Abstract
Background
Interferons (IFNs) are cytokines which possess immunoregulatory properties and have been used to successfully treat a number of chronic inflammatory disorders. It has been postulated that Type I IFNs may be able to re‐establish the Th1/Th2 balance in Th2 predominant diseases like ulcerative colitis.
Objectives
To systematically evaluate the efficacy and safety of type I IFN therapy for induction of remission in ulcerative colitis.
Search methods
We searched MEDLINE, EMBASE, CENTRAL, the Cochrane IBD/FBD group specialised register, and ClinicalTrials.gov from inception to August 8, 2014. Reference lists of trials and review articles, as well as recent proceedings from major gastroenterology meetings were manually searched.
Selection criteria
Randomised controlled trials of type I IFNs for induction of remission in UC were included. The study population included patients of any age with active ulcerative colitis. There were no exclusions based on type, dose or duration of IFN treatment.
Data collection and analysis
Two independent authors reviewed studies for eligibility, extracted the data and assessed study quality using the Cochrane risk of bias tool. The overall quality of the evidence supporting the outcomes was evaluated using the GRADE criteria. The primary outcome was induction of remission of ulcerative colitis. Secondary outcomes included: time to remission, mean change in disease activity index score, clinical, histological or endoscopic improvement, improvement in quality of life, and adverse events. We calculated the risk ratio (RR) and corresponding 95% confidence interval (CI) for dichotomous outcomes. We calculated the mean difference and corresponding 95% confidence interval for continuous outcomes. Meta‐analysis was performed using RevMan 5.3.5 software.
Main results
Six studies were eligible for inclusion (517 patients). Five studies compared type I IFNs to placebo injections (485 patients) and a single study compared IFNs to prednisolone enemas in patients with left‐sided colitis (32 patients). The active comparator study was rated as high risk of bias due to an open‐label design. Three studies were rated as unclear risk of bias for random sequence generation and allocation concealment. Two studies described as double blind were rated as unclear risk of bias for blinding. There was no significant benefit of type I IFNs over placebo for inducing clinical remission or improvement in patients with active ulcerative colitis. Thirty‐six per cent (87/242) of patients in the type I IFNs group achieved clinical remission by 8 to 12 weeks compared to 30% (36/120) of placebo patients (RR 1.16, 95% CI 0.84 to 1.58; 4 studies, 362 patients). A GRADE analysis indicated that the overall quality of the evidence supporting the outcome clinical remission was moderate due to sparse data (123 events). Fifty‐six per cent (149/264) of patients in the type I IFNs group improved clinically by 8 to 12 weeks compared to 48% (77/161) of placebo patients (RR 1.16, 95% CI 0.96 to 1.40; 4 studies, 425 patients). A GRADE analysis indicated that the overall quality of the evidence supporting the outcome clinical improvement was moderate due to sparse data (226 events). Patients who received type I IFNs were significantly more likely to withdraw from the studies due to adverse events than those who received placebo. Seven per cent (18/42) of type I IFNs patients withdrew due to adverse events compared to 2% (3/152) of placebo patients (RR 3.16, 95% CI 1.06 to 9.40). A GRADE analysis indicated that the overall quality of the evidence supporting the outcome withdrawal due to adverse events was low due to very sparse data (21 events). The study comparing type I IFNs to prednisolone enemas found no difference between the treatment groups in quality of life or disease activity scores. Common adverse events included headaches, arthralgias, myalgias, fatigue, back pain, nausea, application site reactions, rigors, and fevers. There were no statistically significant differences in the other secondary outcomes.
Authors' conclusions
Moderate quality evidence suggests that type I IFNs are not effective for the induction of remission in UC. In addition, there are concerns regarding the tolerability of this class of treatment.
Plain language summary
Type I interferons for treatment of active ulcerative colitis
What is ulcerative colitis?
Ulcerative colitis (UC) is a long‐term (chronic) inflammatory bowel disease characterized by abdominal pain, bloody diarrhea, and a need to hurry to the toilet to pass feces (fecal urgency).
What are type I interferons?
Interferons (IFNs) are drugs that regulate the immune system and have been used to successfully treat a number of chronic inflammatory disorders. People with UC who are experiencing disease symptoms have ‘active’ disease, periods when the symptoms stop are called ‘remission’.
What did the researchers investigate?
The researchers investigated whether type I IFNs results in remission in people with active ulcerative colitis, and whether it causes any harms (side effects). The researchers searched the medical literature extensively up to August 8, 2014.
What did the researchers find?
The researchers identified six studies that included a total of 517 participants. Five studies (total 485 participants) compared type I IFNs to placebo (fake medicine) injections. One small (32 participants) low quality study compared types I IFNs to prednisolone (a steroid drug) enemas. This study did not measure remission and found no difference between the treatment groups in quality of life or disease activity scores. There was no difference between type I interferons and placebo treatment groups for the number of people who achieved remission or improvement of their symptoms. These results suggest that type I IFNs do not produce remission from ulcerative colitis. Common side effects included headaches, arthralgias (joint pain), myalgias (muscle pain), fatigue, back pain, nausea, injection site reactions, rigors (cold and shivering), and fevers.
At present, the results from medical trials do not support the use of type I IFNs for the production of remission in active ulcerative colitis.
Summary of findings
Summary of findings for the main comparison. Type I Interferons compared to placebo for induction of remission in ulcerative colitis.
| Type I Interferons compared to placebo for induction of remission in ulcerative colitis | ||||||
| Patient or population: patients with induction of remission in ulcerative colitis Settings: Outpatient Intervention: Type I Interferons Comparison: Placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Type I Interferons | |||||
| Remission Follow‐up: 8‐12 weeks | 300 per 10001 | 348 per 1000 (252 to 474) | RR 1.16 (0.84 to 1.58) | 362 (4 studies) | ⊕⊕⊕⊝ moderate2 | |
| Clinical improvement Follow‐up: 8‐12 weeks | 478 per 10001 | 554 per 1000 (459 to 669) | RR 1.16 (0.96 to 1.40) | 425 (4 studies) | ⊕⊕⊕⊝ moderate3 | |
| Serious adverse events Follow‐up: 8‐12 weeks | 46 per 10001 | 34 per 1000 (6 to 190) | RR 0.74 (0.13 to 4.14) | 468 (4 studies) | ⊕⊕⊝⊝ low4 | |
| Withdrawals due to adverse events Follow‐up: 8‐12 weeks | 20 per 10001 | 62 per 1000 (21 to 186) | RR 3.16 (1.06 to 9.4) | 394 (4 studies) | ⊕⊕⊝⊝ low5 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Control group risk comes from control arm of study 2 Downgraded one level due to sparse data (123 events) 3 Dowgraded one level due to sparse data (226 events) 4 Downgraded two levels due to very sparse data (20 events) 5 Downgraded two levels due to very sparse data (21 events)
Background
Ulcerative colitis Ulcerative colitis (UC) is characterized by chronic or recurrent contiguous inflammation of the colonic mucosa resulting in symptoms such as bloody diarrhoea, abdominal discomfort, urgency and tenesmus. Inflammation typically arises in the distal colon and extends in a proximal direction. The disease can be subcategorised by anatomic extent. Left sided colitis is defined by inflammation that does not extend beyond the splenic flexure, and pancolitis where there is more proximal involvement. UC varies in severity and patients often experience cycles of relapses and remissions. While the exact cause of the disorder remains to be determined, environmental factors are thought to modify disease presentation in genetically predisposed individuals (Ek 2014; Hansen 2010; Ko 2014; Zhang 2014; Hanauer 2006). From an immunological perspective, the naive T cells (Th0) preferentially differentiate into Th2 (T‐helper) lymphocytes, which express a predominant interleukin‐4 (IL‐4), IL‐5, IL‐13 cytokine profile (Geremia 2014; Bouma 2003). However, UC represents an 'atypical' Th2 disease as increased circulating levels of interferon‐γ and TNF‐α (tumour necrosis factor‐α) have also been identified (Tsukada 2002; Heller 2005; Jovanovic 2014). It is hypothesized that this imbalance in circulating pro‐inflammatory and anti‐inflammatory cytokines underlies the chronic disease state (Geremia 2014; Baumgart 2007). The mainstay of UC treatment comprises anti‐inflammatory agents in the form of 5‐aminosalicylic acid compounds (Travis 2006), glucocorticoids (Truelove 1955), immunosuppressives (Timmer 2012), and more recently monoclonal antibodies against tumour necrosis factor‐alpha (TNF‐ɑ) (Rutgeerts 2005) and integrin ɑ4ß7 (Feagan 2013). These standard medical therapeutic options vary in efficacy, and importantly, toxicity (Bernstein 2015). While surgery may be considered 'curative' by some, this is often not ideal, rendering some patients with permanent ostomies and others with ongoing symptomatology, including that associated with suboptimal ileoanal pouch function. Therefore, alternative treatments are continually being evaluated.
Interferons Interferons (IFNs) are cytokines which may be released in response to viruses, bacteria, parasites and tumour cells. Interferons possess immunoregulatory, antiviral and anti‐cancer properties (George 2012). IFNs have been used to successfully treat a number of chronic inflammatory disorders including multiple sclerosis (Kasper 2014; Anonymous 1998; Anonymous 2001), and chronic viral hepatitis (Koretz 2013; Hoofnagle 2006). There are two main classes of IFNs: type I IFNs (which include α, β, ε, Ω, κ isoforms) (Ivashkiv 2014; Ludigs 2012), and type II IFNs (γ isoform) (Ghosh 2006). More recently a new family of antiviral cytokines, the type III IFNs (λ isoform) has been identified (Durbin 2013).
This systematic review will focus on the use of type I IFNs for the induction of remission of UC, specifically IFN‐α and IFN‐β which are marketed in standard recombinant or pegylated forms. IFN‐α has been shown to enhance human Th1 responses. This helps to re‐establish the Th1/Th2 balance in Th2 predominant diseases by down‐regulating Th2 cytokines such as IL‐4, IL‐5 and IL‐13 (Shibuya 2005; Brassard 2002). IFN‐β increases the expression of anti‐inflammatory IL‐10, inhibits IFN‐γ, TNF‐α, and enhances regulatory T lymphocyte and NK (natural killer) cell activity (Graber 2007). As IFN‐α and IFN‐β share a cellular surface receptor, they both induce IL‐1Ra.
Initial interest in IFNs for UC arose from an incidental observation of a patient with known UC who experienced a dramatic improvement in his UC symptoms when he was treated with IFN‐α2a for concurrent chronic hepatitis B (Sümer 1995). This led to a small prospective open‐label study by the same investigators in which 28 inpatients who had failed to respond to 5‐aminosalicylic acid compounds and oral or topical corticosteroids were treated with IFN‐α2a therapy for 6 to 12 months. Eighty‐two percent of patients responded to therapy within 15 days and were in complete clinical endoscopic remission after 6 months of therapy (Sümer 1995). Subsequently, Musch 2002 trialed IFN‐β‐1a in 25 steroid refractory UC patients in an open label study. The decision to use IFN‐β rather than IFN‐α occurred in response to its clinical utility in the chronic inflammatory disorder, multiple sclerosis. Furthermore, in vitro studies had suggested that IFN‐β, in contrast to IFN‐α or IFN‐γ did not enhance the production of inflammatory metabolites of arachidonic acid or leukotriene B4. A total of 88% of patients entered remission with a mean time to response of 21 days (Musch 2002).
The adverse effect profile of IFNs includes flu‐like symptoms including fever, headache, malaise, alopecia and arthralgias. Other potential adverse events include skin rashes, psychological disturbances, and perturbations in the haematological profile. IFNs may also induce autoimmune complications including thyroid disorders, diabetes mellitus and alopecia (Borg 2007; Okanoue 1996). Of concern, there have been isolated case reports documenting the association between IFNs and the induction of ischaemic colitis (Okanoue 1996; Sparano 1991; Tada 1996), and ulcerative colitis (Tursi 2007; Watanabe 2006; Sprenger 2005; Mavrogiannis 2001). In contrast, Bargiggia 2005 conducted a case control study of IFN‐α therapy in patients with concomitant inflammatory bowel disease and chronic active hepatitis C and determined that no patients developed an IBD relapse during IFN treatment or in the following 12 month follow‐up. Therefore, there is a need to critically evaluate if IFN therapy results in improvement or detriment to patients with UC.
Commercially available type I IFNs have different pharmacokinetic profiles, and consequently there is variation in the frequency and mode of administration. IFNs can be administered on alternate days, thrice weekly, or once a week by subcutaneous or intramuscular injection. Some of the IFNs are available in pegylated forms to reduce clearance. Pegylation is the process by which the biologically inert polyethylene glycol chains are cross linked to the active moiety, in this case the IFN protein, to optimise overall pharmacokinetics (Foster 2004). Currently available type I IFNs include IFN α‐2a (Roferon A® ‐ Roche), IFN α‐2b (Intron A® ‐ Schering), pegylated IFN α‐2a (Pegasys® ‐ Roche), pegylated IFN α‐2b (Pegatron® ‐ Schering), IFN β‐1a (Rebif® ‐ Pfizer, EMD Serono), IFN β‐1a (Avonex® ‐ Biogen Idec), IFN β‐1a (CinnoVex® ‐ CinnaGen, Fraunhofer Gessellschaff Institute), and IFN β‐1b (Betaseron® ‐ Bayer HealthCare).
Importance of this review In an effort to improve the management of UC, alternative therapeutic options need to be evaluated. Laboratory studies suggest that IFN treatment may attenuate chronic colitis. However, there is limited clinical information on the use of IFN therapy for UC, and there are concerns regarding its adverse effect profile. Furthermore, there is variation in the types of IFN (IFN‐α, IFN‐β), formulations (standard versus pegylated), doses and dosing schedules used in clinical practice. Therefore, a systematic review was planned to assess the role of type I IFN therapy for induction of remission in UC. This systematic review is an update of a previously published Cochrane systematic review (New Reference).
