Abstract
A variety of contemporary techniques were used to investigate the vertical distribution of thermophilic unicellular cyanobacteria, Synechococcus spp., and their activity within the upper 1-mm-thick photic zone of the mat community found in an alkaline siliceous hot spring in Yellowstone National Park in Wyoming. Detailed measurements were made over a diel cycle at a 61°C site. Net oxygenic photosynthesis measured with oxygen microelectrodes was highest within the uppermost 100- to 200-μm-thick layer until midmorning, but as the day progressed, the peak of net activity shifted to deeper layers, stabilizing at a depth of 300 μm from midday throughout the afternoon. Examination of vertical thin sections by bright-field and autofluorescence microscopy revealed the existence of different populations of Synechococcus which form discrete bands at different vertical positions. Denaturing gradient gel electrophoresis analysis of PCR-amplified 16S rRNA gene segments from horizontal cryosections obtained at 100-μm-thick vertical intervals also suggested vertical stratification of cyanobacterial, green sulfur bacterium-like, and green nonsulfur bacterium-like populations. There was no evidence of diel migration. However, image analysis of vertical thin sections revealed the presence of a narrow band of rod-shaped Synechococcus cells in which the cells assumed an upright position. These upright cells, located 400 to 800 μm below the surface, were observed only in mat samples obtained around noon. In mat samples obtained at other time points, the cells were randomly oriented throughout the mat. These combined observations reveal the existence of a highly ordered structure within the very thin photic zone of this hot spring microbial mat, consisting of morphologically similar Synechococcus populations that are likely to be differentially adapted, some co-occurring with green sulfur bacterium-like populations, and all overlying green nonsulfur bacterium-like populations.
Hot spring microbial mats are ideally suited as model systems for studying general principles of microbial diversity and community ecology (49) because their apparent complexity is low compared to other environments. The lack of predation and grazing by eukaryotic organisms makes this system stable and relatively uniformly layered or laminated. Hot spring microbial mats are also excellent habitats in which to apply and evaluate various contemporary molecular, microscopic, and microsensor approaches. For example, 16S rRNA-based analyses of hot spring mat communities have revealed the presence of numerous populations that cannot easily be differentiated microscopically (11, 33, 50, 52). The well-studied Octopus Spring mat in Yellowstone National Park in Wyoming contains two predominant morphotypes: rod-shaped (Synechococcus-like) cyanobacteria and filamentous (Chloroflexus-like) bacteria. However, at least 11 genetically distinct cyanobacterial populations inhabit the mat (49), 4 of which are known to share a common Synechococcus-like morphology (11, 51). It is likely that all the cyanobacterial populations are Synechococcus shaped, since no other cyanobacterial cell type is observed at the elevated temperatures where these populations are detected. Several green nonsulfur bacterium-like 16S rRNA sequences have also been detected, and the cells harboring these sequences may possess similar filamentous morphologies. We are interested in how these populations are distributed in the mat and how they function.
By correlating the distribution of genetic variants and essential physical and chemical parameters in the environment, it has been possible to gain insight into the ecophysiological adaptations of these populations. Ferris et al. (9, 12) showed that the diversity revealed by denaturing gradient gel electrophoresis (DGGE) analysis of rRNA gene segments from samples taken at different temperatures was likely due to highly ordered temperature-adapted populations of (i) Synechococcus sp. (consistent with earlier work on thermophilic strains by Peary and Castenholz [37]) and (ii) green nonsulfur bacterium-like bacteria (consistent with the work of Bauld and Brock [2]). It is thus apparent that temperature is a controlling factor regulating species distribution along the effluent channels where the spring water slowly cools.
We hypothesized that the vertical distribution of bacterial populations might show similar zonation. Microbial mats are laminated structures that are believed to be the modern analogs of stromatolite-forming mats that dominated shallow water communities during the Precambrian era (6). Their layered or laminated appearance is evident, even macroscopically, when viewed in cross section (53). Microelectrode analyses of microbial mats have demonstrated that strong vertical gradients of physical and chemical parameters such as oxygen or hydrogen sulfide concentration and light, exist at scales relevant to microorganisms (24, 27, 42–45). Further, it has been shown that the microscopic biota change in a physiologically meaningful fashion along these gradients and their diel variation (3, 5, 8, 16, 40). Unfortunately, these gradients are interconnected so that it is difficult to evaluate which of the many parameters that change with depth (light intensity and spectral composition, electron donors and acceptors, carbon sources, etc.) are controlling the vertical distribution.
In this study we combined microsensor, microscopic, and molecular techniques to investigate the distribution, orientation, and function of microbial populations within vertical strata of the Mushroom Spring Synechococcus mat during a diel cycle. Measurement of vertical oxygen concentration profiles every 4 h with a microelectrode revealed zonation of net photosynthetic activity and gave evidence of photoinhibition of surface populations. Microscopy and DGGE analysis of 16S rRNA gene segments performed on cryosections gave evidence of stratification of genetically distinct Synechococcus populations. Computer-assisted image analysis of transects through thin vertical slices revealed the presence of oriented Synechococcus cells in a narrow band residing 400 to 800 μm below the mat surface in all midday samples. This upright orientation was not observed at any other time points.
MATERIALS AND METHODS
Study site.
This study was conducted on 11 and 12 June 1996 at the microbial mat located in the effluent channel of Mushroom Spring, in the Lower Geyser Basin, Yellowstone National Park in Wyoming (1). Mushroom Spring is located approximately 0.5 km from the better-studied Octopus Spring (49). The effluent waters of the two springs have a very similar composition (N. Ramsing and D. Ward, unpublished data), and the microbial mats have a very similar appearance. For this study we chose to work in Mushroom Spring, as the Octopus Spring mat was severely damaged, presumably by a hailstorm shortly before the investigation. The microbial mats in the effluent channel of Mushroom Spring were largely unperturbed, possibly because a thicker water layer above the mat (>5 cm in many places) shielded it from the impact of hailstones. The temperature, oxygen concentration, and pH of the running water above the mat were monitored during the 26-h observation period. Downwelling scalar irradiance of photosynthetically active radiation of the incident light was measured with a Lutron model LX-101 lux meter and converted to microeinsteins per square meter per second after calibration with a LICOR scalar irradiance sensor positioned over a black surface.
Microelectrode measurements.
Measurements of oxygen concentration profiles were performed as described by Revsbech and Ward (45). The O2 concentration was measured with a Clark-type O2 microsensor with a guard cathode as previously described by Revsbech (41). The electrode had a tip diameter of 10 μm, a stirring sensitivity of <1 to 2% and a 90% response time of <1 s. The sensor current was measured with a picoammeter coupled to a strip chart recorder and an analog/digital (A/D) converter. Oxygen concentration was also determined in water samples from the effluent channel by Winkler titration at different time points during the diel cycle (21). The oxygen concentration in the effluent channel measured by Winkler titration and a zero reading in the deep anaerobic part of the mat was used to make a linear calibration of the electrodes. The electrode drift during the 26-h measurement period was <1% of the signal.
Net metabolic rate estimates from oxygen concentration profiles.
Rates of oxygenic photosynthesis and aerobic respiration were estimated from the curvature of oxygen profiles. For the sake of simplicity, all metabolic rates pertaining to oxygen will be counted as positive if oxygen is being produced and negative if oxygen is being consumed [i.e., if the rates of photosynthesis, P(z), are positive, rates of respiration, R(z), are negative, and the resulting net metabolism of oxygen is a sum of photosynthesis and respiration M(z) = P(z) + R(z)]. Using this convention, Fick's second law of one-dimensional diffusion (7) becomes:
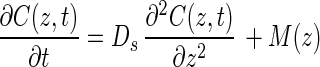 |
1 |
where C(z, t) is the oxygen concentration at depth z and time t, Ds is the diffusivity of oxygen, and M(z) is the net metabolic rate of oxygen production and consumption. An assumption of this formula is that Ds does not change within the depth range being investigated. For our study, a Ds value of 1.44 × 10−3 mm2 s−1 determined in a 1- to 2-mm-thick gelatinous epilithic mat at 25°C (19) was extrapolated to the value expected at 60°C (3.52 × 10−3 mm2 s−1) using published equations (29, 31). Under steady-state conditions, equation 1 becomes
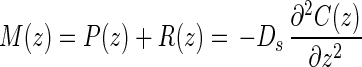 |
2 |
The net metabolic rate is directly proportional to the second derivative of the concentration with respect to depth, that is, to the curvature of the profile. As the measured microprofiles consist of discrete data points with inherent small variations, differentiation of the raw data often leads to a very noisy pattern. To correct for this, we compared the measured concentration profiles to simulated concentration profiles calculated from activity profiles by integration of equation 2. The activity profile consists of a number of zones with constant activity in each zone. A simple optimization method was used to find the activity profile that minimized the difference between the theoretically calculated concentration profile and the measured concentration profile. This procedure was used to find a set of activity profiles, with an increasing number of zones that described the concentration profile best. Each of these solutions was tested by evaluating the sensitivity of the solution to additions of noise to the concentration profile. The activity profile with the highest number of zones yet still robust towards noise addition was finally chosen (J. Berntsen and N. B. Ramsing, unpublished data). The uppermost zone of oxygen consumption is not due to microbial activity but to efflux by molecular diffusion through the diffusive boundary (25). The position of the mat surface was thus resolved empirically as the lower limit of the uppermost consumption zone. This normally corresponded to the inflection point of the first derivative, except in the afternoon sample where the upper portion of the mat exhibited a net oxygen consumption. The determined surface position was confirmed by a marked concurrent stabilization of the sensor signal during measurement (data not shown).
