Abstract
Retinol-binding protein 2 (RBP2, also known as cellular retinol-binding protein 2 (CRBP2)) is a member of the fatty acid-binding protein family and has been extensively studied for its role in facilitating dietary vitamin A (retinol) uptake and metabolism within enterocytes of the small intestine. RBP2 is present in highest concentrations in the proximal small intestine where it constitutes approximately 0.1–0.5% of soluble protein. Recent reports have established that RBP2 binds monoacylglycerols (MAGs) with high affinity, including the canonical endocannabinoid 2-arachidonoylglycerol (2-AG). Crystallographic studies reveal that retinol, 2-AG, or other long-chain MAGs alternatively can bind in the retinol-binding pocket of RBP2. It also has been demonstrated recently that Rbp2-deficient mice are more susceptible to developing obesity and associated metabolic phenotypes when exposed to a high fat diet, or as they age when fed a conventional chow diet. When subjected to an oral fat challenge, the Rbp2-deficient mice release into the circulation significantly more, compared to littermate controls, of the intestinal hormone glucose-dependent insulinotropic polypeptide (GIP). These new findings regarding RBP2 structure and actions within the intestine are the focus of this review.
Keywords: Retinol-binding proteins, RBP2, Cellular retinol-binding protein 2, CRBP2, 2-monoacylglycerol, Retinoids, All-trans-retinoic acid, Vitamin A, Incretin, Glucose-dependent insulinotropic polypeptide (GIP), Obesity
1. Introduction
Retinol-binding protein 2 (RBP2) (also known as cellular retinol-binding protein 2 or CRBP2) was first identified in the mid-1980s by Ong who showed that exogenous retinol added to a tissue homogenate co-purified with RBP2 [1,2]. A few other lipids, including cholesterol and unesterified fatty acids (FFAs), were tested for binding but none were found to bind to RBP2 [1,2]. This was taken to indicate that retinol and its oxidized form, retinaldehyde, which was subsequently shown to bind RBP2 tightly in vitro, are the endogenous ligands for RBP2 [1,2]. In the adult, RBP2 is expressed most prominently in the proximal small intestine where it represents 0.1–0.5% of soluble protein [2,12]; but it also is expressed throughout the remainder of the small intestine and in the colon, albeit at lower levels. RBP2 is not thought to be expressed outside of the adult intestine [2,4]. Since its identification, RBP2 has been studied almost exclusively in the context of its actions to mediate dietary retinoid absorption and metabolism within enterocytes [1–7]. The molecular role of RBP2 identified from these studies is that RBP2 metabolically channels dietary retinol/retinaldehyde towards retinyl ester formation for packaging into nascent chylomicrons [1–7] (Fig. 1). There are several published reports which indicated that RBP2 may also act physiologically to facilitate biosynthesis of the potent transcriptional regulator all-trans-retinoic acid (ATRA), which is used locally to maintain proliferative processes and normal gut immunity as well as other essential processes within the intestine [8–11]. Overall, the literature supporting a role for RBP2 in channeling retinoid metabolism within enterocytes in the small intestine, specifically towards retinyl ester formation, is both extensive and convincing. Its role in channeling ATRA synthesis is less well studied. This will be considered below.
Fig. 1.
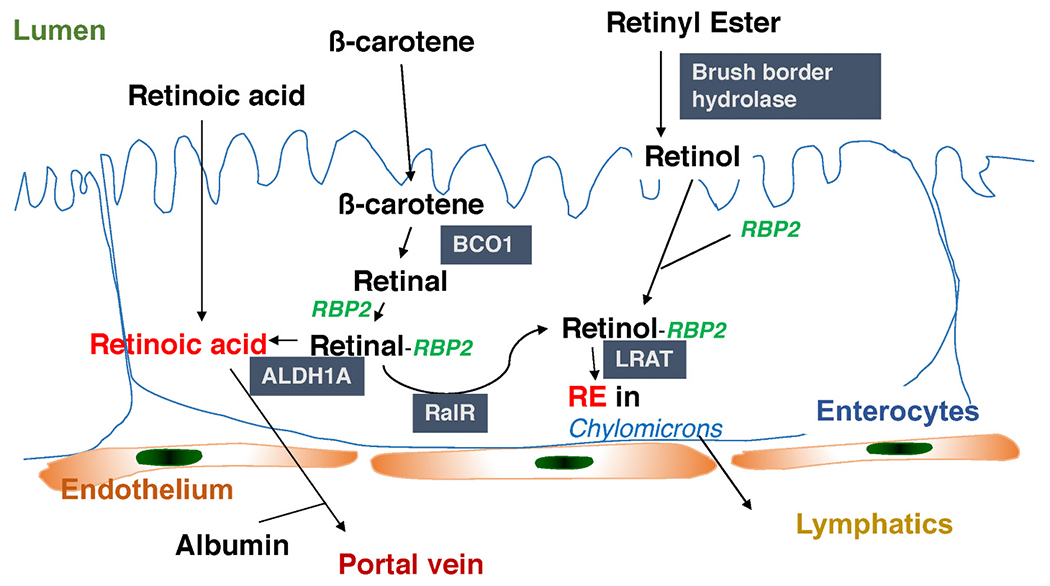
RBP2 acts to facilitate retinoid metabolism within enterocytes. Dietary preformed vitamin A (retinol) or provitamin A carotenoids (like β-carotene) are taken up by enterocytes. Dietary retinyl esters must first be hydrolyzed to retinol before uptake. Retinol binds RBP2, which channels it to lecithin:retinol acyltransferase (LRAT), for retinyl ester formation and packaging into nascent chylomicrons. Provitamin A carotenoids are absorbed as such and cleaved within the enterocyte by β-carotene monooxygenase 1 (BCO1) to retinal (retinaldehyde) which then binds RBP2 which channels it to retinal reductase (RalR) for reduction to retinol, allowing for its esterification and packaging into chylomicrons. It also has been reported that RBP2-bound retinol can be channeled to retinol dehydrogenases (RDHs) that catalyze its oxidation to retinal (not shown). RBP2-bound retinal can then be oxidized to retinoic acid by aldehyde dehydrogenases, ALDH1A enzymes. Retinoic acid is needed and used locally to regulate retinoic acid receptor (RAR)-mediated gene transcription.
In the past 3 years, understanding of RBP2 has undergone a marked transformation with regards to RBP2’s physiological actions, as well as its structural and biochemical properties [12,13]. The present review will focus on these new advances in our understanding of RBP2. The reader is referred to a recent review article on RBP2 [4] for an in-depth consideration of earlier work.
2. Recent advances in understanding the physiologic actions of RBP2
While studying dietary retinoid uptake in RBP2-deficient (Rbp2−/− mice [7], we noted that these mice gained more body weight as they aged, compared to age-, sex-and genetic background-matched wild type (WT) mice. Systematic investigation of this observation established that when 2-month-old male Rbp2−/− mice were placed on a high fat diet (HFD), within 6 weeks of diet administration, they gain significantly more body weight and acquire more body fat than WT mice [12]. In addition, Rbp2−/− mice gain more weight compared to WT mice by 6–7 months of age, even when they were fed a chow diet [12]. These observations indicate that RBP2 plays a role in the maintenance of body weight and protects against obesity development.
