Abstract
Cartilage injury and degeneration are hallmarks of osteoarthritis (OA), the most common joint disease. OA is a major contributor to pain, loss of function, and reduced quality of life. Over the last decade, considerable research efforts have focused on cell-based therapies, including several stem cell-derived approaches to reverse the cartilage alterations associated with OA. Although several tissue sources for deriving cell-based therapies have been identified, none of the resident stem cell populations have adequately fulfilled the promise of curing OA. Indeed, many cell products do not contain true stem cells. As well, issues with aggressive marketing efforts, combined with a lack of evidence regarding efficacy, lead the several national regulatory bodies to discontinue the use of stem cell therapy for OA until more robust evidence becomes available. A review of the evidence is timely to address the status of cell-based cartilage regeneration. The promise of stem cell therapy is not new and has been used successfully to treat non-arthritic diseases, such as hematopoietic and muscle disorders. These fields of regenerative therapy have the advantage of a considerable foundation of knowledge in the area of stem cell repair mechanisms, the role of the stem cell niche, and niche-supporting cells. This foundation is lacking in the field of cartilage repair. So, where should we look for the ideal stem cell to regenerate cartilage? It has recently been discovered that cartilage itself may contain a population of SC-like progenitors. Other potential tissues include stem cell-rich dental pulp and the adolescent growth plate, the latter of which contains chondrocyte progenitors essential for producing the cartilage scaffold needed for bone growth. In this article, we review the progress on stem cell therapies for arthritic disorders, focusing on the various stem cell populations previously used for cartilage regeneration, successful cases of stem cell therapies in muscle and hemopoietic disorders, some of the reasons why these other fields have been successful (i.e., “lessons learned” to be applied to OA stem cell therapy), and finally, novel potential sources of stem cells for regenerating damaged cartilage in vivo.
Keywords: cartilage, regenerative medicine, growth plate, osteoarthritis, musculoskeletal health, stem cells
Introduction
Osteoarthritis (OA) is a major health concern, affecting more than 50% of adults over the age of 65 (Loeser, 2010). OA contributes significantly to pain, disability, and rising healthcare costs (Cross et al., 2014). The average annual cost per person afflicted with OA is as high as €23,000, a massive sum considering the millions of individuals affected with OA worldwide. In the United States, the annual cost of OA is >$16.5 billion, accounting for >4% of the combined costs for all hospitalizations (CDC Prevention, 2021). OA of the hip and knee contribute the most to OA burden, often resulting in joint replacement surgery, including >1 million annual joint replacements in the United States and roughly 1,60,000 in the United Kingdom (Centers for Disease Control and Prevention, 2010; Cross et al., 2014; Registry, 2020). It is predicted that OA will soon be the fourth most disabling chronic disease in the world, and OA is the fastest growing major health condition (Silverwood et al., 2015). The burden of OA has, therefore, become an urgent international healthcare issue.
OA is a total joint disease, but the end result is the complete loss of articular cartilage. The presence of early cartilage defects is a strong risk factor for OA progression (Guermazi et al., 2017; Everhart et al., 2019). In the OA-affected joint, the products of cartilage breakdown that are released into the synovial fluid are phagocytosed by synovial cells, amplifying synovial inflammation (Sellam and Berenbaum, 2010). In turn, activated synovial cells in the inflamed synovium produce catabolic and pro-inflammatory mediators, such as interleukins 1 and 6 and tumor necrosis factor-α (Sellam and Berenbaum, 2010). This inflammatory response is amplified by activated synovial T cells, B cells, and infiltrating macrophages (Sellam and Berenbaum, 2010). The resulting inflammatory milieu leads to the secretion of matrix-degrading enzymes from chondrocytes, further propagating tissue breakdown and creating a positive feedback loop as joint degeneration continues and progresses (Sellam and Berenbaum, 2010; Mata et al., 2017). To date, there are no accepted disease-modifying OA drugs (DMOADs) to slow OA progression; therefore, treatment has been aimed at reducing symptoms (McAlindon et al., 2014). Analgesic medications such as acetaminophen and nonsteroidal anti-inflammatory drugs do not alter OA-related degeneration. Though some studies evaluating cartilage-based treatment with nutritional supplements such as glucosamine and chondroitin sulfate have suggested these treatments reduce pain and delay in structural progression, other studies have shown equivocal results (Towheed et al., 2009; Bruyère et al., 2016), generating equipoise as to whether these nutritional supplements should be recommended (Hochberg et al., 2012; McAlindon et al., 2014). Antibodies targeting pain pathways, such as tanezumab, have shown some benefits in clinical trials but are believed to contribute to rapidly-progressive OA in a notable proportion of treated individuals, thus they are presently excluded from OA treatment guidelines (Schnitzer et al., 2019; Berenbaum et al., 2020). In addition to changes in cartilage, other articular tissues are affected by OA, including the synovium, ligament, and bone (Barr et al., 2015). Treatments, such as strontium ranelate, directed at preventing pathologic bone alterations have shown some positive effects on clinical outcomes such as pain and disability (Reginster et al., 2013; Bruyère et al., 2016), and possibly structural progression (Roubille et al., 2015); however, these results have not been widely accepted, nor has strontium ranelate been approved for OA treatment (Hochberg et al., 2012; McAlindon et al., 2014).
Surgical strategies to halt the progression from cartilage defect to the development of OA have been limited and prone to failure (Palmer et al., 2019; Zamborsky and Danisovic, 2020). Marrow-stimulation procedures, such as microfracture, rely on the development of a primitive mesenchymal blood clot that often forms fibrous tissue with variable patient outcomes (Haleem et al., 2010). Osteochondral grafting limitations include donor site availability, morbidity, and fibrocartilage formation between osteochondral plugs (Redman et al., 2005; Haleem et al., 2010). Once OA is established, treatment is essentially palliative (McAlindon et al., 2014). As such, research has turned to stem cell-based therapies to slow and/or reverse OA, an area of research that has expanded dramatically over the last decade (Koelling and Miosge, 2009; Yubo et al., 2017; Whittle et al., 2019; Zhang et al., 2019).
Recruiting and Stimulating Endogenous Stem Cells to Treat Osteoarthritis
The development of OA in the joint represents a failure of the endogenous articular repair system to maintain healthy osteochondral units (Goldring and Goldring, 2016). Specific to articular cartilage, native chondrocytes are unable to maintain the extracellular matrix, resulting in cartilage fibrillation, fissuring, and thinning (Pritzker et al., 2006; Sulzbacher, 2013). Over time, cartilage may erode completely, exposing the subchondral bone (Moisio et al., 2009). A complete understanding of the mechanism(s) by which cartilage regeneration fails is lacking; however, the reparative function of the chondrocyte may be disrupted by pathologic changes in the OA joint microenvironment (Sellam and Berenbaum, 2010; Jayasuriya et al., 2016). These changes include increased inflammation along with the production of reactive oxygen species and pro-degradation proteins, including matrix metalloproteinases and ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) (Sellam and Berenbaum, 2010; Jayasuriya et al., 2016). In addition, the subchondral bone becomes more permeable, allowing bone morphogenetic proteins (BMPs) and transforming growth factor β (TGF-β) to diffuse into cartilage from the bone, favoring the terminal differentiation of chondrocytes and osteophyte formation (Zhen et al., 2013; Jayasuriya et al., 2016). As a result, the OA joint microenvironment becomes catabolic, with little support for endogenous cartilage repair. Biomechanical influences also play a role, with overloaded joint compartments experiencing accelerated OA-related structural changes such as osteophyte formation, bone attrition, and deformity, as well as microenvironmental OA alterations and greater overall susceptibility to cartilage injury and loss (Sharma et al., 2001; Guilak, 2011; Chen et al., 2013). Under such circumstances, additional aid must arrive at the site of damage to assist the failing chondrocytes. Mesenchymal stem cells (MSCs) have been proposed as strong candidates for enhancing the articular repair process (McGonagle et al., 2017). MSCs are clonogenic progenitor cells capable of differentiating into mesoderm-derived cells such as osteoblasts, chondrocytes, and adipocytes (Prockop, 1997). One attractive feature of MSCs is that they are found in many tissues of the synovial joints including the bone, synovium, and adipose, representing about 1% of the total cell population (Jones et al., 2010a). As well, endogenous MSCs play a supportive role in the immune system, providing immunomodulation that can either enhance or dampen the inflammatory cascade of the OA articular milieu by adapting their immunoregulatory properties to the local immunological environment (Hoogduijn, 2015; Song et al., 2020a). Through the excretion of cytokines, growth factors, chemokines, and cell–cell contact, MSCs exert their immunomodulatory effect on immune cells, such as T and B cells, natural killer (NK) cells, macrophages, monocytes, dendritic cells (DCs), and neutrophils, thus exerting a potentially potent effect on the local immune response (Song et al., 2020a). With this in mind, exogenous MSCs have been used to treat inflammatory conditions such as graft-versus-host disease, graft rejection, and autoimmune diseases (Müller et al., 2021). To our knowledge, however, capitalizing on the immunomodulatory capabilities of joint-resident MSCs has not yet been attempted for the treatment of OA.
MSCs were shown to accumulate in greater numbers in the regions of the damaged OA bone (Campbell et al., 2016). Directing such subchondral MSC populations to effectively restore the joint microenvironment and repair OA-associated cartilage damage would present a powerful therapeutic target to slow or halt OA progression, particularly if initiated early in the disease (McGonagle et al., 2017). McGonagle and Jones reviewed the potential origins of such reparative MSCs, noting that the MSC native environment of origin (niche) is critical to their function (McGonagle et al., 2017). As an example, synovium-derived MSCs showed superior chondrogenic potential as compared to those derived from bone or subcutaneous fat (Sakaguchi et al., 2005; Mochizuki et al., 2006; Koga et al., 2008). Another attractive characteristic of synovial-resident MSCs is that they have direct access to the synovial fluid, which in turn gives facile migratory access to the superficial layer of cartilage. Early OA-associated damage occurring in the superficial cartilage layers create an anatomic challenge for bone marrow-resident MSCs to reach the site of injury; as it requires their migration through the deeper, undamaged cartilage layers to reach the site in need of repair (McGonagle et al., 2017). Synovial MSCs, on the other hand, would have direct access to the site of injury via the articular space in order to initiate repair. This repair pathway was supported by experiments in a canine model showing that synovium-derived MSCs are able to adhere to the areas of cartilage injury (Wood et al., 2012).
Perhaps the most obvious endogenous progenitor cell population for cartilage repair is that which resides within cartilage itself, as recently reviewed by Rikkers et al.( 2022). Termed articular cartilage-derived progenitor cells (ACPCs), these cells most likely reside in the superficial zone in healthy cartilage and will migrate toward the sites of cartilage injury (Grogan et al., 2009; Williams et al., 2010). These cells show similar markers to those of MSCs (CD90, CD105, CD73, and CD166) (Rikkers et al., 2022), possibly distinguished phenotypically by an increased expression of CD44 and enhanced expression of fibronectin and integrin-α5β1 (Dowthwaite et al., 2004; Levato et al., 2017), suggesting a unique progenitor cell population. Correspondingly, OA joint-derived ACPCs were shown to form more colonies in vitro compared to those from healthy human cartilage, suggesting greater proliferation capacity with increasing OA severity (Wang et al., 2020; Rikkers et al., 2022). An increase in progenitor markers, such as CD271 (Wang et al., 2020), CD105 (Zhang et al., 2016), and VCAM (Grogan et al., 2009) at sites of trauma or in OA cartilage were also observed. Though these data suggest the involvement of ACPCs in cartilage repair, in vivo models outlining corresponding mechanisms are lacking, leaving the role(s) of these progenitor cells in cartilage repair and homeostasis unclear at this time (Rikkers et al., 2022).
