Abstract
To examine the effects of overexpression of trigger factor (TF) on recombinant proteins produced in Escherichia coli, we constructed plasmids that permitted controlled expression of TF alone or together with the GroEL-GroES chaperones. The following three proteins that are prone to aggregation were tested as targets: mouse endostatin, human oxygen-regulated protein ORP150, and human lysozyme. The results revealed that TF overexpression had marked effects on the production of these proteins in soluble forms, presumably through facilitating correct folding. Whereas overexpression of TF alone was sufficient to prevent aggregation of endostatin, overexpression of TF together with GroEL-GroES was more effective for ORP150 and lysozyme, suggesting that TF and GroEL-GroES play synergistic roles in vivo. Although coexpression of the DnaK-DnaJ-GrpE chaperones was also effective for endostatin and ORP150, coexpression of TF and GroEL-GroES was more effective for lysozyme. These results attest to the usefulness of the present expression plasmids for improving protein production in E. coli.
Recombinant proteins produced in Escherichia coli often aggregate or degrade rapidly because of their inability to form correct tertiary structures due to anomalies in protein folding. In some cases, overexpression of molecular chaperones, such as GroEL-GroES and DnaK-DnaJ-GrpE, facilitate protein folding and enhance production of active proteins (16, 18). We previously constructed a series of versatile plasmids for controlled expression of these chaperones and described their utility for stabilizing or preventing aggregation of certain recombinant proteins (9). However, these plasmids are not always effective; there are many instances in which production of active proteins is not improved by the chaperones and seems to require additional factors or conditions. Thus, we examined new chaperonelike factors, including E. coli trigger factor (TF), whose role in protein folding was implicated by the results of several in vitro experiments. This 50-kDa protein exhibits peptidyl-prolyl cis/trans isomerase (PPIase) activity and has a domain structure that is conserved in the FK506-binding protein family (14, 17). TF was originally identified as a protein that can bind to certain precursor proteins and facilitate their transport into membrane vesicles (1). The results of subsequent studies, however, failed to support hypothesis that TF plays a role in protein transport (2) and instead suggested that it may play a role in protein folding because of its association with nascent polypeptides, as well as the 50S ribosome (3, 17). Moreover, TF was found to be associated with GroEL and can strengthen GroEL-substrate binding to facilitate protein folding (5) or degradation (6).
In this study, we constructed plasmids with which we could easily manipulate the production of TF, alone or together with GroEL-GroES chaperones, and examined the effects of coexpression of these molecules on the production of several recombinant proteins. The target proteins employed were mouse endostatin (molecular mass, 20 kDa), human oxygen-regulated protein ORP150 (150 kDa), and human lysozyme (14 kDa), which contained 8, 58, and 2 proline residues, respectively, and formed inclusion bodies when they were expressed in E. coli.
MATERIALS AND METHODS
Bacterial strains and plasmids.
E. coli K-12 strains JM109 {recA1 endA1 gyrA96 thi hsdR17 supE44 relA1 Δ(lac-proAB)/F′[traD36 proAB+ lacIq lacZΔM15]} and BL21 (F− ompT r−B m−B) were used throughout this study. An expression vector for human ORP150, pORP4, was constructed as follows. A 3.2-kb DNA fragment containing the coding region for the ORP150 mature protein (4) was inserted into the pTrc99A vector (Pharmacia Biotech) just downstream of its ATG initiation codon. Mouse endostatin expression vector pTB01#8 was kindly donated by Thomas Boehm (10), and human lysozyme expression vector pLY-46 was donated by Yasushi Matsuki (Sumitomo Chemical).
Media, chemicals, and culture conditions.
