Abstract
Blunted stress reactivity resulting from early exposure to stress during childhood and adolescence may increase vulnerability to addiction. Early life adversity (ELA) affects brain structure and function and results in blunted stress axis reactivity. In this review, we focus on the underlying neurobiological mechanisms associated with a blunted response to stress, ELA, and risk for addictive disorders. ELA and blunted reactivity are accompanied by unstable mood regulation, impulsive behaviors, and reduced cognitive function. Neuroimaging studies reveal cortical and subcortical changes in persons exposed to ELA and those who have a genetic disposition for addiction. We propose a model in which blunted stress reactivity may be a marker of risk for addiction through an altered motivational and behavioral reactivity to stress that contribute to disinhibited behavioral reactivity and impulsivity leading in turn to increased vulnerability for substance use. Evidence supporting this hypothesis in the context of substance use initiation, maintenance, and risk for relapse is presented. The effects of ELA on persons at risk for addiction may lead to early experimentation with drugs of abuse. Early adoption of drug intake may alter neuroregulation in such vulnerable persons leading to a permanent dysregulation of motivational responses consistent with dependence.
This article is part of the special issue on ‘Vulnerabilities to Substance Abuse’.
Keywords: Stress reactivity, Early life adversity, Addiction risk, Neurobiological mechanisms, HPA axis
1. Introduction
Exposure to adverse circumstances during childhood and adolescence may lead to poor health outcomes in adulthood. In the behavioral realm, ELA is associated with future maladaptive behaviors including abuse of alcohol and other substances (SUD), compulsive gambling, and risk-taking behaviors (Carroll et al., 2017; Duffy et al., 2018; Ellis et al., 2020; Schilling et al., 2007; Sinha 2008). In this review, we focus on the underlying neurobiological mechanisms associated with a blunted response to stress, early life adversity (ELA), and risk for addictive disorders. ELA broadly denotes exposure early stressors including low socioeconomic status, physical and sexual abuse, significant family disruption, and lack of nurturing (Lovallo et al., 2012). The pathways leading from ELA to poor health behaviors and addiction are not well understood, although dysregulation of the stress axis appears to have a central role. In addition, there is evidence indicating that ELA exerts its impact on behavioral and mental health by triggering changes in the developing brain (Acheson et al., 2014b; Lim et al., 2020; Meaney et al., 2002; Miguel et al., 2019; Tomalski and Johnson 2010). Evidence points to diminished communication between the prefrontal cortex and brain reward centers along with the hypothalamus and brainstem leading to modification of stress reactivity, behavioral control, and regulation of affect. These modifications collectively appear to increase the likelihood of behavioral risk-taking resulting in increased risk for substance use disorders (SUD). We note that not all persons exposed to ELA have adverse outcomes and some that manifest protective characteristics including adaptive coping and problem-solving skills as noted in recent papers (Ellis et al., 2020; Hoffmeister et al., 2019; Malhi et al., 2019; Romeo 2015; Rutter 2013).
Substantial evidence has emerged supporting ELA as a precursor to blunted stress reactivity (Carroll et al., 2017). Evidence is also emerging that specific genetic polymorphisms may confer vulnerabilities to ELA that confer enhanced risk of SUD. Behavioral and mechanistic evidence indicates that ELA can permanently modify neurobiological processes including communication pathways to and from the hypothalamus and brainstem resulting in abnormal regulation of the stress axis (Kataoka et al., 2020). Observational studies show that blunted stress reactivity resulting from ELA is associated with a tendency toward high-risk behaviors including drug use. Other evidence suggests that ELA, blunted stress reactivity, and poor emotional and behavioral regulation involve impaired prefrontal communication with reward centers in ways that may increase the reward value of abused drugs (Koob and Le Moal 2008; Koob and Schulkin 2019). At present the nature of the above relationships are understood at a correlational level but the precise mechanisms are not fully developed. We review the current state of work, point to areas in need of attention, and provide a heuristic model to aid in our focus on these questions as described in Fig. 1.
Fig. 1.
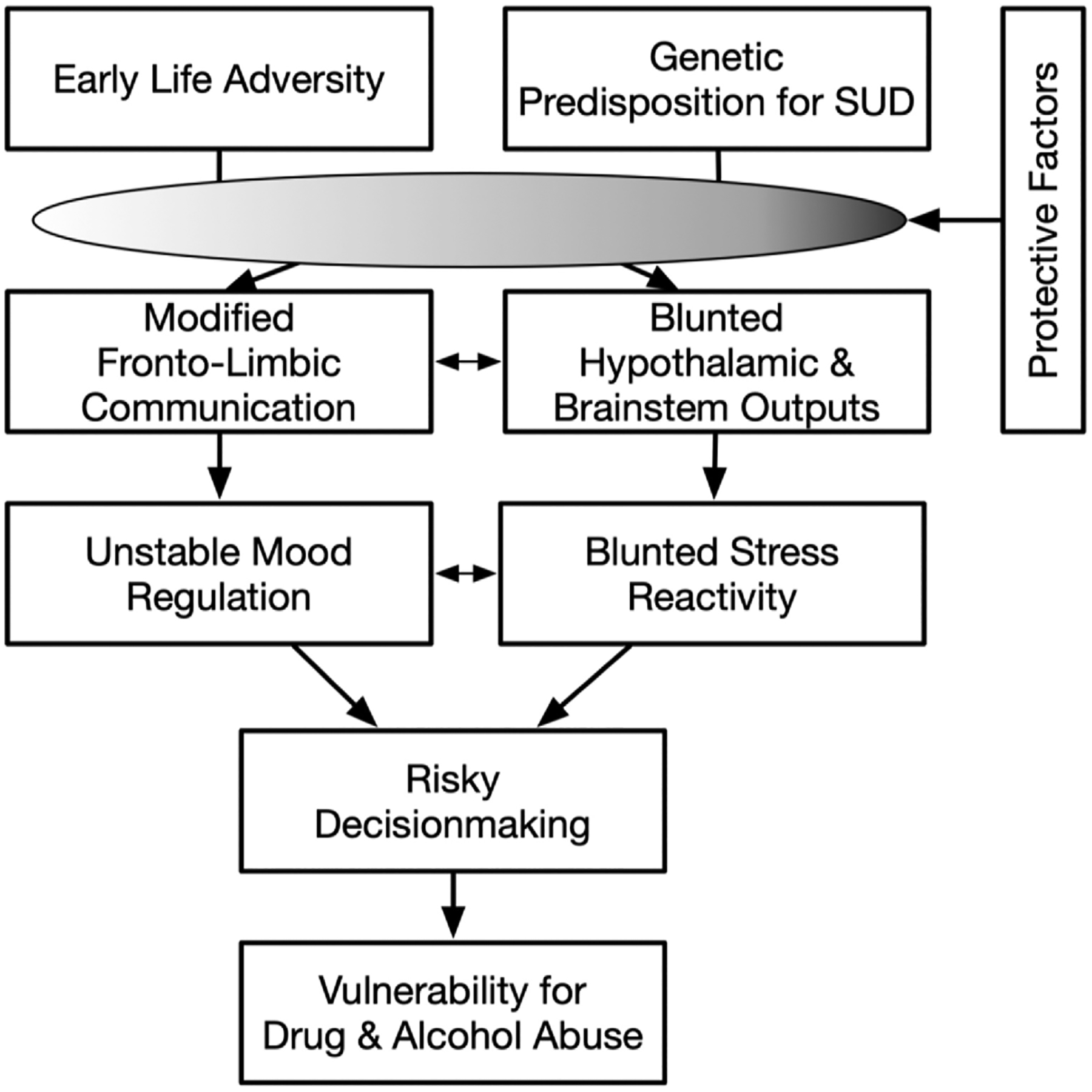
The influence of early life stress and genetic predisposition in modifying brain development and stress response systems leading to poor decision making and increased vulnerability for substance use. The gradient oval indicates that a range of protective factors may confer either low or high levels of protection to individuals, with the result that risk for substance use disorders is manifested to different degrees in downstream outputs.
2. Stress response and regulation
Activation of the stress axis by physical and psychological challenges is represented by rapid increases of catecholamines, driven by sympathetic nervous system activity, and of cortisol and beta-endorphin, controlled by the hypothalamic-pituitary-adrenocortical axis (HPA) (Akil et al., 1984; al’Absi et al., 2006a,b; al’Absi et al., 2004). Although cortisol and beta-endorphin release are key signals of HPA response to threats to homeostasis, it is often overlooked that both substances also regulate the stress axis by preventing excessive activation of the central nervous system and peripheral systems (de Kloet et al., 2005). By extension, blunted stress axis reactivity to stressor exposure can impair the ability of the system as a whole to regulate the impact of those same challenges.
Dysregulation of the stress axis is likely to represent long term modification of structures including the nucleus accumbens, amygdala and hippocampus along with their connections to key brainstem structures including the locus coeruleus, raphe nuclei, and ventral tegmental nuclei, all of which receive opioid neuronal projections leading to the stimulation of dopaminergic neurons, inhibition of CRF activity neurons within the PVN and adrenergic neurons within the locus coeruleus (Bruijnzeel 2009; Drolet et al., 2001; Zhou et al., 2008). The interaction of these systems represents a nexus that could help us understand the role of stress and reward regulation in risk for substance use (Chong et al., 2006; Oswald and Wand 2004; Wand et al., 2002; Wand and Schumann 1998). In particular, emerging research is providing insights into the means by which ELA leads to risk-related behavioral tendencies (Gianoulakis 1996, 2004). In light of the complex network involving the endogenous opioid system, we have explored the role of the opioid system in understanding the blunted stress response in tobacco addiction (al’Absi and Bongard, 2006; al’Absi et al., 2004; al’Absi et al., 2008; Ceballos et al., 2007; France et al., 2005a; b; France et al., 2007; Shaw and al’Absi 2010) using the opioid blocker, naltrexone.
