Abstract
Cannabidiol (CBD) is a major non-euphoric cannabis-derived compound that has become popular in its over-the-counter utilization. CBD possesses low affinity for cannabinoid receptors, while the primary molecular target(s) by which CBD mediates biological activity remain poorly defined. Individuals commonly self-medicate using CBD products with little knowledge of its specific immunopharmacological effects on the human immune system; however, research has established primarily in rodent models that CBD possesses immune modulating properties. The objective of this study was to evaluate whether CBD modulates the innate immune response by human primary monocytes activated through toll-like receptors (TLR) 1–9. Monocytes were activated through each TLR and treated with CBD (0.5–10μM) for 22 hours. Monocyte secretion profiles for 13 immune mediators were quantified including: IL-4, IL-2, IP-10, IL-1β, TNFα, MCP-1, IL-17a, IL-6, IL-10, IFNγ, IL-12p70, IL-8, and TGF-β1. CBD treatment significantly suppressed secretion of proinflammatory cytokine IL-1β by monocytes activated through most TLRs, apart from TLRs 3 and 8. Additionally, CBD treatment induced significant modulation of IL-6 production by monocytes activated through most TLRs, except for TLRs 1 and 3. Most other monocyte-derived factors assayed were refractory to CBD modulation. Overall, CBD selectively altered monocyte-derived IL-1β and IL-6 when activated through most TLRs. This study is of particular importance as it provides a direct and comprehensive assessment of the effects of CBD on TLR-activated primary human monocytes at a time when CBD containing products are being widely used by the public.
Keywords: cannabidiol (CBD), cannabinoid, monocyte, TLR, IL-1β, IL-6
1. Introduction
Cannabidiol (CBD) is a cannabis-derived compound that has recently drawn attention in the research community and public domain for its potential therapeutic effects. CBD, although non-euphoric, shares structural similarity with Δ9-tetrahydrocannabinol (THC), the main psychotropic phytocannabinoid (Gaoni and Mechoulam 1964). Medical grade CBD has been approved by the FDA in 2018 (Epidiolex®) for the treatment of seizures associated with Lennox-Gastaut syndrome, Dravet syndrome, as well as tuberous sclerosis complex (Devinsky et al. 2017; Devinsky et al. 2018; Thiele et al. 2021). In states where cannabis use has been legalized, or decriminalized for recreational use, CBD has become widely available to the public through its commercialization as oil, edible, and tincture products. As a result, these products have become widely popular for self-medication purposes as anti-inflammatory agents, anxiolytics, sleep aids, as well as analgesics (McFadden and Malone 2021; Piper et al. 2017; Reiman et al. 2017). Consequently, there has been an increase in research to determine its efficacy and mechanism of action in mediating these effects, of which particular interest is the putative anti-inflammatory activity of CBD. In vivo studies provide evidence that CBD treatment decreases inflammation in various murine disease models for carrageenan-induced inflammation (Lodzki et al. 2003), rheumatoid arthritis (Hammell et al. 2016), and colitis (Borrelli et al. 2009). Furthermore, ex vivo murine studies have provided evidence that CBD can suppress splenocyte IFN-γ production in response to phorbol 12-myristate 12-acetate/ionomycin (PMA/Io) and CD3/CD28 (Showalter et al. 1996). However, there is relatively little published research on the immune modulating and anti-inflammatory activity of CBD concerning the human immune system.
Research has suggested that CBD may mediate its biological activity via a number of molecular targets. Unlike THC, which exerts much of its biological activity through binding CB1 and CB2 receptors (Matsuda et al. 1990; Munro et al. 1993), CBD possesses low binding affinity to both CB1 and CB2 (Kaplan et al. 2008; Matsuda et al. 1990; Pertwee 2008). Although some studies have proposed that CBD acts as an antagonist at CB1 and CB2 receptors, other studies suggest its actions are through negative allosteric modulation (Laprairie et al. 2015; Martínez-Pinilla et al. 2017; Tham et al. 2019). Further research has identified additional targets by which CBD mediates its effects, such as its inhibitory activity on fatty acid amide hydrolase (Bisogno et al. 2001; Elmes et al. 2015) and GPR55 (Chiurchiù et al. 2015; Ryberg et al. 2007; Whalley et al. 2018), as well as its simultaneous agonistic activity on various members of the transient receptor potential (TRP) family (Bisogno et al. 2001; De Petrocellis et al. 2011), adenosine A2 receptors (Carrier et al. 2006; Castillo et al. 2010), peroxisome proliferator-activated receptor gamma (PPAR-γ) (Hind et al. 2016; Sonego et al. 2018), and 5-HT receptors (Russo et al. 2005). Most of these targets are thought to have the potential of contributing to the immune modulating effects of CBD at various concentrations from 0.6 to 20 μM, while also providing the rationale for investigating its anti-inflammatory properties.
Inflammation is the body’s natural line of defense against pathogens and injury; however, persistent inflammation can be associated with or the direct cause of a plethora of disease states (Furman et al. 2019). Inflammation is generally associated as a response of the innate immune system, which utilizes various pattern recognition receptors (PRRs) as one of the first lines of defense by detecting molecular signatures commonly associated with foreign pathogens (Amarante-Mendes et al. 2018). Toll-like receptors (TLRs) are a class of PRRs that play a critical role in responding to various bacterial and viral pathogen associated molecular patterns (PAMPs), as well as self-derived damage associated molecular patterns (DAMPs) (Kawasaki and Kawai 2014). Upon pathogen-receptor binding, downstream signaling pathways become activated, resulting in cytokine release, triggering host defense responses such as inflammation, innate immune system activation, as well as recruitment of the adaptive immune system (Kawasaki and Kawai 2014). There are currently 10 members of the TLR family identified in humans, with each member possessing a unique binding pocket facilitating specific binding of PAMPs to signal detection of invasion (Kawasaki and Kawai 2014). TLRs are categorized by their cellular location into two subfamilies: intracellular and extracellular TLRs. Extracellular TLRs (1, 2, 4, 5, and 6) are expressed on the plasma membrane of cells to interact with microbial pathogenic components. Meanwhile, intracellular TLRs (3, 7, 8, and 9) are associated with the endosomal and lysosomal compartments and have the ability to recognize internalized pathogenic DNA and RNA as well as intracellular pathogens (Chaturvedi and Pierce 2009). This family of receptors is widely expressed in innate immune cells, such as dendritic cells, macrophages, and monocytes but is also expressed by adaptive immune cells (e.g., T cells and non-immune cells (e.g., epithelial cells) (Kawasaki and Kawai 2014). Not surprisingly, monocytes, a crucial population of sentinel cells, express all TLRs to some degree (Hornung et al. 2002), which have the ability to initiate monocytes to produce cytokines such as IL-1β, IL-6, TNF-α, and other interleukins (ILs), making this a therapeutic target for influencing innate immunity. A growing body of evidence has demonstrated that cannabinoids have the ability to alter monocyte functions through the suppression of TLR 4- and 7-induced pro-inflammatory cytokines such as IL-1β, IL-6, MCP-1, TNF-α, and IL-8 (Gu et al. 2019; Rizzo et al. 2019). Likewise, CBD can also suppress TLR 4-mediated microglial activation, reactive oxygen species production (Sonego et al. 2018), and cytokine secretion (Kozela et al. 2010).
Although research supports the premise that CBD has immune modulating activity, including altered release of pro and anti-inflammatory cytokines, currently much of this evidence stems from experiments conducted in rodent models (Nichols and Kaplan 2020a) and are generally centered at determining the effects of CBD on adaptive immunity, rather than on the innate immune system. While animal models represent a valuable tool, it is presently unclear how much of the information gathered is translatable to humans. Specifically, rodents have been shown to experience differential inflammatory responses to exogenous agents as compared to humans, and poorly predict human inflammatory disease due to the extreme sensitivity of humans to massive inflammation compared to the murine counterpart (Seok et al. 2013). Thus, the effects of CBD on primary human innate immune cells represents a major data gap. Therefore, the overall objective of this study was to evaluate the direct effects of CBD on TLR-induced pro and anti-inflammatory cytokine and chemokine secretion by human primary monocytes.
2. Material and methods
2.1. Chemicals and reagents
Roswell Park Memorial Institute Medium (RPMI) 1640 (Catalog #: 31800-022) was purchased from Gibco™ by Life Technologies (Carlsbad, CA). Lyophilized CBD (CAS#: 13956-29-1) was obtained from the National Institute on Drug Abuse Drug Repository, Bethesda, MD. Anti-CD45-Pacific Blue (clone: H130, Cat. #:304029), anti-CD14-PeCy7 (M5E2, 301,814), anti-CD16 -APC (3G8, 302,012), anti-CD56-PerCP (HCD56, 318,342), and anti-CD57-PerCP/Cy5.5 (HNK-1, 359,622) antibodies were purchased from BioLegend (San Diego, CA). Toll-like receptor (TLR) agonists, with the exception of lipopolysaccharides (LPS) were purchased from InvivoGen (San Diego, CA) and included: synthetic triacylated lipopeptide (Pam3CysSerLys4) (product ID: tlrl-pms), heat killed listeria monocytogenes (HKLM) (product ID: tlrl-hklm), polyinosine-polycytidylic acid (poly(I:C) LMW) (product ID: tlrl-picw), flagellin from Bacillus subtilis (product ID: tlrl-bsfla), synthetic diacylated lipoprotein (FSL-1) (product ID: tlrl-fsl), Imiquimod (R837) (product ID: tlrl-imq), HIV-1 LTsR-derived ssRNA (product ID: tlrl-lrna40), and stimulatory CpG ODN2395 (class C) (product ID: tlrl-2395). LPS isolated from Salmonella typhosa (LPS) was purchased from Sigma-Aldrich (Cat #: L2387).
2.2. Human leukocyte blood packs and monocyte purification
Human enriched leukocyte blood packs, collected from anonymous donors, were obtained from Gulf Coast Regional Laboratories (Houston, TX). Leukocyte packs that tested negative for HIV, HBV, HCV, HTLV, and SARS-CoV-2 antibodies were shipped at 4°C overnight. Peripheral blood mononuclear cells were isolated from each blood pack by density gradient centrifugation using Ficoll-Paque PLUS (GE Healthcare Life Sciences, Pittsburgh, PA). Briefly, centrifugation was at 1300 × g for 25 min with low acceleration and low brake. Buffy coats were isolated, washed and counted, followed by pan monocyte isolation by negative selection (Human Pan Monocyte Isolation Kit from Miltenyi Biotec (Bergisch Gladbach, Germany) per the manufacturer’s instructions. Immediately after isolation, monocyte purity was assessed by flow cytometry using BD FACS Canto II and FACS Diva Software (BD Bioscience). Data analysis was performed using FlowJo software (Version 10, Treestar Software, Ashland, OR). Only isolations with pan monocyte purity ≥90% were utilized in the following studies. For purity determination cells were first determined by the expression of CD45 (leukocyte common antigen), and lack of CD56 and CD57 (Natural Killer cell markers). Pan monocytes were identified based on cell surface expression of CD14 and CD16. Monocyte subpopulations include classical (CD14++, CD16−), intermediate (CD14+CD16+), and non-classical (CD14-CD16++).
2.3. Monocyte culture and treatment
After pan monocyte isolation, as described in section 2.2, cells were seeded in a 96 well plate at 1 ×105 cells/well. After seeding, monocytes were activated using agonists directed at toll-like receptors (TLRs) 1–9. The agonists were optimized for each TLR and are listed as: Pam3CysSerLys4 (TLR1) (100 ng/mL), HKLM (TLR2) (107 cells/mL), annealed low molecular weight poly(I:C) (TLR 3) (100 μg/mL), LPS (TLR 4) (10 ng/mL), flagellin (TLR5) (5μg/mL), FSL-1 (TLR6) (1 ng/mL), R837 (TLR7) (10 μg/mL), ssRNA (TLR 8) (0.5 μg/mL), and CpG ODN (TLR9 agonist) (15 μg /mL). Prior to evaluation of immune modulating activity of CBD, pilot studies were conducted to identify the optimal concentration required for each TLR to robustly activate human primary monocytes. Concurrent to TLR agonist addition, monocytes were treated with CBD (0, 0.5, 1, 5, or 10 μM) and cultured in RPMI 1640 (Gibco™) supplemented with 5% human AB serum (Sigma-Aldrich, St. Louis, MO), 100 U/mL Penicillin (Gibco™), and 100 μg/mL streptomycin (Gibco™) and incubated at 37°C and 5% CO2 for 22 hours. CBD concentrations utilized within these studies are relevant to human use according to the pharmacokinetic studies conducted in support of Epidiolex® new drug application (Center for Drug Evaluation and Research 2018). Lyophilized CBD was dissolved in 100% ethanol and diluted in RPMI 1640. The vehicle concentration for each treatment was 0.032% ethanol. There is a total of 3 sets of experimental groups within these studies: unstimulated (U.S.), TLR activation only, and TLR activated monocytes treated with either vehicle (Vh) or CBD.
2.4. Quantifying monocyte cytokine production
After a 22-hour incubation of treated monocytes, supernatants were transferred into a new 96 well culture plate and stored at −80°C. Monocyte supernatants were quantified for immune specific cytokines using a 13-plex LEGENDplex™ HU Essential Immune Response Panel (BioLegend, Cat. #: 740930), per the manufacturer’s instructions using the BD FACS Canto II. Cytokine determinations in this LEGENDplex panel include IL-4, IL-2, CXCL10 (IP-10), IL-1β, TNF-α, CCL2 (MCP-1), IL-17A, IL-6, IL-10, IFN-γ, IL-12p70, CXCL8 (IL-8), and TGF-β1. ELISAmax™ technology was utilized to quantify IL-6 and IL-8 levels in supernatants per the manufacturer’s direction when measurements were oversaturated through LEGENDplex™.
2.5. Statistical analysis
Statistical analysis was performed using Prism 7 (GraphPad, San Diego, CA). Non-stimulated naïve, TLR stimulation only, and vehicle groups (Vhwere compared by repeated measures one-way ANOVA, followed by a Sidak’s multiple comparisons post hoc test to compare the mean of each unstimulated control with TLR stimulation only control, and the stimulation only control with the vehicle control. Due to the variation among donors, the measurements in the vehicle and CBD treated groups were normalized to the TLR stimulation only group of the same donor. For multiple comparisons, a repeated-measures one-way ANOVA was performed, followed by a Dunnett’s multiple comparisons post hoc test to compare the mean of each CBD-treatment group with the vehicle treated group. Significant differences are indicated by * p < 0.05, ** p < 0.01, *** p< 0.001, and **** p< 0.0001. Error bars represent the standard error of the mean for each group.
