Abstract
Background and Purpose:
The peptide hormone vasopressin regulates water transport in the renal collecting duct largely via the V2 receptor, which triggers a cAMP-mediated activation of a protein kinase A (PKA)-dependent signaling network. The protein kinases downstream from PKA have not been fully identified or mapped to regulated phosphoproteins.
Experimental Approach:
We carried out systems-level analysis of large-scale phosphoproteomic data quantifying vasopressin-induced changes in phosphorylation in aquaporin-2-expressing cultured collecting duct cells (mpkCCD). Quantification was done using stable isotope labeling (SILAC method).
Key Results:
9640 phosphopeptides were quantified. Stringent statistical analysis identified significant changes in response to vasopressin in 429 of these phosphopeptides. The corresponding phosphoproteins were mapped to known vasopressin-regulated cellular processes. The vasopressin-regulated sites were classified according to the sequences surrounding the phosphorylated amino acids giving 11 groups. Among the vasopressin-regulated phosphoproteins were 25 distinct protein kinases. Among these, six plus PKA appeared to account for phosphorylation of about 81% of the 313 vasopressin-regulated phosphorylation sites. The six downstream kinases were salt-inducible kinase 2 (Sik2), cyclin-dependent kinase 18 (Cdk18/PCTAIRE-3), calmodulin-dependent kinase kinase 2 (Camkk2), protein kinase D2 (Prkd2), mitogen-activated kinase 3 (Mapk3/ERK1), and myosin light chain kinase (Mylk).
Conclusion and Implications:
In V2 receptor-mediated signaling, PKA is at the head of a complex network that includes at least 6 downstream vasopressin-regulated protein kinases that are prime targets for future study. The extensive phosphoproteomic data reported in this study is provided as a web-based data resource for future studies of G-protein coupled receptors.
Keywords: mpkCCD, GPCR signaling, V2 receptor signaling, Sik2, Prkd2, Cdk18, Camkk2
INTRODUCTION
G-protein coupled receptors (GPCRs) are central to the regulation of a variety of physiological processes and are frequently targeted for pharmacological treatments (Insel et al., 2012). One such GPCR is the vasopressin V2 receptor (V2R), expressed in renal collecting duct cells where it regulates the transport of water, sodium and urea (Fenton & Knepper, 2007). Signaling through the V2R controls water transport through effects on the water channel, aquaporin-2 (AQP2). With vasopressin binding, the heterotrimeric G-protein α subunit, Gαs, activates adenylyl cyclase 6 and increases cAMP production. Cyclic AMP levels can be independently regulated in cellular nanodomains to result in localized activation of effectors including protein kinase A (PKA) (Bers, Xiang & Zaccolo, 2019).
Loss of this signaling pathway results in diabetes insipidus. Central diabetes insipidus is managed clinically through use of a V2R-selective vasopressin analog 1-deamino-8-D-arginine vasopressin (dDAVP), often referred to as ‘desmopressin’ (Sawyer, Acosta, Balaspiri, Judd & Manning, 1974). Desmopressin is also commonly employed for treatment of enuresis in children. Defects in the V2R signaling pathway can also result in abnormal renal water retention, e.g. the syndrome of inappropriate antidiuresis, characterized by dilutional hyponatremia. Hyponatremia of all causes accounts for as much as 30% of hospitalized patients in tertiary care centers (Upadhyay, Jaber & Madias, 2006). Treatment employs a drug class known as ‘vaptans’ (e.g. tolvaptan), which function as V2R antagonists (Schrier et al., 2006). The clinical use of vaptans has recently expanded because of FDA-approval of vaptans in autosomal dominant polycystic kidney disease (Torres et al., 2012).
The functional effects of vasopressin at a cellular level in collecting duct principal cells are shown in Figure 1, which summarizes an extensive literature on the topic (Salhadar et al., 2020). Most of these downstream effects are believed to be mediated by PKA through cAMP (Isobe et al., 2017) (Figure 1). Almost all vasopressin-mediated phosphorylation changes in murine immortalized cortical collecting duct (mpkCCD) cells were ablated when both PKA catalytic genes were deleted (PKA-null cell) with CRISPR-Cas9 genome editing (Datta et al., 2020). In addition, an inhibitor of PKA and other basophilic kinases, H-89, blocked a large array of phosphorylation events in PKA-intact mpkCCD cells that were not blocked in PKA-null cells (Limbutara, Kelleher, Yang, Raghuram & Knepper, 2019). Understanding of vasopressin signaling beyond PKA activation by cAMP remains incomplete.
Figure 1.
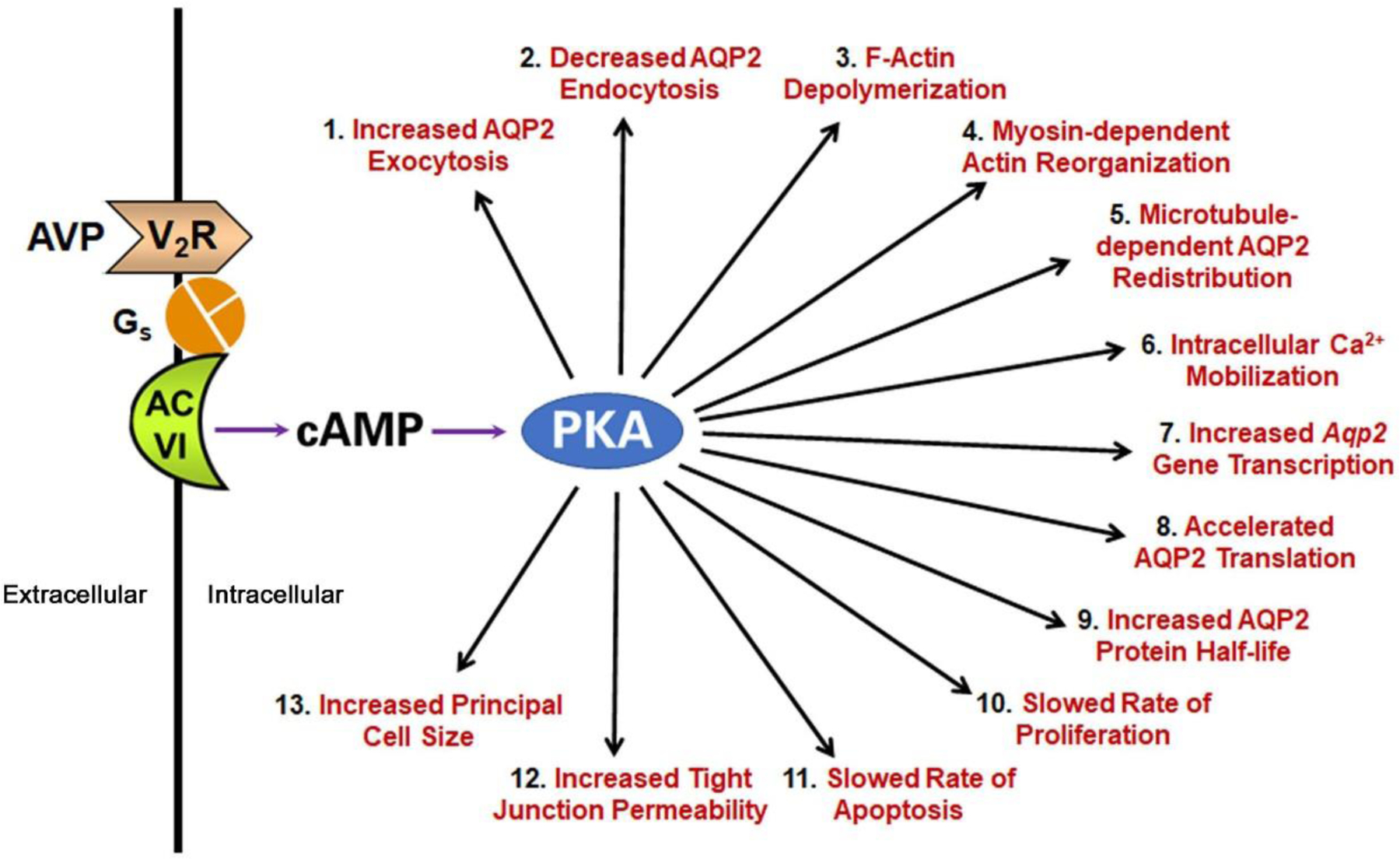
The physiologic responses at the cellular level elicited by vasopressin in the collecting duct epithelial cells as documented in prior literature (Salhadar et al., 2020).
Here, we carried out systems-level analysis of large-scale phosphoproteomic data quantifying vasopressin-induced changes in phosphorylation in AQP2-expressing cultured mpkCCD cells. The data were used (a) to construct a publicly accessible web resource useful for the study of signaling by Gαs-coupled GPCRs; and (b) to identify a set of protein kinases downstream from PKA that are likely responsible for many of the phosphorylation changes triggered by vasopressin in collecting duct cells. We propose that the new information presented in this paper will be useful, not only for understanding water balance disorders, but also for understanding regulation by other Gαs-coupled receptors in other tissues.
METHODS
The raw data used for this paper were reported as control experiments in our prior publication about PKA-independent signaling in collecting duct cells (Datta et al., 2020), but were not analyzed bioinformatically with respect to physiological mechanisms. For the reader’s convenience, we provide the methods used in a supplemental file (Supplemental Information – Methods). Briefly, mpkCCD, a collecting duct cell line, was grown on permeable supports using the Stable Isotope Labeling with Amino acids in Cell culture (SILAC) protocol for labeling (Datta et al., 2020). The cells were exposed to the vasopressin analog dDAVP (0.1 nM basolateral side only for 30 min) versus vehicle in three pairs of SILAC experiments. Previous studies demonstrated that 30 minutes is required for a steady state short-term response to vasopressin (Nielsen & Knepper, 1993). Additional prior characterization of the concentration-response relationship and time course of response for dDAVP in mpkCCD cells was carried out by Yu and colleagues (https://esbl.nhlbi.nih.gov/data/Concentration-Response/) (Yu et al., 2009). The two members of each pair, labeled with heavy and light amino acids, were mixed and subjected to our total and phosphoproteomic analysis protocol. Phosphopeptides were enriched using TiO2 and Ferric nitrilotriacetate (Fe-NTA) columns. The enriched samples were analyzed using LC-MS/MS by Higher-Energy Collision Dissociation (HCD) and Electron-Transfer/Higher-Energy Collision Dissociation (EThcD) fragmentation. The raw data was analyzed using Proteome Discoverer 1.4 (version 1.4.0.288, Research Resource Identifier (RRID):SCR_014477). The peptide False Discovery Rate was set to <0.01(Datta et al., 2020).
Phosphoproteomics Data Integration across Biological Replicates
For phosphoproteomics, the phosphopeptides having an area (MS1 scan) of at least 1.0E7 in each of the replicate cell clones along with a corresponding median ratio calculated from TiO2 and Fe-NTA results were considered for further analysis. The data files obtained from HCD and EThcD fragmentation were combined. For duplicate peptides, the ones with lowest standard deviation among three replicate clones were selected. Co-efficient of variation (CV) was calculated to provide an estimate of the biological variation among three independent clones. Phosphopeptides were considered changed in abundance if they met the following criteria:|Log2(dDAVP/vehicle)|>0.4 and (−log10(CV))≥0.4. The threshold of 0.4 for |Log2(dDAVP/vehicle)| was fixed to encompass 95% of log2(vehicle/vehicle) values (95% confidence interval for vehicle-to-vehicle comparisons) as described earlier (Schenk et al., 2012). Amino acid sequences (13-mer) of phosphopeptides were centralized around the phosphorylation site using PTM Centralizer (https://hpcwebapps.cit.nih.gov/ESBL/PtmCentralizer/).
Generation of Sequence Logos
PTM-Logo (https://hpcwebapps.cit.nih.gov/PTMLogo/) was used to generate sequence logos (Saethang et al., 2019). The inputs were centralized sequences of specific curated groups of phosphorylation sites. Background sequences and chi-square alpha values for generation of each logo are noted in the figure legends.
Bioinformatics Analysis
External Data Sources.
Known substrates of various kinases and their regulated phosphorylation sites were downloaded from PhosphoSitePlus (https://www.phosphosite.org/homeAction, RRID:SCR_001837). Known effects of phosphorylation changes were downloaded from PhosphoSitePlus (https://www.phosphosite.org/staticDownloads). Additional information about the effects of specific phosphorylation events was obtained from Kinexus PhosphoNET (http://www.phosphonet.ca/, RRID:SCR_013070) and through direct PubMed (RRID:SCR_004846) searches. A list of PDZ domain-containing mouse proteins was downloaded from the SMART database (http://smart.embl.de/, RRID:SCR_005026) (Letunic & Bork, 2018). Abdesigner (https://esbl.nhlbi.nih.gov/AbDesigner/) was used to visualize the domain organization of proteins relative to the regulated phosphorylation sites (Pisitkun, Hoffert, Saeed & Knepper, 2012).
Network Construction.
