Abstract
Alzheimer’s disease (AD) is a chronic neurodegenerative disease characterized by the formation of intracellular neurofibrillary tangles (NFTs) and extracellular amyloid plaques. Growing evidence has suggested that AD pathogenesis is not only limited to the neuronal compartment but also strongly interacts with immunological processes in the brain. On the other hand, aggregated and misfolded proteins can bind with pattern recognition receptors located on astroglia and microglia and can, in turn, induce an innate immune response, characterized by the release of inflammatory mediators, ultimately playing a role in both the severity and the progression of the disease. It has been reported by genome-wide analysis that several genes which elevate the risk for sporadic AD encode for factors controlling the inflammatory response and glial clearance of misfolded proteins. Obesity and systemic inflammation are examples of external factors which may interfere with the immunological mechanisms of the brain and can induce disease progression. In this review, we discussed the mechanisms and essential role of inflammatory signaling pathways in AD pathogenesis. Indeed, interfering with immune processes and modulation of risk factors may lead to future therapeutic or preventive AD approaches.
Keywords: Neuroinflammation, Alzheimer’s disease, inflammatory cytokine, astroglia, microglia, disease-associated microglia
1. INTRODUCTION
Alzheimer's disease (AD) is a progressive neurodegenerative disease that is the most common cause of dementia characterized by neuronal loss and cognitive impairment [1, 2]. Worldwide, about 50 million people have AD or associated dementia, and this figure is expected to reach 82 million in 2030 and 152 million in 2050 [3]. Presently, over 5 million Americans are affected by AD [4]. In addition, as life expectancy increases, it is also projected that by 2050, around 13.8 million Americans will be affected by AD [5]. AD-related financial burden exceeds about 230 billion US dollars and is also predicted to reach 1.1 trillion US dollars by the year 2050 [6]. Considering the AD-related financial and clinical burden, it has become essential to identify novel mechanisms which are accountable for AD pathogenesis and also detect potential therapeutic targets.
The main characteristics of AD pathology include the presence of amyloid-beta (Aβ) and neurofibrillary tangles (NFTs) [7]. The pathology of Aβ is found to arise from the inappropriate amyloid precursor protein (APP) cleavage, which can result in aggregation of Aβ monomers leading to Aβ oligomer formation and, ultimately, to the aggregation of Aβ plaques and fibrils [8, 9]. Tau regulates microtubule dynamics and interacts with various proteins that can cause adverse gains of function [10]. The fast kiss-and-hop interaction reveals why tau, although binding to microtubules, does not hinder axonal transport [11]. On the other hand, it is known that tau hyperphosphorylation can result in the formation of NFTs [12, 13]. Furthermore, in the case of AD, tau is found to be phosphorylated at various sites, which appears to modulate its interaction with microtubules [14-16]. Hyper-phosphorylated soluble tau contributes to neuronal dysfunction prior to its deposition in the brain [17]. Highly phosphorylated tau interferes with neuronal activities like axonal transport and mitochondrial respiration [18,19]. Moreover, hyperphosphorylated tau can aggregate into paired helical fragments, which can further result in the formation of NFTs [20]. Loss of neuronal functions can occur due to compromised cellular function and buildup of hyperphosphorylated tau tangles, eventually leading to apoptosis [15].
In the past several years, a third main AD characteristic has emerged that might provide a better understanding regarding the pathogenesis of AD. It has been confirmed through multiple studies that along with the NFTs and Aβ plaques, the presence of sustained neuroinflammatory response was observed in AD brains [21-23]. Additionally, the inflammatory response has also been examined in several studies involving postmortem tissues of AD individuals, and this response was also often observed in preclinical AD models [24-30]. Various studies now support that neuroinflammation might be the main neuropathological event causing neurodegeneration in AD. Multiple reports have indicated the presence of an elevated level of cytokines in animal models of AD [31-33] as well as in the brain of AD patients [34, 35]. It has also been revealed that glial cell (i.e., including astrocytes and microglia) activation has a significant contribution in inducing neurodegeneration-related inflammatory signaling mechanisms [36, 37]. In the case of AD individuals, reactive astrocytes and microglia are predominantly found in increased numbers near the senile plaques [38-40], which is further suggesting a contribution of these immune cells in AD pathogenesis.
Although noteworthy progress has been made in the current research, there is still a debate regarding whether the glial-facilitated inflammatory response noticed in AD is a consequence or a cause of neurodegeneration. A better understanding regarding the pathophysiological processes of AD is essential to facilitate the development of new therapeutic approaches. In this review, a comprehensive outline of the role of neuroinflammation in AD pathogenesis has been provided based on recent findings.
2. INFLAMMATORY REACTIONS IN ALZHEIMER’S BRAIN PATIENTS
There are numerous evidence that inflammation plays an intrinsic role in pathogenic events leading to the progression of AD, like Aβ accumulation, neuronal damage, and cognitive deficits [32, 41-43]. Activation of the glial cells may take place at the early stage of AD, even before the accumulation of Aβ [44]. Some studies have also reported that the activation of astrocytes takes place following microglial activation owing to cytokine expression in AD [45, 46]. Works carried out with human AD brains (post-mortem brain tissues) and brains of APP transgenic animals have shown elevated levels of inflammatory molecules such as interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, and IL-6 [47-51]. IFN-γ and TNF-α are toxic for neurons, and these molecules reduce the levels of insulin-degrading enzymes, which serve as a key Aβ degrading protease. This process is considered as an alternative mechanism by which the inflammatory process could induce amyloid deposition. In addition, IFN-γ and TNF-α also induce the release of Aβ and reduce the ability of microglial cells to degrade Aβ [49].
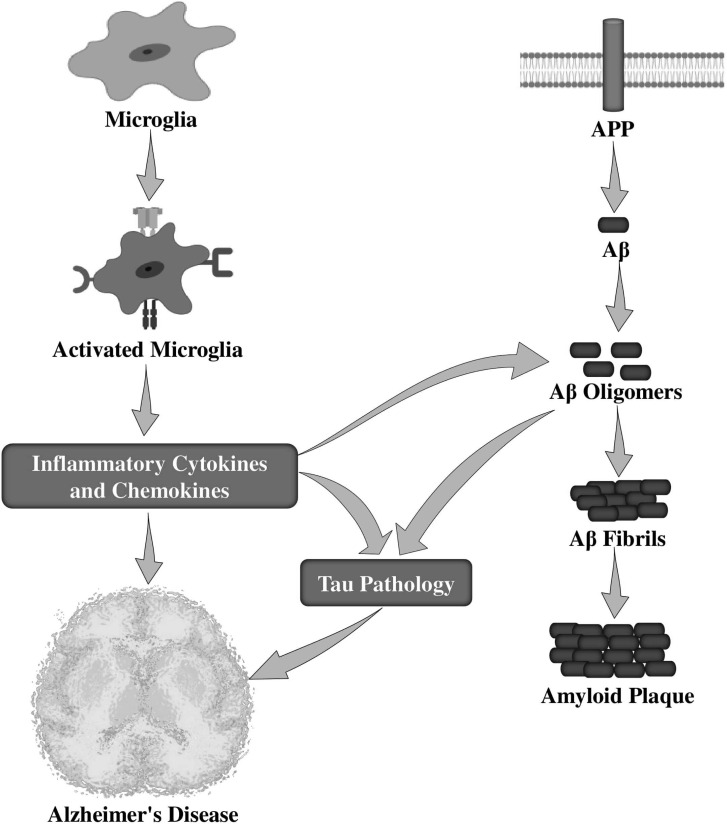
Microglia in AD can respond to a variety of stimuli, such as Aβ peptides, their APP, and NFTs [52]. Numerous works have studied the interrelationship between microglial activation and amyloid deposition (Fig. 1) that triggers the production of several pro-inflammatory mediators [53-56]. Microglial stimulation induced by Aβ via the Fc receptors, CD36 receptor [57], toll-like receptors (TLRs) [58], and receptor for advanced glycation end products (RAGE) [59], can increase Aβ formation while decreasing Aβ clearance, then leading to AD progression. Interestingly, fibrillar Aβ (fAβ) has been reported to activate microglia and thus stimulates the production and secretion of several proinflammatory cytokines, including IL-1β, IL-6, TNF-α, TGF-β, macrophage inflammatory protein 1α (MIP1α), MIP1β, and monocyte chemoattractant protein-1 (MCP-1) [47, 60, 61]. It has also been observed that Aβ-stimulated microglia produces reactive oxygen species (ROS) [62, 63].
Fig. (1).
Role of activated microglia in the pathological events of Alzheimer’s disease. APP, amyloid precursor protein; Aβ, amyloid-beta.
The effects of the sustained overexpression of IL-1β were investigated in an APPswe/PS1dE9 mouse model of AD [64]. Surprisingly, a significant reduction of amyloid deposition was observed in the hippocampus of mouse models. It was also reported that the over-expression of IL-1β induces a robust neuroinflammatory response in the mouse hippocampus characterized by activation of astrocytes and microglia [64]. Chakrabarty et al. [65] revealed in an animal model of AD that there is an increased level of IL-6 in the brain, which further diminishes amyloid deposition. Indeed, inflammation is a critical factor that severely damages the brain, and numerous molecules implied in this process may play a role in the AD-related pathology.
3. MEDIATORS AND MODULATORS OF NEUROINFLAMMATORY SIGNALING IN ALZHEIMER’S DISEASE
It has been confirmed that the deregulation of several mediators and modulators of neuroinflammation, including cytokines, chemokines, caspases, prostanoids, and neuroprotectin D1, and complement system are involved in the AD pathogenesis.
3.1. Cytokines
3.1.1. TNF-α
TNF-α is an inflammatory cytokine which is found to be increased in both the plasma and brain of AD individuals and is proximal to amyloid plaques on autopsy, clarifying its role in the inflammatory milieu [66]. A study revealed that single-nucleotide polymorphisms in the promoter regions of TNF-α and IL-10 are found to be linked with cerebral inflammatory response and increase late-onset AD risk [67]. When neuron-specific TNF-α is recurrently overexpressed in triple-transgenic AD (3xTg-AD) mice using adeno-associated virus (AAV) vectors, there was an elevated level of intracellular Aβ in the short-term and, in the long-term, an induced inflammation and tau pathology, resulting in neuronal cell death [68]. Additionally, the study indicated that overexpression of TNF-α signaling could increase AD-related pathology and is harmful to neuronal viability [68]. Nevertheless, TNF-α is also found to be associated with a detrimental effect triggered by Aβ on inducing memory and learning impairments as well as synaptic memory mechanisms in AD [66, 69]. On the other hand, TNF-α inhibition decreases the Aβ-induced impairment on recognition memory through long-term potentiation (LTP) [70]. Pharmacological TNF-α inhibition suppressed cognitive impairment in mouse models [71]. Collectively, these findings indicate that selective prevention of neuronal TNF-α signaling through the targeted blocking of receptor expression might preserve neurons in AD.
Tumor necrosis factor receptor-1 (TNFR1) and TNFR2 are the two cognate transmembrane receptors for TNF-α [72]. Their biological actions have been found to be differentially expressed and controlled. Interestingly, it has been observed that signaling through the cognate TNFR induces various cellular responses, including cell migration, cell proliferation, and apoptosis, these processes being mediated via the activation of several downstream signal transduction cascades involving nuclear factor kappa light chain enhancer of activated B cells (NF-κB), c-Jun N-terminal kinase (JNK), p38, and ceramide-sphingomyelinase pathways [73]. Interestingly, it has been observed that TNFR1 gene deletion in APP transgenic mice decreases the deposition of plaque and also improves cognitive impairment by the down-regulating activity of the BACE1 promoter [74]. A study has revealed that TNFR1 knockout could lead to suppression of the AD-associated amyloid pathology [75]. It was also observed that inhibition of soluble TNF signaling can avert, on a short-term basis, the AD-related amyloid pathology [75]. Nevertheless, deletion of TNFR1 and TNFR2 signaling worsens the hallmarks of amyloid and NFTs pathology without any cell type or stage specificity in the 3xTg mouse model [76]. This aforesaid result indicates that particular attention should be taken when anti-TNF-α is used on a long-term basis. Recently, researchers have observed that prolonged TNFR2 knockdown in hippocampal neurons through recombinant AAV serotype 2 (rAAV2) vector-mediated short interfering ribonucleic acid (siRNA) delivery can result in an increased deposition of Aβ plaques and formation of paired helical filaments [73], further suggesting that TNFR2 may exert protective effects that might be essential to counteract TNFR1-driven signal transduction.
However, similar neuroprotective properties have been ascribed to TNF-α. It was observed that pretreatment with TNF-α and Aβ peptides in dissociated neuronal cultures spared cells from Aβ-mediated neuronal death, iron toxicity, and intracellular accumulation of calcium (Ca2+) ions through the NF-κB-dependent process [77]. In enriched cultures of primary neurons, TNF-α was found to be trophic to rat hippocampal neurons, protecting them from free radicals, glutamate, and Aβ toxicity [47]. Furthermore, it has also been revealed that intrathecal TNF-α levels are inversely associated in a marked fashion, with neuronal degradation and intracerebral apoptosis [78]. Moreover, the generation of B-cell lymphoma 2 (Bcl-2), a molecule that decreases neuronal apoptosis, was observed owing to the incubation of human neuronal cells with TNF-α. Collectively, these findings suggest the complex nature of TNF-mediated signaling mechanisms; more studies are thus required for a better understanding of the cell-specific activities of TNF-α in AD.
3.1.2. IL-1β
IL-1β, an IL-1 cytokine family member, is an important pro-inflammatory cytokine which plays a crucial role in the progression of AD pathology [79, 80]. This cytokine is generated and released in response to various stimuli via astrocytes and microglia in pro-forms (pro-IL-1β) in the cytoplasm. However, in order to be produced as a bioactive and mature form, pro-IL-1β needs to be cleaved by the protease caspase-1 (activated via inflammasomes) [81, 82]. It has recently been observed that soluble oligomeric Aβ elevates the conversion of pro-IL-1β into mature IL-1β through ROS-induced activation of NLR family pyrin domain containing 3 (NLRP3) inflammation [83].
It was reported that overexpression of cytokine IL-1β, which is secreted via astrocytes and microglia adjacent to Aβ plaques, occurs in the brain of AD animal models [84, 85]. However, IL-1β generation relies on mitogen-activated protein kinase (MAPK) and also NF-κB signaling mechanisms. In vitro studies have demonstrated that IL-1β plays a role in APP processing. Several studies also suggest that IL-1β overexpression worsens phosphorylation of tau and the formation of tangles via an abnormal activation of glycogen synthase kinase 3 (GSK3) and p38-MAPK [86, 87], which can eventually influence the synaptic plasticity, suppress LTP, and then learning and memory [88]. Blocking IL-1β in the AD mouse model, significantly prevents cognitive deficits, reduces tau pathology, and decreases the generation of fAβ and S100 [87]. Furthermore, fAβ can trigger the activation of microglia, which can further result in the enhanced generation and secretion of neurotoxic secretory substances, including pro-inflammatory cytokines (for example, ROS and IL-1β) [89]. From these results, it can be assumed that Aβ deposits can be both a consequence and a cause of IL-1β expression in AD individuals. Moreover, an enhanced expression of IL-1β impairs the Aβ clearance activity of microglia [90] and increases the blood-brain barrier (BBB) penetrability, further mediating brain Aβ accumulation [91]. It was demonstrated that IL-1β might have a significant contribution to reducing AD pathology [64]. Moreover, a prolonged overexpression of IL-1β decreases Aβ-associated pathology via the control of the microglia-mediated plaque degradation or mediation of the non-amyloidogenic cleavage of APP in cell cultures and AD mouse models [92-94]. Therefore, IL-1β might have a multifaceted function in AD pathogenesis.
3.1.3. IL-6
IL-6, a pleiotropic inflammatory cytokine, is primarily formed via stimulated astrocytes and microglia in various brain areas [95]. Additionally, IL-6 might induce astrocytes and microglia to release acute-phase proteins as well as pro-inflammatory cytokines, including the C-reactive protein (CRP) [96]. In AD individuals and animal models, increased IL-6 levels have been observed in the plasma, cerebrospinal fluid, and brain, particularly around the Aβ plaques. Thus, it is anticipated that IL-6 is associated with AD pathophysiology with chronic or acute inflammatory constituents.
Numerous studies have explored the molecular and cellular mechanisms underlying the relationship of IL-6 with AD involving Aβ and tau [97]. It has been reported that IL-6 generation via human neurons is induced through glycation end product-modulated Aβ and tau. Previous studies have revealed the function of IL-6, which participates in APP generation and processing in primary rat cortical neuronal cells [98]. As a result, fAβ was found to activate microglia, which further leads to the increased generation and secretion of IL-6 [99]. Trans-signaling is the process of IL-6 signaling involving its soluble form, IL-6R (sIL-6R), which is assumed to be responsible for the pro-inflammatory effects of IL-6. A study has reported that Toll-like receptor 2 (TLR2) activation in primary human peripheral blood mononuclear cells and THP-1 cells, causes IL-6 trans-signaling via metalloproteases like ADAM10 and ADAM17 [100]. IL-6-treated hippocampal cells may play a role in the formation of NFTs via stimulating tau phosphorylation by cyclin dependent kinase 5 (Cdk5)/p35 dysregulation [101]. IL-6 can also cause activation of Janus kinase/signal transducers and activators of transcription (JAK/STAT), N-methyl-D-aspartate (NMDA) receptor, and MAPK-p38 MAPK-p38 protein kinases, both being found associated with tau hyperphosphorylation [98]. Nonetheless, IL-6 overexpression stimulated via AAV1 in transgenic APP mouse models and nontransgenic littermates was shown to exert a significant inhibition of Aβ deposition, which did not markedly modify the levels of APP [65]. Therefore, it can be said that IL-6 reduces Aβ-plaque pathology, possibly by the activation of the microglia M2 phenotype that enhances Aβ phagocytosis [65].
