Abstract
Great progress has been made in specifically identifying the central neural circuits (CNCs) of the core body temperature (Tcore), sleep-wakefulness states (SWs), and general anesthesia states (GAs), mainly utilizing optogenetic or chemogenetic manipulations. We summarize the neuronal populations and neural pathways of these three CNCs, which gives evidence for the orchestration within these three CNCs, and the integrative regulation of these three CNCs by different environmental light signals. We also outline some transient receptor potential (TRP) channels that function in the CNCs-Tcore and are modulated by some general anesthetics, which makes TRP channels possible targets for addressing the general-anesthetics-induced-hypothermia (GAIH). We suggest this review will provide new orientations for further consummating these CNCs and elucidating the central mechanisms of GAIH.
Keywords: Central neural circuits, body temperature regulation, sleep-wakefulness states, general anesthesia, TRP channels, intrinsically photosensitive retinal ganglion cells, optogenetics, chemogenetics
1. INTRODUCTION
Keeping the homeostasis of the core body temperature (Tcore) and sleep-wakefulness states (SWs) is essential for mammals’ normal metabolic activities [1, 2]. In recent decades, studies have been made to establish the central neural circuits (CNCs) of Tcore and SWs, respectively. Part of the existing findings on the central regulation of Tcore [3-6] and SWs [7-9] have been summarized in some reviews, respectively. Although the preoptic area (POA) has been put forward as an important regulator of both Tcore and SWs for a long time [10-15], only recently, combining with optogenetic or chemogenetic manipulations, the relative neuronal populations, as well as neural pathways, have been identified in detail. By comparing the CNCs-Tcore with CNCs-SWs, it turned out to find that they share largely overlapped neuronal populations as well as neural pathways. For instance, the preoptic area (POA) [1, 2, 16-19] and lateral hypothalamus (LH) [1, 20-22] have been reported to be co-regulators between CNCs-Tcore and CNCs-SWs in some latest studies. It is imperative to review the CNCs-Tcore and CNCs-SWs to find out more possible interactions between them.
Ever since the advent of ether in 1846, general anesthesia has undergone continuous development in order to bring comfort to surgical patients. Mild hypothermia is a common and serious complication of general anesthesia, which promotes postoperative wound infections and myocardial events, increases perioperative blood loss, impairs immune function, delays postoperative recovery, and prolongs the effect of almost all anesthetics [23-25]. To address this side effect, many interventions have been used, such as forced-air warming [24, 26-28] as well as amino acids [28, 29], which may attenuate the hypothermia to some extent but are not suited to all conditions [30] and cannot entirely solve this problem [31], thus making it essential to identify the central thermoregulatory mechanisms during general anesthesia.
Recent studies have revealed the largely overlapped CNCs between SWs and general anesthesia states (GAs), both anatomically and functionally, which has been reviewed elsewhere [32, 33]. Together with the orchestration between the CNCs-Tcore and CNCs-SWs as well as some latest findings of the central coordinators of Tcore and GAs [17, 34], there emerge the possible central mechanisms of general-anesthetics-induced-hypothermia (GAIH).
The transient receptor potential (TRP) channels have long been deemed as important thermo-sensors [35, 36]. We also outlined the evidence that favors some major TRP channels to function in the CNCs-Tcore, which may guide for new interventions to address GAIH.
2. CENTRAL THERMOREGULATORY CIRCUITS
The two mechanisms of thermoregulation: physiological regulation and behavioral regulation, have been reviewed elsewhere [3, 4]. The former is involuntary and autonomic, including thermogenic responses and heat loss reactions, while the latter is motivated and goal-oriented. Since the behavioral regulation is inhibited during general anesthesia [23], here, we mainly focus on the CNCs-Tcore of physiological responses. Given that the afferent pathways involving the lateral parabrachial nucleus (LPB), from the periphery to the POA have been thoroughly summarized in some reviews [3-5], here we focus on the downstream central thermoregulatory pathways from the LPB.
2.1. TRP Channels that Might Function in the Central Thermoregulatory Circuits
It has been found that TRP channels play an important role in peripheral body temperature sensation [3, 4, 35, 36]. Among TRP channels, the heat-activated transient receptor potential vanilloid-1 (TRPV1) to TRPV4 and transient receptor potential melastatin-2 (TRPM2) as well as the cold-activated TRPM8 have attracted major attention. Their roles as peripheral temperature sensors are still controversial [4], which may be due to the possibility that studies on the role of individual TRP channel have been compensated for by other subtypes of TRP channels, or the unknown compensatory mechanism in the growth and development of TRP channel knockout (KO) mice [37]. It is also possible that due to the universally low expression of TRP channels in the brain [38, 39] and the limitations of current detection techniques, the studies on the central thermoregulatory mechanisms of the TRP channels remain to be scarce. In this part, we focus on the TRP channels mentioned above and make a brief discussion on their possible roles in the CNCs-Tcore.
2.1.1. TRPV1
Capsaicin (CAP) is a classic agonist for TRPV1. Subcutaneous administration of CAP [40] or oral gavage of CAP [41] activates neurons in the POA, demonstrating the peripheral thermoregulatory role of TRPV1. Central administration of CAP can also cause hypothermia [42]. In a study, by pharmacological binding TRPV1 to detect its expression in the brain of monkey, it has been found that TRPV1 has a high density in locations such as the POA and locus coeruleus (LC) [43]. However, other studies have shown that TRPV1 is not expressed in the brain of mice [38] or extremely low among different species while not existing in the POA [44]. The role that TRPV1 plays in central thermoregulation at the level of the POA needs to be further confirmed.
In addition to the POA, in the review of Szolcsanyi (2015), it is suggested that TRPV1 may also act on the CNCs-Tcore at the level of the LC and nucleus of the solitary tract (NTS) [45]. The noradrenergic LC (LCNE) neurons can be activated by cutaneous thermal stimuli, which is reduced in capsaicin-desensitized rats [46]. In vitro brain slices, CAP can evoke spontaneous excitatory postsynaptic currents (sEPSCs) of LC neurons, while this effect can be abolished by selective TRPV1 antagonist [47]. And the central PGE2-induced thermogenesis can be attenuated in LC-lesioned rats [48]. Combined with the reported high density of TRPV1 in the LC of monkeys [43], the thermoregulatory role of TRPV1 in LC neurons needs further demonstrations. In vitro NTS slices, TRPV1 channels of the glutamatergic nerve terminals of unmyelinated afferents stay active at normal temperatures, and their activities increase dramatically when the tissue temperature is increased between 30°C and 42°C [45]. Oral gavage of CAP induces Fos expression in the NTS, which is scarcely observed in TRPV1-KO mice [41]. It has also been found that activation of TRPV1 channels in the NTS of intact vagal afferent pathway rats can lower Tcore [49]. These studies reveal a possible thermoregulatory role of TRPV1 at the level of NTS. It has been shown that proopiomelanocortin (POMC) neurons in the arcuate nucleus of the hypothalamus (ARH) express TRPV1, and elevated hypothalamic temperature can activate the TRPV1-like receptors in POMC ARH neurons [50], which makes the ARH another location where the thermoregulatory TRPV1 might function. Furthermore, bilateral electrolytic lesions of the rostral ventrolateral medulla (RVLM) of rats can greatly alleviate thermogenesis caused by subcutaneous injection of CAP, and thermogenesis can be caused by unilateral microinjection of CAP into the RVLM [51]. Similarly, the microinjection of CAP into the caudal ventrolateral medulla (CVLM) increases the body temperature of rats during endotoxemia [52]. It seems that TRPV1 also plays a role in thermoregulation at the level of the ventrolateral medulla (VLM), but apart from the above two studies, no other studies on the thermoregulatory role of TRPV1 at the VLM level have been reported.
2.1.2. TRPV2
TRPV2 has been found to play a peripheral role in the thermogenesis of brown adipose tissue (BAT) [53]. The expression of TRPV2 in the medial preoptic area (MPO/MPA), paraventricular nucleus of the hypothalamus (PVH), NTS, RVLM, and other brain regions related to the regulation of Tcore has been confirmed [54, 55]. PVH plays an important role in the febrile response caused by lipopolysaccharide (LPS) [56] and stress [57]. Bilateral lesions of PVH by ibotenic acid (IBO) can relieve fever induced by LPS and bradykinin [58]. It has been shown that oxytocin stimulates extracellular Ca2+ influx through TRPV2 channels of PVH, thereby exerting its anxiolytic effects [59]. Oxytocin has been shown to decrease Tcore [60, 61]. TRPV2 may participate in the CNCs-Tcore; from one perspective, oxytocin may act on TRPV2 of the PVH to alleviate the febrile response as well as anxiety.
Besides, it is found that TRPV2 is co-expressed with prokineticin 2 receptor (PKR2) in the suprachiasmatic nucleus (SCN) neurons, and TRPV2 enhances the signal of PKR2 in calcium mobilization or ion current conductance, and TRPV2 may be involved in the regulation of circadian rhythms by prokineticin 2 (PK2) [62]. PK2 mRNA is shown to express in many brain locations apart from the SCN, such as the median preoptic nucleus (MnPO) and PVH [63], both of which are related to the CNCs-Tcore and are under circadian control. The effect of the circadian input signal of the SCN onto the CNCs-Tcore has been outlined previously [2]. The SCN neurons expressing TRPV2 may enhance the signal of PKR2 and subsequently modulating the downstream thermoregulatory pathways.
2.1.3. TRPV3 and TRPV4
The activation of TRPV3 from different species sources by innocuous warmth [64-66], combined with the presence of impaired thermo-sensation in TRPV3-KO mice [67], provides evidence for the role of TRPV3 in peripheral temperature sensation. The expression of TRPV3 in peripheral locations (especially the skin keratinocytes) [38, 64] and central locations [38, 66] has been confirmed. However, studies on the central thermoregulatory role of TRPV3, if possible, remain scarce. A selective TRPV3 agonist, incensole acetate, is found to have anxiolytic effects in the elevated plus-maze and antidepressant-like effects in the forced swim test, effects that are absent in TRPV3-KO mice [68]. It has been proposed that the serotonergic (5-HT) neurons in the interfascicular part of the dorsal raphe nucleus (DRI) can regulate emotional behavior through thermosensitive TRPV3 and TRPV4 [69]. Given that the dorsal raphe nucleus (DRN) is important in modulating Tcore [34] (Table 1), TRPV3 and TRPV4 might act in the CNCs-Tcore at the level of the DRN.
Table 1.
Central locations or pathways regulating core body temperature or(and) sleep-wakefulness states.
| Locations/ Pathways | Cell Type Marker | Experimental Manipulations | Neural Activity | Thermoregulatory Responses | Sleep-wakefulness States | Food Intake | Other Changes | Refs. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Opto-/Chemo-genetic Manipulations | Other Handlings# | Core Temperature | Tail Vasodilation | BAT Temperature | Locomotion | NREM Total Time (s) | REM Total Time (s) | Wakefulness Total Time (s) | ||||||
| POA | TRPM2 | hM3Dq | Inactive | ↑ | ↓ | ↑ | ↓ | N/A | N/A | N/A | N/A | [37] | ||
| POA | TRPM2 | hM4Di | Inactive | ↓ | ↑ | ND | ND | N/A | N/A | N/A | N/A | [37] | ||
| POA | Vglut2 | hM3Dq | Inactive | ↑ | ↓ | N/A | N/A | N/A | N/A | N/A | N/A | [37] | ||
| POA | Vgat | hM3Dq | Inactive | ↑ | ND | N/A | N/A | N/A | N/A | N/A | N/A | [37] | ||
| POA | Ptgds | hM3Dq | Inactive | ↑ | ↓ | N/A | N/A | N/A | N/A | N/A | N/A | [99] | ||
| POA | Ptgds | hM3Dq | Active | ↑ | ND | N/A | N/A | N/A | N/A | N/A | N/A | [99] | ||
| POA | Ptgds | hM4Di | Inactive | ↓ | ↑ | N/A | N/A | N/A | N/A | N/A | N/A | [99] | ||
| POA | Ptgds | hM4Di | Active | ↓ | ND | N/A | N/A | N/A | N/A | N/A | N/A | [99] | ||
| vLPO | Vgat | ChR | No | ↑ | ↓ | N/A | N/A | ↓ | N/A | N/A | N/A | [95] | ||
| vLPO | Vgat | hGtACR1 | No | ↓ | ↑ | N/A | N/A | ↑ | N/A | N/A | N/A | [95] | ||
| vLPO | Vglut2 | ChR | No | ↑ | ↓ | N/A | N/A | ↓ | N/A | N/A | N/A | [95] | ||
| vLPO | Vglut2 | hM3Dq | No | ↑ | ↓ | N/A | N/A | N/A | N/A | N/A | N/A | [95] | ||
| VLPO | Gal | ChR | Tamb=22°C, active | ↑ | ND | N/A | N/A | N/A | ↑ | ND | N/A | N/A | N/A | [16] |
| VLPO | Gal | ArCH | Tamb=22°C, active and inactive | ↓ | ND | N/A | N/A | N/A | ↓ | ND | N/A | N/A | N/A | [16] |
| VLPO | Gal | hM3Dq | Tamb=22°C, active and inactive | ↑ | ↓ | N/A | N/A | N/A | ↑ | ↓ | N/A | N/A | N/A | [16] |
| VLPO | Gal | hM3Dq | Tamb=29°C, activea | ↑ | ↓ | N/A | N/A | N/A | ↑ | ↓↓ | N/A | N/A | N/A | [16] |
| VLPO | Gal | hM3Dq | Tamb=36°C, activea | ↑ | ND | N/A | N/A | N/A | ↑ | ↓ | N/A | N/A | N/A | [16] |
| VLPO | Gal | hM3Dq | Tamb=29°C, inactivea | ↑ | ↓ | N/A | N/A | N/A | N/A | ↑^ | N/A | N/A | N/A | [16] |
| VLPO | Gal | hM3Dq | Tamb=22°C, inactive, cage exchangeb | ↑ | SIH ↓ | N/A | N/A | N/A | ↑ | ND | SIW ↓ | N/A | N/A | [16] |
| LPO | Gal | hM3Dq | active | ↑ | ↓ | N/A | N/A | N/A | ↑ | ND | ↓ | N/A | N/A | [17] |
| MPO | Vgat | ChR | no | ↑ | ND | N/A | N/A | ND | N/A | N/A | N/A | [95] | ||
| MnPO, VMPO | LepRb | hM3Dq | inactive | ↑ | ↓ | N/A | N/A | ↓ | N/A | N/A | postural extension↑ |
[87] | ||
| MnPO, VMPO | Vglut2 | hM3Dq | inactive | ↑ | ↓ | N/A | N/A | N/A | N/A | N/A | N/A | [87] | ||
| MnPO, VMPO | Vgat | hM3Dq | inactive | ↑ | ND | N/A | N/A | N/A | N/A | N/A | N/A | [87] | ||
| MnPO, VMPO | PACAP | SSFO | no | ↑ | ↓ | ↑ | ↓ | N/A | N/A | N/A | cold seeking↑, nest building↓ |
[85] | ||
| MnPO, VMPO | BDNF | SSFO | no | ↑ | ↓ | ↑ | ↓ | N/A | N/A | N/A | cold seeking↑, nest building↓ |
[85] | ||
| MnPOe | Vglut2 | ChR | Tamb=21°C, active and inactive | ↑ | ↓↓ | ↑ | N/A | N/A | ↓ | ND | ↑ | N/A | HR↓↓, drinking in subset↑ |
[86] |
| MnPO | Vglut2 | ChR | Tamb=31°C, active and inactivea | ↑ | ↓ | ↑ | N/A | N/A | N/A | N/A | HR↓ | [86] | ||
| MnPO, MPO | Nos1 | hM3Dq | Tamb=22°C, active | ↑ | ↓ | N/A | N/A | N/A | ↑ | N/A | ↓ | N/A | N/A | [18] |
| MnPO, MPO | Vgat | hM3Dq | Tamb=22°C, active | ↑ | ND | N/A | N/A | N/A | ↑ | N/A | ↓ | N/A | N/A | [18] |
| AVPe/MPA | QRFP | hM3Dq | Tamb=22°C | ↑ | ↓ (>48h) | N/A | ↓ (>48h) | hibernation-like immobility | A hibernation-like state with EEG amplitude↓ | VO2↓, HR↓, RR↓ | [93] | |||
| AVPe/MPA | QRFP | hM4Di | Tamb=22°C | ↓ | N/A | N/A | N/A | N/A | N/A | N/A | N/A | [93] | ||
| AVPe/MPA | QRFP | SSFO | Tamb=22°C | ↑ | ↓(~30min) | ↑(~30min) | ↓(~30min) | N/A | N/A | N/A | N/A | [93] | ||
| POA→DMH | vLPOVgat | ChR | no | ↑ | ↓ | N/A | N/A | ↓ | N/A | N/A | N/A | [95] | ||
| POA→DMH | POAPACAP | ChR | no | ↑ | ↓ | ND | ↓ | N/A | N/A | N/A | cold seeking ND |
[85] | ||
| AVPe/MPA→DMH | AVPe/MPA Q | SSFO | Tamb=22°C | ↑ | ↓ | ↑ | ↓ | N/A | N/A | N/A | N/A | [93] | ||
| AVPe/MPA→RPa | AVPe/MPA Q | SSFO | Tamb=22°C | ↑ | ↓ | ↑ | ↓ (subtle) | N/A | N/A | N/A | N/A | [93] | ||
| DMH | ChAT | ChR | no | ↑ | ↓ | N/A | ↓ | N/A | N/A | N/A | N/A | [106] | ||
| DMH | ChAT | ArCH | no | ↓ | ↑ | N/A | ↑ | N/A | N/A | N/A | N/A | [106] | ||
| DMH | LepRb | hM3Dq | no | ↑ | ↑ | N/A | ↑ | ↑ | N/A | N/A | N/A | [107] | ||
| DMH | Vglut2 | ChR | no | ↑ | ↑ | N/A | ↑ | N/A | N/A | N/A | N/A | [85] | ||
| DMH | Vglut2 | hM3Dq | no | ↑ | ↑ | N/A | ↑ | N/A | N/A | N/A | N/A | [85] | ||
| DMH | Vglut2 | hM3Dq | no | ↑ | ↑ | N/A | N/A | ↑ | N/A | N/A | N/A | [95] | ||
| DMH | Vglut2 | hGtACR1 | no | ↓ | ↓ | N/A | N/A | ↓ | N/A | N/A | N/A | [95] | ||
| DHA | Vglut2 | hGlyR | no | ↓ | ↓ | N/A | N/A | ND | N/A | N/A | N/A | [101] | ||
| DHA | Vglut2 | hGlyR | cage exchangeb | ↓ | SIH ↓ | N/A | N/A | SIL ND | N/A | N/A | N/A | [101] | ||
| DHA | Vglut2 | hM3Dq | no | ↑ | ↑ | ↑ | ↑ | ↑ | N/A | N/A | N/A | [101] | ||
| DHA | Vglut2 | hM3Dq | cage exchangeb | ↑ | SIH ND | ↓ | ↑ | SIL ND | N/A | N/A | N/A | [101] | ||
| DMH | Vgat | ChR | no | ↑ | ↑ | N/A | N/A | ↑ | N/A | N/A | N/A | [95] | ||
| DMH | Vgat | hM3Dq | no | ↑ | ↑ | N/A | N/A | ↑ | N/A | N/A | N/A | [95] | ||
| DMH | Vgat | hGtACR1 | no | ↓ | ↓ | N/A | N/A | ↓ | N/A | N/A | N/A | [95] | ||
| DMH | Brs3 | hM3Dq | Tamb=22°C | ↑ | ↑ | N/A | N/A | ND | N/A | ND | TEE↑, BG↑ | [94] | ||
| DMH | Brs3 | hM4Di | Tamb=22°C | ↓ | ↓ | N/A | N/A | ND | N/A | N/A | TEE↓ | [94] | ||
