Abstract
Objective
This review aimed to systematically determine the optimal nasal saline regimen for different types of sinonasal diseases.
Data Sources
PubMed, Embase, SCOPUS, Cochrane Library, Web of Science, ClinicalTrials.gov. The last search was on December 6, 2021.
Review Methods
Study selection was done by 2 independent authors. Randomized controlled trials and meta-analyses were included. The effects of nasal saline treatment through various devices, saline tonicities, and buffer statuses were evaluated in patients with allergic and nonallergic rhinitis, acute and chronic rhinosinusitis (CRS), CRS with cystic fibrosis, and postoperative care, including septoplasty/turbinoplasty and endoscopic sinus surgery.
Results
Sixty-nine studies were included: 10 meta-analyses and 59 randomized controlled trials. For allergic rhinitis, large-volume devices (≥60 mL) were effective for treating adults, while low-volume devices (5-59 mL) were effective for children. Isotonic saline was preferred over hypertonic saline due to fewer adverse events. For acute rhinosinusitis, saline irrigation was beneficial in children, but it was an option for adults. Large-volume devices were more effective, especially in the common cold subgroup. For CRS, large-volume devices were effective for adults, but saline drop was the only regimen that had available data in children. Buffered isotonic saline was more tolerable than nonbuffered or hypertonic saline. The data for CRS with cystic fibrosis and nonallergic rhinitis were limited. For postoperative care, buffered isotonic saline delivered by large-volume devices was effective.
Conclusion
Nasal saline treatment is recommended for treating most sinonasal diseases. Optimal delivery methods for each condition should be considered to achieve therapeutic effects of saline treatment.
Keywords: irrigation, nose, recommendation, saline, sinusitis
Intranasal saline treatment is effective, inexpensive, and safe. 1 It mechanically washes out mucus, secretory aggregation, and inflammatory cytokines from the nose. In addition, it promotes mucociliary clearance and helps reduce mucosal edema. Currently, it is recommended as an adjunct therapy for sinonasal diseases, such as rhinosinusitis, rhinitis, and upper respiratory tract infections. 2 It is unclear whether nasal saline treatment benefits all or only specific types of sinonasal diseases. There are several kinds of devices, such as spray, drop, syringe, pot, aerosol, and squeeze bottle. These devices deliver the saline solution into the nose and paranasal sinuses with different volumes and pressure. Different solutions (buffered or nonbuffered saline) and tonicities (hypertonic or isotonic) are used. These factors contribute to the difference in treatment outcomes.
There are limited systematic reviews that determine the optimal device and regimen of nasal saline treatment for different sinonasal diseases. This systematic review aimed to assess the therapeutic effects and safety of nasal saline treatment for sinonasal diseases and identify an optimal delivery method for each sinonasal disease.
Methods
A systematic search was conducted through databases for relevant publications: PubMed, Embase, SCOPUS, Cochrane Database Library, Web of Science, and ClinicalTrials.gov. The date of the last search was December 6, 2021. The search strategy employed combinations of the following Medical Subject Headings keywords: nose, nasal, irrigation, spray, inhalation, atomization, vaporization, saline, and sodium chloride. Randomized controlled trials (RCTs) and meta-analyses of systematic reviews were included. The search terms are provided in the Supplemental File (available online).
Study selection was performed independently by 2 authors using a web-based application for systematic review (covidence.org). Titles and abstracts were screened. Nonrelevant studies and duplicate studies were excluded. Full text of the screened articles was assessed for eligibility. Any disagreement on the study selection process was resolved by discussions until a final consensus was reached. Study selection was based on the following inclusion criteria: (1) RCT or meta-analysis conducted in humans, (2) patients with any sinonasal disease and any age, (3) intranasal saline treatment with any delivery method (tonicity, buffered, pH, temperature, volume, and device), (4) comparison between saline treatment and no-saline treatment or between 2 delivery methods, and (5) any clinical outcomes.
Studies were excluded per the following criteria: (1) systematic reviews without meta-analysis, (2) conference abstracts without complete data, (3) non-English articles, (4) delivery methods unavailable in the market, (5) medicated saline, and (6) comparison between saline and other medications. Data from studies with mixed populations were excluded when not reported separately. The meta-analyses were thoroughly cross-checked for the included studies, duplication, and analyses.
The device was categorized according to the volume of saline delivered into the nose: very low (<5 mL), low (5-59 mL), and large (≥60 mL). 3 Very low-volume devices included spray, drop, and aerosol; low-volume devices, syringe and jet flow; and large-volume devices, pot and squeeze bottles.
Sinonasal diseases were categorized into 3 groups:
Rhinitis: allergic rhinitis (AR) and nonallergic rhinitis (NAR)
Rhinosinusitis: acute rhinosinusitis (ARS), chronic rhinosinusitis (CRS), and chronic rhinosinusitis with cystic fibrosis (CRS-CF)
Postoperative care: after septoplasty/turbinoplasty and endoscopic sinus surgery (ESS)
The effects of saline treatment in each group were evaluated between saline and no-saline treatment, among different devices, between hypertonic and isotonic solution, and between buffered and nonbuffered saline.
Results
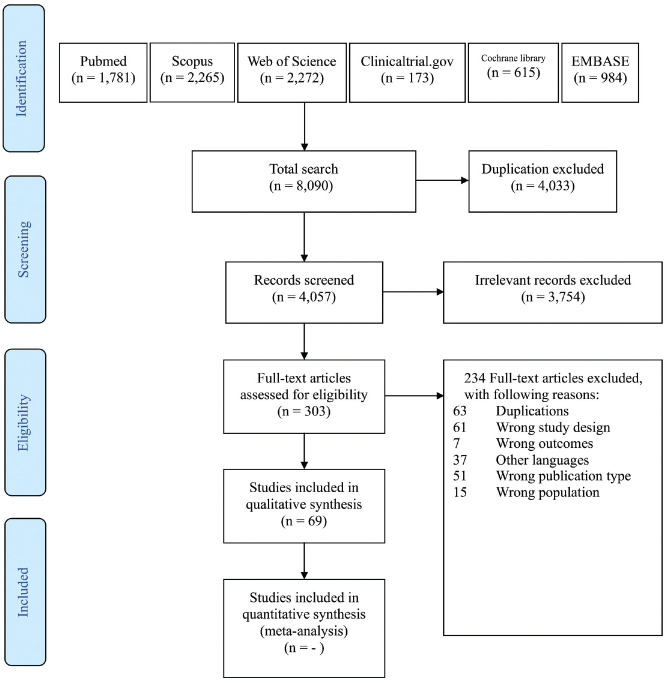
A total of 8090 publications were retrieved, of which 69 were included for review. The PRISMA flowchart (Preferred Reporting Items for Systematic Reviews and Meta-analyses) is displayed in Figure 1 . There were 10 meta-analyses and 59 RCTs (1 study by Ural et al 4 assessed 3 patient subgroups). Twenty-three studies assessed nasal saline treatment in patients with rhinitis (23 AR and 0 NAR). Thirty-five studies assessed rhinosinusitis (17 ARS, 17 CRS, and 1 CRS-CF). Thirteen studies assessed postoperative care (2 septoplasty/turbinoplasty and 11 ESS).
Figure 1.
Diagram of study selections based on the 2009 PRISMA statement (Preferred Reporting Items for Systematic Reviews and Meta-analyses).
Rhinitis
Allergic Rhinitis
Of the 23 studies that assessed the effects of nasal saline treatment in patients with AR, 19 were RCTs and 4 were meta-analyses. Eleven RCTs assessed adult AR,4-14 and 8 RCTs assessed pediatric AR.15-22 Three meta-analyses pooled the data from adult and pediatric AR,1,3,23 and 1 meta-analysis examined data only from children. 24
In adult AR, 4 RCTs compared the effects of saline treatment with no-saline treatment.6,7,10,13 Duration of treatment ranged from 2 to 6 weeks. Three studies favored the saline treatment in symptom reduction.6,7,10 The benefits were demonstrated after 2 to 3 weeks and continued until 4 to 6 weeks. Patients in the saline treatment group used fewer antihistamines.7,10,13 The meta-analyses showed that saline treatment was superior in symptom improvements.3,23 Adverse effects were not different from control.
In pediatric AR, 6 RCTs compared the effects of saline treatment with no-saline treatment.15,17,19-22 All RCTs reported benefits of nasal saline treatment over control. Decreases in symptom score and antihistamine usage favored the nasal saline treatment at 4 weeks. 19 The addition of saline spray to nasal steroid showed beneficial effects, such as reduced dosage of intranasal steroid spray, at 8 to 12 weeks.17,22 In children with asthma, quality of life (QoL) improvement was shown at 12 weeks. 15 The meta-analyses showed decreases in symptoms3,23 and antihistamine usage. 24 However, the disease-specific health-related QoL was not affected. 3 Temporary otalgia and epistaxis were noted. 15
Devices
In adult patients, 1 RCT showed that nasal saline treatment with a squeeze bottle (240 mL) was better than a syringe (20 mL) in reducing symptoms. 12 There were no adverse effects in either device. 12 Spray was effective when compared with baseline.9,10 Yet, there were no comparisons between spray and other devices.
No study directly compared the effects among different devices in children. Many devices (spray, atomizer device, or large-volume syringe) provided beneficial effects. Minor adverse events, such as otalgia, ear fullness, and epistaxis, occurred in 30% of the patients who used a large-volume device 15 but none in very low and low-volume devices.17,19-21
Tonicity
In adult AR, 5 RCTs compared hypertonic saline (range, 1.8%-3%) and isotonic saline.4,5,9,11,14 Symptom reduction and QoL improvement favored the hypertonic solution at the duration of 1 to 8 weeks.5,9,11 Three meta-analyses with a mixed population of adults and children reported greater benefits of hypertonic saline.1,3,23 Isotonic saline shortened the saccharin transit time (STT), but hypertonic saline did not. 4 In 1 study, 2 of 40 patients (5%) noted nasal discomfort (light pain sensation) from the 3% hypertonic saline. 14
In pediatric AR, 3 RCTs compared the effects between hypertonic and isotonic saline.16,18,19 The meta-analysis, which included these RCTs, favored the hypertonic saline over the isotonic saline. The antihistamine usage was not different between the tonicities. Adverse effects were reported without statistical differences. 24
Buffer
One RCT assessed the effects of buffered saline in adult AR among 3 groups: mild alkalinity (pH 7.2-7.4), high alkalinity (pH8.2-8.4), and nonbuffered saline. 8 There were no differences among the groups in nasal symptoms, mucociliary clearance time, and nasal patency, although the mild alkaline buffered saline was preferred by the patients.
