Abstract
Glucose is a key substrate for supporting sperm energy production and function. Previous studies have demonstrated that sperm glucose uptake is facilitated by several isoforms of the glucose transporters (GLUT). Here, we report that sperm also expresses the Na+-dependent sodium glucose cotransporter (SGLT). This was first suggested by our observation that genetic deletion of the testis-specific Na,K-ATPase α4, which impairs the sperm plasma membrane Na+ gradient, reduces glucose uptake and ATP production. Immunoblot analysis revealed the presence of an SGLT in sperm, with specific expression of isoform 1 (SGLT-1), but not of isoform 2 (SGLT-2). Immunocytochemistry identified SGLT-1 in the mid- and principal piece of the sperm flagellum. Inhibition of SGLT-1 with the isotype-selective inhibitor phlorizin significantly reduced glucose uptake, glycolytic activity, and ATP production in noncapacitated and capacitated sperm from wild-type mice. Phlorizin also decreased total sperm motility, as well as other parameters of sperm movement. In contrast, inhibition of SGLT-1 had no significant effect on sperm hyperactivation, protein tyrosine phosphorylation, or acrosomal reaction. Importantly, phlorizin treatment impaired the fertilizing capacity of sperm. Altogether, these results demonstrate that mouse sperm express a functional SGLT transport system that is important for supporting sperm energy production, motility, and fertility.
Keywords: SGLT, sperm, energetics, glycolysis, glucose uptake, sperm capacitation, sperm motility, acrosomal reaction
The Na+-dependent glucose cotransporter type 1 (SGLT-1) is expressed in mouse spermatozoa and plays an important role in sperm physiology.
Graphical abstract

Introduction
After mammalian sperm are released into the female reproductive tract, they perform the immense task of transporting and delivering the male’s genetic material to the oocyte [1]. To accomplish this, sperm must first undergo a series of biochemical and physiological changes known as capacitation [2]. Sperm capacitation is accompanied by the activation of specific plasma membrane ion transport systems, changes in electrolyte composition, and an increase in the activity of several key enzymes. This initiates a signaling cascade of protein phosphorylation [3]. Ultimately, sperm change their motility pattern to reach the hyperactivated state and undergo the exocytotic release of the acrosome contents [4, 5]. These steps only occur if sperm have an adequate supply of energy [6–8]. Accordingly, low energy production in sperm has been associated with asthenospermia and male infertility [9–11]. The close relationship between energy supply and fertility in sperm highlights the need for a better understanding of the molecular mechanisms involved in the regulation of sperm energetics and function.
Sperm obtain energy through glycolysis and oxidative phosphorylation (OXPHOS) [8, 12]. The relative contribution of aerobic and anaerobic pathways varies between species; with mouse sperm relying on glycolysis as the primary energy producing pathway. Pharmacological inhibition or genetic knockdown of glycolytic enzymes results in varying degrees of infertility due to impaired energy production [7, 13–15]. However, mouse sperm are energetically flexible and can alternate between glycolysis and OXPHOS depending on substrate availability [16].
As sperm traverse the female reproductive tract, they are exposed to high levels of glucose in the uterus [17, 18]. Accordingly, glucose uptake increases during capacitation and this glucose is important for proper sperm movement, protein tyrosine phosphorylation, and hyperactive motility [8, 19]. Glucose is transported into the sperm cytoplasm through facilitated diffusion via glucose transporters (GLUTs). Several variants of GLUTs exist in spermatozoa; including the GLUT 1, 2, 3, 8, 9a, and 9b isoforms, which present a distinct pattern of expression in the principal and mid-piece of the flagellum, as well as in the sperm head [20]. Aside from the GLUT proteins, the sodium glucose cotransporter (SGLT) is another transport system that also participates in glucose uptake in different cell types. SGLT belongs to the SLC5 family of transporters and is expressed as two main isoforms in mammals, SGLT-1 and SGLT-2 [18]. A third isoform, SGLT-3, acts as a glucose sensor and does not transport either glucose or Na+ [21, 22]. Both SGLT-1 and SGLT-2 are symporters, which utilize the inward electrochemical sodium gradient to drive the secondary active transport of glucose into the cell. However, while SGLT-1 transports two Na+ per each glucose internalized, SGLT-2 only transports one. Structurally, SGLT-1 and SGLT-2 are transmembrane proteins, with hydrophilic intra- and extracellular regions that contain the binding site for Na+ and glucose [23]. SGLT-2 plays an essential role in glucose reabsorption in the kidney, where it is expressed in the apical membrane of the renal tubular cells. SGLT-1 is also found in the kidney but is mainly expressed in the brush border of the intestinal epithelium, where it is important for the absorption of glucose in the gut [21, 24]. The dependency of SGLT on Na+ is what allows the effective glucose uptake by this transporter and functionally distinguishes SGLT from the GLUT proteins. The maintenance of an appropriate Na+ gradient across the plasma membrane is essential for SGLT function and depends on the activity of the Na,K-ATPase. The link between SGLT and the Na,K-ATPase has been well established in tissues such as the kidney and small intestine [25, 26].
The Na,K-ATPase is an integral protein of the plasma membrane of most mammalian cells, which exchanges 3 Na+ from the cell cytoplasm for 2 K+ from the extracellular space. This ion translocation is coupled to the hydrolysis of ATP, catalyzed by the enzymatic action of Na,K-ATPase [27, 28]. Na,K-ATPase is composed of two primary polypeptides, the α and β subunits, both of which are expressed as different isoforms [27, 29]. We and others have shown that two α polypeptides are expressed in sperm: the ubiquitous α1 subunit and the testis-specific α4 isoform. Na,K-ATPase α4 isoform (ATP1A4) is found primarily in the flagellum of differentiated sperm and exhibits unique functional properties [30, 31]. Deletion of the ATP1A4 gene results in complete infertility of male, but not female mice. This is due to a severe reduction in total and hyperactive sperm motility. In addition, ATP1A4 knockout (KO) mice show increased intracellular Na+ and a depolarized plasma membrane, demonstrating the important role that the Na,K-ATPase α4 transporter plays in maintaining the transmembrane Na+ gradient in sperm [32].
By exploring additional processes that could depend on the Na+ gradient created by Na,K-ATPase α4, we discovered that sperm express a functional SGLT. Prior to this study, the presence of a Na+-dependent glucose transporter had only been shown in canine sperm. However, this work did not investigate the expression of different SGLT isoforms or whether SGLT is functionally relevant for sperm physiology [33]. We found that mouse sperm express SGLT, and specifically the SGLT-1 isoform of this transporter. Our studies revealed that SGLT-1 is important for sperm function by mediating glucose uptake, glycolytic activity, ATP production, and by supporting motility and fertility of these cells.
Results
Sperm devoid of Na,K-ATPase α4 exhibit lower glucose uptake and ATP levels
Our lab previously generated a mouse model in which the ATP1A4 gene that encodes for Na,K-ATPase α4 was deleted. Sperm from these mice display elevated levels of intracellular Na+ and alterations in several functional parameters associated with the reduction of the transmembrane Na+ gradient [32]. Here, we explored whether deletion of ATP1A4 had any effect on sperm glucose uptake. Glucose uptake was measured in sperm from ATP1A4 KO and wild-type (WT) mice, using the fluorescent glucose analog, 6-(N-(7-Nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-6-Deoxyglucose (6-NBDG). As shown in Figure 1A, non-capacitated (NC) sperm from ATP1A4 KO mice displayed significantly reduced glucose uptake compared with WT sperm, with an overall decrease of ~25%.
Figure 1.
