Abstract
Four of five apple cultivars (Golden Delicious, Red Delicious, McIntosh, Macoun, and Melrose) inoculated with Escherichia coli O157:H7 promoted growth of the bacterium in bruised tissue independent of the date of harvest (i.e., degree of apple ripening) or the source of the apple (i.e., tree-picked or dropped fruit). Apple harvest for this study began 4 September 1998 and ended 9 October, with weekly sampling. Throughout this study, freshly picked (<2 days after harvest) McIntosh apples usually prevented the growth of E. coli O157:H7 for 2 days. Growth of E. coli O157:H7 did occur following 6 days of incubation in bruised McIntosh apple tissue. However, the maximum total cell number was approximately 80-fold less than the maximum total cell number recovered from Red Delicious apples. When fruit was stored for 1 month at 4°C prior to inoculation with E. coli O157:H7, all five cultivars supported growth of the bacterium. For each apple cultivar, the pH of bruised tissue was significantly higher and °Brix was significantly lower than the pH and °Brix of undamaged tissue regardless of the source. In freshly picked apples, changes in the pH did not occur over the harvest season. Bruised Golden Delicious, McIntosh, and Melrose apple tissue pHs were not significantly different (tree-picked or dropped), and the °Brix values of McIntosh, Macoun, and Melrose apple tissue were not significantly different. Single-cultivar preparations of cider did not support growth of E. coli, and the cell concentration of inoculated cider declined over an 11-day test period. The rate of decline in E. coli cell concentration in the McIntosh cider was greater than those in the other ciders tested. The findings of this study suggested that the presence of some factor besides, or in addition to, pH inhibited E. coli growth in McIntosh apples.
On several occasions, apple cider (defined for this article as a fresh unfermented juice extracted from apples that has not been heat treated) has been strongly implicated or found responsible for outbreaks of disease caused by Escherichia coli O157:H7. The first reported outbreak of disease presumably caused by this pathogen and associated with the consumption of apple cider occurred in 1980 in Canada (27). In the United States, three reported outbreaks of disease associated with the consumption of apple cider have occurred in the last decade (3, 5, 6). The first of these outbreaks occurred in Massachusetts in 1991 and resulted in 23 reported cases of E. coli O157:H7 infection. The next two outbreaks (Connecticut and Washington State) occurred in the fall of 1996. These two outbreaks resulted in a total of 78 reported cases and the first reported death associated with consumption of this product and E. coli O157:H7 infection (5, 6).
An important unanswered question in regard to these outbreaks is the source(s) of E. coli O157:H7 for contamination of the manufactured cider. Cattle, sheep, deer, birds, and many other animals can serve as reservoirs for this microbe (4, 9, 17, 24, 26, 28). Insects such as the lesser mealworm (Alphitobius diaperinus Panzer) and the house fly (Musca domestica) have also been shown to carry this pathogen (15, 21). Fruit flies (Drosophila melanogaster Meigen) have recently been shown to have the potential to transmit this microbe to apples (16). Apples obtained from the ground beneath the tree (i.e., dropped apples) are highly suspect as the source of E. coli-contaminated apple cider (6, 14), and wild deer have been considered to be a major source for fecal contamination of dropped apples. However, no direct link between E. coli in deer and E. coli in apple cider has been documented. Also, no direct evidence linking the use of dropped apples to fecal contamination of cider has been presented. Cider manufactured using only tree-picked (i.e., obtained directly from the tree) fruit has been found to contain E. coli (8).
One issue seemingly overlooked in discussions on the source of E. coli O157:H7 in cider is the distinction between surface contamination of the fruit due to fecal contact and internal contamination of the fruit due to bacterial growth. Discussions (11) have focused on surface contamination of apples as the source for cider contamination, and regulations to require washing and brushing of apples prior to cider production are being implemented (12). Although the potential for this pathogen to contaminate cider when surface-contaminated apples that have been washed and brushed are used is unknown, the ability of E. coli O157:H7 to grow in damaged apple tissue would present an increased potential for this bacterium to contaminate cider. Recently, Janisiewicz and associates (16) reported the growth of E. coli O157:H7 in damaged apple tissue. For that study, the damaged tissue was produced on Golden Delicious apples without reference to the time of apple harvest. Growth of this microbe in damaged tissue of several different apple cultivars and throughout the harvest season would further extend the potential risk for cider contamination by this pathogen.
This article reports the results of an investigation to determine whether the susceptibility of damaged (i.e., bruised) apple tissue to promote the growth of E. coli O157:H7 varies with the apple cultivar, the degree of apple maturity at harvest, and the source (tree-picked or dropped fruit) of harvested fruit. A weekly analysis of apple cultivar pH and °Brix (soluble sugars) was also done to determine whether changes in these parameters occurred and whether such changes might affect susceptibility.
MATERIALS AND METHODS
Collection and bruising of apple cultivars.
Beginning 4 September 1998, apples were collected on a weekly schedule from a commercial apple orchard located in central Connecticut. Five different apple cultivars (Golden Delicious, Red Delicious, McIntosh, Macoun, and Melrose) were collected on each visit. The same trees were used for each collection date, and apples of similar sizes were harvested. During each collection period, two tree-picked and two dropped apples were obtained for each cultivar and were placed into separate plastic bags. The collected apples were transported to the laboratory and stored at 4°C. One day after collection, each apple was bruised by dropping the fruit onto a tile floor from a height of about 1 m and catching the apple on the rebound. The surface of the apple was not ruptured, and all bruises were similar in size. Two bruises (on opposite sides) were produced on each apple, and the fruit was placed into Nalgene (Nalge Nunc International, Rochester, N.Y.) tubs lined with plastic bags and incubated at room temperature (20 to 25°C). In addition to collecting fresh samples on a weekly basis, tree-picked and dropped apples of each cultivar were harvested on 25 September and stored at 4°C for 1 month prior to bruising.
Inoculation of apples with E. coli O157:H7.