3. Results
3.1. Cell surface TLRs
Extracellular membrane bound TLRs play a critical role in recognizing extracellular microbial membrane components such as lipids, proteins, and lipoproteins, in addition to cell wall components (Kawasaki and Kawai 2014). TLRs found on the cell surface include TLR 1, 2, 4, 5, and 6. Each TLR individually possess unique pathogen recognition patterns and PAMP sensitivities that aid in the identification of invading pathogens by innate immune cells (Kawasaki and Kawai 2014). Here we investigated the effects of CBD on monocyte activation via surface expressed TLRs. Activation of monocytes through these various cell surface TLRs resulted in a varied range of responses as demonstrated by the profile of cytokine and chemokine release. Table 1 depicts a summarization of how CBD modulates monocyte secretion of cytokines/chemokines as a result of activation through each cell surface TLR. Table 1 does not depict results involving IFN-γ and IL-17A, which were below the level of detection in supernatants. Cytokines that were significantly modulated by CBD treatment are presented in the main text as figures for closer inspection of the directionality and level of modulation.
Table 1.
A comprehensive overview of how CBD modulates the secretion of 11 cytokines and chemokines from monocytes activated through extracellular TLRs (1, 2, 4, 5, and 6). Activation columns indicate whether there was a significant difference in cytokine secretion between unstimulated and TLR agonist treated monocytes. CBD effect columns denote whether cytokine production was modulated by CBD treatment, in addition to directionality and concentrations at which significant modulation was observed, (n = 6). Cytokine production below detection is denoted by “−”. Activation columns are denoted by Y (yes) if statistically significant change was observed upon agonist treatment, or N (no). CBD effect columns are denoted by concentrations where CBD produced statistically significant difference, or NE (no effect).
| Cytokine | IL-4 |
LL-2 |
IP-10 |
IL-lβ |
TNFα |
|||||
| Treatment | Activation | CBD effect | Activation | CBD effect | Activation | CBD effect | Activation | CBD effect | Activation | CBD effect |
|
| ||||||||||
| TLR 1 | Y | NE | Y | ↑5 | Y | NE | Y | ↓0.5, 1, 5, 10 | Y | ↑10 |
| TLR 2 | Y | NE | Y | NE | Y | ↓1, 5, 10 | Y | ↓5, 10 | Y | NE |
| TLR 4 | — | — | N | NE | Y | ↓10 | Y | ↓0.5, 1, 5, 10 | Y | NE |
| TLR 5 | Y | NE | Y | NE | Y | NE | Y | ↓1, 5, 10 | Y | ↑10 |
| TLR 6 | Y | NE | N | NE | Y | NE | Y | ↓1, 5, 10 | Y | NE |
|
| ||||||||||
| Cytokine | MCP-1 |
IL-6 |
IL-10 |
LL-12p70 |
IL-8 |
|||||
| Treatment | Activation | CBD effect | Activation | CBD effect | Activation | CBD effect | Activation | CBD effect | Activation | CBD effect |
|
| ||||||||||
| TLR 1 | N | ↑10 | Y | NE | Y | NE | Y | ↑10 | Y | NE |
| TLR 2 | Y | NE | Y | ↓5 | Y | NE | Y | NE | N | NE |
| TLR 4 | N | NE | Y | ↓0.5, 1, 5, 10 | Y | ↓0.5, 1, 10 | Y | NE | Y | NE |
| TLR 5 | Y | ↑10 | Y | ↓1, 5, 10 | N | NE | Y | NE | Y | NE |
| TLR 6 | Y | ↑l, 5 | Y | ↓1, 5, 10 | Y | NE | Y | NE | Y | NE |
|
| ||||||||||
| Cytokine | TGF-β1 |
|||||||||
| Treatment | Activation | CBD effect | ||||||||
|
| ||||||||||
| TLR 1 | Y | NE | ||||||||
| TLR 2 | Y | ↓10 | ||||||||
| TLR 4 | Y | ↓0.5, 1, 5, 10 | ||||||||
| TLR 5 | Y | NE | ||||||||
| TLR 6 | Y | NE | ||||||||
TLR 1 engages in the recognition of triacylated lipoproteins from gram-negative bacteria and mycoplasma through heterodimerization with TLR 2 (El-Zayat et al. 2019). In this experiment, monocytes were treated with a synthetically derived TLR 1 agonist, PAM3CSK4, which mimics the acylated amino terminus of bacterial triacylated lipoproteins. After a 22-hour stimulation of monocytes with the TLR 1 agonist, PAM3CSK4, significant induction above background levels was observed in a number of cytokines. Specifically, TLR 1 ligation upregulated the production of IL-2, IL-4, IP-10, IL-12p70, IL-8, and TGF-β1 in a statistically significant manner, that did not exceed 2-fold above background (Table 1, Fig. 1A and L, Supplemental fig. 1A, C, G, and I). By contrast, PAM3CSK4 treatment induced monocyte secretion of IL-1β, TNF-α, IL-6, and IL-10 ranging 7 – 13-fold above background (Fig. 1C, E, and I, Supplemental fig. 1E). The chemokine MCP-1 was the only regulatory factor in our panel that was not induced above background levels by TLR 1 ligation (Fig. 1G). It is noteworthy that a statistically significant vehicle effect was detected regarding IL-12p70 secretion by PAM3CSK4 activated monocytes (Fig. 1L). Of the TLR 1-induced cytokines that were measured, significant CBD modulation was observed in IL-2, IL-1β, TNF-α, MCP-1, and IL-12p70 (Fig. 1B, D, F, H, and M). CBD treatment significantly suppressed TLR 1-induced monocyte IL-1β production in a concentration-dependent manner, interestingly even at our lowest CBD concentration (0.5 μM), where cytokine production was suppressed by 20%, compared to the vehicle control (Fig. 1D). Furthermore, the 10 μM treatment of CBD suppressed IL-1β production as much as 37%. In contrast, TNF-α production was enhanced by CBD treatment from vehicle in a concentration-dependent manner; however, statistical significance was only observed at the 10 μM concentration, which induced a 27% increase in TNF-α release (Fig. 1F). Monocyte IL-2 production was very modestly increased compared to the vehicle with 5 μM CBD treatment (Fig. 1B). CBD treatment at 10 μM very modestly increased IL-12p70 production in TLR1 activated monocytes compared to vehicle (Fig. 1M). Although the TLR 1 agonist did not significantly alter monocyte MCP-1 production, CBD treatment was able to modulate basal levels of this chemokine (Fig. 1H). CBD modestly upregulated TLR 1 treated monocyte production of MCP-1 compared to the vehicle control with statistical significance only observed at the 10 μM CBD treatment, where the level of chemokine production was induced by 32%. Interestingly, CBD treatment did not influence monocyte IL-6 production (Fig. 1J).
Fig. 1. The effect of CBD on TLR 1 activated monocytes.
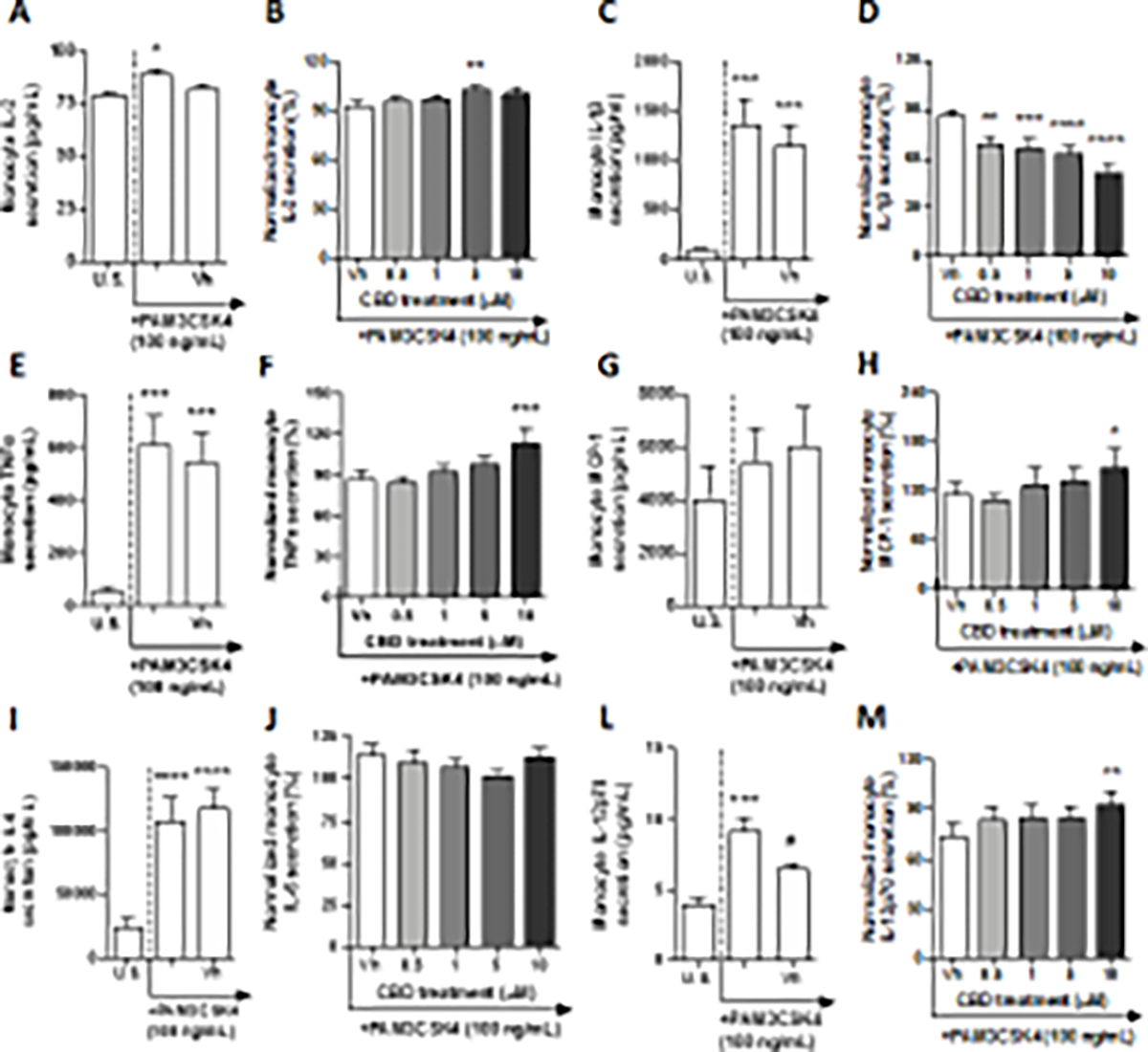
Monocytes were either unstimulated (U.S.), activated with TLR 1 agonist, PAM3CSK4, at 100 ng/mL (activation only control), or activated with PAM3CSK4 in combination with vehicle (0.03% ethanol; Vh), then cultured for 22 hours (A, C, E, G, I, and L). These graphs present the unnormalized concentration of each cytokine and chemokine. Figures were statistically analyzed by repeated measures one-way ANOVA with Sidak’s post-hoc test. Asterisks denote statistically significant differences compared to U.S. (*p < 0.05, ***p < 0.001, ****p < 0.0001) as determined by repeated measures one-way ANOVA with Sidak’s post-hoc test. Pound symbols denote statistically significant differences between activated and Vh treated cells (#p < 0.05). Monocytes were activated with PAM3CSK4 and treated with Vh or CBD (0.5, 1, 5, or 10 μM), then cultured for 22 hours (B, D, F, H, J, and M). After quantification of cytokine and chemokine, values were normalized to the PAM3CSK4 activation only control and presented as a percentage. Asterisks denote statistically significant differences from the Vh control in each graph (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001) as determined by repeated measures one-way ANOVA with Dunnett’s post-hoc test. All graphs are means S.E.M. (A-M) (n = 6).
TLR 2 has the ability to distinguish between 2 forms of lipopeptides, diacylated and triacylated, depending on the heterodimeric complex that it forms with either TLRs 1 or 6 (Monie 2017). While the TLR2/TLR1 heterodimer is able to recognize triacylated lipoproteins, the TLR2/TLR6 heterodimer forms a unique binding pocket that enhances recognition of diacylated lipoproteins (Schenk et al. 2009). In this set of experiments, monocyte TLR 2 was activated using a heat-killed preparation of Listeria monocytogenes (HKLM), a gram-positive bacterium known to synthesize diacylated lipoproteins through post-translational modifications. After a 22-hour culture period with HKLM, a significant increase in monocyte produced IL-4, IL-2, IP-10, IL-1β, TNF-α, IL-6, IL-10, IL-12p70, and TGF-β1 from basal production was observed (Table 1, Fig. 2A, C, E, and G, Supplementary fig. 2A, E, I, and K). Although the TLR 2 agonist induced monocyte secretion of IL-4 and IL-2 in a statistically significant manner, induction was less than 2-fold when compared to background (i.e., no TLR stimulation) production (Table 1, Supplementary fig 2A and C). In contrast, monocyte activation by HKLM induced IP-10, IL-1β, TNF-α, IL-6, IL-10, IL-12p70, and TGF-β1 that was greater than 2-fold (Table 1, Fig. 2A, C, E, and G, Supplementary fig. 2E, I, and K). Specifically, the upregulation of IL-1β, TNF-α, and IL-10 were induced at a range between 90 – 113-fold above background. Likewise, IP-10, IL-6, IL-12p70, and TGF-β1 were upregulated between 2.7 – 7-fold above background. Monocyte secretion of chemokine IL-8 and MCL-1 were not affected by HKLM treatment (Table 1, Supplementary fig. 2G and M). Of the 13 secretory factors measured, modest modulation by CBD was observed in 4 cytokines or chemokines. Monocyte secretion of IL-1β was suppressed modestly in a concentration-dependent manner, at 5 and 10 μM CBD treatment (Fig. 2D). The 10 μM CBD treatment suppressed monocyte IL-1β by 25% compared to the vehicle control. IP-10 production by TLR 2-activated monocytes was also modestly suppressed in a concentration-dependent manner. Significant differences compared to the vehicle control were observed at 1, 5, and 10 μM CBD treatment, with the greatest level of suppression observed at the 10 μM treatment, where IP-10 production was inhibited by 23% (Fig. 2B). Furthermore, IL-6 and TGF-β1 production were also modestly inhibited at only 5 and 10 μM CBD treatments, respectively (Fig. 2F and H).
