Abstract
Hearing impairment commonly co-occurs with dementia. Audiologists, therefore, need to be prepared to address the specific needs of people living with dementia (PwD). PwD have needs in terms of dementia-friendly clinical settings, assessments, and rehabilitation strategies tailored to support individual requirements that depend on social context, personality, background, and health-related factors, as well as audiometric HL and experience with hearing assistance. Audiologists typically receive limited specialist training in assisting PwD and professional guidance for audiologists is scarce. The aim of this review was to outline best practice recommendations for the assessment and rehabilitation of hearing impairment for PwD with reference to the current evidence base. These recommendations, written by audiology, psychology, speech-language, and dementia nursing professionals, also highlight areas of research need. The review is aimed at hearing care professionals and includes practical recommendations for adapting audiological procedures and processes for the needs of PwD.
Keywords: Audiology, Cognitive impairment, Dementia, Hearing loss
INTRODUCTION
Aging populations and increasing numbers of people living with dementia (PwD) means the caseload of audiologists includes many older people with hearing impairment and cognitive difficulties (Kricos, 2006). Hearing impairment is highly prevalent among PwD. One survey identified hearing impairment (>40 dB HL over 0.5 to 4.0 kHz in the better hearing ear) in 87% of PwD in the general community (Allen et al. 2003). Hearing impairment may be even more common among PwD living in aged care facilities (Weinstein, 1986; Cohen-Mansfield & Taylor, 2004). However, hearing impairment often remains unrecognized and unmanaged among PwD (Allen et al. 2003; Nirmalasari et al. 2017). This is in part due to a lack of interdisciplinary working between the audiology and dementia care professions (Leroi et al. 2019).
Discriminating hearing difficulties from cognitive difficulties may be challenging. Difficulty following conversations, forgetfulness, and withdrawal from social gatherings are symptoms of both hearing and cognitive problems (Kricos, 2009). PwD may lack insight and not be able to verbalize their hearing difficulties or communication challenges, and families or carers may miss-attribute communication difficulties as related to dementia rather than being due to potentially remediable hearing problems (Hopper & Hinton, 2012; Slaughter et al. 2014). If they are aware of hearing problems, carers may assume that hearing cannot be reliably assessed among PwD. Hearing care professionals may lack the confidence to work with PwD (Wright et al. 2014), or feel that PwD will not be able to use or benefit from hearing interventions. However, reliable hearing assessment is possible for most PwD, provided that assessment is adapted to the needs of PwD (Bott et al. 2019) and systematic reviews indicate that PwD do benefit from hearing intervention (Dawes et al. 2019b; Mamo et al. 2018).
Under-identification and lack of support for hearing impairment among PwD are problematic because hearing impairment exacerbates the impact of dementia¸ with lower quality of life, increased depression, functional decline, social isolation, increased dependency, and carer burden all linked to untreated hearing impairment among PwD (Resnick et al. 1997; Dawes et al. 2019b; Mamo et al. 2018). Behavioral and psychological symptoms associated with dementia including agitation, hallucinations, and aggression are also exacerbated with untreated hearing impairment (Haque et al. 2012; El Haj et al. 2017).
International guidance has recently been produced for the identification and management of hearing and vision impairment in PwD (Leroi, Constantinidou, et al. 2020; Littlejohn et al. 2021), but the recommendations do not state specifically how hearing care professionals should modify their practice to best support PwD. Surveys of hearing care professionals suggested that although most clinicians had seen PwD, they did not feel adequately supported to provide hearing care and would welcome specific guidance on working with PwD (Wright et al. 2014; Leroi et al. 2019). Thus, the purpose of this paper is to provide person-centered recommendations to support any professional in the context of providing hearing healthcare to PwD, including audiologists, audiometrists, and otolaryngologists.
Dementia
Dementia is a syndrome describing progressive deterioration in cognitive function which interferes with a person’s ability to maintain activities of daily living (World Health Organisation 2020). There are different types of dementia with different brain pathology, the main ones being Alzheimer’s disease (60–80% of cases), vascular dementia (5–10%), dementia with Lewy bodies (5–10%), frontotemporal dementia (5–10%), and “others” including mixed dementia (combination of more than one cause) and Parkinson’s and Huntington’s dementia (Prince et al. 2015). The specific cognitive impairments, symptoms, and difficulties experienced by an individual person depend on which disease is present, where disease pathology is expressed throughout the brain and how the disease progresses over time. Symptoms of dementia may include problems with memory, visuospatial skills, attention and concentration, executive functions, receptive and expressive language, psychomotor skills, and social and emotional difficulties (Oyebode & Clare 2014). The clinical presentation of dementia occurs and interacts with the context of personal history, personality, and social environment that is different for each person. Supporting the hearing and communication needs of a PwD must therefore be individualized to consider the unique strengths and difficulties of every person, as described in the VIPS model in the following section.
The VIPS Model: Understanding Dementia from a Person-centered Perspective
The pioneering work of Kitwood (1997, 2020) and others have been influential in shifting understanding of dementia from a narrow biomedical perspective (which focuses on identification and management of cognitive impairment) to a broader holistic one (which considers a person’s individual circumstances). Kitwood’s person-centered perspective draws attention to a wide range of factors that can affect a person’s experience of living with dementia, including their neurological impairment, health (including sensory impairments), biography, personality, and social environment. The “social environment” factor includes how people, including clinicians, communicate with PwD. Kitwood challenged the view that there was little that others could do to improve the well-being of PwD, and argued that clinicians should concern themselves with the person, rather than dementia. Brooker and Latham (2016) draw on Kitwood’s holistic approach to providing care for PwD in their “VIPS” model of person-centered dementia care (Table 1). The VIPS model informs national guidelines for dementia care (e.g., the United Kingdom’s dementia guidelines [National Institute for Health and Care Excellence 2018]). We refer to hearing healthcare in the context of the VIPS model in the remainder of this document.
TABLE 1.
The VIPS model for person-centered dementia care (adapted from Brooker and Latham 2016)
| VIPS Component | Key Principles | Examples of Hearing healthcare Practice |
|---|---|---|
| V – Valuing people with dementia and those who care for them | • Valuing people with dementia, regardless of their level of cognitive impairment • Promoting the rights and citizenship of people with dementia and their families/supporters. • Rooting out the practice that might discriminate against people with dementia. |
• Ensuring there is access to hearing care services to meet hearing needs regardless of the level of cognitive impairment • Staff training/awareness to promote knowledge and understanding of dementia • Supporting staff to make positive changes to practice to meet the needs of people with dementia |
| I – Seeing the individuality of people living with dementia | • No two people with dementia are alike. • ‘One-size-fits-all’ approaches and interventions will not work for everyone |
• Practices that are flexible and draw on knowledge and understanding of the person to find means of “connecting” and building trust and rapport, using preferred modes of communication and interaction. • Assessment and rehabilitation approaches tailored to individual needs. |
| P – Looking at the world from the perspective of the person | • An individual’s perception of their current situation and their immediate surroundings may be very different from how others perceive it. • Impairments to memory and perception may mean the person misinterprets what is happening. |
• Recognize that a hearing assessment may be perceived as something confusing and frightening by the person with dementia. • Be attentive to the person’s words as well as their nonverbal communication including behaviors that may communicate a need or emotion. • Consider the physical environment and how it may be perceived by and impact the person with dementia. |
| S – A social environment that supports relationships and interactions that promote well-being. | • How we enable the person with dementia to feel safe, respected, and included. • Meeting of social and psychological needs as well as physical needs. |
• Developing skills in communication to build trusting relationships. • Aiming for familiarity and emotional support by enabling the presence of a family member/important other at appointments/assessments where the person with dementia agrees. • Always addressing and involving the person with dementia even where communication is difficult and a carer/significant other is present. |
The Clinical Setting
This section describes how VIPS principles could be applied in the context of the clinical setting, with consideration of the physical clinical environment, staff training, making appointments, and capacity to consent.
Physical Environment
PwD report that when environments are poorly adapted to cognitive and perceptual difficulties that occur in dementia, the environment can feel hostile and has a negative impact on the person’s mood, causing stress and anxiety (Houston et al. 2020). Problematic features of the environment include settings with medical equipment and staff in laboratory coats that may make someone who is not aware of where they are fearful about experiencing unpleasant medical procedures (Waller & Masterson 2015). Hearing clinics that are more homely and less hospital-like are desirable. Lack of clear color difference between walls and floors, patterned floors, highly reflective surfaces, and abrupt color changes in flooring are also problematic; they may contribute to disorientation, be perceived as holes in the floor or as pools of water (Waller & Masterson 2015). Barrett et al. (2019) provide a detailed review, describing dementia-friendly design including clear signage; environmental cues to aid navigation and familiarity; accessible toilets; appropriate use of color; and visual information to inform people of the function of the setting. Dementia-friendly design principles can also be applied to home, healthcare settings including audiology and ENT clinics as well as aged care settings. Creating a dementia-friendly clinical environment may also provide an important benefit in facilitating access to, use of, and benefit from clinical facilities by older people in general (c.f. “Universal design;” Carr et al. 2013). Although a detailed discussion of dementia-friendly design (e.g., Calkins et al. 2011; Fleming et al. 2012; Van Hoof & O’Brien 2014) is beyond the scope of this document, there are resources to support dementia-friendly design available, such as the Dementia Services Design Centre’s audit tool (https://dementia.stir.ac.uk/). Hearing care service providers could also consider inviting comments from PwD on the design and layout of clinics (Houston et al. 2020). Local and national organizations (Appendix, Supplemental Digital Content 1, http://links.lww.com/EANDH/A985) may be able to assist with putting clinicians in touch with people who could provide the perspective of PwD. Whereas extensive redecorating of hearing clinics may be prohibitive in terms of cost and practicality, small changes such as clear signage or putting up pictures to make the environment less “clinical” may be feasible and have a useful impact in making clinics a more supportive environment for PwD. When renovations or a new clinical facility are being planned, hearing health professionals should capitalize on the opportunity to make their clinic dementia-friendly.