Sample collection.
Microbial mat samples were collected at different time points using a no. 4 cork borer (ca. 8-mm diameter). To preserve the vertical structure, each core was rapidly frozen by immediately placing it into a small volume of isopentane that had been precooled in liquid nitrogen (46). The cores were stored on dry ice during transit and maintained at −20°C in the laboratory and throughout the thin-sectioning process.
Cryosectioning.
A cryostat (2800 Frigcut; Reichert Scientific Instruments, Vashon, Wash.) was used to collect 100-μm-thick horizontal sections (i.e., cut parallel to the mat surface) of the mat cores for DGGE analyses. Frozen cores were mounted for sectioning using a small amount of O.C.T. embedding compound (Miles Inc. Elkhart, Ind.). The uppermost mat layer (ca. 3 mm) was oriented toward the blade and was not coated with O.C.T., since initial attempts to isolate DNA in the presence of O.C.T. failed. Sections that are 50 μm thick were shaved from the upper surface of the core and transferred into 1.5-ml screw-cap microcentrifuge tubes, and two adjacent 50-μm-thick sections were combined in each tube. In this fashion, the first 10 100-μm-thick sections of the vertical interval of mat were prepared for nucleic acid extraction, PCR amplification, and DGGE analysis. For microscopy, 15- or 40-μm-thick vertically oriented (i.e., perpendicular to the mat surface) cryosections of a duplicate mat core were placed on a microscope slide, fixed with formalin, and mounted in immersion oil as described previously (40). The slicing was done with the edge of the knife of the cryotome perpendicular to the surface of the mat sample to avoid spatial distortions in the vertical plane.
Light microscopy and image acquisition.
An Axioskop epifluorescence microscope (Zeiss, Oberkochen, Germany) equipped with plan NEOFLUAR objectives, a HB50 mercury arc lamp, and a Zeiss video camera was used to obtain microscopic images. The autofluorescence of the cyanobacterial chlorophyl was clearly visible without staining using a standard epifluorescence filter set (set 15): green excitation (546 nm) and red emission (>590 nm). Digital images from the video camera were acquired with the built-in frame grabber of a PowerMac 7500 (Apple, Cupertino, Calif.) controlled by the program NIH Image version 1.59. NIH Image, written by Wayne Rasband, National Institutes of Health (NIH), Bethesda, Md., is a freeware program available on the internet from the anonymous FTP site zippy.nimh.nih.gov. The image acquisition subroutines used to acquire images are available on request from N. B. Ramsing. Selected images were further processed for publication using Adobe Photoshop 3.0 and Canvas 5.0 software.
Analysis of cell orientation.
Image-processing methods were used to quantify changes in cell orientation. The brief description here is expanded upon in Results where illustrations are provided to further explain the method. Several images collected along a vertical transect through a mat slice were combined into one continuous picture of 3,000 by 500 pixels. Small subregions (256 by 256 pixels) of the combined picture were subjected to a two-dimensional fast Fourier transformation (FFT). The amplitude pattern of the Fourier transform reflects the predominant intensity variations in the region that was evaluated to determine the average orientation of the principal objects. This was done (i) by thresholding the amplitude pattern of the two-dimensional Fourier transform and measuring the orientation of the resulting object by evaluating the best-fitting ellipsoid or (ii) by determining the ratio of the vertical and horizontal standard deviations of the frequency distribution of the Fourier transform. The resulting algorithm was implemented in the program NIH image and tested on different sample images. The custom-made subroutines for measuring cell orientation are available on request from N. B. Ramsing.
Scanning electron microscopy.
The block of microbial mat left over after vertical slicing for light microscopy was evaluated by scanning electron microscopy. Excess O.C.T. embedding medium was removed with a scalpel. The frozen blocks were then fixed in 2% glutaraldehyde, dehydrated in ethanol, and critical point dried by sublimation as described previously (36). The specimen was then coated by Pt sputtering and observed through a JEOL scanning electron microscope, model 840.
DGGE analysis.
Cells were lysed by bead beating, and nucleic acids were extracted with buffered phenol and chloroform mixtures followed by ethanol precipitation overnight at −20°C as described elsewhere (9, 12). The extracted nucleic acids were suspended in 15 μl of TRIS-Cl buffer, pH 8.0 (Sigma). Conditions for PCR amplification of positions 1070 to 1392 of the 16S rRNA gene, DGGE analyses, and band sequencing were as described previously (12). Twenty microliters of each PCR mixture was loaded onto each lane of the denaturing gradient gels. A DGGE analysis of the intact mat (upper ca. 2 mm) was also performed as previously described (9, 12). Photographic negatives of DGGE gels were scanned at a resolution of 300 dots per inch (dpi) as previously reported (12), and images were analyzed using a gel analysis subroutine within the program NIH Image. The subroutine was based on the “Gel plotting macro” included in the NIH package. The macro was modified to include the “rolling rod” background subtraction algorithm (diameter, 50 pixels) and to automate analysis. The gel analysis subroutines are available on request from N. B. Ramsing. Intensities of both homoduplex and heteroduplex bands were quantified. Half the intensity value of heteroduplex bands was added to the intensity of each homoduplex band (12) to obtain a total intensity for each sequence type.
RESULTS
Diel variations in water chemistry.
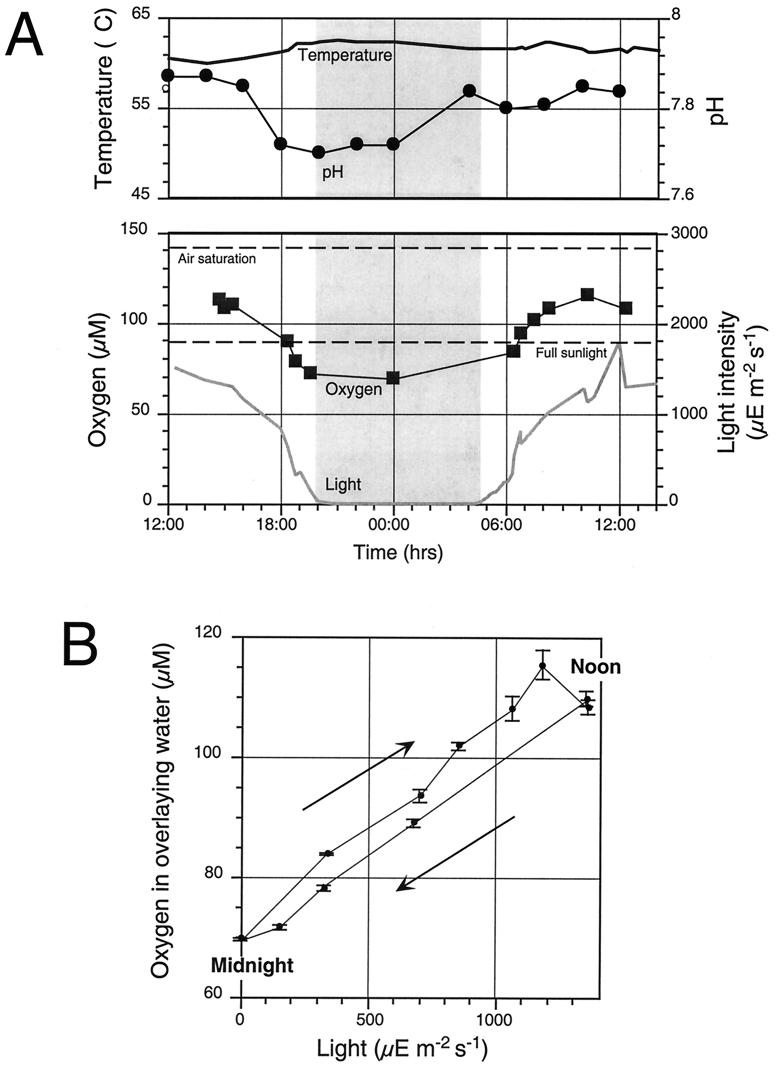
Diel variations in the physical and chemical parameters of the effluent water above the sampling site are depicted in Fig. 1A. The flow within the effluent channel was constant and showed no trace of the periodic surges known from Octopus Spring (30, 49). The temperature at the sampling site remained relatively constant between 60.6 and 62.6°C throughout the investigation. One notable difference from the Octopus Spring system was a lower temperature within the large source pool (70°C in Mushroom Spring compared to 92°C in Octopus Spring) which is covered with a thick carpet-like photosynthetic mat. The net photosynthesis within the source pool and effluent channel upstream of the sampling area apparently increase the oxygen concentration within the effluent channels dramatically during the day (Fig. 1A). However, the oxygen concentration remained well below the saturation point for atmospheric air at 61°C (approximately 140 μmol liter−1) at all times. The correlation between incident light intensity and oxygen concentration in the effluent channel is depicted in Fig. 1B. The diel cycle of corresponding measurements shows a notable hysteresis effect in that the afternoon oxygen concentrations were lower than the morning ones at a similar light intensity. The pH in the effluent channel was likewise increased during daylight hours as would be expected from photosynthetic CO2 uptake, but the direct correlation with light intensities was less obvious.
