Abstract
Electroconvulsive therapy is one of the oldest and most effective forms of neurostimulation, where electrical current is used to elicit brief, generalized seizures under general anesthesia. When electrodes are positioned to target fronto-temporal cortex, ECT is arguably the most effective treatment for severe major depression, with response rates and times superior to other available antidepressant therapies. Neuroimaging research has been pivotal in improving the field’s mechanistic understanding of ECT, with a growing number of MRI studies demonstrating hippocampal plasticity after ECT, in line with evidence of upregulated neurotrophic processes in the hippocampus in animal models. However, the precise roles of the hippocampus and other brain regions in antidepressant response to ECT remain unclear. Seizure physiology may also play a role in antidepressant response to ECT, as indicated by early PET/SPECT and EEG research, and corroborated by recent ECT-MRI studies. In this review, we discuss the evidence supporting neuroplasticity in the hippocampus and other brain regions during and after ECT, and their associations with antidepressant response. We also offer a mechanistic, circuit- level model which proposes that core mechanisms of antidepressant response to ECT involve thalamocortical and cerebellar networks that are active during seizure generalization and termination over repeated ECT sessions, and their interactions with corticolimbic circuits that are dysfunctional prior to treatment and targeted with the electrical stimulus.
Keywords: Electroconvulsive therapy, neuroimaging, seizure, depression, MRI, Antidepressant
Introduction
Electroconvulsive therapy (ECT) is a highly effective treatment for severe treatment-refractory depression, with efficacy as high as 80–90% in some trials (1,2) and rapid reduction in symptoms occurring in as little as 2 or 3 treatments (3). In ECT, pulsed electrical current is passed between two electrodes on the head to elicit brief, generalized tonic-clonic seizures (Figure 1). The bitemporal electrode configuration effective at treating depressive symptoms, and is also used to treat catatonia, schizophrenia, and other conditions (4). However, bitemporal ECT is associated with greater risk of cognitive side effects, typically memory difficulties around the time of treatment (5–7). In recent years, the right unilateral configuration is used more frequently, which may be less effective for some patients but is associated with fewer cognitive side effects (8,9), presumably due to reduced temporal-lobe engagement (particularly in the left hemisphere). Recent and ongoing efforts have been made to improve ECT, including the introduction of brief and ultra-brief pulse stimulus delivery (7,10), exploration of other electrode configurations targeting frontal cortex (11,12), and application of electromagnetic energy in magnetic seizure therapy (13,14). Further improvements to ECT (and antidepressant therapies more generally) could be informed by studying its underlying mechanisms, which are not completely understood.
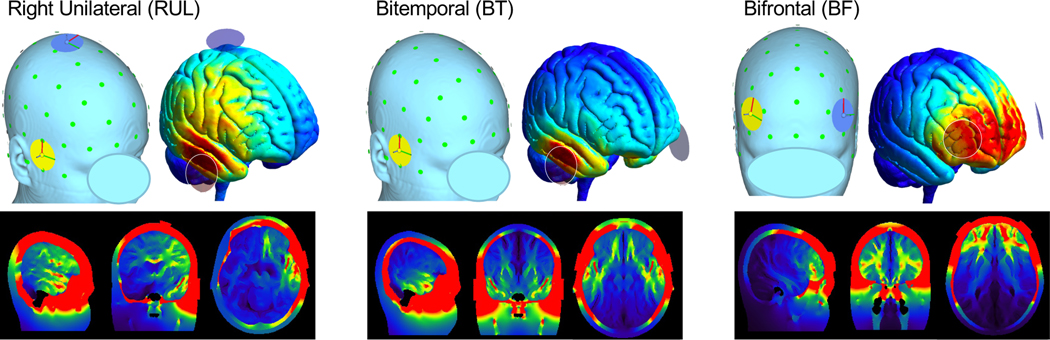
Figure 1.
ECT Electrode Positions and Estimated Electric Fields (E Fields). Right unilateral (RUL), bitemporal (BT), and bifrontal (BF) electrode positions are displayed in relation to the skin surface and pial surface of an MNI-space template head in top row (green circles marked estimated 10–10 EEG positions). On pial surfaces in top row, and gray-matter images in bottom row, color indicate the magnitude of E field estimated for each electrode position using SimNIBS software (https://simnibs.github.io/simnibs; warm colors indicate higher magnitude). Note that left electrode is not visible for BT electrode position in panel displaying skin surface.
In recent years, longitudinal ECT-MRI studies have improved our understanding of neuroplasticity associated with ECT. Reports of hippocampal gray-matter increases after treatment are robust and highly replicated (15–35), consistent with studies reporting increased trophic factors like BDNF in blood and CSF after treatment (36,37) and with animal models reporting increased hippocampal neurogenesis (38,39) and other markers of cellular plasticity (40–42). However, the nature of the link between hippocampal plasticity and antidepressant response to ECT is unclear, and is complicated by confounding effects of age and aspects of treatment dose like number of sessions, electrode placement, and charge (25,35,43,44). Other ECT-MRI studies controlling for these potential confounds have implicated specific hippocampal subregions with antidepressant response to ECT (45‒48), as well as other corticolimbic regions like subgenual and dorsal anterior cingulate cortex (ACC), left dorsolateral prefrontal cortex (DLPFC) and other structures (17,43,49‒51), all of which factor heavily into neurobiological models of depression (52,53). In earlier PET/SPECT and EEG studies, changes in thalamus and frontotemporal cortex activity were reported during and after ECT-induced seizures (54‒56) similar to changes associated with antidepressant response after treatment in fMRI (45), supporting the idea that seizure physiology may be integral to antidepressant response to ECT (57,58). Taken together, existing ECT neuroimaging literature provides evidence supporting potentially significant roles for the hippocampus and other MTL structures, networks relevant to seizure physiology, and regions previously linked with depressive neurobiology. A comprehensive understanding of how these regions and circuits/networks interact during ECT will be key to understanding the mechanisms of antidepressant response to ECT and other treatments.
In this review, we discuss neuroimaging evidence for neuroplastic change after ECT, and contextualize this evidence within mechanistic models of antidepressant response to ECT. We discuss evidence suggesting that some changes (particularly large, macro-anatomical changes) in hippocampus and medial temporal lobe (MTL) reflect the nonspecific effects of the electrical stimulus (and resulting electric field in the head). By contrast, evidence most closely related to antidepressant response to ECT implicates plasticity within specific hippocampal subregions, as well as corticolimbic regions and networks previously associated with depressive neurobiology (52,53). We discuss neuroimaging studies measuring brain activity during ECT-induced seizures, and how seizure physiology relates to antidepressant response in therapies like ECT. Regional and circuit-level plasticity after ECT is readily measured by neuroimaging and is therefore the focus of this review; however, these regional and circuit-level changes are supported by molecular/cellular processes like upregulation of trophic factors resulting in increased synapses, neurons, glial, and blood vessels, changes in neurotransmitter and receptor availability, and other factors, all of which are implicated in ECT and depression (38‒40,42,59). Other reviews have discussed molecular/cellular evidence in more detail (60‒62), and we hope that the systems-level evidence and model discussed here will motivate future research leading to truly comprehensive mechanistic models of the antidepressant mechanisms of ECT.
Hippocampal Plasticity after ECT: Epiphenomenon or Core Mechanism?
Increased hippocampal gray matter after ECT is robust and highly replicated across independent MRI studies (15‒29) and meta-/mega-analyses (30‒33,35), with larger effect sizes compared to other brain structures (35). Neuroimaging studies have also noted changes in hippocampal function using BOLD and ASL fMRI, PET and SPECT (45,47,63,64), as well as changes in metabolites with MRS (18,65,66) and associated white matter tracts with diffusion MRI (48,67). These results are consistent with the neurotrophic theory of depression (59,68), which is supported by evidence of downregulated trophic factors (59,68,69) and associated hippocampal and cortical atrophy (70) in depressed patients. Animal models of ECT also report upregulation of a number of trophic processes, including the creation of new synapses, neurons, glia, and blood vessels (38‒41), and upregulation of BDNF and other trophic factors (36,37,42). Correspondingly, human ECT studies also report increased BDNF and other trophic factors after ECT (36,37), as well as modulation of cytokines (71), both of which are markers of cellular plasticity that likely correspond with macroanatomical changes noted in MRI research [(72), cf. (73)]. Thus, increased hippocampal gray-matter and other forms of neuroplasticity after ECT are consistent with current neurotrophic theories of depression and antidepressant response (52,53), and the robustness and reproducibility of these findings constitute a promising advancement in neuropsychiatry research. Indeed, ECT-MRI research is in an excellent position to elucidate the role of the hippocampus in the neurobiology of depression and antidepressant response.