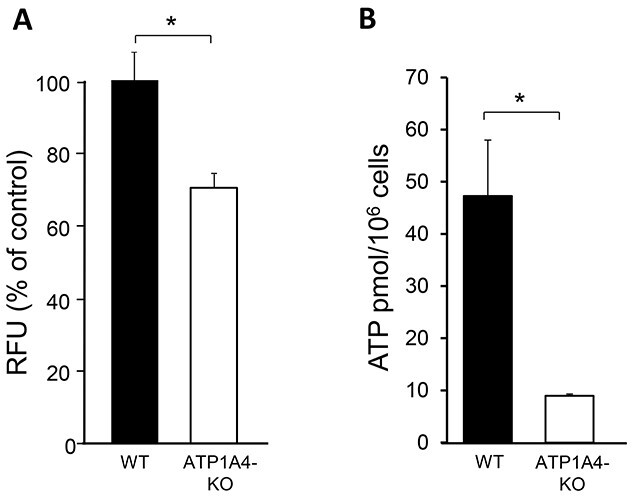
Sperm from ATP1A4 KO mice display decreased glucose uptake and ATP levels compared with WT sperm. (A) Cells were collected in NC media and glucose uptake was measured using the fluorescent glucose analog, 6-NBDG. Results were expressed as relative fluorescent units (RFUs) as a percent of the WT controls. (B) ATP content was determined using an ATP Determination Kit. Experimental samples were compared with the standard using a Biotek plate reader and normalized to cell number. The data are expressed as the mean ± SEM of three different experiments. Asterisks indicate statistically significant differences, with *P < 0.05.
To assess whether the reduced internalization of glucose was correlated with impaired energetics in ATP1A4 KO sperm, we measured ATP levels in NC sperm from WT and ATP1A4 KO mice, using an ATP Bioluminescent Assay. As shown in Figure 1B, sperm lacking ATP1A4 exhibited almost a 5-fold decrease in ATP content compared with WT sperm. These results indicate a link between the function of Na,K-ATPase α4 and glucose internalization in sperm. Since Na,K-ATPase α4 is not directly involved in glucose transport, these data suggest that a Na+-dependent glucose uptake system is operational in sperm and that disruption of the transmembrane Na+ gradient, due to Na,K-ATPase α4 deletion, affects glucose transport.
SGLT-1 is expressed in mouse sperm
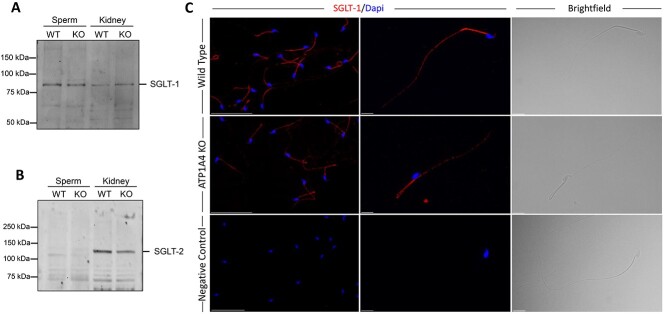
We explored the potential presence of a Na+-dependent glucose uptake system in mouse sperm, by determining the expression of SGLT-1 and SGLT-2 using immunoblot analysis. We found that SGLT-1 is present in mouse spermatozoa. The SGLT-1 protein expressed in sperm migrated at a relatively similar size to that of the kidney SGLT-1, which was used as a control (Figure 2A). In contrast to SGLT-1, we were unable to detect expression of SGLT-2 (Figure 2B). In addition, we determined the expression of SGLT-1 in sperm from ATP1A4 KO mice. When normalized to total protein levels, the amount of SGLT-1 was similar between sperm from ATP1A4 KO and WT mice (data not shown).
Figure 2.
SGLT-1 is expressed in WT and ATP1A4 KO sperm. (A, B) Protein from WT and ATP1A4 KO sperm was isolated and separated on a SDS/polyacrylamide gel. Membranes were probed with antibodies against SGLT-1 or SGLT-2 and a horseradish peroxidase secondary antibody. A kidney protein homogenate was used as a positive control for SGLT-1 and SGLT-2. (C) Sperm from WT and ATP1A4 KO mice were probed with SGLT-1 antibody followed by a goat-anti rabbit Alexa 594 conjugated secondary antibody. Cells were co-stained with DAPI and imaged using lower and higher magnification. The scale bars on the left panels represents 50 μM, while the scale bar on the middle and right panels represents 10 μM.
We were also able to identify SGLT-1 by immunocytochemical analysis. SGLT-1 was found to be localized to the sperm flagellum, with expression in the mid- and principal piece (Figure 2C). This observation was confirmed using another antibody against SGLT-1, which recognizes a different epitope within the protein (data not shown). We also found some sporadic SGLT-1 label at the sperm head. This appeared in only few cells and at levels much lower than those found in the sperm flagellum. In contrast to SGLT-1, we were unable to detect SGLT-2 in our immunocytochemical analysis of isolated sperm (data not shown).
Altogether, these results demonstrate that mouse sperm express isoform 1 of the Na+-dependent glucose cotransporter, but not the SGLT-2 isoform.
SGLT-1 contributes to sperm glucose uptake, glycolytic activity, and ATP production
To determine whether SGLT-1 function is important for supporting sperm energetics, we studied the contribution of this transporter to different parameters that are relevant indicators of energy production in sperm. We started by measuring the overall glucose uptake in sperm from WT mice, using the fluorescent nonhydrolyzable glucose analog, 6-NBDG, and flow cytometry [34]. Experiments were performed under NC and capacitated conditions (CAP) and in the presence or absence of the SGLT-1 inhibitor, phlorizin. This compound is a plant derivative that competes with glucose for its binding site in SGLT, thus inhibiting its transport [35]. Two different concentrations of phlorizin were used (10 and 100 μM), which are known to effectively inhibit SGLT-1 transport in other cell types [36–38]. Propidium iodide (PI) was included as a viability marker to exclude dead cells. Glucose uptake by SGLT was determined by the mean fluorescent intensity of 6-NBDG in PI negative cells and that were sensitive to phlorizin. As observed in Figure 3, 100 μM phlorizin significantly decreased glucose uptake in the NC (Figure 3A) as well as in the capacitated (Figure 3B) sperm, by ~25 and 15%, respectively. The noninhibitable component of glucose uptake in the cells is independent of SGLT function and is likely due to the activity of the GLUT transporters [20]. These results show that in addition to glucose uptake via GLUTs, sperm can also bring glucose into the cell through the Na+-dependent glucose cotransporter, SGLT-1, under both NC and CAP conditions.
Figure 3.
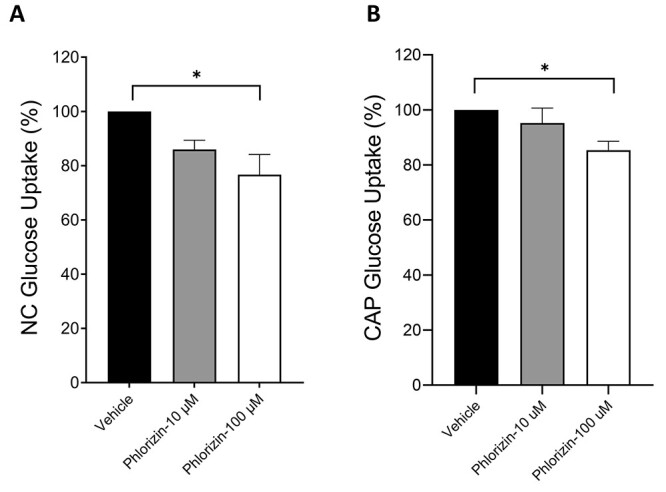
The SGLT-1 inhibitor, phlorizin, reduced glucose uptake in NC and CAP WT sperm. (A, B) WT sperm were collected in NC or CAP media containing no glucose and incubated with either 10 or 100 μM phlorizin before adding 6-NBDG (100 μM). PI (30 nM) was added immediately before flow cytometric analysis using the BD-LSR II. Data were expressed as the percentage of the mean fluorescent intensity (MFI) of the vehicle control. Results are shown as mean ± SEM of three different experiments. Asterisks indicate a statistically significant difference, with *P < 0.1.
