Significance
The assembly of immature HIV-1 particles is initiated by targeting of the Gag polyproteins to the plasma membrane (PM). Gag binding to the PM is mediated by the N-terminally myristoylated matrix (myrMA) domain. Formation of a Gag lattice on the PM is obligatory for the assembly of immature HIV-1 and envelope (Env) incorporation. The structure of the myrMA lattice presented here provided insights on the molecular factors that stabilize the lattice and hence favor Env incorporation. Our data support a mechanism for Gag binding to the PM during the assembly of immature particles and upon maturation. These findings advance our understanding of a critical step in HIV-1 assembly.
Keywords: HIV-1, retrovirus, Gag, matrix, envelope
Abstract
During the late phase of HIV type 1 (HIV-1) infection cycle, the virally encoded Gag polyproteins are targeted to the inner leaflet of the plasma membrane (PM) for assembly, formation of immature particles, and virus release. Gag binding to the PM is mediated by interactions of the N-terminally myristoylated matrix (myrMA) domain with phosphatidylinositol 4,5-bisphosphate. Formation of a myrMA lattice on the PM is an obligatory step for the assembly of immature HIV-1 particles and envelope (Env) incorporation. Atomic details of the myrMA lattice and how it mediates Env incorporation are lacking. Herein, we present the X-ray structure of myrMA at 2.15 Å. The myrMA lattice is arranged as a hexamer of trimers with a central hole, thought to accommodate the C-terminal tail of Env to promote incorporation into virions. The trimer–trimer interactions in the lattice are mediated by the N-terminal loop of one myrMA molecule and α-helices I–II, as well as the 310 helix of a myrMA molecule from an adjacent trimer. We provide evidence that substitution of MA residues Leu13 and Leu31, previously shown to have adverse effects on Env incorporation, induced a conformational change in myrMA, which may destabilize the trimer–trimer interactions within the lattice. We also show that PI(4,5)P2 is capable of binding to alternating sites on MA, consistent with an MA–membrane binding mechanism during assembly of the immature particle and upon maturation. Altogether, these findings advance our understanding of a key mechanism in HIV-1 particle assembly.
During the late phase of HIV type 1 (HIV-1) infection cycle, the virally encoded Gag polyproteins are targeted to the inner leaflet of the plasma membrane (PM) for assembly of the immature particle and subsequent budding and release (1–9). Concomitant or subsequent to particle budding, the Gag protein is cleaved by the viral protease into matrix (MA), capsid (CA), nucleocapsid, and short peptides (SP1, SP2, and P6), leading to formation of mature particles (reviewed in refs. 1 and 2). HIV-1 Gag binding to the PM is mediated by the MA domain, which contains an N-terminal myristoyl (myr) group and a highly basic region (HBR). Efficient HIV-1 Gag binding to the PM is dependent on the myr group and HBR and is regulated by multiple factors, such as Gag multimerization, cellular and viral RNA, and the type of lipids and degree of acyl chain saturation (1, 2, 10–17). Proper targeting and membrane binding of HIV-1 Gag is also dependent on specific MA interactions with phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] (9), a PM component that functions as a substrate for numerous signaling proteins (18).
Despite advances in our understanding of the structure and function of the monomeric MA protein, the structural details of MA multimers and how the myr group mediates multimerization are not known. Previous X-ray crystallography studies of HIV-1 unmyristoylated MA [myr(–)MA] have shown that the protein adopts a trimeric arrangement (19), while NMR studies indicated that the protein is monomer in solution (20). In contrast, the biologically relevant myristoylated MA (myrMA) protein existed in a monomer–trimer equilibrium in solution, demonstrating the role of the myr group in promoting oligomerization (21). The myr group can be sequestered in a hydrophobic pocket or exposed, and exposure can be increased by factors that promote protein self-association, such as increasing protein concentration and inclusion of the CA domain (21).
NMR studies have shown that binding of PI(4,5)P2 containing truncated (tr) acyl chains [tr-PI(4,5)P2] to HIV-1 myrMA induced a conformational change that triggered myr exposure (22). The structure of myrMA bound to tr-PI(4,5)P2 showed that the 2′-acyl chain is sequestered into a preexisting hydrophobic cleft, whereas the inositol group is packed against the HBR of MA (22). Cryoelectron diffraction data obtained from two-dimensional (2D) crystals of myrMA grown on a lipid monolayer containing PI(4,5)P2 suggested that the protein organizes as hexamers of trimers (23–25). The hexamer of trimers lattice is used as a model to explain the mechanism of envelope (Env) incorporation into virus particles (26–29). Genetic and biochemical studies suggested that incorporation of the Env protein into virus particles is mediated by interactions between the myrMA domain of Gag and the cytoplasmic tail of Env (gp41CT) (30–34). How the gp41CT domain of Env interacts with the Gag lattice is still unknown. It is strongly suggested that myrMA trimerization is considered an obligatory step in HIV-1 Env incorporation (28), and that myrMA–gp41(CT) interactions and Env incorporation are mediated by formation of higher-order organization (hexamers of trimers) of myrMA on the membrane (25, 26, 28, 35).
Atomic details of myrMA arrangement in the lattice, how PI(4,5)P2 mediates myrMA binding to the PM during assembly and maturation, and how single residues in the MA can control Env incorporation are still lacking. A recent cryoelectron tomography study revealed that the myrMA protein undergoes dramatic structural changes to allow the formation of distinct hexameric lattices in immature and mature particles (36). These studies indicated that while PI(4,5)P2 was bound in the pocket in the mature form, it was absent in the immature form. These results suggested that myrMA also undergoes a structural transformation in the immature/mature transition, perhaps priming it for virus entry and uncoating. Based on these findings, a model was proposed in which HIV-1 not only achieves assembly of the CA core surrounding the RNA genome, but it also extends to repurpose the myrMA lattice for an entry or postentry function (36).
Here, we report the X-ray structure of the HIV-1 myrMA protein at 2.15 Å. In the crystal lattice, the myrMA protein is arranged as a hexamer of trimers with a threefold rotation axis at the central hole, which is thought to accommodate Env gp41CT to promote incorporation into virions. The trimer–trimer interactions in the lattice are mediated by the N-terminal residues, indicating that this motif plays a role not only in regulating the myr switch mechanism but also in stabilizing the myrMA lattice. We show that MA mutants defective of Env incorporation induced a conformational change in myrMA, which appear to disrupt formation of the lattice. We also provide evidence that support an alternating PI(4,5)P2 binding mechanism during assembly and maturation. These findings advance our understanding of a key mechanism in HIV-1 particle assembly.
Results
Structure Determination of the HIV-1 myrMA Trimer.
NMR studies have shown that the HIV-1 myrMA protein is predominantly globular and composed of five α-helical domains, a short 310 helix, and a β-hairpin (19, 21, 22). The C-terminal α-helix of the MA extends away from the globular region, progressively transitioning into a random coil, which serves as the linker between the MA and CA domains in the immature Gag polyprotein. Due to technical challenges, determination of the structure of the HIV-1 myrMA trimer has been elusive. Herein, to facilitate crystal screening conditions, we generated an MA construct lacking the unstructured amino acids 113 to 132 (myrMA112). Crystallization conditions were obtained and optimized for myrMA112, which allowed for determination of the high-resolution structure by X-ray crystallography at 2.15 Å. Atomic coordinates and structure factors have been deposited in the Protein Data Bank (PDB ID code 7TBP). Data collection and refinement statistics are shown in SI Appendix, Table S1.