| DMH | Brs3 | hM4Di | LPSc | ↓ | LIH ↓ | N/A | N/A | LIL ND | N/A | N/A | N/A | [94] | ||
| DMH | Brs3 | hM4Di | cage exchangeb | ↓ | SIH ↓ | N/A | N/A | SIL ND | N/A | N/A | N/A | [94] | ||
| DMH | Brs3 | hM4Di | Tamb=34°Ca | ↓ | ND | N/A | N/A | ND | N/A | N/A | N/A | [94] | ||
| DMH | non- specific |
ChR | Tamb=22°C | ↑ | ↑↑ | N/A | ↑ | ↑ | N/A | N/A | N/A | [94] | ||
| DMH | Brs3 | ChR | Tamb=22°C | ↑ | ↑ | N/A | ↑ | ND | N/A | N/A | HR↑, MAP↑ | [94] | ||
| DMH | non-Brs3 | ChR | Tamb=22°C | ↑ | ↑ | N/A | ↑ | ↑ | N/A | N/A | N/A | [94] | ||
| DMH→RPa | DMHBrs3 | ChR | Tamb=22°C | ↑ | ↑ | N/A | N/A | ND | N/A | N/A | N/A | [94] | ||
| DMH→rMR | non- specific |
ChR | inactive | ↑ | ND | N/A | ↑ | N/A | N/A | N/A | HR↑, MAP↑ | [57] | ||
| DMH→PAG | non- specific |
hM3Dq | inactive | ↑ | ↑ | ↑ | N/A | ↑ | N/A | N/A | N/A | [102] | ||
| DHA→RPa | DHAVglut2 | ArCH | no | ↓ | ↓ | N/A | N/A | N/A | N/A | N/A | N/A | [101] | ||
| DHA→RPa | DHAVglut2 | ArCH | cage exchangeb | ↓ | SIH ↓ | N/A | N/A | N/A | N/A | N/A | N/A | [101] | ||
| PVH | Brs3 | hM3Dq | no | ↑ | ND | N/A | N/A | ND | N/A | ↓ | TEE ND, BG ND | [94] | ||
| PVH | Brs3 | hM4Di | inactive | ↓ | ND | N/A | N/A | ND | N/A | ↑ | N/A | [94] | ||
| LH | Vglut2 | hM3Dq | no | ↑ | N/A | N/A | ↑ | N/A | N/A | N/A | N/A | [120] | ||
| LH | MCH | hM3Dq | active and inactive | ↑ | ND | N/A | N/A | ND | ND | ↑ | ↓* | N/A | N/A | [22] |
| LH | MCH | DTR | active and inactive | ablation | ND (inactive), ↑ (active) | N/A | N/A | ND (inactive), ↑ (active) | alteration of the diurnal distributions of REM and wakefulness (inactive) | ↓ | weight↓ | [22] | ||
| LH | MCH | hM3Dq | inactive | ↑ | ND | N/A | N/A | ND | ND | ↑ | ND | N/A | N/A | [20] |
| LH | Vglut2-KO MCH | hM3Dq | inactive | ↑ | ND | N/A | N/A | ND | ND | ↑ | ND | N/A | N/A | [20] |
| LH | Nts | ChR | inactive | ↑ | ↑ | N/A | N/A | ND | NREM-wake but not REM-wake transitions | N/A | EMG↑ | [21] | ||
| LH | Nts | hM3Dq | inactive | ↑ | ↑ | N/A | N/A | ↑ | a potential NREM rebound | a REM sleep rebound | Wakefulness for 4-6h | N/A | N/A | [21] |
| LH | Nts | hM3Dq | active | ↑ | ↑↑ | N/A | N/A | ↑↑ | ND | ND | ND | N/A | N/A | [21] |
| LH | Nts | hM4Di | active and inactive | ↓ | ND | N/A | N/A | ND | ND | ND | ND | N/A | N/A | [21] |
| LH | Nts | hM4Di | inactive, cage changeb | ↓ | SIH ↓ | N/A | N/A | SIL ↓ | ↑ | ↑ | SIW ↓ | N/A | N/A | [21] |
| LH | Nts | hM4Di | active, acute fastingd | ↓ | FIH ↓ | N/A | N/A | FIL ↑ | ↓ | ND | FIW ↑ | N/A | N/A | [21] |
| LH | QRFP | hM3Dq | Tamb=22°C | ↑ | N/A | N/A | N/A | N/A | N/A | N/A | N/A | [93] | ||
| ARH | Rip-Cre | hM3Dq | no | ↑ | ↑ | N/A | ↑ | N/A | N/A | ND | VO2↑, subcutaneous flank temperature ND | [111] | ||
| ARH | Vgat KO | hM3Dq | no | ↑ | ↑ | N/A | ND | N/A | N/A | ND | VO2 ND | [111] | ||
| ARH | Rip-Cre + Vgat KO | hM3Dq | no | ↑ | ↑ | N/A | ND | N/A | N/A | ND | VO2 ND | [111] | ||
| ARH | Kiss1 | hM3Dq | active, female | ↑ | ↓ | ↑ | N/A | ↓ | N/A | N/A | N/A | [115] | ||
| ARH | Kiss1 | hM3Dq | active, male | ↑ | ↓ | ↑ | N/A | N/A | N/A | N/A | N/A | [115] | ||
| ARH | Kiss1 | ChR | active, female | ↑ | N/A | ↑ | N/A | N/A | N/A | N/A | N/A | [115] | ||
| ARH | Kiss1 | ChR | active, male | ↑ | N/A | ↑ | N/A | N/A | N/A | N/A | N/A | [115] | ||
| ARH | Kiss1 | hM3Dq | active, female, ovariectomy | ↑ | N/A | ↑ (more sensitive) | N/A | N/A | N/A | N/A | N/A | [115] | ||
| ARH | Kiss1 | hM3Dq | active, female, NKBR antagonist into the POA | ↑ | N/A | ↓ | N/A | N/A | N/A | N/A | N/A | [115] | ||
| ARH→POA | ARHKiss1 | ChR | active, female | ↑ | ↓ | ↑ | N/A | ↓ | N/A | N/A | N/A | [115] | ||
| ARH→PVH | ARHRip-Cre | ChR | no (in vitro) | ↑ | N/A | N/A | N/A | IPSCs | [111] | |||||
| ARH→PVH | ARHAgRP | ChR | no (in vitro) | ↑ | N/A | N/A | N/A | No IPSCs | [111] | |||||
| NTS→RPa | NTSVgat | ChR | no (in vitro) | ↑ | N/A | N/A | N/A | IPSCs | [111] | |||||
| DRN | Vgat | hM3Dq | inactive | ↑ | ↓ | ND | ↓ | ↓ | N/A | N/A | TEE↓ | [34] | ||
| DRN | Vgat | hM3Dq | Inactive+ ISO (1-1.25%) | ↑ | ↓ | ND | ↓ | - | N/A | N/A | N/A | [34] | ||
| DRN | Vgat | hM4Di | inactive | ↓ | ↑ | ND | ND | ↑ | N/A | N/A | TEE↑ | [34] | ||
| DRN→RPa | DRNVgat | ChR | inactive | ↑ | ↓ | N/A | ↓ | N/A | N/A | ND | N/A | [34] | ||
| DRN→BNST | DRNVgat | ChR | inactive | ↑ | ↓ | N/A | ↓ | N/A | N/A | ↑ | N/A | [34] | ||
| DRN→DMH | DRNVgat | ChR | inactive | ↑ | ↓ | N/A | ↓ | N/A | N/A | ↑ | N/A | [34] | ||
| DRN→MPO | DRNVgat | ChR | inactive | ↑ | ↓ | N/A | ↓ | N/A | N/A | ND | N/A | [34] | ||
| DRN→BNST | DRNVgat | hM4Di | inactive | ↓ | ↑(transiently) | N/A | N/A | N/A | N/A | N/A | N/A | [34] | ||
| DRN→DMH | DRNVgat | hM4Di | inactive | ↓ | ↑(transiently) | N/A | N/A | N/A | N/A | N/A | N/A | [34] | ||
| DRN | SERT | hM3Dq | active and inactive | ↑ | ND | N/A | N/A | ↓* | ↑ | ↑ | ↓ | N/A | anxiolysis | [127] |
| IRt/PCRt | Gad2 | hM3Dq | no | ↑ | ↓ | N/A | ↓ | N/A | N/A | N/A | HR↓ | [132] | ||
| BLA | Glutamatergic | NpHR | footshock stress | ↓ | SIH ND | N/A | N/A | N/A | ND | ↑ | N/A | N/A | N/A | [152] |
| ipRGCs | Brn3b | hM3Dq | active | ↑ | ↓ | N/A | N/A | N/A | N/A | N/A | N/A | [161] | ||
Abbreviations: POA, the preoptic area; vLPO, the ventral part of the lateral preoptic nucleus; VLPO, the ventrolateral preoptic nucleus; LPO, the lateral preoptic area; MPO/MPA, the medial preoptic area; MnPO, the median preoptic nucleus; VMPO, the ventromedial preoptic nucleus; AVPe, the anteroventral periventricular nucleus; DMH, the dorsomedial hypothalamus; dDMH, the dorsal part of the dorsomedial hypothalamus; DHA, the dorsal hypothalamic area; RPa, the raphe pallidus; rRPa, the rostral raphe pallidus; rMR, the rostral medullary raphe area; PAG, the periaqueductal gray; PVH, the paraventricular nucleus of the hypothalamus; LH, the lateral hypothalamus; ARH, the arcuate nucleus of the hypothalamus; NTS, the nucleus of the solitary tract; DRN, the dorsal raphe nucleus; BNST, the bed nucleus of the stria terminalis; IRt/PCRt, the intermediate and parvicellular reticular nuclei; BLA, the basolateral nucleus of the amygdala; ipRGCs, the intrinsically photosensitive retinal ganglion cells; Ptgds, the gene that encodes lipocalin-type prostaglandin-D synthase; Gal, galanin; LepRb, leptin receptors; PACAP, pituitary adenylate cyclase-activating polypeptide; BDNF, brain-derived neurotrophic factor; Nos1, nitric oxide synthase 1; QRFP, pyroglutamylated RFamide peptide; Q, QRFP neurons; Brs3, Bombesin-like receptor 3; MCH, melanin-concentrating hormone; Nts, neurotensin; Rip-Cre, the rat insulin-2 promoter-Cre recombinase; Kiss1, kisspeptin/ neurokinin B; NKBR, neurokinin B receptor; ISO, isoflurane; SERT, the serotonin transporter; Brn3b, a transcription factor expressed in the M1 subtype of ipRGCs;
N/A, not assessed; ND, no difference; BAT, brown adipose tissue; NREM, non-rapid eye movement sleep; REM, rapid eye movement sleep; Tamb, ambient temperature; active/inactive, during the animals’ active/inactive cycles; HR, heart rate; VO2, oxygen consumption; RR, respiratory rate; TEE, total energy expenditure; BG, blood glucose; MAP, mean arterial pressure; EMG, electromyography; IPSCs, inhibitory postsynaptic currents.
Remarks:
#: for this column, “no” means under normal ambient temperature (about 22°C) with the experimental time (during whether the animals’ active or inactive cycles) unspecified; the normal ambient temperature is not specified unless the study involves different ambient temperature;
^: ↓ for 4h, then ↑ for the next 12h↓ until fully recovered;
*: slightly, no statistical difference;
a: these groups are compared to the control group (under the same ambient temperature but without optogenetic or chemogenetic manipulations), respectively;
b: these groups were compared to the control group (cage exchange but without optogenetic or chemogenetic manipulations), respectively, and the outcome was defined as whether the stress-induced hyperthermia (SIH), stress-induced locomotion (SIL) or stress-induced wakefulness (SIW) was changed;
c: this group was compared to the control group (LPS administration but without chemogenetic manipulation), and the outcome was defined as whether LPS-induced hyperthermia (LIH) or LPS-induced locomotion (LIL) was changed;
d: this group was compared to the control group (fasting but without chemogenetic manipulation), and the outcome was defined as whether fasting-induced hyperthermia (FIH), fasting-induced locomotion (FIL) or fasting-induced wakefulness (FIW) was changed;
e: for this study, there was a weak correlation between the hypothermic mice and the drinking-increasing mice, while the wakefulness-promoting mice showed no correlation to the hypothermic mice; the increase of drinking was more during active cycles.
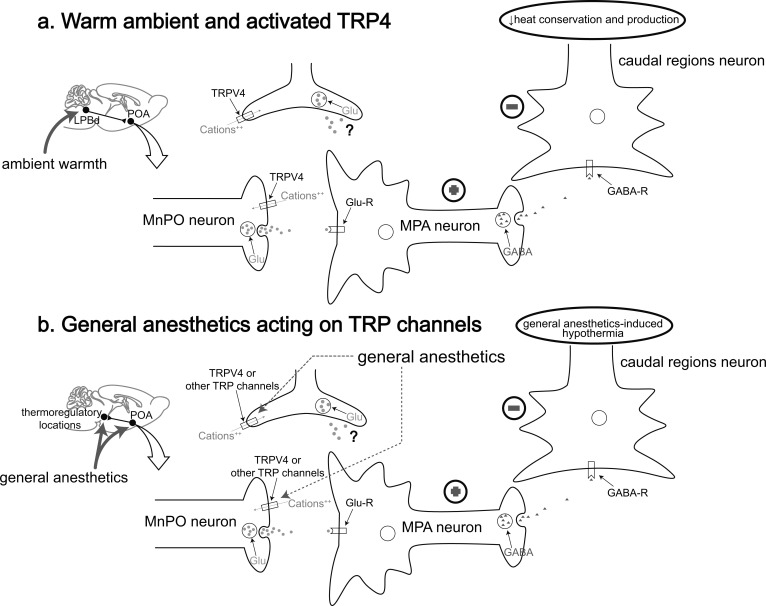
Previous studies have demonstrated that TRPV4 acts as a warm sensor in the periphery [70-72], and the presence of TRPV4 expression in the POA has been confirmed by immunohistochemistry [73], especially in the MPA and MnPO [70]. Microinjection of TRPV4 agonist into the POA can lower Tcore of rats, while microinjection of TRPV4 antagonist into that can increase Tcore [73]. Similarly, microinjection of the TRPV4 antagonist into the MPA leads to elevated Tcore in rats in warm environments, and the possible role of TRPV4+ POA neurons in the CNCs-Tcore is proposed: under warm conditions, TRPV4, located in the synaptic terminals of glutamatergic MnPO neurons con-nected to MPA, is activated, which subsequently activates the postsynaptic GABAergic MPA neurons to inhibit the caudal region neurons, thereby reducing the heat con-servation and production; alternatively, blocking TRPV4 in the presynaptic glutamatergic MnPO neurons has the opposite effect, leading to an increase in heat conservation and production [74] (Fig. 1a).
Fig. (1).
A model of TRPV4 and other possible TRP channels functioning in the central thermoregulatory pathway under warm ambient and general anesthesia. (a) Ambient warmth activates the LPBd→MnPO pathway, subsequently activating TRPV4 located in the synaptic terminals of the MnPO and causing an influx of cations to contribute to the glutamate release from these neurons. Then the postsynaptic GABAergic MPA neurons are activated (indicated by “plus sign” signal), thus inhibiting the neurons in caudal regions (indicated by ”minus sign” signal) to cause a reduction in heat conservation and production. Alternatively, TRPV4 may present in the astrocytes and regulate these thermoregulatory responses. (b) Under sedation or general anesthesia, the POA as well as other possible central thermoregulatory locations is activated by general anesthetics, TRPV4 or other possible TRP channels, such as heat-activated TRPV1 and cold-activated TRPM8, may be the target for general anesthetics. Similar as the procedures represented above, general anesthetics may activate the heat-activated TRP channels or inhibit the cold-activated TRP channels (not shown in this model), thereby causing hypothermia. LPBd, the dorsal part of lateral parabrachial nucleus; Glu, glutamate; Glu-R, glutamate receptor; GABA-R, GABA receptor. (Adapted from [74]).
2.1.4. TRPM2
TRPM2 has been shown to play a role in peripheral thermo-sensation [75]. Chemogenetic activation or inhibition of the POA neurons that express TRPM2 can decrease or increase body temperature, respectively. Moreover, chemo-genetically activating the Vesicular glutamate transporter 2 positive (Vglut2+) neurons, rather than the Vesicular GABA transporter positive (Vgat+) neurons in the POA, can recapitulate the hypothermia induced by activating TRPM2+ POA neurons, and activate the Corticotropin-releasing hormone positive (Crh+) PVH neurons, but not Thyrotropin-releasing hormone positive (Trh+) or magnocellular arginine vasopressin positive (AVP+) and oxytocin+ PVH neurons [37] (Table 1). Crh+ PVH neurons have been demonstrated to be part of the hypothalamic-pituitary-adrenal (HPA) stress response system [56]. Conclusively, TRPM2 may play a role in the CNCs-Tcore: excessive febrile response activates TRPM2+/Vglut2+ neurons in the POA and promotes the release of glutamate to the Crh+ PVH neurons, thus leading to increased heat loss and hypothermia.
2.1.5. TRPM8
TRPM8 is a major peripheral cold sensor, and its important role in thermoregulation has been summarized in some reviews [3, 4]. Intravenous administration of TRPM8 antagonist is more effective in decreasing body temperature in rats than intrathecal or intracerebroventricular admin-istration, which tends to indicate the peripheral thermo-regulatory effects of TRPM8 [76]. Given that the expression level of TRPM8 in the brain is extremely low [38], it is proposed that the central thermoregulation effects of TRPM8 might be dissembled. Recently, the immunohistochemistry of transgenic TRPM8 mice and rats shows that in the hypothalamus of the rodents, almost all TRPM8+ neurons are concentrated in the POA, with their projections widely distributed in other brain regions, such as the MnPO, LH, PVH, the dorsomedial hypothalamus (DMH), lateral habenula (LHb), DRN and caudal lateral and ventrolateral periaqueductal gray (clPAG and vlPAG) [77], which suggests a potential role for central TRPM8+ neurons in thermo-regulation, as well as in other non-thermal homeostatic functions, of rodents.
The TRPM8-KO mice have lower Tcore, an increase in tail heat loss, delayed obesity and metabolic disorders upon mild cooling, which reveals that TRPM8 plays an important role not only in thermoregulation, but also in the regulation of ingestive behavior and metabolic fuel selection during mild cooling [78]. This finding can hardly be explained by the peripheral effects of TRPM8; rather, it is more convincing that it is caused by the dysfunction of TRPM8 in some central locations, such as the LH, a potential orchestrator of Tcore regulation [79-81] and energy metabolism [1, 82-84]. Given that the LH may receive projections from TRPM8+ POA neurons [77], it seems reasonable to make an infer: TRPM8+ POA neurons receive signals from changes in Tcore and energy metabolism and then transmit them to the LH to regulate Tcore and energy metabolism.
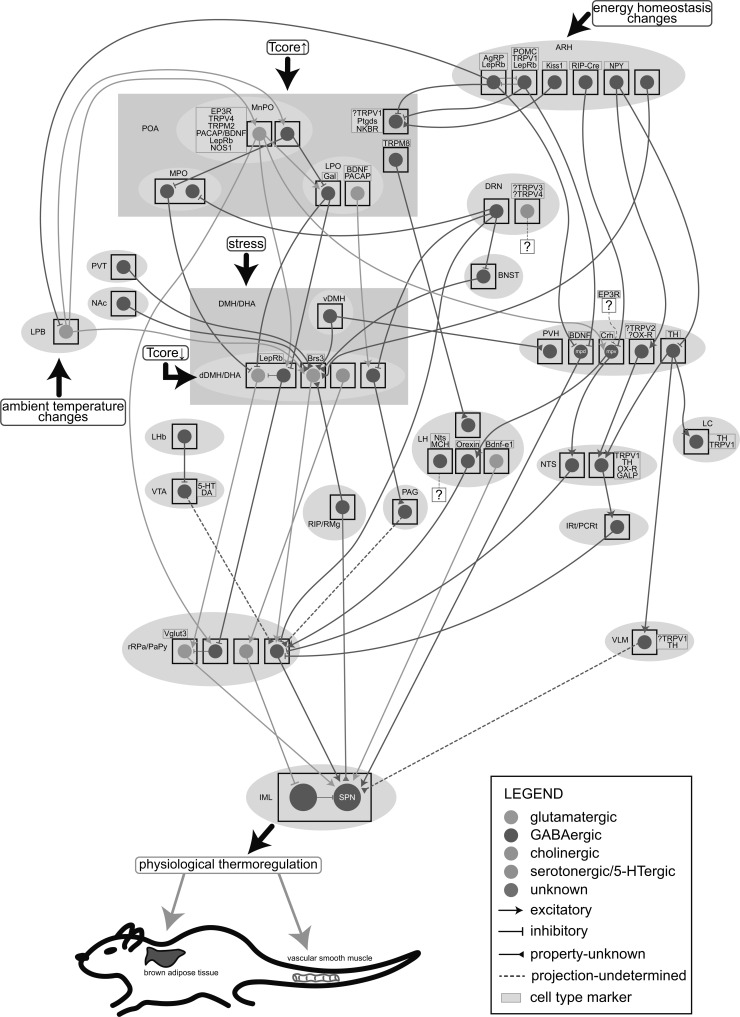
It is precise because of the complexity of the TRP channels’ functions in the thermoregulatory mechanisms that uncovering their roles in the CNCs-Tcore becomes more than imperative. With their possible expression in some thermoregulatory locations been depicted (Fig. 2), it may offer new ideas for further study.
Fig. (2).
A schematic of the specific central thermoregulatory neuronal populations and neural pathways. The signals of ambient temperature changes, energy homeostasis changes and stress are input from the LPB, ARH and DMH/DHA, respectively. The POA is heat-activated and the dDMH/DHA is cold-activated. The glutamatergic MnPO neurons reported to co-express some specific cell type markers (presented in the yellow box) send projections to some downstream locations, including the LPO, dDMH/DHA, rRPa/PaPy and PVH, while the GABAergic MnPO neurons locally inhibit the MPO and LPO. The Brs3+ dDMH/DHA neurons, which receive inputs from the POA (uncharted), vDMH, ARH, BNST, PVT, NAc and RIP/RMg, are glutamatergic and project to the rRPa/PaPy, together with the dDMH/DHALepRb→rRPa/PaPy→sympathetic preganglionic neurons (SPN), dDMH/DHAAch→rRPa/PaPy5-HT→spinal intermediolateral nucleus (IML) and dDMH/DHA→PAG pathways. The rRPa/PaPy may also receive projections from LPOVglut2 neurons (uncharted) and the LHbGABA innervating VTA neurons which are proposed to be dopaminergic or serotonergic. The metabolism-related ARH send inhibitory projections to the LPB, POA and PVH, thus modulating the canonical thermoregulatory circuits and regulating the neuroendocrine outputs from Crh+ PVHmpv neurons to the orexin neurons or NTSGABA neurons. PVHTH neurons project to downstream NTS, LC and VLM. PVHmpdBDNF neurons, receiving projections from ARHAgRP and ARHPOMC neurons, directly project to the SPN. The PVH is also regulated by the stress signals input from the vDMH. The IRt/PCRtGABA neurons in the downstream hypothalamic neuropeptide Y (NPY)-related pathways inhibit the rRPa/PaPy and subsequently the BAT thermogenesis. In the LH, receiving projections from TRPM8+ POA neurons, the orexin neurons project to the rRPa/PaPy and the Bdnf-e1 glutamatergic neurons directly innervate the SPN. The DRN is another important thermoregulatory location with GABAergic projections to the MPO, dDMH/DHA, rRPa/PaPy and BNST. The DRN5-HT neurons proposed to co-express TRPV3/TRPV4 may also be involved in thermoregulation. All these pathways cooperatively regulate the physiological thermo-responses which especially characterized by the brown adipose tissue (BAT) thermogenesis and tail vasomotor responses. Tcore, core body temperature; the question mark in the yellow box means that cell type marker is uncertain.
