Abstract
Major depressive disorder is a common and serious mood illness. The molecular mechanisms underlying the pathogenesis and symptomatology of depression are poorly understood at present. Multiple neurotransmitter systems are believed to be implicated in depression. Increasing evidence supports glutamatergic transmission as a critical element in depression and antidepressant activity. In this study, we investigated adaptive changes in expression of AMPA receptors in a key limbic reward structure, the striatum, in response to an anhedonic model of depression. Prolonged social isolation in adult rats caused anhedonic/depression- and anxiety-like behavior. In these depressed rats, surface levels of AMPA receptors, mainly GluA1 and GluA3 subunits, were reduced in the nucleus accumbens (NAc). Surface GluA1/A3 expression was also reduced in the caudate putamen (CPu) following chronic social isolation. No change was observed in expression of presynaptic synaptophysin, postsynaptic density-95, and dendritic microtubule-associated protein 2 in the striatum. Noticeably, chronic treatment with the metabotropic glutamate (mGlu) receptor 5 antagonist MTEP reversed the reduction of AMPA receptors in the NAc and CPu. MTEP also prevented depression- and anxiety-like behavior induced by social isolation. These data indicate that adulthood prolonged social isolation induces the adaptive downregulation of GluA1/A3-containing AMPA receptor expression in the limbic striatum. mGlu5 receptor activity is linked to this downregulation, and antagonism of mGlu5 receptors produces an antidepressant effect in this anhedonic model of depression.
Abbreviations: AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid;; ANOVA, analysis of variance; CDH2, Cadherin-2; CPu, caudate putamen; MAP-2, microtubule-associated protein 2; mGlu, metabotropic glutamate; MTEP, 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]-pyridine; NAc, nucleus accumbens; NCAD, neural cadherin; PFC, prefrontal cortex; PSD-95, postsynaptic density-95
Keywords: Caudate putamen, Nucleus accumbens, GluA1, Metabotropic glutamate receptor, MTEP, Antidepressant, Social isolation
Highlights
-
•
Social isolation (SI) induced depression-like behavior and reduced surface expression of AMPA receptors in the striatum.
-
•
Blockade of metabotropic glutamate (mGlu) receptor 5 reversed the reduction of AMPA receptor expression.
-
•
An mGlu5 antagonist also reversed the SI-induced a set of depressive behaviors.
-
•
Thus, SI downregulates AMPA receptor levels and induces depressive behavior via a mechanism involving mGlu5.
1. Introduction
Glutamate is a neurotransmitter in the central nervous system and interacts with ionotropic and metabotropic glutamate receptors to achieve its action. The α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor is one of the ionotropic glutamate receptors and serves as a ligand-gated ion channel processing the fast excitatory synaptic transmission. AMPA receptors usually become functional upon homo- or heterotetrameric assembly of four subunits, GluA1–4 (formerly known as GluR1–4) (Greger et al., 2017). As a glutamate receptor enriched in broad regions of the brain, the AMPA receptor is critical for the regulation of normal neural and synaptic activities and is linked to the pathogenesis of a variety of neuropsychiatric and neurological disorders (Traynelis et al., 2010).
Major depressive disorder is a common mental illness. While the specific neurological mechanisms for this illness are far from clear, many neurotransmitters play a role. In addition to the relatively well characterized monoaminergic system, glutamate homeostasis is implicated in depression (Paul and Skolnick, 2003, Bleakman et al., 2007, Tokita et al., 2012; Vose and Stanton, 2017). In particular, the AMPA receptor is an important molecular target based on accumulating evidence. For instance, in response to repeated restraint or unpredictable stress in rats, GluA1/A2 surface expression was reduced in pyramidal neurons of the prefrontal cortex (PFC), an adaptive change critical for depression-like behavior (Yuen et al., 2012). Chronic mild stress also reduced GluA1 expression in the PFC and hippocampus of adult rats, although GluA1 expression was increased in the anterior part of the nucleus accumbens (NAc) of adult but not young rats (Toth et al., 2008). Chronic antidepressant treatment elevated GluA1 and GluA2/3 expression in the hippocampus and striatum (Martinez-Turrillas et al., 2005, Tan et al., 2006). AMPA receptor potentiators generally exhibited antidepressant-like action in various animal models of depression and in patients (Bleakman et al., 2007, Nations et al., 2012, Tokita et al., 2012, Jaso et al., 2017). In addition, AMPA receptors are positively regulated by phosphorylating GluA1 at S831 and S845. Mice bearing point mutations at both sites showed an increase in immobility (Svenningsson et al., 2007). Similarly, mice lacking the GluR-A (GluA1) subunit represented a model of depression with good face and construct validity (Chourbaji et al., 2008).
Prolonged period (10–12 weeks) of social isolation in adult rats is an animal paradigm modeling depression in adulthood (Wallace et al., 2009). Rats subjected to this chronic passive stress exhibited anhedonia-like symptoms, i.e., decreases in natural reward-related behaviors (such as deficits in sucrose preference and sexual reward) (Wallace et al., 2009, Mao and Wang, 2018). Various brain areas are implicated in this depressive disorder. Central among them is the limbic reward system. The striatum containing two subdivisions, the NAc and the caudate putamen (CPu), is a key structure within the limbic reward circuit. A number of studies have demonstrated the role of the NAc in anhedonic behavior and antidepressant-like activity (Zacharko and Anisman, 1991, Nestler and Carlezon, 2006). Of note, AMPA receptors are abundant in the striatum (Bernard et al., 1997, Kondo et al., 2000, Reimers et al., 2011), although to date, little is known about the responsivity of striatal AMPA receptors to chronic adulthood social isolation.
This study explored possible adaptive changes in AMPA receptor expression in the striatum following social isolation in adult rats. As a unique chronic stress, prolonged social isolation is particularly useful for monitoring the long-lasting neuroadaptation of a given receptor in relation to enduring anhedonic behavior (Krishnan and Nestler, 2011). In addition, metabotropic glutamate (mGlu) receptor 5 antagonists produced the antidepressant-like effect in several models of depression (Tatarczynska et al., 2001, Pilc et al., 2002, Palucha et al., 2005, Li et al., 2006, Belozertseva et al., 2007). We thus investigated whether an mGlu5 antagonist produces similar behavioral effects in our model of depression and whether it has any impact on responses of striatal AMPA receptors to social isolation.
2. Methods
2.1. Animals and prolonged social isolation at adulthood
In this study, we used Wistar male rats. Rats were 7–8 weeks of age (200–225 g) at arrival and acclimated to the animal facility for 5 days prior to the experiments. Animals were housed in standard rat cages (46 × 24 × 20 cm) with corncob bedding and at 23 ºC and humidity of 50 ± 10 % with a 12-h/12-h light/dark cycle. Water and food were available ad libitum. Rats were kept in accordance with the US National Institutes of Health Guide for the Care and Use of Laboratory Animals. Rat care and use were approved by the Institutional Animal Care and Use Committee. The Animal Research: Reporting In Vivo Experiments (ARRIVE) guidelines have been followed. This study was not pre-registered.