STRING (https://string-db.org/, RRID:SCR_005223) was used to create a relational network for proteins with increased and decreased phosphorylation sites using default settings to construct a preliminary signaling network. This network was the starting point for manual curation based on knowledge gleaned from prior literature to classify vasopressin signaling into various cellular processes, functions, or components. Cytoscape 3.7.1 (https://cytoscape.org/, RRID:SCR_015784) was used for visualization of the resulting networks.
Kinase Tree Annotation and Substrate Motif Generation.
KinMap (Eid, Turk, Volkamer, Rippmann & Fulle, 2017) (http://kinhub.org/kinmap/) was used to locate vasopressin-regulated protein kinases to the Manning dendrogram (Manning, Whyte, Martinez, Hunter & Sudarsanam, 2002). Curated target sequences for each kinase (downloaded from PhosphoSitePlus) along with the kinases on the same sub-branch of the dendrogram were put into PTM-Logo to identify target sequence preferences for individual kinase neighborhoods. Background sequences for this analysis were the list of all phosphorylation sites in the PhosphositePlus mouse database. This analysis was visualized using CORAL (http://phanstiel-lab.med.unc.edu/CORAL/) (Metz et al., 2018). CORAL is an interactive web application for kinome annotation and visualization that is capable of generating vector graphics (SVG) images of the Manning kinome tree with customizable branch color and node color.
Gene Ontology (GO) Analysis.
GO terms, annotated protein domains, and FASTA sequences were obtained using ABE (Automated Bioinformatics Extractor; http://helixweb.nih.gov/ESBL/ABE/). Chi-square analysis was used to identify the association between regulated proteins and various annotation terms that were obtained through ABE. InterPro database in the functional annotation tool, DAVID (https://david.ncifcrf.gov/, RRID:SCR_001881) was used to determine the enrichment of specific protein domains. DAVID uses a modified Fisher Exact test (EASE score) to test the enrichment of annotation terms. The full list of unique accession numbers identified from the current phospho-proteomics experiment was used as a background for DAVID analysis.
Rule-based Classification of Regulated Phosphosites.
All regulated phosphorylation sites were classified based on two criteria: 1) direction of change in phosphorylation following dDAVP stimulation; 2) the presence of specific amino acids in different positions upstream or downstream of altered phosphosites as described in the Results section.
Immunoblot Analysis
For short-term vasopressin stimulation, dDAVP-conditioned mpkCCD cells on 6-well Transwell plates in simple media were kept in the absence of dDAVP for 2 hours (wash-out phase) on the day of experiment. They were subsequently treated with either 0.1 nM dDAVP or vehicle for 30 minutes (in the basolateral medium only). The experiments were performed in triplicate.
After incubation, the cells were rapidly washed with ice-cold PBS and extracted with a solubilization buffer (1.5% SDS,10 mM Tris, pH 6.8, 1X Halt protease and phosphatase inhibitor cocktail, EDTA-free (Thermo Scientific, NY)). Protein concentration was measured using the BCA assay. The denatured samples were subjected to 4–12% SDS/PAGE. The proteins were transferred to nitrocellulose membranes and probed with primary antibodies from Cell Signaling Technology (Danvers, MA) at indicated dilutions: anti-CaMKK2 (D8D4D) (1:1000, Rabbit monoclonal, Cat. No. 16810, Ref: 04/2020 Lot No. 1, RRID:AB_2798771), anti-Phospho-CaMKK2 (Ser511) (1:1000, Rabbit polyclonal, Cat. No. 12818, Ref: 01/2020, Lot No. 2, RRID:AB_2798034). REVERT total protein stain, blocking buffer and infrared fluorescence-conjugated secondary antibodies were obtained from LI-COR. Fluorescence images were visualized by a LI-COR Odyssey System (ODY-0428). Band intensities were analyzed by LI-COR Image Studio software. Procedures follow journal standards for immunoblotting (https://bpspubs.onlinelibrary.wiley.com/hub/journal/14765381/journal-resources/policy-editorials.html).
Data Availability
The raw files (.raw) can be accessed at www.ebi.ac.uk/pride/archive/ with the dataset identifier PXD015719. The curated data are available at https://hpcwebapps.cit.nih.gov/ESBL/Database/mpkCCD-AVP/.
Nomenclature of Targets and Ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in https://www.guidetopharmacology.org/, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander et al., 2019a; Alexander et al., 2019b).
RESULTS
To identify phosphorylation responses to the V2R-selective vasopressin analog dDAVP (0.1 nM, basolateral side for 30min) in mouse renal mpkCCD epithelial cells, we used mass spectrometry (SILAC labeling) for proteome-wide quantification of phosphopeptides in mpkCCD cells. Earlier, we showed that 30 minutes is required for the steady-state response to vasopressin in collecting duct cells (Nielsen & Knepper, 1993). The full dataset is provided at https://hpcwebapps.cit.nih.gov/ESBL/Database/mpkCCD-AVP/.
Figure 2A shows a general view of the phosphoproteomic responses following dDAVP stimulation. Of the 9640 quantified phosphopeptides (2649 phosphoproteins), 429 (295 phosphoproteins) were classified as showing high probability abundance changes in response to dDAVP based on dual criteria (−Log10(CV)≥0.4 and |Log2(dDAVP/Vehicle)|>0.4) where CV means coefficient of variation (Supplemental Table 1). In the same experiments, we also carried out total protein quantification for 10,128 proteins in mpkCCD cells, of which 7,339 were quantified in all three pairs of samples (Supplementary Table 2). In general, treatment of the cells for 30 minutes with dDAVP resulted in few changes in protein abundances, none of which impinge on phosphopeptide quantification.
Figure 2.
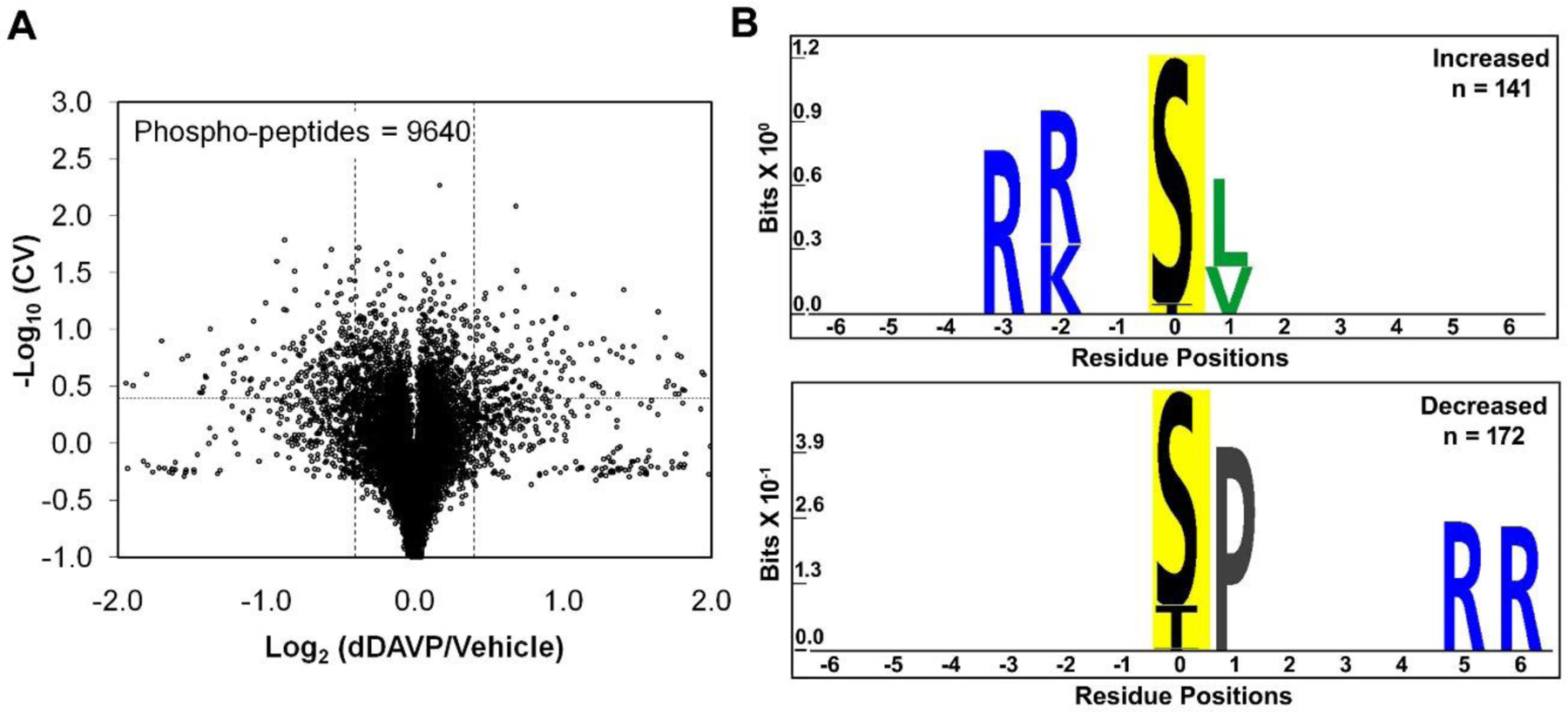
Vasopressin effects on phosphoproteome of mpkCCD cells. A. Torch plot for phosphopeptides quantified across all three pairs (dDAVP vs. vehicle) of mpkCCD cell clones. Vertical dashed lines show log2(dDAVP/Vehicle) of 0.4, and −0.4, while the horizontal line represents −log10(CV) of 0.4 (CV=0.4). These phosphopeptides are found in 295 distinct phosphoproteins. Out of 9640 quantified phosphopeptides, 429 were altered (increased, 187; decreased, 242) according to the thresholds defined above. These phosphopeptides consisted of 332 mono-phosphopeptides (increased, 153; decreased, 179) and 97 multi-phosphorylated peptides. B. Motif analysis shows the amino acid residues over-represented in sets of unique mono-phosphopeptides whose abundances are significantly increased (141 phospho-sites, corresponds to 153 mono-phosphopeptides; upper panel) and decreased (172 phospho-sites, corresponds to 179 mono-phosphopeptides; lower panel). PTM-Logo was used with whole mouse dataset as background and Chi-square cutoff was set at 0.0001.
To identify general sequence preferences among phosphorylation sites that respond to vasopressin, we used PTM-Logo (Figure 2B). The increased phosphorylation sites (n=141) identified a motif typical of PKA and other basophilic protein kinases [R-(R/K)-X-p(S/T)] (Figure 2B, upper). The logo generated from the decreased sites (n=172) identified a preference for a proline in position +1 relative to the phosphorylated amino acid (Figure 2B, lower).
Supplemental Table 3A–B compares the dDAVP-mediated responses in this paper with data from Isobe et al. showing effects of deletion of both PKA catalytic subunits (Isobe et al., 2017) in mpkCCD cells. In general, many of the identified PKA targets show reciprocal changes with dDAVP in the current study and these dDAVP-responses were ablated when PKA was deleted (Datta et al., 2020).
We mapped all altered phosphoproteins to specific cellular functions using STRING and GO term annotations (Figure 3). Many of the functional groups identified correspond closely to known cellular level actions of vasopressin in collecting duct cells (Figure 1). Some categories of proteins define other possible roles of vasopressin not previously identified in the literature, e.g., “Desmosome”, “mRNA Stability”, “mRNA Processing”, and “Nuclear Envelope”. A particularly large number of phosphoproteins are involved in “Gene Transcription”.
Figure 3.
