PURPOSE
Oncogenic fibroblast growth factor receptor (FGFR) gene alterations have been described in patients with cholangiocarcinoma (CCA). This post hoc analysis assessed progression-free survival (PFS) in patients who had received first- or second-line systemic therapy for advanced/metastatic CCA before enrollment in the phase II FIGHT-202 study (ClinicalTrials.gov identifier: NCT02924376).
PATIENTS AND METHODS
Patients with locally advanced or metastatic CCA with FGFR2 fusions/rearrangements (n = 107), other FGF/FGFR alterations (n = 20), or no FGF/FGFR alterations (n = 18) and documented disease progression after at least one systemic cancer therapy before enrollment in FIGHT-202 were assessed. Prior therapy and disease response data were collated from electronic case report forms. PFS was calculated for each prior line of systemic cancer therapy.
RESULTS
Among patients with FGFR2 fusions/rearrangements, other FGF/FGFR alterations, and no FGF/FGFR alterations, respectively, the median PFS with prior first-line systemic therapy was 5.5 months (95% CI, 4.0 to 8.0; n = 102), 4.4 months (2.7 to 7.1; n = 19), and 2.8 months (1.6 to 11.3; n = 16); the median PFS with prior second-line systemic therapy was 4.2 months (3.0 to 5.3; n = 39), 3.0 months (1.1 to 9.9; n = 8), and 5.9 months (2.4 to 12.5; n = 6). The median PFS was 7.0 months (4.9 to 11.1) for patients with FGFR2 fusions/rearrangements (n = 65) with second-line pemigatinib received during the FIGHT-202 trial.
CONCLUSION
In patients with CCA and FGFR2 fusions or rearrangements, second-line treatment with pemigatinib may be associated with longer PFS compared with second-line treatment with systemic therapy received before study enrollment; however, a prospective controlled trial is required to confirm this. The results support the therapeutic potential of pemigatinib previously demonstrated in FIGHT-202.
INTRODUCTION
Cholangiocarcinoma (CCA) is the most common primary malignancy of the bile duct and comprises a heterogeneous group of tumors, classified as intrahepatic (iCCA) or extrahepatic (eCCA; including perihilar and distal) on the basis of location in the biliary tract.1,2 For the time period 2008-2012, national age-standardized incidence rates worldwide were in the range of 0.26-2.80 per 100,000 person-years for iCCA and 0.08-2.24 per 100,000 person-years for eCCA.3 Most patients with CCA are diagnosed with advanced disease and are ineligible for surgical resection, which is currently the only potentially curable treatment option.4 First-line, standard-of-care systemic therapy for patients with advanced/metastatic biliary tract cancer (BTC) not amenable to surgery is gemcitabine plus cisplatin chemotherapy.4,5 After progression on first-line chemotherapy, there is no standard-of-care second-line systemic therapy, and current second-line systemic chemotherapy is associated with limited survival outcomes, despite continued attempts.6-9 The prognosis and treatment outcomes of patients with BTC may differ according to the tumor location within the biliary tract.9 In a recent retrospective study of second-line chemotherapy in patients with advanced BTCs, including CCA, median overall survival (OS) was longer for patients with iCCA (13.4 months; 95% CI, 10.9 to 17.9 months) compared with gallbladder cancer (9.4 months; 95% CI, 7.2 to 12.3 months) or eCCA (6.8 months; 95% CI, 5.0 to 10.6 months). No difference was observed in median time to treatment failure among the three groups.9 Molecular profiling studies on the basis of next-generation sequencing have revealed distinct patterns of genetic alterations in patients with iCCA versus those with eCCA.10,11 Notably, these studies report potential associations between these genetic alterations and clinical outcomes, which highlight the importance of molecular profiling in addition to the differential diagnosis of CCA types to inform treatment decisions.
CONTEXT
Key Objective
The effects of FGFR2 fusions or rearrangements on progression-free survival (PFS) in patients with cholangiocarcinoma are not fully characterized. This retrospective post hoc analysis assesses the effect of FGFR2 fusions/rearrangements on PFS in patients with cholangiocarcinoma who had received first- or second-line systemic therapy before enrolling in FIGHT-202 to receive pemigatinib.
Knowledge Generated
Median PFS in patients with FGFR2 fusions/rearrangements who received pemigatinib as their second-line treatment in FIGHT-202 was longer than that observed in patients with FGFR2 fusions/rearrangements who had received first- or second-line systemic therapy (other than pemigatinib) immediately before enrolling in FIGHT-202 (7.0 v 4.2 months).
Relevance
The results suggest a PFS advantage of pemigatinib versus other systemic therapies in patients with FGFR2 fusions/rearrangements in both first-line and second-line and highlight the importance of genomic testing to identify patients with genomic alterations who might benefit from targeted therapy.
Among patients with BTC, fusions or rearrangements involving the FGFR2 gene occur almost exclusively in patients with iCCA.11,12 The natural history of CCA in patients with FGFR alterations and the potential prognostic role of FGFR alterations are not fully characterized. In a retrospective study of 377 patients with CCA, patients with FGFR alterations (mainly FGFR2 fusions) were more likely to be younger and have earlier stage disease at presentation and have longer OS, compared with those who did not have FGFR alterations.13 In the same analysis, progression-free survival (PFS) for 177 patients who received first-line chemotherapy was similar between those with and without FGFR alterations.