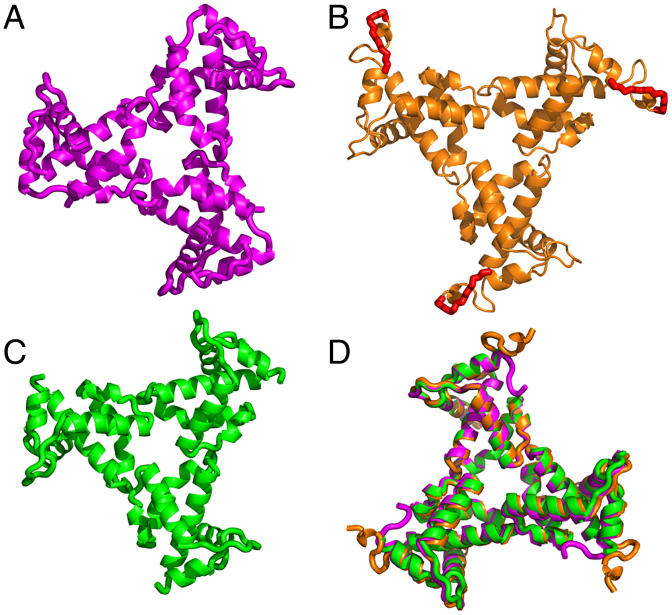
The asymmetric unit of the crystal structure contained three independent copies of myrMA112 (chains a, b, and c). Like the WT myrMA protein (21, 22), the myrMA112 structure consists of five α-helices, a 310 helix, and a β-hairpin. Residues 109 to 112 (chain a), 110 to 112 (chain b), and 108 to 112 (chain c) lacked electron density consistent with a disordered conformation, as previously shown by the NMR structural studies of the WT myrMA protein (21, 22). Interestingly, the complete N terminus, including the myr group and linkage to the N-terminal glycine, were well-defined in the electron density map encompassing chain b (SI Appendix, Fig. S1). For chains a and c, the electron density for the myr group as well as the N-terminal 2–5 (chain a) and 2–8 (chain c) residues was not observed presumably due to disorder. Each chain (a, b, and c) forms an independent trimer through crystallographic symmetry (Fig. 1). The trimeric arrangement of the three chains is very similar to each other, and to that observed for myr(–)MA, with only slight orientation differences of monomers in the trimeric assembly (SI Appendix, Fig. S2) (19). In summary, we report a structural snapshot of the myrMA and trimeric assemblies at high-resolution, which allowed for observation of the attached myr group.
Fig. 1.
X-ray structure of HIV-1 myrMA112. Three independent monomeric chains (a, b, and c) of MA112 were found in the asymmetric unit of the crystal. Trimeric assemblies are shown of chain a (A), chain b (B), and chain c (C). These assembled units are generated by imposing crystallographic symmetry. In B, the complete N terminus of myrMA112 is shown with attached myr group (red) and linkage to the N-terminal glycine shown in stick model. For chain b, well-defined electron density was observed for the entire N terminus (SI Appendix, Fig. S1). For chains a and c, electron density for the myr group as well as residues 2–5 and 2–9, respectively, was not observed presumably due to flexibility of the N terminus. (D) Overlay of the three trimers showing that the structures of the MA molecules and the trimer arrangements are nearly identical.
Myristoyl Swapping.
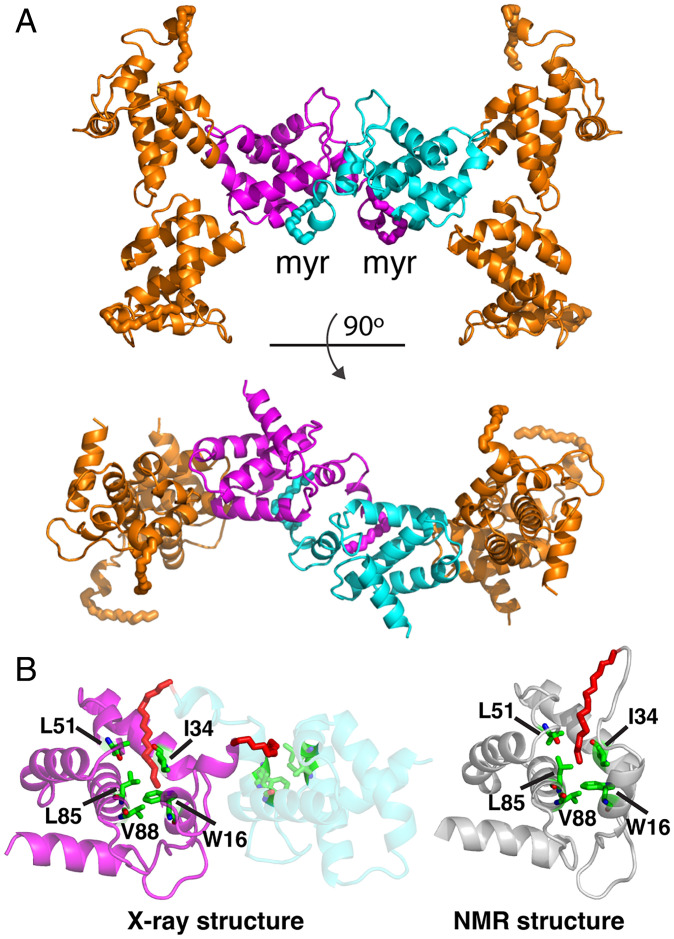
The X-ray structure of myrMA112 revealed that for chain b, in addition to trimeric associations, each myrMA within the trimer has a dimeric interaction with an adjacent copy of myrMA from another trimer. This interaction is symmetrical, with a twofold rotational symmetry, and shows the myr group of one subunit to insert in the hydrophobic cavity of the subunit across the twofold axis (Fig. 2A), introducing a myristoyl swap. Although it is possible that “myristoyl swapping” between the neighboring asymmetric units is a consequence of crystal packing, a few important structural details have emerged. First, chain b presents the myr group in an exposed conformation as related to self-association observed for WT myrMA in solution (21, 22) (SI Appendix, Fig. S3). Second, the myr group of myrMA112 adopts almost an identical conformation to that observed for the self-associated and myr-sequestered WT myrMA (21, 22), and is inserted into a cavity formed by the same exact residues of the adjacent molecule (Fig. 2B). These conformations reveal myrMA in myr-exposed conformation (no longer self-associated) and myr-sequestered conformation (to build a broad lattice). In the trimeric structure, the exposed myr groups are quite distant from each other (51 Å) (SI Appendix, Fig. S3). Third, the structure of the myrMA112 trimer is virtually identical to that observed for the myr(–)MA trimer (SI Appendix, Fig. S2) (19), demonstrating that although the myr group was found to promote MA self-association in solution (22, 37, 38), the trimer arrangement appears to be an intrinsic property of the protein.
Fig. 2.
Myristoyl swap. (A) Cartoon representation of myrMA112 chain b showing the dimeric interface formed by two trimers through a myr swapping mode (cyan and magenta). The two trimers are aligned in an antiparallel mode. (B) Cartoon representation showing two myrMA112 molecules (cyan and magenta) in which the myr group (red) is sequestered in a preexisting hydrophobic cavity of an adjacent molecule. For comparison, the NMR structure of the monomeric myrMA protein (gray; PDB ID code 2H3I) is shown with the myr group buried in an identical hydrophobic cavity formed by the side chains of residues Trp16, Ile34, Leu51, Leu85, and Val88.
Lattice Formation: Hexamer of Trimers.