In children, there was no study that compared the buffered and nonbuffered saline. Buffered and nonbuffered saline showed benefits over the no-saline group.15,17,19-21
Summary
Nasal saline treatment decreased symptoms of AR. The duration of treatment was at least 2 weeks in adult patients and 4 weeks in pediatric patients. There was a slight chance of local nasal irritation in pediatric patients. A large-volume device (≥60 mL) was more effective and recommended in adult patients, while a very low- to low-volume device (<60 mL) was recommended in children. Hypertonic saline treatment was more effective in adults and children with AR. However, adverse events were reported in a small number of patients. Therefore, isotonic saline should be used first. Buffered and nonbuffered saline can be used in adults and children. A summary of the studies in AR is shown in Table 1 .
Table 1.
Studies of Patients With Allergic Rhinitis. a
| First Author | Year | Study type | Patients b | Saline type c | Buffered | Devices c | Treatment duration | Outcomes | Main result |
|---|---|---|---|---|---|---|---|---|---|
| Adult | |||||||||
| Yata 14 | 2021 | RCT | 80: adult, AR | I: Hypertonic saline 3% (40). II: Isotonic saline (40) | I: No. II: No | Syringe. 20 mL × 2/side | 2 times/d, 2 wk | Symptom VAS, inferior turbinate size, PNEF | No difference in any outcomes between groups. |
| Piromchai 12 | 2020 | RCT | 116: adult, AR | Isotonic saline (116) | No | I: Squeeze bottle 240 mL (58). II: Syringe 20 mL (58) | 2 times/d, 4 wk | Symptoms VAS, LKES, ease of use (Likert) | Squeeze bottle reduced symptoms more than syringe. |
| Sansila 11 | 2020 | RCT | 78: adult, AR | I: Hypertonic saline 1.8% (35). II: Isotonic saline (43). | I: Yes. II: No | Syringe 20 mL (80 mL/side) | 2 times/d, 4 wk | RCQ36, TNSS | Hypertonic saline is superior to isotonic on RCQ36 and congestion. |
| Wang 23 | 2020 | SRMA (12 studies: adult 6, children 6) | 819: adult (342); children (477), AR | Saline vs no saline. Saline + medication vs medication. Hypertonic vs isotonic saline | Any | Any | 1-12 wk | Symptom scores | Saline showed significant efficacy vs no saline. Saline was a safe adjunct. Hypertonic was superior in children. |
| Head 3 | 2018 | SRMA (14 studies: adult 7, children 7) | 747: adult (248); children (499), AR | Saline vs no saline. Saline + medication vs medication | Any | Very low <5 mL, low 5-59 mL, high ≥60 mL | 1-6 mo | Patient-reported symptom score, disease specific HRQL, adverse effects | Saline improved symptoms vs no saline. Uncertain add-on benefit as adjunct. |
| Di Berardino 10 | 2017 | RCT | 40: adult, AR | I: Hypertonic saline (20). II: No saline (20) | I: Yes. II: NA | Spray. 1 sprays/side | 3 times/d, 4 wk | Symptom score, STT, use of antihistamines | Saline reduced symptom and antihistamine use vs no saline. |
| Singh 9 | 2016 | RCT | 60: adult, AR | I: Hypertonic seawater 2.2% (30). II: Isotonic saline (30) | I: Yes. II: No | Spray. 2 sprays/side | 3 times/d, 2 mo | Symptom score | Hypertonic seawater improved symptoms more than isotonic saline. |
| Chusakul 8 | 2013 | RCT (crossover) | 36: adult, AR | I: Isotonic saline (36). II: Isotonic saline, mild alkalinity (36). III: Isotonic saline, alkaline (36) | I: No. II: Yes (pH 7.2-7.4). III: Yes (pH 8.2-8.4) | Squeeze bottle. 240 mL | 2 times/d, 10 d | Symptoms, STT, ARM | No difference between groups. |
| Hermelingmeier 1 | 2012 | SRMA (10 studies: adult 7, children 3) | 400: adult (314), children (86), pregnancy, AR | Saline vs no saline. Medicine consumption. | Any | Spray or irrigation | Varied:, up to 7 wk | Symptom score, medicine consumption, STT, QoL | Saline irrigation reduced symptom and medication use vs no saline. |
| Garavello 7 | 2010 | RCT | 45: adult, pregnancy, AR | I: Hypertonic saline 3% (22). II: No saline (23) | I: No. II: NA | Syringe. 10 mL/side | 3 times/d, 6 wk | TNSS, RMM, use of antihistamines | Saline irrigation reduced nasal symptoms and antihistamine use. |
| Ural 4 | 2009 | RCT | 21: adult; AR/ARS/CRS | I: Hypertonic saline 3% (11). II: Isotonic saline (10) | I: No. II: No | Syringe. 4 mL/side | 2 times/d, 10 d | STT | Hypertonic saline improved STT in CRS. Isotonic saline improved STT in AR and ARS. |
| Rogkakou 6 | 2005 | RCT | 14: adult, AR | I: Hypertonic saline (7). II: No saline (7) | I: No. II: NA | Spray. (unknown volume) | 4 times/d, 4 wk | Symptoms, QoL, ARM | Saline spray improved symptoms and QoL vs control. |
| Cordray 5 | 2005 | RCT | 15: adult, AR | I: Hypertonic Dead Sea saline (5). II: Isotonic saline (5). III: Nasal steroid (5) | I: Yes. II: No. III: NA | Spray. 2 sprays/side | 3 times/d, 7 d | RQLQ | Hypertonic saline and nasal steroid improved RQLQ, while isotonic saline did not. |
| Klimek 13 | 2001 | RCT | 75: adult, AR | I: Emser isotonic solution (38). II: No saline (37) | I: Yes. II: NA | Squeeze bottle. 250 mL | 2-3 times/d, 2-4 wk | Allergic drug usage, symptoms, ECP and tryptase in nasal secretions | No difference in any outcomes except the lower allergic drug use in saline group. |
| Children | |||||||||
| Jung 15 | 2019 | RCT | 20: children, AR/asthma | I: Isotonic saline (10). II: No saline (10) | I: No. II: NA | Syringe. 60-150 mL. | 2 times/d, 12 wk | AR severity, QoL, eosinophil in nasal secretion | Saline irrigation improved severity and QoL, but not in the control group |
| Li 24 | 2019 | SRMA (4 studies) | 351: children, AR | Saline vs no saline. Hypertonic 1.25%-3% vs isotonic saline | Any | 2.5, 5, 20, 240 mL | 2-3 times/d, 3-6 wk | Symptom score, use of antihistamines, adverse effects | Hypertonic saline irrigation improved symptoms more than isotonic or no saline |
| Malizia 16 | 2017 | RCT | 30: children, AR | I: Hypertonic saline 3% (15). II: Isotonic saline (15) | I: Yes. II: No | Atomizer. 5 mL | 2 times/d, 3 wk | Symptom score, RQLQ, PSQI, NCC | Hypertonic saline improved symptoms and NCC, more than isotonic saline |
| Chen 17 | 2014 | RCT | 61: children, AR | I: Combined isotonic seawater. and nasal steroid (26). II: Nasal steroid alone (17). III: Isotonic seawater alone (18) | I: Yes. II: NA. III: Yes | Spray. 4-6 sprays | 2 times/d, 8-12 wk | Symptoms and signs of AR, eosinophils in the nasal secretions | The combination improved symptom and signs more than steroid or saline alone |
| Satdhabudha 18 | 2012 | RCT | 81: children, AR | I: Hypertonic saline 1.25% (40). II: Isotonic saline (41) | I: Yes. II: No | Syringe. (20 mL x6/side) | 2 times/d, 4 wk | STT, TNSS | Hypertonic saline improved STT and TNSS more than isotonic saline |
| Marchisio 19 | 2012 | RCT | 220: children, AR | I: Hypertonic saline 2.7% (80). II: Isotonic saline (80). III: No saline (60) | I: No. II: No. III: NA | Syringe. 20 mL. | 2 times/d, 4 wk | Symptoms, oral antihistamine use | Hypertonic saline reduced all symptoms. Isotonic only reduced rhinorrhea and sneezing. None reduced in no-saline group |
| Li 22 | 2009 | RCT | 26: children, AR | I: Isotonic saline alone (8). II: Combined saline and. nasal steroid (12). III: Nasal steroid alone (6) | I: No. II: No. III: NA | Unknown. 500 mL | 2 times/d, 12 wk | Symptoms and signs, MCC, nasal secretion sICAM-1 | The combination was superior to either steroid or saline alone |
| Garavello 20 | 2005 | RCT | 40: children, AR | I: Hypertonic saline 3% (20). II: No saline (20). | I: No. II: NA | Spray. 3 sprays/side | 3 times/d, 7 wk | Nasal and ocular symptoms, oral antihistamine use | Saline reduced symptoms and oral antihistamine intake vs control |
| Garavello 21 | 2003 | RCT | 20: children, AR | I: Hypertonic saline 3% (10). II: No saline (10). | I: No. II: NA | Syringe. 2.5 mL/side | 3 times/d, 6 wk | Symptoms, oral antihistamine use | Saline reduced symptoms and oral antihistamine intake vs control |
Abbreviations: AR, allergic rhinitis; ARM, acoustic rhinometry; ARS, acute rhinosinusitis; CRS, chronic rhinosinusitis; ECP, eosinophilic cationic protein; HRQL, health-related quality of life; LKES, Lund-Kennedy Endoscopic Score; MCC, mucociliary clearance; NA, not applicable; NCC, nasal cytology count; PNEF, peak nasal expiratory flow rate; QoL, quality of life; PSQI, Pittsburgh Sleep Quality Index; RCQ36, 36-Item Rhinoconjunctivitis Quality of Life Questionnaire; RQLQ, Rhinoconjunctivitis Quality of Life Questionnaire; RMM, rhinomanometry; sICAM-1, soluble intercellular adhesion molecule; SRMA, systematic review and meta-analysis; STT, saccharin transit time; TNSS, Total Nasal Symptom Score; VAS, visual analog scale.
Roman numerals indicate patients groups.
Patient total number, age group (number per group), and disease.
Number per group in parentheses.