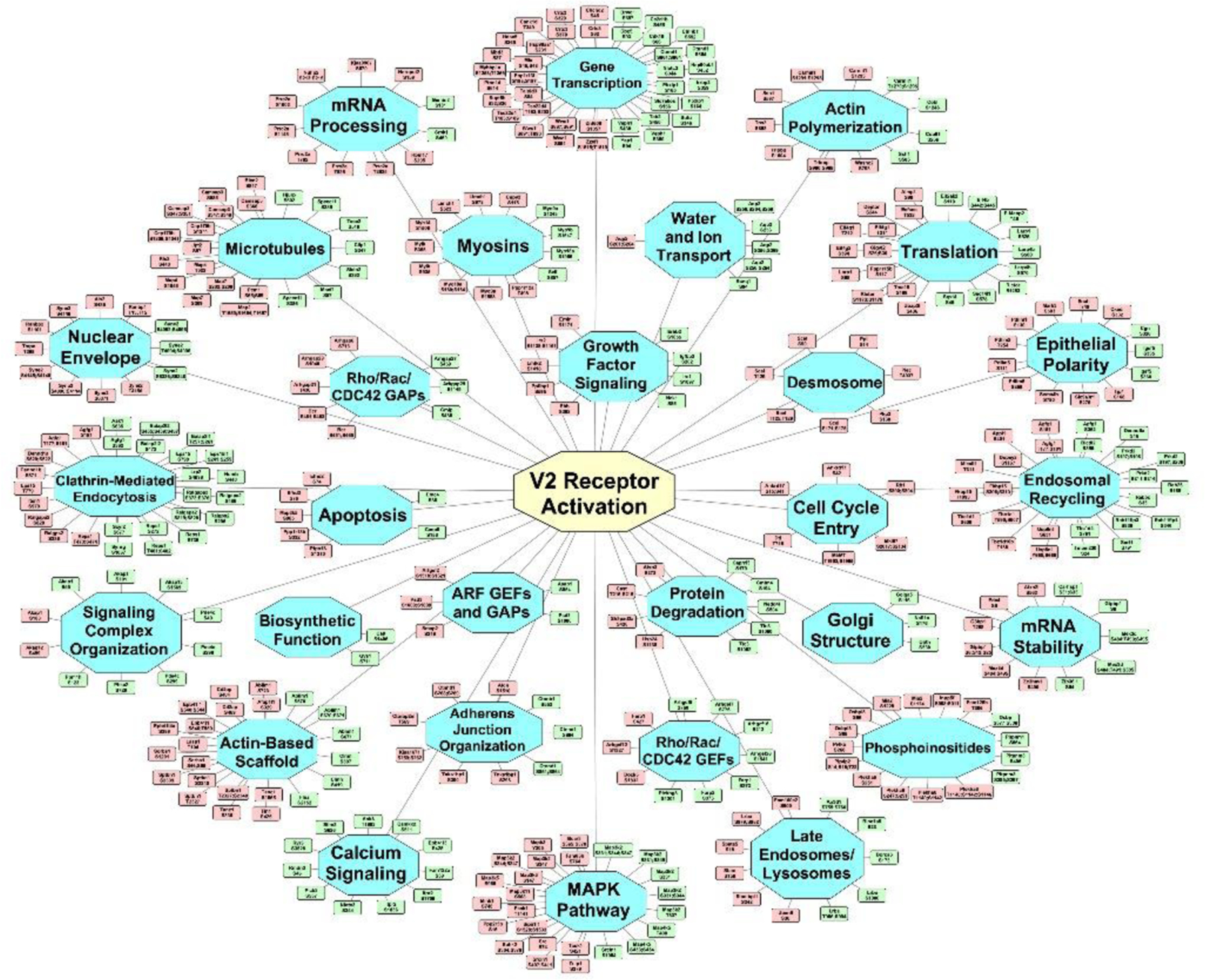
Vasopressin signaling network. Undirected graph depicting the mapping of proteins containing altered phosphorylation sites to specific cellular functions or structures [blue nodes]. The green and red nodes indicate specific phosphorylation sites altered in response to dDAVP administration (green, increased; red, decreased). A vector-graphic, zoomable version is provided at https://hpcwebapps.cit.nih.gov/ESBL/Database/Vasopressin-Network/ to aid in viewing details. Network was constructed initially in STRING and manually extended to the full data set using GO terms and PubMed entries. Cytoscape was used for visualization.
Vasopressin-Responsive Protein Kinases
Of the 295 vasopressin-regulated phosphoproteins, 25 are themselves protein kinases that are candidates for downstream vasopressin signaling (Table 1). These kinases are mapped to a KinMap-based dendrogram (Figure 4), classifying kinases on the basis of similarities of their catalytic regions. Different branches define kinase families that typically share target sequence preferences. Several of the sites that changed have known effects on the catalytic activity, namely Mapk3 at Y205, Src at S74, Lmtk2 at S1418, Camkk2 at S511 and Mylk at S355 and S935. Also, of interest are phosphorylation changes in multiple MAP kinase kinase kinases (Map3k2, Mapk3k3, Map3k5 and Map3k11) which can be upstream from MAP kinase activation (p38, JNK and extracellular signal-regulated kinase (ERK) proteins). Immunoblotting confirming the large increase in phosphorylation of Camkk2 at S511 is shown in Figure 5.
Table 1.
Protein kinases with vasopressin-responsive phosphopeptides in mpkCCD renal epithelial cells¶
| Gene Symbol | Mod. Site(s) | Annotation | Peptide Sequence | Centralized Sequence | Log2 (dDAVP/Vehicle) | −log10 (CV) |
|---|---|---|---|---|---|---|
| Sik2 | S358 | Serine/threonine-protein kinase SIK2 | RPS*TIAEQTVAK | GRQRRPS*TIAEQT | 2.10 | 0.40 |
| Nrbp2 | S359 | Nuclear receptor-binding protein 2 | YS*EVSFLELDK | PMQWRYS*EVSFLE | 1.94 | 0.62 |
| Eif2ak2 | S110 | Interferon-induced, double-stranded RNA-activated protein kinase | LS*VNYEQCEPNSELPQR | AQKKKLS*VNYEQC | 1.80 | 0.47 |
| Camkk2 | S511 | Calcium/calmodulin-dependent protein kinase kinase 2 | SLS*APGNLLTK | REERSLS*APGNLL | 1.71 | 0.54 |
| Map4k5 | S433, S434 | Mitogen-activated protein kinase kinase kinase kinase 5 | RQS*S*PSCVPVAETSSSIGNGDGISK | - | 1.36 | 0.79 |
| Map3k2 | T337 | Mitogen-activated protein kinase kinase kinase 2 | RRGSDIDNPT*LTVTDISPPSR | SDIDNPT*LTVTDI | 1.13 | 0.42 |
| Scyl2 | S677 | SCY1-like protein 2 | RGS*LTLEEK | GKQKRGS*LTLEEK | 1.04 | 0.75 |
| Map4k5 | T400 | Mitogen-activated protein kinase kinase kinase kinase 5 | VNT*YPEDSLPDEEK | PKPRVNT*YPEDSL | 0.95 | 1.35 |
| Cdk18 | S66 | Cyclin-dependent kinase 18 | RFS*MEDLNK | QNQRRFS*MEDLNK | 0.88 | 0.42 |
| Map3k2 | S331 | Mitogen-activated protein kinase kinase kinase 2 | RRGS*DIDNPTLTVTDISPPSR | RIRRRGS*DIDNPT | 0.78 | 0.84 |
| Map3k2 | S331, S344, S347 | Mitogen-activated protein kinase kinase kinase 2 | RRGS*DIDNPTLTVTDIS*PPS*RSPR | - | 0.70 | 0.77 |
| Aak1 | S635 | AP2-associated protein kinase 1 | ILS*DVTHSAVFGVPASK | GHRRILS*DVTHSA | 0.62 | 0.48 |
| Map3k2 | S331, S344 | Mitogen-activated protein kinase kinase kinase 2 | RRGS*DIDNPTLTVTDIS*PPSRSPR | - | 0.62 | 0.48 |
| Map3k2 | S331, S349 | Mitogen-activated protein kinase kinase kinase 2 | RGS*DIDNPTLTVTDISPPSRS*PR | - | 0.54 | 1.01 |
| Prkd2 | S211, S214 | Serine/threonine-protein kinase D2 | LGS*SES*LPCTAEELSR | - | 0.47 | 1.27 |
| Srpk1 | S450 | SRSF protein kinase 1 | ADTPS*GDEQEPNGALDSK | IRADTPS*GDEQEP | 0.44 | 0.66 |
| Prkd2 | S197, S206 | Serine/threonine-protein kinase D2 | RLS*STSLASGHS*VR | - | 0.42 | 0.66 |
| Prkd2 | S197, S198 | Serine/threonine-protein kinase D2 | RLS*S*TSLASGHSVR | - | 0.42 | 1.11 |
| Nek4 | S430 | Serine/threonine-protein kinase Nek4 | RSS*DGGDGEGSELVKPLYPSNK | LLPRRSS*DGGDGE | 0.41 | 0.59 |
| Erbb2 | S1055 | Receptor tyrosine-protein kinase erbB-2 | S*GGGELTLGLEPSEEEPPR | RSSSARS*GGGELT | 0.40 | 0.61 |
| Src | S74 | Neuronal proto-oncogene tyrosine-protein kinase Src | LFGGFNSSDTVTS*PQR | SSDTVTS*PQRAGP | −0.40 | 0.40 |
| Map3k3 | S147 | Mitogen-activated protein kinase kinase kinase 3 | IKPSQS*AGDINTIYQAPEPR | RIKPSQS*AGDINT | −0.42 | 0.60 |
| Mapk3 | Y205 | Mitogen-activated protein kinase 3 | IADPEHDHTGFLTEY*VATR | TGFLTEY*VATRWY | −0.44 | 1.44 |
| Taok1 | S421 | Serine/threonine-protein kinase TAO1 | ASDPQS*PPQVSR | RASDPQS*PPQVSR | −0.44 | 0.54 |
| Map3k5 | S965 | Mitogen-activated protein kinase kinase kinase 5 | LSALSTGS*NEYLR | SALSTGS*NEYLRS | −0.48 | 0.47 |
| Map3k11 | S508 | Mitogen-activated protein kinase kinase kinase 11 | ITVQAS*PGLDR | RITVQAS*PGLDRR | −0.54 | 0.66 |
| Csnk1d | T349 | Casein kinase I isoform delta | GTQEVAPPTPLTPT*SHTANTSPRPVSGMER | PTPLTPT*SHTANT | −0.60 | 0.48 |
| Mark3 | S583 | MAP/microtubule affinity-regulating kinase 3 | TATYNGPPAS*PSLSHEATPLSQTR | YNGPPAS*PSLSHE | −0.70 | 0.98 |
| Mylk | S355 | Myosin light chain kinase, smooth muscle | VPAIGSFS*PGEDRK | PAIGSFS*PGEDRK | −0.73 | 0.59 |
| Mylk | S935 | Myosin light chain kinase, smooth muscle | KVHS*PQQVDFR | EERKVHS*PQQVDF | −0.76 | 0.62 |
| Mylk | S355 | Myosin light chain kinase, smooth muscle | VPAIGSFS*PGEDR | PAIGSFS*PGEDRK | −0.79 | 0.56 |
| Mink1 | S746 | Misshapen-like kinase 1 | LDSS*PVLSPGNK | STKLDSS*PVLSPG | −0.84 | 0.42 |
| Peak1 | T1141 | Inactive tyrosine-protein kinase PEAK1 | TDQEGLNASQPT*PPPLPK | LNASQPT*PPPLPK | −0.88 | 0.41 |
| Lmtk2 | S1418 | Serine/threonine-protein kinase LMTK2 | YFS*PPPPAR | QTSKYFS*PPPPAR | −0.93 | 0.53 |
| Map3k2 | S344, S347 | Mitogen-activated protein kinase kinase kinase 2 | GSDIDNPTLTVTDIS*PPS*RSPR | - | −1.45 | 0.45 |
| Map3k2 | S347 | Mitogen-activated protein kinase kinase kinase 2 | GSDIDNPTLTVTDISPPS*RSPR | TDISPPS*RSPRAP | −1.57 | 0.74 |
The complete list of 9640 phosphopeptides was filtered based on dual criteria (−Log10(CV)≥0.4 and |Log2(dDAVP/Vehicle) |>0.4) to shortlist 25 protein kinases (36 phosphopeptides) with high probability abundance changes in response to dDAVP. This table contains mono, and multi-phosphorylated peptides. For multi-phosphorylated peptides, no centralized sequence is presented. The mean ratio from three clones of mpkCCD cells used for three SILAC experiments were presented. CV = Coefficient of variation in vasopressin response among three clones of mpkCCD cells.
Figure 4.