IL-6 exerts a significant role in neurodegeneration and neuroinflammation via the control of cognitive functions [102]. It was observed that an increased level of IL-6 is linked with an age-associated cognitive deficit in humans [103]. Various studies have demonstrated that upon inflammatory situations, excessive levels of IL-6 via activation of the NADPH oxidase in neuronal cells, which is mediated through the aging process or inflammation, might damage spatial learning and memory [104]. In contrast, it was also found that under basal inflammation conditions, IL-6 and JAK/STAT downstream signaling facilitates cognitive flexibility [105].
3.1.4. IL-18
IL-18, also known as interferon-γ (IFN-γ) inducing factor, is a pro-inflammatory cytokine produced from an inactive precursor protein called pro-IL-18 (24-kDa) and cleaved via proteinase-3 and caspase-1 to produce a biologically active and mature protein of 18 kDa molecular mass [106, 107]. However, the main sources of IL-18 in the central nervous system (CNS) include activated microglia, ependymal cells and astrocytes, and neurons. It has been reported that an increased level of IL-18 expression may result in a vicious inflammation cycle [108]. IL-18 is generated from an inactive 24 kDa precursor protein. The intracellular cysteine proteinase caspase-1 cleaves pro-IL-18 to a mature and bioactive molecule of 18 kDa [109, 110].
Growing evidence has confirmed that IL-18 might be associated with various features of AD-related neurodegeneration [108, 111]. Studies have also confirmed that IL-18 probably has a direct effect on synaptic plasticity and neuron survival [112, 113]. However, IL-18 is required to bind with its receptor to activate NF-κB and thus increasing the formation and release of numerous cytokines and chemokines [114]. IL-18 can activate both Fas and Fas-L promoter activities via NF-κB activation [115], suggesting that IL-18 could be one of the apoptosis-inducing agents which can play a role in the progression of AD pathogenesis. IL-18 also activates the phosphoinositide 3-kinase/AKT serine/threonine kinase (PI3K/ AKT) and MAPKs signaling in certain cell types, resulting in the production and release of proinflammatory cytokines [114]. Furthermore, IL-18 was found to possibly exert cytotoxic effects in cardiomyocytes leading to elevated levels of intracellular Ca2+, and also dysregulation of calcium, which play an essential role in AD pathogenesis [116]. Indeed, IL-18 suppresses the LTP stimulation in the dentate gyrus region, which is essential for the cellular processes underlying memory and learning [112]. Nonetheless, lower levels of IL-18 associated with polymorphism in the cytokine gene might be related to enhanced physical functioning in healthy aged men, indicating that IL-18 might possess neuroprotective activities [117]. However, more studies are required to find out the precise role of IL-18 in AD pathogenesis.
Various studies have examined the molecular mechanisms underlying the role of IL-18 in AD, although the precise incidence of IL-18 in AD pathogenesis remains obscure. It was observed that IL-18 might enhance APP generation and its Thr668 phosphorylation in neuron-like differentiated human SH-SY5Y cells [108]. Similarly, it can also upregulate the amyloidogenic APP processing to generate Aβ through stimulation of the expression of BACE1 and N-terminal fragment of presenilin (PSEN-1) as well as a portion of the functional γ-secretase complex [118]. It has thus been suggested that increased or extended IL-18 levels might play a role in AD by elevating Aβ levels. In another study, it was observed that IL-18 might affect tau hyperphosphorylation via glycogen synthase kinase-3β (GSK-3β) and Cdk5/p35 kinases [119]. Besides, elevated levels of IL-18 expression in peripheral blood and brain were found to be linked with cognitive deficit [118].
3.2. Chemokines
Chemokines in the pathogenesis of AD were found to control the migration of microglia to the regions of neuroinflammation, which can further result in local inflammation [120]. Elevated levels of chemokine C-C motif ligand 2 (CCL2), chemokine receptor 5 (CCR5), and CCR3 in reactive microglia were observed in the case of AD [121, 122], however, the chemokine C-C motif ligand 4 (CCL4) being identified in reactive astrocytes near the areas of Aβ plaques [121]. It has been demonstrated through in vitro experiments conducted on human astrocytes and macrophages that Aβ can trigger the production of CCL4, CCL3, CCL2, and CXCL8 (IL-8) [123]. Furthermore, an elevated expression of CCL3, CCL2, and CXCL8 was also observed following experimental Aβ exposure, in microglia cultured from AD autopsies [124]. Modulation of neuronal cell survival [125], load of plaques [126], and cognitive function [127] through the CX3CL1/CX3CR1 have also been reported in a mouse model of AD. The receptors CCR2 [128-130] and CCR5 [99] can also influence the progress of the disease via affecting microglial function and positioning.
3.3. Caspases
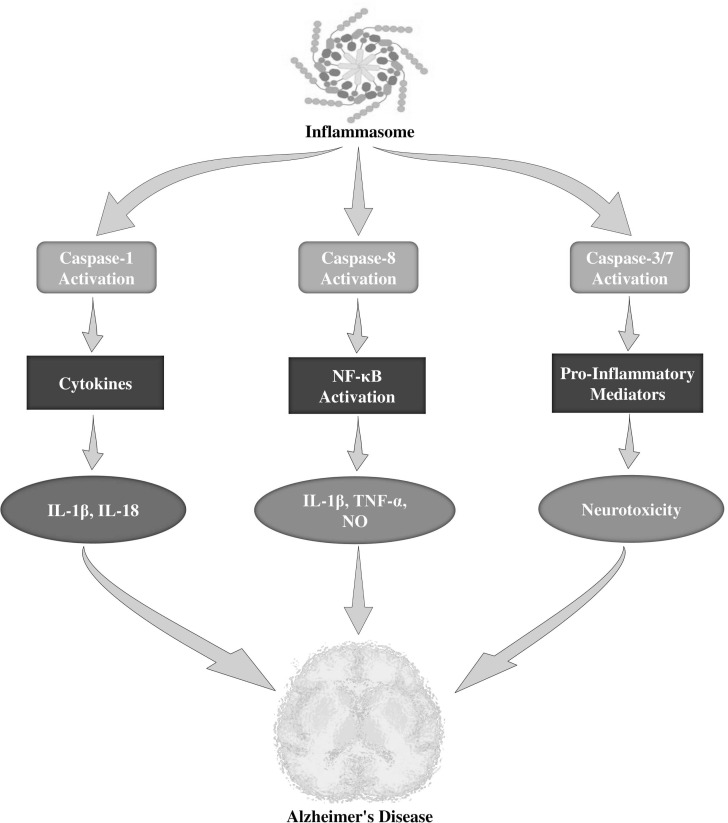
It has been stated that the caspase protein family is a group of intracellular proteases which are well known as crucial mediators of inflammation and apoptosis [131, 132]. However, among the inflammatory caspases, caspase-1 catalytic action is strongly controlled through the signal-mediated auto-activation of the inflammasome, which can induce caspase-1 autocatalytic activation and the subsequent cleavage of IL-18 and IL-1β precursors into bioactive cytokines [133, 134]. In addition, Aβ fibrils were found to activate NRLP3 inflammasomes through lysosomal impairment in microglia of mouse models [135]. It was also observed that increased active caspase-1 levels are present in the brain of AD individuals and APP/PS1 mouse models [136]. Furthermore, APP/PS1 mouse models lacking caspase-1 or NLRP3 were significantly protected from behavioral impairments, hippocampal loss of synaptic plasticity, spatial memory deficit, and other AD-related consequences [136]. In APP/PS1 mouse models, deficiency of caspase 1 or NLRP3 was found to alter microglial cells from a pro-inflammatory M1 phenotype to a neuroprotective M2 phenotype and resulted in the reduced Aβ deposition [136]. Moreover, induction of microglia through multiple inflammogens resulted in the organized activation of the apoptotic caspase-3/7 and caspase-8 (Fig. 2). Once activated, caspase-3 regulates the activation of NF-κB through the protein kinase Cδ and enhances the generation of various neurotoxic pro-inflammatory mediators including TNF-α, IL-1β, and nitric oxide (NO). Suppression of these caspases delayed neurotoxicity and microglial activation [137]. Interestingly, these caspases were found to be activated in microglia in AD individuals [138]. However, therapeutic interventions showed effective neuroprotective properties in AD mice [139, 140].
Fig. (2).
Role of caspases in the neuroinflammation of Alzheimer's disease. IL-1β, interleukin-1β; NF-κB, nuclear factor kappa B; TNFα, tumor necrosis factor-α; NO, nitric oxide.
3.4. Prostanoids
The arachidonic acid derivatives, prostanoids (PG) are synthesized via cyclooxygenase-1 and inducible cyclooxygenase-2 [141]. A study has reported that selective cyclooxygenase-1 (COX-1; localized in microglia) inhibition reduces neuroinflammation, as well as increases cognitive function in an AD mouse model by reducing the expression of pro-inflammatory factors and changing the phenotype of activated microglia to promote phagocytic activity [142]. Furthermore, an increased level of the proinflammatory prostaglandin E2 (PGE2, which binds with EP1-4 receptors) was observed in individuals with probable AD [143]. Generally, EP1-3 receptors are expressed by microglia cells [144]; however, these were also detected in other brain cells (primarily neurons). EP2 receptors were reported to suppress phagocytosis of Aβ and increase the neurotoxic actions of microglia [145]. This last finding is in line with results showing that removal of the prostaglandin EP2 or the EP3 receptor in AD mice reduced neuroinflammation, oxidative stress, BACE expression, and Aβ-burden [146-148]. However, usage of PGE2 EP4 receptor agonists on microglia has shown inhibition of AD-associated inflammation [149]. In contrast, removal of the EP4 receptor in APP/PS1 transgenic mice enhanced plaque burden and the generation of proinflammatory cytokines and chemokines such as Il-1β and CCL3 [149]. EP4 receptor expression is reduced in the cortex regions of AD and mild cognitive impairment (MCI) patients [149], suggesting that this receptor might play a role in AD-related inflammatory processes. Nevertheless, the function of PGE2 in neurodegeneration is possibly far more complex because of the effects of PGE2 on other brain cells, including neurons.
3.5. Neuroprotectin D1
Neuroprotectin D1 (NPD1) is an endogenous anti-inflammatory mediator derived from the polyunsaturated fatty acid, docosahexaenoic acid (DHA), a major component of cell membranes [150], which is reduced in AD [151]. Moreover, NPD1 is an autocrine/paracrine mediator [152] participating in the resolution response during the initial events of neuroinflammation, which reduces amyloidogenic APP processing, pro-expression of inflammatory genes, and stimulates neural cell survival [153]. NPD1 suppresses the shedding of the Aβ42 peptide by activating the α-secretase as well as down-regulating β-secretase (BACE1) [153]. Additional studies suggested that the peroxisome proliferator-activated receptor-γ (PPARγ) is required for NPD1-mediated downregulation of BACE1 and Aβ42 peptide release [153]. Collectively, NPD1 bioactivity effectively down-regulates inflammatory signaling, amyloidogenic APP processing, and apoptosis to abate neurodegeneration.
3.6. Complement System
Within the innate immune system, the complement system is that which plays a main role in the protection from pathogens [154]. The proteolytic complement cascade activation was found to result in opsonization and eventually microbial lysis [155]. Astrocytes and microglia are the major brain cells responsible for the generation of proteins of the complement system [156]. Products of the activated complement components are linked with Aβ deposits in AD [157]. It was reported upon in vitro studies that Aβ activates the complement system through an alternative pathway [158, 159]. The observation that apolipoprotein J (clusterin, CLU) variants might play a role in the regulation of C3 convertase activity and processing as well as in the removal of opsonized immune complexes, which are related to AD, has additionally validated the significance of the complement system in AD [160-162].
4. OXIDATIVE STRESS AND INFLAMMATORY MEDIATORS IN ALZHEIMER’S DISEASE
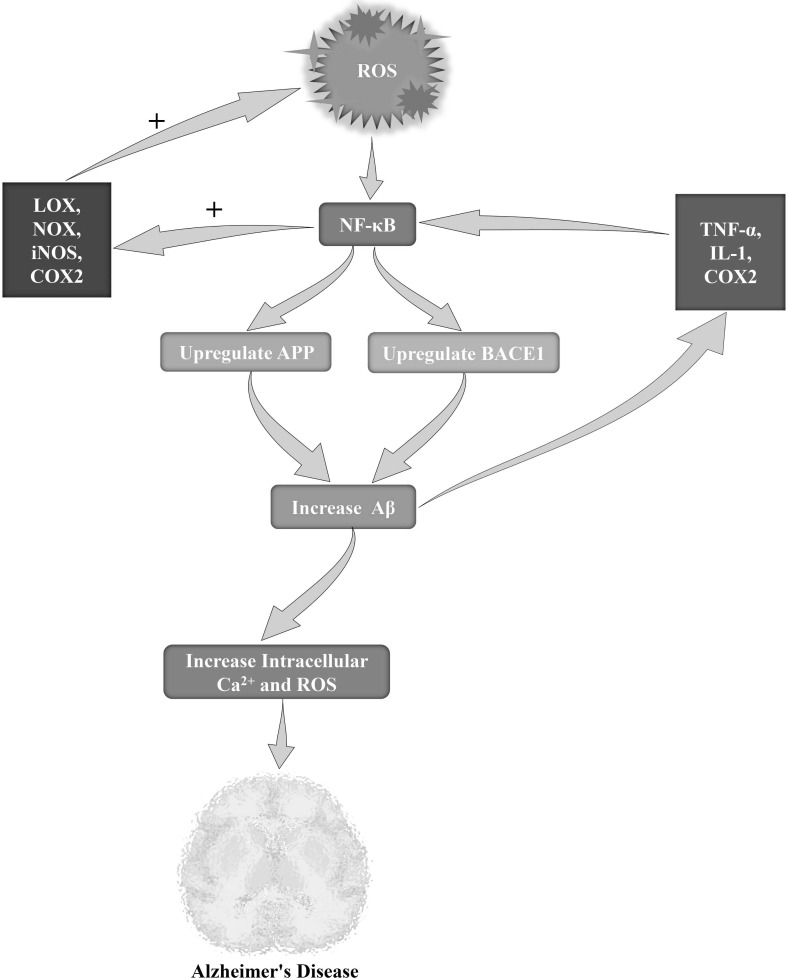
Oxidative stress and inflammatory response are essential components of AD pathogenesis [163, 164]. Several studies have concentrated on the oxidative and the inflammatory elements of AD which are found to be associated with the transcription factor NF-κB, this latter playing a pivotal role in immunity and inflammation [165]. Interestingly, in animal models of neurodegeneration, including AD, activation of NF-κB has also been observed with an elevated level of oxidative stress [166], as shown in Fig. (3). In addition to this,higher NF-κB translocation and its DNA binding have also been reported in multiple tissues during aging, and dietary antioxidants have been found to suppress the nuclear translocation of activated NF-κB [166, 167]. On the other hand, examples of pro-oxidant genes that are under the control of NF-κB include multiple lipoxygenases (LOXs), NADPH oxidase (NOX), inducible nitric oxide synthase (iNOS), and cycloxygenase-2 (COX 2) [168]. In the post-mortem AD brain, several studies have mentioned the presence of an elevated level of NF-κB activity [169-171]. Some experimental studies have strongly supported that this process might take place due to the accumulation of Aβ, inflammatory reactions, and elevated levels of oxidative stress [172, 173]. Therefore, it has been observed in the rat brain cortex that single microinjection of oligomeric Aβ42 can lead to an elevated expression of TNF-α, IL-1 and COX2, through NF-κB activation in reactive astrocytes close to the injection site [174]. On the other hand, in co-cultures of microglia and cortical neurons, Aβ can cause neuronal death and toxicity via glial NF-κB activation, which is found to be associated with the acetylation of lysine 310 of the p65 subunit [175]. Interestingly, it has been observed that Aβ25-35 or Aβ40 can induce cell death together with a rise in intracellular Ca2+ and ROS levels in hippocampal neuronal cultures; also, this process was found to be protected by TNF-β or TNF-α via NF-κB activation [77]. In terms of the association between AD and NF-κB, regulation of Aβ homeostasis is one of the vital factors which is found to be maintained via the transcriptional upregulation of several associated proteins and enzymes, particularly BACE1 and APP [176].
Fig. (3).
Role of ROS in the NF-κB mediated Alzheimer's pathogenesis. Abbreviation: ROS, reactive oxygen species; NF-κB, nuclear factor kappa B; APP, amyloid precursor protein; BACE1, beta-secretase 1. LOX, lypoxygenase; NOX, NADPH oxidase; iNOS, inducible nitric oxide synthase; COX2, cyclooxygenase 2; TNFα, tumor necrosis factor-α; IL-1, interleukin-1.
Cytokines stimulate iNOS in astroglia and microglia, leading to increased generation of NO, which can exert harmful effects in neurons. iNOS levels are increased in AD brains [177], and genetic iNOS knockout was found to be protective in AD mouse models [178]. Similarly, increased levels of PHOX (an NADPH oxidase which is vastly expressed via microglial cells) have been detected in AD [179]. PHOX is rapidly activated through various inflammatory stimuli, including Aβ, yielding the production of hydrogen peroxide that can further mediate microglial activation [180, 181]. Moreover, superoxides derived from PHOX were found to react with iNOS-derived NO to produce peroxynitrite [182]. In AD, elevated iNOS expression was found to result in NO-mediated post-translational modifications [183], including S-nitrosylation, nitration, and dityrosine formation [183]. Furthermore, S-nitrosylation of dynamin-related protein 1 is increased in AD individuals, which might be linked with neurodegeneration [184]. Interestingly, Aβ peptide nitration at tyrosine 10 (Tyr10) was found to elevate the Aβ tendency to aggregate [185]. This altered Aβ triggered the formation of plaques in APP/PS1 mouse models, which is further indicating its pivotal role in the early stage of AD. Indeed, nitrated Aβ significantly inhibited hippocampal LTP as compared to the non-nitrated form of Aβ, suggesting that this post-translational modification causes both structural and functional impairments in AD brains. It has been reported that oxidative stress mediates the generation of this Aβ species [186].
