Abstract
Purpose
Chronic obstructive pulmonary disease (COPD)-related pulmonary hypertension (PH) is one of the most common comorbidities of COPD, and often leads to a worse prognosis. Although the estimated prevalence and risk factors of COPD-related PH have been widely reported, these results have not been well integrated. This study aimed to review the worldwide incidence and prevalence of COPD-related PH and explore possible factors affecting its prevalence.
Patients and Methods
We searched four electronic databases (Web of Science, Embase, Cochrane, and MEDLINE) to identify all observational studies on the prevalence of COPD-related PH from database creation until July 20, 2021. Eligibility screening, quality assessment, and data extraction of the retrieved studies were independently conducted by two reviewers. Meta-analyses were performed to determine the prevalence of PH in the COPD population. Random-effects meta-regression model analyses were conducted to investigate the sources of heterogeneity.
Results
Altogether, 38 articles were included in the meta-analyses. The pooled prevalence was 39.2% (95% CI: 34.0–44.4, I2 = 97.6%) for COPD-related PH. Subgroup analyses showed that the prevalence of PH increased with COPD severity, where the majority (30.2%) had mild PH and the minority had severe PH (7.2%). Furthermore, we found a significant regional difference in the prevalence of COPD-related PH (P = 0.000), which was the highest in Africa (64.0%) and the lowest in Europe (30.4%). However, stratified studies on other factors involving mean age, sex, enrolment time, participant recruitment settings, and PH diagnostic methods showed no significant differences in prevalence (P >0.05).
Conclusion
The global incidence of PH in the COPD population is very high, and there are significant regional and international variations. Patients with COPD should be screened for PH and contributing risk factors to reduce the burden on individuals and society.
Keywords: chronic obstructive pulmonary disease, pulmonary hypertension, prevalence, heterogeneity, meta-analysis
Introduction
Chronic obstructive pulmonary disease (COPD) is a common, preventable, and treatable condition characterized by persistent respiratory symptoms and restricted airflow due to abnormalities in the airways or alveoli, usually caused by high exposure to toxic particles or gases.1,2 COPD is the most common cause of chronic respiratory disease-attributable deaths, which has become a major global health problem because of its high prevalence (approximately 10% in the adult population) and increasing incidence every year (partly related to the aging population), resulting in extremely significant increases in associated personal, social, and economic costs.3,4 The 2017 Global Burden of Disease (GBD) Study estimates that the global mortality of COPD is approximately 41.9 deaths per 100 000 individuals (5.7% of total all-cause deaths). Specifically, 46.7 deaths per 100 000 men, and 37.0 deaths per 100 000 women are attributable to COPD.5 In the United States, hospital expenses for this condition result in more than 13 billion dollars every year.6
In addition to effects on the lungs, patients with COPD exhibit many systemic effects, with mean pulmonary arterial pressure (PAP) >20 mmHg at rest in up to 90% of the patients.7,8 Studies have shown that most patients with severe emphysema have an mPAP of 20 to 25 mmHg.9 In contrast, COPD patients very rarely (approximately 1%) have an mPAP >40 mmHg, provided that no other condition is causing PH.9 Pulmonary hypertension (PH), a serious and common complication in the COPD population, has a dramatic correlation with worsening clinical symptoms and prognosis for which treatment options are proven to be limited.10–12 The current guidelines define PH as a mean pulmonary artery pressure (mPAP) ≥25 mmHg (1 mmHg = 0.133 kPa) measured by right heart catheterization (RHC) in a resting state at sea level.13 Based on data from the Swiss PH registry and the English ASPIRE registry, group 3 PH has a poorer prognosis than that of other PH groups, although its mPAP and pulmonary vascular resistance (PVR) are less elevated on average.14 Studies have shown that mPAP approximately >19 mmHg worsens the prognosis of COPD, which results in a higher proportion of hospitalization and mortality.12,15 Among hemodynamic factors, particularly a high PVR and a low pulmonary oxygen saturation (SvO2) are strongly associated with mortality in COPD.16
The clinical importance of PH associated with COPD has been documented in several studies that demonstrated the independent prognostic role of PH in the COPD population. The prevalence of COPD-related PH cannot be neglected because PH generally increases with COPD severity. As a result, interest in this area of research has increased over the past decade, leading to the publication of numerous studies investigating the prevalence and risk factors of PH in COPD. However, to date, the prevalence of COPD-related PH has proved to be inconclusive due to the COPD classification criteria for historical changes. To our knowledge, only few estimates on the prevalence of COPD-related PH have been consolidated globally despite numerous studies on the same topic. Being informed and updated on the worldwide burden of COPD-related PH is imperative in developing effective strategies for the primary prevention and management of this disease and informing the corresponding stakeholders. The purpose of this study was to review the currently published literature on the incidence and prevalence of COPD-related PH. We aimed to assess the reported prevalence of this condition on a global scale and explore possible factors that may affect it.
Materials and Methods
This review was conducted according to the Meta-analyses of Observational Studies in Epidemiology guidelines (MOOSE).17 The protocol for this systematic review is available on the PROSPERO, with the registration number CRD42021270357.
Data Sources and Search Strategy
We searched in four electronic databases, namely, Web of Science, Embase, Cochrane, and MEDLINE (via PubMed), to identify all observational studies on the prevalence of PH associated with COPD from database creation until July 20, 2021, without setting language restrictions. Our search method adopted the strategy of combining subject words and free words. We used the following Medical Subject Heading terms and free words: “Pulmonary Disease, Chronic Obstructive” OR “Chronic Obstructive Lung Disease” OR “Chronic Obstructive Pulmonary Diseases” OR “COAD” OR “COPD” OR “Chronic Obstructive Airway Disease” OR “Chronic Obstructive Pulmonary Disease” OR “Airflow Obstruction, Chronic” OR “Airflow Obstructions, Chronic” OR “Chronic Airflow Obstructions” OR “Chronic Airflow Obstruction” AND “Hypertension, Pulmonary” OR “Pulmonary Hypertension” AND “Prevalence*” OR “Epidemiology*” OR “Incidence*” OR “Morbidity*” OR “Risk*” OR “Cross Sectional*” OR “Cross-sectional*”. In addition, we manually searched the reference list of each identified article for other relevant publications. Gray literature was also searched through some medical websites. Some authors were contacted by email for further details or to ask for their help in resolving any uncertainties. The study was based entirely on previously published research and did not require the approval of an ethics committee.
Study Selection
After eliminating duplicate studies, two investigators independently screened titles and abstracts to identify eligible publications according to an inclusion and exclusion criteria. Full-text articles were retrieved when at least one investigator considered the abstract eligible for the study. The inclusion criteria in the systematic review were as follows: (1) patients with COPD as research subjects; (2) diagnoses for COPD and PH made through effective and objective methods (ie, spirometry of COPD; RHC or transthoracic echocardiography (TTE) for PH); (3) reported prevalence of patients with COPD-related PH; (4) observational studies, including case-control, cross-sectional, and longitudinal cohort designs. The exclusion criteria were as follows: (1) incomplete research data; (2) non-English articles; (3) a sample size <50; (4) published articles from conference abstracts, reviews, narrative reviews, or case reports.
