Abstract
Introduction:
Prior to 2006, nearly every U.S. child was infected with rotavirus by 5 years of age, and rotavirus was the leading cause of severe childhood gastroenteritis. In February 2006 and June 2008, the Advisory Committee on Immunization Practices recommended a live attenuated pentavalent rotavirus vaccine (RV5) and a monovalent rotavirus vaccine (RV1), respectively, for routine vaccination of infants in the United States.
Areas covered:
We reviewed U.S. data on coverage, vaccine effectiveness (VE), and vaccine impact from 2006 to 2017. National rotavirus vaccine coverage estimates increased since vaccine introduction but plateaued at 71–75% in 2013–2015, a level 15–20% lower than that of other routine childhood vaccines. Pooled VE of full series RV5 and RV1 against rotavirus-associated hospitalizations and emergency department visits were 84% (95% CI: 80–87%) and 83% (95% CI: 72–89%), respectively. Vaccine introduction resulted in a median decline in rotavirus-associated hospitalizations and emergency department visits of 80% and 57%, respectively, along with indirect protection of unvaccinated age groups and a decrease in health-care costs. A biennial pattern in rotavirus detection emerged post-vaccine implementation.
Expert Commentary:
The increasing use of rotavirus vaccines has substantially diminished the burden and changed the epidemiology of rotavirus disease in U.S. children; efforts to increase rotavirus vaccine coverage should continue.
Keywords: Rotavirus, vaccine, gastroenteritis, vaccination program, RotaTeq, Rotarix
1. Introduction
Prior to 2006, nearly every U.S. child was infected with rotavirus by 5 years of age [1–3]. Rotavirus infection was the leading cause of severe gastroenteritis among U.S. children in this age group, resulting in an estimated 205,000–272,000 emergency department (ED) visits, 55,000–70,000 hospitalizations, and 20–60 deaths annually [2–8]. Accounting for both direct and indirect costs, the estimated annual cost of rotavirus gastroenteritis to the U.S. health-care system was nearly US$ 1 billion [9].
In February 2006, the Advisory Committee on Immunization Practices (ACIP) recommended a live attenuated vaccine containing five human-bovine reassortant rotavirus strains – G1P[7], G2P [7], G3P[7], G4P[7], and G6P[1A] (RV5; RotaTeq, Merck and Co, West Point, PA, USA) for routine vaccination of infants in the United States [10]. This three-dose vaccine series is administered orally at ages 2, 4, and 6 months. The ACIP recommendation was updated in June 2008 to include Rotarix, a live attenuated vaccine containing a single attenuated G1P[8] human rotavirus strain (RV1; Rotarix, GlaxoSmithKline Biologicals, Rixensart, Belgium) [11]. This two dose vaccine series is administered orally at ages 2 and 4 months. Rotavirus vaccines in the United States have upper age limits for the administration of the first (14 weeks 6 days) and last (8 months 0 days) doses.
Pre-licensure randomized, double-blind clinical trials in developed countries (United States, Europe, and Latin America) demonstrated high efficacy (85–98%) for both RV5 and RV1 [12–15]. Clinical trials also suggested that RV5 and RV1 vaccines were efficacious against homotypic strains (matched the G-types and P-types included in the vaccine), fully heterotypic (did not match any serotypes in the vaccines), and partially heterotypic strains [13,15]. Limited data from trials were available on the efficacy of a partial vaccine series and the durability of protection over the first few years of life, and no data were available on efficacy of a mixed series [16].
We previously reviewed published data on impact and effectiveness of rotavirus vaccination in the United States 3 years post-licensure (2006–2009)[17] and 7 years post-licensure (2006–2012) [18]. In this report, we provide a comprehensive review of more than a decade of published data on coverage, rotavirus vaccine effectiveness (VE) against rotavirus hospitalization and ED visits, and impact on health-care utilization in the United States. Since we also previously reviewed published data on rotavirus vaccine safety in developing and developed countries, including the United States, 8-year post-licensure (2006–2013) [19], and only very limited additional data are available, we did not further review safety data.
2. Methods
2.1. Data abstraction
We identified U.S. articles published between 1 January 2006 and 17 September 2017 by searching for the terms (1) ‘rotavirus’ and either ‘vaccine*’ or ‘immuni*’ in the PubMed database, or (2) ‘rotavirus’ or ‘rotavirus infections’ and either ‘vaccines, attenuated,’ ‘vaccination’ or ‘rotavirus vaccines,’ or ‘rotavirus’ and ‘vaccine*’ in the PubMed, Medline, and Embase databases.
For the VE meta-analyses, we included post-licensure, observational case-control studies of RV1 or RV5 VE. Cases were children who were hospitalized or were treated in an emergency department for acute gastroenteritis (AGE) and tested positive for rotavirus. Controls were defined as subjects with acute gastroenteritis who tested negative for rotavirus. Studies that used control groups from national insurance claims data were not included in the VE meta-analysis. If studies utilized multiple control groups, only rotavirus-negative AGE controls were included in the VE meta-analysis to optimize comparability between studies.
2.2. Statistical analyses
We did a meta-analysis by vaccine type using a mixed-effects model, and present I2 and Q statistics to describe heterogeneity for specific models. We then conducted three VE sub-analyses: VE of RV5 partial series by number of doses, setting (hospital versus ED visit), and year of life (first versus second). To summarize VE study results in subgroups without sufficient study numbers to conduct meta-analyses, we calculated median VE estimates or described reported findings individually. Statistical analyses were conducted using RStudio version 3.4.0 (The R Foundation for Statistical Computing Platform, Vienna, Austria), and SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) for these analyses.
3. Results
3.1. Uptake of rotavirus vaccine
U.S. national rotavirus vaccine coverage estimates are based on data from the National Immunization Survey (NIS), a random-digitdialed telephone-based survey that estimates vaccination coverage among U.S. children aged 19–35 months [20–27]. From 2009 to 2013, national data showed improvements in rotavirus vaccine coverage, but from 2013 to 2015, NIS-based national coverage estimates plateaued (72.6% [95% CI: 71.1–74.4%] in 2013, 71.7% [95% CI: 70.1–73.2%] in 2014, and 73.2% [95% CI: 71.8–74.6%] in 2015) and remain below the Healthy People 2020 target (80%) (Figure 1). Furthermore, rotavirus vaccine coverage continues to be between 9.5% and 21.8% percentage points lower than diphtheria, tetanus, and acellular pertussis (DTaP), Poliovirus, Haemophilus influenzae type b (Hib), and pneumococcal conjugate vaccination (PCV) coverage in children age 19–35 months [23–28].
Figure 1.
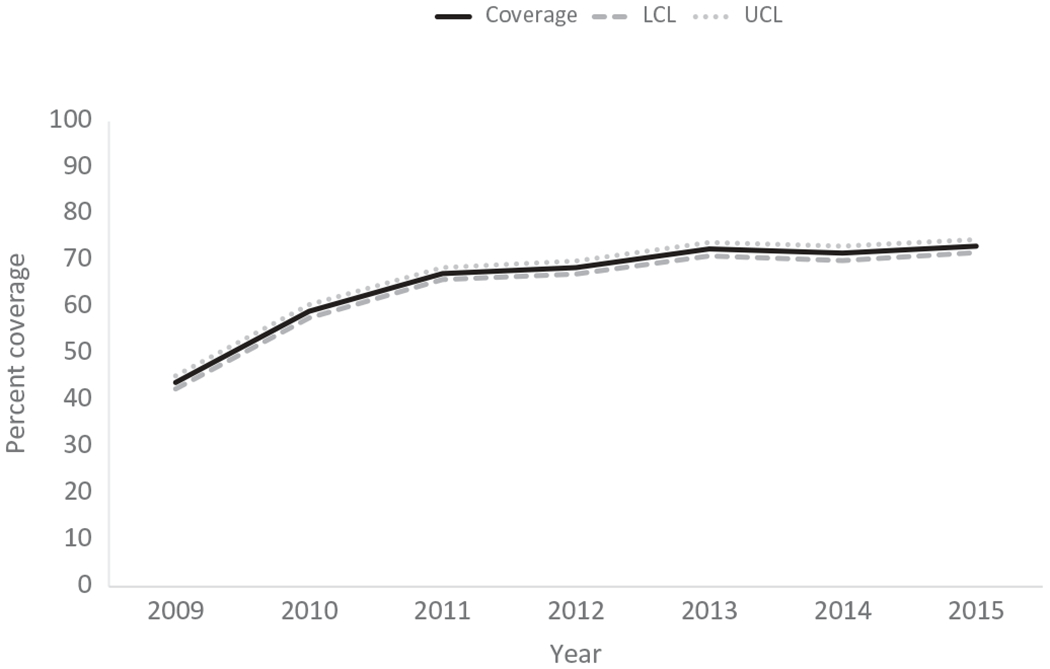
National rotavirus vaccine coverage among children by ages 19–35 months. LCL = 95% lower confidence limit; UCL = 95% upper confidence limit.