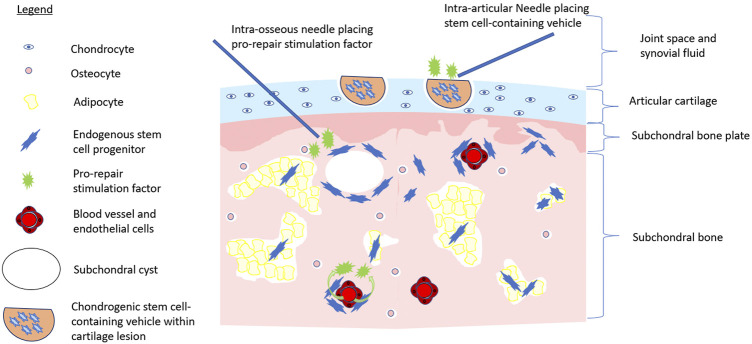
One major hurdle faced by relying on endogenous MSCs to repair cartilage is enhancing their repair capacity. Clearly, the phenotype/reparative potential of these cells in OA patients is inadequate to halt disease progression. Causative factors include irreversible factors such as age. Bone marrow-resident MSCs decline functionally with age (Sethe et al., 2006; Oh et al., 2014), a distinct disadvantage for those with OA, which tends to occur later in life (Felson, 2004). As well, cultured MSCs derived from an inflammatory joint environment have reduced chondrogenic potential in vitro (Jones et al., 2010b). In vivo, the number of MSCs in magnetic resonance imaging (MRI)-determined bone marrow lesions is five-fold greater than non-bone marrow lesion (Campbell et al., 2016); from a functional perspective; however, these MSCs demonstrated reduced proliferative and osteogenic functional capacity, possibly due to cellular fatigue while residing in a chronically damaged trabecular bone niche (Campbell et al., 2016). Restoring the functional capacity of MSCs or ACPCs at the site of tissue damage could provide a large number of repair cells at the site of injury. With respect to cartilage, regrowth following procedures such as microfracture or bone drilling (albeit often suboptimal fibrocartilage) indicates that a population of bone marrow-resident cells exist within the bone marrow that can repair damaged cartilage (Palmer et al., 2019; Zamborsky and Danisovic, 2020). Enhancing the repair capacity of these cells through the intra-osseous application of growth factors or other molecular interventions may represent a viable treatment opportunity (Delgado et al., 2019) (Figure 1). The application of chemotactic factors to the site of injury to augment the recruitment of endogenous MSCs, as well as other cells involved in the cartilage repair and local immune suppression, could be combined with approaches that enhance the chondrogenic function (Song et al., 2020a). Intra-articular treatment targeting synovium-resident MSCs to enhance their chondrogenic capacity and improve their ability to repair cartilage in an inflammatory microenvironment, or the image-guided placement of intra-defect biomaterials that release these factors to reparative cells upon their migration to the cartilage lesion, may be a less invasive option than the intra-osseous treatment of bone marrow-resident MSCs (Figure 1). Strategies such as arthrocentesis that evacuate the pro-inflammatory synovial fluid of the OA joint, followed by the replacement of the synovial fluid with a pro-chondrogenic cellular and molecular cocktail would be ideal. These treatments, in combination with biomechanical interventions that unload the affected joint compartments such as joint distraction or tibial osteotomy, may further help reduce the hostile microenvironment of the OA joint and prevent further direct mechanical injury (McGonagle et al., 2017; Jansen et al., 2021). Such a combination of interventions could provide a better opportunity for tissue-resident MSCs to repair the cartilage damage, than simply relying on cellular function alone. In summary, our understanding of how endogenous MSCs repair or support the repair of damaged tissue, the effect of the local niche, and potential supporting roles of other cell populations in the OA joint is lacking. As a result, strategies utilizing the recruitment and stimulation of endogenous MSCs to repair OA-associated cartilage injury have yet to emerge. Consequentially, many have turned toward the use of exogenous or transplanted MSCs for cartilage repair.
FIGURE 1.
Multistep approach to stem cell-initiated cartilage repair. Image depicts the application of chondrogenic stem cell-containing vehicle (e.g., hyaluronic acid injectate) to a cartilage lesion via intra-articular injection while pro-repair stimulation factors are applied to intra-articular space, via intra-osseous application, or systemically via the bloodstream to activate endogenous repair of the OA joint.
Cell-Based Therapies for Osteoarthritis
Although DMOADs for OA are lacking, cell-based therapies have shown promise in reversing the symptoms and structural alterations of OA (Koelling and Miosge, 2009). Given the chondrogenic potential of MSCs, these cells quickly emerged as candidates. Their enhanced ability to differentiate toward chondrogenic lineages under low oxygen tension (Merceron et al., 2010) is also an advantage, due to the avascular and hypoxic nature of cartilage tissue (Goldring and Goldring, 2016). Cell-based therapies currently proposed for OA include autologous cultured chondrocyte transplantation, co-culture and transplantation of MSCs with chondrocytes or hematopoietic-lineage cells, 3D-MSC cultures, or transplantation of MSC-laden scaffolds made of hyaluronic acid, or other synthetic derivatives (Mamidi et al., 2016; Brittberg et al., 1994). In an effort to reduce heterogeneity in therapeutic MSC populations, the International Society for Cell and Gene Therapy (ISCT) developed a set of minimal criteria to define the MSC phenotype, including adherence to plastic, specific antigen expression (e.g., CD73, CD90, and CD105), and multipotent differentiation potential (Dominici et al., 2006). Early phase I–II clinical trials showed improvement in pain and function following the intra-articular application of MSCs into OA-affected knees (Soler et al., 2016; Jo et al., 2014); however, despite these encouraging findings, exogenous MSCs have yet to emerge as a mainstream player for OA treatment. The drawbacks of MSC therapies include the necessity for cell culture, loss of differentiation capacity ex vivo or with multiple culture passages, and reduced or halted cellular division after multiple population doublings (Mamidi et al., 2016; Jones and Yang, 2011). Such heterogeneity exists in studies using various animal models of OA in preclinical studies (Cope et al., 2019; Wang et al., 2022), as well as clinical trials. Despite these limitations, several clinical trials have recently reported on the efficacy of exogenous MSCs to regenerate cartilage in OA (Table 1) (Emadedin et al., 2018; Kuah et al., 2018; Freitag et al., 2019; Khalifeh Soltani et al., 2019; Lee et al., 2019; Lu et al., 2019; Matas et al., 2019; Shapiro et al., 2019; Anz et al., 2020; Yang et al., 2022). Across these trials, methodologic heterogeneity has hindered a standardized approach to MSC-based therapies, including the use of host source (allogeneic vs. autologous), tissue source (bone marrow, adipose, umbilical cord, and placenta), injectate (tissue concentrates vs. isolated MSCs), whether they were expanded in vitro prior to injection, the dosage used, and the delivery method (e.g., image-guided or not). In addition, not all clinical studies characterized the stem cells being injected, for example, using the ISCT minimal criteria for MSCs (Dominici et al., 2006), making it difficult to ensure what had actually been injected into participants’ joints and impeding the reproducibility of results across studies. Several systematic reviews evaluating the overall efficacy of MSCs for the treatment of OA have been conducted (Kim et al., 2019a; Ha et al., 2019; Song et al., 2020b; Kim et al., 2020) or are underway (Whittle et al., 2019). Although meta-analyses have shown a benefit in pain reduction following MSC intra-articular injection in OA, there is little data supporting their effectiveness for cartilage regeneration. The strength of the evidence supporting their use in clinical practice is, therefore, limited (Kim et al., 2019a; Ha et al., 2019; Song et al., 2020b; Kim et al., 2020). Indeed, many regulatory and scientific bodies, including the United States Food and Drug Administration and Health Canada, have issued position statement warning against the clinical use of these unproven stem cell (SC) therapies for cartilage repair (Marks et al., 2017; CDA, 2019; ISSCR, 2021).
TABLE 1.
Summary of described clinical trials.
| Trial (country) | Sample size | Stem cell source | MSC characterization and laboratory processing | Control | Clinical outcome(s) | Cartilage recovery outcome |
|---|---|---|---|---|---|---|
| Emadedin 2018 (Iran) | 47 | Autologous BM | ISCT criteria tissue culture expansion | Saline | Pain (VAS) function (WOMAC) | NI |
| Kuah 2018 (Aus) | 21 | Allogeneic adipose | No characterization tissue culture expansion | Culture media | Pain (VAS) function (WOMAC) | MRI (MOAKS) |
| Freitag 2019 (Aus) | 30 | Autologous adipose | ISCT criteria tissue culture expansion under hypoxic conditions | Usual care | Pain (NRS) function (KOOS) | MRI (MOAKS) |
| Khalifeh soltani 2019 (Iran) | 20 | Placenta | No characterization tissue culture expansion | Saline | Pain (VAS) function (KOOS) | MRI (cartilage thickness) |
| Lee 2019 (Korea) | 24 | Autologous adipose | Code of federal regulations | Saline | Pain (VAS) function (WOMAC) | MRI (cartilage depth—Noyes grading) |
| Characterization tissue culture expansion | ||||||
| Lu 2019 (China) | 53 | Autologous adipose | ISCT criteria tissue culture expansion | HA | Pain (VAS) function (WOMAC) | MRI (cartilage volume) |
| Matas 2019 (Chile) | 29 | Umbilical cord | ISCT criteria tissue culture expansion | HA | Pain (VAS) function (WOMAC) | MRI (WORMS) |
| Shapiro 2019 (USA) | 25 | Autologous BM | ISCT criteria no processing | Saline | Pain (VAS) | MRI (Mean T2 values) |
| Anz 2020 (USA) | 90 | Autologous bone marrow | No characterization no processing | PRP | Pain (WOMAC) function (WOMAC) | NI |
| Yang 2022 (Korea) | 176 | Umbilical cord | Code of federal regulations | BMAC | Pain (IKDC) function (KOOS) | Arthroscopy (ICRS) |
Aus, Australia; BM, bone marrow; BMAC, bone marrow aspirate concentrate; HA, hyaluronic acid; IKDC, International Knee Documentation Committee questionnaire; ICRS, International Cartilage Repair Society score; KOOS, knee injury and osteoarthritis outcome score; MOAKS, MRI osteoarthritis knee score; MRI, magnetic resonance imaging; NI, not included; NRS, numeric rating scale; PRP, platelet-rich plasma; USA, United States of America; VAS, visual analogue scale; WOMAC, Western Ontario and McMaster Universities osteoarthritis index; WORMS, whole-organ magnetic resonance imaging score.
Despite the aforementioned caveats, clinical studies evaluating MSCs for cartilage repair continue (Clinicaltrial.gov, 2022). Factors such as ease of access, low likelihood of side effects, and potential immune-suppressing characteristics are attractive (Whittle et al., 2019). MSCs also present the opportunity to use allogenic sources, suggesting the possibility of an “off-the-shelf” formulation produced through a standardized good manufacturing process (Sanz-Nogués and O'Brien, 2021). With these attractive features in mind, researchers have sought to enhance MSC function in vivo. Tissue engineering strategies began in the 1990s using scaffolds and matrices to maintain MSCs at the site of injury and potentiate their repair capacity (Mandrycky et al., 2016; Kim et al., 2019b). Three-dimensional encapsulating matrices could safely deliver MSCs to the site of cartilage injury and provide a biologically optimal milieu for MSCs to repair tissue, protecting them from the hostile inflammatory OA joint environment (Kim et al., 2019b). Growth and differentiation factors could be encapsulated within the matrix, providing stimulus toward chondrogenic differentiation (Kim et al., 2019b). Biomaterials using the proteins fibrin and collagen, or polysaccharides such as hyaluronic acid and agarose have the advantage of being derived from endogenous materials, having good biocompatibility and being biodegradable (Li et al., 2015a). Alternatively, synthetic biomaterials such as polylactic acid, polyglycolide, and polyethylene glycol have the advantage of improved mechanical strength and are easier to mold; for example, to match the shape of a cartilage lesion (Matai et al., 2020). In addition, synthetic biomaterials are immunologically-neutral, are not associated with the risk of transmitting pathogens, and have modifiable chemical and mechanical properties as well as the rate of degradation (Mandrycky et al., 2016; Kim et al., 2019b; Matai et al., 2020). Clinical trials have mainly utilized natural biomaterials such as fibrin, and most studies using synthetic materials were performed in animal models. Like MSCs, the ideal scaffold would be available in a standardized off-the-shelf format.