Fig. 2. The effect of CBD on TLR 2 activated monocytes.
Monocytes were either unstimulated (U.S.), activated with TLR 2 agonist, HKLM, at 107 cells/mL (activation only control), or activated with HKLM in combination with vehicle (0.03% ethanol; Vh), then cultured for 22 hours (A, C, E, and G). These graphs present the unnormalized concentration of each cytokine and chemokine. Asterisks denote statistically significant differences compared to U.S. (****p < 0.0001) as determined by repeated measures one-way ANOVA with Sidak’s post-hoc test. Monocytes were activated with HKLM and treated with Vh or CBD (0.5, 1, 5, or 10 μM), then cultured for 22 hours (B, D, F, and H). After quantification of cytokine and chemokine, values were normalized to the HKLM activation only control for each donor and presented as a percentage. Asterisks denote statistically significant differences from the Vh control in each graph (*p < 0.05, **p < 0.01) as determined by repeated measures one-way ANOVA with Dunnett’s post-hoc test. All graphs show the mean S.E.M. (A-H) (n = 6).
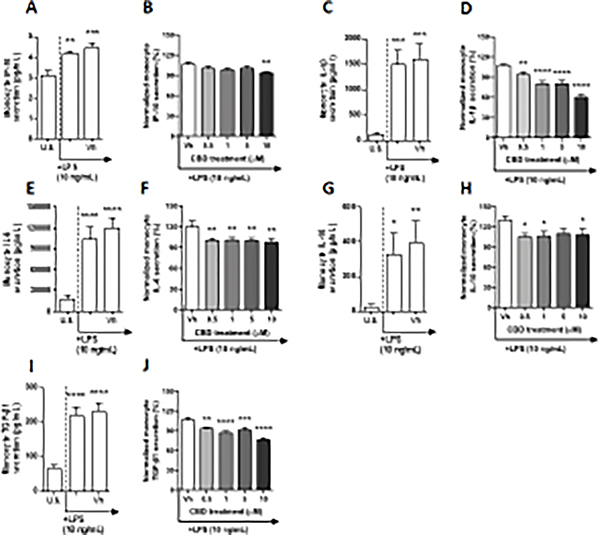
TLR 4 is the most extensively studied of the TLRs and is known to recognize bacterial lipopolysaccharide (LPS), in addition to endogenous ligands associated with tissue damage (i.e. DAMPs), albeit to a lesser degree (Molteni et al. 2016). After a 22-hour culture period of monocytes with LPS, a significant upregulation of IP-10, IL-1β, TNF-α, IL-6, IL-10, IL-12p70, IL-8, and TGF-β1 was observed compared to background levels (Table 1, Fig. 3A, C, E, G, and I, Supplementary fig. 3C, G, and I). Although LPS induced monocyte secretion of IP-10 in a statistically significant manner, induction was less than 2-fold when compared to basal production (Fig. 3A). In contrast, LPS activation of monocytes resulted in an induction of IL-1β, TNF-α, IL-6, IL-10, IL-12p70, IL-8, and TGF-β1 that was greater than 2-fold (Table 1, Fig. 3C, E, G, and I, Supplementary fig. 3 C, G, and I). The upregulation of IL-1β, TNF-α, and IL-10 were induced between 12 – 13-fold above background, while IL-6, IL-12p70, IL-8, and TGF-β1 were upregulated by 3.4 – 5.8-fold above background. Secretion of cytokines/chemokines IL-2 and MCP-1 was not affected by LPS treatment (Table 1, Supplementary fig. 3A and E). Of the 13 factors measured, modulation of cytokine/chemokine production by CBD was observed with IP-10, IL-1β, IL-6, IL-10, and TGF-β1 (Table 1). TLR 4-activated monocyte secretion of IL-1β was significantly suppressed in a concentration-dependent manner by CBD even at the lowest CBD concentration (Fig. 3D). Moreover, monocyte treatment with 10 μM CBD suppressed IL-1β secretion by 47%. TGF-β1 production by monocytes activated via TLR 4 was also suppressed by CBD (Table 1, Fig. 3J), with the greatest suppression observed at the 10 μM treatment, where TGF-β1 production was decreased by 30%. CBD treatment at 10 μM also suppressed the secretion of IP-10 by 16% compared to vehicle (Fig. 3B). Interestingly, CBD treatment significantly suppressed IL-6 production compared to vehicle at all concentrations; however, the level of suppression was not altered with increasing concentrations of CBD (Fig. 3F). Lastly, monocyte-derived IL-10 production was suppressed by 0.5, 1, and 10 μM CBD (Fig. 3H).
Fig. 3. The effect of CBD on TLR 4 activated monocytes.
Monocytes were either unstimulated (U.S.), activated with TLR 4 agonist, LPS, at 10 ng/mL (activation only control), or activated with LPS in combination with vehicle (0.03% ethanol; Vh), then cultured for 22 hours (A, C, E, G, and I). These graphs present the unnormalized concentration of each cytokine and chemokine. Asterisks denote statistically significant differences compared to U.S. (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001) as determined by repeated measures one-way ANOVA with Sidak’s post-hoc test. Monocytes were activated with LPS and treated with Vh or CBD (0.5, 1, 5, or 10 μM), then cultured for 22 hours (B, D, F, H, and J). After quantification of cytokine and chemokine, values were normalized to the LPS activation only control and presented as a percentage. Asterisks denote statistically significant differences from the Vh control in each graph (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001) as determined by repeated measures one-way ANOVA with Dunnett’s post-hoc test. All graphs are means S.E.M. (A-J) (n = 6).
The TLR 5 complex is able to engage in the recognition of flagellin, a key component of flagella, which is expressed on many gram-negative and gram-positive bacteria (Takeda et al. 2003). Monocytes were activated through TLR 5 by flagellin isolated from the gram-positive bacteria Bacillus subtilis. After a 22-hour culture period with flagellin, monocyte production of IL-4, IL-2, IP-10, IL-1β, TNF-α, MCP-1, IL-6, IL-12p70, IL-8, and TGF-β1 were significantly modulated compared to background levels (Table 1, Fig. 4 A, C, E, and G, Supplementary fig. 4A, C, E, I, K, and M). Although flagellin treatment resulted in a statistically significant increase in IL-4, IL-2, IP-10, IL-12p70, IL-8, and TGF-β1, the cytokine induction from the unstimulated group was below 2-fold (Table 1, Supplementary fig. 4 A, C, E, I, K, and M). Additionally, flagellin induced MCP-1 production slightly below 2-fold (Fig. 4E). Furthermore, activation of monocytes via TLR 5 resulted in a 4.4 and 3.3-fold induction of IL-1β and TNF-α from background levels, respectively (Fig. 4 A and C). Interestingly, flagellin treatment resulted in a 2.4-fold suppression of IL-6 production by monocytes (Fig. 4G). No statistically significant change was observed in monocyte IL-10 production in response to flagellin (Supplementary fig. 4G). Of the 13 factors measured, monocyte IL-1β, TNF-α, MCP-1, and IL-6 production were significantly modulated by CBD treatment, compared to the vehicle control (Table 1, Fig. 4B, D, F, and H). Monocyte IL-1β production was suppressed in a concentration-dependent manner by CBD at 1, 5, and 10 μM. CBD was able to suppress IL-1β production up to 33% compared to the vehicle control (Fig. 4B). IL-6 production was also modestly suppressed by CBD treatment (Fig. 4H). In contrast, both TNF-α and MCP-1 production was enhanced by CBD treatment (Fig. 4D and F). A significant difference was observed at the 10 μM CBD treatment group for both cytokines; TNF-α production was induced by 40% compared to the vehicle, while MCP-1 modulation was increased by 43%.
Fig. 4. The effect of CBD on TLR 5 activated monocytes.
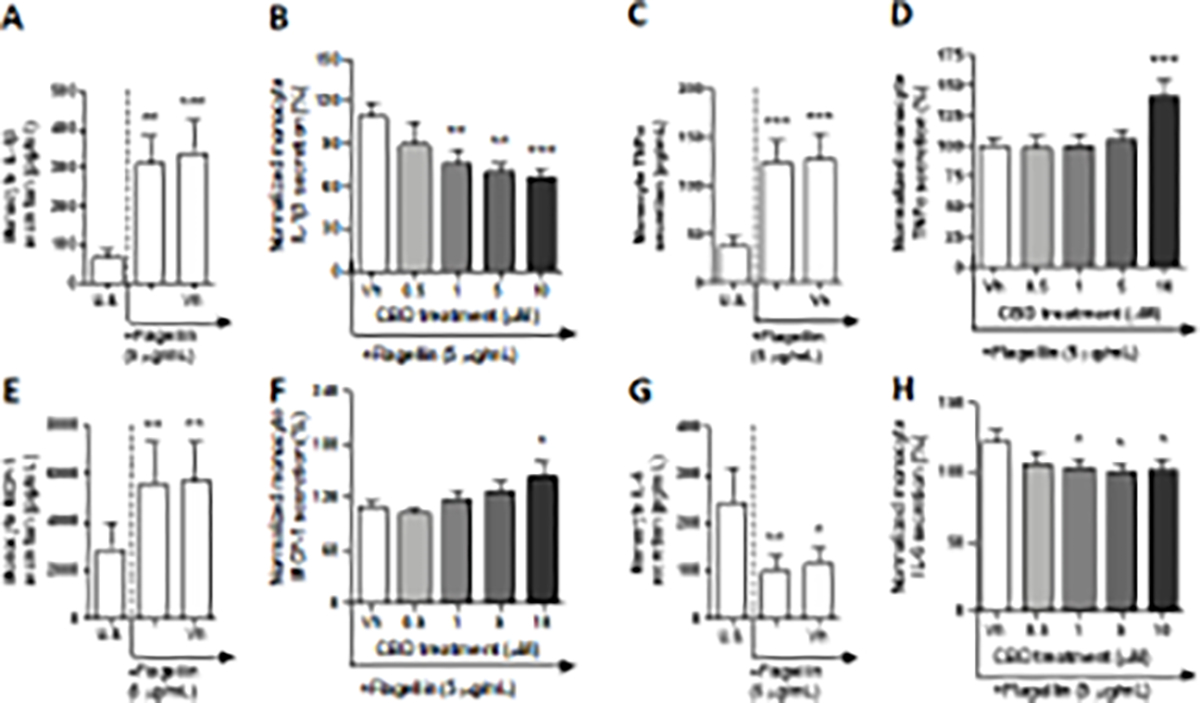
Monocytes were either unstimulated (U.S.), activated with TLR 5 agonist, flagellin, at 5 μg/mL (activation only control), or activated with flagellin in combination with vehicle (0.03% ethanol; Vh), then cultured for 22 hours (A, C, E, and G). These graphs present the unnormalized concentration of each cytokine and chemokine. Asterisks denote statistically signigicant differences compared to U.S. (*p < 0.05, **p < 0.01, ***p < 0.001) as determined by repeated measures one-way ANOVA with Sidak’s post-hoc test. Monocytes were activated with flagellin and treated with Vh or CBD (0.5, 1, 5, or 10 μM), then cultured for 22 hours (B, D, F, and H). After quantification of cytokine and chemokine, values were normalized to the flagellin activation only control and presented as a percentage. Asterisks denote statistically significant differences from the Vh control in each graph (*p < 0.05, **p < 0.01, ***p < 0.001) as determined by repeated measures one-way ANOVA with Dunnett’s post-hoc test. All graphs are means ± S.E.M. (A-H) (n = 6).
TLR6 engages in the recognition of diacylated lipoproteins from gram-positive bacteria and mycoplasma through heterodimerization with TLR 2 (Takeda et al. 2003). In this experiment, monocytes were treated with FSL-1, a synthetic lipopeptide derived from Mycoplasma salivarium, which mimics the acylated amino terminus of bacterial diacylated lipoproteins. After a 22-hour culture period, monocyte production of IL-4, IP-10, IL-1β, TNF-α, MCP-1, IL-6, IL-10, IL-12p70, IL-8, and TGF-β1 were significantly modulated by FSL-1 treatment (Table 1, Fig. 5A, C, and E, Supplementary fig. 5A, E, G, I, K, M, and O). Although monocyte production of IL-4, IP-10, IL-12p70, IL-8, and TGF-β1 were significantly increased, induction was less than 2-fold compared to background levels (Supplementary fig. 5A, E, K, M, and O). Additionally, FSL-1 increased MCP-1 production by 1.9-fold (Fig. 5C). Monocyte activation via TLR 5 increased IL-1β, TNF-α, and IL-10 5.4 – 7.3-fold compared to background (Fig. 5A Supplementary fig. 5G and I). Interestingly, monocyte IL-6 production was suppressed nearly 2-fold by FSL-1 treatment (Fig. 5E). Monocyte IL-2 production was not affected by FSL-1 treatment (Supplemental fig. 5C). Of the 13 cytokines measured, CBD treatment altered production of IL-1β, MCP-1, and IL-6, compared to the vehicle control. CBD treatment at 1, 5, and 10 μM significantly suppressed monocyte IL-1β secretion, with the greatest level of suppression measured at 48% in the 10 μM treatment group, compared to the vehicle control (Fig. 5B). Moreover, CBD significantly increased secretion of MCP-1 in the 1 and 5 μM CBD treated groups by approximately 36%, compared to the vehicle control (Fig. 5D). Additionally, IL-6 production was modestly suppressed by 1, 5, and 10 μM CBD (Fig. 5F).
Fig. 5. The effect of CBD on TLR 6 activated monocytes.
