Abstract
Calcium ions function as a key second messenger ion in eukaryotes. Spatially and temporally defined cytoplasmic Ca2+ signals are shaped through the concerted activity of ion channels, exchangers, and pumps in response to diverse stimuli; these signals are then decoded through the activity of Ca2+‐binding sensor proteins. In plants, Ca2+ signaling is central to both pattern‐ and effector‐triggered immunity, with the generation of characteristic cytoplasmic Ca2+ elevations in response to potential pathogens being common to both. However, despite their importance, and a long history of scientific interest, the transport proteins that shape Ca2+ signals and their integration remain poorly characterized. Here, we discuss recent work that has both shed light on and deepened the mysteries of Ca2+ signaling in plant immunity.
Keywords: calcium, channel, ETI, immunity, PTI
Subject Categories: Microbiology, Virology & Host Pathogen Interaction
This review summarizes how calcium ions, and the proteins which transport them, are integrated into plant immune responses.
The plant immune system
All eukaryotes use immune systems to protect themselves against potential pathogens. The plant immune system consists of two characterized perception layers: one that utilizes cell‐surface pattern recognition receptors (PRRs) to perceive extracellular immunogenic patterns, and another that relies on intracellular nucleotide‐binding leucine‐rich repeat (NLR) receptors that recognize pathogenic effectors inside the cell (Jones & Dangl, 2006).
In the first layer of the plant immune system, apoplastic immunogenic elicitors such as pathogen‐, microbe‐, damage‐, or herbivore‐associated molecular patterns (PAMPs, MAMPs, DAMPs, or HAMPs, respectively) or immune‐modulating peptide phytocytokines are recognized by PRRs, which leads to defense responses termed pattern‐triggered immunity (PTI) (Boller & Felix, 2009; Yu et al, 2017; DeFalco & Zipfel, 2021). All plant PRRs described to date are receptor kinases (RKs) or receptor proteins (RPs) (Boutrot & Zipfel, 2017; Albert et al, 2020). RKs are characterized by a domain structure reminiscent of metazoan receptor tyrosine kinases (RTKs) (DeFalco & Zipfel, 2021); namely, a ligand‐binding extracellular domain (ECD), a single‐span transmembrane helix (TM) and a cytosolic protein kinase domain (Jamieson et al, 2018), while RPs lack a cytoplasmic kinase domain and instead form functional bipartite receptors with adapter RKs (Liebrand et al, 2013; Albert et al, 2015; Postma et al, 2016). Because of their domain architecture, plasma membrane (PM)‐localized PRRs (or their complexes) allow extracellular ligand binding to be communicated across the membrane into cytosolic signaling events. The molecular nature of elicitors varies, including proteins, lipids, and carbohydrates, and can be derived from either the potential pathogen or herbivore (e.g., MAMPs, PAMPs, or HAMPs) or the host plant, as in the case of macromolecules released upon cell damage (DAMPs) or secreted peptide phytocytokines (Gust et al, 2017). PRR ECDs are characterized by a variety of subdomains, including leucine‐rich repeat (LRR), epidermal growth factor‐like (EGF), lectin, and lysin motif (LysM) domains (Boutrot & Zipfel, 2017). The best‐studied PRRs to‐date are the LRR‐RKs FLAGELLIN‐SENSING 2 (FLS2) and EF‐TU RECEPTOR (EFR), which perceive the bacterial PAMPs flg22 and elf18, respectively (Gómez‐Gómez & Boller, 2000; Zipfel et al, 2006). Both FLS2 and EFR form stable ligand‐dependent complexes with common LRR‐RK co‐receptors of the SOMATIC EMBRYOGENESIS RECEPTOR KINASE (SERK) family, such as BRASSINOSTEROID‐INSENSITIVE 1‐ASSOCIATED KINASE 1 (BAK1, also called SERK3) (Chinchilla et al, 2007; Heese et al, 2007; Roux et al, 2011). Complex formation between PRRs and co‐receptors leads to phosphorylation events within the cytoplasmic kinase domains and the activation of receptor‐like cytoplasmic kinases (RLCKs), which directly phosphorylate and regulate target proteins in order to activate PTI (Liang & Zhou, 2018; DeFalco & Zipfel, 2021) (Fig 1A).
Figure 1. PTI and ETI induce cytoplasmic Ca2+ elevations.
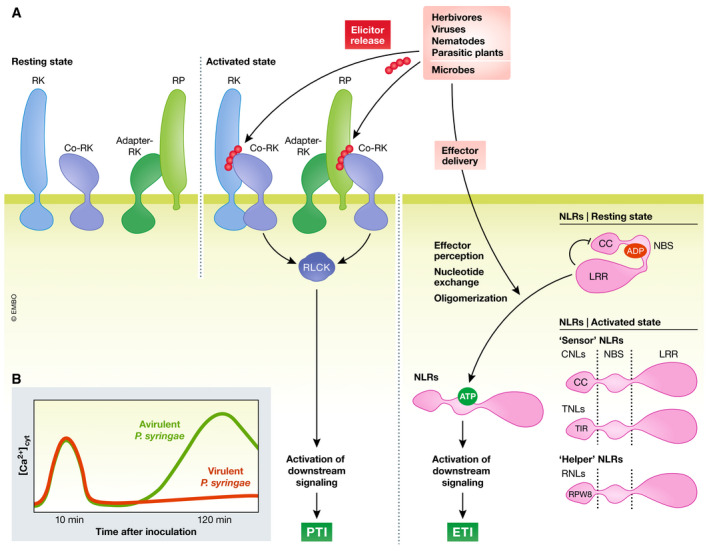
RKs and RPs are PRRs residing at the PM. They form complexes with co‐receptors upon perception of molecular patterns originating from microbes, viruses, herbivores, parasitic plants, or damaged host cells. In turn, RLCKs are activated and released from the complexes to activate downstream signaling to induce pattern‐triggered immunity, of which Ca2+ release within few minutes after ligand perception is one facet. Microbes introduce effector proteins into host cells to disturb and overcome immune responses. Cytoplasmic NLRs sense the presence or activity of effectors to induce ETI. To this end, autoinhibition is released, ADP is changed to ATP and oligomerization of NLRs occurs, leading to downstream signaling and finally ETI (A). A significant cytoplasmic Ca2+ increase has been reported to occur in Arabidopsis leaves starting 1.5 h and peaking at about 2 h after infection with avirulent bacteria (B). Schematic Ca2+ signatures of Arabidopsis plants induced by bacterial infection as reported by Grant et al (2000) (B). RK: receptor kinase; co‐RK: coreceptor kinase; RP: receptor protein; RLCK: receptor like cytoplasmic kinase; NLR: nucleotide‐binding leucine‐rich repeat receptor; CC: coiled‐coil; TIR: toll/interleukin‐related; CNLs: CC‐NLRs; TNLs: TIR‐NLRs; RNLs: RPW8‐NRLs; NBS: nucleotide binding site; LRR: leucine‐rich repeats; PTI: pattern‐triggered immunity; ETI: effector‐triggered immunity, c[Ca2+]: cytoplasmic free Ca2+ concentration.
Pathogens introduce effectors into the host cytoplasm that promote pathogenicity, often by disturbing PTI (Jones & Dangl, 2006). To counteract this, plants rely on a second layer of immunity, in which intracellular NLR‐type receptors sense effectors and/or their activity, leading to effector‐triggered immunity (ETI). Interestingly, plant NLRs share a common architecture with those of animals, featuring a conserved nucleotide‐binding domain (NBD) and LRR domain, with variable accessory domains at both N and C termini (DeYoung & Innes, 2006; Jones et al, 2016; Baggs et al, 2017; van Wersch et al, 2020). NLRs are categorized based on their N‐terminal domains: coiled‐coil (CC)‐NLRs (CNLs), toll/interleukin‐related (TIR)‐NLRs (TNLs), or RPW8‐NLRs (RNLs). Of these NLRs, CNLs and TNLs function as sensors while RNLs function as helpers downstream of TNLs (Baggs et al, 2017; Wu et al, 2017; Jubic et al, 2019; Feehan et al, 2020). NLRs can be present in an inactive state, in which the LRR domain is likely autoinhibitory, and adenosine diphosphate (ADP) is bound to their NBD (Williams et al, 2011; Bernoux et al, 2016). Upon activation, ADP is exchanged to adenosine triphosphate (ATP) and autoinhibition is released (Fig 1A). In animals, NLR activation often leads to oligomerization via N‐terminal domains and the formation of large multimeric structures (Danot et al, 2009). A similar oligomerization mechanism has been long hypothesized for plant NLRs, but has only been recently corroborated by structural data that are discussed in detail below.
PTI and ETI have traditionally been viewed as independent pathways; however, at least some signaling components are shared by both layers of immunity (Thomma et al, 2011). Activation of either layer of the immune system triggers numerous overlapping cell signaling events, including Ca2+ fluxes, production of apoplastic reactive oxygen species (ROS), mitogen‐activated protein kinase (MAPK) cascades, transcriptional reprogramming, and phytohormone biosynthesis (Cui et al, 2015; Yu et al, 2017; Zhou & Zhang, 2020; DeFalco & Zipfel, 2021). ETI is generally also accompanied by a form of programmed cell death termed the hypersensitive response (HR) at the site of infection (DeYoung & Innes, 2006; Jones & Dangl, 2006), although HR‐like cell death is also induced by some forms of PTI (Wang et al, 2020). Recent work has further demonstrated that PTI and ETI are linked at transcriptional and/or molecular levels (Ngou et al, 2021; Pruitt et al, 2021; Tian et al, 2021; Yuan et al, 2021); however, the exact mechanisms governing linkage of these immune pathways remains to be elucidated fully. As changes in intracelluar Ca2+ levels have been well documented downstream of both PRR and NLR activation, Ca2+ signaling is thought to be key to both layers of the plant immune system (Seybold et al, 2014; Moeder et al, 2019).
Ca2+ in immunity
Ca2+ is a universal second messenger in eukaryotes (Clapham, 2007). Owing to its cytotoxicity, cytosolic Ca2+ levels must be maintained at low (~10−8 to 10−7 M) levels in living cells, and thus Ca2+ is sequestered in intracellular stores (in plants, primarily the vacuole and the endoplasmatic reticulum, but also the vesicular compartments, the chloroplasts and mitochondria) or the apoplast via active transport, generating enormous electrochemical potential gradients across membranes (Clapham, 2007; Edel et al, 2017; Costa et al, 2018). Ca2+‐permeable channels can therefore generate rapid, transient increases in Ca2+ concentrations, which are in turn interpreted by a large suite of Ca2+‐binding sensor proteins that regulate diverse cellular processes (DeFalco et al, 2010). Ca2+ signaling is thus summarized in three steps: encoding (via stimulus‐triggered Ca2+ fluxes), decoding (via Ca2+ sensor proteins), and responses (via regulation of downstream cellular processes).
In plants, Ca2+ signaling is involved in all aspects of life, including growth regulation, development, abiotic stress responses, and reproduction (Kudla et al, 2018), as well as the establishment of beneficial plant‐microbe interactions (Tian et al, 2020). In this review, we focus on how cytoplasmic Ca2+ signals are encoded via transport across the PM during immune signaling.
Ca2+ influx and the oxidative burst (Doke, 1983, 1985; Apostol et al, 1989; Keppler et al, 1989) were among the first cellular responses to pathogen infection or elicitor treatment to be described (Atkinson et al, 1996; Levine et al, 1996; Zimmermann et al, 1997; Lecourieux et al, 2002). ROS production during the oxidative burst was eventually attributed to the activity of PM‐localized NADPH oxidases of the RESPIRATORY BURST OXIDASE HOMOLOGUE (RBOH) family (Torres et al, 2002); in the model plant Arabidopsis thaliana (hereafter, Arabidopsis), a single member, RBOHD, is responsible for ROS production in response to elicitors (Nühse et al, 2007; Zhang et al, 2007). In contrast, the molecular nature of the Ca2+ channel(s) involved in plant immunity remained comparably elusive for many years (Seybold et al, 2014).
Cytosolic Ca2+ signals evoked by treatment with various immunogenic elicitors were first measured in plant cell culture using Ca2+ radioisotopes, Ca2+‐sensitive dyes, or electrophysiological approaches (Atkinson et al, 1996; Levine et al, 1996; Gelli et al, 1997; Zimmermann et al, 1997). The development of genetically encoded Ca2+ indicators (GECIs) greatly expanded the possibilities for real‐time, kinetic analysis of Ca2+ fluxes in intact tissues upon infection or elicitor treatment. The first GECI deployed in plants was aequorin (AEQ) from Aequoria victoria (Knight et al, 1991), which forms a holo‐enzyme with its cofactor coelenterazine and emits light upon Ca2+‐binding. When challenged with either virulent or avirulent strains of the pathogenic bacterium Pseudomonas syringae, Arabidopsis plants expressing AEQ showed a first Ca2+ signal peak after ~10 min. A second, stronger, more persistent Ca2+ signal was seen after 1.5–2 h only with avirulent, ETI‐activating P. syringae (Grant et al, 2000; Kang et al, 2010; Hung et al, 2014). The similar kinetics of early Ca2+ elevation induced by P. syringae and that triggered by elicitors (Blume et al, 2000; Lecourieux et al, 2002) and the biphasic nature of the response to ETI‐inducing bacteria suggested that PTI and ETI may induce distinct Ca2+ signals (Fig 1B).
Subsequent analyses of AEQ‐expressing Arabidopsis plants have shown perception of diverse elicitors, including PAMPs, DAMPs, and phytocytokines, to be sufficient to elicit rapid Ca2+ signals (Ranf et al, 2008, 2011; Vadassery et al, 2009; Krol et al, 2010). Such PTI Ca2+ signaling requires functional PRRs and downstream signaling components, including RLCKs such as the RLCK‐VII/ AVRPPHB SUSCEPTIBLE 1 (PBS1)‐LIKE (PBL) family members BOTRYTIS‐INDUCED KINASE 1 (BIK1) and PBL1 (Li et al, 2014; Ranf et al, 2014; Monaghan et al, 2015). More recently, the deployment of fluorescent GECIs in plants has allowed for the analysis of elicitor‐induced Ca2+ signals at the cellular level. Such fluorescent GECIs include ratiometric (e.g., yellow cameleons) and intensiometric (e.g., GCaMPs and GECOs) sensors (Grenzi et al, 2021b; Waadt et al, 2021). Flourescent GECIs have been utilized to show that elicitor‐induced Ca2+ signals in leaves are oscillatory at the single‐cell level (Thor & Peiter, 2014; Keinath et al, 2015) and that in roots both elicitor application and laser ablation‐induced cell damage lead to the formation of Ca2+ transients (Keinath et al, 2015; Marhavý et al, 2019; Waadt et al, 2020).
