Abstract
Selenium (Se) is an essential trace element for humans and other animals. The human body mainly acquires Se from plant foods, especially cereal grains. Rice is the staple food for more than half of the world’s population. Increasing the Se concentration of rice grains can increase the average human dietary Se intake. This review summarizes recent advances in the molecular mechanisms of Se uptake, transport, subcellular distribution, retranslocation, volatilization, and Se-containing protein degradation in plants, especially rice. The strategies for improving Se concentration in rice grains by increasing Se accumulation, reducing Se volatilization, and optimizing Se form were proposed, which provide new insight into Se biofortification in rice by improving the utilization efficiency of Se.
Keywords: Rice (Oryza sativa L.), Selenium, Se biofortification, Se metabolism, Se reutilization
Background
Selenium (Se) is an essential trace element for humans and other animals (Schwarz and Foltz 1957; Rotruck et al. 1973). It was first discovered in 1817 by the Swedish chemist Jons Jakob Berzelius in the residue of sulfuric acid production. Se had been ignored chronically by scientists until its toxicity was revealed (Franke 1934). Since it was discovered that Se could prevent liver necrosis in rats, the essential physiological functions of Se in animals have been gradually revealed (Schwarz and Foltz 1957). Se exerts its antioxidant functions by forming the active site of glutathione peroxidase as selenocysteine (SeCys), thereby protecting animal tissues and cell membranes from oxidative stress damage (Rotruck et al. 1973; Rayman 2000). A low Se status in the human body reduces the immunity to many diseases and increases the susceptibility to cancers, virus infections, and heart diseases (Rayman 2000, 2012). Severe Se deficiency can even cause Keshan disease and Kashin-Beck disease (Moreno-Reyes et al. 1998; Tan et al. 2002; Oropeza-Moe et al. 2015).
Se primarily depends on selenoproteins to exert its functions in humans and animals. The human selenoproteome reveals that many selenoproteins generally participate in antioxidant and anabolic processes (Hatfield and Gladyshev 2002; Kryukov et al. 2003). The level of Se intake in Europe and some parts of China is not adequate for full expression of selenoprotein (Rayman 2007). Approximately one billion people worldwide suffer from insufficient Se intake (Combs 2001). Therefore, an adequate Se intake is crucial to prevent Se deficiency-related diseases in humans.
The human body mainly acquires Se from plant foods, especially cereal grains. Rice is the staple food for more than half of the world’s population. However, approximately 75% of rice grains provide less than 70% of the recommended daily intake of Se (Williams et al. 2009). Therefore, increasing the Se concentration in rice grains is of great importance for improving the human body’s Se intake. The Se concentration of rice grains largely depends on the Se status in the paddy soil where the rice plants are grown. The global Se concentration in soil ranges from 0.01 to 2.0 mg kg−1 with an average of 0.40 mg kg−1. However, it can be as high as 1200 mg/kg in seleniferous soils (Fiona 2007). The soil Se levels were generally divided into five grades based on the concentration range, including Se-deficient (< 0.125 mg/kg), Se-marginal (0.125–0.175 mg/kg), Se-sufficient (0.175–0.40 mg/kg), Se-rich (0.40–3.0 mg/kg), and Se-excessive (> 3.0 mg/kg) (Tan et al. 2002; Dinh et al. 2018). China has a large area of low Se soils. A saddle-shaped Se-deficient belt extends from the northeast to the southwest, and the soil Se concentration is even less than 0.13 mg/kg (Tan et al. 2002; Li et al. 2012). Therefore, it is necessary to increase the Se concentration in rice grains by applying Se in low Se areas.
Se naturally occurs as selenide, elemental Se, thioselenate, selenite, and selenate in soils (Läuchli 1993). The forms of Se are governed by various chemical and physical properties, including pH, chemical and mineralogical composition, adsorbing surfaces, and oxidation–reduction status (Neal et al. 1987). Selenate and selenite are the dominant forms of Se available to plants in soils. The availability of selenite in the soil is usually many times lower than that of selenate due to being adsorbed by organic matter and Fe hydrated oxides (Coppin et al. 2006; Keskinen et al. 2013). In well-aerated alkaline soils, selenate is the dominant Se form. In neutral and acid soils, selenite is the dominant Se form (Neal et al. 1987; Mikkelsen et al. 1989). Under reducing conditions, most of the selenate is readily converted to selenite (Elrashidi et al. 1987). Therefore, rice plants mainly take up selenite under flooded conditions in paddy fields. After selenite is taken up by plants, it can be converted into organic Se such as selenomethionine (SeMet) and unspecifically involved in protein synthesis (Terry et al. 2000; Zhang et al. 2019). Se-containing proteins are degraded by different types of proteases during leaf senescence to release SeMet. SeMet is an analog of methionine (Met) and has a common transporter with Met (Gits and Grenson 1967). Met has been demonstrated to be transported by amino acid transporters (Taylor et al. 2015). Therefore, SeMet can also be transported into grains by amino acid transporters. In addition, Se can be further converted into dimethyl selenides (DMSe) and volatilized, resulting in a decrease in the accumulation of Se in rice grains (Zayed et al. 1998). Se accumulation in rice grains involves a series of complex processes, including uptake, transport, subcellular distribution, and retranslocation. It requires fine cooperation of multiple transporters specifically localized to the cell membranes or subcellular membranes of different organs and tissues. In this review, we summarize the research progresses on the mechanisms of Se uptake, transport, subcellular distribution, retranslocation, volatilization, and Se-containing protein degradation in plants, especially rice, and propose the strategies for improving Se accumulation in rice grains, which is crucial for promoting Se biofortification in rice.
The Se Uptake and Transport in Plant
Selenate Uptake and Transport
Earlier physiological studies revealed that selenate uptake was an active process because respiratory inhibitors and low temperature almost completely inhibited selenate uptake (Ulrich and Shrift 1968; Shrift and Ulrich 1969). Sulfate can largely inhibit selenate uptake, suggesting that selenate shares a common uptake mechanism with sulfate, with both being taken up by proton gradient-driven sulfate transporters (Terry et al. 2000; Hawkesford 2003; Sors et al. 2005). The sulfate transporter gene family could be classified into four distinguishable groups according to phylogenetic analysis of the plant gene or amino acid sequences (Hawkesford 2003; Buchner et al. 2004). Group 1 sulfate transporters are high-affinity transporters responsible for sulfate uptake, and the genes of these transporters are mainly expressed in root tissues and induced by sulfur deficiency (Smith et al. 1995, 1997; Takahashi et al. 2000; Howarth et al. 2003). In Arabidopsis, the high-affinity sulfate transporters Sultr1.1 and Sultr1.2 locate in the root hairs, epidermis, and cortical cell layers. They are responsible for sulfate uptake from soil under sulfur-deficient conditions (Takahashi et al. 2000; Hawkesford 2003; Buchner et al. 2004) (Table 1). Sultr1;2 was identified by screening selenate-resistant mutants, which mediated selenate uptake (Shibagaki et al. 2002; El Kassis et al. 2007). In rice, the high-affinity sulfate transporter genes OsSultr1;1, OsSultr1;2, and OsSultr1;3 were identified, and their expressions in roots are regulated by sulfate status (Buchner et al. 2004; Kumar et al. 2011; Réthoré et al. 2020) (Table 1). OsSultr1;1 was demonstrated to transport sulfate by expressing in heterologous systems yeast and Arabidopsis (Kumar et al. 2019). Due to the highly similar chemical properties between sulfate and selenate, the uptake of selenate by rice roots is most likely through OsSultr1;1, OsSultr1;2, and OsSultr1;3 (Fig. 1, Table 1). However, sulfate transporters are selective in taking up sulfate and selenate (Ferrari and Renosto 1972).
Table 1.
Identified and potential transporters and channels for Se uptake, transport, and subcellular distribution
| Functions | Type of transporters | Tissue or subcellular localization |
|---|---|---|
| Selenate uptake (group 1 sulfate transporters) | AtSultr1;1 | Root hairs, epidermis, and cortical cell layers (Takahashi et al. 2000) |
| Sultrl;2 | Root cortex, root tip and lateral roots (Shibagaki et al. 2002; Yoshimoto et al. 2002) | |
| AtSultr1;3* | Sieve element-companion cell complexes of the phloem in cotyledons and roots (Yoshimoto et al. 2003) | |
| OsSultr1;1-OsSultr1;3 | Root (Kumar et al. 2011; Réthoré et al. 2020) | |
| Selenate transport (group 2 sulfate transporters) | AtSultr2;1 | Xylem parenchyma cells of leaves and roots (Takahashi et al. 2000) |
| AtSultr2;2 | Root phloem and leaf vascular bundle sheath cells (Takahashi et al. 2000) | |
| OsSultr2;1-OsSultr2;2 | Vascular tissues (Dixit et al. 2015) | |
| Selenate transport (channels) | Anion channels | Xylem parenchyma cells (Gilliham and Tester 2005) |
| Selenate subcellular distribution (group 3 sulfate transporters) | AtSultr3;1-AtSultr3;5 | Chloroplast (Hawkesford 2003; Buchner et al. 2004; Cao et al. 2013) |
| OsSultr3;1-OsSultr3;6 | ||
| Selenate subcellular distribution (group 4 sulfate transporters) | AtSultr4;1-AtSultr4;2 | Tonoplast (Hawkesford 2003; Buchner et al. 2004) |
| OsSultr4;1 | ||
| Uptake (group 1 phosphate transporters) | OsPht1;2 | Root epidermal cells and steles in primary and lateral roots (Zhang et al. 2014) |
| OsPht1;8 | Root tips, lateral roots, leaves, stamens, caryopses (Jia et al. 2011) | |
| Uptake (NIP subfamily) | OsNIP2;1 | Plasma membrane of the distal side of both exodermis and endodermis cells (Ma et al. 2006) |
| SeMet transport (PTR family) | OsNRT1.1B | Vascular tissues of roots, leaf sheaths, leaf blades and culms (Hu et al. 2015) |
| Subcellular distribution (group 4 phosphate transporters) | AtPht4;1-AtPht4;5 | Plastid envelope (Guo et al. 2008) |
| OsPht4;1-OsPht4;4 | Inner chloroplast membrane (Li et al. 2020) | |
| Subcellular distribution (group 2 phosphate transporters) | OsPht2;1 | Chloroplast (Liu et al. 2020) |
| Subcellular distribution (group 5 phosphate transporters or SPX-MFS proteins) | AtSPX-MFS 1–3 | Tonoplast (Liu et al. 2020) |
| OsSPX-MFS 1–3 | Tonoplast (Wang et al. 2012, 2015; Xu et al. 2019) |
Italic fonts represent potential transporters and channels. *Represents that AtSultr1;3 is a potential transporter responsible for selenate transport
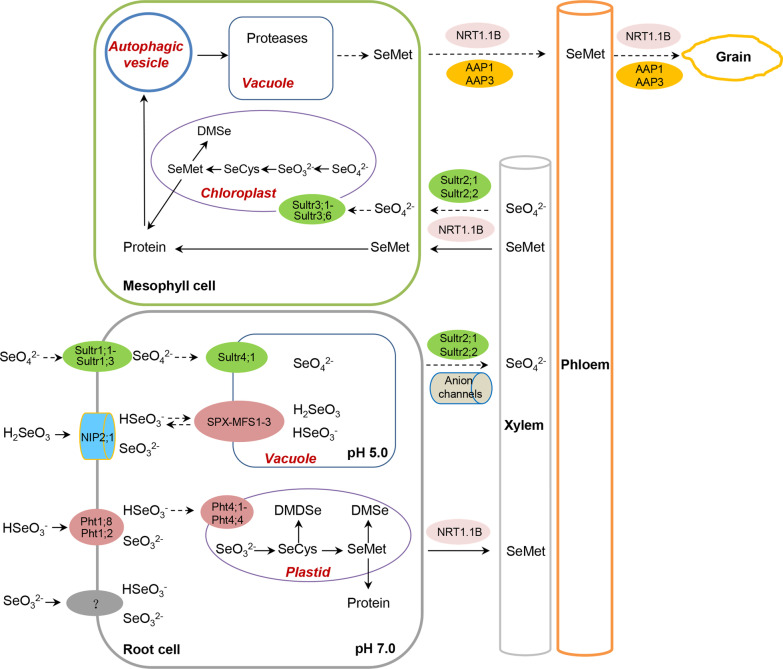
Fig. 1.