Since the only established function for RBP2 is to facilitate intestinal uptake and intracellular transport/metabolic channeling of dietary retinol and retinaldehyde [1–7], we hypothesized that the phenotypic differences seen in Rbp2−/− mice compared to WT mice are because of alterations in tissue retinoid homeostasis and actions. Surprisingly, no significant differences were detected in plasma ATRA levels between 7-month-old Rbp2−/− and WT mice after oral administration of a physiologic dose of retinol (6 μg) dissolved in 100 μL of corn oil [12]. Fasting plasma ATRA levels also did not differ between Rbp2−/− and WT mice fed a high fat diet. In addition, there were no differences in mucosal levels of ATRA of Rbp2−/− and matched WT mice either following a 5 h fast or 2 h after an oral gavage of 100 μL of corn oil [12]. Based on these and other data, it was concluded that differences in retinoid-related parameters cannot account for the excess body weight gain and adipose tissue accrual phenotypic differences observed between Rbp2−/− and WT mice.
RBP2 is a member of the fatty acid-binding protein (FABP) family that comprises 9 known 14–15 kDa intracellular proteins that bind FFAs with high affinity [14–16]. The FABP family additionally contains three intracellular retinol-binding proteins (retinol-binding protein 1 (RBP1), RBP2, and retinol-binding protein 7 (RBP7)) and two intracellular retinoic acid-binding proteins (cellular retinoic acid-binding protein 1 (CRABP1) and cellular retinoic acid-binding protein 2 (CRABP2)) [2–4]. Until recently, none of the retinoid-binding proteins (RBPs) had been shown to bind, with high affinity, ligands other than retinol, retinaldehyde, or ATRA. However, a number of non-retinoid binding FABP family members are reported to bind strongly to 2-monoacylglycerols (2-MAGs), N-acylethanolamides (NEAs), or other lipids, and that this in turn influences the levels of these lipids in cells and tissues [17–21]. Consequently, a question was raised regarding whether RBP2 may have some role in the metabolism of these or other bioactive lipids. To search for alternative ligands for RBP2, recombinant human holo-RBP2 was purified and used in a retinol displacement assay to screen diverse chemical libraries composed of over 900 endogenous lipids [12,13]. From this screen, it was determined that human RBP2 is a novel 2-MAG-binding protein, interacting with the canonical endocannabinoid 2-arachidonoylglycerol (2-AG) and several other 2-MAGs with high affinities (Kd values in the 20–70 nM range; e.g. Kd for 2-AG is 27.1 ± 2.4 nM), comparable to that of retinol (Kd ~10 nM) [13,14]. By contrast, NEAs, including the other canonical endocannabinoid anandamide (AEA) and long chain fatty acids, showed little displacement of RBP2-bound retinol (e.g. Kd for AEA is 663.0 ± 24.0 nM) [12,13]. The structural considerations that give rise to this ligand specificity for RBP2 are discussed immediately below. Chemical structures for retinol, retinoic acid, 1-MAGs, 2-MAGs, and NEAs are provided in Fig. 2.
Fig. 2.
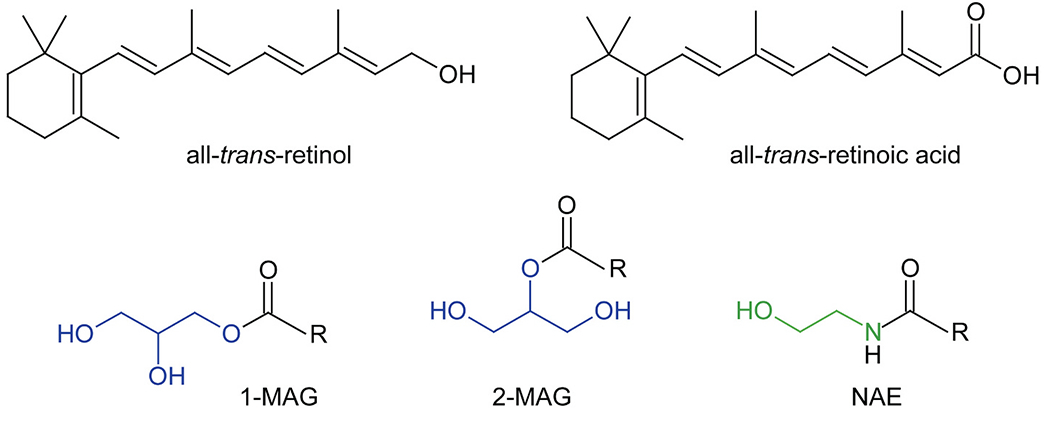
Chemical structures of lipids which interact with selected intracellular retinoid-binding proteins. 1-MAG and 2-MAG stands for 1- and 2-monoacylglicerides. The glycerol backbone in these compounds is colored blue. NAE corresponds to N-acylethanolamides. The ethanolamine moiety is shown in green. R – indicate a fatty acid moiety, including C14 – C22 long acyl chains that can be saturated, mono- or polyunsaturated. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3. Mechanistic basis for the interaction of RBP2 with MAGs
To obtain insight into the molecular aspects of these interactions, several high-resolution crystal structures of human RBP2 were solved in complex with non-retinoid lipids [12,13]. These structural studies provide compelling proof of protein-ligand interaction, as well as a wealth of data regarding the molecular aspect of ligand selectivity. This will be considered in more detail in the section below. However, it is clear from these data that RBP2 links two very potent classes of signaling lipids, 2-MAGs and retinoids, and must influence signaling by both classes of lipids.
3.1. Structure of RBPs
The first information about the overall architecture of RBP2 was derived from the structures of two representative members of fatty acid-binding proteins, rat FABP1 and bovine myelin P2 protein [22–24]. They share a nearly identical protein fold, despite only ~28% sequence identity, suggesting that other members of the lipocalin protein family, including RBP2, have similar structures. The subsequent determination of the first crystallographic structure of rat RBP2 confirmed this assumption [25]. RBP2 is comprised of a ten-stranded anti-parallel β-barrel (β1-β10), the inside of which constitutes the hydrophobic ligand binding cavity (Fig. 3A) [4,13,26,27]. Two short α-helices (αI and αII) cover the open end of the β-barrel. Together with the two loops connecting β-strands 3–4 and 5–6, they form a so-called ‘portal region’ that surrounds the entry way to the ligand binding site. The conformational flexibility of the portal region observed in the apo form of RBP2 enables the entry of ligands into the binding cavity. Upon binding, the ligand causes the stabilization of the closed conformation of the portal region that effectively locks a bound molecule inside the binding pocket. Thus, the interactions of the ligand with the side chains of the portal region play a pivotal role in overall binding affinity. This phenomenon is exemplified by the binding of the canonical ligand for RBP2, retinol [28]. The retinoid moiety is positioned deep inside the narrowing binding pocket (Fig. 3B). The hydroxyl group of retinol interacts with the polar side chains of the conserved residues K40 and E108, forming a network of hydrogen bonds. Consequently, the β-ionone ring of retinol is oriented towards the opening to the binding cavity, making contact with the entry portal region. In the closed configuration of the portal, the hydrophobic side chains of this part of the protein are involved in extensive hydrophobic and van der Waals interactions with the β-ionone ling (Fig. 3B). The ability to limit the dynamic flexibility of the portal region has a direct impact on the dissociation rate of a ligand, disfavoring its release from the binding pocket [29,30]. The functional significance of these interactions is underscored by the observation that even small changes in the amino acid composition of the portal region contribute to significant differences in the binding affinity for retinol between closely related RBPs. For example, the substitution of L78 in RBP2 by isoleucine present in the sequence of RBP1 resulted in ~2.8-fold decrease in the Kd value for retinol [29]. The underlying cause for the change in binding affinity is the strengthened interaction of the β-ionone ring with the mutated residue and the increased free energy gap between the open and closed conformation of the portal that prevents the release of the bound ligand. Therefore, the selectivity of RBPs for lipid ligands depends on two independent factors: the specificity of hydrogen bond formation in the binding cavity and the ability of a ligand to stabilize the closed conformation of the protein via its interaction with the portal site.