FIGHT-202 was an open-label, multicenter, phase II study (ClinicalTrials.gov identifier: NCT02924376) of pemigatinib, a selective, potent, oral fibroblast growth factor receptor (FGFR) 1-3 inhibitor,14 in patients with locally advanced or metastatic CCA with or without FGF/FGFR alterations who had progressed on at least one line of prior systemic therapy.15 In patients with FGFR2 fusions or rearrangements who were treated with pemigatinib, the objective response rate was 37.0% (40 of 108 [95% CI, 27.9 to 46.9]; four complete responses and 36 partial responses) and the median PFS was 7.0 months (95% CI, 6.1 to 10.5). The median OS was 17.5 months (95% CI, 14.4 to 22.9).16 This study provided support for the approval of pemigatinib for patients with previously treated, unresectable locally advanced or metastatic CCA harboring FGFR2fusion or other rearrangements in the United States, Japan, and Europe.17-19
The design of FIGHT-202 provided an opportunity to evaluate post hoc the question of whether FGFR2fusions or rearrangements affect the outcomes to systemic therapy in patients with CCA. This post hoc analysis of data from FIGHT-202 assessed PFS in patients who, before study enrollment, had received first- or second-line systemic therapy for advanced/metastatic CCA harboring FGFR2 fusions or rearrangements, other FGF/FGFR genomic alterations, or no FGF/FGFR genomic alterations.
PATIENTS AND METHODS
Study Design and Patients
Details of the FIGHT-202 study design have been previously reported.15 Briefly, patients with locally advanced or metastatic CCA with documented disease progression after at least one previous systemic cancer therapy were assigned to cohorts on the basis of the presence and type of FGF/FGFR alterations (confirmed by a central laboratory): patients with FGFR2 fusions or rearrangements, patients with other FGF/FGFR alterations, and patients with no FGF/FGFR alterations. Information on exposure to prior therapies was collected per protocol, including start date; best response; date of, and reason for, discontinuation of prior therapies; and dates of disease progression.
Prior therapy and disease response data for this analysis were collated from electronic case report forms transcribed from source documents at each investigational site. Evaluable patients had at minimum the month and year recorded for the initiation and termination of prior treatments.
A prior line of systemic cancer therapy (LOSCT) was a patient-based treatment that was administered orally or intravenously (not locally or regionally) and was considered a treatment for advanced/metastatic CCA and was defined as follows: (1) a therapy with a purpose of treatment reported as first-line, advanced/metastatic, or palliative therapy; (2) a therapy reported as neoadjuvant; (3) a therapy reported as adjuvant treatment if administered within 12 months before disease progression; and (4) a therapy reported as maintenance if administered within 12 months before disease progression. The same therapy or combination regimen administered twice separated by an interval of at least 6 months was considered two different lines of therapy. For LOSCT with multiple agents comprising a combination regimen, the therapies could have different start and end dates. The start date of a LOSCT was the earliest initiation date among any therapy comprising that LOSCT, and the end date of the LOSCT was the latest end date among any therapy comprising on the same LOSCT.
For this post hoc analysis, systemic therapy includes any systemic cancer therapy received before enrollment in FIGHT-202 (eg, chemotherapy, targeted therapy [with the exception of selective FGFR inhibitors, including pemigatinib], and checkpoint inhibitors).
End Points
PFS was calculated for each prior LOSCT received and was defined as the initiation date of that LOSCT until the date of progression. Combination treatment (eg, gemcitabine plus cisplatin) was recorded from multiple records, one for each drug included in the combination regimen. In cases where individual drugs within a combination regimen had different initiation and termination dates, the earliest initiation date and the latest termination date associated with that combination regimen in that line of therapy were used. Best response for each LOSCT was defined as the best response from all the treatments within the same line of therapy. The reason for discontinuation of the last treatment in a combination regimen was recorded as the reason for discontinuation of that line of therapy. If multiple reasons for discontinuation were given and they did not contradict each other, all were included as reasons for discontinuation. The progression date was defined as the first documented date of progression on that line of therapy, which could have been during any of the treatments comprising a combination regimen. When a progression date was not documented for a line of therapy and there were no other indicators of disease progression (eg, reasons for discontinuation or best response), the patient was considered censored at the end date of the LOSCT.
Statistical Analysis
The primary outcome for this post hoc analysis was PFS in patients with FGFR2 fusions or rearrangements receiving second-line treatment before enrollment in FIGHT-202. The Kaplan-Meier method was used to calculate the PFS for each prior LOSCT, and median PFS and associated 95% CIs were estimated. Continuous variables were summarized descriptively using mean (standard deviation), median, and range. Variables collected in binary fashion were described using number and proportion with 95% CI when applicable.
RESULTS
Patients
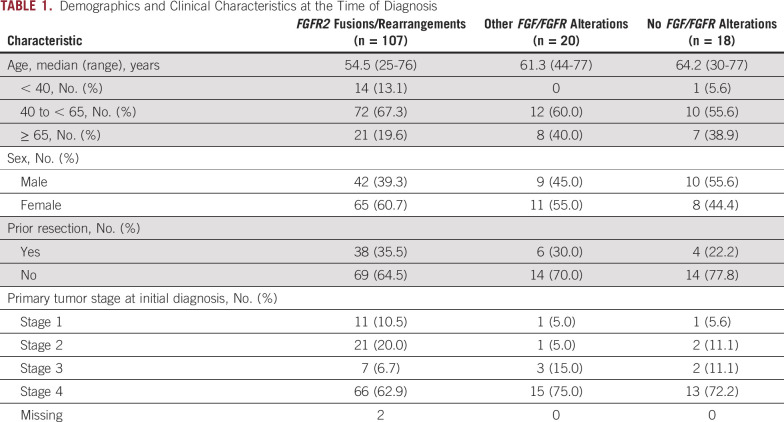
In total, 145 patients with confirmed FGF/FGFR status by central laboratory were enrolled in FIGHT-202: 107 patients with FGFR2 fusions or rearrangements, 20 patients with other FGF/FGFR genomic alterations, and 18 patients with no FGF/FGFR genomic alterations. Detailed demographics and clinical characteristics of patients at the time of enrollment in FIGHT-202 have been previously published.15 Patients with FGFR2 fusions or rearrangements tended to be younger at the time of diagnosis and were diagnosed at an earlier tumor stage compared with those patients with other or no FGF/FGFR genomic alterations (Table 1).
TABLE 1.