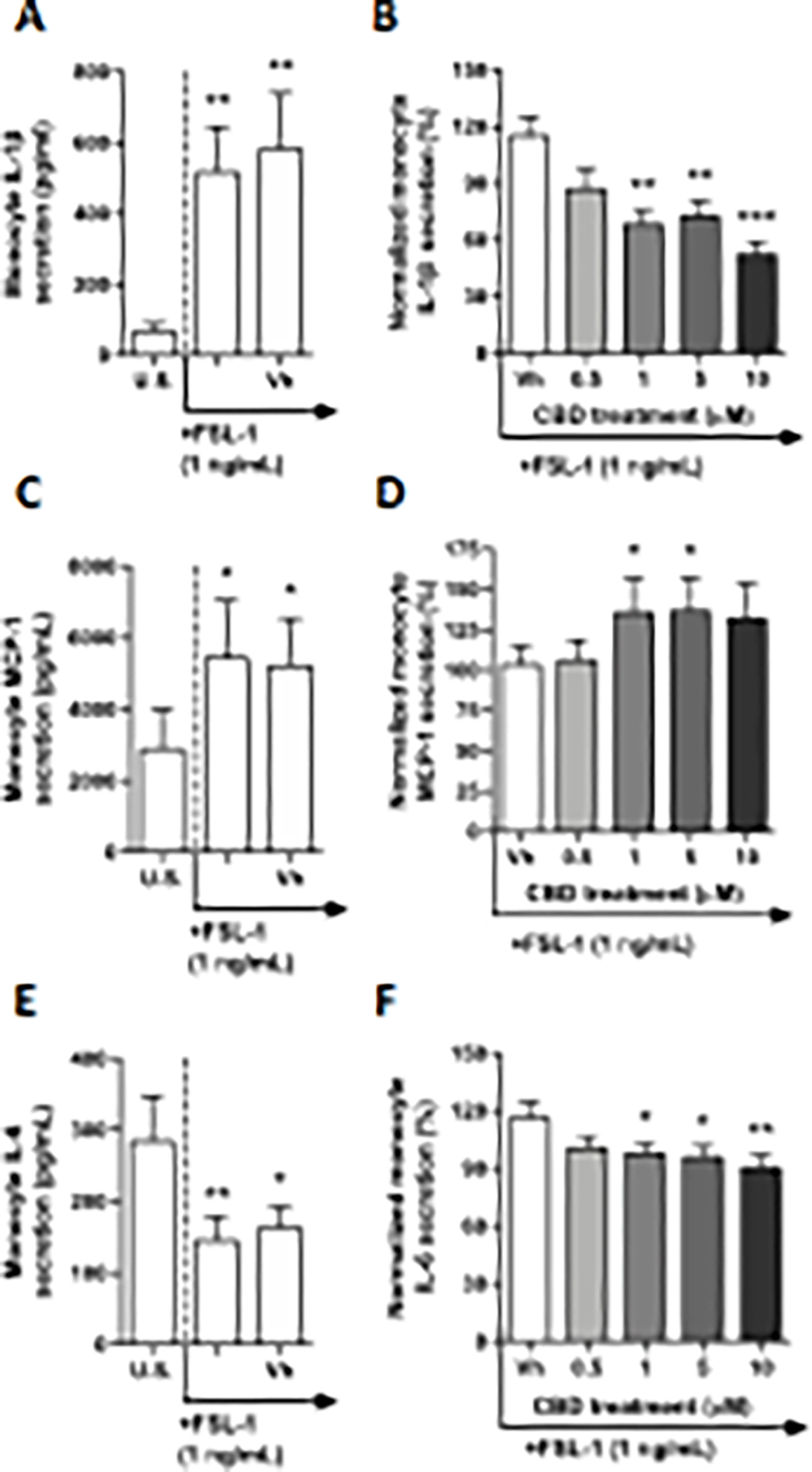
Monocytes were either unstimulated (U.S.), activated with TLR 6 agonist, FSL-1, at 1 ng/mL (activation only control), or activated with FSL-1 in combination with vehicle (0.03% ethanol; Vh), then cultured for 22 hours (A, C and E). These graphs present the unnormalized concentration of each cytokine and chemokine. Asterisks denote statistically significant differences compared to U.S. (*p < 0.05, **p < 0.01) as determined by repeated measures one-way ANOVA with Sidak’s post-hoc test. Monocytes were activated with FSL-1 and treated with Vh or CBD (0.5, 1, 5, or 10 μM), then cultured for 22 hours (B, D, and F). After quantification of cytokine and chemokine, values were normalized to the FSL-1 activation only control and presented as a percentage. Asterisks denote statistically significant differences from the Vh control in each graph (*p < 0.05, **p < 0.01, ***p < 0.001) as determined by repeated measures one-way ANOVA with Dunnett’s post-hoc test. All graphs are means S.E.M. (A-F) (n = 6).
3.2. Intracellular TLRs
Intracellular TLRs found within the endosomal compartments of cells provide the host with detection and immunity against nucleic acids of pathogenic origin: most commonly a signature of viruses, as well as specific intracellular pathogenic bacteria (Kawasaki and Kawai 2014). TLRs confined in the endosomal compartments include TLRs 3, 7, 8, and 9. Each intracellular TLR exhibits specific PAMP sensitivities and activates the innate immune system upon pathogen recognition. Activation of monocytes through the various intracellular TLRs resulted in a varied range of responses through cytokine and chemokine release. Table 2 summarizes how CBD modulates monocyte secretion of cytokines/chemokines resulting from activation through each intracellular TLR. Table 2 does not show results for IFN-γ and IL-17A due to amounts below the level of detection.
Table 2.
A comprehensive overview of how CBD modulates the secretion of 11 cytokines and chemokines from monocytes activated through extracellular TLRs (3, 7, 8, and 9). Activation columns indicate whether there was a significant difference in cytokine secretion between unstimulated and TLR agonist treated monocytes. CBD effect columns denote whether cytokine production was modulated by CBD treatment, in addition to directionality and concentrations at which significant modulation was observed. (n = 6). Cytokine production below detection is denoted by “−”. Activation columns are denoted by Y (yes) if statistically significant change was observed upon agonist treatment, or N (no). CBD effect columns are denoted by concentrations where CBD produced statistically significant difference, or NE (no effect).
| Cytokine | IL-4 |
IL-2 |
IP-10 |
IL-1β |
TNFα |
|||||
| Treatment | Activation | CBD effect | Activation | CBD effect | Activation | CBD effect | Activation | CBD effect | Activation | CBD effect |
|
| ||||||||||
| TLR 3 | Y | NE | Y | NE | Y | ↓5, 10 | N | NE | N | NE |
| TLR 7 | Y | NE | Y | NE | N | NE | Y | ↓5 | Y | ↑10 |
| TLR 8 | — | — | Y | NE | Y | NE | Y | NE | Y | ↑10 |
| TLR 9 | Y | NE | N | NE | Y | NE | N | ↓0.5, 1, 5, 10 | Y | NE |
|
| ||||||||||
| Cytokine | MCP-1 |
IL-6 |
IL-10 |
IL-12p70 |
IL-8 |
|||||
| Treatment | Activation | CBD effect | Activation | CBD effect | Activation | CBD effect | Activation | CBD effect | Activation | CBD effect |
|
| ||||||||||
| TLR 3 | Y | NE | N | NE | N | NE | Y | NE | N | NE |
| TLR 7 | Y | NE | Y | ↑10 | N | NE | Y | NE | Y | NE |
| TLR 8 | Y | NE | Y | ↓0.5, 1, 5, 10 | Y | ↑10 | Y | NE | N | NE |
| TLR 9 | N | NE | Y | ↓0.5, 1, 5, 10 | N | NE | — | — | N | NE |
|
| ||||||||||
| Cytokine | TGF-β1 |
|||||||||
| Treatment | Activation | CBD effect | ||||||||
|
| ||||||||||
| TLR 3 | Y | NE | ||||||||
| TLR 7 | Y | NE | ||||||||
| TLR 8 | Y | NE | ||||||||
| TLR 9 | Y | ↓0.5, 1 | ||||||||
TLR 3 recognizes double stranded RNA (dsRNA), in addition to certain single stranded RNA from specific pathogens (Blasius and Beutler 2010). Monocytes were activated through TLR 3 using low molecular weight Poly (I:C), a synthetic analog of dsRNA, which was annealed in our laboratory prior to monocyte activation. After a 22-hour culture period, monocyte production of IL-4, IL-2, IP-10, MCP-1, IL-12p70, and TGF-β1were significantly modulated by Poly (I:C) compared to background (Table 2, Fig. 6A, Supplementary fig. 6A, C, G, K, and O). Although monocyte production of IL-4 and TGF-β1 were significantly upregulated, induction was less than 2-fold above background levels while induction of MCP-1 and IL-12p70 were slightly above 2-fold (Table 2, Supplementary fig. 6G and K). Monocyte IP-10 was induced by nearly 35-fold above background levels by Poly (I:C) (Fig. 6A). Additionally, Poly (I:C) activation resulted in a slight reduction of IL-2 that was less than 2-fold compared to background (Supplementary fig. 6C). Monocyte IL-1β, TNF-α, IL-6, IL-10, and IL-8 were not significantly affected by Poly (I:C) (Fig. 6C, and E, Supplementary fig. 6E, I, and M). Intriguingly, of the 13 cytokines measured after TLR3 stimulation, CBD only affected monocyte IP-10 secretion (Fig. 6B). IP-10 secretion was suppressed by CBD in a concentration-dependent manner with statistically significant differences observed in the 5 and 10 μM treatment groups. At 10 μM level, CBD suppressed IP-10 production by approximately 70% from vehicle control. Interestingly, Poly (I:C) activated monocyte secretion of IL-1β was not significantly affected by CBD (Fig. 6D).
Fig. 6. The effect of CBD on TLR 3 activated monocytes.
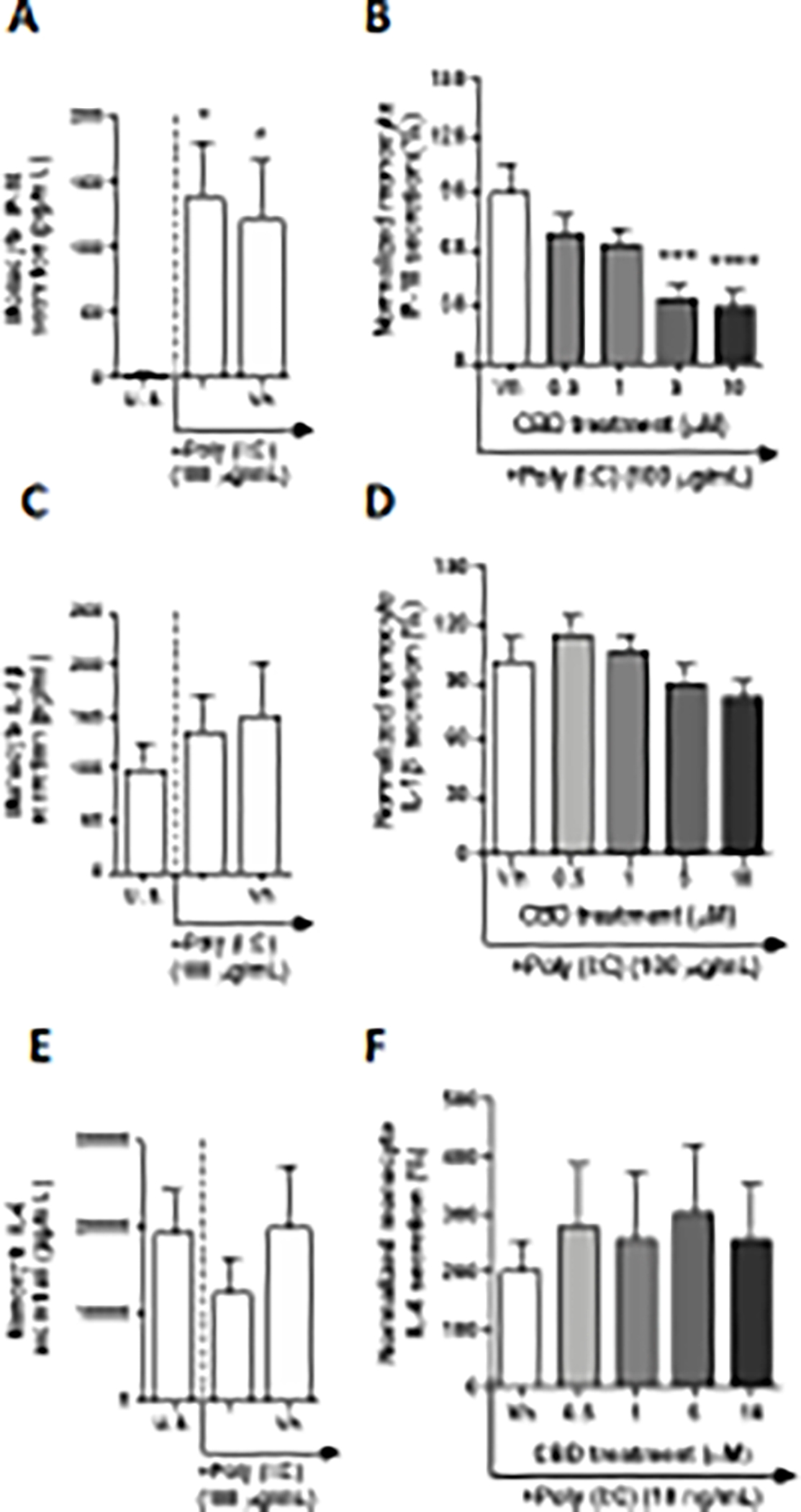
Monocytes were either unstimulated (U.S.), activated with TLR 3 agonist, Poly (I:C), at 100 μg/mL (activation only control), or activated with Poly (I:C) in combination with vehicle (0.03% ethanol; Vh), then cultured for 22 hours (A and C). These graphs present the unnormalized concentration of each cytokine and chemokine. Asterisks denote statistically significant differences compared to U.S. (*p < 0.05) as determined by repeated measures one-way ANOVA with Sidak’s post-hoc test. Monocytes were activated with Poly (I:C) and treated with Vh or CBD (0.5, 1, 5, or 10 μM), then cultured for 22 hours (B and D). After quantification of cytokine and chemokine, values were normalized to the Poly(I:C) activation only control and presented as a percentage. Asterisks denote statistically significant differences from the Vh control in each graph (***P < 0.001, ****P < 0.0001) as determined by repeated measures one-way ANOVA with Dunnett’s post-hoc test. All graphs are means S.E.M. (A-F) (n = 6).