Fig. 3.
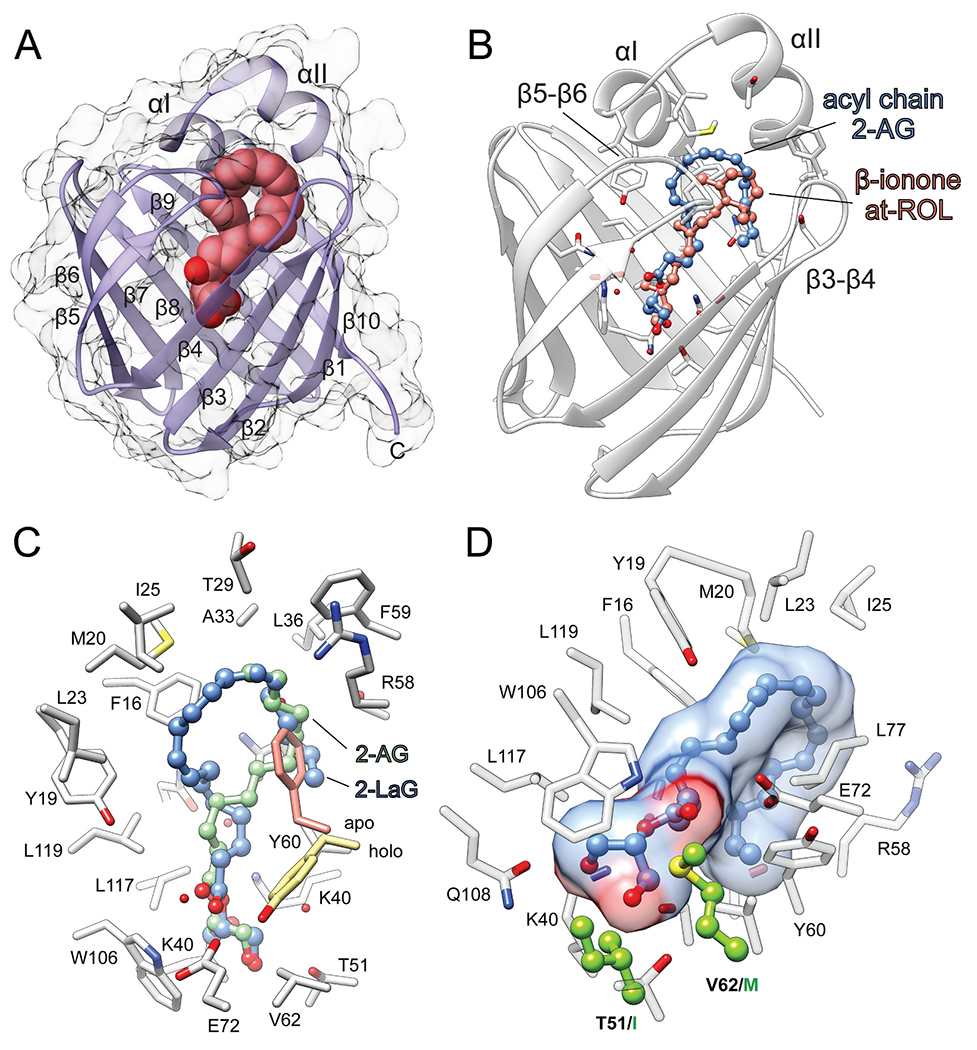
Interaction of human RBP2 with its lipid ligands. (A) Representation of the overall RBP2 fold (PDB: 6BTH). Location of the ligand binding site is marked by a molecule of 2-AG depicted as spheres. (B) Comparison of the spatial positions of all-trans-retinol (at-ROL) and 2-AG in the binding cavity of RBP2 (PDBs: 4QZT and 6BTH, respectively). Geometric configuration of arachidonoyl chain of 2-AG mimics the structure of β-ionone ring of at-ROL, interacting with the portal region of the protein. (C) The opposite orientation of the acyl chains of 2-AG (blue) and 2-lauroylglycerol (2-LaG) (green) (PDB: 7K3I) in the binding site of RBP2. Regardless the differences in the binding modes both ligands cause repositioning of the Y60 side chain as compared with the apo-form of the protein. (D) The key difference in the amino acid composition of the binding pocket of RBP1 that prevents its high-affinity interaction with MAGs. Residues I51 and M62 present in RBP1 are colored green. 2-AG molecule within the binding site is represented in ball and stick style. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2. The principles of the interaction of RBP2 with MAGs
The protein fold present in RBP2 is common for numerous lipid binding proteins and even enzymes. Thus, it represents a versatile structural platform that can be adapted to serve specific biological functions by altering ligand binding specificity even within a family of closely related proteins. A good illustration of this phenomenon is the discovery of the interaction of RBP2 with MAGs [12]. The interaction with MAGs is an intrinsic characteristic of RBP2 that distinguishes it from RBP1 and probably other members of the RBP protein family [4]. As evidenced by the crystal structures of human RBP2 in complex with MAGs, these non-retinoid lipids occupy the same binding site as retinol [12]. Moreover, MAGs can effectively outcompete retinol from the canonical binding cavity of RBP2 due to their high affinity which is comparable to that of retinoids [13]. But how can distinctively different lipid molecules effectively utilize the same binding site as retinoids? A comparison of the binding modes for retinol and MAGs revealed surprising resemblances in the spatial orientations of these ligands. Similar to retinol, the hydroxyl groups of MAGs interact with the polar side chains present at the bottom of the binding cavity of RBP2 (Fig. 3C). Both K40 and E108 form a hydrogen bond with one of the free hydroxyls of MAGs, as they do with the hydroxyl group of bound retinol. However, the additional hydrogen bond donor (−OH) and acceptor (=O) present in MAGs provide an opportunity for more extensive interactions with the protein scaffold. Indeed, the carbonyl oxygen of the ester group is part of an extended network of hydrogen bonds involving the side chains of Q97, E72, and Y60, as well as ordered water molecules, whereas the second MAG hydroxyl group interacts with T51. Importantly, a dramatically lower affinity of RBP2 for AEA (which possesses only one hydroxyl group) or MAG derivatives, which lack the carbonyl oxygen, strongly indicates the significance of hydrogen bonds for overall binding energy and selectivity [13].