A fluorescent and ampicillin (AMP)-resistant strain of E. coli O157:H7 (E. coli O157:H7 gfp-72ec; P. M. Fratamico, Agricultural Research Service, U.S. Department of Agriculture, Eastern Regional Research Center, Wyndmoor, Pa.) (13) was grown in brain heart infusion broth (Difco Laboratories, Detroit, Mich.)-AMP (50 μg/ml; Sigma Chemical Co., St. Louis, Mo.) for 18 h at 37°C for use in apple inoculation. This bacterial culture was diluted into sterile saline prior to inoculation, and the total cell number of this inoculum was determined by serial dilution plating onto brain heart infusion agar plates containing AMP (50 μg/ml). One day after being bruised, one tree-picked apple and one dropped apple from each cultivar were inoculated with 0.1 ml of the E. coli O157:H7 saline dilution per bruise by hypodermic needle injection into the damaged tissue. The holes in the apple cuticle resulting from the injection procedure were sealed with Scotch tape, and the inoculated fruit was returned to the Nalgene tub for incubation at room temperature.
Analysis of apple tissue for the presence of E. coli O157:H7.
For each apple, both bruises were excised into a sterile beaker 2 days ± 1 h after inoculation. A razor blade was used to cut the apple cuticle around the periphery of the bruise, and a stainless steel spoonula (Fisher Scientific, Pittsburgh, Pa.) was used to scrape the damaged tissue from the apple. A second spoonula (bent at a 90° angle) was used to squeeze the juice from the damaged tissue. The razor blade and spoonulas were soaked in antiseptic detergent (Conflikt; Decon Labs, Bryn Mawr, Pa.) and thoroughly cleaned and dried using Kimwipes (Kimberly-Clarke Corp., Roswell, Ga.) between use on each apple. The juice was collected in a 10-ml plastic culture tube, and the volume was measured. Serial dilutions of the juice were made into TPAP broth (20 g of Difco tryptose broth, 2.5 g of Difco yeast extract, 2 g of glucose, 2.5 g of Na2HPO4 · 7H2O, 2.5 g of NaCl, and 1 g of sodium pyruvate/liter) (19) for plating onto TPAP agar plates. Plates were incubated at 37°C, and, 2 days after plating, green fluorescent colonies observed under a UV lamp (365 nm) were counted to obtain total cell numbers in the collected juice.
Analysis of apple tissue for the presence of E. coli O157:H7 over a prolonged time period.
Six tree-picked apples of the cultivars McIntosh and Red Delicious that had been freshly picked (1 to 2 days) were bruised and inoculated with E. coli O157:H7 as described above, placed in Nalgene tubs, and incubated at room temperature for analysis of bacterial growth over several days. Additionally, six apples of these two cultivars that had been harvested on 25 September and stored for 1 month at 4°C were bruised and injected with this microbe. At various times postinjection, damaged tissues were excised and analyzed for the presence of E. coli O157:H7 as described above.
pH and °Brix measurements.
pHs for undamaged and bruised apple tissue of uninoculated apples were determined using an Accumet AP61 portable pH meter and an AccuFet solid-state electrode (Fisher Scientific) which had been standardized using pH 4.0 and 7.0 standard reagents. Measurements were made by inserting the electrode into the tissue after the apple cuticle was cut. The pH of apple juice extracted from inoculated apples was measured using Hydrion pH paper (pH range, 3.0 to 7.5 in 0.5 increments; Micro Essential Laboratory, Brooklyn, N.Y.). °Brix values for juice samples obtained from uninoculated apples were measured using a Fisherbrand (Fisher Scientific) hand-held Brix refractometer standardized using H2O equilibrated to room temperature.
Small-scale production of cider using a single apple cultivar.
Apple ciders were produced using only two tree-picked apples of the same apple cultivar for each cider. Ciders were produced using McIntosh, Red Delicious, Golden Delicious, and Melrose apples. For each cider, the fruit was washed and cut into slices prior to homogenization in a blender (Waring Products Division, New Hartford, Conn.). The resulting apple pomace was transferred onto cheesecloth and wrapped into a ball. The ball of pomace-containing cheesecloth was hand-squeezed over a funnel leading into a sterile bottle. Washed latex gloves were worn during the squeezing process. The bottle containing the collected extract (i.e., cider) was capped and stored at 4°C until needed. Cider was stored no longer than 1 week prior to use. For experiments in which the growth of mold and bacteria in cider was to be inhibited, cycloheximide (1 mg/ml; Sigma Chemical Co.) and AMP (50 μg/ml) were added to the cider immediately after production.
Statistical analysis of data.
Total bacterial cell recovery following inoculation, pH, and °Brix comparisons among the various apple cultivar samples (harvested as tree-picked or dropped apples and used as fresh or stored fruit) were statistically analyzed using the mean and standard error of each sampling. Means and standard errors were calculated using the results of at least two sample measurements. Comparisons using two sample t test distribution analyses and analysis of variance (ANOVA) (Excel; Microsoft Corp., Redmond, Wash.) at a confidence interval of 95% (P ≤ 0.05) were performed to determine the statistical significance of differences between sample means.
RESULTS
Extraction of E. coli O157:H7 from bruised apple tissue.
Prior to monitoring growth of E. coli O157:H7 in bruised apple tissue, it was necessary to know the efficiency with which the protocol used in this investigation (i.e., squeezing bruised tissue to obtain juice and bacteria) would extract this bacterium. Bruised tissue of three apples for each of four apple cultivars (McIntosh, Red Delicious, Melrose, and Macoun) was injected with approximately 105 E. coli O157:H7 cells to determine the extraction efficiency. The apple tissue was analyzed for bacterial presence as outlined in Materials and Methods with the exception that the bruised apple tissue was excised from the apple 10 to 15 min after injection of E. coli. The efficiencies of extraction (calculated as [cell number recovered/cell number injected] × 100%) from the damaged tissue of the McIntosh, Red Delicious, Melrose, and Macoun apples (three samples each) were measured to be 25.8 ± 4.5, 28.0 ± 8.4, 36.4 ± 6.8, and 30.9% ± 4.9%, respectively. These extraction efficiencies were not significantly different (P > 0.6). For the four cultivars tested, the combined efficiency of extraction of this microbe from bruised apple tissue using the procedure described in this article was 30.3% ± 3.0% of the E. coli O157:H7 organisms present. Golden Delicious apples were not tested for this experiment. However, testing of Golden Delicious and Red Delicious apples at a later date (three samples each; September 1999) demonstrated extraction efficiencies of 43.6 ± 2.2 and 29.9% ± 1.8%, respectively.