We observed a consistent reduction of HPA response to opioid blockade during stress in abstinent smokers, suggesting reduced opioid tone during tobacco withdrawal as evidenced by HPA stress response (al’Absi et al., 2020). This seems to be reversed by acute use of tobacco suggesting that nicotine may produce its reinforcing effects in part by its effects in the opioid-HPA stress response regulation.
3. Early life adversity and vulnerability to addiction
Several lines of evidence point to relationships among blunted stress reactivity, ELA, and proneness to addiction. Population studies and national surveys in the USA have shown that exposure to two or more adverse experiences during childhood significantly increases risk for alcohol and drug abuse (Pilowsky et al., 2009) across ethnic and racial groups (Ducci et al., 2009; Koss et al., 2003; Robin et al., 1997). Children exposed to ELA begin use of alcohol and drugs at an earlier age than persons not exposed to ELA (Anda et al., 1999; Dube et al., 2003; Hyman et al., 2006; Hyman and Sinha 2009), and early-onset alcohol and drug use is associated with increased risk for abuse and dependence in adulthood (Agorastos et al. 2018, 2019; Englund et al., 2008; Kessler et al., 2005).
In earlier work, we observed blunted cortisol reactivity to laboratory stressors in a group of male veterans hospitalized for alcoholism (Errico et al., 1993). In a later study, we showed that blunted reactivity was not due to disruption of basal cortisol secretion, suggesting that the HPAC was functionally intact in alcoholic patients, instead suggesting alterations in brain systems mediating responses to the environment including the mesolimbic system (Lovallo et al., 2000). Since the stressors we employed were largely psychological in nature, we surmised that the source of the observed blunted reactivity was likely to be due to diminished communication from the prefrontal cortex to brain regions associated with response to reward and punishment and activation of the stress axis.
We next examined a large group of healthy young adults with a family history of alcoholism (FH+) who were social drinkers and found that they too had blunted cortisol and heart rate responses to psychosocial stressors (Lovallo et al., 2019b). We then found that the largest contributor to blunted reactivity was the degree of prior exposure to ELA (Lovallo et al., 2012). Not surprisingly, FH + persons are more often exposed to ELA than their FH– counterparts (Vincent et al., 2017), and FH + show a greater degree of blunted reactivity following ELA exposure than do FH– (Lovallo et al., 2019b). Research has shown that blunted stress reactivity predicts risk for SUD and proneness to relapse following withdrawal and treatment (al’Absi et al., 2003; Gilbert et al., 1997; Kirschbaum et al., 1994; Kirschbaum et al., 1993a; b; Tsuda et al., 1996). For example, our laboratory has examined stress reactivity during initial phases of a smoking cessation attempt and examined the extent to which these changes predicted risk for relapse Results indicate that blunted HPA (cortisol and ACTH) as well as beta endorphin, prolactin, and blood pressure responses during withdrawal predict shorter time to relapse (al’Absi et al., 2005).
4. Mechanisms of the blunted stress response
Several mechanisms may contribute to the dysregulated stress response, including changes in both central brain processes and peripheral feedback loops that may have resulted from developmental alterations and chronic dysregulation of these systems (al’Absi, 2018; Eddie et al., 2020). These modifications are likely to result is altered motivational and behavioral reactivity to external stimuli.
4.1. Alteration in HPA regulation and signaling
The greater sensitivity to ELA seen in FH + persons may reflect some combination of their increased exposure as well as a genetic diathesis reflecting their family history (Lovallo et al., 2019b). Although gene-by-environment interactions remain to be explored in depth, several findings in the Family Health Patterns Project point to genetic vulnerabilities in relation to FH + status, ELA exposure, and phenotypic tendencies reflecting risk for alcoholism, including early adoption of drinking prior to age 15.
Gene alleles associated with cortisol trafficking in the brain may be associated with poor working memory and blunted HR reactivity in relation to a history of ELA (Lovallo et al. 2016, 2019a). FKBP5 is a molecular cochaperone that contributes to the functional status of the glucocorticoid receptor (GR) and to the quality of corticosteroid signaling that is modified in the G-to-A single nucleotide polymorphism (SNP), rs9296158. Compared with FKBP5, GG homozygotes (N = 118), A-allele carriers (N = 132) without psychiatric morbidity had progressively worse performance on the Stroop color-word task with histories of increasing levels of ELA exposure (Genotype × ELA, F = 5.14, P = 0.007), indicating a G × E interaction on working memory in early adulthood. In addition, heart rate response to mental stress was diminished in AA/AG-allele carriers (F = 5.15, P = 0.024) having greater ELA exposure. Given the greater impact of stress levels of cortisol on GR receptor function in carriers of the A allele, ELA may affect the prefrontal cortex and, by extension, its interactions with external stimuli and subsequent impact on the limbic system and stress axis during development in A-allele carriers in agreement with work by others (Buchmann et al., 2014; Lapp et al., 2019; VanZomeren-Dohm et al., 2015).
Another avenue of vulnerability to ELA appears in studies of polymorphisms in the gene for catechol-O-methyltransferase (COMT) and altered dopaminergic signaling in the prefrontal cortex (Abraham et al., 2020). We have shown that differences in prefrontal DA availability known to occur in met vs. val/val carriers of the COMT val158met polymorphism (rs4680) are differentially reactive in HPA stress reactivity and differentially vulnerable to ELA, and in addition met-carriers are prone to early alcohol intake and smoking, showing that the met polymorphism not only renders individuals sensitive to ELA but that this sensitivity translates into a significant effect on health behaviors (Lovallo et al., 2019c). Neuroimaging of brain response to reward feedback shows greater effects in met-allele carriers exposed to ELA, also suggesting differential sensitivity to ELA in met carriers (Boecker-Schlier et al., 2016). As noted by Goldman, “The higher activity Val158 allele is predicted to reduce dopamine levels in the frontal cortex because levels of the dopamine transporter are low in this region. Consistent with the role of dopamine in the tuning of frontal cortical function, the Val158 allele and Val158 haplotypes have replicably been linked to frontal lobe function” (Goldman et al., 2005). Some work implies a greater increase in frontal DA availability in val homozygotes against their presumed lower baseline (Serrano et al., 2019). Met carriers show a gene-dose effect greater response to unpleasant stimuli in the limbic system (left hippocampus, right amygdala, right thalamus), connected prefrontal areas (bilateral ventrolateral prefrontal cortex, right dorsolateral prefrontal cortex), and the visuospatial attention system (bilateral fusiform gyrus, left inferior parietal lobule) relative to val homozygotes (Smolka et al., 2005). A review of rodent literature shows an impact of ELA on DA availability in the striatal region (Bonapersona et al., 2018). Our work and others agree that one or two copies of the COMT met allele render individuals more sensitive to ELA than their val/val counterparts (Abraham et al., 2020; Boecker-Schlier et al., 2016). These studies point to a gene × environment interaction between the COMT val158met allele and ELA resulting in altered responses to motivational cues that may enhance risk for SUD. The COMT polymorphism seems to warrant more extensive investigation of the impact of ELA on the stress axis and in relation to health behaviors in both animal models and clinical populations (Lonsdorf et al., 2011).
4.2. Network disruption cortical-subcortical integration
4.2.1. Structural evidence
Human neuroimaging studies demonstrate that ELA and blunted stress reactivity may relate to altered structure of the cerebral cortex (Busso et al. 2017a, 2017b). To our knowledge, there have only been two studies examining the associations between cardiovascular stress reactivity and grey matter volume (Gianaros et al., 2008; Trotman et al., 2019). The studies suggest, in healthy populations, higher levels of cardiovascular stress reactivity are associated with lower amygdala and hippocampal volume (Gianaros et al., 2008; Trotman et al., 2019). A more extensive line of research has specifically focused on the influence of ELA and brain structure. Research suggests ELA is associated with alternations in corticolimbic brain structures that have also been associated with neuroendocrine and autonomic control such as the anterior cingulate cortex, prefrontal cortex, amygdala, hippocampus (Beissner et al., 2013; Bremner et al., 1997; Gianaros and Wager 2015; Ginty et al., 2017; Gorka et al., 2014; McLaughlin et al., 2019; Myers 2017; Teicher et al., 2016; Thayer et al., 2012). Both animal and human studies demonstrate that ELA alters the normal course of brain development, particularly for corticolimbic brain structures (Card et al., 2005; Davidson and McEwen 2012; Harden et al., 2016; Malter Cohen et al., 2013; Pechtel and Pizzagalli 2011; Shonkoff 2010; Teicher et al., 2016; Tottenham and Sheridan 2009) and alternations in cortical structures in adulthood (Cassiers et al., 2018; Jensen et al., 2015; Paquola et al., 2016; van Harmelen et al., 2010). In a study comparing individuals with a history of childhood maltreatment to those with no history, maltreatment was associated with decreased structural connectivity from the anterior cingulate with other areas in a network of regions important in emotional regulation, attention, and social cognition (Teicher et al., 2014).
