Abstract
Neurodegenerative diseases are a group of pathological conditions that cause motor inc-ordination (jerking movements), cognitive and memory impairments result from degeneration of neurons in a specific area of the brain. Oxidative stress, mitochondrial dysfunction, excitotoxicity, neuroinflammation, neurochemical imbalance and histone deacetylase enzymes (HDAC) are known to play a crucial role in neurodegeneration. HDAC is classified into four categories (class I, II, III and class IV) depending upon their location and functions. HDAC1 and 2 are involved in neurodegeneration, while HDAC3-11 and class III HDACs are beneficial as neuroprotective. HDACs are localized in different parts of the brain- HDAC1 (hippocampus and cortex), HDAC2 (nucleus), HDAC3, 4, 5, 7 and 9 (nucleus and cytoplasm), HDAC6 & HDAC7 (cytoplasm) and HDAC11 (Nucleus, cornus ammonis 1 and spinal cord). In pathological conditions, HDAC up-regulates glutamate, phosphorylation of tau, and glial fibrillary acidic proteins while down-regulating BDNF, Heat shock protein 70 and Gelsolin. Class III HDACs are divided into seven sub-classes (SIRT1-SIRT7). Sirtuins are localized in the different parts of the brain and neuron -Sirt1 (nucleus), Sirt2 (cortex, striatum, hippocampus and spinal cord), Sirt3 (mitochondria and cytoplasm), Sirt4, Sirt5 & Sirt6 (mitochondria), Sirt7 (nucleus) and Sirt8 (nucleolus). SIRTs (1, 3, 4, and 6) are involved in neuronal survival, proliferation and modulating stress response, and SIRT2 is associated with Parkinsonism, Huntington’s disease and Alzheimer’s disease, whereas SIRT6 is only associated with Alzheimer’s disease. In this critical review, we have discussed the mechanisms and therapeutic targets of HDACs that would be beneficial for the management of neurodegenerative disorders.
Keywords: HDACs, SIRTs, neurodegenerative disorder, neuroprotection and neurotoxic effect, future targets
1. INTRODUCTION
Neurodegenerative diseases are a set of hereditary or sporadic conditions that result in progressive degeneration of neurons in the specific region of the brain [1]. The prevalence of age dependent neurodegeneration is rapidly increasing in the elderly population. Central nervous system (CNS) damage is prone to cell death, demyelination, neuronal and functional deficits. Oxidative stress is involved in the pathogenesis of several neurodegenerative disorders such as Alzheimer’s disease (AD), Parkinson's disease (PD), which leads to the generation of free radicals and initiate neurodegeneration. Reactive oxygen species (ROS) toxicity associated glial cell activation, mitochondrial dysfunction; protein misfolding and cellular apoptosis are well accepted mechanisms of neurodegeneration [2]. Mitochondria are critical in maintaining cellular energy balance, cellular differentiation and cell cycle, etc. Impairment in mitochondrial mechanism leads to reduced oxidative phosphorylation, eventually causing neuronal cell death [3]. Glutamate and aspartate are the major excitatory neurotransmitters present in the brain, which worked through the activation of ligand gated ion channels and some via G-protein coupled receptors (metabotropic glutamate receptors). Increased concentration of extracellular glutamate is responsible for excitotoxicity reported in both in-vitro as well as in-vivo studies [4].
Only symptomatic treatments are available for the management of PD, AD, Huntington disease (HD) and Amyotrophic lateral sclerosis (ALS). Available in-vitro and in-vivo studies showed that histone deacetylases protect neurons and helps in neuronal survival [5]. HDACs are involved in cell division through stabilizing chromatin structure, repression of gene expression and regulation of cellular processes such as cellular differentiation, development and multiplication [6].
Histones are water-soluble proteins around which DNA is packed and contains positively charged amino acids such as lysine, arginine and histidine [7]. Nuclear DNA is highly condensed and wrapped around the histone moiety to pack them inside the nucleus and in condensed form and cause twining of the chromosome during cellular division [8, 9]. Histones are mainly associated with DNA and interaction between histone and DNA is regulated by two enzymes e.g. Histone acetyl transferases (HATs) and HDACs, presents in nucleus and cytoplasm [10]. The positive charge of lysine residue gets neutralized on the addition of acetyl group in HATs and HDACs, which decrease interaction between lysine and phosphate group in DNA, thereby inhibit gene transcription [11], while deacetylation through HDACs resulted in restoration of positive charge in condensation of chromatin followed by turning off gene transcription [12].
In mammals, there are 18 different types of HDAC enzymes, which are classified into four types based on their structural and functional homologies to yeast HDACs. There are several non-histone protein targets, including transcription factors (e.g., p53, STAT1, or STAT3), cellular proteins (e.g., heat shock protein (HSP90), or KU70) involved in various cellular functions such as cell cycle progression, gene expression and necrobiosis pathways [13]. Several non-histone proteins have been recognized as a substrate of HDACs [14].
Classes I, II, and III of HDACs govern the acetylation of lysine of those non histone proteins that possess neuroprotective effects [14]. The HDAC inhibitors in AD prevent the amyloid beta induced hyper phosphorylation of tau protein and the expression of genes that are responsible for cognition loss [15]. Generally, HDACI increases protein transcription, activates heat-shock proteins, anti-apoptotic Bcl-2 family members and free radical scavenger enzymes that prevent degeneration of dopaminergic neurons in PD [16]. Similarly, in HD, the HDAC1and HDAC3 selective inhibitor (RGFP966) prevent corticostriatal-dependent motor learning deficit, suppressed striatal CAG repeat expansions and reduce the accumulation of mutant huntingtin [17]. HDACIs (especially HDAC6 inhibitor) prevent autoimmune demyelination of neurons in Multiple sclerosis [18]. The HDACIs in TBI maintain the cellular functions and the expression of proteins through post translational modification of histone moiety and heat shock chaperons [19].
2. THE HDAC SUPER-FAMILIES/TYPES
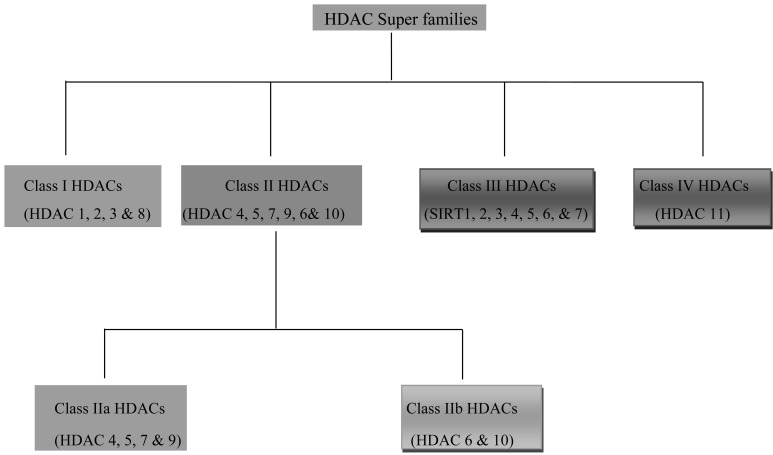
The brief classification of HDAC super-families is described in Fig. (1).
Fig. (1).
Classification of HDAC super families.
2.1. Class I HDACs
HDAC1 and HDAC2 have a sequence similarity index of 82% [20, 21]. The recombinant HDAC1 and HDAC2 are initially inactive because their activation requires cofactors. HDAC1 and HDAC2 protein complexes are necessary for the modulation of the deacetylase activity. The sequence similarity index between HDAC3 and HDAC8 is about 34%. The amino acid region (181-333) of HDAC3 has 93% similarity with HDAC1 and HDAC2. Although, there is only 68% similarity in the corresponding region of HDAC with HDAC1 and HDAC2 [22]. For deacetylation activity and transcriptional repression, the non-conserved C-terminal region of HDAC3 is essential. In addition to NLS, an NES is also present in the 181-333 amino acid regions. HDAC8 is composed of a large catalytic domain along with NLS at the central region [23, 24]. Because of the recent discovery of HDAC8, the involvement co-repressor protein complex is still unknown.
2.2. Class II HDACs: Class II HDACs are Further Subdivided into Two Categories
2.2.1. Class IIa HDACs
This class is further divided into two sub-classes, class IIa and class IIb. Class IIa includes HDAC4, HDAC5, HDAC7 and HDAC9, while class IIb contains HDAC6 and HDAC10. Class IIa HDACs have large N-terminal extensions with conserved binding sites for the transcription factor like myocyte-specific enhancer factor 2A (MEF2) and 14-3-3 protein which are required for HDAC signaling, followed by phosphorylation through kinases, such as calcium/calmodulin dependent protein kinase (CaMK) and protein kinase D shuttle from the nucleus to the cytoplasm (10). It is hypothesized that class IIa (especially HDAC4 &5) activity is regulated by the hippocampal neurons. However, the nuclear exportation of HDAC4 is done through electrical signals but not that of HDAC5 in hippocampal neurons [25]. Nuclear exportation of HDAC5 was demonstrated to be induced via stimulation of calcium flux through NMDA receptor or L-type calcium channels [26].
2.2.2. Class IIb HDACs
HDAC6 and HDAC10 belong to the class IIb HDAC family. HDAC6 is the main cytoplasmic deacetylase rather than HDAC10 towards histone substrates in mammalian cells [10]. HDAC6 is different from all other HDACs, as it refuses deacetylase domains and a C-terminal zinc finger protein [27]. HDAC6 possesses two non-identical catalytic domains in tandem and also has a lack of N-terminal extension [28]. Iwata et al. disclosed that HDAC6 is required for the autophagic degradation of aggregated huntingtin (affected protein in HD) [29]. It has the ability to associate with spinocerebellar ataxia type 3 protein (Ataxin 3), which is a neurodegenerative protein involved in the regulation of aggresomal formation [26].
2.3. Class III HDACs
Class III HDACs are the adenine dinucleotide dependence HDACs, which are further sub-classified as SIRT1, SIRT2, SIRT3, SIRT4, SIRT5, SIRT6, and SIRT7. All sirtuins have their own specific functions (neuroprotection and metabolic processes). Sirtuins are involved in various age related neurodegenerative diseases (Alzheimer’s disease, Parkinson’s disease dementia, cerebrovascular disease). Sirtuins may regulate various pathophysiological pathways (mitochondrial dysfunction, stress response, oxidative stress and inflammatory processes) which are associated with neurodegenerative diseases [30].
2.4. Class IV HDACs
HDAC11 belongs to the class IV HDAC super family, expressed in the brain, heart, muscles, kidneys and testes [27]. It consists of a deacetylase domain that looks like class I and II HDAC domains, and has small N- and C- terminal extensions [31, 32].
3. PHYSIOLOGICAL ROLE AND CLASS I HDACS
3.1. HDAC1
Lower expression of HDAC1 has been seen in the brain as compared to HDAC3 & HDAC11. HDAC1 is primarily present in neuronal cells like glial cells, including astrocytes and oligodendrocytes [33, 34]. Montgomery et al. described that HDAC1 lacking mice died before embryonic day and showed severe proliferation defects and general growth retardation [35]. HDAC lacks embryonic stem cells (ES) are associated with increased expression of cyclin-dependent kinase inhibitors (p21, p27) and the proliferation defects can also be observed in the particular type of cells (HDACs lack embryonic stem cells) [36]. Several studies revealed that 5% of genes (the peg3, H19 and Plag11 gene) are upregulated and 3% genes (Igf2 and p57genes) are down regulated in HDAC1 null embryonic stem cells, and they suggested that global repressor of transcription is not simply functioned by HDAC1, but they also functioned by the repressing or activating certain promoters in specific gene programs [37]. HDAC1 lacking zebrafish found defects within neuronal and skeletal muscle but yet these genes have not been identified [38]. The morpholino mediated HDAC3 knockdown zebrafish shows disruption in liver development [35, 39].
3.1.1. Role of HDAC1 in Neurotoxicity
HDAC1 plays a crucial role in neurotoxicity by affecting axon transport and reducing mitochondrial activity. HDAC1 binds to motor protein, such as kinesin and α-tubulin in cytosol, and decreases their ability to form complex with cargo protein followed by neurotoxicity. The cytosolic HDAC1 is expressed in the patient’s brain suffering from autoimmune disease associated with demyelination of axons, such as multiple sclerosis [35]. The acetylation of p53 gene is regulated by HDAC1 and HDAC2 resulting in neurotoxic effects. HDAC1 is also involved in the pathogenesis of an autosomal dominant and progressive neurodegenerative disease [40, 41].
3.1.2. HDAC1 as Neuroprotective in Various Neurological Disorders
HDAC1 formed a neurotoxic complex in association with HDAC3, whereas HDAC1 interacts with HDRP protein (histone deacetylase related protein) showing neuroprotective effects in cultured neurons [42]. SIRT1 binds with HDAC1 and is thought to produce neuroprotective effects by preserving genomic stability [43].
3.2. HDAC2
HDAC2 has various roles in the human body. The activity of HDAC2 is essential in the regulation of pre- and postnatal brain tissue maturation [35]. HDAC2 has a greater number of genes that are responsible for learning, memory and synaptic plasticity. Inhibition of HDAC2 leads to cell death in many cells [44]. For the maturation of T-cell, the role of HDAC2 is essential [45]. Some studies revealed that HDAC2 knockout leads to microglia maturation defects [46]. The study revealed that HDAC2 knockdown mice have birth defect with severe cardiac malformation, including obliteration of the lumen of the right ventricle due to excessive proliferation of cardiomyocytes as well as bradycardia who died within first 24 hours of birth [35, 47].
3.2.1. Role of HDAC2 in Neurotoxicity
HDAC2 has been involved in the origin of various neurological disorders as well as cognitive impairment found in AD. Mutation in the SMN1 gene (survival motor neuron-1) causes fatal motor neuron and autosomal recessive disease by the duplication of SMN locus and SMN2 gene (SMN2), however, SMN1 is also coded by the SMN protein [48]. SMN1 derived protein lacking patients may experience spinal muscular atrophy (characterized by the degeneration of motor neurons in the spinal cord), the expression of that particular gene is decreased by the HDAC inhibitors [49]. Hence, it may be the possible therapeutic target for the development of drugs against spinal muscular atrophy. However, it has been found that the over expression of HDAC2 in hippocampus decrease the transcription of BDNF and c-fos which causes neuronal loss and memory impairment [50].