Growth of E. coli O157:H7 in bruised apple tissue.
To ascertain whether E. coli O157:H7 would grow in bruised apple tissue and whether the apple cultivar, date of apple harvest, or source of the apples (i.e., tree-picked or dropped fruit) would influence the ability of this bacterium to grow in bruised apple tissue, an injection/extraction study as outlined in Materials and Methods was conducted on five different apple cultivars. Throughout this experiment, bacteria other than E. coli O157:H7 were not isolated from the bruised apple tissue.
The results of this study for the cultivars McIntosh and Red Delicious are shown in Table 1. Growth of E. coli O157:H7 in the Golden Delicious, Macoun, and Melrose cultivars was similar to growth in the Red Delicious cultivar. Apples of these four cultivars were susceptible to the growth of E. coli O157:H7 in damaged tissue independent of the date of harvest (i.e., stage of development), and dropped fruit did not differ from tree-picked fruit in promoting the growth of E. coli.
TABLE 1.
Growth of E. coli O157:H7 in damaged apple tissue as influenced by apple cultivar and harvest date
| Harvest date | Total no. of E. coli O157:H7 cells
|
||||
|---|---|---|---|---|---|
| Injected | In bruised tissuea of:
|
||||
| McIntosh
|
Red Delicious
|
||||
| Tree-picked fruit | Dropped fruit | Tree-picked fruit | Dropped fruit | ||
| 4 Sept. | 9.0 × 104 | 4.9 × 104 | 3.0 × 104 | 1.0 × 108 | 8.7 × 107 |
| 11 Sept. | 1.6 × 105 | 4.7 × 104 | 9.9 × 104 | 1.7 × 108 | 4.9 × 107 |
| 18 Sept. | 1.0 × 105 | 2.3 × 105 | 2.7 × 105 | 2.0 × 108 | 1.6 × 108 |
| 25 Sept. | 1.5 × 105 | 9.5 × 105 | 1.0 × 107 | 2.8 × 107 | 1.2 × 108 |
| 2 Oct. | 1.8 × 105 | 4.0 × 104 | 6.5 × 104 | 5.1 × 106 | 5.8 × 106 |
| 9 Oct. | 1.9 × 105 | 9.6 × 104 | 2.1 × 104 | 1.5 × 107 | 1.0 × 107 |
| 27 Oct.b | 1.9 × 105 | 2.3 × 105 | 7.5 × 106 | 5.2 × 106 | 2.2 × 107 |
The total number of cells recovered following juice extraction from the bruised tissue multiplied by 3.3 (correction for the 30% efficiency of bacterial extraction from bruised tissue). Apples harvested were either hand-picked directly from the tree (tree-picked) or obtained from the ground (dropped).
Apple testing date. The apples had been harvested and stored at 4°C for 1 month prior to this date.
Interestingly, bruised tissue of McIntosh apples had an inhibitory effect on the growth of E. coli O157:H7 (Table 1). Four out of six weekly samples of fresh tree-picked and dropped McIntosh apples had bacterial recovery counts less than bacterial injection counts after accounting for a 30% efficiency of extraction. When stored at 4°C for 1 month prior to testing, fruit of all five cultivars supported growth of E. coli O157:H7 (Table 1; data for 27 October) after accounting for a 30% extraction efficiency.
Prolonged exposure of E. coli O157:H7 to bruised tissue of McIntosh and Red Delicious apple cultivars.
Because E. coli O157:H7 growth appeared to be inhibited in bruised McIntosh apple tissue during a 2-day period of exposure (Table 1), an investigation to study the growth pattern of this bacterium in bruised McIntosh tissue over a longer time period was performed. The Red Delicious apple cultivar was used for comparison since E. coli O157:H7 grew well in this cultivar during the 2-day study.
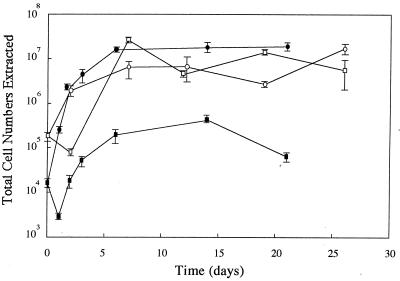
This experiment, described in Materials and Methods, used freshly picked (1 to 2 days) apples and apples which had been stored for 1 month at 4°C. Classical bacterial growth profiles were observed for growth of E. coli O157:H7 in all bruised apple tissues tested (Fig. 1). After an initial drop in cell number 1 to 2 days postinoculation for McIntosh apples, exponential growth of E. coli occurred over a 4- to 5-day period. For all fruit tested, bacterial growth reached a maximum total cell number (i.e., stationary-growth phase) approximately 6 days after inoculation of the bruise and room temperature incubation. The maximum total cell number determined for growth of this pathogen in freshly picked McIntosh apple bruises ([7.4 ± 2.3] × 105 CFU; six samples) was approximately 80-fold less than the maximum total cell number measured for growth in fresh Red Delicious apples ([5.8 ± 0.3] × 107 CFU; seven samples). Maximum total cell number was calculated as the average of the total cell counts obtained from inoculated bruises incubated for 6 days and longer following a correction for the efficiency of bacterial extraction from the tissue. However, the maximum total cell number determined for E. coli O157:H7 growth in bruised tissue of McIntosh apples that had been stored at 4°C for 1 month ([4.2 ± 1.1] × 107 CFU; eight samples) was not significantly different from the maximum total cell number determined for E. coli growth in bruised tissue of fresh (P > 0.2) or stored (P > 0.1) Red Delicious apples. This finding suggested that some property inhibitory to the growth of E. coli was lost from the McIntosh apples during storage.