Data Extraction
Two independent investigators extracted data from the included studies. Disagreements were resolved through discussion or by a third investigator when necessary. The following information was recorded: title, the first author’s name, publication year, study design, study location, sample size, diagnostic criteria, the prevalence of COPD-related PH, and the demographic characteristics of participants. All extracted data were stored in a Microsoft Excel file format.
Quality Assessment
Two investigators independently appraised the quality of the included studies, using a tool for disease prevalence quality developed by Loney et al.18 Any disagreement about the quality of the studies was resolved by a third investigator. The quality assessment of the included studies was based on the Newcastle–Ottawa Scale for observational, non-randomized studies.19,20 A total of 10 points was assigned to the following categories: selection (representativeness of the sample, sample size, non-respondents, and ascertainment of the exposure), comparability (controlling for confounding factors), and outcome (assessment and statistics). Studies awarded 7–10 stars were considered high quality; 5–6 stars, moderate quality; and <5 stars, low quality.
Data Analysis
The proportion of participants with COPD-related PH was extracted from all included studies to calculate the pooled prevalence of the disease. Data extracted from the included publications were entered into the Stata 12.0 software package (StataCorp LLC, College Station, TX, USA) for analysis. Heterogeneity between studies was tested using Cochran’s Q statistics. The I2 statistic was used to assess the degree of heterogeneity, with I2 values of 25%, 50%, and 75% defining low, medium, and high heterogeneity, respectively. When significant heterogeneity was found in Cochran’s Q statistics, a random-effects model was used to calculate the pooled prevalence and 95% confidence intervals (CIs) of COPD-related PH; otherwise, a fixed-effects model would be used (P < 0.05 was considered significant). These findings were illustrated in the form of forest maps. Sensitivity analysis adopted a leave-one-out method, which iteratively deleted a study from the meta-analysis to assess change in the overall effect size. Publication bias was assessed using a funnel plot, the Begg test, and the Egger test (P < 0.1 was considered significant). When publication bias was observed, the trim and fill method was used to account for the publication bias.21
Results
Study Selection
After the initial search, we retrieved 4522 articles, of which 1283 were duplicates. After screening titles and abstracts based on the inclusion and exclusion criteria, we selected 366 articles for further, detailed evaluation. Sixteen studies were excluded due to the lacking list of diagnostic criteria for PH; 25, due to a small sample size (<50); 89, due to lacking target cohort data; and 185, due to type mismatch during the second examination. A total of 51 studies met the screening criteria, of which eight articles had a low-quality score (<5) and five articles used the same data. A total of 38 articles were available for the meta-analysis (Figure 1).
Figure 1.
Flow diagram of the systematic search and selection of studies.
Note: Numbers refer to unique records not datasets, except where otherwise indicated.
Characteristics of the Included Studies
The 38 studies had a total of 16,345 patients with COPD, containing data across 16 countries from 1991 to 2018. In 14 studies that assigned gender distribution, the ratio of males to females was approximately 8:5 (3205 vs 2049). In 30 of the included studies, the mean age of the patients was 61.6 years (n = 15,376). Majority of the surveyed patients with COPD were in a stable phase of their disease. The characteristics of the patients from the 38 studies are summarized in Table 1.
Table 1.
Characteristics of Studies Reporting the Prevalence of COPD-Related PH
| Study | Country | Enrolment Time | Total | Gender (M/F) | Mean Age | Sample Source | Sample Type | Diagnostic Methods for PH | Prevalence |
|---|---|---|---|---|---|---|---|---|---|
| Acharya et al 201846 | India | 2012–2014 | 50 | NA | 61.14±10.33 | NA | Stable | TTE | 54.00% |
| Aksu et al 201347 | Turkey | 2008–2009 | 89 | 77/12 | 60.6±8.5 | NA | Stable | TTE | 23.60% |
| Alkukhun et al 201448 | America | 2004–2011 | 92 | 31/61 | 55.1 | Lung transplants | NA | RHC | 32.60% |
| Andersen et al 201215 | Danish | 1991–2010 | 409 | 140/216 | 54.01 | Lung transplants | Stable | RHC | 48.66% |
| Blanco et al 201949 | Spain | NA | 3105 | 1612/1493 | 59.5 | Lung transplants | NA | RHC | 54.00% |
| Buklioska et al 201950 | Skopje | 2018–2018 | 60 | 52/8 | NA | NA | NA | TTE | 33.33% |
| Chaouat et al 200951 | France | NA | 183 | 160/23 | 67.0–79.0 | NA | Stable | TTE | 21.90% |
| Chen et al 201552 | China | 2013–2014 | 221 | 175/46 | 69±10 | Inpatients | TTE | 25.34% | |
| Fayngersh et al 201153 | America | 2002–2008 | 174 | 155/19 | 40–80 | NA | Stable | TTE | 37.36% |
| Freixa et al 201254 | Spain | 2004–2006 | 342 | 318/24 | 67.9±8.6 | Inpatients | AECOPD | TTE | 19.00% |