Objectives
The primary objective of this review was to systematically evaluate the efficacy of type I IFN therapy (including IFN‐β‐1a, IFN‐β‐1b, IFN‐α‐2a, IFN‐α‐2b and associated pegylated formulations) for induction of remission in ulcerative colitis. The secondary objectives were to determine improvement in disease activity (including quality of life) and to evaluate adverse events associated with IFN therapy.
Methods
Criteria for considering studies for this review
Types of studies
Randomised, double‐blind trials reporting either the primary or secondary objective and published in any language, with the following study designs: parallel arm placebo‐controlled trials and trials comparing two active agents, were considered for this review. Studies published in abstract form were only included if enough data were provided to assess the validity of the study and reported outcomes.
Types of participants
UC is usually diagnosed using a combination of clinical, radiologic, endoscopic and histologic criteria. Patients (both paediatric and adult) with active UC at the time of recruitment were included. It was anticipated that there would be heterogeneity in defining disease activity, therefore the definitions used by the authors of the primary studies were accepted. These included some of the following published disease activity indices: the Colitis Activity Index (CAI) (Rachmilewitz 1989), the Powell‐Tuck Index (Powell‐Tuck 1978), the Simple Clinical Colitis Activity Index (SCCAI) (Walmsley 1998), Beattie's Colitis Symptom Score (Beattie 1996), Lichtiger Symptom Score for Acute Ulcerative Colitis (Lichtiger 1990), the Mayo Index (Schroeder 1987), the Seo Index (Seo 1992), the Truelove and Witt's Index (Truelove 1955), Ulcerative Colitis Scoring System (UCSS) (Schroeder 1987) and the Ulcerative Colitis Endoscopic Index of Severity (UCEIS) (Travis 2012).
Types of interventions
Trials assessing type I IFNs (IFN‐α or IFN‐β) compared to placebo, no treatment, different regimens of interferon or an active comparator were included. Co‐interventions were permitted if the co‐interventions were balanced across the study groups. There were no exclusions based on type, dose or duration of IFN treatment.
Types of outcome measures
Primary outcome The primary outcome was induction of remission of UC. Remission was defined by the primary studies (see disease activity indices described under 'Types of participants') and was expressed as the percentage of patients randomised (intention‐to‐treat analysis).
Secondary outcomes The secondary outcomes included: 1. Time to remission; 2. The mean change in the disease activity index score; 3. Clinical, histological or endoscopic improvement as defined by the authors; 4. Improvement in quality of life as defined by a validated quality of life tool; and 5. Adverse events associated with IFN therapy for the treatment of UC. Four different outcome measures were used to evaluate the safety of type I IFNs:
The percentage of patients experiencing adverse events (which may have included but were not limited to flu‐like symptoms, skin rashes, psychological disturbances, perturbations in the haematological profile, and autoimmune complications such as thyroid disorders, diabetes mellitus and alopecia);
The percentage of patient withdrawals due to adverse events;
The percentage of patients undergoing colectomy; and
Mortality expressed as a percentage.
Search methods for identification of studies
Search sources A. Electronic searching (Please see Appendix 1 for a complete list of search strategies) 1. MEDLINE (1950 ‐ August 2014) 2. EMBASE (1980 ‐ August 2014) 3. Cochrane Central Register of Controlled Trials 4. Cochrane Inflammatory Bowel Disease and Functional Bowel Disorders (IBD/FBD) Group Specialised Trial Register 5. Ongoing trials identified from ClinicalTrials.gov registry
B. Hand searching using reference lists of trials and review articles identified by means of the computer‐assisted search.
C. Proceedings from major gastroenterology meetings (American Gastroenterology Association, British Society of Gastroenterology, United European Gastroenterology Week) were manually searched from 2002 onwards.
D. Pharmaceutical and personal contacts Relevant pharmaceutical companies that have or are involved in the development of the type I IFNs, and leaders in the field of inflammatory bowel disease were contacted to try and identify further unpublished studies.
Data collection and analysis
Study selection All the article abstracts identified by the above search strategies were reviewed for eligibility. The full text articles of potentially relevant studies were independently reviewed by YW and JKM for inclusion in the review. Review articles were also retrieved and reference lists were manually searched. Disagreements were resolved by consensus. Trials published in abstract form were only included if full details of the protocol and results could be obtained from the authors.
Data collection The eligible articles were reviewed in duplicate (YW, JKM) and the results of the trials were abstracted onto specially designed data extraction forms which mandated the following information be recorded:
1. General article information: Study title, first author, and year of publication. 2. Study design: Randomisation process, allocation concealment, and blinding. 3. Patient cohort: Countries in which study was performed, years patients were entered into the study, total number of patients screened, total number of patients randomised, inclusion/exclusion criteria, baseline characteristics (demographics, disease extent, disease severity). 4. Intervention: Type of IFN (α versus β), formulation of IFN (standard versus pegylated), route of administration, dose, and dosing schedule. 5. Control: No treatment, placebo, or details of co‐intervention. 6. Primary outcome: Proportion of patients achieving remission in the intervention and control groups. Where available, the median number of days to remission and the mean change in the disease activity index score will be recorded. 7. Secondary outcomes: Data on other clinical, histologic, endoscopic measures of disease activity; quality of life information; adverse events; withdrawal of participants from either the intervention or control group, where provided.
Assessment of methodological quality of included studies The methodological quality of the included studies was evaluated using the Cochrane risk of bias tool (Higgins 2011). This tool involves rating trials as high, low or unclear risk of bias for each of the following criteria:
Random sequence generation;
Allocation concealment;
Blinding;
Missing data and attrition;
Outcome reporting; and
Other sources of bias.
The overall quality of the evidence supporting the primary and secondary outcomes was evaluated using the GRADE approach (Guyatt 2008; Schünemann 2011). Outcome data are rated as being of high, moderate, low or very low quality evidence. Data from randomised controlled trials begin as high quality but can be downgraded based on the following criteria:
Risk of bias in the included trials;
Indirect evidence;
Inconsistent findings (including unexplained heterogeneity);
Imprecision (i.e. sparse data or wide confidence interval or both); and
Reporting bias.
The different quality ratings are interpreted as the likelihood that future research would affect the estimate of effect. An estimate of effect based on high quality evidence is unlikely to change with further research. If the overall evidence is of moderate quality further research may have an impact on our confidence in the estimate and may change the estimate. Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate when the evidence is rated as low quality. Very low quality research indicates significant uncertainty with the findings (Guyatt 2008; Schünemann 2011).
Statistical analysis
Measures of treatment effect Data were extracted from the original studies and converted into individual 2 x 2 tables (e.g. remission versus no remission x IFN versus control) for each study. The proportion of patients who entered remission was calculated and reported as a relative risk (RR) and 95% confidence interval (95% CI). Where appropriate, the number needed to treat (NNT) and risk difference (RD) was also calculated. This was determined using an intention‐to‐treat analysis, based on the total number of patients randomised to each of the two groups, and the number of patients in remission at the end of follow‐up in each group. For continuous variables, the results were presented as the mean difference (MD) and 95% CI or the standardised mean difference (SMD) when different scales were used to measure the same underlying construct. Where available, individual 2 x 2 tables for strata within studies were also abstracted.
Meta‐analysis For pooled analyses we utilized a random‐effects or fixed‐effect model depending on clinical and statistical heterogeneity.
Assessment of heterogeneity The studies were first independently assessed for clinical or methodological heterogeneity. Then, the I2 measure was calculated to quantify inconsistency. The I2 statistic describes the percentage of total variation across studies that was due to heterogeneity rather than chance. We interpreted I2 as follows: 25% ‐ low heterogeneity, 50% ‐ moderate heterogeneity, 75% ‐ high heterogeneity (Higgins 2003). The Chi2 test was also calculated. Being a relatively insensitive test for the presence of heterogeneity, a P‐value < 0.10 was considered to be statistically significant.
Subgroup analysis A priori subgroup analyses were planned for the different isoforms of type I IFN (IFN‐α versus IFN‐β), different formulations of IFN (standard versus pegylated), different doses, different durations of treatment, paediatric versus adult, and left‐sided colitis versus pancolitis.
Sensitivity analysis We performed sensitivity analyses excluding poor quality studies and studies published in abstract form. There were insufficient eligible trials to construct a 'funnel plot' to assess publication bias (Egger 1997).
Analyses were performed using the Review Manager software (RevMan 5.3.5, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014).
Results
Description of studies
See Table of included studies (Characteristics of included studies), Table of excluded studies (Characteristics of excluded studies).
The initial search yielded 211 non‐duplicated articles. Based on abstract review, all potential controlled trials and review articles were retrieved for full text review. A total of 29 full text manuscripts were obtained of which six randomised controlled trials (21 full‐text articles) were identified by the authors as being eligible for inclusion (See Figure 1). Eight studies were excluded. Agreement among authors regarding the eligibility of the included studies was 100%. Mannon 2010 was published as conference abstracts only. We contacted the lead author of the Mannon 2010 trial but were unable to obtain any additional information about the study.
1.
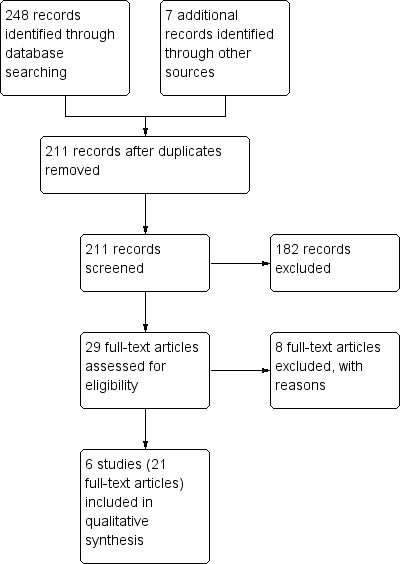
Study flow diagram.
All six included studies (total of 517 patients) were conducted in adult patients aged 18 years and over. The studies can be differentiated by the comparison groups and formulation of interferon used. Five trials compared a type I IFN to placebo in patients with active UC of any anatomic extent (Mannon 2010; Musch 2005; Nikolaus 2003; Pena‐Rossi 2008; Tilg 2003). Of these, three studies compared IFN‐β‐1a injected subcutaneously three times a week to placebo (Musch 2005; Nikolaus 2003; Pena‐Rossi 2008); and one study compared pegylated IFN‐α injected subcutaneously once a week to placebo (Tilg 2003). The remaining trial compared IFN‐α‐2a to prednisolone enemas in patients with left sided UC (Madsen 2001).
Mannon 2010 was a multi‐center phase II study in North America and Eastern Europe sponsored by Biogen Idec. This was a randomised, double blind, placebo‐controlled trial of 123 adult patients who had active ulcerative colitis with a total Mayo score of 6 to 13 points including a Mayo endoscopic subscore of at least 2, indicating moderate to severe endoscopic activity, despite prior or concomitant treatment. Patients were randomly assigned to IFNβ 30 μg intramuscularly twice a week for 12 weeks (n = 62), or placebo injections (n = 61). The primary endpoint was clinical response at week 8, defined as a decrease from baseline in the total Mayo score by 30% and at least a 3 point decrement, accompanied by a decrease in the subscore for rectal bleeding of at least 1 point or an absolute endoscopic subscore of 0 or 1. The secondary endpoints were the safety and tolerability of IFNβ and the percentage of subjects with a decrease in the SCCAI score of ≥ 3 points at week eight.
Musch 2005 was conducted in Germany and the Czech Republic. This was a randomised, double‐blind, placebo‐controlled trial of 91 adult patients with steroid refractory, active UC defined by a CAI score of at least 8 points. Patients were randomly assigned to one of three groups. Patients in group one received three million international units (MIU) of IFN‐β‐1a by subcutaneous injection three times a week (n = 32). Patients in group two received one MIU of IFN‐β‐1a by subcutaneous injection three times a week (n = 30); and group three received placebo injections (n = 29). The total duration of treatment was eight weeks. The primary outcome was the response rate at the end of treatment. Response was defined as a reduction of six or more points on the CAI at week eight compared to baseline. Secondary endpoints included: the number of patients achieving a complete response (reduction of CAI to ≤4 points after 8 weeks of treatment); time until response; reduction of CAI after 4 and 8 weeks; reduction of the endoscopic index after 8 weeks; number of patients receiving colectomy; and reduction of steroid dose.
Nikolaus 2003 compared the effects of IFN‐β‐1a to placebo. The study was conducted in three countries; Belgium, Canada and Germany. This was a randomised, double blind, intra‐individual, dose escalating trial of 17 adult patients with moderately active UC. This was defined by a UCSS score of 6 to 10, with a proctosigmoidoscopy score of 2. Patients were randomly assigned to IFN‐β‐1a by subcutaneous injection three times a week (n = 10) or placebo injections (n = 7). Patients in the IFN‐β‐1a group were started at 22 μg three times a week by subcutaneous injection. Dose escalation was dependent on 'improvement'. Improvement was defined as a decrease of one point in the combined score of UCSS symptoms and physician's global assessment (PGA). If no improvement was observed after six injections, the dose was increased to 44 μg three times a week. If no improvement was observed after six injections at the 44 μg dose, this was increased further to 88 μg three times a week. If improvement was observed after six injections at any dose, the patient entered a maintenance treatment phase of 6 to 12 injections at that dose. If no improvement was observed after six injections at 88 μg, or if remission occurred at any point, treatment was stopped. The maximum duration of treatment was eight weeks, and the minimum duration was four weeks. The primary end point was efficacy, which was defined by treatment response and remission. Treatment response was defined by a decrease of at least three points from baseline in the UCSS symptoms score and PGA (without the proctosigmoidoscopic score) during treatment. Remission was defined as complete resolution of clinical symptoms (all clinical UCSS subscores equal to zero), with a proctosigmoidoscopy score of zero or one at any time during treatment. Secondary end points included overall treatment and end point responses (defined as a decrease in UCSS symptoms score, PGA, and proctosigmoidoscopic scores of at least one point during or at the end of treatment), and clinical end point responses (a decrease of at least one point from baseline in UCSS symptoms scores and PGA, without the proctosigmoidoscopic score). Safety data were also collected.