Nonallergic rhinitis
There was no study evaluating saline treatment in NAR. Tomooka et al investigated a mixed population of NAR, AR, and CRS, but the data could not be extracted separately. 25
Rhinosinusitis
Acute Rhinosinusitis
There were 17 studies that assessed the effects of nasal saline treatment in ARS. Ten RCTs4,26-34 and 1 meta-analysis 35 investigated the effects in adults. Five RCTs36-40 and 1 meta-analysis were conducted in children. 41
In adult patients, 5 RCTs assessed nasal saline vs no-saline treatment.26,27,30,31,33 Duration of treatment ranged from 1 to 4 weeks. There were no differences in symptom reduction,26,30,31,33 QoL improvement,30,31,33 and STT. 27 However, a post hoc analysis of an RCT showed that the common cold subgroup benefited from saline irrigation on symptom reduction and QoL. 31 The duration of illness after saline treatment showed mixed results. One RCT 26 and 1 meta-analysis 35 reported that the nasal saline treatment did not shorten the duration of illness. In contrast, 1 RCT cited decreases in the duration of illness and usage of medications. 30 Minor adverse effects were indicated, such as dry nose and pain/irritation. 26
In children, 4 RCTs assessed nasal saline vs no-saline treatment.36-39 There were no differences between the groups at the duration of 2 days. 36 Symptom reductions (secretion and nasal obstruction) favored the saline at a longer duration (5 days to 3 weeks).37-39 A meta-analysis showed benefits on nasal symptom reduction. 41 Minor adverse effects were noted, such as nosebleed and burning sensation. 37
Device
Three RCTs compared the effects of saline treatment among different devices in adult patients.28,29,32 The saline treatment, either nasal irrigation with a 250-mL hanging bag (large volume, no pressure) or atomized nasal douche at 7 to 8 mL (low volume, high diffusion, pressure), reduced symptoms and improved nasal patency.28,29 One RCT assessed the overall effectiveness of a syringe (10 mL) with and without a silicone tip applicator. The patient-reported outcomes showed that the syringe with a silicone tip was more effective. 32
Two RCTs compared the effects of saline treatment among different devices in pediatric patients.37,40 One study compared a low-volume syringe (20 mL) with a large-volume squeeze bottle (240 mL). The effects favored the squeeze bottle on reducing nasal obstruction and rhinorrhea. 40 The other study compared a very low-volume spray (3 mL) with a low-volume jet flow (9 mL). There were no differences in the benefits and adverse events between the devices. 37
Tonicity
Four RCTs compared the effects of saline treatment among different tonicities in adult patients.4,26,27,34 There were no differences between the hypertonic and isotonic saline for nasal symptom score26,34 and days to resolution. 26 The STT was markedly improved in the isotonic saline group in 1 study 4 but not different in another. 27 Nasal irritation was more frequent in the hypertonic saline group. 26
In children, 1 study compared 2.3% hypertonic saline with isotonic nasal saline drops for 5 days. There was no difference in symptom score reduction and no report of adverse effects in this study. 39
Buffer
There was no comparative study between the buffered and nonbuffered saline. Both solutions were shown to be effective when compared with the baseline data.
Summary
The effects of nasal saline treatment for ARS had mixed results. It should be considered an option for adults but recommended for children with a duration >5 days. Devices with high diffusion, regardless of volume or pressure, were favored for adult patients, while large-volume with positive pressure devices were recommended for children. Isotonic saline is suggested for adults and children due to its low adverse event rate. Characteristics of the studies are displayed in Table 2 .
Table 2.
Studies of Patients With Acute Rhinosinusitis. a
| First Author | Year | Study type | Patients b | Saline type c | Buffered | Devices c | Treatment duration | Outcomes | Main result |
|---|---|---|---|---|---|---|---|---|---|
| Adult | |||||||||
| Chitsuthipakorn 31 | 2021 | RCT | 61: adult, ARS | I: Isotonic saline (30). II: No saline (31) | I: Yes. II: NA | Squeeze bottle. 250 mL. 1 bottle/time | 2 times/d, 2 wk | SNOT-22, rhinologic subscore, symptom VAS, MLKES, % cure rate | No difference between groups in any outcomes. Post hoc analysis showed the benefits of saline irrigation in the viral subgroup. |
| Piromchai 32 | 2021 | RCT (crossover) | 12: adult, ARS | Isotonic saline | Yes | I: Syringe with nasal applicator (12). II: Syringe 10 mL alone (12). | 10 min | Overall effectiveness | The syringe with nasal applicator had better overall effectiveness scores than the syringe alone. |
| Ramalingam 30 | 2019 | RCT | 61: adult, URI | I: Irrigation and gargling with hypertonic seawater 2%-3% (30). II: No irrigation and gargling (31) | I: Yes. II: NA | Not stated. 100 mL | 6 times/d, 2-14 d | Duration of illness, OTC medication used, viral shredding | Duration of illness reduced by 1.9 d and less medication used by 36% in the irrigation/gargling group |
| Chanaseeyotin 33 | 2016 | RCT | 48: adult, ABRS | I: Isotonic saline (25). II: No saline (23) | I: Yes II: NA | Syringe. 20 mL, 5 times/nostril | 2 times/d, 4 wk | Symptom VAS, SNOT-20 | No difference between groups in any outcomes. |
| King 35 | 2015 | SRMA (3 studies) | 542: adult (152), children (390), URI | Isotonic saline or isotonic seawater vs no saline | Any | Spray. 2-3 sprays/nostril | 3-6 times/d, 1-3 wk | Days to resolution, antibiotic usage | No difference between groups |
| Gelardi 29 | 2009 | RCT | 20: adult, ARS | Warm isotonic saline (36 ºC) | No | I: Syringe 10 mL (10). II: Irrigation bag 250 mL (10) | I: 3 times daily. II: 2 times/d, 2 wk | Symptom (VAS), RMM | Irrigation bag improved symptom and nasal resistance better than a syringe |
| Ural 4 | 2009 | RCT | 24: adult, AR/ARS/CRS | I: Hypertonic saline 3% (12). II: Isotonic saline (12) | I: No. II: No | Syringe. 4 mL/side | 2 times/d, 10 d | STT | Isotonic improved STT, but the hypertonic did not |
| Hauptman 34 | 2007 | RCT | 80: adult, ARS | I: Hypertonic saline (40). II: Isotonic saline (40) | I: Yes. II: Yes | Spray. 1 mL/side, 1 time | 10 min | Symptoms, ARM, STT | Not difference between groups for symptom reduction. Hypertonic better improved STT but had worse ARM than isotonic saline. |
| Passàli 28 | 2005 | RCT | 200: adult, ARS | Isotonic saline | No | I: Atomized douche (7-8 mL) (100). II: Syringe 20 mL (100) | 4 times/d, 15 d | GSS, RMM, ARM, STT | Atomized douche improved all outcomes. But the syringe did not |
| Inanli 27 | 2002 | RCT | 60: adult, ARS | I: Hypertonic saline 3.0% (12). II: Isotonic saline (13). III: Nasal steroid spray (14). IV: Oxymetazoline 0.05% (9). V: No saline (12) | I: Yes. II: Yes. III: NA. IV: NA. V: NA | Irrigation 10 mL. | 3 times/d, 3 wk | STT | No difference between groups |
| Adam 26 | 1998 | RCT | 119: adult, ARS | I: Hypertonic saline 1.6% (41). II: Isotonic saline (35). III: no saline (43) | I: Yes. II: No. III: NA | Spray. 2 sprays/nostril | 3 times/d, 1 wk | Third-day nasal symptom score, days to resolution | No difference between groups in any outcomes |
| Children | |||||||||
| Cabaillot 41 | 2020 | SRMA (4 studies) | 489: children, ARS/URI | Hypertonic saline 2.3% or isotonic saline (334) vs no saline (155) | Any | Drop, syringe, spray, jet flow. | 2 d–3 wk | Rhinologic score, health status scores. | Saline irrigation benefited for rhinological symptoms |
| Satdhabudha 40 | 2017 | RCT | 74: children, ARS | Hypertonic saline 1.25% | Yes | I: Squeeze bottle 240 mL (38). II: Syringe 20 mL (36) | 2 times/d, 2 wk | 5S, satisfaction score, medication used | Squeeze bottle improved in 5S and satisfaction scores more than syringe |
| Köksal 39 | 2016 | RCT | 109: children, URI | I: Isotonic saline (38). II: Hypertonic seawater 2.3% (36). III: No saline (35) | I: No. II: Yes. III: NA | Drop | 3 vials daily, 5 d | Nasal symptoms at days 1 and 7, sleep quality | Saline and seawater drop relieved nasal symptoms and improved sleep quality vs no saline |
| Wang 38 | 2009 | RCT | 69: children, ARS | I: Isotonic saline (30). II: No saline (39) | I: No. II: NA | Syringe. 15-20 mL. | 1-3 times/d, 3 wk | TSS, PRQLQ, nPEFR, nasal smear, Water’s film score | Saline irrigation improved TSS, PRQLQ, nPEFR vs no saline |
| Šlapak 37 | 2008 | RCT | 390: children, common cold | I: Isotonic seawater (99). II: Isotonic seawater (95). III: Isotonic seawater (95). IV: No saline (101) | I: Yes. II: Yes. III: Yes. IV: NA | I: Jet flow 9 mL/side. II: Fine spray 3 mL/side. III: dual formula fine spray 3 mL/side. IV: NA | 6 times/d, 3 wk | Nasal symptoms, medication used | Saline group showed faster resolution of some nasal symptoms and less medication used |
| Bollag 36 | 1984 | RCT | 46: children, URI | I: Isotonic saline (15). II: Phenylephrine drop (16). III: No saline (15) | I: No. II: NA. III: NA | Drop. 4 drops/side. | Every 2 h, 2 d | Nasal symptoms, respiratory symptom | No difference between groups |
Abbreviations: 5S, 5 Symptoms Score; ABRS, acute bacterial rhinosinusitis; AR, allergic rhinitis; ARM, acoustic rhinometry; ARS, acute rhinosinusitis; CRS, chronic rhinosinusitis; GSS, Global Symptom Score; MLKES, Modified Lund-Kennedy Endoscopic Score; NA, not applicable; nPEFR, nasal peak expiratory flow rate; OTC, over the counter; PRQLQ, Pediatric Rhinoconjunctivitis Quality of Life Questionnaire; RCT, randomized controlled trial; RMM, rhinomanometry; SNOT, Sino-nasal Outcome Test; SRMA, systematic review and meta-analysis; STT, saccharin transit time; TSS, Total Symptom Score; URI, upper respiratory tract infection; VAS, visual analog scale.
Roman numerals indicate patients groups.
Patient total number, age group (number per group), and disease.
Number per group in parentheses.