FIG. 1.
Diel variation in water chemistry above the 61°C cyanobacterial mat in Mushroom Spring Yellowstone National Park in Wyoming. (A) Changes in oxygen concentration, pH, temperature, and light intensity over the course of a day. The time span from sunset to sunrise is indicated by gray shading. (B) Correlation between oxygen concentration and light intensity, indicating lower net photosynthetic oxygen production in the afternoon. Reference time points are noted on the graph. Arrows indicate direction of progression during the diel cycle. Error bars show 95% confidence limits for the mean values reported. μE, microeinsteins.
Diel variation in oxygen metabolism within the mat.
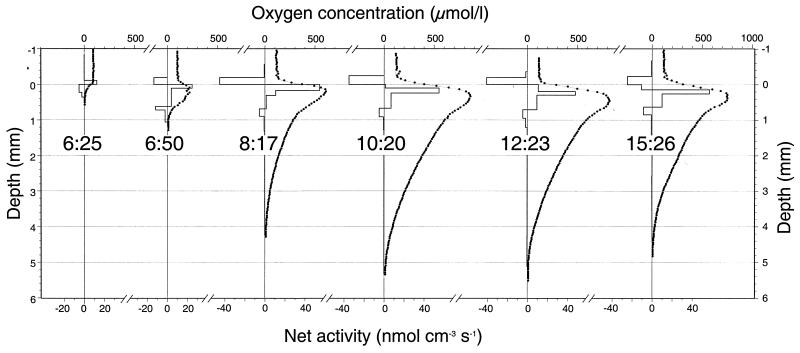
Oxygen microelectrode concentration profiles indicated that oxygen production and photosynthesis were occurring at high levels in the surface layer of the mat during most of the day. Representative oxygen profiles and derived net rates of oxygen consumption and production are depicted in Fig. 2. In the early morning, when light intensities were still below 500 microeinsteins m−2 s−1, there was a net oxygen consumption within the mat and oxygen penetration was only 0.2 to 0.4 mm (Fig. 2, profile at 6:25 in the morning). However, as the light intensity increased, the surface mat layer displayed a strong peak of net oxygen production and a gradual buildup of oxygen within the mat followed (Fig. 2, profile at 6:50 in the morning). One and a half hours later, oxygen penetration had increased fivefold and extended to a depth of about 4 mm; the net photosynthesis within the mat peaked at around 55 nmol cm−3 s−1 (Fig. 2, 8:17 profile). As the light intensity increased further, the maximum oxygen concentration within the mat increased to a value close to or slightly exceeding the ebullition point for pure oxygen at 61°C (approximately 700 μmol liter−1) (Fig. 2, 10:20 profile). From then on, the net photosynthesis in the surface layer was inhibited; however, the net photosynthetic activity of deeper layers increased (Fig. 2, 12:23 profile), so that the net photosynthesis of the photic zone remained remarkably constant (Table 1). As the zone of maximum activity shifted down into the mat, the vertical concentration gradient out of the mat became less steep and the oxygen efflux through the diffusive boundary layer above the mat was reduced. The net export of oxygen from the mat to the overlaying water was thus reduced somewhat as the day progressed (Table 1). The maximum oxygen export recorded was 0.9 nmol cm−2 s−1 at 8:17 decreasing to 0.7 nmol cm−2 s−1 at 15:26. It should be noted that the uppermost consumption zone, which corresponds to efflux by molecular diffusion through the boundary layer, has a constant thickness. This agrees well with the constant water flow above the mat and confirms the correct identification of the position of the mat surface.
FIG. 2.
Vertical stratification of metabolic processes within the Mushroom Spring 61°C Synechococcus mat from early morning to midafternoon. The small solid circles are the measurement points of in situ oxygen concentration profiles recorded at different times of the day with microelectrodes. The bars show the calculated net rates of oxygen production and consumption based on the observed concentration profiles and a simple diffusion model using a constant diffusion coefficient. Negative values indicate net consumption. The thin lines show the modeled concentration profiles according to the activity zonation indicated by the bars.
TABLE 1.
Net oxygen production within the photic zone, efflux to the overlaying water, and export to the underlaying mat from daybreak to the early afternoon
| Time of daya | Net photosynthesis within photic zone (nmol cm−2 s−1)b | Flux of oxygen from photic zone
|
|
|---|---|---|---|
| To overlaying water (nmol cm−2 s−1) | To underlaying mat (nmol cm−2 s−1) | ||
| 6:25 | −1.4c | −0.14d | − |
| 6:50 | 0.46 | 0.27 | 0.19 |
| 8:17 | 1.11 | 0.91 | 0.20 |
| 10:20 | 1.08 | 0.85 | 0.23 |
| 12:23 | 1.09 | 0.81 | 0.27 |
| 15:26 | 1.04 | 0.75 | 0.29 |
Listed time points are corrected for daylight-saving time, such that the sun was at the zenith around 12 noon.
The photic zone is defined as the zone where there is a net photosynthesis (i.e., the second derivative of the oxygen concentration profile is negative).
The light intensity at daybreak was too low to give rise to a net photosynthesis. The net photosynthesis is thus the net formation rate within the mat, which is negative, as oxygen is being consumed by respiration.
The flux of oxygen from the mat to the overlaying water is negative, indicating a net flux of oxygen from the overlaying water to the mat.
Microscopy of vertical cryosections.
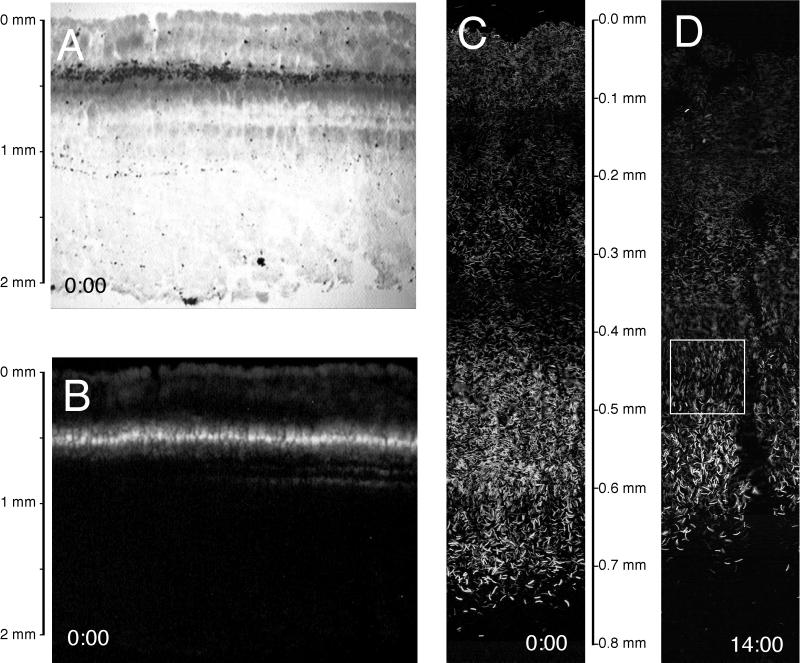
Microscopic examination of the green surface layer revealed a dense population of Synechococcus-shaped cyanobacterial cells with an abundance of nonfluorescent filamentous cell forms resembling Chloroflexus sp. To evaluate the vertical distribution of these cells at different times of the day, we prepared a series of six 40-μm-thick vertical slices of mat material obtained every 2 h by cryosectioning rapidly frozen samples. Figure 3A shows a representative microscale bright-field photograph of a cross section of the upper 2 mm of the mat. Figure 3B is the same field viewed by epifluorescence microscopy. Sections (15 μm thick) of the mat samples were evaluated at higher magnifications. The enlarged views of the upper 900-μm portions of the autofluorescence images at 00:00 (Fig. 3C) and at 14:00 (Fig. 3D) indicate that lamination was present throughout this interval. Prominent features included a diffuse layer of dimly autofluorescent Synechococcus-shaped cyanobacteria extending from the top of the mat to a depth of about 400 μm. A layer of an apparent abiotic mineral precipitate, extending from 400 to 500 μm was also visible in bright-field images (Fig. 3A). Particularly notable was a densely packed layer of brightly autofluorescent Synechococcus-shaped cyanobacterial cells extending from 400 to 700 μm. Deeper, thin autofluorescent layers were also apparent in some areas of some vertical sections (e.g., Fig. 3B).