Training for Staff
A positive, accessible, and enabling physical environment is important but cannot alone create a positive therapeutic milieu (Chaudhury et al. 2018; Hebert & Scales 2019). In addition to a supportive physical environment, the VIPS model highlights the need for a supportive social environment. It is the person-centered values and interactions of the hearing health professionals and supporting staff that has the potential to make the biggest positive – or negative – impact on the health and wellbeing of PwD utilizing their service. Training and support for staff are therefore of major importance.
Staff training in dementia awareness and effective communication is useful for everyone working within hearing services, from custodial staff to reception staff to clinicians and clinic managers (Skills for Care & Skills for Health and Department of Health 2011). Dementia awareness training focuses on the “VIP” elements of the VIPS model. There are various courses in dementia awareness available globally, pitched at different levels of staff (i.e., from informal interaction to clinical interactions, up to the level of those who make service-level decisions on care for PwD). Some courses are free (Appendix, Supplemental Digital Content 1, http://links.lww.com/EANDH/A985). The quality of education and training programs vary. It is advisable to consider the learning outcomes of courses, the appropriateness of the course for those undertaking the training in terms of their roles and responsibilities working with PwD, and how the learning outcomes of the course link to any national standards on dementia training. In the UK, this is the Dementia Training Standards Framework (https://www.hee.nhs.uk/our-work/dementia-awareness/core-skills). Dementia training standards vary between states in the USA (https://justiceinaging.org/our-work/healthcare/dementia-training-requirements/dementia-training-requirements-state-by-state/). Australian aged care quality standards include dementia training (https://www.agedcarequality.gov.au/providers/standards).
Making Appointments
PwD often have difficulties with comprehension and memory, even in the early stages of the syndrome. Any appointment information should be provided in a simple and accessible format. Guidance on writing “dementia-friendly” information has been produced by PwD (https://www.dementiavoices.org.uk/deep-guides/for-organisations-and-communities/). The following information should be stated in simple language:
When the assessment/appointment will take place.
Where it will take place, with clear guidance regarding travel, maps, and physical access.
Who the appointment will be with.
What the appointment will entail. This may help the PwD to understand the purpose of the appointment and what to expect. Pictorial representations of equipment and service staff can be helpful.
A recommendation for the PwD to be accompanied to the appointment by a family member/significant other. This will provide help in respect of (i) the provision of emotional support; (ii) reminding the PwD of the purpose of the appointment; (iii) assisting the clinician to establish hearing functionality and hearing needs in daily life; and (iv) providing information on hearing diagnosis and treatment options.
A person’s level of functioning may fluctuate. Particularly in the middle and later stages of dementia, some people may become more confused, aggressive, or agitated during late afternoon and evening (sometimes referred to as “sundowning”), and function better earlier in the day (Canevelli et al. 2016). It is useful to establish the best time of day to see the PwD and plan appointments accordingly. A person’s functioning may also fluctuate from day to day, so it may also be necessary to re-schedule or provide additional appointments to see the PwD when the PwD has a “good” day.
Capacity to Consent
Most people with mild dementia and many with moderate dementia can participate in decisions about their healthcare (Moye et al. 2004). Active involvement in clinical decisions helps PwD retain their independence. To have the capacity to make decisions about healthcare, a person must be able to understand the information relevant to the decision, remember the information long enough to make a decision, evaluate the information and communicate their decision (Moye & Marson 2007). If a person is unable to do all these things, they lack the capacity to make a specific decision. Assessment of capacity is done by an appropriately trained health professional who is part of the clinical care team for that person (Lamont et al. 2013). If it is not possible for the person to participate in clinical decision-making due to a lack of capacity to make decisions or consent to treatment, you may need to seek additional support from the person’s family or caregiver, or from advocacy services for PwD. One should check a person’s case notes for legal guardianship/conservatorship/power of attorney for healthcare or advice directives. If such an arrangement is in place, discussing medical care with the person directly may be distressing and legally inappropriate. Capacity and consent are covered by different legal arrangements in different countries (Buchanan 2004; HM Government 2005; Moye & Marson 2007; Alzheimer Europe 2016). The general principles are that:
1. You must assume a person has capacity unless it is established that they lack capacity. It is helpful to have a care partner at the clinical appointment, but you should always obtain consent from the PwD before discussing the patient’s condition and clinical care with anyone other than the PwD. Record the name of the person who accompanies the patient and record the name of any person that the PwD consents to receive the results from the examination and treatment recommendations. Address the PwD first when you present the results and offer treatment options.
2. A person should not be treated as being unable to make a decision unless all practical steps to help them have been taken without success. You should always explain the purpose of any clinical tests and obtain consent from the PwD before proceeding. A PwD’s capacity to understand and consent may vary so you may have to assess them on another occasion. It may be useful to program and fit hearing aid for the PwD to try out, to facilitate informed consent. Tools such as those developed by the Ida Institute (Gregory 2013) may facilitate informed, patient-centered decision-making. Record the reasons for any limitations on the examination and results obtained.
3. A person should not be treated as unable to make a decision just because they make an unwise decision (in the opinion of a clinician or anyone else; for example, by turning down a hearing aid).
4) If a person does lack capacity, then any act done or decision made on behalf of that person must be made in their best interests. You must consider past wishes, beliefs, and values that would likely have influenced their decisions if they had the capacity. You should consider the possibility that the care partner’s wishes may not coincide with those of the PwD, and the PwD’s wishes take precedent.
5) Before making any decision on behalf of someone, you must consider if the outcome can be achieved in a way that is less restrictive of the person’s right to self-determination and freedom of action. For example, if the PwD declines a hearing aid, you may instead optimize communication by focusing on communication tactics with care partners and improving the environment (see the following section).
Protection and Duty of Care
PwD are vulnerable to neglect (including “self-neglect” – not being able to take care of themselves properly) and abuse. One systematic review concluded that 6% of older adults in general and around a quarter of vulnerable adults experience abuse (Cooper et al. 2008). If you have any doubt about the PwD’s circumstances, you should report your concerns to the PwD’s general medical practitioner and be prepared to discuss these if necessary.
The vulnerability of PwD extends to multi-morbidity. Given that dementia is strongly age-associated, PwD are likely to live with multiple long-term health conditions (Banerjee 2015; Vassilaki et al. 2015) and are likely to require additional support services. You may suggest that PwD discuss these with their general medical practitioner or nurse. Formal referral of PwD should involve discussion with the patient and their care partner, as appropriate.
Identifying People With Cognitive Impairment
History taking Referral sources may mention a formal diagnosis of dementia, but referral information cannot be relied on with respect to a person’s dementia status; up to 40% of people do not receive a formal diagnosis of dementia (Amjad et al. 2018). Even if a person has received a diagnosis of dementia, the referral source may overlook or not see fit to mention this in the referral for hearing care. As dementia is progressive and may have a rapid onset due to acute injury or illness, a person may not have a diagnosis of dementia identified by their referral source (e.g., 6 months ago), but may be experiencing dementia-related difficulties by the time they attend their audiology appointment.
History taking gives an opportunity to ask about the cognitive status. Discussions regarding difficulties understanding speech may suggest underlying cognitive problems, for example, difficulties with memory for recent events or word finding. With appropriate consent from the PwD, involving friends and family members in history taking may provide helpful information on how much the person’s abilities have deteriorated. Aspects of cognition including attention, working memory and cognitive flexibility may or may not be apparent in conversation, and some PwD are adept at compensating for cognitive difficulties (Tomaszewski Farias et al. 2018). It is therefore unlikely that hearing health professionals could reliably identify dementia only based on their clinical impression. Some clinicians explicitly ask about memory problems and dementia (Wright et al. 2014). Unfortunately, up to 20% of people do not report having had a dementia diagnosis (based on self- or proxy-report) despite having had received one (Amjad et al. 2018). Information from referrals and history taking should not be neglected but may provide an incomplete impression of a person’s cognitive status. An alternative option is to formally screen cognitive functioning.
Cognitive screening There are various screening tools to assess cognitive function, which may give insight into the presence and severity of cognitive impairment (Shen et al. 2016) and inform hearing assessment and rehabilitation. These screening tools include the General Practitioner assessment of cognition (Brodaty et al. 2002), the Mini-Mental State Examination (Folstein et al. 1975), or the Montreal Cognitive Assessment (Nasreddine et al. 2005). However, cognitive screening by hearing care professionals should be approached with caution. Many other factors outside of cognitive function may influence test performance, including hearing and vision impairment (Pye et al. 2017), age, level of education, and mood. Adequate training and expertise are required to administer, interpret and discuss the results of a cognitive screen with patients. Recent surveys of hearing professionals in the United Kingdom (UK) reported that awareness of cognitive screening tools is limited and training in the use of cognitive screening tools is lacking (Leroi et al. 2019; Wright et al. 2014). Half of UK audiologists who completed an online survey were aware of cognitive screening tests that could be used in the clinic, but only 20% of the sample reported they felt they had the training and expertise needed to administer tests and interpret the results (Leroi et al. 2019). If cognitive difficulties were identified, audiology professionals did not feel confident in using the results to adapt hearing assessment and management. Evidence to inform practice is lacking, and audiologists report being uncertain how the results of cognitive screening would inform audiological care, including how to choose the right hearing aid for the PwD, how to select hearing aid features, and how to motivate PwD to wear hearing aids (Wright et al. 2014). Simple pass/fail results on a cognitive screening test may be of limited utility in relation to these questions.
Further, there is no gold-standard cognitive screening for hearing clinics (Ladduwahetty et al. 2013). Cognitive screening tests for use in hearing clinics should reliably identify cognitive impairment, be quick enough to be practical to use in hearing clinics, and be resistant to the confounding effects of hearing impairment. Unfortunately, most cognitive screening tests rely on the test-taker having a good hearing function. Simulated hearing impairment impacts performance on the Mini-Mental State Examination (Jorgensen et al. 2016), one of the most widely used cognitive screening tools, and other cognitive measures (Füllgrabe 2020). People with hearing impairment may fail a cognitive screen due to not having heard the questions properly. Adapted versions of cognitive screening tools for hearing impairment exist, but the reliability of these tools for identifying cognitive impairment has generally not been established (Pye et al. 2017), or is unsatisfactory (Al-Yawer et al. 2019). A version of the Montreal Cognitive Assessment adapted for and validated with people with hearing loss has recently been released (Dawes et al. 2019a), and is freely available to registered users (https://www.mocatest.org/). An alternative to direct assessment of cognition for people with a care partner (or someone who has known the person for several years) is to use a proxy assessment, such as the informant interview version of the General Practitioner assessment of cognition (http://gpcog.com.au/index/informant-interview).