Diffusion tensor imaging (DTI) studies have also shown reduced fractional anisotropy in individuals with a history of ELA (Choi et al., 2009; Lim et al., 2020; Teicher et al., 2010; Tendolkar et al., 2018). In a study examining individuals exposed to ELA, participants who developed post-traumatic stress disorder symptoms had decreased FA compared to those who did not develop symptoms (Fani et al., 2012), suggesting white matter integrity may be an important risk marker for psychopathology. DTI measures also show impaired integrity of myelinated fiber pathways in the PFC (anterior corona radiata) in relation to impulsive tendencies (Peper et al., 2013) and in studies of FH + subjects from two of our data sets (Acheson et al., 2014b). In FH + persons with no psychiatric history, the degree of myelinated fiber functional impairment was associated independently with FH + history in these studies, one on children 11–14 years of age and the other in young adults averaging 23.5 years of age, with each sample being significantly correlated with the number of alcoholic first-degree relatives (rs = 0.4) (Acheson et al. 2014a, 2014b). Although the foregoing studies did not have sufficient statistical power to examine ELA in the FH + population, a recent meta-analysis of ELA exposure showed widespread white matter microstructural abnormalities in the fornix, corpus callosum, and optic radiations linking fronto-limbic connections to areas presumably involved in conveying and processing aversive experiences (Lim et al., 2020). White matter abnormalities suggest a source of further work examining a direct impact of ELA on brain development and a source of interaction between ELA exposure on vulnerable genotypes affecting development of glial cells responsible for myelinated pathways. We suspect that further work on larger subject samples will allow an examination of white matter structural abnormalities and behavioral and emotional dysregulation contributing to SUD risk. There is also a role for sex-related hormones with changes in cortical-subcortical communication. For example, high levels of testosterone are associated with reduced cortico-subcortical communication (Peper et al., 2011). For example, high testosterone levels over time may attenuate white matter tracts between frontal and striatal regions, which may have chronic effects on reward and threat processing (e.g. increased impulsivity) (Peper et al., 2013).
4.2.2. Resting state functional evidence
Studies of ELA and resting state functional connectivity compliment studies of white matter abnormalities. Both sets of studies suggest that ELA alters connectivity in areas associated with stress circuitry. ELA is associated with reduced functional connectivity between the prefrontal cortex and limbic regions, including the amygdala, in humans (Birn et al., 2014; Grant et al., 2014; Herringa et al., 2013; Kraynak et al., 2019) and animals (Guadagno et al., 2018; Yan et al., 2017).
4.3. Blunted reactivity and cortico-limbic function
Reviews and meta-analyses have confirmed cortical and limbic areas associated with visceral control (anterior cingulate cortex, medial prefrontal cortex, hippocampus, insula, and amygdala) are core components of a network of forebrain systems involved in stressor-evoked cardiovascular reactivity (Gianaros and Wager 2015; Ginty et al., 2017; Myers 2017; Shoemaker and Goswami 2015; Thayer et al., 2012) and addiction reward circuitry (Eddie et al., 2020). Three studies in healthy young adults demonstrate lower cardiovascular reactivity in relation to reduced activation in these same brain regions. Lower blood pressure reactivity was associated with reduced stressor-evoked amygdala activation (Gianaros et al., 2008) and anterior cingulate cortex activation (Gianaros et al., 2005) in these samples. In a study comparing individuals with blunted and exaggerated cardiovascular reactivity, we demonstrated that blunted reactivity was associated with reduced stressor-evoked activation in the anterior mid-cingulate cortex and insula and greater deactivation in the amygdala and posterior cingulate (Ginty et al., 2013).
In the context of psychological stressors, it is proposed that cortical and subcortical circuits associated with autonomic and neuroendocrine function generate anticipatory visceromotor commands to engender the cardiovascular and neuroendocrine system, via subcortical and brainstem cell groups, to prepare individuals to cope with the stressors (Gianaros and Wager 2015; Ginty et al., 2017). Individual differences in these forebrain appraisals may explain individual differences in cardiovascular and neuroendocrine stress responses. Failure to mount a stressor-evoked cardiovascular or neuroendocrine response may be caused by a ‘visceral prediction error’ or a mis-calibration between anticipatory commands and actual behavioral needs to cope with the stressor (Gianaros and Wager 2015; Ginty et al., 2017). Previous work has proposed a cardiovascular response to acute psychological stress is a marker of active coping (Obrist et al., 1978). It is possible that blunted cardiovascular responses to acute psychological stress are a result of forebrain appraisals predicting that less support is needed than is actually needed to cope with the stressor. Indeed, as mentioned earlier in this review, blunted reactivity has been associated with disorders associated with reduced motivation (Ginty et al., 2020).
While there is a substantial body of literature examining the impact of ELA on functional activation during emotion processing, inhibitory control, and reward processing tasks (for review see (Kraaijenvanger et al., 2020), there is scant research examining whether ELA is related to alternations in stressor-evoked activity within the brain regions shown to regulate cardiovascular and cortisol responses to stress. A study of 155 healthy adults demonstrated that childhood physical abuse was associated with reduced stressor-evoked activity in the subgenual anterior cingulate cortex, bed nucleus of stria terminalis, amygdala, and paraventricular nucleus of the hypothalamus (Banihashemi et al., 2015). Foster/adopted children with ELA had significantly less activation in the dorsal anterior cingulate cortex and dorsolateral prefrontal cortex compared with matched controls during a social stressor (Puetz et al., 2014). These two studies provide evidence that ELA is associated with altered stressor-evoked neural activity in areas associated with cardiovascular and neuroendocrine control. Given that ELA is associated with reduced cardiovascular and neuroendocrine activity and alterations in stressor-evoked neural activity, it is possible that ELA alters the impact of forebrain appraisals of stress or threatening stimuli and results in ‘visceral prediction errors’ which result in reduced cardiovascular and neuroendocrine activity needed to behaviorally cope with the stressor. In the context of early life adversity, such alterations may be an adaptive “survival” response to external conditions that are out of the individual’s control and cannot be escaped. However, these hypotheses have yet to be tested.
4.4. Genetic diathesis
The above studies implicating ELA, blunted stress reactivity, addiction risk, and addiction raise questions about genetic polymorphisms that may point to increased risk for addiction (Mueller et al., 2012). A number of such studies point toward genotypes possibly affecting neurochemical systems and brain regions associated with reward processes and behavioral regulation more generally (Blum et al., 2000; Coplan et al., 2011; Mueller et al., 2012; Ouellet-Morin et al., 2008). We have examined potential clustering of genetic polymorphisms in FH + young adults in relation to phenotypes associated with heightened risk for SUD. We found an association between ELA and neuroticism, harm avoidance, and symptoms of depression in persons carrying the gain-of-function polymorphism of the serotonin transporter gene SCL6A4 (Lovallo et al., 2014) consistent with other work showing enhanced startle reactivity in carriers of the short allele of the serotonin transporter gene (Armbruster et al., 2009). Other analyses showed impaired working memory and blunted heart rate stress reactivity in persons carrying the rs9296158 polymorphism of the gene for the molecular cochaperone, FKBP5, an allele that impairs glucocorticoid signaling in the central nervous system (Lovallo et al., 2016). Persons exposed to ELA who carry the high-activity polymorphism of the gene for COMT (rs4680, val158met) display blunted stress reactivity and begin drinking at an earlier age and experimenting with more drugs during adolescence (Lovallo et al. 2017, 2019c). Collectively, these studies suggest the value of examining these and related genotypes for main effects and interactions with FH status, ELA exposure, blunted stress reactivity and addictions (Lovallo et al., 2019c).
4.5. Sex differences in the stress response and risk for addiction
Research has demonstrated significant sex differences in different systems involved in regulating the stress response including the sympathetic system and HPA axis (al’Absi et al., 1999; al’Absi et al., 2004; Kirschbaum et al., 1995; RC et al., 2014). Sex steroids, such as estradiol, may mediate these differences (Allen and Matthews 1997; Kirschbaum et al., 1999; Steptoe et al., 1996). For example, suggested by observations showing that cortisol response to stress was similar between men and women in the luteal phase of the menstrual cycle, while women in the follicular phase showed a blunted response relative to men (Kirschbaum et al., 1999). Research also indicated sex differences in the pattern and level of regulation on the HPA stress response exerted by the endogenous opioid regulation (al’Absi et al., 2004; Chong et al., 2007; Uhart et al., 2006). Findings in humans extend preclinical evidence indicating sex differences in the sensitivity, quantity, and ratio of the different classes of opioid receptors (Hammer 1985; Hammer et al., 1994; Sershen et al., 1998) and observation in humans showing higher μ-opioid binding in women compared to men (Zubieta et al., 2002).
Research examining the interaction between sex and early life adversity on stress responses has produced mixed results, however. One study suggests there is a significant association between exposure to violence and blunted cortisol reactivity in males, but no significant association in females (Peckins et al., 2012). While other studies have demonstrated that although men tend to have a higher cortisol response to stress than females overall, there are no sex by ELA interactions (Lovallo et al., 2012; Trickett et al., 2014). A recent study in smokers reported that higher levels of ELA were associated with blunted stress responses in females and heightened responses in males (Hood et al., 2020). The general lack of consensus could be due to a number of factors. First, many studies examining the impact of ELA on stress reactivity use female only samples (Heim et al., 2001; Klumpers et al., 2004; MacMillan et al., 2009; Mielock et al., 2017; Voellmin et al., 2015). Second, ELA differentially impacts males and females depending on the timing of the adverse exposure; with males being more vulnerable than females to adversity that occur early in life (Hodes and Epperson 2019). Future research should aim to use samples that include both males and females and that record timing of ELA.