5. INFLAMMATORY CHANGES IN THE NEUROVASCULAR UNIT OF ALZHEIMER’S DISEASE
Numerous neuropathological, clinical, and epidemiological studies have revealed that vascular pathology is a crucial risk factor for AD development [187, 188]. Additionally, it has been observed that AD is linked with various functional, inflammatory, and morphological changes of perivascular neurons and glia as well as cerebral blood vessels. These early-onset and chronic alterations are indeed stimulated via collective actions of vascular Aβ deposits and soluble Aβ oligomers [189, 190], which eventually result in diminished cerebral blood flow and impaired functional hyperemia due to an increased local blood flow linked to neuronal activation [191].
In AD animal models and patients, chronic cerebral hypoxia was induced by various blood-borne factors [192]. Furthermore, a combination of inflammation of the neurovascular unit, mild hypoxia, and chronic accumulation of Aβ in brain parenchyma can increase the upregulation of the receptor for advanced glycation end products (RAGE), thus facilitating Aβ transport through the BBB into the brain [193]. Besides, hypoxia can induce amyloidogenic APP processing through several pathways, including γ-secretase, neprilysin, β-secretase [194]. In AD, chronic hypoxia directly triggers neuronal damage; however, it also increases neurodegeneration through stimulating amyloidogenic processing and reducing Aβ clearance from the brain.
6. DISEASE-ASSOCIATED MICROGLIA IN ALZHEIMER’S DISEASE
In recent times, our knowledge has grown about the role of disease-associated microglia (DAM), which may enable further understanding of the beneficial or harmful effects of microglia [195]. DAM are categorized as immune cells that express classic microglial markers, including Hexb, Cst3, and Iba1, along with downregulation of homeostatic microglial genes, such as Tmem119, CD33, Cx3cr1, P2ry13, and P2ry12 [196]. Furthermore, DAM mediates the upregulation of various genes associated with lipid metabolism as well as the phagocytic and lysosomal pathways, comprising various known risk factors for AD, including triggering receptor expressed on myeloid cells 2 (TREM2), transmembrane immune signaling adaptor (TYROBP), lipoprotein lipase (LPL), cathepsin D (CTSD) and apolipoprotein E (APOE) [197]. It has been reported in TREM2-deficient 5XFAD and APP/PS1 mouse models, that DAM differentiation is a consecutive two steps mechanism. In the first step, the transition of DAM is essential to promote the second stage of DAM activation [198, 199]. Although the TREM2 signal is required for the transition from the first stage to the second stage of DAM, the transition from homeostatic microglia to the first stage of the DAM is not dependent on TREM. Factors that facilitate this stage are still not known. DAM is linked with Aβ plaques in AD brains. It has been revealed by in vitro studies that TREM2 binds Aβ with nanomolar affinity [200, 201]. In TREM2-NFAT (nuclear factor of activated T cell) reporter cells, the TREM2 signaling pathway was found to be activated by various negatively-charged phospholipids (i.e., phosphatidylcholine, phosphatidylinositol) and possibly others which associate with Aβ and accumulate during Aβ deposition in the brain [202-204]. In addition, TREM2 also binds with several lipoproteins (i.e., CLU, APOE), forming complexes with Aβ aggregates to mediate their uptake via microglia [205-207]. Indeed, the existence of this wide range of ligands confirms the role of TREM2 as a pattern recognition receptor of CNS injury. Interestingly, rare human TREM2 variants displaying an increased risk of neurodegeneration exhibit weakened ability in vitro and in vivo to bind with numerous ligands [200, 204, 205, 207, 208].
It has been revealed that a rare mutation (R47H) in TREM2 can elevate AD risk by 3- to 4-fold, which is further suggesting that microglia dysfunction might play a role in AD [209, 210]. Song et al. [208] have introduced a human TREM2 version containing the R47H mutation in 5XFAD mouse models and confirmed the impaired activity of microglia [208]. Since TREM2 is required for full DAM activation in mouse models, these results, therefore, suggest that DAM activity might provide protection against disease progression. TREM2 was reported to form a receptor-signaling complex with TYROBP [211]. Moreover, TYROBP is upregulated in the case of DAM and is associated with presenile dementia [212].
More studies are required by utilizing mouse models lacking genes associated with the DAM phenotype as well as extensive analyses of individuals carrying mutations in DAM-associated genes in order to reveal the action of DAM in specific disease settings. In this regard, deficiency in CX3CR1, for instance, modified the activation of microglia and decreased Aβ deposition in TgCRND8, APP/PS1, and R1.40 mouse models for AD [126, 213], but worsened AD pathogenesis in a tau model for AD [214]. Silencing TREM2 in the P301S-tau model exacerbated tau pathology and spatial cognitive deficits [215]. In contrast, deficiency of TREM2 in Aβ pathology containing models (APP/PS1 or 5XFAD) revealed conflicting findings, indicating that the consequence of DAM might be dependent on the different stages of AD pathology [216]. In AD, microglial activation causes widespread shedding of soluble TREM2, which interacts with plaques and neurons. In humanized TREM2-5XFAD mouse models, it was shown that the R47H mutation reduces the interaction of microglia with Aβ plaques [208].
7. TARGETING NEUROINFLAMMATION AS A THERAPEUTIC STRATEGY FOR ALZHEIMER'S DISEASE
It is assumed that neuroinflammation only takes place at late to end AD stages and probably suggests simply an epiphenomenon. Activation of glial cells was supposed to merely accompany rather than considerably play a role in amyloid pathology [46, 177]. Nonetheless, the impact of glial cell activities and other immune-related alterations is not yet fully revealed in AD, and more studies are required in this regard. Bioinformatic, genetic, and preclinical findings have revealed that activation of the immune system plays an active role in AD pathogenesis [217]. Detection of risky variants in genes encoding for molecules of the immune system led to the re-evaluation of former results reporting that levels of inflammatory chemokines, cytokines, and various other immune mediators, are elevated in the body fluids and tissues of AD patients or at the prodromal stages of AD [218, 219]. Nevertheless, correlative studies of the clinical symptoms preceding AD (i.e., MCI) and the existence of inflammatory modifications have suggested a much earlier implication of the immune system [218, 219]. Furthermore, it was reported that systemic immune challenges by the viral mimic polyriboinosinic polyribocytidilic acid, intermittently induces the development of AD-like neuropathology (i.e., tau aggregation and Aβ plaques), reactive gliosis, and microglia activation in wild-type mouse models [220]. The modulation of neurodegenerative disorders via certain immune molecules as reported in preclinical studies as well as the upregulation of inflammatory genes in tissues derived from individuals with CNS diseases, indicate a link between neurodegeneration including AD and inflammation and suggest the early involvement of immune responses in the pathogenesis [221-231]. As per the preclinical findings obtained on AD individuals, several signaling pathways or immune molecules have been identified as auspicious therapeutic targets [232]. Table 1 represents a summary of some attractive immune targets for AD.
Table 1.
Possible immune targets to combat Alzheimer’s disease pathology.
| Immune Targets | Major Functions | Levels in Alzheimer’s Models/Patients | Probable Therapeutic Intervention in Alzheimer’s Disease | References |
|---|---|---|---|---|
| Interleukin (IL)-12/IL-23 | Exerts Aβ-mediated inflammatory response | Increased | Downregulation or inhibition of IL-12 and/or IL-23 | [225, 233-236] |
| IL-6 | Exerts Aβ-mediated inflammatory response | - | Downregulation or inhibition of IL-6 | [65, 237-239] |
| Triggering receptor expressed on myeloid cells 2 (TREM2) or Transmembrane immune signaling adaptor TYROBP (TYROBP) | Promotes Aβ clearance | Increased | Inconsistent, needs further studies | [204, 209, 210, 217, 240-242] |
| Sialic acid binding Ig-like lectin 3 (CD33) | Promotes Aβ accumulation | Increased | Downregulation or inhibition of CD33 | [243, 244] |
| Complement receptor 1 (CR1) | Promotes phagocytosis of immune complexes and Aβ | - | Upregulation or activation of CR1 | [160, 245, 246] |
| Peroxisome proliferator-activated receptor-γ (PPARγ) or Retinoid X receptor (RXR) |
Promotes Aβ clearance | - | Upregulation or activation of PPARγ or RXR | [247, 248] |
| NLR family pyrin domain containing 3 (NLRP3) | Regulates Aβ clearance, mediates caspase-1 activation and pro-inflammatory cytokine secretion | - | Downregulation or inhibition of NLRP3 | [136] |
| Cluster of differentiation 36 (CD36) | Regulates NLRP3 activation as well as binds with Aβ | - | Inconsistent, needs further studies | [247, 249, 250] |
| CD14 | Regulates microglial inflammatory response | Increased | Downregulation or inhibition of CD14 | [251-253] |
| CX3C chemokine receptor 1 (CX3CR1) | Modulates glial activation | - | Inconsistent, needs further studies | [126, 254-256] |
| P2X7 receptor (P2X7R) | Controls APP processing and mediates microglial inflammatory responses | Increased | Downregulation or inhibition of P2X7R | [257, 258] |
| Scavenger receptor class A member 1 (SCARA1) | Promotes Aβ clearance | Reduced | Upregulation or activation of SCARA1 | [259] |
| Transforming growth factor beta 1 (TGFβ1) | Suppresses glial and T cell-mediated neuroinflammation | Reduced | Upregulation or activation of TGFβ1 | [260, 261] |
IL-12 and IL-23 are therapeutically important immune targets for AD since they display elevated levels in the cerebrospinal fluid of patients with AD and/or MCI [233, 262]. The peripheral activity of IL-12 and IL-23 is usually induced by natural killers and T cells. However, in AD, the CNS activity of IL-12 and IL-23 is mediated by a process independent of natural killers and T cells [233]. Furthermore, in case of AD, IL-12 and IL-23 play direct roles on astrocytes (which express the respective receptors) in the CNS [233]. Therefore, in AD, current biologicals that suppress these ILs might be equally effective. A different and attractive approach to regulate AD-related immune responses could be the targeting of the NLRP3 inflammasome. Right now, no FDA-approved drug can specifically and exclusively target NLRP3, though the recent discovery of an inhibitor of NLRP3 [263] as well as the identification of CD36 serve as an important upstream controller of NLRP3 activation [249], and might overcome this issue. In an AD mouse model, PPARγ agonists, for example, pioglitazone, induced the clearance of Aβ via triggering microglial Aβ uptake just like a CD36-facilitated manner [247]. This finding suggests that CD36 downregulation might not only decrease inflammation but also reduce Aβ clearance, which is further indicating the need for in vivo studies regarding CD36 action in AD animal models. Regulation of the microglial CX3C chemokine receptor 1 (CX3CR1) expression exerted positive activities in mouse models displaying amyloid deposition, along with an intense deterioration of tau pathology [126, 254, 255].
Similarly, it has been shown through in vitro and in vivo studies that macrophage scavenger receptors types I and II (SCARA1) are associated with the clearance of soluble Aβ via myeloid cells [259], therefore suggesting other therapeutically attractive immune targets. The transforming growth factor β1 (TGFβ1) has been reported as a crucial regulatory cytokine suppressing the activation of microglia. In AD, TGFβ1 levels were found to be increased in the brain, cerebrospinal fluid, and plasma [234, 264-267]. Transgenic TGFβ1 overexpression reduced the Aβ burden by mediating microglial Aβ clearance in an AD mouse model [265]. However, TGFβ1 blocking and downstream SMAD2/3 signaling, mainly in CD11c-positive myeloid cells, have decreased Aβ-like pathology in a mouse model of AD [266].
CONCLUSION
Neuroinflammation plays a significant role in the progression and pathogenesis of AD. Astrocytes and microglia have an important contribution to neuroinflammation. Nevertheless, activation of astrocytes and microglia may differ depending on the disease phase though the mechanisms are still not clear. Although imaging of activated astrocytes and microglia is possible nowadays, further studies are required to assess whether they possess protective activities at the initial stage or if, at a later stage, they exert a detrimental impact on neurodegeneration. It is now assumed that the Aβ-induced inflammatory response might trigger the pathology of tau. Therefore, to characterize potential targets for the treatment of AD, identification of proper mechanisms through which inflammatory responses can take place and approaches on how to modify these responses, have gathered attention in recent times. Considering the multifactorial feature of AD, not all AD patients exhibit neuroinflammation at all-time points in the course of the disease. Eventually, combination therapeutic strategies targeting both neuropathological hallmarks (i.e. Aβ, tau) of AD and modulating neuroinflammation, may be a way to considerably reduce the progression of the disease. Therefore, more preclinical and clinical studies are required to assess the neuroinflammatory signaling pathways and their molecular underpinnings in AD. Future research should focus on deciphering the complex pathways of the neuroinflammatory process and determining the proper moment to intervene. The discovery of DAM paved the way for the development of a therapeutic that targets the pathogenic mechanisms linked to AD. The strategy of treatments should be taking into account the patient's medical history and lifestyle risk factors tracking central and peripheral neuroinflammation. Together with hopes of not just reducing but also reversing AD progression, intensive research for potential biomarkers-drug co-development pipelines is strongly suggested.
ACKNOWLEDGEMENTS
Declared none.