Because up to half of PwD do not receive a formal diagnosis (Connolly et al. 2011) and because hearing impairment is a marker of risk for dementia (Livingston et al. 2020), a potential advantage of cognitive screening of people presenting at hearing clinics could be to identify the presence of cognitive impairment and inform the process of physician assessment for dementia. Timely dementia diagnosis is beneficial for access to therapies and support services, and patients and their families can prepare better for the future (Prince et al. 2011). However, it may not be feasible or appropriate to screen for possible cases of dementia in hearing clinics. A lack of appropriate dementia assessments, effective treatments, and support from dementia care professionals means that population screening for dementia in asymptomatic persons is not currently advised (Lin et al. 2013; Public Health England 2019). Within hearing clinics, practical limitations such as additional time required to undertake the screening, discuss the results, and address further questions may not be possible within a busy clinic. Hearing clinicians need to feel comfortable and have appropriate training in delivering a cognitive test and explaining the purpose of the test and its results. Cognitive testing may be met with resistance from people who are not there for reasons to do with their cognitive health. The acceptability of cognitive screening in hearing clinics is uncertain.
A final consideration is that if cognitive screening is done in hearing clinics, it is essential that there are clear and appropriate referral pathways to memory services in place. Before cognitive screening is implemented, hearing clinics need to be aware of local memory services and a referral protocol including when and where to refer and what information is included in the referral should be established (Shen et al. 2016).
Hearing Assessment
There is emerging evidence that some people with mild dementia can reliably complete behavioral hearing tests, provided tests are adapted to support the needs of PwD in the ways described below (i.e., an individualized approach consistent with the VIPS model). When standard behavioral hearing tests are unsuccessful, clinicians could consider using functional assessments or objective tests, such as auditory evoked potentials or Distortion Product Otoacoustic Emissions (DPOAEs). However, more information is needed to establish the feasibility and reliability of hearing assessments for people with more severe dementia.
Behavioral Assessment – Pure-Tone Audiometry
Behavioral assessments, such as pure-tone audiometry and speech audiometry, form part of the standard audiological diagnostic tests used to diagnose the degree of hearing impairment and site of lesion in adults. Some PwD may be unable to complete these behavioral tests (at least if administered in a standard way), presenting challenges to hearing care professionals in determining how to identify hearing impairment for PwD. One study reported that binaural pure-tone thresholds could be obtained in 32% (98 of 307) of PwD in residential aged care (Burkhalter et al. 2009). However, only 5% of people could complete a comprehensive audiometric evaluation (air and bone conduction thresholds, speech reception threshold, speech recognition, most comfortable level) and 37% could not complete any of the assessments. Because the hearing assessments were carried out as part of a research study (on the effect of recordings of environmental sounds or soothing voice on behavioral and psychological symptoms of dementia), the assessments in Burkhalter et al.’s study were carried out “by the book” without adjustment for the individual needs of PwD.
A recent systematic review reported that 56% to 59% of PwD could complete pure-tone audiometry (Bott et al. 2019). Only three studies met the criteria for inclusion, and studies included people with mild to severe dementia. Although up to 60% of PwD could complete at least some audiometry in Bott et al.’s review, the reliability of audiometric assessment was not examined. Bott et al. were not able to report on the type or use of modifications to audiometric assessments, because modifications were not reported in the papers included in the review. Bott and colleagues concluded that most people with mild dementia could be audiometrically assessed reliably at a minimum of three frequencies in both ears. McClannahan et al. (2021) reported comparable test re-test reliability of pure-tone audiometry, tests of middle ear function, speech perception, and self-reported hearing handicap measures for people with mild dementia as for people with normal cognition. Some adults with moderate and severe dementia are also able to be assessed with pure-tone audiometry, but many PwD may benefit from adaptations to standard audiometric procedures.
Based on clinical experience, some authors offer the following suggestions for modifying hearing assessment to support individual PwD (Durrant et al. 1991; Palmer et al. 1998; Vance & Johns 2003; Burk & Wiley 2004; Dancer & Watkins 2006; Kricos 2009; Lemke 2011; Hopper & Hinton 2012; McClannahan et al. 2021):
Be prepared. A PwD may or may not be cooperative with the traditional hearing test, in which case you may try strategies outlined below to understand the person’s hearing abilities.
Include family members, caregivers, and assistive living or nursing practitioners in the evaluation and treatment process. They may help calm the person, and you will be able to share information with and receive information from the caregiver.
PwD tend to function better in familiar environments. If possible, arrange for a hearing assessment at the person’s home (or own room in an aged care facility).
Plan to make several visits to succeed in helping the person if you are working with people in a facility or home.
Understand that the PwD may be anxious or irritable and may repeatedly ask the same questions. Be patient and smile.
Reduce background noise as much as possible.
Let the person know your name and who you are, even if you have seen them many times before.
Speak slowly, clearly, calmly, and gently with simple words and sentences. Then give the person time to respond. Use simple gestures when the person seems confused. Demonstrate what is required using relevant objects.
Asking the person to repeat what they are supposed to do to be sure that they understand what is expected.
Give examples, demonstrate the test, and give practice runs.
Slow down the pace of the assessment (e.g., slow the presentation of stimuli).
Use pulsed tones rather than continuous ones to assist with difficulties with alertness and orientation to the stimuli.
Reducing test duration may reduce inaccuracies from limited concentration.
Test strategies such as testing 4 kHz immediately after 1 kHz and testing only 1 or 2 frequencies in each ear may provide an essential minimal level of audiometric information to inform management. The priority should be a small number of accurate thresholds, rather than a complete audiogram of questionable reliability.
Give frequent reminders about the task requirements and encouragement.
Allow the partner/family member/friend to accompany the PwD to the testing appointment and into the test booth. Having family and carers within sight may support the PwD to continue with the test. Arrangement of the clinic room is important to ensure that the PwD can see family/and or carers. Seeing familiar faces can also alleviate consternation if the person does not know where they are or who the hearing professional is.
Having the person respond with “yes” (or any other consistent behavioral response) rather than a button press. PwD may mimic the tone or respond with a description, for example, “that’s a quiet one” or “that’s better.” Look for repeatable nonverbal or activity responses such as head turns, eye movements, or any other behavioral changes.
If an audiologist is unable to obtain reliable audiometric thresholds despite adapting audiometric assessment, it may be possible to estimate the likely current level of hearing based on extrapolation from past audiometric results (if available). If past audiometric results are not available and there is insufficient information on which to safely base amplification targets, consider fitting a personal amplification device instead.
Electrophysiological Tests
If modifications to pure-tone audiometry do not facilitate reliable hearing thresholds and if previous hearing test results are unavailable, electrophysiological tests could be considered. Audiologists should be aware of the limitations of electrophysiological tests and consider the capacity to consent and VIPS principles when using electrophysiological tests with PwD.
Otoacoustic emissions index cochlear function; absent emissions indicate a likely hearing loss of >25 to 30 dB HL. Otoacoustic emissions testing does not provide an estimate of the severity of hearing impairment. Otoacoustic emissions testing is quick (<1 minute per ear), does not require a behavioral response, and can be carried out by people with a basic level of training (e.g., nursing home staff). Jupiter (2012) evaluated the feasibility of DPOAEs testing to identify cases of hearing impairment in 100 nursing home residents of age 65 to 108 years. Most (90%) of residents failed the DPOAE screening at 40 dB HL, consistent with the results of pure-tone audiometric testing. All one hundred residents completed DPOAE testing, with cognitive ability (indexed by the Mini-Mental State Examination) of the sample of residents ranging between the normal and severely cognitively impaired range. However, an unspecified number of residents were excluded from the study due to severe cognitive impairment or being judged to be uncooperative or agitated. Jupiter (2012) concluded that DPOAEs can be used to identify cases of hearing impairment in nursing home populations that include PwD.
To date, two studies have examined the feasibility of using auditory evoked potentials for hearing threshold estimation with PwD. Bott, Hickson, et al. (2020) examined a late auditory evoked potential test – cortical automatic threshold estimation (CATE), while Villeneuve et al. (2017) assessed the auditory steady-state response (ASSR) as alternatives to pure-tone audiometry for PwD. Both studies found that CATE and ASSR were strongly correlated (r = 0.5–0.9, depending on frequency) with pure-tone audiometry results based on small samples of PwD (n = 6 in each study). Both studies included people with mild dementia, who are not the target population for an electrophysiological hearing assessment. The testing time of CATE and ASSR is approximately 60 minutes which may be impractical for busy clinicians and unacceptable to many PwD. In Bott et al.’s study, 5 of 16 (31%) of participants did not complete CATE due to refusal to wear the electrodes or discontinued due to the test being too long. There is dubious benefit in using intrusive, time-consuming electrophysiological testing to estimate audiometric thresholds in people with more severe dementia who are in any case probably not good candidates for hearing aids. A pragmatic alternative to obtaining audiometric thresholds required to program a hearing aid is to trial a personal amplification device (see below), which does not require an audiogram to be fitted.