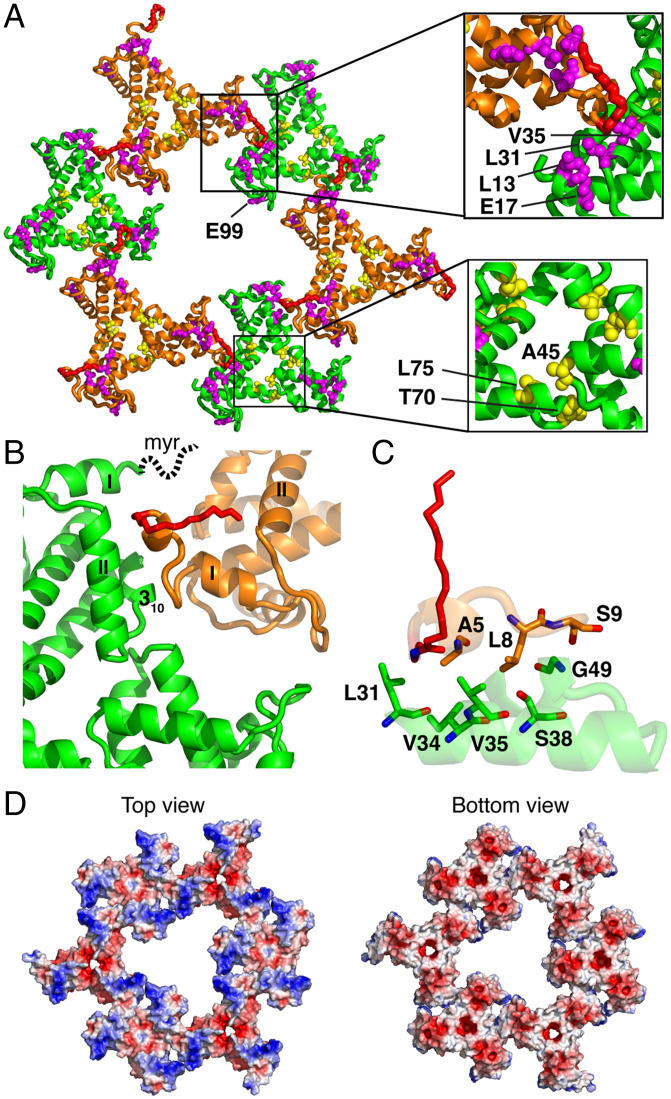
A continuous 2D lattice of myrMA is observed in the crystal, building from monomer to trimer and spatially arranged trimers to yield higher level hexameric arrangements. Individual spatial arrangements of trimers formed by chain c are observed in the lattice with a threefold rotation symmetry. Layered within the crystal lattice, three sets of trimers of chain b and three sets of trimers of chain c yielded a tightly packed hexamer of trimers (lattice b/c) with a threefold rotation axis (Fig. 3A and SI Appendix, Fig. S4). In this structure, all six sets of trimers are aligned in an equivalent top-to-bottom orientation and the myr groups of trimers b are present in the myr-exposed conformation. The structure of the myrMA112 lattice (b/c) revealed several unique features. First, the trimer–trimer interactions are mediated by the N-terminal loop of one myrMA molecule and α-helices I–II, as well as the 310 helix of a molecule from an adjacent trimer (Fig. 3B). Several N-terminal residues—including Ala5, Leu8, and Ser9—of one molecule are in close proximity and interact with residues Leu31, Ile34, Val35, Ser38, and Gly49 of an adjacent molecule (Fig. 3C). Second, trimer–trimer packing places the myr groups in juxtaposition (Fig. 3B). Third, MA residues Leu13, Glu17, Leu31, and Val35 implicated in Env incorporation are not exposed toward the central hole but are located in the trimer–trimer interface close to the N-terminal residues of myrMA (Fig. 3A) (30–33). These residues are involved in hydrophobic interactions that stabilize the myrMA structure, which likely stabilize the trimer–trimer interface and therefore the lattice (see Impact of Env-Defective MA Mutations on the Lattice Structure). Of note, hydrophobic interactions between the side chains of Leu13 and Leu31 within the same molecule stabilize packing of α-helices I–II (Fig. 3A), which is key for stabilizing the trimer–trimer interface within the lattice. Fourth, Ala45, Thr70, and Leu75 that were found to play a role in Env incorporation (28) are located in the MA–MA trimer interface (Fig. 3A), consistent with their role in the stabilization of the trimer structure and therefore the hexamer of trimers lattice. Last, the structure of the lattice displays the HBR surface that plays a key role in membrane binding (Fig. 3D). These structural features are consistent with a recent model of the immature particle based on cryotomography data (36). A lattice resembling that described in the HIV-1 mature particle has not been captured in our crystallization studies. Altogether, these structural data are consistent with the hypothesis that residues that stabilize the trimer structure, trimer–trimer interface, and therefore the lattice appear to strongly impact Env incorporation.
Fig. 3.
Hexamer of trimer lattice formation. (A) Hexamer of trimer is formed by trimers b and c. In this lattice, the myr group (red) is extruded and projecting out-of-plane. All six trimers are in a parallel mode with a threefold rotation axis. Residues implicated in Env incorporation (Glu17, Leu13, Leu31, and Val35; magenta) are located in the trimer–trimer interface. Glu99, which was also shown to impact Env incorporation, is located in the interior of the central hole of the hexamer lattice. Other residues that have been shown to impact Env incorporation by stabilizing the myrMA trimer are shown as yellow spheres. (B) Trimer–trimer interactions are mediated by the N-terminal residues of one molecule (orange) and α-helices I–II and 310 helix of an adjacent molecule (green). The myr group in chain c, which is not observed due to its dynamic nature, is drawn as a dotted line. (C) A close-up view of the residues involved in the trimer–trimer interface. (D) Electrostatic surface potential maps of the myrMA112 lattice. The blue (+5 kT/e) (e, electron charge; k, Boltzmann constant; T, temperature) and red (−5 kT/e) colors indicate positively and negatively charged electric potentials, respectively.
Impact of Env-Defective MA Mutations on the Lattice Structure.
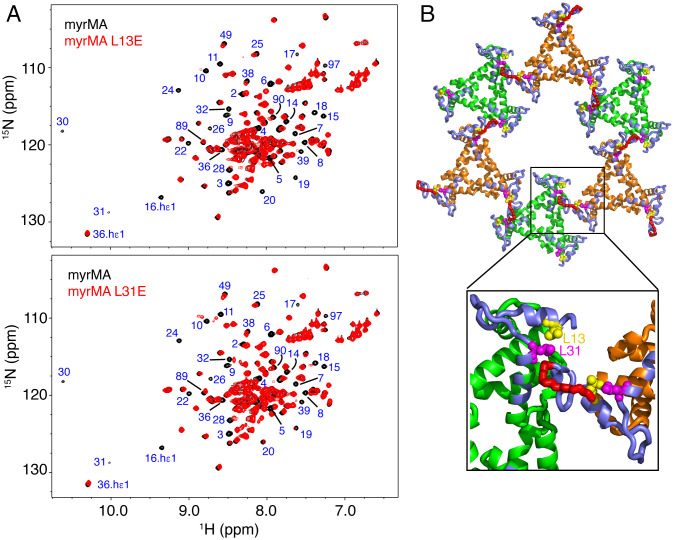
As discussed in the Introduction, the hexamer of trimers lattice of myrMA is used as a model to explain the mechanism of Env incorporation into virus particles (26–29). Point mutations of MA residues Leu13, Glu17, Leu31, Val35, and Glu99 were previously found to impair Env incorporation without affecting virus particle formation (30–33). However, it is not known whether these residues interact directly with gp41CT by accommodation in the central hole or if they play an indirect role in MA-mediated Env incorporation. The structure of the myrMA112 lattice revealed that except for Glu99, these residues are not projecting toward the central hole but rather reside in the MA intra- and intertrimer interfaces (Fig. 3A). Herein, we employed an NMR chemical shift perturbation (CSP) assay to assess the effect of the L13E and L31E mutations on the structure of myrMA. Typically, only a few signals corresponding to amino acids in the vicinity of the mutation site exhibit major CSPs in the 1H–15N HSQC spectrum. Our NMR data show that, compared with the spectrum of the WT myrMA protein, numerous residues exhibited substantial CSPs for both the L13E and L31E mutants (Fig. 4A). NMR signals corresponding to residues 2 to 40 were significantly shifted or severely broadened, indicating that L13E and L31E mutations induced structural/conformation changes in the myrMA protein. Mapping the CSPs on the structure of the myrMA112 lattice revealed that the changes are localized to the N-terminal region and α-helices I–II (Fig. 4B). These are the same motifs involved in the trimer–trimer interactions and appear to stabilize the lattice (Fig. 3). Surprisingly, the 2D 1H–15N HSQC spectra of myrMA L13E and L31E mutants are nearly identical (SI Appendix, Fig. S5), indicating that substitution of either of these two leucine residues causes a similar conformational/structural change in the protein.