3.3. HDAC3
HDAC3 has a structural resemblance with HDAC8. HDAC3 is also functionally related to other class I HDACs, but HDAC3 possesses multisubunit complexes which are different from other available complexes. For the activation of HDAC3, SMRT ((silencing mediator for retinoic acid and thyroid hormone receptors) and N-CoR (nuclear receptor co-repressor) are the essential required factors. Both these factors are structurally and functionally similar and act as co-repressors (Class II histone deacetylases: structure, function, and regulation. Isolation of a novel histone deacetylase reveal that class I and class II deacetylases promote SMRT-mediated repression). The conserved deacetylase-activating domain is present in SMRT and N-CoR for HDAC3 activation [51]. HDAC3 has the ability to form oligomers with the rest of HDACs both in vivo and in vitro [22]. Some studies have shown that HDAC3 can co-precipitate with HDACs 4, 5, and 7 via SMRT and N-CoR complex formation [22, 52]. HDAC3 is also found to have complex formation with HDAC related protein (HDRP) [53, 54]. In addition, HDAC3 also shares the ability of HDAC1 to form Rb–RbAp48 interaction, which showed the essential role in the cell cycle process [55, 56].
3.4. HDAC8
Less information is available on the HDAC8 role in brain development and function. Investigation of HDAC8 expression in mice and chicks during embryonic development demonstrated that in mice HDAC8 is solely expressed in midbrain and forebrain regions, while in chicks, it displays a broader pattern of expression [57]. HDAC8 has a similarity index of 34% with HDAC3 [58]. HDAC8 enzyme is involved in the organization of chromosomes and regulation of cell structure during cell division [59]. Cells copy their chromosomes during the S phase of cell division and arranged the DNA into two identical structures referred to as sister chromatids obtained from each chromosome [60]. Sister chromatids are attached with each other with the group of a protein called cohesion complex during the early stage of cell division; cohesion complex is removed in a later phase of cell division by a chemical reaction carried by HDAC8 that is involved in the removal of cohesion complex, so the sister chromatids are separated and moved to newly forming cell and further recycled for future cell divisions [60]. Due to its current discovery, only two different transcripts of 2.0 kb and 2.4 kb have been observed.
4. PHYSIOLOGICAL ROLE AND CLASS IIA HDACS
4.1. HDAC4
HDAC4 has a similarity index of 70% with HDAC5 and 58% with HDAC7. HDAC4 also has the ability to interact with SMRT/N-CoR, and co-repressor BCoR and CtBP [58]. HDAC4 is highly expressed in the rodent’s brain during early postnatal life [61]. Accumulation of HDAC4 is seen in the cytoplasm of neurons through histochemical analysis of brain along with CRH, oxytocin, vasopressin, orexin, histamine, serotonin and noradrenalin [62]. HDACs are involved in the various signaling pathways of neurons in the brain. Nuclear translocation of HDAC4 is suppressed during the treatment with BDNF (Brain derived neurotropic factor), while the nuclear accumulation of HDAC4 is stimulated by the CaMK inhibitors. It is hypothesized that the nuclear localization of HDAC4 leads to neuronal apoptosis in various neurological disorders, whereas its inhibitors and SiRNA (small interfering RNA) decrease its expression and increases cell survival [63]. HDAC4 is required for the learning & memory and synaptic plasticity in mice as well as essential for normal cognition in mice, drosophila and humans [64, 65]. Earlier it was reported that HDAC inhibition is a negative regulator of learning, memory and synaptic plasticity [66, 67]. However, due to the majority of central neurons having the tendency to retain HADC4 in the cytoplasm; a study demonstrated that there is no detectable abnormalities seen in mRNA levels in the brain in HDAC4 deficient mice [68]. Arnold et al. reported that impairments in motor coordination, hyperactivity and decreased anxiety- like behaviors were seen in Hdac4bko mice [69]. Nevertheless, a recent report revealed that HDAC4 irreversibly enters neuronal nuclei under pathological conditions [70], but physiological significance of the nuclear import of HDAC4 remains to be unexplored.
4.2. HDAC5
HDAC5 is expressed in the whole part of the brain as well as peripheral tissues [71]. It is partially involved in neurodegeneration, but it has a more protective role compared with its harmful (neurodegeneration) effect. The study revealed that phosphorylation of the HDAC5 by the protein kinase C leads to the injury of the sensory neurons [72]. A study demonstrated that HDAC5 lacking mice are hypersensitive to stress results from the neurohumoral signaling. Neurohumoral signaling often activate the CaMK-PKD and calcineurin pathways which further regulate the phosphorylation of class IIa HDACs and promote their nuclear exportation [73]. Agis et al. reported that HDAC5 is important for the consolidation of context and tone-dependent fear memory. Hence, the author suggested that HDAC5 is crucial for the formation of fear memory but not involved in spatial memory [74].
4.3. HDAC7
HDAC7 appears in the nucleus and cytoplasm of the striatum neurons and is also found in the cortex and cerebellum. HDAC7 is expressed especially in the endothelial cells, which are responsible for the formation of the inner lining of CVS system; if the HDAC7 is deleted by the gene silencing techniques, the offspring having embryonic lethality as well as loss of integrity of endothelial cells [75, 76]. HDAC7 has a high degree of sequence similarities with HDAC5. It is transported from the nucleus to cytoplasm during muscle cell differentiation [77]. The location of HDAC7 is less clear and remains to be explored except for muscle cells [58, 78]. The overexpression of HDAC7 protects the cell from death via the inhibition of the expression of c-Jun in cultured neurons [79].
4.4. HDAC9
The catalytic domain of HDAC9 is present on the N-terminus. Mainly, three types of splice variants are available, including HDAC9a, HDAC9b and HDRP/HDAC9c, but HDAC9c/HDRP does not have any catalytic domain [80]. Additionally, HDAC9 also has a role in interacting with myocyte enhancer factor 2 (MFE2), which confirms the function in muscle differentiation [78]. HDAC9 plays an important role in the differentiation and development of many types of cells, including regulatory T-cells. Disorganization of HDAC9 gene involved in the congenital defect of eye in the part of anterior chamber. Genetically, HDAC9 along with HDAC11, SIRT4 and SIRT5 had shown an effect on the brain in multiple sclerosis patients [81, 82].
5. PHYSIOLOGICAL ROLE AND CLASS IIB HDACS
5.1. HDAC6
HDAC6 plays an important role in the brain in various neurological disorders. It has a role in various cellular function and deacetylase the target proteins like tubulin, cortactin, Hsp90 (DmHsp83) and Bruchpilot [83-85]. In-vivo study explored that the inhibition of HDAC6 increased neuronal survival and regeneration through reduction of oxidative stress. Further, some studies have also shown that axonal degeneration from kainic acid is related to a decrease in alpha tubulin acetylation [86]. In the Drosophila larva model, researchers examined that N-terminal deacetylase domain of HDAC6 is imperative for memory continuation and any mutation in HDAC troubled homeostatic synaptic plasticity [86, 87]. Human HDAC6 consists of 1215 amino acid residues arranged in space to form catalytic domain with a zinc finger ubiquitin-binding domain located at the C-terminus [88]. HDAC6 acts as a putative therapeutic agent in amyotrophic lateral sclerosis. Several ALS associated proteins have been found to have a relationship with HDAC6 [89].
5.2. HDAC 10
Of all class II HDACs, HDAC10 is discovered recently. HDAC10 has two splice variants [76]. A protein sequence study showed that HDAC10 has 37% overall similarity with HDAC6 [90]. The catalytic domain of HDAC10 is present on its N-terminus, and a putative second catalytic domain on the C-terminus region. HDAC10 has a regulatory role on the cell cycle as two putative Rb binding domains are present on it. The role of HDAC10 is reported in inflammatory condition like in arthritis, through elevation of cytokine (IL-1β, IL-6) [91]. These markers are well reported in neurological disorders like PD, AD, HD, and MS. However, these lines of evidence indicate that an inhibitor of HDAC10 could be beneficial in inflammation and hyper-immune associated neurodegeneration. So far, the exact role of HDAC10 in neurological disorder is not established, but it could be a useful future perspective against neurodegeneration [91, 92].
6. PHYSIOLOGICAL ROLE AND CLASS III HDACS
The silent information regulator (SIRT) belongs under the category of HDAC. There are seven classes of sirtuin proteins, denoted as SIRT1 to SIRT7, present with different enzymatic activities and localization within the mammalian cells. Sirtuins act as NAD+- dependent deacetylases for a target protein, which is required for different biological functions [93].
6.1. SIRT1
Sirtuin incorporates a protective role from environmental stress in mammalian cells [94]. During starvation, the extent of SIRT1 is increased in mammalian cells. SIRT1 controls the metabolic process in several tissues through the activation of the nuclear receptor peroxisome-proliferator activated receptor- gamma (PPAR-γ) and its transcriptional co-activator. The cell survival, proliferation and stress response are regulated by several non-histone substrates of SIRT1 like tumor suppressor p53, forkhead transcription factor FOXO3a and NF-kβ [95]. Deacetylation of DNA repair factor Ku70 (an inhibitor of Bax-mediated apoptosis) increases cell survival promoted by calorie restriction (CR) [96, 97]. A recent study showed that SIRT1 over-expression increases longevity and delays the aging in mice, and also improved cell survival and level of BDNF [98].
6.2. SIRT2
The NAD+ dependent SIRT2 was the first sirtuin to be discovered, and it is essential for transcriptional silencing in vitro [99]. SIRT2 is mainly a cytoplasmic protein localized in the outer loops of the myelin sheath of neurons. There are several cytoplasmic proteins, such as OLN-93, oligodendrocytes; alpha-tubulin is the predominant substrate of SIRT2 deacetylase [100]. The level of SIRT2 is upregulated by calorie restriction (CR) as well as by oxidative stress [101]. HDAC6 has a binding affinity towards SIRT2 but the evidence is still doubtful whether SIRT2 deacetylates soluble or polymerized alpha tubulin or it can be considered as microtubule deacetylase. SIRT2 deacetylase has forkhead transcription factors of class O, FOXO1 and FOXO2 that are involved in various cellular processes such as DNA repairing mechanism, metabolism and aging [102]. The depletion of sirt2 results in an increase in acetylation of mitochondrial proteins and changes in the nature of mitochondria, leads to oxidative stress [103]. The potent inhibitor of SIRT2 (Sirtinol, AGK2, Tenovin-1 and Salermide) reduces the α-synuclein toxicity [104].
6.3. SIRT3
Sirt3 is mostly present in mitochondria and has an emerging role in the regulation of mitochondrial function and prevention of oxidative stress [105]. It also regulates mitophagy to remove altered mitochondria [106]. The extension of the lifespan of neuronal cell is directly linked to SIRT3. Null SIRT3 mice do not show any reduction of cell survival but display alteration in the fatty acid oxidation under the condition of caloric restriction (CR) [107]. Over-expression of SIRT3 demonstrates the phosphorylation of CREB, stimulating the expression of PGC alpha and its target UCP1, which shows a decrease in ROS production through a positive feedback mechanism. Expression of SIRT3 has a protective role in dopaminergic neuronal cell death [108, 109].
6.4. SIRT4
SIRT4 has different functions (i.e. energy metabolism and regulation of TCA cycle) in different tissues; its role in the brain remains unexplored. The first reported SIRT4 was Glutamate dehydrogenase (GDH), which regulates amino acid flux into energy production [110]. A recent study showed that up-regulation of SIRT4 is facilitated by the treatment with a neurotoxin (kainic acid). SIRT4 has a protecting and promoting effect on ageing by the regulation of reactive oxygen species. SIRT4 acts as a neuroprotective agent by maintaining the level of glutamate in the synapse [111].
6.5. SIRT5
Sirt5 mainly localizes in the mitochondrial matrix and mostly expressed in the brain, heart, liver, muscle and kidney [112]. It modulates the function of mitochondrial targets and catalyze NAD+ dependent deacetylation (demalonylation, deacetylation and desuccinylation) to maintain the metabolic status in mammalian cells [113]. Another study demonstrated that SIRT5 is an important regulator in kainate induced neurodegeneration as well as in oxidative stress [114].
6.6. SIRT6
SIRT6 is a predominant nuclear protein, which is highly associated with the heterochromatic regions. It is involved in deacetylation of various proteins, including H3-lysine 9 (H3K9) and H3-lysine 56 (H3K56) needed for the formation of telomers [115]. A study demonstrated that SIRT6 showed its protective role in neurodegenerations, those mammals lacking this protein may undergo premature ageing like processes (ostopenia, lymphopenia, lordokyphosis and metabolic defects) and also leads to the accumulation of telomere damage [116]. The phenotypic observations on SIRT6 knockout mice demonstrated that this protein is thought to regulate both DNA base excision repair (BER) and spontaneous genomic stability [117].
6.7. SIRT7
SIRT7 is located in the nucleus and nucleolus of the mitotically active cells. It interacts with ribosomal RNA and regulates RNA polymerase-I [118]. The role of SIRT7 is not fully explored in neurodegeneration. Basically, SIRT7 is anticipated to possess a major role in numerous neuronal pathways; it regulates rRNA synthesis and assembly of ribosome via the changes in NAD+/NADH ratio [119]. Similarly, Sirt7 regulates the genomic stability of cells by promoting the repair mechanism of non-homologous DNA damage [120].
7. PHYSIOLOGICAL ROLE AND CLASS IV HDACS
7.1. HDAC11
Phylogenetic analysis showed that HDAC11 has a structural resemblance with HDAC3 and HDAC8, which confirms the relation of HDAC11 with class I HDACs rather than class II HDACs [121]. The catalytic domain of HDAC11 is present on N-terminus. This enzyme does not have interaction with any HDAC complexes (Sin3, N-CoR/SMRT) [122]. HDAC11 elevates the gene expression of IL-10 both in humans and animals, which leads to the activation and release of CD4+ and T-cell expression, although hyper-immune activation is well demonstrated to promote neuronal loss in Parkinson's disease, which stays in accordance with the role of HDAC11. The blockade of HDAC11 via its specific inhibitors will be a novel approach for the treatment of Parkinson's disease [123].