Rotavirus vaccine coverage in specific sociodemographic groups and geographic locations showed additional disparities. In 2015, children in families with income below the federal poverty level (FPL) had lower rotavirus vaccine coverage than children in families at or above the FPL (66.8%; 95% CI: 64.1–69.5% versus 76.8%; 95% CI: 75.2–78.4%) [26]. Children of mothers with educational attainment of a high school degree had lower rotavirus vaccine coverage (68.4%, 95% CI: 64–72.8%) than children of mothers with advanced degrees (84%, 95% CI: 81.7–86.3%). Also, uninsured children had lower vaccination coverage (56.5%, 95% CI: 49.5–63.5%) than children with private health insurance (79.1%, 95% CI: 77.6–80.6%) [29]. Compared with non-Hispanic white (white) children, non-Hispanic black (black) children had lower rotavirus series coverage (75.6%, 95% CI: 73.9–77.3% versus 69.7%, 95% CI: 66.1–73.3%, respectively) but these differences were not statistically significant after adjusting for poverty status. Significant unadjusted disparities between American Indian (AI)/Alaskan Native (AN) children and white children were also noted in 2015 (vaccine coverage was 61.5%; 95% CI: 52.5–70.5%, for AI/AN children). Also, coverage in children living in metropolitan areas versus non-metropolitan areas (as measured by the Metropolitan Statistical Area status, a measure of urbanicity) was 72.7% (95% CI: 70.6–74.8%) as compared to 68.6% (95% CI: 65.6–71.6%), respectively. Vaccination coverage also differed by state, ranging from a high of 87.6% in Rhode Island to a low of 63.8% in Florida [24].
The most recent NIS coverage estimates available for foreign-born children are also lower than for non-foreign born children (in 2013: 18.3%; 95% CI: 8.2–28.4%% versus 73.3%, 95% CI: 71.9–74.7%, respectively). One study of NIS data from 2010–2012 found that not only did differences in rotavirus vaccination coverage among foreign-born and non-foreign-born children persist after controlling for demographic, access-to care, poverty, and language effects (adjusted vaccination coverage 15.6%; 95% CI: 11–21.6%, versus 65.7%; 95% CI: 64.9–66.4%, respectively,) but being foreign-born was the strongest predictor for not having completed the rotavirus vaccination series [28].
Studies examining barriers to rotavirus vaccine coverage are limited, with the majority focusing on the impact of upper age restrictions. Based on a retrospective review of preterm infants admitted to the Neonatal Intensive Care Unit (NICU) at two urban hospitals from 2011 to 2013, an estimated one in five hospitalized infants would be ineligible for vaccination upon NICU discharge [30]. Studies to estimate the impact of upper age restrictions on coverage have compared rotavirus vaccine timing and coverage to other infant vaccines with similar schedules but without upper age restrictions, or catchup options (DTaP-containing vaccines and PCV). In a U.S. study of infants using data from Immunization Information System (IIS) sentinel sites from 2006 to 2009, site-specific rotavirus vaccine coverage was 13 percentage points lower than DTaP and PCV7 [21]. A later IIS-based study from infants in the 2012 birth cohort found that on average, 79–81% of infants received at least one dose of rotavirus vaccine, while 87–90% of infants received at least one dose of DTaP. Only one-third of this difference in coverage could be attributed to the maximum age restriction [31].
Provider-level safety concerns have been hypothesized to contribute to missed opportunity for rotavirus vaccination. A small increased risk of intussusception (1–6 cases per 100,000 doses) has been found with both RV5 and RV1 vaccinations in U.S. post-licensure studies [32–35].
Provider-level knowledge, attitude, and practice surveys have demonstrated mixed findings. In one study of privately insured U.S. infants from 2006 to 2010, visiting a pediatrician versus a family physician was independently associated with initiation and completion of the rotavirus vaccine series [36]. A survey of U.S. physicians in 2007 found financial barriers to be the most commonly cited barrier by pediatrician respondents (19%) and safety barriers to be the most commonly cited barrier among family medicine physician respondents (25%) [37]. It remains unclear whether this financial barrier related to rotavirus vaccination only, or also applied to other infant vaccinations. A follow-up survey of a sample U.S. pediatricians (n = 289 respondents) and family medicine physicians (n = 243 respondents) from 2010 to 2011 demonstrated that all reported barriers to rotavirus vaccination had decreased compared to 2007, though 31% of family physicians and 5% of pediatricians still reported concerns about safety of rotavirus vaccine as somewhat or definitely a barrier to use. However, only 2% of family medicine physicians and 1% of pediatricians reported that they had stopped or would stop giving rotavirus vaccine due to risk of intussusception [38].
3.2. Rotavirus vaccine effectiveness
Seventeen VE studies were reviewed, including 3 studies using large administrative databases and 14 case-control studies. All studies used rotavirus-associated hospitalization and/or ED visit as the outcome of interest. Of the 14 case-control studies, 7 were conducted using data from the New Vaccine Surveillance Network (NVSN), a regionally diverse active surveillance program in the United States.
VEs against rotavirus-associated hospitalization or ED visit ranged from 68% to 100%. Among the 10 unique case-control studies, the median full-dose VE against rotavirus-associated hospitalizations or ED visits was 84% (25%, 75% Interquartile Range (IQR): 83, 91), ranging from 70% to 98% [39–51] (Supplemental materials: Table 1). To further examine the VE data, we conducted a meta-analysis to estimate the full-dose effectiveness of RV5 and RV1, of a partial vaccine series, and against hospitalizations and ED visits.
3.2.1. RV5 versus RV1 (full series)
Nine unique RV5 VE and four unique RV1 VE studies were published. RV5 studies tended to be larger than RV1 studies, with a median subject number of 324 (25%, 75% Interquartile Range (IQR): 200, 624), as compared to 215.5 (IQR: 177, 385), respectively. VEs for a full series of RV5 and RV1 from mixed-effect models were similar at 84% (95% CI: 80–87%) and 83% (95% CI: 72–89%), respectively (Figure 2). In studies that compared VE of full-series RV5 to RV1, no statistically significant difference was noted [48,49].
Figure 2.
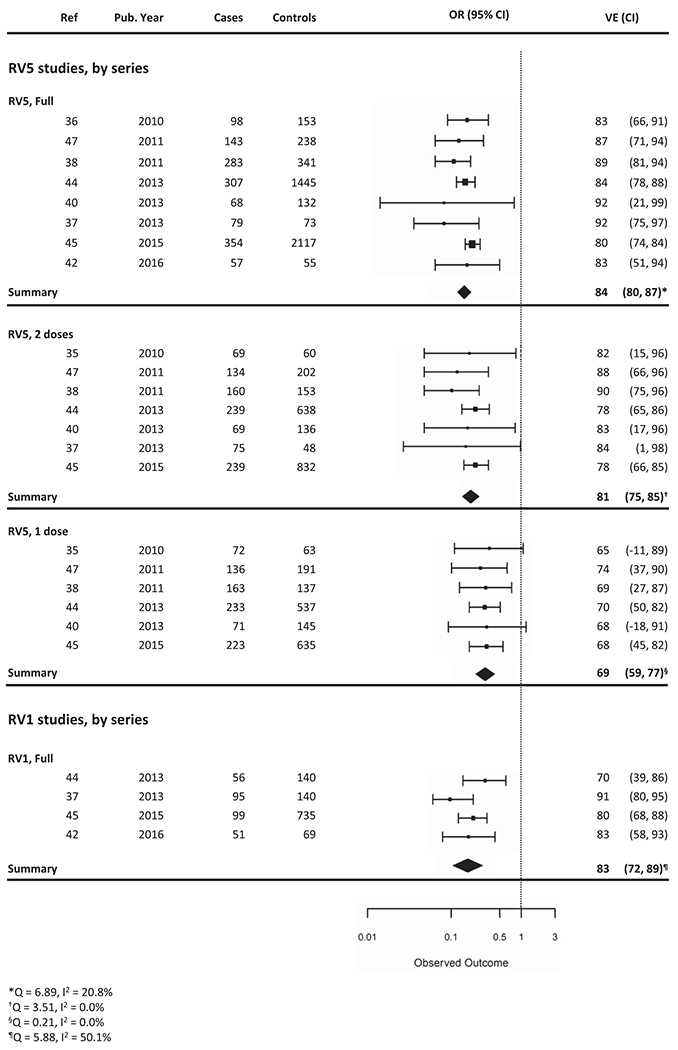
Vaccine effectiveness of full series or partial series of RV5 and RV1 in the hospital or ED setting.
3.2.2. Partial vaccination
Ten case-control studies calculated the VE of partial rotavirus vaccine series (1 or 2 doses of RV5, or 1 dose of RV1). Eight studies conducted RV5 partial vaccination analyses, specifying number of doses administered; one study grouped 1 or 2 doses of RV5 together, and one study grouped partial vaccine series of RV5 or RV1 together. Of the RV5 partial vaccination studies that specified number of doses administered, VE from mixed-effect models for 1 dose was 69% (95% CI: 59–77%) and for 2 doses was 81% (95% CI: 75–85%) (Figure 2). Less information can be gleaned regarding partial vaccination with RV1, given paucity of data. In a study from 2009 to 2011, the VE for 1 dose of RV1 was 57% (95% CI: −45% to 87%) compared with 70% (95% CI: 39–86%) for a full series [48]. In a later study from 2012 to 2013, the VE for 1 dose of RV1 was 96% (95% CI: 67–99%), as compared to 80% (95% CI: 68–88%) for full series [49].