Fig. 4.
Structural analysis of myrMA mutants that impair Env incorporation. (A) Overlay of 2D 1H–15N HSQC spectra for WT (black) and mutant L13E (Top) and L31E (Bottom) myrMA proteins collected at 120 μM (red). Substantial CSPs or severe broadening of signals corresponding to residues 2 to 40 are observed in the NMR spectra of myrMA L13E and L31E mutants when compared with the spectrum of the WT myrMA protein. (B) CSPs induced by the L13E and L31 mutants are mapped to the N-terminal loop and α-helices I–II of myrMA (slate). These CSPs are suggestive of a conformational change in the packing of α-helices, which also altered the position of the myr group (red) and ultimately lattice formation.
Next, we examined whether L13E and L31E mutations had any effect on the position of the myr group. Previous NMR studies of the WT myrMA protein have shown that a subset of 1H and 15N resonances for amino acid residues 3–18, Val35, Trp36, Arg39, Gly49, Glu52, His89, and Gln90 are sensitive to the position of the myr group and shift progressively toward the corresponding frequencies observed for myr(–)MA upon increasing protein concentration, indicating exposure of the myr group and a concomitant shift in the monomer–trimer equilibrium toward the trimeric species (21). Similar CSPs were observed upon changing the protonation state of His89 by changing the solution pH to 5, which stabilized the salt bridge formed between the side chains of His89 and Glu12, leading to exposure of the myr group and a shift in the equilibrium from monomer to trimer (37). As shown in the 2D 1H–15N HSQC data for the L13E and L31E mutants (Fig. 4 and SI Appendix, Fig. S6), signals corresponding to residues 3–18, Val35, Trp36, Arg39, Gly49, Glu52, His89, and Gln90 shift toward the corresponding frequencies observed for myr(–)MA, suggesting that L13E and L31E mutations caused a perturbation in the position of the myr group and a shift in equilibrium to the myr-exposed state.
A possible explanation of the CSPs observed for signals of residues 2 to 40 is that these changes are caused by changes in the conformation and position of the myr group only. To test this hypothesis, we collected 2D 1H–15N HSQC data for the myr(–)MA L13E and L31E proteins (SI Appendix, Fig. S7). Similar to the corresponding myrMA proteins, numerous signals corresponding to residues in the N-terminal loop and α-helices I–II exhibited significant CSPs or severe broadening, indicating a conformational change in these motifs (SI Appendix, Fig. S7). Taken together, our NMR data indicated that substitution of Leu13 or Leu31 to glutamate induced a conformational change in the N-terminal loop and α-helices I–II, which likely destabilized the trimer–trimer interface and therefore the hexamer lattice.
Alternate Binding of PI(4,5)P2 to myrMA.
Previous NMR-based structural studies revealed that binding of tr-PI(4,5)P2 to HIV-1 myrMA induced a conformational change that triggered myristate exposure, and that tr-PI(4,5)P2 adopted an “extended lipid” conformation in which the inositol head group and 2′-acyl acid chain bind to a hydrophobic cleft, and the 1′-acyl acid and exposed myr group bracket the HBR implicated in membrane binding (22). NMR-based liposome binding studies with native lipids, however, suggested that the acyl chains may not be involved in myrMA–PI(4,5)P2 interaction, and that interaction is mediated predominantly by electrostatic interactions between the HBR and the headgroup of PI(4,5)P2 (39). Yet, recent cryotomography data suggested that PI(4,5)P2 may bind to myrMA differently in the immature and mature HIV-1 particles (36). The lack of electron density corresponding to PI(4,5)P2 in the immature particle reconstruction suggested that interactions may be limited to the headgroup of PI(4,5)P2 and the HBR of myrMA (36). However, electron density attributed to the PI(4,5)P2 acyl chain inserted in a cleft in myrMA was observed in the mature particle (36), consistent with the NMR structural studies (22). How PI(4,5)P2 binds to MA in the immature particle is not clear.
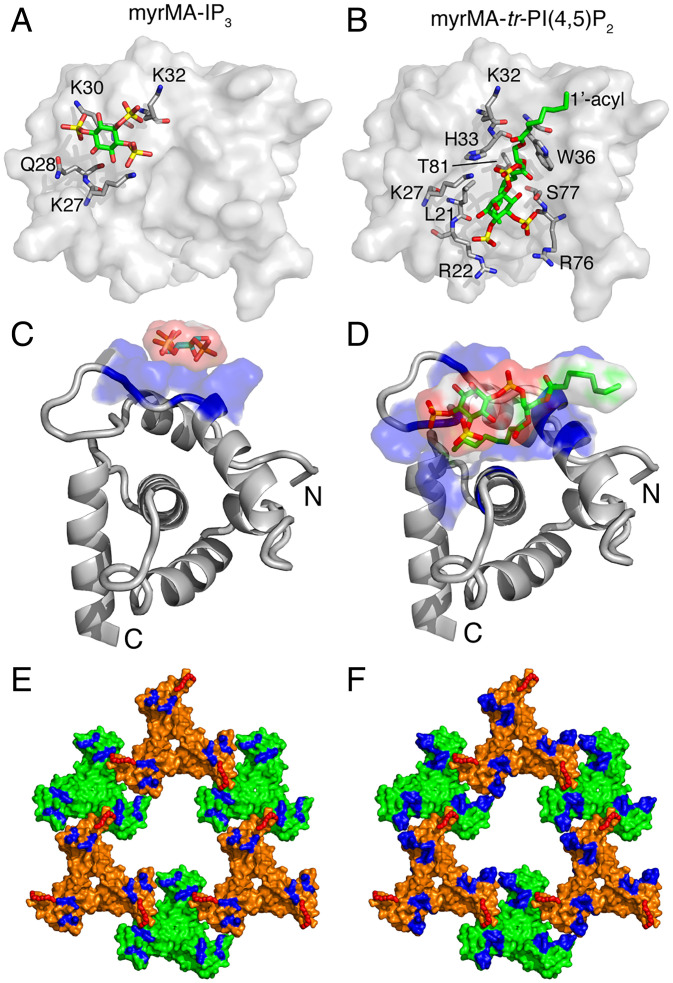
Here, we assessed how myrMA may utilize an alternating PI(4,5)P2 binding mechanism during assembly and maturation. We obtained 2D 1H–15N HSQC NMR data on the WT myrMA protein as titrated with inositol 1,4,5-trisphosphate (IP3), the headgroup of PI(4,5)P2. As shown in SI Appendix, Fig. S8, a subset of signals corresponding to residues Lys27, Gln28, Lys30, and Lys32 (group 1) exhibited significant CSPs upon addition of IP3. Interestingly, these residues are different from those involved in binding of tr-PI(4,5)P2 (22). Mapping the CSPs on the structure of the myrMA112 protein revealed that these interacting residues are located in the HBR and are not part of the hydrophobic cleft (Fig. 5). Of note, the 1H–15N resonances for Arg22, Trp36, Arg76, Thr81, and Ser77 were unaffected by IP3 titration; the latter four residues were previously shown to interact with the 2′-acyl chain of tr-PI(4,5)P2 (22). These results indicated that the headgroup of PI(4,5)P2 binds to myrMA via a different site (Fig. 5 A–D). Additionally, a second group of signals corresponding to residues 2–18, Arg39, Leu75, and His89 (group 2) that are well removed from group 1 residues in the myrMA structure also exhibited IP3-dependent CSPs and shifted progressively toward values observed in the myr(−)MA spectrum (SI Appendix, Fig. S8). Similar changes were observed previously upon binding of tr-PI(4,5)P2 to myrMA, indicative of a shift in the myr switch equilibrium from a predominantly myr-sequestered to myr-exposed state. These results indicated that binding of the headgroup of PI(4,5)P2 is sufficient for triggering myr exposure. Altogether, these findings indicate that PI(4,5)P2 is capable of binding to myrMA via an alternate mechanism during assembly and maturation.
Fig. 5.