Culture-expanded MSCs may exert a therapeutic effect through immune modulation and via trophic actions on local joint cells through secreted factors without directly participating in new cartilage formation (Mak et al., 2016; Zhang et al., 2022). Consequently, non-cellular biologically-based strategies harnessing the MSC secretome have been explored for cartilage and bone repair (Kalluri and LeBleu, 2020; To et al., 2020; Zhang et al., 2022). MSC-derived extracellular vesicles (MSC-EVs) are MSC-produced nanovesicles ranging from 10 nm to several μm in diameter that contain components such as messenger RNA, microRNA, lipids, and bioactive proteins that produce regenerative paracrine effects within damaged tissue (To et al., 2020). Compared to MSCs, MSC-EVs have the advantage of low toxicity and immunogenicity with repeated transplantation and can be stored for potential off-the-shelf applications (To et al., 2020). A systematic review evaluating the use of MSC-derived extracellular vesicles (MSC-EVs) in preclinical studies for cartilage regeneration showed reduced cartilage loss across a variety of animal models of OA (To et al., 2020). MSC-EVs have been evaluated in preclinical studies for their potential toward bone healing (Kirkham et al., 2021), and a systematic review by Kirkham et al. (2021) reported a promising potential (Kirkham et al., 2021). Though the review focused on fracture healing, pathologic bone changes in the osteochondral unit are well-characterized as a part of OA progression, suggesting that this type of treatment could someday have a role in treating both cartilage and bone-related OA alterations.
In summary, despite important advances in MSC therapy for cartilage regeneration over the last decade, the hunt is still on for the optimal regenerative cell-based approach to repair the cartilage damage associated with OA. In pursuit of the ideal approach, we look to lessons learned from the successes seen in other fields of regenerative medicine, as well as at emerging discoveries of novel chondrogenic stem cell populations, that can be applied to the treatment of OA.
Hematopoietic Stem Cell Therapy: The Importance of Tissue Microenvironments and Parallels for Cartilage Regeneration
Transplantation of donor-derived hematopoietic stem and progenitor cells to regenerate the hematopoietic system has a long and established history that provided a starting point for future generations of cell therapies (Passweg et al., 2021). Cell product characterization ensured an adequate dose and viability of blood-forming stem cells for the recipient (Gauntner et al., 2021). Extensive regulations and standards protect donors and patients and ensure optimal outcomes following allogeneic transplantation. The processes, standards, and regulations that guide the procurement and transplantation of hematopoietic cell products can be leveraged for other cell-based therapies. The use of autologous cells can also rescue hematopoiesis in recipients following the high-dose chemotherapy with lower risks of transmission of infections, and avoids issues such as graft-versus-host disease. Whether allogeneic or autologous cells are used is an important aspect of cell collection and characterization. The cells from young allogeneic donors can reduce transplant complications and improve survival compared with cells from older allogeneic donors (Shaw et al., 2018). The more robust regenerative capacity of younger cells likely accounts for the improved outcomes. Autologous cells in patients who suffer from disease and/or its treatment may yield cell products with compromised function (Choudhery et al., 2012; Yang et al., 2019) and this should be considered in applications such as cartilage regeneration for OA.
A critical aspect of successful hematopoietic engraftment following transplantation relates to the function and status of the bone marrow microenvironment (Morrison and Scadden, 2014). Recipient age, prior therapy, and disease-induced changes in the marrow microenvironment can impair hematopoiesis. MSCs, a chief component of the marrow microenvironment, that are derived from patients with acute myeloid leukemia are abnormal with skewed differentiation potential and reduced ability to support normal hematopoiesis (Chandran et al., 2015; Le et al., 2016). Understanding the tissue microenvironment will be paramount in cell therapies for cartilage regeneration. Strategies restoring the health of the tissue microenvironment may augment the success of cell-based therapies. Exercise, for instance, was shown in a mouse model to accelerate hematopoietic engraftment following transplantation (De Lisio et al., 2013) and nutritional status including the essential amino acids is crucial for robust hematopoiesis (Wilkinson et al., 2018).
A global network of registries of healthy volunteer donors exists to facilitate the collection of blood stem cells to support unrelated allogeneic hematopoietic transplantation. The process of identifying HLA-compatible donors who are healthy and free of transmissible disease is well-established through the World Marrow Donor Association and its connected network of international registries and collection centers (Bochtler et al., 2011). Whether these donors could provide cells and tissues to support other forms of cellular therapy is intriguing. In a recent survey of registrants on the Stem Cell Registry at Canadian Blood Services, many registrants were willing to donate cells for uses other than blood cell transplants (Liao et al., 2020).
Leveraging the clinical experience from hematopoietic transplantation in the areas of product characterization and donor cell procurement may accelerate the translation of stem cell therapy to cartilage degeneration in OA.
Muscle Stem Cell Therapy
The development of cell-replacement therapies for the treatment of muscle wasting diseases has received much attention (Dao et al., 2020). Most efforts focused on the muscle-resident stem cell, termed satellite cells, juxtaposed between the basal membrane, and muscle fiber (Aziz et al., 2012). Since their discovery in the early 1960s (Katz, 1961; Mauro, 1961), considerable knowledge was gained on the role of satellite cells in muscle repair, the influence of the niche in which they reside, as well as the role of other niche-supporting cells (Sousa-Victor et al., 2021). These critical aspects of the repair process are less-well described for cartilage repair (McGonagle et al., 2017). For example, in normal resting muscle, the majority of satellite cells are maintained in a long-lived Pax7-expressing quiescent (G0 reversible arrest) state (Aziz et al., 2012; García-Prat et al., 2016). To ensure tissue homeostasis, satellite cells will repair small myofiber defects by re-entering the cell cycle, undergoing a single asymmetric cell division that generates one differentiating daughter cell that will contribute to the myofiber and one daughter stem cell to replenish the stem cell pool (Aziz et al., 2012; Sousa-Victor et al., 2021). Only after enough progenitor cells have been made to repair the myofiber will a small number of progenitors return to the quiescent state to repopulate the stem cell niche (Cutler et al., 2021; Robinson et al., 2021).
Myogenic regulatory factors (MRFs) contribute to establishing the myogenic cell identity, to the subsequent differentiation and formation of muscle fibers during muscle regeneration in postnatal life (Sousa-Victor et al., 2021). Molecular mechanisms governing the transition between quiescence and activation have been studied and include transcriptional and post-translational, epigenetic (Sousa-Victor et al., 2021), as well as metabolic and proteostatic regulation (Sousa-Victor et al., 2021). In addition, several extrinsic factors, including epidermal growth factor (Roe et al., 1989), hepatocyte growth factor (Miller et al., 2000), angiopoietin 1 (Abou-Khalil et al., 2009), nitric oxide (Wehling et al., 2001), fibroblast growth factor (Bischoff, 1986), and insulin-like growth factor (Allen and Boxhorn, 1989), are known to modulate satellite cell quiescence, activation, expansion, self-renewal, and differentiation (Kuang et al., 2008; Aziz et al., 2012). Further modulation of satellite cells occurs through other cells within the satellite cell niche, including macrophages, neutrophils and other white blood cells, fibroadipogenic progenitors (FAPs), and endothelial cells (Sousa-Victor et al., 2021). The satellite cell crosstalks with other constituents of the niche through signaling pathways such as TGFβ, Notch, and Wnt further refines the satellite cell function and fate (Sousa-Victor et al., 2021).
This breadth of data regarding the phenotype, function, and niche of satellite cells has provided a foundation of knowledge toward stem cell therapy for muscle-related conditions, including age-related sarcopenia (García-Prat et al., 2016; Lukjanenko et al., 2019) and muscular dystrophies (Périé et al., 2014; Campbell and Puymirat, 2021). Like those faced by MSC therapies, ongoing challenges with the use of satellite cell-derived myoblasts include cell culture-passaging limitations that restrict their expansion potential in vitro, and the development of specific cell differentiation protocols (Chal and Pourquié, 2017; Magli et al., 2017; Xi et al., 2017). The continuously evolving knowledge of satellite cell repair mechanisms, the role of their niche, and niche-supporting cells provides great optimism for their therapeutic use for muscle disease in the future. Such a foundation of knowledge may contribute to the stem cell regenerative approach to cartilage repair in OA.
Future Avenues for Stem Cell Therapy for Cartilage Regeneration
Stimulating native articular stem cells to enhance their ability to repair cartilage in vivo constitutes a viable avenue for future research. Among MSCs, a significant amount of heterogeneity exists (Jones and Schafer, 2015). Factors such as culture age, donor sex, and health status play a role in the MSC function and surface receptor expression (Murphy et al., 2002; Baxter et al., 2004; Astudillo et al., 2008; Zhen et al., 2013; Jones and Schafer, 2015). Of these factors, the niche from which MSCs are isolated is believed to have a decisive influence on their function and differentiation capacity (Risbud et al., 2006; Jones and McGonagle, 2011; de Sousa et al., 2014; Jones and Schafer, 2015). Bone marrow-derived MSCs are believed to have a more optimal capacity for chondrogenic differentiation than those derived from adipose tissue, while adipose-derived MSCs have a higher proliferative capacity (Li et al., 2015b). Even among bone-derived MSCs, the particular bone and the topographic region, therein, can influence differentiation capacity and surface marker expression (Risbud et al., 2006; Ackema and Charite, 2008; Tormin et al., 2011; Jones and Schafer, 2015). Therefore, selecting an appropriate endogenous stem cell target and niche to assist in OA joint repair will be a crucial factor toward positive outcomes. Similarly, the tissue of origin of exogenous MSCs transplanted into the site of cartilage injury will also be a primary factor toward successful cartilage regeneration. Interestingly, while studies have compared stem cell chondrogenic capacity from different niches in vitro (Mochizuki et al., 2006; Koga et al., 2008), and studies have compared stem cells to implanted chondrocytes (Nejadnik et al., 2010) and bone marrow concentrate (Yang et al., 2022) in vivo, we are not aware of any in vivo studies directly comparing chondrogenic repair capacities of different stem cell populations derived from different tissue sources. To date, clinical trials evaluating MSCs have shown some evidence of reducing OA-associated pain and can produce cartilage in vitro, but there is little evidence that current MSC treatment regenerates damaged cartilage in vivo. In sum, the hunt is still on for the most potent stem cell population capable of mediating cartilage repair in vivo. This begs the question: where would such a population exist?
Beyond MSCs derived from adipose sources and articular tissues, other potential candidates for cartilage repair are also being pursued. Dental-derived MSCs, such as dental pulp stem cells (DPSCs) originating from neural crest mesenchyme in the dental pulp, can be extracted with minimal donor site morbidity (Ibarretxe et al., 2012; Lo Monaco et al., 2020; Li et al., 2021). The dental pulp provides a protective environment for DPSCs during a person’s lifetime, preserving their stem cell capacity (Fernandes et al., 2018). In vitro, DPSCs can be differentiated into cartilage-producing cells and can secrete several chondrogenic growth factors (Bronckaers et al., 2013; Ahmed Nel et al., 2016). Like bone-derived MSCs, they also have immunomodulatory capabilities that may be beneficial in the inflamed OA joint (Li et al., 2014; Lo Monaco et al., 2020). Preclinical models evaluated DPSC chondrogenic capacity. Lei et al. (2014) transplanted human DPSC cell pellets into the dorsal surface of immunodeficient mice where they maintained their chondrogenic capacity (Lei et al., 2014). Lo Monaco et al. (2020) showed an improved in vitro pro-survival effect when immature murine chondrocytes were cultured with DPSC-conditioned media (Lo Monaco et al., 2020). Mata et al. (2017) evaluated the ability of DPSCs to repair osteochondral defects in rabbits using alginate matrix-embedded DPSCs and alginate-embedded rabbit chondrocytes. Compared to controls, both rabbit chondrocytes and human DPSCs showed an improved quantity of in vivo cartilage regeneration and collagen fiber alignment (Mata et al., 2017). Fernandes et al. (2018) demonstrated that the DPSCs in a biomaterial scaffold showed a thicker deep layer of cartilage with less fibroblastic tissue, as compared to scaffold-alone (Fernandes et al., 2018). Overall, though DPSCs remain in the preclinical stage of experimentation for cartilage regeneration, they have shown potential to repair cartilage lesions in vivo and to promote native chondrocyte survival. These encouraging data may eventually benefit patients with OA.