AUTHORS’ CONTRIBUTION
MSU conceived the original idea and designed the outlines of the study. MSU, MTK, MJ, and MAR wrote the draft of the manuscript. MSU prepared the figures for the manuscript. PJ edited the whole manuscript and improved the draft. TB, AA, GMA, MMA-D, AP, and GMA performed the literature review and aided in revising the manuscript. All authors have read and agreed to the published version of the manuscript.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This research was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the Fast-Track Research Funding Program.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Sharma P., Sharma A., Fayaz F., Wakode S., Pottoo F.H. Biological Signatures of Alzheimer’s Disease. Curr. Top. Med. Chem. 2020;20(9):770–781. doi: 10.2174/1568026620666200228095553. [DOI] [PubMed] [Google Scholar]
- 2.Uddin M.S., Al Mamun A., Ashraf G.M. Neurotoxic Aβ: Linking Extracellular and Intracellular Aβ in Alzheimer’s Disease. Curr. Protein Pept. Sci. 2021;22:442–448. doi: 10.2174/1389203722666210122144437. [DOI] [PubMed] [Google Scholar]
- 3.WHO Dementia Available at: . https://www.who.int/news-room/fact-sheets/detail/dementia (accessed January 14, 2021)
- 4.Mayeux R., Stern Y. Epidemiology of Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012;2(8):2. doi: 10.1101/cshperspect.a006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tarawneh R., Holtzman D.M. The clinical problem of symptomatic Alzheimer disease and mild cognitive impairment. Cold Spring Harb. Perspect. Med. 2012;2(5):a006148–a006148. doi: 10.1101/cshperspect.a006148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kinney J.W., Bemiller S.M., Murtishaw A.S., Leisgang A.M., Salazar A.M., Lamb B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement. (N. Y.) 2018;4:575–590. doi: 10.1016/j.trci.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uddin M.S., Kabir M.T., Rahman M.S., Behl T., Jeandet P., Ashraf G.M., Najda A., Bin-Jumah M.N., El-Seedi H.R., Abdel-Daim M.M. Revisiting the amyloid cascade hypothesis: From anti-aβ therapeutics to auspicious new ways for alzheimer’s disease. Int. J. Mol. Sci. 2020;21(16):5858. doi: 10.3390/ijms21165858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gzil F. Alzheimer’s disease: Psychiatric or neurological disorder? Poiesis Prax. 2009;6:13–26. doi: 10.1007/s10202-008-0061-3. [DOI] [Google Scholar]
- 9.Zaplatic E., Bule M., Shah S.Z.A., Uddin M.S., Niaz K. Molecular mechanisms underlying protective role of quercetin in attenuating Alzheimer’s disease. Life Sci. 2019;224:109–119. doi: 10.1016/j.lfs.2019.03.055. [DOI] [PubMed] [Google Scholar]
- 10.Chang C.W., Shao E., Mucke L. Tau: Enabler of diverse brain disorders and target of rapidly evolving therapeutic strategies. Science (80-. )., 2021, 371. [DOI] [PMC free article] [PubMed]
- 11.Janning D., Igaev M., Sündermann F., Brühmann J., Beutel O., Heinisch J.J., Bakota L., Piehler J., Junge W., Brandt R. Single-molecule tracking of tau reveals fast kiss-and-hop interaction with microtubules in living neurons. Mol. Biol. Cell. 2014;25(22):3541–3551. doi: 10.1091/mbc.e14-06-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harilal S., Jose J., Parambi D.G.T., Kumar R., Mathew G.E., Uddin M.S., Kim H., Mathew B. Advancements in nanotherapeutics for Alzheimer’s disease: current perspectives. J. Pharm. Pharmacol. 2019;71(9):1370–1383. doi: 10.1111/jphp.13132. [DOI] [PubMed] [Google Scholar]
- 13.Mamun A.A., Uddin M.S., Mathew B., Ashraf G.M. Toxic tau: structural origins of tau aggregation in Alzheimer’s disease. Neural Regen. Res. 2020;15(8):1417–1420. doi: 10.4103/1673-5374.274329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanisch U-K., Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 2007;10(11):1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 15.Sierra A., Abiega O., Shahraz A., Neumann H. Janus-faced microglia: beneficial and detrimental consequences of microglial phagocytosis. Front. Cell. Neurosci. 2013;7:6. doi: 10.3389/fncel.2013.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzumura A. Neuron-microglia interaction in neuroinflammation. Curr. Protein Pept. Sci. 2013;14(1):16–20. doi: 10.2174/1389203711314010004. [DOI] [PubMed] [Google Scholar]
- 17.Santacruz K., Lewis J., Spires T., Paulson J., Kotilinek L., Ingelsson M., Guimaraes A., DeTure M., Ramsden M., McCowan E., Forster C., Yue M., Orne J., Janus C., Mariash A., Kuskowski M., Hyman B., Hutton M., Ashe K.H. Tau suppression in a neurodegenerative mouse model improves memory function.Science (80-.), . 2005;309:476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mondragón-Rodríguez S., Perry G., Zhu X., Moreira P.I., Acevedo-Aquino M.C., Williams S. Phosphorylation of tau protein as the link between oxidative stress, mitochondrial dysfunction, and connectivity failure: implications for alzheimer’s disease.Oxid. Med. Cell. Longev., 2013, 2013. [DOI] [PMC free article] [PubMed]
- 19.Müller W.E., Eckert A., Kurz C., Eckert G.P., Leuner K. Mitochondrial dysfunction: common final pathway in brain aging and Alzheimer’s disease--therapeutic aspects. Mol. Neurobiol. 2010;41(2-3):159–171. doi: 10.1007/s12035-010-8141-5. [DOI] [PubMed] [Google Scholar]
- 20.Kigerl K.A., de Rivero Vaccari J.P., Dietrich W.D., Popovich P.G., Keane R.W. Pattern recognition receptors and central nervous system repair. Exp. Neurol. 2014;258:5–16. doi: 10.1016/j.expneurol.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holmes C., Boche D., Wilkinson D., Yadegarfar G., Hopkins V., Bayer A., Jones R.W., Bullock R., Love S., Neal J.W., Zotova E., Nicoll J.A. Long-term effects of Abeta42 immunisation in Alzheimer’s disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372(9634):216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- 22.Hong S., Beja-Glasser V.F., Nfonoyim B.M., Frouin A., Li S., Ramakrishnan S., Merry K.M., Shi Q., Rosenthal A., Barres B.A., Lemere C.A., Selkoe D.J., Stevens B. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016;352(6286):712–716. doi: 10.1126/science.aad8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Czirr E., Castello N.A., Mosher K.I., Castellano J.M., Hinkson I.V., Lucin K.M., Baeza-Raja B., Ryu J.K., Li L., Farina S.N., Belichenko N.P., Longo F.M., Akassoglou K., Britschgi M., Cirrito J.R., Wyss-Coray T. Microglial complement receptor 3 regulates brain Aβ levels through secreted proteolytic activity. J. Exp. Med. 2017;214(4):1081–1092. doi: 10.1084/jem.20162011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGeer P.L., Akiyama H., Itagaki S., McGeer E.G. Immune system response in Alzheimer’s disease. Can. J. Neurol. Sci. 1989;16(4) Suppl.:516–527. doi: 10.1017/S0317167100029863. [DOI] [PubMed] [Google Scholar]
- 25.Heneka M.T., Sastre M., Dumitrescu-Ozimek L., Hanke A., Dewachter I., Kuiperi C., O’Banion K., Klockgether T., Van Leuven F., Landreth G.E. Acute treatment with the PPARgamma agonist pioglitazone and ibuprofen reduces glial inflammation and Abeta1-42 levels in APPV717I transgenic mice. Brain. 2005;128(Pt 6):1442–1453. doi: 10.1093/brain/awh452. [DOI] [PubMed] [Google Scholar]
- 26.Rodríguez J.J., Witton J., Olabarria M., Noristani H.N., Verkhratsky A. Increase in the density of resting microglia precedes neuritic plaque formation and microglial activation in a transgenic model of Alzheimer’s disease. Cell Death Dis. 2010;1:e1–e1. doi: 10.1038/cddis.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung C.K.E., Keppler K., Steinbach S., Blazquez-Llorca L., Herms J. Fibrillar amyloid plaque formation precedes microglial activation. PLoS One. 2015;10(3):e0119768. doi: 10.1371/journal.pone.0119768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jana M., Palencia C.A., Pahan K. Fibrillar amyloid-beta peptides activate microglia via tlR2: Implications for alzheimer’s disease. J. Immunol. (Baltimore, Md. 1950) 2008;181:7254–7262. doi: 10.4049/jimmunol.181.10.7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richard K.L., Filali M., Préfontaine P., Rivest S. Toll-like receptor 2 acts as a natural innate immune receptor to clear amyloid beta 1-42 and delay the cognitive decline in a mouse model of Alzheimer’s disease. J. Neurosci. 2008;28(22):5784–5793. doi: 10.1523/JNEUROSCI.1146-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reed-Geaghan E.G., Savage J.C., Hise A.G., Landreth G.E. CD14 and toll-like receptors 2 and 4 are required for fibrillar Abeta-stimulated microglial activation. J. Neurosci. 2009;29(38):11982–11992. doi: 10.1523/JNEUROSCI.3158-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Apelt J., Schliebs R. Beta-amyloid-induced glial expression of both pro- and anti-inflammatory cytokines in cerebral cortex of aged transgenic Tg2576 mice with Alzheimer plaque pathology. Brain Res. 2001;894(1):21–30. doi: 10.1016/S0006-8993(00)03176-0. [DOI] [PubMed] [Google Scholar]
- 32.Patel N.S., Paris D., Mathura V., Quadros A.N., Crawford F.C., Mullan M.J. Inflammatory cytokine levels correlate with amyloid load in transgenic mouse models of Alzheimer’s disease. J. Neuroinflammation. 2005;2(1):9. doi: 10.1186/1742-2094-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benzing W.C., Wujek J.R., Ward E.K., Shaffer D., Ashe K.H., Younkin S.G., Brunden K.R. Evidence for glial-mediated inflammation in aged APP(SW) transgenic mice. Neurobiol. Aging. 1999;20(6):581–589. doi: 10.1016/S0197-4580(99)00065-2. [DOI] [PubMed] [Google Scholar]
- 34.Salminen A., Kauppinen A., Suuronen T., Kaarniranta K., Ojala J. ER stress in Alzheimer’s disease: a novel neuronal trigger for inflammation and Alzheimer’s pathology. J. Neuroinflammation. 2009;6:41. doi: 10.1186/1742-2094-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morimoto K., Horio J., Satoh H., Sue L., Beach T., Arita S., Tooyama I., Konishi Y. Expression profiles of cytokines in the brains of Alzheimer’s disease (AD) patients compared to the brains of non-demented patients with and without increasing AD pathology. J. Alzheimers Dis. 2011;25(1):59–76. doi: 10.3233/JAD-2011-101815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agostinho P., Cunha R.A., Oliveira C. Neuroinflammation, oxidative stress and the pathogenesis of Alzheimer’s disease. Curr. Pharm. Des. 2010;16(25):2766–2778. doi: 10.2174/138161210793176572. [DOI] [PubMed] [Google Scholar]
- 37.Wang W-Y., Tan M-S., Yu J-T., Tan L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med. 2015;3(10):136. doi: 10.3978/j.issn.2305-5839.2015.03.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGeer P.L., Itagaki S., Tago H., McGeer E.G. Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neurosci. Lett. 1987;79(1-2):195–200. doi: 10.1016/0304-3940(87)90696-3. [DOI] [PubMed] [Google Scholar]
- 39.Zotova E., Holmes C., Johnston D., Neal J.W., Nicoll J.A., Boche D. Microglial alterations in human Alzheimer’s disease following Aβ42 immunization. Neuropathol. Appl. Neurobiol. 2011;37(5):513–524. doi: 10.1111/j.1365-2990.2010.01156.x. [DOI] [PubMed] [Google Scholar]
- 40.Shao Y., Gearing M., Mirra S.S. Astrocyte-apolipoprotein E associations in senile plaques in Alzheimer disease and vascular lesions: a regional immunohistochemical study. J. Neuropathol. Exp. Neurol. 1997;56(4):376–381. doi: 10.1097/00005072-199704000-00006. [DOI] [PubMed] [Google Scholar]
- 41.Uddin M.S., Kabir M.T., Mamun A.A., Barreto G.E., Rashid M., Perveen A., Ashraf G.M. Pharmacological approaches to mitigate neuroinflammation in Alzheimer’s disease. Int. Immunopharmacol. 2020;84:106479. doi: 10.1016/j.intimp.2020.106479. [DOI] [PubMed] [Google Scholar]
- 42.Ibrahim A.M., Pottoo F.H., Dahiya E.S., Khan F.A., Kumar J.B.S. Neuron-glia interactions: Molecular basis of alzheimer’s disease and applications of neuroproteomics. Eur. J. Neurosci. 2020;52(2):2931–2943. doi: 10.1111/ejn.14838. [DOI] [PubMed] [Google Scholar]
- 43.Kabir M.T., Uddin M.S., Zaman S., Rahman M.S., Behl T., Ahmad A., Hafeez A., Perveen A., Ashraf G.M. Exploring the anti-neuroinflammatory potential of steroid and terpenoid-derived phytochemicals to combat alzheimer’s disease. Curr. Pharm. Des. 2021;27(22):2635–2647. doi: 10.2174/1381612826666210101152352. [DOI] [PubMed] [Google Scholar]
- 44.Kummer M.P., Hammerschmidt T., Martinez A., Terwel D., Eichele G., Witten A., Figura S., Stoll M., Schwartz S., Pape H-C., Schultze J.L., Weinshenker D., Heneka M.T., Urban I. Ear2 deletion causes early memory and learning deficits in APP/PS1 mice. J. Neurosci. 2014;34(26):8845–8854. doi: 10.1523/JNEUROSCI.4027-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gahtan E., Overmier J.B. Inflammatory pathogenesis in Alzheimer’s disease: biological mechanisms and cognitive sequeli. Neurosci. Biobehav. Rev. 1999;23(5):615–633. doi: 10.1016/S0149-7634(98)00058-X. [DOI] [PubMed] [Google Scholar]
- 46.Ii M., Sunamoto M., Ohnishi K., Ichimori Y. β-Amyloid protein-dependent nitric oxide production from microglial cells and neurotoxicity. Brain Res. 1996;720(1-2):93–100. doi: 10.1016/0006-8993(96)00156-4. [DOI] [PubMed] [Google Scholar]
- 47.Akiyama H., Barger S., Barnum S., Bradt B., Bauer J., Cole G.M., Cooper N.R., Eikelenboom P., Emmerling M., Fiebich B.L., Finch C.E., Frautschy S., Griffin W.S., Hampel H., Hull M., Landreth G., Lue L., Mrak R., Mackenzie I.R., McGeer P.L., O’Banion M.K., Pachter J., Pasinetti G., Plata-Salaman C., Rogers J., Rydel R., Shen Y., Streit W., Strohmeyer R., Tooyoma I., Van Muiswinkel F.L., Veerhuis R., Walker D., Webster S., Wegrzyniak B., Wenk G., Wyss-Coray T. Inflammation and Alzheimer’s disease. Neurobiol. Aging. 2000;21(3):383–421. doi: 10.1016/S0197-4580(00)00124-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heneka M.T., O’Banion M.K. Inflammatory processes in Alzheimer’s disease. J. Neuroimmunol. 2007;184(1-2):69–91. doi: 10.1016/j.jneuroim.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 49.Rojo L.E., Fernández J.A., Maccioni A.A., Jimenez J.M., Maccioni R.B. Neuroinflammation: implications for the pathogenesis and molecular diagnosis of Alzheimer’s disease. Arch. Med. Res. 2008;39(1):1–16. doi: 10.1016/j.arcmed.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 50.Hoozemans J.J.M., Veerhuis R., Rozemuller J.M., Eikelenboom P. Neuroinflammation and regeneration in the early stages of Alzheimer’s disease pathology. Int. J. Dev. Neurosci. 2006;24(2-3):157–165. doi: 10.1016/j.ijdevneu.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 51.Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat. Med. 2006;12(9):1005–1015. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]
- 52.Clayton K.A., Van Enoo A.A., Ikezu T. Alzheimer’s disease: The role of microglia in brain homeostasis and proteopathy. Front. Neurosci. 2017;11:680. doi: 10.3389/fnins.2017.00680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Versijpt J.J., Dumont F., Van Laere K.J., Decoo D., Santens P., Audenaert K., Achten E., Slegers G., Dierckx R.A., Korf J. Assessment of neuroinflammation and microglial activation in Alzheimer’s disease with radiolabelled PK11195 and single photon emission computed tomography. A pilot study. Eur. Neurol. 2003;50(1):39–47. doi: 10.1159/000070857. [DOI] [PubMed] [Google Scholar]
- 54.Edison P., Archer H.A., Gerhard A., Hinz R., Pavese N., Turkheimer F.E., Hammers A., Tai Y.F., Fox N., Kennedy A., Rossor M., Brooks D.J. Microglia, amyloid, and cognition in Alzheimer’s disease: An [11C](R)PK11195-PET and [11C]PIB-PET study. Neurobiol. Dis. 2008;32(3):412–419. doi: 10.1016/j.nbd.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 55.Wiley C.A., Lopresti B.J., Venneti S., Price J., Klunk W.E., DeKosky S.T., Mathis C.A. Carbon 11-labeled Pittsburgh Compound B and carbon 11-labeled (R)-PK11195 positron emission tomographic imaging in Alzheimer disease. Arch. Neurol. 2009;66(1):60–67. doi: 10.1001/archneurol.2008.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Venneti S., Lopresti B.J., Wang G., Hamilton R.L., Mathis C.A., Klunk W.E., Apte U.M., Wiley C.A. PK11195 labels activated microglia in Alzheimer’s disease and in vivo in a mouse model using PET. Neurobiol. Aging. 2009;30(8):1217–1226. doi: 10.1016/j.neurobiolaging.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bamberger M.E., Harris M.E., McDonald D.R., Husemann J., Landreth G.E. A cell surface receptor complex for fibrillar β-amyloid mediates microglial activation. J. Neurosci. 2003;23(7):2665–2674. doi: 10.1523/JNEUROSCI.23-07-02665.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carty M., Bowie A.G. Evaluating the role of Toll-like receptors in diseases of the central nervous system. Biochem. Pharmacol. 2011;81(7):825–837. doi: 10.1016/j.bcp.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 59.Arancio O., Zhang H.P., Chen X., Lin C., Trinchese F., Puzzo D., Liu S., Hegde A., Yan S.F., Stern A., Luddy J.S., Lue L.F., Walker D.G., Roher A., Buttini M., Mucke L., Li W., Schmidt A.M., Kindy M., Hyslop P.A., Stern D.M., Du Yan S.S. Rage potentiates Abeta-induced perturbation of neuronal function in transgenic mice. EMBO J. 2004;23(20):4096–4105. doi: 10.1038/sj.emboj.7600415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koenigsknecht-Talboo J., Landreth G.E. Microglial phagocytosis induced by fibrillar β-amyloid and IgGs are differentially regulated by proinflammatory cytokines. J. Neurosci. 2005;25(36):8240–8249. doi: 10.1523/JNEUROSCI.1808-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.El Khoury J.B., Moore K.J., Means T.K., Leung J., Terada K., Toft M., Freeman M.W., Luster A.D. CD36 mediates the innate host response to beta-amyloid. J. Exp. Med. 2003;197(12):1657–1666. doi: 10.1084/jem.20021546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lue L.F., Brachova L., Civin W.H. Rogers, J. Inflammation, A beta deposition, and neurofibrillary tangle formation as correlates of Alzheimer’s disease neurodegeneration. J. Neuropathol. Exp. Neurol. 1996;55(10):1083–1088. doi: 10.1097/00005072-199655100-00008. [DOI] [PubMed] [Google Scholar]