Alternate PI(4,5)P2–binding mechanism. (A and C) Surface and cartoon representation, respectively, of the HIV-1 myrMA structure (PDB ID code 2H3I) highlighting basic residues that exhibited substantial chemical-shift changes upon binding of IP3. The IP3 molecule was modeled on the myrMA structure using PyMOL. (B and D) Surface and cartoon representation, respectively, of the HIV-1 myrMA structure bound to tr-P(4,5)P2 (PDB ID code 2H3V). Residues involved in tr-P(4,5)P2 binding are shown in sticks. Structures in A and B, and those in C and D, are viewed in identical orientations. The myr group is not shown in A–D. (E and F) Surface representation of the myrMA112 hexamer of trimer lattice highlighting residues that exhibited CSPs upon binding of IP3 and tr-PI(4,5)P2, respectively. The myr groups are shown as red sticks in E and F.
Discussion
Structural and biophysical studies of retroviral MA proteins and their interactions with phospholipids and membrane mimetics (bicelles, micelles, liposomes, and lipid nanodiscs) provided invaluable insights on key molecular determinants of MA-mediated Gag assembly on the PM (2, 22, 39–52). In this report, we provide high-resolution structural details of the myrMA trimer and hexamer lattice, the impact of myrMA mutations defective of Env incorporation on the myrMA structure and lattice formation, and provide evidence to support an alternating PI(4,5)P2 binding mechanism during assembly and maturation. We provide evidence that myrMA is capable of forming a well-ordered hexamer of trimers lattice even in the absence of PI(4,5)P2 or membrane. Previous cryoelectron tomography data revealed that the HIV-1 Gag lattice manifests as a cage of interconnected hexamers that cover most of the inner surface of the viral envelope (53, 54). Furthermore, cryoelectron diffraction studies obtained from 2D crystals of myrMA grown on a lipid monolayer containing PI(4,5)P2 suggested that myrMA organizes as hexamers of trimers (23). The hexamer of trimers lattice is increasingly used as a model to explain the mechanism of Env incorporation into virus particles (26–29). These findings led us to hypothesize that forces stabilizing the myrMA lattice are intrinsic to the protein and that binding to lipids and membranes may play a role in altering the orientation or conformation of the lattice.
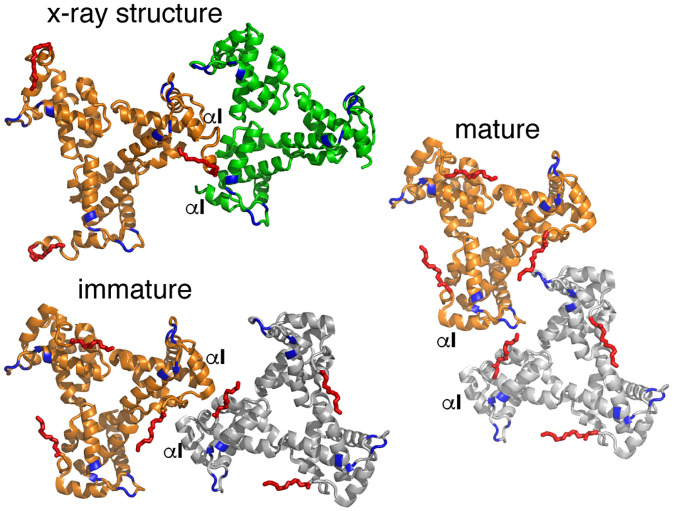
Recent cryotomography data revealed distinct trimer–trimer packing in the immature and mature HIV-1 particles (Fig. 6) (36). Subtomogram averaging analysis of the immature particle revealed a hexamer of myrMA trimer lattice with large holes at the sixfold axis (Fig. 7A). Similar analysis of the mature particle revealed large patches of a hexagonal lattice of myrMA trimers also with a sixfold rotation axis, but with a significantly smaller central hole (Fig. 7B). Because of the low resolution of the cryotomography data, atomic details and the type of residues involved in the trimer–trimer interface were not clearly identified. In the immature particle, the reconstruction model suggested that trimer–trimer contacts are mediated by the N-terminal residues, α-helix I, and the 310 helix (Fig. 6) (36). In the mature particle, the HBR loop faces acidic residues in the N terminus of α-helix IV and the 310 helix of an adjacent myrMA monomer, to form a dimeric interface that links trimers together (Fig. 6). The PI(4,5)P2 binding cleft adjacent to the HBR is positioned at the center of the dimer interface (Fig. 7A) (36). Although it offers a different arrangement (threefold vs. sixfold symmetry), the X-ray structure has several features similar to that of the immature lattice in which the N-terminal residues of myrMA are directly involved in trimer–trimer contact, placing the myr groups of MA subunits in juxtaposition (Figs. 6 and 7). These findings indicate that the N-terminal loop (residues 2 to 10) of myrMA is a key motif not only in regulating the myr switch mechanism but also in stabilizing the MA lattice.
Fig. 6.
Comparison of trimer–trimer contacts in the lattice. Cartoon illustration of the HIV-1 myrMA trimer–trimer units obtained by the X-ray structure and those reconstructed from the cryotomography data of the immature (PDB ID code 7OVQ) and mature (PDB ID code 7OVR) particles. The orange trimers are in an identical orientation. As shown, the trimer–trimer relationship and interface in the X-ray structure is relatively similar to that of the immature particle. The HBR and myr groups are shown in blue and red, respectively.
Fig. 7.
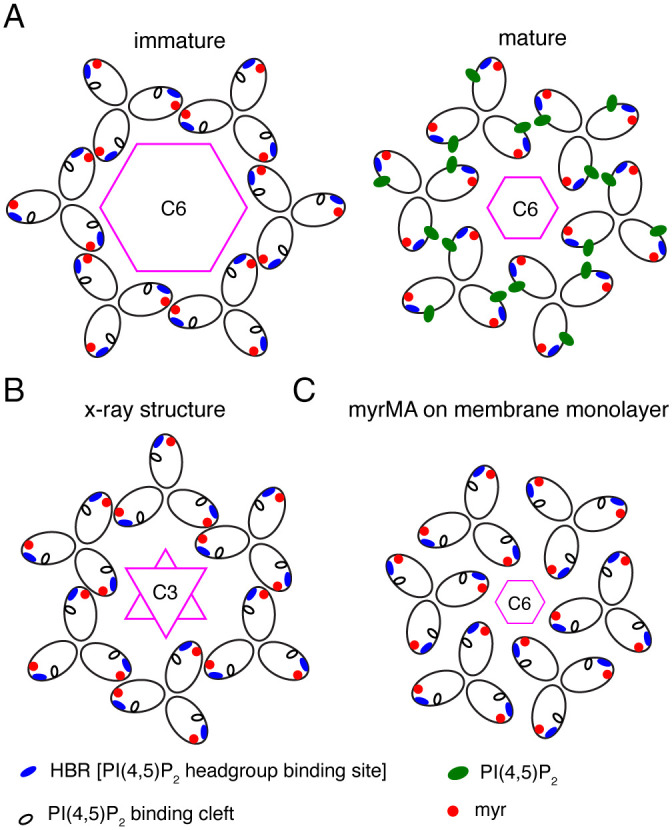
Comparison of myrMA lattices. (A) Illustration of the myrMA lattice for the immature and mature particles based on the cryotomography data (36). The trimer–trimer interactions are mediated by the N-terminal domain in the vicinity of the myr group, while the PI(4,5)P2 binding pocket is empty. In the mature myrMA lattice, PI(4,5)P2 is bound to the cleft and myrMA trimer–trimer interactions are formed by the HBR and PI(4,5)P2. (B) Schematic illustration of the myrMA lattice based on the X-ray structure of myrMA112. In this lattice, myrMA–myrMA interaction at the trimer–trimer interface is mediated by the N-terminal residues. Of note, myrMA–myrMA interaction at the trimer–trimer interface places the myr groups (red) in juxtaposition. The HBR and PI(4,5)P2 binding cleft are also shown. (C) A schematic representation of the myrMA lattice based on the assembly of the protein on a membrane monolayer containing PI(4,5)P2 (23).