The growth plate is the site of long bone growth in youth, through the process of endochondral bone growth (Kronenberg, 2003; Berendsen and Olsen, 2015). This process involves the longitudinal growth and ossification of a cartilage matrix, which is initiated by the action of chondrocytes in the proliferative zone of the growth plate (Koelling et al., 2009). These, in turn, are derived from small and relatively inactive stem cells located in the reserve zone close to the secondary ossification center (Koelling et al., 2009). Endochondral ossification and bone growth, therefore, rely on a yet-uncharacterized population of undifferentiated skeletal stem cells (SSCs) with highly robust chondrogenic functional capacity that resides in a protected niche within the growth plate (Chan et al., 2015). In 2015, Chan isolated SSCs from the femoral head growth plate of mice (Yin et al., 2013). The characterization of these skeletal stem cells showed a robust ability to differentiate in vitro to chondrocytes, osteoblasts, or bone marrow stromal cells. Although the chondrogenic potential of these murine SSCs was not directly compared to that of MSCs, studies from muscle (and other tissues) suggest that tissue-embedded stem cell populations are more efficient at repairing damaged tissue compared to MSCs (i.e., MSCs sacrifice efficiency for versatility while tissue-specific stem cells are more efficient at repairing, but show limited versatility) (Jankowski et al., 2002; Chan et al., 2018). In 2018, a human SSC population demonstrating self-renewal and multilineage differentiation to the bone, cartilage, and stroma was isolated from the growth plate of human embryonic femoral bones (Murphy et al., 2020). Within this growth plate population, several subpopulations were described that were able to produce cartilage in vitro, identified by surface marker expression PDPN+CD146−. The cells that were CD146+, a marker commonly associated with MSCs, displayed reduced colony size and frequency compared to PDPN+CD146− SSCs, as observed by light microscopy and flow cytometry (Murphy et al., 2020). As well, two of the three chondrogenic SSC subpopulations did not express CD73, a marker included in the ISCT MSC definition, further suggesting these cells are not MSCs and are indeed a distinct and functionally unique stem cell population. The SSC with greatest functional diversity, including high chondrogenic potential, was identified as PDPN+CD146−CD73+CD164+ with the highest expression of these marker transcripts isolated from the proliferative and pre-hypertrophic zone of fetal bones (Murphy et al., 2020). The authors developed a monocyte-derived induced pluripotent stem cell (iPSC) line that also expressed PDPN+CD146−CD73+CD164+ and produced cartilage in vivo, an important finding that would allow laboratory-based production of a cartilage-generating cell line not requiring access to fetal tissue (Murphy et al., 2020). We are unaware of clinical trials evaluating SSCs applied exogenously for cartilage repair.
In line with enhancing the endogenous stem cell population toward repairing cartilage defects in vivo, Murphy et al. evaluated the response of SSCs to microfracture in both the distal femur of mice that had undergone the destabilization of the medial meniscus (a well-described model of OA), as well as human fetal phalangeal bone (Csobonyeiova et al., 2021). Following the articular surface microfracture in mice, the authors identified a proliferation of SSCs; however, the resulting regenerative tissue appeared to be morphologically heterogeneous, containing both fibrotic and chondrogenic tissue (Csobonyeiova et al., 2021). The addition of a hydrogel containing BMP2 and a VEGF antagonist enhanced cartilage tissue formation that approximated the structural properties of native cartilage. Similar results were achieved following the microfracture of the human phalangeal articular surface with BMP2 and anti-VEGF treatment, suggesting that endogenous SSCs could be stimulated toward cartilage regeneration following microfracture intervention. Whether such potent cartilage regeneration can be achieved in adult OA-affected articular remains to be determined.
Recently, research has emerged evaluating the differentiation of iPSCs toward chondrocytes for the purpose of treating OA (Takahashi et al., 2007). iPSCs were initially generated from somatic cells by the viral transfection of key reprogramming factors (Oct3/4, Sox2, c-myc, and Klf4) into the donor cells (Koyama et al., 2013). Like embryonic stem cells, iPSCs are pluripotent and have similar cell morphology, gene expression, and proliferation capability; however, iPSCs are derived from somatic cells, avoiding the ethical issues related to collecting cells from embryos (Takahashi et al., 2007). iPSCs may be differentiated toward the chondrocyte lineage using media supplemented with growth factors such as TGF-β, BMP, WNT3A, and FGF-2 (Takahashi et al., 2007). The four main chondrogenic differentiation approaches used to date include 1) the generation of MSC-like iPSCs with further differentiation into chondrocytes (Takahashi et al., 2007; Qu et al., 2013; Nejadnik et al., 2015); 2) co-culture of iPSC-derived MSCs with primary chondrocytes (Takahashi et al., 2007; Rim et al., 2018); 3) through the formation of three-dimensional cellular aggregates (Takahashi et al., 2007; Yamashita et al., 2015); and 4) culturing of iPSCs in a series of media which mimics physiological developmental pathways (Takahashi et al., 2007; Murphy et al., 2018). Using the appropriate cell culture conditions, iPSCs can also be differentiated towards potent chondrogenic progenitors, such as SSCs (Murphy et al., 2020), suggesting that they could generate a pool of precursors mimicking the functionality of a chondrogenic stem cell population. The drawbacks of iPSCs, however, includes genomic instability, difficulties in obtaining uniform mature cell populations, and tumor formation, owing to the risks of insertional mutagenesis and reactivation of transgenes caused by the integration of the viral genome used to create these cells (Takahashi et al., 2007; Martel-Pelletier et al., 2016). Strategies for overcoming these issues, as well as improving differentiation protocol efficiency of the phenotype of the ideal stem cell population for cartilage regeneration is an ongoing and exciting area of research (Takahashi et al., 2007; Martel-Pelletier et al., 2016).
Conclusion
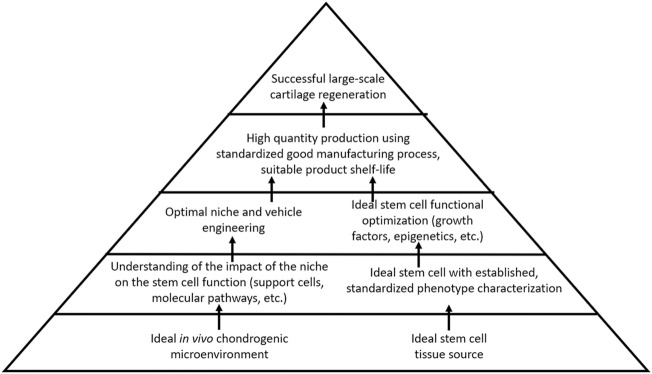
Current strategies to regenerate cartilage in the OA joint are limited to surgical interventions that may fail or yield fibrocartilaginous tissue with insufficient biomechanical properties for joint load distribution. Furthermore, such surgical treatments are less ideal for larger, non-focal areas of cartilage loss, as seen in the later stages of OA. Regenerative medicine thus remains an attractive option for OA treatment. Although stem cell therapy holds promise for cartilage regeneration in OA, safe and reliable strategies to either optimize endogenous stem cells toward cartilage repair, to apply exogenous stem cells into damaged joints, or both simultaneously, have yet to emerge. The research study has emphasized the importance of the stem cell source niche, which proffers the characteristics that stem cells bring to the table with respect to their regenerative capacity. Despite this, however, there remains a lack of standardization across clinical studies regarding stem cell tissue source and phenotypic characterization. A well-characterized cell product that can be reproducibly isolated or manufactured, and that can reliably produce healthy cartilage in the avascular cartilage microenvironment—that is, standardization of stem cells used for treatment and evidence-based outcome measures—will be an essential factor for moving the field of cartilage regeneration forward. The effective means of contending with the catabolic microenvironment of the OA joint and maintaining cellular chondrogenic functional capacity in a region primed toward cartilage destruction will also be an important challenge to overcome. The lessons from other areas of regenerative medicine can be applied to the field of cartilage regeneration. Hematopoietic therapies benefit from a deep understanding of the impact of the microenvironment on stem cell activity, not only the stem cells, but also the intricate role played by other support cells within the hematopoietic niche. A well-established international infrastructure responsible for thoroughly characterizing, standardizing, and distributing hematopoietic stem cells represents a major advantage. The field of muscle regeneration also takes the advantage of decades of data describing the mechanism of regeneration as well as the impact on the microenvironment on the satellite cells, additionally benefiting from a strong understanding of molecular pathways by which satellite cells develop, replicate, and respond to external function-altering stimuli. As compared to these other areas of regenerative medicine, our current understanding of the optimal cartilage-producing stem cell, its niche, the identity and role of niche-supporting cells, and the molecular mechanisms governing functional potential remains limited. Further research is needed to address these fundamental factors (Figure 2).
FIGURE 2.
Suggested directions for cell-based therapy research for cartilage regeneration.
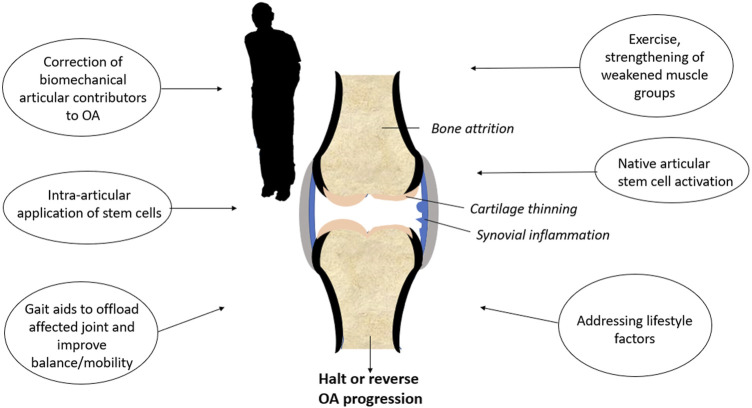
Even though stem cell therapy will likely 1 day become a valuable tool for cartilage regeneration, OA remains a whole-joint disease (Martel-Pelletier et al., 2016). Articular tissues including bone, synovium, and the joint capsule are all affected by OA, as well as peri-articular tissues such as muscle (Goldring and Goldring, 2016; Martel-Pelletier et al., 2016). Protocols, such as stem cell injections used in isolation, are unlikely to halt or reverse OA if the numerous factors contributing to joint degeneration are not addressed. Multimodal approaches (Figure 3), such as compartmental or total joint unloading to remove excess biomechanical stress, physiotherapy for muscle reconditioning, and lifestyle changes such as weight control and regular exercise will give the ideal stem cell population a fighting chance to regenerate the cartilage in people suffering from OA.
FIGURE 3.
Multimodal treatment of the osteoarthritis-affected knee.