The structurally distinct β-ionone ring that is a characteristic of retinoids might be considered an important contributor to the binding specificity by RBPs. However, an analysis of the modes of interaction of RBP2 with MAGs indicates that the β-ionone ring does not provide selectivity for the interactions with RBPs per se [12,13]. This notion is also supported by the binding of cannabinoid-based small molecule inhibitors to RBP1, which are not chemically related to retinoids [31]. Nevertheless, it is critical for the high-affinity ligands to maintain strong interactions with the residues of the portal region, particularly α-helix II and the β3-β4 loop. This is evident in the structure of RBP2 in complex with 2-AG (PDB: 6BTH) and related ligands where the acyl chain adapts an orientation similar to that observed for the β-ionone ring in retinol (Fig. 3B). Mechanistically, the interaction of high-affinity lipids with the portal region plays an important role in the stabilization of the ‘closed’ (ligand-bound) conformation of RBP2. Thus, the apparent increase in the Kd values for 2-MAGs (20–70 nM) and retinol (~10 nM) is most likely a result of the extent to which the acyl chains of MAGs and the β–ionone ring of retinol interact with the hydrophobic side chains of the portal region.
Seemingly, the same principles of ligand binding apply to all RBPs. However, an extensive screening for non-retinoid binding partners for RBP1 did not result in the identification of any other endogenous ligands besides retinoids raising a question about the molecular determinants that so dramatically alter the binding specificity [13,31]. A comparison of the binding sites in human RBP1 and RBP2 revealed a highly conserved 3D architecture with just a few amino acid substitutions characteristic for each of the proteins [13]. Two residues, I51 and M62, present in sequence of RBP1 are not directly involved in the interaction with retinol. However, they are located in the direct vicinity of the key residues involved in the interaction of RBP2 with MAGs (Fig. 3D). Thus, they may interfere with the binding of MAGs by RBP1. To test this hypothesis, a RBP2 double mutant in which T51 and V62 were replaced with isoleucine and methionine residues was generated and examined for its ability to bind MAGs [13]. Although the (RBP2T51I/V62M) mutant protein revealed spectral properties identical to the wild-type protein upon binding retinol, it displayed a > 16-fold increase in the Kd value for 2-AG. This dramatic change in the affinity of RBP2T51I/V62M for MAGs is caused by the elimination of the hydrogen bond acceptor, the increased hydrophobicity of the binding site near the polar hydroxyl group of MAGs, and the steric hindrance of the large side chain of methionine as compared to a valine residue. Interestingly, in an earlier study, Jakoby et al. observed a similar phenomenon [33]. A single substitution of Arg106 for glutamine in FABP1, which is an equivalent of Gln108 in RBP2, enabled the high-affinity binding of retinol by the mutated protein [32]. Thus, the ligand selectivity of RBPs and FABPs can be interchangeably switched by the exchange of key residues within the binding cavity.
3.3. Selectivity of RBP2 towards MAGs
The biochemical and structural analysis of RBP2 in complex with retinol, 2-AG, and AEA provides strong evidence that ligand selectivity is achieved by a combination of specific hydrogen bond interactions in the binding pocket and the ability of a ligand to stabilize the closed configuration of the protein. Therefore, the gain-of-function (i.e. the ability to bind MAGs) observed in RBP2 should preserve ligand selectivity, enabling the interaction only with an explicit subset of MAGs. A systematic evaluation of the MAG structure/binding relationships by Silvaroli et al. revealed molecular determinants that govern the binding selectivity in the context of the protein structure [13].
Based on the overall size of the binding pocket and the position of 2-AG, the maximum length of the acyl chains in MAGs that bind to RBP2 is 20 to 22 carbons. If the hypothesis regarding the importance of the interaction with the portal region is true, a sharp decline in the affinity should be observed for MAGs with shorter acyl chains. Indeed, the Kd value for MAGs with acyl chain containing 12 carbons increased >6-fold, whereas the Kd value for those with 10 carbons was even higher (>28-fold) as compared to 2-AG. Much less profound changes (~2-fold) were reported for the affinities of MAGs containing C18 and C16 acyl chains. The examination of the X-ray crystallography of RBP2 bound to 2-AG (PDB: 6BTH), 2-oleoylglycerol (2-OG) (PDB: 7JWR), and 2-linoleoylglycerol (2-LG) (PDB: 7JWD) revealed that despite the two-carbon difference in length, the aliphatic ends of the fatty acid chains for each of these ligands are positioned exactly in the same place, providing an identical contact point with the hydrophobic side chains of the portal region. This spatial compensation for the difference in length of acyl chains was achieved by a more extended conformation of the acyl chains in 2-LG and 2-OG as compared to 2-AG [14]. Interestingly, palmitoyl and lauroyl moieties are deemed to be too short to secure the same degree of interaction with the portal region as 2-OG and 2-LG. Yet, RBP2 revealed a comparably high affinity for interactions with 2-palmitoylglycerol (2-PG) and 2-lauroylglucerol (2-LaG). This may be due to an unexpected change in the spatial position of the palmitoyl chain as compared with longer acyl moieties. The longer acyl chains in 2-AG, 2-LG, or 2-OG bend inside the binding cavity making contacts with the β5-β6 loop, α-helices I and II, and finally the β3-β4 loop. The palmitoyl and lauroyl groups in 2-PG and 2-LaG are too short to adopt the same position without losing the key interactions with α-helices II and the β3-β4 loop, which stabilize the closed configuration of holo-RBP2. Consequently, the palmitoyl and lauroyl moieties bind in an inverse orientation, facing towards the β3-β4 loop first before interacting with α-helices II and I (Fig. 3C). Another interesting aspect related to the inverse orientations of the acyl chains in 2-PG and 2-LaG is the outward rotation of the side chain of Y60 (Fig. 3C). This conformational change of Y60 observed in holo-RBPs is significant for two reasons: i) it eliminates the bulky hydroxyphenyl ring from the ligand binding cavity. ii) it allows for a favorable position of the bound molecule, enhancing its interaction with the portal region [13,31,33]. Thus, the differences in the spatial positions of long and short-chains of MAGs occur to maximize their interactions with α-helix II and the β3-β4 loop, that in turn stabilize RBP2 in the ligand-bound state.
Both the sn-1 and sn-2 isomers of MAGs can be tightly bound to RBP2. However, as evidenced by fluorescence titrations, the affinity for MAGs with an acyl chain in the sn-2 position is ~2-fold higher as compared to their sn-1 isomers [13]. Despite the geometrical differences between 1-AG and 2-AG, the mode of interaction for both molecules with RBP2 is nearly identical [14]. Inside the binding cavity, the arachidonoyl chains of both ligands are superimposable, and the location of the carboxyl oxygen atoms is identical for both isomers. Moreover, the free hydroxyl groups of both ligands are engaged in similar networks of hydrogen bonds with the side chains of K40 and Q108, as well as T51. The same mode of binding for isomers with different positions of the acyl chains is possible by altered positions of the glycerol moiety in the binding site. This causes a shift of the sn-3 hydroxyl group in 1-AG towards the location normally occupied by an ordered water observed when 2-AG is bound. The main consequence of this repositioning of the hydroxyl group and elimination of the water molecule is the inability to form an extended network of hydrogen bonds characteristic for 2-AG that connects 2-AG with Q108, the hydroxyl of the water molecule, and the T51 side chain. The limited number and rearrangement of the hydrogen bonds is most likely responsible for the lower affinity of the sn-1 isomers.