Adulthood social isolation was carried out as described previously (Wallace et al., 2009, Mao and Wang, 2018). Briefly, we randomly divided rats into two groups: socially isolated rats and control rats. Socially isolated rats were housed in home cages individually (one per cage) for 10–12 weeks. Control rats were housed two animals per cage for the same period of time. After 10–12 weeks of social isolation, behavioral assessments were performed. Rats were anesthetized next day by an intraperitoneal injection of sodium pentobarbital (55–60 mg/kg) and sacrificed. Brains were dissected for following neurochemical assays. Of note, pentobarbital induced rapid anesthesia and had no effect on surface GluA1–3 expression in the mouse cortex at 5 min after injection (Carino et al., 2012).
We employed a computer-generated randomization table (GraphPad software/QuickCalcs, La Jolla, CA) to randomly divide animals into different groups. Sample size was calculated by alpha = 0.05 and beta = 0.2 (80 % power) with no difference in sample size between the beginning and end of the experiments. The health state of animals was used as the criteria for inclusion/exclusion. The healthy animals showed no signs of illness as evaluated by body weight and visual observations.
2.2. Drug administration
Vehicle or the mGlu5 selective antagonist 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]-pyridine (MTEP) was administered intraperitoneally (i.p.) at a dose of 1 mg/kg once daily for 14 consecutive days (isolation for 8–10 weeks with 2 additional weeks of isolation with MTEP treatment). Selection of the MTEP dose was based on the finding that chronic administration of MTEP (1 mg/kg, i.p., once daily for 14 days) attenuated the hyperactivity in a rat olfactory bulbectomy model of depression, while MTEP itself did not cause any behavioral changes in the sham-operated rats (Palucha et al., 2005). Behavioral assessments were performed 24 h after the last dose of a drug.
2.3. Sucrose preference test
Sucrose preference was tested as an operational index of anhedonic behavior. To this end, a two-bottle-choice paradigm was performed as described previously (Wallace et al., 2009, Mao and Wang, 2018). Briefly, rats were initially habituated to two bottles of water for five days. Unlimited access to two bottles containing tap water or 1 % (w/v) sucrose was then given to rats for a period of 24 h. The consumed amounts of water and sucrose solutions were measured. We calculated preference for sucrose as the percentage of the volume of sucrose consumed (ml per 24 h) divided by the total fluid (sucrose + water) intake (ml per 24 h).
2.4. Elevated plus maze
We performed the elevated plus maze test to assess anxiety-like behavior (Walf and Frye, 2007). Four arms (two enclosed and two open arms) were arranged in a plus shape (61 cm from the floor). We placed rats onto the central area of the plus maze with rats facing an open arm. Behavior activity was recorded and scored in a blind manner. Anxiety-like behavior as assessed by the elevated plus maze was analyzed as (1) the time spent in the open arms (s), and (2) the ratio of open arm entries to the total number of entries (expressed as a percentage) during a 5-min freely exploring period.
2.5. Surface protein biotinylation
Surface protein biotinylation was performed on CPu and NAc slices as described previously (Dennis et al., 2011, Jin et al., 2017) with minor modifications. Briefly, rats were anesthetized and decapitated. Brains were removed and cut into slices (300 µm) using a vibratome (VT1200S). The CPu and NAc were dissected using a biopsy punch (1.5 mm, ThermoFisher, Waltham, MA) and incubated in ice-cold artificial cerebrospinal fluid containing (in mM) 10 glucose, 124 NaCl, 3 KCl, 1.25 KH2PO4, 26 NaHCO3, 2 MgSO4, 2 CaCl2, bubbled with 95% O2-5% CO2, pH 7.4, and 1 mg/ml EZ-LINK-Sulfo-NHS-SS-Biotin (ThermoFisher) for 45 min. Slices were washed and quenched by glycine (100 mM). Slices were then homogenized by sonication in ice-cold lysis buffer containing 20 mM HEPES, pH 7.4, 150 mM NaCl, 2 mM EDTA, 1 % Triton X-100, 0.5 % sodium deoxycholate, 0.1 % SDS, and a protease/phosphatase inhibitor cocktail (ThermoFisher). Homogenized samples were centrifuged at 800 g (10 min, 4 °C). The supernatant was collected as the total protein fraction. An aliquot of total proteins was further incubated with neutrAvidin resin (ThermoFisher) overnight. Precipitation of biotinylated proteins (i.e., surface proteins) was achieved by centrifugation. Surface proteins were then eluted with a lithium dodecyl sulfate sample buffer. Proteins of interest in surface and total fractions were analyzed by immunoblot.
2.6. Western blot analysis
This was performed as described previously (Jin et al., 2013). Protein samples were separated on 4–12% Tris-glycine gels (Invitrogen, Carlsbad, CA). Separated proteins were transferred to polyvinylidene fluoride membranes. A primary antibody was used in incubation of membranes overnight at 4 °C. A goat anti-mouse or anti-rabbit secondary antibody was then used. An enhanced chemiluminescence reagent (GE Healthcare Life Sciences, Piscataway, NJ) was used to visualize immunoblots. The density of immunoblots was measured using the NIH ImageJ (RRID:SCR_003073; Bethesda, MD). Values of total and surface AMPA receptor levels were normalized to total protein load as determined by β-actin blot (Clem et al., 2010).
2.7. Antibodies and pharmacological drugs
Rabbit primary antibodies used in this study include those against GluA1 (RRID:AB_1977216; Millipore, Bedford, MA; 1:2000), synaptophysin (RRID:AB_2286949; Abcam, Cambridge, MA; 1:2000), microtubule-associated protein 2 (MAP-2, RRID:AB_91939; Millipore; 1:2000), and β-actin (RRID:AB_476693; Sigma-Aldrich, St. Louis, MO; 1:2500). Mouse antibodies used include those against GluA2 (RRID:AB_2113875; Millipore; 1:1500), GluA3 (RRID:AB_2113897; Millipore; 1:2000), postsynaptic density-95 (PSD-95, RRID:AB_325399; ThermoFisher; 1:4000), and N-cadherin (RRID:AB_10610922; Santa Cruz Biotechnology, Dallas, TX: 1:2000). Data for the validation of these antibodies are available from the companies. MTEP was purchased from Tocris (Minneapolis, MN) and was suspended in 10% Tween 80 in sterile water.
2.8. Statistics
To statistically analyze our data, we used GraphPad Prism 6 (RRID:SCR_002798; GraphPad software, La Jolla, CA) with no blinding. The normality of data was tested. No test for outliers on the data was performed, and no animals were excluded from the study. We used either two-way analysis of variance (ANOVA) followed by a post hoc test for comparing multiple groups or two-tailed unpaired Student's t-test for comparing two groups. A level of P < 0.05 was defined as a statistically significant level.
3. Results
3.1. Effects of social isolation on expression of total AMPA receptors
We first explored the effect of social isolation on total AMPA receptor expression in the striatum. To this end, rats were sacrificed after 10–12 weeks of social isolation. Changes in cellular levels of total AMPA receptor subunit proteins were examined in the NAc and CPu using immunoblot. We focused on three subunits (GluA1/A2/A3) since they are the principal subunits in the striatum (Boudreau et al., 2007). In the NAc, a significant decrease in total GluA1 levels was seen in socially isolated rats as compared to double-housed control rats (Fig. 1A). Levels of total GluA2 proteins were not significantly altered. Total GluA3 levels were reduced following social isolation. Likewise, in the CPu, expression of the two subunits (GluA1 and GluA3) but not GluA2 was reduced in this region of socially isolated rats (Fig. 1B). These results demonstrate that prolonged social isolation in adulthood downregulates cellular expression of total GluA1 and GluA3 subunits in the striatum. Of note, body weight was not significantly altered in isolated rats as compared to control rats (control rats: 504.83 ± 7.90 g versus isolated rats: 478.01 ± 10.30 g; P > 0.05).