8. ROLE OF HDAC IN VARIOUS NEUROLOGICAL DISORDERS
8.1. Alzheimer’s Disease
Alzheimer’s disease (AD) is a progressive neurodegenerative disease characterized by loss of memory and impairment in cognition [124]. AD is the most common form of dementia. Neurofibrillary tangles (NFTs), insoluble β-amyloid (Aβ) plaque and neuron loss are the reasons behind AD [125]. On the basis of hypothesis at present, “Amyloid cascade hypothesis,” it is suggested that the accumulation of β-amyloid leads to an intricate cascade of neuronal apoptosis and thus results in the development of AD. Acetylation of histone and deacetylation of histone is catalyzed by two different enzymes, histone acetyltransferase (HATs) and histone deacetylase (HDACs), respectively. The level of acetylation of histone has a significant role in the regulation of chromatin condensation and transcription of genes. Histone deacetylases regulate the level of histone acetylation and furthermore cause the downstream of gene expression. Pathology of AD is associated with the abnormality of histone acetylation. HDAC proteins may be the target for the prevention of AD [15, 40].
The study revealed that HDAC2 knockdown AD mouse shows improved hippocampus dependent memory performance and synaptic plasticity. It may become a possible target for designing drugs to treat Alzheimer's disease [126]. A study reported that HDAC2 knockdown mice have birth defect with severe cardiac malformation including obliteration of the lumen of the right ventricle due to excessive proliferation of cardiomyocytes as well as bradycardia died within the first 24 hours of birth [35]. McQuown et al. observed that HDAC3-Flox-modified mice (deletion of HDAC3 in the CA1 area of the hippocampus) or the mice treated with a selective inhibitor of HDAC3 (RGF136) increases the acetylation of histone and considerably increase the cognition and the expression of the nuclear receptor gene subfamily 4, group A, member 2(Nr4a2) and c-Fos is involved in cognition [127]. Bardai FH and D’Mello also found that the protein HDAC3 has a strong neurotoxic effect and this neurotoxicity directly affects cellular mechanism [15, 128]. Another study revealed that it has a protective role in AD, and down regulation of SIRT6 leads to hyper-phosphorylation of tau levels in the Alzheimer’s disease [129] (Fig. 2).
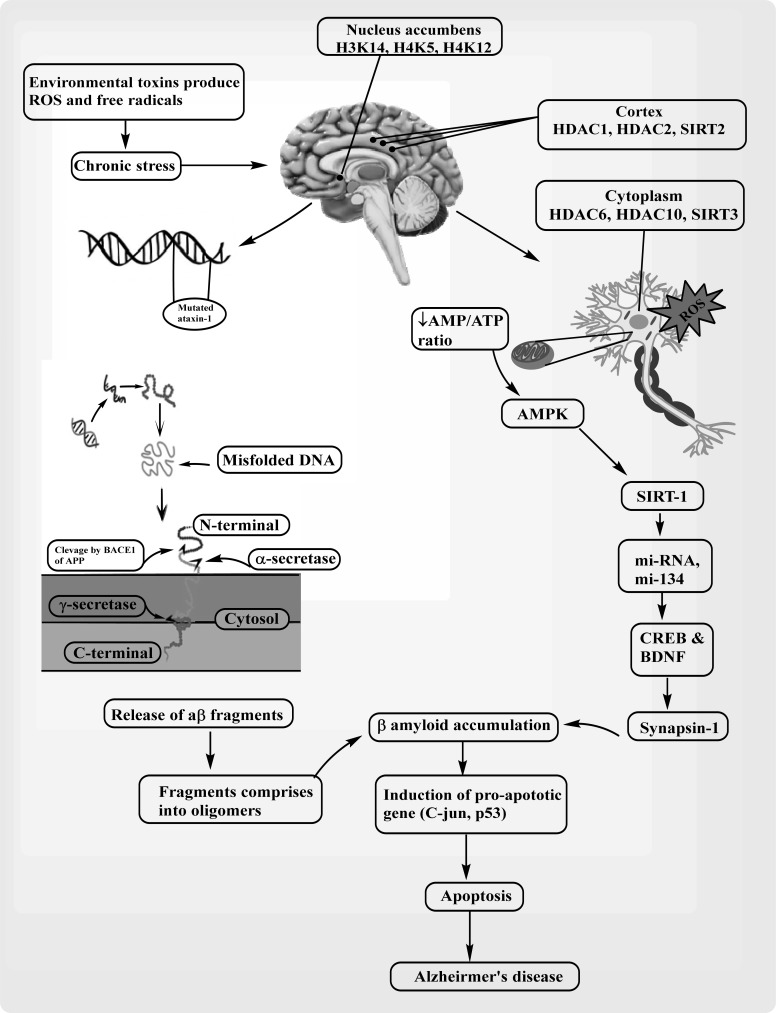
Fig. (2).
HDACs and SIRTs mediated pathological mechanism of Alzheimer’s disease. Histone proteins present in nucleus accumbens and cortex causes mutation on ataxin 1 through chronic stress. Mutation on ataxin 1 causes misfolded DNA which further releases aβ fragments, result beta amyloid accumulation. On the other side, in the brain neuron, presence of HDAC causes ROS production which activates AMPK followed by SIRT1 activation. SIRT1 further increases the level of CREB and BDNF. Activation of Synapsin1 leads to beta amyloid accumulation and further causes Alzheirmer’s disease.
8.2. Parkinson’s Disease
Parkinson’s disease (PD) is the most prevalent CNS disorder and also a second most common neurodegenerative disease mainly associated with reduced motor performance and memory impairment and affecting more than 4 million peoples worldwide. PD is characterized by the progressive hypokinesia followed by the slowness of movement, loss of postural reflex, rigidity and development of resting tremor. The prevalence of PD is 0.3-1% with age>50 years and even in greater age [130]. Histone proteins are known to provide neuroprotection via histone remodeling and cause acetylation, deacetylation of deoxyribonucleic acid (DNA) [32, 131]. Studies demonstrated that histone deacetylase inhibitors (HDACIs) are efficacious in PD, but how the histone acetylation prevents the neurodegeneration is still not well enlightened [132]. HDAC reduces the deacetylation of histone by using HDAC inhibitors (i.e. valproic acid, sodium butyrate and phenyl butyrate), which leads to chromatin relaxation and activation of multiple genes [133]. For example, brain derived neurotrophic factor, glial derived neurotrophic factor (GDNF) αsyn, Bcl-2, Bcl-XL, heat shock protein (HSP70), p21 and gelsolin have been shown to be induced upon HDAC inhibitors treatment [32, 134].
However, non-transcriptional effect of HDACs like non-histone targets of HDAC catalyzed deacetylation are also involved in neuroprotection [135]. Acetylation of α- tubulin is increased by the inhibition of HDACs, while non-histone target increases microtubule stabilization and axonal transportation with the release of BDNF [136, 137]. HDAC inhibition is also associated with the reduction of astrocyte and T-cell mediated inflammation [32, 138]. The study revealed that the depletion of SIRT5 showed motor deficits, dopaminergic degradation in substantia nigra and antioxidant activities of mitochondria in MPTP-induced PD mouse model [139]. HDACIs are beneficial therapeutic targets in neuroscience to treat various neurological disorders (Fig. 3).
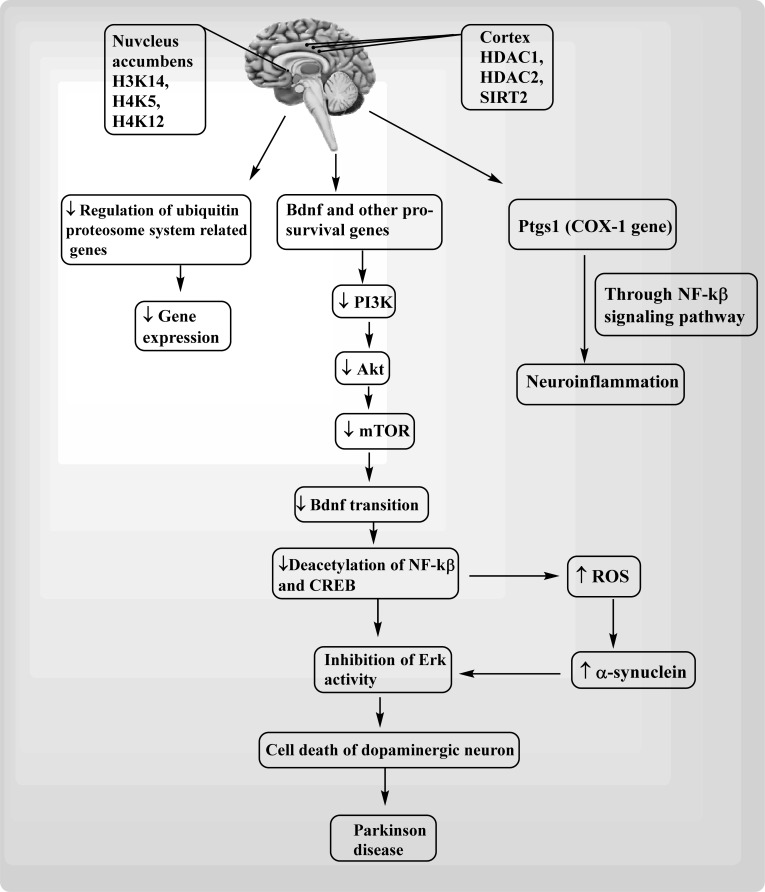
Fig. (3).
HDACs and SIRTs mediated pathological mechanism of Parkinson disease. Histone proteins present in nucleus accumbens and cortex causes activation of COX-1. Gene (ptgs1, Prostaglandin-Endoperoxide Synthase 1) causes neuroinflammation through NF-kβ signalling pathway.
8.3. Huntington’s Disease
Huntington’s disease is an autosomal inherited neurodegenerative disorder caused by trinucleotide Cytosine-Adenine-Guanosine (CAG) repeats (>36) in an HD gene. Normal people have CAG repeats of 7-34. The CAG repeats length fluctuates with ageing and the repeats of CAG >100, causes juvenile onset [140]. HD is characterized by problems in movements; cognition impairment and function of the behavior [141]. Recent studies showed that transcriptional dysregulation is a mechanism of the pathogenesis of Huntington's disease. The eukaryotic gene expression depends on the alteration of histone protein. Acetylation and deacetylation of histone moiety play a crucial role in gene expression. The mutation in the IT15 gene (mHTT), that is located on the short arm of the chromosome 4(4p 16.3), which leads to an alteration in histone acetyl transferase (HATs) activity and believe that the change in HAT activity may be the mechanism of transcriptional dysregulation in Huntington’s disease [142, 143].
Gardian and Colleagues observed the neuroprotective activity of HDAC inhibitor (phenylbutyrate). The administration of phenylbutyrate at 75 days of age in transgenic HD mice (HD-N171-82Q) increased survival rate and decreased the degradation of the striatum and ventricular enlargement, but there was no effect seen on motor coordination. With the following phenylbutyrate treatment, there was an increased acetylation of histone H3 & H4 in the striatum and concurrently there was a decrease in methylation of histone H3 [144]. Jia et al. described that class I inhibitors have shown neuroprotective effects in different HD models via targeting HDAC1 and HDAC3 [145]. In-vivo evidence suggested that down regulation of HDAC1 prevents cognitive decline and motor dysfunction in HD mouse model [76] (Fig. 4).
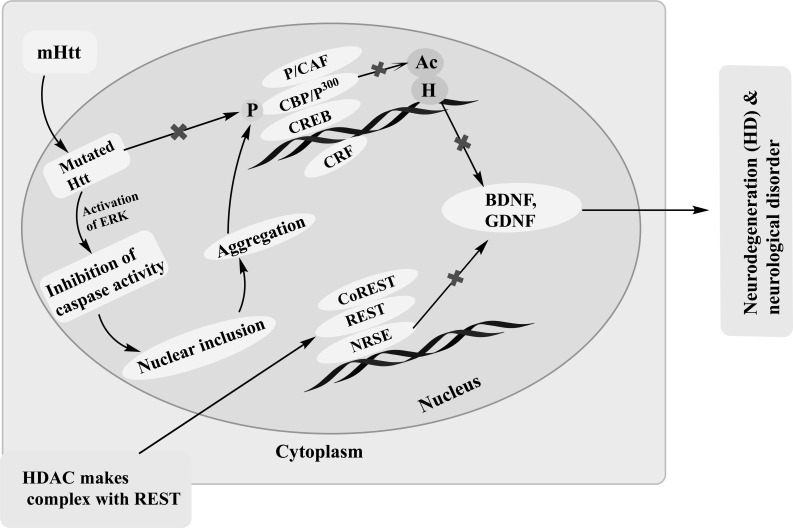
Fig. (4).
Mutated huntingtin (mHtt) mediated Huntington pathology through making complex with HDACs. The complex formed between CREB, REST and mutant huntingtin (mHtt). Normally, Htt bonded with REST, a transcriptional repressor in the cytoplasm. Htt does not intrude with CREB phosphorylation and acetyltransferase activity of CBP which results transcriptional activation followed by neuroprotective activity. In HD, CREB phosphorylation and CBP acetyltransferase activity is inhibited by mutant huntingtin. In the nucleus, REST interacts with NRSE and BDNF transcription is repressed which results neuronal death. Whereas HDACIs are responsible for increase the CREB phosphorylation and histone acetyltransferase activity with addition of restoring BDNF expression and shows neuroprotection.
8.4. Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis (ALS) is a serious neurodegenerative disorder caused by the loss of neurons in the motor cortex, brainstem. ALS is associated with the paralysis of voluntary muscles. Symptoms of motor neurons come in middle age, and death occurs within 2-5 years after symptoms expression. ALS can be either genetic or non-genetic. Superoxide dismutase 1(SOD1), TAR-DNA binding protein 43 (TDP-43) encodes the mutant gene, which is responsible for ALS [146, 147].