ROS and Ca2+—tightly linked second messengers
There is extensive interplay between Ca2+ and ROS signaling (Gilroy et al, 2016); however, the initial PTI‐related Ca2+ signal triggered by P. syringae was shown to be only mildly reduced by treatment with the NADPH oxidase inhibitor DPI or catalase, while there was no effect on the longer‐term, effector‐triggered signal (Grant et al, 2000). Similarly, rbohd mutants showed a slight, quantitative defect in elicitor‐triggered Ca2+ signals when measured in seedlings (Ranf et al, 2011). In contrast, elicitor‐induced ROS production can be severely attenuated by treatment with Ca2+ channel blockers (Ranf et al, 2011). Elicitor perception can directly activate RBOHD via phosphorylation by BIK1 (Kadota et al, 2014; Li et al, 2014), suggesting a complex relationship between Ca2+ and ROS in immune signaling and a model wherein, upon elicitor perception, initial activation of RBOHD through PRR‐mediated phosphorylation primes the system for subsequent activation through Ca2+ signaling (Kadota et al, 2015) (Fig 2). Ca2+ not only activates RBOHD directly via its cytoplasmic Ca2+‐binding EF‐hand domains but also indirectly via Ca2+‐regulated kinase‐mediated RBOHD phosphorylation (Ogasawara et al, 2008; Dubiella et al, 2013). Interestingly, BIK1 and CALCIUM DEPENDENT PROTEIN KINASE 5 (CPK5) activate RBOHD through phosphorylation at distinct sites (Dubiella et al, 2013; Kadota et al, 2014; Li et al, 2014). While target residues have been described to be strictly required for PTI‐induced ROS bursts (Nühse et al, 2007), individual contribution from other phosphorylation sites and the impact of certain phosphorylation patterns remain to be uncovered.
Figure 2. Ca2+ and ROS signals are tightly interconnected.
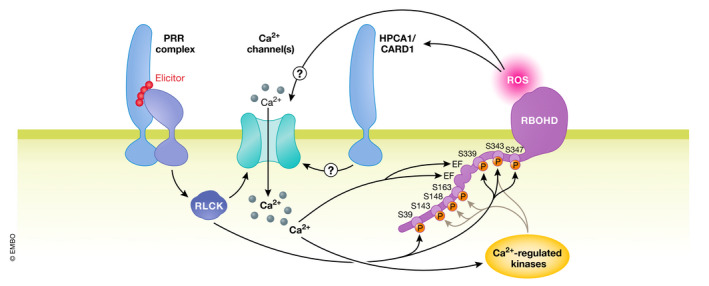
Upon activation of PRR complexes during PTI, RLCKs activate Ca2+ channels leading to cytoplasmic Ca2+ signals. Ca2+ ions can directly activate the NADPH‐oxidase RBOHD through binding to its N‐terminal EF‐hands, but also induce the activity of Ca2+‐regulated kinases that phosphorylate the cytoplasmic N terminus of RBOHD (indicated by grey arrows targeting RBOHD p‐sites). In addition, RLCKs directly phosphorylate the N terminus and thereby activate RBOHD (indicated by black arrows targeting RBOHD p‐sites). Reactive oxygen species derived from RBOHD activity can be perceived by cysteine pairs of the RK HPCA1/CARD1. This is required for H2O2 induced Ca2+ signals in Arabidopsis, the signaling pathway downstream of HPCA1 activation is not known.
A recent AEQ‐based screen for impaired H2O2‐induced Ca2+ signaling identified an LRR‐RK, HYDROGEN PEROXIDE INDUCED Ca2+ INCREASE 1 (HPCA1), as a putative ROS sensor (Wu et al, 2020a). Interestingly, HPCA1 was independently identified as CANNOT RESPOND TO DMBQ 1 (CARD1), which showed a loss of response to the quinone compound 2,6‐dimethoxy‐1,4‐benzoquinone (DMBQ), which regulates interactions with parasitic plants and also triggers HPCA1/CARD1‐dependent Ca2+ signaling (Laohavisit et al, 2020). Both the nature of the channel(s) that are regulated by HPCA1/CARD1, as well as the exact role of ROS in regulating Ca2+ signaling via such sensor(s) remain unclear. Interestingly, AEQ‐measured calcium signals in response to H2O2 were reduced in cngc2 and cngc4 mutants (Tian et al, 2019), suggesting that these channels may function downstream of ROS perception.
Shaping immune signals via Ca2+ efflux
Ca2+ signals are generated via the coordinated action of channels and active transporters and involve influx from the apoplast and release from intracellular stores (Spalding & Harper, 2011; Edel et al, 2017; Resentini et al, 2021). In addition, plants possess three major families of proteins that mediate active Ca2+ transport out of the cytosol: Ca2+/H+ exchangers (CAXs), autoinhibited Ca2+‐ATPases (ACAs) and ER Ca2+‐ATPases (Geisler et al, 2000; Shigaki & Hirschi, 2000; García Bossi et al, 2020). ACA autoinhibition can be relieved by Ca2+/CaM‐binding, which allows for rapid feedback regulation of Ca2+ signals (Geisler et al, 2000). The PM‐localized ACA8 and its homolog ACA10 were identified as interactors of FLS2, and aca8 aca10 mutants displayed quantitative defects in flg22‐induced calcium signals and compromised resistance to P. syringae infection (Frei dit Frey et al, 2012), as well as disturbed stomatal closure upon PAMP perception (Yang et al, 2017), suggesting that Ca2+ efflux across the PM to the apoplast shapes Ca2+ signaling during PTI.
Two tonoplast‐localized ACAs, ACA4 and ACA11, have also been implicated in immunity, as aca4 aca11 mutants display autoimmune phenotypes and spontaneous cell death (Boursiac et al, 2010). Although aca4 aca11 mutants have wildtype total calcium content (Boursiac et al, 2010), subsequent work has revealed that basal cytosolic calcium levels are elevated in aca4 aca11 (Hilleary et al, 2020). Elicitor‐induced calcium signals also show elevated peaks in aca4 aca11 mutants (Fig 3), which can be rescued by mis‐localization of PM ACAs to the tonoplast (Hilleary et al, 2020), indicating that transport of Ca2+ into the vacuole is critical to maintain Ca2+ homeostasis and modulate signaling during PTI.
Figure 3. Disturbance of the Ca2+ efflux machinery impairs plant immunity.

Ca2+ exchangers (CAX) and autoinhibited Ca2+‐ATPase (ACAs) reside at the PM or tonoplast and establish low cytoplasmic Ca2+ concentrations and rapid termination of Ca2+ signals through export of the Ca2+ ions into the apoplast or vacuolar lumen (A). This function is disturbed in Arabidopsis aca4 aca11 mutants, which consequently show an autoimmune phenotype (B). PTI induced Ca2+ signatures are compromised in those lines, with slower onset of the signal, and higher peak concentration and retarded reduction of the Ca2+ signals. Schematic Ca2+ signatures as reported by Hilleary et al (2020) (C).
Plasma membrane‐localized Ca2+ channels involved in immunity
Extensive work has demonstrated that elicitor‐induced Ca2+ signals strictly require PM‐localized, Ca2+‐permeable channels, as treatment with blockers such as Gd3+ or La3+ abolishes such signals (Blume et al, 2000; Grant et al, 2000; Lecourieux et al, 2002; Kwaaitaal et al, 2011; Ranf et al, 2011; Maintz et al, 2014; DeFalco et al, 2017). While such studies clearly implicate Ca2+‐permeable channels as components of immune signaling, their nature has remained hidden. However, recent work has started to decipher how Ca2+ signals are generated upon immune activation, and the defense‐related roles of several classes of plant Ca2+ channels have begun to be characterized. Below, we discuss immunity‐related channel candidates by their phylogenetic groups rather than following a chronological order of identification or a strict PTI/ETI dichotomy.
CNGCs—from strong phenotypes to complex regulation
One of the first families of potential Ca2+ channels identified in plants were the tetrameric cyclic nucleotide‐gated channels (CNGCs) (Köhler & Neuhaus, 1998). Plant CNGCs comprise large gene families (e.g., 20 members in Arabidopsis) (Mäser et al, 2001) and are named for their topology and domain organization, which are reminiscent of mammalian cyclic nucleotide‐gated (CNG) and hyperpolarization‐activated cyclic nucleotide‐modulated (HCN) families (Kaupp & Seifert, 2002; Matulef & Zagotta, 2003). Individual CNGCs have six transmembrane helices and cytosolic N and C termini, with the cyclic nucleotide‐binding domain (CNBD) located within the CNGC C terminus (Kaplan et al, 2007). While previous reports have indicated that the CNBDs of plant CNGCs may bind cyclic nucleotides (Baxter et al, 2008), and some electrophysiological analyses have indicated that application of cAMP or cGMP can promote CNGC activity (Leng et al, 2002; Zhang et al, 2007; Gao et al, 2014, 2016; Meena et al, 2019), it remains unclear whether cyclic nucleotides are bona fide agonists for plant CNGCs in planta. Furthermore, the existence of guanylate and adenylate cyclases (GCs and ACs) in plant proteomes is still under debate and will not be discussed in detail here. Indeed, while studies suggest multiple plant proteins, including RKs, to display GC activity (Qi et al, 2010; Turek & Irving, 2021), the low determined in vitro activities of the putative GCs and the position of their putative active sites within the kinase domains of RKs argues against a physiological relevance for such potential GC activity (Ashton, 2011; Bojar et al, 2014).
Nevertheless, extensive electrophysiological work over the past two decades has shown that at least some CNGCs form Ca2+‐permeable, non‐selective cation channels (Jarratt‐Barnham et al, 2021). CNGCs are directly regulated by the conserved Ca2+ sensor calmodulin (CaM), with one or more CaM‐binding domains (CaMBDs) found within the cytosolic C termini of all CNGCs examined to date (Arazi et al, 1999; Köhler & Neuhaus, 2000; Hua et al, 2003; Fischer et al, 2013, 2017; DeFalco et al, 2016a) as well as the N terminus of some CNGC isoforms (DeFalco et al, 2016a). Ca2+/CaM regulation of CNGCs is complex (DeFalco et al, 2016b) as a Ca2+‐independent IQ motif CaMBD at the C‐terminal end of the channel is essential for CNGC function (DeFalco et al, 2016a; Pan et al, 2019), with additional Ca2+‐dependent CaMBDs providing negative (feedback) regulation (DeFalco et al, 2016a; Pan et al, 2019; Tian et al, 2019).
Plant CNGCs are divided into four subfamilies based on phylogeny, with group IV CNGCs further divided into groups IVa and IVb (Mäser et al, 2001). The best‐studied CNGCs to‐date are the two Arabidopsis group IVb members, CNGC2 and CNGC4, which were first isolated as the defense, no death (dnd) or HR‐like lesion mimic (hlm) mutants dnd1 and dnd2/hlm1 (null mutants of CNGC2 and CNGC4, respectively) (Clough et al, 2000; Balagué et al, 2003; Jurkowski et al, 2004). The dnd mutants were initially described to be defective in the induction of HR, despite still being able to carry out ETI to avirulent pathogens (Yu et al, 1998). These dnd mutants display numerous phenotypic defects, including dwarf morphology, delayed flowering, elevated concentrations of the phytohormone salicylic acid (SA), spontaneous cell death, and dis‐regulated auxin signaling (Clough et al, 2000; Balagué et al, 2003; Chan et al, 2003; Jurkowski et al, 2004; Chin et al, 2013; Chakraborty et al, 2021). In keeping with the immune‐related phenotypes of dnd1/cngc2 mutants, CNGC2 was also suggested to be a mediator of Ca2+ fluxes in plant immunity, as production of the signaling molecule nitric oxide (NO) was reported to be reduced in cngc2 mutants compared to WT plants after treatment with the PAMP lipopolysaccharide (LPS) (Ali et al, 2007). The same study used pharmacological inhibitors to implicate CaM, Ca2+ channels, and a NO synthase (NOS)‐type protein to be required for this process. Given the lack of mammalian‐type NOS enzymes in land plants (Santolini et al, 2017) and the myriad functions of CaM (DeFalco et al, 2010), results from such pharmacological studies must however be interpreted cautiously. Subsequent work using AEQ reporter lines suggested that CNGC2 is required for full Ca2+ signals in response to some but not all elicitors (Ma et al, 2012). Given the convergence of signaling downstream of diverse PRRs (Couto & Zipfel, 2016; Bjornson et al, 2021), it remains unclear how such specificity may be achieved. Interestingly, virus‐induced gene silencing (VIGS) of IVb isoforms in tomato compromised ROS production in response to flg22, further suggesting that these CNGCs may positively regulate PTI (Saand et al, 2015).
Recently, loss‐of‐function cngc2 and cngc4 mutants were each isolated in an AEQ‐based forward genetic screen for compromised Ca2+ signaling upon flg22 treatment (Tian et al, 2019). Both mutants displayed defects in Ca2+ influx and ROS production after treatment with flg22 and exhibited compromised resistance to P. syringae. Remarkably, these phenotypes were however strictly dependent on high Ca2+ concentrations in the growth media, as cngc2 and cngc4 responses under low Ca2+ growth were indistinguishable from those of WT plants. Interestingly, PRR signaling mutants, such as bik1, do not display such conditional phenotypes (Li et al, 2014; Ranf et al, 2014; Monaghan et al, 2015). Detailed electrophysiological characterization of the heterologously expressed channels in Xenopus laevis oocytes found the single subunits to be inactive, while CNGC2‐CNGC4 heteromers produce strong currents (Tian et al, 2019), in keeping with a model wherein these channel subunits function together (Chin et al, 2013). CNGC2‐CNGC4 currents were inhibited by CaM; further experiments suggested that phosphorylation of the CNGC4 C terminus by BIK1 can partially release this negative regulation (Tian et al, 2019) (Fig 4A). This work further highlights the complex regulation to which CNGCs are likely subject, including by CaM, phosphorylation, and, potentially, ligand‐binding (Jarratt‐Barnham et al, 2021).
Figure 4. CNGCs fulfil diverse roles in plant immune signaling.
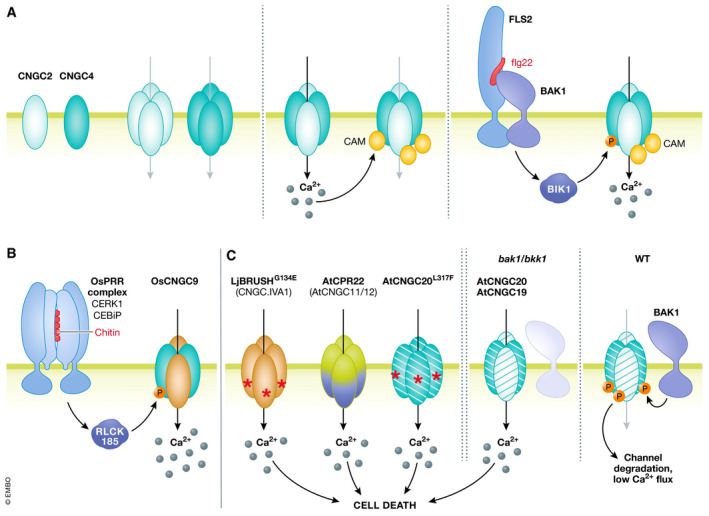
CNGCs form homo‐ or heterotertramers at the PM. Arabidopsis CNGC2 and CNGC4 homotetramers are inactive, but heterotetramers allow cation fluxes into the cytosol. Ca2+‐bound Calmodulin (CAM) inhibits those channels, generating a negative feedback loop. Upon initiation of PTI, activated BIK1 phosphorylates CNGC4 to release CAM‐mediated inhibition and to induce Ca2+ influx (A). In rice, PRR complexes activate RLCK185 upon ligand perception, which phosphorylates and thereby activates OsCNGC9. If the OsCNGC9 containing tetramer is homomeric or heteromeric is not known (B). In Arabidopsis, CNGC activity can lead to the induction of cell death via to date not resolved signaling pathways. CNGC19 and CNGC20 form complexes at the PM, and are phosphorylated by BAK1, which initiates degradation of the channels. In bak1/bkk1 coRK mutants, accumulation of CNGC19/CNGC20 channels leads to Ca2+ influx, ultimately causing cell death.