The uptake, transport, subcellular distribution, retranslocation, and volatilization of Se in rice. Selenate is taken up through Sultr1;1, Sultr1;2, and Sultr1;3, located in the root epidermal cell membrane, a small part of selenate enters the vacuole through Sultr4;1 located in the tonoplast, and most of it is transported to the leaves through Sultr2;1 and Sultr2;2 located in the parenchyma cell membrane of the xylem, finally enters the chloroplast through Sultr3;1, Sultr3;2, Sultr3;3, Sultr3;4, Sultr3;5, and Sultr3;6 located in the chloroplast membrane, where they are converted into SeCys and SeMet to participate in protein synthesis in a non-specific manner, and can also be further converted into DMSe and volatilized. Rice roots can take up HSeO3− and H2SeO3 through OsPht1;2 (OsPT2) and OsNIP2;1, respectively. After selenite enters the cytoplasm, it mainly exists in the form of HSeO3− and SeO32−; part of the selenite enters the vacuole through OsSPX-MFS1/3 and OsVPE1/OsVPE2 located in the tonoplast. Selenite mainly exists in the form of H2SeO3 and HSeO3− in the vacuole; most of it is transported to the plastid by OsPHT4;1-OsPHT4;4 and OsPHT2;1 and converted into SeCys and SeMet, and then participate in protein synthesis non-specifically, and can also be further converted into DMSe and volatilizes. Part of SeMet is transported to shoots through NRT1.1B and participates in protein synthesis. In senescent leaves, protein is encapsulated in autophagic vesicles and transported to vacuoles, degraded into SeMet by proteases, and transported to rice grains through OsAAP1, OsAAP3, and OsNRT1.1B. Sulfate transporters,
 ; OsNIP2;1,
; OsNIP2;1,
 ; Phosphate transporters,
; Phosphate transporters,
 ; NRT1.1B,
; NRT1.1B,
 ; Amino acid transporters,
; Amino acid transporters,
 ; Anion channels,
; Anion channels,
 Solid lines correspond to identified transporters and dashed lines correspond to potential transporters
Solid lines correspond to identified transporters and dashed lines correspond to potential transporters
Similar to sulfate, selenate is taken up by root epidermal cells and transported radially to the stele via the apoplastic and symplasmic pathways (Takahashi et al. 2000). Apoplastic transport to the stele is restricted by Casparian bands in the endodermal cell wall, limiting the uptake of ions from the apoplast by endodermal cells (White 2001; Moore et al. 2002). The symplasmic pathway plays a key role in delivering most ions to the xylem, with radial transport of ions to adjacent root cells via the plasmodesmata (Lucas and Wolf 1993). Likewise, selenate is also transported in the symplast via plasmodesmata. After radial transport of selenate to the stele via the symplasm, selenate is released into xylem vessels across the plasma membrane of xylem parenchymal cells.
Group 2 sulfate transporters, including Sultr 2;1 and Sultr 2;2, are mainly located in the vascular tissues and are responsible for sulfate transport (Takahashi et al. 2000). Sultr 2;1, located in the xylem parenchyma cells of leaves and roots, participates in sulfate transport from roots to shoots via the xylem (Takahashi et al. 2000). Therefore, selenate may be loaded into the stele via Sultr 2;1 (Fig. 1, Table 1). Furthermore, since anion channels can facilitate the movement of sulfate from the cytoplasm of the xylem parenchyma cells to the xylem vessels along the electrochemical gradient, selenate may also be loaded into the xylem in a similar manner (Gilliham and Tester 2005) (Fig. 1, Table 1). Sultr2;1 is colocalized with Sultr 3;5 in xylem parenchyma and pericycle cells in roots. Co-expression of Sultr2;1 and Sultr3;5 provides a maximum capacity of sulfate transport, which facilitates retrieval of apoplastic sulfate to the xylem parenchyma cells in the vasculature of roots and may contribute to the root-to-shoot transport of sulfate (Kataoka et al. 2004a). Sulfate released from the xylem is retrieved by Sultr 2;1 to xylem parenchyma cells and vascular bundle sheath cells and enters mesophyll cells through plasmodesmata (Kataoka et al. 2004a). Sultr2;2, expressed in the root phloem and leaf vascular bundle sheath cells, participates in the transport of sulfate released from xylem vessels into the mesophyll cells (Takahashi et al. 2000). Therefore, Sultr 2;1 and Sultr 2;2 may also retrieve selenate from xylem vessels and transport it into mesophyll cells (Fig. 1). Group 2 sulfate transporters in rice include OsSultr 2;1 and OsSultr 2;2. The selenate taken up by the roots is most likely to be transported from roots to shoots by these transporters, which remains to be experimentally confirmed (Fig. 1, Table 1).
Selenite Uptake and Transport
Earlier physiological studies revealed that respiratory inhibitors and low temperature could largely inhibit selenite uptake at pH 4.0, suggesting that selenite uptake was an active process (Ulrich and Shrift 1968). However, later studies indicated that the respiratory inhibitors and low temperature could only slightly inhibit selenite uptake, indicating that selenite uptake was a passive process (Arvy 1993). Therefore, previous studies on the physiological mechanism of selenite uptake by plants were inconsistent. The conflicting results of previous studies should be attributed to the neglect of the existence of different Se forms in selenite solutions, which vary with pH. At low selenite concentrations, H2SeO3, SeO32−, and HSeO3− coexist in aqueous solutions and can also be converted into each other with pH to maintain ion balance (Zhang et al. 2006a). According to the ionization constant of H2SeO3 (K1 = 2.7 × 10–3, K2 = 2.5 × 10–7), the proportion of different Se species in different pH media can be calculated. For example, H2SeO3 and HSeO3− constitute about 27% and 73% at pH 3.0; HSeO3−, SeO32−, and H2SeO3 constituted about 97.2%, 2.4%, and 0.04% at pH 5.0; SeO32− and HSeO3− constitute about 96.2% and 3.8% at pH 8.0. The H2SeO3, HSeO3−, or SeO32− species represented the dominant forms of selenite in the solution at different pH, respectively (Zhang et al. 2006a, 2010b). Respiratory inhibitors and low temperature largely inhibited selenite uptake at pH 5.0, suggesting that selenite (HSeO3−) uptake at pH 5.0 was coupled with energy metabolism. Furthermore, the excellent fit of selenite (HSeO3−) uptake data to a Michaelis–Menten equation suggested that selenite was taken up by a transporter-mediated process involving selective membrane binding sites (Zhang et al. 2006a, 2010b). Selenite uptake was inhibited by phosphate in hydroponic experiments (Hopper and Parker 1999). Physiological experiments further revealed that selenite uptake could be regulated by phosphate transporters (Li et al. 2008). OsPHT1;2 (OsPT2) is a phosphate transporter responsible for Pi transport in rice (Ai et al. 2009). OsPT2 is localized in root epidermal cells and steles in primary and lateral roots (Ai et al. 2009; Zhang et al. 2014). Interestingly, OsPT2-overexpressing and knock-down plants displayed significantly increased and decreased rates of selenite uptake, demonstrating that OsPT2 is involved in selenite uptake (Zhang et al. 2014). OsPHT1;8 (OsPT8) is a high-affinity Pi transporter, expressed in various tissues and organs, including root tips, lateral roots, leaves, stamens, caryopsis, and germinated seeds, which is involved in Pi homeostasis in rice (Jia et al. 2011). Overexpression of OsPT8 increased Se concentration in tobacco plants, suggesting that OsPT8 is also involved in selenite uptake and transport (Song et al. 2017) (Fig. 1, Table 1).
Respiratory inhibitors and low temperature had little effect on selenite uptake at pH 3.0 and pH 8.0, suggesting that selenite uptake was an energy-independent process at these pHs (Zhang et al. 2010b). The amount of selenite taken up increased linearly in proportion to the increasing Se concentration in the absorption solution, suggesting that selenite uptake was possibly a passive process at pH 3.0 or pH 8.0 (Zhang et al. 2006a, 2010b). The result was partly consistent with previous results (Arvy 1993). HgCl2 and AgNO3 are well-known aquaporin blockers, which could inhibit the uptake of selenite largely at pH 3.0 in rice and maize (Niemietz and Tyerman 2002; Zhang et al. 2006a, 2010b). The inhibition of selenite uptake by HgCl2 or AgNO3 might be related to the inhibition of aquaporin activity, suggesting that H2SeO3 is taken up through aquaporin (Zhang et al. 2006a, 2010b). Furthermore, a silicon influx transporter OsNIP2;1 (Lsi1) identified in rice belongs to the nodulin 26-like intrinsic membrane protein (NIP) subfamily of aquaporins (Ma et al. 2006). OsNIP2;1 is localized to the plasma membrane of the distal side of both exodermis and endodermis cells and is constitutively expressed in roots. Expression of OsNIP2;1 in yeast enhanced selenite uptake at pH 3.5 and 5.5, but not at pH 7.5. Defect of Si efflux transporter OsNIP2;2 did not affect selenite uptake, demonstrating that Si influx transporter OsNIP2;1 is permeable to selenite (Zhao et al. 2010) (Fig. 1, Table 1).
Previous studies indicated that after selenite was taken up by plant roots, only a small amount of Se was transported to the shoots, and most of the Se remained in the roots (Arvy 1993; Zayed et al. 1998; Li et al. 2008). When rice seedlings were supplied with selenite, large amounts of SeMet were detected in the roots, only a small amount of MeSeCys was found, suggesting that selenite is mainly converted to SeMet in plastids (Zhang et al. 2019). In addition, SeMet is mainly detected in leaves and sheaths, indicating that SeMet is the dominant form of transport when supplied with selenite (Zhang et al. 2019). OsPT2 and OsPT6 are both highly expressed in stele cells of rice roots and play essential roles in Pi root-to-shoot translocation and Pi homeostasis in the plant (Ai et al. 2009). Although more selenite was present in roots, no selenite was detected in leaf blades and leaf sheaths, indicating that selenite was not transported to shoots by OsPT2 and OsPT6 (Zhang et al. 2019). Selenite is also not transported into the shoots of pakchoi after being taken up by the roots (Yu et al. 2019). The reason why selenite cannot be transported to shoots should be that selenite has been converted to organic Se before being transported (Yu et al. 2019; Zhang et al. 2019).
Organic Se Uptake and Transport in Plant
Organic Se is also one of the forms of Se available to plants (Abrams and Burau 1989). Organic Se accounts for about 40% of the total Se in the soil and 50% of the soluble Se extracted from the soil. It is stable in the soil and does not change appreciably with the variation of soil conditions (Yamada et al. 1998). Organic Se may be derived from decomposing plant tissues or incorporating into the organic fraction from inorganic Se abiotically or microbiological activity (Abrams and Burau 1989). SeMet is the dominant organic Se identified in soils (Abrams and Burau 1989; Abrams et al. 1990a). SeMet uptake by wheat seedlings followed Michaelis–Menten kinetics and was coupled to metabolism evidenced by inhibition of metabolic inhibitors and by anaerobic conditions (Abrams et al. 1990b). NRT1.1B, a member of the PTR family, encodes a protein containing a peptide-transporter domain. NRT1.1B is predominantly expressed in the vascular tissues of rice roots, leaf sheaths, leaves, and culms. Together with its plasma membrane localization, NRT1.1B was demonstrated to be involved in root-to-shoot nitrate transport (Hu et al. 2015). Furthermore, NRT1.1B has SeMet transport activity and also mediates the root-to-shoot translocation of SeMet in rice (Fig. 1, Table 1). NRT1.1B overexpression significantly improved not only the Se concentrations in shoots but also in grains (Zhang et al. 2019).