Fig. 1.
Effects of chronic social isolation on expression of total AMPA receptors in the rat striatum. (A) Effects of social isolation on total AMPA receptor expression in the NAc. (B) Effects of social isolation on total AMPA receptor expression in the CPu. Note that the amounts of total cellular GluA1 and GluA3 proteins in the NAc (A) and CPu (B) were reduced in socially isolated (SI) rats compared to control (Con) rats as detected by immunoblots (IB). Representative immunoblots are shown to the left of the quantified data. Data were statistically analyzed using Student’s t-test (n = 6 per group) with ‘n′ equal to the number of animals. * P < 0.05 versus double-housed control animals. GluA1-NAc [t(10) = 3.435, P = 0.006], GluA2-NAc [t(10) = 1.151, P = 0.277], GluA3-NAc [t(10) = 2.879, P = 0.016], GluA1-CPu [t(10) = 2.829, P = 0.017], GluA2-CPu [t(10) = 0.399, P = 0.699], GluA3-CPu [t(10) = 4.524, P = 0.001].
3.2. Effects of social isolation on surface expression of AMPA receptors
We next examined the effect of social isolation on surface expression of AMPA receptors in the striatum. The NAc and CPu slices were cut from socially isolated rats and control rats. The abundance of GluA1, GluA2, and GluA3 subunits in a specific subcellular compartment, i.e., the surface membrane fraction, was assayed by surface biotinylation. We found that surface GluA1 and GluA3 levels in the NAc were markedly reduced in socially isolated rats as compared to control rats (Fig. 2A). A tendency of a decrease in surface GluA2 levels was also seen, although it did not reach a statistically significant level. Similar results were observed in the CPu. GluA1 and GluA3 but not GluA2 exhibited a lower level of surface expression in socially isolated rats than that of their control rats (Fig. 2B). These results indicate that striatal surface expression of GluA1 and GluA3 subunits is subjected to the downregulation in response to chronic social isolation.
Fig. 2.
Effects of chronic social isolation on surface expression of AMPA receptors in the rat striatum. (A) Effects of social isolation on surface expression of AMPA receptors in the NAc. (B) Effects of social isolation on surface expression of AMPA receptors in the CPu. Note that chronic social isolation caused a decrease in the amounts of GluA1 and GluA3 proteins in the surface fraction isolated from the NAc (A) and CPu (B) of socially isolated (SI) rats compared to control (Con) rats as detected by immunoblots (IB). Representative immunoblots are shown to the left of the quantified data. Data were statistically analyzed using Student’s t-test (n = 6 per group) with ‘n′ equal to the number of animals. * P < 0.05 versus double-housed control animals. GluA1-NAc [t(10) = 4.846, P = 0.001], GluA2-NAc [t(10) = 2.148, P = 0.057], GluA3-NAc [t(10) = 3.124, P = 0.009], GluA1-CPu [t(10) = 3.595, P = 0.005], GluA2-CPu [t(10) = 1.465, P = 0.174], GluA3-CPu [t(10) = 2.929, P = 0.015].
N-cadherin, also known as Cadherin-2 (CDH2) or neural cadherin (NCAD), is a classical cell adhesion protein with its extracellular domains carrying out cell-cell adhesion. We used this protein as a surface protein marker for the surface biotinylated fraction, whereas an intracellular protein marker, β-actin, was used as a negative control for the biotinylated fraction. No significant change in total N-cadherin levels was found in the NAc between socially isolated and control rats (Fig. 3A). Similarly, surface expression of NAc N-cadherin showed a minimal change. Total β-actin levels remained unchanged following social isolation, while β-actin was not detected in the surface biotinylated fraction. Similar results were observed in the CPu (Fig. 3B). These data support the selectivity and efficiency of biotinylation assays in analyzing the distinct pool of surface proteins.
Fig. 3.
Effects of chronic social isolation on N-cadherin expression in the rat striatum. (A) Effects of social isolation on total and surface expression of N-cadherin in the NAc. (B) Effects of social isolation on total and surface expression of N-cadherin in the CPu. Total and surface protein fractions were prepared from the NAc (A) and CPu (B) of socially isolated (SI) rats and control (Con) rats and were analyzed by immunoblots (IB). Representative immunoblots are shown to the left of the quantified data. Data were statistically analyzed using Student’s t-test (n = 6 per group) with ‘n′ equal to the number of animals.
3.3. Effects of social isolation on expression of synaptic proteins
To determine effects of social isolation on expression of major synaptic proteins, we monitored changes in expression levels of a representative presynaptic vesicle membrane protein (synaptophysin), a postsynaptic protein (PSD-95), and a dendritic protein (MAP-2) in striatal lysates. In the NAc, synaptophysin showed little change in its abundance between the two groups (socially isolated rats versus control rats, Fig. 4A). Expression of PSD-95 and MAP-2 also remained stable after social isolation. In the CPu, all three markers were not altered in their levels in socially isolated rats relative to control rats (Fig. 4B). Thus, there is no general loss of synaptic and dendritic components in the striatum after social isolation as evidenced by the stable expression of synaptic and dendritic marker proteins in response to social isolation.
Fig. 4.
Effects of chronic social isolation on expression of synaptic and dendritic proteins in the rat striatum. (A) Effects of social isolation on expression of synaptophysin, PSD-95, and MAP-2 in the NAc. (B) Effects of social isolation on expression of synaptophysin, PSD-95, and MAP-2 in the CPu. Protein samples were prepared from the NAc (A) and CPu (B) of socially isolated (SI) rats and control (Con) rats and were analyzed by immunoblots (IB). Representative immunoblots are shown to the left of the quantified data. Data were statistically analyzed using Student’s t-test (n = 5–6 per group) with ‘n′ equal to the number of animals.
3.4. Effects of mGlu5 antagonism on the social isolation-induced loss of striatal AMPA receptors
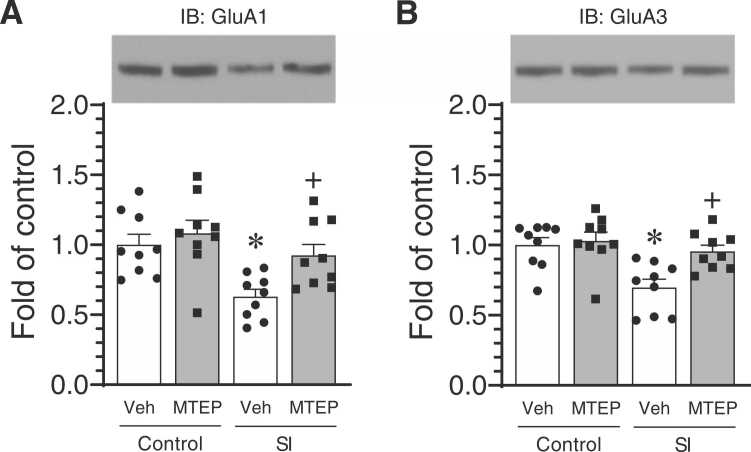
Pharmacological antagonism of mGlu5 receptors by an acute or chronic systemic injection of an mGlu5 antagonist consistently produced an antidepressant effect (Tatarczynska et al., 2001, Pilc et al., 2002, Palucha et al., 2005, Li et al., 2006, Belozertseva et al., 2007). To determine the impact of an mGlu5 antagonist on the loss of AMPA receptors in the striatum in response to chronic social isolation in adult rats, we examined changes in surface expression of striatal AMPA receptors in isolated rats in the presence of an mGlu5 antagonist. In detail, the selective mGlu5 antagonist MTEP was administered to socially isolated and control rats (1 mg/kg, i.p., once daily for the last 14 days of isolation). Rats were then sacrificed for testing changes in surface expression of GluA1 and GluA3 subunits in striatal slices. Chronic MTEP treatment had an insignificant effect on basal surface expression of GluA1 and GluA3 in the striatum of control rats (Figs. 5A and 5B). Significant decreases in surface levels of GluA1 and GluA3 were seen in socially isolated rats as compared to control rats. Remarkably, the decreases in surface levels of these subunits in isolated rats were reversed by MTEP, indicating that chronic administration of the mGlu5 antagonist can reverse the loss of GluA1 and GluA3 subunits in striatal neurons induced by prolonged social isolation.