3.2.3. Mixed series (RV5 and RV1 in a 3-dose combination)
One case-control study calculated the VE of a full-dose (3-dose) mixed series of RV5 and RV1. In this study of children enrolled in NVSN, the VE of a full-dose mixed series was 80% (95% CI: 51–92%) [50].
3.2.4. Clinical setting (hospitalization versus emergency room)
Seven case-control studies calculated the VE of RV5 against hospitalization alone, and five of these studies also calculated the VE of RV5 against ED visits alone. One of these studies combined partial and full series in calculating VE against hospitalizations [48]. RV5 VE from mixed-effects models against hospitalization was 89% (95% CI: 82–93%), and against ED visit was 79% (95% CI: 75–83%) (Figure 3).
Figure 3.
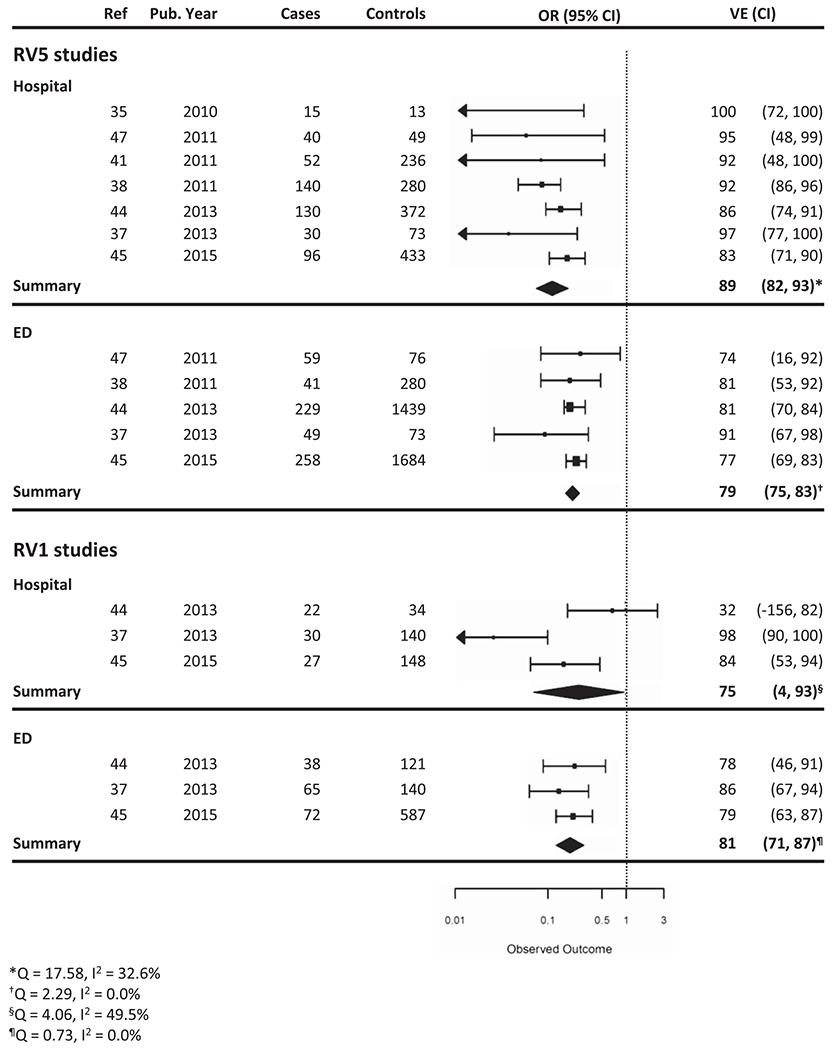
Vaccine effectiveness of RV5 and RV1 by clinical setting.
Three of the seven studies also calculated the VE of RV1 for different clinical settings. Of these three studies, RV1 VE against hospitalizations ranged from 32% to 98%, and RV1 VE against ED visits ranged from 78% to 86% [48,49,52].
Three studies estimated VEs against outpatient visits but they were all ecologic studies. Of these three studies, one study also calculated the VE against triage center outpatient calls, and one study calculated VE against AGE-associated outpatient encounters only [53–55]. Overall VE estimates were lower than against inpatient and ED visits, but paucity of data limits further conclusions.
3.2.5. Disease severity
Along with need for hospitalization, intravenous fluids (IVF) administration and high clinical severity score using the 20-point modified Vesikari Severity Score (modified-VSS) have been used as indicators of rotavirus infection severity. The modified-VSS has been validated for estimating severity of AGE in the U.S. pediatric population, with mild, moderate, and severe defined as a score of ≤10, 11–15, and ≥16, respectively [56].
Due to low numbers of post-licensure studies, VEs against severe rotavirus infection were analyzed descriptively. Three studies calculated RV5 VE against IVF, and VEs ranged from 92% to 97%. Two studies estimating the RV1 VE against IVF found VEs of 90% and 95%, respectively [40,41,46]. VE estimates against severe rotavirus infection best approximated vaccine efficacy estimates from clinical trials, but paucity of data limits further conclusions [57].
The one study that estimated RV5 VE against rotavirus infections categorized as mild, moderate, and severe found increasing VEs with increasing severity score (67%; 95% CI: 48–79%, 78%; 95% CI: 70–85, and 84%; 95% CI: 71–92%, respectively) [49].
3.2.6. Duration of protection
Five studies examined waning immunity by year of life of a full dose series. Overall, statistically significant rotavirus VE was observed through at least the fourth year of life for RV5 (five studies) and through the third year of life for RV1 (two studies) [41,42,46,51,58]. RV5 VE from mixed-effects models against hospitalizations and ED visits within the first year of life was 91% (95% CI: 85–95%) and within the second year of life was 86% (95% CI: 78–91%) (Figure 4).
Figure 4.
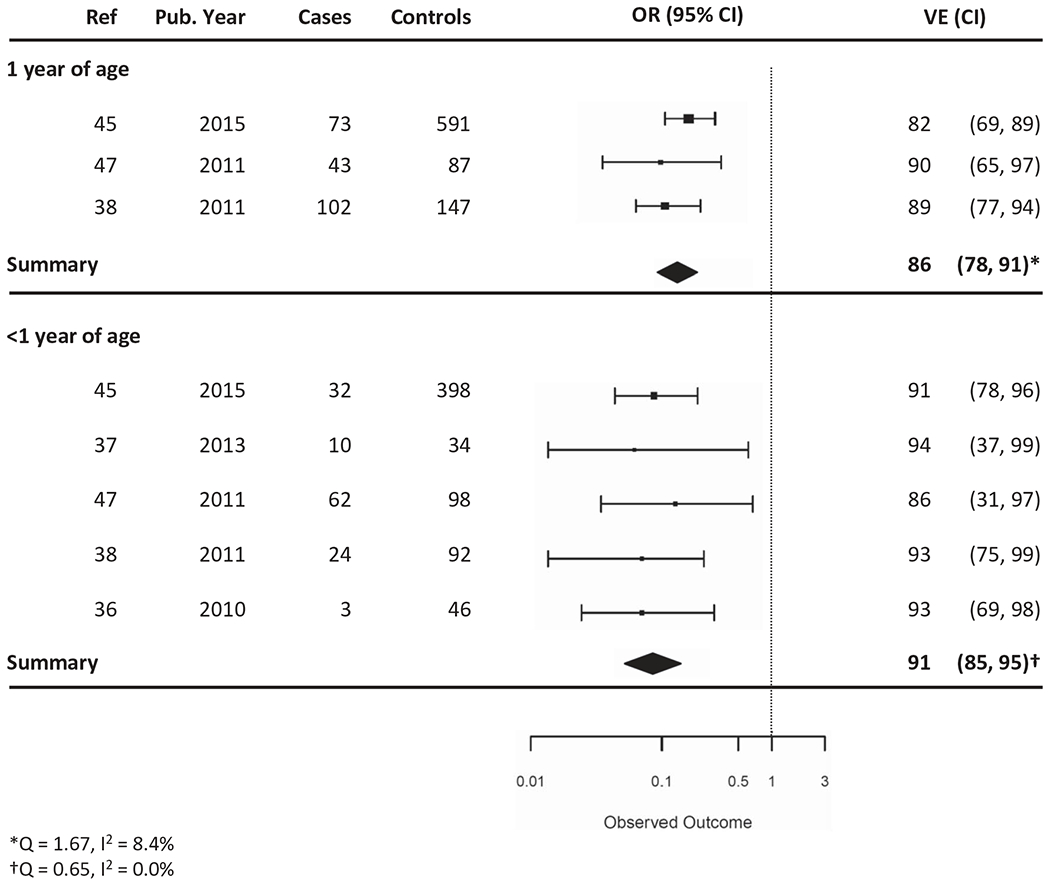
Vaccine effectiveness of RV5 vaccine in the hospital or ED setting, stratified by year of life (<1 versus 1 year of life).
Of the two studies of full dose RV1 by year of life, RV1 VE against hospitalizations and ED visits during the first year of life was 82% and 85% and during the second year of life was 86% and 91%. In both studies, VE estimate confidence intervals in the first and second year of life overlapped.