L broth was used for most experiments. To express lysozyme, RM medium (1× M9 salts, 2% Casamino Acids, 1% glycerol, 1 mM MgCl2) was used. All chemicals were purchased from Wako Pure Chemicals (Osaka, Japan) and Nacalai Tesque (Kyoto, Japan). Strains harboring a pair of expression plasmids (one for a recombinant protein and the other for chaperones) were grown in medium containing 50 μg of ampicillin per ml and 20 μg of chloramphenicol per ml at 37°C with constant aeration. To induce chaperone expression, l-arabinose and/or tetracycline was added to the medium at the concentrations indicated below. When a culture reached the mid-log phase (optical density at 600 nm, 0.4 to 0.6), expression of the recombinant protein was induced and the culture was incubated for an additional 1 to 4 h.
Construction of plasmids.
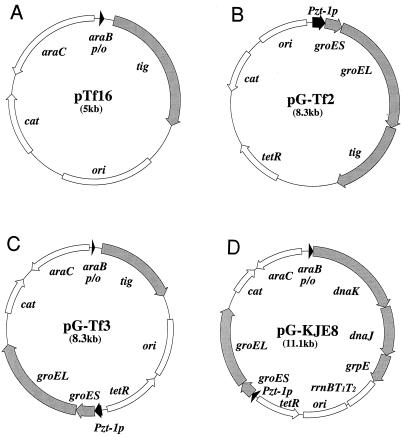
TF expression plasmid pTf16 with the l-arabinose-inducible (araB) promoter was constructed by cutting out a 1.7-kb DNA fragment containing the TF coding region (tig gene) from λ clone 148 of the Kohara genomic library (8) (digested with XmnI and NdeI) and inserting it just downstream of the araB promoter in vector pAR3 (11) that had been treated with PstI and BglII. Both the tig gene and the vector were treated with T4 DNA polymerase to form blunt ends before ligation. Plasmid pG-Tf2, which could express TF and GroEL-GroES together under the tetracycline-inducible promoter (Pzt-1), was constructed by cutting out a 2.5-kb Bsp1286I-NruI fragment containing the tig gene from λ clone 148 (8) and inserting it just downstream of groEL in GroEL-GroES expression plasmid pGro11 (9) that had been cut with SmaI. The resulting plasmid, pG-Tf1, was partially digested with BspHI and then with AflII; this was followed by isolation of the 8.3-kb fragment and self-ligation to remove a 0.3-kb fragment containing part of clpP gene, which yielded pG-Tf2. Plasmid pG-Tf3, which could express TF from the araB promoter and GroEL-GroES from the Pzt-1 promoter independently from each other, was constructed by inserting a 3.3-kb DNA fragment containing the groES-groEL operon under the control of the Pzt-1 promoter and the tetR repressor (prepared as described previously [9]) into plasmid pTf16 that had been cut at the XmnI site (located between ori and cat). Plasmid pG-KJE8, which could express the DnaK-DnaJ-GrpE and GroEL-GroES chaperones from the araB and Pzt-1 promoters, respectively, was a derivative of plasmid pG-KJE6 (9) into which the rrnBT1T2 terminator from plasmid pTrc99A was inserted downstream of grpE.
Protein extraction and analysis.
Culture samples were harvested and treated with trichloroacetic acid, and whole-cell proteins from samples having equivalent optical densities were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) by using a 10 or 12.5% polyacrylamide gel as described previously (19). Proteins were detected by staining the gel with Coomassie brilliant blue. To examine the extent of aggregation of recombinant proteins, cells were disrupted by sonication (model XL2020 ultrasonic liquid processor; Heat Systems Inc.) for 30 s, which was followed by centrifugation to remove debris; then the resulting preparation was separated into soluble and insoluble fractions by centrifugation at 8,200 × g for 10 min (9). Protein contents were determined with a MicroBCA protein assay reagent kit (Pierce Chemical Co.). Fractions obtained from extracts having equivalent protein contents (approximately 7 μg) were analyzed by SDS-PAGE. Protein bands were quantified with an Intelligent Quantifier apparatus (BioImage Systems Co., Tokyo, Japan).
RESULTS
Plasmids for controlled expression of TF and GroEL-GroES chaperones.