2.2. Thermoregulatory Neuronal Populations in the POA
The key role of the POA as the primary integrative site in the CNCs-Tcore has been elucidated in several reviews [3, 4], here we do not give unnecessary details about it but focus on recent progress in the identification of different cell types in the POA, which are specifically involved in thermo-regulation.
2.2.1. Glutamatergic and GABAergic Neurons in the POA
A warm-sensitive neuronal subpopulation in the anterior ventromedial preoptic area (VMPO) and MnPO co-expressed the neuropeptides pituitary adenylate cyclase-activating polypeptide (PACAP) and brain-derived neurotrophic factor (BDNF) (MnPOPACAP/BDNF neurons) is shown to induce hypothermia when optogenetic activated [85] (Table 1). Approximately two-thirds of MnPOPACAP/BDNF neurons express GAD2, and they are consequently supposed to be GABAergic [85]. However, there are studies revealing that optogenetic or chemogenetic stimulation of glutamatergic (Vglut2+) POA neurons [86], rather than GABAergic (Vgat+) POA neurons, induces hypothermia [18, 19, 37, 87] (Tables 1 and 2). Consistently, MnPO neurons that express the EP3 prostaglandin receptor (EP3R) and mediate LPS-induced fever responses are Vglut2+, not Vgat+ [88]. It is also reported that many neurons co-express GAD2 with Vglut2, rather than Vgat, in the POA [89, 90], as well as other regions of the brain [90-92]. Based on the results above, it has been proposed that the MnPOPACAP/BDNF neurons that express GAD2 in the research of Tan et al. [85] are probably part of the Vglut2+ MnPO neurons that participate in fever responses [88]. Recently, neurons that express pyroglutamylated RFamide peptide (QRFP) in the anteroventral periventricular nucleus (AVPe), MPA and periventricular nucleus (AVPe/MPA Q neurons) are reported to induce a hibernation-like state in rodents [93]. Many AVPe/MPA Q neurons constitute a unique subpopulation of MnPOPACAP/BDNF neurons, and AVPe/MPA Q neurons express both Vglut2 and Vgat [93], suggesting that MnPOPACAP/BDNF neurons express both Vglut2 and Vgat.
Table 2.
Central locations or pathways regulating general anesthesia states or(and) sleep-wakefulness states.
| Locations/ Pathways (Animals) | Cell Type Marker |
Experimental Mani-
pulations |
Experimental Period | Body Temperature Control | Neural Activity | Anesthetic-states Changes | Sleep-wakefulness States Changes | Other Changes | Ref. | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anesthetics | Time to LORR (s) | Time to RORR (s) | ED50 of LORR (%) | ED50 of RORR (%) | BSR (%) | Power Spectrum (%) | Other Changes | NREM | REM | wakefulness | |||||||||||||||||
| Total Time (s) | Bouts Number | Bout Duration (s) | Power Spectrum (%) | Total Time (s) | Bouts Number | Bout Duration (s) | Power Spectrum (%) | Total Time (s) | Bout Number | Bout Duration (s) | Power Spectrum (%) | ||||||||||||||||
| MnPO (M) | Vgat | hM3Dq | inactive | 37-38°C | ↑ | ISO (1.5% OR 1.2%) | ND | ND | N/A | N/A | N/A | N/A | wake-like episodes (1.2%) | ↑ | ↓ | ↑ | N/A | ↓ | ↓ | N/A | N/A | ND | ↓ | N/A | N/A | Tc ND | [19] |
| MnPO (M) | Vglut2 | hM3Dq | inactive | 37-38°C | ↑ | ISO (1.5% OR 1.2%) | ND | ND | N/A | N/A | N/A | N/A | wake-like episodes (1.2%) | ↑* | N/A | N/A | N/A | ↓ | N/A | N/A | N/A | ↑* | N/A | N/A | N/A | Tc ↓ | [19] |
| VLPO (M) | Vgat | hM3Dq | inactive | 37-38°C | ↑ | ISO (1.5% OR 1.2%) | ND | ND | N/A | N/A | N/A | N/A | N/A | sleep-wakefulness states ND | N/A | [19] | |||||||||||
| VLPO (M) | Vglut2 | hM3Dq | inactive | 37-38°C | ↑ | ISO (1.5% OR 1.2%) | ND | ND | N/A | N/A | N/A | N/A | N/A | ↓ | N/A | N/A | N/A | ↓ | N/A | N/A | N/A | ↑ | N/A | N/A | N/A | N/A | [19] |
| SON (M)a | AANs | hM3Dq | active | N/A | ↑ | N/A | ↑ | ND | ↑ | N/A | ND | N/A | N/A | N/A | ↓ | N/A | N/A | N/A | N/A | [136] | |||||||
| SON (M)a | AANs | hM3Dq | active | N/A | ↑ | N/A | ↑ | N/A | N/A | N/A | ND | N/A | N/A | N/A | ↓ | N/A | N/A | N/A | N/A | [136] | |||||||
| SON (M)a | AANs | GFP (control) | active | N/A | ND | N/A | ↓ | N/A | N/A | N/A | ND | N/A | N/A | N/A | ↑ | N/A | N/A | N/A | N/A | [136] | |||||||
| SON (M)a | AANs | ChR | active | N/A | ↑ | ISO (1.0%) | ND (induction time) | ↑ (fully awake time) | N/A | N/A | N/A | N/A | duration time ↑ | ↑(long-lasting) | N/A | N/A | δ↑ | ND | N/A | N/A | N/A | ↓ | N/A | N/A | N/A | N/A | [136] |
| SON (M)a | AANs | DTR | whole day | N/A | ablation (chronic) | N/A | ↓ | ND | ↓ | δ↓ | ↓ | ↓ | ND | θ↓ | ↓ | N/A | N/A | N/A | N/A | [136] | |||||||
| SON (M)a | AANs | eArch3.0 | active | N/A | ↓ | ISO (1.0%) | ND (induction time) | ↓ (fully awake time) | N/A | N/A | N/A | N/A | duration time ↓ | N/A | N/A | [136] | |||||||||||
| LH (M) | orexin | hM3Dq | N/A | 35–37°C | ↑ | ISO (2.0%) | N/A | ↓ | N/A | N/A | N/A | β↓ (2.0%); δ↓, α↑, β↑ (ISO off) | EMG↑ (0.5%), pain tolerance↑ | N/A | N/A | [179] | |||||||||||
| LH→TRN (M)b | LHGABA | ChETA | N/A | N/A | ↑ | ISO (0-1.2%) (burst-suppression) | no LORR (2 of 4 animals) | emergence (2 of 4 animals) | N/A | N/A | ↓ | N/A | burst duration (s)↑ | NREM-wake but not REM-wake transitions | N/A | [145] | |||||||||||
| ISO (0-1.2%) (iso-electric) | ND | ND | N/A | N/A | N/A | N/A | burst duration (s) ND | ||||||||||||||||||||
| LH→LC (M) | LHGABA | ChETA | N/A | N/A | ↑ | N/A | NREM-wake and REM-wake transitions | N/A | [145] | ||||||||||||||||||
| LH→PVT (M) | LHGABA | ChETA | N/A | N/A | ↑ | N/A | NREM-wake but not REM-wake transitions | N/A | [145] | ||||||||||||||||||
| LH→VTA (R) | LHorexin | ChR | inactive | 35–37°C | ↑ | ISO (1.4%) | N/A | ↓ | N/A | N/A | ↓ | δ↓, α↑, β↑, γ↑ (0.8%) | behavioral arousal | N/A | N/A | [180] | |||||||||||
| LH→VTA (R) | LHorexin | NpHR | inactive | 35–37°C | ↓ | ISO (1.4%) | N/A | ↑ | N/A | N/A | ND | θ↓, β↓ (0.8%) | no behavioral arousal | N/A | N/A | [180] | |||||||||||
| LHb (M) | Grm2-Cre | hM3Dq | active | N/A | ↑ | N/A | ND | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | locomotion↓ | [158] | |||||||
| LHb (M) | Grm2-Cre | GFP (control) | active | N/A | ND | PRO (7 mg/kg, i.v.) | N/A | N/A | N/A | N/A | N/A | δ and frequencies range from >10Hz to extending γ↑ | locomotion↓ | N/A | N/A | [158] | |||||||||||
| LHb (M) | Grm2-Cre | TeLC | active | N/A | neurotransmission blocked | PRO (7 mg/kg, i.v.) | ↑ | N/A | ↑ | N/A | N/A | δ and higher frequencies↑ (slightly) | attenuation of the locomotion↓ | N/A | N/A | [158] | |||||||||||
| LHb (M) | Grm2-Cre | TeLC | active or inactive | N/A | neurotransmission blocked | N/A | ↓ | ↑ | N/A | δ↓ | ND | ND | N/A | REM-wake transitions↑ | ↓ | ↑ (particularly when inactive) | N/A | N/A | N/A | [158] | |||||||
| LHb (M) | Grm2-Cre | TeLC+ dual orexin-R antagonist | active | N/A | neurotransmission blocked+ orexinergic inhibition n |
N/A | ↑ | N/A | N/A | N/A | ND | N/A | N/A | N/A | ↓ | N/A | N/A | N/A | N/A | [158] | |||||||
| PVT (M)c | Glutamatergic | hM4Di | active | N/A | ↓ | N/A | ↑ | ↑ | ND | N/A | ↑ | ↑ | ND | N/A | ↓ | ↑ | ↓ | δ (2-4Hz) ↑; micr0-arousal number↑ | N/A | [157] | |||||||
| PVT (M)c | Glutamatergic | hM4Di | inactive | N/A | ↓ | N/A | sleep-wakefulness states ND | N/A | [157] | ||||||||||||||||||
| PVT (M)c | Glutamatergic | DTA | whole day | N/A | ablation (chronic) | N/A | ↑ | ↑ | ND | α↓ | ND | ND | ND | N/A | ↓ | ↑ | ↓ | high↑, low θ↑, α↓; micro-arousal number↑ | sleep-wakefulness states ND (inactive) | [157] | |||||||
| PVT (M)c | Glutamatergic | IBO | whole day | N/A | lesion (acute) | N/A | ↑ | ↑ | ND | N/A | ↑ | ND | ND | N/A | ↓ | ↑ | ↓ | high δ↑, α↓; micro-arousal number↑ | locomotion and exploratory activities↓ (active); sleep-wakefulness states ND (inactive) | [157] | |||||||
| PVT (M)c | Glutamatergic | ChR | inactive | N/A | ↑ | ISO | N/A | ↓ | N/A | N/A | ↓ | δ↑, θ↓ | burst duration (s)↑ | NREM-wake and REM-wake transitions | N/A | [157] | |||||||||||
| PVT→NAc (M)c | PVTGlu | ChR | inactive | N/A | ↑ | N/A | NREM-wake and REM-wake transitions | N/A | [157] | ||||||||||||||||||
| PVT→NAc (M)c | PVTGlu | hM4Di | active | N/A | ↓ | N/A | ↑ | ↑ | ND | N/A | ND | ND | ND | N/A | ↓ | ↑ | ↓ | N/A | N/A | [157] | |||||||
| LH→PVT (M) | LHorexin | hM4Di | active | N/A | ↓ | N/A | ↑ | ↑ | N/A | N/A | ND | ND | N/A | N/A | ↓ | N/A | N/A | N/A | N/A | [157] | |||||||
| LH→PVT (M) | LHorexin | ChR | inactive | N/A | ↑ | N/A | sleep-wake transition | N/A | [157] | ||||||||||||||||||
| LH (M) | orexin | hM3Dq | N/A | N/A | ↑ | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | ↑# | N/A | N/A | N/A | N/A | [157] | |||||||
| TRN (M)d | Vgat | ChR | N/A | N/A | ↑ | ISO | N/A | N/A | N/A | N/A | N/A | broadband (0.5-50Hz) ↓ | the cortex is shifted into a deeper state | ↑ | N/A | N/A | δ↑, spindle ↓ | ND | N/A | N/A | N/A | ↓ | N/A | N/A | δ↑ | EMG↓, locomotion↓ | [164] |
| TRN (M) | Vgat | eNpHR | stimulation time was shuffled | N/A | ↓ | N/A | N/A | N/A | N/A | δ↓ | N/A | N/A | N/A | N/A | N/A | N/A | N/A | δ ND | locomotion ND | [164] | |||||||
| VTA (M)e | DAT | YFP (control) | N/A | normothermia | ND | ISO | N/A | N/A | N/A | N/A | N/A | ND | no behavioral arousal | N/A | N/A | [156] | |||||||||||
| VTA (M)e | DAT | ChR | N/A | normothermia | ↑ | ISO | N/A | N/A | N/A | N/A | N/A | <5Hz↓, 6-17Hz↓ | behavioral arousal, propensity for righting↑ | N/A | N/A | [156] | |||||||||||
| VTA (M)e | DAT | ChR+ D1-R antagonist | N/A | normothermia | ↑ | ISO | N/A | N/A | N/A | N/A | N/A | 6-17Hz↓ | no behavioral arousal | N/A | N/A | [156] | |||||||||||
| VTA (M) | Vgat | ChR | N/A | N/A | ↑ | ISO (0.8%, or 1.0%) | N/A | N/A | N/A | N/A | ↑ (1.0%) | δ↑, β↓, γ↓ (0.8%) | N/A | N/A | N/A | [165] | |||||||||||
| VTA (M) | Vgat | eNpHR | N/A | N/A | ↓ | ISO (0.8%, or 1.0%) | N/A | N/A | N/A | N/A | ↓ (1.0%) | δ↓, θ↓, α↓, γ↑ (0.8%) | N/A | N/A | N/A | [165] | |||||||||||
| VTA (M) | Vgat | hM3Dq | N/A | N/A | ↑ | ISO (1.4%) | ↓ | ↑ | ↓ | ↓ | N/A | N/A | N/A | N/A | N/A | [165] | |||||||||||
| VTA (M) | Vgat | hM4Di | N/A | N/A | ↓ | ISO (1.4%) | ↑ | ↓ | ↑ | ↑ | N/A | N/A | N/A | N/A | N/A | [165] | |||||||||||
| VTA→LH (M) | VTAVgat | ChR | N/A | N/A | ↑ | ISO (0.8%, or 1.0%) | N/A | N/A | N/A | N/A | ↑ (1.0%) | δ↑, β ND, γ↓ (0.8%) | N/A | N/A | N/A | [165] | |||||||||||
| VTA→LH (M) | VTAVgat | eNpHR | N/A | N/A | ↓ | ISO (0.8%, or 1.0%) | N/A | N/A | N/A | N/A | ↓ (1.0%) | δ↓, θ ND, α↓, γ↑ (0.8%) | N/A | N/A | N/A | [165] | |||||||||||
| VTA→LH (M) | VTAVgat | ChR | N/A | N/A | ↑ | ISO (1.4%) | ↓ | ↑ | N/A | N/A | N/A | N/A | N/A | N/A | N/A | [165] | |||||||||||
| VTA→LH (M) | VTAVgat | eNpHR | N/A | N/A | ↓ | ISO (1.4%) | ↑ | ND | N/A | N/A | N/A | N/A | N/A | N/A | N/A | [165] | |||||||||||
| PBN (M) | Vglut2 | hM3Dq | N/A | N/A | ↑ | SEVO (2.0%) | ↑ | ↓ | ↑ | N/A | N/A | N/A | N/A | N/A | N/A | [167] | |||||||||||
| PBN (M) | Vglut2 | hM4Di | N/A | N/A | ↓ | SEVO (2.0%) | ND | ↑ | ↓ | N/A | N/A | N/A | N/A | N/A | N/A | [167] | |||||||||||
| PBN (M) | Vglut2 | ChR | N/A | N/A | ↑ | SEVO (2.0%) | N/A | N/A | N/A | N/A | N/A | low δ↓, θ↑ | N/A | N/A | N/A | [167] | |||||||||||
| LC (R) | PRSx8 | hM3Dq | active | 36.0-37.5°C | ↑ | ISO (2.0%) | ↑ | ↓ | N/A | N/A | ↓ | δ↓, θ↑ | Locomotion ND | N/A | N/A | [170] | |||||||||||
| LC→TRN (M) | LCTH | hM3Dq | active | 36.0-37.5°C | ↑ | PRO (90 mg/kg, i.p.) | ND | ↑ | ND | ↓ | N/A | δ↑ | N/A | N/A | N/A | [171] | |||||||||||
| LC→TRN (M) | LCTH | hM3Dq+ prazosin |
active | 36.0-37.5°C | ↑ | PRO (90 mg/kg, i.p.) | N/A | ↓ | N/A | N/A | N/A | reversal of the δ↑ | N/A | N/A | N/A | [171] | |||||||||||
Abbreviations: SON, the supraoptic nucleus; TRN, thalamic reticular nucleus; LC, the locus coeruleus; PVT, the paraventricular thalamus; VTA, the ventral tegmental area; LHb, the lateral habenula; NAc, the nucleus accumbens; PBN, the parabrachial nucleus;
AANs, anesthesia-activated neurons; Grm2-Cre, the metabotropic glutamate receptor 2-Cre recombinase; TeLC, tetanus toxin light chain; DTA, diphtheria toxin A; IBO, ibotenic acid; DAT, dopamine transporter; PRSx8, the synthetic dopamine β hydroxylase (DBH) promoter; TH, tyrosine hydroxylase; prazosin, the selective a1-adrenoceptor blocker;
M, mice; R, rats; ND, no difference; N/A, not assessed; active/inactive, during active/inactive cycles; ISO, isoflurane; PRO, propofol; SEVO, sevoflurane; the percentage in bracket is referred to the concentration of the volatile anesthetic; Tc, core body temperature.
Remarks:
*: no statistical difference;
#: with or without chemogenetic inhibition of the PVT;
a: for this study, the NREM is referred to SWS (slow-wave sleep); the time of Induction was determined by the onset of strong slow-wave power in EEG and minimal muscle activity in EMG; the time point of Fully Awake was determined by diminished slow-wave power and re-appearance of movement continuously for at least 1 min; the total Duration of unconscious time was calculated by the time point of Fully Awake minus the time point of Induction;
b: for this study, the optogenetic stimulation was given once stable burst-suppression mode was recorded or during iso-electric activity;
c: for this study, the cell type of the PVT was non-specific, but primarily deemed to be glutamatergic; ISO was 2% for induction and 1.5% after the induction
d: for this study, ISO was 1.5% for induction, 0.6-1% for maintaining lightly anesthetized range;
e: for this study, ISO was 2.5% for 20 min and then 0.8-0.9% for maintenance; behavior arousal was assessed by arousal score.
Specific ablation of either Vglut2+ or Vgat+ AVPe/MPA Q neurons attenuated the hibernation-like hypothermia, suggesting that the hibernation-like state is a synergistic effect of both Vglut2+ and Vgat+ AVPe/MPA Q neurons [93]. Vgat+ AVPe/MPA Q neurons may promote tail vasodilation by inhibiting the circuit between Vglut2+ neurons in the dorsal part of the DMH (dDMH) and the raphe pallidus (RPa), while Vglut2+ AVPe/MPA Q neurons may inhibit thermogenesis by activating another neuronal subpopulation in the DMH [93]. Consistently, genetic ablation of either Vglut2+ or Vgat+ MnPO neurons has an effect on thermoregulation during behavioral, warm or cold stress [88]. Likewise, it is also proposed that both Vglut2+ and Vgat+ POA neurons send thermoregulatory projections to a specific neuronal subpopulation in the dDMH/the dorsal hypothalamic area (DHA) that connects to the RPa [94]. Based on the results above, it is time to discard our “glutamatergic or GABAergic” single-action model.
Additionally, in the ventral part of the lateral preoptic nucleus (vLPO), optogenetic activation of either Vglut2+ or Vgat+ neurons causes hypothermia, with the Vgat+ vLPO neurons supposed to receive afferent signals from other parts of the POA and project to the dDMH/DHA [95] (Table 1). Only by lesions of both the MnPO and dorsolateral preoptic (DLPO) neurons, but not either alone, can cause hyperthermia [96], the DLPO appears to be in the location of the vLPO. Taken together, it is shown that the POA cannot be regarded as a whole part since there are different neuronal populations in the intra-POA circuitry, which has also been outlined elsewhere [3].
Another concerning is that optogenetic and chemogenetic manipulations per se may affect the results. Firstly, it’s hard to control the injection sites and the diffusion of the viral vectors in optogenetic and chemogenetic experiments, which could have an effect on the degree of thermoregulatory responses. As has been shown, the injection sites that caused the greatest fall in temperature were confined to the core and dorsal extended of the ventrolateral preoptic nucleus (VLPO), specifically the location that contains vLPO, with little or no involvement of the medial, median, or periventricular preoptic areas [16]. Moreover, it is reported that optogenetic and chemogenetic manipulations cause different thermoregulatory responses, possibly due to the differences in the firing rate/pattern [16] or in the sensitivity [93] of neurons under different kinds of manipulations (Table 1). It seems particularly important to identify these confounding factors brought by optogenetic and chemogenetic manipulations per se in further studies.