| Gartman et al 201255 | America | 2008–2010 | 142 | 84/58 | 59 | Lung transplants | NA | RHC | 63.38% |
| Gupta et al 201856 | India | 2015–2016 | 109 | 72/27 | 58.04 | NA | NA | TTE | 62.40% |
| Halvani et al 201957 | Islam | NA | 142 | NA | 67.5–70.8 | Outpatients | Stable | TTE | 63.38% |
| Hayes et al 201758 | America | 2005–2013 | 86 | 31/55 | 60.86 | Outpatients | Stable | TTE | 63.00% |
| Hilde et al 201659 | Norway | NA | 100 | 49/51 | 63±7 | NA | Stable | RHC | 26.00% |
| Jethani et al 201660 | India | NA | 50 | 49/1 | 35–80 | NA | NA | TTE | 48.00% |
| Kwon et al 201061 | Korea | 2009 | 108 | 82/26 | 71.79 | NA | NA | TTE | 53.70% |
| Malinovschi et al 201462 | Italy | 2011–2012 | 276 | 186/90 | 67.76 | Inpatients | Stable | RHC | 47.80% |
| Matsuyama et al 200163 | Japan | NA | 65 | NA | 65.64 | Inpatients | NA | RHC | 32.31% |
| Mohamed et al 201664 | Netherlands | 2004–2014 | 65 | 33/32 | 59.34 | Lung transplants | NA | RHC | 58.46% |
| Mohamed et al 201965 | Egypt | 2017–2018 | 228 | NA | 63.30±9.22 | Outpatients | Stable | TTE | 63.00% |
| Nakahara et al 201666 | Japan | 2007–2013 | 503 | NA | 69.9±6.8 | Inpatients | Stable | RHC | 16.70% |
| Nakayama et al 202067 | Japan | 2010–2012 | 105 | 57/48 | 68.14 | NA | NA | TTE | 60.00% |
| Nathan et al 201268 | America | 2005–2018 | 6572 | 3252/3320 | 60.4±6.3 | Lung transplants | NA | RHC | 52.40% |
| Portillo et al 201569 | Spain | NA | 139 | 134/5 | 63±8 | NA | Stable | RHC | 18.00% |
| Sertogullarindan et al 201270 | Turkey | 2000–2010 | 600 | 336/264 | 67±10 | Inpatients | Stable | TTE | 54.17% |
| Seyhan et al 201371 | Turkey | 2007–2009 | 270 | 207/63 | 61±7.3 | NA | Stable | TTE | 48.00% |
| Shabrawy et al 201772 | Egypt | 2012–2014 | 252 | 163/89 | 58.46 | NA | AECOPD | TTE | 64.80% |
| Shin et al 201473 | America | 1998–2012 | 148 | 118/30 | 63.39 | NA | Stable | RHC | 39.00% |
| Sims et al 200974 | America | 1991–2003 | 362 | NA | 55.95 | Lung transplants | NA | RHC | 23.00% |
| Skjorten et al 201375 | Norway | 2006 | 96 | 48/48 | 63.47 | Outpatients | Stable | RHC | 26.00% |
| Sridhara et al 202076 | India | NA | 106 | NA | NA | NA | NA | RHC | 16.00% |
| Stolz et al 200877 | Switzerland | NA | 123 | NA | NA | Inpatients | AECOPD | TTE | 22.80% |
| Sun et al 201979 | China | 2016–2018 | 106 | 97/9 | 69.5±10.1 | NA | Stable | TTE | 22.60% |
| Takahashi et al 201879 | Japan | 2006–2016 | 131 | NA | NA | NA | NA | TTE | 12.98% |
| Xiong et al 201880 | China | 2015–2017 | 97 | 49/48 | 67.5±10.5 | Lung transplants | Stable | TTE | 23.71% |
| Xiong et al 202081 | China | NA | 513 | 432/81 | 68.02 | Outpatients | Stable | TTE | 29.24% |
| Yazici et al 201982 | Turkey | 2015–2018 | 126 | 119/7 | 66.73±9.76 | Outpatients | NA | TTE | 26.19% |
Abbreviations: AECOPD, acute exacerbation of chronic obstructive pulmonary disease; M/F, the number of males/the number of females; NA, not available or applicable; PH, pulmonary hypertension; TTE, transthoracic echocardiography; RHC, right heart catheterization.
Pooled Prevalence of COPD-Related PH
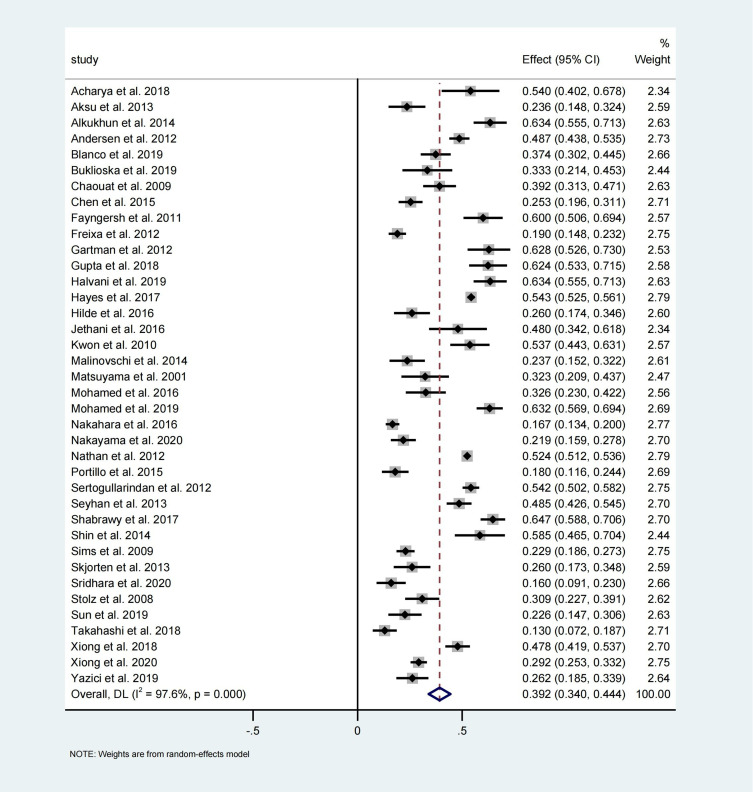
In the 38 studies available for meta-analysis, the prevalence of COPD-related PH ranged from 12.98% to 64.80%. Based on the random-effects model–based meta-analysis conducted on all data points, the overall COPD-related PH prevalence was approximately 39.2% (95% CI: 34.0–44.4, I2 = 97.6%, P = 0.000) (Figure 2).
Figure 2.
Forest plot of COPD-related PH prevalence.
Stratified Prevalence of COPD-Related PH by Age, Sex, Enrolment Time, Participant Recruitment Settings, Geographic Area, COPD Severity, PH Grades, and PH Diagnostic Methods
The estimated pooled prevalence acquired in the subgroup analyses is shown in Table 2. The prevalence of COPD-related PH widely varied partly due to differences in COPD severity and geographic regions. The collected data were from patients across four continents. The calculated prevalence rates of COPD-related PH were 30.4%, 32.6%, 52.6%, and 64.0%, in Europe, Asia, North America, and Africa, respectively (P = 0.000). Based on COPD classification, the pooled prevalence rates of PH associated with mild, moderate, severe, and very severe COPD were 24.5%, 34.1%, 38.6%, and 61.5%, respectively (P = 0.024). Based on gender and age, the COPD-related PH prevalence rates were approximately 42.6% in men, 43.5% in women, 44.5% in those <65 years, and 35.3% in those >65 years (P > 0.05). Based on participant recruitment settings, the calculated PH prevalence rates were 40.5% in outpatients, 32.3% in inpatients, and 44.5% in lung transplant evaluation patients (P = 0.247), with 38.1% in a stable phase and 38.2% in an acute exacerbation of COPD (AECOPD) (P = 0.998). The estimated prevalence rates of COPD-related PH were 41.8%, 41.2%, and 42.7% at different enrolment times (P = 0.992). Based on TTE and RHC, which are two different PH diagnostic methods, the estimated prevalence rates of COPD-related PH were 40.7% and 37.0%, respectively (P = 0.494). Finally, based on PH grade, a significant difference was noted in the prevalence rates of COPD-related PH, which were 30.2%, 10.0%, and 7.2% for mild, moderate, and severe grades, respectively (P = 0.000).