FIG. 1.
Profile of E. coli O157:H7 gfp-72ec recovery from bruised apple tissue following injection of the bacterium and prolonged incubation. Bruised tissues of Red Delicious (circles) and McIntosh (squares) cultivars were injected with bacteria, and total cell numbers extracted from the tissues were measured after incubation for various periods of time. Total cell numbers were not corrected for efficiency of extraction. Solid symbols, freshly picked (1 to 2 days) fruit; open symbols, fruit that had been stored at 4°C for 1 month prior to injection. Data points are the averages obtained from counting three plates, and error bars represent 1 standard deviation from the norm. When in close proximity to another data point, data points have been slightly offset from the sampling date.
The maximum total cell count determined for growth of E. coli in Red Delicious apples that had been stored at 4°C for 1 month ([2.4 ± 0.5] × 107 CFU; 11 samples) was approximately 2.5-fold less than the maximum total cell count recovered from fresh Red Delicious apples. A statistical t test inferential analysis determined this to be a significant difference (P < 0.001). For Red Delicious apples, storage lowered the potential of the fruit to promote growth of E. coli O157:H7.
The pHs of the juices extracted from the inoculated McIntosh and Red Delicious apples (fresh or stored) were tested by Hydrion paper and found to be approximately 4.5 and 5.5, respectively. These pH values did not show any major change over the course of this experiment.
Apple pH and °Brix over the harvest season.
For the five apple cultivars used in this study, Table 2 shows the averaged results of weekly pH and °Brix measurements performed on undamaged and bruised apple tissue obtained from fresh apples (4 September to 9 October) that were harvested as either tree-picked or dropped fruit. Also shown are the average pH and °Brix values for undamaged and bruised apple tissue measured following storage of these apples for 1 month at 4°C (27 October data).
TABLE 2.
pH and °Brix measurements of apple tissue obtained as tree-picked and dropped applesc
| Cultivar | Harvest conditiond | pH (mean ± SE)a for:
|
°Brix (mean ± SE)b for:
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Tree-picked fruit
|
Dropped fruit
|
Tree-picked fruit
|
Dropped fruit
|
||||||
| Undamaged | Bruised | Undamaged | Bruised | Undamaged | Bruised | Undamaged | Bruised | ||
| Golden Delicious | Fresh | 3.55 ± 0.05 | 3.73 ± 0.05 | 3.74 ± 0.06 | 4.05 ± 0.08 | 14.9 ± 0.3 | 13.3 ± 0.3 | 15.6 ± 0.4 | 14.0 ± 0.4 |
| Stored | 3.62 ± 0.17 | 4.00 ± 0.03 | 3.68 ± 0.06 | 4.00 ± 0.09 | 14.2 ± 1.0 | 14.0 ± 0.4 | 8.9 ± 0.4 | 8.8 ± 0.4 | |
| McIntosh | Fresh | 3.50 ± 0.05 | 3.62 ± 0.04 | 3.58 ± 0.06 | 3.84 ± 0.10 | 11.9 ± 0.3 | 11.0 ± 0.3 | 12.5 ± 0.3 | 11.6 ± 0.2 |
| Stored | 3.33 ± 0.01 | 3.54 ± 0.07 | 3.49 ± 0.01 | 3.69 ± 0.06 | 12.8 ± 0.4 | 12.3 ± 0.3 | 11.9 ± 0.6 | 11.2 ± 0.2 | |
| Red Delicious | Fresh | 3.98 ± 0.05 | 4.57 ± 0.11 | 4.15 ± 0.07 | 4.90 ± 0.09 | 10.7 ± 0.3 | 9.7 ± 0.3 | 12.2 ± 0.3 | 11.0 ± 0.3 |
| Stored | 3.83 ± 0.04 | 4.65 ± 0.05 | 4.10 ± 0.05 | 4.77 ± 0.28 | 13.7 ± 0.3 | 11.9 ± 0.7 | 13.9 ± 0.7 | 12.8 ± 1.0 | |
| Macoun | Fresh | 3.56 ± 0.04 | 3.92 ± 0.03 | 3.84 ± 0.06 | 4.33 ± 0.19 | 12.8 ± 0.2 | 11.3 ± 0.3 | 12.6 ± 0.3 | 10.9 ± 0.3 |
| Stored | 3.43 ± 0.02 | 3.96 ± 0.10 | 3.70 ± 0.13 | 4.28 ± 0.12 | 12.7 ± 0.7 | 11.8 ± 0.0 | 11.4 ± 0.0 | 11.9 ± 0.9 | |
| Melrose | Fresh | 3.59 ± 0.05 | 3.78 ± 0.09 | 3.69 ± 0.06 | 3.91 ± 0.08 | 12.6 ± 0.4 | 11.3 ± 0.3 | 12.0 ± 0.4 | 10.9 ± 0.5 |
| Stored | 3.36 ± 0.03 | 3.62 ± 0.03 | 3.53 ± 0.04 | 3.83 ± 0.00 | 14.9 ± 0.1 | 13.7 ± 0.7 | 14.9 ± 0.3 | 13.1 ± 0.3 | |
Calculated using 18 fresh undamaged samples, 12 fresh bruised samples, 3 stored undamaged samples, and 2 stored bruised samples.
Calculated using 12 fresh fruit samples and 2 stored fruit samples.
Apples harvested were either hand-picked directly from the tree (tree-picked fruit) or obtained from the ground (dropped fruit) and were either healthy (undamaged) or had been dropped onto a tile floor to damage the tissue (bruised).
Apples were harvested on a weekly basis (4 September to 9 October) and tested within 1 to 2 days of harvest (fresh) or had been harvested and stored at 4°C for 1 month prior to testing on 27 October (stored).