2.2.2. Other Thermoregulatory Neuronal Populations in the POA
Apart from glutamatergic and GABAergic POA neurons, many studies have identified other neuronal subpopulations that regulate thermogenesis using optogenetic and(or) chemogenetic manipulations.
It has been found that the Vglut2+ neurons in the MnPO and VLPO highly colocalize with PACAP and project directly to the RPa [88], which makes a hint that, apart from the MnPOPACAP/BDNF neurons, the Vglut2+ VLPOPACAP neurons might synergic contribute to the warmth-induced hypothermia. Chemogenetic stimulation of MnPO neurons that express Leptin receptors (LepRb) is sufficient to induce hypothermia, and it is possible that MnPOLepRb neurons are glutamatergic [87] (Table 1). The neuronal nitric oxide synthase (NOS1)-positive glutamatergic neurons in the MnPO-MPO are activated by ambient warmth, and chemogenetic activation of them can reduce the Tcore [18] (Table 1).
Chemogenetic activation of galanin VLPO (VLPOGal) neurons markedly reduces Tcore during both the light and dark periods [16] (Table 1). Similarly, chemogenetic activation of galanin neurons in the lateral preoptic (LPO) also reduces Tcore, and chronic ablation of LPOGal neurons largely elevates Tcore [17] (Table 1). But here comes the question again, although it has been found that VLPOGal neurons mainly co-express GAD [97], is there any possibility that VLPOGal neurons are glutamatergic? And taking the injection sites into consideration, where is the specific POA division that the thermoregulatory galanin neurons lie in, more specifically, the NREM sleep-promoting VLPO [16, 17, 97] (Table 1) or the thermoregulatory vLPO?
Ptgds, which encodes lipocalin-type prostaglandin-D synthase (L-PTGDS) that generates prostaglandin D2 (PGD2) [98], is a genetic marker for thermosensitive POA neurons [99]. Chemogenetic manipulations on the PTGDS-Cre transgenic mice in the daytime, but not the nighttime, cause changes in body temperature [99] (Table 1). The result reveals that POA neurons that express Ptgds are involved in thermoregulation, and it is possible that POAPtgds neurons also receive circadian inputs from the SCN, which functions as the central circadian clock in the anterior hypothalamus [100]. A caveat here is that the circadian regulation of body temperature is unavoidable that may interfere with the results during optogenetic or chemogenetic manipulations on CNCs-Tcore. The possible correlation of the SCN and CNCs-Tcore will be reviewed later.
2.3. Central Thermoregulatory Locations Other Than the POA
Although the POA→dDMH/DHA→rRPa pathway has been recognized as the most canonical part of the CNCs-Tcore [3, 4], there still exist other locations that play an important role. In this part, we focus on some newly identified neuronal populations and locations in the CNCs-Tcore, mostly demonstrated by optogenetic and(or) chemogenetic manipulations.
2.3.1. DMH/DHA and RPa
The dDMH/DHA plays an important role in thermoregulation by receiving afferent signals from MnPOPACAP/BDNF neurons [85], vLPOVgat neurons [95] and AVPe/MPA Q neurons [93] and making efferent projections to the RPa [94, 101], rostral medullary raphe area (rMR) [57] and PAG [102]. Using optogenetic and chemogenetic manipulations, it is demonstrated that both dDMH/DHAVglut2 neurons and dDMH/DHAVgat neurons function in CNCs-Tcore [85, 95, 101] (Table 1).
It has been found that chemogenetic activation of the DHAVglut2 neurons can replicate a hyperthermic response with the amplitude and duration of stress-induced hyperthermia (SIH), accompanying with increased BAT thermogenesis and reflex tail vasodilation. However, chemogenetic inhibition of the DHAVglut2 neurons or optogenetic inhibition of the DHAVglut2→RPa terminals is only able to reduce SIH by about one-third [101] (Table 1). It is known that activation of BAT and cutaneous vasoconstriction (CVC) is ordinarily coordinated during stressful conditions [103], and vasoconstriction is thought to be mediated by RPaVglut3 neurons [104, 105]. Conclusively, there are supposed to be two groups of RPaVglut3 neurons: the BAT-thermogenesis-activating one is innervated by the DHAVglut2 neurons, while another tail-vasoconstriction-promoting one receives no DHAVglut2 input [101].
Optogenetic stimulation of cholinergic DMH (DMHAch) neurons, which are excited by warm ambient temperature (36°C), decreases BAT activity with reduced Tcore, possibly through activation of M2 muscarinic ACh receptors (mAChRs) on serotonergic RPa (RPa5-HT) neurons [106] (Table 1), giving evidence of the DMHACh→RPa5-HT pathway mediating heat-defense responses during warm ambient temperature.
Chemogenetic activation of dDMH/DHALepRb neurons increases Tcore, BAT thermogenesis, and locomotor activity [107] (Table 1). Supposed to be overlapped with dDMH/ DHALepRb neurons, the dDMH/DHA neurons that express Bombesin-like receptor 3 (dDMH/DHABrs3 neurons) have been identified to play a necessary role in the regulation of Tcore and energy expenditure [94] (Table 1). Optogenetic activation of the dDMH/DHABrs3→ RPa pathway is sufficient to increase Tcore, most likely through glutamatergic projections [94] (Table 1). However, it is also clarified that the dDMH/DHALepRb neurons contain both glutamatergic and GABAergic subpopulations, with the majority being glutamatergic [95]. It seems that the dDMH/DHALepRb neurons are more than just the dDMH/DHABrs3 neurons, which needs further demonstration.
It is found that the LPB can directly input to the dDMH/DHABrs3 neurons projecting to the RPa (dDMH/DHABrs3→RPa neurons) [94], independent of the LPB→POA pathway [3, 4]. It’s reported that both dDMH/DHAVglut2 neurons and dDMH/DHAVgat neurons are cold-activated, possibly resulting from periphery sensory input [95]. The LPB→dDMH/DHA pathway possibly exists to work parallelly with the LPB→POA pathway as well as receiving input from the latter simultaneously. It is also found that the raphe interpositus nucleus and the raphe magnus (RIP/RMg) neurons project to dDMH/DHABrs3→RPa neurons [94], while the RIP/RMg→dDMH/DHA pathway has not been previously reported [108]. The RMg has also been identified to innervate BAT [109], and spinally projecting neurons of the RMg are considered to be part of the BAT sympathetic premotor neurons (SPN) [3]. The RIP/RMg have been demonstrated to be part of the CNCs-Tcore whether by the direct RMg→SPN pathway or by the indirect RIP/RMg→dDMH/DHABrs3→RPa pathway, while the specific neuronal population(s) and the afferent pathway(s) of the RIP/RMg remain to be further established.
In addition, it is reported that substantial numbers of neurons in the ventral part of the DMH (vDMH) and posterior DMH send inputs to the dDMH/DHABrs3→RPa neurons, which suggests an intra-DMH/DHA circuitry [94]. As has been proposed, the inhibitory interneurons in the DMH might innervate the glutamatergic hyperther- mic neurons in the DHA [88], which gives a direction for identifying the intra-DMH/DHA circuitry.
2.3.2. PVH and ARH
There are now three divisions of the PVH that form polysynaptic connections to interscapular BAT in rats: dorsal medial parvicellular part (PVHmpd), ventral medial parvicellular part (PVHmpv), and posterior part [109, 110]. It is reported that the RIP-Cre GABAergic neurons in the arcuate nucleus of the hypothalamus (ARH)→PVHmpv neurons→GABAergic NTS neurons→RPa neurons→→→BAT circuit is required for BAT thermogenesis in response to high-fat diet ingestion [111] (Table 1), while the BDNF neurons in the PVMmpd and posterior PVH→spinal intermediolateral nucleus (IML)→sympathetic ganglion→BAT circuit drives adaptive thermogenesis in response to cold exposure [112] (Table 1). It is recently shown that acting through the agouti-related peptide (AgRP) expressing ARHLepRb neurons and POMC ARHLepRb neurons→BDNF PVH neurons→IML→sympathetic ganglion→BAT circuit, chronic leptin treatment of ob/ob mice that have mutations in the leptin gene, can restore reductions of sympathetic innervation of BAT and defects in thermogenesis [113]. Taken together, there are now two main circuits linking the ARH and PVH to BAT thermogenesis: (1) RIP-Cre GABAergic ARH neurons→PVHmpv neurons→GABAergic NTS neurons→RPa neurons→→→BAT circuit; (2) AgRP ARHLepRb neurons and POMC ARHLepRb neurons→BDNF neurons in the PVMmpd and posterior PVH→IML→sympathetic ganglion→BAT circuit.
In the ARH, it has been concluded that the AgRP neurons have opposite functions against the RIP-Cre neurons, with the former inhibit energy expenditure and stimulate food intake, but the latter stimulate energy expenditure and have no effect on food intake [111] (Table 1). The neuropeptide Y (NPY) ARH neurons are suggested to stimulate energy expenditure and reduce the tyrosine hydroxylase (TH) expression in the PVH with subsequent reduction of TH expression in the LC, NTS, and VLM [114]. Taken together, the NPY ARH neurons may overlap with the RIP-Cre GABAergic ARH neurons [111] and share common thermoregulatory pathways with the latter, leading to energy expenditure, BAT thermogenesis and Tcore increase, which needs to be further demonstrated.
Chemogenetic and optogenetic activation of the ARH neurons expressing kisspeptin (also known as neurokinin B) (ARHKiss1 neurons) causes a reduction of Tcore and evokes a heat-dissipation response as tail vasodilation in both female and male mice. And optogenetic stimulation of ARHKiss1 terminals in the rostral POA, mostly overlapped with the MnPO, reduces Tcore and promotes tail vasodilation in female mice, through the neurokinin B receptor (NKBR) in the POA [115] (Table 1). The result is consistent with the hypothermia induced by microinjection of NKBR agonist into the MnPO [116]. The ARHKiss1→MnPO-NKBR pathway is possible to be part of the CNCs-Tcore, but the interactions between this pathway and the reproductive axis remain to be further established.
The leptin signaling is also proposed to act on the ARH [2]. Leptin is an important hormone in the co-regulation of body temperature and energy homeostasis [117, 118], which plays an integrative role in the CNCs-Tcore that contains the ARH, PVH, POA and dDMH/DHA [117] (Fig. 2).
The upstream location(s) of these two PVH circuits, except the ARH, hasn’t been identified so far. It has been found that chemogenetic stimulation of the TRPM2+/ Vglut2+ POA neurons can decrease Tcore, partly by activating Crh+ PVH neurons [37]. Given that administration of glutamate into the PVH would increase thermogenesis through BDNF neurons in the PVMmpd and posterior PVH, but decrease thermogenesis through PVHmpv neurons [112], the Crh+ PVH neurons activated by TRPM2+/Vglut2+ POA neurons may be overlapped with PVHmpv neurons. Moreover, given that PGE2, via actions of presynaptic EP3R, can depress GABA release onto parvocellular neuroendocrine cells (PNCs) in the PVH to disinhibit the LPS-induced responses [119], it seems reasonable to propose that the upstream GABAergic neurons that express inhibitory EP3R could send input to disinhibit the PVH and promote LPS-induced hyperthermia. Further studies are needed to test our hypothesis and explore the possible afferent locations of the PVH.
Chemogenetic manipulations of the PVHBrs3 neurons can regulate food intake without affecting Tcore or locomotor activity [94] (Table 1). Given that the BDNF neurons in the anterior PVH regulate not BAT thermogenesis but food intake and locomotor activity [112], it seems probable that the PVHBrs3 neurons mostly overlap with them. However, these two studies seem to be contradictory considering the different results of locomotor activity. It’s worth noticing that the BDNF neurons in the anterior PVH do affect the locomotor activity during the dark cycle but not during the light cycle [112], which indeed is consistent with the result of Piñol et al. since the chemogenetic manipulations of the PVHBrs3 neurons were done during the light cycle [94] (Table 1). Taking the circadian rhythms into consideration, it hasn’t been addressed whether the PVHBrs3 neurons would affect Tcore during the dark cycle.
2.3.3. LH
Genetic inhibiting the expression of Bdnf promoter I (Bdnf-e1-/-), but not that of the promoter IV (Bdnf-e4-/-) or VI (Bdnf-e6-/-) results in severe deficits in thermogenesis. And only in the LH, but not the VMPO, DMH, the ventromedial hypothalamus (VMH), RPa, or PVH, the BAT-connected neurons co-labeled with Bdnf-e1-derived BDNF [120]. The BAT-connected Bdnf-e1-expressing LH neurons are Vglut2+, but not Vgat+, orexin+, or few melanin-concentrating hormone-positive (MCH+). And chemogenetic activation of the Vglut2+ LH neurons increases the BAT thermogenesis [120] (Table 1). This study revealed a specific population of LH neurons important for regulating BAT thermogenesis. But there still stand other possibilities: (1) promoters other than e1, e4, and e6 may also participate in thermoregulation of the PVH; (2) promoters other than e1 may act on that of the VMPO, DMH/VMH, and RPa; (3) some other brain regions may also participate in thermoregulation through Bdnf-e1-derived BDNF expression [120].
It is reported that mice lacking MCH1 receptor (MCH-R1) exhibit higher Tcore during baseline than wildtype littermates [121]. Selective deletion of MCH neurons can increase body temperature and locomotor activity while decrease food intake during the dark cycle [22] (Table 1). Contrarily, the blockade of hypothalamic MCH expression by injecting antisense MCH oligonucleotide into the lateral ventricle (LV) increases the BAT mass and uncoupling protein 1 (UCP-1) expression in BAT, while do not increase the body temperature of cold-exposed rats [122]. More recently, chemogenetic activation of neither LHMCH neurons [20, 22] (Table 1) nor Vglut2-KO LHMCH neurons [20] (Table 1) has an effect on the body temperature or locomotor activity. It seems that chronic deletion of MCH, rather than acute manipulations of MCH, can induce some unknown complementary mechanism(s) that causes the increase of Tcore. With the paradox remains, here we make two inferences: (1) given that the orexin system and the MCH system have potentially opposing effects [1], acute activation of either one may be concealed by another; (2) given that the appearance is different between the light and dark cycles after chronic ablation of the MCH neurons [22] (Table 1), the CNCs-SWs or circadian rhythms may affect the Tcore-regulating mechanisms of LHMCH neurons.
As for LHorexin neurons, it is reasonable that they send orexinergic projections to the RPa to regulate thermogenesis [5, 80, 123]. It is also suggested that the Crh+ PVH neurons, activated by the hypothermia-promoting TRPM2+/Vglut2+ POA neurons [37], may activate the orexin neurons by inputs to them [124]. Recently, orexin neurons are reported to be involved in the thermoregulation under stress related to exercise conditions, which is independent of the locomotor activity [125]. It is also proposed that factors other than orexin in the orexin-expressing neurons may contribute to adaptive thermogenesis regulation [120], one possible scenario is that orexin neurons may interact with the Bdnf-e1-expressing Vglut2+ LH neurons to modulate thermogenesis indirectly [120].
There are some LH neurons apart from MCH and orexin neurons that also participate in the CNCs-Tcore. Microinjection of the GABAA agonist muscimol into the LH to inhibit the LH neurons can promote vasoconstriction [126]. Likely to be neither orexin nor MCH, the specific neuronal population(s) involved in this vasoconstrictor response needs to be further unraveled. In addition, optogenetic and chemogenetic activation of the cold-sensitive neurotensin (Nts)-expressing LH (LHNts) neurons which are distinct from both orexin and MCH, can increase the Tcore [21] (Table 1).
2.3.4. DRN
It has been found that heat-activated GABAergic neurons in the DRN, more specifically, in the DRN and bordering ventral portion of vlPAG, regulate energy expenditure through changes in thermogenesis and locomotor activity, by directly projecting to the RPa to regulate BAT thermogenesis and indirectly innervating the MPA, DMH, and bed nucleus of the stria terminalis (BNST) [34] (Table 1). The identification of the DRN as an upstream thermoregulator of the POA highlights the truth that it is time to confront the canonical model that deems the POA as the central core of the CNCs-Tcore [6]. Given that the TRPM8-expressing POA neurons project to the DRN [77], the interactions between the POA and DRN may be another focus for further research.
It has been proposed that the DRI5-HT neurons might interactively regulate Tcore and emotional behavior through TRPV3 or TRPV4 [69]. However, chemogenetic activation of the DRN5-HT neurons facilitates sleep through anxiolysis, but doesn’t significantly reduce the stress-induced thermo- genesis or locomotion [127] (Table 1). It has been clarified that the two highly complementary parallel DRN5-HT neuro- nal pathways: the DRN5-HT→the orbitofrontal cortex (OFC) pathway and the DRN5-HT→the central nucleus of the amygdala (CeA) pathway, are each activated by reward but show opposite responses to aversive stimuli, with the former one having anxiolytic effects and the latter one promoting anxiety [128]. Further studies are needed to identify the interrelation of the thermoregulatory DRNVgat neurons and the emotional-related DRN5-HT neurons, and to demonstrate whether thermosensitive TRPV3 or TRPV4 participates in the functions of these two DRN subpopulations.
2.3.5. VTA
The thermoregulatory vasomotor role of the ventral tegmental area (VTA) has been proposed [129]. Heat exposure induces heat loss and promotes 5-HT releasing from the VTA, and consistently, increasing extracellular 5-HT in the VTA promotes heat loss by Tcore decrease and tail temperature increase [130]. Using anesthetized rats, it is found that the LHb promotes BAT thermogenesis by sending inhibitory GABAergic projections to the VTA, possibly to the dopaminergic VTA neurons that are responsible for regulating BAT thermogenesis [131]. Since anesthesia may be another factor that affects thermoregulation [23], the role that the VTA plays in CNCs-Tcore needs further studies.
2.3.6. Other Possible Locations
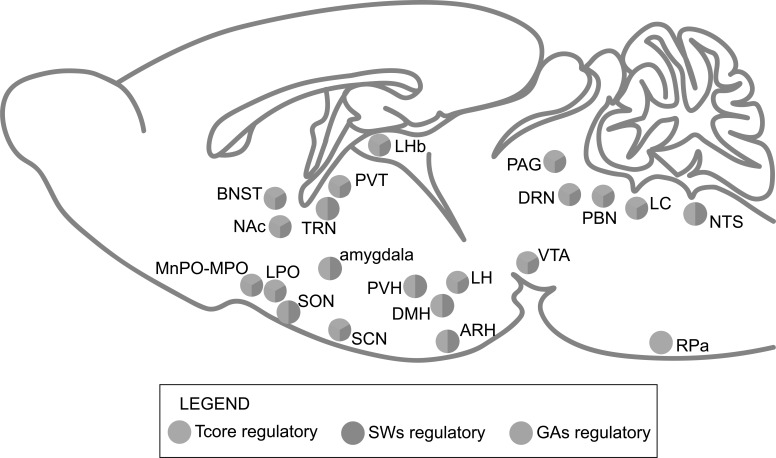
There still exists many other thermoregulatory neuronal populations, such as the TRPV1 expressing LC neurons [45], the hypothalamic NPY-activated GABAergic neurons in the intermediate and parvicellular reticular nuclei (IRt/PCRt) of the medulla oblongata [132], the thermoregulatory vasomotor neurons in the rostral ventrolateral periaqueductal gray (rvlPAG) [129], the oxytocin receptor-expressing NTS neurons involved in high-fat diet-induced thermogenesis [133] and the NTS neurons participating in Galanin-Like Peptide (GALP)-induced hyperthermia [134]. The specific neuronal populations and neural pathways of the CNCs-Tcore are summarized in Fig. (2).
3. CENTRAL CO-REGULATION OF CORE BODY TEMPERATURE AND SLEEP-WAKEFULNESS STATES
3.1. Central Coordinators Between Core Body Temperature and Sleep-Wakefulness States
It has long been hypothesized about the interactions between sleep and thermoregulation, mostly depending on the sleep-regulating and thermosensitive properties of POA neurons [11-13]. In more recent reviews, neurons in the MnPO [1, 2, 14, 15], VLPO [15], DMH [15], PBN, LH, and PAG [1] are also considered to be reasonable candidates for the interactions between the regulation of Tcore and SWs, but left with several concerns. Firstly, the specific neuronal populations in the above-mentioned brain regions and their innervations involved in the coordination of Tcore and SWs need to be clarified. On the other hand, given that both CNCs-Tcore and CNCs-SWs haven't been completely elucidated so far, it cannot be ruled out the possibility that other brain regions may also be involved in the orchestration of Tcore and SWs. In this part, we will briefly discuss the possible coordinators in the CNCs of both Tcore (Fig. 3) and SWs (Fig. 4).
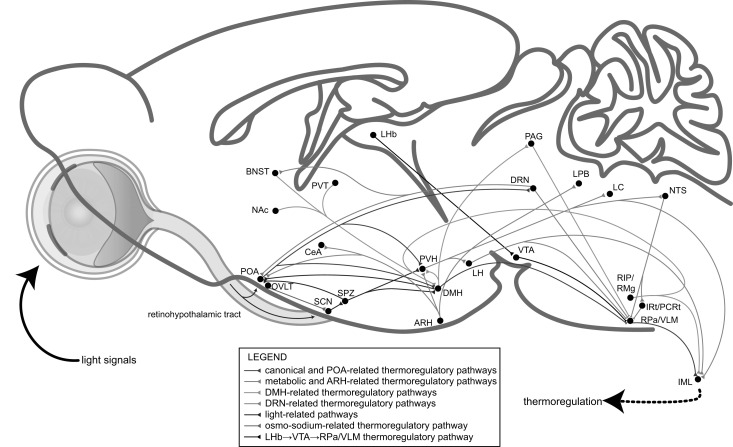
Fig. (3).