Pena‐Rossi 2008 was an European phase II clinical trial sponsored by EMD Serono. The study was randomised, multi‐centred, double‐blinded, and placebo‐controlled. The trial involved 194 patients with moderately active ulcerative colitis, defined by a UCSS score between 6 and 10 with a UCSS PGA of less than three and a proctosigmoidoscopy score of two or three. Patients were randomised using stochastic minimization to one of three trial arms: IFN‐β‐1a 44 μg (n = 65), IFN‐β‐1a 66 μg (n = 65), or matching (same excipients but no IFN‐β‐1a) placebo (n = 64). All study drugs were given by subcutaneous injection three times a week for eight weeks and there was a four week follow‐up period.The primary objective was to identify the best dose of IFN‐β‐1a for the induction of endoscopically confirmed remission and examine the safety profile of this dose. Safety, tolerability, quality of life and biological markers were also assessed as secondary outcomes.
Tilg 2003 compared the effects of pegylated IFN‐α (PegIFN) to placebo. The study was conducted in five university hospitals in four countries including Austria, Belgium, Germany and France. This was a multicentre double‐blind randomised controlled trial of 60 adults with UC. Patients were randomly assigned to PegIFN 0.5 μg/kg (n = 19); PegIFN 1.0 μg/kg (n = 21); or placebo (n = 20). All therapies were administered by subcutaneous injection once a week, for a period of 12 weeks. Patients were eligible if they had evidence of clinical activity despite oral or topical 5‐aminosalicylate maintenance therapy, stable doses of steroids or azathioprine. Active disease was defined by a CAI score of greater than six, and endoscopic activity was defined by a Rachmilewitz endoscopy score of greater than four. Clinical evaluation was performed before the start of treatment (day ‐8), then on days 0, +8, +15, +29, +43, +57, and at the end of treatment (day +85). Patients underwent sigmoidoscopy and/or colonoscopy on days ‐1, +29, and +85. The primary outcome was safety. All adverse events were recorded and classified as serious or non‐serious, and likely or unlikely to be related to treatment. Other outcomes included disease remission (defined as a CAI score of less than or equal to four), endoscopic remission (defined as a score of less than four), and histological activity (graded on a scale from zero to three). These markers of remission were measured at week 12. Changes in serological inflammatory indices including haemoglobin, white cell count, platelet count, C reactive protein, α‐1 acid glycoprotein, creatinine, liver function tests, and albumin were also recorded.
Madsen 2001 compared the effects of IFN‐α‐2a to prednisolone enemas. The study was conducted in Denmark. This was an open‐label, randomised controlled trial of 32 adult patients. The patient cohort was restricted to those with active left‐sided UC. Patients were randomly assigned to IFN‐α‐2a therapy by subcutaneous injection three times a week (n = 16) for 12 weeks; or prednisolone enemas 100 mL (25 mg) daily for 30 consecutive days (n = 16). The dose of IFN used was 9 MIU for the first week, 6 MIU for the second week, and 3 MIU from weeks 3 to 12 inclusive. All patients were treated with sulfasalazine or mesalazine compounds with a median daily dose of 2.4 g (range 1.2 to 3.6 g), and were not allowed dose adjustments for at least four weeks before entry. The patients receiving enemas were evaluated after one week, two weeks and on completion of the trial; while the IFN‐α‐2a group had appointments after one week, two weeks, four weeks, eight weeks, and after completion of the trial. Clinical and endoscopic disease activity was graded at each visit by semi‐quantitative scales. Clinical activity assessment was based on the patient filling out a five point symptom scale daily; while a physician evaluated abdominal tenderness, enquired about limitations in the patient's daily activities, adverse events and extraintestinal manifestations at each clinic visit. Endoscopic evaluation involved proctoscopy or colonoscopy at each clinic visit, and histological assessment of disease activity (rectal biopsies) were obtained at entry and after treatment. The combined clinical and endoscopic Powell‐Tuck Index was also calculated. Secondary outcomes included an assessment of quality of life, and tolerability of treatment. Remission was not defined by the authors, rather, the change in the activity indices were measured.
Risk of bias in included studies
The risk bias assessment results are summarized in Figure 2. A table detailing withdrawals or drop outs is provided (Table 2). While none of the studies were excluded on this basis, the studies have to be interpreted with caution given the substantial proportion of withdrawals or drop outs.
2.
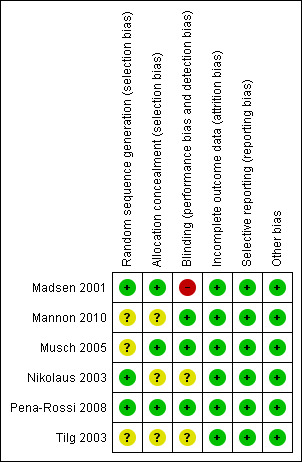
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
1. Overall withdrawals (%); Placebo versus Type I IFNs.
| Study | Placebo | IFN Total | High dose IFN | Low dose IFN |
| Mannon 2010 | 13.1 | 9.68 | ||
| Musch 2005 | 24.1 | 14.5 | 12.5 | 16.7 |
| Nikolaus 2003 | 28.6 | 60.0 | ||
| Pena‐Rossi 2008 | 17.2 | 20.8 | 20.0 | 21.5 |
| Tilg 2003 | 55.0 | 40.0 | 47.6 | 31.6 |
Mannon 2010 did not report on methods used for randomisation or allocation concealment. These items were rated as unclear. Patient drop‐out data were not included in the abstract publications, but analyses were conducted on an intention‐to‐treat basis. Based on ClinicalTrial.gov (NCT00616434), 10% (6/62) of patients in the IFNβ‐1a 30 μg treatment group and 13% (8/61) of patients in the placebo group did not complete the study. Musch 2005 was reported as randomised but the methods used to generate the random sequence were not described. There was an adequate description of the blinding process as well as allocation concealment. The drop‐out rate was 12% (4/32) in the 3 MIU IFN‐β‐1a group, 17% (5/30) in the 1 MIU IFN‐β‐1a group, and 24% (7/29) in the placebo group. Nikolaus 2003 was a well designed but small study. However, there was a substantial drop out rate particularly in the intervention (IFN‐β‐1a) group where 60% (6/10) of patients withdrew. The withdrawal rate in the control group was 29% (2/7). There was a detailed description of the use of a centralised, computer generated list for randomisation. Study subjects were stratified by centre with a block size of three (a ratio of 2 to 1: IFN‐β‐1a to placebo). Allocation concealment was not described. The study design was described as double blind, but further details on how this was achieved were not reported. An intention‐to‐treat analysis was utilised. Pena‐Rossi 2008 provided adequate descriptions regarding randomisation, allocation concealment, blinding and outcome data. Twenty per cent (13/65) of patients in 66 μg IFN‐β‐1a treatment group, 22% (14/65) of patients in 44 μg IFN‐β‐1a treatment group, and 17% (11/64) of patients in placebo group withdrew from the study. All efficacy endpoints were analysed using intention‐to‐treat (ITT) populations. Tilg 2003 was described as randomised, but there was no information provided on the generation of the randomisation sequence, nor on blinding or allocation concealment. Thirty‐two per cent (6/19) of patients in the PegIFN‐α 0.5 μg/kg group withdrew from the study, compared to 48% (10/21) of patients in the PegIFN‐α 1.0 μg/kg group, and 55% (11/20) of patients in the control group. The results were interpreted with caution given the high drop out rate. Madsen 2001 was an open‐label study. There was an adequate description of the randomisation process (using a computer generated random number generator), as well as allocation concealment. All 16 patients in the prednisolone enema (control) group completed the trial compared to a withdrawal rate of 19% (3/16) in the IFN‐α‐2a group.
Effects of interventions
See: Table 1
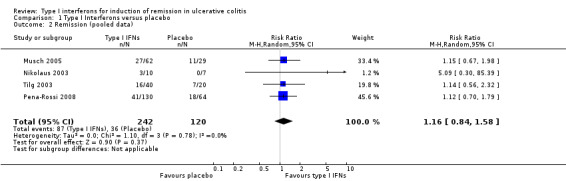
Primary Outcome The primary outcome was induction of remission of UC. Of the six studies satisfying the inclusion criteria, four studies reported on the proportion of patients achieving remission at the end of treatment (Musch 2005; Nikolaus 2003; Pena‐Rossi 2008; Tilg 2003). There were three studies that compared IFN‐β‐1a versus placebo albeit using different formulations and doses (Musch 2005; Nikolaus 2003; Pena‐Rossi 2008), and one study comparing PegIFN‐α versus placebo (Tilg 2003).
After 8 weeks of treatment in the Musch 2005 study, 56% (18/32) of patients in the IFN‐β‐1a 3 MIU group achieved remission, compared to 30% (9/30) of the 1 MIU group and 38% (11/29) in the placebo group. The difference between the 3 MIU and the 1 MIU group was statistically significant with a P = 0.04, but not significant when compared with placebo. The authors concluded that IFN‐β‐1a was not more effective than placebo in steroid‐refractory UC. The non‐pooled dose‐dependent data are presented in Figure 3.
3.
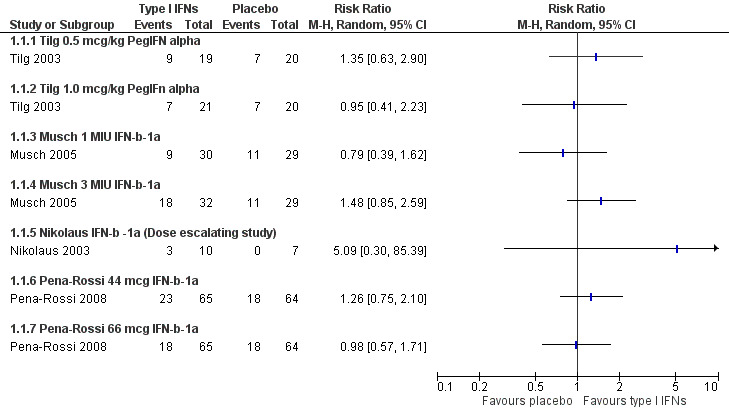
Forest plot of comparison: 1 Type I Interferons versus placebo, outcome: 1.1 Remission (non pooled data).
In the IFN‐β‐1a dose escalating study by Nikolaus 2003, 30% (3/10) of the intervention group achieved remission compared to 0% in the placebo group. One patient received 44 μg IFN‐β‐1a injections whilst two were treated with 88 μg IFN‐β‐1a injections. The authors concluded that patients treated with escalating doses of IFN‐β‐1a tended to show a higher remission rate than those in the placebo group, however, the difference between the groups was not statistically significant.
In the Pena‐Rossi 2008 study clinical remission was achieved by 35.4% (23/65) of patients treated in the 44μg IFN‐β‐1a group, 27.7% (18/65) of those in the 66μg IFN‐β‐1a group, and 28.1% (18/64) of patients in the placebo group. The differences between the groups were not statistically significant. Please note, only percentages were reported in the original publication, absolute numbers in brackets were calculated using the intention to treat population.
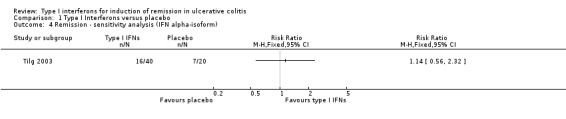
Tilg 2003 used a CAI of ≤ 4 to define remission. At week 12, 7/21 (33.3%) of the 1.0 μg/kg PegIFN‐α group, 9/19 (47.4%) of the 0.5 μg/kg and 7/20 (35.0%) in the placebo group achieved remission. The authors concluded that there was no significant advantage of PegIFN over placebo.
Data from four studies were pooled in a meta‐analysis. While three of the studies used IFN‐β‐1a (Musch 2005; Nikolaus 2003; Pena‐Rossi 2008), and one study used PegIFN‐α (Tilg 2003), the authors felt there was sufficient clinical homogeneity across both IFN preparations in immunological action, such that the data could be meta‐analysed. There was no statistically significant difference in remission rates between IFNs and placebo. Thirty‐six per cent (87/242) of IFNs patients achieved remission compared to 30% (36/120) of placebo patients (RR 1.16, 95% CI 0.84 to 1.58; P = 0.37). A GRADE analysis indicated that the quality of evidence supporting the outcome clinical remission was moderate due to sparse data (See Table 1). However, these results should be interpreted with caution given variability in dosing, treatment duration, and the timing of outcome assessment. Table 3 documents the comparative doses of IFN‐b‐1a used in the Musch 2005, Nikolaus 2003 and Pena‐Rossi 2008 trials. A 44 μg dose is approximately equivalent to 12 MIU (Antonetti 2002). Although the Musch 2005 trial suggested that remission may be dose dependent, the dosing was still lower than that used in the Nikolaus 2003 trial preventing comparisons of high versus low dose IFN across studies. The comparison was further confounded by differing durations of treatment; 8 weeks (56 days) in the Musch 2005 and Pena‐Rossi 2008 trials compared to approximately 5 weeks (35.5 days) in the Nikolaus 2003 study.