Subcellular Distribution of Different Forms of Se
Plastids, especially chloroplasts, are the main site of reductive assimilation of sulfur and Se in plants (Terry et al. 2000). Group 3 sulfate transporters in Arabidopsis, including Sultr3;1, Sultr3;2, Sultr3;3, Sultr3;4, and Sultr3;5, are localized in the chloroplasts responsible for sulfate transport into chloroplasts (Cao et al. 2013; Chen et al. 2019) (Table 1). Single knockout mutants of group 3 sulfate transporters showed reduced chloroplast sulfate uptake, suggesting that these sulfate transporters may also be involved in chloroplastic sulfate transport, the contribution of sulfate influx into chloroplasts by Sultr3;2, Sultr3;3, and Sultr3;4 was estimated at 74%, 66%, and 69% of the wild type, respectively (Cao et al. 2013). Sulfate uptake by chloroplasts of the quintuple mutant was reduced by more than 50% compared with the wild type (Chen et al. 2019). However, sulfate uptake was hardly detectable with Sultr3;5 expression alone, whereas cells coexpressing both Sultr3;5 and Sultr2;1 exhibited substantial uptake activity that was considerably higher than with Sultr2;1 expression alone (Kataoka et al. 2004a). Due to the highly similar properties of selenate and sulfate, selenate in the chloroplasts is most likely transported from the cytoplasm by group 3 sulfate transporters. In rice, group 3 sulfate transporters include OsSultr3;1, OsSultr3;2, OsSultr3;3, OsSultr3;4, OsSultr3;5, and OsSultr3;6 (Buchner et al. 2004) (Table 1). Overexpression of OsSultr3;3 in yeast and Xenopus oocytes revealed that OsSultr3;3 had no sulfate transporter activity. However, disruption of OsSultr3;3 reduces sulfate and cysteine concentrations, whereas no significant differences in total S concentration were observed (Zhao et al. 2016). Although these transporters have not been demonstrated to transport selenate, cytosolic selenate is most likely transported by them into the chloroplasts (Fig. 1).
The efflux of sulfate from the vacuoles maintains cytosolic sulfate homeostasis and promotes transport toward the xylem vessels. In group 4, there are two sulfate transporters Sultr4;1 and Sultr4;2 in Arabidopsis but only one OsSultr4;1 in rice (Buchner et al. 2004) (Table 1). Sultr4;1 and Sultr4;2 are tonoplast-localizing transporters in Arabidopsis (Kataoka et al. 2004b). Sultr4;1 is the main transporter facilitating the unloading of vacuolar sulfate reserve in the roots, and Sultr4;2 may play similar and supplementary roles in supporting the Sultr4;1 function at the tonoplast. The contribution of Sultr4;2 was estimated 15% of the Sultr4;1 function (Kataoka et al. 2004b). OsSultr4;1 may be located in the tonoplast of rice and is responsible for the transport of selenate to the vacuole, but further confirmation is needed (Fig. 1, Table 1).
After selenite is taken up by the roots, it is readily converted to SeMet catalyzed by sulfur-metabolizing enzymes (Terry et al. 2000). Since GSH reductase, Cys synthase, cystathionine-γ-synthase, and cystathionine-β-lyase are primarily present in plastids, suggesting that selenite enters plastids soon after being taken up by the root (Takahashi and Saito 1996; Terry et al. 2000). When rice seedlings were supplied with selenite for 3 d, large amounts of selenite were still detected in the roots (Zhang et al. 2019). Since GSH, O-acetylserine, and NADPH are present in the cytoplasm (Foyer et al. 2001; Chai et al. 2006; Hider and Kong 2011; Li et al. 2022), so selenite is readily reduced to selenol (GS-SeH) by GSH and NADPH (Terry et al. 2000). Therefore, a large amount of selenite is unlikely to exist in the cytoplasm for a long time but in the vacuole.
The distribution of selenite in organelles largely depends on the pH in the cytosol. At neutral pH in the cytosol, there are mainly two forms of Se, SeO32− and HSeO3−, which account for 71.4% and 28.6%, respectively (Zhang et al. 2006a, 2010b) (Fig. 1). Previous studies indicated that SeO32− could enter the root passively at a slow speed (Zhang et al. 2006a, 2010b). Likewise, SeO32− also slowly enters the vacuole and plastid. Under pH-neutral conditions, SeO32− and HSeO3− coexist in a specific ratio in the selenite solution. The two chemical forms of Se can be converted into each other to maintain ion balance. Since the uptake rate of HSeO3− is much higher than that of SeO32−, the ion balance between SeO32− and HSeO3− is broken, resulting in more SeO32− being converted to HSeO3−. Therefore, selenite in the cytosol is mainly transported in the form of HSeO3− into organelles such as vacuoles and plastids.
Previous studies have demonstrated that Pi transporters have transport activities for selenite (Li et al. 2008; Zhang et al. 2014; Song et al. 2017). The Arabidopsis PHT4 proteins mediate Pi transport in yeast with high specificity. PHT4;1-PHT4;5 localize to the plastid envelope and regulates Pi entry into the plastid (Guo et al. 2008). Rice OsPHT4;1-OsPHT4;4 localize to the inner chloroplast membrane and are involved in the distribution of Pi between the cytoplasm and chloroplast (Li et al. 2020) (Table 1). In addition, the low-affinity Pi transporter OsPHT2;1 functions as a chloroplast-localized low-affinity Pi transporter, mediating Pi entry into the chloroplast (Liu et al. 2020). The Arabidopsis SPX-MFS protein, designated as PHOSPHATE TRANSPORTER 5 family (PHT5), also named Vacuolar Phosphate Transporter (VPT), functions as vacuolar Pi transporters (Liu et al. 2015, 2016). Rice SPX-MFS family contains four genes, including OsSPX-MFS1, OsSPX-MFS2, SPX-MFS3, and SPX-MFS4 (Secco et al. 2012). OsSPX-MFS1, OsSPX-MFS1, and SPX-MFS3 localize to the tonoplast and are responsible for vacuolar Pi influx or efflux (Wang et al. 2012, 2015; Xu et al. 2019) (Fig. 1, Table 1). Therefore, the selenite in the form of HSeO3− in the cytosol may enter the vacuole and the plastid through these Pi transporters located in the tonoplast and chloroplast membrane (Fig. 1, Table 1).
The Release of SeMet from Protein Degradation
The leaves mainly accumulated SeMet when rice seedlings were supplied with selenite (Zhang et al. 2019). A large amount of SeMet non-specifically replaces Met to participate in protein synthesis in the leaves. During leaf senescence, Se-containing proteins are degraded by proteases, and SeMet is released and retranslocated to the developing grains. Se concentration in brown rice was positively correlated with the total Se in shoots (Zhang et al. 2006,b). Therefore, the higher the Se concentration in leaf blades, the more SeMet released by protein degradation during leaf senescence, the more SeMet transported to the grain, and the higher the grain Se concentration. Previous studies have revealed that most of the nitrogen in the grains mainly derives from the reutilization of nitrogen in shoots (Palta and Fillery 1995; Kichey et al. 2007; Gregersen et al. 2008). Protein degradation in leaves is a prerequisite for nitrogen reutilization (Gregersen et al. 2008). Up to 75% of the proteins in the leaves are in the chloroplast, including thylakoid membrane proteins and matrix proteins, most of which are ribulose-1,5-bisphosphate carboxylase (RuBisCO, EC 4.1.1.39). The nitrogen released from senescent leaves mainly comes from the degradation of RuBisCO and other proteins in chloroplasts (Otegui 2018). Improving the reutilization capacity of RuBisCO can enhance the efficiency of nitrogen utilization (Desclos et al. 2009; Girondé et al. 2015). Since Se and nitrogen coexist in proteins in the form of SeMet and various amino acids, respectively, protein degradation in senescent leaves may simultaneously affect the reutilization of Se and nitrogen. Regulating the degradation of proteins, especially chloroplast proteins, may also improve the reutilization efficiency of Se.
Protein degradation in senescent leaves is closely associated with protease activities (Roberts et al. 2012; Diaz-Mendoza et al. 2016). During the senescence of leaves, a large number of proteases, including cysteine proteases, serine proteases, aspartic proteases, metalloproteases, and threonine proteases, are induced (Guo et al. 2004; Roberts et al. 2012). At least one or more proteases are distributed in the cytoplasm, nucleus, chloroplast, mitochondria, endoplasmic reticulum, Golgi apparatus, and cell wall (Diaz-Mendoza et al. 2016). The vacuole is the main compartment where proteins are hydrolyzed into amino acids (Masclaux-Daubresse et al. 2017). Autophagy is considered to be an important mechanism for the selective degradation of chloroplasts in the vacuole (Xie et al. 2015; Otegui 2018; Zhuang and Jiang 2019). Chloroplasts are encapsulated in different vesicles by autophagy and then are transported to vacuoles for complete degradation (Fig. 1). Cysteine proteases and serine proteases are the main proteases in vacuoles. During plant senescence, the expressions of cysteine protease genes are greatly increased (Bhalerao et al. 2003; Guo et al. 2004; Diaz-Mendoza et al. 2016). Under continuous dark conditions, at least four vacuolar cysteine protease genes in senescent wheat leaves were induced (Martínez et al. 2007). In Arabidopsis and soybean leaves, cysteine protease activity is high in senescence-associated vacuoles (Otegui et al. 2005; Martínez et al. 2007). The cysteine protease SAG12 is highly expressed in natural aging tissues and exists in senescence-associated vacuoles (Guo et al. 2004; Otegui et al. 2005; Parrott et al. 2007). Cysteine protease in senescent wheat leaves could degrade the large subunit of RuBisCO into 50 kDa fragments (Thoenen et al. 2007). Serine proteases are the most abundant proteases in plants (Van der Hoorn 2008; Roberts et al. 2012). During the senescence of wheat leaves, serine protease genes are induced and play a vital role in the reutilization of nitrogen (Chauhan et al. 2009; Roberts et al. 2011). In barley senescent leaves induced by girding, the expression of serine protease is induced to accelerate leaf senescence and promote the degradation of RuBisCO and membrane proteins (Parrott et al. 2007). In senescent leaves, a large number of protease genes are induced to express, and the enzyme activities are increased, thereby accelerating protein degradation. Increasing the activity of serine protease and cysteine protease can increase the efficiency of nitrogen reutilization (Poret et al. 2019). Therefore, mining the proteases related to Se reutilization, especially serine proteases and cysteine proteases, is expected to promote protein degradation by regulating their gene expression to release more SeMet, thereby increasing grain Se concentrations.