Table 2.
Subgroup Analyses of COPD-Related PH Prevalence
| Subgroups | Number of Included Studies | COPD-Related PH | |||||
|---|---|---|---|---|---|---|---|
| Prevalence | Sample (n) | 95% CI | I2 | P value | P Within Groups | ||
| Continents | |||||||
| Africa | 2 | 64.0% | 372 | 59.4–68.3 | 0.0% | 0.728 | 0.000 |
| Asia | 18 | 32.6% | 3648 | 28.4–44.3 | 96.5% | 0.000 | |
| Europe | 11 | 30.4% | 1780 | 23.3–37.6 | 91.0% | 0.000 | |
| North America | 7 | 52.6% | 10 437 | 45.3–60.0 | 96.9% | 0.000 | |
| Enrolment time | |||||||
| −2010 | 10 | 41.8% | 2467 | 31.1–52.5 | 96.9% | 0.000 | 0.992 |
| 2010–2015 | 4 | 41.2% | 706 | 18.6–63.8 | 97.7% | 0.000 | |
| 2015- | 6 | 42.7% | 905 | 28.4–57.0 | 95.2% | 0.000 | |
| Diagnostic methods for PH | |||||||
| TTE | 23 | 40.7% | 4165 | 33.3–48.2 | 96.4% | 0.000 | 0.494 |
| RHC | 15 | 37.0% | 12 180 | 29.0–44.9 | 98.3% | 0.000 | |
| Mean age | |||||||
| >65 | 14 | 35.3% | 3387 | 26.7–43.9 | 96.8% | 0.000 | 0.082 |
| <65 | 17 | 44.5% | 12 172 | 38.7–50.2 | 96.5% | 0.000 | |
| COPD classifications | |||||||
| I | 4 | 24.5% | 104 | 2.4–46.7 | 83.1% | 0.030 | 0.024 |
| II | 7 | 34.1% | 437 | 15.6–52.6 | 95.9% | 0.000 | |
| III | 8 | 38.6% | 563 | 23.0–54.2 | 92.3% | 0.000 | |
| IV | 8 | 61.5% | 358 | 46.2–76.8 | 90.2% | 0.000 | |
| Gender | |||||||
| Female | 14 | 43.5% | 2110 | 38.0–49.1 | 81.5% | 0.000 | 0.720 |
| Male | 14 | 42.6% | 3205 | 33.5–51.8 | 95.7% | 0.000 | |
| PH grades | |||||||
| Mild | 5 | 30.2% | 543 | 22.3–38.0 | 73.9% | 0.000 | 0.000 |
| Moderate | 5 | 10.0% | 543 | 5.7–14.3 | 64.3% | 0.000 | |
| Severe | 5 | 7.2% | 543 | 1.4–13.0 | 90.1% | 0.015 | |
| Sample source | |||||||
| Lung transplants | 8 | 44.5% | 10 844 | 37.3–51.7 | 97.2% | 0.000 | 0.247 |
| Outpatients | 6 | 40.5% | 1191 | 33.9–47.2 | 98.2% | 0.000 | |
| Inpatients | 7 | 32.3% | 2130 | 19.4–45.2 | 97.8% | 0.000 | |
| Sample type | |||||||
| Stable phase | 19 | 38.1% | 4209 | 30.6–45.7 | 96.6% | 0.000 | 0.998 |
| AECOPD | 3 | 38.2% | 717 | 30.9–45.4 | 98.7% | 0.012 | |
Abbreviations: AECOPD, acute exacerbation of chronic obstructive pulmonary disease; TTE, transthoracic echocardiography; PH, pulmonary hypertension; RHC, right heart catheterization.
Publication Bias and Sensitivity Analyses
The funnel plot, Egger test, and Begg test showed no publication bias (Figure 3). The sensitivity analyses showed that the pooled prevalence of COPD-related PH varied from 38.55% (95% CI: 33.26–43.84) to 39.94% (95% CI: 34.77–45.10) after deleting any one study (Figure 4); however, no study had an undue effect on the pooled prevalence.
Figure 3.
Funnel plot, Egger test, and Begg test for assessing publication bias.
Figure 4.
Sensitivity analysis of the COPD-related PH pooled prevalence.
Discussion
This meta-analysis was designed to provide an updated estimate of COPD-related PH prevalence combined with data from 1991 to 2018. To our knowledge, this is the first comparative systematic review and meta-analysis to assess differences in COPD-related PH prevalence rates to date. In this study, we systematically collected available evidence and reckoned the overall prevalence of COPD-related PH in 16 regions. Based on the meta-analysis involving 38 studies and 16 345 participants, the pooled prevalence of COPD-related PH was 39.2% (95% CI: 34.0–44.4). Heterogeneity was observed between the included studies, explained by differences in definitions and assessed by subgroup analyses.
The current meta-analysis suggested that the pooled prevalence of COPD-related PH varied with COPD severity (P = 0.024) or PH grades (P = 0.000). As the severity of COPD increased, the prevalence of PH gradually elevated, of which most were mild (30.2% incidence) and few were severe (7.2% incidence), consistent with the general understanding of the disease. In fact, the Global Initiative for Chronic Obstructive Pulmonary Disease (GOLD) conducted a study on Phase 4 patients, suggesting that up to 90% of them may have an abnormal mPAP (>20 mmHg), most of which may range between 20 and 35 mmHg; only about 1% to 5% of the patients with COPD have a resting mPAP greater than 35 to 40 mmHg.22 PH in COPD is caused by pulmonary vascular remodeling, which comprise toxic effects of cigarette smoke and is characterized by the following factors that interact either additively or independently: intimal proliferation of poorly differentiated smooth muscle cells, deposition of elastic and collagen fibers, a decrease in pulmonary vascular surface, parenchymal loss, inflammation, and alveolar hypoxia.23–26 However, the specific pathophysiological mechanism, explaining why some patients with COPD develop severe PH, remains unclear.
In addition to being associated with COPD severity, significant regional differences were found in the incidence of PH (P = 0.000). The prevalence of PH was the lowest in Europe (30.4%) and the highest in Africa (64.0%). These data were similar to the results of a meta-analysis focused on the prevalence of PH in an African population, in which 51 patients with COPD had a PH prevalence of 62.7% (95% CI: 49.0–74.7).27 In fact, many of the known PH risk factors, such as schistosomiasis, human immunodeficiency virus, homozygous sickle cell disease, and poor medical conditions, are highly prevalent in Africa; although some have not been fully explored, these risk factors may contribute to the high incidence of PH.28–31 PH can be caused by a multitude of conditions and comorbidities highly prevalent in low- and middle-income countries and may complicate majority of respiratory and cardiovascular diseases, leading to excess morbidity and mortality in multimorbid patients. Approximately 80% of the global burden of COPD-related PH occurs in low- and middle-income countries.31,32 Based on these statements, the prevalence of COPD-related PH in Africa is likely to be largely underestimated. Since the majority of the included studies were from Asia or Europe, while the rest were from North America or Africa, we were unable to examine the prevalence of COPD-related PH in South America and Oceania due to the lack of data from these regions. Therefore, in the future, population-based studies are needed to estimate and compare the prevalence of COPD-related PH in different geographic regions.