Acknowledgments
The authors thank Dee Cao for contributing to the graphical design of the manuscript figures.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was supported by funding from the Bruyère Academic Medical Organization Incentive Fund, The Ottawa Hospital Department of Medicine Translational Research Grant, and the University of Ottawa Translational Research Grant.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Abou-Khalil R., Le Grand F., Pallafacchina G., Valable S., Authier F. J., Rudnicki M. A., et al. (2009). Autocrine and Paracrine Angiopoietin 1/Tie-2 Signaling Promotes Muscle Satellite Cell Self-Renewal. Cell Stem Cell 5, 298–309. 10.1016/j.stem.2009.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackema K. B., Charite J. (2008). Mesenchymal Stem Cells from Different Organs Are Characterized by Distinct Topographic Hox Codes. Stem Cells Dev. 17, 979–991. 10.1089/scd.2007.0220 [DOI] [PubMed] [Google Scholar]
- Ahmed Nel M., Murakami M., Hirose Y., Nakashima M. (2016). Therapeutic Potential of Dental Pulp Stem Cell Secretome for Alzheimer's Disease Treatment: An In Vitro Study. Stem Cells Int. 2016, 8102478. 10.1155/2016/8102478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen R. E., Boxhorn L. K. (1989). Regulation of Skeletal Muscle Satellite Cell Proliferation and Differentiation by Transforming Growth Factor-Beta, Insulin-like Growth Factor I, and Fibroblast Growth Factor. J. Cell Physiol. 138, 311–315. 10.1002/jcp.1041380213 [DOI] [PubMed] [Google Scholar]
- Anz A. W., Hubbard R., Rendos N. K., Everts P. A., Andrews J. R., Hackel J. G. (2020). Bone Marrow Aspirate Concentrate Is Equivalent to Platelet-Rich Plasma for the Treatment of Knee Osteoarthritis at 1 year: A Prospective, Randomized Trial. Orthop. J. Sports Med. 8, 2325967119900958. 10.1177/2325967119900958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astudillo P., Rios S., Pastenes L., Pino A. M., Rodriguez J. P. (2008). Increased Adipogenesis of Osteoporotic Human-Mesenchymal Stem Cells (MSCs) Characterizes by Impaired Leptin Action. J. Cell Biochem. 103, 1054–1065. 10.1002/jcb.21516 [DOI] [PubMed] [Google Scholar]
- Aziz A., Sebastian S., Dilworth F. J. (2012). The Origin and Fate of Muscle Satellite Cells. Stem Cell Rev. Rep. 8, 609–622. 10.1007/s12015-012-9352-0 [DOI] [PubMed] [Google Scholar]
- Barr A. J., Campbell T. M., Hopkinson D., Kingsbury S. R., Bowes M. A., Conaghan P. G. (2015). A Systematic Review of the Relationship between Subchondral Bone Features, Pain and Structural Pathology in Peripheral Joint Osteoarthritis. Arthritis Res. Ther. 25, 228. 10.1186/s13075-015-0735-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter M. A., Wynn R. F., Jowitt S. N., Wraith J. E., Fairbairn L. J., Bellantuono I. (2004). Study of Telomere Length Reveals Rapid Aging of Human Marrow Stromal Cells Following In Vitro Expansion. Stem Cells 22, 675–682. 10.1634/stemcells.22-5-675 [DOI] [PubMed] [Google Scholar]
- Berenbaum F., Blanco F. J., Guermazi A., Miki K., Yamabe T., Viktrup L., et al. (2020). Subcutaneous Tanezumab for Osteoarthritis of the Hip or Knee: Efficacy and Safety Results from a 24-week Randomised Phase III Study with a 24-week Follow-Up Period. Ann. Rheum. Dis. 79, 800–810. 10.1136/annrheumdis-2019-216296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendsen A. D., Olsen B. R. (2015). Bone Development. Bone 80, 14–18. 10.1016/j.bone.2015.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff R. (1986). Proliferation of Muscle Satellite Cells on Intact Myofibers in Culture. Dev. Biol. 115, 129–139. 10.1016/0012-1606(86)90234-4 [DOI] [PubMed] [Google Scholar]
- Bochtler W., Maiers M., Bakker J. N., Oudshoorn M., Marsh S. G., Baier D., et al. (2011). World Marrow Donor Association Framework for the Implementation of HLA Matching Programs in Hematopoietic Stem Cell Donor Registries and Cord Blood Banks. Bone Marrow Transpl. 46, 338–343. 10.1038/bmt.2010.132 [DOI] [PubMed] [Google Scholar]
- Brittberg M., Lindahl A., Nilsson A., Ohlsson C., Isaksson O., Peterson L. (1994). Treatment of Deep Cartilage Defects in the Knee with Autologous Chondrocyte Transplantation. N. Engl. J. Med. 331, 889–895. 10.1056/nejm199410063311401 [DOI] [PubMed] [Google Scholar]
- Bronckaers A., Hilkens P., Fanton Y., Struys T., Gervois P., Politis C., et al. (2013). Angiogenic Properties of Human Dental Pulp Stem Cells. PLoS One 8, e71104. 10.1371/journal.pone.0071104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruyère O., Altman R. D., Reginster J. Y. (2016). Efficacy and Safety of Glucosamine Sulfate in the Management of Osteoarthritis: Evidence from Real-Life Setting Trials and Surveys. Semin. Arthritis Rheum. 45, S12–S17. 10.1016/j.semarthrit.2015.11.011 [DOI] [PubMed] [Google Scholar]
- Campbell C., Puymirat J. (2021). Transplantation of Myoblasts to Duchenne Muscular Dystrophy (DMD) Patients. NIH. [Google Scholar]
- Campbell T. M., Churchman S. M., Gomez A., McGonagle D., Conaghan P. G., Ponchel F., et al. (2016). Mesenchymal Stem Cell Alterations in Bone Marrow Lesions in Hip Osteoarthritis. Arthritis Rheumatol. 68, 1648–1659. 10.1002/art.39622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDA (2019). Health Canada Policy Position Paper – Autologous Cell Therapy Products. Ottawa, ON: Health Canada. [Google Scholar]
- Cdc Prevention (2021). Cost Statistics: Cost of Osteoarthritis. Atlanta, GA: CDC. [Google Scholar]
- Centers for Disease Control and Prevention (2010). National Hospital Discharge Survey. Atlanta, GA: CDC. [Google Scholar]
- Chal J., Pourquié O. (2017). Making Muscle: Skeletal Myogenesis In Vivo and In Vitro . Development 144, 2104–2122. 10.1242/dev.151035 [DOI] [PubMed] [Google Scholar]
- Chan C. K. F., Gulati G. S., Sinha R., Tompkins J. V., Lopez M., Carter A. C., et al. (2018). Identification of the Human Skeletal Stem Cell. Cell 175, 43–56. e21. 10.1016/j.cell.2018.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C. K., Seo E. Y., Chen J. Y., Lo D., McArdle A., Sinha R., et al. (2015). Identification and Specification of the Mouse Skeletal Stem Cell. Cell 160, 285–298. 10.1016/j.cell.2014.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandran P., Le Y., Li Y., Sabloff M., Mehic J., Rosu-Myles M., et al. (2015). Mesenchymal Stromal Cells from Patients with Acute Myeloid Leukemia Have Altered Capacity to Expand Differentiated Hematopoietic Progenitors. Leuk. Res. 39, 486–493. 10.1016/j.leukres.2015.01.013 [DOI] [PubMed] [Google Scholar]
- Chen C., Tambe D. T., Deng L., Yang L. (2013). Biomechanical Properties and Mechanobiology of the Articular Chondrocyte. Am. J. Physiol. Cell Physiol. 305, C1202–C1208. 10.1152/ajpcell.00242.2013 [DOI] [PubMed] [Google Scholar]
- Choudhery M. S., Khan M., Mahmood R., Mehmood A., Khan S. N., Riazuddin S. (2012). Bone Marrow Derived Mesenchymal Stem Cells from Aged Mice Have Reduced Wound Healing, Angiogenesis, Proliferation and Anti-apoptosis Capabilities. Cell Biol. Int. 36, 747–753. 10.1042/cbi20110183 [DOI] [PubMed] [Google Scholar]
- Clinicaltrial.gov (2022). Search: Mesenchymal Stem Cells for Osteoarthritis: Recruiting Studies. [Google Scholar]
- Cope P. J., Ourradi K., Li Y., Sharif M. (2019). Models of Osteoarthritis: the Good, the Bad and the Promising. Osteoarthr. Cartil. 27, 230–239. 10.1016/j.joca.2018.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross M., Smith E., Hoy D., Nolte S., Ackerman I., Fransen M., et al. (2014). The Global Burden of Hip and Knee Osteoarthritis: Estimates from the Global Burden of Disease 2010 Study. Ann. Rheum. Dis. 73, 1323–1330. 10.1136/annrheumdis-2013-204763 [DOI] [PubMed] [Google Scholar]
- Csobonyeiova M., Polak S., Nicodemou A., Zamborsky R., Danisovic L. (2021). iPSCs in Modeling and Therapy of Osteoarthritis. Biomedicines 9, 186. 10.3390/biomedicines9020186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler A. A., Palikowski B., Wheeler J. R., Dalla Betta N. C., Antwine T., O'Rourke R., et al. (2021). The Regenerating Skeletal Muscle Niche Guides Muscle Stem Cell Self-Renewal. Cell Rep. 1, 1. [Google Scholar]
- Dao T., Green A. E., Kim Y. A., Bae S. J., Ha K. T., Gariani K., et al. (2020). Sarcopenia and Muscle Aging: A Brief Overview. Endocrinol. Metab. Seoul. 35, 716–732. 10.3803/enm.2020.405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lisio M., Baker J. M., Parise G. (2013). Exercise Promotes Bone Marrow Cell Survival and Recipient Reconstitution Post-bone Marrow Transplantation, Which Is Associated with Increased Survival. Exp. Hematol. 41, 143–154. 10.1016/j.exphem.2012.10.003 [DOI] [PubMed] [Google Scholar]
- de Sousa E. B., Casado P. L., Moura Neto V., Duarte M. E. A. (2014). Synovial Fluid and Synovial Membrane Mesenchymal Stem Cells: Latest Discoveries and Therapeutic Perspectives. Stem Cell Res. Ther. 5, 112. 10.1186/scrt501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado D., Garate A., Vincent H., Bilbao A. M., Patel R., Fiz N., et al. (2019). Current Concepts in Intraosseous Platelet-Rich Plasma Injections for Knee Osteoarthritis. J. Clin. Orthop. Trauma 10, 36–41. 10.1016/j.jcot.2018.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., et al. (2006). Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 8, 315–317. 10.1080/14653240600855905 [DOI] [PubMed] [Google Scholar]
- Dowthwaite G. P., Bishop J. C., Redman S. N., Khan I. M., Rooney P., Evans D. J., et al. (2004). The Surface of Articular Cartilage Contains a Progenitor Cell Population. J. Cell Sci. 117, 889–897. 10.1242/jcs.00912 [DOI] [PubMed] [Google Scholar]
- Emadedin M., Labibzadeh N., Liastani M. G., Karimi A., Jaroughi N., Bolurieh T., et al. (2018). Intra-articular Implantation of Autologous Bone Marrow-Derived Mesenchymal Stromal Cells to Treat Knee Osteoarthritis: a Randomized, Triple-Blind, Placebo-Controlled Phase 1/2 Clinical Trial. Cytotherapy 20, 1238–1246. 10.1016/j.jcyt.2018.08.005 [DOI] [PubMed] [Google Scholar]
- Everhart J. S., Abouljoud M. M., Kirven J. C., Flanigan D. C. (2019). Full-Thickness Cartilage Defects Are Important Independent Predictive Factors for Progression to Total Knee Arthroplasty in Older Adults with Minimal to Moderate Osteoarthritis. J. Bone Jt. Surg. 101, 56–63. 10.2106/jbjs.17.01657 [DOI] [PubMed] [Google Scholar]
- Felson D. T. (2004). An Update on the Pathogenesis and Epidemiology of Osteoarthritis. Radiologic Clin. N. Am. 42, 1–9. 10.1016/s0033-8389(03)00161-1 [DOI] [PubMed] [Google Scholar]
- Fernandes T. L., Shimomura K., Asperti A., Pinheiro C. C. G., Caetano H. V. A., Oliveira C., et al. (2018). Development of a Novel Large Animal Model to Evaluate Human Dental Pulp Stem Cells for Articular Cartilage Treatment. Stem Cell Rev. Rep. 14, 734–743. 10.1007/s12015-018-9820-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag J., Bates D., Wickham J., Shah K., Huguenin L., Tenen A., et al. (2019). Adipose-derived Mesenchymal Stem Cell Therapy in the Treatment of Knee Osteoarthritis: a Randomized Controlled Trial. Regen. Med. 14, 213–230. 10.2217/rme-2018-0161 [DOI] [PubMed] [Google Scholar]