Altogether, the binding of MAGs by RBP2 is enabled by a combination of the discrete hydrogen bond network formed by these lipids inside the binding pocket and their hydrophobic and van der Waals contacts with the side chains of the portal region. These two types of interactions define the chemical boundaries for tightly bound ligands. Consequently, even a partial loss of just one of these interactions by a ligand leads to the decrease of its affinity to RBP2.
4. Physiological significance of the RBP2 interaction with MAGs
To understand the physiological relevance of these binding data, the concentrations of different 2-MAGs were measured by LC/MS/MS in mucosal scrapings of jejunums (where RBP2 is most highly expressed) obtained from 6–7-month-old chow-fed Rbp2−/− and WT mice [12]. Mucosal samples were collected at two time points, after 12 h fasting and then 2 h following administration of an oral challenge with 100 μL of corn oil. Levels of 2-AG were significantly higher, by approximately 50%, in mucosal scrapings from Rbp2−/− mice as compared to WT mice (Fig. 4A). Similarly, mucosal levels of 2-LG and 2-PG were also significantly elevated in mucosa from Rbp2−/− mice (Fig. 4A and B). No statistically significant differences in 2-OG levels were observed (Fig. 4B), nor were differences in AEA levels between Rbp2−/− and WT intestines detected. Thus, RBP2, which localizes anatomically primarily to the jejunum, influences 2-MAG levels in the proximal small intestine following a fat challenge. For the same mice employed for 2-MAG measurements, levels of glucose-dependent insulinotropic polypeptide (GIP) (Fig. 4C), glucagon-like peptide-1 (GLP-1) (Fig 4D), cholecystokinin 8 (CCK8) (Fig. 4E), and insulin (Fig. 4F) were determined in the plasma 2 h after the fat challenge. Plasma levels of GIP for the Rbp2−/− mice were elevated by approximately 2-fold compared to the matched WT mice (p < 0.05). This was accompanied by a 3-fold elevation in insulin concentrations (p < 0.05). Plasma levels of GLP-1 and CCK8 were not different between matched Rbp2−/− and WT mice. One week prior to mucosal sample collection, blood was obtained from the Rbp2−/− and WT mice after fasting and re-feeding. While GIP levels were significantly elevated for Rbp2−/− mice (p < 0.05) in both fasted (Fig. 4H) and fed (Fig 4G) states, GLP-1 and CCK8 levels were not different between Rbp2−/− and WT mice.
Fig. 4.
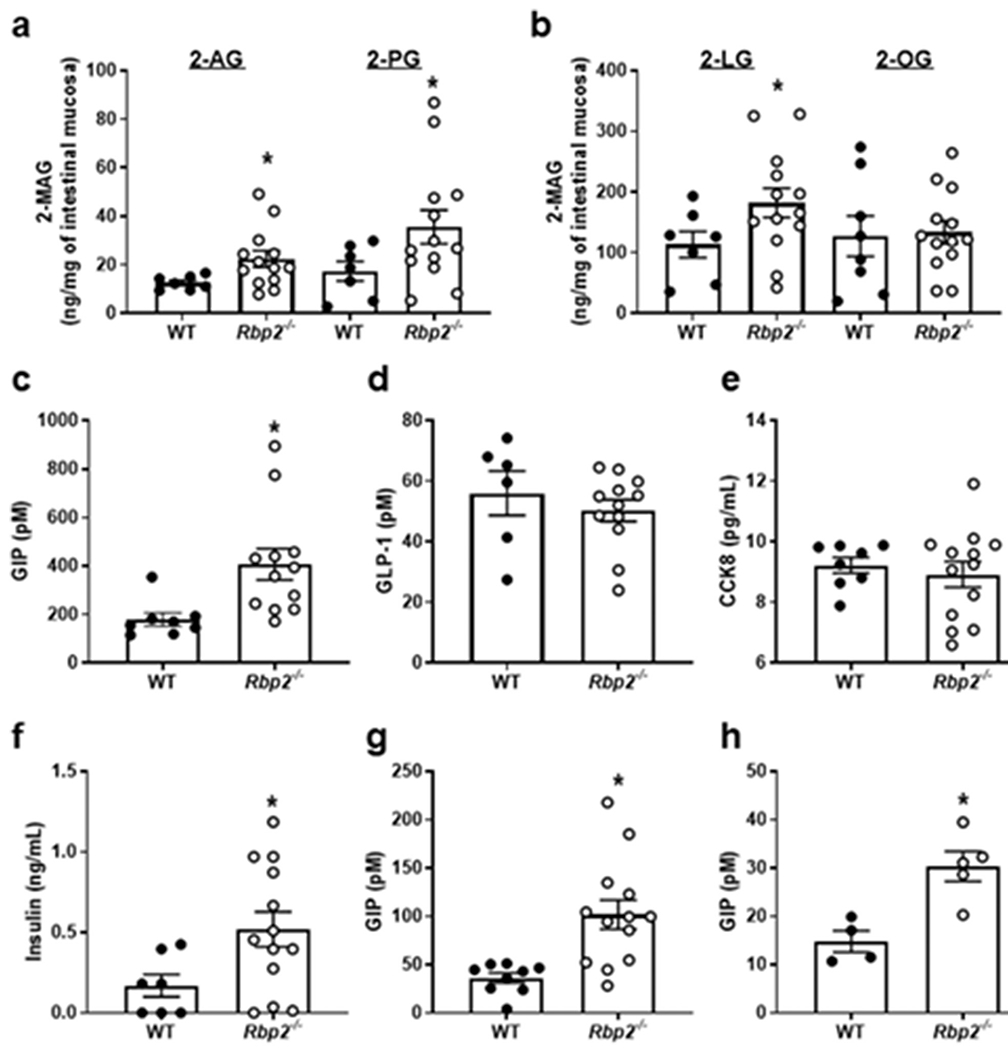
Following an oral fat challenge to chow-fed Rbp2−/− and WT mice of approximately 7-months-of-age, levels of 2-AG, 2-PG, and 2-LG but not 2-OG were significantly elevated in mucosal scrapings from Rbp2−/− mice (A and B). Plasma levels of GIP (C) and insulin (F) but not GLP-1 (D) or CCK8 (E) were significantly elevated for Rbp2−/− mice receiving the corn oil challenge. GIP levels in plasma of both fed (G) and fasted (H) Rbp2−/− mice were also significantly elevated compared to those of matched WT mice. Data are provided as means ± SEM with N = 6 or more for each group, *, p < 0.05. Taken directly from Lee et al. [12].
Similar effects of a fat challenge on plasma GIP levels were observed in 2–3-month-old Rbp2−/− mice fed a HFD for 6 weeks [12]. Again, administration of the fat challenge was not found to affect in HFD-fed Rbp2−/− mice plasma GLP-1 levels. Importantly, a glucose challenge (1 mg/g) did not lead to elevated GIP levels. Thus, the absence of Rbp2 specifically affects GIP responses to a fat challenge but not to a glucose challenge. This was taken to indicate that the altered GIP response may be driven by changes in 2-MAGs levels [12]. Control experiments established that the absence of Rbp2 affect only the magnitude of the GIP response but not the time course of GIP release from the intestine [12].