FIG. 3.
Images from 40-μm-thick vertical slices through a 61°C Mushroom Spring microbial mat sample at midnight (0:00) shown under bright-field (A) and epifluorescence (B) microscopy. (C) Enlarged view of epifluorescence image at midnight (0:00). (D) Enlarged view of epifluorescence image at 14:00. The boxed area in panel D is shown at a higher magnification in Fig. 5A.
It was evident that the surface of the mat, as well as the deeper laminations, were not completely planar, though we tried to select sampling sites with a smooth surface and a homogenous mat. The surface topography can be evaluated from vertical slices, such as that shown in Fig. 3A. This topography imposes a problem when attempting to obtain horizontal mat slices corresponding to a given depth by use of a cryostat. The surface topography and unevenness of the underlying laminations reduce the vertical resolution obtained in individual horizontal mat slices, as each slice will contain mixtures of cells from different vertical layers.
Vertical stratification of genotypes.
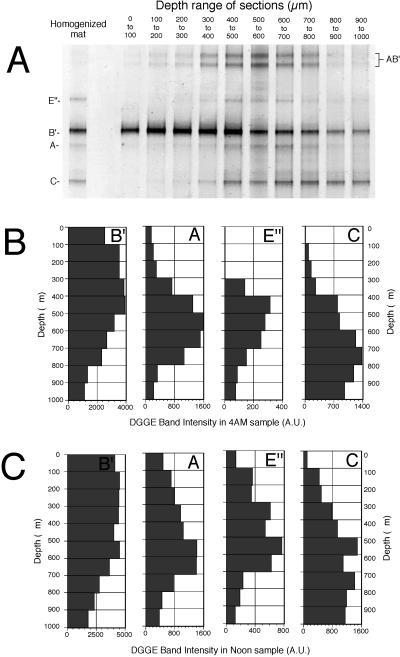
The results of the DGGE analyses of the horizontal cryosections presented in Fig. 4A reveal stratification of 16S rRNA-defined populations. The lane labeled homogenized mat shows the DGGE profile generated using DNA extracted from the intact 2.0-mm surface layer. The major bands identified by 16S rRNA sequencing are indicated along the sides of the gel. All sequences detected in this section of Mushroom Spring mat matched those that have been previously detected in similar temperature regions of the Octopus Spring mat (9, 12). The sequences of bands A and B′ are cyanobacterial, that of band C is green nonsulfur bacteria-like, and that of band E" is green sulfur bacteria-like. Bands AB′, near the top of the gel, are heteroduplex PCR products that form during the PCR by annealing between single strands of types A and B′, which have high sequence similarity (12). The adjacent lanes (Fig. 4A) are DGGE profiles generated from 100-μm-thick cryostat sections through the vertical aspect of the mat. The B′ band appeared more intense in the upper sections of the mat, while the others appeared more intense with depth. The differences were readily visible when plotting the intensity curve of each lane in a scan of a gel image (not shown). Individual bands were displayed as prominent peaks whose area could be quantified. Digitized images not only permitted quantification of band intensities but also summing of homo- and heteroduplex bands to account for all PCR products of a specific sequence type (12). Figure 4B presents graphs of the summed intensities of DGGE bands containing A, B′, C, and E" 16S rRNA gene segments along the vertical interval through the top 1 mm of mat sampled at 04:00. The intensity of cyanobacterial band B′ was highest in the upper 500 μm of the mat and then decreased gradually below 500 μm (Fig. 4B). The intensity of the cyanobacterial band A was high in the interval from 400 to 800 μm and was maximal in the 500-to-600-μm interval. The highest intensities of band A coincided with the interval over which the intensely fluorescent lower cyanobacterial layer was observed (compare Fig. 3B and C with Fig. 4B). The relative distributions of A and B′ bands are nicely reflected in the two heteroduplex bands AB′ on Fig. 4A. The maximum intensity of the heteroduplex bands is found at depths where both B′ and A populations co-occur. The green sulfur bacteria-like band E" was below detection in the first 300-μm interval, but its intensity increased to a maximum in the 400-to-500-μm interval and gradually decreased with depth. The green nonsulfur bacterium-like band C was below detection in the upper 100-μm interval, but its intensity gradually increased to a maximum over the 700-to-800-μm interval and decreased slightly over the 800-to-1,000-μm interval.
FIG. 4.
Vertical distribution of 16S rRNA-defined genotypes in the 61°C Mushroom Spring microbial mat. (A) DGGE gel of the upper 2-mm cyanobacterial layer and of 100-μm-thick horizontal cryostat sections through the upper 1 mm of the mat's surface layer sampled at 04:00. Single letters correspond to Octopus Spring 16S rRNA sequence types: A and B′, cyanobacterial; C, green nonsulfur bacteria-like; E", green sulfur bacteria-like. AB′ bands are heteroduplex molecules (see text). (B and C) Bar graphs show the intensities of individual DGGE bands shown in panel A with depth from samples collected at night (04:00) (B) and at noon (12:00) (C). The intensities are depicted in arbitrary units (A.U.).
Does diel migration occur?
The stratification of cyanobacterial morpho- and genotypes was remarkably constant during the 26 h of investigation (as exemplified in day-night comparisons in Fig. 3 and 4). Only minor differences between individual samples were observed, but no large-scale changes in the banding patterns could be discerned. We were thus unable to detect any reproducible diel migration of cyanobacterial morpho- or genotypes within the 26-h investigation.
Vertical midday reorientation of cyanobacteria in a defined band within the mat.
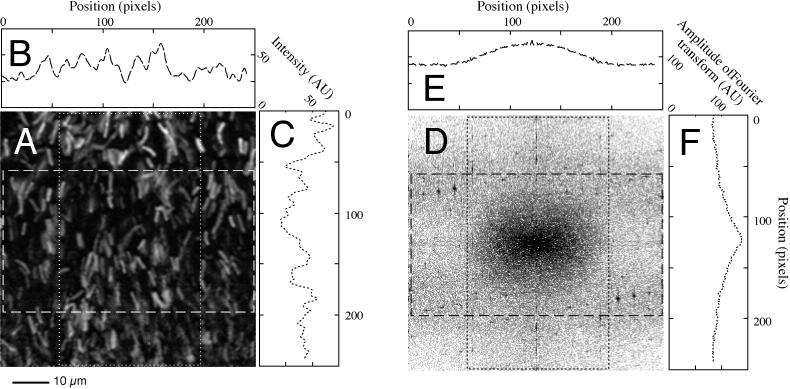
A close inspection of vertical transects through the mat assembled from a continuous collage of microscopic images revealed an apparent reorientation of the cells within a narrow band found at a depth of 400 to 800 μm at midday. The cyanobacteria were randomly oriented at all positions within the mat, except for the four midday transects (obtained from 10:00 to 14:00). In these transects, a 100- to 200-μm-thick subsurface band of vertically oriented Synechococcus cells (e.g., Fig. 3D) was observed. The degree of orientation and the exact depth interval (ranging from 400 to 800 μm below the surface) at which the band was found differed between the investigated transects. The prominent band of vertically oriented cells was not observed in any of the seven transects obtained at other times of day. To objectively evaluate the cell orientation, we used quantitative image analysis. It was impossible to delineate single cells and measure their individual orientation using Marr-Hildreth or other conventional detection algorithms (e.g., see reference 38) due to the thickness of the evaluated mat slices (20 μm). Instead, we developed a novel algorithm based on an evaluation of the two-dimensional FFT of randomly chosen areas of 256 by 256 pixels along the original transect. The method is illustrated in Fig. 5. Panel A is an enlarged area from the transect shown in Fig. 3D. The average intensity profiles of the central region of the investigated area in horizontal and vertical directions are shown along their appropriate axes. Figure 5B shows the horizontal intensity profile of the rectangular area delineated by long white dashes. Figure 5C shows the vertical intensity profile of the area delineated by short dashes. Note how the horizontal intensity profile (Fig. 5B) contains many high-frequency oscillations, as it is perpendicular to the predominant cell orientation. The vertical intensity profile (Fig. 5C) contains more low-frequency oscillations as it is parallel to the cell orientation. The FFT of the investigated area (Fig. 5D) thus has a higher amplitude in the high-frequency domain along the horizontal axis than along the vertical axis. The amplitude profiles of the FFT in horizontal and vertical directions (corresponding to the black rectangles in the figure) are shown in Fig. 5E (long dashes) and F (short dashes), respectively. A predominant vertical cell orientation is thus reflected in a larger variance in the amplitude distribution along the horizontal axis (Fig. 5E) than along the vertical axis (Fig. 5F). We can therefore evaluate the average cell orientation without delineating single cells by thresholding the FFT picture and measuring the orientation of the resulting ellipsoid. If the cells are pointing in random directions, the ellipsoid will be circular; however, if the cells are mostly pointing in a particular direction, then the ellipsoid will be oriented perpendicular to the cell orientation. Alternatively, if we are interested only in discriminating between vertically and horizontally oriented cells, we can use the ratio between the standard deviation of the FFT in the vertical direction to that in the horizontal direction. Clearly, the width of the central peak in the FFT amplitude profile in Fig. 5E is greater than that of Fig. 5F. This can be quantified by calculating the standard deviation of the peak in each amplitude profile. If the cells are predominantly vertically oriented, then the standard deviation of the vertical profile (Fig. 5F) divided by the standard deviation of the horizontal profile (Fig. 5E) becomes less than one. Both analysis methods were employed and gave similar results showing almost randomly oriented cells at all depths except for a 100- to 200-μm-thick band found within the depth range from 400 to 800 μm in all midday samples. Within this band, the cells appeared to be predominantly vertically oriented (i.e., standing on ends within the mat).