3.3. Vaccine impact
3.3.1. Health-care utilization
Rotavirus vaccine introduction has dramatically decreased health-care utilization and associated costs in the hospital, ED, and outpatient settings. This has best been demonstrated in studies using national, state, or local-level administrative databases to estimate rate reductions in rotavirus-associated health-care encounters in children under 5 years of age in the post-vaccine period (Supplemental materials: Table 2).
Of the 10 studies comparing rotavirus-coded hospitalization rates in children in the pre-vaccine period as compared to the post-vaccine period, the median rate reduction was 80% (IQR: 63.3%, 82%) (Figure 5a). Six of these studies were based on data from large national administrative claims databases (MarketScan, Healthcare Cost and Utilization Project (HCUP), or SDI Health, LLC) [59–64]. Two studies used data from less representative multi-regional administrative databases (the United States military health system’s M2 database and Indian Health Service data) [65,66], one study used state-level data [67], and one study used local-level data [68]. Eight of 10 studies reported rotavirus-coded hospitalization rate reductions by year, with significant variability in the years studied. Overall, an increase in the median rate reduction over time was observed, but a paucity of data limits further analyses. The most recent study examined rotavirus-coded hospitalization rates using hospitalization discharge data from 26 State Inpatient Databases of the HCUP over 12 years (2000–2012). Compared with the pre-vaccine annual mean rotavirus-associated hospitalization rate of 16 per 10,000 children <5 years old, rates of rotavirus-coded hospitalizations declined by 79% (95% CI: 79–80%) in 2011, and by 94% (95% CI: 94–95%) in 2012. A biennial pattern was also noted, with lower seasonal peaks in 2010 and 2012, as compared to 2009 and 2011 [64].
Figure 5.
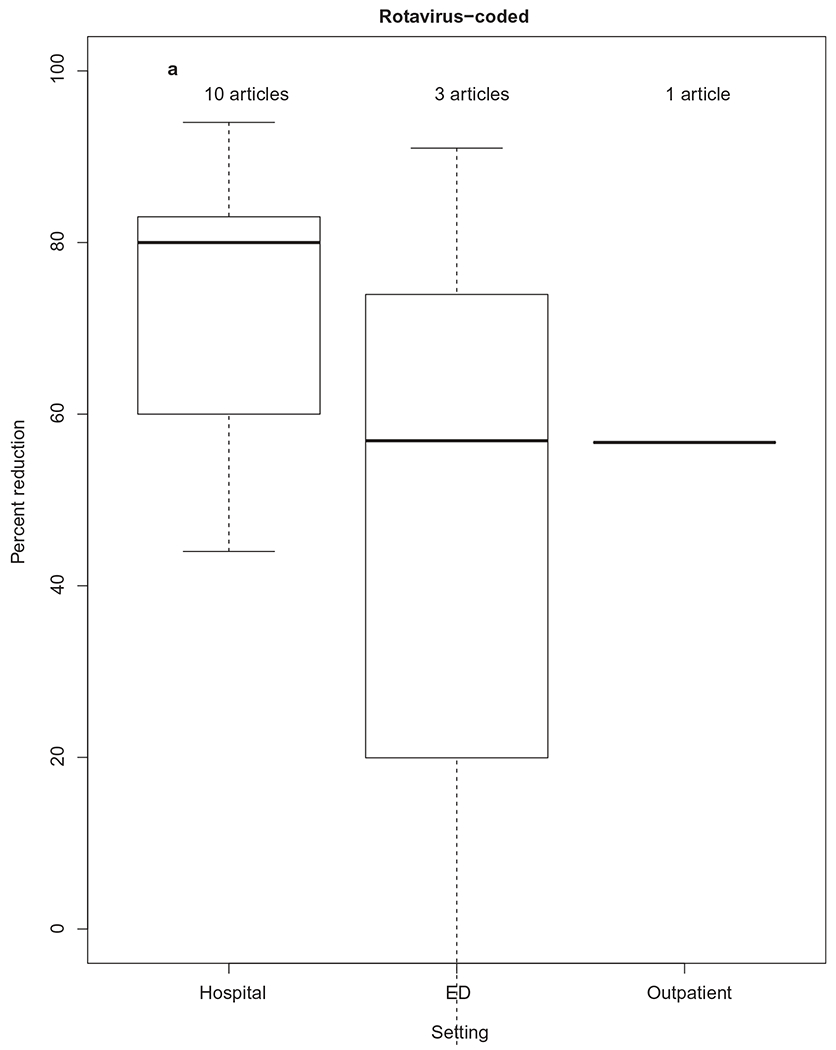
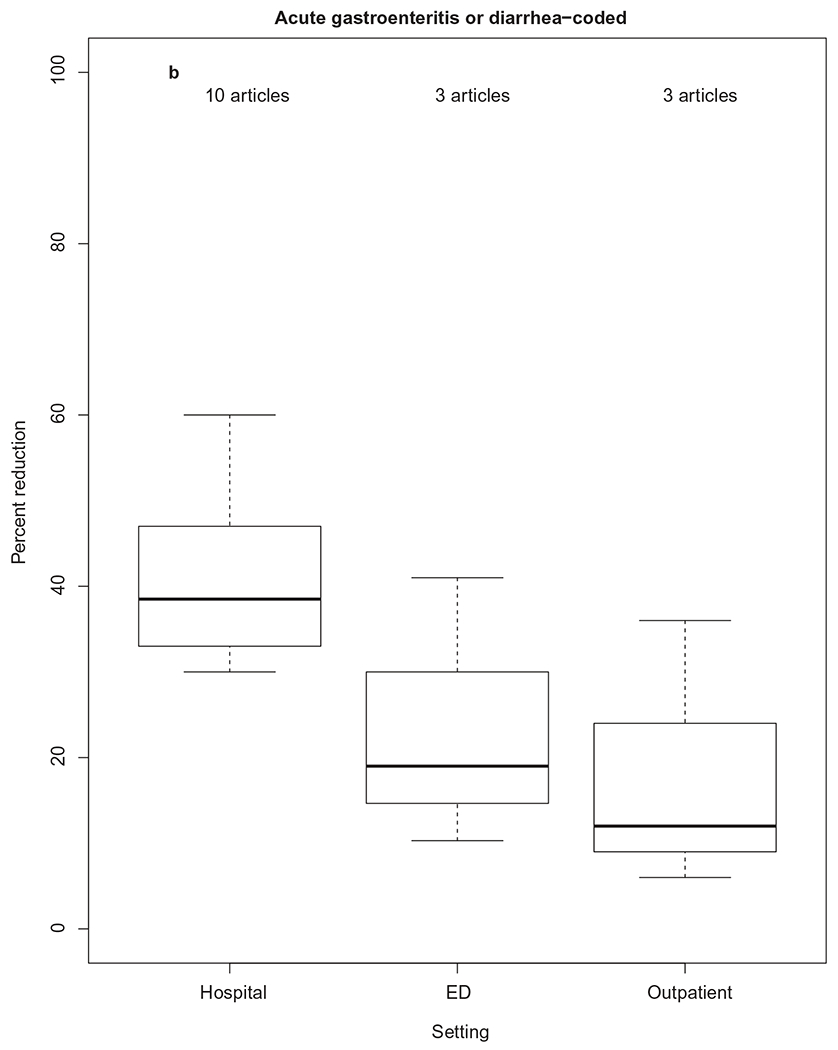
Percent reduction in rotavirus-coded or acute gastroenteritis-coded health-care utilization, by health-care setting. The thick horizontal lines represent the median percent reduction, by health-care setting. The upper and lower limits of each box represent 75th and 25th interquartile ranges of the data by health-care setting.
Of the three studies comparing rotavirus-coded ED visit rates in the pre-vaccine period to the post-vaccine period, median rate reduction was generally lower than that for hospitalizations (57%, IQR: 20%, 74%) (Figure 5a). All three of these studies used data on children less than 5 years old, but only one was nationally representative [62]. Compared with the pre-vaccine annual mean rotavirus-associated ED visit rate of 13.2 per 10,000 person-years (2003–2006), rates of rotavirus-coded ED visits declined to 4.85 (95% CI: 4.66–5.06), signifying a rate reduction of 57% [62].
A nationally representative study of rotavirus-coded outpatient visits compared post-vaccine introduction rates from 2008–2010 to pre-vaccine study period rates (2003–2006). Compared with the pre-vaccine annual mean rotavirus-associated outpatient visit rate of 13.2 per 10,000 person-years, post-vaccine rates of rotavirus-coded outpatient visits declined to 6.96 (95% CI: 6.75–7.2), signifying a rate reduction of 57%.
A decline in all-cause AGE health-care encounters has also been observed in hospitals, EDs, and outpatient settings following rotavirus vaccine introduction in the United States [69,70]. Sixteen studies compared AGE-coded health-care encounter rates in the pre-vaccine period to the post-vaccine period; of the 10 studies looking specifically at AGE-coded hospitalization rates, the median rate reduction was 38.5% (IQR: 33.25, 46.5) (Figure 5b). Three studies each compared AGE-coded ED visits and outpatient visits in the pre-vaccine period to the post-vaccine period; median rate reductions of 19% (IQR: 15, 30) and 12% (IQR: 9, 24), respectively, were observed.