5. Discussion and conceptual integration
The cascade of ELA, blunted stress reactivity, impairments of motivated behavior (a phrase that broadly encompasses poor regulation of affect, attraction for immediate rewards, antisocial behavior, and poor cognitive function) are all associated with elevated risk for addictive behaviors. These risk factors may also vary as a function of genetic predisposition, as indicated by recent studies demonstration gene × environment interaction in shaping responses to motivationally relevant stimuli as well as the stress response and early adoption of drinking (Lovallo et al. 2016, 2019a). While it is currently unknown how these reactivity and behavioral propensities interact, we present here a model where we propose that ELA through initial changes in the brain can influence the manner by which information is processed and learning is developed. This also influence evaluative and emotional elaboration of stress as well as decision making and risk for impulsivity. Previous research has shown that traits such as impulsivity, sensation seeking, and disinhibition can influence the manner by which individuals respond to acute challenges and also increase propensity to engage in high-risk behaviors, such as substance use (Scarpa, 2015; Ginty et al., 2013; Lovallo et al., 2012; Phillips et al., 2013; Phillips et al., 2011).
Level of risk may be exacerbated or mitigated by various environmental and biological conditions and circumstances (Fig. 1). One of the outcomes of these upstream changes associated with ELA is dysregulation of the stress response (Carpenter et al. 2007, 2011; Lovallo et al., 2019b; Voellmin et al., 2015). Related work indicates that ELA and blunted reactivity may place otherwise healthy young adults at risk for drug abuse or for increased risk to relapse following cessation. Multiple mechanisms may mediate these effects including poor regulation of motivated behavior.
In conditions of ongoing stress and long-term effects of ELA on reward and stress response circuitry, drug use may have a potent reinforcing effect caused by its role in modulating the stress response. These effects, acutely, may also be facilitated by modifications in brain structures involved in emotional regulation and stress response. Chronically, this may then lead to long-term effects on brain’s motivational systems leaded to increased risk for maintenance of substance use. Our discussion here is therefore geared towards understanding the role of the chronic effects of ELA in altering the stress response and therefore increasing the risk for addiction. Indeed, much work in the field of substance use disorders has focused on studies of patients in treatment and in brain changes in animal models exposed to alcohol or other substances. The perspective presented here shows that similar changes in brain function and response to motivational stimuli may occur in healthy individuals thereby contributing to drug exposure, maintenance, and relapse (Koob and Le Moal 2008; Koob and Schulkin 2019; Zorrilla et al., 2014).
Our model assumes that in the face of stress and risk for drug use a robust stress and well-regulated recovery can be considered markers of resilience and the individual’s ability to buffer the impact of early stress (al’Absi, 2018). This argument takes note of cortisol’s important role as a regulator of brain function in the immediate aftermath of acute stress episodes. Elements involved in the stress response, biologically or behaviorally, may constitute immediate and delayed defense against stress-related threats, and therefore dysregulated response may reflect structural and functional problems in how we cope with demands that can be manifested by, for example, a disruption in integrating informational, physiological, and behavioral processes. The disruption of this systems coordination provides the impetus for subsequent risk for mental and substance use problems. Indeed, other research has linked dysregulated stress response and ELA with increased risk for engagement in risky behaviors such as smoking, binge drinking, risky driving, engaging in early sexual activity, and early and unintended pregnancy (Anda et al., 1999; Anderson 2017; Bentley and Widom 2009; Bogaert 2008; Culpin et al., 2014; Dietz et al., 1999; Gaydosh et al., 2018; Graber et al., 1995; Haller et al., 2014; Shin et al., 2013).
One important consideration in understanding the role of ELA in altering the stress response and risk for addiction, is the timing of exposure to ELA, in addition to frequency and intensity. Indeed, research has demonstrated that different types of adversity are linked to specific psychological and behavioral consequences, and these in turn are mediated by specific rain mechanisms (Teicher and Khan 2019; Teicher and Parigger 2015). The type of adversity may also impact the trajectory of risk as well as severity (Teicher et al., 2016). Similarly age of exposure to adversity (i.e., timing) also determine the type of risk and severity of the consensus of this risk (Khan, McCormack, et al., 2015). The stress-response profile however has not been evaluated directly to examine the impact of these parameters and requires greater attention in the context of substance use disorders.
We note that our model focuses on the extant literature on adverse childhood experiences, such as physical and sexual abuse, but there remain other factors during early childhood that may impact risk and still need to be developed in the literature less attention has been paid to environmental factors including crowding, noise, and neighborhood safety. Indeed, recent work has proposed that active trauma (e.g., abuse) may have differential effects compared with trauma caused by environmental factors (e.g., SES; for reviews see: (Duffy et al., 2018; McLaughlin et al., 2014; McLaughlin et al., 2019). We have acknowledged this issue and added it as and topic for future research.
6. Future directions
An outgrowth of the discussion above is to identify resilience factors (see Fig. 1) that may buffer the impact of early life stress on vulnerability to addiction. Recent research provides encouraging data. Insight into the question of vulnerability and resilience comes from a limited number of analyses addressing the question of who avoids developing alcohol use disorders despite drinking relatively heavily. Resistance to addiction (Kendler and Myers 2015) was seen in persons with minimal exposure to ELA and who scored in the highly socialized range of CPI-So scores. Individuals who reported greater emotional stability, norm adherence, risk avoidance, and fewer family members with substance use disorders were more resistant to AUD despite higher alcohol intake (Hoffmeister et al., 2019). This analysis agreed in large measure with two analyses of larger groups of persons examined in an epidemiological study of risk for psychiatric disorders (Cooke et al., 2017; Dick et al., 2013).
Future research should also better define the amount of addiction vulnerability conferred by environmental factors during early life adversity, genetic risk factors, and the interaction of these two factors. To that end, although research over the last decade has advanced our understanding of clinical and neuronal markers of risk associated with the ELA, there is still much to be discovered about the specific trajectories of developmental risk across age and areas of the brain, both structurally and functionally, most influenced by this risk and predictive of addiction vulnerability. A host of phenotypes and endophenotypes have been linked to ELA and have also been shown to be related to drug use risk. Yet, the extent to which these markers mediate the influence of ELA on risk for addiction remains to be defined. In addition, these factors may also be influenced by early exposure to substance use, introducing another layer of complexity yet to be defined. Such efforts would provide a substantial growth in translating these rich data sets into the clinical context. Identification of early risk pathways provides a means of better understanding the progression toward changes in reward pathways that represent responses to consistent exposure to drugs of abuse (Koob and Schulkin 2019).
In light of the observation that blunted stress response is implicated in different stages of the addiction cycle, and research showing the link of dysregulated stress response with post-traumatic-stress disorder (PTSD), it is intriguing to propose that blunted stress response may be a nexus of risk for SUD-PTSD comorbidity, although no direct evidence available at this time. This may therefore be a fruitful line of research into understanding SUD-PTSD.
The literature to date points to additional directions focusing on the importance of developing targeted behavioral and psychosocial interventions and programs to mitigate the impact of exposure to high levels of early adversity. Such program may also increase bolstering components within existing intervention to specifically target factors mediating the impact of early life adversity on risk for substance use disorders or mental health comorbidity. For example, efforts to manage psychological reaction to emotional and environmental cues associated with history of adv cues associated with history of adversity should be investigated as potential preventive measures. To that end, we note the need for additional intervention to address intermediate risk pheno-types, such as blunted stress reactivity, in the context of early life adversity. This may take integrated intervention approaches including pharmacological and psychological approaches targeting stress-related regulation processes.
7. Conclusions
Recent studies have shown an impact of ELA on blunted stress reactivity in conjunction with vulnerability to addiction. The mechanisms connecting life history, blunted reactivity, and consequences of drug exposure aid our understanding of pathways to dependence in vulnerable persons and resilience in others. Our review here highlights the role of dysregulated stress response and the impact of ELA on vulnerability to drug use. Changes in specific stress and reward-related pathways provide avenues through which ELA may lead to blunted stress response, and how this dysregulated response may translate into increased risk for drug use. The recognition of the role of stress in initiating, maintaining, and increasing risk for drug relapse is now well accepted. This work must therefore be complemented by effort to develop intervention strategies that seek to mitigate this ongoing and delayed cost stress.
Acknowledgement
This paper was supported in part by grants from the National Institutes of Health: R01DA016351 and R01DA027232 (MA), K01HL145021 (ATG); the US Department of Veterans Affairs, CX000252, and the National Institutes of Health, AA12207 (WRL).