Demographics and Clinical Characteristics at the Time of Diagnosis
For first-line systemic therapy received before enrollment in FIGHT-202, data were available for 102 patients with FGFR2 fusions or rearrangements, 19 with other FGF/FGFR genomic alterations, and 16 with no FGF/FGFR genomic alterations; for second-line systemic therapy received before enrollment, data were available for 39 patients with FGFR2 fusions or rearrangements, eight patients with other FGF/FGFR genomic alterations, and six patients with no FGF/FGFR genomic alterations (Fig 1). During FIGHT-202, pemigatinib was received as second-line therapy by 65 patients with FGFR2 fusions or rearrangements, 12 patients with other FGF/FGFR genomic alterations, and 12 patients with no FGF/FGFR genomic alterations (Fig 1). Overall, the most frequently reported systemic therapies received before enrollment in FIGHT-202 were pyrimidine analogs (99.3%) and platinum compounds (93.8%). The most common combination regimen received was gemcitabine plus cisplatin (77.9%), which was received primarily in first-line (67.6%). Across treatment lines, gemcitabine was received as monotherapy in 6.9% of patients; no patients received cisplatin as monotherapy.
FIG 1.
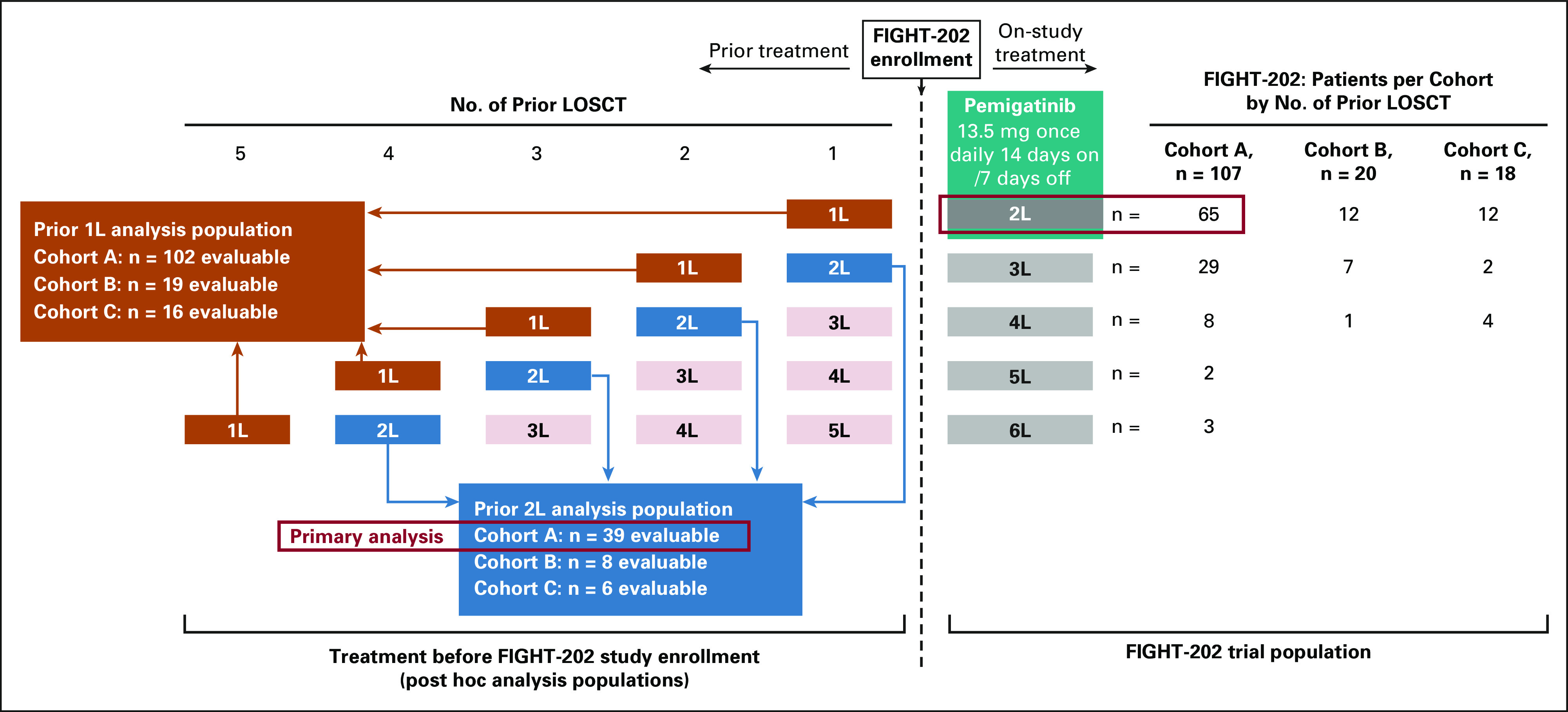
Distribution of patients by line of therapy. Cohort A, patients with FGFR2 fusions or rearrangements. Cohort B, patients with other FGF/FGFR alterations. Cohort C, patients with no FGF/FGFR alterations. 1L, first-line; 2L, second-line; LOSCT, line of systemic cancer therapy.
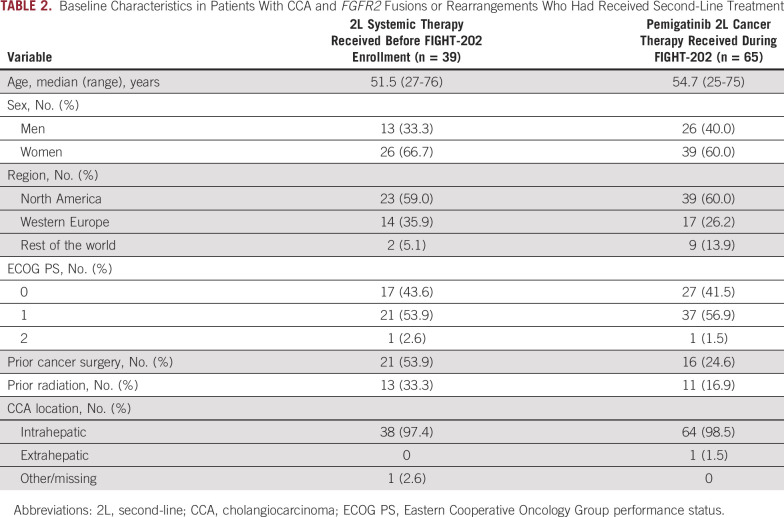
Demographic and disease characteristics of patients with FGFR2 fusions or rearrangements who received a second-line systemic therapy before enrollment in FIGHT-202 and of patients who received pemigatinib as second-line therapy as part of FIGHT-202 are summarized in Table 2. Characteristics were generally similar; however, patients receiving a second-line systemic therapy before enrollment in FIGHT-202 were more likely to have received prior cancer surgery (54% v 25%) and prior radiation (33% v 17%) compared with patients receiving pemigatinib second-line as part of the FIGHT-202 study (Table 2).