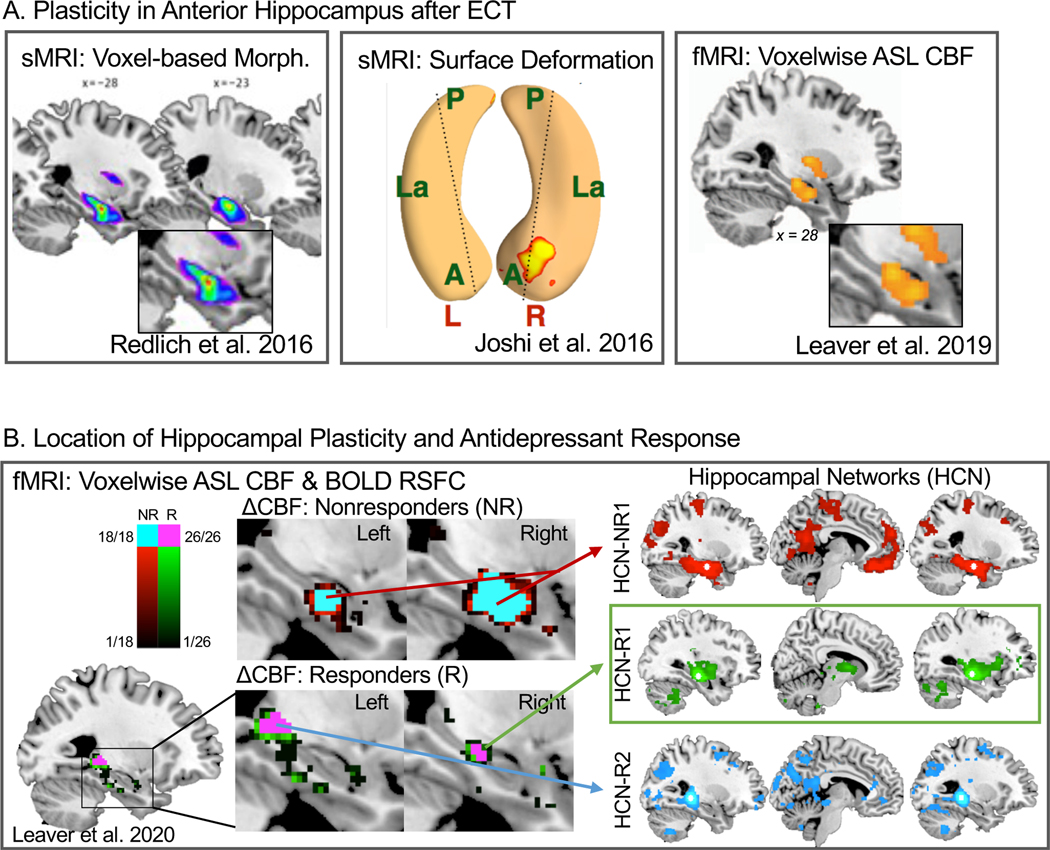
There is well-established, systematic heterogeneity along the anterior-posterior axis of the hippocampus in connectivity, function, gene expression, cell subtypes, and other factors, even within its canonical subfields (i.e., cornu ammonis, dentate gyrus, subiculum, etc.) (74–80). Correspondingly, the hippocampus is unlikely to be affected uniformly by depressive neurobiology or by ECT and other antidepressant therapies, and thus measuring the entire volume of the hippocampus or its subfields may be suboptimal, as has been done in previous studies reporting decreased total hippocampal volume in depressed patients and increases after ECT and other treatments. Indeed, some ECT-MRI studies have demonstrated that plasticity may localize to anterior hippocampus (e.g., Redlich et al. in Figure 2A) when taking a voxel-wise approach ((17,20,21,26,81,82), cf. (24,83) where regional specificity is more difficult to assess). Consistent with these results, Joshi et al. 2016 identified subtle, regional changes in hippocampal shape after ECT in a circumscribed region of right anterior hippocampus using a novel surface-deformation approach (15). In a follow-up analysis, Leaver et al. 2019 measured voxel-wise changes in cerebral blood flow (CBF) measured with ASL fMRI after ECT, and reported increased CBF in right anterior hippocampus near the same region noted in Joshi et al. (45). Together, these studies suggest that hippocampal plasticity after ECT may be regionally specific, and by extension the role of the hippocampus in depressive neurobiology may demonstrate such regional specificity as well (84).
Figure 2.
Hippocampal Plasticity after ECT. In A, representative studies showing that hippocampal plasticity is not uniform after ECT, and occurs preferentially in anterior hippocampal regions. Left panel is adapted with permission from Joshi et al. 2016, which showed significant deformation in the shape of the right anterior hippocampus, progressively increasing after two treatments and after treatment course. Middle panel is adapted with permission (pending) from Redlich et al. 2016, where voxelwise markers of gray-matter volume increased after ECT course in right anterior hippocampus and adjacent regions. In left panel adapted with permission from Leaver et al. 2019, cerebral blood flow (CBF) measured with arterial spin-labelled (ASL) fMRI increased after treatment course in a similar region to the middle and left panels. B is adapted with permission (pending) from Leaver et al. 2020, and displays preliminary evidence that the location(s) of hippocampal plasticity may be different in responders and nonresponders to ECT. Left panels displays a leave-one-out subsampling cross-validation analysis confirming the location of peak change in CBF after ECT separately for nonresponders and responders. Each subregion at left corresponded with separable hippocampal networks, shown at right.
Statistical approach also matters. ECT and other treatments are likely to change the brain regardless of antidepressant response. Tnerefore, studies that examine effects of ECT a priori while testing effects of antidepressant response post hoc are more likely to capture the nonspecific effects of ECT rather than precise effects related to the antidepressant response (35). Correspondingly, studies analyzing the longitudinal effects of ECT on anterior hippocampus or total hippocampal volume (i.e., with time or treatment as the independent variable of interest) have reported inconsistent relationships with antidepressant response measured post hoc. Indeed, some of these studies found that such correlations between symptom change and change in total hippocampal volume (or e.g., total dentate gyms volume) could be more readily explained by other factors also linked with antidepressant response to ECT like age, electrode placement (BT vs. RUL), and number of treatments (25,30,35,43). By contrast, studies targeting antidepressant response to ECT as the primary independent variable (e.g., measuring correlations between symptom change and pre-treatment neurobiology or post-treatment-course plasticity) were much more likely to identify brain regions previously linked to neurological models of depression (52,53) like anterior cingulate (17,50,85), left dorsolateral prefrontal cortex (49), and other corticolimbic regions (17,43,49,85‒91).
Taken together, these studies suggest that the role of the hippocampus in antidepressant response may be best studied with direct a-priori examination of antidepressant response, and when measuring regional change within the hippocampus and its subfields (i.e., rather than total volume). For example, recent preliminary evidence from our group suggests the location of hippocampal plasticity differs when responders and nonresponders to ECT were analyzed separately (45,47) (Figure 2B). Nonresponders (i.e., <50% improvement in depression scores) showed increased CBF in bilateral anterior hippocampus overlapping the amygdala (45,47), and widespread bilateral increased hippocampal gray matter volume (47). By contrast, responders (>50% improvement) showed increased CBF in right mid and left posterior hippocampus, and increased gray matter volume isolated to right amygdala and anterior hippocampus (47). These locations of regional CBF change associated with different functional networks measured with resting-state BOLD fMRI, one of which was preferentially modulated during and after ECT in responders, connecting the thalamus and basal ganglia with mid-anterior hippocampus (47). In diffusion MRI, microstructural markers in hippocampal tracts originating in right mid-anterior hippocampus also correlated with antidepressant response to ECT (48). These results corroborate reports from other groups that connectivity within subregional hippocampal circuits correlate with antidepressant response to ECT (46,92).
In sum, some aspects of hippocampal plasticity after ECT (e.g., total volume change) may be epiphenoma reflecting the nonspecific effects of the treatment itself. However, core antidepressant mechanisms of ECT may involve neuroplastic change within specific subregions of the hippocampus and corresponding extrinsic connections, which vary along the length of the structure. In other words, hippocampal plasticity in antidepressant response (and hippocampal dysfunction/atrophy in the neurobiology of depression) is unlikely to affect the hippocampus uniformly and is unlikely to occur in isolation from the other brain regions and networks. Given the robustness and replicability of total hippocampal volume (and total subfield volume) changes after ECT (15‒35), future independent studies that measure regional changes within the hippocampus and MTL (e.g., using standard voxelwise or shape-deformation methods, ultra-high resolution MRI, and/or rodent and nonhuman primate research (93)), concurrent with changes occurring elsewhere within hippocampal/corticolimUic circuits, and while examining antidepressant response directly (i.e., rather than pre- vs. post-treatment effects) are in an excellent position to elucidate the role of the hippocampus in the neurobiology of depression.
The hippocampus and MTL are also involved in memory for past events and other information, as well as other cognitive processes like spatial navigation, planning, and sequence processing (94). ECT is associated with transient cognitive difficulties during treatment, and in some cases episodic memory complaints persist after treatment course, particularly after bitemporal treatment (5‒7). To our knowledge, robust, consistent relationships between hippocampal change and cognitive side effects have not been identified in ECT (47,95,96), perhaps because relatively few such studies exist and cognitive measures vary across studies. The great majority of patients in these naturalistic studies also receive RUL ECT, which confers lower risk of cognitive side effects. However, it is worth noting that the same issues that complicate studying antidepressant outcomes (as described above) may also apply when assessing the neurobiology of cognitive outcomes. Studies that target correlations between cognitive/memory changes after ECT and granular, regional changes within hippocampus/MTL and associated networks are needed.
Relevance of Seizure Physiology
ECT and other seizure therapies are unique amongst brain stimulation treatments, because in addition to the exogenous electrical (or other) stimulus, they activate a kind of endogenous stimulus, a generalized tonic-clonic (convulsive) seizure. Thus, each ECT session involves a cascade of neurofunctional events, beginning with general anesthesia, application of the electrical stimulus, the initiation, generalization, and termination of the seizure, and ending with post-ictal recovery. Each of these has profound effects on brain activity, and may play independent and/or interdependent roles in long-term neuroplasticity associated with ECT outcomes like improved symptoms or cognitive side effects. Thus, understanding the antidepressant mechanisms of ECT requires understanding the neurophysiological consequences of each part of the treatment session itself. In this section, we discuss evidence describing which brain regions and networks are engaged during each seizure stage and discuss parallels with longitudinal MRI research.
Overview.