Functional Hearing Assessment
If it is not possible to estimate hearing thresholds using behavioral or electrophysiological methods, audiologists may use functional assessment to index the PwD’s hearing function, their communicative needs, abilities and limitations, and the potential to benefit from intervention. Functional assessments could also be used to test the impact of communicative interventions. Self-report measures include the Self-Assessment of Communication (SAC) (Schow & Nerbonne 1982) or the screening version of the Hearing Handicap Inventory for the Elderly (HHIE-S) (Ventry & Weinstein 1982). If a person is unable to reliably report on their own communication, the assessment could be completed by a carer or someone who knows the PwD well, for example, the Significant Other Assessment of Communication (SOAC) (Schow & Nerbonne 1982). The Parents’ Evaluation of Aural/Oral Performance of Children (PEACH) is a parent-completed checklist for evaluating the effectiveness of amplification for infants and children with hearing impairment (Ching & Hill 2007). The PEACH is used by Australian audiologists (Emma Scanlan, personal communication, April 2021) as a carer-completed checklist of a PwD’s ability to hear in different situations and indicate the need for intervention. Audiologists may supplement self/proxy reported communication measures with informal suprathreshold speech recognition testing. American Speech-Language-Hearing Association (ASHA) (1997) guidelines for audiology service delivery in nursing homes recommend using “functionally relevant” test materials (e.g., the speech of carers, friends or family, music, television, or radio). Functional assessment could be carried out using the PwDs’ own personal items (e.g., photo albums) supplemented with information from caregivers (or anyone else who knows the PwD well) concerning their background, interests, functional strengths and limitations that may inform audiological interventions, consistent with the VIPS model of person-centered dementia care.
Interventions for Hearing Impairment
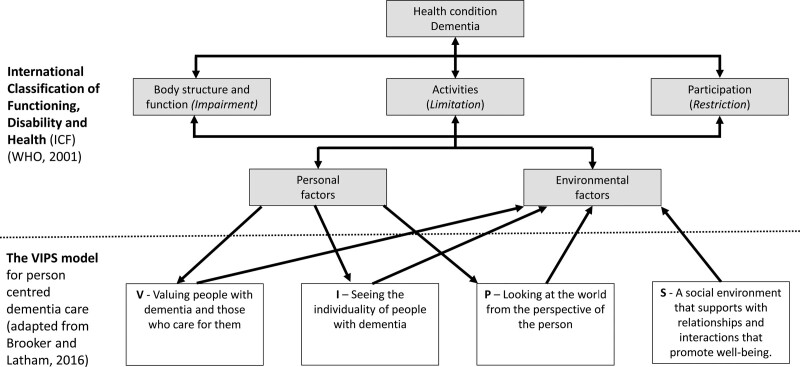
Hearing intervention involves addressing the hearing impairment and its impact on a person’s functioning in everyday life. Hearing intervention is often conceptualized in the context of the International Classification of Functioning, Disability and Health (ICF) (WHO 2001), a biopsychosocial framework for understanding the interaction between hearing impairment and contextual factors related to physical, social, and attitudinal environments in which the person lives, personal factors, and overall functioning and disability in communication. The ICF model’s emphasis on a holistic weighing of individual and contextual factors is complementary with the VIPS model for dementia care described above (Brooker & Latham 2016), which takes a similar conceptual approach (Figure 1). The advantage of considering the VIPS as an alternative (or addition to) the ICF model is that the VIPS model was specifically developed for dementia care. The VIPS focuses on the aspects of communication and social relationships that are particularly critical in dementia care. A further advantage is that although the ICF model implies a need for “person-centered care,” the model does not specify the key elements of a person-centered key in the way that the VIPS model does (i.e., four key elements; (i) valuing those with dementia and their caregivers, (ii) recognizing the individuality of those with dementia, (iii) acknowledging the perspective of those with dementia, and (iv) promoting an environment that facilitates well-being for those with dementia). Hearing assessment and interventions have traditionally followed a medical model, predominantly focusing on assessing and addressing the hearing impairment (i.e., fitting hearing aids based on audiogram information) (Hopper 2003). Effective hearing interventions for PwD draw on holistic aspects of the ICF and VIPS models, not only focusing on device-based attempts to address the hearing impairment but maximizing communication via attention to factors including communication partners’ attitudes and behaviors and the communication environment. Systematic reviews suggest that hearing interventions are effective in improving hearing-related quality of life and may have benefits for other outcomes including reducing behavioral and psychological symptoms of dementia, functioning in daily life, and rate of cognitive decline (Dawes et al. 2019a; Mamo et al. 2018). But the evidence base for hearing interventions for PwD is limited. The hearing interventions included in these systematic reviews mostly involved hearing aids, and evaluations of hearing interventions for PwD were of low to moderate quality. More evidence from robustly designed studies is required in relation to the benefits of hearing interventions on hearing-specific as well as broader health and well-being outcomes for PwD. More evidence is also needed for interventions besides hearing aids, including interventions that take a holistic approach to hearing intervention drawing on ICF/VIPS models than ones that focus on addressing hearing impairment by providing amplification.
Fig. 1.
The VIPS model for person-centered dementia care and the International Classification of Functioning, Disability and Health (ICF).
As dementia involves progressive declines in cognition, one might speculate that people may be better able to participate in hearing assessment and subsequently use, adapt to, benefit from, and continue to use hearing interventions if hearing impairment could be identified and treated during earlier stages of cognitive impairment rather than in later stages. There is some evidence for the benefits of early intervention for both pharmacological (i.e., acetylcholinesterase inhibitors and N-methyl-D-aspartic acid receptor antagonists) and nonpharmacological (e.g., cognitive stimulation therapy) interventions (Robinson et al. 2015). Hearing aids may work better for PwD who were experienced aid users before the onset of dementia. Evidence is lacking for the benefits of early hearing intervention in the context of dementia, but it is probably desirable to identify hearing impairment and initiate intervention at the earliest opportunity.
Hearing Aids
Hearing aids are effective in improving hearing function by amplifying speech and other sounds. Hearing aids may also have a cognitive benefit via reducing cognitively effortful listening experienced by people with hearing impairment (Desjardins & Doherty 2014; McGarrigle et al. 2014). However, hearing aids are of limited benefit in reducing the deleterious effects of reverberation, rapid speech, or a background of multiple talkers on comprehension (Armero & Jerger 2018), and impaired cognition may still impact on communication. PwD, families and carers should therefore have realistic expectations of benefits to be expected from hearing aids. Amplification in combination with other management approaches including communication training and environmental modifications may be necessary for optimal communication outcomes.
Selecting the Hearing Aid
Physical and cognitive difficulties of PwD in being able to independently manage their hearing aids present challenges to hearing clinicians in determining which features and hearing aid style is most appropriate for the PwD. Hearing aids are designed to be as small and discrete as possible. Unfortunately, difficulties with spatial cognition and comorbid long-term conditions including visual impairments and arthritis in the hands commonly co-occur with dementia (Bunn et al. 2014). These cognitive, visual, and physical difficulties make it difficult for some PwD to manage small-sized hearing aids (Bruhn & Dammeyer 2018). Larger hearing aids or assistive listening devices (below) may be options for people who have difficulties managing small hearing aids.
Evidence is limited and inconsistent in relation to individualizing hearing aid features according to dementia status. In adults with intact cognitive function, some researchers have reported that adults with greater working memory capacity had better-aided speech recognition with fast compression speeds than people with lower working memory capacity (Souza & Sirow 2014). Another study reported no interactions between working memory capacity and either noise reduction or fast compression aided speech recognition (Yumba 2017). Ghiringhelli and Iorio (2013) compared hearing aid benefit at 4 months for hearing aids with either fast or slow release times. Participants were people with Alzheimer’s disease and mild to moderate symmetrical bilateral sensorineural hearing loss. The severity of dementia was not reported. There was no difference in hearing aid benefit for fast versus slow release times (improvement of 70% to 25% for the fast subgroup and 71% to 26% for the slow release time subgroup in HHIE scores).
Clinicians also have differing opinions over an optimal choice of hearing aid features for PwD (Bott, Meyer et al. 2020). A pragmatic solution is that if advanced features are activated, these features should not present a barrier to use for the PwD. Activation of directional microphones, programmed change, and telecoil could be automatic. Souza (2014) recommended turning off audible alerts, except perhaps essential notifications for battery changes, to minimize the need for the PwD to interpret and act on the signals. Souza also recommended activating verbal prompts (“change the battery”) rather than tone beeps. It may also be helpful to match aid controls, style, settings, and features with any successful aid fittings in the past (Catherine Hart, personal communication, April 2021).
Some hearing aid styles and features may be appropriate for one PwD, but not appropriate for another. Clinicians should bear in mind the VIPS model, including issues of capacity and consent, and involve PwD in decisions about hearing aid options as much as possible. It may also be useful to offer trials of alternative hearing aid options to identify the appropriate choice for each person.
Hearing Aid Fitting and Verification
Clinical guidelines for adult hearing aid fitting recommend probe tube verification of hearing aid amplification (e.g., National Institute for Health and Care Excellence 2018a). Systematic reviews suggest a small statistically significant benefit of probe tube verification on speech recognition performance, although it is unclear whether these benefits are clinically relevant (Almufarrij et al. 2021). In the absence of evidence for the clinical benefits of hearing aid verification, one might therefore adopt a “click and fit” approach (i.e., relying on hearing aid manufacturer’s computerized fitting algorithms to provide optimal levels of amplification, without measuring and adjusting the actual levels of amplification within the ear canal) for PwD who may find it difficult to tolerate probe tube verification and the additional time that verification involves. In addition, PwD’s self-reported experience becomes more limited and inaccurate with more severe cognitive impairment (Farias et al. 2005), which may affect the adjustments to amplification required for personal preference following verification. It may therefore be beneficial to work with a family member or carer who can support the PwD in expressing their opinion (Alsawy et al. 2017). Alternatively, one could verify amplification levels via fitting the ear mold and hearing aid to an appropriate coupler, followed by validation of audibility with the PwD wearing the hearing aid in a clinical setting (e.g., via free field aided speech/warble threshold measures, aided behavioral observational testing or free field aided cortical testing), and home visits to observe responses to sounds in a familiar environment. One should also consider measuring Maximum Power Output in the coupler to minimize any distress an individual may experience with loud sounds and ensure safe levels of input.