Comparison of our X-ray structure with the MA lattice models constructed from the low-resolution reconstruction data (7 to 22 Å) (23, 36) revealed a few intriguing differences. While models of the immature and mature particles (Fig. 7A), as well as that of myrMA assembled on a membrane monolayer containing PI(4,5)P2 (Fig. 7C), adopt a hexameric arrangement with C6 rotation axis, they differ in the arrangement of the trimeric units, trimer–trimer contact points, and size of the central hole (Fig. 7). Our X-ray structure of the myrMA lattice offers yet a different arrangement with a threefold rotation axis but relatively similar trimer–trimer contact points to the model of the immature particle (Figs. 6 and 7). These findings lead us to propose a “structural plasticity” feature of the myrMA lattice in which the trimer units are capable of adopting different orientations, while maintaining a relatively unchanged myrMA structure.
Despite the accumulating evidence for an interplay between Gag assembly on the PM and the incorporation of Env into virus particles, this mechanism is not fully understood. There is mounting evidence that Env incorporation may require formation of a Gag lattice (hexamers of trimers) on the membrane (25, 26, 28, 35). The hypothesis of an interplay between myrMA and gp41CT is supported by the finding that point mutations in the MA—such as L13E, E17K, L31E, V35E, and E99V—had adverse effects on Env incorporation without affecting virus particle formation (30–34). Likewise, nonconservative mutations in the trimer interface of the MA (A45E, T70R, and L75G) (Fig. 3) were found to impair Env incorporation and infectivity, indicating that MA trimerization is an obligatory step for Env incorporation (28). The trimer interface was characterized by hydrogen–deuterium exchange mass spectrometry and structural studies of a compensatory mutant that rescues Env incorporation (51, 55). Nanoscale single-particle tracking of Env on the PM has demonstrated that Env immobilization at sites of Gag assembly is regulated by gp41CT and specific MA residues such as Leu13, and that Env is restricted in subviral regions within the Gag lattice (56). Collectively, these studies led to a model for Env incorporation in which the gp41CT domain is accommodated in the central aperture formed by the hexamer of trimers (27, 28). Our structural data show that Leu13, Glu17, Leu31, and Val35 reside in the myrMA intra- and intertrimer interfaces, whereas Glu99 is projecting toward the central hole (Fig. 3).
Furthermore, we provide evidence that substitution of Leu13 or Leu31 to glutamate induced a conformational change in the N-terminal loop and α-helices I–II, leading to perturbation of the myr group position, destabilization of the trimer–trimer interface, and therefore the lattice. Most importantly, NMR data show that both mutations induced a similar structural change in the myrMA protein, suggesting that the defect in Env incorporation caused by these mutations in is a consequence of a structural change in the MA and destabilization of the lattice. It has yet to be determined whether these mutations have any effect on MA–membrane binding. Previous studies have shown that substitution of Val35 to Ile compensated for the effects of L13E mutation and rescued Env incorporation defect (29, 31). As shown in Fig. 3C, Val35 is located in a relatively hydrophobic nest (Ala5, Leu8, Leu31), which stabilizes the trimer–trimer interface and consequently the lattice. We hypothesize that although this mutation is conservative (Val to Ile), the more branched hydrophobic side chain of Ile may stabilize the lattice by making intermolecular contacts with hydrophobic residues on the adjacent molecule.
We also provide evidence that support a molecular switch mechanism for MA–membrane binding during virus assembly and maturation. The HBR in MA (Fig. 5) is highly conserved in almost all retroviral MA proteins and serves as the binding site for acidic phospholipids in the inner leaflet of the PM (1, 57). A few models of the HIV-1 myrMA trimer bound to membranes have been proposed based on structural, biochemical, biophysical, and functional studies (2, 22, 39). Our data indicate that, in addition to the previously described mechanism of PI(4,5)P2 binding to myrMA (22), the protein is capable of biding to the headgroup of PI(4,5)P2 via a second site in the HBR, also promoting myr exposure. These findings support an alternating PI(4,5)P2 binding mechanism in the immature and mature particles, as described recently (Fig. 5) (36). Interestingly the absence of electron density for PI(4,5)P2 in the cryotomography reconstruction of the immature myrMA lattice suggested that interactions of myrMA with PI(4,5)P2 are mediated by interactions of the HBR and the headgroup of PI(4,5)P2 (36). We have shown that the headgroup of PI(4,5)P2 can interact directly with Lys27, Lys30, and Lys32, which are not directly involved in binding of tr-PI(4,5)P2 (Fig. 5) (22) but are considered key residues for Gag binding to the PM, virus assembly, and particle release (39, 58, 59). On the other hand, the observation of electron density for an extended acyl chain attributed to the PI(4,5)P2 ligand in the subtomogram reconstruction of the mature particle led to the suggestion that maturation of Gag results in the partial removal of up to ∼2,500 of the ∼150,000 lipids in the inner leaflet of the viral membrane (36). Interestingly, in a recent X-ray structure of the HIV-1 myr(–)MA protein in complex with inositol hexakisphosphate (IP6), the asymmetric unit contained three types of structures in which the IP6 ligand interacted with various residues in the HBR (60). One of the three structures is almost identical to that described for myrMA bound to IP3 (Fig. 5 A and C), consistent with the hypothesis that MA contains alternate PI(4,5)P2 binding sites and that the conformational switch of myrMA during maturation may also involve lipid/membrane reorganization.
In conclusion, we provide an atomic view for the HIV-1 myrMA lattice, which reveals invaluable structural insights on the arrangement of the myrMA subunits, trimers, trimer–trimer interface, myr swapping, impact of MA mutations defective of Env incorporation on the structure of myrMA, and consequently lattice formation. Our data also support an alternating MA–PI(4,5)P2 binding mechanism during virus assembly and maturation. These findings fill a major gap in our understanding of the mechanisms of Gag assembly on the PM and Env incorporation into virus particles. In a very recent study by Moderna and the NIH, it was shown that messenger RNA vaccine coexpressing membrane-anchored HIV-1 Env and simian immunodeficiency virus Gag proteins to generate virus-like particles induced antibodies capable of broad neutralization and reduces the risk of infection in rhesus macaques (61). The findings described here may facilitate the development of new therapeutic agents that inhibit Gag assembly, Env incorporation, and ultimately virus production.
Materials and Methods
Plasmid Construction.
A plasmid encoding for HIV-1 MA (132 amino acid residues; pNL4-3 strain) and yeast N-terminal myristoyl transferase was provided by Michael Summers, Howard Hughes Medical Institute, University of Maryland Baltimore County, Baltimore, MD. Using a QuikChange Lightning site-directed mutagenesis kit (Agilent Technologies), the MA gene was modified by inserting a stop codon after amino acid residue 112 codon, yielding an MA gene encoding for untagged MA112. (Note: The N-terminal Met, which is absent in the myristoylated protein, is designated as residue 1.) The L13E and L31E MA mutant constructs were generated using a QuikChange Lightning site-directed mutagenesis kit. Forward and reverse primers (Integrated DNA Technologies) extended 15 base pairs on either side of the mutation codon. DNA sequences were verified by plasmid sequencing at the Heflin Genomics Core at the University of Alabama at Birmingham.
Protein Expression and Purification.