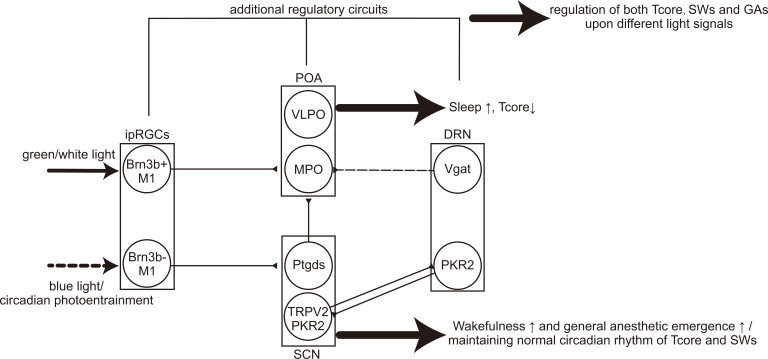
A model of the central thermoregulatory circuits controlled by environmental light signals. The light signal as well as the circadian photoentrainment is transmitted to the SCN and POA through the retinohypothalamic tract and then the downstream subparaventricular zone (SPZ), DMH and PVH. The POA→DMH→RPa/VLM pathway is canonical, functioning together with other thermoregulatory circuits, including the ARH-, DMH-, DRN-, osmo-sodium-related and other possible pathways.
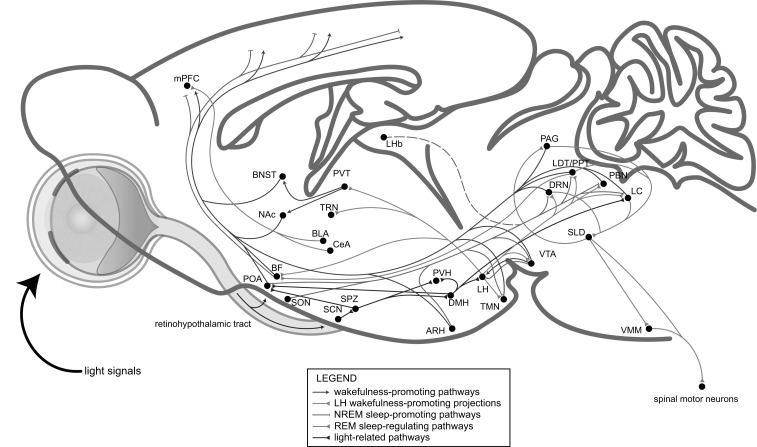
Fig. (4).
A model of the central neural circuits of sleep-wakefulness states controlled by environmental light signals. The light-related pathway also regulates the LH and LC, both of which are wakefulness-promoting. The LH sends innervations to extensive locations, including the basal forebrain (BF), TRN, PVT, TMN, VTA, DRN, laterodorsal tegmental area (LDT)/ pedunculopontine tegmental area (PPT), PBN and LC. The arousal-promoting system, includes the LC, PBN, LDT/PPT, DRN, VTA, TMN, BF, PVT, NAc and BNST, while the NREM sleep-promoting pathways involves the inhibition of the wakefulness-promoting pathways by the POA, the inhibition of the cortex by the basal forebrain (BF), and the inhibition of the LH by VTA. The LHb is also NREM sleep-promoting, while the related pathway(s) is not clear (indicated by dotted line). The DRN can be wakefulness-promoting when hungry while sleep-permissive during satiety. Additionally, the latest finding suggests that the VLPOVglut2 neurons are wakefulness-promoting whilst the MnPOVgat neurons are NREM sleep-promoting. The DRN, PAG and LC participate in the REM sleep-regulating pathways to mutually regulate the muscle paralysis of REM sleep by directly inhibiting the excitatory sublaterodorsal nucleus (SLD)→VMM pathway or indirectly inhibit the excitatory LDT/PPT→SLD pathway, and the PAG receives inhibitory projections from the SLD. The LDT/PPT→BF pathway may help drive the typical fast EEG activity of REM sleep. And the BLA/CeA→medial prefrontal cortex (mPFC) pathway may also regulate REM sleep. (Adapted from [8]).
3.1.1. POA
It has been found that the NOS1+ glutamatergic neurons in the MnPO-MPO region induced both body cooling and non-rapid eye movement (NREM) sleep, whereas GABAergic neurons in the MPO induce only NREM sleep but not hypothermia [18] (Table 1). It is proposed that NOS1+ glutamatergic MnPO-MPO neurons may overlap with LepRb+ POA neurons [2]. Given that the leptin signaling appears to have a direct role in regulating SWs [2], it is reasonable to infer that the thermoregulatory leptin pathways may also play an important role in sleep homeostasis.
The VLPOGal neurons are important for NREM-promoting by providing inhibitory inputs to the tuberomam-millary nucleus (TMN) and other components of the ascending monoaminergic arousal system [97]. Recently, chronic ablation of LPOGal neurons largely elevates Tcore and abolishes the homeostatic sleep rebound following sleep deprivation [17], which reveals the potential role of VLPO/LPO Gal neurons in the integrative regulation of Tcore and SWs.
As has been discussed in 2.2.2, chemogenetic activation of the POAPtgds neurons can promote hypothermia, by activating the DP1 receptor in the VMPO neurons [99] (Table 1). PGD2 has been identified as one of the most potent endogenous sleep-promoting molecules by stimu-lating the DP1 receptors on the ventral surface from the basal forebrain to the hypothalamus [135]. Recently, neuro-endocrine cells in the supraoptic nucleus (SON) and paraSON (including the VLPO and POA) have been proved to strongly promote slow-wave sleep (SWS) of NREM [136], with the VLPO temperature-dependently producing PGD2 [97] and the SON highly expressing PTGDS [137] and activated by endogenous PGD2 [138]. It seems reasonable that the PTGDS-expressing neurons in the SON/POA co-potentiate NREM sleep and hypothermia. Interestingly, in support of this, systemic administration of nicotinic acid, a common regulator for TRPV1-4 [139], can elicit robust NREM sleep increase, Tcore decrease and hot flush, which can be completely abolished by the cyclooxygenase inhibitor indomethacin, indicating the involvement of the endogenous prostaglandins, mostly PGD2 [140].
3.1.2. LH
Apart from the POA, the LH is another integrative location under hot discussion. It has been proposed that orexin-expressing neurons may modulate the effects of the ambient temperature on SWs, while genetic ablation of orexin-expressing neurons doesn’t prevent the occurrence of these effects [141], indicating that orexin-expressing neurons may not be essential for the coordination of Tcore and SWs. Furthermore, given that factors other than orexin in the orexin-expressing neurons may also contribute to the regulation of adaptive thermogenesis [120] and SWs [142, 143], it deserves further studies on identifying the specific neuronal subpopulation(s) of orexin-expressing neurons involved in the coordination of Tcore and SWs.
The mice lacking the MCH-R1 have a higher Tcore as well as an increase of wakefulness and a decrease of NREM sleep [121]. Ablation of MCH neurons alters the circadian pattern of the locomotor activity and Tcore as well as that of wakefulness and rapid eye movement (REM) sleep, with the bouts number of REM sleep increased and the bout duration of wakefulness decreased during the light period [22] (Table 1). However, chemogenetic activation of MCH neurons [20, 22] or that of Vglut2-KO MCH neurons [20] increases REM sleep without affecting Tcore (Table 1). The differences among the deletion of MCH-R1, chronic ablation of MCH neurons, and acute activation of MCH neurons, again lead us to our hypothesis raised in 2.3.3 that warrants further studies.
In addition to the orexin and MCH population, there exist other neuronal population(s) in the LH involved in the modulation of SWs [126]. Recently, the LHNts neuron, a newly-identified neuronal population, which has no overlap with either orexin or MCH population, is proved to be critical for promoting NREM-to-wakefulness transitions, hyperactivity, and hyperthermia and to be important for orchestrating SWs and thermoregulatory responses to acute stress exposure with the PVH participating in this process [21] (Table 1). The effects of the specific cold-sensitive and arousal-promoting LHNts neurons are consistent with the results of Cerri et al. [126]. It is suggested that the LHNts→VTADA being a possible regulatory pathway for locomotor activity [21]. Furthermore, LHNts neurons receive inputs from the PVH, BNST, and amygdala, and project to the VTA, vlPAG, PBN, LC, RPa, VLM, and LPO [21], all of which participate either singly or simultaneously in the CNCs of Tcore and SWs. For the first time, the specific neuronal population in the LH that coordinates both thermogenesis and SWs has been identified and proposed as a “master orchestrator” to modulate the activity of orexin and MCH neurons through intra-LH circuits [21].
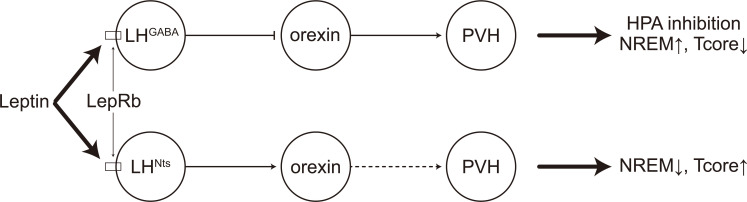
The LH may be another target regulated by the metabolic leptin signals to coordinate Tcore and SWs. Orexin neurons receive GABAergic innervation by LepRb+ LH neurons, and the leptin signals can inhibit the orexin→PVH pathway to decrease corticosterone release in response to stress [144]. Furthermore, optogenetic activation of the GABAergic LH neurons expressing LepRb can promote NREM sleep [145]. It is reasonable to infer that the LHGABA→orexin→PVH pathway may promote NREM sleep through inhibition of the HPA, accompanying with a decrease of Tcore. Given that a subset of LHNts neurons expresses LepRb and LHNts neurons are activated by leptin in brain slices [146, 147], it is possible that the leptin signaling may also regulate the LHNts neurons to promote hyperthermia and NREM-to-wakefulness transitions, which is opposite to the effects of the LHGABA→orexin→PVH pathway (Fig. 5). Further studies are needed to verify our model, especially the intra-LH circuits among LHNts neurons, LHGABA neurons, and orexin neurons.
Fig. (5).
A possible model of leptin signals co-regulating core body temperature and sleep-wakefulness states. The leptin signals activate the LepRb of both the LHGABA neurons and LHNts neurons, with the former inhibit the orexin neurons that innervate the PVH to subsequently induce inhibition of the hypothalamic-pituitary-adrenal (HPA), increase of NREM sleep and decrease of Tcore, while the latter contrarily activate the orexin neurons and downstream location(s), possibly the PVH (indicated by dotted arrow), to reduce NREM sleep and elevate Tcore.
3.1.3. DMH
Indeed, the glutamatergic DMH neurons strongly innervate wake-promoting brain regions including the LH, TMN, LC, VTA, and DRN, whereas GABAergic DMH neurons innervate sleep-promoting regions including the VLPO and MnPO [8]. Together with the thermoregulatory DMHVglut2 and DMHVgat neurons discussed in 2.3.1, it seems possible that these two neuronal populations may co-regulate Tcore and SWs. Besides, the thermoregulatory dDMH/DHABrs3 neurons may also participate in the coordination of Tcore and SWs. The dDMH/DHABrs3 neurons receive afferent projections from and send efferent projections to extensive locations in the brain, such as the PVH, POA, vlPAG, nucleus accumbens (NAc), LC, BNST, paraventricular thalamus (PVT), ARH and so on [94], most of which are involved in the CNCs-SWs.
3.1.4. PVH and ARH
As has been discussed in 2.3.2, the PVH is involved in the CNCs-Tcore by direct neural connections [21, 109-112, 114] and indirect secretory responses [37, 119, 133, 144]. Furthermore, it is known that the PVH, which locates in the pathway that regulates the secretion of melatonin, might coordinate with the SCN to regulate sleep onset by entraining circadian rhythms [8]. The specific role that the PVH plays in the orchestration of thermogenesis and sleep-wake homeostasis remains to be further established.
It has been proposed by Harding et al. that different neuronal populations in the ARH, more specifically, ARHPOMC neurons, ARHAgRP neurons, and ARHNPY neurons, interact with each other to regulate sleep onset, thermo-genesis, and energy homeostasis, possibly controlled by the leptin signaling [2].
3.1.5. DRN, PAG, BNST and Amygdala
It has been reported that the DRN5-HT neurons [148], as well as the vPAGDA neurons just located lateral to the DRN [149], can promote wakefulness. Contrarily, as has been mentioned in 2.3.4, chemogenetic activation of the DRN5-HT neurons facilitates sleep through anxiolytic effects, without effects on Tcore or locomotor activity [127] (Table 1). The DRNVgat neurons (including the bordering ventral portion of vlPAG) have been identified to regulate thermogenesis and locomotion by indirectly projecting to the MPA, DMH, and BNST [34] (Table 1). Taken together, the DRN appears to be critical in regulating both Tcore and SWs, with the interactions among wake-promoting DRN5-HT neurons, anxiolytic DRN5-HT neurons and hypothermia-promoting DRNVgat neurons remains to be settled. As the PAG has also been proposed to involve in thermoregulation [129], considering the anatomical contiguity of the DRN and PAG, it would be better to study these two locations discretely. Furthermore, it is intriguing that the BNST is wake-promoting [150, 151], which makes the BNST another coordinator of Tcore and SWs.
It is also reported that optogenetic inhibition of the neurons in the basolateral nucleus of the amygdala (BLA) that specifically overlay with glutamatergic neurons, can facilitate REM sleep under fearful and unfearful conditions while not affecting SIH [152] (Table 1). It has been shown that the DRN5-HT→CeA pathway increases anxiety [127], while the possible BLAGlu→GABAergic neurons in the medial prefrontal cortex (mPFC)→DRN pathway underlies the increases in REM sleep after footshock stress [152]. The BLAGlu→GABAergic neurons in the intercalated cell masses (ICM) or lateral CeA neurons→CeA intra-amygdala pathway underlies the opposite roles between the BLA and CeA [153]. Moreover, given that the thermoregulatory ARHNPY neurons [2] project to the CeA [154], it cannot be ruled out the possibility that these amygdala-related pathways may be involved in thermoregulation, especially under specific emotional states.
3.1.6. VTA
It is known that the VTA dopaminergic neurons play an important role in promoting wakefulness [155, 156]. As has been discussed in 2.3.5, the VTA is part of the CNCs-Tcore. Moreover, the hyperthermia-promoting and arousal-promoting LHNts neurons densely project to the VTA and the LHNts→VTADA pathway possibly mediate locomotor activity [21]. To conclude, the VTA should be regarded as another coordinator of Tcore and SWs.
3.1.7. Other Possible Locations
There also exist other potential coordinators, among which the LC, NTS, LHb, PVT, and NAc are most possible. All these locations play important roles in the regulation of SWs [7-9, 157, 158] (Table 2), with the LCNE neurons activated by cutaneous thermal stimuli [46], the NTS regulating thermogenesis through oxytocin [133] or GALP [147], the GABAergic LHb neurons innervating VTA to promote BAT thermogenesis [131], the PVT and NAc projecting to the thermoregulatory dDMH/DHABrs3 neurons [94]. The major CNCs-Tcore and CNCs-SWs are summarized in Figs. (3 and 4), respectively.
3.2. Different Environmental Light Signals Coordinating Core Body Temperature and the Sleep-Wakefulness States
As has been discussed in 2.1.2, the SCN neurons that co-express TRPV2 with PKR2 may be involved in the circadian oscillation of Tcore and SWs. It is also been reported that the DRN expresses PKR2, and the DRN is likely to have mutual connections with the SCN [63]. Given that the DRN plays an important role in the regulation of both Tcore [6, 34, 69] and SWs [7-9, 127, 148, 149], the circadian signals may be input from the SCN to the DRN to co-regulate Tcore and SWs.
In addition, it is suggested that the PGD2 signaling is situated in the POAPtgds upstream, possibly located in the SCN [99]. It is also revealed that the SCN→POA pathway involves in sleep modulation [159]. Ablation of the SCN abolishes the circadian rhythms in both Tcore and SWs [160]. It is reasonable to infer that the SCN→POA pathway co-regulate the circadian oscillation of both Tcore and SWs, possibly partly by the PGD2 signaling.
Recently, the intrinsically photosensitive retinal ganglion cells (ipRGCs) have been presented as an important neuronal population in mediating both Tcore and SWs towards different light conditions. Genetic ablation of the Brn3b+ ipRGCs, which have widespread projections that largely avoid the SCN, does not affect normal circadian photoentrainment of Tcore and SWs, but attenuates light's acute effects on decreasing Tcore as well as on increasing time spent asleep [161]. Moreover, chemogenetic activation of Brn3b+ ipRGCs significantly decreases Tcore, demonstrating that Brn3b+ ipRGCs mediate light’s acute effects on Tcore though extra-SCN projection(s), while Brn3b- ipRGCs mediate circadian photoentrainment of Tcore possibly by projecting to SCN-involving pathway(s) [161] (Table 1). And it is also proposed that the POA may be involved in the “acute” Brn3b+ ipRGCs→extra-SCN pathway, since the MPO and VLPO have been demonstrated to receive projections from ipRGCs and these projections are lost in animals with genetic ablation of Brn3b [161]. This work highlights the possibility that acute light exposure is another condition regulated independently by the pathway(s) aside from the SCN, and that both Tcore and SWs can be modulated by not only circadian photoentrainment but also acute light signals.
4. CENTRAL MECHANISMS REGULATING GENERAL ANESTHESIA
4.1. Relations Between Central Regulation of the Sleep-Wakefulness States and that of General Anesthesia States
General anesthesia is an artificially-induced unconscious state, which does share many similarities with natural sleep, not only in the electroencephalogram (EEG) manifestations [162] but also in the functionally and anatomically shared CNCs [32] (Table 2).
As has been reviewed elsewhere [32, 33, 163], many different kinds of anesthetics act on the CNCs-SWs to subsequently induce sleep-like cortical neural EEG dynamics, mainly characterized by enhancement of slow-delta (0.5–4 Hz) oscillations [162, 164]. In recent decades, optogenetic and chemogenetic manipulations enable us to specifically identify the neuronal populations and neural pathways related to states induced by general anesthesia (Table 2). The most noteworthy is some non-classical locations that are found to co-regulate SWs and GAs, such as the thalamic reticular nucleus (TRN) [164], PVT [157], LHb [158], VTA [155, 156, 165, 166] and PBN [167] as well as the neuroendocrine general-anesthesia-activated neurons (AANs) in the SON [136].
However, there still stand many questions. Firstly, the different EEG signatures produced by different kinds of anesthetics [33, 162] as well as the different extents the AANs in the SON re-activated by different kinds of anesthetics [136] suggest that different anesthetics may act on different neuronal populations or neural pathways [163].
Besides, albeit the VLPO has long been deemed as an important location that co-promote sleep and anesthetic-induced unconsciousness [136, 168, 169], it's recently been reported that chemogenetic stimulation of neither GABAergic nor glutamatergic neurons in the VLPO affects the anesthetic state transitions, more strikingly, chemogenetic activation of the glutamatergic VLPO neurons promotes wakefulness [19] (Table 2). A caveat is that, since the range of hM3Dq expression has extended beyond the VLPO [19], the pathways in intra-POA may be involved to interfere with the results. Given that a few transient wake-like episodes are observed under 1.2% isoflurane (ISO) but not under 1.5% ISO during chemogenetic activation of MnPOVgat neurons or MnPOVglut2 neurons [19] (Table 2), neural circuits promoting emergence may be activated to a different extent under the different depth of general anesthesia. What's more, the time to give the stimulation may also affect the outcomes: optogenetic activating GABAergic LH→TRN projections promotes emergence during burst-suppression mode while has no effect during iso-electric activity [145] (Table 2). What is certain is that the CNCs-SWs and CNCs-GAs may not exclusively overlap with each other [19, 162].
Furthermore, there still exist many inconsistencies among different studies or conditions: the heterogeneous manifestations between the emergence-promoting noradrenergic LC neurons [170] and the emergence-delaying noradrenergic LC→TRN projections [171], the inhibition of the emergence-promoting glutamatergic PBN neurons inducing no decrease of the induction time [167], the non-differential manifestations of the induction time when optogenetic manipulates the SON AANs [136] as well as the non-differential manifestations of the emergence time when optogenetic manipulates the GABAergic VTA→LH projections [165]. The widespread GAs-regulating neurons may interact with each other, thus inducing these inconsistent results.
Moreover, different light signals may also cause side effects. As has been reported, acute blue light exposure can promote wakefulness, while acute green light exposure promotes sleep in mice [172]. Consistently, we find that acute blue light exposure can promote arousal from sevoflurane anesthesia by activation of the SCN [173]. Seeming to be contradictory, genetic ablation of the Brn3b+ ipRGCs, with projections largely avoiding the SCN, can attenuate the increase of time spent asleep induced by acute light exposure [161]. However, it is noteworthy that the white light source, 6500K CFL bulb, used in this study [161] is more comparable with the sleep-promoting green light [172]. It is also revealed that non-M1 ipRGCs, which have been suggested to promote sleep by projections to the VLPO [172], express Brn3b. Taken together, acute green or white light exposure potentiates sleep through the Brn3b+ ipRGCs→VLPO pathway, while acute blue light exposure, promoting wakefulness and anesthesia emergence, is regulated through Brn3b- ipRGCs→SCN pathway. Together with the discussion in 3.2, we hypothesize a model of the CNCs regulated by environmental light signals (Fig. 6). It is also suggested that the optogenetic stimulation per se may activate the ipRGCs and promote arousal [136, 161]. Apart from acute light exposure, the circadian rhythms [16, 17, 99, 112, 157, 158] and body temperature [16] may also make differences among different studies (Tables 1 and 2), which underscore the necessity to maintain the consistency of circadian and thermo factors during experiments.