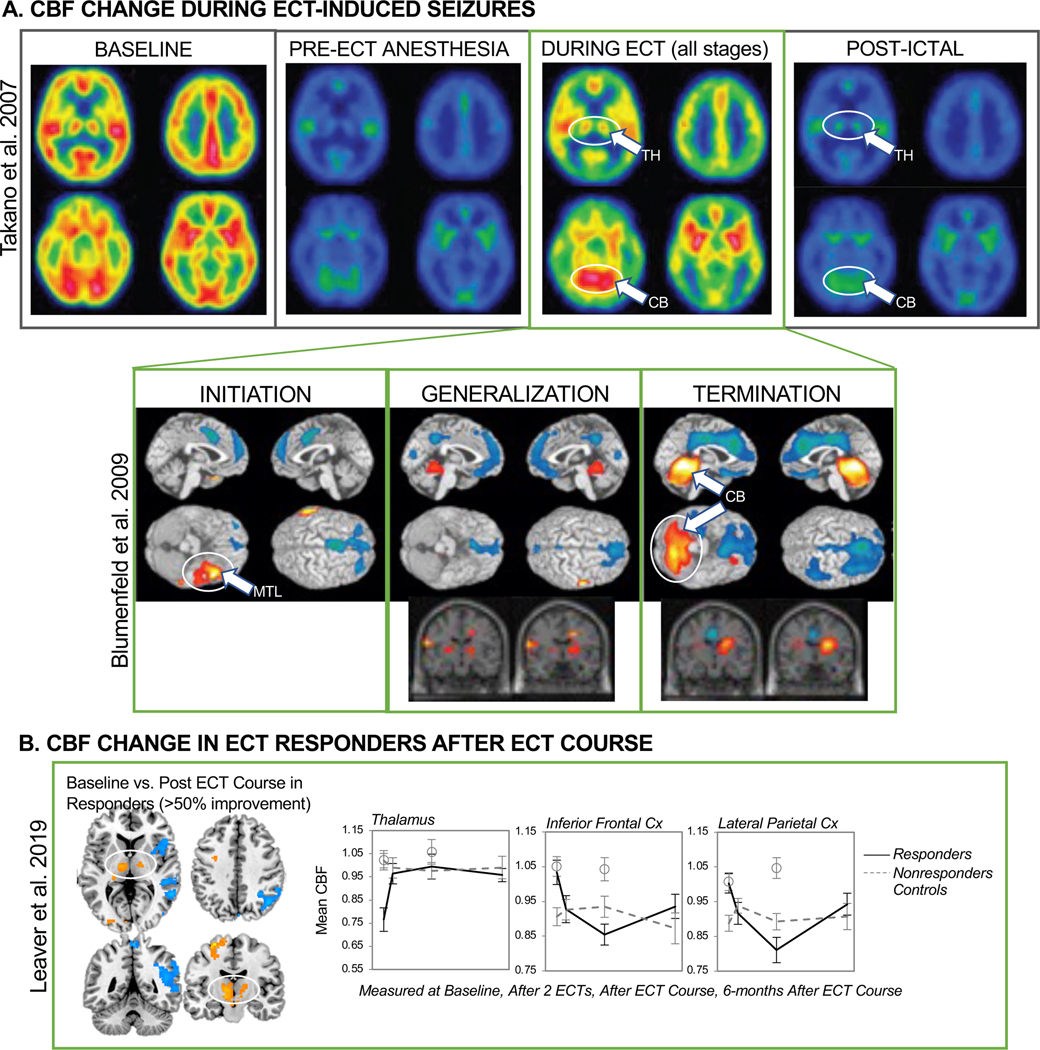
Ictal neuroimaging studies have shown a consistent progression of brain activity during the ECT session and during generalized seizures in epilepsy, using electroencephalography (EEG), positron emission tomography (PET), and single photon emission computed tomography (SPECT). PET/SPECT studies have demonstrated widespread decreased brain activity immediately prior to ECT stimulation associated with generalized anesthesia, corresponding with widespread decreased EEG amplitude across channels (55). In the seconds during and immediately after delivery of the ECT stimulus, increased brain activity has been noted in medial temporal lobe(s) near electrode sites (97,98). This is interpreted as reflecting the site of seizure initiation and is supported by similar studies with radiotracer contrast injections applied at the start of secondarily generalized seizures in patients with epilepsy (where location associates with seizure focus; (56,99)). When PET/SPECT radiotracer injections are given during seizure generalization, activity in thalamus and adjacent subcortical structures increases (55,56,97,98,100,101), and somewhat widespread increases in cortical activity are noted (55,97,98,102). This corresponds well with the timing of increased spike-and-wave discharges across most or all EEG recording electrodes and motor symptoms associated with seizure generalization. During seizure termination, increased activity has been noted in the cerebellum in PET/SPECT studies (55,56). Decreased cortical activity has also been reported during seizure termination in PET/SPECT studies (97,98,103,104), which extends into the post-ictal period corresponding with post-ictal depression measured with EEG (55,104,105). The following sections discuss ictal neuroimaging findings with respect to longitudinal ECT-MRI findings.
Seizure Initiation.
Early ictal activity during RUL and BT ECT measured with SPECT occurs in MTL near electrodes (97,98), corresponding with early ictal activity measured with SPECT in secondarily generalized seizures in patients with epilepsy (i.e., seizure focus (56)). Notably, these locations also overlap locations of post-ECT plasticity in MTL measured with MRI discussed above, as well as computer simulations of electric fields (E-fields) applied during ECT and MST (106,107) (Figure 1). Indeed, there is evidence that greater E-field strength may associate with greater plasticity after treatment in some regions (108) and with antidepressant response (44), though other treatment-related factors like seizure duration may also play a role (109). Taken together, these studies suggest that electrode placement may determine the site(s) of seizure initiation, and E-field estimations may be a way of understanding and even controlling where seizures are initiated during ECT. Future studies are needed to establish whether the location and/or intensity of early ictal brain activity correlates with post-treatment change, and whether these phenomena can be manipulated to improve outcomes (e.g., patient-tailored dosing including electrode position, stimulus amplitude, and/or waveform (110‒112)).
Seizure Generalization.
Robust seizure generalization appears to be important for successful antidepressant response to RUL and BT ECT, as stimulation at or below the seizure threshold is not sufficient to ameliorate depressive symptoms (4,113). During generalized seizures, spike-and-wave discharges are noted at all (or most) EEG channels (114), and increased cortical activity has been noted in PET/SPECT studies (100,102), with spatial distributions varying with seizure focus (56,99,104) or ECT electrode placement (97,98,115). Ictal PET/SPECT studies have also consistently noted increased thalamic and upper-brainstem activity during seizure generalization (which EEG is less able to detect) (54‒,56,97,98,100,101), extending into the post-ictal and inter-ictal periods (54‒56). This pattern matches CBF change noted after ECT in Leaver et al. 2019, where responders to ECT exhibited decreased frontoparietal activity with increased activity in bilateral thalamus (45). Notably, in Leaver et al. 2019 thalamic CBF was much lower in responders prior to treatment, which suggests that robust recruitment of the thalamus during seizure generalization may be important for outcome, and that low thalamic CBF prior to treatment may be a predictive biomarker for treatment response to ECT (45,116) and a potential target for pre-treatment manipulation to improve outcome. Of note, sub-anesthetic ketamine alters thalamocortical activity in depressed and nondepressed volunteers both during infusion (117) and after treatment (118,119); studies exploring overlap in the systems-level mechanisms of ketamine and seizure therapy could be informative. Taken together, this evidence supports the idea that the thalamus, adjacent subcortical structures like the hypothalamus (120), and thalamocortical networks may be important in seizure generalization (e.g., centrencephalic theory, (121)) and in successful antidepressant response to ECT (54,58) and other fast acting antidepressant therapies.
Seizure Termination.
It is well documented that cortical activity decreases after therapeutic seizures in ECT and after pathological seizures in epilepsy (103), a phenomenon called post-ictal suppression. This is evidenced by decreased EEG amplitude and decreased cortical activity in PET/SPECT measured after seizure termination (97,98,103,104), and decreased consciousness or awareness in patients. Some studies have linked antidepressant response with the strength of post-ictal suppression measured with PET (115) and EEG (114), motivating the development of the “anticonvulsant model” or “GABA hypothesis” of ECT (57). Indeed, our own work mirrors this effect, reporting decreased CBF in frontoparietal cortex after a treatment course in RUL-ECT responders (45). However, though the original theory was initially linked with increased seizure threshold over treatment course (57), larger scale studies have not replicated this association (122,123). Furthermore, GABA metabolites measured with magnetic resonance spectroscopy (MRS) have also not consistently linked GABA with antidepressant response to ECT (124,125), and post-ictal suppression and decreased brain activity in PET/SPECT are not as apparent in MST (126,127) cf. (128). Together, these studies suggest that widespread cortical inhibitory or GABAergic processes may not be of primary relevance to antidepressant response, and other modulatory “anticonvulsant” processes might be at play (14).
For example, brain regions like the cerebellum and thalamus may be involved in stopping seizures. In animal models of epilepsy, the cerebellum is active during seizure termination (129), and cerebellar stimulation stops ongoing seizure activity in animal models (130,131) and has been studied as a treatment for intractable epilepsy in patients (132–134). This corresponds well with ECT and epilepsy neuroimayiny studies that reported increased cerebellar activity during seizure termination and post-ictally (56,99). Blumenfeld et al. also reported a correlation between increased cerebellar activity and decreased prefrontal activity postictally (56), while Deppiny et al. reported structural changes in the cerebellum after ECT course that correlated with antidepressant response (135). Increased thalamic activity also appears to persist during seizure termination and the post-ictal period (54–56,97,98,100,101), and increased thalamic CBF after treatment course correlates with antidepressant response to ECT (45). Thalamus DBS has also been studied to prevent or stop seizure activity in intractable epilepsy (136,137). Together, these studies suggest that circuits involviny the thalamus, cerebellum, and cortex may be important to both seizure termination and antidepressant response to ECT. The cerebellum and cortex are connected in a series of topographically organized “closed-loop” circuits via thalamic and cerebellar nuclei, where a specific cerebellar region will both send and receive projections to a specific cortical region (138,139). Thus, the cerebellum may inhibit generalized seizure activity in frontoparietal cortex via its efferent connections with the thalamus (138,139) during ECT, and the efficiency or strength of these circuit-level “anti-convulsant” processes may be important for successful antidepressant response to ECT. Indeed, both the cerebellum and the thalamus have been previously implicated in depressive neurobiology (140–142); whether the role of these structures in seizure physiology overlaps with their dysfunction in depression (and/or temporal lobe epilepsy) may be an interesting avenue of future research.