2. Comparative IFN‐b‐1a Doses used in the Nikolaus, Musch and Pena‐Rossi Trials.
| Dose of IFN‐b‐1a | Remission (n) | Total (N) | % |
| Nikolaus 2003: Median treatment duration 35.5 days | |||
| 88mcg t.i.w | 2 | 4 | 50 |
| 44mcg t.i.w. | 1 | 2 | 50 |
| 22mcg t.i.w. | 0 | 4 | 0 |
| TOTAL (Nikolaus) | 3 | 10 | 30 |
| Musch 2005: Treatment duration: 56 days | |||
| 3MIU t.i.w. = 11mcg t.i.w. | 18 | 32 | 56 |
| 1MIU t.i.w. = 3.7mcg t.i.w. | 9 | 30 | 30 |
| TOTAL (Musch) | 27 | 62 | 43.5 |
| [12MIU = 44mcg] Reference: Antonetti 2002 | |||
| Pena‐Rossi 2008: Treatment duration: 56 days | |||
| 66mcg t.i.w. | 18 | 65 | 27.7 |
| 44mcg t.i.w. | 23 | 65 | 35.4 |
| TOTAL (Pena‐Rossi) | 41 | 130 | 31.5 |
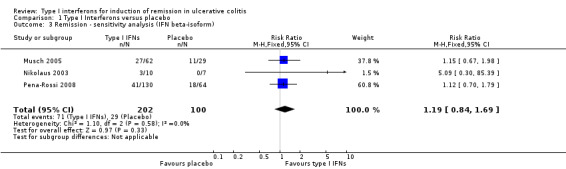
A priori subgroup and sensitivity analyses were planned for the different IFN isoforms and according to study quality. The analysis was repeated excluding the Tilg 2003 study as patients were treated with the α−isoform preparation and this was the only study that was judged to be low quality. Analysis of the three IFN‐β‐1a, moderate quality studies (Musch 2005; Nikolaus 2003; Pena‐Rossi 2008), did not change the results (RR 1.19, 95% CI 0.84 to 1.69).
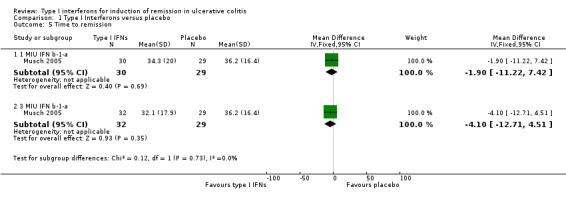
Secondary outcomes 1. Time to remission (in days) Two studies using IFN‐β‐1a reported on the mean number of days to remission (Musch 2005; Nikolaus 2003). In the Musch 2005 study, the time to complete response (remission) was 32.1 ± 17.9 days in the 3 MIU group; 34.3 ± 20.0 days in the 1 MIU group and 36.2 ± 16.4 days in the placebo group. The differences in mean time to remission were not statistically significant between the groups. Nikolaus 2003 reported the mean time to remission was 52 ± 7 days in the IFNs group. There were no patients in the placebo group who achieved remission within the study time frame. Pena‐Rossi 2008 did not provide sufficient data for comparison. Mannon 2010, Tilg 2003 and Madsen 2001 did not report on this outcome.
2. Change in the disease activity index score The change in disease activity index scores could not be pooled given the heterogeneity of indices and the differing time of outcome measurement used.
Musch 2005 reported the median change in CAI at four and eight weeks. All patients started with a CAI score of 10; at 4 weeks, there was a reduction of 5 points in the CAI in the 3 MIU group, 3 points in the 1 MIU group, and 4 points in the placebo group. The median change in CAI at eight weeks was six points in the 3 MIU group, three points in the 1 MIU group, and four points in the placebo group. There was no statistically significant difference between the three groups at either time point. Tilg 2003 measured the CAI at baseline and weeks 1, 2, 4, 6, 8 and 12. Results were reported as the mean score and standard deviation. Using the Follmann 1992 method, the mean change in the CAI and standard deviation were calculated. The mean reduction in CAI from baseline to week 12 was 4.0 ± 2.5 in the 1.0 μg/kg group, 4.9 ± 2.3 in the 0.5 μg/kg group and 6.3 ± 2.8 in the placebo group.
Both Musch 2005 and Tilg 2003 reported on end of treatment change in endoscopic index scores. Musch 2005 provided both the mean and median reduction of the endoscopic index score at eight weeks, while Tilg 2003 reported on endoscopic index values at baseline, week 4 (day 29), and at week 12 (day 85). Using the Follmann 1992 method, the mean change in endoscopic index score and standard deviation were calculated for the Tilg 2003 study. The mean reduction in scores in the Musch 2005 study was 4.4 ± 3.4 points in the 3 MIU group, 3.3 ± 4.1 in the 1 MIU group and 3.6 ± 3.4 points in the placebo group. The mean reduction in scores in the Tilg 2003 study was 3.6 ± 2.4 in the 1.0 μg/kg PegIFN‐α group, 3.2 ± 3.6 in the 0.5 μg/kg group, and 4.0 ± 3.2 in the placebo group. The change in endoscopic index scores was not statistically significant in either study.
There were no statistically significant differences in any of the UCSS subscores between the IFN‐β‐1a and placebo groups four weeks after the end of treatment in the Nikolaus 2003 study. The change in UCSS symptom scores are as reported as follows: UCSS subscore type, change in score for IFN‐β‐1a group, change in score for the placebo group. Stool frequency, ‐1.0 ± 1.2, ‐0.14 ± 0.89; rectal bleeding, ‐0.4 ± 1.1, ‐0.42 ± 0.79; physician global assessment ‐0.56 ± 1.2, ‐0.38 ± 0.92; proctosigmoidoscopy score ‐0.8 ± 1.1, 0.14 ± 0.69.
Madsen 2001 reported a statistically significant improvement from baseline in the Powell‐Tuck Index in the IFN‐α‐2a group (P = 0.0002), and in the prednisolone enema group (P = 0.0009). The median score in the IFN‐α‐2a group at baseline was 9.0 (95% CI 7.2 to 10.4), and post treatment 1.5 (95% CI 1.2 to 4.5). For the prednisolone enema group, the baseline score was 8 (95% CI 6.5 to 9.0) and post treatment 3 (95% CI 1.9 to 5.6). There was no statistically significant difference in Powell‐Tuck scores between the intervention groups.
Mannon 2010 and Pena‐Rossi 2008 did not provide sufficient data for comparison.
3. Clinical, histological or endoscopic improvement Mannon 2010 reported the percentage of participants with a clinical response, defined as a decrease from baseline in the total Mayo score of at least 3 points and at least 30%, accompanied by a decrease in the subscore for rectal bleeding of at least 1 point or an absolute subscore of 1 or less. 53% (33/62) in IFN‐β‐1a group and 44% (27/61) in placebo group achieved a clinical response. There was no statistically significant difference between the groups.
Musch 2005 reported the proportion of patients achieving a clinical response at the end of treatment, defined by a reduction of 6 or more CAI points after the 8 week treatment period. In the 3 MIU IFN‐β‐1a group, 18/32 (56%) achieved a clinical response, this was achieved by 11/30 (36%) in the 1 MIU group, and 10/29 (34%) in the placebo group. There was no statistically significant difference between the groups.
Nikolaus 2003 reported the percentage of patients who achieved a clinical response (distinct from remission), which was defined as a decrease of at least 3 points in the UCSS. This was achieved by 5/10 (50%) in the IFN‐β‐1a group compared to 1/7 (15%) in the placebo group (P = 0.14).
Pena‐Rossi 2008 reported the percentage of patients who achieved a clinical response (≥3‐point decrease in UCSS and PGA scores), which was achieved in 64.6% (42/65) of IFN‐β‐1a 44 μg group, 61.5% (40/65) of IFN‐β‐1a 66 μg group, and 60.9% (39/64) of placebo group.
Data from four studies were pooled in a meta‐analysis. There was no statistically significant difference in clinical improvement remission rates between IFNs and placebo. Fifty‐six per cent (149/264) of IFN treated patients improved clinically compared to 48% (77/161) of placebo patients (RR 1.16, 95% CI 0.96 to 1.40; P = 0.13). A GRADE analysis indicated that the quality of evidence supporting this outcome was moderate due to sparse data (See Table 1).
Tilg 2003 reported the proportion of patients achieving endoscopic remission (defined by an endoscopic activity score of < 4 at 12 weeks). In the 1.0 μg/kg PegIFN‐α group, 20% achieved this secondary endpoint, compared to 18% in the 0.5 μg/kg group and 26% in the placebo group. The authors reported that there was no difference in endoscopic activity between the three treatment groups at any time point. Rectal histological activity was assessed in a subgroup of patients, however at the end of treatment at week 12 (day 85), the data were too limited to allow comparison.
Madsen 2001 utilised clinical and endoscopic activity scores to document disease but these scoring systems were semi‐quantitative and not validated.
4. Improvement in quality of life Madsen 2001 used the Inflammatory Bowel Disease Questionnaire (IBDQ) to assess quality of life (Guyatt 1989). The following results represent an ITT analysis in patients with disease extension exceeding the rectum. The IBDQ has not been validated in patients with proctitis alone. Of the 12 patients randomised to IFN‐α‐2a, the baseline median IBDQ (total) score was 166 (95% CI 145.3 to 181.4), and post treatment was 193.5 (95% CI 181.0 to 204.6). The difference in scores from baseline for the IFNs group was statistically significant (P = 0.002). In the prednisolone enema group, there was no statistically significant difference before and after treatment (P = 0.055). The baseline median IBDQ score in the prednisolone enema group was 181 (95% CI 165.3 to 197.2) and the post treatment score was 207 (95% CI 178.5 to 215.3). There was no statistically significant difference in IBDQ scores between the treatment groups.
Pena‐Rossi 2008 reported IBDQ scores as a secondary endpoint. The IBDQ improved meaningfully (≥15‐point improvement) in 41% (26/64) of patients in the placebo group compared to 48% (31/65) of the IFN‐β‐1a 44 μg group, and 44.6% (29/65) of the IFN‐β‐1a 66 μg group.
5. Adverse events a. Adverse eventsThe most common adverse events experienced by patients were consistent with the known side effect profile of IFNs and included headaches, arthralgias, myalgias, fatigue, back pain, nausea, application site reactions, rigors, and fevers. The adverse event profile of the different isoforms of IFN appeared similar. It was not possible to meta‐analyse these adverse events due to variability in reporting. For example, a large number of patients experienced adverse events in the Nikolaus 2003 study, 100% of patients experienced at least one adverse event and 97% of these events were graded as mild or moderate in severity. In the Musch 2005 trial, 68.1% of patients experienced adverse events. However adverse events were not reported according to treatment allocation. Tilg 2003 tabulated all adverse events but did not report them per patient. There were 13 adverse effects in the placebo group, 47 in the PegIFN 0.5 mg/kg group, and 45 in the PegIFN 1.0 mg/kg group. Madsen 2001 reported that adverse events only occurred in the IFNs group. A table of adverse events was provided on a time line, using a semi‐quantitative scale. However, this does not allow one to determine the total number of patients suffering an adverse event and the tolerability of treatment was assessed by the patient rather than the physician. The largest number of adverse events was reported during week one, where 35 events were reported by an undisclosed number of patients in the IFN‐a‐2a group. The authors commented that the adverse events were generally mild to moderate with the most common being flu‐like symptoms. Mannon 2010 reported that 84% (52/62) of the IFN‐β‐1a group experienced at least one adverse event compared to 57% (35/61) of placebo patients. A more detailed breakdown of the adverse events is reported on ClinicalTrials.gov. Pena‐Rossi 2008 listed common (>10% of patients) and less common (<10% of patients) adverse events and tabulated data for some mild to moderate adverse effects (e.g. headache, fever, influenza‐like symptoms, and application‐site disorders) and serious adverse effects.
Serious adverse events were more clearly documented. Musch 2005 reported 7 serious adverse events in 5 patients; 1/32 (3.1%) in the 3 MIU IFN‐β‐1a group experienced chest pain while 4/29 (13.8%) in the placebo group experienced a variety of adverse events including worsening UC symptoms, infection, and a respiratory disorder. There were no serious adverse events documented in the 1 MIU IFN group. Tilg 2003 reported that 3/21 (14.3%) in the 1.0 μg/kg group and 3/19 (15.8%) in the 0.5 μg/kg group experienced serious adverse events. The three adverse events in the higher dose (1.0 μg/kg IFN) group included a disease flare, thrombosis of the brachiocephalic vein, and a grand mal seizure. All serious adverse events in the 0.5 μg/kg group were related to lack of efficacy, resulting in hospitalisation and intensification of treatment in the three patients. There were no serious adverse events in the placebo group. Mannon 2010 found 4 serious adverse events, one (1/62, 1.61%) with worsening of ulcerative colitis in the IFN‐β‐1a group, and three in the placebo group (3/61, 4.92%), of which, two patients experienced worsening of UC and one patients had a tibial fracture. Pena‐Rossi 2008 reported five serious adverse events (one event in the placebo group and two in each of the IFN‐β‐1a groups), A pooled analysis of four studies showed no statistically significant difference in the proportion of patients who experienced a serious adverse event. Four per cent (12/294) of IFN patients had a serious adverse event compared to 5% (8/174) placebo patients (RR 0.74, 95% CI 0.13 to 4.14). A GRADE analysis indicated that the quality of evidence supporting this outcome was low due to very sparse data (See Table 1). A sensitivity analysis excluding an abstract publication did not change the results (RR 1.03, 95% 0.10 to 10.87).
b. Withdrawals (including those due to adverse events)
Eighteen per cent (16/91) of patients withdrew from the Musch 2005 study. Withdrawal was most common in the placebo group at 24% followed by 17% in the low dose IFNs group and 12% in the high dose IFNs group. Reasons for discontinuation included two withdrawals due to worsening health and one withdrawal due to intolerable adverse events. However, the authors did not report which study medication these patients received. Nikolaus 2003 reported a 29% (2/7) withdrawal rate amongst placebo treated patients, and a substantial 60% (6/10) non‐completion rate amongst IFNs treated individuals. Both of the placebo patients who withdrew and four of six IFNs patients withdrew due to progressive disease. One IFNs patient withdrew due to an adverse event (influenza like symptoms). There were no withdrawals due to adverse events in the placebo group. A high withdrawal rate was documented in the Tilg 2003 study. More than half (11/20 = 55.0%) of the placebo patients dropped out, compared to 48% (10/21) of the high dose IFNs group, and 32% (6/19) in the low dose IFNs group. Of these, one individual (1/20 = 5%) in the placebo group and 2 patients (2/21 = 10%) in the high dose IFNs group withdrew due to adverse events (fever in the placebo treated patients, and unspecified 'serious' adverse events in the IFNs patients), while the remaining individuals withdrew due to disease progression. In the Madsen 2001 study, there were 3/16 (19%) withdrawals in the IFNs group, two of these patients were noted to have abnormal liver biochemistry and one experienced progressive disease. No patients withdrew from the prednisolone enema group. Mannon 2010 reported that 8% (5/62) of IFNs patients withdrew due to adverse events, compared to 3% (2/61) of placebo patients. Details of the adverse events were not reported. In Pena‐Rossi 2008 study, ten patients withdrew from the study because of adverse events ‐ four in the IFN‐β‐1a 44 μg group, six in the IFN‐β‐1a 66 μg group, and none in the placebo group. In the IFN‐β‐1a 44 μg group, two patients withdrew because of constitutional symptoms and one withdrew due to severe thrombocytopenia, anemia and macrohematuria concurrent with an exacerbation of ulcerative colitis. In the IFN‐β‐1a 66 μg group, three patients withdrew because of constitutional symptoms and one patient discontinued because of worsening of UC.
