paul MC, Dainese R, et al. Mast cells and cellularity of the colonic mucosa correlated with fatigue and depression in irritable bowel syndrome. Gut. 2008;57(4):468–473. doi: 10.1136/gut.2007.127068 [DOI] [PubMed] [Google Scholar]
- 21.Klooker TK, Braak B, Koopman KE, et al. The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut. 2010;59(9):1213–1221. doi: 10.1136/gut.2010.213108 [DOI] [PubMed] [Google Scholar]
- 22.Lee H, Park JH, Park DI, et al. Mucosal mast cell count is associated with intestinal permeability in patients with diarrhea predominant irritable bowel syndrome. J Neurogastroenterol Motil. 2013;19(2):244–250. doi: 10.5056/jnm.2013.19.2.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bashashati M, Moossavi S, Cremon C, et al. Colonic immune cells in irritable bowel syndrome: A systematic review and meta-analysis. Neurogastroenterol Motil. 2018;30(1). doi: 10.1111/nmo.13192 [DOI] [PubMed] [Google Scholar]
- 24.Lobo B, Ramos L, Martínez C, et al. Downregulation of mucosal mast cell activation and immune response in diarrhoea-irritable bowel syndrome by oral disodium cromoglycate: A pilot study. United European Gastroenterol J. 2017;5(6):887–897. doi: 10.1177/2050640617691690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buhner S, Li Q, Vignali S, et al. Activation of human enteric neurons by supernatants of colonic biopsy specimens from patients with irritable bowel syndrome. Gastroenterology. 2009;137(4):1425–1434. doi: 10.1053/j.gastro.2009.07.005 [DOI] [PubMed] [Google Scholar]
- 26.Stefanini GF, Saggioro A, Alvisi V, et al. Oral cromolyn sodium in comparison with elimination diet in the irritable bowel syndrome, diarrheic type. Multicenter study of 428 patients. Scand J Gastroenterol. 1995;30(6):535–541. doi: 10.3109/00365529509089786 [DOI] [PubMed] [Google Scholar]
- 27.Theoharides TC, Valent P, Akin C. Mast cells, mastocytosis, and related disorders. N Engl J Med. 2015;373(2):163–172. doi: 10.1056/NEJMra1409760 [DOI] [PubMed] [Google Scholar]
- 28.Theoharides TC, Tsilioni I, Ren H. Recent advances in our understanding of mast cell activation - or should it be mast cell mediator disorders? Expert Rev Clin Immunol. 2019;15(6):639–656. doi: 10.1080/1744666X.2019.1596800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valent P, Akin C, Nedoszytko B, et al. Diagnosis, classification and management of mast cell activation syndromes (MCAS) in the era of personalized medicine. Int J Mol Sci. 2020;21(23). doi: 10.3390/ijms21239030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monnier J, Georgin-Lavialle S, Canioni D, et al. Mast cell sarcoma: new cases and literature review. Oncotarget. 2016;7(40):66299–66309. doi: 10.18632/oncotarget.11812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Georgin-Lavialle S, Lhermitte L, Dubreuil P, Chandesris M-O, Hermine O, Damaj G. Mast cell leukemia. Blood. 2013;121(8):1285–1295. doi: 10.1182/blood-2012-07-442400 [DOI] [PubMed] [Google Scholar]
- 32.Sokol H, Georgin-Lavialle S, Canioni D, et al. Gastrointestinal manifestations in mastocytosis: a study of 83 patients. J Allergy Clin Immunol. 2013;132(4):866–73.e1. doi: 10.1016/j.jaci.2013.05.026 [DOI] [PubMed] [Google Scholar]
- 33.Maurer M, Abuzakouk M, Bérard F, et al. The burden of chronic spontaneous urticaria is substantial: Real-world evidence from ASSURE-CSU. Allergy. 2017;72(12):2005–2016. doi: 10.1111/all.13209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aitella E, De Bartolomeis F, Savoia A, Fabiani M, Romano M, Astarita C. The overlap syndrome of urticaria and gastroesophageal reflux disease. PLoS ONE. 2018;13(11):e0207602. doi: 10.1371/journal.pone.0207602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Afrin LB, Self S, Menk J, Lazarchick J. Characterization of mast cell activation syndrome. Am J Med Sci. 2017;353(3):207–215. doi: 10.1016/j.amjms.2016.