The myrMA112 protein was overexpressed in Escherichia coli BL21-CodonPlus (DE3)-RIL cells (Agilent Technologies). Cells were grown at 37 °C in Luria–Bertani broth medium containing 100 mg/L ampicillin. Media were supplemented with 60 μM myristic acid (Sigma Aldrich) when A600 reached ∼0.2. At A600 0.7 to 0.8, cells were induced with 0.5 mM isopropyl β-d-1-thiogalactopyranoside (Gold Biotechnology). Cells were then grown at 37 °C for 4 h, spun down, and stored at –80 °C. The cell pellet was resuspended in lysis buffer containing 20 mM phosphates (pH 8.0), 1 M ammonium sulfate, 2 mM DTT, and 1× inhibitor mixture (EMD Millipore). Cells were then sonicated, and lysate was separated from pellet by spinning down at 35,000 × g for 30 min at 4 °C. The supernatant was loaded into a HiTrap Butyl FF column (Cytiva) equilibrated in lysis buffer (excluding protease inhibitors). Resin was then washed with lysis buffer (excluding protease inhibitors). The myrMA112 protein was eluted via a gradient using a buffer containing 20 mM sodium phosphates (pH 8) and 2 mM DTT. Protein purity was verified by SDS/PAGE. Fractions containing the protein were pooled and dialyzed in a buffer containing 20 mM phosphates (pH 8.0), 1 M ammonium sulfate, and 2 mM DTT. Purification steps described above were repeated to remove minor contaminants. The protein was then run on a size-exclusion column (HiLoad 16/600 Superdex 75, Cytiva) in a buffer containing 20 mM Hepes (pH 8) and 100 mM NaCl. 15N–labeled WT myrMA, WT myr(–)MA, myrMA L13E, myrMA L31E, myr(–)MA L13E, and myr(–)MA L31E samples were prepared as described previously (21, 22, 40).
X-Ray Crystallography and Structure Determination.
The HIV-1 myrMA112 protein (20 mg/mL) in 20 mM Hepes (pH 8) and 100 mM NaCl was subjected to crystallization trials. Diffraction quality crystals were obtained using hanging-drop vapor diffusion in a solution of 40% PEG 400 (Hampton Research) and 0.1 M Bis-Tris-propane buffer (pH 4.7). Crystals were cryocooled in the same conditions. X-ray diffraction data were collected at the Advance Photon Source, Southeast Regional Collaborative Access Team (SER-CAT) beamline 22-ID. Raw intensity data were processed with the HKL2000 software package (62). The initial electron density map was generated via molecular replacement with PHASER (63) using the previously solved structure of the WT myr(–)MA protein (PDB ID code 1HIW) (19). The structure was then iteratively refined with PHENIX (64) and with manual manipulation in Coot (65). Visualization of structures was performed using PyMOL (66).
NMR Spectroscopy.
NMR data were collected at 35 °C on a Bruker Avance III (600 MHz 1H) equipped with a cryogenic triple-resonance probe, processed with NMRPIPE (67) and analyzed with NMRVIEW (68). The 15N–labeled protein samples were prepared at 120 μM in 50 mM sodium phosphates (pH 5.5), 100 mM NaCl, 1 mM EDTA, and 2 mM TCEP.
Lipid NMR Titrations.
1H–15N HSQC NMR titrations with IP3 (Echelon Biosciences) were conducted with a 100-μM sample of 15N-labeled myrMA in 50 mM sodium phosphates (pH 5.5) and 2 mM TCEP. A stock solution of IP3 was prepared in deionized water at 20 mM.
Supplementary Material
Acknowledgments
We thank Dr. Peter E. Prevelige for the feedback on the manuscript. This work was supported by NIH Grant 9 R01 AI150901-10 (to J.S.S.). The High-Field NMR facility at the University of Alabama at Birmingham was established through NIH Grant 1S10RR026478 and is currently supported through the O’Neal Comprehensive Cancer Center (National Cancer Institute Grant P30 CA013148). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2200794119/-/DCSupplemental.
Data Availability
The atomic coordinates of myrMA112 have been deposited in the Protein Data Bank, PDB ID code 7TBP.
References
- 1.Freed E. O., HIV-1 assembly, release and maturation. Nat. Rev. Microbiol. 13, 484–496 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy R. E., Saad J. S., The interplay between HIV-1 Gag binding to the plasma membrane and Env incorporation. Viruses 12, 548 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mücksch F., Laketa V., Müller B., Schultz C., Kräusslich H. G., Synchronized HIV assembly by tunable PIP2 changes reveals PIP2 requirement for stable Gag anchoring. eLife 6, e25287 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hendrix J., et al. , Live-cell observation of cytosolic HIV-1 assembly onset reveals RNA-interacting Gag oligomers. J. Cell Biol. 210, 629–646 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gousset K., et al. , Real-time visualization of HIV-1 GAG trafficking in infected macrophages. PLoS Pathog. 4, e1000015 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jouvenet N., et al. , Plasma membrane is the site of productive HIV-1 particle assembly. PLoS Biol. 4, e435 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Welsch S., et al. , HIV-1 buds predominantly at the plasma membrane of primary human macrophages. PLoS Pathog. 3, e36 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chukkapalli V., Hogue I. B., Boyko V., Hu W.-S., Ono A., Interaction between HIV-1 Gag matrix domain and phosphatidylinositol-(4,5)-bisphosphate is essential for efficient Gag-membrane binding. J. Virol. 82, 2405–2417 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ono A., Ablan S. D., Lockett S. J., Nagashima K., Freed E. O., Phosphatidylinositol (4,5) bisphosphate regulates HIV-1 Gag targeting to the plasma membrane. Proc. Natl. Acad. Sci. U.S.A. 101, 14889–14894 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thornhill D., Murakami T., Ono A., Rendezvous at plasma membrane: Cellular lipids and tRNA set up sites of HIV-1 particle assembly and incorporation of host transmembrane proteins. Viruses 12, 842 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chukkapalli V., Inlora J., Todd G. C., Ono A., Evidence in support of RNA-mediated inhibition of phosphatidylserine-dependent HIV-1 Gag membrane binding in cells. J. Virol. 87, 7155–7159 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dick R. A., Goh S. L., Feigenson G. W., Vogt V. M., HIV-1 Gag protein can sense the cholesterol and acyl chain environment in model membranes. Proc. Natl. Acad. Sci. U.S.A. 109, 18761–18766 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalton A. K., Ako-Adjei D., Murray P. S., Murray D., Vogt V. M., Electrostatic interactions drive membrane association of the human immunodeficiency virus type 1 Gag MA domain. J. Virol. 81, 6434–6445 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barros M., et al. , Membrane binding of HIV-1 matrix protein: Dependence on bilayer composition and protein lipidation. J. Virol. 90, 4544–4555 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaines C. R., et al. , HIV-1 matrix protein interactions with tRNA: Implications for membrane targeting. J. Mol. Biol. 430, 2113–2127 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wen Y., Vogt V. M., Feigenson G. W., PI(4,5)P2 clustering and its impact on biological functions. Annu. Rev. Biochem. 90, 681–707 (2021). [DOI] [PubMed] [Google Scholar]
- 17.Bou-Nader C., et al. , HIV-1 matrix-tRNA complex structure reveals basis for host control of Gag localization. Cell Host Microbe 29, 1421–1436.e7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolay S., Basu U., Raghu P., Control of diverse subcellular processes by a single multi-functional lipid phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2]. Biochem. J. 473, 1681–1692 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill C. P., Worthylake D., Bancroft D. P., Christensen A. M., Sundquist W. I., Crystal structures of the trimeric HIV-1 matrix protein: Implications for membrane association. Proc. Natl. Acad. Sci. U.S.A. 93, 3099–3104 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Massiah M. A., et al. , Three-dimensional structure of the human immunodeficiency virus type 1 matrix protein. J. Mol. Biol. 244, 198–223 (1994). [DOI] [PubMed] [Google Scholar]