The data were meta‐analysed in two ways: firstly, comparing the overall withdrawal rate amongst type I IFNs against placebo; then comparing the withdrawal rate due to adverse events for the same groups. A fixed‐effect model was used for the overall withdrawal rate as there was minimal heterogeneity (P = 0.40, I2=2.0%). All five placebo‐controlled studies were used for the analysis, with a total of 485 patients (Mannon 2010; Musch 2005; Nikolaus 2003; Pena‐Rossi 2008; Tilg 2003). There was no statistically significant difference in withdrawal rates. Twenty‐one per cent (64/304) of patients in the IFNs group withdrew before the end of the study compared to 22% (39/181) of placebo patients (RR 0.91, 95% CI 0.65 to 1.29; P = 0.90). A sensitivity analysis excluding an abstract publication did not change the results (RR 0.95, 95% CI 0.66 to 1.36). A fixed‐effect model was used for withdrawal due to adverse events as there was no evidence of statistical heterogeneity (P = 0.62, I2 = 0%). Four trials with a total of 394 patients were included in this comparison (Mannon 2010; Nikolaus 2003; Pena‐Rossi 2008; Tilg 2003). There was a statistically significant difference in withdrawals due to adverse events. Seven per cent (18/242) of patients in the IFNs group withdrew due to adverse events compared to 2% (3/152) of placebo patients (RR 3.16, 95% CI 1.06 to 9.40; P = 0.03). A GRADE analysis indicated that the quality of evidence supporting this outcome was low due to very sparse data (See Table 1). c. Colectomy In the Musch 2005 study, two patients underwent colectomy, 1/32 (3.1%) in the 1 MIU IFN‐β‐1a group at 8 weeks, and 1/29 (3.4%) in the placebo group 3 weeks into the trial. Madsen 2001 reported 1/16 (6.2%) patients in the IFN‐α‐2a group required a colectomy. There were no colectomies in the prednisolone enema control group (Madsen 2001). Mannon 2010, Nikolaus 2003, Pena‐Rossi 2008 and Tilg 2003 did not report this outcome.
d. Mortality There were no deaths reported in any of the six included studies.
Discussion
Moderate quality evidence suggests that type I IFNs are not effective for the treatment of patients with active UC (Madsen 2001; Mannon 2010; Musch 2005; Nikolaus 2003; Pena‐Rossi 2008; Tilg 2003). Meta‐analysis of placebo‐controlled studies showed no difference in clinical remission or response rates. There was no difference in the time to remission nor a difference in endoscopic activity index scores following treatment with IFN compared to placebo. The only study to show a statistically significant improvement in a clinical activity index and quality of life compared treatment with IFN‐α‐2a to prednisolone enemas in patients with left‐sided colitis (Madsen 2001). The response to therapy may reflect the limited extent of the disease in these patients. The results of this study should be interpreted with caution due to risk of bias (i.e. open‐label design) and the small number of patients enrolled.
The most common adverse events related to IFN therapy included headaches, arthralgias, myalgias, fatigue, back pain, nausea, application site reactions, rigors, and fever. There did not appear to be a difference in the adverse event profile of the different isoforms of IFN. There were no differences in the proportion of patients experiencing 'serious' adverse events which were defined as those that required specific treatment, including but not limited to escalation of therapy for UC, hospitalisation, or any symptoms which led to withdrawal from the trial. Based on the pooled data from four trials, there was an increased rate of withdrawals due to adverse events in IFN treated patients.
The above data could not fully address concerns raised by a handful of case reports that suggest that IFN therapy may exacerbate UC (Tursi 2007; Watanabe 2006; Sprenger 2005; Mavrogiannis 2001). This would need to be studied by reviewing controlled clinical trials of type I IFNs in patients who are in remission.
The results of this review should be interpreted with caution due to methodological concerns with the included studies. Even with data from the new included studies Pena‐Rossi 2008 and Mannon 2010, there were only 362 patients in the pooled analysis for clinical remission and only 425 patients in the pooled analysis for clinical response. These sample sizes likely have suboptimal power to detect a difference in treatment effect should one exist. A sample size calculation was performed based on the magnitude of the observed treatment effect in the pooled analysis. Using the pooled proportion of patients achieving remission in the IFN group of 36.0% (87/242) and 30.0% (36/120) in the placebo group, α=0.05, β=0.8, a case sample size of 1954 individuals would be required assuming a 1:1 case:control ratio for two independent populations of UC patients and no cross‐overs. Furthermore, this estimate is conservative and does not take into account the potential yet substantial withdrawal rates demonstrated in the existing trials.
There was clinical heterogeneity with variability in the isoform of IFN used (α versus β) and even in the studies using the same preparation of IFN‐β‐1a, there were marked differences in the dose used and the overall duration of treatment. It is possible that there may be isoform dependent and dose dependent effects.
The use of different clinical indices for measuring UC activity limited comparison of treatment efficacy data. The use of standardised validated clinical activity indices should be emphasised. The use of the same endoscopic activity index permitted comparisons. However, the length of treatment and follow‐up may have been insufficient to allow for maximal endoscopic response.
Based on the existing literature, the current evidence does not support the use of type I IFNs for induction of remission in patients with UC. Furthermore, there are concerns regarding the tolerability of this class of treatment.
Authors' conclusions
Implications for practice.
Moderate quality evidence suggests that type I IFNs are not effective for induction of remission in UC. Furthermore, there are concerns regarding the tolerability of this class of treatment.
Implications for research.
Several well‐designed studies that were included in this review, did not demonstrate any benefit for type I IFNs therapy in patients with ulcerative colitis. Further research is unlikely to yield any drastically different results.
What's new
| Date | Event | Description |
|---|---|---|
| 8 August 2014 | New search has been performed | New literature search was performed on August 8, 2014. |
| 8 August 2014 | New citation required but conclusions have not changed | Substantively updated review with new authors |
History
Protocol first published: Issue 4, 2007 Review first published: Issue 3, 2008
| Date | Event | Description |
|---|---|---|
| 7 July 2009 | Amended | Contact details updated |
| 28 April 2008 | Amended | Converted to new review format. |
| 25 March 2008 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Funding for the IBD/FBD Review Group (September 1, 2010 to August 31, 2015) has been provided by the Canadian Institutes of Health Research (CIHR) Knowledge Translation Branch (CON ‐ 105529) and the CIHR Institutes of Nutrition, Metabolism and Diabetes (INMD); and Infection and Immunity (III) and the Ontario Ministry of Health and Long Term Care (HLTC3968FL‐2010‐2235).
Miss Ila Stewart has provided support for the IBD/FBD Review Group through the Olive Stewart Fund.
We thank Mrs. Claire Parker for her invaluable guidance and assistance during the literature search process.
Appendices
Appendix 1. Search strategies
MEDLINE search strategy
1. random$.tw.
2. factorial$.tw.
3. (crossover$ or cross over$ or cross‐over$).tw.
4. placebo$.tw.
5. single blind.mp.
6. double blind.mp.
7. triple blind.mp.
8. (singl$ adj blind$).tw.
9. (double$ adj blind$).tw.
10. (tripl$ adj blind$).tw.
11. assign$.tw.
12. allocat$.tw.
13. crossover procedure/
14. double blind procedure/
15. single blind procedure/
16. triple blind procedure/
17. randomized controlled trial/
18. or/1‐17
19. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.)
20. 18 not 19
21. Colitis, Ulcerative/ or Inflammatory Bowel Diseases/
22. exp Interferons or interferons (nm) or interferon: .mp.
23. 21 and 22
24. 20 and 23
EMBASE search strategy
1. random$.tw.
2. factorial$.tw.
3. (crossover$ or cross over$ or cross‐over$).tw.
4. placebo$.tw.
5. single blind.mp.
6. double blind.mp.
7. triple blind.mp.
8. (singl$ adj blind$).tw.
9. (double$ adj blind$).tw.
10. (tripl$ adj blind$).tw.
11. assign$.tw.
12. allocat$.tw.
13. crossover procedure/
14. double blind procedure/
15. single blind procedure/
16. triple blind procedure/
17. randomized controlled trial/
18. or/1‐17
19. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.)
20. 18 not 19
21. Ulcerative Colitis/ or (inflammatory adj5 bowel).ti,ab.
22. exp INTERFERON/ or interferon:.mp. or 76543‐88‐9.rn.
23. 21 and 22
24. 20 and 23
Cochrane Central Library search strategy
1. Ulcerative Colitis OR Inflammatory Bowel Disease
2. "Interferon" or "76543‐88‐9" or "type 1 IFN" or "IFN" or "Interleukin 28A" or "Interleukin 29" or "Interleukin 6"
3. #1 and #2
SR‐IBD search strategy
Interferon AND ulcerative colitis
Data and analyses
Comparison 1. Type I Interferons versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Remission (non pooled data) | 4 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Tilg 0.5 mcg/kg PegIFN alpha | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Tilg 1.0 mcg/kg PegIFn alpha | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Musch 1 MIU IFN‐b‐1a | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Musch 3 MIU IFN‐b‐1a | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Nikolaus IFN‐b ‐1a (Dose escalating study) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Pena‐Rossi 44 mcg IFN‐b‐1a | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.7 Pena‐Rossi 66 mcg IFN‐b‐1a | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Remission (pooled data) | 4 | 362 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.84, 1.58] |
| 3 Remission ‐ sensitivity analysis (IFN beta‐isoform) | 3 | 302 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.84, 1.69] |
| 4 Remission ‐ sensitivity analysis (IFN alpha‐isoform) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Time to remission | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 1 MIU IFN b‐1‐a | 1 | 59 | Mean Difference (IV, Fixed, 95% CI) | ‐1.90 [‐11.22, 7.42] |
| 5.2 3 MIU IFN b‐1‐a | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | ‐4.10 [‐12.71, 4.51] |
| 6 Clinical improvement | 4 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.96, 1.40] |
| 7 Clinical improvement ‐ sensitivity analysis published manuscripts only (fixed‐effect model) | 3 | 302 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.91, 1.43] |
| 8 Endoscopic activity index score | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 1 MIU IFN b‐1‐a | 1 | 59 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐2.22, 1.62] |
| 8.2 3 MIU IFN b‐1‐a | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐0.91, 2.51] |
| 8.3 0.5 mcg/kg IFN b‐1‐a | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐2.94, 1.34] |
| 8.4 1.0 mcg/kg IFN b‐1‐a | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐2.14, 1.34] |
| 9 Serious adverse events | 4 | 468 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.13, 4.14] |
| 10 Serious adverse events ‐ sensitivity analysis published manuscripts only (random‐effects model) | 3 | 345 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.10, 10.87] |
| 11 Overall withdrawals | 5 | 485 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.65, 1.29] |
| 12 Overall withdrawals ‐ sensitivity analysis published manuscripts only (fixed‐effect model) | 4 | 362 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.66, 1.36] |
| 13 Withdrawals due to adverse events | 4 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.16 [1.06, 9.40] |
1.1. Analysis.
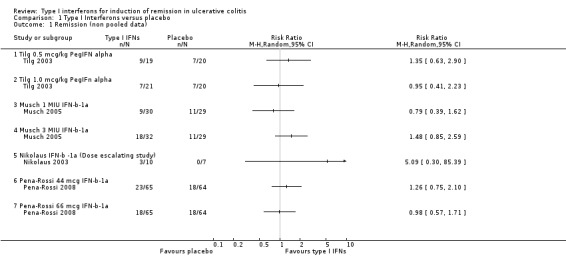
Comparison 1 Type I Interferons versus placebo, Outcome 1 Remission (non pooled data).
1.2. Analysis.
Comparison 1 Type I Interferons versus placebo, Outcome 2 Remission (pooled data).
1.3. Analysis.
Comparison 1 Type I Interferons versus placebo, Outcome 3 Remission ‐ sensitivity analysis (IFN beta‐isoform).
1.4. Analysis.
Comparison 1 Type I Interferons versus placebo, Outcome 4 Remission ‐ sensitivity analysis (IFN alpha‐isoform).
1.5. Analysis.
Comparison 1 Type I Interferons versus placebo, Outcome 5 Time to remission.
1.6. Analysis.
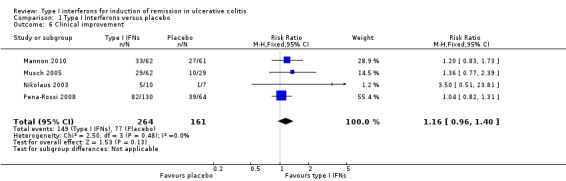
Comparison 1 Type I Interferons versus placebo, Outcome 6 Clinical improvement.
1.7. Analysis.
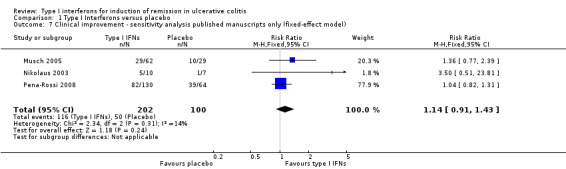
Comparison 1 Type I Interferons versus placebo, Outcome 7 Clinical improvement ‐ sensitivity analysis published manuscripts only (fixed‐effect model).