Fig. 5.
Effects of MTEP on the downregulated surface expression of AMPA receptors in the striatum of socially isolated rats. (A and B) Effects of MTEP on the loss of surface GluA1 (A) and GluA3 (B) subunits in the striatum following social isolation. Chronic i.p. treatment with vehicle (Veh) or MTEP (1 mg/kg, once daily for last 14 days during 10–12 weeks of social isolation) was given to socially isolated (SI) rats and control rats. Surface GluA1 and GluA3 proteins were isolated by biotinylation and were analyzed by immunoblots (IB). Data were analyzed by two-way ANOVA followed by a post hoc test (n = 9 per group): GluA1: vehicle versus MTEP, F(1,32) = 6.073, P = 0.019, control versus SI, F(1,32) = 11.97, P < 0.001, and interaction, F(1,32) = 1.917, P = 0.175; GluA3: vehicle versus MTEP, F(1,32) = 6.906, P = 0.013, control versus SI, F(1,32) = 11.90, P < 0.001, and interaction, F(1,32) = 4.296, P = 0.046. * P < 0.05 versus vehicle in double-housed control animals. + P < 0.05 versus vehicle in socially isolated animals.
3.5. Effects of MTEP on depression- and anxiety-like behavior
After prolonged adulthood social isolation, rats showed depression-like behavior, e.g., a decrease in sucrose intake (Wallace et al., 2009, Mao and Wang, 2018). As a core symptom of anhedonic depression that can be objectively measured in rodents, we monitored changes in the sucrose intake behavior in isolated versus control rats. As expected, after chronic social isolation, rats exhibited a significant decrease in sucrose intake relative to control rats (Fig. 6A). The value of sucrose preference was also lower in socially isolated rats than that in control rats (Fig. 6B). Noticeably, in socially isolated rats chronically treated with MTEP, the decreases in sucrose intake and preference were reversed. No significant differences in both parameters were observed between socially isolated and control rats in the presence of MTEP. These behavioral results provide evidence supporting a critical role played by mGlu5 receptors in the anhedonic behavior induced by prolonged adulthood social isolation.
Fig. 6.
Effects of MTEP on depression- and anxiety-like behavior induced by social isolation. (A) Effects of MTEP on the social isolation-induced decrease in sucrose intake. (B) Effects of MTEP on the social isolation-induced decrease in sucrose preference. (C) Effects of MTEP on the social isolation-induced decrease in time spent in open arms. (D) Effects of MTEP on the social isolation-induced decrease in open arm entries. Note that social isolation did not induce a significant decrease in sucrose intake (A), sucrose preference (B), time spent in open arms (C), and open arm entries (D) in the rats treated with MTEP as compared to the rats treated with vehicle. After behavioral experiments, the same rats were used for surface receptor expression assays. Data were analyzed by two-way ANOVA followed by a post hoc test (n = 9 per group). * P < 0.05 versus vehicle in double-housed control animals. + P < 0.05 versus vehicle in socially isolated (SI) animals.
Anxiety usually occurs together with depression as a characteristic of depression. Indeed, anxiety-like behavior was also seen in socially isolated rats (Wallace et al., 2009, Mao and Wang, 2018). In this study, we examined the effect of MTEP on anxiety-like behavior in socially isolated rats. Using an elevated plus maze test, we found that adulthood social isolation caused significant decreases in the time spent in open arm exploration (Fig. 6C) and the number of open arm entries (Fig. 6D). In the rats treated with MTEP, the decreases in the two parameters were reversed. Thus, the mGlu5 antagonist possesses the ability to reverse anxiety-like behavior in parallel with the reduction of depression-like behavior.
4. Discussion
Expression of AMPA receptors was altered in response to depression (Freudenberg et al., 2015). At the mRNA level, GluA1 and GluA3 mRNAs were reduced in the perirhinal cortex and hippocampus of depressed patients (Beneyto et al., 2007, Duric et al., 2013). Similar results were observed in the rat hippocampus after chronic unpredictable stress (Duric et al., 2013). At the protein level, repeated restraint or unpredictable stress reduced total and surface GluA1 and GluA2 levels in the PFC (Yuen et al., 2012). Similarly, chronic mild stress reduced GluA1 expression in the PFC and hippocampus of adult rats (Toth et al., 2008). Postnatal or postweaning social isolation induced a decline of total or surface GluA1 or GluA2 expression in the rat PFC or hippocampus (Hermes et al., 2011, Hsiao et al., 2011, Sestito et al., 2011). Neonatal social isolation prevented experience-dependent synaptic trafficking of AMPA receptors in the rat barrel cortex (Miyazaki et al., 2012, Miyazaki et al., 2013) and medial PFC (Tada et al., 2016). It appears that expression of AMPA receptors in the PFC and hippocampus is generally downregulated in depressed animals, although chronic social isolation for 4 weeks upregulated GluA1 and GluA2 protein levels in the mouse amygdala (Shimizu et al., 2016) and chronic restraint stress elevated surface GluA1 but not GluA2 expression in the mouse basolateral amygdala (Zhou et al., 2019).
In the striatum, postnatal social isolation for 30 days caused no change in the number of GluA1 immunostaining neurons in the rat NAc (Wood et al., 2005). Sprague-Dawley rats subjected to a set of several different and unpredictable chronic mild stressors showed an increase in GluA1 expression in the anterior but not the posterior NAc of adult but not young rats as detected by immunohistochemistry, while these stressors decreased GluA1 expression in the PFC (Toth et al., 2008). Additionally, social defeat stress caused different changes in GluA1 and/or GluA2 expression in the NAc of susceptible mice (Vialou et al., 2010, Yang et al., 2016; Jiang et al., 2022). In this study, we found that total and surface GluA1/3 expression in the CPu and NAc was downregulated in adult Wistar rats after prolonged social isolation. It appears that AMPA receptor expression in the striatum could be differentially regulated in response to depression-inducing stressors, depending on the AMPA receptor subunits, striatal subdivisions, animal strains, and stressor types and paradigms utilized.