Interestingly, when 70-day-old Rbp2−/− received the same fat challenge that was administered to older animals, the same elevation in GIP plasma levels compared to matched WT mice was observed [12]. At this young age, Rbp2−/− and WT mice do not show differences in body weights, the elevated GIP response cannot arise as the result of an elevation in body weight. Thus, the altered GIP response in Rbp2−/− mice precedes the metabolic and weight phenotypes [12].
Since RBP2 binds tightly to 2-MAGs, expression levels of genes encoding proteins that are involved in 2-MAG metabolism and actions within the small intestine were examined [12]. To this end, expression levels in the proximal small intestine were determined for the cannabinoid receptors Cb1 and Cb2; enzymes involved in NAE synthesis (N-acylphosphatidyl ethanolamine phospholipase D (Nape-pld)) and degradation (fatty acid amide hydrolase (Faah)); 2-MAG synthesis (diacylglycerol lipase b (Dgl-b)) and degradation (monoglyceride lipase (Mgl)); and a number of cell surface and nuclear receptors that are known to bind FFA, NAEs or 2-MAGs, including Cd36, Gpr119, Trpv1, and Ppara. mRNA expression of Dgl-b and Faah were significantly reduced (p < 0.05) for Rbp2−/− mice. No statistically significant differences were observed for the other transcripts. Interestingly, both Fabp1 and Fabp2 mRNA in the proximal small intestine of Rbp2−/− mice were elevated by approximately 1.6-fold. These data are summarized in Table I.
Table I.
Rbp2 absence significantly affects mRNA expression levels of some genes encoding proteins that mediate 2-MAG metabolism and actions within the small intestine.
| Protein name (gene) | Expression change |
|---|---|
| Cannabinoid receptor Cb1 (Cb1) | NSa |
| Cannabinoid receptor Cb2 (Cb2) | NS |
| N-acylphosphatidyl ethanolamine phospholipase D (Nape-pld) | NS |
| Fatty acid amide hydrolase (Faah) | Decreased by 60% |
| Diacylglycerol lipase b (Dgl-b) | Decreased by 50% |
| Monoglyceride lipase (Mgl) | NS |
| Cluster of differentiation 36, CD36 (Cd36) | NS |
| G protein-coupled receptor 119, GPR119 (Gpr119) | NS |
| Transient receptor potential cation channel subfamily V member 1, TrpV1 (Trpv1) | NS |
| Peroxisome proliferator-activated receptor-alpha, PPAR-α, (Ppara) | NS |
| Fatty acid-binding protein 1, FABP1 (Fabp1) | Increased by 1.6-fold |
| Fatty acid-binding protein 2, FABP2 (Fabp2) | Increased by 1.6-fold |
NS = No Statistically Significant Differences.
The possible contribution of the hypothalamus, the area within the central nervous system responsible for regulating energy expenditure and body weight [34,35], was also assessed. Although no Rbp2 mRNA expression in whole hypothalamus obtained from chow fed WT mice was detected, the absence of Rbp2 expression affected the expression of hypothalamic genes involved in controlling food intake and energy expenditure. Interestingly, expression of proopiomelanocortin (Pomc), a gene known to inhibit food intake and to facilitate weight loss, was significantly reduced in chow-fed 6-to 7-month-old male Rbp2−/− mice. In contrast, expression of an orexigenic gene, Agouti-related peptide (Agrp), was not different between Rbp2−/− and WT mice. These data suggest the possible involvement of the hypothalamus in mediating the excessive weight gain phenotype of Rbp2−/− mice.
As mentioned above, RBP2 is a member of the FABP family that is highly expressed in the proximal small intestine [2,3,14–16]. RBP2 is unique in that it binds lipids other than retinoids, unlike other RBPs that are members of the FABP family that bind only retinoids. A number of FABPs have been reported to bind strongly to 2-MAGs, NEAs, or other lipids [17–21], and this in turn has been found to influence the levels of these compounds in cells and tissues. However, FFA-binding FABPs are not known to bind retinoids. The expression pattern of RBP2 in the small intestine overlaps completely with those of FABP1 (also known as liver fatty acid-binding protein or LFABP) and FABP2 (also known as intestinal fatty acid binding protein or IFABP). Like RBP2, FABP1 strongly binds 2-MAGs with a Kd for 2-OG of 65 nM [20]. Thus, within the same anatomic region of the small intestine and indeed the same cell type, two FABP family members (FABP1 and RBP2) that bind 2-MAGs and two FABP family members that bind FFAs (FABP1 and FABP2) are colocalized. This raises an interesting question as to the functional significance of this apparent redundancy. Do these proteins and/or the ligands they bind have different metabolic functions? And/or do they have distinct signaling activities within the small intestine?
5. Potential role of RBP2 in ATRA synthesis in intestine
As mentioned earlier, the role of RBP2 in channeling retinoid metabolism has been studied mainly in enterocytes present within the small intestine. While RBP2 can channel retinol to retinyl ester or ATRA, its role in retinyl ester formation has been much more extensively studied, as it is needed for dietary vitamin A absorption in small intestine. Because of the relative lack on information on the role of RBP2 in ATRA synthesis, we have explored this in the small intestine. We found that the proximal small intestine has a higher capacity for ATRA synthesis compared to the distal small intestine (Fig. 5A), similar to the pattern of RBP2 expression (higher proximally and lower distally). Because the colon does not participate in nutrient absorption, we hypothesize that RBP2 expressed in this organ may have a role in channeling retinol for ATRA synthesis rather than towards retinyl ester synthesis. Indeed, while the colon expresses very low levels of Lrat which is needed for retinyl ester synthesis, it expresses at high levels both Rbp2 and the ATRA synthesizing enzyme aldehyde dehydrogenase 1A1 (Aldh1a1) (Fig. 5B). In agreement with this, colonic mucosa from Aldh1a1−/− mice produced significantly lower levels of ATRA compared to that obtained from WT mice (Fig. 5C), suggesting that ALDH1A1 is the main contributor of ATRA synthesis in the colonic mucosa. Considering that the colon does not significantly contribute to nutrient absorption, it appears likely that RBP2 acts in the colon to channel ATRA biosynthesis to meet local needs, producing ATRA to regulate transcriptional activity. Further studies will be needed to decipher the role of RBP2 in channeling ATRA synthesis in the intestine, especially in colon.
Fig. 5.