Network Model of Seizure Therapy
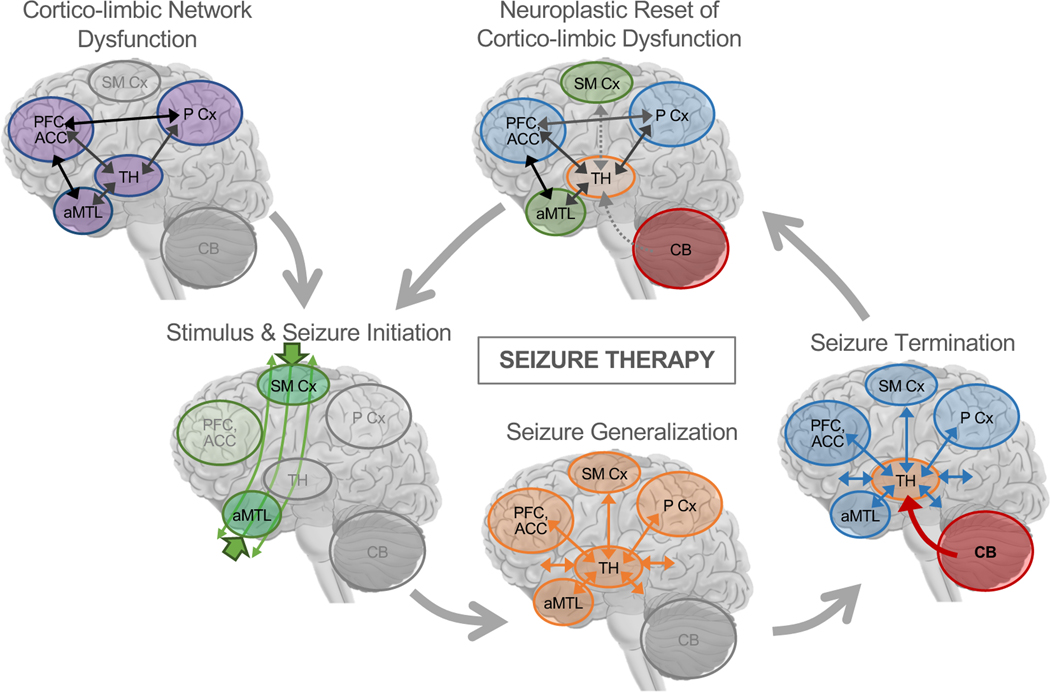
The neurobiology of depression is widely thought to involve circuit-level dysfunction in corticolimbic networks, including structures like hippocampus and amygdala (52,53). Based on the neuroimaging evidence discussed here, we propose that seizure therapies improve symptoms by correcting or “resetting” this dysfunction, through the action of cerebellar-thalamo-cortical circuits on these dysfunctional corticolimbic circuits (Figure 4). During seizure initiation, the electrical stimulus passes through the head (106,107) and initiates seizure activity in antero-hippocampal corticolimbic circuits (97,98), which are dysfunctional due to disease state (52,53). This seizure activity spreads to corticothalamic networks during seizure generalization (54–56,97,98,100,101), and ends when the cerebellum inhibits generalized cortical seizure activity (56,99) via its connections with the thalamus (138,139), perhaps with greater inhibitory control needed in corticolimbic regions/networks where seizure activity initiated. Over repeated sessions of seizure therapy, these processes constitute a neuroplastic correction or reset of corticolimbic dysfunction in depression. Under this hypothesized network model of ECT, successful outcomes would occur when the “correct” antero-hippocampal corticolimUic networks are targeted by the electrical stimulus and efficient seizure activity is initiated there, and when subsequent seizure generalization and termination in thalamocortical networks and cerebellum are effective (e.g., seizure activity and/or seizure-termination signals are neither too weak nor too strong). Poor outcomes could occur at any point in this putative series of events, each offering different opportunities for intervention and/or further study. For example:
Figure 4.
A Mechanistic Network Model of Therapeutic Seizures. Prior to treatment, patients may have network-level dysfunction in corticolimbic circuits involving anterior medial temporal lobe (aMTL) structures like hippocampus and amygdala, medial and lateral prefrontal cortex (PFC), subgenual and dorsal anterior cingulate cortex (ACC), medial and lateral Parietal Cortex (P Cx), thalamus, and other related structures like ventral striatum previously implicated in neurobiological models and studies of depression (52,53). At the beginning of ECT, the electrical stimulus passes through the head, and localized seizure activity begins near electrode sites. During seizure generalization, synchronized brain activity increases in thalamocortical networks across the brain, perhaps most strongly in regions near electrodes. During seizure termination, cerebellar circuits inhibit generalized seizure activity in thalamo-cortical networks, again perhaps with greater inhibitory control needed in brain regions near electrodes. Over repeated sessions of seizure therapy, these processes constitute a neuroplastic correction or “reset” of corticolimbic dysfunction 1 in depression. When used to target dysfunction in other networks, therapeutic seizure processes in cerebellar-thalamocortical networks may have similar effects on dysfunction in these other networks (e.g., bifrontal ECT to target prefrontal network dysfunction in schizophrenia or depression). Figure adapted with permission [pending] from Leaver et al. 2020 Molecular Psychiatry.
Inefficient seizure initiation could occur if the path of electrical current does not reach the “correct” regions effectively (e.g., due to inter-patient variability in head size, skull thickness, or cortical morphology). For example, if primary dysfunction or depression “biotype” (143‒145) occurs outside antero-hippocampal circuits, even adequate stimulus delivery may not have the desired effects (i.e., if primary dysfunction occurs elsewhere in the brain). Or, engagement of certain MTL circuits may confer greater risk of lasting cognitive/memory side effects. In these cases, adjusting electrode position or size could improve seizure induction in target antero-hippocampal or other networks (110,111).
Inefficient seizure initiation could also occur if target corticolimbic tissue is resistant (or hyper-responsive) to the electrical stimulus, for example due to inter-patient variability in neurofunctional or neurochemical state prior to treatment (e.g., due to past/current medications or other factors). Here, interventions that modulate excitability (e.g., noninvasive neurostimulation, pharmacological, or behavioral) could make target networks more (or less) amenable to seizure induction, improving clinical outcomes.
Ineffective seizure generalization and/or termination could also occur. High thalamic activity prior to treatment (45) could prevent thalamic activity from increasing during seizure generalization in nonresponders (i.e., given ictal neuroimaging evidence of increased thalamic activity during seizure generalization and termination (54‒56,97,98.100,101)). Lowering thalamic activity prior to treatment, or perhaps personalizing stimulus waveform to each patient’s thalamocortical oscillation frequency (112), could improve seizure morphology and clinical outcomes. Similar hypotheses could be applied to the cerebellum and its efferent connections with thalamus and cortex (138); further study of cerebellar plasticity and antidepressant response to ECT will be informative.
Notably, this model could explain the success of different methods of seizure induction for the same disorder (146,147), as well as the success of seizure therapy across different disorders. These processes also require coordination of many different events on different scales (molecular/cellular/regional/systems), and a thorough assessment of these other factors is a topic of future review and/or research, e.g., regarding centrencephalic neurotransmitter systems, neurotransmitter receptor expression across the brain, regional upregulation of trophic factors or markers of neurogenesis in hippocampal subregions in animal models, and other phenomena.
Conclusions
In this review, we have discussed evidence suggesting that subregional changes within MTL and associated cortical-limbic networks could be core mechanisms of successful antidepressant response to ECT (46–48,92). We also discussed evidence that networks relevant to seizure physiology could be important to ECT outcomes, particularly networks involving the thalamus and cerebellum (45,56,99,135). Despite the questions that remain, it is notable and potentially significant that such a well-replicated finding, i.e., structural plasticity in hippocampus and MTL after ECT, is tied to such an effective psychiatric treatment. The ECT-MRI field is poised to make major contributions to our understanding of antidepressant response not just in the context of therapeutic seizures, but with respect to systems- and network-level changes occurring with the panoply of antidepressant interventions. This is particularly true given the highly collaborative nature of the field as evidenced by the creation and success of the Global ECT MRI Consortium and the new genetics ECT consortium (148,149). We hope that the evidence and questions presented here will inform future studies where treatment parameters are examined and adjusted to improve outcome using a personalized medicine approach (111,150), or where other types of brain stimulation are developed to similar effect in depression and other disorders.
Figure 3.
Evidence of thalamo-cortical and cerebellar changes during ECT-induced seizures and after ECT course. In A, top row is adapted with permission from Takano et al. 2007, who showed increased cerebral blood flow in thalamus and cerebellum during ECT-induced seizures that persisted post-ictally using PET. Bottom row is adapted from Blumenfeld et al. 2009, who used radiotracer injections near the beginning, middle, and end of secondarily generalized pathological seizures to capture changes in CBF associated with seizure initiation, generalization, and termination, respectively using SPECT in patients with epilepsy. They showed increased CBF in MTL at initiation, increased thalamic CBF at generalization and termination, and increased (decreased) CBF in cerebellum (cortex) during seizure termination. In B, this pattern thalamocortical modulation in CBF is apparent in patients who improved after ECT course, after two treatments, after treatment course, and 6 months after treatment. Leaver et al. 2019 did not analyze cerebellar CBF. Panel B was adapted with permission from Leaver et al. 2019.