FIG. 5.
Estimating average cell orientation in the Mushroom Spring 61°C mat at 14:00 based on a two-dimensional fast Fourier transformation and subsequent analysis of the frequency distribution. (A) High magnification of part of a vertical transect through a 14:00 h sample (same as the boxed region of Fig. 3D). (B) Horizontal intensity profile through the part of panel A delineated by the horizontal rectangle. (C) Vertical intensity profile through the part of panel A delineated by the vertical rectangle. (D) Amplitude of Fourier transform of panel A. (E) Horizontal amplitude profile through horizontal rectangle in Fourier transform shown in panel D. (F) Vertical intensity profile through vertical rectangle in Fourier transform shown in panel D. For more extensive explanation, see Results.
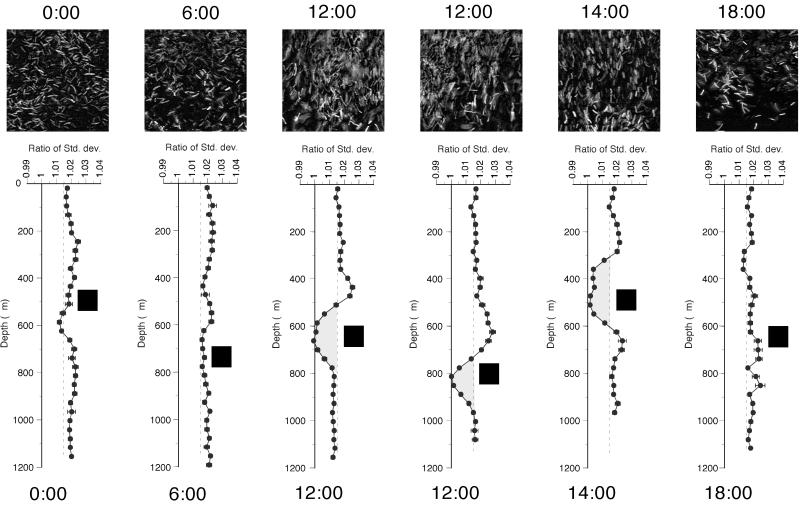
The average ratios at different positions down through the mat are depicted in Fig. 6, with error bars indicating 95% confidence limits. The measured ratio is very stable with an average value of 1.015 at most depths. The average value is significantly different from the ratio of 1.0 expected for randomly oriented cells. Three explanations for this finding can be envisioned: (i) horizontal noise in the acquired digital images of the biofilm due to interlacing of TV frames, (ii) a general slight preference for a horizontal cell orientation for all cells in the mat environment, and (iii) cutting artifact due to a cutting angle perpendicular to the mat surface. The stability of the ratio enabled us to identify layers where the cells are not randomly oriented. The predominantly vertical orientation of cells in deeper layers in the midday samples is reflected in a conspicuously low ratio of standard deviations. The change in ratio is small, but analysis of artificial test pictures with strongly oriented rectangular-shaped objects (e.g., vertical ±30°) showed an equivalent and moderate change in the ratio. In some noon transects, we found evidence of a layer of horizontally oriented cells above the vertically oriented cells. A total of 11 transects from different mat samples were analyzed. Of these, only the four samples obtained around noon showed signs of vertically oriented cells (one at 10:00, two at 12:00, and one at 14:00). The remaining samples (two at 0:00, two at 6:00, one at 16:00, and two at 18:00) gave no strong indication of oriented cells at any depth. We have included transects through two different samples obtained at 12:00 in order to show the sample to sample variation in band position.
FIG. 6.
Orientation of Synechococcus cells with depth in the Mushroom Spring 61°C mat expressed as the standard deviation of the Fourier transform in the vertical plane divided by the standard deviation of the Fourier Transform in the horizontal plane. Two different transects through a noon sample (12:00) show sample-to-sample variation. The micrographs above the analysis result are sample pictures taken at the depths indicated by black squares in the representative analysis profiles. The dashed lines represent the overall average ratio of 1.015. Values of <1.015, accentuated by the shaded area in the midday transects, denote the presence of vertically oriented cells.
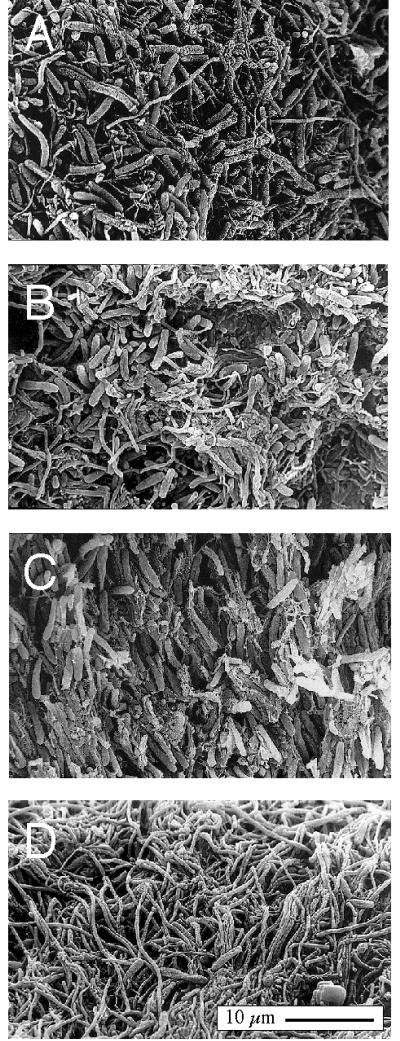
We confirmed the orientation of the cells by scanning electron microscopy as shown in Fig. 7. Figure 7A shows the surface of the mat containing sausage-shaped Synechococcus and filamentous cells viewed from above. Figure 7B shows randomly oriented Synechococcus cells in a cut mat ca. 100 μm beneath the surface. Figure 7C shows vertically oriented Synechococcus cells 700 μm below the surface. Finally, Fig. 7D shows the undermat, seen from below. It is evident how the filaments closely knit the mat together and provide tensile strength.
FIG. 7.
Scanning electron microscopy picture of vertical cryosections of samples from the Mushroom Spring 61°C mat collected at noon. (A) Surface view with randomly oriented sausage-shaped Synechococcus cells. (B) Area near the surface (approximately 100 μm deep) also showing randomly oriented cells. (C) Vertically oriented Synechococcus cells found 700 μm below surface (D) Network of filamentous Chloroflexus-like organisms in undermat viewed from below. The scale bar in panel D applies to all panels.