For each apple cultivar examined, the pH of bruised-apple tissue was significantly higher than the pH of undamaged apple tissue regardless of the apple source (i.e., tree-picked or dropped) or age (i.e., fresh or stored). Comparisons within the cultivars showed that (whether fresh or stored apples) undamaged tree-picked fruit had a pH less than the pH of undamaged dropped fruit for these five cultivars. Also, Red Delicious apples had tissue pHs that were significantly higher than those of the other cultivars independent of the apple source or whether the tissue was undamaged or bruised.
For undamaged tree-picked fruit (fresh or stored), the pHs of Golden Delicious, McIntosh, Macoun, and Melrose apples were not significantly different based on ANOVA comparisons of the cultivars (Table 3). Additionally, the pHs of Golden Delicious, McIntosh, and Melrose apples were found to be not significantly different regardless of whether undamaged dropped fruit (fresh or stored) or bruised apple tissue (from tree-picked or dropped fresh apples) was compared. When the pHs of bruised apple tissue (from tree-picked or dropped apples) for stored apples were compared, it was found that McIntosh and Melrose were not significantly different. Within each cultivar grouping, statistical comparisons of pH between fresh and stored fruit of undamaged tree-picked apples did not demonstrate significant differences. For bruised tree-picked fruit and dropped fruit (undamaged or bruised) of McIntosh, Melrose, and Red Delicious apples, comparisons of the pH values between fresh and stored fruit showed that the values were also not significantly different.
TABLE 3.
Summary of ANOVA comparing cultivar groupings for pH and °Brix
| Variable | Comparative grouping characteristics
|
ANOVA dataa
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Tissueb | Sourcec | Typed | Apple cultivarse | Variation | df | MS | F | Fcrit | |
| pH | Undamaged | TP | Fresh | GD, Mc, Ma, and Me | Between cultivars | 3 | 0.024 | 0.57 | 2.74 |
| Within cultivars | 68 | 0.041 | |||||||
| TP | Stored | GD, Mc, Ma, and Me | Between cultivars | 3 | 0.052 | 2.27 | 4.07 | ||
| Within cultivars | 8 | 0.023 | |||||||
| D | Fresh | GD, Mc, and Me | Between cultivars | 2 | 0.123 | 1.93 | 3.19 | ||
| Within cultivars | 51 | 0.064 | |||||||
| D | Stored | GD, Mc, Ma, and Me | Between cultivars | 3 | 0.033 | 1.97 | 4.07 | ||
| Within cultivars | 8 | 0.017 | |||||||
| Bruised | TP | Fresh | GD, Mc, and Me | Between cultivars | 2 | 0.077 | 1.61 | 3.28 | |
| Within cultivars | 33 | 0.048 | |||||||
| D | Fresh | GD, Mc, and Me | Between cultivars | 2 | 0.141 | 1.53 | 3.28 | ||
| Within cultivars | 33 | 0.092 | |||||||
| °Brix | Undamaged | TP | Fresh | Mc, Ma, and Me | Between cultivars | 2 | 2.556 | 2.33 | 3.28 |
| Within cultivars | 33 | 1.095 | |||||||
| TP | Stored | GD, RD, Mc, Ma, and Me | Between cultivars | 4 | 1.795 | 2.67 | 5.19 | ||
| Within cultivars | 5 | 0.673 | |||||||
| D | Fresh | RD, Mc, Ma, and Me | Between cultivars | 3 | 0.889 | 0.63 | 2.82 | ||
| Within cultivars | 44 | 1.401 | |||||||
| D | Stored | RD, Mc, Ma, and Me | Between cultivars | 3 | 4.411 | 7.58 | 9.28 | ||
| Within cultivars | 3 | 0.582 | |||||||
| Bruised | TP | Fresh | Mc, Ma, and Me | Between cultivars | 2 | 0.380 | 0.33 | 3.28 | |
| Within cultivars | 33 | 1.145 | |||||||
| TP | Stored | GD, RD, Mc, Ma, and Me | Between cultivars | 4 | 2.127 | 4.73 | 5.19 | ||
| Within cultivars | 5 | 0.450 | |||||||
| D | Fresh | RD, Mc, Ma, and Me | Between cultivars | 3 | 1.344 | 1.01 | 2.83 | ||
| Within cultivars | 42 | 1.331 | |||||||
| D | Stored | RD, Mc, Ma, and Me | Between cultivars | 3 | 1.535 | 1.68 | 6.59 | ||
| Within cultivars | 4 | 0.913 | |||||||
Data showing values for degrees of freedom (df), mean square (MS), F, and Fcrit (value above which the null hypothesis is rejected at the 95% confidence level).
Apples were either healthy (undamaged) or had been dropped onto a tile floor to damage the tissue (bruised).
Harvested either as hand-picked directly from the tree (tree-picked; TP) or obtained from the ground (dropped; D).
Harvested on a weekly basis (4 September to 9 October) and tested within 1 to 2 days of harvest (fresh) or harvested and stored at 4°C for 1 month prior to testing (stored).
RD, Red Delicious; GD, Golden Delicious; Mc, McIntosh; Ma, Macoun; Me, Melrose.
Bruised tissue had a significantly lower °Brix than undamaged tissue for fresh apples regardless of the apple cultivar or source (Table 2). However, comparisons between the °Brix values of bruised and undamaged apple tissue for stored apples, regardless of the apple cultivar or source, did not demonstrate significant differences. Except for stored Golden Delicious and fresh Red Delicious apples, the °Brix of tree-picked fruit was found to be not significantly different from the °Brix of the corresponding dropped fruit cultivar in undamaged, bruised, fresh, and stored apple comparisons.
In comparisons between apple cultivars, the °Brix values of fresh tree-picked McIntosh, Macoun, and Melrose apples (whether undamaged or with bruised tissue) were not significantly different (Table 3). Golden Delicious apples generally had °Brix values that were significantly higher than those for the other cultivars independent of the apple source or whether the tissue was undamaged or bruised. Intercultivar comparisons of dropped apples (undamaged or bruised) produced °Brix values that were not significantly different for all but the Golden Delicious. The °Brix values of stored tree-picked fruit (undamaged or bruised) for the five cultivars were not significantly different.