ACKNOWLEDGEMENTS
This work was supported by the NIH, including R01MH092301 and U01MH110008 to K.L.N and R.E., R03MH121769 to A.M.L., K24MH102743 to K.L.N., and K99MH119314 to B.W., Muriel Harris Chair (Geriatric Psychiatry) to R.E., and Brain and Behavior Research Foundation including 2015 & 2020 Young Investigator award to A.M.L. and 2018 Young Investigator award to B.W.
Footnotes
CONFLICT OF INTEREST
All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Dierckx B, Heijnen WT, van den Broek WW, Birkenhäger TK (2012): Efficacy of electroconvulsive therapy in bipolar versus unipolar major depression: a meta-analysis. Bipolar Disord 14: 146–150. [DOI] [PubMed] [Google Scholar]
- 2.Fink M (2014): What was learned: studies by the consortium for research in ECT (CORE) 1997–2011. Acta Psychiatr Scand 129: 417–426. [DOI] [PubMed] [Google Scholar]
- 3.Husain MM, Rush AJ, Fink M, Knapp R, Petrides G, Rummans T, et al. (2004): Speed of response and remission in major depressive disorder with acute electroconvulsive therapy (ECT): a Consortium for Research in ECT (CORE) report. J Clin Psychiatry 65: 485–491. [DOI] [PubMed] [Google Scholar]
- 4.Kellner CH, Pritchett JT, Beale MD, Coffey CE (1997): Handbook of ECT. Washington, D.C.: American Psychiatric Press. [Google Scholar]
- 5.Lisanby SH, Maddox JH, Prudic J, Devanand DP, Sackeim HA (2000): The Effects of Electroconvulsive Therapy on Memory of Autobiographical and Public Events. Arch Gen Psychiatry 57: 581–590. [DOI] [PubMed] [Google Scholar]
- 6.Sackeim HA, Prudic J, Fuller R, Keilp J, Lavori PW, Olfson M (2007): The Cognitive Effects of Electroconvulsive Therapy in Community Settings [no. 1]. Neuropsychopharmacology 32: 244–254. [DOI] [PubMed] [Google Scholar]
- 7.Sackeim HA, Prudic J, Nobler MS, Fitzsimons L, Lisanby SH, Payne N, et al. (2008): Effects of pulse width and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. Brain Stimulat 1: 71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sackeim HA, Prudic J, Devanand DP, Nobler MS, Lisanby SH, Peyser S, et al. (2000): A prospective, randomized, double-blind comparison of bilateral and right unilateral electroconvulsive therapy at different stimulus intensities. Arch Gen Psychiatry 57: 425. [DOI] [PubMed] [Google Scholar]
- 9.Kellner CH, Husain MM, Knapp RG, McCall WV, Petrides G, Rudorfer MV, et al. (2016): Right Unilateral Ultrabrief Pulse ECT in Geriatric Depression: Phase 1 of the PRIDE Study. Am J Psychiatry 173: 1101–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loo CK, Sainsbury K, Sheehan P, Lyndon B (2008): A comparison of RUL ultrabrief pulse (0.3 ms) ECT and standard RUL ECT. Int J Neuropsychopharmacol 11: 883–890. [DOI] [PubMed] [Google Scholar]
- 11.Kellner CH, Knapp R, Husain MM, Rasmussen K, Sampson S, Cullum M, et al. (2010): Bifrontal, bitemporal and right unilateral electrode placement in ECT: randomised trial. Br J Psychiatry J Ment Sci 196: 226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bailine SH, Rifkin A, Kayne E, Selzer JA, Vital-Herne J, Blieka M, Pollack S (2000): Comparison of Bifrontal and Bitemporal ECT for Major Depression. Am J Psychiatry 157: 121–123. [DOI] [PubMed] [Google Scholar]
- 13.Lisanby SH, Peterchev AV (2007): Magnetic Seizure Therapy for the Treatment of Depression. Transcranial Brain Stimul Treat Psychiatr Disord 23: 155–171. [Google Scholar]
- 14.Lisanby SH, Deng Z-D (2015): Magnetic Seizure Therapy for the Treatment of Depression. Brain Stimulation. John Wiley & Sons, Ltd, pp 123–148. [Google Scholar]
- 15.Joshi SH, Espinoza RT, Pirnia T, Shi J, Wang Y, Ayers B, et al. (2015): Structural plasticity of the hippocampus and amygdala induced by electroconvulsive therapy in major depression. Biol Psychiatry 79: 282–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tendolkar I, Beek M, Oostrom I, Mulder M, Janzing J, Voshaar RO, Eijndhoven P (2013): Electroconvulsive therapy increases hippocampal and amygdala volume in therapy refractory depression: a longitudinal pilot study. Psychiatry Res 214: 197–203. [DOI] [PubMed] [Google Scholar]
- 17.Redlich R, Opel N, Grotegerd D, Dohm K, Zaremba D, Bürger C, et al. (2016): Prediction of Individual Response to Electroconvulsive Therapy via Machine Learning on Structural Magnetic Resonance Imaging Data. JAMA Psychiatry 73: 557. [DOI] [PubMed] [Google Scholar]
- 18.Cano M, Martínez-Zalacaín I, Bernabéu-Sanz Á, Contreras-Rodriguez O, Hernández-Ribas R, Via E, et al. (2017): Brain volumetric and metabolic correlates of electroconvulsive therapy for treatment-resistant depression: a longitudinal neuroimaging study. Transl Psychiatry 7: e1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yrondi A, Nemmi F, Billoux S, Giron A, Sporer M, Taib S, et al. (2019): Grey Matter changes in treatment-resistant depression during electroconvulsive therapy. J Affect Disord 258: 42–49. [DOI] [PubMed] [Google Scholar]
- 20.Bouckaert F, De Winter F-L, Emsell L, Dols A, Rhebergen D, Wampers M, et al. (2016): Grey matter volume increase following electroconvulsive therapy in patients with late life depression: a longitudinal MRI study. J Psychiatry Neurosci JPN 41: 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dukart J, Regen F, Kherif F, Colla M, Bajbouj M, Heuser I, et al. (2014): Electroconvulsive therapy-induced brain plasticity determines therapeutic outcome in mood disorders. Proc Natl Acad Sci U S A 111: 1156–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gbyl K, Rostrup E, Raghava JM, Andersen C, Rosenberg R, Larsson HBW, Videbech P (2021): Volume of hippocampal subregions and clinical improvement following electroconvulsive therapy in patients with depression. Prog Neuropsychopharmacol Biol Psychiatry 104: 110048. [DOI] [PubMed] [Google Scholar]
- 23.Nuninga JO, Mandl RCW, Boks MP, Bakker S, Somers M, Heringa SM, et al. (2019): Volume increase in the dentate gyrus after electroconvulsive therapy in depressed patients as measured with 7T. Mol Psychiatry. 10.1038/s41380-019-0392-6 [DOI] [PubMed] [Google Scholar]
- 24.Ota M, Noda T, Sato N, Okazaki M, Ishikawa M, Hattori K, et al. (2015): Effect of electroconvulsive therapy on gray matter volume in major depressive disorder. J Affect Disord 186: 186–191. [DOI] [PubMed] [Google Scholar]
- 25.Takamiya A, Plitman E, Chung JK, Chakravarty M, Graff-Guerrero A, Mimura M, Kishimoto T (2019): Acute and long-term effects of electroconvulsive therapy on human dentate gyrus. Neuropsychopharmacology. 10.1038/s41386-019-0312-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolf RC, Nolte HM, Hirjak D, Hofer S, Seidl U, Depping MS, et al. (2016): Structural network changes in patients with major depression and schizophrenia treated with electroconvulsive therapy. Eur Neuropsychopharmacol 26: 1465–1474. [DOI] [PubMed] [Google Scholar]
- 27.Sartorius A, Demirakca T, Böhringer A, Clemm von Hohenberg C, Aksay SS, Bumb JM, et al. (2016): Electroconvulsive therapy increases temporal gray matter volume and cortical thickness. Eur Neuropsychopharmacol 26: 506–517. [DOI] [PubMed] [Google Scholar]
- 28.Nordanskog P, Dahlstrand U, Larsson MR, Larsson E-M, Knutsson L, Johanson A (2010): Increase in hippocampal volume after electroconvulsive therapy in patients with depression: a volumetric magnetic resonance imaging study. J ECT 26: 62–67. [DOI] [PubMed] [Google Scholar]
- 29.Cao B, Luo Q, Fu Y, Du L, Qiu T, Yang X, et al. (2018): Predicting individual responses to the electroconvulsive therapy with hippocampal subfield volumes in major depression disorder. Sci Rep 8: 5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oltedal L, Narr KL, Abbott C, Anand A, Argyelan M, Bartsch H, et al. 1 (2018): Volume of the Human Hippocampus and Clinical Response Following Electroconvulsive Therapy. Biol Psychiatry 84: 574–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilkinson ST, Sanacora G, Bloch MH (2017): Hippocampal Volume Changes Following Electroconvulsive Therapy: A Systematic Review and Meta-analysis. Biol Psychiatry Cogn Neurosci Neuroimaging 2: 327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gbyl K, Videbech P (2018): Electroconvulsive therapy increases brain volume in major depression: a systematic review and meta-analysis. Acta Psychiatr Scand 138: 180–195. [DOI] [PubMed] [Google Scholar]
- 33.Takamiya A, Chung JK, Liang K, Graff-Guerrero A, Mimura M, Kishimoto T (2018): Effect of electroconvulsive therapy on hippocampal and amygdala volumes: systematic review and meta-analysis. Br J Psychiatry 212: 19–26. [DOI] [PubMed] [Google Scholar]
- 34.Enneking V, Leehr EJ, Dannlowski U, Redlich R (2020): Drain structural effects of treatments for depression and biomarkers of response: a systematic review of neuroimaging studies. Psychol Med 50: 187–209. [DOI] [PubMed] [Google Scholar]
- 35.Ousdal OT, Argyelan M, Narr KL, Abbott C, Wade B, Vandenbulcke M, et al. (2020): Brain Changes Induced by Electroconvulsive Therapy Are Broadly Distributed. Biol Psychiatry 87: 451–461. [DOI] [PubMed] [Google Scholar]