Retranslocation of SeMet from Senescent Leaves to Grains
Plant leaf senescence and nutrient reutilization are closely related processes. Senescence is also a process of nutrient retranslocation from leaves to reproductive organs. The protein degradation products in senescent leaves are primarily transported into the grain in the form of amino acids and small peptides (Roberts et al. 2012). Since leaf Se is predominantly present in protein as SeMet, like other amino acids, SeMet is released from protein degradation in senescent leaves before it can be transported to developing grains. The expression of multiple amino acid and peptide transporter (AAP and PTR subfamily transporters) genes was up-regulated in senescent leaves of Arabidopsis (Marmagne et al. 2016). AtAAP1 is responsible for the uptake of amino acids by the embryo in Arabidopsis and is important for storage protein synthesis and seed yield (Sanders et al. 2009). Among the AAP subfamily members whose expression was up-regulated, AtAAP2 was involved in the reutilization of nitrogen in senescent leaves, and grain nitrogen concentration was reduced in aap2 mutants (Zhang et al. 2010a). AtAAP8 is expressed in the phloem of leaves and is located to the plasma membrane of mesophyll cells. In the aap8 mutants, the amino acid concentrations in phloem and grains were decreased, indicating that AtAAP8 plays an important role in the source-to-sink partitioning of nitrogen (Santiago and Tegeder 2016). In rice, OsAAP1 is localized to the plasma membrane and nuclear membrane and is highly expressed in roots, axillary buds, leaves, and panicles. Overexpression of OsAAP1 may promote the transport of neutral amino acids from straw to grains (Ji et al. 2020). OsAAP3 was mainly expressed in roots, leaves, leaf sheaths, culms, and panicles (Lu et al. 2018). Elevated expression of OsAAP3 leads to amino acid accumulation in rice grains (Lu et al. 2018). OsAAP6 is expressed in the vascular bundles in the flag leaves of rice at the heading stage. OsAAP6 can increase the concentration of grain storage protein and total amino acids, suggesting that OsAAP6 is involved in the transport of amino acids from leaves to grains (Peng et al. 2014). Improved amino acid transport from senescent leaves to grains can increase the grain protein concentration (Kade et al. 2005). SeMet, an analog of Met, shares a common transporter with Met (Gits and Grenson 1967). OsAAP1, OsAAP3, OsAAP7, and OsAAP16 have transport activity for Met (Taylor et al. 2015). Therefore, it is very likely that OsAAP1 and OsAAP3 are involved in the transport of SeMet from senescent leaves to grains. In addition, the peptide transporter NRT1.1B is expressed in the vascular tissues of leaf sheaths, leaves and culms, and is not only involved in the transport of SeMet from roots to shoots, but may also be involved in the transport of SeMet from leaves to grains and increases the Se concentration in grains (Zhang et al. 2019) (Fig. 1).
Plant Se Volatilization
Plant Se Volatilization is Ubiquitous
Se volatilization in plants is a ubiquitous phenomenon during growth, harvest, drying, and storage stages. The loss of Se was as high as 66% during the air-drying process after the plants were collected, and it varied with seasons and growth stages (Beath et al. 1937). Plant volatile Se was mainly released through leaves, and the volatilization rate varied throughout the day (Lewis et al. 1966). 0.5–3.0% of the total Se in the shoots and roots supplied with selenate and selenite were released in volatile form when dried at 70 °C for 48 h (Evans and Johnson 1967), while dry heating of cereal grains led to 7–23% losses of Se (Higgs et al. 1972). Se losses reached 4–73% when the grains of barley, corn, and wheat were stored for 3 to 5 years (Moxon and Rhian 1938). The release of volatile Se compounds has been demonstrated during plant growth and development (Lewis et al. 1966; Evans et al. 1968; Zayed et al. 1998; de Souza et al. 2000). Se volatilization is greatly affected by soil Se concentrations, plant species, growth stage, organs, physiological state, and Se-supplied forms (Beath et al. 1937; Zayed et al. 1998; de Souza et al. 2002). Se hyperaccumulators growing in seleniferous soils can accumulate and volatilize large amounts of Se (Beath et al. 1937). Se non-hyperaccumulators could also volatilize and release Se, which varies with plant species and Se forms. The effect of Se forms on Se volatilization rate may be attributed to be in different stages of Se metabolism (Zayed et al. 1998).
Plant Volatile Se Forms
After selenate or selenite is converted to SeCys in the chloroplast, it can be further converted to MeSeCys and MeSeMet, and finally to volatile Se compounds such as DMSe and dimethyldiselenide (DMDSe) (Terry et al. 2000; Kubachka et al. 2007). The volatile Se compounds are mainly DMSe, which is released directly from the intermediate metabolites of SeMet, namely MeSeMet and dimethylselenoniopropionate (DMSeP) (Terry et al. 2000). The DMSe arises from MeSeMet cleaved by S-methyl-Met hydrolase, suggesting that MeSeMet is a precursor of DMSe (Lewis et al. 1974). DMSeP is an analog of dimethylsulfoniopropionate (DMSP). MeSeMet is converted to DMSeP-aldehyde, further converted to DMSeP by transamination by the chloroplast enzyme β-aldehyde dehydrogenase (Terry et al. 2000). DMSP is degraded by DMSP lyase to produce dimethylsulfide (DMS) (Diaz et al. 1992; Ledyard et al. 1993; Van Boekel and Stefels 1993; De Souza and Yoch 1995; Yoch 2002). DMSeP is postulated to be cleaved by DMSP lyase to DMSe. DMSeP-supplied plants volatilized Se at a rate of 113 times higher than that measured from plants supplied with selenate, 38 times higher than from selenite, and 6 times higher than from SeMet, suggesting DMSeP is the most likely precursor of DMSe (de Souza et al. 2000). Since MeSeMet and DMSeP are only found in Se non-hyperaccumulators, they are the precursors of DMSe volatilized by these plants. In contrast, DMDSe could likely be derived from MeSeCys selenoxide, an intermediate metabolite of MeSeCys, mainly found in Se hyperaccumulators (Terry et al. 2000). Se hyperaccumulators can accumulate large amounts of MeSeCys, γ-glutamyl-methyl-SeCys, or selenocystathionine. Therefore, DMDSe is mainly produced from MeSeCys in Se hyperaccumulators (Fig. 2).
Fig. 2.
The metabolism of selenate and selenite in chloroplasts or plastids (Terry et al. 2000). Selenate is first reduced to adenosine 5-phosphoselenate (APSe) by ATP sulfurylase (EC: 2.7.7.4) and then further reduced nonenzymatically to GSH-conjugated selenite (GS-selenite). Selenite is also reduced nonenzymatically to GS-selenite. The GS-selenite is reduced to selenodiglutathione (GS-Se-SG) by GSH, and GS-Se-SG is further reduced to selenol (GS-SeH) by NADPH and subsequently to GSH-conjugated selenide (GS-Se−) by GSH reductase. SeCys is synthesized from GS-Se− and O-acetylserine catalyzed by Cys synthase. SeMet may be synthesized from SeCys via SeCystathionine and SeHomoCys catalyzed by cystathionine-γ-synthase, cystathionine-β-lyase, and Met synthase. SeCys is methylated to methyl-SeMet by Cys methyltransferase. SeMet is methylated to methyl-SeMet, and is further converted into dimethylselenonium propionate (DMSeP) by DMSeP lyase, and then cleaved to DMSe by S-methylMet hydrolase and volatilized. R represents the rate-limiting step
Rate-Limiting Enzymes for Se Volatilization
After selenate is taken up by roots via the sulfate transporters, it is transported to the leaves and enters chloroplasts, where it is metabolized by the enzymes of sulfate assimilation (Terry et al. 2000). Selenate is first reduced to adenosine 5-phosphoselenate (APSe) by ATP sulfurylase (EC: 2.7.7.4) and then further reduced nonenzymatically to GSH-conjugated selenite (GS-selenite). The GS-selenite is reduced to selenodiglutathione (GS-Se-SG) by GSH, and GS-Se-SG is further reduced to selenol (GS-SeH) by NADPH, and subsequently to GSH-conjugated selenide (GS-Se−) by GSH reductase (Ng and Anderson 1979). APSe could also be converted to selenite with GSH by APS reductase (EC: 1.8.99.2), and then to selenide by sulfite reductase (EC: 1.8.7.1) (Bick and Leustek 1998). SeCys is synthesized from GS-Se− and O-acetylserine catalyzed by Cys synthase (Ng and Anderson 1978, 1979). In the cytoplasm, due to the presence of GSH, O-acetylserine, NADPH, and cysteine synthase, selenite may also be further converted to SeCys by Cys synthase after being reduced to GS-Se− by GSH and NADPH in the cytoplasm (Nakamura et al. 1999; Foyer et al. 2001; Chai et al. 2006; Hider and Kong 2011; Li et al. 2022). SeMet is synthesized from SeCys via SeCystathionine and SeHomoCys catalyzed by cystathionine-γ-synthase, cystathionine-β-lyase, and Met synthase (Dawson and Anderson 1988). SeMet is methylated to MeSeMet and further cleaved to DMSe by S-methyl-Met hydrolase. DMSeP is most likely the precursor of DMSe, which is cleaved to DMSe by DMSP lyase (de Souza et al. 2000) (Fig. 2).
The conversion from selenate to DMSe involves at least three rate-limiting steps. The reduction of selenate is the first rate-limiting step. ATP sulfurylase catalyzes the reduction of selenate as well as sulfate in plants (Shaw and Anderson 1972; Dilworth and Bandurski 1977; Burnell 1981). The overexpression of the ATP sulfurylase gene could promote selenate reduction and accumulate more organic Se (Pilon-Smits et al. 1999). SeMet synthesis is the second rate-limiting step of selenate reduction. Se volatilization rate from the SeCys-supplied plants was almost fivefold lower than those from SeMet-supplied plants, suggesting that it is a rate-limiting step to synthesize SeMet, and Met synthase is a rate-limiting enzyme for Se volatilization. The conversion of SeMet to DMSeP is the third rate-limiting step. SeMet is first methylated by Met methyltransferase to MeSeMet, then cleaved by DMSeP lyase to DMSeP, and ultimately cleaved to DMSe by S-methyl-Met hydrolase. The rate of Se volatilization from DMSeP-supplied plants was fivefold higher than that from SeMet-supplied plants, even though the roots of plants supplied with SeMet accumulated eightfold more Se than DMSeP-supplied plants, suggesting that the conversion of SeMet to DMSeP is also a rate-limiting step and DMSeP lyase is a rate-limiting enzyme (Fig. 2). The overexpression of rate-limiting enzymes can accelerate Se volatilization (Pilon-Smits et al. 1999). Therefore, inhibiting the activity of rate-limiting enzymes may inhibit Se volatilization to a large extent.
The Speciations of Se Compounds in Plants
Plants mainly take up selenate, selenite, and a small amount of SeMet from the soil. Selenate and selenite are mainly converted to SeCys, SeMet, and intermediate metabolites in most plants and converted to MeSeCys in a few plants such as broccoli, garlic, onion, etc. (Ávila et al. 2003; Cai et al. 1995; Lyi et al. 2005; Zhang et al. 2019). Therefore, humans can acquire Se in the form of selenate, selenite, SeCys, SeMet, MeSeCys, and intermediate metabolites from plant foods. The speciation of Se compounds has obvious different effects on preventing cancers with relative efficacy ranging from high to low as MeSeCys > selenite > SeCys > dimethylselenoxide (Ip et al. 1991). High-Se garlic has obvious anticancer efficacies. The function of high-Se garlic to prevent cancer primarily depends on the effect of Se (Ip and Lisk 1995). High-Se garlic contains Se in the form of MeSeCys (Cai et al. 1995). Feeding mice a carcinogen containing Se-enriched garlic results in a reduction in the incidence of mammary tumors in mice. This result is associated with enhanced levels of SeCys and MeSeCys in these plants (Ip et al. 1992; Cai et al. 1995). MeSeCys exhibits greater efficacy as a chemopreventive agent than several previously used Se compounds in experimental models of breast cancer (Medina et al. 2001). Rats fed supranutritional amounts of Se as broccoli exhibited greater colon cancer protection than rats fed low Se broccoli supplemented with the same amount of selenite or selenate. MeSeCys offers selective protection against organ-specific toxicity induced by clinically active agents and enhances further antitumour activity, resulting in an improved therapeutic index (Cao et al. 2014). MeSeCys is powerful in ameliorating Alzheimer’s disease-related neuropathology and cognitive deficits via modulating oxidative stress, metal homeostasis, and extracellular signal-regulated kinase activation (Xie et al. 2018). Therefore, the production of Se-enriched plant edible products mainly containing MeSeCys could improve cancer prevention. SeCys methyltransferase (SMT) in plants is responsible for the methylation of SeCys to form MeSeCys. The transgenic plants accumulated more MeSeCys by overexpressing the gene SMT from the Se hyperaccumulator Astragalus bisulcatus in Arabidopsis, Indian mustard, tobacco, and tomato than the wild type (LeDuc et al. 2004; McKenzie et al. 2009; Brummell et al. 2011). Therefore, a large amount of the cancer-preventing compound, MeSeCys, can be produced in plants by overexpressing SMT to meet human nutritional requirements.