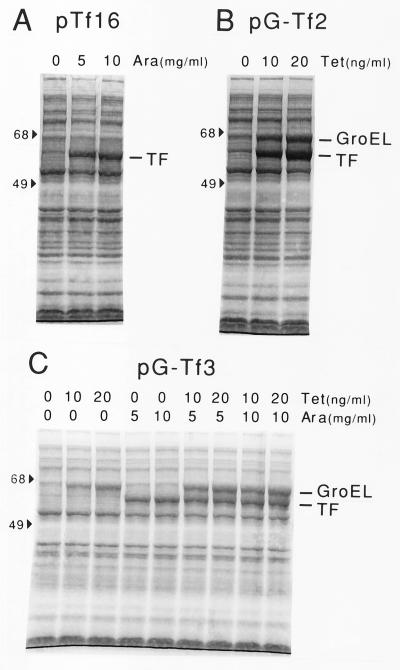
We constructed several TF expression plasmids in which the tig gene encoding TF was placed under the control of the araB promoter inducible with l-arabinose or the Pzt-1 promoter inducible with tetracycline. The plasmid that could express TF alone and the plasmid that could express TF together with GroEL-GroES were designated pTf16 and pG-Tf2, respectively (Fig. 1A and B). Another plasmid, which could express TF and GroEL-GroES from two separate promoters, was also constructed and was designated pG-Tf3 (Fig. 1C). All of these plasmids were derivatives of pACYC184 that were compatible with the ColE1 type of plasmids widely used for expression of recombinant proteins and carried the chloramphenicol resistance gene (cat) for selection. In addition, the times and levels of TF and/or GroEL-GroES expression could be controlled independently from the times and levels of expression of the recombinant proteins induced by IPTG (isopropyl-β-d-thiogalactopyranoside). When E. coli cells harboring these plasmids were induced, they could produce TF or GroEL-GroES at levels that were up to 10 times the levels produced by the wild type (Fig. 2). GroES was produced coordinately with GroEL, which was verified separately (data not shown). Overexpression of the chaperones had little effect on cell growth under the conditions used. We then examined the effects of overexpression of TF, either alone or in combination with GroEL-GroES, on preventing aggregation of three mammalian proteins, endostatin, ORP150, and lysozyme.
FIG. 1.
Structures of TF and other chaperone expression plasmids used, including pTf16 (A), pG-Tf2 (B), pG-Tf3 (C), and pG-KJE8 (D). ori, replication origin of pACYC184; cat, chloramphenicol acetyltransferase gene; araB p/o, araB promoter-operator; araC, araC repressor gene; Pzt-1p, Pzt-1 promoter; tetR, tetR repressor gene; tig, gene encoding trigger factor.
FIG. 2.
Overexpression of TF and GroEL induced by l-arabinose or tetracycline in strain JM109 harboring TF expression plasmid pTf16 (A), pG-Tf2 (B), or pG-Tf3 (C). Cells were grown in L broth containing 20 μg of chloramphenicol per ml at 37°C, and l-arabinose (Ara) and/or tetracycline (Tet) was added to the medium as indicated. Cultures were grown to the mid-log phase, and proteins were analyzed by SDS-PAGE (10% polyacrylamide gel); the gel was stained with Coomassie brilliant blue. The positions of molecular mass markers (in kilodaltons) are indicated on the left.
Overexpression of TF but not GroEL-GroES prevents aggregation of endostatin.