References
- Abraham E, Scott MA, Blair C, 2020. Catechol-O-methyltransferase val(158)Met genotype and early-life family adversity interactively affect attention-deficit hyperactivity symptoms across childhood. Front. Genet 11, 724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheson A, Wijtenburg SA, Rowland LM, Bray BC, Gaston F, Mathias CW, Fox PT, Lovallo WR, Wright SN, Hong LE, McGuire S, Kochunov P, Dougherty DM, 2014a. Combining diffusion tensor imaging and magnetic resonance spectroscopy to study reduced frontal white matter integrity in youths with family histories of substance use disorders. Hum. Brain Mapp 35, 5877–5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheson A, Wijtenburg SA, Rowland LM, Winkler AM, Gaston F, Mathias CW, Fox PT, Lovallo WR, Wright SN, Hong LE, Dougherty DM, Kochunov P, 2014b. Assessment of whole brain white matter integrity in youths and young adults with a family history of substance-use disorders. Hum. Brain Mapp 35, 5401–5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agorastos A, Pervanidou P, Chrousos GP, Baker DG, 2019. Developmental trajectories of early life stress and trauma: a narrative review on neurobiological aspects beyond stress system dysregulation. Front. Psychiatr 10, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agorastos A, Pervanidou P, Chrousos GP, Kolaitis G, 2018. Early life stress and trauma: developmental neuroendocrine aspects of prolonged stress system dysregulation. Hormones (Basel) 17, 507–520. [DOI] [PubMed] [Google Scholar]
- Akil H, Watson SJ, Young E, Lewis ME, Khachaturian H, Walker JM, 1984. Endogenous opioids: biology and function. Annu. Rev. Neurosci 7, 223–255. [DOI] [PubMed] [Google Scholar]
- al’Absi M, 2018. Stress and addiction: when a robust stress response indicates resiliency. Psychosom. Med 80, 2–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al’Absi M, Bongard S, 2006. Neuroendocrine and behavioral mechanisms mediating the relationship between anger expression and cardiovascular risk: assessment considerations and improvements. J. Behav. Med 29, 573–591. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Buchanan TW, Marrero A, Lovallo WR, 1999. Sex differences in pain perception and cardiovascular responses in persons with parental history for hypertension. Pain 83, 331–338. [DOI] [PubMed] [Google Scholar]
- al’Absi M, France C, Harju A, France J, Wittmers L, 2006a. Adrenocortical and Nociceptive Responses to Opioid Blockade in Hypertension-Prone Men and Women Psychosomatic Medicine, pp. 292–298. United States. [DOI] [PubMed] [Google Scholar]
- al’Absi M, France C, Harju A, France J, Wittmers L, 2006b. Adrenocortical and nociceptive responses to opioid blockade in hypertension-prone men and women. Psychosom. Med 68, 292–298. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Hatsukami D, Davis GL, 2005. Attenuated adrenocorticotropic responses to psychological stress are associated with early smoking relapse. Psychopharmacol. (Berl.) 181, 107–117. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Nakajima M, DeAngelis B, Grant J, King A, Grabowski J, Hatsukami D, Allen S, 2020. Blunted opioid regulation of the HPA stress response during nicotine withdrawal: therapeutic implications. Stress 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al’Absi M, Wittmers LE, Ellestad D, Nordehn G, Kim SW, Kirschbaum C, Grant JE, 2004. Sex differences in pain and hypothalamic-pituitary-adrenocortical responses to opioid blockade. Psychosom. Med 66, 198–206. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Wittmers LE, Erickson J, Hatsukami DK, Crouse B, 2003. Attenuated adrenocortical and blood pressure responses to psychological stress in ad libitum and abstinent smokers. Pharmacol. Biochem. Behav 74, 401–410. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Wittmers LE, Hatsukami D, Westra R, 2008. Blunted opiate modulation of hypothalamic-pituitary-adrenocortical activity in men and women who smoke. Psychosom. Med 70, 928–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen MT, Matthews KA, 1997. Hemodynamic responses to laboratory stressors in children and adolescents: the influences of age, race, and gender. Psychophysiology 34, 329–339. [DOI] [PubMed] [Google Scholar]
- Anda RF, Croft JB, Felitti VJ, Nordenberg D, Giles WH, Williamson DF, Giovino GA, 1999. Adverse childhood experiences and smoking during adolescence and adulthood. J. Am. Med. Assoc 282, 1652–1658. [DOI] [PubMed] [Google Scholar]
- Anderson KG, 2017. Adverse childhood environment: relationship with sexual risk behaviors and marital status in a large American sample. Evol. Psychol 15, 1474704917710115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster D, Moser DA, Strobel A, Hensch T, Kirschbaum C, Lesch KP, Brocke B, 2009. Serotonin transporter gene variation and stressful life events impact processing of fear and anxiety. Int. J. Neuropsychopharmacol 12, 393–401. [DOI] [PubMed] [Google Scholar]
- Banihashemi L, Sheu LK, Midei AJ, Gianaros PJ, 2015. Childhood physical abuse predicts stressor-evoked activity within central visceral control regions. Soc. Cognit. Affect Neurosci 10, 474–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beissner F, Meissner K, Bär KJ, Napadow V, 2013. The autonomic brain: an activation likelihood estimation meta-analysis for central processing of autonomic function. J. Neurosci 33, 10503–10511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley T, Widom CS, 2009. A 30-year follow-up of the effects of child abuse and neglect on obesity in adulthood. Obesity 17, 1900–1905. [DOI] [PubMed] [Google Scholar]
- Birn RM, Patriat R, Phillips ML, Germain A, Herringa RJ, 2014. Childhood maltreatment and combat posttraumatic stress differentially predict fear-related fronto-subcortical connectivity. Depress. Anxiety 31, 880–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K, Braverman ER, Holder JM, Lubar JF, Monastra VJ, Miller D, Lubar JO, Chen TJ, Comings DE, 2000. Reward deficiency syndrome: a biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. J. Psychoact. Drugs 32 (Suppl. l), 1–112 i-iv. [DOI] [PubMed] [Google Scholar]
- Boecker-Schlier R, Holz NE, Buchmann AF, Blomeyer D, Plichta MM, JennenSteinmetz C, Wolf I, Baumeister S, Treutlein J, Rietschel M, Meyer-Lindenberg A, Banaschewski T, Brandeis D, Laucht M, 2016. Interaction between COMT Val(158)Met polymorphism and childhood adversity affects reward processing in adulthood. Neuroimage 132, 556–570. [DOI] [PubMed] [Google Scholar]
- Bogaert AF, 2008. Menarche and father absence in a national probability sample. J. Biosoc. Sci 40, 623–636. [DOI] [PubMed] [Google Scholar]
- Bonapersona V, Joels M, Sarabdjitsingh RA, 2018. Effects of early life stress on biochemical indicators of the dopaminergic system: a 3 level meta-analysis of rodent studies. Neurosci. Biobehav. Rev 95, 1–16. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, Capelli S, McCarthy G, Innis RB, Charney DS, 1997. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse—a preliminary report. Biol. Psychiatr 41, 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, 2009. kappa-Opioid receptor signaling and brain reward function. Brain Res. Rev 62, 127–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmann AF, Holz N, Boecker R, Blomeyer D, Rietschel M, Witt SH, Schmidt MH, Esser G, Banaschewski T, Brandeis D, Zimmermann US, Laucht M, 2014. Moderating role of FKBP5 genotype in the impact of childhood adversity on cortisol stress response during adulthood. Eur. Neuropsychopharmacol 24, 837–845. [DOI] [PubMed] [Google Scholar]
- Busso DS, McLaughlin KA, Brueck S, Peverill M, Gold AL, Sheridan MA, 2017a. Child abuse, neural structure, and adolescent psychopathology: a longitudinal study. J. Am. Acad. Child Adolesc. Psychiatry 56, 321–328 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busso DS, McLaughlin KA, Sheridan MA, 2017b. Dimensions of adversity, physiological reactivity, and externalizing psychopathology in adolescence: deprivation and threat. Psychosom. Med 79, 162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card JP, Levitt P, Gluhovsky M, Rinaman L, 2005. Early experience modifies the postnatal assembly of autonomic emotional motor circuits in rats. J. Neurosci 25, 9102–9111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, Anderson GM, Wilkinson CW, Price LH, 2007. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biol. Psychiatr 62, 1080–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Shattuck TT, Tyrka AR, Geracioti TD, Price LH, 2011. Effect of childhood physical abuse on cortisol stress response. Psychopharmacol. (Berl.) 214, 367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll D, Ginty AT, Whittaker AC, Lovallo WR, de Rooij SR, 2017. The behavioural, cognitive, and neural corollaries of blunted cardiovascular and cortisol reactions to acute psychological stress. Neurosci. Biobehav. Rev 77, 74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassiers LLM, Sabbe BGC, Schmaal L, Veltman DJ, Penninx B, Van Den Eede F, 2018. Structural and functional brain abnormalities associated with exposure to different childhood trauma subtypes: a systematic review of neuroimaging findings. Front. Psychiatr 9, 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceballos NA, France CR, al’Absi M, 2007. Influence of Naltrexone Administration on Dehydroepiandrosterone Sulfate Levels in Male and Female Participants Biological Psychology, pp. 414–416. Netherlands. [DOI] [PubMed] [Google Scholar]
- Choi J, Jeong B, Rohan ML, Polcari AM, Teicher MH, 2009. Preliminary evidence for white matter tract abnormalities in young adults exposed to parental verbal abuse. Biol. Psychiatr 65, 227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong RY, Oswald L, Yang X, Uhart M, Lin PI, Wand GS, 2006. The Micro-opioid receptor polymorphism A118G predicts cortisol responses to naloxone and stress. Neuropsychopharmacology 31, 204–211. [DOI] [PubMed] [Google Scholar]