TABLE 2.
Baseline Characteristics in Patients With CCA and FGFR2 Fusions or Rearrangements Who Had Received Second-Line Treatment
Progression-Free Survival
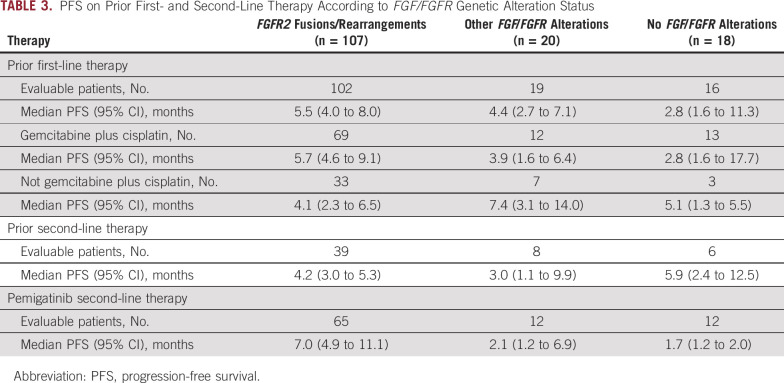
The median PFS with first-line systemic therapy received before enrollment in FIGHT-202 was 5.5 months (95% CI, 4.0 to 8.0) for patients with FGFR2 fusions or rearrangements (n = 102), 4.4 months (95% CI, 2.7 to 7.1) for patients with other FGF/FGFR alterations (n = 19), and 2.8 months (95% CI, 1.6 to 11.3) for patients with no FGF/FGFR alterations (n = 16; Table 3). Two thirds of patients with FGFR2 fusions or rearrangements (69 of 102, 67.6%) had received a combination regimen that included gemcitabine plus cisplatin as first-line treatment. The median PFS for patients with FGFR2 fusions or rearrangements with first-line gemcitabine plus cisplatin was 5.7 months (95% CI, 4.6 to 9.1) and 4.1 months (95% CI, 2.3 to 6.5) for patients with FGFR2 fusions or rearrangements who had received a regimen other than gemcitabine plus cisplatin as first-line treatment before enrollment in FIGHT-202 (including gemcitabine as monotherapy or in combination with oxaliplatin or a fluorouracil-containing regimen). The median PFS with first-line gemcitabine plus cisplatin was 3.9 months (95% CI, 1.6 to 6.4) for patients with other FGF/FGFR genomic alterations (n = 12) and 2.8 months (95% CI, 1.6 to 17.7) for patients with no FGF/FGFR genomic alterations (n = 13; Table 3).
TABLE 3.
PFS on Prior First- and Second-Line Therapy According to FGF/FGFR Genetic Alteration Status
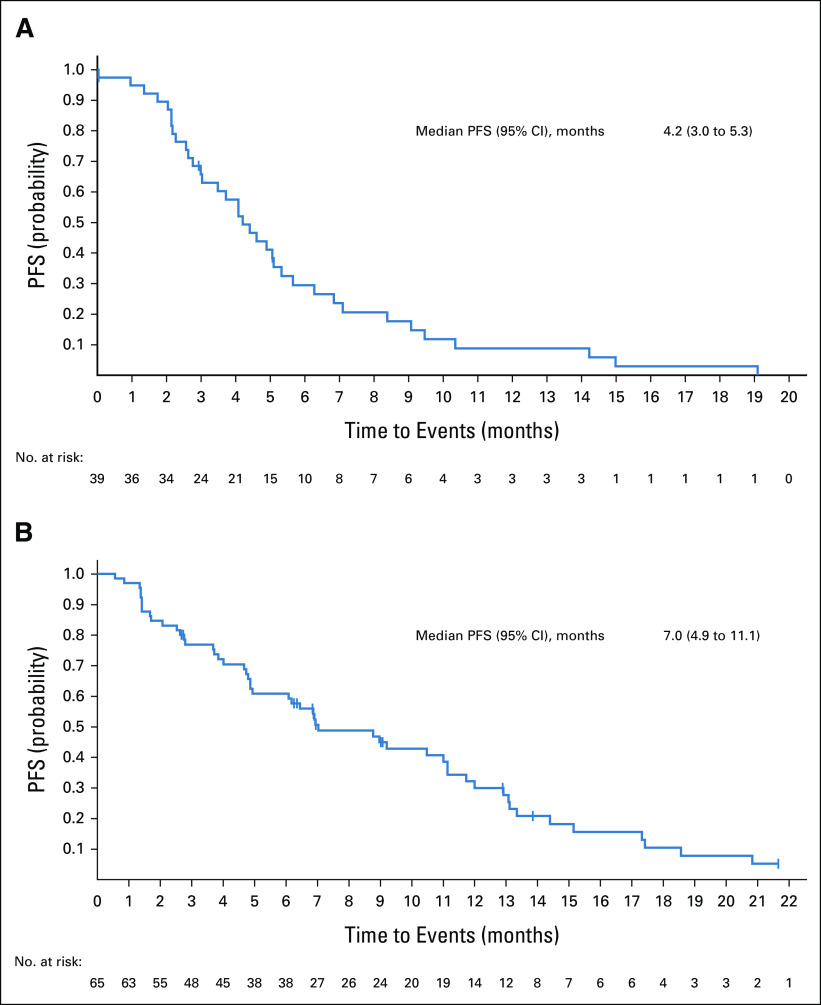
Among patients with FGFR2 fusions or rearrangements, 93% (38 of 41) received second-line chemotherapy (most commonly gemcitabine plus cisplatin [17%], infusional combination of fluorouracil, leucovorin calcium [folinic acid], and oxaliplatin [FOLFOX; 17%], or fluorouracil plus oxaliplatin [12%]) before enrollment in FIGHT-202. Seven percent (3 of 41) received second-line antiprogrammed cell death protein 1 or targeted therapy (nivolumab, pazopanib, or ponatinib) before enrollment in FIGHT-202. The median PFS with second-line therapy before enrollment in FIGHT-202 among these patients was 4.2 months (95% CI, 3.0 to 5.3; n = 39; Fig 2; Table 3). Among patients with other or no FGF/FGFR genomic alterations, the median PFS with prior second-line therapy was 3.0 months (95% CI, 1.1 to 9.9; n = 8) and 5.9 months (95% CI, 2.4 to 12.5; n = 6), respectively. Given the small numbers of patients with other FGF/FGFR genomic alterations or no FGF/FGFR genomic alterations enrolled in FIGHT-202 who had received a first- or second-line regimen, these data should be interpreted with caution.