Both cngc2 and cngc4 mutants are hypersensitive to Ca2+ concentration in growth media (Chan et al, 2003; Chin et al, 2013), and their pleiotropic dnd phenotypes have been suggested to be caused by the mutant’s inability to take up Ca2+ from the apoplast into the cells in the vicinity of vasculature (Wang et al, 2017). Over‐accumulation of apoplastic Ca2+ and the resulting perturbations of both tissue‐ and cellular Ca2+ homeostasis may thus (at least partially) cause cngc2 (and cngc4) phenotypes, though this will require further study to resolve fully. Given that PTI is not affected in cngc2 and cngc4 mutants grown at low Ca2+ concentrations (Tian et al, 2019), at such growth conditions—under which no growth defects also occur—other, currently unknown Ca2+ channels must also contribute to PTI (Dietrich et al, 2020). Recent studies reported a member of CNGC subfamily II, AtCNGC6, to be involved in the generation of Ca2+ signals during immunity after perception of the DAMP eATP, supporting the possibility of diverse CNGC subunits playing specific roles in plant immune responses (Duong et al, 2022).
CNGCs and cell death
A genetic screen in rice (Oryza sativa, Os) recently identified loss‐of‐function mutants of OsCNGC9 (a group III CNGC and homolog of Arabidopsis CNGC18) that displayed compromised resistance to rice blast disease and lesion‐mimic phenotypes after flowering (Wang et al, 2019b). PAMP‐induced Ca2+ currents across the PM were found to be strongly diminished in Oscngc9 mesophyll cells compared to WT controls and, using an elegant heterologous reconstitution assay in mammalian cell culture, the authors demonstrated activation of the channel by OsRLCK185, a rice member of the RLCK‐VII/PBL family that functions downstream of chitin perception (Wang et al, 2019b) (Fig 4B). The autoimmune phenotypes of Oscngc9 mutants are reminiscent of Arabidopsis cngc2 and cngc4, it will therefore be interesting to determine whether such autoimmune phenotypes are due to these channels being guarded by NLRs and/or through perturbed Ca2+ homeostasis.
In contrast to the loss‐of‐function mutants described above, several gain‐of‐function CNGC mutants have also been isolated from genetic screens. These include several instances of (semi‐) dominant gain‐of‐function mutations that trigger autoimmunity such as cpr22 (caused by expression of an in‐frame CNGC11/12 chimera) (Yoshioka et al, 2006; Urquhart et al, 2007) and cngc20‐4 (caused by a leucine to phenylanaline mutation within one of the transmembrane helices of CNGC20) (Zhao et al, 2021) of Arabidopsis and the brush mutant of Lotus japonicus (hereafter, Lotus), which is caused by an N‐terminal glycine to glutamic acid mutation in the Lotus homolog of Arabidopsis CNGC19 (Chiasson et al, 2017) (Fig 4C). While such gain‐of‐function mutants must be interpreted cautiously, detailed study of these mutants suggests that dis‐regulated CNGCs can induce Ca2+‐ and SA‐dependent immunity and HR‐like cell death (Yoshioka et al, 2006; Urquhart et al, 2007; DeFalco et al, 2016a; Zhao et al, 2021), suggesting possible roles in ETI signaling and immunity more generally (Moeder et al, 2019).
CNGC20 has also recently been identified as a positive regulator of a specific form of autoimmunity (Yu et al, 2019). Loss of the SERK family co‐receptors BAK1/SERK3 and BAK1‐LIKE 1 (BKK1/SERK4) triggers constitutive cell death and seedling lethality (He et al, 2007; Kemmerling et al, 2007; Schwessinger et al, 2011). VIGS‐based screen revealed that this cell death is dependent on CNGC20 and to a lesser extent its close IVa homolog CNGC19 (Yu et al, 2019). This study further proposed a mechanism wherein SERKs phosphorylate the C terminus of CNGC20 to destabilize the channel in the absence of immunogenic stimuli, thereby precluding detrimental Ca2+ influx and cell death (Yu et al, 2019), adding an interesting component to the regulation of Ca2+ fluxes in immunity (Fig 4C). Whether or not recruitment of BAK1 into PRR complexes after elicitor recognition permits CNGC20 phosphorylation and therefore induces CNGC20 activity should be addressed in future studies. Recent work suggests a more complex role for Ca2+ in BAK1‐related cell death, as NLRs that mediate bak1 bkk1 autoimmunity have been identified, including the NLR CONSTITUTIVE SHADE‐AVOIDANCE 1 (CSA1) (preprint: Schulze et al, 2021) and helper NLRs of the ACTIVATED DISEASE RESISTANCE 1 (ADR1)‐like family (Wu et al, 2020b). Strikingly, these ADR1‐type helper NLRs have recently been proposed to themselves act as Ca2+‐permeable cation channels (see detailed discussion below).
The other CNGC‐IVa member in Arabidopsis, CNGC19, was identified in a screen for genes whose expression was upregulated by mechanical wounding, and cngc19 mutants were found to be more susceptible to Spodoptera littoralis caterpillars, likely due to impaired jasmonate (JA) and alipathic glucosinolate production (Meena et al, 2019). Interestingly, Ca2+ signals in response to the DAMP AtPep1 were also reduced in cngc19 mutants (Meena et al, 2019), though other work using cngc19 cngc20 protoplasts expressing GCaMP3 suggested that group IVa CNGCs are not required for the Ca2+ signals in response to flg22 (Yu et al, 2019). CNGC19 was also implicated in responses to the root‐colonizing mutualist endophytic fungus Piriformospora indica (Jogawat et al, 2020). cngc19 mutants display clear phenotypes with respect to the symbiosis‐induced gain in growth rate; however, only minor defects in cytoplasmic Ca2+ rises after treatment with cell wall extracts from P. indica have been reported. This indicates the involvement of additional channel(s) (Jogawat et al, 2020).
An intriguing aspect of plant immune signaling is the propagation of electrical and second messenger‐based signals through the plant body, despite the obvious lack of any neurons or nervous system tissues in plants. In addition to reduced AtPep1 responses, cngc19 mutants also displayed reduced systemic Ca2+ signals after mechanical wounding in the vasculature (Meena et al, 2019). Such signaling has long been associated with glutamate receptor‐like (GLR) channels, which are discussed below.
GLRs – the long road to plant immunity
GLRs form a family of PM‐localized, ligand‐gated Ca2+ channels. Plant GLRs are named for their homology to metazoan ionotropic glutamate receptors (iGluRs), which are ligand‐regulated, homo‐ or heterotetrameric cation channels functioning in animal nervous systems. In Arabidopsis, GLRs form a 20‐member family that is sub‐divided into three clades: GLR1s, GLR2s and GLR3s; (Lam et al, 1998); individual members of the family have been implicated in various physiological processes (Wudick et al, 2018). GLRs feature a large, extracellular N‐terminal domain, which perceives amino acid ligands, three transmembrane helices and a short, cytosolic C terminus (Alfieri et al, 2020).
GLRs were first implicated in the generation of Ca2+ signals upon elicitor perception by pharmacological experiments using iGluR inhibitors, which reduced PAMP‐induced Ca2+ signals (Kwaaitaal et al, 2011). Arabidopsis glr3.3 mutants were subsequently found to have compromised immunity toward the oomycete pathogen Hyaloperonospora arabidopsidis. In the same study, however, Ca2+ measurements in AEQ‐expressing glr3.3 lines did not show reduced signals after treatment with oligogalacturonides (OGs), products of hydrolyzed host cell walls that act as DAMPs during H. arabidopsidis colonization (Manzoor et al, 2013). Similarly, glr3.3 was also found to be more susceptible to P. syringae (Li et al, 2013), although formation of Ca2+ signals was not analyzed in that study. Together, such findings suggested a role for GLR3.3 in plant immunity which is distinct from its role in mediating the formation of the early Ca2+ signal. Indeed, ground‐breaking work from the labs of Ted Farmer and Simon Gilroy instead unraveled the role of GLR3s in the propagation of long‐distance signals and the formation of systemic immune responses. Multiple clade 3 GLRs were initially found to be required for the generation of electrical signals necessary for the induction of defense responses in distal tissues of plants after mechanical wounding or larval feeding on local leaves (Mousavi et al, 2013). Using Arabidopsis plants stably expressing GCaMP3, another study found that GLR3.3 and GLR3.6 are required for the generation of Ca2+ signals that propagate through the vasculature upon wounding or feeding (Toyota et al, 2018), and, correspondingly, glr3.3 glr3.6 plants were shown to be more susceptible to S. littoralis (Nguyen et al, 2018). Simultaneous measurements of the electrical signals and cytoplasmic Ca2+ concentrations revealed that membrane depolarization preceded the rise of Ca2+ levels, a temporal sequence that was also observed in mesophyll cells after perceptrion of flg22 (Nguyen et al, 2018; Li et al, 2021). Such results highlight the specific role of clade 3 GLRs in systemic signaling. Taken together, those studies support a model wherein interconnected electrical, Ca2+ and ROS signals, as well as activity of the tonoplast‐localized cation channel TWO‐PORE CHANNEL 1 (TPC1) are required for effective long‐distance signal propagation in plants (Steinhorst & Kudla, 2014; Evans et al, 2016; Choi et al, 2017; Farmer et al, 2020; Johns et al, 2021).
It remains unclear whether clade 3 (or other) GLRs are also direct or indirect targets of PRR‐activated signaling pathways. It has been proposed that OG perception involves RKs of the WALL‐ASSOCIATED KINASE (WAK) family of epidermal growth factor (EGF)‐motif containing RKs (Brutus et al, 2010; Kohorn & Kohorn, 2012), while local Ca2+ signals in response to aphid feeding are BAK1‐ as well as GLR3.3‐ and GLR3.6‐dependent (Vincent et al, 2017). This suggests that the PRR signaling machinery may regulate GLR activity. Extracellular glutamate, which can act as a DAMP upon cell disruption, is also capable of inducing Ca2+ signals that are abolished in glr3.3 glr3.6 mutants (Toyota et al, 2018; Shao et al, 2020), suggesting apoplastic amino acid(s) may act as agonist ligands for GLRs. The direct binding of glutamate to GLRs was further resolved through structural analysis of GLR3 ectodomains (Alfieri et al, 2020; Gangwar et al, 2021; Green et al, 2021). The role of clade 3 GLRs in systemic signaling has been recently reviewed in detail (Grenzi et al, 2021a), while the details of how ligand‐gating and/or PRR signaling coordinate the activation of GLR3 channel activity remain to be fully resolved.
While clade 3 GLRs and their specific role in intercellular and long‐distance signaling are to date the best studied, other GLRs have also been recently found to play roles in the immune system. The Arabidopsis clade 2 GLRs GLR2.7 and GLR2.9 were recently identified in a large‐scale transcriptomic analyses as so‐called “core immunity response” (CIR) genes, which were transcriptionally upregulated in response to a panel of elicitors but not abiotic stresses (Bjornson et al, 2021) (Fig 5). GLR2.7 and GLR2.9 form a tandemly‐arranged, closely‐related cluster along with GLR2.8, and glr2.7 glr2.8 glr2.9 triple mutants displayed defects in Ca2+ responses upon treatment with a variety of elicitors and reduced immunity against P. syringae (Bjornson et al, 2021). In keeping with their identification as CIR genes, these GLR2s were not found to contribute to Ca2+ signals during abiotic stress, suggesting that PTI involves common signaling components downstream of diverse elicitor/PRR complexes, but distinct from those involved in abiotic stress responses. As with GLR3s, how PRR complex activation mechanistically triggers rapid Ca2+ fluxes involving these GLR2s remains to be uncovered, as does the potential role for amino‐acid binding in this process.
Figure 5. PRR signaling controls GLR2 abundance.
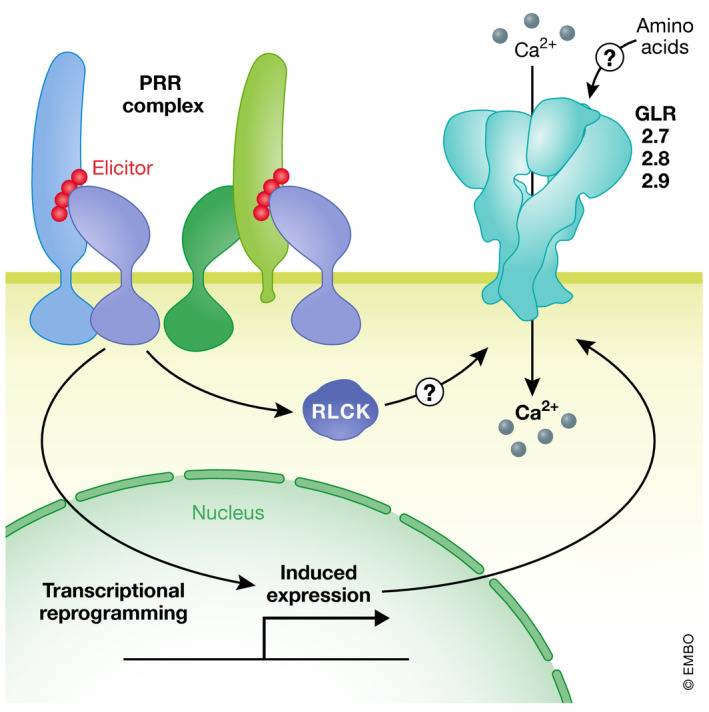
Upon perception of various elicitors, transcription of clade 2 GLRs is strongly induced. Activity of GLR2.7, GLR2.8, and GLR2.9 is required for complete PTI induced rapid Ca2+ influx, arguing for direct regulation of those channels in that process. The signaling pathway leading to this activation has not been resolved yet.
OSCAs, Ca2+ and stomatal gatekeeping
While the CNGC and GLR families of proteins were annotated shortly following release of the first sequenced plant genomes, the REDUCED HYPEROSMOLALITY, INDUCED CA2+ INCREASE (OSCA) family was only recently identified. OSCAs have nine transmembrane helices, with a short extracellular N terminus and a larger C terminus, and constitute a 15‐member family in Arabidopsis (Yuan et al, 2014). OSCA1.1 was identified in an AEQ‐based screen for regulators of Ca2+ signaling in response to osmotic stress (Yuan et al, 2014) and its homolog OSCA1.2 (also named CALCIUM PERMEABLE STRESS‐GATED CATION CHANNEL 1, CSC1) was identified through a heterologous screening of uncharacterized Arabidopsis transmembrane proteins for Ca2+ channel activity (Hou et al, 2014). Both OSCA1.1 and OSCA1.2/CSC1 were shown to be Ca2+‐permeable channels (Hou et al, 2014; Yuan et al, 2014), while subsequent structural, electrophysiological, and bioinformatic studies have revealed that OSCAs represent an evolutionarily conserved family of mechanosensitive, Ca2+‐permeable cation channels (Jojoa‐Cruz et al, 2018; Liu et al, 2018; Murthy et al, 2018).