The nerve-skeletal muscle interaction through HDAC4 may be the potential target in ALS. In a mouse model, the silencing of HDAC4 in skeletal muscle caused early onset of ALS, suggesting a potential risk in the use of inhibitor of HDAC for the treatment of ALS [148]. There are several combinations of drugs available to treat ALS, like combination treatment with SAHA (a pan HDACI), RGFP109 (HDAC1/3 inhibitor), and arimoclomol decreases the loss of nuclear hallmark of ALS (FUS). ALS associated FUS mutation rescued by HDACI through DNA repair mechanism [149] (Table 1).
Table 1.
Mechanistic role of HDAC family, in various neurological disorders and their localization in brain.
| Class | Sub Class | Modulat-or | Sub Cellular Localization |
Pathophysiological
Role/Disease |
Drug Target | References | |||
|---|---|---|---|---|---|---|---|---|---|
| Specific Drugs | Examples | ||||||||
| Gene/ Protein Upregulated | Gene/ Protein Downregulated | ||||||||
| Class I | HDAC1 (Neurotoxic/ protective) |
HDAC3 (-ve modulator) AD |
Hippocampus, cortex |
Parkinsonism
|
Sodium butyrate, Phenyl butyrate |
Butyric acid, Valproic acid Tubastatin A Lacosamide |
[27, 150-154] |
||
| NF-kB, p21ras P21rac Glutamate |
BDNF GDNF HSP70 Alpha- synuclein Bcl2 Gelsolin |
||||||||
|
Alzheimer’s Disease
| |||||||||
| HSP90 Alpha-synuclein Beta-catenin Tau protein H4k8 acetylation Acetylation (H3k9, H3k14, H4k5,&H4k12) |
gluR1, PSD95 Neprilysin Tubulin acetylation |
||||||||
| Specific Drugs | Examples | ||||||||
| Gene/ Protein Upregulated | Gene/ Protein Downregulated | ||||||||
| - | - | - | - | Huntington’s Disease | - | - | - | ||
| Glial fibrillary acidic protein |
|
||||||||
| HDAC2 (Neuro-toxic/protective –NR) |
- | Nucleus | Parkinsonism- | Butyric acid Valproic acid |
- |
[151, 154] |
|||
| NF-kB, p21ras P21rac Glutamate P53 E2F1 GATA4 |
BDNF GDNF HSP70 |
||||||||
| HDAC3 (Neuro-toxic) HDAC 8(Neuro-toxic/protective- (NR) |
- | Nucleus/ cytoplasm Nucleus |
Alzheimer’s- |
Valproic acid RGFP963 EGCG VorinostatTSA RGFP966 Phenyl butyrate ACY-738 or tubastatin, Sodium phenylbutyrate, MC1568 |
[152, 154-158] [17, 154, 159-161] |
||||
| Aβ peptide GSK3β Tau phosphorylation Tau acetylation β secretase cleavage of APP amyloid β(1-42) accumulation |
BDNF PSD95 GluR1 HSP acetylation |
||||||||
|
Huntington’s –
| |||||||||
| Implicate aberrant transcriptional patterns Helps fuel CAG repeats expression Negative regulation of gene expression Histone methylation caspases Site- specific acetylation of mutant huntigtin Histone acetylation Huntington’s – Negative regulation of gene expression Histone methylation caspases Site- specific acetylation of mutant huntigtin Histone acetylation Amyotrophic lateral sclerosis | |||||||||
| Mir206/FGFBP1-mediated muscle reinnervation | α- tubulin acetylation histone skeletal muscle electrical potentials myogenic genes expression |
||||||||
| Specific Drugs | Examples | ||||||||
| Gene/ Protein Upregulated | Gene/ Protein Downregulated | ||||||||
| Class II (a) | HDAC 4 (Neuro-protective) S |
- | Nucleus/cytoplasm |
Amyotrophic lateral sclerosis- |
ACY-738 or tubastatin, Sodium phenylbutyrate, MC1568 |
Valproic acid Trichostatin A BML-210 |
[154, 161, 162] |
||
| Mir206/FGFBP1-mediated muscle reinnervation |
Histone acetylation α- tubulin acetylation myogeneic genes expression |
||||||||
| HDAC5 (Neuro-toxic/protective) | - | Nucleus/cytoplasm |
Alzheimer’s- |
Valproic acid EGCG |
Trichostatin A BML-210 |
[14, 27, 152, 154] |
|||
| plays a role in the consolidation of contextual and tone dependent fear memories Alpha-synuclein Beta-catenin Tau protein |
PSD95 Neprilysin Tubulin acetylation |
||||||||
| - | - | - | - | Parkinsonism- |
Phenyl butyrate |
Valproic acid |
|
||
| Essential for spatiotemporal control of ERG (early response gene) programme during memory encoding Histone H3 lysine 9 Acetylation | |||||||||
| HDAC7 (neuroprotective) | - | Nucleus/cytoplasm |
Parkinsonism
|
Valproate, RGFP109 |
TSA, Vorinostat, sodium phenyl butyrate |
[163] |
|||
| Decrease beta catenin activity in chondrocytes interact with Bcl6 Decrease BDNF and GDNF expression | |||||||||
| HDAC9 (Neuro-toxic/protective-NR) |
- | Nucleus/cytoplasm |
Amyotrophic lateral sclerosis |
MC1568 ACY-738 |
Valproic acid Trichostatin A BML-210 |
[154, 159-161] |
|||
| FGFBP1-mediated muscle reinnervation | Skeletal muscle electrical potentials, myogenic genes |
||||||||
| Class II(b) | HDAC6 (Neuro-protective /toxic) |
- | Cytoplasm | Huntington’s Disease |
Vorinostat |
TSA Phenylbutyrate RGFP966 |
[154, 157] |
||
| Accumulation of misfolded protein Glial fibrillary |
Decrease tubulin acetylation Histone acetylation |
||||||||
| - | - | - | Alzheimer’s Disease |
Tubastatin A MPT0G211 |
4-Phenylbutyrate Tubacin EGCG |
[152, 154] |
|||
| HDAC6-HSP90 binding Tau hyperphosphorylation |
Tubulin acetylation |
||||||||
| Specific Drugs | Examples | ||||||||
| Gene/ Protein Upregulated | Gene/ Protein Downregulated | ||||||||
| - | - | - | - | Parkinsonism |
Valproate |
TSA, Vorinostat, sodium phenyl butyrate |
[27, 154] |
||
| Deacetylation of Prxl and Prxl2 (peroxiredoxin) Oxidative cell injury | BDNF& GDNF expression |
||||||||
| HDAC10 (Neuro-Toxic/protective-NR) |
- | Cytoplasm | Alzheimer’s Disease |
Valproic acid EGCG |
Trichostatin A BML-210 |
[154] |
|||
| plays a role in the consolidation of contextual and tone dependant fear memories | Tubulin acetylation |
||||||||
| Class III (sirtuins) | Sirt 1 | - | Nucleus | Alzheimer’s Disease |
Crebinostat Salisistate |
Valproate RGFP963 EGCG |
[154] |
||
| Fear learning& memory in wild type mice on treatment, Granulin expression Synepsin1 expression |
mi-RNA mi-134 expression CREB& BDNF expression |
||||||||
| Sirt 2 | - | Cortex, striatum, hippocampus, spinal cord | Parkinsonism |
AGK-2 |
Trichostatin A BML-210 |
[154, 164, 165] |
|||
| Regulate microtubule function, cell cycle, oxidative stress, Autophagy, overexp- ession increases α synuclein aggregation | Glutamate transporter 1 and glutamate/ aspartate transporter |
||||||||
| - | - | - | - | Huntington’s Disease |
Vorinostat |
Trichostatin A BML-210 |
[154, 157] |
||
| Increase accumulation of polyglutamine in the N terminus of neuron | Histone acetylation | ||||||||
| - | - | - | Alzheimer’s Disease | - |
Vorinostat, sodium phenyl butyrate Trichostatin A |
[166] |
|||
| Abnormally overexpressed and deacetylates tubulin which results microtubule stabilization | |||||||||
| Sirt 3 | - | Mitochondria/Cytoplasm |
Alzheimer’s Disease | - |
[167] |
||||
| Dysregulation of sirt 3 linked to mitochondrial dysfunction, Over expression restored the expression of ND2 and ND4 and increase mitochondrial oxygen consumption by repressing mito p53 activity, Shows strong deacetylaseactivity | |||||||||
| Gene/ Protein Upregulated | Gene/ Protein Downregulated | ||||||||
| - | - | - | - | Parkinsonism- | - | - |
[168, 169] |
||
| Sirt 3 acts indirectly on PINK1 and the sirt3-FOXO3 pathway activates mitophagy via PINK1 parkin pathway Decrease level of superoxide (SOD-2) and glutathione peroxidase expression in MPTP induce SIRT KO mice in vivo, Clinically not reported | |||||||||
| Sirt 4 | - | Mitochondria | Alzheimer’s Disease | - |
Vorinostat Quicinostat s |
[111, 169] |
|||
| Specific role of Sirt 4 is not well reported but shows various physiological role in various tissues. Recent study shows Sirt 4 is up-regulated in brain on treatment with kainic acid decrease the level of glutamate leads to neuro-degeneration. | |||||||||
| Sirt 5 | - | Mitochondria | Parkinsonism | - | Valproic acid |
[169] |
|||
| Expression of sirt5 seen in MPTP induced nigrostriatal degeneration in SIRT5 KO mice. | |||||||||
| Sirt 6 | - | Mitochondria | Alzheimer’s Disease | - |
Vorinostat Quicinostat s |
[169, 170] |
|||
| Recent study shows that sirt 6 increase the stability of tau protein while decrease level of sirt6 increase the hyperphosrylated tau levels | |||||||||
| Sirt7 | - | Nucleus/Nucleolus | Specific role of sirt7 in neurodegenerative diseases and brain not well reported | - |
[30, 118] |
||||
| - | Sirt8 | - | Nucleolus | Expression of Sirt8 is not well reported in neurodegenerative disorders. | - | - |
[170] |
||
| Class IV | HDAC11 (Neurotoxic/protective-NR) |
- | Nucleus (cornu Ammonis1)/spinal cord |
Schizophrenia | - | - |
[171] |
||
| EGF FGF Interleukin-10 |
BDNF GDNF Nerotrophin-3(NT-3) |
||||||||
CONCLUSION
This review established the current knowledge about HDAC and its inhibitors. The role of several HDAC inhibitors is represented in various neuronal insult and neuroprotection in different cell lines, in vivo preclinical animal models and information on clinical evolution. This review also focused on the application of HDAC inhibitors in cognition, neuroplasticity and memory. The role of HDAC inhibitors is toxic rather than being neuroprotective in certain cases. Different classes of HDACs have different functions, while class 1 HDACI has been shown to reduce atrophy and fibrosis proliferation, class 2 HDACI shows interference with delayed maturation. In vitro studies on primary cell culture shows the regulation of neuroprotection as well as neuronal cell death by HDACs. The functions of different HDACs are correlated with each other- HDAC1 has been known to be protective when it interacts with HDAC9, whereas neurological insult during interaction with HDAC3. In some specific tissues, HDAC shows acute toxicity. Expression of HDAC3 in healthy mice induces death of cerebral neurons and rat cortical neurons. Inhibition of GSK3β pathway has been shown to be effective against neuro toxicity caused by HDAC3, which is phosphorylated by GSK3β.
Class III HDACs (SIRTs) achieved both neuro-protection and neuro-toxic effects in neuronal cells. Overexpression of SIRT1 and SIRT3 render protection to neurons. There are several problems associated with HDAC inhibitors because of their lack of specificity and isoform selectivity. The lack of knowledge of off-target specificity of HDAC inhibitors raised a concern to find out the exact drug action. In the upcoming years, the novel drug design with HDAC inhibitors may increase its specificity and effectiveness by overcoming problems associated with HDAC inhibitors currently. Future research on HDACIs is expected to have a potential neuroprotection activity against neurodegenerative disorders.
FUTURE PERSPECTIVE
Available reports demonstrated that hypoacetylation of histones and transcriptional changes are involved in various neurodegenerative disorders. In the present scenario, treatment with class I and class II HDACIs overcome these deficiencies and prevent neuronal degeneration [132]. HDAC inhibition-induced neurotrophins in neuronal tissue as well as in microglial cells, is believed to be a significant target as therapeutics. Class I and class II inhibitors reduce microglia activation in-vivo and in-vitro conditions that would be protective strategy to combat neurological disorders by overcoming neuroinflammation [172].
Non-transcriptional cascades, like microtubule stabilization through acetylation, have been implicated as neuroprotective in various neurodegenerative diseases [173]. In PD, transcription of free radical scavenger, heat shock proteins and apoptotic proteins (Bcl-xL, caspases) are up regulated by the expression of HDACs (HDAC1, 2, 5, 7 & 11) in dopaminergic neurons, result dopaminergic neuronal loss in SNPc of brain and showed PD like symptoms and shows PD like symptoms [174]. In Huntington's disease, HDAC (especially HDAC 3) causes corticostriatal dependent motor learning deficit and also increases the CAG repeat expansion in striatum. The mHTT undergoes proteolysis and fragmented into oligomeric form which is accumulated into the nucleus and cytoplasm. In cytoplasm, it interacts with mitochondria and altered respiratory chain and ATP production [175]. Thus, the inhibition of HDACs, especially HDAC 3 by the specific inhibitor, takes a critical role in the treatment of HD.
This review focuses on the important functions of sirtuins and HDAC in modulating neurodegeneration in central nervous system [176]. This review focuses on important functions of sirtuins and HDAC in modulating neurodegeneration in the central nervous system [176]. The exact functions of sirtuins are still unexplored, but some evidence suggest that sirtuins are important player in several brain disorders (AD, PD and HD) [177, 178]. However, further study is needed to explore the molecular role of sirtuins which may help to develop novel strategies against neurodegenerative diseases. The sirtuin information regulator provides a significant role in alleviating neurodegenerative disorders [176].