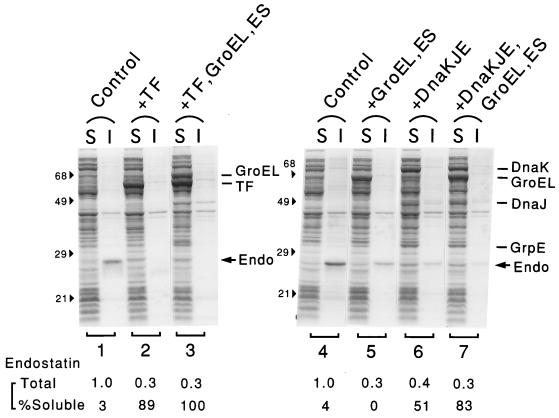
Derivatives of strain BL21 harboring a pair of compatible plasmids for expression of endostatin from a T7 promoter and for expression of TF and/or other chaperones from the araB or Pzt-1 promoter were used to examine the effects of TF overexpression on production of endostatin. To avoid the deleterious effects of constitutive expression of endostatin on cell growth, cells lacking T7 RNA polymerase were grown to the log phase and were infected with λCE6 phage carrying the polymerase gene to induce endostatin synthesis. As shown in Fig. 3, when a large amount of endostatin was expressed in E. coli at 37°C, it was found mostly in the insoluble fraction (Fig. 3, lanes 1 and 4). However, when TF was overexpressed prior to endostatin production, it was recovered mostly in the soluble fraction, suggesting that overproduced TF could effectively prevent aggregation of the recombinant protein, although the total amount was reduced appreciably (by about 50% based on several independent experiments) (lanes 2). Control experiments revealed that endostatin production clearly depended on λCE6 infection and that production of soluble endostatin resulted from coexpression of TF and not from other effects of the arabinose used to induce TF (data not shown). In contrast, overexpression of GroEL-GroES hardly increased the amount in the soluble fraction and reduced the total yield (lanes 5), whereas overexpression of both TF and GroEL-GroES gave results similar to the results obtained with expression of TF alone (lanes 3). When the DnaK-DnaJ-GrpE chaperones were overexpressed, endostatin was partially converted from the insoluble fraction to the soluble fraction, and the yield was reduced (lanes 6); this conversion was accelerated by coexpression of both DnaK-DnaJ-GrpE and GroEL-GroES chaperones (lanes 7). Thus, aggregation of endostatin produced in E. coli could be effectively prevented by prior overexpression of TF or the combined members of two major chaperone teams (DnaK-DnaJ-GrpE and GroEL-GroES) but was less effectively prevented by the members of either team alone. Similar effects of TF and other chaperones that occurred concomitantly with reduced yield were observed in experiments performed with human endostatin (whose amino acid sequence was ∼85% identical to the amino acid sequence of the mouse protein) (data not shown). To minimize possible degradation of endostatin when chaperone overexpression occurred, we repeated the experiments by using a multiple protease mutant lacking Lon, Clp, and HslVU proteases (7) as the host. However, a similar decrease in endostatin yield occurred even with this mutant (data not shown). Moreover, mouse endostatin expressed in cells with coexpressing TF and mouse endostatin expressed in cells without coexpressing TF were both quite stable (data not shown). No further attempt was made to improve production of soluble endostatin by optimizing the amounts of coexpressing chaperones.
FIG. 3.
Effects of coexpression of TF and other chaperones on production of mouse endostatin. Strain BL21 harboring endostatin expression plasmid pTB01#8 together with pTf16 (lanes 1 and 2), pG-Tf2 (lanes 3), or pG-KJE8 (lanes 4 through 7) was grown at 37°C in L broth with or without l-arabinose and/or tetracycline (see below), and the cells were infected with λCE6 phage (2 × 109 PFU/ml) to induce endostatin at the mid-log phase. After 1 h of incubation, cells were harvested, disrupted, and separated into soluble (S) and insoluble (I) fractions; this was followed by an SDS-PAGE analysis (12.5% polyacrylamide gel). The protein bands for endostatin (Endo), TF, and other chaperones and the positions of molecular mass markers (in kilodaltons) are indicated. The amounts of endostatin were quantified and corrected for the background due to control cells carrying no plasmid (data not shown), and total amounts (relative to the control) and the percentages of the soluble fraction are shown at the bottom. The chaperone inducers used were as follows: none (lanes 1 and 4), 10 mg of l-arabinose per ml (lanes 2 and 6), 10 ng of tetracycline per ml (lanes 3), 20 ng of tetracycline per ml (lanes 5), and 20 ng of tetracycline per ml and 5 mg of l-arabinose per ml (lanes 7).
Simultaneous overexpression of TF and GroEL-GroES prevents aggregation of ORP150.