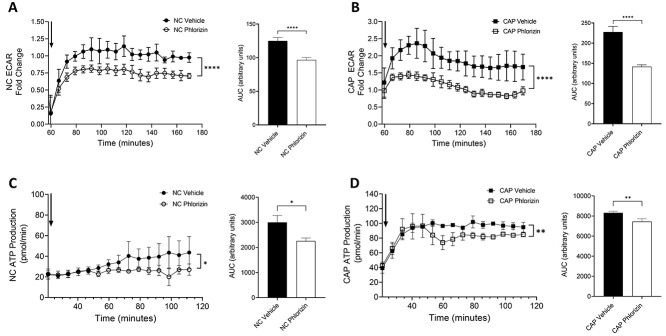
To determine whether the glucose uptake mediated by SGLT-1 contributes to the production of energy in sperm, we measured glycolytic activity in the cells using a Seahorse Extracellular Flux (XF) Analyzer. Assays were performed in NC and CAP sperm from WT mice and in the absence or presence of 100 μM phlorizin. Glycolytic activity was assessed as the extracellular acidification rate (ECAR) of the media due to the release of H+ from the conversion of pyruvate to lactate. As shown in Figure 4A, ECAR was significantly reduced when NC sperm were treated with phlorizin. Similar results were obtained for CAP sperm (Figure 4B). By quantifying and comparing the area under the curve (AUC) between control samples and those treated with phlorizin, we found that SGLT-1 inhibition caused a greater reduction in the ECAR in CAP than in NC sperm, over the 110-min incubation period tested (Figure 4A and B). These results indicate that the glucose uptake mediated by SGLT-1 is an important contributor to the glycolytic activity of sperm.
Figure 4.
SGLT-1 inhibition reduces ECAR and ATP production of NC and CAP sperm. (A, B) Sperm were swam up and plated in a 96-well plate at a concentration of 1 × 106 per well. The plate was sequentially injected with a mock media injection (Port A), vehicle or phlorizin (Port B), and NC or CAP media, containing db-cAMP/IBMX (Port C). The ECAR data presented on the graph corresponds to the values collected 110 min after the last injection, as indicated by the black arrow (Port C). Each data point is represented as the fold change from the mean of the three data points before the last injection. The data were quantified as the AUC. AUCs were compared using an unpaired t-test. The data shown represent the mean ± SEM of four different experiments. Asterisks indicate significance, with ****P < 0.0001. (C, D) Sperm were swam up in NC 5 mM HEPES, no glucose media and then preincubated ±100 μM phlorizin. Cells were then plated and subjected to a mock injection (Port A) and then media containing 5.6 mM glucose ± db-cAMP/IBMX (Port B). The arrow represents the Port B injection. The data were expressed as the AUC, and analyzed using an unpaired t-test. The data represent the mean ± SEM of three different experiments. Asterisks indicate statistically significant differences, with **P < 0.01 and *P < 0.05.
The continuous production of ATP is essential for sperm function, so to determine whether SGLT-1-mediated glucose uptake influences energy production in sperm, we measured ATP production using the Seahorse XF Analyzer ATP Rate Assay. This method provides real-time quantification of ATP production, allowing the discrimination of ATP produced from glycolysis or mitochondrial OXPHOS. Figure 4C and D shows the time course of ATP production in sperm under NC and CAP conditions, respectively. AUC quantification showed that SGLT-1 inhibition significantly decreased the glycolytic production of ATP in NC and CAP spermatozoa (Figure 4C and D). These results further confirm that SGLT-1 mediated glucose uptake is important for the glycolytic production of ATP in sperm.
SGLT-1-mediated glucose uptake is important for supporting sperm motility
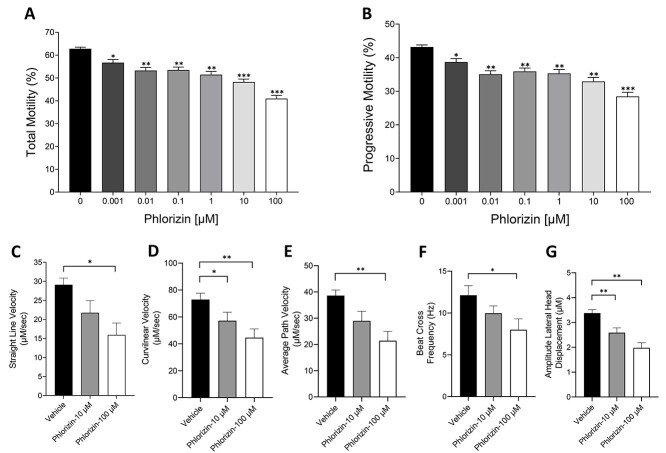
Sperm motility is an important marker of sperm function and is highly dependent on ATP production. In mice, glycolysis appears to be the primary pathway that supplies the energy needed for sperm movement. Thus, deletion or inhibition of glycolytic enzymes has a more drastic effect on sperm function than mitochondrial inhibition, and renders sperm infertile [39]. To determine whether SGLT function supports sperm motility, we assessed the effect of phlorizin on sperm movement using computer-assisted sperm analysis (CASA). Sperm were treated with phlorizin concentrations ranging from 1 nM to 100 μM and total and progressive motility, as well as different parameters of sperm motility, were determined (Figure 5). Increasing concentrations of phlorizin caused a dose-dependent decrease in sperm motility. Treatment with 10 and 100 μM phlorizin caused a significant reduction in total and progressive sperm motility by ~25 and ~35%, respectively (Figure 5A). Similarly, several other sperm motility parameters were affected by SGLT-1 inhibition, including straight line velocity (VSL), curvilinear velocity (VCL), average path velocity (VAP), beat cross frequency (BCF), and amplitude of lateral head displacement (ALH) (Figure 5C–G). We also determined the effect of SGLT-1 inhibition on sperm motility in medium containing methylcellulose, which more closely resembles the viscous environment of the female reproductive tract [40]. Under this condition, phlorizin-treated sperm also displayed a significant reduction in total and progressive motility compared with the vehicle-treated control (Supplemental Figure S1). This demonstrates that the function of SGLT-1 is important for supporting full motility of these cells.
Figure 5.
SGLT-1 inhibition causes a significant reduction in sperm motility parameters. (A, B) WT sperm were treated in the absence and presence of different concentrations of phlorizin (1–100 μM) and total and progressive motility were measured using CASA. (C–G) WT sperm were treated with 10 or 100 μM phlorizin and VSL, VCL, VAP, BCF, and ALH were measured. The data are expressed as the mean ± SEM of three separate experiments. Asterisks indicate significance, with **P < 0.01, **P < 0.01 and *P < 0.05.
As a secondary active transporter, SGLT-1 requires a steep transmembrane Na+ gradient to transport glucose into the cytoplasm. To confirm that the glucose uptake occurring through SGLT-1 was Na+ dependent, we performed motility experiments in the absence of extracellular Na+. In media containing no Na+, sperm were still motile (Figure 6A and B), albeit at a lower degree than in the presence of Na+ (Figure 5A and B). In media without Na+, treatment with 10 and 100 μM phlorizin did not affect either total or progressive sperm motility (Figure 6A and B). These results demonstrate that SGLT-1 is not functional in the absence of Na+ and that the transmembrane Na+ gradient is important for SGLT-1 glucose uptake and sperm motility. To further explore whether the decreased motility caused by phlorizin was due directly to a decrease in glucose uptake, we investigated the effect of phlorizin on sperm motility in the absence of glucose in the medium. Under glucose-deprived conditions, only a minimal or no effect on total and progressive sperm motility was observed in the presence of 10 and 100 μM phlorizin, respectively (Figure 6C and D). This is in agreement with the idea that glucose uptake in sperm is driven, at least in part, through SGLT-1.
Figure 6.