Few studies have examined vaccine impact on health-care utilization using data from active surveillance with laboratory testing to confirm rotavirus infection in AGE patients [53,71]. One study of children less than 3 years old hospitalized at three NVSN sites from 2006 to 2009 demonstrated a hospitalization rate reduction of 87% in the 6–11-month-old age group, 96% in the 12–23-month-old age group, and 92% in the 24–35-month-old age group [71].
3.3.2. Indirect protection – herd immunity
Herd immunity is the reduction of disease in unvaccinated susceptible individuals due to decreased disease burden in the vaccinated individuals leading to decreased transmission within a population. Studies have demonstrated herd immunity due to rotavirus vaccination in a variety of ways (Supplemental materials: Table 3). One study of administrative claims data of commercially insured U.S. children less than 5 years old found that rotavirus-coded hospitalization rates among unvaccinated children decreased in post-vaccine years from 2007 to 2010 compared with pre-vaccine years from 2001 to 2006, though the post-vaccine reductions varied by year to year (50% [95% CI: 36–62%] in 2007–2008, 2% [95% CI: −17% to 18%] in 2008–2009, 77% [95% CI: 65–85%] in 2009–2010, and 25% [95% CI: 4–41%] in 2010–2011 [63]. A similar study using HCUP’s Nationwide Inpatient Sample (NIS) found significant decreases in rotavirus-coded hospitalization rates in 2008 as compared to the pre-vaccine period in all children and adults ages 5–64, which was most pronounced in the 5–14 and 15–24-year-old age groups (71% [95% CI: 555–81%] and 65% [95% CI: 18–85%], respectively). Cause-unspecified AGE discharges in these age groups (29% [95% CI: 22–35%], and 8% [95% CI: 2–14%], respectively) were also reduced [72]. Unvaccinated children 5–14 years of age of Hispanic ethnicity had the highest rotavirus-coded hospitalization rate reductions overall as compared to any other racial or ethnic group studied (White, Black, and Other). A study of all U.S. military dependents and a study of New York state hospitals also found significant rate reductions in unimmunized individuals ranging from 57% to 70% [66,67]. These findings were supported by a later study using data from Quest Diagnostics clinical reference laboratory from September 2003 to August 2014. Of children who were ‘unlikely vaccinated’ (defined as born on or before July 31st, 2006) in the post-vaccine period (2008–2014) as compared to the pre-vaccine period (2003–2006), the overall rotavirus test positivity rate decreased from 21% (95% CI: 90.7–21.2%) in the pre-vaccine period to 6.3% (95% CI: 6–6.5%) in the post-vaccine period (P < 0.001) [73].
Rotavirus active surveillance programs have studied the indirect protection of unvaccinated children and adults using alternative approaches. One study of children less than 3 years of age enrolled in NVSN from 2006 to 2009 compared estimated expected rotavirus associated hospitalization rate reduction (NVSN vaccination coverage estimates multiplied by the post-licensure estimates of rotavirus VE) and found that observed hospitalization rates were much lower than expected among children 12–23 months of age, suggesting substantial indirect benefit from vaccination [71].
3.3.3. Seasonal trends
CDC’s National Respiratory and Enteric Virus Surveillance System (NREVSS), a passive sentinel laboratory reporting system for respiratory and enteric viruses, including rotavirus, has been the primary national data source for detecting shifts and trends in rotavirus activity. Before the introduction of the rotavirus vaccine, rotavirus disease showed annual winter–spring seasonality, beginning in the West during December–January and ending in the Northeast during April–May [17]. Post vaccine introduction, four significant seasonal trends emerged: a biennial pattern, season onset delay, decreased duration, and a disruption of the regular West to East pattern, which have been attributed to a decrease in the number of susceptible children to support annual and sustained rotavirus transmission.
Comparing the pre-vaccine introduction period (2000–2006) to the post-vaccine introduction period (2007–2014), the percent of tests positive for rotavirus decreased overall from a pre-vaccine average of 43.1% to post-vaccine season estimates ranging from 10.9% to 27.3%, following a biennial ‘peak’ pattern [74]. Furthermore, regional changes were noted in the onset of the rotavirus season; in the South, season onset and duration were comparable with median values from the pre-vaccine years, but in all other regions (West, Midwest, and North) season onset was delayed or absent, and duration was shorter.
3.3.4. Genotype
Multi-regional rotavirus strain surveillance in the United States has demonstrated strain variation from year to year [71,75,76]. In one study of children <36 months old enrolled in NVSN at three regionally diverse sites (Ohio, New York, and Tennessee) during the period 2006–2009, the predominant strain of G1P[8] (91% of strains in 2006) shifted to G3P [3] (43%) in 2009 [71]. This was consistent with findings from the National Rotavirus Strain Surveillance System, and single center rotavirus strain surveillance studies, which also showed a shift from G1 dominance to G3 dominance during this time period [77–80]. NVSN data continued to show G3 dominance leading into the 2012 season, when the predominant strain shifted to G12P[8] (70%) and G12P[8] persisted in the 2013 season (68%) [81].
3.3.5. Reduction in health-care costs
Since vaccine introduction, studies using national commercial claims data of children under 5 years of age, from pre-vaccine season up to 2011, have estimated mean annual health-care (direct) cost savings from rotavirus-associated and AGE-associated health-care utilization, with results ranging from $121 to $231 million, depending on years and outcomes studied [59,63,72,82] (Table 1).
Table 1.
Select U.S. studies examining cost associated with rotavirus vaccination programs.
| Study | Study type | Database | Age | Study period | Outcome | Measure | Economic impact |
|---|---|---|---|---|---|---|---|
| [83] | Ecologic | Truven Commercial Claims and Truven Medicaid Claims | <5 years old | Commercial: 2000–2011 Medicaid: 2002–2010 | Diarrhea and rotavirus-coded H, ED, OP; | Cost reduction in 2012 assuming all vaccinated; Mean cost difference for first RV episode per completely vaccinated versus contemporarily unvaccinated | $60 million for 2012 birth cohort; Commercial: $15.33 (95% CI $12.99–$18.03) Medicaid: $4.26 (95% CI $2.34–$6.35) |
| [63] | Ecologic | Truven Commercial Claims | <5 years old | 2001–2006 versus 2007–2011 | Diarrhea and rotavirus-coded H, ED, OP | Cost reduction for the first 4-year period after vaccine introduction | $924 million |
| [62] | Ecologic | Truven Commercial Claims and Truven Medicaid Claims | <5 years old | Commercial: 2000–2010 Medicaid: 2002–2009 | Diarrhea and rotavirus-coded H, ED, OP; | Per patient per month cost differences of first RV episode compared to controls without RV | Commercially insured: $3363 (95% CI: $3308–$3418 Medicaid: $1831 (95% CI: $1768–$1887) |
| [84] | Cohort | NVSN surveillance in 3 US sites | <3 years old | 2006–2009 | RV-associated H, ED | Annual cost reduction after vaccine introduction | $187 million annually |
| [82] | Ecologic | State Inpatient Databases | <5 years old | 2000–2009 | Diarrhea and RV-coded H | Hospital cost reduction | $242 million in 2008–2009 |
| [68] | Ecologic | Utah healthcare system | <5 years old | 2003–2007 versus 2008–2010 | Lab-confirmed RV disease-associated H, ED | Annual cost savings at each hospital from ED visits and hospitalizations for RVGE after vaccine introduction | Costs decreased by 79% and 72%, respectively, with annual savings of $790,000 at children’s hospital and $140,000 at a community hospital a |
| [72] | Ecologic | National Inpatient Sample | All | 2000–2006 versus 2008 | RV-coded or cause-unspecified gastroenteritis-coded H | Averted direct medical costs attributable to the RV vaccination program | $204 million in 2008; 20% of cost averted among unvaccinated 5–24 year old |
| [59] | Ecologic | MarketScan | <5 years old | 2001–2006 v 2007–2009 | Diarrhea and RV-coded H, ED, OP | Averted cost of diarrhea-associated hospitalization post vaccine introduction | $278 million in 2007–2009 |
| [67] | Ecologic | New York state hospitals | <1 year old | 2003–2006 v. 2008 | RV-associated H | Statewide hospital cost reduction for RV hospitalizations post vaccine introduction | $10 million in 2008 |
H = Hospital; ED = Emergency Department; OP = Outpatient; RV = Rotavirus vaccine.
A study using claims data of commercially insured that U. S. children aged <5 years from 2007 to 2011 estimated 176,587 hospitalizations, 242,335 ED visits, and 1,116,869 outpatient visits for diarrhea were averted, resulting in a reduction in health-care utilization of $924 million for that 4-year period [63]. Other national commercial and Medicaid claims-based studies have also demonstrated direct cost savings due to decreased rotavirus-associated or diarrhea-associated health-care utilization in children <5 years [62,83], and also in unvaccinated 5–24 year olds [72]. An active population-based rotavirus surveillance-based study of children <3 years of age enrolled at one of three NVSN sites from 2006 to 2009 estimated similar annual medical cost reductions ($187 million) [84].