Fig. (6).
A model of central neural circuits regulated by the acute light exposure/circadian photoentrainment. The acute green/white light stimuli may be transmitted by the Brn3b+ ipRGCs→POA pathway to promote sleep and decrease Tcore, while the blue light may stimulate the Brn3b- ipRGCs→SCN pathway to promote wakefulness as well as emergence from general anesthesia. The circadian rhythm of Tcore and SWs may also be regulated by the Brn3b- ipRGCs→SCN pathway. The SCN neurons that co-express TRPV2 and PKR2 are mutually innervated with the PKR2+ DRN neurons to regulate the circadian rhythm. The Ptgds+ SCN neurons may release thermoregulatory PGD2 to the POA to regulate the circadian oscillation of Tcore. The DRNVgat neurons inhibit the MPO neurons to modulate Tcore. Together with other regulatory circuits, different light signals are able to regulate Tcore, SWs and general anesthetic states (GAs). Full lines represent light-related/circadian projections and dotted line represents simplex thermoregulatory projection.
4.2. Central Coordinators Between Core Body Temperature and the General Anesthesia States
All general anesthetics can dose-dependently decrease Tcore [23]. It is suggested that the hypothermia induced by general anesthesia results from the redistribution of body heat [24, 174, 175], caused by anesthetic inhibition of vasoconstriction [23, 24] and shivering thermogenesis [23]. It has also been suggested that these two major cold defenses, vasoconstriction, and shivering, may share an identical central regulator [23], which suggests that Tcore under general anesthesia may be regulated by central neural circuits. Based on two sound facts: (1) the orchestration between central regulation of Tcore and SWs [1, 2] (Table 1) and (2) the largely shared CNCs, anatomically and functionally, between SWs and GAs [32, 33] (Table 2), here we reasonably infer that GAIH is caused by general anesthetics acting on the common central regulatory neuronal coordinators for both GAs and Tcore (Fig. 7).
Fig. (7).
Evidence for central orchestration among core body temperature, sleep-wakefulness states and general anesthesia states. A sagittal view of the rodent brain shows the approximate locations of the nuclei known to play a role in regulating Tcore and(or) SWs, which may also be associated with regulation of GAs.
Favoring for our inference, recently, it has been identified that the LPOGal neurons are required for the sedation and hypothermia induced by dexmedetomidine (Dex), an α2A adrenergic agonist. And it is suggested that the Dex-induced sedation, which characteristically accompanies a delta NREM sleep rebound, is not restorative like normal sleep and is possibly the consequence of deep hypothermia [17]. However, it is proposed that both the NREM sleep increase and hypothermia are parallel consequences of activation of the VLPOGal neurons, the range of which more specifically overlaps with the LPOGal neurons [16]. Taken together, these results give a hint that Dex may act on other central coordinator(s), apart from LPOGal neurons, to synergistically potentiate Dex-induced sedation and Dex-induced hypothermia.
Chemogenetic activation of DRNVgat neurons reduces Tcore, BAT thermogenesis, and locomotor activity [34]. To verify whether the suppressed locomotion accounts for the hypothermia, ~1%–1.25% ISO is used to suppress the locomotor activity, after which the decrease of BAT thermogenesis and body temperature still exists [34]. This experiment largely mimics the clinical sedation or general anesthesia condition, under which patients lack of locomotion and bear hypothermia. Therefore, we may conclude that the suppressed locomotion is not decisive for GAIH. In addition, it cannot be ruled out the possibility that ISO can directly act on the CNCs-Tcore, including the DRN, to induce GAIH.
4.3. Thermo-TRP Channels Involving in General Anesthesia-Induced Hypothermia
It is reported that TRPV1 antagonists can dose-dependently prevent the hypothermia induced by either ISO or ketamine in wild-type but not TRPV1-KO rodents, possibly through activation of BAT thermogenesis and changes in vasomotor responses [176].
Volatile anesthetics, under clinical concentrations, produce a two-phase modulation on TRPM8 in vitro. Firstly, volatile anesthetics transiently potentiate TRPM8, giving explanations for the shivering and cooling sensation during the beginning of general anesthesia. Secondly, it follows by a sustained inhibition of TRPM8, possibly leading to the GAIH. And consistently, the hypothermia induced by volatile anesthetics are partially abolished in TRPM8-KO mice [177].
Given that TRPV1 and TRPM8 putatively function in the CNCs-Tcore, which has been discussed in 2.1.1 and 2.1.5, respectively, these results again evidence that general anesthetics may act on the CNCs-Tcore to induce GAIH, possibly through TRP channels. Based on the model raised by Scarpellini et al. [74], the possible central mechanism of GAIH through TRP channels is depicted (Fig. 1b), which may in turn provide promising targets for treating GAIH.
CONCLUSION AND PERSPECTIVES
Recently, it’s reported that GAD-expressing neurons in the organum vasculosum lamina terminalis (OVLT) relay osmo-sodium signals to vasopressin-expressing neurons in the SCN, resulting in the activating of the latter. And optogenetic activation of the OVLTGAD→SCNAVP pathway not only decreases BAT thermogenesis and Tcore, but also significantly phase-advances the circadian locomotion onset [178] (Fig. 3). These findings demonstrate that one non-photic physiological factor can act through the circadian pathway to modulate another physiological response, which further support the orchestration among CNCs of different physiological responses, aiming to keep the overall homeostasis in response to various environmental signals.
The orchestrations among the CNCs-Tcore, CNCs-SWs, and CNCs-GAs has been established by sufficient evidence. And we hope this review will prompt the beginning for further studies to find more solid proofs that favoring orchestrations among different CNCs, which in turn may provide new directions for further consummating these CNCs. For example, the DRN, which is an important regulator of both CNCs-Tcore [6, 34] and CNCs-SWs [127, 148], whereas its role in CNCs-GAs, if possible, hasn’t been identified. Also, it’s reported that the DRNVgat neurons project to the BNST to regulate thermogenesis [34], while the efferent pathway(s) from the BNST is pendent. By comparison with the possible BNSTGABA→LHOrexin or BNSTGABA→LCNE pathway in CNCs-SWs [150], we may find the downstream target(s) of the thermoregulatory BNST neurons.
Apart from the classic neurotransmitters like glutamate and GABA, some neuroendocrine cells such as the SON AANs [136] and the Crh+ PVH neurons [37] as well as the longer-acting neuromodulators like orexin and MCH may also play important roles in the orchestration and act as coordinators during a longer period.
Furthermore, based on the model we put forward (Fig. 6), the environmental light signals, both the circadian rhythms and acute light exposure, may also co-regulate Tcore and SWs as well as affecting GAs, with different wavelength light activating different neural pathways. But there still remain questions to be addressed. For instance, to what extent is the DRN function in these pathways? Besides, additional regulatory pathways that may participate are yet clear.
Moreover, the energy homeostasis has also been shown to strongly link to thermoregulation as well as SWs [1, 2], during which leptin signaling may play an important role. It needs to be further identified whether the central regulation of energy homeostasis, especially that of the leptin signaling, may affect GAs.
In addition, this review gives a possible reason why it presents contradictory conclusions [19] on the canonical NREM-promoting and general anesthesia-promoting VLPO neurons, which may be brought by (1) different injection sites that may activate different intra-POA pathways, (2) different time to give the stimulation or (3) different experimental light signals or circadian periods and different experimental controlling of Tcore.
A caveat is that, the complex coordination among these CNCs as well as the multiple projections brought by heterogeneous neuronal populations reminds us never to regard a sole neuronal population, central location, or neural pathway to be the only central core that functions to regulate these mysterious and dynamic physiological responses.
As for GAIH, the orchestration reveals a possibility that general anesthetics may act on the CNCs-Tcore, thus resulting in it. For the moment, given that TRPV1 [176] and TRPM8 [177] can regulate GAIH, it’s reasonable to deem the superfamily of TRP channels to be promising therapeutic targets for GAIH based on their roles in CNCs-Tcore. Speak of clinical transformation, given that BAT thermogenesis works as a primary thermoregulatory response in rodents while it is not particularly important in unanesthetized adults and relatively insignificant in perioperative patients [23], it needs to be addressed to what extent the CNCs-Tcore in rodents can be conserved in humans.
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This work was supported by the National Natural Science Foundation of China (No: 81873793 and 81571357).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Latifi B., Adamantidis A., Bassetti C., Schmidt M.H. Sleep-wake cycling and energy conservation: role of hypocretin and the lateral hypothalamus in dynamic state-dependent resource optimization. Front. Neurol. 2018;9:790. doi: 10.3389/fneur.2018.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harding E.C., Franks N.P., Wisden W. The temperature dependence of sleep. Front. Neurosci. 2019;13:336. doi: 10.3389/fnins.2019.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morrison S.F., Nakamura K. Central mechanisms for thermoregulation. Annu. Rev. Physiol. 2019;81:285–308. doi: 10.1146/annurev-physiol-020518-114546. [DOI] [PubMed] [Google Scholar]
- 4.Tan C.L., Knight Z.A. Regulation of body temperature by the nervous system. Neuron. 2018;98(1):31–48. doi: 10.1016/j.neuron.2018.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morrison S.F., Madden C.J., Tupone D. Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metab. 2014;19(5):741–756. doi: 10.1016/j.cmet.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piñol R.A., Reitman M.L. Cool(ing) brain stem GABA neurons. Cell Res. 2019;29(10):785–786. doi: 10.1038/s41422-019-0223-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weber F., Dan Y. Circuit-based interrogation of sleep control. Nature. 2016;538(7623):51–59. doi: 10.1038/nature19773. [DOI] [PubMed] [Google Scholar]
- 8.Scammell T.E., Arrigoni E., Lipton J.O. Neural circuitry of wakefulness and sleep. Neuron. 2017;93(4):747–765. doi: 10.1016/j.neuron.2017.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones B.E. Arousal and sleep circuits. Neuropsychopharmacology. 2020;45(1):6–20. doi: 10.1038/s41386-019-0444-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parmeggiani P.L. Interaction between sleep and thermoregulation: an aspect of the control of behavioral states. Sleep. 1987;10(5):426–435. doi: 10.1093/sleep/10.5.426. [DOI] [PubMed] [Google Scholar]
- 11.Alam M.N., McGinty D., Szymusiak R. Neuronal discharge of preoptic/anterior hypothalamic thermosensitive neurons: relation to NREM sleep. Am. J. Physiol. 1995;269(5 Pt 2):R1240–R1249. doi: 10.1152/ajpregu.1995.269.5.R1240. [DOI] [PubMed] [Google Scholar]
- 12.McGinty D., Alam M.N., Szymusiak R., Nakao M., Yamamoto M. Hypothalamic sleep-promoting mechanisms: coupling to thermoregulation. Arch. Ital. Biol. 2001;139(1-2):63–75. [PubMed] [Google Scholar]
- 13.Parmeggiani P.L. Thermoregulation and sleep. Front. Biosci. 2003;8:s557–s567. doi: 10.2741/1054. [DOI] [PubMed] [Google Scholar]
- 14.McKinley M.J., Yao S.T., Uschakov A., McAllen R.M., Rundgren M., Martelli D. The median preoptic nucleus: front and centre for the regulation of body fluid, sodium, temperature, sleep and cardiovascular homeostasis. Acta Physiol. (Oxf.) 2015;214(1):8–32. doi: 10.1111/apha.12487. [DOI] [PubMed] [Google Scholar]
- 15.Szymusiak R. Body temperature and sleep. Handb. Clin. Neurol. 2018;156:341–351. doi: 10.1016/B978-0-444-63912-7.00020-5. [DOI] [PubMed] [Google Scholar]
- 16.Kroeger D., Absi G., Gagliardi C., Bandaru S.S., Madara J.C., Ferrari L.L., Arrigoni E., Münzberg H., Scammell T.E., Saper C.B., Vetrivelan R. Galanin neurons in the ventrolateral preoptic area promote sleep and heat loss in mice. Nat. Commun. 2018;9(1):4129. doi: 10.1038/s41467-018-06590-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma Y., Miracca G., Yu X., Harding E.C., Miao A., Yustos R., Vyssotski A.L., Franks N.P., Wisden W. Galanin neurons unite sleep homeostasis and α2-Adrenergic Sedation. Curr. Biol. 2019;29(19):3315–3322.e3. doi: 10.1016/j.cub.2019.07.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harding E.C., Yu X., Miao A., Andrews N., Ma Y., Ye Z., Lignos L., Miracca G., Ba W., Yustos R., Vyssotski A.L., Wisden W., Franks N.P. A neuronal hub binding sleep initiation and body cooling in response to a warm external stimulus. Curr. Biol. 2018;28(14):2263–2273.e4. doi: 10.1016/j.cub.2018.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vanini G., Bassana M., Mast M., Mondino A., Cerda I., Phyle M., Chen V., Colmenero A.V., Hambrecht-Wiedbusch V.S., Mashour G.A. Activation of Preoptic GABAergic or glutamatergic neurons modulates sleep-wake architecture, but not anesthetic state transitions. Curr. Biol. 2020;30(5):779–787.e4. doi: 10.1016/j.cub.2019.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naganuma F., Bandaru S.S., Absi G., Chee M.J., Vetrivelan R. Melanin-concentrating hormone neurons promote rapid eye movement sleep independent of glutamate release. Brain Struct. Funct. 2019;224(1):99–110. doi: 10.1007/s00429-018-1766-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naganuma F., Kroeger D., Bandaru S.S., Absi G., Madara J.C., Vetrivelan R. Lateral hypothalamic neurotensin neurons promote arousal and hyperthermia. PLoS Biol. 2019;17(3):e3000172. doi: 10.1371/journal.pbio.3000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vetrivelan R., Kong D., Ferrari L.L., Arrigoni E., Madara J.C., Bandaru S.S., Lowell B.B., Lu J., Saper C.B. Melanin-concentrating hormone neurons specifically promote rapid eye movement sleep in mice. Neuroscience. 2016;336:102–113. doi: 10.1016/j.neuroscience.2016.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sessler D.I. Temperature monitoring and perioperative thermoregulation. Anesthesiology. 2008;109(2):318–338. doi: 10.1097/ALN.0b013e31817f6d76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buhre W., Rossaint R. Perioperative management and monitoring in anaesthesia. Lancet. 2003;362(9398):1839–1846. doi: 10.1016/S0140-6736(03)14905-7. [DOI] [PubMed] [Google Scholar]
- 25.Kurz A. Thermal care in the perioperative period. Baillieres. Best Pract. Res. Clin. Anaesthesiol. 2008;22(1):39–62. doi: 10.1016/j.bpa.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Conahan T.J., III, Williams G.D., Apfelbaum J.L., Lecky J.H. Airway heating reduces recovery time (cost) in outpatients. Anesthesiology. 1987;67(1):128–130. doi: 10.1097/00000542-198707000-00028. [DOI] [PubMed] [Google Scholar]
- 27.Andrzejowski J., Hoyle J., Eapen G., Turnbull D. Effect of prewarming on post-induction core temperature and the incidence of inadvertent perioperative hypothermia in patients undergoing general anaesthesia. Br. J. Anaesth. 2008;101(5):627–631. doi: 10.1093/bja/aen272. [DOI] [PubMed] [Google Scholar]
- 28.Gupta N., Bharti S.J., Kumar V., Garg R., Mishra S., Bhatnagar S. Comparative evaluation of forced air warming and infusion of amino acid-enriched solution on intraoperative hypothermia in patients undergoing head and neck cancer surgeries: A prospective randomised study. Saudi J. Anaesth. 2019;13(4):318–324. doi: 10.4103/sja.SJA_839_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selldén E., Brundin T., Wahren J. Augmented thermic effect of amino acids under general anaesthesia: a mechanism useful for prevention of anaesthesia-induced hypothermia. Clin. Sci. (Lond.) 1994;86(5):611–618. doi: 10.1042/cs0860611. [DOI] [PubMed] [Google Scholar]
- 30.Kanazawa M., Watanabe M., Suzuki T. Reduction in amino-acid-induced anti-hypothermic effects during general anesthesia in ovariectomized rats with progesterone replacement. J. Anesth. 2016;30(1):123–131. doi: 10.1007/s00540-015-2075-z. [DOI] [PubMed] [Google Scholar]
- 31.Alfonsi P., Bekka S., Aegerter P. Prevalence of hypothermia on admission to recovery room remains high despite a large use of forced-air warming devices: Findings of a non-randomized observational multicenter and pragmatic study on perioperative hypothermia prevalence in France. PLoS One. 2019;14(12):e0226038. doi: 10.1371/journal.pone.0226038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelz M.B., Mashour G.A. The biology of general anesthesia from paramecium to primate. Curr. Biol. 2019;29(22):R1199–R1210. doi: 10.1016/j.cub.2019.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hemmings H.C., Jr, Riegelhaupt P.M., Kelz M.B., Solt K., Eckenhoff R.G., Orser B.A., Goldstein P.A. Towards a Comprehensive understanding of anesthetic mechanisms of action: a decade of discovery. Trends Pharmacol. Sci. 2019;40(7):464–481. doi: 10.1016/j.tips.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schneeberger M., Parolari L., Das Banerjee T., Bhave V., Wang P., Patel B., Topilko T., Wu Z., Choi C.H.J., Yu X., Pellegrino K., Engel E.A., Cohen P., Renier N., Friedman J.M., Nectow A.R. Regulation of energy expenditure by brainstem gaba neurons. Cell. 2019;178(3):672–685.e12. doi: 10.1016/j.cell.2019.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romanovsky A.A., Almeida M.C., Garami A., Steiner A.A., Norman M.H., Morrison S.F., Nakamura K., Burmeister J.J., Nucci T.B. The transient receptor potential vanilloid-1 channel in thermoregulation: a thermosensor it is not. Pharmacol. Rev. 2009;61(3):228–261. doi: 10.1124/pr.109.001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Voets T. TRP channels and thermosensation. Handb. Exp. Pharmacol. 2014;223:729–741. doi: 10.1007/978-3-319-05161-1_1. [DOI] [PubMed] [Google Scholar]
- 37.Song K., Wang H., Kamm G.B., Pohle J., Reis F.C., Heppenstall P., Wende H., Siemens J. The TRPM2 channel is a hypothalamic heat sensor that limits fever and can drive hypothermia. Science. 2016;353(6306):1393–1398. doi: 10.1126/science.aaf7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kunert-Keil C., Bisping F., Krüger J., Brinkmeier H. Tissue-specific expression of TRP channel genes in the mouse and its variation in three different mouse strains. BMC Genomics. 2006;7:159. doi: 10.1186/1471-2164-7-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jang Y., Lee Y., Kim S.M., Yang Y.D., Jung J., Oh U. Quantitative analysis of TRP channel genes in mouse organs. Arch. Pharm. Res. 2012;35(10):1823–1830. doi: 10.1007/s12272-012-1016-8. [DOI] [PubMed] [Google Scholar]
- 40.Nakayama T., Suzuki M., Ishikawa Y., Nishio A. Effects of capsaicin on hypothalamic thermo-sensitive neurons in the rat. Neurosci. Lett. 1978;7(2-3):151–155. doi: 10.1016/0304-3940(78)90159-3. [DOI] [PubMed] [Google Scholar]
- 41.Inagaki H., Kurganov E., Park Y., Furube E., Miyata S. Oral gavage of capsaicin causes TRPV1-dependent acute hypothermia and TRPV1-independent long-lasting increase of locomotor activity in the mouse. Physiol. Behav. 2019;206:213–224. doi: 10.1016/j.physbeh.2019.04.015. [DOI] [PubMed] [Google Scholar]
- 42.Hori T. Capsaicin and central control of thermoregulation. Pharmacol. Ther. 1984;26(3):389–416. doi: 10.1016/0163-7258(84)90041-X. [DOI] [PubMed] [Google Scholar]
- 43.Szabo T., Biro T., Gonzalez A.F., Palkovits M., Blumberg P.M. Pharmacological characterization of vanilloid receptor located in the brain. Brain Res. Mol. Brain Res. 2002;98(1-2):51–57. doi: 10.1016/S0169-328X(01)00313-8. [DOI] [PubMed] [Google Scholar]