For each of these five cultivars, the pH did not significantly change over the course of the study (September to October). The °Brix did demonstrate a trend toward increasing for each of the cultivars over this time period. However, the pH and °Brix values of these five cultivars were not important factors influencing a change in the ability of E. coli O157:H7 to grow in the fruit over the time period of this study.
Growth of E. coli O157:H7 in cider produced from an individual apple cultivar.
Apple cider is generally produced from a blend of different apple cultivars. In New England, McIntosh varieties are predominant components of this blend (personal observation). Although E. coli O157:H7 remains viable in cider, the bacterium does not appear to grow in cider (10, 29). Suppression of bacterial growth in cider is reportedly due to the low pH (22, 23). Experiments in which the pH of cider has been adjusted to 7.0 readily permitted E. coli to grow in cider (data not presented). However, other factors may also work to suppress growth of this bacterium in cider. Cider fermentation effectively destroys E. coli (25).
To determine whether use of McIntosh apples in the production of cider might be affecting the ability of this organism to grow or survive in cider, ciders (30 ml each) that had been produced using apples of a single cultivar were inoculated with E. coli O157:H7 (2.2 × 104 CFU) and incubated at room temperature. At selected times, samples were removed from the inoculated ciders and measured for E. coli cell concentration. Fruit (McIntosh, Red Delicious, Golden Delicious, and Melrose) used to produce these ciders had been stored at 4°C for more than 1 month prior to juice extraction. Macoun apples were not tested.
Table 4 shows that E. coli O157:H7 did not grow in single-cultivar cider produced from any of the fruit tested. Inhibition of mold and bacterial growth in the cider by the presence of cycloheximide and AMP also did not induce growth of this microbe. The strain of E. coli O157:H7 used was resistant to AMP. These findings demonstrated that the suppression of the growth of E. coli in these ciders was due to a factor(s) other than the presence of McIntosh apples in the cider or the metabolic activity of other microbes. Interestingly, the rapid drop in the percentage of E. coli cells present in the McIntosh cultivar cider in comparison to the other ciders (Table 4) suggested that some property other than pH affected the survival of this bacterium in the McIntosh cider. The pH of the McIntosh cider was not significantly different from the pHs of the Golden Delicious and Melrose ciders.
TABLE 4.
Survival profile of E. coli O157:H7 in apple cider produced from a single apple cultivara
| Apple cultivar (additivesd) | Ciderb
|
% of E. coli O157:H7 cells recoveredc on day:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| pH | °Brix | 0.3 | 1 | 2 | 4 | 5 | 7 | 11 | |
| McIntosh | 3.65 | 11.8 | 75.0 | 31.4 | 17.7 | 1.4 | |||
| McIntosh (C + A) | 3.76 | 11.6 | 72.2 | 51.3 | 56.5 | 7.4 | <1.0 | ||
| Red Delicious | 4.17 | 14.0 | 60.0 | 57.3 | 38.2 | ||||
| Red Delicious (C + A) | 4.05 | 13.4 | 65.7 | 57.4 | 59.1 | 11.3 | 30.0 | ||
| Golden Delicious | 3.72 | 15.8 | 70.9 | 50.5 | 30.0 | 20.5 | |||
| Golden Delicious (C + A) | 3.73 | 16.8 | 65.7 | 55.7 | 57.0 | 34.8 | 19.1 | ||
| Melrose | 3.68 | 13.2 | 53.2 | 61.4 | 31.4 | 31.4 | |||
| Melrose (C + A) | 3.57 | 13.5 | 73.0 | 42.2 | 53.0 | 60.4 | 40.4 | ||
The apples were stored at 4°C for greater than 1 month prior to cider extraction.
pH and °Brix determinations of the cider were made prior to inoculation.
Calculated as [(recovery day CFU/ml)/(CFU/ml immediately after cider inoculation on day 0)] × 100%.
C, cycloheximide (1 mg/ml); A, AMP (50 μg/ml). Additives were added to the cider immediately after production.
DISCUSSION
With the exception of Melrose, the apple cultivars used in this study are among the varieties that are predominantly used in the production of apple cider (18). Since this investigation examined apple susceptibility to E. coli growth as influenced by apple maturity, Melrose was included to represent late-maturing apples. All of the cultivars tested promoted growth of E. coli O157:H7 in damaged apple tissue independent of the apple source (i.e., harvested as tree-picked or dropped fruit; Table 1). Red Delicious, Golden Delicious, Macoun, and Melrose apples (fresh and stored) were susceptible to the growth of this bacterium throughout the study period (early September through late October). Fresh McIntosh apples generally inhibited the growth of E. coli O157:H7 following a 2-day incubation period at room temperature (Table 1) throughout the study, and growth was approximately 80-fold less than growth in fresh Red Delicious apples in prolonged growth experiments (Fig. 1). Maximum growth of E. coli in the bruised tissue of Red Delicious and stored McIntosh apples was in the range of 2 × 107 to 6 × 107 CFU (Fig. 1). This was higher than the values reported by Janisiewicz and associates (16), who found that the recovery of E. coli O157:H7 from exposed Golden Delicious apple tissue was in the range of 105 to 107 CFU per wound. Fresh McIntosh apples occasionally supported growth of the bacterium following a 2-day incubation at room temperature (Table 1), and fruit stored at 4°C for 1 month prior to testing promoted E. coli growth to the same extent as freshly picked or stored Red Delicious apples (Fig. 1).
In a previous report (8), I have shown that the appearance of E. coli in apple cider manufactured in Connecticut occurred primarily within a window of time from late October to early November and did not occur early in the production season when cider output was at maximum. Except for fresh McIntosh apples, bruised tissue of the apples tested in this investigation promoted growth of E. coli O157:H7 independent of the time of harvest (Table 1). Therefore, why didn't the presence of E. coli in cider manufactured in Connecticut occur early in the production season?