FIG 2.
Second-line PFS in patients with FGFR2 fusions or rearrangements after (A) systemic therapy received before FIGHT-202 enrollment and (B) pemigatinib received during FIGHT-202. PFS, progression-free survival.
In patients who had progressed after only one line of systemic therapy before enrollment into FIGHT-202 and received pemigatinib as second-line therapy, the median PFS with pemigatinib was 7.0 months (95% CI, 4.9 to 11.1; n = 65) in those with FGFR2 fusions or rearrangements, 2.1 months (95% CI, 1.2 to 6.9; n = 12) in those with other FGF/FGFR alterations, and 1.7 months (95% CI, 1.2 to 2.0; n = 12) in those with no FGF/FGFR alterations (Fig 2; Table 3).
DISCUSSION
Few studies have assessed response to therapy in patients with CCA with specific genomic alterations. The FIGHT-202 study evaluated the efficacy and safety of pemigatinib in patients with advanced, unresectable, or metastatic CCA who had progressed on one or more prior lines of systemic therapy.15 As part of FIGHT-202, information regarding prior treatments and response data for enrolled patients was collected by the investigators, providing an opportunity to retrospectively investigate the response to systemic therapy in patients with FGFR2 fusions or rearrangements, patients with other FGF/FGFR alterations, and patients with no FGF/FGFR alterations.
The results of this post hoc analysis show that median PFS with first-line systemic therapy received before enrollment in FIGHT-202 was longer for patients with FGFR2 fusions or rearrangements than with other FGF/FGFR alterations (5.5 v 4.4 months) or with no FGF/FGFR alterations (2.8 months). A similar pattern in PFS was observed across cohorts for patients receiving gemcitabine plus cisplatin as first-line therapy (FGFR2 fusions or rearrangements, 5.7 months; other FGF/FGFR alterations, 3.9 months; and no FGF/FGFR alterations, 2.8 months). In a retrospective analysis of patients with iCCA receiving first-line chemotherapy at Memorial Sloan Kettering, the median PFS was 6.2 months for patients with FGFR2 fusions or rearrangements and 7.2 months for patients with no FGFR2 alterations.20 The retrospective study by Jain et al reported that the median PFS in patients with FGFR alterations receiving first-line chemotherapy for CCA was numerically longer compared with those without FGFR alterations (33.9 v 25.4 weeks); however, this difference did not reach statistical significance (P = .07).13 Another retrospective, observational study based on real-world data from 571 patients with advanced CCA compared OS in patients with FGFR2 fusions/rearrangements versus those without FGFR2 alterations.21 Although patients were not selected by therapy received or by therapy line, median OS was numerically longer for patients with FGFR2 fusions/rearrangements compared with those without FGFR2 alterations (12.1 v 7.1 months). However, this difference did not attain significance (log-rank P = .184), and FGFR2 status was not reported to be a significant predictor of OS in multivariate models.21 In general, because of the divergent study designs (eg, prospective versus retrospective), patient populations, and small sample sizes, caution should be exercised in drawing conclusions from comparisons across these studies.
Previous studies evaluating the efficacy of systemic chemotherapy in patients with advanced BTC, including CCA, have typically been conducted in molecularly unselected patient populations, which have generally yielded a longer median PFS than observed here.5,22 First-line gemcitabine plus cisplatin was associated with a median PFS of 8.0 months in the ABC-02 trial that was conducted in molecularly unselected patients with primary tumors in gallbladder and ampulla of Vater, as well as CCA.5 In a pooled post hoc analysis of 66 molecularly unselected patients with iCCA receiving first-line gemcitabine plus cisplatin in the ABC-01, -02, and -03 trials, the median PFS was 8.4 months (95% CI, 5.9 to 8.9).22 Comparisons of PFS in molecularly selected versus unselected patients is confounded by differences in patient populations and in study design, especially the frequency of radiologic evaluations.
The median PFS in 107 patients with FGFR2 fusions or rearrangements receiving pemigatinib in FIGHT-202 as second-line or later therapy was 6.9 months.15 In the present analysis, the median PFS in patients with FGFR2 fusions or rearrangements who received second-line pemigatinib in FIGHT-202 was 7.0 months. This median PFS is longer than that observed in patients with CCA and FGFR2 fusions or rearrangements who had received second-line systemic therapy immediately before enrolling in FIGHT-202 (4.2 months). These data suggest that pemigatinib is associated with a meaningful clinical benefit over other systemic therapy (predominantly chemotherapy) in the second-line setting for patients with advanced or metastatic CCA harboring FGFR2 fusions or rearrangements.