The sensitivity of AMPA receptors to a variety of stressors that induce depression indicates their roles in the pathogenesis and/or symptomatology of depression. One theory is that exposure to stress causes the long-lasting adaptive downregulation of AMPA receptors in their expression and function in the brain regions critical for depression, which then leads to the development of depression. Several lines of evidence support this notion. First, GluA1-/- mice displayed behavioral and neurochemical features of depression (Chourbaji et al., 2008). In addition, AMPA receptor activity is positively regulated by the phosphorylation of GluA1 at S831 and S845 sites. Point mutations of GluA1 to prevent its phosphorylation at S831 and S845 induced depression-like behavior (Svenningsson et al., 2007). Second, AMPA receptor potentiators are of an antidepressant value in most models of depression in experimental animals and patients (Bleakman et al., 2007, Nations et al., 2012, Tokita et al., 2012, Jaso et al., 2017). Finally, restoration of GluA1 and GluN1 (an NMDA receptor subunit) expression in the PFC prevented cognitive impairment in repeatedly stressed rats (Yuen et al., 2012).
Several standard antidepressant medications elevate AMPA receptor phosphorylation. For instance, the selective serotonin reuptake inhibitor fluoxetine (Prozac) increased GluA1 S845 phosphorylation in the PFC, hippocampus, and striatum of normal mice (Svenningsson et al., 2002). The classical tricyclic antidepressant imipramine increased mouse hippocampal S845 phosphorylation, while total GluA1 levels remained unchanged (Du et al., 2007). Another antidepressant tianeptine enhanced S831 phosphorylation in the mouse frontal cortex and hippocampus, elevated S845 phosphorylation in the hippocampus, and produced the antidepressant effect (Svenningsson et al., 2007). In addition to phosphorylation, chronic treatment with the antidepressant paroxetine elevated GluA1 and GluA2/3 protein expression in the hippocampus of normal rats (Martinez-Turrillas et al., 2005). Chronic administration of the antidepressant maprotiline also increased GluA1 and GluA2/3 expression in the normal mouse hippocampus and striatum (Tan et al., 2006). Since maprotiline caused no change or decrease in GluA2 expression (Tan et al., 2006), the antidepressant likely induced an increase in GluA2-lacking, Ca2+-permeable AMPA receptors in limbic/striatal brain regions, leading to a higher level of synaptic activity and plasticity in these circuits. In depressed rats, fluoxetine reversed the reduction of GluA1 mRNA expression in the hippocampus (Duric et al., 2013). Of note, ketamine after a single intravenous infusion at a subanesthetic dose produced a robust, rapid, and sustained antidepressant effect in patients with treatment-resistant depression. Accumulating evidence suggests that facilitation of the AMPA receptor-mediated synaptic transmission contributes to the ketamine effect (reviewed in Dutta et al., 2015; Aleksandrova et al., 2017).
In addition to AMPA receptors, mGlu5 receptors play a role in depression (Pilc et al., 2008). The mGlu5 receptor is a Gαq-coupled receptor (Niswender and Conn, 2010) and is enriched in striatal neurons (Testa et al., 1994, Tallaksen-Greene et al., 1998). Pharmacological studies revealed the antidepressant effect of mGlu5 antagonists and negative allosteric modulators (Tatarczynska et al., 2001, Pilc et al., 2002, Wieronska et al., 2002, Palucha et al., 2005, Li et al., 2006, Molina-Hernandez et al., 2006, Belozertseva et al., 2007, Pomierny-Chamiolo et al., 2010, Liu et al., 2012, Kato et al., 2015). mGlu5 knockout mice also exhibited antidepressant features (Li et al., 2006). How mGlu5 receptors play a role in chronic stress-induced depression remains unclear. Recently, we found an increase in mGlu5 receptor expression and signaling in the striatum of adult rats subjected to chronic social isolation (Mao and Wang, 2018). In the present study, we further found that the mGlu5 antagonist MTEP reversed the loss of surface AMPA receptors in striatal neurons and antagonized anhedonic/depression- and anxiety-like behavior. Thus, the adaptive upregulation of mGlu5 receptors may contribute to the remodeling of AMPA receptor-involved excitatory synaptic transmission in the limbic striatum, leading to anhedonic behavior. In detail, the enhanced mGlu5 activity may trigger a downstream event, i.e., the weakening of AMPA receptors, to link chronic stress to depression. In fact, stimulation of group I mGlu receptors is known to reduce surface and synaptic expression of AMPA receptors and blunt the AMPA receptor-mediated glutamatergic transmission in striatal and hippocampal neurons (Snyder et al., 2001, Xiao et al., 2001, Zho et al., 2002, Mangiavacchi and Wolf, 2004). Future studies will need to elucidate the causal linkage between upregulated mGlu5 receptors and downregulated AMPA receptors in striatal neurons in response to chronic adulthood social isolation. Of note, the AMPA receptor antagonist NBXQ did not affect the MTEP antidepressant effect as evaluated using the forced swim test in mice (Pomierny-Chamiolo et al., 2010). Thus, accurate roles of the mGlu5-AMPA receptor interaction may differ, depending on the type of stress, the stage and symptom of depression, the neural circuits involved, and the antidepressant treatment regimen.
Within the striatum, PSD-95 transcripts remained stable in depressed subjects (Kristiansen and Meador-Woodruff, 2005). Similarly, sub-chronic variable stress had no effect on PSD-95 puncta density in the NAc of male and female mice, corresponding to little changes in spine density and morphology in this region (Brancato et al., 2017). Acute but not chronic administration of an antidepressant (citalopram) enhanced PSD-95 mRNA expression in the rat CPu and NAc (Tomasetti et al., 2011). However, chronic social defeat stress also enhanced PSD-95 protein levels in the NAc of susceptible mice (Yang et al., 2016, Jiang et al., 2022). Chronic mild stress increased dendritic branching in the rat NAc and spine density in the NAc core but not shell, although it caused no changes in the relative abundance of mature and immature spines in the NAc (Bessa et al., 2013). In the present study, we found insignificant changes in striatal PSD-95 levels in isolated rats. This indicates that PSD-95 expression might not be altered in the striatum in this particular model of depression. However, it could not be ruled out that circuit- or even cell type (D1 versus D2 receptor-bearing projection neurons)-specific changes in expression of postsynaptic proteins such as PSD-95 may occur. Future studies will elucidate the circuit- or cell type-specific changes in striatal pre- and postsynaptic proteins in response to chronic social isolation.
CRediT authorship contribution statement
Limin Mao: Conceptualization, Data curation, Investigation, Methodology, Validation, Roles/Writing – original draft; Nirav Mathur: Formal analysis, Validation, Writing – review & editing; John Wang: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Supervision, Validation, Writing – review & editing.
Conflict of interest
The authors declare that there are no potential conflicts of interest.
Acknowledgments
This work was supported by the National Institutes of Health (NIH, USA) via a grant R01MH61469 (JQW). The authors wish to thank Dr. Daozhong Jin for his technical assistance.