DISCUSSION
We observed several differences in physiology and behavior associated with Synechococcus cells that are distributed at different depths in the Mushroom Spring mat community. Oxygen microprofiles demonstrated the dynamic nature of chemical gradients that develop in a microbial mat during the diel light cycle. By calculating the vertical distribution of net metabolic activities that are required to produce such profiles, these profiles revealed microbial activities (25) that are markedly different in different parts of the mat. Our interpretation of these profiles requires an accurate surface determination, which is difficult to obtain for in situ measurements, yet the chosen criteria were confirmed by a concurrent stabilization of the sensor signal when entering the mat and by the constant thickness of the diffusive boundary layer above the mat surface. The net photosynthesis within the surface layer of the mat increases dramatically with light intensity early in the morning, but around noon, the net activity in the uppermost surface layer (above 300 μm) is strongly reduced. This might be due to UV damage (3, 14, 15, 30) and/or to increased photorespiration as CO2 becomes depleted and the pH and oxygen concentration increase (19). The recent study by Miller et al. (30) using cell suspensions from a comparable site in the nearby Octopus Spring with an average temperature of 70°C indicated that UV inhibition reduced the photosynthetic rate by about a third compared to UV-shielded incubations. This agrees well with our observations. The noon to afternoon inhibition of photosynthesis is reflected in the oxygen contribution by the mat to the overlaying water being higher in the morning than in the afternoon at the same light intensity (i.e. the hysteresis effect in Fig. 1, oxygen concentration profiles in Fig. 2, and efflux to overlaying water in Table 1). The upper 200 μm of the mat developed a high net oxygen production in the early morning (Fig. 2, 8.17 profile) which disappeared around noon (Fig. 2, 10:20 and 12:23) to be replaced by a net oxygen consumption in the early afternoon (Fig. 2, 15:26). As the zone of maximum net photosynthesis shifted down through the mat, the longer diffusive pathway for effusive oxygen through respiring mat layers reduced the oxygen efflux from the mat (Table 1). However, the overall inhibition of net photosynthesis (Table 1) was not nearly as dramatic as the decline in photosynthetic rates in the afternoon observed by Miller et al. (30). The discrepancy may be due to the fact that we studied a thick mat, whereas Miller et al. studied a thin biofilm, or to the approximately 10°C difference in average temperature. It also seems possible that differences might relate to sample treatment. Miller et al. studied homogenized samples, whereas our measurements were made in situ. Our previous DGGE results suggested that in some cases two cyanobacterial populations co-occur in the biofilm (12). If, as in the mat we studied, one of these populations is acclimated or adapted to low light intensity and/or UV irradiation, it may have been inhibited by exposure to high light intensity or UV light during incubation.
The net photosynthetic activity in deeper layers of the mat (below 300 μm) is apparently not inhibited by the high light intensities around noon, possibly due to a more adequate CO2 supply from below (28) and an absence of harmful UV irradiation below the first few hundred micrometers as the UV light is rapidly attenuated by light scatter and absorption (25–27).
Marked stratification of cell morphotypes in recognizable layers revealed by microscopy and the brighter autofluorescence of the cells in the deeper layer also strongly suggest that the Synechococcus cells residing deeper in the mat are different than those nearer the mat surface. Without genetic information, this could be interpreted either as acclimation of one genetic population or as adaptations of more than one genetic population. The vertical variations in cyanobacterial DGGE band intensities correlated well with the phenotypically different Synechococcus populations observed at different depths, suggesting that they are genetically distinct populations that are adapted differently to the unique microenvironments in the upper and lower regions of the 1-mm-thick green layer. The ca. 5% difference between the 16S rRNA sequences of B′ and A populations is sufficiently large to consider them different species, but the ecological uniqueness of these populations seems a more natural way to make such a distinction (48). Two technical problems could weaken the inference that ecologically distinct Synechococcus populations occur at different depths in the mat. One is the known difficulty associated with preferential amplification of certain templates in a complex mixture (i.e., PCR bias [47]), which makes it impossible to enumerate populations in the environment based on absolute intensities of PCR-amplified bands on a DGGE gel. The second problem is that due to the uneven contours of the Mushroom Spring mat laminations (see surface of vertical slice in Fig. 3), cryosectioning did not result in exact separation of extracted nucleic acids originating from visibly prominent layers. Because of the mixing of natural laminations, the intervals over which the populations are actually distributed are probably less broad than the results indicate. The observed correlation between genetic and physiologic populations is nevertheless compelling. A similar correlation has been observed with marine Prochlorococcus populations associated with the surface or with the deep chlorophyll maximum (10) which have been shown to be genetically distinct populations uniquely adapted to high or low light intensities (32).
Since our work was conducted in Mushroom Spring, local spring-to-spring population distributions could be compared. Mushroom Spring is located over a small rise approximately 0.5 km from Octopus Spring. The mats in the effluent channels of the two springs are indistinguishable in most respects. One notable difference is the absence of temperature fluctuations at Mushroom Spring. The temperature at the Mushroom Spring study site in this experiment did not vary by more than 1°C from 61°C. Most sites in the Octopus Spring effluent channel experience cycling on the order of 5° or 10°C over a several-minute interval (9). Still, the DGGE profiles and the 16S rRNA sequences recovered from the Mushroom Spring site were indistinguishable from those detected in a site with temperatures of 53 to 63°C in the Octopus Spring mat (12). Previous DGGE studies on the Octopus Spring effluent channel mat have shown that these same populations occupy defined intervals along the thermal gradient as well (12). Interestingly, two or more cyanobacterial populations were observed at each temperature-defined site in the Octopus Spring study, but because of the periodic temperature fluctuations in this system (see below), the coexistence of multiple cyanobacterial populations (9, 12) might be explained by the short-term availability of adequate growth temperatures for each population. This explanation now seems unlikely, given the co-occurrence of the A and B′ type populations at the stable 61°C site in this experiment. It seems more probable that cyanobacterial populations coexist within the same temperature range because each is adapted to fill niche space created by light and/or chemical gradients within the vertical aspect of the mat. That is, specialization to both temperature and parameters that vary in the vertical aspect of the mat (possibly light intensity) explain the diversity of 16S rRNA-defined genetic populations observed in such mats. There is microelectrode evidence of the existence of multiple temperature-adapted populations of oxygenic photosynthetic populations within the upper 300-μm layer of a single Octopus Spring mat sample (M. Kühl, unpublished data). If, as in Mushroom Spring, there is only one 16S rRNA-defined genotype present in this layer (i.e., type B′), these temperature-adapted populations may have identical 16S rRNA sequences. We have used the intervening transcribed spacer region that separates the 16S and 23S rRNA genes to detect multiple genetic variants with the type B′ 16S rRNA sequence, and we are currently examining their temperature distributions (T. Papke, unpublished data).
Movements of mat microorganisms in response to diel changes in light availability are well-known (3, 16) and we have recently reported on the motility of Synechococcus spp. (39). Hence, we searched for diel variations in the cyanobacterial distribution within the mat, but we were unable to detect any mass migration by molecular methods or by microscopy. Distribution differences between samples retrieved at different time points were observed, but we did not find a consistent pattern of temporal mass migrations. The unexpected detection of vertically oriented cyanobacteria in a defined layer more than 400 μm below the mat surface in all midday samples, but in none of the other samples, may reveal what physicochemical factor controls vertical zonation of Synechococcus populations in the hot spring microbial mats. Diel reorientation of chloroplasts within plant cells to optimize photosynthesis and reduce photic damage at peak light intensities is well established (reviewed in reference 22). In leaves, self-shading and light tunneling can reduce the light intensity impinging on light-saturated photosystems, if the chloroplasts are concentrated on the cell walls parallel to the direction of the incident light. However, even among small unicellular algae, where self-shading effects are presumed to be negligible, an upright orientation of motile epipelic diatoms around noon on sediment surfaces (23) was observed. Absorption of light by the photosystems is more efficient if the incident light is perpendicular to the photosynthetic membrane (22). An organism that reduces the amount of photosynthetic membranes that is perpendicular to the incident light by reorienting itself will reduce the photon load on its photosystems and possibly avoid damage by excessive radiation. The photosynthetic membranes of Synechococcus sp. are oriented parallel to the cell wall (4). When the rod-shaped cells assume an upright position in the mat, most incident photons will be parallel to the photosynthetic membranes and thus less likely to cause photorespiration within photon-saturated photosystems.
Changing cell orientation within a mat matrix is likely to be a very efficient way to avoid photic damage due to excess light. It would be an energy-efficient alternative to vertical mass migration through a gelatinous densely packed mat matrix. It would be well suited for small unicellular organisms that possess a relatively inefficient “wriggling” motion such as has been described for thermophilic Synechococcus sp. (39). Short random walks would eventually reorient the cells into an optimal orientation. We thus propose two mutually exclusive strategies for cyanobacteria to cope with daily changing light intensities in mat communities. One, mass migration, is most effectively accomplished by long filamentous cyanobacteria that sometimes even lay down “tracks” in the form of slime sheaths, that ensure a directional movement. The alternative strategy requires relatively short rod-shaped cells reorienting themselves relative to the incident light field without mass migration. It should be noted that the surface population of Synechococcus sp. does not reorient during the diel cycle, though the surface populations are sensitive to light, as indicated by the hysteresis effect shown in Fig. 1B and the net photosynthesis measurements shown in Fig. 2.
Assuming that the cells in the narrow band between 400 and 800 μm below the surface reorient themselves in noon samples to avoid saturation of their photosystems, we are led to two hypotheses. First, the Synechococcus cells within the band where the cells reorient constitute a unique species compared to overlying Synechococcus populations. Because the light intensity is higher above the band, the cells nearer the surface would have even more reason to reorient at midday. The cell type found at the surface must thus be a different organism. The “reorienting cells” have more light than they can cope with around noon. They would thus have no advantage of living higher in the mat and there is no indication of competition with the species living above it. Second, the distribution of the reorienting cell type is controlled directly by the available light field, and not indirectly by a related parameter such as oxygen or CO2 concentration, as the observed reorientation of the cells would not change the chemical microenvironment to which the cell is exposed.