Multiple registries noted a larger proportion of women with PH, ranging from 56% to 86%, suggesting that the female sex is a risk factor for PH.33–36 Further, women have a higher prevalence rate in at least seven comorbidities and a significantly worse prognosis that correlates with the number of comorbidities; however, on average, women have been reported to show fewer comorbidities.37 The gender-related difference was not found in our study most likely because it is generally male-dominated, and males have a higher degree of exposure to the risk factors. This proportion may change in the future as the number of female smokers increase. Similarly, no difference was observed in population-stratified studies with a 65-year cut-off most likely because the patients in the included studies were generally of advanced age, and the age differences were not significant. Additionally, it may have been affected by the number of smoking years. In summary, age appears to have little effect on PH prevalence in COPD. The prevalence of PH has steadily increased with the increasing PH awareness and population aging; however, we did not find a significant difference in PH prevalence among COPD populations over time; This may be explained by the increasing number of patients with COPD year by year, and its improved treatment delaying the progression of PH.
We also analyzed other possible influencing factors for the prevalence of COPD-related PH, including enrolment time and participant recruitment settings (lung transplant patients, outpatients or inpatients; the acute exacerbation or stable phase of COPD). However, we did not find significant differences between these factors. Cardiovascular comorbidity is common among COPD patients. Some studies have suggested that AECOPD can further aggravate cardiovascular comorbidity with a worse prognosis.38–40 However, there are limited studies on whether AECOPD exacerbates PH compared to that during a stable period. For this reason, the role of AECOPD in PH incidence cannot be determined at present. As some included studies lacked sufficient information for analysis, the results may be biased to some extent. More external validation is needed in the future to support our results.
Considering PH diagnostic methods, TTE remains the most important non-invasive screening tool, and RHC remains a prerequisite for diagnosis.41,42 We also performed subgroup statistics on prevalence for the two diagnostic assessment methods. Nevertheless, no significant differences were found. Previous studies have shown that TTE demonstrates a low sensitivity or specificity in patients with a definite pulmonary disease.43,44 Our results may be interpreted as more suitable for population studies rather than for the definitive diagnosis of individual patients with PH.45
Limitations
Although a comprehensive search strategy, dual review process, and rigorous selection criteria were used in this meta-analysis, several inherent limitations of this study should also be recognized. First, this meta-analysis was based on cases that had different definitions for the diseases and varied from self-reported to spiroscopy-based results, increasing the heterogeneity observed and in turn, influencing the overall prevalence estimated in our study. The diagnostic definition for COPD also varied even in the spirometry-based studies partly due to changes in diagnostic guidelines over time. Second, although we observed heterogeneity in the overall prevalence and conducted subgroup analyses, some studies did not include relevant data, resulting to a small number of studies in some subgroups and possibly producing selection bias. Third, the meta-regression analysis was not performed on other factors, such as BMI, pack years of smoking, and family history, due to limited data. Lastly, only studies in English were included, and articles published in other languages were excluded from the study, limiting the comprehensiveness of the literature Indexed. More studies are needed to overcome these limitations and make a more comprehensive statistics on the prevalence of COPD-related PH.
Conclusion
In summary, our study analyzed the global prevalence of COPD-related PH to date and found a high pooled prevalence of 39.2%. The prevalence of PH increased with COPD severity, with the majority being mild PH, which is associated with increased mortality and morbidity in patients with COPD. These suggest the necessity of raising public awareness on PH associated with COPD because of an enormous personal and social burden caused by its high prevalence and destructive power. Furthermore, the prevalence rates in Africa appear to be significantly higher than those in western countries, which highlights the potential for additional risk factors and the need for more medical attention in Africa. Our study provides an updated summary of COPD-related PH epidemiology in the general population for reference. However, the available evidence of this synthesis cannot fully cover the content of COPD-related PH prevalence worldwide. More epidemiological studies worldwide are needed to better understand the global disease burden of COPD-related PH.
Acknowledgments
Thanks to the authors of the included studies to provide primary data.
Funding Statement
The study was supported by the National Natural Science Foundation of China (No. 21677030), the Key Research and Development Project of Liaoning Province, China (No. 2017225009), and the Science and Technology Project of Shenyang, China (No. 202054021).
Abbreviations
COPD, chronic obstructive pulmonary disease; AECOPD, acute exacerbations of chronic obstructive pulmonary disease; PH, pulmonary hypertension; GBD, Global Burden of Disease; GOLD, Global Initiative for Chronic Obstructive Pulmonary Disease; mPAP, mean pulmonary arterial pressure; PVR, pulmonary vascular resistance; RHC, right heart catheterization; SvO2, pulmonary oxygen saturation; TTE, transthoracic echocardiography.
Data Sharing Statement
There are some amendments to information provided at registration. The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
The present meta-analysis was based entirely on previously published research and did not require the approval of an ethics committee.