- 36.Fernandes B, Gama CS, Massuda R, Torres M, Camargo D, Kunz M, et al. (2009): Serum brain-derived neurotrophic factor (BDNF) is not associated with response to electroconvulsive therapy (ECT): A pilot study in drug resistant depressed patients. Neurosci Lett 453: 195–198. [DOI] [PubMed] [Google Scholar]
- 37.Bocchio-Chiavetto L, Zanardini R, Dortolomasi M, Abate M, Segala M, Giacopuzzi M, et al. (2006): Electroconvulsive Therapy (ECT) increases serum Drain Derived Neurotrophic Factor (DDNF) in drug resistant depressed patients. Eur Neuropsychopharmacol 16: 620–624. [DOI] [PubMed] [Google Scholar]
- 38.Madsen TM, Treschow A, Bengzon J, Bolwig TG, Lindvall O, Tingström A (2000): Increased neurogenesis in a model of electroconvulsive therapy. Biol Psychiatry 47: 1043–1049. [DOI] [PubMed] [Google Scholar]
- 39.Perera TD, Coplan JD, Lisanby SH, Lipira CM, Arif M, Carpio C, et al. (2007): Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. J Neurosci Off J Soc Neurosci 27: 4894–4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen F, Madsen TM, Wegener G, Nyengaard JR (2009): 1 Repeated electroconvulsive seizures increase the total number of synapses in adult male rat hippocampus. Eur Neuropsychopharmacol 19: 329–338. [DOI] [PubMed] [Google Scholar]
- 41.Hellsten J, West MJ, Arvidsson A, Ekstrand J, Jansson L, Wennström M, Tingström A (2005): Electroconvulsive seizures induce angiogenesis in adult rat hippocampus. Biol Psychiatry 58: 871–878. [DOI] [PubMed] [Google Scholar]
- 42.Duman RS, Aghajanian GK, Sanacora G, Krystal JH (2016): Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med 22: 238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mulders PCR, Llera A, Beckmann CF, Vandenbulcke M, Stek M, Sienaert P, et al. (2020): Structural changes induced by electroconvulsive therapy are associated with clinical outcome. Brain Stimulat 13: 696–704. [DOI] [PubMed] [Google Scholar]
- 44.Fridgeirsson EA, Deng Z-D, Denys D, van Waarde JA, van Wingen GA (2021): Electric field strength induced by electroconvulsive therapy is associated with clinical outcome. Neuroimage Clin 30: 102581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leaver AM, Vasavada M, Joshi SH, Wade B, Woods RP, Espinoza R, Narr KL (2019): Mechanisms of Antidepressant Response to Electroconvulsive Therapy Studied With Perfusion Magnetic Resonance Imaging. Biol Psychiatry 85: 466–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bai T, Wei Q, Xie W, Wang A, Wang J, Ji G-J, et al. (2019): Hippocampal-subregion functional alterations associated with antidepressant effects and cognitive impairments of electroconvulsive therapy. Psychol Med 49: 1357–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leaver AM, Vasavada M, Kubicki A, Wade B, Loureiro J, Hellemann G, et al. (2020): Hippocampal subregions and networks linked with antidepressant response to electroconvulsive therapy. Mol Psychiatry 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kubicki A, Leaver AM, Vasavada M, Njau S, Wade B, Joshi SH, et al. (2019): Variations in Hippocampal White Matter Diffusivity Differentiate Response to Electroconvulsive Therapy in Major Depression. Biol Psychiatry Cogn Neurosci Neuroimaging 4: 300–309. [DOI] [PubMed] [Google Scholar]
- 49.Leaver AM, Wade B, Vasavada M, Hellemann G, Joshi SH, Espinoza R, Narr KL (2018): Fronto-Temporal Connectivity Predicts ECT Outcome in Major Depression. Front Psychiatry 9. 10.3389/fpsyt.2018.00092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leaver AM, Espinoza R, Pirnia T, Joshi SH, Woods RP, Narr KL (2016): Modulation of Intrinsic Brain Activity by Electroconvulsive Therapy in Major Depression. Biol Psychiatry Cogn Neurosci Neuroimaging 1: 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pirnia T, Joshi SH, Leaver AM, Vasavada M, Njau S, Woods RP, et al. (2016): Electroconvulsive therapy and structural neuroplasticity in neocortical, limbic and paralimbic cortex. Transl Psychiatry 6: e832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mayberg HS (1997): Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci 9: 471–481. [DOI] [PubMed] [Google Scholar]
- 53.Price JL, Drevets WC (2010): Neurocircuitry of mood disorders. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol 35: 192–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McNally KA, Blumenfeld H (2004): Focal network involvement in generalized seizures: new insights from electroconvulsive therapy. Epilepsy Behav 5: 3–12. [DOI] [PubMed] [Google Scholar]
- 55.Takano H, Motohashi N, Uema T, Ogawa K, Ohnishi T, Nishikawa M, et al. (2007): Changes in regional cerebral blood flow during acute electroconvulsive therapy in patients with depression: Positron emission tomographic study. Br J Psychiatry 190: 63–68. [DOI] [PubMed] [Google Scholar]
- 56.Blumenfeld H, Varghese GI, Purcaro MJ, Motelow JE, Enev M, McNally KA, et al. (2009): Cortical and subcortical networks in human secondarily generalized tonic-clonic seizures. Brain 132: 999–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sackeim HA, Decina P, Prohovnik I, Malitz S, Resor SR (1983): Anticonvulsant and antidepressant properties of electroconvulsive therapy: a proposed mechanism of action. Biol Psychiatry 18: 1301–1310. [PubMed] [Google Scholar]
- 58.Bolwig TG (2011): How does electroconvulsive therapy work? Theories on its mechanism. Can J Psychiatry Rev Can Psychiatr 56: 13–18. [DOI] [PubMed] [Google Scholar]
- 59.Duman RS, Heninyer GR, Nestler EJ (1997): A Molecular and Cellular Theory of Depression. Arch Gen Psychiatry 54: 597–606. [DOI] [PubMed] [Google Scholar]
- 60.Sharma AN, da Costa e Silva BFB, Soares JC, Carvalho AF, Quevedo J (2016): Role of trophic factors GDNF, IGF-1 and VEGF in major depressive disorder: A comprehensive review of human studies. J Affect Disord 197: 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kato N (2009): Neurophysiological mechanisms of electroconvulsive therapy for depression. Neurosci Res 64: 3–11. [DOI] [PubMed] [Google Scholar]
- 62.Fosse R, Read J (2013): Electroconvulsive Treatment: Hypotheses about Mechanisms of Action. Front Psychiatry 4. 10.3389/fpsyt.2013.00094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abbott CC, Jones T, Lemke NT, Gallegos P, McClintock SM, Mayer AR, et al. (2014): Hippocampal structural and functional changes associated with electroconvulsive therapy response. Transl Psychiatry 4: e483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McCormick LM, Boles Ponto LL, Pierson RK, Johnson HJ, Magnotta V, Brumm MC (2007): Metabolic Correlates of Antidepressant and Antipsychotic Response in Patients With Psychotic Depression Undergoing Electroconvulsive Therapy. J ECT 23: 265–273. [DOI] [PubMed] [Google Scholar]
- 65.Njau S, Joshi SH, Espinoza R, Leaver AM, Vasavada M, Marquina A, et al. (2017): Neurochemical correlates of rapid treatment response to electroconvulsive therapy in patients with major depression. J Psychiatry Neurosci JPN 42: 6–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Njau S, Joshi SH, Leaver AM, Vasavada M, Fleet J, Espinoza R, Narr KL (2016): Variations in myo-inositol in fronto-limbic regions and clinical response to electroconvulsive therapy in major depression. J Psychiatr Res 80: 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lyden H, Espinoza RT, Pirnia T, Clark K, Joshi SH, Leaver AM, et al. (2014): Electroconvulsive therapy mediates neuroplasticity of white matter microstructure in major depression. Transl Psychiatry 4: e380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Molendijk ML, Spinhoven P, Polak M, Bus B a. A, Penninx BWJH, Elzinga BM (2014): Serum BDNF concentrations as peripheral manifestations of depression: evidence from a systematic review and meta-analyses on 179 associations (N=9484). Mol Psychiatry 19: 791–800. [DOI] [PubMed] [Google Scholar]
- 69.Diniz BS, Teixeira AL, Machado-Vieira R, Talib LL, Gattaz WF, Forlenza OV (2013): Reduced serum nerve growth factor in patients with late-life depression. Am J Geriatr Psychiatry Off J Am Assoc Geriatr Psychiatry 21: 493–496. [DOI] [PubMed] [Google Scholar]
- 70.Sheline YI, Sanghavi M, Mintun MA, Gado MH (1999): Depression Duration But Not Age Predicts Hippocampal Volume Loss in Medically Healthy Women with Recurrent Major Depression. J Neurosci 19: 5034–5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kruse JL, Congdon E, Olmstead R, Njau S, Breen EC, Narr KL, et al. (2018): Inflammation and Improvement of Depression Following Electroconvulsive Therapy in Treatment-Resistant Depression. J Clin Psychiatry 79: 17m11597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Van Den Bossche MJA, Emsell L, Dols A, Vansteelandt K, De Winter F-L, Van den Stock J, et al. (2019): Hippocampal volume change following ECT is mediated by rs699947 in the promotor region of VEGF. Transí Psychiatry 9: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bouckaert F, Dols A, Emsell L, De Winter F-L, Vansteelandt K, Claes L, et al. (2016): Relationship Between Hippocampal Volume, Serum BDNF, and Depression Severity Following Electroconvulsive Therapy in Late-Life Depression. Neuropsychopharmacol Off Publ Am Coíí Neuropsychopharmacol 41: 2741–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Strange BA, Witter MP, Lein ES, Moser EI (2014): Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci 15: 655–669. [DOI] [PubMed] [Google Scholar]