To evaluate the proposed hypotheses, we need more information on the available light field within the mat at different intensities and depths below the mat surface. Direct measurements of the light field within the mat, made at high spatial resolutions using fiber-optic microprobes that measure radiance, irradiance, and scalar irradiance must be obtained before correlations between light gradients and population distributions can be evaluated (27). Detecting UV screening molecules, such as scytonemin (13) or mycosporine-like compounds (14) among cultivated Octopus Spring isolates might also provide evidence to support the hypothesis that these populations are differentially adapted to light (15, 39).
Vertical stratifications of cyanobacterial and other bacterial populations have been demonstrated at the microscale in microbial mats where cell shape permits populations to be differentiated, such as those in the hypersaline mats of Guerrero Negro, Baja California Sur, Mexico (3, 8, 34). Additional layers in the Mushroom Spring mat beneath the green surface layer, which appeared orange or peach in color, were evident in the bright-field image (Fig. 3A). It has been reported that Chloroflexus aurantiacus commonly forms an orange, photoheterotrophic layer under the cyanobacterial layer in many nonsulfidic hot spring mats (18, 44). Although the 16S rRNA sequence of C. aurantiacus was not observed in this study, another sequence, Octopus Spring type C, belonging to the phylogenetic lineage containing the green nonsulfur bacteria, was detected. The intensity of the type C DGGE band was greatest at depths from about 700 to 1,000 μm, which was below the cyanobacterial layers. This agrees well with the zonation observed by scanning electron microscopy indicating an increasing abundance of filamentous bacteria with depth which become predominant below 1 mm (Fig. 7). A similar pattern over a 1-mm interval, showing multiple stratified cyanobacterial populations above deeper Chloroflexus populations, has been observed in electron microscopy profiles of the Guerrero Negro hypersaline mats as well (8). Perhaps the presence of the type C population at depths below that of the cyanobacterial layers indicates adaptation to infrared light. The green sulfur bacterium-like population E" distribution appears to be similar to that of the type A cyanobacterial population. It is virtually absent from the uppermost mat samples. The peak band intensity is found between 300 and 600 μm below the surface and the intensity decreases at greater depth. The physiological role of this population cannot be inferred, as the sequence falls just outside the monophyletic clade including all known green sulfur bacteria (35). Nevertheless, it is interesting that similar vertical positioning of (16S rRNA-detected) oxygenic phototrophic bacteria, green sulfur bacterium-like, and green nonsulfur bacterium-like bacteria has been observed in the marine (open ocean) environment, albeit over a much greater depth interval (17, 20).
Our results reveal a highly ordered vertical structure in the 60°C cyanobacterial mat in Mushroom Spring, with distinct species occurring within different microenvironments in the upper 1-mm depth interval. In contrast to mats constructed by motile filamentous cyanobacteria, Synechococcus populations with unique adaptations to light appear to be spatially fixed at different vertical intervals, though at least one low-light-adapted population is able to change cell orientation to minimize light exposure. These results indicate that both light and temperature specialization may explain the adaptive radiations of closely related cyanobacterial populations inhabiting such mats, previously attributed to temperature specialization alone (12). In interpreting the significance of clusters of closely related genetic populations, it is wise to keep an open mind as to the variety of environmental variables that might have driven diversification. Similar vertical patterns have been observed in preliminary microscopic studies of lower and higher temperature sites in the Mushroom Spring cyanobacterial mat, but additional studies are needed to establish whether light adaptations explain the co-occurrence of other cyanobacterial populations detected by their 16S rRNA sequences in samples from these temperatures. One consequence of analyses of microscale vertical microbial mat structure is that the position at which photosynthesis occurs varies with light intensity during a diel cycle, which in turn affects the transport of O2 between the mat and the water above. Another is that experimental approaches that disrupt such natural positioning may lead to measurement error.
ACKNOWLEDGMENTS
We thank Mary Bateson, Michael Friedrich, Michael Kühl, and Steve Nold for helpful discussions.
This work was funded in part by NASA (NAGW-5-3652), NSF (BSR-9708136), the Danish National Science Foundation, and the Carlsberg Foundation (980406/40-839).
REFERENCES
- 1.Bateson M M, Ward D M. Photoexcretion and fate of glycolate in a hot spring cyanobacterial mat. Appl Environ Microbiol. 1988;54:1738–1743. doi: 10.1128/aem.54.7.1738-1743.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauld J, Brock T D. Ecological studies of Chloroflexus, a gliding photosynthetic bacterium. Arch Microbiol. 1973;92:267–284. [Google Scholar]
- 3.Bebout B M, Garcia-Pichel F. UV B-induced vertical migrations of cyanobacteria in a microbial mat. Appl Environ Microbiol. 1995;61:4215–4222. doi: 10.1128/aem.61.12.4215-4222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brock T D. Thermophilic microorganisms and life at high temperature. New York, N.Y: Springer Verlag; 1978. [Google Scholar]
- 5.Castenholz R W. Ecology of blue-green algae in hot springs. In: Carr N G, Whitton B A, editors. The biology of blue-green algae. Vol. 1. Oxford, United Kingdom: Blackwell; 1973. pp. 379–414. [Google Scholar]
- 6.Cohen Y, Rosenberg E, editors. Microbial mats: physiological ecology of benthic communities. Washington, D.C.: American Society for Microbiology; 1989. [Google Scholar]
- 7.Crank J. The mathematics of diffusion. Oxford, United Kingdom: Clarendon Press; 1983. [Google Scholar]
- 8.D'Amelio E D, Cohen Y, Marais D J D. Comparative functional ultrastructure of two hypersaline submerged cyanobacterial mats: Guerrero Negro, Baja California Sur, Mexico and Solar Lake, Sinai, Egypt. In: Cohen Y, Rosenberg E, editors. Microbial mats: physiological ecology of benthic communities. Washington, D.C.: American Society for Microbiology; 1989. pp. 97–113. [Google Scholar]
- 9.Ferris M J, Muyzer G, Ward D M. Denaturing gradient gel electrophoresis profiles of 16S rRNA-defined populations inhabiting a hot spring microbial mat community. Appl Environ Microbiol. 1996;62:340–346. doi: 10.1128/aem.62.2.340-346.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferris M J, Palenik B. Niche adaptation in ocean cyanobacteria. Nature. 1998;396:226–228. [Google Scholar]
- 11.Ferris M J, Ruff-Roberts A L, Kopczynski E D, Bateson M M, Ward D M. Enrichment culture and microscopy conceal diverse thermophilic Synechococcus populations in a single hot spring mat habitat. Appl Environ Microbiol. 1996;62:1045–1050. doi: 10.1128/aem.62.3.1045-1050.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferris M J, Ward D M. Seasonal distributions of dominant 16S rRNA-defined populations in a hot spring microbial mat examined by denaturing gradient gel electrophoresis. Appl Environ Microbiol. 1997;63:1375–1381. doi: 10.1128/aem.63.4.1375-1381.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Pichel F, Castenholz R W. Characterization and biological implications of scytonemin, a cyanobacterial sheath pigment. J Phycol. 1991;27:395–409. [Google Scholar]
- 14.Garcia-Pichel F, Castenholz R W. Occurrence of UV-absorbing, mycosporine-like compounds among cyanobacterial isolates and an estimate of their screening capacity. Appl Environ Microbiol. 1993;59:163–169. doi: 10.1128/aem.59.1.163-169.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia-Pichel F, Castenholz R W. On the significance of solar ultraviolet radiation for the ecology of microbial mats. In: Stahl L J, Caumette P, editors. Microbial mats: structure, development and environmental significance. Heidelberg, Germany: Springer-Verlag; 1994. pp. 77–84. [Google Scholar]
- 16.Garcia-Pichel F, Mechling M, Castenholz R W. Diel migrations of microorganisms in a hypersaline microbial mat. Appl Environ Microbiol. 1994;60:1500–1511. doi: 10.1128/aem.60.5.1500-1511.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giovannoni S J, Rappe M S, Vergin K L, Adair N L. 16S rRNA genes reveal stratified open ocean bacterioplankton populations related to the green non-sulfur bacteria. Proc Natl Acad Sci USA. 1996;93:7979–7984. doi: 10.1073/pnas.93.15.7979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giovannoni S J, Revsbech N P, Ward D M, Castenholz R W. Obligately phototrophic Chloroflexus: primary production in anaerobic hot spring microbial mats. Arch Microbiol. 1987;147:80–87. [Google Scholar]