In addition to systemic and long‐distance immune signaling, Ca2+ signaling also occurs at the single cell level in stomatal immunity. Stomata are gas‐exchange pores in the leaf epidermis that are formed by pairs of guard cells, with stomatal aperture controlled by changes in guard cell turgor (Lawson & Matthews, 2020). Aside from controlling gas exchange, stomata also serve as key points of entry for foliar pathogens (Melotto et al, 2017), and elicitor perception leads to rapid stomatal closure (Melotto et al, 2006; Desikan et al, 2008; Zeng & He, 2010). Stomatal closure is controlled by activation of SLOW ANION CHANNEL‐ASSOCIATED 1 (SLAC1) and/or SLAC1 HOMOLOGUE 3 (SLAH3), which mediate guard cell anion efflux, and which can be activated by Ca2+‐dependent or ‐independent phosphorylation cascades (reviewed in (Jezek & Blatt, 2017)). PTI signaling involves Ca2+ influx in guard cells (Thor & Peiter, 2014), and recently Arabidopsis osca1.3 osca1.7 loss‐of‐function mutants were found to be defective in elicitor‐induced stomatal closure (Thor et al, 2020). The mechanism of the underlying core signaling pathway was duly unraveled, as OSCA1.3 was identified as a direct substrate of BIK1, which phosphorylates the channel on its N‐terminal cytosolic loop, providing a direct molecular connection from the activated PRR complex to the Ca2+ signal generation in guard cells (Fig 6A). PAMP treatment triggered phosphorylation of this BIK1‐dependent phosphosite (Benschop et al, 2007; Thor et al, 2020), and phosphorylation was found to promote the channel activity of OSCA1.3 in heterologous electrophysiological measurements (Thor et al, 2020). Ca2+ signaling defects in osca1.3 osca1.7 mutants were also specific to guard cells, as signals in seedlings and epidermal tissues were unaffected. This study indicates a specific role of OSCA1.3 and OSCA1.7 in guard cells and stomatal immunity, and future studies may reveal whether other members of this family play additional roles in immunity, as well as how their potential mechano‐regulation contributes to such functions. Remarkably, another route of PRR signaling required for stomatal immunity was recently identified. Upon perception of chitin by CHITIN‐ELICITOR RECEPTOR KINASE 1 (CERK1)/ LYSM‐CONTAINING RECEPTOR‐LIKE KINASE 5 (LYK5) complexes, PBL27 directly phosphorylates the anion channel SLAH3 (Liu et al, 2019) (Fig 6B). Why different elicitors activate specific pathways to achieve the same physiological response, and whether the chitin induced pathway indeed functions without contribution of Ca2+ signaling, remains to be resolved.
Figure 6. PRR signaling controls Ca2+‐dependent and Ca2+‐independent pathways leading to stomatal immunity.
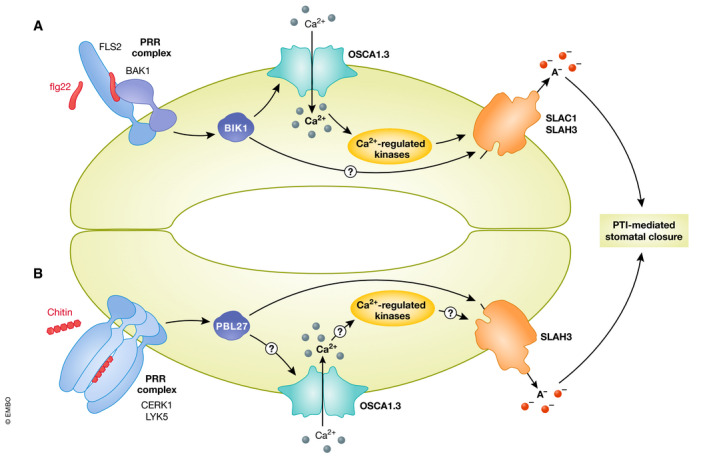
Perception of bacterial flg22 leads to activation of BIK1 and phosphorylation of the Ca2+ channel OSCA1.3. Subsequent Ca2+ influx into the cytosol is required for guard cell closure. This closure is likely achieved through the activation of Ca2+‐regulated kinases, which in turn phosphorylate SLAC1 or SLAH3 anion channels. Upon channel activation of those channels, resulting ion fluxes cause turgor loss in the guard cell and stomatal closure (A). Perception of chitin by CERK1/LYK5 complexes activates the RLCK PBL27, which directly phosphorylates SLAH3, leading to stomatal closure (B).
In addition to the osca mutants defective in elicitor‐induced Ca2+ influx, disturbance of Ca2+ signals through loss of the ACA 8 and 10 cause loss of pathogen‐induced stomatal closure (Yang et al, 2017). Mutations in either of those Ca2+ pumps or their interactor BONZAI 1 (BON1) caused enhanced steady state Ca2+ signals and additionally failed to generate stimulus dependent stomatal Ca2+ oscillations due to retarded Ca2+ efflux after initial influx (Yang et al, 2017). Interestingly, the effect on guard cell Ca2+ fluxes in osca1.3 osca1.7 mutants after flg22 application was quantitative (Thor et al, 2020), in contrast to the near‐complete loss of flg22‐induced stomatal closure in these mutants, while the defects in ACA8 and ACA10 activity still allowed the generation of Ca2+ signals but nevertheless prevented stomatal closure (Yang et al, 2017). Together, these studies suggest that minor perturbations in Ca2+ signals can trigger detrimental effects on downstream physiological processes.
ANNs—atypical Ca2+ channels?
Annexins (ANNs) are small proteins occurring in both prokaryotes and eukaryotes and form a family of eight members in Arabidopsis (Laohavisit & Davies, 2011; Clark et al, 2012). Unlike other Ca2+‐permeable channels, ANNs are soluble proteins that lack transmembrane helices and instead reversibly bind negatively charged phospholipids, a process that is controlled by Ca2+ (Laohavisit & Davies, 2011). ANNs have previously been suggested either to regulate Ca2+ fluxes or provide Ca2+ transport activity themselves in response to H2O2 and salt stress (Laohavisit et al, 2012; Ma et al, 2019).
Recently, Arabidopsis ANN1 was identified as a positive regulator of local and systemic Ca2+ responses that are induced upon mechanical wounding and perception of S. littoralis oral secretions, with ANN1 loss‐of‐function or overexpression lines displaying enhanced or decreased susceptibility toward S. littoralis, respectively (Malabarba et al, 2021). Furthermore, ann1 mutants were compromised in both transcriptional responses and JA production—phenotypes remarkably reminiscent of those reported for cngc19 mutants (Meena et al, 2019). In this context, it will be an interesting target of future studies to parse how ANN1‐ and CNGC19‐mediated Ca2+ influx is able to distinguish between the induction of local and long‐distance signals.
In addition to those wound‐induced signals, ANN1 was also found to be involved in the generation of Ca2+ signals upon treatment of Arabidopsis with eATP (Mohammad‐Sidik et al, 2021), which is perceived as a DAMP by the L‐type lectin RK DOES NOT RESPOND TO NUCLEOTIDES 1/P2 RECEPTOR KINASE 1 (DORN1/P2K1) (Choi et al, 2014). The quantitative defect in eATP‐induced Ca2+ in ann1 mutants suggests ANN1 as part of the signaling pathway downstream of PRR activation; however, it remains unclear whether ANN1 itself acts as a Ca2+ transporter, as well as how such activity is regulated. Furthermore, ANN1 was reported to interact with the chitin‐perceiving PRR CERK1 and thereby connects chitin perception and salt stress responses, a process in which ANN1 was previously characterized (Laohavisit et al, 2013; Espinoza et al, 2017). However, the underlying molecular mechanism remains to be resolved. Interestingly, ANN1 was independently identified as a mediator of Arabidopsis cold stress tolerance and was shown to positively regulate Ca2+ signals after cold shock (Liu et al, 2021). In this case, ANN1 Ca2+ transport activity was documented using electrophysiological characterization in X. laevis oocytes, with phosphorylation by the kinase OST1 having a positive effect on this activity (Liu et al, 2021). Whether similar regulatory phosphorylation of ANNs occurs in the context of immune signaling remains to be discovered, as does the mechanism by which activity of ANNs and GLRs are coordinated in the formation of long‐distance Ca2+ signals upon wounding.
Ankyrin repeat domain proteins – a new class of Ca2+‐permeable channels in immunity?
Recently, LR14a, a wheat six‐transmembrane PM intrinsic protein with a N‐terminal cytoplasmic domain containing 12 ankyrin repeats was found to confer resistance to leaf rust in wheat (Kolodziej et al, 2021). Silencing of LR14a led to increased growth of the causal fungal pathogen Puccinia triticina and reduced induction of HR flecks. Interestingly, LR14a shares structural similarity with the mammalian protein TRANSIENT RECEPTOR POTENTIAL CHANNEL SUBFAMILY A MEMBER1 (TRPA1) (Suo et al, 2020; Kolodziej et al, 2021). TRPs are Ca2+‐permeable cation channel, suggesting a similar function of LR14a. LR14a was found to be required for the transcriptional induction of 160 genes upon infection with P. tricitina which were associated with the gene ontology term “Ca2+‐binding”. Overexpression of LR14a in Nicotiana benthamiana leaves induced a water‐soaking like phenotype indicative for osmotic disbalance, which could be prevented by the application of the Ca2+ channel blocker La3+ (Kolodziej et al, 2021). These findings support the possibility that LR14a acts as a Ca2+ channel, although electrophysiological characterization of the protein remains lacking.
Interestingly, another ankyrin repeat domain containing protein, Arabidopsis ACCELERATED CELL DEATH 6 (ACD6), is a positive regulator of cell death, as multiple acd6 alleles were found to induce varying degrees of autoimmunity and have been subject of research for over 20 years (Rate et al, 1999; Lu et al, 2003). While the molecular basis of ACD6 action remained largely elusive, a recent study has documented ACD6‐induced ion channel activity upon heterologous expression in X. laevis oocytes (preprint: Zhu et al, 2021). Furthermore, autoimmunity of the acd6‐1 allele could be abolished by growth at low [Ca2+], suggesting similar perturbances of Ca2+ homeostasis as reported for the dnd mutants (Chan et al, 2003; Chin et al, 2013; Wang et al, 2017; preprint: Zhu et al, 2021). ACD6 had been previously found to be associated with multiple RKs (Tateda et al, 2014; Zhang et al, 2017); however if and how this contributes to its regulation during immune responses has not been resolved.
NLRs – wheels of death
PTI signaling immediately downstream of elicitor perception by PRRs involves a characteristic rapid and transient Ca2+ signal. Understanding elicitor‐triggered Ca2+ fluxes has been the focus of most studies of immunity‐related Ca2+ channels. ETI signaling, by contrast, involves long‐term, sustained Ca2+ signals (as discussed above). As outlined previously, PTI and ETI induce qualitatively similar signaling outputs, some of which (e.g., ROS, MAPK activation) have been shown to involve the same molecular components in both pathways (Kadota et al, 2019). It was thus reasonable to expect that similar channels were involved in Ca2+ signaling during both PTI and ETI, a supposition reinforced by the ETI‐like autoimmune and cell death phenotypes of several Ca2+ channel mutants, as discussed above. However, the landscape of Ca2+ channels in immunity was recently revealed to be more complex than previously thought.
Plant NLRs have been long hypothesized to form large, multimeric complexes (as it the case in animals, (Jones et al, 2016)). This was finally shown to be the case with structural analysis of the complex of the CNL HOPZ‐ACTIVATED RESISTANCE 1 (ZAR1) and its RLCK interactors RESISTANCE‐RELATED KINASE 1 (RKS1) and PBL2 (Wang et al, 2019a, 2019c). Using cryo‐electron microscopy (cryo‐EM), the authors were able to resolve how activation of ZAR1 via uridylation of the decoy PBL2 by the bacterial effector AvrAC triggers subsequent exchange of ADP to dADP in the ZAR1 NBD, leading the complex to take a radially symmetrical, pentameric structure, termed a resistosome (Fig 7). Interestingly, the N‐terminal α‐helical domains of ZAR1 formed a funnel‐like domain within the resistosome, which was hypothesized to embed into membranes (Wang et al, 2019a). The overall resistosome structure resembled that of mammalian inflammasomes, and of the fungal toxin HET‐S, both of which create pores in membranes upon activation through terminal helical domains and thereby allow ion transport (highlighted in Dangl & Jones, 2019; Mermigka & Sarris, 2019), suggesting that this may be the case in plants. Subsequent work using a combination of detailed electrophysiological characterization and in planta Ca2+ measurements revealed the nature of the ZAR1 resistosome as a non‐selective cation‐channel with permeability to Ca2+ (Bi et al, 2021). This ion permeability is required for ZAR1‐induced cell death, which occurs through disintegration of the PM and cellular rupture (Bi et al, 2021).
Figure 7. The ZAR1 resistosome forms a Ca2+‐permeable pore upon activation.

In the native state, ADP‐bound ZAR1 binds the RLCK RKS1. After delivery of the bacterial effector protein AvrAC, the RLCK PBL2 gets uridylated, which is in turn bound by the ZAR1‐RKS1 complex. Structural rearrangements lead to ADP exchange to ATP and relocalization of the CC domain. Pentamerization of ZAR1‐RKS1‐PBL2UMP complexes leads to the assembly of the resitosome multiprotein complex. The center of the complex is formed by the helices of the five CC domains and displays a funnel‐like form with a central pore. The funnel inserts into the PM and allows cation influx from the apoplast into the cytoplasm. This process is required for the initiation of the hypersensitive response. If cell death is achieved through Ca2+ toxicity, active Ca2+ signaling or a loss of membrane potential through the leak created by the resistosome, is not resolved yet. Besides ZAR1, also RNLs were found to form Ca2+ permeable pores after activation.
In addition to the CNL ZAR1, TNLs have since been shown to also assemble into resistosome‐like structures (Ma et al, 2020; Martin et al, 2020), though no evidence yet indicates that these also form pores in membranes. Instead, helper NLRs of the RNL type such as ADR1 and NRG1.1, which function downstream of TNL sensors, were recently found to form oligomers and constitute ion pores through assembly of their α‐helical N‐terminal domains (Jacob et al, 2021). Auto‐activated forms of both NRG1.1 and ADR1 were found to function as Ca2+‐permeable channels in planta and to induce cell death upon controlled over‐expression. Similar to what had been reported for the ZAR1 resistosome (Bi et al, 2021), the cation permeability of NRG1.1 and ADR1 was dependent of the presence of negatively charged residues within the pore region of the protein complexes (Jacob et al, 2021).