Strategies for Se Biofortification
The accumulation of Se in rice grains involves the uptake, transport, subcellular distribution, metabolism, and retanslocation of Se in rice plants. Each of these processes is crucial for grain Se accumulation. Therefore, the following strategies are proposed to improve Se accumulation in rice grains by improve Se utilization efficiency. Firstly, improving the capability of roots to take up selenite by secreting more protons. The H+-ATPases located in the cell membrane of the root cells are responsible for the secretion of protons. Regulating the H+-ATPases activity to secrete more protons can reduce the pH of the apoplastic space and the rhizosphere soil, resulting in more Se rapidly entering the root cells in the form of H2SeO3 through aquaporins. Secondly, the accumulation of Se in grains mainly involves phosphate transporters, amino acid transporters, and peptide transporters. Mining key transporters for Se accumulation and increasing the expression of these transporter genes are effective strategies to increase Se concentration in rice grains. Thirdly, regulating the distribution of Se in organelles is expected to improve the utilization efficiency of Se. When rice roots take up selenite, large amounts of Se enter the vacuole and are stored. Only when this part of Se is transported from the vacuole to the cytoplasm and then into the plastid can it be converted into organic Se and transported to the shoots. Therefore, mining the transporters responsible for Se influx and efflux located in the tonoplast and modulating their gene expression can enhance the influx of Se from the vacuole to the cytoplasm and increase the transport of more Se into the plastid. Fourthly, inhibition of Se volatilization can largely increase the Se concentration in rice grains. Se volatilization results in a large amount of Se loss from plants. Several key enzymes such as ATP sulfurylase, Met synthase, DMSeP lyase, Met methyltransferase, and S-methylMet hydrolase control the conversion from selenate to DMSe. Without interfering with normal sulfur metabolism, inhibition of certain enzyme activities is beneficial to reducing the formation of DMSe and increasing the Se concentration in rice grains. Fifthly, Se is mainly present in proteins in leaves as SeMet. Different proteases coordinate their actions to regulate protein degradation jointly during leaf senescence. It is crucial to identify the key proteases that degrade proteins to release more SeMet. Finally, the speciation of Se in plants should be optimized to make MeSeCys the main Se form in rice grains. Se exists in rice plants in the form of selenate, selenite, SeCys, SeMet, MeSeCys, and intermediate metabolites, of which MeSeCys is the most potent anticancer form. Therefore, the synthesis of MeSeCys in rice plants can be greatly enhanced by overexpressing the gene encoding SeCys methyltransferase or by gene editing its promoters by CRISPR/Cas9 to increase gene expression, thereby predominantly accumulating MeSeCys in rice grains.
In summary, enhanced Se accumulation in rice grains can be achieved by improving the efficiency of Se uptake, transport, distribution, and reutilization, and by inhibiting Se volatilization. In addition, we can also optimize the Se species that are more beneficial for human health, providing new insights and directions for the future biofortification of Se in rice.
Acknowledgements
Not applicable.
Abbreviations
- AAP
Amino acid transporter
- APSe
Adenosine 5-phosphoselenate
- Cys
Cysteine
- DMDSe
Dimethyldiselenide
- DMSe
Dimethylselenide
- DMSeP
Dimethylselenoniopropionate
- DMSP
Dimethylsulfoniopropionate
- GS-selenite
GSH-conjugated selenite
- GS-Se-SG
Selenodiglutathione
- Met
Methionine
- MFS
Major facilitator superfamily
- NIP
Nodulin 26-like intrinsic membrane protein
- PT2
PHT1;2
- PT8
PHT1;8
- PTR
Peptide transporter
- RuBisCO
Ribulose-1,5-bisphosphate carboxylase
- Se
Selenium
- SeCys
Selenocysteine
- SeMet
Selenomethionine
- SMT
SeCys methyltransferase
- SPX
SYG1/Pho81/XPR1
- VPT
Vacuolar phosphate transporter
Author Contributions
LHZ wrote the manuscript. CCC made valuable suggestions and revised the manuscript. All authors read and approved the final manuscript.
Funding
The work was supported by NSFC (National Natural Science Foundation of China) -Henan Joint Fund (U1904114) and the Major Program of Guangdong Basic and Applied Research (2019B030302006).
Declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare that they have no competing interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lianhe Zhang, Email: lhzhang2007@126.com.
Chengcai Chu, Email: ccchu@scau.edu.cn.
References
- Abrams M, Burau R. Fractionation of selenium and detection of selenomethionine in a soil extract. Commun Soil Sci Plant Anal. 1989;20:221–237. [Google Scholar]
- Abrams M, Burau R, Zasoski R. Organic selenium distribution in selected California soils. Soil Sci Soc Am J. 1990;54:979–982. [Google Scholar]
- Abrams MM, Shennan C, Zasoski R, Burau R. Selenomethionine uptake by wheat seedlings. Agron J. 1990;82:1127–1130. [Google Scholar]
- Ai P, Sun S, Zhao J, Fan X, Xin W, Guo Q, Yu L, Shen Q, Wu P, Miller AJ. Two rice phosphate transporters, OsPht1; 2 and OsPht1; 6, have different functions and kinetic properties in uptake and translocation. Plant J. 2009;57:798–809. doi: 10.1111/j.1365-313X.2008.03726.x. [DOI] [PubMed] [Google Scholar]
- Arvy M. Selenate and selenite uptake and translocation in bean plants (Phaseolus vulgaris) J Exp Bot. 1993;44:1083–1087. [Google Scholar]
- Ávila FW, Faquin V, Yang Y, Ramos SJ, Guilherme LR, Thannhauser TW, Li L. Assessment of the anticancer compounds Se-methylselenocysteine and glucosinolates in Se-biofortified broccoli (Brassica oleracea L. var. italica) sprouts and florets. J Agric Food Chem. 2003;61:6216–6223. doi: 10.1021/jf4016834. [DOI] [PubMed] [Google Scholar]
- Beath O, Eppson H, Gilbert C. Selenium distribution in and seasonal variation of type vegetation occurring on seleniferous soils. J Am Dent Assoc (1912) 1937;26:394–405. [Google Scholar]
- Bhalerao R, Keskitalo J, Sterky F, Erlandsson R, Björkbacka H, Birve SJ, Karlsson J, Gardeström P, Gustafsson P, Lundeberg J. Gene expression in autumn leaves. Plant Physiol. 2003;131:430–442. doi: 10.1104/pp.012732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bick JA, Leustek T. Plant sulfur metabolism-the reduction of sulfate to sulfite. Curr Opin Plant Biol. 1998;1:240–244. doi: 10.1016/s1369-5266(98)80111-8. [DOI] [PubMed] [Google Scholar]
- Brummell DA, Watson LM, Pathirana R, Joyce NI, West PJ, Hunter DA, McKenzie MJ. Biofortification of tomato (solanum lycopersicum) fruit with the anticancer compound methylselenocysteine using a selenocysteine methyltransferase from a selenium hyperaccumulator. J Agric Food Chem. 2011;59:10987–10994. doi: 10.1021/jf202583f. [DOI] [PubMed] [Google Scholar]
- Buchner P, Takahashi H, Hawkesford MJ. Plant sulphate transporters: co-ordination of uptake, intracellular and long-distance transport. J Exp Bot. 2004;55:1765–1773. doi: 10.1093/jxb/erh206. [DOI] [PubMed] [Google Scholar]
- Cai XJ, Block E, Uden PC, Zhang X, Quimby BD, Sullivan JJ. Allium chemistry: identification of selenoamino acids in ordinary and selenium-enriched garlic, onion, and broccoli using gas chromatography with atomic emission detection. J Agric Food Chem. 1995;43:1754–1757. [Google Scholar]
- Cao MJ, Wang Z, Wirtz M, Hell R, Oliver DJ, Xiang CB. SULTR 3; 1 is a chloroplast-localized sulfate transporter in Arabidopsis thaliana. Plant J. 2013;73:607–616. doi: 10.1111/tpj.12059. [DOI] [PubMed] [Google Scholar]
- Cao S, Durrani FA, Toth K, Rustum YM. Se-methylselenocysteine offers selective protection against toxicity and potentiates the antitumour activity of anticancer drugs in preclinical animal models. Br J Cancer. 2014;110:1733–1743. doi: 10.1038/bjc.2014.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai MF, Wei PC, Chen QJ, An R, Chen J, Yang S, Wang XC. NADK3, a novel cytosolic source of NADPH, is required under conditions of oxidative stress and modulates abscisic acid responses in arabidopsis. Plant J. 2006;47:665–674. doi: 10.1111/j.1365-313X.2006.02816.x. [DOI] [PubMed] [Google Scholar]
- Chauhan S, Srivalli S, Nautiyal A, Khanna-Chopra R. Wheat cultivars differing in heat tolerance show a differential response to monocarpic senescence under high-temperature stress and the involvement of serine proteases. Photosynthetica. 2009;47:536–547. [Google Scholar]
- Chen Z, Zhao PX, Miao ZQ, Qi GF, Wang Z, Yuan Y, Ahmad N, Cao MJ, Hell R, Wirtz M. SULTR3s function in chloroplast sulfate uptake and affect ABA biosynthesis and the stress response. Plant Physiol. 2019;180:593–604. doi: 10.1104/pp.18.01439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs GF. Selenium in global food systems. British J Nutr. 2001;85:517–547. doi: 10.1079/bjn2000280. [DOI] [PubMed] [Google Scholar]
- Coppin F, Chabroullet C, Martin-Garin A, Balesdent J, Gaudet JP. Methodological approach to assess the effect of soil ageing on selenium behaviour: first results concerning mobility and solid fractionation of selenium. Biol Fertil Soils. 2006;42:379–386. [Google Scholar]
- Dawson J, Anderson J. Incorporation of cysteine and selenocysteine into cystathionine and selenocystathionine by crude extracts of spinach. Phytochemistry. 1988;27:3453–3460. [Google Scholar]
- De Souza M, Yoch DC. Purification and characterization of dimethylsulfoniopropionate lyase from an Alcaligenes-like dimethyl sulfide-producing marine isolate. Appl Environ Microbiol. 1995;61:21–26. doi: 10.1128/aem.61.1.21-26.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza MP, Lytle CM, Mulholland MM, Otte ML, Terry N. Selenium assimilation and volatilization from dimethylselenoniopropionate by Indian mustard. Plant Physiol. 2000;122:1281–1288. doi: 10.1104/pp.122.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza MP, Pickering IJ, Walla M, Terry N. Selenium assimilation and volatilization from selenocyanate-treated Indian mustard and muskgrass. Plant Physiol. 2002;128:625–633. doi: 10.1104/pp.010686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desclos M, Etienne P, Coquet L, Jouenne T, Bonnefoy J, Segura R, Reze S, Ourry A, Avice JC. A combined 15N tracing/proteomics study in Brassica napus reveals the chronology of proteomics events associated with N remobilisation during leaf senescence induced by nitrate limitation or starvation. Proteomics. 2009;9:3580–3608. doi: 10.1002/pmic.200800984. [DOI] [PubMed] [Google Scholar]