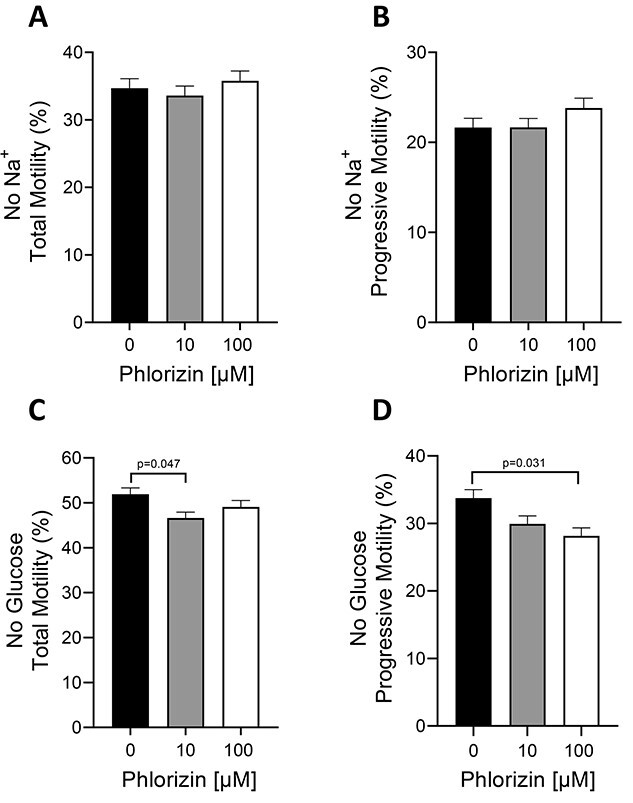
Phlorizin has minimal or no effect on sperm motility in the absence of Na+ or glucose. (A–D) WT sperm were treated with ±10 and 100 μM phlorizin for 1 h in Whitten’s media containing no Na+ (A, B) or in Whitten’s media containing no glucose (C, D). Sperm total and progressive motility were assessed using CASA. Results are expressed as the mean ± SEM of three individual experiments. P-values are indicated on top of bars.
Role of SGLT-1 on sperm capacitation and fertility
To investigate the potential role of SGLT-1 in sperm capacitation, we assessed several events known to be associated with this process. During capacitation, activation of the sperm soluble adenylyl cyclase results in a downstream wave of protein phosphorylation [41]. We focused our attention on whole cell protein tyrosine phosphorylation in WT sperm treated in the presence or absence of 10 and 100 μM phlorizin. After 90 min of capacitation, we found that phlorizin treatment had no significant effect on the level or pattern of sperm protein phosphorylation (Figure 7A and B).
Figure 7.

SGLT-1 inhibition does not significantly affect sperm capacitation. (A) WT sperm were collected and incubated with 10 or 100 μM phlorizin for 90 min in CAP media. Protein was isolated and run on a SDS/polyacrylamide gel. Membranes were probed with p-Tyr. For normalization, the blot was stripped and reprobed with α-Tubulin. (B) Quantification of the blots was performed using Image J software and results were expressed as percent of the signal obtained in the CAP medium. The data represent the mean ± SEM of three different experiments, NC and CAP. (C) WT sperm were collected and treated with 10 or 100 μM phlorizin for 90 min in capacitation media and hyperactive motility was assessed using CASA. (D) WT sperm were collected and incubated with 10 or 100 μM phlorizin for 90 min in capacitation media. Sperm were stained with FITC-PNA and imaged using the Olympus IX-81 microscope. At least 300 cells were counted for each condition. Graphs represent the mean ± SEM of three different experiments.
We also tested the effect of phlorizin on sperm hyperactivation. After treating the cells with or without phlorizin and inducing capacitation, we measured hyperactivation using CASA. SGLT-1 inhibition had no effect on the ability of sperm to undergo the hyperactivated pattern of motility (Figure 7C).
Finally, we measured the effect of SGLT inhibition on sperm acrosomal reaction by labeling the sperm acrosome with fluorescein isothiocyanate (FITC)-conjugated peanut agglutinin (PNA). Representative images of the cells are shown in Supplemental Figure S2), while quantification of the data from different experiments is presented in Figure 7D. As shown, after sperm incubation in capacitating media, there was a decrease in the number of cells that stained positive for FITC-PNA, consistent with an increase in the number of acrosome reacted sperm. Treatment with phlorizin at 10 and 100 μM had no significant effect on the number of cells that underwent the acrosomal reaction, displaying similar levels of FITC-PNA staining as compared with the untreated controls (Figure 7D and Supplemental Figure S S2). Altogether, these experiments suggested that the glucose uptake occurring via SGLT-1 is not a requirement for supporting the biochemical and physiological changes typically associated with sperm capacitation.
To further investigate the potential relevance of SGLT-1 for sperm fertility, we performed in vitro fertilization assays (IVF), using sperm from WT mice treated with ±10 or 100 μM phlorizin. Fertilization was assessed by quantifying the number of two-cell embryos that developed after overnight coincubation of the oocytes and sperm. Figure 8A shows representative images of the fertilized/unfertilized eggs, while Figure 8B shows the quantification of the compiled experiments. The number of fertilized two-cell embryos vs the unfertilized one-cell eggs was higher in the vehicle-treated sperm than in the phlorizin-treated sperm. Conversely, the number of one-cell unfertilized eggs vs the two-cell embryos was greater in the phlorizin-treated sperm compared with the vehicle-treated controls (Figure 8B). These results suggest that the function of SGLT-1 is important for the fertilizing capacity of sperm.
Figure 8.
SGLT-1 inhibition affects sperm fertility in vitro. WT sperm collected from male mice were treated with ±10 or 100 μM phlorizin and incubated with oocytes for several hours. Oocytes were then washed and cultured overnight at 37 °C. Light microscopic images were taken of the embryos the following day (A). Fertilization was determined by the number of embryos that had developed to the two-cell stage (B). The data represent two separate experiments. For each experiment, sperm were collected from four male mice, and oocytes from two to three females. Statistical significance was determined using a chi-square test, with **P < 0.01.
Discussion
Previous work has established the presence of several GLUT isoforms in mouse spermatozoa [20]. In this work, we show that mouse sperm express another transporter involved in the uptake of glucose, SGLT-1. To our knowledge, there has only been one report that described the presence of a SGLT in dog spermatozoa [33]. However, that work did not investigate the presence of other SGLT isotypes or their role in sperm. Here, we found that mouse sperm express SGLT-1, but not SGLT-2.
SGLT-1 is mainly found in the sperm flagellum, where it is present in both the flagellar mid- and principal piece. The sperm flagellum is the primary site of ATP production in sperm, with most glycolytic enzymes anchored to the fibrous sheath in the principal piece [42]. Thus, the localization of SGLT-1 coincides with the role of the transporter in delivering glucose directly to the glycolytic machinery of sperm. Additionally, SGLT-1 expression overlaps with that of Na,K-ATPase α4, which is also found in the flagellum and primarily the sperm mid-piece. The shared localization of SGLT-1 and ATP1A4 may facilitate the functional coupling of both transporters to catalyze Na+-dependent glucose movement into the cytoplasm.
Our results also show that expression levels of SGLT-1 are comparable between sperm from WT and ATP1A4 KO mice. This suggests that the decrease in glucose uptake observed in sperm devoid of Na,K-ATPase α4 is not due to reduced expression levels of SGLT-1, but rather the decline in the Na+-transmembrane gradient that drives SGLT-1 function. Moreover, our finding that phlorizin was ineffective in reducing sperm motility in media without Na+ provides additional evidence for a functional SGLT in spermatozoa.
Interestingly, sperm express two isoforms of the Na,K-ATPase. Our results show that deletion of Na, K-ATPase α4 is sufficient to reduce glucose uptake and ATP production in sperm. This indicates the functional association between SGLT-1 and Na,K-ATPase α4. While we cannot discard the participation of Na, K-ATPase α1 in SGLT-1 function, Na, K-ATPase α4 possesses unique functional properties with respect to Na+ that make it best suited to regulate SGLT-1 in sperm. Na,K-ATPase α4 has a significantly higher affinity for Na+ than Na,K-ATPase α1 [30]. This allows Na,K-ATPase α4 to be the main controller of sperm intracellular Na+ levels and maintain a steeper Na+ gradient between the cell cytoplasm and the environment. This gradient provides the chemical force that drives SGLT-1 activity.