Author Contributions
LMZ and YJL drafted the manuscript. LMZ and GYJ contributed to the study concept or design. SZ and ZW acquired the data. XZW supervised and coordinated the study. All authors made a significant contribution to the work reported in terms of execution, analysis, and interpretation; took part in revising the manuscript; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted and to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Lareau SC, Fahy B, Meek P, et al. Chronic obstructive pulmonary disease (COPD). Am J Respir Crit Care Med. 2019;199(1):P1–P2. doi: 10.1164/rccm.1991P1 [DOI] [PubMed] [Google Scholar]
- 2.Nici L, Mammen MJ, Charbek E, et al. Pharmacologic management of chronic obstructive pulmonary disease. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201(9):e56–e69. doi: 10.1164/rccm.202003-0625ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halpin DMG, Criner GJ, Papi A, et al. Global initiative for the diagnosis, management, and prevention of chronic obstructive lung disease. The 2020 GOLD science committee report on COVID-19 and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2021;203(1):24–36. doi: 10.1164/rccm.202009-3533SO [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2019;394(10204):1145–1158. doi: 10.1016/S0140-6736(19)30427-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soriano JB, Kendrick PJ, Paulson KR; GBD Chronic Respiratory Disease Collaborators. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet Respir Med. 2020;8(6):585–596. doi: 10.1016/S2213-2600(20)30105-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feetham L, van Dorn A. Chronic obstructive pulmonary disease (COPD). Lancet Respir Med. 2017;5(1):18–19. doi: 10.1016/S2213-2600(16)30442-8 [DOI] [PubMed] [Google Scholar]
- 7.Scharf SM, Iqbal M, Keller C, Criner G, Lee S, Fessler HE. Hemodynamic characterization of patients with severe emphysema. Am J Respir Crit Care Med. 2002;166(3):314–322. doi: 10.1164/rccm.2107027 [DOI] [PubMed] [Google Scholar]
- 8.Thabut G, Dauriat G, Stern JB, et al. Pulmonary hemodynamics in advanced COPD candidates for lung volume reduction surgery or lung transplantation. Chest. 2005;127(5):1531–1536. doi: 10.1378/chest.127.5.1531 [DOI] [PubMed] [Google Scholar]
- 9.Chaouat A, Bugnet AS, Kadaoui N, et al. Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172(2):189–194. doi: 10.1164/rccm.200401-006OC [DOI] [PubMed] [Google Scholar]
- 10.Medrek SK, Sharafkhaneh A, Spiegelman AM, Kak A, Pandit LM. Admission for COPD exacerbation is associated with the clinical diagnosis of pulmonary hypertension: results from a retrospective longitudinal study of a veteran population. COPD. 2017;14(5):484–489. doi: 10.1080/15412555.2017.1336209 [DOI] [PubMed] [Google Scholar]
- 11.Wheaton AG, Cunningham TJ, Ford ES, Croft JB. Centers for Disease Control and Prevention (CDC). Employment and activity limitations among adults with chronic obstructive pulmonary disease–United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64(11):289–295. [PMC free article] [PubMed] [Google Scholar]
- 12.Olschewski H, Behr J, Bremer H, et al. Pulmonary hypertension due to lung diseases: updated recommendations from the cologne consensus conference 2018. Int J Cardiol. 2018;272S:63–68. doi: 10.1016/j.ijcard.2018.08.043 [DOI] [PubMed] [Google Scholar]
- 13.Hirani N, Brunner NW, Kapasi A, et al. Canadian Cardiovascular Society/Canadian Thoracic Society position statement on pulmonary hypertension. Can J Cardiol. 2020;36(7):977–992. doi: 10.1016/j.cjca.2019.11.041 [DOI] [PubMed] [Google Scholar]
- 14.Mueller-Mottet S, Stricker H, Domenighetti G, et al. Long-term data from the Swiss pulmonary hypertension registry. Respiration. 2015;89(2):127–140. doi: 10.1159/000370125 [DOI] [PubMed] [Google Scholar]
- 15.Andersen KH, Iversen M, Kjaergaard J, et al. Prevalence, predictors, and survival in pulmonary hypertension related to end-stage chronic obstructive pulmonary disease. J Heart Lung Transplant. 2012;31(4):373–380. doi: 10.1016/j.healun.2011.11.020 [DOI] [PubMed] [Google Scholar]
- 16.Hurdman J, Condliffe R, Elliot CA, et al. Pulmonary hypertension in COPD: results from the ASPIRE registry. Eur Respir J. 2013;41(6):1292–1301. doi: 10.1183/09031936.00079512 [DOI] [PubMed] [Google Scholar]
- 17.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 18.Loney PL, Chambers LW, Bennett KJ, Roberts JG, Stratford PW. Critical appraisal of the health research literature: prevalence or incidence of a health problem. Chronic Dis Can. 1998;19(4):170–176. [PubMed] [Google Scholar]
- 19.Wells GA, Shea B, O’Connell D, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed March 15, 2021.
- 20.Luchini C, Stubbs B, Solmi M, et al. Assessing the quality of studies in meta-analyses: advantages and limitations of the Newcastle Ottawa scale. World J Meta-Anal. 2017;5(4):80–84. doi: 10.13105/wjma.v5.i4.80 [DOI] [Google Scholar]
- 21.Mavridis D, Salanti G. How to assess publication bias: funnel plot, trim-and-fill method and selection models. Evid Based Ment Health. 2014;17(1):30. doi: 10.1136/eb-2013-101699 [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann J, Wilhelm J, Olschewski A, Kwapiszewska G. Microarray analysis in pulmonary hypertension. Eur Respir J. 2016;48(1):229–241. doi: 10.1183/13993003.02030-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Opitz I, Ulrich S. Pulmonary hypertension in chronic obstructive pulmonary disease and emphysema patients: prevalence, therapeutic options and pulmonary circulatory effects of lung volume reduction surgery. J Thorac Dis. 2018;10(Suppl 23):S2763–S2774. doi: 10.21037/jtd.2018.07.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rahaghi FN, Argemí G, Nardelli P, et al. Pulmonary vascular density: comparison of findings on computed tomography imaging with histology. Eur Respir J. 2019;54(2):1900370. doi: 10.1183/13993003.00370-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Washko GR, Nardelli P, Ash SY, et al. Arterial vascular pruning, right ventricular size, and clinical outcomes in chronic obstructive pulmonary disease a longitudinal observational study. Am J Respir Crit Care Med. 2019;200(4):454–461. doi: 10.1164/rccm.201811-2063OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blanco I, Tura-Ceide O, Peinado VI, Barberà JA. Updated perspectives on pulmonary hypertension in COPD. Int J Chron Obstruct Pulmon Dis. 2020;15:1315–1324. doi: 10.2147/COPD.S211841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bigna JJ, Noubiap JJ, Nansseu JR, Aminde LN. Prevalence and etiologies of pulmonary hypertension in Africa: a systematic review and meta-analysis. BMC Pulm Med. 2017;17(1):183. doi: 10.1186/s12890-017-0549-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murray CJ, Ortblad KF, Guinovart C, et al. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990–2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2014;384(9947):1005–1070. doi: 10.1016/S0140-6736(14)60844-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet. 2014;383(9936):2253–2264. doi: 10.1016/S0140-6736(13)61949-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bigna JJ, Nansseu JR, Um LN, et al. Prevalence and incidence of pulmonary hypertension among HIV-infected people in Africa: a systematic review and meta-analysis. BMJ Open. 2016;6(8):e011921. doi: 10.1136/bmjopen-2016-011921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoeper MM, Humbert M, Souza R, et al. A global view of pulmonary hypertension. Lancet Respir Med. 2016;4(4):306–322. doi: 10.1016/S2213-2600(15)00543-3 [DOI] [PubMed] [Google Scholar]