- Chong RY, Uhart M, Wand GS, 2007. Endogenous opiates, addiction, and stress response. In: al’Absi M (Ed.), Stress and Addiction: Biological and Psychological Mechanisms. Academic Press/Elsevier, London, pp. 85–104. [Google Scholar]
- Cooke ME, Neale ZE, Barr PB, Myers J, Dick DM, Kendler KS, Edwards AC, 2017. The role of social, familial, and individual-level factors on multiple alcohol use outcomes during the first year of university. Alcohol Clin. Exp. Res 41, 1783–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coplan JD, Abdallah CG, Kaufman J, Gelernter J, Smith EL, Perera TD, Dwork AJ, Kaffman A, Gorman JM, Rosenblum LA, Owens MJ, Nemeroff CB, 2011. Early-life stress, corticotropin-releasing factor, and serotonin transporter gene: a pilot study. Psychoneuroendocrinology 36, 289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culpin I, Heron J, Araya R, Melotti R, Lewis G, Joinson C, 2014. Father absence and timing of menarche in adolescent girls from a UK cohort: the mediating role of maternal depression and major financial problems. J. Adolesc 37, 291–301. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, McEwen BS, 2012. Social influences on neuroplasticity: stress and interventions to promote well-being. Nat. Neurosci 15, 689–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER, Sibug RM, Helmerhorst FM, Schmidt MV, 2005. Stress, genes and the mechanism of programming the brain for later life. Neurosci. Biobehav. Rev 29, 271–281. [DOI] [PubMed] [Google Scholar]
- Dick DM, Aliev F, Latendresse SJ, Hickman M, Heron J, Macleod J, Joinson C, Maughan B, Lewis G, Kendler KS, 2013. Adolescent alcohol use is predicted by childhood temperament factors before age 5, with mediation through personality and peers. Alcohol Clin. Exp. Res 37, 2108–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz PM, Spitz AM, Anda RF, Williamson DF, McMahon PM, Santelli JS, Nordenberg DF, Felitti VJ, Kendrick JS, 1999. Unintended pregnancy among adult women exposed to abuse or household dysfunction during their childhood. J. Am. Med. Assoc 282, 1359–1364. [DOI] [PubMed] [Google Scholar]
- Drolet G, Dumont EC, Gosselin I, Kinkead R, Laforest S, Trottier JF, 2001. Role of endogenous opioid system in the regulation of the stress response. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 25, 729–741. [DOI] [PubMed] [Google Scholar]
- Dube SR, Felitti VJ, Dong M, Chapman DP, Giles WH, Anda RF, 2003. Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: the adverse childhood experiences study. Pediatrics 111, 564–572. [DOI] [PubMed] [Google Scholar]
- Ducci F, Roy A, Shen PH, Yuan Q, Yuan NP, Hodgkinson CA, Goldman LR, Goldman D, 2009. Association of substance use disorders with childhood trauma but not African genetic heritage in an African American cohort. Am. J. Psychiatr 166, 1031–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy KA, McLaughlin KA, Green PA, 2018. Early life adversity and health-risk behaviors: proposed psychological and neural mechanisms. Ann. N. Y. Acad. Sci 1428, 151–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddie D, Bates ME, Buckman JF, 2020. Closing the brain-heart loop: towards more holistic models of addiction and addiction recovery. Addiction Biol, e12958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis BJ, Abrams LS, Masten AS, Sternberg RJ, Tottenham N, Frankenhuis WE, 2020. Hidden talents in harsh environments. Dev. Psychopathol 1–19. [DOI] [PubMed] [Google Scholar]
- Englund MM, Egeland B, Oliva EM, Collins WA, 2008. Childhood and adolescent predictors of heavy drinking and alcohol use disorders in early adulthood: a longitudinal developmental analysis. Addiction 103 (Suppl. 1), 23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errico AL, Parsons OA, King AC, Lovallo WR, 1993. Attenuated cortisol response to biobehavioral stressors in sober alcoholics. J. Stud. Alcohol 54, 393–398. [DOI] [PubMed] [Google Scholar]
- Fani N, King TZ, Jovanovic T, Glover EM, Bradley B, Choi K, Ely T, Gutman DA, Ressler KJ, 2012. White matter integrity in highly traumatized adults with and without post-traumatic stress disorder. Neuropsychopharmacology 37, 2740–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- France CR, al’absi M, Ring C, France JL, Brose J, Spaeth D, Harju A, Nordehn G, Wittmers LE, 2005a. Assessment of Opiate Modulation of Pain and Nociceptive Responding in Young Adults with a Parental History of Hypertension Biological Psychology, pp. 168–174. Netherlands. [DOI] [PubMed] [Google Scholar]
- France CR, al’absi M, Ring C, France JL, Brose J, Spaeth D, Harju A, Nordehn G, Wittmers LE, 2005b. Assessment of opiate modulation of pain and nociceptive responding in young adults with a parental history of hypertension. Biol. Psychol 70, 168–174. [DOI] [PubMed] [Google Scholar]
- France CR, al’Absi M, Ring C, France JL, Harju A, Wittmers LE, 2007. Nociceptive flexion reflex and pain rating responses during endogenous opiate blockade with naltrexone in healthy young adults. Biol. Psychol 75, 95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaydosh L, Belsky DW, Domingue BW, Boardman JD, Harris KM, 2018. Father absence and accelerated reproductive development in non-hispanic white women in the United States. Demography 55, 1245–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Derbyshire SW, May JC, Siegle GJ, Gamalo MA, Jennings JR, 2005. Anterior cingulate activity correlates with blood pressure during stress. Psychophysiology 42, 627–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Sheu LK, Matthews KA, Jennings JR, Manuck SB, Hariri AR, 2008. Individual differences in stressor-evoked blood pressure reactivity vary with activation, volume, and functional connectivity of the amygdala. J. Neurosci 28, 990–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Wager TD, 2015. Brain-body pathways linking psychological stress and physical health. Curr. Dir. Psychol. Sci 24, 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianoulakis C, 1996. Implications of endogenous opioids and dopamine in alcoholism: human and basic science studies. Alcohol Alcohol Suppl. 1, 33–42. [PubMed] [Google Scholar]
- Gianoulakis C, 2004. Endogenous opioids and addiction to alcohol and other drugs of abuse. Curr. Top. Med. Chem 4, 39–50. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, Meliska CJ, Plath LC, 1997. Noise stress does not modulate effects of smoking/nicotine on ⋅-endorphin, cortisol, ACTH, glucose, and mood. Psychopharmacology 130, 197–202. [DOI] [PubMed] [Google Scholar]
- Ginty AT, Gianaros PJ, Derbyshire SW, Phillips AC, Carroll D, 2013. Blunted cardiac stress reactivity relates to neural hypoactivation. Psychophysiology 50, 219–229. [DOI] [PubMed] [Google Scholar]
- Ginty AT, Hurley PE, Young DA, 2020. Diminished cardiovascular stress reactivity is associated with higher levels of behavioral disengagement. Biol. Psychol 107933. [DOI] [PubMed] [Google Scholar]
- Ginty AT, Kraynak TE, Fisher JP, Gianaros PJ, 2017. Cardiovascular and autonomic reactivity to psychological stress: neurophysiological substrates and links to cardiovascular disease. Auton. Neurosci 207, 2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D, Oroszi G, Ducci F, 2005. The genetics of addictions: uncovering the genes. Nat. Rev. Genet 6, 521–532. [DOI] [PubMed] [Google Scholar]
- Gorka AX, Hanson JL, Radtke SR, Hariri AR, 2014. Reduced hippocampal and medial prefrontal gray matter mediate the association between reported childhood maltreatment and trait anxiety in adulthood and predict sensitivity to future life stress. Biol. Mood Anxiety Disord 4, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graber JA, Brooks-Gunn J, Warren MP, 1995. The antecedents of menarcheal age: heredity, family environment, and stressful life events. Child Dev. 66, 346–359. [DOI] [PubMed] [Google Scholar]
- Grant MM, White D, Hadley J, Hutcheson N, Shelton R, Sreenivasan K, Deshpande G, 2014. Early life trauma and directional brain connectivity within major depression. Hum. Brain Mapp 35, 4815–4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadagno A, Wong TP, Walker CD, 2018. Morphological and functional changes in the preweaning basolateral amygdala induced by early chronic stress associate with anxiety and fear behavior in adult male, but not female rats. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 81, 25–37. [DOI] [PubMed] [Google Scholar]
- Haller J, Harold G, Sandi C, Neumann ID, 2014. Effects of adverse early-life events on aggression and anti-social behaviours in animals and humans. J. Neuroendocrinol 26, 724–738. [DOI] [PubMed] [Google Scholar]
- Hammer RP Jr., 1985. The sex hormone-dependent development of opiate receptors in the rat medial preoptic area. Brain Res. 360, 65–74. [DOI] [PubMed] [Google Scholar]
- Hammer RP Jr., Zhou L, Cheung S, 1994. Gonadal steroid hormones and hypothalamic opioid circuitry. Horm. Behav 28, 431–437. [DOI] [PubMed] [Google Scholar]
- Harden BJ, Buhler A, Parra LJ, 2016. Maltreatment in infancy: a developmental perspective on prevention and intervention. Trauma Violence Abuse 17, 366–386. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB, 2001. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am. J. Psychiatr 158, 575–581. [DOI] [PubMed] [Google Scholar]