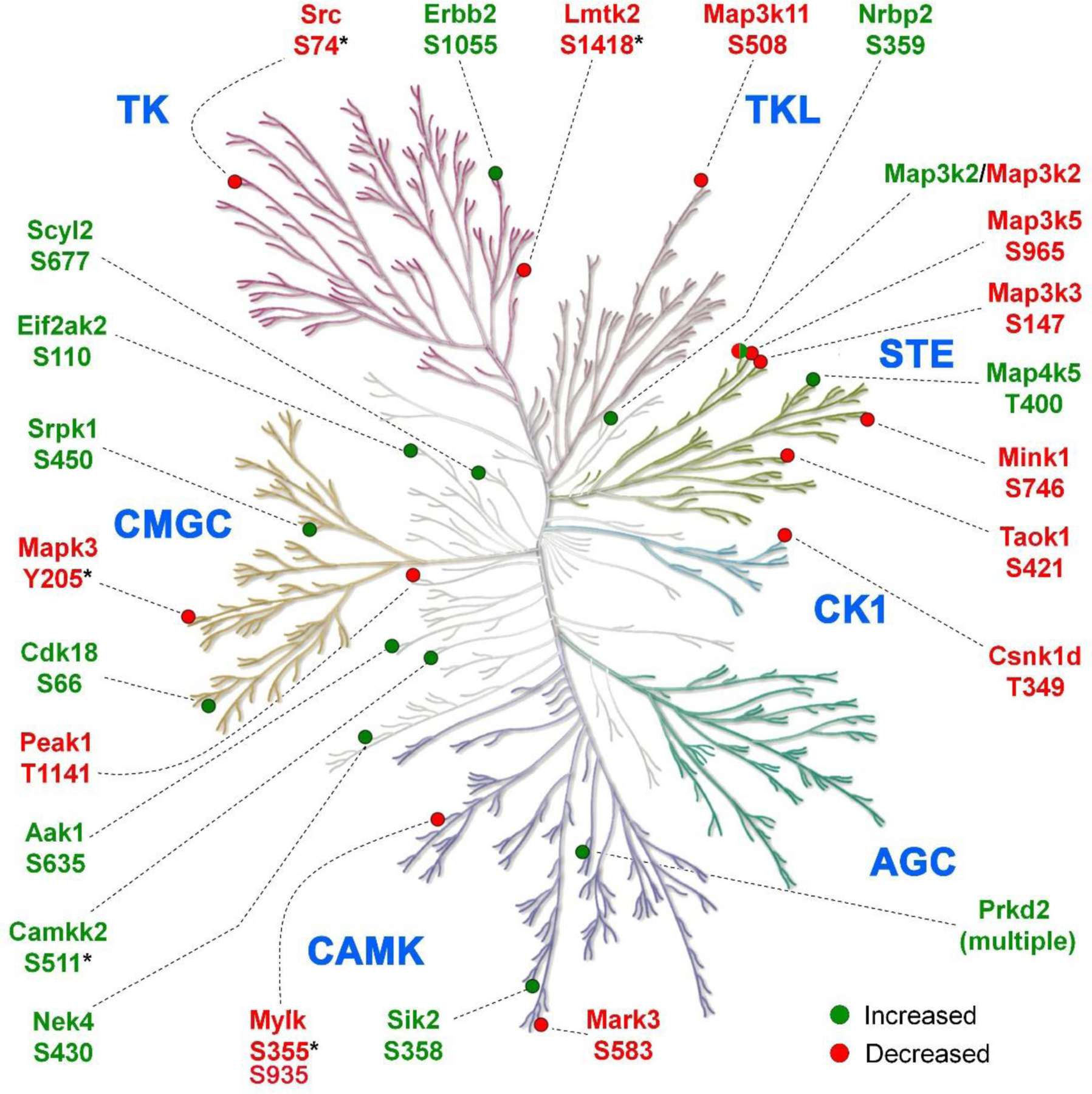
Vasopressin responsive phosphorylation sites mapped to protein kinases. Green and red bullets indicate increased and decreased phosphorylation sites respectively following dDAVP treatment. None of the kinases that undergo phosphorylation changes are in the AGC category, but instead are chiefly members of the CAMK, CMGC, TLK, CK1, STE, and TK families as well as a few that are unclassified. KinMap was used to generate the kinome tree. *, phosphorylation sites with known effects on catalytic activity.
Figure 5.
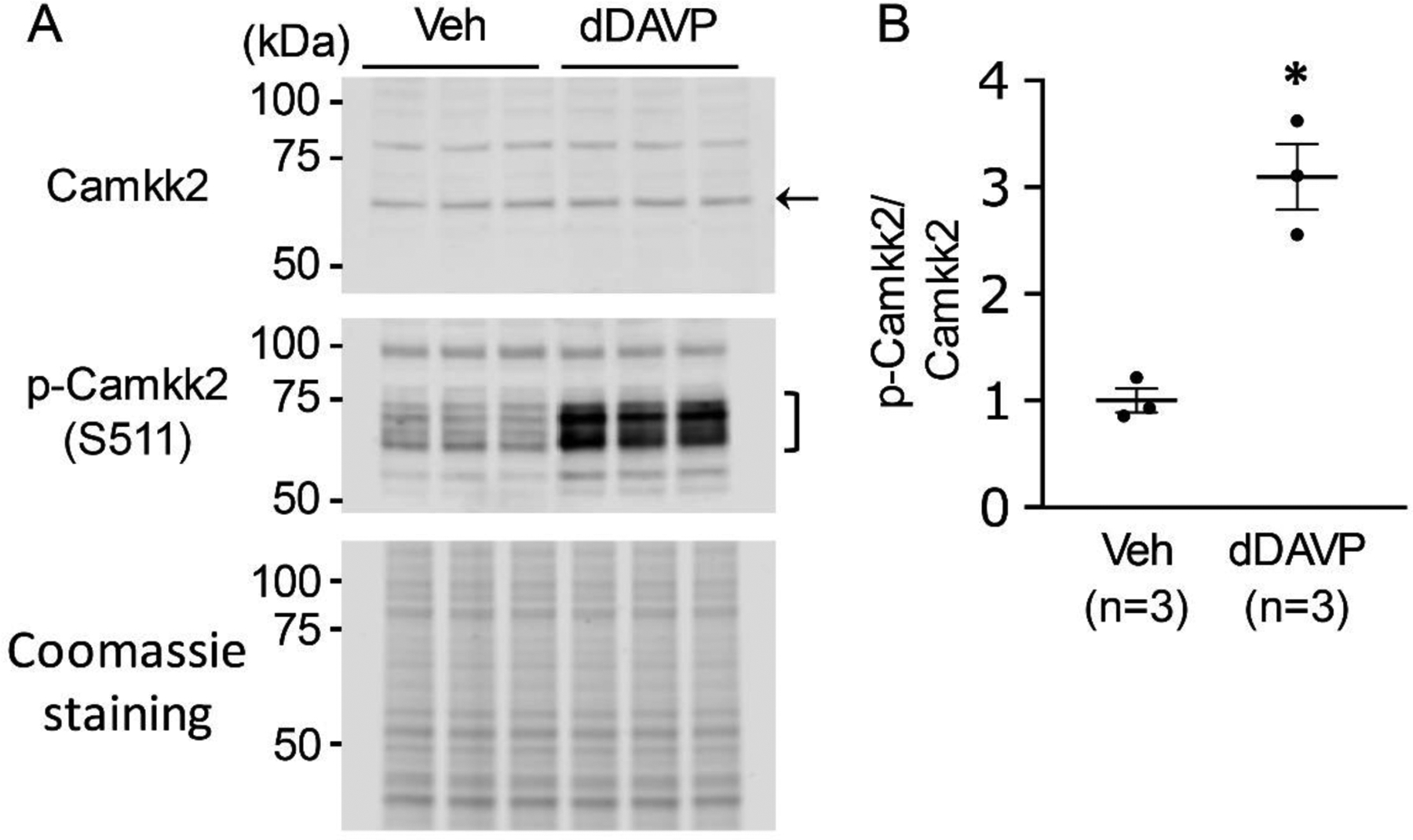
Immunoblot analysis to detect dDAVP-induced phosphorylation on Camkk2. A. Camkk2 and pS511-Camkk2 immunoblot from dDAVP- and vehicle-treated mpkCCD cells. The gels were stained with Coomassie stain to check equal loading among different samples. B. Separated scatter plot showing the ratio of normalized immuno-reactivities (mean ± SEM in arbitrary units) of pS511-Camkk2 and Camkk2. Unpaired t-test was used to determine significant difference between dDAVP and vehicle-treated samples. dDAVP caused a significant increase in phosphorylation of S511 of Camkk2 in mpkCCD cells. n, experimental replicate for vasopressin stimulation experiment. *P < .05. A number of immunoblots using phospho-specific antibodies have been reported previously (https://esbl.nhlbi.nih.gov/ESBL/WB-data/).
Target Motifs of Vasopressin-Responsive Protein Kinases
To derive target motifs for each regulated kinase we used the sequences of known targets of all kinases (data downloaded from PhosphoSitePlus) on a particular branch of the kinase dendrogram in Figure 4. For example, since Sik2 and Mark3 (SNF1-subfamily kinases) underwent a change in phosphorylation, we collected the centralized sequences of known targets of Mark3, Sik2, plus 11 other kinases (274 sequences, Supplemental Table 4) from the same branch of the dendrogram and used PTM-Logo to create a sequence preference prediction for Mark3/Sik2-related kinases. The resulting logos are shown on Figure 6. Of note, several vasopressin-regulated kinases were excluded from this analysis due to an insufficient number (i.e. less than 25) of known substrates, viz., Nrbp2, Camkk2, Aak1, Lmtk2, Peak1 and Scyl2 (Supplemental Table 4). Conversely, although PKA did not undergo phosphorylation changes, it was included due to its central role in the V2R signaling. Note that the substrates of Protein Kinase D (Prkd2)-related kinases share a motif [L-X-R-(R/H)-X-p(S/T)] that resembles the PKA target motif.
Figure 6.
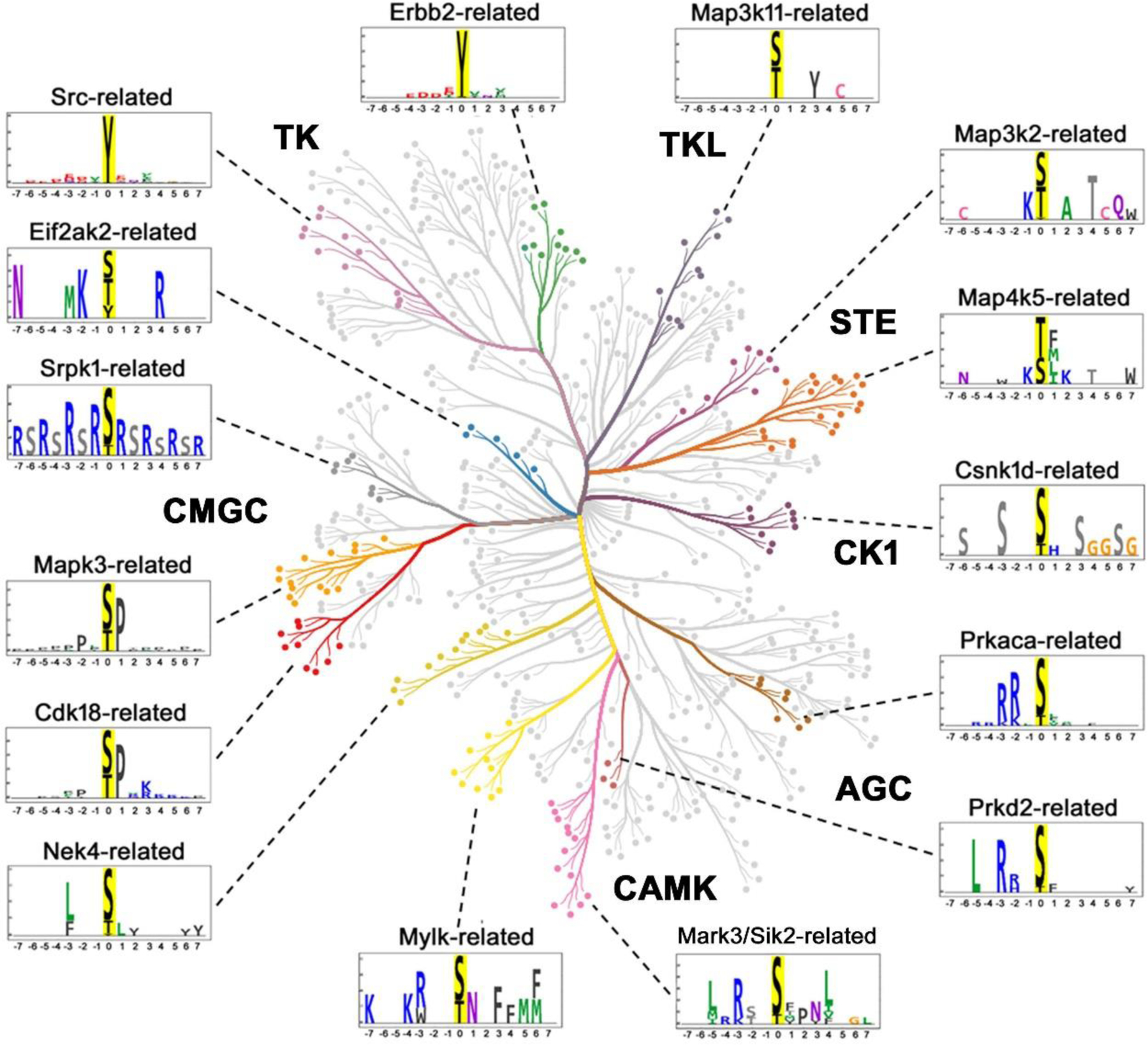
Substrate motifs corresponding to different branches of kinase dendrogram (color-coded) that contains one or more vasopressin responsive protein kinase as depicted in Figure 4. Prkaca (PKA) being the central kinase in V2R signaling was added although altered phosphorylation of PKA was not detected in the current study. The logos were generated after combining known substrates of all kinases in a particular branch that are available in the PhosphoSitePlus database. The list of kinases for different branches, total number and the list of centralized sequences of substrates for each of these branches that were retrieved from the PhosphoSitePlus database are provided in Supplemental Table 4. CORAL was used to generate the color-coded kinome tree. PTM-Logo was used for logo generation with 15-mer centralized peptide sequences. Whole mouse proteome was used as background for motif analysis. Chi-square = 0.001
Rule-Based Classification of Regulated Phosphorylation Sites
Figure 7 (top panel) presents a rule-based classification of the 313 regulated phosphorylation sites based on the sequence surrounding the phosphorylated S or T and the direction of change, yielding 11 subgroups (Supplemental Table 5). The over-representation of specific amino acids (arginine (R), lysine (K), and proline (P)) in the motif analysis (Figure 2B) guided this classification. The objective was to assign one or more protein kinases from Figure 4 to individual regulated phosphorylation sites. The substrate logos generated in Figure 6 for vasopressin-regulated kinase sub-groups were used to find the candidate upstream kinases (Figure 7, bottom). A total of 133 out of the 313 phosphosites possessed a P in position +1. A majority of these sites (91%, n=121, sub-group 1) displayed reduced phosphorylation following dDAVP treatment (predicted kinase: Mapk3). However, the presence of at least 12 of these sites with increased phosphorylation (sub-group 2) indicates likely increased activity of one or more proline-directed kinase(s) (predicted kinase: Cdk18). The phosphosites without P at position +1 were next divided into two groups; phosphosites with (n= 110) and without (n=70) positively charged amino acids (R/K/H) in position −3. This step was done to separate substrates of nominally basophilic kinases from non-basophilic kinases. Out of these 110 phosphosites, 93 had increased phosphorylation (85%), while 17 (sub-group 3) had decreased phosphorylation (15%) in response to vasopressin. The 93 increased sites were subclassified based on the presence of R/K/H in position −2. Among these, 63 (68%, sub-group 4) had R/K/H in position −2. These are likely target sites of PKA or Prkd2. Interestingly, in more than half (16 out of 30, sub-group 5) of the sites that do not have R/K/H in position −2, R/K is present in position −5. This indicates possible roles of Sgk1, Akt1 or Lats1 (Miller et al., 2008) although none of these kinases underwent significant changes in phosphorylation in the current study. Among the upregulated 36 sites that do not contain R/K/H in position −3, two major sub-groups can be identified based on whether R/K is present in position −2. Sixteen sites have R/K in position −2 (44%, sub-group 7, no predicted kinase). Out of the remaining 20 sites that do not have R/K in position −2, 15 had S/D/E in position −3 (sub-group 8, no predicted kinase). Sub-group 10 contains 12 out of 34 (33%) down-regulated sites that contain S/T in position −3 instead of R/K/H (predicted kinase: Csnk1d). Overall, about 81% (Figure 7, 255/313) of the phosphorylation sites were tentatively mapped to candidate upstream kinases.