- Diaz MR, Visscher PT, Taylor BF. Metabolism of dimethylsulfoniopropionate and glycine betaine by a marine bacterium. Fems Microbiol Lett. 1992;96:61–65. [Google Scholar]
- Diaz-Mendoza M, Velasco-Arroyo B, Santamaria ME, González-Melendi P, Martinez M, Diaz I. Plant senescence and proteolysis: two processes with one destiny. Genet Mol Biol. 2016;39:329–338. doi: 10.1590/1678-4685-GMB-2016-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh QT, Cui Z, Huang J, Tran TAT, Wang D, Yang W, Zhou F, Wang M, Yu D, Liang D. Selenium distribution in the Chinese environment and its relationship with human health: a review. Environ Int. 2018;112:294–309. doi: 10.1016/j.envint.2017.12.035. [DOI] [PubMed] [Google Scholar]
- Dixit G, Singh AP, Kumar A, Singh PK, Kumar S, Dwivedi S, Trivedi PK, Pandey V, Norton GJ, Dhankher OP, Tripathi RD. Sulfur mediated reduction of arsenic toxicity involves efficient thiol metabolism and the antioxidant defense system in rice. J Hazard Mater. 2015;298:241–251. doi: 10.1016/j.jhazmat.2015.06.008. [DOI] [PubMed] [Google Scholar]
- El Kassis E, Cathala N, Rouached H, Fourcroy P, Berthomieu P, Terry N, Davidian JC. Characterization of a selenate-resistant arabidopsis mutant. Root growth as a potential target for selenate toxicity. Plant Physiol. 2007;143:1231–1241. doi: 10.1104/pp.106.091462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrashidi MA, Adriano DC, Workman SM, Lindsay WL. Chemical equilibria of selenium in soils: a theoretical development. Soil Sci. 1987;144:141–152. [Google Scholar]
- Evans CAC, Johnson C. Collection and partial characterization of volatile selenium compounds from Medicago sativa L. Saudi J Biol Sci. 1967;20:737–748. [Google Scholar]
- Evans CS, Asher C, Johnson C. Isolation of dimethyl diselenide and other volatile selenium compounds from Astragalus racemosus (Pursh.) Saudi J Biol Sci. 1968;21:13–20. [Google Scholar]
- Ferrari G, Renosto F. Regulation of sulfate uptake by excised barley roots in the presence of selenate. Plant Physiol. 1972;49:114–116. doi: 10.1104/pp.49.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiona F. Selenium geochemistry and health. Ambio. 2007;36:94–97. doi: 10.1579/0044-7447(2007)36[94:sgah]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Theodoulou FL, Delrot S. The functions of inter-and intracellular glutathione transport systems in plants. Trends Plant Sci. 2001;6:486–492. doi: 10.1016/s1360-1385(01)02086-6. [DOI] [PubMed] [Google Scholar]
- Franke KW. A new toxicant occurring naturally in certain samples of plant foodstuffs. 1. Results obtained in preliminary feeding trials. J Nutr. 1934;8:597–608. [Google Scholar]
- Gilliham M, Tester M. The regulation of anion loading to the maize root xylem. Plant Physiol. 2005;137(3):819–828. doi: 10.1104/pp.104.054056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girondé A, Etienne P, Trouverie J, Bouchereau A, Le Cahérec F, Leport L, Orsel M, Niogret M-F, Nesi N, Carole D, Soulay F, Masclaux-Daubresse C, Avice JC. The contrasting N management of two oilseed rape genotypes reveals the mechanisms of proteolysis associated with leaf N remobilization and the respective contributions of leaves and stems to N storage and remobilization during seed filling. BMC Plant Biol. 2015;15:59. doi: 10.1186/s12870-015-0437-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gits J, Grenson M. Multiplicity of the amino acid permeases in Saccharomyces cerevisiae: III. Evidence for a specific methionine-transporting system. Biochim Biophys Acta Biomembr. 1967;135:507–516. doi: 10.1016/0005-2736(67)90040-5. [DOI] [PubMed] [Google Scholar]
- Gregersen PL, Holm PB, Krupinska K. Leaf senescence and nutrient remobilisation in barley and wheat. Plant Biol (stuttg) 2008;10(Suppl 1):37–49. doi: 10.1111/j.1438-8677.2008.00114.x. [DOI] [PubMed] [Google Scholar]
- Guo Y, Cai Z, Gan S. Transcriptome of arabidopsis leaf senescence. Plant Cell Environ. 2004;27:521–549. [Google Scholar]
- Guo B, Jin Y, Wussler C, Blancaflor E, Motes C, Versaw WK. Functional analysis of the Arabidopsis PHT4 family of intracellular phosphate transporters. New Phytol. 2008;177:889–898. doi: 10.1111/j.1469-8137.2007.02331.x. [DOI] [PubMed] [Google Scholar]
- Hatfield DL, Gladyshev VN. How selenium has altered our understanding of the genetic code. Mol Cell Biol. 2002;22:3565–3576. doi: 10.1128/MCB.22.11.3565-3576.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkesford MJ. Transporter gene families in plants: The sulphate transporter gene family-redundancy or specialization? Physiol Plant. 2003;117:155–163. [Google Scholar]
- Hider RC, Kong XL. Glutathione: a key component of the cytosolic labile iron pool. Biometals. 2011;24:1179–1187. doi: 10.1007/s10534-011-9476-8. [DOI] [PubMed] [Google Scholar]
- Higgs DJ, Morris VC, Levander OA. Effect of cooking on selenium content of foods. J Agric Food Chem. 1972;20:678–680. doi: 10.1021/jf60181a019. [DOI] [PubMed] [Google Scholar]
- Hopper JL, Parker DR. Plant availability of selenite and selenate as influenced by the competing ions phosphate and sulfate. Plant Soil. 1999;210:199–207. [Google Scholar]
- Howarth JR, Fourcroy P, Davidian JC, Smith FW, Hawkesford MJ. Cloning of two contrasting high-affinity sulfate transporters from tomato induced by low sulfate and infection by the vascular pathogen Verticillium dahliae. Planta. 2003;218:58–64. doi: 10.1007/s00425-003-1085-5. [DOI] [PubMed] [Google Scholar]
- Hu B, Wang W, Ou S, Tang J, Li H, Che R, Zhang Z, Chai X, Wang H, Wang Y. Variation in NRT1. 1B contributes to nitrate-use divergence between rice subspecies. Nat Genet. 2015;47:834–838. doi: 10.1038/ng.3337. [DOI] [PubMed] [Google Scholar]
- Ip C, Lisk DJ. Efficacy of cancer prevention by high-selenium garlic is primarily dependent on the action of selenium. Carcinogenesis. 1995;16:2649–2652. doi: 10.1093/carcin/16.11.2649. [DOI] [PubMed] [Google Scholar]
- Ip C, Hayes C, Budnick RM, Ganther HE. Chemical form of selenium, critical metabolites, and cancer prevention. Cancer Res. 1991;51:595–600. [PubMed] [Google Scholar]
- Ip C, Lisk D, Stoewsand G. Mammary cancer prevention by regular garlic and selenium-enriched garlic. Nutr Cancer. 1992;17(3):279–286. doi: 10.1080/01635589209514197. [DOI] [PubMed] [Google Scholar]
- Ji Y, Huang W, Wu B, Fang Z, Wang X. The amino acid transporter AAP1 mediates growth and grain yield by regulating neutral amino acid uptake and reallocation in Oryza sativa. J Exp Bot. 2020;71:4763–4777. doi: 10.1093/jxb/eraa256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H, Ren H, Gu M, Zhao J, Sun S, Zhang X, Chen J, Wu P, Xu G. The phosphate transporter gene OsPht1; 8 is involved in phosphate homeostasis in rice. Plant Physiol. 2011;156:1164–1175. doi: 10.1104/pp.111.175240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kade M, Barneix AJ, Olmos S, Dubcovsky J. Nitrogen uptake and remobilization in tetraploid ‘Langdon’ durum wheat and a recombinant substitution line with the high grain protein gene Gpc-B1. Plant Breed. 2005;124:343–349. [Google Scholar]
- Kataoka T, Hayashi N, Yamaya T, Takahashi H. Root-to-shoot transport of sulfate in arabidopsis. Evidence for the role of SULTR3; 5 as a component of low-affinity sulfate transport system in the root vasculature. Plant Physiol. 2004;136:4198–4204. doi: 10.1104/pp.104.045625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka T, Watanabe-Takahashi A, Hayashi N, Ohnishi M, Mimura T, Buchner P, Hawkesford MJ, Yamaya T, Takahashi H. Vacuolar sulfate transporters are essential determinants controlling internal distribution of sulfate in arabidopsis. Plant Cell. 2004;16:2693–2704. doi: 10.1105/tpc.104.023960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keskinen R, Yli-Halla M, Hartikainen H. Retention and uptake by plants of added selenium in peat soils. Commun Soil Sci Plant Anal. 2013;44:3465–3482. [Google Scholar]
- Kichey T, Hirel B, Heumez E, Dubois F, Le Gouis J. In winter wheat (Triticum aestivum L.), post-anthesis nitrogen uptake and remobilisation to the grain correlates with agronomic traits and nitrogen physiological markers. Field Crops Res. 2007;102:22–32. [Google Scholar]
- Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigó R, Gladyshev VN. Characterization of mammalian selenoproteomes. Science. 2003;300:1439–1443. doi: 10.1126/science.1083516. [DOI] [PubMed] [Google Scholar]
- Kubachka KM, Meija J, LeDuc DL, Terry N, Caruso JA. Selenium volatiles as proxy to the metabolic pathways of selenium in genetically modified Brassica juncea. Environ Sci Technol. 2007;41:1863–1869. doi: 10.1021/es0613714. [DOI] [PubMed] [Google Scholar]
- Kumar S, Asif MH, Chakrabarty D, Tripathi RD, Trivedi PK. Differential expression and alternative splicing of rice sulphate transporter family members regulate sulphur status during plant growth, development and stress conditions. Funct Integr Genomic. 2011;11:259–273. doi: 10.1007/s10142-010-0207-y. [DOI] [PubMed] [Google Scholar]
- Kumar S, Khare R, Trivedi PK. Arsenic-responsive high-affinity rice sulphate transporter, OsSultr1; 1, provides abiotic stress tolerance under limiting sulphur condition. J Hazard Mater. 2019;373:753–762. doi: 10.1016/j.jhazmat.2019.04.011. [DOI] [PubMed] [Google Scholar]
- Läuchli A. Selenium in plants: uptake, functions, and environmental toxicity. Botanica Acta. 1993;106:455–468. [Google Scholar]