Because apple cultivars ripen at different rates (7), the use of a particular cultivar in cider production will vary throughout the cider production season. Fresh McIntosh apples (maturing approximately 135 to 140 days from bloom [i.e., in mid-August] with maximum harvest from early September through mid-October) are used early in the cider production season. Red and Golden Delicious apples (maturing approximately 150 to 155 days from bloom) and Macoun apples are harvested from mid-September through late October, and Melrose apples are harvested in late October. As the cider production season progresses (i.e., early October through late November), the use of fresh McIntosh apples decreases and the use of other cultivars increases. Also, early in the production season (i.e., late August through mid-October) the harvested apples are used very quickly (stored less than 7 days after harvest) due to the demand for apple cider. As the season progresses, apples that have been stored at 4°C for 1 month or longer are predominantly used.
Overall, as the cider production season progresses, predominant usage of an apple cultivar that is less favorable to the growth of E. coli O157:H7 in damaged tissue is replaced by usage of apple cultivars that readily promote the growth of this organism. Therefore, it can be speculated that the time-limited appearance of E. coli in cider manufactured in Connecticut which was reported earlier (8) may have occurred as a result of the cultivar usage pattern and fruit storage practices used in the manufacture of cider.
It is not known why growth of E. coli O157:H7 was inhibited in bruised tissue of fresh McIntosh apples. The limiting pH (i.e., growth/no growth interface) for E. coli M23 at 20°C with no lactic acid is reported to be within the range of 3.6 to 3.8 (23). Except for Red Delicious and Macoun apples, the average pH values of bruised tissue in the fresh apples used in this study were within this limiting range. Although the pH and °Brix values of the bruised McIntosh tissue (Table 3) were not significantly different from the values of bruised tissue that promoted growth of E. coli (i.e., that of Golden Delicious, Macoun, and Melrose), the overall average pH values observed for the McIntosh cultivar were lower than the average values for the other cultivars (Table 2). It is possible that this lower average pH accounted for the difference in growth properties. However, bruised tissue of stored McIntosh apples (tree picked and dropped) had lower average pH values than bruised tissue of fresh apples and promoted growth of E. coli (Table 2). This observation would indicate that some factor besides, or in addition to, pH and °Brix accounted for inhibition of E. coli growth in McIntosh apples.
The McIntosh cultivar, first propagated in 1870 as a chance seedling on the John McIntosh farm in Ontario, Canada (2), might lack some physical property of the bruise that is present in cultivars propagated from other sources. It has been suggested that the ability of this microbe to grow in apple tissue is due to the development of a favorable microenvironment within the fruit (16). The failure of E. coli O157:H7 to grow in cider produced from a single apple cultivar (Table 4), while it was able to grow in damaged tissue of the same cultivar (Table 1), would support the idea that some property (i.e., a microenvironment) within bruised tissue permitted growth of the microbe. Development of such a microenvironment may not occur in bruised tissue of fresh McIntosh apples. However, growth in bruised tissue of stored McIntosh apples and the occasional growth in fresh apples (Table 1) indicated otherwise.
Alternatively, the inhibition of E. coli growth in McIntosh apples may have been due to some compound(s) not present in the other cultivars. The observation that McIntosh apples demonstrated good growth of E. coli after storage (Fig. 1) supports the possibility of the presence of some compound(s) that is unstable or volatile and lost upon storage. Also, the fast decline in E. coli cell concentration in cider made from McIntosh apples in comparison to the declines in cider made from the other cultivars (Table 4) supports the presence of such a compound(s). The presence of an inhibitory compound(s) would not negate the effect of low pH or the necessity for the existence of a microenvironment to overcome the inhibitory effects of pH. Research to determine whether an inhibiting factor exists in the McIntosh apple and to characterize this factor will be performed.
Regardless of the source of E. coli for contamination of apples, the ability of this pathogen to grow in damaged fruit will, in comparison to surface contamination of fruit, considerably lower the number of E. coli cells initially needed to contaminate cider. Also, growth of E. coli O157:H7 in the apple will protect the pathogen from certain sanitation practices (i.e., washing and brushing of fruit) and, due to growth in the moderate pH of the bruise, will likely predispose the bacterium for survival in the low pH of cider (1, 19, 20). Based on current cider production practices, the growth of E. coli in damaged apples poses a greater risk for contamination of cider than surface contamination of apples only.
ACKNOWLEDGMENTS
I thank S. M. Douglas for helpful suggestions in the preparation of the manuscript and Cindy Musante for technical assistance. I also thank Susan Fratamico for kindly providing the green fluorescent protein-containing E. coli strain and the owner and operator of the Connecticut apple orchard for agreeing to participate in this study.