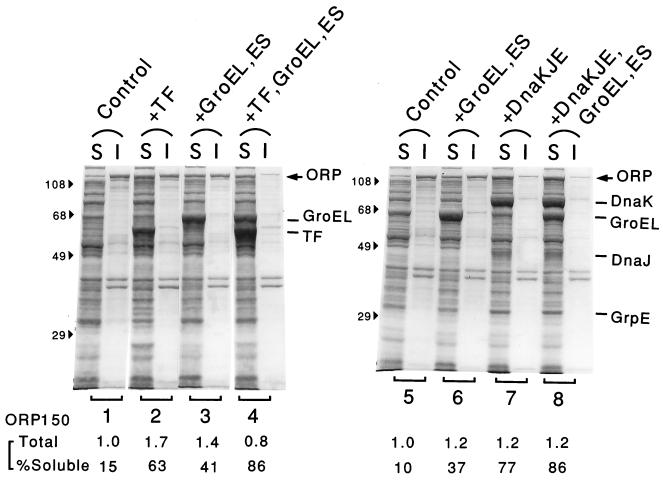
Similar experiments carried out with JM109 cells harboring a pair of expression plasmids for ORP150 and TF and/or chaperones yielded results that apparently differed from the results obtained for endostatin. As shown in Fig. 4, the ORP150 protein that for the most part was insoluble in the control cells at 37°C (lanes 1 and 5) was only partially converted to the soluble form when it was coexpressed with either TF or GroEL-GroES alone (lanes 2, 3, and 6). l-Arabinose (10 mg/ml) significantly enhanced the solubility of ORP150 in the control strain harboring no TF expression plasmid, although TF coexpression had a much stronger effect (data not shown). In contrast, most ORP150 was found in the soluble fraction when both TF and GroEL-GroES were overexpressed (lanes 4). Essentially the same results were obtained with another plasmid, pG-Tf3, in which expression of TF and expression of GroEL-GroES were controlled by separate promoters, although induction of TF with l-arabinose enhanced GroEL expression to some extent (data not shown). These results strongly suggested that TF and GroEL-GroES worked cooperatively in preventing aggregation of ORP150 under the conditions used. Coexpression of the DnaK-DnaJ-GrpE chaperones alone or together with GroEL-GroES was also quite effective in solubilizing this protein (lanes 7 and 8), suggesting that there is a partial functional overlap between TF and DnaK-DnaJ-GrpE. Thus, both simultaneous coexpression of TF and GroEL-GroES and coexpression of the DnaK-DnaJ-GrpE chaperone team were very effective for producing ORP150 in soluble states.
FIG. 4.
Effects of coexpression of TF and other chaperones on production of human ORP150. Strain JM109 harboring ORP150 expression plasmid pORP4 together with pTf16 (lanes 1 and 2), pGro11 (lanes 3), pG-Tf2 (lanes 4), or pG-KJE8 (lanes 5 through 8) was grown as described in the legend to Fig. 3, and 1 mM IPTG was added to induce ORP150 at the mid-log phase. After 2 h of incubation, cells were harvested, disrupted, and separated into soluble (S) and insoluble (I) fractions; this was followed by an SDS-PAGE analysis (10% polyacrylamide gel). The protein bands for ORP150 (ORP) and chaperones and the positions of molecular mass markers (in kilodaltons) are indicated. The amounts of ORP150 were quantified and corrected, and the total amounts (relative to the control) and percentages of the soluble fraction are indicated as described in the legend to Fig. 3. The chaperone inducers used were as follows: none (lanes 1 and 5), 10 mg of l-arabinose per ml (lanes 2 and 7), 50 ng of tetracycline per ml (lanes 3 and 4), 20 ng of tetracycline per ml (lanes 6), and 20 ng of tetracycline per ml and 10 mg of l-arabinose per ml (lanes 8).