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leru PM, Anton VF, Ureche C, Zurac S, Bratu O, Neagoe CD. Mast cell activation syndromes - evaluation of current diagnostic criteria and laboratory tools in clinical practice (Review). Exp Ther Med. 2020;20(3):2348–2351. doi: 10.3892/etm.2020.8947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valent P, Akin C, Bonadonna P, et al. Proposed Diagnostic Algorithm for Patients with Suspected Mast Cell Activation Syndrome. J Allergy Clin Immunol Pract. 2019;7(4):1125–1133.e1. doi: 10.1016/j.jaip.2019.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.IBM Explorys Solutions | Watson Health | IBM. https://www.explorys.com/about-us.html. Accessed February 3, 2021. [Google Scholar]
- 39.Kaelber DC, Foster W, Gilder J, Love TE, Jain AK. Patient characteristics associated with venous thromboembolic events: a cohort study using pooled electronic health record data. J Am Med Inform Assoc. 2012;19(6):965–972. doi: 10.1136/amiajnl-2011-000782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tobin MC, Moparty B, Farhadi A, DeMeo MT, Bansal PJ, Keshavarzian A. Atopic irritable bowel syndrome: a novel subgroup of irritable bowel syndrome with allergic manifestations. Ann Allergy Asthma Immunol. 2008;100(1):49–53. doi: 10.1016/S1081-1206(10)60404-8 [DOI] [PubMed] [Google Scholar]
- 41.MedCalc’s Odds ratio calculator. https://www.medcalc.org/calc/odds_ratio.php. Accessed February 3, 2021.
- 42.Akin C Mast cell activation syndromes. J Allergy Clin Immunol. 2017;140(2):349–355. doi: 10.1016/j.jaci.2017.06.007 [DOI] [PubMed] [Google Scholar]
- 43.Nautiyal KM, Ribeiro AC, Pfaff DW, Silver R. Brain mast cells link the immune system to anxiety-like behavior. Proc Natl Acad Sci USA. 2008;105(46):18053–18057. doi: 10.1073/pnas.0809479105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kempuraj D, Selvakumar GP, Thangavel R, et al. Mast Cell Activation in Brain Injury, Stress, and Post-traumatic Stress Disorder and Alzheimer’s Disease Pathogenesis. Front Neurosci. 2017;11:703. doi: 10.3389/fnins.2017.00703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Unal M, Kucuk A, Akyurek F, Islamoglu Z. Evaluation of frequency of irritable bowel syndrome in patients with chronic urticaria. J Turgut Ozal Med Cent. 2018:1. doi: 10.5455/jtomc.2018.02.026 [DOI] [Google Scholar]
- 46.Shalom G, Magen E, Babaev M, et al. Chronic urticaria and irritable bowel syndrome: a cross-sectional study of 11 271 patients. Br J Dermatol. 2018;178(3):e204–e206. doi: 10.1111/bjd.15997 [DOI] [PubMed] [Google Scholar]
- 47.Roussos A, Koursarakos P, Patsopoulos D, Gerogianni I, Philippou N. Increased prevalence of irritable bowel syndrome in patients with bronchial asthma. Respir Med. 2003;97(1):75–79. doi: 10.1053/rmed.2001.1409 [DOI] [PubMed] [Google Scholar]
- 48.Cohen S, Berkman N, Picard E, et al. Co-morbidities and cognitive status in a cohort of teenagers with asthma. Pediatr Pulmonol. 2016;51(9):901–907. doi: 10.1002/ppul.23443 [DOI] [PubMed] [Google Scholar]
- 49.Kaya İslamoğlu ZG, Unal M, Küçük A. Atopic Dermatitis in Adults and Irritable Bowel Syndrome: A Cross-sectional Study. Indian J Dermatol. 2019;64(5):355–359. doi: 10.4103/ijd.IJD_490_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Loo EXL, Wang DY, Siah KTH. Association between Irritable Bowel Syndrome and Allergic Diseases: To Make a Case for Aeroallergen. Int Arch Allergy Immunol. 2020;181(1):31–42. doi: 10.1159/000503629 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Features of the IBS+U cohort by manual chart review.
Supplementary Table 2. Features of the IBS+IU patients identified via manual. chart review
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