- 63.Schilling T., Eder C. Amyloid-β-induced reactive oxygen species production and priming are differentially regulated by ion channels in microglia. J. Cell. Physiol. 2011;226(12):3295–3302. doi: 10.1002/jcp.22675. [DOI] [PubMed] [Google Scholar]
- 64.Shaftel S.S., Kyrkanides S., Olschowka J.A., Miller J.N., Johnson R.E., O’Banion M.K. Sustained hippocampal IL-1 beta overexpression mediates chronic neuroinflammation and ameliorates Alzheimer plaque pathology. J. Clin. Invest. 2007;117(6):1595–1604. doi: 10.1172/JCI31450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chakrabarty P., Jansen-West K., Beccard A., Ceballos-Diaz C., Levites Y., Verbeeck C., Zubair A.C., Dickson D., Golde T.E., Das P. Massive gliosis induced by interleukin-6 suppresses Abeta deposition in vivo: evidence against inflammation as a driving force for amyloid deposition. FASEB J. 2010;24(2):548–559. doi: 10.1096/fj.09-141754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Decourt B., Lahiri D.K., Sabbagh M.N. Targeting tumor necrosis factor alpha for alzheimer’s disease. Curr. Alzheimer Res. 2017;14(4):412–425. doi: 10.2174/1567205013666160930110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ramos E.M., Lin M.T., Larson E.B., Maezawa I., Tseng L.H., Edwards K.L., Schellenberg G.D., Hansen J.A., Kukull W.A., Jin L.W. Tumor necrosis factor α and interleukin 10 promoter region polymorphisms and risk of late-onset Alzheimer disease. Arch. Neurol. 2006;63(8):1165–1169. doi: 10.1001/archneur.63.8.1165. [DOI] [PubMed] [Google Scholar]
- 68.Janelsins M.C., Mastrangelo M.A., Park K.M., Sudol K.L., Narrow W.C., Oddo S., LaFerla F.M., Callahan L.M., Federoff H.J., Bowers W.J. Chronic neuron-specific tumor necrosis factor-alpha expression enhances the local inflammatory environment ultimately leading to neuronal death in 3xTg-AD mice. Am. J. Pathol. 2008;173(6):1768–1782. doi: 10.2353/ajpath.2008.080528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yin Y., Ren Y., Wu W., Wang Y., Cao M., Zhu Z., Wang M., Li W. Protective effects of bilobalide on Aβ(25-35) induced learning and memory impairments in male rats. Pharmacol. Biochem. Behav. 2013;106:77–84. doi: 10.1016/j.pbb.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 70.Tobinick E. Tumour necrosis factor modulation for treatment of alzheimerʼs disease. CNS Drugs. 2009;23:713–725. doi: 10.2165/11310810-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 71.Shin J-W., Cheong Y-J., Koo Y-M., Kim S., Noh C-K., Son Y-H., Kang C., Sohn N-W. α-asarone ameliorates memory deficit in lipopolysaccharide-treated mice via suppression of pro-inflammatory cytokines and microglial activation. Biomol. Ther. (Seoul) 2014;22(1):17–26. doi: 10.4062/biomolther.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bremer E. Targeting of the tumor necrosis factor receptor superfamily for cancer immunotherapy. ISRN Oncol. 2013;2013:371854. doi: 10.1155/2013/371854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Montgomery S.L., Narrow W.C., Mastrangelo M.A., Olschowka J.A., O’Banion M.K., Bowers W.J. Chronic neuron- and age-selective down-regulation of TNF receptor expression in triple-transgenic Alzheimer disease mice leads to significant modulation of amyloid- and Tau-related pathologies. Am. J. Pathol. 2013;182(6):2285–2297. doi: 10.1016/j.ajpath.2013.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.He P., Zhong Z., Lindholm K., Berning L., Lee W., Lemere C., Staufenbiel M., Li R., Shen Y. Deletion of tumor necrosis factor death receptor inhibits amyloid β generation and prevents learning and memory deficits in Alzheimer’s mice. J. Cell Biol. 2007;178(5):829–841. doi: 10.1083/jcb.200705042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McAlpine F.E., Lee J-K., Harms A.S., Ruhn K.A., Blurton-Jones M., Hong J., Das P., Golde T.E., LaFerla F.M., Oddo S., Blesch A., Tansey M.G. Inhibition of soluble TNF signaling in a mouse model of Alzheimer’s disease prevents pre-plaque amyloid-associated neuropathology. Neurobiol. Dis. 2009;34(1):163–177. doi: 10.1016/j.nbd.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Montgomery S.L., Mastrangelo M.A., Habib D., Narrow W.C., Knowlden S.A., Wright T.W., Bowers W.J. Ablation of TNF-RI/RII expression in Alzheimer’s disease mice leads to an unexpected enhancement of pathology: implications for chronic pan-TNF-α suppressive therapeutic strategies in the brain. Am. J. Pathol. 2011;179(4):2053–2070. doi: 10.1016/j.ajpath.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barger S.W., Hörster D., Furukawa K., Goodman Y., Krieglstein J., Mattson M.P. Tumor necrosis factors alpha and beta protect neurons against amyloid beta-peptide toxicity: evidence for involvement of a kappa B-binding factor and attenuation of peroxide and Ca2+ accumulation. Proc. Natl. Acad. Sci. USA. 1995;92(20):9328–9332. doi: 10.1073/pnas.92.20.9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tarkowski E., Blennow K., Wallin A., Tarkowski A. Intracerebral production of tumor necrosis factor-alpha, a local neuroprotective agent, in Alzheimer disease and vascular dementia. J. Clin. Immunol. 1999;19(4):223–230. doi: 10.1023/A:1020568013953. [DOI] [PubMed] [Google Scholar]
- 79.Kaneko N., Kurata M., Yamamoto T., Morikawa S., Masumoto J. The role of interleukin-1 in general pathology. Inflamm. Regen. 2019;39:12. doi: 10.1186/s41232-019-0101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shaftel S.S., Griffin W.S.T., O’Banion M.K. The role of interleukin-1 in neuroinflammation and Alzheimer disease: an evolving perspective. J. Neuroinflammation. 2008;5:7. doi: 10.1186/1742-2094-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lamkanfi M., Dixit V.M. Inflammasomes and their roles in health and disease. Annu. Rev. Cell Dev. Biol. 2012;28:137–161. doi: 10.1146/annurev-cellbio-101011-155745. [DOI] [PubMed] [Google Scholar]
- 82.Liu L., Martin R., Chan C. Palmitate-activated astrocytes via serine palmitoyltransferase increase BACE1 in primary neurons by sphingomyelinases. Neurobiol. Aging. 2013;34(2):540–550. doi: 10.1016/j.neurobiolaging.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Parajuli B., Sonobe Y., Horiuchi H., Takeuchi H., Mizuno T., Suzumura A. Oligomeric amyloid β induces IL-1β processing via production of ROS: implication in Alzheimer’s disease. Cell Death Dis. 2013;4:e975–e975. doi: 10.1038/cddis.2013.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Boutajangout A., Wisniewski T. The innate immune system in Alzheimer’s disease. Int. J. Cell Biol. 2013;2013:576383. doi: 10.1155/2013/576383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hunter J.M., Kwan J., Malek-Ahmadi M., Maarouf C.L., Kokjohn T.A., Belden C., Sabbagh M.N., Beach T.G., Roher A.E. Morphological and pathological evolution of the brain microcirculation in aging and Alzheimer’s disease. PLoS One. 2012;7(5):e36893. doi: 10.1371/journal.pone.0036893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sheng J.G., Zhu S.G., Jones R.A., Griffin W.S.T., Mrak R.E. Interleukin-1 promotes expression and phosphorylation of neurofilament and tau proteins in vivo. Exp. Neurol. 2000;163(2):388–391. doi: 10.1006/exnr.2000.7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kitazawa M., Cheng D., Tsukamoto M.R., Koike M.A., Wes P.D., Vasilevko V., Cribbs D.H., LaFerla F.M. Blocking IL-1 signaling rescues cognition, attenuates tau pathology, and restores neuronal β-catenin pathway function in an Alzheimer’s disease model. J. Immunol. 2011;187(12):6539–6549. doi: 10.4049/jimmunol.1100620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pickering M., O’Connor J.J. Pro-inflammatory cytokines and their effects in the dentate gyrus. 2007. [DOI] [PubMed]
- 89.Rubio-Perez J.M., Morillas-Ruiz J.M.A. A review: inflammatory process in Alzheimer’s disease, role of cytokines. ScientificWorldJournal. 2012;2012:756357. doi: 10.1100/2012/756357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Heneka M.T., Nadrigny F., Regen T., Martinez-Hernandez A., Dumitrescu-Ozimek L., Terwel D., Jardanhazi-Kurutz D., Walter J., Kirchhoff F., Hanisch U-K., Kummer M.P. Locus ceruleus controls Alzheimer’s disease pathology by modulating microglial functions through norepinephrine. Proc. Natl. Acad. Sci. USA. 2010;107(13):6058–6063. doi: 10.1073/pnas.0909586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang Y., Jin S., Sonobe Y., Cheng Y., Horiuchi H., Parajuli B., Kawanokuchi J., Mizuno T., Takeuchi H., Suzumura A. Interleukin-1β induces blood-brain barrier disruption by downregulating Sonic hedgehog in astrocytes. PLoS One. 2014;9(10):e110024. doi: 10.1371/journal.pone.0110024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shaftel S.S., Kyrkanides S., Olschowka J.A., Miller J.N., Johnson R.E., O’Banion M.K. Sustained hippocampal IL-1 β overexpression mediates chronic neuroinflammation and ameliorates Alzheimer plaque pathology. J. Clin. Invest. 2007;117(6):1595–1604. doi: 10.1172/JCI31450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ghosh S., Wu M.D., Shaftel S.S., Kyrkanides S., LaFerla F.M., Olschowka J.A., O’Banion M.K. Sustained interleukin-1β overexpression exacerbates tau pathology despite reduced amyloid burden in an Alzheimer’s mouse model. J. Neurosci. 2013;33(11):5053–5064. doi: 10.1523/JNEUROSCI.4361-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tachida Y., Nakagawa K., Saito T., Saido T.C., Honda T., Saito Y., Murayama S., Endo T., Sakaguchi G., Kato A., Kitazume S., Hashimoto Y. Interleukin-1 β up-regulates TACE to enhance α-cleavage of APP in neurons: resulting decrease in Abeta production. J. Neurochem. 2008;104(5):1387–1393. doi: 10.1111/j.1471-4159.2007.05127.x. [DOI] [PubMed] [Google Scholar]
- 95.Wu Y.Y., Hsu J.L., Wang H.C., Wu S.J., Hong C.J., Cheng I.H.J. Alterations of the neuroinflammatory markers il-6 and trail in alzheimer’s disease. Dement. Geriatr. Cogn. Disord. Extra. 2015;5(3):424–434. doi: 10.1159/000439214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Querfurth H.W., LaFerla F.M. Alzheimer’s disease. N. Engl. J. Med. 2010;362(4):329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 97.Huell M., Strauss S., Volk B., Berger M., Bauer J. Interleukin-6 is present in early stages of plaque formation and is restricted to the brains of Alzheimer’s disease patients. Acta Neuropathol. 1995;89(6):544–551. doi: 10.1007/BF00571510. [DOI] [PubMed] [Google Scholar]
- 98.Spooren A., Kolmus K., Laureys G., Clinckers R., De Keyser J., Haegeman G., Gerlo S. Interleukin-6, a mental cytokine. Brain Res. Brain Res. Rev. 2011;67(1-2):157–183. doi: 10.1016/j.brainresrev.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 99.Vukic V., Callaghan D., Walker D., Lue L-F., Liu Q.Y., Couraud P-O., Romero I.A., Weksler B., Stanimirovic D.B., Zhang W. Expression of inflammatory genes induced by beta-amyloid peptides in human brain endothelial cells and in Alzheimer’s brain is mediated by the JNK-AP1 signaling pathway. Neurobiol. Dis. 2009;34(1):95–106. doi: 10.1016/j.nbd.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Flynn C.M., Garbers Y., Lokau J., Wesch D., Schulte D.M., Laudes M., Lieb W., Aparicio-Siegmund S., Garbers C. Activation of Toll-like Receptor 2 (TLR2) induces Interleukin-6 trans-signaling. Sci. Rep. 2019;9(1):7306. doi: 10.1038/s41598-019-43617-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Quintanilla R.A., Orellana D.I., González-Billault C., Maccioni R.B. Interleukin-6 induces Alzheimer-type phosphorylation of tau protein by deregulating the cdk5/p35 pathway. Exp. Cell Res. 2004;295(1):245–257. doi: 10.1016/j.yexcr.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 102.Yirmiya R., Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav. Immun. 2011;25(2):181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 103.Weaver J.D., Huang M-H., Albert M., Harris T., Rowe J.W., Seeman T.E. Interleukin-6 and risk of cognitive decline: MacArthur studies of successful aging. Neurology. 2002;59(3):371–378. doi: 10.1212/WNL.59.3.371. [DOI] [PubMed] [Google Scholar]
- 104.Dugan L.L., Ali S.S., Shekhtman G., Roberts A.J., Lucero J., Quick K.L., Behrens M.M. IL-6 mediated degeneration of forebrain GABAergic interneurons and cognitive impairment in aged mice through activation of neuronal NADPH oxidase. PLoS One. 2009;4(5):e5518. doi: 10.1371/journal.pone.0005518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Donegan J.J., Girotti M., Weinberg M.S., Morilak D.A. A novel role for brain interleukin-6: Facilitation of cognitive flexibility in rat orbitofrontal cortex. J. Neurosci. 2014;34(3):953–962. doi: 10.1523/JNEUROSCI.3968-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sivakumar P.V., Westrich G.M., Kanaly S., Garka K., Born T.L., Derry J.M.J., Viney J.L. Interleukin 18 is a primary mediator of the inflammation associated with dextran sulphate sodium induced colitis: blocking interleukin 18 attenuates intestinal damage. Gut. 2002;50(6):812–820. doi: 10.1136/gut.50.6.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dinarello C.A. Interleukin-18 and the pathogenesis of inflammatory diseases. Semin. Nephrol. 2007;27(1):98–114. doi: 10.1016/j.semnephrol.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 108.Sutinen E.M., Pirttilä T., Anderson G., Salminen A., Ojala J.O. Pro-inflammatory interleukin-18 increases Alzheimer’s disease-associated amyloid-β production in human neuron-like cells. J. Neuroinflammation. 2012;9:199. doi: 10.1186/1742-2094-9-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ghayur T., Banerjee S., Hugunin M., Butler D., Herzog L., Carter A., Quintal L., Sekut L., Talanian R., Paskind M., Wong W., Kamen R., Tracey D., Allen H. Caspase-1 processes IFN-γ-inducing factor and regulates LPS-induced IFN-γ production. Nature. 1997;386(6625):619–623. doi: 10.1038/386619a0. [DOI] [PubMed] [Google Scholar]
- 110.Fantuzzi G., Dinarello C.A. Interleukin-18 and interleukin-1 β: Two cytokine substrates for ICE (caspase-1). J. Clin. Immunol. 1999;19(1):1–11. doi: 10.1023/A:1020506300324. [DOI] [PubMed] [Google Scholar]
- 111.Ojala J., Alafuzoff I., Herukka S.K., van Groen T., Tanila H., Pirttilä T. Expression of interleukin-18 is increased in the brains of Alzheimer’s disease patients. Neurobiol. Aging. 2009;30(2):198–209. doi: 10.1016/j.neurobiolaging.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 112.Curran B., O’Connor J.J. The pro-inflammatory cytokine interleukin-18 impairs long-term potentiation and NMDA receptor-mediated transmission in the rat hippocampus in vitro. Neuroscience. 2001;108(1):83–90. doi: 10.1016/S0306-4522(01)00405-5. [DOI] [PubMed] [Google Scholar]
- 113.Alboni S., Cervia D., Sugama S., Conti B. Interleukin 18 in the CNS. J. Neuroinflammation. 2010;7:9. doi: 10.1186/1742-2094-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rex D.A.B., Agarwal N., Prasad T.S.K., Kandasamy R.K., Subbannayya Y., Pinto S.M. A comprehensive pathway map of IL-18-mediated signalling. J. Cell Commun. Signal. 2020;14(2):257–266. doi: 10.1007/s12079-019-00544-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chandrasekar B., Valente A.J., Freeman G.L., Mahimainathan L., Mummidi S. Interleukin-18 induces human cardiac endothelial cell death via a novel signaling pathway involving NF-kappaB-dependent PTEN activation. Biochem. Biophys. Res. Commun. 2006;339(3):956–963. doi: 10.1016/j.bbrc.2005.11.100. [DOI] [PubMed] [Google Scholar]