4. Conclusion
The introduction of RotaTeq and Rotarix in the United States have resulted in dramatic decline in rotavirus disease, as evidenced by declines in national laboratory-based rotavirus surveillance, rotavirus-coded hospitalizations and ED visits, and consistently high VE estimates across studies. As of 2015, rotavirus vaccination coverage estimates had not reached the national Healthy People 2020 target of 80%, and estimates plateaued from 2013 to 2015.
5. Expert commentary
5.1. Uptake
U.S. full-dose rotavirus vaccination coverage estimates are lower than that of other countries with low child mortality rates [85]. Continued efforts are needed to expand rotavirus vaccine coverage on a national scale; while rotavirus vaccination coverage gradually increased after vaccine introduction, coverage estimates plateaued starting in 2013 and have remained unchanged.
Disparities in rotavirus vaccine coverage persist and are concerning, especially in children of families living below the FPL and foreign-born children. This lower coverage in foreign-born children is likely related in part to the ongoing lack of worldwide access to rotavirus vaccine, and no recommendation for catchup series. However, other barriers to vaccination, such as inadequate access to preventive health care, language services, or provider-level factors, may also be playing a role. Optimal public health interventions may vary based on characteristics of populations with low rotavirus coverage. Continued rotavirus coverage assessment will be important to identify barriers to rotavirus vaccination and appropriate public health response.
5.2. VE
VE of full-dose RV5 and RV1 against hospitalization and ED visits was high (mixed-effect models estimating VEs of 84% and 83%, respectively), and found to be durable over the first few years of life. A partial vaccine series was effective (pooled RV5 VE, 81%; 95% CI: 75–85%) but had a comparably lower VE than a full series. These findings, in combination with unknown durability of partial vaccine series effectiveness, supports the current U.S. ACIP recommendation to allow for the completion of a 3-dose rotavirus vaccination series with either RV5 or RV1 if a single vaccine is not available for the full series. VE estimates by genotype are limited, with pooled results from four studies demonstrating no significant difference by genotype strain matching. However, a nonsignificant trend favoring matching strains was noted, and only one RV1 VE study by genotype was included. Continued rotavirus surveillance and future genotype-specific VE studies will help inform our understanding of rotavirus vaccine cross-protection and its evolution.
5.3. Impact
Direct protection of vaccinated children and indirect protection of unvaccinated children and adults against hospitalization and ED visits have also been observed in multiple large nationally representative studies. Comparing rotavirus-coded hospitalization rates in children in the pre-vaccine period to the post-vaccine period, the median rate reduction was 80% (IQR: 63.3%, 82%). Studies investigating indirect protection of unvaccinated children and adults against rotavirus-coded hospitalizations were heterogeneous in both scale and age strata studied, but estimates ranged from 50% to 70%. These results indicate an indirect protective effect of rotavirus vaccination against population-level rotavirus-associated hospitalizations among unvaccinated children and adults. It will be important to continue to monitor rotavirus-coded hospitalizations in both vaccinated and unvaccinated children over time, as residual disease may indicate another source of infection [57].
A continual decline in rotavirus test positivity, decreased season duration, delayed onset, and biennial pattern initially noted in the immediate post-vaccine period have persisted, with differences in percent test positive decreasing in recent years. The reasons for this biennial pattern are not fully understood and continued monitoring of seasonal trends is needed. Variations in genotype have been observed from year to year, likely as a result of natural secular variation, since high VE has been observed against a range of vaccine-type and non-vaccine-type strains. However, continued monitoring of circulating genotypes over time in the post-vaccine introduction era will be important.
6. Five-year view
Over the last decade, large, representative studies have consistently demonstrated high VE of both RV5 and RV1. Additionally, large reductions in both rotavirus-coded and AGE-coded health-care utilization rates have occurred after vaccine introduction. However, national rotavirus coverage is not at goal (80%), and a significant gap of 5–15% between rotavirus vaccine coverage and other infant vaccines still exists.
Future studies should continue to explore the relative contributions of age restrictions, safety concerns, and financial concerns in explaining this coverage gap, both overall and by subpopulations (e.g. poverty level, country of origin, and urbanicity of residence). Implementation science studies are also needed to investigate how operational barriers to implementing rotavirus vaccination programs in specific subpopulations can be overcome.
The shift in annual to biennial rotavirus peak activity after rotavirus vaccine introduction, attributed to insufficient numbers of rotavirus-infected children to support annual transmission, is not well understood. Ongoing laboratory-based surveillance will be important for following future seasonal trends, as this biennial pattern may diminish if numbers of rotavirus-infected individuals continue to decrease with increased vaccine coverage.
Regionally diverse and nationally representative VE studies will be important for continuing to evaluate VE, particularly for RV1 given limited existing data, VE by strain, mixed series, and durability of protection. Finally, incorporating the Vesikari clinical severity score and other markers of severe disease, such as need for IVF, will improve our understanding of rotavirus VE against severe disease, which is essential for understanding the benefit of a national public health vaccination program.
Supplementary Material
Key issues.
Now over a decade post-rotavirus vaccine introduction in the United States, dramatic decreases in rotavirus-associated health-care utilization have been demonstrated in multiple national and multi-regional studies.
As of 2015, rotavirus vaccination coverage estimates (73.2%) have not reached the national Healthy People 2020 target of 80%, with no significant improvement in coverage noted since 2013 (72.6%).
Disparities in rotavirus vaccine coverage persist, especially in children of families living below the FPL and foreign-born children.
Vaccine effectiveness of RV5 and RV1 against hospitalization and ED visits is high and consistent with clinical trials, with an overall RV5 VE of 84%, and RV1 VE of 83%.
Rotavirus vaccination directly protects vaccinated children, and indirectly protects unvaccinated children and adults, against hospitalization and ED visits.
Changes in seasonal rotavirus detection patterns from pre-vaccine to post-vaccine seasons have persisted, with a shorter season duration and delayed onset, and emergence of biennial pattern.
Footnotes
Supplemental data for this article can be accessed here.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
One of the reviewers on the manuscript disclosed work as a Principle investigator of studies of RotaTeq and Rotarix vaccines (equally).
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Glass RI, Kilgore PE, Holman RC, et al. The epidemiology of rotavirus diarrhea in the United States: surveillance and estimates of disease burden. J Infect Dis. 1996;174(Suppl 1):S5–11. [DOI] [PubMed] [Google Scholar]
- 2.Jin S, Kilgore PE, Holman RC, et al. Trends in hospitalizations for diarrhea in United States children from 1979 through 1992: estimates of the morbidity associated with rotavirus. Pediatr Infect Dis J. 1996;15(5):397–404. [DOI] [PubMed] [Google Scholar]
- 3.Kilgore PE, Holman RC, Clarke MJ, et al. Trends of diarrheal disease-associated mortality in US children, 1968 through 1991. Jama. 1995;274(14):1143–1148. [PubMed] [Google Scholar]
- 4.Charles MD, Holman RC, Curns AT, et al. Hospitalizations associated with rotavirus gastroenteritis in the United States, 1993–2002. Pediatr Infect Dis J. 2006;25(6):489–493. [DOI] [PubMed] [Google Scholar]
- 5.Esposito DH, Holman RC, Haberling DL, et al. Baseline estimates of diarrhea-associated mortality among United States children before rotavirus vaccine introduction. Pediatr Infect Dis J. 2011;30(11):942–947. [DOI] [PubMed] [Google Scholar]
- 6.Fischer TK, Viboud C, Parashar U, et al. Hospitalizations and deaths from diarrhea and rotavirus among children <5 years of age in the United States, 1993–2003. J Infect Dis. 2007;195(8):1117–1125. [DOI] [PubMed] [Google Scholar]
- 7.Malek MA, Curns AT, Holman RC, et al. Diarrhea- and rotavirus-associated hospitalizations among children less than 5 years of age: United States, 1997 and 2000. Pediatrics. 2006;117(6):1887–1892. [DOI] [PubMed] [Google Scholar]
- 8.Parashar UD, Holman RC, Clarke MJ, et al. Hospitalizations associated with rotavirus diarrhea in the United States, 1993 through 1995: surveillance based on the new ICD-9-CM rotavirus-specific diagnostic code. J Infect Dis. 1998;177(1):13–17. [DOI] [PubMed] [Google Scholar]