TLR 7 engages in the recognition of guanosine-rich single-stranded RNA (ssRNA), as well as some siRNAs (Blasius and Beutler 2010). In this series of experiments, monocytes were activated with a synthetically derived imidazoquinolinone amine analog to guanosine, imiquimod (R-837). After a 22-hour culture period of monocytes with R-837, a significant upregulation of IL-4, IL-2, IL-1β, MCP-1, IL-6, IL-12p70, IL-8, and TGF-β1 was detected from background levels (Table 2, Fig. 7A, C, and E, and Supplementary fig. 7A, C, G, K, M, and O) Although R-837 treatment produced a significant upregulation of monocyte IL-2 and IL-8, induction was less than 2-fold above background levels (Supplementary fig. 7C and M). The induction of IL-4, MCP-1, IL-6, IL-12p70, and TGF-β1 ranged from 2.9 – 9-fold above background (Fig. 7E, and Supplementary fig. 7 A, G, K, and O). Furthermore, the R-837-mediated induction of monocyte IL-1β was 212-fold above background levels (Fig. 7A). Induction of TNF-α was 46.5-fold above basal production, however, was not statistically significant due to high variation (Fig. 7C). R-837 did not significantly affect monocyte secretion of IP-10 and IL-10 (Supplementary fig. 7M and I). Of the 13 cytokines measured, monocyte secretion of IL-1β, TNF-α, and IL-6 were significantly modulated by CBD treatment (Fig. 7). CBD (5 μM) caused a significant downregulation of monocyte IL-1β secretion by 23% compared to the vehicle control (Fig. 7B). In contrast, TLR 7 activated monocyte secretion of TNF-α and IL-6 were increased by 10 μM CBD treatment by 145% and 26%, respectively (Fig. 7D and F).
Fig. 7. The effect of CBD on TLR 7 activated monocytes.
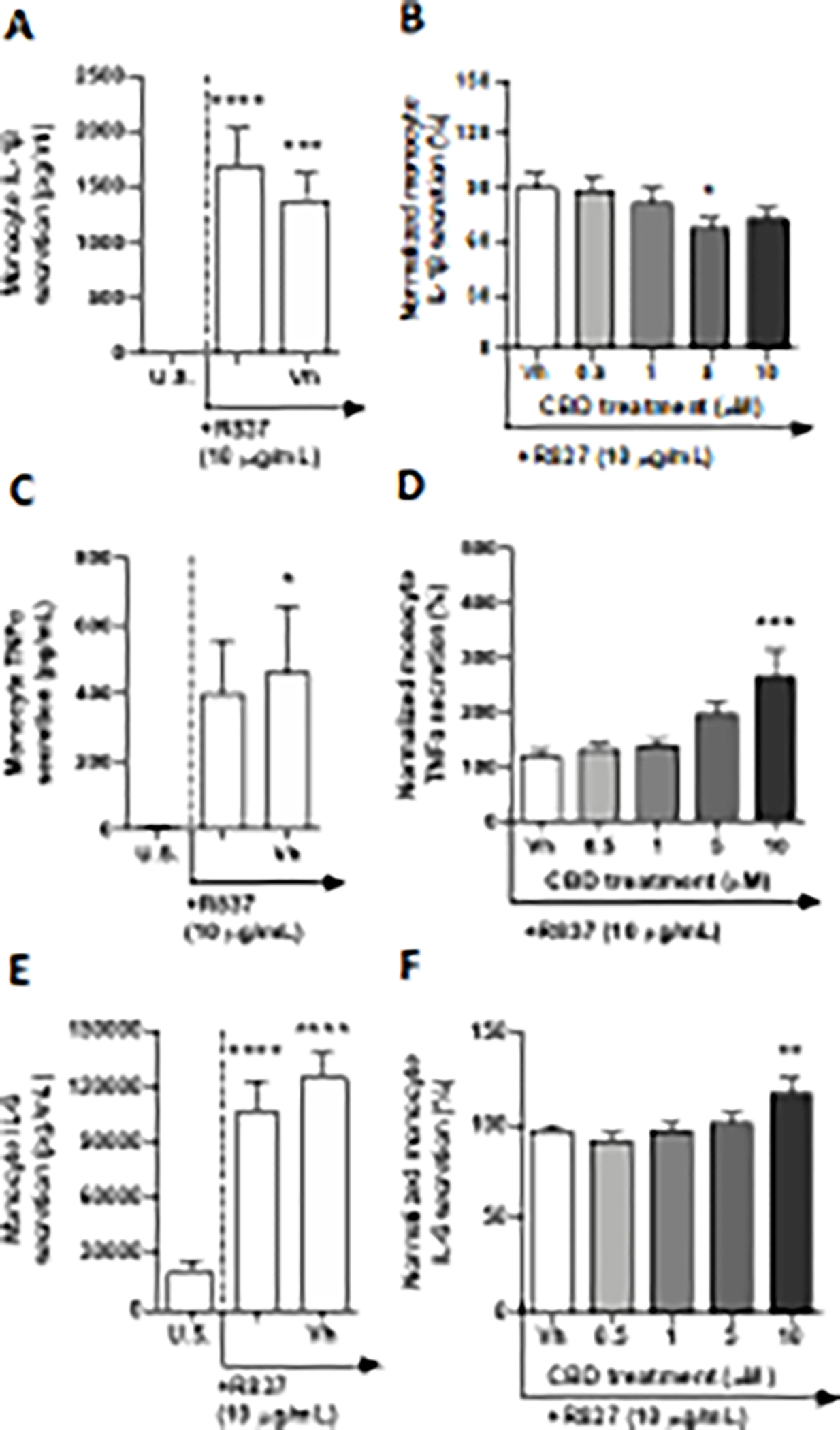
Monocytes were either unstimulated (U.S.), activated with TLR 7 agonist, R-837, at 10 μg/mL (activation only control), or activated with R837 in combination with vehicle (0.03% ethanol; Vh), then cultured for 22 hours (A, C, and E). These graphs present the unnormalized concentration of each cytokine and chemokine. Asterisks denote statistically significant differences compared to U.S. (*p < 0.05,***p < 0.001, ****p < 0.0001) as determined by repeated measures one-way ANOVA with Sidak’s post-hoc test. Monocytes were activated with R-837 and treated with Vh or CBD (0.5, 1, 5, or 10 μM), then cultured for 22 hours (B, D, and F). After quantification of cytokine and chemokine, values were normalized to the R-837 activation only control and presented as a percentage. Asterisks denote statistically significant differences from the Vh control in each graph (*p < 0.05, ***p < 0.001) as determined by repeated measures one-way ANOVA with Dunnett’s post-hoc test. All graphs are means S.E.M. (A-F) (n = 6).
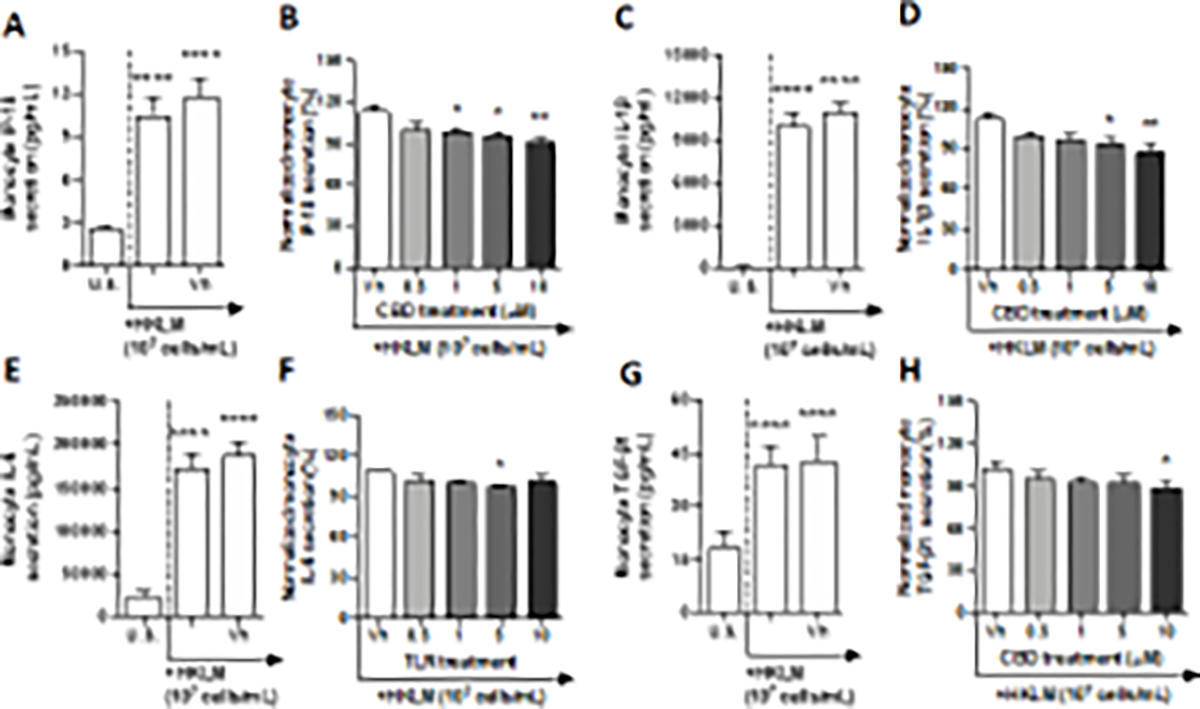
TLR 8 shares a high degree of homology, as well as similarity in function with TLR 7. It is known to recognize pathogenic uridine-rich ssRNA, as well as degradation products of ssRNA (Tanji et al. 2015). In these experiments, monocytes were activated through TLR 8 by ssRNA40, a uridine-rich ssRNA derived from the HIV-1 long terminal repeat. After a 22-hour stimulation of monocytes with TLR 8 agonist, ssRNA40, significant induction above background levels was observed in a number of cytokines. Specifically, TLR 8 ligation increased the production of IL-2, IP-10, IL-1β, TNF-α, MCP-1, IL-6, IL-10, IL-12p70, and TGF-β1(Table 2, Fig. 8A, C, E, and G, and Supplementary fig. 8A, C, E, G, and K). Although ssRNA40 treatment increased monocyte secretion of IL-2, induction was less than 2-fold above background levels (Supplementary fig. 8A). TLR 8 stimulation resulted in an upregulation of IP-10, MCP-1, IL-6, IL-10, and TGF-β1 ranging from 5 – 17-fold above background (Fig. 8E and G, and Supplementary fig. 8C, E, and K). Additionally, monocyte activation upregulated the secretion of IL-1β, TNF-α, and IL-12p70 by 153, 134, and 240-fold, respectively (Fig. 8A and C, and Supplementary fig. 8G). ssRNA40 had no statistically significant effect on monocyte secretion of IL-8 (Supplementary fig. 8I). Of the 13 cytokines measured, CBD affected the secretion of ssRNA40-activated monocyte TNF-α, IL-6, and IL-10 (Table 2 and Fig. 8D, F, and H). CBD (10 μM) treatment induced the secretion of ssRNA mediated monocyte TNF-α and IL-10 by 42% and 45%, respectively, compared to vehicle control (Fig. 8D and H). Furthermore, monocyte secretion of IL-6 was suppressed by all concentrations of CBD, with maximal suppression of 19% achieved with treatment as low as 0.5 μM (Fig. 8F). In congruence with TLR3, CBD treatment had no effect on TLR 8-activated monocyte secretion of IL-1β (Fig. 8B).
Fig. 8. The effect of CBD on TLR 8 activated monocytes.
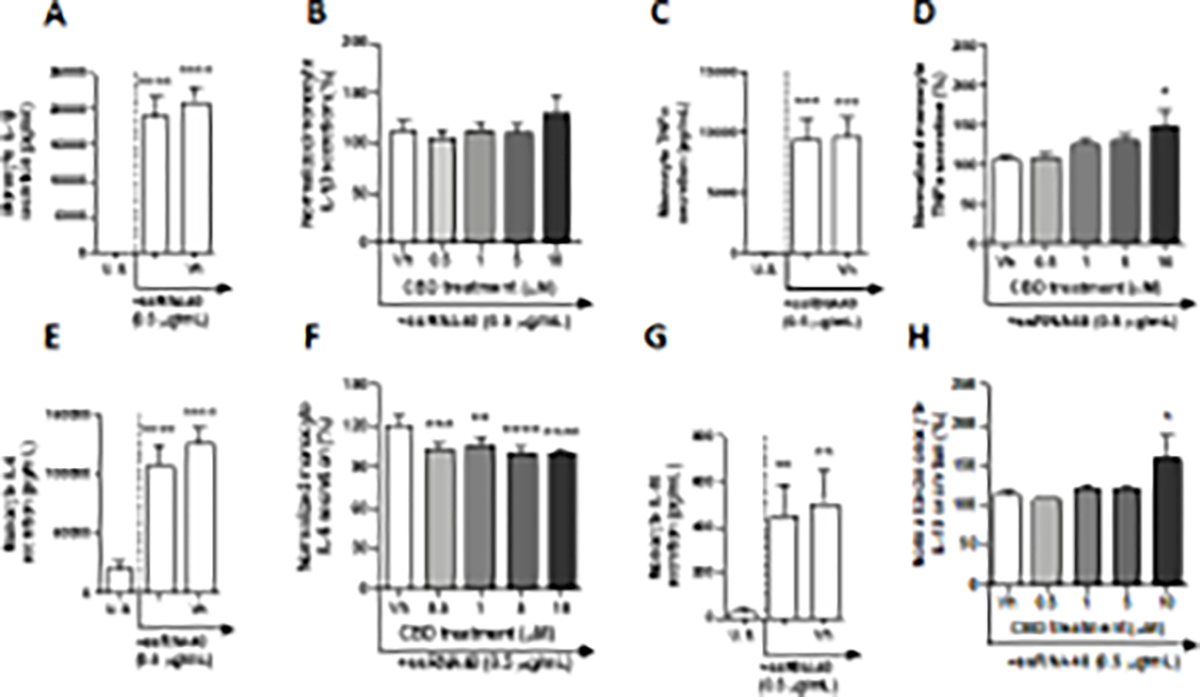
Monocytes were either unstimulated (U.S.), activated with TLR 8 agonist, ssRNA40, at 0.5 μg/mL (activation only control), or activated with ssRNA40 in combination with vehicle (0.03% ethanol; Vh), then cultured for 22 hours (A, C, E, and G). These graphs present the unnormalized concentration of each cytokine and chemokine. Asterisks denote statistical differences compared to U.S. (**p < 0.01, ***p < 0.001, ****p < 0.0001) as determined by repeated measures one-way ANOVA with Sidak’s post-hoc test. Monocytes were activated with ssRNA40 and treated with Vh or CBD (0.5, 1, 5, or 10 μM), then cultured for 22 hours (B, D, F, and H). After quantification of cytokine and chemokine, values were normalized to the ssRNA40 activation only control and presented as a percentage. Asterisks denote statistically significant differences from the Vh control in each graph (*p < 0.05) as determined by repeated measures one-way ANOVA with Dunnett’s post-hoc test. All graphs are means S.E.M. (A-H) (n = 6).