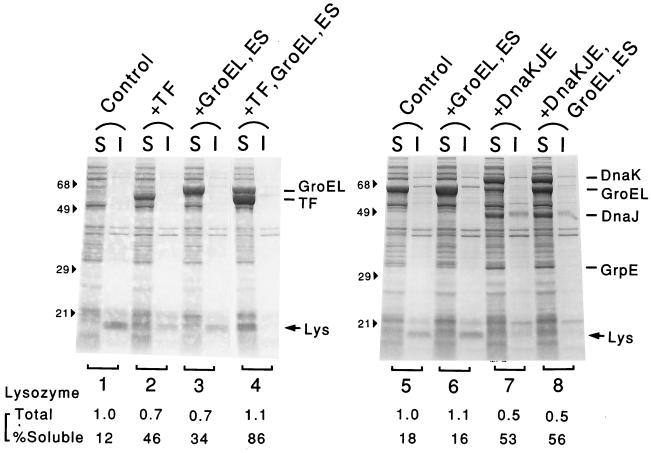
Overexpression of both TF and GroEL-GroES is required for production of soluble lysozyme.
Similar chaperone coexpression experiments were carried out for human lysozyme by using strain JM109 cells harboring appropriate plasmids that were grown in RM medium, which supported much higher levels of expression of lysozyme than L broth supported. The effects of TF and chaperones observed for lysozyme were similar to, but slightly different from, the effects for ORP150. As shown in Fig. 5, most of the lysozyme produced in the control cells was insoluble, but some of it was converted to the soluble form upon coexpression of TF, although there was a slight decrease in the total yield (lanes 1 and 2). Whereas coexpression of GroEL-GroES alone was less effective in solubilizing lysozyme (lanes 3 and 6), coexpression of both TF and GroEL-GroES was quite effective, and most of the lysozyme was soluble without a significant decrease in yield (lanes 4). Such cooperativity was also confirmed by using another plasmid, pG-Tf3 (data not shown). Our results indicated that overexpression of both TF and GroEL-GroES was needed to prevent aggregation of lysozyme effectively under the conditions employed. Lysozyme was also made partially soluble by coexpression of DnaK-DnaJ-GrpE (lanes 7), although the total yield appeared to be reduced and the effects were not affected by simultaneous coexpression of GroEL-GroES (lanes 8) or TF (data not shown). These results also indicated that TF and GroEL-GroES had specific synergistic effects in preventing aggregation of lysozyme. Control experiments revealed that the reduced yield of lysozyme was largely due to the l-arabinose used to induce TF or other chaperones (data not shown). Lysozyme enzyme activity (as determined by a turbidimetric assay) was detected in the soluble fraction prepared with the strain that coexpressed TF and GroEL-GroES, although the specific activity was much lower than the specific activity of purified lysozyme obtained from human milk (data not shown). On the other hand, no activity was detected in the strain without coexpressing chaperones, suggesting that at least some of the soluble lysozyme obtained was folded correctly.
FIG. 5.
Effects of coexpression of TF and other chaperones on production of human lysozyme. Strain JM109 harboring lysozyme expression plasmid pLY-46 and TF or another chaperone expression plasmid was grown as described in the legend to Fig. 4 (except that RM medium was used), and 1 mM IPTG was added to induce lysozyme at the mid-log phase. After 4 h of incubation, cells were harvested, disrupted, and separated into soluble (S) and insoluble (I) fractions; this was followed by an SDS-PAGE analysis (12.5% polyacrylamide gel). The protein bands for lysozyme (Lys) and chaperones and the positions of molecular mass markers (in kilodaltons) are indicated. The amounts of lysozyme were quantified and corrected, and the total amounts (relative to the control) and percentages of the soluble fraction are indicated at the bottom. The chaperone inducers used were as follows: none (lanes 1 and 5), 10 mg of l-arabinose per ml (lanes 2), 5 ng of tetracycline per ml (lanes 3, 4, and 6), 5 mg of l-arabinose per ml (lanes 7), and 5 ng of tetracycline per ml and 5 mg of l-arabinose per ml (lanes 8).