In a recent retrospective analysis reporting outcomes with second-line chemotherapy in patients with advanced or metastatic CCA harboring FGFR2 fusions who previously received gemcitabine-based chemotherapy,23 the median PFS was 4.6 months, which is similar to the 4.2 months observed in our study in patients with FGFR2 fusions or rearrangements receiving second-line therapy before enrollment in FIGHT-202. In the retrospective analysis of patients with iCCA receiving second-line chemotherapy at Memorial Sloan Kettering, the median PFS was 5.6 months for patients with FGFR2 fusions or rearrangements and 3.7 months for patients with no FGFR2 alterations.20
Various systemic chemotherapy regimens have been investigated in the second-line setting for molecularly unselected advanced BTC, in both retrospective studies6,9,24-26 and prospective clinical trials.7,8 A systematic review of second-line chemotherapy in advanced BTC reported a weighted mean PFS of only 3.2 months and a weighted mean OS of 7.2 months27; another systematic review and meta-analysis of second-line treatments for advanced BTC reported a weighted median PFS of 2.6 months and a weighted median OS of 6.5 months.28 The randomized ABC-06 trial comparing active symptom control (ASC) plus FOLFOX with ASC only in molecularly unselected patients with locally advanced or metastatic BTC with disease progression on first-line gemcitabine plus cisplatin reported a median OS of 6.2 months and 5.3 months, respectively.8 In patients with iCCA, the median OS was 5.7 months and 5.2 months, respectively. For patients receiving ASC plus FOLFOX, the median PFS was 4.0 months for all patients with BTC and 3.3 months for patients with iCCA.8 The randomized phase II, NIFTY trial compared the efficacy and safety of liposomal irinotecan plus fluorouracil and leucovorin with that of fluorouracil and leucovorin alone in 174 molecularly unselected patients with metastatic BTC who had progressed on gemcitabine plus cisplatin.29 Liposomal irinotecan plus fluorouracil and leucovorin was associated with a median PFS that was significantly longer than that of fluorouracil and leucovorin alone (7.1 v 1.4 months; P = .0019).29
In FIGHT-202, patients with FGFR2 fusions or rearrangements tended to be diagnosed with CCA at a younger age and at an earlier disease stage compared with patients with other types or no FGF/FGFR alterations.15 This is consistent with a retrospective analysis of 377 patients with CCA treated at four major US cancer centers, which found that, compared with patients with CCA who did not have any FGFR genomic alterations, a higher proportion of patients with CCA with FGFR genomic alterations were younger than 40 years of age at presentation and presented with early stage disease.13 Contributions of patient demographic and disease characteristics to the association of FGFR2 status with PFS on chemotherapy are likely highly confounded, and further studies would be required to delineate these factors. Regardless, in the current study in patients with FGFR2 fusions or rearrangements, second-line treatment with pemigatinib suggested an improvement in median PFS compared with patients who had received second-line therapy before enrollment in FIGHT-202.
Limitations on the interpretation of the results of this analysis include the fact that it was based on post hoc assessment of retrospective, investigator-reported medical histories, and responses to prior systemic therapy. Thus, progression on systemic therapy before enrollment in FIGHT-202 was assessed locally by the investigators, whereas these assessments made during the FIGHT-202 study were based on confirmed central radiologic review. In addition, because of the small population of patients with other or no FGF/FGFR genomic alterations, numerical differences between cohorts should be interpreted with caution, and any trends observed await confirmation in future prospective, randomized studies.
Given the paucity of information regarding the response to chemotherapy in patients with CCA harboring FGFR2 fusions or rearrangements, this post hoc analysis adds to the recent retrospective studies mentioned above.13,20,21,23 The results suggest that second-line treatment with pemigatinib is associated with longer PFS when compared with either first- or second-line treatment with other systemic therapy in patients with CCA harboring FGFR2 fusions or rearrangements. Additional insight into appropriate treatment sequencing with pemigatinib will come from the ongoing phase III study of first-line pemigatinib versus gemcitabine plus cisplatin in patients with unresectable or metastatic CCA harboring FGFR2 fusions or rearrangements (FIGHT-302; ClinicalTrials.gov identifier: NCT03656536).30
Our results also highlight the importance of genomic testing to identify patients with advanced CCA harboring FGFR or other actionable genomic alterations who might benefit from targeted therapies.31 Further research is warranted to prospectively characterize the disease course and response to chemotherapy in patients with and without FGFR2 fusions and, in the real-world setting, to characterize the second-line treatment outcomes of patients with FGFR2 alterations outside of a clinical trial.
ACKNOWLEDGMENT
The authors wish to thank the participants and their families, investigators, and site personnel who participated in the FIGHT-202 study. FIGHT-202 and this post hoc analysis were sponsored by Incyte Corporation (Wilmington, DE). Medical writing assistance was provided by Abigail Marmont, PhD, CMPP, of Envision Pharma Group (Philadelphia, PA) and funded by Incyte Corporation.
Kristen Bibeau
Employment: Incyte
Stock and Other Ownership Interests: Incyte
Research Funding: Incyte
Luis Féliz
Employment: Incyte
Stock and Other Ownership Interests: Incyte
Christine F. Lihou
Employment: Incyte
Stock and Other Ownership Interests: Incyte
Research Funding: Incyte (Inst)
Haobo Ren
Stock and Other Ownership Interests: Incyte
Ghassan K. Abou-Alfa
Consulting or Advisory Role: Silenseed (I), Eisai, Ipsen, Merck Serono, AstraZeneca, CytomX Therapeutics (I), BeiGene, Genoscience Pharma, RedHill Biopharma, SOBI (I), Yiviva, Flatiron Health, Roche/Genentech, Autem Medical, Berry Genomics, Incyte, Vector Health, Helio Health, Alnylam, Adicet Bio, Exelixis, Nerviano Medical Sciences, QED Therapeutics, Center for Emerging & Neglected Diseases (CEND) (I), Celgene (I), Lilly (I), Rafael Pharmaceuticals (I), Servier
Research Funding: Bayer (Inst), AstraZeneca (Inst), Bristol Myers Squibb (Inst), Incyte (Inst), Agios (Inst), Polaris (Inst), Puma Biotechnology (Inst), QED Therapeutics (Inst), Arcus Ventures (Inst), BioNtech (Inst), Celgene (Inst), Flatiron Health (Inst), Genentech/Roche (Inst), Genoscience Pharma (Inst), QED Therapeutics (Inst), Silenseed (Inst), Yiviva (Inst)
Travel, Accommodations, Expenses: Polaris
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at ASCO-Gastrointestinal Cancers Symposium (ASCO-GI), San Francisco, CA, January 23-25, 2020, and ESMO World Congress on Gastrointestinal Cancer, virtual meeting, June 30-July 3, 2021.