Figure 7.
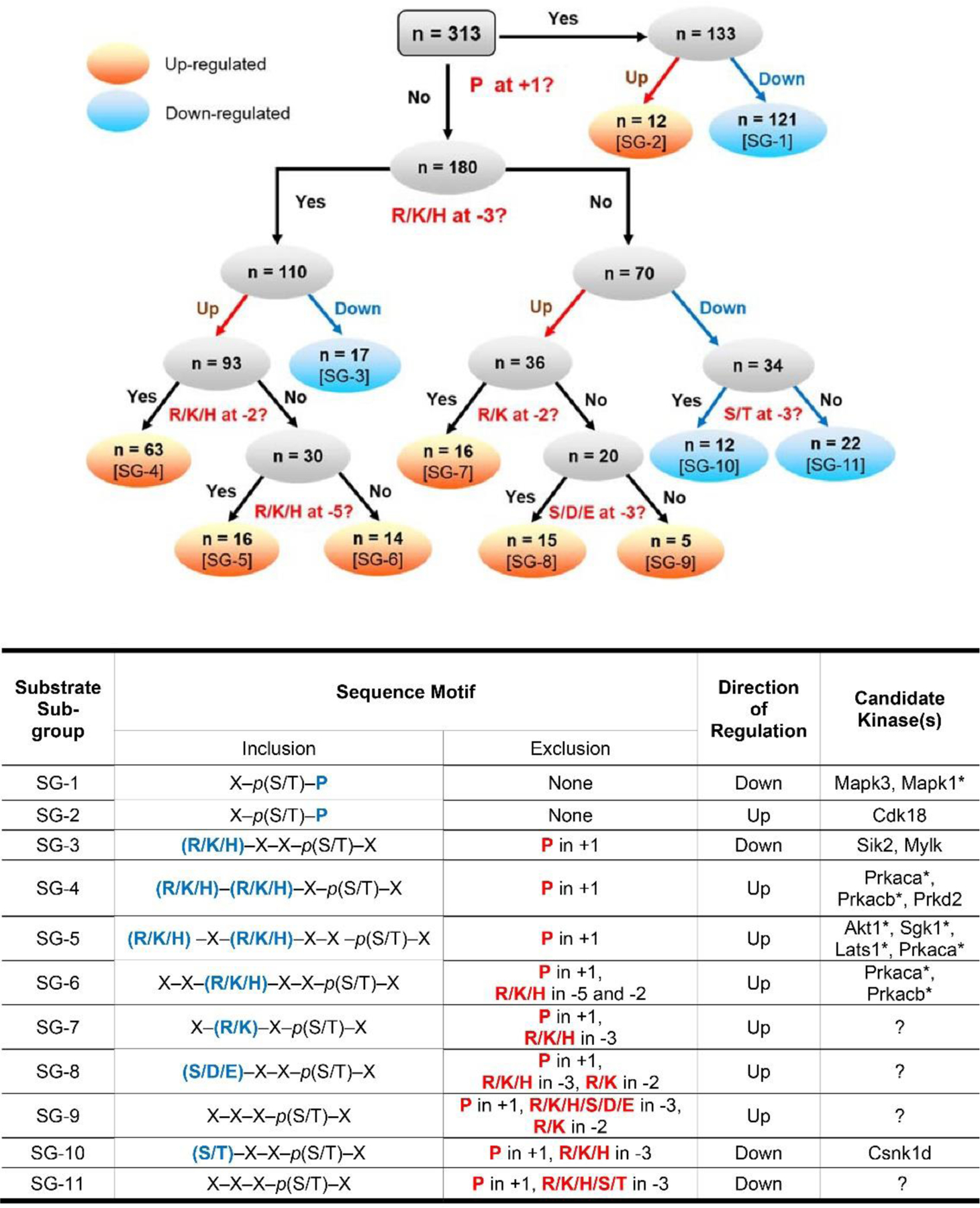
Rule-based classification of vasopressin-regulated phosphorylation sites. Top panel: All 313 regulated single phosphorylation sites were classified into 11 sub-groups (numbered SG-1 to SG-11 in square brackets). This classification is based on the direction of change (up-regulated or down-regulated) and sequence surrounding the phosphorylated S or T in position +1, −2, −3 and −5. Seven subgroups contain up-regulated phospho-sites (orange) while 4 sub-groups contain down-regulated phospho-sites (blue). Bottom panel: The table presents the sequence motifs in the ‘inclusion’ column (amino acids in blue color) for all 11 sub-groups of substrates along with the candidate kinases. Amino acid residues that are excluded from specific positions are indicated in the exclusion column. *Kinases that are not listed in Table 1.’?’, no known kinase.
Co-localization of Kinase and Target Substrates
Aside from the target motif, a second factor is important in determining targets of a given protein kinase, namely localization within the cells. Intuitively, for a phosphorylation reaction to take place, a protein kinase and its substrate must be in the same sub-cellular compartment. Figure 8 shows a heat-map representation showing relative abundances of the regulated kinases from Table 1 in subcellular fractions from dDAVP-treated mpkCCD cells. The data for regulated substrates is reported in Supplemental Table 6. As can be seen in Figure 8, some kinases are highly restricted regarding subcellular localization. For example, Camkk2 may not be a good candidate for phosphorylation of AQP2, which is present in different fractions.
Figure 8.
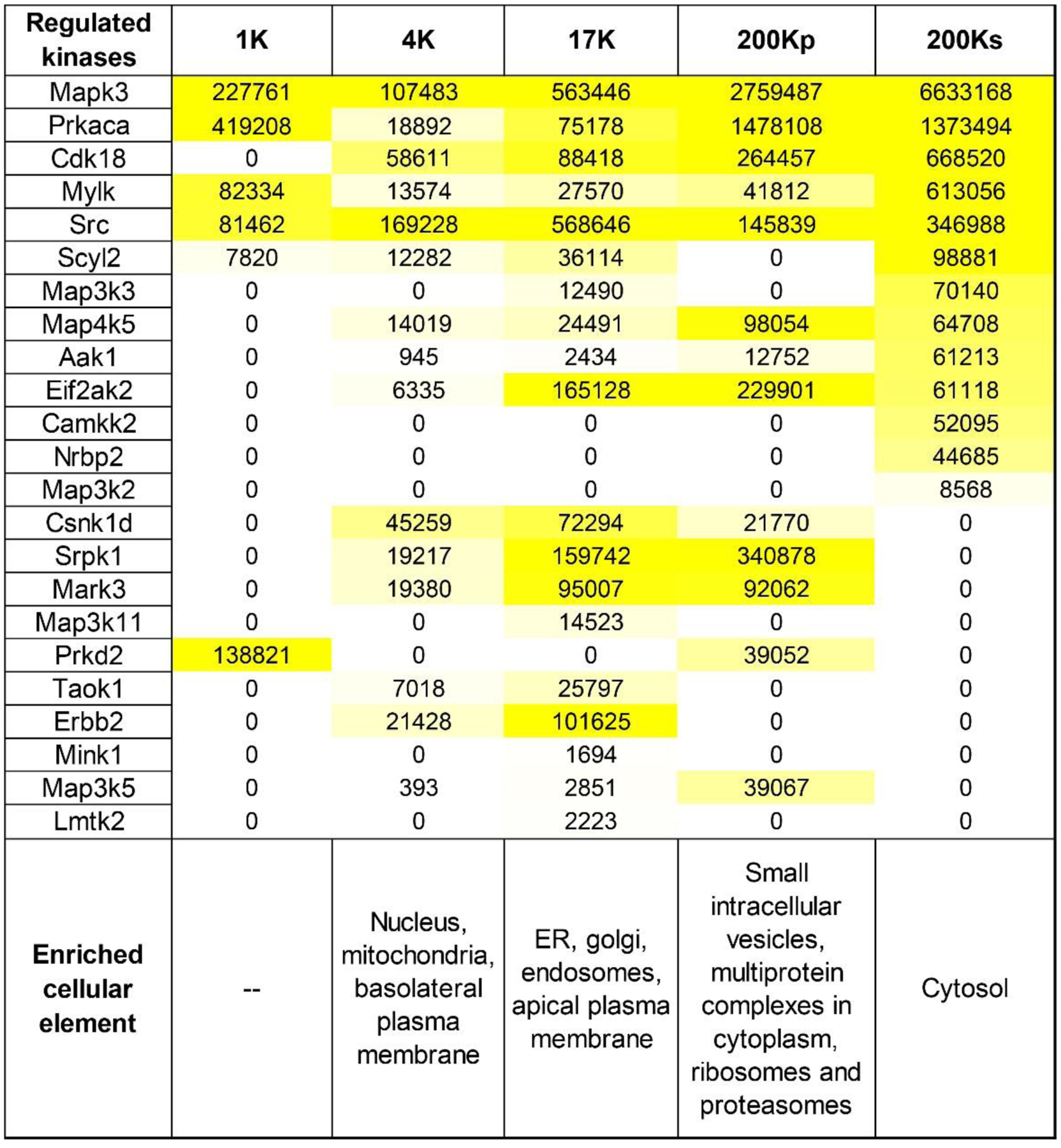
Protein abundances of altered kinases in differentially centrifuged fractions from mouse mpkCCD cells after treatment with 0.1 nM dDAVP for 30 min. Abundances were calculated from total areas under the reconstructed chromatograms. The area was normalized with the molecular weight of the respective protein kinase and presented in arbitrary units. The values were color-coded to relative abundance level to aid visualization. The data was obtained from a web resource generated by Yang et al. (Yang, Raghuram, Emamian, Sandoval & Knepper, 2015; Yang, Tongyoo, Emamian, Sandoval, Raghuram & Knepper, 2015). Vasopressin-regulated Nek4, Peak1 and Sik2 were not included in the table as they were not detected in the source study. These fractions were loosely assigned to various elements of the cell based on prior literature evidence and distribution of the marker proteins in the above two studies.1K, 1000 Xg; 4K, 4000 Xg; 17K, 17,000 Xg; 200Kp, 200,000 Xg-pellet; 200Ks, 200,000 Xg-supernatant.
Subcellular Targeting Mechanisms for Kinases and their Substrates
Some protein kinases such as PKC isoforms are preferentially active at membrane surfaces (Steinberg, 2008). We identified “membrane-associated proteins” as those with GO Cellular Component terms containing the strings “membrane”, “vesicle” or “endosome”. 93 of 137 phosphoproteins with increased phosphorylation were membrane-associated proteins (68%) compared with 1163/2512 (46%) of all phosphoproteins that did not contain upregulated phosphopeptides (Chi-square, P=1.00E-06), consistent with preferential phosphorylation at membrane surfaces (see Supplemental Spreadsheet 1 for protein lists). Similar findings were obtained with phosphoproteins that contain phosphopeptides decreased in response to dDAVP. 109 proteins out of 183 (60%) were membrane-associated (Chi-square, P=0.001, Supplemental Spreadsheet 1). We conclude that phosphoproteins altered in V2R signaling are preferentially membrane-associated.