DISCUSSION
We found that overexpression of TF can effectively prevent aggregation of recombinant proteins when they are coexpressed in E. coli. Previous findings that TF binds to ribosomes and to nascent polypeptides suggested that this molecule acts as a folding catalyst (3, 17), and our results provide in vivo evidence that supports this notion. TF exhibits PPIase activity and is known to catalyze the PPIase-dependent refolding of RCM-T1 (a reduced and carboxymethylated variant of RNase T1) in vitro (12, 14). On the other hand, the binding of TF to some protein substrates has been reported to be independent of proline residues (13). Whether the PPIase activity is needed for the chaperonelike activity of TF found in this study has not been determined.
It seems remarkable that all three recombinant proteins that have distinct molecular masses and structures and are prone to aggregation in E. coli were rescued by overexpression of TF to appreciable extents, particularly when the GroEL-GroES chaperone was simultaneously overproduced (Fig. 3 through 5). In contrast, coexpression of either the GroEL-GroES chaperone team or the DnaK-DnaJ-GrpE chaperone team was relatively inefficient. It is likely that the TF coexpression system described here will provide a useful way to study and improve production of various recombinant proteins that are difficult to obtain in the native states.
The effects of TF, however, may differ depending on the specific target protein used. Overexpression of TF alone appeared to be sufficient for preventing aggregation of endostatin but was only partially effective for ORP150 and for lysozyme. In the case of the latter two proteins, simultaneous overexpression of TF and GroEL-GroES proved to be more effective than overexpression of TF alone (Fig. 4 and 5). These results clearly indicate that TF and GroEL-GroES play cooperative roles in assisting folding at least for some proteins. Such cooperativity is in good agreement with previous findings that TF binds to GroEL and increases its affinity for certain proteins in order to promote their folding or degradation (5, 6).
The effect of overexpression of TF was apparently similar to the effect of the DnaK-DnaJ-GrpE chaperone team in preventing aggregation of both endostatin and ORP150. These results suggest that TF and DnaK-DnaJ-GrpE have similar functions during protein folding, which is consistent with recent data that indicated that the functions of DnaK and TF in folding nascent polypeptides overlap partially (15).
ACKNOWLEDGMENTS
We express our gratitude to T. Boehm (Harvard Medical School) for providing an endostatin expression vector and to Y. Matsuki (Sumitomo Chemical) for providing a lysozyme expression vector. We are grateful to M. Nakayama, S. Takahara, and T. Yoshifusa for providing technical assistance.
REFERENCES
- 1.Crooke E, Wickner W. Trigger factor: a soluble protein that folds pro-OmpA into a membrane-assembly-competent form. Proc Natl Acad Sci USA. 1987;84:5216–5220. doi: 10.1073/pnas.84.15.5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guthrie B, Wickner W. Trigger factor depletion or overproduction causes defective cell division but does not block protein export. J Bacteriol. 1990;172:5555–5562. doi: 10.1128/jb.172.10.5555-5562.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hesterkamp T, Hauser S, Lutcke H, Bukau B. Escherichia coli trigger factor is a prolyl isomerase that associates with nascent polypeptide chains. Proc Natl Acad Sci USA. 1996;93:4437–4441. doi: 10.1073/pnas.93.9.4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ikeda J, Kaneda S, Kuwabara K, Ogawa S, Kobayashi T, Matsumoto M, Yura T, Yanagi H. Cloning and expression of cDNA encoding the human 150kDa oxygen-regulated protein, ORP150. Biochem Biophys Res Commun. 1997;230:94–99. doi: 10.1006/bbrc.1996.5890. [DOI] [PubMed] [Google Scholar]