- 44.Cavanaugh D.J., Chesler A.T., Jackson A.C., Sigal Y.M., Yamanaka H., Grant R., O’Donnell D., Nicoll R.A., Shah N.M., Julius D., Basbaum A.I. Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J. Neurosci. 2011;31(13):5067–5077. doi: 10.1523/JNEUROSCI.6451-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szolcsányi J. Effect of capsaicin on thermoregulation: an update with new aspects. Temperature. 2015;2(2):277–296. doi: 10.1080/23328940.2015.1048928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hajós M., Engberg G., Elam M. Reduced responsiveness of locus coeruleus neurons to cutaneous thermal stimuli in capsaicin-treated rats. Neurosci. Lett. 1986;70(3):382–387. doi: 10.1016/0304-3940(86)90584-7. [DOI] [PubMed] [Google Scholar]
- 47.Khakpay R., Polster D., Köles L., Skorinkin A., Szabo B., Wirkner K., Illes P. Potentiation of the glutamatergic synaptic input to rat locus coeruleus neurons by P2X7 receptors. Purinergic Signal. 2010;6(3):349–359. doi: 10.1007/s11302-010-9198-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Almeida M.C., Steiner A.A., Coimbra N.C., Branco L.G. Thermoeffector neuronal pathways in fever: a study in rats showing a new role of the locus coeruleus. J. Physiol. 2004;558(Pt 1):283–294. doi: 10.1113/jphysiol.2004.066654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mohammed M., Madden C.J., Andresen M.C., Morrison S.F. Activation of TRPV1 in nucleus tractus solitarius reduces brown adipose tissue thermogenesis, arterial pressure, and heart rate. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018;315(1):R134–R143. doi: 10.1152/ajpregu.00049.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jeong J.H., Lee D.K., Liu S.M., Chua S.C., Jr, Schwartz G.J., Jo Y.H. Activation of temperature-sensitive TRPV1-like receptors in ARC POMC neurons reduces food intake. PLoS Biol. 2018;16(4):e2004399. doi: 10.1371/journal.pbio.2004399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Osaka T., Lee T.H., Kobayashi A., Inoue S., Kimura S. Thermogenesis mediated by a capsaicin-sensitive area in the ventrolateral medulla. Neuroreport. 2000;11(11):2425–2428. doi: 10.1097/00001756-200008030-00017. [DOI] [PubMed] [Google Scholar]
- 52.Koulchitsky S.V. Are the capsaicin-sensitive structures of ventral medulla involved in the temperature response to endotoxin in rats? Neurosci. Lett. 1998;244(2):112–114. doi: 10.1016/S0304-3940(98)00128-1. [DOI] [PubMed] [Google Scholar]
- 53.Sun W., Uchida K., Suzuki Y., Zhou Y., Kim M., Takayama Y., Takahashi N., Goto T., Wakabayashi S., Kawada T., Iwata Y., Tominaga M. Lack of TRPV2 impairs thermogenesis in mouse brown adipose tissue. EMBO Rep. 2016;17(3):383–399. doi: 10.15252/embr.201540819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wainwright A., Rutter A.R., Seabrook G.R., Reilly K., Oliver K.R. Discrete expression of TRPV2 within the hypothalamo-neurohypophysial system: Implications for regulatory activity within the hypothalamic-pituitary-adrenal axis. J. Comp. Neurol. 2004;474(1):24–42. doi: 10.1002/cne.20100. [DOI] [PubMed] [Google Scholar]
- 55.Nedungadi T.P., Dutta M., Bathina C.S., Caterina M.J., Cunningham J.T. Expression and distribution of TRPV2 in rat brain. Exp. Neurol. 2012;237(1):223–237. doi: 10.1016/j.expneurol.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matsuoka Y., Furuyashiki T., Bito H., Ushikubi F., Tanaka Y., Kobayashi T., Muro S., Satoh N., Kayahara T., Higashi M., Mizoguchi A., Shichi H., Fukuda Y., Nakao K., Narumiya S. Impaired adrenocorticotropic hormone response to bacterial endotoxin in mice deficient in prostaglandin E receptor EP1 and EP3 subtypes. Proc. Natl. Acad. Sci. USA. 2003;100(7):4132–4137. doi: 10.1073/pnas.0633341100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kataoka N., Hioki H., Kaneko T., Nakamura K. Psychological stress activates a dorsomedial hypothalamus-medullary raphe circuit driving brown adipose tissue thermogenesis and hyperthermia. Cell Metab. 2014;20(2):346–358. doi: 10.1016/j.cmet.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 58.Caldeira J.C., Franci C.R., Pelá I.R. Bilateral lesion of hypothalamic paraventricular nucleus abolishes fever induced by endotoxin and bradykinin in rats. Ann. N. Y. Acad. Sci. 1998;856:294–297. doi: 10.1111/j.1749-6632.1998.tb08342.x. [DOI] [PubMed] [Google Scholar]
- 59.van den Burg E.H., Stindl J., Grund T., Neumann I.D., Strauss O. Oxytocin stimulates extracellular Ca2+ influx through trpv2 channels in hypothalamic neurons to exert its anxiolytic effects. Neuropsychopharmacology. 2015;40(13):2938–2947. doi: 10.1038/npp.2015.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ring R.H., Malberg J.E., Potestio L., Ping J., Boikess S., Luo B., Schechter L.E., Rizzo S., Rahman Z., Rosenzweig-Lipson S. Anxiolytic-like activity of oxytocin in male mice: behavioral and autonomic evidence, therapeutic implications. Psychopharmacology (Berl.) 2006;185(2):218–225. doi: 10.1007/s00213-005-0293-z. [DOI] [PubMed] [Google Scholar]
- 61.Hicks C., Ramos L., Reekie T., Misagh G.H., Narlawar R., Kassiou M., McGregor I.S. Body temperature and cardiac changes induced by peripherally administered oxytocin, vasopressin and the non-peptide oxytocin receptor agonist WAY 267,464: a biotelemetry study in rats. Br. J. Pharmacol. 2014;171(11):2868–2887. doi: 10.1111/bph.12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burton K.J., Li X., Li J.D., Hu W.P., Zhou Q.Y. Rhythmic Trafficking of TRPV2 in the suprachiasmatic nucleus is regulated by prokineticin 2 signaling. J. Circadian Rhythms. 2015;13:2. doi: 10.5334/jcr.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burton K.J., Li X., Li B., Cheng M.Y., Urbanski H.F., Zhou Q.Y. Expression of prokineticin 2 and its receptor in the macaque monkey brain. Chronobiol. Int. 2016;33(2):191–199. doi: 10.3109/07420528.2015.1125361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peier A.M., Reeve A.J., Andersson D.A., Moqrich A., Earley T.J., Hergarden A.C., Story G.M., Colley S., Hogenesch J.B., McIntyre P., Bevan S., Patapoutian A. A heat-sensitive TRP channel expressed in keratinocytes. Science. 2002;296(5575):2046–2049. doi: 10.1126/science.1073140. [DOI] [PubMed] [Google Scholar]
- 65.Xu H., Ramsey I.S., Kotecha S.A., Moran M.M., Chong J.A., Lawson D., Ge P., Lilly J., Silos-Santiago I., Xie Y., DiStefano P.S., Curtis R., Clapham D.E. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature. 2002;418(6894):181–186. doi: 10.1038/nature00882. [DOI] [PubMed] [Google Scholar]
- 66.Smith G.D., Gunthorpe M.J., Kelsell R.E., Hayes P.D., Reilly P., Facer P., Wright J.E., Jerman J.C., Walhin J.P., Ooi L., Egerton J., Charles K.J., Smart D., Randall A.D., Anand P., Davis J.B. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature. 2002;418(6894):186–190. doi: 10.1038/nature00894. [DOI] [PubMed] [Google Scholar]
- 67.Moqrich A., Hwang S.W., Earley T.J., Petrus M.J., Murray A.N., Spencer K.S., Andahazy M., Story G.M., Patapoutian A. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science. 2005;307(5714):1468–1472. doi: 10.1126/science.1108609. [DOI] [PubMed] [Google Scholar]
- 68.Moussaieff A., Rimmerman N., Bregman T., Straiker A., Felder C.C., Shoham S., Kashman Y., Huang S.M., Lee H., Shohami E., Mackie K., Caterina M.J., Walker J.M., Fride E., Mechoulam R. Incensole acetate, an incense component, elicits psychoactivity by activating TRPV3 channels in the brain. FASEB J. 2008;22(8):3024–3034. doi: 10.1096/fj.07-101865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lowry C.A., Lightman S.L., Nutt D.J. That warm fuzzy feeling: brain serotonergic neurons and the regulation of emotion. J. Psychopharmacol. 2009;23(4):392–400. doi: 10.1177/0269881108099956. [DOI] [PubMed] [Google Scholar]
- 70.Güler A.D., Lee H., Iida T., Shimizu I., Tominaga M., Caterina M. Heat-evoked activation of the ion channel, TRPV4. J. Neurosci. 2002;22(15):6408–6414. doi: 10.1523/JNEUROSCI.22-15-06408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee H., Iida T., Mizuno A., Suzuki M., Caterina M.J. Altered thermal selection behavior in mice lacking transient receptor potential vanilloid 4. J. Neurosci. 2005;25(5):1304–1310. doi: 10.1523/JNEUROSCI.4745.04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vizin R.C. Scarpellini, Cda.S.; Ishikawa, D.T.; Correa, G.M.; de Souza, C.O.; Gargaglioni, L.H.; Carrettiero, D.C.; Bícego, K.C.; Almeida, M.C. TRPV4 activates autonomic and behavioural warmth-defence responses in Wistar rats. Acta Physiol. (Oxf.) 2015;214(2):275–289. doi: 10.1111/apha.12477. [DOI] [PubMed] [Google Scholar]
- 73.Yadav R., Jaryal A.K., Mallick H.N. Participation of preoptic area TRPV4 ion channel in regulation of body temperature. J. Therm. Biol. 2017;66:81–86. doi: 10.1016/j.jtherbio.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 74.Scarpellini C.D.S., Cristina-Silva C., Biancardi V., Gargaglioni L.H., Almeida M.C., Bícego K.C. Hypothalamic TRPV4 channels participate in the medial preoptic activation of warmth-defence responses in Wistar male rats. Pflugers Arch. 2019;471(9):1191–1203. doi: 10.1007/s00424-019-02303-1. [DOI] [PubMed] [Google Scholar]
- 75.Tan C.H., McNaughton P.A. The TRPM2 ion channel is required for sensitivity to warmth. Nature. 2016;536(7617):460–463. doi: 10.1038/nature19074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Almeida M.C., Hew-Butler T., Soriano R.N., Rao S., Wang W., Wang J., Tamayo N., Oliveira D.L., Nucci T.B., Aryal P., Garami A., Bautista D., Gavva N.R., Romanovsky A.A. Pharmacological blockade of the cold receptor TRPM8 attenuates autonomic and behavioral cold defenses and decreases deep body temperature. J. Neurosci. 2012;32(6):2086–2099. doi: 10.1523/JNEUROSCI.5606-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ordas P., Hernandez-Ortego P., Vara H., Fernandez-Pena C., Reimundez A., Morenilla-Palao C., Guadano-Ferraz A., Gomis A., Hoon M., Viana F., Senaris R. Expression of the cold thermoreceptor TRPM8 in rodent brain thermoregulatory circuits. J. Comp. Neurol. 2019 doi: 10.1002/cne.24694. [DOI] [PubMed] [Google Scholar]
- 78.Reimúndez A., Fernández-Peña C., García G., Fernández R., Ordás P., Gallego R., Pardo-Vazquez J.L., Arce V., Viana F., Señarís R. Deletion of the cold thermoreceptor TRPM8 increases heat loss and food intake leading to reduced body temperature and obesity in mice. J. Neurosci. 2018;38(15):3643–3656. doi: 10.1523/JNEUROSCI.3002-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang W., Sunanaga J., Takahashi Y., Mori T., Sakurai T., Kanmura Y., Kuwaki T. Orexin neurons are indispensable for stress-induced thermogenesis in mice. J. Physiol. 2010;588(Pt 21):4117–4129. doi: 10.1113/jphysiol.2010.195099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tupone D., Madden C.J., Cano G., Morrison S.F. An orexinergic projection from perifornical hypothalamus to raphe pallidus increases rat brown adipose tissue thermogenesis. J. Neurosci. 2011;31(44):15944–15955. doi: 10.1523/JNEUROSCI.3909-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Takahashi Y., Zhang W., Sameshima K., Kuroki C., Matsumoto A., Sunanaga J., Kono Y., Sakurai T., Kanmura Y., Kuwaki T. Orexin neurons are indispensable for prostaglandin E2-induced fever and defence against environmental cooling in mice. J. Physiol. 2013;591(22):5623–5643. doi: 10.1113/jphysiol.2013.261271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cai X.J., Widdowson P.S., Harrold J., Wilson S., Buckingham R.E., Arch J.R., Tadayyon M., Clapham J.C., Wilding J., Williams G. Hypothalamic orexin expression: modulation by blood glucose and feeding. Diabetes. 1999;48(11):2132–2137. doi: 10.2337/diabetes.48.11.2132. [DOI] [PubMed] [Google Scholar]
- 83.Kong D., Vong L., Parton L.E., Ye C., Tong Q., Hu X., Choi B., Brüning J.C., Lowell B.B. Glucose stimulation of hypothalamic MCH neurons involves K(ATP) channels, is modulated by UCP2, and regulates peripheral glucose homeostasis. Cell Metab. 2010;12(5):545–552. doi: 10.1016/j.cmet.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Milbank E., López M. Orexins/Hypocretins: Key Regulators of Energy Homeostasis. Front. Endocrinol. (Lausanne) 2019;10:830. doi: 10.3389/fendo.2019.00830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tan C.L., Cooke E.K., Leib D.E., Lin Y.C., Daly G.E., Zimmerman C.A., Knight Z.A. Warm-sensitive neurons that control body temperature. Cell. 2016;167(1):47–59.e15. doi: 10.1016/j.cell.2016.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Abbott S.B.G., Saper C.B. Median preoptic glutamatergic neurons promote thermoregulatory heat loss and water consumption in mice. J. Physiol. 2017;595(20):6569–6583. doi: 10.1113/JP274667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yu S., Qualls-Creekmore E., Rezai-Zadeh K., Jiang Y., Berthoud H.R., Morrison C.D., Derbenev A.V., Zsombok A., Münzberg H. Glutamatergic preoptic area neurons that express leptin receptors drive temperature-dependent body weight homeostasis. J. Neurosci. 2016;36(18):5034–5046. doi: 10.1523/JNEUROSCI.0213-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Machado N.L.S., Bandaru S.S., Abbott S.B.G., Saper C.B. EP3R-expressing glutamatergic preoptic neurons mediate inflammatory fever. J. Neurosci. 2020;40(12):2573–2588. doi: 10.1523/JNEUROSCI.2887-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Moffitt J.R., Bambah-Mukku D., Eichhorn S.W., Vaughn E., Shekhar K., Perez J.D., Rubinstein N.D., Hao J., Regev A., Dulac C., Zhuang X. Molecular, spatial, and functional single-cell profiling of the hypothalamic preoptic region. Science. 2018;362(6416):eaau5324. doi: 10.1126/science.aau5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Leib D.E., Zimmerman C.A., Poormoghaddam A., Huey E.L., Ahn J.S., Lin Y.C., Tan C.L., Chen Y., Knight Z.A. The forebrain thirst circuit drives drinking through negative reinforcement. Neuron. 2017;96(6):1272–1281.e4. doi: 10.1016/j.neuron.2017.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Romanov R.A., Zeisel A., Bakker J., Girach F., Hellysaz A., Tomer R., Alpár A., Mulder J., Clotman F., Keimpema E., Hsueh B., Crow A.K., Martens H., Schwindling C., Calvigioni D., Bains J.S., Máté Z., Szabó G., Yanagawa Y., Zhang M.D., Rendeiro A., Farlik M., Uhlén M., Wulff P., Bock C., Broberger C., Deisseroth K., Hökfelt T., Linnarsson S., Horvath T.L., Harkany T. Molecular interrogation of hypothalamic organization reveals distinct dopamine neuronal subtypes. Nat. Neurosci. 2017;20(2):176–188. doi: 10.1038/nn.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chee M.J., Arrigoni E., Maratos-Flier E. Melanin-concentrating hormone neurons release glutamate for feedforward inhibition of the lateral septum. J. Neurosci. 2015;35(8):3644–3651. doi: 10.1523/JNEUROSCI.4187-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Takahashi T.M., Sunagawa G.A., Soya S., Abe M., Sakurai K., Ishikawa K., Yanagisawa M., Hama H., Hasegawa E., Miyawaki A., Sakimura K., Takahashi M., Sakurai T. A discrete neuronal circuit induces a hibernation-like state in rodents. Nature. 2020;583(7814):109–114. doi: 10.1038/s41586-020-2163-6. [DOI] [PubMed] [Google Scholar]
- 94.Piñol R.A., Zahler S.H., Li C., Saha A., Tan B.K., Škop V., Gavrilova O., Xiao C., Krashes M.J., Reitman M.L. Brs3 neurons in the mouse dorsomedial hypothalamus regulate body temperature, energy expenditure, and heart rate, but not food intake. Nat. Neurosci. 2018;21(11):1530–1540. doi: 10.1038/s41593-018-0249-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhao Z.D., Yang W.Z., Gao C., Fu X., Zhang W., Zhou Q., Chen W., Ni X., Lin J.K., Yang J., Xu X.H., Shen W.L. A hypothalamic circuit that controls body temperature. Proc. Natl. Acad. Sci. USA. 2017;114(8):2042–2047. doi: 10.1073/pnas.1616255114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yoshida K., Li X., Cano G., Lazarus M., Saper C.B. Parallel preoptic pathways for thermoregulation. J. Neurosci. 2009;29(38):11954–11964. doi: 10.1523/JNEUROSCI.2643-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sherin J.E., Elmquist J.K., Torrealba F., Saper C.B. Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J. Neurosci. 1998;18(12):4705–4721. doi: 10.1523/JNEUROSCI.18-12-04705.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Smith W.L., Urade Y., Jakobsson P.J. Enzymes of the cyclooxygenase pathways of prostanoid biosynthesis. Chem. Rev. 2011;111(10):5821–5865. doi: 10.1021/cr2002992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang T.A., Teo C.F., Åkerblom M., Chen C., Tynan-La Fontaine M., Greiner V.J., Diaz A., McManus M.T., Jan Y.N., Jan L.Y. Thermoregulation via temperature-dependent PGD2 production in mouse preoptic area. Neuron. 2019;103(2):309–322.e7. doi: 10.1016/j.neuron.2019.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hastings M.H., Maywood E.S., Brancaccio M. Generation of circadian rhythms in the suprachiasmatic nucleus. Nat. Rev. Neurosci. 2018;19(8):453–469. doi: 10.1038/s41583-018-0026-z. [DOI] [PubMed] [Google Scholar]
- 101.Machado N.L.S., Abbott S.B.G., Resch J.M., Zhu L., Arrigoni E., Lowell B.B., Fuller P.M., Fontes M.A.P., Saper C.B. A Glutamatergic hypothalamomedullary circuit mediates thermogenesis, but not heat conservation, during stress-induced hyperthermia. Curr. Biol. 2018;28(14):2291–2301.e5. doi: 10.1016/j.cub.2018.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.de Git K.C.G., van Tuijl D.C., Luijendijk M.C.M., Wolterink-Donselaar I.G., Ghanem A., Conzelmann K.K., Adan R.A.H. Anatomical projections of the dorsomedial hypothalamus to the periaqueductal grey and their role in thermoregulation: a cautionary note. Physiol. Rep. 2018;6(14):e13807. doi: 10.14814/phy2.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mohammed M., Ootsuka Y., Blessing W. Brown adipose tissue thermogenesis contributes to emotional hyperthermia in a resident rat suddenly confronted with an intruder rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014;306(6):R394–R400. doi: 10.1152/ajpregu.00475.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nakamura K., Matsumura K., Hübschle T., Nakamura Y., Hioki H., Fujiyama F., Boldogköi Z., König M., Thiel H.J., Gerstberger R., Kobayashi S., Kaneko T. Identification of sympathetic premotor neurons in medullary raphe regions mediating fever and other thermoregulatory functions. J. Neurosci. 2004;24(23):5370–5380. doi: 10.1523/JNEUROSCI.1219-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lkhagvasuren B., Nakamura Y., Oka T., Sudo N., Nakamura K. Social defeat stress induces hyperthermia through activation of thermoregulatory sympathetic premotor neurons in the medullary raphe region. Eur. J. Neurosci. 2011;34(9):1442–1452. doi: 10.1111/j.1460-9568.2011.07863.x. [DOI] [PubMed] [Google Scholar]
- 106.Jeong J.H., Lee D.K., Blouet C., Ruiz H.H., Buettner C., Chua S., Jr, Schwartz G.J., Jo Y.H. Cholinergic neurons in the dorsomedial hypothalamus regulate mouse brown adipose tissue metabolism. Mol. Metab. 2015;4(6):483–492. doi: 10.1016/j.molmet.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rezai-Zadeh K., Yu S., Jiang Y., Laque A., Schwartzenburg C., Morrison C.D., Derbenev A.V., Zsombok A., Münzberg H. Leptin receptor neurons in the dorsomedial hypothalamus are key regulators of energy expenditure and body weight, but not food intake. Mol. Metab. 2014;3(7):681–693. doi: 10.1016/j.molmet.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Thompson R.H., Swanson L.W. Organization of inputs to the dorsomedial nucleus of the hypothalamus: a reexamination with Fluorogold and PHAL in the rat. Brain Res. Brain Res. Rev. 1998;27(2):89–118. doi: 10.1016/S0165-0173(98)00010-1. [DOI] [PubMed] [Google Scholar]
- 109.Cano G., Passerin A.M., Schiltz J.C., Card J.P., Morrison S.F., Sved A.F. Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J. Comp. Neurol. 2003;460(3):303–326. doi: 10.1002/cne.10643. [DOI] [PubMed] [Google Scholar]
- 110.Oldfield B.J., Giles M.E., Watson A., Anderson C., Colvill L.M., McKinley M.J. The neurochemical characterisation of hypothalamic pathways projecting polysynaptically to brown adipose tissue in the rat. Neuroscience. 2002;110(3):515–526. doi: 10.1016/S0306-4522(01)00555-3. [DOI] [PubMed] [Google Scholar]