- Herringa RJ, Birn RM, Ruttle PL, Burghy CA, Stodola DE, Davidson RJ, Essex MJ, 2013. Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proc. Natl. Acad. Sci. U. S. A 110, 19119–19124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes GE, Epperson CN, 2019. Sex differences in vulnerability and resilience to stress across the life span. Biol. Psychiatr 86, 421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmeister JR, Cohoon AJ, Sorocco KH, Acheson A, Lovallo WR, 2019. Addiction resistance to alcohol: what about heavy drinkers who avoid alcohol problems? Drug Alcohol Depend. 204, 107552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood CO, Tomko RL, Baker NL, Tuck BM, Flanagan JC, Carpenter MJ, Gray KM, Saladin ME, McClure EA, 2020. Examining sex, adverse childhood experiences, and oxytocin on neuroendocrine reactivity in smokers. Psychoneuroendocrinology 120, 104752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SM, Garcia M, Sinha R, 2006. Gender specific associations between types of childhood maltreatment and the onset, escalation and severity of substance use in cocaine dependent adults. Am. J. Drug Alcohol Abuse 32, 655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SM, Sinha R, 2009. Stress-related factors in cannabis use and misuse: implications for prevention and treatment. J. Subst. Abuse Treat 36, 400–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen SK, Dickie EW, Schwartz DH, Evans CJ, Dumontheil I, Paus T, Barker ED, 2015. Effect of early adversity and childhood internalizing symptoms on brain structure in young men. JAMA Pediatr. 169, 938–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka N, Shima Y, Nakajima K, Nakamura K, 2020. A central master driver of psychosocial stress responses in the rat. Science 367, 1105–1112. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Myers J, 2015. Addiction resistance: definition, validation and association with mastery. Drug Alcohol Depend. 154, 236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE, 2005. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch. Gen. Psychiatr 62, 593–602. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Klauer T, Filipp SH, Hellhammer DH, 1995. Sex specific effects of social support on cortisol, heart rate, and subjective responses to acute psychological stress. Pschyosomatic Med. 57, 23–31. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH, 1999. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom. Med 61, 154–162. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Scherer G, Strasburger CJ, 1994. Pituitary and adrenal hormone responses to pharmacological, physical, and psychological stimulation in habitual smokers and nonsmokers. Clin. Invest 72, 804–810. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Strasburger CJ, Langkrar J, 1993a. Attenuated cortisol response to psychological stress but not to CRH or ergometry in young habitual smokers. Pharmacol. Biochem. Behav 44, 527–531. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Strasburger CJ, Langkrar J, 1993b. Attenuated cortisol response to psychological stress but not to CRH or ergometry in young habitual smokers. Pharmacol. Biochem. Behav 44, 527–531. [DOI] [PubMed] [Google Scholar]
- Klumpers UM, Tulen JH, Timmerman L, Fekkes D, Loonen AJ, Boomsma F, 2004. Responsivity to stress in chronic posttraumatic stress disorder due to childhood sexual abuse. Psychol. Rep 94, 408–410. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M, 2008. Addiction and the brain antireward system. Annu. Rev. Psychol 59, 29–53. [DOI] [PubMed] [Google Scholar]
- Koob GF, Schulkin J, 2019. Addiction and stress: an allostatic view. Neurosci. Biobehav. Rev 106, 245–262. [DOI] [PubMed] [Google Scholar]
- Koss MP, Yuan NP, Dightman D, Prince RJ, Polacca M, Sanderson B, Goldman D, 2003. Adverse childhood exposures and alcohol dependence among seven Native American tribes. Am. J. Prev. Med 25, 238–244. [DOI] [PubMed] [Google Scholar]
- Kraaijenvanger EJ, Pollok TM, Monninger M, Kaiser A, Brandeis D, Banaschewski T, Holz NE, 2020. Impact of early life adversities on human brain functioning: a coordinate-based meta-analysis. Neurosci. Biobehav. Rev 113, 62–76. [DOI] [PubMed] [Google Scholar]
- Kraynak TE, Marsland AL, Hanson JL, Gianaros PJ, 2019. Retrospectively reported childhood physical abuse, systemic inflammation, and resting corticolimbic connectivity in midlife adults. Brain Behav. Immun 82, 203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapp HE, Ahmed S, Moore CL, Hunter RG, 2019. Toxic stress history and hypothalamic-pituitary-adrenal axis function in a social stress task: genetic and epigenetic factors. Neurotoxicol. Teratol 71, 41–49. [DOI] [PubMed] [Google Scholar]
- Lim L, Howells H, Radua J, Rubia K, 2020. Aberrant structural connectivity in childhood maltreatment: a meta-analysis. Neurosci. Biobehav. Rev 116, 406–414. [DOI] [PubMed] [Google Scholar]
- Lonsdorf TB, Golkar A, Lindstom KM, Fransson P, Schalling M, Ohman A, Ingvar M, 2011. 5-HTTLPR and COMTval158met genotype gate amygdala reactivity and habituation. Biol. Psychol 87, 106–112. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Acheson A, Cohoon AJ, Sorocco KH, Vincent AS, Hodgkinson CA, Goldman D, 2019a. Working memory reflects vulnerability to early life adversity as a risk factor for substance use disorder in the FKBP5 cortisol cochaperone polymorphism, rs9296158. PloS One 14, e0218212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, Cohoon AJ, Acheson A, Sorocco KH, Vincent AS, 2019b. Blunted stress reactivity reveals vulnerability to early life adversity in young adults with a family history of alcoholism. Addiction 114, 798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, Cohoon AJ, Sorocco KH, Vincent AS, Acheson A, Hodgkinson CA, Goldman D, 2019c. Early-life adversity and blunted stress reactivity as predictors of alcohol and drug use in persons with COMT (rs4680) Val158Met genotypes. Alcohol Clin. Exp. Res 43, 1519–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, Dickensheets SL, Myers DA, Thomas TL, Nixon SJ, 2000. Blunted stress cortisol response in abstinent alcoholic and polysubstance-abusing men. Alcohol Clin. Exp. Res 24, 651–658. [PubMed] [Google Scholar]
- Lovallo WR, Enoch MA, Acheson A, Cohoon AJ, Sorocco KH, Hodgkinson CA, Vincent AS, Goldman D, 2016. Early-life adversity interacts with FKBP5 genotypes: altered working memory and cardiac stress reactivity in the Oklahoma family health patterns project. Neuropsychopharmacology 41, 1724–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, Enoch MA, Sorocco KH, Vincent AS, Acheson A, Cohoon AJ, Hodgkinson CA, Goldman D, 2017. Joint impact of early life adversity and COMT Val158Met (rs4680) genotypes on the adult cortisol response to psychological stress. Psychosom. Med 79, 631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, Enoch MA, Yechiam E, Glahn DC, Acheson A, Sorocco KH, Hodgkinson CA, Kim B, Cohoon AJ, Vincent AS, Goldman D, 2014. Differential impact of serotonin transporter activity on temperament and behavior in persons with a family history of alcoholism in the Oklahoma Family Health Patterns Project. Alcohol Clin. Exp. Res 38, 1575–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, Farag NH, Sorocco KH, Cohoon AJ, Vincent AS, 2012. Lifetime adversity leads to blunted stress axis reactivity: studies from the Oklahoma Family Health Patterns Project. Biol. Psychiatr 71, 344–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan HL, Georgiades K, Duku EK, Shea A, Steiner M, Niec A, Tanaka M, Gensey S, Spree S, Vella E, Walsh CA, De Bellis MD, Van der Meulen J, Boyle MH, Schmidt LA, 2009. Cortisol response to stress in female youths exposed to childhood maltreatment: results of the youth mood project. Biol. Psychiatr 66, 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi GS, Das P, Bell E, Mattingly G, Mannie Z, 2019. Modelling resilience in adolescence and adversity: a novel framework to inform research and practice. Transl. Psychiatry 9, 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malter Cohen M, Jing D, Yang RR, Tottenham N, Lee FS, Casey BJ, 2013. Early-life stress has persistent effects on amygdala function and development in mice and humans. Proc. Natl. Acad. Sci. U. S. A 110, 18274–18278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Lambert HK, 2014. Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience. Neurosci. Biobehav. Rev 47, 578–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Weissman D, Bitrán D, 2019. Childhood adversity and neural development: a systematic review. Annu. Rev. Dev. Psychol 1, 277–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Brake W, Gratton A, 2002. Environmental regulation of the development of mesolimbic dopamine systems: a neurobiological mechanism for vulnerability to drug abuse? Psychoneuroendocrinology 27, 127–138. [DOI] [PubMed] [Google Scholar]
- Mielock AS, Morris MC, Rao U, 2017. Patterns of cortisol and alpha-amylase reactivity to psychosocial stress in maltreated women. J. Affect. Disord 209, 46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel PM, Pereira LO, Silveira PP, Meaney MJ, 2019. Early environmental influences on the development of children’s brain structure and function. Dev. Med. Child Neurol 61, 1127–1133. [DOI] [PubMed] [Google Scholar]
- Mueller A, Strahler J, Armbruster D, Lesch KP, Brocke B, Kirschbaum C, 2012. Genetic contributions to acute autonomic stress responsiveness in children. Int. J. Psychophysiol 83, 302–308. [DOI] [PubMed] [Google Scholar]
- Myers B, 2017. Corticolimbic regulation of cardiovascular responses to stress. Physiol. Behav 172, 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrist PA, Gaebelein CJ, Teller ES, Langer AW, Grignolo A, Light KC, McCubbin JA, 1978. The relationship among heart rate, caratid dP/dt, and blood pressure in humans as a function of the type of stress. Psychophysiology 15, 102–115. [DOI] [PubMed] [Google Scholar]
- Oswald LM, Wand GS, 2004. Opioids and alcoholism. Physiol. Behav 81, 339–358. [DOI] [PubMed] [Google Scholar]
- Ouellet-Morin I, Boivin M, Dionne G, Lupien SJ, Arseneault L, Barr RG, Perusse D, Tremblay RE, 2008. Variations in heritability of cortisol reactivity to stress as a function of early familial adversity among 19-month-old twins. Arch. Gen. Psychiatr 65, 211–218. [DOI] [PubMed] [Google Scholar]
- Paquola C, Bennett MR, Lagopoulos J, 2016. Understanding heterogeneity in grey matter research of adults with childhood maltreatment-A meta-analysis and review. Neurosci. Biobehav. Rev 69, 299–312. [DOI] [PubMed] [Google Scholar]