- 19.Glud R N, Ramsing N B, Revsbech N P. Photosynthesis and photosynthesis-coupled respiration in natural biofilms measured by use of oxygen microsensors. J Phycol. 1992;28:51–60. [Google Scholar]
- 20.Gordon D A, Giovannoni S J. Detection of stratified microbial populations related to Chlorobium and Fibrobacter species in the Atlantic and Pacific ocean. Appl Environ Microbiol. 1996;62:1171–1177. doi: 10.1128/aem.62.4.1171-1177.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grasshoff K. Determination of oxygen. In: Grasshoff K, Ehrhardt M, Kremling K, editors. Methods of seawater analysis. Basel, Switzerland: Verlag Chemie; 1983. p. 419. [Google Scholar]
- 22.Haupt W, Scheuerlein R. Chloroplast movement. Plant Cell Environ. 1990;13:595–614. [Google Scholar]
- 23.Jönsson B, Sundbäck K, Nilsson C. An upright life-form of an epipelic motile diatom: on the behavior of Gyrosigma balticum. Eur J Phycol. 1994;29:11–15. [Google Scholar]
- 24.Jørgensen B B. Light penetration, absorption, and action spectra in cyanobacterial mats. In: Cohen Y, Rosenberg E, editors. Microbial mats: physiological ecology of benthic microbial communities. Washington, D.C.: American Society for Microbiology; 1989. pp. 123–137. [Google Scholar]
- 25.Kühl M, Glud R N, Ploug H, Ramsing N B. Microenvironmental control of photosynthesis and photosynthesis-coupled respiration in an epilithic cyanobacterial biofilm. J Phycol. 1996;32:799–812. [Google Scholar]
- 26.Kühl M, Jørgensen B B. Spectral light measurements in microbenthic phototrophic communities with a fiber-optic microprobe coupled to a sensitive diode array detector. Limnol Oceanogr. 1992;37:1813–1823. [Google Scholar]
- 27.Kühl M, Lassen C, Jørgensen B B. Optical properties of microbial mats: light measurements with fiber-optic microprobes. In: Stal L J, Caumette P, editors. Microbial mats: structure, development and environmental significance. G35. Berlin, Germany: Springer Verlag; 1994. pp. 149–166. [Google Scholar]
- 28.Lassen C, Glud R N, Ramsing N B, Revsbech N P. A method to improve the spatial resolution of photosynthetic rates obtained by oxygen microsensors. J Phycol. 1998;34:89–93. [Google Scholar]
- 29.Li Y-H, Gregory S. Diffusion of ions in sea water and in deep-sea sediments. Geochim Cosmochim Acta. 1974;38:703–714. [Google Scholar]
- 30.Miller S R, Wingard C E, Castenholz R W. Effects of visible light and UV radiation on photosynthesis in a population of a hot spring cyanobacterium, a Synechococcus sp., subjected to high-temperature stress. Appl Environ Microbiol. 1998;64:3893–3899. doi: 10.1128/aem.64.10.3893-3899.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Millero F J. Appendix Table 25. In: Skirrow J P R G, editor. Chemical oceanography. 2nd ed. Vol. 1. London, United Kingdom: Academic Press; 1974. p. 576. [Google Scholar]
- 32.Moore L R, Rocap G, Chisholm S W. Physiology and molecular phylogeny of coexisting Prochlorococcus ecotypes. Nature. 1998;393:464–467. doi: 10.1038/30965. [DOI] [PubMed] [Google Scholar]
- 33.Nold S C, Ward D M. Diverse Thermus species inhabit a single hot spring microbial mat. System Appl Microbiol. 1995;18:274–278. doi: 10.1016/s0723-2020(11)80398-x. [DOI] [PubMed] [Google Scholar]
- 34.Nübel U, Garcia-Pichel F, Kühl M, Muyzer G. Quantifying microbial diversity: morphotypes, 16S rRNA genes, and carotenoids of oxygenic phototrophs in microbial mats. Appl Environ Microbiol. 1999;65:422–430. doi: 10.1128/aem.65.2.422-430.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Overmann J, Tuschak C. Phylogeny and molecular fingerprinting of green sulfur bacteria. Arch Microbiol. 1997;167:302–309. doi: 10.1007/s002030050448. [DOI] [PubMed] [Google Scholar]
- 36.Pearl H W, Shimp S L. Preparation of filtered plankton and detritus for study with scanning electron microscopy. Limnol Oceanogr. 1973;18:802–804. [Google Scholar]
- 37.Peary J A, Castenholz R W. Temperature strains of a thermophilic blue-green algae. Nature. 1964;202:720–721. [Google Scholar]
- 38.Ramsing N B, Ferdelman T, Fossing H, Thamdrup B, Andersen F. Distribution of bacterial populations in a stratified fjord (Mariager Fjord, Denmark) quantified by in situ hybridization and related to chemical gradients in the water column. Appl Environ Microbiol. 1996;62:1391–1404. doi: 10.1128/aem.62.4.1391-1404.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramsing N B, Ferris M J, Ward D M. Light-induced motility of thermophilic Synechococcus isolates from Octopus Spring, Yellowstone National Park. Appl Environ Microbiol. 1997;63:2347–2354. doi: 10.1128/aem.63.6.2347-2354.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramsing N B, Kühl M, Jørgensen B B. Distribution of sulfate-reducing bacteria, H2S, and O2 in photosynthetic biofilm determined by oligonucleotide probes and microelectrodes. Appl Environ Microbiol. 1993;59:3840–3849. doi: 10.1128/aem.59.11.3840-3849.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Revsbech N P. An oxygen microelectrode with a guard cathode. Limnol Oceanogr. 1989;34:474–478. [Google Scholar]
- 42.Revsbech N P. Analysis of microbial mats by use of electrochemical microsensors: recent advances. In: Stal L J, Caumette P, editors. Microbial mats: structure, development, and environmental significance. Berlin, Germany: Springer-Verlag; 1994. pp. 135–147. [Google Scholar]
- 43.Revsbech N P, Christensen B P, Nielsen L P. Microelectrode analysis of photosynthetic and respiratory processes in microbial mats. In: Cohen Y, Rosenberg E, editors. Microbial mats: physiological ecology of benthic microbial communities. Washington, D.C.: American Society for Microbiology; 1989. pp. 153–162. [Google Scholar]
- 44.Revsbech N P, Ward D M. Microelectrode studies of interstitial water chemistry and photosynthetic activity in a hot spring microbial mat. Appl Environ Microbiol. 1984;48:270–275. doi: 10.1128/aem.48.2.270-275.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Revsbech N P, Ward D M. Microprofiles of dissolved substances and photosynthesis in microbial mats measured with microelectrodes. In: Cohen Y, Castenholz R W, Halvorson H O, editors. Microbial mats: stromatolites. New York, N.Y: Alan R. Liss, Inc.; 1984. pp. 171–188. [Google Scholar]
- 46.Schulz H, Jørgensen B B, Fossing H, Ramsing N B. Community structure of filamentous, sheath-building sulfur bacteria, Thioploca spp., off the coast of Chile. Appl Environ Microbiol. 1996;62:1855–1862. doi: 10.1128/aem.62.6.1855-1862.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suzuki M T, Giovannoni S J. Bias caused by template annealing in the amplification of mixtures of 16S rRNA genes by PCR. Appl Environ Microbiol. 1996;62:625–630. doi: 10.1128/aem.62.2.625-630.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ward D M. A natural species concept for procaryotes. Curr Opin Microbiol. 1998;1:271–277. doi: 10.1016/s1369-5274(98)80029-5. [DOI] [PubMed] [Google Scholar]
- 49.Ward D M, Ferris M J, Nold S C, Bateson M M. A natural view of microbial biodiversity within hot spring cyanobacterial mat communities. Microbiol Mol Biol Rev. 1998;62:1353–1370. doi: 10.1128/mmbr.62.4.1353-1370.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ward D M, Ferris M J, Nold S C, Bateson M M, Kopczynski E D, Ruff-Roberts A L. Species diversity in hot spring microbial mats as revealed by both molecular and enrichment culture approaches—relationship between biodiversity and community structure. In: Stal L J, Caumette P, editors. Microbial mats: structure, development and environmental significance. G. 1996. pp. 33–44. 35. Springer Verlag, Berlin, Germany. [Google Scholar]
- 51.Ward D M, Santegoeds C M, Nold S C, Ramsing N B, Ferris M J, Bateson M M. Biodiversity within hot spring microbial mat communities: molecular monitoring of enrichment cultures. Antonie Leeuwenhoek. 1997;71:143–150. doi: 10.1023/a:1000131426164. [DOI] [PubMed] [Google Scholar]
- 52.Ward D M, Weller R, Bateson M M. 16S rRNA sequences reveal numerous uncultivated microorganisms in a natural environment. Nature. 1990;345:63–65. doi: 10.1038/345063a0. [DOI] [PubMed] [Google Scholar]
- 53.Ward D M, Weller R, Shiea J, Castenholz R W, Cohen Y. Hot spring microbial mats: anoxygenic mats of possible evolutionary significance. In: Cohen Y, Rosenberg E, editors. Microbial mats: physiological ecology of benthic microbial communities. Washington, D.C.: American Society for Microbiology; 1989. pp. 3–15. [Google Scholar]