The neuronal morphology is mainly affected by SIRT1. In the future, in the treatment of diabetes and obesity, SIRT1 may be a novel approach [179, 180]. In the case of AD, SIRT1 modulation may be a valuable strategy to facilitate aggregate clearance [181]. Reduction of SIRT1/3 miRNA/protein levels observed in pathological condition of AD. The expression of SIRT1 to SIRT7 undergoes changes in ageing brain [182]. Especially SIRT1, 2, and 3 have a role in AD and other categories are still unknown [183]. In the case of PD, SIRT1 in response to resveratrol may render MPTP induced neurodegerative mouse model in addition, also SIRT5 have a protective role against PD mouse model [184].
In case of HD, modulation of SIRT1 with treatment may alleviate the expression of glutamine repeat toxicity. In the future, inhibition of SIRT2 will be much more consistent with the current scenario [185]. In the future, targeting SIRT3 in HD might improve mitochondrial insult and oxidative stress [186]. In ALS, dietary treatment with resveratrol may not be sufficient to show effect, whereas intraperitoneal injection may show symptomatic relief and neuro survival. SIRT3 protect ALS patient through prevention from mitochondrial stress and cell death [187]. So, from this review, we hypothesized that in the coming years, drug targets to HDACs and SIRTs would be grateful to alleviate neurodegenerative disorders.
ACKNOWLEDGEMENTS
We are highly thankful to ISF College of Pharmacy Moga, Punjab, department of pharmacology, Chairman Mr. Praveen Garg sir, and Director Dr. GD Gupta sir for guiding us to finalize this article.
LIST OF ABBREVIATIONS
- HDAC
Histone deacetylase
- HDACI
Histone deacetylase inhibitor
- DNA
Deoxyribonucleic Acid
- HAT
Histone acetyl transferase
- STAT
Signal transducers and activators of transcription
- HSP
heat shock protein
- PD
Parkinson's disease
- Bcl
B-cell lymphoma
- HD
Huntington's disease
- CAG
cytosine-adenine-guanine
- MS
Multiple Sclerosis
- TBI
Traumatic Brain Injury
- NAD
Nicotinamide adenine dinucleotide
- RPD
RNA polymerase domain
- Sin
serine recombinase
- CoREST
conserved neuronal co-repressor
- PRC
Polycomb repressive complex
- NuRD
Nucleosome remodeling and histones deacetylation
- MEF
Myocyte-specific enhancer factor
- CaMK
Calcium/calmodulin dependant protein kinase
- SIRT
Sirtuins
- ES
Embryonic stem cell
- HDRP
Histone deacetylase related protein
- HOP
Homeodomain protein
- SMN
Survival of motor neuron
- CRH
Corticotropin releasing hormone
- BDNF
Brain derived neurotrophic factor
- SiRNA
Small interfering RNA
- ALS
Amyotrophic lateral sclerosis
- IL
Interleukin
- PPARγ
Peroxisome proliferator activated receptor gamma
- FOXO
class O of forkhead box transcription factors
- NF-kB
Nuclear Factor kappa-light-chain-enhancer of activated B cells
- CR
Calorie restriction
- CREB
cAMP response element-binding protein
- ROS
Reactive Oxygen Species
- MPTP-1
methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- H3K9
lysine 9 of histone 3
- BER
Base excision repair
- rRNA
Ribosomal RNA
- NADH
nicotinamide adenine dinucleotide hydrogen
- CD4+
cluster of differentiation 4
- NFTs
Neurofibrillary tangles
- Aβ
Amyloid Beta
- αsyn
Alpha synuclein
- Bcl-xL
B-cell lymphoma-extralarge
- mHTT
Mutant huntingtin
- SOD
Superoxide dismutase
- TAR-DNA
trans-activation response element DNA
- TDP
Ribothymidine 5′-diphosphate
- SAHA
Suberoylanilide hydroxamic acid
- FGFBP
Fibroblast growth factor binding protein
- EGF
Epidermal growth factor
- FGF
Fibroblast growth factor
- REST
Repressor element-1 (RE1) silencing transcription factor
- NRSE
Neuron restrictive silencer element
- mTOR
mammalian target of rapamycin
- Erk
Extracellular signal regulated kinase
- P/CAF
p300/CBP associated factor
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Gitler A.D., Dhillon P., Shorter J. Neurodegenerative disease: models, mechanisms, and a new hope. The Company of Biologists Ltd; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jomova K., Vondrakova D., Lawson M., Valko M. Metals, oxidative stress and neurodegenerative disorders. Mol. Cell. Biochem. 2010;345(1-2):91–104. doi: 10.1007/s11010-010-0563-x. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y., Xu E., Musich P.R., Lin F. Mitochondrial dysfunction in neurodegenerative diseases and the potential countermeasure. CNS Neurosci. Ther. 2019;25(7):816–824. doi: 10.1111/cns.13116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewerenz J., Maher P. Chronic glutamate toxicity in neurodegenerative diseases—what is the evidence? Front. Neurosci. 2015;9:469. doi: 10.3389/fnins.2015.00469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D’Mello S.R. Histone deacetylases as targets for the treatment of human neurodegenerative diseases. Drug News Perspect. 2009;22(9):513–524. doi: 10.1358/dnp.2009.22.9.1437959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Simone A., Milelli A. Histone deacetylase inhibitors as multitarget ligands: new players in Alzheimer’s disease drug discovery? ChemMedChem. 2019;14(11):1067–1073. doi: 10.1002/cmdc.201900174. [DOI] [PubMed] [Google Scholar]
- 7.Annunziato A.T., Hansen J.C. Role of histone acetylation in the assembly and modulation of chromatin structures. Gene Expr. 2000;9(1-2):37–61. doi: 10.3727/000000001783992687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alberts B. Molecular biology of the cell. Garland science. 2008.
- 9.Alberts B., Johnson A., Lewis J., Raff M., Roberts K., Walter P. Drosophila and the molecular genetics of pattern formation: Genesis of the body plan.Molecular Biology of the Cell. 4th ed. Garland Science; 2002. [Google Scholar]
- 10.Seto E., Yoshida M. Erasers of histone acetylation: the histone deacetylase enzymes. Cold Spring Harb. Perspect. Biol. 2014;6(4):a018713. doi: 10.1101/cshperspect.a018713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bannister A.J., Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21(3):381–395. doi: 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parbin S., Kar S., Shilpi A., Sengupta D., Deb M., Rath S.K., Patra S.K. Histone deacetylases: a saga of perturbed acetylation homeostasis in cancer. J. Histochem. Cytochem. 2014;62(1):11–33. doi: 10.1369/0022155413506582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh B.N., Zhang G., Hwa Y.L., Li J., Dowdy S.C., Jiang S-W. Nonhistone protein acetylation as cancer therapy targets. Expert Rev. Anticancer Ther. 2010;10(6):935–954. doi: 10.1586/era.10.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang X-J., Grégoire S. Class II histone deacetylases: from sequence to function, regulation, and clinical implication. Mol. Cell. Biol. 2005;25(8):2873–2884. doi: 10.1128/MCB.25.8.2873-2884.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu K., Dai X-L., Huang H-C., Jiang Z-F. Targeting HDACs: A promising therapy for Alzheimer’s disease. Oxidative medicine and cellular longevity. 2011 doi: 10.1155/2011/143269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y., Wang X., Liu L., Wang X. HDAC inhibitor trichostatin A-inhibited survival of dopaminergic neuronal cells. Neurosci. Lett. 2009;467(3):212–216. doi: 10.1016/j.neulet.2009.10.037. [DOI] [PubMed] [Google Scholar]
- 17.Suelves N., Kirkham-McCarthy L., Lahue R.S., Ginés S. A selective inhibitor of histone deacetylase 3 prevents cognitive deficits and suppresses striatal CAG repeat expansions in Huntington’s disease mice. Sci. Rep. 2017;7(1):6082. doi: 10.1038/s41598-017-05125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LoPresti P. The selective HDAC6 inhibitor ACY-738 impacts memory and disease regulation in an animal model of multiple sclerosis. Front. Neurol. 2019;10:519. doi: 10.3389/fneur.2019.00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu J., Frerich J.M., Turtzo L.C., Li S., Chiang J., Yang C., Wang X., Zhang C., Wu C., Sun Z., Niu G., Zhuang Z., Brady R.O., Chen X. Histone deacetylase inhibitors are neuroprotective and preserve NGF-mediated cell survival following traumatic brain injury. Proc. Natl. Acad. Sci. USA. 2013;110(26):10747–10752. doi: 10.1073/pnas.1308950110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wade P.A. Transcriptional control at regulatory checkpoints by histone deacetylases: molecular connections between cancer and chromatin. Hum. Mol. Genet. 2001;10(7):693–698. doi: 10.1093/hmg/10.7.693. [DOI] [PubMed] [Google Scholar]
- 21.Cress W.D., Seto E. Histone deacetylases, transcriptional control, and cancer. J. Cell. Physiol. 2000;184(1):1–16. doi: 10.1002/(SICI)1097-4652(200007)184:1<1:AID-JCP1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 22.Yang W-M., Tsai S-C., Wen Y-D., Fejér G., Seto E. Functional domains of histone deacetylase-3. J. Biol. Chem. 2002;277(11):9447–9454. doi: 10.1074/jbc.M105993200. [DOI] [PubMed] [Google Scholar]
- 23.Buggy J.J., Sideris M.L., Mak P., Lorimer D.D., McIntosh B., Clark J.M. Cloning and characterization of a novel human histone deacetylase, HDAC8. Biochem. J. 2000;350(Pt 1):199–205. doi: 10.1042/bj3500199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marks P., Rifkind R.A., Richon V.M., Breslow R., Miller T., Kelly W.K. Histone deacetylases and cancer: causes and therapies. Nat. Rev. Cancer. 2001;1(3):194–202. doi: 10.1038/35106079. [DOI] [PubMed] [Google Scholar]
- 25.Chawla S., Vanhoutte P., Arnold F.J., Huang C.L.H., Bading H. Neuronal activity-dependent nucleocytoplasmic shuttling of HDAC4 and HDAC5. J. Neurochem. 2003;85(1):151–159. doi: 10.1046/j.1471-4159.2003.01648.x. [DOI] [PubMed] [Google Scholar]
- 26.Majdzadeh N, Morrison BE, D'Mello SR. Class IIA HDACs in the regulation of neurodegeneration.Frontiers in bioscience: a journal and virtual library. 2008;13:1072. doi: 10.2741/2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haberland M., Johnson A., Mokalled M.H., Montgomery R.L., Olson E.N. Genetic dissection of histone deacetylase requirement in tumor cells. Proc. Natl. Acad. Sci. USA. 2009;106(19):7751–7755. doi: 10.1073/pnas.0903139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mottamal M., Zheng S., Huang T.L., Wang G. Histone deacetylase inhibitors in clinical studies as templates for new anticancer agents. Molecules. 2015;20(3):3898–3941. doi: 10.3390/molecules20033898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwata A., Riley B.E., Johnston J.A., Kopito R.R. HDAC6 and microtubules are required for autophagic degradation of aggregated huntingtin. J. Biol. Chem. 2005;280(48):40282–40292. doi: 10.1074/jbc.M508786200. [DOI] [PubMed] [Google Scholar]
- 30.Yalcin G. Sirtuins and neurodegeneration. Journal of Neurology & Neuromedicine. 2018;3(1) doi: 10.29245/2572.942X/2017/1.1168. [DOI] [Google Scholar]
- 31.Majdzadeh N., Wang L., Morrison B.E., Bassel-Duby R., Olson E.N., D’Mello S.R. HDAC4 inhibits cell-cycle progression and protects neurons from cell death. Dev. Neurobiol. 2008;68(8):1076–1092. doi: 10.1002/dneu.20637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chuang D-M., Leng Y., Marinova Z., Kim H-J., Chiu C-T. Multiple roles of HDAC inhibition in neurodegenerative conditions. Trends Neurosci. 2009;32(11):591–601. doi: 10.1016/j.tins.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas E.A. Involvement of HDAC1 and HDAC3 in the pathology of polyglutamine disorders: therapeutic implications for selective HDAC1/HDAC3 inhibitors. Pharmaceuticals (Basel) 2014;7(6):634–661. doi: 10.3390/ph7060634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blander G., Guarente L. The Sir2 family of protein deacetylases. Annu. Rev. Biochem. 2004;73(1):417–435. doi: 10.1146/annurev.biochem.73.011303.073651. [DOI] [PubMed] [Google Scholar]
- 35.Montgomery R.L., Davis C.A., Potthoff M.J., Haberland M., Fielitz J., Qi X., Hill J.A., Richardson J.A., Olson E.N. Histone deacetylases 1 and 2 redundantly regulate cardiac morphogenesis, growth, and contractility. Genes Dev. 2007;21(14):1790–1802. doi: 10.1101/gad.1563807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKinsey T.A., Zhang C-L., Lu J., Olson E.N. Signal-dependent nuclear export of a histone deacetylase regulates muscle differentiation. Nature. 2000;408(6808):106–111. doi: 10.1038/35040593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zupkovitz G., Tischler J., Posch M., Sadzak I., Ramsauer K., Egger G., Grausenburger R., Schweifer N., Chiocca S., Decker T., Seiser C. Negative and positive regulation of gene expression by mouse histone deacetylase 1. Mol. Cell. Biol. 2006;26(21):7913–7928. doi: 10.1128/MCB.01220-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamaguchi M., Tonou-Fujimori N., Komori A., Maeda R., Nojima Y., Li H., Okamoto H., Masai I. Histone deacetylase 1 regulates retinal neurogenesis in zebrafish by suppressing Wnt and Notch signaling pathways. Development. 2005;132(13):3027–3043. doi: 10.1242/dev.01881. [DOI] [PubMed] [Google Scholar]