- 116.Yu J-T., Chang R.C-C., Tan L. Calcium dysregulation in Alzheimer’s disease: from mechanisms to therapeutic opportunities. Prog. Neurobiol. 2009;89(3):240–255. doi: 10.1016/j.pneurobio.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 117.Frayling T.M., Rafiq S., Murray A., Hurst A.J., Weedon M.N., Henley W., Bandinelli S., Corsi A-M., Ferrucci L., Guralnik J.M., Wallace R.B., Melzer D. An interleukin-18 polymorphism is associated with reduced serum concentrations and better physical functioning in older people. J. Gerontol. A Biol. Sci. Med. Sci. 2007;62(1):73–78. doi: 10.1093/gerona/62.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bossù P., Ciaramella A., Salani F., Vanni D., Palladino I., Caltagirone C., Scapigliati G. Interleukin-18, from neuroinflammation to Alzheimer’s disease. Curr. Pharm. Des. 2010;16(38):4213–4224. doi: 10.2174/138161210794519147. [DOI] [PubMed] [Google Scholar]
- 119.Ojala J.O., Sutinen E.M., Salminen A., Pirttilä T. Interleukin-18 increases expression of kinases involved in tau phosphorylation in SH-SY5Y neuroblastoma cells. J. Neuroimmunol. 2008;205(1-2):86–93. doi: 10.1016/j.jneuroim.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 120.Savarin-Vuaillat C., Ransohoff R.M. Chemokines and chemokine receptors in neurological disease: Raise, retain, or reduce? Neurotherapeutics. 2007;4(4):590–601. doi: 10.1016/j.nurt.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xia M.Q., Qin S.X., Wu L.J., Mackay C.R., Hyman B.T. Immunohistochemical study of the β-chemokine receptors CCR3 and CCR5 and their ligands in normal and Alzheimer’s disease brains. Am. J. Pathol. 1998;153(1):31–37. doi: 10.1016/S0002-9440(10)65542-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ishizuka K., Kimura T., Igata-yi R., Katsuragi S., Takamatsu J., Miyakawa T. Identification of monocyte chemoattractant protein-1 in senile plaques and reactive microglia of Alzheimer’s disease. Psychiatry Clin. Neurosci. 1997;51(3):135–138. doi: 10.1111/j.1440-1819.1997.tb02375.x. [DOI] [PubMed] [Google Scholar]
- 123.Smits H.A., Rijsmus A., van Loon J.H., Wat J.W.Y., Verhoef J., Boven L.A., Nottet H.S.L.M. Amyloid-beta-induced chemokine production in primary human macrophages and astrocytes. J. Neuroimmunol. 2002;127(1-2):160–168. doi: 10.1016/S0165-5728(02)00112-1. [DOI] [PubMed] [Google Scholar]
- 124.Lue L.F., Walker D.G., Rogers J. Modeling microglial activation in Alzheimer’s disease with human postmortem microglial cultures. Neurobiol. Aging. 2001;22(6):945–956. doi: 10.1016/S0197-4580(01)00311-6. [DOI] [PubMed] [Google Scholar]
- 125.Fuhrmann M., Bittner T., Jung C.K.E., Burgold S., Page R.M., Mitteregger G., Haass C., LaFerla F.M., Kretzschmar H., Herms J. Microglial Cx3cr1 knockout prevents neuron loss in a mouse model of Alzheimer’s disease. Nat. Neurosci. 2010;13(4):411–413. doi: 10.1038/nn.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lee S., Varvel N.H., Konerth M.E., Xu G., Cardona A.E., Ransohoff R.M., Lamb B.T. CX3CR1 deficiency alters microglial activation and reduces beta-amyloid deposition in two Alzheimer’s disease mouse models. Am. J. Pathol. 2010;177(5):2549–2562. doi: 10.2353/ajpath.2010.100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cho S-H., Sun B., Zhou Y., Kauppinen T.M., Halabisky B., Wes P., Ransohoff R.M., Gan L. CX3CR1 protein signaling modulates microglial activation and protects against plaque-independent cognitive deficits in a mouse model of Alzheimer disease. J. Biol. Chem. 2011;286(37):32713–32722. doi: 10.1074/jbc.M111.254268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kiyota T., Yamamoto M., Xiong H., Lambert M.P., Klein W.L., Gendelman H.E., Ransohoff R.M., Ikezu T. CCL2 accelerates microglia-mediated Abeta oligomer formation and progression of neurocognitive dysfunction. PLoS One. 2009;4(7):e6197. doi: 10.1371/journal.pone.0006197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Semple B.D., Frugier T., Morganti-Kossmann M.C. CCL2 modulates cytokine production in cultured mouse astrocytes. J. Neuroinflammation. 2010;7:67. doi: 10.1186/1742-2094-7-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.El Khoury J., Toft M., Hickman S.E., Means T.K., Terada K., Geula C., Luster A.D. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat. Med. 2007;13(4):432–438. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- 131.Van Opdenbosch N., Lamkanfi M. Caspases in cell death, inflammation, and disease. Immunity. 2019;50(6):1352–1364. doi: 10.1016/j.immuni.2019.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Venero J.L., Burguillos M.A., Joseph B. Caspases playing in the field of neuroinflammation: Old and new players. Proc. Devel. Neurosci. 2013;35:88–101. doi: 10.1159/000346155. [DOI] [PubMed] [Google Scholar]
- 133.Schroder K., Tschopp J. The inflammasomes. Cell. 2010;140(6):821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 134.van de Veerdonk F.L., Netea M.G., Dinarello C.A., Joosten L.A.B. Inflammasome activation and IL-1β and IL-18 processing during infection. Trends Immunol. 2011;32(3):110–116. doi: 10.1016/j.it.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 135.Halle A., Hornung V., Petzold G.C., Stewart C.R., Monks B.G., Reinheckel T., Fitzgerald K.A., Latz E., Moore K.J., Golenbock D.T. The NALP3 inflammasome is involved in the innate immune response to amyloid-β. Nat. Immunol. 2008;9(8):857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Heneka M.T., Kummer M.P., Stutz A., Delekate A., Schwartz S., Vieira-Saecker A., Griep A., Axt D., Remus A., Tzeng T-C., Gelpi E., Halle A., Korte M., Latz E., Golenbock D.T. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493(7434):674–678. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fricker M., Vilalta A., Tolkovsky A.M., Brown G.C. Caspase inhibitors protect neurons by enabling selective necroptosis of inflamed microglia. J. Biol. Chem. 2013;288(13):9145–9152. doi: 10.1074/jbc.M112.427880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Burguillos M.A., Deierborg T., Kavanagh E., Persson A., Hajji N., Garcia-Quintanilla A., Cano J., Brundin P., Englund E., Venero J.L., Joseph B. Caspase signalling controls microglia activation and neurotoxicity. Nature. 2011;472(7343):319–324. doi: 10.1038/nature09788. [DOI] [PubMed] [Google Scholar]
- 139.Rohn T.T., Kokoulina P., Eaton C.R., Poon W.W. Caspase activation in transgenic mice with Alzheimer-like pathology: results from a pilot study utilizing the caspase inhibitor, Q-VD-OPh. Int. J. Clin. Exp. Med. 2009;2(4):300–308. [PMC free article] [PubMed] [Google Scholar]
- 140.Biscaro B., Lindvall O., Tesco G., Ekdahl C.T., Nitsch R.M. Inhibition of microglial activation protects hippocampal neurogenesis and improves cognitive deficits in a transgenic mouse model for Alzheimer’s disease. Neurodegener. Dis. 2012;9(4):187–198. doi: 10.1159/000330363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ricciotti E., FitzGerald G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011;31(5):986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Choi S-H., Aid S., Caracciolo L., Minami S.S., Niikura T., Matsuoka Y., Turner R.S., Mattson M.P., Bosetti F. Cyclooxygenase-1 inhibition reduces amyloid pathology and improves memory deficits in a mouse model of Alzheimer’s disease. J. Neurochem. 2013;124(1):59–68. doi: 10.1111/jnc.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Montine T.J., Sidell K.R., Crews B.C., Markesbery W.R., Marnett L.J., Roberts L.J., II, Morrow J.D. Elevated CSF prostaglandin E2 levels in patients with probable AD. Neurology. 1999;53(7):1495–1498. doi: 10.1212/WNL.53.7.1495. [DOI] [PubMed] [Google Scholar]
- 144.Slawik H., Volk B., Fiebich B., Hüll M. Microglial expression of prostaglandin EP3 receptor in excitotoxic lesions in the rat striatum. Neurochem. Int. 2004;45(5):653–660. doi: 10.1016/j.neuint.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 145.Shie F-S., Montine K.S., Breyer R.M., Montine T.J. Microglial EP2 as a new target to increase amyloid beta phagocytosis and decrease amyloid beta-induced damage to neurons. Brain Pathol. 2005;15(2):134–138. doi: 10.1111/j.1750-3639.2005.tb00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liang X., Wang Q., Hand T., Wu L., Breyer R.M., Montine T.J., Andreasson K. Deletion of the prostaglandin E2 EP2 receptor reduces oxidative damage and amyloid burden in a model of Alzheimer’s disease. J. Neurosci. 2005;25(44):10180–10187. doi: 10.1523/JNEUROSCI.3591-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shi J., Wang Q., Johansson J.U., Liang X., Woodling N.S., Priyam P., Loui T.M., Merchant M., Breyer R.M., Montine T.J., Andreasson K. Inflammatory prostaglandin E2 signaling in a mouse model of Alzheimer disease. Ann. Neurol. 2012;72(5):788–798. doi: 10.1002/ana.23677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xiang Z., Ho L., Yemul S., Zhao Z., Qing W., Pompl P., Kelley K., Dang A., Qing W., Teplow D., Pasinetti G.M. Cyclooxygenase-2 promotes amyloid plaque deposition in a mouse model of Alzheimer’s disease neuropathology. Gene Expr. 2002;10(5-6):271–278. doi: 10.3727/000000002783992352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Woodling N.S., Wang Q., Priyam P.G., Larkin P., Shi J., Johansson J.U., Zagol-Ikapitte I., Boutaud O., Andreasson K.I. Suppression of Alzheimer-associated inflammation by microglial prostaglandin-E2 EP4 receptor signaling. J. Neurosci. 2014;34(17):5882–5894. doi: 10.1523/JNEUROSCI.0410-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bazan N.G., Molina M.F., Gordon W.C. Docosahexaenoic acid signalolipidomics in nutrition: significance in aging, neuroinflammation, macular degeneration, Alzheimer’s, and other neurodegenerative diseases. Annu. Rev. Nutr. 2011;31:321–351. doi: 10.1146/annurev.nutr.012809.104635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lukiw W.J., Cui J-G., Marcheselli V.L., Bodker M., Botkjaer A., Gotlinger K., Serhan C.N., Bazan N.G. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J. Clin. Invest. 2005;115(10):2774–2783. doi: 10.1172/JCI25420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bazan N.G. Neuroprotectin D1-mediated anti-inflammatory and survival signaling in stroke, retinal degenerations, and Alzheimer’s disease. J. Lipid Res. 2009;50(Suppl.):S400–S405. doi: 10.1194/jlr.R800068-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhao Y., Calon F., Julien C., Winkler J.W., Petasis N.A., Lukiw W.J., Bazan N.G. Docosahexaenoic acid-derived neuroprotectin D1 induces neuronal survival via secretase- and PPARγ-mediated mechanisms in Alzheimer’s disease models. PLoS One. 2011;6(1):e15816. doi: 10.1371/journal.pone.0015816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Alawieh A., Langley E.F., Weber S., Adkins D., Tomlinson S. Identifying the role of complement in triggering neuroinflammation after traumatic brain injury. J. Neurosci. 2018;38(10):2519–2532. doi: 10.1523/JNEUROSCI.2197-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dunkelberger J.R., Song W.C. Complement and its role in innate and adaptive immune responses. Cell Res. 2010;20(1):34–50. doi: 10.1038/cr.2009.139. [DOI] [PubMed] [Google Scholar]
- 156.Veerhuis R., Nielsen H.M., Tenner A.J. Complement in the brain. Mol. Immunol. 2011;48(14):1592–1603. doi: 10.1016/j.molimm.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Strohmeyer R., Ramirez M., Cole G.J., Mueller K., Rogers J. Association of factor H of the alternative pathway of complement with agrin and complement receptor 3 in the Alzheimer’s disease brain. J. Neuroimmunol. 2002;131(1-2):135–146. doi: 10.1016/S0165-5728(02)00272-2. [DOI] [PubMed] [Google Scholar]
- 158.Yakupova E.I., Bobyleva L.G., Vikhlyantsev I.M., Bobylev A.G. Complement system activation by amyloid aggregates of Aβ(1-40) and Aβ(1-42) peptides: facts and hypotheses. Biophys. Russian Fed. 2020;65:18–21. [Google Scholar]
- 159.Emmerling M.R., Watson M.D., Raby C.A., Spiegel K. The role of complement in Alzheimer’s disease pathology. Biochim. Biophys. Acta. 2000;1502(1):158–171. doi: 10.1016/S0925-4439(00)00042-9. [DOI] [PubMed] [Google Scholar]
- 160.Lambert J-C., Heath S., Even G., Campion D., Sleegers K., Hiltunen M., Combarros O., Zelenika D., Bullido M.J., Tavernier B., Letenneur L., Bettens K., Berr C., Pasquier F., Fiévet N., Barberger-Gateau P., Engelborghs S., De Deyn P., Mateo I., Franck A., Helisalmi S., Porcellini E., Hanon O., de Pancorbo M.M., Lendon C., Dufouil C., Jaillard C., Leveillard T., Alvarez V., Bosco P., Mancuso M., Panza F., Nacmias B., Bossù P., Piccardi P., Annoni G., Seripa D., Galimberti D., Hannequin D., Licastro F., Soininen H., Ritchie K., Blanché H., Dartigues J-F., Tzourio C., Gut I., Van Broeckhoven C., Alpérovitch A., Lathrop M., Amouyel P., Amouyel P. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat. Genet. 2009;41(10):1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- 161.Harold D., Abraham R., Hollingworth P., Sims R., Gerrish A., Hamshere M.L., Pahwa J.S., Moskvina V., Dowzell K., Williams A., Jones N., Thomas C., Stretton A., Morgan A.R., Lovestone S., Powell J., Proitsi P., Lupton M.K., Brayne C., Rubinsztein D.C., Gill M., Lawlor B., Lynch A., Morgan K., Brown K.S., Passmore P.A., Craig D., McGuinness B., Todd S., Holmes C., Mann D., Smith A.D., Love S., Kehoe P.G., Hardy J., Mead S., Fox N., Rossor M., Collinge J., Maier W., Jessen F., Schürmann B., Heun R., van den Bussche H., Heuser I., Kornhuber J., Wiltfang J., Dichgans M., Frölich L., Hampel H., Hüll M., Rujescu D., Goate A.M., Kauwe J.S.K., Cruchaga C., Nowotny P., Morris J.C., Mayo K., Sleegers K., Bettens K., Engelborghs S., De Deyn P.P., Van Broeckhoven C., Livingston G., Bass N.J., Gurling H., McQuillin A., Gwilliam R., Deloukas P., Al-Chalabi A., Shaw C.E., Tsolaki M., Singleton A.B., Guerreiro R., Mühleisen T.W., Nöthen M.M., Moebus S., Jöckel K-H., Klopp N., Wichmann H-E., Carrasquillo M.M., Pankratz V.S., Younkin S.G., Holmans P.A., O’Donovan M., Owen M.J., Williams J. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat. Genet. 2009;41(10):1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Uddin M.S., Kabir M.T., Begum M.M., Islam M.S., Behl T., Ashraf M.G.M. Exploring the role of clu in the pathogenesis of Alzheimer’s disease. Neurotox. Res. 2020;38(4):1062. doi: 10.1007/s12640-020-00279-w. [DOI] [PubMed] [Google Scholar]
- 163.Cheignon C., Tomas M., Bonnefont-Rousselot D., Faller P., Hureau C., Collin F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018;14:450–464. doi: 10.1016/j.redox.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Uddin M.S., Upaganlawar A.B. Oxidative stress and antioxidant defense: Biomedical value in health and diseases. USA: Nova Science Publishers; 2019. [Google Scholar]
- 165.Jones S.V., Kounatidis I. Nuclear factor-kappa b and alzheimer disease, unifying genetic and environmental risk factors from cell to humans. Front. Immunol. 2017;8:1805. doi: 10.3389/fimmu.2017.01805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tilstra J.S., Clauson C.L., Niedernhofer L.J., Robbins P.D. NF-κB in Aging and Disease. Aging Dis. 2011;2(6):449–465. [PMC free article] [PubMed] [Google Scholar]
- 167.Thakurta I.G., Chattopadhyay M., Ghosh A., Chakrabarti S. Dietary supplementation with N-acetyl cysteine, α-tocopherol and α-lipoic acid reduces the extent of oxidative stress and proinflammatory state in aged rat brain. Biogerontology. 2012;13(5):479–488. doi: 10.1007/s10522-012-9392-5. [DOI] [PubMed] [Google Scholar]
- 168.Morgan M.J., Liu Z.G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011;21(1):103–115. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kaltschmidt B., Uherek M., Volk B., Baeuerle P.A., Kaltschmidt C. Transcription factor NF-kappaB is activated in primary neurons by amyloid beta peptides and in neurons surrounding early plaques from patients with Alzheimer disease. Proc. Natl. Acad. Sci. USA. 1997;94(6):2642–2647. doi: 10.1073/pnas.94.6.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kitamura Y., Shimohama S., Ota T., Matsuoka Y., Nomura Y., Taniguchi T. Alteration of transcription factors NF-kappaB and STAT1 in Alzheimer’s disease brains. Neurosci. Lett. 1997;237(1):17–20. doi: 10.1016/S0304-3940(97)00797-0. [DOI] [PubMed] [Google Scholar]
- 171.Terai K., Matsuo A., McGeer P.L. Enhancement of immunoreactivity for NF-kappa B in the hippocampal formation and cerebral cortex of Alzheimer’s disease. Brain Res. 1996;735(1):159–168. doi: 10.1016/0006-8993(96)00310-1. [DOI] [PubMed] [Google Scholar]
- 172.Cai Z., Zhao B., Ratka A. Oxidative stress and β-amyloid protein in Alzheimer’s disease. Neuromol. Med. 2011;13(4):223–250. doi: 10.1007/s12017-011-8155-9. [DOI] [PubMed] [Google Scholar]