- LeDuc DL, Tarun AS, Montes-Bayon M, Meija J, Malit MF, Wu CP, AbdelSamie M, Chiang CY, Tagmount A, deSouza M, Neuhierl B, Böck A, Caruso J, Terry N. Overexpression of selenocysteine methyltransferase in arabidopsis and Indian mustard increases selenium tolerance and accumulation. Plant Physiol. 2004;135:377–383. doi: 10.1104/pp.103.026989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledyard KM, DeLong EF, Dacey JW. Characterization of a DMSP-degrading bacterial isolate from the Sargasso Sea. Arch Microbiol. 1993;160:312–318. [Google Scholar]
- Lewis BG, Johnson C, Delwiche C. Release of volatile selenium compounds by plants. Collection procedures and preliminary observations. J Agric Food Chem. 1966;14:638–640. [Google Scholar]
- Lewis B, Johnson C, Broyer T. Volatile selenium in higher plants the production of dimethyl selenide in cabbage leaves by enzymatic cleavage of Se-methyl selenomethionine selenonium salt. Plant Soil. 1974;40:107–118. [Google Scholar]
- Li HF, McGrath SP, Zhao FJ. Selenium uptake, translocation and speciation in wheat supplied with selenate or selenite. New Phytol. 2008;178:92–102. doi: 10.1111/j.1469-8137.2007.02343.x. [DOI] [PubMed] [Google Scholar]
- Li S, Xiao T, Zheng B. Medical geology of arsenic, selenium and thallium in China. Sci Total Environ. 2012;421:31–40. doi: 10.1016/j.scitotenv.2011.02.040. [DOI] [PubMed] [Google Scholar]
- Li RI, Wang JL, Xu L, Sun ML, Yi KK, Zhao HY. Functional analysis of phosphate transporter OsPHT4 family members in rice. Rice Sci. 2020;27:493–503. [Google Scholar]
- Li Y, Huang F, Tao Y, Zhou Y, Bai A, Yu Z, Xiao D, Zhang C, Liu T, Hou X. BcGR1. 1, a cytosolic localized glutathione reductase, enhanced tolerance to copper stress in arabidopsis thaliana. Antioxidants. 2022;11:389. doi: 10.3390/antiox11020389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yang L, Luan M, Wang Y, Zhang C, Zhang B, Shi J, Zhao FG, Lan W, Luan S. A vacuolar phosphate transporter essential for phosphate homeostasis in arabidopsis. Proc Natl Acad Sci USA. 2015;112:6571–6578. doi: 10.1073/pnas.1514598112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TY, Huang TK, Yang SY, Hong YT, Huang SM, Wang FN, Chiang SF, Tsai SY, Lu WC, Chiou TJ. Identification of plant vacuolar transporters mediating phosphate storage. Nat Commun. 2016;7(1):1–11. doi: 10.1038/ncomms11095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XL, Wang L, Wang XW, Yan Y, Yang XL, Xie MY, Hu ZS, Xing AH, Lin HH, Xu GH, Yang J, Sun SB. Mutation of the chloroplast-localized phosphate transporter OsPHT2; 1 reduces flavonoid accumulation and UV tolerance in rice. Plant J. 2020;102(1):53–67. doi: 10.1111/tpj.14611. [DOI] [PubMed] [Google Scholar]
- Lu K, Wu B, Wang J, Zhu W, Nie H, Qian J, Huang W, Fang Z. Blocking amino acid transporter OsAAP3 improves grain yield by promoting outgrowth buds and increasing tiller number in rice. Plant Biotechnol J. 2018;16:1710–1722. doi: 10.1111/pbi.12907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas WJ, Wolf S. Plasmodesmata: the intercellular organelles of green plants. Trends Cell Biol. 1993;3:308–315. doi: 10.1016/0962-8924(93)90013-q. [DOI] [PubMed] [Google Scholar]
- Lyi SM, Heller LI, Rutzke MR, Welch ML, Kochian V, Li L. Molecular and biochemical characterization of the selenocysteine Se-methyltranferase gene and Se-methylselenocysteine synthesis in broccoli. Plant Physiol. 2005;138:409–420. doi: 10.1104/pp.104.056549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JF, Tamai K, Yamaji N, Mitani N, Konishi S, Katsuhara M, Ishiguro M, Murata Y, Yano M. A silicon transporter in rice. Nature. 2006;440:688–691. doi: 10.1038/nature04590. [DOI] [PubMed] [Google Scholar]
- Marmagne A, Chardon F, Havé M, Masclaux-Daubresse C. Nitrogen remobilization during leaf senescence: lessons from arabidopsis to crops. J Exp Bot. 2016;68:2513–2529. doi: 10.1093/jxb/erw365. [DOI] [PubMed] [Google Scholar]
- Martínez DE, Bartoli CG, Grbic V, Guiamet JJ. Vacuolar cysteine proteases of wheat (Triticum aestivum L.) are common to leaf senescence induced by different factors. J Exp Bot. 2007;58:1099–1107. doi: 10.1093/jxb/erl270. [DOI] [PubMed] [Google Scholar]
- Masclaux-Daubresse C, Chen Q, Havé M. Regulation of nutrient recycling via autophagy. Curr Opin Plant Biol. 2017;39:8–17. doi: 10.1016/j.pbi.2017.05.001. [DOI] [PubMed] [Google Scholar]
- McKenzie MJ, Hunter DA, Pathirana R, Watson LM, Joyce NI, Matich AJ, Rowan DD, Brummell DA. Accumulation of an organic anticancer selenium compound in a transgenic solanaceous species shows wider applicability of the selenocysteine methyltransferase transgene from selenium hyperaccumulators. Transgenic Res. 2009;18:407. doi: 10.1007/s11248-008-9233-0. [DOI] [PubMed] [Google Scholar]
- Medina D, Thompson H, Ganther H, Ip C. Se-methylselenocysteine: a new compound for chemoprevention of breast cancer. Nutr Cancer. 2001;40:12–17. doi: 10.1207/S15327914NC401_5. [DOI] [PubMed] [Google Scholar]
- Mikkelsen R, Page AL, Bingham FT. Factors affecting selenium accumulation by agricultural crops. In: Jacobs LW, editor. Selenium in agriculture and the environment. Amsterdam: Elsevier; 1989. pp. 65–94. [Google Scholar]
- Moore CA, Bowen HC, Scrase-Field S, Knight MR, White PJ. The deposition of suberin lamellae determines the magnitude of cytosolic Ca2+ elevations in root endodermal cells subjected to cooling. Plant J. 2002;30:457–465. doi: 10.1046/j.1365-313x.2002.01306.x. [DOI] [PubMed] [Google Scholar]
- Moreno-Reyes R, Suetens C, Mathieu F, Begaux F, Zhu D, Rivera MT, Boelaert M, Nève J, Perlmutter N, Vanderpas J. Kashin-Beck osteoarthropathy in rural Tibet in relation to selenium and iodine status. N Engl J Med. 1998;339:1112–1120. doi: 10.1056/NEJM199810153391604. [DOI] [PubMed] [Google Scholar]
- Moxon A, Rhian M. Loss of selenium by various grains during storage. Proc S Dakota Acad Sci. 1938;18:20–22. [Google Scholar]
- Nakamura T, Yamaguchi Y, Sano H. Four rice genes encoding cysteine synthase: isolation and differential responses to sulfur, nitrogen and light. Gene. 1999;229:155–161. doi: 10.1016/s0378-1119(99)00019-0. [DOI] [PubMed] [Google Scholar]
- Neal RH, Sposito G, Holtzclaw K, Traina S. Selenite adsorption on alluvial soils: I. Soil composition and pH effects. Soil Sci Soc Am J. 1987;51:1161–1165. [Google Scholar]
- Ng BH, Anderson JW. Synthesis of selenocysteine by cysteine synthases from selenium accumulator and non-accumulator plants. Phytochemistry. 1978;17:2069–2074. [Google Scholar]
- Ng BH, Anderson JW. Light-dependent incorporation of selenite and sulphite into selenocysteine and cysteine by isolated pea chloroplasts. Phytochemistry. 1979;18:573–580. [Google Scholar]
- Niemietz CM, Tyerman SD. New potent inhibitors of aquaporins: silver and gold compounds inhibit aquaporins of plant and human origin. FEBS Lett. 2002;531:443–447. doi: 10.1016/s0014-5793(02)03581-0. [DOI] [PubMed] [Google Scholar]
- Oropeza-Moe M, Wisløff H, Bernhoft A. Selenium deficiency associated porcine and human cardiomyopathies. J Trace Elem Med Bio. 2015;31:148–156. doi: 10.1016/j.jtemb.2014.09.011. [DOI] [PubMed] [Google Scholar]
- Otegui MS. Vacuolar degradation of chloroplast components: autophagy and beyond. J Exp Bot. 2018;69:741–750. doi: 10.1093/jxb/erx234. [DOI] [PubMed] [Google Scholar]
- Otegui MS, Noh YS, Martínez DE, Vila Petroff MG, Andrew Staehelin L, Amasino RM, Guiamet JJ. Senescence-associated vacuoles with intense proteolytic activity develop in leaves of Arabidopsis and soybean. Plant J. 2005;41:831–844. doi: 10.1111/j.1365-313X.2005.02346.x. [DOI] [PubMed] [Google Scholar]
- Palta J, Fillery I. N application enhances remobilization and reduces losses of pre-anthesis N in wheat grown on a duplex soil. Aust J Agric Resour Econ. 1995;46:519–531. [Google Scholar]
- Parrott DL, McInnerney K, Feller U, Fischer AM. Steam-girdling of barley (Hordeum vulgare) leaves leads to carbohydrate accumulation and accelerated leaf senescence, facilitating transcriptomic analysis of senescence-associated genes. New Phytol. 2007;176:56–69. doi: 10.1111/j.1469-8137.2007.02158.x. [DOI] [PubMed] [Google Scholar]
- Peng B, Kong H, Li Y, Wang L, Zhong M, Sun L, Gao G, Zhang Q, Luo L, Wang G, Xie W, Chen J, Yao W, Peng Y, Lei L, Lian X, Xiao J, Xu C, Li X, He Y. OsAAP6 functions as an important regulator of grain protein content and nutritional quality in rice. Nat Commun. 2014;5:4847. doi: 10.1038/ncomms5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilon-Smits EA, Hwang S, Mel Lytle C, Zhu Y, Tai JC, Bravo RC, Chen Y, Leustek T, Terry N. Overexpression of ATP sulfurylase in Indian mustard leads to increased selenate uptake, reduction, and tolerance. Plant Physiol. 1999;119:123–132. doi: 10.1104/pp.119.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poret M, Chandrasekar B, van der Hoorn RAL, Déchaumet S, Bouchereau A, Kim TH, Lee BR, Macquart F, Hara-Nishimura I, Avice JC. A genotypic comparison reveals that the improvement in nitrogen remobilization efficiency in oilseed rape leaves is related to specific patterns of senescence-associated protease activities and phytohormones. Front Plant Sci. 2019;10:46. doi: 10.3389/fpls.2019.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayman MP. The importance of selenium to human health. The Lancet. 2000;356:233–241. doi: 10.1016/S0140-6736(00)02490-9. [DOI] [PubMed] [Google Scholar]
- Rayman MP. Selenium in cancer prevention: a review of the evidence and mechanism of action. Proc Nutr Soc. 2007;64:527–542. doi: 10.1079/pns2005467. [DOI] [PubMed] [Google Scholar]
- Rayman MP. Selenium and human health. The Lancet. 2012;379:1256–1268. doi: 10.1016/S0140-6736(11)61452-9. [DOI] [PubMed] [Google Scholar]
- Réthoré E, Ali N, Yvin J-C, Hosseini SA. Silicon regulates source to sink metabolic homeostasis and promotes growth of rice plants under sulfur deficiency. Int J Mol Sci. 2020;21:3677. doi: 10.3390/ijms21103677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts IN, Caputo C, Kade M, Criado MV, Barneix AJ. Subtilisin-like serine proteases involved in N remobilization during grain filling in wheat. Acta Physiol Plant. 2011;33:1997–2001. [Google Scholar]