Personal Amplification Devices
Cognitive impairment, cost of hearing aids, manual dexterity, and lifestyle factors may limit successful hearing aid use. Personal amplifiers (e.g., the Pocketalker, by Williams AV, Eden Prairie MN; numerous similar products are available) may have advantages over hearing aids in terms of cost (US$100–350 versus $3,000–$5,000 for a hearing aid), ease of fitting (do not require fitting by an audiologist) and availability (can be bought over the counter). Personal amplifiers also have a larger-sized design that may be easier to physically manage and less easy to lose than a hearing aid. Several studies report on the use of personal amplifiers for PwD (Weinstein & Amsel 1986; Leverett 1991; Hopper & Hinton 2012; Hopper et al 2016; Jupiter 2016; Mamo et al. 2017a,b; Bott, Meyer, et al. 2020). There were reported improvements in communication and quality of life as well as reduced anxiety, agitation, and reduced use of psychotropic medication in some studies (Leverett 1991; Hopper & Hinton 2012; Mamo et al. 2017a,b; Bott, Meyer, et al. 2020). Jupiter (2016) reported no improvement in quality-of-life in a small group of PwD (n = 7), and Hopper et al. (2016) reported no improvements in communication with personal amplifier use. Both studies were conducted in nursing home settings. Hopper et al. (2016) suggested the lack of improvement was because participants had mild hearing loss and were tested with and without amplification in quiet conditions; there may have been no additional benefit of amplification on communication under these conditions. Some PwD went on to use hearing aids after having tried personal amplifiers (Hopper & Hinton 2012; Jupiter 2012). Studies generally reported that personal amplifiers were accepted by PwD and carers, although personal amplifiers were reported to be unfamiliar to nursing home staff and residents (Bott, Meyer, et al. 2020), and sometimes disliked by PwD due to the heaviness of the devices (Leverett 1991; Jupiter 2016).
Communicative Environment
The communicative environment is a critical component of any holistic hearing intervention program and may be particularly important for PwD (c.f. the VIPS model; Brooker & Latham 2016). The communicative environment includes both the communication partner(s) and the physical environment. Because of the memory limitations that PwD experience and impaired ability to retain new information, communication training necessarily involves the communication partners of the PwD (Jordan et al. 1993; Mamo et al. 2017). Communication skills training for professional and informal caregiver communication partners in residential and home care settings is associated with improved quality of life and well-being and increased positive interactions (Eggenberger et al. 2013). Communication skills training is designed primarily to compensate for the cognitive limitations of dementia (i.e., difficulties with memory and attention). However, the strategies that communication skills training typically include are likely to be helpful in the context of hearing impairment as well as cognitive impairment. Example communication tactics suggested by Hubbard et al. (2018) include:
Speaking clearly
Facing the person when speaking
Saying the person’s name before starting a conversation
Using written and graphic cues to supplement spoken language
Staying on topic, provide clear transition statements between topics
Reducing distractions
Using nonverbal communication (gestures, actions, facial expression) to help comprehension of speech.
A handful of studies exist to support aural rehabilitation programs for PwD that involve communication skills training. Palmer et al. (2017) reported on a program that involved the use of a communication facilitator trained and supervised by an off-site audiologist, to provide daily audiology services and communication assistance to residents in an assisted living facility. The authors reported that the communication facilitator reduced the need for trips to the audiology clinic for hearing aid repairs, increased use of hearing aids and hearing technology by residents, and increased awareness of residents, staff, and families of the importance of communication for healthy aging. Similarly, Mamo et al. (2017) found improvements in behaviors and psychological symptoms of dementia and social engagement following intervention comprised of training in communication strategies for family and professional carers and use of personal amplification devices for PwD in aged care settings.
Interventions may also optimize the physical environment to facilitate communication. Hubbard et al. (2018) suggested the following approaches:
Reducing noise—turn down televisions, avoid loud talking/yelling during staff-to-staff communication
Using sound-absorbing acoustical tiles; soft fabrics and sound absorbent surfaces
Providing private spaces for conversation.
Leroi, Simkin, et al. (2020) reported that a hearing and vision intervention for PwD delivered in people’s homes led to better quality of life, more social engagement, reduced dependence on carers, improved functional ability, and communication. The intervention included assessment and management of sensory deficits, support with adherence and maintenance of devices, communication training for carers, sensory enhancement of the home environment (e.g., strategies suggested by Hubbard et al. above), and signposting to additional support. The intervention was individualized according to the needs and preferences of each PwD.
Understanding Behavior as Communication
“Behavioral and psychological symptoms of dementia (BPSD)” and “challenging behavior” are two of the more commonly used terms used to refer to depression/anxiety, repetitive behavior, social inappropriateness, wandering, delusions, hallucinations, sleep disturbances, and physical aggression among PwD. The terminology is controversial due to debate about causes and the view that such terminology is unhelpful and serves to objectify people’s experiences (Wolverson et al. 2019). The term “responsive behaviors” has been adopted by some agencies (Alzheimer Society of Canada, https://alzheimer.ca/en/help-support/im-caring-person-living-dementia/understanding-symptoms/responsive-reactive-behaviours) to emphasize that the behaviors have meaning and that there are physical, emotional or physical environmental factors that influence behavior. Changing the language that describes these behaviors also changes the potential management strategies that carers use when these behaviors occur (Dupuis et al. 2012). We acknowledge that the term is problematic, although, in the absence of consensus for suitable alternative terminology, we use the term “BPSD” here because it is arguably the most commonly used term.
Up to 97% of PwD in the general community experience BPSD (Cloak & Al Khalili 2020). BPSD increases the likelihood of institutionalization, impacts caregiver well-being, and can make clinical interaction and delivery of care challenges. BPSDs may result in over-prescription of psychotropic medications and adverse outcomes including falls, morbidity, and hospitalization (Reus et al. 2016). The etiology of BPSD is a subject of ongoing debate, although it is likely a combination of the impacts of brain changes due to dementia and a reaction to unmet needs in the context of someone who may not be able to communicate them due to the impacts of dementia (Macfarlane et al. 2021a). For example, around two-thirds of PwD experience chronic or acute pain due to comorbid health conditions (Atee et al. 2021). If a person cannot communicate that they are experiencing pain, behavioral and psychological stress reactions may result. In a clinical audiological interaction, a PwD may not know who the audiologist is, why they are in a frightening clinical setting, how they got there and what might be about to happen to them. In such circumstances, a PwD may quite reasonably be hostile toward their audiologist.
The recommended therapeutic approach for BPSD is a comprehensive assessment of possible causes of BPSD (e.g., Jackman & Beatty 2015) followed by the multimodal person-centered nonpharmacological intervention (e.g., Macfarlane et al. 2021), with pharmacological therapies then systematically trialed only if needed. Effective management of BPSD requires audiologists to work with carers and a coordinated multi-disciplinary healthcare team. Audiologists may have a role in addressing sensory under-stimulation and disorientation that may underlie some BPSDs by providing amplification and optimizing hearing function (Haque et al. 2012; Leverett 1991). Audiologists also have a role involving understanding (and helping others to understand) the perspective of the PwD (consistent with the VIPS model) and that some BPSDs are responses to unmet needs, discomfort, or attempts to communicate. Some BPSDs may therefore be addressed by responding in ways that address the cause of the behavior (Cloak & Al Khalili 2020). In the example above of a PwD being aggressive toward their audiologist, the audiologist might respond by calmly explaining where the PwD is, who the audiologist is, and why they are there (even if these things have been explained several times previously).
A Long-term Care Plan
Dementia is a progressive, degenerative condition involving deterioration in cognition and functional abilities. Audiologists should therefore plan periodic reviews of PwD’s hearing and communication needs. We are not aware of specific guidance, although given the rate of functional decline in dementia, it may be desirable to plan audiological reviews at shorter time intervals (i.e., at least annually) than for hearing impaired adults with normal cognition. Principles of patient-centered long-term care planning include (i) regular, comprehensive person-centered assessment of needs; (ii) approaching assessments as opportunities for information gathering, relationship-building, education, and support; (iii) a collaborative approach to assessment and care planning with other healthcare professionals and carers, (iv) using documentation to facilitate communication of person-centered information between healthcare professionals; and (v) long-term planning to maximize physical, psychosocial and financial wellbeing and to increase awareness of care options (Molony et al. 2018). Long-term planning for audiological care could involve implementing caregiver communication training and environmental modification (in addition to amplification) that could continue to support communication after the PwD is no longer able to use a hearing aid.
With respect to end-of-life care, the primary aims are to maximize the quality of life and comfort of the PwD (Gove et al. 2010). Audiological care has a key role to play in end-of-life care via optimizing communication with healthcare professions and connections with loved ones. Guidelines recommend facilitating communication with PwD, ensuring the PwD is consulted and kept informed about care and treatment issues as much as possible (Gove et al. 2010). All healthcare professionals and those involved in palliative care should have appropriate training in dementia awareness and communication with PwD. There should be coordination of health and nonhealthcare professionals (e.g., religious counselors, social workers) with the healthcare team (including audiologists) being aware of the needs of the PwD, changes in the person’s condition, and how supports are being provided to meet the changing needs of the PwD (Gove et al. 2010).
Collaborative care models are recommended for individuals with chronic and complex medical needs, including those that occur in dementia. Galvin et al. (2014) define collaborative care models as team-based, multicomponent interventions to improve person-centered care. Collaborative care is transdisciplinary care that focuses on shared decision-making between members of the healthcare team, including informal caregivers and PwD. Galvin et al. specify the following components as foundational for successful collaborative care in dementia: open communication between team members, teamwork, collegiality, trust, and respect for each team member’s expertise, and clarity around each member’s scope of practice.
Regarding specific strategies to facilitate collaborative, team approaches to care planning for PwD, Galvin et al. (2014) and Molony et al. (2018) recommend the identification of a care coordinator to integrate, document and share relevant information and to avoid redundancy and potentially conflicting advice from multiple providers. During transitions of care, Hirschman and Hodgson (2018) further emphasize the need to ensure complete and timely communication of information between, across, and within settings. They recommend standardized ways to share medical records and essential clinical information (e.g., linking electronic medical records across settings) whenever possible and assisting PwD and their caregivers in accessing and sharing information. Information on hearing and communication abilities should be included in medical records and considered as essential clinical information for quality care of the PwD, at every stage of the care continuum.