- 5.Kandror O, Sherman M, Moerschell R, Goldberg A L. Trigger factor associates with GroEL in vivo and promotes its binding to certain polypeptides. J Biol Chem. 1997;272:1730–1734. doi: 10.1074/jbc.272.3.1730. [DOI] [PubMed] [Google Scholar]
- 6.Kandror O, Sherman M, Rhode M, Goldberg A L. Trigger factor is involved in GroEL-dependent protein degradation in Escherichia coli and promotes binding of GroEL to unfolded proteins. EMBO J. 1995;14:6021–6027. doi: 10.1002/j.1460-2075.1995.tb00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanemori M, Nishihara K, Yanagi H, Yura T. Synergistic roles of HslVU and other ATP-dependent proteases in controlling in vivo turnover of ς32 and abnormal proteins in Escherichia coli. J Bacteriol. 1997;179:7219–7225. doi: 10.1128/jb.179.23.7219-7225.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kohara Y, Akiyama K, Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987;50:495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- 9.Nishihara K, Kanemori M, Kitagawa M, Yanagi H, Yura T. Chaperone coexpression plasmids: differential and synergistic roles of DnaK-DnaJ-GrpE and GroEL-GroES in assisting folding of an allergen of Japanese cedar pollen, Cryj2, in Escherichia coli. Appl Environ Microbiol. 1998;64:1694–1699. doi: 10.1128/aem.64.5.1694-1699.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Reilly M S, Boehm T, Shing Y, Fukai N, Vasios G, Lane W S, Flynn E, Birkhead J R, Olsen B R, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 11.Pélez-Pélez J, Gutiérrez J. An arabinose-inducible expression vector, pAR3, compatible with ColE1-derived plasmids. Gene. 1995;158:141–142. doi: 10.1016/0378-1119(95)00127-r. [DOI] [PubMed] [Google Scholar]
- 12.Scholz C, Stoller G, Zarnt T, Fischer G, Schmid F X. Cooperation of enzymatic and chaperone functions of trigger factor in the catalysis of protein folding. EMBO J. 1997;16:54–58. doi: 10.1093/emboj/16.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scholz C, Mücke M, Rape M, Pecht A, Pahl A, Bang H, Schmid F X. Recognition of protein substrates by the prolyl isomerase trigger factor is independent of proline residues. J Mol Biol. 1998;277:723–732. doi: 10.1006/jmbi.1997.1604. [DOI] [PubMed] [Google Scholar]
- 14.Stoller G, Rücknagel K P, Nierhaus K N, Schmid F X, Fischer G, Rahfeld J U. A ribosome-associated peptidyl-prolyl cis/trans isomerase identified as the trigger factor. EMBO J. 1995;14:4939–4948. doi: 10.1002/j.1460-2075.1995.tb00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teter S A, Houry W A, Ang D, Tradler T, Rockabrand D, Fischer G, Blum P, Georgopoulos C, Hartl F U. Polypeptide flux through bacterial Hsp70: DnaK cooperates with trigger factor in chaperoning nascent chains. Cell. 1999;97:755–765. doi: 10.1016/s0092-8674(00)80787-4. [DOI] [PubMed] [Google Scholar]
- 16.Thomas J G, Ayling A, Baneyx F. Molecular chaperones, folding catalysts, and the recovery of active recombinant proteins from E. coli. Appl Biochem Biotechnol. 1997;66:197–238. doi: 10.1007/BF02785589. [DOI] [PubMed] [Google Scholar]
- 17.Valent Q A, Kendall D A, High S, Kusters R, Oudega B, Luirink J. Early events in preprotein recognition in E. coli: interaction of SRP and trigger factor with nascent polypeptides. EMBO J. 1995;14:5494–5505. doi: 10.1002/j.1460-2075.1995.tb00236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wall J G, Plückthun A. Effects of overexpressing folding modulators on the in vivo folding of heterologous proteins in Escherichia coli. Curr Opin Biotechnol. 1995;6:507–516. doi: 10.1016/0958-1669(95)80084-0. [DOI] [PubMed] [Google Scholar]
- 19.Yano R, Imai M, Yura T. The use of operon fusions in studies of the heat-shock response: effects of altered sigma32 on heat-shock promoter function in Escherichia coli. Mol Gen Genet. 1987;207:24–28. doi: 10.1007/BF00331486. [DOI] [PubMed] [Google Scholar]