The above results prompted us to ask, how do phosphoproteins downstream from V2R activation localize to membranes? We used DAVID to identify proteins with lipid-binding or protein-binding domains that are enriched in upregulated phosphopeptides. This analysis identified the pleckstrin homology (PH)-like domain, (found in 22 of the 137 upregulated phosphoproteins, EASE=4.40E-05; 28 out of 183 downregulated phosphoproteins, EASE= 2.50E-06). PH domains mediate membrane binding of proteins through interactions with inositol phospholipids (Harlan, Hajduk, Yoon & Fesik, 1994), but can also mediate binding to certain proteins such as protein kinase C (Yao, Kawakami & Kawakami, 1994). Prkd2 is a PH domain containing kinase identified as vasopressin-regulated in the current study (Figure 9A).
Figure 9.
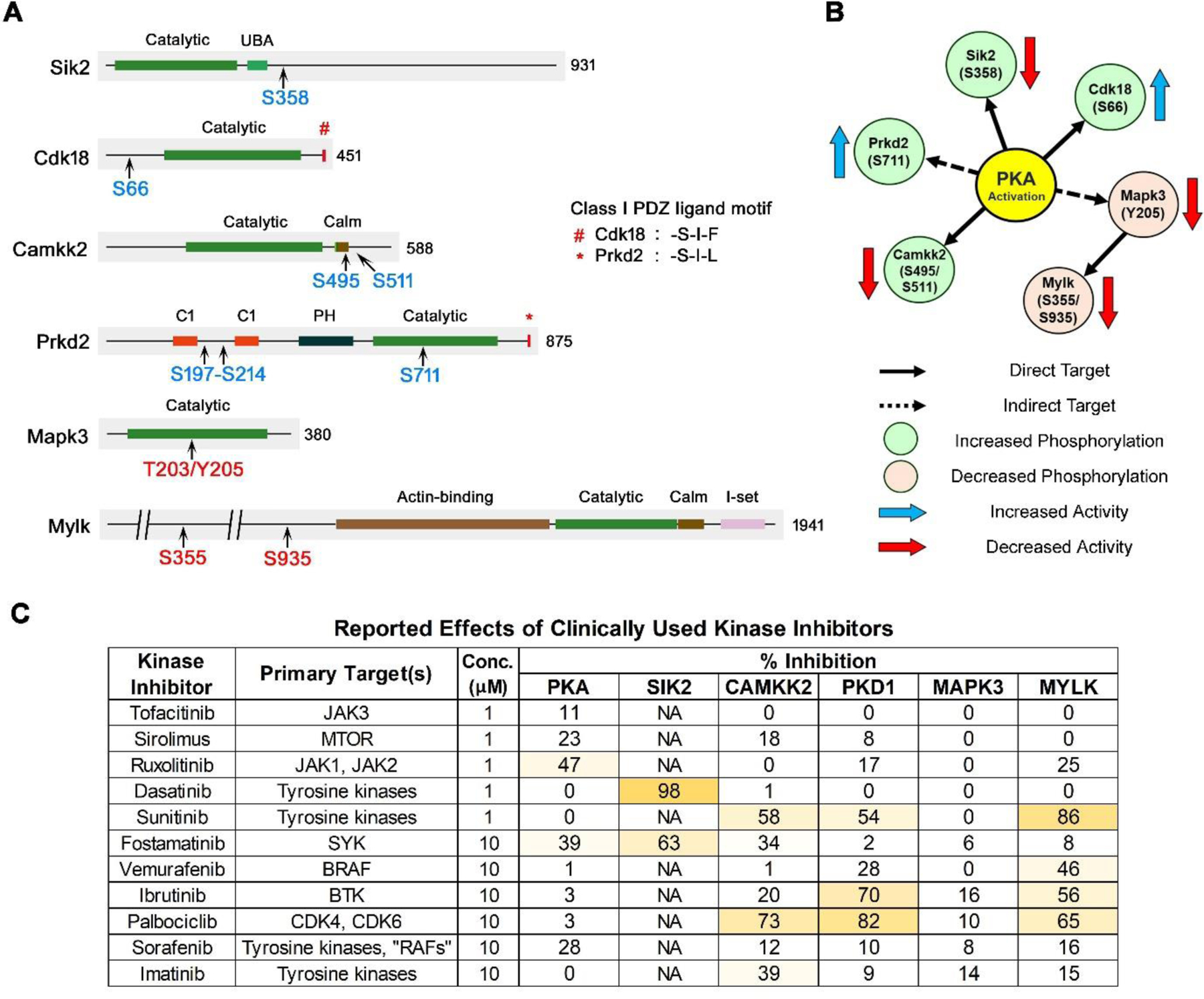
Protein kinases that undergo phosphorylation as either direct or indirect targets of PKA. A. Locations of vasopressin-responsive phosphorylation sites are indicated in blue (increased) or red (decreased). Class I PDZ ligand motif on the carboxyl terminal is shown. The total number of amino acid residues in each kinase is shown. Calm, calmodulin-binding domain; C1, conserved region-1/ diacylglycerol-binding domain; PH, plekstrin homology phosphoinositide binding domain; UBA, ubiquitin-associated domain; I-set, Immunoglobulin I-set domain. B. The relationship between PKA and downstream kinases are indicated using continuous (direct target) or dashed (indirect target) arrows. The direction of change in phosphorylation (blue, increased; red, decreased) and the resultant effect on the activity of the target kinase (blue arrow, increased; red arrow, decreased) are shown. Of note, increased phosphorylation can cause increase or decrease in the activity of a kinase and vice versa. C. Inhibition of protein kinases involved in vasopressin signaling by clinically utilized protein kinase inhibitors. Kinase inhibitor data were downloaded from the International Centre for Kinase Profiling (http://www.kinase-screen.mrc.ac.uk/kinase-inhibitors). Data for an expanded list of kinases are shown in Supplemental Table 7.
Another means of membrane targeting of proteins is interaction between PDZ domain-containing proteins and proteins with COOH-terminal PDZ ligand motifs, which are often integral membrane proteins (Moyer et al., 2000). It is noteworthy that a Class I COOH-terminal ligand motif is present in PKA catalytic subunit alpha (-T-E-F). Consequently, we analyzed the COOH-terminal sequences of upregulated and membrane-associated phosphoproteins to explore possible roles of PDZ interactions in membrane localization. The class I PDZ ligand motif was defined as -(S/T)-X- Ø where S/T indicates serine or threonine, X indicates any amino acid and Ø indicates any hydrophobic amino acid (W/F/L/I/V/A/M) (Dunn & Ferguson, 2015). A total of 13 out of 93 (14%) membrane-associated phosphoproteins with upregulated phosphosites possessed COOH-terminal Class I PDZ ligand motifs compared with 91 of 1158 (8%) of non-upregulated membrane-associated phosphoproteins (Chi-square, P=0.040, Supplemental Spreadsheet 2). Among the vasopressin-regulated kinases in Table 1, Cdk18 (-S-I-F) and Prkd2 (-S-I-L) were found to possess Class I PDZ ligand motifs on their carboxyl termini (Figure 9A). In addition, nine PDZ domain proteins underwent changes in phosphorylation namely, Afadin (Afdn), ZO-1 (Tjp1), Deptor, Pdlim1, Pdlim2, Pdlim5, Ptpn13, Sipa1l1 and NHERF1 (Slc9a3r1). Remarkably, all exhibited substantial decreases in phosphorylation and 8 out of 9 of these decreases were at sites with P in position +1 relative to the phosphorylated amino acid, consistent with a potential role of ERK.
A related question is the role of A-kinase anchoring proteins (AKAPs) in compartmentalization of protein kinase activities. Several AKAPs have been identified as playing roles in vasopressin action in the renal collecting duct (Vukićević, Schulz, Faust & Klussmann, 2016). Six different phosphorylated AKAPs were identified here, namely Akap1, Akap2, Akap8, Akap9, Akap12 and Akap13. Among these, Akap1, Akap12 and Akap13 underwent changes in phosphorylation in response to dDAVP (Figure 3). AKAPs not only bind to PKA regulatory subunits but also other protein kinases, protein phosphatases, cyclic AMP phosphodiesterases, adenylyl cyclases, phospholipases and other signaling proteins (Bucko & Scott, 2020). Among these, the analysis detected vasopressin-induced changes in phosphorylation of cyclic nucleotide phosphodiesterase Pde4c, protein phosphatase Ppm1h, protein phosphatase slingshot 1 (Ssh1), phospholipase C beta (Plcb3), protein kinase Src, Prkd2, and multiple protein phosphatase regulatory subunits. Several of these changes were in basophilic sites compatible with phosphorylation by PKA. For example, Pde4c underwent increases in phosphorylation at two putative PKA target sites, S40 and S299.
DISCUSSION
Vasopressin exerts its physiological effects in collecting duct cells through a GPCR that signals largely through Gαs-mediated activation of adenylyl cyclase, production of cAMP and activation of PKA. Indeed, we previously showed that almost all vasopressin-mediated phosphorylation changes in mpkCCD cells are ablated when the two PKA catalytic genes (Prkaca and Prkacb) are deleted via CRISPR-Cas9 (PKA-null cell) (Datta et al., 2020). In the vasopressin signaling network, a majority of downstream actions can result from PKA-mediated regulation of other protein kinases from various kinase subfamilies that have different target sequence specificities and different localizations in the cell. Based on the direction of phosphorylation change and the sequence surrounding the phosphorylated amino acid, all 313 phosphorylation sites were classified into sub-groups that could be mapped to the regulated kinases (Figure 7). About 81% of phosphorylation changes are potentially mediated by just a few protein kinases. In this Discussion, we focus on the six protein kinases that fit with prior literature regarding vasopressin signaling in the collecting duct, viz. Sik2, Cdk18, Camkk2, Prkd2, Mapk3 (ERK1), and Mylk. Figure 9A shows the location of phosphorylation sites relative to the major protein domains in each of these six PKA-downstream kinases. This list is based on phosphorylation changes alone and several other kinases are known to be regulated by other mechanisms.
Salt-inducible Kinase 2 (Sik2).
In our prior study in PKA-null cells (Isobe et al., 2017), two sites in Sik2 showed striking decreases in phosphorylation, namely S358 and S587, both of them with sequences compatible with direct PKA phosphorylation. In this study, only S358 showed a significant increase in response to vasopressin (more than 4-fold), presumably PKA-mediated. PKA was previously demonstrated to phosphorylate S358 of Sik2 in adipocytes (Henriksson et al., 2012), reducing its enzymatic activity (Henriksson et al., 2015) via 14–3-3 binding (Sonntag, Vaughan & Montminy, 2018). Thus, Sik2 activity is likely reduced by vasopressin in collecting duct cells. Sik2 is an AMP-activated protein kinase-like kinase with a basophilic target motif as illustrated in Figure 6. Among its targets are CREB-related transcriptional coregulators Crebbp, Ep300, Crtc1, Crtc2 and Crtc3. All three of the CRTC proteins showed large increases in phosphorylation at Sik2 sites in response to deletion of both PKA catalytic genes (Isobe et al., 2017). In this study, Crtc3 showed a marked decrease in phosphorylation at three recognized Sik2 sites, viz. S62, S329 and S370 (Clark et al., 2012). The CRTCs activate transcription of cAMP-responsive target genes by interacting with one or more of the three cAMP response element-binding protein (CREB) transcription factors (ATF1, CREB1 or CREM) (Sonntag, Vaughan & Montminy, 2018). Therefore, the PKA-Sik2-Crtc3 regulatory pathway has potential relevance to the regulation of Aqp2 gene transcription by vasopressin.
Cyclin-dependent Kinase 18.
Cdk18 is a proline-directed kinase (CMGC Family) (Figure 4) that we have identified in previous studies as a target for regulation by vasopressin (Deshpande, Kao, Raghuram, Datta, Chou & Knepper, 2019; Sandoval, Claxton, Lee, Saeed, Hoffert & Knepper, 2016). The regulation is of two types. First, vasopressin treatment markedly accelerates transcription of the Cdk18 gene (Sandoval, Claxton, Lee, Saeed, Hoffert & Knepper, 2016). This effect is likely PKA-dependent because Cdk18 protein abundance was markedly decreased in PKA-null cells (Isobe et al., 2017). In addition, in native rat inner medullary collecting duct (IMCD) cells, short term exposure to vasopressin resulted in a marked increase in phosphorylation at S66 (Bansal et al., 2010), a site compatible with phosphorylation by PKA. Here, vasopressin increased S66 phosphorylation, a response not seen in PKA-null cells (Datta et al., 2020) providing additional evidence that Cdk18 could be an important downstream target of PKA. In HEK293T cells, PKA mediated phosphorylation has been shown to activate Cdk18, with phosphorylation at three sites including S66 (Matsuda et al., 2014). If PKA does the same in collecting duct cells, Cdk18 activation would provide a likely explanation for the many phosphorylation sites with proline in position +1 that increase in response to vasopressin (Figure 7, bottom). Cdk18 expression in collecting duct has recently been confirmed by Dema et al (Dema et al., 2020) who proposed a role in regulation of AQP2 degradation. We found that Cdk18 is very abundant in nuclear fractions in both mpkCCD (Schenk et al., 2012) and native IMCD cells (Pickering et al., 2016) and speculate that it could be involved in regulation of transcriptional events in collecting duct cells as well.