Chronic Rhinosinusitis
There were 17 studies that assessed the effects of nasal saline treatment. Sixteen articles (13 RCTs and 3 meta-analyses) assessed adult CRS or mixed adults and children with CRS.4,42-57 One RCT was conducted in children. 58
Four RCTs compared the effects of nasal saline with no-saline treatment.42,43,46,52 The benefits of 2-week nasal saline treatment were not shown. 43 One RCT showed significant symptom improvement in the saline irrigation group over no saline at 4 weeks. 52 Taccariello et al found that saline douche and saline spray for 8 weeks improved the QoL and nasal endoscopic appearance. 42 One RCT 46 and 2 meta-analyses47,50 did not provide additional data. Adverse effects were indicated in the nasal saline group, including irritation, burning, tearing, and nosebleeds, 50 with a prevalence of 0% to 23%.43,59
No study assessed the effects of saline irrigation vs no saline in children. There was 1 RCT by Shoseyov et al that assessed the effects among different tonicities in children. 58
Device
Two RCTs assessed the effects of different devices.42,43 One study demonstrated that the nasal douche (60 mL) improved endoscopic appearances but not QoL, whereas the very low-volume spray had the opposite effects. 42 The other study compared 2 large-volume devices (bulb syringe vs irrigation pot) and showed no difference in the 31-item Rhinosinusitis Outcome Measure. 43
In children, there was no direct comparison of devices. One study showed that nasal drops relieved nasal symptoms with acceptable tolerability. 58
Tonicity
Nine studies (8 RCTs4,44,46,48,49,54,56,57 and 1 meta-analysis 55 ) assessed different tonicities of nasal saline treatment in adult CRS. The data from 1 RCT could not be used. 46 Hypertonic saline was more effective than isotonic saline in improving symptoms (nasal congestion48,49,54,56,57 and nasal discharge),48,49,54,56,57 the Lund-Mackay computed tomography score, 56 and STT. 4 In contrast, 1 RCT reported similar effectiveness between hypertonic and isotonic saline. 44
The risks of adverse events in the hypertonic saline group was significantly higher than the isotonic saline. Most events were irritation and burning sensation. 55
One RCT assessed hypertonic saline (3.5%) vs isotonic saline (0.9%) drops in children. 58 Both tonicities showed significant improvements in postnasal drip, cough, and radiologic score from the baseline. Hypertonic saline significantly reduced cough and radiologic score better than isotonic saline but not the postnasal discharge score. Adverse effects in the pediatric group, such as itching and burning sensation, were indicated in the high tonicity group in the first 3 to 4 days. 58 Two meta-analyses did not provide additional data.47,50
Buffer
One RCT compared buffered saline with nonbuffered saline. 45 Buffered saline improved symptoms and the Rhinoconjunctivitis Quality of Life Questionnaire scores. No adverse event was reported from the study.
No study compared buffered and nonbuffered saline in children.
Summary
Nasal saline treatment for at least 4 weeks is recommended for adults. The saline should be buffered and delivered in a large-volume device. Due to its higher adverse events, hypertonic saline is suggested only after isotonic saline has failed to improve the symptoms. Nasal drop can be used in pediatric CRS, preferably with isotonic saline. A summary of the studies in patients with CRS is displayed in Table 3 .
Table 3.
Studies of Patients With Chronic Rhinosinusitis. a
| First Author | Year | Study type | Patients b | Saline type c | Buffered | Devices | Treatment duration | Outcomes | Main result |
|---|---|---|---|---|---|---|---|---|---|
| Adult | |||||||||
| Liu 55 | 2020 | SRMA (7 studies) | 454: adult, CRS | Hypertonic saline vs isotonic saline | Any | Any | Any | Symptom score, radiologic score, STT | Hypertonic saline improved symptoms and STT more than isotonic saline. |
| Muthubabu 54 | 2020 | RCT | 60: adult, CRS | I: Hypertonic saline 3.5% (30). II: Isotonic saline (30) | I: No. II: No | Squeeze bottle, 100 mL | 3 times/d, 6 wk | SNOT-20 subscores (obstruction, discharge, postnasal drip, ear pain, facial pain) | Hypertonic saline showed significant improvement in symptoms more than isotonic saline. |
| Rachana 52 | 2019 | RCT | 400: adult, CRS | I: Isotonic saline (200). II: No saline (200) | I: Yes. II: NA | Syringe, 20 mL/side | 2 times/d, 4 wk | Nasal symptoms, RSDI | Saline group showed more improvement on symptoms than no saline. |
| Nimsakul 51 | 2018 | RCT | 23: adult, CRS | I: Heated isotonic saline (12). II: Nonheated isotonic saline (11). III: Heated isotonic saline (9 healthy) | I: No. II: No. III: No | Squeeze bottle, 250 mL | One time | Nasal symptom, STT, PNIF, ARM | No difference between heated and nonheated saline |
| Chong 50 | 2016 | SRMA (1 study) | 76: adult, CRS | Saline irrigation vs no saline | Any | Any | Any | HRQL, patient-reported disease severity, endoscopic score, CT score, adverse event | Saline improved QoL and symptoms vs no saline. |
| Nikakhlagh 46 | 2016 | RCT | 185: adult, CRS | I: No saline (not stated). II: Hypertonic saline (not stated). III: Isotonic saline (not stated). IV: Hypotonic saline (not stated) | I: NA. II: No. III: No. IV: No | Not stated | Not stated, 3 wk | Nasal symptoms | Isotonic saline showed better effect than hypertonic saline on nasal congestion. |
| Sudhakaran 56 | 2016 | RCT | 46: adult, CRS | I: Hypertonic saline 3% (23). II: Isotonic saline (23) | I: No. II: No | Drop, 10 drops/nostril | 3 times/d, 4 wk | LM score, symptom VAS | Hypertonic saline was more effective than isotonic saline in symptoms VAS reduction and radiologic score. |
| Kumar 57 | 2013 | RCT | 42: adult, CRS | I: Hypertonic saline 3.5% (21). II: Isotonic saline (21) | I: No. II: No | Drop, 10 drops | 3 times/d, 4 wk | Symptoms, radiologic score | Hypertonic saline was more effective than isotonic saline in symptom reduction and radiologic score. |
| Berjis 49 | 2011 | RCT | 114: adult, CRS | I: Hypertonic saline 3% (57). II: Isotonic saline (57) | I: No. II: No | Drop, 4-5 drops | Frequency, not stated, 1 mo | Symptoms, patient satisfaction | Hypertonic saline irrigation was more effective than isotonic saline in symptom reduction and patient satisfaction |
| Čulig 48 | 2010 | RCT | 60: adult, CRS | I: Hypertonic seawater 2.1% (30). II: Isotonic seawater (30) | I: Yes. II: Yes | Spray, 3 s of continuous spray/side | 3-6 times/d, 2 wk | Nasal symptoms, medication used | Hypertonic seawater improved all symptoms while isotonic seawater improved only congestion and rhinorrhea |
| Ural 4 | 2009 | RCT | 42: adult, AR/ARS/CRS | I: Hypertonic saline 3% (18). II: Isotonic saline (24) | I: No. II: No | Syringe, 4 mL/side | 2 times/d, 10 d | STT | Hypertonic saline improved STT, but isotonic saline did not. |
| Harvey 47 | 2007 | SRMA (6 studies) | 334: adult-children, CRS | Saline vs no treatment, Saline vs placebo, Hypertonic vs isotonic saline | Any | Any | Any | QoL measures, symptom scores, adverse events, radiologic scores, endoscopic score | Saline irrigations improve CRS symptoms vs no-saline irrigation. |
| Friedman 45 | 2006 | RCT | 42: adult, CRS | I: Hypertonic Dead Sea solution 1.8% (22). II: Hypertonic saline 1.8% (20) | I: Yes. II: No | Irrigation (volume not stated) and spray | 2 times/d, 1 mo | Nasal symptoms, RQLQ | Hypertonic Dead Sea solution is more effective in reducing RQLQ and symptom score than the hypertonic saline. |
| Heatley 43 | 2001 | RCT (crossover) | 128: adult, CRS | I: Hypertonic saline 2.7% (43). II: Hypertonic saline 2.7% (39). III: No treatment (reflexology, 46) | I: No. II: No. III: NA | I: Bulb syringe. II: Irrigation pot (volume not stated for both). III: NA | Once daily, 2 wk then crossover between 1 and 2 | RSOM31, SF36, patient satisfaction, medication use | There was no difference between the irrigation groups and reflexology after 2 wk |
| Bachmann 44 | 2000 | RCT | 40: adult, CRS | I: Emser hypertonic saline 1.1% (20). II: Isotonic saline (20) | I: Yes. II: No | Nasal irrigator, 200 mL | 2 times/d, 7 d | Symptoms, endoscopic finding, STT, olfactometry, RMM | No difference between Emser salt hypertonic solution and isotonic irrigation at 7 d |
| Taccariello 42 | 1999 | RCT | 62: adult, CRS | I: Isotonic seawater (21). II: Hypertonic alkaline saline (19). III: No saline (22) | I: Yes. II: Yes. III: NA | I: Spray. II: Douche 60 mL. III: NA | 2 times/d, 8 wk | STT, CBF, endoscopic score, ARM, QoL, nasal score diary card | Both saline groups showed significant improvements in endoscopic score or QoL, while no improvement was found in the control group at 8 wk |
| Children | |||||||||
| Shoseyov 58 | 1998 | RCT | 30: children, CRS | I: Isotonic saline (15). II: Hypertonic saline 3.5% (15) | I: No. II: No | Drop, 10 drops | 3 times/d, 4 wk | Cough/postnasal drip, radiologic score | The hypertonic saline significantly better than normal saline for cough and radiologic score. |
Abbreviations: AR, allergic rhinitis; ARM, acoustic rhinometry; ARS, acute rhinosinusitis; CBF, ciliary beat frequency; CRS, chronic rhinosinusitis; CT, computed tomography; HRQL, health-related quality of life; LM, Lund-Mackay; NA, not applicable; PNIF, peak nasal inspiratory flow; QoL, quality of life; RCT, randomized controlled trial; RMM, rhinomanometry; RQLQ, Rhinoconjunctivitis Quality of Life Questionnaire; RSDI, Rhinosinusitis Disability Index; RSOM31, 31-item Rhinosinusitis Outcome Measure; SF36, 36-item Medical Outcomes Study Short Form; SNOT-20, 20-item Sino-nasal Outcome Test; SRMA, systematic review and meta-analysis; STT, saccharin transit time; VAS, visual analog score.
Roman numerals indicate patients groups.
Patient total number, age group (number per group), and disease.
Number per group in parentheses.
Chronic Rhinosinusitis With Cystic Fibrosis
No study evaluated the effects of saline vs no saline in CRS-CF. Just 1 study by Mainz et al was identified. 60 It compared hypertonic saline (6.0%) with isotonic saline via a randomized crossover study design.
Device
The study utilized an atomizer delivering a very low volume (1 mL per nostril) of saline once daily for 28 days. Benefits were shown when compared with the baseline. 60 Other devices were not assessed.