The striking overall similarities found in the ZAR1 resistosome and the channels formed by NRG1.1 and ADR1 raise the question if the formation of ion‐permeable pores is indeed the general function of all helper NLRs. The physiological role of those channels will have to be analyzed in detail in future studies to resolve several open questions regarding channel‐like NLR functions in immunity. After strong, induced overexpression of (auto‐) activated helper NLRs, massive ion fluxes and rapid cell death have been documented (Jacob et al, 2021). This cell death is likely to be a consequence of the loss of ion homeostasis and resulting PM destabilization rather than Ca2+ signaling per se. It therefore remains to be seen whether, under natural infection conditions, effector‐triggered activation of NLR‐formed Ca2+ channels induces bona fide Ca2+ signals that are perceived by Ca2+ sensors to in turn induce physiological responses other than HR. Similarly, it will be critical to resolve how the channel‐like activities of NLRs are interwoven with those of classical Ca2+ channels, given both the ETI‐like phenotypes of numerous channel mutants and the interdependence of the BAK1‐related cell death on both ADR1‐type RNLs (Wu et al, 2020b) and CNGC20 (Yu et al, 2019).
Our understanding of NLR function continues to evolve rapidly, and recent parallel studies have reported NADase activity of the TIR domain of TNLs upon their activation (Horsefield et al, 2019; Wan et al, 2019; Ma et al, 2020). The mechanistic basis of this activity has been resolved with the structure of the TNL RPP1, wherein the tetrameric protein complex was found to form a holoenzyme (Ma et al, 2020). A similar tetramerization upon activation was also been recently reported for the N. benthamiana NLR RECOGNITION OF XopQ 1 (ROQ1) (Martin et al, 2020). How NADase activity regulates downstream signaling pathways remains to be fully characterized; however, it will be of great interest to determine if and how the resulting products (nicotinamide, adenosine diphosphate ribose (ADPR), and a variant of cyclic ADPR (v‐cADPR)) may modulate and/or induce Ca2+ fluxes. The same holds true for another recently‐reported enzymatic activity of TIR domain containing proteins: RESPONSE TO THE BACTERIAL TYPE III EFFECTOR PROTEIN HOPBA1 (RBA1) was recently found to produce 2′,3′‐cAMP/cGMP through hydrolysis of RNA and DNA molecules (preprint: Yu et al, 2021). Production of 2′,3′‐cAMP/cGMP appears to be required for TIR mediated signaling and cell death, but the exact function of those molecules will require further study.
Recently, plant genomes were found to encode proteins with similarities to necroptosis‐inducing MIXED LINEAGE KINASE‐DOMAIN LIKE (MLKL) proteins (Mahdi et al, 2020). In animals, those MLKL proteins are phosphorylated upon necroptosis to induce oligomerization. This causes them to translocate through membrane insertion of an N‐terminal four helix bundle called HeLo domain, which ultimately disturbs membrane integrity and causes cell death (Petrie et al, 2019). Interestingly, Arabidopsis MLKL3 and 4 were found to form tetramers, and loss of MLKL function led to severe defects in immunity toward the obligate biotrophic fungus Golovinomyces orontii via a TNL‐dependent pathway that does not involve the induction of cell death (Mahdi et al, 2020). Remarkably, chemical oligomerization of MLKL HeLo domains was found to be sufficient for the induction of cell death in Arabidopsis (Mahdi et al, 2020). How Arabidopsis MLKLs are regulated during immune responses, if their action also induces Ca2+ fluxes across the PM, and to what extent their functional mechanism is similar to that of the ZAR1 resistosome or the ADR1 type RNLs will be interesting topics for future studies.
Conclusions and outlook: answers, yet more questions
The molecular basis of Ca2+ signaling during immune responses has been a major scientific question within plant biology for decades. As outlined in this review, numerous candidate channel proteins have been identified in recent years as contributing to PTI and/or ETI. However, despite this rapid increase in knowledge, critical questions remain unanswered, and the fact remains that the channel(s) responsible for the early Ca2+ transient during PTI is/are still largely unknown.
The study of immunity and Ca2+ signaling continues to benefit from tool development, and the modern, ever‐growing GECI repertoire has allowed for ever‐more detailed analyses of Ca2+ signals in vivo. However, we must remember that our conceptualization of Ca2+ signaling is at least partially defined by the GECIs we use, and may be too broad. It is possible that loss of individual Ca2+ channels evokes loss of individual Ca2+ signals within micro‐ and nanodomains, which are simply not resolved even by state‐of‐the‐art Ca2+ measurements.
It is remarkable that numerous channels from different families appear to contribute quantitatively to the rapid Ca2+ signal upon elicitor perception. These results beg the question of whether PRR‐mediated signaling cascades indeed target and regulate such a high number of individual channels. One possibility is that such a dividing and reunifying signaling architecture may allow for genetic robustness, although this remains to be explored. It is also possible that individual channels function in cell type‐specific manners, as has been at least suggested in the case of OSCAs in guard cells. It is also possible that each of the Ca2+ channels currently identified are indeed either quantitative contributors and/or regulators, while the channel(s) mediating the major influx still await identification. Indeed, the recent identification of channel families (e.g., OSCAs) or novel characterization of known proteins as potential channels (e.g., NLRs, ankyrin repeat domain proteins) indicates that there remains much to be discovered regarding Ca2+‐permeable channels in plants.
With regards to NLRs functioning as cation channels in ETI, future studies will have to find if they indeed generate Ca2+ signals that evoke specific downstream responses, or if their channel activity rather represents the loss of membrane impermeability, with Ca2+ influx just being a fellow traveler of cell death’s onset. In either case, it will be as well critical to resolve the role of “classical” Ca2+ channels in ETI signaling.
Author contributions
Philipp Köster: Visualization; Writing—original draft; Writing—review & editing. Thomas A DeFalco: Visualization; Writing – original draft; Writing—review & editing. Cyril Zipfel: Funding acquisition; Writing—review & editing.
Disclosure and competing interests statement
The authors declare that they have no conflict of interest.
Acknowledgements
The authors thank all members of the Zipfel lab for feedback on the manuscript. C.Z. has received generous funding from the Gatsby Charitable Foundation, the Biotechnology and Biological Research Council (BB/P012574/1), the European Research Council under the European Union (EU)'s Horizon 2020 Research and Innovation Programme (grant agreements No 309858, project “PHOSPHinnATE” and No 773153, project “IMMUNO‐PEPTALK”), the University of Zürich, and the Swiss National Science Foundation grant no. 31003A_182625. P.K. is supported by the EU Horizon 2020 Research and Innovation Program Marie Skłodowska‐Curie Actions (grant agreement no. 892398). T.A.D. was supported by postdoctoral fellowships from the European Molecular Biology Organization (EMBO LTF 100‐2017) and the Natural Sciences and Engineering Research Council of Canada (NSERC PDF‐532561‐2019).
The EMBO Journal (2022) 41: e110741
References
- Albert I, Böhm H, Albert M, Feiler CE, Imkampe J, Wallmeroth N, Brancato C, Raaymakers TM, Oome S, Zhang H et al (2015) An RLP23‐SOBIR1‐BAK1 complex mediates NLP‐triggered immunity. Nat Plants 1: 15140 [DOI] [PubMed] [Google Scholar]
- Albert I, Hua C, Nürnberger T, Pruitt RN, Zhang L (2020) Surface sensor systems in plant immunity. Plant Physiol 182: 1582–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfieri A, Doccula FG, Pederzoli R, Grenzi M, Bonza MC, Luoni L, Candeo A, Romano Armada N, Barbiroli A, Valentini G et al (2020) The structural bases for agonist diversity in an Arabidopsis thaliana glutamate receptor‐like channel. Proc Natl Acad Sci USA 117: 752–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali R, Ma W, Lemtiri‐Chlieh F, Tsaltas D, Leng Q, Von Bodman S, Berkowitz GA (2007) Death don’t have no mercy and neither does calcium: Arabidopsis CYCLIC NUCLEOTIDE GATED CHANNEL2 and innate immunity. Plant Cell 19: 1081–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostol I, Heinstein PF, Low PS (1989) Rapid stimulation of an oxidative burst during elicitation of cultured plant cells: role in defense and signal transduction. Plant Physiol 90: 109–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arazi T, Sunkar R, Kaplan B, Fromm H (1999) A tobacco plasma membrane calmodulin‐binding transporter confers Ni2+ tolerance and Pb2+ hypersensitivity in transgenic plants. Plant J 20: 171–182 [DOI] [PubMed] [Google Scholar]
- Ashton AR (2011) Guanylyl cyclase activity in plants? Proc Natl Acad Sci USA 108: E96–E98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson MM, Midland SL, Sims JJ, Keen NT (1996) Syringolide 1 triggers Ca2+ influx, K+ efflux, and extracellular alkalization in soybean cells carrying the disease‐resistance gene Rpg4. Plant Physiol 112: 297–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggs E, Dagdas G, Krasileva KV (2017) NLR diversity, helpers and integrated domains: making sense of the NLR identity. Curr Opin Plant Biol 38: 59–67 [DOI] [PubMed] [Google Scholar]
- Balagué C, Lin B, Alcon C, Flottes G, Malmström S, Köhler C, Neuhaus G, Pelletier G, Gaymard F, Roby D (2003) HLM1, an essential signaling component in the hypersensitive response, is a member of the cyclic nucleotide‐gated channel ion channel family. Plant Cell 15: 365–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter J, Moeder W, Urquhart W, Shahinas D, Chin K, Christendat D, Kang H‐G, Angelova M, Kato N, Yoshioka K (2008) Identification of a functionally essential amino acid for Arabidopsis cyclic nucleotide gated ion channels using the chimeric AtCNGC11/12 gene. Plant J 56: 457–469 [DOI] [PubMed] [Google Scholar]
- Benschop JJ, Mohammed S, O’Flaherty M, Heck AJR, Slijper M, Menke FLH (2007) Quantitative phosphoproteomics of early elicitor signaling in Arabidopsis . Mol Cell Proteomics 6: 1198–1214 [DOI] [PubMed] [Google Scholar]
- Bernoux M, Burdett H, Williams SJ, Zhang X, Chen C, Newell K, Lawrence GJ, Kobe B, Ellis JG, Anderson PA et al (2016) Comparative analysis of the flax immune receptors L6 and L7 suggests an equilibrium‐based switch activation model. Plant Cell 28: 146–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi G, Su M, Li N, Liang YU, Dang S, Xu J, Hu M, Wang J, Zou M, Deng Y et al (2021) The ZAR1 resistosome is a calcium‐permeable channel triggering plant immune signaling. Cell 184: 3528–3541.e12 [DOI] [PubMed] [Google Scholar]
- Bjornson M, Pimprikar P, Nürnberger T, Zipfel C (2021) The transcriptional landscape of Arabidopsis thaliana pattern‐triggered immunity. Nat Plants 7: 579–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume B, Nürnberger T, Nass N, Scheel D (2000) Receptor‐mediated increase in cytoplasmic free calcium required for activation of pathogen defense in parsley. Plant Cell 12: 1425–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojar D, Martinez J, Santiago J, Rybin V, Bayliss R, Hothorn M (2014) Crystal structures of the phosphorylated BRI1 kinase domain and implications for brassinosteroid signal initiation. Plant J 78: 31–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller T, Felix G (2009) A renaissance of elicitors: perception of microbe‐associated molecular patterns and danger signals by pattern‐recognition receptors. Annu Rev Plant Biol 60: 379–406 [DOI] [PubMed] [Google Scholar]
- Boursiac Y, Lee SM, Romanowsky S, Blank R, Sladek C, Chung WS, Harper JF (2010) Disruption of the vacuolar calcium‐ATPases in Arabidopsis results in the activation of a salicylic acid‐dependent programmed cell death pathway. Plant Physiol 154: 1158–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutrot F, Zipfel C (2017) Function, discovery, and exploitation of plant pattern recognition receptors for broad‐spectrum disease resistance. Annu Rev Phytopathol 55: 257–286 [DOI] [PubMed] [Google Scholar]
- Brutus A, Sicilia F, Macone A, Cervone F, De Lorenzo G (2010) A domain swap approach reveals a role of the plant wall‐associated kinase 1 (WAK1) as a receptor of oligogalacturonides. Proc Natl Acad Sci USA 107: 9452–9457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty S, Toyota M, Moeder W, Chin K, Fortuna A, Champigny M, Vanneste S, Gilroy S, Beeckman T, Nambara E et al (2021) CYCLIC NUCLEOTIDE‐GATED ION CHANNEL 2 modulates auxin homeostasis and signaling. Plant Physiol 187: 1690–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CWM, Schorrak LM, Smith RK, Bent AF, Sussman MR (2003) A cyclic nucleotide‐gated ion channel, CNGC2, is crucial for plant development and adaptation to calcium stress. Plant Physiol 132: 728–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiasson DM, Haage K, Sollweck K, Brachmann A, Dietrich P, Parniske M (2017) A quantitative hypermorphic CNGC allele confers ectopic calcium flux and impairs cellular development. Elife 6: e25012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin K, DeFalco TA, Moeder W, Yoshioka K (2013) The Arabidopsis cyclic nucleotide‐gated ion channels AtCNGC2 and AtCNGC4 work in the same signaling pathway to regulate pathogen defense and floral transition. Plant Physiol 163: 611–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinchilla D, Zipfel C, Robatzek S, Kemmerling B, Nürnberger T, Jones JDG, Felix G, Boller T (2007) A flagellin‐induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 448: 497–500 [DOI] [PubMed] [Google Scholar]
- Choi J, Tanaka K, Cao Y, Qi Y, Qiu J, Liang Y, Lee SY, Stacey G (2014) Identification of a plant receptor for extracellular ATP. Science 343: 290–294 [DOI] [PubMed] [Google Scholar]