- García-Prat L., Martínez-Vicente M., Perdiguero E., Ortet L., Rodríguez-Ubreva J., Rebollo E., et al. (2016). Autophagy Maintains Stemness by Preventing Senescence. Nature 529, 37–42. [DOI] [PubMed] [Google Scholar]
- Gauntner T. D., Brunstein C. G., Cao Q., Weisdorf D., Warlick E. D., Jurdi N. E., et al. (2021). Association of CD34 Cell Dose with 5-Year Overall Survival after Peripheral Blood Allogeneic Hematopoietic Cell Transplantation in Adults with Hematologic Malignancies. Transpl. Cell Ther. 28, 88–95. 10.1016/j.jtct.2021.11.004 [DOI] [PubMed] [Google Scholar]
- Goldring S. R., Goldring M. B. (2016). Changes in the Osteochondral Unit during Osteoarthritis: Structure, Function and Cartilage-Bone Crosstalk. Nat. Rev. Rheumatol. 12, 632–644. 10.1038/nrrheum.2016.148 [DOI] [PubMed] [Google Scholar]
- Grogan S. P., Miyaki S., Asahara H., D'Lima D. D., Lotz M. K. (2009). Mesenchymal Progenitor Cell Markers in Human Articular Cartilage: Normal Distribution and Changes in Osteoarthritis. Arthritis Res. Ther. 11, R85. 10.1186/ar2719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guermazi A., Hayashi D., Roemer F. W., Niu J., Quinn E. K., Crema M. D., et al. (2017). Brief Report: Partial- and Full-Thickness Focal Cartilage Defects Contribute Equally to Development of New Cartilage Damage in Knee Osteoarthritis: The Multicenter Osteoarthritis Study. Arthritis & Rheumatology 69, 560–564. 10.1002/art.39970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilak F. (2011). Biomechanical Factors in Osteoarthritis. Best Practice & Research. Clin. Rheumatol. 25, 815–823. 10.1016/j.berh.2011.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha C. W., Park Y. B., Kim S. H., Lee H. J. (2019). Intra-articular Mesenchymal Stem Cells in Osteoarthritis of the Knee: A Systematic Review of Clinical Outcomes and Evidence of Cartilage Repair. Arthroscopy 35, 277–288.e2. 10.1016/j.arthro.2018.07.028 [DOI] [PubMed] [Google Scholar]
- Haleem A. M., Singergy A. A., Sabry D., Atta H. M., Rashed L. A., Chu C. R., et al. (2010). The Clinical Use of Human Culture-Expanded Autologous Bone Marrow Mesenchymal Stem Cells Transplanted on Platelet-Rich Fibrin Glue in the Treatment of Articular Cartilage Defects: A Pilot Study and Preliminary Results. Cartilage 1, 253–261. 10.1177/1947603510366027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg M. C., Altman R. D., April K. T., Benkhalti M., Guyatt G., McGowan J., et al. (2012). American College of Rheumatology 2012 Recommendations for the Use of Nonpharmacologic and Pharmacologic Therapies in Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res. Hob. 64, 465–474. 10.1002/acr.21596 [DOI] [PubMed] [Google Scholar]
- Hoogduijn M. J. (2015). Are Mesenchymal Stromal Cells Immune Cells? Arthritis Res. Ther. 17, 88. 10.1186/s13075-015-0596-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarretxe G., Crende O., Aurrekoetxea M., García-Murga V., Etxaniz J., Unda F. (2012). Neural Crest Stem Cells from Dental Tissues: a New Hope for Dental and Neural Regeneration. Stem Cells Int. 2012, 103503. 10.1155/2012/103503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isscr (2021). Preventing the Marketing of Unproven Stem Cell Treatments. Chicago, IL: ISSCR. [Google Scholar]
- Jankowski R. J., Deasy B. M., Huard J. (2002). Muscle-derived Stem Cells. Gene Ther. 9, 642–647. 10.1038/sj.gt.3301719 [DOI] [PubMed] [Google Scholar]
- Jansen M. P., Boymans T., Custers R. J. H., Van Geenen R. C. I., Van Heerwaarden R. J., Huizinga M. R., et al. (2021). Knee Joint Distraction as Treatment for Osteoarthritis Results in Clinical and Structural Benefit: A Systematic Review and Meta-Analysis of the Limited Number of Studies and Patients Available. Cartilage 13, 1113s–1123s. 10.1177/1947603520942945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasuriya C. T., Chen Y., Liu W., Chen Q. (2016). The Influence of Tissue Microenvironment on Stem Cell-Based Cartilage Repair. Ann. N. Y. Acad. Sci. 1383, 21–33. 10.1111/nyas.13170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo C. H., Lee Y. G., Shin W. H., Kim H., Chai J. W., Jeong E. C., et al. (2014). Intra-articular Injection of Mesenchymal Stem Cells for the Treatment of Osteoarthritis of the Knee: a Proof-Of-Concept Clinical Trial. Stem Cells 32, 1254–1266. 10.1002/stem.1634 [DOI] [PubMed] [Google Scholar]
- Jones E., Churchman S. M., English A., Buch M. H., Horner E. A., Burgoyne C. H., et al. (2010). Mesenchymal Stem Cells in Rheumatoid Synovium: Enumeration and Functional Assessment in Relation to Synovial Inflammation Level. Ann. Rheum. Dis. 69, 450–457. 10.1136/ard.2008.106435 [DOI] [PubMed] [Google Scholar]
- Jones E., English A., Churchman S. M., Kouroupis D., Boxall S. A., Kinsey S., et al. (2010). Large-scale Extraction and Characterization of CD271+ Multipotential Stromal Cells from Trabecular Bone in Health and Osteoarthritis: Implications for Bone Regeneration Strategies Based on Uncultured or Minimally Cultured Multipotential Stromal Cells. Arthritis Rheum. 62, 1944–1954. 10.1002/art.27451 [DOI] [PubMed] [Google Scholar]
- Jones E., McGonagle D. (2011). Synovial Mesenchymal Stem Cells In Vivo: Potential Key Players for Joint Regeneration. World J. Rheumatol. 1, 4–11. 10.5499/wjr.v1.i1.4 [DOI] [Google Scholar]
- Jones E., Schafer R. (2015). Where Is the Common Ground between Bone Marrow Mesenchymal Stem/stromal Cells from Different Donors and Species? Stem Cell Res. Ther. 6, 143. 10.1186/s13287-015-0144-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E., Yang X. (2011). Mesenchymal Stem Cells and Bone Regeneration: Current Status. Injury 42, 562–568. 10.1016/j.injury.2011.03.030 [DOI] [PubMed] [Google Scholar]
- Kalluri R., LeBleu V. S. (2020). The Biology, Function, and Biomedical Applications of Exosomes. Science 367, eaau6977. 10.1126/science.aau6977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B. (1961). The Terminations of the Afferent Nerve Fibre in the Muscle Spindle of the Frog. Philosophical Trans. R. Soc. Lond. Biol. 243, 221–240. [Google Scholar]
- Khalifeh Soltani S., Forogh B., Ahmadbeigi N., Hadizadeh Kharazi H., Fallahzadeh K., Kashani L., et al. (2019). Safety and Efficacy of Allogenic Placental Mesenchymal Stem Cells for Treating Knee Osteoarthritis: a Pilot Study. Cytotherapy 21, 54–63. 10.1016/j.jcyt.2018.11.003 [DOI] [PubMed] [Google Scholar]
- Kim H., Bae C., Kook Y. M., Koh W. G., Lee K., Park M. H. (2019). Mesenchymal Stem Cell 3D Encapsulation Technologies for Biomimetic Microenvironment in Tissue Regeneration. Stem Cell Res. Ther. 10, 51. 10.1186/s13287-018-1130-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. H., Djaja Y. P., Park Y. B., Park J. G., Ko Y. B., Ha C. W. (2020). Intra-articular Injection of Culture-Expanded Mesenchymal Stem Cells without Adjuvant Surgery in Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Am. J. Sports Med. 48, 2839–2849. 10.1177/0363546519892278 [DOI] [PubMed] [Google Scholar]
- Kim S. H., Ha C. W., Park Y. B., Nam E., Lee J. E., Lee H. J. (2019). Intra-articular Injection of Mesenchymal Stem Cells for Clinical Outcomes and Cartilage Repair in Osteoarthritis of the Knee: a Meta-Analysis of Randomized Controlled Trials. Arch. Orthop. Trauma Surg. 139, 971–980. 10.1007/s00402-019-03140-8 [DOI] [PubMed] [Google Scholar]
- Kirkham A. M., Bailey A. J. M., Tieu A., Maganti H. B., Montroy J., Shorr R., et al. (2021). MSC-derived Extracellular Vesicles in Preclinical Animal Models of Bone Injury: A Systematic Review and Meta-Analysis. Stem Cell Rev. Rep. 18, 1054–1066. 10.1007/s12015-021-10208-9 [DOI] [PubMed] [Google Scholar]
- Koelling S., Kruegel J., Irmer M., Path J. R., Sadowski B., Miro X., et al. (2009). Migratory Chondrogenic Progenitor Cells from Repair Tissue during the Later Stages of Human Osteoarthritis. Cell Stem Cell 4, 324–335. 10.1016/j.stem.2009.01.015 [DOI] [PubMed] [Google Scholar]
- Koelling S., Miosge N. (2009). Stem Cell Therapy for Cartilage Regeneration in Osteoarthritis. Expert Opin. Biol. Ther. 9, 1399–1405. 10.1517/14712590903246370 [DOI] [PubMed] [Google Scholar]
- Koga H., Muneta T., Nagase T., Nimura A., Ju Y. J., Mochizuki T., et al. (2008). Comparison of Mesenchymal Tissues-Derived Stem Cells for In Vivo Chondrogenesis: Suitable Conditions for Cell Therapy of Cartilage Defects in Rabbit. Cell Tissue Res. 333, 207–215. 10.1007/s00441-008-0633-5 [DOI] [PubMed] [Google Scholar]
- Koyama N., Miura M., Nakao K., Kondo E., Fujii T., Taura D., et al. (2013). Human Induced Pluripotent Stem Cells Differentiated into Chondrogenic Lineage via Generation of Mesenchymal Progenitor Cells. Stem Cells Dev. 22, 102–113. 10.1089/scd.2012.0127 [DOI] [PubMed] [Google Scholar]
- Kronenberg H. M. (2003). Developmental Regulation of the Growth Plate. Nature 423, 332–336. 10.1038/nature01657 [DOI] [PubMed] [Google Scholar]
- Kuah D., Sivell S., Longworth T., James K., Guermazi A., Cicuttini F., et al. (2018). Safety, Tolerability and Efficacy of Intra-articular Progenza in Knee Osteoarthritis: a Randomized Double-Blind Placebo-Controlled Single Ascending Dose Study. J. Transl. Med. 16, 49. 10.1186/s12967-018-1420-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang S., Gillespie M. A., Rudnicki M. A. (2008). Niche Regulation of Muscle Satellite Cell Self-Renewal and Differentiation. Cell Stem Cell 2, 22–31. 10.1016/j.stem.2007.12.012 [DOI] [PubMed] [Google Scholar]
- Le Y., Fraineau S., Chandran P., Sabloff M., Brand M., Lavoie J. R., et al. (2016). Adipogenic Mesenchymal Stromal Cells from Bone Marrow and Their Hematopoietic Supportive Role: Towards Understanding the Permissive Marrow Microenvironment in Acute Myeloid Leukemia. Stem Cell Rev. Rep. 12, 235–244. 10.1007/s12015-015-9639-z [DOI] [PubMed] [Google Scholar]
- Lee W. S., Kim H. J., Kim K. I., Kim G. B., Jin W. (2019). Intra-Articular Injection of Autologous Adipose Tissue-Derived Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis: A Phase IIb, Randomized, Placebo-Controlled Clinical Trial. Stem Cells Transl. Med. 8, 504–511. 10.1002/sctm.18-0122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei M., Li K., Li B., Gao L. N., Chen F. M., Jin Y. (2014). Mesenchymal Stem Cell Characteristics of Dental Pulp and Periodontal Ligament Stem Cells after In Vivo Transplantation. Biomaterials 35, 6332–6343. 10.1016/j.biomaterials.2014.04.071 [DOI] [PubMed] [Google Scholar]
- Levato R., Webb W. R., Otto I. A., Mensinga A., Zhang Y., van Rijen M., et al. (2017). The Bio in the Ink: Cartilage Regeneration with Bioprintable Hydrogels and Articular Cartilage-Derived Progenitor Cells. Acta Biomater. 61, 41–53. 10.1016/j.actbio.2017.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Ouchi T., Cao Y., Zhao Z., Men Y. (2021). Dental-Derived Mesenchymal Stem Cells: State of the Art. Front. Cell Dev. Biol. 9, 654559. 10.3389/fcell.2021.654559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. Y., Wu X. Y., Tong J. B., Yang X. X., Zhao J. L., Zheng Q. F., et al. (2015). Comparative Analysis of Human Mesenchymal Stem Cells from Bone Marrow and Adipose Tissue under Xeno-free Conditions for Cell Therapy. Stem Cell Res. Ther. 13, 55. 10.1186/s13287-015-0066-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Meng H., Liu Y., Lee B. P. (2015). Fibrin Gel as an Injectable Biodegradable Scaffold and Cell Carrier for Tissue Engineering. ScientificWorldJournal 2015, 685690. 10.1155/2015/685690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Jiang C. M., An S., Cheng Q., Huang Y. F., Wang Y. T., et al. (2014). Immunomodulatory Properties of Dental Tissue-Derived Mesenchymal Stem Cells. Oral Dis. 20, 25–34. 10.1111/odi.12086 [DOI] [PubMed] [Google Scholar]