- Roberts IN, Caputo C, Criado MV, Funk C. Senescence-associated proteases in plants. Physiol Plant. 2012;145:130–139. doi: 10.1111/j.1399-3054.2012.01574.x. [DOI] [PubMed] [Google Scholar]
- Rotruck J, Pope A, Ganther HE, Swanson A, Hafeman DG, Hoekstra W. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- Sanders A, Collier R, Trethewy A, Gould G, Sieker R, Tegeder M. AAP1 regulates import of amino acids into developing Arabidopsis embryos. Plant J. 2009;59:540–552. doi: 10.1111/j.1365-313X.2009.03890.x. [DOI] [PubMed] [Google Scholar]
- Santiago JP, Tegeder M. Connecting source with sink: the role of Arabidopsis AAP8 in phloem loading of amino acids. Plant Physiol. 2016;171:508–521. doi: 10.1104/pp.16.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz K, Foltz CM. Selenium as an integral part of factor 3 against dietary necrotic liver degeneration. J Am Chem Soc. 1957;79:3292–3293. [PubMed] [Google Scholar]
- Secco D, Wang C, Arpat BA, Wang Z, Poirier Y, Tyerman SD, WuP SH, Whelan J. The emerging importance of the SPX domain-containing proteins in phosphate homeostasis. New Phytol. 2012;193:842–851. doi: 10.1111/j.1469-8137.2011.04002.x. [DOI] [PubMed] [Google Scholar]
- Shibagaki N, Rose A, McDermott JP, Fujiwara T, Hayashi H, Yoneyama T, Davies JP. Selenate-resistant mutants of Arabidopsis thaliana identify sultr1;2, a sulfate transporter required for efficient transport of sulfate into roots. Plant J. 2002;29:475–486. doi: 10.1046/j.0960-7412.2001.01232.x. [DOI] [PubMed] [Google Scholar]
- Shrift A, Ulrich JM. Transport of selenate and selenite into Astragalus roots. Plant Physiol. 1969;44:893–896. doi: 10.1104/pp.44.6.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith FW, Ealing PM, Hawkesford MJ, Clarkson DT. Plant members of a family of sulfate transporters reveal functional subtypes. Proc Nat Acad Sci USA. 1995;92:9373–9377. doi: 10.1073/pnas.92.20.9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith FW, Hawkesford MJ, Ealing PM, Clarkson DT, Vanden Berg PJ, Belcher AR, Warrilow AG. Regulation of expression of a cDNA from barley roots encoding a high affinity sulphate transporter. Plant J. 1997;12:875–884. doi: 10.1046/j.1365-313x.1997.12040875.x. [DOI] [PubMed] [Google Scholar]
- Song Z, Shao H, Huang H, Shen Y, Wang L, Wu F, Han D, Song J, Jia H. Overexpression of the phosphate transporter gene OsPT8 improves the Pi and selenium contents in Nicotiana tabacum. Environ Exp Bot. 2017;137:158–165. [Google Scholar]
- Sors TG, Ellis DR, Salt DE. Selenium uptake, translocation, assimilation and metabolic fate in plants. Photosynth Res. 2005;86:373–389. doi: 10.1007/s11120-005-5222-9. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Saito K. Subcellular localization of spinach cysteine synthase isoforms and regulation of their gene expression by nitrogen and sulfur. Plant Physiol. 1996;112:273–280. doi: 10.1104/pp.112.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Watanabe-Takahashi A, Smith FW, Blake-Kalff M, Hawkesford MJ, Saito K. The roles of three functional sulphate transporters involved in uptake and translocation of sulphate in Arabidopsis thaliana. Plant J. 2000;23:171–182. doi: 10.1046/j.1365-313x.2000.00768.x. [DOI] [PubMed] [Google Scholar]
- Tan T, Zhu W, Wang W, Li R, Hou S, Wang D, Yang L. Selenium in soil and endemic diseases in China. Sci Total Environ. 2002;284:227–235. doi: 10.1016/s0048-9697(01)00889-0. [DOI] [PubMed] [Google Scholar]
- Taylor MR, Reinders A, Ward JM. Transport function of rice amino acid permeases (AAPs) Plant Cell Physiol. 2015;56:1355–1363. doi: 10.1093/pcp/pcv053. [DOI] [PubMed] [Google Scholar]
- Terry N, Zayed A, De Souza M, Tarun A. Selenium in higher plants. Annu Rev Plant Biol. 2000;51:401–432. doi: 10.1146/annurev.arplant.51.1.401. [DOI] [PubMed] [Google Scholar]
- Thoenen M, Herrmann B, Feller U. Senescence in wheat leaves: is a cysteine endopeptidase involved in the degradation of the large subunit of Rubisco? Acta Physiol Plant. 2007;29:339–350. [Google Scholar]
- Ulrich JM, Shrift A. Selenium absorption by excised Astragalus roots. Plant Physiol. 1968;43:14–20. doi: 10.1104/pp.43.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Boekel J, Stefels W. Production of DMS from dissolved DMSP in axenic cultures of the marine phytoplankton species Phaeocystis sp. Mar Ecol Prog Ser. 1993;97:11–18. [Google Scholar]
- Van der Hoorn RA. Plant proteases: from phenotypes to molecular mechanisms. Annu Rev Plant Biol. 2008;59:191–223. doi: 10.1146/annurev.arplant.59.032607.092835. [DOI] [PubMed] [Google Scholar]
- Wang C, Huang W, Ying Y, Li S, Secco D, Tyerman S, Whelan J, Shou H. Functional characterization of the rice SPX-MFS family reveals a key role of OsSPX-MFS1 in controlling phosphate homeostasis in leaves. New Phytol. 2012;196(1):139–148. doi: 10.1111/j.1469-8137.2012.04227.x. [DOI] [PubMed] [Google Scholar]
- Wang C, Yue W, Ying Y, Wang S, Secco D, Liu Y, Whelan J, Tyerman SD, Shou H. Rice SPX-major facility superfamily3, a vacuolar phosphate efflux transporter, is involved in maintaining phosphate homeostasis in rice. Plant Physiol. 2015;169(4):2822–2831. doi: 10.1104/pp.15.01005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PJ. The pathways of calcium movement to the xylem. J Exp Bot. 2001;52:891–899. doi: 10.1093/jexbot/52.358.891. [DOI] [PubMed] [Google Scholar]
- Williams PN, Lombi E, Sun GX, Scheckel K, Zhu YG, Feng X, Zhu J, Carey AM, Adomako E, Lawgali Y, Deacon C, Meharg AA. Selenium characterization in the global rice supply chain. Environ Sci Technol. 2009;43:6024–6030. doi: 10.1021/es900671m. [DOI] [PubMed] [Google Scholar]
- Xie Q, Michaeli S, Peled-Zehavi H, Galili G. Chloroplast degradation: one organelle, multiple degradation pathways. Trends Plant Sci. 2015;20:264–265. doi: 10.1016/j.tplants.2015.03.013. [DOI] [PubMed] [Google Scholar]
- Xie Y, Liu Q, Zheng L, Wang B, Qu X, Ni J, Zhang Y, Du X. Se-Methylselenocysteine ameliorates neuropathology and cognitive deficits by attenuating oxidative stress and metal dyshomeostasis in alzheimer model mice. Mol Nutr Food Res. 2018;62:e1800107. doi: 10.1002/mnfr.201800107. [DOI] [PubMed] [Google Scholar]
- Xu L, Zhao H, Wan R, Liu Y, Xu Z, Tian W, Ruan WY, Wang F, Deng MJ, Wang JM, Dolan L, Luan S, Xue SW, Yi K. Identification of vacuolar phosphate efflux transporters in land plants. Nat Plants. 2019;5(1):84–94. doi: 10.1038/s41477-018-0334-3. [DOI] [PubMed] [Google Scholar]
- Yamada H, Kang Y, Aso T, Uesugi H, Fujimura T, Yonebayashi K. Chemical forms and stability of selenium in soil. Soil Sci Plant Nutr. 1998;44:385–391. [Google Scholar]
- Yoch DC. Dimethylsulfoniopropionate: its sources, role in the marine food web, and biological degradation to dimethylsulfide. Appl Environ Microbiol. 2002;68:5804–5815. doi: 10.1128/AEM.68.12.5804-5815.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto N, Takahashi H, Smith FW, Yamaya T, Saito K. Two distinct high-affinity sulfate transporters with different inducibilities mediate uptake of sulfate in Arabidopsis roots. Plant J. 2002;29(4):465–473. doi: 10.1046/j.0960-7412.2001.01231.x. [DOI] [PubMed] [Google Scholar]
- Yoshimoto N, Inoue E, Saito K, Yamaya T, Takahashi H. Phloem-localizing sulfate transporter, sultr1;3, mediates re-distribution of sulfur from source to sink organs in Arabidopsis. Plant Physiol. 2003;131:1511–1517. doi: 10.1104/pp.014712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Liu Z, Luo LY, Fu PN, Wang Q, Li HF. Selenium uptake and biotransformation in Brassica rapa supplied with selenite and selenate: a hydroponic work with HPLC speciation and RNA-sequencing. J Agric Food Chem. 2019;67:12408–12418. doi: 10.1021/acs.jafc.9b05359. [DOI] [PubMed] [Google Scholar]
- Zayed A, Lytle CM, Terry N. Accumulation and volatilization of different chemical species of selenium by plants. Planta. 1998;206:284–292. [Google Scholar]
- Zhang LH, Shi WM, Wang XC. Difference in selenite absorption between high- and low-selenium rice cultivars and its mechanism. Plant Soil. 2006;282:183–193. [Google Scholar]
- Zhang LH, Shi WM, Wang XC, Zhou XB. Genotypic differences in selenium accumulation in rice seedlings at early growth stage and analysis of dominant factors influencing selenium content in rice seeds. J Plant Nutr. 2006;29:1601–1618. [Google Scholar]
- Zhang L, Tan Q, Lee R, Trethewy A, Lee YH, Tegeder M. Altered xylem-phloem transfer of amino acids affects metabolism and leads to increased seed yield and oil content in Arabidopsis. Plant Cell. 2010;22:3603–3620. doi: 10.1105/tpc.110.073833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LH, Yu FY, Shi WM, Li YJ, Miao YF. Physiological characteristics of selenite uptake by maize roots in response to different pH levels. J Soil Sci Plant Nutr. 2010;173:417–422. [Google Scholar]
- Zhang LH, Hu B, Li W, Che RH, Deng K, Li H, Yu FY, Ling HQ, Li YJ, Chu CC. OsPT2, a phosphate transporter, is involved in the active uptake of selenite in rice. New Phytol. 2014;201:1183–1191. doi: 10.1111/nph.12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LH, Hu B, Deng K, Gao XK, Sun GX, Zhang ZL, Li P, Wang W, Li H, Zhang ZH, Fu ZH, Yang JY, Gao SP, Li LG, Yu FY, Li YJ, Ling HQ, Chu CC. NRT1.1B improves selenium concentrations in rice grains by facilitating selenomethinone translocation. Plant Biotechnol J. 2019;17:1058–1068. doi: 10.1111/pbi.13037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XQ, Mitani N, Yamaji N, Shen RF, Ma JF. Involvement of silicon influx transporter OsNIP2; 1 in selenite uptake in rice. Plant Physiol. 2010;153:1871–1877. doi: 10.1104/pp.110.157867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Frank T, Tan Y, Zhou C, Jabnoune M, Arpat AB, Cui H, Huang J, He Z, Poirier Y. Disruption of Os SULTR 3; 3 reduces phytate and phosphorus concentrations and alters the metabolite profile in rice grains. New Phytol. 2016;211:926–939. doi: 10.1111/nph.13969. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Jiang L. Chloroplast degradation: multiple routes into the vacuole. Front Plant Sci. 2019;10:359. doi: 10.3389/fpls.2019.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]