DISCUSSION
Due to aging populations and high comorbidity of hearing loss and dementia, audiologists face increasing demands to support the hearing needs of people with cognitive impairment. Evidence to inform practice is building, although more evidence is required with respect to the effectiveness of specific adaptations to support PwD (e.g., with respect to the reliability of audiometric testing), how hearing interventions could be individualized according to cognitive status (e.g., advanced hearing aid features) and the effectiveness of hearing interventions on improving quality of life and other dementia-related outcomes. Ideally, this evidence would be informed by PwD and carers via co-development of appropriate assessments, interventions, and care pathways (Miah et al. 2020) as well as closer collaboration between dementia and social care professionals with audiologists. Audiologists typically follow a passive model of care, dependent on referrals from other healthcare workers and patients approaching audiologists and attending clinics for help with hearing needs. As hearing loss tends to be substantially under-detected and unsupported among PwD, especially in long-term care settings, audiologists should seek to proactively engage with PwD and their carers in the community and in long-term care settings to identify and support the hearing needs of PwD.
There is an urgent imperative to make audiology a dementia-friendly profession. Dementia-friendly person-centered care involves not only focusing on assessing and addressing the level of hearing impairment by providing hearing aids, but on a holistic approach to care considering social context, personality, background, and health-related factors. The VIPS model is a helpful adjunct to the ICF model because it is specifically developed in the context of dementia care and is a synthesis of the key “person-centered” elements that can be operationalized in audiological care: valuing those with dementia and their caregivers, recognizing the individuality of those with dementia, acknowledging the perspective of those with dementia, and promoting an environment that facilitates well-being for those with dementia. Making audiology a dementia-friendly profession involves staff who have appropriate training in providing support for PwD as well as dementia-friendly physical environments in audiology clinics. PwD may benefit from hearing aids (although hearing aids may not be suitable for some people), but the focus should be on optimizing communication via interventions that include personal amplification devices, communication training, and environmental modifications. Unfortunately, clinical audiology mostly follows a medical model of intervention, with a focus on characterizing the impairment (via pure-tone audiometric assessment) and addressing it with a technological solution (i.e., a hearing aid). Audiology has tended to follow a narrow scope of practice, with an emphasis on numbers of hearing aids dispensed or sold, rather than on patient-centered outcomes. This tendency probably adversely affects the quality of care, particularly for people with complex needs including those living with dementia. We recognize that current care paradigms, audiological training programs, scope of practice conventions, and funding arrangements limit what is possible with respect to the provision of holistic person-centered hearing care for PwD. Audiologists should do what is possible within existing constraints and strive to change practice to improve the quality of hearing care for PwD. A key global challenge is to optimize the quality of life for PwD. Audiologists have a key role to play in meeting this challenge.
ACKNOWLEDGMENTS
P.D. and J.L. were supported in part by the NIHR Manchester Biomedical Research Centre. P.D. and J.L. were supported in part by SENSE-Cog project, which has received funding from the European Union’s Horizon 2020 research and innovation program under Grant agreement no. 668648. Thank you to Dr Rebecca Millman and Catherine Hart for their comments on this article. All authors discussed the scope of the paper, contributed to the writing, and commented on the article at all stages.
Supplementary Material
Footnotes
published online ahead of print December 23, 2021.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and text of this article on the journal’s Web site (www.ear-hearing.com).
REFERENCES
- Al-Yawer F., Pichora-Fuller M. K., Phillips N. A. (2019). The montreal cognitive assessment after omission of hearing-dependent subtests: Psychometrics and clinical recommendations. J Am Geriatr Soc, 67, 1689–1694. [DOI] [PubMed] [Google Scholar]
- Allen N. H., Burns A., Newton V., Hickson F., Ramsden R., Rogers J., Butler S., Thistlewaite G., Morris J. (2003). The effects of improving hearing in dementia. Age Ageing, 32, 189–193. [DOI] [PubMed] [Google Scholar]
- Almufarrij I., Dillon H., Munro K. J. (2021). Does probe-tube verification of real-ear hearing aid amplification characteristics improve outcomes in adults? A systematic review and meta-analysis. Trends Hear, 25, 2331216521999563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsawy S., Mansell W., McEvoy P., Tai S. (2017). What is good communication for people living with dementia? A mixed-methods systematic review. Int Psychogeriatr, 29, 1785–1800. [DOI] [PubMed] [Google Scholar]
- Alzheimer Europe. (2016). Decision making and legal capacity in dementia. Retrieved from https://www.alzheimer-europe.org/Policy-in-Practice2/Country-comparisons/2016-Decision-making-and-legal-capacity-in-dementia
- American Speech-Language-Hearing Association. (1997). Guidelines for audiology service delivery in nursing homes. Ad Hoc Committee on Audiology Service Delivery in Home Care and Institutional Settings. Retrieved from https://www.asha.org/policy/gl1997-00004/ [PubMed]
- Amjad H., Roth D. L., Sheehan O. C., Lyketsos C. G., Wolff J. L., Samus Q. M. (2018). Underdiagnosis of Dementia: An observational study of patterns in diagnosis and awareness in US Older adults. J Gen Intern Med, 33, 1131–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armero O., & Jerger J. (2018). Aural rehabilitation for older adults. Hearing Review, 25, 12–16. [Google Scholar]
- Atee M., Morris T., Macfarlane S., Cunningham C. (2021). Pain in Dementia: Prevalence and association with neuropsychiatric behaviors. J Pain Symptom Manage, 61, 1215–1226. [DOI] [PubMed] [Google Scholar]
- Banerjee S. (2015). Multimorbidity-older adults need health care that can count past one. Lancet, 385, 587–589. [DOI] [PubMed] [Google Scholar]
- Barrett P., Sharma M., Zeisel J. (2019). Optimal spaces for those living with dementia: Principles and evidence. Building Research & Information, 47, 734–746. [Google Scholar]
- Bott A., Hickson L., Meyer C., Bardy F., Van Dun B., Pachana N. A. (2020). Is cortical automatic threshold estimation a feasible alternative for hearing threshold estimation with adults with dementia living in aged care? Int J Audiol, 59, 745–752. [DOI] [PubMed] [Google Scholar]
- Bott A., Meyer C., Hickson L., Pachana N. A. (2019). Can adults living with dementia complete pure-tone audiometry? A systematic review. Int J Audiol, 58, 185–192. [DOI] [PubMed] [Google Scholar]
- Bott A., Meyer C., Hickson L., Pachana N. A. (2020). “It’s Huge, in a Way.” Conflicting stakeholder priorities for managing hearing impairment for people living with dementia in residential aged care facilities. Clin Gerontol, 1–15. [DOI] [PubMed] [Google Scholar]
- Brodaty H., Pond D., Kemp N. M., Luscombe G., Harding L., Berman K., Huppert F. A. (2002). The GPCOG: A new screening test for dementia designed for general practice. J Am Geriatr Soc, 50, 530–534. [DOI] [PubMed] [Google Scholar]
- Brooker D., & Latham I. (2016). Person-Centred Dementia Care: Making Services Better with the VIPS Framework. (2nd ed.). Jessica Kingsley Publishers. [Google Scholar]
- Bruhn P., & Dammeyer J. (2018). Assessment of Dementia in individuals with dual sensory loss: Application of a tactile test battery. Dement Geriatr Cogn Dis Extra, 8, 12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan A. (2004). Mental capacity, legal competence and consent to treatment. J R Soc Med, 97, 415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn F., Burn A. M., Goodman C., Rait G., Norton S., Robinson L., Schoeman J., Brayne C. (2014). Comorbidity and dementia: A scoping review of the literature. BMC Med, 12, 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk M. H., & Wiley T. L. (2004). Continuous versus pulsed tones in audiometry. Am J Audiol, 13, 54–61. [DOI] [PubMed] [Google Scholar]
- Burkhalter C. L., Allen R. S., Skaar D. C., Crittenden J., Burgio L. D. (2009). Examining the effectiveness of traditional audiological assessments for nursing home residents with dementia-related behaviors. J Am Acad Audiol, 20, 529–538. [DOI] [PubMed] [Google Scholar]
- Calkins M., Sanford J., Proffitt M. (2011). Design for dementia: Challenges and lessons for universal design. Preiser W. & Ostroff E. (Eds.), In: Universal design handbook (2nd ed., pp. 22.21–22.24). McGraw-Hill. [Google Scholar]
- Canevelli M., Valletta M., Trebbastoni A., Sarli G., D’Antonio F., Tariciotti L., de Lena C., Bruno G. (2016). Sundowning in Dementia: Clinical relevance, pathophysiological determinants, and therapeutic approaches. Front Med (Lausanne), 3, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr K., Weir P. L., Azar D., Azar N. R. (2013). Universal design: A step toward successful aging. J Aging Res, 2013, 324624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury H., Cooke H. A., Cowie H., Razaghi L. (2018). The Influence of the physical environment on residents with dementia in long-term care settings: A review of the empirical literature. Gerontologist, 58, e325–e337. [DOI] [PubMed] [Google Scholar]
- Ching T. Y., & Hill M. (2007). The Parents’ Evaluation of Aural/Oral Performance of Children (PEACH) scale: Normative data. J Am Acad Audiol, 18, 220–235. [DOI] [PubMed] [Google Scholar]
- Cloak N., & Al Khalili Y. (2020). Behavioral and psychological symptoms in Dementia. StatPearls [Internet]. [PubMed] [Google Scholar]
- Cloak N., & Al Khalili Y. (2020). Behavioral and Psychological Symptoms in Dementia. StatPearls Publishing. [PubMed] [Google Scholar]
- Cohen-Mansfield J., & Taylor J. W. (2004). Hearing aid use in nursing homes. Part 1: Prevalence rates of hearing impairment and hearing aid use. J Am Med Dir Assoc, 5, 283–288. [PubMed] [Google Scholar]
- Connolly A., Gaehl E., Martin H., Morris J., Purandare N. (2011). Underdiagnosis of dementia in primary care: Variations in the observed prevalence and comparisons to the expected prevalence. Aging Ment Health, 15, 978–984. [DOI] [PubMed] [Google Scholar]
- Cooper C., Selwood A., Livingston G. (2008). The prevalence of elder abuse and neglect: a systematic review. Age Ageing, 37, 151–160. [DOI] [PubMed] [Google Scholar]
- Dancer J., & Watkins P. (2006). Remember me? A guide to Alzheimers Disease and hearing loss. Audiology Online [serial online]. Retrieved from http://www.audiologyonline.com/articles/remember-me-guide-to-alzheimer-1008.