- 9.Widdowson M-A, Meltzer MI, Zhang X, et al. Cost-effectiveness and potential impact of rotavirus vaccination in the United States. Pediatrics. 2007;119(4):684–697. [DOI] [PubMed] [Google Scholar]
- 10.Parashar UD, Alexander JP, Glass RI. Prevention of rotavirus gastroenteritis among infants and children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. Recommendations and Reports: Morbidity and Mortality Weekly Report. Recommendations and Reports. 2006;55(Rr–12):1–13. [PubMed] [Google Scholar]
- 11.Cortese MM, Parashar UD. Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. Recommendations and Reports: Morbidity and Mortality Weekly Report. Recommendations and Reports. 2009;58(Rr–2):1–25. [PubMed] [Google Scholar]
- 12.Goveia MG, Rodriguez ZM, Dallas MJ, et al. Safety and efficacy of the pentavalent human-bovine (WC3) reassortant rotavirus vaccine in healthy premature infants. Pediatr Infect Dis J. 2007;26(12):1099–1104. [DOI] [PubMed] [Google Scholar]
- 13.Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354(1):11–22. [DOI] [PubMed] [Google Scholar]
- 14.Vesikari T, Itzler R, Matson DO, et al. Efficacy of a pentavalent rotavirus vaccine in reducing rotavirus-associated health care utilization across three regions (11 countries). International J Infectious Diseases: IJID: Official Publication of the International Society for Infectious Diseases. 2007;11(Suppl 2):S29–35. [DOI] [PubMed] [Google Scholar]
- 15.Vesikari T, Matson DO, Dennehy P, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354(1):23–33. [DOI] [PubMed] [Google Scholar]
- 16.Dennehy PH, Vesikari T, Matson DO, et al. Efficacy of the pentavalent rotavirus vaccine, rotateq(R) (RV5), between doses of a 3-dose series and with less than 3 doses (incomplete regimen). Hum Vaccin. 2011;7 (5):563–568. [DOI] [PubMed] [Google Scholar]
- 17.Tate JE, Cortese MM, Payne DC, et al. Uptake, impact, and effectiveness of rotavirus vaccination in the United States: review of the first 3 years of postlicensure data. Pediatr Infect Dis J. 2011;30(1 Suppl):S56–60. [DOI] [PubMed] [Google Scholar]
- 18.Rha B, Tate JE, Payne DC, et al. Effectiveness and impact of rotavirus vaccines in the United States – 2006–2012. Expert Rev Vaccines. 2014;13 (3):365–376. [DOI] [PubMed] [Google Scholar]
- 19.Rha B, Tate JE, Weintraub E, et al. Intussusception following rotavirus vaccination: an updated review of the available evidence. Expert Rev Vaccines. 2014;13(11):1339–1348. [DOI] [PubMed] [Google Scholar]
- 20.National, state, and local area vaccination coverage among children aged. 19–35 months — United States, 2009. MMWR Morb Mortal Wkly Rep. 2010;59(36):1171–1177. [PubMed] [Google Scholar]
- 21.Rotavirus vaccination coverage among infants aged. 5 months – immunization information system sentinel sites, United States, June 2006–June 2009. MMWR Morb Mortal Wkly Rep. 2010;59(17):521–524. [PubMed] [Google Scholar]
- 22.National and state vaccination coverage among children aged. 19–35 months – United States, 2010. MMWR Morb Mortal Wkly Rep. 2011;60(34):1157–1163. [PubMed] [Google Scholar]
- 23.National, state, and local area vaccination coverage among children aged. 19–35 months – United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:689–696. [PubMed] [Google Scholar]
- 24.National, state, and local area vaccination coverage among children aged. 19–35 months – United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62(36):733–740. [PMC free article] [PubMed] [Google Scholar]
- 25.Elam-Evans LD, Yankey D, Singleton JA, et al. National, state, and selected local area vaccination coverage among children aged 19–35 months – United States, 2013. MMWR Morb Mortal Wkly Rep. 2014;63(34):741–748. [PMC free article] [PubMed] [Google Scholar]
- 26.Hill HA, Elam-Evans LD, Yankey D, et al. Vaccination coverage among children aged 19–35 months – United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(39):1065–1071. [DOI] [PubMed] [Google Scholar]; • Most recent national coverage estimates using National Immunization Survey data demonstrated incremental improvements in rotavirus vaccine coverage from 2009 to 2012, with estimates plateauting from 71.7%-73.2% between 2013 and 2015.
- 27.Hill HA, Elam-Evans LD, Yankey D, et al. National, state, and selected local area vaccination coverage among children aged 19–35 months – United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64(33):889–896. [DOI] [PubMed] [Google Scholar]
- 28.Varan A, Rodriguez-Lainz A, Hill H, et al. Vaccination coverage disparities between foreign-born and U.S.-born children aged 19–35 months, United States, 2010–2012. J Immigrant Minor Health. 2017;19(4):779–789. [DOI] [PubMed] [Google Scholar]
- 29.U.S. Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Healthy People 2020. Washington, DC: U.S. Department of Health and Human Services; 2010. (ODPHP Publication No. B0132). Available from: https://www.healthypeople.gov/sites/default/files/HP2020_brochure_with_LHI_508_FNL.pdf. [Google Scholar]
- 30.Thrall S, Doll MK, Nhan C, et al. Evaluation of pentavalent rotavirus vaccination in neonatal intensive care units. Vaccine. 2015;33 (39):5095–5102. [DOI] [PubMed] [Google Scholar]
- 31.Pringle K, Cardemil CV, Pabst LJ, et al. Uptake of rotavirus vaccine among US infants at Immunization Information System Sentinel Sites. Vaccine. 2016;34(50):6396–6401. [DOI] [PubMed] [Google Scholar]
- 32.Weintraub ES, Baggs J, Duffy J, et al. Risk of intussusception after monovalent rotavirus vaccination. N Engl J Med. 2014;370(6):513–519. [DOI] [PubMed] [Google Scholar]
- 33.Yih WK, Lieu TA, Kulldorff M, et al. Intussusception risk after rotavirus vaccination in U.S. infants. N Engl J Med. 2014;370(6):503–512. [DOI] [PubMed] [Google Scholar]
- 34.Haber P, Patel M, Pan Y, et al. Intussusception after rotavirus vaccines reported to US VAERS, 2006–2012. Pediatrics. 2013;131 (6):1042–1049. [DOI] [PubMed] [Google Scholar]
- 35.Haber P, Parashar UD, Haber M, et al. Intussusception after monovalent rotavirus vaccine – United States, Vaccine Adverse Event Reporting System (VAERS), 2008–2014. Vaccine. 2015;33(38):4873–4877. [DOI] [PubMed] [Google Scholar]
- 36.Panozzo CA, Becker-Dreps S, Pate V, et al. Patterns of rotavirus vaccine uptake and use in privately-insured US infants, 2006–2010. PLoS One. 2013;8(9):e73825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kempe A, Patel MM, Daley MF, et al. Adoption of rotavirus vaccination by pediatricians and family medicine physicians in the United States. Pediatrics. 2009;124(5):e809–816. [DOI] [PubMed] [Google Scholar]
- 38.O’Leary ST, Parashar UD, Crane LA, et al. Adoption of rotavirus vaccine by U.S. physicians: progress and challenges. Am J Prev Med. 2013;44(1):56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boom JA, Tate JE, Sahni LC, et al. Effectiveness of pentavalent rotavirus vaccine in a large urban population in the United States. Pediatrics. 2010;125(2):e199–207. [DOI] [PubMed] [Google Scholar]
- 40.Boom JA, Tate JE, Sahni LC, et al. Sustained protection from pentavalent rotavirus vaccination during the second year of life at a large, urban United States pediatric hospital. Pediatr Infect Dis J. 2010;29(12):1133–1135. [DOI] [PubMed] [Google Scholar]
- 41.Cortese MM, Immergluck LC, Held M, et al. Effectiveness of monovalent and pentavalent rotavirus vaccine. Pediatrics. 2013;132(1):e25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cortese MM, Leblanc J, White KE, et al. Leveraging state immunization information systems to measure the effectiveness of rotavirus vaccine. Pediatrics. 2011;128(6):e1474–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Desai SN, Esposito DB, Shapiro ED, et al. Effectiveness of rotavirus vaccine in preventing hospitalization due to rotavirus gastroenteritis in young children in Connecticut, USA. Vaccine. 2010;28(47):7501–7506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Donauer S, Payne DC, Edwards KM, et al. Determining the effectiveness of the pentavalent rotavirus vaccine against rotavirus hospitalizations and emergency department visits using two study designs. Vaccine. 2013;31(24):2692–2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guh AY, Hadler JL. Use of the state immunization information system to assess rotavirus vaccine effectiveness in Connecticut, 2006–2008. Vaccine. 2011;29(37):6155–6158. [DOI] [PubMed] [Google Scholar]
- 46.Immergluck LC, Parker TC, Jain S, et al. Sustained effectiveness of monovalent and pentavalent rotavirus vaccines in children. J Pediatr. 2016;172(116–120):e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mohammed A, Immergluck L, Parker TC, et al. Association between mixed rotavirus vaccination types of infants and rotavirus acute gastroenteritis. Vaccine. 2015;33(42):5670–5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Payne DC, Boom JA, Staat MA, et al. Effectiveness of pentavalent and monovalent rotavirus vaccines in concurrent use among US children <5 years of age, 2009–2011. Clin Infect Dis. 2013;57(1):13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Payne DC, Selvarangan R, Azimi PH, et al. Long-term consistency in rotavirus vaccine protection: RV5 and RV1 vaccine effectiveness in US children, 2012–2013. Clin Infect Dis. 2015;61(12):1792–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• RV5 and RV1 vaccine effectiveness study in children enrolled in 1 of 7 active surveillance sites using multiple strata, including vaccine type, partial series, year of life, and healthcare setting. RV5 and RV1 VEs overall were similar (80%; 95% CI: 74%-84%, and 80%; 95% CI 68%-88%, respectively.).