- Choi W‐G, Miller G, Wallace I, Harper J, Mittler R, Gilroy S (2017) Orchestrating rapid long‐distance signaling in plants with Ca2+, ROS and electrical signals. Plant J 90: 698–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE (2007) Calcium signaling. Cell 131: 1047–1058 [DOI] [PubMed] [Google Scholar]
- Clark GB, Morgan RO, Fernandez MP, Roux SJ (2012) Evolutionary adaptation of plant annexins has diversified their molecular structures, interactions and functional roles. New Phytol 196: 695–712 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Fengler KA, Yu IC, Lippok B, Smith RK, Bent AF (2000) The Arabidopsis dnd1 ‘defense, no death’ gene encodes a mutated cyclic nucleotide‐gated ion channel. Proc Natl Acad Sci USA 97: 9323–9328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A, Navazio L, Szabo I (2018) The contribution of organelles to plant intracellular calcium signalling. J Exp Bot 69: 4175–4193 [DOI] [PubMed] [Google Scholar]
- Couto D, Zipfel C (2016) Regulation of pattern recognition receptor signalling in plants. Nat Rev Immunol 16: 537–552 [DOI] [PubMed] [Google Scholar]
- Cui H, Tsuda K, Parker JE (2015) Effector‐triggered immunity: From pathogen perception to robust defense. Annu Rev Plant Biol 66: 487–511 [DOI] [PubMed] [Google Scholar]
- Dangl JL, Jones JDG (2019) A pentangular plant inflammasome. Science 364: 31–32 [DOI] [PubMed] [Google Scholar]
- Danot O, Marquenet E, Vidal‐Ingigliardi D, Richet E (2009) Wheel of life, wheel of death: a mechanistic insight into signaling by stand proteins. Structure 17: 172–182 [DOI] [PubMed] [Google Scholar]
- DeFalco TA, Bender KW, Snedden WA (2010) Breaking the code: Ca2+ sensors in plant signalling. Biochem J 425: 27–40 [DOI] [PubMed] [Google Scholar]
- DeFalco TA, Marshall CB, Munro K, Kang H‐G, Moeder W, Ikura M, Snedden WA, Yoshioka K (2016a) Multiple calmodulin‐binding sites positively and negatively regulate Arabidopsis CYCLIC NUCLEOTIDE‐GATED CHANNEL12. Plant Cell 28, 1738–1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFalco TA, Moeder W, Yoshioka K (2016b) Opening the gates: insights into cyclic nucleotide‐gated channel‐mediated signaling. Trends Plant Sci 21: 903–906 [DOI] [PubMed] [Google Scholar]
- DeFalco TA, Toyota M, Phan V, Karia P, Moeder W, Gilroy S, Yoshioka K (2017) Using GCaMP3 to study Ca2+ signaling in nicotiana species. Plant Cell Physiol 58: 1173–1184 [DOI] [PubMed] [Google Scholar]
- DeFalco TA, Zipfel C (2021) Molecular mechanisms of early plant pattern‐triggered immune signaling. Mol Cell 81: 3449–3467 [DOI] [PubMed] [Google Scholar]
- Desikan R, Horák J, Chaban C, Mira‐Rodado V, Witthöft J, Elgass K, Grefen C, Cheung M‐K, Meixner AJ, Hooley R et al (2008) The histidine kinase AHK5 integrates endogenous and environmental signals in Arabidopsis guard cells. PLoS One 3: e2491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeYoung BJ, Innes RW (2006) Plant NBS‐LRR proteins in pathogen sensing and host defense. Nat Immunol 7: 1243–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich P, Moeder W, Yoshioka K (2020) Plant cyclic nucleotide‐gated channels: new insights on their functions and regulation1. Plant Physiol 184: 27–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doke N (1983) Generation of superoxide anion by potato tuber protoplasts during the hypersensitive response to hyphal wall components of Phytophthora infestans and specific inhibition of the reaction by suppressors of hypersensitivity. Physiol Plant Pathol 23: 359–367 [Google Scholar]
- Doke N (1985) NADPH‐dependent O2 − generation in membrane fractions isolated from wounded potato tubers inoculated with Phytophthora infestans . Physiol Plant Pathol 27: 311–322 [Google Scholar]
- Dubiella U, Seybold H, Durian G, Komander E, Lassig R, Witte C‐P, Schulze WX, Romeis T (2013) Calcium‐dependent protein kinase/NADPH oxidase activation circuit is required for rapid defense signal propagation. Proc Natl Acad Sci USA 110: 8744–8749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong HN, Cho S‐H, Wang L, Pham AQ, Davies JM, Stacey G (2022) Cyclic nucleotide‐gated ion channel 6 is involved in extracellular ATP signaling and plant immunity. Plant J 109: 1386–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edel KH, Marchadier E, Brownlee C, Kudla J, Hetherington AM (2017) The evolution of calcium‐based signalling in plants. Curr Biol 27: R667–R679 [DOI] [PubMed] [Google Scholar]
- Espinoza C, Liang Y, Stacey G (2017) Chitin receptor CERK1 links salt stress and chitin‐triggered innate immunity in Arabidopsis . Plant J 89: 984–995 [DOI] [PubMed] [Google Scholar]
- Evans MJ, Choi WG, Gilroy S, Morris RJ (2016) A ROS‐assisted calcium wave dependent on the AtRBOHD NADPH oxidase and TPC1 cation channel propagates the systemic response to salt stress. Plant Physiol 171: 1771–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer EE, Gao YQ, Lenzoni G, Wolfender JL, Wu Q (2020) Wound‐ and mechanostimulated electrical signals control hormone responses. New Phytol 227: 1037–1050 [DOI] [PubMed] [Google Scholar]
- Feehan JM, Castel B, Bentham AR, Jones JD (2020) Plant NLRs get by with a little help from their friends. Curr Opin Plant Biol 56: 99–108 [DOI] [PubMed] [Google Scholar]
- Fischer C, Defalco TA, Karia P, Snedden WA, Moeder W, Yoshioka K, Dietrich P (2017) Calmodulin as a Ca2+‐sensing subunit of Arabidopsis cyclic nucleotide‐gated channel complexes. Plant Cell Physiol 58: 1208–1221 [DOI] [PubMed] [Google Scholar]
- Fischer C, Kugler A, Hoth S, Dietrich P (2013) An IQ domain mediates the interaction with calmodulin in a plant cyclic nucleotide‐gated channel. Plant Cell Physiol 54: 573–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei dit Frey N, Mbengue M, Kwaaitaal M, Nitsch L, Altenbach D, Häweker H, Lozano‐Duran R, Njo MF, Beeckman T, Huettel B et al (2012) Plasma membrane calcium ATPases are important components of receptor‐mediated signaling in plant immune responses and development. Plant Physiol 159: 798–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangwar SP, Green MN, Michard E, Simon AA, Feijó JA, Sobolevsky AI (2021) Structure of the Arabidopsis glutamate receptor‐like channel GLR3.2 ligand‐binding domain. Structure 29: 161–169.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q‐F, Fei C‐F, Dong J‐Y, Gu L‐L, Wang Y‐F (2014) Arabidopsis CNGC18 is a Ca2+‐permeable channel. Mol Plant 7: 739–743 [DOI] [PubMed] [Google Scholar]
- Gao Q‐F, Gu L‐L, Wang H‐Q, Fei C‐F, Fang X, Hussain J, Sun S‐J, Dong J‐Y, Liu H, Wang Y‐F (2016) Cyclic nucleotide‐gated channel 18 is an essential Ca2+ channel in pollen tube tips for pollen tube guidance to ovules in Arabidopsis . Proc Natl Acad Sci USA 113: 3096–3101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García Bossi J, Kumar K, Barberini ML, Domínguez GD, Rondón Guerrero YDC, Marino‐Buslje C, Obertello M, Muschietti JP, Estevez JM (2020) The role of P‐type IIA and P‐type IIB Ca2+‐ATPases in plant development and growth. J Exp Bot 71: 1239–1248 [DOI] [PubMed] [Google Scholar]
- Geisler M, Axelsen KB, Harper JF, Palmgren MG (2000) Molecular aspects of higher plant P‐type Ca2+‐ATPases. Biochim Biophys Acta ‐ Biomembr 1465: 52–78 [DOI] [PubMed] [Google Scholar]
- Gelli A, Higgins VJ, Blumwald E (1997) Activation of plant plasma membrane Ca2+‐permeable channels by race‐specific fungal elicitors. Plant Physiol 113: 269–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilroy S, Białasek M, Suzuki N, Górecka M, Devireddy AR, Karpiński S, Mittler R (2016) ROS, calcium, and electric signals: key mediators of rapid systemic signaling in plants. Plant Physiol 171: 1606–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez‐Gómez L, Boller T (2000) FLS2: An LRR receptor‐like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis . Mol Cell 5: 1003–1011 [DOI] [PubMed] [Google Scholar]
- Grant M, Brown I, Adams S, Knight M, Ainslie A, Mansfield J (2000) The RPM1 plant disease resistance gene facilitates a rapid and sustained increase in cytosolic calcium that is necessary for the oxidative burst and hypersensitive cell death. Plant J 23: 441–450 [DOI] [PubMed] [Google Scholar]
- Green MN, Gangwar SP, Michard E, Simon AA, Portes MT, Barbosa‐Caro J, Wudick MM, Lizzio MA, Klykov O, Yelshanskaya MV et al (2021) Structure of the Arabidopsis thaliana glutamate receptor‐like channel GLR3.4. Mol Cell 81: 3216–3226.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenzi M, Bonza MC, Alfieri A, Costa A (2021a) Structural insights into long‐distance signal transduction pathways mediated by plant glutamate receptor‐like channels. New Phytol 229: 1261–1267 [DOI] [PubMed] [Google Scholar]
- Grenzi M, Resentini F, Vanneste S, Zottini M, Bassi A, Costa A (2021b) Illuminating the hidden world of calcium ions in plants with a universe of indicators. Plant Physiol 187: 550–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gust AA, Pruitt R, Nürnberger T (2017) Sensing danger: key to activating plant immunity. Trends Plant Sci 22: 779–791 [DOI] [PubMed] [Google Scholar]
- He K, Gou X, Yuan T, Lin H, Asami T, Yoshida S, Russell SD, Li J (2007) BAK1 and BKK1 regulate brassinosteroid‐dependent growth and brassinosteroid‐independent cell‐death pathways. Curr Biol 17: 1109–1115 [DOI] [PubMed] [Google Scholar]
- Heese A, Hann DR, Gimenez‐Ibanez S, Jones AME, He K, Li J, Schroeder JI, Peck SC, Rathjen JP (2007) The receptor‐like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc Natl Acad Sci USA 104: 12217–12222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilleary R, Paez‐Valencia J, Vens C, Toyota M, Palmgren M, Gilroy S (2020) Tonoplast‐localized Ca2+ pumps regulate Ca2+ signals during pattern‐triggered immunity in Arabidopsis thaliana . Proc Natl Acad Sci USA 117: 18849–18857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsefield S, Burdett H, Zhang X, Manik MK, Shi Y, Chen J, Qi T, Gilley J, Lai JS, Rank MX et al (2019) NAD+ cleavage activity by animal and plant TIR domains in cell death pathways. Science 365: 793–799 [DOI] [PubMed] [Google Scholar]
- Hou C, Tian W, Kleist T, He K, Garcia V, Bai F, Hao Y, Luan S, Li L (2014) DUF221 proteins are a family of osmosensitive calcium‐permeable cation channels conserved across eukaryotes. Cell Res 24: 632–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua BG, Mercier RW, Zielinski RE, Berkowitz GA (2003) Functional interaction of calmodulin with a plant cyclic nucleotide gated cation channel. Plant Physiol Biochem 41: 945–954 [Google Scholar]
- Hung CY, Aspesi P, Hunter MR, Lomax AW, Perera IY (2014) Phosphoinositide‐signaling is one component of a robust plant defense response. Front Plant Sci 5: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob P, Kim NH, Wu F, El‐Kasmi F, Chi Y, Walton WG, Furzer OJ, Lietzan AD, Sunil S, Kempthorn K et al (2021) Plant “helper” immune receptors are Ca2+‐permeable nonselective cation channels. Science 373: 420–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson PA, Shan L, He P (2018) Plant cell surface molecular cypher: receptor‐like proteins and their roles in immunity and development. Plant Sci 274: 242–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarratt‐Barnham E, Wang L, Ning Y, Davies JM (2021) The complex story of plant cyclic nucleotide‐gated channels. Int J Mol Sci 22: 1–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezek M, Blatt MR (2017) The membrane transport system of the guard cell and its integration for stomatal dynamics. Plant Physiol 174: 487–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jogawat A, Meena MK, Kundu A, Varma M, Vadassery J (2020) Calcium channel CNGC19 mediates basal defense signaling to regulate colonization by Piriformospora indica in Arabidopsis roots. J Exp Bot 71: 2752–2768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns S, Hagihara T, Toyota M, Gilroy S (2021) The fast and the furious: rapid long‐range signaling in plants. Plant Physiol 185: 694–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jojoa‐Cruz S, Saotome K, Murthy SE, Tsui CCA, Sansom MSP, Patapoutian A, Ward AB (2018) Cryo‐EM structure of the mechanically activated ion channel OSCA1.2. Elife 7: e41845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JDG, Dangl JL (2006) The plant immune system. Nature 444: 323–329 [DOI] [PubMed] [Google Scholar]
- Jones JD, Vance RE, Dangl JL (2016) Intracellular innate immune surveillance devices in plants and animals. Science 354: aaf6395 [DOI] [PubMed] [Google Scholar]
- Jubic LM, Saile S, Furzer OJ, El Kasmi F, Dangl JL (2019) Help wanted: helper NLRs and plant immune responses. Curr Opin Plant Biol 50: 82–94 [DOI] [PubMed] [Google Scholar]
- Jurkowski GI, Smith RK, Yu IC, Ham JH, Sharma SB, Klessig DF, Fengler KA, Bent AF (2004) Arabidopsis DND2, a second cyclic nucleotide‐gated ion channel gene for which mutation causes the ‘defense, no death’ phenotype. Mol Plant Microbe Interact 17: 511–520 [DOI] [PubMed] [Google Scholar]
- Kadota Y, Liebrand TWH, Goto Y, Sklenar J, Derbyshire P, Menke FLH, Torres M‐A, Molina A, Zipfel C, Coaker G et al (2019) Quantitative phosphoproteomic analysis reveals common regulatory mechanisms between effector‐ and PAMP‐triggered immunity in plants. New Phytol 221: 2160–2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadota Y, Shirasu K, Zipfel C (2015) Regulation of the NADPH oxidase RBOHD during plant immunity. Plant Cell Physiol 56: 1472–1480 [DOI] [PubMed] [Google Scholar]
- Kadota Y, Sklenar J, Derbyshire P, Stransfeld L, Asai S, Ntoukakis V, Jones J, Shirasu K, Menke F, Jones A et al (2014) Direct regulation of the NADPH oxidase RBOHD by the PRR‐associated kinase BIK1 during plant immunity. Mol Cell 54: 43–55 [DOI] [PubMed] [Google Scholar]
- Kang HG, Oh CS, Sato M, Katagiri F, Glazebrook J, Takahashi H, Kachroo P, Martin GB, Klessig DF (2010) Endosome‐associated CRT1 functions early in resistance gene‐mediated defense signaling in Arabidopsis and tobacco. Plant Cell 22: 918–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan B, Sherman T, Fromm H (2007) Cyclic nucleotide‐gated channels in plants. FEBS Lett 581: 2237–2246 [DOI] [PubMed] [Google Scholar]