References
- Aleksandrova L.R., Phillips A.G., Wang Y.T. Antidepressant effects of ketamine and roles of AMPA glutamate receptors and other mechanisms beyond NMDA receptor antagonism. J. Psychiatry Neurosci. 2017;42:222–229. doi: 10.1503/jpn.160175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belozertseva I.V., Kos T., Popik P., Danysz W., Bespalov A.Y. Antidepressant-like effects of mGluR1 and mGluR5 antagonists in the rat forced swim and the mouse tail suspension tests. Eur. Neuropsychopharmacol. 2007;17:172–179. doi: 10.1016/j.euroneuro.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Beneyto M., Kristiansen L.V., Oni-Orisan A., McCullumsmith R.E., Meador-Woodruff J.H. Abnormal glutamate receptor expression in the medial temporal lobe in schizophrenia and mood disorders. Neuropsychopharmacology. 2007;32:1888–1902. doi: 10.1038/sj.npp.1301312. [DOI] [PubMed] [Google Scholar]
- Bernard V., Somogyi P., Bolam J.P. Cellular, subcellular, and subsynaptic distribution of AMPA-type glutamate receptor subunits in the neostriatum of the rats. J. Neurosci. 1997;17:819–833. doi: 10.1523/JNEUROSCI.17-02-00819.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessa J.M., Morais M., Marques F., Pinto L., Palha J.A., Almeida O.F.X., Sousa N. Stress-induced anhedonia is associated with hypertrophy of medium spiny neurons of the nucleus accumbens. Transl. Psychiatry. 2013;3 doi: 10.1038/tp.2013.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleakman D., Alt A., Witkin J.M. AMPA receptors in the therapeutic management of depression. CNS Neurol. Discord. Drug Targets. 2007;6:117–126. doi: 10.2174/187152707780363258. [DOI] [PubMed] [Google Scholar]
- Boudreau A.C., Reimers J.M., Milovanovic M., Wolf M.E. Cell surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize after cocaine challenge in association with altered activation of mitogen-activated protein kinases. J. Neurosci. 2007;27 doi: 10.1523/JNEUROSCI.2163-07.2007. (1062–1035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancato A., Bregman D., Ahn H.F., Pfau M.L., Menard C., Cannizzaro C., Russo S.J., Hodes G.E. Sub-chronic variable stress induces sex-specific effects on glutamatergic synapses in the nucleus accumbens. Neuroscience. 2017;350:180–189. doi: 10.1016/j.neuroscience.2017.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carino C., Fibuch E.E., Mao L.M., Wang J.Q. Dynamic loss of surface-expressed AMPA receptors in mouse cortical and striatal neurons during anesthesia. J. Neurosci. Res. 2012;90:315–323. doi: 10.1002/jnr.22749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chourbaji S., Vogt M.A., Fumagalli F., Sohr R., Frasca A., Brandwein C., Hortnagl H., Riva M.A., Sprengel R., Gass P. AMPA receptor subunit 1 (GluR-A) knockout mice model the glutamate hypothesis of depression. FASEB J. 2008;22:3129–3134. doi: 10.1096/fj.08-106450. [DOI] [PubMed] [Google Scholar]
- Clem R.L., Anggono V., Huganir R.L. PICK1 regulates incorporation of calcium-permeable AMPA receptors during cortical synaptic strengthening. J. Neurosci. 2010;30:6360–6366. doi: 10.1523/JNEUROSCI.6276-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis S.H., Jaafari N., Cimarosti H., Hanley J.G., Henley J.M., Mellor J.R. Oxygen-glucose deprivation induces reduction in synaptic AMPA receptors on hippocampal CA3 neurons mediated by mGluR1 and adenosine A3 receptors. J. Neurosci. 2011;31:11941–11952. doi: 10.1523/JNEUROSCI.1183-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J., Suzuki K., Wei Y., Wang Y., Blumenthal R., Chen Z., Falke C., Zarate C.A., Jr, Manji H.K. The anticonvulsants lamotrigine, riluzole, and valproate differentially regulate AMPA receptor membrane localization: relationship to clinical effects in mood disorders. Neuropsychopharmacology. 2007;32:793–802. doi: 10.1038/sj.npp.1301178. [DOI] [PubMed] [Google Scholar]
- Duric V., Banasr M., Stockmeier C.A., Simen A.A., Newton S.S., Overholser J.C., Jurjus G.J., Dieter L., Duman R.S. Altered expression of synapse and glutamate related genes in post-mortem hippocampus of depressed subjects. Int. J. Neuropsychopharmacol. 2013;16:69–82. doi: 10.1017/S1461145712000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta A., McKie S., Deakin J.F. Ketamine and other potential glutamate antidepressants. Psychiatry Res. 2015;225:1–13. doi: 10.1016/j.psychres.2014.10.028. [DOI] [PubMed] [Google Scholar]
- Freudenberg F., Celikel T., Reif A. The role of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors in depression: central mediators of pathophysiology and antidepressant activity? Neurosci. Biobehav. Rev. 2015;52:193–206. doi: 10.1016/j.neubiorev.2015.03.005. [DOI] [PubMed] [Google Scholar]
- Greger I.H., Watson J.F., Cull-Candy S.G. Structural and functional architecture of AMPA-type glutamate receptors and their auxiliary proteins. Neuron. 2017;94:713–730. doi: 10.1016/j.neuron.2017.04.009. [DOI] [PubMed] [Google Scholar]
- Hermes G., Li N., Duman C., Duman R. Post-weaning chronic social isolation produces profound behavioral dysregulation with decreases in prefrontal cortex synaptic-associated expression in female rats. Physiol. Behav. 2011;104:354–359. doi: 10.1016/j.physbeh.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao Y.H., Chen P.S., Chen S.H., Gean P.W. The involvement of Cdk5 activator p35 in social isolation-triggered onset of early Alzheimer's disease-related cognitive deficit in the transgenic mice. Neuropsychopharmacology. 2011;36:1848–1858. doi: 10.1038/npp.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaso B.A., Niciu M.J., Iadarola N.D., Lally N., Richards E.M., Park M., Ballard E.D., Nugent A.C., Machado-Vieira R., Zarate C.A. Therapeutic modulation of glutamate receptors in major depressive disorder. Curr. Neuropharmacol. 2017;15:57–70. doi: 10.2174/1570159X14666160321123221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L., Zhang H., He Y., Liu H., Li S., Chen R., Han S., Zhou Y., Zhang J., Wan X., Xu R., Wang S., Gu H., Wei Q., Qin F., Zhao Y., Chen Y., Li H., Wang L., Wang X., Wang Y., Dai Y., Li M., Chen Y., Zhang H., Hu Y., Bu Q., Zhao Y., Cen X. Synapse differentiation-induced gene 1 regulates stress-induced depression through interaction with the AMPA receptor GluA2 subunit of nucleus accumbens in male mice. Neuropharmacology. 2022;213 doi: 10.1016/j.neuropharm.2022.109076. [DOI] [PubMed] [Google Scholar]
- Jin D.Z., Guo M.L., Xue B., Fibuch E.E., Choe E.S., Mao L.M., Wang J.Q. Phosphorylation and feedback regulation of metabotropic glutamate receptor 1 by calcium/calmodulin-dependent protein kinase II. J. Neurosci. 2013;33:3402–3412. doi: 10.1523/JNEUROSCI.3192-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, D.Z., Mao, L.M., Wang, J.Q. , 2017. An essential role of Fyn in the modulation of metabotropic glutamate receptor 1 in neurons. eNeuro 4 ENEURO.0096–17.2017. [DOI] [PMC free article] [PubMed]