- 39.Farooq M., Sulochana K.N., Pan X., To J., Sheng D., Gong Z., Ge R. Histone deacetylase 3 (hdac3) is specifically required for liver development in zebrafish. Dev. Biol. 2008;317(1):336–353. doi: 10.1016/j.ydbio.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 40.Thomas E.A., D’Mello S.R. Complex neuroprotective and neurotoxic effects of histone deacetylases. J. Neurochem. 2018;145(2):96–110. doi: 10.1111/jnc.14309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jacob C., Christen C.N., Pereira J.A., Somandin C., Baggiolini A., Lötscher P., Ozçelik M., Tricaud N., Meijer D., Yamaguchi T., Matthias P., Suter U. HDAC1 and HDAC2 control the transcriptional program of myelination and the survival of Schwann cells. Nat. Neurosci. 2011;14(4):429–436. doi: 10.1038/nn.2762. [DOI] [PubMed] [Google Scholar]
- 42.Lebrun-Julien F., Suter U. Combined HDAC1 and HDAC2 depletion promotes retinal ganglion cell survival after injury through reduction of p53 target gene expression. ASN Neuro. 2015;7(3):1759091415593066. doi: 10.1177/1759091415593066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morrison B.E., Majdzadeh N., Zhang X., Lyles A., Bassel-Duby R., Olson E.N., D’Mello S.R. Neuroprotection by histone deacetylase-related protein. Mol. Cell. Biol. 2006;26(9):3550–3564. doi: 10.1128/MCB.26.9.3550-3564.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou H., Cai Y., Liu D., Li M., Sha Y., Zhang W., Wang K., Gong J., Tang N., Huang A., Xia J. Pharmacological or transcriptional inhibition of both HDAC1 and 2 leads to cell cycle blockage and apoptosis via p21Waf1/Cip1 and p19INK4d upregulation in hepatocellular carcinoma. Cell Prolif. 2018;51(3):e12447. doi: 10.1111/cpr.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boucheron N., Tschismarov R., Goeschl L., Moser M.A., Lagger S., Sakaguchi S., Winter M., Lenz F., Vitko D., Breitwieser F.P., Müller L., Hassan H., Bennett K.L., Colinge J., Schreiner W., Egawa T., Taniuchi I., Matthias P., Seiser C., Ellmeier W. CD4(+) T cell lineage integrity is controlled by the histone deacetylases HDAC1 and HDAC2. Nat. Immunol. 2014;15(5):439–448. doi: 10.1038/ni.2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Datta M, Staszewski O, Raschi E, Frosch M, Hagemeyer N. Tay, TL Histone deacetylases 1 and 2 regulate microglia function during development, homeostasis, and neurodegeneration in a context- dependent manner. Immunity, 2018, 48(3), 514-529. e6. [DOI] [PubMed]
- 47.Gräff J., Rei D., Guan J-S., Wang W-Y., Seo J., Hennig K.M., Nieland T.J., Fass D.M., Kao P.F., Kahn M., Su S.C., Samiei A., Joseph N., Haggarty S.J., Delalle I., Tsai L.H. An epigenetic blockade of cognitive functions in the neurodegenerating brain. Nature. 2012;483(7388):222–226. doi: 10.1038/nature10849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lorson C.L., Rindt H., Shababi M. Spinal muscular atrophy: mechanisms and therapeutic strategies. Hum. Mol. Genet. 2010;19(R1):R111–R118. doi: 10.1093/hmg/ddq147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kernochan L.E., Russo M.L., Woodling N.S., Huynh T.N., Avila A.M., Fischbeck K.H., Sumner C.J. The role of histone acetylation in SMN gene expression. Hum. Mol. Genet. 2005;14(9):1171–1182. doi: 10.1093/hmg/ddi130. [DOI] [PubMed] [Google Scholar]
- 50.Sun X-Y., Zheng T., Yang X., Liu L., Gao S-S., Xu H-B., Song Y.T., Tong K., Yang L., Gao Y., Wu T., Hao J.R., Lu C., Ma T., Gao C. HDAC2 hyperexpression alters hippocampal neuronal transcription and microglial activity in neuroinflammation-induced cognitive dysfunction. J. Neuroinflammation. 2019;16(1):249. doi: 10.1186/s12974-019-1640-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guenther M.G., Barak O., Lazar M.A. The SMRT and N-CoR corepressors are activating cofactors for histone deacetylase 3. Mol. Cell. Biol. 2001;21(18):6091–6101. doi: 10.1128/MCB.21.18.6091-6101.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fischle W., Dequiedt F., Fillion M., Hendzel M.J., Voelter W., Verdin E. Human HDAC7 histone deacetylase activity is associated with HDAC3 in vivo. J. Biol. Chem. 2001;276(38):35826–35835. doi: 10.1074/jbc.M104935200. [DOI] [PubMed] [Google Scholar]
- 53.Dequiedt F., Van Lint J., Lecomte E., Van Duppen V., Seufferlein T., Vandenheede J.R., Wattiez R., Kettmann R. Phosphorylation of histone deacetylase 7 by protein kinase D mediates T cell receptor-induced Nur77 expression and apoptosis. J. Exp. Med. 2005;201(5):793–804. doi: 10.1084/jem.20042034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li J., Lin Q., Wang W., Wade P., Wong J. Specific targeting and constitutive association of histone deacetylase complexes during transcriptional repression. Genes Dev. 2002;16(6):687–692. doi: 10.1101/gad.962502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brehm A., Miska E.A., McCance D.J., Reid J.L., Bannister A.J., Kouzarides T. Retinoblastoma protein recruits histone deacetylase to repress transcription. Nature. 1998;391(6667):597–601. doi: 10.1038/35404. [DOI] [PubMed] [Google Scholar]
- 56.Nicolas E., Ait-Si-Ali S., Trouche D. The histone deacetylase HDAC3 targets RbAp48 to the retinoblastoma protein. Nucleic Acids Res. 2001;29(15):3131–3136. doi: 10.1093/nar/29.15.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Katayama S., Morii A., Makanga J.O., Suzuki T., Miyata N., Inazu T. HDAC8 regulates neural differentiation through embryoid body formation in P19 cells. Biochem. Biophys. Res. Commun. 2018;498(1):45–51. doi: 10.1016/j.bbrc.2018.02.195. [DOI] [PubMed] [Google Scholar]
- 58.Ruijter, AJd; GENNIP, AHv; Caron, HN; Kemp, S; KUILENBURG, ABv Histone deacetylases (HDACs): Characterization of the classical HDAC family. Biochem. J. 2003;370(3):737–749. doi: 10.1042/bj20021321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chakrabarti A., Oehme I., Witt O., Oliveira G., Sippl W., Romier C., Pierce R.J., Jung M. HDAC8: a multifaceted target for therapeutic interventions. Trends Pharmacol. Sci. 2015;36(7):481–492. doi: 10.1016/j.tips.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 60.Alam N., Zimmerman L., Wolfson N.A., Joseph C.G., Fierke C.A., Schueler-Furman O. Structure-based identification of HDAC8 non-histone substrates. Structure. 2016;24(3):458–468. doi: 10.1016/j.str.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takase K., Oda S., Kuroda M., Funato H. Monoaminergic and neuropeptidergic neurons have distinct expression profiles of histone deacetylases. PLoS One. 2013;8(3):e58473. doi: 10.1371/journal.pone.0058473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mielcarek M., Zielonka D., Carnemolla A., Marcinkowski J.T., Guidez F. HDAC4 as a potential therapeutic target in neurodegenerative diseases: a summary of recent achievements. Front. Cell. Neurosci. 2015;9:42. doi: 10.3389/fncel.2015.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Korzus E., Rosenfeld M.G., Mayford M. CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron. 2004;42(6):961–972. doi: 10.1016/j.neuron.2004.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fitzsimons H.L., Schwartz S., Given F.M., Scott M.J. The histone deacetylase HDAC4 regulates long-term memory in Drosophila. PLoS One. 2013;8(12):e83903. doi: 10.1371/journal.pone.0083903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhu Y., Huang M., Bushong E., Phan S., Uytiepo M., Beutter E., Boemer D., Tsui K., Ellisman M., Maximov A. Class IIa HDACs regulate learning and memory through dynamic experience-dependent repression of transcription. Nat. Commun. 2019;10(1):3469. doi: 10.1038/s41467-019-11409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sando R., III, Gounko N., Pieraut S., Liao L., Yates J., III, Maximov A. HDAC4 governs a transcriptional program essential for synaptic plasticity and memory. Cell. 2012;151(4):821–834. doi: 10.1016/j.cell.2012.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fischer A., Sananbenesi F., Wang X., Dobbin M., Tsai L-H. Recovery of learning and memory is associated with chromatin remodelling. Nature. 2007;447(7141):178–182. doi: 10.1038/nature05772. [DOI] [PubMed] [Google Scholar]
- 68.Kim M-S., Akhtar M.W., Adachi M., Mahgoub M., Bassel-Duby R., Kavalali E.T., Olson E.N., Monteggia L.M. An essential role for histone deacetylase 4 in synaptic plasticity and memory formation. J. Neurosci. 2012;32(32):10879–10886. doi: 10.1523/JNEUROSCI.2089-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arnold M.A., Kim Y., Czubryt M.P., Phan D., McAnally J., Qi X., Shelton J.M., Richardson J.A., Bassel-Duby R., Olson E.N. MEF2C transcription factor controls chondrocyte hypertrophy and bone development. Dev. Cell. 2007;12(3):377–389. doi: 10.1016/j.devcel.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 70.Lang C, Campbell KR, Ryan BJ, Carling P, Attar M, Vowles J. Single-cell sequencing of iPSC-dopamine neurons reconstructs disease progression and identifies HDAC4 as a regulator of Parkinson cell phenotypes.Cell stem cell, 2019;24(1):93-106–e6. doi: 10.1016/j.stem.2018.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cho Y., Cavalli V. HDAC5 is a novel injury-regulated tubulin deacetylase controlling axon regeneration. EMBO J. 2012;31(14):3063–3078. doi: 10.1038/emboj.2012.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cho Y., Sloutsky R., Naegle K.M., Cavalli V. Injury-induced HDAC5 nuclear export is essential for axon regeneration. Cell. 2013;155(4):894–908. doi: 10.1016/j.cell.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haberland M., Montgomery R.L., Olson E.N. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat. Rev. Genet. 2009;10(1):32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Agis-Balboa R.C., Pavelka Z., Kerimoglu C., Fischer A. Loss of HDAC5 impairs memory function: implications for Alzheimer’s disease. J. Alzheimers Dis. 2013;33(1):35–44. doi: 10.3233/JAD-2012-121009. [DOI] [PubMed] [Google Scholar]
- 75.Ma C., D’Mello S.R. Neuroprotection by histone deacetylase-7 (HDAC7) occurs by inhibition of c-jun expression through a deacetylase-independent mechanism. J. Biol. Chem. 2011;286(6):4819–4828. doi: 10.1074/jbc.M110.146860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jia H., Pallos J., Jacques V., Lau A., Tang B., Cooper A., Syed A., Purcell J., Chen Y., Sharma S., Sangrey G.R., Darnell S.B., Plasterer H., Sadri-Vakili G., Gottesfeld J.M., Thompson L.M., Rusche J.R., Marsh J.L., Thomas E.A. Histone deacetylase (HDAC) inhibitors targeting HDAC3 and HDAC1 ameliorate polyglutamine-elicited phenotypes in model systems of Huntington’s disease. Neurobiol. Dis. 2012;46(2):351–361. doi: 10.1016/j.nbd.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kao H-Y., Verdel A., Tsai C-C., Simon C., Juguilon H., Khochbin S. Mechanism for nucleocytoplasmic shuttling of histone deacetylase 7. J. Biol. Chem. 2001;276(50):47496–47507. doi: 10.1074/jbc.M107631200. [DOI] [PubMed] [Google Scholar]
- 78.Bertos N.R., Wang A.H., Yang X-J. Class II histone deacetylases: structure, function, and regulation. Biochem. Cell Biol. 2001;79(3):243–252. doi: 10.1139/o01-032. [DOI] [PubMed] [Google Scholar]
- 79.Benn C.L., Butler R., Mariner L., Nixon J., Moffitt H., Mielcarek M., Woodman B., Bates G.P. Genetic knock-down of HDAC7 does not ameliorate disease pathogenesis in the R6/2 mouse model of Huntington’s disease. PLoS One. 2009;4(6):e5747. doi: 10.1371/journal.pone.0005747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou X., Marks P.A., Rifkind R.A., Richon V.M. Cloning and characterization of a histone deacetylase, HDAC9. Proc. Natl. Acad. Sci. USA. 2001;98(19):10572–10577. doi: 10.1073/pnas.191375098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Foolad F., Khodagholi F., Javan M. Sirtuins in Multiple Sclerosis: The crossroad of neurodegeneration, autoimmunity and metabolism. Mult. Scler. Relat. Disord. 2019;34:47–58. doi: 10.1016/j.msard.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 82.He H., Hu Z., Xiao H., Zhou F., Yang B. The tale of histone modifications and its role in multiple sclerosis. Hum. Genomics. 2018;12(1):31. doi: 10.1186/s40246-018-0163-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kovacs J.J., Murphy P.J., Gaillard S., Zhao X., Wu J-T., Nicchitta C.V., Yoshida M., Toft D.O., Pratt W.B., Yao T.P. HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Mol. Cell. 2005;18(5):601–607. doi: 10.1016/j.molcel.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 84.Zhang X., Yuan Z., Zhang Y., Yong S., Salas-Burgos A., Koomen J., Olashaw N., Parsons J.T., Yang X.J., Dent S.R., Yao T.P., Lane W.S., Seto E. HDAC6 modulates cell motility by altering the acetylation level of cortactin. Mol. Cell. 2007;27(2):197–213. doi: 10.1016/j.molcel.2007.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rivieccio M.A., Brochier C., Willis D.E., Walker B.A., D’Annibale M.A., McLaughlin K., Siddiq A., Kozikowski A.P., Jaffrey S.R., Twiss J.L., Ratan R.R., Langley B. HDAC6 is a target for protection and regeneration following injury in the nervous system. Proc. Natl. Acad. Sci. USA. 2009;106(46):19599–19604. doi: 10.1073/pnas.0907935106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Perry S., Kiragasi B., Dickman D., Ray A. The role of histone deacetylase 6 in synaptic plasticity and memory. Cell Rep. 2017;18(6):1337–1345. doi: 10.1016/j.celrep.2017.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Finnin M.S., Donigian J.R., Cohen A., Richon V.M., Rifkind R.A., Marks P.A., Breslow R., Pavletich N.P. Structures of a histone deacetylase homologue bound to the TSA and SAHA inhibitors. Nature. 1999;401(6749):188–193. doi: 10.1038/43710. [DOI] [PubMed] [Google Scholar]