1.8. Analysis.
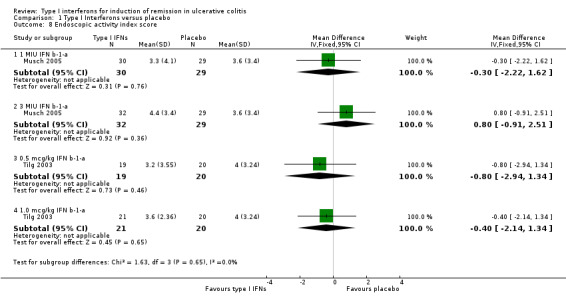
Comparison 1 Type I Interferons versus placebo, Outcome 8 Endoscopic activity index score.
1.9. Analysis.
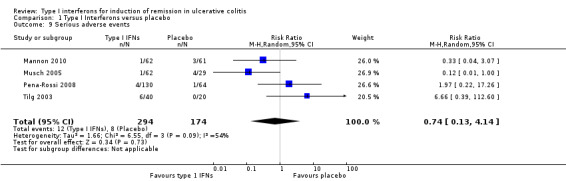
Comparison 1 Type I Interferons versus placebo, Outcome 9 Serious adverse events.
1.10. Analysis.
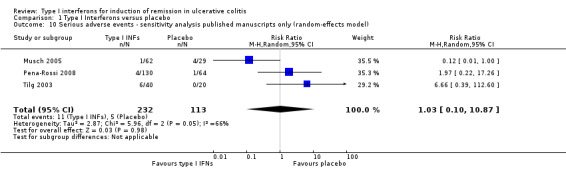
Comparison 1 Type I Interferons versus placebo, Outcome 10 Serious adverse events ‐ sensitivity analysis published manuscripts only (random‐effects model).
1.11. Analysis.
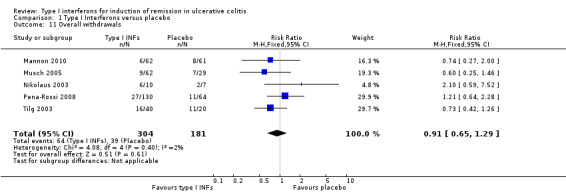
Comparison 1 Type I Interferons versus placebo, Outcome 11 Overall withdrawals.
1.12. Analysis.
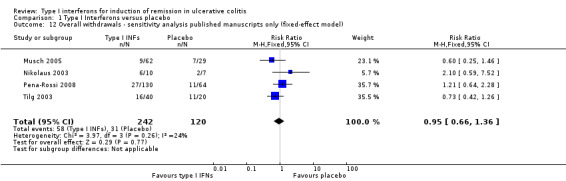
Comparison 1 Type I Interferons versus placebo, Outcome 12 Overall withdrawals ‐ sensitivity analysis published manuscripts only (fixed‐effect model).
1.13. Analysis.
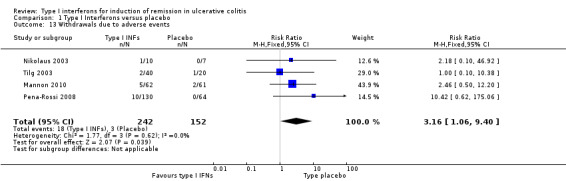
Comparison 1 Type I Interferons versus placebo, Outcome 13 Withdrawals due to adverse events.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Madsen 2001.
| Methods | Open‐label, randomised controlled trial | |
| Participants | 32 adult patients with active left‐sided UC Group 1: n=16, M:F = 8:8, median age = 47 yrs (19‐73), median disease duration 9.5 yrs (1‐25), median Powell‐Tuck Index score = 9 (5‐16) Group 2: n=16, M:F = 11:5, median age = 49 yrs (29‐68), median disease duration 3.5 yrs (0‐34), median Powell‐Tuck Index score = 8 (4‐12) | |
| Interventions | Group 1: IFN‐a‐2a therapy by SC injection t.i.w. for a total duration of 12 weeks. 9 MIU t.i.w. for week 1, 6 MIU t.i.w. for week 2, and 3 MIU t.i.w. from weeks 3‐12 inclusive Group 2: Prednisolone enemas 100mL (25mg) daily for 30 days | |
| Outcomes | Clinical, endoscopic and histological assessment of disease activity
Quality of life assessment Tolerability of treatment |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The allocation schedule using random numbers was generated using a computer program |
| Allocation concealment (selection bias) | Low risk | Each 'treatment' was consecutively numbered on concealed envelopes, which were only opened after the patient had given informed consent to participate. |
| Blinding (performance bias and detection bias) All outcomes | High risk | The study was an open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were accounted for 3/16 (18.8%) patients withdrew from IFN ‐a‐2A group and 0/16 (0%) withdrew from the prednisolone enema group |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | No other issues were found |
Mannon 2010.
| Methods | Double‐blind, multicenter, randomised, placebo‐controlled trial (Biogen Trial) | |
| Participants | 123 patients (18‐65 year old) with moderate to severe UC (modified Mayo Score 6‐13) Group 1: n=62, M:F = 43:19, median age = 41.1 yrs (21‐64); Group 2: n=61, M:F = 35:26, median age = 41.0 yrs (20‐65) |
|
| Interventions | Group 1: IFNβ‐1a 30 μg IM twice a week for 12 weeks Group 2: placebo IM twice per week for 12 weeks |
|
| Outcomes | Primary endpoint: clinical response at week 8, defined as a decrease from baseline in the total Mayo score of at least 3 points and at least 30%, accompanied by a decrease in the subscore for rectal bleeding of at least 1 point or absolute subscore of 0 or 1 Secondary endpoints: safety and tolerability of IFNb and the percentage of subjects with a decrease in the Short Clinical Activity Index (SCCAI) score of ≥3 points at week 8 |
|
| Notes | It is a conference abstract publication, efforts to locate the full journal article in the literature and to contact the trial lead author were unsuccessful | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described in the publication |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind (subject, caregiver) ‐ based on information published on clinicaltrials.gov; trial ID: NCT00616434 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 123 enrolled patients were included in the intention to treat (ITT) population, 61 in placebo and 62 in IFNβ. 6/62 (9.68%) patients in the IFNβ‐1a 30 μg treatment group and 8/61 (13.1%) patients in the placebo group did not complete the study |
| Selective reporting (reporting bias) | Low risk | Pre‐defined outcomes were reported |
| Other bias | Low risk | No other issues were identified |
Musch 2005.
| Methods | Randomised, double‐blind, placebo‐controlled, multicenter trial | |
| Participants | 91 adult patients with active UC defined by a Rachmilewitz CAI score of at least 8 points Group 1: n=32, M:F = 21:11, median age = 38.0 yrs, median disease duration 7.5 yrs, median Rachmilewitz CAI score = 10, mean endoscopic index score = 10 Group 2: n=30, M:F = 18:12, median age = 34.5 yrs, median disease duration 6.7 yrs, median Rachmilewitz CAI score = 10, mean endoscopic index score = 9 Group 3: n=29, M:F = 15:14, median age = 38.0 yrs, median disease duration 3.2 yrs, median Rachmilewitz CAI score = 10, median endoscopic index score = 9 | |
| Interventions | Group 1: 3 MIU IFN‐β‐1a by SC injection t.i.w Group 2: 1 MIU by SC injection IFN‐β‐1a t.i.w Group 3: placebo injections Duration of treatment 8 weeks | |
| Outcomes | Primary outcome was response rate at the end of treatment. Response was defined as reduction of 6 or more CAI points at week 8 compared with baseline Secondary endpoints: 1. Number of patients with complete response ‐ reduction of CAI to 4 points of less after 8 weeks of treatment; 2. Time until response; 3. Reduction of CAI after 4 and 8 weeks; 4. Reduction of the endoscopic index after 8 weeks; 5. Number of patients receiving colectomy; 6. Reduction of steroid dose |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Patients were assigned by a centralised randomisation schedule |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The study was double‐blind in design Supervision of the clinical trial was performed by a steering committee of investigators who were blinded from the results throughout the trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/32 patients in 3 MIU group, 5/30 patients in 1 MIU group, 7/29 patients in placebo group dropped out during treatment |
| Selective reporting (reporting bias) | Low risk | All pre‐defined outcomes were reported |
| Other bias | Low risk | No other issues were identified |
Nikolaus 2003.
| Methods | Randomised, double blind, intra‐individual, dose escalating study | |
| Participants | 17 adult patients with moderately active UC defined by a UCSS score of 6‐10, with a proctosigmoidoscopy score of 2 Group 1: n=10, M:F = 4:6, median age = 42.2 yrs (32‐68), median disease duration 9.8 yrs (2.6‐14.2), median UCSS score = 9 (7‐10), left sided colitis n=5 Group 2: n=7, M:F = 2:5, median age = 35 yrs (30‐63), median disease duration 9.0 yrs (2.6‐40.3), median UCSS score = 9 (7‐12), left sided colitis n=5 | |
| Interventions | Group 1: IFN‐β‐1a by SC injection t.i.w. at variable dose for a variable duration of treatment. Started at 22mcg (t.i.w.) Improvement was defined as a decrease of 1 point in the combined score of UCSS symptoms and PGA. If no improvement was observed after six injections, dose increased to 44 mcg t.i.w., and increased further to 88 mcg t.i.w., if no improvement was observed after six injections at 44 mcg dose If improvement was observed after six injections at any dose, the patient entered a maintenance treatment phase of 6‐12 injections at that dose If no improvement was observed after six injections at 88 mcg, or if remission occurred at any point, treatment was stopped Group 2: placebo The maximum duration of treatment was eight weeks, and the minimum duration was four weeks |
|
| Outcomes | Efficacy end points were treatment response and remission Treatment response was defined as a decrease of at least 3 points from baseline in the UCSS symptoms score and PGA (without the proctosigmoidoscopic score) during treatment Remission was defined as complete resolution of clinical symptoms (all clinical UCSS subscores equal to 0), with a proctosigmoidoscopy score of 0 or 1 at any time during treatment Secondary end points included overall treatment and end point responses, and clinical end point responses Safety data were also collected |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible patients were randomised by means of a computer generated list Randomisation was stratified by centre with block size of three (2:1 IFN‐β‐1a: placebo) |
| Allocation concealment (selection bias) | Unclear risk | Details were not provided in the published report |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The trial is double blind in design but the methods used for blinding were not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All enrolled patients (n=18) were accounted for. One patients was excluded a priori (before the code was broken) from the analysis, because of misallocation of study drug. 6/10 (60%) of IFN‐β‐1a group and 2/7 (28.6%) of control group stopped the treatment early |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | No other issues were identified |
Pena‐Rossi 2008.
| Methods | Multicentre, double‐blind, randomised placebo‐controlled trial (Serono Trial) | |
| Participants | 194 adult patients with diagnosed moderately active UC Group 1: n = 65, M:F = 37:28, mean age = 39.9 yrs (SD=14.0), mean disease duration 7.2 yrs (SD=6.5), mean UCSS score = 7.7 (SD=1.2), length of colonic involvement = 53.5 (SD=28.0); Group 2: n = 65, M:F = 30:35, mean age = 40.7 yrs (SD=13.3), mean disease duration 4.9 yrs (SD=5.1), median UCSS score = 7.8 (SD=1.2), length of colonic involvement = 50.2 (SD=27.5); Group 3: n = 64, M:F = 31:33, mean age = 41.1 yrs (SD=12.6), mean disease duration 5.6 yrs (SD=5.5), median UCSS score = 7.9 (SD=1.1), length of colonic involvement = 52.4 (SD=24.2) |
|
| Interventions | Group 1: 44 μg IFN‐β‐1a Group 2: 66 μg IFN‐β‐1a Group 3: matching placebo All study drugs were given sc t.i.w. for 8 weeks with 4‐week follow‐up period |
|
| Outcomes | Primary outcome: identify the best dose of IFN‐β‐1a for the induction of ECR and examine the safety profile of this dose Secondary outcomes: 1. investigate the safety and tolerability of IFN‐β‐1a; 2. establish the dose needed to enable clinical response and a change in disease severity; 3. estimate disease‐related quality of life on IBDQ; 4. assess changes in biological markers of inflammation. Safety and antibodies to IFN‐β were also evaluated |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised centrally using stochastic minimization, which considered the overall balance, centre, region and current use of maintenance therapy as minimization factors |
| Allocation concealment (selection bias) | Low risk | Following central randomisation, each patient was assigned a treatment kit number corresponding to a blinded treatment kit containing sufficient medication for the 8 weeks of treatment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The solutions of study drug were physically indistinguishable from one another, prepared and administered using the same technique for all patients and the labelling and packaging were designed so as to preserve the blinded nature of the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised patients were accounted for. 13/65 (20.0%) patients in 66 μg IFN‐β‐1a treatment group, 14/65 (21.5%) patient in 44 μg IFN‐β‐1a treatment group, and 11/64 (17.2%) patients in control group withdrew from the study |
| Selective reporting (reporting bias) | Low risk | Clinically relevant outcomes were reported |
| Other bias | Low risk | No other issues were identified |
Tilg 2003.