- Kato T., Takata M., Kitaichi M., Kassai M., Inoue M., Ishikawa C., Hirose W., Yoshida K., Shimizu I. DSR-98776, a novel selective mGlu5 receptor negative allosteric modulator with potent antidepressant and antimanic activity. Eur. J. Pharm. 2015;757:11–20. doi: 10.1016/j.ejphar.2015.03.024. [DOI] [PubMed] [Google Scholar]
- Kondo M., Okabe S., Sumino R., Okado H. A high GluR1:GluR2 expression ratio is correlated with expression of Ca2+-binding proteins in rat forebrain neurons. Eur. J. Neurosci. 2000;12:2812–2822. doi: 10.1046/j.1460-9568.2000.00167.x. [DOI] [PubMed] [Google Scholar]
- Krishnan V., Nestler E.J. Animal models of depression: molecular perspectives. Curr. Top. Behav. Neurosci. 2011;7:121–147. doi: 10.1007/7854_2010_108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen L.V., Meador-Woodruff J.H. Abnormal striatal expression of transcripts encoding NMDA interacting PSD proteins in schizophrenia, bipolar disorder and major depression. Schizophr. Res. 2005;78:87–93. doi: 10.1016/j.schres.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Li X., Need A.B., Baez M., Witkin J.M. Metabotropic glutamate 5 receptor antagonism is associated with antidepressant-like effects in mice. J. Pharm. Exp. Ther. 2006;319:254–259. doi: 10.1124/jpet.106.103143. [DOI] [PubMed] [Google Scholar]
- Liu C.Y., Jiang X.X., Zhu Y.H., Wei D.N. Metabotropic glutamate receptor 5 antagonist 2-methyl-6-(phenylethynyl)pyridine produces antidepressant effects in rats: role of brain-derived neurotrophic factor. Neuroscience. 2012;223:219–224. doi: 10.1016/j.neuroscience.2012.08.010. [DOI] [PubMed] [Google Scholar]
- Mangiavacchi S., Wolf M.E. Stimulation of N-methyl-D-aspartate receptors, AMPA receptors or metabotropic glutamate receptors leads to rapid internalization of AMPA receptor in cultured nucleus accumbens neurons. Eur. J. Neurosci. 2004;20:649–657. doi: 10.1111/j.1460-9568.2004.03511.x. [DOI] [PubMed] [Google Scholar]
- Mao L.M., Wang J.Q. Alterations in mGlu5 receptor expression and function in the striatum in a rat depression model. J. Neurochem. 2018;145:287–298. doi: 10.1111/jnc.14307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Turrillas R., Del Rio J., Frechilla D. Sequential changes in BDNF mRNA expression and synaptic levels of AMPA receptor subunits in rat hippocampus after chronic antidepressant treatment. Neuropharmacology. 2005;49:1178–1188. doi: 10.1016/j.neuropharm.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Miyazaki T., Takase K., Nakajima W., Tada H., Ohya D., Sano A., Goto T., Hirase H., Malinow R., Takahashi T. Disrupted cortical function underlies behavior dysfunction due to social isolation. J. Clin. Investig. 2012;122:2690–2701. doi: 10.1172/JCI63060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki T., Kunii M., Jitsuki S., Sano A., Kuroiwa Y., Takahashi T. Social isolation perturbs experience-driven synaptic glutamate receptor subunit 4 delivery in the developing rat barrel cortex. Eur. J. Neurosci. 2013;37:1602–1609. doi: 10.1111/ejn.12188. [DOI] [PubMed] [Google Scholar]
- Molina-Hernandez M., Tellez-Alcantara N.P., Perez-Garcia J., Olivera-Lopez J.I., Jaramillo M.T. Antidepressant-like and anxiolytic-like actions of the mGlu5 receptor antagonist MTEP, microinjected into lateral septal nuclei of male Wistar rats. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2006;30:1129–1135. doi: 10.1016/j.pnpbp.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Nations K.R., Dogterom P., Bursi R., Schipper J., Greenwald S., Zraket D., Gertsik L., Johnstone J., Lee A., Pande Y., Ruigt G., Ereshefsky L. Examination of Org 26576, an AMPA receptor positive allosteric modulator, in patients diagnosed with major depressive disorder: an exploratory, randomized, doubled-blind, placebo-controlled trial. J. Psychopharmacol. 2012;26:1525–1539. doi: 10.1177/0269881112458728. [DOI] [PubMed] [Google Scholar]
- Nestler E.J., Carlezon W.A., Jr. The mesolimbic dopamine reward circuit in depression. Biol. Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Niswender C.M., Conn P.J. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu. Rev. Pharm. Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palucha A., Branski P., Szewczyk B., Wieronska J.M., Klak K., Pilc A. Potential antidepressant-like effect of MTEP, a potent and highly selective mGluR5 antagonist. Pharm. Biochem. Behav. 2005;81:901–906. doi: 10.1016/j.pbb.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Paul I.A., Skolnick P. Glutamate and depression: clinical and preclinical studies. Ann. NY Acad. Sci. 2003;1003:250–272. doi: 10.1196/annals.1300.016. [DOI] [PubMed] [Google Scholar]
- Pilc A., Klodzinska A., Branski P., Nowak G., Palucha A., Szewczyk B., Tatarczynska E., Chojnacka-Wojcik E., Wieronska J.M. Multiple MPEP administrations evoke anxiolytic- and antidepressant-like effects in rats. Neuropharmacology. 2002;43:181–187. doi: 10.1016/s0028-3908(02)00082-5. [DOI] [PubMed] [Google Scholar]
- Pilc A., Chaki S., Nowak G., Witkin J.M. Mood disorders: regulation by metabotropic glutamate receptors. Biochem. Pharm. 2008;75:997–1006. doi: 10.1016/j.bcp.2007.09.021. [DOI] [PubMed] [Google Scholar]
- Pomierny-Chamiolo L., Poleszak E., Pilc A., Nowak G. NMDA but not AMPA glutamatergic receptors are involved in the antidepressant-like activity of MTEP during the forced swim test in mice. Pharm. Res. 2010;62:1186–1190. doi: 10.1016/s1734-1140(10)70381-9. [DOI] [PubMed] [Google Scholar]
- Reimers J.M., Milovanovic M., Wolf M.E. Quantitative analysis of AMPA receptor subunit composition in addiction-related brain regions. Brain Res. 2011;1367:223–233. doi: 10.1016/j.brainres.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestito R.S., Trindade L.B., de Souza R.G., Kerbauy L.N., Iyomasa M.M., Rosa M.L. Effect of isolation rearing on the expression of AMPA glutamate receptors in the hippocampal formation. J. Psychopharmacol. 2011;25:1720–1729. doi: 10.1177/0269881110385595. [DOI] [PubMed] [Google Scholar]
- Shimizu K., Kurosawa N., Seki K. The role of the AMPA receptor and 5-HT(3) receptor on aggressive behavior and depressive-like symptoms in chronic social isolation-reared mice. Physiol. Behav. 2016;153:70–83. doi: 10.1016/j.physbeh.2015.10.026. [DOI] [PubMed] [Google Scholar]
- Snyder E.M., Philpot B.D., Huber K.M., Dong X., Fallon J.R., Bear M.F. Internalization of ionotropic glutamate receptors in response to mGluR activation. Nat. Neurosci. 2001;4:1079–1085. doi: 10.1038/nn746. [DOI] [PubMed] [Google Scholar]