- 88.Ganai S.A. A Ganai S. Small-molecule modulation of HDAC6 activity: the propitious therapeutic strategy to vanquish neurodegenerative disorders. Curr. Med. Chem. 2017;24(37):4104–4120. doi: 10.2174/0929867324666170209104030. [DOI] [PubMed] [Google Scholar]
- 89.Guo W, Van Den Bosch L. Therapeutic potential of HDAC6 in amyotrophic lateral sclerosis.Cell stress, 2018;2(1) doi: 10.15698/cst2018.01.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guardiola A.R., Yao T-P. Molecular cloning and characterization of a novel histone deacetylase HDAC10. J. Biol. Chem. 2002;277(5):3350–3356. doi: 10.1074/jbc.M109861200. [DOI] [PubMed] [Google Scholar]
- 91.Wang L., Zheng S., Zhang L., Xiao H., Gan H., Chen H., Zhai X., Liang P., Zhao J., Li Y. Histone deacetylation 10 alleviates inflammation after intracerebral hemorrhage via the PTPN22/NLRP3 pathway in rats. Neuroscience. 2020;432:247–259. doi: 10.1016/j.neuroscience.2020.02.027. [DOI] [PubMed] [Google Scholar]
- 92.Liao W., Sun J., Liu W., Li W., Jia J., Ou F., Su K., Zheng Y., Zhang Z., Sun Y. HDAC10 upregulation contributes to interleukin 1β-mediated inflammatory activation of synovium-derived mesenchymal stem cells in temporomandibular joint. J. Cell. Physiol. 2019;234(8):12646–12662. doi: 10.1002/jcp.27873. [DOI] [PubMed] [Google Scholar]
- 93.Michan S., Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem. J. 2007;404(1):1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kroemer G., Mariño G., Levine B. Autophagy and the integrated stress response. Mol. Cell. 2010;40(2):280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nemoto S., Fergusson M.M., Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1α. J. Biol. Chem. 2005;280(16):16456–16460. doi: 10.1074/jbc.M501485200. [DOI] [PubMed] [Google Scholar]
- 96.Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. science, 2004;305(5682):390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- 97.Qadir MI, Anwar S. Sirtuins in brain aging and neurological disorders.Critical Reviews™ in Eukaryotic Gene Expression, 2017;27(4) doi: 10.1615/CritRevEukaryotGeneExpr.2017019532. [DOI] [PubMed] [Google Scholar]
- 98.Jeong H., Cohen D.E., Cui L., Supinski A., Savas J.N., Mazzulli J.R., Yates J.R., III, Bordone L., Guarente L., Krainc D. Sirt1 mediates neuroprotection from mutant huntingtin by activation of the TORC1 and CREB transcriptional pathway. Nat. Med. 2011;18(1):159–165. doi: 10.1038/nm.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Imai S., Armstrong C.M., Kaeberlein M., Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403(6771):795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- 100.Li W., Zhang B., Tang J., Cao Q., Wu Y., Wu C., Guo J., Ling E.A., Liang F. Sirtuin 2, a mammalian homolog of yeast silent information regulator-2 longevity regulator, is an oligodendroglial protein that decelerates cell differentiation through deacetylating α-tubulin. J. Neurosci. 2007;27(10):2606–2616. doi: 10.1523/JNEUROSCI.4181-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Guarente L. Calorie restriction and sirtuins revisited. Genes Dev. 2013;27(19):2072–2085. doi: 10.1101/gad.227439.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Donmez G., Outeiro T.F. SIRT1 and SIRT2: emerging targets in neurodegeneration. EMBO Mol. Med. 2013;5(3):344–352. doi: 10.1002/emmm.201302451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee I.H. Mechanisms and disease implications of sirtuin-mediated autophagic regulation. Exp. Mol. Med. 2019;51(9):1–11. doi: 10.1038/s12276-019-0302-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang F., Wang S., Gan L., Vosler P.S., Gao Y., Zigmond M.J., Chen J. Protective effects and mechanisms of sirtuins in the nervous system. Prog. Neurobiol. 2011;95(3):373–395. doi: 10.1016/j.pneurobio.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang J., Xiang H., Liu J., Chen Y., He R-R., Liu B. Mitochondrial Sirtuin 3: New emerging biological function and therapeutic target. Theranostics. 2020;10(18):8315–8342. doi: 10.7150/thno.45922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vernucci E., Tomino C., Molinari F., Limongi D., Aventaggiato M, Sansone L. Mitophagy and oxidative stress in cancer and aging: focus on sirtuins and nanomaterials. Oxidative medicine and cellular longevity. 2019 doi: 10.1155/2019/6387357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ye X., Li M., Hou T., Gao T., Zhu W.G., Yang Y. Sirtuins in glucose and lipid metabolism. Oncotarget. 2017;8(1):1845–1859. doi: 10.18632/oncotarget.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Morin N., Jourdain V.A., Di Paolo T. Modeling dyskinesia in animal models of Parkinson disease. Exp. Neurol. 2014;256:105–116. doi: 10.1016/j.expneurol.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 109.Salvatori I., Valle C., Ferri A., Carrì M.T. SIRT3 and mitochondrial metabolism in neurodegenerative diseases. Neurochem. Int. 2017;109:184–192. doi: 10.1016/j.neuint.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 110.Han Y., Zhou S., Coetzee S., Chen A. SIRT4 and its roles in energy and redox metabolism in health, disease and during exercise. Front. Physiol. 2019;10:1006. doi: 10.3389/fphys.2019.01006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shih J., Liu L., Mason A., Higashimori H., Donmez G. Loss of SIRT4 decreases GLT-1-dependent glutamate uptake and increases sensitivity to kainic acid. J. Neurochem. 2014;131(5):573–581. doi: 10.1111/jnc.12942. [DOI] [PubMed] [Google Scholar]
- 112.Schlicker C., Gertz M., Papatheodorou P., Kachholz B., Becker C.F., Steegborn C. Substrates and regulation mechanisms for the human mitochondrial sirtuins Sirt3 and Sirt5. J. Mol. Biol. 2008;382(3):790–801. doi: 10.1016/j.jmb.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 113.Wang C-H., Wei Y-H. Roles of Mitochondrial Sirtuins in Mitochondrial Function, Redox Homeostasis, Insulin Resistance and Type 2 Diabetes. Int. J. Mol. Sci. 2020;21(15):5266. doi: 10.3390/ijms21155266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li F., Liu L. SIRT5 Deficiency Enhances susceptibility to kainate-induced seizures and exacerbates hippocampal neurodegeneration not through mitochondrial antioxidant enzyme SOD2. Front. Cell. Neurosci. 2016;10:171. doi: 10.3389/fncel.2016.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tennen R.I., Chua K.F. Chromatin regulation and genome maintenance by mammalian SIRT6. Trends Biochem. Sci. 2011;36(1):39–46. doi: 10.1016/j.tibs.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang Y., He J., Liao M., Hu M., Li W., Ouyang H., Wang X., Ye T., Zhang Y., Ouyang L. An overview of Sirtuins as potential therapeutic target: Structure, function and modulators. Eur. J. Med. Chem. 2019;161:48–77. doi: 10.1016/j.ejmech.2018.10.028. [DOI] [PubMed] [Google Scholar]
- 117.Mostoslavsky R., Chua K.F., Lombard D.B., Pang W.W., Fischer M.R., Gellon L., Liu P., Mostoslavsky G., Franco S., Murphy M.M., Mills K.D., Patel P., Hsu J.T., Hong A.L., Ford E., Cheng H.L., Kennedy C., Nunez N., Bronson R., Frendewey D., Auerbach W., Valenzuela D., Karow M., Hottiger M.O., Hursting S., Barrett J.C., Guarente L., Mulligan R., Demple B., Yancopoulos G.D., Alt F.W. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006;124(2):315–329. doi: 10.1016/j.cell.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 118.Ford E., Voit R., Liszt G., Magin C., Grummt I., Guarente L. Mammalian Sir2 homolog SIRT7 is an activator of RNA polymerase I transcription. Genes Dev. 2006;20(9):1075–1080. doi: 10.1101/gad.1399706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fan B., Lu K-Y., Reymond Sutandy F.X., Chen Y-W., Konan K., Zhu H., Kao C.C., Chen C.S. A human proteome microarray identifies that the heterogeneous nuclear ribonucleoprotein K (hnRNP K) recognizes the 5′ terminal sequence of the hepatitis C virus RNA. Mol. Cell. Proteomics. 2014;13(1):84–92. doi: 10.1074/mcp.M113.031682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vazquez B.N., Thackray J.K., Serrano L. Sirtuins and DNA damage repair: SIRT7 comes to play. Nucleus. 2017;8(2):107–115. doi: 10.1080/19491034.2016.1264552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.George S., Palli S.R. Histone deacetylase 11 knockdown blocks larval development and metamorphosis in the red flour beetle, Tribolium castaneum. Front. Genet. 2020;11:683. doi: 10.3389/fgene.2020.00683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gao L., Cueto M.A., Asselbergs F., Atadja P. Cloning and functional characterization of HDAC11, a novel member of the human histone deacetylase family. J. Biol. Chem. 2002;277(28):25748–25755. doi: 10.1074/jbc.M111871200. [DOI] [PubMed] [Google Scholar]
- 123.Licciardi P.V, Karagiannis T.C. Regulation of immune responses by histone deacetylase inhibitors. International Scholarly Research Notices. 2012 doi: 10.5402/2012/690901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kelley B.J., Petersen R.C. Alzheimer’s disease and mild cognitive impairment. Neurol. Clin. 2007;25(3):577–609. doi: 10.1016/j.ncl.2007.03.008. v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Karantzoulis S., Galvin J.E. Distinguishing Alzheimer’s disease from other major forms of dementia. Expert Rev. Neurother. 2011;11(11):1579–1591. doi: 10.1586/ern.11.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Guan J-S., Haggarty S.J., Giacometti E., Dannenberg J-H., Joseph N., Gao J., Nieland T.J., Zhou Y., Wang X., Mazitschek R., Bradner J.E., DePinho R.A., Jaenisch R., Tsai L.H. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009;459(7243):55–60. doi: 10.1038/nature07925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.McQuown S.C., Barrett R.M., Matheos D.P., Post R.J., Rogge G.A., Alenghat T., Mullican S.E., Jones S., Rusche J.R., Lazar M.A., Wood M.A. HDAC3 is a critical negative regulator of long-term memory formation. J. Neurosci. 2011;31(2):764–774. doi: 10.1523/JNEUROSCI.5052-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bardai F.H., D’Mello S.R. Selective toxicity by HDAC3 in neurons: regulation by Akt and GSK3β. J. Neurosci. 2011;31(5):1746–1751. doi: 10.1523/JNEUROSCI.5704-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Szegő É.M., Outeiro T.F., Kazantsev A.G. Sirtuins in brain and neurodegenerative disease. Introductory Review on Sirtuins in Biology, Aging, and Disease. Elsevier; 2018. pp. 175–195. [Google Scholar]
- 130.de Lau L.M., Breteler M.M. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5(6):525–535. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 131.Dietz K.C., Casaccia P. HDAC inhibitors and neurodegeneration: at the edge between protection and damage. Pharmacol. Res. 2010;62(1):11–17. doi: 10.1016/j.phrs.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shukla S., Tekwani B.L. Histone deacetylases inhibitors in neurodegenerative diseases, neuroprotection and neuronal differentiation. Front. Pharmacol. 2020;11:537. doi: 10.3389/fphar.2020.00537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Harrison I.F., Powell N.M., Dexter D.T. The histone deacetylase inhibitor nicotinamide exacerbates neurodegeneration in the lactacystin rat model of Parkinson’s disease. J. Neurochem. 2019;148(1):136–156. doi: 10.1111/jnc.14599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wu X., Chen P.S., Dallas S., Wilson B., Block M.L., Wang C-C., Kinyamu H., Lu N., Gao X., Leng Y., Chuang D.M., Zhang W., Lu R.B., Hong J.S. Histone deacetylase inhibitors up-regulate astrocyte GDNF and BDNF gene transcription and protect dopaminergic neurons. Int. J. Neuropsychopharmacol. 2008;11(8):1123–1134. doi: 10.1017/S1461145708009024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Glozak MA, Sengupta N, Zhang X, Seto E. Acetylation and deacetylation of non-histone proteins. gene, 2005;363:15–23. doi: 10.1016/j.gene.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 136.Dompierre J.P., Godin J.D., Charrin B.C., Cordelières F.P., King S.J., Humbert S., Saudou F. Histone deacetylase 6 inhibition compensates for the transport deficit in Huntington’s disease by increasing tubulin acetylation. J. Neurosci. 2007;27(13):3571–3583. doi: 10.1523/JNEUROSCI.0037-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang Y., Li N., Caron C., Matthias G., Hess D., Khochbin S., Matthias P. HDAC-6 interacts with and deacetylates tubulin and microtubules in vivo. EMBO J. 2003;22(5):1168–1179. doi: 10.1093/emboj/cdg115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Suh H-S., Choi S., Khattar P., Choi N., Lee S.C. Histone deacetylase inhibitors suppress the expression of inflammatory and innate immune response genes in human microglia and astrocytes. J. Neuroimmune Pharmacol. 2010;5(4):521–532. doi: 10.1007/s11481-010-9192-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu L., Peritore C., Ginsberg J., Shih J., Arun S., Donmez G. Protective role of SIRT5 against motor deficit and dopaminergic degeneration in MPTP-induced mice model of Parkinson’s disease. Behav. Brain Res. 2015;281:215–221. doi: 10.1016/j.bbr.2014.12.035. [DOI] [PubMed] [Google Scholar]