REFERENCES
- 1.Arnold K W, Kaspar C W. Starvation- and stationary-phase-induced acid tolerance in Escherichia coli O157:H7. Appl Environ Microbiol. 1995;61:2037–2039. doi: 10.1128/aem.61.5.2037-2039.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beach S A, Booth N O, Taylor O M. The apples of New York. Vol. 2. Albany, N.Y: New York Agricultural Experiment Station; 1903. [Google Scholar]
- 3.Besser R E, Lett S M, Weber J T, Doyle M P, Barret T J, Wells J G, Griffin P M. An outbreak of diarrhea and hemolytic uremic syndrome from Escherichia coli O157:H7 in fresh-pressed apple cider. JAMA. 1993;269:2217–2220. [PubMed] [Google Scholar]
- 4.Beutin L, Knollmann-Schanbacher G, Rietschel W, Seeger H. Animal reservoirs of Escherichia coli O157:[H7] Vet Rec. 1996;139:70–71. doi: 10.1136/vr.139.3.70. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Outbreak of Escherichia coli O157:H7 infections associated with drinking unpasteurized commercial apple juice—British Columbia, California, Colorado, and Washington, October 1996. Morbid Mortal Weekly Rep. 1996;45:975. [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Outbreaks of Escherichia coli O157:H7 infection and cryptosporidiosis associated with drinking unpasteurized apple cider—Connecticut and New York, October 1996. Morbid Mortal Weekly Rep. 1997;46:4–8. [PubMed] [Google Scholar]
- 7.Childers N F. Modern fruit science. Gainesville, Fla: Horticultural Publications; 1983. pp. 123–146. [Google Scholar]
- 8.Dingman D W. Prevalence of Escherichia coli in apple cider manufactured in Connecticut. J Food Prot. 1999;62:567–573. doi: 10.4315/0362-028x-62.6.567. [DOI] [PubMed] [Google Scholar]
- 9.Doyle M P, Schoeni J L. Isolation of Escherichia coli O157:H7 from retail fresh meats and poultry. Appl Environ Microbiol. 1987;53:2394–2396. doi: 10.1128/aem.53.10.2394-2396.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher T L, Golden D A. Survival of Escherichia coli O157:H7 in apple cider as affected by dimethyl dicarbonate, sodium bisulfate, and sodium benzoate. J Food Sci. 1998;63:904–906. [Google Scholar]
- 11.Food and Drug Administration. Current science and technology on fresh juices; notice of meeting. Fed Regist. 1996;61:60290. [Google Scholar]
- 12.Food and Drug Administration. Draft guidance for industry: guide to minimize microbial food safety hazards for fresh fruits and vegetables. Fed Regist. 1998;63:18029–18030. [Google Scholar]
- 13.Fratamico P M, Deng M Y, Strobaugh T P, Palumbo S A. Construction and characterization of Escherichia coli O157:H7 strains expressing firefly luciferase and green fluorescent protein and their use in survival studies. J Food Prot. 1997;60:1167–1173. doi: 10.4315/0362-028X-60.10.1167. [DOI] [PubMed] [Google Scholar]
- 14.Goverd K A, Beech F W. The occurrence and survival of coliforms and salmonellas in apple juice and cider. J Appl Bacteriol. 1979;46:521–530. doi: 10.1111/j.1365-2672.1979.tb00851.x. [DOI] [PubMed] [Google Scholar]
- 15.Iwasa M, Makino S, Asakura H, Kobori H, Morimoto Y. Detection of Escherichia coli O157:H7 from Musca domestica (Diptera: Muscidae) at a cattle farm in Japan. J Med Entomol. 1999;36:108–112. doi: 10.1093/jmedent/36.1.108. [DOI] [PubMed] [Google Scholar]
- 16.Janisiewicz W J, Conway W S, Brown M W, Sapers G M, Fratamico P, Buchanan R L. Fate of Escherichia coli O157:H7 on fresh-cut apple tissue and its potential for transmission by fruit flies. Appl Environ Microbiol. 1999;65:1–5. doi: 10.1128/aem.65.1.1-5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kudva I T, Hatfield P G, Hovde C J. Escherichia coli O157:H7 in microbial flora of sheep. J Clin Microbiol. 1996;34:431–433. doi: 10.1128/jcm.34.2.431-433.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuhn G D, Barden C L. Cider production. In: Serotkin N, editor. Pennsylvania tree fruit production guide 1998–1999. University Park: Pennsylvania State University; 1998. pp. 223–233. [Google Scholar]
- 19.Leyer G J, Wang L, Johnson E A. Acid adaptation of Escherichia coli O157:H7 increases survival in acidic foods. Appl Environ Microbiol. 1995;61:3752–3755. doi: 10.1128/aem.61.10.3752-3755.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin J, Smith M P, Chapin K C, Baik H S, Bennett G N, Foster J W. Mechanisms of acid resistance in enterohemorrhagic Escherichia coli. Appl Environ Microbiol. 1996;62:3094–3100. doi: 10.1128/aem.62.9.3094-3100.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McAllister J C, Steelman C D, Skeeles J K, Newberry L A, Gbur E E. Reservoir competence of Alphitobius diaperinus (Coleoptera: Tenebrionidae) for Escherichia coli (Eubacteriales: Enterobacteriaceae) J Med Entomol. 1996;33:983–987. doi: 10.1093/jmedent/33.6.983. [DOI] [PubMed] [Google Scholar]
- 22.Miller L G, Kaspar C W. Escherichia coli O157:H7 acid tolerance and survival in apple cider. J Food Prot. 1994;57:460–464. doi: 10.4315/0362-028X-57.6.460. [DOI] [PubMed] [Google Scholar]
- 23.Presser K A, Ross T, Ratkowsky D A. Modelling the growth limits (growth/no growth interface) of Escherichia coli as a function of temperature, pH, lactic acid concentration, and water activity. Appl Environ Microbiol. 1998;64:1773–1779. doi: 10.1128/aem.64.5.1773-1779.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rice D H, Hancock D D, Besser T E. Verotoxigenic E. coli O157 colonization of wild deer and range cattle. Vet Rec. 1995;137:524. doi: 10.1136/vr.137.20.524. [DOI] [PubMed] [Google Scholar]
- 25.Semanchek J J, Golden D A. Survival of Escherichia coli O157-H7 during fermentation of apple cider. J Food Prot. 1996;59:1256–1259. doi: 10.4315/0362-028X-59.12.1256. [DOI] [PubMed] [Google Scholar]
- 26.Shere J A, Bartlett K J, Kaspar C W. Longitudinal study of Escherichia coli O157:H7 dissemination on four dairy farms in Wisconsin. Appl Environ Microbiol. 1998;64:1390–1399. doi: 10.1128/aem.64.4.1390-1399.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steele B T, Murphy N, Arbus G S, Rance C P. An outbreak of hemolytic uremic syndrome associated with ingestion of fresh apple juice. Clin Lab Observ. 1982;101:963–965. doi: 10.1016/s0022-3476(82)80021-8. [DOI] [PubMed] [Google Scholar]
- 28.Wallace J S, Cheasty T, Jones K. Isolation of vero cytotoxin-producing Escherichia coli O157:H7 from wild birds. J Appl Microbiol. 1997;82:399–404. doi: 10.1046/j.1365-2672.1997.00378.x. [DOI] [PubMed] [Google Scholar]
- 29.Zhao T, Doyle M P, Besser R E. Fate of enterohemorrhagic Escherichia coli O157:H7 in apple cider with and without preservatives. Appl Environ Microbiol. 1993;59:2526–2530. doi: 10.1128/aem.59.8.2526-2530.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]