Tonicity
Both tonicities showed improvements from baseline in 20-item Sino-nasal Outcome Test (SNOT-20) score and nasal symptoms. The improvements were not different between the tonicities. Minor adverse events were noted in both tonicities. 60
Buffer
There was no comparison between buffered and nonbuffered saline treatment. Nonbuffered saline demonstrated improvements from the baseline. 60
Summary
Limited data showed that saline treatment could be used for symptom control and QoL improvement. The saline may be hypertonic or isotonic and delivered by a very low–volume device (atomizer). Only data of nonbuffered saline were available.
Postoperative Care
After Septoplasty/Turbinoplasty Surgery
Two RCTs evaluated the effects of saline treatment after septoplasty/turbinoplasty.61,62 None of these studies used the no-saline treatment as control. Saline irrigation decreased the crusting and improved nasal obstruction, which was assessed by visual analog scale 62 and anterior rhinomanometry. 61 Benefits were shown during postoperative days 7 to 15. 62 The STT showed mixed results.61,62 Minor adverse effects were noted. There was no eligible study in pediatric patients.
Device
None of these studies compared different devices. Kurtaran et al studied the effects of a large-volume device (60 mL). 62 The improvements in crusting, nasal obstruction, and STT were reported on postoperative day 15. Süslü et al did not state the volume of nasal saline. 61
Tonicity
Two RCTs compared the effects of 2 tonicities.61,62 Hypertonic and isotonic saline improved the crusting and nasal obstruction greater than tap water. 62 Hypertonic saline was more effective than isotonic saline in improving the crusting, nasal obstruction, and nasal patency.61,62 However, hypertonic saline caused more adverse effects, such as burning sensation. 61
Buffer
Süslü et al compared buffered with nonbuffered isotonic saline. 61 Nasal patency improvement was not different between the groups. Yet, nonbuffered saline (pH 5.5) caused more burning sensation than buffered saline (pH 7.4). The authors believed that the burning sensation might be caused by the acidity of the solution. 61
Summary
With minor adverse events reported, nasal saline irrigation may be used after septoplasty/turbinoplasty. The large-volume saline is suggested to reduce crusting. Although hypertonic saline showed greater beneficial effects than isotonic saline, it was more likely to cause undesirable effects. Therefore, isotonic saline is recommended. The effects of buffered and nonbuffered saline were comparable, although nonbuffered saline caused more irritation. The buffered saline is recommended due to its better tolerability. Characteristics of the studies are displayed in Table 4 .
Table 4.
Studies in Postoperative Period After Septoplasty/Turbinoplasty: Adults. a
| First Author | Year | Study type | Patients b | Saline type c | Buffered | Devices | Treatment duration | Outcomes | Main result |
|---|---|---|---|---|---|---|---|---|---|
| Kurtaran 62 | 2018 | RCT | 120 adults, septoplasty and RF | I: Tap water (30). II: Isotonic saline (30). III: Isotonic saline with xylitol (30). IV: Hypertonic seawater 2.3% (30) | I: No. II: Yes. III: Yes. IV: Yes | 60 mL (30 mL/side; device not stated) | 3 times/d, 15 d | STT, VAS (crusting, dryness, obstruction) at days 7 and 15 | Hypertonic and isotonic saline improved the crusting and nasal obstruction greater than tap water. Hypertonic saline was more effective than isotonic saline in reducing the crusting and improving nasal obstruction and patency. |
| Süslü 61 | 2009 | RCT | 45 adults, septoplasty | I: Hypertonic seawater 2.3% (15). II: Isotonic saline (15). III: Isotonic saline (15) | I: Yes. II: Yes. III: No | Instillation (volume not stated) | 6 times/d, 20 d | STT, ARM, VAS (burning sensation) at days 5 and 20 | No significant difference in STT on the postoperative day 5 among the 3 groups. On day 20, the hypertonic saline improved STT and nasal patency more than isotonic saline. |
Abbreviations: ARM, acoustic rhinometry; RCT, randomized controlled trial; RF, radiofrequency tissue reduction of inferior turbinate; STT, saccharin transit time; VAS, visual analog scale.
Roman numerals indicate patients groups.
Patient total number, age group (number per group), and disease.
Number per group in parentheses.
After Endoscopic Sinus Surgery
There were 10 RCTs and 1 meta-analysis that assessed post-ESS saline treatment.63-73 All RCTs assessed adult populations, of which 5 evaluated nasal saline vs no-saline treatment.63-67 The study duration ranged from 5 days to 12 months.
One RCT evaluated the outcomes at the first 5 postoperative days. The saline spray did not show benefit on nasal symptoms. 63 At 3 weeks, Freeman et al demonstrated the benefits of atomized douching on nasal discharge reduction. 64 At 3 months, 4 RCTs reported mixed results.64-67 One RCT cited no benefits on adhesion, polyps, crusting, mucosal edema, and nasal discharge improvements. 64 Another study indicated no benefit of saline treatment at 3 months on the SNOT-20, minimal cross-sectional area, smell test, STT, and endoscopic scores. 66 In contrast, 2 RCTs reported beneficial effects of saline treatment on nasal symptoms,65,67 nasal endoscopy, 65 and QoL. 67 Clinical outcomes were significantly improved after 3 months and up to 12 months. 67 When the benefits were assessed by severity of CRS, Liang et al found that the benefits were shown in the mild CRS subgroup with computed tomography scores ≤12. 65
There was no study in the pediatric population.
Device
Two RCTs compared the effects of saline treatment between 2 devices.68,72 Salib et al randomly assigned nasal saline treatment via 2 puffs of saline spray into 1 nostril and a squeeze bottle (120 mL) into the other nostril in the same patient. The large-volume irrigation showed better endoscopic findings at 2 and 4 weeks. Nasal endoscopic findings were not different at 3 months. The benefits assessed by the patient’s perception favored the large volume throughout the follow-up period. 68 The other RCT evaluated the feasibility of a multicenter trial and recruited a small number of patients. 72
Five other studies that utilized large-volume devices (240 mL) for saline treatment showed improvements from the baseline.65-67,69,70 Very low-volume devices used in 2 studies did not show beneficial results.63,64
Tonicity
Five RCTs compared hypertonic with isotonic saline.63,66,69-71 There were no differences between hypertonic and isotonic saline in improving symptoms,63,69,71 SNOT-20 scores,66,69,71 endoscopic scores, 66 minimal cross-sectional area, 66 smell threshold, 66 and STT.66,69,71 However, hypertonic saline demonstrated more benefits than isotonic saline during the first 6 weeks,69,71 but these differences did not persist in the longer follow-up period. 71 One meta-analysis compared different solutions with isotonic saline and reported no differences in symptoms or endoscopic scores. 73
More patients receiving hypertonic saline refused or discontinued the treatments than isotonic saline. 66 The adverse events, as reported in the hypertonic saline group, were irritation, 66 increased mucous secretion, and pain. 63
Buffer
Three studies compared the effectiveness between the buffered and nonbuffered saline.63,68,70 All were inconclusive due to discrepancies of other factors between the experimental arms. Pinto et al 63 and Perić et al 70 compared buffered hypertonic with nonbuffered isotonic saline. Salib et al compared buffered isotonic saline spray with nonbuffered isotonic saline irrigation using a bottle. 68 Although the differences between buffered and nonbuffered saline were inconclusive, both solutions revealed beneficial effects as compared with baseline.
Summary
The benefits at a short postoperative period were not demonstrated. Saline treatment is recommended when the duration of treatment is >3 weeks and up to 3 months after ESS. A large-volume device is preferred. Hypertonic and isotonic saline showed benefits on subjective and objective outcomes. Due to the potential of increasing pain and irritations caused by hypertonic saline, isotonic saline is recommended for the postoperative period after ESS. Buffered and nonbuffered saline can be used. Characteristics of the studies are displayed in Table 5 .
Table 5.