- Liao G., Gilmore K., Steed A., Elmoazzen H., Allan D. S. (2020). Willingness of Volunteers from Canadian Blood Service's Stem Cell Registry to Donate Blood, Marrow, and Other Tissues for Regenerative Therapy. Transfusion 60, 582–587. 10.1111/trf.15695 [DOI] [PubMed] [Google Scholar]
- Lo Monaco M., Gervois P., Beaumont J., Clegg P., Bronckaers A., Vandeweerd J. M., et al. (2020). Therapeutic Potential of Dental Pulp Stem Cells and Leukocyte- and Platelet-Rich Fibrin for Osteoarthritis. Cells 9, 980. 10.3390/cells9040980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeser R. F. (2010). Age-related Changes in the Musculoskeletal System and the Development of Osteoarthritis. Clin. geriatric Med. 26, 371–386. 10.1016/j.cger.2010.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Dai C., Zhang Z., Du H., Li S., Ye P., et al. (2019). Treatment of Knee Osteoarthritis with Intra-articular Injection of Autologous Adipose-Derived Mesenchymal Progenitor Cells: a Prospective, Randomized, Double-Blind, Active-Controlled, Phase IIb Clinical Trial. Stem Cell Res. Ther. 10, 143. 10.1186/s13287-019-1248-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukjanenko L., Karaz S., Stuelsatz P., Gurriaran-Rodriguez U., Michaud J., Dammone G., et al. (2019). Aging Disrupts Muscle Stem Cell Function by Impairing Matricellular WISP1 Secretion from Fibro-Adipogenic Progenitors. Cell Stem Cell 24, 433–446. e7. 10.1016/j.stem.2018.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magli A., Incitti T., Kiley J., Swanson S. A., Darabi R., Rinaldi F., et al. (2017). PAX7 Targets, CD54, Integrin α9β1, and SDC2, Allow Isolation of Human ESC/iPSC-Derived Myogenic Progenitors. Cell Rep. 19, 2867–2877. 10.1016/j.celrep.2017.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak J., Jablonski C. L., Leonard C. A., Dunn J. F., Raharjo E., Matyas J. R., et al. (2016). Intra-articular Injection of Synovial Mesenchymal Stem Cells Improves Cartilage Repair in a Mouse Injury Model. Sci. Rep. 6, 23076. 10.1038/srep23076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamidi M. K., Das A. K., Zakaria Z., Bhonde R. (2016). Mesenchymal Stromal Cells for Cartilage Repair in Osteoarthritis. Osteoarthr. Cartil. 24, 1307–1316. 10.1016/j.joca.2016.03.003 [DOI] [PubMed] [Google Scholar]
- Mandrycky C., Wang Z., Kim K., Kim D. H. (2016). 3D Bioprinting for Engineering Complex Tissues. Biotechnol. Adv. 34, 422–434. 10.1016/j.biotechadv.2015.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks P. W., Witten C. M., Califf R. M. (2017). Clarifying Stem-Cell Therapy's Benefits and Risks. N. Engl. J. Med. 376, 1007–1009. 10.1056/nejmp1613723 [DOI] [PubMed] [Google Scholar]
- Martel-Pelletier J., Barr A. J., Cicuttini F. M., Conaghan P. G., Cooper C., Goldring M. B., et al. (2016). Osteoarthritis. Nat. Rev. Dis. Prim. 2, 16072. 10.1038/nrdp.2016.72 [DOI] [PubMed] [Google Scholar]
- Mata M., Milian L., Oliver M., Zurriaga J., Sancho-Tello M., de Llano J. J. M., et al. (2017). In Vivo Articular Cartilage Regeneration Using Human Dental Pulp Stem Cells Cultured in an Alginate Scaffold: A Preliminary Study. Stem Cells Int. 2017, 8309256. 10.1155/2017/8309256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matai I., Kaur G., Seyedsalehi A., McClinton A., Laurencin C. T. (2020). Progress in 3D Bioprinting Technology for Tissue/organ Regenerative Engineering. Biomaterials 226, 119536. 10.1016/j.biomaterials.2019.119536 [DOI] [PubMed] [Google Scholar]
- Matas J., Orrego M., Amenabar D., Infante C., Tapia-Limonchi R., Cadiz M. I., et al. (2019). Umbilical Cord-Derived Mesenchymal Stromal Cells (MSCs) for Knee Osteoarthritis: Repeated MSC Dosing Is Superior to a Single MSC Dose and to Hyaluronic Acid in a Controlled Randomized Phase I/II Trial. Stem Cells Transl. Med. 8, 215–224. 10.1002/sctm.18-0053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro A. (1961). Satellite Cell of Skeletal Muscle Fibers. J. Biophysical Biochem. Cytol. 9, 493–495. 10.1083/jcb.9.2.493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlindon T. E., Bannuru R. R., Sullivan M. C., Arden N. K., Berenbaum F., Bierma-Zeinstra S. M., et al. (2014). OARSI Guidelines for the Non-surgical Management of Knee Osteoarthritis. Osteoarthr. Cartil. 22, 363–388. 10.1016/j.joca.2014.01.003 [DOI] [PubMed] [Google Scholar]
- McGonagle D., Baboolal T. G., Jones E. (2017). Native Joint-Resident Mesenchymal Stem Cells for Cartilage Repair in Osteoarthritis. Nat. Rev. Rheumatol. 13, 719–730. 10.1038/nrrheum.2017.182 [DOI] [PubMed] [Google Scholar]
- Merceron C., Vinatier C., Portron S., Masson M., Amiaud J., Guigand L., et al. (2010). Differential Effects of Hypoxia on Osteochondrogenic Potential of Human Adipose-Derived Stem Cells. Am. J. Physiol. Cell Physiol. 298, C355–C364. 10.1152/ajpcell.00398.2009 [DOI] [PubMed] [Google Scholar]
- Miller K. J., Thaloor D., Matteson S., Pavlath G. K. (2000). Hepatocyte Growth Factor Affects Satellite Cell Activation and Differentiation in Regenerating Skeletal Muscle. Am. J. Physiol. Cell Physiol. 278, C174–C181. 10.1152/ajpcell.2000.278.1.c174 [DOI] [PubMed] [Google Scholar]
- Mochizuki T. M., Sakaguchi Y., Nimura A., Yokoyama A., Koga H., Sekiya I. (2006). Higher Chondrogenic Potential of Fibrous Synovium- and Adipose Synovium-Derived Cells Compared with Subcutaneous Fat-Derived Cells: Distinguishing Properties of Mesenchymal Stem Cells in Humans. Arthritis Rheum. 54, 843–853. 10.1002/art.21651 [DOI] [PubMed] [Google Scholar]
- Moisio K., Eckstein F., Chmiel J. S., Guermazi A., Prasad P., Almagor O., et al. (2009). Denuded Subchondral Bone and Knee Pain in Persons with Knee Osteoarthritis. Arthritis Rheum. 60, 3703–3710. 10.1002/art.25014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison S. J., Scadden D. T. (2014). The Bone Marrow Niche for Haematopoietic Stem Cells. Nature 505, 327–334. 10.1038/nature12984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller L., Tunger A., Wobus M., von Bonin M., Towers R., Bornhäuser M., et al. (2021). Immunomodulatory Properties of Mesenchymal Stromal Cells: An Update. Front. Cell Dev. Biol. 9, 637725. 10.3389/fcell.2021.637725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy C., Mobasheri A., Táncos Z., Kobolák J., Dinnyés A. (2018). The Potency of Induced Pluripotent Stem Cells in Cartilage Regeneration and Osteoarthritis Treatment. Adv. Exp. Med. Biol. 1079, 55–68. 10.1007/5584_2017_141 [DOI] [PubMed] [Google Scholar]
- Murphy J. M., Dixon K., Beck S., Fabian D., Feldman A., Barry F. (2002). Reduced Chondrogenic and Adipogenic Activity of Mesenchymal Stem Cells from Patients with Advanced Osteoarthritis. Arthritis Rheum. 46, 704–713. 10.1002/art.10118 [DOI] [PubMed] [Google Scholar]
- Murphy M. P., Koepke L. S., Lopez M. T., Tong X., Ambrosi T. H., Gulati G. S., et al. (2020). Articular Cartilage Regeneration by Activated Skeletal Stem Cells. Nat. Med. 26, 1583–1592. 10.1038/s41591-020-1013-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejadnik H., Diecke S., Lenkov O. D., Chapelin F., Donig J., Tong X., et al. (2015). Improved Approach for Chondrogenic Differentiation of Human Induced Pluripotent Stem Cells. Stem Cell Rev. Rep. 11, 242–253. 10.1007/s12015-014-9581-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejadnik H., Hui J. H., Feng Choong E. P., Tai B. C., Lee E. H. (2010). Autologous Bone Marrow-Derived Mesenchymal Stem Cells versus Autologous Chondrocyte Implantation: an Observational Cohort Study. Am. J. sports Med. 38, 1110–1116. 10.1177/0363546509359067 [DOI] [PubMed] [Google Scholar]
- Oh J., Lee Y. D., Wagers A. J. (2014). Stem Cell Aging: Mechanisms, Regulators and Therapeutic Opportunities. Nat. Med. 20, 870–880. 10.1038/nm.3651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J. S., Monk A. P., Hopewell S., Bayliss L. E., Jackson W., Beard D. J., et al. (2019). Surgical Interventions for Symptomatic Mild to Moderate Knee Osteoarthritis. Cochrane database Syst. Rev. 7, Cd012128. 10.1002/14651858.CD012128.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passweg J. R., Baldomero H., Chabannon C., Basak G. W., de la Cámara R., Corbacioglu S., et al. (2021). Hematopoietic Cell Transplantation and Cellular Therapy Survey of the EBMT: Monitoring of Activities and Trends over 30 Years. Bone Marrow Transpl. 56, 1651–1664. 10.1038/s41409-021-01227-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Périé S., Trollet C., Mouly V., Vanneaux V., Mamchaoui K., Bouazza B., et al. (2014). Autologous Myoblast Transplantation for Oculopharyngeal Muscular Dystrophy: a Phase I/IIa Clinical Study. Mol. Ther. 22, 219–225. 10.1038/mt.2013.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritzker K. P., Gay S., Jimenez S. A., Ostergaard K., Pelletier J. P., Revell P. A., et al. (2006). Osteoarthritis Cartilage Histopathology: Grading and Staging. Osteoarthr. Cartil. 14, 13–29. 10.1016/j.joca.2005.07.014 [DOI] [PubMed] [Google Scholar]
- Prockop D. J. (1997). Marrow Stromal Cells as Stem Cells for Nonhematopoietic Tissues. Science 276, 71–74. 10.1126/science.276.5309.71 [DOI] [PubMed] [Google Scholar]
- Qu C., Puttonen K. A., Lindeberg H., Ruponen M., Hovatta O., Koistinaho J., et al. (2013). Chondrogenic Differentiation of Human Pluripotent Stem Cells in Chondrocyte Co-culture. Int. J. Biochem. Cell Biol. 45, 1802–1812. 10.1016/j.biocel.2013.05.029 [DOI] [PubMed] [Google Scholar]
- Redman S. N., Oldfield S. F., Archer C. W. (2005). Current Strategies for Articular Cartilage Repair. Eur. cells Mater. 9, 23–32. 10.22203/ecm.v009a04 [DOI] [PubMed] [Google Scholar]
- Reginster J. Y., Badurski J., Bellamy N., Bensen W., Chapurlat R., Chevalier X., et al. (2013). Efficacy and Safety of Strontium Ranelate in the Treatment of Knee Osteoarthritis: Results of a Double-Blind, Randomised Placebo-Controlled Trial. Ann. Rheum. Dis. 72, 179–186. 10.1136/annrheumdis-2012-202231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Registry N. J. (2020). National Joint Registry 17th Annual Report. [PubMed] [Google Scholar]
- Rikkers M., Korpershoek J. V., Levato R., Malda J., Vonk L. A. (2022). The Clinical Potential of Articular Cartilage-Derived Progenitor Cells: a Systematic Review. NPJ Regen. Med. 7, 2. 10.1038/s41536-021-00203-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rim Y. A., Nam Y., Park N., Lee J., Park S. H., Ju J. H. (2018). Repair Potential of Nonsurgically Delivered Induced Pluripotent Stem Cell-Derived Chondrocytes in a Rat Osteochondral Defect Model. J. Tissue Eng. Regen. Med. 12, 1843–1855. 10.1002/term.2705 [DOI] [PubMed] [Google Scholar]