While SGLT-1 is not the only glucose uptake system in sperm, its contribution to glucose movement into the cell is relevant. This can be concluded from our experiments using genetic ablation of Na,K-ATPase α4 and the pharmacological inhibition of SGLT-1 with phlorizin, which showed a decrease in glucose transport, ranging from one-fourth to one-seventh of the total glucose uptake in NC and CAP sperm, respectively. This shows that while SGLT-1 is important for glucose uptake, most of the glucose enters sperm via Na+ independent mechanisms, presumably through the well-described GLUT proteins [20]. Nevertheless, the glucose uptake associated with Na+ is relevant to sperm energetics and function. This is not surprising considering that glucose is the preferred energy substrate for ATP generation in mouse spermatozoa [12, 43–45].
The role of SGLT in sperm energetics is supported by the reduction in ATP levels (~80%) that we observe in sperm from ATP1A4 KO mice. This decrease in cellular ATP is relatively more pronounced than the ~25% reduction in Na+-dependent glucose uptake that we measured in these KO sperm. This discrepancy could be due to the different information that the glucose uptake and ATP assays provide. Thus, glucose uptake is a direct indicator of transport of the sugar at the specific time of the assay, while ATP levels reflect the accumulated changes in this energy substrate over time. In agreement with this idea are the experiments in which ATP production was measured continuously in WT sperm treated with phlorizin. Therefore, the discrepancy in the magnitude of the changes may just reflect differences between an acute vs a chronic impairment of SGLT-1 function. Alternatively, sperm from the ATP1A4 KO mouse may also exhibit lower ATP levels not only due to impaired glycolysis, but also a deficiency in OXPHOS, which could be a consequence of the electrochemical disturbance observed in ATP1A4 KO sperm [46]. Future experiments are planned to follow up on this hypothesis.
In addition, the reduction in ATP content that we observed in sperm from the ATP1A4 KO mouse is more pronounced than the decrease in ATP caused by inhibition of SGLT-1 with phlorizin. This again may reflect the more drastic and long-lasting effect that the genetic deletion of ATP1A4 has compared with the more acute pharmacological model. In any case, our results show that there is a correlation between Na+-dependent glucose uptake and sperm energetics, which supports the presence of a functional SGLT in mouse sperm.
Measurements with the Seahorse XF Analyzer also indicated that SGLT-1-mediated glucose uptake is important for supporting the glycolytic production of ATP in both NC and CAP sperm. The basal ECAR readings that we observed are in agreement with previous measurements reported by Balbach et al. [47]. The phlorizin-sensitive generation of ATP through glycolysis reinforces the physiological relevance of SGLT-1 for glucose transport and sperm metabolism. The decrease in ECAR that we observe in the presence of phlorizin shows that SGLT-1 contributes to ~1/3 to 1/5 of the total sperm ATP derived from glycolysis in NC and CAP sperm, respectively. Despite this difference, the absolute values of ATP levels generated were higher in the CAP than in the NC state.
The decrease in ECAR and ATP production is more pronounced in phlorizin-treated CAP vs NC sperm. This could suggest that SGLT function is more important during sperm capacitation. However, the decrease in glucose uptake in sperm treated with phlorizin displays an opposite trend, with the NC sperm showing a greater sensitivity to SGLT-1 inhibition than CAP sperm. Again, these discrepancies are likely due to differences in the information provided by the Seahorse XF Analyzer, which shows additive effects, vs the glucose uptake which provides an endpoint measurement.
Our findings are physiologically relevant in the context of the environments of male and female reproductive tracts. In contrast to the epididymis where sperm are quiescent, the female reproductive tract is rich in glucose and contains a much higher concentration of Na+. These conditions are optimal for SGLT-1-mediated glucose transport, where the high extracellular Na+ creates a steep Na+ transmembrane gradient that SGLT-1 can utilize to bring glucose into the cytoplasm. Thus, it is likely that SGLT-1 plays an important role as sperm transit through the female reproductive tract and need to increase glucose uptake and energy production to support their arduous journey. Additionally, SGLT-1 activity is known to be subjected to regulatory mechanisms that are different from that of the GLUT proteins [24]. Therefore, glucose uptake through SGLT-1 could provide an alternate pathway for glucose internalization, which is subjected to regulation distinct from that of the GLUT proteins [48]. This could represent a refined mechanism to adjust glucose uptake and metabolism to the sperm’s energetic needs.
We also show that SGLT-1 is involved in an essential parameter of sperm function, sperm motility. Phlorizin caused a decrease in total, as well as several aspects of sperm motility, including progressive motility, VCL, VSL, VAP, BCF, and ALH. This impairment in motility was observed not only in the aqueous medium, but in medium of higher viscosity, which is closer in fluidity to the environment of the female genital tract. The reduction in motility produced by phlorizin reached ~30% in both media conditions. While one may expect that the higher resistance of the high viscosity medium would have unveiled a greater effect of phlorizin, it is possible that the overall contribution of SGLT-1 to sperm motility reaches a maximum, with the remaining motility being supported by other glucose uptake mechanisms, such as the GLUTs. In any case, our experiments indicate a clear role of SGLT-1 in supporting sperm movement. Experiments in the absence of Na+ showed that phlorizin has no effect on sperm motility. This indicates that when the transmembrane Na+ gradient is impaired, SGLT-1 is nonfunctional, and this precludes the effects of phlorizin on sperm motility. These results further support the dependency of SGLT-1 on the transmembrane Na+ gradient. The capacity of sperm to sustain normal levels of motility in the absence of Na+ also supports the notion that sperm can use other proteins to catalyze the Na+ independent transport of energetic substrates into the cytoplasm. Also, in media containing no glucose, minimal effect of phlorizin on sperm motility was observed. Without the transported substrate glucose, SGLT-1 function is diminished, negating any effect of phlorizin on glucose uptake. This further confirmed that glucose uptake via SGLT-1 is a key contributor to sperm motility.
While blockage of SGLT-1 affected sperm movement, phlorizin has no significant effect on sperm hyperactive motility or on protein tyrosine phosphorylation, both events known to accompany sperm capacitation. In addition, phlorizin does not modify the number of cells that undergo acrosomal reaction. This could be due to differences in the amount of energy required for motility vs capacitation. Although phlorizin reduced glycolytic activity and ATP production in CAP sperm, it was not completely diminished. It is possible that the remaining glycolytic activity is enough to support capacitation, whereas motility requires maximal levels of glycolytic activity. The fact that motility is dependent on SGLT-1 also coincides with the idea that glucose is the primary fuel for sperm movement [10–19]. Therefore, SGLT-1 activity in sperm may be harnessed to serve specific functional demands that require a greater amount of energy.
Despite our observations that phlorizin did not affect sperm capacitation, we found that it caused a significant reduction in the fertilizing capacity of WT sperm. It is possible that other factors, such as the combined effects of SGLT-1 inhibition on sperm glucose uptake, glycolytic activity, energy production, and motility, affect the ability of sperm to bind and fertilize the oocyte.
In conclusion, our studies identified for the first time the expression and function of SGLT-1 as an additional pathway for glucose entry in mouse sperm. The presence of SGLT along with the GLUT proteins in sperm is not a redundancy, but rather expands the diversity of transporters in the sperm plasma membrane, providing them an additional mechanism with different functional properties to better serve the function and energetic demands of these cells. These findings also further advance our understanding of the molecular mechanisms underlying sperm physiology and men’s reproductive health.
Methods
Reagents
Reagents for Whitten’s Media were obtained from Sigma-Aldrich. SGLT-1 antibody was purchased form Millipore Sigma (07-1417) and Thermofisher (PA5-77460). The p-Tyr antibody was purchased from Millipore Sigma. PI, 6-NBDG, concanavalin A, antimycin A, rotenone, oligomycin, and 3-isobutyl-1-methylxanthine (IBMX) were obtained from Sigma-Aldrich, phlorizin from Cayman Chemical, dibutyl cAMP from Selleckchem, and α-tubulin antibody from Cell Signaling Technologies. The SGLT-1 antibody against the extracellular epitope was obtained from Thermofisher (PA5-77460).