SUPPORT
Funding was provided by Incyte Corporation.
DATA SHARING STATEMENT
Incyte Corporation (Wilmington, DE) is committed to data sharing that advances science and medicine while protecting patient privacy. Qualified external scientific researchers may request anonymized data sets owned by Incyte for the purpose of conducting legitimate scientific research. Researchers may request anonymized data sets from any interventional study (except phase I studies) for which the product and indication have been approved on or after January 1, 2020, in at least one major market (eg, the United States, the European Union, and Japan). Data will be available for request after the primary publication or 2 years after the study has ended. Information on Incyte's clinical trial data sharing policy and instructions for submitting clinical trial data requests are available at: https://www.incyte.com/Portals/0/Assets/Compliance%20and%20Transparency/clinical-trial-data-sharing.pdf?ver=2020-05-21-132838-960.
AUTHOR CONTRIBUTIONS
Conception and design: Kristen Bibeau, Luis Féliz, Christine F. Lihou, Ghassan K. Abou-Alfa
Financial support: Kristen Bibeau, Luis Féliz, Haobo Ren
Administrative support: Kristen Bibeau, Haobo Ren
Provision of study materials or patients: Kristen Bibeau, Luis Féliz, Christine F. Lihou
Collection and assembly of data: Kristen Bibeau, Luis Féliz, Christine F. Lihou, Ghassan K. Abou-Alfa
Data analysis and interpretation: Luis Féliz, Christine F. Lihou, Haobo Ren, Ghassan K. Abou-Alfa
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Kristen Bibeau
Employment: Incyte
Stock and Other Ownership Interests: Incyte
Research Funding: Incyte
Luis Féliz
Employment: Incyte
Stock and Other Ownership Interests: Incyte
Christine F. Lihou
Employment: Incyte
Stock and Other Ownership Interests: Incyte
Research Funding: Incyte (Inst)
Haobo Ren
Stock and Other Ownership Interests: Incyte
Ghassan K. Abou-Alfa
Consulting or Advisory Role: Silenseed (I), Eisai, Ipsen, Merck Serono, AstraZeneca, CytomX Therapeutics (I), BeiGene, Genoscience Pharma, RedHill Biopharma, SOBI (I), Yiviva, Flatiron Health, Roche/Genentech, Autem Medical, Berry Genomics, Incyte, Vector Health, Helio Health, Alnylam, Adicet Bio, Exelixis, Nerviano Medical Sciences, QED Therapeutics, Center for Emerging & Neglected Diseases (CEND) (I), Celgene (I), Lilly (I), Rafael Pharmaceuticals (I), Servier
Research Funding: Bayer (Inst), AstraZeneca (Inst), Bristol Myers Squibb (Inst), Incyte (Inst), Agios (Inst), Polaris (Inst), Puma Biotechnology (Inst), QED Therapeutics (Inst), Arcus Ventures (Inst), BioNtech (Inst), Celgene (Inst), Flatiron Health (Inst), Genentech/Roche (Inst), Genoscience Pharma (Inst), QED Therapeutics (Inst), Silenseed (Inst), Yiviva (Inst)
Travel, Accommodations, Expenses: Polaris
No other potential conflicts of interest were reported.
REFERENCES
- 1.Rizvi S, Borad MJ: The rise of the FGFR inhibitor in advanced biliary cancer: The next cover of time magazine? J Gastrointest Oncol 7:789-796, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louis C, Papoutsoglou P, Coulouarn C: Molecular classification of cholangiocarcinoma. Curr Opin Gastroenterol 36:57-62, 2020 [DOI] [PubMed] [Google Scholar]
- 3.Florio AA, Ferlay J, Znaor A, et al. : Global trends in intrahepatic and extrahepatic cholangiocarcinoma incidence from 1993 to 2012. Cancer 126:2666-2678, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan SA, Davidson BR, Goldin RD, et al. : Guidelines for the diagnosis and treatment of cholangiocarcinoma: An update. Gut 61:1657-1669, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Valle J, Wasan H, Palmer DH, et al. : Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 362:1273-1281, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Brieau B, Dahan L, De Rycke Y, et al. : Second-line chemotherapy for advanced biliary tract cancer after failure of the gemcitabine-platinum combination: A large multicenter study by the Association des Gastro-Enterologues Oncologues. Cancer 121:3290-3297, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Kim ST, Oh SY, Lee J, et al. : Capecitabine plus oxaliplatin as a second-line therapy for advanced biliary tract cancers: A multicenter, open-label, phase II trial. J Cancer 10:6185-6190, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamarca A, Palmer DH, Wasan HS, et al. : Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): A phase 3, open-label, randomised, controlled trial. Lancet Oncol 22:690-701, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lowery MA, Goff LW, Keenan BP, et al. : Second-line chemotherapy in advanced biliary cancers: A retrospective, multicenter analysis of outcomes. Cancer 125:4426-4434, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Churi CR, Shroff R, Wang Y, et al. : Mutation profiling in cholangiocarcinoma: Prognostic and therapeutic implications. PLoS One 9:e115383, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lowery MA, Ptashkin R, Jordan E, et al. : Comprehensive molecular profiling of intrahepatic and extrahepatic cholangiocarcinomas: Potential targets for intervention. Clin Cancer Res 24:4154-4161, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arai Y, Totoki Y, Hosoda F, et al. : Fibroblast growth factor receptor 2 tyrosine kinase fusions define a unique molecular subtype of cholangiocarcinoma. Hepatology 59:1427-1434, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Jain A, Borad MJ, Kelley RK, et al. : Cholangiocarcinoma with FGFR genetic aberrations: A unique clinical phenotype. JCO Precis Oncol 10.1200/PO.17.00080, 2018 [DOI] [PubMed] [Google Scholar]