| Methods | Multicentre, double‐blind, randomised, placebo controlled trial | |
| Participants | 60 adults with UC Group 1: n=19, M:F = 9:10, mean age = 36.90 yrs (13.19), mean disease duration 107.30 mths (64.11), mean Rachmilewitz CAI score = 8.0 (2.1), mean endoscopic index score = 8.3 (2.1) Group 2: n=21, M:F = 11:10, mean age = 40.90 yrs (10.84), mean disease duration 106.88 mths (71.83), mean Rachmilewitz CAI score = 7.9 (2.1), mean endoscopic index score = 8.3 (2.0), left sided colitis n=12 Group 3: n=20, M:F = 10:10, mean age = 46.95 yrs (14.43), mean disease duration 142.90 mths (132.01), mean Rachmilewitz CAI score = 8.7 (2.5), mean endoscopic index score = 8.2 (2.3), left sided colitis n=14 | |
| Interventions | Group 1: PegIFN 0.5 μg/kg Group 2: PegIFN 1.0 μg/kg Group 3: placebo All therapies were administered by subcutaneous injection once a week, for a period of 12 weeks | |
| Outcomes | The primary outcome was safety
Secondary outcomes were disease remission, (which was defined as a Rachmilewitz CAI score of <=4), endoscopic remission (which was defined as a score of <4), and histological activity (graded on a scale from 0 to 3) Outcomes were measured at week 12 Changes in serological inflammatory indices were also recorded |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | At entry, all patients were randomised to receive placebo, PegIFN 0.5 μg/kg, or PegIFN 1.0 μg/kg body weight |
| Allocation concealment (selection bias) | Unclear risk | Details were not provided in the published report |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The study was double blind in design |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All enrolled patients were accounted for. 6/19 (31.6%) in the PegIFN‐α 0.5 μg/kg group, 10/21 (47.6%) in the PegIFN‐α 1.0 μg/kg group, and 11/20 (55.0%) in the control group withdrew from the study |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | No other issues were identified |
CAI: Clinical Activity Index
Mean (standard deviation in brackets)
Median (range in brackets)
MIU: million international units
mths: months
PGA: Physician's global assessment
SC: subcutaneous
t.i.w.: three times a week
UCSS: Ulcerative colitis scoring system
yrs: years
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bargiggia 2005 | Single centre case control study of 513 IBD patients with mildly active disease or disease in remission 21 IBD patients (11 with UC) had detectable hepatitis C antibodies, and were subsequently treated with IFN‐alpha 6 MIU t.i.w. for 12 months Primary endpoint was related to treatment of hepatitis C (biochemical and virological response) |
| Hadziselimovic 1995 | Single centre prospective cohort study of 8 children and adolescents with IBD treated with IFN‐alpha‐2a t.i.w. for variable treatment periods No comparison group is provided Only 2 of the 8 patients had UC |
| Maev 2002 | Randomised controlled trial of 113 patients with UC The interventions were orally administered IFN inducers (amixin or cycloferon) rather than IFN itself |
| Mannon 2011 | Open label pilot study enrolled 18 UC patients (SCCAI of 6) to receive interferon‐β‐1a 30 μg IM per week for 12 weeks. with 24‐week follow‐up Main outcomes included clinical response (SCCAI score drop ≥3 point for at least two consecutive visits), and effects on cytokine production No control group (NIAID trial) |
| Musch 2002 | Open‐label pilot study of IFN‐beta in 25 adult patients Patients were administered 0.5 MIU human natural IFN‐beta i.v. (n = 18) or 1 MIU recombinant IFN‐beta‐1a s.c. (n = 7) daily with the goal of induction of remission Subsequent maintenance treatment was provided at the same dose, t.i.w. for 52.0 +/‐ 78.8 weeks (range 4‐336 weeks). No control group |
| Musch 2007 | Non‐randomised open‐label study of IFN‐beta in 46 adult patients Patients were administered 0.5 MIU human natural IFN‐beta i.v. (n = 18) or 1 MIU recombinant IFN‐beta‐1a s.c. (n = 28) daily for 8 weeks Patients who achieved complete remission (CAI ≤ 4) during induction period received maintenance therapy at the same dose three times a week Remission, maintenance of remissions and safety were evaluated No control group |
| Sümer 1995 | Open‐label study of IFN‐alpha‐2a in 28 adult patients Patients received 6 to 12 months of IFN therapy by subcutaneous injection three times a week No control group |
IBD: Inflammatory bowel disease MIU: Million international units t.i.w.: Three times a week i.v.: Intravenous s.c.: Subcutaneous
Contributions of authors
The following authors participated in the updated review:
YW was responsible for the updating the literature search, selecting and reviewing the studies, performing the analyses and updating the manuscript. JKM was responsible for the updating the literature search, selecting and reviewing the studies, performing the analyses and updating the manuscript. EIB provided IBD expert opinion and reviewed the manuscript. AMG provided IBD expert opinion and reviewed the manuscript. AHS provided IBD expert opinion and reviewed the manuscript. RP provided IBD expert opinion and reviewed the manuscript. CHS was responsible for updating the literature search, performing the analyses, and updating the manuscript.
The following authors were involved in the original review:
CHS was responsible for the literature search, selecting and reviewing the studies, performing the analyses and writing the manuscript. EIB acted as co‐reviewer of the studies, was involved in the analyses and reviewed the manuscript. AHS provided methodological expertise, IBD expert opinion and reviewed the manuscript. AMG provided IBD expert opinion and reviewed the manuscript.
Declarations of interest
YW: None known.
JKM: None known.
EIB: None known.
AMG: Anne Marie Griffiths has received fee(s) from Johnson and Johnson for Board membership; fee(s) from Janssen, Abbvie and Ferring for consultancy; grants or grants pending from Johnson and Johnson and Abbive; lecture fee(s) from: Abbvie and Merck and payment for development of educational presentations from Ferring. All of these activities are outside the submitted work.
AHS: Hillary Steinhart has received fee(s) from Janssen, Abbvie, Shire, Pendopharm, Pfizer, and Takeda for consultancy; and lecture fee(s) from: Janssen, Abbvie, Shire, Warner Chilcott, Aptalis, and Takeda. His institution has received grants or grants pending from Janssen, Abbvie, Pfizer, Amgen, Takeda and Actavis. All of these activities are outside the submitted work. AHS was a collaborator on the paper "Interferon β‐1a in ulcerative colitis: a placebo controlled, randomised, dose escalating study." (Gut 2003;52:1286‐1290) and Mount Sinai Hospital, Toronto, Ontario, CANADA was one of the participating study centres in the clinical trial.
RP: Remo Panaccione has received fee(s) from Abbott, Abbvie, Allergan, Actavis, Biogen Idec, Celgene, Eisai, Elan, Ferring, Genentech, Janssen, Merck, Nestle, Osiris, Prometheus, Qu Biologics, Roche, Salix, Takeda, Teva, Vertex, Warner Chilcott for consultancy; grants or grants pending from Abbvie, Janssen; lecture fee(s) from: Abbott, Abbvie, Ferring, Janssen, Shire, Takeda; and payment for development of educational presentations from Abbvie, Janssen, Takeda, Shire. All of these activities are outside the submitted work. RP was a collaborator on the paper "Interferon‐ß‐1a in active moderate to severe ulcerative colitis: Efficacy and safety from a phase IIa multicenter study." (American Journal of Gastroenterology 2010;105:S446).
CHS: Cynthia Seow has served as a consultant and on advisory boards for Janssen Pharmaceuticals, Abbvie, Takeda, Shire and Actavis. She previously held a grant through Janssen Pharmaceuticals. Dr. Seow has also provided lectures for Janssen Pharmaceuticals and Warner Chilcott. All of these activities are outside the submitted work.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Madsen 2001 {published data only}
- Madsen SM, Schlichting P, Davidsen B, Nielsen OH, Federspiel B, Riis P, et al. An open, randomized study comparing systemic interferon‐a‐2A and prednisolone enemas in the treatment of left‐sided ulcerative colitis. Gut 1999;45(Suppl V):A286. [DOI] [PubMed] [Google Scholar]
- Madsen SM, Schlichting P, Davidsen B, Nielsen OH, Federspiel B, Riis P, et al. An open‐labeled, randomized study comparing systemic interferon‐alpha‐2A and prednisolone enemas in the treatment of left‐sided ulcerative colitis. American Journal of Gastroenterology 2001;96(6):1807‐15. [DOI] [PubMed] [Google Scholar]
Mannon 2010 {published data only}
- Mannon P, Feagan B, Xi Y, McAllister A, Cataldi F. Mucosal healing an essential efficacy measure in ulcerative colitis: Effect of country‐enrollment site in a phase IIa study with interferon‐ß‐1a. Journal of Crohn's and Colitis 2011;5(1):S102‐3. [Google Scholar]
- Mannon P, Miner P, Lukas M, McAllister A, Cataldi F. Secondary efficacy analysis from a phase IIa multicenter study with interferon‐ß‐1a for induction of clinical response in active moderate to severe ulcerative colitis. American Journal of Gatroenterology 2010;105:S468‐9. [Google Scholar]
- Mannon P, Panaccione R, Miner P, McAllister A, O'Gorman J, Cataldi F. Interferon‐ß‐1a in active moderate to severe ulcerative colitis: Efficacy and safety from a phase IIa multicenter study. American Journal of Gastroenterology 2010;105:S446. [Google Scholar]
- Mannon P, Reinisch W, Miner P, McAllister A, Xi Y, Cataldi F. Different scoring methods affect the accuracy of clinical outcomes in ulcerative colitis. Post‐hoc analysis in a phase 2a study with interferon‐ß‐1a. Journal of Crohn's and Colitis 2011;5(1):S69‐70. [Google Scholar]
- Mannon P, Reinisch W, Miner P, Xi Y, McAllister A, Cataldi F. Are the results of multicenter trials hampered by country variability? Comparison between two endpoints used in a study with interferon‐ß‐1a in ulcerative colitis. Journal of Crohn's and Colitis 2011;5(1):S94‐5. [Google Scholar]
- Mannon P, Reinisch W, Miner P, Xi Y, McAllister A, Cataldi F. Is there a difference between self‐assessed patient‐reported outcome and physician evaluation in ulcerative colitis? Results from a study with interferon‐ß‐1a. Journal of Crohn's and Colitis 2011;5(1):S84‐5. [Google Scholar]
- Miner P, Lembo A, Mannon P, Xi Y, McAllister A, Cataldi F. Stool frequency a subjective endpoint in ulcerative colitis and irritable bowel syndrome clinical trials. Comparison between stool frequency and mucosal healing from study with Interferon‐B‐1a. Inflammatory Bowel Diseases 2011;17:S54‐5. [Google Scholar]
- Miner P, Mannon P, Rajagopalan K, McAllister A, Xi Y, Cataldi F. Improvement in work productivity: Analysis from a proof of concept in ulcerative colitis with Interferon‐B‐1a. Inflammatory Bowel Diseases 2011;17:S55. [Google Scholar]
- Miner P, Mannon P, Zakko S, McAllister A, O'Gorman J, Cataldi F. Is the partial Mayo score predictive of efficacy in ulcerative colitis trials? Comparison between the partial and the total Mayo score from a study with Interferon‐B‐1a in ulcerative colitis. Inflammatory Bowel Diseases 2011;17:S36. [Google Scholar]
- Miner P, Zakko S, McAllister A, Cataldi F. Safety analysis from a phase IIa multicenter study with interferon‐ß‐1a in active moderate to severe ulcerative colitis. American Journal of Gastroenterology 2010;105:S433‐4. [Google Scholar]
- NCT00616434. A multicenter, randomized, double‐blind, placebo‐controlled study to evaluate the safety, tolerability, and efficacy of Avonex® in subjects with moderate to severe ulcerative colitis. https://clinicaltrials.gov/ct2/show/study/NCT00616434 (accessed 8 August 2014). [NCT00616434]
- Reinisch W, Mannon P, Miner P, McAllister A, Xi Y, Cataldi F. Is elevated CRP a predictor of efficacy in ulcerative colitis? Post‐hoc analysis from a placebo‐controlled study with interferon‐ß‐1a. Journal of Crohn's and Colitis 2011;5(1):S72‐3. [Google Scholar]
Musch 2005 {published data only}
- Musch E, Andus T, Kruis W, Raedler A, Spehlmann M, Schreiber S, et al. Interferon‐beta‐1a for the treatment of steroid‐refractory ulcerative colitis: a randomized, double‐blind, placebo‐controlled trial. Clinical Gastroenterology and Hepatology 2005;3(6):581‐6. [DOI] [PubMed] [Google Scholar]
- Musch E, Raedler A, Andus T, Kruis W, Schreiber S, Lorenz A, et al. A phase II placebo‐controlled, randomized multicenter study to evaluate efficacy and safety of interferon beta‐1a in patients with ulcerative colitis. Gastroenterology 2002;122(4 Suppl 1):A431. [Google Scholar]
- Musch E, Raedler A, Andus T, Kruis W, Schreiber S, Lorenz A, et al. Results of a randomised, double‐blind, placebo‐controlled, multi‐centre, phase‐II study to test the effectivity and tolerance of Interferon‐beta‐1a (CHO‐beta) [rIFN‐beta‐1a] in patients with ulcerative colitis. Zeitschrift fur Gastroenterologie 2002;40(8):705. [Google Scholar]
Nikolaus 2003 {published data only}
- Nikolaus S, Rutgeerts P, Fedorak R, Steinhart AH, Wild GE, Theuer D, et al. Interferon beta‐1a in ulcerative colitis: a placebo controlled, randomised, dose escalating study. Gut 2003;52(9):1286‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaus S, Rutgeerts P, Fedorak RN, Steinhart H, Wild GE, Theuer D, et al. Recombinant human interferon‐beta (IFNb‐1a) induces remission and is well tolerated in moderately active ulcerative colitis (UC). Gastroenterology 2001;120(5 Suppl 1):A454. [Google Scholar]
Pena‐Rossi 2008 {published data only}
- Pena‐Rossi C, Schreiber S, Golubovic G, Mertz‐Nielsen A, Panes J, Rachmilewitz D, et al. Clinical trial: a multicentre, randomized, double‐blind, placebo‐controlled, dose‐finding, phase II study of subcutaneous interferon‐beta‐la in moderately active ulcerative colitis. Alimentary Pharmacology and Therapeutics 2008;28(6):758‐67. [DOI] [PubMed] [Google Scholar]
Tilg 2003 {published data only}