- 140.Reuter I., Tai Y.F., Pavese N., Chaudhuri K.R., Mason S., Polkey C.E., Clough C., Brooks D.J., Barker R.A., Piccini P. Long-term clinical and positron emission tomography outcome of fetal striatal transplantation in Huntington’s disease. J. Neurol. Neurosurg. Psychiatry. 2008;79(8):948–951. doi: 10.1136/jnnp.2007.142380. [DOI] [PubMed] [Google Scholar]
- 141.Beal M.F., Ferrante R.J., Swartz K.J., Kowall N.W. Chronic quinolinic acid lesions in rats closely resemble Huntington’s disease. J. Neurosci. 1991;11(6):1649–1659. doi: 10.1523/JNEUROSCI.11-06-01649.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Valor L.M. Understanding histone deacetylation in Huntington’s disease. Oncotarget. 2017;8(4):5660–5661. doi: 10.18632/oncotarget.13924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sadri-Vakili G., Cha J-H.J. Histone deacetylase inhibitors: a novel therapeutic approach to Huntington’s disease (complex mechanism of neuronal death). Curr. Alzheimer Res. 2006;3(4):403–408. doi: 10.2174/156720506778249407. [DOI] [PubMed] [Google Scholar]
- 144.Gardian G., Browne S.E., Choi D-K., Klivenyi P., Gregorio J., Kubilus J.K., Ryu H., Langley B., Ratan R.R., Ferrante R.J., Beal M.F. Neuroprotective effects of phenylbutyrate in the N171-82Q transgenic mouse model of Huntington’s disease. J. Biol. Chem. 2005;280(1):556–563. doi: 10.1074/jbc.M410210200. [DOI] [PubMed] [Google Scholar]
- 145.Jia H., Morris C.D., Williams R.M., Loring J.F., Thomas E.A. HDAC inhibition imparts beneficial transgenerational effects in Huntington’s disease mice via altered DNA and histone methylation. Proc. Natl. Acad. Sci. USA. 2015;112(1):E56–E64. doi: 10.1073/pnas.1415195112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wijesekera L.C., Leigh P.N. Amyotrophic lateral sclerosis. Orphanet J. Rare Dis. 2009;4(1):3. doi: 10.1186/1750-1172-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rossaert E., Pollari E., Jaspers T., Van Helleputte L., Jarpe M., Van Damme P., De Bock K., Moisse M., Van Den Bosch L. Restoration of histone acetylation ameliorates disease and metabolic abnormalities in a FUS mouse model. Acta Neuropathol. Commun. 2019;7(1):107. doi: 10.1186/s40478-019-0750-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pigna E., Simonazzi E., Sanna K., Bernadzki K.M., Proszynski T., Heil C., Palacios D., Adamo S., Moresi V. Histone deacetylase 4 protects from denervation and skeletal muscle atrophy in a murine model of amyotrophic lateral sclerosis. EBioMedicine. 2019;40:717–732. doi: 10.1016/j.ebiom.2019.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kuta R., Larochelle N., Fernandez M., Pal A., Minotti S., Tibshirani M., St Louis K., Gentil B.J., Nalbantoglu J.N., Hermann A., Durham H.D. Depending on the stress, histone deacetylase inhibitors act as heat shock protein co-inducers in motor neurons and potentiate arimoclomol, exerting neuroprotection through multiple mechanisms in ALS models. Cell Stress Chaperones. 2020;25(1):173–191. doi: 10.1007/s12192-019-01064-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lagger G., O’Carroll D., Rembold M., Khier H., Tischler J., Weitzer G., Schuettengruber B., Hauser C., Brunmeir R., Jenuwein T., Seiser C. Essential function of histone deacetylase 1 in proliferation control and CDK inhibitor repression. EMBO J. 2002;21(11):2672–2681. doi: 10.1093/emboj/21.11.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yang W-M., Yao Y-L., Sun J-M., Davie J.R., Seto E. Isolation and characterization of cDNAs corresponding to an additional member of the human histone deacetylase gene family. J. Biol. Chem. 1997;272(44):28001–28007. doi: 10.1074/jbc.272.44.28001. [DOI] [PubMed] [Google Scholar]
- 152.Longworth M.S., Laimins L.A. Histone deacetylase 3 localizes to the plasma membrane and is a substrate of Src. Oncogene. 2006;25(32):4495–4500. doi: 10.1038/sj.onc.1209473. [DOI] [PubMed] [Google Scholar]
- 153.Hu E., Chen Z., Fredrickson T., Zhu Y., Kirkpatrick R., Zhang G-F., Johanson K., Sung C.M., Liu R., Winkler J. Cloning and characterization of a novel human class I histone deacetylase that functions as a transcription repressor. J. Biol. Chem. 2000;275(20):15254–15264. doi: 10.1074/jbc.M908988199. [DOI] [PubMed] [Google Scholar]
- 154.Ganai S.A. Histone Deacetylase Inhibitors-Epidrugs for Neurological Disorders. Springer; 2019. [DOI] [Google Scholar]
- 155.Rumbaugh G., Sillivan S.E., Ozkan E.D., Rojas C.S., Hubbs C.R., Aceti M., Kilgore M., Kudugunti S., Puthanveettil S.V., Sweatt J.D., Rusche J., Miller C.A. Pharmacological selectivity within class I histone deacetylases predicts effects on synaptic function and memory rescue. Neuropsychopharmacology. 2015;40(10):2307–2316. doi: 10.1038/npp.2015.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jeong J.H., An J.Y., Kwon Y.T., Rhee J.G., Lee Y.J. Effects of low dose quercetin: cancer cell-specific inhibition of cell cycle progression. J. Cell. Biochem. 2009;106(1):73–82. doi: 10.1002/jcb.21977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hockly E., Richon V.M., Woodman B., Smith D.L., Zhou X., Rosa E., Sathasivam K., Ghazi-Noori S., Mahal A., Lowden P.A., Steffan J.S., Marsh J.L., Thompson L.M., Lewis C.M., Marks P.A., Bates G.P. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor, ameliorates motor deficits in a mouse model of Huntington’s disease. Proc. Natl. Acad. Sci. USA. 2003;100(4):2041–2046. doi: 10.1073/pnas.0437870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Chang, JK Tolfenamic acid: A potential modifier of tau protein in Alzheimer's disease. 2015 (Thesis).
- 159.Guo W., Naujock M., Fumagalli L., Vandoorne T., Baatsen P., Boon R., Ordovás L., Patel A., Welters M., Vanwelden T., Geens N., Tricot T., Benoy V., Steyaert J., Lefebvre-Omar C., Boesmans W., Jarpe M., Sterneckert J., Wegner F., Petri S., Bohl D., Vanden B.P., Robberecht W., Van Damme P., Verfaillie C., Van Den Bosch L. HDAC6 inhibition reverses axonal transport defects in motor neurons derived from FUS-ALS patients. Nat. Commun. 2017;8(1):861. doi: 10.1038/s41467-017-00911-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cudkowicz M.E., Andres P.L., Macdonald S.A., Bedlack R.S., Choudry R., Brown R.H., Jr, Zhang H., Schoenfeld D.A., Shefner J., Matson S., Matson W.R., Ferrante R.J. Northeast ALS and National VA ALS Research Consortiums. Phase 2 study of sodium phenylbutyrate in ALS. Amyotroph. Lateral Scler. 2009;10(2):99–106. doi: 10.1080/17482960802320487. [DOI] [PubMed] [Google Scholar]
- 161.Buonvicino D., Felici R., Ranieri G., Caramelli R., Lapucci A., Cavone L., Muzzi M., Di Pietro L., Bernardini C., Zwergel C., Valente S., Mai A., Chiarugi A. Effects of class II-selective histone deacetylase inhibitor on neuromuscular function and disease progression in SOD1-ALS mice. Neuroscience. 2018;379:228–238. doi: 10.1016/j.neuroscience.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 162.Grozinger C.M., Hassig C.A., Schreiber S.L. Three proteins define a class of human histone deacetylases related to yeast Hda1p. Proc. Natl. Acad. Sci. USA. 1999;96(9):4868–4873. doi: 10.1073/pnas.96.9.4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wu L-P., Wang X., Li L., Zhao Y., Lu S., Yu Y., Zhou W., Liu X., Yang J., Zheng Z., Zhang H., Feng J., Yang Y., Wang H., Zhu W.G. Histone deacetylase inhibitor depsipeptide activates silenced genes through decreasing both CpG and H3K9 methylation on the promoter. Mol. Cell. Biol. 2008;28(10):3219–3235. doi: 10.1128/MCB.01516-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Maxwell P.H., Wiesener M.S., Chang G-W., Clifford S.C., Vaux E.C., Cockman M.E., Wykoff C.C., Pugh C.W., Maher E.R., Ratcliffe P.J. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399(6733):271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 165.Liu Y., Zhang Y., Zhu K., Chi S., Wang C., Xie A. Emerging role of Sirtuin 2 in Parkinson’s disease. Front. Aging Neurosci. 2020;11:372. doi: 10.3389/fnagi.2019.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhang L., Hou X., Ma R., Moley K., Schedl T., Wang Q. Sirt2 functions in spindle organization and chromosome alignment in mouse oocyte meiosis. FASEB J. 2014;28(3):1435–1445. doi: 10.1096/fj.13-244111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Meng H., Yan W-Y., Lei Y-H., Wan Z., Hou Y-Y., Sun L-K., Zhou J.P. SIRT3 regulation of mitochondrial quality control in neurodegenerative diseases. Front. Aging Neurosci. 2019;11:313. doi: 10.3389/fnagi.2019.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhang J-Y., Deng Y-N., Zhang M., Su H., Qu Q-M. SIRT3 acts as a neuroprotective agent in rotenone-induced Parkinson cell model. Neurochem. Res. 2016;41(7):1761–1773. doi: 10.1007/s11064-016-1892-2. [DOI] [PubMed] [Google Scholar]
- 169.Moraes D.S., Moreira D.C., Andrade J.M.O., Santos S.H.S. Sirtuins, brain and cognition: A review of resveratrol effects. IBRO Rep. 2020;9:46–51. doi: 10.1016/j.ibror.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Min S-W., Sohn P.D., Cho S-H., Swanson R.A., Gan L. Sirtuins in neurodegenerative diseases: an update on potential mechanisms. Front. Aging Neurosci. 2013;5:53. doi: 10.3389/fnagi.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kaluski S., Portillo M., Besnard A., Stein D., Einav M., Zhong L., Ueberham U., Arendt T., Mostoslavsky R., Sahay A., Toiber D. Neuroprotective functions for the histone deacetylase SIRT6. Cell Rep. 2017;18(13):3052–3062. doi: 10.1016/j.celrep.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Durham B.S., Grigg R., Wood I.C. Inhibition of histone deacetylase 1 or 2 reduces induced cytokine expression in microglia through a protein synthesis independent mechanism. J. Neurochem. 2017;143(2):214–224. doi: 10.1111/jnc.14144. [DOI] [PubMed] [Google Scholar]
- 173.Pirooznia S.K., Elefant F. Targeting specific HATs for neurodegenerative disease treatment: translating basic biology to therapeutic possibilities. Front. Cell. Neurosci. 2013;7:30. doi: 10.3389/fncel.2013.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bournival J., Quessy P., Martinoli M-G. Protective effects of resveratrol and quercetin against MPP+ -induced oxidative stress act by modulating markers of apoptotic death in dopaminergic neurons. Cell. Mol. Neurobiol. 2009;29(8):1169–1180. doi: 10.1007/s10571-009-9411-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mochel F., Haller R.G. Energy deficit in Huntington disease: why it matters. J. Clin. Invest. 2011;121(2):493–499. doi: 10.1172/JCI45691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chandramowlishwaran P., Vijay A., Abraham D., Li G., Mwangi S.M., Srinivasan S. Role of Sirtuins in modulating neurodegeneration of the Enteric Nervous system and Central Nervous system. Front. Neurosci. 2020;14:614331. doi: 10.3389/fnins.2020.614331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hildreth K.L., Van Pelt R.E., Schwartz R.S. Obesity, insulin resistance, and Alzheimer’s disease. Obesity (Silver Spring) 2012;20(8):1549–1557. doi: 10.1038/oby.2012.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Singh P., Hanson P.S., Morris C.M. SIRT1 ameliorates oxidative stress induced neural cell death and is down-regulated in Parkinson’s disease. BMC Neurosci. 2017;18(1):46. doi: 10.1186/s12868-017-0364-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Qiao Y., Sun J., Xia S., Tang X., Shi Y., Le G. Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity. Food Funct. 2014;5(6):1241–1249. doi: 10.1039/c3fo60630a. [DOI] [PubMed] [Google Scholar]
- 180.Green K.N., Steffan J.S., Martinez-Coria H., Sun X., Schreiber S.S., Thompson L.M., LaFerla F.M. Nicotinamide restores cognition in Alzheimer’s disease transgenic mice via a mechanism involving sirtuin inhibition and selective reduction of Thr231-phosphotau. J. Neurosci. 2008;28(45):11500–11510. doi: 10.1523/JNEUROSCI.3203-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Donmez G. The effects of SIRT1 on Alzheimer’s disease models. Int. J. Alzheimers Dis. 2012;2012:509–529. doi: 10.1155/2012/509529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cacabelos R., Carril J.C., Cacabelos N., Kazantsev A.G., Vostrov A.V., Corzo L., Cacabelos P., Goldgaber D. Sirtuins in Alzheimer’s disease: SIRT2-related genophenotypes and implications for pharmacoepigenetics. Int. J. Mol. Sci. 2019;20(5):1249. doi: 10.3390/ijms20051249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lalla R., Donmez G. The role of sirtuins in Alzheimer’s disease. Front. Aging Neurosci. 2013;5:16. doi: 10.3389/fnagi.2013.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Herskovits A.Z., Guarente L. SIRT1 in neurodevelopment and brain senescence. Neuron. 2014;81(3):471–483. doi: 10.1016/j.neuron.2014.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Duan W. Targeting sirtuin-1 in Huntington’s disease: rationale and current status. CNS Drugs. 2013;27(5):345–352. doi: 10.1007/s40263-013-0055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Naia L., Carmo C., Campesan S., Lopes C., Valero J., Rosenstock T.R. SIRT3, a modifier of mitochondrial function in Huntington’s disease. Free Radic. Biol. Med. 2018;120:S17. doi: 10.1016/j.freeradbiomed.2018.04.072. [DOI] [PubMed] [Google Scholar]
- 187.Pasinetti G.M., Bilski A.E., Zhao W. Sirtuins as therapeutic targets of ALS.Cell Res., 2013;23(9):1073–1074. doi: 10.1038/cr.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]