- Pechtel P, Pizzagalli DA, 2011. Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacol. (Berl.) 214, 55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckins MK, Dockray S, Eckenrode JL, Heaton J, Susman EJ, 2012. The longitudinal impact of exposure to violence on cortisol reactivity in adolescents. J. Adolesc. Health 51, 366–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper JS, Mandl RC, Braams BR, de Water E, Heijboer AC, Koolschijn PC, Crone EA, 2013. Delay discounting and frontostriatal fiber tracts: a combined DTI and MTR study on impulsive choices in healthy young adults. Cerebr. Cortex 23, 1695–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper JS, van den Heuvel MP, Mandl RC, Hulshoff Pol HE, van Honk J, 2011. Sex steroids and connectivity in the human brain: a review of neuroimaging studies. Psychoneuroendocrinology 36, 1101–1113. [DOI] [PubMed] [Google Scholar]
- Phillips AC, Ginty AT, Hughes BM, 2013. The other side of the coin: blunted cardiovascular and cortisol reactivity are associated with negative health outcomes. Int. J. Psychophysiol 90, 1–7. [DOI] [PubMed] [Google Scholar]
- Phillips AC, Hunt K, Der G, Carroll D, 2011. Blunted cardiac reactions to acute psychological stress predict symptoms of depression five years later: evidence from a large community study. Psychophysiology 48, 142–148. [DOI] [PubMed] [Google Scholar]
- Pilowsky DJ, Keyes KM, Hasin DS, 2009. Adverse childhood events and lifetime alcohol dependence. Am. J. Publ. Health 99, 258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puetz VB, Kohn N, Dahmen B, Zvyagintsev M, Schüppen A, Schultz RT, Heim CM, Fink GR, Herpertz-Dahlmann B, Konrad K, 2014. Neural response to social rejection in children with early separation experiences. J. Am. Acad. Child Adolesc. Psychiatry 53, 1328–1337 e8. [DOI] [PubMed] [Google Scholar]
- Rc B, At G, Ac P, D C, 2014. A tale of two mechanisms: a meta-analytic approach toward understanding the autonomic basis of cardiovascular reactivity to acute psychological stress. Psychophysiology 51. [DOI] [PubMed] [Google Scholar]
- Robin RW, Chester B, Rasmussen JK, Jaranson JM, Goldman D, 1997. Prevalence, characteristics, and impact of childhood sexual abuse in a Southwestern American Indian tribe. Child Abuse Negl. 21, 769–787. [DOI] [PubMed] [Google Scholar]
- Romeo RD, 2015. Perspectives on stress resilience and adolescent neurobehavioral function. Neurobiol. Stress 1, 128–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, 2013. Annual research review: resilience–clinical implications. J. Child Psychol. Psychiatr 54, 474–487. [DOI] [PubMed] [Google Scholar]
- Scarpa A, 2015. Physiological arousal and its dysregulation in child maladjustment. Curr. Dir. Psychol. Sci 24, 345–351. [Google Scholar]
- Schilling EA, Aseltine RH Jr., Gore S, 2007. Adverse childhood experiences and mental health in young adults: a longitudinal survey. BMC Publ. Health 7, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano JM, Banks JB, Fagan TJ, Tartar JL, 2019. The influence of Val158Met COMT on physiological stress responsivity. Stress 22, 276–279. [DOI] [PubMed] [Google Scholar]
- Sershen H, Hashim A, Lajtha A, 1998. Gender differences in kappa-opioid modulation of cocaine-induced behavior and NMDA-evoked dopamine release. Brain Res. 801, 67–71. [DOI] [PubMed] [Google Scholar]
- Shaw D, al’Absi M, 2010. Blunted opiate modulation of prolactin response in smoking men and women. Pharmacol. Biochem. Behav 95, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SH, Miller DP, Teicher MH, 2013. Exposure to childhood neglect and physical abuse and developmental trajectories of heavy episodic drinking from early adolescence into young adulthood. Drug Alcohol Depend. 127, 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker JK, Goswami R, 2015. Forebrain neurocircuitry associated with human reflex cardiovascular control. Front. Physiol 6, 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff JP, 2010. Building a new biodevelopmental framework to guide the future of early childhood policy. Child Dev. 81, 357–367. [DOI] [PubMed] [Google Scholar]
- Sinha R, 2008. Chronic stress, drug use, and vulnerability to addiction. Ann. NY Acad. Sci 1141, 105–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolka MN, Schumann G, Wrase J, Grusser SM, Flor H, Mann K, Braus DF, Goldman D, Buchel C, Heinz A, 2005. Catechol-O-methyltransferase val158met genotype affects processing of emotional stimuli in the amygdala and prefrontal cortex. J. Neurosci 25, 836–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Fieldman G, Evans O, Perry L, 1996. Cardiovascular risk and responsivity to mental stress: the influence of age, gender and risk factors. J. Cardiovasc. Risk 3, 83–93. [PubMed] [Google Scholar]
- Teicher MH, Anderson CM, Ohashi K, Polcari A, 2014. Childhood maltreatment: altered network centrality of cingulate, precuneus, temporal pole and insula. Biol. Psychiatr 76, 297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Khan A, 2019. Childhood maltreatment, cortical and amygdala morphometry, functional connectivity, laterality, and psychopathology. Child. Maltreat 24, 458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Parigger A, 2015. The ‘Maltreatment and Abuse Chronology of Exposure’ (MACE) scale for the retrospective assessment of abuse and neglect during development. PloS One 10, 1–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, Anderson CM, Ohashi K, 2016. The effects of childhood maltreatment on brain structure, function and connectivity. Nat. Rev. Neurosci 17, 652–666. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, Sheu YS, Polcari A, McGreenery CE, 2010. Hurtful words: association of exposure to peer verbal abuse with elevated psychiatric symptom scores and corpus callosum abnormalities. Am. J. Psychiatr 167, 1464–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tendolkar I, Mårtensson J, Kühn S, Klumpers F, Fernández G, 2018. Physical neglect during childhood alters white matter connectivity in healthy young males. Hum. Brain Mapp 39, 1283–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Ahs F, Fredrikson M, Sollers JJ 3rd, Wager TD, 2012. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci. Biobehav. Rev 36, 747–756. [DOI] [PubMed] [Google Scholar]
- Tomalski P, Johnson MH, 2010. The effects of early adversity on the adult and developing brain. Curr. Opin. Psychiatr 23, 233–238. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Sheridan MA, 2009. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Front. Hum. Neurosci 3, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trickett PK, Gordis E, Peckins MK, Susman EJ, 2014. Stress reactivity in maltreated and comparison male and female young adolescents. Child. Maltreat 19, 27–37. [DOI] [PubMed] [Google Scholar]
- Trotman GP, Gianaros PJ, Veldhuijzen van Zanten J, Williams SE, Ginty AT, 2019. Increased stressor-evoked cardiovascular reactivity is associated with reduced amygdala and hippocampus volume. Psychophysiology 56, e13277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda A, Steptoe A, West R, Fieldman G, Kirschbaum C, 1996. Cigarette smoking and psychophysiological stress responsiveness: effects of recent smoking and temporary abstinence. Psychopharmacol. (Berl.) 126, 226–233. [DOI] [PubMed] [Google Scholar]
- Uhart M, Chong RY, Oswald L, Lin PI, Wand GS, 2006. Gender differences in hypothalamic-pituitary-adrenal (HPA) axis reactivity. Psychoneuroendocrinology 31, 642–652. [DOI] [PubMed] [Google Scholar]
- van Harmelen AL, van Tol MJ, van der Wee NJ, Veltman DJ, Aleman A, Spinhoven P, van Buchem MA, Zitman FG, Penninx BW, Elzinga BM, 2010. Reduced medial prefrontal cortex volume in adults reporting childhood emotional maltreatment. Biol. Psychiatr 68, 832–838. [DOI] [PubMed] [Google Scholar]
- VanZomeren-Dohm AA, Pitula CE, Koss KJ, Thomas K, Gunnar MR, 2015. FKBP5 moderation of depressive symptoms in peer victimized, post-institutionalized children. Psychoneuroendocrinology 51, 426–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent AS, Sorocco KH, Carnes B, Cohoon AJ, Lovallo WR, 2017. Antisocial characteristics and early life adversity predict substance use disorders in young adults: the Oklahoma Family Health Patterns Project. J. Substance Abuse Alcoholism 5, 1059. [Google Scholar]
- Voellmin A, Winzeler K, Hug E, Wilhelm FH, Schaefer V, Gaab J, La Marca R, Pruessner JC, Bader K, 2015. Blunted endocrine and cardiovascular reactivity in young healthy women reporting a history of childhood adversity. Psychoneuroendocrinology 51, 58–67. [DOI] [PubMed] [Google Scholar]
- Wand GS, McCaul M, Yang X, Reynolds J, Gotjen D, Lee S, Ali A, 2002. The muopioid receptor gene polymorphism (A118G) alters HPA axis activation induced by opioid receptor blockade. Neuropsychopharmacology 26, 106–114. [DOI] [PubMed] [Google Scholar]
- Wand GS, Schumann H, 1998. Relationship between plasma adrenocorticotropin, hypothalamic opioid tone, and plasma leptin. J. Clin. Endocrinol. Metab 83, 2138–2142. [DOI] [PubMed] [Google Scholar]
- Yan CG, Rincón-Cortés M, Raineki C, Sarro E, Colcombe S, Guilfoyle DN, Yang Z, Gerum S, Biswal BB, Milham MP, Sullivan RM, Castellanos FX, 2017. Aberrant development of intrinsic brain activity in a rat model of caregiver maltreatment of offspring. Transl. Psychiatry 7, e1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Zhu G, Hariri AR, Enoch MA, Scott D, Sinha R, Virkkunen M, Mash DC, Lipsky RH, Hu XZ, Hodgkinson CA, Xu K, Buzas B, Yuan Q, Shen PH, Ferrell RE, Manuck SB, Brown SM, Hauger RL, Stohler CS, Zubieta JK, Goldman D, 2008. Genetic Variation in Human NPY Expression Affects Stress Response and Emotion Nature, pp. 997–1001. England. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Logrip ML, Koob GF, 2014. Corticotropin releasing factor: a key role in the neurobiology of addiction. Front. Neuroendocrinol 35, 234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS, 2002. mu-opioid receptor-mediated antinociceptive responses differ in men and women. J. Neurosci 22, 5100–5107. [DOI] [PMC free article] [PubMed] [Google Scholar]