Calcium/calmodulin-Dependent Protein Kinase Kinase 2.
Camkk2 is a calcium/calmodulin-dependent protein kinase known to phosphorylate various downstream targets including AMP-activated kinase (Woods et al., 2005). Our early phosphoproteomics findings in suspensions of native rat IMCD cells identified Camkk2 as a component of vasopressin signaling (Hoffert, Pisitkun, Saeed, Song, Chou & Knepper, 2012). Those studies demonstrated that vasopressin increases phosphorylation at two sites, S494 and S510 (equivalent to S495 and S511 in mouse). Prior studies showed that deletion of PKA decreases phosphorylation at both sites, with almost total ablation of phosphorylation at S495 (Isobe et al., 2017). This confirmed the conclusion from studies in COS-7 cells that both S495 and S511 phosphorylation are PKA-dependent (Wayman, Tokumitsu & Soderling, 1997). Our current results are consistent with the prior findings, providing evidence for vasopressin-mediated phosphorylation at both sites with the greatest increase in S511 phosphorylation. Vasopressin potentially has two effects on Camkk2 activity in the collecting duct: 1) activation via vasopressin-induced calcium mobilization (demonstrated in native collecting duct) (Figure 3) (Chou et al., 2000); and 2) inhibition via S511 phosphorylation, which inhibits Camkk2 by creating a binding site for 14–3-3 proteins (Langendorf et al., 2020) (Figure 9A). Camkk2 is found in nuclear fractions in mpkCCD cells (Schenk et al., 2012) and native rat IMCD cells (Pickering et al., 2016) and therefore is likely to phosphorylate nuclear proteins. For example, Camkk2 has been found to be required for activation of the transcription factor cyclic AMP-responsive element binding protein (CREB1) in the hippocampus (Peters, Mizuno, Ris, Angelo, Godaux & Giese, 2003) and if true in collecting duct cells such a response could be involved in vasopressin-mediated regulation of Aqp2 gene transcription.
Protein Kinase D2 (Prkd2).
Prkd2 is a diacylglycerol regulated kinase that underwent increases in phosphorylation at several sites. Previously, in PKA-null cells, we found markedly decreased phosphorylation at S197 (Isobe et al., 2017), suggesting that it could be a direct PKA target site (centralized sequence: ARKRRLS*STSLAS). Here, we found increases in two phospho-S197-containing double phospho-peptides. Also in prior studies in native rat IMCD cells, we found a moderate increase at another site, S711 (Deshpande, Kao, Raghuram, Datta, Chou & Knepper, 2019), which matches a moderate increase in the present experiments. Phosphorylation of this site has been reported to activate Prkd2 (Konopatskaya et al., 2011).Thus, although the evidence is not ironclad, it suggests that vasopressin increases Prkd2 activity (Figure 9B). The Prkd2 target motif is similar to that of PKA (Figure 6), Thus, some increases in phosphorylation attributed to PKA could also be due to Prkd2 activation.
Mitogen-activated Protein Kinase 3 (Mapk3).
Mapk3 (ERK1) is a serine/threonine kinase which along with Mapk1 (ERK2) plays an important role as the endpoint of the classic Raf-MEK-ERK cascade. ERK stimulates cell proliferation and dedifferentiation through phosphorylation of AP1 and Ets family transcription factors (Cohen, 1999; Kelly & Siebenlist, 1995). It also has many effects on cytoplasmic processes. In this study, a decrease was observed in active site phosphorylation of ERK1, with a smaller response in ERK2. This confirms earlier observations of decreases in active site phosphorylation in response to vasopressin in cultured MDCK cells (Yamada et al., 1995), rat IMCD (Pisitkun, Jacob, Schleicher, Chou, Yu & Knepper, 2008) and mpkCCD cells (Rinschen et al., 2010). These responses can help to explain the anti-proliferative, pro-differentiation effects of vasopressin in normal collecting duct cells (Yamaguchi, Hempson, Reif, Hedge & Wallace, 2006). In PKA-null cells, active site phosphorylation was increased compared to PKA-intact mpkCCD cells indicating that the decreases seen with vasopressin are likely indirectly dependent on PKA activation (Isobe et al., 2017). ERK1 and ERK2 are proline-directed kinases with a target motif, X-p(S/T)-P (Figure 6). The vasopressin-induced decrease in ERK activity provides an explanation for the general decrease in the phosphorylation of proline-directed sites in our current dataset that includes kinases like Src (S74), Mylk (S355, S935), Mark3 (S583) and many others (Table 1). Based on the analysis shown in Figure 7, 39% (Sub-group-1, n= 121) of phosphorylation changes in response to vasopressin in this study could be due to downregulated ERK1 activity.
In addition to heterotrimeric G-protein signaling, GPCRs like the vasopressin V2R can also signal through β-arrestin, which can result in ERK activation (Lefkowitz & Shenoy, 2005). Since ERK1 activation was decreased in the present study, not increased, we believe that β-arrestin signaling does not play a major role in these cells. The main PKA-independent effects of vasopressin as seen in PKA-null cells, which would be expected to reveal arrestin-dependent signaling, were consistent with the activation of SNF1 family kinases, but not MAP kinases (Datta et al., 2020).
Myosin Light Chain Kinase (Mylk).
Mylk is a calcium/calmodulin-dependent protein kinase named for its ability to phosphorylate myosin regulatory light chains (Myl9, Myl12a and Myl12b), thereby activating conventional myosins Myh9 and Myh10 in a variety of cell types. Aside from its canonical target, Mylk has been shown to phosphorylate a variety of other substrates in mpkCCD cells (Isobe, Raghuram, Krishnan, Chou, Yang & Knepper, 2020). In prior studies in collecting duct cells, Mylk has been shown to be regulated by vasopressin in three ways: 1) long-term vasopressin exposure triggers a greater-than 50% decrease in the total cellular abundance of Mylk in mpkCCD cells (Khositseth et al., 2011); 2) vasopressin increases intracellular calcium causing a Mylk-dependent short-term increases in osmotic water permeability in isolated perfused collecting ducts presumably through regulation of AQP2 trafficking (Chou et al., 2000); 3) short-term vasopressin exposure triggers a large decrease in phosphorylation at S355 of Mylk, a putative ERK phosphorylation site, in the nucleus of mpkCCD cells (Bolger, Hurtado, Hoffert, Saeed, Pisitkun & Knepper, 2012). In the present study, we confirmed the decrease at S355 and also found a similar decrease in phosphorylation at S935, also a likely ERK target. In general, ERK mediated phosphorylation of Mylk is associated with increased activity (Huang, Jacobson & Schaller, 2004; Klemke, Cai, Giannini, Gallagher, de Lanerolle & Cheresh, 1997), so decreases in phosphorylation in the present study would quite possibly be associated with decreased catalytic activity (Figure 9B), at least in the nucleus. Isobe et al. proposed that the effect of vasopressin on Mylk activity may be different in the nucleus versus the cytoplasm, with Ca-calmodulin-stimulated increases in cytoplasm and phosphorylation associated decreases in the nucleus (Isobe, Raghuram, Krishnan, Chou, Yang & Knepper, 2020).
Future Studies.
Figure 9B summarizes the relationships between PKA activation by vasopressin in collecting duct principal cells and regulation of the six downstream protein kinases highlighted in Figure 9A. A future goal is to carry out experiments to match these kinases to the target substrates shown in Figure 7 and to the physiological responses shown in Figure 1. Such studies will use CRISPR-Cas9 to delete the kinases, as already done for Mylk (Isobe, Raghuram, Krishnan, Chou, Yang & Knepper, 2020), and to observe the ensuing changes in phosphorylation and function. In addition to V2R, collecting duct principle cells express two other Gαs-coupled GPCRs, viz. the prostaglandin EP4 receptor (Ptger4) and the calcitonin receptor-like receptor (Calcrl) that mediates adrenomedullin signaling (Chen et al., 2017). These GPCRs could provide a means of bypassing the mutant V2R in X-linked nephrogenic diabetes insipidus (Li et al., 2009). Ptger4 and Calcrl might signal through the same pathways as V2R but could signal independently if they exist in different microdomains. Future phosphoproteomic studies will be needed to test this possibility.
Clinical Relevance.
Figure 9C lists several clinically utilized protein kinase inhibitors that have undergone kinase inhibitor profiling (International Centre for Kinase Profiling, http://www.kinase-screen.mrc.ac.uk/kinase-inhibitors) and their inhibitory activity against PKA and the kinases that are shown in Figure 9A. The data show that several of the vasopressin-regulated kinases identified in this study can be inhibited by clinically utilized kinase inhibitor drugs. Thus, effects of these drugs on systemic water balance are likely and are potential topics for future studies.
Supplementary Material
BULLET POINT SUMMRY.
What is already known?
Vasopressin regulates water transport in kidney collecting duct by activating a cAMP-PKA-dependent signaling network.
Defects of this signaling pathway result in water balance disorders such as nephrogenic diabetes insipidus.
What this study adds?
Comprehensive phosphoproteomics identifies signaling via the vasopressin V2 receptor in renal collecting duct cells.
PKA is at the head of an arborized phosphorylation network involving several downstream protein kinases.
What is the clinical significance?
Several clinically utilized protein kinase inhibitors have inhibitory activity toward vasopressin-regulated protein kinases.
Six protein kinases (Sik2, Cdk18, Camkk2, Prkd2, Mapk3, Mylk) are possible therapeutic targets for water-balance disorders.
ACKNOWLEDGEMENTS
The work was primarily funded by the Division of Intramural Research, National Heart, Lung, and Blood Institute (project ZIA-HL001285 and ZIA-HL006129, M.A.K.). Protein mass spectrometry was done in the NHLBI Proteomics Core Facility (Marjan Gucek, Director). The authors thank Angel Aponte and Guanghui Wang of the NHLBI Proteomics Core Facility for mass spectrometry assistance. We thank Kavee Limbutara and Lihe Chen for helpful discussions.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigor of preclinical research as stated in the BJP guidelines for Design & Analysis, Immunoblotting and Immunochemistry, and Animal Experimentation, and as recommended by funding agencies, publishers, and other organizations engaged with supporting research.
Abbreviations for specialized terms:
- AKAP
A-kinase anchoring protein
- AMPK
5’-AMP-activated protein kinase
- AQP2
aquaporin-2
- CV
Co-efficient of variation
- dDAVP
1-deamino-8-D-arginine vasopressin
- ERK
extracellular signal-regulated kinase
- EThcD
Electron-Transfer/Higher-Energy Collision Dissociation
- Fe-NTA
Ferric nitrilotriacetate
- GO
Gene Ontology
- GPCR
G-protein coupled receptor
- H-89
N-[2-[[3-(4-Bromophenyl)-2-propenyl]amino]ethyl]-5-isoquinolinesulfonamide
- HCD
Higher-energy Collision Dissociation
- IMCD
inner medullary collecting duct
- mpkCCD
murine immortalized cortical collecting duct
- PDZ
domain present in PSD-95, Dlg, and ZO-1
- PH
pleckstrin homology
- PKA
protein kinase A
- RRID
Research Resource Identifier
- SILAC
Stable Isotope Labeling with Amino acids in Cell culture
- V2R
type 2 vasopressin receptor
Footnotes
Official gene symbols used to specify individual proteins can be identified at https://www.uniprot.org/.
CONFLICT OF INTEREST
Authors declare that there is no conflict of interest.