Sperm isolation
All animal experiments were approved by the University of Kansas Medical Center Institutional Animal Care and Use Committee. Adult 12–20 week old C57Bl/6 J WT and ATP1A4 KO mice were euthanized and the right and left cauda were placed in 37 °C NC Whitten’s media to obtain the swim up, as previously described [46]. Sperm concentration was assessed before all experiments. Spermatozoa were incubated in either NC Whitten’s media containing 100 mM NaCl, 4.7 mM KCl, 1.2 mM KH2 PO4, 1.2 mM MgSO4, 5.5 mM glucose, 0.8 mM pyruvic acid, 4.8 mM lactic acid, 20 mM hepes, and 1.7 mM CaCl or CAP Whitten’s media supplemented with 12.5 mM sodium bicarbonate and 2.5 mg/ml bovine serum albumin (BSA). Sperm were incubated in capacitated media for ~90 min in 5% CO2 at 37 °C. In some experiments, the incubation medium contained no sodium or glucose to assess the dependency of these ligands on SGLT-1 function. To eliminate contaminating sodium, sperm were swam up in medium in the absence of the cation, washed once by centrifugation at 1800 revolutions per minute (RPM), and resuspended in Whitten’s medium without sodium.
Immunoblot
WT and ATP1A4 KO SGLT-1 protein expression was analyzed via sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE; 8% gel) and immunoblotting, as described previously. Membranes were probed using the polyclonal SGLT-1 antibody (Millipore Sigma, 07-1417) and polyclonal SGLT-2 antibody (Abcam, ab37296) [32]. For whole cell phosphorylation experiments, sperm were lysed using 1 mM orthovanadate, 1 mM EDTA, 1 mM fluoride, PhosStop (Roche), 1% NP-40, 1% deoxycholate, and 0.1% SDS. Samples were ran on a 10% SDS-PAGE, and transferred to nitrocellulose. Immunoblot was performed as described in ref. [49]. Briefly, blocking of unspecific binding was performed with 5% BSA in Tris buffered saline. Incubation with the anti-p-Tyr antibody (G410, Millipore-Sigma) was performed overnight at 4 °C. After washing with TBST, a horseradish peroxidase-conjugated secondary antibody was applied for 1 h. Chemiluminescence was used for detection. Blots were stripped and reprobed with α-tubulin, which was used as a loading control.
Immunocytochemistry
For SGLT-1 staining, WT and ATP1A4 KO sperm were swam up in NC media and washed in saline three times by centrifuging at 1000 RPM. The pellet was resuspended in 500 μl of 4% paraformaldehyde for 15 min. Sperm were washed with saline and ~10 μl of the sperm suspension was air-dried on a clean slide. Cells were permeabilized with 0.3% Triton-X for 10 min, washed, and blocked in 2% BSA for 1 h at room temperature. SGLT-1 (1:500) antibody (Thermofisher, PA5-77460) in 2% BSA was added to slides and incubated overnight at 4 °C in a humid chamber. The next day, slides were incubated with goat anti-rabbit Alexa Flour 594 conjugated secondary antibody (1:1000) for 1 h and washed with phosphate-buffered saline (PBS). The slides were air dried and mounted with 10 μl of DAPI. For acrosome staining, WT sperm were swam up in NC media, incubated in either NC or CAP media ±10 or 100 μM phlorizin, centrifuged at 1000 RPM, and resuspended in PBS. After 10 μl of sperm suspension was air dried on a clean slide, cells were incubated with 50 μl of FITC-PNA at 37 °C for 30 min in the dark, washed with PBS, and mounted using DAPI as a nuclear counterstain. All slides were imaged using a fluorescence microscope (Olympus IX-81, Japan). Approximately 300 cells were counted per condition.
ATP content
Intracellular ATP content in sperm lysates was measured using the ATP Determination Kit (A22066, ThermoFisher, Waltham, MA) following the manufacturer’s instructions. Briefly, sperm were collected as previously described and flashed frozen in liquid nitrogen. Aliquoted (100 μl) samples were thawed on ice, centrifuged at 16000 × g for 20 min at 4 °C, and the supernatant was used for the assay. Working in the dark, a standard reaction solution (8.9 ml dH2O, 0.5 ml 20x Reaction Buffer, 0.1 ml 0.1 M DTT, 0.5 ml 10 mM D-luciferin, and 2.5 μl 5 mg/ml firefly luciferase) was aliquoted into a 96-well plate and warmed to 37 °C for 5 min. ATP (for the standard) and the sample were added to the reaction solution and measurements were made using a BioTek Synergy HT plate reader (Bio-Tek, Winooski, VT). The ATP content was normalized to pmol/106 cells.
Sperm-motility assay
WT sperm were swam up in NC media and treated with different concentrations of phlorizin (0.001, 0.01. 0.1, 1, 10, and 100 μM) for 1 h at 37 °C. Sperm motility was analyzed using CASA (version 3.9.8, Penetrating Innovations), as described previously [46]. Total, progressive, and different parameters of sperm motility were evaluated, as defined elsewhere [46]. For motility assays in media of higher viscosity, an equal volume of sperm was added to a solution of 2% methylcellulose in the NC medium, to obtain a final concentration of 1% methylcellulose. Sperm motility was also measured under media without sodium or glucose, to assess the effect of these transported substrates on SGLT function.
Glucose uptake assay
Determination of glucose internalization was performed following two procedures, fluorometric and flow cytometric analysis. For fluorometric determinations, sperm were swam up in NC media containing no glucose, and counted. Approximately 10 million sperm in 500 μl of media were incubated with 150 μM of the fluorescent glucose analog, 6-deoxy-6- [(7-nitro-2,1,3-benzoxadiazol-4-yl)amino]-D-glucose, or 6-NBDG. After 15 min of incubation with the dye, sperm were centrifuged for 5 min at 300×g. The supernatant was removed and sperm were resuspended in 250 μl of media. Approximately 100 μl of the sperm suspension was added to a cuvette containing 2.4 ml of media. Fluorescence was measured using an excitation of 465 nm and emission of 540 nm.
For the flow cytometry measurements, sperm were swam up in NC Whitten’s media with no glucose. Approximately 0.2–0.4 × 106 cells were treated with 10 or 100 μM phlorizin for 1 h at 37 °C. Then, 100 μM 6-NBDG was added to sperm in either NC or CAP media for 15 min. Sperm were centrifuged at 1000 RPM for 5 min and washed with Whitten’s media devoid of glucose twice. The final pellet was resuspended in 350 μl of medium and sperm were treated with 30 nM PI immediately before sorting. Glucose uptake analysis was performed using the BD LSR II flow cytometer (BD Biosciences, Franklin Lake, New Jersey).
Seahorse XF analysis
Glycolytic activity was measured using the Seahorse XF Analyzer. Approximately 72 h before the assay, a 96-well plate was coated with 45 μl of Concanavalin A (0.5 mg/mL) and allowed to air dry. The night before the assay, the sensor cartridge was loaded with 200 μl of calibrant and placed in a 37 °C incubator. The day of the experiment, mice were euthanized, and sperm were swam up in media containing 138 mM NaCl, 4.7 mM KCl, 1.7 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 5.6 mM glucose, and 1 mM hepes. The sensor cartridge was loaded with the corresponding compounds. Port A was reserved for a mock injection of media, as reported elsewhere [47]. Phlorizin was dissolved directly in TYH (Toyoda, Yokoyama, and Hoshi) media at a concentration of 1 mM (final 100 μM) and loaded into Port B. To capacitate sperm, 10 mM db-cAMP (final 1 mM) and 5 mM IBMX (final 500 μM) were dissolved in media and loaded into Port C. After sperm were swam up for ~20 min, cells were washed twice and resuspended in media ± BSA. BSA has been shown to clog the injection ports and therefore is added to the resuspension media. Approximately 180 μl of the sperm suspension was added to each well of the 96-well plate, for about 1 × 106 million per well. For each assay, at least 4 wells were reserved for media alone, to correct for background. After calibration with the sensor cartridge, the cell plate was loaded into the Seahorse Analyzer. The settings for 1 cycle were 3 min of mixing and 3 min of reading. The basal reading was 2 cycles, the mock injection 4 cycles, ± phlorizin 3 cycles, and ± db-cAMP/IBMX 18 cycles. Cells were stained with Hoechst to allow for normalization to cell number. The data were analyzed using Wave software and statistical analysis was performed using Graph Pad Prism.