- Risbud M. V., Shapiro I. M., Guttapalli A., Di Martino A., Danielson K. G., Beiner J. M., et al. (2006). Osteogenic Potential of Adult Human Stem Cells of the Lumbar Vertebral Body and the Iliac Crest. Spine 31, 83–89. 10.1097/01.brs.0000193891.71672.e4 [DOI] [PubMed] [Google Scholar]
- Robinson D. C. L., Ritso M., Nelson G. M., Mokhtari Z., Nakka K., Bandukwala H., et al. (2021). Negative Elongation Factor Regulates Muscle Progenitor Expansion for Efficient Myofiber Repair and Stem Cell Pool Repopulation. Dev. Cell 56, 1014–1029. e7. 10.1016/j.devcel.2021.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe J. A., Harper J. M., Buttery P. J. (1989). Protein Metabolism in Ovine Primary Muscle Cultures Derived from Satellite Cells-Eeffects of Selected Peptide Hormones and Growth Factors. J. Endocrinol. 122, 565–571. 10.1677/joe.0.1220565 [DOI] [PubMed] [Google Scholar]
- Roubille C., Martel-Pelletier J., Raynauld J. P., Abram F., Dorais M., Delorme P., et al. (2015). Meniscal Extrusion Promotes Knee Osteoarthritis Structural Progression: Protective Effect of Strontium Ranelate Treatment in a Phase III Clinical Trial. Arthritis Res. Ther. 17, 82. 10.1186/s13075-015-0579-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi Y., Sekiya I., Yagishita K., Muneta T. (2005). Comparison of Human Stem Cells Derived from Various Mesenchymal Tissues: Superiority of Synovium as a Cell Source. Arthritis Rheum. 52, 2521–2529. 10.1002/art.21212 [DOI] [PubMed] [Google Scholar]
- Sanz-Nogués C., O'Brien T. (2021). Current Good Manufacturing Practice Considerations for Mesenchymal Stromal Cells as Therapeutic Agents. Biomater. Biosyst. 2, 100018. 10.1016/j.bbiosy.2021.100018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer T. J., Easton R., Pang S., Levinson D. J., Pixton G., Viktrup L., et al. (2019). Effect of Tanezumab on Joint Pain, Physical Function, and Patient Global Assessment of Osteoarthritis Among Patients with Osteoarthritis of the Hip or Knee: A Randomized Clinical Trial. Jama 322, 37–48. 10.1001/jama.2019.8044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellam J., Berenbaum F. (2010). The Role of Synovitis in Pathophysiology and Clinical Symptoms of Osteoarthritis. Nat. Rev. Rheumatol. 6, 625–635. 10.1038/nrrheum.2010.159 [DOI] [PubMed] [Google Scholar]
- Sethe S., Scutt A., Stolzing A. (2006). Aging of Mesenchymal Stem Cells. Ageing Res. Rev. 5, 91–116. 10.1016/j.arr.2005.10.001 [DOI] [PubMed] [Google Scholar]
- Shapiro S. A., Arthurs J. R., Heckman M. G., Bestic J. M., Kazmerchak S. E., Diehl N. N., et al. (2019). Quantitative T2 MRI Mapping and 12-Month Follow-Up in a Randomized, Blinded, Placebo Controlled Trial of Bone Marrow Aspiration and Concentration for Osteoarthritis of the Knees. Cartilage 10, 432–443. 10.1177/1947603518796142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma L., Song J., Felson D. T., Cahue S., Shamiyeh E., Dunlop D. D. (2001). The Role of Knee Alignment in Disease Progression and Functional Decline in Knee Osteoarthritis. Jama 286, 188–195. 10.1001/jama.286.2.188 [DOI] [PubMed] [Google Scholar]
- Shaw B. E., Logan B. R., Spellman S. R., Marsh S. G. E., Robinson J., Pidala J., et al. (2018). Development of an Unrelated Donor Selection Score Predictive of Survival after HCT: Donor Age Matters Most. Biol. Blood Marrow Transpl. 24, 1049–1056. 10.1016/j.bbmt.2018.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverwood V., Blagojevic-Bucknall M., Jinks C., Jordan J. L., Protheroe J., Jordan K. P. (2015). Current Evidence on Risk Factors for Knee Osteoarthritis in Older Adults: a Systematic Review and Meta-Analysis. Osteoarthr. Cartil. 23, 507–515. 10.1016/j.joca.2014.11.019 [DOI] [PubMed] [Google Scholar]
- Soler R., Orozco L., Munar A., Huguet M., López R., Vives J., et al. (2016). Final Results of a Phase I-II Trial Using Ex Vivo Expanded Autologous Mesenchymal Stromal Cells for the Treatment of Osteoarthritis of the Knee Confirming Safety and Suggesting Cartilage Regeneration. Knee 23, 647–654. 10.1016/j.knee.2015.08.013 [DOI] [PubMed] [Google Scholar]
- Song N., Scholtemeijer M., Shah K. (2020). Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends Pharmacol. Sci. 41, 653–664. 10.1016/j.tips.2020.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y., Zhang J., Xu H., Lin Z., Chang H., Liu W., et al. (2020). Mesenchymal Stem Cells in Knee Osteoarthritis Treatment: A Systematic Review and Meta-Analysis. J. Orthop. Transl. 24, 121–130. 10.1016/j.jot.2020.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa-Victor P., García-Prat L., Muñoz-Cánoves P. (2021). Control of Satellite Cell Function in Muscle Regeneration and its Disruption in Ageing. Nat. Rev. Mol. Cell Biol. 23, 204–226. 10.1038/s41580-021-00421-2 [DOI] [PubMed] [Google Scholar]
- Sulzbacher I. (2013). Osteoarthritis: Histology and Pathogenesis. Wien Med. Wochenschr 163, 212–219. 10.1007/s10354-012-0168-y [DOI] [PubMed] [Google Scholar]
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K. (2007). Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 131, 861–872. 10.1016/j.cell.2007.11.019 [DOI] [PubMed] [Google Scholar]
- To K., Romain K., Mak C., Kamaraj A., Henson F., Khan W. (2020). The Treatment of Cartilage Damage Using Human Mesenchymal Stem Cell-Derived Extracellular Vesicles: A Systematic Review of In Vivo Studies. Front. Bioeng. Biotechnol. 8, 580. 10.3389/fbioe.2020.00580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tormin A., Li O., Brune J. C., Walsh S., Schütz B., Ehinger M., et al. (2011). CD146 Expression on Primary Non-hematopoietic Bone Marrow Stem Cells Correlates to In Situ Localization. Blood 117, 5067–5077. 10.1182/blood-2010-08-304287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towheed T. E., Maxwell L., Anastassiades T. P., Shea B., Houpt J., Robinson V., et al. (2009). Glucosamine Therapy for Treating Osteoarthritis. Cochrane Database Syst. Rev. 18, CD002946. 10.1002/14651858.CD002946.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Xing D., Liu W., Zhu Y., Liu H., Yan L., et al. (2022). Preclinical Studies and Clinical Trials on Mesenchymal Stem Cell Therapy for Knee Osteoarthritis: A Systematic Review on Models and Cell Doses. Int. J. Rheum. Dis. 1, 1. 10.1111/1756-185x.14306 [DOI] [PubMed] [Google Scholar]
- Wang Y. X., Zhao Z. D., Wang Q., Li Z. L., Huang Y., Zhao S., et al. (2020). Biological Potential Alterations of Migratory Chondrogenic Progenitor Cells during Knee Osteoarthritic Progression. Arthritis Res. Ther. 22, 62. 10.1186/s13075-020-2144-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehling M., Spencer M. J., Tidball J. G. (2001). A Nitric Oxide Synthase Transgene Ameliorates Muscular Dystrophy in Mdx Mice. J. Cell Biol. 155, 123–131. 10.1083/jcb.200105110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S. L., Johnston R. V., McDonald S., Worthley D., Campbell T. M., Buchbinder R. (2019). Stem Cell Injections for Osteoarthritis of the Knee. Cochrane Database Syst. Rev. 5, CD013342. 10.1002/14651858.CD013342 [DOI] [Google Scholar]
- Wilkinson A. C., Morita M., Nakauchi H., Yamazaki S. (2018). Branched-chain Amino Acid Depletion Conditions Bone Marrow for Hematopoietic Stem Cell Transplantation Avoiding Amino Acid Imbalance-Associated Toxicity. Exp. Hematol. 63, 12–16.e1. 10.1016/j.exphem.2018.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R., Khan I. M., Richardson K., Nelson L., McCarthy H. E., Analbelsi T., et al. (2010). Identification and Clonal Characterisation of a Progenitor Cell Sub-population in Normal Human Articular Cartilage. PLoS One 5, e13246. 10.1371/journal.pone.0013246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. A., Chung D. J., Park S. A., Zwingenberger A. L., Reilly C. M., Ly I., et al. (2012). Periocular and Intra-articular Injection of Canine Adipose-Derived Mesenchymal Stem Cells: an In Vivo Imaging and Migration Study. J. Ocul. Pharmacol. Ther. 28, 307–317. 10.1089/jop.2011.0166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi H., Fujiwara W., Gonzalez K., Jan M., Liebscher S., Van Handel B., et al. (2017). In Vivo Human Somitogenesis Guides Somite Development from hPSCs. Cell Rep. 18, 1573–1585. 10.1016/j.celrep.2017.01.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita A., Morioka M., Yahara Y., Okada M., Kobayashi T., Kuriyama S., et al. (2015). Generation of Scaffoldless Hyaline Cartilaginous Tissue from Human iPSCs. Stem Cell Rep. 4, 404–418. 10.1016/j.stemcr.2015.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Yang Y., Ma L., Zhang G. X., Shi F. D., Yan Y., et al. (2019). Study of the Cytological Features of Bone Marrow Mesenchymal Stem Cells from Patients with Neuromyelitis Optica. Int. J. Mol. Med. 43, 1395–1405. 10.3892/ijmm.2019.4056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H. Y., Song E. K., Kang S. J., Kwak W. K., Kang J. K., Seon J. K. (2022). Allogenic Umbilical Cord Blood-Derived Mesenchymal Stromal Cell Implantation Was Superior to Bone Marrow Aspirate Concentrate Augmentation for Cartilage Regeneration Despite Similar Clinical Outcomes. Knee Surg. Sports Traumatol. Arthrosc. 30, 208–218. 10.1007/s00167-021-06450-w [DOI] [PubMed] [Google Scholar]
- Yin H., Price F., Rudnicki M. A. (2013). Satellite Cells and the Muscle Stem Cell Niche. Physiol. Rev. 93, 23–67. 10.1152/physrev.00043.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yubo M., Yanyan L., Li L., Tao S., Bo L., Lin C. (2017). Clinical Efficacy and Safety of Mesenchymal Stem Cell Transplantation for Osteoarthritis Treatment: A Meta-Analysis. PLoS One 12, e0175449. 10.1371/journal.pone.0175449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamborsky R., Danisovic L. (2020). Surgical Techniques for Knee Cartilage Repair: An Updated Large-Scale Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. Arthroscopy 36, 845–858. 10.1016/j.arthro.2019.11.096 [DOI] [PubMed] [Google Scholar]
- Zhang B., Tian X., Qu Z., Hao J., Zhang W. (2022). Hypoxia-Preconditioned Extracellular Vesicles from Mesenchymal Stem Cells Improve Cartilage Repair in Osteoarthritis. Membr. (Basel) 12, 225. 10.3390/membranes12020225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Shi J., Li Y., Jiang Y., Tao T., Li W., et al. (2016). Chondrogenic Cells Respond to Partial-Thickness Defects of Articular Cartilage in Adult Rats: an In Vivo Study. J. Mol. Histol. 47, 249–258. 10.1007/s10735-016-9668-1 [DOI] [PubMed] [Google Scholar]
- Zhang R., Ma J., Han J., Zhang W., Ma J. (2019). Mesenchymal Stem Cell Related Therapies for Cartilage Lesions and Osteoarthritis. Am. J. Transl. Res. 11, 6275–6289. [PMC free article] [PubMed] [Google Scholar]
- Zhen G., Wen C., Jia X., Li Y., Crane J. L., Mears S. C., et al. (2013). Inhibition of TGF-β Signaling in Mesenchymal Stem Cells of Subchondral Bone Attenuates Osteoarthritis. Nat. Med. 19, 704–712. 10.1038/nm.3143 [DOI] [PMC free article] [PubMed] [Google Scholar]