TLR 9 recognizes pathogenic DNA rich in unmethylated CpG-DNA motifs (Kawasaki and Kawai 2014). The TLR 9 signaling pathway was activated in monocytes with CpG ODN, a synthetically derived oligonucleotide containing unmethylated CpG dinucleotides. Following a 22-hour stimulation of monocytes with CpG ODN, we detected a significant increase in monocyte secretion of IL-4, IP-10, TNF-α, IL-6, and TGF-β1 (Table 2, Fig. 9C and E, and Supplementary fig. 9A, E, G). Although the TLR 9 agonist induced monocyte secretion of IL-4, IP-10, and TGF-β1 in a statistically significant manner, induction was less than 2-fold compared to background production. Additionally, TLR 9 stimulation resulted in an upregulation of TNF-α and IL-6 by 7.4 and 3.8-fold above background. CpG ODN had no statistically significant effect on monocyte secretion of IL-2, IL-1β, MCP-1, IL-10, IL-8 (Fig. 9A, and Supplementary fig. 9C, I, K, and M). Of the 13 cytokines measured, CBD treatment resulted in a suppression of TLR 9-activated monocyte secretion of IL-6 and TGF-β1 (Fig. 9D and F). Monocyte IL-6 production was suppressed by all concentrations of CBD, with maximal suppression (30%) observed at 0.5 μM CBD. TLR 9-activated monocyte TGF-β1 secretion was similarly suppressed by 0.5 and 1 μM CBD; however, no effect was seen at the two highest concentrations. Furthermore, even though CpG ODN treatment did not result in a statistically significant increase in IL-1β production by monocyte, all concentrations of CBD treatment resulted in suppression of monocyte IL-1β secretion, with the greatest level of suppression observed at 10 μM CBD, upon which IL-1β levels were impaired by 63% compared to vehicle control (Fig. 9B).
Fig. 9. The effect of CBD on TLR 9 activated monocytes.
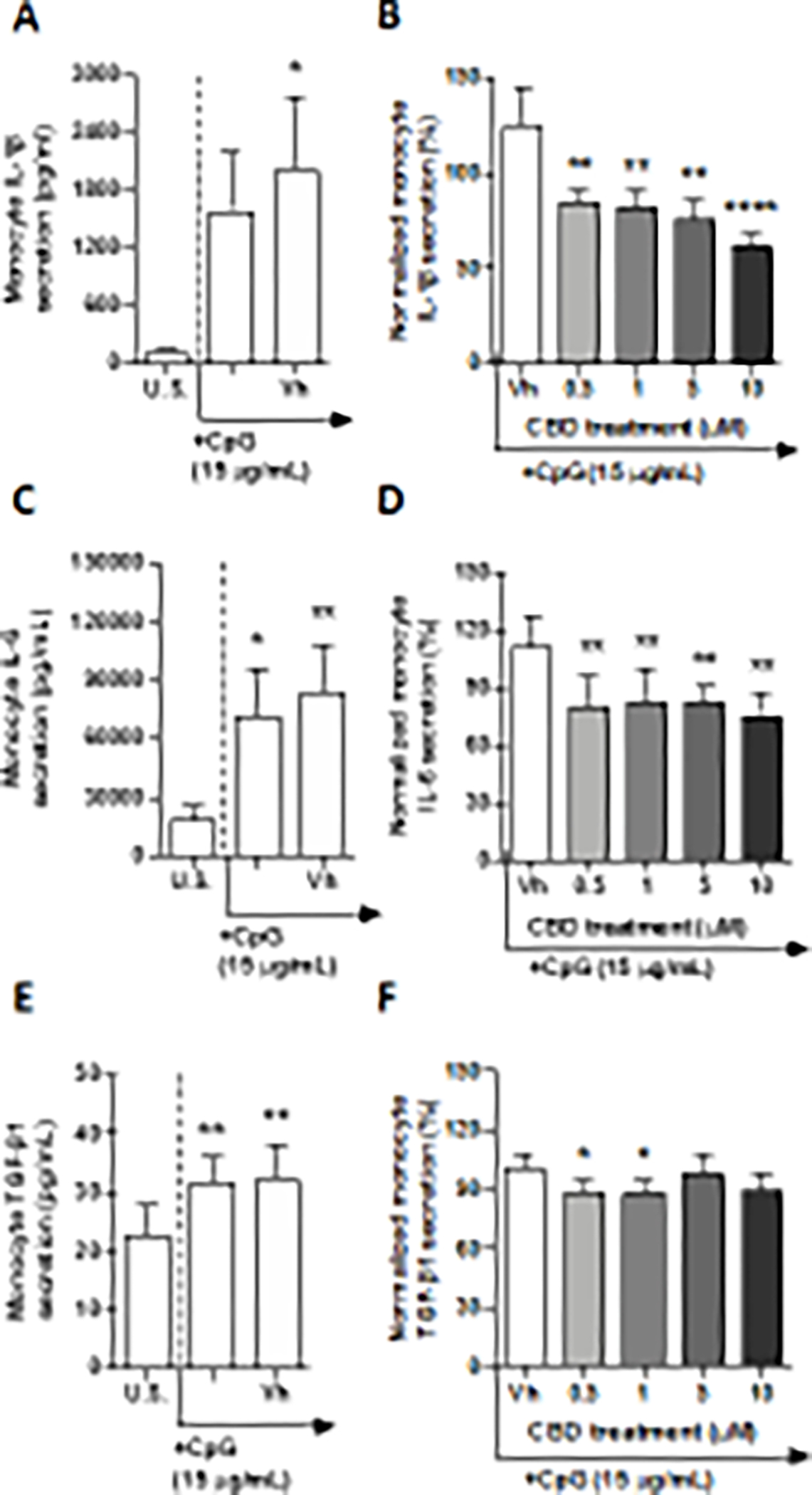
Monocytes were either unstimulated (U.S.), activated with TLR 9 agonist, CpG ODN, at 15 μg/mL (activation only control), or activated with CpG in combination with vehicle (0.03% ethanol; Vh), then cultured for 22 hours (A,C, and E). These graphs present the unnormalized concentration of each cytokine and chemokine. Asterisks denote statistically significant differences compared to U.S. (*p< 0.05, **p < 0.01)) as determined by repeated measures one-way ANOVA with Sidak’s post-hoc test. Monocytes were activated with Cpg ODN and treated with Vh or CBD (0.5, 1, 5, or 10 μM), then cultured for 22 hours (B, D, and F). After quantification of cytokine and chemokine, values were normalized to the CpG ODN activation only control and presented as a percentage. Asterisks denote statistically significant differences from the Vh control in each graph (*p < 0.05, **p < 0.01, ****p < 0.0001) as determined by repeated measures one-way ANOVA with Dunnett’s post-hoc test. All graphs are means S.E.M. (A-F) (n = 6).
4. Discussion
As the popularity of CBD containing products for self-medicinal use becomes more prevalent in the United States, there is a growing need and interest in assessing the toxicological effects of cannabis-derived compounds. Here the effects of CBD were evaluated on the activated human innate immune system. Specifically, human monocytes isolated from healthy donors and activated with TLR 1–9 were treated with increasing concentrations of CBD. In this investigation we demonstrated that CBD treatment resulted in the specific suppression of IL-1β in monocytes activated through all TLRs, individually, with the exception of TLR 3 and 8. Similarly, CBD treatment also suppressed the secretion of IL-6 from monocytes activated through TLRs 2, 4, 5, 6, 8, and 9. Interestingly, CBD upregulated IL-6 release from monocytes activated through TLR 7 and had no effect on secretion by monocytes activated through TLRs 1 and 3. Besides IL-1β and IL-6, significant modulation by CBD treatment was observed in a TLR specific manner in 7 additional cytokines: TNF-α, IL-2, IP-10, MCP-1, IL-10, IL-12p70, and TGF-β1. Although consistent trends were not observed for these other cytokines, some trends did exist. For example, TNF-α and MCP-1 were consistently upregulated by CBD where significant modulation was observed. Likewise, TGF-β and IP-10 were consistently downregulated when modulation by CBD occurred. Furthermore, specific suppression of monocyte derived pro-inflammatory cytokines IL-1β and IL-6 by CBD may provide anti-inflammatory relief to users depending on the context of disease state by putative suppression of monocytes.
The results shown here support previously published studies conducted in murine models and monocytic cell lines showing the immunosuppresive activity of CBD through the repression of IL-1β and IL-6 mRNA expression, as well as the downregulation of pro-inflammatory mediators such as IL-6, IL-8, MCP-1, and TNF-α following LPS stimulation, respectively (Lee et al. 2014; Muthumalage and Rahman 2019). However, our study shows that CBD treatment of primary monocytes activated through TLR 4 does not significantly modulate monocyte-derived IL-8, MCP-1, and TNF-α. The discrepancy observed between our findings and those reported by Muthumalage and coworkers may be due to the differences in cell model (primary vs cell-line) and the utilization of LPS concentrations 100-fold higher compared to the present investigation. To our knowledge this is the first comprehensive evaluation of the effects of CBD on TLR receptor (1–9)-activated human primary peripheral blood monocytes. This is of particular importance as the vast majority of studies evaluating the immune modulating activity of CBD have been conducted in animal models, most often mice. Although studies involving in vivo murine models provide important insights into the putative mechanism responsible for some of the biological effects produced by CBD, there are limitations when extrapolating these results to humans, which includes but is not limited to genetic variability, diet, lifestyle differences, differences in age, and known differential inflammatory responses to exogenous agents between species (Seok et al. 2013).
IL-1β is a potent cytokine, critical in regulating the host-defense and inflammatory response to infection, through PAMP and DAMP signaling, and is mainly produced by cells of the myeloid lineage (Lopez-Castejon and Brough 2011). It is noteworthy that IL-1β also acts to bridge the innate and adaptive immune system by stimulating T cell-dependent antibody production, as well as promoting Th17 differentiation (Dinarello 2009). Due to its function as a potent pyrogenic and pro-inflammatory mediator, it is not surprising that there can be crucial pathophysiological consequences due to chronic overproduction of IL-1β, such as rheumatoid arthritis, neuroinflammation, cancer, as well as neurodegenerative diseases, such as Alzheimer’s disease and HIV associated dementia (Jin et al. 1997; Kaneko et al. 2019; Kay 2004; Proescholdt et al. 2002; Shaftel et al. 2008; Stanley et al. 1994).
Similar to IL-1β, IL-6 is also a potent pro-inflammatory mediator involved in the induction of the inflammatory acute phase response (Kramer et al. 2008). One of the most extensively characterized functions of IL-6 is its influential role in stimulating the production of acute-phase proteins, which function as a survival response to restore homeostasis in the face of inflammatory conditions (Gabay and Kushner 1999). Additionally, IL-6 plays an important role in bridging innate and adaptive immunity. Like IL-1β, IL-6 is an important factor in stimulating CD4+ T cell differentiation into Th17 cells and has been implicated in suppressing TGF-β-induced regulatory T cell differentiation (Bettelli et al. 2006; Korn et al. 2009). Not surprisingly, chronically elevated levels of circulating IL-6 have also been implicated in inflammatory and autoimmune diseases such as rheumatoid arthritis, systemic lupus, psoriasis, Chron’s disease, and systemic juvenile idiopathic arthritis (Gabay 2006; Nakahara et al. 2003).
In light of the intricate role both IL-1β and IL-6 play in mediating inflammation, the ability of CBD to suppress monocyte production of these cytokines may be beneficial for those experiencing acute inflammatory responses, chronic inflammation and autoimmune diseases. Considering monocytes are an important source of these potent cytokines, the modulatory effects of CBD on monocyte production of IL-1β and IL-6 may have downstream influences related to the direct modulation of the innate immune response, as well as indirect effects on the adaptive immune system. However, it is important to emphasize that whether CBD exerts beneficial or adverse effects is highly dependent on the context in which an individual is exposed to this cannabinoid. As such, those responding to an infectious organism could experience a tempered immune response due to CBD, which could result in an adverse or deleterious effect, whereas someone suffering from persistent or chronic inflammation may derive beneficial effects from the anti-inflammatory properties through suppression of IL-1β and IL-6 by CBD.
Although immune modulation by CBD has been investigated by numerous laboratories, the specific mechanism and molecular target(s) has remained elusive. Several receptors have been implicated by which CBD may exert its biological activity and include the TRP family receptors, 5-HT receptors, GPR55, adenosine A2 receptors, and PPARs, all of which are expressed on monocytes (Chiurchiù et al. 2015; Haskó et al. 2007; Palmqvist et al. 2016; Santoni et al. 2018). Therefore, signaling initiated by CBD through one or more of these receptors in addition to its direct interactions with the TLR signaling pathways at this time cannot be ruled out and may contribute to altered TLR-induced monocyte production of IL-1β and IL-6. For example, CBD has been identified as an agonist at the TRPV1 ion channel (Bisogno et al. 2001), which becomes rapidly desensitized upon activation, thus preventing further Ca2+ influx. The influx of extracellular Ca2+ has been implicated in mediating some TLR signaling pathways that result in cytokine production (Tang et al. 2017). Therefore, it is possible that CBD is activating and rapidly desensitizing TRPV1 receptors on the monocyte membrane, preventing Ca2+influx and downstream TLR signaling. Additionally, PPARγ has been identified to basally suppress the pro-inflammatory immune response mediated by TLRs through repression of NF-κB-mediated inflammatory signaling (Necela et al. 2008). CBD has also been reported to activate PPARγ (O’Sullivan et al. 2009), which could putatively enhance repression on NF-κB signaling to suppress pro-inflammatory cytokine transcription.