- 111.Kong D., Tong Q., Ye C., Koda S., Fuller P.M., Krashes M.J., Vong L., Ray R.S., Olson D.P., Lowell B.B. GABAergic RIP-Cre neurons in the arcuate nucleus selectively regulate energy expenditure. Cell. 2012;151(3):645–657. doi: 10.1016/j.cell.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.An J.J., Liao G.Y., Kinney C.E., Sahibzada N., Xu B. Discrete BDNF neurons in the paraventricular hypothalamus control feeding and energy expenditure. Cell Metab. 2015;22(1):175–188. doi: 10.1016/j.cmet.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang P., Loh K.H., Wu M., Morgan D.A., Schneeberger M., Yu X., Chi J., Kosse C., Kim D., Rahmouni K., Cohen P., Friedman J. A leptin-BDNF pathway regulating sympathetic innervation of adipose tissue. Nature. 2020;583(7818):839–844. doi: 10.1038/s41586-020-2527-y. [DOI] [PubMed] [Google Scholar]
- 114.Shi Y.C., Lau J., Lin Z., Zhang H., Zhai L., Sperk G., Heilbronn R., Mietzsch M., Weger S., Huang X.F., Enriquez R.F., Baldock P.A., Zhang L., Sainsbury A., Herzog H., Lin S. Arcuate NPY controls sympathetic output and BAT function via a relay of tyrosine hydroxylase neurons in the PVN. Cell Metab. 2013;17(2):236–248. doi: 10.1016/j.cmet.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 115.Padilla S.L., Johnson C.W., Barker F.D., Patterson M.A., Palmiter R.D. A neural circuit underlying the generation of hot flushes. Cell Rep. 2018;24(2):271–277. doi: 10.1016/j.celrep.2018.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dacks P.A., Krajewski S.J., Rance N.E. Activation of neurokinin 3 receptors in the median preoptic nucleus decreases core temperature in the rat. Endocrinology. 2011;152(12):4894–4905. doi: 10.1210/en.2011-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rezai-Zadeh K., Münzberg H. Integration of sensory information via central thermoregulatory leptin targets. Physiol. Behav. 2013;121:49–55. doi: 10.1016/j.physbeh.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang Y., Kerman I.A., Laque A., Nguyen P., Faouzi M., Louis G.W., Jones J.C., Rhodes C., Münzberg H. Leptin-receptor-expressing neurons in the dorsomedial hypothalamus and median preoptic area regulate sympathetic brown adipose tissue circuits. J. Neurosci. 2011;31(5):1873–1884. doi: 10.1523/JNEUROSCI.3223-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Khazaeipool Z., Wiederman M., Inoue W. Prostaglandin E2 depresses GABA release onto parvocellular neuroendocrine neurones in the paraventricular nucleus of the hypothalamus via presynaptic receptors. J. Neuroendocrinol. 2018;30(11):e12638. doi: 10.1111/jne.12638. [DOI] [PubMed] [Google Scholar]
- 120.You H., Chu P., Guo W., Lu B. A subpopulation of Bdnf-e1-expressing glutamatergic neurons in the lateral hypothalamus critical for thermogenesis control. Mol. Metab. 2020;31:109–123. doi: 10.1016/j.molmet.2019.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ahnaou A., Dautzenberg F.M., Huysmans H., Steckler T., Drinkenburg W.H. Contribution of melanin-concentrating hormone (MCH1) receptor to thermoregulation and sleep stabilization: evidence from MCH1 (-/-) mice. Behav. Brain Res. 2011;218(1):42–50. doi: 10.1016/j.bbr.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 122.Pereira-da-Silva M., Torsoni M.A., Nourani H.V., Augusto V.D., Souza C.T., Gasparetti A.L., Carvalheira J.B., Ventrucci G., Marcondes M.C., Cruz-Neto A.P., Saad M.J., Boschero A.C., Carneiro E.M., Velloso L.A. Hypothalamic melanin-concentrating hormone is induced by cold exposure and participates in the control of energy expenditure in rats. Endocrinology. 2003;144(11):4831–4840. doi: 10.1210/en.2003-0243. [DOI] [PubMed] [Google Scholar]
- 123.Berthoud H.R., Patterson L.M., Sutton G.M., Morrison C., Zheng H. Orexin inputs to caudal raphé neurons involved in thermal, cardiovascular, and gastrointestinal regulation. Histochem. Cell Biol. 2005;123(2):147–156. doi: 10.1007/s00418-005-0761-x. [DOI] [PubMed] [Google Scholar]
- 124.Saito Y.C., Maejima T., Nishitani M., Hasegawa E., Yanagawa Y., Mieda M., Sakurai T. Monoamines inhibit GABAergic neurons in ventrolateral preoptic area that make direct synaptic connections to hypothalamic arousal neurons. J. Neurosci. 2018;38(28):6366–6378. doi: 10.1523/JNEUROSCI.2835-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Martin T., Dauvilliers Y., Koumar O.C., Bouet V., Freret T., Besnard S., Dauphin F., Bessot N. Dual orexin receptor antagonist induces changes in core body temperature in rats after exercise. Sci. Rep. 2019;9(1):18432. doi: 10.1038/s41598-019-54826-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cerri M., Del Vecchio F., Mastrotto M., Luppi M., Martelli D., Perez E., Tupone D., Zamboni G., Amici R. Enhanced slow-wave EEG activity and thermoregulatory impairment following the inhibition of the lateral hypothalamus in the rat. PLoS One. 2014;9(11):e112849. doi: 10.1371/journal.pone.0112849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Venner A., Broadhurst R.Y., Sohn L.T., Todd W.D., Fuller P.M. Selective activation of serotoninergic dorsal raphe neurons facilitates sleep through anxiolysis. Sleep . 2020;43(2):zsz231. doi: 10.1093/sleep/zsz231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ren J., Friedmann D., Xiong J., Liu C.D., Ferguson B.R., Weerakkody T., DeLoach K.E., Ran C., Pun A., Sun Y., Weissbourd B., Neve R.L., Huguenard J., Horowitz M.A., Luo L. Anatomically defined and functionally distinct dorsal raphe serotonin sub-systems. Cell. 2018;175(2):472–487.e20. doi: 10.1016/j.cell.2018.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ootsuka Y., Tanaka M. Control of cutaneous blood flow by central nervous system. Temperature. 2015;2(3):392–405. doi: 10.1080/23328940.2015.1069437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ishiwata T., Hasegawa H., Greenwood B.N. Involvement of serotonin in the ventral tegmental area in thermoregulation of freely moving rats. Neurosci. Lett. 2017;653:71–77. doi: 10.1016/j.neulet.2017.05.030. [DOI] [PubMed] [Google Scholar]
- 131.Brizuela M., Swoap S.J., Ang J., Blessing W.W., Ootsuka Y. Neurons in ventral tegmental area tonically inhibit sympathetic outflow to brown adipose tissue: possible mediation of thermogenic signals from lateral habenula. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019;316(1):R6–R12. doi: 10.1152/ajpregu.00256.2018. [DOI] [PubMed] [Google Scholar]
- 132.Nakamura Y., Yanagawa Y., Morrison S.F., Nakamura K. Medullary reticular neurons mediate neuropeptide y-induced metabolic inhibition and mastication. Cell Metab. 2017;25(2):322–334. doi: 10.1016/j.cmet.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ong Z.Y., Bongiorno D.M., Hernando M.A., Grill H.J. Effects of endogenous Oxytocin receptor signaling in nucleus tractus solitarius on satiation-mediated feeding and thermogenic control in male rats. Endocrinology. 2017;158(9):2826–2836. doi: 10.1210/en.2017-00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sergeant L., Rodriguez-Dimitrescu C., Barney C.C., Fraley G.S. Injections of Galanin-Like Peptide directly into the nucleus of the tractus solitarius (NTS) reduces food intake and body weight but increases metabolic rate and plasma leptin. Neuropeptides. 2017;62:37–43. doi: 10.1016/j.npep.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 135.Urade Y., Hayaishi O. Prostaglandin D2 and sleep/wake regulation. Sleep Med. Rev. 2011;15(6):411–418. doi: 10.1016/j.smrv.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 136.Jiang-Xie L.F., Yin L., Zhao S., Prevosto V., Han B.X., Dzirasa K., Wang F. A common neuroendocrine substrate for diverse general anesthetics and sleep. Neuron. 2019;102(5):1053–1065.e4. doi: 10.1016/j.neuron.2019.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mure L.S., Le H.D., Benegiamo G., Chang M.W., Rios L., Jillani N., Ngotho M., Kariuki T., Dkhissi-Benyahya O., Cooper H.M., Panda S. Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science. 2018;359(6381):eaao0318. doi: 10.1126/science.aao0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Scammell T., Gerashchenko D., Urade Y., Onoe H., Saper C., Hayaishi O. Activation of ventrolateral preoptic neurons by the somnogen prostaglandin D2. Proc. Natl. Acad. Sci. USA. 1998;95(13):7754–7759. doi: 10.1073/pnas.95.13.7754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ma L., Lee B.H., Clifton H., Schaefer S., Zheng J. Nicotinic acid is a common regulator of heat-sensing TRPV1-4 ion channels. Sci. Rep. 2015;5:8906. doi: 10.1038/srep08906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Szentirmai É., Kapás L. Nicotinic acid promotes sleep through prostaglandin synthesis in mice. Sci. Rep. 2019;9(1):17084. doi: 10.1038/s41598-019-53648-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lo Martire V., Silvani A., Bastianini S., Berteotti C., Zoccoli G. Effects of ambient temperature on sleep and cardiovascular regulation in mice: the role of hypocretin/orexin neurons. PLoS One. 2012;7(10):e47032. doi: 10.1371/journal.pone.0047032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ferrari L.L., Park D., Zhu L., Palmer M.R., Broadhurst R.Y., Arrigoni E. Regulation of lateral hypothalamic orexin activity by local GABAergic Neurons. J. Neurosci. 2018;38(6):1588–1599. doi: 10.1523/JNEUROSCI.1925-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Schöne C., Apergis-Schoute J., Sakurai T., Adamantidis A., Burdakov D. Coreleased orexin and glutamate evoke nonredundant spike outputs and computations in histamine neurons. Cell Rep. 2014;7(3):697–704. doi: 10.1016/j.celrep.2014.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bonnavion P., Jackson A.C., Carter M.E., de Lecea L. Antagonistic interplay between hypocretin and leptin in the lateral hypothalamus regulates stress responses. Nat. Commun. 2015;6:6266. doi: 10.1038/ncomms7266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Herrera C.G., Cadavieco M.C., Jego S., Ponomarenko A., Korotkova T., Adamantidis A. Hypothalamic feedforward inhibition of thalamocortical network controls arousal and consciousness. Nat. Neurosci. 2016;19(2):290–298. doi: 10.1038/nn.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Leinninger G.M., Opland D.M., Jo Y.H., Faouzi M., Christensen L., Cappellucci L.A., Rhodes C.J., Gnegy M.E., Becker J.B., Pothos E.N., Seasholtz A.F., Thompson R.C., Myers M.G., Jr Leptin action via neurotensin neurons controls orexin, the mesolimbic dopamine system and energy balance. Cell Metab. 2011;14(3):313–323. doi: 10.1016/j.cmet.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Leinninger G.M. Lateral thinking about leptin: a review of leptin action via the lateral hypothalamus. Physiol. Behav. 2011;104(4):572–581. doi: 10.1016/j.physbeh.2011.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ito H., Yanase M., Yamashita A., Kitabatake C., Hamada A., Suhara Y., Narita M., Ikegami D., Sakai H., Yamazaki M., Narita M. Analysis of sleep disorders under pain using an optogenetic tool: possible involvement of the activation of dorsal raphe nucleus-serotonergic neurons. Mol. Brain. 2013;6:59. doi: 10.1186/1756-6606-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lu J., Jhou T.C., Saper C.B. Identification of wake-active dopaminergic neurons in the ventral periaqueductal gray matter. J. Neurosci. 2006;26(1):193–202. doi: 10.1523/JNEUROSCI.2244-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kodani S., Soya S., Sakurai T. Excitation of GABAergic neurons in the bed nucleus of the stria terminalis triggers immediate transition from non-rapid eye movement sleep to wakefulness in mice. J. Neurosci. 2017;37(30):7164–7176. doi: 10.1523/JNEUROSCI.0245-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hua R., Wang X., Chen X., Wang X., Huang P., Li P., Mei W., Li H. Calretinin neurons in the midline thalamus modulate starvation-induced arousal. Curr. Biol. 2018;28(24):3948–3959.e4. doi: 10.1016/j.cub.2018.11.020. [DOI] [PubMed] [Google Scholar]
- 152.Machida M., Wellman L.L., Fitzpatrick Bs M.E., Hallum Bs O., Sutton Bs A.M., Lonart G., Sanford L.D. Effects of optogenetic inhibition of BLA on sleep brief Optogenetic inhibition of the basolateral amygdala in mice alters effects of stressful experiences on rapid eye movement sleep. Sleep . 2017;40(4):zsx020. doi: 10.1093/sleep/zsx020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rosenkranz J.A., Buffalari D.M., Grace A.A. Opposing influence of basolateral amygdala and footshock stimulation on neurons of the central amygdala. Biol. Psychiatry. 2006;59(9):801–811. doi: 10.1016/j.biopsych.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 154.Hwang Y.G., Lee H.S., Neuropeptide Y., Neuropeptide Y. NPY) or cocaine- and amphetamine-regulated transcript (CART) fiber innervation on central and medial amygdaloid neurons that project to the locus coeruleus and dorsal raphe in the rat. Brain Res. 2018;1689:75–88. doi: 10.1016/j.brainres.2018.03.032. [DOI] [PubMed] [Google Scholar]
- 155.Eban-Rothschild A., Rothschild G., Giardino W.J., Jones J.R., de Lecea L. VTA dopaminergic neurons regulate ethologically relevant sleep-wake behaviors. Nat. Neurosci. 2016;19(10):1356–1366. doi: 10.1038/nn.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Taylor N.E., Van Dort C.J., Kenny J.D., Pei J., Guidera J.A., Vlasov K.Y., Lee J.T., Boyden E.S., Brown E.N., Solt K. Optogenetic activation of dopamine neurons in the ventral tegmental area induces reanimation from general anesthesia. Proc. Natl. Acad. Sci. USA. 2016;113(45):12826–12831. doi: 10.1073/pnas.1614340113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ren S., Wang Y., Yue F., Cheng X., Dang R., Qiao Q., Sun X., Li X., Jiang Q., Yao J., Qin H., Wang G., Liao X., Gao D., Xia J., Zhang J., Hu B., Yan J., Wang Y., Xu M., Han Y., Tang X., Chen X., He C., Hu Z. The paraventricular thalamus is a critical thalamic area for wakefulness. Science. 2018;362(6413):429–434. doi: 10.1126/science.aat2512. [DOI] [PubMed] [Google Scholar]
- 158.Gelegen C., Miracca G., Ran M.Z., Harding E.C., Ye Z., Yu X., Tossell K., Houston C.M., Yustos R., Hawkins E.D., Vyssotski A.L., Dong H.L., Wisden W., Franks N.P. Excitatory pathways from the lateral habenula enable propofol-induced sedation. Curr. Biol. 2018;28(4):580–587.e5. doi: 10.1016/j.cub.2017.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Deurveilher S., Semba K. Indirect projections from the suprachiasmatic nucleus to major arousal-promoting cell groups in rat: implications for the circadian control of behavioural state. Neuroscience. 2005;130(1):165–183. doi: 10.1016/j.neuroscience.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 160.Saper C.B., Scammell T.E., Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437(7063):1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 161.Rupp A.C., Ren M., Altimus C.M., Fernandez D.C., Richardson M., Turek F., Hattar S., Schmidt T.M. Distinct ipRGC subpopulations mediate light’s acute and circadian effects on body temperature and sleep. eLife. 2019;8:8. doi: 10.7554/eLife.44358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Akeju O., Brown E.N. Neural oscillations demonstrate that general anesthesia and sedative states are neurophysiologically distinct from sleep. Curr. Opin. Neurobiol. 2017;44:178–185. doi: 10.1016/j.conb.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Brown E.N., Purdon P.L., Van Dort C.J. General anesthesia and altered states of arousal: a systems neuroscience analysis. Annu. Rev. Neurosci. 2011;34:601–628. doi: 10.1146/annurev-neuro-060909-153200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lewis L.D., Voigts J., Flores F.J., Schmitt L.I., Wilson M.A., Halassa M.M., Brown E.N. Thalamic reticular nucleus induces fast and local modulation of arousal state. eLife. 2015;4:e08760. doi: 10.7554/eLife.08760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yin L., Li L., Deng J., Wang D., Guo Y., Zhang X., Li H., Zhao S., Zhong H., Dong H. Optogenetic/chemogenetic activation of GABAergic neurons in the ventral tegmental area facilitates general anesthesia via projections to the lateral hypothalamus in mice. Front. Neural Circuits. 2019;13:73. doi: 10.3389/fncir.2019.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yu X., Li W., Ma Y., Tossell K., Harris J.J., Harding E.C., Ba W., Miracca G., Wang D., Li L., Guo J., Chen M., Li Y., Yustos R., Vyssotski A.L., Burdakov D., Yang Q., Dong H., Franks N.P., Wisden W. GABA and glutamate neurons in the VTA regulate sleep and wakefulness. Nat. Neurosci. 2019;22(1):106–119. doi: 10.1038/s41593-018-0288-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wang T.X., Xiong B., Xu W., Wei H.H., Qu W.M., Hong Z.Y., Huang Z.L. Activation of parabrachial nucleus glutamatergic neurons accelerates reanimation from sevoflurane anesthesia in mice. Anesthesiology. 2019;130(1):106–118. doi: 10.1097/ALN.0000000000002475. [DOI] [PubMed] [Google Scholar]
- 168.Han B., McCarren H.S., O’Neill D., Kelz M.B. Distinctive recruitment of endogenous sleep-promoting neurons by volatile anesthetics and a nonimmobilizer. Anesthesiology. 2014;121(5):999–1009. doi: 10.1097/ALN.0000000000000383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.McCarren H.S., Chalifoux M.R., Han B., Moore J.T., Meng Q.C., Baron-Hionis N., Sedigh-Sarvestani M., Contreras D., Beck S.G., Kelz M.B. α2-Adrenergic stimulation of the ventrolateral preoptic nucleus destabilizes the anesthetic state. J. Neurosci. 2014;34(49):16385–16396. doi: 10.1523/JNEUROSCI.1135-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Vazey E.M., Aston-Jones G. Designer receptor manipulations reveal a role of the locus coeruleus noradrenergic system in isoflurane general anesthesia. Proc. Natl. Acad. Sci. USA. 2014;111(10):3859–3864. doi: 10.1073/pnas.1310025111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhang Y., Fu B., Liu C., Yu S., Luo T., Zhang L., Zhou W., Yu T. Activation of noradrenergic terminals in the reticular thalamus delays arousal from propofol anesthesia in mice. FASEB J. 2019;33(6):7252–7260. doi: 10.1096/fj.201802164RR. [DOI] [PubMed] [Google Scholar]
- 172.Pilorz V., Tam S.K., Hughes S., Pothecary C.A., Jagannath A., Hankins M.W., Bannerman D.M., Lightman S.L., Vyazovskiy V.V., Nolan P.M., Foster R.G., Peirson S.N. Melanopsin regulates both sleep-promoting and arousal-promoting responses to light. PLoS Biol. 2016;14(6):e1002482. doi: 10.1371/journal.pbio.1002482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Liu D., Li J., Wu J., Dai J., Chen X., Huang Y., Zhang S., Tian B., Mei W. Monochromatic blue light activates suprachiasmatic nucleus neuronal activity and promotes arousal in mice under sevoflurane anesthesia. Front. Neural Circuits. 2020;14:55. doi: 10.3389/fncir.2020.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Matsukawa T., Sessler D.I., Sessler A.M., Schroeder M., Ozaki M., Kurz A., Cheng C. Heat flow and distribution during induction of general anesthesia. Anesthesiology. 1995;82(3):662–673. doi: 10.1097/00000542-199503000-00008. [DOI] [PubMed] [Google Scholar]
- 175.Deakin C.D. Changes in core temperature compartment size on induction of general anaesthesia. Br. J. Anaesth. 1998;81(6):861–864. doi: 10.1093/bja/81.6.861. [DOI] [PubMed] [Google Scholar]
- 176.Garami A., Ibrahim M., Gilbraith K., Khanna R., Pakai E., Miko A., Pinter E., Romanovsky A.A., Porreca F., Patwardhan A.M. Transient receptor potential vanilloid 1 antagonists prevent anesthesia-induced hypothermia and decrease postincisional opioid dose requirements in rodents. Anesthesiology. 2017;127(5):813–823. doi: 10.1097/ALN.0000000000001812. [DOI] [PubMed] [Google Scholar]
- 177.Vanden Abeele F., Kondratskyi A., Dubois C., Shapovalov G., Gkika D., Busserolles J., Shuba Y., Skryma R., Prevarskaya N. Complex modulation of the cold receptor TRPM8 by volatile anaesthetics and its role in complications of general anaesthesia. J. Cell Sci. 2013;126(Pt 19):4479–4489. doi: 10.1242/jcs.131631. [DOI] [PubMed] [Google Scholar]
- 178.Gizowski C., Bourque C.W. Sodium regulates clock time and output via an excitatory GABAergic pathway. Nature. 2020;583(7816):421–424. doi: 10.1038/s41586-020-2471-x. [DOI] [PubMed] [Google Scholar]
- 179.Zhou W., Cheung K., Kyu S., Wang L., Guan Z., Kurien P.A., Bickler P.E., Jan L.Y. Activation of orexin system facilitates anesthesia emergence and pain control. Proc. Natl. Acad. Sci. USA. 2018;115(45):E10740–E10747. doi: 10.1073/pnas.1808622115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Li J., Li H., Wang D., Guo Y., Zhang X., Ran M., Yang C., Yang Q., Dong H. Orexin activated emergence from isoflurane anaesthesia involves excitation of ventral tegmental area dopaminergic neurones in rats. Br. J. Anaesth. 2019;123(4):497–505. doi: 10.1016/j.bja.2019.07.005. [DOI] [PubMed] [Google Scholar]