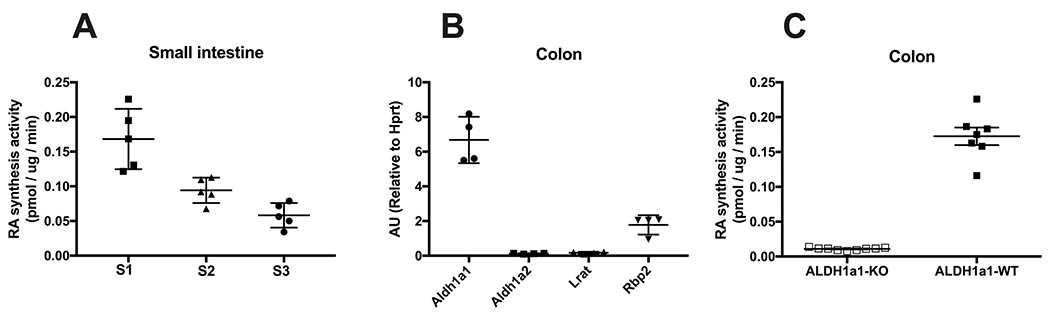
Potential involvement of RBP2 in ATRA synthesis in intestine. (A)Three equal length sections of small intestine (SI 1–3, proximal to distal) were taken to prepare mucosal scrapings. For these scrapings, all-trans-retinoic acid (ATRA) synthesis activity was determined upon incubation with 1 μM all-trans-retinaldehyde and 15 μg protein and is expressed as ATRA synthesis rate (pmol ATRA/μg protein/min). (B) Relative mRNA expression levels of ATRA (Aldh1a1 and Aldh1a2) or retinyl ester (Lrat) synthesizing enzymes, and Rbp2 in colon. (C) ATRA synthesis capacity of colon for Aldh1a1-KO and WT mice.
6. Summary and future research
At present, it is still too early to understand fully RBP2’s physiological actions in the intestine. The unexpected finding that Rbp2−/− mice develop, as they age, obesity and associated metabolic diseases even when they are maintained on a conventional chow diet points to a role for RBP2 in regulating energy homeostasis. Since RBP2 binds 2-AG and the other 2-MAGs with very high affinity and 2-MAGs are potent regulators of energy homeostasis [36,37], this may help explain the origins of the obesity and metabolic phenotypes. We doubt, however, that this will be found to be the sole factor that is responsible for the phenotypes. Retinoids are potent regulators of cell differentiation and proliferation [38–40] and these processes too will likely be found to contribute to the phenotypes and hormonal profiles observed for Rbp2−/− mice. There is a clear need for future research that provides insight into the specific roles of 2-MAGs and of retinoids in the development of metabolic disease.
The finding that RBP2 is able to bind either 2-MAGs or retinoids, but not both simultaneously, is also very intriguing. This suggests that RBP2 sits on a node that links the signaling pathways that utilize either 2-MAGs or retinoids. This had not been suspected previously. Thus, there also is a clear need for better understanding of the physiological significance of this and also of the biochemical factors and processes that coordinate the two signaling pathways.
Acknowledgments
The authors wish to acknowledge grant support from National Institutes of Health grants R01 DK068437 (WSB and MG), R01 DK122071 (WSB and MG), R01 EY023948 (MG), and R21 AA028110 (WSB).
Abbreviations
- 2-AG
2-arachidonoylglycerol
- 2-MAG
2-monoacylglycerol
- Aldh1a1
aldehyde dehydrogenase 1a1
- ATRA
all-trans-retinoic acid
- CCK8
cholecystokinin 8
- CRABP1
cellular retinoic acid-binding protein 1
- CRABP2
cellular retinoic acid-binding protein 2
- CRBP2
cellular retinol-binding protein 2
- FABP
fatty acid-binding protein
- FABP1
fatty acid-binding protein 1
- FABP2
fatty acid-binding protein 2
- GIP
glucose-dependent insulinotropic polypeptide
- GLP-1
glucagon-like peptide-1
- HFD
high fat diet
- MAG
monoacylglycerol
- NAE
N-acylethanolamide
- POMC
proopiomelanocortin
- RAR
retinoic acid receptor
- RBP
retinol-binding protein
- RBP1
retinol-binding protein 1
- RBP2
retinol-binding protein 2
- RBP7
retinol-binding protein 7
Footnotes
This article is part of a Special Issue entitled Intestinal Lipid Metabolism in Health and Disease edited by Dr. Kimberly Buhman.
Declaration of competing interest
None of the authors of this review article have any conflicts of interest that have influenced the writing of the review.
CRediT authorship contribution statement
All authors participated in writing and editing the first draft of the manuscript. MG, JP and WSB were responsible for the final editing of the manuscript and for making revisions to the originally submitted manuscript. MG, JP and WSB provided financial support allowing for undertaking of the writing of the manuscript.
References
- [1].Ong DE, A novel retinol-binding protein from rat. Purification and partial characterization, J. Biol. Chem 259 (1984) 1476–1482. [PubMed] [Google Scholar]
- [2].Ong DE, Cellular transport and metabolism of vitamin a: roles of the cellular retinoid-binding proteins, Nutr. Rev 52 (1994) S24–S31. [DOI] [PubMed] [Google Scholar]
- [3].Napoli JL, Functions of intracellular retinoid binding-proteins, Subcell Biochem. 81 (2016) 21–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Blaner WS, Brun PJ, Calderon RM, Golczak M, Retinol-binding protein 2 (RBP2): biology and pathobiology, Crit. Rev. Biochem. Mol. Biol 55 (2020) 197–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Napoli JL, Cellular retinoid binding-proteins CRBP, CRABP, FABP5: effects on retinoid metabolism, function and related diseases, Pharmacol. Ther 173 (2017) 19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhang XEL, Lu J, Tso P, Blaner WS, Levin MS, Li E, Increased neonatal mortality in mice lacking cellular retinol-binding protein II, J. Biol. Chem 277 (2002) 36617–36623. [DOI] [PubMed] [Google Scholar]
- [7].Wongsiriroj N, Piantedosi R, Palczewski K, Goldberg IJ, Johnston TP, Li E, Blaner WS, The molecular basis of retinoid absorption: a genetic dissection, J. Biol. Chem 283 (2008) 13510–13519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dodson BD, Wang JL, Swietlicki EA, Rubin DC, Levin MS, Analysis of cloned cDNAs differentially expressed in adapting remnant small intestine after partial resection, Am. J. Phys 271 (1996) G347–G356. [DOI] [PubMed] [Google Scholar]
- [9].McDonald KG, Leach MR, Brooke KWM, Wang C, Wheeler LW, Hanly EK, Rowley CW, Levin MS, Wagner M, Li E, Newberry RD, Epithelial expression of the cytosolic retinoid chaperone cellular retinol binding protein II is essential for in vivo imprinting of local gut dendritic cells by lumenal retinoids, Am. J. Pathol 180 (2012) 984–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Takase S, Goda T, Shinohara H, Adaptive changes of intestinal cellular retinol-binding protein, type II following jejunum-bypass operation in the rat, Biochim. Biophys. Acta 1156 (1993) 223–231. [DOI] [PubMed] [Google Scholar]
- [11].Hebiguchi T, Mezaki Y, Morii M, Watanabe R, Yoshikawa K, Miura M, Imai K, Senoo H, Yoshino H, Massive bowel resection upregulates the intestinal mRNA expression levels of cellular retinol-binding protein II and apolipoprotein A-IV and alters the intestinal vitamin a status in rats, Int. J. Mol. Med 35 (2015) 724–730. [DOI] [PubMed] [Google Scholar]