- Svenningsson P., Tzavara E.T., Witkin J.M., Fienberg A.A., Nomikos G.G., Greengard P. Involvement of striatal and extrastriatal DAPRR-32 in biochemical and behavioral effects of fluoxetine (Prozac) Proc. Natl. Acad. Sci. USA. 2002;99:3182–3187. doi: 10.1073/pnas.052712799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P., Bateup H., Qi H., Takamiya K., Huganir R.L., Spedding M., Roth B.L., McEwen B.S., Greengard P. Involvement of AMPA receptor phosphorylation in antidepressant actions with special reference to tianeptine. Eur. J. Neurosci. 2007;26:3509–3517. doi: 10.1111/j.1460-9568.2007.05952.x. [DOI] [PubMed] [Google Scholar]
- Tada H., Miyazaki T., Takemoto K., Takase K., Jitsuki S., Nakajima W., Koide M., Yamamoto N., Komiya K., Suyama K., Sano A., Taguchi A., Takahashi T. Neonatal isolation augments social dominance by altering actin dynamics in the medial prefrontal cortex. Proc. Natl. Acad. Sci. USA. 2016;113:E7097–E7105. doi: 10.1073/pnas.1606351113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallaksen-Greene S.J., Kaatz K.W., Romano C., Albin R.L. Localization of mGluR1a-like immunoreactivity and mGluR5a-like immunoreactivity in identified population of striatal neurons. Brain Res. 1998;780:210–217. doi: 10.1016/s0006-8993(97)01141-4. [DOI] [PubMed] [Google Scholar]
- Tan C.H., He X., Yang J., Ong W.Y. Changes in AMPA subunit expression in the mouse brain after chronic treatment with the antidepressant maprotiline: a link between noradrenergic and glutamatergic function? Exp. Brain Res. 2006;170:448–456. doi: 10.1007/s00221-005-0228-2. [DOI] [PubMed] [Google Scholar]
- Tatarczynska E., Klodzinska A., Chojnacka-Wojcik E., Palucha A., Gasparini F., Kuhn R., Pilc A. Potential anxiolytic- and antidepressant-like effects of MPEP, a potent, selective and systemically active mGlu5 receptor antagonist. Br. J. Pharm. 2001;132:1423–1430. doi: 10.1038/sj.bjp.0703923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa C.M., Standaert D.G., Young A.B., Penney J.B., Jr. Metabotropic glutamate receptor mRNA expression in the basal ganglia of the rat. J. Neurosci. 1994;14:3005–3018. doi: 10.1523/JNEUROSCI.14-05-03005.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokita K., Yamaji T., Hashimoto K. Roles of glutamate signaling in preclinical and/or mechanistic models of depression. Pharm. Biochem. Behav. 2012;100:688–704. doi: 10.1016/j.pbb.2011.04.016. [DOI] [PubMed] [Google Scholar]
- Tomasetti C., Dell’Aversano C., Iasevoli F., Marmo F., de Bartolomeis A. The acute and chronic effects of combined antipsychotic-mood stabilizing treatment on the expression of cortical and striatal postsynaptic density genes. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2011;35:184–197. doi: 10.1016/j.pnpbp.2010.10.025. [DOI] [PubMed] [Google Scholar]
- Toth E., Gersner R., Wilf-Yarkoni A., Raizel H., Dar D.E., Richter-Levin G., Levit O., Zangen A. Age-dependent effects of chronic stress on brain plasticity and depressive behavior. J. Neurochem. 2008;107:522–532. doi: 10.1111/j.1471-4159.2008.05642.x. [DOI] [PubMed] [Google Scholar]
- Traynelis S.F., Wollmuth L.P., McBain C.J., Menniti F.S., Vance K.M., Ogden K.K., Hansen K.B., Yuan H., Myers S.J., Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharm. Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialou V., Robison A.J., LaPlant Q.C., Covington H.E., III, Dietz D.M., Ohnishi Y.N., Mouzon E., Rush A.J., III, Watts E.L., Wallace D.L., Iñiguez S.D., Ohnishi Y.H., Steiner M.A., Warren B.L., Krishnan V., Bolaños C.A., Neve R.L., Ghose S., Berton O., Tamminga C.A., Nestler E.J. ΔFosB in brain reward circuits mediate resilience to stress and antidepressant responses. Nat. Neurosci. 2010;13:745–754. doi: 10.1038/nn.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vose L.R., Stanton P.K. Synaptic plasticity, metaplasticity and depression. Curr. Neuropharmacol. 2017;15:71–86. doi: 10.2174/1570159X14666160202121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf A.A., Frye C.A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007;2:322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace D.L., Han M.H., Graham D.L., Green T.A., Vialou V., Iniguez S.D., Cao J.L., Kirk A., Chakravarty S., Kumar A., Krishnan V., Neve R.L., Cooper D.C., Bolanos C.A., Barrot M., McClung C.A., Nestler E.J. CREB regulation of nucleus accumbens excitability mediates social isolation-induced behavioral deficits. Nat. Neurosci. 2009;12:200–209. doi: 10.1038/nn.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieronska J.M., Szewczyk B., Branski P., Palucha A., Pilc A. Antidepressant-like effect of MPEP, a potent, selective and systemically active mGlu5 receptor antagonist in the olfactory bulbectomized rats. Amino Acids. 2002;23:213–216. doi: 10.1007/s00726-001-0131-5. [DOI] [PubMed] [Google Scholar]
- Wood D.A., Buse J.E., Wellman C.L., Rebec G.V. Differential environmental exposure alters NMDA but not AMPA receptor subunit expression in nucleus accumbens core and shell. Brain Res. 2005;1042:176–183. doi: 10.1016/j.brainres.2005.02.029. [DOI] [PubMed] [Google Scholar]
- Xiao M.Y., Zhou Q., Nicoll R.A. Metabotropic glutamate receptor activation causes a rapid redistribution of AMPA receptors. Neuropharmacology. 2001;41:664–671. doi: 10.1016/s0028-3908(01)00134-4. [DOI] [PubMed] [Google Scholar]
- Yang B., Zhang J.C., Han M., Yao W., Yang C., Ren Q., Ma M., Chen Q.X., Hashimoto K. Comparison of R-ketamine and rapastinel antidepressant effects in the social defeat stress model of depression. Psychopharmacology. 2016;233:3647–3657. doi: 10.1007/s00213-016-4399-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen E.Y., Wei J., Liu W., Zhong P., Li X., Yan Z. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron. 2012;73:962–977. doi: 10.1016/j.neuron.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharko R.M., Anisman H. Stressor-induced anhedonia in the mesocorticolimbic system. Neurosci. Biobehav. Rev. 1991;15:391–405. doi: 10.1016/s0149-7634(05)80032-6. [DOI] [PubMed] [Google Scholar]
- Zho W.M., You J.L., Huang C.C., Hsu K.S. The group I metabotropic glutamate receptor agonist (S)-3,5-dihydroxyphenylglycine induces a novel form of depotentiation in the CA1 region of the hippocampus. J. Neurosci. 2002;22:8838–8849. doi: 10.1523/JNEUROSCI.22-20-08838.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H.Y., He J.G., Hu Z.L., Xue S.G., Xu J.F., Cui Q.Q., Gao S.Q., Zhou B., Wu P.F., Long L.H., Wang F., Chen J.G. A-kinase anchoring protein 150 and protein kinase A complex in the basolateral amygdala contributes to depressive-like behaviors induced by chronic restraint stress. Biol. Psychiatry. 2019;86:131–142. doi: 10.1016/j.biopsych.2019.03.967. [DOI] [PubMed] [Google Scholar]