REFERENCES
- Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Mathie A, Peters JA, et al. (2019a). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: G protein-coupled receptors. British Journal of Pharmacology 176: S21–S141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Mathie A, Peters JA, Veale EL, et al. (2019b). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Enzymes. British Journal of Pharmacology 176: S297–S396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal AD, Hoffert JD, Pisitkun T, Hwang S, Chou CL, Boja ES, et al. (2010). Phosphoproteomic profiling reveals vasopressin-regulated phosphorylation sites in collecting duct. Journal of the American Society of Nephrology : JASN 21: 303–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers DM, Xiang YK, & Zaccolo M (2019). Whole-Cell cAMP and PKA Activity are Epiphenomena, Nanodomain Signaling Matters. Physiology (Bethesda) 34: 240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger SJ, Hurtado PA, Hoffert JD, Saeed F, Pisitkun T, & Knepper MA (2012). Quantitative phosphoproteomics in nuclei of vasopressin-sensitive renal collecting duct cells. Am J Physiol Cell Physiol 303: C1006–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucko PJ, & Scott JD (2020). Drugs that Regulate Local Cell Signaling: AKAP Targeting as a Therapeutic Option. Annu Rev Pharmacol Toxicol. [DOI] [PubMed] [Google Scholar]
- Chen L, Lee JW, Chou CL, Nair AV, Battistone MA, Păunescu TG, et al. (2017). Transcriptomes of major renal collecting duct cell types in mouse identified by single-cell RNA-seq. Proceedings of the National Academy of Sciences of the United States of America 114: E9989–e9998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou CL, Yip KP, Michea L, Kador K, Ferraris JD, Wade JB, et al. (2000). Regulation of aquaporin-2 trafficking by vasopressin in the renal collecting duct. Roles of ryanodine-sensitive Ca2+ stores and calmodulin. The Journal of biological chemistry 275: 36839–36846. [DOI] [PubMed] [Google Scholar]
- Clark K, MacKenzie KF, Petkevicius K, Kristariyanto Y, Zhang J, Choi HG, et al. (2012). Phosphorylation of CRTC3 by the salt-inducible kinases controls the interconversion of classically activated and regulatory macrophages. Proceedings of the National Academy of Sciences of the United States of America 109: 16986–16991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen DM (1999). Signalling and gene regulation by urea and NaCl in the renal medulla. Clin Exp Pharmacol Physiol 26: 69–73. [DOI] [PubMed] [Google Scholar]
- Datta A, Yang CR, Limbutara K, Chou CL, Rinschen MM, Raghuram V, et al. (2020). PKA-independent vasopressin signaling in renal collecting duct. Faseb j 34: 6129–6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dema A, Faust D, Lazarow K, Wippich M, Neuenschwander M, Zühlke K, et al. (2020). Cyclin-Dependent Kinase 18 Controls Trafficking of Aquaporin-2 and Its Abundance through Ubiquitin Ligase STUB1, Which Functions as an AKAP. Cells 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande V, Kao AR, Raghuram V, Datta A, Chou C-L, & Knepper MA (2019). Phosphoproteomic Identification of Vasopressin V2 Receptor-Dependent Signaling in the Renal Collecting Duct. American Journal of Physiology-Renal Physiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn HA, & Ferguson SS (2015). PDZ Protein Regulation of G Protein-Coupled Receptor Trafficking and Signaling Pathways. Molecular pharmacology 88: 624–639. [DOI] [PubMed] [Google Scholar]
- Eid S, Turk S, Volkamer A, Rippmann F, & Fulle S (2017). KinMap: a web-based tool for interactive navigation through human kinome data. BMC Bioinformatics 18: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton RA, & Knepper MA (2007). Mouse models and the urinary concentrating mechanism in the new millennium. Physiological reviews 87: 1083–1112. [DOI] [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S, et al. (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic acids research 46: D1091–d1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan JE, Hajduk PJ, Yoon HS, & Fesik SW (1994). Pleckstrin homology domains bind to phosphatidylinositol-4,5-bisphosphate. Nature 371: 168–170. [DOI] [PubMed] [Google Scholar]
- Henriksson E, Jones HA, Patel K, Peggie M, Morrice N, Sakamoto K, et al. (2012). The AMPK-related kinase SIK2 is regulated by cAMP via phosphorylation at Ser358 in adipocytes. The Biochemical journal 444: 503–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksson E, Sall J, Gormand A, Wasserstrom S, Morrice NA, Fritzen AM, et al. (2015). SIK2 regulates CRTCs, HDAC4 and glucose uptake in adipocytes. Journal of cell science 128: 472–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffert JD, Pisitkun T, Saeed F, Song JH, Chou CL, & Knepper MA (2012). Dynamics of the G protein-coupled vasopressin V2 receptor signaling network revealed by quantitative phosphoproteomics. Molecular & cellular proteomics : MCP 11: M111.014613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Jacobson K, & Schaller MD (2004). MAP kinases and cell migration. Journal of cell science 117: 4619–4628. [DOI] [PubMed] [Google Scholar]
- Insel PA, Snead A, Murray F, Zhang L, Yokouchi H, Katakia T, et al. (2012). GPCR expression in tissues and cells: are the optimal receptors being used as drug targets? Br J Pharmacol 165: 1613–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isobe K, Jung HJ, Yang CR, Claxton J, Sandoval P, Burg MB, et al. (2017). Systems-level identification of PKA-dependent signaling in epithelial cells. Proceedings of the National Academy of Sciences of the United States of America 114: E8875–E8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isobe K, Raghuram V, Krishnan L, Chou CL, Yang CR, & Knepper MA (2020). CRISPR-Cas9/phosphoproteomics identifies multiple noncanonical targets of myosin light chain kinase. American journal of physiology Renal physiology 318: F600–f616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly K, & Siebenlist U (1995). Immediate-early genes induced by antigen receptor stimulation. Curr Opin Immunol 7: 327–332. [DOI] [PubMed] [Google Scholar]
- Khositseth S, Pisitkun T, Slentz DH, Wang G, Hoffert JD, Knepper MA, et al. (2011). Quantitative protein and mRNA profiling shows selective post-transcriptional control of protein expression by vasopressin in kidney cells. Molecular & cellular proteomics : MCP 10: M110.004036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemke RL, Cai S, Giannini AL, Gallagher PJ, de Lanerolle P, & Cheresh DA (1997). Regulation of cell motility by mitogen-activated protein kinase. J Cell Biol 137: 481–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopatskaya O, Matthews SA, Harper MT, Gilio K, Cosemans JM, Williams CM, et al. (2011). Protein kinase C mediates platelet secretion and thrombus formation through protein kinase D2. Blood 118: 416–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langendorf CG, O’Brien MT, Ngoei KRW, McAloon LM, Dhagat U, Hoque A, et al. (2020). CaMKK2 is inactivated by cAMP-PKA signaling and 14–3-3 adaptor proteins. The Journal of biological chemistry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz RJ, & Shenoy SK (2005). Transduction of receptor signals by beta-arrestins. Science 308: 512–517. [DOI] [PubMed] [Google Scholar]
- Letunic I, & Bork P (2018). 20 years of the SMART protein domain annotation resource. Nucleic acids research 46: D493–d496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JH, Chou CL, Li B, Gavrilova O, Eisner C, Schnermann J, et al. (2009). A selective EP4 PGE2 receptor agonist alleviates disease in a new mouse model of X-linked nephrogenic diabetes insipidus. J Clin Invest 119: 3115–3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limbutara K, Kelleher A, Yang CR, Raghuram V, & Knepper MA (2019). Phosphorylation Changes in Response to Kinase Inhibitor H89 in PKA-Null Cells. Scientific reports 9: 2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning G, Whyte DB, Martinez R, Hunter T, & Sudarsanam S (2002). The protein kinase complement of the human genome. Science 298: 1912–1934. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Kominato K, Koide-Yoshida S, Miyamoto K, Isshiki K, Tsuji A, et al. (2014). PCTAIRE kinase 3/cyclin-dependent kinase 18 is activated through association with cyclin A and/or phosphorylation by protein kinase A. The Journal of biological chemistry 289: 18387–18400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz KS, Deoudes EM, Berginski ME, Jimenez-Ruiz I, Aksoy BA, Hammerbacher J, et al. (2018). Coral: Clear and Customizable Visualization of Human Kinome Data. Cell Syst 7: 347–350.e341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller ML, Jensen LJ, Diella F, Jørgensen C, Tinti M, Li L, et al. (2008). Linear motif atlas for phosphorylation-dependent signaling. Science signaling 1: ra2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer BD, Duhaime M, Shaw C, Denton J, Reynolds D, Karlson KH, et al. (2000). The PDZ-interacting domain of cystic fibrosis transmembrane conductance regulator is required for functional expression in the apical plasma membrane. The Journal of biological chemistry 275: 27069–27074. [DOI] [PubMed] [Google Scholar]
- Nielsen S, & Knepper MA (1993). Vasopressin activates collecting duct urea transporters and water channels by distinct physical processes. The American journal of physiology 265: F204–213. [DOI] [PubMed] [Google Scholar]
- Peters M, Mizuno K, Ris L, Angelo M, Godaux E, & Giese KP (2003). Loss of Ca2+/calmodulin kinase kinase beta affects the formation of some, but not all, types of hippocampus-dependent long-term memory. J Neurosci 23: 9752–9760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering CM, Grady C, Medvar B, Emamian M, Sandoval PC, Zhao Y, et al. (2016). Proteomic profiling of nuclear fractions from native renal inner medullary collecting duct cells. Physiol Genomics 48: 154–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisitkun T, Hoffert JD, Saeed F, & Knepper MA (2012). NHLBI-AbDesigner: an online tool for design of peptide-directed antibodies. Am J Physiol Cell Physiol 302: C154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisitkun T, Jacob V, Schleicher SM, Chou CL, Yu MJ, & Knepper MA (2008). Akt and ERK1/2 pathways are components of the vasopressin signaling network in rat native IMCD. American journal of physiology Renal physiology 295: F1030–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinschen MM, Yu MJ, Wang G, Boja ES, Hoffert JD, Pisitkun T, et al. (2010). Quantitative phosphoproteomic analysis reveals vasopressin V2-receptor-dependent signaling pathways in renal collecting duct cells. Proceedings of the National Academy of Sciences of the United States of America 107: 3882–3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saethang T, Hodge K, Yang CR, Zhao Y, Kimkong I, Knepper MA, et al. (2019). PTM-Logo: a program for generation of sequence logos based on position-specific background amino-acid probabilities. Bioinformatics 35: 5313–5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salhadar K, Matthews A, Raghuram V, Limbutara K, Yang CR, Datta A, et al. (2020). Phosphoproteomic Identification of Vasopressin/cAMP/PKA-Dependent Signaling in Kidney. Molecular pharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval PC, Claxton JS, Lee JW, Saeed F, Hoffert JD, & Knepper MA (2016). Systems-level analysis reveals selective regulation of Aqp2 gene expression by vasopressin. Scientific reports 6: 34863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer WH, Acosta M, Balaspiri L, Judd J, & Manning M (1974). Structural changes in the arginine vasopressin molecule that enhance antidiuretic activity and specificity. Endocrinology 94: 1106–1115. [DOI] [PubMed] [Google Scholar]
- Schenk LK, Bolger SJ, Luginbuhl K, Gonzales PA, Rinschen MM, Yu MJ, et al. (2012). Quantitative proteomics identifies vasopressin-responsive nuclear proteins in collecting duct cells. Journal of the American Society of Nephrology : JASN 23: 1008–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrier RW, Gross P, Gheorghiade M, Berl T, Verbalis JG, Czerwiec FS, et al. (2006). Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med 355: 2099–2112. [DOI] [PubMed] [Google Scholar]
- Sonntag T, Vaughan JM, & Montminy M (2018). 14–3-3 proteins mediate inhibitory effects of cAMP on salt-inducible kinases (SIKs). Febs j 285: 467–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg SF (2008). Structural basis of protein kinase C isoform function. Physiological reviews 88: 1341–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, et al. (2012). Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 367: 2407–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay A, Jaber BL, & Madias NE (2006). Incidence and prevalence of hyponatremia. Am J Med 119: S30–35. [DOI] [PubMed] [Google Scholar]
- Vukićević T, Schulz M, Faust D, & Klussmann E (2016). The Trafficking of the Water Channel Aquaporin-2 in Renal Principal Cells-a Potential Target for Pharmacological Intervention in Cardiovascular Diseases. Front Pharmacol 7: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman GA, Tokumitsu H, & Soderling TR (1997). Inhibitory cross-talk by cAMP kinase on the calmodulin-dependent protein kinase cascade. The Journal of biological chemistry 272: 16073–16076. [DOI] [PubMed] [Google Scholar]
- Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, et al. (2005). Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab 2: 21–33. [DOI] [PubMed] [Google Scholar]
- Yamada T, Terada Y, Homma MK, Nonoguchi H, Sasaki S, Yuasa Y, et al. (1995). AVP inhibits EGF-stimulated MAP kinase cascade in Madin-Darby canine kidney cells. Kidney Int 48: 745–752. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Hempson SJ, Reif GA, Hedge AM, & Wallace DP (2006). Calcium restores a normal proliferation phenotype in human polycystic kidney disease epithelial cells. Journal of the American Society of Nephrology : JASN 17: 178–187. [DOI] [PubMed] [Google Scholar]
- Yang CR, Raghuram V, Emamian M, Sandoval PC, & Knepper MA (2015). Deep proteomic profiling of vasopressin-sensitive collecting duct cells. II. Bioinformatic analysis of vasopressin signaling. Am J Physiol Cell Physiol 309: C799–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CR, Tongyoo P, Emamian M, Sandoval PC, Raghuram V, & Knepper MA (2015). Deep proteomic profiling of vasopressin-sensitive collecting duct cells. I. Virtual Western blots and molecular weight distributions. Am J Physiol Cell Physiol 309: C785–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, Kawakami Y, & Kawakami T (1994). The pleckstrin homology domain of Bruton tyrosine kinase interacts with protein kinase C. Proceedings of the National Academy of Sciences of the United States of America 91: 9175–9179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu MJ, Miller RL, Uawithya P, Rinschen MM, Khositseth S, Braucht DW, et al. (2009). Systems-level analysis of cell-specific AQP2 gene expression in renal collecting duct. Proceedings of the National Academy of Sciences of the United States of America 106: 2441–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw files (.raw) can be accessed at www.ebi.ac.uk/pride/archive/ with the dataset identifier PXD015719. The curated data are available at https://hpcwebapps.cit.nih.gov/ESBL/Database/mpkCCD-AVP/.