- 50.Payne DC, Sulemana I, Parashar UD. Evaluation of effectiveness of mixed rotavirus vaccine course for rotavirus gastroenteritis. JAMA Pediatrics. 2016;170(7):708–710. [DOI] [PubMed] [Google Scholar]; • A study of children with acute gastroenteritis enrolled in 1 of 6 active surveillance sites demonstrated good VE of a 3-dose mixed series (80%; 95% CI 51%-92%).
- 51.Staat MA, Payne DC, Donauer S, et al. Effectiveness of pentavalent rotavirus vaccine against severe disease. Pediatrics. 2011;128(2): e267–275. [DOI] [PubMed] [Google Scholar]
- 52.Cortese MM, Dahl RM, Curns AT, et al. Protection against gastroenteritis in US households with children who received rotavirus vaccine. J Infect Dis. 2015;211(4):558–562. [DOI] [PubMed] [Google Scholar]
- 53.Nolan SM, Prasad P, Fiks AG, et al. Effect of rotavirus vaccine on reducing acute gastroenteritis in a large outpatient pediatric network. Arch Pediatr Adolesc Med. 2012;166(3):232–239. [DOI] [PubMed] [Google Scholar]
- 54.Wang FT, Mast TC, Glass RJ, et al. Effectiveness of the pentavalent rotavirus vaccine in preventing gastroenteritis in the United States. Pediatrics. 2010;125(2):e208–213. [DOI] [PubMed] [Google Scholar]
- 55.Wang FT, Mast TC, Glass RJ, et al. Effectiveness of an incomplete RotaTeq (RV5) vaccination regimen in preventing rotavirus gastroenteritis in the United States. Pediatr Infect Dis J. 2013;32(3):278–283. [DOI] [PubMed] [Google Scholar]
- 56.Wikswo M, Payne D, Parashar U Validation of a modified Vesikari Score in US children with gastroenteritis. In: 11th International Rotavirus Symposium. (Ed.^(Eds) (New Delhi, 2014) [Google Scholar]
- 57.Standaert B, Strens D, Alwan A, et al. Medium- to long-term impact of rotavirus vaccination on hospital care in Belgium: a 7-year follow-up of the rotavirus Belgium impact study (RotaBIS). Infect Dis Ther. 2016;5(1):31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Payne DC, Baggs J, Klein NP, et al. Does preventing rotavirus infections through vaccination also protect against naturally occurring intussusception over time? Clin Infect Dis. 2015;60(1):163–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cortes JE, Curns AT, Tate JE, et al. Rotavirus vaccine and health care utilization for diarrhea in U.S. children. N Engl J Med. 2011;365 (12):1108–1117. [DOI] [PubMed] [Google Scholar]
- 60.Cortese MM, Tate JE, Simonsen L, et al. Reduction in gastroenteritis in United States children and correlation with early rotavirus vaccine uptake from national medical claims databases. Pediatr Infect Dis J. 2010;29(6):489–494. [DOI] [PubMed] [Google Scholar]
- 61.Gastanaduy PA, Curns AT, Parashar UD, et al. Gastroenteritis hospitalizations in older children and adults in the United States before and after implementation of infant rotavirus vaccination. Jama. 2013;310(8):851–853. [DOI] [PubMed] [Google Scholar]
- 62.Krishnarajah G, Demissie K, Lefebvre P, et al. Clinical and cost burden of rotavirus infection before and after introduction of rotavirus vaccines among commercially and Medicaid insured children in the United States. Hum Vaccin Immunother. 2014;10 (8):2255–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leshem E, Moritz RE, Curns AT, et al. Rotavirus vaccines and health care utilization for diarrhea in the United States (2007-2011). Pediatrics. 2014;134(1):15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leshem E, Tate JE, Steiner CA, et al. Acute gastroenteritis hospitalizations among US children following implementation of the rotavirus vaccine. Jama. 2015;313(22):2282–2284. [DOI] [PubMed] [Google Scholar]
- 65.Desai R, Haberling D, Holman RC, et al. Impact of rotavirus vaccine on diarrhea-associated disease burden among American Indian and Alaska Native children. Pediatrics. 2012;129(4):e907–913. [DOI] [PubMed] [Google Scholar]
- 66.Eberly MD, Gorman GH, Eide MB, et al. The effect of rotavirus immunization on rotavirus gastroenteritis hospitalization rates in military dependents. Vaccine. 2011;29(4):650–659. [DOI] [PubMed] [Google Scholar]
- 67.Chang HG, Smith PF, Tserenpuntsag B, et al. Reduction in hospitalizations for diarrhea and rotavirus infections in New York state following introduction of rotavirus vaccine. Vaccine. 2010;28(3):754–758. [DOI] [PubMed] [Google Scholar]
- 68.Guerra AH, Stockmann C, Pavia AT, et al. Laboratory-confirmed rotavirus disease in Utah children: clinical and economic impact of rotavirus vaccination. J Pediatric Infect Dis Soc. 2012;1(4):268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mast TC, Walter EB, Bulotsky M, et al. Burden of childhood rotavirus disease on health systems in the United States. Pediatr Infect Dis J. 2010;29(2):e19–25. [DOI] [PubMed] [Google Scholar]
- 70.Payne DC, Staat MA, Edwards KM, et al. Active, population-based surveillance for severe rotavirus gastroenteritis in children in the United States. Pediatrics. 2008;122(6):1235–1243. [DOI] [PubMed] [Google Scholar]
- 71.Payne DC, Staat MA, Edwards KM, et al. Direct and indirect effects of rotavirus vaccination upon childhood hospitalizations in 3 US Counties, 2006–2009. Clin Infect Dis. 2011;53(3):245–253. [DOI] [PubMed] [Google Scholar]
- 72.Lopman BA, Curns AT, Yen C, et al. Infant rotavirus vaccination may provide indirect protection to older children and adults in the United States. J Infect Dis. 2011;204(7):980–986. [DOI] [PubMed] [Google Scholar]
- 73.Kaufman HW, Chen Z. Trends in laboratory rotavirus detection: 2003 to 2014. Pediatrics. 2016;138:4. [DOI] [PubMed] [Google Scholar]
- 74.Aliabadi N, Tate JE, Haynes AK, et al. Centers for Disease C, Prevention. Sustained decrease in laboratory detection of rotavirus after implementation of routine vaccination – United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2015;64(13):337–342. [PMC free article] [PubMed] [Google Scholar]; • Most recent passive laboratory-based rotavirus surveillance report, describing a persistent biennial “peak pattern trend from 2007 to 2012, and decreasing percent of tests positive overall.
- 75.Payne DC, Parashar UD. Epidemiological shifts in severe acute gastroenteritis in US children: will rotavirus vaccination change the picture? J Pediatrics. 2008;153(6):737–738. [DOI] [PubMed] [Google Scholar]
- 76.Payne DC, Szilagyi PG, Staat MA, et al. Secular variation in United States rotavirus disease rates and serotypes: implications for assessing the rotavirus vaccination program [Erratum appears in Pediatr Infect Dis J. 2010 Mar;29(3):287-8;PMID: 20190618]. Pediatr Infect Dis J. 2009;28(11):948–953. [DOI] [PubMed] [Google Scholar]
- 77.Hull JJ, Teel EN, Kerin TK, et al. United States rotavirus strain surveillance from 2005 to 2008: genotype prevalence before and after vaccine introduction. Pediatr Infect Dis J. 2011;30(1 Suppl):S42–47. [DOI] [PubMed] [Google Scholar]
- 78.Sanzone AM, Begue RE. Rotavirus hospital surveillance in the era of immunization. Open Vaccine Journal. 2010;3(1):89–95. [Google Scholar]
- 79.Clark HF, Lawley D, Mallette LA, et al. Decline in cases of rotavirus gastroenteritis presenting to The Children’s Hospital of Philadelphia after introduction of a pentavalent rotavirus vaccine. Clin Vaccine Immunol. 2009;16(3):382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Clark HF, Lawley D, DiStefano D, et al. Distribution of rotavirus genotypes causing nosocomial and community-acquired acute gastroenteritis at the Children’s Hospital of Philadelphia in the new rotavirus vaccine era. Hum Vaccin. 2011;7(11):1118–1123. [DOI] [PubMed] [Google Scholar]
- 81.Bowen MD, Mijatovic-Rustempasic S, Esona MD, et al. Rotavirus strain trends during the postlicensure vaccine era: United States, 2008–2013. J Infect Dis. 2016;214(5):732–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Desai R, Curns AT, Steiner CA, et al. All-cause gastroenteritis and rotavirus-coded hospitalizations among US children, 2000–2009. Clin Infect Dis. 2012;55(4):e28–34. [DOI] [PubMed] [Google Scholar]
- 83.Krishnarajah G, Duh MS, Korves C, et al. Public health impact of complete and incomplete rotavirus vaccination among commercially and Medicaid insured children in the United States. PLoS One. 2016;11(1):e0145977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kilgore A, Donauer S, Edwards KM, et al. Rotavirus-associated hospitalization and emergency department costs and rotavirus vaccine program impact. Vaccine. 2013;31(38):4164–4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.UNICEF. Child survival: under-five mortality current progress and status. New York, NY: UNICEF; [updated 2018 Feb; cited 2018 Jun 21]. Available from: https://data.unicef.org/topic/child-survival/under-five-mortality/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