Studies of Saline Treatment During Postoperative Period After Endoscopic Sinus Surgery: Adults. a
| First Author | Year | Study type | Patients b | Saline type c | Buffered | Devices c | Treatment duration | Outcomes | Main result |
|---|---|---|---|---|---|---|---|---|---|
| Wang 71 | 2020 | RCT | 93: adult, CRSwNP | I: Hypertonic seawater 2.0% (48). II: Isotonic seawater (45) | I: Yes. II: Yes | Spray. 5 spray/nostril | 3 times/d, 24 wk | SNOT-22, symptoms VAS, LKES, STT at 2, 8, 16, and 24 wk | Hypertonic saline improved edema, crusting, STT more than isotonic saline spray at 8 wk |
| Peric 70 | 2019 | RCT | 30: adult, CRSwNP/AERD | I: Hypertonic seawater 2.3% (15). II: Isotonic saline (15) | I: Yes. II: No | Not mentioned. 60 mL (30 mL/nostril) | 3 times/d, 4 wk | Symptom score, endoscopic score at 1, 7, 14, 21, and 28 d | Hypertonic seawater improved symptoms and/or endoscopic score more than isotonic saline at 7-28 d |
| Chen 73 | 2018 | SRMA (3 studies) | 233: adult, CRS | I: Electrolyzed acid water (1.1%), hypertonic saline 2.7%, Ringer lactate solution. II: Isotonic saline | I: No. II: No | Any | 6 wk to 2 mo | SNOT-22, symptoms VAS, endoscopic score, computed tomography score, eosinophil count, adverse events | No difference in symptom or endoscopic scores among various solutions |
| Giotakis 67 | 2016 | RCT | 158: adult, CRSwNP | I: Emser salt solution 1.175% or isotonic mineral salt mixture (110). II: No saline (48) | I: Yes. II: NA | Squeeze bottle. 250 mL | 2 times/d, 12 mo, | Nasal symptom score, general QoL scores, endoscopic score, missed workday at 3, 6, 9, and 12 mo | Saline irrigation improved symptoms but not nasal mucosa vs no-saline group |
| Macdonald 72 | 2015 | RCT | 86: adult, CRS (55 CRSwNP,31 CRSsNP) | Isotonic saline | Yes | I: Squeeze bottle 240 mL (43). II: Spray, 2 spray/nostril (43) | 2 times/d, 1 mo | SNOT-22, NSS, POSE at 1 mo | Both devices showed significant improvement in all outcomes |
| Jiang 66 | 2014 | RCT | 110: adult, CRS | I: Electrolyzed acid water (1.1%) (36). II: Isotonic saline (35). III: No saline (39) | I: No. II: No. III: NA | Pulsatile nasal irrigator. 250 mL | Once daily, 2 mo (during 1-3 mo postoperative) | SNOT-20, ARM, UPSIT, endoscopic score, VAS (comfortable of device), bacterial culture, STT at 3 mo | No difference among 3 groups |
| Low 69 | 2014 | RCT | 63: adult, CRS (31 CRSwNP, 32 CRSsNP) | I: Isotonic saline (22). II: Hypertonic saline 2.7% (21). III: Ringer lactate solution (20) | I: No. II: No. III: No | Squeeze bottle. 240 mL | 3 times/d, 6 wk | SNOT-20, STT, VAS, endoscopic score at 1, 3, and 6 wk | Ringer solution irrigation showed better SNOT-20 and VAS than both saline types. Hypertonic saline reduced polypoid mucosa more than isotonic saline. |
| Salib 68 | 2013 | RCT | 25: adult, CRS (17 CRSwNP, 8 CRSsNP) | I: Isotonic seawater (25). II: Isotonic saline (25). Act as own control | I: Yes. II: No | I: Spray 2 spray/side (25). II: Squeeze bottle 120 mL (25). Use I and II in separate nostril | 3 times/d, 12 wk | SNOT-22, modified LKES at 2, 4, and 12 wk | Squeeze bottle showed significantly better LKES than spray at 2 and 4 wk |
| Liang 65 | 2008 | RCT | 77: adult-children, CRS | I: Isotonic saline (44). II: No saline (33) | I: Yes. II: NA | Pulsatile nasal irrigator. 250 mL/nostril | Once daily, 3 mo | Symptom score, endoscopic score at 2 wk, 1, 2, and 3 mo | Saline irrigation benefited mild CRS |
| Freeman 64 | 2008 | RCT | 17: adult, CRS | I: Isotonic saline on one nostril (17). II: No saline on the other nostril (17) | I: No. II: NA | Atomized douching device. 2 mL into the one side | 3 times/d, 6 wk | Endoscopic finding: adhesions, polyps, crusting, discharge, edema at 3 wk and 3 mo | Saline douching reduced nasal discharge; no difference in long-term endoscopic findings |
| Pinto 63 | 2006 | RCT | 60: adult, CRS | I: Isotonic saline (20). II: Hypertonic saline 3.0% (20). III: No saline (20) | I: No. II: Yes. III: NA | Spray. 2 sprays/nostril | 4 times/d, 5 d | Nasal symptoms, medication used at everyday from 1 to 5 | No difference of saline spray vs no saline |
Abbreviations: AERD, aspirin-exacerbated respiratory disease; ARM, acoustic rhinometry; CRS, chronic rhinosinusitis; CRSsNP, chronic rhinosinusitis without nasal polyp; CRSwNP, chronic rhinosinusitis with nasal polyp; LKES, Lund-Kennedy Endoscopic Score; NA, not applicable; NSS, Nasal and Sinus Symptom Score; POSE, perioperative sinus endoscopy; QoL, quality of life; RCT, randomized controlled trial; SNOT, Sino-nasal Outcome Test; STT, saccharin transit time; UPSIT, University of Pennsylvania Smell Identification Test; VAS, visual analog scale.
Roman numerals indicate patients groups.
Patient total number, age group (number per group), and disease.
Number per group in parentheses.
Discussion
Intranasal saline treatment may not be a one-size-fits-all for the different sinonasal diseases. In patients with AR, the saline treatment showed benefits on symptom reduction after 2 to 4 weeks. A large-volume device was recommended for adult AR, but it could cause adverse events in some children. Therefore, a low-volume device was recommended for children. Isotonic saline was suggested due to its effectiveness and tolerability. There were no differences between the buffered and nonbuffered saline. Therefore, either could be chosen.
In adult patients with ARS, the benefits of saline treatment were not revealed in most RCTs. However, symptom and QoL improvements were demonstrated in the common cold subgroup through large-volume saline irrigation. In addition, Ramalingam et al showed a reduction in duration of the common cold. 30 Due to this conflict in high-level evidence, the saline treatment was suggested as an option in adult ARS. The improvements in nasal obstruction and rhinorrhea were demonstrated in children after 5 days of saline treatment and up to 3 weeks. These findings were supported by the majority of RCTs and 1 meta-analysis. Thus, it was recommended for pediatric ARS, and isotonic saline delivered via a large-volume device was suggested.
Improvements in symptoms, QoL, and nasal endoscopic findings were demonstrated in adults with CRS after 4 weeks of nasal saline treatment. Therefore, it was recommended for CRS and should be administered for at least 4 weeks. Buffered isotonic saline delivered by a large-volume device was suggested. The evidence in children was limited to nasal saline drops.
The nasal saline treatment enhances mucociliary function, which benefits patients with CRS-CF. Just saline atomizer was recommended because it was the only device used in 1 study. Nasal saline irrigation after septoplasty/turbinoplasty and ESS reduced crusting and nasal obstruction. Isotonic saline delivered by a large-volume device effectively cleared out the clotted blood, mucous, and debris in the postoperative cavity. Beneficial effects after ESS were demonstrated up to 3 months, and then mixed results were reported. Effects of saline for healing and re-epithelization should be clearly seen during the first 3 months.
This review identified knowledge gaps and research opportunities. The beneficial effects of saline treatment are still unclear in adults with NAR, ARS, and CRS-CF. There is insufficient evidence of saline for pediatric CRS, CRS-CF, and postoperative care. Optimal devices for saline treatment for children with CRS require further studies.
This review had limitations. The recommendations suffered from heterogeneity of delivery methods and timing of evaluations. For instance, the outcomes of nasal saline spray was assessed at 5 days after ESS, while other studies evaluated the larger-volume device after 2 weeks or later. In addition, there were other confounding factors, such as the endotype of CRS that might affect a later stage of postoperative healing.
This review showed different levels of evidence among conditions. AR had very strong evidence supporting the saline treatment, whereas others conditions had relatively much weaker evidence. This review did not intend to discourage the saline usage in those without firmly supportive data. It rather informed how much the evidence currently existed and which delivery methods provided the best possible outcomes based on the available data. Future research is likely to provide or change the answers to a certain topic and/or change the recommendations. Physicians can always use their discrete decision to use the saline treatment or to wait until more evidence becomes available.
Conclusion
Evidence supported the use of the nasal saline treatment for AR, ARS, CRS, CRS-CF, and postoperative patients, but there were no data for NAR. For AR, large-volume devices were effective for treating adults, but low-volume devices were effective for children. Isotonic saline had fewer adverse events than hypertonic saline. For ARS, the evidence supported the use of saline irrigation in children, but there was weak evidence for adults. Large-volume devices were more effective, especially in the common cold subgroup. For CRS, large-volume devices were effective for adults, but saline drop was the only available data in children. Buffered isotonic saline was more tolerable than nonbuffered or hypertonic saline. There were limited data for CRS-CF. For postoperative care, buffered isotonic saline delivered by large-volume devices was effective.
Supplemental Material
Supplemental material, sj-docx-1-opn-10.1177_2473974X221105277 for Optimal Device and Regimen of Nasal Saline Treatment for Sinonasal Diseases: Systematic Review by Wirach Chitsuthipakorn, Dichapong Kanjanawasee, Minh P. Hoang, Kachorn Seresirikachorn and Kornkiat Snidvongs in OTO Open: The Official Open Access Journal of the American Academy of Otolaryngology-Head and Neck Surgery Foundation
Footnotes
Author Contributions: Wirach Chitsuthipakorn, material preparation, study screening, data collection, evidence evaluation, manuscript drafting; Dichapong Kanjanawasee, material preparation, study screening, data collection; Minh P. Hoang, evidence evaluation, manuscript review; Kachorn Seresirikachorn, evidence evaluation, manuscript review; Kornkiat Snidvongs, study concept and design, evidence evaluation, manuscript preparation. All authors approved the final manuscript.
Disclosures: Competing interests: None.
Sponsorships: None.
Funding source: Kornkiat Snidvongs has served on the speaker’s bureau for Merck Sharp Dolme, Viatris, AstraZeneca, and Menarini.