- 32.Mocumbi AO, Thienemann F, Sliwa K. A global perspective on the epidemiology of pulmonary hypertension. Can J Cardiol. 2015;31(4):375–381. doi: 10.1016/j.cjca.2015.01.030 [DOI] [PubMed] [Google Scholar]
- 33.Hoeper MM, Huscher D, Ghofrani HA, et al. Elderly patients diagnosed with idiopathic pulmonary arterial hypertension: results from the COMPERA registry. Int J Cardiol. 2013;168(2):871–880. doi: 10.1016/j.ijcard.2012.10.026 [DOI] [PubMed] [Google Scholar]
- 34.Valverde AB, Soares JM, Viana KP, Gomes B, Soares C, Souza R. Pulmonary arterial hypertension in Latin America: epidemiological data from local studies. BMC Pulm Med. 2018;18(1):106. doi: 10.1186/s12890-018-0667-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kjellström B, Nisell M, Kylhammar D, et al. Sex-specific differences and survival in patients with idiopathic pulmonary arterial hypertension 2008–2016. ERJ Open Res. 2019;5(3):00075–2019. doi: 10.1183/23120541.00075-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheron C, McBride SA, Antigny F, et al. Sex and gender in pulmonary arterial hypertension. Eur Respir Rev. 2021;30(162):200330. doi: 10.1183/16000617.0330-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grabicki M, Kuźnar-Kamińska B, Rubinsztajn R, et al. COPD course and comorbidities: are there gender differences? Adv Exp Med Biol. 2019;1113:43–51. [DOI] [PubMed] [Google Scholar]
- 38.Almagro P, López García F, Cabrera FJ, et al. Comorbidity and gender-related differences in patients hospitalized for COPD. The ECCO study. Respir Med. 2010;104(2):253–259. doi: 10.1016/j.rmed.2009.09.019 [DOI] [PubMed] [Google Scholar]
- 39.Chung LP, Winship P, Phung S, Lake F, Waterer G. Five-year outcome in COPD patients after their first episode of acute exacerbation treated with non-invasive ventilation. Respirology. 2010;15(7):1084–1091. doi: 10.1111/j.1440-1843.2010.01795.x [DOI] [PubMed] [Google Scholar]
- 40.Pavasini R, d’Ascenzo F, Campo G, et al. Cardiac troponin elevation predicts all-cause mortality in patients with acute exacerbation of chronic obstructive pulmonary disease: systematic review and meta-analysis. Int J Cardiol. 2015;191:187–193. doi: 10.1016/j.ijcard.2015.05.006 [DOI] [PubMed] [Google Scholar]
- 41.Frost A, Badesch D, Gibbs JSR, et al. Diagnosis of pulmonary hypertension. Eur Respir J. 2019;53(1):1801904. doi: 10.1183/13993003.01904-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang WC, Hsu CH, Sung SH, et al. 2018 TSOC guideline focused update on diagnosis and treatment of pulmonary arterial hypertension. J Formos Med Assoc. 2019;118(12):1584–1609. doi: 10.1016/j.jfma.2018.12.009 [DOI] [PubMed] [Google Scholar]
- 43.Ni JR, Yan PJ, Liu SD, et al. Diagnostic accuracy of transthoracic echocardiography for pulmonary hypertension: a systematic review and meta-analysis. BMJ Open. 2019;9(12):e033084. doi: 10.1136/bmjopen-2019-033084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keir GJ, Wort SJ, Kokosi M, et al. Pulmonary hypertension in interstitial lung disease: limitations of echocardiography compared to cardiac catheterization. Respirology. 2018;23(7):687–694. doi: 10.1111/resp.13250 [DOI] [PubMed] [Google Scholar]
- 45.D’Alto M, Bossone E, Opotowsky AR, Ghio S, Rudski LG, Naeije R. Strengths and weaknesses of echocardiography for the diagnosis of pulmonary hypertension. Int J Cardiol. 2018;263:177–183. doi: 10.1016/j.ijcard.2018.04.024 [DOI] [PubMed] [Google Scholar]
- 46.Acharya V, Mugularao S, Seshadri S, Shetty RK. Assessment of diastolic dysfunction parameters and cardiac chamber size in smokers with COPD: a case control study. J Clin Diagn Res. 2018;12(1):45. [Google Scholar]
- 47.Aksu F, Capan N, Aksu K, et al. C-reactive protein levels are raised in stable Chronic obstructive pulmonary disease patients independent of smoking behavior and biomass exposure. J Thorac Dis. 2013;5(4):414–421. doi: 10.3978/j.issn.2072-1439.2013.06.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alkukhun L, Baumgartner M, Budev M, Dweik RA, Tonelli AR. Electrocardiographic differences between COPD patients evaluated for lung transplantation with and without pulmonary hypertension. COPD. 2014;11(6):670–680. doi: 10.3109/15412555.2014.898047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blanco I, Valeiro B, Torres-Castro R, et al. Effects of pulmonary hypertension on exercise capacity in patients with chronic obstructive pulmonary disease. Arch Bronconeumol. 2020;56(8):499–505. doi: 10.1016/j.arbr.2019.10.018 [DOI] [PubMed] [Google Scholar]
- 50.Buklioska-Ilievska D, Minov J, Kochovska-Kamchevska N, et al. Cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: echocardiography changes and their relation to the level of airflow limitation. Open Access Maced J Med Sci. 2019;7(21):3568–3573. doi: 10.3889/oamjms.2019.848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chaouat A, Savale L, Chouaid C, et al. Role for interleukin-6 in COPD-related pulmonary hypertension. Chest. 2009;136(3):678–687. doi: 10.1378/chest.08-2420 [DOI] [PubMed] [Google Scholar]
- 52.Chen X, Liu K, Wang Z, et al. Computed tomography measurement of pulmonary artery for diagnosis of COPD and its comorbidity pulmonary hypertension. Int J Chron Obstruct Pulmon Dis. 2015;10:2525–2533. doi: 10.2147/COPD.S94211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fayngersh V, Drakopanagiotakis F, Dennis McCool F, Klinger JR. Pulmonary hypertension in a stable community-based COPD population. Lung. 2011;189(5):377–382. doi: 10.1007/s00408-011-9315-2 [DOI] [PubMed] [Google Scholar]
- 54.Freixa X, Portillo K, Paré C, et al. Echocardiographic abnormalities in patients with COPD at their first hospital admission. Eur Respir J. 2013;41(4):784–791. doi: 10.1183/09031936.00222511 [DOI] [PubMed] [Google Scholar]
- 55.Gartman EJ, Blundin M, Klinger JR, Yammine J, Roberts MB, Dennis McCool F. Initial risk assessment for pulmonary hypertension in patients with COPD. Lung. 2012;190(1):83–89. doi: 10.1007/s00408-011-9346-8 [DOI] [PubMed] [Google Scholar]