CBD may also putatively mediate monocyte cytokine modulation through the dysregulation of transcription factors downstream of TLR signaling pathways. Following recognition of specific PAMPs, TLR signaling commences through the recruitment of specific toll/interleukin-1 receptor (TIR) domain containing adaptor proteins, myeloid differentiation factor 88 (MyD88) and/or TIR-domain-containing adapter-inducing interferon beta (TRIF) (Kawasaki and Kawai 2014). These adaptor proteins initiate signaling pathways that result in the activation of mitogen-activated protein kinases (MAPK) and transcription factors such as NF-κB, AP-1, and interferon regulatory factor (IRF) to regulate cytokine, chemokine, and type 1 interferon production (Kawasaki and Kawai 2014). There is great overlap between TLR signaling pathways, wherein all pathways, apart from TLR 3, are initiated by the recruitment of MyD88, resulting in the activation of transcription factors NF-κB and AP-1, which are responsible for the transcription of IL-1β and IL-6 (Nichols and Kaplan 2020b). TLR 3 is the only TLR that solely relies on the TRIF adaptor protein, which has been reported to activate IRF (Kawasaki and Kawai 2014). Various previously published studies have established that CBD treatment results in the downregulation in activity of both NF-κB and AP-1in in vivo murine models, as well as in vitro murine microglial cell lines (Kaplan et al. 2008; Kozela et al. 2010; Mukhopadhyay et al. 2011; Rajesh et al. 2010). Further research has established that CBD treatment can also result in the downregulation of p38 MAPKs and upregulation of the NF-κB inhibitor, IκBα (McKallip et al. 2006; Rajesh et al. 2010). Moreover, p38 MAPKs are particularly important in promoting NF-κB transcriptional activity (Saha et al. 2007), and are also crucial for the phosphorylation of activating transcription factor 2 and c-Jun, thus regulation of AP-1 activation (Backert and Naumann 2010). The suppression of these transcription factors is particularly interesting because it may explain why CBD is only able to downregulate cytokines in MYD88-dependent TLR signaling pathways, and not that of TLR 3. In addition, a putative explanation as to why CBD did not inhibit IL-1β in monocytes activated through TLR 8, as seen with TLR 7 may be, at least in part, that the TLR 8 signaling cascade activates the NF-κB pathway more strongly compared to TLR 7 (Bender et al. 2020), suggesting that CBD is not as efficacious in impairing the strong TLR 8-mediated induction of IL-1β. As observed in this paper, TLR 8 activation resulted in IL-1β production that was 10-fold higher than in monocytes stimulated through TLR 7.
Indeed, it is also possible that CBD may alter IL-1β secretion through a post translational mechanism, by inhibition of the inflammasome. IL-1β is initially produced as a precursor proprotein that is proteolytically cleaved by inflammasome-mediated activated caspase-1, resulting in bioactive mature IL-1β (Afonina et al. 2015; Franchi et al. 2009). Furthermore, some recent studies have implicated CBD treatment in inhibiting inflammasome activation (Huang et al. 2019; Libro et al. 2016). The role that IL-1β plays in the transcriptional regulation of IL-6 is also of particular importance. IL-1β not only induces IL-6 promoter activity on the transcriptional level, but also enhances IL-6 protein secretion in an AP-1 and NF-κB dependent manner (Szabo-Fresnais et al. 2008). Thus, it is possible that the suppression of the IL-1β response by CBD through inflammasome suppression may be indirectly mediating IL-6 production in a paracrine fashion, contributing to the suppression of IL-6 as observed in the current investigation.
It is critical to note that the concentrations of CBD used in this series of studies are relevant to human exposure. It has been recently reported that nearly two-thirds of CBD products on the market do not contain the concentration of CBD that is indicated on the product label (Bonn-Miller et al. 2017). Therefore, the majority of consumers are using unknown quantities of CBD and perhaps in a manner that is not in concordance with labels, which further complicates accurately estimating human exposure levels. However, pharmacokinetic clinical studies for Epidiolex® have demonstrated that peak CBD plasma levels reached over 1μM of the parent compound following 20 mg/kg/day dosing, which falls within our concentration response curve (Center for Drug Evaluation and Research 2018). Additionally, Epidiolex® clinical studies also detailed that there is a 50-fold higher peak plasma concentration of the active metabolite 7-COOH-CBD compared to the parent compound. Since monocytes do not express CYP3A4 or CYP2c19, the cytochrome-P450 isozymes responsible for CBD metabolism, the immunomodulating effect of CBD metabolites were not assessed in our study. Given the aforementioned caveats and the broad CBD concentration range evaluated here, the concentrations of CBD utilized in the current study are concordant with those estimated for human consumption. However, it is important to note that those utilizing very low doses of over-the-counter CBD products will not achieve blood plasma concentrations utilized within this study, however, as CBD is very lipophilic, it is possible that CBD accumulates within the body with daily use wherein these concentrations may be reached over time. These studies are critical in detailing the concentrations at which CBD can affect monocyte effector function.
There was a limitation within this study, which involves the fact that because TLR 2 monomers can homodimerize, as well as heterodimerize with TLR 6 and TLR 1, there is no feasible way to isolate the CBD effect on monocytes activated in a solely TLR 1, 2, or 6 dependent manner. However, on a physiological level these TLRs work in conjunction with one another, thus it is not necessarily relevant to determine how CBD modulates the response of monocytes activated through these receptors individually.
In summary, this paper provides a comprehensive overview of the effects produced by CBD on cytokine/chemokine production in monocytes activated through TLRs. Specifically, we demonstrate that CBD can modulate the production of monocyte-derived proinflammatory cytokines IL-1β and IL-6, both of which are critical mediators in immune activation, inflammation, and recruitment of the adaptive immune system. This study provides further evidence that CBD has a major anti-inflammatory effect on the innate immune system, specifically on monocytes activated via most TLRs, thus exerting an indirect effect on the adaptive immune system through the dampening of the innate immune response. Perhaps CBD use has the potential of partially mitigating inflammation associated with certain disease states. Further studies will be required to ascertain the role of CBD metabolites and their contribution, if any, on the immune modulating properties of CBD. Last and as stated above, it is important to consider the context within which CBD is used in order to ascertain whether the effects produced on the immune system are beneficial or detrimental to health.
Supplementary Material
5. Funding:
This work was supported in part by NIH R01 DA047180 and the Center for Research on Ingredient Safety at Michigan State University.
Non-standard abbreviations:
- 5-HT
5-hydroxytryptamine
- 7-COOH-CBD
7-carboxy cannabidiol
- ANOVA
analysis of variance
- AP-1
activator protein 1
- CB
cannabinoid receptor
- CBD
cannabidiol
- CpG ODN
cytosine-phosphorothioate-guanine oligodeoxynucleotides
- DAMP
damage-associated molecular pattern
- EAM
experimental autoimmune myocarditis
- FSL-1
synthetic diacylated lipoprotein
- GPR55
G-protein coupled receptor 55
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HIV
human immunodeficiency disorder
- HKLM
heat killed listeria monocytogenes
- HTLV
human T-lymphotropic virus
- IFN-γ
interferon gamma
- IL
interleukin
- IP-10
interferon gamma-induced protein 10
- IRF
interferon regulating factor
- LMW
low molecular weight
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- MCP-1
monocyte chemoattractant protein 1
- MyD88
myeloid differentiation factor 88
- NF-κβ
nuclear factor kappa-light-chain-enhancer of activated B cells
- NLRP3
NLR family pyrin domain containing 3
- PAMP
pathogen-associated molecular pattern
- PMA/Io
phorbol 12-myristate 12-acetate/ionomycin
- poly(I:C)
polyinosine-polycytidylic acid
- PPAR-γ
peroxisome proliferator-activated receptor gamma
- PPR
pattern recognition receptor
- R837
imiquimod
- RPMI
Roswell Park Memorial Institute Medium
- TGF-β1
transforming growth factor beta 1
- THC: Δ9
tetrahydrocannabinol
- TIR
toll/interleukin-1 receptor
- TLR
toll-like receptor
- TNF-α
tumor necrosis factor alpha
- TRIF
TIR-domain-containing adapter-inducing interferon beta
- TRP
transient receptor potential
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6 References:
- Afonina, Müller C, Seamus and Beyaert 2015. Proteolytic Processing of Interleukin-1 Family Cytokines: Variations on a Common Theme. Immunity 42, 991–1004. [DOI] [PubMed] [Google Scholar]
- Amarante-Mendes GP, Adjemian S, Branco LM, Zanetti LC, Weinlich R and Bortoluci KR 2018. Pattern Recognition Receptors and the Host Cell Death Molecular Machinery. Frontiers in Immunology 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backert S and Naumann M 2010. What a disorder: proinflammatory signaling pathways induced by Helicobacter pylori. Trends in Microbiology 18, 479–486. [DOI] [PubMed] [Google Scholar]
- Bender AT, Tzvetkov E, Pereira A, Wu Y, Kasar S, Przetak MM, Vlach J, Niewold TB, Jensen MA and Okitsu SL 2020. TLR7 and TLR8 Differentially Activate the IRF and NF-κB Pathways in Specific Cell Types to Promote Inflammation. ImmunoHorizons 4, 93–107. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Hanuš L, De Petrocellis L, Tchilibon S, Ponde DE, Brandi I, Moriello AS, Davis JB, Mechoulam R and Di Marzo V 2001. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. British Journal of Pharmacology 134, 845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasius AL and Beutler B 2010. Intracellular Toll-like Receptors. Immunity 32, 305–315. [DOI] [PubMed] [Google Scholar]
- Bonn-Miller MO, Loflin MJE, Thomas BF, Marcu JP, Hyke T and Vandrey R 2017. Labeling Accuracy of Cannabidiol Extracts Sold Online. JAMA 318, 1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrelli F, Aviello G, Romano B, Orlando P, Capasso R, Maiello F, Guadagno F, Petrosino S, Capasso F, Di Marzo V and Izzo AA 2009. Cannabidiol, a safe and non-psychotropic ingredient of the marijuana plant Cannabis sativa, is protective in a murine model of colitis. Journal of Molecular Medicine 87, 1111–1121. [DOI] [PubMed] [Google Scholar]
- Carrier EJ, Auchampach JA and Hillard CJ 2006. Inhibition of an equilibrative nucleoside transporter by cannabidiol: A mechanism of cannabinoid immunosuppression. Proceedings of the National Academy of Sciences 103, 7895–7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo A, Tolón MR, Fernández-Ruiz J, Romero J and Martinez-Orgado J 2010. The neuroprotective effect of cannabidiol in an in vitro model of newborn hypoxic–ischemic brain damage in mice is mediated by CB2 and adenosine receptors. Neurobiology of Disease 37, 434–440. [DOI] [PubMed] [Google Scholar]
- Center for Drug Evaluation and Research. 2018. Non-clinical reviews. US FDA Report. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210365Orig1s000PharmR.pdf. Accessed August 5, 2021. [Google Scholar]
- Chaturvedi A and Pierce SK 2009. How Location Governs Toll-Like Receptor Signaling. Traffic 10, 621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiurchiù V, Lanuti M, De Bardi M, Battistini L and Maccarrone M 2015. The differential characterization of GPR55 receptor in human peripheral blood reveals a distinctive expression in monocytes and NK cells and a proinflammatory role in these innate cells. International Immunology 27, 153–160. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Ligresti A, Moriello AS, Allarà M, Bisogno T, Petrosino S, Stott CG and Di Marzo V 2011. Effects of cannabinoids and cannabinoid-enrichedCannabisextracts on TRP channels and endocannabinoid metabolic enzymes. British Journal of Pharmacology 163, 1479–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Cross JH, Laux L, Marsh E, Miller I, Nabbout R, Scheffer IE, Thiele EA and Wright S 2017. Trial of Cannabidiol for Drug-Resistant Seizures in the Dravet Syndrome. New England Journal of Medicine 376, 2011–2020. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Patel AD, Cross JH, Villanueva V, Wirrell EC, Privitera M, Greenwood SM, Roberts C, Checketts D, VanLandingham KE and Zuberi SM 2018. Effect of Cannabidiol on Drop Seizures in the Lennox–Gastaut Syndrome. New England Journal of Medicine 378, 1888–1897. [DOI] [PubMed] [Google Scholar]
- Dinarello CA 2009. Immunological and Inflammatory Functions of the Interleukin-1 Family. Annual Review of Immunology 27, 519–550. [DOI] [PubMed] [Google Scholar]
- El-Zayat SR, Sibaii H and Mannaa FA 2019. Toll-like receptors activation, signaling, and targeting: an overview. Bulletin of the National Research Centre; 43. [Google Scholar]
- Elmes MW, Kaczocha M, Berger WT, Leung K, Ralph BP, Wang L, Sweeney JM, Miyauchi JT, Tsirka SE, Ojima I and Deutsch DG 2015. Fatty Acid-binding Proteins (FABPs) Are Intracellular Carriers for Δ9-Tetrahydrocannabinol (THC) and Cannabidiol (CBD). Journal of Biological Chemistry 290, 8711–8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi L, Eigenbrod T, Muñoz-Planillo R and Nuñez G 2009. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nature Immunology 10, 241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, Ferrucci L, Gilroy DW, Fasano A, Miller GW, Miller AH, Mantovani A, Weyand CM, Barzilai N, Goronzy JJ, Rando TA, Effros RB, Lucia A, Kleinstreuer N and Slavich GM 2019. Chronic inflammation in the etiology of disease across the life span. Nature Medicine 25, 1822–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay C 2006. Arthritis Research & Therapy 8, S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay C and Kushner I 1999. Acute-Phase Proteins and Other Systemic Responses to Inflammation. New England Journal of Medicine 340, 448–454. [DOI] [PubMed] [Google Scholar]