ORCID iD: Wirach Chitsuthipakorn  https://orcid.org/0000-0002-9787-6767
https://orcid.org/0000-0002-9787-6767
Supplemental Material: Additional supporting information is available at http://journals.sagepub.com/doi/suppl/10.1177/2473974X18[article ID]
References
- 1. Hermelingmeier KE, Weber RK, Hellmich M, Heubach CP, Mösges R. Nasal irrigation as an adjunctive treatment in allergic rhinitis: a systematic review and meta-analysis. Am J Rhinol Allergy. 2012;26(5):e119-e125. doi: 10.2500/ajra.2012.26.3787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fokkens WJ, Lund VJ, Hopkins C, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020;58(suppl S29):1-464. doi: 10.4193/Rhin20.600 [DOI] [PubMed] [Google Scholar]
- 3. Head K, Snidvongs K, Glew S, et al. Saline irrigation for allergic rhinitis. Cochrane Database Syst Rev. 2018;6:CD012597. doi: 10.1002/14651858.CD012597.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ural A, Oktemer TK, Kizil Y, Ileri F, Uslu S. Impact of isotonic and hypertonic saline solutions on mucociliary activity in various nasal pathologies: clinical study. J Laryngol Otol. 2009;123(5):517-521. doi: 10.1017/S0022215108003964 [DOI] [PubMed] [Google Scholar]
- 5. Cordray S, Harjo JB, Miner L. Comparison of intranasal hypertonic dead sea saline spray and intranasal aqueous triamcinolone spray in seasonal allergic rhinitis. Ear Nose Throat J. 2005;84(7):426-430. [PubMed] [Google Scholar]
- 6. Rogkakou A, Guerra L, Massacane P, et al. Effects on symptoms and quality of life of hypertonic saline nasal spray added to antihistamine in persistent allergic rhinitis—a randomized controlled study. Eur Ann Allergy Clin Immunol. 2005;37(9):353-356. [PubMed] [Google Scholar]
- 7. Garavello W, Somigliana E, Acaia B, Gaini L, Pignataro L, Gaini RM. Nasal lavage in pregnant women with seasonal allergic rhinitis: a randomized study. Int Arch Allergy Immunol. 2010;151(2):137-141. doi: 10.1159/000236003 [DOI] [PubMed] [Google Scholar]
- 8. Chusakul S, Warathanasin S, Suksangpanya N, et al. Comparison of buffered and nonbuffered nasal saline irrigations in treating allergic rhinitis: nasal saline irrigations in allergic rhinitis. Laryngoscope. 2013;123(1):53-56. doi: 10.1002/lary.23617 [DOI] [PubMed] [Google Scholar]
- 9. Singh R, Galagali JR, Kumar S, Bahurupi Y, Chandrachood M. Comparative study of intranasal hypertonic seawater saline versus intranasal normal saline in allergic rhinitis. Int J Otorhinolaryngol Head Neck Surg. 2016;3(1):104. doi: 10.18203/issn.2454-5929.ijohns20164810 [DOI] [Google Scholar]
- 10. Di Berardino F, Zanetti D, D’Amato G. Nasal rinsing with an atomized spray improves mucociliary clearance and clinical symptoms during peak grass pollen season. Am J Rhinol Allergy. 2017;31(1):40-43. doi: 10.2500/ajra.2016.30.4383 [DOI] [PubMed] [Google Scholar]
- 11. Sansila K, Eiamprapai P, Sawangjit R. Effects of self-prepared hypertonic nasal saline irrigation in allergic rhinitis: a randomized controlled trial. Asian Pac J Allergy Immunol. 2020;38(3):200-207. doi: 10.12932/AP-090618-0331 [DOI] [PubMed] [Google Scholar]
- 12. Piromchai P, Kasemsiri P, Reechaipichitkul W. Squeeze bottle versus syringe nasal saline irrigation for persistent allergic rhinitis—a randomized controlled trial. Rhinology. Published online May 19, 2020. doi: 10.4193/Rhin19.308 [DOI] [PubMed] [Google Scholar]
- 13. Klimek L, Johannssen V, Hundorf I, Hommel G, Hormann K. A nasal rinsing with isoosmotic Emser brine solution is able to reduce drug use in seasonal allergic rhinitis. Allergologie. 2001;24(7):309-315. [Google Scholar]
- 14. Yata K, Srivanitchapoom C. The comparison of nasal irrigation outcome between 3% NaCl and 0.9% NaCl in adults majority with intermittent allergic rhinitis: a randomized double-blind study. Asian Pac J Allergy Immunol. 2021;39(1):9-14. doi: 10.12932/AP-140520-0844 [DOI] [PubMed] [Google Scholar]
- 15. Jung M, Lee JY, Ryu G, et al. Beneficial effect of nasal saline irrigation in children with allergic rhinitis and asthma: a randomized clinical trial. Asian Pac J Allergy Immunol. Published online April 23, 2019. doi: 10.12932/AP-070918-0403 [DOI] [PubMed] [Google Scholar]
- 16. Malizia V, Fasola S, Ferrante G, et al. Efficacy of buffered hypertonic saline nasal irrigation for nasal symptoms in children with seasonal allergic rhinitis: a randomized controlled trial. Int Arch Allergy Immunol. 2017;174(2):97-103. doi: 10.1159/000481093 [DOI] [PubMed] [Google Scholar]
- 17. Chen JR, Jin L, Li XY. The effectiveness of nasal saline irrigation (seawater) in treatment of allergic rhinitis in children. Int J Pediatr Otorhinolaryngol. 2014;78(7):1115-1118. doi: 10.1016/j.ijporl.2014.04.026 [DOI] [PubMed] [Google Scholar]
- 18. Satdhabudha A, Poachanukoon O. Efficacy of buffered hypertonic saline nasal irrigation in children with symptomatic allergic rhinitis: a randomized double-blind study. Int J Pediatr Otorhinolaryngol. 2012;76(4):583-588. doi: 10.1016/j.ijporl.2012.01.022 [DOI] [PubMed] [Google Scholar]
- 19. Marchisio P, Varricchio A, Baggi E, et al. Hypertonic saline is more effective than normal saline in seasonal allergic rhinitis in children. Int J Immunopathol Pharmacol. 2012;25(3):721-730. doi: 10.1177/039463201202500318 [DOI] [PubMed] [Google Scholar]
- 20. Garavello W, Di Berardino F, Romagnoli M, Sambataro G, Gaini RM. Nasal rinsing with hypertonic solution: an adjunctive treatment for pediatric seasonal allergic rhinoconjunctivitis. Int Arch Allergy Immunol. 2005;137(4):310-314. doi: 10.1159/000086462 [DOI] [PubMed] [Google Scholar]
- 21. Garavello W, Romagnoli M, Sordo L, Gaini RM, Di Berardino C, Angrisano A. Hypersaline nasal irrigation in children with symptomatic seasonal allergic rhinitis: a randomized study. Pediatr Allergy Immunol. 2003;14(2):140-143. doi: 10.1034/j.1399-3038.2003.00021.x [DOI] [PubMed] [Google Scholar]
- 22. Li H, Sha Q, Zuo K, et al. Nasal saline irrigation facilitates control of allergic rhinitis by topical steroid in children. ORL. 2009;71(1):50-55. doi: 10.1159/000178165 [DOI] [PubMed] [Google Scholar]
- 23. Wang Y, Jin L, Liu SX, Fan K, Qin ML, Yu SQ. Role of nasal saline irrigation in the treatment of allergic rhinitis in children and adults: a systematic analysis. Allergol Immunopathol (Madr). 2020;48(4):360-367. doi: 10.1016/j.aller.2020.01.002 [DOI] [PubMed] [Google Scholar]
- 24. Li CL, Lin HC, Lin CY, Hsu TF. Effectiveness of hypertonic saline nasal irrigation for alleviating allergic rhinitis in children: a systematic review and meta-analysis. JCM. 2019;8(1):64. doi: 10.3390/jcm8010064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tomooka LT, Murphy C, Davidson TM. Clinical study and literature review of nasal irrigation. Laryngoscope. 2000;110(7):1189-1193. [DOI] [PubMed] [Google Scholar]
- 26. Adam P, Stiffman M, Blake RL. A clinical trial of hypertonic saline nasal spray in subjects with the common cold or rhinosinusitis. Arch Fam Med. 1998;7(1):39-43. doi: 10.1001/archfami.7.1.39 [DOI] [PubMed] [Google Scholar]
- 27. Inanli S, Oztürk O, Korkmaz M, Tutkun A, Batman C. The effects of topical agents of fluticasone propionate, oxymetazoline, and 3% and 0.9% sodium chloride solutions on mucociliary clearance in the therapy of acute bacterial rhinosinusitis in vivo. Laryngoscope. 2002;112(2):320-325. doi: 10.1097/00005537-200202000-00022 [DOI] [PubMed] [Google Scholar]
- 28. Passàli D, Damiani V, Passàli FM, Passàli GC, Bellussi L. Atomized nasal douche vs nasal lavage in acute viral rhinitis. Arch Otolaryngol Head Neck Surg. 2005;131(9):788-790. doi: 10.1001/archotol.131.9.788 [DOI] [PubMed] [Google Scholar]
- 29. Gelardi M, Mezzoli A, Fiorella ML, Carbonara M, Di Gioacchino M, Ciprandi G. Nasal irrigation with lavonase as ancillary treatment of acute rhinosinusitis: a pilot study. J Biol Regul Homeost Agents. 2009;23(2):79-84. [PubMed] [Google Scholar]
- 30. Ramalingam S, Graham C, Dove J, Morrice L, Sheikh A. A pilot, open labelled, randomised controlled trial of hypertonic saline nasal irrigation and gargling for the common cold. Sci Rep. 2019;9(1):1015. doi: 10.1038/s41598-018-37703-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chitsuthipakorn W, Thanaphiphatsatja A, Doungbuppha P, Lawpoolsri S, Seresirikachorn K, Snidvongs K. Effects of large volume, isotonic nasal saline irrigation for acute rhinosinusitis: a randomized controlled study. Int Forum Allergy Rhinol. Published online May 7, 2021. doi: 10.1002/alr.22807 [DOI] [PubMed] [Google Scholar]
- 32. Piromchai P, Phannikul C, Thanaviratananich S. Syringe with nasal applicator versus syringe alone for nasal irrigation in acute rhinosinusitis: a matched-pair randomized controlled trial. BMH. 2021;6(1):25-29. doi: 10.1159/000512664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chanaseeyotin C, Jitaroon K, Ungkhara G, Kulthaveesup A. The effectiveness of intranasal saline irrigation compared with non-irrigation on sinonasal symptoms in acute bacterial rhinosinusitis (ABRS) of adult patient in Department of Otolaryngology Head and Neck Surgery of Faculty of Medicine Vajira Hospital, Navamindradhiraj University. Vajira Med J. 2016;60(2):105-116. doi: 10.14456/vmj.2016.10 [DOI] [Google Scholar]
- 34. Hauptman G, Ryan MW. The effect of saline solutions on nasal patency and mucociliary clearance in rhinosinusitis patients. Otolaryngol Head Neck Surg. 2007;137(5):815-821. doi: 10.1016/j.otohns.2007.07.034 [DOI] [PubMed] [Google Scholar]
- 35. King D, Mitchell B, Williams CP, Spurling GKP. Saline nasal irrigation for acute upper respiratory tract infections. Cochrane Database Syst Rev. 2015;4:CD006821. doi: 10.1002/14651858.CD006821.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bollag U, Albrecht E, Wingert W. Medicated versus saline nose drops in the management of upper respiratory infection. Helv Paediatr Acta. 1984;39(4):341-345. [PubMed] [Google Scholar]
- 37. Šlapak I, Skoupá J, Strnad P, Horník P. Efficacy of isotonic nasal wash (seawater) in the treatment and prevention of rhinitis in children. Arch Otolaryngol Head Neck Surg. 2008;134(1):67. doi: 10.1001/archoto.2007.19 [DOI] [PubMed] [Google Scholar]