To measure ATP production in real time, the Seahorse ATP Rate Assay was used. For the ATP Rate Assay, the buffer capacity of custom-made media must be determined and found to be within a specific range (1.8–4.0 mM/pH). To reach the appropriate range, 5 mM HEPES was added to TYH media containing no glucose. The plate and sensor cartridge were prepared as described above. The day of the experiment, mice were euthanized, and sperm were allowed to swim up for ~20 min and counted. The sperm suspension was then preincubated in NC or CAP media ±100 μM phlorizin for 30 min. During this time, the sensor cartridge was loaded. Port A was loaded with 18 μl of 5 mM HEPES no glucose TYH media (mock injection). Port B was loaded with 20 μl of 5 mM HEPES TYH media containing 56 mM glucose (final 5.6 mM glucose) ± db-cAMP/IBMX. Port C was loaded with 22 μl of 30 μM oligomycin (final 3 μM). Port D was loaded with 25 μl of 5 μM antimycin A/rotenone (final 0.5 μM). The basal reading was 1 cycle, the mock injection 2 cycles, ± db-cAMP/IBMX 18 cycles, oligomycin 3 cycles, and antimycin A/rotenone 3 cycles. The data were analyzed using Wave, where the buffer capacity of the media was adjusted, and values were normalized to cell count. The data were exported to excel and uploaded to the ATP Rate Assay Report Generator. Statistical analysis was performed using Graph Pad Prism.
In vitro fertilization
Female C57B/6 J mice were superovulated with 5 IU of PG 600 and 5 IU of human chorionic gonadotrophin and cumulus-oocyte masses were collected in CARD medium (Cosmo Bio USA, KYD-003-EX). Spermatozoa were collected from the cauda epididymis and treated ±10 μM and 100 μM phlorizin for 30 min. Sperm were centrifuged at 300×g for 5 min and resuspended in 30 μl of Fertiup (Cosmo Bio USA, KYD-002-05-EX). Approximately 1 × 106 sperm were incubated with oocytes for 4 h at 37 °C in 6% CO2 and 5% O2. Oocytes were then washed in EmbryoMax Advanced KSOM (Millipore Sigma, MR-101-D) and cultured overnight. Fertilization was assessed by counting the number of one-cell vs two-cell stage embryos.
Authors’ Contributions
G.S. was responsible for obtaining the glucose uptake data in Figure 1A. J.M. measured the ATP contents of WT and ATP1A4 KO sperm in Figure 1B. S.N. designed and executed all subsequent experiments and performed experimental analysis and interpretation. S.N. generated all the figures for the manuscript and wrote the manuscript. A.M. aided with the staining technique to measure the acrosomal reaction. G.B. assisted with experimental design and interpretation as well as with the revisions of the manuscript.
Funding
This work was funded by National Institutes of Health grant HD102623.
Supplementary Material
Acknowledgment
We would like to acknowledge the Flow Cytometry Core Laboratory for their assistance with flow cytometric analysis on spermatozoa. Additionally, we would like to thank Dr Heather Wilkins from the Mitochondrial Genomics and Metabolism Core Facility for her assistance with the Seahorse XF Analyzer experiments and Dr Melissa Larson for her help with the IVF experiments.
Contributor Information
September Numata, Department of Molecular and Integrative Physiology, University of Kansas Medical Center, Kansas City, KS, USA.
Jeff P McDermott, Department of Molecular and Integrative Physiology, University of Kansas Medical Center, Kansas City, KS, USA.
Gladis Sanchez, Department of Molecular and Integrative Physiology, University of Kansas Medical Center, Kansas City, KS, USA.
Amrita Mitra, Department of Molecular and Integrative Physiology, University of Kansas Medical Center, Kansas City, KS, USA.
Gustavo Blanco, Department of Molecular and Integrative Physiology, University of Kansas Medical Center, Kansas City, KS, USA.
Conflicts of interest
The authors have declared that no conflict of interest exists.
Data Availability
The data presented are available in the article along with supplemental data.
References
- 1. Bucci D, Spinaci M, Galeati G, Tamanini C. Different approaches for assessing sperm function. Anim Reprod 2020; 16:72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rickard JP, de Graaf SP. Sperm surface changes and their consequences for sperm transit through the female reproductive tract. Theriogenology 2020; 150:96–105. [DOI] [PubMed] [Google Scholar]
- 3. Stival C, Puga Molina LC, Paudel B, Buffone MG, Visconti PE, Krapf D. Sperm capacitation and acrosome reaction in mammalian sperm. Adv Anat Embryol Cell Biol 2016; 220:93–106. [DOI] [PubMed] [Google Scholar]
- 4. Ickowicz D, Finkelstein M, Breitbart H. Mechanism of sperm capacitation and the acrosome reaction: role of protein kinases. Asian J Androl 2012; 14:816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hirohashi N, Yanagimachi R. Sperm acrosome reaction: its site and role in fertilization. Biol Reprod 2018; 99:127–133. [DOI] [PubMed] [Google Scholar]
- 6. Tourmente M, Villar-Moya P, Rial E, Roldan ER. Differences in ATP generation via glycolysis and oxidative phosphorylation and relationships with sperm motility in mouse species. J Biol Chem 2015; 290:20613–20626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. du Plessis SS, Agarwal A, Mohanty G, van der Linde M. Oxidative phosphorylation versus glycolysis: what fuel do spermatozoa use? Asian J Androl 2015; 17:230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Visconti PE. Sperm bioenergetics in a nutshell. Biol Reprod 2012; 87:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nowicka-Bauer K, Lepczynski A, Ozgo M, Kamieniczna M, Fraczek M, Stanski L, Olszewska M, Malcher A, Skrzypczak W, Kurpisz MK. Sperm mitochondrial dysfunction and oxidative stress as possible reasons for isolated asthenozoospermia. J Physiol Pharmacol 2018; 69:403–417. [DOI] [PubMed] [Google Scholar]
- 10. Benkhalifa M, Ferreira YJ, Chahine H, Louanjli N, Miron P, Merviel P, Copin H. Mitochondria: participation to infertility as source of energy and cause of senescence. Int J Biochem Cell Biol 2014; 55:60–64. [DOI] [PubMed] [Google Scholar]
- 11. Dias TR, Alves MG, Silva BM, Oliveira PF. Sperm glucose transport and metabolism in diabetic individuals. Mol Cell Endocrinol 2014; 396:37–45. [DOI] [PubMed] [Google Scholar]
- 12. Ferramosca A, Zara V. Bioenergetics of mammalian sperm capacitation. Biomed Res Int 2014; 2014:902953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mukai C, Okuno M. Glycolysis plays a major role for adenosine triphosphate supplementation in mouse sperm flagellar movement. Biol Reprod 2004; 71:540–547. [DOI] [PubMed] [Google Scholar]