- 173.Sivandzade F., Prasad S., Bhalerao A., Cucullo L. NRF2 and NF-қB interplay in cerebrovascular and neurodegenerative disorders: Molecular mechanisms and possible therapeutic approaches. Redox Biol. 2019;21:101059. doi: 10.1016/j.redox.2018.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Carrero I., Gonzalo M.R., Martin B., Sanz-Anquela J.M., Arévalo-Serrano J., Gonzalo-Ruiz A. Oligomers of β-amyloid protein (Aβ1-42) induce the activation of cyclooxygenase-2 in astrocytes via an interaction with interleukin-1β, tumour necrosis factor-α, and a nuclear factor κ-B mechanism in the rat brain. Exp. Neurol. 2012;236(2):215–227. doi: 10.1016/j.expneurol.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 175.Chen J., Zhou Y., Mueller-Steiner S., Chen L-F., Kwon H., Yi S., Mucke L., Gan L. SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J. Biol. Chem. 2005;280(48):40364–40374. doi: 10.1074/jbc.M509329200. [DOI] [PubMed] [Google Scholar]
- 176.Chen X-F., Zhang Y.W., Xu H., Bu G. Transcriptional regulation and its misregulation in Alzheimer’s disease. Mol. Brain. 2013;6:44. doi: 10.1186/1756-6606-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Vodovotz Y., Lucia M.S., Flanders K.C., Chesler L., Xie Q.W., Smith T.W., Weidner J., Mumford R., Webber R., Nathan C., Roberts A.B., Lippa C.F., Sporn M.B. Inducible nitric oxide synthase in tangle-bearing neurons of patients with Alzheimer’s disease. J. Exp. Med. 1996;184(4):1425–1433. doi: 10.1084/jem.184.4.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Nathan C., Calingasan N., Nezezon J., Ding A., Lucia M.S., La Perle K., Fuortes M., Lin M., Ehrt S., Kwon N.S., Chen J., Vodovotz Y., Kipiani K., Beal M.F. Protection from Alzheimer’s-like disease in the mouse by genetic ablation of inducible nitric oxide synthase. J. Exp. Med. 2005;202(9):1163–1169. doi: 10.1084/jem.20051529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ansari M.A., Scheff S.W. NADPH-oxidase activation and cognition in Alzheimer disease progression. Free Radic. Biol. Med. 2011;51(1):171–178. doi: 10.1016/j.freeradbiomed.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Jekabsone A., Mander P.K., Tickler A., Sharpe M., Brown G.C. Fibrillar beta-amyloid peptide Abeta1-40 activates microglial proliferation via stimulating TNF-alpha release and H2O2 derived from NADPH oxidase: a cell culture study. J. Neuroinflammation. 2006;3:24. doi: 10.1186/1742-2094-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Choi S-H., Aid S., Kim H-W., Jackson S.H., Bosetti F. Inhibition of NADPH oxidase promotes alternative and anti-inflammatory microglial activation during neuroinflammation. J. Neurochem. 2012;120(2):292–301. doi: 10.1111/j.1471-4159.2011.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mander P., Brown G.C. Activation of microglial NADPH oxidase is synergistic with glial iNOS expression in inducing neuronal death: a dual-key mechanism of inflammatory neurodegeneration. J. Neuroinflammation. 2005;2:20. doi: 10.1186/1742-2094-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Butterfield D.A., Reed T.T., Perluigi M., De Marco C., Coccia R., Keller J.N., Markesbery W.R., Sultana R. Elevated levels of 3-nitrotyrosine in brain from subjects with amnestic mild cognitive impairment: implications for the role of nitration in the progression of Alzheimer’s disease. Brain Res. 2007;1148:243–248. doi: 10.1016/j.brainres.2007.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Cho D.-H., Nakamura T., Fang J., Cieplak P., Godzik A., Gu Z., Lipton S.A. S-nitrosylation of drp1 mediates β-amyloid-related mitochondrial fission and neuronal injury. Science (80-. ), 2009, 324, 102-105. [DOI] [PMC free article] [PubMed]
- 185.Kummer M.P., Hermes M., Delekarte A., Hammerschmidt T., Kumar S., Terwel D., Walter J., Pape H.C., König S., Roeber S., Jessen F., Klockgether T., Korte M., Heneka M.T. Nitration of tyrosine 10 critically enhances amyloid β aggregation and plaque formation. Neuron. 2011;71(5):833–844. doi: 10.1016/j.neuron.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 186.Thiabaud G., Pizzocaro S., Garcia-Serres R., Latour J-M., Monzani E., Casella L. Heme binding induces dimerization and nitration of truncated β-amyloid peptide Aβ16 under oxidative stress. Angew. Chem. Int. Ed. Engl. 2013;52(31):8041–8044. doi: 10.1002/anie.201302989. [DOI] [PubMed] [Google Scholar]
- 187.Rius-Pérez S., Tormos A.M., Pérez S., Taléns-Visconti R. Vascular pathology: Cause or effect in Alzheimer disease? Neurologia (Engl Ed) 2018;33(2):112–120. doi: 10.1016/j.nrleng.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 188.Attems J., Jellinger K.A. The overlap between vascular disease and Alzheimer’s disease--lessons from pathology. BMC Med. 2014;12:206. doi: 10.1186/s12916-014-0206-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat. Rev. Neurosci. 2004;5(5):347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 190.Shin H.K., Jones P.B., Garcia-Alloza M., Borrelli L., Greenberg S.M., Bacskai B.J., Frosch M.P., Hyman B.T., Moskowitz M.A., Ayata C. Age-dependent cerebrovascular dysfunction in a transgenic mouse model of cerebral amyloid angiopathy. Brain. 2007;130(Pt 9):2310–2319. doi: 10.1093/brain/awm156. [DOI] [PubMed] [Google Scholar]
- 191.Petzold G.C., Murthy V.N. Role of astrocytes in neurovascular coupling. Neuron. 2011;71(5):782–797. doi: 10.1016/j.neuron.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 192.Jarre A., Gowert N.S., Donner L., Münzer P., Klier M., Borst O., Schaller M., Lang F., Korth C., Elvers M. Pre-activated blood platelets and a pro-thrombotic phenotype in APP23 mice modeling Alzheimer’s disease. Cell. Signal. 2014;26(9):2040–2050. doi: 10.1016/j.cellsig.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 193.Deane R., Du Yan S., Submamaryan R.K., LaRue B., Jovanovic S., Hogg E., Welch D., Manness L., Lin C., Yu J., Zhu H., Ghiso J., Frangione B., Stern A., Schmidt A.M., Armstrong D.L., Arnold B., Liliensiek B., Nawroth P., Hofman F., Kindy M., Stern D., Zlokovic B. RAGE mediates amyloid-β peptide transport across the blood-brain barrier and accumulation in brain. Nat. Med. 2003;9(7):907–913. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- 194.Zlokovic B.V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 2011;12(12):723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Aguzzi A., Barres B.A., Bennett M.L. Microglia: Scapegoat, saboteur, or something else? Science (80- ), 2013, 339, 156-161. [DOI] [PMC free article] [PubMed]
- 196.Butovsky O., Jedrychowski M.P., Moore C.S., Cialic R., Lanser A.J., Gabriely G., Koeglsperger T., Dake B., Wu P.M., Doykan C.E., Fanek Z., Liu L., Chen Z., Rothstein J.D., Ransohoff R.M., Gygi S.P., Antel J.P., Weiner H.L. Identification of a unique TGF-β-dependent molecular and functional signature in microglia. Nat. Neurosci. 2014;17(1):131–143. doi: 10.1038/nn.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lambert J-C., Ibrahim-Verbaas C.A., Harold D., Naj A.C., Sims R., Bellenguez C., DeStafano A.L., Bis J.C., Beecham G.W., Grenier-Boley B., Russo G., Thorton-Wells T.A., Jones N., Smith A.V., Chouraki V., Thomas C., Ikram M.A., Zelenika D., Vardarajan B.N., Kamatani Y., Lin C.F., Gerrish A., Schmidt H., Kunkle B., Dunstan M.L., Ruiz A., Bihoreau M.T., Choi S.H., Reitz C., Pasquier F., Cruchaga C., Craig D., Amin N., Berr C., Lopez O.L., De Jager P.L., Deramecourt V., Johnston J.A., Evans D., Lovestone S., Letenneur L., Morón F.J., Rubinsztein D.C., Eiriksdottir G., Sleegers K., Goate A.M., Fiévet N., Huentelman M.W., Gill M., Brown K., Kamboh M.I., Keller L., Barberger-Gateau P., McGuiness B., Larson E.B., Green R., Myers A.J., Dufouil C., Todd S., Wallon D., Love S., Rogaeva E., Gallacher J., St George-Hyslop P., Clarimon J., Lleo A., Bayer A., Tsuang D.W., Yu L., Tsolaki M., Bossù P., Spalletta G., Proitsi P., Collinge J., Sorbi S., Sanchez-Garcia F., Fox N.C., Hardy J., Deniz Naranjo M.C., Bosco P., Clarke R., Brayne C., Galimberti D., Mancuso M., Matthews F., Moebus S., Mecocci P., Del Zompo M., Maier W., Hampel H., Pilotto A., Bullido M., Panza F., Caffarra P., Nacmias B., Gilbert J.R., Mayhaus M., Lannefelt L., Hakonarson H., Pichler S., Carrasquillo M.M., Ingelsson M., Beekly D., Alvarez V., Zou F., Valladares O., Younkin S.G., Coto E., Hamilton-Nelson K.L., Gu W., Razquin C., Pastor P., Mateo I., Owen M.J., Faber K.M., Jonsson P.V., Combarros O., O’Donovan M.C., Cantwell L.B., Soininen H., Blacker D., Mead S., Mosley T.H., Jr, Bennett D.A., Harris T.B., Fratiglioni L., Holmes C., de Bruijn R.F., Passmore P., Montine T.J., Bettens K., Rotter J.I., Brice A., Morgan K., Foroud T.M., Kukull W.A., Hannequin D., Powell J.F., Nalls M.A., Ritchie K., Lunetta K.L., Kauwe J.S., Boerwinkle E., Riemenschneider M., Boada M., Hiltuenen M., Martin E.R., Schmidt R., Rujescu D., Wang L.S., Dartigues J.F., Mayeux R., Tzourio C., Hofman A., Nöthen M.M., Graff C., Psaty B.M., Jones L., Haines J.L., Holmans P.A., Lathrop M., Pericak-Vance M.A., Launer L.J., Farrer L.A., van Duijn C.M., Van Broeckhoven C., Moskvina V., Seshadri S., Williams J., Schellenberg G.D., Amouyel P., Lathrop M., Pericak-Vance M.A., Launer L.J., Farrer L.A., van Duijn C.M., Van Broeckhoven C., Moskvina V., Seshadri S., Williams J., Schellenberg G.D., Amouyel P. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat. Genet. 2013;45(12):1452–1458. doi: 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Friedman B.A., Srinivasan K., Ayalon G., Meilandt W.J., Lin H., Huntley M.A., Cao Y., Lee S.H., Haddick P.C.G., Ngu H., Modrusan Z., Larson J.L., Kaminker J.S., van der Brug M.P., Hansen D.V. Diverse brain myeloid expression profiles reveal distinct microglial activation states and aspects of Alzheimer’s disease not evident in mouse models. Cell Rep. 2018;22(3):832–847. doi: 10.1016/j.celrep.2017.12.066. [DOI] [PubMed] [Google Scholar]
- 199.Keren-Shaul H., Spinrad A., Weiner A., Matcovitch-Natan O., Dvir-Szternfeld R., Ulland T.K., David E., Baruch K., Lara-Astaiso D., Toth B., Itzkovitz S., Colonna M., Schwartz M., Amit I. A unique microglia type associated with restricting development of alzheimer’s disease. Cell. 2017;169(7):1276–1290.e17. doi: 10.1016/j.cell.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 200.Zhao Y., Wu X., Li X., Jiang L.L., Gui X., Liu Y., Sun Y., Zhu B., Piña-Crespo J.C., Zhang M., Zhang N., Chen X., Bu G., An Z., Huang T.Y., Xu H. TREM2 is a receptor for β-amyloid that mediates microglial function. Neuron. 2018;97(5):1023–1031.e7. doi: 10.1016/j.neuron.2018.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhong L., Wang Z., Wang D., Wang Z., Martens Y.A., Wu L., Xu Y., Wang K., Li J., Huang R., Can D., Xu H., Bu G., Chen X.F. Amyloid-beta modulates microglial responses by binding to the triggering receptor expressed on myeloid cells 2 (TREM2). Mol. Neurodegener. 2018;13(1):15. doi: 10.1186/s13024-018-0247-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ahyayauch H., Raab M., Busto J.V., Andraka N., Arrondo J.R., Masserini M., Tvaroska I., Goñi F.M. Binding of β-amyloid (1-42) peptide to negatively charged phospholipid membranes in the liquid-ordered state: modeling and experimental studies. Biophys. J. 2012;103(3):453–463. doi: 10.1016/j.bpj.2012.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Nagarathinam A., Höflinger P., Bühler A., Schäfer C., McGovern G., Jeffrey M., Staufenbiel M., Jucker M., Baumann F. Membrane-anchored Aβ accelerates amyloid formation and exacerbates amyloid-associated toxicity in mice. J. Neurosci. 2013;33(49):19284–19294. doi: 10.1523/JNEUROSCI.2542-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wang Y., Cella M., Mallinson K., Ulrich J.D., Young K.L., Robinette M.L., Gilfillan S., Krishnan G.M., Sudhakar S., Zinselmeyer B.H., Holtzman D.M., Cirrito J.R., Colonna M. TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell. 2015;160(6):1061–1071. doi: 10.1016/j.cell.2015.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Song W., Hooli B., Mullin K., Jin S.C., Cella M., Ulland T.K., Wang Y., Tanzi R.E., Colonna M. Alzheimer’s disease-associated TREM2 variants exhibit either decreased or increased ligand-dependent activation. Alzheimers Dement. 2017;13(4):381–387. doi: 10.1016/j.jalz.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Terwel D., Steffensen K.R., Verghese P.B., Kummer M.P., Gustafsson J.Å., Holtzman D.M., Heneka M.T. Critical role of astroglial apolipoprotein E and liver X receptor-α expression for microglial Aβ phagocytosis. J. Neurosci. 2011;31(19):7049–7059. doi: 10.1523/JNEUROSCI.6546-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Yeh F.L., Wang Y., Tom I., Gonzalez L.C., Sheng M. TREM2 binds to apolipoproteins, including apoe and clu/apoj, and thereby facilitates uptake of amyloid-beta by microglia. Neuron. 2016;91(2):328–340. doi: 10.1016/j.neuron.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 208.Song W.M., Joshita S., Zhou Y., Ulland T.K., Gilfillan S., Colonna M. Humanized TREM2 mice reveal microglia-intrinsic and -extrinsic effects of R47H polymorphism. J. Exp. Med. 2018;215(3):745–760. doi: 10.1084/jem.20171529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Guerreiro R., Wojtas A., Bras J., Carrasquillo M., Rogaeva E., Majounie E., Cruchaga C., Sassi C., Kauwe J.S.K., Younkin S., Hazrati L., Collinge J., Pocock J., Lashley T., Williams J., Lambert J-C., Amouyel P., Goate A., Rademakers R., Morgan K., Powell J., St George-Hyslop P., Singleton A., Hardy J. TREM2 variants in Alzheimer’s disease. N. Engl. J. Med. 2013;368(2):117–127. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Jonsson T., Stefansson H., Steinberg S., Jonsdottir I., Jonsson P.V., Snaedal J., Bjornsson S., Huttenlocher J., Levey A.I., Lah J.J., Rujescu D., Hampel H., Giegling I., Andreassen O.A., Engedal K., Ulstein I., Djurovic S., Ibrahim-Verbaas C., Hofman A., Ikram M.A., van Duijn C.M., Thorsteinsdottir U., Kong A., Stefansson K. Variant of TREM2 associated with the risk of Alzheimer’s disease. N. Engl. J. Med. 2013;368(2):107–116. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Mori Y., Yoshino Y., Ochi S., Yamazaki K., Kawabe K., Abe M., Kitano T., Ozaki Y., Yoshida T., Numata S., Mori T., Iga J., Kuroda N., Ohmori T., Ueno S. TREM2 mrna expression in leukocytes is increased in Alzheimer’s disease and schizophrenia. PLoS One. 2015;10(9):e0136835. doi: 10.1371/journal.pone.0136835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Paloneva J., Kestilä M., Wu J., Salminen A., Böhling T., Ruotsalainen V., Hakola P., Bakker A.B.H., Phillips J.H., Pekkarinen P., Lanier L.L., Timonen T., Peltonen L. Loss-of-function mutations in TYROBP (DAP12) result in a presenile dementia with bone cysts. Nat. Genet. 2000;25(3):357–361. doi: 10.1038/77153. [DOI] [PubMed] [Google Scholar]
- 213.Liu Z., Condello C., Schain A., Harb R., Grutzendler J. CX3CR1 in microglia regulates brain amyloid deposition through selective protofibrillar amyloid-β phagocytosis. J. Neurosci. 2010;30(50):17091–17101. doi: 10.1523/JNEUROSCI.4403-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Bhaskar K., Konerth M., Kokiko-Cochran O.N., Cardona A., Ransohoff R.M., Lamb B.T. Regulation of tau pathology by the microglial fractalkine receptor. Neuron. 2010;68(1):19–31. doi: 10.1016/j.neuron.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Jiang T., Zhang Y.D., Chen Q., Gao Q., Zhu X.C., Zhou J.S., Shi J.Q., Lu H., Tan L., Yu J.T. TREM2 modifies microglial phenotype and provides neuroprotection in P301S tau transgenic mice. Neuropharmacology. 2016;105:196–206. doi: 10.1016/j.neuropharm.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 216.Jay T.R., Hirsch A.M., Broihier M.L., Miller C.M., Neilson L.E., Ransohoff R.M., Lamb B.T., Landreth G.E. Disease progression-dependent effects of trem2 deficiency in a mouse model of Alzheimer’s disease. J. Neurosci. 2017;37(3):637–647. doi: 10.1523/JNEUROSCI.2110-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zhang B., Gaiteri C., Bodea L-G., Wang Z., McElwee J., Podtelezhnikov A.A., Zhang C., Xie T., Tran L., Dobrin R., Fluder E., Clurman B., Melquist S., Narayanan M., Suver C., Shah H., Mahajan M., Gillis T., Mysore J., MacDonald M.E., Lamb J.R., Bennett D.A., Molony C., Stone D.J., Gudnason V., Myers A.J., Schadt E.E., Neumann H., Zhu J., Emilsson V. Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer’s disease. Cell. 2013;153(3):707–720. doi: 10.1016/j.cell.2013.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Tarkowski E., Andreasen N., Tarkowski A., Blennow K. Intrathecal inflammation precedes development of Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry. 2003;74(9):1200–1205. doi: 10.1136/jnnp.74.9.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Brosseron F., Krauthausen M., Kummer M., Heneka M.T. Body fluid cytokine levels in mild cognitive impairment and Alzheimer’s disease: A comparative overview. Mol. Neurobiol. 2014;50(2):534–544. doi: 10.1007/s12035-014-8657-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Krstic D., Madhusudan A., Doehner J., Vogel P., Notter T., Imhof C., Manalastas A., Hilfiker M., Pfister S., Schwerdel C., Riether C., Meyer U., Knuesel I. Systemic immune challenges trigger and drive Alzheimer-like neuropathology in mice. J. Neuroinflammation. 2012;9:151. doi: 10.1186/1742-2094-9-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Gandy S., Heppner F.L. Microglia as dynamic and essential components of the amyloid hypothesis. Neuron. 2013;78(4):575–577. doi: 10.1016/j.neuron.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Perry V.H., Holmes C. Microglial priming in neurodegenerative disease. Nat. Rev. Neurol. 2014;10(4):217–224. doi: 10.1038/nrneurol.2014.38. [DOI] [PubMed] [Google Scholar]