- 38. Wang YH, Yang CP, Ku MS, Sun HL, Lue KH. Efficacy of nasal irrigation in the treatment of acute sinusitis in children. Int J Pediatr Otorhinolaryngol. 2009;73(12):1696-1701. doi: 10.1016/j.ijporl.2009.09.001 [DOI] [PubMed] [Google Scholar]
- 39. Köksal T, Çizmeci MN, Bozkaya D, et al. Comparison between the use of saline and seawater for nasal obstruction in children under 2 years of age with acute upper respiratory infection. Turk J Med Sci. 2016;46(4):1004-1013. doi: 10.3906/sag-1507-18 [DOI] [PubMed] [Google Scholar]
- 40. Satdhabudha A, Utispan K, Monthanapisut P, Poachanukoon O. A randomized controlled study comparing the efficacy of nasal saline irrigation devices in children with acute rhinosinusitis. Asian Pac J Allergy Immunol. 2017;35(2):102-107. doi: 10.12932/AP0753 [DOI] [PubMed] [Google Scholar]
- 41. Cabaillot A, Vorilhon P, Roca M, Boussageon R, Eschalier B, Pereirad B. Saline nasal irrigation for acute upper respiratory tract infections in infants and children: a systematic review and meta-analysis. Paediatr Respir Rev. 2020;36:151-158. doi: 10.1016/j.prrv.2019.11.003 [DOI] [PubMed] [Google Scholar]
- 42. Taccariello M, Parikh A, Darby Y, Scadding G. Nasal douching as a valuable adjunct in the management of chronic rhinosinusitis. Rhinology. 1999;37(1):29-32. [PubMed] [Google Scholar]
- 43. Heatley DG, McConnell KE, Kille TL, Leverson GE. Nasal irrigation for the alleviation of sinonasal symptoms. Otolaryngol Head Neck Surg. 2001;125(1):44-48. doi: 10.1067/mhn.2001.115909 [DOI] [PubMed] [Google Scholar]
- 44. Bachmann G, Hommel G, Michel O. Effect of irrigation of the nose with isotonic salt solution on adult patients with chronic paranasal sinus disease. Eur Arch Otorhinolaryngol. 2000;257(10):537-541. doi: 10.1007/s004050000271 [DOI] [PubMed] [Google Scholar]
- 45. Friedman M, Vidyasagar R, Joseph N. A randomized, prospective, double-blind study on the efficacy of dead sea salt nasal irrigations. Laryngoscope. 2006;116(6):878-882. doi: 10.1097/01.mlg.0000216798.10007.76 [DOI] [PubMed] [Google Scholar]
- 46. Nikakhlagh S, Abshirini H, Lotfi M, Mohammad S, Saki N. A comparison between the effects of nasal lavage with hypertonic, isotonic and hypotonic saline solutions for the treatment of chronic sinusitis. J Glob Pharma Technol. 2016;8(12):68-73. [Google Scholar]
- 47. Harvey R, Hannan SA, Badia L, Scadding G. Nasal saline irrigations for the symptoms of chronic rhinosinusitis. Cochrane Database Syst Rev. 2007;3:CD006394. doi: 10.1002/14651858.CD006394.pub2 [DOI] [PubMed] [Google Scholar]
- 48. Čulig J, Leppée M, Vceva A, Djanic D. Efficiency of hypertonic and isotonic seawater solutions in chronic rhinosinusitis. Med Glas (Zenica). 2010;7(2):116-123. [PubMed] [Google Scholar]
- 49. Berjis N, Sonbolastan SM, Okhovat SH, Narimani A, Razmjui JR. Normal saline versus hypertonic 3% saline: it’s efficacy in non-acute rhinosinusitis. Iran J Otorhinolaryngol. 2011;23(1):23-28. doi: 10.22038/IJORL.2011.618 [DOI] [Google Scholar]
- 50. Chong LY, Head K, Hopkins C, et al. Saline irrigation for chronic rhinosinusitis. Cochrane Database Syst Rev. 2016;4:CD011995. doi: 10.1002/14651858.CD011995.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nimsakul S, Ruxrungtham S, Chusakul S, Kanjanaumporn J, Aeumjaturapat S, Snidvongs K. Does heating up saline for nasal irrigation improve mucociliary function in chronic rhinosinusitis? Am J Rhinol Allergy. 2018;32(2):106-111. doi: 10.1177/1945892418762872 [DOI] [PubMed] [Google Scholar]
- 52. Rachana R, Santhi T. Efficacy of saline nasal irrigation in chronic rhinosinusitis. Int J Sci Stud. 2019;7(6):71-77. [Google Scholar]
- 53. Perkasa MF, Ahmad A, Kadir A, Bahar B. Benefits of standard therapy with nasal irrigation using NACL 0.9% on chronic rhinosinusitis patients without polyp. Indian Journal of Public Health Research and Development. 2019;10(8):1357. doi: 10.5958/0976-5506.2019.02085.0 [DOI] [Google Scholar]
- 54. Muthubabu K, Srinivasan MK, Alagammai S, et al. A comparative study in the management of chronic rhinosinusitis by nasal douching with hypertonic saline vs isotonic saline. Indian J Otolaryngol Head Neck Surg. Published online February 8, 2020. doi: 10.1007/s12070-020-01811-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Liu L, Pan M, Li Y, Tan G, Yang Y. Efficacy of nasal irrigation with hypertonic saline on chronic rhinosinusitis: systematic review and meta-analysis. Braz J Otorhinolaryngol. 2020;86(5):639-646. doi: 10.1016/j.bjorl.2020.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sudhakaran SM, Tharayil U, Kallidanthil SD. Comparison of efficacy of hypertonic saline versus normal saline in the treatment of chronic rhinosinusitis with the help of CT: PNS. J Evolution Med Dent Sci. 2016;5(16):790-794. doi: 10.14260/jemds/2016/183 [DOI] [Google Scholar]
- 57. Kumar RA, Viswanatha B, Krishnamurthy N, Jayanna N, Shetty DR. Efficacy of hypertonic saline and normal saline in the treatment of chronic sinusitis. Int J Otorhinolaryngol Head Neck Surg. 2013;2(3):90-96. doi: 10.4236/ijohns.2013.23022 [DOI] [Google Scholar]
- 58. Shoseyov D, Bibi H, Shai P, Shoseyov N, Shazberg G, Hurvitz H. Treatment with hypertonic saline versus normal saline nasal wash of pediatric chronic sinusitis. J Allergy Clin Immunol. 1998;101(5):602-605. doi: 10.1016/S0091-6749(98)70166-6 [DOI] [PubMed] [Google Scholar]
- 59. Rabago D, Zgierska A, Mundt M, Barrett B, Bobula J, Maberry R. Efficacy of daily hypertonic saline nasal irrigation among patients with sinusitis: a randomized controlled trial. J Fam Pract. 2002;51(12):1049-1055. [PubMed] [Google Scholar]
- 60. Mainz JG, Schumacher U, Schädlich K, et al. Sino nasal inhalation of isotonic versus hypertonic saline (6.0%) in CF patients with chronic rhinosinusitis—results of a multicenter, prospective, randomized, double-blind, controlled trial. J Cyst Fibros. 2016;15(6):e57-e66. doi: 10.1016/j.jcf.2016.05.003 [DOI] [PubMed] [Google Scholar]
- 61. Süslü N, Bajin MD, Süslü AE, Öğretmenoğlu O. Effects of buffered 2.3%, buffered 0.9%, and non-buffered 0.9% irrigation solutions on nasal mucosa after septoplasty. Eur Arch Otorhinolaryngol. 2009;266(5):685-689. doi: 10.1007/s00405-008-0807-5 [DOI] [PubMed] [Google Scholar]
- 62. Kurtaran H, Ugur KS, Yilmaz CS, et al. The effect of different nasal irrigation solutions following septoplasty and concha radiofrequency: a prospective randomized study. Braz J Otorhinolaryngol. 2018;84(2):185-190. doi: 10.1016/j.bjorl.2017.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pinto JM, Elwany S, Baroody FM, Naclerio RM. Effects of saline sprays on symptoms after endoscopic sinus surgery. Am J Rhinol. 2006;20(2):191-196. [PubMed] [Google Scholar]
- 64. Freeman SRM, Sivayoham ESG, Jepson K, de Carpentier J. A preliminary randomised controlled trial evaluating the efficacy of saline douching following endoscopic sinus surgery. Clin Otolaryngol. 2008;33(5):462-465. doi: 10.1111/j.1749-4486.2008.01806.x [DOI] [PubMed] [Google Scholar]
- 65. Liang KL, Su MC, Tseng HC, Jiang RS. Impact of pulsatile nasal irrigation on the prognosis of functional endoscopic sinus surgery. J Otolaryngol Head Neck Surg. 2008;37(2):148-153. [PubMed] [Google Scholar]
- 66. Jiang RS, Liang KL, Wu SH, Su MC, Chen WK, Lu FJ. Electrolyzed acid water nasal irrigation after functional endoscopic sinus surgery. Am J Rhinol Allergy. 2014;28(2):176-181. doi: 10.2500/ajra.2014.28.4015 [DOI] [PubMed] [Google Scholar]
- 67. Giotakis AI, Karow EM, Scheithauer MO, Weber R, Riechelmann H. Saline irrigations following sinus surgery—a controlled, single blinded, randomized trial. Rhinology. 2016;54(4):302-310. doi: 10.4193/Rhin16.026 [DOI] [PubMed] [Google Scholar]
- 68. Salib RJ, Talpallikar S, Uppal S, Nair SB. A prospective randomised single-blinded clinical trial comparing the efficacy and tolerability of the nasal douching products Sterimar and Sinus Rinse following functional endoscopic sinus surgery. Clin Otolaryngol. 2013;38(4):297-305. doi: 10.1111/coa.12132 [DOI] [PubMed] [Google Scholar]
- 69. Low THH, Woods CM, Ullah S, Carney AS. A double-blind randomized controlled trial of normal saline, lactated Ringer’s, and hypertonic saline nasal irrigation solution after endoscopic sinus surgery. Am J Rhinol Allergy. 2014;28(3):225-231. doi: 10.2500/ajra.2014.28.4031 [DOI] [PubMed] [Google Scholar]
- 70. Perić A, Kovačević SV, Barać A, Gaćeša D, Perić AV, Jožin SM. Efficacy of hypertonic (2.3%) sea water in patients with aspirin-induced chronic rhinosinusitis following endoscopic sinus surgery. Acta Otolaryngol. 2019;139(6):529-535. doi: 10.1080/00016489.2019.1605454 [DOI] [PubMed] [Google Scholar]
- 71. Wang J, Shen L, Huang ZQ, et al. Efficacy of buffered hypertonic seawater in different phenotypes of chronic rhinosinusitis with nasal polyps after endoscopic sinus surgery: a randomized double-blind study. Am J Otolaryngol. 2020;41(5):102554. doi: 10.1016/j.amjoto.2020.102554 [DOI] [PubMed] [Google Scholar]
- 72. Macdonald KI, Wright ED, Sowerby LJ, et al. Squeeze bottle versus saline spray after endoscopic sinus surgery for chronic rhinosinusitis: a pilot multicentre trial. Am J Rhinol Allergy. 2015;29(1):e13-e17. doi: 10.2500/ajra.2015.29.4125 [DOI] [PubMed] [Google Scholar]
- 73. Chen XZ, Feng SY, Chang LH, et al. The effects of nasal irrigation with various solutions after endoscopic sinus surgery: systematic review and meta-analysis. J Laryngol Otol. 2018;132(8):673-679. doi: 10.1017/S0022215118000919 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-opn-10.1177_2473974X221105277 for Optimal Device and Regimen of Nasal Saline Treatment for Sinonasal Diseases: Systematic Review by Wirach Chitsuthipakorn, Dichapong Kanjanawasee, Minh P. Hoang, Kachorn Seresirikachorn and Kornkiat Snidvongs in OTO Open: The Official Open Access Journal of the American Academy of Otolaryngology-Head and Neck Surgery Foundation