- Gaoni Y and Mechoulam R 1964. Isolation, Structure, and Partial Synthesis of an Active Constituent of Hashish. Journal of the American Chemical Society 86, 1646–1647. [Google Scholar]
- Gu Z, Singh S, Niyogi RG, Lamont GJ, Wang H, Lamont RJ and Scott DA 2019. Marijuana-Derived Cannabinoids Trigger a CB2/PI3K Axis of Suppression of the Innate Response to Oral Pathogens. Frontiers in Immunology 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammell DC, Zhang LP, Ma F, Abshire SM, McIlwrath SL, Stinchcomb AL and Westlund KN 2016. Transdermal cannabidiol reduces inflammation and pain-related behaviours in a rat model of arthritis. European Journal of Pain 20, 936–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskó G, Pacher P, Deitch EA and Vizi ES 2007. Shaping of monocyte and macrophage function by adenosine receptors. Pharmacology & Therapeutics 113, 264–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hind WH, England TJ and O’Sullivan SE 2016. Cannabidiol protects an in vitro model of the blood-brain barrier from oxygen-glucose deprivation via PPARγ and 5-HT1A receptors. British Journal of Pharmacology 173, 815–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdörfer B, Giese T, Endres S and Hartmann G 2002. Quantitative Expression of Toll-Like Receptor 1–10 mRNA in Cellular Subsets of Human Peripheral Blood Mononuclear Cells and Sensitivity to CpG Oligodeoxynucleotides. The Journal of Immunology 168, 4531–4537. [DOI] [PubMed] [Google Scholar]
- Huang Y, Wan T, Pang N, Zhou Y, Jiang X, Li B, Gu Y, Huang Y, Ye X, Lian H, Zhang Z and Yang L 2019. Cannabidiol protects livers against nonalcoholic steatohepatitis induced by high fat high cholesterol diet via regulating NF κB and NLRP3 inflammasome pathway. Journal of Cellular Physiology 234, 21224–21234. [DOI] [PubMed] [Google Scholar]
- Jin L, Yuan RQ, Fuchs A, Yao Y, Joseph A, Schwall R, Schnitt SJ, Guida A, Hastings HM, Andres J, Turkel G, Polverini PJ, Goldberg ID and Rosen EM 1997. Expression of interleukin-1β in human breast carcinoma. Cancer 80, 421–434. [DOI] [PubMed] [Google Scholar]
- Kaneko N, Kurata M, Yamamoto T, Morikawa S and Masumoto J 2019. The role of interleukin-1 in general pathology. Inflammation and Regeneration 39, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan BLF, Springs AEB and Kaminski NE 2008. The profile of immune modulation by cannabidiol (CBD) involves deregulation of nuclear factor of activated T cells (NFAT). Biochemical Pharmacology 76, 726–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T and Kawai T 2014. Toll-Like Receptor Signaling Pathways. Frontiers in Immunology 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay J 2004. The role of interleukin-1 in the pathogenesis of rheumatoid arthritis. Rheumatology 43, iii2–iii9. [DOI] [PubMed] [Google Scholar]
- Kozela E, Pietr M, Juknat A, Rimmerman N, Levy R and Vogel Z 2010. Cannabinoids Δ9-Tetrahydrocannabinol and Cannabidiol Differentially Inhibit the Lipopolysaccharide-activated NF-κB and Interferon-β/STAT Proinflammatory Pathways in BV-2 Microglial Cells. Journal of Biological Chemistry 285, 1616–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer F, Torzewski J, Kamenz J, Veit K, Hombach V, Dedio J and Ivashchenko Y 2008. Interleukin-1β stimulates acute phase response and C-reactive protein synthesis by inducing an NFκB- and C/EBPβ-dependent autocrine interleukin-6 loop. Molecular Immunology 45, 2678–2689. [DOI] [PubMed] [Google Scholar]
- Laprairie RB, Bagher AM, Kelly MEM and Denovan-Wright EM 2015. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. British Journal of Pharmacology 172, 4790–4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Choi EK, Seo KW, Bae JU, Park SY and Kim CD 2014. TLR4-Mediated Expression of Mac-1 in Monocytes Plays a Pivotal Role in Monocyte Adhesion to Vascular Endothelium. PLoS ONE 9, e104588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libro R, Scionti D, Diomede F, Marchisio M, Grassi G, Pollastro F, Piattelli A, Bramanti P, Mazzon E and Trubiani O 2016. Cannabidiol Modulates the Immunophenotype and Inhibits the Activation of the Inflammasome in Human Gingival Mesenchymal Stem Cells. Frontiers in Physiology 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodzki M, Godin B, Rakou L, Mechoulam R, Gallily R and Touitou E 2003. Cannabidiol—transdermal delivery and anti-inflammatory effect in a murine model. Journal of Controlled Release 93, 377–387. [DOI] [PubMed] [Google Scholar]
- Lopez-Castejon G and Brough D 2011. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev 22, 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Pinilla E, Varani K, Reyes-Resina I, Angelats E, Vincenzi F, Ferreiro-Vera C, Oyarzabal J, Canela EI, Lanciego JL, Nadal X, Navarro G, Borea PA and Franco R 2017. Binding and Signaling Studies Disclose a Potential Allosteric Site for Cannabidiol in Cannabinoid CB2 Receptors. Frontiers in Pharmacology 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC and Bonner TI 1990. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346, 561–564. [DOI] [PubMed] [Google Scholar]
- McFadden BR and Malone T 2021. Homegrown perceptions about the medical use and potential abuse of CBD and THC. Addictive Behaviors 115, 106799. [DOI] [PubMed] [Google Scholar]
- McKallip RJ, Jia W, Schlomer J, Warren JW, Nagarkatti PS and Nagarkatti M 2006. Cannabidiol-Induced Apoptosis in Human Leukemia Cells: A Novel Role of Cannabidiol in the Regulation of p22phox and Nox4 Expression. Molecular Pharmacology 70, 897–908. [DOI] [PubMed] [Google Scholar]
- Molteni M, Gemma S and Rossetti C 2016. The Role of Toll-Like Receptor 4 in Infectious and Noninfectious Inflammation. Mediators of Inflammation 2016, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monie TP 2017. Integrated Innate Immunity—Combining Activation and Effector Functions. Elsevier, pp. 121–169. [Google Scholar]
- Mukhopadhyay P, Rajesh M, Horváth B, Bátkai S, Park O, Tanchian G, Gao RY, Patel V, Wink DA, Liaudet L, Haskó G, Mechoulam R and Pacher P 2011. Cannabidiol protects against hepatic ischemia/reperfusion injury by attenuating inflammatory signaling and response, oxidative/nitrative stress, and cell death. Free Radical Biology and Medicine 50, 1368–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S, Thomas KL and Abu-Shaar M 1993. Molecular characterization of a peripheral receptor for cannabinoids. Nature 365, 61–65. [DOI] [PubMed] [Google Scholar]
- Muthumalage T and Rahman I 2019. Cannabidiol differentially regulates basal and LPS-induced inflammatory responses in macrophages, lung epithelial cells, and fibroblasts. Toxicol Appl Pharmacol 382, 114713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahara H, Song J, Sugimoto M, Hagihara K, Kishimoto T, Yoshizaki K and Nishimoto N 2003. Anti-interleukin-6 receptor antibody therapy reduces vascular endothelial growth factor production in rheumatoid arthritis. Arthritis & Rheumatism 48, 1521–1529. [DOI] [PubMed] [Google Scholar]
- Necela BM, Su W and Thompson EA 2008. Toll-like receptor 4 mediates cross-talk between peroxisome proliferator-activated receptor γ and nuclear factor-κB in macrophages. Immunology 125, 344–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols JM and Kaplan BLF 2020a. Immune Responses Regulated by Cannabidiol. Cannabis Cannabinoid Res 5, 12–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols JM and Kaplan BLF 2020b. Immune Responses Regulated by Cannabidiol. Cannabis and Cannabinoid Research 5, 12–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan SE, Kendall DA and Randall MD 2009. Time-Dependent Vascular Effects of Endocannabinoids Mediated by Peroxisome Proliferator-Activated Receptor Gamma (PPAR). PPAR Research 2009, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmqvist N, Siller M, Klint C and Sjödin A 2016. A human and animal model-based approach to investigating the anti-inflammatory profile and potential of the 5-HT2B receptor antagonist AM1030. Journal of Inflammation 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG 2008. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9 -tetrahydrocannabinol, cannabidiol and Δ9 -tetrahydrocannabivarin. British Journal of Pharmacology 153, 199–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper BJ, DeKeuster RM, Beals ML, Cobb CM, Burchman CA, Perkinson L, Lynn ST, Nichols SD and Abess AT 2017. Substitution of medical cannabis for pharmaceutical agents for pain, anxiety, and sleep. Journal of Psychopharmacology 31, 569–575. [DOI] [PubMed] [Google Scholar]
- Proescholdt MG, Chakravarty S, Foster JA, Foti SB, Briley EM and Herkenham M 2002. Intracerebroventricular but not intravenous interleukin-1β induces widespread vascular-mediated leukocyte infiltration and immune signal mRNA expression followed by brain-wide glial activation. Neuroscience 112, 731–749. [DOI] [PubMed] [Google Scholar]
- Rajesh M, Mukhopadhyay P, Bátkai S, Patel V, Saito K, Matsumoto S, Kashiwaya Y, Horváth B, Mukhopadhyay B, Becker L, Haskó G, Liaudet L, Wink DA, Veves A, Mechoulam R and Pacher P 2010. Cannabidiol Attenuates Cardiac Dysfunction, Oxidative Stress, Fibrosis, and Inflammatory and Cell Death Signaling Pathways in Diabetic Cardiomyopathy. Journal of the American College of Cardiology 56, 2115–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman A, Welty M and Solomon P 2017. Cannabis as a Substitute for Opioid-Based Pain Medication: Patient Self-Report. Cannabis and Cannabinoid Research 2, 160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo MD, Crawford RB, Bach A, Sermet S, Amalfitano A and Kaminski NE 2019. Δ9-Tetrahydrocannabinol Suppresses Monocyte-Mediated Astrocyte Production of Monocyte Chemoattractant Protein 1 and Interleukin-6 in a Toll-Like Receptor 7–Stimulated Human Coculture. Journal of Pharmacology and Experimental Therapeutics 371, 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo EB, Burnett A, Hall B and Parker KK 2005. Agonistic Properties of Cannabidiol at 5-HT1a Receptors. Neurochemical Research 30, 1037–1043. [DOI] [PubMed] [Google Scholar]
- Ryberg E, Larsson N, Sjögren S, Hjorth S, Hermansson NO, Leonova J, Elebring T, Nilsson K, Drmota T and Greasley PJ 2007. The orphan receptor GPR55 is a novel cannabinoid receptor. British Journal of Pharmacology 152, 1092–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha RN, Jana M and Pahan K 2007. MAPK p38 Regulates Transcriptional Activity of NF-κB in Primary Human Astrocytes via Acetylation of p65. The Journal of Immunology 179, 7101–7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoni G, Morelli MB, Amantini C, Santoni M, Nabissi M, Marinelli O and Santoni A 2018. “Immuno-Transient Receptor Potential Ion Channels”: The Role in Monocyte- and Macrophage-Mediated Inflammatory Responses. Frontiers in Immunology 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk M, Belisle JT and Modlin RL 2009. TLR2 Looks at Lipoproteins. Immunity 31, 847–849. [DOI] [PubMed] [Google Scholar]
- Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, López CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG, Inflammation, t. and Host Response to Injury, L.S.C.R.P. 2013. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proceedings of the National Academy of Sciences 110, 3507–3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaftel SS, Griffin WST and O’Banion MK 2008. The role of interleukin-1 in neuroinflammation and Alzheimer disease: an evolving perspective. Journal of Neuroinflammation 5, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showalter VM, Compton DR, Martin BR and Abood ME 1996. Evaluation of binding in a transfected cell line expressing a peripheral cannabinoid receptor (CB2): identification of cannabinoid receptor subtype selective ligands. Journal of Pharmacology and Experimental Therapeutics 278, 989–999. [PubMed] [Google Scholar]
- Sonego AB, Prado DS, Vale GT, Sepulveda-Diaz JE, Cunha TM, Tirapelli CR, Del Bel EA, Raisman-Vozari R and Guimarães FS 2018. Cannabidiol prevents haloperidol-induced vacuos chewing movements and inflammatory changes in mice via PPARγ receptors. Brain, Behavior, and Immunity 74, 241–251. [DOI] [PubMed] [Google Scholar]
- Stanley LC, Mrak RE, Woody RC, Perrot LJ, Zhang S, Marshak DR, Nelson SJ and Griffin WST 1994. Glial Cytokines as Neuropathogenic Factors in HIV Infection: Pathogenic Similarities to Alzheimer’s Disease. Journal of Neuropathology & Experimental Neurology 53, 231–238. [DOI] [PubMed] [Google Scholar]
- Szabo-Fresnais N, Blondeau J-P and Pomérance M 2008. Activation of the cAMP pathway synergistically increases IL-1-induced IL-6 gene expression in FRTL-5 thyroid cells: Involvement of AP-1 transcription factors. Molecular and Cellular Endocrinology 284, 28–37. [DOI] [PubMed] [Google Scholar]
- Takeda K, Kaisho T and Akira S 2003. Toll-Like Receptors. Annual Review of Immunology 21, 335–376. [DOI] [PubMed] [Google Scholar]
- Tang S, Chen T, Yang M, Wang L, Yu Z, Xie B, Qian C, Xu S, Li N, Cao X and Wang J 2017. Extracellular calcium elicits feedforward regulation of the Toll-like receptor-triggered innate immune response. Cellular & Molecular Immunology 14, 180–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji H, Ohto U, Shibata T, Taoka M, Yamauchi Y, Isobe T, Miyake K and Shimizu T 2015. Toll-like receptor 8 senses degradation products of single-stranded RNA. Nature Structural & Molecular Biology 22, 109–115. [DOI] [PubMed] [Google Scholar]
- Tham M, Yilmaz O, Alaverdashvili M, Kelly MEM, Denovan-Wright EM and Laprairie RB 2019. Allosteric and orthosteric pharmacology of cannabidiol and cannabidiol-dimethylheptyl at the type 1 and type 2 cannabinoid receptors. British Journal of Pharmacology 176, 1455–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele EA, Bebin EM, Bhathal H, Jansen FE, Kotulska K, Lawson JA, O’Callaghan FJ, Wong M, Sahebkar F, Checketts D, Knappertz V and Group GS 2021. Add-on Cannabidiol Treatment for Drug-Resistant Seizures in Tuberous Sclerosis Complex: A Placebo-Controlled Randomized Clinical Trial. JAMA Neurology 78, 285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalley BJ, Bazelot M, Rosenberg E and Tsien R 2018. A role of GPR55 in the antiepileptic properties of cannabidiol (CBD) (P2.277). Neurology 90, P2.277. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.