- [12].Lee SA, Yang KJZ, Bran PJ, Silvaroli JA, Yuen JJ, Shmarakov I, Jiang H, Feranil JB, Li X, Lackey AI, Krezel W, Leibel RL, Libien J, Storch J, Golczak M, Blaner WS, Retinol-binding protein 2 (RBP2) binds monoacylglycerols and modulates gut endocrine signaling and body weight, Sci. Adv 6 (2020) eaay8937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Silvaroli JA, Plau J, Adams CH, Banerjee S, Widjaja-Adhi MAK, Blaner WS, Golczak M, Molecular basis for the interaction of cellular retinol binding protein 2 (CRBP2) with nonretinoid ligands, J. Lipid Res 62 (2021), 100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Storch J, Corsico B, The emerging functions and mechanisms of mammalian fatty acid-binding proteins, Annu. Rev. Nutr 28 (2008) 73–95. [DOI] [PubMed] [Google Scholar]
- [15].Storch J, Thumser AE, Tissue-specific functions in the fatty acid-binding protein family, J. Biol. Chem 285 (2010) 32679–32683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hotamisligil GS, Bernlohr DA, Metabolic functions of FABPs–mechanisms and therapeutic implications, Nat. Rev. Endocrinol 11 (2015) 592–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kaczocha M, Vivieca S, Sun J, Glaser ST, Deutsch DG, Fatty acid-binding proteins transport N-acylethanolamines to nuclear receptors and are targets of endocannabinoid transport inhibitors, J. Biol. Chem 287 (2012) 3415–3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kaczocha M, Glaser ST, Deutsch DG, Identification of intracellular carriers for the endocannabinoid anandamide, Proc. Natl. Acad. Sci. U. S. A 106 (2009) 6375–6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].McDonald KG, Leach MR, Brooke KW, Wang C, Wheeler LW, Hanly EK, Rowley CW, Levin MS, Wagner M, Li E, Newberry RD, Epithelial expression of the cytosolic retinoid chaperone cellular retinol binding protein II is essential for in vivo imprinting of local gut dendritic cells by lumenal retinoids, Am. J. Pathol 180 (2012) 984–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lagakos WS, Guan X, Ho SY, Sawicki LR, Corsico B, Kodukula S, Murota K, Stark RE, Storch J, Liver fatty acid-binding protein binds monoacylglycerol in vitro and in mouse liver cytosol, J. Biol. Chem 288 (2013) 19805–19815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Elmes MW, Kaczocha M, Berger WT, Leung K, Ralph BP, Wang L, Sweeney JM, Miyauchi JT, Tsirka SE, Ojima I, Deutsch DG, Fatty acid-binding proteins (FABPs) are intracellular carriers for Delta9-tetrahydrocannabinol (THC) and cannabidiol (CBD), J. Biol. Chem 290 (2015) 8711–8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sacchettini JC, Said B, Schulz H, Gordon JI, Rat heart fatty acid-binding protein is highly homologous to the murine adipocyte 422 protein and the P2 protein of peripheral nerve myelin, J. Biol. Chem 261 (1986) 8218–8223. [PubMed] [Google Scholar]
- [23].Jones TA, Bergfors T, Sedzik J, Unge T, The three-dimensional structure of P2 myelin protein, EMBO J. 7 (1988) 1597–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sacchettini JC, Gordon JI, Banaszak LJ, The structure of crystalline Escherichia coli-derived rat intestinal fatty acid-binding protein at 2.5-a resolution, J. Biol. Chem 263 (1988) 5815–5819. [PubMed] [Google Scholar]
- [25].Sacchettini JC, Meininger TA, Lowe JB, Gordon JI, Banaszak LJ, Crystallization of rat intestinal fatty acid binding protein. Preliminary X-ray data obtained from protein expressed in Escherichia coli, J. Biol. Chem 262 (1987) 5428–5430. [PubMed] [Google Scholar]
- [26].Winter NS, Bratt JM, Banaszak LJ, Crystal structures of holo and apo-cellular retinol-binding protein II, J. Mol. Biol 230 (1993) 1247–1259. [DOI] [PubMed] [Google Scholar]
- [27].Lu J, Lin CL, Tang C, Ponder JW, Kao JL, Cistola DP, Li E, The structure and dynamics of rat apo-cellular retinol-binding protein II in solution: comparison with the X-ray structure, J. Mol. Biol 286 (1999) 1179–1195. [DOI] [PubMed] [Google Scholar]
- [28].Nossoni Z, Assar Z, Yapici I, Nosrati M, Wang W, Berbasova T, Vasileiou C,Borhan B, Geiger J, Structures of holo wild-type human cellular retinol-binding protein II (hCRBPII) bound to retinol and retinal, Acta Crystallogr. D Biol. Crystallogr 70 (2014) 3226–3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Estarellas C, Scaffidi S, Saladino G, Spyrakis F, Franzoni L, Galdeano C,Bidon-Chanal A, Gervasio FL, Luque FJ, Modulating ligand dissociation through methyl isomerism in accessory sites: binding of retinol to cellular carriers, J. Phys. Chem. Lett 10 (2019) 7333–7339. [DOI] [PubMed] [Google Scholar]
- [30].Lu J, Lin CL, Tang C, Ponder JW, Kao JL, Cistola DP, Li E, Binding of retinol induces changes in rat cellular retinol-binding protein II conformation and backbone dynamics, J. Mol. Biol 300 (2000) 619–632. [DOI] [PubMed] [Google Scholar]
- [31].Silvaroli JA, Widjaja-Adhi MAK, Trischman T, Chelstowska S, Horwitz S,Banerjee S, Kiser PD, Blaner WS, Golczak M, Abnormal cannabidiol modulates vitamin a metabolism by acting as a competitive inhibitor of CRBP1, ACS Chem. Biol 14 (2019) 434–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Jakoby MG, Miller KR, Toner JJ, Bauman A, Cheng L, Li E, Cistola DP, Ligand-protein electrostatic interactions govern the specificity of retinol- and fatty acid-binding proteins, Biochemistry 32 (1993) 872–878. [DOI] [PubMed] [Google Scholar]
- [33].Silvaroli JA, Arne JM, Chelstowska S, Kiser PD, Banerjee S, Golczak M, Ligand binding induces conformational changes in human cellular retinol-binding protein 1 (CRBP1) revealed by atomic resolution crystal structures, J. Biol. Chem 291 (2016) 8528–8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mayer EA, Nance K, Chen S, The gut-brain Axis, Annu. Rev. Med 73 (2021) 439–453. [DOI] [PubMed] [Google Scholar]
- [35].Nogueiras R, Mechanisms in endocrinology: the gut-brain axis: regulating energy balance independent of food intake, Eur. J. Endocrinol 185 (2021) R75–R91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Cristino L, Becker T, Di Marzo V, Endocannabinoids and energy homeostasis: an update, Biofactors 40 (2014) 389–397. [DOI] [PubMed] [Google Scholar]
- [37].Silvestri C, Di Marzo V, The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders, Cell Metab. 17 (2013) 475–490. [DOI] [PubMed] [Google Scholar]
- [38].Balmer JE, Blomhoff R, Gene expression regulation by retinoic acid, J. Lipid Res 43 (2002) 1773–1808. [DOI] [PubMed] [Google Scholar]
- [39].Tang XH, Gudas LJ, Retinoids, retinoic acid receptors, and cancer, Annu. Rev. Pathol 6 (2011) 345–364. [DOI] [PubMed] [Google Scholar]
- [40].Al Tanoury Z, Piskunov A, Rochette-Egly C, Vitamin a and retinoid signaling: genomic and nongenomic effects, J. Lipid Res 54 (2013) 1761–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]