- 21.Tang C., et al. , Entropic switch regulates myristate exposure in the HIV-1 matrix protein. Proc. Natl. Acad. Sci. U.S.A. 101, 517–522 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saad J. S., et al. , Structural basis for targeting HIV-1 Gag proteins to the plasma membrane for virus assembly. Proc. Natl. Acad. Sci. U.S.A. 103, 11364–11369 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alfadhli A., Barklis R. L., Barklis E., HIV-1 matrix organizes as a hexamer of trimers on membranes containing phosphatidylinositol-(4,5)-bisphosphate. Virology 387, 466–472 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alfadhli A., Huseby D., Kapit E., Colman D., Barklis E., Human immunodeficiency virus type 1 matrix protein assembles on membranes as a hexamer. J. Virol. 81, 1472–1478 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alfadhli A., et al. , Trimer enhancement mutation effects on HIV-1 matrix protein binding activities. J. Virol. 90, 5657–5664 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tedbury P. R., Ablan S. D., Freed E. O., Global rescue of defects in HIV-1 envelope glycoprotein incorporation: Implications for matrix structure. PLoS Pathog. 9, e1003739 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tedbury P. R., Freed E. O., The role of matrix in HIV-1 envelope glycoprotein incorporation. Trends Microbiol. 22, 372–378 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tedbury P. R., Novikova M., Ablan S. D., Freed E. O., Biochemical evidence of a role for matrix trimerization in HIV-1 envelope glycoprotein incorporation. Proc. Natl. Acad. Sci. U.S.A. 113, E182–E190 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tedbury P. R., et al. , HIV-1 matrix trimerization-impaired mutants are rescued by matrix substitutions that enhance envelope glycoprotein incorporation. J. Virol. 94, e01526-19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dorfman T., Mammano F., Haseltine W. A., Göttlinger H. G., Role of the matrix protein in the virion association of the human immunodeficiency virus type 1 envelope glycoprotein. J. Virol. 68, 1689–1696 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freed E. O., Martin M. A., Domains of the human immunodeficiency virus type 1 matrix and gp41 cytoplasmic tail required for envelope incorporation into virions. J. Virol. 70, 341–351 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freed E. O., Martin M. A., Virion incorporation of envelope glycoproteins with long but not short cytoplasmic tails is blocked by specific, single amino acid substitutions in the human immunodeficiency virus type 1 matrix. J. Virol. 69, 1984–1989 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu X., Yuan X., Matsuda Z., Lee T.-H., Essex M., The matrix protein of human immunodeficiency virus type 1 is required for incorporation of viral envelope protein into mature virions. J. Virol. 66, 4966–4971 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cosson P., Direct interaction between the envelope and matrix proteins of HIV-1. EMBO J. 15, 5783–5788 (1996). [PMC free article] [PubMed] [Google Scholar]
- 35.Alfadhli A., et al. , Analysis of HIV-1 matrix-envelope cytoplasmic tail interactions. J. Virol. 93, e01079-19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qu K., et al. , Maturation of the matrix and viral membrane of HIV-1. Science 373, 700–704 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fledderman E. L., et al. , Myristate exposure in the human immunodeficiency virus type 1 matrix protein is modulated by pH. Biochemistry 49, 9551–9562 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salazar M., Champoux J. J., Reid B. R., Sugar conformations at hybrid duplex junctions in HIV-1 and Okazaki fragments. Biochemistry 32, 739–744 (1993). [DOI] [PubMed] [Google Scholar]
- 39.Mercredi P. Y., et al. , Structural and molecular determinants of membrane binding by the HIV-1 matrix protein. J. Mol. Biol. 428, 1637–1655 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saad J. S., et al. , Point mutations in the HIV-1 matrix protein turn off the myristyl switch. J. Mol. Biol. 366, 574–585 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vlach J., Saad J. S., Trio engagement via plasma membrane phospholipids and the myristoyl moiety governs HIV-1 matrix binding to bilayers. Proc. Natl. Acad. Sci. U.S.A. 110, 3525–3530 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saad J. S., et al. , Structure of the myristylated HIV-2 MA protein and the role of phosphatidylinositol-(4,5)-bisphosphate in membrane targeting. J. Mol. Biol. 382, 434–447 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vlach J., et al. , Structural basis for targeting avian sarcoma virus Gag polyprotein to the plasma membrane for virus assembly. J. Biol. Chem. 293, 18828–18840 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamard-Peron E., et al. , Targeting of murine leukemia virus gag to the plasma membrane is mediated by PI(4,5)P2/PS and a polybasic region in the matrix. J. Virol. 84, 503–515 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stansell E., et al. , Basic residues in the Mason-Pfizer monkey virus gag matrix domain regulate intracellular trafficking and capsid-membrane interactions. J. Virol. 81, 8977–8988 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prchal J., Srb P., Hunter E., Ruml T., Hrabal R., The structure of myristoylated Mason-Pfizer monkey virus matrix protein and the role of phosphatidylinositol-(4,5)-bisphosphate in its membrane binding. J. Mol. Biol. 423, 427–438 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown L. A., et al. , NMR structure of the myristylated feline immunodeficiency virus matrix protein. Viruses 7, 2210–2229 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anraku K., et al. , Highly sensitive analysis of the interaction between HIV-1 Gag and phosphoinositide derivatives based on surface plasmon resonance. Biochemistry 49, 5109–5116 (2010). [DOI] [PubMed] [Google Scholar]
- 49.Shkriabai N., et al. , Interactions of HIV-1 Gag with assembly cofactors. Biochemistry 45, 4077–4083 (2006). [DOI] [PubMed] [Google Scholar]
- 50.Fernandes F., et al. , Phosphoinositides direct equine infectious anemia virus gag trafficking and release. Traffic 12, 438–451 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murphy R. E., et al. , Structural and biophysical characterizations of HIV-1 matrix trimer binding to lipid nanodiscs shed light on virus assembly. J. Biol. Chem. 294, 18600–18612 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Herrmann D., et al. , Structural insights into the mechanism of human T-cell Leukemia virus type 1 Gag targeting to the plasma membrane for assembly. J. Mol. Biol. 433, 167161 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wright E. R., et al. , Electron cryotomography of immature HIV-1 virions reveals the structure of the CA and SP1 Gag shells. EMBO J. 26, 2218–2226 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Briggs J. A., et al. , Structure and assembly of immature HIV. Proc. Natl. Acad. Sci. U.S.A. 106, 11090–11095 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eastep G. N., Ghanam R. H., Green T. J., Saad J. S., Structural characterization of HIV-1 matrix mutants implicated in envelope incorporation. J. Biol. Chem. 296, 100321 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pezeshkian N., Groves N. S., van Engelenburg S. B., Single-molecule imaging of HIV-1 envelope glycoprotein dynamics and Gag lattice association exposes determinants responsible for virus incorporation. Proc. Natl. Acad. Sci. U.S.A. 116, 25269–25277 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olety B., Ono A., Roles played by acidic lipids in HIV-1 Gag membrane binding. Virus Res. 193, 108–115 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chukkapalli V., Oh S. J., Ono A., Opposing mechanisms involving RNA and lipids regulate HIV-1 Gag membrane binding through the highly basic region of the matrix domain. Proc. Natl. Acad. Sci. U.S.A. 107, 1600–1605 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ono A., Freed E. O., Cell-type-dependent targeting of human immunodeficiency virus type 1 assembly to the plasma membrane and the multivesicular body. J. Virol. 78, 1552–1563 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ciftci H., et al. , Structural insight into host plasma membrane association and assembly of HIV-1 matrix protein. Sci. Rep. 11, 15819 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang P., et al. , A multiclade env-gag VLP mRNA vaccine elicits tier-2 HIV-1-neutralizing antibodies and reduces the risk of heterologous SHIV infection in macaques. Nat. Med. 27, 2234–2245 (2021). [DOI] [PubMed] [Google Scholar]
- 62.Otwinowski Z., Minor W., Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997). [DOI] [PubMed] [Google Scholar]
- 63.McCoy A. J., et al. , Phaser crystallographic software. J. Appl. Cryst. 40, 658–674 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Adams P. D., et al. , PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Emsley P., Lohkamp B., Scott W. G., Cowtan K., Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.DeLano W. L., The PyMOL Molecular Graphics System (DeLano Scientific, San Carlos, CA, 2002). [Google Scholar]
- 67.Delaglio F., et al. , NMRPipe: A multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 6, 277–293 (1995). [DOI] [PubMed] [Google Scholar]
- 68.Johnson B. A., Blevins R. A., NMR view: A computer program for the visualization and analysis of NMR data. J. Biomol. NMR 4, 603–614 (1994). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The atomic coordinates of myrMA112 have been deposited in the Protein Data Bank, PDB ID code 7TBP.