- 223.Heneka M.T., Kummer M.P., Latz E. Innate immune activation in neurodegenerative disease. Nat. Rev. Immunol. 2014;14(7):463–477. doi: 10.1038/nri3705. [DOI] [PubMed] [Google Scholar]
- 224.Cunningham C. Microglia and neurodegeneration: the role of systemic inflammation. Glia. 2013;61(1):71–90. doi: 10.1002/glia.22350. [DOI] [PubMed] [Google Scholar]
- 225.Sudduth T.L., Schmitt F.A., Nelson P.T., Wilcock D.M. Neuroinflammatory phenotype in early Alzheimer’s disease. Neurobiol. Aging. 2013;34(4):1051–1059. doi: 10.1016/j.neurobiolaging.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Krstic D., Knuesel I. Deciphering the mechanism underlying late-onset Alzheimer disease. Nat. Rev. Neurol. 2013;9(1):25–34. doi: 10.1038/nrneurol.2012.236. [DOI] [PubMed] [Google Scholar]
- 227.Hickman S.E., El Khoury J. TREM2 and the neuroimmunology of Alzheimer’s disease. Biochem. Pharmacol. 2014;88(4):495–498. doi: 10.1016/j.bcp.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Perry V.H. Contribution of systemic inflammation to chronic neurodegeneration. Acta Neuropathol. 2010;120(3):277–286. doi: 10.1007/s00401-010-0722-x. [DOI] [PubMed] [Google Scholar]
- 229.Holmes C., Cunningham C., Zotova E., Woolford J., Dean C., Kerr S., Culliford D., Perry V.H. Systemic inflammation and disease progression in Alzheimer disease. Neurology. 2009;73(10):768–774. doi: 10.1212/WNL.0b013e3181b6bb95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Cribbs D.H., Berchtold N.C., Perreau V., Coleman P.D., Rogers J., Tenner A.J., Cotman C.W. Extensive innate immune gene activation accompanies brain aging, increasing vulnerability to cognitive decline and neurodegeneration: a microarray study. J. Neuroinflammation. 2012;9:179. doi: 10.1186/1742-2094-9-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Schwartz M., Shechter R. Systemic inflammatory cells fight off neurodegenerative disease. Nat. Rev. Neurol. 2010;6(7):405–410. doi: 10.1038/nrneurol.2010.71. [DOI] [PubMed] [Google Scholar]
- 232.Lo A.W., Ho C., Cummings J., Kosik K.S. Parallel discovery of Alzheimer’s therapeutics. Sci. Transl. Med. 2014;6(241):241cm5. doi: 10.1126/scitranslmed.3008228. [DOI] [PubMed] [Google Scholar]
- 233.Vom Berg J., Prokop S., Miller K.R., Obst J., Kälin R.E., Lopategui-Cabezas I., Wegner A., Mair F., Schipke C.G., Peters O., Winter Y., Becher B., Heppner F.L. Inhibition of IL-12/IL-23 signaling reduces Alzheimer’s disease-like pathology and cognitive decline. Nat. Med. 2012;18(12):1812–1819. doi: 10.1038/nm.2965. [DOI] [PubMed] [Google Scholar]
- 234.Hu W.T., Holtzman D.M., Fagan A.M., Shaw L.M., Perrin R., Arnold S.E., Grossman M., Xiong C., Craig-Schapiro R., Clark C.M., Pickering E., Kuhn M., Chen Y., Van Deerlin V.M., McCluskey L., Elman L., Karlawish J., Chen-Plotkin A., Hurtig H.I., Siderowf A., Swenson F., Lee V.M.Y., Morris J.C., Trojanowski J.Q., Soares H. Plasma multianalyte profiling in mild cognitive impairment and Alzheimer disease. Neurology. 2012;79(9):897–905. doi: 10.1212/WNL.0b013e318266fa70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Tan M.S., Yu J.T., Jiang T., Zhu X.C., Guan H.S., Tan L. IL12/23 p40 inhibition ameliorates Alzheimer’s disease-associated neuropathology and spatial memory in SAMP8 mice. J. Alzheimers Dis. 2014;38(3):633–646. doi: 10.3233/JAD-131148. [DOI] [PubMed] [Google Scholar]
- 236.Liu Y., Yu J.T., Zhang W., Zong Y., Lu R.C., Zhou J., Tan L. Interleukin-23 receptor polymorphisms are associated with Alzheimer’s disease in Han Chinese. J. Neuroimmunol. 2014;271(1-2):43–48. doi: 10.1016/j.jneuroim.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 237.Papassotiropoulos A., Bagli M., Jessen F., Bayer T.A., Maier W., Rao M.L., Heun R. A genetic variation of the inflammatory cytokine interleukin-6 delays the initial onset and reduces the risk for sporadic Alzheimer’s disease. Ann. Neurol. 1999;45(5):666–668. doi: 10.1002/1531-8249(199905)45:5<666:AID-ANA18>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 238.Cojocaru I.M., Cojocaru M., Miu G., Sapira V. Study of interleukin-6 production in Alzheimer’s disease. Rom. J. Intern. Med. 2011;49(1):55–58. [PubMed] [Google Scholar]
- 239.Ng A., Tam W.W., Zhang M.W., Ho C.S., Husain S.F., McIntyre R.S., Ho R.C. IL-1β, IL-6, TNF- α and CRP in Elderly Patients with Depression or Alzheimer’s disease: Systematic Review and Meta-Analysis. Sci. Rep. 2018;8(1):12050. doi: 10.1038/s41598-018-30487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Kleinberger G., Yamanishi Y., Suárez-Calvet M., Czirr E., Lohmann E., Cuyvers E., Struyfs H., Pettkus N., Wenninger-Weinzierl A., Mazaheri F., Tahirovic S., Lleó A., Alcolea D., Fortea J., Willem M., Lammich S., Molinuevo J.L., Sánchez-Valle R., Antonell A., Ramirez A., Heneka M.T., Sleegers K., van der Zee J., Martin J.J., Engelborghs S., Demirtas-Tatlidede A., Zetterberg H., Van Broeckhoven C., Gurvit H., Wyss-Coray T., Hardy J., Colonna M., Haass C. TREM2 mutations implicated in neurodegeneration impair cell surface transport and phagocytosis. Sci. Transl. Med. 2014;6(243):243ra86. doi: 10.1126/scitranslmed.3009093. [DOI] [PubMed] [Google Scholar]
- 241.Jay T.R., Miller C.M., Cheng P.J., Graham L.C., Bemiller S., Broihier M.L., Xu G., Margevicius D., Karlo J.C., Sousa G.L., Cotleur A.C., Butovsky O., Bekris L., Staugaitis S.M., Leverenz J.B., Pimplikar S.W., Landreth G.E., Howell G.R., Ransohoff R.M., Lamb B.T. TREM2 deficiency eliminates TREM2+ inflammatory macrophages and ameliorates pathology in Alzheimer’s disease mouse models. J. Exp. Med. 2015;212(3):287–295. doi: 10.1084/jem.20142322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Baleriola J., Walker C.A., Jean Y.Y., Crary J.F., Troy C.M., Nagy P.L., Hengst U. Axonally synthesized ATF4 transmits a neurodegenerative signal across brain regions. Cell. 2014;158(5):1159–1172. doi: 10.1016/j.cell.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Bradshaw E.M., Chibnik L.B., Keenan B.T., Ottoboni L., Raj T., Tang A., Rosenkrantz L.L., Imboywa S., Lee M., Von Korff A., Morris M.C., Evans D.A., Johnson K., Sperling R.A., Schneider J.A., Bennett D.A., De Jager P.L. CD33 Alzheimer’s disease locus: altered monocyte function and amyloid biology. Nat. Neurosci. 2013;16(7):848–850. doi: 10.1038/nn.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Naj A.C., Jun G., Beecham G.W., Wang L.S., Vardarajan B.N., Buros J., Gallins P.J., Buxbaum J.D., Jarvik G.P., Crane P.K., Larson E.B., Bird T.D., Boeve B.F., Graff-Radford N.R., De Jager P.L., Evans D., Schneider J.A., Carrasquillo M.M., Ertekin-Taner N., Younkin S.G., Cruchaga C., Kauwe J.S.K., Nowotny P., Kramer P., Hardy J., Huentelman M.J., Myers A.J., Barmada M.M., Demirci F.Y., Baldwin C.T., Green R.C., Rogaeva E., St George-Hyslop P., Arnold S.E., Barber R., Beach T., Bigio E.H., Bowen J.D., Boxer A., Burke J.R., Cairns N.J., Carlson C.S., Carney R.M., Carroll S.L., Chui H.C., Clark D.G., Corneveaux J., Cotman C.W., Cummings J.L., DeCarli C., DeKosky S.T., Diaz-Arrastia R., Dick M., Dickson D.W., Ellis W.G., Faber K.M., Fallon K.B., Farlow M.R., Ferris S., Frosch M.P., Galasko D.R., Ganguli M., Gearing M., Geschwind D.H., Ghetti B., Gilbert J.R., Gilman S., Giordani B., Glass J.D., Growdon J.H., Hamilton R.L., Harrell L.E., Head E., Honig L.S., Hulette C.M., Hyman B.T., Jicha G.A., Jin L.W., Johnson N., Karlawish J., Karydas A., Kaye J.A., Kim R., Koo E.H., Kowall N.W., Lah J.J., Levey A.I., Lieberman A.P., Lopez O.L., Mack W.J., Marson D.C., Martiniuk F., Mash D.C., Masliah E., McCormick W.C., McCurry S.M., McDavid A.N., McKee A.C., Mesulam M., Miller B.L., Miller C.A., Miller J.W., Parisi J.E., Perl D.P., Peskind E., Petersen R.C., Poon W.W., Quinn J.F., Rajbhandary R.A., Raskind M., Reisberg B., Ringman J.M., Roberson E.D., Rosenberg R.N., Sano M., Schneider L.S., Seeley W., Shelanski M.L., Slifer M.A., Smith C.D., Sonnen J.A., Spina S., Stern R.A., Tanzi R.E., Trojanowski J.Q., Troncoso J.C., Van Deerlin V.M., Vinters H.V., Vonsattel J.P., Weintraub S., Welsh-Bohmer K.A., Williamson J., Woltjer R.L., Cantwell L.B., Dombroski B.A., Beekly D., Lunetta K.L., Martin E.R., Kamboh M.I., Saykin A.J., Reiman E.M., Bennett D.A., Morris J.C., Montine T.J., Goate A.M., Blacker D., Tsuang D.W., Hakonarson H., Kukull W.A., Foroud T.M., Haines J.L., Mayeux R., Pericak-Vance M.A., Farrer L.A., Schellenberg G.D. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nat. Genet. 2011;43(5):436–441. doi: 10.1038/ng.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Thambisetty M., An Y., Nalls M., Sojkova J., Swaminathan S., Zhou Y., Singleton A.B., Wong D.F., Ferrucci L., Saykin A.J., Resnick S.M. Effect of complement CR1 on brain amyloid burden during aging and its modification by APOE genotype. Biol. Psychiatry. 2013;73(5):422–428. doi: 10.1016/j.biopsych.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Mahmoudi R., Feldman S., Kisserli A., Duret V., Tabary T., Bertholon L.A., Badr S., Nonnonhou V., Cesar A., Neuraz A., Novella J.L., Cohen J.H.M. Inherited and acquired decrease in complement receptor 1 (cr1) density on red blood cells associated with high levels of soluble cr1 in alzheimer’s disease. Int. J. Mol. Sci. 2018;19(8):19. doi: 10.3390/ijms19082175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Yamanaka M., Ishikawa T., Griep A., Axt D., Kummer M.P., Heneka M.T. PPARγ/RXRα-induced and CD36-mediated microglial amyloid-β phagocytosis results in cognitive improvement in amyloid precursor protein/presenilin 1 mice. J. Neurosci. 2012;32(48):17321–17331. doi: 10.1523/JNEUROSCI.1569-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Cramer P.E., Cirrito J.R., Wesson D.W., Lee C.Y.D., Karlo J.C., Zinn A.E., Casali B.T., Restivo J.L., Goebel W.D., James M.J., Brunden K.R., Wilson D.A., Landreth G.E. ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in ad mouse models.Science (80-.), 2012;335:1503–1506. doi: 10.1126/science.1217697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Sheedy F.J., Grebe A., Rayner K.J., Kalantari P., Ramkhelawon B., Carpenter S.B., Becker C.E., Ediriweera H.N., Mullick A.E., Golenbock D.T., Stuart L.M., Latz E., Fitzgerald K.A., Moore K.J. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat. Immunol. 2013;14(8):812–820. doi: 10.1038/ni.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Ries M., Sastre M. Mechanisms of aβ clearance and degradation by glial cells. Front. Aging Neurosci. 2016;8:160. doi: 10.3389/fnagi.2016.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Fassbender K., Walter S., Kühl S., Landmann R., Ishii K., Bertsch T., Stalder A.K., Muehlhauser F., Liu Y., Ulmer A.J., Rivest S., Lentschat A., Gulbins E., Jucker M., Staufenbiel M., Brechtel K., Walter J., Multhaup G., Penke B., Adachi Y., Hartmann T., Beyreuther K. The LPS receptor (CD14) links innate immunity with Alzheimer’s disease. FASEB J. 2004;18(1):203–205. doi: 10.1096/fj.03-0364fje. [DOI] [PubMed] [Google Scholar]
- 252.Reed-Geaghan E.G., Reed Q.W., Cramer P.E., Landreth G.E. Deletion of CD14 attenuates Alzheimer’s disease pathology by influencing the brain’s inflammatory milieu. J. Neurosci. 2010;30(46):15369–15373. doi: 10.1523/JNEUROSCI.2637-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Pase M.P., Himali J.J., Beiser A.S., DeCarli C., McGrath E.R., Satizabal C.L., Aparicio H.J., Adams H.H.H., Reiner A.P., Longstreth W.T., Jr, Fornage M., Tracy R.P., Lopez O., Psaty B.M., Levy D., Seshadri S., Bis J.C. Association of CD14 with incident dementia and markers of brain aging and injury. Neurology. 2020;94(3):e254–e266. doi: 10.1212/WNL.0000000000008682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Chen P., Zhao W., Guo Y., Xu J., Yin M. CX3CL1/CX3CR1 in alzheimer’s disease: A target for neuroprotection. Biomed Res. Int. 2016;2016:8090918. doi: 10.1155/2016/8090918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Nash K.R., Lee D.C., Hunt J.B., Jr, Morganti J.M., Selenica M.L., Moran P., Reid P., Brownlow M., Guang Yu Yang, C.; Savalia, M.; Gemma, C.; Bickford, P.C.; Gordon, M.N.; Morgan, D. Fractalkine overexpression suppresses tau pathology in a mouse model of tauopathy. Neurobiol. Aging. 2013;34(6):1540–1548. doi: 10.1016/j.neurobiolaging.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Hickman S.E., Allison E.K., Coleman U., Kingery-Gallagher N.D., El Khoury J. Heterozygous cx3cr1 deficiency in microglia restores neuronal β-amyloid clearance pathways and slows progression of alzheimer’s like-disease in ps1-app mice. Front. Immunol. 2019;10:2780. doi: 10.3389/fimmu.2019.02780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Ryu J.K., McLarnon J.G. Block of purinergic P2X(7) receptor is neuroprotective in an animal model of Alzheimer’s disease. Neuroreport. 2008;19(17):1715–1719. doi: 10.1097/WNR.0b013e3283179333. [DOI] [PubMed] [Google Scholar]
- 258.Diaz-Hernandez J.I., Gomez-Villafuertes R., León-Otegui M., Hontecillas-Prieto L., Del Puerto A., Trejo J.L., Lucas J.J., Garrido J.J., Gualix J., Miras-Portugal M.T., Diaz-Hernandez M. In vivo P2X7 inhibition reduces amyloid plaques in Alzheimer’s disease through GSK3β and secretases. Neurobiol. Aging. 2012;33(8):1816–1828. doi: 10.1016/j.neurobiolaging.2011.09.040. [DOI] [PubMed] [Google Scholar]
- 259.Frenkel D., Wilkinson K., Zhao L., Hickman S.E., Means T.K., Puckett L., Farfara D., Kingery N.D., Weiner H.L., El Khoury J. Scara1 deficiency impairs clearance of soluble amyloid-β by mononuclear phagocytes and accelerates Alzheimer’s-like disease progression. Nat. Commun. 2013;4:2030. doi: 10.1038/ncomms3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Chen J-H., Ke K-F., Lu J-H., Qiu Y-H., Peng Y-P. Protection of TGF-β1 against neuroinflammation and neurodegeneration in Aβ1-42-induced Alzheimer’s disease model rats. PLoS One. 2015;10(2):e0116549. doi: 10.1371/journal.pone.0116549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Juraskova B., Andrys C., Holmerova I., Solichova D., Hrnciarikova D., Vankova H., Vasatko T., Krejsek J. Transforming growth factor beta and soluble endoglin in the healthy senior and in Alzheimer’s disease patients. J. Nutr. Health Aging. 2010;14(9):758–761. doi: 10.1007/s12603-010-0325-1. [DOI] [PubMed] [Google Scholar]
- 262.Guerreiro R.J., Santana I., Brás J.M., Santiago B., Paiva A., Oliveira C. Peripheral inflammatory cytokines as biomarkers in Alzheimer’s disease and mild cognitive impairment. Neurodegener. Dis. 2007;4(6):406–412. doi: 10.1159/000107700. [DOI] [PubMed] [Google Scholar]
- 263.Coll R.C., Robertson A.A.B., Chae J.J., Higgins S.C., Muñoz-Planillo R., Inserra M.C., Vetter I., Dungan L.S., Monks B.G., Stutz A., Croker D.E., Butler M.S., Haneklaus M., Sutton C.E., Núñez G., Latz E., Kastner D.L., Mills K.H.G., Masters S.L., Schroder K., Cooper M.A., O’Neill L.A.J. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 2015;21(3):248–255. doi: 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.van der Wal E.A., Gómez-Pinilla F., Cotman C.W. Transforming growth factor-beta 1 is in plaques in Alzheimer and down pathologies. Neuroreport. 1993;4(1):69–72. doi: 10.1097/00001756-199301000-00018. [DOI] [PubMed] [Google Scholar]
- 265.Wyss-Coray T., Lin C., Yan F., Yu G.Q., Rohde M., McConlogue L., Masliah E., Mucke L. TGF-β1 promotes microglial amyloid-β clearance and reduces plaque burden in transgenic mice. Nat. Med. 2001;7(5):612–618. doi: 10.1038/87945. [DOI] [PubMed] [Google Scholar]
- 266.Town T., Laouar Y., Pittenger C., Mori T., Szekely C.A., Tan J., Duman R.S., Flavell R.A. Blocking TGF-β-Smad2/3 innate immune signaling mitigates Alzheimer-like pathology. Nat. Med. 2008;14(6):681–687. doi: 10.1038/nm1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Swardfager W., Lanctôt K., Rothenburg L., Wong A., Cappell J., Herrmann N. A meta-analysis of cytokines in Alzheimer’s disease. Biol. Psychiatry. 2010;68(10):930–941. doi: 10.1016/j.biopsych.2010.06.012. [DOI] [PubMed] [Google Scholar]