- 14.Liu PCC, Koblish H, Wu L, et al. : INCB054828 (pemigatinib), a potent and selective inhibitor of fibroblast growth factor receptors 1, 2, and 3, displays activity against genetically defined tumor models. PLoS One 15:e0231877, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abou-Alfa GK, Sahai V, Hollebecque A, et al. : Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol 21:671-684, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abou-Alfa GK, Sahai V, Hollebecque A, et al. : Pemigatinib for previously treated locally advanced/metastatic cholangiocarcinoma (CCA): Update of FIGHT-202. J Clin Oncol 39, 2021. (abstr 4086) [Google Scholar]
- 17.PEMAZYRE (Pemigatinib) Prescribing Information. https://www.pemazyre.com/pdf/prescribing-information.pdf [Google Scholar]
- 18.Pemazyre (Pemigatinib) European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/EPAR/pemazyre [Google Scholar]
- 19.Incyte announces approval of Pemazyre® (pemigatinib) in Japan for the treatment of patients with unresectable biliary tract cancer (BTC) with a fibroblast growth factor receptor 2 (FGFR2) fusion gene, worsening after cancer chemotherapy. https://investor.incyte.com/press-releases/press-releases/2021/Incyte-Announces-Approval-of-Pemazyre-pemigatinib-in-Japan-for-the-Treatment-of-Patients-with-Unresectable-Biliary-Tract-Cancer-BTC-with-a-Fibroblast-Growth-Factor-Receptor-2-FGFR2-Fusion-Gene-Worsening-After-Cancer-Chemotherapy/default.aspx
- 20.Abou-Alfa GK, Bibeau K, Schultz N, et al. : Effect of FGFR2 alterations on survival in patients receiving systemic chemotherapy for intrahepatic cholangiocarcinoma. J Clin Oncol 39, 2021. (suppl 3; abstr 303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shroff RT, Rearden J, Li A, et al. : Natural history of patients (pts) with advanced cholangiocarcinoma (CCA) with FGFR2 gene fusion/rearrangement or wild-type (WT) FGFR2. J Clin Oncol 39, 2021. (abstr 4089) [Google Scholar]
- 22.Lamarca A, Ross P, Wasan HS, et al. : Advanced intrahepatic cholangiocarcinoma: Post hoc analysis of the ABC-01, -02, and -03 clinical trials. J Natl Cancer Inst 112:200-210, 2020 [DOI] [PubMed] [Google Scholar]
- 23.Javle MM, Sadeghi S, El-Khoueiry AB, et al. : A retrospective analysis of post second-line chemotherapy treatment outcomes for patients with advanced or metastatic cholangiocarcinoma and FGFR2 fusions. J Clin Oncol 38, 2020. (abstr 4591) [Google Scholar]
- 24.Mizrahi JD, Gunchick V, Mody K, et al. : Multi-institutional retrospective analysis of FOLFIRI in patients with advanced biliary tract cancers. World J Gastrointest Oncol 12:83-91, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rogers JE, Law L, Nguyen VD, et al. : Second-line systemic treatment for advanced cholangiocarcinoma. J Gastrointest Oncol 5:408-413, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schweitzer N, Kirstein MM, Kratzel AM, et al. : Second-line chemotherapy in biliary tract cancer: Outcome and prognostic factors. Liver Int 39:914-923, 2019 [DOI] [PubMed] [Google Scholar]
- 27.Lamarca A, Hubner RA, David Ryder W, et al. : Second-line chemotherapy in advanced biliary cancer: A systematic review. Ann Oncol 25:2328-2338, 2014 [DOI] [PubMed] [Google Scholar]
- 28.Ying J, Chen J: Combination versus mono-therapy as salvage treatment for advanced biliary tract cancer: A comprehensive meta-analysis of published data. Crit Rev Oncol Hematol 139:134-142, 2019 [DOI] [PubMed] [Google Scholar]
- 29.Yoo C, Kim KP, Jeong JH, et al. : Liposomal irinotecan plus fluorouracil and leucovorin versus fluorouracil and leucovorin for metastatic biliary tract cancer after progression on gemcitabine plus cisplatin (NIFTY): A multicentre, open-label, randomised, phase 2b study. Lancet Oncol 22:1560-1572, 2021 [DOI] [PubMed] [Google Scholar]
- 30.Bekaii-Saab TS, Valle JW, Van Cutsem E, et al. : FIGHT-302: First-line pemigatinib vs gemcitabine plus cisplatin for advanced cholangiocarcinoma with FGFR2 rearrangements. Future Oncol 16:2385-2399, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bekaii-Saab TS, Bridgewater J, Normanno N: Practical considerations in screening for genetic alterations in cholangiocarcinoma. Ann Oncol 32:1111-1126, 2021 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Incyte Corporation (Wilmington, DE) is committed to data sharing that advances science and medicine while protecting patient privacy. Qualified external scientific researchers may request anonymized data sets owned by Incyte for the purpose of conducting legitimate scientific research. Researchers may request anonymized data sets from any interventional study (except phase I studies) for which the product and indication have been approved on or after January 1, 2020, in at least one major market (eg, the United States, the European Union, and Japan). Data will be available for request after the primary publication or 2 years after the study has ended. Information on Incyte's clinical trial data sharing policy and instructions for submitting clinical trial data requests are available at: https://www.incyte.com/Portals/0/Assets/Compliance%20and%20Transparency/clinical-trial-data-sharing.pdf?ver=2020-05-21-132838-960.