- Kaupp UB, Seifert R (2002) Cyclic nucleotide‐gated ion channels. Physiol Rev 82: 769–824 [DOI] [PubMed] [Google Scholar]
- Keinath NF, Waadt R, Brugman R, Schroeder JI, Grossmann G, Schumacher K, Krebs M (2015) Live cell imaging with R‐GECO1 sheds light on flg22‐ and chitin‐induced transient [Ca2+]cyt patterns in Arabidopsis . Mol Plant 8: 1188–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemmerling B, Schwedt A, Rodriguez P, Mazzotta S, Frank M, Qamar SA, Mengiste T, Betsuyaku S, Parker JE, Müssig C et al (2007) The BRI1‐associated kinase 1, BAK1, has a brassinolide‐independent role in plant cell‐death control. Curr Biol 17: 1116–1122 [DOI] [PubMed] [Google Scholar]
- Keppler LD, Baker CJ, Atkinson MM (1989) Active oxygen production during a bacteria‐induced hypersensitive reaction in tobacco suspension cells. Phytopathology 79: 974–978 [Google Scholar]
- Knight MR, Campbell AK, Smith SM, Trewavas AJ (1991) Transgenic plant aequorin reports the effects of touch and cold‐shock and elicitors on cytoplasmic calcium. Nature 352: 524–526 [DOI] [PubMed] [Google Scholar]
- Köhler C, Neuhaus G (1998) Cloning and partial characterization of two putative cyclic nucleotide‐regulated ion channels from Arabidopsis thaliana, designated CNGC1 and CNGC2. Plant Physiol 116: 1604 [Google Scholar]
- Köhler C, Neuhaus G (2000) Characterisation of calmodulin binding to cyclic nucleotide‐gated ion channels from Arabidopsis thaliana . FEBS Lett 471: 133–136 [DOI] [PubMed] [Google Scholar]
- Kohorn BD, Kohorn SL (2012) The cell wall‐associated kinases, WAKs, as pectin receptors. Front Plant Sci 3: 88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodziej MC, Singla J, Sánchez‐Martín J, Zbinden H, Šimková H, Karafiátová M, Doležel J, Gronnier J, Poretti M, Glauser G et al (2021) A membrane‐bound ankyrin repeat protein confers race‐specific leaf rust disease resistance in wheat. Nat Commun 12: 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol E, Mentzel T, Chinchilla D, Boller T, Felix G, Kemmerling B, Postel S, Arents M, Jeworutzki E, Al‐Rasheid KAS et al (2010) Perception of the Arabidopsis danger signal peptide 1 involves the pattern recognition receptor AtPEPR1 and its close homologue AtPEPR2. J Biol Chem 285: 13471–13479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudla J, Becker D, Grill E, Hedrich R, Hippler M, Kummer U, Parniske M, Romeis T, Schumacher K (2018) Advances and current challenges in calcium signaling. New Phytol 218: 414–431 [DOI] [PubMed] [Google Scholar]
- Kwaaitaal M, Huisman R, Maintz J, Reinstadler A, Panstruga R, Reinstädler A, Panstruga R, Reinstädler A, Panstruga R (2011) Ionotropic glutamate receptor (iGluR)‐like channels mediate MAMP‐induced calcium influx in Arabidopsis thaliana . Biochem J 440: 355–365 [DOI] [PubMed] [Google Scholar]
- Lam HM, Chiu J, Hsieh MH, Meisel L, Oliveira IC, Shin M, Coruzzi G (1998) Glutamate‐receptor genes in plants. Nature 396: 125–126 [DOI] [PubMed] [Google Scholar]
- Laohavisit A, Davies JM (2011) Annexins. New Phytol 189: 40–53 [DOI] [PubMed] [Google Scholar]
- Laohavisit A, Richards SL, Shabala L, Chen C, Colaço RDDR, Swarbreck SM, Shaw E, Dark A, Shabala S, Shang Z et al (2013) Salinity‐induced calcium signaling and root adaptation in Arabidopsis require the calcium regulatory protein annexin1. Plant Physiol 163: 253–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laohavisit A, Shang Z, Rubio L, Cuin TA, Véry AA, Wang A, Mortimer JC, Macpherson N, Coxon KM, Battey NH et al (2012) Arabidopsis annexin1 mediates the radical‐activated plasma membrane Ca2+‐and K+‐permeable conductance in root cells. Plant Cell 24: 1522–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laohavisit A, Wakatake T, Ishihama N, Mulvey H, Takizawa K, Suzuki T, Shirasu K (2020) Quinone perception in plants via leucine‐rich‐repeat receptor‐like kinases. Nature 587: 92–97 [DOI] [PubMed] [Google Scholar]
- Lawson T, Matthews J (2020) Guard cell metabolism and stomatal function. Annu Rev Plant Biol 71: 273–302 [DOI] [PubMed] [Google Scholar]
- Lecourieux D, Mazars C, Pauly N, Ranjeva R, Pugin A (2002) Analysis and effects of cytosolic free calcium increases in response to elicitors in nicotiana plumbaginifolia cells. Plant Cell 14: 2627–2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng Q, Mercier RW, Hua BG, Fromm H, Berkowitz GA (2002) Electrophysiological analysis of cloned cyclic nucleotide‐gated ion channels. Plant Physiol 128: 400–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A, Pennell RI, Alvarez ME, Palmer R, Lamb C (1996) Calcium‐mediated apoptosis in a plant hypersensitive disease resistance response. Curr Biol 6: 427–437 [DOI] [PubMed] [Google Scholar]
- Li F, Wang J, Ma C, Zhao Y, Wang Y, Hasi A, Qi Z (2013) Glutamate receptor‐like channel3.3 is involved in mediating glutathione‐triggered cytosolic calcium transients, transcriptional changes, and innate immunity responses in Arabidopsis . Plant Physiol 162: 1497–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Prada J, Damineli DSC, Liese A, Romeis T, Dandekar T, Feijó JA, Hedrich R, Konrad KR (2021) An optimized genetically encoded dual reporter for simultaneous ratio imaging of Ca2+ and H+ reveals new insights into ion signaling in plants. New Phytol 230: 2292–2310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Li M, Yu L, Zhou Z, Liang X, Liu Z, Cai G, Gao L, Zhang X, Wang Y et al (2014) The FLS2‐associated kinase BIK1 directly phosphorylates the NADPH oxidase RbohD to control plant immunity. Cell Host Microbe 15: 329–338 [DOI] [PubMed] [Google Scholar]
- Liang X, Zhou JM (2018) Receptor‐like cytoplasmic kinases: central players in plant receptor kinase‐mediated signaling. Annu Rev Plant Biol 69: 267–299 [DOI] [PubMed] [Google Scholar]
- Liebrand TWH, van den Berg GCM, Zhang Z, Smit P, Cordewener JHG, America AHP, Sklenar J, Jones AME, Tameling WIL, Robatzek S et al (2013) Receptor‐like kinase SOBIR1/EVR interacts with receptor‐like proteins in plant immunity against fungal infection. Proc Natl Acad Sci USA 110: 10010–10015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Ding Y, Shi Y, Ma L, Wang YI, Song C, Wilkins KA, Davies JM, Knight H, Knight MR et al (2021) The calcium transporter ANNEXIN1 mediates cold‐induced calcium signaling and freezing tolerance in plants. EMBO J 40: e104559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Wang J, Sun L (2018) Structure of the hyperosmolality‐gated calcium‐permeable channel OSCA1.2. Nat Commun 9: 5060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Maierhofer T, Rybak K, Sklenar J, Breakspear A, Johnston MG, Fliegmann J, Huang S, Roelfsema MRG, Felix G et al (2019) Anion channel SLAH3 is a regulatory target of chitin receptor‐associated kinase PBL27 in microbial stomatal closure. Elife 8: e44474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Rate DN, Song JT, Greenberg JT (2003) ACD6, a novel ankyrin protein, is a regulator and an effector of salicylic acid signaling in the Arabidopsis defense response. Plant Cell 15: 2408–2420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Ye J, Yang Y, Lin H, Yue L, Luo J, Long YU, Fu H, Liu X, Zhang Y et al (2019) The SOS2‐SCaBP8 complex generates and fine‐tunes an AtANN4‐dependent calcium signature under salt stress. Dev Cell 48: 697–709.e5 [DOI] [PubMed] [Google Scholar]
- Ma S, Lapin D, Liu LI, Sun Y, Song W, Zhang X, Logemann E, Yu D, Wang J, Jirschitzka J et al (2020) Direct pathogen‐induced assembly of an NLR immune receptor complex to form a holoenzyme. Science 370: eabe3069 [DOI] [PubMed] [Google Scholar]
- Ma Y, Walker RK, Zhao Y, Berkowitz GA (2012) Linking ligand perception by PEPR pattern recognition receptors to cytosolic Ca2+ elevation and downstream immune signaling in plants. Proc Natl Acad Sci USA 109: 19852–19857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdi LK, Huang M, Zhang X, Nakano RT, Kopp LB, Saur IML, Jacob F, Kovacova V, Lapin D, Parker JE et al (2020) Discovery of a family of mixed lineage kinase domain‐like proteins in plants and their role in innate immune signaling. Cell Host Microbe 28: 813–824.e6 [DOI] [PubMed] [Google Scholar]
- Maintz J, Cavdar M, Tamborski J, Kwaaitaal M, Huisman R, Meesters C, Kombrink E, Panstruga R (2014) Comparative analysis of MAMP‐induced calcium influx in arabidopsis seedlings and protoplasts. Plant Cell Physiol 55: 1813–1825 [DOI] [PubMed] [Google Scholar]
- Malabarba J, Meents AK, Reichelt M, Scholz SS, Peiter E, Rachowka J, Konopka‐Postupolska D, Wilkins KA, Davies JM, Oelmüller R et al (2021) ANNEXIN1 mediates calcium‐dependent systemic defense in Arabidopsis plants upon herbivory and wounding. New Phytol 231: 243–254 [DOI] [PubMed] [Google Scholar]
- Manzoor H, Kelloniemi J, Chiltz A, Wendehenne D, Pugin A, Poinssot B, Garcia‐Brugger A (2013) Involvement of the glutamate receptor AtGLR3.3 in plant defense signaling and resistance to Hyaloperonospora arabidopsidis . Plant J 76: 466–480 [DOI] [PubMed] [Google Scholar]
- Marhavý P, Kurenda A, Siddique S, Dénervaud Tendon V, Zhou F, Holbein J, Hasan MS, Grundler FM, Farmer EE, Geldner N (2019) Single‐cell damage elicits regional, nematode‐restricting ethylene responses in roots. EMBO J 38: e100972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R, Qi T, Zhang H, Liu F, King M, Toth C, Nogales E, Staskawicz BJ (2020) Structure of the activated ROQ1 resistosome directly recognizing the pathogen effector XopQ. Science 370: eabd9993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäser P, Thomine Sébastien, Schroeder JI, Ward JM, Hirschi K, Sze H, Talke IN, Amtmann A, Maathuis FJM, Sanders D et al (2001) Phylogenetic relationships within cation transporter families of Arabidopsis . Plant Physiol 126: 1646–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matulef K, Zagotta WN (2003) Cyclic nucleotide‐gated ion channels. Annu Rev Cell Dev Biol 19: 23–44 [DOI] [PubMed] [Google Scholar]
- Meena MK, Prajapati R, Krishna D, Divakaran K, Pandey Y, Reichelt M, Mathew MK, Boland W, Mithöfer A, Vadassery J (2019) The Ca2+ channel CNGC19 regulates Arabidopsis defense against spodoptera herbivory. Plant Cell 31: 1539–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melotto M, Underwood W, Koczan J, Nomura K, He SY (2006) Plant stomata function in innate immunity against bacterial invasion. Cell 126: 969–980 [DOI] [PubMed] [Google Scholar]
- Melotto M, Zhang L, Oblessuc PR, He SY (2017) Stomatal defense a decade later. Plant Physiol 174: 561–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermigka G, Sarris PF (2019) The rise of plant resistosomes. Trends Immunol 40: 670–673 [DOI] [PubMed] [Google Scholar]
- Moeder W, Phan V, Yoshioka K (2019) Ca2+ to the rescue ‐ Ca2+ channels and signaling in plant immunity. Plant Sci 279: 19–26 [DOI] [PubMed] [Google Scholar]
- Mohammad‐Sidik A, Sun J, Shin R, Song Z, Ning Y, Matthus E, Wilkins KA, Davies JM (2021) Annexin 1 is a component of eATP‐induced cytosolic calcium elevation in Arabidopsis thaliana roots. Int J Mol Sci 22: 494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan J, Matschi S, Romeis T, Zipfel C (2015) The calcium‐dependent protein kinase CPK28 negatively regulates the BIK1‐mediated PAMP‐ induced calcium burst. Plant Signal. Behav 10: e1018497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi SAR, Chauvin A, Pascaud F, Kellenberger S, Farmer EE (2013) GLUTAMATE RECEPTOR‐LIKE genes mediate leaf‐to‐leaf wound signalling. Nature 500: 422–426 [DOI] [PubMed] [Google Scholar]
- Murthy SE, Dubin AE, Whitwam T, Jojoa‐Cruz S, Cahalan SM, Mousavi SAR, Ward AB, Patapoutian A (2018) OSCA/TMEM63 are an evolutionarily conserved family of mechanically activated ion channels. Elife 7: e41844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngou BPM, Ahn H‐KK, Ding P, Jones JDGG (2021) Mutual potentiation of plant immunity by cell‐surface and intracellular receptors. Nature 592: 110–115 [DOI] [PubMed] [Google Scholar]
- Nguyen CT, Kurenda A, Stolz S, Chételat A, Farmer EE (2018) Identification of cell populations necessary for leaf‐to‐leaf electrical signaling in a wounded plant. Proc Natl Acad Sci USA 115: 10178–10183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nühse TS, Bottrill AR, Jones AME, Peck SC (2007) Quantitative phosphoproteomic analysis of plasma membrane proteins reveals regulatory mechanisms of plant innate immune responses. Plant J 51: 931–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara Y, Kaya H, Hiraoka G, Yumoto F, Kimura S, Kadota Y, Hishinuma H, Senzaki E, Yamagoe S, Nagata K et al (2008) Synergistic activation of the Arabidopsis NADPH oxidase AtrbohD by Ca2+ and phosphorylation. J Biol Chem 283: 8885–8892 [DOI] [PubMed] [Google Scholar]
- Pan Y, Chai X, Gao Q, Zhou L, Zhang S, Li L, Luan S (2019) Dynamic interactions of plant CNGC subunits and calmodulins drive oscillatory Ca2+ channel activities. Dev Cell 48: 710–725.e5 [DOI] [PubMed] [Google Scholar]
- Petrie EJ, Czabotar PE, Murphy JM (2019) The structural basis of necroptotic cell death signaling. Trends Biochem Sci 44: 53–63 [DOI] [PubMed] [Google Scholar]
- Postma J, Liebrand TWH, Bi G, Evrard A, Bye RR, Mbengue M, Kuhn H, Joosten MHAJ, Robatzek S (2016) Avr4 promotes Cf‐4 receptor‐like protein association with the BAK1/SERK3 receptor‐like kinase to initiate receptor endocytosis and plant immunity. New Phytol 210: 627–642 [DOI] [PubMed] [Google Scholar]
- Pruitt RN, Locci F, Wanke F, Zhang L, Saile SC, Joe A, Karelina D, Hua C, Fröhlich K, Wan W‐L et al (2021) The EDS1‐PAD4‐ADR1 node mediates Arabidopsis pattern‐triggered immunity. Nature 598: 495–499 [DOI] [PubMed] [Google Scholar]
- Qi Z, Verma R, Gehring C, Yamaguchi Y, Zhao Y, Ryan CA, Berkowitz GA (2010) Ca2+ signaling by plant Arabidopsis thaliana Pep peptides depends on AtPepR1, a receptor with guanylyl cyclase activity, and cGMP‐activated Ca2+ channels. Proc Natl Acad Sci USA 107: 21193–21198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranf S, Eschen‐Lippold L, Pecher P, Lee J, Scheel D (2011) Interplay between calcium signalling and early signalling elements during defence responses to microbe‐ or damage‐associated molecular patterns. Plant J 68: 100–113 [DOI] [PubMed] [Google Scholar]