- 75.Poppenk J, Evensmoen HR, Moscovitch M, Nadel L (2013): Long-axis specialization of the human hippocampus. Trends Cogn Sci 17: 230–240. [DOI] [PubMed] [Google Scholar]
- 76.Dalton MA, McCormick C, Maguire EA (2019): Differences in functional connectivity along the anterior-posterior axis of human hippocampal subfields. Neuroimage 192: 38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Poppenk J (2020): Anatomically guided examination of extrinsic connectivity gradients in the human hippocampus. Cortex 128: 312–317. [DOI] [PubMed] [Google Scholar]
- 78.Przezdźik I, Faber M, Fernández G, Beckmann CF, Haak KV (2019): The functional organisation of the hippocampus along its long axis is gradual and predicts recollection. Cortex 119: 324–335. [DOI] [PubMed] [Google Scholar]
- 79.Flores R de, Berron D, Ding S-L, Ittyerah R, Pluta JB, Xie L, et al. (2020): Characterization of hippocampal subfields using ex vivo MRI and histology data: Lessons for in vivo segmentation. Hippocampus 30: 545–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Duvernoy Cattin F (2005): The Human Hippocampus: Functional Anatomy, Vascularization and Serial Sections with MRI., 3rd ed. Springer. [Google Scholar]
- 81.Thomann PA, Wolf RC, Nolte HM, Hirjak D, Hofer S, Seidl U, et al. (2017): Neuromodulation in response to electroconvulsive therapy in schizophrenia and major depression. Brain Stimulat 10: 637–644. [DOI] [PubMed] [Google Scholar]
- 82.Gyger L, Regen F, Ramponi C, Marquis R, Mall J-F, Swierkosz-Lenart K, et al. (2021): Gradient of electroconvulsive therapy’s antidepressant effects along the longitudinal hippocampal axis. Transl Psychiatry 11: 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Qiu H, Li X, Zhao W, Du L, Huang P, Fu Y, et al. (2016): Electroconvulsive Therapy-Induced Brain Structural and Functional Changes in Major Depressive Disorders: A Longitudinal Study. Med Sci Monit Int Med J Exp Clin Res 22: 4577–4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Neumeister A, Wood S, Bonne O, Nugent AC, Luckenbaugh DA, Young T, et al. (2005): Reduced hippocampal volume in unmedicated, remitted patients with major depression versus control subjects. Biol Psychiatry 57: 935–937. [DOI] [PubMed] [Google Scholar]
- 85.Argyelan M, Lencz T, Kaliora S, Sarpal DK, Weissman N, Kingsley PB, et al. (2016): Subgenual cingulate cortical activity predicts the efficacy of electroconvulsive therapy. Transl Psychiatry 6: e789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.van Waarde JA, Verwey B, van der Mast RC (2009): Meta-analysis of initial seizure thresholds in electroconvulsive therapy. Eur Arch Psychiatry Clin Neurosci 259: 467. [DOI] [PubMed] [Google Scholar]
- 87.Wade BSC, Sui J, Hellemann G, Leaver AM, Espinoza RT, Woods RP, et al. (2017): Inter and intra-hemispheric structural imaging markers predict depression relapse after electroconvulsive therapy: a multisite study. Transl Psychiatry 7: 1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tsolaki E, Narr KL, Espinoza R, Wade B, Hellemann G, Kubicki A, et al. (2020): Subcallosal Cingulate Structural Connectivity Differs in Responders and Nonresponders to Electroconvulsive Therapy. Biol Psychiatry Cogn Neurosci Neuroimaging. 10.1016/7j.bpsc.2020.05.010 [DOI] [PubMed] [Google Scholar]
- 89.Schmitgen MM, Kubera KM, Depping MS, Nolte HM, Hirjak D, Hofer S, et al. (2020): Exploring cortical predictors of clinical response to electroconvulsive therapy in major depression. Eur Arch Psychiatry Clin Neurosci 270: 253–261. [DOI] [PubMed] [Google Scholar]
- 90.Sun H, Jiang R, Qi S, Narr KL, Wade BS, Upston J, et al. (2020): Preliminary prediction of individual response to electroconvulsive therapy using whole-brain functional magnetic resonance imaging data. Neuroimage Clin 26: 102080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Waarde JA, Scholte HS, Oudheusden LJB, Verwey B, Denys D, Wingen GA (2015): A functional MRI marker may predict the outcome of electroconvulsive therapy in severe and treatment-resistant depression. Mol Psychiatry 20: 609–614. [DOI] [PubMed] [Google Scholar]
- 92.Takamiya A, Kishimoto T, Hirano J, Nishikata S, Sawada K, Kurokawa S, et al. (2020): Neuronal network mechanisms associated with depressive symptom improvement following electroconvulsive therapy. Psychol Med 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Peterchev AV, Krystal AD, Rosa MA, Lisanby SH (2015): Individualized Low-Amplitude Seizure Therapy: Minimizing Current for Electroconvulsive Therapy and Magnetic Seizure Therapy. Neuropsychopharmacology 40: 2076–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lisman J, Buzsáki G, Eichenbaum H, Nadel L, Ranganath C, Redish AD (2017): Viewpoints: how the hippocampus contributes to memory, navigation and cognition. Nat Neurosci 20: 1434–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Laroy M, Bouckaert F, Vansteelandt K, Obbels J, Dols A, Emsell L, et al. (2019): Association between hippocampal volume change and change in memory following electroconvulsive therapy in late-life depression. Acta Psychiatr Scand 140: 435–445. [DOI] [PubMed] [Google Scholar]
- 96.Nordanskog P, Larsson MR, Larsson E-M, Johanson A (2014): Hippocampal volume in relation to clinical and cognitive outcome after electroconvulsive therapy in depression. Acta Psychiatr Scand 129: 303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Blumenfeld H, Westerveld M, Ostroff RB, Vanderhill SD, Freeman J, Necochea A, et al. (2003): Selective frontal, parietal, and temporal networks in generalized seizures. NeuroImage 19: 1556–1566. [DOI] [PubMed] [Google Scholar]
- 98.Blumenfeld H, McNally KA, Ostroff RB, Zubal IG (2003): Targeted prefrontal cortical activation with bifrontal ECT. Psychiatry Res 123: 165–170. [DOI] [PubMed] [Google Scholar]
- 99.Varghese GI, Purcaro MJ, Motelow JE, Enev M, McNally KA, Levin AR, et al. (2009): Clinical use of ictal SPECT in secondarily generalized tonic-clonic seizures. Brain 132: 2102–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Enev M, McNally KA, Varghese G, Zubal IG, Ostroff RB, Blumenfeld H (2007): Imaging onset and propagation of ECT-induced seizures. Epilepsia 48: 238–244. [DOI] [PubMed] [Google Scholar]
- 101.Takano H, Motohashi N, Uema T, Ogawa K, Ohnishi T, Nishikawa M, Matsuda H (2011): Differences in cerebral blood flow between missed and generalized seizures with electroconvulsive therapy: A positron emission tomographic study. Epilepsy Res 97: 225–228. [DOI] [PubMed] [Google Scholar]
- 102.Bajc M, Medved V, Basic M, Topuzovic N, Babic D, Ivancevic D (1989): Acute effect of electroconvulsive therapy on brain perfusion assessed by Tc99m-hexamethylpropyl-eneamineoxim and single photon emission computed tomography. Acta Psychiatr Scand 80: 421–426. [DOI] [PubMed] [Google Scholar]
- 103.Mazziotta JC, Engel J (1984): The Use and Impact of Positron Computed Tomography Scanning in Epilepsy. Epilepsia 25: S86–S104. [DOI] [PubMed] [Google Scholar]
- 104.Engel J, Kuhl DE, Phelps ME (1982): Patterns of human local cerebral 1 glucose metabolism during epileptic seizures. Science 218: 64–66. [DOI] [PubMed] [Google Scholar]
- 105.Nobler MS, Oquendo MA, Kegeles LS, Malone KM, Campbell CC, Sackeim HA, Mann JJ (2001): Decreased regional brain metabolism after ect. Am J Psychiatry 158: 305–308. [DOI] [PubMed] [Google Scholar]
- 106.Lee WH, Lisanby SH, Laine AF, Peterchev AV (2016): Comparison of electric field strength and spatial distribution of electroconvulsive therapy and magnetic seizure therapy in a realistic human head model. Eur Psychiatry J Assoc Eur Psychiatr 36: 55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bai S, Martin D, Guo T, Dokos S, Loo C (2019): Computational comparison of conventional and novel electroconvulsive therapy electrode placements for the treatment of depression. Eur Psychiatry J Assoc Eur Psychiatr 60: 71–78. [DOI] [PubMed] [Google Scholar]
- 108.Argyelan M, Oltedal L, Deng Z-D, Wade B, Bikson M, Joanlanne A, et al. (2019): Electric field causes volumetric changes in the human brain. eLife 8: e49115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Takamiya A, Bouckaert F, Laroy M, Blommaert J, Radwan A, Khatoun A, et al. (2021): Biophysical mechanisms of electroconvulsive therapy-induced volume expansion in the medial temporal lobe: A longitudinal in vivo human imaging study. Brain Stimulat 14: 1038–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Spellman T, Peterchev AV, Lisanby SH (2009): Focal Electrically Administered Seizure Therapy: A Novel form of ECT Illustrates the Roles of Current Directionality, Polarity, and Electrode Configuration in Seizure Induction. Neuropsychopharmacology 34: 2002–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Abbott CC, Quinn D, Miller J, Ye E, Iqbal S, Lloyd M, et al. (2021): Electroconvulsive Therapy Pulse Amplitude and Clinical Outcomes. Am J Geriatr Psychiatry 29: 166–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zaehle T, Rach S, Herrmann CS (2010): Transcranial Alternating Current Stimulation Enhances Individual Alpha Activity in Human EEG. PLOS ONE 5: e13766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Folkerts H (1996): The ictal electroencephalogram as a marker for the efficacy of electroconvulsive therapy. Eur Arch Psychiatry Clin Neurosci 246: 155–164. [DOI] [PubMed] [Google Scholar]