- Tilg H, Vogelsang H, Ludwiczek O, Lochs H, Colombel JF, Rutgeerts P, et al. A randomized placebo‐controlled trial of pegylated interferon alpha in active ulcerative colitis. Gastroenterology 2003;124(4 Suppl 1):A62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilg H, Vogelsang H, Ludwiczek O, Lochs H, Kaser A, Colombel JF, et al. A randomised placebo controlled trial of pegylated interferon alpha in active ulcerative colitis. Gut 2003;52(12):1728‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Bargiggia 2005 {published data only}
- Bargiggia S, Thorburn D, Anderloni A, Ardizzone S, Giorgi A, Porro GB, et al. Is interferon‐alpha therapy safe and effective for patients with chronic hepatitis C and inflammatory bowel disease? A case‐control study. Alimentary Pharmacology and Therapeutics 2005;22(3):209‐15. [DOI] [PubMed] [Google Scholar]
Hadziselimovic 1995 {published data only}
- Hadziselimovic F, Schaub U, Emmons LR. Interferon alpha‐2A (roferon) as a treatment of inflammatory bowel disease in children and adolescents. Advances in Experimental Medicine and Biology 1995;371B:1323‐6. [PubMed] [Google Scholar]
Maev 2002 {published data only}
- Maev IV, Grigorian SS, Gadzhieva MG, Ovchinnikova NI, Ospel'nikova TP, Kaziulin AN. [Interferon status of patients with non‐specific ulcerous colitis and its correction with interferon inducers]. Terapevticheskiĭ Arkhiv 2002;74(2):31‐5. [PubMed] [Google Scholar]
Mannon 2011 {published data only}
- Cataldi F, Mannon P, Fuss I, Groden C, Strober W. A phase IIa study of AVONEX (interferon‐B‐1a) in ulcerative colitis (UC): Integrating basic immunology and human pilot study results. Journal of Crohn's and Colitis Supplements 2010;4(1):33. [Google Scholar]
- Mannon PJ, Hornung RL, Yang Z, Yi C, Groden C, Friend J, et al. Suppression of inflammation in ulcerative colitis by interferon‐ß‐1a is accompanied by inhibition of IL‐13 production. Gut 2011;60(4):449‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Musch 2002 {published data only}
- Musch E, Andus T, Malek M. Induction and maintenance of clinical remission by interferon‐beta in patients with steroid‐refractory active ulcerative colitis ‐ an open long‐term pilot trial. Alimentary Pharmacology and Therapeutics 2002;16(7):1233‐9. [DOI] [PubMed] [Google Scholar]
Musch 2007 {published data only}
- Musch E, Andus T, Malek M, Chrissafidou A, Schulz M. Successful treatment of steroid refractory active ulcerative colitis with natural interferon‐beta‐‐an open long‐term trial. Zeitschrift für Gastroenterologie 2007;45(12):1235‐40. [DOI] [PubMed] [Google Scholar]
Sümer 1995 {published data only}
- Sümer N, Palabiyikoğlu M. Induction of remission by interferon‐alpha in patients with chronic active ulcerative colitis. European Journal of Gastroenterology and Hepatology 1995;7(7):597‐602. [PubMed] [Google Scholar]
Additional references
Anonymous 1998
- Anonymous. Randomised double‐blind placebo‐controlled study of interferon beta‐1a in relapsing/remitting multiple sclerosis. PRISMS (Prevention of Relapses and Disability by Interferon beta‐1a Subcutaneously in Multiple Sclerosis) Study Group. Lancet 1998;352(9139):1498‐504. [PubMed] [Google Scholar]
Anonymous 2001
- Anonymous. PRISMS‐4: Long‐term efficacy of interferon‐beta‐1a in relapsing MS. Neurology 2001;56(12):1628‐36. [DOI] [PubMed] [Google Scholar]
Antonetti 2002
- Antonetti F, Finocchiaro O, Mascia M, Terlizzese MG, Jaber A. A comparison of the biologic activity of two recombinant IFN‐beta preparations used in the treatment of relapsing‐remitting multiple sclerosis. Journal of Interferon & Cytokine Research 2002;22(12):1181‐4. [DOI] [PubMed] [Google Scholar]
Baumgart 2007
- Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet 2007;369(9573):1627‐40. [DOI] [PubMed] [Google Scholar]
Beattie 1996
- Beattie RM, Nicholls SW, Domizio P, Williams CB, Walker‐Smith JA. Endoscopic assessment of the colonic response to corticosteroids in children with ulcerative colitis. Journal of Pediatric Gastroenterology and Nutrition 1996;22(4):373‐9. [DOI] [PubMed] [Google Scholar]
Bernstein 2015
- Bernstein CN. Treatment of IBD: where we are and where we are going. American Journal of Gastroenterology 2015;110(1):114‐26. [DOI] [PubMed] [Google Scholar]
Borg 2007
- Borg FA, Isenberg DA. Syndromes and complications of interferon therapy. Current Opinion in Rheumatology 2007;19(1):61‐6. [DOI] [PubMed] [Google Scholar]
Bouma 2003
- Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nature reviews. Immunology 2003;3(7):521‐33. [DOI] [PubMed] [Google Scholar]
Brassard 2002
- Brassard DL, Grace MJ, Bordens RW. Interferon‐alpha as an immunotherapeutic protein. Journal of Leukocyte Biology 2002;71(4):565‐81. [PubMed] [Google Scholar]
Durbin 2013
- Durbin RK, Kotenko SV, Durbin JE. Interferon induction and function at the mucosal surface. Immunological Reviews 2013;255(1):25‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ek 2014
- Ek WE, D'Amato M, Halfvarson J. The history of genetics in inflammatory bowel disease. Annals of Gastroenterology 2014;27(4):294‐303. [PMC free article] [PubMed] [Google Scholar]
Feagan 2013
- Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. New England Journal of Medicine 2013;369(8):699‐710. [DOI] [PubMed] [Google Scholar]
Follmann 1992
- Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. Journal of Clinical Epidemiology 1992;45(7):769‐73. [DOI] [PubMed] [Google Scholar]
Foster 2004
- Foster GR. Review article: pegylated interferons: chemical and clinical differences. Alimentary Pharmacology and Therapeutics 2004;20(8):825‐30. [DOI] [PubMed] [Google Scholar]
George 2012
- George PM, Badiger R, Alazawi W, Foster GR, Mitchell JA. Pharmacology and therapeutic potential of interferons. Pharmacology & Therapeutics 2012;135(1):44‐53. [DOI] [PubMed] [Google Scholar]
Geremia 2014
- Geremia A, Biancheri P, Allan P, Corazza GR, Sabatino A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmunity Reviews 2014;13(1):3‐10. [DOI] [PubMed] [Google Scholar]
Ghosh 2006
- Ghosh S, Chaudhary R, Carpani M, Playford R. Interfering with interferons in inflammatory bowel disease. Gut 2006;55(8):1071‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Graber 2007
- Graber JJ, Ford D, Zhan M, Francis G, Panitch H, Dhib‐Jalbut S. Cytokine changes during interferon‐beta therapy in multiple sclerosis: correlations with interferon dose and MRI response. Journal of Neuroimmunology 2007;185(1‐2):168‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 1989
- Guyatt G, Mitchell A, Irvine EJ, Singer J, Williams N, Goodacre R, et al. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology 1989;96(3):804‐10. [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hanauer 2006
- Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflammatory Bowel Diseases 2006;12 Suppl 1:S3‐9. [DOI] [PubMed] [Google Scholar]
Hansen 2010
- Hansen R, Thomson JM, El‐Omar EM, Hold GL. The role of infection in the aetiology of inflammatory bowel disease. Journal of Gastroenterology 2010;45(3):266‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heller 2005
- Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, et al. Interleukin‐13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology 2005;129(2):550‐64. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Hoofnagle 2006
- Hoofnagle JH, Seeff LB. Peginterferon and ribavirin for chronic hepatitis C. New England Journal of Medicine 2006;355(23):2444‐51. [DOI] [PubMed] [Google Scholar]
Ivashkiv 2014
- Ivashkiv LB, Donlin LT. Regulation of type I interferon responses. Nature reviews. Immunology 2014;14(1):36‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jovanovic 2014
- Jovanovic K, Siebeck M, Gropp R. The route to pathologies in chronic inflammatory diseases characterized by T helper type 2 immune cells. Clinical and Experimental Immunology 2014;178(2):201‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kasper 2014
- Kasper LH, Reder AT. Immunomodulatory activity of interferon‐beta. Annals of Clinical and Translational Neurology 2014;1(8):622‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ko 2014
- Ko Y, Butcher R, Leong RW. Epidemiological studies of migration and environmental risk factors in the inflammatory bowel diseases. World Journal of Gastroenterology 2014;20(5):1238‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koretz 2013
- Koretz RL, Pleguezuelo M, Arvaniti V, Barrera Baena P, Ciria R, Gurusamy KS, et al. Interferon for interferon nonresponding and relapsing patients with chronic hepatitis C. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD003617.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lichtiger 1990
- Lichtiger S, Present DH. Preliminary report: cyclosporin in treatment of severe active ulcerative colitis. Lancet 1990;336(8706):16‐9. [DOI] [PubMed] [Google Scholar]
Ludigs 2012
- Ludigs K, Parfenov V, Du Pasquier RA, Guarda G. Type I IFN‐mediated regulation of IL‐1 production in inflammatory disorders. Cellular and Molecular Life Sciences 2012;69(20):3395‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mavrogiannis 2001
- Mavrogiannis C, Papanikolaou IS, Elefsiniotis IS, Psilopoulos DI, Karameris A, Karvountzis G. Ulcerative colitis associated with interferon treatment for chronic hepatitis C. Journal of Hepatology 2001;34(6):964‐5. [DOI] [PubMed] [Google Scholar]
Okanoue 1996
- Okanoue T, Sakamoto S, Itoh Y, Minami M, Yasui K, Sakamoto M, et al. Side effects of high‐dose interferon therapy for chronic hepatitis C. Journal of Hepatology 1996;25(3):283‐91. [DOI] [PubMed] [Google Scholar]
Powell‐Tuck 1978
- Powell‐Tuck J, Bown RL, Lennard‐Jones JE. A comparison of oral prednisolone given as single or multiple daily doses for active proctocolitis. Scandinavian Journal of Gastroenterology 1978;13(7):833‐7. [DOI] [PubMed] [Google Scholar]
Rachmilewitz 1989
- Rachmilewitz D. Coated mesalazine (5‐aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: a randomised trial. BMJ 1989;298(6666):82‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rutgeerts 2005
- Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. New England Journal of Medicine 2005;353(23):2462‐76. [DOI] [PubMed] [Google Scholar]
Schroeder 1987
- Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5‐aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. New England Journal of Medicine 1987;317(26):1625‐9. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Seo 1992
- Seo M, Okada M, Yao T, Ueki M, Arima S, Okumura M. An index of disease activity in patients with ulcerative colitis. American Journal of Gastroenterology 1992;87(8):971‐6. [PubMed] [Google Scholar]
Shibuya 2005
- Shibuya H, Hirohata S. Differential effects of IFN‐alpha on the expression of various TH2 cytokines in human CD4+ T cells. Journal of Allergy and Clinical Immunology 2005;116(1):205‐12. [DOI] [PubMed] [Google Scholar]
Sparano 1991
- Sparano JA, Dutcher JP, Kaleya R, Caliendo G, Fiorito J, Mitsudo S, et al. Colonic ischemia complicating immunotherapy with interleukin‐2 and interferon‐alpha. Cancer 1991;68(7):1538‐44. [DOI] [PubMed] [Google Scholar]
Sprenger 2005
- Sprenger R, Sagmeister M, Offner F. Acute ulcerative colitis during successful interferon/ribavirin treatment for chronic hepatitis. Gut 2005;54(3):438‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tada 1996
- Tada H, Saitoh S, Nakagawa Y, Hirana H, Morimoto M, Shima T, et al. Ischemic colitis during interferon‐alpha treatment for chronic active hepatitis C. Journal of Gastroenterology 1996;31(4):582‐4. [DOI] [PubMed] [Google Scholar]
Timmer 2012
- Timmer A, McDonald JWD, Tsoulis DJ, MacDonald JK. Azathioprine and 6‐mercaptopurine for maintenance of remission in ulcerative colitis. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD000478.pub3] [DOI] [PubMed] [Google Scholar]
Travis 2006
- Travis SP. Review article: induction therapy for patients with active ulcerative colitis. Alimentary Pharmacology and Therapeutics 2006;24 Suppl 1:10‐6. [DOI] [PubMed] [Google Scholar]
Travis 2012
- Travis SP, Schnell D, Krzeski P, Abreu MT, Altman DG, Colombel JF, et al. Developing an instrument to assess the endoscopic severity of ulcerative colitis: the Ulcerative Colitis Endoscopic Index of Severity (UCEIS). Gut 2012;61(4):535‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Truelove 1955
- Truelove SC, Witts LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. British Medical Journal 1955;2(4947):1041‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsukada 2002
- Tsukada Y, Nakamura T, Iimura M, Iizuka BE, Hayashi N. Cytokine profile in colonic mucosa of ulcerative colitis correlates with disease activity and response to granulocytapheresis. American Journal of Gastroenterology 2002;97(11):2820‐8. [DOI] [PubMed] [Google Scholar]
Tursi 2007
- Tursi A. Rapid onset of ulcerative colitis after treatment with PEG‐interferon plus ribavirin for chronic hepatitis C. Inflammatory Bowel Diseases 2007;13(9):1189‐90. [DOI] [PubMed] [Google Scholar]
Walmsley 1998
- Walmsley RS, Ayres RC, Pounder RE, Allan RN. A simple clinical colitis activity index. Gut 1998;43(1):29‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Watanabe 2006
- Watanabe T, Inoue M, Harada K, Homma N, Uchida M, Ogata N, et al. A case of exacerbation of ulcerative colitis induced by combination therapy with PEG‐interferon alpha‐2b and ribavirin. Gut 2006;55(11):1682‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2014
- Zhang YZ, Li YY. Inflammatory bowel disease: pathogenesis. World Journal of Gastroenterology 2014;20(1):91‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Seow 2008
- Seow CH, Benchimol EI, Griffiths AM, Steinhart AH. Type I interferons for induction of remission in ulcerative colitis. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD006790.pub2] [DOI] [PubMed] [Google Scholar]