- Ranf S, Eschen‐Lippold L, Fröhlich K, Westphal L, Scheel D, Lee J (2014) Microbe‐associated molecular pattern‐induced calcium signaling requires the receptor‐like cytoplasmic kinases, PBL1 and BIK1. BMC Plant Biol 14: 374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranf S, Wünnenberg P, Lee J, Becker D, Dunkel M, Hedrich R, Scheel D, Dietrich P (2008) Loss of the vacuolar cation channel, AtTPC1, does not impair Ca2+ signals induced by abiotic and biotic stresses. Plant J 53: 287–299 [DOI] [PubMed] [Google Scholar]
- Rate DN, Cuenca JV, Bowman GR, Guttman DS, Greenberg JT (1999) The gain‐of‐function Arabidopsis acd6 mutant reveals novel regulation and function of the salicylic acid signaling pathway in controlling cell death, defenses, and cell growth. Plant Cell 11: 1695–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resentini F, Ruberti C, Grenzi M, Bonza MC, Costa A (2021) The signatures of organellar calcium. Plant Physiol 187: 1985–2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux M, Schwessinger B, Albrecht C, Chinchilla D, Jones A, Holton N, Malinovsky FG, Tör M, de Vries S, Zipfel C (2011) The Arabidopsis leucine‐rich repeat receptor‐like kinases BAK1/SERK3 and BKK1/SERK4 are required for innate immunity to hemibiotrophic and biotrophic pathogens. Plant Cell 23: 2440–2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saand MA, Xu YP, Munyampundu JP, Li W, Zhang XR, Cai XZ (2015) Phylogeny and evolution of plant cyclic nucleotide‐gated ion channel (CNGC) gene family and functional analyses of tomato CNGCs. DNA Res 22: 471–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santolini J, André F, Jeandroz S, Wendehenne D (2017) Nitric oxide synthase in plants: where do we stand? Nitric Oxide ‐ Biol Chem 63: 30–38 [DOI] [PubMed] [Google Scholar]
- Schulze S, Yu L, Ehinger A, Kolb D, Saile SC, Stahl M, Franz‐Wachtel M, Li L, Elkasmi F, Cevik V et al (2021) The TIR‐NBS‐LRR protein CSA1 is required for autoimmune cell death in Arabidopsis pattern recognition co‐receptor bak1 and bir3 mutants. bioRxiv 10.1101/2021.04.11.438637 [PREPRINT] [DOI]
- Schwessinger B, Roux M, Kadota Y, Ntoukakis V, Sklenar J, Jones A, Zipfel C (2011) Phosphorylation‐dependent differential regulation of plant growth, cell death, and innate immunity by the regulatory receptor‐like kinase BAK1. PLOS Genet 7: e1002046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seybold H, Trempel F, Ranf S, Scheel D, Romeis T, Lee J (2014) Ca2+ signalling in plant immune response : from pattern recognition receptors to Ca2+ decoding mechanisms. New Phytol 204: 782–790 [DOI] [PubMed] [Google Scholar]
- Shao Q, Gao Q, Lhamo D, Zhang H, Luan S (2020) Two glutamate‐ and pH‐regulated Ca2+ channels are required for systemic wound signaling in Arabidopsis . Sci Signal 13: eaba1453 [DOI] [PubMed] [Google Scholar]
- Shigaki T, Hirschi K (2000) Characterization of CAX‐like genes in plants: implications for functional diversity. Gene 257: 291–298 [DOI] [PubMed] [Google Scholar]
- Spalding EP, Harper JF (2011) The ins and outs of cellular Ca2+ transport. Curr Opin Plant Biol 14: 715–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhorst L, Kudla J (2014) Signaling in cells and organisms ‐ calcium holds the line. Curr Opin Plant Biol 22: 14–21 [DOI] [PubMed] [Google Scholar]
- Suo Y, Wang Z, Zubcevic L, Hsu AL, He Q, Borgnia MJ, Ji R‐R, Lee S‐Y (2020) Structural insights into electrophile irritant sensing by the human TRPA1 channel. Neuron 105: 882–894.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateda C, Zhang Z, Shrestha J, Jelenska J, Chinchilla D, Greenberg JT (2014) Salicylic acid regulates Arabidopsis microbial pattern receptor kinase levels and signaling. Plant Cell 26: 4171–4187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomma BPHJ, Nürnberger T, Joosten MHAJ (2011) Of PAMPs and effectors: the blurred PTI‐ETI dichotomy. Plant Cell 23: 4–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thor K, Jiang S, Michard E, George J, Scherzer S, Huang S, Dindas J, Derbyshire P, Leitão N, DeFalco TA et al (2020) The calcium‐permeable channel OSCA1.3 regulates plant stomatal immunity. Nature 585: 569–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thor K, Peiter E (2014) Cytosolic calcium signals elicited by the pathogen‐associated molecular pattern flg22 in stomatal guard cells are of an oscillatory nature. New Phytol 204: 873–881 [DOI] [PubMed] [Google Scholar]
- Tian H, Wu Z, Chen S, Ao K, Huang W, Yaghmaiean H, Sun T, Xu F, Zhang Y, Wang S et al (2021) Activation of TIR signalling boosts pattern‐triggered immunity. Nature 598: 500–503 [DOI] [PubMed] [Google Scholar]
- Tian W, Hou C, Ren Z, Wang C, Zhao F, Dahlbeck D, Hu S, Zhang L, Niu QI, Li L et al (2019) A calmodulin‐gated calcium channel links pathogen patterns to plant immunity. Nature 572: 131–135 [DOI] [PubMed] [Google Scholar]
- Tian W, Wang C, Gao Q, Li L, Luan S (2020) Calcium spikes, waves and oscillations in plant development and biotic interactions. Nat Plants 6: 750–759 [DOI] [PubMed] [Google Scholar]
- Torres MA, Dangl JL, Jones JDG (2002) Arabidopsis gp91phox homologues Atrbohd and Atrbohf are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci USA 99: 517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyota M, Spencer D, Sawai‐Toyota S, Jiaqi W, Zhang T, Koo AJ, Howe GA, Gilroy S (2018) Glutamate triggers long‐distance, calcium‐based plant defense signaling. Science 361: 1112–1115 [DOI] [PubMed] [Google Scholar]
- Turek I, Irving H (2021) Moonlighting proteins shine new light on molecular signaling niches. Int J Mol Sci 22: 1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urquhart W, Gunawardena AHLAN, Moeder W, Ali R, Berkowitz GA, Yoshioka K (2007) The chimeric cyclic nucleotide‐gated ion channel ATCNGC11/12 constitutively induces programmed cell death in a Ca2+ dependent manner. Plant Mol Biol 65: 747–761 [DOI] [PubMed] [Google Scholar]
- Vadassery J, Ranf S, Drzewiecki C, Mithöfer A, Mazars C, Scheel D, Lee J, Oelmüller R (2009) A cell wall extract from the endophytic fungus Piriformospora indica promotes growth of Arabidopsis seedlings and induces intracellular calcium elevation in roots. Plant J 59: 193–206 [DOI] [PubMed] [Google Scholar]
- Vincent TR, Avramova M, Canham J, Higgins P, Bilkey N, Mugford ST, Pitino M, Toyota M, Gilroy S, Miller AJ et al (2017) Interplay of plasma membrane and vacuolar ion channels, together with BAK1, elicits rapid cytosolic calcium elevations in Arabidopsis during aphid feeding. Plant Cell 29: 1460–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waadt R, Köster P, Andrés Z, Waadt C, Bradamante G, Lampou K, Kudla J, Schumacher K (2020) Dual‐reporting transcriptionally linked genetically encoded fluorescent indicators resolve the spatiotemporal coordination of cytosolic abscisic acid and second messenger dynamics in Arabidopsis . Plant Cell 32: 2582–2601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waadt R, Kudla J, Kollist H (2021) Multiparameter in vivo imaging in plants using genetically encoded fluorescent indicator multiplexing. Plant Physiol 187: 537–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan L, Essuman K, Anderson RG, Sasaki Y, Monteiro F, Chung EH, Nishimura EO, DiAntonio A, Milbrandt J, Dangl JL et al (2019) TIR domains of plant immune receptors are NAD+‐cleaving enzymes that promote cell death. Science 365: 799–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Liang X, Bao Y, Yang S, Zhang X, Yu H, Zhang Q, Xu G, Feng X, Dou D (2020) A malectin‐like receptor kinase regulates cell death and pattern‐triggered immunity in soybean. EMBO Rep 21: e50442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Hu M, Wang J, Qi J, Han Z, Wang G, Qi Y, Wang H‐W, Zhou J‐M, Chai J (2019a) Reconstitution and structure of a plant NLR resistosome conferring immunity. Science 364: eaav5870 [DOI] [PubMed] [Google Scholar]
- Wang J, Liu XI, Zhang AN, Ren Y, Wu F, Wang G, Xu Y, Lei C, Zhu S, Pan T et al (2019b) A cyclic nucleotide‐gated channel mediates cytoplasmic calcium elevation and disease resistance in rice. Cell Res 29: 820–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wang J, Hu M, Wu S, Qi J, Wang G, Han Z, Qi Y, Gao N, Wang HW et al (2019c) Ligand‐triggered allosteric ADP release primes a plant NLR complex. Science 364: eaav5868 [DOI] [PubMed] [Google Scholar]
- Wang Y, Kang Y, Ma C, Miao R, Wu C, Long Y, Ge T, Wu Z, Hou X, Zhang J et al (2017) CNGC2 Is a Ca2+ influx channel that prevents accumulation of apoplastic Ca2+ in the leaf. Plant Physiol 173: 1342–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wersch S, Tian L, Hoy R, Li X (2020) Plant NLRs: the whistleblowers of plant immunity. Plant Commun 1: 100016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SJ, Sornaraj P, deCourcy‐Ireland E, Menz RI, Kobe B, Ellis JG, Dodds PN, Anderson PA (2011) An autoactive mutant of the M flax rust resistance protein has a preference for binding ATP, whereas wild‐type M protein binds ADP. Mol Plant Microbe Interact 24: 897–906 [DOI] [PubMed] [Google Scholar]
- Wu CH, Abd‐El‐Haliem A, Bozkurt TO, Belhaj K, Terauchi R, Vossen JH, Kamoun S (2017) NLR network mediates immunity to diverse plant pathogens. Proc Natl Acad Sci USA 114: 8113–8118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Chi Y, Jiang Z, Xu Y, Xie L, Huang F, Wan DI, Ni J, Yuan F, Wu X et al (2020a) Hydrogen peroxide sensor HPCA1 is an LRR receptor kinase in Arabidopsis . Nature 578: 577–581 [DOI] [PubMed] [Google Scholar]
- Wu Y, Gao Y, Zhan Y, Kui H, Liu H, Yan L, Kemmerling B, Zhou J‐M, He K, Li J (2020b) Loss of the common immune coreceptor BAK1 leads to NLR‐dependent cell death. Proc Natl Acad Sci USA 117: 27044–27053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wudick MM, Michard E, Oliveira Nunes C, Feijó JA (2018) Comparing plant and animal glutamate receptors: common traits but different fates? J Exp Bot 69: 4151–4163 [DOI] [PubMed] [Google Scholar]
- Yang D‐L, Shi Z, Bao Y, Yan J, Yang Z, Yu H, Li Y, Gou M, Wang S, Zou B et al (2017) Calcium pumps and interacting BON1 protein modulate calcium signature, stomatal closure, and plant immunity. Plant Physiol 175: 424–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka K, Moeder W, Kang H‐G, Kachroo P, Masmoudi K, Berkowitz G, Klessig DF (2006) The chimeric Arabidopsis CYCLIC NUCLEOTIDE‐GATED ION CHANNEL11/12 activates multiple pathogen resistance responses. Plant Cell 18: 747–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Song W, Tan EYJ, Liu L, Cao Y, Jirschitzka J, Li E, Logemann E, Xu C, Huang S et al (2021) TIR domains of plant immune receptors are 2′,3′‐cAMP/cGMP synthetases mediating cell death. bioRxiv 10.1101/2021.11.09.467869 [PREPRINT] [DOI] [PubMed]
- Yu IC, Parker J, Bent AF, Ent ANFB, Yu IC, Parker J, Bent AF (1998) Gene‐for‐gene disease resistance without the hypersensitive response in Arabidopsis dnd1 mutant. Proc Natl Acad Sci USA 95: 7819–7824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Feng B, He P, Shan L (2017) From chaos to harmony: responses and signaling upon microbial pattern recognition. Annu Rev Phytopathol 55: 109–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Xu G, Li BO, de Souza Vespoli L, Liu H, Moeder W, Chen S, de Oliveira MVV, Ariádina de Souza S, Shao W et al (2019) The receptor kinases BAK1/SERK4 regulate Ca2+ channel‐mediated cellular homeostasis for cell death containment. Curr Biol 29: 3778–3790.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan F, Yang H, Xue Y, Kong D, Ye R, Li C, Zhang J, Theprungsirikul L, Shrift T, Krichilsky B et al (2014) OSCA1 mediates osmotic‐stress‐evoked Ca2+ increases vital for osmosensing in Arabidopsis . Nature 514: 367–371 [DOI] [PubMed] [Google Scholar]
- Yuan M, Jiang Z, Bi G, Nomura K, Liu M, Wang Y, Cai B, Zhou J‐MM, He SY, Xin X‐FF (2021) Pattern‐recognition receptors are required for NLR‐mediated plant immunity. Nature 592: 105–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W, He SY (2010) A prominent role of the flagellin receptor FLAGELLIN‐SENSING2 in mediating stomatal response to Pseudomonas syringae pv tomato DC3000 in Arabidopsis . Plant Physiol 153: 1188–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Shao F, Li Y, Cui H, Chen L, Li H, Zou Y, Long C, Lan L, Chai J et al (2007) A Pseudomonas syringae effector inactivates MAPKs to suppress PAMP‐induced immunity in plants. Cell Host Microbe 1: 175–185 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Tateda C, Jiang S‐C, Shrestha J, Jelenska J, Speed DJ, Greenberg JT (2017) A suite of receptor‐like kinases and a putative mechano‐sensitive channel are involved in autoimmunity and plasma membrane‐based defenses in Arabidopsis . Mol Plant Microbe Interact 30: 150–160 [DOI] [PubMed] [Google Scholar]
- Zhao C, Tang Y, Wang J, Zeng Y, Sun H, Zheng Z, Su R, Schneeberger K, Parker JE, Cui H (2021) A mis‐regulated cyclic nucleotide‐gated channel mediates cytosolic calcium elevation and activates immunity in Arabidopsis . New Phytol 230: 1078–1094 [DOI] [PubMed] [Google Scholar]
- Zhou J‐M, Zhang Y (2020) Plant immunity: danger perception and signaling. Cell 181: 978–989 [DOI] [PubMed] [Google Scholar]
- Zhu W, Li L, Neuhäuser B, Thelen M, Wang M, Chen J, Wei L, Venkataramani K, Exposito‐Alonso M, Liu C et al (2021) Small peptides modulate the immune function of the ion channel‐like protein ACD6 in Arabidopsis thaliana . bioRxiv 10.1101/2021.01.25.428077 [PREPRINT] [DOI]
- Zimmermann S, Nurnberger T, Frachisse J‐MJ‐M, Wirtz W, Guern J, Hedrich R, Scheel D, Nürnberger T, Frachisse J‐MJ‐M, Wirtz W et al (1997) Receptor‐mediated activation of a plant Ca2+‐permeable ion channel involved in pathogen defense. Proc Natl Acad Sci USA 94: 2751–2755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones JDG, Boller T, Felix G (2006) Perception of the bacterial PAMP EF‐Tu by the receptor EFR restricts Agrobacterium‐mediated transformation. Cell 125: 749–760 [DOI] [PubMed] [Google Scholar]