- 114.Perera TD, Luber B, Nobler MS, Prudic J, Anderson C, Sackeim HA (2004): Seizure expression duringelectroconvulsive therapy: relationships with clinical outcome and cognitive side effects. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol 29: 813–825. [DOI] [PubMed] [Google Scholar]
- 115.Nobler MS (1994): Regional Cerebral Blood Flow in Mood Disorders, III. Arch Gen Psychiatry 51: 884. [DOI] [PubMed] [Google Scholar]
- 116.Takamiya A, Kishimoto T, Liang K, Terasawa Y, Nishikata S, Tarumi R, et al. (2019): Thalamic volume, resting-state activity, and their association with the efficacy of electroconvulsive therapy. J Psychiatr Res 117: 135–141. [DOI] [PubMed] [Google Scholar]
- 117.Deakin JFW, Lees J, McKie S, Hallak JEC, Williams SR, Dursun SM (2008): Glutamate and the Neural Basis of the Subjective Effects of Ketamine: A Pharmaco‒Magnetic Resonance Imaging Study. Arch Gen Psychiatry 65: 154–164. [DOI] [PubMed] [Google Scholar]
- 118.Gonzalez S, Vasavada MM, Njau S, Sahib AK, Espinoza R, Narr KL, Leaver AM (2020): Acute changes in cerebral blood flow after single-infusion ketamine in major depression: A pilot study. Neurol Psychiatry Brain Res 38: 5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Evans JW, Szczepanik J, Brutsché N, Park LT, Nugent AC, Zarate CA (2018): Default Mode Connectivity in Major Depressive Disorder Measured Up to 10 Days After Ketamine Administration. Biol Psychiatry 84: 582–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ferland RJ, Applegate CD (1998): The role of the ventromedial nucleus of the hypothalamus in epileptogenesis. Neuroreport 9: 3623–3629. [DOI] [PubMed] [Google Scholar]
- 121.Penfield W, Jasper H (1954): Epilepsy and the Functional Anatomy of the Human Brain. Oxford, England: Little, Brown & Co., pp xv, 896. [Google Scholar]
- 122.Duthie AC, Perrin JS, Bennett DM, Currie J, Reid IC (2015): Anticonvulsant Mechanisms of Electroconvulsive Therapy and Relation to Therapeutic Efficacy. J ECT 31: 173–178. [DOI] [PubMed] [Google Scholar]
- 123.Coffey CE, Lucke J, Weiner RD, Krystal AD, Aque M (1995): Seizure threshold in electroconvulsive therapy (ECT) II. The anticonvulsant effect of ECT. Biol Psychiatry 37: 777–788. [DOI] [PubMed] [Google Scholar]
- 124.Erchinger VJ, Miller J, Jones T, Kessler U, Bustillo J, Haavik J, et al. (2020): Anterior cingulate gamma-aminobutyric acid concentrations and electroconvulsive therapy. Brain Behav 10: e01833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Knudsen MK, Near J, Blicher AB, Videbech P, Blicher JU (2019): Magnetic resonance (MR) spectroscopic measurement of Y-aminobutyric acid (GABA) in major depression before and after electroconvulsive therapy. Acta Neuropsychiatr 31: 17–26. [DOI] [PubMed] [Google Scholar]
- 126.Lisanby SH, Luber B, Schlaepfer TE, Sackeim HA (2003): Safety and Feasibility of Magnetic Seizure Therapy (MST) in Major Depression: Randomized Within-Subject Comparison with Electroconvulsive Therapy [no. 10]. Neuropsychopharmacology 28: 1852–1865. [DOI] [PubMed] [Google Scholar]
- 127.Kayser S, Bewernick B, Matusch A, Hurlemann R, Soehle M, Schlaepfer T (2014) Magnetic seizure therapy in treatment-resistant depression: clinical, neuropsychological and metabolic effects. Psychol Med. 10.1017/S0033291714002244 [DOI] [PubMed] [Google Scholar]
- 128.Kayser S, Bewernick BH, Soehle M, Switala C, Gippert SM, Dreimueller N, Schlaepfer TE (2017): Degree of Postictal Suppression Depends on Seizure Induction Time in Magnetic Seizure Therapy and Electroconvulsive Therapy. J ECT 33: 167–175. [DOI] [PubMed] [Google Scholar]
- 129.Salgado-Benftez A, Briones R, FernanUez-GuarUiola A (1982): Purkinje Cell Responses to a Cerebral Penicillin-Induced Epileptogenic Focus in the Cat. Epilepsia 23: 597–606. [DOI] [PubMed] [Google Scholar]
- 130.Krook-Magnuson E, Szabo GG, Armstrong C, Oijala M, Soltesz I (2014): Cerebellar Directed Optogenetic Intervention Inhibits Spontaneous Hippocampal Seizures in a Mouse Model of Temporal Lobe Epilepsy. eNeuro 1. 10.1523/ENEURO.0005-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Streny ML, Krook-Magnuson E (2020): Excitation, but not inhibition, of the fascial nucleus provides powerful control over temporal lobe seizures. J Physiol 598: 171–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cooper IS, Amin I, Riklan M, Waltz JM, Poon TP (1976): Chronic Cerebellar Stimulation in Epilepsy: Clinical and Anatomical Studies. Arch Neurol 33: 559–570. [DOI] [PubMed] [Google Scholar]
- 133.Cooper IS, Crighel E, Amin I (1973): Clinical and Physiological Effects of Stimulation of the Paleocerebellum in Humans. J Am Geriatr Soc 21: 40–43. [DOI] [PubMed] [Google Scholar]
- 134.Wright GD, McLellan DL, Brice JG (1984): A double-blind trial of chronic cerebellar stimulation in twelve patients with severe epilepsy. J Neurol Neurosurg Psychiatry 47: 769–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Depping MS, Nolte HM, Hirjak D, Palm E, Hofer S, Stieltjes B, et al. (2017): Cerebellar volume change in response to electroconvulsive therapy in patients with major depression. Prog Neuropsychopharmacol Biol Psychiatry 73: 31–35. [DOI] [PubMed] [Google Scholar]
- 136.Upton ARM, Amin I, Garnett S, Springman M, Nahmias C, Cooper IS (1987): Evoked Metabolic Responses in the LimbicStriate System Produced by Stimulation of Anterior Thalamic Nucleus in Man. Pacing Clin Electrophysiol 10: 217–225. [DOI] [PubMed] [Google Scholar]
- 137.Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R, et al. (2010): Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia 51: 899–908. [DOI] [PubMed] [Google Scholar]
- 138.Bostan AC, Strick PL (2018): The basal ganglia and the cerebellum: nodes 1 in an integrated network. Nat Rev Neurosci 19: 338–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kelly RM, Strick PL (2003): Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci Off J Soc Neurosci 23: 8432–8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Depping MS, Schmitgen MM, Kubera KM, Wolf RC (2018): Cerebellar Contributions to Major Depression. Front Psychiatry 9: 634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Escalona PR, Early B, McDonald WM, Doraiswamy PM, Shah SA, Husain MM, et al. (1993): Reduction of cerebellar volume in major depression: A controlled MRI study. Depression 1: 156–158. [Google Scholar]
- 142.Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, et al. (2007): Resting-State Functional Connectivity in Major Depression: Abnormally Increased Contributions from Subgenual Cingulate Cortex and Thalamus. Biol Psychiatry 62: 429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wade BSC, Hellemann G, Espinoza RT, Woods RP, Joshi SH, Redlich R, et al. (2020): Depressive Symptom Dimensions in Treatment-Resistant Major Depression and Their Modulation With Electroconvulsive Therapy. J ECT 36: 123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dinga R, Schmaal L, Penninx BWJH, van Tol MJ, Veltman DJ, van Velzen L, et al. (2019): Evaluating the evidence for biotypes of depression: Methodological replication and extension of Drysdale et al. (2017). NeuroImage Clin 22: 101796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, et al. (2017): Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med 23: 28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fink M (2014): The seizure, not electricity, is essential in convulsive therapy: the flurothyl experience. J ECT 30: 91–93. [DOI] [PubMed] [Google Scholar]
- 147.Cooper K, Fink M (2014): The chemical induction of seizures in psychiatric therapy: were flurothyl (indoklon) and pentylenetetrazol (metrazol) abandoned prematurely? J Clin Psychopharmacol 34: 602–607. [DOI] [PubMed] [Google Scholar]
- 148.Soda T, McLoughlin DM, Clark SR, Oltedal L, Kessler U, Haavik J, et al. (2020): International Consortium on the Genetics of Electroconvulsive Therapy and Severe Depressive Disorders (Gen-ECT-ic). Eur Arch Psychiatry Clin Neurosci 270: 921–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Oltedal L, Bartsch H, Sørhaug OJE, Kessler U, Abbott C, Dols A, et al. (2017): The Global ECT-MRI Research Collaboration (GEMRIC): Establishing a multi-site investigation of the neural mechanisms mechanisms underlying response to electroconvulsive therapy. NeuroImage Clin 14: 422–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ousdal OT, Brancati GE, Kessler U, Erchinger V, Dale AM, Abbott C, Oltedal L (2021): The neurobiological effects of electroconvulsive therapy studied through magnetic resonance – what have we learnt and where do we go? Biol Psychiatry 0. 10.1016/j.biopsych.2021.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]