- 14. Miki K, Qu W, Goulding EH, Willis WD, Bunch DO, Strader LF, Perreault SD, Eddy EM, O'Brien DA. Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme, is required for sperm motility and male fertility. Proc Natl Acad Sci U S A 2004; 101:16501–16506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Odet F, Duan C, Willis WD, Goulding EH, Kung A, Eddy EM, Goldberg E. Expression of the gene for mouse lactate dehydrogenase C (Ldhc) is required for male fertility. Biol Reprod 2008; 79:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Odet F, Gabel S, London RE, Goldberg E, Eddy EM. Glycolysis and mitochondrial respiration in mouse LDHC-null sperm. Biol Reprod 2013; 88:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Martikainen P, Sannikka E, Suominen J, Santti R. Glucose content as a parameter of semen quality. Arch Androl 1980; 5:337–343. [DOI] [PubMed] [Google Scholar]
- 18. Deng D, Yan N. GLUT, SGLT, and SWEET: structural and mechanistic investigations of the glucose transporters. Protein Sci 2016; 25:546–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Balbach M, Gervasi MG, Hidalgo DM, Visconti PE, Levin LR, Buck J. Metabolic changes in mouse sperm during capacitation. Biol Reprod 2020; 103:791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bucci D, Rodriguez-Gil JE, Vallorani C, Spinaci M, Galeati G, Tamanini C. GLUTs and mammalian sperm metabolism. J Androl 2011; 32:348–355. [DOI] [PubMed] [Google Scholar]
- 21. Poulsen SB, Fenton RA, Rieg T. Sodium-glucose cotransport. Curr Opin Nephrol Hypertens 2015; 24:463–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ferrannini E. Sodium-glucose co-transporters and their inhibition: clinical physiology. Cell Metab 2017; 26:27–38. [DOI] [PubMed] [Google Scholar]
- 23. Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev 2011; 91:733–794. [DOI] [PubMed] [Google Scholar]
- 24. Sano R, Shinozaki Y, Ohta T. Sodium-glucose cotransporters: functional properties and pharmaceutical potential. J Diabetes Investig 2020; 11:770–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee SH, Pritchard JB. Role of the electrochemical gradient for Na+ in D-glucose transport by mullet kidney. Am J Physiol 1983; 244:F278–F288. [DOI] [PubMed] [Google Scholar]
- 26. Storelli C, Vilella S, Cassano G. Na-dependent D-glucose and L-alanine transport in eel intestinal brush border membrane vesicles. Am J Physiol 1986; 251:R463–R469. [DOI] [PubMed] [Google Scholar]
- 27. Clausen MV, Hilbers F, Poulsen H. The structure and function of the Na,K-ATPase isoforms in health and disease. Front Physiol 2017; 8:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jorgensen PL, Hakansson KO, Karlish SJ. Structure and mechanism of Na,K-ATPase: functional sites and their interactions. Annu Rev Physiol 2003; 65:817–849. [DOI] [PubMed] [Google Scholar]
- 29. Geering K. The functional role of the beta-subunit in the maturation and intracellular transport of Na,K-ATPase. FEBS Lett 1991; 285:189–193. [DOI] [PubMed] [Google Scholar]
- 30. Blanco G, Melton RJ, Sánchez G, Mercer RW. Functional characterization of a testes-specific alpha-subunit isoform of the sodium/potassium adenosinetriphosphatase. Biochemistry 1999; 38:13661–13669. [DOI] [PubMed] [Google Scholar]
- 31. McDermott JP, Sánchez G, Chennathukuzhi V, Blanco G. Green fluorescence protein driven by the Na,K-ATPase α4 isoform promoter is expressed only in male germ cells of mouse testis. J Assist Reprod Genet 2012; 29:1313–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jimenez T, McDermott JP, Sánchez G, Blanco G. Na,K-ATPase alpha4 isoform is essential for sperm fertility. Proc Natl Acad Sci U S A 2011; 108:644–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rigau T, Rivera M, Palomo MJ, Fernández-Novell JM, Mogas T, Ballester J, Peña A, Otaegui PJ, Guinovart JJ, Rodríguez-Gil JE. Differential effects of glucose and fructose on hexose metabolism in dog spermatozoa. Reproduction 2002; 123:579–591. [PubMed] [Google Scholar]
- 34. Barros LF, Bittner CX, Loaiza A, Ruminot I, Larenas V, Moldenhauer H, Oyarzún C, Alvarez M. Kinetic validation of 6-NBDG as a probe for the glucose transporter GLUT1 in astrocytes. J Neurochem 2009; 109:94–100. [DOI] [PubMed] [Google Scholar]
- 35. Ehrenkranz JR, Lewis NG, Kahn CR, Roth J. Phlorizin: a review. Diabetes Metab Res Rev 2005; 21:31–38. [DOI] [PubMed] [Google Scholar]
- 36. Meng L, Uzui H, Guo H, Tada H. Role of SGLT1 in high glucose level-induced MMP-2 expression in human cardiac fibroblasts. Mol Med Rep 2018; 17:6887–6892. [DOI] [PubMed] [Google Scholar]
- 37. Hirose M, Shibazaki T, Nakada T, Kashihara T, Yano S, Okamoto Y, Isaji M, Matsushita N, Taira E, Yamada M. Phlorizin prevents electrically-induced ventricular tachyarrhythmia during ischemia in langendorff-perfused Guinea-pig hearts. Biol Pharm Bull 2014; 37:1168–1176. [DOI] [PubMed] [Google Scholar]
- 38. Vormann MK, Gijzen L, Hutter S, Boot L, Nicolas A, van den Heuvel A, Vriend J, Ng CP, Nieskens TTG, van Duinen V, de Wagenaar B, Masereeuw R et al. Nephrotoxicity and kidney transport assessment on 3D perfused proximal tubules. AAPS J 2018; 20:90. [DOI] [PubMed] [Google Scholar]
- 39. Amaral A, Lourenço B, Marques M, Ramalho-Santos J. Mitochondria functionality and sperm quality. Reproduction 2013; 146:R163–R174. [DOI] [PubMed] [Google Scholar]
- 40. Suarez SS, Katz DF, Owen DH, Andrew JB, Powell RL. Evidence for the function of hyperactivated motility in sperm. Biol Reprod 1991; 44:375–381. [DOI] [PubMed] [Google Scholar]
- 41. Naz RK, Rajesh PB. Role of tyrosine phosphorylation in sperm capacitation/acrosome reaction. Reprod Biol Endocrinol 2004; 2:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Krisfalusi M, Miki K, Magyar PL, O'Brien DA. Multiple glycolytic enzymes are tightly bound to the fibrous sheath of mouse spermatozoa. Biol Reprod 2006; 75:270–278. [DOI] [PubMed] [Google Scholar]
- 43. Urner F, Sakkas D. Glucose participates in sperm-oocyte fusion in the mouse. Biol Reprod 1996; 55:917–922. [DOI] [PubMed] [Google Scholar]
- 44. Goodson SG, Qiu Y, Sutton KA, Xie G, Jia W, O'Brien DA. Metabolic substrates exhibit differential effects on functional parameters of mouse sperm capacitation. Biol Reprod 2012; 87:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mannowetz N, Wandernoth PM, Wennemuth G. Glucose is a pH-dependent motor for sperm beat frequency during early activation. PLoS One 2012; 7:e41030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jimenez T, Sánchez G, Wertheimer E, Blanco G. Activity of the Na,K-ATPase alpha4 isoform is important for membrane potential, intracellular Ca2+, and pH to maintain motility in rat spermatozoa. Reproduction 2010; 139:835–845. [DOI] [PubMed] [Google Scholar]
- 47. Balbach M, Buck J, Levin LR. Using an extracellular flux Analyzer to measure changes in glycolysis and oxidative phosphorylation during mouse sperm capacitation. J Vis Exp 2020. 10.3791/60815. [DOI] [PubMed] [Google Scholar]
- 48. Thorens B, Mueckler M. Glucose transporters in the 21st century. Am J Physiol Endocrinol Metab 2010; 298:E141–E145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wagoner K, Sanchez G, Nguyen AN, Enders GC, Blanco G. Different expression and activity of the alpha1 and alpha4 isoforms of the Na,K-ATPase during rat male germ cell ontogeny. Reproduction 2005; 130:627–641. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented are available in the article along with supplemental data.