- 56.Gupta KK, Roy B, Chaudhary SC, et al. Prevalence of pulmonary artery hypertension in patients of chronic obstructive pulmonary disease and its correlation with stages of chronic obstructive pulmonary disease, exercising capacity, and quality of life. J Family Med Prim Care. 2018;7(1):53–57. doi: 10.4103/jfmpc.jfmpc_18_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Halvani A, Haddad H. Comparison of the factors influencing pulmonary arterial pressure in smoker and non-smoker COPD patients with pulmonary hypertension. Tanaffos. 2019;18(1):41–46. [PMC free article] [PubMed] [Google Scholar]
- 58.Hayes D Jr, Tumin D, Budev MM, et al. Adverse outcomes associated with pulmonary hypertension in chronic obstructive pulmonary disease after bilateral lung transplantation. Respir Med. 2017;128:102–108. doi: 10.1016/j.rmed.2017.04.010 [DOI] [PubMed] [Google Scholar]
- 59.Hilde JM, Skjørten I, Hansteen V, et al. Assessment of right ventricular afterload in COPD. COPD. 2016;13(2):176–185. doi: 10.3109/15412555.2015.1057275 [DOI] [PubMed] [Google Scholar]
- 60.Jethani V, Sindhwani G. Chronic obstructive pulmonary disease and cardiac comorbidities: a cross-sectional study. Lung India. 2016;33(6):697. doi: 10.4103/0970-2113.192866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kwon YS, Chi SY, Shin HJ, et al. Plasma C-reactive protein and endothelin-1 level in patients with chronic obstructive pulmonary disease and pulmonary hypertension. J Korean Med Sci. 2010;25(10):1487–1491. doi: 10.3346/jkms.2010.25.10.1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Malinovschi A, Masoero M, Bellocchia M, et al. Severe vitamin D deficiency is associated with frequent exacerbations and hospitalization in COPD patients. Respir Res. 2014;15(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matsuyama W, Ohkubo R, Michizono K, et al. Usefulness of transcutaneous Doppler jugular venous echo to predict pulmonary hypertension in COPD patients. Eur Respir J. 2001;17(6):1128–1131. doi: 10.1183/09031936.01.00088401 [DOI] [PubMed] [Google Scholar]
- 64.Mohamed Hoesein FA, Besselink T, Pompe E, et al. Accuracy of CT pulmonary artery diameter for pulmonary hypertension in end-stage COPD. Lung. 2016;194(5):813–819. doi: 10.1007/s00408-016-9926-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mohamed MF, Ali A, Abbas A, Awad MS, Gouda M, Sediq AM. Mean platelet volume as a predictor of pulmonary hypertension in patients with stable COPD. Int J Chron Obstruct Pulmon Dis. 2019;14:1099–1108. doi: 10.2147/COPD.S176413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nakahara Y, Taniguchi H, Kimura T, et al. Exercise hypoxaemia as a predictor of pulmonary hypertension in COPD patients without severe resting hypoxaemia. Respirology. 2017;22(1):120–125. doi: 10.1111/resp.12863 [DOI] [PubMed] [Google Scholar]
- 67.Nakayama S, Chubachi S, Sakurai K, et al. Characteristics of chronic obstructive pulmonary disease patients with pulmonary hypertension assessed by echocardiography in a three-year observational cohort study. Int J Chron Obstruct Pulmon Dis. 2020;15:487–499. doi: 10.2147/COPD.S230952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nathan SD, Barnett SD, King CS, et al. Impact of the new definition for pulmonary hypertension in patients with lung disease: an analysis of the United Network for organ sharing database. Pulm Circ. 2021;11(2):2045894021999960. doi: 10.1177/2045894021999960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Portillo K, Torralba Y, Blanco I, et al. Pulmonary hemodynamic profile in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2015;10:1313–1320. doi: 10.2147/COPD.S78180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sertogullarindan B, Gumrukcuoglu HA, Sezgi C, Akil MA. Frequency of pulmonary hypertension in patients with COPD due to biomass smoke and tobacco smoke. Int J Med Sci. 2012;9(6):406–412. doi: 10.7150/ijms.4715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Seyhan EC, Özgül MA, Tutar N, Ömür I, Uysal A, Altin S. Red blood cell distribution and survival in patients with chronic obstructive pulmonary disease. COPD. 2013;10(4):416–424. doi: 10.3109/15412555.2012.758697 [DOI] [PubMed] [Google Scholar]
- 72.El-Shabrawy M, Eldamanhory AS. Study of cardiovascular diseases in hospitalized AECOPD patients. Egypt J Chest Dis Tuberc. 2017;66(1):17–25. doi: 10.1016/j.ejcdt.2016.08.008 [DOI] [Google Scholar]
- 73.Shin S, King CS, Brown AW, et al. Pulmonary artery size as a predictor of pulmonary hypertension and outcomes in patients with chronic obstructive pulmonary disease. Respir Med. 2014;108(11):1626–1632. doi: 10.1016/j.rmed.2014.08.009 [DOI] [PubMed] [Google Scholar]
- 74.Sims MW, Margolis DJ, Localio AR, Panettieri RA, Kawut SM, Christie JD. Impact of pulmonary artery pressure on exercise function in severe COPD. Chest. 2009;136(2):412–419. doi: 10.1378/chest.08-2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Skjørten I, Hilde JM, Melsom MN, Hansteen V, Steine K, Humerfelt S. Pulmonary artery pressure and PaO2 in chronic obstructive pulmonary disease. Respir Med. 2013;107(8):1271–1279. doi: 10.1016/j.rmed.2013.03.021 [DOI] [PubMed] [Google Scholar]
- 76.Sridhara VSHK, Acharya V. Comorbidities of chronic obstructive pulmonary disease and their affect on hospitalization of patients in a tertiary care hospital. J Community Hosp Intern Med Perspect. 2021;11(1):120–123. doi: 10.1080/20009666.2020.1843823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stolz D, Christ-Crain M, Morgenthaler NG, et al. Plasma pro-adrenomedullin but not plasma pro-endothelin predicts survival in exacerbations of COPD. Chest. 2008;134(2):263–272. [DOI] [PubMed] [Google Scholar]
- 78.Sun WL, Wang JL, Jia GH, et al. Impact of obstructive sleep apnea on pulmonary hypertension in patients with chronic obstructive pulmonary disease. Chin Med J. 2019;132(11):1272–1282. doi: 10.1097/CM9.0000000000000247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Takahashi T, Yoshihisa A, Sugimoto K, et al. Associations between diabetes mellitus and pulmonary hypertension in chronic respiratory disease patients. PLoS One. 2018;13(10):e0205008. doi: 10.1371/journal.pone.0205008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xiong W, Zhao Y, Gong S, Zhao Q, Liu J. Prophylactic function of excellent compliance with LTOT in the development of pulmonary hypertension due to COPD with hypoxemia. Pulm Circ. 2018;8(2):2045894018765835. doi: 10.1177/2045894018765835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xiong M, Guo M, Huang D, Li J, Zhou Y. TRPV1 genetic polymorphisms and risk of COPD or COPD combined with PH in the Han Chinese population. Cell Cycle. 2020;19(22):3066–3073. doi: 10.1080/15384101.2020.1831246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yazici O, Tas Gulen S, Eryilmaz U, Omurlu IK. The evaluation of cardiac functions according to chronic obstructive pulmonary disease groups. Aging Male. 2020;23(2):106–111. doi: 10.1080/13685538.2019.1606191 [DOI] [PubMed] [Google Scholar]