- Dawes P., Pye A., Reeves D., Yeung W. K., Sheikh S., Thodi C., Charalambous A. P., Gallant K., Nasreddine Z., Leroi I. (2019a). Protocol for the development of versions of the Montreal Cognitive Assessment (MoCA) for people with hearing or vision impairment. BMJ Open, 9, e026246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes P., Wolski L., Himmelsbach I., Regan J., Leroi I. (2019b). Interventions for hearing and vision impairment to improve outcomes for people with dementia: a scoping review. Int Psychogeriatr, 31, 203–221. [DOI] [PubMed] [Google Scholar]
- Desjardins J. L., & Doherty K. A. (2014). The effect of hearing aid noise reduction on listening effort in hearing-impaired adults. Ear Hear, 35, 600–610. [DOI] [PubMed] [Google Scholar]
- Dupuis S. L., Wiersma E., Loiselle L. (2012). Pathologizing behavior: Meanings of behaviors in dementia care. J Aging Studies, 26, 162–173. [Google Scholar]
- Durrant J. D., Gilmartin K. J., Holland A., Kamerer D. B. (1991). Hearing disorder management in Alzheimer’s disease patients. Hearing Instruments, 42, 32–35. [Google Scholar]
- Eggenberger E., Heimerl K., Bennett M. I. (2013). Communication skills training in dementia care: a systematic review of effectiveness, training content, and didactic methods in different care settings. Int Psychogeriatr, 25, 345–358. [DOI] [PubMed] [Google Scholar]
- El Haj M., Roche J., Jardri R., Kapogiannis D., Gallouj K., Antoine P. (2017). Clinical and neurocognitive aspects of hallucinations in Alzheimer’s disease. Neurosci Biobehav Rev, 83, 713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias S. T., Mungas D., Jagust W. (2005). Degree of discrepancy between self and other-reported everyday functioning by cognitive status: dementia, mild cognitive impairment, and healthy elders. Int J Geriatr Psychiatry, 20, 827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming R., Crookes P., Sum S. (2012). Dementia design audit tool. In Part 3: literature review. A review of the empirical literature on the design of physical environments for people with dementia (3rd ed.). Dementia Services Development Centre, University of Stirling. [Google Scholar]
- Folstein M. F., Folstein S. E., McHugh P. R. (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res, 12, 189–198. [DOI] [PubMed] [Google Scholar]
- Füllgrabe C. (2020). On the possible overestimation of cognitive decline: The impact of age-related hearing loss on cognitive-test performance. Frontiers in Neuroscience, 14, 454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvin J. E., Valois L., Zweig Y. (2014). Collaborative transdisciplinary team approach for dementia care. Neurodegener Dis Manag, 4, 455–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiringhelli R., & Iorio M. C. (2013). Hearing aids and recovery times: a study according to cognitive status. Braz J Otorhinolaryngol, 79, 177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gove D., Sparr S., Dos Santos Bernardo A. M., Cosgrave M. P., Jansen S., Martensson B., Pointon B., Tudose C., Holmerova I. (2010). Recommendations on end-of-life care for people with dementia. J Nutr Health Aging, 14, 136–139. [DOI] [PubMed] [Google Scholar]
- Gregory M. (2013). The Ida Institute toolbox: Tools to enhance collaborative self-management of hearing problems. Perspectives on Aural Rehabilitation and Its Instrumentation, 20, 22–36. [Google Scholar]
- Haque R., Abdelrehman N., Alavi Z. (2012). ‘There’s a monster under my bed’: hearing aids and dementia in long-term care settings. Annals of Long-Term Care: Clinical Care and Aging, 20, 28–33. [Google Scholar]
- Hebert C. A., & Scales K. (2019). Dementia friendly initiatives: A state of the science review. Dementia (London), 18, 1858–1895. [DOI] [PubMed] [Google Scholar]
- Hirschman K. B., & Hodgson N. A. (2018). Evidence-based interventions for transitions in care for individuals living with dementia. Gerontologist, 58(suppl_1), S129–S140. [DOI] [PubMed] [Google Scholar]
- HM Government. (2005). The Mental Capacity Act. The Stationery Office. [Google Scholar]
- Hopper T. L. (2003). “They’re just going to get worse anyway”: perspectives on rehabilitation for nursing home residents with dementia. J Commun Disord, 36, 345–359. [DOI] [PubMed] [Google Scholar]
- Hopper T. L., & Hinton P. (2012). Hearing loss among individuals with Dementia: Barriers and facilitators to care. Canadian J Speech-Language Pathology Audiology, 36. [Google Scholar]
- Hopper T., Slaughter S. E., Hodgetts B., Ostevik A., Ickert C. (2016). Hearing loss and cognitive-communication test performance of long-term care residents with Dementia: Effects of amplification. J Speech Lang Hear Res, 59, 1533–1542. [DOI] [PubMed] [Google Scholar]
- Houston A., Mitchell W., Ryan K., Hullah N., Hitchmough P., Dunne T., Dunne J., Edwards B., Marshall M., Christie J., Cunningham C. (2020). Accessible design and dementia: A neglected space in the equality debate. Dementia (London), 19, 83–94. [DOI] [PubMed] [Google Scholar]
- Hubbard H. I., Mamo S. K., Hopper T. (2018). Dementia and Hearing Loss: Interrelationships and Treatment Considerations. Semin Speech Lang, 39, 197–210. [DOI] [PubMed] [Google Scholar]
- Jackman L., & Beatty A. (2015). Using the Newcastle Model to understand people whose behaviour challenges in dementia care. Nurs Older People, 27, 32–39. [DOI] [PubMed] [Google Scholar]
- Jordan F. M., Worrall L. E., Hickson L. M., Dodd B. J. (1993). The evaluation of intervention programmes for communicatively impaired elderly people. Eur J Disord Commun, 28, 63–85. [DOI] [PubMed] [Google Scholar]
- Jorgensen L. E., Palmer C. V., Pratt S., Erickson K. I., Moncrieff D. (2016). The effect of decreased audibility on MMSE performance: A measure commonly used for diagnosing dementia. J Am Acad Audiol, 27, 311–323. [DOI] [PubMed] [Google Scholar]
- Jupiter T. (2012). Cognition and screening for hearing loss in nursing home residents. J Am Med Dir Assoc, 13, 744–747. [DOI] [PubMed] [Google Scholar]
- Jupiter T. (2016). Does hearing assistive technology provide benefit to nursing home residents with Dementia? A Pilot Study. J Academy Rehabilitative Audiology, 49, 34–39. [Google Scholar]
- Kitwood T. (1997). Dementia Reconsidered The Person Comes First (1st ed.): Open University Press. [Google Scholar]
- Kitwood T. (2020). Brooker D. (Ed.), In: Dementia Reconsidered: The Person Still Comes First (2nd ed.). Open University Press. [Google Scholar]
- Kricos P. B. (2006). Audiologic management of older adults with hearing loss and compromised cognitive/psychoacoustic auditory processing capabilities. Trends Amplif, 10, 1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kricos P. (2009). Providing hearing rehabilitation to people with dementia presents unique challenges. Hearing J, 62, 39–40, 42–43. [Google Scholar]
- Ladduwahetty S., Dowell R. C., Winton E. (2013). Dementia and cochlear implant outcomes: Can we screen for dementia effectively using the mini mental state examination in implant candidates? Australian and New Zealand J Audiology, 33, 88–102. [Google Scholar]
- Lamont S., Jeon Y. H., Chiarella M. (2013). Assessing patient capacity to consent to treatment: an integrative review of instruments and tools. J Clin Nurs, 22, 2387–2403. [DOI] [PubMed] [Google Scholar]
- Lemke U. (2011). Hearing impairment in dementia - how to reconcile two intertwined challenges in diagnostic screening. Audiol Res, 1, e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroi I., Constantinidou F., Langenbahn D., Heyn P., Yeung W. K., Dawes P. (2020). Hearing and vision impairment in people with dementia: A guide for clinicians. Arch Phys Med Rehabil, 101, 1667–1670. [DOI] [PubMed] [Google Scholar]
- Leroi I., Himmelsbach I., Wolski L., Littlejohn J., Jury F., Parker A., Charalambous A. P., Dawes P., Constantinidou F., Thodi C.; (SENSE-Cog Expert Reference Group). (2019). Assessing and managing concurrent hearing, vision and cognitive impairments in older people: an international perspective from healthcare professionals. Age Ageing, 48, 580–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroi I., Simkin Z., Hooper E., Wolski L., Abrams H., Armitage C. J., Camacho E., Charalambous A. P., Collin F., Constantinidou F., Dawes P., Elliott R., Falkingham S., Frison E., Hann M., Helmer C., Himmelsbach I., Hussain H., Marié S., Montecelo S., et al. (2020). Impact of an intervention to support hearing and vision in dementia: The SENSE-Cog Field Trial. Int J Geriatr Psychiatry, 35, 348–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leverett M. (1991). Approaches to problem behaviors in dementia. Physical & Occupational Therapy In Geriatrics, 9(3–4), 93–105. [Google Scholar]
- Lin J. S., O’Connor E., Rossom R. C., Perdue L. A., Eckstrom E. (2013). Screening for cognitive impairment in older adults: A systematic review for the U.S. Preventive Services Task Force. Ann Intern Med, 159, 601–612. [DOI] [PubMed] [Google Scholar]
- Livingston G., Huntley J., Sommerlad A., Ames D., Ballard C., Banerjee S., Brayne C., Burns A., Cohen-Mansfield J., Cooper C., Costafreda S. G., Dias A., Fox N., Gitlin L. N., Howard R., Kales H. C., Kivimäki M., Larson E. B., Ogunniyi A., Orgeta V., et al. (2020). Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet, 396, 413–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlejohn J., Bowen M., Constantinidou F., Dawes P., Dickinson C., Heyn P., Langenbahn D. (2021). International Practice recommendations for the recognition and management of hearing and vision impairment in people with dementia. Gerontology, 1–15. 10.1159/000515892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane S., Atee M., Morris T., Cunningham C. (2021a). When responsive and reactive meet organic? Treatment implications of language use in the era of# BanBPSD. In: Wiley Online Library. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane S., Atee M., Morris T., Whiting D., Healy M., Alford M., Cunningham C. (2021b). Evaluating the clinical impact of National Dementia Behaviour support programs on neuropsychiatric outcomes in Australia. Front Psychiatry, 12, 652254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamo S. K., Nirmalasari O., Nieman C. L., McNabney M. K., Simpson A., Oh E. S., Lin F. R. (2017a). Hearing care intervention for persons with Dementia: A pilot study. Am J Geriatr Psychiatry, 25, 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamo S. K., Nirmalasari O., Nieman C. L., McNabney M. K., Simpson A., Oh E. S., Lin F. R. (2017b). Hearing care intervention for persons with Dementia: A pilot study. Am J Geriatr Psychiatry, 25, 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamo S. K., Oh E., Lin F. R. (2017c). Enhancing communication in adults with Dementia and age-related hearing loss. Semin Hear, 38, 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamo S. K., Reed N. S., Price C., Occhipinti D., Pletnikova A., Lin F. R., Oh E. S. (2018). Hearing loss treatment in older adults with cognitive impairment: A systematic review. J Speech Lang Hear Res, 61, 2589–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClannahan K. S., Chiu Y. F., Sommers M. S., Peelle J. E. (2021). Test-retest reliability of audiometric assessment in individuals with mild Dementia. JAMA Otolaryngol Head Neck Surg, 147, 442–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarrigle R., Munro K. J., Dawes P., Stewart A. J., Moore D. R., Barry J. G., Amitay S. (2014). Listening effort and fatigue: what exactly are we measuring? A British Society of Audiology Cognition in Hearing Special Interest Group ‘white paper’. Int J Audiol, 53, 433–440. [DOI] [PubMed] [Google Scholar]
- Miah J., Parsons S., Lovell K., Starling B., Leroi I., Dawes P. (2020). Impact of involving people with dementia and their care partners in research: a qualitative study. BMJ Open, 10, e039321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molony S. L., Kolanowski A., Van Haitsma K., Rooney K. E. (2018). Person-centered assessment and care planning. Gerontologist, 58(suppl 1), S32–S47. [DOI] [PubMed] [Google Scholar]
- Moye J., Karel M. J., Azar A. R., Gurrera R. J. (2004). Capacity to consent to treatment: empirical comparison of three instruments in older adults with and without dementia. Gerontologist, 44, 166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moye J., & Marson D. C. (2007). Assessment of decision-making capacity in older adults: an emerging area of practice and research. J Gerontol B Psychol Sci Soc Sci, 62, P3–P11. [DOI] [PubMed] [Google Scholar]
- Nasreddine Z. S., Phillips N. A., Bédirian V., Charbonneau S., Whitehead V., Collin I., Cummings J. L., Chertkow H. (2005). The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc, 53, 695–699. [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence. (2018a). Heraing loss in adults: Assessment and management. Retrieved from https://www.nice.org.uk/guidance/ng98/evidence/full-guideline-pdf-4852693117 [PubMed]
- National Institute for Health and Care Excellence. (2018b). [NG97] Dementia: assessment, management and support for people living with dementia and their carers. In [NG97] Dementia: assessment, management and support for people living with dementia and their carers. National Institute for Health and Care Excellence. [PubMed] [Google Scholar]
- Nirmalasari O., Mamo S. K., Nieman C. L., Simpson A., Zimmerman J., Nowrangi M. A., Lin F. R., Oh E. S. (2017). Age-related hearing loss in older adults with cognitive impairment. Int Psychogeriatr, 29, 115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyebode J., & Clare L. (2014). Supporting Cognitive Abilities. Downs M. & Bowers B. (Eds.), In: Excellence in Dementia Care: Research into Practice (2nd ed.). Open University Press. [Google Scholar]
- Palmer C. V., Adams S. W., Durrant J. D., Bourgeois M., Rossi M. (1998). Managing hearing loss in a patient with Alzheimer disease. J Am Acad Audiol, 9, 275–284. [PubMed] [Google Scholar]
- Palmer C. V., Mulla R., Dervin E., Coyan K. C. (2017). HearCARE: Hearing and Communication Assistance for Resident Engagement. Semin Hear, 38, 184–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince M., Bryce R., Ferri C. (2011). World Alzheimer report 2011. The benefits of early diagnosis and intervention. Retrieved from www.alz.co.uk/worldreport2011.
- Prince M., Wimo A., Guerchet M., Ali G.-C., Wu Y.-T., Prina M. (2015). The Global Impact of Dementia: an analysis of prevalence, incidence, cost and trends. Retrieved from World Alzheimer Report 2015: https://www.alz.co.uk/research/WorldAlzheimerReport2015.pdf
- Public Health England. (2019). Screening for dementia. Retrieved from London: https://legacyscreening.phe.org.uk/dementia
- Pye A., Charalambous A. P., Leroi I., Thodi C., Dawes P. (2017). Screening tools for the identification of dementia for adults with age-related acquired hearing or vision impairment: a scoping review. Int Psychogeriatr, 29, 1771–1784. [DOI] [PubMed] [Google Scholar]
- Resnick H. E., Fries B. E., Verbrugge L. M. (1997). Windows to their world: the effect of sensory impairments on social engagement and activity time in nursing home residents. J Gerontol B Psychol Sci Soc Sci, 52, S135–S144. [DOI] [PubMed] [Google Scholar]
- Reus V. I., Fochtmann L. J., Eyler A. E., Hilty D. M., Horvitz-Lennon M., Jibson M. D., Lopez O. L., Mahoney J., Pasic J., Tan Z. S., Wills C. D., Rhoads R., Yager J. (2016). The American Psychiatric Association Practice Guideline on the Use of Antipsychotics to Treat Agitation or Psychosis in Patients With Dementia. Am J Psychiatry, 173, 543–546. [DOI] [PubMed] [Google Scholar]
- Robinson L., Tang E., Taylor J. P. (2015). Dementia: Timely diagnosis and early intervention. BMJ, 350, h3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schow R. L., & Nerbonne M. A. (1982). Communication screening profile: use with elderly clients. Ear Hear, 3, 135–147. [DOI] [PubMed] [Google Scholar]
- Shen J., Anderson M. C., Arehart K. H., Souza P. E. (2016). Using Cognitive Screening Tests in Audiology. Am J Audiol, 25, 319–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skills for Care & Skills for Health and Department of Health. (2011). Common Core Principles for Supporting People with Dementia. A guide to training the social care and health workforce. Retrieved from https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/215562/dh_127587.pdf
- Slaughter S. E., Hopper T., Ickert C., Erin D. F. (2014). Identification of hearing loss among residents with dementia: perceptions of health care aides. Geriatr Nurs, 35, 434–440. [DOI] [PubMed] [Google Scholar]
- Souza P. (2014). Hearing Loss and Aging: Implications for Audiologists. ASHA. Retrieved from http://www.asha.org/Articles/Hearing-Loss-and-Aging-Implications-for-Audiologists/
- Souza P. E., & Sirow L. (2014). Relating working memory to compression parameters in clinically fit hearing AIDS. Am J Audiol, 23, 394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaszewski Farias S., Schmitter-Edgecombe M., Weakley A., Harvey D., Denny K. G., Barba C., Gravano J. T., Giovannetti T., Willis S. (2018). Compensation strategies in older adults: Association with cognition and everyday function. Am J Alzheimers Dis Other Demen, 33, 184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hoof J., & O’Brien D. (2014). Designing for dementia. Evid Based Des J, 1, 1–38. [Google Scholar]
- Vance D. E., & Johns R. N. (2003). Montessori improved cognitive domains in adults with Alzheimer’s Disease. Physical & Occupational Therapy In Geriatrics, 20, 19–33. [Google Scholar]
- Vassilaki M., Aakre J. A., Cha R. H., Kremers W. K., St Sauver J. L., Mielke M. M., Geda Y. E., Machulda M. M., Knopman D. S., Petersen R. C., Roberts R. O. (2015). Multimorbidity and Risk of Mild Cognitive Impairment. J Am Geriatr Soc, 63, 1783–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventry I. M., & Weinstein B. E. (1982). The hearing handicap inventory for the elderly: a new tool. Ear Hear, 3, 128–134. [DOI] [PubMed] [Google Scholar]
- Villeneuve A., Hommet C., Aussedat C., Lescanne E., Reffet K., Bakhos D. (2017). Audiometric evaluation in patients with Alzheimer’s disease. Eur Arch Otorhinolaryngol, 274, 151–157. [DOI] [PubMed] [Google Scholar]
- Weinstein B. (1986). Hearing loss and senile dementia in the institutionalized elderly. Clinical Gerontologist, 4, 3–15. [Google Scholar]
- Waller S., & Masterson A. (2015). Designing dementia-friendly hospital environments. Future Hosp J, 2, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein B., & Amsel L. (1986). The relationship between dementia and hearing impairment in the institutionalized elderly. Cin Gerontol, 4, 3–15. [Google Scholar]
- WHO. (2001). International Classification of Functioning, Disability and Health (ICF). Retrieved from http://www.who.int/classifications/icf/en/
- Wolverson E., Birtles H., Moniz-Cook E., James I., Brooker D., Duffy F. (2019). Naming and framing the behavioural and psychological symptoms of dementia (BPSD) paradigm: professional stakeholder perspectives. OBM Geriatrics, 3, 1–19. [Google Scholar]
- World Health Organisation. (2020). Dementia: Key Facts. Retrieved from https://www.who.int/news-room/fact-sheets/detail/dementia. Accessed April 06, 2020.
- Wright N., Stickley T., Mulla I., Bradshaw E., Buckley L., Archbold S. (2014). Hearing loss and dementia: an exploratory study of the views of audiologists. Quality in Ageing and Older Adults, 15, 220–231. [Google Scholar]
- Yumba W. K. (2017). Cognitive Processing Speed, Working Memory, and the Intelligibility of Hearing Aid-Processed Speech in Persons with Hearing Impairment. Front Psychol, 8, 1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.